Abstract
Alkaloids have been utilized by humans for years. They have diverse applications in pharmaceuticals. They have been proven to be effective in treating a number of diseases. They also form an important part of regular human diets, as they are present in food items, food supplements, diet ingredients and food contaminants. Despite their obvious importance, these alkaloids are toxic to humans. Their toxicity is dependent on a range of factors, such as specific dosage, exposure time and individual properties. Mild toxic effects include nausea, itching and vomiting while chronic effects include paralysis, teratogenicity and death. This review summarizes the published studies on the toxicity, analytical methods, occurrence and risk assessments of six major alkaloid groups that are present in food, namely, ergot, glycoalkaloids, purine, pyrrolizidine, quinolizidine and tropane alkaloids.
Keywords: Alkaloids, Food, Toxicity, Analytical methods, Occurrence, Risk assessments
Introduction
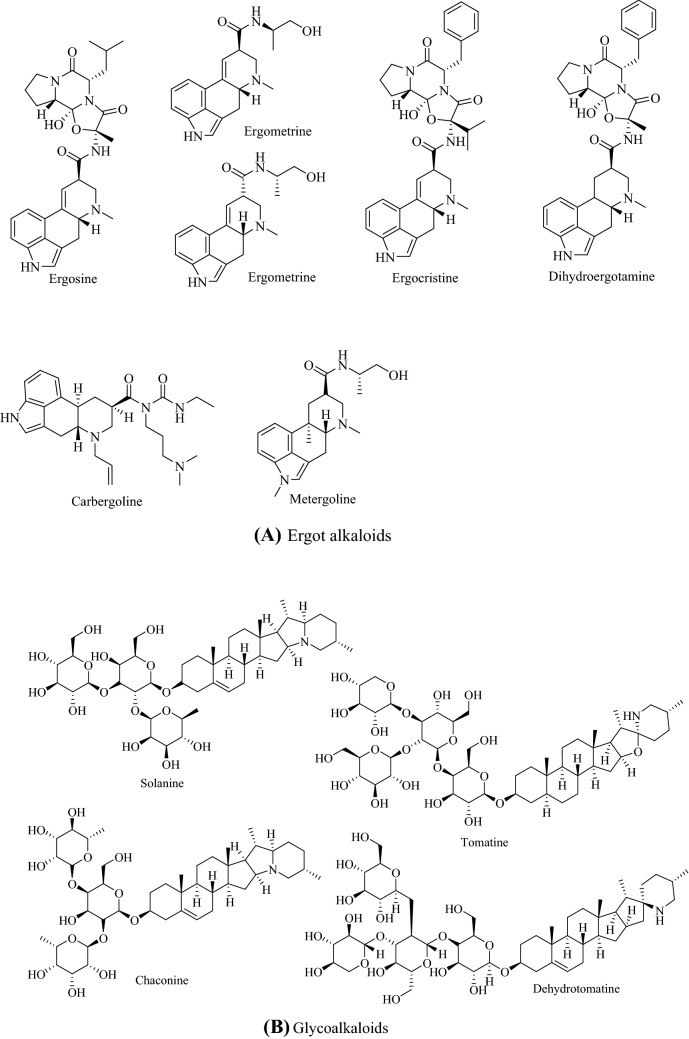
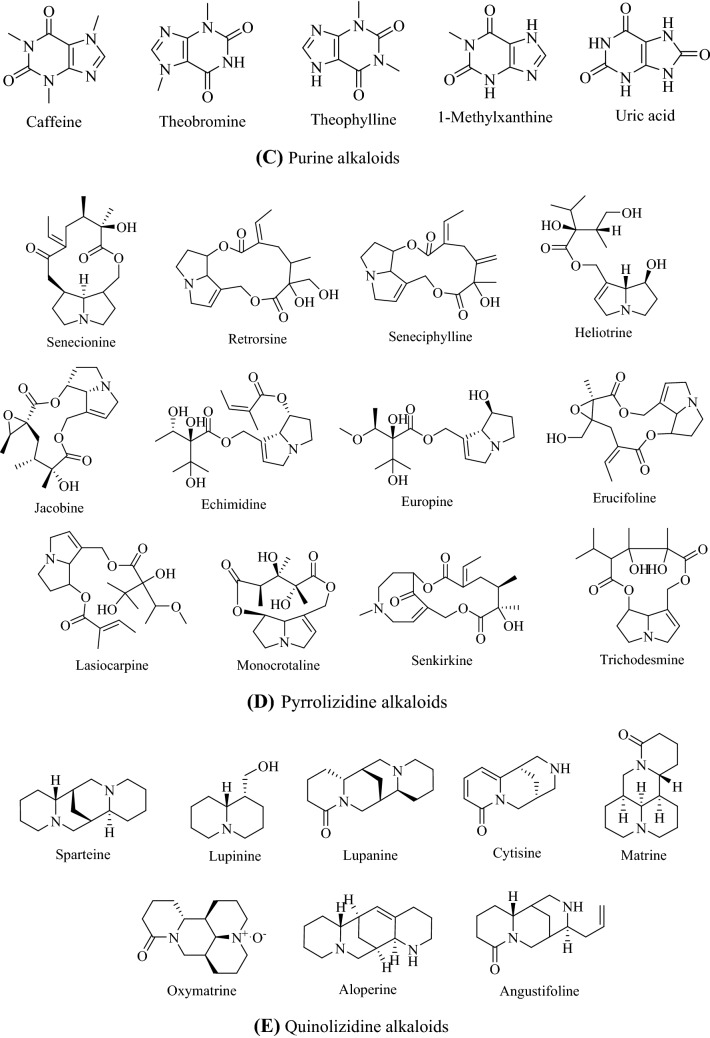
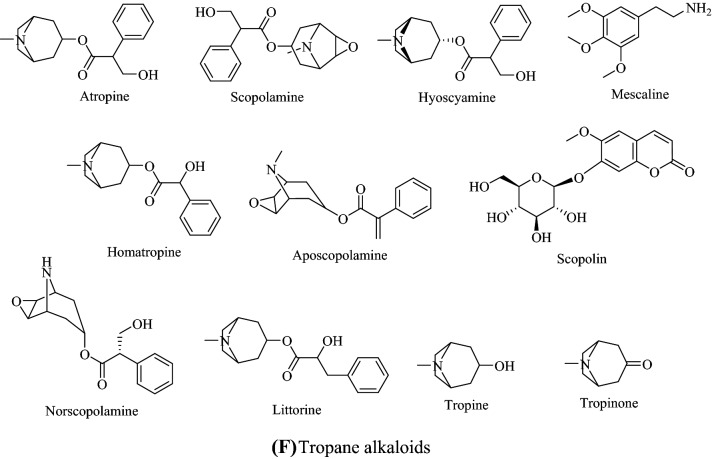
Alkaloids are naturally occurring nitrogen-containing compounds that are found in plants, fungi, bacteria and animals (Cushnie et al., 2014). The presence of nitrogen atoms in their structures is responsible for their alkaline nature, and Fig. 1 shows the structures of some main alkaloids. Alkaloids are found primarily in plants as secondary metabolites, secreted to assist in their survival and reproduction (Acamovic et al., 2004; Jing et al., 2014). They are found in many higher plants, such as Papaveraceae (poppy), Ranunculaceae (buttercups) and Solanaceae (nightshades) (Aniszewski, 2007; Jing et al., 2014).
Fig. 1.
Structures of some a ergot alkaloids, b glycoalkaloids, c purine alkaloids, d pyrrolizidine alkaloids, e quinolizidine alkaloids, f tropane alkaloid
Humans have made use of alkaloids for various purposes, such as stimulants, narcotics, aphrodisiacs, poisons and medicines. Additionally, plants containing alkaloids form a regular part of human diets, both in food and drinks (Koleva et al., 2012). Common examples of alkaloids that are found in human diets include caffeine from coffee seeds, theobromine and caffeine from cacao seeds, theophylline and caffeine from tea leaves, tomatine from tomatoes, solanine from potatoes and caffeine from Coca-Cola (Kurek, 2019).
Alkaloids possess a broad spectrum of biological activities, such as antiparasitic (Fernandez et al., 2010), antiplasmodial (Frédérich et al., 2002), anticorrosive (Capasso et al., 2002), antioxidative (Czapski et al., 2014), antibacterial (Karou et al., 2006), anti-HIV (Zhang et al., 2015)and insecticidal activities (Ge et al., 2015). They have also been found to have good applications in human and animal health. Morphine, strychnine, quinine, ephedrine, colchicine and nicotine are common alkaloids that are particularly useful in clinical settings (Kurek, 2019). However, a number of alkaloids have toxic effects on humans and animals. Commonly abused drugs include alkaloids, such as morphine, cocaine, caffeine and nicotine (Beyer et al., 2009). Ergot alkaloid contamination results in direct smooth muscle stimulation, central sympatholytic activity and peripheral alpha-adrenergic blockade (Merhoff Portel, 1974). Toxic symptoms from ingesting quinolizidine alkaloids, which results from the consumption of lupin seeds that were not debittered, include blurry vision, facial flushing, dry mouth and confusion (Koleva et al., 2012). A number of pyrrolizidine alkaloids have been found to be hepatotoxic and carcinogenic (Cushnie et al., 2014).
Therefore, this review focuses on the analytical methods and toxicity of some these alkaloid groups, as well as their occurrence and risk assessments in food.
Toxicity
A wide range of toxic effects have been associated with alkaloids being present in food. Toxicity could vary from mild effects, such as vomiting and nausea, to more chronic effects, such as teratogenicity and sudden death. A summary of some of the toxic effects associated with these alkaloids is presented in Table 1.
Table 1.
Toxic effects of alkaloids
| Alkaloids | Toxic Effects | References |
|---|---|---|
| Ergot Alkaloids | Decreased body weight gain, increased glycogen storage, increased lipogenesis, decreased glucose and thyroxin levels, increased organ weights, enlarged parathymal lymph nodes |
Peters-Volleberg et al. (1996); Janssen et al. (2000a) |
| Developmental toxicity, Genotoxicity |
Kraicer and Strauss (1970); Carpent and Desclin (1969); Witters et al. (1975); Grauwiler and Schon (1973); Sommer and Buchanan (1955); Schaar and Clemens (1972); Roberts and Rand (1977a); Roberts and Rand (1977b); Roberts and Rand (1978); |
|
| Glycoalkaloids |
Irritation, congestion in the epididymis and testes, gut bleeding, gastric glandular mucosal necrosis, intestinal mucosal necrosis, gastric distension lesions, severe renal and hepatic congestion, leukocytic infiltration, increased haemoglobin and haemocrit, decreased liver weight, increased white and red blood cells, developmental toxicity, genotoxicity |
Al Chami et al. (2003); Phillips et al. (1996); Baker et al. (1989); Sharma et al. (1979); Phillips et al. (1996); Langkilde et al. (2008); Langkilde et al. (2009); Langkilde et al. (2012); Dixit and Gupta (1982); Gupta and Dixit 2002; Dixit et al. (1989); Gaffield and Keeler (1993), Gaffield and Keeler (1996); Renwick et al. (1984); Munari et al. (2012); Freidman and Henika (1992); Almeida et al. (2010). |
| Purine Alkaloids | Genotoxicity, carcinogenicity | Kulmann Fromme (1968); Choudhury and Palo (2004); Haynes et al. (1996); Aeschbacher et al. (1986); NTP (1998); Yu et al. (2011) |
| Pyrrolizidine Alkaloids | Vasoconstriction and medial hypertrophy of pulmonary arteries, cytoplasmic and pulmonary edema, chronic lung lesions, liver damage, hemangiosarcoma, alveolar/bronchiolar neoplasms, hepatopathy, hepatocytomegaly, developmental toxicity, genotoxicity, carcinogenicity | Wagenvoort et al. (1974); Miller et al. (1978); Culvenor et al. (1976); Schoental and Magee (1959); Chan et al. (1994); Chan et al. (2003); Peterson and Jago (1980); Green and Christie (1961); Schoental (1959); Silva-Neto et al. (2010); Petry et al. (1986); Hirono et al. (1977), Hirono et al. (1979); Rao and Reddy (1978) |
|
Quinolizidine Alkaloids |
Haemocrit, trembling, tonic–clonic convulsions, tonic–clonic spasms, liver problem, squinting, bilateral pupillary dilation, reddening of the eyelids, genotoxicity | Pothier et al. (1998); Yovo et al. (1984); Butler et al. (1996); Stanek et al. (2015); NTP (1978); Silva et al. (2014); Winks et al. (1998). |
| Tropane Alkaloids | Genotoxicity; carcinogenicity | McCann et al. (1975); Waskell (1978); EFSA Journal (2013); Cabello et al. (2001); Tatsuka et al. (1989). |
Acute toxicity
Ergot alkaloids
LD50 values have been determined for several ergot alkaloids, including ergometrine, ergotamine, ergosine and ergostine, via intravenous, subcutaneous or oral administration in rabbits and mice (Griffith et al., 1978). Generally, acute toxicity displayed by these alkaloids are low following oral administration, but the orally administered LD50 values are always higher than intravenous LD50 values for similar animal species, indicating low absorption and high pre-systemic metabolism subsequent to oral administration (Griffith et al., 1978).
Glycoalkaloids
When studied for toxicity in rats, α-solamargine has been found to have an intraperitoneal LD50 of 42 mg/kg bw. At up to 50 mg/kg bw dose, it causes slight irritation and congestion in the epididymis and testes (Chami et al., 2003).
Poisoning from potato glycoalkaloids (PGA), particularly α-solanine and α-chaconine, can be attributed to the consumption of green potatoes (Phillips et al., 1996). In one study, a 25 mg/kg bw intraperitoneal injection of a mixture of α-solanine and α-chaconine (1:1, w/w) that was administered to hamsters had a lethal effect, caused by bleeding in the gut, but a 50 mg/kg bw oral dose of the same mixture had no effect (Phillips et al., 1996).
In another study, the oral administration of ground plant materials from Solanum plant species was shown to have lethal effects on some Syrian hamsters. These deaths were characterized by mucosal necrosis in the stomach and small intestine with little inflammation. Some animals survived but developed lesions that were characteristic of abdominal enlargement (Baker et al., 1989).
Another study reported increased levels of serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) and the decreased activity of serum cholinesterase (ChE) and microsomal enzymes, including cytochrome P-450 following the intraperitoneal administration of a 250 mg/kg dose of solanine to male rats (Dalvi, 1985). The histopathological effects of α-solanine and α-chaconine (which have intraperitoneal LD50 of 32.3 mg/kg and 19.2 mg/kg, respectively) include severe renal and hepatic congestion and leukocytic infiltration (Sharma et al., 1979).
Pyrrolizidine alkaloids
Several pyrrolizidine alkaloids have been reported to cause acute toxicity. Senecionine and adonifoline have an acute oral toxicity corresponding to 57 mg/kg b.w. and 163 mg/kg b.w., respectively (Wang et al., 2011). Oral or intraperitoneal LD50 values range from 34 to 38 mg/kg for retrorsine administration to male rats, while its N-oxide has LD50 values of 48 mg/kg and 250 mg/kg for oral and intraperitoneal administration, respectively (Mattocks, 1971). Single oral doses of heliotrine, lasiocarpine and its N-oxide, retrorsine, riddelliine and seneciphylline produced significant toxicity in rats (Schoental and Magee, 1959).
Symptoms associated with acute toxicity from pyrrolizidine alkaloids include vasoconstriction, hypertrophy of the right ventricles and pulmonary arteries, pulmonary edema, cytoplasmic edema, lung lesions leading to chronic liver lesions and chronic liver damage (Culvenor et al., 1976; Miller et al., 1978; Schoental and Magee, 1959; Wagenvoort et al., 1974).
Quinolizidine alkaloids
LD50 values associated with quinolizidine alkaloids include 100 mg/kg for sparteine, 250 mg/kg for lupanine and lupine extracts (Pothier et al., 1998), a range of 750–4000 mgkg−1 body weight for several fractions of Lupinus angustifolius seeds (Stobiecki et al., 1993), 2279 mg/kg as a result of the oral administration of L. angustifolius, 1464 mg/kg and 177 mg/kg for the oral and intraperitoneal administration of lupanine, respectively, 199 mg/kg for the intraperitoneal injection of 13-hydroxylupanine (Petterson et al., 1987) and 36 mg/kg and 175 mg/kg for the oral administration and 220 mg/kg and 410 mg/kg for the intraperitoneal administration of sparteine and lupanine. (Yovo et al., 1984). At these lethal doses, the symptoms of intoxication include trembling, tonic–clonic convulsions and death by breathing arrest (Pothier et al., 1998; Yovo et al., 1984).
Repeated dose toxicity
Ergot alkaloids
A 28–32-day study on the subacute dietary toxicity of α-ergocryptine in rats showed body weight loss, body weight gain, food intake and food deficiency in U-shaped dose–response curves with a NOAEL of 4 mg/kg food (Janssen et al., 2000b). U-shaped dose–response relationships were also observed for hormonal and metabolic changes, which resulted in increased glycogen storage and lipogenesis. This situation could lead to an increase in protein catabolism (Janssen et al., 2000a).
