Abstract
Background
Rheumatoid arthritis (RA) represents a primary public health challenge, which is a major source of pain, disability, and socioeconomic effects worldwide. Several factors contribute to its pathogenesis. Infections are an important concern in RA patients, which play a key role in mortality risk. Despite major advances in the clinical treatment of RA, long‐term use of disease‐modifying anti‐rheumatic drugs can cause serious adverse effects. Therefore, effective strategies for developing novel prevention and RA‐modifying therapeutic interventions are sorely needed.
Objective
This review investigates the available evidence on the interplay between various bacterial infections, particularly oral infections and RA, and focuses on some potential interventions such as probiotics, photodynamic therapy, nanotechnology, and siRNA that can have therapeutic effects.
Keywords: anti‐citrullinated protein antibodies, inflammation, microbiota; immune dysregulation; infections; periodontitis; probiotics, nanoparticles, siRNA; rheumatoid arthritis
This study aimed to investigate the available evidence on the interplay between various bacterial infections, particularly oral infections and rheumatoid arthritis and focuses on some potential interventions such as probiotics, photodynamic therapy, nanotechnology, and siRNA that can have therapeutic effects. Different bacterial pathogens alone or in inter‐bacterial interactions directly associate with the pathogenesis of RA. Potential interventions such as probiotics, photodynamic therapy, nanotechnology, and siRNA can be considered for rheumatoid arthritis treatment.

1. BACKGROUND
Rheumatoid arthritis (RA) is an inflammatory joint disease, with an overall prevalence of 0.46% worldwide. 1 The disease is characterized by bone erosion, loss of articular cartilage, chronic synovial inflammation, causing joint pain, swelling, stiffness, and disability in performing physical functions. 2 , 3 Although all joints are subject to the disease, RA usually affects the joints of the feet, knees, and hands. 4 The clinical manifestations of RA are usually not limited to joints but can turn into a systemic disorder involving the blood vessels, kidneys, lungs, heart, and liver. Furthermore, RA patients may suffer from nonspecific complications such as weight loss, fatigue, and malaise. 5 The presence of either rheumatoid factor (RF) or anti‐citrullinated peptide antibodies (ACPAs) has usually been used as the hallmark of RA. 6 This disease is multifactorial lying on genetic as well as environmental factors triggering its pathogenesis. 7 Environmental factors such as obesity and diet, smoking, infections, and microbiota have been recognized as risk factors to develop RA in the predisposed population. 8 HLA and some non‐HLA genes were also associated with susceptibility to RA. It was established that HLA alleles HLA‐DRB1*01, HLA‐DRB1*04, HLA‐DRB1*13, and DRB1*15 are linked to RA susceptibility. 9
Infections have long been known as a main player in the pathogenesis of RA. Some of the pathogens can involve multiple routes of the immune system, potentially triggering several immune processes. 10 Bacteria‐derived lipopolysaccharide (LPS) could spread into the blood and be transported to the joints. Hence, LPS could stimulate innate immune receptors in the synovium, cartilage, and bone. 11 The development of biofilms is considered to be a driving force behind persistent infections. LPS has a significant role in biofilm formation. 12 Moreover, endogenous and microbial ligands can activate toll‐like receptors (TLRs) and trigger an immune response in patients' derived cells. In particular, bacterial peptidoglycan and LPS induced the expression of chemokines IL‐6 and (C‐X‐C motif) ligand 8 (CXCL8), via TLR‐2 binding, in RA synovial fibroblast. ILs are a key element in infiltrating and maintaining the inflammatory cells within the synovium membrane. 13 Furthermore, macrophages with a high‐level expression of TLR‐2 resulted in an improper response to bacterial cell wall peptidoglycan. 14
Persuasive research has suggested a possible link between the immune response against oral infections and the development of RA through the production of enzymes by oral pathogens. 6 , 15 , 16 , 17 Pathobiology of the disease itself, immunocompromising comorbidities, lifestyle, and immunosuppressive drugs appear to play an important role in creating the conditions for the development of this correlation as well. 18 Qiao et al. reported that patients infected by periodontal pathogens had a 69% greater risk for RA than individuals without periodontal disease. 19
The utilization of disease‐modifying antirheumatic drugs (DMARDs) like methotrexate (MTX) is applied as first‐line therapy for RA, but long‐term use of MTX could not continue because of adverse effects and drug resistance. 20 , 21 To overcome the side effects, many researchers have investigated techniques to develop targeted delivery strategies. However, intra‐articular injection provides the best‐targeted therapy, frequent joint needling is usually necessary. Hence, this treatment choice might intensify the risk of infection. Also, RA involves all joints throughout the whole body. Therefore, local administration of inflamed joints is not a suitable option. 22 The new approach for tissue‐specific delivery of drugs, as well as enhanced drug bioavailability in the inflamed synovium while reducing off‐target unwanted adverse effects, are very favorable. 23 A detailed understanding of the pathogenesis of RA will help us to develop complementary therapies such as probiotics, photodynamic therapy (PDT), nanostructures, and siRNA against RA in the pharmaceutical market.
This review aims to achieve a comprehensive understanding of bacterial populations in the oral cavity, which may support the idea that oral infections are a main modifiable risk factor for life‐threatening diseases. Furthermore, the new therapeutic options will also be discussed how targeting RA.
