Abstract
Chronic graft-versus-host disease (cGVHD) is a major complication of allogeneic hematopoietic cell transplantation and a leading cause of long-term morbidity, non-relapse mortality, and impaired health-related quality of life. The skin is commonly affected and presents heterogeneously, making the role of dermatologists critical in both diagnosis and treatment. In addition, new clinical classification and grading schemes inform treatment algorithms, which now includes 3 FDA approved therapies, and evolving transplant techniques are changing disease epidemiology. Part I reviews the epidemiology, pathogenesis, clinical manifestations, and diagnosis of cGVHD. Part II discusses disease grading and therapeutic management.
Keywords: graft-versus-host disease, medical dermatology, transplantation
Introduction
Hematopoietic cell transplantation (HCT) utilizes hematopoietic progenitor cells to (re)establish hematopoietic and immune function. Indications for HCT include malignant and nonmalignant hematologic diseases, diseases of the immune system, some solid tumors, and inherited disorders of metabolism. Over 1.5 million HCTs have been performed worldwide to date (1) and >21,000 HCTs were performed in the United States (US) in 2018 (2). Due to advances in transplant regimens and supportive care, patients are surviving longer after HCT. The projected prevalence of survivors in the US is expected to double to over 500,000 in the next 10 years (3).
HCT can broadly be divided into autologous HCT, which uses the patient’s own progenitor cells, and allogeneic HCT, which uses donor cells. While graft-versus-host disease (GVHD) can occur rarely following solid organ transplantation, autologous HCT, or blood product transfusions (4), the focus of this CME will be GVHD following allogeneic HCT.
GVHD is classified into acute and chronic forms, which are increasingly recognized as distinct entities (5). In 2005, the National Institutes of Health (NIH) Consensus Development Project established the current classification scheme based on clinical features (Figure 1) (6). Patients with chronic GVHD (cGVHD) are further classified into (1) classic cGVHD (no features of acute GVHD) or (2) overlap cGVHD (concurrent acute GVHD features). The latter has been associated with poorer prognosis (7-10).
Figure 1. Graft-versus-host disease classification.
Acute GVHD is defined by characteristic clinical features of the skin, liver, and gastrointestinal tract and can occur as classic acute GVHD (prior to day 100) or late acute GVHD (after day 100). Chronic GVHD is defined by 2014 NIH Diagnostic Criteria of involved organs and can occur as classic chronic GVHD (without acute GVHD features) or chronic overlap GVHD (with acute GVHD features).
Adapted from Lee SJ Classification systems for chronic graft-versus-host disease, Blood, 2017. 129(1): 30-37
Epidemiology and Risk factors for Chronic GVHD
Key points
Chronic GVHD is a leading cause of morbidity, non-relapse mortality, and impaired health-related quality of life after HCT
The skin and mucous membranes are the organ systems most frequently involved in cGVHD
Chronic GVHD is a multisystem syndrome that occurs in 30-70% of allogeneic HCT recipients (11-15). The skin (60-80%), oral (60%) and ocular (50%) mucosal surfaces, and genital area (20-50%) are frequently involved, with the latter likely under-reported (16-19).
Chronic GVHD Morbidity and Mortality
Among long-term (≥ 2 years) allogeneic HCT survivors, cGVHD is a substantial contributor to morbidity and a leading cause of death, exceeded only by relapse of primary disease (14, 20-24). Non-relapse mortality (NRM) in cGVHD patients is 22% at 5 years and 40% at 12 years (25). Higher cGVHD skin scores are associated with an increased risk of NRM (HR 1.9, p=0.002) and this association persists even when controlling for patient age, with cGVHD resulting in a 30-40% increased risk of NRM in all age groups and lower overall survival (OS) (26).
Mortality is attributable to cGVHD complications as well as immunosuppressive therapy. Allogeneic HCT is associated with increased risk of second cancers (27-30), with risk further increased 2.4-fold in patients with cGVHD (30). Keratinocyte carcinomas (10-year cumulative incidence of 15.5% (29, 31)) and squamous cell carcinomas of the lip, tongue, and mouth (13-fold risk (30)) (32) are amongst the highest but lung, liver, brain, soft tissue cancers, and melanoma are also seen (29, 30). Chronic GVHD increases the risk of cardiovascular disease through accelerated arterial disease, dyslipidemia, and hyperglycemia (33), which are further exacerbated by immunosuppressive therapies (24, 33-35). Infections, primarily from bacterial pathogens, are a leading cause of late deaths in adults and children with cGVHD (36), as well as pulmonary complications from bronchiolitis obliterans syndrome (37).
Chronic GVHD is associated with significant healthcare burden (38). In a US-based claims analysis, patients had a mean of 21 visits in the first year after cGVHD diagnosis and generated costs of nearly $300,000 per patient/year (13). Patients living with cGVHD are almost two-fold more likely to develop another severe or life-threatening illness (39) and also experience poor functional status, psychosocial distress, poor social functioning, anxiety, and less resilience (40-42). Skin severity alone correlates with worse health-related quality of life (hrQoL) (43, 44).
Chronic GVHD Risk Factors
The most important predictor of GVHD is the degree of HLA match between donor and recipient (45-48), but several other important risk factors influence the likelihood of cGVHD (Table 1). Use of peripheral blood stem cells (PBSC) as the graft source significantly increases the risk of cGVHD (19, 49-52). One study reported severe skin cGVHD in one-third of PBSC recipients (52) and two studies have demonstrated PBSC as a risk factor for skin sclerosis (53, 54). Total body irradiation (TBI) is an additional risk factor for cGVHD (55) and the development of sclerosis (53, 54). Vitamin D deficiency has been identified as a possible risk factor for cGVHD with repletion shown to decrease incidence and/or severity in some studies (56).
Table 1:
Risk Factors for Chronic GVHD
| Risk Factor | Reference | |
|---|---|---|
| Donor-related Factors | PBSCsa | (5, 19, 49-52, 145-147) |
| Female donor/male recipient | (5, 145, 148-150) | |
| Parity of female donor | (5, 149-151) | |
| HLA mismatch | (5, 45-48, 147) | |
| Unrelated donor | (5, 145, 152) | |
| Older donor age | (5, 149, 153) | |
| Recipient-related Factors | Older patient age | (5, 145, 149, 150) |
| CML | (149, 150) | |
| High intensity conditioning | (154) | |
| TBIa | (53-55) | |
| Busulfanb | (37) | |
| Prior acute GVHD | (5, 57, 147, 149, 150) | |
| DLI | (155) |
Risk factors for skin cGVHD
Risk factor for pulmonary cGVHD
Abbreviations: PBSCs, peripheral blood stem cells; HLA, human leukocyte antigens; CML, chronic myelogenous leukemia; TBI, total body irradiation; GVHD, graft-versus-host disease; DLI, donor lymphocyte infusion
Chronic GVHD Prophylaxis Strategies
There has been significant focus on improving GVHD prophylaxis to reduce GVHD incidence. Facilitating or augmenting graft-versus-tumor effect while minimizing GVHD has been a longstanding, but elusive, goal of allogeneic HCT and is an ongoing research priority (57). Calcineurin inhibitor/methotrexate has been the standard of care for GVHD prophylaxis (58, 59); however, in a recent 3-arm trial compared two calcineurin-free regimens (post-transplant cyclophosphamide [PTCy] alone or CD34 selection) to tacrolimus/methotrexate, CD34-selected grafts led to significantly less cGVHD but higher mortality, while PTCy had similar cGVHD to controls (60). Other forms of T cell depletion (antithymocyte globulin (ATG) (61-63), alemtuzumab (64-66)) have reduced cGVHD, but at the expense of increased relapse and infections. Graft engineering and targeting of specific donor T cell subsets offers the potential for better control of GVHD in the future (a full discussion of these approaches can be found in a recent excellent summary (57)).
