Abstract
Background
Bloodstream infections (BSIs) produced by antibiotic-resistant bacteria (ARB) cause a substantial disease burden worldwide. However, most estimates come from high-income settings and thus are not globally representative. This study quantifies the excess mortality, length of hospital stay (LOS), intensive care unit (ICU) admission, and economic costs associated with ARB BSIs, compared to antibiotic-sensitive bacteria (ASB), among adult inpatients in low- and middle-income countries (LMICs).
Methods and findings
We conducted a systematic review by searching 4 medical databases (PubMed, SCIELO, Scopus, and WHO’s Global Index Medicus; initial search n = 13,012 from their inception to August 1, 2022). We only included quantitative studies. Our final sample consisted of n = 109 articles, excluding studies from high-income countries, without our outcomes of interest, or without a clear source of bloodstream infection. Crude mortality, ICU admission, and LOS were meta-analysed using the inverse variance heterogeneity model for the general and subgroup analyses including bacterial Gram type, family, and resistance type. For economic costs, direct medical costs per bed-day were sourced from WHO-CHOICE. Mortality costs were estimated based on productivity loss from years of potential life lost due to premature mortality. All costs were in 2020 USD. We assessed studies’ quality and risk of publication bias using the MASTER framework. Multivariable meta-regressions were employed for the mortality and ICU admission outcomes only. Most included studies showed a significant increase in crude mortality (odds ratio (OR) 1.58, 95% CI [1.35 to 1.80], p < 0.001), total LOS (standardised mean difference “SMD” 0.49, 95% CI [0.20 to 0.78], p < 0.001), and ICU admission (OR 1.96, 95% CI [1.56 to 2.47], p < 0.001) for ARB versus ASB BSIs. Studies analysing Enterobacteriaceae, Acinetobacter baumanii, and Staphylococcus aureus in upper-middle-income countries from the African and Western Pacific regions showed the highest excess mortality, LOS, and ICU admission for ARB versus ASB BSIs per patient. Multivariable meta-regressions indicated that patients with resistant Acinetobacter baumanii BSIs had higher mortality odds when comparing ARB versus ASB BSI patients (OR 1.67, 95% CI [1.18 to 2.36], p 0.004). Excess direct medical costs were estimated at $12,442 (95% CI [$6,693 to $18,191]) for ARB versus ASB BSI per patient, with an average cost of $41,103 (95% CI [$30,931 to $51,274]) due to premature mortality. Limitations included the poor quality of some of the reviewed studies regarding the high risk of selective sampling or failure to adequately account for relevant confounders.
Conclusions
We provide an overview of the impact ARB BSIs in limited resource settings derived from the existing literature. Drug resistance was associated with a substantial disease and economic burden in LMICs. Although, our results show wide heterogeneity between WHO regions, income groups, and pathogen–drug combinations. Overall, there is a paucity of BSI data from LMICs, which hinders implementation of country-specific policies and tracking of health progress.
Kasim Allel and colleagues systematically review published literature from low- and middle-income countries and meta-analyse data extracted from 109 articles to explore the impact of bloodstream infections caused by antibiotic-resistant bacteria.
Author summary
Why was this study done?
Bloodstream infections (BSIs) caused by antibiotic-resistant bacteria (ARB) have multifaceted impacts, including higher admission to intensive care units (ICUs), prolonged hospitalisations, and high economic and societal costs worldwide.
Despite the global burden, most evidence on the excess burden of ARB BSIs has been derived from high-income countries; comparatively, there are limited data from low- and middle-income countries (LMICs).
What did the researchers do and find?
We employed a systematic literature review and subsequent meta-analysis of 109 individual studies to quantify the impact of ARB BSIs in hospitalised patients from LMICs.
Based mostly on crude data comparisons ignoring the possible influence of confounding factors, we found that ARB BSIs, compared to BSIs caused by antibiotic-sensitive bacteria (ASB), were associated with substantially longer stays in hospitals and ICUs, higher mortality, and increased direct medical and productivity costs.
What do these findings mean?
Our findings highlight the excess morbidity, mortality, and costs associated with ARB BSIs and the sparsity of data from LMICs.
Targeted strategies to improve the prevention, detection, and treatment of resistant BSIs in LMICs are required to reduce the economic and disease burden.
Introduction
Antibiotic-resistant bacteria (ARB) constitute a global health priority, particularly where resistance proportion is highest in low- and middle-income countries (LMICs) [1]. Resource-limited hospital infrastructure, poor health system capacity, and inadequate sanitation and hygiene infrastructure partly explain the spread and impact of ARB in LMICs [1,2]. Ameliorating health inequities is hampered by the feedback caused by ARB infections resulting in increased morbidity and mortality, more complicated treatments due to the use of reserved antibiotics, and prolonged hospitalisations, all of which exacerbate costs to countries’ health systems and society [1,3]. Recent figures from the World Health Organization (WHO) Global Antimicrobial Resistance and Surveillance System (GLASS) report show that the proportion of Escherichia coli bloodstream infections (BSIs) caused by third-generation cephalosporins resistant E. coli was more than triple in LMICs compared to high-income countries, (58.3% and 17.53%, respectively) [4]. A similar trend was observed for other WHO critical- and high-priority BSI pathogens, including Klebsiella pneumoniae and Staphylococcus aureus [4,5].
BSIs are one of the most lethal infections, having an estimated overall crude mortality of 15% to 30% [4,6]. BSIs are intrinsically more deadly as pathogens can spread quickly via blood, producing multiple infections and leading to organ damage and dysfunction. Extensive literature has examined the excess burden of ARB BSIs in specific locations [7–13]. For example, compared to their sensitive counterparts, carbapenem-resistant Klebsiella spp. [12] and methicillin-resistant Staphylococcus aureus (MRSA) [11] BSIs are associated with 9.08 (95% CI [1.17 to 70.51]) and 2.23 (95% CI [1.14 to 4.37]) times greater mortality, respectively. Higher admission to the intensive care units (ICUs), (OR 8.57; 95% CI [3.99 to 18.38]), greater length of hospital stay (LOS), (4.89 additional days; 95% CI [0.56 to 11.52]) and sizeable hospital costs ($23,318, 95% CI [$858 to $57,090]) have been linked to vancomycin-resistant versus -sensitive Enterococci BSIs [13]. Studies conducted in high-income countries contribute disproportionately to these estimates [14–16]; data from LMICs are scant. This comprises a critical gap in our understanding of the impact of drug-resistant BSI in countries with higher underlying health risks (e.g., cancer, neutropenia and haematological malignancies, pneumonia, and diabetes) [17].
Here, we present a systematic review and meta-analysis of the literature on the impact (i.e., LOS, mortality, and ICU admission) and excess economic costs per patient associated with ARB BSI compared with antibiotic-sensitive (ASB) BSI among hospitalised patients in LMICs.
Methods
This study is reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (S1 Checklist) [18] and was prospectively registered with PROSPERO (id number: CRD42021264056).
Search strategy
We searched the literature for studies examining the burden of ARB BSIs compared with ASB BSIs among inpatients from LMICs. PubMed, SCIELO, Scopus, and WHO’s Global Index Medicus (Latin American and Caribbean Health Sciences Literature “LILACs” and African Index Medicus “AIM”) were searched without restrictions to language or year of publication using a family of keywords related to antibiotic/drug-resistance, bloodstream infections/bacteraemia, and burden measures among inpatients. We searched articles published through August 1, 2022. The complete list of terms, abbreviations, and Boolean connectors used by search engine can be found in the Supporting information (S1 Text, section 1).
Study selection
We selected articles according to a step-guided protocol. First, articles were excluded if carried out in high-income countries; these were defined according to the 2021 World Bank classification list (i.e., gross national income “GNI” per capita > $12,696) [19]. Second, studies were only included if BSIs were presented based on laboratory-confirmed positive blood cultures. Either primary or secondary BSIs were included. Articles that analysed patients with different culture types (e.g., blood, urine, wound, nasal) were removed unless BSI episodes were clearly detailed. Third, articles were included if the ASB and ARB groups were identified among adult patients presenting BSIs in the hospital. Fourth, participants with chronic or severe diseases (e.g., HIV, cancer) were removed unless they were present in the ARB and ASB groups (e.g., studies were withdrawn if HIV–positive patients having ARB BSIs were compared with HIV–negative patients having ASB BSIs). Finally, studies were removed if they did not present our selected outcomes (i.e., mortality, ICU admission, LOS, or costs). Experimental and observational articles were included. We removed correspondence letters or opinions, short reports without data analysis, literature reviews, and single-case studies.
Studies were analysed only when the number of patients was reported. We only included the adult population (average ≥18 years of age) because (i) the number of studies focusing on children was limited (n = 4) after looking at the provisional results; and (ii) children’s inherent behaviour and exposure level differ from adults [3]. Only data on WHO-priority pathogens were retained [20]. The Results section (PRISMA chart) and Table A in S1 Text present the complete list of search criteria used.
To avoid our study hinging only on published articles’ results, we systematically reviewed the grey literature and other current literature reviews analysing similar topics. Four referees resolved any disagreement presented at any stage of study selection through scholarly discussion. Two native Spanish speakers fluent in Portuguese and English, a native English speaker, and a native Chinese speaker fluent in English conducted the screening and consecutive data extraction. Papers written in any other language were translated to English using Google Translate PDF (<1% of the included articles). We used the Rayyan free online tool (https://rayyan.ai/) to screen, select, and decide which articles were included. Double article screening for eligibility was employed, and discrepancies were resolved via scholarly dialogue.
Data extraction
We extracted data including authors, publication year, country, study setting, population characteristics, bacterium type, resistance type, and sample sizes (for cases and control groups). We classified pathogen resistance based on the specific pathogen-resistance profiles evaluated in each study (e.g., cephalosporin-resistant Acinetobacter baumanii). For completeness, we also collated data on ESBL+ and non-ESBL (ESBL-) groups for gram-negative pathogens. For the analysis, the case group comprised infections with resistant strains (ARB), whereas the control group comprised sensitive-strain infections (ASB). Selected studies were organised using unique identifiers (e.g., 1, 2, 3), and sub-studies within the primary articles were classified using consecutive numbers separated by a dot (e.g., 1.1, 1.2, 1.3) if they presented bacterium- or resistance type-specific information (S1 Data).
We extracted the following outcomes by case/control group: mortality (crude 30-day mortality, whenever available, or overall crude mortality if timing was not reported), LOS (average total days and standard deviation), and ICU admission (patients admitted). We also collected data on demographics and underlying conditions: average age, previous surgery and hospitalisation, community- or hospital-acquired BSI, any underlying condition (diabetes, hypertension, cardiovascular or heart diseases, solid tumour or malignancy, liver or kidney disease, pulmonary/respiratory diseases, and any hematologic disease), and BSI source (urinary tract, intravenous or catheter, pulmonary, and intrabdominal or gastrointestinal). Pitt bacteraemia score, APACHE II, and CHARLSON scores were collected if presented. We compared ARB and ASB groups by comparing variables’ proportion or mean using McNemar’s χ2 or T-tests for binary and continuous data, respectively. Additionally, we classified the studies by World Bank income level, WHO region, WHO Global Priority Pathogens List, bacterium family and antibiotic class, pathogen strain, and bacterium Gram type. We used Microsoft Excel 2022 to compile and extract included articles’ data. We used double data extraction reviewing, and inconsistencies (14% disagreement) were resolved through scholarly discussion.
Study quality and risk assessment
We used a unified framework to evaluate the methodological quality of analytic study designs (MASTER scale) [21]. This framework comprises 36 questions classified into 7 domains concerning equal recruitment, retention, implementation, prognosis, ascertainment, sufficient analysis, and temporal precedence. Each question was scored independently by 2 reviewers as 1 if the study complied with the domain or 0 if it did not. Therefore, a higher score indicates higher study quality. Two independent reviewers performed a risk of bias assessment. Conflicts were addressed through scholarly discussion.
Statistical analysis
Firstly, we employed population-weighted descriptive statistics of the health and demographic characteristics collated by studies’ patients having ARB and ASB BSIs to contrast both groups and check whether mean differences across patient features existed. Secondly, the overall estimates for excess mortality, ICU admission, and LOS associated with resistant strains compared to their sensitive counterparts were meta-analysed using the inverse variance heterogeneity model [22]. The heterogeneity was calculated using the I2 statistics; I2 values were classified as high (>75%), moderate (50% to 75%), and low (<50%) heterogeneity. All results were computed using odds ratios (ORs) for mortality and ICU admission rates, and the standardised mean difference (SMD) for LOS. We estimated ORs based on studies’ crude numbers or unadjusted ORs provided. Forest plots and meta-analyses were computed by outcome and subgroups of variables, including bacterial family, Gram type, reported resistance type, most common antibiotic-resistant microbial strains, World Bank income group, and WHO region. P-values (p) were reported using a two-tailed t test (p < 0.05) for the ORs for mortality and ICU admissions and LOS’s standardised mean difference. We also analysed and compared, whenever reported, the unadjusted and confounder-adjusted ORs, for studies reporting univariate and multivariable regression analyses.
As a secondary analysis, we used univariate and multivariable meta-regressions to explore the main determinants of mortality and ICU admission (LOS was not included because of a small sample size). We included the bacterial family and resistance profile, demographics, and underlying health condition variables in the univariate regression. Variables were transformed to odds between ARB and ASB groups. We evaluated the associations with the original and fully imputed observations. Multiple imputations were performed using fully completed data as factors and with 1,000 repetitions following a multivariable normal regression design. Variables associated with our outcomes in the univariate analysis with p < 0.05 using non-imputed data were included in the fully imputed multivariable model.
Excess economic costs per patient (i.e., costs associated with ARB BSI minus costs associated with ASB BSI) were computed only for excess length of stay, separated by ICU and non-ICU wards. Hospital-day costs included all the inpatient hospitality costs per patient stay for primary and secondary level and teaching hospitals and were calculated based on WHO-CHOICE costs [23]. ICU costs were calculated per patient stay for tertiary/teaching hospitals and were retrieved from the literature for countries with available information [24–36], or by using an approximation ratio between hospital and ICU costs [37–39]. Direct medical costs comprised hospital-day and ICU admission costs per patient, adjusted to their respective patients’ LOS in the hospitalised or ICU services. We also calculated excess productivity losses per patient associated with premature mortality from ARB BSIs (compared to ASB BSIs) using the life expectancy at death and human capital approaches [40]. Excess productivity losses associated with premature mortality costs were computed by multiplying the years of life lost, based on the reference standard life expectancy at the average age of death [41] from ARB BSI (i.e., costs associated with ARB BSI minus costs associated with ASB BSI), using the study-weighted average age for all patients over all studies, without age-weights and a 5% time discount [42]. All costs were expressed in 2020 USDs, adjusting for inflation using US GDP implicit price deflators. Due to a lack of data, we excluded direct and indirect nonmedical costs (e.g., travel). Cost computations and methods are detailed in S1 Text, section 4.
Small-study effects
The Doi [43] plots and the LFK index were used to evaluate small-study effects when there were at least 5 studies in the meta-analysis. Leave-one-out cross-validation [44] was used to estimate the generalisation performance of our main meta-analyses to cross-validate the results’ sensitivity.
Sensitivity analyses
We evaluated whether our main meta-analysis results varied by location. Due to the large proportion of studies from China (N = 41), we assessed our meta-analyses by separating our sampled studies into those performed in China and other LMICs.
All statistical analyses included studies and sub-studies according to their specific population features and were performed in Stata 17, College Station, TX: StataCorp LLC.
Results
Yield of the search strategy
Our search strategy identified 13,012 articles: 4,720 through PubMed, 8,193 in Scopus, 55 in SCIELO, and 44 in AIM and LILACs (Fig 1). Of these, 1,076 were duplicated (8.3%; 1,076/13,012), and 10,948 were performed in high-income countries (84.1%; 10,948/13,012) and hence removed. In total, 988 articles were full-text screened, resulting in the inclusion of 109 studies (N = 22,756 patients).
Fig 1. Flowchart detailing systematic review according to PRISMA guidelines.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [18]. HICs: High-income countries. PRISMA checklist is provided in S1 Text. ARB, antibiotic-resistant bacteria; ASB, antibiotic-sensitive bacteria; BSI, bloodstream infections; WHO, World Health Organization.
Characteristics of included studies
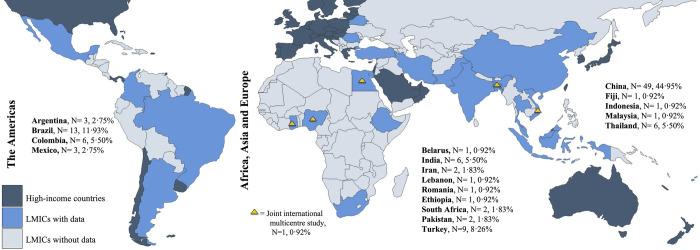
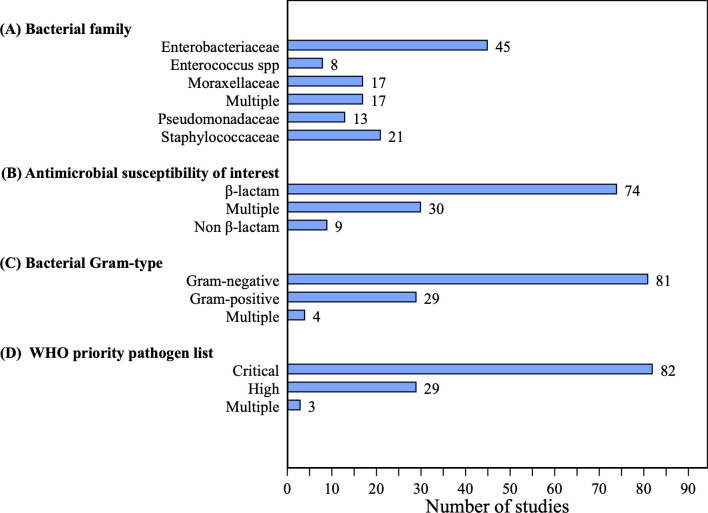
Of the 109 articles, 100 (91.7%; 100/109) studies reported the impacts of ARB BSIs on mortality, 42 on hospital LOS, but only 18 displayed the average LOS with its standard deviation (16.5%; 18/109) and 52 (47.7%; 52/109) reported on ICU admission (Table 1). Studies were primarily conducted in China (44.9%; 49/109, N = 12,092 patients), Brazil (11.9%; 13/109, N = 1,559 patients), and Turkey (8.3%; 9/109, N = 2,190 patients) (Fig 2). Most studies collected data from the Western Pacific region according to the WHO classification (46.8%; 51/109) and 88% (96/109) were from upper-middle-income countries (S1 Text, section 2). The majority of the studies reported on gram-negative bacteria, mainly Enterobacteriaceae (41.3%; 45/109), Moraxellaceae or Acinetobacter baumanii (15.6%; 17/109), and Pseudomonas aeruginosa (11.9%, 13/109) (Fig 3). The main gram-positive pathogens reported were Staphylococcus aureus (19.3%; 21/109) and Enterococcus spp. (7.3%; 8/109); 75.2% (82/109) of the pathogens reported were classified as a critical priority following the WHO criteria (Fig 3). β-lactam antibiotics were among the most tested antibiotic class within the studies (67.9%; 74/109), 71.6% (53/74) of which were carbapenems or cephalosporins (Fig 3). The total number of patients and most prevalent features per country’s studies are reported in Table E in S1 Text. Table F in S1 Text presents the weighted unadjusted differences for sociodemographic and health variables among ARB and ASB groups. We found no statistically significant difference between ARB and ASB groups for most of these variables (χ2 test p > 0.05). S1 Text section 2 describes the distribution of our studies by WHO region, World Bank income group, year, and outcomes densities per ARB/ASB group.
