SUMMARY
Plasmodium falciparum infections have been implicated in immune deficiencies resulting in ineffective control of Epstein–Barr virus, thereby increasing the risk of endemic Burkitt lymphoma in children. However, the impact of Epstein–Barr virus infections on the development of immunity to P. falciparum has not been studied in depth. In this review, we examine novel findings from animal co-infection models and human immuno-epidemiologic studies to speculate on the impact of acute gammaherpesvirus co-infection on malarial disease severity. Children are often concurrently or sequentially infected with multiple pathogens, and this has implications for understanding the development of protective immunity as well as in the evaluation of vaccine efficacy.
Keywords: animal models, co-infection, epidemiologic studies, immunity
INTRODUCTION
CO-INFECTION WITH EPSTEIN–BARR VIRUS AND PLASMODIUM FALCIPARUM IS UBIQUITOUS IN SUB-SAHARAN AFRICA
More than 200 million individuals worldwide are infected with Plasmodium on a yearly basis, and of these infections, approximately 1 million individuals die, largely children under 5 years of age and pregnant women (1). In sub-Saharan Africa, Plasmodium falciparum is responsible for over 90% of infections. Disease severity is spectral, ranging from mild febrile illness to life-threatening conditions such as cerebral malaria (CM) and severe malarial anaemia (SMA) (2). As suggested by the age of fatalities, the most complicated forms of malaria are largely restricted to childhood (3) although not all children living in endemic areas suffer severe disease. Multiple factors contribute to disease severity including host genetics, parasite virulence and transmission intensity (4). Immune responses to each stage of the parasite life cycle that contribute to the development of protective immunity have been described in more detail elsewhere (5).
Disease severity associated with malarial infection in areas endemic for Plasmodium transmission is confounded by the high prevalence of concomitant infections with other pathogens. Children in sub-Saharan Africa experience their primary Epstein–Barr virus (EBV) infection within the first years of life, with the majority of children becoming seropositive by 12 months of age (6-10). Contrary to more affluent settings where primary EBV infection is characterized by the symptomatic manifestation infectious mononucleosis (IM) occurring mainly during late adolescence and early adulthood (8), infections of young children are typically asymptomatic. EBV and malarial co-infection is postulated to negatively impact the generation of immune responses that effectively control EBV replication and limit the proportion of B cells latently infected with EBV (reviewed in Ref. 11). Deficiencies in immune control over latent EBV infections are thought to be permissive to the development of endemic Burkitt lymphoma (eBL), a B-cell tumour that occurs predominantly in children 5–9 years of age in Equatorial Africa, first described by Dennis Burkitt in 1958 (12). Studies from children with eBL suggest that this EBV-associated tumour results, in part, from a loss of EBV-specific T-cell responses in children after chronic exposure to P. falciparum (13-17) that consequently reactivates latent EBV (18-20). In this review, we examine the converse hypothesis that EBV may also have an impact on immunity to and severity of malarial infections.
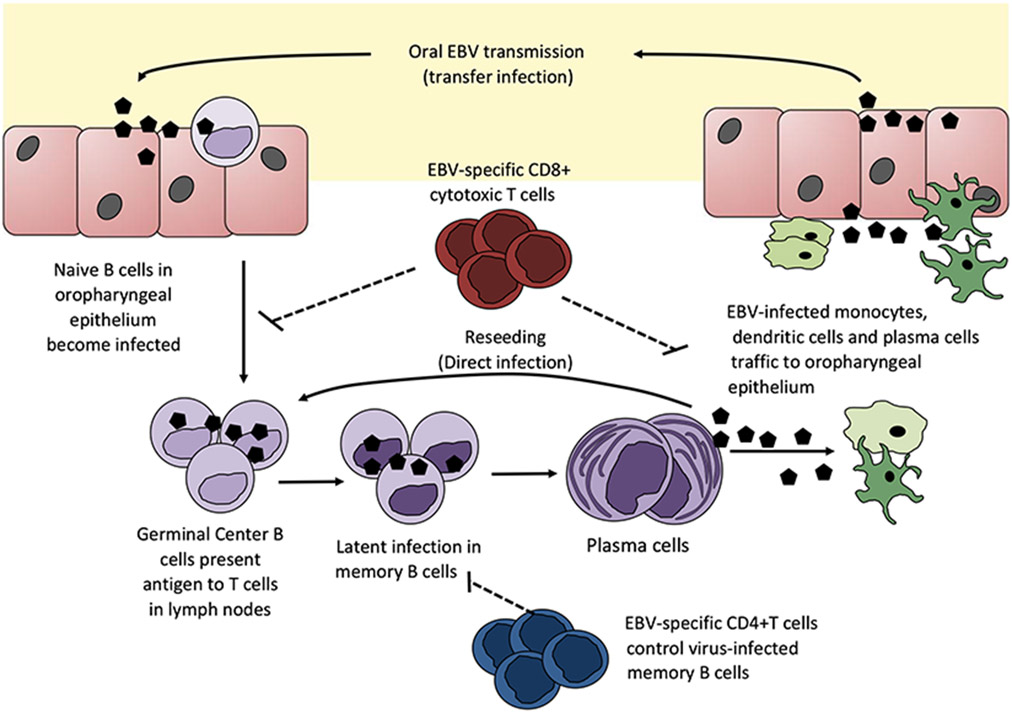
EBV infection has distinct lytic and latent life cycles and maintains lifelong persistence within the human host. Gammaherpesviruses such as EBV are lymphotropic and primarily infect and maintain latency in B cells (Figure 1). Humoral immunity has been shown to play a key role in clearance of circulating malaria-infected red blood cells (iRBCs) (21, 22). Given that the B-cell compartment is the primary niche for EBV persistence, it is plausible that the humoral immune response to malaria may be altered during EBV co-infection. Asymptomatic primary EBV infection, defined serologically by IgM+ viral capsid antigen (VCA) antibodies, resulted in a 4-week-long humoral immune suppression in a 2·5-year-old infant (23). The same virally induced humoral suppression was observed during symptomatic infection in young adults with IM (24). Additionally, seropositivity for EBV but not cytomegalovirus (CMV) in young infants immunized with meningococcal polysaccharide and measles vaccines showed reduced efficiency in the generation of vaccine-specific antibody responses (25). Collectively, these studies indicate that primary EBV infection in a naïve host may substantially suppress the humoral immune response. The consequences of an impaired humoral response have been associated with increased risk for severe malarial anaemia (26, 27). These observations provide compelling evidence that implicate EBV co-infection in altering immunity to malaria. Indeed, one study noted higher EBV loads were associated with increased susceptibility and frequency of malarial attacks in children in Gabon (28).
Figure 1.
The life cycle of Epstein–Barr virus. EBV is thought to be able to infect B cells directly as lytic virions or epithelial cells during oral transmission. However, one theory suggests that EBV may initially infect B cells even before infecting epithelial cells because X-linked agammaglobulinemia patients are not infected with EBV. EBV efficiently infects naïve B cells that express both CD21 and CD35. However, activated B cells only express CD21, but no or limited CD35 which explains why a significant number of B cells become refractory to EBV infection.
