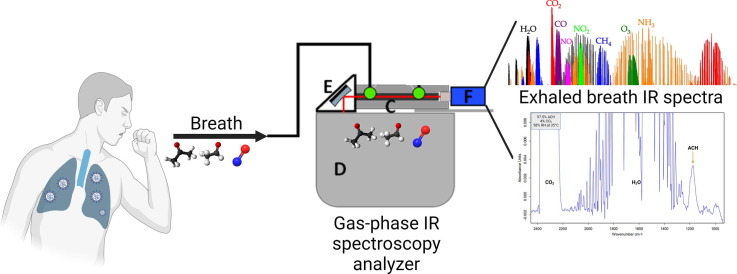
Graphical abstract
Keywords: Gas-phase infrared spectroscopy, Substrate-integrated hollow waveguide, iHWG, Exhaled breath, VOCs, COVID-19, Screening
Abstract
The COVID-19 pandemic remains a global challenge now with the long-COVID arising. Mitigation measures focused on case counting, assessment and determination of variants and their likely targets of infection and transmission, the pursuit of drug treatments, use and enhancement of masks, social distancing, vaccination, post-infection rehabilitation, and mass screening. The latter is of utmost importance given the current scenario of infections, reinfections, and long-term health effects. Research on screening platforms has been developed to provide more sensitive, specific, and reliable tests that are accessible to the entire population and can be used to assess the prognosis of the disease as well as the subsequent health follow-up of patients with sequelae of COVID-19. Therefore, the aim of the present study was the simulation of exhaled breath of COVID-19 patients by evaluation of three identified COVID-19 indicator breath biomarkers (acetone (ACE), acetaldehyde (ACH) and nitric oxide (NO)) by gas-phase infrared spectroscopy as a proof-of-concept principle for the detection of infected patients’ exhaled breath fingerprint and subsequent follow-up. The specific fingerprints of each of the compounds and the overall fingerprint were obtained. The synthetic exhaled breath evaluation concept revealed a linearity of r = 0.99 for all compounds, and LODs of 6.42, 13.81, 9.22 ppm, and LOQs of 42.26, 52.57, 69.23 ppm for NO, ACE, and ACH, respectively. This study proves the fundamental feasibility of gas-phase infrared spectroscopy for fingerprinting lung damage biomarkers in exhaled breath of patients with COVID-19. This analysis would allow faster and cheaper screening and follow-up of infected individuals, which could improve mass screening in POC settings.
1. Introduction
The ongoing pandemic of COVID-19 has affected all countries. Efforts by several research groups focused on addressing the mitigation strategies established by health agencies within each country. Strategies include counting cases, evaluating and determining the variants, and their likely targets of infection and transmission, searching for pharmacological treatments, the use and improvement of masks, social distancing, vaccination, post-infection rehabilitation, and mass screening [1], [2]. The latter being of utmost importance given the present scenario of infections, re-infections, and long-term health effects now so-called the ‘long COVID’.
Among the technologies described for screening COVID-19 cases and post-infection monitoring are RTq-PCR tests as the gold standard [3] and rapid antigen and antibody tests [4], some have been evaluated and accepted by the Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA) as acceptance tests for case detection, depending on the temporality of infection [5]. However, these tests are only indicative of latent SARS-CoV-2 virus infection or in the case of antibody tests of past recent infection. In this context, research on screening platforms has been developed to offer more sensitive, specific, reliable tests that are accessible to the entire population and can be used to evaluate the disease prognosis as well as the subsequent monitoring of patients with long-COVID.
Despite SARS-CoV-2 being considered a respiratory disease, it has been shown that the virus can use the olfactory nerve to infiltrate and damage the nervous system by activating T lymphocytes which, in turn, activate microglia and inflammatory mediators. Damage to the nervous system has been associated with disease-specific metabolites and unusual chemical biomarkers that are emitted into the exhaled breath from soft tissue and body cavities, such as the lungs or nasal cavity, which can be detected in a person's breath [6]. These biomarkers are volatile organic compounds (VOCs) and represent important metabolic endpoints that can be evaluated for the clinical and non-clinical status of an individual, and thus provide relevant health status information. VOCs have been studied in different infectious and non-infectious lung diseases including COVID-19 [7], [8], [9], [10]. Gould et al. state that the virus-host interaction alters cellular metabolism at different levels, triggering oxidative stress processes, that result in changes in the composition and concentration of certain VOCs in exhaled breath and other biological matrices [11]. On April 2022, the FDA approved the first COVID-19 diagnostic test using exhaled breath samples, this test is a portable GC–MS and is based on the detection of five VOCs from the ketone and aldehyde families associated with SARS-CoV-2 infection [12]. Nevertheless, these techniques require complex sample treatment schemes [13].
The need for sensitive, specific, portable, and low-cost techniques for the determination of VOCs in exhaled breath that can be applied in point-of-care (POC) settings has led researchers to propose emerging analytical technologies such as gas-phase infrared spectroscopy (IR). Among all the IR regions, the mid-IR region (λ = 400–4000 cm−1) has been applied to biological studies because the range of λ = 900–1800 cm−1 is the 'fingerprint region’ of biological samples [14], [15]. Several biomarkers have been assessed in exhaled breath for disease detection by this technique, however further studies are required in the development of technologies that can detect differences between exhaled breath fingerprints of patients with infectious diseases. Previous studies of our research group have developed strategies for the detection of compounds in exhaled breath via this technology [13], [16], thus demonstrating its usefulness for the detection of chemical fingerprints of VOCs in human exhaled breath.
Some of the most reported VOCs in exhaled breath of COVID-19-infected individuals are ketones, aldehydes, alcohols, and nitric oxide. Studies in Edinburgh and Dortmund indicated concentrations of acetone and methanol as biomarkers that could discriminate between patients infected with SARS-CoV-2 and non-infected [17]. Acetone is generated from the decarboxylation of acetoacetate in hepatocytes, which derives from lipolysis or lipid peroxidation, and it has been associated with liver damage because of hypoxia. The liver anomalies caused by COVID-19 are also likely to cause hypoxia and therefore, acetone in breath could be possibly related to the infection.
Regarding acetaldehyde, a recent study reported a simultaneous increase in acetaldehyde and acetone determined in exhaled breath of COVID-19-positive patients through GC–MS [18]. Also, in another study on exhaled breath of children with COVID-19, they reported that the breath abundance of acetaldehyde, propanal and n-propyl acetate increased during acute infection and decreased as infection resolved [19].
On the other hand, Nitric oxide (NO) indicates oxidative stress in our body, the imbalance of NO leads to more than a dozen pathophysiological conditions like hypertension, stroke, cardiac failure, CNS disorder, diabetes mellitus, and many others. The origin of NO is found in the airway epithelium, and its increased level can determine several lung diseases [20]. Analysis of peripheral blood indicated that viral infection often caused a significant increase in pro-inflammatory cytokines and chemokines and developed into a strong cytokine storm. When high inflammation persists for a long time, it causes damage to multiple tissues and organs. In addition, high inflammation causes a severe imbalance of NO/ROS in the body, which in turn leads to oxidative stress [21].
