Abstract
Background
Electronic cigarette (e-cigarette) vapour is gaining popularity as an alternative to tobacco smoking and can induce acute lung injury. However, the specific role of nicotine in e-cigarette vapour and its long-term effects on the airways, lung parenchyma and vasculature remain unclear.
Results
In vitro exposure to nicotine-containing e-cigarette vapour extract (ECVE) or to nicotine-free e-cigarette vapour extract (NF ECVE) induced changes in gene expression of epithelial cells and pulmonary arterial smooth muscle cells (PASMCs), but ECVE in particular caused functional alterations (e.g. a decrease in human and mouse PASMC proliferation by 29.3±5.3% and 44.3±8.4%, respectively). Additionally, acute inhalation of nicotine-containing e-cigarette vapour (ECV) but not nicotine-free e-cigarette vapour (NF ECV) increased pulmonary endothelial permeability in isolated lungs. Long-term in vivo exposure of mice to ECV for 8 months significantly increased the number of inflammatory cells, in particular lymphocytes, compared to control and NF ECV in the bronchoalveolar fluid (BALF) (ECV: 853.4±150.8 cells·mL−1; control: 37.0±21.1 cells·mL−1; NF ECV: 198.6±94.9 cells·mL−1) and in lung tissue (ECV: 25.7±3.3 cells·mm−3; control: 4.8±1.1 cells·mm−3; NF ECV: 14.1±2.2 cells·mm−3). BALF cytokines were predominantly increased by ECV. Moreover, ECV caused significant changes in lung structure and function (e.g. increase in airspace by 17.5±1.4% compared to control), similar to mild tobacco smoke-induced alterations, which also could be detected in the NF ECV group, albeit to a lesser degree. In contrast, the pulmonary vasculature was not significantly affected by ECV or NF ECV.
Conclusions
NF ECV components induce cell type-specific effects and mild pulmonary alterations, while inclusion of nicotine induces significant endothelial damage, inflammation and parenchymal alterations.
Short abstract
E-cigarette use, particularly with nicotine-containing vapour, is a harmful alternative to tobacco smoking. Nicotine-containing e-cigarette vapour increases pulmonary endothelial permeability, induces inflammation and causes airway and parenchymal alterations. https://bit.ly/40s24n9
Introduction
Chronic exposure to cigarette smoke (CS) is a major trigger of chronic obstructive pulmonary disease (COPD), which affects more than 174 million people worldwide [1]. COPD comprises pulmonary inflammation, airway obstruction, pulmonary emphysema and often pulmonary hypertension (PH) [2]. Aside from nicotine, numerous ingredients in tobacco smoke are responsible for increased oxidative stress and activation of immune cells, leading to airway destruction and chronic inflammation in the lung [3]. Therefore, the substitution of traditional tobacco smoking with electronic cigarettes (e-cigarettes) containing liquids that optionally include nicotine and/or different flavours to produce an aerosol (commonly referred to as vapour or e-cigarette vapour) for inhalation has grown in popularity as a healthier alternative to cigarette smoking, especially among young people [4].
Despite a growing number of studies in this area, there is currently no consensus on the effects of e-cigarette smoking on human health. The large variability of e-liquid content (e.g. flavours, nicotine), technical specifications of e-cigarettes (e.g. voltage, temperature) and user habits (e.g. puff duration, number of puffs) and the relatively short period that e-cigarettes have been in use hamper robust scientific conclusions, in particular those concerning their long-term effects [5]. Accordingly, studies in humans report discrepant results with a decrease [6], an increase [7] or no change [8] in inflammatory markers after short-term use of e-cigarettes. Although, until recently, studies in humans had not found severe effects of e-cigarette use on health [5], more than 2600 cases of acute e-cigarette or vaping-product-associated lung injury have been reported in recent years [9].
Owing to these variabilities and limited experience with long-term effect in humans, it is necessary to assess the effects of e-cigarette vapour in a clearly defined animal model. Previously, discrepant results from in vivo studies concerning the development of CS-like lung structural alterations, such as emphysema, have been reported: some studies show signs of emphysema after 4 months [10] and others demonstrate no effect after 8 months [11] of exposure to nicotine-containing (18 mg·mL−1) e-cigarette vapour. Furthermore, cell type-specific effects on primary lung cells and the impacts of e-cigarette vapour on pulmonary vasculature remain to be fully addressed. In this regard, previous in vitro experiments suggested cell type-specific cytotoxic effects of different e-cigarette preparations depending on their ingredients [12–16].
Therefore, we investigated 1) the in vitro effects of nicotine-containing e-cigarette vapour extract (ECVE) and nicotine-free e-cigarette vapour extract (NF ECVE) on different human and murine pulmonary cell types; 2) the short-term ex vivo effects of nicotine-containing e-cigarette vapour (ECV) and nicotine-free e-cigarette vapour (NF ECV) on endothelial permeability in isolated perfused and ventilated mouse lungs; and 3) the long-term in vivo effects of ECV and NF ECV on pulmonary inflammation, function, structure and the vasculature.
Methods
For detailed methods, please see the supplementary material.
Animal experiments
Wild-type C57BL/6J mice were obtained from Charles River Laboratories (Sulzfeld, Germany). Animals were housed under controlled conditions of a 14/10 h daylight/night cycle with food and water supplied ad libitum. All experiments were approved by the governmental ethics committee for animal welfare (Regierungspräsidium Giessen, Germany, GI 20/10, Nr. 105/2014, GI 20/10, Nr. 74/2016, GI 20/10, number 115/2014).
Cell culture
Primary mouse alveolar type II (mATII) cells were isolated, as described previously, by negative selection of CD16/32+ cells (553142, BD Biosciences, Franklin Lakes, NJ, USA) and CD45+ cells (553076, BD Biosciences) [17]. Primary mouse pulmonary arterial smooth muscle cells (mPASMCs) were isolated from precapillary pulmonary arterial vessels as described previously [18]. Primary human pulmonary arterial smooth muscle cells (hPASMCs) were isolated from pulmonary arteries of donor lung transplants by dissection of the medial layer. The studies with hPASMCs were approved by the Ethics Committee of the Faculty of Medicine at Justus-Liebig University Giessen (AZ 58/15, AZ 10/06). A549 cells were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and human bronchial epithelial cells (HBEpC) from PromoCell (Heidelberg, Germany). All cells were cultured in a humidified atmosphere of 5% CO2 at 37°C in a cell incubator. 24 h after seeding, the cell culture medium was replaced with a medium containing different doses of ECVE, NF ECVE or cigarette smoke extract (CSE) (for preparation, see below). The medium bubbled with room air served as the control medium. For detailed isolation protocols and functional assays, please refer to the supplementary material.
Preparation of ECVE and CSE for in vitro assays
100% ECVE or NF ECVE was produced by bubbling the vapour from an e-cigarette (2.2 Ohm, 3.3 V, Joyetech eGo-C, Shenzhen, China) filled with 0.8 mL e-cigarette liquid (60% propylene glycol, 30% glycerol and 10% water; Riccardo Retail GmbH, Neubrandenburg, Germany) either containing 18 mg·mL−1 nicotine or without nicotine through 10 mL of culture medium. The puffing condition was 15 puffs of 4 s duration with 20 s intervals between them. 100% CSE was freshly prepared by bubbling 10 mL of basal cell culture medium (without fetal calf serum) with mainstream smoke of one 3R4F cigarette (University of Kentucky, Lexington, USA). After pH adjustment to 7.4, the medium was sterile-filtered through a 0.22-µm pore filter and the CSE concentration was determined spectrophotometrically (absorbance 290 nm). For control experiments, 100% medium for the respective cell type was bubbled with room air for 5 min.
