Abstract
The investigation of chirality at the nanoscale is important to bridge the gap between molecular and macroscopic chirality. Atomically precise metal nanoclusters provide an ideal platform for this research, while their enantiopure preparation poses a challenge. Here, we describe an efficient approach to enantiopure metal nanoclusters via asymmetric transformation, that is, achiral Au23(SC6H11)16 nanoclusters are converted into chiral and enantiopure Au24(L)2(SC6H11)16 nanoclusters by a chiral inducer phosphoramidite (L). Two enantiomers of Au24(L)2(SC6H11)16 are obtained and the crystal structures reveal their hierarchical chirality, which originates from the two introduced chiral L molecules, the transformation-triggered asymmetric rearrangement of the staple motifs on the surface of the gold core, and the helical arrangement of nanocluster molecules. The construction of this type of enantiomerically pure nanoclusters is achieved based on the easy-to-synthesize and modular L. Lastly, the chirality-related chiroptical performance was investigated, revealing a negative nonlinear CD-ee dependence.
Subject terms: Organic molecules in materials science, Structural properties, Nanoparticles
Atomically precise metal nanoclusters provide an ideal platform for studying chirality at the nanoscale. Here, the authors use a phosphoramidite ligand as a chiral inducer to asymmetrically transform achiral into chiral gold nanoclusters with negative nonlinear CD-ee dependence.
Introduction
Chirality is a fundamental characteristic in nature and can be found at various scales, from small organic molecules to macroscopic materials1–4. The same component materials with different chiroptical activities show distinct performance5. As a typical example, the S and R enantiomers of organic molecules with asymmetric sp3 carbons display different even completely opposite biological activities6. That is why the asymmetric synthesis is so important in medicinal chemistry. Based on the analysis of small organic molecules’ structures, we can clarify the origin of their chirality and achieve efficient enantiomer synthesis. However, when the scale of materials comes to nanometers or even micrometers (e.g., organic ligand-protected metal nanoparticles), things become complex7–10. The chirality of nanoparticles can originate from the chiral organic ligands, the asymmetric arrangements of metal atoms or metal-ligand motifs, as well as the particles’ asymmetric packing at a higher scale11,12. The complex chirality and the undefined structure of nanoparticles make the determination of chirality origin, the achievement of enantiopure isomers, and the investigation of the related performance challenging.
The atomically precise metal nanocluster constitutes an ideal platform for the research of complex systems’ chirality at atomic level13. Moreover, chiral metal nanoclusters show unique properties and potential applications in catalysis, sensing, as well as biomedicine14,15. Thus, the investigation of the chirality of metal nanoclusters has received tremendous attention16–20. In spite of the significant progress, the achievement of enantiopure metal nanoclusters is desired but remains a great challenge. Based on the previous reports, some methods were developed for acquiring metal nanoclusters with enantiomeric excess. These methods include: (1) enantioseparation of racemic mixtures of metal nanoclusters by chiral high-performance liquid chromatography (HPLC)21–24 or resolving agents;25–27 (2) enantioselective phase transfer of chiral nanoclusters;28,29 (3) direct synthesis of enantiopure nanoclusters by using chiral organic ligands as the precursors30–37. However, the method for transforming an achiral metal nanocluster into a chiral one is limited. There are a few reports on the chiral ligand exchange of achiral metal nanoclusters38,39, in which the chirality generally comes from the chiral ligands on the surface. In view of hundreds of achiral metal nanoclusters being reported13 and the wide application of asymmetric organic transformations in pharmaceutical synthesis and industry chemistry40,41, we envisioned the possibility of using a chiral inducer to realize the asymmetric transformation of achiral metal nanoclusters. This strategy would be developed into a general protocol for the construction of enantiopure metal nanoclusters.
We have a long-standing interest in the chirality transfer of organic molecules42–45 as well as the construction of functional metal nanoclusters46,47. We developed a surface phosphorization method48 and realized the structural evolution of thiolate-protected gold nanoclusters, which has been proven to be effective to modify the nanoclusters’ structure and performance48,49. Inspired by these achievements, in this work, we realized the asymmetric transformation of an achiral gold nanocluster by using an easily synthesized and modular phosphine inducer. The transformation is enantiodivergent and the two optical pure enantiomers of the as-transformed nanoclusters can be obtained respectively. More interestingly, this strategy not only introduces the chirality of the phosphine inducer but also triggers the asymmetric arrangement of -S-Au-S- and -S-Au-S-Au-S-Au-S- staple motifs that were originally symmetric on the surface of the gold nanocluster. An intriguing helical arrangement of nanocluster molecules was also discovered. Thus, the asymmetric transformation leads to a hierarchically chiral structure of the gold nanocluster.