The identity of the biomarker compounds is consistent with COVID-19 derangement of breath-biochemistry by ketosis, gastrointestinal effects, and inflammatory processes. In this regard, breath analysis is attractive because it offers: (i) POC location; (ii) rapid results (<10 min) without dependency on reagents; (iii) non-invasive sampling with a low biosecurity burden; (iv) and (v) usability in a worldwide range of scenarios, including low-resourced environments such as community or primary care settings.
Development and validation of technologies that address disease-specific biomarkers are of paramount importance since this approach may allow rapid diagnosis of COVID-19 in the coming endemic flu seasons. Therefore, the objective of the present study was a preliminary evaluation of VOCs reported in the breath of patients with COVID-19 (i.e., acetone (ACE), acetaldehyde (ACH), and nitric oxide (NO)) by gas-phase infrared spectroscopy via synthetic exhaled breath as a proof-of-concept principle study for future detection and monitoring of COVID-19 cases and long-COVID as a perspective.
2. Materials and methods
2.1. Calibration curves and validation
The choice of representative biomarkers in exhaled breath of people infected by the SARS-CoV-2 virus was made based on the existing literature up to the time of the beginning of the study, acetone (ACE), acetaldehyde (ACH) and nitric oxide (NO). For each of the selected biomarkers, validation was performed by establishing calibration curves, first separately for each compound and then for the three compounds in the mixture. Exhaled human breath was simulated by the addition of 4% CO2 (4–6% of CO2 is reported in exhaled breath) [22]. Additionally, for ACH a high humidity background was also added (58% r.h. @ 36 °C) to simulate the real exhaled breath fingerprint and to show that these measurements are also possible with humid gases.
The method validation was based on the Guide for the Validation of Analytical Methods for the Determination of Organic Compounds in trace levels AOAC/FAO/IAEA/IUPAC [23], evaluating the following parameters: limit of detection (LOD) and limit of quantification (LOQ), linearity (r), sensitivity (m), and precision (repeatability and reproducibility). For linearity, the five-point calibration curve for ACE and ACH was plotted at concentrations of 15 to 100 ppm and for NO at three points from 250 to 750 ppm. For sensitivity, the slope of the curve was evaluated. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard curve according to the blank method. The precision was measured by the coefficient of variation. The repeatability was measured by three calibration curves that were obtained on the same day. Reproducibility was measured by the coefficient of variation of seven calibration curves performed on three different days.
2.2. Measurement procedure
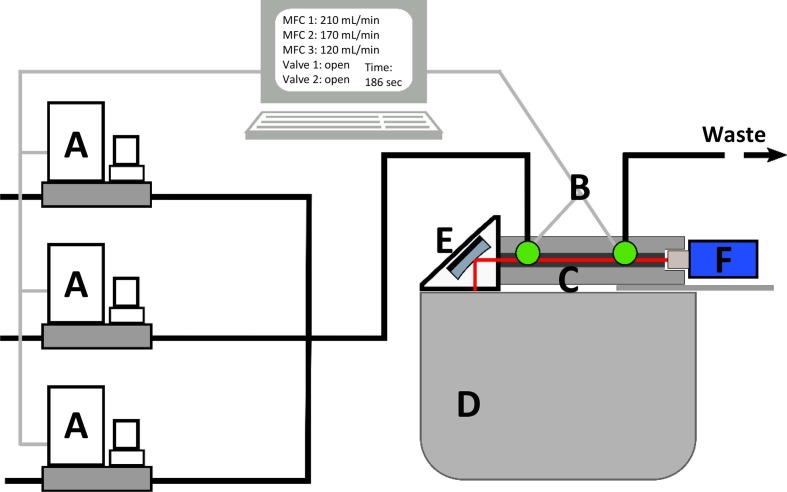
The experimental setup is schematically shown in Fig. 1 . For the gas supply, a system of Bronkhorst and Vögtlin mass flow controllers (MFCs) (A) with different flow capacities for each of the gases was used. The MFCs were operated automatically with the use of a self-written LabVIEW program to accurately generate the calculated flows and mixtures. The total gas flow was kept at 500 mL/min.
Fig. 1.
Measurement setup with MFCs (A), valves (B), iHWG (C), infrared spectrometer (D), OAPM (E), and infrared detector (F).
All measurements were performed in a stopped-flow mode with a software-controlled valve (B) on each side of the custom 14 cm long substrate-integrated hollow waveguide (iHWG) (C) containing a 12.5 cm light guiding channel with a quadratic cross-section of 4 mm, which was closed on both sides with infrared transparent zinc selenide windows.
As a light source, a Bruker Alpha II (Bruker Optics GmbH, Ettlingen, Germany) FTIR spectrometer (D) was used. The infrared light was directed by a 1′' off-axis parabolic mirror (OAPM) (E) and focused onto the gas channel of the iHWG. After interaction with the molecules in the light pathway, the infrared signal was sensed by the infrared detector (MIP-10–1 M−F−M4, VIGO Systems S.A., Poznanska, Poland) (F).
Before each measurement, the tubes and the iHWG, which were used as a gas cell, were purged for 3 min using synthetic air (20.5% O2, 79.5% N2, MTI IndustrieGase AG, Neu-Ulm, Germany) at a flow rate of 500 mL/min. After closing both valves, an IR background spectrum was recorded. Immediately after finishing the measurement, the valves opened again and a defined gas mixture from the gas mixing system was purged through the iHWG for 3 min. Then the valves closed again, and repeated sample measurements started. After recording five IR spectra of the sample gas, the gas flow was instantly turned only to synthetic air to get to the purging step again.
2.3. Data acquisition
The IR data acquisition was based on previous studies [16]. For IR data acquisition and processing, OPUS 8.5 (Bruker Optik GmbH, Ettlingen, Germany) software package was used. Each IR spectrum was recorded in the spectral range of 4000 to 700 cm−1 with a spectral resolution of 2 cm−1 by using a Blackman–Harris 3-term apodization function averaging 64 scans.
For IR spectra evaluation of acetone, acetaldehyde, and nitric oxide, an integration method was developed with selected spectral regions.
