Abstract
Classification of the Class Echinoidea is under significant revision in light of emerging molecular phylogenetic evidence. In particular, the sister-group relationships within the superorder Luminacea (Echinoidea: Irregularia) have been considerably updated. However, the placement of many families remains largely unresolved due to a series of incongruent evidence obtained from morphological, paleontological, and genetic data for the majority of extant representatives. In this study, we investigated the phylogenetic relationships of 25 taxa, belonging to eleven luminacean families. We proposed three new superfamilies: Astriclypeoidea, Mellitoidea, and Taiwanasteroidea (including Dendrasteridae, Taiwanasteridae, Scutellidae, and Echinarachniidae), instead of the currently recognized superfamily Scutelloidea Gray, 1825. In light of the new data obtained from ten additional species, the historical biogeography reconstructed shows that the tropical western Pacific and eastern Indian Oceans are the cradle for early sand dollar diversification. Hothouse conditions during the late Cretaceous and early Paleogene were coupled with diversification events of major clades of sand dollars. We also demonstrate that Taiwan fauna can play a key role in terms of understanding the major Cenozoic migration and dispersal events in the evolutionary history of Luminacea.
Subject terms: Ecology, Evolution, Systems biology, Zoology, Ecology
Introduction
The irregular echinoids of the order “Clypeasteroida” sensu A. Agassiz 1872–1874 or “sand dollars” sensu lato, constitute a morphologically well-defined group of burrowing sea urchins living on sandy bottoms from the intertidal to the deep-sea1–3. Because of their characteristic flat body, they are commonly known as sand dollars or sea biscuits and were previously subdivided into two suborders “Scutellina” and “Clypeasterina”, respectively. However, the monophyly of this traditionally defined morpho-group has been challenged since the advent of molecular studies4–9. Members of the former suborder “Scutellina” were resolved as sister group of irregular echinoids of the suborders Cassiduloida + Echinolampadoida7, and together, they were sister to the “Clypeasterina”7,8. Based on molecular evidence, Mongiardino Koch et al.8 proposed an elevation of the suborder “Scutellina” to "Scutelloida" and restriction of usage of the order "Clypeasteroid" solely to member of the former suborder "Clypeasterina". To date, four modern irregular echinoid clades, Clypeasteroida, Scutelloida, Cassiduloida, and Echinolampadoida are recognized; these taxa together constitute the clade Luminacea10. Luminacea first appeared in the Middle to Late Jurassic7. As bioturbators, they provide significant services to the marine ecosystem. Dead Luminacea also contribute an important part of shallow marine sediments (e.g., in form of sand dollar mass deposits11) and have indirectly influenced the ecosystem functions responding to global changes, in modern time as well as in the past.
Due to their good fossil record, the Luminacea were considered one of the best examples to document echinoderm spatio-temporal evolution12. Originating in the Early Cretaceous13, the Cassiduloida and Echinolampadoida were extremely diverse. Their diversity peaked during the Eocene7,14, in which 60% of all echinoids found from this period belong to these groups15. Since then, the number of living species dramatically decreased. At present, there are 28 extant species and hundreds of fossil species described from the suborders Cassiduloida and Echinolampadoida. Compared to the cassiduloids and echinolampadoids, the diversification of the other luminacean clades: Scutelloida and Clypeasteroida, are relatively recent, with no known fossil record before the Eocene1,6,13,16, which is in conflict with molecular phylogenetics based hypothesis, suggesting that the order Clypeasteroida is sister to all other luminacean echinoids. As the fossils of Luminacea exhibit high preservation potential, they can be easily identified and classified at least to order level. The incongruence between paleontological evidence and recent phylogenetic hypothesis based on molecules, highlights the need for further investigation to resolve the early evolution of the Luminacea5,7,8,13.
To date, there are 173 extant species of Luminacea17, which are globally distributed with considerably more species in the tropical Indo-West Pacific region, especially in southeast Asian waters18. Extant species are currently classified under three orders and 14 families. The Cassiduloida comprises three families: Cassidulidae, Eurhodiidae, and Neolampadidae7. The Echinolampadoida comprises a single family: Echinolampadidae; while Clypeasteroida includes two families: Arachnoididae and Clypeasteridae, respectively. The Scutelloida includes nine extant families. Based on morphology, the included Fibulariidae and Laganidae can be further grouped into an infraorder Laganiformes whereas Echinarachniidae, Taiwanasteridae, Astriclypeidae, Dendrasteridae, Mellitidae, and Scutellidae are grouped into another infraorder, Scutelliformes13,15,18–22. The classification of the remaining family Rotulidae is still unsettled. Some studies placed it within the Laginiformes15,18,21,22, whereas others consider it as a member of the Scutelliformes13,19,20.
Regarding the distribution of modern Clypeasteroida and Scutelloida, the seas around Taiwan are unique due to mixing of surface ocean currents. The warm, Kuroshio Branch Current coming from the tropical zone and the cold, China Coastal Current coming from the temperate region meet in the Taiwan Strait23. Consequently, a mixture of warm- (e.g., Arachnoides placenta24) and cold-water (e.g., Scaphechinus mirabilis23 and Astriclypeus mannii; Table 1) species occur in Taiwan. Based on the global distributions of sand dollars sensu lato, three biogeographic patterns seem to intersect at this area (Fig. 1A–C). Pattern 1, exemplified by Peronella lesueuri (Fig. 1A), which exhibits a longitudinal distribution ranging from the Northwest Pacific to South Australia. Other taxa with similar distributions include Arachnoides placenta, Clypeaster virescens, and Fibularia plateia. Pattern 2, exemplified by Sculpsitechinus auritus (Fig. 1B), exhibits a latitudinal distribution, covering the Indo-West Pacific. Other taxa with similar distributions include Clypeaster reticulatus, Laganum fudsiyama and Echinocyamus megapetalus. Pattern 3, exemplified by Astriclypeus mannii (Fig. 1C), shows a narrow distribution, restricted to the region of Japan, South Korea and Taiwan. Other taxa in this group include Sinaechinocyamus mai and Scaphechinus mirabilis. High diversity and disparity of sand dollars sensu lato at both subtidal and intertidal coastal regions, combined with three distinct biogeographic patterns (Fig. 1A–C), indicate that Taiwanese waters could be a major migration juncture for the Luminacea12,25.
Table 1.
Global occurrences of clypeasteroids reported from Taiwan waters.
| Faunal provinces | Taxa | Data sources |
|---|---|---|
| West Pacific | Clypeaster virescens Döderlein, 1885 | https://doi.org/10.15468/dl.7i7hje |
| Arachnoides placenta (Linnaeus, 1758) | https://doi.org/10.15468/dl.wswvtk | |
| Peronella lesueuri (L. Agassiz, 1841) | https://doi.org/10.15468/dl.uftmga | |
| Fibularia plateia H.L. Clark, 1928 | https://www.gbif.org/occurrence/download/0005913-190621201848488 | |
| Indo-West Pacific | Sculpsitechinus auritus (Leske, 1778) | https://doi.org/10.15468/dl.hbqzud |
| Clypeaster reticulatus (Linnaeus, 1758) | https://www.gbif.org/occurrence/download/0005918-190621201848488 | |
| Laganum fudsiyama Döderlein, 1885 | https://www.gbif.org/occurrence/download/0005919-190621201848488 | |
| Echinocyamus megapetalus H.L. Clark, 1914 | https://www.gbif.org/occurrence/download/0005914-190621201848488 | |
| Endemic to East Asia | Astriclypeus mannii Verrill, 1867 | https://doi.org/10.15468/dl.jdvqfb |
| Sinaechinocyamus mai (Wang, 1984) | Refs26–28 | |
| Scaphechinus mirabilis A. Agassiz, 1864 | https://doi.org/10.15468/dl.yfwncs |
Figure 1.
