Abstract
Introduction:
Integrins are a family of multi-functional cell-adhesion molecules, heterodimeric receptors that connect extracellular matrix (ECM) to actin cytoskeleton in the cell cortex, thus regulating cellular adhesion, migration, proliferation, invasion, survival, and apoptosis. Consequently, integrins play a role in inflammation, angiogenesis and fibrosis.
Areas covered:
This review examines individual anti-integrin agents in terms of their chemical nature, route of administration, and anti-integrin action. It also provides a summary of preclinical and clinical studies. Current clinical candidates include risuteganib, THR-687, and SF-0166, which have shown promise in treating diabetic macular edema (DME) and/or age-related macular degeneration (AMD) in early clinical studies. Preclinical candidates include SB-267268, AXT-107, JNJ-26076713, Cilengitide and Lebecetin, which exhibit a decrease in retinal permeability, angiogenesis and/or choroidal neovascularization (CNV).
Expert opinion:
Anti-integrin therapies show potential in treating retinal diseases. Anti-integrin agents tackle the multi-factorial nature of diabetic retinopathy (DR) and AMD and show promise as injectable and topical agents in preclinical and early clinical studies. Integrin inhibition has potential to serve as primary therapy, adjunctive therapy to anti-vascular endothelial growth factor agents, or secondary therapy in refractory cases.
Keywords: Age-related macular degeneration, diabetic retinopathy, diabetic macular edema, vitreomacular traction, angiogenesis, integrin, extracellular matrix, Risuteganib, THR-687, SF-0166, SB-267268, AXT-107, JNJ-26076713, Cilengitide, Lebecetin
1. Introduction
Since their discovery in the 1980s, integrins have been shown to play a key role in multiple diseases, including cancer, diabetic eye diseases, and age-related macular degeneration (AMD). Named for their function as ‘integrators’ of cell surface and extracellular matrix (ECM), integrins are a family of multi-functional cell-adhesion molecules that connect ECM molecules to actin in the cell cortex. Composed of alpha and beta subunits and serving as cell adhesion and cell signaling receptors, they regulate the shape, orientation, and movement of cells. Integrins also regulate a variety of cellular interactions with their microenvironment, such as adhesion, spreading migration, proliferation, invasion, survival, and apoptosis [1]. Consequently, integrins play a role in pathologic processes, such as inflammation, angiogenesis and fibrosis. This report reviews the role of integrins in vitreoretinal diseases and investigational retinal treatments targeting integrins.
2. Integrins
Integrins are heterodimeric receptors that exist in 24 unique combinations of non-covalently interacting α (18 types) and β (8 types) subunits. While some subunits appear only in a single heterodimer, 12 integrins contain β1 subunits, and five integrins contain αv subunits. This heterogeneity in integrins facilitates binding to a variety of ECM components and cell surface receptors [1]. Both α and β integrins are type-1 transmembrane proteins consisting of a large extracellular domain, a single-pass transmembrane helix and a short cytoplasmic domain. The integrin α and β subunits are heterodimerized in the endoplasmic reticulum and are expressed as obligatory heterodimers on the cell surface [2].
Integrins bind to a variety of ligands, including ECM proteins such as fibronectin, vitronectin, laminin, collagen, bone matrix protein, thrombospondin and von Willebrand factor. The Arginine-Glycine-Aspartate (RGD) sequence on ECM proteins, for example, fibronectin, vitronectin and fibrinogen, is the primary binding site for 8 of the 24 known integrins [3]. In this way, integrins play a primary role in cell-to-cell and cell-to-extracellular matrix interactions. In addition, integrins may serve as counter-receptors where they help in mediating cell-cell interactions, and some microorganisms may even utilize integrins to gain entry into cells [4]. For integrins to initiate signal transduction and affect cellular function, binding to a ligand is necessary. Integrins provide a transmembrane link by coupling extracellular and intracellular ligands, and this link enables the bidirectional transmission of mechanical force and biochemical signals across the plasma membrane [5]. The cell regulates integrin signaling by controlling the integrin signaling inside out. Signals generated inside a cell can either enhance or inhibit the ability of integrins to bind to their ligands outside the cell. This is, in particular, relevant to platelet and leukocyte signaling where integrin activation is essential prior to their adhesion. In the other cell types, integrins are maintained in a competent adhesion state. Because of the lack of enzymatic activity in integrins, the signaling is instead induced by the assembly of signaling complex on the face of the plasma membrane via increasing molecular interactions and induction of conformational changes [6].
3. Vitreoretinal diseases and pathophysiology
Although integrins likely play a role in a variety of vitreoretinal conditions, such as vitreomacular traction (VMT), macular pucker, macular hole, and proliferative vitreoretinopathy, this review will focus on diabetic retinopathy (DR) and AMD, the leading causes of irreversible legal blindness in the industrialized world. Relentlessly rising globally, diabetes now afflicts 34.2 million Americans, approximating 10.5% of the US population, and DR afflicts 7.7 million Americans, with 14.6 million projected by 2050. Currently, 11.7% of all American diabetics report vision disability, including blindness [7]. While DR represents the leading cause of legal blindness among working-age adults, AMD represents the leading cause of legal blindness among seniors, affecting 14% of those aged 80 or older [8–10]. By 2050, AMD will affect 5.44 million Americans [11].
While AMD and DR pathophysiology is beyond the scope of the review, integrins play some common roles in both disorders through the vitreous interphase and at neovascular fronds. Notably, targeting vitreous using anti-integrin therapy can promote vitreous liquefication and posterior vitreous detachment, to limit the vitreous scaffolding that normally facilitates retinal neovascularization, vitreoretinal traction, and traction retinal detachment. Similarly, targeting neovascular fronds with anti-integrin agents may reduce proteolytic degradation of ECM and tissue remodeling, to limit new vessel formation in both AMD and DR.
3.1. Integrins and the vitreoretinal interface
The human vitreous, which occupies 80% of the eye, is composed of well-organized collagen fibers, along with water and hyaluronic acid [12]. The vitreoretinal interface consists of an internal limiting membrane (ILM), posterior vitreous cortex, and intervening ECM. The ILM mediates adhesion between the neural retina and vitreous body [13], and comprises the basement membrane of retinal Müller cells, with a smooth anterior side and an irregular posterior surface [14]. The ILM consists of the three distinct strata: lamina rara interna (immediately adjacent to the Müller cell end feet); the lamina densa and lamina rara externa (adjoining with the vitreous cortex). During aging, there is physiologic liquefication and separation of vitreous, as well as changes in the internal limiting membrane and extracellular matrix, all leading to posterior vitreous detachment (PVD) [13] However, VMT, which results from incomplete PVD, can exacerbate DR and exudative AMD [15].
Integrins are intimately involved with the ILM, which consists of ECM, and are critical for cell-cell and cell-matrix interactions. The ECM is comprised of components such as fibronectin, laminin, collagen type I and IV. The fibronectin and laminin play a vital role in the attachment of vitreous to ILM on the anterior side and Müller cells to ILM on the posterior side [13]. While these glycoproteins are expressed throughout the ILM, there are some topographical differences in intensity and pattern. Interestingly, immunofluorescence studies demonstrate alterations in laminin-fibronectin in aged eyes, which could precipitate PVD. Some integrins are implicated in systemic diseases such as cancer and auto-immune disorders, while some are closely associated with vitreolysis, choroidal and pre-retinal angiogenesis, as well as ocular surface diseases [16]. Consequently, targeting integrins in the eye has the potential to halt vision loss, independent of anti-vascular endothelial growth factor (VEGF) therapies.
