Abstract
Aims
Use of an absorbable antibacterial envelope during implantation prevents cardiac implantable electronic device infections in patients with a moderate-to-high infection risk. Previous studies demonstrated that an envelope is cost-effective in high-risk patients within German, Italian, and English healthcare systems, but these analyses were based on limited data and may not be generalizable to other healthcare settings.
Methods and results
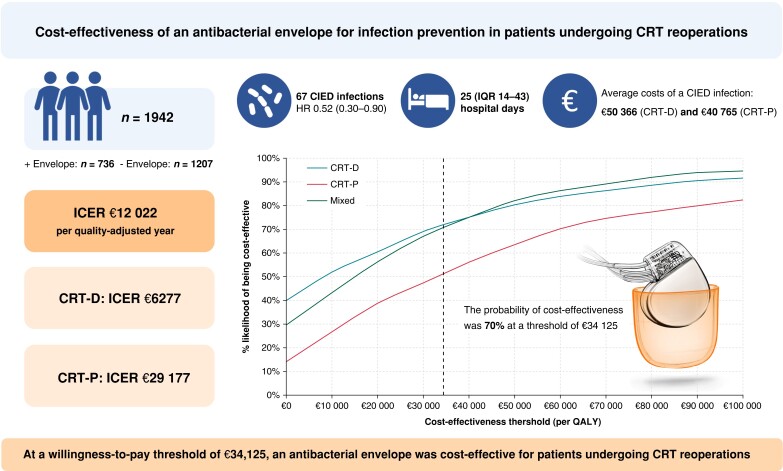
A previously published decision-tree-based cost-effectiveness model was used to compare the costs per quality-adjusted life year (QALY) associated with adjunctive use of an antibacterial envelope for infection prevention compared to standard-of-care intravenous antibiotics. The model was adapted using data from a Danish observational two-centre cohort study that investigated infection-risk patients undergoing cardiac resynchronization therapy (CRT) reoperations with and without an antibacterial envelope (n = 1943). We assumed a cost-effectiveness threshold of €34 125/QALY gained, based on the upper threshold used by the National Institute for Health and Care Excellence (£30 000). An antibacterial envelope was associated with an incremental cost-effectiveness ratio (ICER) of €12 022 per QALY in patients undergoing CRT reoperations, thus indicating that the envelope is cost-effective when compared with standard of care. A separate analysis stratified by device type showed ICERS of €6227 (CRT defibrillator) and €29 177 (CRT pacemaker) per QALY gained.
Conclusions
Cost-effectiveness ratios were favourable for patients undergoing CRT reoperations in the Danish healthcare system, and thus are in line with previous studies. Results from this study can contribute to making the technology available to Danish patients and align preventive efforts in the pacemaker and ICD area.
Keywords: Cardiac resynchronization therapy, Infection, Cost-effectiveness analysis, Antibacterial envelope, Pacemaker, Implantable cardioverter defibrillator
Graphical Abstract
Graphical Abstract.
CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy (-P, pacemaker; -D, defibrillator); ICER, incremental cost-effectiveness ratio; HR, hazard ratio; IQR, interquartile range; QALY, quality-adjusted life year.
What’s New.
Use of an antibacterial envelope has been associated with favourable cost-effectiveness ratios for patients at high-risk of developing cardiac implantable electronic device infections in Germany, Italy, England, and North America, but never within the Danish healthcare system.
Unlike prior analyses, which were based on smaller patient samples, we included a complete and consecutive cohort (n = 1943) of patients who underwent a cardiac resynchronization therapy reoperation with or without an antibacterial envelope in two tertiary centres in Denmark. The baseline infection rate was 4.1%.
An antibacterial envelope was associated with incremental cost-effectiveness ratios (ICERs) below accepted willingness-to-pay thresholds. The ICER was €12 022 per quality-adjusted life year, and the probability of cost-effectiveness was 70% at a willingness-to-pay threshold of €34 125.