Table 1. Details of all studies included in the systematic literature review (N = 109).
| ID⁂ | Author/year | Country setting | Bacterium family | Group comparison | Group N of obs. | Mortality, n (%) | LOS (mean) | ICU admission, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | ||||
| 1 | Abhilash, 2010 [46] | India | Enterobacteriaceae | ESBL+ | ESBL- | 96 | 35 | 24(25) | 9(26) | ||||
| 2 | Abolghasemi, 2018 [47] | Iran | Moraxellaceae | XDR | non-XDR | 16 | 14 | 13(81) | 1(7) | 8(50) | 0(0) | ||
| 3 | Akhtar, 2016 [48] | Pakistan | Enterococcus spp. | VRE | VSE | 46 | 65 | 29(63) | 28(43) | 28.5 | 13.2 | 23(50) | 9(14) |
| 4 | Anggraini, 2022 [49] | Indonesia | Moraxellaceae | CRAB | CSAB | 72 | 72 | 41(57) | 35(49) | 17 | 13 | 60(83) | 49(68) |
| 5 | Anunnatsiri, 2011 [50] | Thailand | Moraxellaceae | MDR | non-MDR | 24 | 25 | 22(92) | 12(48) | 21.5 | 14 | 9(38) | 3(12) |
| 6 | Arias-Ortiz, 2016 [51] | Colombia | Staphylococcaceae | MRSA | MSSA | 186 | 186 | 105(56) | 89(48) | ||||
| 7 | Atmaca, 2014 [52] | Turkey | Staphylococcaceae | MRSA | MSSA | 99 | 99 | 70.84 | 14 | 25(25) | 6(6) | ||
| 8 | Barrero, 2014 [53] | Colombia | Staphylococcaceae | MRSA | MSSA | 102 | 102 | 62(61) | 46(45) | 30 | 21 | 64(63) | 54(53) |
| 9.1 | Braga, 2013 [54] | Brazil | Staphylococcacea | MRSA | MSSA | 12 | 44 | 7(58) | 25(57) | ||||
| 9.2 | Braga, 2013 [54] | Brazil | Pseudomonadaceae | CRPA | CSPA | 14 | 42 | 13(93) | 19(45) | ||||
| 9.3 | Braga, 2013 [54] | Brazil | Enterobacteriaceae | CREN | CSEN | 3 | 53 | 2(67) | 30(57) | ||||
| 9.4 | Braga, 2013 [54] | Brazil | Enterobacteriaceae | CERKP | CESKP | 5 | 51 | 4(80) | 28(55) | ||||
| 10 | Castillo 2012 [55] | Colombia | Staphylococcaceae | MRSA | MSSA | 186 | 186 | 62(33) | 48(26) | 105(56) | 90(48) | ||
| 11 | Carena, 2020 [56] | Argentina | Multiple | MDR | non-MDR | 168 | 226 | 58(35) | 36(16) | 54(32) | 43(19) | ||
| 12 | Cetin, 2021 [57] | Turkey | Multiple gram-negative | CRGN | CSGN | 54 | 157 | 29(54) | 31(20) | 45 | 20 | ||
| 13 | Chang, 2020 [58] | China | Enterobacteriaceae | CRKP | CSKP | 46 | 239 | 27(59) | 37(15) | 26(57) | 33(14) | ||
| 14 | Chen, 2022 [59] | China | Enterobacteriaceae | CRKP | CSKP | 29 | 223 | 14(48) | 13(6) | 21(72) | 38(17) | ||
| 15 | Chen, 2012 [60] | China | Staphylococcaceae | MRSA | MSSA | 75 | 43 | 25(33) | 8(19) | 55 | 38.7 | ||
| 16 | Chusri 2019 [61] | Thailand | Moraxellaceae | CRAB | CSAB | 31 | 11 | 20(65) | 2(18) | 89 | 57 | 20(65) | 6(55) |
| 17 | Conterno 1998 [62] | Brazil | Staphylococcaceae | MRSA | MSSA | 90 | 46 | 44(49) | 9(20) | 54(60) | 13(28) | ||
| 18 | Dantas 2017 [63] | Brazil | Pseudomonadaceae | MDR | non-MDR | 67 | 90 | 39(58) | 35(39) | ||||
| 19 | Deodhar 2015 [64] | India | Staphylococcaceae | MRSA | MSSA | 40 | 61 | 8(20) | 13(21) | ||||
| 20 | De-Oliveira 2002 [65] | Brazil | Staphylococcaceae | MRSA | MSSA | 159 | 92 | 73(46) | 19(21) | ||||
| 21 | Deris, 2011 [66] | Malaysia | Moraxellaceae | IRAB | ISAB | 15 | 41 | 6(40) | 9(22) | 32.3 | 32.8 | 11(73) | 20(49) |
| 22 | Dramowski, 2022 [67] | South Africa | Enterobacteriaceae | CEREN | CESEN | 62 | 115 | 27(44) | 33(29) | 10.5 | 9 | ||
| 23 | Durdu, 2016 [68] | Turkey | Enterobacteriaceae | CRKP | CRSKP | 46 | 63 | 23(50) | 23(37) | ||||
| 24 | Ergönül, 2016 [69] | Turkey | Multiple | CRGN | CSGN | 379 | 452 | 236(62) | 135(30) | ||||
| 25 | Ferreira, 2018 [70] | Brazil | Multiple | MDR | non-MDR | 25 | 37 | 10(40) | 3(8) | ||||
| 26 | Fu, 2015 [71] | China | Moraxellaceae | XDR | non-XDR | 39 | 86 | 31(79) | 38(44) | 36.7 | 36.1 | 31(79) | 45(52) |
| 27 | Furtado, 2006 [72] | Brazil | Enterococcus spp. | VRE | VSE | 34 | 55 | 57.7 | 29 | 13(38) | 18(33) | ||
| 28 | Garnica, 2009 [73] | Brazil | Multiple | MDR | non-MDR | 10 | 44 | 4(40) | 4(9) | ||||
| 29 | Gaytán, 2006 [74] | Mexico | Enterobacteriaceae | CiREC | CiSEC | 26 | 24 | 4(15) | 3(13) | ||||
| 30 | Ghafur, 2014 [75] | India | Multiple | MDR | non-MDR | 44 | 97 | 28(64) | 37(38) | ||||
| 31.1 | Goda, 2022 [76] | India | Multiple | MDR | non-MDR | 8 | 22 | 1(13) | 8(36) | ||||
| 31.2 | Goda, 2022 [76] | India | Multiple | XDR | non-XDR | 20 | 10 | 8(40) | 1(10) | ||||
| 32 | González, 2014 [77] | Colombia | Pseudomonadaceae | MDR | non-MDR | 92 | 141 | ||||||
| 33 | Guo, 2016 [78] | China | Moraxellaceae | MDR | non-MDR | 64 | 23 | 38(59) | 1(4) | 51(80) | 5(22) | ||
| 34 | Hincapié, 2020 [45] | Colombia | Staphylococcaceae | MRSA | MSSA | 292 | 909 | 219(75) | 71(8) | 239(82) | 84(9) | ||
| 35.1 | Islas-Muñoz, 2018 [79] | Mexico | Enterobacteriaceae | ESBL+ | ESBL- | 123 | 148 | 37(30) | 35(24) | ||||
| 35.2 | Islas-Muñoz, 2018 [79] | Mexico | Multiple gram-negative | MDR | non-MDR | 9 | 34 | 6(67) | 5(15) | ||||
| 35.3 | Islas-Muñoz, 2018 [79] | Mexico | Multiple gram-positive | MDR | non-MDR | 6 | 43 | 2(33) | 4(9) | ||||
| 36 | Jafari, 2020 [80] | Iran | Enterococcus spp. | VRE | VSE | 52 | 21 | 30(57) | 6(29) | 36.6 | 22.32 | 30(58) | 5(24) |
| 37 | Jamulitrat, 2009 [81] | Thailand | Moraxellaceae | IRAB | ISAB | 67 | 131 | 35(52) | 26(20) | 37 | 27 | ||
| 38 | Kalam, 2014 [82] | Pakistan | Multiple | MDR | non-MDR | 117 | 126 | 54(46) | 34(27) | 32(27) | 36(29) | ||
| 39 | Li, 2019 [83] | China | Enterobacteriaceae | CRKP | CSKP | 19 | 21 | 8(42) | 2(10) | 21 | 18 | 11(58) | 5(24) |
| 40 | Li, 2017 [84] | China | Enterobacteriaceae | MDR | non-MDR | 76 | 28 | 23(30) | 3(11) | ||||
| 41 | Li, 2018 [85] | China | Pseudomonadaceae | CRPA | CSPA | 63 | 63 | 17(27) | 8(13) | 30 | 21 | ||
| 42 | Li, 2017 [86] | China | Enterobacteriaceae | CREN | CSEN | 26 | 122 | 17(65) | 21(17) | 25.4 | 21 | 20(77) | 10(8) |
| 43 | Li, 2020 [87] | China | Enterobacteriaceae | CRKP | CSKP | 164 | 328 | 72(44) | 49(15) | 31 | 19 | 116(71) | 58(18) |
| 44 | Liang, 2021 | China | Enterobacteriaceae | CRKP | CSKP | 56 | 47 | 22(39) | 9(19) | 28.5 | 28 | 20(36) | 13(28) |
| 45.1 | Lim, 2016 [88] | Thailand | Staphylococcaceae | MDR | non-MDR | 2017 | 299* | ||||||
| 45.2 | Lim, 2016 [88] | Thailand | Enterobacteriaceae | MDR | non-MDR | 144 | 20* | ||||||
| 45.3 | Lim, 2016 [88] | Thailand | Enterobacteriaceae | MDR | non-MDR | 288 | 7* | ||||||
| 45.4 | Lim, 2016 [88] | Thailand | Pseudomonadaceae | MDR | non-MDR | 94 | 4* | ||||||
| 45.5 | Lim, 2016 [88] | Thailand | Moraxellaceae | MDR | non-MDR | 864 | 351* | ||||||
| 46 | Lima, 2020 [89] | Brazil | Multiple | CR | CS | 60 | 30 | 30(50) | 12(40) | 26.5 | 15 | ||
| 47 | Lipari, 2020 [90] | Argentina | Enterobacteriaceae | CREN | CSEN | 42 | 42 | 22(52) | 7(17) | 32(76) | 12(29) | ||
| 48 | Liu, 2019 [91] | China | Enterobacteriaceae | CRKP | CSKP | 20 | 69 | 11(55) | 11(16) | ||||
| 49 | Liu, 2015 [92] | China | Moraxellaceae | MDR | non-MDR | 182 | 59 | 50(27) | 3(5) | 109(60) | 7(12) | ||
| 50 | Liu, 2019 [93] | China | Enterobacteriaceae | CRKP | CSKP | 70 | 28 | 30(43) | 12(43) | ||||
| 51 | Liu, 2020 [94] | China | Moraxellaceae | CRAB | CSAB | 229 | 88 | 60(26) | 4(5) | 129(56) | 26(30) | ||
| 52 | Loftus, 2022 [95] | Fiji | Enterobacteriaceae | CREN | CSEN | 66 | 96 | 20(30) | 16(17) | 13 | 8 | ||
| 53.1 | Lopez-Luis, 2020 [96] | Mexico | Enterococcus spp | VRE | VSE | 107 | 85 | 34(32) | 11(13) | 41(38) | 11(13) | ||
| 53.2 | Lopez-Luis, 2020 [96] | Mexico | Enterococcus spp | ARE | ASE | 18 | 129 | 5(28) | 23(18) | 4(22) | 22(17) | ||
| 54 | Ma, 2017 [97] | China | Enterobacteriaceae | ESBL+ | ESBL- | 70 | 43 | 15(21) | 6(14) | ||||
| 55 | Marra, 2006 [98] | Brazil | Enterobacteriaceae | ESBL+ | ESBL- | 56 | 52 | 18(32) | 8(15) | 31(55) | 18(35) | ||
| 56 | Meneküe 2019 [99] | Turkey | Enterobacteriaceae | CRKP | CSKP | 111 | 99 | 77(69) | 44(44) | ||||
| 57 | Metan, 2009 [100] | Turkey | Moraxellaceae | CRAB | CSAB | 54 | 46 | 41(76) | 22(48) | ||||
| 58 | Moghnieh, 2015 [101] | Lebanon | Multiple | MDR | non-MDR | 7 | 68 | 4(57) | 3(4) | ||||
| 59 | Moreira, 1998 [102] | Brazil | Staphylococcaceae | ORSA | OSSA | 71 | 71 | 40(56) | 8(11) | 32.7 | 29.7 | ||
| 60 | Najmi, 2019 [103] | India | Enterobacteriaceae | ESBL+ | ESBL- | 101 | 81 | 29(29) | 19(24) | ||||
| 61 | Niu, 2018 [104] | China | Moraxellaceae | CRAB | CSAB | 242 | 51 | 84(35) | 2(4) | ||||
| 62.1 | Palavutitotai, 2018 [105] | Thailand | Pseudomonadaceae | MDR | non-MDR | 32 | 167 | 12(38) | 38(23) | ||||
| 62.2 | Palavutitotai, 2018 [105] | Thailand | Pseudomonadaceae | XDR | non-XDR | 56 | 199 | 23(41) | 50(25) | 53.5 | 45.5 | 8(14) | 42(21) |
| 63 | Porto, 2013 [106] | Brazil | Staphylococcaceae | MRSA | MSSA | 61 | 169 | 44(71) | 36(21) | 43.2 | 20.5 | ||
| 64 | Rao 2020 [107] | India | Enterococcus spp. | VRE | VSE | 73 | 100 | 27(37) | 33(33) | 34.47 | 26.25 | 21(29) | 41(41) |
| 65 | Seboxa, 2015 [108] | Ethiopia | Enterobacteriaceae | CEREC | CESEC | 10 | 6 | 10(100) | 0(0) | ||||
| 66 | Serefhanoglu 2009 [109] | Turkey | Enterobacteriaceae | MDR | non-MDR | 30 | 64 | 7(23) | 12(19) | ||||
| 67 | Shi, 2009 [110] | China | Multiple | MDR | non-MDR | 70 | 82 | 27(39) | 12(15) | ||||
| 68.1 | Shi, 2022 [111] | China | Multiple | CRGN | CSGN | 65 | 953 | 29(45) | 79(8) | ||||
| 68.2 | Shi, 2022 [111] | China | Multiple | ESBL+ | ESBL- | 347 | 671 | 33(10) | 75(11) | ||||
| 68.3 | Shi, 2022 [111] | China | Multiple | MDR | non-MDR | 412 | 606 | 56(14) | 52(9) | ||||
| 69.1 | Sirijatuphat, 2018 [112] | Thailand | Enterobacteriaceae | CREC | CSEC | 106 | 100 | 23(22) | 18(18) | ||||
| 69.2 | Sirijatuphat, 2018 [112] | Thailand | Enterobacteriaceae | CRKP | CSKP | 45 | 65 | 23(51) | 22(34) | ||||
| 69.3 | Sirijatuphat, 2018 [112] | Thailand | Pseudomonadaceae | CRPA | CSPA | 21 | 47 | 10(48) | 19(40) | ||||
| 69.4 | Sirijatuphat, 2018 [112] | Thailand | Moraxellaceae | CRAB | CSAB | 57 | 24 | 38(67) | 3(13) | ||||
| 69.5 | Sirijatuphat, 2018 [112] | Thailand | Enterobacteriaceae | FRS | FSS | 2 | 2 | 0(0) | 1(50) | ||||
| 69.6 | Sirijatuphat, 2018 [112] | Thailand | Staphylococcaceae | MRSA | MSSA | 16 | 47 | 9(56) | 13(28) | ||||
| 69.7 | Sirijatuphat, 2018 [112] | Thailand | Enterococcus spp. | VRE | VSE | 9 | 20 | 6(67) | 12(60) | ||||
| 70 | Soares, 2022 [113] ⍴ | Brazil | Enterobacteriaceae | CRKP | CSKP | 28 | 79 | ||||||
| 71 | Steinhaus, 2018 [114] a | South Africa | Staphylococcaceae | MRSA | MSSA | 23 | 75 | ||||||
| 72 | Stewardson, 2019 [115] | Multiple LMICs ☨ | Enterobacteriaceae | CREN | CSEN | 123 | 174 | 43(35) | 35(20) | 3.7* | 54(44) | 51(29) | |
| 73.1 | Stoma, 2016 [116] | Belarus | Multiple | CR | CS | 23 | 112 | 17(74) | 25(22) | ||||
| 73.2 | Stoma, 2016 [116] | Belarus | Enterobacteriaceae | ESBL+ | ESBL- | 24 | 111 | 6(25) | 36(32) | ||||
| 73.3 | Stoma, 2016 [116] | Belarus | Staphylococcaceae | MRSA | MSSA | 15 | 120 | 4(27) | 38(32) | ||||
| 74 | Tang, 2021 [117] | China | Multiple | CRGN | CSGN | 78 | 757 | 27(35) | 79(10) | ||||
| 75 | Tian, 2016 [118] | China | Enterobacteriaceae | CRKP | CSKP | 33 | 81 | 14(42) | 16(20) | 50 | 24 | ||
| 76 | Topeli, 2000 [119] | Turkey | Staphylococcaceae | MRSA | MSSA | 46 | 55 | 27(59) | 17(31) | 50.3 | 32.7 | 20(43) | 13(24) |
| 77 | Traverso, 2010 [120] | Argentina | Staphylococcaceae | MRSA | MSSA | 17 | 22 | 12(71) | 8(36) | ||||
| 78 | Tu, 2018 [121] | China | Enterobacteriaceae | MDR | non-MDR | 55 | 145 | 9(16) | 19(13) | 16(29) | 18(12) | ||
| 79 | Tuon, 2012 [122] | Brazil | Pseudomonadaceae | CRPA | CSPA | 29 | 48 | 13(45) | 26(54) | 43 | 43.1 | 24(83) | 25(52) |
| 80 | Valderrama, 2016 [123] | Colombia | Pseudomonadaceae | CRPA | CSPA | 42 | 126 | 24(57) | 45(36) | 26 | 16 | 26(62) | 73(58) |
| 81 | Wang, 2016 [124] | China | Enterobacteriaceae | CREN | CSEN | 94 | 93 | 33(35) | 11(12) | 40 | 26 | 49(52) | 33(35) |
| 82 | Wang, 2018 [125] | China | Enterobacteriaceae | CRKP | CSKP | 48 | 48 | 23(48) | 2(4) | 84 | 33 | 25(52) | 3(6) |
| 83 | Wei, 2020 [126] | China | Pseudomonadaceae | CRPA | CSPA | 23 | 58 | 14(61) | 10(17) | ||||
| 84.1 | Wu, 2021 [127] | China | Enterobacteriaceae | CRKP | CSKP | 24 | 55 | 10(42) | 12(22) | ||||
| 84.2 | Wu, 2021 [127] | China | Enterobacteriaceae | ESBL+ | ESBL- | 24 | 55 | 9(38) | 15(27) | ||||
| 84.3 | Wu, 2021 [127] | China | Enterobacteriaceae | MDR | non-MDR | 36 | 43 | 12(33) | 12(28) | ||||
| 85 | Xiao, 2018 [128] | China | Enterobacteriaceae | CRKP | CSKP | 135 | 293 | 52(39) | 26(9) | ||||
| 86 | Xiao, 2020 [129] | China | Enterobacteriaceae | CRKP | CSKP | 104 | 267 | 58(56) | 37(14) | 35 | 23 | ||
| 87 | Xie, 2018 [130] | China | Multiple | MDR | non-MDR | 186 | 322 | 59(32) | 72(22) | 42(23) | 40(12) | ||
| 88 | Xu, 2015 [131] | China | Enterococcus spp. | VRE | VSE | 31 | 54 | 21(68) | 24(44) | ||||
| 89 | Yang, 2018 [132] | China | Moraxellaceae | CRAB | CSAB | 84 | 34 | 23(27) | 2(6) | 55(65) | 6(18) | ||
| 90 | Yang, 2021 [133] | China | Pseudomonadaceae | CRPA | CSPA | 65 | 155 | 17(26) | 29(19) | 38 | 24 | 34(52) | 46(30) |
