Abstract
Spinal cord injury (SCI) remains a severe condition with an extremely high disability rate. The challenges of SCI repair include its complex pathological mechanisms and the difficulties of neural regeneration in the central nervous system. In the past few decades, researchers have attempted to completely elucidate the pathological mechanism of SCI and identify effective strategies to promote axon regeneration and neural circuit remodeling, but the results have not been ideal. Recently, new pathological mechanisms of SCI, especially the interactions between immune and neural cell responses, have been revealed by single-cell sequencing and spatial transcriptome analysis. With the development of bioactive materials and stem cells, more attention has been focused on forming intermediate neural networks to promote neural regeneration and neural circuit reconstruction than on promoting axonal regeneration in the corticospinal tract. Furthermore, technologies to control physical parameters such as electricity, magnetism and ultrasound have been constantly innovated and applied in neural cell fate regulation. Among these advanced novel strategies and technologies, stem cell therapy, biomaterial transplantation, and electromagnetic stimulation have entered into the stage of clinical trials, and some of them have already been applied in clinical treatment. In this review, we outline the overall epidemiology and pathophysiology of SCI, expound on the latest research progress related to neural regeneration and circuit reconstruction in detail, and propose future directions for SCI repair and clinical applications.
Subject terms: Regeneration and repair in the nervous system, Trauma
Introduction
SCI is defined as damage to the spinal cord that causes temporary or permanent changes in its function, and this condition has a high incidence, high costs, a high disability rate and a low age of onset.1 SCI can be caused by high-intensity injuries, such as traffic accidents, falling injuries and violent injuries, or by infections, tumors, vertebral column degenerative disorders, ischemia–reperfusion injuries, and vascular causes.2,3 Serious SCI represents a significant physical, psychological and financial burden for patients and their families. It was reported that there are 759,302 patients with traumatic SCI in total and 66,374 new cases annually in China.4 In addition, data from the United States showed that the annual incidence of SCI is approximately 17,000 people per year, and the first-year expenses of one high tetraplegia patient are more than $1 million.1
According to the cause of injury, SCI can be divided into traumatic and nontraumatic SCI. According to the pathophysiology, acute SCI can be divided into primary and secondary injuries.5 According to the severity, SCI can be divided into complete or incomplete injury, and incomplete SCI can manifest as central core syndrome, Brown-Séquard syndrome, anterior cord syndrome, and posterior core syndrome.5 SCI has always been a research hotspot in the field of neural regeneration and repair. We selected the Web of Science (core collection) as the data source, and 35,567 documents related to SCI research were retrieved from January 1, 2012, to January 1, 2022. The retrieval strategy was: ((TS=spinal cord injury) OR (TS= spinal cord injuries) OR (TS= spinal cord traum*) OR (TS=spinal traum*)). From January 1, 2012 to January 1, 2022, after deleting repetition, 35,567 documents were retrieved. The document type mainly included “Article” and “Review”. Then the retrieval results were input into the bibliometric analysis software to further analyze. The Bibiliometrix R package provided a suite of tools for bibliometric studies, which was selected as the analysis software in bibliometric analysis.6 It was an open-source statistical programming environment, based on R language with a large number of efficient high-quality statistical algorithms and integrated visualization tools. Among these published articles, pathological mechanism studies and comprehensive studies, including surgical, cell transplantation and materials construction studies, and clinical trials for potential clinical translation were the most common.
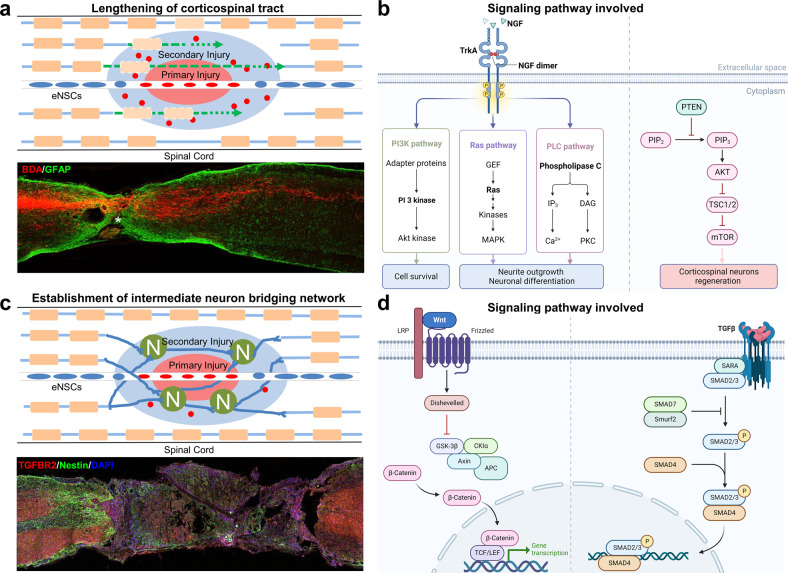
The recovery of spinal cord function depends on the remodeling and integrity of neural circuits. After SCI, the breakage of neuronal axons and the death of neurons cause dysfunction of neural circuits. The plasticity of neural circuits is the basis of the recovery of neural function. The traditional repair principle is to promote the regeneration and extension of the corticospinal tract (CST) and re-establish the connection with distal neurons, including reducing the production of regenerative-related inhibitors, such as chondroitin sulfate proteoglycans (CSPG)/NogoA/myelin-associated glycoprotein (MAP)/oligodendrocyte myelin glycoprotein (OMG), and even lipid metabolites, in the microenvironment during the early stage of SCI or promoting axon regeneration by exploiting the intrinsic growth ability.7–10 Phosphatase and tensin homolog (PTEN) deletion effectively enhanced the regenerative ability of adult corticospinal neurons and promoted the recovery of motor function after SCI.11–13 However, regenerated axons can hardly be reconnected to distal effectors because of the long distance. Recently, interneurons, which may provide bridges to proximal and distal neurons and form new neural circuits, have become an important strategy for SCI repair. These crucial interneurons may be derived from primitive interneurons in the spinal cord or cells differentiated from transplanted stem cells and endogenous NSCs (eNSCs) (Fig. 1).14–23
Fig. 1.
Two main repair strategies and related signaling pathway of neural circuit reconstruction after SCI. a Type I: CST extension. PTEN and suppressor of cytokine signaling 3 (SOCS3) deletion could effectively enhance the CST traced with biotin dextran amine (BDA), which showed the regenerative ability of adult corticospinal neurons.13,513 b Signaling pathways involved such as PI3K pathway, Ras pathway, PLC pathway, and PTEN/mTOR pathway. The figure was created with BioRender.com. c Type II: establishment of intermediate neuron bridging network. SCI mice were significantly improved by LDH-NT3 implantation.34 d Signaling pathways involved such as Wnt/β-Catenin pathway and TGFβ/SMAD pathway. The figure was created with BioRender.com
The remodeling of neural circuit is extremely difficult, and the analysis of pathological mechanism of SCI helps to identify more key intervention targets. The primary injury of SCI is unpredictable and irreversible, while the secondary injury is a target for therapeutic intervention and should be regarded as an important regulatory period for the treatment of SCI.24–27 However, the pathological mechanisms of SCI are like a “black box” and are still not entirely clear. Furthermore, the role of each pathological mechanism in SCI, such as the immune response and astrocyte scar formation, is controversial. Recently, transcriptome analyses, weighted gene coexpression network analysis (WGCNA) and single-cell sequencing technology have been widely used in SCI research and have provided better tools for clarifying pathological mechanisms.18,28–31 By using single-cell RNA sequencing, Frisén found that neural stem cells (NSCs) in the ependymal region of the spinal cord had latent lineage potential to differentiate into oligodendrocytes, which could be realized by regulating the expression of OLIG2 in NSCs.32 Our previous work revealed through transcriptomic analyses and WGCNA that a steady state of immune deficiency potentially led to CNS hyperconnectivity and then revealed using single-cell sequencing that temporal and spatial cellular and molecular pathological alterations occurred in the adult spinal cord after injury.28,33 Some researchers established a detailed transcriptomic profile of every major cell type in a mouse SCI model at the single-cell level and revealed novel insights into myeloid cell heterogeneity and specific signaling pathways by which unique myeloid subtypes contribute to the wound-healing process in the CNS.31
Whether transplanted stem cells or eNSCs, in the process of neural circuit remodeling after SCI, they are similar to “seeds”, requiring good regeneration microenvironment and necessary means of cell fate regulation, such as “soil” and “fertilizer”. To date, there have been many studies on the regulation of the injury microenvironment by bioactive materials, which combined with single or multiple neurotrophic factors to not only reduce the local immune response but also mobilize eNSCs to repair SCI.23,34,35 In terms of cell fate regulation, novel biomolecules and physical regulation have received more attention and have become noninvasive means for SCI repair.36–38 Indeed, most bioactive materials and physical regulation methods aim to repair SCI by promoting the plasticity of intermediate neural network.23,34,39
This review will summarize the latest research progress on SCI, including epidemiology and pathophysiology, the current research progress on neural regeneration and SCI repair, the progress of clinical trials and the prospects of clinical translation research on SCI.
Overall incidence and demographic characteristics
Since the 1980s, the incidence of SCI in different countries or regions has been reported.40 When we summarized the incidence of SCI for the five continents, we found that there was no significant difference in the incidence of SCI between continents. Comparing the studies from the Americas and Asia with the largest sample sizes, the incidence of SCI was 54 cases per million people in the United States,1 as a representative country in the Americas, and 66.4 cases per million people in China,4 as a representative county in Asia. Germany41 and Australia42 in Europe and Oceania, respectively, had 74.8 and 32.3 cases per million people. In the few African43–45 countries that reported the incidence of SCI, there were no significant differences in the rates of SCI in these countries. Regarding the etiology and demographic characteristics of SCI, motor vehicle accidents (MVAs) and falls are the most common causes of injury, while an age younger than 50 years and male sex are demographic risk factors for SCI.1,46–54 Table 1 shows a summary of epidemiologic investigations and demographics of SCI patients. Overall, a wide range of SCI incidence rates among different countries has been observed, and some developing countries show a low SCI incidence rate, while some developed countries show a high SCI incidence rate.46,55,56
Table 1.
The synthetic view of epidemiologic investigation and demographics of SCI patients
| Location | Research period | Incidence | Prevalence | Leading causes | Mean age | Sex ratio |
|---|---|---|---|---|---|---|
| Australia42 | 1921–2011 | 32.3 | 490 | N/A | N/A | 4.00:1 |
| Austria481 | 2002–2012 | 17 | N/A | Falls | N/A | 1.86:1 |
| Botswana384 | 2011–2013 | 13 | N/A | MVCs | N/A | 2.45:1 |
| Brazil482 | 1997–2006 | 26.1 | N/A | Falls | 36.75 | 7.35:1 |
| Cambodia483 | 2013–2014 | N/A | N/A | MVCs | 37 | 5.20:1 |
| Canada484 | 2000–2011 | 16.9 | N/A | MVCs | 46.2 | 3.95:1 |
| China4 | 2010–2013 | 66.4 | 759.3 | Falls | 43.7 | 1.01:1 |
| Egypt485 | 2009–2012 | N/A | 180 | MVCs | 40 | 5.00:1 |
| Estonia486 | 1997–2007 | 39.7 | N/A | Falls | 39 | 5.45:1 |
| Ethiopia45 | 2008–2012 | N/A | N/A | MVCs | 31.7 | 7.59:1 |
| Europe487 | 1988–2009 | N/A | N/A | MVCs | 44.5 | 1.85:1 |
| Finland488 | 2007–2011 | 27 | N/A | Falls | N/A | N/A |
| Germany41 | 2002–2012 | 74.8 | N/A | MVCs | 48.9 | N/A |
| Ghana44 | 2012–2014 | N/A | N/A | MVCs | 36.3 | 3.2:1 |
| Iceland489 | 1975–2009 | 33.5 | 526 | MVCs | 38 | 2.57:1 |
| India490 | 2000–2008 | N/A | N/A | Falls | N/A | 4.20:1 |
| Iran491 | 2003–2008 | 44‘ | 440 | MVCs | 31 | 1.00:1 |
| Ireland492 | 2000 | 13.1 | N/A | MVCs | 37 | 6.69:1 |
| Italy493 | 2011–2022 | 26.5 | N/A | MVCs | 59.2 | 2.15:1 |
| Japan494 | 2011–2012 | 121.4 | N/A | Falls | 67.6, 64.3 | 2.65, 2.75:1 |
| Korea495 | 2004–2008 | N/A | N/A | MVCs | 43.6 | 2.86:1 |
| Kuwait496 | 2010–2015 | N/A | N/A | MVCs | 36.4 | 4.3:1 |
| Macedonia497 | 2015–2016 | 13 | 180 | MVCs | 43 | 5.3:1 |
| Malaysia498 | 2006–2009 | N/A | N/A | MVCs | 39 | 3.35:1 |
| Nepal499 | 2008–2011 | N/A | N/A | Falls | N/A | 2.77:1 |
| Netherlands49 | 2010 | 14 | N/A | Falls | 62 | 2.85:1 |
| Nigeria500 | 2009–2012 | N/A | N/A | MVCs | 36.1 | 4.31:1 |
| Norway501 | 2009–2012 | 16.5 | N/A | N/A | 51 | 2.2:1 |
| Pakistan502 | 2008–2012 | 10.23 | N/A | MVCs | 20–29 | 4.25:1 |
| Russia503 | 2012–2016 | 16.6 | N/A | MVCs | 42.1 | 2.4:1 |
| Saudi Arabia504 | 2003–2008 | N/A | N/A | MVCs | 29.5 | 7.53:1 |
| South Africa43 | 2013–2014 | 20 | N/A | MVCs | 48.0 | 3.33:1 |
| Spain505 | 2001–2015 | 9.3 | N/A | Falls | 42.8 | 4.03:1 |
| Switzerland506 | 2005–2012 | 18 | N/A | Falls | 48 | 2.90:1 |
| Tanzania507 | 2010–2012 | 26 | N/A | Falls | 39.1 | 4.19:1 |
| Turkey52 | 2013–2014 | 21.3 | N/A | Falls | 38.3 | 2.31:1 |
| USA1 | 2015–2022 | 54 | 299 | MCVs | 43 | 3.55:1 |
Note. Only the most representative study with the largest sample size in each region is retained in the table. Incidence rate represents the frequency of new cases of TSCI occurring in a given population within a given period of time, while the prevalence rate is the proportion of a population found to have the TSCI condition
Pathophysiological mechanism of SCI
The pathophysiology of SCI includes primary injury and secondary injury; the former is usually a mechanical injury to the cord, and the latter is the consequence of cell and biological reactions to the primary injury, which involve the immune system, nervous system, vascular system, etc., including hemorrhage, ischemia, oxidative stress, inflammatory reaction, neural cell death, demyelination, scar formation and so on. At present, there are many animal models mimics human SCI, including zebrafish, rodents, large animals and primates. They have their own advantages and disadvantages in the process of studying SCI. Among them, zebrafish has strong nerve regeneration ability and is suitable for studying transverse injury models. Studies have shown that the mechanism of nerve regeneration in the zebrafish SCI model may be related to extracellular matrix Cthrc1 or pro-regenerative macrophages.57,58 Rodents, such as mice and rats, are most widely used because of its good repeatability, and are suitable for contusion or crush or suction models.59 Large animals and primates are generally suitable for the study of spinal cord hemisection model due to nursing difficulties.60 In a study on the pathological mechanism of the model of spinal cord hemisection injury in nonhuman primates, it reported that activated microglia/macrophages were found both within the injury center and the peri lesion area, and in contrast to rodent, substantial reactive astrocytic responsesat the lesion border were not observed in the monkey. Conversely, a deposit of robust fibrotic scar was observed at the injury epicenter, which filled the space originally created by the hemisection.61
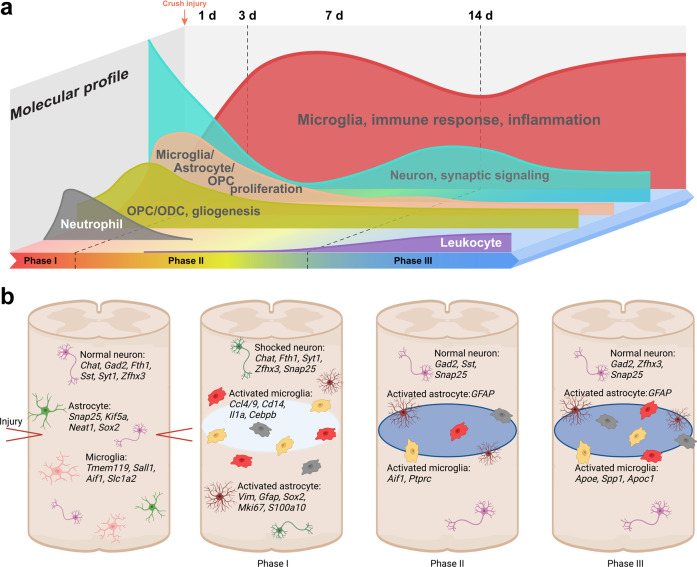
Our previous studies reported temporal molecular and cellular changes in crush-injured adult mouse spinal cord using single-cell transcriptomic analyses combined with classic anatomical, behavioral, electrophysiological analyses.33 We found that most dynamic changes occur at 3 days post injury, and by day-14 the second wave of microglial activation emerged, accompanied with changes in various cell types including neurons, indicative of the second round of attacks. By day-38, major cell types are still substantially deviated from uninjured states, demonstrating prolonged alterations.33 It was reported that the spinal ischemia, vasogenic edema and glutamate excitotoxicity were mean involved in the acute stage of SCI, while neuroinflammatory, mitochondrial phosphorylation, production of NOS in the subacute stage, and apoptosis and necrosis, axon degeneration, axon remyelination, axon remodeling, glial scar formation in the chronic stage (Fig. 2).62
Fig. 2.
A schematic illustration of molecular (a) and cellular (b) changes post SCI.33
The cell response is the basic unit in the pathophysiology of SCI and has temporal and spatial characteristics.33,63 The elucidation of the cellular response, especially the response mechanisms of different cell subsets, is of great significance for finding effective intervention targets for SCI. The pathological mechanism of SCI discussed in this review mainly focuses on rodents.
Immune responses at the peri-injury site
The immune response is a critical pathological mechanism that may determine the prognosis of SCI. When SCI occurs, local microglial reactivity and the destruction of the blood‒spinal cord barrier provide opportunities for blood-derived immune cells, including neutrophils, monocyte macrophages, and lymphocytes, to enter spinal cord tissue. These cells secrete proinflammatory or immunomodulatory factors to participate in the immune response. The immune response has been thought to have destructive effects and is not conducive to SCI repair. However, the roles of the immune response in SCI damage expansion and regeneration and repair are still controversial.