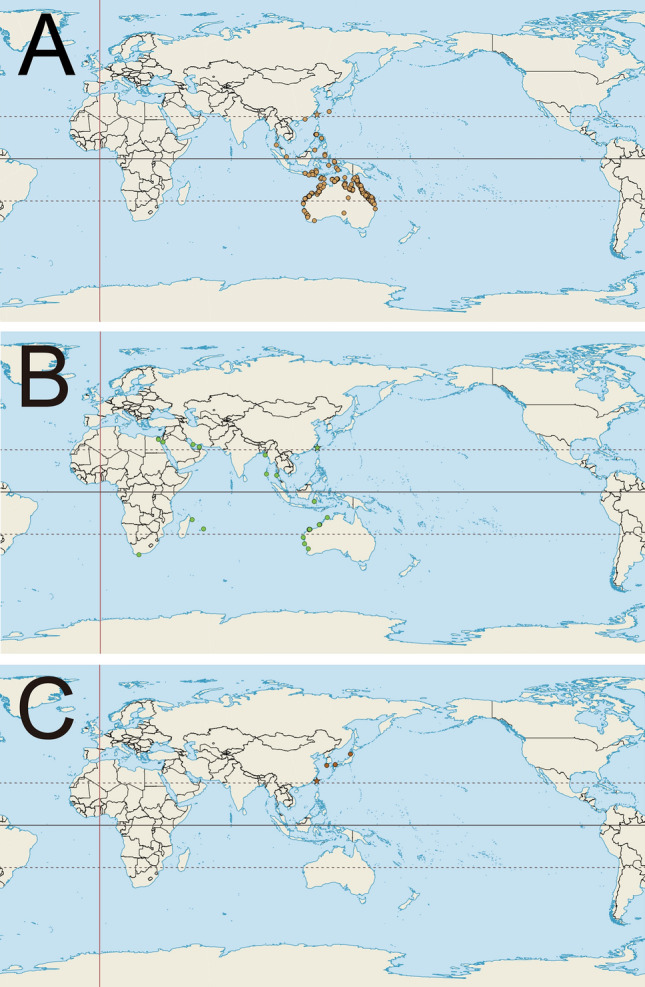
Three types of global distribution patterns based on occurrence data recorded in the Global Biodiversity Information Facility (GBIF) (Table 1). (A) Occurrences of Peronella lesueuri (L. Agassiz, 1841) showing a longitudinal distribution in the Pacific-West, at both northern and southern hemispheres (GBIF; https://doi.org/10.15468/dl.uftmga; Table 1). (B) Occurrences of Sculpsitechinus auritus (Leske, 1778) showing an Indo-West-Pacific (IWP) distribution, including the Red Sea and Persian Gulf (GBIF; https://doi.org/10.15468/dl.hbqzud; Table 1). (C) Occurrences of Astriclypeus mannii Verrill, 1867 showing endemism in the region of Japan, South Korea and Taiwan (GBIF; https://doi.org/10.15468/dl.jdvqfb; Table 1). Maps were created with QGIS (https://qgis.org/, version 3.0.3).
Luminacea includes irregular echinoids with spectacular diversification of mobile marine faunas during the Mesozoic Marine Revolution that now constitutes one of the most important components of echinoid fauna in modern seas29. This superorder, however, is in need of taxonomic revision, because its classification (see Electronic Supplementary Material 1) is not fully resolved yet7. In this study, we inferred the phylogeny of extant Luminacea based on a multi-gene dataset with denser taxon-sampling in Clypeasteroida and Scutelloida compared to previous DNA-based studies. A time-calibrated phylogenetic tree based on a relaxed Bayesian molecular clock and eight robust fossil calibration points was reconstructed to provide a timescale for the origin and diversification of the Luminacea and its main lineages. This time tree was then used as a framework to test existing hypotheses regarding the biogeographical history of the Luminacea.
Results
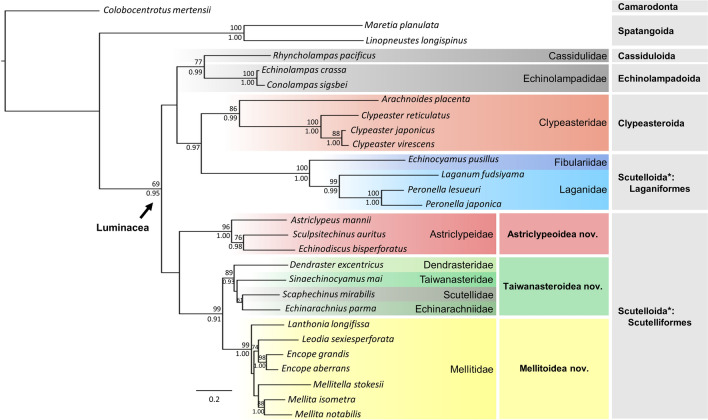
Phylogenetic reconstruction
We reconstructed the phylogeny of the Luminacea based on a combined dataset containing DNA sequences from both mitochondrial (cox1 and 16S) and nuclear (28S and H3) gene fragments from 28 echinoderm taxa. The topologies of inferred Maximum Likelihood (ML) and Bayesian Inference (BI) trees (Fig. 2 and Fig. S1, respectively) were largely identical. Within the Luminacea, five well- to moderately-supported clades were resolved: Cassiduloida + Echinolampadoida clade, Clypeasteroida clade, Laganiformes clade, the Astriclypeidae clade, and the clade containing Dendrasteridae, Echinarachniidae, Mellitidae, Scutellidae, and Taiwanasteridae. These phylogenetic results confirmed the monophyly of the Cassiduloida + Echinolampadoida and Clypeasteroida (sensu Mongiardino Koch et al.8, 7) yet rejected that of Scutelloida. The Scutelloida is composed of two distinct clades with one clade (Laganiformes) resolving as sister to the Clypeasteroida and the other (Scutelliformes) as sister to the clade containing the Cassiduloida, Echinolampadoida, Clypeasteroida and Laganiformes in our analysis. In accordance with the results of Mongiardino Koch et al.10, within the Scutelliformes, the families Dendrasteridae, Taiwanasteridae, Scutellidae, and Echinarachniidae formed a monophyletic group, which was sister to the Mellitidae (Fig. 2).
Figure 2.
Phylogenetic relationships of the Luminacea inferred using partitioned Maximum Likelihood analysis based on 3301 bp long concatenated multi-gene sequences. Asterisk represents polyphyletic Scutelloida. Nodal supports are shown as bootstrap (BS) values in percentage (above) and posterior probabilities (PP) (below). Values below 60% in BS and 0.8 in PP are not shown. Colored rectangles highlight the resolved main clades. Figure was made with Microsoft PowerPoint (https://www.microsoft.com/, version 2016).
