Abstract
Due to changes in climate, numerous mosquito species are continuously extending their geographical distributions, posing potential new public health threats as arbovirus infections emerge in these new areas. During probing and feeding on the vertebrate host, a mosquito can inject both arbovirus and saliva into the skin of the host. The presence of mosquito saliva in the host skin during arbovirus transmission contributes to high viral titers in the skin, enhanced viremia, and rapid dissemination of the virus to target organs. This enhanced phenotype effectuated by the presence of mosquito saliva in the skin can be partly ascribed to a polarization of the local immune balance towards a Th2 response, an increased permeability of the dermal endothelium, and the influx of virus-susceptible immune cells to the bite site. However, the complete identification and characterization of immunomodulatory salivary proteins from different mosquito species and the mechanisms by which these salivary proteins exert their effects synergistically or antagonistically remains to be further explored. Moreover, the effect of new virus-vector combinations on the outcome of arbovirus infection in a new host is limited. Here, we review the immunomodulatory effects of mosquito saliva in the skin and the proposed mechanisms by which mosquito saliva enhances arbovirus pathogenesis in the vertebrate host, and discuss potential differences between Aedes and Culex mosquito species, the main vectors for medically important arboviruses. Gaining more insight into the effect of mosquito saliva in the vector-virus-host triad aids in predicting the potential transmission risk and disease severity of emerging vector-borne diseases.
Keywords: Arbovirus, Mosquito saliva, Transmission, Pathogenesis
1. Arboviruses and their mosquito vectors
Arthropod-borne (arbo) viruses comprise a range of different virus families and are transmitted primarily by arthropod vectors such as mosquitoes or ticks. Approximately 3.9 billion people in the tropics and sub-tropics are at risk of arboviral infections. Medically important mosquito-borne viruses include yellow fever virus (YFV), Zika virus (ZIKV), dengue virus (DENV), chikungunya virus (CHIKV), and West Nile virus (WNV) [1]. Mosquito species that play a major role in the transmission of these arboviruses include Aedes (Ae.) aegypti and Ae. Albopictus as well as Culex (Cx.) species. Arbovirus infection in humans is often asymptomatic but can lead to serious disease including encephalitis, arthralgia, haemorrhagic fever, and death [2].
Over the past few decades, numerous mosquito species have expanded their geographical range due to climate change, deforestation, urbanisation, increased travel and global trade [[3], [4], [5], [6], [7], [8], [9], [10]]. For example, the origins of Ae. aegypti and Ae. albopictus trace back to tropical forest areas. However, these species are now established throughout the world, in particular Brazil and the USA, but there are also occurrences in Asia, Africa, Oceania, and southern Europe [7,[11], [12], [13], [14]]. Regular incursions of these mosquito species are reported in non-endemic areas, including the Netherlands. While it is unlikely for Ae. aegypti to become established in northern latitudes with temperate climates in the near future, such as north-western Europe [9,15], Ae. albopictus is more tolerant to colder temperatures and is capable of readily adapting to new (man-made) environments [6,14,[16], [17], [18], [19], [20]], thus could potentially establish itself in northern latitudes [6,16,17].
Simultaneously, arboviruses are emerging in areas where suitable mosquito vectors are already present, such as the recent emergence of WNV in north-western Europe, vectored by Cx. pipiens (also known as the common house mosquito) [21]. WNV is now widespread in Europe and causes neuroinvasive disease in humans. An arbovirus related to WNV is the less-studied Usutu virus (USUV), which shares a similar transmission cycle between vectors and birds as their reservoir host species as WNV. USUV first emerged in 2001 in Austria [22], but has since caused mass die-offs in birds in the majority of Western European countries, including recent outbreaks in the Netherlands, Belgium, France and Germany in 2016–2018, and continues to spread across Europe [23].
The main vector of both WNV and USUV is Cx. pipiens. Culex spp. mosquitoes are distributed throughout the world and are primary vectors for a wide array of (neurotropic) arboviruses including WNV, USUV, and Japanese encephalitis virus (JEV). However, other mosquito species are also suggested to be competent vectors for WNV, including Ae. albopictus [24,25]. The opportunistic feeding behaviour of Ae. albopictus biting both mammals and birds may render this mosquito species an excellent bridge vector transferring endemic arboviruses such as WNV from a sylvatic cycle to the human population [26,27]. In addition, the possibility of arboviruses to naturally adapt to a new mosquito species and expand their global distribution cannot be ruled out. For example, a single mutation in the CHIKV genome shifted its specificity from its typical vector Ae. aegypti to Ae. albopictus, leading to CHIKV outbreaks in areas where Ae. aegypti is absent [28,29].
During arbovirus transmission, the mosquito bite itself is of crucial importance; the saliva injected in the skin during the bite can markedly shape the establishment of arbovirus infection and disease development in the vertebrate host [30]. For example, the presence of mosquito saliva during arbovirus infection enhances or prolongs viremia in in vivo studies when compared to inoculation of virus alone [[31], [32], [33], [34], [35], [36], [37], [38], [39]]. An alteration in host viremia could have implications for the transmission dynamics of circulating arboviruses. Higher host viremia levels increases the chances of a mosquito to pick up the virus while taking a bloodmeal and subsequently transmit the virus to a new host [40,41].
While mosquito saliva is naturally present during arbovirus transmission from the mosquito vector to humans, it is unknown whether saliva from exotic mosquito species that are not primarily associated with specific endemic arboviruses, differentially affect host viremia and clinical outcome. This is of concern, considering the potential for new combinations of vectors and viruses due to their geographic expansion. The virus-enhancing effect of mosquito saliva has been most extensively studied for Ae. aegypti, possibly due to the fact that it is the primary vector for arboviruses that are affecting the highest number of people worldwide [42]. Regardless, the effect of mosquito saliva from Ae. albopictus has only once been included in a recent study, despite the fact that it can also be considered a primary vector for medically important arboviruses such as DENV and CHIKV [43]. In addition, there are only a few studies where the effect of saliva from different Aedes and Culex mosquito species were compared side by side [34,35]. It is therefore unknown whether the effects on pathogenesis of arboviruses are a general feature of saliva for all haematophagous mosquito species.
Here we reviewed the current knowledge on the effects of mosquito saliva on arbovirus transmission and pathogenesis and identified key gaps in knowledge. For this review paper, the PubMed and Scopus databases were used and we included the following search terms: [Vector OR Culex OR Aedes OR Anopheles]; [Arbovirus OR flavivirus OR mosquito-borne virus OR arthropod-borne virus]; [Transmission OR mosquito bite]; [Skin OR dermis OR skin cells]; [Immune system OR immune cells OR immunity]; [Mosquito saliva OR salivary proteins OR mosquito bite]; [Europe]; [Climate change] AND [Vector competence] AND [mosquito feeding behaviour] AND [temperature];[Pathogenesis OR tissue tropism OR neuroinvasion];[Mosquito bite AND allergy]. Exclusion criteria included non-English written papers; papers not focussing on arboviruses.
2. Establishment of infection and antiviral responses in the skin
During arbovirus transmission, mosquitoes deposit virus-loaded saliva into the skin while probing and feeding [44]. The skin serves as the initial site of arbovirus replication prior to the virus reaching the bloodstream and disseminating to other organs. The skin consists of the dermis and epidermis and is composed of different non-hematopoietic skin cells and skin-resident immune cells. The main cellular components of the skin are keratinocytes in the granular layer of the epidermis [45,46] and fibroblasts in the dermal layer. Mosquitoes are able to probe through the entire dermis up until the hypodermis (the fat layer), where bites are detectable as small haemorrhagic spots [47].