In situ immune cells
Microglia
When SCI occurs, microglia change their cell morphology and protein expression profile, making it easier to phagocytize and remove debris, and they change to a proinflammatory state overall and mediate further secondary injury.64 When microglia are eliminated long-term in the injured spinal cord, the transcriptional inflammatory response is reduced.65 The microglial response began in the early stage after SCI and lasted for up to 1 year in a rat transection SCI model.66 There may be two peaks of microglial activation. Nguyen et al. reported that in a contusion model of SCI in rats, activated microglia were detected with two peak times, one at 7 dpi and one at 60 dpi.67 In our previous studies, we found similar characteristics of microglia, with two peaks in a mouse SCI model.33 As in spatial distribution, in a pathological study of human SCI, it was reported that microglia rapidly disappeared in the lesion core. In contrast, at the peripheral margin, the number of TMEM119+ microglia is maintained through local proliferation and exhibits a major proinflammatory phenotype.68 These results were consistent with that reported in other literature.69
The function of microglia depends on their phenotype, which changes in response to the microenvironment. Microglia have two main phenotypes: the M1 phenotype, which tends to promote the inflammatory response and aggravate neuroinflammation, and the M2 phenotype, which tends to exert anti-inflammatory effects and promote tissue repair. It was reported that transplantation of M2-polarized microglia could promote recovery of motor function in a mouse SCI model.70 However, more subtypes of microglia may exist in the injured spinal cord. In our previous studies, we found a total of 8 clusters of microglial subpopulations in SCI mice at single-cell resolution, and each subpopulation had different characteristics; this topic needs further research.33 The best time window for the conversion of reactive microglia to their neuroprotective phenotypes may be the first week post SCI.71 The phenotypic transformation of microglia is dynamic and regulated by many factors in the injured microenvironment. Recently, researchers showed that LRCH1 alleviates the activation of p38 MAPK and Erk1/2 signaling and negatively regulates microglia-mediated neuroinflammation after SCI.72 Downregulation of ubiquitin-specific protease 4 expression promotes microglial activation through NF-κB by attenuating the deubiquitination of TRAF6.73
Interestingly, more and more studies have found that microglia may become the key target cells for the repair of SCI. Microglia are involved in the formation of corralling and glial scarring, which reduce parenchymal immune infiltrates and reduce the apoptosis of neurons and oligodendrocytes in the first two weeks after SCI.74–76 It has also been reported that in a neonatal mouse SCI model, the spinal cord can heal without scarring and allow long projection axons to grow through the lesion, while the depletion of microglia destroys this healing and prevents axon regeneration.77 The authors point out that microglia temporarily secrete fibronectin and its binding protein to form an extracellular matrix bridge connecting the broken ends of axons. In addition, the unique role of neonatal microglia is related to the expression of peptidase inhibitors. Both adult microglia and neonatal microglia treated with peptidase inhibitors can significantly improve healing and axon regeneration after transplantation in the setting of adult SCI.77 Our previous study also found that there are microglial subsets in the adult spinal cord, which are similar to those in neonatal mice, but their ability to promote regeneration is reduced due to the expression of higher levels of CD68 and lower levels of p2ry12.33
Blood-derived immune cells
Neutrophils
Neutrophils are considered to be one triggering factor of the secondary injury process after SCI. Their recruitment are facilitated by interleukin 1a (IL-1a), IL-β, IL-8, tumor necrosis factor (TNF), granulocyte colony-stimulating factor, CCL3, CXCL1, CXCL2, and CXCL5, which are secreted by resident cells of the spinal cord, after detecting the damage. Meanwhile, the neutrophils could recognize these pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) through pattern recognition receptors (PRRs) and its downstream signaling pathways such as nuclear factor kB (NF-kB) pathways78 Neutrophils can be detected in the spinal cord 2 h after injury and peak at 1 dpi in rats or 3 dpi in mice.25,67
Neutrophils generally participate in the pathological mechanism of SCI in a harmful role. On the one hand, they release a variety of proinflammatory mediators, including reactive oxygen species (ROS), lysosomal enzymes, proteolytic enzymes (such as elastase and matrix metalloproteinase-9) and oxidative enzymes (myeloperoxidase; MPO).79–81 It was reported that spleen tyrosine kinase could facilitate neutrophil activation and worsen long-term neurologic deficits after SCI.82 On the other hand, neutrophils can produce neutrophil extracellular traps (NETs), which aggravate secondary injury by promoting neuroinflammation and blood-spinal cord barrier destruction in SCI.80,83–85 However, Neutrophils can also be divided into subsets. Recently, research revealed a new subset of neutrophils, CD14+ly6glow granulocytes, that can promote spinal cord repair, partly due to secretion of the growth factors NGF and IGF-143.82,86
Recent studies have shown that inhibiting leukocyte infiltration contributes to functional recovery after SCI, and a high neutrophil-to-lymphocyte ratio is associated with poor outcomes in patients with acute cervical SCI.87–89 However, the early infiltration of neutrophils not only accurately guides circulating macrophages by secreting enzymes and other factors but also creates conditions that promote macrophage phagocytosis. In this regard, the role of neutrophils also has a favorable side.78,90
Myeloid monocytes
Monocyte-derived macrophages (MDMs) also have two main subgroups, M1 and M2 macrophages. In a contusion SCI model, M1 macrophages were detected at an early stage and maintained at a high level, while M2 macrophages were briefly detected high levels and returned to preinjury levels after 1 week.91 In related studies on the mechanism of macrophage activation after SCI, an increase in intracellular iron and myelin debris could regulate the polarization of macrophages, promoting a harmful M1 phenotype.92,93 The lipid catabolic pathway is another target for regulating the important functions of macrophages, which was revealed by detecting the specific transcription profile of macrophages after SCI.30
To distinguish the role of microglia from that of MDMs, the lysozyme M EGFP-knockin mouse provides a better tool for macrophage research in combination with some specific markers, such as P2ry12, Siglec H, TGFBR1, and Tmem119.94–98 As in time distribution, it was reported that microglia were the main type of macrophages during the early response to SCI, and infiltrating macrophages later became the main cells in contact with degenerative axons, which lasted for 42 days.95 As in spatial distribution, the MDMs were distributed at the center of the site of injury, while microglia were distributed at the edge of the lesion.93 This had also been confirmed in pathological studies of human SCI, which reported that in the lesion core, microglia were rapidly lost while intermediate (co-expressing pro- as well as anti-inflammatory molecules) blood-borne macrophages dominated.68 Interestingly, the distribution characteristics of different subsets of macrophages are also different. It was reported that Cx3Cr1hi macrophages are present in the glial scar, whereas Cx3Cr1lo macrophages are present in fibrotic scar, and this may drived distinct physiological processes.69
However, MDMs also participate in wound-healing activities after infiltrating the injury site. For example, they promote corralling and recovery via Plexin-B2, forming a closed loop surrounded by astrocytes, and Plexin-B2 ablation in myeloid cells impairs motor sensory recovery.74 Also, MDMs could provide a regulatory mechanism by inhibiting microglia-mediated phagocytosis and inflammation.99
After SCI, a large number of myelin sheath fragments and necrotic tissue are produced locally, which need to be cleared by phagocytes. Compared with activated microglial cells, blood-derived macrophages have stronger phagocytic capacity. However, after phagocytosis of a large amount of lipid-rich myelin sheath tissue, macrophages will form foam macrophages, which will not only reduce the phagocytosis ability, but also cause damage to neural tissue.100 It was reported that endogenous glucocorticoid receptors (GRs) signaling was a key pathway that normally inhibits mechanisms of macrophage-mediated repair after SCI through regulation of lipid and myelin phagocytosis and foamy macrophage formation.101
Lymphocytes
T lymphocytes and B lymphocytes are the main cell types involved in adaptive immunity. In human SCI, lymphocyte numbers were low and mainly consisted of CD8+ T cells.68 In the animal SCI model, T cells can be detected at 1 dpi after SCI and peak at 7 dpi, and sustained T-cell responses are observed at 180 dpi.67 Cytotoxic CD8+CD28+ T cells were dominant in the first two weeks, which means those two weeks after SCI, survival time can be prolonged and the proportion of CD8+ regulatory T cells can be increased.102 It was also reported that γδ T cells, a subgroup of T cells, provided an early source of IFN-γ, which aggravated the inflammatory response after SCI.103,104
Adaptive immunity is unfavorable to nerve recovery because T-cell- and B-cell-immunodeficient SCI models showed better neurological recovery.28,105–107 It has been reported that perforin derived from CD8 T cells damages the CNS by increasing the permeability of the blood-spinal cord barrier, resulting in the infiltration of inflammatory cells and related cytokines.108 In addition, T-cell infiltration and signal transduction in the dorsal spinal cord of adults are the main causes of neuropathic pain, such as hypersensitivity.109 However, myelin basic protein-activated T cells play a beneficial role in the repair of the CNS, and an increase in the number of Th2 cells promotes the transformation of Th1 and M1 cells to Th2 and M2 cells, respectively, thus changing the local microenvironment and facilitating the repair of SCI.110
Neural cell-related responses and interactions
Astrocytes undergo long-term and large-scale activation and proliferation after SCI occurs. Due to the proliferation of astrocytes and the infiltration of inflammatory cells, neurons, oligodendrocytes and NSCs/ neural progenitor cells (NPCs) are vulnerable to degeneration and death. During the pathophysiological process of SCI, all neural functional cells produce pathological responses, and there is also abnormally active neural communication between neural cells and inflammatory cells. Analyses of the responses and communication mechanisms of these important functional cells are helpful for understanding the pathological mechanism of SCI and providing key regulatory targets.
Neurons and NSCs/NPCs
The loss of mature neurons is the main cause of functional defects after SCI, and this is directly involved in the induction of programmed cell death (PCD) in neural cells, including apoptosis, necroptosis, autophagy, and ferroptosis.111 It was reported that apoptosis in neurons could be detected at 4 h and peaked at 8 h after SCI, the total number of axons at the injured site decreased immediately, and the downward trend continued during the subacute phase and reached a minimum during the chronic phase.112,113 The results showed that cytoplasmic Nissl material was decreased in neurons within a few minutes after mild SCI, and the lesion area expanded and cavitated within the next 7 days.112 Oxidative stress is an important cause for ongoing neuronal damage long after initial trauma. In one study of human SCI pathology, oxidative neuronal cell body and axonal damage can be observed through the intracellular accumulation of amyloid precursor protein (APP) and oxidized phospholipid (e06), which occurs early in the lesion core and decreases over time. In contrast, within the peripheral margin, significant neuronal APP+/e06+ axonal dendritic damage was detected, which remained significantly elevated for months/years.68
Neuronal apoptosis has a close relationship with cell autophagy. Autophagy disorder causes the accumulation of neurotoxic proteins and subsequent neuronal cell death.114 Neuronal apoptosis is also closely related to regulatory proteins, such as the RNA-binding protein src-associated in mitosis (Sam68), IGFBP6, a member of the insulin-like growth factor-binding protein family, HS1-associated protein X-1 (HAX1) and TNF receptor-associated factor 7 (TRAF7).115–118 Ferroptosis is a novel type of iron-dependent cell death, and it has a strong correlation with secondary injury after SCI.119 The inhibition of ferroptosis could promote the recovery of neurological function by enhancing neuronal survival.120 Ferroptosis is different from other forms of cell death, and it may be a novel direction for further research on acute CNS injuries.121
After primary SCI injury, the rostral end of the axon retracts, and the caudal end loses the support of the cell body, resulting in degeneration and disintegration. However, at the same time, the axon starts regeneration and repair, but the molecular mechanism is unclear. It was reported that axonal sprouting was observed within 6 h after SCI, which may be supported by calpain activation and protein synthesis in axons.122 The author further reported that the accumulation of damaged axon glial complexes (AGCs) was an obstacle for axon regeneration at the injury site. When the author surgically eliminated the AGC, the regenerated axons successfully penetrated the lesion site within 4 h after surgery.122
Neurogenesis in adult mammals mainly occurs in the subependymal area of the lateral wall of the ventricle and the subgranular area of the hippocampus. It has been proven that the spinal cord contains endogenous NSCs/NPCs within the ependymal cell population.23,123–126 After SCI, NSCs/NPCs are activated, migrate into the lesion site, and produce newborn astrocytes and oligodendrocytes.124–129 It was reported that the ependyma of the adult spinal cord is a latent stem cell niche that can be activated and contribute to glial scar formation.124 Interestingly, more effective endogenous regeneration was found in zebrafish after CNS injury, which was related to pluripotency via regulation of the key factors pou5f1 and sox2.130 In addition, connexin signaling in the ependyma changes after SCI, functionally resembling the immature active stem cell niche of neonatal animals, suggesting that connexins in ependymal cells are potential targets to improve self-repair of the spinal cord.131
Astrocytes
Astrocytes are the most abundant supporting cells of the nervous system. In response to SCI, astrocytes become activated and transform into reactive astrocytes, which protect the uninjured spinal cord from inflammatory cell infiltration and minimize initial damage at an early stage but later form a glial scar that is a physical barrier to nerve regeneration. There are two main subtypes of reactive astrocytes: neurotoxic astrocytes (A1 cells) induced by inflammation and neuroprotective astrocytes (A2 cells) induced by ischemia, and this functional transformation involves a variety of substances and intracellular signaling pathways.132 It was reported that astrocytes transform into A1 cells (with C3 as a marker) through the NF-κB pathway and into A2 cells (with S100A10 as a marker) through the STAT3 pathway.133 Recently, the Deneen group identified five subpopulations of astrocytes, named Populations A, B, C, D, and E, based on combinatorial expression of CD51/CD71/CD63 and found that population C possessed significantly enhanced synaptogenic properties in vitro. However, the astrocyte subpopulation distribution showed that Population A was dominant in the spinal cord in the presence or absence of injury. Therefore, subpopulation switching would be a promising repair target for SCI.134
Interestingly, microglia may be the most important cell type that triggers reactive astrogliosis. It was reported that A1 astrocytes are induced by three factors, Il-1α, TNFα, and C1q, which are all produced by activated microglia. The authors found that these three factors should be simultaneously present for LPS-induced in vitro polarization; if not, microglia do not induce astrocyte polarization.135 Additionally, type I collagen, which is expressed in the injured spinal cord, induces N-cadherin-mediated adhesion and is directly involved in the transformation of reactive astrocytes, as well as in astrocytic scar formation, after SCI.136
Astrocytes are neural parenchymal cells tile the whole mammalian CNS.137 The astrocytes provide multiple functions essential for the CNS functions, such as maintenance of the molecular, systemic and metabolic homeostasis,138 provision of metabolites to neurons,139 modulation of local blood flow,140 etc. In response to SCI, astrocytes exhibit morphological, molecular and functional changes, referred to as reactive astrocytes.141–144 Reactive astrocytes are highly heterogeneous, range from subtle and reversible alterations in gene expression and morphology to permanent astrocyte scar formation, depend on the distance from the injury and types of injury.143,145,146 Though reactive astrocytes were long regarded as functional passive, numerous newly researches provide evidences of their positive aspects,146–148 and may influence the outcome of SCI.143
Transcriptome analysis can divide reactive astrocytes into different clusters or subtypes according to their molecular signatures.33,135,149,150 For example, high-profile subtypes of A1 neurotoxic astrocytes and A2 neuroprotective astrocytes,135,149 which may represent new therapeutic potential. However, the function of these marker genes are not well known,137 and no experimental evidence proved the A1/A2 astrocytes marker genes exert either toxic or protective functions.144 Thus, the meaningful definition of reactive astrocytes subtypes should not solely based on the molecular signatures.137
Oligodendrocytes and OPCs
Oligodendrocyte apoptosis was detected in the white matter after 24 h and reached its highest level at 8 dpi.151 Oligodendrocytes may also have subtypes and different responses to SCI. In one study, six mature oligodendrocyte (MOL) subpopulations were described, and they presented different responses to SCI.152 During the acute phase, the responses of MOL2 and MOL5/6 to injury were similar, but during the chronic phase, the response of MOL2 at the injury site was decreased, while MOL5/6 reached a higher level.152 The overexpression of p53 can enhance the endoplasmic reticulum–mitochondria interaction and trigger the E2F1-mediated apoptosis pathway.153 However, it was also reported that inflammatory cells or their mediators had no significant destructive effect on oligodendrocytes during the early stage of SCI.154
Oligodendrocyte precursor cells (OPCs) are a subgroup accounting for 5–8% of the cells in the CNS, and they are a potential source of oligodendrocyte replacement after SCI.155 Traditionally, OPCs have been identified by their expression of NG2 and PDGFRα. After SCI, OPCs are activated, proliferate, differentiate into new oligodendrocytes and Schwann cells to regenerate axons, and participate in the formation of astrocyte scars.156
Endogenous OPCs have been proven to effectively and spontaneously repair the myelin sheath after SCI through genetic fate mapping.157,158 It was reported that OPCs (PDGFRα+) are responsible for in 30% of the new myelin sheath at the epicenter of SCI.157 However, the results showed that oligodendrocyte remyelination is not required for spontaneous recovery of stepping.158 This was contrary to the conclusions of other research.159–162
Regarding remyelination, the regenerative capacity of OPCs is conspicuously restricted by the hostile microenvironment of SCI, which includes factors such as scar-associated chondroitin sulfate proteoglycans (CSPGs)/microglial activation/Nrg-1.163 OPC proliferation and oligodendrocyte maturation following remyelination could be enhanced by neuronal activity and improved hindlimb motor function.164 In addition, it was shown that the proinflammatory reaction process is needed for the degradation of myelin debris and the generation of new oligodendrocytes.165
Interactions between immune cells and neural cells
As mentioned above, the immune microenvironment may indirectly lead to neural cell death. Meanwhile, the microglia-neuron interaction is an important factor in chronic pain after SCI. It was reported that the upstream regulator of prostaglandin E2 (PGE2) release, phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2), was specifically localized in microglia, while the PGE2 receptor E-prostanoid 2 (EP2) was localized in neuronal cells. Blocking the EP2 receptor resulted in a decrease in the hyperresponsiveness of dorsal horn neurons.166 In SCI in zebrafish, there is one macrophage subtype with high expression of TNFα that has direct communication with spinal progenitor cells and promotes neurogenesis.58
Microglia are essential for restoring tissue homeostasis and achieving optimal recovery after SCI. Microglia play these beneficial roles by regulating the transcriptional fate, function and intercellular crosstalk of various nonneuronal cell types.167 It was reported that microglia are indispensable in the process of astrocyte scarring.75 Reactive astrocytes interact with microglia within the glial scar via fibronectin, a major ligand of β1R, and enhance microglia-mediated immune inflammation.168
Glial scar formation and function
The SCI lesion is composed of three main compartments: a nonneural lesion core, an astrocyte scar surrounding the lesion core, and spare but reactive neural tissue.169–172 The cellular components of these three compartments are quite different. Blood-borne cells such as fibroblasts and other immune cells leak from the disrupted blood‒brain barrier in the injured spinal cord. Local pericytes and fibroblasts also start proliferating. They produce extracellular matrix (ECM) components and form fibrotic scars, and almost no neural cells can be found in this toxic environment. Local astrocytes are activated by inflammatory reactions secondary to SCI and form a narrow astrocyte scar surrounding the fibrotic scar, protecting adjacent spare neural cells.147,148,173,174 In the distal area of the lesion, continuous with the astrocyte scar, the spare but reactive neural tissue contains neurons, astrocytes, oligodendrocytes, OPCs, and microglia.172,175 The features of this compartment include reactive astrocytes and OPCs with a hypertrophic cell morphology, and this area can be surprisingly large.141
Astrocyte scars
After SCI, resident astrocytes are activated by many molecules produced by all cell types in the spinal cord tissue.176 After mild injury, astrocytes upregulate GFAP, an intermediate filament protein, with hypertrophy of the cell body and processes, but preserve their original numbers without proliferation.145 After severe injury, astrocytes proliferate, migrate and organize around the severely damaged lesion center. They intertwine with their cell processes and form a dense scar tissue corral around the inflamed lesion center, which is named the astrocyte scar.141,145,170,177,178 The astrocyte scar is narrow, with only several cell layers separating the spare neural tissue from the nonneural lesion core, and its layers are continuous with the spare but reactive neural tissue.141,171,177 Experimental evidence revealed the beneficial aspects of the astrocyte scar. Astrocyte scars isolate and sequester the harmful lesion center from the neighboring spare neural tissue, which limits the lesion size to activate neuroprotection and regulate spinal cord homeostasis.141,179 After using GFAP-TK transgenic mice and ganciclovir administration to ablate dividing reactive astrocytes, failure of blood‒brain barrier repair, leukocyte infiltration, demyelination, neural cell death, and functional deficits were observed.174 In STAT3 conditional knockout transgenic mice, the astrocytic reaction after SCI was inhibited, and inflammatory cell infiltration increased, leading to worse functional outcomes.180 Additionally, no axon regeneration was observed when astrocyte scar formation was prevented or chronic astrocyte scars were ablated.147
Conversely, robust axon regeneration occurred despite the presence of an astrocyte scar under appropriate conditions (activation of neuron intrinsic growth capacity, growth supportive substrate and chemoattractive factors).147 The reason is that scar-forming astrocytes express laminin, an axon growth-supporting matrix protein, and laminin-integrin binding blockade attenuates axon regeneration.147 Axon growth-inhibitive CSPGs are produced by many cell types after SCI, and reactive astrocyte ablation cannot reduce CSPG levels. Scarce-forming astrocytes express numerous permissive molecules for axonal regeneration.147
Although there are also other cell types within the astrocyte scar compartment, such as OPCs, the mature astrocyte scar after SCI consists primarily of newly generated astrocytes.177,181,182
Fibrotic scars
After injury, the lesion center of the spinal cord undergoes hemorrhage, edema, etc. Blood-borne cells such as fibroblasts invade the spinal cord and secrete ECMs such as Type IV collagen, fibronectin, laminin and proteoglycan.170,172 Pericytes are also recruited by innate inflammation. They proliferate and form the fibrotic scar. Pericyte-derived cellular components of scar tissue are important for regaining tissue integrity. By using Glast-CreER transgenic mice and a Rosa26-YFP reporter mouse line, a subtype of pericytes named type A pericytes was labeled.183 Blocking the progeny of type A pericytes results in failure to seal the injured spinal cord.183 However, this kind of scar tissue is considered a barrier to axon regeneration.184 When a specific transgenic mouse line (Glast-CreERT2 Rasless, Rosa26-YFP) was used, the proliferation of type A pericytes was inhibited, and fibrotic scarring and ECM deposition were reduced. Enhanced axon regeneration and functional recovery were observed.185 Thus, due to the dual functions of the fibrotic scar, balancing the beneficial and detrimental effects of fibrotic scars is fundamental in treatment strategies targeting fibrotic scarring.