Pathophysiology
Key points
Chronic GVHD pathophysiology can be conceptualized into three phases: 1) tissue injury and early inflammation, 2) dysregulated B and T cell immunity, and 3) fibrosis
Therapeutic targets are broad in scope; the FDA-approved therapies target B and T cell signaling
Phase 1: Tissue Injury and Early Inflammation
The earliest phase of cGVHD is characterized by activation of the innate immune system following damage to the host tissue (67). Pre-transplantation conditioning regimens, post-transplantation infections, and acute GVHD result in tissue injury, leading to release of damage associated molecular patterns (DAMPs) and pathogen associated molecular patterns (PAMPs) (68, 69). DAMPs and PAMPs activate toll-like receptors (TLR), nucleotide-binding oligomerization domain-like receptors (NOD-R), and the NOD-like receptor 3 (NLRP3) inflammasome. Tissue damage is subsequently amplified by innate immune cells that produce reactive oxygen species and matrix metalloproteinases (70). TLR and NOD-R activation further stimulates antigen presentation, initiating the adaptive immune response that characterizes phase 2. Endothelial cells damaged from tissue injury release von Willebrand factor and activate platelets, resulting in microvascular damage. Intimal arteritis and loss of small and medium-sized arteries have been observed in histopathologic examination of skin (71). TLRs, NLRP3, and NOD-R are current experimental therapeutic targets (67).
Phase 2: Chronic Inflammation and Dysregulated B and T Cell Immunity
B and T cells are activated via a specific ligand-receptor interaction with antigen presenting cells (APCs). The generation of autoreactive B and T cells is primarily driven by loss of central and peripheral tolerance, and results in reactivity to antigens common to both donor and recipient (72, 73). This process mimics autoimmunity and diverges from acute GVHD, which is driven by alloreactivity. Central tolerance is compromised by thymic injury caused by medications and immune insults (74-76). Malfunction of the thymus allows escape of autoreactive CD4+ T cells (77-80). In humans, thymic production of mature T cells is greatly impaired (81). Loss of peripheral tolerance is driven by impairment in regulatory T cells (Tregs), amongst others (82-90).
Autoreactive T cells differentiate into Th1, Th2, and TH17 subsets, and dysregulated production of cytokines such as IL-17 can drive chronic inflammation (91-93). In skin samples of lichenoid cGVHD, a Th1/Th17 signature is seen, including elevation of cytokine and chemokine transcripts associated with IL-17 producing T cells (94). Activated follicular helper T cells (FhT cells) release IL-21 and lead to B cell somatic hypermutation with germinal center formation and production of immunoglobulins associated with skin cGVHD (95). Indeed, mature autoreactive B cells are necessary for cGVHD development and for autoreactive T and B cell amplification (96-98). The majority of existing therapies for cGVHD, including the three FDA-approved medications, target the adaptive phase (Phase 2) (99).
Phase 3: Aberrant Tissue Repair with Fibrosis
Activated macrophages produce transforming growth factor-beta (TGF-β) and platelet-derived growth factor-alpha (PDGF-α), which stimulate fibroblasts to produce extracellular matrix collagen and biglycan, resulting in tissue fibrosis (67, 100, 101). Th17 cells that escape immune regulation maintain the fibrotic process and are found in higher concentrations in fibrotic human liver tissue (93, 101). B-cell isotype switching and Ig deposition, driven by B-cell activating factor (BAFF), leads to further tissue fibrosis (102-104). Anti-fibrotic therapies include the PDGF-α receptor inhibitor imatinib (105-108) and the TGF-β inhibitor, pirfenidone (109).
Diagnostic Criteria and Clinical Manifestations of Chronic GVHD
Chronic skin GVHD
Key points
Diagnostic manifestations of skin cGVHD include poikiloderma, lichen planus-like, deep sclerosis, morphea-like, and lichen sclerosus-like features
Sclerotic cGVHD confers significant morbidity, including impaired mobility and cutaneous ulceration
The 2014 NIH diagnostic criteria divides cGVHD skin manifestations into 1) diagnostic: sufficient for diagnosis and not requiring biopsy, 2) distinctive: insufficient for diagnosis and requiring biopsy, 3) other: acknowledged as a cGVHD feature if diagnosis confirmed elsewhere, 4) common: seen in both acute and cGVHD (Table 2) (6). A survey of 72 transplant centers in 3 countries supported the NIH recommendations that skin biopsy is indicated in suspected skin cGVHD lacking diagnostic features, recommending punch biopsy for nonsclerotic disease and incisional biopsy for sclerotic disease (110). However, in the authors’ experience, a 5-6mm punch biopsy is typically sufficient to render the diagnosis of sclerotic cGVHD when a clinical diagnosis is uncertain. The histology of skin cGVHD evolves over time, is modified by treatment, and can overlap with features of acute GVHD. Histologic criteria for nonsclerotic GVHD include apoptosis in the basilar layer, vacuolar changes, lichenoid inflammation, and lymphocyte satellitosis (Figure 2A), but biopsy may not be diagnostic (111). Sclerosis can present with thickened collagen bundles at any level of the dermis or subcutis (Figure 2B). The common features category assists clinicians in correctly classifying patients with overlap cGVHD (6).
Table 2:
Criteria for Mucocutaneous Chronic Graft-versus-host Diseasea
| Organ Site | Diagnosticb | Distinctivec | Other featuresd | Commone |
|---|---|---|---|---|
| Skin | Poikiloderma Lichen planus-Sclerosis Morphea-like Lichen sclerosus |
Depigmentation Papulosquamous lesions |
Sweat impairment Ichthyosis Keratosis pilaris Hypopigmentation Hyperpigmentation |
Erythema Maculopapular rash Pruritus |
| Nails | Dystrophy Longitudinal ridging Onycholysis Pterygium unguis Nail loss (symmetric, most nails) |
|||
| Scalp and hair | Scarring or nonscarring alopecia Loss of body hair Scaling |
Thinning scalp hair Premature gray hair |
||
| Mouth | Lichen planus | Xerostomia Mucoceles Mucosal atrophy Ulcers Pseudomembranes |
Gingivitis Mucositis Erythema Pain |
|
| Eyes | Dry eye Cicatricial conjunctivitis Keratoconjunctivitis sicca Confluent areas of punctate keratopathy |
Photophobia Periorbital hyperpigmentation Blepharitis |
||
| Genitalia | Lichen planus Lichen sclerosus Females: vaginal scarring or clitoral/labial agglutination Males: Phimosis or urethral scarring |
Erosions Fissures Ulcers |
Jagasia MH. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-host Disease: The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 2015,21:389-401
Sufficient to establish a diagnosis of chronic GVHD
Seen in chronic GVHD but insufficient to establish diagnosis. Must exclude infection, drug reaction, malignancy, and other causes with confirmatory testing.
