Abstract
Background
Chimeric antigen receptor T (CAR-T) cells have proven to be a game changer for treating several hematologic malignancies. Randomized controlled trials have highlighted potential life-threatening adverse drug reactions (ADRs), including cytokine release syndrome (CRS). Acute renal failure (ARF) has also been reported in 20% of the patients treated. However, an analysis of renal safety supported by large-scale real-life data seems warranted.
Patients and methods
We queried VigiBase® for all reports of the Standardised MedDRA Query “acute renal failure” (ARF) involving a CAR-T cell, registered until 24 July 2022. Disproportionality for this ADR was analyzed through calculation of the Information Component [IC (95% confidence interval)]. A positive lower end of the 95% confidence interval of the IC is the threshold used in statistical signal detection in VigiBase®. The same analysis was carried out for various hydroelectrolytic disorders.
Results
We gathered 224 reports of ARF, and 125 reports of hydroelectrolytic disorders involving CAR-T cells. CAR-T cells were disproportionately reported with ARF [IC 1.5 (1.3–1.7)], even after excluding reports mentioning CRS. A significant disproportionate reporting was also found for hypernatremia [IC 3.1 (2.2–3.8)], hyperphosphatemia [IC 3.1 (1.8–3.9)], hypophosphatemia [IC 2.0 (0.6–2.9)], metabolic acidosis [IC 1.8 (1.2–2.2)], hyponatremia [IC 1.6 (1.1–2.0)], and hypercalcemia [IC 1.4 (0.5–2.1)]. There was no disproportionate reporting of dyskalemia.
Conclusions
This study is limited by the inherent flaws of pharmacovigilance approaches. Nonetheless, our findings suggest that ARF and an array of hydroelectrolytic disorders are potential ADRs of CAR-T cell therapy, in real-life settings and in a nonselected population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40259-023-00599-1.
Key Points
| Acute renal failure has been reported in around 20% of patients treated with CAR-T cells in randomized clinical trials. |
| There is a significant safety signal regarding the association between CAR-T cells and acute renal failure in a real-life setting, even when reports of cytokine release syndrome are excluded. Dysnatremia, metabolic acidosis, and hypercalcemia were also disproportionately reported, unlike dyskalemia. |
| Close monitoring of kidney function and hydroelectrolytic disorders could improve the management of patients treated with CAR-T cells. |
Introduction
Chimeric antigen receptor T (CAR-T) cells are autologous T cells genetically engineered ex vivo to express a chimeric receptor [1]. The latter consists of an intracellular activation domain, added to an extracellular binding domain that is derived from the variable fragment of an antibody directed against a selected antigen (e.g., CD-19). After reinfusion to the patient, CAR-T cells can activate and kill any target cell expressing the chosen antigen.
The first successful clinical uses of CAR-T cells were achieved in hematology [2, 3]. Indeed, CAR-T cells have proven to be a game changer for treating several hematologic malignancies that were hitherto refractory to almost any therapy. The two frontrunners of this new drug class were directed against CD-19: tisagenlecleucel (tisa-cel), initially approved for relapsed and refractory acute lymphoblastic leukemia in 2017 [4, 5], and axicabtagene ciloleucel (axi-cel), approved the same year for the treatment of diffuse large B-cell lymphoma [6, 7]. Since then, other CAR-T cells directed against CD-19 have been granted marketing authorizations, namely brexucabtagene ciloleucel (brexu-cel) and lisocabtagene maraleucel (liso-cel) [8]. As their indications keep expanding, novel CAR-T cells keep getting developed. Two of them are directed against B-cell maturation antigen (BCMA) and indicated in multiple myeloma: idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) [9]. CAR-T cells are now being investigated in some solid tumor malignancies[10] as well as autoimmune diseases [11].
Despite their undeniable efficacy, the safety profile of CAR-T cells remains largely unexplored, due to their recent availability and unprecedented mechanism of action. Randomized controlled trials have highlighted potential life-threatening adverse drug reactions (ADRs) [12, 13], including cytokine release syndrome (CRS). The latter results from the release of cytokines (interleukin-6 inter alia), and can lead to multiorgan failure. Another frequent ADR is immune effector cell-associated neurotoxicity syndrome (ICANS).
Acute renal failure (ARF) has been reported in 10–20% of the patients treated, from randomized clinical trials [14, 15]. This incidence has been confirmed in a small cohort of patients, treated in a real-life setting [16, 17]. Hydroelectrolytic disorders (HEDs) also emerged as a possible ADR of CAR-T cells. However, an analysis of renal safety supported by large-scale real-life data seems warranted. We aimed, therefore, to characterize and refine the signal regarding the association between CAR-T cell therapy and ARF in the World Health Organization (WHO) safety database. We also aimed to provide an overview of the HEDs reported in patients treated with CAR-T cells.
Material and Methods
Database
The WHO Safety Database (VigiBase®) is managed by the Uppsala Monitoring Center (UMC) [18]. Since 1967, VigiBase® gathers Individual Case Safety Reports issued from the national pharmacovigilance networks of more than 172 countries. These postmarketing reports can originate from healthcare professionals, patients, and pharmaceutical companies. The anonymity of both patients and reporters is preserved. Each report contains administrative information (country, reporter qualification), characteristics of the patients (sex, age), drugs (indication, start and cessation dates, dose, concomitant drugs), and ADRs (effects, seriousness, onset, outcome).
