Abstract
Ischemic stroke in rodents is usually induced by intraluminal middle cerebral artery occlusion (MCAO) via the common carotid artery plugging filament invented by Koizumi et al. (MCAO-KM), or the external carotid artery plugging filament created by Zea Longa et al. (MCAO-LG). A systematic review of the distinctions between them is currently lacking. Here, we performed a meta-analysis in terms of model establishment, cerebral blood flow (CBF), and cerebral ischemia–reperfusion injury (CIRI) between them, Weighted Mean Differences and Standardized Mean Difference were used to analyze the combined effects, Cochrane's Q test and the I2 statistic were applied to determine heterogeneity, sensitivity analysis and subgroup analysis were performed to explore the source of heterogeneity. Literature mining suggests that MCAO-KM brings shorter operation time (p = 0.007), higher probability of plugging filament (p < 0.001) and molding establishment (p = 0.006), lower possibility of subarachnoid hemorrhage (p = 0.02), larger infarct volume (p = 0.003), severer brain edema (p = 0.002), and neurological deficits (p = 0.03). Nevertheless, MCAO-LG shows a more adequate CBF after ischemia–reperfusion (p < 0.001), a higher model survival rate (p = 0.02), and a greater infarct rate (p = 0.007). In conclusion, the MCAO-KM method is simple to operate with a high modeling success rate, and is suitable for the study of brain edema under long-term hypoperfusion, while the MCAO-LG method is highly challenging for novices, and is suitable for the study of CIRI caused by complete ischemia–reperfusion. These findings are expected to benefit the selection of intraluminal filament MCAO models before undertaking ischemic stroke preclinical effectiveness trials.
Subject terms: Diseases, Neurology, Pathogenesis
Introduction
Stroke is one of the leading causes of death and long-term disability across the world, a reliable stroke model is essential to produce an effective therapy for the destructive cerebrovascular accident1–3. Of note, about 87% of strokes in humans are ischemic strokes, and 70% of cerebral infarcts are caused by occlusion of the middle cerebral artery (MCA) and its branches4. The MCAO model conducted by intraluminal filament is considered as the most clinically relevant surgical model to mimic human ischemic stroke with the advantage of minor craniotomy trauma, stable controllability of reperfusion, as well as high reproducibility, etc5,6. This model was first reported by Jin-ichi Koizumi in 19867, and modified by Enrique Zea Longa in 19898, it has produced a profound influence on the research of ischemic stroke.
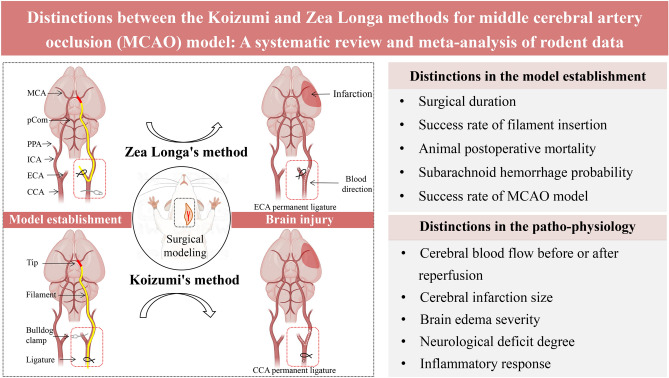
The main distinctions between Koizumig's method (MCAO-KM) and Zea Longa's method (MCAO-LG) reside in the route of filament insertion to the cerebral artery and, subsequently, the CBF resupply degree (Fig. 1). Specifically, for MCAO-LG, the occlusion of the MCA by filament is accomplished via inserting the external carotid artery (ECA), and the ischemic tissue is reperfused by bilateral common carotid arteries (CCA). But, for MCAO-KM, the filament-blocked MCA is achieved by plugging through the CCA, and the reperfusion is accomplished via contralateral CCA using the circle of Willis9.
Figure 1.
Distinctions in model establishment and patho-physiology between the Koizumi (MCAO-KM) and Zea Longa (MCAO-LG) methods for MCAO model. MCA middle cerebral artery, pCom posterior communicating artery, PPA pterygopalatine artery, ICA internal carotid artery, ECA external carotid artery, CCA common carotid artery.
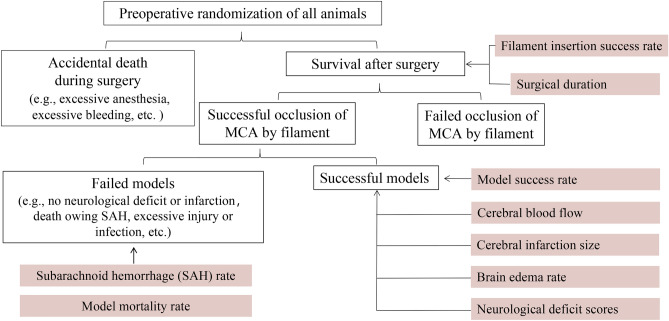
The rodent intraluminal filament model is frequently employed in the research of preclinical neuroprotective medicines, however, there are numerous parameters influencing modeling performance, which is a significant obstacle for novices (Fig. 2). In addition, some scholars have conducted comparative studies on the two methods, but the results of previous studies were rooted in a relatively small sample, and there is no relevant meta-analysis to systematically compare their distinctions. Methodically reviewing and meta-analysis of all relevant studies in an objective and quantitative manner provide us with much credible and solid evidence to demonstrate the unique characteristics of each method. Therefore, in this study, we conducted a meta-analysis to determine the distinctions of modeling establishment and brain injury between the two methods, aiming to provide a reference for practitioners in this field.
Figure 2.
Experimental flowchart for MCAO modeling. The success rate of filament insertion = Animals with filament successfully inserted into the skull and blocked the MCA/animals involved in filament insertion; Postoperative mortality rate = Model animals that died after surgery (animals that died during surgery are not included)/model animals with successful insertion filament; Subarachnoid hemorrhage rate = Model animals with postoperative subarachnoid hemorrhage/model animals with successful insertion filament; MCAO model success rate = Successful model animals/animals involved in surgical modeling (successful model animal must meet one of the following characteristics: (1) TTC staining or MRI arise a significant infarct, or (2) neurological defect score (Zea Longa score) is 1 to 3 marks, and did not sacrifice during the test period).
Methods
Search strategy and study inclusion
The review was registered on the PROSPERO database before initiation (registration number CRD42022331652). A comprehensive search strategy was conducted in Web of Science, PubMed, Chinese National Knowledge Infrastructure (CNKI) and Wanfang database from their inceptions to July 2022 with the search terms: (Zea Longa's method OR external carotid artery insertion OR bilateral common carotid reperfusion) AND (Koizumi's method OR common carotid artery insertion OR unilateral common carotid reperfusion) AND (intraluminal filament model OR middle cerebral artery occlusion OR focal cerebral ischemia).
We included articles if (1) animal models: MCAO model was built by intraluminal filament method, without the restriction of ischemia and reperfusion duration; (2) interventions: the route of filament into the brain (CCA insertion or ECA insertion), or a surgical method (Koizumi's method or Zea Longa's method), or a reperfusion mode (bilateral common carotid reperfusion or unilateral common carotid reperfusion); (3) Outcomes: operation duration, inserting filament success rate, SAH probability, model animal mortality rate and modeling success rate, cerebral infarction size, brain edema rate, neurological deficit score (NDS).
Data extraction and quality assessment
Two independent reviewers assessed related articles for eligibility, and extracted the following details: (1) document elements: first author and year of publication; (2) animal data: strains, sex, and weight (or age); (3) surgical details: anesthetic, ischemia duration, and reperfusion duration; (4) experimental outcomes: mean value, standard deviation, and sample size. WebPlotDigitizer (Version 4.4. 2020) was used for data extrapolation from graphs of published articles. In addition, any divergences were resolved by a senior author.
Quality of evidence in included studies was conducted based on a ten-item modified scale10,11: (a) peer-reviewed publication; (b) random allocation; (c) control of physiological parameter; (d) blinded conduct of the experiments; (e) blinded assessment of outcome; (f) use of anesthetic without significant neuroprotective activity; (g) co-morbidities (aged, diabetic and hypertensive etc.); (h) sample size calculation; (i) compliance with animal welfare regulations; (j) statement of potential conflict of interests. Among them, items (b), (c), (d), (e), (f), (g) and (h) may directly affect the model success rate.
Statistical analysis
RevMan (version 5.4) was used for meta-analysis. Relative risk (RR) was estimated for all dichotomous variables. The weighted mean differences (WMD/MD) was calculated as a summary statistic if the continuous-type variable outcomes adopted the same scale, and the standardized mean difference (SMD) was used if the indexes were measured by various methods or techniques. Heterogeneity between studies was assessed via a standard chi-square test and I2 statistic, and p ≥ 0.01, I2 ≤ 50% and p < 0.01, I2 > 50% are considered low and high heterogeneity, respectively. If no statistical evidence of heterogeneity existed, the fixed effect (FE) model was performed with a 95% confidence interval (CI); if statistical heterogeneity was found, the sensitivity analysis and subgroup analysis were used. Statistical significance for all analyses was considered if p < 0.05. Egger’s tests were employed to detect publication bias, which was completed by Stata (version 15.1).
