Abstract
Owing to the advances in genome editing technologies, research on human pluripotent stem cells (hPSCs) have recently undergone breakthroughs that enable precise alteration of desired nucleotide bases in hPSCs for the creation of isogenic disease models or for autologous ex vivo cell therapy. As pathogenic variants largely consist of point mutations, precise substitution of mutated bases in hPSCs allows researchers study disease mechanisms with “disease-in-a-dish” and provide functionally repaired cells to patients for cell therapy. To this end, in addition to utilizing the conventional homologous directed repair system in the knock-in strategy based on endonuclease activity of Cas9 (i.e., ‘scissors’ like gene editing), diverse toolkits for editing the desirable bases (i.e., ‘pencils’ like gene editing) that avoid the accidental insertion and deletion (indel) mutations as well as large harmful deletions have been developed. In this review, we summarize the recent progress in genome editing methodologies and employment of hPSCs for future translational applications.
Keywords: Human pluripotent stem cells, Disease modeling, Ex vivo therapy, Isogenic pair, Base editors, Prime editor, Base substitution, Cas9
Background
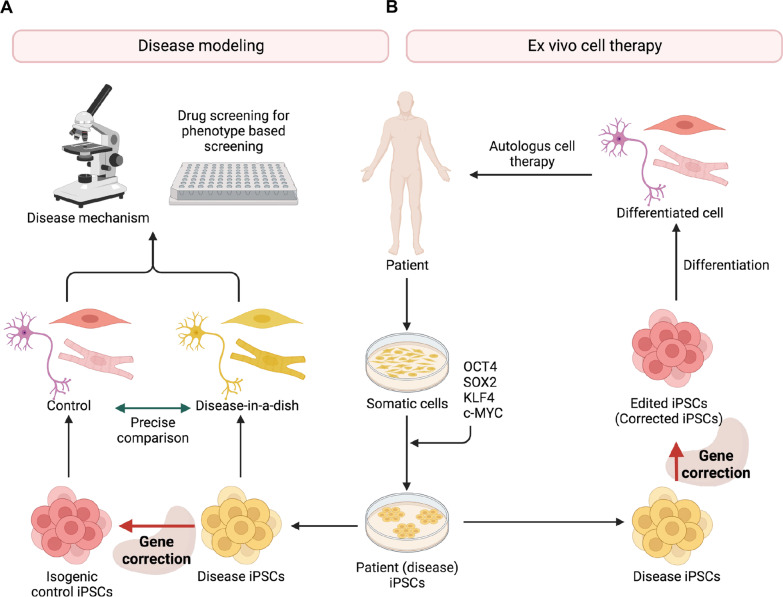
The establishment of induced pluripotent stem cells (iPSCs) from human somatic cells [1] was a breakthrough not only for regenerative medicine to enable the autologous stem cell therapy but also for generating cells of any type with pathogenic phenotypes for drug discovery [2]. Thus, soon after the discovery of iPSCs, patient iPSCs harboring pathogenic mutations have been established [3] with the aims of (i) understanding the underlying mechanisms of disease and (ii) utilizing the cellular platform to assess candidate drugs based on disease phenotypes (i.e., phenotype-based drug screening; Fig. 1A) [4]. The new terminology “diseases-in-a-dish” was coined to indicate the cell-type specificity of the cells derived from patient iPSCs to reveal the pathogenic characteristics (or pathogenic phenotypes) [5]. However, the differences in cellular characteristics originating from differences in genetic backgrounds of individual patients are frequently more robust than those associated with the disease itself, which complicates the process of comparative analysis. Thus, genome editing techniques capable of specifically targeting desired sequences are essential for the establishment of isogenic pairs of disease and control human pluripotent stem cells (hPSCs) to enable “precise comparison” [6]. Furthermore, the success of the first autologous stem cell therapy utilizing cells derived from iPSCs for the Parkinson’s disease [7] opens a new chapter for autologous stem cell therapy [8]. In parallel with autologous stem cell therapy for degenerative diseases, functionally intact (i.e., devoid of mutations) cells derived from the edited forms of iPSCs initially obtained from the patients constitute a promising source to treat diverse genetic diseases through ex vivo cell therapy (Fig. 1B). Therefore, soon after their development, the efficacy and safety of new genome editing techniques have been extensively validated in hPSCs for their potential in translational applications [9–12].
Fig. 1.
Application of patient derived iPSCs for disease modeling and cell therapy (A) Establishment of patient derived iPSCs (or disease iPSCs) allows the production of somatic cells with pathogenic phenotypes (i.e., “Disease-in-a-dish”), which would be ultimate cell source to study the molecular mechanism to underlying disease and to screen small molecules to revert the phenotypes. Gene correction is critical to produce the isogenic control iPSCs to enable the precise comparison to avoid the variation from the different genetic background. (B) Autologous cell therapy from the patient with a genetic disease is achieved by gene correction of pathogenic mutations from disease iPSCs. The functionally intact somatic cells from the edited iPSCs serve as ideal cell source for reconstitution of specific organ with disease phenotype. Created with BioRender.com
Toolbox for precise genome editing in hPSCs
Point mutations (58%) and deletions (25%) account for the majority of pathogenic variants associated with human genetic diseases [13]. Thus, various genome editing tools for the precise correction of pathogenic mutations and for the insertion of missing sequences have been developed for potential clinical applications.
Development of programmable nucleases
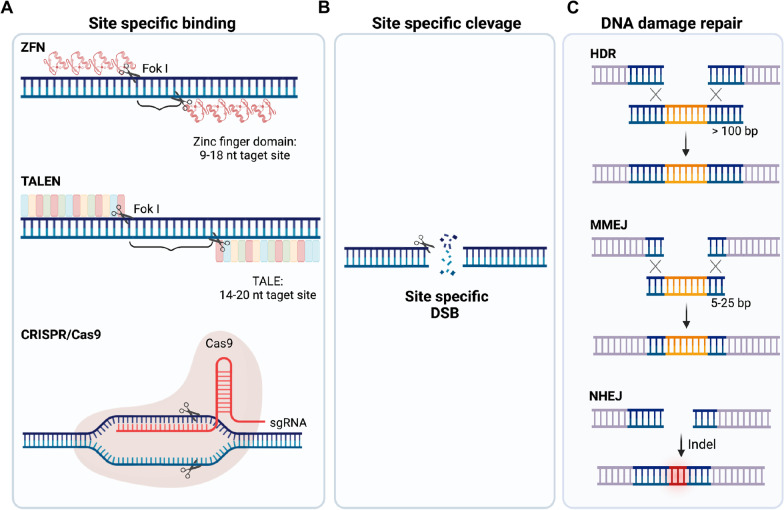
In order to manipulate genomic sequences in a programmable manner, various nucleases such as zinc finger nucleases (ZFNs) [14], transcription activator-like effector nucleases (TALENs) [15], and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) system have been developed [16–19]. These programmable nuclease systems (i.e., editing tools) consist of a “DNA binding module” to guide the system to a specific DNA sequence and a “DNA-cleavage module” to cleave the target DNA sequence [20]. Upon the recruitment of “DNA-cleavage module” to the target site by the “‘DNA binding module” (Fig. 2A), site-specific cleavage occurs inducing a double strand break (DSB) through the action of “DNA-cleavage module” (Fig. 2B).
