Abstract
This review is made up of two parts; the first part discussing intellectual disability (ID) in general, while the second part covers the pain associated with intellectual disability and the challenges and practical tips for the management of pain associated with (ID). Intellectual disability is characterized by deficits in general mental abilities, such as reasoning, problem solving, planning, abstract thinking, judgment, academic learning, and learning from experience. ID is a disorder with no definite cause but has multiple risk factors, including genetic, medical, and acquired. Vulnerable populations such as individuals with intellectual disability may experience more pain than the general population due to additional comorbidities and secondary conditions, or at least the same frequency of pain as in the general population. Pain in patients with ID remains largely unrecognized and untreated due to barriers to verbal and non-verbal communication. It is important to identify patients at risk to promptly prevent or minimize those risk factors. As pain is multifactorial, thus, a multimodal approach using both pharmacotherapy and non-pharmacological management is often the most beneficial. Parents and caregivers should be oriented to this disorder, given adequate training and education, and be actively involved with the treatment program. Significant work to create new pain assessment tools to improve pain practices for individuals with ID has taken place, including neuroimaging and electrophysiological studies. Recent advances in technology-based interventions such as virtual reality and artificial intelligence are rapidly growing to help give patients with ID promising results to develop pain coping skills with effective reduction of pain and anxiety. Therefore, this narrative review highlights the different aspects regarding the current status of the pain associated with intellectual disability, with more emphasis on the recent pieces of evidence for the assessment and management of pain among populations with intellectual disability.
Keywords: Intellectual disability, Pain assessment with intellectual disability, Prevention of pain with intellectual disability, Pharmacotherapy, Non-pharmacological treatment, Pain management with intellectual disability
Key Summary Points
| Why carry out this study? |
| To highlight the current status, prevalence, and risk factors for painful conditions associated with intellectual disability. |
| To change the wrong beliefs that patients with intellectual disability cannot recognize, understand, or even experience pain like the comparable normal populations. |
| To identify the challenges of pain assessment and practical tips for the management of pain among patients with intellectual disability. |
| To focus on the recent evidence for the assessment and management of pain among populations with intellectual disability with more emphasis on the use of new technology tools. |
| What was learned from the study? |
| Pain in intellectual disability is associated with significant functional and psychological impacts and has gained increasing recognition as a disability in and of itself. |
| Quantitative sensory testing and previous reports showed that individuals with intellectual disability experienced pain similar to or even more than the comparable normal populations, with different responses to pain according to the type and extent of disability. |
| Evidence is promising regarding the incorporation of new tools for the assessment and management of pain that can aid in overcoming the communication barriers in individuals with intellectual disability. |
| More research focusing on current challenges and active knowledge translation as well as new ideas for future attention is required. |
Introduction
Developmental delay is a disorder associated with significant limitations in intellectual functioning and adaptive behaviors. Currently, it is widely referred to as intellectual disability (ID). Intellectual disability is defined as a neurodevelopmental disorder that manifests during the developmental period and affects an individual’s intellectual abilities (e.g., abstractive thinking, language, memory) and adaptive functioning (e.g., communication, independent living) [1, 2]. Developmental delay includes gross or fine motor skills, speech and language, cognition, personal–social, and activities of daily living [3, 4]. The incidence of childhood disability disorders varies between different studies. It ranges between 5% and 10%. Most of these disorders improve, while the condition persists in about 1% of adults [1].
Pain management in individuals with ID, an important category of the most vulnerable group, is an important health issue. Accordingly, the International Association for the Study of Pain (IASP) has revised the definition of pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [5]. Therefore, it also applies to children with disability who are unable to express their pain experience.
Living with acute or chronic pain associated with an ID is a real challenge [6]. In 2018, the US Court of Appeals for the Federal Circuit recognized that “pain is enough” for a veteran to be eligible for disability compensation, even when the claimant is unable to establish the underlying cause of their pain. The associated burdens of chronic pain, such as functional, personal, and social impacts, should be sufficient to meet the definition of disability under any of the legal schemes for disability [7, 8].
Clinical reports and sensory testing showed that individuals with ID experienced pain similar to the comparable normal populations with different responses to pain according to the type and extent of disability. Moreover, several imaging techniques, endocrine responses, and brain-evoked potentials recorded during noxious events have identified that individuals with intellectual disability showed increased responses compared with controls [9, 10].
As pain is a multidimensional and subjective experience, taking into consideration the recognition of the complexity of pain perception, all these issues make pain experience and expression by those with intellectual disability more challenging. Therefore, the pain associated with an ID is frequently underestimated and poorly managed [6].
This review article aims to provide a broad description of the intellectual disability and challenges of the assessment and management of pain in individuals with intellectual disability in an evidence-based manner, in addition to exploring the updated and recent tools for the assessment and management of pain with intellectual disability.
Methodology
This review is made up of two parts; the first part focuses on intellectual disability (ID) in general, such as the prevalence, risk factors, causes, diagnosis, and possible management, while the second part covers the pain associated with intellectual disability, as well as sources, types, and challenges in the assessment and management of pain in the presence of communication barriers, and finally the practical tips for the management of pain associated with (ID).
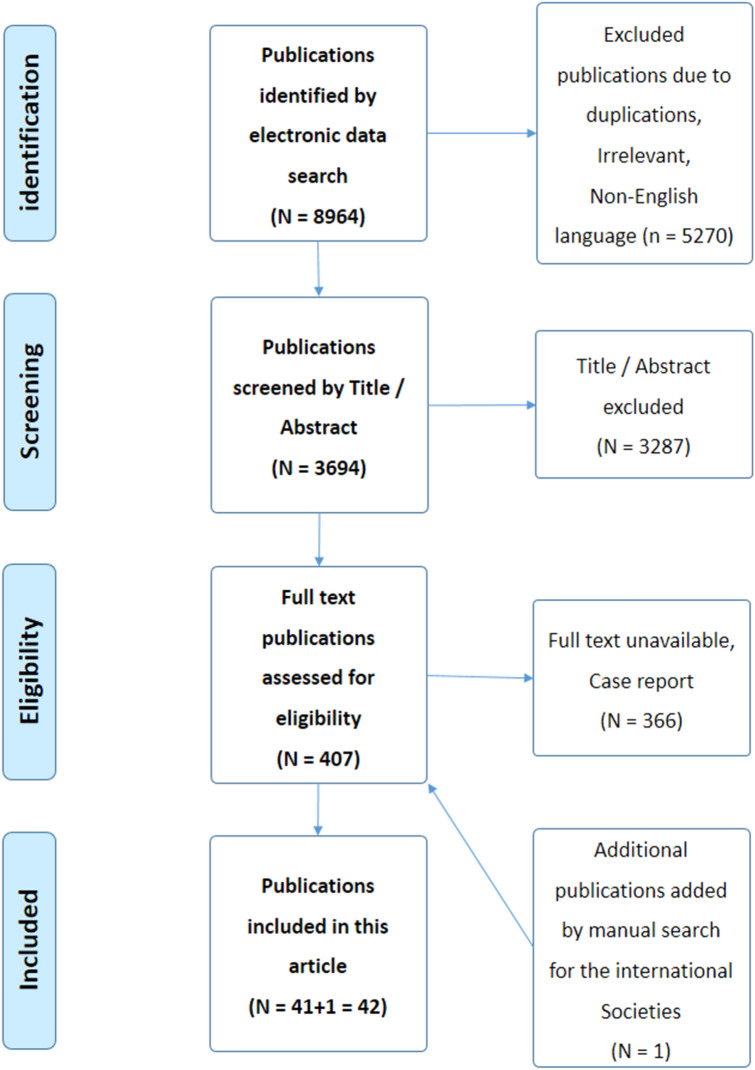
A computer search was conducted including literature from PubMed, Scopus, MEDLINE, Web of Science, and EMBASE databases. Manual screening of references from relevant sites was also conducted, and additional references were added. The search strategy included articles that were published from January 2010 to March 2023. The previously mentioned keywords were used for the search. Articles that met the inclusion criteria, such as articles published in the English language, relevant to the condition, presented information on the pain with ID, and involving both pediatric and adult patients, were included. The search strategy included observational, cross-sectional, cohort, case–control, longitudinal studies, systematic reviews, and meta-analyses. The exclusion criteria included non-English language articles, failure to get the full articles, case reports, editorials, or expert opinions. The selected articles were screened by two independent reviewers using the same method of evaluation. The final reviewing strategy of the literature search results in a total of [41] articles included in this review (Fig. 1) [11]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Fig. 1.
Flow chart of the included studies (PRISMA, 2009) [11]
Intellectual Disability
Intellectual disability involves problems with general mental abilities that affect both intellectual functioning (such as learning and reasoning) and adaptive functioning (activities of daily living such as communication and independent living) [1]. Childhood disability disorders range between 5% and 10%. Most of these disorders improve, and the condition persists in about 1% of adults [6], while the prevalence of children with profound intellectual and multiple disability is estimated to be between 0.4% and 1.3% [12].
Causes of Intellectual Disability
The causes of intellectual disability are heterogeneous; the cause can be congenital or environmental. Congenital causes include those such as genetic (e.g., fragile X syndrome, trisomy 21), exposure to teratogens in utero, congenital hypothyroidism, infections (e.g., toxoplasmosis, rubella), perinatal hypoxia, or trauma [13]. Acquired causes include those such as CNS infections (e.g., viral encephalitis, meningitis), malignancies (e.g., neuroblastomas), traumatic brain injury, toxins (e.g., lead, mercury), or malnutrition [14].