Query
In the Medical Dictionary for Regulatory Activities (MedDRA, version 25.0 [19]), a Standardised MedDRA Query (SMQ) is an exhaustive, validated, predetermined set of Preferred Terms (PT) intended to help regulatory agencies and pharmaceutical companies to address issues pertaining to drug safety [20]. A PT is defined as the distinct descriptor for a single medical concept [21].
Firstly, VigiBase® was queried for all reports containing the SMQ “acute renal failure” (ARF, narrow) registered until 24 July 2022, and involving one of the following CAR-T cells as a suspect drug: axi-cel, brexu-cel, cilta-cel, ide-cel, liso-cel, or tisa-cel.
Secondly, VigiBase® was queried for all reports involving any of the same six CAR-T cells and mentioning one of the following HEDs: hyponatremia, hypernatremia, hypokalemia, hyperkalemia, hypocalcemia, hypercalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, hypermagnesemia, alkalosis, metabolic acidoses (excluding diabetic acidoses).
Quantitative variables were described in terms of medians with interquartile ranges (IQR) and/or minimum–maximum ranges (min–max). Qualitative variables were described with numbers and proportions. Characteristics of patients were compared using Pearson’s chi-square test with Yates correction for categorical variables, or Student’s t-test for continuous and normally distributed variables. p < 0.05 was considered to be statistically significant. Statistical analyses were performed using Jamovi (version 2.2.5).
Disproportionality Analysis
Disproportionality is a case/non-case analysis used to detect pharmacovigilance signals [22, 23]. If the proportion of reports with a specific ADR and a given drug (cases) is greater than the proportion of reports with the same ADR and other drugs (non-cases), an association between this drug and the ADR is suggested. Disproportionality can be assessed by the Information Component (IC), derived from a Bayesian confidence propagation neural network [24]. The IC is a tool validated by UMC. It compares observed and expected numbers of reports with an ADR–drug combination. This tool allows more accurate detection of potential pharmacovigilance signals (lowering the risk of false positive signals) compared with other measures, such as the reporting odds ratio [25]. A positive lower end of the 95% confidence interval (CI) of the IC is the common threshold used in statistical signal detection at UMC.
We used disproportionality analyses to detect whether ARF or any of the HEDs was reported differentially with CAR-T cells, as compared with all other combinations of ADRs and active ingredients in VigiBase®. Specifically, we also calculated the IC for the combination of each of the six studied CAR-T cells with ARF and any of the HED. To support the accuracy of our disproportionality analysis, we included a positive and a negative control. As a positive control, we calculated the IC for the association between ARF and cisplatin, the latter being widely recognized as a nephrotoxic drug. As a negative control, we calculated the IC for the association between ARF and procarbazine, which is not commonly considered a nephrotoxic drug. Besides, taking into account the potential confounding factors arising from the disease itself (often in patients with polymorbidity, leading to an indication bias), the IC was also calculated for rituximab, cyclophosphamide, doxorubicin, and vincristine (RCHO). RCHO is a treatment protocol routinely used in patients with B-cell lymphoma. Then, we calculated the comparative IC of CAR-T cells, using RCHO as a background (instead of the whole database). Furthermore, as CRS could per se foster ARF, we sought to determine whether CAR-T cells were still disproportionately associated with ARF after exclusion of reports mentioning CRS. This additional disproportionality analysis aimed to mitigate the impact of this potential intermediate factor, and to increase the specificity of any possible findings regarding a direct nephrotoxicity of CAR-T cells.
Results
Acute Renal Failure
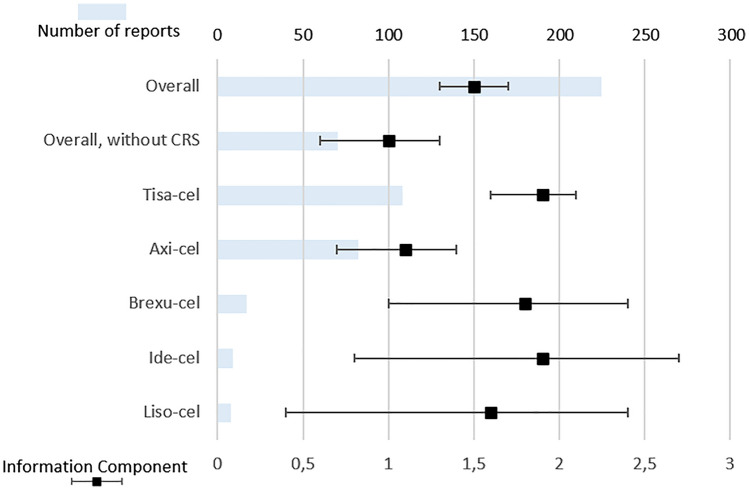
As of 24 July 2022, 224 reports involving CAR-T cells in VigiBase® were gathered in the SMQ “acute renal failure” (Fig. 1), accounting for 3.3% of the 6832 reports involving CAR-T cells. After exclusion of reports mentioning CRS, 70 cases of ARF (2.2%) were collected among the remaining 3149 reports involving CAR-T cells. Most patients were male (137, 61.2%), with a median age of 60 years (IQR 27–70, min–max 4–76). The USA issued most reports (177, 79.0%), followed by France (14, 6.3%) and Germany (12, 5.4%). Among healthcare professionals, physicians were the most frequent reporters (114, 50.9%) followed by pharmacists and others (85, 37.9%). The three most reported CAR-T cells were tisa-cel (108 reports, 48.2%, including 36 pediatric reports), axi-cel (82, 36.6%), and brexu-cel (17, 7.6%). The characteristics of the respective reports are compared in Table 1. We found respectively nine (4.0%) and eight (3.6%) reports with ide-cel and liso-cel, while no report was associated with cilta-cel.