Although the majority of children in Equatorial Africa would have been exposed to both pathogens, not all will display exacerbated malarial or EBV-associated pathologies. This may vary depending on entomological inoculation rates for malaria and the viral shedding status of the mother. More importantly, timing of primary EBV infections may lead to diverse outcomes with respect to the generation of antimalarial immunity and disease severity. We currently do not understand to what extent EBV exposure precedes Plasmodium infection in young children of sub-Saharan Africa and the role transplacentally acquired maternal antibodies might play in modulating an infant’s response to EBV infection. As such, it is difficult to speculate on the proportion of children that may be at a higher risk of severe malarial disease as a result of EBV modulation of the host immune response. Additionally, there are currently no reports in the literature that examine the age of acquisition of EBV infection and the status of P. falciparum co-infection and disease severity. Consequently, the hypotheses proposed in this review speculate on the possible outcomes of EBV-mediated immunomodulation in contributing to severe malarial disease based on novel findings using mouse models of co-infection.
As suggested by epidemiologic studies, we propose that the risk of developing severe malaria in young nonimmune children is dependent on the force of infection (FOI), defined as the rate of infection of a susceptible host with a pathogen per unit of time, with EBV and/or malaria, as suggested by epidemiological studies for the risk of developing eBL. Malaria FOI exhibits seasonality and is correlated with age (29). The peak FOI occurs in children 2–5 years of age coinciding with the age range of highest malarial morbidity (30). In fact, EBV infection was shown to occur at a younger age in children living in areas holoendemic for P. falciparum. This earlier infection rate geographically correlated with an increased risk in developing eBL later in childhood (9). The time between primary EBV infection and the development of eBL provides an opportunity for the virus to replicate during malarial co-infections (18). We and others speculate that this repeated interplay between pathogens results in progressively diminished EBV-specific T-cell control, which has previously been proposed as one of the mechanisms by which malaria facilitates progression to eBL (31).
CAN GAMMAHERPESVIRUSES ALTER THE PATHOGENESIS OF MALARIAL INFECTION?
To our knowledge, there are currently no published studies that have investigated the impact of a primary EBV infection of a naïve host on the development of severe malaria. However, it is currently feasible to examine whether a gammaherpesvirus infection could exacerbate the pathogenesis of malaria using well-defined rodent models.
Rodent models of malarial infection
There are several well-described models of malarial infection that were isolated from thicket rats in the 1960s (32) that collectively demonstrate different aspects of the pathogenesis of human malarial infections, and that share several key characteristics of immune responses to human Plasmodium parasites. The Plasmodium chabaudi AS and Plasmodium yoelii XNL models of malaria are nonlethal in most strains of mice, whereas Plasmodium berghei ANKA infections of C57BL/6 or CBA mice mimic some features of cerebral malaria (CM), a syndrome commonly referred to as experimental cerebral malaria (ECM). Immunologically, Plasmodium infection induces inflammatory responses within hours of infection in both mice and humans. Reports on human Plasmodium infection indicate a positive correlation between IL-12 levels and protection from severe malaria (33-36). Although there are contradicting results regarding the ability of P. falciparum to activate dendritic cells (DCs) (37-40), activation of rodent DCs exposed to iRBCs of rodent malarial parasites (41-44) has been repeatedly shown. In general, DC activation is thought to lead to a Th1 response and this can be measured in peripheral blood mononuclear cells (PBMCs) of individuals infected with malaria and is also a feature of rodent Plasmodium models. Interferon-γ (IFN-γ) can be produced from NK cells, γδ-T cells, CD4+ and CD8+ T cells both in humans (45-48) and in rodents (49-54) infected with malaria. Antibodies are thought to be a primary mechanism of controlling the erythrocytic stage of malarial infection (21, 55), and multiple immunodominant parasite antigens have been identified. In fact, in one study, the breadth of the antibody response with respect to reactivity to multiple antigens has been correlated with protection against developing clinical malaria (55). The requirement for antibody to control the primary peak of parasitaemia in the P. chabaudi and P. yoelii models of nonlethal malaria differs, with CD4+ T-cell-mediated immunity being sufficient to control P. chabaudi infection (56). Although the development of humoral immunity against Plasmodium infection appears to require constant challenge infections in humans, the P. chabaudi model of malaria has been used to demonstrate that memory B cells can form in rodent malarial infections (57) and contribute to protection against secondary challenge (58, 59).
The MHV68 rodent model of gammaherpesvirus infection
The murine gammaherpesvirus 68 (MHV68) has been extensively used to understand gammaherpesvirus infection in humans, which exhibits restricted host tropism. The two known human gammaherpesviruses include EBV and Kaposi’s sarcoma-associated herpesvirus (KSHV). Gammaherpesviruses are capable of maintaining lytic and latent infection in the host. Of importance, analysis of MHV68 infection in the mouse has been instrumental in understanding the lytic phase of infection, which is difficult to assess in human subjects. Viral transmission is postulated to occur orally as evidenced by the enumeration of EBV titres in the saliva of patients suffering from IM. In the laboratory setting, intranasal infection with a luciferase expressing MHV68 indicated viral replication and presence in the cervical draining lymph nodes, the lungs, the spleen (splenomegaly) and vagina during female C57BL/6 infections (60). Splenomegaly is observed in EBV-infected patients experiencing IM (61), and viral titres have also been detected in female and male genital secretions (62, 63). Intranasal infection of C57BL/6 mice consists of an acute lytic replication in the lungs, peaking around 4–6 days and cleared by day 12 post-infection. Acute lytic replication in the lung seeds B cells travelling to the spleen, which initiates germinal centre responses and splenomegaly (64). Nearly 80% of virus-infected B cells show a germinal centre phenotype around day 18 post-infection (65). By day 16–18 post-infection, the virus reaches peak latency levels, with a reduction in productive viral replication, and 1 : 100 cells being latently infected with virus. Frequency of viral reactivation is approximately 1 : 1000 at this time point and is almost undetectable by day 42 post-infection.
The virus maintains latency predominantly in the memory B-cell (66) reservoir but is capable of infecting macrophages and DCs (67). Of importance are the two distinct phases of viral replication. In this review, the initial lytic infection of the virus in a naïve host which consists of fulminant viral replication in the lung and inflammatory response induction will be referred to as acute primary infection. The strong inflammatory response induced by acute infection generates memory to a primary viral infection. The second phase of viral infection is reactivation from latency and is considered to be a distinct aspect of the viral life cycle. Low-level reactivating virus does not generate potent inflammatory responses in an immune-competent host and is required for seeding the latency reservoir. In the mouse, the second phase of viral reactivation from latency can be assessed by monitoring the levels of preformed virus in the lung and spleen 18 days post-infection. If viral levels persist, the infection is not considered to be properly controlled. The equivalent of this type of infection in humans, using our definition, is the asymptomatic primary infection of a naïve child who has lost maternal antibody protection against EBV. This primary infection, although asymptomatic, will generate a memory response, the formation of which is dependent on the innate inflammatory response. This immune response is also distinct from viral reactivation from latency in which stimuli that expand the latently infected pool will induce virus production. In an immune-competent host, the antiviral response is considered to be effective and will ensure proper control of reactivating virus (67). An ELISA-based technique can discriminate between a primary, recent primary or reactivating EBV infection in children (68). This assay feasibly could be used to evaluate the stage of EBV infection present in children experiencing severe malaria.