Fig. 2.
Gene editing procedure of typical programmable genome editing tools (A) The zinc-finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), or CRISPR/Cas9 nuclease recognize target sequence in genome (i.e., “site specific binding”) by zinc-finger domain, transcription activator-like effector (TALE), or single guide-RNA (sgRNA) respectively. (B) The ZFN / TALEN and CRISPR/Cas9 induce “site specific cleavage” of DNA via FokI nuclease and Cas9 endonuclease respectively. (C) Upon DNA damage by activity of endonucleases, innate DNA damage repair system repair DNA. Site specific gene insertion from donor DNA for knock-in is achieved by homology directed repair (HDR) and micro-homology mediated end joining (MMEJ). Insertion or deletion (Indel), leading to functional knock-out occurs by non-homologous end joining (NHEJ). Created with BioRender.com
ZFN and TALENs commonly use FokI endonuclease for inducing a DSB at target sites, which is led by specific binding to target sequence of either zinc finger domain [14] or transcription activator-like effector (TALE) protein, respectively [15]. Similarly, the site-specific DNA cleavage in CRISPR/Cas system (like scissors) is conducted by single guide RNA (sgRNA) and conjugated Cas9 endonucleases [16–19]. Gene editing occurs at the site of DNA cleavage by the Cas9 endonuclease activity during the process of DNA damage repair (Fig. 2C). The desired DNA sequences from the accompanied donor DNA are inserted into the damaged DNA site [achieving knock-in (KI)] through the innate homology directed repair (HDR) or microhomology-mediated end joining (MMEJ) pathways [21, 22]. In parallel with HDR, non-homologous end joining (NHEJ) repair, an error-prone DSB repair mechanism dominantly occurring upon DSB produces random insertion or deletion (indel) mutations, leading to functional knock-out (KO) due to frame-shift (Fig. 2C). It is well-documented that 75% of DSBs are repaired by NHEJ and the remaining 25% are repaired by HR. This overall 3:1 ratio between NHEJ and HR [23] in the mammalian cells, which is altered in a cell cycle-dependent manner [24], accounts for the majority of NHEJ-associated indel mutations over HDR mediated KI by Cas9. Thus, the inevitable indel mutations for precise genome editing (base substitution or insertion) in hPSCs require the additional laborious clonal selection [21]. Alternatively, newly developed editing tools to be programmed precisely editing the desired bases (like pencil) without inducing DSBs, rather than just cutting the target DNA (like scissors), are highlighted.
Base editors
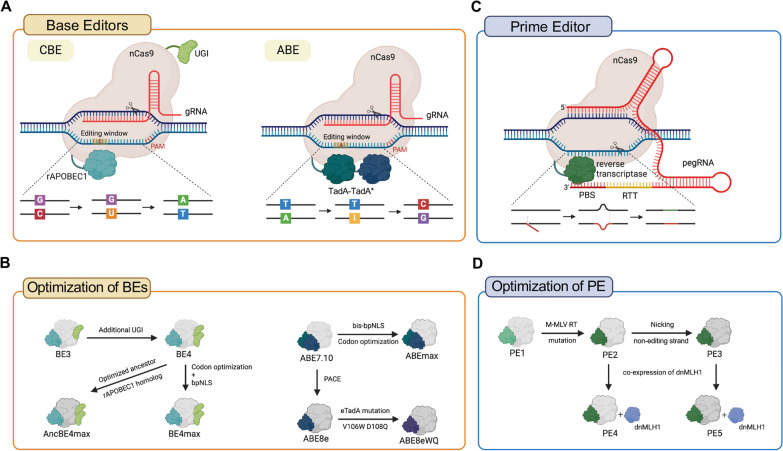
Base editors (BEs) use a deaminase linked to modified Cas proteins (unable to induce DSBs due to lack of endonuclease activity) for the site-specific base substitution [25]. Cytosine base editor (CBE) produces C:G to T:A base substitution through the action of cytosine deaminase (e.g., rat APOBEC1 [rAPOBEC1]) conjugated to nickase Cas9 (nCas9) [26]. The original version of CBE (BE3) is further optimized by adding uracil glycosylase inhibitor (UGI) resulting in BE4 for improved efficiency and product purity [27]. Additional optimization and improvement based on BE4 is continuously carried out. For example, the updated versions of CBE (BE4max and AncBE4max [28]) are produced by codon optimization or adoption of optimized ancestor rAPOBEC1 homolog (Fig. 3A). Adenine base editor (ABE) induces A:T to G:C point mutation by deaminating A via engineered adenine deaminase (e.g., TadA7.10) linked to nCas9 [29]. The original version, ABE7.10, is upgraded by the replacement of the nuclear localization sequence (NLS) with a bipartite NLS linked to both N-terminus and C-terminus (bis-bpNLS) and codon optimization (ABEmax) [28]. The adenine deaminase TadA7.10 is also improved by phage-assisted non-continuous and continuous evolution (PACE) to produce ABE8e and ABE8eWQ by introducing further point mutations in TadA8e (V106W and D108Q) [30] (Fig. 3A). In addition to transition mutations, C-to-G base substitution is achieved by C-to-G base editors (CGBE1) composed of an E. coli-derived uracil DNA glycosylase (eUNG) and mutant rAPOBEC1 fused to nCas9 [31] through the induction of apurinic/apyrimidinic site (AP site) by UNG activity.
Fig. 3.
Molecular modules of BEs and PE (A) Base editors consist of nickase Cas9 (nCas9) and deaminase. CBE adopts rAPOBEC deaminase for cytosine deamination. For further improvement, uracil DNA glycosylase inhibitor (UGI) is conjugated. ABE adopt two deaminases (TadA-TadA*) composed of wild type TadA and engineered TadA (TadA*). (B) Editing efficiency and product purity of BEs are continuously improved by optimization of BEs. The original version of CBE (BE3) is further optimized to BE4, BE4max or AncBE4max with additional UGI, codon optimization and/or adoption of ancestor rAPOBEC1 homolog. The original version of ABE, ABE7.10, is optimized to ABEmax by codon optimization and adoption of bis-bpNLS. Further engineering TadA* by PACE or induction of specific mutations (e.g., V106W and D108Q) produces ABE8e and ABE8eWQ. (C) PE is composed of engineered reverse transcriptase (i.e., M-MLV RT) linked to nCas9 and PE guide RNA (pegRNA). M-MLV RT synthesizes DNA strand containing desired edit sequences. The edit strand is inserted into the target sequence. (D) The original version of PE (i.e., PE1) is optimized to PE2 by induction of mutation on M-MLV RT. PE3 is developed by nicking non-editing strand. Co-expression of dnMLH1 with PE2 and PE3, to further improve the efficiency produces PE4 and PE5 respectively. Created with BioRender.com
Prime editors
Unlike BEs, which can induce only certain types of point mutations (transition and C-to-G mutations), prime editors (PEs) can induce not only all 12 types of transition/transversion point mutations but also insertions and deletions without inducing DSB and requiring donor DNA [32]. PEs conduct precise genome editing by synthesizing DNA with desired mutation on the target site via PE gRNA (pegRNA) and engineered Moloney murine leukemia virous (M-MLV) reverse transcriptase (RT) [32]. After nCas9 induces DNA single strand break (SSB), primer binding site (PBS) of pegRNA binds to cleaved single strand DNA and allows RT to synthesize the DNA strand complementary to reverse transcriptase template (RTT) containing the editing information [32]. Nicking non-editing strand during prime editing (PE3) dramatically increases PE efficiency. Furthermore, co-expression of dominant negative MLH1(MutL Homolog 1) is applied to PE system (in PE4 and PE5) resulting in a significant increase in PE efficiency (Fig. 3B) [33, 34].