Fig. 1.
Reports of acute renal failure involving CAR-T cells. Upper horizontal axis: absolute number of reports. Lower horizontal axis: information component with its 95% confidence interval
Table 1.
Characteristics of reports of acute renal failure involving CAR-T cells, and comparison between the three most frequently involved.
| Overall | Tisa-cel | Axi-cel | Brexu-cel | Fatal cases | |
|---|---|---|---|---|---|
| Number of reports (%) | 224 (100) | 108 (48.2) | 82 (36.6) | 17 (7.6) | 121 (54.0) |
| Male (%) | 137 (61.2) | 64 (61.0) | 43 (56.6) | 17 (100) | 71 (58.7) |
| Age (%) | |||||
| < 18 years | 36 (1.3) | 36 (33.3) | 0 | 0 | 24 (19.8) |
| 18–44 years | 28 (12.5) | 22 (20.4) | 5 (6.1) | 1 (5.9) | 18 (14.9) |
| 45–64 years | 66 (29.5) | 12 (11.1) | 38 (46.3) | 6 (35.3) | 30 (24.8) |
| 65–74 years | 47 (21.0) | 14 (13.0) | 24 (29.3) | 6 (35.3) | 25 (20.7) |
| ≥ 75 years | 20 (8.9) | 7 (6.5) | 7 (8.5) | 2 (11.8) | 10 (8.3) |
| Unknown | 27 (12.1) | 17 (15.7) | 8 (9.8) | 2 (11.8) | 14 (11.6) |
| Median age (IQR) | 60 (27–70) | 21 (12–62) | 64 (55–71) | 65 (56–73) | 55 (18–69) |
| Fatal cases (%) | 121 (54.0) | 69 (63.9) | 41 (50.0) | 5 (29.4) | 121 (100) |
IQR, interquartile range
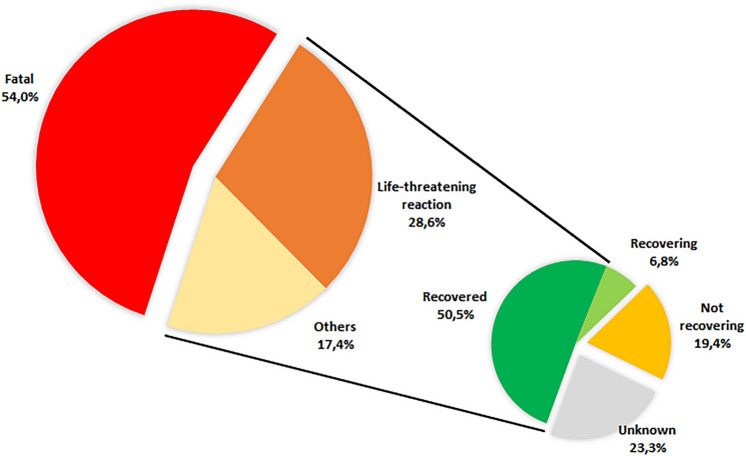
The median time to onset was 7 days (IQR 3–18, min–max 2–526 days). The most frequently co-reported MedDRA terms (Table 2) were cytokine release syndrome (154, 68.8%), pyrexia (85, 37.9%), neurotoxicity (72, 32.1%), hypotension (69, 30.8%), and hypoxia (46, 20.5%). Hemophagocytic lymphohistiocytosis was mentioned in 19 (8.5%) reports, and tumor lysis syndrome in 11 (4.9%) reports. Two reports (0.9%) mentioned an effect belonging to the proteinuria SMQ, both involving tisa-cel. Suspect co-reported active ingredients reported in more than five cases included only three drugs: cyclophosphamide (22, 9.8%), fludarabine (21, 9.4%), and tocilizumab (14, 6.3%). After exclusion of reports with CRS, the active ingredients reported in more than five reports were cyclophosphamide and fludarabine, each in nine (12.9%) reports. All reports were deemed serious, including 121 deaths (54.0%) and 64 life-threatening reactions (28.6%). Among surviving patients, 59 recovered or were recovering (57.3%) and 20 did not recover (19.4%). The outcome was unavailable in 24 reports (Fig. 2). Among reports without CRS, displaying an available outcome and involving surviving patients, 14 patients recovered from ARF (66.7%).
Table 2.