| 91 | Ye, 2014 [134] | China | Multiple | rESKAPE | sESKAPE | 39 | 32 | 22(56) | 12(38) | ||||
| 92 | Yilmaz, 2016 [135] | Turkey | Staphylococcaceae | MRSA | MSSA | 100 | 145 | 22(22) | 7(5) | ||||
| 93 | Yuan, 2020 [136] | China | Enterobacteriaceae | CRKP | CSKP | 98 | 141 | 7(7) | 2(1) | 55 | 51 | 82(84) | 44(31) |
| 94 | Zhang, 2020 [137] | China | Enterobacteriaceae | CRKP | CSKP | 108 | 388 | 41(38) | 34(9) | 24.5 | 26 | 85(79) | 155(40) |
| 95 | Zhang, 2019 [138] | China | Enterobacteriaceae | ESBL+ | ESBL- | 160 | 164 | 39(24) | 32(20) | ||||
| 96 | Zhang, 2017 [139] | China | Enterobacteriaceae | CEREC | CESEC | 51 | 197 | 13(25) | 24(12) | 29.88 | 30.98 | 4(8) | 23(12) |
| 97 | Zhang, 2017 [140] | China | Enterococcus spp. | VRE | VSE | 7 | 217 | 2(29) | 52(24) | ||||
| 98 | Zhang, 2020 [141] | China | Pseudomonadaceae | CRPA | CSPA | 40 | 29 | 30(75) | 12(41) | ||||
| 99 | Zhao, 2022 [142] | China | Enterobacteriaceae | ESBL+ | ESBL- | 159 | 205 | 29(18) | 24(12) | ||||
| 100.1 | Zhao, 2020 [143] | China | Pseudomonadaceae | CRPA | CSPA | 55 | 238 | 11(20) | 14(6) | 29 | 26 | ||
| 100.2 | Zhao, 2020 [143] | China | Pseudomonadaceae | MDR | non-MDR | 38 | 255 | 11(29) | 14(5) | 27 | 26 | ||
| 101 | Zheng, 2018 [144] | China | Enterobacteriaceae | CRKP | CSKP | 59 | 230 | 32(54) | 45(20) | 28(47) | 47(20) | ||
| 102 | Zheng, 2017 [145] | China | Enterobacteriaceae | CRKP | CSKP | 31 | 17 | 19(61) | 8(47) | 31.74 | 21.47 | ||
| 103 | Zhou, 2019 [146] | China | Moraxellaceae | MDR | non-MDR | 274 | 64 | 161(59) | 8(13) | 29 | 22.5 | 184(67) | 12(19) |
| 104 | Zhu, 2016 [147] | China | Staphylococcaceae | MRSA | MSSA | 22 | 42 | 6(27) | 6(14) | 25.7 | 15.3 | ||
| 105 | Zhu, 2021 [148] | China | Enterobacteriaceae | CREN | CSEN | 152 | 727 | 87(57) | 133(18) | 35 | 20 | 98(64) | 135(19) |
| 106 | Zlatian, 2018 [149] | Romania | Staphylococcaceae | MRSA | MSSA | 23 | 40 | 14(61) | 19(48) | ||||
| 107 | Zou, 2020 [150] | China | Enterobacteriaceae | CREC | CSEC | 31 | 367 | 17(55) | 39(11) | 20(65) | 61(17) | ||
| 108 | Zhang, 2018 [151] | China | Enterobacteriaceae | MDR | non-MDR | 77 | 33 | 10(13) | 10(30) | ||||
| 109 | Zhang, 2017 [152] | China | Moraxellaceae | CRAB | CSAB | 49 | 29 | 40(82) | 6(21) | 10(20) | 12(41) | ||
Full information can be found in S1 Data.
*Reported as excess mortality or length of stay. Empty cells did not reported values for the outcomes.
aThis study reported unadjusted and adjusted ORs rather than raw values for outcome variables.
⁂Studies ID comprised the main articles and articles’ sub-studies if information on the outcomes by comparison group was reported separately for more than 1 bacterium or resistance-type according to their specific populations.
☨LMICs included in the study were India, Egypt, Nigeria, Colombia, Ghana, Pakistan, Lebanon, Vietnam, and Bangladesh.
⍴Odds ratios were reported only.
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; MDR, multi-drug resistance; CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-sensitive Klebsiella pneumoniae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; CSPA, carbapenem-sensitive Pseudomonas aeruginosa; CRAB, carbapenem-resistant Acinetobacter baumannii; CSAB, carbapenem-sensitive Acinetobacter baumannii; CREC, carbapenem-resistant Escherichia coli; CSEC, carbapenem-sensitive Escherichia coli; IRAB, imipenem-resistant Acinetobacter baumannii; ISAB, imipenem-sensitive Acinetobacter baumannii; ESBL, extended-spectrum β-lactamases; VRE, Vancomycin-resistant Enterococcus spp; VRE, Vancomycin-sensitive Enterococcus spp.; CERKP, Cephalosporins-resistant Klebsiella pneumoniae; CESKP, Cephalosporins-sensitive Klebsiella pneumoniae; CiREC, Ciprofloxacin-resistant Escherichia coli; CiSEC, Ciprofloxacin-sensitive Escherichia coli; CRGN, Carbapenem-resistant gram-negative bacteria; CSGN, Carbapenem sensitive gram-negative bacteria; CR, Carbapenem resistance; CS, Carbapenem sensitive; CREN, Carbapenem-resistant Enterobacteriaceae; CSEN, Carbapenem-sensitive Enterobacteriaceae; ARE, Ampicillin-resistant Enterococcus spp.; ASE, Ampicillin-sensitive Enterococcus spp.; ORSA, Oxacillin-resistant Staphylococcus aureus; OSSA, Oxacillin-sensitive Staphylococcus aureus; CEREC, Cephalosporins-resistant Escherichia coli; CESEC, Cephalosporins-sensitive Escherichia coli; FRS, Fluoroquinolone-resistant Salmonella spp.; FSS, Fluoroquinolone-sensitive Salmonella spp.; XDR, Extensive drug-resistance. rESKAPE: Vancomycin-resistant E. faecium, methicillin-resistant S. aureus (MRSA), extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae, carbapenem-resistant A. baumannii, carbapenem- and quinolone-resistant P. aeruginosa, and de-repressed chromosomal β-lactam and ESBL-producing Enterobacter species. sESKAPE: sensitive ESKAPE; ICU: intensive care unit; LOS: length of stay.
Fig 2. Distribution of the included studies according to country (N = 109 articles).
Maps indicate the country where studies came from with their respective number (N) of studies included and the percentage of studies per country of the total studies analysed. Joint studies used cross-country designs (i.e., analysed ARB BSIs in more than 1 country). White areas represent high-income countries or missing LMICs. Maps were computed in QGIS Development Team (2020), Geographic Information System, version 3.16: Open-Source Geospatial Foundation Project. http://qgis.osgeo.org. ARB, antibiotic-resistant bacteria; BSI, bloodstream infection; LMIC, low- and middle-income country; QGIS, Quantum Geographic Information System.
Fig 3. Number of included studies categorised by microbiological features †.
(A) Number of included studies by bacterial family (B) Number of included studies by antimicrobial susceptibility of interest (C) Number of included studies by bacterial Gram-type (D) Number of included studies by WHO priority pathogen list. Enterobacteriaceae included Escherichia coli and Klebsiella pneumoniae. Enterococcus spp. stands for Enterococcus species pluralis (multiple species), which included Enterococcus faecalis and faecium. The multiple categories stand for either multiple bacteria or antibiotics analysed throughout our selected studies, which were not reported disaggregated by bacterial family, biological strain, gram type, or WHO priority pathogen list. † Studies could include more than 1 subcategory per biological feature (i.e., a study might report Enterobacteriaceae and Pseudomonadaceae species separately in their analyses, or altogether, in which case it was classified as “Multiple,” meaning no clear distinction between subcategories). Categories might not be exclusive per study. WHO, World Health Organization.
Quantitative results
The odds of health outcomes
The crude OR for mortality of ARB versus ASB BSIs was 1.58 (95% CI [1.35 to 1.80], p < 0.001); we obtained similar values for gram-negative or WHO critical priority pathogens (OR 1.59, 95% CI [1.34 to 1.83], p < 0.001) (Table 2, section I). The highest OR of crude mortality for resistant pathogens was for carbapenem-resistant Enterobacteriaceae (OR 1.97, 95% CI [1.37 to 2.56], p < 0.001) (Table 3). The impact seemed to be lower among gram-positive bacteria, with an OR of 1.51 (95% CI [0.76 to 2.26], p 0.13) for MRSA and an OR of 1.31 (95% CI [1.01 to 1.60], p 0.02) for vancomycin-resistant Enterococcus species. Compared to ASB BSIs, ARB BSIs in upper-middle-income countries (OR 1.64, 95% CI [1.36 to 1.92], p < 0.001) from Europe and Western Pacific WHO regions (OR 1.79, 95% CI [1.49 to 2.11], p < 0.001, and OR 1.66, 95% CI [1.18 to 2.14], p < 0.001, respectively) had the highest excess mortality (Table G in S1 Text). Among priority pathogens defined by the WHO, crude excess mortality from carbapenem-resistant K. pneumoniae was substantially higher than for other pathogens (OR 1.79, 95% CI [1.15 to 2.43], p 0.002; Table 3), compared to sensitive counterparts. Among studies reporting both adjusted and unadjusted ORs for mortality (N = 12), we found 1.35 and 1.57 times higher unadjusted and adjusted mortality figures, respectively, for patients having BSIs caused by ARB versus ASB (Fig AJ in S1 Text). We found lower mortality estimates among studies reporting adjusted ORs for gram-negative ARB BSIs (OR = 1.88), specifically for Enterobacteriaceae and Moraxellaceae species (OR 1.91 and OR 1.73, respectively), compared to the same unadjusted estimates (OR 2.95 and OR 3.28, respectively) (Figs AK and AL in S1 Text). However, and surprisingly for the most part, adjusted ORs for mortality among ARB versus ASB BSI patients reflected greater odds compared to unadjusted ORs. This is explained by a single, highly influential study [45] among unadjusted estimates displaying a smaller OR (although confidence intervals overlap between unadjusted and adjusted ORs, and study’s weight is lower among adjusted estimates).
Table 2. Main results of the meta-analysis comparing outcomes between patients with drug-resistant and drug-sensitive infections, overall and per bacterial family and WHO priority list classification (N = 109 studies‡).
| Outcome variables | OR/SMD | 95% CI | P-value | tau2 | N of patients | N of studies |
|---|---|---|---|---|---|---|
| I. Mortality a | OR | |||||
| Overall | 1.58 | 1.35, 1.80 | <0.001 | 0.39 | 19,597 | 93 |
| WHO classification | ||||||
| Critical priority pathogens (gram-negative) | 1.59 | 1.34, 1.83 | <0.001 | 0.36 | 15,206 | 72 |
| High-priority pathogens (gram-positive) | 1.47 | 0.94, 2.00 | 0.045 | 0.48 | 4,472 | 22 |
| Bacterial family | ||||||
| Enterobacteriaceae | 1.49 | 1.09, 1.90 | 0.005 | 0.61 | 8,646 | 40 |
| Enterococcus spp. | 1.32 | 1.02, 1.61 | 0.017 | 0.00 | 949 | 6 |
| Moraxellaceae | 1.59 | 1.16, 2.02 | <0.001 | 0.12 | 2,297 | 16 |
| Pseudomonadaceae | 1.37 | 1.04, 1.69 | 0.011 | 0.10 | 1,353 | 10 |
| Staphylococcaceae | 1.52 | 0.76, 2.28 | 0.135 | 0.80 | 3,566 | 17 |
| II. ICU admission b | OR | |||||
| Overall | 1.96 | 1.56, 2.47 | <0.001 | 0.33 | 12,005 | 52 |
| WHO classification | ||||||
| Critical priority pathogens (gram-negative) | 2.02 | 1.62, 2.52 | <0.001 | 0.21 | 8,488 | 38 |
| High-priority pathogens (gram-positive) | 1.82 | 0.99, 3.37 | 0.055 | 0.68 | 3,517 | 14 |
| Bacterial family | ||||||
| Enterobacteriaceae | 2.59 | 1.95, 3.45 | <0.001 | 0.16 | 4,841 | 18 |
| Enterococcus spp. | 1.48 | 0.90, 2.41 | 0.119 | 0.27 | 870 | 6 |
| Moraxellaceae | 1.57 | 1.02, 2.41 | 0.039 | 0.20 | 1,625 | 12 |
| Pseudomonadaceae | 1.37 | 1.05, 1.77 | 0.018 | 0.05 | 877 | 5 |
| Staphylococcaceae | 1.91 | 0.86, 4.25 | 0.112 | 0.82 | 2,647 | 8 |
| III. LOS c | SMD | |||||
| Overall | 0.49 | 0.20, 0.78 | <0.001 | 0.27 | 3,185 | 18 |
| WHO classification | ||||||
| Critical priority pathogens (gram-negative) | 0.37 | 0.17, 0.57 | <0.001 | 0.06 | 2,097 | 11 |
| High-priority pathogens (gram-positive) | 0.71 | 0.03, 1.39 | 0.040 | 0.66 | 1,088 | 7 |
| Bacterial family | ||||||
| Enterobacteriaceae | 0.43 | 0.14, 0.73 | 0.004 | 0.06 | 1,175 | 5 |
| Enterococcus spp. | 0.25 | −0.05, 0.55 | 0.102 | - | 173 | 1 |
| Moraxellaceae | 0.16 | −0.06, 0.38 | 0.155 | 0.00 | 379 | 3 |
| Pseudomonadaceae | 0.14 | −0.11, 0.39 | 0.276 | 0.00 | 332 | 2 |
| Staphylococcaceae | 0.82 | 0.01, 1.63 | 0.047 | 0.78 | 915 | 6 |
WHO, World Health Organization. Where the numbers of studies seem inconsistent, this is attributable to several studies reporting on multiple categories (WHO) or combined pathogens simultaneously. ICU stands for intensive care unit. Fully disaggregated results, including their respective forest plots, are shown in S1 Text, section 3. OR, odds ratio; SMD, standardised mean difference; CI, Confidence interval; N, number.
aFrom the total 109 studies included in the systematic review, 9 were excluded as they had missing data; one study was excluded as it only reported excess deaths for ARB BSIs at the country level [88]; and, 6 studies evaluated mortality by comparison group but reported different bacteria for the sample of individuals and therefore were excluded from the overall analysis but had sufficient information to be retained for the subgroup analyses.
bOne study [96] reported data on demographics and ARB BSI for 2 different pathogens and with non-duplicate episodes, which were included as separate sub-studies.
cThe number of studies/sub-studies differs from Table F in S1 Text because some studies did not report the standard deviation of LOS, so the SMD could not be computed.
‡One study was excluded from the N = 109 initial sample because it only reported excess mortality. P-values (p) were reported using a two-sided z-test (α = 5%) for the log-transformed mortality and ICU admission ratios and LOS’s SMD.
ARB, antibiotic-resistant bacteria; BSI, bloodstream infection; LOS, length of hospital stay.