Results
Study search and selection
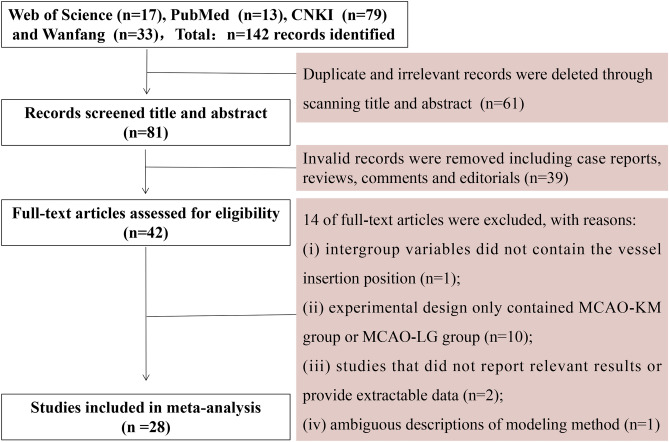
After an initial search from English databases (Web of Science and PubMed) and Chinese databases (CNKI and Wanfang), a total of 142 potentially eligible studies were identified, and 61 studies were ruled out due to duplication and irrelevance. Further, thirty-nine studies were excluded owing to invalid records, and 14 studies were removed from the remaining 42 full-text articles post careful investigation. Ultimately, a total of 28 studies were included in the systematic meta-analysis (Fig. 3).
Figure 3.
Flowchart of literature search.
Study characteristics
All the experimental animals were male-dominated rodents, except for one study sexes in half. Isoflurane was frequently used as anesthetic, others, including ketamine, pentobarbital sodium and fentanyl, etc. The treatment and plugging depth of the filament were reported in almost all literature. Cerebral ischemic injury in the included studies was induced by transient MCAO (tMCAO) or permanent MCAO (pMCAO). The characteristics of the included studies are provided in Table 1.
Table 1.
Characteristics of the included study.
| Study (Author, years) | Rodents | Anesthetic | Filament | Ischaemia time and hemisphere | Measurement indicators |
|---|---|---|---|---|---|
| Laing, R.J. 199316 | Wistar rats (no report, 300–400 g) | Fentanyl citrate + fluanisone + midazolam |
1. Processing: tip coated with silicone (MCAO-KM)/tip burnt blunt round (MCAO-LG) 2. Diameter: no report 3. Insertion depth: 17–20 mm |
No report, right |
1. CBF 2. Success rate of filament insertion |
| Qu Qiumin 200034 | SD rats (male, 280–320 g) | Halothane + nitrous oxide |
1. Processing: no report 2. Diameter: no report 3. Insertion depth: no report |
2 h, left |
1. Model success rate 2. Brain edema rate 3. Infarction rate |
| Cao Yongjun 200137 | Wistar rats (male, 200–250 g) | Chloral hydrate |
1. Processing: tip burnt blunt round 2. Diameter: 4–0 nylon thread (0.2 mm) 3. Insertion depth: about 19.5–20.5 mm |
6 h, right |
1. rCBF 2. NDS (Zea Longa score) 3. Infarction size |
|
Xi Gangming 2004 |
Kunming mice (Male, 28–42 g) |
Chloral hydrate |
1. Processing: tip burnt blunt round and coated with polylysine 2. Diameter: 0.078–0.1 mm 3. Insertion depth: 8–10 mm |
Permanent, right |
1. Physiological parameters 2. Model mortality 3. Neurological scores 4. Infarction volume |
| Sun Guobing 200638 | SD rats (male, 250–300 g) | Chloral hydrate |
1. Processing: tip burnt blunt round 2. Diameter: 4–0 nylon thread 3. Insertion depth: about 18 mm |
2 h, right |
1. Infarction rate 2. NF-κB expression |
| Johannes Woitzik 200635 | SD rats (male, 290–350 g) | Isoflurane |
1. Processing: tip burnt blunt round (MCAO-LG) or coated with silicone (MCAO-KM) 2. Diameter: 5–0 polyamide filament (0.445–0.455 mm) 3. Insertion depth: 19 mm (based on CBF signals) |
Permanent, right |
1. Physiological parameters 2. CBF 3. Infarction rate 4. Brain edema rate 5. NDS |
| Hiroaki Sakai 200740 | Wistar rats (male, 10 weeks-age) | Isoflurane |
1. Processing: tip coated with silicone 2. Diameter: 0.38 mm 3. Insertion depth: 19–20 mm |
50 min, right |
1. Infarction volume 2. NDS |
| Cam Ertugrul 200819 | Wistar rats (male, 285–370 g) | Isoflurane |
1. Processing: abrading the tip with sandpaper 2. Diameter: 0.23 mm 3. Insertion depth: 18 mm |
Permanent, left |
1. Physiological parameters 2. CBF 3. Model mortality 4. Model success rate 5. Subarachnoid hemorrhage rate 6. Infarction volume |
| Sun Yu 200812 | SD rats (male, 240–280 g) | Chloral hydrate |
1. Processing: tip burnt blunt round 2. Diameter: 4–0 filaments (0.22 mm) 3. Insertion depth: 19–20 mm |
2 h, right |
1. Operation duration 2. Model success rate 3. Model mortality 4. Infarction rate 5. NDS (Zea Longa score) 6. HE staining |
| Xiang Heng 200820 | SD rats (male, 300–350 g) | Chloral hydrate |
1. Processing: tip coated with silicone 2. Diameter: 0.26–0.30 mm 3. Insertion depth: a feel of slight resistance |
Permanent, left |
1. Model success rate 2. Model mortality 3. Infarction rate 4. NDS (Zea Longa score) |
| Yang Zanzhang 200821 | Wistar rats (sex in half, 260–300 g) | Pentobarbital sodium |
1. Processing: tip burnt blunt round 2. Diameter: fishing thread (0.235 mm) 3. Insertion depth: about 18 mm (a feel of slight resistance) |
Permanent, left |
1. Model success rate 2. Infarction rate 3. NDS (Zea Longa score) |
| Yang Debing 200917 | Wistar rats (male, 230–280 g) | No report |
1. Processing: no report 2. Diameter: no report 3. Insertion depth: a feel of slight resistance |
2 h, right |
1. Model success rate 2. Model mortality 3. NDS (Zea Longa score) |
| Trueman, R, C 201122 | Wistar rats (male, 10–12 weeks-age) | Isoflurane |
1. Processing: tip coated with silicone 2. Diameter: 0.39–0.41 μm 3. Insertion depth: depending on changes of CBF |
1 h, right |
1. Model success rate 2. Model mortality 3. CBF 4. Infarction rate 5. Body weight change 6. Behavioral testing 7. Survival rate of neurons |
| Liu Jianren 201223 | SD rats (male, 200–250 g) | Chloral hydrate |
1. Processing: tip coated with silicone 2. Diameter: no report 3. Insertion depth: no report |
1.5 h, right |
1. Physiological parameters 2. CBF 3. Infarction volume 4. Brain edema rate 5. NDS (Berdeson/Garcia score) |
| Zhao Kai 201232 | Wistar rats (male, 280–300 g) | Chloral hydrate |
1. Processing: tip burnt blunt round or cut into a flat end 2. Diameter: 0.26 mm 3.insertion depth: 18–20 mm (a feel of slight resistance) |
100 min, right |
1. Model success rate 2. NDS (Zea Longa score) 3. Infarction rate 4. HE staining |
| Tang Qiqiang 201333 | SD rats (male, 250–300 g) | Chloral hydrate |
1. Processing: tip coated with silicone 2. Diameter: 0.21–0.22 mm or 0.27–0.28 mm or 0.28–0.29 mm; 3. Insertion depth: 16–20 mm |
Permanent, right |
1. Physiological parameters 2. Success rate of filament insertion 3. NDS (Zea Longa score) 4. Infarction rate 5. Brain edema rate 6. HE staining |
| Fan Ruijuan 201414 | SD rats (male, 250–300 g) | Chloral hydrate |
1. Processing: tip coated with polylysine 2. Diameter: 0.34–0.38 mm 3. Insertion depth: 19–20 mm (A feel of slight resistance) |
Transient (no ischaemia time), right |
1. Operation duration 2. Success rate of filament insertion 3. Model success rate 4. Model mortality 5. Subarachnoid hemorrhage rate |
| Morris, G.P. 201515 | C57BL/6 mice (male, 8–13 weeks-age) | Ketamine + xylazine |
1. Processing: silicone coated for 9–10 mm (MCAO-KM)) or 2–3 mm (MCAO-LG) 2. Diameter: 0.19–0.23 mm 3. Insertion depth: depending on changes of CBF |
1 h, right |
1. CBF 2. Infarction rate 3. Body weight change |
| Smith, H.K. 201524 | C57BL/6 mice (male, 25–29 g) | Ketamine/xylazine |
1. Processing: tip coated with silicone 2. Diameter: 0.18 mm 3. Insertion depth: a feel of slight resistance |
0.5 h, no report |
1. CBF 2. Model mortality 3. Infarction rate 4. NDS (mNSS score) 5. Inflammation |
| Zheng Jianfeng 201625 | SD rats (male, 280–320 g) | Pentobarbital sodium |
1. Processing: tip coated with polylysine 2. Diameter: 0.26–0.28 mm 3. Insertion depth: 18–20 mm |
2 h/permanent, right |
1. Model mortality 2. Infarction rate 3. NDS (Zea Longa score) |
| Zuo Xialin 201326 | SD rats (male, 250–280 g) | Chloral hydrate |
1. Processing: tip coated with solid paraffin; 2. Diameter: 0.28 mm 3. Insertion depth: about 18 mm |
1 h, left |
1. Model mortality 2. Infarction rate 3. BBB integrity 4. NDS (Zea Longa score) 5. BDNF expression |
| Cai Qiang 201613 | C57BL/6 mice (male, 20–25 g) | Isoflurane |
1. Processing: tip coated with silicone 2. Diameter: 0.21–0.22 mm 3. Insertion depth: a feel of slight resistance |
1 h, right |
1. CBF 2. Operation duration 3. Model mortality 4. Infarction rate 5. NDS (Zea Longa score) |
| Melissa, T.L. 201736 | C57BL/6 mice (male, 24–31 g) | Isoflurane |
1. Processing: tip coated with silicone 2. Diameter: 0.18–0.20 mm 3. Insertion depth: depending on changes of CBF |
1 h, right |
1. rCBF 2. Infarction volume |
| Wang Dongliang 201927 | C57BL/6 mice (male, 20–22 g) | Isoflurane |
1. Processing: tip coated with silicone 2. Diameter: no report 3. Insertion depth: 8–12 mm or a feel of slight resistance |
1 h, right |
1. Model mortality 2. Model success rate 3. Infarction rate 4. Brain water content 5. Neuronal apoptosis rate |
| Onufriev, M.V. 202128 | Wistar rats (male, 200–300 g) | Isoflurane |
1. Processing: tip burnt blunt round 2. Diameter: 3–0 nylon monofilament 3. Insertion depth: no report |
1 h, left |
1. Model mortality 2. NDS 3. Infarction rate 4. Inflammation |
| Wang Wenxiu 202229 | SD rats (male, 250–300 g) | Chloral hydrate |
1. Processing: tip burnt blunt round and coated with polylysine 2. Diameter: 0.34–0.38 mm 3. Insertion depth: 21–22 mm |
2 h, right |
1. Success rate of filament insertion 2. Model mortality 3. NDS (Zea Longa score) |
| Yang Zhong 202230 | C57Bl6 mice (male, 8–10 weeks-age) | Isoflurane |
1. Processing: no report 2. Diameter: no report 3. Insertion depth: no report |
1.5 h, right |
1. CBF 2. NDS 3. Infarction volume 4. Body weight change 5. Inflammation 6. Neuronal apoptosis rate |
| Helena, J. 202231 | C57Bl6 mice (male, 3–6 months old) | Isoflurane |
1. Processing: tip coated with silicone 2. Diameter: 0.19–0.21 mm 3. Insertion depth: depending on changes of CBF |
0.5 h, left |
1. CBF 2. Infarction volume 3. Body weight change 4. NDS |
BDNF brain derived neurotrophic factor, CBF cerebral blood flow, NDS neurological deficit score, BBB blood–brain barrier, HE staining hematoxylin and eosin staining.