Arboviruses are able to infect a range of skin cells including keratinocytes [[48], [49], [50], [51], [52], [53]], fibroblasts [48,50,54,55], (immature) dendritic cells (DCs) [48,52,56,57], Langerhans cells (DC population which resides in the epidermis) [52,[57], [58], [59], [60]], mast cells [52,61], and macrophages [52,56,57,59]. Cells involved in the skin immune system include macrophages, neutrophils, DCs, mast cells, and lymphocytes [46,62,63], and aid in protecting the host from microbial pathogens and allergens. However, non-immune skin cells like keratinocytes also exert immune-regulating effects upon infection [52,64] by expressing pathogen recognition receptors such as toll-like receptor 3/7 [[65], [66], [67]] and interacting with skin-resident immune cells to induce immune responses [68]. Upon recognition of viral RNA by endosomal RNA sensors, virus infection generally triggers host innate immune responses to rapidly control viral replication and spread [[69], [70], [71], [72], [73], [74]]. For example, the expression of interferon (IFN)ß [50,54,75] and tumor-necrosis factor (TNF)α [54] is upregulated in fibroblasts and keratinocytes upon infection [50,66]. In fact, keratinocytes are thought to play a valuable role in inciting cutaneous inflammation [45,76]. Infection of keratinocytes leads to an increased production of cytokines interleukin (IL)1ß [52,77], IL6, TNFα [78], IFNß, IFNγ [49], and chemokines CXCL-1, 2, 8, 10, and CCL20 [78] which are critical for recruiting local immune cells and establishing an antiviral immune state shortly after an infectious mosquito bite.
Activated Langerhans cells are able to extend their dendrites up until right below the stratum corneum (the outermost layer of the epidermis), penetrating keratinocyte tight-junctions to scan for, and take up, external antigens [79] followed by maturation into potent immunostimulatory DCs [53,80,81]. Langerhans cells require signals from IL1ß [82] and TNFα [83] for migration to the draining lymph nodes (dLNs) [52,59,84,85] to present viral antigen [86,87], followed by a leukocyte influx into the dLNs [87,88]. Langerhans cell-susceptibility to arbovirus infection coincidentally allows virus migration to the dLNs [60] and consequent viral spread to distant organs. Likewise, infection of dermal DCs and macrophages leads to the recruitment of monocytes from the blood to the dermis, which subsequently differentiate into DCs that can also become infected and migrate to the dLN [56,57].
Another route arboviruses may take to travel to the dLNs is via infection of mast cells and subsequent transport from the infection site to the dLNs in extracellular mast cell granules, although this has so far only been studied for DENV [61]. In addition, infected mast cells signal to dermal endothelial cells to increase the expression of intercellular adhesion molecule and vascular cell adhesion molecule [61], which mediate the adhesion and migration of leukocytes through the endothelium of blood vessels [89]. Neutrophils are one of the first immune cells recruited to the site of infection [90] but may be susceptible to infection, as is shown for WNV [91]. The recruitment of immune cells to the bite site may thus inadvertently provide extra targets for arbovirus replication in the skin and migration to the dLNs and beyond.
Considering that dermal cells initiate antiviral immune responses but simultaneously facilitate viral replication and systemic spread, the initiation of an inflammatory response can result in both a protective or pathogenic outcome [92]. Efficient early peripheral replication contributes to the capacity of neurotropic arboviruses to cause neuroinvasion and mortality [69,93,94]. As such, the dampening of antiviral T-helper (Th)1 responses in the skin following a mosquito bite creates an immune environment that partly favours peripheral viral replication before dissemination to major target organs such as, in case of neurotropic arboviruses, the brain.
3. Mosquito saliva: Skewing the immune balance
Mosquitoes probe their host for 1–7 min depending on the mosquito species [44,95], mosquito age and infection status [47], and host species [95]. Mosquito saliva is retained in the host's skin for 4–18 h after feeding [47,96], where it initially exerts vasodilatory and anti-coagulatory functions to aid the mosquito in successfully taking up a blood meal straight from a capillary or from resulting blood pools [30,47,95,[97], [98], [99], [100]]. The skin is rich in capillaries, veins, and arteries and when stimulated by mosquito saliva dermal microvascular endothelial cell permeability is induced. This results in plasma extravasation [101,102] and the ensuing appearance of oedema following the bite of a mosquito [103]. This is regulated by mast cell activation and degranulation [101], the subsequent release of histamine [102], or through a direct effect of mosquito saliva [104,105]. Concurrently, mosquito saliva polarizes the skin towards a Th2 immune response as it induces the production of high levels of IL4 [33,[106], [107], [108]] and IL10 [33,107,109,110], along with a decreased amount of IFNß [109], and IFNγ [107,108,110,111]. A Th2-dominated immune milieu at the bite site results in a classic type I allergic reaction mediated by IgE [112,113], IL10, and mast cells [110,114].
The presence of mosquito saliva at the bite site promotes homing of immune cells to the skin and includes eosinophils, monocytes, mast cells, CD4+ T-cells [115], and neutrophils [101,103,109,115]. Recruited neutrophils initiate innate immune responses and express the chemoattractant CXCL2, which stimulates the migration of monocytic cells from the bloodstream into the skin [103]. Under the influence of local inflammatory cytokines, monocytes differentiate into macrophages and DCs [116]. Mosquito saliva consists of a myriad of different proteins for many of which the immunomodulatory properties still need to be elucidated. Only a subset of specific salivary proteins, mostly those of Ae. aegypti, have been studied in vivo for their effect on arbovirus pathogenesis [[117], [118], [119], [120], [121], [122], [123]] (Table 1). For example, the Ae. aegypti salivary protein NeSt1 induces IL1ß and CXCL2 expression at the inoculation site, which activates neutrophils, induces macrophage infiltration into the bite site, and enhances viral pathogenesis [117]. Likewise, the Ae. aegypti salivary protein SAAG-4 reduces in vitro CD4+ T-cell expression of IFNγ while simultaneously programming T-cells to express the Th2 cytokine IL4 [124], which creates a Th2-dominant environment that can further stimulate naïve CD4+ T-cells to differentiate into Th2 cells [125].
Table 1.