Time-calibrated phylogenetic tree
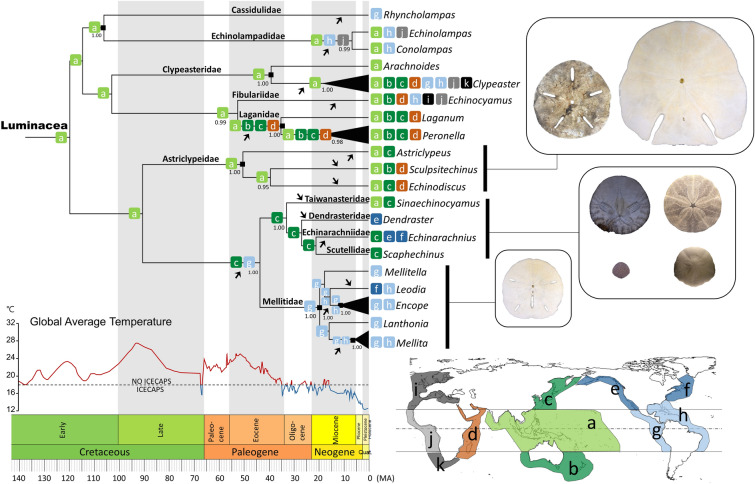
A BI time-calibrated phylogenetic tree based on a concatenated gene sequence dataset (cox1,16S, 28S and H3) was reconstructed using a relaxed log-normal clock model with eight well-documented fossil calibration points (see Table S1). The resulting topology was nearly identical to the ML and BI tree (Figs. 2 and 3) except a few shallow branching nodes with weak or without supports (e.g., within the Mellitidae). The divergent time to the most recent common ancestor (MRCA) of the crown group of Luminacea is estimated to be 121.05 million years ago (Ma) with a 95% highest posterior density (95% HPD) of 107.69–136.61 Ma. The origin of modern Cassiduloida + Echinolampadoida is estimated to be 108.72 Ma (102.5–123.79 Ma). The divergent time to the MRCA of the Clypeasteroida and Laganiformes is 111.11 Ma (74.89–166.83 Ma), while that of the Scutelliformes is 91.09 Ma (66.06–112.32 Ma). Although these estimations were older than their oldest fossil records (Eocene) (Fig. 3), they are closer to their oldest known fossil records than those ones estimated in previous studies7,13.
Figure 3.
Most-likely ancestral range reconstruction of the Luminacea using the Dispersal–Extinction–Cladogenesis (DEC) model on the simplified Bayesian phylogenetic tree inferred by BEAST v.2.6.730 based on cox1, 16S, 28S, and H3 data. Outgroups were omitted from this analysis. Nodes represent the median divergence times. Values near nodes represent posterior probabilities (PP). PP values below 0.95 are not shown. Shapes of lunule from the corresponding scutelloid clades are shown to the right. Colored, lettered boxes on the nodes represent the most likely areas of origin (lower right; a. Tropical eastern Indian Ocean and western Pacific Ocean (EIWP); b. Southern Australia and New Zealand (SANZ); c. Northwestern Pacific (NWP); d. Tropical western Indian Ocean (WIO); e. Northeastern Pacific (NEP); f. Northwestern Atlantic (NWA); g. Tropical eastern Pacific (EP); h. Tropical western Atlantic and Caribbean Sea (WA); i. Northeastern Atlantic and Mediterranean (NEA); j. Tropical East Atlantic (EA); k. South Africa (SAFR)) reconstructed by RASP v.4.231. Filled squares represent the constrained and assigned age prior nodes. Black arrows indicate inferred events of range expansions. Graph of Phanerozoic paleotemperatures was modified from Scotese et al.32. Figure was made with Microsoft PowerPoint (https://www.microsoft.com/, version 2016). Image credit: Jih-Pai Lin.
Ancestral area reconstruction
Ancestral area reconstruction based on the time-calibrated tree using the Dispersal–Extinction–Cladogenesis (DEC) model implementing in RASP ver. 4.231 suggests that the Luminacea most likely originated and diversified in the tropical eastern Indian Ocean and western Pacific (EIWP) region during the early Cretaceous before undergoing multiple expansions to other areas (Fig. 3). The ancestral Laganidae (Scutelloida: Laganiformes) were likely widespread in the entire Indo-West Pacific, south of southern Australia and New Zealand, and north to the northwestern Pacific during the Eocene. The common ancestor of the clade containing Taiwanasteridae, Dendrasteridae, Echinarachniidae, Scutellidae, and Mellitidae was likely distributed to the northwestern Pacific and tropical eastern Pacific before the Eocene. Wide-spread genera such as Clypeaster, Echinocyamus, Echinodiscus, and Echinarachnius likely underwent multiple range expansions either from the area of origin or by secondary migrations. The broadest distributed genus Clypeaster may have had its range expansion by the earliest Miocene (Fig. 3). The full DEC analysis result is shown in Electronic Supplementary Material 1.
Discussion
Based on the most dense taxon sampling to date, comprising nine out of ten families and 18 out of 29 extant genera of sand dollars sensu lato, our phylogenetic results reject the sister-group relationship between Clypeasteroida and Scutelloida, which is congruent with previous DNA-based studies4,5,7,8. The monophyly of the currently used superfamily Scutelloidea Gray, 182517 (including the families Astriclypeidae, Dendrasteridae, Mellitidae, Scutellidae) as well as the Scutelloida7,8,10 in other usage should be re-examined. Among the Scutelloida, the sister-group relationship between Laganiformes and Scutelliformes was often suggested by previous studies, yet the phylogenetic inferences were either based on fewer representative genera4,5,7,8 or unbalanced character-sampling of a molecular vs. morphology data in combined analyses7,13. This relationship is rejected by the present study. We argue that the attributed morphological similarity in these two groups could be the result of convergent evolution.
The phylogenetic position of the family Taiwanasteridae, represented by Sinaechinocyamus mai, has been reviewed recently9,13,21,33,34. It is closely related to Dendraster, Echinarachnius, and Scaphechinus (Fig. 2). Among them, the eccentric apical disc is an adaptation to the feeding strategy of Dendraster and its feeding posture35. Other than that, the four groups are similar—non-lunulate, with similar petals (Figs. S3, S4). Sinaechinocyamus is a relatively long ranging genus with good fossil records since the late Miocene (~ 8 Ma) in Taiwan36. Furthermore, new occurrences of Sinaechinocyamus have been found in coastal regions in Japan (Fig. S5), extending the geographic distribution outside of Taiwan Strait.
The new superfamily Taiwanasteroidea is proposed here to include families of Dendrasteridae, Echinarachniidae, Scutellidae, and Taiwanasteridae. Descriptions of Taiwanasteroidea together with other two new superfamilies Astriclypeoidea and Mellitoidea are given at the end of this section.
With the new phylogeny of the Luminacea reconstructed, we demonstrate important evidence on solving the mystery of lunule origins. Seilacher25 stated that lunule evolved at least six times in sand dollars. Combining molecular and fossil evidence, Mongiardino Koch and Thompson7 proposed a lunulate clade consisting of Astriclypeus and Mellita (as well as other fossil forms). In our study, lunule belong to two distinct clades (Astriclypeoidea and Mellitoidea) separated by the non-lunulate clade (Taiwanasteroidea) (Figs. 2 and 3). This indicates independent origins for the lunule in the Luminacea. The formation of lunule in Mellitidae consists of plates with a festooned arrangement and that is significantly different from the ones in Astriclypeidae with a cross-linked arrangement (see ref.25). In a broader sense, the macroevolutionary trend of Echinoidea suggested on the basis of molecular evidence9 similarly reflects the “Dollo’s law of irreversibility”37, a hypothetical scenario showing: (A) innovation of lantern in early echinoids during the late Paleozoic; (B) loss of lantern at adult stage among early irregular echinoids during the Mesozoic; and (C) independent re-innovation of the lantern with modifications33 in adult clypeasteroids and scutelliformes during the Cenozoic.
With new biogeographic analyses presented here (Fig. 4A–C), hypotheses12,18,25,38 on the origin(s) of the modern groups of sand dollars can be tested. Smith12 suggested that modern sand dollars have three biogeographical radiation hotspots to explain their present-day pattern of diversification. First, the diverse Arachnoidinae (including the genera Arachnoides and Fellaster) within the Clypeasteroida originated in the Australian region. Second, the ancestral Scutelloida emerged in the Caribbean Sea/ Mediterranean Sea. Third, the ancestral Rotulidae arose in tropical West Africa. Based on the abundant, solid fossil evidence, Seilacher38 further noted that Taiwan could be another radiation hotspot where modern Astriclypeidae emerged.