Introduction
Cardiac implantable electronic device (CIED) infections are associated with significant patient disability, increased mortality, and substantial economic costs.1–4 A disproportionate increase in infection rates has been observed in the aftermath of rising implantation rates,5 and hence, the clinical and economic impact of CIED infections is growing. Pre-procedural intravenous antibiotics are standard of care (SoC) for infection prevention,6,7 and in 2019, the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) demonstrated that an absorbable antibacterial envelope reduces the incidence of infections in patients undergoing CIED reoperations and de novo cardiac resynchronization therapy (CRT) defibrillator (-D) implantations—albeit at low overall infection rates (0.7% and 1.2% with and without an envelope).8 Subsequent economic evaluations of the envelope for infection prevention within Canadian,9 North American,10 and selected European healthcare systems1,11 indicate that an envelope may not be economically favourable in patients with low infection risk. Some suggest that an envelope is cost-effective at baseline infection rates below 2–3%,1,12 while others report favourable cost-effectiveness ratios only when infection rates exceed 6%.9 Importantly, sub-analyses in higher-risk subsets were based on small or diverse patient samples, and context-specific economic assessments are not easily generalized to other healthcare settings.1 Even so, health economic evaluations are needed to inform and support decision-making in healthcare to maximize the benefit of an intervention to society and patients.13,14
In this study, we present an economic evaluation of the absorbable antibacterial envelope for patients undergoing CRT reoperations within the Danish healthcare system.15
Methods
Study population and cardiac implantable electronic device infections
In Denmark for primary prophylaxis, we used CRT pacemaker (-P) for most patients with non-ischaemic cardiomyopathy, especially if older than 70 years, and CRT-D for patients with ischaemic cardiomyopathy as well as for the younger patients with non-ischaemic heart failure. This study was based on data from a Danish, observational two-centre cohort study. The main results—including a detailed study description—are reported previously.15 In brief, the study population comprised 1943 consecutive patients who underwent a CIED CRT reoperation at either Odense or Aarhus University Hospital between January 2008 and November 2019. Patients were followed from time of implantation for a maximum of 2 years. Device, procedural, and clinical data were obtained from the Danish Pacemaker and ICD Register and a systematic medical chart review. During the study period, preprocedural intravenous antibiotics were SoC in both centres. The absorbable antibacterial envelope (TYRX™, Medtronic, USA) was introduced in Denmark in 2015 and was used as an adjunct to SoC for patients with multiple risk factors for infection. The median Prevention of Arrhythmia Device Infection (PADIT) risk score was 7 (IQR 6–8) in this population.16 A PADIT score of 7 corresponds to an estimated infection risk at 1 year of 4.3% using SoC antibiotics.
A total of 67 (3.4%) (predominantly local) CIED infections were observed over a maximum follow-up of 2 years, and the use of an absorbable antibacterial envelope was associated with a hazard ratio (HR) for infection of 0.52 [95% confidence interval (CI) 0.30–0.90]. Fifty (4.1%) CIED infections occurred in patients treated with SoC alone. Baseline clinical characteristics are shown in Table 1, and infections are briefly described in Table 1.
Table 1.
Selected baseline characteristics and clinical outcomes data for patients who developed cardiac implantable electronic device (CIED) infections (complete cohort of infected patients)
| CRT-P (n = 20) | CRT-D (n = 47) | Total (n = 67) | |||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | |||||||
| Age, mean ± SD | 71 | ± 10 | 70 | ± 9 | 70 | ± 9 | |
| Female, n (%) | − | − | − | − | 4 | 6% | |
| PADIT score, median (IQR) | 8 | IQR 5–9 | 7 | IQR 6–9 | 7 | IQR 6–9 | |
| Previous procedures, n (%) | |||||||
| 1 | 12 | 52% | 24 | 54.5% | 36 | 54% | |
| ≥2 | 11 | 48% | 20 | 45.5% | 31 | 46% | |
| Procedure type, n (%) | |||||||
| Replacements | 15 | 65% | 29 | 66% | 44 | 66% | |
| Upgrades | 8 | 35% | 15 | 34% | 23 | 34% | |
| Clinical outcomes data | |||||||
| Infection type, n (%) | |||||||
| 17 | 74% | 30 | 68% | 47 | 70% | ||
| Systemic | 6 | 26% | 14 | 32% | 20 | 30% | |
| Hospitalization (days), median (IQR) | 22 | IQR 12–37 | 26 | IQR 15–67 | 25 | IQR 14–43 | |
| ICU stay, n (%) | − | − | − | − | 12 | 18% | |
| Days in the ICU, median (IQR) | − | − | − | − | 8.5 | IQR 3–11.5 | |
| In-hospital mortality, n (%) | − | − | − | − | 7 | 10% | |
Subgroups with <4 patients are not reported due to patient discretion considerations.
CRT-P/-D, cardiac resynchronization therapy pacemaker/-defibrillator; PADIT, prevention of arrhythmia device infection; ICU, intensive care unit.
The Danish healthcare system
Danish healthcare is universally tax-financed. All residents have free access to healthcare, and examinations and treatments (including CIED procedures) are free of charge. Financing is administered in block grants and balanced at a regional level.17 Danish hospitals operate with global budget plans, and no specific reimbursement for medical devices exists. Hence, the decision to utilize an envelope is left to individual physicians, departments, and/or hospitals.