The comparison of model establishment, CBF and ischemic brain injury was the focus of our study. Among them, the success rate of plugging filament, incidence of SAH, model mortality rate and model success rate were analyzed as dichotomous variables. While, the surgical operation duration, cerebral infarction size, brain edema rate and neurological deficit score were analyzed as continuous variables.
Quality of included studies
Study quality scores for 28 studies counted in this meta-analysis were summarized in Table 2. The quality scores varied from 3 to 8 with an average of 5.46. All the studies were peer-reviewed publications. Twenty-four studies declared randomization of group allocation, and 21 studies described the monitoring of physiological parameters. Besides, one study masked the details of experimental designs, and 14 studies reported a blinded assessment of the outcome. Thirteen studies avoided the use of anesthetics with marked neuroprotective properties. None of the studies reported the application of co-morbidities in animals. Twenty-five studies reported sample size calculation. Among all of them, 18 studies stated compliance with animal welfare regulations, and 9 studies addressed the conflict of interests.
Table 2.
Quality evaluation of included studies.
| Year | Lead author | A | B | C | D | E | F | G | H | I | J | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | Laing, R.J.16 | + | + | + | 3 | |||||||
| 2000 | Qu Qiumin34 | + | + | + | 3 | |||||||
| 2001 | Cao Yongjun37 | + | + | + | + | + | 5 | |||||
| 2004 | Xi Gangming18 | + | + | + | + | + | 5 | |||||
| 2006 | Sun Guobing38 | + | + | + | + | 4 | ||||||
| 2006 | Johannes Woitzik35 | + | + | + | + | + | + | 6 | ||||
| 2007 | Hiroaki Sakai40 | + | + | + | + | + | + | 6 | ||||
| 2008 | Cam Ertugrul19 | + | + | + | + | + | 5 | |||||
| 2008 | Sun Yu12 | + | + | + | + | 4 | ||||||
| 2008 | Xiang Heng20 | + | + | + | + | + | 5 | |||||
| 2008 | Yang Zanzhang21 | + | + | + | + | + | 5 | |||||
| 2009 | Yang Debing17 | + | + | + | 3 | |||||||
| 2011 | Trueman, R, C.22 | + | + | + | + | + | + | + | 7 | |||
| 2012 | Liu Jianren23 | + | + | + | + | + | + | + | + | 8 | ||
| 2012 | Zhao Kai32 | + | + | + | + | 4 | ||||||
| 2013 | Tang Qiqiang33 | + | + | + | + | + | + | + | 7 | |||
| 2014 | Fan Ruijuan14 | + | + | + | + | + | 5 | |||||
| 2015 | Morris, G.P.15 | + | + | + | + | + | + | + | 7 | |||
| 2015 | Smith, H.K.24 | + | + | + | + | 4 | ||||||
| 2016 | Zheng Jianfeng25 | + | + | + | + | + | + | 6 | ||||
| 2016 | Zuo Xialin26 | + | + | + | + | + | + | + | 7 | |||
| 2016 | Cai Qiang13 | + | + | + | + | + | 5 | |||||
| 2017 | Melissa, T.L.36 | + | + | + | + | + | + | + | 7 | |||
| 2019 | Wang Dongliang27 | + | + | + | + | + | 5 | |||||
| 2021 | Onufriev, M.V.28 | + | + | + | + | + | + | + | 7 | |||
| 2022 | Wang Wenxiu29 | + | + | + | + | + | + | 6 | ||||
| 2022 | Yang Zhong30 | + | + | + | + | + | + | + | 7 | |||
| 2022 | Helena, Justic31 | + | + | + | + | + | + | + | 7 |
Study quality items are A, Peer-reviewed publication; B, Random grouping; C, Monitoring of physiological parameters (e.g. temperature, blood pressure, gases); D, Blinded conduct of ischemia; E, Blinded assessment of outcomes; F, Use of anesthetic without marked intrinsic neuroprotective properties (e.g., isoflurane, ketamine, halothane); G, Animal with co-morbidities (e.g., diabetic, aged, or hypertensive); H, Sample size calculation; I, Statement of compliance with animal welfare regulations; J, Statement of potential conflict of interests.
Comparisons between the two methods in model establishment
Distinction on operation duration
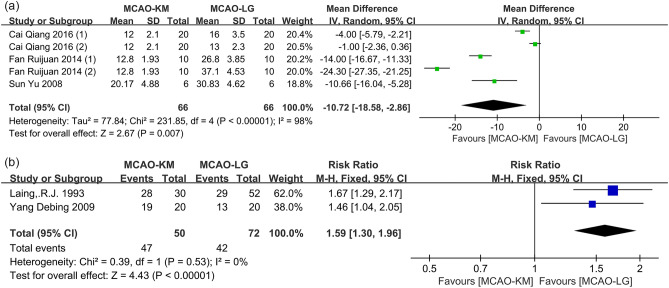
Four studies12–15 reported the duration of surgical operation, and one15 was excluded due to the absence of an extractable date. Overall, the operation duration of MCAO-KM was significantly shorter than that of MCAO-LG (MD = − 10.72, 95% CI [− 18.58, − 2.86], p = 0.007, heterogeneity I2 = 98%, p < 0.00001) (Fig. 4a). The separation and ligation of the pterygopalatine artery (PPA), as well as the surgical proficiency, may be important sources of heterogeneity. Of note, the study15 also pointed out that the operation time of MCAO-KM is shorter than MCAO-LG, which is consistent with the pooled MD estimation.
Figure 4.
Comparison of (a) operation duration and (b) success rate of filament insertion between the MCAO-KM and MCAO-LG.
Distinction on probability of plugging filament
The success rates of filament insertion were reported in three studies14,16,17. Overall, the MCAO-KM shows a higher probability of plugging filament than MCAO-LG (RR = 1.43, 95% CI [1.20, 1.70], p < 0.0001, heterogeneity I2 = 84%, p = 0.002) (Supplementary Fig. 1), and one study14 should be the source of heterogeneity through sensitivity analyses. After the removal of this study, the pooled result between the two methods remained similar and the heterogeneity was decreased (RR = 1.59, 95% CI [1.30, 1.96], p < 0.00001, heterogeneity I2 = 0%, p = 0.53) (Fig. 4b).