Results
Asymmetric transformation process
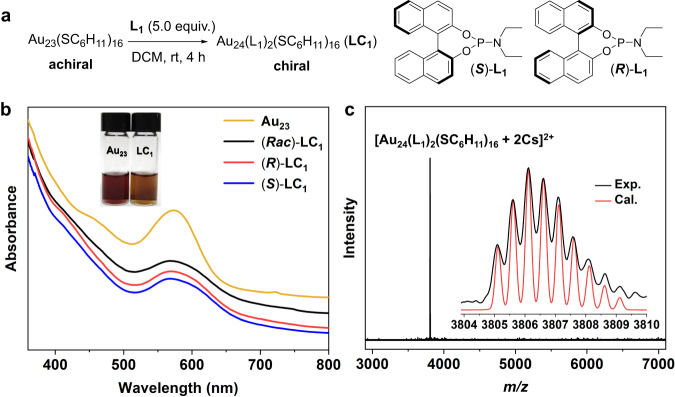
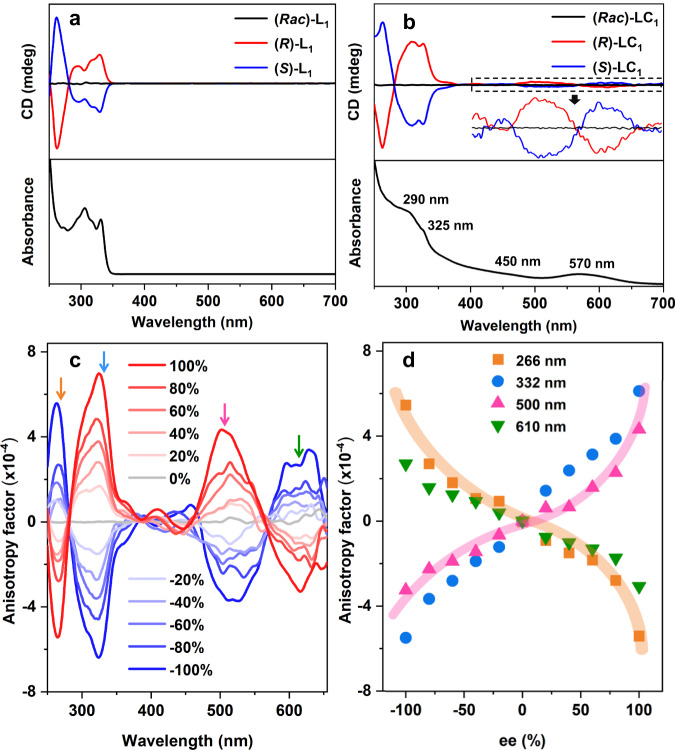
A facilely synthesized Au23(SC6H11)16 (abbreviated as Au23) was used as the model nanocluster for the investigation. It is an achiral gold nanocluster, bearing an Au13 kernel with symmetrically arranged two -S-Au-S-Au-S-Au-S- and four -S-Au-S- staple motifs on the surface50. Meanwhile, it is a representative thiolate-protected gold nanocluster, which has been applied as a typical example for the studies of structural anatomy, metal doping, and structure-property correlation51–53. We initiated our studies by screening different kinds of chiral phosphine ligands to react with Au23 (Supplementary Table 1). Butane-2,3-diybis(diphenylphosphine) with two chiral sp3 carbons was reactive to Au23 but showed poor selectivity and resulted in wide product distribution (Supplementary Fig. 1a). Axially chiral 2,2’-bis(diphenylphosphino)–1,1’-binaphthyl (BINAP) with sterically hindered phosphine sites displayed low activity to Au23 instead, and led to the recovery of the starting materials (Supplementary Fig. 1b). Chiral phosphoric acid resulted in the decomposition of Au23, probably due to its strong acidity. Delightedly, a “privileged” ligand phosphoramidite48 was found to demonstrate satisfactory reactivity as well as good selectivity (Supplementary Fig. 2). This ligand was first introduced by Feringa and coworkers54,55 and Alexakis and coworkers56 in the late 1990s for copper-catalyzed asymmetric conjugate additions. They are modular, easy to synthesize, and widely applied as a versatile and readily accessible class of chiral ligands in asymmetric catalysis56. Racemic phosphoramidite with a diethylamine module (Rac)-L1 was prepared initially to react with Au23 at room temperature (Fig. 1a). This reaction is efficient, giving an exclusive nanocluster product in high yield. The obtained nanocluster showed a different polarity to Au23 based on the preparative thin layer chromatography (PTLC), demonstrating that a different nanocluster (LC1) was formed (Supplementary Fig. 2). Meanwhile, the ultraviolet and visible (UV-vis) spectrum of LC1 is quite similar to that of Au23, and only a slight blueshift of the characteristic peaks at 460 and 575 nm was observed during the transformation (Fig. 1b). This result suggests that the structure of LC1 would be relevant to that of Au23. (R)-L1 and (S)-L1 reacted with Au23, affording (R)-LC1 and (S)-LC1 with the same UV-vis spectra (Fig. 1b). Electrospray ionization mass spectrometry (ESI-MS) was used to determine the composition of the as-obtained nanocluster. A single peak at m/z = 3806.15 was observed on the positive mode with the addition of CsOAc. This peak is assigned to [Au24(L1)2(SC6H11)16 + 2Cs]2+ (Fig. 1c), and the experimental isotope patterns match very well with the calculated one (Fig. 1c, inset). The ESI-MS result suggests that the as-obtained nanocluster is charge-neutral and determines the molecular formula of LC1 to be Au24(L1)2(SC6H11)16.
Fig. 1. Procedure and characterizations of asymmetric transformation.
a Procedure of the asymmetric transformation. b UV-vis spectra of Au23 (yellow trace), (Rac)-LC1 (black trace), (R)-LC1 (red trace) and (S)-LC1 (blue trace). Inset: the photographs of Au23 and LC1 in DCM. c ESI-MS spectrum of LC1. Inset: the experimental (black trace) and calculated (red trace) isotope patterns. Source data are provided as a Source Data file.
Crystal structures analysis
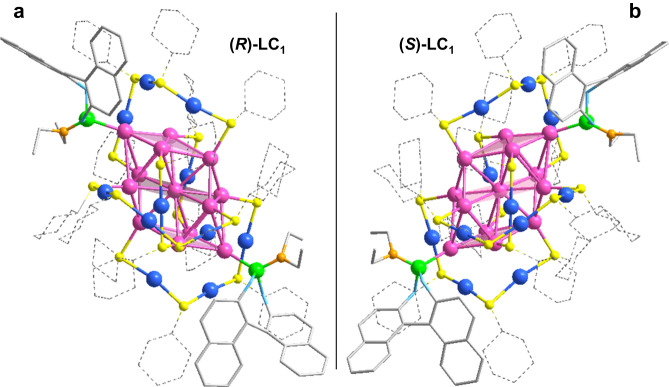
Single crystals of (R)-LC1 and (S)-LC1 were obtained in the mixed solvents of dichloromethane and hexane. From the crystal structures revealed by single-crystal X-ray diffraction (SCXRD), (R)-LC1 and (S)-LC1 all crystallized in the enantiomorphic P21 space group, which is different from the centrosymmetric Ccca space group of Au2344. The (R)-LC1 and (S)-LC1 nanocluster molecules take a similar ‘ABCD’ stacking sequence along the [100], [010] and [001] directions based on the observation of their crystallographic arrangements (Supplementary Figs. 4 and 5). The total structures of (R)-LC1 and (S)-LC1 demonstrate that the as-transformed nanoclusters are composed of 24 gold atoms, 16 cyclohexanethiols, and two phosphoramidites L1 (Fig. 2), which is consistent with the molecular formula revealed by ESI-MS. (R)-LC1 and (S)-LC1 cannot overlap with each other, and show near-perfect mirror images (Fig. 2), indicating that the (R)- and (S)-phosphoramidite-induced transformations of achiral Au23 leads to a pair of enantiomeric nanoclusters.
Fig. 2. Crystal structures.
a Structure of (R)-LC1. b Structure of (S)-LC1. Color label: Au = pink, blue; S = yellow; P = green; N = orange; O = light blue; C = gray. H atoms are omitted for clarity.