Acknowledged as part of chronic GVHD if diagnosis confirmed
Seen in acute and chronic GVHD
Figure 2: Histologic features of chronic skin graft-versus-host disease.
A) Nonsclerotic-type in a patient with lichen planus-like involvement (see Figure 3B for clinical pathologic correlation) demonstrating vacuolar interface changes at the dermal-epidermal junction, with scattered apoptotic basal epidermal keratinocytes, and focal detachment of the epidermis from the subjacent dermis (hematoxylin and eosin, 20x). B) Sclerotic-type in a patient with deep-seated involvement resembling eosinophilic fasciitis demonstrating thickening of collagen bundles in the deep reticular dermis near the border with the subcutaneous fat and marked thickening of the fat septae. The epidermis and papillary dermis appear relatively normal (hematoxylin and eosin, 2x).
Significant efforts are underway to identify early disease. Candidate diagnostic biomarkers are in early discovery and include soluble B cell activating factor (sBAFF), chemokine (C-X-C motif) ligand 9/10 (CXCL9/10), and suppression of tumorigenicity 2 (ST2) (112, 113). Clinician and patient education, telehealth, and electronic reporting tools have been implemented to improve diagnostic accuracy (15). The skin presents early in the disease course of cGVHD and dermatologists play a critical role in establishing the diagnosis of cGVHD.
Nonsclerotic chronic GVHD
Nonsclerotic cGVHD refers to all skin manifestations without superficial or deep tissue fibrosis (Table 2). Nonsclerotic cGVHD presents earlier and is more common than sclerotic disease, and carries prognostic significance (17, 114). Lichenoid changes at presentation (115), extent of erythema (116), and patient-reported pruritus (117) have been associated with worse OS. It remains unclear whether nonsclerotic disease is a direct driver of mortality or merely a marker of overall disease activity. Regardless, early recognition and diagnosis of nonsclerotic disease can risk stratify patients for early referral to clinical trials.
Lichen planus (LP)-like GVHD has long been recognized as a diagnostic manifestation of nonsclerotic cGVHD (6) and involves atypical areas such as the face, neck, palms, soles and may have a blaschkoid appearance (Figure 3A,B) (118, 119). Papulosquamous lesions, although common in GVHD, are considered distinctive (not diagnostic) and, therefore, require biopsy for confirmation. Patients may present with eczematous features, ichthyosis, exfoliative dermatitis, keratosis pilaris-like involvement or pruritus (120-122). Dyspigmentation has been reported in up to 96% of patients with skin cGVHD and may cause significant psychological impact (123) (Figure 3C) (124). Skin of color patients with a history of skin cGVHD reported more skin bother from dyspigmentation than from active sclerosis (125).
Figure 3. Nonsclerotic features of chronic skin graft-versus-host disease.
A) Lichen planus-like, back, B) Lichen planus-like, forehead, C) Dyspigmentation, D) Nail dystrophy, pterygium
Vitiligo and alopecia areata have been reported in 5-20% of patients (123, 126, 127). The use of a female donor for male recipient was an independent risk factor in multivariable regression models. Cutaneous lupus erythematosus (128), dermatomyositis (129), and epidermolysis bullosa acquisita-like presentations have also been described (130). While autoantibodies were previously implicated as a risk factor for cGVHD, two recent large cross-sectional studies found no association between the presence of autoantibodies and cGVHD (54, 131). Nail dystrophy (Figure 3D) and diffuse alopecia are also common, reported in up to 20% of patients with skin cGVHD. In a case-control study, hair and nail involvement were associated with oral and ocular involvement (132).
Sclerotic chronic GVHD
Sclerotic cGVHD encompasses all cutaneous disease manifestations with superficial and/or deep sclerosis (Table 2). In a cohort of 977 patients with cGVHD, sclerotic cGVHD was present in 7% of patients at initial diagnosis and increased to 20% at 3 years after transplant (53). Sclerotic disease can occur de novo, concurrently with nonsclerotic disease, or following prior nonsclerotic disease (17, 53). In a cohort of 423 allogeneic HCT patients, only 36.4% of patients with sclerotic GVHD had a history of nonsclerotic disease (133). In 2 large observational studies, sclerotic cGVHD was not associated with OS, NRM, or malignancy relapse (53, 54).
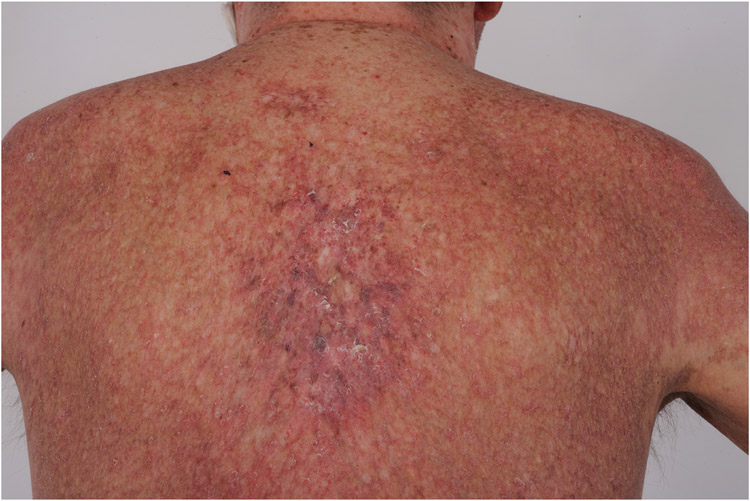
Lichen sclerosus (LS)-like lesions of cGVHD occur most commonly on the neck and upper back (Figure 4A) (134). Morphea-like cGVHD can cause contractures of overlying joints (Figure 4B) and can localize to sites of skin injury (isomorphic response) including ultraviolet radiation, radiotherapy, viral infection (e.g. herpes zoster) and pressure (Figure 4C) (135, 136). Deep sclerosis and fascial disease (Figure 4D) (134, 137) resembles subcutaneous morphea and eosinophilic fasciitis and may be insidious in onset. Joint contractures may develop without epidermal skin changes. The presence of new onset limb edema in HCT patients, particularly in the absence of other potential etiology, should prompt evaluation for new onset skin sclerosis. In contrast to systemic sclerosis, sclerotic cGVHD often clinically spares acral skin. Sclerotic cGVHD confers significant morbidity and is often refractory to treatment. Standard photographic range of motion (PROM) assessment can help monitor for worsening joint mobility (Figure 4E) (6). Chest wall restriction may reductions lung vital capacity (Figure 4F) (54). Sclerosis predisposes patients to poorly healing wounds (Figure 4G). Vascular proliferations (GVHD-associated angiomatosis) (Figure 4H) (138) and cutaneous calcifications may also develop (Figure 4I).