2. RA IMMUNOPATHOGENESIS
RA is a chronic inflammatory disease involving impaired function of both the innate and adaptive immune systems. 24 Macrophages, neutrophils, mast cells, and natural killer (NK) cells are part of the innate immune system involved in the development of the inflammatory process in the joint. Antigen‐presenting cells (APCs), such as macrophages, and dendritic cells (DCs), stimulate inflammation and release pro‐inflammatory products, such as tumor necrosis factor‐alpha (TNF‐α), cytokines interleukin‐1B, 6, 18, 23, reactive oxygen species (ROS), and matrix‐degrading enzymes involved in cartilage and bone destruction. 25 Especially TNF‐α is produced during the inflammatory process, mainly by activated monocytes/ macrophages. 26
Neutrophils have been known as a key contributing factor in the pathogenesis of RA. They increase inflammatory activity and tissue destruction by releasing pro‐inflammatory cytokines, enhanced oxidative stress, granules containing destructive enzymes, and release of neutrophil extracellular trap (NET) and leading to cartilage and bone destruction. 24 Furthermore, TLRs activation plays a major role in RA pathogenesis. In RA patients, the aberrant activation of TLRs renders chronic inflammatory processes. 14 The TLR system recognizes LPS (TLR4), peptidoglycans and lipoteichoic acid (TLR2), and unmethylated CpG DNA (TLR9) from bacteria. 27 In addition, macrophages express TLR‐2, resulting in an improper response to the bacterial peptidoglycan. 14 Many of these observations could be explained in RA patients, with upregulated responses to TLR‐2 and TLR‐4 ligands of blood monocytes and synovial macrophages. 28 Additionally, type 17 helper T‐cells (Th17) have a crucial role in RA pathogenesis. IL‐17A cytokines can also mediate fibroblast‐like synoviocytes (FLS) and osteoclast activation, recruitment, and activation of neutrophils, synovial macrophages to secrete osteoclastogenic factors such as TNF‐α and IL1‐β. 29
3. The ROLE OF PERIODONTAL INFECTIONS IN RA
Periodontitis as a common infectious disease has attracted much attention in the field of public health. Periodontitis leads to progressive damage such as gingival recession, alveolar bone resorption, progressive resorption of periodontal supporting tissues, and eventual tooth loss. 30 A recent meta‐analysis found that patients with periodontitis had a 69% greater risk of RA compared to healthy groups (OR = 1.70, 95%CI: 0.75–3.85, p < 0.001). 19
Porphyromonas gingivalis is a major periodontal pathogen that may be an important threat due to disturbing host immune homeostasis. LPS, gingipains, collagenase, proteases, fimbriae, lectins, capsule, and superoxide dismutase (SOD) are among the strategies employed by this pathogen to increase bacterial colonization and destroy host periodontal structures. In addition, virulence factors enhance the adhesion of P. gingivalis with other bacteria and biofilm build‐up. Bacterial virulence factors also modulate or interfere with inflammatory response and disrupt the host immune response to escape bacterial clearance. 31 , 32 P. gingivalis expresses the citrullinating enzyme peptidyl arginine deiminase (PAD). Citrullination, a post‐translational modification, leads to loss of tolerance to self‐proteins in genetically susceptible individuals, inducing an immune response driving RA onset. Therefore, P. gingivalis infection causing protein citrullination could induce the generation of ACPAs and subsequent RA development. The presence of ACPA has been related to anti‐P. gingivalis titres in RA patients. 33 Interestingly, this pathogen may alter the TLR response, subvert IL‐8, or alter the complement cascade. 34
Neutrophils as the primary effectors of the innate immune system, immobilize and kill a broad range of microbes via NET formation. NETs are networks composed of chromatin, intracellular granules, and antimicrobial peptides with high bactericidal potential that form an extracellular matrix‐like structure. 35 Although the antimicrobial defense structures of NETs are beneficial, accumulated NETs or delayed clearance of NETs might intensify the tissue damage and represent a source of autoantigens; indeed, ACPA‐positive RA reacts strongly with histones found in NETs. 36 The potential immunogenic role of NETs is associated with the PAD4 activity, which arises the citrullination of histones and Eno, leading to a breakdown of immune tolerance to citrullinated epitopes. 37 Additionally, P. gingivalis induces NETosis in a gingipain‐dependent manner. 38
Prevotella intermedia, part of the sub‐gingival microbiota, is an anaerobic Gram‐negative bacterium. P. intermedia plays a role in the initiation and progression of periodontitis by inducing a variety of pro‐inflammatory cytokines such as IL‐8, IL1‐β, proteases, and matrix metalloproteinases (MMPs), and macrophage inflammatory proteins. 39 P. intermedia does not express the PAD gene but facilitates human PAD activity via the induction of activated neutrophils as the main source of citrullinated antigens. 40 Two genes from P. intermedia have been characterized, nucA and nucD, encoding secreted nucleases. 41 Nucleases can block phagocytosis by neutrophils, which could lead to increased periodontal pathogenicity and the release of endogenous PAD. 40 In addition, this bacterium is known to induce the production of MMP‐1 and MMP‐8 in human periodontal ligament cells. These MMPs are also notably present in RA individuals. 42 , 43 P. intermedia increases the synthesis of prostaglandin E2 from arachidonic acid via inducing cyclooxygenase‐2 (COX‐2). In particular, overexpression of the COX‐2 enzyme was detected in RA. 44
Fusobacterium nucleatum is a periodontal anaerobic bacterium associated with various forms of human diseases such as RA. The innate immune cells are made upby cells such as macrophages, neutrophils, mast cells, and NK cells contribute to the development of inflammatory responses in joints. 27 The binding of NK cells to F. nucleatum activates a wide range of inflammatory mediators implicated in the pathogenesis of the periodontal disease. 45 The balance between pro‐/anti‐inflammatory cytokines expression is maintained during periodontal full health. F. nucleatum exacerbates inflammation after the release outside the oral cavity or during dysbiosis. For example, F. nucleatum induces inflammatory responses through the TLR4 pathway. 45 TLRs activation is very critical in the onset and progression of autoimmune diseases, such as RA. Patients with RA have exhibited raised levels of TLR2 and TLR4 in leukocytes. 46 Moreover, F. nucleatum has a powerful stimulatory effect on inflammatory cytokines, IL‐6, IL‐8, and TNF‐α. 45 APCs population, such as macrophages, and effector cells, increase inflammation and contribute to bone and cartilage destruction by releasing a variety of pro‐inflammatory factors. 25 In particular, TNF‐α found crucial in the pathogenesis of the disease by increasing inflammatory cytokine levels, activating macrophages and lymphocytes. 47