Distinction on postoperative mortality
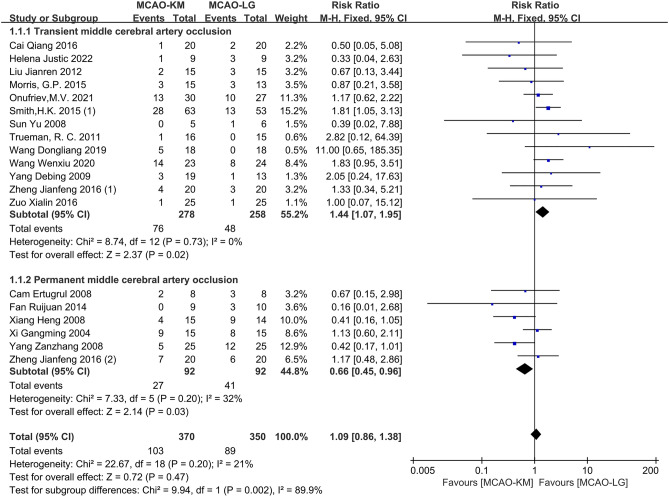
Nineteen studies12–15,17–31 reported rodent mortality after plugging filament, and one study30 was excluded due to a lack of extractable data. Overall, and, no significant difference between the two methods was observed (RR = 1.09, 95% CI [0.86, 1.38], p = 0.47, heterogeneity I2 = 21%, p = 0.20) (Fig. 5). Of note, sub-analyses show that modeling animals induced by MCAO-KM exhibited higher mortality than that by MCAO-LG in the tMCAO subgroup (RR = 1.44, 95% CI [1.07, 1.95], p = 0.02, heterogeneity I2 = 0%, p = 0.73), whereas, the opposite result was found in the pMCAO subgroup (RR = 0.66, 95% CI [0.45, 0.96], p = 0.03, heterogeneity I2 = 32%, p = 0.20). The p-value of Egger's regression test in the tMCAO subgroup was 0.291, indicating no significant publication bias (Supplementary Fig. 2).
Figure 5.
Comparison of postoperative mortality between MCAO-KM and MCAO-LG.
Distinction on occurence of SAH
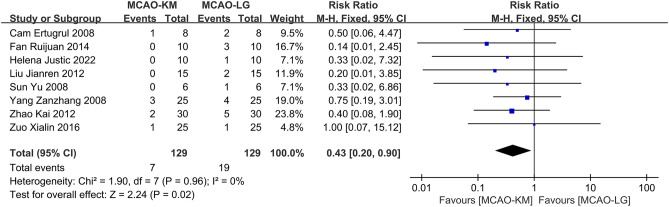
Nine studies12,14,19,21,23,26,31–33 reported the occurrence of SAH after the operation, and one study33 was excluded due to a lack of detailed data. Overall, the meta-analysis results indicate that the modeling method of MCAO-KM causes a lower probability of SAH compared to the MCAO-LG with no substantial heterogeneity (RR = 0.43, 95% CI [0.20, 0.90], p = 0.02, heterogeneity I2 = 0%, p = 0.96) (Fig. 6).
Figure 6.
Comparison of postoperative SAH between MCAO-KM and MCAO-LG.
Distinction on success rate of MCAO models
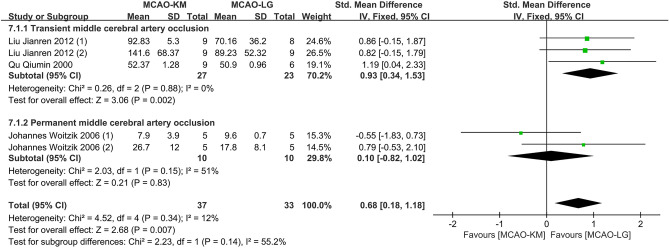
The success rates of MCAO models were assessed in 16 studies12,14,15,17,19–23,25,27–29,32–34. Overall, the MCAO-KM brings a statistically higher probability of model success than the MCAO-LG (RR = 1.14, 95% CI [1.03, 1.27], p = 0.01, heterogeneity I2 = 45%, p = 0.02). Subgroup analyses suggest that the MCAO-KM supports higher model success rate in the pMCAO subgroup (RR = 1.34, 95% CI [1.09, 1.66], p = 0.006, heterogeneity I2 = 0%, p = 0.51), but not tMCAO (RR = 1.06, 95% CI [0.94, 1.20], p = 0.33, heterogeneity I2 = 46%, p = 0.04) (Supplementary Fig. 3a).
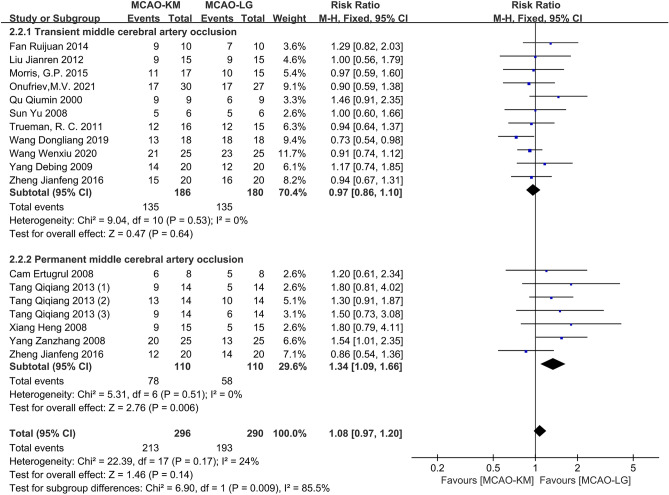
Sensitivity analysis of the tMCAO subgroups revealed one study32 may be the source of heterogeneity. After deletion of this study, no significant difference between the two methods was found in overall effect (RR = 1.08, 95% CI [0.97, 1.20], p = 0.14, heterogeneity I2 = 24%, p = 0.17) as well as tMCAO subgroup (RR = 0.97, 95% CI [0.86, 1.10], p = 0.64, heterogeneity I2 = 0%, p = 0.53). However, the MCAO-KM method's success rate remains higher in the pMCAO subgroup (Fig. 7). The p-value of Egger's regression test in the tMCAO subgroup was 0.089, indicating no significant publication bias (Supplementary Fig. 3b,c).
Figure 7.
Comparison of modeling success rates between MCAO-KM and MCAO-LG.
Comparisons between the two methods in CBF and brain injury
Distinction on CBF after ischemia or ischemia–reperfusion
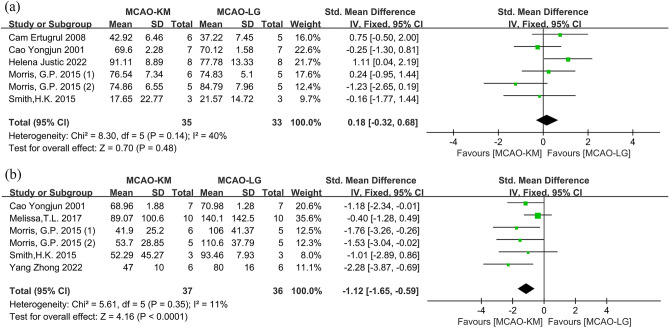
Eight studies13,15,24,30,31,35–37 with 9 comparisons were applied to evaluate CBF after cerebral ischemia or ischemia–reperfusion between MCAO-KM and MCAO-LG, and one study 13 was excluded due to lack of sample size, and another study35 was also excluded because it did not reperfuse by removing the filament (Table 3). Overall, there was no significant difference in CBF between the two methods during ischemia (SMD = 0.18; 95% CI [− 0.32, 0.68], p = 0.48; heterogeneity I2 = 40%, p = 0.14) (Fig. 8a). Interestingly, after reperfusion, the CBF achieved by MCAO-KM was dramatically lower than that by MCAO-LG (SMD = − 1.34; 95% CI [− 1.85, − 0.83], p < 0.00001; heterogeneity I2 = 60%, p = 0.02) (Supplementary Fig. 4). After the exclusion of one study31 as a potential source of heterogeneity, the CBF outcomes between the two methods remained similar and the heterogeneity was significantly reduced (SMD = − 1.12; 95% CI [− 1.65, − 0.59], p < 0.0001; heterogeneity I2 = 11%, p = 0.35) (Fig. 8b).
Table 3.
Details of cerebral blood flow test.
| Lead author | Year | Test method | Ischemia duration | Test time |
|---|---|---|---|---|
| Cai Qiang13 | 2016 | Laser Doppler flowmetry | 1 h | 5 min after reperfusion |
| Cam Ertugrul19 | 2008 | Laser Doppler flowmetry | Permanent | 20 min after occlusion |
| Cao Yongjun37 | 2001 | Hydrogen clearance method | 6 h | 0.5 h/1 h/2 h after reperfusion |
| Melissa, T.L.36 | 2017 | Laser Doppler flowmetry | 1 h | 5 min after reperfusion |
| Helena Justic31 | 2022 | Laser Doppler flowmetry | 0.5 h | No report |
| Johannes Woitzik35 | 2006 | Laser Doppler flowmetry | Permanent | 240 min after occlusion |
| Morris, G.P.15 (thin filament) | 2015 | Laser Doppler flowmetry | 1 h | 4 h after reperfusion |
| Morris,.G.P.15 (thick filament) | 2016 | Laser Doppler flowmetry | 1 h | 4 h after reperfusion |
| Smith, H.K.24 | 2015 | Laser Doppler flowmetry | 0.5 h | 5 min after reperfusion |
| Yang Zhong30 | 2022 | Laser Doppler flowmetry | 1.5 h | 10 min after reperfusion |
Figure 8.
Comparison of CBF between MCAO-KM and MCAO-LG after (a) cerebral ischemia and (b) ischemia–reperfusion.