3. Results
3.1. Validation of the analytical method
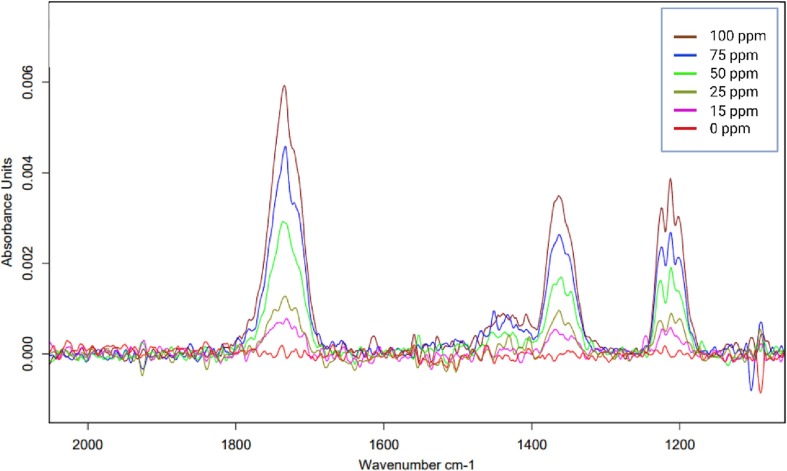
The validation parameters for each one of the VOCs are presented in Table 1 . For ACE from the IR spectrum obtained, a single characteristic peak was selected for integration. The wavelength range chosen for this integration was 1397–1327 cm−1. The calibration curves were performed at concentrations of 100, 75, 50, 50, 25, and 15 ppm (Fig. 2 ).
Table 1.
Validation parameters for each of the investigated biomarkers.
| Parameter | Values |
|||
|---|---|---|---|---|
| NO | ACE | ACH | ||
| LOD (ppm) | 6.42 | 13.81 | 9.22 | |
| LOQ (ppm) | 42.26 | 52.57 | 69.23 | |
| Linearity (r) | 0.998 | 0.999 | 0.999 | |
| Sensitivity (CI 95%) | 0.0001 ± 0.01 | 0.001 ± 0.001 | 0.002 ± 0.001 | |
| Precision | Repeatability (%RSD) | 3.9–10.4 | 2.1–17-6 | 3.4–13.4 |
| Reproducibility (%RSD) | 8.6–10.6 | 3.1–11.8 | 3.1–12.4 | |
CI 95 %: Confidence Interval at 95 %; %RSD: Relative Standard Deviation.
Fig. 2.
A representative IR spectrum for establishing the calibration function for acetone.
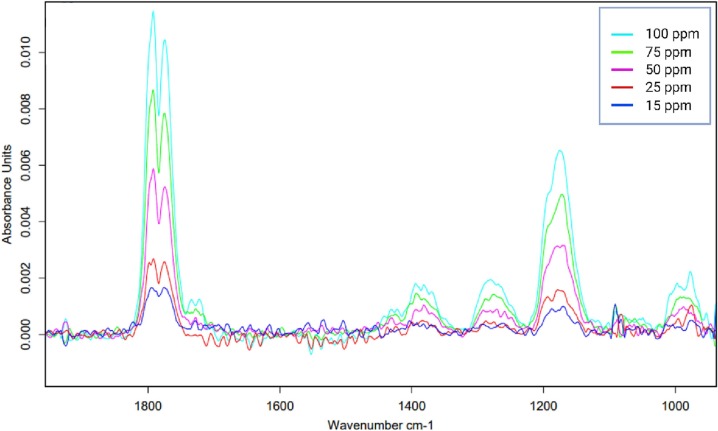
For ACH, the validation parameters are presented in Table 1. From the IR spectrum obtained, a single characteristic peak was selected for integration; the wavelengths used for integration were 1239–1137 cm−1. The calibration curves were established in the concentrations range of 100, 75, 50, 50, 25, and 15 ppm (Fig. 3 ).
Fig. 3.
A representative IR spectrum for establishing the calibration function for acetaldehyde.
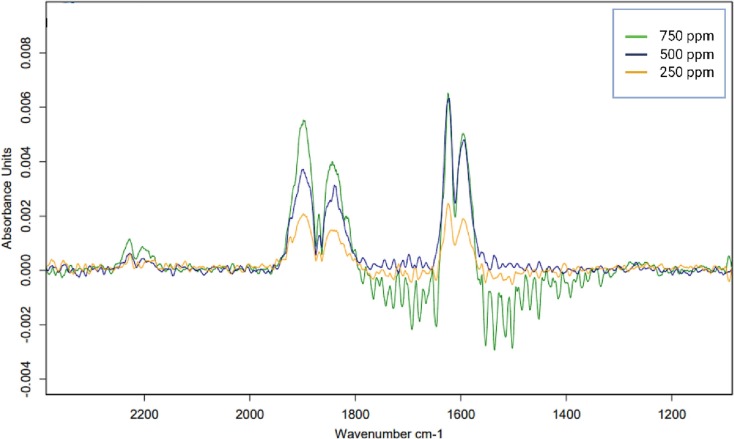
For NO, from the IR spectrum obtained, a characteristic double peak was selected for integration; the wavelength regime for this integration was 1638.5–1577 cm−1. The calibration curves were established via the analysis of concentrations in the range 750, 500, and 250 ppm (Table 1) (Fig. 4 ).
Fig. 4.
A representative IR spectrum for establishing the calibration function for nitric oxide.
3.2. VOC mixtures
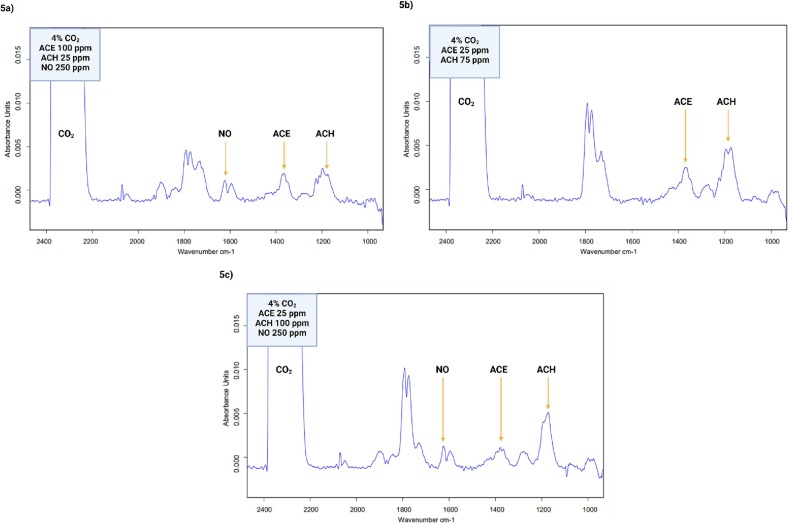
For a better understanding and representation of the exhaled breath biological matrix, the CO2 concentration equivalent to the concentration found in naturally exhaled breath (4%) was added. Thirty-one measurements of the mixture containing different concentrations of each of the gases were performed in duplicate (Fig. 5 a), to accurately quantify the biomarkers in the mixture and to establish the least variability between measurements. Representative spectra of two different concentrations of the biomarkers are shown in Fig. 5b and c. It is important to note that the quantification of the complex gas mixture was successfully performed, even with the presence of CO2. This demonstrates the capacity of the applied technology for the determination and quantification of gases in a corresponding mixture.