Specific salivary proteins expressed in the salivary glands of Ae. aegypti that are studied for their effect on arbovirus pathogenesis in vivo (mice). ZIKV = Zika virus, DENV = dengue virus, SFV = Semliki forest virus.
| Salivary factor | Effect in vivo | Proposed mechanism | Reference |
|---|---|---|---|
|
Aedes aegypti Venom allergen-1 (AaVA-1) |
Promotes ZIKV and DENV infection |
Activation of immune cell autophagy |
[121] |
| LTRIN |
Enhanced ZIKV pathogenesis |
Inhibiting LTβR signalling |
[118] |
| Neutrophil stimulation factor 1 (NeSt1) |
Enhanced ZIKV pathogenesis |
Activation of neutrophils and recruitment of macrophages to the bite site |
[117] |
|
Ae. aegypti bacteria-responsive protein 1(AgBR1) |
Enhanced ZIKV pathogenesis |
Induction of neutrophil infiltration to the bite site |
[122] |
| Aegyptin |
Lower DENV pathogenesis |
Augmentation of cytokine concentrations in the inoculation site |
[119] |
| Sialokinin |
Enhanced SFV pathogenesis |
Induction of blood vascular barrier leakage |
[105] |
Of note, mosquito salivary protein transcripts are differentially expressed upon blood meal digestion, as opposed to sugar feeding. Some salivary gland proteins are constitutively expressed, but blood-feeding versus sugar-feeding modulates the expression levels [126,127]. Their activity can be either abrogated [128] or induced upon blood feeding [118,129], suggesting that the feeding status of a mosquito can influence the immunomodulatory properties of mosquito saliva as a whole. For example, Aedes D7 proteins and apyrase are upregulated upon blood-feeding [126]. The D7 proteins of Ae. albopictus and Cx. quinquefasciatus inhibit the recruitment of eosinophils and neutrophils [130], and facilitate blood feeding to the mosquito by inhibiting platelet aggregation [100,130] and antagonizing vasoconstriction [131]. Apyrase is an enzyme that inhibits platelet aggregation during blood-feeding [132] and prevents neutrophil activation [133]. Also the activity of Ae. aegypti salivary enzyme adenosine deaminase is upregulated in the salivary glands after a blood meal. Upregulation of its activity could lead to the inhibition of platelet aggregation, inhibition of proinflammatory cytokine production, and inhibition of mast cell degranulation [126,134]. Adenosine deaminase is known to be present in the salivary glands of Ae. aegypti and Cx. quinquefasciatus, and it appears that only Ae. aegypti secretes adenosine deaminase in its saliva [134]. Almost all proteins that are upregulated in the salivary glands of blood-fed mosquitoes seem to have an important role in successful blood-feeding. The proteins that are downregulated in blood-fed mosquitoes (and upregulated in sugar-fed mosquitoes) tend to have housekeeping functions [126]. On the whole, the immunogenic properties of many mosquito salivary proteins remain undetermined, including any possible synergistic or antagonistic effects salivary proteins might exert at the vector-host interface.
4. Effect of mosquito saliva on arbovirus pathogenesis
A substantial amount of in vivo data, using experimental mouse models, shows that co-inoculation of virus with mosquito saliva, inoculation via an infectious mosquito bite, or feeding of uninfected mosquitoes prior to virus inoculation generally leads to a higher virus titer in the skin [31,38,103,104,129], higher and/or longer-lasting viremia [[31], [32], [33], [34], [35], [36], [37], [38], [39]], higher tissue titers and/or earlier spread to other tissues [31,38,39,103], and higher or accelerated mortality rates [31,35,38,103,104,121,135] compared to needle-inoculation. The bite of even one mosquito already enhances viral infection when compared to needle-inoculation [37,39], however, enhanced viremia is sustained for a longer time when mice are probed by more mosquitoes [37]. The effect of mosquito saliva is dose-dependent [37] and local, meaning that mosquito saliva deposited away from the bite site does not augment viral pathogenesis [34,37,39]. It is also timing-dependent; mosquito saliva enhances viremia when injected from 24 h before to 12 h after virus inoculation [37].
Most virus is injected extravascularly during probing and feeding by the mosquito [44,[136], [137], [138]]. This initially leaves the virus confined to the bite site rather than rapidly disseminating via the circulatory system [136] following the bite of a mosquito [103,139]. Surgical removal of the virus inoculation site in the absence of mosquito saliva improves survival chances of the host [136], an effect that is achieved up until (at least) 4 h after virus inoculation [104]. However, when Ae. aegypti saliva is present at the bite site, removal of the skin 4 h after inoculation does not have any protective effect, suggesting that arboviruses disseminate to the dLNs and beyond more rapidly in the presence of mosquito saliva at the bite site [104]. In contrast, another study found that the presence of Ae. aegypti saliva during arbovirus infection results in a higher viral load in the skin in conjunction with significantly lower virus titers in the dLNs 3 and 6 h post-infection, yet from 24 h onwards the opposite is observed. Furthermore, the presence of saliva results in earlier and higher viral titers in remote LNs, i.e. away from the bite site, as well as in the brain, compared to inoculation of virus alone [103]. This indicates that the enhancing effect of mosquito saliva is not attributed to early rapid dissemination of virus from the skin to the dLNs but rather suggests retention and efficient replication of the virus at the bite site, before subsequently disseminating to remote LNs and organs.
Neutrophil recruitment to the bite site, brought about by the presence of Ae. aegypti saliva, is observed as early as 3 h post-bite along with an increased level of dermal vascular leakage [103], while an influx of monocytic cells is seen between 2 and 16 h post-bite [104,105]. These findings suggest that the mechanism by which viruses disseminate to distant organs more rapidly due to the presence of mosquito saliva at the bite site partially occurs through first confining the virus at the bite site for (at least) 6 h. This is followed by increased viral titers in the skin as a result of the influx of neutrophils and (susceptible) myeloid cells 3 to 16 h post-bite. Subsequently, the virus disseminates to the dLNs, remote LNs, and distant organs [103]. This likely occurs in combination with hampering early viral clearance through the downregulation of Th1 cytokines, shifting the immune balance towards a Th2 response [107,140], and aided by an increase in dermal microvascular permeability [101,104,105] (Fig. 1). Overall, an alteration in immune cell populations as well as cytokine and chemokine signalling effectuated by the presence of mosquito saliva in the skin contributes to the dysregulation of antiviral signalling by antigen-presenting cells, ultimately influencing arbovirus pathogenesis [109].
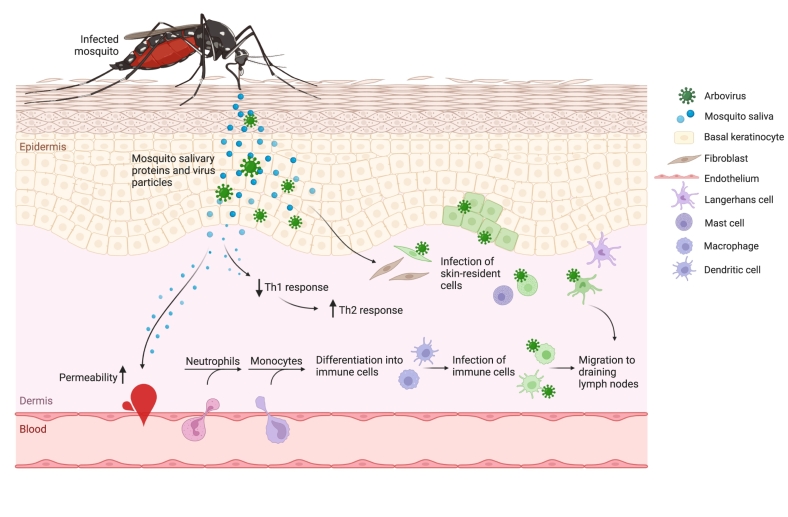
Fig. 1.