Distinction on cerebral infarction size
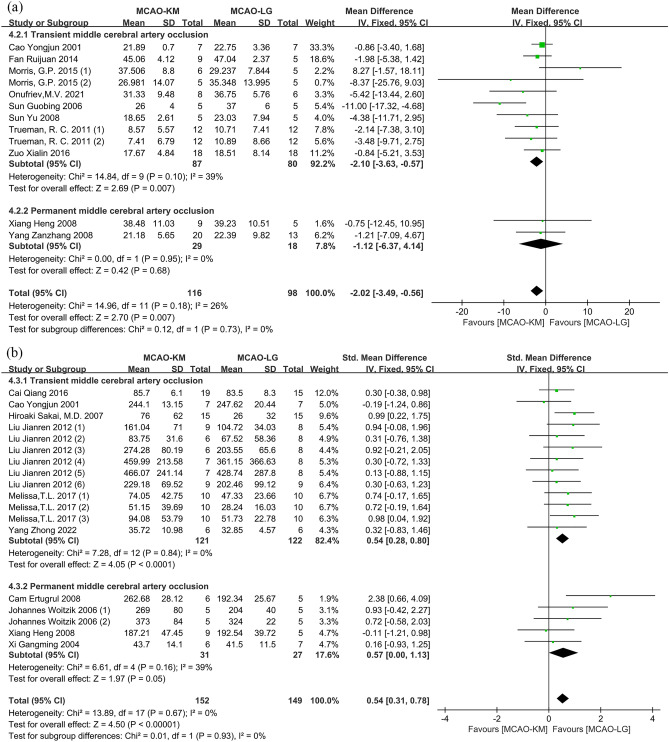
Eleven studies12,14,15,21,24,26–28,37–39 evaluated the cerebral infarction rates between two modeling methods (Table 4), and one study27 was excluded due to lack of sample size. Overall, the cerebral infarction rates of the MCAO-KM were markedly lower than the MCAO-LG (MD = − 3.37; 95% CI [− 4.71, − 2.04], p < 0.00001; heterogeneity I2 = 82%, p < 0.00001). And sub-analyses suggest that the MCAO-KM gets low cerebral infarction rates in the tMCAO subgroup, but not in the pMCAO subgroup (MD = − 3.53, 95% CI [− 4.91, − 2.15], p < 0.00001, heterogeneity I2 = 84%, p < 0.00001; and MD = − 1.12; 95% CI [− 6.37, 4.14]; p = 0.68 heterogeneity I2 = 0%, p = 0.95, respectively) (Supplementary Fig. 5a). After the removal of one study24, the distinction of cerebral infarction rates remained significant between the two methods, and the heterogeneity of the overall effect as well as the tMCAO subgroup was eliminated (SMD = − 2.10, 95% CI [− 3.63, − 0.57], p = 0.007, heterogeneity I2 = 39%, p = 0.10 in tMCAO subgroup; and SMD = − 2.02, 95% CI [− 3.49, − 0.56], p = 0.007, heterogeneity I2 = 26%, p = 0.18 in overall effect) (Fig. 9a). The p-value of Egger's regression test in the tMCAO subgroup was 0.390, verifying no significant publication bias (Supplementary Fig. 5b,c).
Table 4.
Detailed table of cerebral infarction rates test.
| Lead author | Year | Test method | Ischemia duration | Reperfusion duration |
|---|---|---|---|---|
| Cao Yongjun37 | 2001 | TTC staining | 6 h | 18 h |
| Fan Ruijuan14 | 2014 | TTC staining | 20 min | 1 day |
| Morris, G.P.15 (thin filament) | 2015 | TTC staining | 1 h | 4 h |
| Morris, G.P.15 (thick filament) | 2015 | TTC staining | 1 h | 4 h |
| Onufriev, M.V.28 | 2021 | TTC staining | 1 h | 3 days |
| Smith, H.K.24 (1) | 2015 | TTC staining | 0.5 h | 1 day |
| Smith, H.K.24 (2) | 2015 | TTC staining | 0.5 h | 7 days |
| Sun Guobing38 | 2006 | TTC staining | 2 h | 1 day |
| Sun Yu12 | 2008 | TTC staining | 2 h | 1 day |
| Trueman, R. C.22 | 2011 | Magnetic resonance imaging | 1 h | 1 day |
| Trueman, R. C.22 | 2011 | Magnetic resonance imaging | 1 h | 1 day |
| Wang Dongliang27 | 2019 | TTC staining | 1 h | 1 day |
| Zuo Xialin26 | 2016 | TTC staining | 1 h | 1 day |
| Xiang Heng20 | 2008 | TTC staining | Permanent | 7 days |
| Yang Zanzhang21 | 2008 | TTC staining | Permanent | 1 day |
Figure 9.
Comparison of cerebral infarction size between MCAO-KM and MCAO-LG split by (a) infarction rate and (b) lesion volume.
Twelve studies13,18–20,23,25,30,31,35–37,40 evaluated the distinction of cerebral lesion volumes between the two modeling methods (Table 5). Meta-analysis suggests that the average cerebral lesion volumes of the MCAO-KM rodents were higher than the MCAO-LG rodents (SMD = 0.68; 95% CI [0.46, 0.91], p < 0.00001; heterogeneity I2 = 59%, p = 0.003). And sub-analyses suggest that the MCAO-KM animals show a higher average cerebral infarction volume than MCAO-LG in both tMCAO and pMCAO subgroups (SMD = 0.61; 95% CI [0.36, 0.86], p < 0.00001; heterogeneity I2 = 57%, p = 0.003; and SMD = 0.96, 95% CI [0.47, 1.45], p = 0.0001, heterogeneity I2 = 66%, p = 0.001, respectively) (Supplementary Fig. 6a). Sensitivity analyses pointed out that two studies25,31 should be the source of heterogeneity. After removing these studies, the heterogeneity was significantly reduced. Sub-analyses suggest that the MCAO-KM animals showed a higher average cerebral lesion volume in tMCAO but not pMCAO subgroup (SMD = 0.54; 95% CI [0.28, 0.80]; p < 0.0001, heterogeneity I2 = 0%, p = 0.84 in tMCAO; SMD = 0.57, 95%CI [0.00, 1.13], p = 0.05, heterogeneity I2 = 39%, p = 0.16 in pMCAO; and SMD = 0.54, 95%CI [0.31, 0.78], p < 0.00001, heterogeneity I2 = 0%, p = 0.67 in overall effect) (Fig. 9b). The p-value of Egger's regression test in the tMCAO subgroup was 0.679, indicating no significant publication bias (Supplementary Fig. 6b,c).
Table 5.
Detailed table of cerebral infarction volume test.
| Lead author | Year | Test method | Ischemia duration | Reperfusion duration |
|---|---|---|---|---|
| Cai Qiang13 | 2016 | TTC staining | 1 h | 7 days |
| Cao Yongjun37 | 2001 | TTC staining | 6 h | 18 h |
| Helena Justic31 | 2022 | Magnetic resonance imaging | 0.5 h | 2 days |
| Hiroaki Sakai40 | 2007 | Succinate dehydrogenase staining | 50 min | 14 days |
| Liu Jianren (1)23 | 2012 | T2-weighted MRI | 1.5 h | 1 day |
| Liu Jianren (2)23 | 2012 | Diffusion weighted imaging | 1.5 h | 1 h |
| Liu Jianren (3)23 | 2012 | Diffusion weighted imaging | 1.5 h | 1 day |
| Liu Jianren (4)23 | 2012 | Perfusion-weighted imaging | 1.5 h | 1 h |
| Liu Jianren (5)23 | 2012 | Perfusion-weighted imaging | 1.5 h | 1 day |
| Liu Jianren (6)23 | 2012 | TTC staining | 1.5 h | 1 day |
| Melissa, T.L. (1)36 | 2017 | T2-weighted MRI | 1 h | 2 days |
| Melissa, T.L. (2)36 | 2017 | Diffusion weighted imaging | 1 h | 2 days |
| Melissa, T.L. (3)36 | 2017 | TTC staining | 1 h | 2 days |
| Yang Zhong30 | 2022 | TTC staining | 1.5 h | 28 days |
| Zheng Jianfeng (1)25 | 2016 | TTC staining | 2 h | 1 day |
| Cam Ertugrul19 | 2008 | Toluidine blue staining | Permanent | 1 day |
| Johannes Woitzik (1)35 | 2006 | TTC staining | Permanent | 8 h |
| Johannes Woitzik (2)35 | 2006 | TTC staining | Permanent | 1 day |
| Xiang Heng20 | 2008 | TTC staining | Permanent | 7 days |
| Xi Gangming18 | 2004 | TTC staining | Permanent | 7 days |
| Zheng Jianfeng (2)25 | 2016 | TTC staining | Permanent | 1 day |
Distinction on brain edema
The assessments of the brain edema rates were performed in six studies23,27,31,33–35, and two studies27,33 were excluded due to a lack of detailed data (Table 6). Overall, the MCAO-KM animals produced severer brain edema compared to the MCAO-LG with marked heterogeneity (SMD = 0.53, 95% CI [0.03, 1.02], p = 0.04, heterogeneity I2 = 71%, p = 0.005) (Supplementary Fig. 7). Subgroup analysis indicates that significant differences occur in the tMCAO but not in the pMCAO subgroup (SMD = 0.70, 95% CI [0.12, 1.28], p = 0.02, heterogeneity I2 = 78%, p = 0.003, and SMD = 0.10, 95% CI [− 0.82, 1.02], p = 0.83, heterogeneity I2 = 51%, p = 0.15, respectively). Sensitivity analysis uncovered that one study31 is a potential source of heterogeneity. After the removal of this study, the pooled estimation of brain edema between the two MCAO animals remained similar and the heterogeneity was eliminated (SMD = 0.93, 95% CI [0.34, 1.53], p = 0.002, heterogeneity I2 = 0%, p = 0.88 in tMCAO subgroup; SMD = 0.10, 95% CI [− 0.82, 1.02], p = 0.83, heterogeneity I2 = 51%, p = 0.15 in pMCAO subgroup; and SMD = 0.68, 95% CI [0.18, 1.18], p = 0.007, heterogeneity I2 = 12%, p = 0.34 in overall effect) (Fig. 10).