Unique cellular characteristic of hPSCs affecting genome editing outcome
The maintenance of genome integrity, highly developed in human embryonic stem cells (hESCs), is one of the most distinct cellular characteristics of hESCs compared to somatic cells [35]. Thus, spontaneous mutation frequency in hESCs during in vitro culturing is 40-fold lower than those in other somatic cells [36]. This unique feature is achieved by drastic sensitivity to DNA damage stress and highly developed DNA damage repair systems in hESCs [35]. It is noteworthy that iPSCs of which most of cellular characteristics share those of hESCs [1, 37], showing similar DNA damage responses such as hypersensitivity [38] and active DNA damage repair [39, 40]. The common cellular characteristics of hESCs and iPSCs (i.e., hPSCs) upon DNA damage are well summarized in multiple review articles [35, 37, 41, 42]. As various types of DNA damage, including DSB, single strand break (SSB), or mismatch, inevitably occurs by genome editing procedure, the editing outcomes in hPSCs would not be identical to those in somatic cell lines.
High susceptibility to DNA damage stimuli
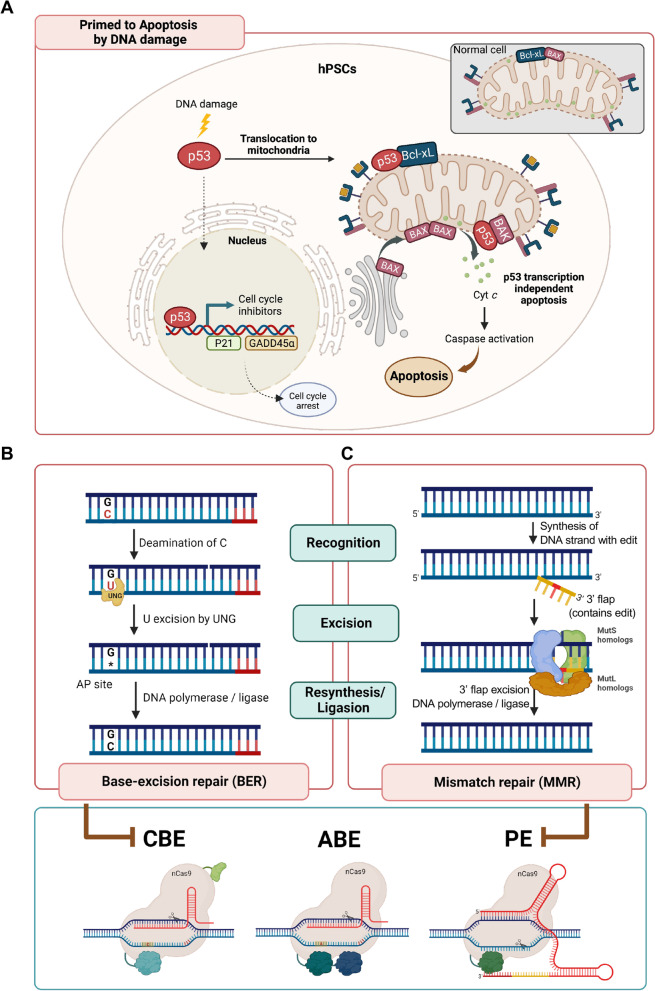
A well-characterized tumor suppressor mediating diverse stress responses, p53, is readily stabilized by genotoxic stress and triggers either apoptosis or cell cycle arrest in a transcription-dependent manner [43]. Unlike somatic cells, which induce cell cycle arrest through p53-dependent gene expression of cell cycle inhibitors, hESCs tend to undergo massive cell death upon even slight genotoxic stress through the action of p53 [44]. In particular, p53 is preferably translocated into the mitochondria to prime apoptosis in hESCs [45] and iPSCs [38]. The following disruption of the mitochondrial membrane permeability (MMP) by direct interaction to BAK [46] or BCL-xL [47] to activate BAX activation [48], which leads to cytochrome C (Cyt C) release to trigger mitochondria-dependent apoptosis in hESCs upon DNA damage (i.e., p53 transcription-independent apoptosis [47]) [38, 45] (Fig. 4A). Furthermore, elevated expression of pro-apoptotic factors [38] as well as prompt translocation of active BAX, a pro-apoptotic member of BCL2 family, to mitochondria [49] accounts for the high susceptibility to DNA damage in hPSCs [50]. Accordingly, p53 activation in response to DSB induction by Cas9 endonuclease activity [51] leads to massive cell death in hPSCs, which accounts for the lower editing efficiency in hPSCs [52]. Of note, p53 activation in hPSCs also occurs as a result of nCas9 activity, which induces single strand break. Thus, editing efficiencies of BEs (both ABE and CBE) and PEs are enhanced upon genetic perturbation of TP53 in both hESCs and iPSCs [11, 53].
Fig. 4.