Arbovirus transmission from the mosquito vector to a vertebrate host. Schematic overview of the early events in the skin upon the bite of an infectious mosquito. During probing and feeding, a mosquito injects both saliva and virus particles into the host skin. Arboviruses infect a range of resident skin-cells including dermal fibroblasts, epidermal keratinocytes, mast cells, and Langerhans cells. Simultaneously, mosquito salivary proteins induce permeability of the endothelium of dermal capillaries while also dampening antiviral Th1 immune responses, resulting in a local Th2-dominant immune response. Both virus infection in the skin and an increased permeability of the endothelium allows for an influx of neutrophils to the bite site followed by an influx of monocytes, where these cells differentiate into dendritic cells or macrophages. Subsequently, the infected immune cells in the skin migrate to the draining lymph nodes followed by dissemination to distant organs. Green cells represent infected cells.
Collectively, the most studied vector-virus pairing in vivo is DENV in combination with Ae. aegypti (Table 2), where it is repeatedly shown that DENV pathogenesis is enhanced when transmitted via infectious Ae. aegypti bites [32,33,141], pre-exposure to Ae. aegypti probing prior to virus inoculation [36], or co-inoculation of Ae. aegypti saliva and virus [104]. The effect of Ae. albopictus or Ae. japonicus saliva on DENV pathogenesis in vivo remains unexplored, even though both species are considered competent vectors for DENV [43,142]. It is possible that the Ae. aegypti salivary proteins responsible for the observed enhanced DENV pathogenesis are conserved within the Aedes genus or even across species [131,143], which would allow extrapolation of data from studies with Ae. aegypti and DENV to other arbovirus pathogenesis-enhancing effects that saliva of other members from the Aedes (or even Culex) genus might have. This is supported by the comparable effects of saliva from Cx. tarsalis [37,39,129] and Ae. aegypti [38,109,135] on WNV infection in mice, where the presence of saliva results in enhanced viremia, higher viral load at the inoculation site, and earlier neuroinvasion. Likewise, Cache valley virus viremia is enhanced to the same extent by saliva from Ae. aegypti, Ae. triseratius, or Cx. pipiens [34]. However, while saliva of both Ae. triseriatus and Ae. aegypti increase Rift valley fever virus (RVFV) tissue titers, viremia, and mortality rates in a uniform manner, saliva of Cx. pipiens does not have an enhancing effect on RVFV infection [35]. Therefore, differences in the effect of mosquito saliva on arbovirus pathogenesis may indeed differ among species. Whether the effects of mosquito saliva on the pathogenesis of distinct (arbo)viruses differs also remains a gap in knowledge, for example there is so far no data available on the effect of mosquito saliva on the pathogenesis of JEV and USUV in a vertebrate host.
Table 2.
Overview of available in vivo data on different vector-virus pairings studying the effect of mosquito saliva on arbovirus pathogenesis. Includes data from either an infectious mosquito bite, saliva co-inoculation with virus, or feeding/probing of uninfected mosquitoes prior to virus inoculation. Cx. = Culex, Ae. = Aedes. WNV = West Nile virus, ZIKV = Zika virus, DENV = dengue virus, JEV = Japanese encephalitis virus, USUV = Usutu virus, CHIKV = chikungunya virus, SFV = Semliki forest virus, VEEV = Venezuelan equine encephalitis virus, RVFV = Rift valley fever virus, LACV = La Crosse virus, CVV = Cache Valley virus. (+) indicates enhanced pathogenesis, referring to one or more of the following parameters: higher (early) and/or longer-lasting viremia, higher viral load at inoculation site and/or remote tissues, earlier neuroinvasion, higher or accelerated mortality rates, increased morbidity. (-) indicates no effect on pathogenesis, blank indicates no in vivo data available on this vector-virus pairing.
| Ae. aegypti | Ae. albopictus | Ae. vexans | Ae. triseriatus | Ae. taeniorhynchus | Cx. pipiens | Cx. tarsalis | Cx. quinquefasciatus | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
Flaviviridae Genus Flavivirus |
WNV |
+ |
+ |
- |
[37,38,39,130,135,147] |
|||||
| ZIKV | + | + | + | [105,117,118,121] | ||||||
| DENV | + | [32,33,36,104,129,141] | ||||||||
|
Togaviridae Genus Alphavirus |
CHIKV |
+ |
[31] |
|||||||
| SFV | + | + | + | [103,105] | ||||||
| VEEV | - | [136] | ||||||||
| Bunyaviridae Genus Phlebovirus | RVFV | + | + | - | [35] | |||||
| Bunyaviridae Genus Orthobunyavirus |
LACV |
+ |
[148] |
|||||||
| CVV | + | + | + | [34] | ||||||
In addition to the mosquito salivary proteins that enhance arbovirus pathogenesis, some salivary proteins may in fact protect the host against development of arbovirus disease. The presence of mosquito salivary protein D7 can inhibit DENV infection [144], and neutralizing the D7 protein through vaccination resulted in enhanced mortality after WNV infection in mice [120]. The D7 salivary protein family is conserved across mosquito species (Aedes, Culex, and Anopheles) and other blood-feeding insects such as sandflies [131]. In addition to modulating the host response, factors within mosquito saliva have also been shown to interact with the virus directly, affecting its infectivity. The D7 protein of Ae. aegypti inhibits DENV infection in mice, possibly through the direct interaction of D7 with the envelope protein of DENV [144]. In addition to D7 binding the DENV envelope protein, three other Ae. aegypti salivary proteins are shown to bind to the ZIKV envelope protein, two of which have anti-thrombotic or anti-platelet aggregation functions [145]. Although the exact mechanism by which the binding of mosquito salivary proteins to the virus envelope protein mediates viral infectivity is unknown, it may have implications for binding of the virus to host cell receptors.
5. Discussion and future perspectives
The transmission of arboviruses from a mosquito vector to a vertebrate host invariably involves mosquito saliva. Mosquito saliva consists of a cocktail of bioactive compounds that aid the mosquito in successfully taking up a blood meal through halting blood clotting of dermal vessels, inducing vasodilation and promoting cutaneous oedema [30,95,[97], [98], [99], [100]]. It is suggested that the extent of the host immune response following a mosquito bite partially dictates the severity of arboviral disease in the vertebrate host [103], however the detailed mechanism by which mosquito saliva enhances viral replication and pathogenesis remains to be further unravelled.
The most important parameter driving arbovirus outbreaks is suggested to be the host-feeding preference of mosquitoes, which is, among other things, dependent on the (seasonal) abundance of reservoir host species [149]. Most mosquito species that display a strong inherent anthropophilic host-preference belong to Aedes spp., the vectors that account for transmitting nearly all medically important arboviruses to humans. It is therefore speculated that host-preference has co-evolved with the evolution of arboviruses with their host [150]. The salivary protein transcripts may thus vary between mosquito species showing distinct host-feeding preferences. For example, the blood clotting mechanism of birds is different from that of humans in terms of coagulation time, which is longer for birds compared to mammals [[151], [152], [153], [154]]. It may therefore be redundant for strictly ornithophilic mosquito species, such as some of those belonging to the Culex genus, to have evolved salivary factors that rapidly antagonize coagulation in order to facilitate blood meal acquisition.