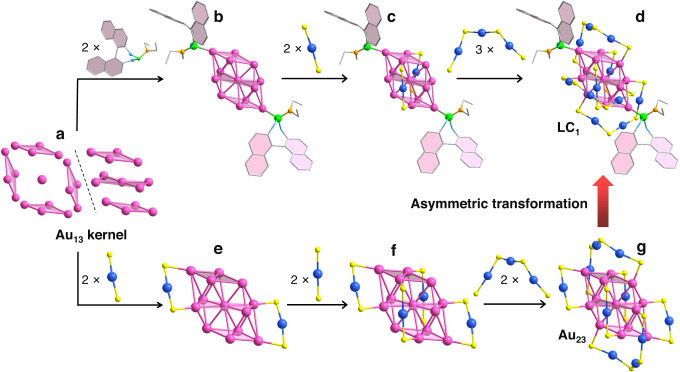
One of the enantiomers (R)-LC1 was selected as an example for further structural anatomy. As shown in Fig. 3, LC1 consists of an Au13 kernel, which can be seen as an Au4-Au5-Au4 sandwich structure, and also a central gold atom surrounded by four common-vertex rhombic blocks (Fig. 3a). The central gold atom is associated with the surrounded ten gold atoms with the average Au–Au bond of 2.84 Å (Supplementary Fig. 6) except for the two gold atoms on the vertices (which are far away from the central gold atom with the distances of 4.28 and 4.30 Å). The two vertices are coordinated with the phosphine sites of phosphoramidites L1 (Fig. 3b), and the other ten gold atoms are covered by two -S-Au-S- and three -S-Au-S-Au-S-Au-S- staple motifs (Fig. 3c, d). Notably, LC1 and Au23 nanoclusters have similar sandwich Au13 kernels. A careful comparison of the Au–Au distances on the upper and lower Au4 layers reveals that the Au13 kernel in Au23 is completely centrosymmetric (Supplementary Fig. 7a). The analysis of Au–Au distances between the Au4 and Au5 layers further confirms this conclusion (Supplementary Fig. 7b). The examination of the Au4-Au5-Au4 sandwich kernel of LC1 indicates that its Au13 kernel is slightly twisted (Supplementary Fig. 7c, d). Despite the tiny distortion of the Au13 kernel in LC1, the main difference between LC1 and Au23 comes from the introduced phosphoramidites (L1) ligands and the arrangements of -S-Au-S- and -S-Au-S-Au-S-Au-S- motifs on the surface of the Au13 kernel. Specifically, Au23 has four -S-Au-S- and two -S-Au-S-Au-S-Au-S- staple motifs (Fig. 3e–g), in which two of the -S-Au-S- motifs take the same arrangement as that in LC1, bridging the upper and lower Au4 layers of the Au4-Au5-Au4 sandwich kernel (Fig. 3c, f). The other two -S-Au-S- and all of the -S-Au-S-Au-S-Au-S- motifs of Au23 locate between the layers of the middle Au5 and the upper (or lower) Au4 (Fig. 3e, g). The total six staple motifs on the surface of the Au13 kernel are also completely centrosymmetric in Au23 (Fig. 3g). In contrast, the arrangement of -S-Au-S-Au-S-Au-S- motifs in LC1 is more diversified. One of the -S-Au-S-Au-S-Au-S- motifs twists around the middle Au5 layer, and the other two locate between the middle Au5 layer and the (upper or lower) Au4 layer (Fig. 3d).
Fig. 3. Structural anatomy of LC1 and Au23.
a The Au13 kernel. b Au13 kernel with two phosphoramidites L1. c Au13 kernel with two phosphoramidites L1 and two -S-Au-S- motifs. d The total structure of LC1. e Au13 kernel with two -S-Au-S- motifs. f Au13 kernel with four -S-Au-S- motifs. g The total structure of Au23. Color label: Au = pink, blue; S = yellow; P = green; N = orange; O = light blue; C = gray. H atoms are omitted for clarity.
Asymmetric transformation mechanism
Based on the experimental results and crystal structures of LC1 and Au23, a proposed transformation mechanism from Au23 to LC1 was shown in Supplementary Fig. 8. The two vertex Au atoms of the sandwich Au13 kernel of Au23 constitute two open sites (Supplementary Fig. 8a), which are easily attacked by the phosphine site of ligand L1. The Au–P interaction would trigger the cleavage of Au–S bonds and the further dissociation of the two corresponding -S-Au-S- staple motifs (Supplementary Fig. 8b). A subsequential rearrangement of a -S-Au-S-Au-S-Au-S- motif (Supplementary Fig. 8c) and the association of the two vacant Au sites with another -S-Au-S-Au-S-Au-S- would generate asymmetrically arranged staple motifs on the Au13 kernel and give LC1 (Supplementary Fig. 8d). During the transformation, the Au13 kernel is retained and only slightly twisted, while two short -S-Au-S- staple motifs are replaced by two phosphine ligands L1 and a long -S-Au-S-Au-S-Au-S- staple motifs. Therefore, LC1 contains one more Au atom and two L1 while possessing the same number of thiol ligands, when compared to Au23. We carefully analyzed the product components of the reaction between Au23 and L1 (Supplementary Table 2). Apart from a few undesired complexes and nanoparticles, LC1 was isolated as the major product with a yield of 65% (based on the Au atom). According to the whole process proposed in Supplementary Fig. 8, the mole ratio of the Au atom of two decomposed -S-Au-S- and one generated -S-Au-S-Au-S-Au-S- is 2/3, which is basically consistent with the yield (65%) of LC1 observed. This result suggests that 3 equivalents of Au23 would produce 2 equivalents of LC1, and the additional Au atom of LC1 probably comes from the decomposed -S-Au-S- staple motifs of Au23. The Au23 that are not transformed into LC1 during the reaction would decompose or aggregate into complexes or nanoparticles.
Hierarchical chirality
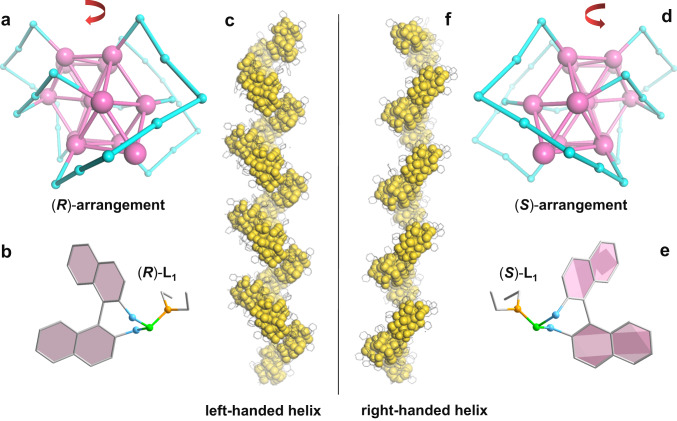
Further analysis of the two enantiomers’ structures reveals the origin of the chirality of the LC1 nanocluster (Fig. 4). First, (R)-LC1 and (S)-LC1 bear the same Au13 kernel that can overlap completely (Supplementary Fig. 9), demonstrating that the Au13 kernel is achiral. Second, the two short -S-Au-S- and three long -S-Au-S-Au-S-Au-S- motifs that surround around the sandwich Au13 kernel of (R)-LC1 cannot overlap with that of (S)-LC1. Moreover, the arrangements of these motifs in (R)-LC1 and (S)-LC1 are completely mirror-symmetric (Fig. 4a, d), indicating that the chirality of LC1 partially comes from the enantiotropic arrangement (tentatively defined as (R)- and (S)-arrangements) of surface motifs. Third, the (R)-L1 and (S)-L1 at the vertices of the Au13 kernels of (R)-LC1 and (S)-LC1 are enantiomeric (Fig. 4b, e), which also contribute to the chirality of the nanocluster. More interestingly, we found that R)-LC1 and (S)-LC1 nanocluster molecules take the left-handed helix and right-handed helix arrangements, respectively, based on the observation of the crystallographic arrangements of the two enantiomeric nanoclusters (Supplementary Fig. 10). One of the helical and enantiotropic chains of (R)-LC1 and (S)-LC1 molecular arrangements were picked out and shown in Fig. 4c, f. The intermolecular hydrogen bonds might play an important role in the helical arrangement of nanocluster molecules (Supplementary Fig. 11). Based on the above analysis, LC1 demonstrates a hierarchically chiral structure, which originates from three aspects: (1) the asymmetric arrangement of the -S-Au-S- and -S-Au-S-Au-S-Au-S- motifs on the surface of the Au13 kernel; (2) the intrinsic chirality of phosphoramidite ligand at the two vertices of the Au13 kernel; (3) the helical arrangement of nanocluster molecules. Thus, the phosphoramidite-induced asymmetric transformation from Au23 to LC1 not only introduces the intrinsic chirality of phosphoramidite itself but also triggers the asymmetric rearrangements of the staple motifs and nanocluster molecules that were originally symmetric in Au23.