Fig. 5.
a. A representative IR spectrum of the VOC mixture fingerprint including CO2. CO2 at 4%, acetone at 100 ppm, acetaldehyde at 25 ppm and nitric oxide at 250 ppm. b. A representative IR spectrum of the VOC mixture fingerprint with CO2. CO2 at 4%, acetone at 25 ppm and acetaldehyde at 75 ppm. c. A representative IR spectrum of the VOC mixture fingerprint with CO2. CO2 at 4%, acetone at 25 ppm, acetaldehyde at 100 ppm and NO 250 ppm.
3.3. COVID-19-infected exhaled breath simulation
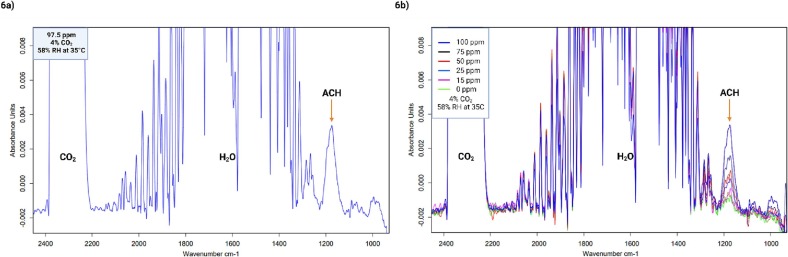
For the representation of the synthetic exhaled breath of people with lung damage caused by SARS-CoV-2 infection, a calibration curve with acetaldehyde, 4% CO2, and 58% relative humidity at a temperature of 35 °C was performed. Only the quantification of acetaldehyde was possible due to the contribution of H2O, which dominates in the mixture and overlaps with the other biomarkers. Nevertheless, as a perspective from these results the contribution from the humidity of the sample will be reduced. Fig. 6 a shows a representative IR spectrum of the analysis, where the peaks obtained for CO2, H2O, and the peak quantified for acetaldehyde are shown. The calibration curve was established using the following concentrations of acetaldehyde: 100, 75, 50, 25, and 15 ppm (Fig. 6b).
Fig. 6.
a. A representative IR spectrum of synthetic exhaled breath with 95.5 ppm ACH, 4% CO2 and 58% relative humidity at 35 °C. b. IR spectra used for the acetaldehyde calibration curve with 4% CO2 and 58% relative humidity at 35 °C.
4. Discussion
In the present study, a proof-of-concept principle, for the evaluation of specific VOC fingerprints indicative of SARS-CoV-2 virus infection, in synthetic exhaled breath by application of gas-phase infrared spectroscopy is presented. The public health system's capacity relies on detecting, testing, contact tracing, and isolating those who are or might be sick or have been exposed to known or suspected COVID-19 cases. The principal challenge lies in the identification of the cases, currently, RTq-PCR remains the gold standard in the detection of those infected, in the same way, the application of antigen and antibody tests for mass screening has been established. These technologies have certain disadvantages such as low specificity, high percentages of false positives and negatives, and invasiveness in the sample collection, which decreases its acceptance, but also, they are not accessible to all people due to the high economic cost [24], [25]. With the continuous occurrence of new SARS-CoV-2 virus variants, and the fact that they behave differently in the host and among the same populations, it is necessary to develop and apply sensitive techniques that can establish and determine specific chemical fingerprints based on the metabolism of the virus interaction with the host and discriminate between other infections, thus providing an efficient, accurate and rapid result for case counting, also considering the long-COVID scenario. It is of outmost importance to develop techniques which could discriminate between long-COVID, COVID-19, other lung diseases and infections, and could serve as a continuous monitoring of these patients. It is hypothesized that the breath fingerprint will change in the course of the infection and therefore in the course of long-COVID development and subsequent rehabilitation. The study of volatile organic compounds in exhaled breath is of great relevance in the present context. This has been performed by different technologies; however, these represent high costs, complex and time-consuming sample preparation processes, sensitivity and specificity issued as well as the need for a highly qualified analysts for their performance and the subsequent analysis of the results. In addition, they are difficult to transport to POC settings and remote locations, with the need for a laboratory and certain conditions for the analysis and the analyst [26], [27].
There is strong evidence that the analysis of trace components in exhaled breath could provide a complementary approach to non-invasive monitoring of inflammation, oxidative stress, and other processes in the airways and lungs [26]. Optical analytical techniques with inherently high sensitivity and specificity are ideally suited to fill this gap. A significant advantage of gas-phase infrared spectroscopy is the ability to perform online measurements next to exquisite molecular selectivity [28], [29]. In this context exhaled breath is analyzed during exhalation in real-time, whereas offline techniques rely on collecting breath samples in a bag or a sorbent trap for subsequent analysis. The potential issues of offline methods, like reproducibility of breath sample collection, contamination during sample storage, and the inability to allow for instantaneous feedback can be avoided using online methods. Additionally, information on the concentration during different exhalation phases is directly accessible via fast online studies, whereas offline methods integrate across a complete exhalation cycle or require an extra effort to separate exhaled gas coming from the lungs from gas that originates from the upper airways (i.e., dead space air) [30], [31], [32].
There are several clinical applications where real-time breath analysis would have an immediate application [32], [33] such as monitoring the progress of the infection and the long-term effects in long-COVID scenarios.
Spectroscopy in the mid-infrared spectral region, therefore, appears most advantageous, since most of the gaseous compounds of biomedical interest are molecular gases that have characteristic and pronounced absorption bands in this spectral region. Addressing these fingerprint spectra, therefore, allows sensitive, specific and rapid monitoring of gas mixtures [34], [35], [36], [37]. This technology has been applied in COVID-19 screening scenario, Liang et al., 2023, presented an approach of breath analysis by ultra-sensitive broadband laser spectroscopy, which in turn works in the same range -MIR-; with a total of 170 individual breath samples they reported great discrimination with an area under the receiver-operating-characteristics curve of 0.849(4), nevertheless in their approach they still transport and process samples offline [38]. This demonstrates the feasibility of application of gas-phase IR spectroscopy in disease screening with breath sampling.
The VOCs selected in the present study have been reported as relevant biomarkers in the breath of people with pulmonary conditions such as COPD, lung cancer, and COVID-19. In this context, exhaled ACH reflects aspects of oxidative stress and metabolism, the relationship of oxygenated compounds such as ketones and aldehydes with oxidative stress and the regulation of cell proliferation is well established. In the case of acute COVID-19, an inflammatory process occur due to the hyperactivity of the immune system which involves a state of cellular hyperinflammation [39], but also this state may remain in the long-COVID stage until the patient's rehabilitation [40]. ACH is derived, along with hydrocarbons, from lipid peroxidation and inflammatory processes and has been reported widely in a range of respiratory conditions [11], [17], [41]. In this context, oxidative stress in COVID-19 patients may be caused when the organism interacts with the virus, generating local inflammation and thus releasing the compound in the exhaled breath.