Porphyromonas endodontalis is an anaerobic, gram‐negative, black‐pigmented, asaccharolytic bacteria associated with periodontitis and infected root canals. P. endodontalis has a lower ability to bind gingival cells than P. gingivalis because it does not harbor the PAD gene. 6 , 48 P. endodontalis also does not possess a trypsin‐like enzyme associated with periodontal tissue destruction and does not display hemagglutination activity. 49 However, P. endodontalis produces collagenases and protease enzymes that can also play a key role during tissue destruction. 48 Another major virulence factor of P. endodontalis is LPS, which releases the secretion of inflammatory cytokines network and promotes bone destruction. 50 P. endodontalis was dramatically increased in the saliva microbiota profiles of patients with early‐onset RA. 6
Tannerella forsythia is an obligate anaerobe related to periodontal disease. 51 T. forsythia does not contain fimbriae, and would therefore not colonize and invade periodontal tissue. However, it expresses BspA, a surface adhesion protein, in the absence of fimbriae. The BspA could utilize the P. gingivalis fimbrial protein‐like domains and induces the strong production of inflammatory cytokines in mammalian cells. These features provide a greater correlation between P. endodontalis and T. forsythia. 52 Moreover, BspA has a role in a strong coaggregation with F. nucleatum. 53 In cellular activation, BspA stimulates the release of bone‐resorbing pro‐inflammatory cytokines and IL‐8 from monocytes and gingival epithelial cells (GECs), respectively in a TLR‐2‐dependent manner. 51 In addition, the lipoprotein fractions containing ester‐bound fatty acids can stimulate human gingival fibroblasts and monocytic cells to trigger pro‐inflammatory cytokines, notably IL −6 and TNF‐α. 54 Overexpression of TNF‐α in joint synovial fluid leads to induce lymphocytes to aggregate into the inflamed joint site. Overexpressed TNF‐α stimulates the elevation of pro‐inflammatory cytokines such as IL‐1β and IL‐6, and enhances the production of collagenase and MMPs, which cause changes in subchondral bone and cartilage destruction. IL‐6 can also mediate the production of autoantibodies and RF in RA patients. In the joint, IL‐6 activates endothelial cells, resulting in the production of IL‐8, monocyte chemokine, and highly expresses adhesion molecules. Accumulation of adhesion molecules increases the leukocytes in the inflamed area. IL‐6 can also stimulate synovial cell proliferation and osteoclast activation, and finally lead to pannus formation. IL‐6 and IL‐1β, along with an increased synthesis of MMPs, are principal in articular cartilage damage. 55 Likewise, P. intermedia and T. forsythia have been detected in synovial fluid from RA patients using the checkerboard DNA–DNA hybridization. 56
Aggregatibacter actinomycetemcomitans is a facultative anaerobic oral bacterium associated with the pathogenesis of aggressive and chronic periodontitis. 57 This bacterium expresses several virulence factors, including LPS, adherence factors, biofilm, and toxins. 58 Leukotoxin (LtxA) is an important protein toxin produced by A. actinomycetemcomitans and plays a critical role in the subversion of the host immune response. 58 A. actinomycetemcomitans induces hypercitrullination in human neutrophils through the activity of LtxA, the main target of autoantibodies in RA. 59 Furthermore, LtxA induces the production of IL‐1β and IL‐18 cytokine by macrophages. These cytokines are the principal mediators in the immunopathogenesis of RA. 60 As well, LtxA promotes NET release via a CD11/CD18 (LFA‐1) route. LFA‐1, a cell surface receptor for LtxA on neutrophils, is involved in a downstream signaling cascade that may also participate in ROS generation. The increased production of ROSs is necessary for NET release. 61 The increased ROS following NETosis can activate the receptor activator of the nuclear factor kappa‐B ligand (RANKL) expression on osteoblast and stimulate osteoclast activation. 62 The role of periodontal bacteria in ACPAs generation and RA development is depicted in Figure 1A, B.
FIGURE 1.

Role of periodontal bacteria in ACPAs generation and RA development. (A) Porphyromonas gingivalis citrullinate proteins including host proteins by the combined action of gingipains followed by citrullination by PPAD. Alternatively, the citrullinated proteins are produced through LtxA‐mediated Netosis by Aggregatibacter actinomycetemcomitans. Thus, both pathogens can lead to an RA‐specific ACPAs response. ACPAs: anti‐citrullinated protein antibodies, PPAD: peptidyl‐arginine deiminase, LtxA: leukotoxin A, RA: rheumatoid arthritis, APC: antigen‐presenting cell. (B) In the periodontal pockets, receptors on GECs and phagocytes, such as DCs recognize Porphyromonas gingivalis‐associated virulence factors. Bacteria–host cell interactions stimulate cytokines and chemokines that activate the complement pathway, RANKL, and the differentiation of T helper cells, which contribute toward joint destruction. RANKL, receptor activator for nuclear factor‐κB ligand; GECs, Gingival epithelial cells; DCs, Dendritic cells; Pg, Porphyromonas gingivalis; Aa, Aggregatibacter actinomycetemcomitans.
Peptostreptococcus micros is an anaerobic Gram‐positive bacteria predominated in periodontitis and infected dental root canals. 63 Yoshioka, et al. report that LPS released from A. actinomycetemcomitans can attach to P. micros, resulting in a population of bacteria that could be a potent stimulant for human macrophages. P. micros might help cytokine induction in a periodontal lesion, thus favoring inflammation. 64 P. micros can adhere to host epithelial cells and interact with periodontopathogenic bacteria, including F. nucleatum and P. gingivalis. 65 P. micros cell wall induced the secretion of a high level of IL‐6 cytokine by macrophages. IL‐6 enhances bone resorption. 63
Treponema denticola, an anaerobic oral spirochete, could be the main contributor to periodontal disease. 66 T. denticola Eno is a virulence factor, with higher degrees of homology with the human Eno. It is also served as a plasminogen‐binding receptor, which assists in inflammatory cell invasion. 67 Interaction between anti‐ Eno antibodies and Eno‐positive cells that express in synovial fluid and peripheral blood mononuclear cells may contribute to the elevated TNFα serum levels in patients with RA, and the circulating TNFα may contribute to the progression of periodontal disease. 68 , 69 In addition, P. gingivalis, T. forsythia, F. nucleatum, and A. actinomycetemcomitans contain human Eno‐homologous proteins. 70
Filifactor alocis is a Gram‐positive anaerobic bacterium and has been detected in endodontic infections, and periodontal disease. 71 It interacts with other bacteria, specifically P. gingivalis. This interaction increases invasive capability and biofilm formation. F. alocis expresses various virulence factors and can promote the expression of pro‐inflammatory factors and proteases. 72 F. alocis can convert the arginine to ornithine via an enzymatic pathway without the citrulline production step. 73 The L‐ornithine‐converting enzyme was increased approximately 1.5‐fold in early‐onset RA patients' microbiota, supporting a potential association between F. alocis and RA progression. 6 The link between oral dysbiosis with the pathogenesis of RA is depicted in Figure 2.
FIGURE 2.