3.2. Integrins and diabetic retinopathy
Capillary basement membrane thickening is a histologic hallmark of DR. Furthermore, the basement membrane in DR is modified by advanced glycation end (AGEs) products that disturb cellular interaction with the basement membrane [17]. The thickened capillary basement membrane in DR leads to an increase in integrin expression [18]. In the eye, two integrin dependent pathways were proposed to be involved in angiogenesis; (i) in corneal or chorioallantoic angiogenesis models involving basic fibroblast growth factor (bFGF) and tumor necrosis factor α (TNF-α), angiogenesis is mediated by αvβ3 integrin and (ii) angiogenesis initiated by transforming growth factor α (TGFα) or VEGF is mainly mediated by αvβ5 integrin [19]. The αvβ3 integrins are expressed on both basal and luminal surfaces of endothelial cells, as well as on actively proliferating endothelial cells [20,21] and inflamed cells in DR [20]. Activation of αvβ3 integrin maintains macrophage inflammatory response processes [22]. Increased levels of αvβ3, αvβ5 and α5 integrins have been reported in fibrovascular membranes (FVM) of individuals with proliferative diabetic retinopathy (PDR) [23]. Therefore, the αv β3 and αvβ5 integrins may play key roles in the pathogenesis of DR (Figure 1) and therapies that inhibit the binding of vascular endothelial cells to ECM may hold promise.
Figure 1.
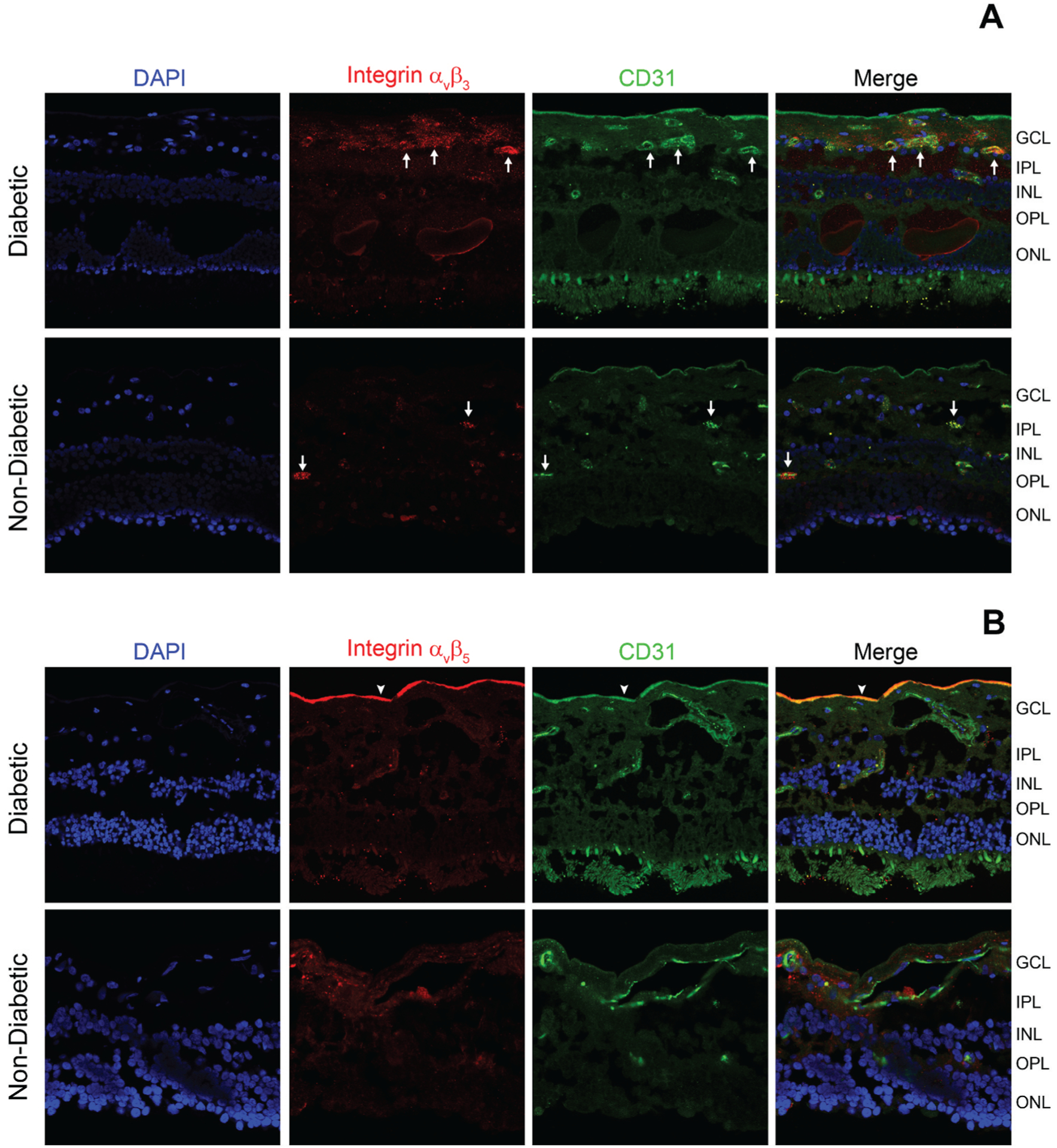
Frozen ocular sections of human donors (non-diabetic and diabetes with macular edema with non-proliferative diabetic retinopathy (NPDR)) were stained with DAPI (highlighting cell bodies), integrin αvβ3 or αvβ5 (red, Millipore MAB1976 or MAB1961, 1:100) antibodies, and CD31 antibodies (highlighting endothelial cells, green, Abcam ab28364, 1:100). (A) Representative photomicrographs are showing an increase in the staining for αvβ3 in the ganglion cell layer and around the vessels (white arrows) from a diabetic donor (B) Representative photomicrographs are showing more intense αvβ5 staining near the internal limiting membrane (arrowhead) in a diabetic donor. GLC-ganglion cell layer, IPL-inner plexiform layer, INL-inner nuclear layer, OPL-outer plexiform layer, ONL-outer nuclear layer.
Previous studies demonstrate a higher abundance of β1 integrin in the capillaries and thickened basement membrane among individuals with at least 8 years of diabetes. It is suggested that an increase in β1integrin is mediated via the upregulation of TGFβ1 induced basement membrane thickening [24,25]. Increased expression of α1β1 and α2β1 integrins have been reported on non-endothelial regions of FVMs [23]. The α5 integrins under pathological conditions promote angiogenesis and inflammation [26].
Integrins may play a role in inflammation-mediated microvascular changes, based on preclinical DR models, demonstrating integrin-mediated adherence of leukocytes to the retinal vasculature [27,28]. Ultimately, migration of leukocytes to the neural retina contributes to the early retinal vascular changes of DR [29,30]. When cellular adhesion inhibitors such as anti-ICAM-1 or anti-β2-integrin are used to treat diabetic rats, there is decreased leukocyte adhesion, less endothelial cell death, and limited blood-retinal barrier breakdown [31]. Furthermore, several studies show that integrin-blocking agents prevent retinal angiogenesis in preclinical models [32–35].
3.3. Integrins and age-related macular degeneration
AMD is classified into early, intermediate and advanced forms based on the severity of the disease; the early and intermediate forms of AMD are characterized by an accumulation of yellowish-lipid rich protein content (drusen) in between retinal pigment epithelial cells (RPE) and Bruch’s membrane. Drusen formation leads to functional loss of retinal photoreceptors. The more advanced form of non-neovascular AMD is associated with geographic atrophy (GA), thinning in the areas of RPE cell and underlying choriocapillaris, which leads to photoreceptor degeneration and subsequent loss of the visual function [36]. Alternatively, in neovascular AMD (nAMD), choroidal neovascularization develops, which is responsible for approximately 90% of the cases of severe vision loss due to AMD [37]. Anti-VEGF treatments are effective in treating the neovascular form of the disease. For non-neovascular AMD, there are no approved treatments, except for nutritional supplements; a large study, Age-Related Eye Disease Study (AREDS) and the follow-on study AREDS2, conducted by National Eye Institute, demonstrated that vitamin supplement contained Vitamins E and C, Lutein, Zeaxanthin and Zinc were beneficial in reducing the risk intermediate to advanced AMD [38].