Unique cellular characteristic of hPSCs affecting genome editing outcome (A) hPSCs are highly susceptible to DNA damage (Primed to apoptosis). Upon DNA damage, p53 preferably translocates to mitochondria disrupting the mitochondrial membrane permeability (MMP) by direct interaction to BCL2-xL or BAK. Disrupted MMP induce cytochrome C (Cyt C) release into cytosol, which provokes mitochondrial dependent apoptosis. The transcription of cell cycle inhibitors by p53 to induce cell cycle arrest is markedly attenuated in hPSCs. (B) Deamination of C, producing U activates BER. U is readily recognized and removed to produce AP site by DNA glycosylase such as UNG. The high BER activity in hPSCs affects CBE outcomes. (C) Prime editor (PE) synthesizes DNA strand containing edit (3’ flap). The 3’ flap bound to non-editing strand is recognized by MutS and MutL homologs, major components of mismatch repair (MMR). Highly active MMR determines PE efficiency. Created with BioRender.com
Active DNA repair systems
As the genome editing is achieved by DNA damage and consequent activity of DNA damage repair systems, the highly activated DNA damage repair pathway in hPSCs [54, 55] affects the genome editing outcomes. In particular, base excision repair (BER) targets DNA damage formed by spontaneous deamination, alkylation, or oxidation of bases [56]. These damaged bases are recognized and removed by diverse types of DNA glycosylases, including UNG, TDG, and MBD4 [57] (Fig. 4B). C-to-U deamination, the most frequent spontaneous alteration occurring in somatic cells, is a significant cause of somatic C-to-T mutations [58]. To minimize the formation of C-to-T mutations, presence of U is promptly recognized by multiple DNA glycosylases (UNG, MBD4, and TDG) to produce an AP site. Unlike UNG, which mainly recognizes G:U and A:U mismatches, TDG and MBD4 also recognize G:T mismatches [57]. Importantly, the intermediate deaminated DNA products such as U:G from C:G (by CBE) and I:T from A:T (by ABE) are recognized and removed by UNG, MBD4, TDG [57, 59, 60] and MPG [61], respectively. Recent studies have revealed that the frequency of C-to-T transition with CBE is significantly lower than that of A-to-G transition with ABE exclusively in hPSCs. Among the three typical DNA glycosylases UNG, TDG, and MBD4, which exhibit downregulated expression levels during differentiation of hPSCs, UNG has been identified as the main player to impede the editing outcome of CBE (i.e., editing efficiency and product purity) [11] (Fig. 4B).
Similarly, short nucleotide sequences produced by reverse transcriptase (RT) conjugated with nCas9 in PEs (e.g., PE2 [32]) trigger mismatch repair (MMR) activation [32]. The intermediate product formed by the annellation of 3’-flap to non-editing strand and excision of the original strand (5’-flap) is recognized by three human MutS homologs (hMSH2, hMSH3, and hMSH6), initiating mismatch repair (MMR) activation (Fig. 4C). Thus, transient interference of MMR activity by inhibition of MutL homologs improves the editing outcome of PEs [33]. Accordingly, high expression levels of MSH2 and MSH6 reflecting the activity of MutSα (MSH2-MSH6 complex) and MutSβ (MSH2-MSH3 complex) in hPSCs serve as major determinants of editing outcome of PE in hPSCs [62].
Applications of “pencil” in hPSCs
As the significance of gene editing in hPSCs is highlighted [63–65], HDR-mediated KI with Cas9 has been extensively applied to hPSCs soon after its development. The low efficiency of HDR mediated KI in hPSCs has also been improved by a number of methodologies [10, 66, 67]. As a result, Cas9 has become a standardized approach for gene perturbation or correction in hPSCs as evidenced by numerous review articles [68–70]. However, the recently developed pencil like-editing tools (i.e., BEs and PEs) have not been widely utilized in hPSCs in comparison to HDR-mediated KI with Cas9. In this section, we have summarized a few examples of their usage in hPSCs (Table 1).
Table 1.
Base substitution in hPSCs with base editing tools
| Disease | Cell type | Editing tool | Mutations | Phenotype | Reference | |
|---|---|---|---|---|---|---|
| Long QT (LQT) | hESCs, hiPSCs | ABE | L114P, R190Q | KCNQ1 | Prolonged QT beating interval | Qi et al. [72] |
| Y616C, Y475C | KCNH2 | |||||
| GNE myopathy | hESCs, hiPSCs | ABE | I329T, I588T | GNE | Reduced sialic acid production in hPSCs and myoblasts | Park et al. [9] |
| CBE | R160Q, V727M | |||||
| Recessive Dystrophic Epidermolysis Bullosa (RDEB) | Patient-derived iPSC | ABE | R185* (*non-sense mutation) | COL7A1 | Deposition of C7 at the dermal–epidermal junction | Osborn et al. [74] |
| Duchenne muscular dystrophy (DMD) | Patient-derived iPSC | ABE | Exon 50 skipping | DMD | Restoration of dystrophin protein level in differentiated cardiomyocyte | Chemello et al. [76], Yuan et al. [75], Wang et al. [79] Eberherr et al. [77] |
| PE | Exon 52 reframing | |||||
| (GT insertion) | ||||||
| CBE | Modulating mRNA splicing | |||||
| DMD hiPSCs established by CRISPR-Cas9 gene editing | ABE | Exon 55 skipping | ||||
| STAT3-Hyperimmuno-globulin | Patient-derived iPSC | ABE | R382W | STAT3 | Restoration of STAT3 downstream signaling | |
| E syndrome (STAT3-HIES) | ||||||
| Parkinson’s disease (PD) | Patient-derived iPSC | ABE | G2019S | LRRK2 | Reduced LPRRK2 kinase activity, decreased phospho-α-synuclein expression, mitigated neurite shrinkage, apoptosis and restored impaired neurite outgrowth in differentiated dopaminergic neuron | Chang et al. [78] |
| iPSCs, hESCs | PE |
G2019S A30P |
LRRK2 SNCA |
n.a | Li et al. [80] | |
| Dilated cardiomyopathy (DCM) | iPSCs | ABE | R634Q | RBM20 | Normal distribution of RBM20 in cardiomyocytes, TTN splicing pattern, and expression of N2B | Nishiyama et al. [81] |
| PE | R636S | |||||
Disease modeling in hPSCs starts with the introduction of point mutations into normal hPSCs. Once the disease iPSCs harboring pathogenic mutations are established, the pathogenic phenotypes are determined in cell types of interest after differentiation, in comparison to the isogenic control cells. It is also noteworthy that point mutations of which pathogenicity has not been fully characterized (i.e., variants of uncertain significance; VUS) could be experimentally examined by the comparison of disease models with clear pathogenic phenotypes. For example, hPSCs with point mutations occurring in patients of GNE myopathy (also known as hereditary inclusion body myopathy; HIBM), an autosomal recessive degenerative skeletal muscle disorder, were established using base editors [9]. As decreased sialic acid production, a final product of GNE (glucosamine UDP-N-acetyl-2-epimerase/N-acetylmannosamine kinase) due to loss of function mutations in epimerase or kinase domain of GNE, is closely associated with the pathogenicity of GNE myopathy, the levels of sialic acid production in each mutant hPSCs or myoblasts derived from these mutant iPSCs (including one VUS) have been used to predict the clinical significance [9].
Congenital long QT syndrome (LQTS), classified into LQT1, LQT2, and LQT3, arises from the mutations in KCQN1, KCNH2, and SCN5A, respectively [71]. A recent study has established five LQTS disease hPSC models including two LQT1, two LQT2, and one LQT3 and characterized the pathogenic phenotypes of LQTS from cardiomyocytes from hPSCs. Of note, one LQT3 model with a novel mutation identified in a Brugada syndrome (BrS) patient recapitulates BrS phenotypes at the cellular level [72]. Also, an independent protocol article has been published describing the generation of hPSCs carrying pathogenic LQTS mutations using base editors [73].