Fig. 4. Representation of hierarchically chiral structures of (R)-LC1 and (S)-LC1.
a, d The asymmetric arrangement of -S-Au-S- and -S-Au-S-Au-S-Au-S- motifs on the surface of the Au13 kernel. b, e The intrinsic chirality of phosphoramidite ligand at the two vertices of the Au13 kernel. c, f The helical arrangement of nanocluster molecules. Color label: Au13 kernel = rose red; -S-Au-S- and -S-Au-S-Au-S-Au-S- staple motifs = cyan; Helical nanocluster molecules = yellow; P = green; N = orange; O = light blue; C = gray. H atoms are omitted for clarity.
Negative nonlinear CD-ee dependence
The asymmetric transformation process is enantiodivergent, and the as-obtained (R)-LC1 and (S)-LC1 are enantiopure, displaying nearly perfect mirror-symmetric circular dichroism (CD) signals from 250 to 700 nm. Specifically, (R)-LC1 gave positive Cotton effects at 310, 328, 405 and 500 nm with negative Cotton effects at 260, 425 and 600 nm, while (S)-LC1 demonstrated the completely opposite Cotton effects (Fig. 5b). Based on the combined CD-absorption spectra, the zero-crossing points at 450 and 570 nm in the CD spectrum are consistent with the characteristic absorptions in the UV-vis spectrum, indicating that the exciton coupling happens. Compared with the chiral inducer (R)-L1 and (S)-L1 that only showed simple CD signals from 250 to 350 nm (Fig. 5a), the CD spectra of (R)-LC1 and (S)-LC1 displayed much more abundant signals. The anisotropy factors (g = ΔAbs/Abs) of (R)-L1 and (S)-L1 were calculated in the wavelength ranging from 250 to 700 nm (Fig. 5c), showing a maximum value (gmax) of 0.75 × 10−3 at 328 nm.
Fig. 5. Negative nonlinear dependence between chiroptical activity and enantiomeric excess.
a Combined CD-absorption spectra of (Rac)-L1 (black trace), (R)-L1 (red trace) and (S)-L1 (blue trace). b Combined CD-absorption spectra of (Rac)-LC1 (black trace), (R)-LC1 (red trace) and (S)-LC1 (blue trace). Inset: the enlarged view from 400 to 700 nm, and the magnification factor is 5. c Anisotropy factors of LC1 prepared by different ee values of L1. d The negative nonlinear CD-ee dependence between the chiroptical activity of LC1 and enantiomeric excess of L1 based on anisotropy. The solid and broader highlighted colored lines in (d) are merely guides to the eye. Source data are provided as a Source Data file.
To gain a deeper insight into the asymmetric transformation of Au23 induced by phosphoramidite, the relationship between the chiroptical activity of nanocluster LC1 and the enantiomeric excess (ee) of inducer L1 was investigated. As shown in Fig. 5c, with the increment of ee values of L1, the anisotropy of LC1 increase accordingly. However, the CD-ee dependence is not linear based on the data collected at 266, 332, 500, and 610 nm of the anisotropy factors. Interestingly, we tried to obtain clusters containing one (R)-L1 and one (S)-L1 in the ligand shell by the reaction of Au23 with (Rac)-L1 (ee = 0%). However, the crystals suitable for single-crystal X-ray diffraction test were determined to be either (R)-LC1 or (S)-LC1. Considering that (Rac)-LC1 showed none of the CD signal (Fig. 5b), the racemic clusters are likely composed of equimolar (R)-LC1 and (S)-LC1. This result is consistent with the nonlinear CD-ee dependence and suggests that the clusters with one (R)-L1 and one (S)-L1 in the ligand shell are thermodynamically unfavorable products. Notably, because other chiral nanoclusters than LC1 exist in the crude product (Supplementary Table 2), the LC1 nanocluster showed slightly different CD spectra from that of the crude product which was directly obtained from the reaction of Au23 and L1 (Supplementary Fig. 14). This might make the should-be linear CD-ee dependence change into nonlinear one. As we know, there are three types of CD-ee dependence for the chiral auxiliaries-induced chirality of materials: linear, positive nonlinear, and negative nonlinear57. The linear CD-ee dependence is quite common, and the positive nonlinear CD-ee dependence (also known as “majority rules effect”) representing the chiral amplification phenomenon has only been found in a minority of the cases. In sharp contrast, the negative nonlinear CD-ee dependence has been much less reported. Herein, this dependence was found in metal nanoclusters. The CD-ee dependence of the asymmetric transformation process is nonlinear, indicating that more than one chiral auxiliary is involved in the intermediate of the asymmetric transformation of Au23, providing support for the proposed mechanism (Supplementary Fig. 8). The negative nonlinear CD-ee dependence secures a steeper slope at the ee region close to 100%, which would be applied in the future for the accurate determination of enantiopurity of molecules at the high ee region58. Such determination is important in practical applications, such as the optimization of asymmetric catalyst performance.
Asymmetric transformation application
Apart from the introduction of hierarchical chirality, the phosphoramidite-induced transformation of Au23 also leads to the improvement of stability and photoluminescence of the gold nanocluster. Time-dependent UV-vis spectra showed that LC1 was stable even at 80 °C under the ambient atmosphere (Supplementary Fig. 15b). In contrast, Au23 was quickly decomposed under the same conditions (Supplementary Fig. 15a). Based on the molecular formula (Au24(L1)2(SC6H11)16) of LC1, it bears an eight-electron closed-shell (24 × 1 − 16 × 1 = 8) structure. Moreover, differential pulse voltammetry (dpv) reveals that the oxidation (0.22 V) and reduction (−1.65 V) barriers of LC1 are higher than that of Au23 (0.07 V and −1.34 V, Supplementary Fig. 16). The stable electronic structure and the relatively large electrochemical gap all contribute to the high stability of LC1. The fluorescence emission peaks of Au23 and LC1 locate at a similar wavelength of 720 nm based on the photoluminescence spectra (Supplementary Fig. 17). However, the emission intensity of LC1 is almost five times higher than that of Au23. LC1 dissolved in DCM displayed stronger red emission than Au23 by keeping their concentrations the same (Supplementary Fig. 17, inset). Considering that LC1 and Au23 bear the basically same Au13 kernel, the fluorescence enhancement probably originates from the asymmetric transformation-resulted structural modification on the surface.