Co-reported MedDRA terms mentioned in at least ten reports
| Co-reported preferred terms (MedDRA) | Number of reports (%) |
|---|---|
| Cytokine release syndrome | 154 (68.8) |
| Pyrexia | 85 (37.9) |
| Neurotoxicity | 72 (32.1) |
| Hypotension | 69 (30.8) |
| Hypoxia | 46 (20.5) |
| Immune effector cell-associated neurotoxicity syndrome | 45 (20.1) |
| Encephalopathy | 43 (19.2) |
| Multiple organ dysfunction syndrome | 38 (17.0) |
| Respiratory failure | 33 (14.7) |
| Thrombocytopenia | 32 (14.3) |
| Tachycardia | 31 (13.8) |
| Neutropenia | 29 (12.9) |
| Cytopenia | 28 (12.5) |
| Malignant neoplasm progression | 26 (11.6) |
| Sepsis | 25 (11.2) |
| Confusional state | 22 (9.8) |
| Coagulopathy | 21 (9.4) |
| Fatigue | 21 (9.4) |
| Disseminated intravascular coagulation | 20 (8.9) |
| Pancytopenia | 19 (8.5) |
| Hemophagocytic lymphohistiocytosis | 19 (8.5) |
| Pleural effusion | 18 (8.0) |
| Somnolence | 18 (8.0) |
| Atrial fibrillation | 17 (7.6) |
| Febrile neutropenia | 17 (7.6) |
| Hypogammaglobulinemia | 17 (7.6) |
| Platelet count decreased | 17 (7.6) |
| White blood cell count decreased | 17 (7.6) |
| Hemoglobin decreased | 16 (7.1) |
| Acute respiratory failure | 15 (6.7) |
| Death | 15 (6.7) |
| Diffuse large B-cell lymphoma | 15 (6.7) |
| Depressed level of consciousness | 14 (6.3) |
| Tachypnea | 14 (6.3) |
| Tremor | 14 (6.3) |
| Agitation | 13 (5.8) |
| Aphasia | 13 (5.8) |
| Blood creatinine increased | 13 (5.8) |
| Hepatic failure | 13 (5.8) |
| Hepatotoxicity | 13 (5.8) |
| Seizure | 13 (5.8) |
| Mental status changes | 13 (5.8) |
| Anemia | 12 (5.4) |
| Dyspnea | 12 (5.4) |
| Hypertension | 12 (5.4) |
| Neutrophil count decreased | 12 (5.4) |
| Septic shock | 12 (5.4) |
| Serum ferritin increased | 12 (5.4) |
| Acute lymphocytic leukemia recurrent | 12 (5.4) |
| Infection | 11 (4.9) |
| Tumor lysis syndrome | 11 (4.9) |
| Candida infection | 11 (4.9) |
| Cardiac arrest | 10 (4.5) |
| Delirium | 10 (4.5) |
| Hematocrit decreased | 10 (4.5) |
| Headache | 10 (4.5) |
| Hypervolemia | 10 (4.5) |
| Lactic acidosis | 10 (4.5) |
| Transaminases increased | 10 (4.5) |
| Staphylococcal infection | 10 (4.5) |
| Pulmonary toxicity | 10 (4.5) |
Fig. 2.
Outcome of acute renal failure reports
Disproportionality analyses are displayed in Fig. 1. The positive control, cisplatine, yielded a statistically significant IC of 1.9 (95% CI 1.85–1.93), whereas the negative control, procarbazine, showed a nonstatistically significant IC of 0.1 (95% CI −0.2 to 0.5). As a whole, CAR-T cells were significantly associated with ARF, with an IC of 1.5 (95% CI 1.3–1.7). Specifically, acute renal failure was disproportionately reported with ide-cel (IC 1.9, 95% CI 0.8–2.7), tisa-cel (IC 1.9, 95% CI 1.6–2.1), brexu-cel (IC 1.8, 95% CI 1.0–2.4), liso-cel (IC 1.6, 95% CI 0.4–2.4), and axi-cel (IC 1.1, 95% CI 0.7–1.4). ARF was mentioned in 4.2% of the total 2570 reports with tisa-cel (3.7% of the total 1945 adult reports), whereas it was mentioned in 2.4% of the 3464 reports with axi-cel (p < 0.001).
ARF was still disproportionately reported with tisa-cel after exclusion of pediatric reports (IC 1.6, 95% CI 1.3–1.9). Besides, rituximab, cyclophosphamide, doxorubicin, and vincristine (RCHO) were significantly associated with ARF (IC 0.7, 95% CI 0.66–0.74). The comparative IC of ARF with CAR-T cells (using RCHO as the background comparator) was still statistically significant (IC 0.8, 95% CI 0.6–1.0).
After exclusion of all reports mentioning CRS, there was still a significant disproportionate reporting of ARF with CAR-T cells (IC 1.0, 95% CI 0.6–1.3). Cases of ARF co-reported with CRS were compared with cases of ARF without CRS. There was no significant difference between the ages of patients suffering from ARF with and without CRS (respectively 47.8 and 52.8, p = 0.19), nor between their fatality rates (p = 0.12). However, patients presenting with ARF without CRS were more likely to be men (75.8% versus 58.4% of patients with CRS, p < 0.05). The comparison between ARF reports mentioning or not CRS is detailed in Table 3.
Table 3.
Comparison between reports of acute renal failure involving CAR-T cells, with and without cytokine release syndrome (CRS)
| Overall | With CRS | Without CRS | |
|---|---|---|---|
| Number of reports (%) | 224 (100) | 154 (68.8) | 70 (31.3) |
| Male (%) | 137 (61.2) | 87 (58.4) | 50 (75.8) |
| Median age, years (IQR) | 60 (27–70) | 57 (20–70) | 60 (44–68) |
| Median time to onset, days | 7 (3–18) | 4 (3–8) | 15 (6–146) |
| Outcome | |||
| Fatal cases (%) | 121 (54.0) | 89 (57.8) | 32 (45.7) |
| Life-threatening reaction (%) | 64 (28.6) | 55 (35.7) | 9 (12.9) |
| Others (%) | 39 (17.4) | 10 (6.5) | 29 (41.4) |
Hydroelectrolytic Disorders
As of 24 July 2022, 125 reports involving CAR-T cells mentioned at least one of the queried hydroelectrolytic disorders. Most patients were male (72, 63.2%), with a median age of 59 years (IQR 18–68, min–max 4–79). Most cases were reported in the USA (106, 84.8%), specifically by physicians or other health professionals (118, 94.4%). Tisa-cell (63 reports, 50.4%), and axi-cel (50, 40.0%) were the most frequently reported CAR-T cells. No report involved cilta-cel.