Control of MHV68 infection is not exclusively dependent on CD4+ T cells, CD8+ T cells or B cells during the acute phase. However, all three subsets perform a nonredundant role in host protection during latency and are required to control chronic viral replication (69-73). MHV68 induces a potent type I interferon (IFN) response that is required for proper control of acute infection and prevention of host lethality (74). The virus can be detected by Toll-like receptor 9 (TLR9) which senses hypomethylated CpG motifs in the viral DNA (75). Infection in TLR9−/− animals results in slightly higher levels of viral titre in the lungs, but does not significantly alter clearance of the infection or establishment of latency (75). In contrast, MyD88−/− animals clear acute infection normally, but have a defect in the establishment of latency (76) which could be due the requirement of TLR signalling in B cells for the generation and maintenance of germinal centre responses during an infection (77). Recent work also indicates that the unique M2 gene of MHV68 contains an interferon-sensitive response element (ISRE) to the transcriptionally repressive interferon response factor 2 (IRF2) (78). This ISRE allows the reactivating virus to be responsive to type I IFN signals and negatively regulate the viral life cycle, minimizing host lethality while maintaining a latency reservoir.
Co-infection with MHV68 and malaria in rodents
We, and others, have tested the impact of acute MHV68 infection on the pathogenesis of erythrocytic nonlethal P. yoelii XNL and P. chabaudi AS or lethal P. berghei ANKA co-infections in C57BL/6 mice. Acute MHV68 infection resulted in deficiencies in the generation of malaria-specific CD8+ T-cell responses (FH Amante and CR Engwerda, unpublished data) and the maintenance of T follicular helper cells, thus impacting the sustained output of plasma cells in the spleen (79). The net effect of this suppression reduced the CD8+ T-cell-mediated pathology associated with ECM in the P. berghei ANKA model (FH Amante and CR Engwerda, unpublished data) and transformed a nonlethal P. yoelii XNL into a lethal infection (80). In the P. yoelii co-infection model, this resulted in poor antimalarial humoral immunity, loss of control of the primary peak of parasitaemia and the development of lethal SMA (79). Acute MHV68 co-infection with P. chabaudi resulted in similar deficiencies in humoral immunity (79). However, no measurable effect on the primary peak parasitaemia in mice infected with P. chabaudi AS was noted (Table 1), which supports previous findings that B-cell-deficient mice are capable of controlling the primary malarial infection (56).
Table 1.
Plasmodium infection and outcomes modified by gammaherpesvirus co-infection
| Effect of type 1 interferon |
Requirement for cell-mediated immunity |
Requirement for humoral responses |
Effect of overlapping acute viral infection (less than 16 days post-viral infection) |
Effect of a latent viral infection (more than 16 days post-viral infection) |
|
|---|---|---|---|---|---|
|
Plasmodium yoelii XNL (79) C57BL/6 mice |
Inhibits infection by suppressing reticulocytosis (103) | Requires CD4+ T cells to control the primary peak of infection (140) CD8+ T cells may provide some protection against P. yoelii XL (51) |
Requires antibody for control of primary peak of infection (56) | Nonlethal infection becomes lethal (80) due to uncontrolled primary peak of infection | Immunosuppressive effects on humoral responses wanes after 60 days (79) |
|
Plasmodium chabaudi AS (79) C57BL/6 mice |
Suppresses protective IFN-γ production (100) | Requires CD4+ T cells to control the primary peak of infection (140) CD8+ T cells may provide some protection against erythrocytic malaria (120, 142) |
Does not require antibody for control of acute peak of infection (56) Less able to control secondary peaks of infection (59) |
Nonlethal infection remains nonlethal across primary peak of infection | No data |
|
Plasmodium berghei ANKA (FH Amante and CR Engwerda, unpublished data) C57BL/6 mice |
Suppresses CD4+ T-cell responses and control of erythrocytic parasitaemia (100) | CD4+ T cells required for the development of ECM (142) CD8+ T cells trafficking to the brain are pathogenic (121, 122, 144) |
Required for the control of iRBCs for related species Plasmodium berghei NK65 (145) | Protection from death during the inflammatory phase of experimental cerebral malaria (during or just after lytic infection) | No measureable effect on death from ECM (6 weeks post-infection) |
| Plasmodium falciparum | Certain polymorphisms in the IFNAR1 gene have been found to confer protection from cerebral malaria (146) and enhanced susceptibility to severe malarial anaemia (147) | CD4+ T-cell IFN-γ responses are associated with protection from severe disease (148-151) | Antibody response is associated with protection from severe malarial anaemia and control of blood-stage parasitaemia (21, 22, 26, 27) | No data | No data |
Immunologically, it is clear from all three mouse models that MHV68 infection is associated with an immune suppression of specific subsets in the adaptive immune response. C57BL/6 mice acutely infected with MHV68 lose their ability to induce antibody responses to secondary challenge of an unrelated antigen (81). This transient effect has also been observed in young adults experiencing acute EBV infection (23, 24). Importantly, for the MHV68 malarial co-infection studies described above, the effects of MHV68 are dependent on acute and not latent infection, with the detrimental effects of dissipating after 30 days post-infection. It has been demonstrated for the P. chabaudi and P. yoelii nonlethal rodent co-infection models that latent MHV68 infection has very little impact on control of the parasite infection (79, 80). In this respect, malaria differs from other infections such as intracellular bacteria Listeria monocytogenes (82) and Yersinia pestis (83) which show that MHV68 latency, but not acute infection, could protect against infection by maintaining a basal secretion of IFN-γ and activation of macrophages. Given that monocyte and macrophage activation is also considered to play a protective role in phagocytosis of iRBCs and free merozoites (84, 85), it is surprising that a bigger effect of latent MHV68 infection on control of malarial infection is not seen. This could arise if iRBCs are more efficient at evading macrophage clearance to establish infection which may be executed by sequestration of iRBCs to avoid splenic clearance from the circulation (86). The data in rodent co-infections described above demonstrate that primary gammaherpesvirus infection can alter immunity generated to a secondary Plasmodium infection which may result in altered pathogenesis of malarial disease.
GAMMAHERPESVIRUS-INDUCED SUPPRESSION OF THE INNATE IMMUNE RESPONSE TO MALARIA
Although the effects of acute MHV68 infection on malarial pathogenesis appear to stem from alterations in the antimalarial adaptive immune response, adaptive immune responses are generally driven by innate immunity. The ability of gammaherpesviruses to specifically modulate innate immunity may therefore have interesting implications on the immunobiology of malaria during co-infection (Figure 2). Key mechanisms of immune evasion to ensure that the innate immune response does not sterilize the viral infection have been documented in both the murine and human gammaherpesviruses (reviewed in Ref. 87). Down-stream effectors of the type I IFN response include the interferon regulatory factors (IRFs). IRFs are integral to TLR - and NF-κB-mediated signalling. The MHV68-encoded ORF36 can directly inhibit cellular IRF3 (88), and the latency-associated protein of MHV68, ORF73 (LANA), can directly inhibit NF-κB signalling by targeting the p65 subunit to ubiquitin-mediated proteasomal degradation (89, 90). Such features are not unique to this rodent gammaherpesvirus as KSHV can negatively regulate IFN signalling through the expression of homolog viral IRF (vIRF) and v-IL-6 proteins (91-94). The immediate early gene product of EBV, BZLF1, is capable of inhibiting IRF7 (95), and BGLF4 is an inhibitor of IRF3 signalling (96). Collectively, these immune modulating mechanisms are required for proper initiation of the viral life cycle of gammaherpesviruses and thus are primarily employed during acute rather than latent infection (reviewed in Ref. 97). Inhibition of IRF impedes the production of key inflammatory cytokines such as the type I IFNs, IFN-α and IFN-β which limit viral replication, promote apoptosis of the infected cell and activate antigen-presenting cells (APCs) to stimulate the antigen-specific T-cell helper responses. This virus-induced immune regulation may negatively impact secondary adaptive immune responses.
Figure 2.