Model structure
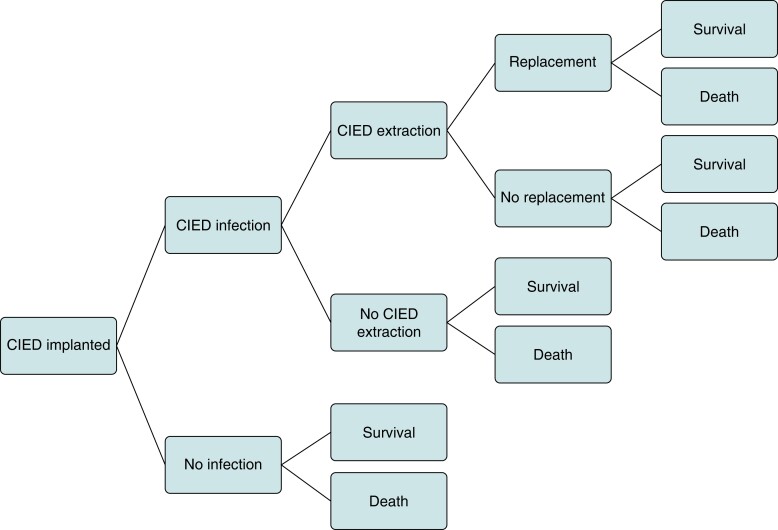
A UK-based decision-tree model developed in Microsoft Excel by Kay et al.12 was adapted to the Danish healthcare setting (Figure 1). Previous adaptations of this model to Germany, Italy, and England have been published.1 The model compared patients who received an antibacterial envelope + SoC to SoC alone. Types of intravenous antibiotics used and CIED infection and device types were assumed the same in both arms of the model. After implantation, all patients were at risk of infection, which could result in either complete extraction (with or without a replacement device) or no extraction. Death and survival at 24 months were end nodes for all arms of the decision tree. Lifetime pay-offs were used to capture lifetime costs and quality-adjusted life years (QALYs) for the remainder of the patient’s life expectancy.18 Model inputs used in the base-case analysis are shown in Table 2.
Figure 1.
Decision-tree model adapted from Kay et al.12 CIED, cardiac implantable electronic device.
Table 2.
Model inputs for the base-case scenario in all-comers
| Parameter | CRT-P | CRT-D | Source |
|---|---|---|---|
| Infection rates up to 24 months (CRT-D), SoC | 4.14% | 4.14% | Frausing et al.15 |
| HR of infection with an envelope up to 24 months | 0.52 | 0.52 | Frausing et al.15 |
| Re-infection following no extraction | 71.40% | 71.40% | Pichlmaier et al.19 |
| Re-infection following complete extraction | 4.80% | 4.80% | Ahmed et al.3 |
| Mortality no infection | 11.00% | 11.00% | WRAP-IT8 |
| Lifetime costs without CIED | €8662 | €8662 | NICE18 |
| Lifetime costs with CIED | €20 679 | €23 107 | NICE18 |
| Lifetime QALYs without CIED | 2.78 | 2.78 | WRAP-IT8 |
| Lifetime QALYs with CIED | 3.47 | 3.88 | WRAP-IT8 |
| Baseline utility after treatment with CIED | 0.76 | 0.81 | WRAP-IT8 |
| Disutility with major CIED infection for 6 months | −0.10 | −0.10 | WRAP-IT8 |
| Infection cost no extraction, SoC | €40 765 | €50 366 | Calculated (DRGs) |
CRT-P/-D, cardiac resynchronization therapy pacemaker/-defibrillator; QALY, quality-adjusted life year; SoC, standard of care.
Model outputs
The model was used to generate incremental costs per QALYs gained (incremental cost-effectiveness ratio, ICERs) for each individual device type (CRT-P or CRT-D) as well as a combined CRT-P and CRT-D device population. In absence of any formal Danish willingness-to-pay threshold value, a conservative approach was applied, and the threshold used in these analyses is the upper limit of £30 000 per QALY gained commonly used by the National Institute for Health and Care Excellence (NICE) converted to a Euro equivalent (€34 125).20 In line with the clinical study that informed the efficacy parameters, results have been generated for an all-comers/high-risk population (the base case) as well as for the following patient subgroups: individuals undergoing revisions/upgrades, those that have only had one previous CIED procedure, those that have had at least two previous CIED procedures, and those that underwent replacement surgery.