Molecular mechanism of neural circuit damage
Primary SCI injury causes irreversible mechanical damage to the neural circuit, and subsequent axonal disruption, degeneration, demyelination, and neuronal death lead to more severe neurological dysfunction. Local injury of the spinal cord causes changes in the sensitivity and excitability of neurons, which may lead to pathological pain and even cause neurodegeneration of the spinal cord remotely from the injury site.
Acute phase
During the acute stage of SCI, the excitability of sensory and motor neural circuits is altered, in addition to mechanical damage to the spinal cord, which may be the pathophysiological mechanism of spinal cord concussion or spinal cord shock in SCI patients. One study that dynamically detected neural circuit changes in lamprey with SCI showed that spinal cord excitability was significantly reduced above and below the lesion site, and excitatory synaptic inputs to motor neurons recovered earlier than those to sensory neurons.186 Interestingly, the change in interneuron excitability in spinal cord tissue was related to functional recovery after SCI.187,188 It was reported that neonatal mice with SCI showed spontaneous recovery because they maintained the excitatory phenotype of glutamatergic interneurons, with the induction of synaptic sprouting to facilitate excitation. In contrast, SCI in adult mice promotes neurotransmitter switching of spatially defined excitatory interneurons to an inhibitory phenotype.188 The excitability of spinal cord inhibitory interneurons may be the crucial factor limiting the integration of descending inputs into relay circuits after SCI.187
Chronic phase
Chronic phase changes in the spinal cord neural circuit are mainly reflected by functional remodeling based on compensatory mechanisms in the brain or spinal cord, including cortical compensation mechanisms and synaptic plasticity through spared axonal sprouting.189–191 The spared tissue and spontaneous repair of the corticospinal tract mediated by interneurons combine to form a new neural circuit and are an important basis for rehabilitative treatment.192,193
During the chronic stage of SCI, corresponding degenerative changes are present in the injured spinal cord194,195 and even the brain.196,197 Yokota et al. used a complete SCI model and found that atrophic changes were widely observed in the injured spinal cord both rostral and caudal to the lesion, but the decrease in area was mainly in the white matter in the rostral spinal cord, while both the white and gray matter showed a decreased area in the caudal spinal cord. However, the motor neurons in the caudal part of the injured spinal cord showed good potential for synaptogenesis, with high expression of acetylcholine-related molecules.195 Azzarito et al. used quantitative MRI and found that in patients with SCI, the cord area and left-right width of the remote cervical spinal cord were decreased, and atrophy of the cerebral cortex was sustained when spinal cord atrophy became slower. The degree of atrophy of the spinal cord and corticospinal tract at 6 months after SCI was closely related to motor function recovery at the 2-year follow-up.196
SCI can induce chronic neuropathic pain, cognitive deficits and physiological depression, which may be involved in chronic inflammation of the brain through sustained induction of M1-type microglia.198 It has also been reported that CNS injury can trigger APP and Tau cleavage by delta-secretase (AEP) and mediate Alzheimer’s disease pathology.197
Sequential study on pathological mechanism of SCI provides intervention targets for sequential treatment.33,199,200 In general, the acute stage of SCI is dominated by neuroprotection and neuroinflammatory regulation, including the use of neuroprotective drugs, reducing the infiltration of inflammatory cells, and reducing oxidative stress; In subacute stage, it mainly regulates scar formation, angiogenesis and promotes nerve regeneration, including materials, cell transplantation and the application of small molecular compounds; In the chronic phase, the compensatory recovery of neural function can be promoted by means of physical regulation, the neural circuit can also be reconstructed by removing glial scar, combining biomaterials and/or stem cells, and astrocytes can even be transdifferentiated into neurons by means of reverse transcription.35,201,202 It is worth mentioning that neuroimmunity participates in the whole process of SCI pathology, especially the role of microglia. We have previously confirmed that the immune response is negatively related to nerve regeneration, and the immune-deficient mice show better neural function recovery.28
Intervention and repair of SCI
A spontaneous repair mechanism exists after SCI, but it faces many difficulties and external intervention is needed to further improve the repair ability. Small biological molecules can provide nutritional factors for neural regeneration or regulate cell metabolism.203 Stem cells can effectively differentiate to replace apoptotic neural cells. Bioactive materials and physical regulation approaches can regulate cell fate at the site of SCI (Fig. 3). These approaches can promote the generation of newborn neurons and the formation of intermediate neural network, which is conducive to the function of SCI.34,204
Fig. 3.
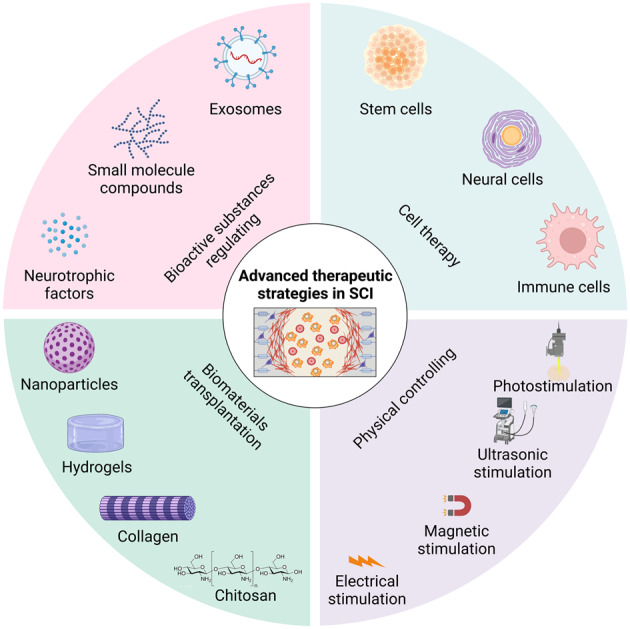
Schematic depiction of various advanced therapeutic strategies for repairing SCI based on the studies. Strategies including bioactive substances regulating, cell therapy, biomaterials transplantation, and physical controlling, are applied to repair SCI from different perspectives. Meanwhile, the combined use of these strategies has also received increasing attention from researchers. The figure is generated from BioRender.com
Bioactive substances
At present, bioactive substances such as neurotrophic factors, small-molecule compounds, and exosomes are widely used in SCI research, and they all show the ability to promote functional recovery after SCI through axon regeneration and microenvironment improvement.205 Traditional Chinese medicines, such as ginsenosides, genistein and tanshinone, also mediate neuroprotection and promote neural function recovery of SCI.206–209
Neurotrophic factors
The roles of different neurotrophic factors vary. Brain-derived neurotrophic factor (BDNF) has functions in axonal regeneration, neurogenesis protection, remyelination, synaptic reformation and synaptic transmission in different neuronal populations after SCI.210 Neurotrophin-3 (NT⁃3) has recently received substantial attention because it can promote oligodendrocyte proliferation and neuronal survival and does not cause side effects such as pain or cramping.211 In addition, many studies have demonstrated that NT3 plays a key role in promoting neural circuit remodeling.23,34,212 Ciliary neurotrophic factor (CNTF) has been proven to promote neuronal development and increase the survival rate of severed axons.213 Fibroblast growth factor (FGF) is involved in stimulating axonal growth, promoting angiogenesis, and exerting anti-inflammatory and neuroprotective effects in inflammatory cells.214 Glial cell-derived neurotrophic factor (GDNF) can promote axonal regeneration in the CNS and PNS after SCI.215 A new factor called nerve growth factor inducible (VGF) was recently identified by our team, and we found that VGF-mediated oligodendrogenesis could benefit SCI repair.216 Although the use of such factors is very promising, there are disadvantages, such as unstable physicochemical properties and high costs, that severely limit their application and remain to be addressed.
Small-molecule compounds
Small-molecule compounds have the unique advantages of high cell permeability, reversibility and ease of manipulating cell fate regulation; thus, this approach is a promising new strategy to regulate cell fate. It was reported that minocycline can reduce the volume of necrotic tissue after SCI and improve the motor function score in animals.217,218 A selective type 2 lysophosphatidic acid receptor (LPA2) antagonist was reported to effectively improve the inflammatory microenvironment after mouse SCI via lipid metabolism regulation.219 It was also reported that the potassium-chloride cotransporter-2 (KCC2) agonist CLP290 can restore stepping ability in paralyzed mice by reducing the excitability of spinal cord inhibitory interneurons.187 An interesting study showed that small-molecule peptides could mediate intercellular signal transmission, enhance supramolecular movement, and activate signaling pathways related to neural regeneration and repair after SCI.220
Exosomes
Exosomes (Exos) have been gradually found to play an important role in signal transmission in various physiological and pathological states, including SCI. Exos are vesicles with a diameter of approximately 40–120 nm that are continuously released into the extracellular environment by cells.221 They are formed by endosomes resulting from membrane endocytosis, which bud into the lumen to form multiple vesicles, and then the multiple vesicles fuse with the membrane and are released into the extracellular matrix. Therefore, exosomes contain large numbers and different kinds of proteins, lipids, RNAs and other biologically active factors.222,223 Exosomes can effectively promote functional recovery after SCI through their immunomodulatory, anti-inflammatory, and anti-apoptotic effects as well as their ability to promote vascular and axon regeneration. Meng et al. proved the presence of large amounts of granulocyte–macrophage colony-stimulating factor (GM-CSF) in exosomes, which have the potential to enhance immunomodulatory function and benefit SCI repair.224 Zhou et al. showed that anti-inflammatory microglia-derived M2-Exos had a better ability to promote the recovery of functional behavior, increasing axon regeneration and reducing the level of pyroptosis in spinal cord neurons after SCI. M2-Exos rich in miR-672-5p could inhibit the AIM2/ASC/caspase-1 signaling pathway by inhibiting AIM2 activity to inhibit neuronal pyroptosis and ultimately promote the recovery of functional behavior in mice with SCI.225 Pan et al. revealed that Schwann cell-derived exosomes can promote functional recovery of mice post SCI by decreasing CSPG deposition via an increase in TLR2 expression on astrocytes through the NF-kappaB/PI3K signaling pathway.226
Advanced cell therapy for SCI repair and regeneration
Mature neurons are difficult to regenerate after damage, and it is crucial to find an ideal, simple, safe, effective and feasible repair strategy to promote axonal regeneration, remyelination and functional recovery. Cell transplantation has emerged as the most promising therapeutic approach for SCI. Cells transplanted into the site of SCI have the potential to differentiate, secrete a variety of cytokines and growth factors, regulate the inflammatory response, provide nutritional support, and promote axonal regeneration and nerve repair. Direct or indirect interactions between transplanted and host cells respond to the microenvironment at the site of spinal cord damage and are also able to influence the microenvironment at the lesion site in the spinal cord and alter the interactions between transplanted and host cells, thus affecting tissue and functional outcomes after SCI.
Stem cells
Stem cells are a class of multipotent cells with self-replication and differentiation abilities; they play important roles in tissue repair and regeneration, and they are expected to be important therapeutic tools for neurological diseases as seed cells. In recent years, stem cell transplantation has been widely used in SCI repair, and a variety of stem cells have been applied in clinical practice. Stem cells can differentiate into neural precursor cells, oligodendrocytes, astrocytes, and neurons, which can promote axonal regeneration, bridge the diseased lumen, and promote functional recovery by replacing missing cells or regulating the microenvironment at the site of injury.
Embryonic stem cells
Embryonic stem cells (ESCs) are derived from endocytic clusters in the blastocyst stage, have a high differentiation potential, and can be induced to differentiate into almost any cell type. If transplanted in undifferentiated form, they are prone to form teratomas in vivo, which severely limit the application of ESCs. Currently, ESCs are generally differentiated into specific cell types, such as neural precursor cells, specific neurons or glial lineages, and then transplanted.
Human ESC-derived neural crest cells can promote remodeling of descending raphespinal projections and contribute to the partial recovery of forelimb motor function in SCI animal models.227 Kim et al. evaluated the efficacy and safety of human polysialylated neural cell adhesion molecule (PSA-NCAM)-positive neural precursor cells (hNPCs (PSA-NCAM+)) as a treatment for SCI.204 hNPCs (PSA-NCAM+) differentiated into neural cells and successfully integrated into the host tissue with no evidence of tumor formation, which also significantly improved locomotor function.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are obtained by reprogramming of genes such as Oct3/4, Sox2, Klf4, and c⁃Myc via transduction into mouse or human fibroblasts and are expected to be the preferred cell source for human SCI therapy because of ethical issues.
Gong et al. showed that spinal GABA interneurons efficiently generated from iPSCs could form synapses with host spinal neurons and mitigate the spasticity-like response of the rat hindlimbs and locomotion deficits within 3 months.228 Wertheim et al. reported an approach to recapitulate the embryonic development of the spinal cord by using iPSCs, which were further encapsulated in ECM-based hydrogels, and the implants enriched the targeted region with biochemical and mechanical cues to attract progenitor cells, supported cell survival and engraftment, reduced inflammation and gliosis at the lesion site, and overall improved the locomotion of the treated animals.229
Neural stem cells/neural progenitor cells
NSCs/NPCs are pluripotent stem cells with self-renewal ability that are able to differentiate into neurons, astrocytes and oligodendrocytes and can replace damaged cells at the injury site and secrete a variety of neurotrophic molecules. NSCs/NPCs have the ability to reduce cell death, reduce lesion volume, inhibit scar formation, exert anti-inflammatory effects, and promote electrophysiological and motor function recovery.26,230,231 They have advantages in forming effective neural networks at the injured site.232–235 For NSCs/NPCs, there are two different strategies, namely, transplantation of exogenous NSCs and activation of endogenous NSCs.
For exogenous NSC transplantation, many studies have applied materials for better cell transplantation. For example, Zou et al. proved that collagen sponge-based 3D-cultured NSCs cultured in a rotary cell culture system had better therapeutic effects than those cultured in a traditional cell culture environment, and this novel and effective method shows promise for application in NSC-based therapy for SCI.236 Liu et al. showed that collagen scaffolds combined with each type of NSC could markedly restore the motor function of the hindlimbs, as indicated by Basso-Beattie-Bresnahan (BBB) scoring, and further proposed that allogeneic NSC transplantation promotes functional recovery after SCI predominantly via the secretion of neurotrophic factors, not via direct neuronal replacement with neurons differentiated from transplanted cells.237
For the activation of endogenous NSCs, many methods, including biomaterials, pharmaceuticals, and electrical stimulation, have been applied. Zhu et al. demonstrated the in vivo behavior of LDH nanoparticles and LDH/NT3 in mice with complete spinal cord transection, both of which could contribute to the proliferation and differentiation of endogenous NSCs, reduce the inflammatory response at the injured site and improve the microenvironment to promote regeneration. These findings support an immunomodulatory strategy to recruit native NSCs as a potential acute care intervention for SCI.34 In this study, a combination treatment with pioglitazone (PGZ) and granulocyte colony-stimulating factor (GCSF) was applied in a rat T9 contusion model of SCI, and PGZ and GCSF treatment synergistically enhanced NSC numbers and improved functional recovery after SCI, which proved that this treatment can support NSCs directly and provide a sustainable microenvironment.22 Electrical stimulation has generated promising evidence as a novel approach to activate NSCs to facilitate neural repair, and more recently, clinically focused therapies aimed at improving outcomes following SCI have investigated the application of epidural electric stimulation. To date, this has been proven to be a promising rehabilitation strategy when used in conjunction with physiotherapy.21,238
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are obtained from a wide range of sources, such as bone (BMSCs), adipose tissue (AT-MSCs), umbilical cord (UCMSCs), and dental pulp (DP-MSCs). They are pluripotent stem cells that can self-renew and directionally differentiate into other types of cells239,240 and have low immunogenicity and multiple differentiation potential, making them popular in the stem cell and regenerative medicine fields.241–245 These cells can secrete colony-stimulating factor, stem cell factor, nerve growth factor, and other cytokines.246 In terms of promoting neuronal regeneration and restoring neuronal pathways, MSCs have obvious advantages in regulating the injury microenvironment and providing neurotrophic factors.221,247,248
After transplantation in the SCI model, MSCs mainly protect neurons in the following ways. First, MSCs play an immunomodulatory role in the microenvironment by inhibiting inflammation. MSC transplantation can inhibit the activities of various inflammatory factors (IL-1α, IL-1β, and TNF-α) and inflammatory cells (T cells, B cells, and macrophages) and reduce the inflammatory response in the lesion area after SCI. Studies have shown that transplantation of MSCs into SCI rat contusion models can significantly increase the number of M2 macrophages and decrease the number of M1 macrophages at the injury site, which might contribute to the recovery of motor function, increased retention of axons and myelin sheaths and reduced glial scar formation after injury.249 Second, MSCs secrete a variety of neurotrophic factors, such as BDNF, NT3, NGF, and GDNF, to play a neuroprotective role.250 On the other hand, MSCs can also act on T lymphocytes, B lymphocytes, natural killer cells (NK cells), antigen-presenting cells and other immune cells in various ways to affect the immune state of the body, reduce the immune response of the body and promote the repair of SCI by inhibiting their proliferation, differentiation and activation.251 Recently, it was reported that the mechanism by which BMSCs reduce neuronal apoptosis after SCI may involve the transfer of mitochondria to neurons via gap junctions.252
Oligodendrocyte progenitor cells
Oligodendrocyte progenitor cells (OPCs) are adult stem cells widely distributed in the central nervous system. As precursor cells of oligodendrocytes (OLs), OPCs can migrate to affected areas and differentiate into OLs under the action of a variety of chemokines to promote the formation and regeneration of the myelin sheath. They are beneficial to the repair of demyelinating lesions. Results have shown that OPCs can survive in the spinal cord of rats with SCI after transplantation and differentiate into neurons such as OLs and astrocytes, which promote myelination of the injured site, repair the damaged spinal cord tissue, and improve the motor ability and evoked potential generation of rats.253 It was reported that when human EMC-derived OPCs were transplanted into the cervical spinal cord 1 week after injury in rats, they significantly improved locomotor performance with no adverse clinical effects.254
Neural cells
Olfactory ensheathing cells
Olfactory ensheathing cells (OECs) exist in both the peripheral nervous system and central nervous system, have regeneration ability, and are unique glial cells that show promise for the treatment of SCI.255 Barbour et al. found that OEC transplantation significantly increased neuronal survival by approximately sixfold in rats with SCI (T10) in the subacute stage.256 Combined transplantation of OECs and NSCs in rats with SCI showed that OECs could guide axonal lengthening through the glial scar and promote myelination. A key ability of OECs is migration from the peripheral nerve to the central nervous system, which enables the enhancement of axon extension after SCI and contributes to nerve regeneration.257
Schwann cells
Schwann cells (SCs) act as structural scaffolds for the peripheral nervous system and can promote a microenvironment favorable to neuronal regeneration.258 In the central nervous system, they can regenerate demyelinated axons by secreting a variety of growth factors and depositing growth-promoting proteins in the extracellular stroma. Barbour et al. performed local injection and transplantation of SCs in an acute (14 d after injury) rat model of moderate SCI and observed that the SC cell injection group showed an increased number of supraspinal fibers, a reduced appearance of cavities and enhanced tissue integrity 4 months later, indicating improved anatomical outcomes after SCI.256 Autologous activated Schwann cell (ASC) transplantation for the treatment of SCI was carried out in China ten years ago and showed some signs of functional improvement.259
Astrocyte lineage
Astrocyte transplantation was thought to be an important strategy for SCI repair because of the formation of local cavities after SCI and the important supporting role of astrocytes in the regeneration and extension of neuronal axons.260 Lepore et al. transplanted cultured lineage-restricted astrocyte progenitors into animals with cervical SCI and found that these cells could survive for a long time and differentiate into astrocytes, promote motor neuron axon regeneration, and improve diaphragm function.261
Immune cells
Macrophages
The proportion of proinflammatory/anti-inflammatory immune cells at the SCI site can be adjusted by transplanting macrophages with immunomodulatory effects. It was reported that transplantation of M2-deviated microglia induced by IL-4 could improve the recovery of motor function in mouse SCI.70 In addition, M2-phenotype (M2) macrophages induced by tauroursodeoxycholic acid had a similar effect.201
Advanced biomaterials for SCI repair and regeneration
In recent years, nanotechnology has made great advancements in the treatment of SCI. Nanomaterials can be used as nanocarriers for targeted drug delivery, and increasing the cycle time can improve the bioavailability of drugs. Recently, many biomaterials have been designed and have shown advantages in eNSC activation, mobilization, and controlled differentiation.34,262–266 Some microenvironment-responsive biomaterials have shown good immunoregulatory effects.267 In research on the repair and regeneration of SCI, a number of natural and composite materials have been utilized, including nanoparticles, hyaluronic acid, alginate, collagen, agarose, polylactic acid, PLGA, etc. The key issues that need to be addressed are to reduce inflammatory infiltration, reduce scar tissue, improve the regenerative capacity of neurons and axons, and guide the axons to the appropriate areas for regeneration.268 Here, we focus on several biomaterials with clinical application prospects.