The median time to onset was 7 days (IQR 3–16, min–max 2–21). The most frequently co-reported terms were cytokine release syndrome (88, 70.4%), pyrexia (63, 50.4%), neurotoxicity (42, 33.6%), and hypotension (41, 32.8%). Hydroelectrolytic disorders and ARF occurred concomitantly in 35 reports (Fig. 3). Suspect co-reported active ingredients reported in more than five cases included cyclophosphamide (10, 8.0%) and fludarabine (10, 8.0%). All reports were deemed serious, including 57 deaths (45.6%) and 32 life-threatening reactions (25.6%).
Fig. 3.
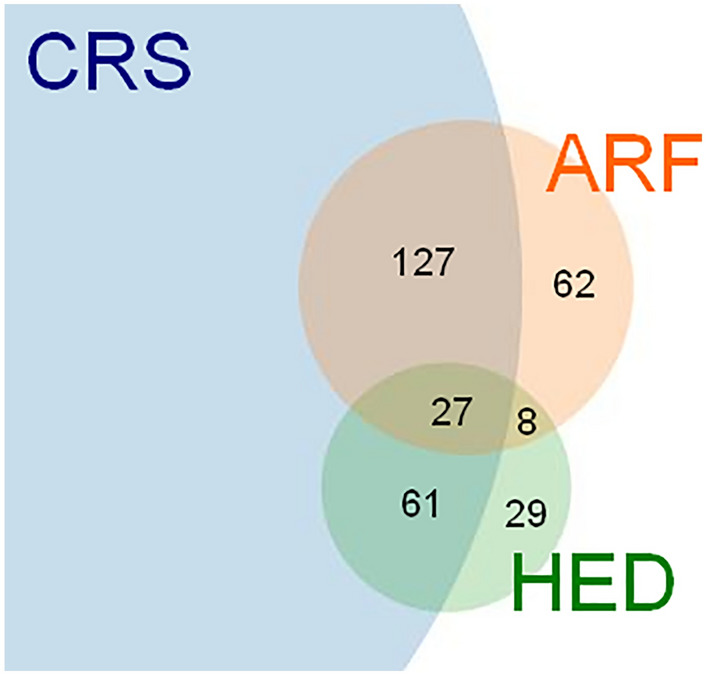
Overlap between reports of acute renal failure (ARF), hydroelectrolytic disorders (HED), and cytokine release syndrome (CRS). Numbers of reports are shown for each section
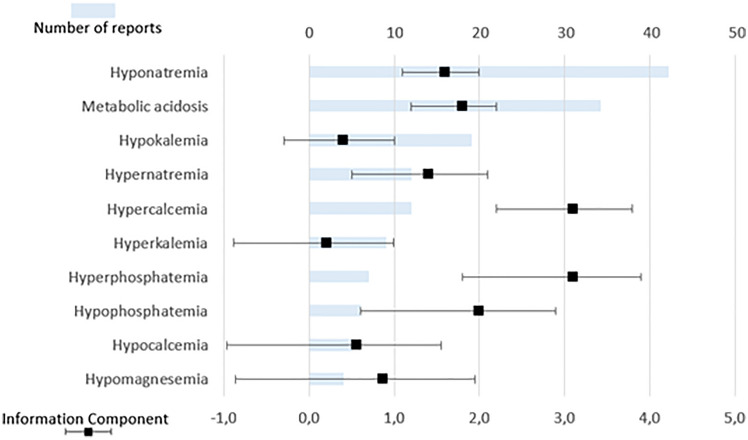
Details on the reported HED are available in Fig. 4. The most frequently reported HED was hyponatremia (42, 39.6%), followed by metabolic acidosis (34, 27.2%) and hypokalemia (19, 15.2%). CAR-T cells were involved in 0.4% and 0.3% of all reports of hyperphosphatemia and hypernatremia, respectively. They accounted for 0.1% of all reports of each hypophosphatemia and hypercalcemia, while they were involved in less than 0.1% of all reports of the other queried HEDs.
Fig. 4.
Reports of hydroelectrolytic disorders involving CAR-T cells, ranked by number of reports. Upper horizontal axis: absolute number of reports. Lower horizontal axis: information component with its 95% confidence interval
Significantly disproportionate reporting was found for hypernatremia (IC 3.1, 95% CI 2.2–3.8), hyperphosphatemia (IC 3.1, 95% CI 1.8–3.9), hypophosphatemia (IC 2.0, 95% CI 0.6–2.9), metabolic acidosis (IC 1.8, 95% CI 1.2–2.2), hyponatremia (IC 1.6, 95% CI 1.1–2.0), and hypercalcemia (IC 1.4, 95% CI 0.5–2.1). There were no reports of hypomagnesemia, nor metabolic alkalosis involving CAR-T cells. Hyperphosphatemia occurred concomitantly with CRS in all cases, and with tumor lysis syndrome in three out of seven (42.9%) cases. Otherwise, tumor lysis syndrome was mentioned in two reports of hyperkalemia, and one case of hypocalcemia.