E-cigarette vapour application in the isolated ventilated and perfused mouse lung system
The isolated ventilated and perfused mouse lung system was used to investigate the effects of repetitive intra-tracheal application of ECV and NF ECV on hypoxic pulmonary vasoconstriction and the capillary filtration coefficient (Kfc) [19]. A detailed description is provided within the supplementary material.
Animals and e-cigarette vapour exposure
To generate e-cigarette vapour for animal exposure, Joyetech eVic-VTC Mini e-cigarettes (coil resistance: 0.15 Ohm, 4.1 V, 15–50 W, Riccardo Retail GmbH) were integrated into a custom-made “inExpose” inhalation exposure chamber (SCIREQ Scientific Respiratory Equipment Inc., Montreal, Canada). This automated system (FlexiWare 6.1, SCIREQ Scientific Respiratory Equipment Inc.) was set to inject one 60 mL puff·min−1 with a flow of 3 L·min−1 into the whole-body exposure chamber. Mice were randomly allocated to the control group or the ECV or NF ECV exposure groups for 6 h·day−1, 5 days·week−1 for 8 months. Control animals were housed under otherwise identical conditions to those of the ECV/NF ECV-exposed mice and were age- and sex-matched. Commercially available e-liquids (60% propylene glycol, 30% glycerol and 10% water (Avoria GmbH, Nuremberg, Germany)) with (18 mg·mL−1) or without (0 mg·mL−1) nicotine or flavouring were used. A MicroDust Pro (UT-CEL712, Casella Measurements, Bedford, UK) device was integrated into the vapour exposure system for real-time monitoring of aerosols. For conventional CS, the mice were exposed as previously described [17].
In vivo haemodynamics, lung function, micro-computed tomography imaging and echocardiography
Lung function and structure and pulmonary vascular function and structure were determined as described previously [17, 20]. Heart function was measured by transthoracic echocardiography using a VEVO770 or VEVO2100 system (Visualsonics, Toronto, Canada). In vivo micro-computed tomography (micro-CT) images were acquired using a Quantum GX microCT Imaging System (PerkinElmer, Waltham, MA, USA) as previously described [20].
Flow cytometry
Flow cytometry was performed with an LSRII flow cytometer (BD Biosciences) using the DIVA software (BD Biosciences) as previously described [21, 22]. For detailed methods and the gating strategy, please refer to the supplementary material.
Immunohistochemistry
Immunohistochemical staining was performed using 3-μm sections of mouse lungs [23]. CD3+ cells were counted per section area of the lung. CD45+ cells were counted around vessels, septa and bronchi in randomly selected fields according to the following scale: 0: small number of CD45+ cells; 1: moderate number of CD45+ cells; and 2: many CD45+ cells. A detailed description is provided within the supplementary material.
Multiplex assay
A custom-made mouse magnetic bead-based multiplex assay was used to analyse the levels of selected inflammatory mediators in bronchoalveolar lavage fluid (BALF) according to the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Data are reported as mean±sem. Statistical analyses using t-test or one-way ANOVA with Tukey's post hoc test were carried out using GraphPad Prism (GraphPad Software, San Diego, CA, USA). The combined p-value of the in vivo lung function measurements was calculated by a meta-analysis using Fisher's method and the BisRNA R package (R Foundation, Vienna, Austria). Categorical analysis was done using pair-wise Wilcoxon tests with continuity correction to evaluate the data from immunohistochemical staining of CD45+ cells. For comparison of the in vivo effects of ECV and NF ECV, binominal analysis was performed using Binom.Dist in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). For this analysis, parameters were categorised as alterations either compatible or incompatible with pathological changes seen in smoke-induced emphysema or PH using differences in mean values of the different parameters.
Results
ECVE and NF ECVE differentially affected metabolic activity and proliferation of murine and human cultured pulmonary cells
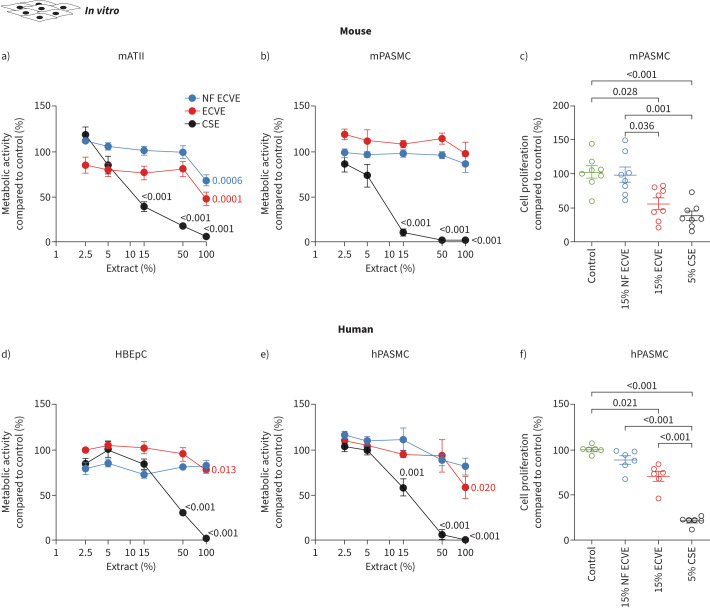
High-dose NF ECVE treatment decreased metabolic activity in isolated mATII cells and A549 cells only (figure 1a, supplementary figure S1a), while ECVE treatment decreased metabolic activity in all epithelial cell types and hPASMCs at high concentrations (100% ECVE; figure 1d, e; supplementary figure S1a). Lower concentrations of ECVE but not NF ECVE (15%) that did not affect metabolic activity did diminish the proliferation of mPASMCs and hPASMCs (figure 1c, f; supplementary figure S1b). Accordingly, only 15% ECVE decreased mPASMC and hPASMC confluence, without affecting migration or the number of dead cells (supplementary figure S1c–g). Moreover, ECVE and NF ECVE did not show any cytotoxic effects at any concentration (supplementary figure S2a–e) or induce apoptosis compared to the untreated controls (supplementary figure S2f–j). In contrast, exposure to CSE decreased the metabolic activity of all cell types (figure 1a, b, d, e; supplementary figure S1a), decreased cell confluence (supplementary figure S1c, d) and migration (supplementary figure S1e, f), induced cellular toxicity and increased apoptosis (supplementary figure S2a–j). The paradoxical reduction in apoptosis in some of these experiments may be explained by faster action of 100% CSE compared to 50% CSE (supplementary figure S2k).
FIGURE 1.
Effect of in vitro nicotine-containing e-cigarette vapour extract (ECVE) or nicotine-free e-cigarette vapour extract (NF ECVE) exposure on metabolic activity and proliferation. a, b) Cell metabolic activity of primary mouse alveolar type II (mATII) cells (a, n=6) and primary mouse pulmonary arterial smooth muscle cells (mPASMCs) (b, n=5) after exposure to different concentrations of either NF ECVE, ECVE or conventional cigarette smoke extract (CSE). Data are presented as percentage of control. c) Cell proliferation of mPASMC (n=8) after exposure to either 15% NF ECVE, 15% ECVE, 5% CSE or control. Data are presented as percentage compared to control. d, e) Cell metabolic activity of primary human bronchial epithelial cells (HBEpCs) (d, n=6) and primary human PASMCs (hPASMCs) (e, n=5) after exposure to different concentrations of either NF ECVE, ECVE or CSE. Data are presented as percentage of control. f) Cell proliferation of hPASMCs (n=6) after exposure to either 15% NF ECVE, 15% ECVE, 5% CSE or control. Data are presented as percentage compared to control. Controls were treated with medium without ECVE, NF ECVE or CSE. Number of mATII cells, mPASMCs and hPASMCs represents independent isolations per group, number of HBEpCs represents independent experiments per group. Statistical analysis was performed by one-way ANOVA with Tukey's post hoc test. Significant p-values in comparison to respective controls are presented. Data are presented as mean±sem.
