Abstract
The obesity paradox is reported to exist in various diseases. However, obesity is a pivotal issue in gastric cancer (GC) patients because of the surgical difficulty related to postoperative abdominal infectious complications (PAIC). This study clarified the existence of the obesity paradox in GC. Between 1997 and 2015, 1536 consecutive patients underwent curative gastrectomy. Of all patients, 18.6% (285/1536) were obese and tended to have a better prognosis (P = 0.073). In patients without PAIC, obesity was a significant prognostic factor for 5-year overall survival (P = 0.017). PAIC was an independent poor prognostic factor in both obese and non-obese patients (P < 0.001; hazard ratio [HR] 4.22 and 1.82). In pStage II–III patients, there was a large and significant prognostic difference between non-PAIC and PAIC obese patients (P = 0.006; 5-year overall survival: 69.7% vs. 43.8%) related to the higher incidence of peritoneal recurrence in PAIC obese patients (P = 0.035; 31% vs. 10%). Whereas, there was a small prognostic difference between non-PAIC and PAIC non-obese patients (P = 0.102; 5-year overall survival: 56.5% vs. 51.9%). Although the obesity paradox is present in GC, PAIC had a more negative prognostic impact through peritoneal recurrence in obese GC patients.
Subject terms: Cancer, Oncology
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third most common cause of death worldwide1–3. Recent developments in diagnostic technology, evidence-based surgical procedures, novel chemotherapy, and immunotherapy have enhanced detection rates and drastically improved the early and long-term outcomes for GC patients. However, GC remains a serious health problem with various clinical issues4–7. In recent years, the obesity rate has been increasing worldwide because of the westernization of lifestyle, especially diet8–10. Obesity is a major cause of various diseases such as cardiovascular disease and metabolic disease. However, various studies have identified that obese patients have a better prognosis compared to underweight patients in several chronic diseases, and this phenomenon is known as the obesity paradox11,12.
In GC patients, obesity has had a negative impact on surgical procedures, leading to increased blood loss, poor lymph node dissection, and postoperative complications13–15. In addition, because postoperative complications lead to a poor oncological prognosis16–20, obese GC patients may have poor prognoses. Therefore, the existence and mechanisms of the obesity paradox in GC remain controversial21.
This study clarified whether the obesity paradox is present in GC from the viewpoint of postoperative complications. Our results may demonstrate the positive impact of obesity on the prognoses of patients with GC.
Results
Clinicopathological characteristics of obese GC patients
There were 285 obese GC patients among 1536 consecutive patients with GC. A comparison of clinicopathological factors between obese and non-obese patients with GC is presented in Table 1. The obese group was significantly associated with a higher incidence of males (P = 0.004; 74.4% vs. 65.6%), cardiovascular disease (P < 0.001; 38.2% vs. 26.9%), and endocrine diseases (P = 0.008; 21.8% vs. 15.1%) than the non-obese group. There was no significant difference in PAIC rate between obese and non-obese patients (10.9% (31/285) vs. 8.7% (108/1251)) or between other surgical factors.
Table 1.
Clinicopathological characteristics of obese and non-obese gastric cancer patients.