Clinical Presentations
According to the American Association on Intellectual and Developmental Disabilities, ID is characterized by significant limitations in intellectual functioning (reasoning, learning, and problem solving) and adaptive behavior (conceptual, social, and practical skills) that originate before the age of 18 years [15]. The clinical diagnosis of ID includes detailed history such as family history, antenatal history, birth history, neonatal history, and postnatal history; physical examination includes growth parameters and full body examination from head-to-toe (e.g., skull, face, spine, hands, and feet); and systematic examination includes neurological, cardiovascular, abdominal, and chest examination. Furthermore, it is important to assess intellectual function and adaptive behavior such as the intelligence quotient test (IQ) [16].
Diagnosis
Diagnosis of intellectual disability can be confirmed by the following three criteria: first, deficits in intellectual functions, such as reasoning, problem solving, planning, abstract thinking, judgment, academic learning, and learning from experience, confirmed by both clinical assessment and standardized intelligence testing. Second, deficits in adaptive functioning that fail to meet developmental and sociocultural standards for personal independence and social responsibility. Without ongoing support, the adaptive deficits limit functioning in one or more activities of daily life, such as communication, social participation, and independent living across multiple environments, such as home, school, work, and community. Third, the onset of intellectual and adaptive deficits occurs during the developmental period [17]. Investigations include biochemistry [e.g., blood, urine, feces, cerebrospinal fluid (CSF)], genetic studies (e.g., baseline and advanced chromosomal studies), and imaging studies [X-ray, computed tomography (CT), and magnetic resonance imaging (MRI)].
Management of Intellectual Disability
Due to the genetic basis of many intellectual disabilities, genetic counseling is crucial for determining the risk of recurrence, offering the possibility of prenatal diagnosis, and relieving families of feelings of guilt. It is important to know that the majority of cases of intellectual disability have behavioral and psychological disorders. Identification of such cases is important to facilitate positive behaviors and enforce appropriate social communication. Psychosocial interventions should be also attempted [1].
Any treatment plan should address the following dimensions, e.g., severity of the intellectual disability, etiology, associated comorbidities, psychiatric problems, and family and social factors. Management of intellectual disability may need a multidisciplinary approach involving pharmacological, non-pharmacological, and psychological interventions. The first line is to deal with preventable or reversible conditions with appropriate management. The second is to identify treatable causes of intellectual disability and potentially treatable symptoms such as hearing impairment with hearing aids, seizures with antiepileptics, and spasticity with anti-spasticity medications. Other treatable cases have to be referred to specialists accordingly for further assessment and proper management [1].
Usually, only a few medications are licensed for pediatric use; accordingly, it is recommended to follow dosing guidelines and to start with low doses and increase gradually, and the child should be monitored continuously [17]. Furthermore, non-pharmacological interventions can focus on skill development and educational purposes. Such interventions can be adapted in the management protocols, with early interventions for children at risk for intellectual disability. Examples of non-pharmacological strategies include taking a lifespan approach, adopting a functional approach, and seeking assistance from specialized centers for integrated child development services [17]. Parents and caregivers should be oriented to the nature and the management of intellectual disability. They should be involved in the management program and fully supported to provide the resources needed for the education, healthcare, and occupational needs for their children [18].
Pain Management in Individuals with Intellectual Disability
Historically, there has been controversy about whether people with an intellectual disability experience pain in the same way as others of average cognitive ability [19]. Clinical studies indicate that chronic pain is as prevalent among people with an intellectual disability as among the general population, but may be underestimated due to the limitations of reporting methods [20].
Prevalence of Pain with Intellectual Disability
The prevalence of chronic pain with intellectual disability is difficult to estimate and shows wide variations because the usual method of self-reporting may not be possible or reliable with the lack of communication [21]. Previously, the commonly held view was that individuals with intellectual disability have decreased sensitivity to pain, because of the relatively little awareness of the problem of pain among caregivers as well as people with an ID [20]. Current evidence suggests that individuals with ID may actually be more sensitive to painful stimuli, have greater pain-evoked potentials, and be more likely to experience chronic pain compared with typically developing peers [22–25]. This inconsistency between previous and current data may be in part attributed to the increased awareness of pain syndromes, advanced pain assessment tools, and better understanding of the individual disabilities [23, 25]. Quantitative sensory testing showed that individuals with ID are equally as sensitive as their healthy normal individuals or may even be more sensitive [10, 26, 27]. Moreover, endocrine responses and brain-evoked potentials recorded during noxious events reveal that individuals with ID showed increased responses compared with controls [9, 28, 29].
According to caregiver reports, previous studies showed the incidence of chronic pain among patients with ID occurring in at least 13% [21] and 15% [20], as in the general population [21], while recent estimates showed that the prevalence of chronic pain in ID is on average 70% (range 38–89%); and these estimates are considerably higher than the general population [30].
The prevalence of chronic pain in patients with ID showed wide variation according to the cause of intellectual disability as well. Schwartz et al. [31] found that 67% of their adult sample reported having chronic pain, with 56% indicating the pain was present daily. Similarly, Turk et al. [32] found that 84% of an adult female sample with cerebral palsy reported a similarly high prevalence of chronic pain. In addition, Houlihan et al. [33] found that pain was present daily in 11% of their participants on the basis of parental reports, and the presence and frequency of pain were positively correlated with the degree of physical disability, while Tervo et al. [34] found that pain was common among children with cerebral palsy and led to a high level of functional interference. According to the degree of disability, McGuire et al. [35] found that chronic pain was reported by 13% of the sample and was 2–2.5 times more prevalent in people with mild intellectual disability than in those with more severe disability. This reflects the fact that individuals with a mild level of disability can typically verbalize and communicate their pain experience so that their caregivers were aware of their pain problem, while those less able to communicate their pain were underrepresented in the reports of chronic pain provided by caregivers in the study [20]. This is actually one of the major challenges in the assessment of, and therefore management of, pain in those populations with ID. Furthermore, Hauer et al. [36] reported pain that occurred weekly in about 44% of children with moderate-to-profound cognitive impairment and almost daily in about 41–42% of children with severe cognitive impairment.
Risk Factors for Pain with Intellectual Disability
The problem of chronic pain among people with ID may be highly prevalent because of the higher frequency of associated physical disability [37]. A variety of factors increase the risk of both acute and chronic pain with ID, including greater risk of accidental injury, reduced involvement in health decision-making, more physical comorbidities, greater musculoskeletal disorders, low levels of physical activity, reduced use of pain management services, and age-related changes associated with greater life expectancy than in previous years of individuals with intellectual disability [20, 38]. A large study also used an informant-based approach and found that 15.4% of the sample was reported to have pain, persisting on average for more than 6 years [39]. Although more women experienced chronic pain, as is found in the general population, the variables of age, communication ability, and level of intellectual disability did not predict the presence of pain. However, the presence of cerebral palsy, other physical disability, and challenging behaviors was more common among people with chronic pain [20]. Patients with cerebral palsy are associated with motor impairments, and presented with increased muscle tone, spasms, and clonus. Spasticity and spasms can cause significant discomfort through waking and sleeping hours [27, 40]. Treatment of such conditions may involve invasive surgical procedures and other painful injections or interventions. Non‐invasive therapies such as regular physical therapy sessions and stretching can also contribute to significant pain [41, 42]. Moreover, children with ID are at increased risk of self-injury such as head banging, self-biting, or damage to the skin. The rates of self-injury ranged between 4% and 10% depending on the degree of ID, autism spectrum disorder, poor communication skills, low mood, high activity levels, and impulsive behavior [43–45].
Challenges and Considerations when Dealing with Intellectual Disability: Table 1
Table 1.
Challenges in the assessment and management of pain with ID
| Challenges | Text |
|---|---|
| Difficulty in discriminating between pain and other symptoms |
Behavioral expressions are inherently consistent across populations and determined by long-established biological dispositions [48] It is difficult to differentiate pain from other conditions such as anxiety, depression, or other emotions due to the overlap of manifestations [49] The signs and symptoms of pain may be mistakenly attributed to the intellectual disability [48, 49] The effects of situational factors such as the immediate surroundings, caregiver behavior, and culture on pain expression in individuals with ID are not well understood [50] Individuals with ID may exhibit typical pain behaviors (e.g., self-injury, moaning, or facial expressions) making it difficult to discriminate between signs of pain from other signs [35, 37] |
| Challenges in the pain assessments |
Pain in individuals with ID is not easy to identify, as is the cause or the source of pain [51] Incorrect beliefs regarding pain insensitivity or indifference lead to underestimation as well as under-treatment of pain [25] Self-reporting scores may not be possible and may be unreliable because of the impaired ability to communicate [35] The underlying neurologic condition and associated functional limitations may confuse the presentation of pain [52, 53] Observers are likely to underestimate patients’ suffering [54, 55] |
| Challenges in pain management |
Individuals with ID are at risk of developing drug-related side effects due to immature nervous systems, functional impairments, and the concurrent use of multiple medications [25] Fear of the risk of respiratory depression linked to opioids is common among prescribers [12] Individuals with ID are given fewer analgesics compared with their cognitively intact populations [56] Many people with an ID are dependent on their caregivers for ongoing management of their healthcare needs [35, 37] Pain medications may mask the sources of pain, and the medications could delay the correct diagnosis of pain [36, 57] |
| Lack of education |
Observers may be subject to old and wrong beliefs about individuals with ID and their decreased capacity to experience pain [25, 53] The skills and the level of education of the nurse and family are important in managing pain in this group [58]. Kankkunen et al. [51] identified that only 8% of the nurses working with individuals with ID had undertaken education for pain management The observers may not blind to the application of the painful stimulus and may overestimate the pain sensation [25] Intrapersonal and interpersonal factors as well as social/cultural factors may influence the caregiver’s ability to provide accurate pain ratings [25] |
| Lack of evidence-based research studies |
Research studies in populations with ID are challenging due to ethical complexity [25] Self-reported methods for pain assessments are not suitable for individuals who are difficult to communicate with [25] The caregiver/parent-reported pain scores are not valid or cannot adequately power studies due to small sample sizes [25] Most studies have been conducted on patients with severe ID who cannot provide informed consent to participate in pain research [59] The evidence on effective pain management practices for individuals with intellectual disability is limited [60] There is an absence of clinical guidelines about the safe and efficient different modalities in the treatment of patients with ID [61, 62] |
According to the IASP definition of pain as a subjective experience, it may be expressed in atypical or unfamiliar ways in those with significant cognitive and communicative difficulties. In some conditions, such as Down syndrome, pain may actually be experienced differently. The evidence suggests that although pain expression appears to be delayed, once pain is registered, there appears to be a magnified pain response. When reaction time is controlled for, there is evidence that people with Down syndrome are more sensitive to pain than average. The pain threshold was affected by the reaction time of the individual [46]. Consequently, pain may not be easily recognized and may go untreated [47].