Correction of pathogenic mutations from patient derived iPSCs further strengthens the advantages of using hPSC for autologous cell therapy due to avoidance of immunological issues. Accordingly, base substitutions are performed in patient-derived iPSCs, followed by validation of restored cellular phenotypes. For example, iPSCs of patients with recessive dystrophic epidermolysis bullosa (RDEB) caused by nonsense mutations in COL7A1 gene are edited using ABE. As nonsense mutations in COL7A1 lead to failure of production of type VII collagen (C7), the phenotypic correction after base editing is readily examined by restoration of C7 expression not only in differentiated cell type but also in teratoma formed in mouse model [74]. Similarly, out-of-frame deletions typically occurring at exon 51 of iPSCs from Duchenne muscular dystrophy (DMD) patients are corrected using base editors. The phenotypic restoration after base correction is assessed by restoration of dystrophin protein expression in cardiomyocytes differentiated from mutation-corrected iPSCs [75]. A similar procedure is carried out in DMD iPSC model (∆Ex51 iPSCs), which is derived from a normal iPSC line. The introduction of a single nucleotide transition at the splice donor site of exon 50 induces exon skipping, and its correction restores dystrophic expression in cardiomyocytes [76]. Furthermore, prime editing is applied in ∆Ex51 iPSC-derived cardiomyocytes directly to achieve the functional recovery of cardiomyocytes [76].
The patient iPSCs from STAT3-Hyperimmunoglobulin E syndrome (HIES), a primary immunodeficiency disease due to heterozygous STAT3 mutation, are base-edited using ABE to restore STAT3 signaling [77]. As previously described [9], base editors, especially ABE, are more efficient for base correction of leucine-rich kinase2 (LRRK2), the dominant gain-of-function mutation in Parkinson’s disease (PD), compared with HDR with no apparent indels or off-target editing [78].
Pros and cons of BEs and PE in hPSCs
Gene pencil rather than gene scissors
Recent studies highlight that application of Cas9 for HDR mediated KI produces large and unexpected deletions even at the chromosome level [82–84], which raises important safety concerns for its clinical applications. Importantly, isogenic pairs established by HDR-mediated KI from the patient iPSCs are later found to be hemizygous (9 out of 27 iPSCs) due to large on-target defects [85]. Similarly, up to 40% of iPSCs show large mono-allelic genomic deletions and loss-of-heterozygosity when edited with HDR-mediated KI [86]. Such large deletions extending over kilobases near the target sites result from DSB formation by Cas9 endonuclease activity [83] as the use of nCas9, which induce SSBs instead of DSBs [26, 32] significantly reduces large on-target defect [87, 88]. Hence, the use of gene editing tools such as BEs and PE based on nickase activity of nCas9 (gene pencils) is considered safer for translational applications of hPSCs as they can avoid on-target and off-target indels as well as chromosomal deletions, which are frequently observed in HDR-mediated KI [87, 88]. As a result, gene pencil would be a more suitable option for genetic manipulation in hPSCs compared to gene scissors. Additionally, it is worth mentioning that a stepwise protocol for BEs in hPSCs has been recently updated, for successful base substitution in hPSCs [89].
Limitation of BEs and PE
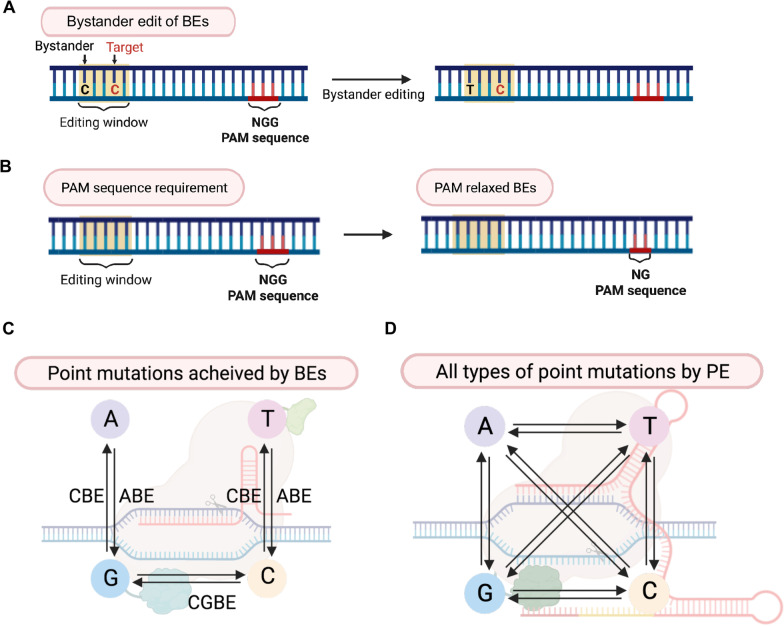
The presence of bystander base(s), a substrate base for deaminase but not a target base, in the editing window (or “activity window”) often produces unintended base substitution, so that laborious clonal selection after base substitution becomes necessary. Base editor variants with narrower activity windows have been developed [90]. Although BEs conduct precise genome editing without introducing DSBs, mutation scope of BEs is confined to specific types of point mutations (e.g., C:G to T:A by CBE, C:G to G:C by CGBE, and A:T to G:C by ABE) [26, 29, 31]. Furthermore, due to the requirement of the PAM sequence at the exact location of the target base, applicability of BEs to point mutations becomes limited [25]. Various versions of BEs with released PAM requirement (e.g., from NGG to NG) or near PAM-less BEs have been developed [91, 92]. PAM-relaxed version of BEs significantly increases the number of pathogenic mutations that can be targeted [9] (Fig. 5A). For example, by replacing BEs (i.e., ABE and CBE with NGG as a PAM) with NG-BEs (i.e., NG-ABE and NG-CBE), accessibility of pathogenic mutations associated with GNE myopathy (OMIM #605,820) extended from 15 to 38% [9]. Similarly, the coverage of mutations associated with Tay-Sachs disease (OMIM#272,800) in NG-BEs (24%) is significantly higher than that in BEs (13%) (unpublished data). Unlike the limited base substitution enabled by currently developed BEs (Fig. 5B), PEs can theoretically replace all types of point mutations as well as indel mutations [32] (Fig. 5C). Unlike HDR-mediated KI, the number of nucleotides inserted by PEs is limited to 44 base pairs [32], which would not be adequate for the targeted integration of a therapeutic gene in patient iPSCs.
Fig. 5.