| Variables | Total | Obese patients (n = 285) | Non-obese patients (n = 1251) | P value |
|---|---|---|---|---|
| Gender | ||||
| Female | 503 |
73 (26%) |
430 (34%) |
0.004 |
| Male | 1033 |
212 (74%) |
821 (66%) |
|
| Age | ||||
| < 75 | 1194 |
220 (77%) |
974 (78%) |
0.813 |
| ≥ 75 | 342 |
65 (23%) |
277 (22%) |
|
| Albumin (g/dl) | ||||
| < 3 | 168 |
25 (9%) |
143 (11%) |
0.208 |
| ≥ 3 | 1368 |
260 (91%) |
1108 (89%) |
|
| Hemoglobin (g/dl) | ||||
| < 8 | 115 |
20 (7%) |
95 (8%) |
0.804 |
| ≥ 8 | 1421 |
265 (93%) |
1156 (92%) |
|
| Cardiovascular disease | ||||
| ( − ) | 1091 |
176 (62%) |
915 (73%) |
< 0.001 |
| ( +) | 445 |
109 (38%) |
336 (27%) |
|
| Digestive disease | ||||
| ( − ) | 1137 |
219 (77%) |
918 (73%) |
0.261 |
| ( +) | 399 |
66 (23%) |
333 (27%) |
|
| Endocrine and autoimmune disease | ||||
| ( − ) | 1285 |
223 (78%) |
1062 (85%) |
0.008 |
| ( +) | 251 |
62 (22%) |
189 (15%) |
|
| Hepatic disease | ||||
| ( − ) | 1442 |
269 (94%) |
1173 (94%) |
0.785 |
| ( +) | 94 |
16 (6%) |
78 (6%) |
|
| Neurological disease | ||||
| ( − ) | 1460 |
277 (97%) |
1183 (95%) |
0.069 |
| ( +) | 76 |
8 (3%) |
68 (5%) |
|
| Renal and urologic disease | ||||
| ( − ) | 1498 |
281 (99%) |
1217 (97%) |
0.289 |
| ( +) | 38 |
4 (1%) |
34 (3%) |
|
| Respiratory disease | ||||
| ( − ) | 1472 |
275 (97%) |
1197 (96%) |
0.624 |
| ( +) | 64 |
10 (3%) |
54 (4%) |
|
| p Stage | ||||
| I | 1037 |
195 (68%) |
842 (67%) |
0.779 |
| II + III | 499 |
90 (32%) |
409 (33%) |
|
| Surgical approach | ||||
| Open | 1051 |
198 (70%) |
853 (68%) |
0.724 |
| Lap | 485 |
87 (30%) |
398 (32%) |
|
| Lymphadenectomy | ||||
| D2 ≤ | 707 |
126 (44%) |
581 (46%) |
0.511 |
| < D2 | 829 |
159 (56%) |
670 (54%) |
|
| PAICa | ||||
| ( − ) | 1396 |
254 (89%) |
1142 (91%) |
0.255 |
| ( +) | 140 |
31 (11%) |
109 (9%) |
|
aPostoperative abdominal infectious complications: anastomotic leakage, pancreatic fistula, and intra-abdominal abscess in grade II or higher of Clavien–Dindo classification.
Multivariate analyses using the Cox’s proportional hazard model in GC patients with or without obesity
Table 2 shows the prognostic factors in patients undergoing curative gastrectomy for GC according to obesity status. In non-obese patients, multivariate analyses using Cox’s proportional hazards model revealed that age ≥ 75 years (P < 0.001; hazard ratio [HR] 2.09), advanced pathological status of pStage II or high (P < 0.001; HR 5.42), open gastrectomy (P < 0.001; HR 3.79), radical D2 lymphadenectomy (P < 0.001; HR 1.95), and PAIC (P < 0.001; HR 1.82) were independent poor prognostic factors. In obese patients, however, advanced pathological status (P < 0.001; HR 5.99) and PAIC (P < 0.001; HR 4.22) were independent poor prognostic factors. PAIC was an independent poor prognostic factor in both obese and non-obese patients, but PAIC was a stronger factor in obese patients.
Table 2.
Multivariate analyses of survival after surgery using the Cox’s proportional hazard model according to obesity status.
| Obese patients | Non-obese patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate a | Multivariate a | ||||||||||||
| HR b | 95% CI c | P value | HR b | 95% CI c | P value | ||||||||
| Age | 75 ≤ | versus | < 75 | 2.09 | 1.58 | – | 2.76 | < 0.001 | |||||
| pStage | II + III | versus | I | 5.99 | 2.96 | – | 12.12 | < 0.001 | 5.42 | 3.92 | – | 7.49 | < 0.001 |
| Surgical approach | Open | versus | Lap | 3.79 | 2.35 | – | 6.11 | < 0.001 | |||||
| Lymphadenectomy | D2 ≤ | versus | < D2 | 1.95 | 1.43 | – | 2.65 | < 0.001 | |||||
| PAIC d | ( +) | versus | ( − ) | 4.22 | 2.11 | – | 8.44 | < 0.001 | 1.82 | 1.26 | – | 2.62 | < 0.001 |
a Multivariate survival analysis was performed using Cox’s proportional hazard model.
b HR: Hazard ratio.
c CI: Confidence interval.
d Postoperative abdominal infectious complications: anastomotic leakage, pancreatic fistula, and intra-abdominal abscess in grade II or high of Clavien–Dindo classification.