Infection risk
The probability of infection with SoC was estimated from the risk of infection reported for all patients in the control group with a common value applied to both device types (Table 3). The treatment effect associated with an envelope was modelled by applying the HR of infection to the baseline risk.15 For simplicity, the HR of infection with an envelope (0.52, 95% CI 0.30–0.90) was applied to all subgroups in the model. Risk of re-infection was sourced from literature: we assumed a re-infection risk of 4.8%21 for patients who underwent a complete device extraction with a replacement, and 71.4% for patients who did not undergo CIED extraction.19
Table 3.
Base-case cost-effectiveness results stratified by device type
| Population | Incremental costs | Incremental QALYs | ICER (per QALY gained) |
|---|---|---|---|
| CRT-D | €65 | 0.01 | €6227 |
| CRT-P | €250 | 0.01 | €29 177 |
| Combined | €118 | 0.01 | €12 022 |
Minor CIED infections included any CIED infection not meeting the criteria for major CIED infection. It was reported as a probability of 0.93% with SoC, and a relative risk of 0.72 with an envelope was applied to cover these events. Again, common values were used for both device types.
Cardiac implantable electronic device infection-related costs in Denmark
Infection-related healthcare costs were estimated at an individual level based on Diagnosis Related Group (DRG) 2022-tariffs with correction for hospital length of stay in case this exceeded the number of days included in each tariff. Cost per day was estimated based on the average cost per bed day at the Department of Cardiology, Aarhus University Hospital (€839 per day). Days in the intensive care unit were added to the tariff when relevant using the national 2022 tariff (€3733 per day). As such, the average costs used in this model indirectly considered both treatment differences and disease severity. Costs associated with prophylactic antibiotics were applied in both arms of the model as one-off costs to all patients (Table 2) (<€5 per dose). In the model, we used a cost of the envelope of €969. Costs related to re-infection were applied to patients who did not have their device extracted, or to patients who underwent a complete extraction with replacement.
Health-related quality of life
Health-related quality of life (HRQoL) values in the first 12 months consisted of the baseline utility valued after treatment with CIED and was based on the EQ-5D analysis from the WRAP-IT.8 These differed between devices (CRT-D 0.81; CRT-P 0.76), and a utility decrement of 0.10 at 6 months was applied for all patients who experienced an infection, regardless of whether an antibacterial envelope was used.
Lifetime costs and benefits
Beyond the initial 24 months, lifetime discounted costs and QALYs were applied based on results reported in National Institute for Health and Care Excellence’s Evidence Review Group analysis (Table 3).18 These depend on the device type and whether a CIED is in place. For example, patients who did not undergo a device re-implantation had fewer QALYs applied than those who had a replacement device. The cost of the initial procedure was subtracted from lifetime costs because these apply to all patients in the model, and not just those surviving beyond 2 years.
Mortality
All-cause mortality data up to 24 months were taken from WRAP-IT.8 The baseline all-cause mortality for patients with and without infection was 19% and 11%, respectively. The mortality rate for patients with infection was assumed to consider management of the infection, such as complete or no extraction, and was applied to both CRT-D and CRT-P devices.
Exchange rate and discounting
Where necessary, costs were converted from Great British Pounds to Euros using an exchange rate of 1.14 and from Danish Kroner to Euros using an exchange rate of 0.13.22 We opted not to apply inflated costs because some costs will have increased over time while others have decreased. Discounting was not applied since it was already applied in the NICE technical appraisal at a rate of 3.5% per annum when calculating the lifetime payoffs.18
Sensitivity analyses
One-way deterministic sensitivity analyses were performed to establish first-order uncertainty around infection rates, mortality, HRQoL, QALYs, lifetime costs, and benefits. Parameters were varied across a plausible range based on clinical expert opinion or 95% CI. All other parameters were varied by +/−20%.
Probabilistic sensitivity analyses were conducted to assess the degree of uncertainty in the model results using 1000 iterations. Gamma distributions were used for costs, lognormal for hazard ratios, and beta for probabilities and utilities.23 When data on variability around the sampling distribution of mean values were not available, the standard error was assumed to be equal to 10% of the mean.
Results
Patients experiencing CIED infection in this population were hospitalized for a median of 25 days (IQR 14–43), and 14 (21%) patients required treatment in the intensive care unit (ICU). The median duration of hospitalization in the ICU was 8.5 days, IQR 3–11.5. We estimated average costs of €50 366 and €40 765 for infections relating to CRT-D and CRT-P devices, respectively (Table 1). Higher cost estimates for CRT-Ds were due to higher costs of the CRT-D devices and longer duration of hospitalizations in this patient group, including more infections with requirement for intensive care (Tables 1 and 2). The in-hospital mortality was 10% (n = 7).