Model of EBV interference with antimalarial immunity. EBV-infected B cells secrete immunosuppressive cytokines (shown in box). The same cytokines are also secreted by EBV-infected pDC, mDC and monocytes – which increases their overall abundance. This immunomodulating cytokine milieu then down-regulates IFN-γ being produced by EBV-specific CD4+ and CD8+ T cells (hashed lines around IFN-γ) – which then in turn prevents monocytes from performing phagocytosis of Plasmodium-infected RBCs or free merozoites and also prevents follicular T cells from providing help to B cells to make antibodies. EBV is represented by the black pentagons. Parasitized red blood cells are represented by red outlined purple circles. Points of interaction are represented by solid lines and inhibition by the dashed lines. BAFF, B-cell-activating factor; BlyS, B lymphocyte stimulator; IDO, indoleamine 2,3 dioxygenase; IFN, interferon; IL, interleukin; iRBCs, malaria-infected red blood cells; mDC, myeloid dendritic cells; pDC, plasmacytoid dendritic cells; TGF, transforming growth factor.
Similar to gammaherpesviruses, Plasmodium induces type I IFN production from APCs via ligation of TLR9 by DNA/protein complexes trapped in haemozoin crystals (98, 99), an insoluble by-product of haemoglobin digestion in RBCs. However, the role that type I IFNs play in antimalarial immunity appears to vary amongst rodent models. Published studies do not agree on the effect of type I IFN on controlling clearance of iRBCs during P. chabaudi infection (100, 101). In contrast, type I IFN may help to control the frequency of iRBCs during P. yoelii infection due to its suppressive effects on erythropoiesis (102) which reduces the release of reticulocytes into the circulation (103), the target cell in a P. yoelii XNL infection. Nevertheless, type I IFN has been shown to promote hematopoietic stem cell exhaustion (104, 105), and in the context of viral infections, type I IFN has been shown to have suppressive properties on hematopoiesis during lymphocytic choriomeningitis virus (LCMV) infections (106). This suggests a potential role for chronic type I IFN signalling in the exacerbation of severe malarial anaemia (SMA), a condition where erythropoietic and hematopoietic suppression is a key feature (107). Indeed, the human immunodeficiency virus (HIV), although not a herpes virus, is associated with induction of type I IFN (108) and published studies suggest that P. falciparum and HIV co-infection can be associated with exacerbation of SMA (109, 110).
Type I IFN can suppress the CD4+ T-cell response in the P. berghei ANKA model of malaria (80). Although the mechanism by which this occurs is not clearly understood, it is likely to be via an effect on APCs which activate T-helper cells. In fact, recent work has demonstrated that MHV68-infected DCs could not up-regulate activation markers and present antigen as efficiently as those not exposed to MHV68 (111). In a similar vein, EBV infection of monocytes prevented their maturation into DCs and promoted apoptosis (112). Of interest, the expression of DC activation markers during an MHV68 and P. berghei co-infection was reduced as compared to a single P. berghei infection (FH Amante and CR Engwerda, unpublished data) supporting a model that implicates virus-induced immune responses as negative regulators of APC activation during a co-infection with malaria.
Gammaherpesviruses have been shown to increase the levels of IL-10 production during an infection. MHV68 does this via the unique gene product M2 (113, 114). Similarly, EBV encodes for a viral IL-10 homolog (115). From the perspective of EBV, virus-induced IL-10 production is postulated to increase B-cell proliferation, thus expanding the latency reservoir. IL-10 is generally regarded as having an immunosuppressive effect on Th1 responses (116), and rodent models of malaria suggest that IL-10 can impair the control of circulating Plasmodium parasitaemia. However, IL-10 seems to play a critical role in protection against over-exuberant inflammatory immune responses which lead to pathogenesis during a malarial infection (49). The effect of virus-induced IL-10 on T-cell activation has not been formally demonstrated either in mouse models of infection or in human EBV infection. Interestingly, the levels of IL-10 production from CD4+ T cells were not altered during MHV68 co-infection with either P. yoelii or P. chabaudi (79).
Indoleamine 2,3 dioxygenase (IDO) is an enzyme that catalyses the breakdown of tryptophan (TRP) via the kynurenine pathways (117) and negatively regulates Th1 CD4+/CD8+ T cells, while activating the CD4+ FOXP3+ T regulatory subset. One of the signals that promotes IDO induction is IL-10. However, there was no evidence that the regulatory CD4+ FOXP3+ T-cell or the Th1 CD4+ T-cell subsets were altered during MHV68 and P. yoelii or P. chabaudi co-infection (79), suggesting IDO is not responsible for the suppressive effects of MHV68 on antimalarial humoral immunity.
MODULATION OF CD8+ T-CELL RESPONSES DURING EBV/P. FALCIPARUM CO-INFECTION: DOES IT MATTER TO MALARIA?
It has been demonstrated that T-cell memory to specific pathogens may be impaired during successive and chronic secondary viral infections (118, 119). Thus, it is postulated that repeated exposure to P. falciparum infection results in the exhaustion of antigen-specific CD8+ T-cell responses. Indeed, the primary molecular link between EBV and P. falciparum co-infection is related to the defective EBV-specific CD8+ T-cell response that fails to prevent the outgrowth of virally transformed B cells and increases the risk of eBL (13–16, 18, 19, 31). Amante et al. have shown that MHV68 and P. berghei co-infection resulted in reduced P. berghei-specific CD8+ T-cell responses (FH Amante and CR Engwerda, unpublished data), suggesting that EBV infection could also limit the generation of malaria-specific CD8+ T cells. Healthy EBV-infected children residing in malarial holoendemic areas and patients with eBL develop T-cell responses to malarial antigens (14), yet children diagnosed with eBL rarely have a history of severe malarial episodes supporting the hypothesis that EBV limits uncontrolled inflammation associated with hyperactive T-cell responses.
CD8+ T cells are expanded in plasmodial infection, but much of the focus on the target and function of these cells has centred on liver-stage malaria whereby CD8+ T-cell-mediated lysis of infected hepatocytes containing developing parasites is considered to be a mechanism of protection against pre-erythrocytic malaria (reviewed in Ref. 5). Studies on the role of CD8+ T-cell responses to erythrocytic malaria with respect to control of iRBCs are scarce. Only one report has described any role for CD8+ T-cell activation in a nonlethal P. chabaudi malarial infection (120), which suggested that CD8+ T-cell activation by CD11c+CD8α+ DCs is essential for control of peripheral parasitaemia and prevention of hyperparasitaemia-induced death that is associated with SMA. Transfer of activated CD8+ T cells from P. yoelii-infected mice was able to provide some protection against a lethal P. yoelii strain (51). The mechanism by which CD8+ T-cell responses play a role in controlling blood-stage parasitaemia during nonlethal infections is unclear; however, IFN-γ production and its contribution in generating cytophilic antibody responses and activating phagocytic macrophages for iRBC clearance are likely to be instrumental.