Inorganic-layered nanomaterials
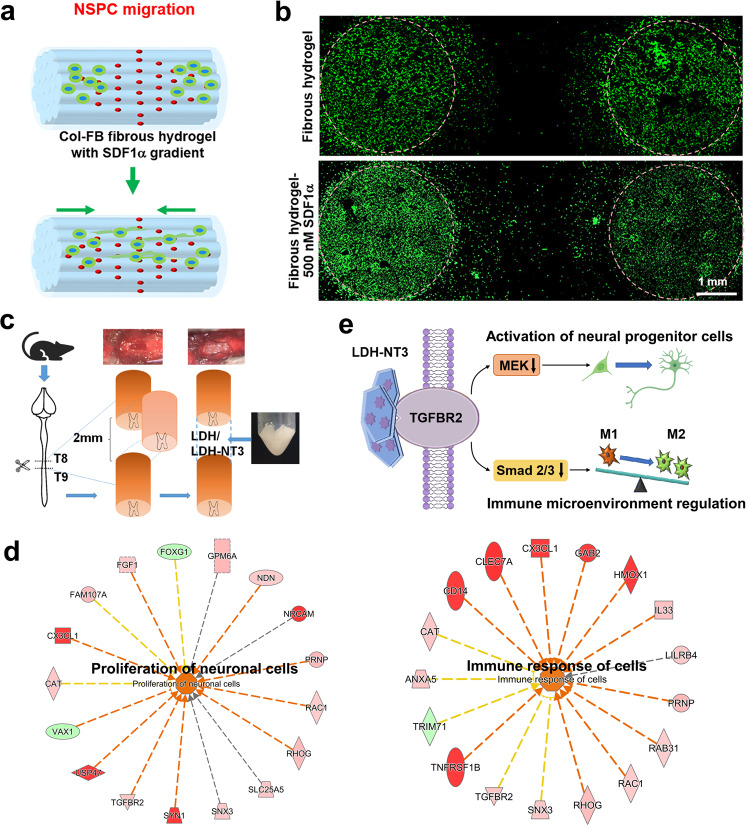
Nanoparticles are increasingly being studied in experimental models for SCI treatment. The composition of these nanoparticles is extremely diverse and includes polymers, metals, metal oxides, silica, and biological molecules.269–271 In our previous study, we used biodegradable Mg/Al LDH as a novel strategy for immune microenvironment amelioration and neural regeneration. The results indicated that both LDH and LDH-NT3 could improve the microenvironment to accelerate NSC migration, neural differentiation, L-Ca2+ channel activation, and inducible action potential generation, which supported the generation of newborn eNSCs at the lesion site.34 In addition, LDH-NT3 performed remarkably well in regulating synaptic transmission and neuron–neuron synaptic transmission. With the improved microenvironment established by LDH/LDH-NT3, neural precursor cell synthesis, axonogenesis and ion channel action-involved signaling pathways were positively regulated to achieve regeneration of neurons and the reconstruction of neural circuits after SCI (Fig. 4).34 Graphene and graphene-based materials have good electrical conductivity, which can make full use of nerve electrical signals in spinal cord tissue to promote axon regeneration.272,273
Fig. 4.
Advanced biomaterials for bioactive molecule delivery and microenvironment regulation. a, b Functionalized aligned Col-FB fibrous hydrogel induced NSPC migration and neuronal differentiation. Adapted with permission from ref. 287 Copyright 2022, American Chemical Society. c–e The LDH/LDH-NT3 transplantation promoted the process of neural regeneration and neural circuit reconstruction in the lesion sites of SCI mice. Adapted with permission from ref. 34 Copyright 2021, American Chemical Society
Hydrogels
As a large category of biological materials, hydrogels can mimic soft tissue environments and have suitable chemical compositions for the integration of extracellular matrix (ECM) molecules and other binding proteins, which can effectively support and guide axon regeneration for SCI repair.34,274–278 Zaviskova et al. modified the hydroxy groups of hyaluronic acid with RGD phenyl derivatives and crosslinked them with enzymes to obtain soft injectable hydrogels.271,279–281 The obtained hydrogel and hydrogel+MSCs were transplanted for the treatment of SCI, which showed strong effects on axon growth at the center of the injury when used in combination. Mukhamedshina et al. used fibronectin-based hydrogels for the culture and transplantation of ADSCs in SCI, and the results showed that the expression of GFAP and Iba1 decreased, with a smaller area of the center cavity.282–285 Dai et al. reported a microenvironment-responsive hydrogel that can effectively inhibit MMP and release the loaded bFGF according to the needs of the SCI microenvironment.286 The author’s other research revealed that one aligned collagen-fibrin (Col-FB) fibrous hydrogels showed good stretchable properties, adhesive behavior, and spatiotemporal delivery capability, and could promote locomotion recovery through recruiting endogenous neural stem/progenitor cells (Fig. 4).287
Collagen
Due to its low immunogenicity, good biocompatibility and biodegradability, appropriate porosity and mechanical strength, collagen has been proven to be one of the most suitable natural polymer materials for SCI repair. SCI triggers a biochemical cascade that creates a microenvironment around the injury site, which inhibits further nerve regeneration. Several signaling molecules in this microenvironment have been found by various scientists to inhibit neuronal axon regeneration. Dai et al. found signaling molecules in the SCI microenvironment that inhibit the differentiation of neural stem cells into neurons, and they have been trying to reconstruct the spinal cord regeneration microenvironment through functional biomaterials for many years. They established rat and beagle models of complete transection SCI to systematically study the effects of biological materials on regeneration in the SCI microenvironment.288–291 Functional biomaterials can reconstitute a regenerative microenvironment and antagonize the inhibitory effects of myelin protein on neural regeneration, thus inducing the differentiation of endogenous or transplanted neural stem cells into neurons. These new neurons form neural bridges through the injury area and transmit neural signals to promote the recovery of nerve function in animals with transection SCI. Research suggests that neural bridges formed by neurons generated from endogenous or exogenous neural stem cells are the main mechanism underlying functional biomaterial-based repair of complete transection SCI.269,292–296
Chitosan scaffolds
Yang et al. implanted an NT3-coupled chitosan biomaterial into a 5 mm space in the thoracic segment of rats in an SCI model, and eNSCs were activated in the injured spinal cord.297 Li et al. prepared a chitosan/ECM/SB216763 scaffold for SCI repair, and after transplanting chitosan/NT3 composites in a rat spinal cord T9 complete transection defect model using chitosan as a substrate, NeuN- and Tuj1-positive neurons appeared in the spinal cord defect area, with effective synaptic connections between neurons, resulting in significant recovery of hindlimb motor function in rats.298 In vivo experiments were conducted in a semitransection SCI model, and the results showed that the BBB score of the chitosan/ECM/SB216763 group was significantly better than those of the other groups.299
Physical regulation and regeneration
Light stimulation
Optogenetics can play a very important role in rebuilding lost neuronal circuits. Ahmad et al. first reported that ChR2 could be expressed in motor neurons or stem cells, thereby stimulating neuronal activation and regeneration, in response to blue light irradiation.300 The p42/p44-MAPK signaling pathway can be modulated optogenetically to counteract the antagonistic effect of p38 MAPK, which can regulate long-term potentiation (LTP) of the mammalian hippocampus.300–302 Another noninvasive method, called photobiomodulation (PBM), can promote functional recovery by reducing neuroinflammation and promoting neuronal axon regeneration after SCI.303 It was reported that photobiomodulation is useful in polarizing macrophages via the NF-kB P65 pathway.304 Neurotoxic microglia and astrocytes atcivation is inhibited by PBM through Lcn2/JAK2-STAT3 crosstalk suppression.305 Additional investigations indicated that PBM therapy at 810 nm upregulates macrophage secretion of neurotrophic factors via PKA-CREB and promote neuronal axon regeneration in vitro, and the activation and secretory function of astrocytes were inhibited by photobiomodulation via alterations in macrophage polarization.306
Ultrasound stimulation
Ultrasound can regulate the proliferation and differentiation of stem cells. The first successful attempt in 1958 revealed that ultrasound could reversibly regulate nerve signal transmission and regulate central nervous system circuits in cats.307 Sangjin et al. showed that focused ultrasound excites primary murine cortical neurons in culture through a primarily mechanical mechanism mediated by specific calcium-selective mechanosensitive ion channels.308 Liao’s study indicated that low-intensity focused ultrasound (LIFU) can alleviate spasticity following SCI by activating spinal neurocircuits and increasing the expression of the neuronal K-Cl cotransporter KCC2.39 A proteomics study analyzed the effect of LIFU on spasticity post SCI and showed that Gap43 protein expression was significantly decreased in the LIFU therapy group.309 Recent works provide a mechanistic explanation for the effect of ultrasound on neurons to facilitate the further development of ultrasonic neuromodulation and sonogenetics as tools for neuroscience research.
Magnetic stimulation
A pulsed magnetic field was used to treat cultured fetal rat dorsal root ganglia in vitro, and it could induce electric fields and promote the growth of axons along the current direction.310 Low-frequency magnetic fields have better potential for promoting neuron proliferation and differentiation and neural circuit remodeling, but the specific parameters are not yet clear.311 It was reported that 50 Hz could affect neural excitability by regulating cortical calcium channels through the AA/LTE4 signaling pathway.312 Xue et al. also found that 50 Hz can enhance the expression of calcium channels on the presynaptic membrane of the mouse brainstem, thereby enhancing the calcium current and promoting the circulation of presynaptic vesicles and synapse touch plasticity.313 The effect of iron oxide nanoparticles (IONPs) along with electromagnetic field (MF) exposure on spontaneous axonal sprouting after SCI was evaluated. Under exposure to MFs (50 Hz, 17.96 μT, and 2 h/day for 5 weeks), the results showed sprouting from mature neurons and axons, significantly less demyelination and more myelinated fibers at the lesion site.314 A mild contusion rat model of SCI suggested that facilitation of sensory-motor recovery occurred after MF exposure, which could be due to attenuation of secondary damage and calcium-mediated excitotoxicity.315
Electric stimulation
The polarity characteristics of electrical stimulation have regulatory effects on the migration and differentiation of stem cells.316,317 It was reported that pulsed DC stimulation (1 V/cm for 12 days) is most effective in enhancing the differentiation of NSCs into neurons.318 Petrella et al. compared the effects of a picosecond pulsed electric field on NSCs and MSCs. Pulsed ES has no influence on MSCs proliferation but improves NSCs proliferation and astrocyte-specific differentiation by upregulating GFAP after 24 h at 40 kV/cm.319 Dong et al. stimulated NSCs with electricity for 3 days at 150 mV/mm, resulting in increased achaete-scute homolog (Ascl1) expression that was further proven to regulate the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway in NSCs.320 To date, the effects of electrical stimulation through 2D or 3D conductive materials on stem cell fate have been thoroughly investigated. Nanopatterned polyurethane-acrylate substrate surfaces and PLGA/GO composite membranes were effective in promoting the proliferation, differentiation and neurite elongation of NSCs.321,322 Stem cells showed improved cell behavior in the 3D culture system. Higher neuronal gene expression levels were observed, and more stem cells differentiated into neural cells with electrical stimulation. The underlying mechanism may be due to the upregulation of neural genes, such as MAP2, βIII-tubulin, and NSE, by electrical stimulation.323,324
The more mature application of electrical stimulation for SCI rehabilitation is functional electrical stimulation technology, whose mode of action is determined by three parameters: pulse amplitude, pulse duration and pulse frequency. The noninvasive nature of this approach makes it possible to use FES very early in the rehabilitation of patients who have had an SCI.
Clinical treatment and research
At present, the clinical treatment methods for SCI include medications, surgery, rehabilitation and nursing. Management in the acute phase aims mainly to stabilize the condition and ensure the survival of patients. Management in the chronic phase aims mainly to restore function, reduce complications, and encourage patients to return to society and work. During this period, it may also be necessary for psychologists to treat patients with psychological disorders. Breakthroughs in clinical treatment have mainly focused on the research and development of new drugs, clinical trials of cell therapies and biomaterial transplantation, new physical regulation approaches, artificial intelligence, etc.
The pathophysiological mechanisms driving the secondary injury after SCI are complex and SCI is a heterogeneous condition.27,33,325 Single treatment may only affect a small portion of mechanisms, whereas combinatorial treatment targeting multiple mechanisms is potentially a better therapeutic selection. In animal studies, while provide a combination of essential factors for axon regeneration-permissive substrates, chemoattractive growth factors and activating intrinsic growth capacity, robust axon regeneration through the astrocytic scar and nonneural lesion core after complete SCI was observed.8,326 Regeneration can also be accentuated by combining cells with biomaterials or neurotrophic factors.327 For instance, Schwann cells combined with neurotrophins, elevation of cyclic AMP levels, olfactory ensheathing cells, a steroid or chondroitinase,328 NSCs expressing embedded into fibrin matrices containing growth factor cocktails,235 combination of Schwann cells, OECs chondroitinase,329 NSCs, fibrin matrices and a cocktail of growth factors and a cell death inhibitor, etc.330 A speculative pharmaceutical cocktail of three commercially available agents including thyrotrophin-releasing hormone, selenium and vitamin E for intravenous and oral administration was proposed as a combination of medications to treat acute SCI based on theoretical benefits such as antagonism of endogenous opioids, petidoleukotrienes, excitoxins, and antioxidant properties. However, no animal or human studies of such cocktails were published, thus this proposal may contentious.331
Clinical treatment
Drugs
Methylprednisolone (MP) is the only FDA-approved drug for the treatment of SCI. The mechanism of MP in SCI mainly involves reducing the secondary inflammatory response, restoring the blood‒spinal cord barrier, improving the spinal cord blood supply, scavenging free radicals, and enhancing neurotrophic factor secretion.332–335 However, recent studies did not recommend the use of MP for the treatment of acute SCI because of substantial clinical evidence for deleterious side effects of MP in acute SCI.336 The latest AO guidelines for SCI recommend 24 h infusion of methylprednisolone only in SCI patients within 8 h of injury but not in patients more than 8 h from injury.337
Clinical trials of new drugs for SCI are constantly being carried out. These studies have focused on neural regeneration and microenvironmental regulation after SCI, including studies of glyburide (NCT02524379), buspirone combined with levodopa-carbidopa (NCT04052776), and vitamin D3 (NCT04400747). Research institutes have also given attention to the drug treatment of SCI-related complications, such as neuropathic pain (Lyrica, NCT00879021; Controlled-Release Morphine, NCT00488969; GW-1000-02, NCT01606202; Amitriptyline, NCT00006428; KAI-1678, NCT01135108), neurogenic bladder (Neostigmine and Glycopyrrolate, NCT02370862; Botox-A, NCT00711087, Fampridine-SR, NCT00041717), and osteoporosis (Alendronate, NCT02195895; Teriparatide, NCT02025179), and a series of clinical trials of drug therapies have achieved some clear clinical effects (Table 2).
Table 2.