A recent cross-sectional study [12] included pediatricians working in Switzerland who provide care to children with ID, and results showed that pain in children with ID was under-evaluated in 95% of children. Around 52% showed that the use of pain scales was more important than with typically developed children. However, more than half 51.2% of the pediatricians did not use pain scales for these children. Regarding pain management, the majority reported that pain in children with ID was under-treated. More than half of the respondents considered pain in children with ID to be more difficult to relieve [12].
Types and Causes of Pain
Patients with intellectual disability may experience any type of pain. A combination of nociceptive, neuropathic pain, and/or mixed types may present in the same patient, and this undoubtedly adds to the complexity of pain assessment as well as management.
Although the majority of post-surgical pain is nociceptive, repeated surgery or direct trauma to the peripheral nerves may result in more difficult and persistent neuropathic pain [63]. Neuropathic pain can be difficult to manage but should be considered in individuals with severe neurological impairments with prolonged pain after an intervention. Another potential source of pain is central in origin (i.e., thalamic injury), where the pain afferents appear to be activated without an ongoing input either from tissue damage or peripheral nerve injury [63, 64]. The major evidence for central pain comes from the observation of pain behavior in children with advancing neurodegenerative diseases [64]. It is important to think about those people who may have pain due to the original primary diseases or the associated comorbidities. For example, patients with epilepsy might have a headache after seizures, and women may have pain when they have their period. Children with cerebral palsy may complain of pain due to joint dislocation, degenerative joint disease, back pain, or the associated spasticity [51]. Moreover, patients with musculoskeletal disorders may experience significant pain due to the associated deformities, muscle spasms, and spasticity. This pain is exaggerated significantly during physical therapy and rehabilitation [65]. Other conditions may require more painful diagnostic or therapeutic interventions such as invasive diagnostic procedures and surgery [66].
The common conditions that require pain management for patients with intellectual disability include the following list of painful conditions: degenerative joint disease, arthritis and immobility, muscle spasms, premenstrual syndrome, cancer pain, traumatic experiences, migraine, and other chronic headaches [51]. Other causes of pain that also require pain management include dental carries/abscesses, otitis media, gastroesophageal reflux, constipation, and urinary tract infection [67]. Poorly managed pain is associated with significant drawbacks such as behavioral changes, psychological distress, impaired sleep, and reduced quality of life [65, 68].
Pain Assessments
Patients with intellectual disability are unable to verbalize their pain experience and self-reported pain scores are not routinely applicable in patients with significant ID. Several alternative systems have been developed, including systems based on behavioral cues or facial expressions [69]. Other researchers have used third-party reports (usually the parents or caregivers) to gather data about pain and other aspects of health in people with an intellectual disability. Accordingly, the pain assessments have focused on two main approaches: behavioral observation methods and caregiver reports. However, patients with a mild disability can verbalize and communicate their pain experience in a recognizable way similar to normal individuals [51]. Ideal pain assessment techniques should follow a logical progression or hierarchy: first, try to obtain a self-report assessment; then search for potential causes, observe behaviors, and obtain information from others (proxy reporting); and finally, attempt a trial of pain medication [70].
Identifying pain in individuals with an intellectual disability is very challenging. They may have difficulties in expressing and differentiating pain as well as understanding the necessary instructions. For example, adults with Down syndrome could define pain location but face difficulty with representations of pain intensity or pain quality [71, 72]. The ability to comprehend and use self-report scales differs according to the type of scale and level of disability, with graphical scales (e.g., faces and pyramids) having the highest usability. The difficulties in self-report, especially among individuals with moderate and severe intellectual disability, necessitate the use of alternative methods [73]. Several observational assessment tools have been developed in which caregivers rate the presence of recognized pain indicators, such as vocalizations, facial and emotional expressions, or motor behaviors. These observational methods have been used to support tools such as theNon-communicating Children’s Pain Checklist [74].
Methods of Pain Assessment in Intellectual Disability
Observational responses to pain and vocalization are perhaps the most useful ways that caregivers use to identify pain in individuals with intellectual disability [75]. Frequent pain behaviors may be reported by the child using special words to express pain, pointing or showing pain location, crying, changes in usual activities, moaning, being uncooperative, or seeking closeness to the parent [76]. In addition, some pain indicators may be observed, such as facial expression, withdrawal, restlessness, disturbed sleep, and behavioral changes [20]. Parents and caregivers can also report emotional responses (e.g., irritability, anger, fear, frustration, and acting out). The following pain scales might be used in patients with ID according to the type and extent of disability:
The Pain Behavior Checklist (PBC)
The PBC was developed to assess pain behaviors in the postoperative setting in children with profound intellectual and motor disability. It is also suitable for detecting pain in daily care situations. It is a score of ten non-verbal items that can be scored positively or negatively [77].
Chronic Pain Scale for Nonverbal Adults with Intellectual Disability (CPS-NAID)
The CPS-NAID consists of 27 items each rated on an ordinal 4-point scale (0–3). It includes six subcategories: vocal expression, emotional reaction, facial expression, body language, protective reactions, and physiological signs. It measures behavior in response to chronic and recurrent pain [78].
The Non-Communicating Children’s Pain Checklist (NCCPC)
The NCCPC is a pain measurement tool that was introduced and specifically designed for children with moderate-to-severe cognitive impairments. The scale discriminates between periods of pain and calm. It consists of 30 items and can be used within a home setting [74, 79, 80]. The NCCPC-postoperative version (PV) is a second version of the original NCCPC, assessing pain behaviors in a postoperative situation. Items related to eating and sleeping were omitted, and the remaining items were then scored on a 4-point scale according to their occurrence. The NCCPC-PV scale was found to have high psychometric values. Assessment of pain in children with cognitive impairments in the postoperative period documents the availability of two tools [NCCPC-PV and Face, Legs, Activity, Cry, Consolability (FLACC) scale] with strong evidence for reliability and validity in this population [79, 81].
The Revised NCCPC-R
The NCCPC-R is a 26-item behavioral scale that was used to measure pain responses and behaviors in children with intellectual and developmental disabilities. It is considered to have the best psychometric properties for acute or chronic pain. Previously omitted items, such as items related to food consumption or sleep duration and quality, are included in this scale [76, 82]. It measures pain responses observed by clinicians, parents, and caregivers regardless of how well they know the child. The NCCPC-R is recommended for use in research studies because it provides a standardized scale that is consistent across children [25, 80]. The NCCPC-R was also tested in home settings with high internal consistency, specificity, and sensitivity [76, 82].
The Pediatric Pain Profile (PPP)
The PPP has [20] behavioral items used for the assessment of pain in children and has been used successfully for adults [83]. It is used for children with significant cognitive and neurological disabilities and who are unable to communicate [84]. The PPP is useful for distinguishing a child’s good days from bad days. This scale is useful for caregivers and parents to add detailed information about the child’s unique pain expression [83].
Revised Faces Legs Activity Cry Consolability Scale (r-FLACC)
This scale includes five categories of behavior (face, legs, activity, cry, and consolability). Each of the five items scored from 0 to 2 on the basis of the detailed descriptors specific to each item, and result in a total score between 0 (no pain) and 10 (worst pain). This scale can be used for children and adults [25].
The Non-Communicating Adults Pain Checklist (NCAPC)
The NCAPC is an 18-item behavioral scale that was recently found to be reliable, valid, sensitive, and clinically feasible to assess pain responses and behavior in adults with intellectual and developmental disabilities. This scale is based on body movements and facial expressions as indicators of discomfort and acute pain [81, 82].
The Universal Pain Assessment Tool (UPAT)
The (UPAT) is used to assess the level of pain in children with limited communication skills and disability. The tool is an adapted version of the Wong–Baker Faces Pain Rating Scale using faces or behavioral observations. The UPAT is useful in assessing pain and detecting the existence of functional jaw pain, and is a valid instrument to score pain intensity associated with temporomandibular disorders in people with ID [85].