On the other hand, ACE is considered an oxygen-containing compound produced from glucose metabolism, and it has been described as a biomarker of lung health in different pathologies such as lung cancer [42], cystic fibrosis [43], asthma [44], and – in the present context – has been described as an indicator VOC in the breath of patients with COVID-19 [17], [27], [41]. On the other hand, it has been shown that in the acute phase of COVID-19, a hypermetabolic phase occur and it demands energy consumption [45]. In a study by Ruskiewicz et al., they report that the identity of the marker compounds identified in this study are consistent with a combination of extrapulmonary metabolic, and gastrointestinal manifestations of COVID-19 within the body as well as airway inflammatory responses [17].
Nitric oxide (NO) plays a major role in pulmonary and cardiovascular physiology. Is a reactive oxygen species (ROS) continually produced by epithelial cells of the paranasal sinuses and nasopharynx via NO synthase (NOS) enzymes [46], [47], [48]. NO diffuses to the bronchi and lungs, where it induces vasodilatory and bronchodilatory effects. This biomarker is involved in several biological roles such as activation of ciliary movements, mucus secretion, antimicrobial effects, and virus inactivation [49], [50], [51]. Concerning COVID-19, a distinctive feature of endothelial dysfunction and thrombotic episodes may be associated with the suppression of endothelial nitric oxide synthase (eNOS) with a concomitant deficiency of NO. In healthy vessels, nitric oxide is released to the endothelium as a vasodilator and antithrombotic factor; on the other hand, in injured vessels, NO is impaired, contributing to hypertension and thrombus formation [52].
Table 2 presents a comparative study of biomarkers in the different pulmonary conditions. At the time of the study, no information was found about the exact concentrations of biomarkers in patients infected with the SARS-CoV-2 virus, however, these concentrations are usually in the ppb to ppm range, so among the limitations considered in the present study are the high concentrations used in this proof of concept; it remains as a research perspective to test and improve the technology at real-life scenarios and also to improve detection limits (sensitivity). Nevertheless, the proposed technology provides a complete fingerprint of the VOCs present in exhaled breath, which expands upon the information provided by addressing only three biomarkers. Hence, another perspective is the analysis of actual exhaled breath in patients infected with the SARS-CoV-2 virus. In this context, to discriminate COVID-19 from other respiratory diseases including long-COVID is not sufficient for clinical test application because other respiratory diseases have similar symptoms and biochemical backgrounds, which could confound differential diagnosis. Therefore, the value of a new test is not only to distinguish COVID-19 patients from healthy people but also to identify patients with other lung diseases. Even though spectroscopic techniques offer sensitivities in the ppb to ppt concentration range, there are still several improvements required to render MIR spectroscopy a useful clinical tool in routine breath gas monitoring. Nevertheless, several studies have shown that multiple rather than individual VOCs give rise to pattern changes across the entire IR spectra, which may be used to be more reliably associated with a particular pathological condition. Also, if this technique is coupled with other orthogonal techniques such as VOCs sensors, it could improve and enhance the obtained information to complement breath fingerprints and generate more sensitive and specific POC devices.
Table 2.
Comparison of exhaled breath biomarkers, concentrations, and assessment techniques.
| Biomarker | Disease | Concentration | Analytical methodology | Reference |
|---|---|---|---|---|
| Nitric Oxide | Asthma | >15 ppb | Chemoluminescence analyzer | [53] |
| Control | 4–9 ppb | |||
| Control | 13 ppb | GC–MS | [54] | |
| COPD | 9.8 ± 0.7 ppb | Chemoluminescence analyzer | [55] | |
| Smokers | 10.3 ± 1.0 ppb | |||
| Control | 5.5 ± 0.4 ppb | |||
| Women | 1.6–21.5 ppb | Chemoluminescence analyzer | [56] | |
| Men | 2.6–28.8 ppb | |||
| Asbestosis | 20 ppb | Chemoluminescence analyzer | [57] | |
| Pulmonary arterial hypertension | 13.9 ± 6.8 ppb | Chemoluminescence analyzer | [58] | |
| Pulmonary Hypertension for COPD | 15.0 ± 9.3 ppb | |||
| Pulmonary Hypertension for Heart disease | 6.7 ± 2.0 ppb | |||
| COPD | 157.3 ppb | Electrochemical voltammetric sensors | [59] | |
| COPD | 10 ppb | Electrochemical sensor-based device | [60] | |
| Acetone | Lung Cancer | 500 ppb | CO2 laser photoacoustic spectrometer | [42] |
| Lung Disease | 350 ppb | |||
| Control | 300 ppb | |||
| Lung Cancer | 250->2500 ppb | Portable gas chromatograph-In2O3 thick film-type gas sensor | [61] | |
| Pulmonary arterial hypertension | 16.54 ± 14.7 nMol/L | Microreactor-FTIR ion cyclotron resonance mass | [62] | |
| Lung Cancer | 6.97 ± 7.02 nMol/L | |||
| ARDS | >300 ppb | IMR-MS | [63] | |
| Lung Cancer | 4.41–96.3 ppb | GC–MS | [64] | |
| Acetaldehyde | Lung Cancer | 0–55 nMol/L | GC–MS | [65] |
| Lung Cancer | 25 ppb | SIFT-MS | [66] | |
| Controls | < 3 ppb | SIFT-MS | [67] | |
| Ventilated patients | 13 ± 5 ppb | MCC–IMS | [68] |
All techniques presented in Table 2 are highly sensitive, however, involve very high instrumental costs, require highly trained personnel, and are laboratory-based tools. An advantage of gas-phase infrared spectroscopy is that the analysis can be performed online and on-site, which provides the user with a technology that can be adapted for POC settings enabling ease of application, accessibility, and rapid and specific analysis that allows for effective monitoring and case tracking.
This virus is not the first and will not be the last to cause a pandemic. In the past, mankind has witnessed many virus-derived pandemics, and the potential for new pandemics in the future due to the emergence of new viruses with mutational and lethal infection capacity cannot be ignored. The lessons learned from this situation include that it is necessary to invest in widespread monitoring for predicting pandemic risks to take early action in avoidance of global pandemic scenarios. The emerging area of IR-spectroscopic exhaled breath sensing is likely to continue to yield promising results as a complementary tool for testing for SARS-CoV-2 infections with the benefits of potential applications for large-scale field screening studies. In this context and with the obtained results, the capability of gas-phase infrared spectroscopy for the detection of global VOC fingerprints in exhaled breath has been demonstrated, even though significantly more work is needed to push the technology into practice.