clinical trials about drug therapy for SCI
| Drugs | Patients condition | Frequency of administration | Study types | Subjects | Phase | ClinicalTrials.gov identifier or refs |
|---|---|---|---|---|---|---|
| Glyburide | Acute SCI | 3.125 mg po on day 1, 2.5 mg for following days to 2 weeks | Interventional | 3 | Phase 1/2 | NCT02524379 |
| Buspirone and Levodopa-Carbidopa | SCI with EES | 40 mg Buspirone, 400 mg/100 mg Levodopa-Carbidopa po. single-dose administration | Interventional | 8 | Phase 1 | NCT04052776 |
| Vitamin D3 | Thoracic-level chronic SCI | 6000 IU per day or 50,000 IU per week for 8 weeks | Interventional | 60 | Not applicable | NCT04400747 |
| Lyrica | Traumatic SCI | 150 mg p.o. Bid for 49 weeks | Interventional | 5 | Phase 3 | NCT00879021 |
| Modified-release morphine | Traumatic SCI | up to 120 mg p.o. for 7 weeks | Interventional | 17 | Phase 2 | NCT00488969 |
| GW-1000-02 | Non-acute SCI |
100 μl (THC 2.7 mg and CBD 2.5 mg)/time 8 times in 3 h, and 48 times in 24 h, 7–21 days. |
Interventional | 116 | Phase 3 | NCT01606202 |
| Amitriptyline | 6 months post SCI | N/A daily dose for 6 weeks | Interventional | 100 | Phase 4 | NCT00006428 |
| KAI-1678 | 1 year post SCI | N/A dose KAI-1678 i.v. | Interventional | 5 | Phase 2 | NCT01135108 |
| Neostigmine and Glycopyrrolate | SCI |
Visit 1: 0.03 mg/kg NEO and 0.006 mg/kg GLY,i.v. Visit 2: 0.05 mg/kg NEO and 0.01 mg/kg GLY, i.v. Visit 3: 0.07 mg/kg NEO and 0.14 mg/kg GLY, i.v. 2–14 days. |
Interventional | 28 | Phase 1 | NCT02370862 |
| BOTOX-A |
T10 or above Thoracic-Level SCI 8 weeks post injury |
100 units BTX-A injections on day 0 and day 90 | Interventional | 1 | Phase 2 | NCT00711087 |
| Fampridine-SR | 18 months post traumatic SCI | 25 mg p.o. bid, 12 weeks | Interventional | 213 | Phase 3 | NCT00041717 |
| Alendronate | Chronic SCI | 70 mg p.o. weekly for 12 months | Interventional | 17 | Phase 2 | NCT02195895 |
| Teriparatide | Chronic SCI | 20 ug daily Sub-Q over 12 months | Interventional | 25 | Phase 2 | NCT02025179 |
Riluzole is known as a neuroprotective agent.5 As a sodium channel blocker, Riluzole blocks the sodium channels and prevents the excessive influx of sodium ions, reduces the intracellular sodium concentration and influx of calcium ions which cause the development of intracellular acidosis and cytotoxic edema.338 In animal studies, riluzole provides histological and functional recovery.339–342 In a prospective, multicenter phase I trial, compared with the control group, the mean motor score showed significant improvement in the riluzole-treated group, and there were no serious adverse events related to riluzole and no deaths.343 But in a prospective, randomized controlled study of acute cervical SCI patients, administration of riluzole did not significantly improve neurological outcome/neuropathic pain.344
Minocycline targets multiple secondary injury mechanisms via its anti-inflammatory, antioxidant, and anti-apoptotic properties.345 Animal experiments showed that administration of minocycline result in preservation of the ultrastructure of spinal cord tissue,346 inhibition of microglial activation,347 significant improvement of motor function,346 reduce oligodendrocyte apoptosis and local inflammation,348,349 thus, improvement of motor function. But a contrary result was also reported.350 In a phase II placebo-controlled randomized trial of minocycline in acute SCI, no difference in motor recovery for thoracic SCI patients. In incomplete cervical SCI patient, functional outcomes exhibited differences but no statistical significance. No severe adverse event related to minocycline.351
Surgery
The aim of surgery for acute SCI is decompression and restoration of spinal alignment and stability.352 Studies have suggested that early decompression surgery may achieve reduced neural injury and improved outcomes, and early surgery may reduce the length of ICU stay and reduce post-injury medical complications.353,354 A multicenter, nonrandomized cohort study showed that early surgery achieves better neurological outcomes at the 6-month follow-up, as indicated by the odds of a 2-grade improvement in the AIS evaluation.355 Recently, a pooled analysis of individual patient data showed that surgical decompression within 24 h after acute SCI caused improved sensorimotor recovery. The first 24–36 h after acute injury is a crucial time window to achieve optimal neurological recovery with surgical decompression.356
In addition to external compression of the spinal cord, internal factors in the spinal cord itself can also affect clinical outcomes after surgery. Hematoma and edema after SCI can lead to increased intraspinal pressure, which may worsen the prognosis of SCI patients.357,358 After bony decompression, the intraspinal pressure remains high due to the tough and nondilated dura mater and has a tamponade effect on the blood vessels of the spinal cord, exacerbating blood supply issues and ultimately causing cytotoxicity and vasogenic edema.62,358–360 Therefore, surgical strategies to reduce intraspinal pressure have also been proposed. In vitro and in vivo animal experiments proved that durotomy can reduce intraspinal pressure and result in better blood perfusion, more neural tissue sparing and improved functional recovery.361–364 In clinical studies, Perkins and Deane performed durotomy in 6 neurologically impaired SCI patients with burst fracture after bony decompression, when the dura mater was noted to be tense and nonpulsatile. After the dura was incised, the return of dural pulsation was observed, and full or partial neurological recovery occurred in all the patients, as evaluated by Frankel grading. The authors believe that durotomy may be of some use in the recovery of spinal cord perfusion.365
Compared with durotomy, duroplasty can enlarge the dural space, reduce the intraspinal pressure and cause fewer complications, such as cerebrospinal fluid leakage, pseudomeningocele, and CNS infection, and it requires only 10–15 min of surgery time to suture an artificial dura to the dural margin.357,358,360 Phang et al conducted an open-label, prospective study comparing laminectomy+duroplasty versus laminectomy alone. The laminectomy+duroplasty group showed a greater increase in intradural space at the injury site, more effective decompression of the spinal cord, a lower intraspinal pressure and higher spinal cord perfusion pressure, and improved radiological and physiological parameters.360 In a retrospective analysis of 16 severe adult SCI cases without radiographic abnormalities, after durotomy with duraplasty, AIS scale and AISA scores improved, and the high level of intraspinal pressure after laminectomy continued to decrease steadily after surgery.366
Myelotomy or spinal cord incision was reported as early as 1911. Allen performed myelotomy in dogs with SCI, and function was restored. The author believed that myelotomy could drain the necrotic tissue.367 Hu et al. performed myelotomy on rats 24 h post-contusion SCI. Compared with those in the control group, significantly improved Basso, Beattie and Bresnahan scores, higher mean angle values in the incline plane test and edema were observed in the myelotomy group.368 Compared with durotomy only, durotomy plus myelotomy promoted spinal tissue formation, elicited a significant beneficial impact on gray matter sparing, increased the preservation of motor neurons and significantly promoted the recovery of hindlimb locomotor function.363 Four acute SCI patients received myelotomy within 24 h, and no patient developed new deficits postoperatively. All patients showed improvement in motor function of the upper extremities, and sensory disturbances also diminished to some degree.369 In another case report, a patient with central cervical SCI was treated by myelotomy. After recovering well from central cord syndrome, the patient developed rapidly progressive myelopathy 2 months after injury due to a new lesion at the C6 level rather than the original lesion at the C7 and T1 levels. Another myelotomy at the C6 level revealed intense gliosis inside the spinal cord. Rapid clinical improvement ensued. The authors concluded that secondary syringomyelia may be an end-stage condition after SCI and trigger a progressive, pathophysiological reaction, leading to central cord necrosis. They believe that in selected cases, myelotomy may interrupt this process.370 However, researchers also reported that compared to SCI-only animals receiving SCI, myelotomy 48 h after injury worsened Basso, Beattie, and Bresnahan score scores and did not improve plantar stepping, ladder climbing, urinary bladder voiding or sensory function, and no expected immunohistochemical changes were found.371 The negative effect after myelotomy may be due to the timing of surgery, as myelotomy itself can cause spinal cord damage, leading to aggravated inflammation in the injured spinal cord, which may cause negative outcomes.357,358,371
Rehabilitation treatment
Traditional rehabilitation
In hyperbaric oxygen (HBO) therapy, 100% oxygen is administered at a pressure onefold to threefold higher than atmospheric pressure. The underlying mechanisms for HBO include decreasing apoptosis and reducing inflammation and edema. Since ischemia is one of the major pathological changes after SCI, a high oxygen pressure, which increases oxygen tension in the spinal cord, may reduce the degree of ischemic injury in the spinal cord and improve clinical outcomes.372 Tan et al. published a retrospective study to assess the therapeutic effect of HBO therapy in the early treatment of acute SCI. Significantly improved ASIA scores and Frankel scores were found in the HBO group, and better results of MRI and electrophysiology tests were also reported.373 Another retrospective study of incomplete cervical SCI treated with and without HBO after surgery showed the safety and efficacy of HBO therapy and indicated that the longer the treatment lasts, the better the effects.374 Asamoto et al also reported that in the HBO group, the improvement rate indicated effectiveness in acute traumatic cervical SCI according to the Neurological Cervical Spine Scale (NCSS).375
Exercise is a noninvasive treatment that provides stimulation to certain regions of the spinal cord and appears to have multiple applications and benefits for SCI.376 Exercise has been proven to preserve muscle mass and strength, restore motor and sensory function, reduce local inflammation of the spinal cord, etc. After aerobic exercise, resistance training and combined exercises and in some studies of gait training and balance training, positive effects were observed.377 A home-based 6-week upper-body exercise improved indices of health-related quality of life in SCI patients, and the improvements were associated with increases in exercise self-efficacy.378
New means of rehabilitation
Neuromodulation technology, such as functional electrical stimulation (NCT03439319), can be used to improve limb function.379 The integrated field of medicine and industry has developed rapidly and innovated constantly, providing more advanced and convenient approaches for the rehabilitation of SCI patients, such as exoskeleton robots and brain-computer interfaces (BCIs). BCI is a new rehabilitation concept that bypasses the relay station of the spinal cord and directly allows an electroencephalogram to control the movement of limbs.380–382 In a primate SCI model, a brain-spine interface restored weight-bearing locomotion of the paralyzed leg on a treadmill and overground.382 A clinical trial on BCI has been carried out, and the results showed that hand motor function and tactility were recovered in patients with clinical complete SCI (NCT01997125).380 Another clinical trial showed that the neural activities of the motor cortex can be decoded into handwritten actions through BCI, significantly improving the writing speed and accuracy of paralyzed hands in patients with SCI (NCT00912041).383
Treatment outcomes and prognostic predictions
In terms of prognosis, it has been reported that the mortality of SCI is still high in recent years. SCI mortality rates in developed countries ranged from 3.1 to 22.2%, whereas mortality rates in developing countries ranged from 1.4 to 20.0%.48,384–388 Most patients can live a long time due to advancements in intervention and support techniques, but due to long-term bed rest or restricted activities, patients often experience unavoidable complications such as nonneuropathic pain, pendant pneumonia, bedsores and urinary tract infection.389–393 The prognosis of SCI is significantly associated with the site and severity of injury. After the basic vital signs have been stabilized, a thorough neurological evaluation is critical for the diagnosis and management of SCI patients. The Neurological and Functional Classification Standard of the American Spinal Injury Association (ASIA) is the preferred tool recommended by current guidelines and is an important tool for initial neurological examination and prognosis follow-up examination.394,395 There is another tool, named the SCI or Dysfunction Quality of Life Rating Scale (SCIDQLRS) (IANR 2022 version), which was designed as a single method to assess various items related to quality of life after SCI.396 It has been reported that 80% of ASIA grade A patients may not recover function and that 54% of ASIA grade B patients and 86% of ASIA grade C-D patients will have varied degrees of neurological recovery.397,398 A combination of assisted physiological tests, such as electrophysiological examination, can better predict the prognosis of SCI patients.399–401 Furthermore, age is also a predictive factor for SCI. It has been reported that 91% of patients with central SCI under 50 years of age regained walking ability, but only 41% of patients over 50 years of age regained walking capacity.400,401 As a result, making a thorough clinical decision based on a number of clinical laboratory test signs and long-term follow-up is required rather than merely predicting the prognosis of patients based on the ASIA scale.395,402,403 In addition, it should be noted that no studies have offered a molecular classification that can predict SCI risk and prognosis.
Advanced technology in clinical trials
Clinical trial of cell transplantation in SCI
Cell transplantation to repair SCI is considered to be the most promising therapeutic strategy, and the cell types transplanted include MSCs, OECs, OPCs, NSCs/NPCs, ESCs, and iPSCs. Autologous stem cell transplantation has low risks of immunogenicity and tumorigenicity, while allogeneic cells are easy to obtain and expand, which is convenient for quality management. The safety, efficiency, cost, and feasibility of large-scale manufacturing should be considered. Several reports have compared different cell sources for SCI therapy.404–406 However, it is still unclear which one is the most effective for SCI therapy. MSCs can participate in immune regulation and neuroprotection to reduce cell loss. OECs/OPCs show advantages in myelin production and tissue modification. NSCs/NPCs may differentiate into neurons and replace the damaged cells to form a local network at the site of injury and form connections with intrinsic neurons. Various types of stem cells have been demonstrated to be safe and effective in rodents, dogs and nonhuman primates.407–411
ESCs and iPSCs are rarely directly used for transplantation because of their tumorigenicity, but their derivatives have been used in clinical trials of SCI. Human ESC-derived OPCs (LCTOPC1; previously known as GRNOPC1 and AST-OPC1) have been approved for clinical trials in the United States, and the results of the first clinical trial in 25 patients with subacute cervical SCI showed that 96% of the patients recovered one or more levels of neurological function and 32% recovered two or more levels.412 The transplantation of iPSC-derived NSCs/NPCs for subacute complete SCI was first approved in Japan (Table 3).413
Table 3.
clinical trials about cell transplantation
| Cell sources | Patients condition | Route of admin | Quantity and times | Subjects | Phase | ClinicalTrials.gov identifier or refs. |
|---|---|---|---|---|---|---|
| AST-OPC1 | Subacute cervical SCI | Intraparenchymal | 1 time; 2×106/1×107/2×107 | 25 | Phase 1/2a | NCT02302157412 |
| hiPSC-NS/PC | Subacute SCI | Intralesional | 1 time; 2×106 | 4 | Phase 1/2 | jRCTa031190228.413 |
| hNSPCs | Traumatic cervical SCI | Intralesional | 1 time; 1×107 | 19 | Phase 1/2 | KCT0000879414 |
| human spinal cord-derived NPCs | Chronic SCI | Intralesional | 1 time; 1.2×106 | 4 | Phase 1 | NCT 01772810416 |
| HuCNS-SC | Chronic cervical SCI | Perilesional intramedullary | 1 time; 4×107 | 52 | Phase 2 | NCT02163876415 |
| Autologous BMSCs | Chronic and subacute SCI | Intrathecal | 2–3 times; 1.2×106/kg | 9 | Phase 1 | NCT02482194.418 |
| Allogeneic hUC-MSCs | Chronic SCI | Subarachnoid | 4 times; 1×106/kg | 143 | Phase 1/2 | 419 |
| BMSCs | Chronic SCI (ASIA grad B) | Intramedullary+ intrathecal | 1 time; 1.6×107 (intramedullary)+ 3.2×107 (intrathecal) | 16 | Phase 3 | NCT01676441421 |
| BMSCs+SCs | Complete SCI (3–12 m,ASI A) | Intrathecal | 5×107 BMSCs+5×107 SCs | 11 | / | 422 |
| Autologous BMSCs | Chronic complete SCI | Intralesional | 1 time; 5×106/cm3 | 14 | Phase 1 | NCT01325103508 |
| BMSCs | Chronic SCI | Intrathecal | 1–8 times; 2×106/kg each month | 70 | Phase 1/2 | NCT00816803509 |
| Autologous ADMCs | Chronic SCI | Intravenous | 1 time; 4×108 | 8 | Phase 1 | NCT01274975510 |
| UCMSCs | SCI | Intralesional | 1 time; 4×107 | 34 | Phase 3 | NCT01393977511 |
| Human UCMSCs | Chronic SCI | Intrathecal | 4 times 1×106/kg each month | 66 | Phase 2 | NCT03521323512 |
| UCMSCs | SCI | Intravenous+ intrathecal | 1 time, 30 ml iv; 3times, 5×104 intrathecal each week | 7 | / | 424 |
| BMMCs | SCI | Intrathecal | 1 time; 2–4 ml cells | 10 | Phase 1/2 | 426 |
| UCB-MNC | Chronic complete SCI | Perilesional intramedullary | 1 time; 1.6×107 ~ 6.4×107 | 28 | Phase 1/2 | NCT01046786/ NCT01354483427 |
NSCs/NPCs for transplantation are mostly obtained from aborted fetuses. A phase I/IIa open-label and nonrandomized controlled clinical trial on the transplantation of human fetal brain-derived NSCs/NPCs into traumatic SCI patients showed that the AIS grade improved in 5 of 19 transplanted patients without serious adverse events.414 NSC transplantation was also reported in chronic SCI. Levi et al. carried out a phase II clinical trial (NCT02163876) using human NSCs (HuCNS-SCs), which were authorized as an investigational new drug (IND-15712) by the United States FDA, and observed recovery in the upper extremities.415 The authors also provided useful data on the surgical safety profile and feasibility of multiple intramedullary perilesional injections of HuCNS-SCs after SCI in another publication.326 Our team is conducting a clinical trial of intrathecal injection of aborted fetus-derived NSCs in the treatment of SCI (ChiCTR2200059595). Transplantation of human fetal spinal cord-derived NPCs (NSI-566) into SCI patients was also carried out, and the results showed that two of four subjects exhibited neurological improvement (Table 3).416
MSCs were used earlier and more widely in clinical trials of SCI due to their immunomodulatory mechanism, low immunogenicity, ease of acquisition, and fewer ethical restrictions.417,418 A phase 1/2 pilot study of 143 SCI patients showed that repeated subarachnoid administration of allogeneic human UCMSCs significantly improved pinprick, light touch, motor and sphincter scores.419 However, there are still questions and limitations in this field.420,421 Combined transplantation of different types of cells, transplantation of MSCs combined with biomaterials, and comprehensive rehabilitation may be strategies to improve the effect of MSCs in clinical trials.422–425 A phase 1/2 clinical trial of cell transplantation combining human autologous Schwann cells and BMSCs was carried out in subacute complete SCI patients and revealed statistically significant improvements in sensory and neurological functions (Table 3).422
Bone marrow mononuclear cells (BMMCs) and umbilical cord blood-derived mononuclear cells (UCB-MNCs) are both useful cell types for repairing SCI, and their function has been verified in clinical research on SCI (Table 3).426,427
Clinical trial of biomaterials in SCI
Biomaterials have been used to replace PNS grafts, which can achieve significant functional recovery by improving axonal regeneration when NPCs are seeded.428 To overcome the solid morphology of scaffolds, hydrogels and self-assembling peptides have been developed to provide injectable scaffolds.429–432 These biomaterials can be modified to deliver drugs or stem cells and have been shown to provide functional recovery in rodent models of SCI.23,433–437
However, there are few clinical studies on functional material transplantation to repair SCI. Dai et al. transplanted NeuroRegen scaffolds combined with UCMSCs into two acute complete SCI patients, and the results showed significant recovery in sensory and motor functions and improvement in bowel and bladder function (NCT02510365).423 Another 3-year clinical study performed by Dai’s team enrolled seven acute complete SCI patients, and neuro-Regen scaffolds loaded with autologous bone marrow mononuclear cells (BMMCs) were implanted into the site after the necrotic spinal cord tissue was surgically cleaned under intraoperative neurophysiological monitoring. No adverse symptoms were observed, and partial shallow sensory and autonomic nervous functional improvements were observed in some patients, but no motor function recovery was observed (NCT02510365).438 These findings indicate that implantation of biomaterials combined with stem cells may serve as a safe and promising clinical treatment for patients with acute complete SCI. However, based on the current development trends, multidimensional therapy based on biomaterials, stem cells, cytokines, physical factors, and rehabilitation has great potential in the regeneration and repair of SCI. To date, some scaffolds based on nanotechnology have entered the clinical trial stage and have yielded some evidence of safety and efficacy, but a large amount of supporting data is still needed before large-scale clinical translation.
Clinical trial of physical regulation in SCI
Ultrasound
Recent clinical trials of ultrasound have mostly focused on the evaluation and complications therapy for SCI. Trials were designed to establish whether the change in ultrasound muscle parameters from the baseline correlates with functional status of SCI patients (in comparison to rehabilitation) (NCT04303728) or to assess blood flow in the injured area of the spinal cord (NCT04056988) or predict deep vein thrombosis in SCI patients (NCT02796235). Ultrasound has also been used to assess neurogenic bladder function after SCI (NCT01299792, NCT01297673).
In addition, ultrasound is helpful for treating complications of SCI. A pilot study evaluated ultrasound/ultraviolet-C and laser use for the treatment of pressure ulcers in patients with SCI and found that ultrasound/ultraviolet-C may decrease healing time and allow faster return to rehabilitation programs, work, and leisure activities among patients with SCI who have pressure ulcers.439 Another trial investigated the effect of one-time shock wave therapy (ESWT) on lower limb spasticity in patients with incomplete SCI (NCT02203994). Furthermore, ultrasound can be used as guidance for corticosteroid injection or microfragmented adipose tissue injection.440,441 In clinical trials, there has been few research on neural regeneration and neural circuit remodeling after ultrasonic stimulation in SCI. One clinical trial involving 82 patients with SCI found that extracorporeal shock waves can change the cell response and reduce neuron loss (NCT04474106).442
Magnetic field control
Most clinical trials in this area have been associated with transcranial magnetic stimulation (NCT02914418, NCT02914418, and NCT04372134). In the context of incomplete SCI, 15 daily sessions of high-frequency rTMS can improve motor scores, walking speed, and spasticity in the lower limbs.443 Another double-blind, randomized sham-controlled crossover trial showed that rTMS produced positive results in treating individuals with physical impairments.444 In addition, recent findings indicate that 10 Hz rTMS over the hand area of the motor cortex could alleviate acute central neuropathic pain during the early phase of SCI and could enhance MEP parameters and modulate BDNF and NGF secretion. The analgesia-enhancing effects of high-frequency rTMS might be related to the amelioration of M1 and PMC hypersensitivity, shedding light upon the clinical treatment of SCI-related neuropathic pain.445,446
Other studies have focused on magnetic stimulation for complications post SCI. A randomized controlled trial showed the effects of repetitive transcranial magnetic stimulation on recovery of lower limb muscle strength and gait function following SCI.447 For bladder dysfunction after SCI, a clinical trial investigated the changes in bladder function in response to long-term bladder conditioning by FMS to further optimize FMS technology and parameters for effective bladder emptying in SCI (NCT00011557). Another trial focused on repetitive transcranial magnetic stimulation and pelvic floor muscle training for female neurogenic bladder dysfunction after SCI (ChiCTR1900026126).448
Neuropathic pain after SCI is also a concern (NCT01932905). Studies have attempted to use a combination of high-frequency noninvasive rTMS and exercise training to enhance motor recovery (NCT01915095) and to investigate the effects of repetitive transcranial magnetic stimulation combined with transspinal electrical stimulation (tsES) intervention on cortical excitability, brain structure, and lower extremity motor ability in individuals with incomplete SCI (NCT04194099).
A brain-computer interface-based medical device based on electromagnetic field (EMF) stimulation was recently invented, and this device is being used to investigate the safety and efficacy of the new advanced electromagnetic field therapy in the management of chronic SCI patients (NCT04050696).
Electric control
Functional electrical stimulation technology (FES), transcutaneous electrical nerve stimulation (TENS) and epidural electrical stimulation (EES) are the most widely used electrical stimulation technologies in clinical trials.
FES is a surface electrical stimulation technology that generates a series of electrical stimuli that trigger action potentials in intact peripheral nerves to further activate muscle contractions.449 FES has advantages in improving muscle status after SCI, but there is no clear report on neural regeneration and nerve remodeling after SCI.450 Advanced weight-bearing mat exercises combined with functional electrical stimulation were shown to improve the ability of wheelchair-dependent people with SCI to transfer and achieve independence in activities of daily living.451 The combination of FES with resistance training may enhance oxygen uptake and ventilatory efficiency independent of mitochondrial complexes after SCI.452
TENS is another noninvasive therapeutic modality that is commonly used in pain control and exerts its effects by stimulating large-diameter mechanosensitive afferent nerve fibers in the skin.453 A clinical study compared the effects of TENS and FES on lower limb spasticity in patients with SCI, and the results suggest that both TENS and FES have the potential to be used as adjunct therapies to relieve spasticity in the clinic, and FES may have better effects on patients presenting with spastic reflexes.450 Another clinical study reported that TENS enabled 8 children with trunk control disorder due to acquired SCI to sit upright (NCT03975634).454 TENS combined with training improves hand strength and manual dexterity in subjects with SCI.455 The lumbosacral spinal networks can be modulated transcutaneously using electrical spinal stimulation to facilitate self-assisted standing after chronic motor and sensory complete paralysis.456 A new pilot clinical trial has been started to explore the efficacy of transcutaneous spinal cord stimulation (TESCoN, SpineX Inc., CA, USA) in mitigating crucial autonomic dysfunctions that impact the health-related quality of life of individuals with SCI (NCT05369520).