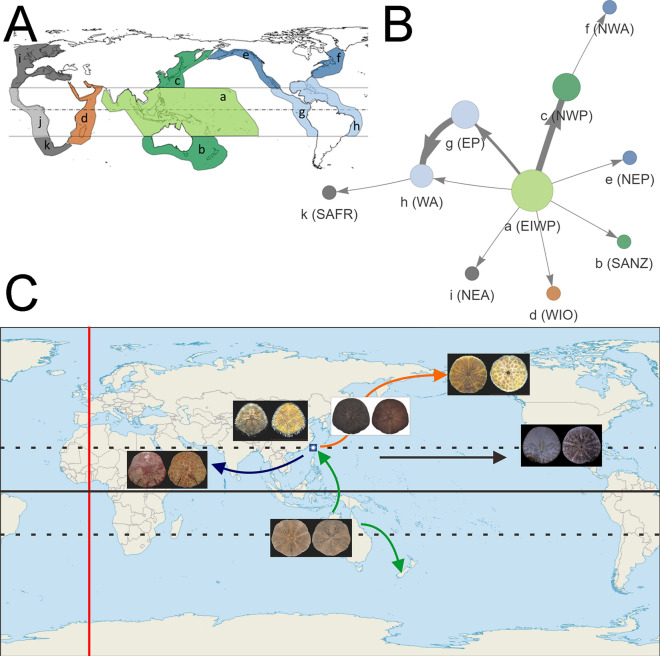
Figure 4.
Biogeography and migration network of Taiwanese fauna. (A) 11 defined biogeographical regions for Luminacea modified from previous studies15,18. a. Tropical eastern Indian Ocean and western Pacific Ocean (EIWP); b. Southern Australia and New Zealand (SANZ); c. Northwestern Pacific (NWP); d. Tropical western Indian Ocean (WIO); e. Northeastern Pacific (NEP); f. Northwestern Atlantic (NWA); g. Tropical eastern Pacific (EP); h. Tropical western Atlantic and Caribbean Sea (WA); i. Northeastern Atlantic and Mediterranean (NEA); j. Tropical East Atlantic (EA); k. South Africa (SAFR). Figure was made with Microsoft PowerPoint (https://www.microsoft.com/, version 2016). (B) StrainHub39 biogeographic network. Connections between geographic provinces is indicated by edges. Arrows indicate direction of migration and thickness of nodes indicates frequency. We used the RASP tree (Fig. S1) and geographic metadata to create the network and calculate the source/hub ratio (SHR). The SHR indicates the relative importance of geographic provinces as sources. Larger circles are more important sources of lineages. Figure was generated with StrainHub v0.2.3 on R v4.1.2. (https://doi.org/10.1093/bioinformatics/btz646). (C) Hypothesized migration trends for key sand dollar species reported in Taiwan waters. Map was created with QGIS (https://qgis.org/, version 3.0.3).
There are 11 geographic provinces defined in this study (Fig. 4A). As such, migration can be well illustrated with the biogeography network visualization software StrainHub39. The tropical eastern Indian and western Pacific Ocean (EIWP) have the highest source/hub ratio (SHR). Tropical eastern Pacific (EP) and northwestern Pacific (NWP), and tropical western Atlantic and Caribbean Sea (WA) have moderate SHR (Fig. 4B). Other nodes have low SHR. In addition, we reconstruct the polarity and frequency of migration events between geographic provinces. EIWP to NWP and EP to WA have a high frequency of migration. EIWP to EP has moderate frequency of migration. The other edges show a low frequency of migration.
Results show that EIWP is one of the key biodiversity and migration centers for shallow marine echinoids (Fig. 4B). In addition to Australia that has been identified previously12,25, the region around Taiwan is an important migration juncture for key echinoids mentioned here (Fig. 4C). Smith12 hypothesized that the clade of Astriclypeidae originated in the Mediterranean Sea based on their common presence in the fossil record of the region. Based on our analyses (Figs. 3 and 4B), however, the clade of modern Astriclypeidae appears to have a reversed migration trend, originating from Asia and then spreading to the Red Sea (Fig. 4C).
The MRCA of Taiwanasteroidea likely started to expand its range from tropical origins to high latitudes along the Pacific coast with warming ocean currents during hothouse conditions. This superfamily gradually gave rise to a cold-water lineage following their range expansion to the northeastern Pacific and northwestern Atlantic during subsequent periods of cooling. A similar model of migration can also be observed in other invertebrates. The range expansions of gastropod superfamilies Turritelloidea and Buccinoidea in the northern Pacific have mainly been influenced by thermal deterioration40. The expansions of boreal Buccinidae, Beringiinae, and Turritellinae were caused by the progressive cooling beginning in the Late Eocene40. In the genus Littorina (Gastropoda: Littorinidae), speciation occurred in response to climatic cooling during the Cenozoic at higher latitudes on the Asian coast41. It should be noted that Ghiold and Hoffman18 mentioned that the living Echinarachnius parma, currently inhabiting the northwestern Atlantic, likely migrated from the Northeast Pacific through the Arctic during the Late Pliocene climatic amelioration (Fig. 3). Extant Sinaechinocyamus, on the other hand, subsequently expanded to tropical area and is nowadays abundant in Taiwan.
The inferred historical biogeography of the Luminacea suggests that the common ancestor of this group originated from the EIWP in the early Cretaceous (Fig. 3). The Cassiduloida and Echinolampadoida, appear to have dominated the world’s echinoid diversity, with 60% of all Eocene echinoids belonging to these groups42. Since then, the number of species decreased dramatically7,14. This implies that the evolution of cassiduloids and echinolampadoids following the Eocene was driven by a series o extinction events. The fossil record thus is fundamental to fully understand their evolutionary histories. Conversely, with dense taxon sampling of extant sand dollars sensu lato, their evolutionary histories are well illustrated.
Echinoids in general, particularly taxa inhabiting shallow subtidal to intertidal habitats, are sensitive to oscillating ocean temperatures12,43. The sand dollar sensu lato likely expanded from the origin tropical EIWP to adjacent areas between the late Cretaceous and Eocene, and before the Late Eocene–Oligocene Cooling. Shallow marine currents that regulate the heat flow and governs sea surface temperature is largely driven by wind directions in the troposphere and climate patterns on Earth44,45. The relatively higher sea temperatures and warm surface currents46 during the late Cretaceous, Paleocene, and Eocene (Fig. 3) drove the dispersal of sand dollars from low- to high-latitudes. Two range expansions likely occurred during this period: the ancestral Laganidae expanded to the south (southern Australia and New Zealand), north (northwest Pacific), and west (tropical western Indian Ocean); and the MRCA of Taiwanasteroidea expanded to the northern Pacific realm (Fig. 3). Vicariance and secondary dispersals likely occurred at various regions as the climate shifted from hothouse to ice-age conditions after the Paleocene–Eocene Thermal Maximum (PETM)47 (Fig. 3). For example, the Dendraster, Echinarachnius, and Scaphechinus seem to have adapted to cold-water and survived through modern northern Pacific and northwestern Atlantic; while the Mellitidae is currently the only group that inhabits tropical America48,49. The widely distributed genus, Clypeaster, likely originated in the western Tethys in the Eocene and has probably undergone several range expansions during the Miocene and spread worldwide.
Systematics
Infraorder Scutelliformes Haeckel, 1896
Diagnosis: One lantern support (auricle) on internal side of single interambulacral plate near peristome; interambulacral column ends with two plates at the apical disc; with lunules and/or noticeable marginal indentations; periproct is on oral side and near peristome.