ECVE and NF ECVE exposure changed gene expression patterns in a cell type-specific manner
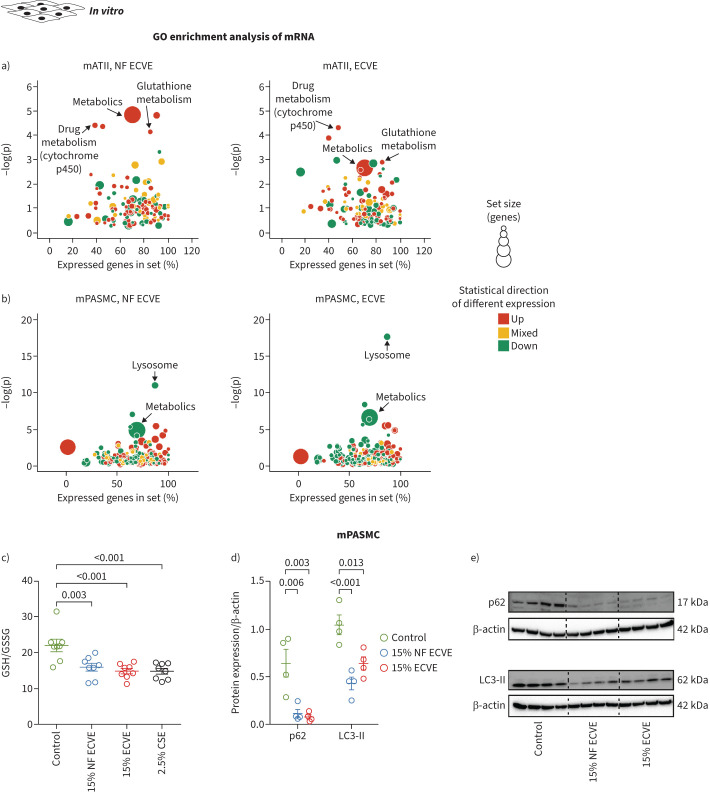
Hypothesis-driven and non-hypothesis-driven approaches were used to study the ECVE and NF EVCE effects on signalling pathways in mPASMCs and mATII cells. First, the expression levels of different genes, which were changed specifically in vessels and/or septa after in vivo CS exposure [24], were investigated in mPASMCs and mATII cells. Only ECVE and not NF ECVE increased the mRNA expression of inducible nitric oxide synthase (Nos2) and cyclin A1 (Ccna1) specifically in mPASMCs (supplementary figure S3a, b). Microarray analysis of ECVE- and NF ECVE-treated mATII and mPASMCs revealed cell type-specific alterations of various pathways in these cells, largely independent of the presence of nicotine in the vapour extract (figure 2a, b; supplementary figures S3c–f, S4a–i and S5a–j). The most consistent regulation of genes after exposure to either ECVE or NF ECVE was an upregulation of DAZ interacting protein 1-like (Dzip1l) in mATII and a downregulation of UDP-N-acetylglucosamine pyrophosphorylase 1-like 1 (Uap1l1) and dipeptidyl peptidase 7 (Dpp7) in mPASMCs (supplementary figure S3c–f). Besides a general effect on metabolic pathways, we found specific upregulation of glutathione metabolism in mATII cells (figure 2a) and downregulation of the lysosomal pathways in mPASMCs (figure 2b). We confirmed these results by showing that the ratio of reduced glutathione (GSH) to oxidised glutathione (GSSG) was decreased by the application of ECVE or NF ECVE in mATII cells (figure 2c) and that protein expression levels of markers of the autophagy-lysosome system, microtubule-associated proteins 1A/1B light chain 3B (LC3-II) and p62, were decreased in mPASMCs (figure 2d, e).
FIGURE 2.
The effects of nicotine-containing e-cigarette vapour extract (ECVE) or nicotine-free e-cigarette vapour extract (NF ECVE) on gene expression patterns, glutathione levels and protein expression of the autophagy-lysosome system. a, b) Gene ontology (GO) enrichment analysis of differentially expressed mRNA transcripts in primary mouse alveolar type II (mATII) cells (a) and primary mouse pulmonary arterial smooth muscle cells (mPASMCs) (b) exposed to either 15% NF ECVE or 15% ECVE. Bubble plots for enrichment analysis of GO terms are presented. The bubble areas indicate the number of genes in the sets, and the colour indicates if most of GO terms were upregulated (red) or downregulated (green). Yellow indicates that both up- and downregulated genes contribute to the enrichment (n=8). The y-axis (−log(p)) displays the significance level, the x-axis the percentage of expressed genes in the respective set of genes for a specific pathway. c) Ratio of reduced glutathione (GSH) to oxidised glutathione (GSSG) in primary mATII cells exposed to either 15% NF ECVE, 15% ECVE, 2.5% cigarette smoke extract (CSE) or control medium without ECVE, NF ECVE or CSE (n=8 each). d, e) Protein expression of p62 and microtubule-associated proteins 1A/1B light chain 3B (LC3-II), normalised to the expression of β-actin, in primary mPASMCs exposed to either 15% NF ECVE, 15% ECVE or control medium without ECVE or NF ECVE (n=4). Numbers represent independent isolations per group. For statistical analysis of c and d, one-way ANOVA with Tukey's post hoc test was used. Data are presented as mean±sem.
ECV and NF ECV inhalation increased endothelial permeability
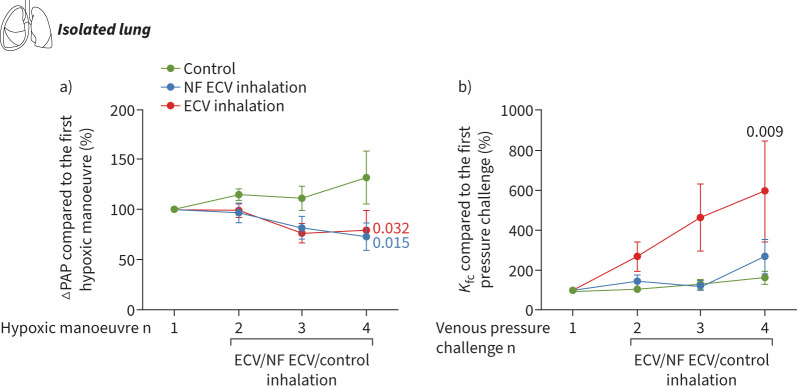
To investigate the effects of ECV and NF ECV on hypoxic pulmonary vasoconstriction and endothelial permeability, we used an isolated perfused and ventilated mouse lung model. Repetitive application of ECV or NF ECV via the trachea attenuated hypoxic pulmonary vasoconstriction in both the ECV and NF ECV groups (figure 3a). However, only the inhalation of ECV (but not of NF ECV) increased the capillary filtration coefficient (Kfc) as a measure of endothelial permeability (figure 3b).