Compared with FES and TENS, EES is an invasive electrical stimulation technique. An electrical stimulation device is surgically placed on the epidural area corresponding to the target area. In 2011, Harkema’s team reported in the Lancet that EES promoted standing in patients with SCI with complete motor impairment.457 Many studies have confirmed that EES promotes functional recovery after SCI and improves the quality of life, including motor functions and hemodynamics.458–460 In one report, four patients with chronic motor complete SCI achieved independent standing and trunk stability via EES combined with rehabilitation.238 In another report, three patients with severe chronic cervical SCI achieved voluntary control of walking with an implanted pulse generator through targeted spinal cord stimulation. All these studies suggested that EES is a very promising method for the treatment of SCI. Deep brain stimulation (DBS) is another invasive technology for locomotion recovery. A clinical trial was carried out on patients with a DBS implant at the site of SCI, and an electrode to stimulate the midbrain motor area was designed (NCT03053791).461 Due to the rapid development of computer technology, electrocorticography (ECoG)-based brain-computer interface systems can measure brain activity using electrodes implanted on the surface of the brain. Preliminary results demonstrate the feasibility of ECoG-based systems for individuals with paralysis (NCT01393444).462 The above information is shown in the table below (Table 4).
Table 4.
clinical trials about physical regulation in SCI
| Physical factors | Patients condition | Stimulus method | Stimulation parameters and frequency | Subjects | Phase | ClinicalTrials.gov identifier or refs |
|---|---|---|---|---|---|---|
| Ultrasound and ultraviolet-C (US/UVC) | Pressure ulcers post SCI | External |
US: 3 MHz, 0.2 W/cm2 (1:4 pulse ratio) for 5 min UVC: (95% emission at 250 nm) calculated level |
20 | N/A | 439 |
| Ultrasound | SCI (AIS C and D) | External |
energy level: 0.030 mJ/mm2, frequency: 4 Hz 2000 pulses per muscle |
20 | N/A | NCT02203994. |
| Ultrasound | Traumatic spinal injuries | External | Energy level: 0.1–0.19 mJ/mm2, frequency: 2–5 Hz | 82 | N/A | NCT04474106442 |
| rTMS | Incomplete SCI | External | 5 Hz, 12 trains of 50 magnetic pulses | 20 | N/A | NCT02899637444 |
| rTMS | Complete and incomplete SCI | External | 10 Hz, 1500 pulses | 48 | N/A | 445 |
| rTMS | Neuropathic pain following SCI | External | 10 Hz, 1200 pulses | 21 | N/A | 446 |
| rTMS | chronic SCI | External | 20 Hz, 1800 pulses | 20 | N/A | 447 |
| FMS | SCI above T10 level, six months post injury | External | N/A | 36 | Phase 2 | NCT00011557 |
| rTMS | Chronic SCI | External | 5 Hz for 20 min | 44 | N/A | ChiCTR1900026126448 |
| rTMS | 6 months after SCI | External | 5 Hz, 10 trains of 15 pulses | 42 | N/A | NCT01915095 |
| rTMS and tsES | Incomplete SCI (AIS B to D) | External |
20 Hz rTMS, 1200 s (Brain) 2.5-mA tsDCS, 600 s (Spinal) |
12 | N/A | NCT04194099 |
| Electromagnetic Field (EMF) | Incomplete SCI (AIS B to D) | External |
BQ, a brain-computer interface device (BCI) |
8 | N/A | NCT04050696 |
| FES | Chronic SCI | External | pulse width 400 µs, frequency 40 Hz, on/off cycle 5/10 s | 16 | N/A | 451 |
| FES | Traumatic SCI | External | pulse width 450 µs, frequency 30 Hz | 23 | N/A | 452 |
| TENS | Chronic SCI | External | pulse width 1 ms, frequency 15–30 Hz. (T11 at 30 Hz, L1 at 15 Hz, and C5 at 30 Hz) | 8 | N/A | NCT03975634454 |
| TENS | Complete cervical SCI | External | 10 kHz with a 16-channel A/D board | 12 | Phase I/II | NCT02313194455 |
| Transcutaneous spinal cord stimulation (TCSCS) | Chronic traumatic SCI | External | frequency between 1 Hz and 90 Hz amplitude 1.0–3.5 mm | 30 | N/A | NCT05369520 |
| EES | Traumatic SCI | Implanted | 2 Hz, 450 μs with 0.1 V intervals ramping incrementally | 4 | N/A | NCT02339233238 |
| EES | Chronic cervical SCI | Implanted | Programmed, 20–100 Hz, 1.8–2.7 mA | 3 | N/A | 458 |
| DBS |
Incomplete SCI AIS C |
Implanted | 20 Hz and 400 µs pulse | 5 | Phase I/II | NCT03053791461 |
| Electrocorticography (ECoG) | Complete cervical SCI | Implanted | Brain-computer interface (BCI) system, 0–200 Hz frequency range (25th order, 10 Hz frequency bands) using 300 ms | 3 | N/A | NCT01393444462 |
Perspectives
With the deepening of the understanding of molecular pathological mechanisms after SCI, different types of intervention strategies, such as controlling inflammation, reducing cell death, modulating scar formation, and regulating neurotrophic factors and angiogenesis, have been validated in animal models in terms of safety and efficacy. However, SCI is still a tough challenge, mainly due to the following reasons. First, various pathological mechanisms of SCI have temporal and spatial characteristics, and they are interlinked and interact with each other, which are difficult to clearly describe and elaborate. Second, the limitation of neural regeneration ability still lacks effective improvement methods; the bottleneck of effective nerve regeneration is still difficult to break. Third, due to the difference of species, it is difficult to translate the research results obtained in animal models into clinical applications.
Although many strategies show reassuring results in animal models of SCI, however, these experiments involved very controlled injuries and recovery conditions in animals while hardly matched for age, weight, gender, species, and genetic background. This obviously dwarfed in comparison to the natural variability that occurs in the acute human SCI. In addition, the stability of animal models is still facing huge challenges. Different modeling methods can only imitate the injury mechanism of SCI to a certain extent, but cannot fully reflect the real molecular pathological timing characteristics of SCI. There are few relevant detection methods for human SCI at present. Blood and cerebrospinal fluid are routine detection methods. In the future, there is an urgent need to develop technical means that can noninvasive or minimally invasive detect the true molecular pathological process of patients with SCI.
The appreciation of the heterogeneity of human SCI is partly the result of the challenges that have been experienced in the execution of clinical trials of novel therapies, particularly in the acute situation. As variability in patients with SCI, the sample size should be increased as much as possible in clinical research to reduce the research bias. Almost all acute SCI studies are faced with the difficulty of recruiting patients. The failure to recruit enough patients within the specified time has led to the suspension or cancellation of many clinical studies on acute SCI. In the future, more network-based technologies need to be developed to overcome this problem to promote clinical research of SCI.
Moreover, it is necessary to further optimize the clinical research of SCI. Due to the high heterogeneity of SCI and the large individual differences, the inclusion window can be appropriately reduced, and the objective biological indicators of the severity of the injury and the more accurate prognosis analysis system of SCI can be used to recruit a precisely defined population.
In the future, the pathological mechanism still needs more in-depth research. Single-cell sequencing technology has already revealed many cell subsets or subtypes, which may become key intervention targets, but there is still a lack of confirmatory research results.33,58,134,463 In addition, quantitative pharmacology, spinal cord organoids, the intestinal flora-mediated “brain gut axis” and quantum mechanics-mediated neurobiology are new perspectives for studying the pathological mechanism of SCI.464
Although stem cell transplantation for SCI is an important therapeutic strategy, many challenges remain. The poor effect and unclear time window of stem cell transplantation, the associated tumorigenic risk, the immune response after transplantation, and the cell survival rate in the human body are issues that need to be carefully considered. Although the safety and efficacy of stem cells have been demonstrated in animal experiments, heterogeneous results remind us that more preclinical studies are needed to evaluate the safety and efficacy of stem cell therapy.407 Future stem cell therapy for patients with SCI should be based on the pathological characteristics of SCI and the cellular and molecular characteristics of the stem cells themselves and should meet the requirements of easy clinical transformation, that is, the ability to promote the reconstruction of neural circuits or the ability to repair the microenvironment.465,466 It is worth mentioning that, from the perspective of neurodevelopment, it is possible to screen the cell types most suitable for the regeneration and repair of SCI.467,468 More importantly, research on the clinical transformation of stem cells requires social, policy and legal support.
The spinal cord tissue is surrounded by cerebrospinal fluid and protected by bony structures in the spinal canal; it is soft in nature and passes up and down to mediate signal connections between the brain and different segments of the spinal cord.469–471 Therefore, considering the current understanding of the characteristics of the spinal cord, and in the future, more excellent biological scaffold need to be developed and should meet the following conditions: (1) good biocompatibility to promote neuron adhesion; (2) a high water content to meet the requirements of cell metabolism; (3) a three-dimensional fiber structure with high permeability and an appropriate orientation for cell migration and axon growth; and (4) excellent flexibility to resist deformation under various stresses. Thus, the mechanical properties and microstructure of such a scaffold still need to meet the requirements for the active repair and regeneration of SCI. To achieve better treatment of SCI with biomaterials, the active combination of cell therapy and biomaterials via tissue engineering is urgently needed, thus providing alternative technologies such as 3D bioprinting and microfluidic devices.472–477
It is difficult to effectively promote recovery after SCI by relying on a single intervention strategy, and future clinical intervention approaches may include multiple synergistic intervention strategies. It is necessary to promote cooperation among all fields of neuroscience to form a comprehensive treatment strategy that integrates surgical and biological rehabilitation.478,479 In addition, attention should be given to psychological interventions for patients with SCI because of its high disability rate. Rehabilitation and regeneration are equally important for patients with SCI. In the future, artificial intelligence will be combined with rehabilitation to make more breakthroughs, such as brain-computer interfaces, intelligent devices, and quantum technology.480
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (No. 2016YFA0100800, 2021YFA1101301), the National Natural Science Foundation of China (Grant Nos. 82225027, 81873994, 81820108013, 81901902, 31727801, 82202702, and 82202351), as well as the State Key Program of the National Natural Science Foundation of China (No. 81330030).
Author contributions
L.M.C. and R.R.Z. conceived and modified the manuscript; X.H., W.X., Y.L.R., Z.J.W., X.L.H., R.Z.H., B.M., J.W.Z., R.R.Z., and L.M.C. prepared the manuscript; X.H., W.X., Y.L.R., Z.J.W., X.L.H. prepared the figures. All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiao Hu, Wei Xu, Yilong Ren, Zhaojie Wang, Xiaolie He
Contributor Information
Rongrong Zhu, Email: rrzhu@tongji.edu.cn.
Liming Cheng, Email: limingcheng@tongji.edu.cn.
References
- 1.Spinal Cord Injury (SCI) 2016. Facts and figures at a glance. J. Spinal Cord Med. 2016;39:493–494. doi: 10.1080/10790268.2016.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh A, et al. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller-Jensen L, et al. Clinical presentation and causes of non-traumatic spinal cord injury: an observational study in emergency patients. Front. Neurol. 2021;12:701927. doi: 10.3389/fneur.2021.701927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang B, et al. Prevalence, incidence, and external causes of traumatic spinal cord injury in China: a nationally representative cross-sectional survey. Front. Neurol. 2021;12:784647. doi: 10.3389/fneur.2021.784647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahuja CS, et al. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 6.Aria M, et al. bibliometrix:An R-tool for comprehensive science mapping analysis. J. Informetr. 2017;11:959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 7.Ewan EE, et al. Ascending dorsal column sensory neurons respond to spinal cord injury and downregulate genes related to lipid metabolism. Sci. Rep. 2021;11:374. doi: 10.1038/s41598-020-79624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Z, et al. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Hilton BJ, et al. An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron. 2022;110:51–69. doi: 10.1016/j.neuron.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, et al. Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury. Brain. 2020;143:1697–1713. doi: 10.1093/brain/awaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du K, et al. Pten deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J. Neurosci. 2015;35:9754–9763. doi: 10.1523/JNEUROSCI.3637-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevan D, et al. Regenerated interneurons integrate into locomotor circuitry following spinal cord injury. Exp. Neurol. 2021;342:113737. doi: 10.1016/j.expneurol.2021.113737. [DOI] [PubMed] [Google Scholar]
- 15.Zholudeva LV, et al. The neuroplastic and therapeutic potential of spinal interneurons in the injured spinal cord. Trends Neurosci. 2018;41:625–639. doi: 10.1016/j.tins.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown AR, et al. From cortex to cord: motor circuit plasticity after spinal cord injury. Neural Regen. Res. 2019;14:2054–2062. doi: 10.4103/1673-5374.262572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazim SF, et al. Corticospinal motor circuit plasticity after spinal cord injury: harnessing neuroplasticity to improve functional outcomes. Mol. Neurobiol. 2021;58:5494–5516. doi: 10.1007/s12035-021-02484-w. [DOI] [PubMed] [Google Scholar]
- 18.Duan H, et al. Transcriptome analyses reveal molecular mechanisms underlying functional recovery after spinal cord injury. Proc. Natl Acad. Sci. USA. 2015;112:13360–13365. doi: 10.1073/pnas.1510176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadoya K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016;22:479–487. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Freria CM, et al. Neural stem cells: promoting axonal regeneration and spinal cord connectivity. Cells. 2021;10:3296. doi: 10.3390/cells10123296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert EAB, et al. Regulating endogenous neural stem cell activation to promote spinal cord injury repair. Cells. 2022;11:846. doi: 10.3390/cells11050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabarsky ZK, et al. Pharmacologic recruitment of endogenous neural stem/progenitor cells for the treatment of spinal cord injury. Spine. 2022;47:505–513. doi: 10.1097/BRS.0000000000004264. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc. Natl Acad. Sci. USA. 2015;112:13354–13359. doi: 10.1073/pnas.1510194112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norenberg MD, et al. The pathology of human spinal cord injury: defining the problems. J. Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 25.Saiwai H, et al. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younsi A, et al. Long-Term effects of neural precursor cell transplantation on secondary injury processes and functional recovery after severe cervical contusion-compression spinal cord injury. Int. J. Mol. Sci. 2021;22:13106. doi: 10.3390/ijms222313106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 28.Luo D, et al. Unbiased transcriptomic analyses reveal distinct effects of immune deficiency in CNS function with and without injury. Protein Cell. 2019;10:566–582. doi: 10.1007/s13238-018-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi LL, et al. Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing. BMC Genomics. 2017;18:173. doi: 10.1186/s12864-017-3532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, et al. Macrophage transcriptional profile identifies lipid catabolic pathways that can be therapeutically targeted after spinal cord injury. J. Neurosci. 2017;37:2362–2376. doi: 10.1523/JNEUROSCI.2751-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milich LM, et al. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J. Exp. Med. 2021;218:e20210040. doi: 10.1084/jem.20210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llorens-Bobadilla E, et al. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science. 2020;370:eabb8795. doi: 10.1126/science.abb8795. [DOI] [PubMed] [Google Scholar]
- 33.Li C, et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct. Target Ther. 2022;7:65. doi: 10.1038/s41392-022-00885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu R, et al. Immunomodulatory layered double hydroxide nanoparticles enable neurogenesis by targeting transforming growth factor-beta receptor 2. ACS Nano. 2021;15:2812–2830. doi: 10.1021/acsnano.0c08727. [DOI] [PubMed] [Google Scholar]
- 35.Tang F, et al. Long-term clinical observation of patients with acute and chronic complete spinal cord injury after transplantation of NeuroRegen scaffold. Sci. China Life Sci. 2021;65:909–926. doi: 10.1007/s11427-021-1985-5. [DOI] [PubMed] [Google Scholar]
- 36.Ayar Z, et al. The effect of low-level laser therapy on pathophysiology and locomotor recovery after traumatic spinal cord injuries: a systematic review and meta-analysis. Lasers Med. Sci. 2021;37:61–75. doi: 10.1007/s10103-021-03301-5. [DOI] [PubMed] [Google Scholar]
- 37.Choi EH, et al. Epidural electrical stimulation for spinal cord injury. Neural Regen. Res. 2021;16:2367–2375. doi: 10.4103/1673-5374.313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang BY, et al. Ultrasound in traumatic spinal cord injury: a wide-open field. Neurosurgery. 2021;89:372–382. doi: 10.1093/neuros/nyab177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao YH, et al. Low-intensity focused ultrasound alleviates spasticity and increases expression of the neuronal K-Cl cotransporter in the L4-L5 sections of rats following spinal cord injury. Front. Cell Neurosci. 2022;16:882127. doi: 10.3389/fncel.2022.882127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bracken MB, et al. Incidence of acute traumatic hospitalized spinal cord injury in the United States, 1970-1977. Am. J. Epidemiol. 1981;113:615–622. doi: 10.1093/oxfordjournals.aje.a113140. [DOI] [PubMed] [Google Scholar]
- 41.Stephan K, et al. Spinal cord injury-incidence, prognosis, and outcome: an analysis of the TraumaRegister DGU. Spine J. 2015;15:1994–2001. doi: 10.1016/j.spinee.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 42.New PW, et al. Estimating the incidence and prevalence of traumatic spinal cord injury in Australia. Arch. Phys. Med. Rehabil. 2015;96:76–83. doi: 10.1016/j.apmr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Phillips J, et al. Another piece to the epidemiological puzzle of traumatic spinal cord injury in Cape Town, South Africa: a population-based study. S Afr. Med. J. 2018;108:1051–1054. doi: 10.7196/SAMJ.2018.v108i12.13134. [DOI] [PubMed] [Google Scholar]
- 44.Ametefe MK, et al. Spinal cord and spine trauma in a large teaching hospital in Ghana. Spinal Cord. 2016;54:1164–1168. doi: 10.1038/sc.2016.57. [DOI] [PubMed] [Google Scholar]
- 45.Lehre MA, et al. Outcome in patients undergoing surgery for spinal injury in an Ethiopian hospital. J. Neurosurg. Spine. 2015;23:772–779. doi: 10.3171/2015.3.SPINE141282. [DOI] [PubMed] [Google Scholar]
- 46.Jazayeri SB, et al. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur. Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 47.Li J, et al. The epidemiological survey of acute traumatic spinal cord injury (ATSCI) of 2002 in Beijing municipality. Spinal Cord. 2011;49:777–782. doi: 10.1038/sc.2011.8. [DOI] [PubMed] [Google Scholar]
- 48.Ning GZ, et al. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J. Spinal Cord Med. 2012;35:229–239. doi: 10.1179/2045772312Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nijendijk JH, et al. Epidemiology of traumatic spinal cord injuries in The Netherlands in 2010. Spinal Cord. 2014;52:258–263. doi: 10.1038/sc.2013.180. [DOI] [PubMed] [Google Scholar]
- 50.Montoto-Marqués A, et al. Epidemiology of traumatic spinal cord injury in Galicia, Spain: trends over a 20-year period. Spinal Cord. 2017;55:588–594. doi: 10.1038/sc.2017.13. [DOI] [PubMed] [Google Scholar]
- 51.Güzelküçük Ü, et al. Demographic and clinical characteristics of patients with traumatic cervical spinal cord injury: a Turkish hospital-based study. Spinal Cord. 2015;53:441–445. doi: 10.1038/sc.2014.211. [DOI] [PubMed] [Google Scholar]
- 52.Taşoğlu Ö, et al. Demographic and clinical characteristics of persons with spinal cord injury in Turkey: One-year experience of a primary referral rehabilitation center. J. Spinal Cord Med. 2018;41:157–164. doi: 10.1080/10790268.2016.1224215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ning GZ, et al. Epidemiology of traumatic spinal cord injury in Tianjin, China. Spinal Cord. 2011;49:386–390. doi: 10.1038/sc.2010.130. [DOI] [PubMed] [Google Scholar]
- 54.Lee BB, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 55.Chiu WT, et al. Review paper: epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac. J. Public Health. 2010;22:9–18. doi: 10.1177/1010539509355470. [DOI] [PubMed] [Google Scholar]
- 56.Kumar R, et al. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–e363. doi: 10.1016/j.wneu.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 57.Tsata V, et al. A switch in pdgfrb(+) cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Dev. Cell. 2021;56:509–524. doi: 10.1016/j.devcel.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 58.Cavone L, et al. A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord. Dev. Cell. 2021;56:1617–1630. doi: 10.1016/j.devcel.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 59.Nardone R, et al. Rodent, large animal and non-human primate models of spinal cord injury. Zoology. 2017;123:101–114. doi: 10.1016/j.zool.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Ko CC, et al. Monkey recovery from spinal cord hemisection: nerve repair strategies for rhesus macaques. World Neurosurg. 2019;129:e343–e351. doi: 10.1016/j.wneu.2019.05.145. [DOI] [PubMed] [Google Scholar]
- 61.Wu W, et al. Axonal and glial responses to a mid-thoracic spinal cord hemisection in the Macaca fascicularis monkey. J. Neurotrauma. 2013;30:826–839. doi: 10.1089/neu.2012.2681. [DOI] [PubMed] [Google Scholar]