Figure 4. Sclerotic features of chronic skin graft-versus-host disease.
A) Lichen sclerosus-like, B) Morphea-like, overlying joint causing contracture, C) Isomorphic (lichen planus-like and morphea-like), buttocks at area of pressure, D) Fasciitis groove sign, E) Sclerosis causing hand contracture, F) Sclerosis causing chest wall restriction, G) Leg ulcers, H) GVHD-associated angiomatosis, I) Cutaneous calcifications
Chronic oral GVHD
Key Points
Oral cGVHD is often preceded by new-onset dry mouth and oral sensitivity to spicy foods
Oral cGVHD initially resemble mucositis, but may evolve into tissue fibrosis
Chronic GVHD affects the oral cavity in three domains: (1) oral mucosa, (2) exocrine salivary gland, and (3) reduced oral opening (139). A diagnosis of oral cGVHD may be made clinically or through biopsy of the oral mucosa (Table 2) (6). Histologic diagnosis may be confounded by immunosuppressive therapy as well as intra-oral infection. The oral mucosa can be examined using a light source, tongue blade and a gauze square to manipulate the tongue. Reduced oral opening is often secondary to perioral skin fibrosis or, less frequently, fibrotic banding of the oral mucosa or involvement of the temporomandibular joint (6).
Symptoms of oral cGVHD include oral sensitivity, particularly to spice, mint, carbonation, acidic, and hard foods, oral pain, difficulty eating, and xerostomia. New-onset xerostomia and sensitivity to spicy foods are frequently the first changes reported by patients.
Clinical signs of oral cGVHD include LP-like changes on the buccal mucosa, vermillion lip and labial mucosa, tongue, palate, or gingiva. Lichenoid oral cGVHD is characterized by white reticular streaks that resemble Wickham’s striae (Figure 5A-B) (6). These can be accompanied by oral ulcers that may be covered by a pseudomembrane, mucosal erythema (Figure 4C), patchy hyperkeratosis including diffuse or dense flat white plaques (Figure 4D), or papillary atrophy of the tongue (Figure 4E). Edema associated with mucosa inflammation can restrict the ductal opening from the minor salivary glands into the oral cavity, which induces mucoceles (Figure 4F).
Figure 5. Chronic oral graft-versus-host disease.
A) Lichen planus-like, vermillion lips, B) Lichen planus-like, buccal mucosa, C) mucosal erythema, gingiva, D) Patchy hyperkeratosis with associated erythema, E) Papillary atrophy and hyperkeratosis of the dorsal tongue, F) Palatal mucoceles
Infectious entities may mimic oral cGVHD and should be ruled out and/or sufficiently treated prior to establishing a diagnosis. Viral pathogens include recrudescent herpes simplex virus, human papillomavirus, cytomegalovirus, and adenovirus. Viral-induced oral lesions are often smaller and more painful than cGVHD-related ulcers, but confirmatory testing should be performed with polymerase chain reaction, histopathology or in situ hybridization. Fungal overgrowth can present as soft white hyperkeratotic material that may be scraped away, erythematous, tender mucosa or superficial oral ulcers. These infections often are accompanied with a burning sensation on the tongue and mucosa.
Chronic Genital GVHD
Key points
Genital cGVHD presents as discomfort, dyspareunia, or vaginal or penile adhesions
Genital cGVHD consists of lichen sclerosus and lichen planus-like features, adhesions and scarring.
Diagnosis of genital cGVHD is based on diagnostic and distinctive features (Table 2) (6). Biopsy can assist in diagnosis if features are non-diagnostic, particularly if cGVHD has not been confirmed at another anatomic site (140), and is also indicated if there is suspicion for neoplasia. However, biopsy may be nondiagnostic even with diagnostic examination findings and healing is often difficult (141). At minimum, external genital involvement can be examined during the dermatology visit by spreading the labia majora and touching the vestibular gland openings, labia minora and majora gently with a cotton swab. If provider and patient are comfortable, palpation of the vaginal walls with a single digit can screen for bands, shortening, narrowing or other signs of vaginal scarring.
Vulvovaginal cGVHD typically presents with pain, burning, dyspareunia, dysuria, or vaginal obstruction, but may also be asymptomatic and found incidentally on examination. Patients may report difficulty with penetration or other vaginal insertion (e.g. sexual intercourse, medication applicators). Moderate to severe pain in the absence of sexual activity suggests more severe disease.
Vulvovaginal cGVHD primarily affects mucosal surfaces, including the medial aspects of the labia majora, labia minora, clitoral hood, and vagina. LS-like (Figure 6A) or LP-like findings may be present, including white patches, hyperkeratosis, or Wickham’s striae. In our experience, cGVHD is not associated with severe vulvar pruritus, severely thickened plaques, or hemorrhagic or bullous lesions, in contrast with idiopathic LS and LP.
Figure 6. Chronic vulvovaginal graft-versus-host disease.
A) Lichen sclerosus-like with hypopigmented, thickened, adhesed left labium minus; fissures at right labium minus and posterior fourchette, B) Vulvar patchy erythema and erosions.
Vulvovaginal cGVHD also shares features with genitourinary syndrome of menopause (GSM), in which hypoestrogenism causes vulvovaginal atrophy and urinary tract changes. Premature ovarian failure occurs in more than 95% of women following HCT and many patients have concurrent GSM and genital cGVHD (142). Erythema (Figure 6B) and fissures (Figure 6A) can occur in both GSM and genital cGVHD; however, erosions (Figure 6B) and ulcers are characteristic of cGVHD. Architectural changes such as resorption or agglutination of the labia minora, adhesions of the clitoral hood, or mild introital narrowing or vaginal shortening can be seen in GSM and genital cGVHD. Dense scarring, tissue retraction, or the presence of intravaginal adhesions favors cGVHD. Vaginal adhesions can preclude access to the cervix for pelvic examination or cervical cancer screening. Vulvovaginal cGVHD has been observed infrequently in prepubertal girls (143).
In penile cGVHD, the glans penis, urethra or meatus may be involved (142), with signs including noninfectious balanoposthitis (Figure 7A), LS-like or LP-like features (Figure 7B), phimosis, or urethra or meatus scarring (Table 2) (144).
Figure 7. Chronic male genital graft-versus-host disease.
A) Noninfectious balanitis with erythema and erosion of the coronal sulcus, B) Lichen planus-like with flat-topped papules with overlying scale
Evaluation of multi-system chronic GVHD
Once a diagnosis of mucocutaneous cGVHD has been established, a thorough review of systems with appropriate specialist referral and diagnostic testing should be performed to evaluate the presence of cGVHD in other organs (Table 3).