A 4-week study on the subacute toxicity of ergometrine maleate in rats showed decreased glucose and thyroxin levels and increased organ weights when administered at high doses and an increase in the incidence of enlarged mediastinal and parathymal lymph nodes in male rats, which was proportional to the administered dose (Peters-Volleberg et al., 1996).
Glycoalkaloids
Langkilde et al. (2008; Langkilde et al. 2009; Langkilde et al. 2012) reported a series of experiments using α-solanine mixed with α-chaconine in female hamsters. Decreases in food consumed and relative weight of the liver and increases in haemoglobin and hematocrit all occurred within a 5-day period following the first day of the gavage administration of 75 mg TGA/kg bw per day of a mixture of the two glycoalkaloids in a 1:2.5 ratio (Langkilde et al., 2008). As with the previous dose, lethality was also observed following the gavage administration of 100 mg TGA/kg bw per day in a quarter of the tested animals due to increased adrenals, red blood cells and serum creatinine and decreased body weight (Langkilde et al., 2008).
In another study, a NOAEL of 10 mg TGAs/kg bw per day was gotten for a 1:3.7 ratio of α-solanine and α-chaconine, at a daily dose of 33 mg TGAs/kg b.w. that was administered via gavage to hamsters. The symptoms, which were mostly associated with the blood occurred within a month of the first day of administration (Langkilde et al., 2009). For a ratio of 1:7 at the same dose, death occurred within 2 h after the gavage administration in one of four treated animals (Langkilde et al., 2009). In a study on the compositional similarity and differential toxicity of a genetically modified potato line (SGT 9–2), the compositional differences observed were of no significance to nutritional value or safety. Additionally, differential toxicity data revealed a few significant differences between potato lines with different α-solanine: α-chaconine ratios, but these differences were determined to pose no safety concerns (Langkilde et al., 2012).
The consumption of green potatoes can lead to poisoning due to the presence of PGA, particularly α-solanine and α-chaconine, which increases in concentration when greening is taking place (Phillips et al., 1996). One study found that a single intraperitoneal injection of a 25 mg/kg bw dose or greater of a mixture of the two PGA (1:1, w/w) was lethal to laboratory animals and led to bleeding in the gut. Generally, the effects of the high concentrations of cytotoxic potato glycoalkaloids that are found in some potato tops have been shown to be minimal in laboratory animals (Phillips et al., 1996).
Pyrrolizidine alkaloids
The toxicity, carcinogenicity and the respective dose–response relationships of riddelliine in mice and rats showed lethal symptoms in rats that were administered 1.0 mg/kg doses. Non–neoplastic lesions were also observed on the livers and kidneys of the rats (Chan et al., 2003). As for the mice, males that were fed with 3.0 mg/kg doses experienced marked increases in hemangiosarcoma in their livers while females that were fed with the same dose experienced significant increases in alveolar/bronchiolar neoplasms (Chan et al., 2003).
In a similar experiment, an inverse relationship was observed between increases in body weight and dose among rats and mice. Dose-related hepatopathy and intravascular macrophage accumulation were observed in rats while mice experienced hepatocytomegaly. (Chan et al., 1994).
In another study, male rats showed more sensitivity than female rats, with increased cases of liver lesions, spleen, lungs and pancreas at a dose of 1.0 mg/kg or higher. The effects were less severe in female rats who received doses of 3.3 mg/kg per day or higher. Increased liver weights and hepatic cytomegaly were the only observed effects in male and female mice. Generally, the mice were considered to be less sensitive (Chan, 1993).
Quinolizidine alkaloids
In one study, feeding a group of rats a diet that was based on lupin flour led to reductions in hematocrit (HCT) and blood cell counts, depending on the dose received. The male group also experienced decreased mean cell volumes (MCVs) (Butler et al., 1996). In a similar experiment, a NOAEL of 330 ppm was reported when considering food intake levels and lower body weights at 1000 ppm and 5000 ppm doses while a NOAEL of 1000 ppm was predicted on the basis of hematology, growth rates, clinical chemistry and histopathological results (Robbins et al., 1996).
In another study, reduced food intake, changes in alanine transaminase activity and the parenchymal degeneration of the liver were all observed in animals that were fed with seeds from blue lupin cultivars (Stanek et al., 2015). Debittered Lupinus mutabilis seeds supplemented with DL-methionine could offer a good alternative source of protein for humans and animals as no harmful effects were observed in any of the parameters measured in a study on rats (Schoeneberger et al., 1987). Another study revealed normal weights in vital organs, such as the liver, kidneys, heart, spleen and adrenals, in rats that were dosed with 20% lupin protein, which was supplemented with DL-methionine. The growth rates and histology of the lungs and kidneys were also normal (Ballester et al., 1980).
The National Toxicology Program (NTP) in the USA reported a series of experiments on the toxicity of (-)-scopolamine hydrobromide trihydrate in rats and mice. In a 16-day study, there were no significant differences between the body weight gain and the final mean body weights of the control and dosed groups. However, death occurred in male and female mice that received the highest dose and a female mouse in the lowest dose group (NTP 1997). Squinting and the bilateral dilation of pupils were observed in all of the treated animals. Bilateral pupillary dilation and the reddening of eyelids were observed in a different 14-week study. In a 2-year study, bilateral dilation of the pupil occurred in rats and mice treated with (-)-scopolamine hydrobromide trihydrate but no changes were observed in their ophthalmic examinations (NTP 1997).
Developmental toxicity
Ergot Alkaloids
Ergocornine blocks ovulation in rats, which is an event that peaks at a time called the critical period. Ergocornine can also inhibit ovulation before the critical period but to a lesser extent. It also causes fluid persistence, which distends the proestrus uterus (Kraicer and Strauss, 1970). A study found that a 1 mg administration of ergocornine to a group of animals on the eighth day of pregnancy disturbed the pregnancy and had teratogenic effects on the fetuses (Carpent and Desclin, 1969). The intraperitoneal administration of elymoclavine, which is produced by Claviceps purpurea, at particular doses has been found to have teratogenic effects on mice and can kill embryos (Witters et al., 1975). The teratogenic effects include vertebral defects and the fusion of ribs.
Another study found that injections of ergotamine or ergotoxine into rats during gestation had adverse effects on lactation by not only diminishing the yield of milk but also the fat content of the secretory material (Sommer and Buchanan, 1955). Pregnant mice, rats and rabbits have been shown to suffer fetal retardation and increases in prenatal mortality as a result of the vasoconstrictive action of ergotamine that was orally administered (Grauwiler and Schon, 1973). In a different study, the subcutaneous administration of ergocornine hydrogenmalienate, ergonovine maleate, ergotamine tartrate and ergocryptine mesylate were found to affect the pituitary gland, which led to depressed levels of serum prolactin, thereby depressing lactation (Shaar and Clemens, 1972).
Glycoalkaloids
In one study, epididymal dysfunction occurred when solasodine was orally administered to dogs, demonstrating the antiandrogenic nature of the compound. Other effects included reductions in total protein, sialic acid, glycogen and acid phosphatase activity (Gupta and Dixit, 2002). Administering solasodine to dogs has also been found to render them infertile as sperm were absent in the cauda epididymis and ductus deferens (Dixit and Gupta, 1982). In another study on rhesus monkeys, solasodine interfered with the spermiogenesis process and led to a marked decrease in cauda sperm count (Dixit et al., 1989).
In a different study, α-solanine, α-chaconine as well as their aglycone, solanidine administered orally to Syrian hamsters resulted in craniofacial malformations (Gaffield and Keeler, 1996). Teratogenicity resulting from the administration of steroidal alkaloids, including solanidanes, to hamsters has been shown to have a direct relationship with or without unsaturation in the C-5 and C-6 positions of the alkaloids (Gaffield and Keeler, 1996). Renwick et al. (1984) reported the teratogenic nature of potato sprouts, which was attributed to the presence of solanidine triglycosides, α-chaconine and higher levels of α-solanine.
Pyrrolizidine alkaloids
Developmental toxicity following intraperitoneal administration of heliotrine and dehydroheliotridine to rats has been linked to distorted ribs, retarded ossification, long bones, cleft palates and foot defects (Green and Chrsitie, 1961; Peterson and Jago, 1980). Lasiocarpine and retrorsine have also been shown to produce significant liver lesions in the offspring of lactating rats but with no significant effects on the mothers (Schoental, 1959).
Genotoxicity
Ergot alkaloids
Some derivatives of ergot alkaloids, such as ergotamine, dihydroergotoxine and methysergide, can induce considerable levels of chromosomal damage in cultured human lymphocytes. These derivatives have been found to induce damage in male mice and early fetal deaths in mice at a dose of 100 mg/kg of dihydroergotoxine and methysergide (Roberts and Rand, 1977a). At all concentrations tested in one study, sister chromatid exchange (SCE) was observed in the ovaries of female hamsters following exposure to ergotamine, ergonovine and methylergonovine. Ergocristine has only been shown to induce SCE at high concentrations while α-ergocryptine has not been found to induce SCE at any tested concentrations (Dighe and Vaidya, 1988). However, in another study, three tested derivatives of ergot alkaloids showed no mutagenic effects in mice or Chinese hamsters at subtoxic or therapeutic doses (Matter, 1982; Matter, 1976,1976).
Glycoalklaoids
One study showed that Solanum lycocarpum fruit extract demonstrated protective activity against genomic and chromosomal damage caused by methanesulfonate, a DNA damage-inducing agent, rather than genotoxic effects in the lung fibroblasts (V79 cells) of Chinese hamsters (Friedman and Henika, 1992). In another study, ethanolic extract of Solanum palinacanthum and purified solamargine were tested using Ames test and showed no mutagenic effects in Salmonella typhimurium strains (Almeida et al., 2010).
Purine alkaloids
Caffeine is mutagenic in Drosophila melanogaster, can cause chromatid breakage in human cells and has dominant lethal effects in male mice (Kuhlmann and Fromme, 1968). At higher doses, it can induce sister chromatid exchange, micronuclei and chromosome aberrations in mice and Chinese hamsters (Choudhury and Palo, 2004; Haynes et al., 1996; Aeschbacher et al., 1986). Since caffeine is metabolized in humans and many mammals at a rapid rate, only high doses (100 mg/kg or higher) can induce chromosomal damage; and therefore, caffeine has low genotoxic potential when used as a flavoring agent (EFSA CONTAM Panel, 2017).
Pyrrolizidine alkaloids
Exposure to monocrotaline and dehydromonocrotaline can induce significant DNA damage and apoptosis in glioblastoma of human GL-15 cell line (Silva-Neto et al., 2010). Monocrotaline and jacobine have been shown to induce DNA crosslinks (DNA–DNA or DNA–protein), which has certain effects in adult male rats (Petry et al., 1984; Petry et al.,1986). These crosslinks have also been found to be induced by eight pyrrolizidine alkaloids and follow a particular potency order (Hincks et al., 1991). Further studies on crosslinks associated with these alkaloids have also been reported (Kim et al., 1995; Pereira et al., 1998; Wang et al., 2005).
Quinolizidine alkaloids
Sparteine (0.5 mM) and extracts from Lupinus mexicanus and Lupinus montanus have shown in vitro genotoxic activity when assessed using alkaline comet assays (Silva et al., 2014). In an experiment where ethanolic extracts of Lupinus termis were subjected to three genotoxic studies, the extracts were found to be non–genotoxic in all of the assays (Santiago Quiles et al., 2010). Additionally, in another study, binding or intercalation with deoxyribonucleic acid (DNA) did not occur for several quinolizidine alkaloids (Winks et al., 1998).
Tropane alkaloids
Atropine sulphate (McCann et al., 1975) and (-)-scopolamine (Waskell, 1978) have shown no mutagenic effects on different Salmonella typhimurium strains. EFSA has since confirmed that atropine and (-)-scopolamine produce no genotoxic effects (EFSA CONTAM Panel, 2013).
Carcinogenicity
Purine alkaloids
When a rats and mice were dosed with 75 mg/kg of theophylline in one study, there were no observed carcinogenic effects (NTP, 1998). Coffee has the potential to reduce the overall incidence of cancer and has inverse relationships with some types of cancers (Yu et al., 2011). The IARC (2016) concluded that “overall coffee drinking was evaluated as inclassifiable (group 3) as to its carcinogenicity to humans”.
Pyrrolizidine alkaloids
Riddelliine is classified as “possibly carcinogenic to human” (group 2B), according to the IARC (2002). The NTP classified it as “reasonably anticipated to be a carcinogen” (NTP, 2003). Liver hemangiosarcoma, hepatocellular adenoma and mononuclear cell leukemia all increased according to a carcinogenicity study on the administration riddelliine to rats and mice (Chan et al., 2003). The IARC classifies lasiocarpine as “possibly carcinogenic to humans” (group 2B) (IARC, 1976). In one study, liver angiosarcoma occurred in experimental animals following the administration of lasiocarpine via their diet, leading to the conclusion that lasiocarpine is carcinogenic in animals (NTP, 1978). The dietary administration of lasiocarpine to rats resulted in liver hemangiosarcoma and hepatocellular carcinoma in one study (Rao and Reddy, 1978) while intraperitoneal administration resulted in liver tumors and hepatocellular carcinoma in another study (Svoboda and Reddy, 1972). Other carcinogenic pyrrolizidine alkaloids include clivorine (Kuhara et al., 1980), petasitenine (Hirono et al., 1977), senkirkine and symphytine (Hirono et al., 1979).
Tropane alkaloids
In separate studies on Sprague–Dawley rats, atropine showed no effects on tumor development (Cabello et al., 2001; Schmäahl and Habs, 1976). However, atropine significantly increased gastric tumors per animal in a study that was conducted to investigate the possibility of atropine promoting experimental carcinogenesis in the stomachs of rats caused by N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) (Tatsuta et al., 1989).
Analytical methods
For most alkaloids, instrumental analyses are usually preceded by extraction and sample clean–up steps. A summary of the various analytical methods that are used for alkaloids is presented in Table 2.
Table 2.