Table 3 presents the summary cost-effectiveness results in each population modelled over a lifetime horizon (Graphical Abstract). In the base-case analysis, the per-patient predicted lifetime incremental costs for CRT-D, CRT-P, and the combined device population were €65, €250, and €118, respectively. The associated incremental costs per QALY gained were €6,227, €29,177, and €12,022, respectively. Hence, all ICERS are below the assumed cost-effectiveness threshold of €34 125 per QALY gained.
The subgroup-specific results are presented in the Supplementary material online, Table S1. Absorbable antibacterial envelopes and a CRT-D device were cost-effective in all subgroups except for patients who only had one previous procedure. Absorbable antibacterial envelopes for CRT-P devices were cost-effective in all patients except those who had either one previous procedure or underwent revision/upgrade surgery. Absorbable antibacterial envelopes provided greater clinical benefit at lower cost in patients who had at least two previous CIED procedures, regardless of which CIED device they were used in combination with.
Sensitivity analyses
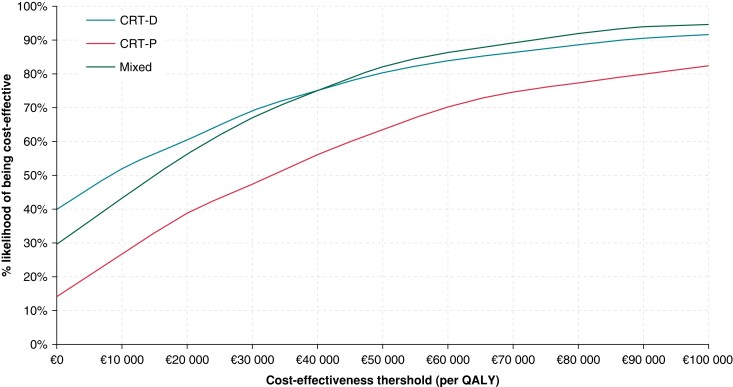
At a willingness-to-pay threshold of €34 125 per QALY gained, the probabilistic sensitivity analyses indicated that the probability that the antibacterial envelope was cost-effective for those who received CRT-D or CRT-P were 72% and 51%, respectively (Figure 2). At the same willingness-to-pay threshold, the probability that an antibacterial envelope was cost-effective in the mixed CRT-P/CRT-D population was 70% (Figure 2). The cost-effectiveness acceptability curves for each of the subgroups of interest are shown in the Supplementary material online, Figure S1, stratified by device type (CRT-D, CRT-P, and mixed CRT-D/P).
Figure 2.
Cost-effectiveness acceptability curves plots for the composite (mixed) population and per device type. CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; QALY, quality-adjusted life year.
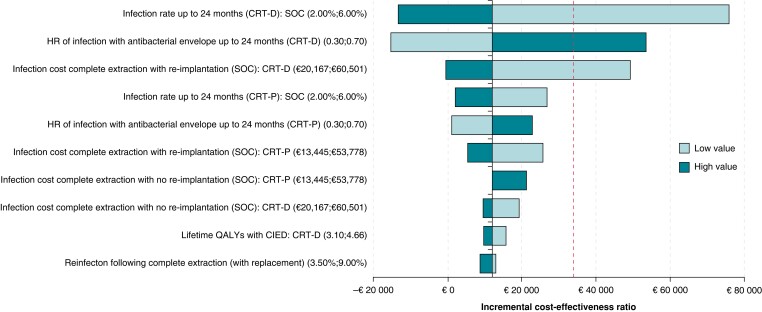
The one-way deterministic sensitivity analyses are reported in Figure 3 (stratified by device type in supplementary material online, Figure S4). Across all three groups, the main drives of the model in all populations were the baseline risk of infection up to 24 months, the HR of infection with an envelope, and the cost of infection with re-implantation. The Tornado plots from all relevant clinical subgroups are presented in the Supplementary material online, Figures S2 and S3.
Figure 3.
Tornado plots for the composite population. The red line corresponds to the relevant incremental cost-effectiveness ratio. CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; QALY, quality-adjusted life year.