Table 3. Meta-analysis subgroup results by the most common antibiotic-resistant microbial strains according to the WHO global priority list of antibiotic-resistant bacteria.
| Outcome | Most common antibiotic-resistant microbial strains* | OR/SMD | 95% CI | P-value | N of studies |
|---|---|---|---|---|---|
| I. Mortality | OR | ||||
| CRAB | 1.46 | 0.80, 2.11 | 0.120 | 10 | |
| CREN | 1.97 | 1.37, 2.56 | <0.001 | 26 | |
| CREC | 1.54 | 0.00, 6.37 | 0.857 | 2 | |
| CRKP | 1.79 | 1.15, 2.43 | 0.002 | 19 | |
| CRPA | 1.36 | 0.89, 1.82 | 0.088 | 9 | |
| MRSA | 1.51 | 0.76, 2.26 | 0.132 | 16 | |
| VRE | 1.31 | 1.01, 1.60 | 0.021 | 6 | |
| II. ICU admission | OR | ||||
| CRAB | 1.36 | 0.85, 2.16 | 0.198 | 6 | |
| CREN | 2.66 | 1.98, 3.57 | <0.001 | 15 | |
| CREC‡ | 3.88 | 2.74, 5.49 | <0.001 | 1 | |
| CRKP | 2.60 | 1.81, 3.75 | <0.001 | 9 | |
| CRPA | 1.39 | 1.02, 1.90 | <0.001 | 3 | |
| MRSA | 1.91 | 0.86, 4.25 | 0.112 | 8 | |
| VRE | 1.48 | 0.87, 2.54 | 0.152 | 6 | |
| III. LOS | SMD | ||||
| CRAB | 0.22 | −0.04, 0.49 | 0.104 | 2 | |
| CREN | 0.53 | 0.39, 0.67 | <0.001 | 4 | |
| CREC‡ | - | - | - | - | |
| CRKP | 0.56 | 0.41, 0.71 | <0.001 | 3 | |
| CRPA‡ | 0.00 | −0.46, 0.46 | 1.000 | 1 | |
| MRSA | 0.82 | 0.00, 1.63 | 0.048 | 6 | |
| VRE‡ | 0.25 | −0.05, 0.55 | 0.102 | 1 | |
*All comparisons and ORs/SMD computations were made concerning their sensitive-specific counterpart. CRAB, Carbapenem-resistant Acinetobacter baumanii; CREN, Carbapenem-resistant Enterobacteriaceae; CREC, Carbapenem-resistant Escherichia coli; CRKP, Carbapenem-resistant Klebsiella pneumoniae; CRPA, Carbapenem-resistant Pseudomonas aeruginosa; MRSA, Methicillin-resistant Staphylococcus aureus; VRE, Vancomycin-resistant Enterococcus faecium/faecalis.
‡Either non or only study-reported estimates for the specific antibiotic-bacterium pair. Full charts, including the studies, can be found in S1 Text, section 7. P-values (p) were reported using a two-sided z-test (α = 5%) for the log-transformed mortality and ICU admission ratios and LOS’s SMD.
ARB, antibiotic-resistant bacteria; CI, confidence interval; ICU, intensive care unit; LOS, length of hospital stay; OR, odds ratio; SMD, standardised mean difference; WHO, World Health Organization.
Overall, the crude odds of ICU admission were 1.96 times higher for ARB compared to ASB BSIs (95% CI [1.56 to 2.47], p < 0.001) (Table 2, section II). Patients with WHO critical priority pathogens resistant to antibiotics were twice as likely to be admitted to ICU (OR 2.02, 95% CI [1.62 to 2.52], p < 0.001), with the highest observed ratio for gram-negative BSIs caused by antibiotic-resistant Enterobacteriaceae (OR 2.59, 95% CI [1.95 to 3.45], p < 0.001). Carbapenem-resistant Enterobacteriaceae in general (OR 2.66, 95% CI [1.98 to 3.57], p < 0.001), and specifically Escherichia coli (OR 3.88, 95% CI [2.74 to 5.49], p < 0.001), accounted for the highest figures (Table 3). Among gram-positive bacteria, Methicillin-resistant Staphylococcus aureus had an OR of 1.91 for ICU admission rate (95% CI [0.86 to 4.25], p 0.11), and vancomycin-resistant Enterococcus faecium/faecalis had an OR of 1.48 (95% CI [0.87 to 2.54], p 0.15) (Table 3). The Western Pacific region had the highest increase in ICU odds (OR 2.42, 95% CI [1.88 to 3.12], p < 0.001), followed by the Americas (OR 1.77, 95% CI [1.08 to 2.89], p 0.02), whereas the Southeast Asia region had the lowest odds of ICU admission of ARB BSIs compared to ASB BSIs (Table G in S1 Text).
The crude SMD for LOS was 0.49 (95% CI [0.20 to 0.78], p < 0.001; Table 2, section III). In other words, the curve representing the distribution of LOS times was shifted to the right by 0.49 standard deviations for the ARB BSIs group (i.e., LOS is approximately 7 days longer for the ARB group; derived from multiplying SMD by LOS’s standard deviation among all patients [0.49*13.91]). The SMD was higher for resistant pathogens classified as WHO high-priority pathogens (or gram-positive, SMD 0.71, 95% CI [0.03 to 1.39], p 0.04) compared with WHO critical priority pathogens (or gram-negative, SMD 0.37, 95% CI [0.17 to 0.57], p 0.13). Studies reporting MRSA accounted for the greatest excess LOS estimated (SMD 0.82; Table 3), compared to methicillin-sensitive S. aureus. The highest excess LOS was observed in studies from Turkey (SMD 1.29). Studies from Europe (SMD 1.29) and Brazil (SMD 0.43) contributed substantially to the greater LOS in ARB BSI patients (Table G in S1 Text).
Full details on the meta-analysis main and subgroup results, including their respective forest plots, can be found in S1 Text, section 3.
Tables W and X in S1 Text show the results of the univariate and multivariable meta-regressions for mortality and ICU admission, respectively. Among the variables selected from the univariate analyses, our multivariable meta-regression showed that patients with resistant Moraxellaceae BSIs and hypertension had higher mortality odds when ARB versus ASB BSI patients were compared (OR 1.67, 95% CI [1.18 to 2.36], p 0.004; OR 1.13, 95% CI [1.00 to 1.28], p 0.035, respectively). Yet, countries from the Southeast Asia WHO region displayed lower mortality odds (OR 0.62, 95% CI [0.46 to 0.85], p 0.004). For the ICU admission multivariable meta-regression, we found a weak negative association between BSIs originating as a secondary infection from the urinary tract and the odds of mortality between patients having ARB and ASB BSIs (OR 0.72, 95% CI [0.51 to 1.02], p 0.06).
Estimated excess costs
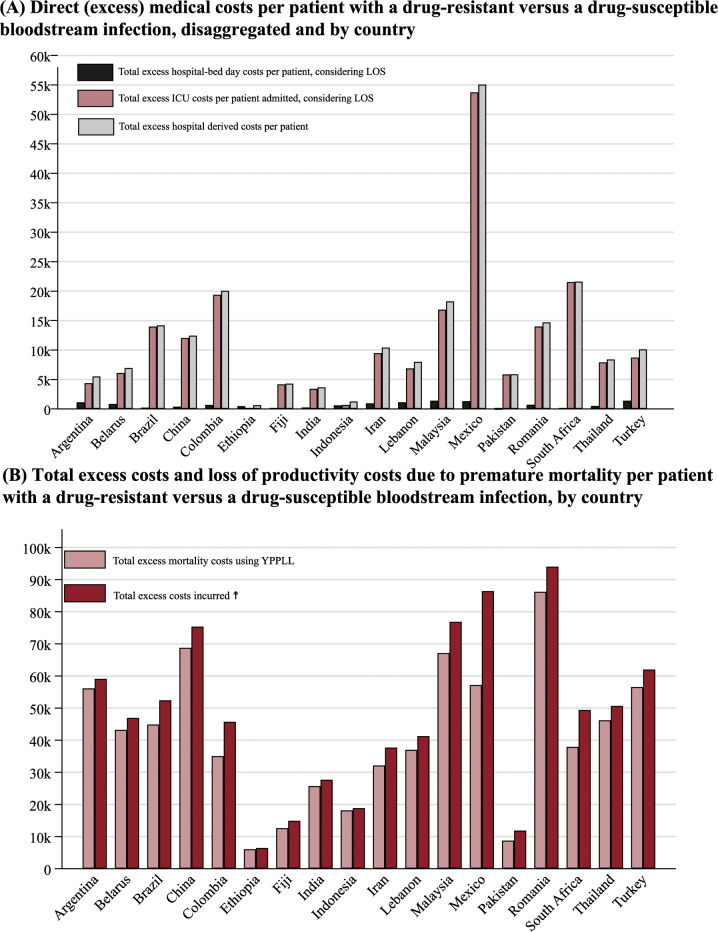
The average excess hospital bed-days cost per ARB BSI patient in tertiary/teaching hospitals, adjusted by the calculated excess LOS from Table 2 and excluding drugs and tests costs, was $812.5 (95% CI [$331.6 to $1,293.3]) (Table J in S1 Text). The excess costs per patient varied considerably between countries, ranging from $30.9, $95.9, and $131.7 (Ethiopia, Pakistan, and India, respectively) to $1,681.7 and $1,683.2 (Mexico and Turkey) (Fig 4, panel A).
Fig 4. Excess costs (in 2020 USD) associated with productivity loss or excess length of stay per patient with a drug-resistant versus a drug-sensitive bloodstream infection.
(A) Direct excess medical costs dissagreggated by ICU and hospital-bed days and by country (B) Total excess costs and productivity lossess due to premature mortality by country. ARB, antibiotic-resistant bacteria; BSI, bloodstream infection; YPLL, years of potential life lost from premature mortality; LOS, length of stay; USD, United States dollars. Full information and data are provided in S1 Text, section 4. ☨ Total excess costs incurred including YPLL and hospital-derived costs per patient with ARB BSI. “k” = thousands. Costs of productivity loss are found in Table L in S1 Text.
We estimated an average excess of productivity loss (indirect costs associated with ARB BSI for an average patient) from years of potential life lost due to premature mortality of $41,102 (95% CI = $30,931 to $51,274) for all bacteria combined (Table L in S1 Text). Romania presented the highest excess producitivity lossess attributed to years of potential life-lost costs per patient, while Ethiopia had the lowest ($86,217 and $6,070, respectively). Mortality costs due to premature mortality using the life expectancy approach had an observed average of $132,560 per patient (95% CI [$99,753 to $165,363]) among all sampled countries (Table L in S1 Text).
The average excess ICU admission costs per patient, multiplied by the calculated ICU LOS, was $11,629 (95% CI [$6,016 to $17,243]) (Table O in S1 Text) for all bacteria combined. The estimates varied, with a middle data dispersion of $5,669 (i.e., third quartile–second quartile). Mexico had the highest costs per patient ($53,747), and Ethiopia had the lowest ($188) (Table O in S1 Text).
Fig 4 displays the direct medical and productivity loss due to premature mortality costs per patient by country (panel B). Direct medical costs (i.e., hospital bed-day costs and bed-day ICU costs per day multiplied by the average hospital and ICU respective LOS) were estimated at $12,442 (95% CI [$6,693 to $18,191]). The average total excess costs for a patient with ARB compared to ASB BSI, comprising direct medical and years of potential life lost, were $53,545 (95% CI [$39,838 to $67,251]). Excess costs for ICU adjusted to ICU’s length of stay were 14 times higher compared with hospital-bed LOS-adjusted among patients with ARB BSIs. Lower middle-income countries had the lowest economic burdens per patient; however, we found substantial between-country differences.
Full details on cost calculation can be found in S1 Text, section 4.
Quality and risk assessment
Using the MASTER scale for methodological assessment, we calculated, on average, 25.1, 23.7, and 23.6 points (out of 36) for the mortality, ICU admission, and length of hospital stay outcomes, respectively (Table 4). Our scores reflect that few studies addressed key confounders (e.g., using statistical methods to control for other correlated risk factors) to account for different prognoses and equal ascertainment (especially for participants, analysts, and caregivers’ blindness towards evaluation; <2% of included studies). Only 37%, 11%, and 13% of the studies incorporated statistical techniques (e.g., regression analyses, stratification, matching, among others) for an equal prognosis for the mortality, ICU admission, and LOS outcomes, respectively (Table 4, equal prognosis scores). Most studies achieved equal retention (e.g., low missing data and null attrition) and sufficient analyses safeguards (e.g., absence of numerical contradictions and data dredging), regardless of the outcome analysed. Full results are found in S1 Text sections 8 and 9 and S1 Data, Master Scale spreadsheet.
Table 4. Assessment of study quality and risk of bias using the MASTER scale.
| Safeguard items and sub-items | Outcomes | ||
|---|---|---|---|
| Mortality | ICU admission | LOS | |
| Equal recruitment | 60.4% | 58.9% | 60.6% |
| 1. Data collected after the start of the study was not used to exclude participants or to select them for the analysis | 38.8% | 39.6% | 40.0% |
| 2. Participants in all comparison groups met the same eligibility requirements and were from the same population and timeframe | 100.0% | 100.0% | 100.0% |
| 3. Determination of eligibility and assignment to treatment group/exposure strategy were synchronised | 17.5% | 11.3% | 12.5% |
| 4. None of the eligibility criteria were common effects of exposure and outcome | 85.4% | 84.9% | 90.0% |
| Equal retention | 96.9% | 97.4% | 96.5% |
| 5. Any attrition (or exclusions after entry) was less than 20% of total participant numbers | 92.2% | 94.3% | 87.5% |
| 6. Missing data was less than 20% | 97.1% | 96.2% | 97.5% |
| 7. Analysis accounted for missing data | 96.1% | 96.2% | 97.5% |
| 8. Exposure variations/treatment deviations were less than 20% | 100.0% | 100.0% | 100.0% |
| 9. The analysis addressed variations in exposure or withdrawals after start of the study | 99.0% | 100.0% | 100.0% |
| Equal ascertainment | 57.1% | 57.4% | 57.1% |
| 10. Procedures for data collection of covariates were reliable and the same for all participants | 100.0% | 100.0% | 100.0% |
| 11. The outcome was objective and/or reliably measured | 100.0% | 100.0% | 100.0% |
| 12. Exposures/interventions were objectively and/or reliably measured | 100.0% | 100.0% | 100.0% |
| 13. Outcome assessor(s) were blinded | 100.0% | 100.0% | 100.0% |
| 14. Participants were blinded | 0.0% | 0.0% | 0.0% |
| 15. Caregivers were blinded | 0.0% | 0.0% | 0.0% |
| 16. Analyst(s) were blinded | 0.0% | 1.9% | 0.0% |
| Equal implementation | 64.6% | 66.4% | 66.3% |
| 17. Care was delivered equally to all participants | 0.0% | 0.0% | 0.0% |
| 18. Cointerventions that could impact the outcome were comparable between groups or avoided | 0.9% | 0.0% | 0.0% |
| 19. Control and active interventions/exposures were sufficiently distinct | 100.0% | 100.0% | 100.0% |
| 20. Exposure/intervention definition was consistently applied to all participants | 87.4% | 98.1% | 97.5% |
| 21. Outcome definition was consistently applied to all participants | 100.0% | 100.0% | 100.0% |
| 22. The period between exposure and outcome was similar across patients and between groups or the analyses adjusted for different lengths of follow-up of patients | 99.0% | 100.0% | 100.0% |
| Equal prognosis | 37.6% | 11.0% | 12.5% |
| 23. Design and/or analysis strategies were in place that addressed potential confounding | 84.5% | 0.0% | 0.0% |
| 24. Key confounders addressed through design or analysis were not common effects of exposure and outcome | 69.9% | 0.0% | 0.0% |
| 25. Key baseline characteristics/prognostic indicators for the study were comparable across groups | 3.9% | 0.0% | 2.6% |
| 26. Participants were randomly allocated to groups with an adequate randomisation process | 4.9% | 9.4% | 10.0% |
| 27. Allocation procedure was adequately concealed | 0.0% | 0.0% | 0.0% |
| 28. Conflict of interests were declared and absent | 62.1% | 56.6% | 62.5% |
| Sufficient analysis | 89.9% | 92.3% | 92.5% |
| 29. Analytic method was justified by study design or data requirements | 84.2% | 88.5% | 90.0% |
| 30. Computation errors or contradictions were absent | 93.2% | 94.3% | 90.0% |
| 31. There was no discernible data dredging or selective reporting of the outcomes | 92.2% | 94.2% | 97.4% |
| Temporal precedence | 100.0% | 100.0% | 100.0% |
| 32. All subjects were selected prior to intervention/exposure and evaluated prospectively | 100.0% | 100.0% | 100.0% |
| 33. Carry-over or refractory effects were avoided or considered in the design of the study or were not relevant | 100.0% | 100.0% | 100.0% |
| 34. The intervention/exposure period was long enough to have influenced the study outcome | 100.0% | 100.0% | 100.0% |
| 35. Dose of intervention/exposure was sufficient to influence the outcome | 100.0% | 100.0% | 100.0% |
| 36. Length of follow-up was not too long or too short in relation to the outcome assessment | 100.0% | 100.0% | 100.0% |
| Average count of safeguard items (raw score out of 36 items) | 25.1 | 23.6 | 23.7 |
| Average percentage of sufficiency considering all 36 items (i.e., average raw score/36) | 69.6% | 65.6% | 65.9% |
Percentage of fulfilment among all included studies, and per outcome, is presented by MASTER’s scale safeguard and items [21].
Small-study effects
We found a medium level of heterogeneity between studies for the mortality outcome (I2 69%, 95% CI [52% to 78%]), and high variation for ICU admission (I2 91%, 95% CI [83% to 94%]) and LOS (I2 90%, 95% CI [75%, 95%]) for the meta-analysis run by specific groups (S1 Text, section 5). Studies reporting ICU admission and LOS were either symmetrical (LFK index ≤1) or slightly asymmetrical (LFK index <3) (Figs AM and AN in S1 Text).
Sensitivity analyses
General mortality estimates from studies in China were not different from studies conducted elsewhere. However, we found larger disaggregated estimates for subgroup meta-analyses, such as Enterobacteriaceae, Moraxellaceae, Pseudomonaceae, and Staphylococcaceae species (8%, 25%, 26%, and 20%, respectively) compared to the average mortality estimates reported in Table 2 for the same subgroups. General LOS SMD was 16% higher among countries other than China, compared to the estimates reported in Table 2, specifically driven by Moraxellaceae and Staphylococcaceae species. Finally, the odds for excess ICU admission were 25% greater in China, with respect to average ICU admission found in all included studies, driven by 27% elevated odds among patients having BSIs caused by gram-negative bacteria. Full results in Tables U and V in S1 Text.
When applying the leave-one-out method to our meta-analyses, we observed that after assessing the effect of every single study on the overall estimates, the numbers presented a relative variation with respect to overall estimates ranging between −2% and 4% for mortality (OR 95% CI [1.57 to 1.58]), −8% and 4% for ICU admission (OR 95% CI [1.95 to 1.97]), and −10% and 4% for LOS (SMD 95% CI [0.48 to 0.50]) (S1 Text, section 6). These results suggest a moderate influence of our studies in the overall estimates if relative variations are compared, especially for ICU admission and LOS.