Analytical methods for the detection of alkaloids
| Alkaloids | Samples | Sample preparation | LOD | LOQ | Analytical method | References |
|---|---|---|---|---|---|---|
|
Ergot Alkaloid |
Cereals and foods | SPE | 0.17–2.78 μg/kg | LC–MS/MS | Krska et al. (2008) | |
| Rye and wheat | SPE |
0.01–1.0 µg/kg (wheat) 0.01–10 µg/kg (rye) |
UPLC-MS/MS | Kokkonen and Jestoi (2010) | ||
| Beer | U-HPLC-Orbitrap MS | Zachariasova et al. (2010) | ||||
| Rye | SPE | 0.25–1.65 μg/kg | 1.10–6.65 μg/kg | HPLC–FLD | Franzmann et al. (2010) | |
| Rye and rye products | Solid-phase filtration | 0.02–1.10 μg/kg | 0.08v3.30 μg/kg | HPLC–FLD | Muller et al. (2009) | |
| 8.1–22.3 μg/kg | 25.5–66.5 μg/kg | |||||
| Claviceps purpurea | GC–MS | Jegorov et al. (1997) | ||||
| Sorghum ergot | 0.01 mg/kg | 0.1 mg | ELISA | Molloy et al. (2003) | ||
| Grasses | ELISA | Tunali et al. (2000) | ||||
| Straw, Seed Digesta | 1.5625 ng/mL | ELISA | Schnitzius et al. (2001 | |||
| Glycoalkaloids | Tomato | 0.39 μg | HPLC–UV | Kozukue et al. (2004) | ||
| 0.94 μg | ||||||
| Potatoes | SPE; C-18 cartridge | RP-HPLC | Houben and Brunt (1994) | |||
| Blood | SPE | 1 μg/L | 2 μg/L | UHPLC-MS/MS | Nara et al. (2019) | |
| Milk | LLE | 0.28 mg/L | GC-NPD | Bushway et al. (1984) | ||
| Potato and tomato | ELISA | Stanker et al. (1994) | ||||
| Potatoes | ELISA | Morgan et al. (1985) | ||||
|
Purine Alkaloids |
Tea, Coffee and Urine | Filtration | 0.10 μg/mL (caffeine) | 0.33 μg/mL | RP-HPLC/UV | Aragao et al.(2005) |
| 0.07 μg/mL (theobromine) | 0.23 μg/mL | |||||
| 0.06 μg/mL (theophylline) | 0.18 μg/mL | |||||
| Tea | Infusion | 0.07–0.47 mg/L | 0.21–1.41 mg/L | HPLC–DAD | Azevedo et al. (2019) | |
| Tea | 0.025 μg/mL (caffeine) | 0.08 μg/mL | UHPLC-MS | Aqel et al. (2019) | ||
| 0.015 μg/L (theobromine) | 0.05 μg/mL | |||||
| 0.01 μg/mL (theophylline) | 0.03 μg/mL | |||||
| Tea | Leaching | HPLC | Jankech et al. (2019) | |||
| Beverage and Food |
Drop-to-drop solvent microextraction |
4.0 ng/mL | GC–MS | Shrivas and Wu (2007) | ||
| Beverage | Ultrasonication | 0.01 mg/L | GC–MS | Zou and Li (2006) | ||
| Soft drinks, tea and coffee | FTIR | Paradkar and Irudayaraj (2002) | ||||
| Yerba mate |
Cellulose acetate membrane |
1.6 × 10–3 mg/kg | 4.2 × 10–3 mg/kg | NIR | Mazur et al. (2014) | |
| Coffee and tea | Infusion |
UV–Visible spectrophotometry |
Lopez-Martinez et al. (2003) | |||
| Pyrrolizidine Alkaloids | Herbal Medicines |
Mixed mode (MCX) cationic exchange SPE cartridge |
0.03–3.37 μg/kg | LC–ESI–MS/MS | Jeong et al. (2021) | |
| Fodder, hay, silage |
SPE, Strata 60 mg, 3 cc cartridges |
3–10 μg/kg | 10 – 30 μg/kg | UHPLC-MS/MS | Mudder et al. (2009) | |
| Plant, milk and urine | SPE | 0.05–0.2 μg/L (milk) | LC–MS/MS | Hoogenboom et al. (2011) | ||
| 0.2v0.5 μg/g (dried plant) | ||||||
| 0.2–0.5 μg/L (urine) | ||||||
| Honey and herbal tea | SPE | 0.5 μg/kg (honey) | 1 μg/kg (honey) | UHPLC-MS/MS | Reinwaldt et al. (2017) | |
| 2.5–10 μg/kg (herbal tea) | 5–20 μg/kg (herbal tea) | |||||
| Senecio inaequidens | Soxhlet | GC–MS | Caniato et al. (1989) | |||
| Senecio alpinus | Soxhlet | GC–MS | Luthy et al. (1981) | |||
| Senecio jacobaea | Extraction with DCM | GC–MS | Pelser et al. (2005) | |||
| Senecio species | Extraction with H2SO4 | GC–MS | Witte et al. (1992) | |||
| Herbal remedies | SPE | GC–MS | Conradie et al. (2005) | |||
| Petasites hybridus | Extraction with methanol | 0.11–1.53 ng/mL | 0.1 ppm | ELISA | Langer et al. (1996) | |
| Senecionine | 0.5–10 ppb | ELISA | Roseman et al. (1996) | |||
| 5–100 ppb | ||||||
| 600–1000 ppb | ||||||
| Antibodies | ELISA | Zundorf et al. (1998) | ||||
| Quinolizidine Alkaloids | Holstein cattle |
Rotary evaporation, Mechanical rotation, DCM |
HPLC–MS | Green et al. (2015) | ||
|
Lupin flours, Lupin-based ingredients and food |
Soxhlet extraction | 2 mg/kg | GC–MS | Resta et al. (2008) | ||
| Lupin seeds | Soxhlet | 2 mg/kg | GC–MS | Boschin et al. (2008) | ||
|
Lupin seeds and Lupin-containing foods |
Centrifugation, silica cartridge |
1 mg/kg | GC–MS/GC-FID | Reinhard et al. (2006) | ||
| 6 mg/kg | ||||||
| 2 mg/kg | ||||||
| Lupinus Species | Centrifugation | GLC, GC-EIMS | Wink et al. (1995) | |||
|
Tropane Alkaloids |
Plasma | Centrifugation | 0.004–0.01 ng/mL | 0.05–0.8 ng/mL | LCI-ESI–MS/MS | John et al. (2010) |
| Atropine | Water dilution | HPLC–UV/MS | Breton et al. (2005) | |||
|
Feedstuff and Biological Samples |
SPE, C-18 cartridge | 12.05 ng (scopolamine) | 37.98 ng (scopolamine) | RP-HPLC/UV | Papadoyannis et al (1993) | |
| 13.25 ng (hyoscyamine) | 38.17 ng (hyoscyamine) | |||||
| Buckwheat | Soxhlet | 0.3 μg/kg (atropine) | 1 μg/kg (atropine) | GC–MS | Caligiani et al. (2011) | |
| 1.0 μg/kg (scopolamine) | 6 μg/kg (scopolamine) | |||||
| Antibodies | Methanol, DCM | 0.1 ng | ELISA | Fliniaux and Jacquin-Dubreuil. (1987) | ||
| Datura |
28% EtOH/NH4OH (9:1), Centrifugation |
0.15 ng | Radioimmunoassay | Savant and Dougall (1990) |
Extraction and sample clean-up
Liquid extraction using various solvent mixtures is a common approach that is applied to extract ergot alkaloids from sample sources. Extraction can be achieved using non–polar solvents or the addition of methanol and ammonium hydroxide to some solvents, such as dichloromethane and ethyl acetate, which raises the pH and increases the solubility of ergot alkaloids in the organic layer (Franzmann et al., 2010; Lauber et al., 2005; Müller et al., 2009). In addition, buffers or dilute acid can be added to polar solvents to lower the pH and facilitate the extraction of ergot alkaloids (Krska et al., 2008; Mulder et al., 2012; Storm et al., 2008). Solid–phase extraction (SPE) using alumina cartridges (Franzmann et al., 2010; Müller et al., 2009), dispersive primary and secondary amine (PSA) SPE cartridges (Krska et al., 2008), cation ion exchange cartridges (Storm et al., 2008) and C18 cartridges (Fajardo et al., 1995; Mohamed et al., 2006) have all been used for sample clean up.
Glycoalkaloid extraction needs to be controlled to avoid the enzymatic degradation of glycoside side–chains by glycosidases that are present in fresh potato tubers (Freidman et al., 1997). The extraction can be achieved by using mixtures of aqueous and acidic solvents (Bodart et al., 2000). Further sample clean–up is needed and can be achieved using solid phase extraction cartridges (Freidman et al., 1997).
Purine alkaloids are mostly extracted using hot water (Aqel et al., 2019; Azevedo et al., 2019; Khanchi et al., 2007; Mazur et al., 2014) or water mixed with organic solvents, such as methanol and acetonitrile (Cimpoiu et al., 2010; Uysal et al., 2009). Extraction can also be achieved using supercritical extraction plants, which are designed for working conditions of up to 690 bar in pressure and 250 °C in temperature (Saldaña et al., 2002).
Extraction of pyrrolizidine alkaloids from sample sources can be achieved by using organic solvents (e.g., methanol) (Langer et al., 1996), sulfuric acid (Reinwaldt et al., 2017) or methanol mixed with sulfuric acid (Jeong et al., 2021). Sample clean–up is achieved using SPE cartridges (Jeong et al., 2021; Reinwaldt et al., 2017).
In some cases, the extraction of quinolizidine alkaloids from powdered lupin seeds is usually preceded by a hexane-defatting step (Boschin et al., 2008; Pothier et al., 1998; Yovo et al., 1984). After alkalinizing the defatted powder using aqueous ammonia, extraction can then be achieved using dichloromethane (Pothier et al., 1998) or chloroform (Yovo et al., 1984). Acidic solutions can also be used to extract quinolizidine alkaloids from sample matrices (Boschin et al., 2008; Kamel et al., 2016).
Pressurized solvent extraction, supercritical fluid extraction and microwave-assisted extraction methods can all be applied for the extraction of tropane alkaloids (Christen et al., 2008). Similar to quinolizidine alkaloids, samples need to first be defatted using hexane, alkalinized and then subjected to static extraction using dichloromethane to obtain tropane alkaloids (Caligiani et al., 2011). Different modifications to the solid phase extraction method have also been employed for extraction and sample clean–up (Long et al., 2012; Mroczek et al., 2006; Papadoyannis et al., 1993).
Chromatographic techniques
Chromatography is an important technique for detecting alkaloids in various sample sources. Methods based on high-performance liquid chromatography, gas chromatography and thin-layer chromatography have all been used to confirm the presence of various alkaloids in different samples.
HPLC-based methods
HPLC and LC–MS/(MS) are commonly to detect alkaloids. LC–MS/MS has been applied to analyze ergot alkaloids in various products, including rye and wheat, and it has the advantage of short analysis times. (Kokkonen and Jestoi, 2010; Krska et al., 2008). Liquid chromatography can be used in combination with various detectors to produce hyphenated methods, such as UHPLC-Orbitrap-MS (Zachariasova et al., 2010) and HPLC–FLD (Franzmann et al., 2010; Müller et al., 2009), which have found applications in the analysis of mycotoxins in beer and ergot alkaloids in rye and rye products.
The HPLC–UV method has been used successfully for the analysis of various glycoalkaloids in food (Friedman et al., 1997; Houben and Brunt, 1994; Kozukue et al., 2004). LC–MS/MS, LC-HRMS and LC-Orbitrap-MS have grown increasingly popular for glycoalkaloid analysis as they can be used in the targeted analysis of glycoalkaloids, exploratory research and metabolomic studies (Caprioli et al., 2014; Hossain et al., 2015; Moco et al., 2007; Nara et al., 2019).
Several purine alkaloids have been analyzed using various modifications to the liquid chromatography techniques. HPLC (Jankech et al., 2019), RP-HPLC/UV (de Aragão et al., 2005), HPLC with a diode array detector (Azevedo et al., 2019) and UHPLC-MS (Aqel et al., 2019) have all found applications in methylxanthine analysis.
The non–destructive nature of liquid chromatography techniques allows for the simultaneous determination of N-oxides and free bases in a single analytical run without the initially reducing the oxides (Crews et al., 2010). This technique has been used widely to analyze pyrrolizidine alkaloids in herbal medicines (Jeong et al., 2021), honey and herbal teas (Reinwaldt et al., 2017) and fodder, hay or silage from Senecio plant parts (Mulder et al., 2009), as well as plants, milk, urine and feces (Hoogenboom et al., 2011).
LC-based methods are uncommon for detecting quinolizidine alkaloids. However, there have been a few reports on the application of this method for determining quinolizidine alkaloids, such as in the serum analysis of Holstein cows after exposure to Lupinus leucophyllus (Green et al., 2015), as well as some conference abstract papers (Mulder and de Nijs, 2015; Vanérková et al., 2014).
UV and MS are common detectors that are used with liquid chromatography to analyze tropane alkaloids. The detection of these alkaloids in plasma (John et al., 2010), fodder and biological samples (Papadoyannis et al., 1993) and the chiral separation of atropine (Breton et al., 2005) have all been carried out using liquid chromatography.
GC-based methods
The determination of ergot alkaloids can be achieved using GC-based methods. The detection of ricinoleic acid, which is a marker for ergot alkaloid contamination in rye and products made from rye (Franzmann et al., 2010), and homoisoleucine, an amino acid present in ergogaline (Jegorov et al., 1997) are examples of GC applications in ergot alkaloid determination.
GC can be applied to glycoalkaloid analysis in two ways. The first method is hydrolyzing glycoalkaloids into their aglycones followed by GC analysis. The second method involves the derivatization and subsequent determination of glycosides (Friedman et al., 1997). These principles have been applied to the glycoalkaloid analysis of bovine milk using a nitrogen–phosphorus detector (Bushway et al., 1984) and the separation of solanidine, demissidine and all other C27–steroidal alkaloids in a certain solanum species (van Gelder, 1985).
A few reports have been published on the use of GC–based methods to analyze purine alkaloids. A method for the rapid determination of caffeine in beverages and food was developed by Shrivas and Wu. This method adopts a drop-to-drop solvent microextraction using gas chromatography–mass spectrometry (Shrivas and Wu, 2007). Zou and Li (2006) also determined caffeine concentrations in beverages using GC–MS. The LOD of this method is 0.001 mg/L.
GC–MS has been widely applied in the determination of pyrrolizidine alkaloids in different samples. There are a few reports on the application of this method for determining quinolizidine alkaloids in different Senecio species (Caniato et al., 1989; Luthy et al., 1981; Pelser et al., 2005; Witte et al., 1992). Edgar and Smith (2000) used GC–MS to study the transfer of pyrrolizidine alkaloids in eggs. There has also been a case report about using GC–MS to identify toxic pyrrolizidine alkaloids in traditional medicines that were administered to two sets of twins in Africa (Conradie et al., 2005).
GC–MS is a common method used to detect quinolizidine alkaloids in different variety of lupin-based products. Lupanine, 13α-hydroxylupanine, angustifoline and other pyrrolizidine alkaloids have been found in lupin seeds, lupin flours and different lupin species (Boschin et al., 2008; Reinhard et al., 2006; Resta et al., 2008; Wink et al., 1995). GC–MS and GC–FID can also be applied in parallel for this analysis (Reinhard et at., 2006).
A number of reviews have been conducted on the GC analysis of tropane alkaloids (Aehle and Dräger, 2010; Drager, 2002). GC–MS in single-ion mode has been used to detect toxic tropane alkaloids in commercial buckwheat (Caligiani et al., 2011). Tropane alkaloids are abundant in Datura stramonium seeds and their presence can be confirmed using GC–MS (El Bazaoui et al., 2009; Philipov and Berkov, 2002).
Immunoassays
Most of the quantitative immunoassays for detecting rye ergot alkaloids that have been described in the literature are insensitive to dihydroergosine (Molloy et al., 2003). An enzyme-linked immunosorbent assay (ELISA) method for measuring dihydroergosine was reported by Molloy et al., which involved using dihydroergosine–specific monoclonal and polyclonal antibodies against dihydroergosine conjugated to bovine serum albumin from mouse and rabbits, respectively (Molloy et al., 2003). Tunali et al. (2000) used ELISA and HPLC to measure levels of ergot alkaloids present that were present in native Turkish grasses. The ELISA method is more desirable for analyzing large numbers of forage samples and can detect the quantities of non–specific ergot alkaloids in tall fescue infected by endophyte (Schnitzius et al., 2001).
In the literature, ELISA assays have also been applied to quantify glycoalkaloids. For example, the total glycoalkaloid content in potato tubers has been determined using ELISA assays. The ELISA methods show good sensitivity and no cross–reactivity with tomatidine derivatives (Morgan et al., 1985). Stanker et al. (1994) was able to develop an ELISA method that helps to distinguish between glycoalkaloids present in potato and tomato and their corresponding aglycons.
Langer et al. developed an immunoassay using antibodies against retrorsine to determine the pyrrolizidine alkaloid senecionine, which is found in Asteraceae (Langer et al., 1996). Roseman et al. (1996) used an ELISA method based on polyclonal antibodies to detect the pyrrolizidine alkaloids retrorsine, monocrotaline and retronecine. A polyclonal antibody-based ELISA has also been applied to detect riddelliine and its N-oxide, with an LOD of 25 µg/L for riddelliine-type pyrrolizidine alkaloids in blood (Lee et al., 2001). In contrast to other references, Zündorf et al. (1998) used a monoclonal antibody-based ELISA for the detection retrorsine.
Savary and Dougall, (1990) developed a sensitive radioimmunoassay to investigate the yields of scopolamine from Datura species cultures. The commercially labeled antigen L-(N-methyl [3H])-scopolamine methyl chloride is adopted in the assay, which measures scopolamine in the range of 0.15–3.0 ng (Savary and Dougall, 1990). The wide specificity of antibodies from immunized rabbits using racemic tropic acid conjugated with bovine serum albumin has been shown to enable the simultaneous analysis of the main tropane alkaloids in Solanaceous plants (Fliniaux and Jacquin-Dubreuil, 1987).
Spectroscopic methods
Spectroscopic methods can also be applied to detect and quantify some alkaloids. Near–infrared (NIR) spectroscopy was used to detect and quantify ergot alkaloids in tall fescue (Roberts et al., 1997) and cereals (Vermeulen et al., 2012). Hyperspectral images have also been adopted to measure ergot contamination in cereals. (Vermeulen et al., 2012).
Paradkar and Irudayaraj, (2002) determined the amount of caffeine in a variety of soft drinks and the total methylxanthine content in tea and coffee using a rapid Fourier–transform–infrared (FT-IR) spectroscopic method in a single calibration model. Two spectral regions (1500–1800 cm−1 and 2800–3000 cm−1) were identified in the FT–IR spectrum of pure caffeine and were used for quantitative estimation. NIR spectroscopy has been used in combination with multivariate calibration techniques to quantify methylxanthines in yerba mate (Ilex paraguariensis) (Mazur et al., 2014). López-Martinez et al. used UV–visible spectrophotometry and the partial least squares method to simultaneously determine methylxanthines in coffee and teas. This method is not statistically significantly different from the standard HPLC technique (López-Martínez et al., 2003).
Nuclear magnetic resonance (NMR) spectroscopy is mainly used for the structural elucidation of pyrrolizidine alkaloids. Comprehensive tables of carbon–13 NMR (Roeder, 1990) and proton NMR (Logie et al., 1994) spectroscopic data for pyrrolizidine alkaloids are available in the literature. Birecka et al., (1981) stoichiometrically reacted protonated pyrrolizidine alkaloids with methyl orange and measured the intensity of the dye spectrophotometrically after the methyl orange had been released from the complex. This method is capable of detecting alkaloids at a concentration of 0.5 µg/mL. Alternatively, Azadbakht and Talavaki (2022) used Ehrlich’s reaction and spectrophotometric detection to measure pyrrolizidine alkaloids in wheat and flour. This method allows for the calculation of pyrrolizidine alkaloids and their N-oxides on the basis of senecionine (Azadbakht and Talavaki (2022).