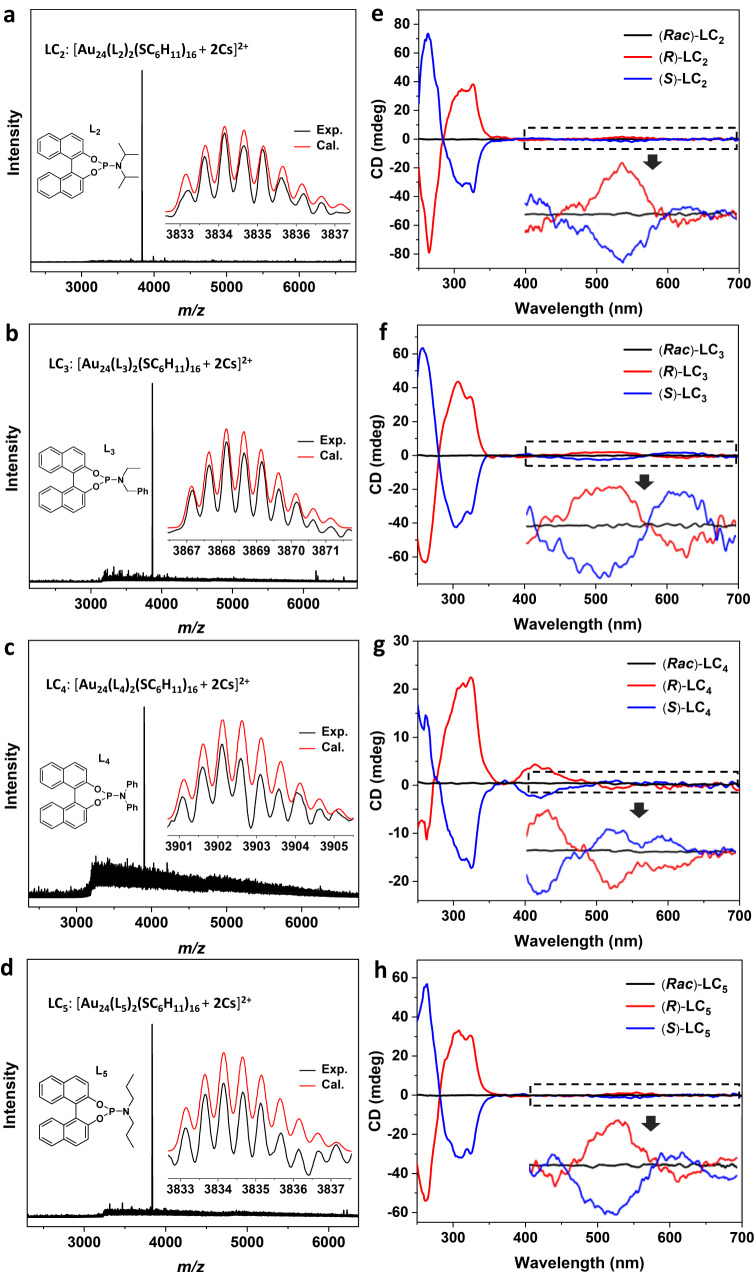
As mentioned above, phosphoramidite is modular and easy to synthesize. Using different amino modules involving sterically hindered, unsymmetric, aryl and alkyl groups, we synthesized the other four enantiopure phosphoramidites (L2-5), which were applied to induce the asymmetric transformations of Au23. These phosphoramidites all reacted well with Au23, affording LC2-5 with racemic, R and S configurations (Fig. 6). The exciton-coupled CD profiles with respect to the UV-vis spectra of LC1-LC5 were carefully analyzed. Based on the combined CD-absorption spectra (Supplementary Fig. 12a–e), LC1, LC2, LC3 and LC5 demonstrate characteristic absorptions at 290, 325 and 570 nm, and a weak peak at about 450 nm. Accordingly, these four clusters showed consistent zero-crossing points at 450 and 570 nm in the CD profiles. LC4 showed a slightly different CD spectrum. This can be explained by a different UV-vis spectrum of ligand L4, compared to that of the other four phosphoramidite ligands (Supplementary Fig. 12f). The distinctive absorption of L4 might originate from the conjugated π system of the two phenyl groups on the nitrogen atom. The UV-vis spectrum of LC4 demonstrates a stronger absorption peak at 410 nm and a bathochromic shift at 580 nm, which are also slightly different from that of LC1, LC2, LC3 and LC5. The absorption at 410 nm is consistent with an obvious Cotton effect at this wavelength. ESI-MS spectra of these nanoclusters confirmed their molecular formulas to be Au24(L2-5)2(SC6H11)16 (Fig. 6a–d). The above analysis combined with the ESI-MS spectra suggest that LC1-LC5 have similar structures.
Fig. 6. Characterizations of LC2-5.
a–d ESI-MS spectra of LC2-5. Inset: the structures of L2-5, and the experimental (black trace) and calculated (red trace) isotope patterns of LC2-5. e–h CD spectra of (Rac)- (black trace), (R)- (red trace), and (S)-LC2-5 (blue trace). Inset: the enlarged view of CD spectra from 400 to 700 nm, the magnification factors of LC2−5 are 5, 4, 3 and 6. Source data are provided as a Source Data file.
Discussion
In summary, we realized the asymmetric transformation of an achiral metal nanocluster in this work for the achievement of enantiopure metal nanoclusters. Phosphoramidites (L) were developed as efficient chiral auxiliaries to induce the enantiodivergent processes, and the enantiomeric nanoclusters (R)- and (S)-Au24(L)2(SC6H11)16 were synthesized separately from achiral Au23. Structural analysis reveals that the asymmetric transformation not only brings the intrinsic chirality of phosphoramidite but also triggers the asymmetric rearrangement of the staple motifs and nanocluster molecules that were originally symmetric in Au23, constituting the hierarchical chirality of Au24(L)2(SC6H11)16. A negative nonlinear CD-ee dependence was found for the relationship between the chiroptical activity of Au24(L)2(SC6H11)16 and the enantiomeric excess of L. The phosphoramidite is modular and a series of substituents can be introduced, leading to the functional diversity of the as-transformed chiral nanoclusters. We expect that our work will stimulate further research on the construction of enantiopure metal nanoclusters and the chirality of complex systems.