Table 3:
Evaluation of Multi-system Chronic Graft-versus-host Diseaseaa
| Organ | ROS | Positive ROS | Negative ROS |
|---|---|---|---|
| Skin | Skin feels tight or hard, increased dryness, pruritus, rash, discoloration? | Dermatology consultation: clinical evaluation, biopsy, photographs, every 3 months or more often | Dermatology consultation: yearly |
| Sweat glands | Inability to sweat or to keep body warm? | ||
| Skin appendages | Loss of hair or nail changes? | ||
| Fasciae/joints | Stiffness or pain in the wrists, fingers, or other joints? | Assessment of range of motion: every visit Physical therapy consultation: every 3 months or more often |
Assessment of range of motion: every visit |
| Eyes | Eye dryness, sensitivity to wind or dry environments, pain? | Ophthalmology consultation: every 3-6 months or more often | Ophthalmology consultation: 100 days after transplant, yearly |
| Mouth | Oral dryness, taste alterations, sensitivities (spicy, carbonate drinks, toothpaste), sores, pain? | Dental or oral medicine consultation: every 3-6 months or more often | Dental or oral medicine consultation: yearly or at symptom onset |
| Esophagus | Food or pills get stuck upon swallowing? | GI consultation for endoscopy | |
| Lungs | Cough, shortness of breath, wheezes? | PFTs: 100 days after transplant, monthly for at least 3 months | PFTs: 100 days after transplant, every 3 months for 1 year, then as needed |
| Genital tract | Vaginal or penile pain, dyspareunia, dysuria, or sense of obstruction? | Gynecology/urogynecology consultation (female), Urology consultation (male) | Gynecology consultation: yearly or at symptom onset |
| Weight loss | Unexplained weight loss or inability to gain weight? | Weight every visit | Weight: every visit |
Adapted from Flowers ME. How we treat chronic graft-versus-host disease. Blood. 2015, 125(4):606-615 Abbreviations: Review of systems, ROS; Gastroenterology, GI; pulmonary function tests, PFTs
Learning Objectives.
After completing this learning activity, participants should be able to identify patient and transplant-related risk factors for chronic graft-versus-host disease, diagnose cutaneous, oral, and genital chronic GVHD, and screen patients for multi-system involvement.
Acknowledgements:
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Dental and Craniofacial Research of the National Institutes of Health.
Abbreviations and Acronyms
- HCT
Hematopoietic cell transplantation
- US
United States
- GVHD
Graft-versus-host disease
- MHC
Major histocompatibility complex
- cGVHD
Chronic graft-versus-host disease
- NRM
Non-relapse mortality
- OS
Overall survival
- PBSC
Peripheral blood stem cells
- TBI
Total body irradiation
- PAMPs
Pathogen associated molecular patterns
- DAMPs
Damage associated molecular patterns
- TLR
Toll like receptor
- NOD-R
Nucleotide-binding oligomerization domain-like receptors
- NLRP3
NOD-like receptor 3
- Ig
Immunoglobulin
- APCs
Antigen presentation cells
- Tregs
T regulatory cells
- FhT cells
Follicular helper T cells
- ATG
Anti-thymocyte globulin
- PTCy
Post-transplant cyclophosphamide
- TGF-B
Transforming growth factor beta
- PDGF-a
Platelet derived growth factor alpha
- BAFF
B cell activation factor
- hrQoL
Health-related quality of life
- LP
Lichen planus
- PROM
Photographic range of motion
- LS
Lichen sclerosus
- GSM
Genitourinary syndrome of menopause
- sBAFF
Soluble B cell activating factor
- CXCL9/10
Chemokine (C-X-C motif) ligand 9/10
- ST2
Suppression of tumorigenicity 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None declared
Patient Consent: Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2020;26(8):e177–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(10):1498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kline J, Subbiah S, Lazarus HM, van Besien K. Autologous graft-versus-host disease: harnessing anti-tumor immunity through impaired self-tolerance. Bone marrow transplantation. 2008;41(6):505–13. [DOI] [PubMed] [Google Scholar]
- 5.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(10):1207–15. [DOI] [PubMed] [Google Scholar]
- 8.Pidala J, Vogelsang G, Martin P, Chai X, Storer B, Pavletic S, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97(3):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora M, Pidala J, Cutler CS, Chai X, Kurland B, Jacobsohn DA, et al. Impact of prior acute GVHD on chronic GVHD outcomes: a chronic graft versus host disease consortium study. Leukemia. 2013;27(5):1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Simón JA, Encinas C, Silva F, Arcos MJ, Díez-Campelo M, Sánchez-Guijo FM, et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: the national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008; 14(10):1163–71. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9(4):215–33. [DOI] [PubMed] [Google Scholar]
- 12.Arora M, Klein JP, Weisdorf DJ, Hassebroek A, Flowers ME, Cutler CS, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood. 2011;117(24):6714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachier CR, Aggarwal SK, Hennegan K, Milgroom A, Francis K, Dehipawala S, et al. Epidemiology and Treatment of Chronic Graft-versus-Host Disease Post-Allogeneic Hematopoietic Cell Transplantation: A US Claims Analysis. Transplant Cell Ther. 2021;27(6):504.e1–.e6. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98(6):1695–700. [DOI] [PubMed] [Google Scholar]
- 15.Kitko CL, Pidala J, Schoemans HM, Lawitschka A, Flowers ME, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplant Cell Ther. 2021;27(7):545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zantomio D, Grigg AP, MacGregor L, Panek-Hudson Y, Szer J, Ayton R. Female genital tract graft-versus-host disease: incidence, risk factors and recommendations for management. Bone marrow transplantation. 2006;38(8):567–72. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsohn DA, Kurland BF, Pidala J, Inamoto Y, Chai X, Palmer JM, et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood. 2012;120(13):2545–52; quiz 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinelli S, Chiodi S, Costantini S, Van Lint MT, Raiola AM, Ravera GB, et al. Female genital tract graft-versus-host disease following allogeneic bone marrow transplantation. Haematologica. 2003;88(10):1163–8. [PubMed] [Google Scholar]
- 19.Flowers ME, Parker PM, Johnston LJ, Matos AV, Storer B, Bensinger WI, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–9. [DOI] [PubMed] [Google Scholar]
- 20.Socié G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–14. [DOI] [PubMed] [Google Scholar]