Occurrence in food
Ergot alkaloids
A comparative study of total ergot alkaloids in rye meals and grains harvested using organic and conventional methods in 2003 and 2004 was conducted by Lauber et al. (2005). Using the HPLC-FLD method, they reported higher levels of ergot alkaloids in 2003 compared to 2004. The same method was adopted to analyze 12 ergot alkaloids in rye and rye products in another study. Of the 39 samples analyzed, the total mean concentrations of ergot alkaloids in rye flour, rye, coarse rye and rye flakes were reported as 137.5 µg/kg, 62.2 µg/kg, 157.7 µg/kg and 26.4 µg/kg, respectively. The maximum LOQ has also been reported for ergometrine (3.3 µg/kg) using the blank method and ergocristine (66.5 µg/kg) using the calibration method.
Crews et al. (2009) used LC–MS/MS to determine six ergot alkaloids and their epimers (i.e., the “ine” and “inine” forms) in 28 rye-based cereal products from the United Kingdom. The analyzed samples included rye crispbread, bread mix and crackers, etc. Other than three samples, ergot alkaloids were present in all the other samples. The highest levels of alkaloids were found in the rye crispbread, with concentrations of 131 µg/kg, 183 µg/kg and 340 µg/kg (Table 3). The LOD and LOQ values of the alkaloids ranged from 0.4–2.7 µg/kg to 0.5–2.8 µg/kg respectively.
Table 3.
Occurrence of alkaloids in food
| Alkaloids | Food | Alkaloid concentration (mg/kg) | References |
|---|---|---|---|
| Mean (Minimum – Maximum) | |||
| Ergot Alkaloids | Rye meals and rye grains | 0.588 (max. 3.28) | Lauber et al., (2005) |
| Rye Products | 0.119 (max. 0.118) | Muller et al., (2009) | |
| Rye-based cereal | 0.4872 (max. 0.340) | Crews et al., (2009) | |
| Rye flour | 0.213 (max. 1.063) | Reinhold and Reinhardt, (2011) | |
| Wheat | 0.444 (0.0103–0.975) | Topi et al., (2017) | |
| Glycoalkaloids | Potatoes | 405.55 (1.3—352.6) | Freidman et al., (2003) |
| Potatoes | 81.05 (33.69—167.79) | Urban et al., (2018) | |
|
Potatoes Tomatoes |
410.35 (94.40—750.0) (42—1498) dehydrotomatine (521—16,285) α-tomatine |
Musita et al., (2020) Kozukue et al., (2004) |
|
| Eggplant | (10.00—221.00) | Sanchez-Mata et al., (2010) | |
|
Purine Alkaloids |
Beverage | (1 × 10–6—350) | Bispo et al., (2002) |
| Leaves, branches and fruits | (400—8600) | Borre et al., (2010) | |
| Teas and coffees | (< 0.06—771.40) | Aragao et al., (2005) | |
| Teas, coffees, energy drinks, chocolates | 80—18,740 | Sanchez, (2017) | |
| Teas | ND—11,064 (ND—17,177.8) | Baek et al., (2022) | |
| Pyrrolizidine Alkaloids | Tea, herbal drugs and honey | 0.05—1.86 (< LOD—5.647) tea | Bodi et al., (2014) |
| ND—0.45 (< LOD—3.098) herbal drugs | |||
| 0.006—0.015 (0.0003—0.234) honey | |||
| Herbal teas and medicines | (< LOQ—7.88) | Chen et al., (2019) | |
| (0.3—1.12) | |||
|
Wheat flour Honey and bee pollen |
(0.045—5.4) 0.026 (0.001—0.267) |
Kakar et al., (2010) Dubecke et al., (2011) |
|
| 1.846 (0.011—37.855) | |||
| Pollen products | 5.17 (1.08—16.35) | Kempf et al., (2010) | |
| Quinolizidine Alkaloids | Lupin | (ND—1664) | Gresta et al., (2010) |
|
Lupin flours, Lupin-based ingredients and food |
(8.45—1247) | Resta et al., (2008) | |
| (< LOQ—86.5) | |||
| (< LOQ—56.3) | |||
| Lupin seed crops | (130 -52,380) | Magalhães et al., (2017) | |
| Lupin species | (20—21,920) | Cortes et al., (2005) | |
| (ND—5670) | |||
| (10—27,580) | |||
| Lupin seeds and foods | (202—19,800) bitter | Reinhard et al., (2006) | |
| (44—2120) sweet | |||
|
Tropane Alkaloids |
Buckwheat | (ND—1283) | Caligiani et al., (2011) |
| Teas and herbal teas | (0.005—4.34) | Romera-Torres et al., (2018) | |
| Cerealsand Solanaceae seeds | (0.003—1.847) | Marin-Saez et al., (2017) | |
| Cereals | (ND—0.0096) | Basle et al., (2020) | |
| Cereal-based food | 0.00037—0.00304 (max. 0.0808) | Mulder et al., (2015) |
Reinhold and Reinhardt, (2011) determined the levels of six ergot alkaloids and their corresponding epimers in 31 samples of rye flour and whole-meal rye flour from stores that sell retail products in Germany. Analysis was done using HPLC–MS/MS, with the concentrations of the alkaloids and the LOQ given as the sums of the alkaloids and their epimers. Individual alkaloid concentrations ranged from 86 µg/kg for ergocornine and its epimer to 365 µg/kg for ergotamine and its epimer.
In another study, the occurrence of twelve ergot alkaloids was determined in seventy-one samples of wheat harvested between 2014 and 2015 in Albania using LC–MS/MS. The concentrations of the alkaloids ranged from 17.3 µg/kg to 975.5 µg/kg in 2014 and from 10.3 µg/kg to 390.5 µg/kg in 2015. LOD and LOQ values for all the ergot alkaloids were 3 µg/kg and 10 µg/kg, respectively (Topi et al., 2017).
Glycoalkaloids
Friedman et al. (2003) analyzed two major glycoalkaloids (α-solanine and α-chaconine) in eight potato cultivars using HPLC. The analysis was carried out on freeze-dried potato, fresh (wet) potato, potato flesh, potato flesh, potato peel and whole potatoes from eight potato cultivars grown in the USA. The highest value for the sum of the two analyzed glycoalkaloids was found in the potato peel of the cultivar from Snowden (3526 mg/kg) while the lowest value was obtained in the wet potato of the cultivar from Russet Norkota.
Urban et al., (2018) reported the effects of genotype, flesh color and environment on the glycoalkaloid contents of potato tubers. They compared the levels of α-chaconine, α-solanine and total glycoalkaloids in 14 cultivars that had red and purple flesh to those in cultivars that had yellow and white flesh. The three-year study revealed that the total glycoalkaloid content in tuber flesh ranged from 33.69 to 167.77 mg/kg fresh matter (FM) while the ratio of α-chaconine to α-solanine ranged from 1.18 to 3.78. The total glycoalkaloid contents of the cultivars were affected by the cultivar genotype, location and weather conditions but were not affected by flesh color. The cultivar with purple flesh from Bora Valley had the highest average total glycoalkaloid content (165 mg/kg FM) while the cultivar with red flesh from Red Emmalie had the lowest total glycoalkaloid level (43.6 mg/kg FM).
A study on the glycoalkaloid levels of commercial potato varieties in Nairobi, Kenya was conducted by Musita et al. (2020) using HPLC. The potatoes were sampled randomly from the varieties that are sold in supermarkets and open-air markets. The highest glycoalkaloid level was found in the Shangi variety while the Royal variety had the lowest glycoalkaloid level.
Kozukue et al. (2004) determined dehydrotomatine and α-tomatine levels in tomatoes and vegetative plant tissues using the HPLC–UV method. In tomatoes, dehydrotomatine and α-tomatine were observed in the ranges of 42–1498 µg/g and 521–16,285 µg/g, respectively.
In one study, liquid chromatography–mass spectrometry was used for the analysis of two glycoalkaloids (α-solasonine and α-solamargine) in Gboma (Solanum macrocarpon L.) and Scarlet (Solanum aethiopicum L.), which are eggplants that are common in traditional sub-Saharan African culture. The results were compared and showed that S. macrocarpon had α-solasonine and α-solamargine in similar proportions (76–89% α-solamargine) unlike S. aethiopicum, which had a more variable composition (48–89% α-solamargine). The implication of the obtained results is that S. macrocarpon is unsafe for human consumption as its glycoalkaloid levels are too high (5–10 times higher than recommended levels in food), but S. aethiopicum has glycoalkaloid levels that are similar to S. melongena and can be consumed by humans (Sánchez-Mata et al., 2010).
Purine alkaloids
Sanchez (2017) determined the methylxanthine contents of some commonly consumed food products in Spain. Of all the samples investigated, caffeine was the most common methylxanthine that was found. In green teas, the mean concentration of caffeine was 13,085 mg/kg, with a range of 8693–18,740 mg/kg. In black teas, the concentration range of caffeine was 7754–19,803 mg/kg, with a mean concentration of 14,530 mg/kg. The concentration levels of caffeine in energy drinks and espresso coffee were similar, with concentration ranges of 80–160 mg. Theobromine was the methylxanthine that was most detected in chocolate, with concentrations ranging between 4000 and 10,000 mg/kg for dark chocolate.
In another investigation, HPLC was used to identify three methylxanthine derivatives in urine and drinks. The concentrations of methylxanthine, caffeine, theobromine, and theophylline were found in the urine and beverages in the ranges of 0.1 pg/mL–350 g/mL, 0.1 pg/mL–32, 0.1 pg/mL–13.2 g/mL, and 0.1 pg/mL–47, 0.1 pg/mL–66.3 g/mL, respectively (Bispo et al., 2002).
Another study used RP-HPLC/UV to measure caffeine, theobromine and theophylline levels simultaneously in tea, coffee and human urine. LOD values were calculated as 0.10 µg/L for caffeine, 0.07 µg/L for theobromine and 0.06 µg/L for theophylline. The highest concentration of caffeine was found in soluble coffee, with a value of 771.40 µg/mL. Mate tea had the highest concentration of theobromine (13.40 µg/mL) while Sene tea (15.50 µg/mL) had the highest concentration of theophylline. The concentrations of these methylxanthines in human urine was between 0.8 to 8.70 µg/mL for caffeine, 1.40 to 52.4 µg/mL for theobromine and from1.20 to 42.30 µg/mL for theophylline (de Aragão et al., 2005).
Baek et al. (2022) determined the methylxanthine contents of different tea types in Korea using HPLC. The mean contents of caffeine, theobromine and theophylline in the 83 leached extracts of 11 different types of tea were 5561.5, 407.3 and 24.8 mg/kg, respectively. The concentrations ranges were ND–17177.8, ND–2220.5 and ND–223.2 mg /kg for caffeine, theobromine and theophylline, respectively. The limit of quantitation values were 0.0254, 0.0144 and 0.0721 mg/kg while the limit of detection values were 0.0084, 0.0047 and 0.0238 mg/kg for caffeine, theobromine and theophylline, respectively.
In a study to compare methylxanthine, phenolic and saponin contents in the leaves, branches and unripe fruits of Ilex paraguariensis A. St.-Hil (Mate), the lowest concentrations of methylxanthines were found in unripe fruits. The highest concentrations of caffeine were in leaves while the highest concentrations of theobromine were in branches. The concentrations of caffeine and theobromine in commercial mate products were 0.79 and 0.30 g/100 g, respectively (Borré et al., 2010).
Pyrrolizidine alkaloids
Bodi et al. (2014) determined the pyrrolizidine alkaloids (PAs) in 274 teas, 41 herbal drugs and 87 honey samples. In the tea samples, the concentrations of pyrrolizidine alkaloids ranged from < LOD to 5647.2 µg/kg while the mean concentrations of the alkaloids ranged from 51.6 to 1856 µg/kg. The maximum PA concentration among the herbal drugs was found in anise, with a value of 3098.8 µg/kg and a mean concentration of 452.6 µg/kg. The honey samples recorded PA concentrations ranging from 0.3 to 234.5 µg/kg and a mean concentration range of 6.1–14.5 µg/kg.
Chen et al. (2019) conducted a risk assessment of pyrrolizidine alkaloid absorption from herbal teas and medicines and determined the concentration range of PAs in the tested samples to be < LOQ–7883 µg/kg for herbal medicines and 30.7–1120 µg/L for herbal teas.
Since pyrrolizidine alkaloids are known to cause hepatic veno-occlusive disease, plant extracts and wheat flour were tested for contamination by pyrrolizidine alkaloids in one study following an outbreak of the disease in Western Afghanistan. In terms of plant matter, lasiocarpine from the affected area (quantified against senecionine) had the highest concentrations of PAs in its stems, leaves, fresh roots and seeds. The flour samples from the affected area had a median PA concentration of 5.6 mg/kg with heliotrine-N-oxide having the highest median value of 5.4 mg/kg (Kakar et al., 2010).
Dübecke et al. (2011) analyzed 3917 honey samples and 119 bee pollen samples using LC–MS/MS. In total, 94% of retail honeys contained pyrrolizidine alkaloids while 66% of raw honeys tested positive for the presence of PAs. PAs were found in 60% of the bee pollen samples, with concentrations ranging from 11 to 37,855 µg/kg.
Pollen products, which are used as food supplements, were also tested for the presence of pyrrolizidine alkaloids in another study. Of the 55 commercial samples tested, 17 (31%) contained pyrrolizidine alkaloids, with a concentration range of 1080 to 16,350 µg/kg. (Kempf et al., 2010).
Quinolizidine alkaloids
Magalhães et al. (2017) identified and quantified alkaloids from lupin derivatives, which are of commercial interest in Europe. The quinolizidine alkaloids that were detected included lupanine, angustifoline, α-isolupanine and sparteine, with the total concentration of alkaloids in the seeds ranging from 690 to 24,950 mg/kg. The concentration range of each alkaloid expressed as percentage of the total quinolizidine alkaloids was 550–20,750 mg/kg for lupanine, 90–3830 mg/kg for angustifoline, 40–550 mg/kg for α-isolupanine and not detected–10 mg/kg for sparteine.
GC–MS/GC-FID was used in parallel for the analysis of the quinolizidine alkaloids lupanine, 13α-hydroxylupanine, α-isolupanine and angustifoline in lupin flours with high alkaloid contents. The total alkaloid concentrations in the bitter varieties ranged from 10,800 to 19,800 mg/kg and those in the sweet varieties ranged from 44 to 2120 mg/kg (Reinhard et al., 2006).
In another study, a total of 27 lupin-based products, including flours, protein isolates and food products, were analyzed for their quinolizidine alkaloid contents using GC–MS (Resta et al., 2008). In total, five alkaloids were detected in L. albus cv Arés (albine, lupanine, 13α-hydroxylupanine, 13α-angeloyloxylupanine and 13α-tigloyloxylupanine) and L. albus cv Typtop (albine, lupanine, multiflorane, 13α-hydroxylupanine and 13α-angeloyloxylupanine). Only lupanine was detected in the protein isolates. The total alkaloid concentrations in the Arés and Typtop were 146 mg/kg and 1247 mg/kg, respectively (Resta et al., 2008).
Additionally, Cortes et al. (2005) investigated variations in alkaloids during germination in different lupin species. The total quinolizidine alkaloids contents in the raw seeds were 1.51 g/100 g for L. angustifolius, 2.36 g/100 g for L. albus and 2.45 g/100 g for L. campestris. In L. albus, lupanine increased during germination while albine and 13-hydroxylupanine decreased significantly.
Sparteine, lupanine, 13α-hydroxylupanine, angustifoline and α-isolupanine were all detected using GC–MS in a study by Gresta et al. (2010), which evaluated the productive and nutritional characteristics of several sweet varieties of lupin seeds. In the Multitalia variety, the total concentration of alkaloids was 1664 mg/kg. Lupanine had a total composition of 1499 mg/kg while the other quinolizidine alkaloid compositions were 99, 34, 9.1 and 2.1 mg/kg for 13α-hydroxylupanine, angustifoline, α-isolupanine and sparteine, respectively. LOQ for the study was 0.2–0.4 mg/kg.
Tropane alkaloids
In one study, the tropane alkaloids atropine and scopolamine were detected in buckwheat fruits, flours and commercial food products using GC–MS. Highest concentration of alkaloids was found in Datura stramonium seeds, with atropine and scopolamine having concentration values of 1283 and 678 mg/kg, respectively (Caligiani et al., 2011).
Romera-Torres et al. used HPLC/Exactive–Orbitrap analyzer to detect tropane alkaloids in teas and herbal teas. The limit of quantitation values for the study was between 5 and 20 µg/kg. Highest concentration of atropine was present in herbal teas (27 µg/kg). Other tropane alkaloids present in the tested samples included physoperuvine, pseudotropine, tropine, homatropine and apoatropine (Romera-Torres et al., 2018).
A similar method was used in another to analyze tropane alkaloids in cereals and Solanaceae seeds. Concentrations of up to 694 µg/kg were obtained for scopolamine in samples contaminated with Brugmansia seeds and 1847 µg/kg for atropine in samples contaminated with Stramonium seeds. Millet flour samples also tested positive for scopolamine, tropinone, atropine, anisodamine and littorine (Marín-Sáez et al., 2017).
Baslé et al. (2020) used LC–MS/MS to determine tropane alkaloids in cereals from Asia and Africa. Of the 95 cereal and cereal-based products that were analyzed, only one sample contained low concentrations of the targeted tropane alkaloids.