- 62.Anjum A, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bighinati A, et al. Time-course changes of extracellular matrix encoding genes expression level in the spinal cord following contusion injury-A data-driven approach. Int. J. Mol. Sci. 2021;22:1744. doi: 10.3390/ijms22041744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orr MB, et al. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, et al. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics. 2020;10:11376–11403. doi: 10.7150/thno.49199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rezvan M, et al. Time-dependent microglia and macrophages response after traumatic spinal cord injury in rat: a systematic review. Injury. 2020;51:2390–2401. doi: 10.1016/j.injury.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen HX, et al. Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method. J. Vis. Exp. 2011;50:2698. doi: 10.3791/2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zrzavy T, et al. Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain. 2021;144:144–161. doi: 10.1093/brain/awaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milich LM, et al. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019;137:785–797. doi: 10.1007/s00401-019-01992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobashi S, et al. Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol. Ther. 2020;28:254–265. doi: 10.1016/j.ymthe.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kisucka A, et al. Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury. Cells. 2021;10:1943. doi: 10.3390/cells10081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen WK, et al. Inhibition of leucine-rich repeats and calponin homology domain containing 1 accelerates microglia-mediated neuroinflammation in a rat traumatic spinal cord injury model. J. Neuroinflamm. 2020;17:202. doi: 10.1186/s12974-020-01884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang X, et al. Downregulation of USP4 promotes activation of microglia and subsequent neuronal inflammation in rat spinal cord after injury. Neurochem. Res. 2017;42:3245–3253. doi: 10.1007/s11064-017-2361-2. [DOI] [PubMed] [Google Scholar]
- 74.Zhou X, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat. Neurosci. 2020;23:337–350. doi: 10.1038/s41593-020-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellver-Landete V, et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019;10:518. doi: 10.1038/s41467-019-08446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu H, et al. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. 2020;11:528. doi: 10.1038/s41419-020-2733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. 2020;587:613–618. doi: 10.1038/s41586-020-2795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zivkovic S, et al. For better or for worse: a look into neutrophils in traumatic spinal cord injury. Front. Cell Neurosci. 2021;15:648076. doi: 10.3389/fncel.2021.648076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donnelly DJ, et al. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolma S, et al. Neutrophil, extracellular matrix components, and their interlinked action in promoting secondary pathogenesis after spinal cord injury. Mol. Neurobiol. 2021;58:4652–4665. doi: 10.1007/s12035-021-02443-5. [DOI] [PubMed] [Google Scholar]
- 81.Ahmed A, et al. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol. Cell Biochem. 2018;441:181–189. doi: 10.1007/s11010-017-3184-9. [DOI] [PubMed] [Google Scholar]
- 82.McCreedy DA, et al. Spleen tyrosine kinase facilitates neutrophil activation and worsens long-term neurologic deficits after spinal cord injury. J. Neuroinflamm. 2021;18:302. doi: 10.1186/s12974-021-02353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 84.Feng Z, et al. Neutrophil extracellular traps exacerbate secondary injury via promoting neuroinflammation and blood-spinal cord barrier disruption in spinal cord injury. Front. Immunol. 2021;12:698249. doi: 10.3389/fimmu.2021.698249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, et al. Role of NETosis in central nervous system injury. Oxid. Med. Cell Longev. 2022;2022:3235524. doi: 10.1155/2022/3235524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sas AR, et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020;21:1496–1505. doi: 10.1038/s41590-020-00813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi J, et al. Neutrophil-targeted engineered prodrug nanoparticles for anti-inflammation. FASEB J. 2020;34:9828–9831. doi: 10.1096/fj.202000978RR. [DOI] [PubMed] [Google Scholar]
- 88.Zhao JL, et al. Circulating neutrophil-to-lymphocyte ratio at admission predicts the long-term outcome in acute traumatic cervical spinal cord injury patients. BMC Musculoskelet. Disord. 2020;21:548. doi: 10.1186/s12891-020-03556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jogia T, et al. Prognostic value of early leukocyte fluctuations for recovery from traumatic spinal cord injury. Clin. Transl. Med. 2021;11:e272. doi: 10.1002/ctm2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neirinckx V, et al. Neutrophil contribution to spinal cord injury and repair. J. Neuroinflamm. 2014;11:150. doi: 10.1186/s12974-014-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen YJ, et al. Temporal kinetics of macrophage polarization in the injured rat spinal cord. J. Neurosci. Res. 2015;93:1526–1533. doi: 10.1002/jnr.23612. [DOI] [PubMed] [Google Scholar]
- 92.Kroner A, et al. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, et al. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia. 2015;63:635–651. doi: 10.1002/glia.22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mawhinney LA, et al. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J. Neuropathol. Exp. Neurol. 2012;71:180–197. doi: 10.1097/NEN.0b013e3182479b41. [DOI] [PubMed] [Google Scholar]
- 95.Greenhalgh AD, et al. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 2014;34:6316–6322. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thawer SG, et al. Temporal changes in monocyte and macrophage subsets and microglial macrophages following spinal cord injury in the Lys-Egfp-ki mouse model. J. Neuroimmunol. 2013;261:7–20. doi: 10.1016/j.jneuroim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 97.David S, et al. Myeloid cell responses after spinal cord injury. J. Neuroimmunol. 2018;321:97–108. doi: 10.1016/j.jneuroim.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Bennett ML, et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greenhalgh AD, et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018;16:e2005264. doi: 10.1371/journal.pbio.2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Broeckhoven J, et al. Macrophage phagocytosis after spinal cord injury: when friends become foes. Brain. 2021;144:2933–2945. doi: 10.1093/brain/awab250. [DOI] [PubMed] [Google Scholar]
- 101.Madalena KM, et al. Genetic deletion of the glucocorticoid receptor in Cx(3)cr1(+) myeloid cells is neuroprotective and improves motor recovery after spinal cord injury. Exp. Neurol. 2022;355:114114. doi: 10.1016/j.expneurol.2022.114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu Y, et al. Temporal kinetics of CD8(+) CD28(+) and CD8(+) CD28(-) T lymphocytes in the injured rat spinal cord. J. Neurosci. Res. 2017;95:1666–1676. doi: 10.1002/jnr.23993. [DOI] [PubMed] [Google Scholar]
- 103.Sun G, et al. gammadelta T cells provide the early source of IFN-gamma to aggravate lesions in spinal cord injury. J. Exp. Med. 2018;215:521–535. doi: 10.1084/jem.20170686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu P, et al. Recruitment of gammadelta T cells to the lesion via the CCL2/CCR2 signaling after spinal cord injury. J. Neuroinflamm. 2021;18:64. doi: 10.1186/s12974-021-02115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu B, et al. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp. Neurol. 2012;237:274–285. doi: 10.1016/j.expneurol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 106.Ankeny DP, et al. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Satzer D, et al. T cell deficiency in spinal cord injury: altered locomotor recovery and whole-genome transcriptional analysis. BMC Neurosci. 2015;16:74. doi: 10.1186/s12868-015-0212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Z, et al. CD8 T cell-derived perforin aggravates secondary spinal cord injury through destroying the blood-spinal cord barrier. Biochem Biophys. Res. Commun. 2019;512:367–372. doi: 10.1016/j.bbrc.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Costigan M, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu JG, et al. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp. Neurol. 2016;277:190–201. doi: 10.1016/j.expneurol.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Shi Z, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54:e12992. doi: 10.1111/cpr.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu XZ, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hassannejad Z, et al. Axonal degeneration and demyelination following traumatic spinal cord injury: A systematic review and meta-analysis. J. Chem. Neuroanat. 2019;97:9–22. doi: 10.1016/j.jchemneu.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 114.Bisicchia E, et al. Autophagy inhibition favors survival of rubrospinal neurons after spinal cord hemisection. Mol. Neurobiol. 2017;54:4896–4907. doi: 10.1007/s12035-016-0031-z. [DOI] [PubMed] [Google Scholar]
- 115.Wang S, et al. The expression of IGFBP6 after spinal cord injury: implications for neuronal apoptosis. Neurochem. Res. 2017;42:455–467. doi: 10.1007/s11064-016-2092-9. [DOI] [PubMed] [Google Scholar]
- 116.Chen X, et al. Expression of Sam68 associates with neuronal apoptosis and reactive astrocytes after spinal cord injury. Cell Mol. Neurobiol. 2017;37:487–498. doi: 10.1007/s10571-016-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu D, et al. Up-regulation of TNF receptor-associated factor 7 after spinal cord injury in rats may have implication for neuronal apoptosis. Neuropeptides. 2018;71:81–89. doi: 10.1016/j.npep.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 118.Lu X, et al. HAX1 is associated with neuronal apoptosis and astrocyte proliferation after spinal cord injury. Tissue Cell. 2018;54:1–9. doi: 10.1016/j.tice.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, et al. The latest view on the mechanism of ferroptosis and its research progress in spinal cord injury. Oxid. Med. Cell Longev. 2020;2020:6375938. doi: 10.1155/2020/6375938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res. 2019;1706:48–57. doi: 10.1016/j.brainres.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 121.Shen L, et al. Ferroptosis in acute central nervous system injuries: the future direction? Front. Cell Dev. Biol. 2020;8:594. doi: 10.3389/fcell.2020.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishio T, et al. Immediate elimination of injured white matter tissue achieves a rapid axonal growth across the severed spinal cord in adult rats. Neurosci. Res. 2018;131:19–29. doi: 10.1016/j.neures.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 123.Luo Y, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161:1175–1186. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sabelstrom H, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 125.Barnabe-Heider F, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 126.Meletis K, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mothe AJ, et al. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Ren Y, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci. Rep. 2017;7:41122. doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang H, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hui SP, et al. Characterization of proliferating neural progenitors after spinal cord injury in adult zebrafish. PLoS ONE. 2015;10:e0143595. doi: 10.1371/journal.pone.0143595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fabbiani G, et al. Connexin signaling is involved in the reactivation of a latent stem cell niche after spinal cord injury. J. Neurosci. 2020;40:2246–2258. doi: 10.1523/JNEUROSCI.2056-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu G, et al. Reactive astrocytes in central nervous system injury: subgroup and potential therapy. Front. Cell Neurosci. 2021;15:792764. doi: 10.3389/fncel.2021.792764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X, et al. Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid. Med. Cell Longev. 2020;2020:9494352. doi: 10.1155/2020/9494352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Allahyari RV HN, et al. Response of astrocyte subpopulations following spinal cord injury. Cells. 2022;11:721. doi: 10.3390/cells11040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hara M, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 137.Sofroniew MV. Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol. 2020;41:758–770. doi: 10.1016/j.it.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verkhratsky A, et al. Physiology of astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bonvento G, et al. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021;33:1546–1564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 140.MacVicar BA, et al. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 2015;7:a020388. doi: 10.1101/cshperspect.a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Shea TM, et al. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017;27:3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liddelow SA, et al. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 143.Burda JE, et al. Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature. 2022;606:557–564. doi: 10.1038/s41586-022-04739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Escartin C, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sofroniew MV. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2015;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bradbury EJ, et al. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019;10:3879. doi: 10.1038/s41467-019-11707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anderson MA, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gu Y, et al. Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav. Immun. 2019;80:394–405. doi: 10.1016/j.bbi.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 149.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hou J, et al. Heterogeneity analysis of astrocytes following spinal cord injury at single-cell resolution. FASEB J. 2022;36:e22442. doi: 10.1096/fj.202200463R. [DOI] [PubMed] [Google Scholar]
- 151.Shuman SL, et al. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J. Neurosci. Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 152.Floriddia EM, et al. Distinct oligodendrocyte populations have spatial preference and different responses to spinal cord injury. Nat. Commun. 2020;11:5860. doi: 10.1038/s41467-020-19453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma L, et al. p53-Mediated oligodendrocyte apoptosis initiates demyelination after compressed spinal cord injury by enhancing ER-mitochondria interaction and E2F1 expression. Neurosci. Lett. 2017;644:55–61. doi: 10.1016/j.neulet.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 154.Frei E, et al. Reactions of oligodendrocytes to spinal cord injury: cell survival and myelin repair. Exp. Neurol. 2000;163:373–380. doi: 10.1006/exnr.2000.7379. [DOI] [PubMed] [Google Scholar]
- 155.Li N, et al. Oligodendrocyte precursor cells in spinal cord injury: a review and update. Biomed. Res. Int. 2015;2015:235195. doi: 10.1155/2015/235195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duncan GJ, et al. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia. 2020;68:227–245. doi: 10.1002/glia.23706. [DOI] [PubMed] [Google Scholar]
- 157.Assinck P, et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J. Neurosci. 2017;37:8635–8654. doi: 10.1523/JNEUROSCI.2409-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duncan GJ, et al. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun. 2018;9:3066. doi: 10.1038/s41467-018-05473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang HF, et al. Effect of glial cells on remyelination after spinal cord injury. Neural Regen. Res. 2017;12:1724–1732. doi: 10.4103/1673-5374.217354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Q, et al. Induced neural activity promotes an oligodendroglia regenerative response in the injured spinal cord and improves motor function after spinal cord injury. J. Neurotrauma. 2017;34:3351–3361. doi: 10.1089/neu.2016.4913. [DOI] [PubMed] [Google Scholar]
- 161.Hassannejad Z, et al. Oligodendrogliogenesis and axon remyelination after traumatic spinal cord injuries in animal studies: a systematic review. Neuroscience. 2019;402:37–50. doi: 10.1016/j.neuroscience.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 162.Papastefanaki F, et al. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63:1101–1125. doi: 10.1002/glia.22809. [DOI] [PubMed] [Google Scholar]
- 163.Alizadeh A, et al. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J. Physiol. 2016;594:3539–3552. doi: 10.1113/JP270895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luo M, et al. Neuronal activity-dependent myelin repair promotes motor function recovery after contusion spinal cord injury. Brain Res. Bull. 2021;166:73–81. doi: 10.1016/j.brainresbull.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 165.Cunha MI, et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J. Exp. Med. 2020;217:e20191390. doi: 10.1084/jem.20191390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao P, et al. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J. Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brennan FH, et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 2022;13:4096. doi: 10.1038/s41467-022-31797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yoshizaki S, et al. Microglial inflammation after chronic spinal cord injury is enhanced by reactive astrocytes via the fibronectin/beta1 integrin pathway. J. Neuroinflamm. 2021;18:12. doi: 10.1186/s12974-020-02059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sofroniew MV, et al. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kawano H, et al. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 2012;349:169–180. doi: 10.1007/s00441-012-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sofroniew MV. Dissecting spinal cord regeneration. Nature. 2018;557:343–350. doi: 10.1038/s41586-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 173.Bush TG, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/S0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 174.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burda JE, et al. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wanner IB, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.White RE, et al. Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. J. Comp. Neurol. 2010;518:1370–1390. doi: 10.1002/cne.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pekny M, et al. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 180.Herrmann JE, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hackett AR, et al. Understanding the NG2 glial scar after spinal cord injury. Front Neurol. 2016;7:199. doi: 10.3389/fneur.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Levine J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2015;1638:199–208. doi: 10.1016/j.brainres.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Goritz C, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 184.Dias DO, et al. Fibrotic scarring following lesions to the central nervous system. Matrix Biol. 2018;68–69:561–570. doi: 10.1016/j.matbio.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 185.Dias DO, et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165. doi: 10.1016/j.cell.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Becker M, Parker D. Time course of functional changes in locomotor and sensory systems after spinal cord lesions in lamprey. J. Neurophysiol. 2019;121:2323–2335. doi: 10.1152/jn.00120.2019. [DOI] [PubMed] [Google Scholar]
- 187.Chen B, et al. Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell. 2018;174:521–535. doi: 10.1016/j.cell.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bertels H, et al. Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury. Nat. Neurosci. 2022;25:617–629. doi: 10.1038/s41593-022-01067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chao ZC, et al. Dynamic reorganization of motor networks during recovery from partial spinal cord injury in monkeys. Cereb. Cortex. 2019;29:3059–3073. doi: 10.1093/cercor/bhy172. [DOI] [PubMed] [Google Scholar]
- 190.Jiang YQ, et al. Neuronal activity and microglial activation support corticospinal tract and proprioceptive afferent sprouting in spinal circuits after a corticospinal system lesion. Exp. Neurol. 2019;321:113015. doi: 10.1016/j.expneurol.2019.113015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wu W, et al. Transhemispheric cortex remodeling promotes forelimb recovery after spinal cord injury. JCI Insight. 2022;7:e158150. doi: 10.1172/jci.insight.158150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zavvarian MM, et al. The functional role of spinal interneurons following traumatic spinal cord injury. Front. Cell Neurosci. 2020;14:127. doi: 10.3389/fncel.2020.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Laliberte AM, et al. Propriospinal neurons: essential elements of locomotor control in the intact and possibly the injured spinal cord. Front. Cell Neurosci. 2019;13:512. doi: 10.3389/fncel.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Crowe MJ, et al. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 195.Yokota K, et al. Pathological changes of distal motor neurons after complete spinal cord injury. Mol. Brain. 2019;12:4. doi: 10.1186/s13041-018-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Azzarito M, et al. Tracking the neurodegenerative gradient after spinal cord injury. Neuroimage Clin. 2020;26:102221. doi: 10.1016/j.nicl.2020.102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wu Z, et al. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog. Neurobiol. 2020;185:101730. doi: 10.1016/j.pneurobio.2019.101730. [DOI] [PubMed] [Google Scholar]
- 198.Wu J, et al. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J. Neurosci. 2014;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hellenbrand DJ, et al. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021;18:284. doi: 10.1186/s12974-021-02337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Begenisic T, et al. Dynamics of biomarkers across the stages of traumatic spinal cord injury— implications for neural plasticity and repair. Restor. Neurol. Neurosci. 2021;39:339–366. doi: 10.3233/RNN-211169. [DOI] [PubMed] [Google Scholar]
- 201.Han GH, et al. Transplantation of tauroursodeoxycholic acid-inducing M2-phenotype macrophages promotes an anti-neuroinflammatory effect and functional recovery after spinal cord injury in rats. Cell Prolif. 2021;54:e13050. doi: 10.1111/cpr.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Puls B, et al. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front. Cell Dev. Biol. 2020;8:591883. doi: 10.3389/fcell.2020.591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dolci S, et al. Therapeutic induction of energy metabolism reduces neural tissue damage and increases microglia activation in severe spinal cord injury. Pharm. Res. 2022;178:106149. doi: 10.1016/j.phrs.2022.106149. [DOI] [PubMed] [Google Scholar]