Self-reporting Tool on Pain in People with Intellectual Disability (STOP-ID!)
The Self-reporting Tool on Pain in People with Intellectual Disability (STOP-ID!) is an online application developed to aid in autonomous self-reporting of pain. It was evaluated in adults with Down syndrome. These comprehension tests assessed four components, e.g., pain location, pain intensity, pain effect, and pain quality. The results provide evidence that the use of tools such as (STOP-ID!) appears to be a promising approach for the reporting of pain information by some adults with Down syndrome in the presence of a trained caregiver [86].
Physiological Pain Assessment
The physiological pain assessment includes a number of physiological measures of pain such as the heart rate, blood pressure, respiration, vagal tone, salivary amylase activity, and intracranial pressure, which has been correlated with acute changes in sympathetic and parasympathetic tones due to pain as well as psychological stress [87–89]. Although physiological measures may be viewed as free of response bias, it is not specific for pain assessments [80]. All these parameters may be subject to changes due to multiple causes other than pain, such as associated medical, physical, or psychological conditions. However, it is useful for research sitting when correlated with other subjective measures.
Hi-Tech Pain Assessments Tools
New technological tools may enable pain assessment and improve accuracy, especially in individuals with moderate-to-severe ID and those who are unable to communicate and report their pain experience, such as geriatric patients with dementia. This technology used pain apps to track episodes of pain, locate the pain sites, and track potentially related factors such as stress, fatigue, and mood [80, 90]. The clinical findings indicated that the use of digital health can reduce bias and can improve the real-time data capture of acute and chronic pain symptoms [91].
Assessing Pain Using Facial Expressions
This involves using an application that relies on facial recognition technology using artificial intelligence (AI). The patient’s facial muscle movements, which are associated with pain, are recorded by directing the camera of a smartphone toward their face. The application then computes a pain score on the basis of this data. However, one potential drawback of this approach is that it may detect behavioral signs of pain when it is absent, potentially resulting in incorrect diagnoses. [80, 92].
Assessing Pain Using Vocal Responses
Pain-related vocal responses, such as non-verbal cries, moans, or screams, which are produced by individuals experiencing pain, may be used as indicators of pain in individuals with ID [93, 94]. A recent study [95] used the acoustic features of vocal expressions to identify pain in adults with ID. Acoustic analyses of spontaneous vocal expressions revealed that pain-related vocalizations were characterized by a higher number of pulses (i.e., segments in which phonation was detected), and higher shimmer values (the cycle-to-cycle amplitude variation) relative to no-pain vocal expressions. These pain-related vocal characteristics may be used to develop objective pain detection means, such as a smartphone app for caregivers [80]. The initial results may prompt further research to explore the possibility to use pain-related vocal output as possible pain monitoring in individuals with ID [95].
Using Smart Shirts as a Base for Pain Assessment
The new technological innovation of Smart Wearable Shirts (SWS) is a wearable medical device enabling continuous monitoring of human physiological signs without any disturbance to the activities of daily living. The (SWS) can collect physiological data including heart rate, respiratory function, and changes in regular respiration, body movements, and pacing; therefore, it might be used as a data collecting device to detect pain signs of individuals with ID. This technology has been used in clinical research during the past few years and has enabled the collection of varied physiological data outside the laboratory. SWS may be used for monitoring and early diagnosis of physical and mental health for individuals with ID. Other accessories or wearable sensors, such as smart electronic bracelets, may be used for this purpose as well [96–98].
Sensing Pain
Pain can be measured through using various body, environmental, and ambient sensors for pain in “smart home” environments [89, 99, 100]. These are wireless and connected devices that collect, transfer, store, and analyze data over a network. Sensor devices can be used to collect more frequent and accurate assessments within a growing digital health ecosystem [101]. These tools may be more accurate and minimize the reporting biases found in other clinical methods. Some medical devices and wearable sensors are used to measure specific aspects of pain sensation, perception, or psychology, while others are used to capture the quality of life. This category of body sensors captures physiological or biometric signals from the body, while wrist-worn devices and epidermal patches are often used to calculate heart rate variability due to pain, as well as psychological stress [102]. Quality of life measures tend to utilize environmental or ambient sensors to monitor movement and activities of daily living [103, 104]. The use of sleep sensors has also been implemented, because pain itself or pain medications may be a cause of disturbed sleep patterns [105, 106]. Finally, facial expression monitoring of pain intensity utilizes multi-camera systems and video recordings to measure “emitted” facial expressions in real time [107, 108].
Imaging Pain
As pain is a complex sensory and/or emotional experience, neuroimaging measurements of pain sensation and perception have also been used to study additional physiological and biological components of pain. Various technologies for imaging and measuring human brain structure and function as it relates to pain are now available [89]. Pain research showed strengthening evidence that acute and chronic pain mechanisms are distinct [109], and shift the field’s focus of attention from somatosensory processing and “the pain matrix” [110] toward brain circuitry related to reward and decision-making [111], emotion [112], and memory [113]. Neuroimaging can be useful and may lead to more effective pain management.
Preventive Strategy
A prevention science approach has been applied for promoting the health and quality of life of individuals with intellectual disability. It may be a particularly effective way to decrease health disparities among individuals with ID by addressing the challenging bio-psycho-social risks that individuals with ID experience during early childhood [114]. Routine annual health assessments are important for the early detection of any medical problem and maintaining good health [68].
Prevention strategies are effective in reducing pain in patients with ID, but the current situation shows that it is underutilized. For example, preventive measures were rarely applied to reduce pain experienced during daily care activities [115]. Similarly, hip dislocation is common in patients with cerebral palsy, which is associated with chronic pain [36, 116]. However, the rate of dislocation can be significantly reduced, to almost zero, if children are included in a surveillance program from an early age [25].
Pain Management in Intellectual Disability
Pain among individuals with ID is often difficult to treat and frequently requires ongoing assessment and titration of drugs before a satisfactory outcome can be achieved [117]. The communication barriers in intellectual disability make it difficult to correlate the source or cause of pain, the method of treatment, and the outcomes [65]. As pain is a multidimensional experience, the effective management of pain should ideally involve multimodal approaches to manage pain and associated comorbid medical, psychological, and psychosocial conditions. Multimodal approaches include pharmacological and non-pharmacological modalities [118].
Pharmacological Treatment
Effective assessment of the type and source of pain is the key to effective management of pain among individuals with ID [119]. A clear pain management plan should be documented and all persons involved in the individual’s care should be familiar with it. The pharmacological treatment of pain in individuals with intellectual disability should be dictated by the individual’s symptoms and based on the same principles applied to those without intellectual disability [119].
When treating individuals with intellectual disability with pharmacotherapy, some unique factors should be considered. For chronic pain patients, long-term medical treatment may be necessary and the non-medication techniques become even more important. Individuals with intellectual disability are at higher risk for the development of drug-related side effects. This is mainly attributed to the immature regulation of autonomic reactions, low nutritional status, impaired renal and liver functions, and the concurrent use of multiple medications possibly increasing the risk of drug-induced side effects [120]. In addition, some types of disability are associated with anatomic and physiologic changes, such as scoliosis and other musculoskeletal deformities. These changes can significantly contribute to hypoventilation and airway obstruction [65, 121].
Non-pharmacological
Non-pharmacological modalities can be used alone or in combination with medical treatment according to the patient’s pain. This multimodal approach aims to treat affective, cognitive, behavioral, and sociocultural dimensions of pain [122].
Social Care
Social care staff can recognize and manage pain in people with learning disabilities. Social care is related to changing or altering the environment, distraction, relaxation, divisional therapy, oils, and supporting the person through any changes in school or work [119]. These techniques serve to promote comfort, relaxation, and engagement of the person with intellectual disability so they may perceive less pain. Distraction works by moving the attention away from the pain to reduce its severity, and methods include listening to music, watching television, or reading a book [122].
A recent systematic review included ten studies that assessed strategies for enhancing social skills for individuals with intellectual disability. This review revealed that social care interventions appeared to be a significantly effective modality. However, it may not be suitable for school settings or self-reported social behavior for individuals with intellectual disability. These strategies have strong relevance for improving the social skills of individuals with intellectual disability [123].
Spiritual Tools
Spiritual tools enable people with intellectual disability to express themselves through a medium that affords pleasure, comfort, peace, and tranquility. This includes listening to music, singing, gardening, art, or religious practices [124].
Psychological Interventions
Pain signal transmission can be influenced by emotions and thoughts. Psychological pain gates can be closed in numerous ways, including by massage, distraction, work, music, leisure activities, biofeedback, breathing exercises, visualization, guided imagery, and relaxation. These techniques are advised to increase control over pain and reduce distress [119].
Psychological interventions for pain management may also have a useful role in pain management among people with an intellectual disability. Psychological modalities aim to increase self-management and behavioral and cogitative change rather than directly eliminating the locus of pain; in fact, it may include relaxation, breathing exercises, visualization, and cognitive behavioral therapy [125].
Cognitive behavioral therapy has become the most widely used psychological intervention for pain management in adults [126] and children [127]. Many review articles have confirmed the efficacy of cognitive behavioral interventions for typically developing adults and children with chronic pain [126, 127]. There is good evidence to support behavioral observation methods for the recognition of pain in people with a limited ability [20]. The most frequently described cognitive techniques were psycho-education and interventions directly aimed at thoughts and beliefs, and most studies reported positive outcomes, although the better-controlled studies tended to report less comprehensive impacts [128].