Superfamily Astriclypeoidea Lin in Lee et al. nov.
Diagnosis: Lunulate scutelliforms up to five lunules; periproct on oral surface; lunules with cross-linked lunule wall.
Remark: This superfamily is proposed here based on the strong molecular support (Fig. 2) and the predicted origination event occurs during the Eocene (Fig. 3).
Family Astriclypeidae Stefanini, 1912
Diagnosis: as for superfamily
Distribution: Indo-Pacific West (Fig. 1B)
Remarks: Astriclypeus, Sculpsitechinus and Echinodiscus are included in this study
Superfamily Taiwanasteroidea Lin in Lee et al. nov.
Diagnosis: Flattened scutelliforms without lunules.
Remark: Wang26 proposed the Superfamily Taiwanasteritida that includes the Family Taiwansteridae and the Family Fibulariidae. Both families are now considered not closely related and this superfamily as defined in Wang26 is polyphyletic. Based on cladistic analyses, Wang50 proposed the Superfamily Dendrasteracea, including Echinarachniidae, Dendrasteridae and Mellitidae, under the Suborder Scutellina, and Fibulariidae and Laganidae under the Suborder Laganina. Mooi51 provided a detailed study on living species of Dendraster. During ontogeny, the position of periproct shifts from the margin toward the peristome in all three living Dendraster species. While the clade is well defined based on molecular evidence (Fig. 2), it is difficult to define morphologic synapomorphies for this superfamily because Dendraster has many autapomorphies. Although Sinaechinocyamus was small in size (< 2 cm), it originates in the Taiwan Strait based on the good fossil records in Taiwan since late Miocene36. The new clade Taiwanasteroidea proposed here include four extant families: Dendrasteridae, Taiwanasteridae, Scutellidae and Echinarachniidae. This is the only clypeasteroid clade that occurs on both West and East Pacific margins.
Superfamily Mellitoidea Lin in Lee et al. nov.
Diagnosis: Lunulate scutelliforms with an anal lunule; with festooned lunule walls25 and/or marginal indentations; periproct positioned between peristome and anal lunule.
Remark: This superfamily is proposed here based on the strong molecular support (Fig. 2) and the predicted origination event occurs during the Miocene (Fig. 3).
Family Mellitidae Stefanini, 1912
Diagnosis: as for superfamily
Distribution: Their main habitats are along the western margins of North America and South America, and around the Caribbean Sea and Gulf of Mexico48.
Remarks: Lanthonia, Leodia, Encope, Mellitella and Mellita are included in this study.
Materials and methods
Ethical approval
No species of echinoderms collected in this study are listed in national laws as protected or endangered. Most of the collected organisms are not subjected to restriction by national or international laws and do not require special permission, except two specimens (see Table 2) collecting in 2017 in Kenting National Park, Taiwan (permit number 1071000145).
Table 2.
Species and sequences used in the current phylogenetic analysis.
| Family | Species | Locality | Voucher | cox1 | 16S | H3 | 28S | |
|---|---|---|---|---|---|---|---|---|
| Camarodonta | Echinometridae | Colobocentrotus mertensii | East coast, Taiwan | Inv-10064 | OQ339142 | OQ308852 | ||
| Spatangoida | Eurypatagidae | Linopneustes longispinus | AJ639918 | AJ639819 | AJ639795 | |||
| Maretiidae | Maretia planulata | Kenting, Taiwan, 21°56.21′N 120º44.82′E | Inv-10069 | OQ339146 | OQ308853 | |||
| Cassiduloida | Cassidulidae | Rhyncholampas pacificus | MN683981 | |||||
| Echinolampadidae | Conolampas sigsbei | AJ639902 | AJ639800 | AJ639777 | ||||
| Echinolampadidae | Echinolampas crassa | DQ073744 | ||||||
| Clypeasteroida | Clypeasteridae | Arachnoides placenta | Changhua, Taiwan | Inv-10063 | MH837529 | OQ308849 | ||
| Clypeaster japonicus | Sagami Bay, Japan | Inv-10067 | MH837530 | OQ308846 | ||||
| Clypeaster reticulatusa | Kenting, Taiwan, 21º56.21′N 120º44.82’E | Inv-10070 | OQ339141 | OQ308848 | OQ308839 | |||
| Clypeaster reticulatusa | Green Island, Taiwan | Inv-10073 | OQ341674 | |||||
| Clypeaster virescens | Keziliao, Taiwan, 22°43.55′N 120°15.25′E | Inv-10072 | OQ339140 | OQ308847 | OQ341673 | |||
| Scutelloida | Echinarachniidae | Echinarachnius parma | HM542173 | |||||
| Taiwanasteridae | Sinaechinocyamus mai | Miaoli, Taiwan | Inv-10065 | OQ339143 | OQ308842 | OQ341670 | OQ308838 | |
| Astriclypeidae | Astriclypeus mannii | Kinmen, Taiwan | Inv-10066 | OQ339144 | OQ308844 | OQ341671 | ||
| Echinodiscus bisperforatusb |
Pra Pas Beach, Ranong, Thailand, 9°21.96'N 98°23.67'E |
Inv-10071 | OQ339147 | OQ308845 | ||||
| Echinodiscus bisperforatusb | DQ073763 | |||||||
| Sculpsitechinus auritus | Keziliao, Taiwan, 22°43.55′N 120°15.25′E | Inv-10062 | OQ339149 | OQ308843 | OQ341669 | |||
| Dendrasteridae | Dendraster excentricus | MK037276 | ||||||
| Fibulariidae | Echinocyamus pusillus | KX458954 | DQ073743 | DQ073762 | ||||
| Laganidae | Laganum fudsiyama | off Yi-Lan, Taiwan, sta. CP4181, 24°36.279′N 122°27.1804’E, 435 m, R/V ORI, French Beam trawl, KAVALAN 2018 expedition | Inv-10068 | OQ339145 | OQ308851 | OQ341672 | ||
| Peronella japonica | LC374903 | |||||||
| Peronella lesueuri | Kinmen, Taiwan | Inv-10061 | OQ339148 | OQ308850 | OQ341668 | |||
| Mellitidae | Encope aberrans | MF616973 | MF617477 | MF617645 | ||||
| Encope grandis | KF204670 | KF205050 | KF205052 | |||||
| Lanthonia longifissa | MF616955 | MF617459 | MF617627 | |||||
| Leodia sexiesperforata | MF616941 | MF617445 | MF617613 | |||||
| Mellita notabilis | KF204742 | KF204931 | KF205124 | |||||
| Mellita isometra | KF204832 | KF205021 | KF205214 | |||||
| Mellitella stokesii | MF616945 | MF617449 | MF617617 | |||||
| Scutellidae | Scaphechinus mirabilisc | Mutsu Bay, Japan | Inv-10074 | OQ 308841 | ||||
| Scaphechinus mirabilisc | JQ341154; | |||||||
| Scaphechinus mirabilisc | AB900169 |
a, b, c: sequences being combined into single taxon in phylogenetic analyses. Voucher specimens are deposited at the marine invertebrate (inv-) collection of the National Taiwan University Museums (NTUM). New sequences generated in this study are indicated in bold.
Sample collection and DNA extraction
A total of 12 specimens of echinoids were newly collected and exanimated in this study (Table 2). The habitat of each collected specimen was shown in Table S2. Collected specimens were directly fixed in 95% ethanol prior to DNA extraction. Genomic DNA was extracted from spine muscle by scratching the oral side of the test using the QIAamp DNA Micro-Kit (Qiagen, Hilden). The specimens were kept as vouchers in National Taiwan University Museums (NTUM) (Table 2).