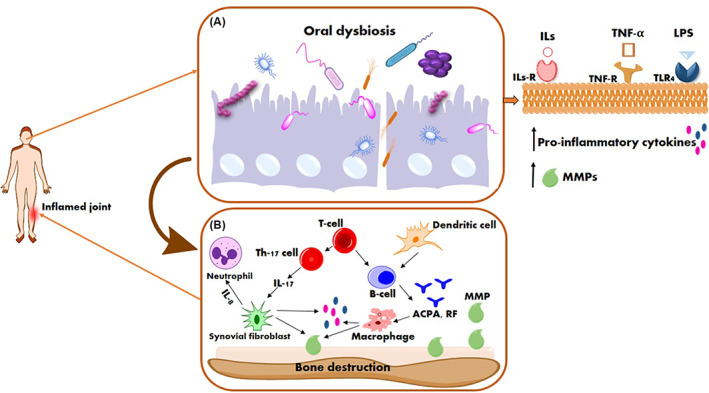
Schematic illustration of the links between rheumatoid arthritis and periodontal Infections. (A) An oral dysbiosis onto the tooth surface initiates innate immunity by producing pro‐inflammatory mediators in response to bacterial LPS (via the toll‐like receptor). (B) Immune cells will produce pro‐inflammatory mediators such as ILs, TNF, and MMPs, which also contribute to the augmentation of the immune response. IL‐17 induces the production of pro‐inflammatory cytokines and MMPs. The interactions among B and T cells and APCs play a major role in immune response activation, leading to the production of autoantibodies and the development of bone resorption. The autoantibodies contributed to the inflammatory process and resulted in bone and cartilage damage. LPS, lipopolysaccharide; ILs, interleukins; TNF, tumor necrosis factor; MMPs, matrix metalloproteinases; APCs, antigen‐presenting cells.
4. THERAPEUTIC INTERVENTIONS
Although drug molecules, predominantly glucocorticoids, and DMARDs, are unchanged as first‐line drug treatment; novel approaches are appealing options to address this challenge. 74 The most common type of adverse effect of DMARDs is an elevated risk of bacterial, fungal, and viral infections. 75 Glucocorticoids accelerated bone resorption by increasing osteoclast precursors. 76 The development of new therapies and drugs for RA has emphasized on inducing the FLS to stop proliferation. This proliferation is related to the activation of specific intracellular signaling pathways. Such inflammation, if not treated at a particular point in time, leads to vascular invasion and cartilage damage by erosion, which causes limited mobility and joint pain. Synovectomy, a standard treatment against RA, is an invasive procedure, destructive, and requires prolonged rehabilitation periods. In recent years, minimally invasive treatments have gained increased attention. 77
Bacteria can also be used as carriers for drug delivery systems. 78 Maddaloni et al. fabricated Lactococcus lactis as a delivery system for IL‐35, a unique anti‐inflammatory cytokine. IL‐35 plays a substantial role in the induction and development of T and B regulatory cells. 79 Various studies have shown treatment intervention with Lactobacillus spp. reduce arthritic scores and pro‐inflammatory cytokines like IL‐17, IL‐1, IL‐6, and TNF‐α, and induce anti‐inflammatory cytokines such as IL‐4 and IL‐10 by inhibiting the COX‐2 enzyme. They decrease oxidative stress, suppressed Th17 cell‐mediated secretion of pro‐inflammatory cytokines, diminish swelling, cartilage damage, and lymphocyte infiltration in joints, and release short‐chain fatty acids. 44 , 80 , 81 A mixture of lactobacillus probiotics (L. rhamnosus GR‐ 1 and L. reuteri RC‐14) showed substantially lower levels of IL‐1α, IL‐6, IL‐12p70, and TNF‐α. 82 So et al. have shown that the oral administration of L. casei displays protective effects against RA in rodents. This micro‐organism induces IL‐10 whilst the expression of pro‐inflammatory cytokines such as IL‐1, IL‐2, IL‐6, IL‐12, IL‐17, interferon‐gamma, COX‐2, and TNF‐α remains restricted (Figure 3A). 83
FIGURE 3.

Schematic illustration of the role of (A) Lactobacillus spp. and (B) nanostructures in rheumatoid arthritis therapy. Photodynamic therapy/siRNA complex nanostructures prevent the expression of pro‐inflammatory cytokines and inhibit bone and cartilage destruction.
The use of complementary therapies such as PDT could improve the sense of well‐being and increase the likelihood of remission. 77 PDT involves a photosensitizing agent, which is excited by visible light at specific wavelengths in the presence of oxygen. Next, the excitation energy gives rise to ROS. 84 Therefore, PDT based on the photosensitizing agents induces cell death in inflammatory cells participating in the joint. Hence, combining PDT with standard treatments can improve the control of bone and cartilage damage in the treated joint. 77 Numerous studies reported that PDT significantly reduced pro‐inflammatory cytokine levels such as IL‐6, IL‐17, and TNF‐α. 13 , 85 , 86
Nanotechnology can be a promising therapeutic strategy for RA. Compared with drugs, nanoparticles (NPs) are more s in circulation and have a longer half‐life, so they are widely applied as carriers for drug delivery. Moreover, the NPs demonstrated good sustained‐release function. They enhance the retention time of drugs in the joint cavity. 87 Chitosan is a natural cationic polysaccharide molecule with similar biological and chemical attributes to components of the bone extracellular matrix. 88 Chitosan NP containing zinc gluconate resulted in reduced expression of pro‐inflammatory cytokines and enhanced levels of SOD expression. 89 Sun et al. formed a self‐assembled structure with entrapped Cu/Zn‐SOD. These polymeric NPs demonstrated good anti‐inflammatory and antioxidant activities in a rat adjuvant‐induced arthritis model (Figure 3B). 90
Macrophages are dynamic cells that play a pivotal role during the pathogenesis of RA. The activated RA synovial macrophages express the folate receptor β (FR‐β). Triptolide (TP) is a molecule with anti‐inflammatory features, but its clinical application to treat RA has been limited due to many disadvantages like poor solubility, low bioavailability, and extremely high toxicity. Liposomes (Lips) are biocompatible spherical vesicles which used as preferred carriers for the delivery of therapeutic agents. 91 The main benefits of Lips include biocompatibility, high stability, high drug loaded to carrier ratios, nontoxicity, and protective features of drugs. 92 Folate‐modified TP‐Lips (FA‐TP‐Lips) target activated macrophages, thereby, improving the safety and effectiveness of treatment in RA. FA‐TP‐Lips with anti‐inflammatory activities inhibited osteoclastogenesis without causing cytotoxic effects. Furthermore, Lips could prolong the circulation lifetime of TP in vivo, as well as show significant anti‐inflammatory and cartilage protective effects with lower toxicity compared with the TP group alone, thereby providing a promising new treatment for RA. 93 Lips as drug delivery systems have helped through the modulation of inflammation and cytokine secretion. A study designed nano‐drug delivery systems for loading avocado soy unsaponifiable (ASU), which synergistically enhanced ASU anti‐inflammatory effects with Lip. 94 , 95 The ASU combination inhibits nuclear factor kappa B (NF‐kB), monocyte migration as well as decreases the side effects due to the co‐delivery of ASU within Lips vesicles. 96 , 97 The loaded Lip with ASU has shown positive effects in controlling and reducing joint inflammation in osteoarthritis models. ASU/liposome combination may be effective in controlling RA joint degradations. However, further investigation is needed on RA models.