- 22.Boyiadzis M, Arora M, Klein JP, Hassebroek A, Hemmer M, Urbano-Ispizua A, et al. Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7,489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin Cancer Res. 2015;21(9):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFilipp Z, Alousi AM, Pidala JA, Carpenter PA, Onstad LE, Arai S, et al. Nonrelapse mortality among patients diagnosed with chronic GVHD: an updated analysis from the Chronic GVHD Consortium. Blood Adv. 2021;5(20):4278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt VR, Wang T, Chen K, Kitko CL, MacMillan ML, Pidala JA, et al. Chronic Graft-versus-Host Disease, Nonrelapse Mortality, and Disease Relapse in Older versus Younger Adults Undergoing Matched Allogeneic Peripheral Blood Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Analysis. Transplant Cell Ther. 2022;28(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tichelli A, Beohou E, Labopin M, Socié G, Rovó A, Badoglio M, et al. Evaluation of Second Solid Cancers After Hematopoietic Stem Cell Transplantation in European Patients. JAMA Oncol. 2019;5(2):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton LM, Saber W, Baker KS, Barrett AJ, Bhatia S, Engels EA, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Subsequent Neoplasms Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(3):367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaar DA, Pirsl F, Holtzman N, Steinberg SM, Nashed J, Ruben C, et al. Subsequent Cancers in Patients Affected with Moderate or Severe Chronic Graft-versus-Host Disease. Transplant Cell Ther. 2021;27(11):937.e1–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105(10):3802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armenian SH, Chemaitilly W, Chen M, Chow EJ, Duncan CN, Jones LW, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Cardiovascular Disease and Associated Risk Factors Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(2):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armenian SH, Yang D, Teh JB, Atencio LC, Gonzales A, Wong FL, et al. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018;2(14):1756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2010;116(8):1197–204. [DOI] [PubMed] [Google Scholar]
- 36.Norkin M, Shaw BE, Brazauskas R, Tecca HR, Leather HL, Gea-Banacloche J, et al. Characteristics of Late Fatal Infections after Allogeneic Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019;25(2):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Jama. 2009;302(3):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schain F, Batyrbekova N, Liwing J, Baculea S, Webb T, Remberger M, et al. Real-world study of direct medical and indirect costs and time spent in healthcare in patients with chronic graft versus host disease. Eur J Health Econ. 2021;22(1):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun CL, Kersey JH, Francisco L, Armenian SH, Baker KS, Weisdorf DJ, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(7):1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Jawahri A What else do I need to worry about when treating graft-versus-host disease? Hematology Am Soc Hematol Educ Program. 2021;2021(1):655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh CA, Yi JC, Rosenberg AR, Crouch MV, Leisenring WM, Syrjala KL. Factors associated with social functioning among long-term cancer survivors treated with hematopoietic stem cell transplantation as adolescents or young adults. Psychooncology. 2020;29(10):1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg AR, Syrjala KL, Martin PJ, Flowers ME, Carpenter PA, Salit RB, et al. Resilience, health, and quality of life among long-term survivors of hematopoietic cell transplantation. Cancer. 2015;121(23):4250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SJ, Kim HT, Ho VT, Cutler C, Alyea EP, Soiffer RJ, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone marrow transplantation. 2006;38(4):305–10. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Onstad L, Chow EJ, Shaw BE, Jim HSL, Syrjala KL, et al. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103(9):1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29(2):79–91. [DOI] [PubMed] [Google Scholar]
- 46.Weisdorf DJ, Nelson G, Lee SJ, Haagenson M, Spellman S, Antin JH, et al. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching reveals more graft-versus-host disease but not less relapse. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(11):1475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–30. [DOI] [PubMed] [Google Scholar]
- 48.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–83. [DOI] [PubMed] [Google Scholar]
- 49.Alousi A, Wang T, Hemmer MT, Spellman SR, Arora M, Couriel DR, et al. Peripheral Blood versus Bone Marrow from Unrelated Donors: Bone Marrow Allografts Have Improved Long-Term Overall and Graft-versus-Host Disease-Free, Relapse-Free Survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019;25(2):270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SJ, Logan B, Westervelt P, Cutler C, Woolfrey A, Khan SP, et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-term Follow-up of a Randomized Clinical Trial. JAMA Oncol. 2016;2(12):1583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23(22):5074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013;121(25):5098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martires KJ, Baird K, Steinberg SM, Grkovic L, Joe GO, Williams KM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118(15):4250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozawa S, Nakaseko C, Nishimura M, Maruta A, Cho R, Ohwada C, et al. Chronic graft-versus-host disease after allogeneic bone marrow transplantation from an unrelated donor: incidence, risk factors and association with relapse. A report from the Japan Marrow Donor Program. British journal of haematology. 2007;137(2):142–51. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Gil A, Carrillo-Cruz E, Marrero-Cepeda C, Rodríguez G, Pérez-Simón JA. Effect of Vitamin D on Graft-versus-Host Disease. Biomedicines. 2022;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams KM, Inamoto Y, Im A, Hamilton B, Koreth J, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2020 Etiology and Prevention Working Group Report. Transplant Cell Ther. 2021;27(6):452–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729–35. [DOI] [PubMed] [Google Scholar]
- 59.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–8. [PubMed] [Google Scholar]
- 60.Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu J, Devine SM, et al. Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor-Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies. J Clin Oncol. 2022;40(4):356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Socié G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–82. [DOI] [PubMed] [Google Scholar]
- 62.Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35(36):4003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baron F, Galimard JE, Labopin M, Yakoub-Agha I, Niittyvuopio R, Kröger N, et al. Allogeneic peripheral blood stem cell transplantation with anti-thymocyte globulin versus allogeneic bone marrow transplantation without anti-thymocyte globulin. Haematologica. 2020;105(4):1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finazzi MC, Boschini C, Craddock C, Rambaldi A, Ward J, Malladi RK. Characteristics of graft-versus-host disease occurring after alemtuzumab-containing allogeneic stem cell transplants: incidence, organ involvement, risk factors and survival. British journal of haematology. 2020;188(4):550–9. [DOI] [PubMed] [Google Scholar]
- 65.Kottaridis PD, Milligan DW, Chopra R, Chakraverty RK, Chakrabarti S, Robinson S, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–25. [PubMed] [Google Scholar]
- 66.Malladi RK, Peniket AJ, Littlewood TJ, Towlson KE, Pearce R, Yin J, et al. Alemtuzumab markedly reduces chronic GVHD without affecting overall survival in reduced-intensity conditioning sibling allo-SCT for adults with AML. Bone marrow transplantation. 2009;43(9):709–15. [DOI] [PubMed] [Google Scholar]
- 67.Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017;377(26):2565–79. [DOI] [PubMed] [Google Scholar]
- 68.Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(2):211–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16(12):1434–8. [DOI] [PubMed] [Google Scholar]
- 70.Schwab L, Goroncy L, Palaniyandi S, Gautam S, Triantafyllopoulou A, Mocsai A, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014;20(6):648–54. [DOI] [PubMed] [Google Scholar]
- 71.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. The American journal of medicine. 1980;69(2):204–17. [DOI] [PubMed] [Google Scholar]