Methods
Characterizations
ESI-MS were acquired on a Waters Q-TOF mass spectrometer equipped with a Z-spray source. All UV-vis absorption measurements were performed on a SPECORD 210 PLUS spectrophotometer. SCXRD data were measured by using a Stoe Stadivari diffractometer. The structures were solved and refined using the SHELXT software. CD spectra were obtained by Circular chromatograph J-1700, and the reference solvent for measurement is DCM. Fluorescence spectra were obtained by a spectrofluorometer FS 5. Electrochemical measurements were performed with a CHI770E electrochemistry workstation in a three-electrode system using an Ag/AgCl electrode as the reference electrode, a glassy carbon electrode as the working electrode and a platinum wire electrode as the auxiliary electrode. NMR spectra were recorded at 400 MHz. Chemical shifts (δ) are reported in ppm, using the residual solvent peak in CDCl3 (7.26 ppm) as the internal standard. Coupling constants (J) are given in Hz.
Synthesis of Au23
HAuCl4·3H2O (0.3 mmol, 118 mg) and tetraoctylammonium bromide (TOAB, 0.35 mmol, 190 mg) were dissolved in methanol (15 mL) in a 100 mL round-bottom flask. After vigorously stirring for 15 min, cyclohexanethiol (1.6 mmol, 196 μL) was added to the mixture at room temperature. After 15 min, NaBH4 (3 mmol, 114 mg dissolved in 6 mL of cold Nanopure water) was rapidly added to the solution under vigorous stirring. The reaction mixture was allowed to stir overnight and finally gave Au23 in 20% yield (purified by recrystallization).
Preparation of phosphoramidite (L1-5)
By using the procedure for the preparation of (S)-L1 as an example: Triethylamine (5.0 eq., 25 mmol, 3.5 mL) was added dropwise to a stirred ice-cooled solution of PCl3 (5 mmol, 436 μL) in CH2Cl2 (35 mL). The ice bath was removed and the solution was warm to room temperature before diethylamine (5 mmol, 517 μL) was added. After 5 h, (S)-binaphthol (5 mmol, 1.43 g) was added to the suspension and the resulting mixture was left to stir for an additional 18 h. The purification of (S)-L1 was achieved via column chromatography on silica gel (eluent: Pentane/EtOAc = 60/1). (Rac)-, (R)- and (S)-L1-5 were prepared following the procedure above.
Synthesis of LC1-5 from Au23
By using the procedure for the synthesis of (S)-LC1 as an example: 5.0 equivalents of (S)-L1 (4.24 mg) were added into the CH2Cl2 (1.5 mL) solution of Au23 (15.0 mg). After the completion of the reaction in 4 h, the mixture was washed with MeOH for 2-3 times to give the crude product (S)-LC1 (precipitate), which was recrystallized in the system of CH2Cl2/n-hexane. Red hexagon crystals were obtained after 7 days, which were suitable for ESI-MS, SCXRD and the other characterizations. (Rac)-, (R)- and (S)-LC1-5 were prepared following the procedure above.
CD-ee dependence studies
First, L1 with different ee (−100%, −80%, −60%, −40%, −20%, 0%, 20%, 40%, 60%, 80% and 100%) were prepared by mixing optical pure (R)-L1 and (S)-L1. The amount of (R)-L1 and (S)-L1 used for each sample was calculated via Eq. (1) and is also shown in Supplementary Table 3. Second, 5.0 equivalents of L1 (5.0 mg) with specific ee was added into 18.0 mg of Au23 (dissolved in 1.5 mL of DCM). After 4 h, the reaction was purified by column chromatography on silica gel (eluent: DCM/MeOH = 20/1) to give LC1. Third, the purified LC1 from the reaction of Au23 and L1 with different ee was used for the CD test. The corresponding anisotropy factors (g) were obtained via Eq. (2). θ and Abs refer to ellipticity and absorbance, respectively.
| 1 |
| 2 |
Stability studies
For this, 5.0 mg of pure Au23 or LC1 was dissolved in 2 mL of toluene. The solution was gently stirred at 80 °C. The time-dependent UV-vis absorption spectra were obtained based on the mixture (Supplementary Fig. 15). 5.0 mg of Au23 or LC1 was dissolved in Bu4NPF6 (70 mg)-DCM (2 mL) solution and the electrochemical property of the nanocluster was measured using an electrochemical workstation. Before the experiment, the working electrode was polished with a mixture of Al2O3 and water and then cleaned sequentially with water and MeOH. The experiment was performed at an amplitude of 0.05 V, a pulse width of 0.05 s, a sampling width of 0.02 s and a pulse period of 0.1 s. The sample was always in a nitrogen atmosphere.
Photoluminescence studies
The excitation and emission spectra of Au23 and LC1 were obtained by dissolving the nanocluster in DCM at room temperature. The concentration of the samples was kept at the same to be 2.3 × 10−5 M. The excitation wavelength was kept at 350 and 360 nm, respectively, for the emission spectra of Au23 and LC1. The data were collected based on the same parameters.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
M.-B.L. acknowledges the financial support from the National Natural Science Foundation of China (92061110), the Anhui Provincial Natural Science Foundation (2108085Y05), the Innovation and Entrepreneurship Project of Overseas Returnees in Anhui Province (2022LCX014), and the Hefei National Laboratory for Physical Sciences at the Microscale (KF2020102).
Source data
Author contributions
M.-B.L. supervised the research and wrote the manuscript. M.-B.L. and S.Z. summarized the data. C.L., T.-S.Z. and C.-B.T. carried out the experiments. Y.Z. and W.F. resolved the structures of metal nanoclusters. All authors contributed to the preparation of the manuscript.
Peer review
Peer review information
Nature Communications thanks Yunbao Jiang, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper. The X-ray crystallographic structures reported in this work have been deposited at the Cambridge Crystallographic Data Center (CCDC) under deposition numbers 2216153 and 2216156 for (R)- and (S)-Au24(L1)2(SC6H11)16, respectively. These data can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chang Liu, Yan Zhao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39462-w.