Discussion
In 2011, Porter et al. published the first attempt to treat a patient with refractory chronic lymphocytic leukemia with CAR-T cells [3]. It turned out that the patient developed ARF in a setting of tumor lysis syndrome. As indications of CAR-T cells keep expanding, their safety profile in real-life setting is getting clearer. Apart from the well-known risks of CRS and neurotoxicity, kidney injury, albeit less frequent, may also be a concern, as suggested by a previous pharmacovigilance study, on the basis of a signal for renometabolic disorders [13]. Our study, based on the WHO drug safety database, provides an overview of the array of renal complications associated with CAR-T cell therapy. A significant safety signal was confirmed and refined regarding the association between CAR-T cells and ARF, with 224 reports analyzed. This signal was further substantiated through Bradford Hill criteria (Supplementary Information), and remains even when reports of CRS are excluded from the analysis. Furthermore, several HED are disproportionally reported in patients treated with CAR-T cells, including dysnatremia, metabolic acidosis, and hypercalcemia.
ARF has been reported in up to 30% of patients of smaller cohort studies, each based on about 15 ARF cases [17, 26, 27]. ARF accounted for around 3% of all ADRs involving CAR-T cells, which is more than previous findings in the European and American databases (up to 1%) [28]. The median time to onset was around 1 week after CAR-T cell infusion, in line with a previous case series [16]. Often overlapping with cases of ARF, severe CRS has been suggested as a likely risk factor for ARF in this setting [13]. The underlying mechanism may involve kidney hypoperfusion following vasodilation, leading to prerenal ARF or, occasionally, tubular necrosis [29]. Accordingly, some authors suggested that axi-cel might increase the risk of ARF through CRS, as this construct is believed to be associated with a higher toxicity when compared with tisa-cel [16, 27, 30, 31]. However, our findings cannot support this assumption: there were fewer reports of ARF with axi-cel, a lesser proportion of ARF among reports involving axi-cel, and a lower IC value for the association of axi-cel and ARF, compared with tisa-cel. The rationale behind this finding is not clear, but might have been influenced by the fact that tisa-cel, contrary to axi-cel, is involved in a significant number of pediatric reports [32]. In fact, among patients treated with CAR-T cells, a higher incidence and severity of ARF has been reported in the pediatric population, when compared with adults [33]. Accordingly, after exclusion of pediatric reports, the confidence intervals of the ICs for ARF with tisa-cel and axi-cel tended to overlap. The discrepancy between the safety profiles of axi-cel and tisa-cel has been previously suggested regarding other ADRs and safety signals [34]. Other hypotheses might involve the costimulatory domain (CD-28 for axi-cel, 4-1BB for tisa-cel), but have yet to be properly investigated [35].
CRS was involved in many but not all reports of ARF, and, in that respect, may not explain all cases of kidney injury. One-quarter of all cases occurred more than 18 days after CAR-T cell infusion, reports without CRS exhibiting a longer time to onset. This finding is consistent with a previous cohort, characterized by a median time to onset of 48 days for ARF, long after most CRS cases have occurred [27]. Moreover, tumor lysis syndrome, although notably nephrotoxic, could not explain most cases, neither do possible concomitant nephrotoxic drugs. Besides, proteinuric kidney injury due to hemophagocytic lymphohistiocytosis does not appear as a leading cause of ARF in the studied reports [31]. Other potential mechanisms for ARF may involve both systemic and renal consequences of sepsis in the context of B-cell aplasia, especially in patients already immunocompromised by their underlying diseases and/or previous treatments [36]. In fact, bacterial infections are reported in up to 40% of patients within 1 month after CAR-T cells treatment [37]. Viral infections are reported in near half of the patients within the first year [38]. Besides, polyomavirus reactivation has been described in patients treated with CAR-T cells [39], and BK virus reactivation is a known adverse event in patients treated with other B-cell-depleting agents, such as rituximab [40, 41]. Hence, the potential role of opportunistic infections in triggering ARF warrants further investigations. Lastly, renal infiltration by CAR-T cells might also contribute to some cases of ARF, as demonstrated by a renal biopsy taken on a kidney transplant recipient [42]. This finding must be confirmed by further renal biopsies, relying on a larger body of evidence. All in all, mechanisms of ARF are probably multifactorial in most cases, overlapping with other consequences of CAR-T cells or the underlying disease [13]. In patients with comorbidity, such as those treated with CAR-T cells, ARF may be induced by several, not mutually exclusive, factors through a “multiple-hit” hypothesis.
The high fatality rate (> 50%) in patients presenting with ARF associated with CAR-T cells is probably due to other systemic complications in high-risk patients, rather than by ARF per se. These complications, such as sepsis, may, in turn, foster the occurrence of ARF. By contrast, more than half of the surviving patients recovered their baseline kidney function, confirming the good intrinsic renal prognosis of ARF associated with CAR-T cell therapy, as suggested by previous cohorts [27].
Regarding HED, previous studies had highlighted the high incidence of hyponatremia in patients treated with CAR-T cells, corroborating package inserts [27, 31, 34, 43, 44]. Interleukin-6 secretion might be pivotal for the occurrence of hyponatremia, through increased central production of vasopressin [45]. We confirm this association, as hyponatremia is the most frequently reported HED, with a significant disproportionate reporting. A possible confounding factor arises from the fact that, before CAR-T cells, most patients received cyclophosphamide, which is a common cause of hyponatremia [46]. Hypophosphatemia has also been frequently reported in previous studies [16, 31], and may be linked with neurotoxicity [47]. Our results suggest a possible safety signal, although the absolute number of reports remains limited. Its pathophysiology remains unclear, and might be related to interleukin-6 release [48] or rapid T-cell expansion [47], or more likely to CRS and/or tumor lysis syndrome. Conversely to previous reports [14, 16, 31, 43], and although hypokalemia was frequently reported, there was no significant disproportionate reporting of dyskalemia, nor hypocalcemia, in patients treated with CAR-T cells.