Aedes mosquitoes have a longer evolutionary linkage with mammals compared to Culex mosquitoes [95]. As such, Cx. quinquefasciatus takes significantly more time finding blood when fed on a human forearm in comparison to Ae. aegypti, while there are no differences between these mosquito species in probing and feeding time when fed on a bird [95], indicating that Culex may indeed not possess a specific anti-clotting salivary protein that optimizes blood-feeding on mammals to the same degree as Aedes. Recently, an Ae. aegypti-specific salivary protein responsible for inducing dermal endothelial permeability in mice has been identified and no homologue of this protein was found in Ae. albopictus, Cx. tarsalis or Cx. quinquefasciatus. This finding implies that the identified salivary protein is aegypti-specific, rather than being specific for anthropophilic mosquito species. However, since both Cx. pipiens and Ae. albopictus enhance arbovirus infection in vivo to a similar amount as Ae. aegypti [105], they most likely possess other factors responsible for the observed enhanced phenotype in vivo (Table 2). For example, while the anti-clotting activity of Cx. quinquefasciatus saliva is significantly lower compared to Ae. aegypti, the anti-platelet activity is found to be the same for both species, while the vasodilatory activity is higher for Cx. quinquefasciatus than for Ae. aegypti [95]. Thus, although the salivary composition of Culex may not be optimally adapted to facilitate feeding on a mammalian hosts, more research into Culex immunomodulatory salivary factors is needed in order to identify and characterize the specific Culex salivary proteins that favour virus replication in a mammalian host.
One important detail to consider is the diverse methods used to isolate mosquito saliva for in vitro and in vivo assays in order to study its pathogenesis-enhancing properties. Most research groups either isolate pure mosquito saliva by employing a forced salivation assay using sugar water or immersion oil, or dissect and homogenize whole mosquito salivary glands. Crude salivary gland extracts presumably contain cellular compounds that in a natural setting would not be injected into the host during probing and feeding, and may therefore be considered a disadvantage of this method. In addition, for both assays it should be taken into account that mosquito salivary protein transcripts are differentially expressed upon blood meal digestion, as opposed to sugar feeding [126,127]. However, a recent paper found comparable enhancing effects in vivo of saliva from blood-fed versus sugar-fed Ae. aegypti [105]. Furthermore, an infected mosquito shows increased probing and biting behaviour [155,156] or changed salivary gland physiology [157], which may eventually increase arbovirus transmission rates [97]. Using uninfected mosquito saliva or probing prior to virus inoculation in an in vivo model may therefore not recapitulate what happens in nature and yield differential results compared to infecting an animal model via an infectious mosquito bite. However, when using infectious mosquitoes it is difficult to know the exact viral dose that is injected after a bite, since it was recently shown that the forced salivation assay that is broadly applied to assess viral load in mosquito saliva may underestimate the actual arbovirus load transmitted to a new host [158]. Overall, such aspects should be considered when interpreting data on the pathogenesis-enhancing properties of mosquito saliva.
Studies on the effect of mosquito saliva on arbovirus pathogenesis in a vertebrate host mainly focus on combinations of an arbovirus in combination with its primary vector, for example DENV and Ae. aegypti. However, numerous mosquito species are continuously expanding their geographical range, which results in new combinations of vectors and viruses. Therefore, the relative contribution of saliva from different mosquito species with regards to arbovirus transmission dynamics and transmission risk should be further elucidated when taking into account different vector-virus pairings. This review highlights a major gap in knowledge on the effects of mosquito saliva from exotic mosquito species on the pathogenesis of endemic viruses and vice versa. Studying this facet of arbovirus transmission could aid in predicting whether different vector-virus pairings will trigger clinical arbovirus disease or change its clinical manifestations in humans. In addition, studying the effect of mosquito saliva on arbovirus transmission will extend the existing vector competence studies as a risk assessment for potential arbovirus transmission or alteration in transmission dynamics. Another major gap in knowledge is the effect environmental (climate) changes may have on the composition of mosquito saliva and thereby its effect on transmission and pathogenesis. While it is known that external factors such as temperature and food abundance can affect mosquito development and host gene expression profiles, data on changes in salivary glands and subsequent saliva composition are largely unavailable. Moving forward, identification and characterization of novel salivary proteins from distinct mosquito species will advance the development of intervention methods such as the establishment of a mosquito saliva-based vaccine [159].
Funding
This work is part of the research program One Health PACT with project number 109986, which is partly financed by the Dutch Research Council (NWO).
Author statement
Individual contributions of authors to this review paper:
Imke Visser: Conceptualization, Visualization, Investigation, Methodology, Writing – original draft, review & editing.
Barry Rockx: Conceptualization, Writing – original draft, review & editing, Funding acquisition.
Constantianus J.M. Koenraadt: Writing – review & editing.
Marion P.G. Koopmans: Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors have declared that no competing interests exist.
Data availability
No data was used for the research described in the article.
References
- 1.WHO Vector-borne diseases. 2020. https://www.who.int/en/news-room/fact-sheets/detail/vector-borne-diseases Available at. (Accessed: 17th June 2022)
- 2.Weaver S.C., Barrett A.D.T. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004;2:789. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell L.P., et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140135. doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Lamballerie X., et al. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol. J. 2008;5:1–4. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould E.A., Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009;103:109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibáñez-Justicia A., et al. Habitat suitability modelling to assess the introductions of Aedes albopictus (Diptera: Culicidae) in the Netherlands. Parasit. Vectors. 2020;13:217. doi: 10.1186/s13071-020-04077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraemer M.U.G., et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reperant L.A., Osterhaus A.D.M.E. AIDS, avian flu, SARS, MERS, Ebola, Zika… what next? Vaccine. 2017;35:4470–4474. doi: 10.1016/j.vaccine.2017.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholte E.J., et al. Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Eurosurveillance. 2010;15:19710. [PubMed] [Google Scholar]
- 10.Weaver S.C., Charlier C., Vasilakis N., Lecuit M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett K.L., et al. Historical environmental change in Africa drives divergence and admixture of Aedes aegypti mosquitoes: a precursor to successful worldwide colonization? Mol. Ecol. 2016;25:4337–4354. doi: 10.1111/mec.13762. [DOI] [PubMed] [Google Scholar]
- 12.Brown J.E., et al. Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc. R. Soc. B Biol. Sci. 2011;278:2446–2454. doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J.E., et al. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution (N. Y). 2014;68(514–525) doi: 10.1111/evo.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Iwamura T., Guzman-Holst A., Murray K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020;11:2130. doi: 10.1038/s41467-020-16010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caminade C., et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J. R. Soc. Interface. 2012;9:2708–2717. doi: 10.1098/rsif.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988;1:1–39. [PubMed] [Google Scholar]