RPE cells undertake multiple functions, including processing and shedding of outer segments of the photoreceptors. In the case of early and intermediate AMD, RPE cells potentially play an important role in drusen formation along with extracellular and serum-derived factors. RPE cells express a variety of factors such as vitronectin, clusterin, serum amyloid P, complement proteins and apolipoprotein E (APOE), which aid in drusen formation. Moreover, RPE cells express a variety of integrins, such as integrin α1–5, αv, and β1 in cell culture studies [39]. In an animal model of AMD, the RPE surface has been shown to express integrin αvβ5, which facilitates interactions between photoreceptors and the RPEs [40]. Mice deficient in β5 integrin subunits lack functional integrin αvβ5, and exhibit a decrease in retinal adhesion and phagocytosis of photoreceptor outer segments, and with aging, show accumulation of autofluorescent storage bodies in the RPE, along with a reduction of retinal photoresponses [40].
The pathogenesis of nAMD is a complex process involving a variety of cytokines and inflammatory molecules, including VEGF, platelet-derived grown factor, FGF, TGF and interleukins. In some cases, nAMD involves persistent attachment of the posterior vitreous cortex to the macula, which may pose a risk for developing nAMD via vitreoretinal traction [41,42]. This vitreomacular adhesion could foster an inflammatory milieu by macular exposure to cytokines, increase in free radicals in vitreous gel, and interference with transvitreal oxygenation, all of which could incite a pro-angiogenic microenvironment leading to nAMD. In such conditions, inducing PVD could potentially provide some prophylactic benefit against nAMD, or facilitate treatment of existing nAMD, as these eyes with vitreomacular adhesion have been shown to require more intense treatment with anti-VEGF agents [43]. Pharmacological vitreolysis with intravitreal RGD peptides has been demonstrated in the rabbit model, in which 24 hr incubation with these integrin-based agents facilitated PVD [44].
The blood vessels of nAMD patients exhibit an increase in αvβ3 integrins [23] and targeting αvβ3 and αvβ5 could potentially represent a useful therapeutic strategy for nAMD [45,46]. In addition, inhibiting α5β1 has been shown to inhibit endothelial cell proliferation and produce regression of choroidal neovascular membranes [47,48]; therefore, it could represent a future therapeutic target. Tenascin-C, a glycoprotein expressed in the extracellular matrix and CNS during developmental stages, has shown to play a role in disease states, including CNV pathogenesis. Tenascin-C levels are upregulated in CNV membranes of AMD patients [49]. Tenascin-C colocalizes with integrin αvβ3 in individuals with AMD and laser-induced CNV. Conversely, genetic deletion of, or small interfering RNA (siRNA) mediated deletion of, Tensacin-C results in a significant reduction in CNV formation [50]. Tenascin-C also promotes retinal neovascularization in PDR patients [51]. Table 1 summarizes different integrins involved in DR and AMD pathogenesis.
Table 1.
Integrins in chorioretinal diseases.
| Disease | Integrin Type | Vitreoretinal location | Study |
|---|---|---|---|
| DR | β1 | Retinal micro- vessels | Roth et al. Proc Natl Acad Sci USA, 1993. 90(20): p. 9640–9644 |
| αvβ3, αvβ5 | FVM tissue | Friedlander, M., et al., Proc Natl Acad Sci U S A, 1996. 93 (18): p. 9764–9 | |
| α5β1 | Matrigel assay | Klein, S., et al. Molecular and cellular biology, 2002. 22(16): p. 5912–5922 | |
| α1β1 and α2β1 | Non-endothelial regions of FVM | Friedlander, M., et al., Proc Natl Acad Sci U S A, 1996. 93 (18): p. 9764–9 | |
| AMD | β5, αvβ5 | Retina, RPE | Nandrot, E.F., et al., The Journal of experimental medicine, 2004. 200(12): p. 1539–1545 |
| αvβ3 | Subretinal vascular cells | Friedlander, M., et al., Proc Natl Acad Sci U S A, 1996. 93 (18): p. 9764–9 | |
| α5β1 | CNV membranes | Ramakrishnan, V., et al. J Exp Ther Oncol, 2006. 5(4): p. 273–86 |
AMD, age-related macular degeneration; DR, diabetic retinopathy, FVM, fibrovascular membrane; RPE, retinal pigment epithelium
4. Anti-integrin strategies
4.1. Clinical candidates
Table 2 describes key features of anti-integrin therapeutic candidates that have been or currently in clinical trials
Table 2.
Summary of major clinical trials of integrin inhibitors for age-related macular degeneration and diabetic macular edema.
| Drug/Company | Target Integrin Receptor(s) | Indication | Trial Stage | Status |
|---|---|---|---|---|
| Risuteganib/Allegro Ophthalmics | αvβ3, αvβ5, α5β1, and α5β3 | DME Dry AMD/GA | Ph 2 | Completed |
| Ph 2 | Completed | |||
| THR-687/Oxurion | αvβ3, αvβ5 and α5β1 | DME | Ph 1 | Completed |
| SF-0166/SciFluor Life Sci. | αvβ3, αvβ6, and αvβ8 | AMD | Ph 1/2 | Completed |
| DME | Ph 1/2 | Completed | ||
| Volociximab/Ophthotech Corporation (now Iveric Bio) | α5β1 | subfoveal CNV secondary to AMD | Ph 1 | No further clinical studies announced |
| JSM-6427/Jerini AG & Shire Pharmaceuticals (now Takeda Pharma) | α5β1 | AMD | Ph 1 | No further clinical studies announced |
DME, diabetic macular edema; AMD, age-related macular degeneration; CNV, choroidal neovascularization.
4.1.1. Risuteganib (Luminate)
Risuteganib (Luminate, Allegro Ophthalmics, CA, USA) is an integrin inhibitor that binds to multiple integrin sites. It is a synthetic RGD class peptide with a molecular weight of less than 1kD. Pharmacokinetic studies show a half-life of risuteganib in the rabbit retina as 21 days, but it localizes in RPE cells for several months [52]. Pharmacodynamically, risuteganib inhibits cell-to-cell and cell-to-endothelial cell membrane adhesion, which otherwise can lead to posterior vitreous detachment. In addition, risuteganib may suppress migration and inflammation under hypoxic conditions as well as improve mitochondrial function in RPE cells [53,54].
Risuteganib has been assessed in phase 2 trials for DME, VMT, and AMD treatment. The phase 2b trial for DME showed promising results, especially in the subgroup of patients that were poor responders to anti-VEGF therapy [55]. The double-masked, placebo-controlled, randomized, multi-center dose-ranging study enrolled 136 subjects and evaluated the safety and non-inferiority of three risuteganib arms (1, 2 and 3 mg) treated with 3 monthly loading intravitreal injections followed by 12 weeks off treatment, compared to an anti-VEGF arm of 6 monthly 1.25 mg bevacizumab injections. In stage 1, this study demonstrated promising visual acuity gains and reduction in central macular thickness (CMT) that were non-inferior to bevacizumab monotherapy, with no drug-related toxicity. In stage 2, this trial evaluated non-inferiority of intravitreal risuteganib (0.5 mg and 1 mg) to bevacizumab as a sequential therapy or in combination therapy at 20 weeks in 80 subjects with DME. For the patients that were inadequate anti-VEGF responders, the mean gain in best-corrected visual acuity (BCVA) was 7.5 letters in the risuteganib (1 mg) with bevacizumab pre-treatment (sequential) group compared to 5.2 letters in the bevacizumab control group. Intravitreal risuteganib was well-tolerated with no drug toxicity or intraocular inflammation and showed 12-week durability after the completion of three loading doses [55].