FIGURE 3.
Nicotine-containing e-cigarette vapour (ECV) inhalation increased endothelial permeability in isolated perfused and ventilated mouse lungs. a) Effect of nicotine-free e-cigarette vapour (NF ECV) or ECV on hypoxic pulmonary vasoconstriction, determined as the maximum increase of pulmonary arterial pressure (ΔPAP) during hypoxic ventilation. b) Effect of NF ECV or ECV on the capillary filtration coefficient (Kfc), which was measured gravimetrically and calculated from the slope of lung weight gain induced by an increase of the pulmonary venous pressure from 2 mmHg to 10 mmHg. Data are provided as percentage change of ΔPAP and Kfc compared to the reference hypoxic manoeuvre or pressure challenge without NF ECV or ECV (n=5–6 isolated mouse lungs per group, control lungs were ventilated without NF ECV or ECV). Statistical analysis was performed by one-way ANOVA with Tukeys post hoc test. Data are presented as mean±sem.
Long-term exposure to ECV caused a pulmonary inflammatory response
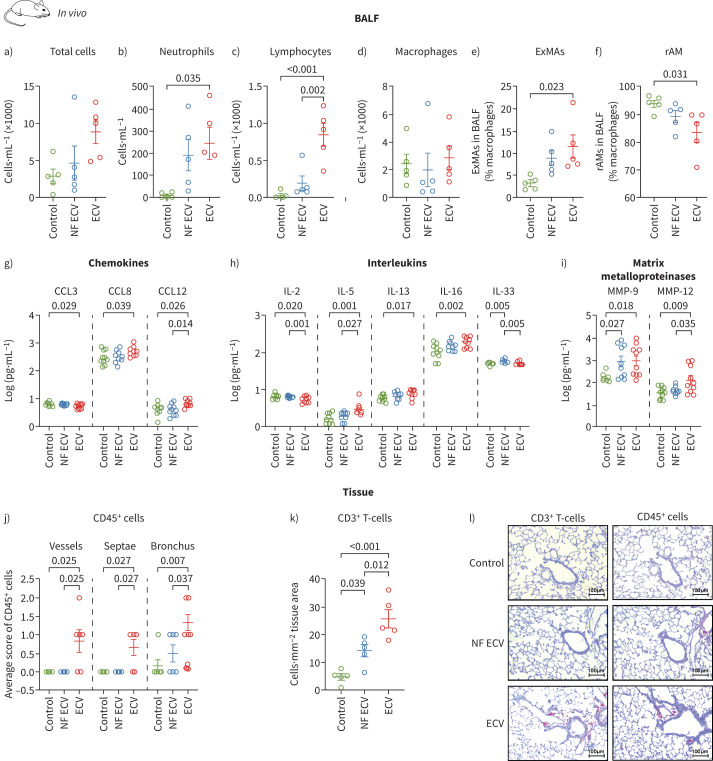
To evaluate the immune response of the lung to ECV and NF ECV exposure, flow cytometry and multiplex immunoassay analysis of BALF from mice exposed to NF ECV or ECV for 8 months were performed (figure 4). The flow cytometry gating strategy is depicted in supplementary figure S6a. Although there was only a trend towards an increase in the total number of cells in BALF from mice exposed to ECV (figure 4a), there was a significant increase in the number of neutrophils (figure 4b, supplementary figure S6b) and lymphocytes (figure 4c, supplementary figure S6b). Moreover, despite an unchanged number of total macrophages after ECV or NF ECV exposure (figure 4d), only ECV significantly shifted the macrophage population from resident macrophages to pro-inflammatory exudative macrophages (figure 4e, f; supplementary figure S6b); NF ECV had no such effect.
FIGURE 4.
Effect of long-term in vivo exposure to nicotine-containing e-cigarette vapour (ECV) or nicotine-free e-cigarette vapour (NF ECV) on pulmonary inflammation. a–d) Total number of cells (a), neutrophils (b), lymphocytes (c) and macrophages (d) in bronchoalveolar lavage fluid (BALF) from mice exposed to NF ECV or ECV for 8 months. e, f) Number of macrophages given for exudate macrophages (ExMAs) (e) and resident macrophages (rAMs) (f). Values are given as the percentage of total macrophages. For a–f, n=5 mice per group. g–i) Levels of selected inflammatory mediators in BALF from mice exposed to NF ECV or ECV for 8 months (n=10 mice per group). j) Number of CD45+ cells located around vessels, alveolar septa or bronchi in lung sections of mice exposed to NF ECV or ECV for 8 months according to the following scale: 0: few CD45+ cells; 1: moderate number of CD45+ cells; 2: many CD45+ cells (n=6 lungs per group). k) Number of CD3+ cells per lung section area in mice exposed to NF ECV or ECV for 8 months (n=5 lungs per group). l) Representative images of lung sections from mice exposed to NF ECV or ECV stained for CD3+ and CD45+ cells. Control animals received room air only. Scale bars: 100 μm. Statistical analysis: a–i, k) one-way ANOVA with Tukey's post hoc test; j) categorical analysis was done using pair-wise Wilcoxon tests with continuity correction. Data are presented as mean±sem.
Multiplex screening of selected inflammatory mediators in BALF showed that ECV altered the levels of C-C motif chemokine ligands (CCLs) in BALF (figure 4g). ECV decreased CCL3 while it increased CCL8 and CCL12 levels, indicating an imbalance of recruitment and activation of different immune cells [25–27]. In addition, ECV increased pro-inflammatory interleukin (IL) levels of IL-5, IL-13 and IL-16 (figure 4h), which promote autoimmune responses as seen in asthma [28], while NF ECV did not. In contrast, ECV exposure decreased the level of IL-2 (figure 4h), the main driver of T-cell proliferation and differentiation, and anti-viral responses [29, 30]. Interestingly, only NF ECV increased the level of IL-33 (figure 4h), which promotes the production of Th2-associated cytokines [31]. Screening of other cytokines and chemokines did not reveal any significant alterations (supplementary figure S7a–c). We also investigated various matrix metalloproteinases (MMP) in BALF, which are known to be associated with increased inflammation in COPD. ECV exposure increased MMP-9 and MMP-12 levels, while NF ECV only increased MMP-9 in BALF from mice exposed to e-cigarette vapour for 8 months (figure 4i). Other MMPs did not reveal any significant alterations (supplementary figure S7d).
To further investigate the effects of ECV and NF ECV on lymphocytes, which showed a change in the initial screening by fluorescence-activated cell sorting (FACS) but were not characterised in detail, we investigated CD45+ cell (leukocyte) and T-cell (CD3+) infiltration in lung sections. The immunohistochemical staining of CD45+ cells revealed that ECV induced a higher accumulation of leukocytes in the different compartments of the lung compared to NF ECV and confirmed the FACS data by showing an increased number of CD3+ T-cells in the lung tissue of ECV-treated mice compared to NF ECV-treated (figure 4j–l).