PCR amplification and sequencing
We performed polymerase chain reactions (PCR) to amplify two mitochondrial genes (cytochrome c oxidase I; cox1 and 16S ribosomal RNA; 16S), and two nuclear genes (28S ribosomal RNA; 28S and Histone H3; H3) from each collected specimen (Table 3).
Table 3.
Primers, annealing temperatures and number of cycles used for PCR and sequencing.
| Gene | Primers | Sequences 5′–3′ | Source | Annealing | Elongation |
|---|---|---|---|---|---|
| cox1 | EchinoF1 | TTT CAA CTA ATC ATA AGG ACA TTG G | Ward et al.52 | 50 °C, 30 s | 72 °C, 1 min |
| HCO | TAA ACT TCA GGG TGA CCA AAA AAT CA | Folmer et al.53 | |||
| COIP2F | GCY ATG AGN GTN ATY ATN CG | Lee et al.24 | 54 °C, 30 s | 72 °C, 1 min | |
| COIP2R | GAG TAT CGY CGN GGC ATT C | Lee et al.24 | |||
| 16S | 16SarL | CGC CTG TTT AAC AAA AAC AT | Palumbi et al.54 | 46 °C, 30 s | 72 °C, 1 min |
| 16SbrH | CCG GTC TGA ACT CAG ATC ACG T | Palumbi et al.54 | |||
| 28S | 28SF1 | AAC CAG GAT TCC YTY AGT AG | This study | 50 °C, 30 s | 72 °C, 1 min. 30 s |
| 28SR1 | GAG GGA ACC AGC TAC TAG | This study | |||
| 28S23L | GAC CTC AGA TCG GAC GAG AC | Stockley et al.55 | 52 °C, 30 s | 72 °C, 1 min. 30 s | |
| 28S1344R | CAA GGC CTC TAA TCA TTC GCT | Stockley et al.55 | |||
| H3 | H3F1 | ATG GCT CGT ACC AAG CAG ACV GC | Colgan et al.56 | 53 °C, 30 s | 72 °C, 40 s |
| H3R1 | ATA TCC TTR GGC ATR ATR GTG AC | Colgan et al.56 |
PCR reactions in a total volume of 25 µl, contained 20–80 ng of DNA template, 0.2 µM of each primer, and 12.5 µl 2 × EmeraldAmp GT PCR Master Mix (TaKaRa Bio.). PCR cycles included an initial denaturation step of 94 °C for 2 min, followed by 35 cycles of denaturation (94 °C, 30 s), annealing and elongation step (Table 3), and a final elongation step at 72 °C for 5 min. The purification and sequencing of PCR products were then carried out at Genomics Company (Taiwan). The obtained DNA sequence chromatograms were assembled using CodonCode Aligner v.6.0.2 (Codoncode Corporation, Dedham, MA, USA). The two overlapping cox1 sequences (cox1 part 1 and part 2) were assembled to form a longer cox1 sequence.
Phylogenetic analysis
Following the current phylogenetic framework of the Echinoidea7, we included 25 species of Luminacea (Cassiduloida, Echinolampadoida, Clypeasteroida, and Scutelloida) and three non-Luminacea species from Spatangoida and Camarodonta in the present phylogenetic analyses. We included Colobocentrotus mertensii (Odontophora) as an outgroup to root the inferred phylogenetic trees. The newly obtained cox1, 16S, 28S, and H3 sequences together with their orthologous sequences from 17 echinoderm taxa retrieved from Genbank were included in the analyses (Table 2). We aligned the cox1 and H3 sequences by eye and 16S and 28S sequences by the MAFFT ver. 7 multiple alignment program, applying default parameters57. The final trimmed alignments consisted of 1,269 bp, 578 bp, 1,145 bp and 309 bp DNA sequences for cox1, 16S, 28S, and H3, respectively. To gain the power in terms of phylogenetic resolution, we adopted an approach with simultaneous analysis for the multi-gene data. The separated gene datasets with a total of 28 common echinoderm taxa were herein combined for the subsequent analyses. Although some of the individual gene sequences of the included taxa in the concatenated dataset may come from different individuals or be missing at a particular gene locus, we do not expect this to cause a significant error in phylogenetic inference at the interfamilial level.
We performed a phylogenetic analysis using partitioned maximum likelihood (ML) method as implemented in RAxML v.8.058 with the GTR + G substitution model based on the compiled multi-gene dataset. The partitions were set by gene and by codon position for protein coding genes. The parameters including nucleotide substitutions and distribution of site rates in each partition were estimated independently. Thus, the estimated alpha value of the gamma distribution varies among partitions, with values of 0.070, 0.045, and 1.281 for three codon position of cox1, respectively, 0.212 for 16S, 0.063 for 28S, 0.888, 0.020, and 0.020 for H3. Nodal support was assessed by bootstrapping59 with 1,000 pseudo-replicates. We also performed a Bayesian Inference (BI) analysis on the combined dataset with MrBayes v.3.2.660 on the CIPRES Science Gateway61. Substitution model for each partition was set following the best-fit partition scheme given by PartitionFinder 262 (Table S3). Four Markov chains were performed in each of two parallel runs for 20,000,000 generations, sampling every thousand generation and a heating temperature of 0.02. Convergence was evaluated using Tracer v.1.730 confirming all Effective Sample Size (ESS) values were over 200. The BI tree was summarized using a 50% majority rule tree. Support values from both ML and BI methods were used to evaluate the robustness of inferred phylogenetic relationships within Luminacea.
Divergence time estimation
Divergence times of lineages were estimated using BEAST v.2.6.763 in a relaxed log-normal clock using the same dataset as for the phylogenetic analyses with eight well-documented echinoderm fossils (see below) for calibration. The Bayesian trees were estimated with a Yule model. We set a single partition for the dataset with GTR + G substitution model. Trees were linked across genes whereas clocks were set unlinked. We followed the previous study of sand dollar phylogeny48, by setting the distributions of priors for mean rates (ucld.mean) uniform, ranging from 0.001 to 1 and standard deviation (ucld.stdev) as exponential distribution with a mean equal to 0.3333. Four independent runs of 100 million MCMC generations each were performed and sampled every 1000th generations. Each run was initiated from a random starting time tree. We checked the parameter log files for convergence with Tracer v.1.7. The resulting trees files from the four independent runs were removed 10% of trees as burn-in in each run, and combined using LogCombiner v.2.6.7. The maximum clade credibility (MCC) tree with mean divergence times generated from BEAST was reconstructed using TreeAnnotator v.2.6.4.
Our phylogenetic tree was time-calibrated using echinoid fossils that provide hard minimum and soft maximum bounds through exponential distributions in which the 95% credibility interval was equal to the maximum age of the strata where the first known fossil excavated, or the maximum bound of the most recent common ancestor (MRCA) that has been estimated in previous studies. The fossils and references used in the analysis were shown in Table S1.
Ancestral area reconstruction
The origin and patterns of geographical diversification of Luminacea taxa were assessed by ancestral area reconstruction using the Dispersal-Extinction-Cladogenesis (DEC) model implemented in RASP v. 4.231 with the time-calibrated consensus tree reconstructed using BEAST (see above). Terminal taxa were regarded as the representatives for their genera (Fig. 3). We defined eleven biogeographical units for the included genera according to previous studies15,18 with minor modifications based on oceanic basin, continental shelf, surface currents, and regional endemicity18 as follows: (a) Tropical eastern Indian and western Pacific Ocean (EIWP); b) Southern Australia and New Zealand (SANZ); (c) Northwestern Pacific (NWP); (d) Tropical western Indian Ocean (WIO); e) Northeastern Pacific (NEP); (f) Northwestern Atlantic (NWA); (g) tropical eastern Pacific (EP); h) tropical western Atlantic and Caribbean Sea (WA); (i) Northeastern Atlantic and Mediterranean (NEA); j) Tropical eastern Atlantic (EA); k) South Africa (SAFR) (Fig. 4A).