- 72.Hess A, Thoburn C, Bright E, Horwitz L. Specificity of effector mechanisms in syngeneic graft-vs-host disease: recognition of the MHC class II invariant chain peptide (CLIP). Transplant Proc. 1997;29(1-2):725–7. [DOI] [PubMed] [Google Scholar]
- 73.Hess AD, Horwitz L, Beschorner WE, Santos GW. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med. 1985;161(4):718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191(1):488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukushi N, Arase H, Wang B, Ogasawara K, Gotohda T, Good RA, et al. Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci U S A. 1990;87(16):6301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105(12):4885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dertschnig S, Hauri-Hohl MM, Vollmer M, Holländer GA, Krenger W. Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood. 2015;125(17):2720–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holländer GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol. 1994;152(4):1609–17. [PubMed] [Google Scholar]
- 79.Teshima T, Reddy P, Liu C, Williams D, Cooke KR, Ferrara JL. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood. 2003;102(2):429–35. [DOI] [PubMed] [Google Scholar]
- 80.Sakoda Y, Hashimoto D, Asakura S, Takeuchi K, Harada M, Tanimoto M, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109(4):1756–64. [DOI] [PubMed] [Google Scholar]
- 81.Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458–66. [DOI] [PubMed] [Google Scholar]
- 82.Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du J, Paz K, Thangavelu G, Schneidawind D, Baker J, Flynn R, et al. Invariant natural killer T cells ameliorate murine chronic GVHD by expanding donor regulatory T cells. Blood. 2017;129(23):3121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107(7):2993–3001. [DOI] [PubMed] [Google Scholar]
- 86.McDonald-Hyman C, Flynn R, Panoskaltsis-Mortari A, Peterson N, MacDonald KP, Hill GR, et al. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood. 2016;128(7):1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110(10):3804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–23. [DOI] [PubMed] [Google Scholar]
- 90.Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood. 2004;103(6):2410–6. [DOI] [PubMed] [Google Scholar]
- 91.Fujiwara H, Maeda Y, Kobayashi K, Nishimori H, Matsuoka K, Fujii N, et al. Programmed death-1 pathway in host tissues ameliorates Th17/Th1-mediated experimental chronic graft-versus-host disease. J Immunol. 2014;193(5):2565–73. [DOI] [PubMed] [Google Scholar]
- 92.Okamoto S, Fujiwara H, Nishimori H, Matsuoka K, Fujii N, Kondo E, et al. Anti-IL-12/23 p40 antibody attenuates experimental chronic graft-versus-host disease via suppression of IFN-γ/IL-17-producing cells. J Immunol. 2015;194(3):1357–63. [DOI] [PubMed] [Google Scholar]
- 93.Forcade E, Paz K, Flynn R, Griesenauer B, Amet T, Li W, et al. An activated Th17-prone T cell subset involved in chronic graft-versus-host disease sensitive to pharmacological inhibition. JCI Insight. 2017;2(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brüggen MC, Klein I, Greinix H, Bauer W, Kuzmina Z, Rabitsch W, et al. Diverse T-cell responses characterize the different manifestations of cutaneous graft-versus-host disease. Blood. 2014;123(2):290–9. [DOI] [PubMed] [Google Scholar]
- 95.Jin H, Ni X, Deng R, Song Q, Young J, Cassady K, et al. Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood. 2016;127(18):2249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone marrow transplantation. 1995;16(2):289–95. [PubMed] [Google Scholar]
- 97.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, et al. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7(8):1353–62. [DOI] [PubMed] [Google Scholar]
- 98.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–27. [DOI] [PubMed] [Google Scholar]
- 99.Zeiser R, Lee SJ. Three US Food and Drug Administration-approved therapies for chronic GVHD. Blood. 2022;139(11):1642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacDonald KP, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–98. [DOI] [PubMed] [Google Scholar]
- 105.Arai S, Pidala J, Pusic I, Chai X, Jaglowski S, Khera N, et al. A Randomized Phase II Crossover Study of Imatinib or Rituximab for Cutaneous Sclerosis after Hematopoietic Cell Transplantation. Clin Cancer Res. 2016;22(2):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zerr P, Distler A, Palumbo-Zerr K, Tomcik M, Vollath S, Dees C, et al. Combined inhibition of c-Abl and PDGF receptors for prevention and treatment of murine sclerodermatous chronic graft-versus-host disease. Am J Pathol. 2012;181(5):1672–80. [DOI] [PubMed] [Google Scholar]
- 107.Baird K, Comis LE, Joe GO, Steinberg SM, Hakim FT, Rose JJ, et al. Imatinib mesylate for the treatment of steroid-refractory sclerotic-type cutaneous chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(6):1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olivieri A, Cimminiello M, Corradini P, Mordini N, Fedele R, Selleri C, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood. 2013;122(25):4111–8. [DOI] [PubMed] [Google Scholar]
- 109.Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood. 2017;129(18):2570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hillen U, Häusermann P, Massi D, Janin A, Wolff D, Lawitschka A, et al. Consensus on performing skin biopsies, laboratory workup, evaluation of tissue samples and reporting of the results in patients with suspected cutaneous graft-versus-host disease. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015;29(5):948–54. [DOI] [PubMed] [Google Scholar]
- 111.Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(4):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paczesny S, Hakim FT, Pidala J, Cooke KR, Lathrop J, Griffith LM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(5):780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bidgoli A, DePriest BP, Saatloo MV, Jiang H, Fu D, Paczesny S. Current Definitions and Clinical Implications of Biomarkers in Graft-versus-Host Disease. Transplant Cell Ther. 2022;28(10):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gandelman JS, Byrne MT, Mistry AM, Polikowsky HG, Diggins KE, Chen H, et al. Machine learning reveals chronic graft-versus-host disease phenotypes and stratifies survival after stem cell transplant for hematologic malignancies. Haematologica. 2019;104(1):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wingard JR, Piantadosi S, Vogelsang GB, Farmer ER, Jabs DA, Levin LS, et al. Predictors of death from chronic graft-versus-host disease after bone marrow transplantation. Blood. 1989;74(4):1428–35. [PubMed] [Google Scholar]
- 116.Curtis LM, Grkovic L, Mitchell SA, Steinberg SM, Cowen EW, Datiles MB, et al. NIH response criteria measures are associated with important parameters of disease severity in patients with chronic GVHD. Bone marrow transplantation. 2014;49(12):1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palmer J, Chai X, Pidala J, Inamoto Y, Martin PJ, Storer B, et al. Predictors of survival, nonrelapse mortality, and failure-free survival in patients treated for chronic graft-versus-host disease. Blood. 2016;127(1):160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aractingi S, Chosidow O. Cutaneous graft-versus-host disease. Archives of dermatology. 1998;134(5):602–12. [DOI] [PubMed] [Google Scholar]
- 119.Beers B, Kalish RS, Kaye VN, Dahl MV. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? Journal of the American Academy of Dermatology. 1993;28(5 Pt 2):888–92. [DOI] [PubMed] [Google Scholar]