References
- 1.Zeng C, Chen Y, Kirschbaum K, Lambright KJ, Jin R. Emergence of hierarchical structural complexities in nanoparticles and their assembly. Science. 2016;354:1580–1584. doi: 10.1126/science.aak9750. [DOI] [PubMed] [Google Scholar]
- 2.Yeom J, et al. Chiral templating of self-assembling nanostructures by circularly polarized light. Nat. Mater. 2015;14:66–72. doi: 10.1038/nmat4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Che S, et al. Synthesis and characterization of chiral mesoporous silica. Nature. 2004;429:281–284. doi: 10.1038/nature02529. [DOI] [PubMed] [Google Scholar]
- 4.Barron LD. Symmetry and molecular chirality. Chem. Soc. Rev. 1986;15:189–223. doi: 10.1039/cs9861500189. [DOI] [Google Scholar]
- 5.Feringa BL, van Delden RA, Koumura N, Geertsema EM. Chiroptical molecular switches. Chem. Rev. 2000;100:1789–1816. doi: 10.1021/cr9900228. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, et al. Identification of a primary target of thalidomide teratogenicity. Nature. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 7.Aida T, Meijer E, Stupp S. Functional supramolecular polymers. Science. 2012;335:813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yashima E, et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016;116:13752–13990. doi: 10.1021/acs.chemrev.6b00354. [DOI] [PubMed] [Google Scholar]
- 9.Magnin Y, Amara H, Ducastelle F, Loiseau A, Bichara C. Entropy-driven stability of chiral single-walled carbon nanotubes. Science. 2018;362:212–215. doi: 10.1126/science.aat6228. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa M, Kawai T. Chirality-controlled syntheses of double-helical Au nanowires. J. Am. Chem. Soc. 2018;140:4991–4994. doi: 10.1021/jacs.8b00910. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Xu J, Wang Y, Chen H. Emerging chirality in nanoscience. Chem. Soc. Rev. 2013;42:2930–2962. doi: 10.1039/C2CS35332F. [DOI] [PubMed] [Google Scholar]
- 12.Lee HE, et al. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature. 2018;556:360–365. doi: 10.1038/s41586-018-0034-1. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Higaki T, Du X, Jin R. Chirality and surface bonding correlation in atomically precise metal nanoclusters. Adv. Mater. 2020;32:1905488. doi: 10.1002/adma.201905488. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty I, Pradeep T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem. Rev. 2017;117:8208–8271. doi: 10.1021/acs.chemrev.6b00769. [DOI] [PubMed] [Google Scholar]
- 15.Knoppe S, Bürgi T. Chirality in thiolate-protected gold clusters. Acc. Chem. Res. 2014;47:1318–1326. doi: 10.1021/ar400295d. [DOI] [PubMed] [Google Scholar]
- 16.Patty JB, et al. Crystal structure and optical properties of a chiral mixed thiolate/stibine-protected Au18 cluster. J. Am. Chem. Soc. 2022;144:478–484. doi: 10.1021/jacs.1c10778. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Acevedo O, Tsunoyama H, Tsukuda T, Häkkinen H, Aikens CM. Chirality and electronic structure of the thiolate-protected Au38 nanocluster. J. Am. Chem. Soc. 2010;132:8210–8218. doi: 10.1021/ja102934q. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, et al. Double-helical assembly of heterodimeric nanoclusters into supercrystals. Nature. 2021;594:380–384. doi: 10.1038/s41586-021-03564-6. [DOI] [PubMed] [Google Scholar]
- 19.Huang J-H, et al. Symmetry breaking of atomically precise fullerene-like metal nanoclusters. J. Am. Chem. Soc. 2021;143:12439–12444. doi: 10.1021/jacs.1c05568. [DOI] [PubMed] [Google Scholar]
- 20.Knoppe S, Dharmaratne AC, Schreiner E, Dass A, Bürgi T. Ligand exchange reactions on Au38 and Au40 clusters: a combined circular dichroism and mass spectrometry study. J. Am. Chem. Soc. 2010;132:16783–16789. doi: 10.1021/ja104641x. [DOI] [PubMed] [Google Scholar]
- 21.Dolamic I, Knoppe S, Dass A, Bürgi T. First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. Nat. Commun. 2012;3:798–804. doi: 10.1038/ncomms1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knoppe S, Dolamic I, Dass A, Bürgi T. Separation of enantiomers and CD spectra of Au40(SCH2CH2Ph)24: spectroscopic evidence for intrinsic chirality. Angew. Chem. Int. Ed. Engl. 2012;51:7589–7591. doi: 10.1002/anie.201202369. [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, Li T, Das A, Rosi NL, Jin R. Chiral structure of thiolate-protected 28-gold-atom nanocluster determined by X-ray crystallography. J. Am. Chem. Soc. 2013;135:10011–10013. doi: 10.1021/ja404058q. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, et al. De novo design of Au36(SR)24 nanoclusters. Nat. Commun. 2020;11:3349–3356. doi: 10.1038/s41467-020-17132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J, et al. Asymmetric synthesis of chiral bimetallic [Ag28Cu12(SR)24]4– nanoclusters via Ion pairing. J. Am. Chem. Soc. 2016;138:12751–12754. doi: 10.1021/jacs.6b08100. [DOI] [PubMed] [Google Scholar]
- 26.Deng G, Teo BK, Zheng N. Assembly of chiral cluster-based metal-organic frameworks and the chirality memory effect during their disassembly. J. Am. Chem. Soc. 2021;143:10214–10220. doi: 10.1021/jacs.1c03251. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, et al. Enantioseparation of Au20(PP3)4Cl4 clusters with intrinsically chiral cores. Angew. Chem. Int. Ed. Engl. 2018;57:9059–9063. doi: 10.1002/anie.201805695. [DOI] [PubMed] [Google Scholar]
- 28.Knoppe S, et al. Chiral phase transfer and enantioenrichment of thiolate-protected Au102 clusters. J. Am. Chem. Soc. 2014;136:4129–4132. doi: 10.1021/ja500809p. [DOI] [PubMed] [Google Scholar]
- 29.Yao H, Iwatsu M. Water-soluble phosphine-protected Au11 clusters: synthesis, electronic structure, and chiral phase transfer in a synergistic fashion. Langmuir. 2016;32:3284–3293. doi: 10.1021/acs.langmuir.6b00539. [DOI] [PubMed] [Google Scholar]
- 30.Man RWY, et al. Synthesis and characterization of enantiopure chiral bis NHC-stabilized edge-shared Au10 nanocluster with unique prolate shape. J. Am. Chem. Soc. 2022;144:2056–2061. doi: 10.1021/jacs.1c11857. [DOI] [PubMed] [Google Scholar]
- 31.Sugiuchi M, Shichibu Y, Konishi K. An inherently chiral Au24 framework with double-helical hexagold strands. Angew. Chem. Int. Ed. Engl. 2018;57:7855–7859. doi: 10.1002/anie.201804087. [DOI] [PubMed] [Google Scholar]
- 32.Shen H, et al. Tertiary chiral nanostructures from C-H···F directed assembly of chiroptical superatoms. Angew. Chem. Int. Ed. Engl. 2021;60:22411–22416. doi: 10.1002/anie.202108141. [DOI] [PubMed] [Google Scholar]