- 18.Medlock J.M., Avenell D., Barrass I., Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J. Vector Ecol. 2006;31:292–304. doi: 10.3376/1081-1710(2006)31[292:aotpfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Romi R., Severini F., Toma L. Cold acclimation and overwintering of female Aedes albopictus in Roma. J. Am. Mosq. Control Assoc. 2006;22:149–151. doi: 10.2987/8756-971X(2006)22[149:CAAOOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Takumi K., et al. Introduction, scenarios for establishment and seasonal activity of aedes albopictus in the Netherlands. Vector-Borne Zoonotic Dis. 2009;9:191–196. doi: 10.1089/vbz.2008.0038. [DOI] [PubMed] [Google Scholar]
- 21.Sikkema R.S., et al. Detection of west nile virus in a common whitethroat (curruca communis) and culex mosquitoes in the Netherlands, 2020. Eurosurveillance. 2020;25:1–6. doi: 10.2807/1560-7917.ES.2020.25.40.2001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissenböck H., et al. Emergence of Usutu virus, an African mosquito-borne Flavivirus of the Japanese encephalitis virus group, Central Europe. Emerg. Infect. Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilibic-Cavlek T., et al. Epidemiology of usutu virus: the european scenario. Pathogens. 2020;9:1–19. doi: 10.3390/pathogens9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortuna C., et al. Evaluation of vector competence for West Nile virus in Italian Stegomyia albopicta (=Aedes albopictus) mosquitoes. Med. Vet. Entomol. 2015;29:430–433. doi: 10.1111/mve.12133. [DOI] [PubMed] [Google Scholar]
- 25.Sardelis M.R., Turell M.J., O’Guinn M.L., Andre R.G., Roberts D.R. Vector competence of three north American strains of Aedes albopictus for West Nile virus. J. Am. Mosq. Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- 26.Hendy A., et al. Into the woods: changes in mosquito community composition and presence of key vectors at increasing distances from the urban edge in urban forest parks in Manaus, Brazil. Acta Trop. 2020;206 doi: 10.1016/j.actatropica.2020.105441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira-Dos-Santos T., Roiz D., Lourenço-De-Oliveira R., Paupy C. A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathog. 2020;9:266. doi: 10.3390/pathogens9040266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsetsarkin K.A., Vanlandingham D.L., McGee C.E., Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:1895–1906. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuffenecker I., et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:1058–1070. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pingen M., Schmid M.A., Harris E., McKimmie C.S. Mosquito biting modulates skin response to virus infection. Trends Parasitol. 2017;33:645–657. doi: 10.1016/j.pt.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A., et al. Mosquito saliva induced cutaneous events augment chikungunya virus replication and disease progression. Infect. Genet. Evol. 2016;40:126–135. doi: 10.1016/j.meegid.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Christofferson R.C., McCracken M.K., Johnson A.M., Chisenhall D.M., Mores C.N. Development of a transmission model for dengue virus. Virol. J. 2013;10:127. doi: 10.1186/1743-422X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox J., Mota J., Sukupolvi-Petty S., Diamond M.S., Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012;86:7637–7649. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards J.F., Higgs S., Beaty B.J. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J. Med. Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 35.Le Coupanec A., et al. Aedes Mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCracken M.K., Christofferson R.C., Chisenhall D.M., Mores C.N. Analysis of early dengue virus infection in mice as modulated by Aedes aegypti probing. J. Virol. 2014;88:1881–1889. doi: 10.1128/JVI.01218-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser L.A., Lim P.-Y., Styer L.M., Kramer L.D., Bernard K.A. Parameters of mosquito-enhanced West Nile virus infection. J. Virol. 2016;90:292–299. doi: 10.1128/JVI.02280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider B.S., et al. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006;19:74–82. doi: 10.1089/vim.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 39.Styer L.M., et al. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 2011;85:1517–1527. doi: 10.1128/JVI.01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lord C.C., Rutledge C.R., Tabachnick W.J. Relationships between host viremia and vector susceptibility for arboviruses. J. Med. Entomol. 2006;43:623. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesla B., et al. Estimating the effects of variation in viremia on mosquito susceptibility, infectiousness, and R0 of Zika in Aedes aegypti. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A global brief on vector-borne diseases. WHO; 2014. [Google Scholar]
- 43.Paupy C., et al. Comparative Role of Aedes albopictus and Aedes aegypti in the Emergence of Dengue and Chikungunya in Central Africa. Vector Borne Zoonotic Dis. 2010;10(3):259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 44.Styer L.M., et al. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262–1270. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker J.N.W.N., et al. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 46.Pivarcsi A., Kemény L., Dobozy A. Innate immune functions of the keratinocytes: a review. Acta Microbiol. Immunol. Hung. 2004;51:303–310. doi: 10.1556/AMicr.51.2004.3.8. [DOI] [PubMed] [Google Scholar]
- 47.Choumet V., et al. Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamel R., et al. Biology of Zika virus infection in human skin cells. J. Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Surasombatpattana P., et al. Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect. Genet. Evol. 2011;11:1664–1673. doi: 10.1016/j.meegid.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Kim J.A., Seong R.K., Son S.W., Shin O.S. Insights into ZIKV-mediated innate immune responses in human dermal fibroblasts and epidermal keratinocytes. J. Invest. Dermatol. 2019;139:391–399. doi: 10.1016/j.jid.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 51.Lim P.-Y., Behr M.J., Chadwick C.M., Shi P.-Y., Bernard K.A. Keratinocytes are cell targets of West Nile virus in vivo. J. Virol. 2011;85:5197–5201. doi: 10.1128/JVI.02692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duangkhae P., et al. Interplay between keratinocytes and myeloid cells drives dengue virus spread in human skin. J. Invest. Dermatol. 2018;138:618–626. doi: 10.1016/j.jid.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Limon-Flores A.Y., et al. Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. Int. J. Exp. Pathol. 2005;86:323–334. doi: 10.1111/j.0959-9673.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bustos-Arriaga J., et al. Activation of the innate immune response against DENV in Normal non-transformed human fibroblasts. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wichit S., et al. Aedes Aegypti saliva enhances chikungunya virus replication in human skin fibroblasts via inhibition of the type I interferon signaling pathway. Infect. Genet. Evol. 2017;55:68–70. doi: 10.1016/j.meegid.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 56.Schmid M.A., Harris E. Monocyte recruitment to the dermis and differentiation to dendritic cells increases the targets for dengue virus replication. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cerny D., et al. Selective susceptibility of human skin antigen presenting cells to productive dengue virus infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu S.J.L., et al. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 59.Castanha P.M.S., et al. Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacDonald G.H., Johnston R.E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Troupin A., et al. A role for human skin mast cells in dengue virus infection and systemic spread. J. Immunol. 2016;197:4382–4391. doi: 10.4049/jimmunol.1600846. [DOI] [PubMed] [Google Scholar]
- 62.Bos J.D., et al. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Invest. Dermatol. 1987;88:569–573. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- 63.Heath W.R., Carbone F.R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 64.Scorza B.M., et al. Differential activation of human keratinocytes by Leishmania species causing localized or disseminated disease. J. Invest. Dermatol. 2017;137:2149–2156. doi: 10.1016/j.jid.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Briant L., Desprès P., Choumet V., Missé D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology. 2014;464–465:26–32. doi: 10.1016/j.virol.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Kalali B.N., et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. 2008;181:2694–2704. doi: 10.4049/jimmunol.181.4.2694. [DOI] [PubMed] [Google Scholar]