In 2019, a phase 2a prospective, randomized, double-masked, placebo-controlled, multi-center study of risuteganib for the treatment of intermediate nonexudative AMD was completed. At baseline, 40 patients were randomized to receive either intravitreal 1 mg risuteganib or sham injection. At 16 weeks, the patients crossed over to receive either of the above treatments. The primary endpoints of the study included the percentage of patients ≥8 letters ETDRS BCVA gain from baseline to week 28 in the risuteganib arm vs baseline. At week 28, 48% individuals met the primary endpoint in risuteganib arm and 7% individuals in the sham group at week 12, gaining ≥8 letters (p = 0.013 for difference) [56]. This threshold of 8 letters exceeds test variation, as a prior study assessing the intersession repeatability of BCVA in AMD showed that only 11% of patients with AMD had a 5-letter reduction or more in visual acuity at the 1 week compared with baseline [57]. In the risuteganib AMD study, the percentage of patients with ≥10 letter and ≥15 letter gain showed similar trends to the outcomes with ≥8 letter threshold, but the difference between the treated and control groups were not statistically significantly different. Overall the risuteganib was found to be safe with no severe adverse drug reactions. Allegro plans a larger Phase 2b/3 study in the U.S.
Risuteganib was also assessed for its safety and efficacy after intravitreal injections in patients with symptomatic focal VMT or vitreomacular adhesion (VMA). In a Phase 2, randomized, prospective clinical trial (NCT02153476) [58], three doses of Risuteganib (2 mg, 2.5 mg and 3.2 mg) and vehicle control (balanced salt solution placebo) group were evaluated in 106 subjects. The intravitreal injections of Risuteganib were found to be well-tolerated with no drug-related toxicity or intraocular inflammation. Ninety days after treatment, 65% eye treated with the highest dose (3.2 mg) achieved the release of VMT or VMA, compared to 9.7% of eye in the placebo control group [59].
4.1.2. THR-687
THR-687 (Oxurion, Leuven, Belgium) is a novel pan integrin receptor antagonist that binds the integrins αvβ3, αvβ5 and α5β1 at a nanomolar concentration. In in vitro studies, THR-687 has prevented cell migration in human umbilical vein endothelial cells (HUVECs) and vascular sprouting in an ex vivo mouse choroidal explant model. Furthermore, in animal studies, THR-687 has decreased retinal vascular permeability and choroidal neovascular-induced vascular leakage [60,61].
The safety and early clinical efficacy of THR-687 were assessed in an open-label, multi-center, single dose-escalation Phase 1 study in 12 DME patients who were responsive to prior anti-VEGF and/or corticosteroid. Three doses (0.4 mg, 1 mg, and 2.5 mg) of THR-687 were administered via a single intravitreal injection in 3, 3, and 6 patients, respectively. In this Phase 1 study, rapid onset of action (mean BCVA improvement of +3 letters) was observed on day 1, with maximal visual improvement at month 1 (+9.2 letters), and maintainance of visual gain at month 3 (+8.3 letters). The greatest effect (+11.2 letters at day 14, and +12.5 letters at month 3) was observed at the highest dose (2.5 mg) of THR-687. Moreover, the highest dose of THR-687 resulted in a decrease in the peak mean central subfield thickness on day 14. In this Phase 1 study, TR-687 was found to be safe and well-tolerated with no dose-limiting toxicities or serious adverse events [62,63].
4.1.3. SF-0166
SF-0166 (SciFluor Life Science, MA, USA) is a small molecule inhibitor of integrin αvβ3. Due to its optimized physico-chemical properties, SF-0166 can distribute to the posterior segment of the eye after topical administration, and the drug concentration was maintained for more than 12 hours [64]. In in vitro studies, SF-0166 was shown to inhibit cell adhesion to vitronectin across a variety of cell lines of rat, rabbit, dog origin, and shown to inhibit integrin-ligand interactions for the human dermal microvascular endothelial cells. In addition to αvβ3, SF-0166 also inhibits integrins αvβ6, and αvβ8 at nanomolar concentrations; however, it does not inhibit binding to αvβ5 or fibronectin via α5 β1 integrins [64]. SF-0166 was found to inhibit neovascularization in the oxygen-induced retinopathy mouse model after topical administration. In the laser-induced CNV animal model, SF-0166 decreased lesion area; this decrease was comparable to bevacizumab. In the rabbit model of VEGF-induced vascular leakage model, topically delivered SF-0166 exhibited a dose-dependent reduction in vascular leakage [64].
A prospective, randomized, double-masked multicenter Phase1/2 clinical study of SF-0166 in 42 AMD patients was completed [65]. The patients were randomized 1:1 to self-administer an eye drop containing 2.5% or a 5% solution of SF-0166 twice-a-day for 28 days. This study reported no drug-related serious adverse events, throughout the 28-day course of treatment or during the 28-day follow up period. Five individuals reported mild to moderate adverse events, with one considered possibly as a drug-related event. Nine out of the 42 individuals demonstrated a decrease in retinal thickness and/or subretinal fluid by spectral-domain optical coherence tomography (SD-OCT). Visual acuity was improved approximately by five letters during the treatment period among the treatment naïve group [66]. A similar clinical trial (NCT02914613) for DME was performed for SF-0166 in 40 subjects with DME [67]. Treatment with SF-0166 was associated with a reduction in retinal thickness of 53% of patients with improvement in visual acuity [68].
4.1.4. Volociximab
Volociximab (Ophthotech Corporation, NY, USA, now Iveric Bio) is a monoclonal antibody that specifically blocks the binding of fibronectin to the α5β1 integrin [69]. The safety and pharmacokinetics of volociximab combined with ranibizumab were evaluated in Phase 1, open-label, multicenter, dose-escalation study of eyes with all subtypes of choroidal neovascularization secondary to AMD (NCT00782093) [70]. Two monthly injections of the combination of volociximab (0.5, 1.25 or 2.5 mg) and ranibizumab (0.5 mg) exhibited a favorable safety profile without dose-limiting toxicity. The mean changes in VA were +9.5 letters and +5.3 letters in treatment-naïve eyes (n=37), and in inadequate anti-VEGF responder eyes (n=11), respectively. Despite early signs of improvement in BCVA and acceptable safety profile in this study, the individual contribution of volociximab could not be delineated. No further clinical trials have been reported to date.
4.1.5. JSM-6427
JSM-6427 (Jerini AG & Shire Pharmaceuticals, now Takeda Pharmaceutical Company, Tokyo, Japan) is a small molecule antagonist of α5β1 integrin receptor. In-vitro, JSM-6427 inhibited the migration of HUVEC, and growth factor-induced tube formation [71]. In in vivo animal models, intravitreal JSM-6427 inhibited proliferation of Müller cells, microglia and macrophages [72], and inhibited laser-induced and growth factor-induced choroidal neovascularization [73]. Although some clinical benefits in Phase 1 clinical trial for exudative AMD (NCT00536016) have been observed [74], no further clinical trials are currently planned.
4.2. Preclinical candidates
Table 3 describes key features of anti-integrin therapeutic candidates that are currently being assessed preclinically
Table 3.