Limiting CD8+ T-cell responses against malaria may have beneficial effects in reducing the development of organ-specific manifestations of malaria. T-cell trafficking to target organs where iRBC sequestration occurs during ECM is thought to be a key event in the development of damage in the brain (121, 122) and lung (123, 124), but not liver (125). CM is a serious condition characterized by encephalopathy, deep coma with seizures that are often fatal, affecting 3 million children worldwide in areas where malaria is endemic (1). iRBCs are thought to sequester and adhere to the brain endothelium via interactions between variant molecules exported to the surface of the infected RBC such as P. falciparum erythrocyte membrane protein-1 (PfEMP-1) and associated families of molecules that can interact with endothelial expressed ICAM-1 (126) and CD36 (127). Localized inflammatory responses to sequestered iRBCs occur and, at least in the P. berghei ANKA model of ECM, IFN-γ driven inflammation is a critical component of adverse outcome for infected mice (121, 122). Both CD4+ and CD8+ T cells are thought to be the important cellular mediators of ECM (128) which result in an inflammatory immune response that causes death between 8 and 10 days post-infection. CD8+ T cells are activated by blood-stage malarial infections by cross-presentation of antigens in the spleen (43, 53, 129). Thus, it is possible that if limiting the expansion of T cells extends to antimalarial CD8+ T cells, the chances of developing CM during acute EBV infection may be reduced, as demonstrated in P. berghei/MHV68 co-infections (FH Amante and CR Engwerda, unpublished data). The mechanism by which EBV infection could alter antimalarial T-cell responses is speculative but may result from the effects of EBV infection of monocytes and in turn macrophage or DC responses to Plasmodium, or alternatively be related to the ability of EBV, in particular EBV type 2, to infect human CD8+ T cells and alter cytokine production (130).
IMPAIRMENT OF THE ANTIMALARIAL HUMORAL IMMUNE RESPONSE BY GAMMAHERPESVIRUS CO-INFECTION
In the context of a co-infection with malaria, some studies suggest that polyclonal B-cell stimulation by P. falciparum results in the expansion of virally infected B cells, thus increasing viral load in the host (18, 131-134). B cells require specific IRF signalling pathways for proper germinal centre responses during infections. T-dependent antigen-specific responses requires TLR stimulation of the B cell in addition to T-helper cell function (77). As EBV is capable of inhibiting IRF and TLR signalling pathways in B cells, it is a logical assumption that virus-mediated regulation of infected B cells may impact humoral responses to secondary unrelated antigenic challenges. The immune-suppressive effects exerted by acute MHV68 and EBV infection on humoral responses have previously been reported in vivo (23, 24, 81), and it appears that the transient immune suppression observed was contingent on acute viral replication and specifically impacted the humoral response. This suppression was still operational in mice deficient in the majority of TLR signalling (MyD88−/−) which presumably abrogated a large portion of the TLR9-dependent type I IFN response to MHV68, as well as in the absence of a stimulator of IFN genes (STING) (135). This suggests that, although type I IFN may be important in suppressing CD4+ T-cell responses against malaria (100), the induction of type I IFN by MHV68 may not explain the deficiency in the generation of antimalarial humoral immunity in the P. yoelii XNL and P. chabaudi AS models (79). Interestingly, the MHV68-induced suppression of the humoral immune response in this study was not dependent on the anti-inflammatory cytokine IL-10 (81), but this does not rule out a dependency on other immunosuppressive molecules such as TGF-β or IDO.
During P. yoelii and MHV68 co-infection, mice showed impaired humoral responses that impacted the control of peripheral parasitaemia. This ultimately resulted in lethality due to SMA (79). The observation that acute MHV68 infection can profoundly suppress humoral immunity to malaria in this mouse model demonstrates that interactions can occur between these pathogens that may be important in the development of clinical malaria. This is supported by evidence for the requirement of antibodies in clearing iRBCs (21, 136) and the established link between insufficient humoral immunity and severe malarial disease in young children (137). MHV68 co-infection with P. chabaudi induced the same humoral suppression (79). However, as humoral immunity is not required for the control of primary peak of infection in this model, the co-infection did not impair acute malarial disease. We speculate that impairment in the development of malaria-specific B-cell responses during primary infection may impact the generation of memory B-cell responses needed to fight subsequent infections (56, 59). Future studies are needed to test this hypothesis.
CONCLUSION
It has been previously demonstrated that immunity to the severe form of malaria is acquired within 1–2 infections in areas of endemic transmission for P. falciparum (138). Yet, despite this minimal number of infections, almost 1 million children die annually as a result of complications associated with severe malaria. This subset of individuals is also restricted to a narrow age bracket consisting of children younger than 5 years of age. Could primary EBV infection impact immune responses to a subsequent malarial infection? The biology of EBV and malarial infections suggests that there are multiple points of immune syndemism that could impact the pathogenesis of malarial infection. Rodent models of EBV and malarial co-infection demonstrate that it is possible for a primary gammaherpesvirus infection to negatively modulate the generation of antimalarial immunity, thus transforming nonlethal infections into lethal ones. However, the suppressive effects of gammaherpesviruses diminish after 30 days of infection. There is no direct human evidence that primary EBV infection negatively impacts the development of Plasmodium-specific adaptive immune responses, nor whether the timing of primary EBV infection in relation to malarial infections leads to more severe malarial disease. The latter could be examined using longitudinal birth cohort studies to determine whether timing of primary EBV infection (as measured by IgM+ VCA+ antibodies in the sera) during the development of an infant’s antimalarial immunity impacts the effectiveness of a response that protects from severe clinical malaria. Case–control studies are confounded by the inability to track relative order of infection with EBV and Plasmodium. A key outcome of longitudinal studies will be to (i) address the long unanswered question of whether children are predominantly infected with EBV prior to being infected with malaria and (ii) whether severe malaria is associated with a narrow time frame of Plasmodium infection following primary exposure to EBV. Current studies have only found a correlation between malarial infection and increased EBV titres. However, these studies fail to demonstrate whether these EBV titres are a result of viral reactivation from latency or normal viral oscillation during the establishment of latency (139). Our studies in mice clearly show that an acute and latent infection with MHV68 have dramatically different outcomes on co-infection with Plasmodium. Addressing the dynamics of this co-infection has implications whether early-age primary EBV infection is a risk factor for the poor generation of antimalarial immunity and development of clinical malaria in infants.