Discussion
In this study, we found that adjunctive use of an absorbable antibacterial envelope for infection prevention was cost-effective when used for all-comers across device types at a cost-effectiveness threshold of €34 125 per QALY gained. Our study is the first economic evaluation of absorbable antibacterial envelopes within the Danish healthcare setting, and the first assessment is based on a complete and consecutive cohort exclusive to patients undergoing CRT reoperations. The baseline 24-month infection risk with SoC was >4% in this population.15 Results of this study represent an update to those presented by Kay et al.12 and Boriani et al.1 Data with a longer follow-up (24 months vs. 12 months) were applied before lifetime costs, and QALYs were added to the analysis. By inclusion of more specific patient subgroups, this study adds to the already published results of adaptations to other European evaluations (Italy, Germany, and England).1
Across subgroups, overall ICERs were more favourable in patients undergoing CRT-D operations. This was primarily related to higher costs of the CRT-D devices, and to more severe courses of disease in this patient group as exemplified by their higher propensity for systemic infections, longer duration of hospitalization, and a more frequent requirement for intensive care during index hospitalization (data not shown).
Key drivers also included the cost of complete extraction with re-implantation, cost of complete extraction without re-implantation, and lifetime QALYs with a CIED. Infection rates up to 24 months, the HR of infection with an envelope, and the costs of complete extraction with re-implantation could cause the envelope to be dominant in all-comers (for CRT-D and combined). On the other hand, for the ≥2 previous procedures subgroup, the infection rates up to 24 months could cause the envelope to become not cost-effective (for CRT-D and CRT-P). Finally, the infection rates up to 24 months, the HR of infection with an envelope, and the cost of complete extraction with re-implantation could cause the envelope to become either not cost-effective or dominant (for all device types).
Compared to the study by Boriani et al., we showed more favourable results in patients with CRT-Ds and ≥2 previous procedures, where an envelope was cost-saving (i.e. dominant) in the current analysis. Furthermore, in the Boriani et al. adaptation, an envelope was cost-effective with ICERs of €18 181 in Germany, €14 371 in Italy, and £16 680 in England. The current model also presents more favourable results for patients with CRT-Ds who undergo replacement procedures, which was dominant over SoC in our analysis but not in the previous evaluation; reported ICERs were €42,912, €39,094, and £37 581 for Germany, Italy, and England, respectively. This difference was primarily driven by the higher risk of infection in this patient subgroup of our population when compared to WRAP-IT (∼7% vs. 4%). Discrepancies in reported infection rates in real-world populations vs. trial populations are well-known, and likely arise from patient selection in randomized controlled trials.
An important detriment to CIED infections not captured in the current analysis relates to challenges when re-implanting the left ventricular (LV) lead following CRT extraction. Often, it is not possible to re-implant the LV lead in the same position. Suboptimal LV lead positioning is established as an important determinant of clinical outcomes after CRT.24 Hence, the clinical benefit from CRT may be less following a CIED infection, which further underscores the importance of preventing infections in this patient group.
In this study, costs were estimated based on DRG tariffs with individual-level corrections for the duration of hospitalization. We estimated average costs of €40 765 for CRT-Ps and €50 366 for CRT-Ds. This was in concordance with previous estimates for costs relating to CIED infections in other european healthcare settings; £41 820 for patients with implanted ICDs and CRTs based in a UK setting,11 and £27 661–£37 633 (UK), €37 168–€42 921 (Germany), and €37 777–€45 560 (Italy) (assuming an extraction was performed) for implanted CRT-Ps and CRT-Ds in an economic evaluation based on a WRAP-IT high-risk sub-cohort.1 Meanwhile, our cost estimates were significantly lower than estimates from the USA ($67 586)10 but comparable to cost inputs from a study based in a Canadian setting (Can$64 809).9 Differences in costs associated with infection and variations in willingness-to-pay thresholds and reimbursement policies hamper extrapolation of results from economic analyses in one country to another. For this reason, local analyses are pivotal.
The estimates for infection-related costs further highlight the significant financial implications of CIED infections. However, an envelope is costly, and even when the feasibility and clinical efficacy of an intervention has been established, there is a growing need to provide assessments of the balance between economic and clinical utility. Results from this analysis indicate that an absorbable antibacterial envelope may be an attractive option for patients undergoing CRT reoperations in Denmark, especially for patients with multiple previous procedures. Efforts should be directed towards development and validation of risk prediction tools to enhance our ability to identify patients at high risk of infection,4,25,26 in whom both the clinical and economical value of an antibacterial envelope is highest.
Limitations
Several limitations must be acknowledged when interpreting results from this analysis. First, the estimated treatment effect of antibacterial envelope was based on observational data, which implied strong assumptions of no unmeasured or unknown confounding. However, results from subgroup analyses in WRAP-IT concur with our results indicating that an envelope reduces infection risk even in higher-risk situations (e.g. the HR for patients receiving high-power devices was 0.51, 95% CI 0.29–0.90). Assuming that late infections are most likely not associated with use of an envelope, follow-up was restricted to 2 years, and any effect beyond this 2-year cut-off would therefore not be captured by our model. Second, we based our subgroup analyses on smaller patient samples with fewer events, which can influence the accuracy of the estimated infection risk in these groups (a main driver of the analysis). This was especially the case for subgroups of patients undergoing revisions and upgrades (n = 23 events) or CRT-P procedures (n = 23 events).