Overall survival curves according to the combination of the PAIC and obesity status
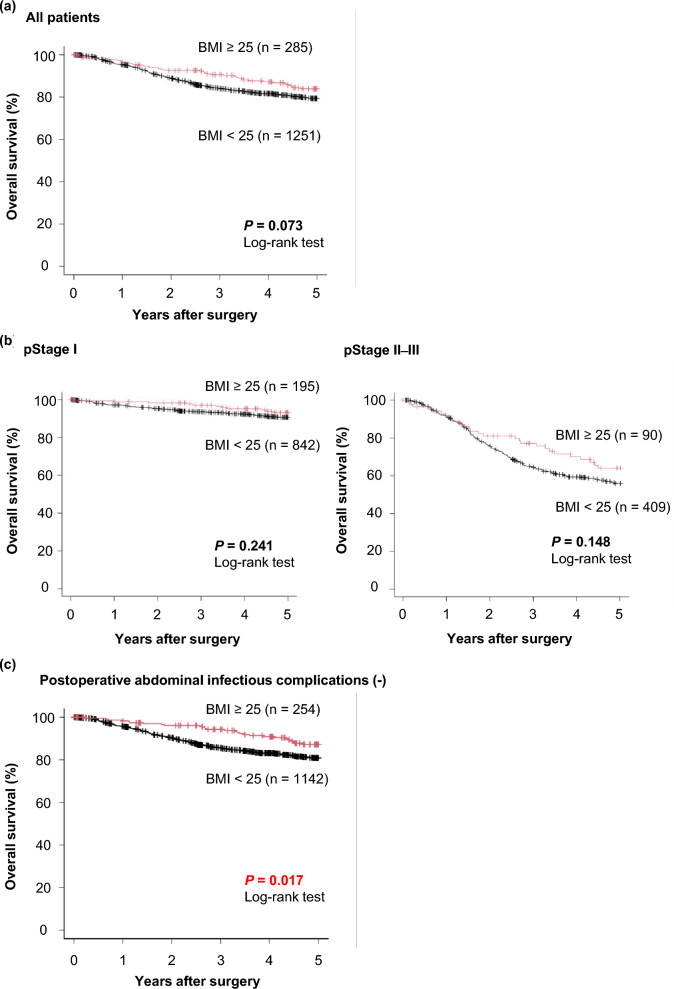
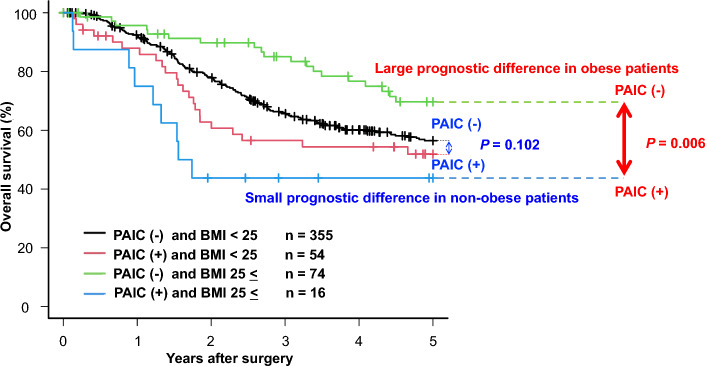
Next, we evaluated the prognostic effects of obesity and PAIC on survival curves. In all patients, there was no prognostic difference between obese and non-obese patients (Fig. 1a) although obesity tended to be a better prognostic factor (P = 0.073). Also, when patients were classified as early pStage (pStage I) or advanced pStage (pStage II–III), there was no difference in prognosis between obese and non-obese patients (Fig. 1b). However, obese patients had a significantly better prognosis than non-obese patients among patients without PAIC (P = 0.017), suggesting the obesity paradox (Fig. 1c). Moreover, the prognostic difference caused by PAIC was small in the non-obese patients with pStage II–III GC (P = 0.102; 5-year overall survival: 56.5% vs. 51.9%), whereas obese patients with PAIC had a significantly extremely poorer prognosis than those without PAIC (P = 0.006; 5-year overall survival: 69.7% vs. 43.8%) (Fig. 2).