This study is limited by the inherent flaws of spontaneous reporting systems and pharmacovigilance approaches. Indeed, underreporting is a well-known issue of drug safety database. Incidence cannot be estimated from spontaneous reporting data. Besides, lacking data are frequent, impeding a thorough qualitative assessment of queried cases. In particular, numerical data regarding ARF events are not available in VigiBase®, so that we could not rely on classical classifications such as KDIGO [49]. Likewise, data regarding kidney biopsies are not available. The heterogeneity in the coding of the various ADRs underpins our choice to rely on SMQ to assess kidney injuries. The large sample size may partly compensate the qualitative heterogeneity of data, and allows the characterization of the studied ADRs across the diversity of a wide spectrum of patients. However, all of these shortcomings and potential biases prevent us from drawing any definite causal conclusion on the suggested safety signals regarding CAR-T cells and ARF or HED, and this study should be considered as a signal refinement approach. The small number of reports involving BCMA-targeted CAR-T cells did not permit their comparison with CD19-targeted CAR-T cells.
All in all, this analysis of the WHO safety database provides new insights on the renal effects of CAR-T cells in real-life settings and in a nonselected population. Beyond CRS and neurotoxicity, our findings tend to confirm that ARF is a potential ADR of CAR-T cell therapy. Close cooperation between nephrologists, hematologists, and clinical pharmacologists is paramount to a deeper understanding of this multifactorial and heterogeneous array of ADRs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the UMC who manages and provided the data used in the present study. Access to VigiBase® is available without fees to Dr Fanny Rocher and the pharmacovigilance center of Nice. The views expressed in this article are the authors’ personal views and may not be understood or quoted as being made on behalf of or reflect the position of the ANSM, the EMA, the WHO, or one of their committees or working parties.
Declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of interest/Competing interests
Alexandre Olivier Gérard, Diane Merino, Alexis Charbinat, Joseph Fournier, Alexandre Destere, Michael Loschi, Thomas Cluzeau, Antoine Sicard, and Milou-Daniel Drici declare that they have no conflicts of interest that might be relevant to the contents of this manuscript. The results presented in this paper have not been published previously in whole or part.
Ethics approval
Ethics committee approval was not required for this observational study because the analysis was carried out on an anonymized pharmacovigilance database.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available because data are owned by UMC, which manages VigiBase®, but are available from the corresponding author on reasonable request.
Code availability
Not applicable
Author contributions
Research idea and study design: AOG, DM, AS, MDD; data acquisition: AOG, DM; data analysis/interpretation: AOG, DM, AD, ML, AC, TC; statistical analysis: AOG, DM; supervision or mentorship: TC, AS, MDD.
Footnotes
Antoine Sicard and Milou-Daniel Drici contributed equally to this work as last authors.
References
- 1.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.First-Ever CAR T-cell Therapy Approved in U.S. Cancer Discov. 2017;7:OF1. [DOI] [PubMed]
- 6.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma [Internet]. FDA. 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma. Accessed 10 Aug 2022.
- 8.Johnson PC, Abramson JS. Engineered T cells: CAR T cell therapy and beyond. Curr Oncol Rep. 2022;24:23–31. doi: 10.1007/s11912-021-01161-4. [DOI] [PubMed] [Google Scholar]
- 9.Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130:2594–2602. doi: 10.1182/blood-2017-06-793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12:81. doi: 10.1186/s13287-020-02128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su M, Zhao C, Luo S. Therapeutic potential of chimeric antigen receptor based therapies in autoimmune diseases. Autoimmun Rev. 2022;21:102931. doi: 10.1016/j.autrev.2021.102931. [DOI] [PubMed] [Google Scholar]
- 12.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolladille C, Ederhy S, Ezine E, Choquet S, Nguyen LS, Alexandre J, et al. Chimeric antigen receptor T-cells safety: a pharmacovigilance and meta-analysis study. Am J Hematol. 2021;96:1101–1111. doi: 10.1002/ajh.26259. [DOI] [PubMed] [Google Scholar]
- 14.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. Elsevier. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Seethapathy H, Strohbehn IA, Frigault MJ, O’Donnell EK, Jacobson CA, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis. 2020;76:63–71. doi: 10.1053/j.ajkd.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Gudsoorkar P, Jhaveri KD. Acute kidney injury in critically ill patients with cancer. Clin J Am Soc Nephrol. 2022;17:1385–1398. doi: 10.2215/CJN.15681221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VigiBase. UMC | Analytics in VigiLyze [Internet]. https://www.who-umc.org/vigibase/vigilyze/analytics-in-vigilyze/. Accessed 11 Oct 2022.
- 19.Welcome to MedDRA | MedDRA [Internet]. https://www.meddra.org/. Accessed 14 Nov 2020.
- 20.Standardised MedDRA Queries | MedDRA [Internet]. https://www.meddra.org/standardised-meddra-queries. Accessed 9 Aug 2020.
- 21.MedDRA. MedDRA Hierarchy | MedDRA [Internet]. https://www.meddra.org/how-to-use/basics/hierarchy. Accessed 3 Sep 2021.