Table 6.
Detailed table of brain edema test.
| Lead author | Year | Ischemia duration | Reperfusion duration | Test method |
|---|---|---|---|---|
| Liu Jianren (1)23 | 2012 | 1.5 h | 1 day | T2-weighted MRI |
| Liu Jianren (2)23 | 2012 | 1.5 h | 1 day | TTC staining |
| Qu Qiumin34 | 2000 | 2 h | 2 days | TTC staining |
| Johannes Woitzik (1)35 | 2006 | Permanent | 8 h | TTC staining |
| Johannes Woitzik (2)35 | 2006 | Permanent | 1 day | TTC staining |
| Helena Justic31 | 2022 | 0.5 h | 2 days | Magnetic resonance imaging |
Figure 10.
Comparison of brain edema in rodents between MCAO-KM and MCAO-LG.
Distinctions on neurological deficit scores
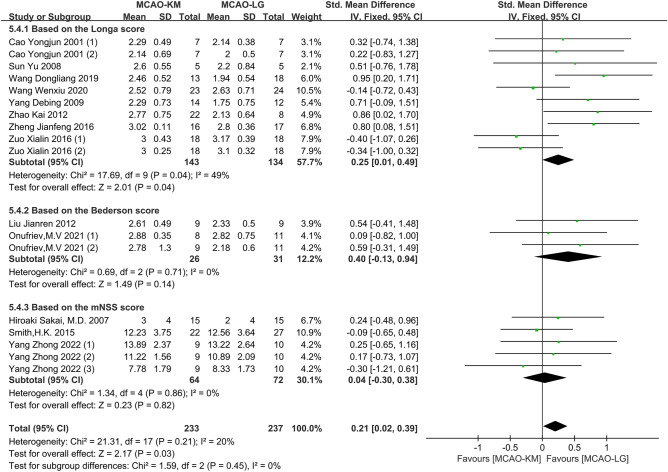
Nineteen studies evaluated neurological deficits via Zea Longa score12,13,17,25–27,29,32,33,35,37, Bederson score23,28, modified neurological severity scores (mNSS) score24,30,40, and Garcia score14,23,31 (Table 7). Among them, three articles13,33,35 were excluded due to the absence of available data. Of note, the higher the the Garcia score, the better the neurobehavior, which is contrary to other scoring methods, thus, these studies using Garcia score were not pooled into the sub-analysis (Supplementary Fig. 8). Overall, the average neurological deficit scores of the MCAO-KM animals were significantly higher than that of MCAO-LG animals without heterogeneity (SMD = 0.21, 95% CI [0.02, 0.39], p = 0.03, heterogeneity I2 = 20%, p = 0.21) (Fig. 11).
Table 7.
Detailed table of neurological deficit scores.
| Lead author | Year | Ischemia duration | Reperfusion duration | Scoring method |
|---|---|---|---|---|
| Cao Yongjun (1)37 | 2001 | 6 h | 6 h | Zea Longa |
| Cao Yongjun (2)37 | 2001 | 6 h | 18 h | Zea Longa |
| Sun Yu12 | 2008 | 2 h | 1 day | Zea Longa |
| Wang Dongliang27 | 2019 | 1 h | 1 day | Zea Longa |
| Wang Wenxiu29 | 2020 | 2 h | 2 h | Zea Longa |
| Yang Debing17 | 2009 | 2 h | 1 day | Zea Longa |
| Zhao Kai32 | 2012 | 100 min | 4 h | Zea Longa |
| Zheng Jianfeng (1)25 | 2016 | 2 h | 1 day | Zea Longa |
| Zheng Jianfeng (2)25 | 2016 | Permanent | 1 day | Zea Longa |
| Zuo Xialin (1)26 | 2016 | 1 h | 6 h | Zea Longa |
| Zuo Xialin (2)26 | 2016 | 1 h | 1 day | Zea Longa |
| Hiroaki Sakai40 | 2007 | 50 min | 14 days | mNSS |
| Smith, H.K. (1)24 | 2015 | 0.5 h | 1 day | mMSS |
| Smith, H.K. (2)24 | 2015 | 0.5 h | 2 days | mMSS |
| Yang Zhong (1)30 | 2022 | 1.5 h | 1 day | mNSS |
| Yang Zhong (2)30 | 2022 | 1.5 h | 7 days | mNSS |
| Yang Zhong (3)30 | 2022 | 1.5 h | 28 days | mNSS |
| Liu Jianren23 | 2012 | 1.5 h | 1 day | Bederson |
| Onufriev, M. V (1)28 | 2021 | 1 h | 1 day | Bederson |
| Onufriev, M. V (2)28 | 2021 | 1 h | 3 days | Bederson |
| Helena Justic (1)31 | 2022 | 0.5 h | 2 days | Garcia |
| Helena Justic (2)31 | 2022 | 0.5 h | 9 days | Garcia |
| Liu Jianren23 | 2012 | 1.5 h | 1 day | Garcia |
| Fan Ruijuan (1)14 | 2014 | No report | 1 day | Garcia |
| Fan Ruijuan (2)14 | 2014 | No report | 1 day | Garcia |
Figure 11.
Comparison of neurological deficit score between MCAO-KM and MCAO-L.
Discussion
The MCAO-KM and MCAO-LG modeling methods are the two most common ways of achieving MCAO as classical surgical approaches in preclinical experiments, and they are invaluable for researchers to understand stroke patho-physiology and develop new therapeutics. However, in preclinical studies, the two classic intraluminal filament approaches for MCAO are considered alternatives, and some researchers even believed that they are interchangeable23,26. Therefore, it is critical to distinguish between them to develop an appropriate stroke model.
Distinctions in model establishment
The fundamental distinction between the MCAO-KM and MCAO-LG methods is the surgical procedure's complexity. Because most anesthetics have neuroprotective benefits against ischemic stroke, the prolonged duration of anesthesia may confuse the therapeutic effect of candidate medications41. According to our experience, the MCAO-KM is easier to perform because it does not require ligating the ECA and its embranchment, which shortens operation time (Fig. 4a) and may decrease eating difficulties caused by injuries of soft tissues and cranial nerves42. Furthermore, CCA is easier to plug a filament than ECA because the former is thicker and straighter than the latter, which explains the reason why MCAO-KM shows a higher success rate of insertion filament and shorter operation time than MCAO-LG.
SAH is one of the leading causes of postoperative mortality in the intraluminal filament MCAO model, usually caused by excessive insertion of the filament43. Generally, the optimal insertion depth depends on the species, and the propulsion of the filament stops when the finger feels a slight resistance after entering the intracranial cavity13,24. Arterioles derive from the ECA are prone to bleeding, thus, the ligature around ECA need to be tied more tightly (Fig. 1), which results in a reduced perception of finger protraction resistance. It may be a direct factor causing elevated SAH in MCAO-LG animals with a higher risk of intracranial vascular puncture (Fig. 6).
Low mortality is advantageous, representing an improved model of MCAO, allowing a reduced number of rodents. There was no overall difference in model mortality between the two surgical methods, but distinct results were found in the subgroup analyses (Fig. 5). In the tMCAO subgroup, the animal mortality of the MCAO-KM was higher than MCAO-LG, which may be related to the long-term hypoperfusion of the ischemic hemisphere caused by permanent ligation of CCA24. Conversely, in the pMCAO subgroup, higher mortality occurs in MCAO-LG, which may be interpreted by the greater surgical injury caused by a relatively complex procedure. Therefore, in the preparation of pMCAO model, inserting suture from CCA is a more sensible choice.
Model success rate, as a comprehensive index, reflects the difficulty of operation. For the tMCAO model, there is no significant difference between the two methods, however, for the pMCAO model, the success rate of MCAO-KM is higher than MCAO-LG (Fig. 7). Given the foregoing, it is reasonable to believe that the MCAO-KM method is simple to model establishment. In other words, the MCAO-LG method is challenging for a fledgling.
Distinctions in CBF
The routine and extent of reperfusion are the main distinctions between the two methods in patho-physiology. In short, the MCAO-LG animals' reperfusion is mainly derived from bilateral CCA, whereas the MCAO-KM animals' reperfusion is primarily derived from contralateral CCA via the circle of Willis because the ipsilateral CCA was permanently ligated (Fig. 1). After plugging in the filament, CBF detection revealed no significant difference between the two groups (Fig. 8a), and the decline was all about 60% of the pre-ischemia level19,35. Interestingly, reperfusion in MCAO-KM animals reached only 50% of baseline levels, while, in MCAO-LG rodents, reperfusion could rapidly restore to near-normal levels (Fig. 8b). Furthermore, high-resolution MR angiography defines the MCAO-KM as an ischemia–reperfusion model with chronic hypoperfusion, whereas the MCAO-LG method achieves ischemia complete reperfusion31.