Biogeography network visualization
For further geographic visualization of biogeography we built StrainHub networks from the phylogenetic data and metadata using the strainhub R package39. The network of connections between geographic provinces is based on the tree used in RASP analysis and the source/hub ratio (SHR). The size of the circles represents the SHR of the nodes in the network (Fig. 4B) with larger, circles indicating the relative importance of a geographic province as a source of lineages. The thickness of the lines indicates the frequency of movement of lineages from one body of water to another.
Equipment and settings
Images of sand dollars in Figs. 3 and S3 were taken by J.-P.L. using a Nikon D750 digital SLR camera fitted with a macro lens (AF-S VR Micro-Nikkor 105 mm f/2.8G IF-ED) and were edited in Adobe Photoshop CS6. Brightness and contrast were adjusted with the software ImageJ.
Supplementary Information
Acknowledgements
We thank Pakorn Tongboonkua and Satoshi Takeda for collecting the samples of Echinodiscus bisperforatus and Scaphechinus mirabilis, respectively. We would also like to thank the participants of the oceanographic cruise KAVALAN 2018 and the crew of the ORI, Taiwan in helping to collect the deep-sea echinoderm samples under the Tropical Deep-Sea Benthos Program. This work is supported by multiple research grants provided by the National Science and Technology Council, Taiwan (110-2116-M-002-016 and 111-2116-M-002-023 to JPL; 110-2611-M-291-005 and 111-2611-M-291-001 to HL; 110-2611-M-002-013 -; 111-2611-M-002-025—to WJC). The authors acknowledge the financial support for open access by the Natural History Museum Vienna.
Author contributions
J.P.L. obtained funding and designed the study. H.L. extracted DNA from echinoid tissues and K.S.L. contributed his PhD research specimens to this study with helps from C.H.H., C.W.L., C.E.L., J.K.W., C.C.T., W.J.C., C.C.H., H.T., T.O. K.S.L. provided images in Fig. 4C; H.T. provided images of Japanese specimens in Fig. S5; A.K. and O.B. provided references on Mediterranean faunas. H.L. curated the data, performed the analyses, and prepared the first draft of the manuscript; W.J.C. provided the resources; D.J. and C.T.F. generated the biogeography network visualizations; J.P.L. wrote the systematic descriptions. All authors reviewed and contributed to writings of the manuscript.
Data availability
New data generated and or analyzed during the current study are available in GenBank under the Accession numbers OQ339140—OQ339149 for cox1, OQ308841—OQ308853 for 16S, OQ341668—OQ341674 for H3 and OQ308838—OQ308839 for 28S.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hsin Lee and Kwen-Shen Lee.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-36848-0.
References
- 1.Smith AB. Probing the cassiduloid origins of clypeasteroid echinoids using stratigraphically restricted parsimony analysis. Paleobiology. 2001;27:392–404. doi: 10.1666/0094-8373(2001)027<0392:PTCOOC>2.0.CO;2. [DOI] [Google Scholar]
- 2.Smith AB. New haven: Peabody museum of natural history. In: Briggs DEG, editor. Evolving Form and Function: Fossils and Development. Yale University Press; 2005. [Google Scholar]
- 3.Hopkins MJ, Smith AB. Dynamic evolutionary change in post-Paleozoic echinoids and the importance of scale when interpreting changes in rates of evolution. PNAS. 2015;112:3758–3763. doi: 10.1073/pnas.1418153112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Littlewood DTJ, Smith AB. A combined morphological and molecular phylogeny for sea urchins (Echinoidea: Echinodermata) Philos. Trans. Biol. Sci. 1995;347:213–234. doi: 10.1098/rstb.1995.0023. [DOI] [PubMed] [Google Scholar]
- 5.Smith AB, et al. Testing the molecular clock: Molecular and paleontological estimates of divergence times in the Echinoidea (Echinodermata) Mol. Biol. Evol. 2006;23:1832–1851. doi: 10.1093/molbev/msl039. [DOI] [PubMed] [Google Scholar]
- 6.Smith AB, Kroh A. Phylogeny of sea urchins. Dev. Aquac. Fish. Sci. 2013;38:1–14. doi: 10.1016/B978-0-12-396491-5.00001-0. [DOI] [Google Scholar]
- 7.Mongiardino Koch N, Thompson JR. A total-evidence dated phylogeny of Echinoidea combining phylogenomic and paleontological data. Syst. Biol. 2021;70:421–439. doi: 10.1093/sysbio/syaa069. [DOI] [PubMed] [Google Scholar]
- 8.Mongiardino Koch N, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol. Biol. 2018;18:189. doi: 10.1186/s12862-018-1300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JP, et al. The first complete mitochondrial genome of the sand dollar Sinaechinocyamus mai (Echinoidea: Clypeasteroida) Genomics. 2020;112:1686–1693. doi: 10.1016/j.ygeno.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongiardino Koch N, et al. Phylogenomic analyses of echinoid diversitification promt a re-evaluation of their fossil reocord. eLife. 2022;11:e72460. doi: 10.7554/eLife.72460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebelsick JH, Kroh A. The stormy path from life to death Assemblages: The formation and preservation of mass accumulations of fossil sand dollars. Palaios. 2002;17:378–393. doi: 10.1669/0883-1351(2002)017<0378:TSPFLT>2.0.CO;2. [DOI] [Google Scholar]
- 12.Smith AB. Echinoid Palaeobiology. George Allen and Unwin; 1984. [Google Scholar]
- 13.Kroh A, Smith AB. The phylogeny and classification of post-Palaeozoic echinoids. J. Syst. Paleontol. 2010;8:147–212. doi: 10.1080/14772011003603556. [DOI] [Google Scholar]
- 14.Souto C, Mooi R, Martins L, Menegola C, Marshall CR. Homoplasy and extinction: The phylogeny of cassidulid echinoids (Echinodermata) Zool. J. Linn. Soc. 2019;187:622–660. doi: 10.1093/zoolinnean/zlz060. [DOI] [Google Scholar]
- 15.Schultz H. Sea Urchins: A Guide to Worldwide Shallow Water Species. Heinke & Peter Schultz Partner Scientific Publications; 2005. [Google Scholar]
- 16.Kier PM. Rapid evolution in echinoids. Palaeontology. 1982;25:1–9. [Google Scholar]
- 17.WoRMS Editorial Board. World Register of Marine Species. Available from https://www.marinespecies.org at VLIZ., 2022).