Physical Therapy
The main goals of physical therapy are to reduce the perception of pain and possibly help people to cope with pain [129]. For individuals with intellectual disability, physical therapy might include muscle strengthening, postural training, seating position, cushioning, splinting, exercises, and rehabilitation programs [130]. The application of simple tools such as ice or heat treatment, hot bath, massage, acupuncture, vibration, transcutaneous electrical nerve stimulation (TENS), and cupping reflect our understanding of Melzack and Wall’s (1965) gate control theory [51].
The use of heat treatment inhibits pain through vasodilatation to reduce pain. Similarly, cold treatment increases the pain threshold and reduces edema, inflammation, and pain experienced [122]. Positioning can be an important treatment especially when a physical disability coexists with gastric reflux. Positioning or repositioning can prevent or reduce pain, increase blood flow, and prevent muscle contractions and spasms. TENS works by transmitting lots of non-painful messages to the same place in the dorsal horn of the spinal cord that receives the pain messages, and in so doing reduces the pain messages that are received and interpreted in the brain. However, Naka et al. [131], in their systematic review, highlight that most studies confirm the usefulness and the analgesic effect of TENS in clinical practice. Massage is asserted to be effective in inducing relaxation and reducing challenging behaviors in people with intellectual disability. However, evidence-based literature demonstrating the effectiveness of massage therapy in supporting clinical practice is extremely limited [132].
Practical Tips for the Management of Pain in Intellectual Disability
The first step in the management of pain is a comprehensive pain assessment that is suitable and applicable according to the type and degree of disability [4].
There is no consistent method for assessing pain in people with ID, various instruments approach assessment differently, and few guidelines are available for training providers to reliably detect pain in non-communicating individuals [133].
Individuals with ID and their parents/caregivers should be included in the assessment and management plan [134].
Educating caregivers in the methods of pain assessment and management tools as well as possible cognitive biases may improve pain management [25].
Creating personal profiles for each patient (e.g., hospital passports, personal cards, health certificates) can assist providers and caregivers in recognizing pain behaviors, and profiles should describe the individuals’ common signs of pain [25].
A prevention approach and routine annual health assessments may be a particularly effective way in reducing pain and decreasing health disparities in patients with ID [68, 114].
Encourage non-pharmacological and physical approaches that raise the pain threshold, increase tolerance, and modify the lifestyle [51].
It is important not to try more than one new modality (e.g., medical treatment or pain intervention) at the same time. This allows for the review and evaluation of each modality separately [135].
It is necessary to be aware of potential drug interactions when multiple medications are needed to manage patients with ID [4].
It is also essential to be aware of the possible genetic variation in drug metabolism and response [4].
Future Plan
Neuroimaging techniques and electrophysiological measures have future potential for the identification and assessment of pain [20].
There has been significant work on developing various pain assessment tools, and projects to improve pain practices for individuals with ID have emerged. As a result, Holland Bloorview’s Chronic Pain Assessment Toolbox for Children with ID was created [136].
Pain education for parents and secondary caregivers of patients with ID to improve their skills and knowledge has begun. Preliminary outcomes have demonstrated improved knowledge and altered beliefs and intention for knowledge application [137, 138].
Technology-based interventions such as the use of artificial intelligence can be applied to develop pain coping skills. AI may have a promising treatment potential in the future [20].
Virtual reality (VR) can be used to distract patients during medical procedures and its use is rapidly growing. Findings suggest that virtual reality effectively reduces pain and anxiety with few side effects [139].
Using technology can support and enhance the learning, independence, and daily living skills of students with intellectual disability. This can be achieved by focusing on two areas: 1) instructional aids, with a focus on reading, writing, and mathematics; and 2) transition and independence [140].
Summary and Conclusions
Individuals with ID are among the most vulnerable populations in terms of developing and experiencing pain due to physical, psychological, and/or verbal impairments. They should be offered the same standard of care as all other individuals. People with intellectual disability are at greater risk to be underestimated and poorly managed for pain due to difficulty in both verbal and non-verbal communication. Identification of the proper methods for pain assessment and effective treatment plans represent a great challenge among those populations with ID. Parents’ and/or caregivers’ training and education should be reinforced to increase their knowledge and awareness as to how to deal with those populations with ID, especially when they have pain. Evidence is promising that incorporation of neuroimaging and electrophysiological tools for the assessment of pain, and new technology such as virtual reality and artificial intelligence for the management of pain among individuals with ID can help to overcome communication barriers. More research is required to identify the possible risk factors, preventive strategies, and new methods for the assessment and management of pain among patients with intellectual disability.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Salah N. El-Tallawy (corresponding author) was responsible for the concept and design, writing, searching, and supervision for all steps; Rania S. Ahmed for searching, study screening, editing, and review; and Mohamed S. Nagiub for searching, study screening, editing, and review.
Disclosures
All authors declare no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Salah N. El-Tallawy, Email: salaheltallawy@yahoo.com
Rania S. Ahmed, Email: raniasalah246890@gmail.com
Mohamed S. Nagiub, Email: hamadasalah13579@gmail.com
References
- 1.Vasudevan P, Suri M. A clinical approach to developmental delay and intellectual disability. Clin Med (Northfield Il) 2017;17(6):558–561. doi: 10.7861/clinmedicine.17-6-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Association on Intellectual and Developmental Disabilities (AAIDD). 2019. https://www.aaidd.org/intellectual-disability/definition. Accessed 27 Sep 2019.
- 3.World Health Organization . International classification of functioning, disability and health (ICF) external icon. Geneva: WHO; 2001. [Google Scholar]
- 4.Raiter A, Merbler A, Burkitt CC, Symons FJ, Oberlander TF. Clinical pain management: a practical guide, chapter 42. 2. Cham: Wiley; 2022. Pain in individuals with intellectual disabilities; pp. 439–449. [Google Scholar]
- 5.Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Defrin R, McGuire BE. International association for the study of pain, fact sheets, pain in individuals with an intellectual disability: scope of the problem and assessment challenges. 2021. https://www.iasp-pain.org/resources/fact-sheets/pain-in-individuals-with-an-intellectual-disability-scope-of-the-problem-and-assessment-challenges/
- 7.Moore KL. Pain is enough: chronic pain as disability. Buffalo Law Rev. 2021;69(5):1471–1540. [Google Scholar]
- 8.Merskey H, Bogduk N. Classification of chronic pain. descriptions of chronic pain syndromes and definitions of pain terms. Int Assoc Study Pain Subcomm Taxon. 1986;3:S1–226. [PubMed] [Google Scholar]
- 9.Aguilar Cordero MJ, Mur Villar N, García GI. Evaluation of pain in healthy newborns and in newborns with developmental problems (Down syndrome) Pain Manag Nurs. 2015;16:267–272. doi: 10.1016/j.pmn.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Benromano T, Pick CG, Granovsky Y, Defrin R. Increased evoked potentials and behavioral indices in response to pain among individuals with intellectual disability. Pain Med. 2017;18(9):1715–1730. doi: 10.1093/pm/pnw349. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Petigas L, Newman CJ. Pediatricians’ views on pain in children with profound intellectual and multiple disabilities. Brain Sci. 2021;11:408. doi: 10.3390/brainsci11030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilissen C, Hehir-Kwa JY, Thung DT, et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 14.Sulkes SB. Intellectual disability. Golisano Children’s Hospital at Strong, University of Rochester School of Medicine and Dentistry (Last review Feb 2022). 2022. http://www.merckmanuals.com/en-ca/professional/pediatrics/learning-and-developmental-disorders/intellectual-disability
- 15.American Association on Intellectual and Developmental Disabilities. Intellectual disability: defining criteria for intellectual disability. AAIDD. 2022. https://www.aaidd.org/intellectual-disability/definition
- 16.Hatton C, Emerson E, Glover G et al. People with learning disabilities in England 2013. London: Public Health England. 2014. http://www.improvinghealthandlives.org.uk/publications/1241/People_with_Learning_Disabilities_in_England_2013