Surge reperfusion observed with the removal of the filament may be like that observed with clinical endovascular thrombectomy44. Considering the insufficient reperfusion of MCAO-KM animals, some scholars believe that the MCAO-LG method with complete reperfusion should be given priority in the exploration of the neuroprotective agents on cerebral ischemia–reperfusion injury44. However, only 7–10% of patients in developed countries receive effective thrombolytic therapy within the therapeutic time window45,46. What's worse, stroke patients—approximately 30.9–72.3%—do not achieve full revascularization despite receiving thrombolytic therapy47–49. Besides, the researchers observed that the brain would provide hypoperfusion blood to the infarcted core via collateral vessels in humans or rodents that did not receive recanalization treatment50,51. Thus, stroke patients with chronic hypoperfusion injury were more common than that with complete reperfusion52. In other words, although the ischemia stroke model prepared by the MCAO-KM method does not achieve complete reperfusion, it may be close to the main situation of no or incomplete thrombolysis.
Distinctions in cerebral infarction size, brain edema rate and neurological deficit score
We evaluated the infarction size in the forms of volume and percentage, interestingly, the results from the two forms were opposites in the tMCAO model, and little difference was observed in the pMCAO model (Fig. 9). As is well known, full ischemia–reperfusion rapidly mobilizes amounts of erythrocytes and hemoglobin53, and mitochondrial dysfunction causes the oxygen transported by this hemoglobin to be converted into amounts of reactive oxygen species54,55. Additionally, via interacting with endothelial cells, a considerable number of circulating immune cells enter the brain parenchyma56. All of the aforementioned factors lead to untimely oxidative stress and inflammatory reactions, which result in secondary CIRI. Another investigation discovered that rats that received quick flow restoration had more infarctions than those who underwent incremental flow restoration57. Thus, it is speculated that the bigger cerebral infarction in the MCAO-LG rodents is caused by the more severe CIRI produced by the remarkable recovery of CBF.
Ligation of a unilateral CCA significantly destroys the blood–brain barrier and increases brain water content in rats58, Similar to this, individuals who have failed recanalization experience an increase and expansion of the edema volume59. Therefore, it may be reasonable to assume that the permanent ligature of CCA explains why the MCAO-KM technique results in more severe brain edema (Fig. 10). In addition, giving that edema-corrected infarction progression is greater than the edema progression, the level of edema is insufficient to account for the overall expansion of infarction volume59. Thus, considering the peak of cerebral edema, a modified formula rather than pure infarction volume should be used when estimating infarct size60.
Neurological abnormalities and cognitive deterioration are linked to chronic cerebral hypoperfusion. In this study, we discovered that neurological impairments in MCAO-KM rodents were more severe owing to the protracted hypoperfusion brought on by unilateral CCA ligation (Fig. 11). Important to keep in mind is that the impact of CCA permanent ligature on cognitive function may negate the effectiveness of emerging treatment options that aim to treat stroke-induced vascular dementia or stroke with Alzheimer's disease30.
Distinctions in inflammation
The development of ischemia stroke is heavily influenced by inflammation, and the inflammatory response brought on by various surgical techniques is time-dependent. As early as 4–6 h after surgery, neutrophils were observed in the ischemic core following ischemia61,62. The interaction between leukocytes and endothelial cells was studied by Smith et al.24, who discovered that both animals' ischemic brain tissue was filled with a significant number of rolling leukocytes. Interestingly, cells remaining stationary within the vessel only occurred in MCAO-LG after urgent reperfusion. Another study27 discovered that NF-κB expression in the ischemic core was considerably greater in the MCAO-LG than the MCAO-KM after 24 h of reperfusion. However, during the subacute stage of ischemic stroke, Onufriev et al.28 discovered that IL-1 together with corticosterone discharge and amass in the MCAO-KM rat hippocampus, indicating that MCAO-KM predisposes the animals to corticosterone-dependent distant neuroinflammatory hippocampal injury. Furthermore, in terms of long-term inflammatory responses, Yang et al.30 found no significant difference between the two modeling approaches in astrocyte and microglial activation, as well as apoptotic neuronal death. Thus, MCAO-LG may have a greater inflammatory response in the acute phase, whereas MCAO-KM may have a stronger inflammatory response in the subacute period.
Distinctions in other aspects
Other subtle differences exist between the two modeling methods, and these discrepancies are important considerations in fundamental research. For example, mice's pterygopalatine artery, the first internal carotid artery branch, is the source of the ocular artery31. The majority of retinal reactions to ischemia coincide with brain tissue in MCAO-KM animals, with substantial atrophy of the inner retinal layers due to permanent CCA ligation, whereas MCAO-LG mice revealed no serious histological retinal abnormalities. Thus, the MCAO-KM is considered to be a better way to study the patho-physiology of cerebral combined retinal ischemia31.
Additionally, equally important is whether the occluding suture covers the posterior communicating artery (pCom). The heat-blunted tip of Zea Longa likely avoids the pCom, but the silicone coating of Koizumi may and frequently does occlude the pCom, particularly in mice35. In this study, we observed that the CBF of MCAO-KM is less than that of MCAO-LG during ischemia although there was no significant difference (Fig. 7a), or that pCom obstruction had no significant effect on CBF. The reason for this may be related to the detected area and the detection time between different studies (Table 3). It may also be that most studies used the same filament for controlling variables (Table 1). In any case, the processing of the filament tip cannot be ignored before model establishment, which is one of the inspirations brought by the two molding methods.
Limitations and outlooks
Even though MCAO-LG and MCAO-KM are extensively utilized across the world, high-quality publications dedicated to a direct comparison of the two approaches are still limited, which is why just 28 pieces of literature were considered in this study. Aside from the vessel insertion position, the processing and insertion depth of the filament may also lead to differences in modeling establishment and brain injury, which may explain the potential heterogeneity of some mete-analysis results. Therefore, more related research is needed to solve this issue, and given the current developments in molecular biotechnology, it is crucial to identify the disparities in molecular biological mechanisms to develop effective therapies for ischemic stroke.
Conclusion
Based on the meta-analysis results, we conclude that the surgical procedure—MCAO-KM or MCAO-LG—should not be chosen arbitrarily, and the filament insertion route has a substantial effect on the model establishment, CBF, and CIRI.
In summary, MCAO-KM has advantages in the model establishment, such as shorter operation time, easier filament insertion, lower risk of SAH, and higher model success rate, it is more appropriate for the study of ischemia-induced brain edema due to chronic hypo-reperfusion with a higher risk of postoperative death and severe neurological deficits. Whereas MCAO-LG is better suited for the study of acute CIRI due to its ability to preserve CCA integrity with a sophisticated and challenging surgical procedure, it has low model mortality, high CBF replenishment, large cerebral infarction, and an acute inflammatory response.
Therefore, instead of arbitrary variables, this discovery may provide scientists with actual parameters for selecting a suitable intraluminal filament MCAO model.
Supplementary Information
Acknowledgements
We appreciate all researchers, some because their literature was included in our meta-analysis, and others because they were involved in this work.
Abbreviations
- BBB
Blood–brain barrier
- CBF
Cerebral blood flow
- CCA
Common carotid artery
- CIRI
Cerebral ischemia–reperfusion injury
- ECA
External carotid artery
- ICA
Internal carotid artery
- MCA
Middle cerebral artery
- MCAO
Middle cerebral artery occlusion
- mNSS
Modified neurological severity scores
- pMCAO
Permanent middle cerebral artery occlusion
- PPA
Pterygopalatine artery
- SAH
Subarachnoid hemorrhage
- TTC staining
2,3,5-Triphenyl tetrazolium chloride staining
- tMCAO
Transient middle cerebral artery occlusion
Author contributions
Y.L., B.D., and L.L. collected relevant documents, ran the statistical analyses, and made the tables and figures, Y.L. and L.H. wrote the first draft of this paper, S.L., L.T., and C.Y., J.G. reviewed the manuscript, and Y.L. submitted the final manuscript.
Funding
This study was funded by grants from National Natural Science Foundation of China (Grant no. 81872959).
Data availability
All the data are available within each publication and in our figures and table. For further inquiries regarding the data extraction, raw data included, and/or analyses, email 3192424728@qq.com.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-37187-w.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chang AR, Muntner P. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation. 2018;137:558. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Owolabi MO, et al. The burden of stroke in Africa: A glance at the present and a glimpse into the future. Cardiovasc. J. Afr. 2015;26:S27. doi: 10.5830/CVJA-2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu SM, et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 4.Bogousslavsky J, Van Melle G, Regli F. The Lausanne stroke registry: Analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.STR.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 5.Sommer CJ. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017;133:245–261. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howells DW, et al. Different strokes for different folks: The rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. Nosotchu. 1986;8:1–8. doi: 10.3995/jstroke.8.1. [DOI] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Ansari, S., Azari, H., McConnell, D. J., Afzal, A. & Mocco, J. Intraluminal middle cerebral artery occlusion (Mcao) model for ischemic stroke with laser doppler flowmetry guidance in mice. J. Vis. Exp. 2879 (2011). [DOI] [PMC free article] [PubMed]
- 10.Landis SC, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macleod MR, O Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 12.Sun, Y., Yan, G. F., Jiang, H. & Zhu, Y. S. A Modified middle cerebral artery occlusion method for establishment of brain reperfusion model in rats. Chin. J. Comp. Med. 8–10 (2008).