Limitation of BEs (A) The existence of multiple substrates in the editing windows causes unintended bystander editing. (B) BEs require PAM sequence (red bases) at proper distance from the target base in editing window (yellow box). The PAM-relaxed BEs (e.g., BEs with NG PAM) to extend the coverage of BEs on target mutations are developed. (C) Typical BEs edit only transition mutations. CGBE enables C:G to G:C base substitution. (D) PE edits transition and transversion point mutations. Created with BioRender.com
The limited editing efficiency of BEs and PEs in hPSCs, caused by their unique DNA damage response characterized by p53-dependent cell death and active DNA damage repair, can be improved through temporary modulation of this response. One approach is the use of dominant negative p53 to interfere temporarily with the p53-dependent cellular response, which has been shown to enhance editing outcomes of CBE and PE in hPSCs [53]. Additionally, temporary inhibition of specific DNA damage repair pathways, such as the BER pathway for CBE with UNG depletion [11] or the MMR pathway for PE with dominant negative MLH1 expression [33], has also been found to improve efficiency in hPSCs.
Conclusions
There have been multiple milestones in more than hundred years of stem cell research. A recent review article published in Stem Cell Reports highlighted twenty-five major discoveries in stem cell research [93], which include “nuclear transfer”, “establishment of embryonic stem cells”, “induced pluripotent stem cells”, and “organoids”. Of note, the successful autologous stem cell therapy toward junctional epidermolysis bullosa (JEB) patients using epidermal stem cells after gene correction (retroviral transduction of LAMB3) [94] needs to be highlighted. The current genome editing technology is capable of directly correcting the pathogenic mutations while avoiding the introduction of a transgene, providing safer therapeutic stem cell sources. Thus, when “hPSCs meet genome editing” [65], further milestones not limited to stem cell research would be expected in future.
Acknowledgements
Not applicable.
Abbreviations
- hPSCs
Human pluripotent stem cells
- iPSCs
Induce pluripotent stem cells
- ZFNs
Zinc finger nucleases
- TALEN
Transcription activator-like effector nucleases
- CRISPR
Clustered regularly interspaced short palindromic repeats
- Cas
CRISPR-associated protein
- DSB
DNA double strand break
- TALE
Transcription activator-like effector
- sgRNA
Single guide RNA
- KI
Knock-in
- HDR
Homology directed repair
- MMEJ
Microhomology-mediated end joining
- NHEJ
Non-homologous end joining
- Indel
Insertion or deletion
- KO
Knock-out
- BEs
Base editors
- CBE
Cytosine base editor
- UGI
Uracil glycosylase inhibitor
- ABE
Adenine base editor
- NLS
Nuclear localization sequence
- PACE
Phage-assisted non-continuous and continuous evolution
- AP site
Apurinic/apyrimidinic site
- UNG
Uracil DNA glycosylase
- PEs
Prime editors
- pegRNA
PE gRNA
- RT
Reverse transcriptase
- SSB
DNA single strand break
- PBS
Primer binding site
- RTT
Reverse transcriptase template
- MMP
Mitochondrial membrane permeability
- Cyt C
Cytochrome C
- MMR
Mismatch repair
- VUS
Variants of uncertain significance
- HIBM
Hereditary inclusion body myopathy
- LQTS
Long QT syndrome
- BrS
Brugada syndrome
- RDEB
Recessive dystrophic epidermolysis bullosa
- C7
Type VII collagen
- DMD
Duchenne muscular dystrophy
- HIES
STAT3-Hyperimmunoglobulin E syndrome
- LRRK2
Leucine-rich kinase2
- PD
Parkinson’s disease
- PAM
Protospacer adjacent motif
Author contributions
HJC and JCP, conceived the overall design of the manuscript. JCP, MJP, and SYL wrote the manuscript. DYK made the figures. KTK and HKJ provided critical feedback. HJC supervised the project. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the National Research Foundation of Korea (NRF-2020R1A2C2005914) and by the Seoul National University Research Grant.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20:377–388. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel G. Diseases in a dish take off. Science. 2010;330:1172–1173. doi: 10.1126/science.330.6008.1172. [DOI] [PubMed] [Google Scholar]
- 6.Soldner F, Jaenisch R. Stem cells, genome editing, and the path to translational medicine. Cell. 2018;175:615–632. doi: 10.1016/j.cell.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 2020;382:1926–1932. doi: 10.1056/NEJMoa1915872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar M, Bjorklund A. From skin to brain: a Parkinson's disease patient transplanted with his own cells. Cell Stem Cell. 2020;27:8–10. doi: 10.1016/j.stem.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Park JC, Kim J, Jang HK, Lee SY, Kim KT, Kwon EJ, Park S, Lee HS, Choi H, Park SY, et al. Multiple isogenic GNE-myopathy modeling with mutation specific phenotypes from human pluripotent stem cells by base editors. Biomaterials. 2022;282:121419. doi: 10.1016/j.biomaterials.2022.121419. [DOI] [PubMed] [Google Scholar]
- 10.Kim KT, Park JC, Jang HK, Lee H, Park S, Kim J, Kwon OS, Go YH, Jin Y, Kim W, et al. Safe scarless cassette-free selection of genome-edited human pluripotent stem cells using temporary drug resistance. Biomaterials. 2020;262:120295. doi: 10.1016/j.biomaterials.2020.120295. [DOI] [PubMed] [Google Scholar]
- 11.Park JC, Jang HK, Kim J, Han JH, Jung Y, Kim K, Bae S, Cha HJ. High expression of uracil DNA glycosylase determines C to T substitution in human pluripotent stem cells. Mol Ther Nucleic Acids. 2022;27:175–183. doi: 10.1016/j.omtn.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habib O, Habib G, Hwang GH, Bae S. Comprehensive analysis of prime editing outcomes in human embryonic stem cells. Nucleic Acids Res. 2022;50:1187–1197. doi: 10.1093/nar/gkab1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith J, Bibikova M, Whitby FG, Reddy AR, Chandrasegaran S, Carroll D. Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res. 2000;28:3361–3369. doi: 10.1093/nar/28.17.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 21.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakade S, Tsubota T, Sakane Y, Kume S, Sakamoto N, Obara M, Daimon T, Sezutsu H, Yamamoto T, Sakuma T, et al. Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat Commun. 2014;5:5560. doi: 10.1038/ncomms6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 2008;7:1765–1771. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao Z, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porto EM, Komor AC, Slaymaker IM, Yeo GW. Base editing: advances and therapeutic opportunities. Nat Rev Drug Discov. 2020;19:839–859. doi: 10.1038/s41573-020-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, Koblan LW, Kim YB, Badran AH, Liu DR. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T: a base editors with higher efficiency and product purity. Sci Adv. 2017;3:eaao4774. doi: 10.1126/sciadv.aao4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, Newby GA, Maianti JP, Raguram A, Liu DR. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol. 2018;36:843–846. doi: 10.1038/nbt.