Figure 1.
(a) Survival curves for all patients. There was no prognostic difference between obese and non-obese patients (P = 0.073). (b) Survival curves according to pStage. There was no prognostic difference between obese and non-obese patients (P = 0.241, P = 0.148). (c) Survival curves for patients without postoperative abdominal infectious complications (PAIC) according to a BMI cut-off value of 25. Obese patients had a significantly better prognosis than non-obese patients (P = 0.017).
Figure 2.
Overall survival curves according to the combination of PAIC and obesity status. In 499 consecutive patients with pStage II–III GC, there was a large and significant prognostic difference between non-PAIC and PAIC obese patients (P = 0.006; 5-year overall survival: 69.7% vs. 43.8%), related to the higher incidence of peritoneal recurrence in PAIC obese patients (P = 0.035; 31.2% vs. 9.5%). In contrast, there was a small prognostic difference between non-PAIC and PAIC non-obese patients (P = 0.102; 5-year overall survival: 56.5% vs. 51.9%).
Recurrence patterns and rates in patients with pStage II–III GC according to the combination of obesity and PAIC
Finally, we investigated the prognostic impact of obesity and PAIC with advanced pStage II–III GC. Obese patients with PAIC were more likely to relapse with peritoneal recurrence than patients without PAIC (P = 0.035; 31% vs. 10%). In contrast, there was no significant correlation between PAIC and peritoneal recurrence in non-obese patients (P = 0.713; 17% vs. 20%) (Table 3 and Fig. 3).
Table 3.
Recurrence patterns and rates in patients with pStage II–III gastric cancer according to the combination of obesity and PAIC.
| Recurrence | Obesity ( +) PAIC ( +) (n = 16) | Obesity ( +) PAIC (-) (n = 74) | P value | Obesity (-) PAIC ( +) (n = 54) | Obesity (-) PAIC (-) (n = 355) | P-value |
|---|---|---|---|---|---|---|
| Total patients | 7 (44%) | 22 (30%) | 0.376 | 20 (37%) | 129 (34%) | 0.882 |
| Recurrence types | ||||||
| Peritoneum | 5 (31%) | 7 (10%) | 0.035 | 9 (17%) | 70 (20%) | 0.713 |
| Local | 1 (6%) | 2 (3%) | 0.448 | 1 (2%) | 11 (3%) | 1.000 |
| Lymph node | 1 (6%) | 7 (10%) | 1.000 | 4 (7%) | 34 (10%) | 0.802 |
| Hematogenous | 2 (13%) | 10 (14%) | 1.000 | 9 (17%) | 37 (10%) | 0.162 |
| Others | 2 (13%) | 1 (1%) | 0.326 | 0 (0.0%) | 9 (3%) | 0.614 |
Figure 3.
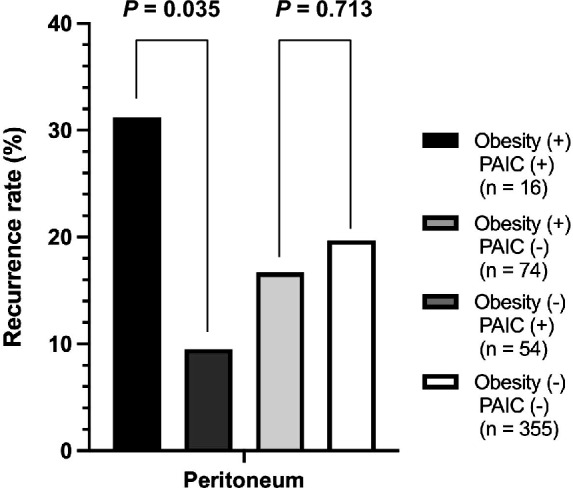
Peritoneal recurrence rates in patients with pStage II–III GC according to the combination of obesity and PAIC. In 499 consecutive patients with pStage II–III GC, obese patients with PAIC were more likely to relapse with peritoneal recurrence than patients without PAIC (P = 0.035; 31.2% vs. 9.5%).