5. Conclusion
In the ongoing COVID-19 pandemic and its sequelae, the development of new platforms for screening large populations is of vital importance. Encouraging data is presented using IR-spectroscopic gas sensing concepts for future faster and cheaper screening of infected individuals. Gas-phase infrared spectroscopy could potentially improve SARS-CoV-2 screening in POC settings, where the use of exhalomics could assist in large-scale diagnosis and further differentiation between lung diseases with the establishment of disease-specific molecular IR fingerprinting providing rapid information on the health status of persons (i.e., infected vs. not infected) and facilitating monitoring and follow-up of cases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was supported by the Ministerium für Wissenschaft, Forschung und Kunst (MWK) in Baden-Württemberg, Germany within the program “Sonderförderlinie COVID-19”.
Author contributions
JG: Conceptualization, Device design, Writing and Editing; BM: Conceptualization, Funding, Writing and Editing; LDLM: Conceptualization, Experimental, Writing and Editing.
Data availability
Data will be made available on request.
References
- 1.Girum T., Lentiro K., Geremew M., Migora B., Shewamare S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop. Med. Health. 2020;48:1–15. doi: 10.1186/s41182-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker P.G.T., Whittaker C., Watson O.J., Baguelin M., Winskill P., Hamlet A., Djafaara B.A., Cucunubá Z., Olivera Mesa D., Green W. The impact of COVID-19 and strategies for mitigation and suppression in low-and middle-income countries. Science. 2020;369:413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaimayo C., Kaewnaphan B., Tanlieng N., Athipanyasilp N., Sirijatuphat R., Chayakulkeeree M., Angkasekwinai N., Sutthent R., Puangpunngam N., Tharmviboonsri T., Pongranweewan O., Chuthapisith S., Sirivatanauksorn Y., Kantakamalakul W., Horthongkham N. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020;17:177. doi: 10.1186/s12985-020-01452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine R., Das S., Hasan A., Abdul Salam S., Augustine P., Dalvi Y.B., Varghese R., Primavera R., Yassine H.M., Thakor A.S. Rapid antibody-based COVID-19 mass surveillance: relevance, challenges, and prospects in a pandemic and post-pandemic world. J Clin. Med. 2020;9:3372. doi: 10.3390/jcm9103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravi N., Cortade D.L., Ng E., Wang S.X. Diagnostics for SARS-CoV-2 detection: a comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.T.C. Miller, S.D. Morgera, S.E. Saddow, A. Takshi, M. Palm, Electronic nose with detection method for alcohol, acetone, and carbon monoxide in coronavirus disease 2019 breath simulation model, IEEE Sens. J. 21 (2021) 15935–15943. https://doi.org/10.1109/JSEN.2021.3076102. [DOI] [PMC free article] [PubMed]
- 7.Dragonieri S., Schot R., Mertens B.J.A., Le Cessie S., Gauw S.A., Spanevello A., Resta O., Willard N.P., Vink T.J., Rabe K.F. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007;120:856–862. doi: 10.1016/j.jaci.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 8.Tirzīte M., Bukovskis M., Strazda G., Jurka N., Taivans I. Detection of lung cancer in exhaled breath with an electronic nose using support vector machine analysis. J. Breath Res. 2017;11:36009. doi: 10.1088/1752-7163/aa7799. [DOI] [PubMed] [Google Scholar]
- 9.de Vries R., Vigeveno R.M., Mulder S., Farzan N., Vintges D.R., Goeman J.J., Bruisten S., van den Corput B., Geelhoed J.J.M., Visser L.G. Ruling out SARS-CoV-2 infection using exhaled breath analysis by electronic nose in a public health setting. MedRxiv. 2021 doi: 10.1101/2021.02.14.21251712. [DOI] [Google Scholar]
- 10.Grassin-Delyle S., Roquencourt C., Moine P., Saffroy G., Carn S., Heming N., Fleuriet J., Salvator H., Naline E., Couderc L.-J. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould O., Ratcliffe N., Król E., de Lacy Costello B. Breath analysis for detection of viral infection, the current position of the field. J. Breath Res. 2020;14:41001. doi: 10.1088/1752-7163/ab9c32. [DOI] [PubMed] [Google Scholar]
- 12.FDA, InspectIR COVID-19 Breathalyzer (for use on PNY-1000), 2022. https://doi.org/10.1088/1752-7163/ab9c32.
- 13.Selvaraj R., Vasa N.J., Nagendra S.M.S., Mizaikoff B. Advances in mid-infrared spectroscopy-based sensing techniques for exhaled breath diagnostics. Molecules. 2020;25:2227. doi: 10.3390/molecules25092227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitole L., Steffens F., Krüger T.P.J., Meyer D. Mid-ATR-FTIR spectroscopic profiling of HIV/AIDS sera for novel systems diagnostics in global health. OMICS J. Integr. Biol. 2014;18:513–523. doi: 10.1089/omi.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.T. Jin, J. Zhou, P.T. Lin, Mid-infrared waveguides for volatile organic compounds detection, in: Opt. Photonics Sens. Environ., Optical Society of America, 2019: p. EW2A. 2. https://opg.optica.org/abstract.cfm?URI=ES-2019-EW2A.2.
- 16.Glöckler J., Jaeschke C., Kocaöz Y., Kokoric V., Tütüncü E., Mitrovics J., Mizaikoff B. iHWG-MOX: a hybrid breath analysis system via the combination of substrate-integrated hollow waveguide infrared spectroscopy with metal oxide gas sensors. ACS Sensors. 2020;5:1033–1039. doi: 10.1021/acssensors.9b02554. [DOI] [PubMed] [Google Scholar]
- 17.Ruszkiewicz D.M., Sanders D., O’Brien R., Hempel F., Reed M.J., Riepe A.C., Bailie K., Brodrick E., Darnley K., Ellerkmann R. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry-a feasibility study. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Török Z.-M., Blaser A.F., Kavianynejad K., de Torrella C.G.M.G., Nsubuga L., Mishra Y.K., Rubahn H.-G., de Oliveira Hansen R. Breath biomarkers as disease indicators: sensing techniques approach for detecting breath gas and COVID-19. Chemosensors. 2022;10:167. doi: 10.3390/chemosensors10050167. [DOI] [Google Scholar]
- 19.Berna A.Z., Akaho E.H., Harris R.M., Congdon M., Korn E., Neher S., M’Farrej M., Burns J., Odom John A.R. Reproducible breath metabolite changes in children with SARS-CoV-2 Infection, ACS. Infect. Dis. 2021;7:2596–2603. doi: 10.1021/acsinfecdis.1c00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurya M.R., Onthath H., Morsy H., Riyaz N.-U.-S., Ibrahim M., Ahmed A.E., Abuznad R., Alruwaili A., Alsaedi F., Kasak P., Sadasivuni K.K. Colorimetry-based detection of nitric oxide from exhaled breath for quantification of oxidative stress in human body. Healthcare. 2021;9:1055. doi: 10.3390/healthcare9081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R.A., Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021;163:153–162. doi: 10.1016/j.freeradbiomed.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.M.A. Anghel, F. Iacobescu, The Influence of Temperature and CO2 in Exhaled Breath, in: 16th Int. Congr. Metrol., EDP Sciences, 2013: p. 10012. https://doi.org/10.1051/metrology/201310012.