Summary of preclinical investigational integrin inhibitors.
| Drug (Company) | Target Integrin Receptor(s) | Preclinical animal models | Key Findings | Study |
|---|---|---|---|---|
| SB-267268 (GlaxoSmithKline) | αvβ3 and αvβ5 | ROP mouse model | Reduced pathologic angiogenesis, and lowered VEGF and VEGFR2 mRNA levels | Wilkinson-Berka, J.L., et al. Invest Ophthalmol Vis Sci, 2006. 47(4): p.1600–5 |
| AXT-107 (AsclepiX Therapeutics) | αvβ3 and α5β1 | Ang2- transgenic mouse model, and LPS-induced inflammation model | Inhibited vascular leakage and inflammation | Mirando, A.C., et al., JCI insight, 2019. 4(4) |
| JNJ-26076713 (Johnson & Johnson Pharmaceutical) | αvβ3 and αvβ5 | ROP mouse model, and diabetic rats | Inhibited retinal neovascularization and retinal vascular permeability | Santulli, R.J., et al., Journal of Pharmacol and Exp Thera, 2008. 324(3): p. 894–901 |
| Cilengitide (Merck-Serono) | αvβ3 | tenascin-C knockout and tenascin-C silenced mouse model | Inhibited CNV formation | Kobayashi, Y., et al., Laboratory investigation, 2016. 96(11): p.1178–1188 |
| Lebecetin (SATT Lutech) | αvβ1, and αv- containing integrins | Mouse CNV and ROP mouse model | Inhibited choroidal and retinal neovascularization | Montassar, F., et al., The FASEB Journal, 2017. 31(3): p. 1107–1119 |
ROP, retinopathy of prematurity; CNV, choroidal neovascularization.
4.2.1. SB-267268
SB-267268 (GlaxoSmithKline) is a small molecule antagonist of αvβ3 and αvβ5 integrins, which exhibits a 2-benzazepine template [75]. In in vitro studies, SB-267268 is found to be 1000-fold selective in binding to αvβ3 and αvβ5 receptors compared to other integrins αIIbβ3, α5β1, and α3β1. In another set of in vitro assays, SB-267268 effectively inhibited vitronectin mediated adhesion and migration of human and rat aortic smooth muscle cells. In an animal model of ROP, SB-267268 reduced pathologic angiogenesis by 50%, along with a decrease in VEGF and VEGFR2 messenger RNA (mRNA) [34]. Although it was reported to be studied in Phase 1 clinical trial for AMD [75], no clinical trial results have been published.
4.2.2. AXT-107
AXT-107 (AsclepiX Therapeutics, NJ, USA) is a collagen IV-derived peptide with strong anti-permeability activity; this effect is mainly mediated by the disruption of α5β1 integrins and activation of Ang2/Tie 2 signaling. AXT-107 stimulates relocation of Tie2 and α5 to cell junction, resulting in the activation of downstream survival signals. This leads to F-actin rearrangement and strengthening of junctions, resulting in a reduction of endothelial permeability, and the potential to address macular edema and related disorders. In addition, AXT-107 inhibits VEGF2 signaling via receptor tyrosine kinase association with specific integrins [76]. AXT-107 inhibited vascular leakage in an Ang2-overexpression transgenic model and an LPS-induced inflammation model via induction of Tie2 phosphorylation. It is therefore hypothesized that targeting α5β1 with AXT-107 may provide an effective treatment for ischemic ocular diseases, such as DME, nAMD and uveitis. An intravitreal formulation, with sustained duration, is being developed for clinical trials in DME and nAMD.
4.2.3. JNJ-26076713
JNJ-26076713 (Johnson & Johnson Pharmaceutical, PA, USA) is a potent, orally bioavailable, α5 integrin antagonist that inhibits αvβ3 and αvβ5 binding to vitronectin in the low nanomolar range. JNJ-26076713 is shown to prevent adhesion to human, rat, and mouse endothelial cells and shown to block cell migration induced by VEGF and FGF. In the chick chorioallantoic membrane model, JNJ-26076713 inhibited FGF-induced angiogenesis. JNJ-26076713 was found to be effective in inhibiting retinal neovascularization and retinal vascular permeability in an animal model of oxygen-induced retinopathy of prematurity, and diabetic rats. Given this profile, JNJ-26076713 is thought to attenuate key pathological processes involved in AMD, DME, and PDR [77].
4.2.4. Cilengitide
Cilengitide (Merck-Serono, Germany), is a cyclic peptide and integrin αvβ3, αvβ5 and α5β1 inhibitor [50], and it has been shown to block tenascin-C induced proliferation, adhesion, migration, and tube formation in human microvascular endothelial cells (HMVECs) [50]. Working in tandem with TGF-β2, Tenascin-C is shown to promote the proliferation of HMVEC and RPE cells in vitro. While tenascin-C promoted CNV in mice by binding to integrin αvβ3, a significant reduction in CNV formation was observed in tenascin-C knockout and tenascin-C mRNA-silenced mice [50]. These in vitro and in vivo studies suggest that tenascin-C-mediated integrin αv β3 modulation could be a potential target for the inhibition of CNV development associated with AMD.
4.2.5. Lebecetin
Lebecetin (LCT), a 30kD C-type lectin, is a heterodimer that is extracted from Macrovipera lebetina venom. It has been reported to interact with α5β1 and αv-containing integrins that are critical for endothelial cell proliferation and stabilization [78,79]. LCT has been shown to reduce human brain microvascular endothelial cell (HBMEC) adhesion, proliferation, and tubulogenesis [80]. In an in vitro, chick chorioallantoic membrane assay, LCT inhibited angiogenesis, but failed to exhibit the same effect in fibroblast growth factor-2 induced angiogenesis in a Matrigel plug assay. LCT inhibited vascular sprouting in ex-vivo assays of aortic and choroidal culture explants [81]. LCT inhibited choroidal and retinal neovascularization in a laser-induced CNV mouse model, and oxygen-induced retinopathy mouse model, respectively, and reported to be targeting only pathological neovascularization, while maintaining integrity of normal retinal vasculature [81].
5. Conclusion
Integrins are heterodimeric receptors that regulate cellular adhesion, migration, proliferation, invasion, survival, and apoptosis. Integrins play a role in the pathogenesis of DR and AMD. Anti-integrin strategies have demonstrated signs of efficacy in both preclinical and early clinical studies of these disorders. With a unique mechanism of action, integrin inhibition has potential to serve as primary therapy, adjunctive therapy to anti-VEGF agents, or secondary therapy in refractory cases.
6. Expert opinion
DR and AMD are leading causes of blindness and visual impairment. While frequent intravitreal anti-VEGF treatments revolutionized disease management, real-world studies highlight the heavy treatment burden of current therapy, as patients receive fewer treatments and experience worse outcomes than noted in clinical trials [82–87]. Consequently, there is a tremendous unmet need for new therapies that address both treatment burden and suboptimal outcomes.
Integrins, with their role as integrators, are crucial in the pathogenesis of DR and AMD due to their intimate association with the cell surface. The upregulation of integrin constitutes an inflammatory milieu in the retina, creating a hostile environment, triggering leukocyte migration and increasing vascular permeability. Several clinical and basic studies demonstrate that integrin expression is upregulated on the cell surface, neovasculature, and FVMs, thus playing a critical role in major complications of DR and AMD, such as neovascularization, vitreoretinal traction, and tractional retinal detachment. Anti-integrin therapy may hold a promise in curtailing these complications by promoting vitreous liquefication and posterior vitreous detachment [42]. This may limit the need for pars plana vitrectomy in DR, or even facilitate vitreous dissection in those severe cases that require surgery. Similarly, vitreous liquefaction and PVD could alleviate vitreomacular adhesions and traction, which have been associated with CNV in nAMD.