Biochemical mechanisms affecting poor prognosis in obese patients with postoperative complications
Next, we examined the causes of the poor prognosis in obese patients with PAIC from the viewpoint of biochemical analysis. We focused on postoperative serum CRP level according to the combination of obesity and PAIC. Obese patients with PAIC had a significantly higher serum CRP level than obese patients without PAIC, non-obese patients with PAIC, and non-obese patients without PAIC (P < 0.001; 18.5 vs. 16.4 vs. 11.8 vs. 11.6 mg/L) on postoperative day 3. We observed similar results on postoperative day 5 (P < 0.001; 14.2 vs. 11.9 vs. 6.3 vs. 5.4 mg/L) and day 7 (P < 0.001; 11.6 vs. 11.3 vs. 3.9 vs. 3.4 mg/L) (Fig. 4).
Figure 4.
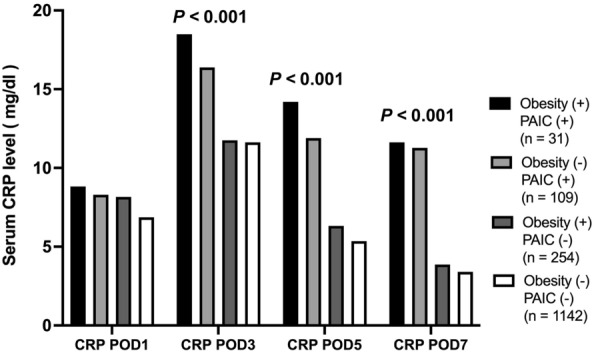
Comparison of postoperative serum CRP level according to the combination of PAIC and obesity status. Obese patients with PAIC had significantly higher serum CRP level than obese patients without PAIC, non-obese patients with PAIC, and non-obese patients without PAIC (P < 0.001; 18.5 vs. 16.4 vs. 11.8 vs. 11.6 mg/L) on postoperative day 3. The results were similar on postoperative day 5 (P < 0.001; 14.2 vs. 11.9 vs. 6.3 vs. 5.4 mg/L) and day 7 (P < 0.001; 11.6 vs. 11.3 vs. 3.9 vs. 3.4 mg/L).
Prognostic analysis and recurrence patterns using a cohort after propensity score matching
Finally, in order to exclude selection bias from potential confounders, we analyzed the prognosis and recurrence patterns using a cohort after propensity score matching. After propensity score matching, there were no significant differences in clinicopathological characteristics between obese and non-obese patients (Supplemental Table 1). The results of prognosis and recurrence patterns were approximately similar to those before propensity score matching. There was no prognostic difference between obese and non-obese patients (Supplemental Fig. 1a, b, c). However, obese patients had a significantly better prognosis than non-obese patients among patients without PAIC (P = 0.032) (Supplemental Fig. 1d). The prognostic difference caused by PAIC was larger in the obese patients than non-obese patients (Supplemental Fig. 2) and was a stronger prognostic factor in obese patients than non-obese patients (HR 4.22 vs. 2.54) (Supplemental Table 2). In recurrence pattern, obese patients with PAIC were more likely to have peritoneal recurrence than those without PAIC. In contrast, there was no significant correlation between PAIC and peritoneal recurrence in non-obese patients (P = 1.000) (Supplemental Table 3).
Discussion
The obesity paradox has been reported in various diseases owing to better nutrition, immunity, and tolerability in treatments. However, it has been unclear and controversial whether the obesity paradox exists in GC. This is because obesity is a pivotal issue of GC patients, affecting the surgical difficulty and postoperative abdominal infectious complications (PAIC), and inducing poor long-term survival. This study clearly demonstrated the obesity paradox in GC patients, particularly in those without PAIC. PAIC might affect a stronger and more negative prognostic impact in obese GC patients through peritoneal recurrence following gastrectomy.