ACKNOWLEDGEMENTS
We would like to thank Drs. Engwerda and Amante for critical reading of the manuscript.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.World Health Organization. Global Malaria Programme. World Malaria Report 2012. Geneva, World Health Organization, 2012: iv, 249 pp. [Google Scholar]
- 2.Miller LH, Baruch DI, Marsh K & Doumbo OK. The pathogenic basis of malaria. Nature 2002; 415: 673–679. [DOI] [PubMed] [Google Scholar]
- 3.Langhorne J, Ndungu FM, Sponaas AM & Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9: 725–732. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood B, Marsh K & Snow R. Why do some African children develop severe malaria? Parasitol Today 1991; 7: 277–281. [DOI] [PubMed] [Google Scholar]
- 5.Riley EM & Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med 2013; 19: 168–178. [DOI] [PubMed] [Google Scholar]
- 6.Biggar RJ, Henle W, Fleisher G, et al. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer 1978; 22: 239–243. [DOI] [PubMed] [Google Scholar]
- 7.Biggar RJ, Henle G, Bocker J, et al. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer 1978; 22: 244–250. [DOI] [PubMed] [Google Scholar]
- 8.Henle G & Henle W. Observations on childhood infections with the Epstein-Barr virus. J Infect Dis 1970; 121: 303–310. [DOI] [PubMed] [Google Scholar]
- 9.Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 2012; 205: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moormann AM, Chelimo K, Sumba OP, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis 2005; 191: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 11.Moormann AM, Snider CJ & Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis 2011; 24: 435–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg 1958; 46: 218–223. [DOI] [PubMed] [Google Scholar]
- 13.Moormann AM, Chelimo K, Sumba PO, et al. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis 2007; 195: 799–808. [DOI] [PubMed] [Google Scholar]
- 14.Moormann AM, Heller KN, Chelimo K, et al. Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-gamma T cell responses. Int J Cancer 2009; 124: 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittle HC, Brown J, Marsh K, et al. T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum malaria. Nature 1984; 312: 449–150. [DOI] [PubMed] [Google Scholar]
- 16.Njie R, Bell AI, Jia H, et al. The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis 2009; 199: 31–38. [DOI] [PubMed] [Google Scholar]
- 17.Snider CJ, Cole SR, Chelimo K, et al. Recurrent Plasmodium falciparum malaria infections in Kenyan children diminish T-cell immunity to Epstein Barr virus lytic but not latent antigens. PLoS One 2012; 7: e31753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chene A, Donati D, Guerreiro-Cacais AO, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog 2007; 3: e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chene A, Nylen S, Donati D, et al. Effect of acute Plasmodium falciparum malaria on reactivation and shedding of the eight human herpes viruses. PLoS One 2011; 6: e26266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piriou E, Kimmel R, Chelimo K, et al. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol 2009; 81: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Mc GI & Carrington S. Gammaglobulin and acquired immunity to human malaria. Nature 1961; 192: 733–737. [DOI] [PubMed] [Google Scholar]
- 22.Sabchareon A, Burnouf T, Ouattara D, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 1991; 45: 297–308. [DOI] [PubMed] [Google Scholar]
- 23.Bowen TJ, Wedgwood RJ, Ochs HD & Henle W. Transient immunodeficiency during asymptomatic Epstein-Barr virus infection. Pediatrics 1983; 71: 964–967. [PubMed] [Google Scholar]
- 24.Junker AK, Ochs HD, Clark EA, Puterman ML & Wedgwood RJ. Transient immune deficiency in patients with acute Epstein-Barr virus infection. Clin Immunol Immunopathol 1986; 40: 436–446. [DOI] [PubMed] [Google Scholar]
- 25.Holder B, Miles DJ, Kaye S, et al. Epstein-Barr virus but not cytomegalovirus is associated with reduced vaccine antibody responses in Gambian infants. PLoS One 2010; 5: e14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobano C, Rogerson SJ, Mackinnon MJ, et al. Differential antibody responses to Plasmodium falciparum merozoite proteins in Malawian children with severe malaria. J Infect Dis 2008; 197: 766–774. [DOI] [PubMed] [Google Scholar]
- 27.Leoratti FM, Durlacher RR, Lacerda MV, et al. Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J 2008; 7: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yone CL, Kube D, Kremsner PG & Luty AJ. Persistent Epstein-Barr viral reactivation in young African children with a history of severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 2006; 100: 669–676. [DOI] [PubMed] [Google Scholar]
- 29.Mueller I, Schoepflin S, Smith TA, et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci USA 2012; 109: 10030–10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith T, Beck HP, Kitua A, et al. Age dependence of the multiplicity of Plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans R Soc Trop Med Hyg 1999; 93(Suppl 1): 15–20. [DOI] [PubMed] [Google Scholar]
- 31.Rochford R, Cannon MJ & Moormann AM. Endemic Burkitt’s lymphoma:a polymicrobial disease? Nat Rev Microbiol 2005; 3: 182–187. [DOI] [PubMed] [Google Scholar]
- 32.Killick-Kendrick R & Peters W. Rodent Malaria. London, Academic Press, 1978. [Google Scholar]
- 33.Perkins DJ, Weinberg JB & Kremsner PG. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis 2000; 182: 988–992. [DOI] [PubMed] [Google Scholar]
- 34.Malaguarnera L, Imbesi RM, Pignatelli S, et al. Increased levels of interleukin-12 in Plasmodium falciparum malaria: correlation with the severity of disease. Parasite Immunol 2002; 24: 387–389. [DOI] [PubMed] [Google Scholar]
- 35.Luty AJ, Perkins DJ, Lell B, et al. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun 2000; 68: 3909–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson MM, Tam MF, Wolf SF & Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol 1995; 155: 2545–2556. [PubMed] [Google Scholar]
- 37.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 1999; 400: 73–77. [DOI] [PubMed] [Google Scholar]
- 38.Giusti P, Urban BC, Frascaroli G, et al. Plasmodium falciparum-infected erythrocytes and beta-hematin induce partial maturation of human dendritic cells and increase their migratory ability in response to lymphoid chemokines. Infect Immun 2011; 79: 2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee P & Chauhan VS. Plasmodium falciparum-free merozoites and infected RBCs distinctly affect soluble CD40 ligand-mediated maturation of immature monocyte-derived dendritic cells. J Leukoc Biol 2008; 84: 244–254. [DOI] [PubMed] [Google Scholar]
- 40.Elliott SR, Spurck TP, Dodin JM, et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect Immun 2007; 75: 3621–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ing R, Segura M, Thawani N, Tam M & Stevenson MM. Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J Immunol 2006; 176: 441–450. [DOI] [PubMed] [Google Scholar]
- 42.Ruedl C, Rieser C, Bock G, Wick G & Wolf H. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer’s patches. Eur J Immunol 1996; 26: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 43.de Walick S, Amante FH, McSweeney KA, et al. Cutting edge: conventional dendritic cells are the critical APC required for the induction of experimental cerebral malaria. J Immunol 2007; 178: 6033–6037. [DOI] [PubMed] [Google Scholar]
- 44.Perry JA, Rush A, Wilson RJ, Olver CS & Avery AC. Dendritic cells from malaria-infected mice are fully functional APC. J Immunol 2004; 172: 475–182. [DOI] [PubMed] [Google Scholar]
- 45.Artavanis-Tsakonas K, Eleme K, McQueen KL, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol 2003; 171: 5396–5405. [DOI] [PubMed] [Google Scholar]
- 46.Chelimo K, Embury PB, Sumba PO, et al. Age-related differences in naturally acquired T cell memory to Plasmodium falciparum merozoite surface protein 1. PLoS One 2011; 6: e24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Ombrain MC, Hansen DS, Simpson KM & Schofield L. gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol 2007; 37: 1864–1873. [DOI] [PubMed] [Google Scholar]
- 48.Troye-Blomberg M, Worku S, Tangteer-awatana P, et al. Human gamma delta T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand J Immunol 1999; 50: 642–650. [DOI] [PubMed] [Google Scholar]
- 49.Freitas do Rosario AP, Lamb T, Spence P, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 2012; 188: 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Ombrain MC, Robinson LJ, Stanisic DI, et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 2008; 47: 1380–1387. [DOI] [PubMed] [Google Scholar]