In addition, NPs can be used to overcome drawbacks and improve the potency and effectiveness of therapeutic agents. 98 , 99 The copper sulfides (Cu7.2S4) NPs plus near‐infrared (NIR) laser irradiation (808 nm, 1 W/cm2) have been applied in PDT against RA. While Cu‐based nanomaterials can serve as photosensitizer agents. The combination of Cu7.2S4 NP with NIR light showed an increase in ROS production. Meanwhile, this combination reduces the level of pro‐inflammatory proteins. 100 Zhao et al. showed PDT using tetra sulphonatophenyl porphyrin‐titanium dioxide nanowhiskers have a strong therapeutic effect in RA. 13
Small interfering RNA (siRNA) showed great potential to decrease main inflammatory cytokines such as TNF‐α and IL‐1β. 21 A siRNA does not easily deliver to cells through passive diffusion due to its high molecular weight, hydrophilicity, and negative charge. Of course, due to susceptibility to endonuclease cleavage, and short half‐life in serum, an efficient delivery system is essential for the clinical application of siRNA. 101 Duan et al. designed the folate‐conjugated PEGylated Lip that could load the siRNA/ calcium phosphate NPs (siRNA/CaP) in its core while MTX will be loaded in the lipid shell of the Lips. An effective combination therapy using NPs inhibited the NF‐kB signaling pathways and reduced the production of pro‐inflammatory cytokines, and also avoid the MTX side effects. 21 In RA, the inflammatory signals induced through TNF‐α and IL‐1β degrade the inhibitory kB protein, resulting in the release of NF‐kB and transfer to the nucleus where they activate multiple inflammatory signals and RA progression. 102 TNF‐α siRNA has revealed evidence of the beneficial therapeutic effects in animal models of RA. Aldayel et al. developed a novel acid‐sensitive sheddable PEGylated solid‐lipid NP comprised of TNF‐α siRNA, lecithin, and cholesterol. This formulation shows a high encapsulation efficiency and a low burst siRNA release and can target inflamed sites after intravenous administration in mouse models that do not respond to MTX. The complexes significantly reduced RA‐induced bone loss, paw thickness, and histopathological scores. 103 Likewise, Lee et al. fabricated anti‐TNF‐α with polymerized siRNA/ thiolated glycol chitosan NP for the treatment of RA. This compound remarkably inhibited inflammation and bone erosion in an animal model. 104
5. CONCLUSIONS
The presented evidence indicates that different bacterial pathogens alone or in inter‐bacterial interactions directly associate with the pathogenesis of RA. The link between microbial dysbiosis and RA should open up interesting therapeutic prospects at a time when we are in urgent need of new alternative treatments. Eventually, clinical trials involving complementary therapies are necessary to provide a definite answer as to the applicability of each approach. In addition, basic and comprehensive researches at the molecular level are needed to fully understand the mechanism of the mentioned therapeutic interventions. Meanwhile, combination therapy should be investigated in detail to increase the retention time and efficiency of therapeutics. Moreover, formulation‐related safety concerns and obstacles should be evaluated systematically.
AUTHOR CONTRIBUTIONS
Literature search, collection, interpretation, and original draft writing; S.A.; Conceptualization, design, and construction of the conceptual framework of the review; S. A, N.C., A.P., R.G.; Review and editing of the manuscript; S.A., A.P. All authors have read and agreed to the submitted final version of the manuscript.
FUNDING INFORMATION
This work was funded by Tehran University of Medical Sciences of Medical Science and Health Services (No. 1401‐4‐209‐64414).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
CONSENT FOR PUBLICATION
All of the authors approved the manuscript for publication.
Afrasiabi S, Chiniforush N, Partoazar A, Goudarzi R. The role of bacterial infections in rheumatoid arthritis development and novel therapeutic interventions: Focus on oral infections. J Clin Lab Anal. 2023;37:e24897. doi: 10.1002/jcla.24897
Contributor Information
Alireza Partoazar, Email: partoazar@tums.ac.ir.
Ramin Goudarzi, Email: ramin.goudarzi@pharminusa.com.
DATA AVAILABILITY STATEMENT
All data are available from the corresponding author.