LC–MS/MS was also used in another study to quantify tropane alkaloids in 113 cereal products for infants and young children from 2011, 2012 and 2014 in the Netherlands. The mean total concentrations of atropine and scopolamine for the three years under consideration were 3.04, 2.37 and 0.37 µg/kg while the maximum total concentrations were 80.8, 57.6 and 3.9 µg/kg (Mulder et al., 2015).
Risk assessments
Ergot alkaloids
Rye and rye products contain high amount of ergot alkaloid. The highest average human chronic exposure to ergot alkaloids was found in “toddlers” and “other children”, with upper bound (UB) estimates of 0.47 and 0.46 µg/kg b.w. per day, respectively. The highest P95 exposure to ergot alkaloids was also observed in “toddlers”. In general, the values derived for the lower bound (LB) estimates were four times lower than the UB estimates, on average (EFSA Journal, 2017). The average acute exposure (MB) was estimated to be in the range of 0.02–0.32 µg/kg bw per day for “infants” and “other children”, respectively while the highest estimated value for the 95th percentile of acute dietary exposure was recorded for “other children” (EFSA, 2017).
Available data indicate that there are no health concerns associated with this alkaloid, based on the determined BMDL10, group ARfD and group TDI values, which are 0.33, 1 and 0.6 µg/kg b.w. per day, respectively (EFSA CONTAM Panel, 2012).
Glycoalkaloids
In one study, the levels of exposure to PGAs, mainly α-solanine and α-chaconine, were determined using occurrence data for raw primary commodities (RPCs) (EFSA CONTAM Panel, 2020). The values obtained for the mean UB and the P95 occurrence were 51.2 mg/kg and 116.8 mg/kg, respectively, with lowest and highest concentrations of 1.1 and 276.9 mg/kg, respectively. Across the conducted surveys, the mean UB potato glycoalkaloid exposure was between 23.3 µg/kg b.w. per day in “adults” to 174.0 µg/kg bw per day in “toddlers” and the P95 exposure ranged from 78.3 µg/kg bw per day in “adults” to 535.1 µg/kg b.w. per day in “toddlers” (EFSA CONTAM Panel, 2020).
The possible health risks resulting from acute exposure to PGAs through food were determined using MOE approach with a LOAEL of 1 mg/kg PGAs adopted as the reference point for acute risk characterization. As shown by the MOE, possible health risks for younger age groups were identified in both food consumption survey with the highest mean exposure and P95 exposure in all surveys. Meanwhile, only the food consumption survey with the highest P95 exposure identified health risks for adult age groups (EFSA CONTAM Panel, 2020).
Purine alkaloids
Using data from the seventh Korean National Health and Nutrition Examination Survey (KNHANES), one study estimated the daily intake values of the purine alkaloids caffeine, theobromine and theophylline to be 30.819, 1.408 and 0.011 µg/kg bw per day, respectively. For caffeine and theobromine, the obtained values were below the recommended daily intake values; therefore, exposure to these alkaloids in teas poses no health concerns (Baek et al., 2022).
The EFSA Panel on Food Contact Materials, Enzymes, Flavorings and Processing Aids estimated the daily exposure through diet to caffeine and theobromine when added as chemically defined flavoring substances. They concluded that caffeine and theobromine do not present any safety concerns based on the estimated intake levels when they are used as flavoring substances (EFSA CONTAM Panel, 2017).
Pyrrolizidine alkaloids
In one study, data regarding the consumption of plant-based food in European populations were used to determine acute dietary exposure to pyrrolizidine alkaloids. Overall, a range of 34.5–48.4 ng/kg b.w. per day (LB–UB) was derived as the mean chronic dietary estimate in toddlers while a range of 154–214 ng/kg b.w. per day (LB–UB) was derived for highly exposed populations (EFSA CONTAM Panel, 2017).
In a more conservative scenario, a mean exposure of up to 311 ng/kg b.w. per day and a P95 exposure of up to 821 ng/kg b.w. per day were the highest estimates, with tea and herbal infusions being the major contributors to exposure to pyrrolizidine alkaloids (EFSA CONTAM Panel, 2017). For honey, the mean chronic exposure range was 0.1–7.4 ng/kg b.w. per day in adult populations and 0.4–18 ng/kg b.w. per day (minimum LB–maximum UB) for high consumers. The average consumption of honey among the younger populations was estimated to be 0.3–27 ng/kg b.w. per day (minimum LB–maximum UB) while it was estimated to be 0.7–31 ng/kg b.w. per day (minimum LB–maximum UB) among high consumers. A chronic exposure level was observed when food with pollen-based supplements was consumed, with exposure ranges of from 0.7 to 12 ng/kg b.w. per day (minimum LB–maximum UB) and 2.8–44 ng/kg b.w. per day (minimum LB–maximum UB) for high consumers only. Exposure to up to 890 ng/kg b.w. per day of pyrrolizidine alkaloids can be reached from the consumption of a 150 mL infusion containing 2 g of selected plant extracts (for example, borage infusion) (EFSA CONTAM Panel, 2017).
On the basis of occurrence data that were limited to honey, the 2011 EFSA CONTAM Panel concluded that the consumption of large amounts of honey could pose health risks to toddlers and children (EFSA CONTAM Panel, 2017). To assess the risks associated with pyrrolizidine alkaloids as possible carcinogens, the Panel used the new reference point of 237 µg/kg b.w. per day and found that pyrrolizidine alkaloid exposure has possible health implications for humans, especially among people that frequently consume teas and herbal infusions (EFSA CONTAM Panel, 2017).
Quinolizidine alkaloids
The EFSA CONTAM Panel limited their risk assessment of quinolizidine alkaloids in food for human and animal health to only the alkaloids contained in Lupinus species. By identifying the lowest single oral effective dose of 0.16 mg sparteine/kg body weight as a reference point, a margin of exposure approach was used to characterize the risks of acute exposure. Dietary exposure could only be estimated for specific scenarios as there were limited data on the occurrence and consumption of this alkaloid. Therefore, it was impossible to fully estimate the risks of exposure to quinolizidine alkaloid for humans. According to the results from the MOE, examples of scenarios that could pose high risks to human health in terms of exposure to quinolizidine alkaloids included the consumption of lupin seeds that had not been debittered, the consumption of lupin seeds that contained high levels of the alkaloid despite debittering and the consumption of lupin-based imitation meat (EFSA CONTAM Panel, 2019).
Tropane alkaloids
Tropane alkaloids are found in plants that have been used in human medicines for centuries, including those used for the treatment of wounds, gout and sleeplessness and as a pre-anesthesia (EFSA CONTAM Panel, 2013). In one study, a mixture of (-)-hyoscyamine and (-)-scopolamine was administered to human volunteers in their food and a NOAEL of 0.16 µg/kg b.w. was determined, which was used to establish a group ARfD. Further observations revealed that a dose of 0.16 µg/kg b.w., resulted in a transient decreased heart rate, which posed no risk to healthy people but could be harmful to vulnerable individuals (EFSA CONTAM Panel, 2013).
Acknowledgements
This work was supported by the Dong-A University research fund.
Abbreviations
- ARfD
Acute reference dose
- BMDL10
Benchmark dose at 10% extra risk
- DNA
Deoxyribonucleic acid
- EFSA
European food safety association
- ELISA
Enzyme-linked immunosorbent assay
- FAB-MS
Fast-atom bombardment mass spectrometry
- FID
Flame ionization detector
- GC
Gas chromatography
- GC-EI-MS
Gas chromatography-electron impact-mass spectrometry
- GC–MS
Gas chromatography–mass spectrometry
- HPLC
High-performance liquid chromatography
- HRGC
High-resolution gas chromatography
- IARC
International agency for research on cancer
- LC–ESI–MS/MS
Liquid chromatography-electrospray ionization-tandem mass spectrometry
- LC-HRMS
Liquid chromatography-high-resolution mass spectrometry
- LC–MS/MS
Liquid chromatography-tandem mass spectrometry
- LC-Orbitrap-MS
Liquid chromatography-orbitrap mass spectrometry
- LD
Lethal dose
- LLE
Liquid–liquid extraction
- LOAEL
Lowest-observed-adverse-effect-level
- LOD
Limit of detection
- LOQ
Limit of quantitation
- MS
Mass spectrometry
- MS/MS
Tandem mass spectrometry
- NOAEL
No-observed-adverse-effect-level
- NTP
National toxicology program
- RP-HPLC
Reverse-phase high-performance liquid chromatography
- TDI
Tolerable daily intake
- TGA
Total glycoalkaloid
- UHPLC
Ultra-high-performance liquid chromatography
- UPLC-MS/MS
Ultra-performance liquid chromatography-tandem mass spectrometry
- UPLC-Q-TOF
Ultra-performance liquid chromatography-quadrupole-time of flight
- UV
Ultraviolet
- WHO
World Health Organization
Declarations
Conflicts of interest
There are no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adebayo J. Akinboye, Email: bayou246@gmail.com
Kiyun Kim, Email: wfw0004@naver.com.
Seyun Choi, Email: seyun015@naver.com.
Inho Yang, Email: ihyang@kmou.ac.kr.
Joon-Goo Lee, Email: jglee@dau.ac.kr.
References
- Acamovic T, Stewart CS, Pennycott TW, editors. Poisonous plants and related toxins. Wallingford: CABI; 2004. [Google Scholar]
- Aehle E, Dräger B. Tropane alkaloid analysis by chromatographic and electrophoretic techniques: an update. Journal of Chromatography b: Analytical Technologies in the Biomedical and Life Sciences. 2010;878:1391–1406. doi: 10.1016/j.jchromb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Aeschbacher HU, Meier H, Jaccaud E. The effect of caffeine in the in vivo SCE and micronucleus mutagenicity tests. Mutation Research Letters. 1986;174:53–58. doi: 10.1016/0165-7992(86)90076-X. [DOI] [PubMed] [Google Scholar]
- Almeida AE, Cardoso CR, Almeida DV, Moreira RR, Silva M, Varanda EA. Mutagenic activity of glycoallkaloids from Solanum palinacanthum Dunal (Solanaceae) found in the Brazilian Cerrado. Latin American Journal of Pharmacy. 2010;29:122–126. [Google Scholar]
- Aniszewski T. Alkaloids-secrets of life: alkaloid chemistry, biological significance, applications and ecological role. Amsterdam: Elsevier; 2007. [Google Scholar]
- Aqel A, Almulla A, Al-Rifai A, Wabaidur SM, Alothman ZA, Badjah-Hadj-Ahmed AY. Rapid and sensitive determination of methylxanthines in commercial brands of tea using ultra-high-performance liquid chromatography-mass spectrometry. International Journal of Analytical Chemistry. 2019 doi: 10.1155/2019/2926580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadbakht M, Talavaki M. Qualitative and quantitative determination of pyrrolizidine alkaloids of wheat and flour contaminated with Senecio in Mazandaran Province farms. Iranian Journal of Pharmaceutical Research. 2022;2:179–183. [Google Scholar]
- de Aragão NM, Veloso MCC, Bispo MS, Ferreira SLC, de Andrade JB. Multivariate optimisation of the experimental conditions for determination of three methylxanthines by reversed-phase high-performance liquid chromatography. Talanta. 2005;67:1007–1013. doi: 10.1016/j.talanta.2005.04.066. [DOI] [PubMed] [Google Scholar]
- Azevedo RSA, Teixeira BS, da Sauthier MC S, Santana MVA, dos Santos WNL, de SantanaD A. Multivariate analysis of the composition of bioactive in tea of the species Camellia sinensis. Food Chemistry. 2019;273:39–44. doi: 10.1016/j.foodchem.2018.04.030. [DOI] [PubMed] [Google Scholar]
- Baek GH, Yang SW, Yun CI, Lee JG, Kim YJ. Determination of methylxanthine contents and risk characterization for various types of tea in Korea. Food Control. 2022;132:108543. doi: 10.1016/j.foodcont.2021.108543. [DOI] [Google Scholar]
- Baker DC, Keeler RF, Gaffield W, Baker DC, Keeler RF, Gaffield W. Pathology in hamsters administered Solanum plant species that contain steroidal alkaloids. Toxicon. 1989;27:1331–1337. doi: 10.1016/0041-0101(89)90065-2. [DOI] [PubMed] [Google Scholar]
- Ballester D, Yáñez E, Garcia R, Erazo S, Lopez F, Haardt E, Cornejo S, Lopez A, Pokniak J, Chichester CO. Chemical composition, nutritive value, and toxicological evaluation of two species of sweet lupine (Lupinus albus and Lupinus luteus) Journal of Agricultural and Food Chemistry. 1980;28:402–405. doi: 10.1021/jf60228a056. [DOI] [PubMed] [Google Scholar]
- Baslé Q, Mujahid C, Bessaire T. Application of a streamlined LC-MS/MS methodology for the determination of atropine and scopolamine in cereals from Asian and African countries. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2020;37:1744–1754. doi: 10.1080/19440049.2020.1800828. [DOI] [PubMed] [Google Scholar]
- El Bazaoui A, Stambouli H, Bellimam MA, Soulaymani A. Determination of tropane alkaloids in seeds of Datura stramonium L. by GC/MS and LC/MS. Annales de Toxicologie Analytique. 2009;21:183–188. [Google Scholar]
- Beyer J, Drummer OH, Maurer HH. Analysis of toxic alkaloids in body samples. Forensic Science International. 2009;185(1–3):1–9. doi: 10.1016/j.forsciint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Birecka H, Catalfamo JL, Eisen RN. A sensitive method for detection and quantitative determination of pyrrolizidine alkaloids. Phytochemistry. 1981;20:343–344. doi: 10.1016/0031-9422(81)85124-2. [DOI] [Google Scholar]
- Bispo MS, Veloso MC, Pinheiro HL, De Oliveira RF, Reis JO, De Andrade JB. Simultaneous determination of caffeine, theobromine, and theophylline by high-performance liquid chromatography. Journal of Chromatographic Science. 2002;40:45–48. doi: 10.1093/chromsci/40.1.45. [DOI] [PubMed] [Google Scholar]
- Bodart P, Kabengera C, Noirfalise A. Determination of a-solanine and a-chaconine in potatoes by high-performance thin-layer chromatography/densitometry. Journal of AOAC International. 2000;83:1468–1473. doi: 10.1093/jaoac/83.6.1468. [DOI] [PubMed] [Google Scholar]
- Bodi D, Ronczka S, Gottschalk C, Behr N, Skibba A, Wagner M, Lahrssen-Wiederholt M, Preiss-Weigert A, These A. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2014;31:1886–1895. doi: 10.1080/19440049.2014.964337. [DOI] [PubMed] [Google Scholar]
- Borré GL, Kaiser S, Pavei C, da Silva FA, Bassani VL, Ortega GG. Comparison of methylxanthine, phenolics and saponin contents in leaves, branches and unripe fruits from ilex paraguariensis A. St.-Hil (mate) Journal of Liquid Chromatography and Related Technologies. 2010;33:362–374. doi: 10.1080/10826070903526055. [DOI] [Google Scholar]
- Boschin G, Annicchiarico P, Resta D, D’Agostina A, Arnoldi A. Quinolizidine alkaloids in seeds of lupin genotypes of different origins. Journal of Agricultural and Food Chemistry. 2008;56:3657–3663. doi: 10.1021/jf7037218. [DOI] [PubMed] [Google Scholar]
- Breton D, Buret D, Clair P, Lafosse M. Chiral separation of atropine by high-performance liquid chromatography. Journal of Chromatography a. 2005;1088(1–2):104–109. doi: 10.1016/j.chroma.2005.02.073. [DOI] [PubMed] [Google Scholar]
- Bushway RJ, McGann DF, Bushway AA. Gas chromatographic method for the determination of solanidine and its application to a study of feed-milk transfer in the cow. Journal of Agricultural and Food Chemistry. 1984;32:548–551. doi: 10.1021/jf00123a033. [DOI] [Google Scholar]
- Butler WH, Fordt GP, Creasy DM. A 90-day Feeding Study of Lupin (Lupinus angustifolius) Flour Spiked with Lupin Alkaloids in the Rat. Food and Chemical Toxicology. 1996;34:531–536. doi: 10.1016/0278-6915(96)00025-7. [DOI] [PubMed] [Google Scholar]