- 33.Wang J-Q, Guan Z-J, Liu W-D, Yang Y, Wang Q-M. Chiroptical activity enhancement via structural control: the chiral synthesis and reversible interconversion of two intrinsically chiral gold nanoclusters. J. Am. Chem. Soc. 2019;141:2384–2390. doi: 10.1021/jacs.8b11096. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M-M, et al. Alkynyl-stabilized superatomic silver clusters showing circularly polarized luminescence. J. Am. Chem. Soc. 2021;143:6048–6053. doi: 10.1021/jacs.1c02098. [DOI] [PubMed] [Google Scholar]
- 35.Kong Y-J, et al. Photoresponsive propeller-like chiral AIE copper(I) clusters. Angew. Chem. Int. Ed. Engl. 2020;59:5336–5340. doi: 10.1002/anie.201915844. [DOI] [PubMed] [Google Scholar]
- 36.Yao L-Y, Lee TK-M, Yam VW-W. Thermodynamic-driven self-assembly: heterochiral self-sorting and structural reconfiguration in gold(I)-sulfido cluster system. J. Am. Chem. Soc. 2016;138:7260–7263. doi: 10.1021/jacs.6b03844. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, et al. Self-assembly of chiral gold clusters into crystalline nanocubes of exceptional optical activity. Angew. Chem. Int. Ed. Engl. 2017;56:15397–15401. doi: 10.1002/anie.201709827. [DOI] [PubMed] [Google Scholar]
- 38.Knoppe S, Dharmaratne AC, Schreiner E, Dass A, Bürgi T. Ligand exchange reactions on Au38 and Au40 clusters: a combined circular dichroism and mass spectrometry study. J. Am. Chem. Soc. 2010;132:16783–16789. doi: 10.1021/ja104641x. [DOI] [PubMed] [Google Scholar]
- 39.Li S, et al. Atom-precise modification of silver(I) thiolate cluster by shell ligand substitution: a new approach to generation of cluster functionality and chirality. J. Am. Chem. Soc. 2018;140:594–597. doi: 10.1021/jacs.7b12136. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Hayashi T. Chiral diene ligands in asymmetric catalysis. Chem. Rev. 2022;122:14346–14404. doi: 10.1021/acs.chemrev.2c00218. [DOI] [PubMed] [Google Scholar]
- 41.Trost BM. Asymmetric catalysis: an enabling science. Proc. Natl Acad. Sci. USA. 2004;101:5348–5355. doi: 10.1073/pnas.0306715101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M-B, Wang Y, Tian S-K. Regioselective and stereospecific cross-coupling of primary allylic amines with boronic acids and boronates through palladium catalyzed C-N bond cleavage. Angew. Chem. Int. Ed. Engl. 2012;51:2968–2971. doi: 10.1002/anie.201109171. [DOI] [PubMed] [Google Scholar]
- 43.Li M-B, et al. Chemodivergent and diastereoselective synthesis of γ-lactones and γ-lactams: a heterogeneous palladium-catalyzed oxidative tandem process. J. Am. Chem. Soc. 2018;140:14604–14608. doi: 10.1021/jacs.8b09562. [DOI] [PubMed] [Google Scholar]
- 44.Li M-B, et al. Silver-triggered activity of a heterogeneous palladium catalyst in oxidative carbonylation reactions. Angew. Chem. Int. Ed. Engl. 2020;59:10391–10395. doi: 10.1002/anie.202001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M-B, et al. Amino-supported palladium catalyst for chemo- and stereoselective domino reactions. Angew. Chem. Int. Ed. Engl. 2021;60:670–674. doi: 10.1002/anie.202011708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan J-Q, Yang Y, Tao C-B, Li M-B. Cadmium-doped and pincer ligand-modified gold nanocluster for catalytic KA2 reaction. Angew. Chem. Int. Ed. Engl. 2023;62:e202215741. doi: 10.1002/anie.202215741. [DOI] [PubMed] [Google Scholar]
- 47.Zhang T-S, et al. Open nitrogen site-induced kinetic resolution and catalysis of a gold nanocluster. Nano Lett. 2023;23:235–242. doi: 10.1021/acs.nanolett.2c04163. [DOI] [PubMed] [Google Scholar]
- 48.Li M-B, Tian S-K, Wu Z, Jin R. Peeling the core-shell Au25 nanocluster by reverse ligand-exchange. Chem. Mater. 2016;28:1022–1025. doi: 10.1021/acs.chemmater.5b04907. [DOI] [Google Scholar]
- 49.Yang Y, et al. An efficient nanocluster catalyst for Sonogashira reaction. J. Catal. 2021;401:206–213. doi: 10.1016/j.jcat.2021.07.023. [DOI] [Google Scholar]
- 50.Das A, et al. Nonsuperatomic [Au23(SC6H11)16]− nanocluster featuring bipyramidal Au15 kernel and trimeric Au3(SR)4 motif. J. Am. Chem. Soc. 2013;135:18264–18267. doi: 10.1021/ja409177s. [DOI] [PubMed] [Google Scholar]
- 51.Li Q, et al. Molecular “surgery” on a 23-gold-atom nanoparticle. Sci. Adv. 2017;3:e1603193. doi: 10.1126/sciadv.1603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, et al. Reconstructing the surface of gold nanoclusters by cadmium doping. J. Am. Chem. Soc. 2017;139:17779–17782. doi: 10.1021/jacs.7b11491. [DOI] [PubMed] [Google Scholar]
- 53.Li Q, et al. A mono-cuboctahedral series of gold nanoclusters: photoluminescence origin, large enhancement, wide tunability, and structure-property correlation. J. Am. Chem. Soc. 2019;141:5314–5325. doi: 10.1021/jacs.8b13558. [DOI] [PubMed] [Google Scholar]
- 54.Teichert JF, Feringa BL. Phosphoramidites: privileged ligands in asymmetric catalysis. Angew. Chem. Int. Ed. Engl. 2010;49:2486–2528. doi: 10.1002/anie.200904948. [DOI] [PubMed] [Google Scholar]
- 55.de Vries AHM, Meetsma A, Feringa BL. Enantioselective conjugate addition of dialkylzinc reagents to cyclic and acyclic enones catalyzed by chiral copper complexes of new phosphorus amidites. Angew. Chem. Int. Ed. Engl. 1996;35:2374–2376. doi: 10.1002/anie.199623741. [DOI] [Google Scholar]
- 56.Alexakis A, Vastra J, Burton J, Benhaim C, Mangeney P. Asymmetric conjugate addition of diethyl zinc to enones with chiral phosphorus ligands derived from TADDOL. Tetrahedron Lett. 1998;39:7869–7872. doi: 10.1016/S0040-4039(98)01761-4. [DOI] [Google Scholar]
- 57.Yan X, Wang Q, Chen X, Jiang Y-B. Supramolecular chiral aggregates exhibiting nonlinear CD-ee dependence. Adv. Mater. 2020;32:1905667. doi: 10.1002/adma.201905667. [DOI] [PubMed] [Google Scholar]
- 58.Chen X-X, Jiang Y-B, Anslyn EV. A racemate-rules effect supramolecular polymer for ee determination of malic acid in the high ee region. Chem. Commun. 2016;52:12669–12671. doi: 10.1039/C6CC06716F. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper. The X-ray crystallographic structures reported in this work have been deposited at the Cambridge Crystallographic Data Center (CCDC) under deposition numbers 2216153 and 2216156 for (R)- and (S)-Au24(L1)2(SC6H11)16, respectively. These data can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.