- 23.Fajgelj A., Ambrus A. In: Principles and Practices of Method Validation. 2000. Guidelines for single-laboratory validation of analytical methods for trace-level concentrations of organic chemicals; pp. 179–252. [DOI] [Google Scholar]
- 24.Dramé M., Teguo M.T., Proye E., Hequet F., Hentzien M., Kanagaratnam L., Godaert L. Should RT-PCR be considered a gold standard in the diagnosis of COVID-19? J Med Virol. 2020 Nov;92(11):2312–2313. doi: 10.1002/jmv.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S. Physicians’ dilemma of false-positive RT-PCR for COVID-19: a case report. SN Compr. Clin. Med. 2021;3:255–258. doi: 10.1007/s42399-020-00655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banik G.D., Mizaikoff B. Exhaled breath analysis using cavity-enhanced optical techniques: a review. J. Breath Res. 2020;14:43001. doi: 10.1088/1752-7163/abaf07. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Aguilar M., Díaz de León-Martínez L., Zamora-Mendoza B.N., Comas-García A., Guerra Palomares S.E., García-Sepúlveda C.A., Alcántara-Quintana L.E., Díaz-Barriga F., Flores-Ramírez R. Comparative analysis of chemical breath-prints through olfactory technology for the discrimination between SARS-CoV-2 infected patients and controls. Clin. Chim. Acta. 2021;519:126–132. doi: 10.1016/j.cca.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson B., Khodabakhsh A., Metsälä M., Ventrillard I., Schmidt F.M., Romanini D., Ritchie G.A.D., te Lintel Hekkert S., Briot R., Risby T., Marzcin N., Harren F.J.M., Critescu S.M. Laser spectroscopy for breath analysis: towards clinical implementation. Appl. Phys. B. 2018;124:161. doi: 10.1007/s00340-018-7030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruderer T., Gaisl T., Gaugg M.T., Nowak N., Streckenbach B., Müller S., Moeller A., Kohler M., Zenobi R. On-line analysis of exhaled breath: focus review. Chem. Rev. 2019;119:10803–10828. doi: 10.1021/acs.chemrev.9b00005. [DOI] [PubMed] [Google Scholar]
- 30.Herbig J., Müller M., Schallhart S., Titzmann T., Graus M., Hansel A. On-line breath analysis with PTR-TOF. J. Breath Res. 2009;3 doi: 10.1088/1752-7155/3/2/027004. [DOI] [PubMed] [Google Scholar]
- 31.Wang C., Sahay P. Breath analysis using laser spectroscopic techniques: breath biomarkers. Spectral Fingerprints, and Detection Limits, Sensors. 2009;9:8230–8262. doi: 10.3390/s91008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boshier P.R., Cushnir J.R., Mistry V., Knaggs A., Španěl P., Smith D., Hanna G.B. On-line, real time monitoring of exhaled trace gases by SIFT-MS in the perioperative setting: a feasibility study. Analyst. 2011;136:3233–3237. doi: 10.1039/C1AN15356K. [DOI] [PubMed] [Google Scholar]
- 33.Metsälä M. Optical techniques for breath analysis: From single to multi-species detection. J. Breath Res. 2018;12:27104. doi: 10.1088/1752-7163/aa8a31. [DOI] [PubMed] [Google Scholar]
- 34.Kim S.-S., Young C., Vidakovic B., Gabram-Mendola S.G.A., Bayer C.W., Mizaikoff B. Potential and challenges for mid-infrared sensors in breath diagnostics. IEEE Sens. J. 2009;10:145–158. doi: 10.1088/1752-7163/aa8a31. [DOI] [Google Scholar]
- 35.Straume T., Loftus D.J., Li J., Coleman M.A., Davis C.E., McMonigal K.A., Piccini M., Singh A.K. Biomarker-detection technologies for comprehensive medical diagnosis during deep-space missions. Recent Patents Sp. Technol. 2013;3:13–23. https://www.ingentaconnect.com/content/ben/rptst/2013/00000003/00000001/art00004 [Google Scholar]
- 36.L. Bodiou, J. Lemaitre, A. Gutierrez, W. El Ayed, Y. Dumeige, I. Hardy, J. Charrier, F. Starecki, E. Baudet, R. Chahal, V. Nazabal, J.L. Doualan, A. Braud, P. Camy and P. Nemec, Rare-earth doped selenides active waveguides for integrated mid-infrared sensing applications, in: 19th Eur. Conf. Integr. Opt. (ECIO 2017), 2017. https://www.ecio-conference.org/wp-content/uploads/2017/03/ECIO_2017_T7.5.pdf.
- 37.Tütüncü E., Mizaikoff B. Cascade laser sensing concepts for advanced breath diagnostics. Anal. Bioanal. Chem. 2019;411:1679–1686. doi: 10.1007/s00216-018-1509-5. [DOI] [PubMed] [Google Scholar]
- 38.Liang Q., Chan Y.-C., Toscano J., Bjorkman K.K., Leinwand L.A., Parker R., Nozik E.S., Nesbitt D.J., Ye J. Breath analysis by ultra-sensitive broadband laser spectroscopy detects SARS-CoV-2 infection. J. Breath Res. 2023;17:36001. doi: 10.1088/1752-7163/acc6e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afrin L.B., Weinstock L.B., Molderings G.J. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020;100:327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H., Qi X., Ma J., Zhang C., Feng H., Yao M. Breath-borne VOC biomarkers for COVID-19. J. Breath Res. 2021;15 doi: 10.1088/1752-7163/ac2e57. [DOI] [PubMed] [Google Scholar]
- 42.Mitrayana D.K., Apriyanto M. Satriawan, CO2 laser photoacoustic spectrometer for measuring acetone in the breath of lung cancer patients. Biosensors. 2020;10:55. doi: 10.3390/bios10060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barker M., Hengst M., Schmid J., Buers H.-J., Mittermaier B., Klemp D., Koppmann R. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur. Respir. J. 2006;27:929–936. doi: 10.1183/09031936.06.00085105. [DOI] [PubMed] [Google Scholar]
- 44.Smolinska A., Klaassen E.M.M., Dallinga J.W., Van De Kant K.D.G., Jobsis Q., Moonen E.J.C., Van Schayck O.C.P., Dompeling E., Van Schooten F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS One. 2014;9:e95668. doi: 10.1371/journal.pone.0095668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakenman P.L.M., Van der Hoven B., Schuijs J.M., Eveleens R.D., van Bommel J., Olieman J.F., Joosten K.F.M. Energy expenditure and feeding practices and tolerance during the acute and late phase of critically ill COVID-19 patients. Clin. Nutr. ESPEN. 2021;43:383–389. doi: 10.1016/j.clnesp.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.P. Sogni, P. Garnier, A. Gadano, R. Moreau, J. Dall’Ava-Santucci, A.T. Dinh-Xuan, D. Lebrec, Endogenous pulmonary nitric oxide production measured from exhaled air is increased in patients with severe cirrhosis, J. Hepatol. 23 (1995) 471–473. https://doi.org/10.1016/0168-8278(95)80207-X. [DOI] [PubMed]
- 47.M. Özkan, R.A. Dweik, Nitric oxide and airway reactivity, Clin. Pulm. Med. 8 (2001) 199–206. https://journals.lww.com/clinpulm/Fulltext/2001/07000/Nitric_Oxide_and_Airway_Reactivity.1.aspx.