- 22.Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting: quantitative signal detection. Pharmacoepidemiol Drug Saf. 2009;18:427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 23.Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–321. doi: 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]
- 25.Park G, Jung H, Heo S-J, Jung I. Comparison of data mining methods for the signal detection of adverse drug events with a hierarchical structure in postmarketing surveillance. Life. 2020;10:E138. doi: 10.3390/life10080138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018;29:2039–2052. doi: 10.1681/ASN.2018050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutgarts V, Jain T, Zheng J, Maloy MA, Ruiz JD, Pennisi M, et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood Marrow Transplant. 2020;26:1071–1076. doi: 10.1016/j.bbmt.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonaldo G, Montanaro N, Alberto V, Motola D. Safety profile of chimeric antigen receptor T-cell immunotherapies (CAR-T) in clinical practice. Eur J Clin Pharmacol. 2021;77:1225–1234. doi: 10.1007/s00228-021-03106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jhaveri KD, Rosner MH. Chimeric antigen receptor T cell therapy and the kidney: what the nephrologist needs to know. Clin J Am Soc Nephrol. 2018;13:796–798. doi: 10.2215/CJN.12871117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4:676–686. doi: 10.1182/bloodadvances.2019000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M, Strohbehn IA, Seethapathy HS, Rusibamayila N, Casey KS, Gupta S, et al. Acute kidney injury after the CAR-T therapy tisagenlecleucel. Am J Kidney Dis. 2021;77:990–992. doi: 10.1053/j.ajkd.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafaniello C, Ferrajolo C, Gaio M, Zinzi A, Scavone C, Sullo MG, et al. Tisagenlecleucel in children and young adults: reverse translational research by using real-world safety data. Pharmaceuticals. 2020;13:258. doi: 10.3390/ph13090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanduri SR, Cheungpasitporn W, Thongprayoon C, Petnak T, Lin Y, Kovvuru K, et al. Systematic review of risk factors and incidence of acute kidney injury among patients treated with CAR-T cell therapies. Kidney Int Rep. 2021;6:1416–1422. doi: 10.1016/j.ekir.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fusaroli M, Isgrò V, Cutroneo PM, Ferrajolo C, Cirillo V, Del Bufalo F, et al. Post-marketing surveillance of CAR-T-cell therapies: analysis of the FDA adverse event reporting system (FAERS) database. Drug Saf [Internet]. 2022. 10.1007/s40264-022-01194-z. Accessed 20 Jul 2022. [DOI] [PMC free article] [PubMed]
- 35.Cappell KM, Kochenderfer JN. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat Rev Clin Oncol. 2021;18:715–727. doi: 10.1038/s41571-021-00530-z. [DOI] [PubMed] [Google Scholar]
- 36.Joseph A, Lafarge A, Azoulay E, Zafrani L. Acute kidney injury in cancer immunotherapy recipients. Cells. 2022;11:3991. doi: 10.3390/cells11243991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph A, Lafarge A, Mabrouki A, Abdel-Nabey M, Binois Y, Younan R, et al. Severe infections in recipients of cancer immunotherapy: what intensivists need to know. Curr Opin Crit Care. 2022;28:540. doi: 10.1097/MCC.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 38.Wudhikarn K, Palomba ML, Pennisi M, Garcia-Recio M, Flynn JR, Devlin SM, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10:1–11. doi: 10.1038/s41408-020-00346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman A, Raschi E, Chapman J, Santomasso BD, Pasquini MC, Perales M-A, et al. Progressive multifocal leukoencephalopathy in patients treated with chimeric antigen receptor T cells. Blood. 2023;141:673–677. doi: 10.1182/blood.2022017386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa D, Kahwaji J, Puliyanda D, Mirocha J, Reinsmoen N, Lai C-H, et al. Polyomavirus BK viremia in kidney transplant recipients after desensitization with IVIG and rituximab. Transplantation. 2014;97:755–761. doi: 10.1097/01.TP.0000437671.78716.f3. [DOI] [PubMed] [Google Scholar]
- 41.Patel SJ, Devos JM, Knight RJ, Dawson KL, Suki WN, Gonzalez JM, et al. Effects of rituximab on the development of viral and fungal infections in renal transplant recipients. Int Sch Res Notes Hindawi. 2013;13:e819025. [Google Scholar]
- 42.de Nattes T, Camus V, François A, Dallet G, Ferrand C, Guerrot D, et al. Kidney transplant T cell-mediated rejection occurring after anti-CD19 CAR T-cell therapy for refractory aggressive Burkitt-like lymphoma with 11q aberration: a case report. Am J Kidney Dis. 2022;79:760–764. doi: 10.1053/j.ajkd.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Uppal NN, Workeneh BT, Rondon-Berrios H, Jhaveri KD. Electrolyte and acid–base disorders associated with cancer immunotherapy. Clin J Am Soc Nephrol. 2022;17:922–933. doi: 10.2215/CJN.14671121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon BN, Daley RJ, Horvat TZ, Buie LW, Hsu M, Latcha S, et al. Risk of hyponatremia and associated clinical characteristics in patients with acute lymphoblastic leukemia after CD19 targeted chimeric antigen receptor (CAR) T-cells. Blood. 2017;130:3584. [Google Scholar]
- 45.Dixon BN, Daley RJ, Buie LW, Hsu M, Park JH, Brentjens RJ, et al. Correlation of IL-6 secretion and hyponatremia with the use of CD19+ chimeric antigen receptor T-cells. Clin Nephrol. 2020;93:42–46. doi: 10.5414/CN109872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008;52:144–153. doi: 10.1053/j.ajkd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Tang JP, Peters CW, Quiros C, Wang X, Nowicki TS. Abstract 2760: Hypophosphatemia due to increased effector cell metabolic activity is associated with neurotoxicity symptoms in anti-CD19 CAR T cell therapy. Cancer Res. 2022;82:2760. doi: 10.1158/1538-7445.AM2022-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94:315–325. doi: 10.1016/j.kint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Khwaja A. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.