Several ongoing clinical and preclinical studies are demonstrating the promise of anti-integrin treatments in DR and AMD. Risuteganib is a leading candidate molecule undergoing Phase 2 studies. Intravitreal treatment using Risuteganib, a synthetic RGD peptide, is promising in treating VMT, DME and AMD with additional benefits of a long half-life and efficacy in VEGF non-responders. The prospect of visual improvement in non-neovascular AMD, as suggested in a clinical trial with risuteganib, is particularly intriguing, and the retina community eagerly looks forward to additional confirmatory trials. Other anti-integrin inhibitors such as THR-687 and SF-0166 are small-molecule antagonists of integrins and are undergoing Phase 1 trial. The Phase 1 DME study of THR-687 shows promise, as there appeared to be a dose-response, as well as an anatomic response reassuringly accompanying a functional response. Specifically, the most significant effect was observed at the highest dose, which also resulted in a decrease in the peak mean central subfield thickness at day 14. In addition, the level of visual improvement in this highest dose cohort was particularly impressive; a Phase 2 trial is planned. Similarly, SF-0166 has shown some preliminary signs of efficacy via a topical route with excellent posterior segment bioavailability, which may enhance patient compliance. This potential for an effective topical agent is particularly exciting because the topical route for retinal therapeutics has been assessed in past clinical trials of multiple agents, including tyrosine kinase inhibitors, with limited success.
There are a series of preclinical candidate anti-integrin molecules in the pipeline, which may ultimately progress to clinical study. Interestingly some of these candidates are distinctive in terms of their mode of action or route of administration. JNJ26076713 is being developed for oral administration, while AXT-107 is administered intravitreally. Cilengitide works on TGF-β2 and tenascin at a cellular level, while AXT-107 affects Ang2/Tie2 and VEGF, validated pathogenic targets in DR and AMD. AXT-107 is particularly intriguing due to both its unique mechanism of action and prolonged durability suggested by preclinical studies. These novel actions for these candidate molecules could potentially broaden the role of anti-integrin therapies in DR and AMD. Overall, anti-integrin treatments in the current Phase 1 studies show no dose-limiting toxicities or serious adverse events.
In summary, based on the pathogenesis of DR and AMD, anti-integrins hold significant promise for treating AMD and DR. Anti-integrins have potential to serve as primary therapy, adjunctive therapy to anti-VEGF-A agents, or potentially play a role in anti-VEGF non-responders, given the unique mechanism of action. Furthermore, integrins currently in development include oral and topical routes of administration, which have the potential to address the treatment burden associated with current regimens of frequent anti-VEGF injection.
Article highlights.
Diabetic retinopathy (DR) and age-related macular degeneration (AMD) represent the leading causes of irreversible legal blindness in the industrialized world. Despite anti-vascular endothelial growth factor (anti-VEGF-A) therapy, there remains a persistent unmet need to improve visual outcomes and to address the treatment burden.
Integrins have been involved in the pathogenesis of multiple diseases, including cancer, DR and AMD. Named for their function as ‘integrators’ of cell surface and extracellular matrix (ECM), integrins are a family of multi-functional cell-adhesion molecules that connect ECM molecules to actin cytoskeleton in the cell cortex.
Composed of α and β subunits and serving as cell adhesion and cell signaling receptors, integrins regulate the shape, orientation, and movement of cells. Integrins also control a variety of cellular interactions with their microenvironment, such as migration, proliferation, invasion, survival, and apoptosis. Consequently, integrins play a role in pathologic processes, such as inflammation, angiogenesis and fibrosis.
Integrins have shared pathogenic roles in both DR and AMD through the vitreous interphase and at neovascular fronds. Intravitreal anti-integrin therapy may promote vitreous liquefication and posterior vitreous detachment, which may limit vitreous scaffolding that normally facilitates retinal neovascularization, vitreoretinal traction, and traction retinal detachment. Similarly, targeting neovascular fronds with anti-integrin agents may interfere with proteolytic degradation of ECM and tissue remodeling, necessary components of new vessel formation and common pathologic features of both AMD and DR.
Anti-integrin therapies that have undergone early clinical study include risuteganib (Allegro Ophthalmics, CA, USA), THR-687 (Oxurion, Leuvin, Belgium), SF-0166 (SciFluor Life Science, MA, USA), Volociximab (Ophthotech Corporation, now Iveric Bio, NY, USA) and JSM-6427 (Jerini AG & Shire Pharmaceuticals, now Takeda Pharmaceutical Company, Tokyo, Japan), all of which have shown some signs of biologic activity.
Anti-integrin candidate therapeutics being assessed preclinically include SB-267268 (GlaxoSmithKline, Brentford, UK) , AXT-107 (AsclepiX Therapeutics, NJ, USA), JNJ-26076713 (Johnson & Johnson Pharmaceutical, PA, USA), and Cilengitide (Merck-Serono, Germany). Integrin inhibition shows promise in early clinical studies of AMD and DME. With a unique mechanism of action, it could serve as primary therapy, adjunctive therapy to anti-VEGF-A agents, or potentially play a role in refractory cases. Further research is warranted.
Acknowledgments
The authors would like to thank Dr. Weiming Mao (Department of Ophthalmology, Indiana University) and Vision First Indiana Lions Eye Bank, Indianapolis, for providing human eyes.
Funding
Research in the Bhatwadekar laboratory is supported by funding from NIH-National Eye Institute (R01EY027779) and by Pilot and Feasibility award from the Center for Diabetes and Metabolic Diseases, Indiana University.
Footnotes
Declaration of interest
T Ciulla has an employment relationship with, and equity ownership in, Clearside Biomedical. This work was undertaken in his role as a Volunteer Clinical Professor at Indiana University School of Medicine and does not reflect any views or opinions of this corporation or its management. V Kansara has an employment relationship with, and equity ownership in, Clearside Biomedical. This work does not reflect any views or opinions of this corporation or its management. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1••.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. [DOI] [PubMed] [Google Scholar]; This is an informative review on integrins covering essential information on integrin and their function.
- 2.Kechagia JZ, Ivaska J, Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 2019;20:457–473. DOI: 10.1038/s41580-019-0134-2 [DOI] [PubMed] [Google Scholar]
- 3••.Kapp TG, Rechenmacher F, Neubauer S, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7(1):39805. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is providing in-depth information on the selectivity profile of ligands for RGD binding integrins.
- 4.Plow EF, Haas TA, Zhang L, et al. Ligand binding to integrins. J Biol Chem. 2000;275(29):21785–21788. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari S, Askari JA, Humphries MJ, et al. Divalent cations regulate the folding and activation status of integrins during their intracellular trafficking. J Cell Sci. 2011;124(10):1672–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderwood DA. Integrin activation. J Cell Sci. 2004;117(5):657–666. [DOI] [PubMed] [Google Scholar]
- 7.Division of diabetes translation | CDC. 2020. [cited 2020 Mar 19]. Available from: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html
- 8.Congdon N, O’Colmain B, Klaver C, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. [DOI] [PubMed] [Google Scholar]
- 10••.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2): e106–e116. [DOI] [PubMed] [Google Scholar]; This is a meta-analysis study providing detailed information on AMD disease burden worldwide.