REFERENCES
- 1. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta‐analysis based on a systematic review. Rheumatol Int. 2021;41(5):863‐877. [DOI] [PubMed] [Google Scholar]
- 2. Christodoulou C, Choy E. Joint inflammation and cytokine inhibition in rheumatoid arthritis. Clin Exp Med. 2006;6(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 3. Zayat AS, Ellegaard K, Conaghan PG, et al. The specificity of ultrasound‐detected bone erosions for rheumatoid arthritis. Ann Rheum Dis. 2015;74(5):897‐903. [DOI] [PubMed] [Google Scholar]
- 4. van Delft MA, Huizinga TW. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun. 2020;110:102392. [DOI] [PubMed] [Google Scholar]
- 5. Grassi W, De Angelis R, Lamanna G, Cervini C. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998;27:S18‐S24. [DOI] [PubMed] [Google Scholar]
- 6. Esberg A, Johansson L, Johansson I, Dahlqvist SR. Oral microbiota identifies patients in early onset rheumatoid arthritis. Microorganisms. 2021;9(8):1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacob N, Jacob CO. Genetics of rheumatoid arthritis: an impressionist perspective. Rheum Dis Clin North Am. 2012;38(2):243‐257. [DOI] [PubMed] [Google Scholar]
- 8. Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I. One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):347‐357. [PubMed] [Google Scholar]
- 9. Kurkó J, Besenyei T, Laki J, Glant TT, Mikecz K, Szekanecz Z. Genetics of rheumatoid arthritis—a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):170‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hussein HM, Rahal EA. The role of viral infections in the development of autoimmune diseases. Crit Rev Microbiol. 2019;45(4):394‐412. [DOI] [PubMed] [Google Scholar]
- 11. Chen B, Zhao Y, Li S, et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci Rep. 2018;8(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyu M, Chen J, Jiang Y, Dong W, Fang Z, Li S. KDiamend: a package for detecting key drivers in a molecular ecological network of disease. BMC Syst Biol. 2018;12(1):89‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao C, Ur Rehman F, Yang Y, et al. Bio‐imaging and photodynamic therapy with tetra sulphonatophenyl porphyrin (TSPP)‐TiO2 nanowhiskers: new approaches in rheumatoid arthritis theranostics. Sci Rep. 2015;5(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll‐like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2192‐2201. [DOI] [PubMed] [Google Scholar]
- 15. Gómez‐Bañuelos E, Mukherjee A, Darrah E, Andrade F. Rheumatoid arthritis‐associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans . J Clin Med. 2019;8(9):1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogrendik M. Rheumatoid arthritis is linked to oral bacteria: etiological association. Mod Rheumatol. 2009;19(5):453‐456. [DOI] [PubMed] [Google Scholar]
- 17. Corrêa JD, Fernandes GR, Calderaro DC, et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. 2019;9(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology. 2013;52(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 19. Qiao Y, Wang Z, Li Y, Han Y, Zhou Y, Cao X. Rheumatoid arthritis risk in periodontitis patients: a systematic review and meta‐analysis. Joint Bone Spine. 2020;87(6):556‐564. [DOI] [PubMed] [Google Scholar]
- 20. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502‐516. [DOI] [PubMed] [Google Scholar]
- 21. Duan W, Li H. Combination of NF‐kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis. J Nanobiotechnol. 2018;16(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Q, Sun X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater Sci. 2017;5(8):1407‐1420. [DOI] [PubMed] [Google Scholar]
- 23. Mitragotri S, Yoo J‐W. Designing micro‐and nano‐particles for treating rheumatoid arthritis. Arch Pharm Res. 2011;34(11):1887‐1897. [DOI] [PubMed] [Google Scholar]
- 24. Cecchi I, de la Rosa IA, Menegatti E, et al. Neutrophils: novel key players in rheumatoid arthritis. Current and future therapeutic targets. Autoimmun Rev. 2018;17(11):1138‐1149. [DOI] [PubMed] [Google Scholar]
- 25. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205‐2219. [DOI] [PubMed] [Google Scholar]
- 26. Moelants EA, Mortier A, Van Damme J, Proost P. Regulation of TNF‐α with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013;91(6):393‐401. [DOI] [PubMed] [Google Scholar]
- 27. Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36(2):271‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kowalski M, Wolska A, Grzegorczyk J, et al. Increased responsiveness to toll‐like receptor 4 stimulation in peripheral blood mononuclear cells from patients with recent onset rheumatoid arthritis. Mediators Inflamm. 2008;2008:132732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim K‐W, Kim H‐R, Kim B‐M, Cho M‐L, Lee S‐H. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am J Pathol. 2015;185(11):3011‐3024. [DOI] [PubMed] [Google Scholar]
- 30. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 31. Xu W, Zhou W, Wang H, Liang S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol. 2020;120:45‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia L, Han N, Du J, Guo L, Luo Z, Liu Y. Pathogenesis of important virulence factors of Porphyromonas gingivalis via toll‐like receptors. Front Cell Infect Microbiol. 2019;9:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ceccarelli F, Orrù G, Pilloni A, et al. Porphyromonas gingivalis in the tongue biofilm is associated with clinical outcome in rheumatoid arthritis patients. Clin Exp Immunol. 2018;194(2):244‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bourgeois D, Inquimbert C, Ottolenghi L, Carrouel F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease—Is there cause for consideration? Microorganisms. 2019;7(10):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532‐1535. [DOI] [PubMed] [Google Scholar]
- 36. Pratesi F, Dioni I, Tommasi C, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis. 2014;73(7):1414‐1422. [DOI] [PubMed] [Google Scholar]
- 37. Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(10):606‐620. [DOI] [PubMed] [Google Scholar]