- 51.Imai T, Shen J, Chou B, et al. Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur J Immunol 2010; 40: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 52.Ing R & Stevenson MM. Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infect Immun 2009; 77: 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundie RJ, de Koning-Ward TF, Davey GM, et al. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc Natl Acad Sci USA 2008; 105: 14509–14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seixas EM & Langhorne J. gammadelta T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol 1999; 162: 2837–2841. [PubMed] [Google Scholar]
- 55.Osier FH, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 2008; 76: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Heyde HC, Huszar D, Woodhouse C, Manning DD & Weidanz WP. The resolution of acute malaria in a definitive model of B cell deficiency, the JHD mouse. J Immunol 1994; 152: 4557–1562. [PubMed] [Google Scholar]
- 57.Ndungu FM, Cadman ET, Coulcher J, et al. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog 2009; 5: e1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stephens R, Ndungu FM & Langhorne J. Germinal centre and marginal zone B cells expand quickly in a second Plasmodium chabaudi malaria infection producing mature plasma cells. Parasite Immunol 2009; 31: 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von der Weid T, Honarvar N & Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol 1996; 156: 2510–2516. [PubMed] [Google Scholar]
- 60.Francois S, Vidick S, Sarlet M, et al. Illumination of murine gammaherpesvirus-68 cycle reveals a sexual transmission route from females to males in laboratory mice. PLoS Pathog 2013; 9: e1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isaacs R. Chronic infectious mononucleosis. Blood 1948; 3: 858–861. [PubMed] [Google Scholar]
- 62.Sixbey JW, Lemon SM & Pagano JS. A second site for Epstein-Barr virus shedding: the uterine cervix. Lancet 1986; 2: 1122–1124. [DOI] [PubMed] [Google Scholar]
- 63.Israele V, Shirley P & Sixbey JW. Excretion of the Epstein-Barr virus from the genital tract of men. J Infect Dis 1991; 163: 1341–1343. [DOI] [PubMed] [Google Scholar]
- 64.Damania B & Pipas JM. DNA Tumor Viruses. New York, Springer Science + Business Media, 2009: xxvi, 794 p., 4 p. of plates p. [Google Scholar]
- 65.Collins CM & Speck SH. Tracking murine gammaherpesvirus 68 infection of germinal center B cells in vivo. PLoS One 2012; 7: e33230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willer DO & Speck SH. Long-term latent murine Gammaherpesvirus 68 infection is preferentially found within the surface immunoglobulin D-negative subset of splenic B cells in vivo. J Virol 2003; 77: 8310–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Week KE, Kim SS, Virgin HI & Speck SH. B cells regulate murine gammaherpesvirus 68 latency. J Virol 1999; 73: 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaade L, Kleines M & Hausler M. Application of virus-specific immunoglobulin M (IgM), IgG, and IgA antibody detection with a polyantigenic enzyme-linked immunosorbent assay for diagnosis of Epstein-Barr virus infections in childhood. J Clin Microbiol 2001; 39: 3902–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardin RD, Brooks JW, Sarawar SR & Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med 1996; 184: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen JP, Cardin RD, Branum KC & Doherty PC. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci USA 1999; 96: 5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dias P, Giannoni F, Lee LN, et al. CD4 T-cell help programs a change in CD8 T-cell function enabling effective long-term control of murine gammaherpesvirus 68: role of PD-1-PD-L1 interactions. J Virol 2010; 84: 8241–8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClellan KB, Gangappa S & Speck SH. Virgin HWt. Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses. PLoS Pathog 2006; 2: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sangster MY, Topham DJ, D’Costa S, et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol 2000; 164: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 74.Barton ES, Lutzke ML & Rochford R. Virgin HWt. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J Virol 2005; 79: 14149–14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guggemoos S, Hangel D, Hamm S, et al. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J Immunol 2008; 180: 438–443. [DOI] [PubMed] [Google Scholar]
- 76.Gargano LM, Moser JM & Speck SH. Role for MyD88 signaling in murine gammaherpesvirus 68 latency. J Virol 2008; 82: 3853–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasare C & Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature 2005; 438: 364–368. [DOI] [PubMed] [Google Scholar]
- 78.Mandal P, Krueger BE, Oldenburg D, et al. A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality. PLoS Pathog 2011; 7: e1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matar CG, Anthony NR, O’Flaherty BM, et al. Gammaherpesvirus co-infection with malaria suppresses anti-parasitic humoral immunity. PLoS Pathog 2011; 7: e1002371. doi: 10.1371/journal.ppat.1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haque A, Rachinel N, Quddus MR, et al. Co-infection of malaria and gamma-herpesvirus: exacerbated lung inflammation or cross-protection depends on the stage of viral infection. Clin Exp Immunol 2004; 138: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Getahun A, Smith MJ, Kogut I, van Dyk LF & Cambier JC. Retention of anergy and inhibition of antibody responses during acute gamma herpesvirus 68 infection. J Immunol 2012; 189: 2965–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yager EJ, Szaba FM, Kummer LW, et al. gamma-Herpesvirus-induced protection against bacterial infection is transient. Viral Immunol 2009; 22: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barton ES, White DW, Cathelyn JS, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007; 447: 326–329. [DOI] [PubMed] [Google Scholar]
- 84.Sponaas AM, Freitas do Rosario AP, Voisine C, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood 2009; 114: 5522–5531. [DOI] [PubMed] [Google Scholar]
- 85.Stevenson MM, Huang DY, Podoba JE & Nowotarski ME. Macrophage activation during Plasmodium chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice. Infect Immun 1992; 60: 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cunnington AJ, Riley EM & Walther M. Stuck in a rut? Reconsidering the role of parasite sequestration in severe malaria syndromes. Trends Parasitol 2013; 29: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paludan SR, Bowie AG, Horan KA & Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 2011; 11: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang S, Kim KS, Flano E, et al. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe 2009; 5: 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodrigues L, Filipe J, Seldon MP, et al. Termination of NF-kappaB activity through a gammaherpesvirus protein that assembles an EC5S ubiquitin-ligase. EMBO J 2009; 28: 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong X & Feng P. Murine-gamma herpesvirus 68 hijacks MAVS and IKKbeta to abrogate NFkappaB activation and antiviral cytokine production. PLoS Pathog 2011; 7: e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zimring JC, Goodbourn S & Offermann MK. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J Virol 1998; 72: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao SJ, Boshoff C, Jayachandra S, et al. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 1997; 15: 1979–1985. [DOI] [PubMed] [Google Scholar]
- 93.Chatterjee M, Osborne J, Bestetti G, Chang Y & Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 2002; 298: 1432–1435. [DOI] [PubMed] [Google Scholar]
- 94.Wies E, Hahn AS, Schmidt K, et al. The Kaposi’s sarcoma-associated Herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J Biol Chem 2009; 284: 8525–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hahn AM, Huye LE, Ning S, Webster-Cyriaque J & Pagano JS. Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1. J Virol 2005; 79: 10040–10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang JT, Doong SL, Teng SC, et al. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J Virol 2009; 83: 1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barton E, Mandal P & Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 2011; 29: 351–397. [DOI] [PubMed] [Google Scholar]
- 98.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci USA 2007; 104: 1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu X, Gowda NM, Kumar S & Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol 2010; 184: 4338–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haque A, Best SE, Ammerdorffer A, et al. Type I interferons suppress CD4(+) T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur J Immunol 2011; 41: 2688–2698. [DOI] [PubMed] [Google Scholar]
- 101.Voisine C, Mastelic B, Sponaas AM & Langhome J. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int J Parasitol 2010; 40: 711–719. [DOI] [PubMed] [Google Scholar]
- 102.Tarumi T, Sawada K, Sato N, et al. Interferon-alpha-induced apoptosis in human erythroid progenitors. Exp Hematol 1995; 23: 1310–1318. [PubMed] [Google Scholar]