The obesity paradox was suggested more than 20 years ago and is a well-known phenomenon in various diseases. Initial studies identified that being overweight could be a favorable prognostic factor in cardiovascular and metabolic diseases11,12. In solid cancers, several studies have identified that overweight patients have a better prognosis compared to normal-weight patients22–25. In esophageal cancer, Kayani et al. demonstrated that obesity was not a risk factor for postoperative complications and that obese patients have better prognoses than non-obese patients22. In colorectal cancer, Walter et al. have also reported that the obesity paradox was present in obese and smaller weight loss patients23. In renal cell carcinoma, Hakimi et al. reported that obese patients had a low malignant tumor compared to non-obese patients24. Moreover, in lung cancer, Kichenadasse et al. reported that overweight non-small cell lung cancer patients had a better prognosis and were more responsive to immune checkpoint inhibitor therapy25. Thus, the obesity paradox could be more likely to exist in various cancers, including gastrointestinal cancers.
Until now, there has been no clear evidence of whether obesity is associated with a better or worse prognosis in patients with GC. This is because obesity has been a negative factor for surgical procedures leading to increased blood loss, poor lymph node dissection, and postoperative complications13–15. Therefore, it has been controversial whether or not the obesity paradox exists. According to the WHO World Health Survey, Japan, South Korea, and Poland have relatively low obesity rates compared to Western countries9. In these countries which have fewer postoperative complications, some studies suggested that obesity was a better prognostic factor26–28. On the other hand, several studies from Western countries suggested that obesity was a poor prognostic factor29. Western countries have a higher incidence of obesity, which is associated with the difficulty of surgery and potentially increased postoperative complications. Thus, these differences might affect whether the prognostic effect of obesity was better or not in GC patients.
In this study, we demonstrated the striking findings that there was a strong and significant prognostic difference between non-PAIC and PAIC obese patients (P = 0.006; 5-year overall survival: 69.7% vs. 43.8%), related to the higher incidence of peritoneal recurrence in PAIC obese patients (P = 0.035) with advanced pStage II–III. In contrast, there was a small prognostic difference between non-PAIC and PAIC non-obese patients (P = 0.102; 5-year overall survival: 56.5% vs. 51.9%).
Adipocytes may be associated with the mechanism of the obesity paradox in GC because they control metabolism and immune interplay relevant to energy homeostasis30, thus contributing to a favorable prognosis. However, adipocytes also produced various hormones and adipokines such as HMGB-131. HMGB-1 has been identified as a proinflammatory mediator and is expressed twofold more in adipose tissue from obese compared to normal-weight individuals32. It was reported to interact with the NF-κB pathway to initiate the transcription of various proinflammatory cytokines including TNF-α, IL-6, and IL-1b to promote inflammation33,34. In clinical settings, Osoegawa et al. demonstrated that higher surgical invasion increased the concentration of postoperative plasma HMGB-135. In our study, serum CRP level, which was the result of inflammation induced by these adipokines, reproduced a similar phenomenon. CRP can be measured more easily than adipokines and reflect the level of postoperative inflammation. We have reported in previous studies that high postoperative CRP level is correlated with obesity and contributes to a poor prognosis36,37. These findings also suggested that obese patients have potentially more adipokines such as HMGB-1 and potentially have a poorer prognosis if postoperative complications occur.
The present study was limited by its retrospective design and small cohort. Additionally, the prolonged recruitment period over which the retrospective analysis was performed at a single institution may have been influenced by possible variations in treatment strategies over time. Therefore, a prospective observational study with several large cohorts or a nationwide clinical database study may be needed to validate the existence of the obesity paradox in GC. In conclusion, the obesity paradox could be present in GC patients without PAIC. However, PAIC might affect a negative impact in obese GC patients through peritoneal recurrence following gastrectomy.