- 38. Bryzek D, Ciaston I, Dobosz E, et al. Triggering NETosis via protease‐activated receptor (PAR)‐2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019;15(5):e1007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karched M, Bhardwaj RG, Qudeimat M, Al‐Khabbaz A, Ellepola A. Proteomic analysis of the periodontal pathogen Prevotella intermedia secretomes in biofilm and planktonic lifestyles. Sci Rep. 2022;12(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwenzer A, Quirke AM, Marzeda AM, et al. Association of distinct fine specificities of anti− citrullinated peptide antibodies with elevated immune responses to Prevotella intermedia in a subgroup of patients with rheumatoid arthritis and periodontitis. Arthritis Rheumatol. 2017;69(12):2303‐2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doke M, Fukamachi H, Morisaki H, Arimoto T, Kataoka H, Kuwata H. Nucleases from Prevotella intermedia can degrade neutrophil extracellular traps. Mol Oral Microbiol. 2017;32(4):288‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guan S‐M, Shu L, Fu S‐M, Liu B, Xu X‐L, Wu J‐Z. Prevotella intermedia upregulates MMP‐1 and MMP‐8 expression in human periodontal ligament cells. FEMS Microbiol Lett. 2009;299(2):214‐222. [DOI] [PubMed] [Google Scholar]
- 43. Mehana E‐SE, Khafaga AF, El‐Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019;234:116786. [DOI] [PubMed] [Google Scholar]
- 44. Paul AK, Paul A, Jahan R, et al. Probiotics and amelioration of rheumatoid arthritis: Significant roles of Lactobacillus casei and Lactobacillus acidophilus . Microorganisms. 2021;9(5):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han YW. Fusobacterium nucleatum: a commensal‐turned pathogen. Curr Opin Microbiol. 2015;23:141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang Q‐Q, Pope RM. The role of toll‐like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11(5):357‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farrugia M, Baron B. The role of TNF‐α in rheumatoid arthritis: a focus on regulatory T cells. J Clin Transl Res. 2016;2(3):84‐90. [PMC free article] [PubMed] [Google Scholar]
- 48. Lombardo Bedran TB, Marcantonio RAC, Spin Neto R, et al. Porphyromonas endodontalis in chronic periodontitis: a clinical and microbiological cross‐sectional study. J Oral Microbiol. 2012;4(1):10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tran T, Flynn MJ, Chen C, Slots J. Porphyromonas endodontalis in subgingival plaque. Clin Infect Dis. 1997;25(Suppl 2):S222‐S223. [DOI] [PubMed] [Google Scholar]
- 50. Guo J, Yang D, Okamura H, et al. Calcium hydroxide suppresses Porphyromonas endodontalis lipopolysaccharide–induced bone destruction. J Dent Res. 2014;93(5):508‐513. [DOI] [PubMed] [Google Scholar]
- 51. Sharma A. Virulence mechanisms of Tannerella forsythia . Periodontol 2000. 2010;54(1):106‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38(1):72‐122. [DOI] [PubMed] [Google Scholar]
- 53. Sharma A, Inagaki S, Sigurdson W, Kuramitsu H. Synergy between Tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol Immunol. 2005;20(1):39‐42. [DOI] [PubMed] [Google Scholar]
- 54. Hasebe A, Yoshimura A, Into T, et al. Biological activities of Bacteroides forsythus lipoproteins and their possible pathological roles in periodontal disease. Infect Immun. 2004;72(3):1318‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Feng N, Guo F. Nanoparticle‐siRNA: A potential strategy for rheumatoid arthritis therapy? J Control Release. 2020;325:380‐393. [DOI] [PubMed] [Google Scholar]
- 56. Moen K, Brun J, Valen M, et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006;24(6):656‐663. [PubMed] [Google Scholar]
- 57. Krueger E, Brown AC. Aggregatibacter actinomycetemcomitans leukotoxin: From mechanism to targeted anti‐toxin therapeutics. Mol Oral Microbiol. 2020;35(3):85‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vega BA, Belinka BA Jr, Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera®): mechanisms of action and therapeutic applications. Toxins. 2019;11(9):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Konig MF, Abusleme L, Reinholdt J, et al. Aggregatibacter actinomycetemcomitans–induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369):369ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mukherjee A, Jantsch V, Khan R, et al. Rheumatoid arthritis‐associated autoimmunity due to Aggregatibacter actinomycetemcomitans and its resolution with antibiotic therapy. Front Immunol. 2018;9:2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hirschfeld J, Roberts HM, Chapple IL, et al. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J Oral Microbiol. 2016;8(1):33070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee YH, Lew PH, Cheah CW, Rahman MT, Baharuddin NA, Vaithilingam RD. Potential mechanisms linking periodontitis to rheumatoid arthritis. J Int Acad Periodontol. 2019;21(3):99‐110. [PubMed] [Google Scholar]
- 63. Tanabe S‐i, Bodet C, Grenier D. Peptostreptococcus micros cell wall elicits a pro‐inflammatory response in human macrophages. J Endotoxin Res. 2007;13(4):219‐226. [DOI] [PubMed] [Google Scholar]
- 64. Yoshioka M, Grenier D, Mayrand D. Binding of Actinobacillus actinomycetemcomitans lipopolysaccharides to Peptostreptococcus micros stimulates tumor necrosis factor α production by macrophage‐like cells. Oral Microbiol Immunol. 2005;20(2):118‐121. [DOI] [PubMed] [Google Scholar]
- 65. Kremer BH, van Steenbergen TM. Peptostreptococcus micros coaggregates with Fusobacterium nucleatum and non‐encapsulated Porphyromonas gingivalis. FEMS Microbiol Lett. 2000;182(1):57‐61. [DOI] [PubMed] [Google Scholar]
- 66. O'Bier NS, Patel DT, Oliver LD Jr, Miller DP, Marconi RT. Development of an FhbB based chimeric vaccinogen that elicits antibodies that block Factor H binding and cleavage by the periopathogen Treponema denticola . Mol Oral Microbiol. 2021;36(1):50‐57. [DOI] [PubMed] [Google Scholar]
- 67. Alam J, Kim YC, Choi Y. Potential role of bacterial infection in autoimmune diseases: a new aspect of molecular mimicry. Immune Netw. 2014;14(1):7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee A, Kim YC, Baek K, et al. Treponema denticola enolase contributes to the production of antibodies against ENO1 but not to the progression of periodontitis. Virulence. 2018;9(1):1263‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bae S, Kim H, Lee N, et al. α‐Enolase expressed on the surfaces of monocytes and macrophages induces robust synovial inflammation in rheumatoid arthritis. J Immunol. 2012;189(1):365‐372. [DOI] [PubMed] [Google Scholar]
- 70. Lee JY, Jung YJ, Jun HK, Choi BK. Pathogenic potential of Tannerella forsythia enolase. Mol Oral Microbiol. 2016;31(2):189‐203. [DOI] [PubMed] [Google Scholar]
- 71. Aruni W, Chioma O, Fletcher H. Filifactor alocis: the newly discovered kid on the block with special talents. J Dent Res. 2014;93(8):725‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nogueira AVB, Nokhbehsaim M, Damanaki A, et al. Filifactor alocis and tumor necrosis factor‐alpha stimulate synthesis of visfatin by human macrophages. Int J Mol Sci. 2021;22(3):1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Uematsu H, Sato N, Hossain M, Ikeda T, Hoshino E. Degradation of arginine and other amino acids by butyrate‐producing asaccharolytic anaerobic Gram‐positive rods in periodontal pockets. Arch Oral Biol. 2003;48(6):423‐429. [DOI] [PubMed] [Google Scholar]