Discussion
Antibiotic resistance imposes substantial morbidity, mortality, and societal costs in LMICs [153]. Bloodstream infections with ARB are among the most lethal, imposing a large disease burden. Examining all available data for hospitalised patients in LMICs, we found that ARB BSIs with WHO critical- and high-priority pathogens were associated with increased mortality (OR 1.58, 95% CI [1.35 to 1.80]), overall length of stay (SMD 0.49, 95% CI [0.20 to 0.78]), and ICU admission (OR 1.96, 95% CI [1.56 to 2.47]).
Our findings on mortality are consistent with the recent estimates by the Global Burden of Disease study [154]. The largest mortality impact was associated with resistant A. baumannii and Enterobacteriaceae. Both bacteria featured in the global top 5 contributors to resistance-associated and -attributable deaths in 2019 [154]. Between a quarter and half of the patients with ARB BSIs caused by Enterobacteriaceae, A. baumannii or P. aureginosa die, corroborating findings from different country settings for Enterobacteriaceae [8,67], P. aeruginosa [155], and large university hospitals in Israel and the US for A. baumanii [156,157].
Our results suggest that patients who acquired ARB BSIs during their hospital stay had an overall hospital stay that is about a week longer than patients that acquired ASB BSIs. However, in our study, we could not distinguish between excess length of stay before or after BSI, and as such this is likely an overestimation. Depending on the pathogen, resistant infections have previously been shown to increase LOS typically by 2.0 to 12.7 days [158]. Longer hospital stay, especially before BSI onset, is a primary risk factor for acquiring a resistant infection due to the cumulative risk of hospital transmission of ARBs [158,159]. We found that MRSA had the greatest impact on LOS (extending stay by 14 days relative to sensitive S. aureus). Others have also shown considerably increased LOS as a result of MRSA compared with sensitive S. aureus: Tsuzuki and colleagues [160] showed an excess overall LOS and LOS after BSI onset of 20 and 7 days, respectively; similarly, Graffunder and colleagues [161] showed MRSA patients presented an overall LOS of 3 weeks longer. Resistant infections are more difficult to treat and increase the rate of ICU admissions. Our analysis showed that resistant Enterobacteriaceae infections more than doubled the odds of ICU admission. This finding is comparable with the 2.69 higher odds of ICU admission previously shown among patients with carbapenem-resistant K. pneumoniae BSIs [162]. Our exploratory analysis for studies performed in China and LMICs other than China exhibited divergent results. We found that China’s patients with antibiotic-resistant gram-negative BSIs (A. baumanii, Enterobacteriaceae, and P. aeruginosa) displayed higher excess mortality, ICU admission, and LOS, compared to the other LMICs with reported data. Large increases in antibiotic consumption and resistance levels over the last 20 years and the rapid development or acquisition of drug resistance among gram-negative pathogens might explain the greater excess mortality and morbidity for ARB BSIs in China [1,163,164]. Correspondingly, inappropriate administration of empirical treatments and low testing rates could increase the burden outcomes for patients with ARB BSIs in these settings [165].
Despite being fundamental to resource allocation for healthcare provision, we found very little data on excess costs associated with ARB BSIs among the reviewed studies. One study conducted in Thailand, reported excess costs associated with hospital-acquired carbapenem-resistant A. baumannii of $5,682 [61]. A study conducted in Colombia, reported excess hospitalisation costs associated with MRSA BSI of $10,212, compared to sensitive S. aureus [53]. We estimated costs associated with mortality, LOS, and ICU admissions from the provider and societal perspective following the WHO-CHOICE standards and human capital approach. We found that the average hospital-related 2020 USD excess costs were $12,442 (95% CI [$6,693 to $18,190]) per ARB BSI patient, compared to ASB, ranging between Ethiopia, with the lowest figures, to Mexico, with the highest. These differences are partly explained by the countries’ disparate economies (Pearson correlation = 0.27 between GDP and hospital costs). Several LMIC-setting studies detailing excess costs of resistant infections were excluded from our review because they did not meet specific inclusion criteria. Cost estimates from these studies include 1 from Turkey in which excess hospital stay and treatment costs were $10,002 [166]. Our estimate for Turkey of $10,403 is similar; however, our estimates did not include therapy/treatment costs. Our estimate for China ($12,516) was higher than a previous study including BSI treatment costs for carbapenem-resistant K. pneumoniae ($10,763) [167]. The average excess total costs comprising direct medical costs and years of potential life lost associated with premature mortality were $53,545 (95% CI [$39,838 to $67,251]) per patient with ARB BSI. WHO [168] recently reported that 58.3% of 22,371 isolates were identified as ARB E. coli, while 33.3% of 23,031 isolates were ARB S. aureus in LMICs, indicating the high relevance of these costs.
This study has limitations. First, the most important limitation is consistent with conclusions from the Global Burden of Diseases study [154]: there is a sparsity of data on ARB from LMICs. Only 18 of the 137 (13%) LMICs published any AMR outcome study. Consistent antibiotic resistance surveillance puts demands on clinical bacteriology, quality control, and data linkage between culture test results and clinical outcomes, which is beyond the capabilities of many LMICs. Applying the leave-one-out method to our meta-analyses (S1 Text, section 6) showed a minor-to-moderate influence of individual studies likely due to the heterogeneity in clinical settings, indicating that our model’s results are robust (assuming countries’ missing information and selection biases are heterogeneously distributed). Future efforts to improve coverage should prioritise WHO’s Africa region, where data were remarkably absent, with no estimates for resistance-associated LOS or ICU admissions. Our results indicate that the studies from the Western Pacific and European areas show the highest excess mortality from ARB BSIs. Studies from Africa show among the lowest but this region has limited data and substantial uncertainty; it is essential to improve epidemiological surveillance of ARB BSIs in this region in particular [169]. Second, some articles were of low quality or reported limited data. Studies often failed to account for confounding factors; hence our analyses relied upon crude estimates. ARB surveillance networks vary in blood culture sampling, potentially overestimating the number of severe cases if selective sampling among patients fulfilling the case definition is present. Third, we did not estimate the total relative harm of ARB BSIs relative to where such infections were prevented (compared to non-infected patients) [170], primarily because of the limited number of studies [171]. While we accounted for some key risk factors when comparing antibiotic-sensitive and antibiotic-resistant groups in the metaregression, others were unavailable. We could not match comparison groups by factors known to impact patients’ underlying health conditions, such as illness severity, prolonged previous hospital stays, or the use of invasive devices. The reported LOS does not distinguish between total LOS and LOS following BSI infection, thus risking reverse causality [172]. This ecological study was designed to identify associations; consequently, our results should be interpreted cautiously. Also, we adjusted WHO-CHOICE country estimates using US GPD implicit price deflators, which may not necessarily reflect price changes in some LMICs, particularly for non-tradable cost components of healthcare. Finally, we may have overestimated the true effect size of the association between ARB BSIs and mortality as indicated by the exploratory analysis of studies’ adjusted—compared to unadjusted—ORs reporting both estimates, specifically among gram-negative species.
Here, we described an updated evaluation of the health impact and excess economic costs of resistant BSIs in low-resourced settings. Our results highlight regions where improved surveillance, expanding microbiology laboratory capacity, and data collection systems are most needed and where the current evidence indicates WHO critical and high-priority drug-resistant pathogens exert the greatest toll on morbidity and mortality.
Supporting information
Text A. Search criteria used by search engine. Table A. Studies inclusion and exclusion criteria. Table B. Years of the studies included. Table C. Number of studies included by WHO region and WB income group. Table D. Correlation between main outcomes and demographic variables. Table E. Most prevalent bacterium family, Gram type, resistance type, and antibiotic-bacterium pair by country among the included studies. Table F. Descriptive statistics of the studies included in the meta-analysis. Table G. Summary of the subgroup meta-analysis results for income level and WHO region by outcome variable. Table H. Costs of hospital bed-day per patient and by country and hospital level (in 2008 USDs). Table I. Costs of total excess hospital bed-days per patient by country and hospital level using estimated SMD and their respective 95% CIs (in 2008 USDs). Table J. Costs of total excess hospital bed-days per patient and by country and hospital level using estimated SMD and their respective 95% CIs (inflated to 2020 USDs). Table K. Calculation of YPLL, YPPLL, and CPL, by country. Table L. Total productivity losses due to premature mortality costs by country using the LE at the age of death and productivity cost approach (age of retirement), discounted. Table M. Intensive care unit costs per patient (daily). Table N. Intensive care unit costs (per patient and daily) adjusted to 2020 USDs (inflated accordingly). Table O. Intensive care unit costs (per day/patient) adjusted to ICU LOS and reported in 2020 USDs (inflated accordingly). Table P. Total excess costs incurred for bloodstream infections caused by antibiotic-resistant bacteria, per patient. Table Q. Statistics calculated for meta-analysis using mortality as an outcome, by model. Table R. Statistics calculated for meta-analysis using ICU admission as an outcome, by model. Table S. Statistics calculated for meta-analysis using the length of stay at hospital as an outcome, by model. Table T. Summary of the subgroup meta-analysis results for specific antibiotic-bacterium combinations declared important by the WHO, by outcome variable. Table U. Meta-analysis subgroup results for bacterium family, and Gram type for those studies carried out in China and other than China, by outcome. Table V. Summary results of meta-analysis results for critical antibiotic-bacterium pathogens for those studies in China and other than China, by outcome. Table W. Meta-regression results for the mortality outcome (univariate and multivariable). Table X. Meta-regression results for the ICU admission outcome (univariate and multivariate). Table Y. Summary results of the meta-analysis for the main outcome variables by separating the studies for low- [LS] and high-scores [HS] obtained from the MASTER scale. Table Z. Checklist of information that should be included in new reports of global health estimates. Table AA. PRISMA Checklist. Fig A. Density of the studies over time. Fig B. Violin and kernel density estimate plots for the main outcomes and by ARB susceptibility. Fig C. Relationship between the main outcomes. Fig D. Meta-analysis using all the studies reporting mortality rates. Fig E. Subgroup meta-analysis using all the studies reporting mortality rates/odds for critical (N = 72) and high-priority (N = 22) pathogens according to the WHO criteria. Fig F. Subgroup meta-analysis using all the studies reporting mortality rates by bacterium’s family name. Fig G. Subgroup meta-analysis using all the studies reporting mortality rates by WHO Region. Fig H. Subgroup meta-analysis using all the studies reporting mortality rates by income level. Fig I. Meta-analysis results using all the studies reporting the mean and SD for the length of stay at the hospital. Fig J. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital for critical and high-priority pathogens according to the WHO. Fig K. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital for Enterococcus spp., Enterobacteriaceae, Moraxellaceae, Pseudomonadaceae, and Staphyloccocaceae. Fig L. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital by income level. Fig M. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital by WHO region. Fig N. Meta-analysis results using all the studies reporting ICU admission rates. Fig O. Subgroup meta-analysis using all the studies reporting ICU admission rates for critical pathogens according to the WHO criteria. Fig P. Subgroup meta-analysis using all the studies reporting ICU admission rates for high-priority pathogens according to the WHO criteria. Fig Q. Subgroup meta-analysis using all the studies reporting ICU admission rates for Enterobacteriaceae. Fig R. Subgroup meta-analysis using all the studies reporting ICU admission rates for Enterobacteriaceae. Fig S. Subgroup meta-analysis using all the studies reporting ICU admission rates for Moraxellaceae. Fig T. Subgroup meta-analysis using all the studies reporting ICU admission rates for Pseudomonadaceae. Fig U. Subgroup meta-analysis using all the studies reporting ICU admission rates for Staphylococcaceae. Fig V. Subgroup meta-analysis using all the studies reporting ICU admission rates by resistance type (ESBL+). Fig W. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Americas. Fig X. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Eastern Mediterranean. Fig Y. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Europe. Fig Z. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Southeast Asia. Fig AA. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Western Pacific region. Fig AB. Subgroup meta-analysis using all the studies reporting ICU admission rates by income level: Low and lower-middle income countries. Fig AC. Subgroup meta-analysis using all the studies reporting ICU admission rates by income level: Upper-middle income countries. Fig AD. Subgroup analysis for studies reporting unadjusted ORs. Fig AE. Subgroup analysis for studies reporting unadjusted ORs, by bacteria’s gram type or WHO criticality category (critical = gram-negative, high-priority = gram-positive in this study). Fig AF. Subgroup analysis for studies reporting unadjusted ORs, by specific bacterium. Fig AG. Subgroup analysis for studies reporting adjusted ORs. Fig AH. Subgroup analysis for studies reporting adjusted ORs, by bacteria’s gram type (critical = gram-negative, high-priority = gram-positive in this study). Fig AI. Subgroup analysis for studies reporting adjusted ORs, by specific bacterium. Fig AJ. Subgroup analysis for studies reporting adjusted and unadjusted ORs simultaneously, general mortality estimates. Fig AK. Subgroup analysis for studies reporting adjusted and unadjusted ORs simultaneously, mortality rates by Gram type or WHO criticality list classification (high = gram-positive, critical = gram-negative). Fig AL. Subgroup analysis for studies reporting adjusted and unadjusted ORs simultaneously, mortality rates by bacterium family. Fig AM. Doi plots for Model 1 (general) and by outcome based on Tables Q, R, and S. Fig AN. Funnel plots for Model 1 (general) and by outcome based on Tables Q, R, and S. Fig AO. Influence analysis for Model 1 using the mortality outcome compared to the general estimates and without subgroup analyses. Fig AP. Influence analysis for Model 1 using the ICU admission outcome compared to the general estimates and without subgroup analyses. Fig AQ. Influence analysis for Model 1 using the length of hospital stay outcome compared to the general estimates and without subgroup analyses. Fig AR. Meta-analysis results disaggregated by specific and prioritised antibiotic-bacterium pairs for mortality. Fig AS. Meta-analysis results disaggregated by carbapenem-resistant Enterobacteriaceae for mortality. Fig AT. Meta-analysis results disaggregated by specific and prioritised antibiotic-bacterium pairs for LOS. Fig AU. Meta-analysis results disaggregated by carbapenem-resistant Enterobacteriaceae for LOS. Fig AV. Meta-analysis results disaggregated by specific and prioritised antibiotic-bacterium pairs for ICU admission. Fig AW. Meta-analysis results disaggregated by carbapenem-resistant Enterobacteriaceae for ICU admission. Fig AX. Graphical results of Table V. Fig AY. Distribution of the Master scale scores by outcome. Fig AZ. Kernel density estimate of the Master scale scores by outcome. Fig BA. Percentage of full completion by MASTER scale main safeguard and outcome.
(PDF)
MasterData spreadsheet. Description and data extracted from each included study. MasterScale spreadsheet. Application of the MASTER scale by outcome and study. Summary MasterScale spreadsheet. Summary statistics per safeguard/item of the application of the MASTER scale.
(XLSX)
Acknowledgments
All authors attest that they meet the ICMJE criteria for authorship and have reviewed and approved the final article. We thank the Royal Society of Tropical Medicine and Hygiene (RSTMH) for its support through the 2021 early career grant award.
Abbreviations
- AIM
African Index Medicus
- ARB
antibiotic-resistant bacteria
- ASB
antibiotic-sensitive bacteria
- BSI
bloodstream infection
- GLASS
Global Antimicrobial Resistance and Surveillance System
- GNI
gross national income
- ICU
intensive care unit
- LMIC
low- and middle-income country
- LOS
length of hospital stay
- MRSA
methicillin-resistant Staphylococcus aureus
- OR
odds ratio
- SMD
standardised mean difference
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was made possible by Asociación Nacional de Investigación y Desarrollo (ANID) Beca de Doctorado en el Extranjero Becas Chile, Chile (Grant number: 73200098 to KA), Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT, Chile (Grant number: 1211933 to EAU), and Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias FONDAP, Chile (Grant number: 1522A0005 to EAU). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
References
- 1.Organisation for Economic Cooperation and Development. Stemming the Superbug Tide: Just a Few Dollars More: OECD; 2019. [Google Scholar]
- 2.Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5(8):481–493. doi: 10.1016/S1473-3099(05)70189-4 [DOI] [PubMed] [Google Scholar]
- 3.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. 2021. [Google Scholar]
- 5.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 6.Hattori H, Maeda M, Nagatomo Y, Takuma T, Niki Y, Naito Y, et al. Epidemiology and risk factors for mortality in bloodstream infections: A single-center retrospective study in Japan. Am J Infect Control. 2018;46(12):e75–e79. doi: 10.1016/j.ajic.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 7.de Kraker ME, Wolkewitz M, Davey PG, Grundmann H. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2011;55(4):1598–1605. doi: 10.1128/AAC.01157-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Kraker M, Wolkewitz M, Davey P, Koller W, Berger J, Nagler J, et al. Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother. 2011;66(2):398–407. doi: 10.1093/jac/dkq412 [DOI] [PubMed] [Google Scholar]
- 9.Thaden JT, Li Y, Ruffin F, Maskarinec SA, Hill-Rorie JM, Wanda LC, et al. Increased costs associated with bloodstream infections caused by multidrug-resistant gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother. 2017;61(3):e01709–e01716. doi: 10.1128/AAC.01709-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wozniak TM, Barnsbee L, Lee XJ, Pacella RE. Using the best available data to estimate the cost of antimicrobial resistance: a systematic review. Antimicrob Resist Infect Control. 2019;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H-Y, Chen C-L, Liu S-Y, Yan Y-S, Chang C-J, Chiu C-H. Impact of molecular epidemiology and reduced susceptibility to glycopeptides and daptomycin on outcomes of patients with methicillin-resistant Staphylococcus aureus bacteremia. PLoS ONE. 2015;10(8):e0136171. doi: 10.1371/journal.pone.0136171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biehle LR, Cottreau JM, Thompson DJ, Filipek RL, O’Donnell JN, Lasco TM, et al. Outcomes and risk factors for mortality among patients treated with carbapenems for Klebsiella spp. bacteremia. PLoS ONE. 2015;10(11):e0143845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheah A, Spelman T, Liew D, Peel T, Howden B, Spelman D, et al. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect. 2013;19(4):E181–E189. doi: 10.1111/1469-0691.12132 [DOI] [PubMed] [Google Scholar]
- 14.Naylor NR, Atun R, Zhu N, Kulasabanathan K, Silva S, Chatterjee A, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ang H, Sun X. Risk factors for multidrug-resistant Gram-negative bacteria infection in intensive care units: A meta-analysis. Int J Nurs Pract. 2018;24(4):e12644. doi: 10.1111/ijn.12644 [DOI] [PubMed] [Google Scholar]
- 16.Saharman YR, Karuniawati A, Severin JA, Verbrugh HA. Infections and antimicrobial resistance in intensive care units in lower-middle income countries: a scoping review. Antimicrob Resist Infect Control. 2021;10(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akova M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence. 2016;7(3):252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Bank. World Bank Country and Lending Groups 2021. [cited 2021 Aug 31]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. [Google Scholar]
- 20.World Health Organization. Antimicrobial resistance: global report on surveillance: World Health Organization. 2014. [Google Scholar]
- 21.Stone JC, Glass K, Clark J, Ritskes-Hoitinga M, Munn Z, Tugwell P, et al. The MethodologicAl STandards for Epidemiological Research (MASTER) scale demonstrated a unified framework for bias assessment. J Clin Epidemiol. 2021. doi: 10.1016/j.jclinepi.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 22.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials. 2015;45:130–138. doi: 10.1016/j.cct.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Choosing interventions that are cost effective (WHO—CHOICE). Cost effectiveness and strategic planning. Available at: http://www.who.int/choice/costs/en/. Accessed March, 2020. 2021.