Methods
Patients and procedures
This study was institutionally approved by the Kyoto Prefectural University of Medicine, and each participant provided written informed consent. A total of 1536 patients who underwent curative surgery for GC at our institute between 1997 and 2015 were included in this study. Furthermore, we retrospectively analyzed the clinicopathological features and prognostic outcomes. Finally, we evaluated the compatibility of our findings with the 8th edition of the AJCC/UICC TNM classification system.
The postoperative follow-up program at our institution comprises a regular physical examination as well as laboratory blood tests and chest X-rays every three or six months. When possible, endoscopy and ultrasonography, or computed tomography, were performed annually for the first five years. All enrolled patients underwent pathological or macroscopic curative resection (R0). Histological types were classified as differentiated (papillary adenocarcinoma, or moderately or well-differentiated adenocarcinoma) or undifferentiated (poorly differentiated or undifferentiated adenocarcinoma, signet-ring cell carcinoma, or mucinous adenocarcinoma) based on the 15th edition of the Japanese Classification of Gastric Carcinom38.
Considering the selection bias from potential confounders, propensity score-matched analysis was performed to compare the outcomes between obese patients and non-obese patients. The variables for calculating the propensity score were gender, age at surgery, presence of cardiovascular disease, presence of endocrine and autoimmune disease, presence of neurological disease, pStage and presence of PAIC, that were clinically important and tended to difference in clinicopathological characteristics between obese and non-obese patients.
Definition of obesity and postoperative abdominal infectious complications
In this study, we defined obese patients as BMI 25 or higher as a previous report39. Regarding the postoperative complications, we focused on PAIC, because previous major studies identified that PAIC has a strong impact on poor prognosis in GC17. In this study, we defined PAIC as anastomotic leakage, pancreatic fistula, and intra-abdominal abscess in grade II or higher of the Clavien–Dindo classification.
Statistical analysis
We used EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) for all analyses. We performed Pearson’s chi-square (χ2) and Fisher’s exact probability tests for categorical variables. For unpaired continuous variables, Student’s t-test and Mann–Whitney’s U test were performed to compare clinicopathological characteristics between the two groups. Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used to assess statistical differences. The data were stratified for the multivariate analysis by using forward and backward stepwise Cox regression methods. P < 0.05 was considered statistically significant40.
Ethical approval
This study was institutionally approved by Kyoto Prefectural University of Medicine.
Supplementary Information
Acknowledgements
This study was approved by ethics committee of Kyoto Prefectural University of Medicine and performed in accordance with relevant guidelines and regulations.
Author contributions
H.K. and S.K. designed this study, and H.K., S.K., K.N., T.O., H.K., A.S., T.K., H.F., and E.O. performed the research and analyzed data, and H.K. and S.K. wrote the paper.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the personal information protection law in Japan but are available after the permission from the institutional review board and the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hajime Kamiya and Shuhei Komatsu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-36968-7.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.The Foundation for Promotion of Cancer Research (FPCR). Cancer Statistics in JAPAN 2018. (2018).
- 3.Ferlay J, et al. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021 doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, et al. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: A meta-analysis. Gastric Cancer. 2016;19:543–552. doi: 10.1007/s10120-015-0500-5. [DOI] [PubMed] [Google Scholar]
- 5.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann. Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/s0140-6736(10)61121-x. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu S, Otsuji E. Essential updates 2017/2018: Recent topics in the treatment and research of gastric cancer in Japan. Ann Gastroenterol Surg. 2019;3:581–591. doi: 10.1002/ags3.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/s0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization, W. (Fact sheet N° 311, 2015: URL: http://www.who.int/mediacentre/factsheets/fs311/en/) (2015).