- 17.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 18.Kishore MT. Disability impact and coping in mothers of children with intellectual disabilities and multiple disabilities. J Intellect Disabil. 2011;15:241. doi: 10.1177/1744629511431659. [DOI] [PubMed] [Google Scholar]
- 19.Blyth FM, Lee L. Giving a voice to the vulnerable. Pain. 2011;152:1937. doi: 10.1016/j.pain.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Zwakhalen SMG, Van Dongen KAJ, Hamers JPH, Abu-Saad HH. Pain assessment in intellectually disabled people: non-verbal indicators. J Adv Nurs. 2004;45(3):236–245. doi: 10.1046/j.1365-2648.2003.02884.x. [DOI] [PubMed] [Google Scholar]
- 21.Walsh M, Morrison TM, McGuire BE. Chronic pain in adults with an intellectual disability: prevalence, impact and health service utilization based on caregiver report. Pain. 2011;152:1951–1957. doi: 10.1016/j.pain.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Nader R, Oberlander TF, Chambers CT, Craig KD. The expression of pain in children with autism. Clin J Pain. 2004;20:88–97. doi: 10.1097/00002508-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 23.May ME, Kennedy CH. Health and problem behavior among people with intellectual disabilities. Behav Anal Pract. 2010;3(2):4–12. doi: 10.1007/BF03391759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riquelme I, Montoya P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin Neurophysiol. 2010;121:1314–1320. doi: 10.1016/j.clinph.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Chantel CB, Randi DA, Ruth D, Lara MG, Brian EM, Frank JS. Challenges in pain assessment and management among individuals with intellectual and developmental disabilities. PAIN Rep. 2020;5(4):821. doi: 10.1097/PR9.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Defrin R, Pick CG, Peretz C, Carmeli E. A quantitative somatosensory testing of pain threshold in individuals with mental retardation. Pain. 2004;108:58–66. doi: 10.1016/j.pain.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Valkenburg AJ, Tibboel D, van Dijk M. Pain sensitivity of children with Down syndrome and their siblings: quantitative sensory testing versus parental reports. Dev Med Child Neurol. 2015;57:1049–1055. doi: 10.1111/dmcn.12823. [DOI] [PubMed] [Google Scholar]
- 28.Ploner M, May ES. Electroencephalography and magnetoencephalography in pain research-current state and future perspectives. Pain. 2018;159(2):206–211. doi: 10.1097/j.pain.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 29.Benromano T, Pick CG, Merick R, Defrin R. Physiological and behavioral responses to calibrated noxious stimuli among individuals with cerebral palsy and intellectual disability. Pain Med. 2017;18:441–453. doi: 10.1093/pm/pnw349. [DOI] [PubMed] [Google Scholar]
- 30.Interagency Pain Research Coordinating Committee . National pain strategy: A comprehensive population health-level strategy for pain. Washington: US Department of Health and Human Services, National Institutes of Health; 2016. p. 36. [Google Scholar]
- 31.Schwartz L, Engel JM, Jensen MP. Pain in persons with cerebral palsy. Arch Phys Med Rehabil. 1999;80:1243–1246. doi: 10.1016/S0003-9993(99)90023-0. [DOI] [PubMed] [Google Scholar]
- 32.Turk MA, Geremski CA, Rosenbaum PF, Weber RJ. The health status of women with cerebral palsy. Arch Phys Med Rehabil. 1997;78:10–17. doi: 10.1016/S0003-9993(97)90216-1. [DOI] [PubMed] [Google Scholar]
- 33.Houlihan CM, O’Donnell M, Conaway M, Stevenson RD. Bodily pain and health-related quality of life in children with cerebral palsy. Dev Med Child Neurol. 2004;46:305–310. doi: 10.1111/j.1469-8749.2004.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 34.Tervo RC, Symons F, Stout J, Novacheck T. Parental report of pain and associated limitations in ambulatory children with cerebral palsy. Arch Phys Med Rehabil. 2006;87:928–934. doi: 10.1016/j.apmr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 35.McGuire BE, Daly P, Smyth F. Chronic pain in people with an intellectual disability: under-recognised and under-treated? J Intellect Disabil Res. 2010;54:240–245. doi: 10.1111/j.1365-2788.2010.01254.x. [DOI] [PubMed] [Google Scholar]
- 36.Hauer JM, Houtrow AJ. Pain assessment and treatment in children with significant impairment of the central nervous system. Pediatrics. 2017;139(6):e20171002. doi: 10.1542/peds.2017-1002. [DOI] [PubMed] [Google Scholar]
- 37.Ho P, Bulsara M, Downs J, Patman S, Bulsara C, Hill AM. Incidence and prevalence of falls in adults with intellectual disability living in the community: a systematic review. JBI Database Syst Rev Implement Rep. 2019;17:390–413. doi: 10.11124/JBISRIR-2017-003798. [DOI] [PubMed] [Google Scholar]
- 38.McGuire BE, Kennedy S. Pain in people with an intellectual disability. Curr Opin Psychol. 2013;26:270–275. doi: 10.1097/YCO.0b013e32835fd74c. [DOI] [PubMed] [Google Scholar]
- 39.Walsh M, Morrison T, McGuire BE. Chronic pain in adults with an intellectual disability: prevalence, impact and health service use based on caregiver report. Pain. 2011;152:1951–1957. doi: 10.1016/j.pain.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Awaad Y, Rizk T, Vrak E. Cerebral palsy: challenges for the future. Intech; 2014. Management of spasticity and cerebral palsy. [Google Scholar]
- 41.Kerem M, Turker D, Ozal C, Kaya Kar O. Physical management of children with cerebral palsy. In: Ch M, editor. Cerebral palsy: challenges for the future [internet] Intech; 2014. [Google Scholar]
- 42.Chang E, Ghosh N, Yanni D, Lee S, Alexanndru D, Mozzaffar T. A review of spasticity treatments: pharmacological and interventional approaches. Crit Rev Phys Rehabil Med. 2013;25(1–2):11–22. doi: 10.1615/CritRevPhysRehabilMed.2013007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper SA, Smiley E, Jackson A, Finlayson J, Allan L, Mantry D, Morrison J. Adults with intellectual disabilities: prevalence, incidence and remission of aggressive behavior and related factors. J Intellect Disabil Res. 2009;53(3):217–232. doi: 10.1111/j.1365-2788.2008.01127.x. [DOI] [PubMed] [Google Scholar]
- 44.Poppes P, Van der Putten AJJ, Vlaskamp C. Frequency and severity of challenging behaviour in people with profound intellectual and multiple disabilities. Res Dev Disabil. 2010;31(6):1269–1275. doi: 10.1016/j.ridd.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Arron K, Oliver C, Moss J, Berg K, Burbidge C. The prevalence and phenomenology of self-injurious and aggressive behaviour in genetic syndromes. J Intellect Disabil Res. 2011;55(2):109–120. doi: 10.1111/j.1365-2788.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- 46.McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9:194. doi: 10.3389/fnbeh.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foley CM, Deely DA, MacDermott EJ, et al. Arthropathy of Down syndrome: an under-diagnosed inflammatory joint disease that warrants a name change. RMD Open. 2019;5:e000890. doi: 10.1136/rmdopen-2018-000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke ZJ, Thompson AR. Parents’ experiences of pain and discomfort in people with learning disabilities. Br J Learn Disabil. 2007;36:84–90. doi: 10.1111/j.1468-3156.2007.00467.x. [DOI] [Google Scholar]
- 49.Kirsh KL. Differentiating and managing common psychiatric comorbidities seen in chronic pain patients. J Pain Palliat Care Pharmacother. 2010;24:39–47. doi: 10.3109/15360280903583123. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill MC, Pillai Riddell R, Garfield H, Greenberg S. Does caregiver behavior mediate the relationship between cultural individualism and infant pain at 12 Months of age? J Pain. 2016;17:1273–1280. doi: 10.1016/j.jpain.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Doody O, Bailey ME. Interventions in pain management for persons with an intellectual disability. J Intellect Disabil. 2019;23(1):132–144. doi: 10.1177/1744629517708679. [DOI] [PubMed] [Google Scholar]
- 52.Oberlander TF, Symons F. The problem of pain in developmental disability. In: Oberlander TF, Symons FJ, editors. Pain in developmental disabilities. Baltimore: Paul H. Brookes Publishing Co. Inc; 2006. [Google Scholar]
- 53.Breau LM, MacLaren J, McGrath PJ, Camfield CS, Finley GA. Caregivers’ beliefs regarding pain in children with cognitive impairment: relation between pain sensation and reaction increases with severity of impairment. Clin J Pain. 2003;19:335–344. doi: 10.1097/00002508-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Prkachin KM, Berzins S, Mercer SR. Encoding and decoding of pain expressions: a judgment study. Pain. 1994;58:253–259. doi: 10.1016/0304-3959(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 55.Lautenbacher S, Niewelt BG, Kunz M. Decoding pain from the facial display of patients with dementia: a comparison of professional and nonprofessional observers. Pain Med. 2013;14:469–477. doi: 10.1111/pme.12050. [DOI] [PubMed] [Google Scholar]
- 56.Boerlage AA, Valkenburg AJ, Scherder EJ, Steenhof G, Effing P, Tibboel D, van Dijk M. Prevalence of pain in institutionalized adults with intellectual disabilities: a cross-sectional approach. Res Dev Disabil. 2013;34:2399–2406. doi: 10.1016/j.ridd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. 2016. www.who.int/medicines/areas/quality_safety/guide_perspainchild/en/. Accessed 15 June 2016. [PubMed]
- 58.Davies RB. Pain in children with Down syndrome, assessment and intervention by parents. Pain Manag Nurs. 2010;11:259–267. doi: 10.1016/j.pmn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Raskoff SZ, Thurm A, Miguel HO, Kim SYH, Quezado ZNM. Pain research and children and adolescents with severe intellectual disability: ethical challenges and imperatives. Lancet. 2022 doi: 10.1016/S2352-4642(22)00346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogtle LK. Pain in adults with cerebral palsy: impact and solutions. Dev Med Child Neurol. 2009;51:113–121. doi: 10.1111/j.1469-8749.2009.03423.x. [DOI] [PubMed] [Google Scholar]