- 74. Prasad P, Verma S, Ganguly NK, Chaturvedi V, Mittal SA. Rheumatoid arthritis: advances in treatment strategies. Mol Cell Biochem. 2022;1‐20:69‐88. [DOI] [PubMed] [Google Scholar]
- 75. Benjamin O, Bansal P, Goyal A, Lappin SL. Disease Modifying Anti‐Rheumatic Drugs (DMARD). StatPearls; 2019. [Google Scholar]
- 76. Eskandarynasab M, Doustimotlagh AH, Takzaree N, et al. Phosphatidylserine nanoliposomes inhibit glucocorticoid‐induced osteoporosis: a potential combination therapy with alendronate. Life Sci. 2020;257:118033. [DOI] [PubMed] [Google Scholar]
- 77. Gallardo‐Villagrán M, Leger DY, Liagre B, Therrien B. Photosensitizers used in the photodynamic therapy of rheumatoid arthritis. Int J Mol Sci. 2019;20(13):3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Han Y, Pang X, Pi G. Biomimetic and bioinspired intervention strategies for the treatment of rheumatoid arthritis. Adv Funct Mater. 2021;31(38):2104640. [Google Scholar]
- 79. Maddaloni M, Kochetkova I, Hoffman C, Pascual DW. Delivery of IL‐35 by Lactococcus lactis ameliorates collagen‐induced arthritis in mice. Front Immunol. 2018;9:2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amdekar S, Singh V, Kumar A, Sharma P, Singh R. Lactobacillus casei and Lactobacillus acidophilus regulate inflammatory pathway and improve antioxidant status in collagen‐induced arthritic rats. J Interferon Cytokine Res. 2013;33(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 81. So J‐S, Lee C‐G, Kwon H‐K, et al. Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol Immunol. 2008;46(1):172‐180. [DOI] [PubMed] [Google Scholar]
- 82. Dorożyńska I, Majewska‐Szczepanik M, Marcińska K, Szczepanik M. Partial depletion of natural gut flora by antibiotic aggravates collagen induced arthritis (CIA) in mice. Pharmacol Rep. 2014;66(2):250‐255. [DOI] [PubMed] [Google Scholar]
- 83. So J‐S, Kwon H‐K, Lee C‐G, et al. Lactobacillus casei suppresses experimental arthritis by down‐regulating T helper 1 effector functions. Mol Immunol. 2008;45(9):2690‐2699. [DOI] [PubMed] [Google Scholar]
- 84. Gallardo‐Villagrán M, Paulus L, Charissoux J‐L, et al. Evaluation of ruthenium‐based assemblies as carriers of photosensitizers to treat rheumatoid arthritis by photodynamic therapy. Pharmaceutics. 2021;13(12):2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Elsadek MF, Farahat MF. Impact of photodynamic therapy as an adjunct to non‐surgical periodontal treatment on clinical and biochemical parameters among patients having mild rheumatoid arthritis with periodontitis. Photodiagnosis Photodyn Ther. 2022;37:102698. [DOI] [PubMed] [Google Scholar]
- 86. Li X, Zhang S, Zhang X, et al. Folate receptor‐targeting semiconducting polymer dots hybrid mesoporous silica nanoparticles against rheumatoid arthritis through synergistic photothermal therapy, photodynamic therapy, and chemotherapy. Int J Pharm. 2021;607:120947. [DOI] [PubMed] [Google Scholar]
- 87. Kumar S, Adjei IM, Brown SB, Liseth O, Sharma B. Manganese dioxide nanoparticles protect cartilage from inflammation‐induced oxidative stress. Biomaterials. 2019;224:119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hu D, Ren Q, Li Z, Zhang L. Chitosan‐based biomimetically mineralized composite materials in human hard tissue repair. Molecules. 2020;25(20):4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ansari MM, Ahmad A, Mishra RK, Raza SS, Khan R. Zinc gluconate‐loaded chitosan nanoparticles reduce severity of collagen‐induced arthritis in Wistar rats. ACS Biomater Sci Eng. 2019;5(7):3380‐3397. [DOI] [PubMed] [Google Scholar]
- 90. Sun X, Yu K, Zhou Y, et al. Self‐assembled pH‐sensitive polymeric nanoparticles for the inflammation‐targeted delivery of Cu/Zn‐superoxide dismutase. ACS Appl Mater Interfaces. 2021;13(15):18152‐18164. [DOI] [PubMed] [Google Scholar]
- 91. Saraf S, Jain A, Tiwari A, Verma A, Panda PK, Jain SK. Advances in liposomal drug delivery to cancer: an overview. J Drug Deliv Sci Technol. 2020;56:101549. [Google Scholar]
- 92. Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9(2):E128‐E147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guo R‐b, Zhang X‐y, Yan D‐k, et al. Folate‐modified triptolide liposomes target activated macrophages for safe rheumatoid arthritis therapy. Biomater Sci. 2022;10(2):499‐513. [DOI] [PubMed] [Google Scholar]
- 94. Goudarzi R, Nasab ME, Saffari PM, Zamanian G, Park CD, Partoazar A. Evaluation of ROCEN on burn wound healing and thermal pain: transforming growth factor‐β1 activation. Int J Low Extrem Wounds. 2021;20(4):337‐346. [DOI] [PubMed] [Google Scholar]
- 95. Partoazar A, Seyyedian Z, Zamanian G, et al. Neuroprotective phosphatidylserine liposomes alleviate depressive‐like behavior related to stroke through neuroinflammation attenuation in the mouse hippocampus. Psychopharmacology (Berl). 2021;238:1531‐1539. [DOI] [PubMed] [Google Scholar]
- 96. Goudarzi R, Partoazar A, Mumtaz F, et al. Arthrocen, an avocado‐soy unsaponifiable agent, improves acetic acid‐induced colitis in rat by inhibition of NF‐kB signaling pathway. J Food Biochem. 2020;44(7):e13244. [DOI] [PubMed] [Google Scholar]
- 97. Taylor JF, Goudarzi R, Yazdi PG, Pedersen BA. In vitro effects of arthrocen, an avocado/soy unsaponifiables agent, on inflammation and global gene expression in human monocytes. Int J Chem. 2017;9(4):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761‐769. [DOI] [PubMed] [Google Scholar]
- 99. Afrasiabi S, Chiniforush N, Barikani HR, Partoazar A, Goudarzi R. Nanostructures as targeted therapeutics for combating oral bacterial diseases. Biomedicine. 2021;9(10):1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lu Y, Li L, Lin Z, et al. A new treatment modality for rheumatoid arthritis: combined photothermal and photodynamic therapy using Cu7. 2S4 nanoparticles. Adv Healthc Mater. 2018;7(14):1800013. [DOI] [PubMed] [Google Scholar]
- 101. Sun X, Dong S, Li X, et al. Delivery of siRNA using folate receptor‐targeted pH‐sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomedicine. 2019;20:102017. [DOI] [PubMed] [Google Scholar]
- 102. Tak PP, Firestein GS. NF‐κB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Aldayel AM, O'Mary HL, Valdes SA, et al. Lipid nanoparticles with minimum burst release of TNF‐α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. J Control Release. 2018;283:280‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee SJ, Lee A, Hwang SR, et al. TNF‐α gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol Ther. 2014;22(2):397‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author.