- 48.Högman M., Thornadtsson A., Liv P., Hua-Huy T., Dinh-Xuan A.-T., Tufvesson E., Dressel H., Janson C., Koskela K., Oksa P. Effects of growth and aging on the reference values of pulmonary nitric oxide dynamics in healthy subjects. J. Breath Res. 2017;11:47103. doi: 10.1088/1752-7163/aa7957. [DOI] [PubMed] [Google Scholar]
- 49.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Åkerström S., Gunalan V., Keng C.T., Tan Y.-J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung K., Gurnani A., Renukaradhya G.J., Saif L.J. Nitric oxide is elicited and inhibits viral replication in pigs infected with porcine respiratory coronavirus but not porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2010;136:335–339. doi: 10.1016/j.vetimm.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green K., Graziadio S., Turner P., Fanshawe T., Allen J. Cent; Evidence-Based Med: 2020. Molecular and antibody point-of-care tests to support the screening, diagnosis and monitoring of COVID-19. https://www.cebm.net/wp-content/uploads/2020/04/POCT-Covid19.pdf. [Google Scholar]
- 53.Hansel T.T., Kharitonov S.A., Donnelly L.E., Erin E.M., Currie M.G., Moore W.M., Manning P.T., Recker D.P., Barnes P.J. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 54.Leone A.M., Gustafsson L.E., Francis P.L., Persson M.G., Wiklund N.P., Moncada S. Nitric oxide is present in exhaled breath in humans: direct GC-MS confirmation. Biochem. Biophys. Res. Commun. 1994;201:883–887. doi: 10.1006/bbrc.1994.1784. [DOI] [PubMed] [Google Scholar]
- 55.Liu J., Sandrini A., Thurston M.C., Yates D.H., Thomas P.S. Nitric oxide and exhaled breath nitrite/nitrates in chronic obstructive pulmonary disease patients. Respiration. 2007;74:617–623. doi: 10.1159/000106379. [DOI] [PubMed] [Google Scholar]
- 56.Olivieri M., Talamini G., Corradi M., Perbellini L., Mutti A., Tantucci C., Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir. Res. 2006;7:94. doi: 10.1186/1465-9921-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehtonen H., Oksa P., Lehtimäki L., Sepponen A., Nieminen R., Kankaanranta H., Saarelainen S., Järvenpää R., Uitti J., Moilanen E. Increased alveolar nitric oxide concentration and high levels of leukotriene B4 and 8-isoprostane in exhaled breath condensate in patients with asbestosis. Thorax. 2007;62:602–607. doi: 10.1136/thx.2006.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carpagnano G.E., Radaeli A., Lacedonia D., Correale M., Carpagnano G., Palmiotti A., Barbaro M.P.F., Di Biase M., Brunetti N., Scioscia G., Malerba M. Exhaled nitric oxide and exhaled breath temperature as potential biomarkers in patients with pulmonary hypertension. Biomed. Res. Int. 2018;2018:7292075. doi: 10.1155/2018/7292045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y., Wang X.-M., Chen Y.-H., Zhu R., Liao C.-C. Exhaled hydrogen sulfide in patients with chronic obstructive pulmonary disease and its correlation with exhaled nitric oxide. Chin. Med. J. (Engl) 2013;126:3240–3244. doi: 10.3760/cma.j.issn.0366-6999.20123064. [DOI] [PubMed] [Google Scholar]
- 60.Shrestha S.K., Shrestha S., Sharma L., Pant S., Neopane A. Comparison of fractional exhaled nitric oxide levels in chronic obstructive pulmonary disease, bronchial asthma and healthy subjects of Nepal. J. Breath Res. 2017;11 doi: 10.1088/1752-7163/aa7e63. [DOI] [PubMed] [Google Scholar]
- 61.Tanda N., Hoshikawa Y., Sato T., Takahashi N., Koseki T. Exhaled acetone and isoprene in perioperative lung cancer patients under intensive oral care: possible indicators of inflammatory responses and metabolic changes. Biomed. Res. 2019;40:29–36. doi: 10.2220/biomedres.40.29. [DOI] [PubMed] [Google Scholar]
- 62.Jalil B.A., Xie Z., Li Q., Fu X.-A., El-Kersh K. B55. USE YOUR IllusIon I ClIn. Res. PAH, American Thoracid Society; 2019. Volatile Organic Compounds in Exhaled Breath of Patients with Pulmonary Arterial Hypertension: A Comparative Analysis, Ain; pp. A3644–A. [DOI] [Google Scholar]
- 63.Meidert A.S., Choukèr A., Praun S., Schelling G., Dolch M.E. Exhaled breath and oxygenator sweep gas propionaldehyde in acute respiratory distress syndrome. Molecules. 2020;26:145. doi: 10.3390/molecules26010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koureas M., Kirgou P., Amoutzias G., Hadjichristodoulou C., Gourgoulianis K., Tsakalof A. Target analysis of volatile organic compounds in exhaled breath for lung cancer discrimination from other pulmonary diseases and healthy persons. Metabolites. 2020;10:317. doi: 10.3390/metabo10080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuchs P., Loeseken C., Schubert J.K., Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer. 2010;126:2663–2670. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- 66.Španěl P., Smith D. Quantification of volatile metabolites in exhaled breath by selected ion flow tube mass spectrometry, SIFT-MS. Clin. Mass Spectrom. 2020;16:18–24. doi: 10.1016/j.clinms.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J., Kumar S., Hanna G.B. Investigation of C3–C10 aldehydes in the exhaled breath of healthy subjects using selected ion flow tube-mass spectrometry (SIFT-MS) J. Breath Res. 2014;8:37104. doi: 10.1088/1752-7155/8/3/037104. [DOI] [PubMed] [Google Scholar]
- 68.Müller-Wirtz L.M., Kiefer D., Ruffing S., Brausch T., Hüppe T., Sessler D.I., Volk T., Fink T., Kreuer S., Maurer F. Quantification of volatile aldehydes deriving from in vitro lipid peroxidation in the breath of ventilated patients. Molecules. 2021;26:3089. doi: 10.3390/molecules26113089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.