- 61.Barney CC, Merbler AM, Simone DA, Walk D, Symons FJ. Investigating the feasibility of a modified quantitative sensory testing approach to profile sensory function and predict pain outcomes following intrathecal baclofen implant surgery in cerebral palsy. Pain Med. 2019;21:109–117. doi: 10.1093/pm/pnz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lonchampt S, Gerber F, Aubry JM, Desmeules J, Kosel M, Besson M. Pain interventions in adults with intellectual disability: a scoping review and pharmacological considerations. Eur J Pain. 2020;24(5):875–885. doi: 10.1002/ejp.1547. [DOI] [PubMed] [Google Scholar]
- 63.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically-induced neuropathic pain: understanding the perioperative process. Ann Surg. 2013;257(3):403–412. doi: 10.1097/SLA.0b013e3182701a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101:259–301. doi: 10.1152/physrev.00045.2019. [DOI] [PubMed] [Google Scholar]
- 65.El-Tallawy SN, Nalamasu R, Salem GI, LeQuang JAK, Pergolizzi JV, Christo PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021;10(1):181–209. doi: 10.1007/s40122-021-00235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKenzie K, Smith M, Purcell A. The reported expression of pain and distress by people with intellectual disability. J Clin Nurs. 2012;22:1833–1842. doi: 10.1111/j.1365-2702.2012.04269.x. [DOI] [PubMed] [Google Scholar]
- 67.Peebles KA, Price TJ. Self-injurious behavior in intellectual disability syndromes: evidence for aberrant pain signaling as a contributing factor. J Intellect Disabil Res. 2012;56(5):441–452. doi: 10.1111/j.1365-2788.2011.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barney CC, Andersen RD, Defrin R, Genik LM, McGuire BE, Symons FJ. Challenges in pain assessment and management among individuals with intellectual and developmental disabilities. Pain Rep. 2020;5(4):e821–e821. doi: 10.1097/PR9.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Symons FJ, Shinde SK, Gilles E. Perspectives on pain and intellectual disability. J Intellect Disabil Res. 2008;52:275–286. doi: 10.1111/j.1365-2788.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 70.Herr K, Coyne P, McCaffery M, Manworren R, Merkel S. Pain assessment in the patient unable to self-report: position statement with clinical practice recommendations. Pain Manag Nurs. 2011;12(4):230–250. doi: 10.1016/j.pmn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Doody O, Bailey ME. Pain and pain assessment in people with intellectual disability: Issues and challenges in practice. Br J Learn Disabil. 2017 doi: 10.1111/bld.12189. [DOI] [Google Scholar]
- 72.de Knegt NC, Lobbezoo F, Schuengel C, Evenhuis HM, Scherder EJA. Self-reporting tool on pain in people with intellectual disabilities (STOP-ID!): a usability study. Augment Altern Commun. 2016;32:1–11. doi: 10.3109/07434618.2015.1100677. [DOI] [PubMed] [Google Scholar]
- 73.Defrin R, McGuire BE. Pain in individuals with an intellectual disability: scope of the problem and assessment challenges: IASP Fact Sheets. 2019. https://www.iasp-pain.org/resources/fact-sheets/pain-in-individuals-with-an-intellectual-disability-scope-of-the-problem-and-assessment-challenges/#
- 74.Breau LM, McGrath PJ, Camfield C, Rosmus C, Finley GA. Preliminary validation of an observational pain checklist for persons with cognitive impairments and inability to communicate verbally. Devel Med Child Neurol. 2000;42:609–616. doi: 10.1017/S0012162200001146. [DOI] [PubMed] [Google Scholar]
- 75.Hennequin M, Morin C, Feine JS. Pain expression and stimulus localisation in individuals with Down’s syndrome. Lancet. 2000;356:1882–1887. doi: 10.1016/S0140-6736(00)03259-1. [DOI] [PubMed] [Google Scholar]
- 76.Breau LM, McGrath PJ, Camfield CS, Finley GA. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain. 2002;99:349–357. doi: 10.1016/S0304-3959(02)00179-3. [DOI] [PubMed] [Google Scholar]
- 77.van der Putten A, Vlaskamp C. Pain assessment in people with profound intellectual and multiple disabilities; a pilot study into the use of the pain behaviour checklist in everyday practice. Res Dev Disabil. 2011;32(5):1677–1684. doi: 10.1016/j.ridd.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 78.Burkitt C, Breau LM, Salsman S, et al. Pilot study of the feasibility of the non-communicating children’s pain checklist revised for pain assessment for adults with intellectual disabilities. J Pain Manag. 2009;2:37–49. [Google Scholar]
- 79.Pizzinato A, Liguoro I, Pusiol A, Cogo P, Palese A, Vidal E. Detection and assessment of postoperative pain in children with cognitive impairment: a systematic literature review and meta-analysis. Eur J Pain. 2022;26:965–979. doi: 10.1002/ejp.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lotan M, Icht M. Diagnosing pain in individuals with intellectual and developmental disabilities: current state and novel technological solutions. Diagnostics. 2023;13:401. doi: 10.3390/diagnostics13030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breau LM, Finley GA, McGrath PJ, Camfield CS. Validation of the non-communicating children’s pain checklist-postoperative version. Anesthesiology. 2002;96:528–535. doi: 10.1097/00000542-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Tudor ME, Walsh CE, Mulder EC, Lerner MD. Pain as a predictor of sleep problems in youth with autism spectrum disorders. Autism. 2015;19(3):292–300. doi: 10.1177/1362361313518994. [DOI] [PubMed] [Google Scholar]
- 83.Hunt A, Goldman A, Seers K, et al. Clinical validation of the paediatric pain profile. Dev Med Child Neurol. 2004;46:9–18. doi: 10.1111/j.1469-8749.2004.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 84.Barney CC, Belew JL, Valkenburg AJ, Symons FJ, Oberlander TF. Pain. In: Rubin IL, Merrick J, Greydanus DE, Patel DR, editors. Health care for people with intellectual and developmental disabilities across the lifespan. Cham: Springer; 2016. [Google Scholar]
- 85.Dugashvili G, Van den Berghe L, Menabde G, Janelidze M, Marks L. Use of the universal pain assessment tool for evaluating pain associated with TMD in youngsters with an intellectual disability. Cir Bucal. 2017;22(1):e88–94. doi: 10.4317/medoral.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Knegt NC. The down side of pain: pain assessment and experience in adults with Down syndrome and the relationship with cognition. [PhD-Thesis - Research and graduation internal, Vrije Universiteit Amsterdam]. 2015. https://research.vu.nl/en/publications/the-down-side-of-pain-pain-assessment-and-experience-in-adults-wi
- 87.Karri J, Zhang L, Li S, Chen YT, Stampas A, Li S. Heart rate variability: a novel modality for diagnosing neuropathic pain after spinal cord injury. Front Physiol. 2017;8:495. doi: 10.3389/fphys.2017.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain. 2016;157:7–29. doi: 10.1097/j.pain.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 89.Berger SE, Baria AT. Assessing pain research: a narrative review of emerging pain methods, their technosocial implications, and opportunities for multidisciplinary approaches. Front Pain Res. 2022;3:896276. doi: 10.3389/fpain.2022.896276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atee M, Hoti K, Parsons R, Hughes JD. A novel pain assessment tool incorporating automated facial analysis: interrater reliability in advanced dementia. Clin Interv Aging. 2018;16(13):1245–1258. doi: 10.2147/CIA.S168024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rejula V, Anitha J, Belfin RV, Peter JD. Chronic pain treatment and digital health era-an opinion. Front Public Health. 2021;9:779328. doi: 10.3389/fpubh.2021.779328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGrath PJ, Rosmus C, Camfield C, Campbell MA, Hennigar AW. Behaviours caregivers use to determine pain in nonverbal, cognitively impaired individuals. Dev Med Child Neurol. 1998;40:340–343. [PubMed] [Google Scholar]
- 93.Herr K, Coyne PJ, McCaffery M, Manworren R, Merkel S. Pain assessment in the patient unable to self–report: position statement with clinical practice recommendations. Pain Manag Nurs. 2011;12:230–250. doi: 10.1016/j.pmn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 94.van Iersel T, Timmerman D, Mullie A. Introduction of a pain scale for palliative care patients with cognitive impairment. Int J Palliat Nurs. 2006;12:54–59. doi: 10.12968/ijpn.2006.12.2.20531. [DOI] [PubMed] [Google Scholar]
- 95.Icht M, Ressistal HW, Lotan M. Can the vocal expression of intellectually disabled individuals be used as a pain indicator? Initial findings supporting a possible novice assessment method. Front Psychol. 2021;12:6555202. doi: 10.3389/fpsyg.2021.655202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Black MH, Milbourn B, Chen NTM, McGarry S, Wali F, Ho ASV, Lee M, Bölte S, Falkmer T, Girdler S. The use of wearable technology to measure and support abilities, disabilities and functional skills in autistic youth: a scoping review. Scand J Child Adolesc Psychiatry Psychol. 2020;8:48–69. doi: 10.21307/sjcapp-2020-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simantiraki O, Giannakakis G, Pampouchidou A, Tsiknakis M. Pervasive computing paradigms for mental health. Cham: Springer; 2016. Stress detection from speech using spectral slope measurements; pp. 41–50. [Google Scholar]