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter MF, Zhao KT, Eton E, Lapinaite A, Newby GA, Thuronyi BW, Wilson C, Koblan LW, Zeng J, Bauer DE, et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol. 2020;38:883–891. doi: 10.1038/s41587-020-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurt IC, Zhou R, Iyer S, Garcia SP, Miller BR, Langner LM, Grunewald J, Joung JK. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol. 2021;39:41–46. doi: 10.1038/s41587-020-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen PJ, Hussmann JA, Yan J, Knipping F, Ravisankar P, Chen PF, Chen C, Nelson JW, Newby GA, Sahin M, et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell. 2021;184:5635–5652 e5629. doi: 10.1016/j.cell.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson JW, Randolph PB, Shen SP, Everette KA, Chen PJ, Anzalone AV, An M, Newby GA, Chen JC, Hsu A, et al. Engineered pegRNAs improve prime editing efficiency. Nat Biotechnol. 2022;40:402–410. doi: 10.1038/s41587-021-01039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissbein U, Benvenisty N, Ben-David U. Quality control: Genome maintenance in pluripotent stem cells. J Cell Biol. 2014;204:153–163. doi: 10.1083/jcb.201310135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuijk E, Jager M, van der Roest B, Locati MD, Van Hoeck A, Korzelius J, Janssen R, Besselink N, Boymans S, van Boxtel R, et al. The mutational impact of culturing human pluripotent and adult stem cells. Nat Commun. 2020;11:2493. doi: 10.1038/s41467-020-16323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puri MC, Nagy A. Concise review: Embryonic stem cells versus induced pluripotent stem cells: the game is on. Stem Cells. 2012;30:10–14. doi: 10.1002/stem.788. [DOI] [PubMed] [Google Scholar]
- 38.Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, Rhee YH, Lee SH, Oh SJ, Lee MY, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110:E3281–3290. doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momcilovic O, Knobloch L, Fornsaglio J, Varum S, Easley C, Schatten G. DNA damage responses in human induced pluripotent stem cells and embryonic stem cells. PLoS ONE. 2010;5:e13410. doi: 10.1371/journal.pone.0013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mujoo K, Pandita RK, Tiwari A, Charaka V, Chakraborty S, Singh DK, Hambarde S, Hittelman WN, Horikoshi N, Hunt CR, et al. Differentiation of human induced pluripotent or embryonic stem cells decreases the DNA damage repair by homologous recombination. Stem Cell Reports. 2017;9:1660–1674. doi: 10.1016/j.stemcr.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ACH, Peng Q, Fong SW, Lee KC, Yeung WSB, Lee YL. DNA damage response and cell cycle regulation in pluripotent stem cells. Genes (Basel) 2021;12:1548. doi: 10.3390/genes12101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong HC, Cho SJ, Lee MO, Cha HJ. Technical approaches to induce selective cell death of pluripotent stem cells. Cell Mol Life Sci. 2017;74:2601–2611. doi: 10.1007/s00018-017-2486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 44.Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J, Li J, Song Z, Qu X, Zhou P, et al. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem. 2007;282:5842–5852. doi: 10.1074/jbc.M610464200. [DOI] [PubMed] [Google Scholar]
- 45.Liu JC, Guan X, Ryan JA, Rivera AG, Mock C, Agrawal V, Letai A, Lerou PH, Lahav G. High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell. 2013;13:483–491. doi: 10.1016/j.stem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 47.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/S1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 48.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 49.Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol Cell. 2012;46:573–583. doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24:268–274. doi: 10.1016/j.tcb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24:927–930. doi: 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 52.Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K, Kommineni S, Chen J, Sondey M, Ye C, et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med. 2018;24:939–946. doi: 10.1038/s41591-018-0050-6. [DOI] [PubMed] [Google Scholar]
- 53.Li M, Zhong A, Wu Y, Sidharta M, Beaury M, Zhao X, Studer L, Zhou T. Transient inhibition of p53 enhances prime editing and cytosine base-editing efficiencies in human pluripotent stem cells. Nat Commun. 2022;13:6354. doi: 10.1038/s41467-022-34045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maynard S, Swistowska AM, Lee JW, Liu Y, Liu ST, Da Cruz AB, Rao M, de Souza-Pinto NC, Zeng X, Bohr VA. Human embryonic stem cells have enhanced repair of multiple forms of DNA damage. Stem Cells. 2008;26:2266–2274. doi: 10.1634/stemcells.2007-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo LZ, Gopalakrishna-Pillai S, Nay SL, Park SW, Bates SE, Zeng X, Iverson LE, O'Connor TR. DNA repair in human pluripotent stem cells is distinct from that in non-pluripotent human cells. PLoS ONE. 2012;7:e30541. doi: 10.1371/journal.pone.0030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beard WA, Horton JK, Prasad R, Wilson SH. Eukaryotic base excision repair: new approaches shine light on mechanism. Annu Rev Biochem. 2019;88:137–162. doi: 10.1146/annurev-biochem-013118-111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs AL, Schar P. DNA glycosylases: in DNA repair and beyond. Chromosoma. 2012;121:1–20. doi: 10.1007/s00412-011-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan BK, Miller JH. Mutagenic deamination of cytosine residues in DNA. Nature. 1980;287:560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- 59.Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morera S, Grin I, Vigouroux A, Couve S, Henriot V, Saparbaev M, Ishchenko AA. Biochemical and structural characterization of the glycosylase domain of MBD4 bound to thymine and 5-hydroxymethyuracil-containing DNA. Nucleic Acids Res. 2012;40:9917–9926. doi: 10.1093/nar/gks714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyons DM, O'Brien PJ. Efficient recognition of an unpaired lesion by a DNA repair glycosylase. J Am Chem Soc. 2009;131:17742–17743. doi: 10.1021/ja908378y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J-C, Kim Y-J, Han JH, Kim D, Park MJ, Kim J, Jang H-K, Bae S, Cha H-J. MutSα and MutSβ as size-dependent cellular determinants for prime editing in human embryonic stem cells. Mol Therapy-Nucleic Acids 2023 [DOI] [PMC free article] [PubMed]
- 63.Hendriks WT, Warren CR, Cowan CA. Genome editing in human pluripotent stem cells: approaches, pitfalls, and solutions. Cell Stem Cell. 2016;18:53–65. doi: 10.1016/j.stem.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hockemeyer D, Jaenisch R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell. 2016;18:573–586. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hotta A, Yamanaka S. From genomics to gene therapy: induced pluripotent stem cells meet genome editing. Annu Rev Genet. 2015;49:47–70. doi: 10.1146/annurev-genet-112414-054926. [DOI] [PubMed] [Google Scholar]