- 103.Vigario AM, Belnoue E, Gruner AC, et al. Recombinant human IFN-alpha inhibits cerebral malaria and reduces parasite burden in mice. J Immunol 2007; 178: 6416–6425. [DOI] [PubMed] [Google Scholar]
- 104.Essers MA, Offner S, Blanco-Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009; 458: 904–908. [DOI] [PubMed] [Google Scholar]
- 105.Sato T, Onai N, Yoshihara H, et al. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med 2009; 15: 696–700. [DOI] [PubMed] [Google Scholar]
- 106.Binder D, Fehr J, Hengartner H & Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med 1997; 185: 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lamikanra AA, Brown D, Potocnik A, et al. Malarial anemia: of mice and men. Blood 2007; 110: 18–28. [DOI] [PubMed] [Google Scholar]
- 108.Hardy GA, Sieg S, Rodriguez B, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One 2013; 8: e56527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davenport GC, Hittner JB, Were T, Ong’echa JM & Perkins DJ. Relationship between inflammatory mediator patterns and anemia in HIV-1 positive and exposed children with Plasmodium falciparum malaria. Am J Hematol 2012; 87: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orish VN, Onyeabor OS, Boampong JN, et al. The effects of malaria and HIV co-infection on hemoglobin levels among pregnant women in Sekondi-Takoradi, Ghana. Int J Gynaecol Obstet 2013; 120: 236–239. [DOI] [PubMed] [Google Scholar]
- 111.Smith CM, Gill MB, May JS & Stevenson PG. Murine gammaherpesvirus-68 inhibits antigen presentation by dendritic cells. PLoS One 2007; 2: e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L, Liu D, Hutt-Fletcher L, et al. Epstein-Barr virus inhibits the development of dendritic cells by promoting apoptosis of their monocyte precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood 2002; 99: 3725–3734. [DOI] [PubMed] [Google Scholar]
- 113.Rangaswamy US & Speck SH. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog 2014; 10: e1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siegel AM, Herskowitz JH & Speck SH. The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog 2008; 4: e1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miyazaki I, Cheung RK & Dosch HM. Viral interleukin 10 is critical for the induction of B cell growth transformation by Epstein-Barr virus. J Exp Med 1993; 178: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saraiva M & O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10: 170–181. [DOI] [PubMed] [Google Scholar]
- 117.Munn DH & Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013; 34: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim SK & Welsh RM. Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J Immunol 2004; 172: 3139–3150. [DOI] [PubMed] [Google Scholar]
- 119.Liu H, Andreansky S, Diaz G, et al. Quantitative analysis of long-term virus-specific CD8+-T-cell memory in mice challenged with unrelated pathogens. J Virol 2003; 77: 7756–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guermonprez P, Helft J, Claser C, et al. Inflammatory Flt3 l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med 2013; 19: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belnoue E, Potter SM, Rosa DS, et al. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol 2008; 30: 544–553. [DOI] [PubMed] [Google Scholar]
- 122.Villegas-Mendez A, Greig R, Shaw TN, et al. IFN-gamma-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol 2012; 189: 968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang WL, Jones SP, Lefer DJ, et al. CD8 (+)-T-cell depletion ameliorates circulatory shock in Plasmodium berghei-infected mice. Infect Immun 2001; 69: 7341–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van den Steen PE, Geurts N, Deroost K, et al. Immunopathology and dexamethasone therapy in a new model for malaria-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2010; 181: 957–968. [DOI] [PubMed] [Google Scholar]
- 125.Haque A, Best SE, Amante FH, et al. High parasite burdens cause liver damage in mice following Plasmodium berghei ANKA infection independently of CD8(+) T cell-mediated immune pathology. Infect Irnmun 2011; 79: 1882–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mayor A, Bir N, Sawhney R, et al. Receptor-binding residues lie in central regions of Duffy-binding-like domains involved in red cell invasion and cytoadherence by malaria parasites. Blood 2005; 105: 2557–2563. [DOI] [PubMed] [Google Scholar]
- 127.Mo M, Lee HC, Kotaka M, et al. The C-terminal segment of the cysteine-rich inter-domain of Plasmodium falciparum erythrocyte membrane protein 1 determines CD36 binding and elicits antibodies that inhibit adhesion of parasite-infected erythrocytes. Infect Immun 2008; 76: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belnoue E, Kayibanda M, Vigario AM, et al. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol 2002; 169: 6369–6375. [DOI] [PubMed] [Google Scholar]
- 129.Piva L, Tetlak P, Claser C, et al. Cutting edge: Clec9A+ dendritic cells mediate the development of experimental cerebral malaria. J Immunol 2012; 189: 1128–1132. [DOI] [PubMed] [Google Scholar]
- 130.Coleman CB, Wohlford EM, Smith NA, et al. Epstein-Barr virus type 2 latently infects T cells, inducing an atypical activation characterized by expression of lymphotactic cytokines. J Virol 2015; 89: 2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Donati D, Zhang LP, Chene A, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun 2004; 72: 5412–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pichyangkul S, Yongvanitchit K, Kum-arb U, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol 2004; 172: 4926–4933. [DOI] [PubMed] [Google Scholar]
- 133.Gabrielsen AA Jr & Jensen JB. Mitogenic activity of extracts from continuous cultures of Plasmodium falciparum. Am J Trop Med Hyg 1982; 31(Pt 1): 441–148. [DOI] [PubMed] [Google Scholar]
- 134.Greenwood BM. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet 1974; 1: 435–436. [DOI] [PubMed] [Google Scholar]
- 135.Ishikawa H & Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008; 455: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T & Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 1990; 172: 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rovira-Vallbona E, Moncunill G, Bassat Q, et al. Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J 2012; 11: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta S, Snow RW, Donnelly CA, Marsh K & Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 1999; 5: 340–343. [DOI] [PubMed] [Google Scholar]
- 139.Hadinoto V, Shapiro M, Greenough TC, et al. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood 2008; 111: 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vinetz JM, Kumar S, Good MF, et al. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. J Immunol 1990; 144: 1069–1074. [PubMed] [Google Scholar]
- 141.Langhorne J, Simon-Haarhaus B & Meding SJ. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett 1990; 25: 101–107. [DOI] [PubMed] [Google Scholar]
- 142.van der Heyde HC, Manning DD, Roopenian DC & Weidanz WP. Resolution of blood-stage malarial infections in CD8+ cell-deficient beta 2-m0/0 mice. J Immunol 1993; 151: 3187–3191. [PubMed] [Google Scholar]
- 143.Yanez DM, Manning DD, Cooley AJ, Weidanz WP & van der Heyde HC. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol 1996; 157: 1620–1624. [PubMed] [Google Scholar]
- 144.Claser C, Malleret B, Gun SY, et al. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS ONE 2011; 6: e18720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nunes JK, Starnbach MN & Wirth DF. Secreted antibody is required for immunity to Plasmodium berghei. Infect Immun 2009; 77: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aucan C, Walley AJ, Hennig BJ, et al. Interferon-alpha receptor-1 (IFNAR1) variants are associated with protection against cerebral malaria in the Gambia. Genes Immun 2003; 4: 275–282. [DOI] [PubMed] [Google Scholar]
- 147.Kempaiah P, Anyona SB, Raballah E, et al. Reduced interferon (IFN)-alpha conditioned by IFNA2 (−173) and IFNA8 (−884) haplotypes is associated with enhanced susceptibility to severe malarial anemia and longitudinal all-cause mortality. Hum Genet 2012; 131: 1375–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 1999; 179: 980–988. [DOI] [PubMed] [Google Scholar]
- 149.John CC, Moormann AM, Sumba PO, et al. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun 2004; 72: 5135–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reece WH, Pinder M, Gothard PK, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med 2004; 10: 406–410. [DOI] [PubMed] [Google Scholar]
- 151.Boyle MJ, Jagannathan P, Bowen K, et al. Effector phenotype of Plasmodium falciparum-specific CD4+ T cells is influenced by both age and transmission intensity in naturally exposed populations. J Infect Dis 2015; 212: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]