- 98.Chen J, Abbod M, Shieh JS. Pain and stress detection using wearable sensors and devices: a review. Sensors. 2021;21:1030. doi: 10.3390/s21041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byrom B, McCarthy M, Schueler P, Muehlhausen W. Brain monitoring devices in neuroscience clinical research: the potential of remote monitoring using sensors, wearables, and mobile devices. Clin Pharmacol Ther. 2018;104:59–71. doi: 10.1002/cpt.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Argüello Prada EJ. The Internet of Things (IoT) in pain assessment and management: an overview. Inform Med Unlocked. 2020;18:100298. doi: 10.1016/j.imu.2020.100298. [DOI] [Google Scholar]
- 101.Kelly JT, Campbell KL, Gong E, Scuffham P. The Internet of Things: impact and implications for health care delivery. J Med Internet Res. 2020;22:e20135. doi: 10.2196/20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Abbod M, Shieh JS. Pain and stress detection using wearable sensors and devices—a review. Sensors. 2021;21:1030. doi: 10.3390/s21041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fritz RL, Wilson M, Dermody G, Schmitter-Edgecombe M, Cook DJ. Automated smart home assessment to support pain management: multiple methods analysis. J Med Internet Res. 2020;22:e23943. doi: 10.2196/23943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grant S, Whitehouse MR, Blom AW, Judge A, Craddock I, Gooberman-Hill R. Using home sensing technology to assess outcome and recovery after joint replacement – findings from the hip and knee study of a sensor platform of healthcare in a residential environment. Osteoarthr Cartil. 2018;26:S342–S343. doi: 10.1016/j.joca.2018.02.681. [DOI] [Google Scholar]
- 105.Andersen ML, Araujo P, Frange C, Tufik S. Sleep disturbance and pain. Chest. 2018;154:1249–1259. doi: 10.1016/j.chest.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 106.Robertson JA, Purple RJ, Cole P, Zaiwalla Z, Wulff K, Pattinson KTS. Sleep disturbance in patients taking opioid medication for chronic back pain. Anaesthesia. 2016;71:1296–1307. doi: 10.1111/anae.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sikka K, Dhall A, Bartlett MS. Classification and weakly supervised pain localization using multiple segment representation. Image Vis Comput. 2014;32:659–670. doi: 10.1016/j.imavis.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lautenbacher S, Hassan T, Seuss D, Loy FW, Garbas JU, Schmid U, et al. Automatic coding of facial expressions of pain: are we there yet? Pain Res Manag. 2022;2022:1–8. doi: 10.1155/2022/6635496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 111.Berger SE, Baria AT, Baliki MN, Mansour A, Herrmann KM, Torbey S, et al. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res Notes. 2014;7:739. doi: 10.1186/1756-0500-7-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tétreault P, Ghantous M, et al. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res. 2016;95:605–612. doi: 10.1177/0022034516638027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berger SE, Vachon-Presseau É, Abdullah TB, Baria AT, Schnitzer TJ, Apkarian AV. Hippocampal morphology mediates biased memories of chronic pain. Neuroimage. 2018;166:86–98. doi: 10.1016/j.neuroimage.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riggs NR, Hepburn SL, Pinks ME, Fidler DJ. A prevention science approach to promoting health and quality of life for individuals with intellectual and developmental disabilities. Inf Child Dev. 2022;31(1):e2278. doi: 10.1002/icd.2278. [DOI] [Google Scholar]
- 115.Bourseul JS, Brochard S, Houx L, Pons C, Bue M, Manesse I, Ropars J, Guyader D, Le Moine P, Dubois A. Care-related pain and discomfort in children with motor disabilities in rehabilitation centres. Ann Phys Rehabil Med. 2016;59:314–319. doi: 10.1016/j.rehab.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 116.Aroojis A, Mantri N, Johari AN. Hip displacement in cerebral palsy: the role of surveillance. Indian J Orthop. 2020;55(1):5–19. doi: 10.1007/s43465-020-00162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taverner T. Neuropathic pain: an overview. Br J Neurosci Nurs. 2014;10:116–123. doi: 10.12968/bjnn.2014.10.3.116. [DOI] [Google Scholar]
- 118.American Academy of Pain Medicine. Use of opioids for the treatment of chronic pain. 2013. http://www.painmed.org/files/use-of-opioids-for-the-treatment-of-chronic-pain.pdf. accessed 11 Jan 2017.
- 119.Breau LM, Lotan M, Koh JL. Pain in individuals with intellectual and developmental disabilities. In: Patel DR, Greydanus DE, Omar HA, Merrick J, editors. Neurodevelopmental disabilities: clinical care for children and young adults. Springer; 2011. pp. 255–276. [Google Scholar]
- 120.Ramstad K, Jahnsen R, Skjeldal OH, Diseth TH. Characteristics of recurrent musculoskeletal pain in children with cerebral palsy aged 8 to 18 years. Dev Med Child Neurol. 2011;53:1013–1018. doi: 10.1111/j.1469-8749.2011.04070.x. [DOI] [PubMed] [Google Scholar]
- 121.Rabach I, Peri F, Minute M, Aru E, Lucafo M, Di Mascio A, Cozzi G, Barbi E. Sedation and analgesia in children with cerebral palsy: a narrative review. World J Pediatr. 2019;15:432–440. doi: 10.1007/s12519-019-00264-0. [DOI] [PubMed] [Google Scholar]
- 122.Demir Y. Non-pharmacological therapies in pain management. In: Racz G, editor. Pain management: current issues and opinions. Rijeka: InTech; 2012. pp. 485–502. [Google Scholar]
- 123.Jacob US, Edozie IS, Pillay J. Strategies for enhancing social skills of individuals with intellectual disability: a systematic review. Front. Rehabil Sci. 2022;3:9614. doi: 10.3389/fresc.2022.968314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Briggs E. Understanding the experience and physiology of pain. Nurs Stand. 2010;25:35–39. doi: 10.7748/ns.25.2.35.s50. [DOI] [PubMed] [Google Scholar]
- 125.Kerns RD, Sellinger J, Goodlin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol. 2011;7:411–434. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- 126.Eccleston C, Williams ACDC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009;2:CD007407. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- 127.Eccleston C, Palermo TM, Williams ACDC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2012;12:CD003968. doi: 10.1002/14651858.CD003968.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dagnan D, Jackson I, Eastlake L. A systematic review of cognitive behavioural therapy for anxiety in adults with intellectual disabilities. J Intellect Disabil Res. 2018;62(11):974–991. doi: 10.1111/jir.12548. [DOI] [PubMed] [Google Scholar]
- 129.El-Tallawy SN, Perglozzi JV, Ahmed RS, Kaki AM, Nagiub MS, LeQuang JK, Hadarah MM. Pain management in the post-COVID era-an update: a narrative review. Pain Ther. 2023;12(2):423–448. doi: 10.1007/s40122-023-00486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Public Health England. How social care staff can recognise and manage pain in people with learning disabilities. PHE publications gateway number: 2017. https://www.gov.uk/government/publications/improving-healthcare-access-for-people-with-learning-disabilities. Accessed 16 Oct 2017.
- 131.Naka A, Keilani M, Loefler S, et al. Does transcutaneous electrical nerve stimulation (TENS) have a clinically relevant analgesic effect on different pain conditions? A literature review. Eur J Transl Myol Basic Appl Myol. 2013;23:95–104. doi: 10.4081/bam.2013.3.95. [DOI] [Google Scholar]
- 132.Chan JS, Tse SH. Massage as therapy for persons with intellectual disabilities: a review of the literature. J Intellect Disabl. 2011;15:47–62. doi: 10.1177/1744629511405105. [DOI] [PubMed] [Google Scholar]
- 133.Rothschild AW, Ricciardi JN, Luiselli JK. Assessing pain in adults with intellectual disability: a descriptive and qualitative evaluation of ratings and impressions among care-providers. J Dev Phys Disabil. 2019;31:219–230. doi: 10.1007/s10882-019-09663-7. [DOI] [Google Scholar]
- 134.Lester PE, Daroowalla F, Harisingani R, et al. Evaluation of house staff knowledge and perception of competence in palliative symptom management. J Palliat Med. 2011;14:139–145. doi: 10.1089/jpm.2010.0305. [DOI] [PubMed] [Google Scholar]
- 135.Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, editor. Patient Safety and Quality: An Evidenced-Based Handbook for Nurses. Rockville: Agency for Healthcare Research and Quality Publication No. 08-0043; 2008. [PubMed] [Google Scholar]
- 136.Orava T, Townley A, Provvidenza C, Adler E, Ami N, Gresley-Jones T, Mankad D, Fay L, Slonim N, Hoffman A, Joachimides N, Hung R, Fehlings D, Kingsnorth S. Chronic pain assessment toolbox for children with disabilities: section 1.0: toolbox background. Toronto: Holland Bloorview Kids Rehabilitation Hospital; 2019. [Google Scholar]
- 137.Genik LM, McMurtry CM, Breau LM, Lewis SP, Freedman-Kalchman T. Pain in children with developmental disabilities: development and preliminary effectiveness of a pain training workshop for respite workers. Clin J Pain. 2018;34:428–437. doi: 10.1097/AJP.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 138.Quinn B, Smolinski M. Improving school nurse pain assessment practices for students with intellectual disability. J Sch Nurs. 2018;34:480–488. doi: 10.1177/1059840517722591. [DOI] [PubMed] [Google Scholar]
- 139.Wittkopf PG, Lloyd DM, Coe O, Yacoobali S, Billington J. The effect of interactive virtual reality on pain perception: a systematic review of clinical studies. Disabil Rehabil. 2020;42(26):3722–3733. doi: 10.1080/09638288.2019.1610803. [DOI] [PubMed] [Google Scholar]
- 140.Bouck EC, Long H, Jakubow L. Using technology to enhance learning for students with intellectual disabilities. In: Bakken JP, Obiakor FE, editors. Using technology to enhance special education (advances in special education) Bingley: Emerald Publishing Limited; 2023. pp. 51–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.