Long-term exposure to ECV resulted in structural and functional pulmonary changes without effects on the lung vasculature
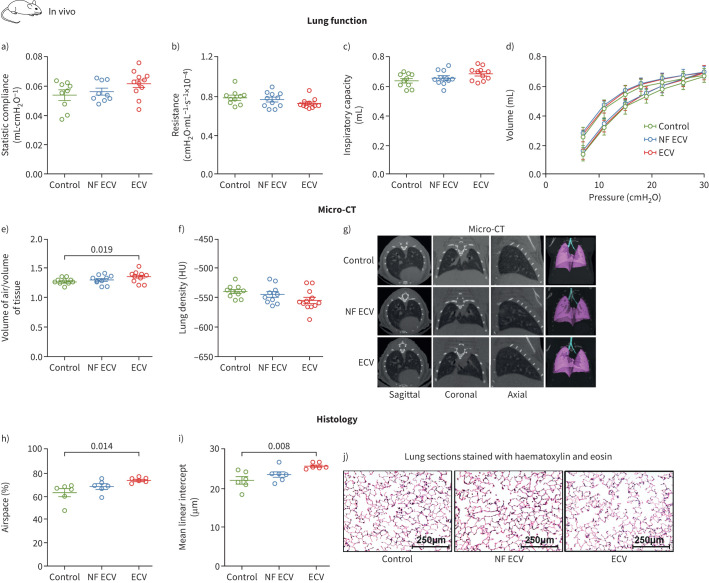
In vivo exposure of mice to ECV in our setup resulted in lower plasma concentrations of nicotine and cotinine compared to conventional CS exposure (nicotine: 3.8±0.9 ng·mL−1 versus 16.0±3.0 ng·mL−1; cotinine: 6.2±0.9 ng·mL−1 versus 50.7±7.4 ng·mL−1; supplementary figure S8a–c). During 8 months of exposure, the mice made similar weight gains in all experimental groups (supplementary figure S8d). The haematocrit was increased in mice after exposure to ECV for 8 months (supplementary figure S8e). Lung function parameters (figure 5a–d) showed a trend for being affected by ECV. Meta-analysis of p-values from lung function (figure 5a: static compliance; figure 5b: resistance; and figure 5c: inspiratory capacity) suggests that ECV induced significant functional alterations similar to CS-induced emphysema-like changes (combined p=0.03); we did not detect significant differences in the NF ECV group. Moreover, only exposure to ECV for 8 months induced significant structural alterations of the pulmonary parenchyma, as determined by in vivo micro-CT imaging (figure 5e–g) and histological analysis (figure 5h–j), while NF ECV exposure did not show this result.
FIGURE 5.
Effect of long-term in vivo exposure to nicotine-containing e-cigarette vapour (ECV) or nicotine-free e-cigarette vapour (NF ECV) on lung function and structure parameters in mice. a–d) Lung function parameters (n=9–11) of static compliance (a), resistance (b), inspiratory capacity (c) and pressure–volume loops (d) of mice exposed to NF ECV, ECV or room air (control) for 8 months. e–j) Lung structure parameters from in vivo micro-computed tomography (CT) measurements (n=9–12) of air/tissue ratio (e), lung density in Hounsfield units (HU) (f) and representative micro-CT imaging (g), or from histological analysis (n=6) of airspace (as a percentage of total area) (h), mean linear intercept (i) and representative pictures (j). Lung sections were stained with haematoxylin and eosin. Scale bars: 250 μm. Statistical analysis: one-way ANOVA with Tukey's post hoc test. Combined p-value from e, f, g, p=0.03 determined by meta-analysis according to Fisher's method using the BisRNA R package. Data are presented as mean±sem.
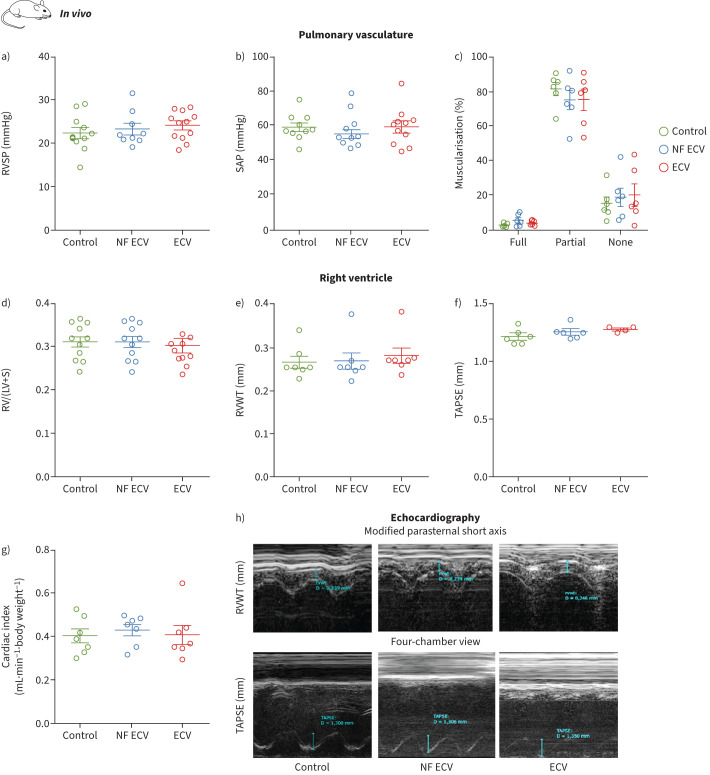
By contrast, after long-term exposure of mice to ECV or NF ECV, we did not detect statistically significant alterations indicating induction of PH, which was assessed by haemodynamic measurements and morphological analysis of the pulmonary vasculature (figure 6a–c). Accordingly, long-term exposure of mice to ECV or NF ECV did not significantly affect the Fulton index (figure 6d) or right ventricular or global heart function evaluated by echocardiography (figure 6e–h). Meta-analysis of p-values also did not indicate any statistically significant effect of ECV or NF ECV on the pulmonary vasculature.
FIGURE 6.
Effect of long-term in vivo exposure to nicotine-containing e-cigarette vapour (ECV) or nicotine-free e-cigarette vapour (NF ECV) on the pulmonary circulation in mice. a, b) Haemodynamic measurements (n=9–12): right ventricular systolic pressure (RVSP) (a) and systolic arterial pressure (SAP) (b). c) Morphological analysis of pulmonary vessels. Data are given for fully, partially or not muscularised vessels as a percentage of total vessel count (n=6). d) Fulton index (ratio of the weight of the right ventricle (RV) to the left ventricle plus septum (LV+S)) (n=10–11). The LV+S weight was not changed between the groups. e–h) Echocardiographic analysis of right ventricular wall thickness (RVWT) (e), tricuspid annular plane systolic excursion (TAPSE) (f) and cardiac index (g) (n=4–7 each) and representative images of echocardiography (h). Data were assessed from mice either exposed to NF ECV, ECV or room air (control) for 8 months. Statistical analysis was performed by one-way ANOVA with Tukey's post hoc test. Data are presented as mean±sem.
Although no significant effect of NF ECV on single measurement parameters was detected, binominal analysis of parameters characterising the pulmonary airways/parenchyma indicated that the NF ECV group was more affected than the control group and less than the ECV group (table 1). Furthermore, we detected a significant difference between the NF ECV and ECV groups when performing a combined analysis of the inflammatory and pulmonary airway/parenchymal parameters (combined p=0.031), supporting the notion that nicotine promotes the effects of e-cigarette vapour on inflammation and airway/parenchymal parameters.
TABLE 1.