- 24.Khwannimit B, Bhurayanontachai R. The direct costs of intensive care management and risk factors for financial burden of patients with severe sepsis and septic shock. J Crit Care. 2015;30(5):929–934. doi: 10.1016/j.jcrc.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 25.Kockaya PD, Kavuncubasi S, Kockaya G. Cost of Intensive Care Stay in Turkey: In the View of Payer and Health Care Provider. Value Health. 2013;16(7):A466. [Google Scholar]
- 26.Mahomed S, Mahomed O. Cost of intensive care services at a central hospital in South Africa. S Afr Med J. 2019;109(1):35–39. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzovici L, Székely A, Csanádi M, Gaál P. Cost assessment of inpatient care episodes of stroke in Romania. Front Public Health. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haque A, Naveed-ur-Rehman Siddiqui RK, Hoda M, Lakahni G, Hooda K. Cost of care in a paediatric intensive care unit of a tertiary-care university hospital of Pakistan. Trauma. 2015;21:14.1. [PubMed] [Google Scholar]
- 29.Velázquez LDS. Análisis de costos en las Unidades de Terapia Intensiva mexicanas. Estudio multicéntrico. Medicina Crítica. 2010;24(4):159–166. [Google Scholar]
- 30.Aung YN, Nur AM, Ismail A, Aljunid SM. Determining the cost and length of stay at intensive care units and the factors influencing them in a teaching hospital in Malaysia. Value Health Reg Issues. 2020;21:149–156. doi: 10.1016/j.vhri.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 31.Soleymani F. Costs of hospital-acquired infection for patients hospitalized in intensive care unit of an Iranian referral hospital. Med J Islam Repub Iran. 2018;32:67. doi: 10.14196/mjiri.32.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter JV, Thomas K, Jeyaseelan L, Yadav B, Sudarsan TI, Christina J, et al. Cost of intensive care in India. Int J Technol Assess Health Care. 2016;32(4):241–245. doi: 10.1017/S0266462316000398 [DOI] [PubMed] [Google Scholar]
- 33.Olivera COE, Urrego KAG, Duque MG, Góngora EM. Costos de atención en UCI de un Hospital universitario de Bogotá DC. Revista Repertorio de Medicina y Cirugía. 2006;15(3):133–142. [Google Scholar]
- 34.Cong Y. Ethical challenges in critical care medicine: a Chinese perspective. J Med Philos. 1998;23(6):581–600. doi: 10.1076/jmep.23.6.581.2558 [DOI] [PubMed] [Google Scholar]
- 35.Sogayar AM, Machado FR, Rea-Neto A, Dornas A, Grion CM, Lobo SM, et al. A multicentre, prospective study to evaluate costs of septic patients in Brazilian intensive care units. Pharmacoeconomics. 2008;26(5):425–434. doi: 10.2165/00019053-200826050-00006 [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal VD, Guzman S, Migone O, Crnich CJ. The attributable cost, length of hospital stay, and mortality of central line-associated bloodstream infection in intensive care departments in Argentina: a prospective, matched analysis. Am J Infect Control. 2003;31(8):475–480. doi: 10.1016/j.ajic.2003.03.002 [DOI] [PubMed] [Google Scholar]
- 37.Tan SS, Bakker J, Hoogendoorn ME, Kapila A, Martin J, Pezzi A, et al. Direct cost analysis of intensive care unit stay in four European countries: applying a standardized costing methodology. Value Health. 2012;15(1):81–86. doi: 10.1016/j.jval.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 38.Evans J, Kobewka D, Thavorn K, D’Egidio G, Rosenberg E, Kyeremanteng K. The impact of reducing intensive care unit length of stay on hospital costs: evidence from a tertiary care hospital in Canada. Can J Anesth. 2018;65(6):627–635. [DOI] [PubMed] [Google Scholar]
- 39.Oostenbrink JB. Buijs-Van der Woude T, van Agthoven M, Koopmanschap MA, Rutten FF. Unit costs of inpatient hospital days. Pharmacoeconomics. 2003;21(4):263–271. doi: 10.2165/00019053-200321040-00004 [DOI] [PubMed] [Google Scholar]
- 40.Springer. Human Capital Approach. In: Kirch W, editor. Encyclopedia of Public Health. Dordrecht: Springer Netherlands; 2008. p. 697–8. [Google Scholar]
- 41.Murray CJ. Comprehensive systematic analysis of global epidemiology: definitions, methods, simplification of DALYs, and comparative results from the Global Burden of Disease Study 2010. Supplement to: Murray CJL, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. [DOI] [PubMed] [Google Scholar]
- 42.Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107–114. doi: 10.1093/heapol/czz127 [DOI] [PubMed] [Google Scholar]
- 43.Furuya-Kanamori L, Barendregt JJ, Doi SA. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Halthc. 2018;16(4):195–203. doi: 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 44.Hastie T, Tibshirani R, Friedman JH, Friedman JH. The elements of statistical learning: data mining, inference, and prediction: Springer; 2009. [Google Scholar]
- 45.Hincapié C, Galeano JA, Tibaduiza MF, Restrepo CA, Garcés D, Caraballo C, et al. Staphylococcemia mortality: Influence of methicillin resistance and site of infection acquisition in a patient’s cohort from Medellin. Colombia. Enferm Infecc Microbiol. 2020;40(1):8–15. [Google Scholar]
- 46.Abhilash K, Veeraraghavan B, Abraham O. Epidemiology and outcome of bacteremia caused by extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in a tertiary care teaching hospital in south India. J Assoc Physicians India. 2010;58(Suppl):13–17. [PubMed] [Google Scholar]
- 47.Abolghasemi S, Madadi Z, Mardani M. Risk Factors for Resistance and Mortality in Patients with Extensively Resistant Acinetobacter Bacteremia in Taleghani Hospital in Tehran, Iran. Arch Pediatr Infect Dis. 2018;6(3). [Google Scholar]
- 48.Akhtar N, Sultan F, Nizamuddin S, Zafar W. Risk factors and clinical outcomes for vancomycin-resistant enterococcus bacteraemia in hospitalised cancer patients in Pakistan: A case-control study. J Pak Med Assoc. 2016;66(7):829–36. Epub 2016/07/19. . [PubMed] [Google Scholar]
- 49.Anggraini D, Santosaningsih D, Endraswari PD, Jasmin N, Siregar FM, Hadi U, et al. Multicenter Study of the Risk Factors and Outcomes of Bloodstream Infections Caused by Carbapenem-Non-Susceptible Acinetobacter baumannii in Indonesia. Trop Med Infect Dis. 2022;7(8):161. doi: 10.3390/tropicalmed7080161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anunnatsiri S, Tonsawan P. Risk factors and clinical outcomes of multidrug-resistant Acinetobacter baumannii bacteremia at a university hospital in Thailand. Southeast Asian J Trop Med Public Health. 2011;42(3):693–703. Epub 2011/06/29. . [PubMed] [Google Scholar]
- 51.Arias-Ortiz PM, Calderón LP, Castillo JS, Moreno J, Leal AL, Cortés JA, et al. Risk factors for methicillin-resistant Staphylococcus aureus bacteremia: A multicenter matched case-control study. Biomedica. 2016;36(4):612–9. doi: 10.7705/biomedica.v36i4.3193 PubMed Central PMCID: PMC27992988. [DOI] [PubMed] [Google Scholar]
- 52.Atmaca Ö, Köşker PZ, Karahan C, Çakir B, Ünal S. Risk factors and antibiotic use in methicillin-resistant Staphylococcus aureus Bacteremia in hospitalized patients at Hacettepe University Adult and Oncology Hospitals (2004–2011) and antimicrobial susceptibilities of the isolates: A nested case-control study. Mikrobiyol Bulteni. 2014;48(4):523–37. doi: 10.5578/mb.8280 PubMed Central PMCID: PMC25492648. [DOI] [PubMed] [Google Scholar]
- 53.Barrero LI, Castillo JS, Leal AL, Sánchez R, Cortés JA, Álvarez CA, et al. Economic burden of methicillin-resistant Staphylococcus aureus bacteremia in critical care patients in hospitals in Bogotá. Biomedica. 2014;34(3):345–53. doi: 10.7705/biomedica.v34i3.1692 PubMed Central PMCID: PMC25504122. [DOI] [PubMed] [Google Scholar]
- 54.Braga IA, Pirett CC, Ribas RM, Gontijo Filho PP, Diogo Filho A. Bacterial colonization of pressure ulcers: assessment of risk for bloodstream infection and impact on patient outcomes. J Hosp Infect. 2013;83(4):314–20. Epub 2013/01/15. doi: 10.1016/j.jhin.2012.11.008 . [DOI] [PubMed] [Google Scholar]
- 55.Castillo Londoño JS, Leal AL, Cortes JA, Alvarez CA, Sanchez R, Buitrago G, et al. Mortality among critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia: A multicenter cohort study in Colombia. Rev Panam Salud Publica Pan Am J Public Health. 2012;32(5):343–50. doi: 10.1590/S1020-49892012001100004 PubMed Central PMCID: PMC23338691. [DOI] [PubMed] [Google Scholar]
- 56.Carena AA, Laborde A, Roccia-Rossi I, Palacios CJ, Jordán R, Valledor A, et al. Proposal of a clinical score to stratify the risk of multidrug-resistant gram-negative rods bacteremia in cancer patients. Braz J Infect Dis. 2020;24(1):34–43. doi: 10.1016/j.bjid.2019.11.001 PubMed Central PMCID: PMC31851901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cetin S, Dokmetas I, Hamidi AA, Bayraktar B, Gunduz A, Sevgi DY. Comparison of risk factors and outcomes in carbapenem-resistant and carbapenem-susceptible Gram-negative bacteremia. Med Bull Sisli Etfal Hospital. 2021;55(3):398. doi: 10.14744/SEMB.2020.49002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang H, Wei J, Zhou W, Yan X, Cao X, Zuo L, et al. Risk factors and mortality for patients with Bloodstream infections of Klebsiella pneumoniae during 2014–2018: Clinical impact of carbapenem resistance in a large tertiary hospital of China. J Infect Public Health. 2020;13(5):784–90. Epub 2019/12/18. doi: 10.1016/j.jiph.2019.11.014 . [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Chen Y, Liu P, Guo P, Wu Z, Peng Y, et al. Risk factors and mortality for elderly patients with Bloodstream infection of Carbapenem resistance Klebsiella pneumoniae: a 10-year longitudinal study in China. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen R, Yan ZQ, Feng D, Luo YP, Wang LL, Shen DX. Nosocomial bloodstream infection in patients caused by Staphylococcus aureus: drug susceptibility, outcome, and risk factors for hospital mortality. Chin Med J (Engl). 2012;125(2):226–9. Epub 2012/02/22. . [PubMed] [Google Scholar]
- 61.Chusri S, Chongsuvivatwong V, Silpapojakul K, Singkhamanan K, Hortiwakul T, Charernmak B, et al. Clinical characteristics and outcomes of community and hospital-acquired Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. 2019;52(5):796–806. Epub 2019/04/30. doi: 10.1016/j.jmii.2019.03.004 . [DOI] [PubMed] [Google Scholar]
- 62.Conterno LO, Wey SB, Castelo A. Risk factors for mortality in Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 1998;19(1):32–7. Epub 1998/02/25. doi: 10.1086/647704 . [DOI] [PubMed] [Google Scholar]
- 63.Dantas RCC, Silva RTE, Ferreira ML, Gonçalves IR, Araújo BF, De Campos PA, et al. Molecular epidemiological survey of bacteremia by multidrug resistant Pseudomonas aeruginosa: The relevance of intrinsic resistance mechanisms. PLoS ONE. 2017;12(5). doi: 10.1371/journal.pone.0176774 PubMed Central PMCID: PMC28481953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deodhar D, Varghese G, Balaji V, John J, Rebekah G, Janardhanan J, et al. Prevalence of Toxin Genes among the Clinical Isolates of Staphylococcus aureus and its Clinical Impact. J Glob Infect Dis. 2015;7(3):97–102. Epub 2015/09/24. doi: 10.4103/0974-777X.162234 ; PubMed Central PMCID: PMC4557147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Oliveira Conterno L, Wey SB, Castelo A. Staphylococcus aureus bacteremia: comparison of two periods and a predictive model of mortality. Braz J Infect Dis. 2002;6(6):288–97. Epub 2003/02/15. doi: 10.1590/s1413-86702002000600004 . [DOI] [PubMed] [Google Scholar]
- 66.Deris ZZ, Shafei MN, Harun A. Risk factors and outcomes of imipenem-resistant Acinetobacter bloodstream infection in North-Eastern Malaysia. Asian Pac J Trop Biomed. 2011;1(4):313–5. Epub 2011/08/01. doi: 10.1016/S2221-1691(11)60050-6 ; PubMed Central PMCID: PMC3614228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dramowski A, Aiken AM, Rehman AM, Snyman Y, Reuter S, Grundmann H, et al. Mortality associated with third-generation cephalosporin resistance in Enterobacteriaceae bloodstream infections at one South African hospital. J Glob Antimicrob Resist. 2022;29:176–184. doi: 10.1016/j.jgar.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durdu B, Hakyemez IN, Bolukcu S, Okay G, Gultepe B, Aslan T. Mortality markers in nosocomial Klebsiella pneumoniae bloodstream infection. Springerplus. 2016;5(1):1892. Epub 2016/11/16. doi: 10.1186/s40064-016-3580-8 ; PubMed Central PMCID: PMC5084144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ergönül Ö, Aydin M, Azap A, Başaran S, Tekin S, Kaya Ş, et al. Healthcare-associated Gram-negative bloodstream infections: antibiotic resistance and predictors of mortality. J Hosp Infect. 2016;94(4):381–5. Epub 2016/11/03. doi: 10.1016/j.jhin.2016.08.012 . [DOI] [PubMed] [Google Scholar]
- 70.Ferreira AM, Moreira F, Guimaraes T, Spadão F, Ramos JF, Batista MV, et al. Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: importance of previous gut colonization. J Hosp Infect. 2018;100(1):83–91. Epub 2018/03/14. doi: 10.1016/j.jhin.2018.03.004 . [DOI] [PubMed] [Google Scholar]
- 71.Fu Q, Ye H, Liu S. Risk factors for extensive drug-resistance and mortality in geriatric inpatients with bacteremia caused by Acinetobacter baumannii. Am J Infect Control. 2015;43(8):857–60. Epub 2015/05/12. doi: 10.1016/j.ajic.2015.03.033 . [DOI] [PubMed] [Google Scholar]
- 72.Furtado GHC, Mendes RE, Campos Pignatari AC, Wey SB, Medeiros EAS. Risk factors for vancomycin-resistant Enterococcus faecalis bacteremia in hospitalized patients: An analysis of two case-control studies. Am J Infect Control. 2006;34(7):447–51. doi: 10.1016/j.ajic.2005.08.015 PubMed Central PMCID: PMC16945692. [DOI] [PubMed] [Google Scholar]
- 73.Garnica M, Maiolino A, Nucci M. Factors associated with bacteremia due to multidrug-resistant Gram-negative bacilli in hematopoietic stem cell transplant recipients. Braz J Med Biol Res. 2009;42(3):289–93. doi: 10.1590/s0100-879x2009000300010 PubMed Central PMCID: PMC19287908. [DOI] [PubMed] [Google Scholar]
- 74.Gaytán JJA, Mancilla GC, Meza HAR, Padilla PAV, González CYA, Lara CEG. Tendency of resistance to ciprofloxacin in bacteriemias due to Escherichia coli. Med Interna Mex. 2006;22(5):386–390. [Google Scholar]
- 75.Ghafur AK, Vidyalakshmi PR, Kannaian P, Balasubramaniam R. Clinical study of carbapenem sensitive and resistant Gram-negative bacteremia in neutropenic and nonneutropenic patients: The first series from India. Indian J Cancer. 2014;51(4):453–5. doi: 10.4103/0019-509X.175362 PubMed Central PMCID: PMC26842159. [DOI] [PubMed] [Google Scholar]
- 76.Goda R, Sharma R, Borkar SA, Katiyar V, Narwal P, Ganeshkumar A, et al. Frailty and Neutrophil Lymphocyte Ratio as Predictors of Mortality in Patients with Catheter-Associated Urinary Tract Infections or Central Line–Associated Bloodstream Infections in the Neurosurgical Intensive Care Unit: Insights from a Retrospective Study in a Developing Country. World Neurosurgery. 2022;162:e187–e197. doi: 10.1016/j.wneu.2022.02.115 [DOI] [PubMed] [Google Scholar]
- 77.González AL, Leal AL, Cortés JA, Sánchez R, Barrero LI, Castillo JS, et al. [Effect of adequate initial antimicrobial therapy on mortality in critical patients with Pseudomonas aeruginosa bacteremia]. Biomedica. 2014;34(Suppl 1):58–66. Epub 2014/06/27. doi: 10.1590/s0120-41572014000500008 . [DOI] [PubMed] [Google Scholar]
- 78.Guo N, Xue W, Tang D, Ding J, Zhao B. Risk factors and outcomes of hospitalized patients with blood infections caused by multidrug-resistant Acinetobacter baumannii complex in a hospital of Northern China. Am J Infect Control. 2016;44(4):e37–9. Epub 2016/01/26. doi: 10.1016/j.ajic.2015.11.019 . [DOI] [PubMed] [Google Scholar]
- 79.Islas-Muñoz B, Volkow-Fernández P, Ibanes-Gutiérrez C, Villamar-Ramírez A, Vilar-Compte D, Cornejo-Juárez P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int J Infect Dis. 2018;71:59–64. Epub 2018/04/13. doi: 10.1016/j.ijid.2018.03.022 . [DOI] [PubMed] [Google Scholar]
- 80.Jafari S, Abdollahi A, Sabahi M, Salehi M, Asadollahi-Amin A, Hasannezhad M, et al. An Update to Enterococcal Bacteremia: Epidemiology, Resistance, and Outcome. Infect Disord Drug Targets. 2020. [DOI] [PubMed] [Google Scholar]
- 81.Jamulitrat S, Pranee Arunpan RN, Parichart Phainuphong RN. Attributable mortality of imipenem-resistant nosocomial Acinetobacter baumannii bloodstream infection. J Med Assoc Thailand. 2009;92(3):413–9. PubMed Central PMCID: PMC19301737. [PubMed] [Google Scholar]
- 82.Kalam K, Qamar F, Kumar S, Ali S, Baqi S. Risk factors for carbapenem resistant bacteraemia and mortality due to gram negative bacteraemia in a developing country. J Pak Med Assoc. 2014;64(5):530–6. Epub 2014/10/03. . [PubMed] [Google Scholar]
- 83.Li H, Zheng Y, Yang X, Zhang P, Xiao W, Yang M. Clinical characteristics and prognosis of carbapenem-resistant klebsiella pneumoniae infection of critical patients. Chin J Evid Based Med. 2019;19(2):129–134. doi: 10.7507/1672-2531.201809113 [DOI] [Google Scholar]