- 120.Ahn HS, Park HJ, Lee JY, Cho BK. A case of chronic cutaneous graft versus host disease with the clinical features of exfoliative dermatitis. Ann Dermatol. 2009;21(3):319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cornejo CM, Kim EJ, Rosenbach M, Micheletti RG. Atypical manifestations of graft-versus-host disease. Journal of the American Academy of Dermatology. 2015;72(4):690–5. [DOI] [PubMed] [Google Scholar]
- 122.Creamer D, Martyn-Simmons CL, Osborne G, Kenyon M, Salisbury JR, Devereux S, et al. Eczematoid graft-vs-host disease: a novel form of chronic cutaneous graft-vs-host disease and its response to psoralen UV-A therapy. Archives of dermatology. 2007;143(9):1157–62. [DOI] [PubMed] [Google Scholar]
- 123.Čeović R, Desnica L, Pulanić D, Serventi Seiwerth R, Ilić I, Grce M, et al. High frequency of cutaneous manifestations including vitiligo and alopecia areata in a prospective cohort of patients with chronic graft-vs-host disease. Croat Med J. 2016;57(3):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(6):984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith ZI, Rosenstein RK, Banerjee S, Pichard DC, Pavletic SZ, Cowen EW. Quality of Life in Patients With Skin of Color and Chronic Graft-vs-Host Disease. JAMA dermatology. 2020;156(5):589–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borg MA, Shalabi RA, Childs R, Wells BC. Alopecia Universalis and Chronic Graft-vs-Host Disease Treated With Ruxolitinib. JAMA dermatology. 2018;154(11):1357–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zuo RC, Naik HB, Steinberg SM, Baird K, Mitchell SA, Kuzmina Z, et al. Risk factors and characterization of vitiligo and alopecia areata in patients with chronic graft-vs-host disease. JAMA dermatology. 2015;151(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu SW, Myskowski PL, Papadopoulos EB, Busam KJ. Chronic cutaneous graft-versus-host disease simulating hypertrophic lupus erythematosus--a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34(6):e81–3. [DOI] [PubMed] [Google Scholar]
- 129.Arin MJ, Scheid C, Hübel K, Krieg T, Groth W, Haerrmann G. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31(1):141–3. [DOI] [PubMed] [Google Scholar]
- 130.Brassat S, Fleury J, Camus M, Monégier du Sorbier C, Guillet G. [Epidermolysa bullosa acquisita and graft-versus-host disease]. Ann Dermatol Venereol. 2014;141(5):369–73. [DOI] [PubMed] [Google Scholar]
- 131.Kuzmina Z, Gounden V, Curtis L, Avila D, Rnp TT, Baruffaldi J, et al. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. American journal of hematology. 2015;90(2):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Naftulin JS, Penzi LR, Manatis-Lornell A, Yasuda MR, Porter ML, Saavedra A, et al. Longstanding alopecia and nail dystrophy are associated with more severe overall chronic graft-versus-host disease in adults. Bone marrow transplantation. 2019;54(3):469–72. [DOI] [PubMed] [Google Scholar]
- 133.Şanlı H, Akay BN, Soydan E, Koçyiğit P, Arat M, ilhan O. Clinical Aspects Of Sclerodermatous Type Graft-Versus-Host Disease After Allogeneic Hematopoietic Cell Transplantation. Turk J Haematol. 2010;27(2):91–8. [DOI] [PubMed] [Google Scholar]
- 134.Schaffer JV, McNiff JM, Seropian S, Cooper DL, Bolognia JL. Lichen sclerosus and eosinophilic fasciitis as manifestations of chronic graft-versus-host disease: expanding the sclerodermoid spectrum. Journal of the American Academy of Dermatology. 2005;53(4):591–601. [DOI] [PubMed] [Google Scholar]
- 135.Martires KJ, Baird K, Citrin DE, Hakim FT, Pavletic SZ, Cowen EW. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury: potential insight into the mechanism of isomorphic and isotopic responses. Archives of dermatology. 2011;147(9):1081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel AR, Pavletic SZ, Turner ML, Cowen EW. The isomorphic response in morphealike chronic graft-vs-host disease. Archives of dermatology. 2008;144(9):1229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel AR, Avila D, Malech HL, Pavletic SZ, Yao L, Cowen EW. Rippled skin, fasciitis, and joint contractures. Journal of the American Academy of Dermatology. 2008;59(6):1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaffenberger BH, Zuo RC, Gru A, Plotner AN, Sweeney SA, Devine SM, et al. Graft-versus-host disease-associated angiomatosis: a clinicopathologically distinct entity. Journal of the American Academy of Dermatology. 2014;71(4):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bassim CW, Fassil H, Mays JW, Edwards D, Baird K, Steinberg SM, et al. Oral disease profiles in chronic graft versus host disease. J Dent Res. 2015;94(4):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frey Tirri B, Häusermann P, Bertz H, Greinix H, Lawitschka A, Schwarze CP, et al. Clinical guidelines for gynecologic care after hematopoietic SCT. Report from the international consensus project on clinical practice in chronic GVHD. Bone marrow transplantation. 2015;50(1):3–9. [DOI] [PubMed] [Google Scholar]
- 141.Smith Knutsson E, Björk Y, Broman AK, Helström L, Levin Jakobsen AM, Nilsson O, et al. Genital chronic graft-versus-host disease in females: a cross-sectional study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(6):806–11. [DOI] [PubMed] [Google Scholar]
- 142.Mertens AC, Ramsay NK, Kouris S, Neglia JP. Patterns of gonadal dysfunction following bone marrow transplantation. Bone marrow transplantation. 1998;22(4):345–50. [DOI] [PubMed] [Google Scholar]
- 143.Carpenter PA, Kitko CL, Elad S, Flowers ME, Gea-Banacloche JC, Halter JP, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(7):1167–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mueller SM, Haeusermann P, Rovó A, Halter JP, Passweg J, Itin P, et al. Genital chronic GVHD in men after hematopoietic stem cell transplantation: a single-center cross-sectional analysis of 155 patients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(11):1574–80. [DOI] [PubMed] [Google Scholar]
- 145.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102(2):470–6. [DOI] [PubMed] [Google Scholar]
- 147.Grube M, Holler E, Weber D, Holler B, Herr W, Wolff D. Risk Factors and Outcome of Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation-Results from a Single-Center Observational Study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22(10):1781–91. [DOI] [PubMed] [Google Scholar]
- 148.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347–52. [DOI] [PubMed] [Google Scholar]
- 149.Carlens S, Ringdén O, Remberger M, Lönnqvist B, Hägglund H, Klaesson S, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone marrow transplantation. 1998;22(8):755–61. [DOI] [PubMed] [Google Scholar]
- 150.Remberger M, Kumlien G, Aschan J, Barkholt L, Hentschke P, Ljungman P, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2002;8(12):674–82. [DOI] [PubMed] [Google Scholar]
- 151.Loren AW, Bunin GR, Boudreau C, Champlin RE, Cnaan A, Horowitz MM, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(7):758–69. [DOI] [PubMed] [Google Scholar]
- 152.Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134(12):924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pidala J, Sigdel TK, Wang A, Hsieh S, Inamoto Y, Martin PJ, et al. A combined biomarker and clinical panel for chronic graft versus host disease diagnosis. J Pathol Clin Res. 2017;3(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schmid C, Labopin M, Schaap N, Veelken H, Brecht A, Stadler M, et al. Long-term results and GvHD after prophylactic and preemptive donor lymphocyte infusion after allogeneic stem cell transplantation for acute leukemia. Bone marrow transplantation. 2022;57(2):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]