- 66.Yu C, Liu Y, Ma T, Liu K, Xu S, Zhang Y, Liu H, La Russa M, Xie M, Ding S, et al. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell. 2015;16:142–147. doi: 10.1016/j.stem.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin RM, Ikeda K, Cromer MK, Uchida N, Nishimura T, Romano R, Tong AJ, Lemgart VT, Camarena J, Pavel-Dinu M, et al. Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated homologous recombination. Cell Stem Cell. 2019;24:821–828 e825. doi: 10.1016/j.stem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Benati D, Leung A, Perdigao P, Toulis V, van der Spuy J, Recchia A. Induced pluripotent stem cells and genome-editing tools in determining gene function and therapy for inherited retinal disorders. Int J Mol Sci. 2022;23:15276. doi: 10.3390/ijms232315276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Sastre D, Wang F. CRISPR/Cas9 genome editing: a promising tool for therapeutic applications of induced pluripotent stem cells. Curr Stem Cell Res Ther. 2018;13:243–251. doi: 10.2174/1574888X13666180214124800. [DOI] [PubMed] [Google Scholar]
- 70.De Masi C, Spitalieri P, Murdocca M, Novelli G, Sangiuolo F. Application of CRISPR/Cas9 to human-induced pluripotent stem cells: from gene editing to drug discovery. Hum Genomics. 2020;14:25. doi: 10.1186/s40246-020-00276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moss AJ. Long QT syndrome. JAMA. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 72.Qi T, Wu FJ, Xie YQ, Gao SQ, Li MM, Pu J, Li DL, Lan F, Wang YM. Base Editing Mediated Generation of Point Mutations Into Human Pluripotent Stem Cells for Modeling Disease. Front Cell Dev Biol. 2020;8:590581. doi: 10.3389/fcell.2020.590581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu F, Guo T, Sun L, Li F, Yang X. Base editing of human pluripotent stem cells for modeling long QT syndrome. Stem Cell Rev Rep. 2022;18:1434–1443. doi: 10.1007/s12015-021-10324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osborn MJ, Newby GA, McElroy AN, Knipping F, Nielsen SC, Riddle MJ, Xia L, Chen W, Eide CR, Webber BR, et al. Base editor correction of COL7A1 in recessive dystrophic epidermolysis bullosa patient-derived fibroblasts and iPSCs. J Invest Dermatol. 2020;140(338–347):e335. doi: 10.1016/j.jid.2019.07.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan J, Ma Y, Huang T, Chen Y, Peng Y, Li B, Li J, Zhang Y, Song B, Sun X. Genetic modulation of RNA splicing with a CRISPR-guided cytidine deaminase. Mol Cell. 2018;72(380–394):e387. doi: 10.1016/j.molcel.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Chemello F, Chai AC, Li H, Rodriguez-Caycedo C, Sanchez-Ortiz E, Atmanli A, Mireault AA, Liu N, Bassel-Duby R, Olson EN. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci Adv. 2021;7:eabg4910. doi: 10.1126/sciadv.abg4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eberherr AC, Maaske A, Wolf C, Giesert F, Berutti R, Rusha E, Pertek A, Kastlmeier MT, Voss C, Plummer M. Rescue of STAT3 function in hyper-IgE syndrome using adenine base editing. CRISPR J. 2021;4:178–190. doi: 10.1089/crispr.2020.0111. [DOI] [PubMed] [Google Scholar]
- 78.Chang KH, Huang CY, Ou-Yang CH, Ho CH, Lin HY, Hsu CL, Chen YT, Chou YC, Chen YJ, Chen Y, et al. In vitro genome editing rescues parkinsonism phenotypes in induced pluripotent stem cells-derived dopaminergic neurons carrying LRRK2 p.G2019S mutation. Stem Cell Res Ther. 2021;12:508. doi: 10.1186/s13287-021-02585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang P, Li H, Zhu M, Han RY, Guo S, Han R. Correction of DMD in human iPSC-derived cardiomyocytes by base-editing-induced exon skipping. Mol Ther Methods Clin Dev. 2023;28:40–50. doi: 10.1016/j.omtm.2022.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, Busquets O, Verma Y, Syed KM, Kutnowski N, Pangilinan GR, Gilbert LA, Bateup HS, Rio DC, Hockemeyer D, et al. Highly efficient generation of isogenic pluripotent stem cell models using prime editing. Elife. 2022;11:e79208. doi: 10.7554/eLife.79208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishiyama T, Zhang Y, Cui M, Li H, Sanchez-Ortiz E, McAnally JR, Tan W, Kim J, Chen K, Xu L, et al. Precise genomic editing of pathogenic mutations in RBM20 rescues dilated cardiomyopathy. Sci Transl Med. 2022;14:eade1633. doi: 10.1126/scitranslmed.ade1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papathanasiou S, Markoulaki S, Blaine LJ, Leibowitz ML, Zhang CZ, Jaenisch R, Pellman D. Whole chromosome loss and genomic instability in mouse embryos after CRISPR-Cas9 genome editing. Nat Commun. 2021;12:5855. doi: 10.1038/s41467-021-26097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–771. doi: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adikusuma F, Piltz S, Corbett MA, Turvey M, McColl SR, Helbig KJ, Beard MR, Hughes J, Pomerantz RT, Thomas PQ. Large deletions induced by Cas9 cleavage. Nature. 2018;560:E8–E9. doi: 10.1038/s41586-018-0380-z. [DOI] [PubMed] [Google Scholar]
- 85.Simkin D, Papakis V, Bustos BI, Ambrosi CM, Ryan SJ, Baru V, Williams LA, Dempsey GT, McManus OB, Landers JE, et al. Homozygous might be hemizygous: CRISPR/Cas9 editing in iPSCs results in detrimental on-target defects that escape standard quality controls. Stem Cell Reports. 2022;17:993–1008. doi: 10.1016/j.stemcr.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weisheit I, Kroeger JA, Malik R, Klimmt J, Crusius D, Dannert A, Dichgans M, Paquet D. Detection of deleterious on-target effects after HDR-mediated CRISPR EDITING. Cell Rep. 2020;31:107689. doi: 10.1016/j.celrep.2020.107689. [DOI] [PubMed] [Google Scholar]
- 87.Song Y, Liu Z, Zhang Y, Chen M, Sui T, Lai L, Li Z. Large-Fragment deletions induced by Cas9 cleavage while not in the BEs system. Mol Ther Nucleic Acids. 2020;21:523–526. doi: 10.1016/j.omtn.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cullot G, Boutin J, Toutain J, Prat F, Pennamen P, Rooryck C, Teichmann M, Rousseau E, Lamrissi-Garcia I, Guyonnet-Duperat V, et al. CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat Commun. 2019;10:1136. doi: 10.1038/s41467-019-09006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park J-C, Kim K-T, Jang H-K, Cha H-J. Transition substitution of desired bases in human pluripotent stem cells with base editors: a step-by-step guide. Int J Stem Cells. 2023;16:234–243. doi: 10.15283/ijsc22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim YB, Komor AC, Levy JM, Packer MS, Zhao KT, Liu DR. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol. 2017;35:371–376. doi: 10.1038/nbt.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang TP, Zhao KT, Miller SM, Gaudelli NM, Oakes BL, Fellmann C, Savage DF, Liu DR. Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat Biotechnol. 2019;37:626–631. doi: 10.1038/s41587-019-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walton RT, Christie KA, Whittaker MN, Kleinstiver BP. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368:290–296. doi: 10.1126/science.aba8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lendahl U. 100 plus years of stem cell research-20 years of ISSCR. Stem Cell Reports. 2022;17:1248–1267. doi: 10.1016/j.stemcr.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.