- 10.Dai H, et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the global burden of disease study. PLoS Med. 2020;17:e1003198. doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–953. doi: 10.1016/s2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujinaka T, et al. Influence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenectomy (JCOG9501) Ann. Surg. Oncol. 2007;14:355–361. doi: 10.1245/s10434-006-9209-3. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa K, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg. Endosc. 2011;25:3825–3830. doi: 10.1007/s00464-011-1798-7. [DOI] [PubMed] [Google Scholar]
- 15.Kunisaki C, et al. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg. Endosc. 2009;23:2085–2093. doi: 10.1007/s00464-008-0247-8. [DOI] [PubMed] [Google Scholar]
- 16.Kubota T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann. Surg. Oncol. 2014;21:891–898. doi: 10.1245/s10434-013-3384-9. [DOI] [PubMed] [Google Scholar]
- 17.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann. Surg. Oncol. 2013;20:1575–1583. doi: 10.1245/s10434-012-2720-9. [DOI] [PubMed] [Google Scholar]
- 18.Kiuchi J, et al. Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer. Int. J. Clin. Oncol. 2016;21:920–926. doi: 10.1007/s10147-016-0987-8. [DOI] [PubMed] [Google Scholar]
- 19.Shimada H, Fukagawa T, Haga Y, Oba K. Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol Surg. 2017;1:11–23. doi: 10.1002/ags3.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda M, et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. 2019;8:5194–5201. doi: 10.1002/cam4.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 22.Kayani B, et al. Does obesity affect outcomes in patients undergoing esophagectomy for cancer? A meta-analysis. World J. Surg. 2012;36:1785–1795. doi: 10.1007/s00268-012-1582-4. [DOI] [PubMed] [Google Scholar]
- 23.Walter V, et al. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am. J. Clin. Nutr. 2016;104:1110–1120. doi: 10.3945/ajcn.116.136531. [DOI] [PubMed] [Google Scholar]
- 24.Hakimi AA, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J. Natl. Cancer Inst. 2013;105:1862–1870. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kichenadasse G, et al. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokunaga M, et al. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann. Surg. Oncol. 2009;16:3245–3251. doi: 10.1245/s10434-009-0645-8. [DOI] [PubMed] [Google Scholar]
- 27.Oh SJ, et al. Effect of being overweight on postoperative morbidity and long-term surgical outcomes in proximal gastric carcinoma. J. Gastroenterol. Hepatol. 2009;24:475–479. doi: 10.1111/j.1440-1746.2008.05704.x. [DOI] [PubMed] [Google Scholar]
- 28.Kulig J, et al. Implications of overweight in gastric cancer: A multicenter study in a Western patient population. Eur. J. Surg. Oncol. 2010;36:969–976. doi: 10.1016/j.ejso.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Bickenbach KA, et al. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann. Surg. Oncol. 2013;20:780–787. doi: 10.1245/s10434-012-2653-3. [DOI] [PubMed] [Google Scholar]
- 30.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 32.Gunasekaran MK, et al. Inflammation triggers high mobility group box 1 (HMGB1) secretion in adipose tissue, a potential link to obesity. Cytokine. 2013;64:103–111. doi: 10.1016/j.cyto.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, et al. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol. Cell. Endocrinol. 2017;454:103–111. doi: 10.1016/j.mce.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Takaki W, et al. Role of extracellular high-mobility group box-1 as a therapeutic target of gastric cancer. Int. J. Mol. Sci. 2022 doi: 10.3390/ijms23063264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osoegawa A, et al. Plasma high-mobility group box 1 as an indicator of surgical stress. Surg. Today. 2011;41:903–907. doi: 10.1007/s00595-010-4371-4. [DOI] [PubMed] [Google Scholar]
- 36.Katsurahara K, et al. Relationship between postoperative CRP and prognosis in thoracic esophageal squamous cell carcinoma. Anticancer Res. 2018;38:6513–6518. doi: 10.21873/anticanres.13016. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara D, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int. J. Clin. Oncol. 2020;25:602–613. doi: 10.1007/s10147-019-01580-1. [DOI] [PubMed] [Google Scholar]
- 38.Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer24, 1-21, doi:10.1007/s10120-020-01042-y (2021). [DOI] [PMC free article] [PubMed]
- 39.New criteria for 'obesity disease' in Japan. Circ J66, 987-992, doi:10.1253/circj.66.987 (2002). [DOI] [PubMed]
- 40.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the personal information protection law in Japan but are available after the permission from the institutional review board and the corresponding author on reasonable request.