- 11.Age-Related Macular Degeneration (AMD) data and statistics | National Eye Institute. 2020. [cited 2020 Mar 19]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/
- 12.Sebag J, Balazs E. Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci. 1989;30(8):1867–1871. [PubMed] [Google Scholar]
- 13.Kohno T, Sorgente N, Ishibashi T, et al. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987;28(3):506–514. [PubMed] [Google Scholar]
- 14.Sebag J Anatomy and pathology of the vitreo-retinal interface. Eye. 1992;6(6):541–552. [DOI] [PubMed] [Google Scholar]
- 15.Tsui I, Pan CK, Rahimy E, et al. Ocriplasmin for vitreoretinal diseases. Biomed Res Int. 2012;2012. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3496214/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser PK. Induciton of pharmacologic vitreolysis with an integrin antagonist. Retina Today. 2016. [Google Scholar]
- 17.Stitt AW. AGEs and diabetic retinopathy. Investigative ophthalmology & visual science. 2010. Oct 1;51(10):4867–4874 [DOI] [PubMed] [Google Scholar]
- 18.Preer K, Kanse S, Hammes H-P. Integrin chatter and vascular function in diabetic retinopathy. Hormone Metab Res. 1997;29(12):643–645. [DOI] [PubMed] [Google Scholar]
- 19••.Friedlander M, Brooks PC, Shaffer RW, et al. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270(5241):1500–1502. [DOI] [PubMed] [Google Scholar]; This study is focussed on key pathways involved in the angiogenic response of integrins.
- 20.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–571. [DOI] [PubMed] [Google Scholar]
- 21.Luna J, Tobe T, Mousa SA, et al. Antagonists of integrin alpha v beta 3 inhibit retinal neovascularization in a murine model. Lab Invest. 1996;75(4):563–573. [PubMed] [Google Scholar]
- 22.Marano RPC, Preer KT, Vilaró S. Fibronectin, laminin, vitronectin and their receptors at newly-formed capillaries in proliferative diabetic retinopathy. Exp Eye Res. 1995;60(1):5–17. [DOI] [PubMed] [Google Scholar]
- 23••.Friedlander M, Theesfeld CL, Sugita M, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93(18):9764–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]; This seminal study highlighted key integrins upregulated in the retina of individuals with diabetic retinopathy.
- 24.Roth T, Podesta F, Stepp MA, et al. Integrin overexpression induced by high glucose and by human diabetes: potential pathway to cell dysfunction in diabetic microangiopathy. Proc Nat Acad Sci. 1993;90(20):9640–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S, Bae E, Amin S, et al. Extracellular matrix, gap junctions, and retinal vascular homeostasis in diabetic retinopathy. Exp Eye Res. 2015;133:58–68. [DOI] [PubMed] [Google Scholar]
- 26.Gerhardinger C, Dagher Z, Sebastiani P, et al. The transforming growth factor-β pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes. 2009;58 (7):1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto K, Khosrof S, Bursell S-E, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96(19):10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barouch FC, Miyamoto K, Allport JR, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41(5):1153–1158. [PubMed] [Google Scholar]
- 29.Joussen AM, Murata T, Tsujikawa A, et al. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158(1):147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto K, Hiroshiba N, Tsujikawa A, et al. In vivo demonstration of increased leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci. 1998;39(11):2190–2194. [PubMed] [Google Scholar]
- 31.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86(4):363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui A, Zhong Y, Demetriades AM, et al. Inhibition of integrin alpha5beta1 ameliorates VEGF-induced retinal neovascularization and leakage by suppressing NLRP3 inflammasome signaling in a mouse model. Graefes Arch Clin Exp Ophthalmol. 2018;256 (5):951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui A, Zhong Y, Demetriades AM, et al. ATN-161 as an integrin alpha5beta1 antagonist depresses ocular neovascularization by promoting new vascular endothelial cell apoptosis. Med Sci Monit. 2018;24:5860–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Wilkinson-Berka JL, Jones D, Taylor G, et al. SB-267268, a nonpeptidic antagonist of alpha(v)beta3 and alpha(v)beta5 integrins, reduces angiogenesis and VEGF expression in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2006;47 (4):1600–1605. [DOI] [PubMed] [Google Scholar]; This preclinical study showed the pharmacological potential of anti-integrin, SB-267268, in retinopathy of prematurity.
- 35.Yoshida T, Gong J, Xu Z, et al. Inhibition of pathological retinal angiogenesis by the integrin alphavbeta3 antagonist tetraiodothyroacetic acid (tetrac). Exp Eye Res. 2012;94(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration. Drugs. 2008;68(8):1029–1036. [DOI] [PubMed] [Google Scholar]
- 38.AREDS/AREDS2 Clinical Trials | national Eye Institute. 2020. [cited 2020 Jun 30]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/
- 39.Zarbin MA. Analysis of retinal pigment epithelium integrin expression and adhesion to aged submacular human Bruch’s membrane. Trans Am Ophthalmol Soc. 2003;101:499. [PMC free article] [PubMed] [Google Scholar]
- 40.Nandrot EF, Kim Y, Brodie SE, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200(12):1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs I, Brannath W, Glittenberg C, et al. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144(5):741–746. e1. [DOI] [PubMed] [Google Scholar]
- 42.Sebag J VI A. Pharmacologic vitreolysis. In: Sebag J, editor. Vitreous. New York, NY: Springer; 2014. p. 799–815. [Google Scholar]
- 43.Ciulla TA, Ying G-S, Maguire MG, et al. Influence of the vitreomacular interface on treatment outcomes in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122(6):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira LB, Meyer CH, Kumar J, et al. RGD peptide-assisted vitrectomy to facilitate induction of a posterior vitreous detachment: a new principle in pharmacological vitreolysis. Curr Eye Res. 2002;25(6):333–340. [DOI] [PubMed] [Google Scholar]
- 45.Yasukawa T, Hoffmann S, Eichler W, et al. Inhibition of experimental choroidal neovascularization in rats by an av-integrin antagonist. Curr Eye Res. 2004;28(5):359–366. [DOI] [PubMed] [Google Scholar]
- 46.Fu Y, Ponce ML, Thill M, et al. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest Ophthalmol Vis Sci. 2007;48(11):5184–5190. [DOI] [PubMed] [Google Scholar]
- 47.Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5(4):273–286. [PubMed] [Google Scholar]
- 48.Umeda N, Kachi S, Akiyama H, et al. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69 (6):1820–1828. [DOI] [PubMed] [Google Scholar]
- 49.Nicolo M, Piccolino FC, Zardi L, et al. Detection of tenascin-C in surgically excised choroidal neovascular membranes. Graefe’s Arch Clin Exp Ophthalmol. 2000;238(2):107–111. [PubMed] [Google Scholar]
- 50.Kobayashi Y, Yoshida S, Zhou Y, et al. Tenascin-C secreted by transdifferentiated retinal pigment epithelial cells promotes choroidal neovascularization via integrin α V. Lab Invest. 2016;96 (11):1178–1188. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi Y, Yoshida S, Zhou Y, et al. Tenascin-C promotes angiogenesis in fibrovascular membranes in eyes with proliferative diabetic retinopathy. Mol Vis. 2016;22:436. [PMC free article] [PubMed] [Google Scholar]
- 52.Retinal Physician - Risuteganib for Intermediate Dry AMD. 2020. [cited 2020 Mar 29]. Available from: https://www.retinalphysician.com/issues/2019/november-2019/risuteganib-for-intermediate-dry-amd
- 53.Yang P, Neal SE, Jaffe GJ. Luminate protects against hydroquinone–induced injury in human RPE cells. Invest Ophthalmol Vis Sci. 2019;60(9):1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nebbioso M, Lambiase A, Cerini A, et al. Therapeutic approaches with intravitreal injections in geographic atrophy secondary to age-related macular degeneration: current drugs and potential molecules. Int J Mol Sci. 2019;20(7):1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allegro Ophthalmics L Risuteganib (LUMINATE®): potential paradigm shift in the treatment of oxidative stress-induced DME. In Ophthalmic Innovations Symposium. Chicago (IL); 2018. [Google Scholar]
- 56.Press releases archives - allegro ophthalmics - from theory to therapy. 2020. [cited 2019 Jun 4]. Available from: https://www.allegroeye.com/allegro-ophthalmics-expands-its-anti-integrin-portfolio-with-new-front-of-the-eye-drug-candidate-alg-1007-for-the-treatment-of-dry-eye-disease-3-2-2/
- 57.Patel PJ, Chen FK, Rubin GS, et al. Intersession repeatability of visual acuity scores in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(10):4347–4352. [DOI] [PubMed] [Google Scholar]
- 58.A safety and efficacy study of Alg-1001 in human subjects with symptomatic focal vitreomacular adhesion - full text view -ClinicalTrials.gov. 2020.