- Cabello G, Valenzuela M, Vilaxa A, Durán V, Rudolph I, Hrepic N, Calaf G. A rat mammary tumor model induced by the organophosphorous pesticide parathion and malathion, possibly through acetylcholinesterase inhibition. Environmental Health Perspectives. 2001;109:471–479. doi: 10.1289/ehp.01109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiani A, Palla G, Bonzanini F, Bianchi A, Bruni R. A validated GC-MS method for the detection of tropane alkaloids in buckwheat (Fagopyron esculentum L.) fruits, flours and commercial foods. Food Chemistry. 2011;127:204–209. doi: 10.1016/j.foodchem.2010.11.141. [DOI] [Google Scholar]
- Caniato R, Tabacchi R, Tsoupras G, Bicchi C. Capillary gas chromatography/positive and negative ion chemical ionization mass spectrometry on pyrrolizidine alkaloids of Senecio inaequidens using ammonia and hydroxyl ions as the reagent species. Journal of Natural Products. 1989;52:32–41. doi: 10.1021/np50061a002. [DOI] [Google Scholar]
- Capasso A, Aquino R, de Tommasi N, Piacente S, Rastrelli L, Pizza C. Neuropharmacology activity of alkaloids from South American Medicinal Plants. Current Medicinal Chemistry-Central Nervous System Agents. 2002;2:1–15. doi: 10.2174/1568015024606600. [DOI] [Google Scholar]
- Caprioli G, Logrippo S, Cahill MG, James KJ. High-performance liquid chromatography LTQ-Orbitrap mass spectrometry method for tomatidine and non-target metabolites quantification in organic and normal tomatoes. International Journal of Food Sciences and Nutrition. 2014;65:942–947. doi: 10.3109/09637486.2014.950205. [DOI] [PubMed] [Google Scholar]
- Carpent G, Desclin L. Effects of ergocornine on the mechanism of gestation and on fetal morphology in the rat. Endocrinology. 1969;84:315–324. doi: 10.1210/endo-84-2-315. [DOI] [PubMed] [Google Scholar]
- Chami Lal, Méndez R, Chataing B, O’Callaghan J, Usubillaga A, LaCruz L. Toxicological effects of α-solamargine in experimental animals. Phytotherapy Research. 2003;17:254–258. doi: 10.1002/ptr.1122. [DOI] [PubMed] [Google Scholar]
- Chan PC, Haseman JK, Prejean JD, Nyska A. Toxicity and carcinogenicity of riddelliine in rats and mice. Toxicology Letters. 2003;144:295–311. doi: 10.1016/S0378-4274(03)00240-6. [DOI] [PubMed] [Google Scholar]
- Chan PC, Mahler J, Bucher JR, Travlos GS, Reid JB. Toxicity and carcinogenicity of riddelliine following 13 weeks of treatment to rats and mice. Toxicon. 1994;32:891–908. doi: 10.1016/0041-0101(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Chan P. NTP technical report on the toxicity studies of Riddelliine (CAS No. 23246–96–0) Administered by Gavage to F344 Rats and B6C3F1 Mice. Toxicity Report Series. 1993;27:1–D9. [PubMed] [Google Scholar]
- Chen L, Mulder PP, Peijnenburg A, Rietjens IM. Risk assessment of intake of pyrrolizidine alkaloids from herbal teas and medicines following realistic exposure scenarios. Food and Chemical Toxicology. 2019;130:142–153. doi: 10.1016/j.fct.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Choudhury RC, Palo AK. Modulatory effects of caffeine on methotrexate-induced cytogenotoxicity in mouse bone marrow. Environmental Toxicology and Pharmacology. 2004;15:79–85. doi: 10.1016/j.etap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Christen P, Bieri S, Veuthey JL. Analysis of tropane alkaloids in biological matrices. Weinheim: Wiley; 2008. pp. 341–367. [Google Scholar]
- Cimpoiu C, Hosu A, Seserman L, Sandru M, Miclaus V. Simultaneous determination of methylxanthines in different types of tea by a newly developed and validated TLC method. Journal of Separation Science. 2010;33:3794–3799. doi: 10.1002/jssc.201000554. [DOI] [PubMed] [Google Scholar]
- Conradie J, Stewart MJ, Steenkamp V. GC/MS identification of toxic pyrrolizidine alkaloids in traditional remedies given to two sets of twins. Annals of Clinical Biochemistry. 2005;42:141–144. doi: 10.1258/0004563053492847. [DOI] [PubMed] [Google Scholar]
- de Cortes S M, Altares P, Pedrosa MM, Burbano C, Cuadrado C, Goyoaga C, Muzquiz M, Jiménez-Martínez C, Dávila-Ortiz G. Alkaloid variation during germination in different Lupin species. Food Chemistry. 2005;90:347–355. doi: 10.1016/j.foodchem.2004.04.008. [DOI] [Google Scholar]
- Crews C, Anderson WAC, Rees G, Krska R. Ergot alkaloids in some rye-based UK cereal products. Food Additives and Contaminants: Part B Surveillance. 2009;2:79–85. doi: 10.1080/02652030903042509. [DOI] [PubMed] [Google Scholar]
- Crews C, Berthiller F, Krska R. Update on analytical methods for toxic pyrrolizidine alkaloids. Analytical and Bioanalytical Chemistry. 2010;396:327–338. doi: 10.1007/s00216-009-3092-2. [DOI] [PubMed] [Google Scholar]
- Culvenor CC, Edgar JA, Jago MV, Outteridge A, Peterson JE, Smith LW. Hepato-and pneumotoxicity of pyrrolizidine alkaloids and derivatives in relation to molecular structure. Chemico-Biological Interactions. 1976;12:299–324. doi: 10.1016/0009-2797(76)90046-6. [DOI] [PubMed] [Google Scholar]
- Cushnie TPT, Cushnie B, Lamb AJ. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. International Journal of Antimicrobial Agents. 2014;44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Czapski GA, Szypuła W, Kudlik M, Wileńska B, Kania M, Danikiewicz W, Adamczyk A. Assessment of antioxidative activity of alkaloids from Huperzia selago and Diphasiastrum complanatum using in vitro systems. Folia Neuropathologica. 2014;52:394–406. doi: 10.5114/fn.2014.47840. [DOI] [PubMed] [Google Scholar]
- Dalvi RR. Comparative assessment of the effect of solanine administered orally and intraperitoneally on hepatic dysfunction in male rats. The Japanese Journal of Veterinary Science. 1985;47:657–659. doi: 10.1292/jvms1939.47.657. [DOI] [PubMed] [Google Scholar]
- Dighe R, Vaidya VG. Induction of Sister Chromatid exchanges by ergot compounds in Chinese hamster ovary cells in vitro. Teratogenesis, Carcinogenesis and Mutagenesis. 1988;8:169–174. doi: 10.1002/tcm.1770080306. [DOI] [PubMed] [Google Scholar]
- Dixit VP, Gupta RS. Antispermatogenic/antiandrogenic properties of solasodine (C27H43O2N) obtained from solanum xanthocarpum berries on the male genital tract of dog (Canis-familiaris). A histophysiological approach. International Journal of Andrology. 1982;5:295–307. doi: 10.1111/j.1365-2605.1982.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Dixit VP, Gupta RS, Gupta S. Antifertility plant products: testicular cell population dynamics following solasodine (C27 H43 O2 N) administration in rhesus monkey (Macaca mulatta) Andrologia. 1989;21:542–546. doi: 10.1111/j.1439-0272.1989.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Drager B. Analysis of tropane and related alkaloids. Journal of Chromatography a. 2002;978(1–2):1–35. doi: 10.1016/S0021-9673(02)01387-0. [DOI] [PubMed] [Google Scholar]
- Dübecke A, Beckh G, Lüllmann C. Pyrrolizidine alkaloids in honey and bee pollen. Food Additives and Contaminants - Part a. 2011;28:348–358. doi: 10.1080/19440049.2010.541594. [DOI] [PubMed] [Google Scholar]
- Edgar JA, Smith LW. Transfer of pyrrolizidine alkaloids into eggs: food safety implications (2000)
- Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl-Kraupp B, EFSA Panel on Contaminants in the Food Chain (CONTAM) Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA Journal. 2017;15:e04908. doi: 10.2903/j.efsa.2017.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Hogstrand C, Hoogenboom L, Leblanc JC, Nebbia CS, Nielsen E, EFSA Panel on Contaminants in the Food Chain (CONTAM) Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA Journal. 2020;18:e06222. doi: 10.2903/j.efsa.2020.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D, Bodin L, Chipman JK, del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom L, Leblanc JC, Nebbia CS, Nielsen E, EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA Journal. 2019;17:e05860. doi: 10.2903/j.efsa.2019.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific opinion on ergot alkaloids in food and feed. EFSA Journal. 2012;10:2798. doi: 10.2903/j.efsa.2012.2798. [DOI] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on Tropane alkaloids in food and feed. EFSA Journal. 2013;11:3386. doi: 10.2903/j.efsa.2013.3386. [DOI] [Google Scholar]
- Silano V, Bolognesi C, Castle L, Cravedi JP, Engel KH, Fowler P, Franz R, Grob K, Gürtler R, Husøy T. Scientific opinion on flavouring group evaluation 49, revision 1 (FGE 49Rev1): xanthine alkaloids from the priority list. EFSA Journal. 2017;15:e04729. doi: 10.2903/j.efsa.2017.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcella D, Gómez Ruiz JA, Innocenti ML, Roldán R, European Food Safety Authority Human and animal dietary exposure to ergot alkaloids. EFSA Journal. 2017;15:e04902. doi: 10.2903/j.efsa.2017.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo J, Dexter J, Roscoe M, Nowicki T. Retention of ergot alkaloids in wheat during processing1,2. Cereal Chemistry. 1995;72:291–298. [Google Scholar]
- Fernandez LS, Sykes ML, Andrews KT, Avery VM. Antiparasitic activity of alkaloids from plant species of Papua New Guinea and Australia. International Journal of Antimicrobial Agents. 2010;36:275–279. doi: 10.1016/j.ijantimicag.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Fliniaux MA, Jacquin-Dubreuil A. A competitive solid-phase enzyme immunoassay for the evaluation of tropane alkaloids in plant material, using anti-DL-tropic acid antibodies. Planta Medica. 1987;53:87–90. doi: 10.1055/s-2006-962629. [DOI] [PubMed] [Google Scholar]
- Franzmann C, Wächter J, Dittmer N, Humpf HU. Ricinoleic acid as a marker for ergot impurities in rye and rye products. Journal of Agricultural and Food Chemistry. 2010;58:4223–4229. doi: 10.1021/jf1006903. [DOI] [PubMed] [Google Scholar]
- Frédérich M, Jacquier MJ, Thépenier P, de Mol P, Tits M, Philippe G, Delaude C, Angenot L, Zèches-Hanrot M. Antiplasmodial activity of alkaloids from various Strychnos species. Journal of Natural Products. 2002;65:1381–1386. doi: 10.1021/np020070e. [DOI] [PubMed] [Google Scholar]
- Friedman M, Henika PR. Absence of genotoxicity of potato alkaloids α-chaconine, α-solanine and solanidine in the Ames Salmonella and adult and fetal erythrocyte micronucleus assays. Food and Chemical Toxicology. 1992;30:689–694. doi: 10.1016/0278-6915(92)90164-G. [DOI] [PubMed] [Google Scholar]
- Friedman M, McDonald GM, Filadelfi-Keszi M. Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Critical Reviews in Plant Sciences. 1997;16:55–132. doi: 10.1080/07352689709701946. [DOI] [Google Scholar]
- Friedman M, Roitman JN, Kozukue N. Glycoalkaloid and calystegine contents of eight potato cultivars. Journal of Agricultural and Food Chemistry. 2003;51:2964–2973. doi: 10.1021/jf021146f. [DOI] [PubMed] [Google Scholar]
- Gaffield W, Keeler RF. Implication of C-5, C-6 unsaturation as a key structural factor in steroidal alkaloid-induced mammalian teratogenesis. Experientia. 1993;49:922–924. doi: 10.1007/BF01952611. [DOI] [PubMed] [Google Scholar]
- Gaffield W, Keeler RF. Induction of terata in hamsters by solanidane alkaloids derived from Solanum tuberosum. Chemical Research in Toxicology. 1996;9:426–433. doi: 10.1021/tx950091r. [DOI] [PubMed] [Google Scholar]
- Ge Y, Liu P, Yang R, Zhang L, Chen H, Camara I, Liu Y, Shi W. Insecticidal constituents and activity of alkaloids from Cynanchum mongolicum. Molecules. 2015;20:17483–17492. doi: 10.3390/molecules200917483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder WMJ. Determination of the total C27-steroidal alkaloid com-position of Solanum species by high-resolution gas chromatography. Journal of Chromatography a. 1985;331:285–293. doi: 10.1016/0021-9673(85)80034-0. [DOI] [Google Scholar]
- Grauwiler J, Schon H. Teratological experiments with ergotamine in mice, rats, and rabbits. Teratology. 1973;7:227–235. doi: 10.1002/tera.1420070303. [DOI] [PubMed] [Google Scholar]
- Green BT, Lee ST, Welch KD, Gardner DR, Stegelmeier BL, Davis TZ. The serum concentrations of lupine alkaloids in orally-dosed Holstein cattle. Research in Veterinary Science. 2015;100:239–244. doi: 10.1016/j.rvsc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Green CR, Christie GS. Malformations in foetal rats induced by the pyrrolizidine alkaloid, heliotrine. British Journal of Experimental Pathology. 1961;42:369. [PMC free article] [PubMed] [Google Scholar]
- Gresta F, Abbate V, Avola G, Magazzà G, Chiofalo B. Lupin seed for the crop-livestock food chain. Italian Journal of Agronomy. 2010;5:333–340. doi: 10.4081/ija.2010.333. [DOI] [Google Scholar]
- Griffith RW, Grauwiler J, Hodel C, Leist KH, Matter B. Ergot–toxicology considerations. Heffter-Heubner’s Handbook of Experimental Pharmacology. Berlin: Springer-Verlag; 1978. pp. 805–851. [Google Scholar]
- Gupta RS, Dixit VP. Effects of short-term treatment of solasodine on cauda epididymis in dogs. Indian Journal of Experimental Biology. 2002;40:169–173. [PubMed] [Google Scholar]
- Haynes P, Lambert TR, Mitchell ID. Comparative in-vivo genotoxicity of antiviral nucleoside analogues; penciclovir, acyclovir, ganciclovir and the xanthine analogue, caffeine, in the mouse bone marrow micronucleus assay. Mutation Research/genetic Toxicology. 1996;369(1–2):65–74. doi: 10.1016/S0165-1218(96)90049-X. [DOI] [PubMed] [Google Scholar]
- Hincks JR, Kim H-Y, Segall HJ, Molyneux RJ, Stermitz FR, Coulombe RA. DNA cross-linking in mammalian cells by pyrrolizidine alkaloids: structure-activity relationships. Toxicology and Applied Pharmacology. 1991;111:90–98. doi: 10.1016/0041-008X(91)90137-4. [DOI] [PubMed] [Google Scholar]
- Hirono I, Haga M, Fujii M, Matsuura S, Matsubara N, Nakayama M, Furuya T, Hikichi M, Takanashi H, Uchida E, Hosaka S. Induction of hepatic tumors in rats by senkirkine and symphytine. Journal of the National Cancer Institute. 1979;63:469–472. [PubMed] [Google Scholar]
- Hirono I, Mori H, Yamada K, Hirata Y, Haga M, Tatematsu H, Kanie S. Carcinogenic activity of petasitenine, a new pyrrolizidine alkaloid isolated from Petasites japonicus Maxim. Journal of the National Cancer Institute. 1977;58:1155–1157. doi: 10.1093/jnci/58.4.1155. [DOI] [PubMed] [Google Scholar]
- Hoogenboom LAP, Mulder PPJ, Zeilmaker MJ, van den Top HJ, Remmelink GJ, Brandon EFA, Klijnstra M, Meijer GAL, Schothorst R, van Egmond HP. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Additives and Contaminants - Part a. 2011;28:359–372. doi: 10.1080/19440049.2010.547521. [DOI] [PubMed] [Google Scholar]
- Hossain MB, Rai DK, Brunton NP. Optimization and validation of ultra-high-performance liquid chromatographic-tandem mass spectrometry method for qualitative and quantitative analysis of potato steroidal alkaloids. Journal of Chromatography b. 2015;997:110–115. doi: 10.1016/j.jchromb.2015.05.033. [DOI] [PubMed] [Google Scholar]
- Houben RJ, Brunt K. Determination of glycoalkaloids in potato tubers by reversed-phase high-performance liquid chromatography. Journal of Chromatography a. 1994;661(1–2):169–174. doi: 10.1016/0021-9673(94)85187-5. [DOI] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, & World Health Organization. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. World Health Organization (2002) [PMC free article] [PubMed]
- International Agency for Research on Cancer. Some naturally occurring substances. International Agency for Research on Cancer (1976)
- International Agency for Research on Cancer. IARC Monographs evaluate drinking coffee, maté, and very hot beverages (2016) [PubMed]
- Jankech T, Maliarová M, Martinka N. Determination of methylxanthines in tea samples by HPLC method. Nova Biotechnologica Et Chimica. 2019;18:124–132. doi: 10.2478/nbec-2019-0015. [DOI] [Google Scholar]