- 67.Lebre M.C., et al. Human keratinocytes express functional toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 68.Lebre M.C., et al. Double-stranded RNA-exposed human keratinocytes promote Th1 responses by inducing a Type-1 polarized phenotype in dendritic cells: role of keratinocyte-derived tumor necrosis factor α, type I interferons, and Interleukin-18. J. Invest. Dermatol. 2003;120:990–997. doi: 10.1046/j.1523-1747.2003.12245.x. [DOI] [PubMed] [Google Scholar]
- 69.Chambers T.J., Diamond M.S. Pathogenesis of flavivirus encephalitis. Adv. Virus Res. 2003;60:273–342. doi: 10.1016/S0065-3527(03)60008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daffis S., et al. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J. Virol. 2008;82:8465–8475. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 72.Samuel M.A., et al. PKR and RNase L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samuel M. Virology, M. D.-J. of & 2005, undefined. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. Am Soc Microbiol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shrestha B., et al. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J. Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurane I., Janus J., Ennis F.A. Dengue virus infection of human skin fibroblasts in vitro production of IFN-β, IL-6 and GM-CSF. Arch. Virol. 1992;124 doi: 10.1007/BF01314622. [DOI] [PubMed] [Google Scholar]
- 76.Nestle F.O., Meglio P. Di, Qin J.-Z., Nickoloff B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009;910(9):679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood L.C., Jackson S.M., Elias P.M., Grunfeld C., Feingold K.R. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J. Clin. Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia M., et al. Innate immune response of primary human keratinocytes to West Nile virus infection and its modulation by mosquito saliva. Front. Cell. Infect. Microbiol. 2018;8:387. doi: 10.3389/fcimb.2018.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubo A., Nagao K., Yokouchi M., Sasaki H., Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnston L.J., Halliday G.M., King N.J. Phenotypic changes in Langerhans’ cells after infection with arboviruses: a role in the immune response to epidermally acquired viral infection? J. Virol. 1996;70 doi: 10.1128/jvi.70.7.4761-4766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schuler G., Steinman R.M. Murine epidermal langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Byrne S.N., Halliday G.M., Johnston L.J., King N.J.C. Interleukin-1β but not tumor necrosis factor is involved in West Nile virus-induced Langerhans cell migration from the skin in C57BL/6 mice. J. Invest. Dermatol. 2001;117:702–709. doi: 10.1046/j.0022-202x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- 83.Cumberbatch M., Dearman R.J., Kimber I. Langerhans cells require signals from both tumour necrosis factor- α and interleukin-1β for migration. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cumberbatch M., Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans’ cell migration. Immunology. 1992;75:257–263. [PMC free article] [PubMed] [Google Scholar]
- 85.Nishibu A., et al. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 86.Allan R.S., et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 87.Allenspach E.J., Lemos M.P., Porrett P.M., Turka L.A., Laufer T.M. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnston L.J., Halliday G.M., King N.J.C. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Invest. Dermatol. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 89.Quarmby S., Kumar P., Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int. J. Cancer. 1999;82:385–395. doi: 10.1002/(sici)1097-0215(19990730)82:3<385::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 90.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;63(6):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 91.Bai F., et al. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ong R.Y., Lum F.M., Ng L.F.P. The fine line between protection and pathology in neurotropic flavivirus and alphavirus infections. Futur. Virol. 2014;9:313–330. [Google Scholar]
- 93.Albrecht P. Pathogenesis of neurotropic arbovirus infections. Curr. Top. Microbiol. Immunol. 1968;43:44–91. doi: 10.1007/978-3-642-46118-7_2. [DOI] [PubMed] [Google Scholar]
- 94.Bryden S.R., et al. Pan-viral protection against arboviruses by activating skin macrophages at the inoculation site. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aax2421. [DOI] [PubMed] [Google Scholar]
- 95.Ribeiro J.M.C. Blood-feeding in mosquitoes: probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex) Med. Vet. Entomol. 2000;14:142–148. doi: 10.1046/j.1365-2915.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto D.S., et al. Visualization and live imaging analysis of a mosquito saliva protein in host animal skin using a transgenic mosquito with a secreted luciferase reporter system. Insect Mol. Biol. 2013;22:685–693. doi: 10.1111/imb.12055. [DOI] [PubMed] [Google Scholar]
- 97.Ribeiro J. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 1987;32 doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 98.Calvo E., et al. An insight into the sialotranscriptome of the West Nile mosquito vector, Culex tarsalis. BMC Genomics. 2010;11:51. doi: 10.1186/1471-2164-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ribeiro J.M.C., Mans B.J., Arcà B. An insight into the sialome of blood-feeding Nematocera. Insect Biochem. Mol. Biol. 2010;40:767–784. doi: 10.1016/j.ibmb.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin-Martin I., et al. ADP binding by the Culex quinquefasciatus mosquito D7 salivary protein enhances blood feeding on mammals. Nat. Commun. 2020;11:2911. doi: 10.1038/s41467-020-16665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Demeure C.E., et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 2005;174:3932–3940. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 102.Ribeiro J.M.C., Francischetti I.M.B. Role of arthropod saliva in blood feeding: Sialome and post-Sialome perspectives. Annu. Rev. Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 103.Pingen M., et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity. 2016;44:1455–1469. doi: 10.1016/j.immuni.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmid M.A., et al. Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and dengue pathogenesis during antibody-dependent enhancement. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lefteri D.A., et al. Mosquito saliva enhances virus infection through sialokinin-dependent vascular leakage. Proc. Natl. Acad. Sci. 2022;119 doi: 10.1073/pnas.2114309119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thangamani S., et al. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeidner N.S., Higgs S., Happ C.M., Beaty B.J., Miller B.R. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 1999;21:35–44. doi: 10.1046/j.1365-3024.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y.L., Simons F.E.R., Peng Z. A Mouse Model of Mosquito Allergy for Study of Antigen–Specific IgE and IgG Subclass Responses, Lymphocyte Proliferation, and IL–4 and IFN–Á Production. Int. Arch. Allergy Immunol. 1998;116:269–277. doi: 10.1159/000023955. [DOI] [PubMed] [Google Scholar]
- 109.Schneider B.S., et al. Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Depinay N., Hacini F., Beghdadi W., Peronet R., Mécheri S. Mast cell-dependent Down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 111.Cross M.L., Cupp E.W., Enriquez F.J. Differential modulation of murine cellular immune responses by salivary gland extract of Aedes aegypti. Am. J. Trop. Med. Hyg. 1994;51:690–696. doi: 10.4269/ajtmh.1994.51.690. [DOI] [PubMed] [Google Scholar]
- 112.Reunala T., Brummer-Korvenkontio H., Palosuo T. Are we really allergic to mosquito bites? Ann. Med. 1994;26:301–306. doi: 10.3109/07853899409147906. [DOI] [PubMed] [Google Scholar]
- 113.Peng Z., Simons F.E.R. Mosquito allergy: immune mechanisms and recombinant salivary allergens. Int. Arch. Allergy Immunol. 2004;133:198–209. doi: 10.1159/000076787. [DOI] [PubMed] [Google Scholar]