- 13.Cai Q, et al. A modification of intraluminal middle cerebral artery occlusion/reperfusion model for ischemic stroke with laser doppler flowmetry guidance in mice. Neuropsychiatr. Dis. Treat. 2016;12:2851–2858. doi: 10.2147/NDT.S118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan RJ, Luo YF, Sun YF. Establishment of new focal cerebral ischemia–reperfusion model in rats by suture method. Chin. J. Ethnomed. Ethnopharm. 2014;23:26–27. [Google Scholar]
- 15.Morris GP, et al. A comparative study of variables influencing ischemic injury in the Longa and Koizumi methods of intraluminal filament middle cerebral artery occlusion in mice. PLoS ONE. 2016;11:e148503. doi: 10.1371/journal.pone.0148503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laing RJ, Jakubowski J, Laing RW. Middle cerebral artery occlusion without craniectomy in rats. Which method works best? Stroke. 1993;24:294–297. doi: 10.1161/01.STR.24.2.294. [DOI] [PubMed] [Google Scholar]
- 17.Yang DB, Zhang LH, Ma C. Development of the middle cerebral artery occlusion model and behavioral assessment improvement. Chin. J. Ethnomed. Ethnopharm. 2009;18:27–28. [Google Scholar]
- 18.Xi, G. M., Wang, H. Q., He, G. H., Huang, C. F. & Wei, G. Y. Evaluation of Murine models of permanent focal cerebral ischemia. Chin. Med. J.117 (2004). [PubMed]
- 19.Cam, E., Kilic, E., Yulug, B. & Ritz, M. F. Occlusion of the middle cerebral artery in rats (2008).
- 20.Heng, X. Modification of focal cerebral ischemia model in rats for long term living [Master thesis]. Tianjin University of Sport (2008).
- 21.Yang, Z. Z. & Chen, H. Improvement and experience in suture methods for rat model of focal cerebral ischemia. Lishizhen Med. Mater. Med. Res. 2189–2190 (2008).
- 22.Trueman RC, et al. A critical re-examination of the intraluminal filament Mcao model: Impact of external carotid artery transection. Transl. Stroke Res. 2011;2:651–661. doi: 10.1007/s12975-011-0102-4. [DOI] [PubMed] [Google Scholar]
- 23.Liu JR, et al. Transient filament occlusion of the middle cerebral artery in rats: Does the reperfusion method matter 24 hours after perfusion? BMC Neurosci. 2012;13:154. doi: 10.1186/1471-2202-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith HK, Russell JM, Granger DN, Gavins FN. Critical differences between two classical surgical approaches for middle cerebral artery occlusion-induced stroke in mice. J. Neurosci. Methods. 2015;249:99–105. doi: 10.1016/j.jneumeth.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Zheng, J. F. Study on the Model of Middle Cerebral Artery Occlusion on Ischemic Stroke. (Fujian Medical University, 2016).
- 26.Zuo XL, Deng HL, Wu P, Xu E. Do different reperfusion methods affect the outcomes of stroke induced by Mcao in adult rats? Int. J. Neurosci. 2015;126:850–855. doi: 10.3109/00207454.2015.1074903. [DOI] [PubMed] [Google Scholar]
- 27.Wang, D. L. et al. Comparison between two types of middle cerebral artery occlusion model in mice by monofilament method. Chin. J. Neurotraumat. Surg. (Electron. Ed). 5, (2019).
- 28.Onufriev MV, Moiseeva YV, Zhanina MY, Lazareva NA, Gulyaeva NV. A comparative study of koizumi and longa methods of intraluminal filament middle cerebral artery occlusion in rats: Early corticosterone and inflammatory response in the hippocampus and frontal cortex. Int. J. Mol. Sci. 2021;22:13544. doi: 10.3390/ijms222413544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang WX, et al. An evaluation of 20-day survival rates of Mcao rat model prepared by modified and traditional suture-occluded methods. West. J. Tradit. Chin. Med. 2019;33:42–44. [Google Scholar]
- 30.Yang Z, et al. Post-ischemia common carotid artery occlusion worsens memory loss, but not sensorimotor deficits, in long-term survived stroke mice. Brain Res. Bull. 2022;183:153–161. doi: 10.1016/j.brainresbull.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Justić H, et al. Redefining the Koizumi model of mouse cerebral ischemia: A comparative longitudinal study of cerebral and retinal ischemia in the koizumi and longa middle cerebral artery occlusion models. J. Cereb. Blood Flow Metab. 2022;42:2080–2094. doi: 10.1177/0271678X221109873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao K, Zhang G, Jia Y, Li N. Improvement and exploration on local cerebral ischemia/reperfusion model in rats by intraluminal suture technique. Chin. J. Stroke. 2012;7:916–921. [Google Scholar]
- 33.Tang QQ, et al. Role of suture diameter and vessel insertion position in the establishment of the middle cerebral artery occlusion rat model. Exp. Ther. Med. 2013;5:1603–1608. doi: 10.3892/etm.2013.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu QM, Cao ZL, Yang JB. Comparison of longa method and Koizumi method in focal cerebral ischemia model of middle cerebral artery occlusion in rats. Chin. J. Neurol. 2000;22:32. [Google Scholar]
- 35.Woitzik J, Schneider UC, Thomé C, Schroeck H, Lothar S. Comparison of different intravascular thread occlusion models for experimental stroke in rats. J. Neurosci. Methods. 2006;151:224–231. doi: 10.1016/j.jneumeth.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Trotman-Lucas M, Kelly ME, Janus J, Fern R, Gibson CL. An alternative surgical approach reduces variability following filament induction of experimental stroke in mice. Dis. Model. Mech. 2017;10:931–938. doi: 10.1242/dmm.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao, Y. J. & Cheng, Y. B. The improvement and discussion of the model of focal cerebral ischemia/reperfusion with suture-occluded method in rats. Chin. J. Appl. Physiol. 95–97 (2001). [PubMed]
- 38.Sun, G. B., Xu, K., Ke, X., Zhao, B. & Li, Z. H. A model of focal cerebral ischemia in rats by reversible middle cerebral artery occlusion. Stroke Nerv. Dis. 342–344 (2006).
- 39.Jianyuan, L., Yazhuo, Z., Weicheng, Y., Songbai, G. & Meizhen, S. A Comparison of two methods in making ischemia-reperfusion model of middle cerebral artery in rats. Acta Aacademiae Medicinae Qingdao Universitatis. 382–384 (2008).
- 40.Sakai H, et al. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Archer DP, Walker AM, McCann SK, Moser JJ, Appireddy RM. Anesthetic neuroprotection in experimental stroke in rodents. Anesthesiology. 2017;126:653–665. doi: 10.1097/ALN.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dittmar M, Spruss T, Schuierer G, Horn M. External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke. 2003;34:2252–2257. doi: 10.1161/01.STR.0000083625.54851.9A. [DOI] [PubMed] [Google Scholar]
- 43.Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: Evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke. 1998;29:2162–2170. doi: 10.1161/01.STR.29.10.2162. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland BA, Neuhaus AA, Couch Y, Balami JS, Buchan AM. The transient intraluminal filament middle cerebral artery occlusion model as a model of endovascular thrombectomy in stroke. J. Cereb. Blood Flow Metab. 2016;36:363–369. doi: 10.1177/0271678X15606722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMeekin P, et al. Estimating the number of Uk stroke patients eligible for endovascular thrombectomy. Eur. Stroke J. 2017;2:319–326. doi: 10.1177/2396987317733343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the number of ischemic strokes potentially eligible for endovascular thrombectomy: A population-based study. Stroke. 2016;47:1377–1380. doi: 10.1161/STROKEAHA.116.013165. [DOI] [PubMed] [Google Scholar]
- 47.Zhang PL, et al. Use of intravenous thrombolytic therapy in acute ischemic stroke patients: Evaluation of clinical outcomes. Cell Biochem. Biophys. 2015;72:11–17. doi: 10.1007/s12013-014-0394-6. [DOI] [PubMed] [Google Scholar]
- 48.Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J. Cereb. Blood Flow Metab. 2012;32:2091–2099. doi: 10.1038/jcbfm.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin, C. Serial Multimodal Imaging Change and its Effects in Acute Ischemic Stroke Patients Who Achieved Recanalization. (Zhejiang University, 2020).
- 50.Wang J, et al. Rapamycin increases collateral circulation in rodent brain after focal ischemia as detected by multiple modality dynamic imaging. Theranostics. 2019;9:4923–4934. doi: 10.7150/thno.32676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 52.Ma R, et al. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. 2020;131:110686. doi: 10.1016/j.biopha.2020.110686. [DOI] [PubMed] [Google Scholar]
- 53.Martha SR, et al. Translational evaluation of acid/base and electrolyte alterations in rodent model of focal ischemia. J. Stroke Cerebrovasc. Dis. 2018;27:2746–2754. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters O, et al. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Pundik S, Xu K, Sundararajan S. Reperfusion brain injury: Focus on cellular bioenergetics. Neurology. 2012;79:S44–S51. doi: 10.1212/WNL.0b013e3182695a14. [DOI] [PubMed] [Google Scholar]
- 56.Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr. Med. Chem. 2015;22:1258–1277. doi: 10.2174/0929867322666150209154036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu WW, et al. Ischemia reperfusion injury after gradual versus rapid flow restoration for middle cerebral artery occlusion rats. Sci. Rep. 2018;8:1638. doi: 10.1038/s41598-018-20095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang W, et al. Effects of acute systemic hypoxia and hypercapnia on brain damage in a rat model of hypoxia-ischemia. PLoS ONE. 2016;11:e167359. doi: 10.1371/journal.pone.0167359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konduri P, et al. The role of edema in subacute lesion progression after treatment of acute ischemic stroke. Front. Neurol. 2021;12:705221. doi: 10.3389/fneur.2021.705221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson RA, et al. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 61.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J. Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perera MN, et al. Inflammation following stroke. J. Clin. Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are available within each publication and in our figures and table. For further inquiries regarding the data extraction, raw data included, and/or analyses, email 3192424728@qq.com.