Binominal analysis of parameters characterising lung airways and parenchyma and the pulmonary vasculature
| Control versus NF ECV | Control versus ECV | NF ECV versus ECV | |
| Lung airways and parenchyma | |||
| Static compliance | Compatible | Compatible | Compatible |
| Resistance | Compatible | Compatible | Compatible |
| Inspiratory capacity | Compatible | Compatible | Compatible |
| Air/tissue, lung density# | Compatible | Compatible | Compatible |
| Airspace | Compatible | Compatible | Compatible |
| MLI | Compatible | Compatible | Compatible |
| Number of compatible changes (n) | 6 | 6 | 6 |
| Total number of analysed parameters (n) | 6 | 6 | 6 |
| p-value | 0.031 | 0.031 | 0.031 |
| Pulmonary vasculature | |||
| Heart ratio | No change | Not compatible | Not compatible |
| RVSP | Compatible | Not compatible | Compatible |
| RVWT | Compatible | Compatible | Compatible |
| TAPSE | Not compatible | Not compatible | Not compatible |
| Degree of muscularisation | Not compatible | Not compatible | Not compatible |
| Number of compatible changes (n) | 2 | 1 | 2 |
| Total number of analysed parameters (n) | 5 | 5 | 5 |
| p-value | >0.99 | >0.99 | >0.99 |
Binominal analysis of parameters characterising lung airways and parenchyma and the pulmonary vasculature. “Compatible” indicates that the parameter was compatible with a certain pathology (smoke-induced emphysema or pulmonary hypertension) (green); “not compatible” indicates that the parameter was not compatible with a certain pathology (red); “no changes” indicates that the parameter was not changed (blue). Binominal analysis was performed by using Binom.Dist in Microsoft Excel. NF ECV: nicotine-free e-cigarette vapour; ECV: nicotine-containing e-cigarette vapour; MLI: mean linear intercept; RVSP: right ventricular systolic pressure; RVWT: right ventricular wall thickness; TAPSE: tricuspid annular plane systolic excursion. #: dependent parameters, regarded as one variable.
Discussion
Our study provides evidence that ECV can induce acute and chronic lung damage. Although both ECV and NF ECV affected functional and gene expression patterns in pulmonary cells in vitro and airway and parenchymal parameters, only ECV significantly increased endothelial permeability ex vivo and promoted inflammation and mild structural and functional pulmonary alterations in vivo, similar to CS-induced alterations after long-term exposure. However, in contrast to CS exposure, we did not detect significant pulmonary vascular alterations.
Our in vitro experiments showed that ECVE and NF ECVE reduce metabolic activity in specific cell types only at high concentrations but are insufficient to induce cytotoxic effects or trigger apoptosis. Furthermore, nicotine promotes the inhibitory effects of e-cigarette vapour on metabolic activity and proliferation, the latter at least in PASMCs. Owing to the lack of general standardisation of e-cigarette vapour generation, we are limited in our ability to compare other published studies with our data [32]. In this regard, some studies reported no effect (regardless of the nicotine content and flavouring) of the pad-collected e-cigarette extracts in CHO-K1/A549 cells [14], while another observed a dose- and flavour-dependent decrease of CALU3 cell viability after e-cigarette vapour exposure [15]. Despite the more pronounced effect of ECVE on cellular functions, microarray analysis showed that exposure to ECVE and NF ECVE changed gene expression patterns differentially in mATII cells compared to mPASMCs but largely independent of nicotine content, suggesting that ECVE and NF ECVE trigger transcriptional alterations with hitherto unknown functional relevance. In line with our microarray data, we showed nicotine-independent downregulation of the GSH/GSSG ratio in mATII cells and of two proteins involved in the autophagy-lysosomal pathway, LC3-II (an active lipid-modified form of LC3) and p62 (autophagy receptor), in mPASMCs. GSH depletion and decreased GSH/GSSG levels occur in many different cell types secondary to CSE-induced oxidative stress [33, 34]. The relevance of the downregulated lysosomal pathway in mATII cells remains unclear; however, alterations of lysosomal pathways have been found to contribute to the pathogenesis of COPD [35].
Because acute respiratory distress syndrome (ARDS) has recently been associated with vaping [36], we investigated the acute effects of ECV inhalation on endothelial permeability in isolated mouse lungs. ECV but not NF ECV increased capillary permeability, indicating the effect of nicotine. Nicotine could directly alter the function of vascular endothelial cells, PASMCs, airway epithelial cells and immune cells expressing nicotinic acetylcholine receptors [37], thus promoting increased capillary permeability. Although using oil-formulated tetrahydrocannabinol or cannabidiol in ECV was suggested to be associated with vaping-induced ARDS, our data indicate that nicotine in e-cigarettes may contribute to ARDS development [6, 7, 36–39]. Similarly, CS increases the risk of ARDS because of alterations in pulmonary vascular permeability and endothelial barrier function [38]. In addition to endothelial permeability, pulmonary vasoreactivity, in response to acute hypoxia, was affected by e-cigarette vapour, independent of the presence of nicotine. This suggests that ECV or NF ECV may alter the endothelial release of vasoactive mediators, similar to inhaled CS that reverses human hypoxic pulmonary vasoconstriction through the NO–cGMP signalling pathway [40].
In accordance with our ex vivo studies, in vivo ECV or NF ECV treatment for 8 months showed different effects related to the presence or absence of nicotine. Only exposure to ECV, not NF ECV, resulted in a significant increase of inflammatory cells in the BALF, as well as of CD45+ in different compartments of the lung. ECV exposure resulted in a significantly higher accumulation of CD3+ T-cells in the lungs compared to NF ECV. In this regard, FACS analysis of BALF may be limited, because lymphocytes were only identified by their scattering properties in our study and were not further characterised. BALF analysis also showed that the presence of nicotine enhanced recruitment and activation of immune cells such as T-cells, eosinophils and macrophages, indicated by increased CCL8 and CCL12 [25–27], and triggers an inflammatory response similar to that seen in asthma, indicated by an increase in IL-5 and IL-13 [41, 42]. Therefore, one could speculate that ECV induces an allergic reaction and predisposes to bronchial hyper-reagibility [28]. In line with our data, previous studies have shown that exposure to nicotine-containing ECV alters lung inflammatory responses in mice after 3 days [43], 2 weeks [44] and 4 months [10] and in humans after 4 weeks [45], leading to macrophage-mediated inflammation [44] and increased levels of pro-inflammatory cytokines [43]. Moreover, the presence of nicotine increases lymphocyte levels in human BALF [44]. In our study, ECV significantly increased MMP-9 and MMP-12 levels in BALF, while NF ECV increased MMP-9 levels only. MMP-9 and MMP-12 are important mediators associated with inflammation and the development of COPD [46, 47]. These findings align with our in vivo study, demonstrating a more pronounced effect of ECV than NF ECV on pulmonary structural alterations, which showed characteristics of CS-induced alterations, albeit less pronounced. Previous in vivo studies suggest the development of emphysema [10], lung function alterations [48] or no effect [11] of long-term ECV treatment in mice. However, differences in mice strains [10], age of mice [48] and daily duration of ECV exposure [11] may explain discrepancies with regard to the severity of lung functional and structural alterations.
Plasma nicotine levels of 3.8±0.9 ng·mL−1 in our study are comparable to low concentrations in human e-cigarette users. In clinical studies, the levels of nicotine in the plasma of e-cigarette users vary from ∼1 ng·mL−1 to ∼50 ng·mL−1 depending on study design, the e-cigarette device etc. [49, 50]. Interestingly, a prospective human study over 3.5 years did not find any functional or structural changes in nine subjects who used e-cigarettes daily [51]; however, the exposure time was much lower compared to the mouse model. Moreover, from our study, we cannot exclude a harmful effect of NF ECV because we found in the distribution analysis of pathological values an effect of NF ECV compared to control. However, we did not find a statistically significant difference for single parameters.