Third, a key limitation is the generalizability and uncertainty of the total cost of infections. To mitigate this limitation, extensive sensitivity analyses were performed; however, due to the small incremental QALYs generated, changing this input could potentially have a large effect on the estimated ICERs. Also, assessment of costs was based on DRG tariffs for diagnosis and procedure codes. The DRG tariffs comprise national averages and hence, do not reflect actual individual-level costs. Moreover, we observed high variation in costs as the course of infection could span from simple extractions with 10-day antibiotic regimens to more severe manifestations with prolonged hospitalizations in the ICU.
Fourth, several model inputs were obtained from external sources including HRQoL (WRAP-IT), mortality with and without infection (WRAP-IT),8 lifetime discounted costs (NICE), QALYs beyond 24 months (NICE),18 and risk of re-infection with and without CIED extraction, which was sourced from literature.19,21 Therefore, these parameters may not reflect conditions specific to our population of patients undergoing CRT reoperations.
Finally, an arbitrary threshold for cost-effectiveness was assumed because no official threshold is established in Denmark. Any interpretation of cost-effectiveness within the Danish healthcare system based on this cut-off value should therefore be carefully considered.
Conclusions
Results from this cost-effectiveness analysis based in the Danish healthcare setting suggest that an absorbable antibacterial envelope for infection prevention in patients undergoing CRT reoperations is cost-effective at a cost-effectiveness threshold of €34 125/QALY gained.
Supplementary Material
Acknowledgements
The authors would like to thank Mrs Judith Shore for her work on the cost-effectiveness model while at York Health Economics Consortium (UK), and Mr Eydfinn Heinesen (Aarhus University Hospital, Denmark) for assisting with the DRG-based calculations of infection-related healthcare costs.
Contributor Information
Maria Hee Jung Park Frausing, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Bvld. 99, 8200 Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Palle Juul-Jensens Bvld. 82, 8200 Aarhus, Denmark.
Jens Brock Johansen, Department of Cardiology, Odense University Hospital, J.B. Winsløws Vej 4, 5000 Odense, Denmark.
Daniela Afonso, York Health Economics Consortium, Enterprise House, Innovation Way, University of York, York Y0105NQ, United Kingdom.
Ole Dan Jørgensen, Department of Cardiac-, Thoracic-, and Vascular Surgery, Odense University Hospital, J.B. Winsløws Vej 4, 5000 Odense, Denmark.
Thomas Olsen, York Health Economics Consortium, Enterprise House, Innovation Way, University of York, York Y0105NQ, United Kingdom.
Christian Gerdes, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Bvld. 99, 8200 Aarhus, Denmark.
Mette Lundsby Johansen, Medtronic Denmark, KLP1, Arne Jacobsens Allé 17, 2300 Copenhagen, Denmark.
Claudia Wolff, Medtronic International Trading Sarl, Route du Molliau 31, CH-1131 Tolochenaz, Switzerland.
Stuart Mealing, York Health Economics Consortium, Enterprise House, Innovation Way, University of York, York Y0105NQ, United Kingdom.
Jens Cosedis Nielsen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Bvld. 99, 8200 Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Palle Juul-Jensens Bvld. 82, 8200 Aarhus, Denmark.
Mads Brix Kronborg, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Bvld. 99, 8200 Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Palle Juul-Jensens Bvld. 82, 8200 Aarhus, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
M.H.J.P.F. was supported by a grant from the Karen Elise Jensen Foundation. Medtronic provided financial support for the cost-effectiveness analyses for this study, but the authors retained control over the study design, analyses, manuscript preparation, and final submission.