- Janssen GB, Beems RB, Elvers LH, Speijers GJA. Subacute toxicity of α-ergocryptine in Sprague-Dawley rats. 2: metabolic and hormonal changes. Food and Chemical Toxicology. 2000;38:689–695. doi: 10.1016/S0278-6915(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Janssen GB, Beems RB, Speijers GJA, van Egmond HP. Subacute toxicity of a-ergocryptine in Sprague-Dawley rats. 1: general toxicological effects. Food and Chemical Toxicology. 2000;38:679–88. doi: 10.1016/S0278-6915(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Jegorov A, Simek P, Heydova A, Cvak L, Minář J. Free and bonded homoisoleucine in sclerotia of the parasitic fungus Claviceps purpurea. Springer-Verlag Amino Acids. 1997;12(1):9–19. doi: 10.1007/BF01373422. [DOI] [Google Scholar]
- Jeong SH, Choi EY, Kim J, Lee C, Kang J, Cho S, Ko KY. LC-ESI-MS/MS simultaneous analysis method coupled with cation-exchange solid-phase extraction for determination of pyrrolizidine alkaloids on five kinds of herbal medicines. Journal of AOAC International. 2021;104:1514–1525. doi: 10.1093/jaoacint/qsab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Liu J, Liu H, Xin H. Histochemical investigation and kinds of alkaloids in leaves of different developmental stages in Thymus quinquecostatus. The Scientific World Journal. 2014 doi: 10.1155/2014/839548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John H, Binder T, Höchstetter H, Thiermann H. LC-ESI MS/MS quantification of atropine and six other antimuscarinic tropane alkaloids in plasma. Analytical and Bioanalytical Chemistry. 2010;396:751–763. doi: 10.1007/s00216-009-3209-7. [DOI] [PubMed] [Google Scholar]
- Kakar F, Akbarian Z, Leslie T, Mustafa ML, Watson J, van Egmond HP, Omar MF, Mofleh J. An outbreak of hepatic veno-occlusive disease in Western Afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. Journal of Toxicology. 2010 doi: 10.1155/2010/313280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel KA, Święcicki W, Kaczmarek Z, Barzyk P. Quantitative and qualitative content of alkaloids in seeds of a narrow-leafed Lupin (Lupinus angustifolius L.) collection. Genetic Resources and Crop Evolution. 2016;63:711–719. doi: 10.1007/s10722-015-0278-7. [DOI] [Google Scholar]
- Karou D, Savadogo A, Canini A, Yameogo S, Montesano C, Simpore J, Colizzi V, Traore AS. Antibacterial activity of alkaloids from Sida acuta. African Journal of Biotechnology. 2006;5:195–200. [Google Scholar]
- Kempf M, Reinhard A, Beuerle T. Pyrrolizidine alkaloids (PAs) in honey and pollen-legal regulation of pa levels in food and animal feed required. Molecular Nutrition and Food Research. 2010;54:158–168. doi: 10.1002/mnfr.200900529. [DOI] [PubMed] [Google Scholar]
- Khanchi AR, Khayatzadeh Mahani M, Hajihosseini M, Maragheh MG, Chaloosi M, Bani F. Simultaneous spectrophotometric determination of caffeine and theobromine in Iranian tea by artificial neural networks and its comparison with PLS. Food Chemistry. 2007;103:1062–1068. doi: 10.1016/j.foodchem.2006.07.035. [DOI] [Google Scholar]
- Kim HY, Stermitz FR, Coulombe RA., Jr Pyrrolizidine alkaloid-induced DNA-protein cross-links. Carcinogenesis. 1995;16:2691–2697. doi: 10.1093/carcin/16.11.2691. [DOI] [PubMed] [Google Scholar]
- Kokkonen M, Jestoi M. Determination of ergot alkaloids from grains with UPLC-MS/MS. Journal of Separation Science. 2010;33:2322–2327. doi: 10.1002/jssc.201000114. [DOI] [PubMed] [Google Scholar]
- Koleva II, van Beek TA, Soffers AE, Dusemund B, Rietjens IM. Alkaloids in the human food chain-natural occurrence and possible adverse effects. Molecular Nutrition and Food Research. 2012;56:30–52. doi: 10.1002/mnfr.201100165. [DOI] [PubMed] [Google Scholar]
- Kozukue N, Han JS, Lee KR, Friedman M. Dehydrotomatine and α-tomatine content in tomato fruits and vegetative plant tissues. Journal of Agricultural and Food Chemistry. 2004;52:2079–2083. doi: 10.1021/jf0306845. [DOI] [PubMed] [Google Scholar]
- Kraicer PF, Strauss JF. Ovulation block produced by an inhibitor of luteotrophin, ergocornine. European Journal of Endocrinology. 1970;65:698–706. doi: 10.1530/acta.0.0650698. [DOI] [PubMed] [Google Scholar]
- Krska R, Stubbings G, MacArthur R, Crews C. Simultaneous determination of six major ergot alkaloids and their epimers in cereals and foodstuffs by LC-MS-MS. Analytical and Bioanalytical Chemistry. 2008;391:563–576. doi: 10.1007/s00216-008-2036-6. [DOI] [PubMed] [Google Scholar]
- Kuhara K, Takanashia H, Hirono I, Furuya T, Asada Y. Carcinogenic activity of clivorine, a pyrrolizidine alkaloid isolated from Ligularia dentata. Cancer Letters. 1980;10:117–122. doi: 10.1016/0304-3835(80)90034-8. [DOI] [PubMed] [Google Scholar]
- Kuhlmann W, Fromme H-G, Heege E-M, Ostertag W. The mutagenic action of caffeine in higher organisms1. Cancer Research. 1968;28(11):2375–2389. [PubMed] [Google Scholar]
- Kurek J. editor. Alkaloids: Their importance in Nature and Human life. BoD–Books on Demand; (2019)
- Langer T, Möstl E, Chizzola R, Gutleb R. A competitive enzyme immunoassay for the pyrrolizidine alkaloids of the senecionine type. Planta Medica. 1996;62:267–271. doi: 10.1055/s-2006-957875. [DOI] [PubMed] [Google Scholar]
- Langkilde S, Mandimika T, Schrøder M, Meyer O, Slob W, Peijnenburg A, Poulsen M. A 28-day repeat dose toxicity study of steroidal glycoalkaloids, α-solanine and α-chaconine in the Syrian Golden hamster. Food and Chemical Toxicology. 2009;47:1099–1108. doi: 10.1016/j.fct.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Langkilde S, Schrøder M, Frank T, Shepherd LVT, Conner S, Davies Hv, Meyer O, Danier J, Rychlik M, Belknap WR, McCue KF, Engel KH, Stewart D, Knudsen I, Poulsen M. Compositional and toxicological analysis of a GM potato line with reduced α-solanine content - A 90-day feeding study in the Syrian Golden hamster. Regulatory Toxicology and Pharmacology. 2012;64:177–185. doi: 10.1016/j.yrtph.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Langkilde S, Schrøder M, Stewart D, Meyer O, Conner S, Davies H, Poulsen M. Acute toxicity of high doses of the glycoalkaloids, α-solanine and α-chaconine, in the Syrian Golden hamster. Journal of Agricultural and Food Chemistry. 2008;56:8753–8760. doi: 10.1021/jf8012794. [DOI] [PubMed] [Google Scholar]
- Lauber U, Schnaufer R, Gredziak M, Kiesswetter Y. Analysis of rye grains and rye meals for ergot alkaloids. Mycotoxin Research. 2005;21:258–262. doi: 10.1007/BF02957588. [DOI] [PubMed] [Google Scholar]
- Lee ST, Schoch TK, Stegelmeier BL, Gardner DR, Than KA, Molyneux RJ. Development of enzyme-linked immunosorbent assays for the hepatotoxic alkaloids riddelliine and riddelliine N-oxide. Journal of Agricultural and Food Chemistry. 2001;49:4144–4151. doi: 10.1021/jf010042m. [DOI] [PubMed] [Google Scholar]
- Logie CG, Grue MR, Liddell JR. Proton NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry. 1994;37:43–109. doi: 10.1016/0031-9422(94)85006-2. [DOI] [Google Scholar]
- Long Z, Wang C, Guo Z, Zhang X, Nordahl L, Zeng J, Zeng J, Liang X. A non-aqueous solid phase extraction method for alkaloid enrichment and its application in the determination of hyoscyamine and scopolamine. Analyst. 2012;137:1451–1457. doi: 10.1039/c2an15824h. [DOI] [PubMed] [Google Scholar]
- López-Martínez L, López-de-Alba PL, García-Campos R, de León-Rodríguez LM. Simultaneous determination of methylxanthines in coffees and teas by UV-Vis spectrophotometry and partial least squares. Analytica Chimica Acta. 2003;493:83–94. doi: 10.1016/S0003-2670(03)00862-6. [DOI] [Google Scholar]
- Luthy J, Zweifel U, Karlhuber B, Schlatter C. Pyrrolizidine alkaloids of Senecio alpinus L. and their detection in feeding stuffs. Journal of Agricultural and Food Chemistry. 1981;29:302–305. doi: 10.1021/jf00104a021. [DOI] [PubMed] [Google Scholar]
- Magalhães SCQ, Fernandes F, Cabrita ARJ, Fonseca AJM, Valentão P, Andrade PB. Alkaloids in the valorization of European Lupinus spp. seeds crop. Industrial Crops and Products. 2017;95:286–295. doi: 10.1016/j.indcrop.2016.10.033. [DOI] [Google Scholar]
- Marín-Sáez J, Romero-González R, Garrido Frenich A. Multi-analysis determination of tropane alkaloids in cereals and solanaceaes seeds by liquid chromatography coupled to single stage Exactive-Orbitrap. Journal of Chromatography a. 2017;1518:46–58. doi: 10.1016/j.chroma.2017.08.052. [DOI] [PubMed] [Google Scholar]
- Matter ΒΕ. Failure to detect chromosome damage in bone-marrow cells of mice and Chinese hamsters exposed in vivo to some ergot derivatives. Journal of International Medical Research. 1976;4:382–392. doi: 10.1177/030006057600400603. [DOI] [PubMed] [Google Scholar]
- Matter BE. Heritable translocation test in mice with triethylenemelamine (TEM) and ergotamine. Mutation Research Letters. 1982;104:177–182. doi: 10.1016/0165-7992(82)90141-5. [DOI] [PubMed] [Google Scholar]
- Mattocks AR. Hepatotoxic effects due to pyrrolizidine alkaloid N-oxides. Xenobiotica. 1971;1:563–565. doi: 10.3109/00498257109041530. [DOI] [PubMed] [Google Scholar]
- Mazur L, Peralta-Zamora PG, Demczuk B, Ribani RH. Application of multivariate calibration and NIR spectroscopy for the quantification of methylxanthines in yerba mate (Ilex paraguariensis) Journal of Food Composition and Analysis. 2014;35:55–60. doi: 10.1016/j.jfca.2014.04.005. [DOI] [Google Scholar]
- McCann J, Choi E, Yamasaki E, Ames BN, Jolley SB, Streitwieser D, Jen G, Donahue V, Stark G, Haroun L, Maron D, Keng T, Matsushima H, Bartsch E, Weisburger J, Miller E, Arcos J, Tomatis L, Rosenkranz H, Poirier L. Detection of carcinogens as mutagens in the Salmonella/microsome test: Assay of 300 chemicals. Proceedings of the National Academy of Sciences. 1975;72:5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhoff GC, Porter JM. Ergot intoxication: Historical review and description of unusual clinical manifestations. Annals of Surgery. 1974;180:773. doi: 10.1097/00000658-197411000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WC, Rice DL, Kreusel RG, Bedrossian CW. Monocrotaline model of noncardiogenic pulmonary edema in dogs. Journal of Applied Physiology. 1978;45:962–965. doi: 10.1152/jappl.1978.45.6.962. [DOI] [PubMed] [Google Scholar]
- Moco S, Capanoglu E, Tikunov Y, Bino RJ, Boyacioglu D, Hall RD, Vervoort J, De Vos RC. Tissue specialization at the metabolite level is perceived during the development of tomato fruit. Journal of Experimental Botany. 2007;58:4131–4146. doi: 10.1093/jxb/erm271. [DOI] [PubMed] [Google Scholar]
- Mohamed R, Gremaud E, Richoz-Payot J, Tabet JC, Guy PA. Quantitative determination of five ergot alkaloids in rye flour by liquid chromatography–electrospray ionisation tandem mass spectrometry. Journal of Chromatography A. 2006;1114:62–72. doi: 10.1016/j.chroma.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Molloy JB, Moore CJ, Bruyeres AG, Murray SA, Blaney BJ. Determination of dihydroergosine in sorghum ergot using an immunoassay. Journal of Agricultural and Food Chemistry. 2003;51:3916–3919. doi: 10.1021/jf0212284. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Coxon DT, Bramham S, Chan HWS, van Gelder WMJ, Allison MJ. Determination of the glycoalkaloid content of potato tubers by three methods including enzyme-linked immunosorbent assay. Journal of the Science of Food and Agriculture. 1985;36:282–288. doi: 10.1002/jsfa.2740360408. [DOI] [Google Scholar]
- Mroczek T, Głowniak K, Kowalska J. Solid-liquid extraction and cation-exchange solid-phase extraction using a mixed-mode polymeric sorbent of Datura and related alkaloids. Journal of Chromatography a. 2006;1107:9–18. doi: 10.1016/j.chroma.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Mulder PPJ, Beumer B, Oosterink E, de Jong J. Dutch survey pyrrolizidine alkaloids in animal forage. Dutch survey pyrrolizidine alkaloids in animal forage. Rikilt-Institute of Food Safety (2009)
- Mulder PP, Van Raamsdonk LW, Voogt HJ, van Brakel MW, van der Horst GM, de Jong J. Dutch survey ergot alkaloids and sclerotia in animal feeds. Rikilt-Institute of Food Safety (2012)
- Mulder PPJ, Pereboom-de Fauw DPKH, Hoogenboom RLAP, de Stoppelaar J, de Nijs M. Tropane and ergot alkaloids in grain-based products for infants and young children in the Netherlands in 2011–2014. Food Additives and Contaminants: Part B Surveillance. 2015;8:284–290. doi: 10.1080/19393210.2015.1089947. [DOI] [PubMed] [Google Scholar]
- Mulder P, de Nijs M. Determination of quinolizidine alkaloids in lupin flour by LC-MS/MS. A promising novel way of analysis. In Proceedings of the XIV International Lupin Conference, Milan, Italy. 21: 26-75 (2015)
- Müller C, Kemmlein S, Klaffke H, Krauthause W, Preiß-Weigert A, Wittkowski R. A basic tool for risk assessment: a new method for the analysis of ergot alkaloids in rye and selected rye products. Molecular Nutrition and Food Research. 2009;53:500–507. doi: 10.1002/mnfr.200800091. [DOI] [PubMed] [Google Scholar]
- Musita CN, Omayio DG, Abong’ GO, Okoth MW. Glycoalkaloids in commercial potato varieties traded in Nairobi, Kenya. F1000Research. 9: 423 (2020)
- Nara A, Saka K, Yamada C, Kodama T, Takagi T. Forensic analysis using ultra-high-performance liquid chromatography–tandem mass spectrometry with solid-phase extraction of α-solanine and α-chaconine in whole blood. Forensic Toxicology. 2019;37:197–206. doi: 10.1007/s11419-018-0452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program Bioassay of lasiocarpine for possible carcinogenicity. National Cancer Institute Carcinogenesis Technical Report Series. 1978;39:1–66. [PubMed] [Google Scholar]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Scopolamine Hydrobromide Trihydrate (CAS No. 6533–68–2) in F344 Rats and B6C3F1 Mice (Gavage Studies). National Toxicology Program Technical Report Series. 445: 1-277 (1997) [PubMed]
- National Toxicology Program. NTP toxicology and carcinogenesis studies of theophylline (CAS No. 58–55–9) in F344/N rats and B6C3F1 mice (feed and gavage studies). National Toxicology Program Technical Report Series. 473: 1-326 (1998) [PubMed]
- National Toxicology Program. Toxicology and carcinogenesis studies of riddelliine (CAS No. 23246–96–0) in F344/N rats and B6C3F1 mice (gavage studies). National Toxicology Program Technical Report Series. 508: 1-280 (2003) [PubMed]
- Papadoyannis IN, Samanidou VF, Theodoridis GA, Vasilikiotis GS, van Kempen GJM, Beelen GM. A simple and quick solid phase extraction and reversed phase HPLC analysis of some tropane alkaloids in feedstuffs and biological samples. Journal of Liquid Chromatography. 1993;16:975–998. doi: 10.1080/10826079308019565. [DOI] [Google Scholar]
- Paradkar MM, Irudayaraj J. A rapid FTIR spectroscopic method for estimation of caffeine in soft drinks and total methylxanthines in tea and coffee. Journal of Food Science. 2002;67:2507–2511. doi: 10.1111/j.1365-2621.2002.tb08767.x. [DOI] [Google Scholar]
- Pereira TN, Webb RI, Reilly PEB, Seawright AA, Prakash AS. Dehydromonocrotaline generates sequence-selective N-7 guanine alkylation and heat and alkali stable multiple fragment DNA crosslinks. Nucleic Acids Research. 1998 doi: 10.1093/nar/26.23.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelser PB, de Vos H, Theuring C, Beuerle T, Vrieling K, Hartmann T. Frequent gain and loss of pyrrolizidine alkaloids in the evolution of Senecio section Jacobaea (Asteraceae) Phytochemistry. 2005;66:1285–1295. doi: 10.1016/j.phytochem.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Peterson JE, Jago MV. Comparison of the toxic effects of dehydroheliotridine and heliotrine in pregnant rats and their embryos. The Journal of Pathology. 1980;131:339–355. doi: 10.1002/path.1711310406. [DOI] [PubMed] [Google Scholar]