In contrast to lung function, airway and alveolar alterations, we did not detect pulmonary vascular alterations indicating development of PH, a frequent comorbidity of COPD [2]. Previous investigations on the impact of ECV on global heart function could not find any ECV-induced alterations after short-term (14 day) [52] and long-term (8 month) [11] exposure in mice. However, 8 months of ECV exposure induced increased aortic stiffness [52]. A recent study using high doses of nicotine induced PH development [53]. Thus, despite ECVE affecting PASMC in vitro, ECV in vivo did not induce pulmonary vascular remodelling in our setting.
A study limitation is the relatively low number in the in vivo experiments and the isolated lung setup, which may mask subtle changes in the different parameters assessed, especially regarding possible differences between NF ECV and ECV. However, the finding of the pronounced effect of ECV in these two independent experimental setups supports the conclusion that nicotine promotes the deleterious effects of e-cigarette vapour.
In summary, we have shown that the presence of nicotine in e-cigarette vapour promotes acute endothelial damage, pulmonary inflammation and chronic pulmonary functional and structural alterations. Although research on e-cigarette use is hampered by the lack of standardisation in methods to produce and apply e-cigarette vapour, this diversity may reflect the variety of human situations in which application depends on e-liquid composition, puffing topography and e-cigarette characteristics [50].
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods ERJ-00951-2022.Supplement (374.2KB, pdf)
Supplementary figure S1 ERJ-00951-2022.Figure_S1 (249.3KB, pdf)
Supplementary figure S2 ERJ-00951-2022.Figure_S2 (674.4KB, pdf)
Supplementary figure S3 ERJ-00951-2022.Figure_S3 (2.5MB, pdf)
Supplementary figure S4 ERJ-00951-2022.Figure_S4 (1.6MB, pdf)
Supplementary figure S5 ERJ-00951-2022.Figure_S5 (2MB, pdf)
Supplementary figure S6 ERJ-00951-2022.Figure_S6 (888.6KB, pdf)
Supplementary figure S7 ERJ-00951-2022.Figure_S7 (687KB, pdf)
Supplementary figure S8 ERJ-00951-2022.Figure_S8 (546.7KB, pdf)
Shareable PDF
Acknowledgement
The authors thank Christine Veith, Nils Schupp, Ingrid Breitenborn-Müller, Carmen Homberger, Elisabeth Kappes, Miriam Wessendorf, Susanne Lich, Christina Vroom and Karin Quanz (Justus Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI), Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany) for technical assistance.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00886-2023
Portions of the doctoral thesis of E.T. Roxlau are incorporated into this report.
Author contributions: E.T. Roxlau, O. Pak, R.P. Brandes, N. Weissmann and N. Sommer contributed to study design, data analysis and interpretation; E.T. Roxlau, O. Pak, A. Pichl, C.F. Garcia-Castro, M. Bednorz, S. Hadzic, D. Spiegelberg, B. Selvakumar, J. Schäffer, K. Schäfer, S. Kraut, S. Herold, M. Gredic, D. Kosanovic, E.M. Zeidan, B. Kojonazarov, I. Strielkov, J. Wilhelm, J. Deutscher and M. Hecker were study investigators who collected and assessed the data; E.T. Roxlau, O. Pak, N. Weissmann, R.P. Brandes and N. Sommer drafted the manuscript; M.M.A. Khalifa, A. Taye, F. Grimminger, R.T. Schermuly, H.A. Ghofrani, R.P. Brandes and W. Seeger critically reviewed the manuscript. All authors reviewed and approved the final manuscript. E.T. Roxlau is designated the first co-author, as she performed all in vitro experiments and completed the project; O. Pak is designated the second co-author, as he performed the in vivo experiments.
Conflict of interest: All authors have nothing to disclose.
Support statement: This work was supported by the German Research Foundation (DFG), project ID 268555672 – SFB 1213, A07 and CP02, and the Balzan prize to the research group of the German Center for Lung Research (DZL): Erika von Mutius, Klaus F. Rabe, Werner Seeger and Tobias Welte. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Finks SW, Rumbak MJ, Self TH. Treating hypertension in chronic obstructive pulmonary disease. N Engl J Med 2020; 382: 353–363. doi: 10.1056/NEJMra1805377 [DOI] [PubMed] [Google Scholar]
- 2.Gredic M, Blanco I, Kovacs G, et al. Pulmonary hypertension in chronic obstructive pulmonary disease. Br J Pharmacol 2021; 178:132–151. doi: 10.1111/bph.14979 [DOI] [PubMed] [Google Scholar]
- 3.Pappas RS. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics 2011; 3: 1181–1198. doi: 10.1039/c1mt00066g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28: 146–151. doi: 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur G, Pinkston R, McLemore B, et al. Immunological and toxicological risk assessment of e-cigarettes. Eur Respir Rev 2018; 27: 170119. doi: 10.1183/16000617.0119-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 2012; 141: 1400–1406. doi: 10.1378/chest.11-2443 [DOI] [PubMed] [Google Scholar]
- 7.Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 2014; 217: 628–637. doi: 10.1016/j.ijheh.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 8.Flouris AD, Chorti MS, Poulianiti KP, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 2013; 25: 91–101. doi: 10.3109/08958378.2012.758197 [DOI] [PubMed] [Google Scholar]
- 9.Cherian SV, Kumar A, Estrada YMRM. E-cigarette or vaping product-associated lung injury: a review. Am J Med 2020; 133: 657–663. doi: 10.1016/j.amjmed.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Arcos I, Geraghty P, Baumlin N, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 2016; 71: 1119–1129. doi: 10.1136/thoraxjnl-2015-208039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olfert IM, DeVallance E, Hoskinson H, et al. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985) 2018; 124: 573–582. doi: 10.1152/japplphysiol.00713.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahl V, Lin S, Xu N, et al. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 2012; 34: 529–537. doi: 10.1016/j.reprotox.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Romagna G, Allifranchini E, Bocchietto E, et al. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol 2013; 25: 354–361. doi: 10.3109/08958378.2013.793439 [DOI] [PubMed] [Google Scholar]
- 14.Misra M, Leverette RD, Cooper BT, et al. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health 2014; 11: 11325–11347. doi: 10.3390/ijerph111111325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowell TR, Reeber SL, Lee SL, et al. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol 2017; 313: L52–L66. doi: 10.1152/ajplung.00392.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie LJ, Vasanthi Bathrinarayanan P, Jackson P, et al. A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal Toxicol 2017; 29: 126–136. doi: 10.1080/08958378.2017.1318193 [DOI] [PubMed] [Google Scholar]
- 17.Seimetz M, Sommer N, Bednorz M, et al. NADPH oxidase subunit NOXO1 is a target for emphysema treatment in COPD. Nat Metab 2020; 2: 532–546. doi: 10.1038/s42255-020-0215-8 [DOI] [PubMed] [Google Scholar]
- 18.Sommer N, Huttemann M, Pak O, et al. Mitochondrial complex IV subunit 4 isoform 2 is essential for acute pulmonary oxygen sensing. Circ Res 2017; 121: 424–438. doi: 10.1161/CIRCRESAHA.116.310482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeger W, Walmrath D, Menger M, et al. Increased lung vascular permeability after arachidonic acid and hydrostatic challenge. J Appl Physiol (1985) 1986; 61: 1781–1789. doi: 10.1152/jappl.1986.61.5.1781 [DOI] [PubMed] [Google Scholar]