- 114.Nagata K., Nishiyama C. IL-10 in mast cell-mediated immune responses: anti-inflammatory and proinflammatory roles. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22094972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Henrique M.O., et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47–59. doi: 10.1111/imm.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hastings A.K., et al. Aedes aegypti NeSt1 protein enhances Zika virus pathogenesis by activating neutrophils. J. Virol. 2019;93 doi: 10.1128/JVI.00395-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jin L., et al. Salivary factor LTRIN from Aedes aegypti facilitates the transmission of Zika virus by interfering with the lymphotoxin-β receptor. Nat. Immunol. 2018;194(19):342–353. doi: 10.1038/s41590-018-0063-9. [DOI] [PubMed] [Google Scholar]
- 119.McCracken M.K., et al. Aedes aegypti salivary protein ‘aegyptin’ co-inoculation modulates dengue virus infection in the vertebrate host. Virology. 2014;468–470:133–139. doi: 10.1016/j.virol.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reagan K.L., Machain-Williams C., Wang T., Blair C.D. Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun P., et al. A mosquito salivary protein promotes flavivirus transmission by activation of autophagy. Nat. Commun. 2020;11 doi: 10.1038/s41467-019-14115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uraki R., Hastings A.K., Brackney D.E., Armstrong P.M., Fikrig E. AgBR1 antibodies delay lethal Aedes aegypti-borne West Nile virus infection in mice. npj Vaccines. 2019;4:1–4. doi: 10.1038/s41541-019-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uraki R., et al. Aedes aegypti AgBR1 antibodies modulate early Zika virus infection of mice. Nat. Microbiol. 2019;4:948–955. doi: 10.1038/s41564-019-0385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boppana V.D., Thangamani S., Adler A.J., Wikel S.K. SAAG-4 is a novel mosquito salivary protein that programmes host CD4 + T cells to express IL-4. Parasite Immunol. 2009;31:287–295. doi: 10.1111/j.1365-3024.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romagnani S. T-cell subsets (Th1 versus Th2) Ann. Allergy Asthma Immunol. 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 126.Wasinpiyamongkol L., et al. Blood-feeding and immunogenic Aedes aegypti saliva proteins. Proteomics. 2010;10:1906–1916. doi: 10.1002/pmic.200900626. [DOI] [PubMed] [Google Scholar]
- 127.Thangamani S., Wikel S.K. Differential expression of Aedes aegypti salivary transcriptome upon blood feeding. Parasit. Vectors. 2009;2:1–8. doi: 10.1186/1756-3305-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wasserman H.A., Singh S., Champagne D.E. Saliva of the yellow fever mosquito, Aedes aegypti, modulates murine lymphocyte function. Parasite Immunol. 2004;26:295–306. doi: 10.1111/j.0141-9838.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 129.Conway M.J., et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J. Virol. 2014;88:164–175. doi: 10.1128/JVI.02235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin-Martin I., et al. Aedes albopictus d7 salivary protein prevents host hemostasis and inflammation. Biomolecules. 2020;10:1–17. doi: 10.3390/biom10101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calvo E., Mans B.J., Andersen J.F., Ribeiro J.M.C. Function and evolution of a mosquito salivary protein family. J. Biol. Chem. 2006;281:1935–1942. doi: 10.1074/jbc.M510359200. [DOI] [PubMed] [Google Scholar]
- 132.Champagne D.E., Smartt C.T., Ribeiro J.M.C., James A.A. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc. Natl. Acad. Sci. U. S. A. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun D., Mcnicol A., James A.A., Peng Z. Expression of functional recombinant mosquito salivary apyrase: a potential therapeutic platelet aggregation inhibitor. Platelets. 2006;17:178–184. doi: 10.1080/09537100500460234. [DOI] [PubMed] [Google Scholar]
- 134.Ribeiro J.M.C., Charlab R., Valenzuela J.G. The salivary adenosine deaminase activity of the mosquitoes Culex quinquefasciatus and Aedes aegypti. J. Exp. Biol. 2001;204:2001–2010. doi: 10.1242/jeb.204.11.2001. [DOI] [PubMed] [Google Scholar]
- 135.Schneider B.S., et al. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith D.R., et al. Venezuelan equine encephalitis virus transmission and effect on pathogenesis. Emerg. Infect. Dis. 2006;12:1190. doi: 10.3201/eid1208.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Turell M.J., Spielman A. Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am. J. Trop. Med. Hyg. 1992;47:190–194. doi: 10.4269/ajtmh.1992.47.190. [DOI] [PubMed] [Google Scholar]
- 138.Turell M.J., Tammariello R.F., Spielman A. Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J. Med. Entomol. 1995;32:563–568. doi: 10.1093/jmedent/32.4.563. [DOI] [PubMed] [Google Scholar]
- 139.Pappas L.G., Pappas C.D., Grossman G.L. Hemodynamics of human skin during mosquito (Diptera: Culicidae) blood feeding. J. Med. Entomol. 1986;23:581–587. doi: 10.1093/jmedent/23.6.581. [DOI] [PubMed] [Google Scholar]
- 140.Wanasen N., Nussenzveig R.H., Champagne D.E., Soong L., Higgs S. Differential modulation of murine host immune response by salivary gland extracts from the mosquitoes Aedes aegypti and Culex quinquefasciatus. Med. Vet. Entomol. 2004;18:191–199. doi: 10.1111/j.1365-2915.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 141.McCracken M.K., et al. Route of inoculation and mosquito vector exposure modulate dengue virus replication kinetics and immune responses in rhesus macaques. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schaffner F., et al. Vector competence of Aedes japonicus for chikungunya and dengue viruses. J. Eur. Mosq. Control Assoc. 2011;29:141–142. [Google Scholar]
- 143.Fontaine A., et al. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit. Vectors. 2011;4:1–17. doi: 10.1186/1756-3305-4-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Conway M.J., et al. Aedes aegypti D7 saliva protein inhibits dengue virus infection. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Valenzuela-Leon P.C., et al. Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein. Viruses. 2022;14:221. doi: 10.3390/v14020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sbrana E., et al. Oral transmission of West Nile virus in a hamster model. Am. J. Trop. Med. Hyg. 2005;72:325–329. [PubMed] [Google Scholar]
- 148.Osorio J.E., Godsey M.S., Defoliart G.R., Yuill T.M. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am. J. Trop. Med. Hyg. 1996;54:338–342. doi: 10.4269/ajtmh.1996.54.338. [DOI] [PubMed] [Google Scholar]
- 149.Simpson J.E., et al. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B Biol. Sci. 2012;279:925–933. doi: 10.1098/rspb.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verhulst. N.O. Host Preferences of Blood-Feeding Mosquitoes. Annu. Rev. Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 151.Bigland C.H. Blood clotting times of five avian species. Poult. Sci. 1964;43:1035–1039. [Google Scholar]
- 152.Strindberg S., et al. Thromboelastography in selected avian species. J. Avian Med. Surg. 2015;29:282–289. doi: 10.1647/2014-034. [DOI] [PubMed] [Google Scholar]
- 153.Schmaier A.A., et al. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood. 2011;118:3661–3669. doi: 10.1182/blood-2011-02-338244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frost C.L., Naudé R.J., Oelofsen W., Jacobson B. Comparative blood coagulation studies in the ostrich. Immunopharmacology. 1999;45:75–81. doi: 10.1016/s0162-3109(99)00058-2. [DOI] [PubMed] [Google Scholar]
- 155.Xiang B.W.W., et al. Dengue virus infection modifies mosquito blood-feeding behavior to increase transmission to the host. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2117589119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grimstad P.R., Ross Q.E., Craig G.B. Aedes Triseriatus (Diptera: Culicidae) and La Crosse virus: II. Modification of mosquito feeding behavior by virus infection. J. Med. Entomol. 1980;17:1–7. doi: 10.1093/jmedent/17.1.1. [DOI] [PubMed] [Google Scholar]
- 157.Sim S., Ramirez J.L., Dimopoulos G. Dengue virus infection of the aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gloria-Soria A., Brackney D.E., Armstrong P.M. Saliva collection via capillary method may underestimate arboviral transmission by mosquitoes. Parasit. Vectors. 2022;15:1–9. doi: 10.1186/s13071-022-05198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Manning J.E., Morens D.M., Kamhawi S., Valenzuela J.G., Memoli M. Mosquito saliva: the Hope for a universal arbovirus vaccine? J. Infect. Dis. 2018;218:7–15. doi: 10.1093/infdis/jiy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.