- 18.Ghiold J, Hoffman A. Biogeography and biogeographic history of clypeasteroid echinoids. J. Biogeogr. 1986;13:183–206. doi: 10.2307/2844920. [DOI] [Google Scholar]
- 19.Solovjev AN. Morphological similarity between echinoid taxa as a result of convergent and parallel evolution. Paleontol. J. 2016;50:1587–1597. doi: 10.1134/S0031030116140094. [DOI] [Google Scholar]
- 20.Kroh A. Sea Urchins: Biology and Ecology. Academic Press; 2020. [Google Scholar]
- 21.Mooi R, Kroh A, Srivastava DK. Phylogenetic re-evaluation of fossil and extant micro-echinoids with revision of Tridium, Cyamidia, and Lenicyamidia (Echinoidea: Clypeasteroida) Zootaxa. 2014;3857:501–526. doi: 10.11646/zootaxa.3857.4.3. [DOI] [PubMed] [Google Scholar]
- 22.Schultz H. Echinoidea, Volume 2: Echinoidea with Bilateral Symmetry. Irregularia. Walter de Gruyter GmbH; 2017. [Google Scholar]
- 23.Ho SL, et al. Changing surface ocean circulation caused the local demise of echinoid Scaphechinus mirabilis in Taiwan during the Pleistocene-Holocene transition. Sci. Rep. 2022;12:8204. doi: 10.1038/s41598-022-11920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, et al. Young colonization history of a widespread sand dollar (Echinodermata; Clypeasteroida) in western Taiwan. Quatern. Int. 2019;528:120–129. doi: 10.1016/j.quaint.2018.12.003. [DOI] [Google Scholar]
- 25.Seilacher A. Constructional morphology of sand dollars. Paleobiology. 1979;5:191–221. doi: 10.1017/S0094837300006527. [DOI] [Google Scholar]
- 26.Wang C-C. New classification of clypeasteroid echinoids. Proc. Geol. Soc. China. 1984;27:119–152. [Google Scholar]
- 27.Liao Y. A new genus of clypeasteroid sea-urchin from Huang Hai. Oceanologia et Limnologia Sinica. 1979;10:67–72. [Google Scholar]
- 28.Liao Y, Clark AM. The Echinoderms of Southern China. Science Press; 1995. [Google Scholar]
- 29.Boivin S, Saucède T, Laffont R, Steimetz E, Neige P. Diversification rates indicate an early role of adaptive radiations at the origin of modern echinoid fauna. PLoS ONE. 2018;13:e0194575. doi: 10.1371/journal.pone.0194575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y, Blair C, He XJ. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2020;37:604–606. doi: 10.1093/molbev/msz257. [DOI] [PubMed] [Google Scholar]
- 32.Scotese CR, Song H, Mills BJW, van der Meer DG. Phanerozoic paleotemperatures: The earth's changing climate during the last 540 million years. Earth Sci. Rev. 2021;215:103503. doi: 10.1016/j.earscirev.2021.103503. [DOI] [Google Scholar]
- 33.Mooi R. Paedomorphosis, Aristotle's lantern, and the origin of the sand dollars (Echinodermata: Clypeasteroida) Paleobiology. 1990;16:25–48. doi: 10.1017/S0094837300009714. [DOI] [Google Scholar]
- 34.Ziegler A, Lenihan J, Zachos LG, Faber C, Mooi R. Comparative morphology and phylogenetic significance of Gregory’s diverticulum in sand dollars (Echinoidea: Clypeasteroida) Org. Divers. Evol. 2016;16:141–166. doi: 10.1007/s13127-015-0231-9. [DOI] [Google Scholar]
- 35.Timko PL. Sand dollars as suspension feeders: a new description of feeding in Dendraster excentricus. Biol. Bull. 1976;151:247–259. doi: 10.2307/1540718. [DOI] [Google Scholar]
- 36.Chu W-C, Chang L-Y. Restudy of fossil specimens of Sinaechinocyamus (Echinoidea; Scutelloida) with new occurrences from Taiwan. Terr. Atmos. Ocean. Sci. J. 2021;32:1–33. [Google Scholar]
- 37.Collin R, Miglietta MP. Reversing opinions on Dollo's Law. Trends Ecol. Evol. 2008;23:602–609. doi: 10.1016/j.tree.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Seilacher A. Evolutionary Innovations. The University of Chicago Press; 1990. [Google Scholar]
- 39.de Bernardi Schneider A, et al. StrainHub: A phylogenetic tool to construct pathogen transmission networks. Bioinformatics. 2020;36:945–947. doi: 10.1093/bioinformatics/btz646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Titova LV. Cenozoic history of Turritelloidea and Buccinoidea (Mollusca: Gastropoda) in the North Pacific. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994;108:319–334. doi: 10.1016/0031-0182(94)90240-2. [DOI] [Google Scholar]
- 41.Reid DG. Trans-Arctic migration and speciation induced by climatic change: the biogeography of Littorina (Mollusca: Gastropoda) Bull. Mar. Sci. 1990;47:35–49. [Google Scholar]
- 42.Kier, P. M. Revision of the cassiduloid echinoids. Smithson. Misc. Collect.114 (1962).
- 43.Smith AB. A functional classification of the coronal pores of regular echinoids. Palaeontology. 1978;21:759–789. [Google Scholar]
- 44.Huang B, et al. Extended reconstructed sea surface temperature, Version 5 (ERSSTv5): Upgrades, validations, and intercomparisons. J. Clim. 2017;30:8179–8205. doi: 10.1175/JCLI-D-16-0836.1. [DOI] [Google Scholar]
- 45.Huang B, Liu C, Ren G, Zhang H-M, Zhang L. The role of buoy and Argo observations in two SST analyses in the global and tropical Pacific Oceans. J. Clim. 2019;32:2517–2535. doi: 10.1175/JCLI-D-18-0368.1. [DOI] [Google Scholar]
- 46.Nunes F, Norris RD. Abrupt reversal in ocean overturning during the Palaeocene/Eocene warm period. Nature. 2006;439:60–63. doi: 10.1038/nature04386. [DOI] [PubMed] [Google Scholar]
- 47.Hansen J, Sato M, Russell G, Kharecha P. Climate sensitivity, sea level and atmospheric carbon dioxide. Phil. Trans. R. Soc. A. 2013;371:20120294. doi: 10.1098/rsta.2012.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coppard SE, Lessios HA. Phylogeography of the sand dollar genus Encope: Implications regarding the Central American Isthmus and rates of molecular evolution. Sci. Rep. 2017;7:11520. doi: 10.1038/s41598-017-11875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coppard SE, Zigler KS, Lessios HA. Phylogeography of the sand dollar genus Mellita: Cryptic speciation along the coasts of the Americas. Mol. Phylogenet. Evol. 2013;69:1033–1042. doi: 10.1016/j.ympev.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 50.Wang, C.-C. Phylogenetic analysis of the clypeasteroid echinoids PhD thesis thesis, Yale University, (1992).
- 51.Mooi R. Sand dollars of the genus Dendraster (Echinoidea: Clypeasteroida): Phylogenetic systematics, heterochrony, and distribution of extant species. Bull. Mar. Sci. 1997;61:343–375. [Google Scholar]
- 52.Ward RD, Holmes BH, O'Hara TD. DNA barcoding discriminates echinoderm species. Mol. Ecol. Resour. 2008;8:1202–1211. doi: 10.1111/j.1755-0998.2008.02332.x. [DOI] [PubMed] [Google Scholar]
- 53.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994;3:294–299. [PubMed] [Google Scholar]
- 54.Palumbi, S. R. Nucleic acids II: The polymerase chain reaction. (1996).
- 55.Stockley B, Smith AB, Littlewood T, Lessios HA, Mackenzie-Dodds JA. Phylogenetic relationships of spatangoid sea urchins (Echinoidea): taxon sampling density and congruence between morphological and molecular estimates. Zoolog. Scr. 2005;34:447–468. doi: 10.1111/j.1463-6409.2005.00201.x. [DOI] [Google Scholar]
- 56.Colgan DJ, et al. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998;46:419. doi: 10.1071/zo98048. [DOI] [Google Scholar]
- 57.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felsenstein J. Confidence-limits on phylogenies - an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 60.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller, M. A., Pfeiffer, W. & Schwartz, T. in Gateway Computing Environments Workshop (GCE) 1 - 8 (New Orleans, LA, 2010).
- 62.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017;34:772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 63.Bouckaert R, et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLos Comput. Biol. 2019;15:e1006650. doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
New data generated and or analyzed during the current study are available in GenBank under the Accession numbers OQ339140—OQ339149 for cox1, OQ308841—OQ308853 for 16S, OQ341668—OQ341674 for H3 and OQ308838—OQ308839 for 28S.