- 84.Li L, Huang H. Risk factors of mortality in bloodstream infections caused by Klebsiella pneumonia: A single-center retrospective study in China. Medicine (Baltimore). 2017;96(35):e7924. Epub 2017/09/01. doi: 10.1097/MD.0000000000007924 ; PubMed Central PMCID: PMC5585510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S, Jia X, Li C, Zou H, Liu H, Guo Y, et al. Carbapenem-resistant and cephalosporin-susceptible pseudomonas aeruginosa: A notable phenotype in patients with bacteremia. Infect Drug Resist. 2018;11:1225–1235. doi: 10.2147/IDR.S174876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li X, Ye H. Clinical and Mortality Risk Factors in Bloodstream Infections with Carbapenem-Resistant Enterobacteriaceae. Can J Infect Dis Med Microbiol. 2017;2017:6212910. Epub 2018/01/31. doi: 10.1155/2017/6212910 ; PubMed Central PMCID: PMC5742906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Li J, Hu T, Hu J, Song N, Zhang Y, et al. Five-year change of prevalence and risk factors for infection and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary hospital in North China. Antimicrob Resist Infect Control. 2020;9(1):79. Epub 2020/06/04. doi: 10.1186/s13756-020-00728-3 ; PubMed Central PMCID: PMC7268443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim C, Takahashi E, Hongsuwan M, Wuthiekanun V, Thamlikitkul V, Hinjoy S, et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife. 2016;5(September). doi: 10.7554/eLife.18082 PubMed Central PMCID: PMC27599374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lima EM, Cid PA, Beck DS, Pinheiro LHZ, Tonhá JPS, Alves MZO, et al. Predictive factors for sepsis by carbapenem resistant Gram-negative bacilli in adult critical patients in Rio de Janeiro: A case-case-control design in a prospective cohort study. Antimicrob Resist Infect Control. 2020;9(1). doi: 10.1186/s13756-020-00791-w PubMed Central PMCID: PMC32795380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lipari FG, Hernández D, Vilaró M, Caeiro JP, Saka HA. Clinical, epidemiological and microbiological characterization of bacteremia produced by carbapenem-resistant enterobacteria in a university hospital in Córdoba, Argentina. Rev Chil Infectol. 2020;37(4):362–70. doi: 10.4067/S0716-10182020000400362 PubMed Central PMCID: PMC33399656. [DOI] [PubMed] [Google Scholar]
- 91.Liu J, Wang H, Huang Z, Tao X, Li J, Hu Y, et al. Risk factors and outcomes for carbapenem-resistant Klebsiella pneumoniae bacteremia in onco-hematological patients. J Infect Dev Ctries. 2019;13(5):357–64. Epub 2020/02/14. doi: 10.3855/jidc.11189 . [DOI] [PubMed] [Google Scholar]
- 92.Liu Q, Li W, Du X, Li W, Zhong T, Tang Y, et al. Risk and prognostic factors for multidrug-resistant Acinetobacter baumannii complex bacteremia: A retrospective study in a tertiary hospital of West China. PLoS ONE. 2015;10(6). doi: 10.1371/journal.pone.0130701 PubMed Central PMCID: PMC26083415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Q, Wu J, Wang Z, Wu X, Wang G, Ren J. Polymicrobial Bacteremia Involving Klebsiella pneumoniae in Patients with Complicated Intra-Abdominal Infections: Frequency, Co-Pathogens, Risk Factors, and Clinical Outcomes. Surg Infect (Larchmt). 2019;20(4):317–25. Epub 2019/02/09. doi: 10.1089/sur.2018.207 . [DOI] [PubMed] [Google Scholar]
- 94.Liu Y, Wang Q, Zhao C, Chen H, Li H, Wang H, et al. Prospective multi-center evaluation on risk factors, clinical characteristics and outcomes due to carbapenem resistance in Acinetobacter baumannii complex bacteraemia: experience from the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections (CARES) Network. J Med Microbiol. 2020;69(7):949–59. Epub 2020/06/26. doi: 10.1099/jmm.0.001222 . [DOI] [PubMed] [Google Scholar]
- 95.Loftus MJ, Young-Sharma TE, Lee SJ, Wati S, Badoordeen GZ, Blakeway LV, et al. Attributable mortality and excess length of stay associated with third-generation cephalosporin-resistant Enterobacterales bloodstream infections: a prospective cohort study in Suva, Fiji. J Glob Antimicrob Resist. 2022;30:286–293. doi: 10.1016/j.jgar.2022.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.López-Luis BA, Sifuentes-Osornio J, Lambraño-Castillo D, Ortiz-Brizuela E, Ramírez-Fontes A, Tovar-Calderón YE, et al. Risk factors and outcomes associated with vancomycin-resistant Enterococcus faecium and ampicillin-resistant Enterococcus faecalis bacteraemia: A 10-year study in a tertiary-care centre in Mexico City. J Glob Antimicrob Resist. 2020;24:198–204. Epub 2020/12/29. doi: 10.1016/j.jgar.2020.12.005 . [DOI] [PubMed] [Google Scholar]
- 97.Ma J, Li N, Liu Y, Wang C, Liu X, Chen S, et al. Antimicrobial resistance patterns, clinical features, and risk factors for septic shock and death of nosocomial e coli bacteremia in adult patients with hematological disease. Medicine. 2017;96(21). doi: 10.1097/MD.0000000000006959 PubMed Central PMCID: PMC28538389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marra AR, Wey SB, Castelo A, Gales AC, Cal RG, Filho JR, et al. Nosocomial bloodstream infections caused by Klebsiella pneumoniae: impact of extended-spectrum beta-lactamase (ESBL) production on clinical outcome in a hospital with high ESBL prevalence. BMC Infect Dis. 2006;6:24. Epub 2006/02/16. doi: 10.1186/1471-2334-6-24 ; PubMed Central PMCID: PMC1382232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menekşe Ş, Çağ Y, Işık ME, Şahin S, Hacıseyitoğlu D, Can F, et al. The effect of colistin resistance and other predictors on fatality among patients with bloodstream infections due to Klebsiella pneumoniae in an OXA-48 dominant region. Int J Infect Dis. 2019;86:208–11. doi: 10.1016/j.ijid.2019.06.008 PubMed Central PMCID: PMC31402295. [DOI] [PubMed] [Google Scholar]
- 100.Metan G, Sariguzel F, Sumerkan B. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur J Intern Med. 2009;20(5):540–4. doi: 10.1016/j.ejim.2009.05.005 PubMed Central PMCID: PMC19712862. [DOI] [PubMed] [Google Scholar]
- 101.Moghnieh R, Estaitieh N, Mugharbil A, Jisr T, Abdallah DI, Ziade F, et al. Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Front Cell Infect Microbiol. 2015;5(FEB). doi: 10.3389/fcimb.2015.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreira M, Medeiros EA, Pignatari AC, Wey SB, Cardo DM. [Effect of nosocomial bacteremia caused by oxacillin-resistant Staphylococcus aureus on mortality and length of hospitalization]. Rev Assoc Med Bras (1992). 1998;44(4):263–8. Epub 1998/12/16. doi: 10.1590/s0104-42301998000400002 . [DOI] [PubMed] [Google Scholar]
- 103.Najmi A, Karimi F, Kunhikatta V, Varma M, Nair S. Resistance Trend, Antibiotic Utilization and Mortality in Patients with E. coli Bacteraemia. Open Access Maced J Med Sci. 2019;7(7):1119–23. Epub 2019/05/03. doi: 10.3889/oamjms.2019.223 ; PubMed Central PMCID: PMC6490482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niu T, Xiao T, Guo L, Yu W, Chen Y, Zheng B, et al. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: Cefoperazone–sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–2030. doi: 10.2147/IDR.S169432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palavutitotai N, Jitmuang A, Tongsai S, Kiratisin P, Angkasekwinai N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS ONE. 2018;13(2). doi: 10.1371/journal.pone.0193431 PubMed Central PMCID: PMC29470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Porto JP, Santos RO, Gontijo Filho PP, Ribas RM. Active surveillance to determine the impact of methicillin resistance on mortality in patients with bacteremia and influences of the use of antibiotics on the development of MRSA infection. Rev Soc Bras Med Trop. 2013;46(6):713–8. Epub 2014/01/30. doi: 10.1590/0037-8682-0199-2013 . [DOI] [PubMed] [Google Scholar]
- 107.Rao C, Dhawan B, Vishnubhatla S, Kapil A, Das B, Sood S. Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium bacteremia from an Indian tertiary hospital. Eur J Clin Microbiol Infect Dis. 2021;40(2):303–14. doi: 10.1007/s10096-020-04030-3 PubMed Central PMCID: PMC32909085. [DOI] [PubMed] [Google Scholar]
- 108.Seboxa T, Amogne W, Abebe W, Tsegaye T, Azazh A, Hailu W, et al. High Mortality from Blood Stream Infection in Addis Ababa, Ethiopia, Is Due to Antimicrobial Resistance. PLoS ONE. 2015;10(12):e0144944. Epub 2015/12/17. doi: 10.1371/journal.pone.0144944 ; PubMed Central PMCID: PMC4682922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Serefhanoglu K, Turan H, Timurkaynak FE, Arslan H. Bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae: Risk factors for multidrug-resistance. Braz J Infect Dis. 2009;13(6):403–7. doi: 10.1590/s1413-86702009000600003 PubMed Central PMCID: PMC20464329. [DOI] [PubMed] [Google Scholar]
- 110.Shi SH, Kong HS, Xu J, Zhang WJ, Jia CK, Wang WL, et al. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transplant Infect Dis. 2009;11(5):405–12. doi: 10.1111/j.1399-3062.2009.00421.x PubMed Central PMCID: PMC19638006. [DOI] [PubMed] [Google Scholar]
- 111.Shi N, Kang J, Wang S, Song Y, Yin D, Li X, et al. Bacteriological Profile and Antimicrobial Susceptibility Patterns of Gram-Negative Bloodstream Infection and Risk Factors Associated with Mortality and Drug Resistance: A Retrospective Study from Shanxi, China. Infect Drug Resist. 2022;15:3561. doi: 10.2147/IDR.S370326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sirijatuphat R, Sripanidkulchai K, Boonyasiri A, Rattanaumpawan P, Supapueng O, Kiratisin P, et al. Implementation of global antimicrobial resistance surveillance system (GLASS) in patients with bacteremia. PLoS ONE. 2018;13(1). doi: 10.1371/journal.pone.0190132 PubMed Central PMCID: PMC29298323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Moraes LS, Magalhaes GLG, Soncini JGM, Pelisson M, Perugini MRE, Vespero EC. High mortality from carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Microb Pathog. 2022;167:105519. doi: 10.1016/j.micpath.2022.105519 [DOI] [PubMed] [Google Scholar]
- 114.Steinhaus N, Al-Talib M, Ive P, Boyles T, Bamford C, Davies MA, et al. The management and outcomes of Staphylococcus aureus bacteraemia at a South African referral hospital: A prospective observational study. Int J Infect Dis. 2018;73:78–84. Epub 2018/06/17. doi: 10.1016/j.ijid.2018.06.004 . [DOI] [PubMed] [Google Scholar]
- 115.Stewardson AJ, Marimuthu K, Sengupta S, Allignol A, El-Bouseary M, Carvalho MJ, et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19(6):601–10. Epub 2019/05/03. doi: 10.1016/S1473-3099(18)30792-8 . [DOI] [PubMed] [Google Scholar]
- 116.Stoma I, Karpov I, Milanovich N, Uss A, Iskrov I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016;51(2):102–6. Epub 2016/07/07. doi: 10.5045/br.2016.51.2.102 ; PubMed Central PMCID: PMC4931927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang Y, Xu C, Xiao H, Wang L, Cheng Q, Li X. Gram-negative bacteria bloodstream infections in patients with hematological malignancies–the impact of pathogen type and patterns of antibiotic resistance: a Retrospective Cohort Study. Infect Drug Resist. 2021;14:3115. doi: 10.2147/IDR.S322812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tian L, Tan R, Chen Y, Sun J, Liu J, Qu H, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48. Epub 2016/11/29. doi: 10.1186/s13756-016-0145-0 ; PubMed Central PMCID: PMC5114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Topeli A, Unal S, Akalin HE. Risk factors influencing clinical outcome in Staphylococcus aureus bacteraemia in a Turkish University Hospital. Int J Antimicrob Agents. 2000;14(1):57–63. Epub 2000/03/16. doi: 10.1016/s0924-8579(99)00147-8 . [DOI] [PubMed] [Google Scholar]
- 120.Traverso F, Peluffo M, Louge M, Funaro F, Suasnabar R, Cepeda R. [Impact of methicillin resistance on mortality and surveillance of vancomycin susceptibility in bacteremias caused by Staphylococcus aureus]. Rev Argent Microbiol. 2010;42(4):274–8. Epub 2011/01/14. doi: 10.1590/s0325-75412010000400007 . [DOI] [PubMed] [Google Scholar]
- 121.Tu B, Bi J, Wu D, Zhao P, Shi L, Xie Y, et al. Bloodstream infection due to Escherichia coli in liver cirrhosis patients: clinical features and outcomes. Oncotarget. 2018;9(87):35780–9. Epub 2018/12/06. doi: 10.18632/oncotarget.23200 ; PubMed Central PMCID: PMC6254670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tuon FF, Gortz LW, Rocha JL. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. Braz J Infect Dis. 2012;16(4):351–6. doi: 10.1016/j.bjid.2012.06.009 PubMed Central PMCID: PMC22846123. [DOI] [PubMed] [Google Scholar]
- 123.Valderrama SL, González PF, Caro MA, Ardila N, Ariza B, Gil F, et al. Risk factors for hospital-acquired bacteremia due to carbapenem-resistant Pseudomonas aeruginosa in a Colombian hospital. Biomedica. 2016;36:69–77. doi: 10.7705/biomedica.v36i2.2784 PubMed Central PMCID: PMC27622627. [DOI] [PubMed] [Google Scholar]
- 124.Wang Q, Zhang Y, Yao X, Xian H, Liu Y, Li H, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis. 2016;35(10):1679–89. Epub 2016/07/13. doi: 10.1007/s10096-016-2710-0 . [DOI] [PubMed] [Google Scholar]
- 125.Wang Z, Qin RR, Huang L, Sun LY. Risk Factors for Carbapenem-resistant Klebsiella pneumoniae Infection and Mortality of Klebsiella pneumoniae Infection. Chin Med J (Engl). 2018;131(1):56–62. Epub 2017/12/23. doi: 10.4103/0366-6999.221267 ; PubMed Central PMCID: PMC5754959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei J, Zhu QL, Sun Z, Wang C. [The impact of carbapenem-resistance Pseudomonas aeruginosa infections on mortality of patients with hematological disorders]. Zhonghua Nei Ke Za Zhi. 2020;59(5):353–9. Epub 2020/05/07. doi: 10.3760/cma.j.cn112138-20191104-00728 . [DOI] [PubMed] [Google Scholar]
- 127.Wu X, Shi Q, Shen S, Huang C, Wu H. Clinical and bacterial characteristics of Klebsiella pneumoniae affecting 30-day mortality in patients with bloodstream infection. Front Cell Infect Microbiol. 2021;11. doi: 10.3389/fcimb.2021.688989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao T, Yu W, Niu T, Huang C, Xiao Y. A retrospective, comparative analysis of risk factors and outcomes in carbapenem-susceptible and carbapenem-nonsusceptible Klebsiella pneumoniae bloodstream infections: tigecycline significantly increases the mortality. Infect Drug Resist. 2018;11:595–606. Epub 2018/05/08. doi: 10.2147/IDR.S153246 ; PubMed Central PMCID: PMC5926074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiao T, Zhu Y, Zhang S, Wang Y, Shen P, Zhou Y, et al. A Retrospective Analysis of Risk Factors and Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Bacteremia in Nontransplant Patients. J Infect Dis. 2020;221(Suppl 2):S174–s83. Epub 2020/03/17. doi: 10.1093/infdis/jiz559 . [DOI] [PubMed] [Google Scholar]
- 130.Xie Y, Tu B, Zhang X, Bi J, Shi L, Zhao P, et al. Investigation on outcomes and bacterial distributions of liver cirrhosis patients with gram-negative bacterial bloodstream infection. Oncotarget. 2018;9(3):3980–95. Epub 2018/02/10. doi: 10.18632/oncotarget.23582 ; PubMed Central PMCID: PMC5790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu X, Wu S, Xie Y, Chen Z, Ma Y, He C, et al. Risk factors of bloodstream infections caused by vancomycin-resistant Enterococcus. Chin J Infect Chemother. 2015;15(5):447–451. [Google Scholar]
- 132.Yang S, Sun J, Wu X, Zhang L. Determinants of Mortality in Patients with Nosocomial Acinetobacter baumannii Bacteremia in Southwest China: A Five-Year Case-Control Study. Can J Infect Dis Med Microbiol. 2018;2018:3150965. Epub 2018/07/06. doi: 10.1155/2018/3150965 ; PubMed Central PMCID: PMC6008754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang K, Xiao T, Shi Q, Zhu Y, Ye J, Zhou Y, et al. Socioeconomic burden of bloodstream infections caused by carbapenem-resistant and carbapenem-susceptible Pseudomonas aeruginosa in China. J Glob Antimicrob Resist. 2021;26:101–107. doi: 10.1016/j.jgar.2021.03.032 [DOI] [PubMed] [Google Scholar]
- 134.Ye QF, Zhao J, Wan QQ, Qiao BB, Zhou JD. Frequency and clinical outcomes of ESKAPE bacteremia in solid organ transplantation and the risk factors for mortality. Transpl Infect Dis. 2014;16(5):767–74. Epub 2014/08/16. doi: 10.1111/tid.12278 . [DOI] [PubMed] [Google Scholar]
- 135.Yilmaz M, Elaldi N, Balkan İ, Arslan F, Batırel AA, Bakıcı MZ, et al. Mortality predictors of Staphylococcus aureus bacteremia: a prospective multicenter study. Ann Clin Microbiol Antimicrob. 2016;15:7. Epub 2016/02/11. doi: 10.1186/s12941-016-0122-8 ; PubMed Central PMCID: PMC4748515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan Y, Wang J, Yao Z, Ma B, Li Y, Yan W, et al. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections and Outcomes. Infect Drug Resist. 2020;13:207–15. Epub 2020/03/12. doi: 10.2147/IDR.S223243 ; PubMed Central PMCID: PMC6985980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang G, Zhang M, Sun F, Zhou J, Wang Y, Zhu D, et al. Epidemiology, mortality and risk factors for patients with K. pneumoniae bloodstream infections: Clinical impact of carbapenem resistance in a tertiary university teaching hospital of Beijing. J Infect Public Health. 2020;13(11):1710–4. Epub 2020/10/22. doi: 10.1016/j.jiph.2020.09.012 . [DOI] [PubMed] [Google Scholar]
- 138.Zhang Q, Gao HY, Li D, Li Z, Qi SS, Zheng S, et al. Clinical outcome of Escherichia coli bloodstream infection in cancer patients with/without biofilm formation: a single-center retrospective study. Infect Drug Resist. 2019;12:359–71. Epub 2019/02/28. doi: 10.2147/IDR.S192072 ; PubMed Central PMCID: PMC6377049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Q, Zhang W, Li Z, Bai C, Li D, Zheng S, et al. Bacteraemia due to AmpC β-lactamase-producing Escherichia coli in hospitalized cancer patients: risk factors, antibiotic therapy, and outcomes. Diagn Microbiol Infect Dis. 2017;88(3):247–51. Epub 2017/04/25. doi: 10.1016/j.diagmicrobio.2017.04.006 . [DOI] [PubMed] [Google Scholar]