Data availability
The Data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Boriani G, Kennergren C, Tarakji KG, Wright DJ, Ahmed FZ, McComb JMet al. Cost-Effectiveness analyses of an absorbable antibacterial envelope for use in patients at increased risk of cardiac implantable electronic device infection in Germany, Italy, and England. Value Health 2021;24:930–8. [DOI] [PubMed] [Google Scholar]
- 2. Boriani G, Vitolo M, Wright DJ, Biffi M, Brown B, Tarakji KGet al. Infections associated with cardiac electronic implantable devices: economic perspectives and impact of the TYRX™ antibacterial envelope. Ep Europace 2021;23:iv33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed FZ, Fullwood C, Zaman M, Qamruddin A, Cunnington C, Mamas MAet al. Cardiac implantable electronic device (CIED) infections are expensive and associated with prolonged hospitalisation: UK retrospective observational study. PLoS One 2019;14:e0213682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sgreccia D, Vitolo M, Valenti AC, Manicardi M, Boriani G. Burden of disease and costs of infections associated with cardiac implantable electronic devices. Expert Rev Pharmacoecon Outcomes Res 2022;22:7–16. [DOI] [PubMed] [Google Scholar]
- 5. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RTet al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 6. de Oliveira JC, Martinelli M, Nishioka SADO, Varejão T, Uipe D, Pedrosa AAAet al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- 7. Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran Pet al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018;72:3098–109. [DOI] [PubMed] [Google Scholar]
- 8. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss Eet al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 9. Rennert-May E, Raj SR, Leal J, Exner DV, Manns BJ, Chew DS. Economic evaluation of an absorbable antibiotic envelope for prevention of cardiac implantable electronic device infection. EP Europace 2021;23:767–74. [DOI] [PubMed] [Google Scholar]
- 10. Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GRet al. Cost-Effectiveness of an antibacterial envelope for cardiac implantable electronic device infection prevention in the US healthcare system from the WRAP-IT trial. Circ Arrhythm Electrophysiol 2020;13:e008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burnhope E, Rodriguez-Guadarrama Y, Waring M, Guilder A, Malhotra B, Razavi Ret al. Economic impact of introducing TYRX amongst patients with heart failure and reduced ejection fraction undergoing implanted cardiac device procedures: a retrospective model based cost analysis. J Med Econ 2019;22:464–70. [DOI] [PubMed] [Google Scholar]
- 12. Kay G, Eby EL, Brown B, Lyon J, Eggington S, Kumar Get al. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ 2018;21:294–300. [DOI] [PubMed] [Google Scholar]
- 13. Boriani G, Vitolo M, Svennberg E, Casado-Arroyo R, Merino JL, Leclercq C. Performance-based risk-sharing arrangements for devices and procedures in cardiac electrophysiology: an innovative perspective. EP Europace 2022;24:1541–7. [DOI] [PubMed] [Google Scholar]
- 14. Gottschalk S, Kany S, König HH, Crijns HJ, Vardas P, Camm AJet al. Cost-effectiveness of early rhythm control vs. usual care in atrial fibrillation care: an analysis based on data from the EAST-AFNET 4 trial. Europace 2023;25(5):euad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frausing MHJP, Nielsen JC, Johansen JB, Jørgensen OD, Gerdes C, Olsen Tet al. Rate of device-related infections using an antibacterial envelope in patients undergoing cardiac resynchronization therapy reoperations. EP Europace 2022;24:421–9. [DOI] [PubMed] [Google Scholar]
- 16. Birnie DH, Wang J, Alings M, Philippon F, Parkash R, Manlucu Jet al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol 2019;74:2845–54. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SAJ, Adelborg K, Sundboll J, Laugesen K, Ehrenstein Vet al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute for Health and Care Excellence (NICE) . Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure [TA314]. National Institute for Health and Care Excellence; 2014.
- 19. Pichlmaier M, Knigina L, Kutschka I, Bara C, Oswald H, Klein Get al. Complete removal as a routine treatment for any cardiovascular implantable electronic device-associated infection. J Thorac Cardiovasc Surg 2011;142:1482–90. [DOI] [PubMed] [Google Scholar]
- 20. NICE health technology evaluations: The Manual. The National Institute for Health and Care Excellence; 2022.
- 21. Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000;133:604–8. [DOI] [PubMed] [Google Scholar]
- 22.https://www.xe.com/ XE. [Available from: , [cited January 1, 2023]
- 23. Briggs AKC, Sculpher M. Decision modelling for health economic evaluation: Oxford: Oxford University Press; 2006. [Google Scholar]
- 24. Sommer A, Kronborg MB, Nørgaard BL, Poulsen SH, Bouchelouche K, Böttcher Met al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Eur J Heart Fail 2016;18:1365–74. [DOI] [PubMed] [Google Scholar]
- 25. Callahan TD, Tarakji KG, Wilkoff BL. Antibiotic eluting envelopes: evidence, technology, and defining high-risk populations. EP Europace 2021;23:iv28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhry U, Borgquist R, Smith JG, Mörtsell D. Efficacy of the antibacterial envelope to prevent cardiac implantable electronic device infection in a high-risk population. EP Europace 2022;24:1973–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Data underlying this article will be shared on reasonable request to the corresponding author.