- 20.Kojonazarov B, Hadzic S, Ghofrani HA, et al. Severe emphysema in the SU5416/hypoxia rat model of pulmonary hypertension. Am J Respir Crit Care Med 2019; 200: 515–518. doi: 10.1164/rccm.201902-0390LE [DOI] [PubMed] [Google Scholar]
- 21.Peteranderl C, Morales-Nebreda L, Selvakumar B, et al. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest 2016; 126: 1566–1580. doi: 10.1172/JCI83931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herold S, Tabar TS, Janssen H, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 2011; 183: 1380–1390. doi: 10.1164/rccm.201009-1431OC [DOI] [PubMed] [Google Scholar]
- 23.Gredic M, Wu CY, Hadzic S, et al. Myeloid-cell-specific deletion of inducible nitric oxide synthase protects against smoke-induced pulmonary hypertension in mice. Eur Respir J 2022; 59: 2101153. doi: 10.1183/13993003.01153-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011; 147: 293–305. doi: 10.1016/j.cell.2011.08.035 [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Yang G, Xiong X, et al. Age-related CCL12 aggravates intracerebral hemorrhage-induced brain injury via recruitment of macrophages and T lymphocytes. Aging Dis 2020; 11: 1103–1115. doi: 10.14336/AD.2019.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia GQ, Gonzalo JA, Lloyd C, et al. Distinct expression and function of the novel mouse chemokine monocyte chemotactic protein-5 in lung allergic inflammation. J Exp Med 1996; 184: 1939–1951. doi: 10.1084/jem.184.5.1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang P, Chen W, Xu H, et al. Correlation of CCL8 expression with immune cell infiltration of skin cutaneous melanoma: potential as a prognostic indicator and therapeutic pathway. Cancer Cell Int 2021; 21: 635. doi: 10.1186/s12935-021-02350-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res 2018; 10: 428–447. doi: 10.4168/aair.2018.10.5.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol 2018; 36: 411–433. doi: 10.1146/annurev-immunol-042617-053352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blattman JN, Grayson JM, Wherry EJ, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 2003; 9: 540–547. doi: 10.1038/nm866 [DOI] [PubMed] [Google Scholar]
- 31.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011; 8: 22. doi: 10.1186/1476-9255-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.E-cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA, Centers for Disease Control and Prevention, 2016. [Google Scholar]
- 33.Dalle-Donne I, Garavaglia ML, Colombo G, et al. Cigarette smoke and glutathione: focus on in vitro cell models. Toxicol In Vitro 2020; 65: 104818. doi: 10.1016/j.tiv.2020.104818 [DOI] [PubMed] [Google Scholar]
- 34.Bazzini C, Rossetti V, Civello DA, et al. Short- and long-term effects of cigarette smoke exposure on glutathione homeostasis in human bronchial epithelial cells. Cell Physiol Biochem 2013; 32: 129–145. doi: 10.1159/000356633 [DOI] [PubMed] [Google Scholar]
- 35.Zhao J, Zhang Y, Sisler JD, et al. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J Hazard Mater 2018; 344: 549–557. doi: 10.1016/j.jhazmat.2017.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilly CM, Khan S, Waksmundzki-Silva K, et al. Vaping-associated respiratory distress syndrome: case classification and clinical guidance. Crit Care Explor 2020; 2: e0081. doi: 10.1097/CCE.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diabasana Z, Perotin JM, Belgacemi R, et al. Nicotinic receptor subunits atlas in the adult human lung. Int J Mol Sci 2020; 21: 7446. doi: 10.3390/ijms21207446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rounds S, Lu Q. Cigarette smoke alters lung vascular permeability and endothelial barrier function (2017 Grover Conference Series). Pulm Circ 2018; 8: 2045894018794000. doi: 10.1177/2045894018794000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christiani DC. Vaping-induced acute lung injury. N Engl J Med 2020; 382: 960–962. doi: 10.1056/NEJMe1912032 [DOI] [PubMed] [Google Scholar]
- 40.Dupuy PM, Lancon JP, Francoise M, et al. Inhaled cigarette smoke selectively reverses human hypoxic vasoconstriction. Intensive Care Med 1995; 21: 941–944. doi: 10.1007/BF01712337 [DOI] [PubMed] [Google Scholar]
- 41.Horikawa K, Takatsu K. Interleukin-5 regulates genes involved in B-cell terminal maturation. Immunology 2006; 118: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015; 75: 68–78. doi: 10.1016/j.cyto.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 2015; 10: e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 2015; 10: e0116861. doi: 10.1371/journal.pone.0116861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song MA, Reisinger SA, Freudenheim JL, et al. Effects of electronic cigarette constituents on the human lung: a pilot clinical trial. Cancer Prev Res (Phila) 2020; 13: 145–152. doi: 10.1158/1940-6207.CAPR-19-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells JM, Parker MM, Oster RA, et al. Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPDGene. JCI Insight 2018; 3: e123614. doi: 10.1172/jci.insight.123614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baggio C, Velazquez JV, Fragai M, et al. Therapeutic targeting of MMP-12 for the treatment of chronic obstructive pulmonary disease. J Med Chem 2020; 63: 12911–12920. doi: 10.1021/acs.jmedchem.0c01285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larcombe AN, Janka MA, Mullins BJ, et al. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am J Physiol Lung Cell Mol Physiol 2017; 313: L67–L79. doi: 10.1152/ajplung.00203.2016 [DOI] [PubMed] [Google Scholar]
- 49.Yingst JM, Foulds J, Veldheer S, et al. Nicotine absorption during electronic cigarette use among regular users. PLoS One 2019; 14: e0220300. doi: 10.1371/journal.pone.0220300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eaton DL, Kwan LY, Stratton K, eds. Public Health Consequences of E-cigarettes. Washington (DC), National Academies Press, 2018. [PubMed] [Google Scholar]
- 51.Polosa R, Cibella F, Caponnetto P, et al. Health impact of e-cigarettes: a prospective 3.5-year study of regular daily users who have never smoked. Sci Rep 2017; 7: 13825. doi: 10.1038/s41598-017-14043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi H, Fan X, Horton A, et al. The effect of electronic-cigarette vaping on cardiac function and angiogenesis in mice. Sci Rep 2019; 9: 4085. doi: 10.1038/s41598-019-40847-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oakes JM, Xu J, Morris TM, et al. Effects of chronic nicotine inhalation on systemic and pulmonary blood pressure and right ventricular remodeling in mice. Hypertension 2020; 75: 1305–1314. doi: 10.1161/HYPERTENSIONAHA.119.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods ERJ-00951-2022.Supplement (374.2KB, pdf)
Supplementary figure S1 ERJ-00951-2022.Figure_S1 (249.3KB, pdf)
Supplementary figure S2 ERJ-00951-2022.Figure_S2 (674.4KB, pdf)
Supplementary figure S3 ERJ-00951-2022.Figure_S3 (2.5MB, pdf)
Supplementary figure S4 ERJ-00951-2022.Figure_S4 (1.6MB, pdf)
Supplementary figure S5 ERJ-00951-2022.Figure_S5 (2MB, pdf)
Supplementary figure S6 ERJ-00951-2022.Figure_S6 (888.6KB, pdf)
Supplementary figure S7 ERJ-00951-2022.Figure_S7 (687KB, pdf)
Supplementary figure S8 ERJ-00951-2022.Figure_S8 (546.7KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00951-2022.Shareable (439.3KB, pdf)