- 59.Allegro ophthalmics announces positive topline results from phase 2 trial evaluating luminate® in patients with vitreomacular traction or vitreomacular adhesion - allegro ophthalmics - from theory to therapy. 2015. [cited 2015 Jul 13]. Available from: https://www.allegroeye.com/allegro-ophthalmics-announces-positive-topline-results-from-phase-2-trial-evaluating-luminate-in-patients-with-vitreomacular-traction-or-vitreomacular-adhesion/
- 60.<OXURION_CorpPrez April 2020(2).pdf>. 2020. Jun. https://www.oxurion.com/sites/default/files/documents/OXURION_CorpPrez%20June%202020.pdf
- 61.Hu -T-T, Vanhove M, Porcu M, et al. The potent small molecule integrin antagonist THR-687 is a promising next-generation therapy for retinal vascular disorders. Exp Eye Res. 2019;180:43–52. [DOI] [PubMed] [Google Scholar]
- 62.Oxurion NV – Expert Presentation of Positive Topline Data from a Phase 1 Study evaluating THR-687 for the treatment of DME, at Angiogenesis, Exudation, and Degeneration 2020 Conference. Miami, FL; 2020. [Google Scholar]
- 63.A phase 1 study of thr 687: an integrin antagonist for the treatment of Diabetic Macular Edema (DME). 2020. [cited 2020 Jun 26]. Available from: https://www.oxurion.com/content/phase-1-study-thr-687-integrin-antagonist-treatment-diabetic-macular-edema-dme [DOI] [PMC free article] [PubMed]
- 64•.Askew BC, Furuya T, Edwards DS. Ocular distribution and pharmacodynamics of SF0166, a topically administered αvβ3 integrin antagonist, for the treatment of retinal diseases. J Pharmacol Exp Ther. 2018;366(2):244–250. [DOI] [PubMed] [Google Scholar]; This study tested pharmacological action of SF-0166 to show that SF-0166 is effective as an anti-integrin after topical administration.
- 65.http://www.scifluor.com/media-center/docs/SciFlour_US-AMD-release-12.18.2017-Final.pdf - Google search. 2020. [cited 2020 Jun 4]. Available from: http://www.scifluor.com/media-center/docs/SciFlour_US-AMD-release-12.18.2017-Final.pdf
- 66.Safety and exploratory efficacy study of SF0166 in the treatment of neovascular Age-Related Macular Degeneration (AMD) - full text view - ClinicalTrials.gov. 2020.
- 67.Safety and exploratory efficacy study of SF0166 for the treatment of Diabetic Macular Edema (DME) - full text view - ClinicalTrials.gov. 2020.
- 68.http://www.scifluor.com/media-center/docs/SciFluor_SF0166-Phase-I_IIDME-results-9-27-2017-Final.pdf - Google search. 2020. [cited 2020 Jun 4]. Available from: http://www.scifluor.com/media-center/docs/SciFluor_SF0166-Phase-I_II%20DME-results-9-27-2017-Final.pdf
- 69.Kuppermann B, Group OS. Inhibition of 5β1 integrin in neovascular AMD-A phase 1 study. Invest Ophthalmol Vis Sci. 2010;51(13):1252. [Google Scholar]
- 70.A phase 1 ascending and parallel group trial to establish the safety, tolerability and pharmacokinetics profile of volociximab (Alpha 5 Beta 1 integrin antagonist) in subjects with neovascular age-related macular degeneration - full text view - ClinicalTrials.gov. 2020.
- 71.Maier A-KB, Kociok N, Zahn G, et al. Modulation of hypoxia-induced neovascularization by JSM6427, an integrin α5β 1 inhibiting molecule. Current Eye Research. 2007;32(9):801–812. [DOI] [PubMed] [Google Scholar]
- 72.Zahn G, Volk K, Lewis GP, et al. Assessment of the integrin alpha5-beta1 antagonist JSM6427 in proliferative vitreoretinopathy using in vitro assays and a rabbit model of retinal detachment. Invest Ophthalmol Vis Sci. 2010;51(2):1028–1035. [DOI] [PubMed] [Google Scholar]
- 73.Zahn G, Vossmeyer D, Stragies R, et al. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch Ophthalmol. 2009;127(10):1329–1335. [DOI] [PubMed] [Google Scholar]
- 74.A phase 1 safety study of single and repeated doses of JSM6427 (intravitreal injection) to treat AMD - full text view - ClinicalTrials. gov. 2020.
- 75.SB 267268 - AdisInsight. 2020. [cited 2020 Apr 7]. Available from: https://adisinsight.springer.com/drugs/800013716
- 76.Mirando AC, Shen J, Silva RLE, et al. A collagen IV–derived peptide disrupts α5β1 integrin and potentiates Ang2/Tie2 signaling. JCI Insight. 2019;4(4). DOI: 10.1172/jci.insight.122043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santulli RJ, Kinney WA, Ghosh S, et al. Studies with an orally bioavailable αV integrin antagonist in animal models of ocular vasculopathy: retinal neovascularization in mice and retinal vascular permeability in diabetic rats. J Pharmacol Exp Ther. 2008;324 (3):894–901. [DOI] [PubMed] [Google Scholar]
- 78.Sarray S, Srairi N, Hatmi M, et al. Lebecetin, a potent antiplatelet C-type lectin from Macrovipera lebetina venom. BBA-Proteins Proteom. 2003;1651(1–2):30–40. [DOI] [PubMed] [Google Scholar]
- 79.Sarray S, Delamarre E, Marvaldi J, et al. Lebectin and lebecetin, two C-type lectins from snake venom, inhibit α5β1 and αv-containing integrins. Matrix Biol. 2007;26(4):306–313. [DOI] [PubMed] [Google Scholar]
- 80.Pilorget A, Conesa M, Sarray S, et al. Lebectin, a Macrovipera lebetina venom-derived C-type lectin, inhibits angiogenesis both in vitro and in vivo. J Cell Physiol. 2007;211(2):307–315. [DOI] [PubMed] [Google Scholar]
- 81.Montassar F, Darche M, Blaizot A, et al. Lebecetin, a C-type lectin, inhibits choroidal and retinal neovascularization. Faseb J. 2017;31 (3):1107–1119. [DOI] [PubMed] [Google Scholar]
- 82.Ciulla TA, Bracha P, Pollack J, et al. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2(12):1179–1187. [DOI] [PubMed] [Google Scholar]
- 83.Cantrell RA, Lum F, Chia Y, et al. Treatment patterns for diabetic macular edema: an intelligent research in sight (IRIS®) registry analysis. Ophthalmology. 2020;127(3):427–429. [DOI] [PubMed] [Google Scholar]
- 84.Ciulla TA, Pollack JS, Williams DF. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol. 2020: bjophthalmol-2020–315933. DOI: 10.1136/bjophthalmol-2020-315933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rao P, Lum F, Wood K, et al. Real-World vision in age-related macular degeneration patients treated with single Anti–VEGF drug type for 1 year in the iris registry. Ophthalmology. 2018;125 (4):522–528. [DOI] [PubMed] [Google Scholar]
- 87.Ciulla TA, Hussain RM, Pollack JS, et al. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4(1):19–30. [DOI] [PubMed] [Google Scholar]