- 140.Zhang Y, Du M, Chang Y, Chen LA, Zhang Q. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp. bloodstream infections in a tertiary-care hospital in Beijing, China: a four-year retrospective study. Antimicrob Resist Infect Control. 2017;6:73. Epub 2017/07/07. doi: 10.1186/s13756-017-0231-y ; PubMed Central PMCID: PMC5496248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Li Y, Zeng J, Chang Y, Han S, Zhao J, et al. Risk Factors for Mortality of Inpatients with Pseudomonas aeruginosa Bacteremia in China: Impact of Resistance Profile in the Mortality. Infect Drug Resist. 2020;13:4115–23. Epub 2020/11/20. doi: 10.2147/IDR.S268744 ; PubMed Central PMCID: PMC7669529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao S, Wu Y, Dai Z, Chen Y, Zhou X, Zhao J. Risk factors for antibiotic resistance and mortality in patients with bloodstream infection of Escherichia coli. Eur J Clin Microbiol Infect Dis. 2022;41(5):713–721. doi: 10.1007/s10096-022-04423-6 [DOI] [PubMed] [Google Scholar]
- 143.Zhao Y, Lin Q, Liu L, Ma R, Chen J, Shen Y, et al. Risk Factors and Outcomes of Antibiotic-resistant Pseudomonas aeruginosa Bloodstream Infection in Adult Patients With Acute Leukemia. Clin Infect Dis. 2020;71(Supplement_4):S386–s93. Epub 2020/12/29. doi: 10.1093/cid/ciaa1522 . [DOI] [PubMed] [Google Scholar]
- 144.Zheng SH, Cao SJ, Xu H, Feng D, Wan LP, Wang GJ, et al. Risk factors, outcomes and genotypes of carbapenem-nonsusceptible Klebsiella pneumoniae bloodstream infection: a three-year retrospective study in a large tertiary hospital in Northern China. Infect Dis (Lond). 2018;50(6):443–51. Epub 2018/01/06. doi: 10.1080/23744235.2017.1421772 . [DOI] [PubMed] [Google Scholar]
- 145.Zheng X, Wang JF, Xu WL, Xu J, Hu J. Clinical and molecular characteristics, risk factors and outcomes of Carbapenem-resistant Klebsiella pneumoniae bloodstream infections in the intensive care unit. Antimicrob Resist Infect Control. 2017;6:102. Epub 2017/10/14. doi: 10.1186/s13756-017-0256-2 ; PubMed Central PMCID: PMC5625719 Institutional Review Board of the First Affiliated Hospital, College of Medicine, Zhejiang University. This research was conducted in compliance with the tenets of the Helsinki Declaration. CONSENT FOR PUBLICATION: Not applicable. COMPETING INTERESTS: The authors declare that they have no competing interests. PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou H, Yao Y, Zhu B, Ren D, Yang Q, Fu Y, et al. Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: A retrospective study from a Chinese hospital. Medicine (Baltimore). 2019;98(13):e14937. Epub 2019/03/29. doi: 10.1097/MD.0000000000014937 ; PubMed Central PMCID: PMC6456023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu C, Liu C, Wu B, Wu Q, Huang D. Analysis of antibiotic resistance in the staphylococcus aureus strains isolated from bloodstream infections and associated patient outcome. Chin J Infect Chemother. 2016;16 (1):1–4. doi: 10.16718/j.1009-7708.2016.01.001 [DOI] [Google Scholar]
- 148.Zhu Y, Xiao T, Wang Y, Yang K, Zhou Y, Luo Q, et al. Socioeconomic Burden of Bloodstream Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Infect Drug Resist. 2021;14:5385. doi: 10.2147/IDR.S341664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, et al. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp Ther Med. 2018;16(6):4499–4510. doi: 10.3892/etm.2018.6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zou XL, Feng DY, Wu WB, Yang HL, Zhang TT. Blood urea nitrogen to serum albumin ratio independently predicts 30-day mortality and severity in patients with Escherichia coli bacteraemia. Med Clin (Barc). 2020. Epub 2020/10/17. doi: 10.1016/j.medcli.2020.06.060 . [DOI] [PubMed] [Google Scholar]
- 151.Zhang WL, Huang J, Wu SY, Liu Y, Long F, Xiao YL, et al. [Antibiotic Resistance and Risk Factors for Mortality of Blood Stream Infections (BSIs) with Escherichia coli in Patients with Hematological Malignancies]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49(1):133–5. Epub 2018/05/08. . [PubMed] [Google Scholar]
- 152.Zhang Y, Zhu W, Zhang J, Chen B. The risk factors associated with bloodstream infections caused by multi-drug resistant acinetobacter baumannii. Chin J Infect Chemother. 2017;17(2):134–139. doi: 10.16718/j.1009-7708.2017.02.003 [DOI] [Google Scholar]
- 153.Jit M, Ng DHL, Luangasanatip N, Sandmann F, Atkins KE, Robotham JV, et al. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: rapid methodological review, conceptual framework and recommendations for future studies. BMC Med. 2020;18(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y, Chen X-L, Huang A-W, Liu S-L, Liu W-J, Zhang N, et al. Mortality attributable to carbapenem-resistant Pseudomonas aeruginosa bacteremia: a meta-analysis of cohort studies. Emerg Microbes Infect. 2016;5(1):1–6. doi: 10.1038/emi.2016.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paul M, Weinberger M, Siegman-Igra Y, Lazarovitch T, Ostfeld I, Boldur I, et al. Acinetobacter baumannii: emergence and spread in Israeli hospitals 1997–2002. J Hosp Infect. 2005;60(3):256–260. doi: 10.1016/j.jhin.2005.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chopra T, Marchaim D, Awali RA, Krishna A, Johnson P, Tansek R, et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. 2013;57(12):6270–6275. doi: 10.1128/AAC.01520-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca-Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017. doi: 10.1093/cid/cix411 [DOI] [PubMed] [Google Scholar]
- 159.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Supplement_2):S82–S89. doi: 10.1086/499406 [DOI] [PubMed] [Google Scholar]
- 160.Tsuzuki S, Yu J, Matsunaga N, Ohmagari N. Length of stay, hospitalisation costs and in-hospital mortality of methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia in Japan. Public Health. 2021;198:292–296. doi: 10.1016/j.puhe.2021.07.046 [DOI] [PubMed] [Google Scholar]
- 161.Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother. 2002;49(6):999–1005. doi: 10.1093/jac/dkf009 [DOI] [PubMed] [Google Scholar]
- 162.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x [DOI] [PubMed] [Google Scholar]
- 163.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- 164.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qu J, Huang Y, Lv X. Crisis of antimicrobial resistance in China: now and the future. Front Microbiol. 2019;10:2240. doi: 10.3389/fmicb.2019.02240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gulen TA, Guner R, Celikbilek N, Keske S, Tasyaran M. Clinical importance and cost of bacteremia caused by nosocomial multi drug resistant Acinetobacter baumannii. Int J Infect Dis. 2015;38:32–35. doi: 10.1016/j.ijid.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 167.Huang W, Qiao F, Zhang Y, Huang J, Deng Y, Li J, et al. In-hospital medical costs of infections caused by carbapenem-resistant Klebsiella pneumoniae. Clin Infect Dis. 2018;67(suppl_2):S225–S230. doi: 10.1093/cid/ciy642 [DOI] [PubMed] [Google Scholar]
- 168.World Health Organization. Sustainable Development Goals (SDGs) AMR indicator 2022. [cited 2022 Mar 29]. Available from: https://www.who.int/data/gho/data/themes/topics/global-antimicrobial-resistance-surveillance-system-glass/sustainable-development-goals-amr-indicator. [Google Scholar]
- 169.MAAP: Mapping AMR and AMU partnership. Incomplete antimicrobial resistance (AMR) data in Africa: The crisis within the crisis. 2022. [Google Scholar]
- 170.de Kraker ME, Lipsitch M. Burden of antimicrobial resistance: compared to what? Epidemiol Rev. 2021;43(1):53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pezzani MD, Tornimbene B, Pessoa-Silva C, de Kraker M, Rizzardo S, Salerno ND, et al. Methodological quality of studies evaluating the burden of drug-resistant infections in humans due to the WHO Global Antimicrobial Resistance Surveillance System target bacteria. Clin Microbiol Infect. 2021;27(5):687–696. doi: 10.1016/j.cmi.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.De Angelis G, Murthy A, Beyersmann J, Harbarth S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin Microbiol Infect. 2010;16(12):1729–1735. doi: 10.1111/j.1469-0691.2010.03332.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text A. Search criteria used by search engine. Table A. Studies inclusion and exclusion criteria. Table B. Years of the studies included. Table C. Number of studies included by WHO region and WB income group. Table D. Correlation between main outcomes and demographic variables. Table E. Most prevalent bacterium family, Gram type, resistance type, and antibiotic-bacterium pair by country among the included studies. Table F. Descriptive statistics of the studies included in the meta-analysis. Table G. Summary of the subgroup meta-analysis results for income level and WHO region by outcome variable. Table H. Costs of hospital bed-day per patient and by country and hospital level (in 2008 USDs). Table I. Costs of total excess hospital bed-days per patient by country and hospital level using estimated SMD and their respective 95% CIs (in 2008 USDs). Table J. Costs of total excess hospital bed-days per patient and by country and hospital level using estimated SMD and their respective 95% CIs (inflated to 2020 USDs). Table K. Calculation of YPLL, YPPLL, and CPL, by country. Table L. Total productivity losses due to premature mortality costs by country using the LE at the age of death and productivity cost approach (age of retirement), discounted. Table M. Intensive care unit costs per patient (daily). Table N. Intensive care unit costs (per patient and daily) adjusted to 2020 USDs (inflated accordingly). Table O. Intensive care unit costs (per day/patient) adjusted to ICU LOS and reported in 2020 USDs (inflated accordingly). Table P. Total excess costs incurred for bloodstream infections caused by antibiotic-resistant bacteria, per patient. Table Q. Statistics calculated for meta-analysis using mortality as an outcome, by model. Table R. Statistics calculated for meta-analysis using ICU admission as an outcome, by model. Table S. Statistics calculated for meta-analysis using the length of stay at hospital as an outcome, by model. Table T. Summary of the subgroup meta-analysis results for specific antibiotic-bacterium combinations declared important by the WHO, by outcome variable. Table U. Meta-analysis subgroup results for bacterium family, and Gram type for those studies carried out in China and other than China, by outcome. Table V. Summary results of meta-analysis results for critical antibiotic-bacterium pathogens for those studies in China and other than China, by outcome. Table W. Meta-regression results for the mortality outcome (univariate and multivariable). Table X. Meta-regression results for the ICU admission outcome (univariate and multivariate). Table Y. Summary results of the meta-analysis for the main outcome variables by separating the studies for low- [LS] and high-scores [HS] obtained from the MASTER scale. Table Z. Checklist of information that should be included in new reports of global health estimates. Table AA. PRISMA Checklist. Fig A. Density of the studies over time. Fig B. Violin and kernel density estimate plots for the main outcomes and by ARB susceptibility. Fig C. Relationship between the main outcomes. Fig D. Meta-analysis using all the studies reporting mortality rates. Fig E. Subgroup meta-analysis using all the studies reporting mortality rates/odds for critical (N = 72) and high-priority (N = 22) pathogens according to the WHO criteria. Fig F. Subgroup meta-analysis using all the studies reporting mortality rates by bacterium’s family name. Fig G. Subgroup meta-analysis using all the studies reporting mortality rates by WHO Region. Fig H. Subgroup meta-analysis using all the studies reporting mortality rates by income level. Fig I. Meta-analysis results using all the studies reporting the mean and SD for the length of stay at the hospital. Fig J. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital for critical and high-priority pathogens according to the WHO. Fig K. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital for Enterococcus spp., Enterobacteriaceae, Moraxellaceae, Pseudomonadaceae, and Staphyloccocaceae. Fig L. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital by income level. Fig M. Subgroup meta-analysis using all the studies reporting the mean and SD for the length of stay at the hospital by WHO region. Fig N. Meta-analysis results using all the studies reporting ICU admission rates. Fig O. Subgroup meta-analysis using all the studies reporting ICU admission rates for critical pathogens according to the WHO criteria. Fig P. Subgroup meta-analysis using all the studies reporting ICU admission rates for high-priority pathogens according to the WHO criteria. Fig Q. Subgroup meta-analysis using all the studies reporting ICU admission rates for Enterobacteriaceae. Fig R. Subgroup meta-analysis using all the studies reporting ICU admission rates for Enterobacteriaceae. Fig S. Subgroup meta-analysis using all the studies reporting ICU admission rates for Moraxellaceae. Fig T. Subgroup meta-analysis using all the studies reporting ICU admission rates for Pseudomonadaceae. Fig U. Subgroup meta-analysis using all the studies reporting ICU admission rates for Staphylococcaceae. Fig V. Subgroup meta-analysis using all the studies reporting ICU admission rates by resistance type (ESBL+). Fig W. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Americas. Fig X. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Eastern Mediterranean. Fig Y. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Europe. Fig Z. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Southeast Asia. Fig AA. Subgroup meta-analysis using all the studies reporting ICU admission rates by WHO region: Western Pacific region. Fig AB. Subgroup meta-analysis using all the studies reporting ICU admission rates by income level: Low and lower-middle income countries. Fig AC. Subgroup meta-analysis using all the studies reporting ICU admission rates by income level: Upper-middle income countries. Fig AD. Subgroup analysis for studies reporting unadjusted ORs. Fig AE. Subgroup analysis for studies reporting unadjusted ORs, by bacteria’s gram type or WHO criticality category (critical = gram-negative, high-priority = gram-positive in this study). Fig AF. Subgroup analysis for studies reporting unadjusted ORs, by specific bacterium. Fig AG. Subgroup analysis for studies reporting adjusted ORs. Fig AH. Subgroup analysis for studies reporting adjusted ORs, by bacteria’s gram type (critical = gram-negative, high-priority = gram-positive in this study). Fig AI. Subgroup analysis for studies reporting adjusted ORs, by specific bacterium. Fig AJ. Subgroup analysis for studies reporting adjusted and unadjusted ORs simultaneously, general mortality estimates. Fig AK. Subgroup analysis for studies reporting adjusted and unadjusted ORs simultaneously, mortality rates by Gram type or WHO criticality list classification (high = gram-positive, critical = gram-negative). Fig AL. Subgroup analysis for studies reporting adjusted and unadjusted ORs simultaneously, mortality rates by bacterium family. Fig AM. Doi plots for Model 1 (general) and by outcome based on Tables Q, R, and S. Fig AN. Funnel plots for Model 1 (general) and by outcome based on Tables Q, R, and S. Fig AO. Influence analysis for Model 1 using the mortality outcome compared to the general estimates and without subgroup analyses. Fig AP. Influence analysis for Model 1 using the ICU admission outcome compared to the general estimates and without subgroup analyses. Fig AQ. Influence analysis for Model 1 using the length of hospital stay outcome compared to the general estimates and without subgroup analyses. Fig AR. Meta-analysis results disaggregated by specific and prioritised antibiotic-bacterium pairs for mortality. Fig AS. Meta-analysis results disaggregated by carbapenem-resistant Enterobacteriaceae for mortality. Fig AT. Meta-analysis results disaggregated by specific and prioritised antibiotic-bacterium pairs for LOS. Fig AU. Meta-analysis results disaggregated by carbapenem-resistant Enterobacteriaceae for LOS. Fig AV. Meta-analysis results disaggregated by specific and prioritised antibiotic-bacterium pairs for ICU admission. Fig AW. Meta-analysis results disaggregated by carbapenem-resistant Enterobacteriaceae for ICU admission. Fig AX. Graphical results of Table V. Fig AY. Distribution of the Master scale scores by outcome. Fig AZ. Kernel density estimate of the Master scale scores by outcome. Fig BA. Percentage of full completion by MASTER scale main safeguard and outcome.
(PDF)
MasterData spreadsheet. Description and data extracted from each included study. MasterScale spreadsheet. Application of the MASTER scale by outcome and study. Summary MasterScale spreadsheet. Summary statistics per safeguard/item of the application of the MASTER scale.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.