- Peters-Volleberg GWM, Beems RB, Speijers GJA. Subacute toxicity of ergometrine maleate in rats. Food and Chemical Toxicology. 1996;34:951–958. doi: 10.1016/S0278-6915(96)00060-9. [DOI] [PubMed] [Google Scholar]
- Petry TW, Bowden GT, Buhler DR, Glenn Sipe KG. Genotoxicity of the pyrrolizidine alkaloid jacobine in rats. Toxicology Letters. 1986;32:275–281. doi: 10.1016/0378-4274(86)90119-0. [DOI] [PubMed] [Google Scholar]
- Petry TW, Bowden GT, Huxtable RJ, Sipes IG. Characterization of hepatic DNA damage induced in rats by the pyrrolizidine alkaloid monocrotaline. Cancer Research. 1984;44:1505–1509. [PubMed] [Google Scholar]
- Petterson DS, Ellis ZL, Harris DJ, Spadek ZE. Acute toxicity of the major alkaloids of cultivated lupinus angustifolius Seed to rats. Journal of Applied Toxicology. 1987;7:51–53. doi: 10.1002/jat.2550070109. [DOI] [PubMed] [Google Scholar]
- Philipov S, Berkov S. GC-MS Investigation of Tropane Alkaloids in Datura stramonium. Zeitschrift Für Naturforschung c. 2002;57:559–561. doi: 10.1515/znc-2002-5-627. [DOI] [PubMed] [Google Scholar]
- Phillips BJ, Hughes JA, Phillips JC, Walters DG, Anderson D, Tahourdint CSM. A study of the toxic hazard that might be associated with the consumption of green potato tops. Food and Chemical Toxicology. 1996;34:439–448. doi: 10.1016/0278-6915(96)87354-6. [DOI] [PubMed] [Google Scholar]
- Pothier J, Galand N, Dormeau C, Viel C. A Comparative study of the effects of sparteine, lupanine and lupin extract on the central nervous system of the mouse. Journal of Pharmacy and Pharmacology. 1998;50:949–954. doi: 10.1111/j.2042-7158.1998.tb04013.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Reddy JK. Malignant neoplasms in rats fed lasiocarpine. British Journal of Cancer. 1978;37:289–293. doi: 10.1038/bjc.1978.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard H, Rupp H, Sager F, Streule M, Zoller O. Quinolizidine alkaloids and phomopsins in lupin seeds and lupin containing food. Journal of Chromatography a. 2006;1112:353–360. doi: 10.1016/j.chroma.2005.11.079. [DOI] [PubMed] [Google Scholar]
- Reinhold L, Reinhardt K. Mycotoxins in foods in lower Saxony (Germany): results of official control analyses performed in 2009. Mycotoxin Research. 2011;27:137–143. doi: 10.1007/s12550-011-0086-7. [DOI] [PubMed] [Google Scholar]
- Reinwaldt C, Bittner M, Kempe G, Glauner T. Quantitation of pyrrolizidine alkaloids in honey and herbal teas by UHPLC/MS/MS. Agilent Technologies Incorporation. (2017)
- Renwick JH, Claringbold WDB, Earthy ME, Few JD, Caroline A, Mclean S. Neural-tube defects produced in Syrian hamsters by potato glycoalkaloids. Teratology. 1984;30:371–381. doi: 10.1002/tera.1420300309. [DOI] [PubMed] [Google Scholar]
- Resta D, Boschin G, D’Agostina A, Arnoldi A. Evaluation of total quinolizidine alkaloids content in lupin flours, lupin-based ingredients, and foods. Molecular Nutrition and Food Research. 2008;52:490–495. doi: 10.1002/mnfr.200700206. [DOI] [PubMed] [Google Scholar]
- Robbins MC, Petterson DS, Brantom PG. A 90-day feeding study of the alkaloids of Lupinus angustifolius in the Rat. Food and Chemical Toxicology. 1996;34:679–686. doi: 10.1016/0278-6915(96)00040-3. [DOI] [PubMed] [Google Scholar]
- Roberts CA, Joost RE, Rottinghaus GE. Quantification of ergovaline in tall fescue by near infrared reflectance spectroscopy. Crop Science. 1997;37:281–284. doi: 10.2135/cropsci1997.0011183X003700010051x. [DOI] [Google Scholar]
- Roberts GT, Rand MJ. Chromosomal damage induced by some ergot derivatives in vitro. Mutation Research/fundamental and Molecular Mechanisms of Mutagenesis. 1977;48:205–214. doi: 10.1016/0027-5107(77)90162-2. [DOI] [PubMed] [Google Scholar]
- Roberts GT and Rand MJ. Effects of some ergot derivatives in bone marrow of mice. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 56: 59-67 (1977b) [DOI] [PubMed]
- Roberts GT, Rand MJ. The dominant lethal effects of some ergot alkaloids. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 50: 317-325 (1978) [DOI] [PubMed]
- Roeder E. Carbon-13 NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry. 1990;29(1):11–29. doi: 10.1016/0031-9422(90)89003-R. [DOI] [Google Scholar]
- Romera-Torres A, Romero-González R, Martínez Vidal JL, Garrido Frenich A. Simultaneous analysis of tropane alkaloids in teas and herbal teas by liquid chromatography coupled to high-resolution mass spectrometry (Orbitrap) Journal of Separation Science. 2018;41:1938–1946. doi: 10.1002/jssc.201701485. [DOI] [PubMed] [Google Scholar]
- Roseman DM, Wu X, Kurth MJ. Enzyme-linked immunosorbent assay detection of pyrrolizidine alkaloids: Immunogens based on quaternary pyrrolizidinium salts. Bioconjugate Chemistry. 1996;7:187–195. doi: 10.1021/bc950084e. [DOI] [PubMed] [Google Scholar]
- Saldaña MDA, Zetzl C, Mohamed RS, Brunner G. Extraction of methylxanthines from guaraná seeds, maté leaves, and cocoa beans using supercritical carbon dioxide and ethanol. Journal of Agricultural and Food Chemistry. 2002;50:4820–4826. doi: 10.1021/jf020128v. [DOI] [PubMed] [Google Scholar]
- Sanchez JM. Methylxanthine content in commonly consumed foods in Spain and determination of its intake during consumption. Foods. 2017;6:109. doi: 10.3390/foods6120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Mata MC, Yokoyama WE, Hong YJ, Prohens J. α-Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplants. Journal of Agricultural and Food Chemistry. 2010;58:5502–5508. doi: 10.1021/jf100709g. [DOI] [PubMed] [Google Scholar]
- Santiago Quiles MR, Oquendo-Jiménez I, Herreño-Saénz D, Antoun MD. Genotoxicity of alkaloid-rich extract from lupinus termis seeds. Pharmaceutical Crops. 2010 doi: 10.2174/2210290601001010018. [DOI] [Google Scholar]
- Savary BJ, Dougall DK. Scopolamine radioimmunoassay for tissue cultures of datura. Phytochemistry. 1990;29:1567–1569. doi: 10.1016/0031-9422(90)80123-X. [DOI] [Google Scholar]
- Schmähl D, Habs M. Life-span investigations for carcinogenicity of some immune-stimulating, immunodepressive and neurotropic substances in Sprague-Dawley-rats Zeitschrift. fur Krebsforschung und Klinische Onkologie. Cancer Research and Clinical Oncology. 1976;86:77–84. doi: 10.1007/BF00304936. [DOI] [PubMed] [Google Scholar]
- Schnitzius JM, Hill NS, Thompson CS, Craig AM. Semiquantitative determination of ergot alkaloids in seed, straw, and digesta samples using a competitive enzyme-linked immunosorbent assay. Journal of Veterinary Diagnostic Investigation. 2001;13:230–237. doi: 10.1177/104063870101300307. [DOI] [PubMed] [Google Scholar]
- Schoeneberger H, Moron S, Gross R. Safety evaluation of water debittered Andean lupins (Lupinus mutabilis): 12-week rat feeding study. Plant Foods for Human Nutrition. 1987;37:169–182. doi: 10.1007/BF01092053. [DOI] [Google Scholar]
- Schoental R. Liver lesions in young rats suckled by mothers treated with the pyrrolizidine (Senecio) alkaloids, lasiocarpine and retrorsine. Journal of Pathology and Bacteriology. 1959;77:485–495. doi: 10.1002/path.1700770220. [DOI] [PubMed] [Google Scholar]
- Schoental R, Magee PN. Further observations on the subacute and chronic liver changes in rats after a single dose of various pyrrolizidine (Senecio) alkaloids. Journal of Pathology and Bacteriology. 1959;78:471–482. doi: 10.1002/path.1700780213. [DOI] [PubMed] [Google Scholar]
- Shaar CJ, Clemens JA. Inhibition of lactation and prolactin secretion in rats by ergot alkaloids. Endocrinology. 1972;90:285–288. doi: 10.1210/endo-90-1-285. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Willhite CC, Shupe JL, Salunkhe DK. Acute toxicity and histopathological effects of certain glycoalkaloids and extracts of Alternaria solani or Phytophthora infestans in mice. Toxicology Letters. 1979;3:349–355. doi: 10.1016/0378-4274(79)90146-2. [DOI] [Google Scholar]
- Shrivas K, Wu HF. Rapid determination of caffeine in one drop of beverages and foods using drop-to-drop solvent microextraction with gas chromatography/mass spectrometry. Journal of Chromatography a. 2007;1170:9–14. doi: 10.1016/j.chroma.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Silva MR, Alvarez CM, García PM, Ruiz MA. Assessing the genotoxicities of sparteine and compounds isolated from Lupinus mexicanus and L. montanus seeds by using comet assay. Genetics and Molecular Research. 2014;13:10510–10517. doi: 10.4238/2014.December.12.12. [DOI] [PubMed] [Google Scholar]
- Silva-Neto JP, Barreto RA, Pitanga BP, Souza CS, Silva VD, Silva AR, Velozo ED, Cunha SD, Batatinha MJ, Tardy M, Ribeiro CS. Genotoxicity and morphological changes induced by the alkaloid monocrotaline, extracted from Crotalaria retusa, in a model of glial cells. Toxicon. 2010;55:105–117. doi: 10.1016/j.toxicon.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Sommer AF, Buchanan AR. Effects of ergot alkaloids on pregnancy and lactation in the albino rat. American Journal of Physiology-Legacy Content. 1955;180:296–300. doi: 10.1152/ajplegacy.1955.180.2.296. [DOI] [PubMed] [Google Scholar]
- Stanek M, Rotkiewicz T, Sobotka W, Bogusz J, Otrocka-Domagała I, Rotkiewicz A. The effect of alkaloids present in blue lupine (Lupinus angustifolius) seeds on the growth rate, selected biochemical blood indicators and histopathological changes in the liver of rats. Acta Veterinaria Brno. 2015;84:55–62. doi: 10.2754/avb201585010055. [DOI] [Google Scholar]
- Stanker LH, Kamps-Holtzapple C, Friedman M. Development and characterization of monoclonal antibodies that differentiate between potato and tomato glycoalkaloids and aglycons. Journal of Agricultural and Food Chemistry. 1994;42:2360–2366. doi: 10.1021/jf00046a053. [DOI] [Google Scholar]
- Stobiecki M, Blaszczyk B, Kowalczyk-Bronisz SH, Gulewicz K. The toxicity of seed extracts and their fractions from Lupinus angustifolius L. and Lupinus albus L. Journal of Applied Toxicology. 1993;13:347–352. doi: 10.1002/jat.2550130509. [DOI] [PubMed] [Google Scholar]
- Storm ID, Rasmussen PH, Strobel BW, Hansen HCB. Ergot alkaloids in rye flour determined by solid-phase cation-exchange and high-pressure liquid chromatography with fluorescence detection. Food Additives and Contaminants - Part a. 2008;25:338–346. doi: 10.1080/02652030701551792. [DOI] [PubMed] [Google Scholar]
- Svoboda DJ, Reddy JK. Malignant tumors in rats given lasiocarpine1. Cancer Research. 1972;32:908–913. [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Baba M. Inhibition by neostigmine and isoproterenol and promotion by atropine of experimental carcinogenesis in rat stomach by N-methyl-N'-nitro-N-nitrosoguanidine. International Journal of Cancer. 1989;44:188–189. doi: 10.1002/ijc.2910440134. [DOI] [PubMed] [Google Scholar]
- Topi D, Jakovac-Strajn B, Pavšič-Vrtač K, Tavčar-Kalcher G. Occurrence of ergot alkaloids in wheat from Albania. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2017;34:1333–1343. doi: 10.1080/19440049.2017.1307528. [DOI] [PubMed] [Google Scholar]
- Tunali B, Shelby R, Morgan-Jones G, Kodan M. Endophytie fungi and ergot alkaloids in native turkish grasses. Phytoparasitica. 2000;28:375–377. doi: 10.1007/BF02981832. [DOI] [Google Scholar]
- Urban J, Hamouz K, Lachman J, Pulkrábek J, Pazderů K. Effect of genotype, flesh colour and environment on the glycoalkaloid content in potato tubers from integrated agriculture. Plant, Soil and Environment. 2018;64:186–191. doi: 10.17221/166/2018-PSE. [DOI] [Google Scholar]
- Uysal UD, Aturki Z, Raggi MA, Fanall S. Separation of catechins and methylxanthines in tea samples by capillary electrochromatography. Journal of Separation Science. 2009;32:1002–1010. doi: 10.1002/jssc.200800634. [DOI] [PubMed] [Google Scholar]
- Vaněrková D, Marková L, Portychová L. Methodology for the safety assessment of lupin in terms of alkaloids content. In Horna A 14th International Nutrition & Diagnostics Conference 2014. pp. 7-82 (2014)
- Vermeulen P, Fernández Pierna JA, van Egmond HP, Dardenne P, Baeten V. Online detection and quantification of ergot bodies in cereals using near infrared hyperspectral imaging. Food Additives and Contaminants - Part a. 2012;29:232–240. doi: 10.1080/19440049.2011.627573. [DOI] [PubMed] [Google Scholar]
- Wagenvoort CA, Wagenvoort N, Dijk HJ. Effect of fulvine on pulmonary arteries and veins of the rat. Thorax. 1974;29:522–529. doi: 10.1136/thx.29.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li Y, Gao J, He Y, Xiong A, Yang L, Cheng X, Ma Y, Wang Z. The comparative pharmacokinetics of two pyrrolizidine alkaloids, senecionine and adonifoline, and their main metabolites in rats after intravenous and oral administration by UPLC/ESIMS. Analytical and Bioanalytical Chemistry. 2011;401:275–287. doi: 10.1007/s00216-011-5075-3. [DOI] [PubMed] [Google Scholar]
- Wang YP, Fu PP, Chou MW. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, retrorsine, leading to DNA adduct formation in vivo. International Journal of Environmental Research and Public Health. 2005;2:74–79. doi: 10.3390/ijerph2005010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskell L. A study of the mutagenicity of anesthetics and their metabolites. Mutation Research/fundamental and Molecular Mechanisms of Mutagenesis. 1978;57:141–153. doi: 10.1016/0027-5107(78)90261-0. [DOI] [PubMed] [Google Scholar]
- Wink M, Meibner C, Witte L. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry. 1995;38:139–153. doi: 10.1016/0031-9422(95)91890-D. [DOI] [Google Scholar]
- Winks M, Schmeller T, Latz-Brüning B. Modes of action of allelochemical alkaloids: interaction with neuroreceptors, DNA and other molecular targets. Journal of Chemical Ecology. 1998;24:1881–1937. doi: 10.1023/A:1022315802264. [DOI] [Google Scholar]
- Witte L, Ernst L, Adam H, Hartmannt T. Chemotypes of two pyrrolizidine alkaloid-containing Senecio species. Phytochemistry. 1992;31:559–565. doi: 10.1016/0031-9422(92)90038-R. [DOI] [Google Scholar]
- Witters WL, Wilms RA, Hood RD. Prenatal effects of elymoclavine administration and temperature stress. Journal of Animal Science. 1975;41:1700–1705. doi: 10.2527/jas1975.4161700x. [DOI] [PubMed] [Google Scholar]
- Yovo K, Huguet F, Pothier J, Durand M, Breteau M, Narcisse G. Comparative pharmacological study of sparteine and its ketonic derivative lupanine from seeds of Lupinus albus. Planta Medica. 1984;50:420–424. doi: 10.1055/s-2007-969753. [DOI] [PubMed] [Google Scholar]
- Yu X, Bao Z, Zou J, Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:1–11. doi: 10.1186/1471-2407-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariasova M, Cajka T, Godula M, Malachova A, Veprikova Z, Hajslova J. Analysis of multiple mycotoxins in beer employing (ultra)-high-resolution mass spectrometry. Rapid Communications in Mass Spectrometry. 2010;24:3357–3367. doi: 10.1002/rcm.4746. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang CR, Han YS, Wainberg MA, Yue JM. New Securinega alkaloids with anti-HIV activity from Flueggea virosa. RSC Advances. 2015;5:107045–107053. doi: 10.1039/C5RA22191A. [DOI] [Google Scholar]
- Zou J, Li N. Simple and environmental friendly procedure for the gas chromatographic-mass spectrometric determination of caffeine in beverages. Journal of Chromatography a. 2006;1136:106–110. doi: 10.1016/j.chroma.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Zündorf I, Wiedenfeld H, Röder E, Dingermann T. Generation and characterization of monoclonal antibodies against the pyrrolizidine alkaloid retrorsine. Planta Medica. 1998;64:259–263. doi: 10.1055/s-2006-957421. [DOI] [PubMed] [Google Scholar]