- 204.Kim DH, et al. Transplantation of PSA-NCAM-positive neural precursors from human embryonic stem cells promotes functional recovery in an animal model of spinal cord injury. Tissue Eng. Regen. Med. 2022;19:1349–1358. doi: 10.1007/s13770-022-00483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Anderson MA, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang T, et al. Potential therapeutic mechanism of traditional Chinese medicine monomers on neurological recovery after spinal cord injury. Chin. Med J. 2021;134:1681–1683. doi: 10.1097/CM9.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qi L, et al. Mechanisms of ginsenosides exert neuroprotective effects on spinal cord injury: a promising traditional Chinese medicine. Front. Neurosci. 2022;16:969056. doi: 10.3389/fnins.2022.969056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li XW, et al. Genistein protects against spinal cord injury in mice by inhibiting neuroinflammation via TLR4-mediated microglial polarization. Appl. Bionics Biomech. 2022;2022:4790344. doi: 10.1155/2022/4790344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 209.Luo D, et al. Sodium tanshinone IIA sulfonate promotes spinal cord injury repair by inhibiting blood spinal cord barrier disruption in vitro and in vivo. Drug Dev. Res. 2022;83:669–679. doi: 10.1002/ddr.21898. [DOI] [PubMed] [Google Scholar]
- 210.Sieck GC, et al. Acute intrathecal BDNF enhances functional recovery after cervical spinal cord injury in rats. J. Neurophysiol. 2021;125:2158–2165. doi: 10.1152/jn.00146.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cong Y, et al. NT-3 promotes oligodendrocyte proliferation and nerve function recovery after spinal cord injury by inhibiting autophagy pathway. J. Surg. Res. 2020;247:128–135. doi: 10.1016/j.jss.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 212.Han Q, et al. Descending motor circuitry required for NT-3 mediated locomotor recovery after spinal cord injury in mice. Nat. Commun. 2019;10:5815. doi: 10.1038/s41467-019-13854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xie Y, et al. Application of the sodium hyaluronate-CNTF scaffolds in repairing adult rat spinal cord injury and facilitating neural network formation. Sci. China Life Sci. 2018;61:559–568. doi: 10.1007/s11427-017-9217-2. [DOI] [PubMed] [Google Scholar]
- 214.Kwon J, et al. Antifibrosis treatment by inhibition of VEGF, FGF, and PDGF receptors improves bladder wall remodeling and detrusor overactivity in association with modulation of C-fiber afferent activity in mice with spinal cord injury. Neurourol. Urodyn. 2021;40:1460–1469. doi: 10.1002/nau.24704. [DOI] [PubMed] [Google Scholar]
- 215.Khazaei M, et al. GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci. Transl. Med. 2020;12:eaau3538. doi: 10.1126/scitranslmed.aau3538. [DOI] [PubMed] [Google Scholar]
- 216.He X, et al. Biocompatible exosome-modified fibrin gel accelerates the recovery of spinal cord injury by VGF-mediated oligodendrogenesis. J. Nanobiotechnol. 2022;20:360. doi: 10.1186/s12951-022-01541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xu J, et al. Effects of minocycline on motor function recovery and expression of glial fibrillary acidic protein and brain-derived neurotrophic factor after spinal cord injury in rats. J. Pharm. Pharm. 2021;73:332–337. doi: 10.1093/jpp/rgaa041. [DOI] [PubMed] [Google Scholar]
- 218.Squair JW, et al. Minocycline reduces the severity of autonomic dysreflexia after experimental spinal cord injury. J. Neurotrauma. 2018;35:2861–2871. doi: 10.1089/neu.2018.5703. [DOI] [PubMed] [Google Scholar]
- 219.Khiar-Fernandez N, et al. Novel antagonist of the type 2 lysophosphatidic acid receptor (LPA2), UCM-14216, ameliorates spinal cord injury in mice. J. Med. Chem. 2022;65:10956–10974. doi: 10.1021/acs.jmedchem.2c00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alvarez Z, et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science. 2021;374:848–856. doi: 10.1126/science.abh3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nakazaki M, et al. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-beta upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J. Extracell. Vesicles. 2021;10:e12137. doi: 10.1002/jev2.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sun G, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 223.Zhou Y, et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen. Res. 2022;17:194–202. doi: 10.4103/1673-5374.314323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Meng, S. H. et al. Isolation of exosome-enriched extracellular vesicles carrying granulocyte-macrophage colony-stimulating factor from embryonic stem cells. J. Vis. Exp. 177, 10.3791/60170 (2021). [DOI] [PMC free article] [PubMed]
- 225.Zhou Z, et al. Exosome-shuttled miR-672-5p from anti-inflammatory microglia repair traumatic spinal cord injury by inhibiting AIM2/ASC/caspase-1 signaling pathway mediated neuronal pyroptosis. J. Neurotrauma. 2022;39:1057–1074. doi: 10.1089/neu.2021.0464. [DOI] [PubMed] [Google Scholar]
- 226.Pan D, et al. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J. Neuroinflamm. 2021;18:172. doi: 10.1186/s12974-021-02215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jones I, et al. Human embryonic stem cell-derived neural crest cells promote sprouting and motor recovery following spinal cord injury in adult rats. Cell Transpl. 2021;30:963689720988245. doi: 10.1177/0963689720988245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gong C, et al. Human spinal GABA neurons alleviate spasticity and improve locomotion in rats with spinal cord injury. Cell Rep. 2021;34:108889. doi: 10.1016/j.celrep.2021.108889. [DOI] [PubMed] [Google Scholar]
- 229.Wertheim L, et al. Regenerating the injured spinal cord at the chronic phase by engineered iPSCs-derived 3D neuronal networks. Adv. Sci. 2022;9:e2105694. doi: 10.1002/advs.202105694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Giraldo E, et al. A rationally designed self-immolative linker enhances the synergism between a polymer-rock inhibitor conjugate and neural progenitor cells in the treatment of spinal cord injury. Biomaterials. 2021;276:121052. doi: 10.1016/j.biomaterials.2021.121052. [DOI] [PubMed] [Google Scholar]
- 231.Xue W, et al. Upregulation of Apol8 by Epothilone D facilitates the neuronal relay of transplanted NSCs in spinal cord injury. Stem Cell Res. Ther. 2021;12:300. doi: 10.1186/s13287-021-02375-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ceto S, et al. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell. 2020;27:430–440. doi: 10.1016/j.stem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kumamaru H, et al. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods. 2018;15:723–731. doi: 10.1038/s41592-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 234.Poplawski GHD, et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581:77–82. doi: 10.1038/s41586-020-2200-5. [DOI] [PubMed] [Google Scholar]
- 235.Lu P, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zou YL, et al. Transplantation of collagen sponge-based three-dimensional neural stem cells cultured in a RCCS facilitates locomotor functional recovery in spinal cord injury animals. Biomater. Sci. 2022;10:915–924. doi: 10.1039/D1BM01744F. [DOI] [PubMed] [Google Scholar]
- 237.Liu S, et al. Collagen scaffold loaded allogeneic neural stem cells promoted locomotion recovery of spinal cord injury mainly through secreting neurotrophic factors. MaterDesign. 2022;219:110804. [Google Scholar]
- 238.Angeli CA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018;379:1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 239.Cho SR, et al. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone marrow in a rat model of spinal cord injury. Cell Transpl. 2016;25:1423. doi: 10.3727/096368916X692078. [DOI] [PubMed] [Google Scholar]
- 240.Luo H, et al. Neural differentiation of bone marrow mesenchymal stem cells with human brain-derived neurotrophic factor gene-modified in functionalized self-assembling peptide hydrogel in vitro. J. Cell Biochem. 2019;120:2828–2835. doi: 10.1002/jcb.26408. [DOI] [PubMed] [Google Scholar]
- 241.Ye Y, et al. The treatment of spinal cord injury in rats using bone marrow-derived neural-like cells induced by cerebrospinal fluid. Neurosci. Lett. 2018;666:85–91. doi: 10.1016/j.neulet.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 242.Gu C, et al. Bone marrow mesenchymal stem cells decrease CHOP expression and neuronal apoptosis after spinal cord injury. Neurosci. Lett. 2017;636:282–289. doi: 10.1016/j.neulet.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 243.Wang GD, et al. The SDF-1/CXCR4 axis promotes recovery after spinal cord injury by mediating bone marrow-derived from mesenchymal stem cells. Oncotarget. 2017;8:11629–11640. doi: 10.18632/oncotarget.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kim M, et al. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J. Tissue Eng. Regen. Med. 2018;12:e1034–e1045. doi: 10.1002/term.2425. [DOI] [PubMed] [Google Scholar]
- 245.Liu WZ, et al. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res. Ther. 2021;12:102. doi: 10.1186/s13287-021-02153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.James SL, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lim M, et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res. Ther. 2018;9:129. doi: 10.1186/s13287-018-0888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang R, et al. Umbilical cord-derived mesenchymal stem cell therapy for neurological disorders via inhibition of mitogen-activated protein kinase pathway-mediated apoptosis. Mol. Med. Rep. 2015;11:1807–1812. doi: 10.3892/mmr.2014.2985. [DOI] [PubMed] [Google Scholar]
- 249.Nakajima H, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang S, et al. HUCMSCs transplantation combined with ultrashort wave therapy attenuates neuroinflammation in spinal cord injury through NUR77/ NF-kappaB pathway. Life Sci. 2021;267:118958. doi: 10.1016/j.lfs.2020.118958. [DOI] [PubMed] [Google Scholar]
- 251.Fu Q, et al. Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. Am. J. Transl. Res. 2017;9:3950–3966. [PMC free article] [PubMed] [Google Scholar]
- 252.Li H, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9:2017–2035. doi: 10.7150/thno.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jeffery N, et al. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur. J. Neurosci. 1999;11:1508–1514. doi: 10.1046/j.1460-9568.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 254.Manley NC, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl. Med. 2017;6:1917–1929. doi: 10.1002/sctm.17-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ursavas S, et al. Olfactory ensheathing cells: unique glial cells promising for treatments of spinal cord injury. J. Neurosci. Res. 2021;99:1579–1597. doi: 10.1002/jnr.24817. [DOI] [PubMed] [Google Scholar]
- 256.Barbour HR, et al. Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion. BMC Neurosci. 2013;14:106. doi: 10.1186/1471-2202-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hutson TH, et al. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15:732–745. doi: 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- 258.Gaudet AD, et al. Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics. 2018;15:554–577. doi: 10.1007/s13311-018-0630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhou XH, et al. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transpl. 2012;21:S39–S47. doi: 10.3727/096368912X633752. [DOI] [PubMed] [Google Scholar]
- 260.Zheng X, et al. Astrocyte transplantation for repairing the injured spinal cord. J. Biomed. Res. 2022;36:312–320. doi: 10.7555/JBR.36.20220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goulao M, et al. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia. 2019;67:452–466. doi: 10.1002/glia.23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ma W, et al. The biological applications of DNA nanomaterials: current challenges and future directions. Signal Transduct. Target Ther. 2021;6:351. doi: 10.1038/s41392-021-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Qian Y, et al. Preclinical assessment on neuronal regeneration in the injury-related microenvironment of graphene-based scaffolds. NPJ Regen. Med. 2021;6:31. doi: 10.1038/s41536-021-00142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rathnam C, et al. Hybrid SMART spheroids to enhance stem cell therapy for CNS injuries. Sci. Adv. 2021;7:eabj2281. doi: 10.1126/sciadv.abj2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yao X, et al. Electroactive nanomaterials in the peripheral nerve regeneration. J. Mater. Chem. B. 2021;9:6958–6972. doi: 10.1039/D1TB00686J. [DOI] [PubMed] [Google Scholar]
- 266.Yari-Ilkhchi A, et al. Design of graphenic nanocomposites containing chitosan and polyethylene glycol for spinal cord injury improvement. RSC Adv. 2021;11:19992–20002. doi: 10.1039/D1RA00861G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xi K, et al. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat. Commun. 2020;11:4504. doi: 10.1038/s41467-020-18265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Slotkin JR, et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials. 2017;123:63–76. doi: 10.1016/j.biomaterials.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 269.Sun X, et al. Neurotrophin-3-loaded multichannel nanofibrous scaffolds promoted anti-inflammation, neuronal differentiation, and functional recovery after spinal cord injury. ACS Biomater. Sci. Eng. 2020;6:1228–1238. doi: 10.1021/acsbiomaterials.0c00023. [DOI] [PubMed] [Google Scholar]
- 270.Chen C, et al. Hyaluronic acid-coated nanoparticles for the localized delivery of methylprednisolone to the injured spinal cord. J. Nanomater. 2021;2021:5358046. doi: 10.1155/2021/5358046. [DOI] [Google Scholar]
- 271.Jeong HJ, et al. Biomaterials and strategies for repairing spinal cord lesions. Neurochem. Int. 2021;144:104973. doi: 10.1016/j.neuint.2021.104973. [DOI] [PubMed] [Google Scholar]
- 272.Wang SX, et al. Graphene and graphene-based materials in axonal repair of spinal cord injury. Neural Regen. Res. 2022;17:2117–2125. doi: 10.4103/1673-5374.335822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang B, et al. Graphene oxide-composited chitosan scaffold contributes to functional recovery of injured spinal cord in rats. Neural Regen. Res. 2021;16:1829–1835. doi: 10.4103/1673-5374.306095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chen X, et al. Electrospinning multilayered scaffolds loaded with melatonin and Fe3O4 magnetic nanoparticles for peripheral nerve regeneration. Adv. Funct. Mater. 2020;30:2004537. doi: 10.1002/adfm.202004537. [DOI] [Google Scholar]
- 275.Chiang MY, et al. 4D spatiotemporal modulation of biomolecules distribution in anisotropic corrugated microwrinkles via electrically manipulated microcapsules within hierarchical hydrogel for spinal cord regeneration. Biomaterials. 2021;271:120762. doi: 10.1016/j.biomaterials.2021.120762. [DOI] [PubMed] [Google Scholar]
- 276.Cho Y, et al. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010;213:1513–1520. doi: 10.1242/jeb.035162. [DOI] [PubMed] [Google Scholar]
- 277.Colello RJ, et al. The incorporation of growth factor and chondroitinase ABC into an electrospun scaffold to promote axon regrowth following spinal cord injury. J. Tissue Eng. Regen. Med. 2016;10:656–668. doi: 10.1002/term.1805. [DOI] [PubMed] [Google Scholar]
- 278.Cui S, et al. Novel design and combination strategy of minocycline and OECs-loaded CeO2 nanoparticles with SF for the treatment of spinal cord injury: In vitro and in vivo evaluations. Green. Process Synth. 2021;10:614–627. doi: 10.1515/gps-2021-0038. [DOI] [Google Scholar]
- 279.Huang J, et al. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. A. 2010;93:164–174. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 280.Ji Y, et al. Biological potential of polyethylene glycol (PEG)-functionalized graphene quantum dots in in vitro neural stem/progenitor cells. Nanomaterials. 2021;11:1446. doi: 10.3390/nano11061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jin Y, et al. Magnetic control of axon navigation in reprogrammed neurons. Nano Lett. 2019;19:6517–6523. doi: 10.1021/acs.nanolett.9b02756. [DOI] [PubMed] [Google Scholar]
- 282.Kumar R, et al. Graphene‐based nanomaterials for neuroengineering: recent advances and future prospective. Adv. Funct. Mater. 2021;31:2104887. doi: 10.1002/adfm.202104887. [DOI] [Google Scholar]
- 283.Kurian AG, et al. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022;8:267–295. doi: 10.1016/j.bioactmat.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kushchayev SV, et al. Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. J. Neurosurg. Spine. 2016;25:114–124. doi: 10.3171/2015.9.SPINE15628. [DOI] [PubMed] [Google Scholar]
- 285.Lai BQ, et al. A modular assembly of spinal cord-like tissue allows targeted tissue repair in the transected spinal cord. Adv. Sci. 2018;5:1800261. doi: 10.1002/advs.201800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fan C, et al. Restoration of spinal cord biophysical microenvironment for enhancing tissue repair by injury-responsive smart hydrogel. Biomaterials. 2022;288:121689. doi: 10.1016/j.biomaterials.2022.121689. [DOI] [PubMed] [Google Scholar]
- 287.Chen Z, et al. Adhesive, stretchable, and spatiotemporal delivery fibrous hydrogels harness endogenous neural stem/progenitor cells for spinal cord injury repair. ACS Nano. 2022;16:1986–1998. doi: 10.1021/acsnano.1c06892. [DOI] [PubMed] [Google Scholar]
- 288.He XL, et al. Solid lipid nanoparticles loading with curcumin and dexanabinol to treat major depressive disorder. Neural Regen. Res. 2021;16:537–542. doi: 10.4103/1673-5374.293155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hor JH, et al. Cell cycle inhibitors protect motor neurons in an organoid model of Spinal Muscular Atrophy. Cell Death Dis. 2018;9:1100. doi: 10.1038/s41419-018-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Houweling DA, et al. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp. Neurol. 1998;153:49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 291.Hsu SH, et al. Peripheral nerve regeneration using a microporous polylactic acid asymmetric conduit in a rabbit long-gap sciatic nerve transection model. Biomaterials. 2011;32:3764–3775. doi: 10.1016/j.biomaterials.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 292.Sun S, et al. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry. 2006;69:133–141. doi: 10.1016/j.bioelechem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 293.Wang J, et al. FGL-functionalized self-assembling nanofiber hydrogel as a scaffold for spinal cord-derived neural stem cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015;46:140–147. doi: 10.1016/j.msec.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 294.Wu GH, et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials. 2018;181:15–34. doi: 10.1016/j.biomaterials.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 295.Wu W, et al. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials. 2014;35:2355–2364. doi: 10.1016/j.biomaterials.2013.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Xu C, et al. Two‐dimensional‐germanium phosphide‐reinforced conductive and biodegradable hydrogel scaffolds enhance spinal cord injury repair. Adv. Funct. Mater. 2021;31:2104440. doi: 10.1002/adfm.202104440. [DOI] [Google Scholar]
- 297.Yang L, et al. Effective modulation of CNS inhibitory microenvironment using bioinspired hybrid-nanoscaffold-based therapeutic interventions. Adv. Mater. 2020;32:e2002578. doi: 10.1002/adma.202002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rao JS, et al. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc. Natl Acad. Sci. USA. 2018;115:E5595–E5604. doi: 10.1073/pnas.1804735115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jian R, et al. Repair of spinal cord injury by chitosan scaffold with glioma ECM and SB216763 implantation in adult rats. J. Biomed. Mater. Res. A. 2015;103:3259–3272. doi: 10.1002/jbm.a.35466. [DOI] [PubMed] [Google Scholar]
- 300.Ahmad A, et al. Optogenetics applications for treating spinal cord injury. Asian Spine J. 2015;9:299–305. doi: 10.4184/asj.2015.9.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Winder DG, et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/S0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- 302.Gudasheva TA, et al. A novel dimeric dipeptide mimetic of the BDNF selectively activates the MAPK-Erk signaling pathway. Dokl. Biochem. Biophys. 2017;476:291–295. doi: 10.1134/S1607672917050027. [DOI] [PubMed] [Google Scholar]
- 303.Zheng Q, et al. Photobiomodulation promotes neuronal axon regeneration after oxidative stress and induces a change in polarization from M1 to M2 in macrophages via stimulation of CCL2 in neurons: relevance to spinal cord injury. J. Mol. Neurosci. 2021;71:1290–1300. doi: 10.1007/s12031-020-01756-9. [DOI] [PubMed] [Google Scholar]
- 304.Li K, et al. Attenuation of the inflammatory response and polarization of macrophages by photobiomodulation. Lasers Med. Sci. 2020;35:1509–1518. doi: 10.1007/s10103-019-02941-y. [DOI] [PubMed] [Google Scholar]
- 305.Wang X, et al. Photobiomodulation inhibits the activation of neurotoxic microglia and astrocytes by inhibiting Lcn2/JAK2-STAT3 crosstalk after spinal cord injury in male rats. J. Neuroinflamm. 2021;18:256. doi: 10.1186/s12974-021-02312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhang J, et al. Low-level laser therapy 810-nm up-regulates macrophage secretion of neurotrophic factors via PKA-CREB and promotes neuronal axon regeneration in vitro. J. Cell Mol. Med. 2020;24:476–487. doi: 10.1111/jcmm.14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fry FJ, et al. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127:83–84. doi: 10.1126/science.127.3289.83. [DOI] [PubMed] [Google Scholar]
- 308.Yoo S, et al. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat. Commun. 2022;13:493. doi: 10.1038/s41467-022-28040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wang B, et al. Proteomics reveals the effect of low-intensity focused ultrasound on spasticity after spinal cord injury. Turk. Neurosurg. 2023;33:77–86. doi: 10.5137/1019-5149.JTN.37469-21.2. [DOI] [PubMed] [Google Scholar]
- 310.Macias MY, et al. Directed and enhanced neurite growth with pulsed magnetic field stimulation. Bioelectromagnetics. 2000;21:272–286. doi: 10.1002/(SICI)1521-186X(200005)21:4<272::AID-BEM4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 311.Abbasnia K, et al. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat. Cell Biol. 2015;48:104–113. doi: 10.5115/acb.2015.48.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cui Y, et al. Exposure to extremely low-frequency electromagnetic fields inhibits T-type calcium channels via AA/LTE4 signaling pathway. Cell Calcium. 2014;55:48–58. doi: 10.1016/j.ceca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 313.Sun ZC, et al. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci. Rep. 2016;6:21774. doi: 10.1038/srep21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pal A, et al. Neuroregenerative effects of electromagnetic field and magnetic nanoparticles on spinal cord injury in rats. J. Nanosci. Nanotechnol. 2018;18:6756–6764. doi: 10.1166/jnn.2018.15820. [DOI] [PubMed] [Google Scholar]
- 315.Bhattacharyya S, et al. Effect of low intensity magnetic field stimulation on calcium-mediated cytotoxicity after mild spinal cord contusion injury in rats. Ann. Neurosci. 2020;27:49–56. doi: 10.1177/0972753120950072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Morimoto J, et al. Electrical stimulation enhances migratory ability of transplanted bone marrow stromal cells in a rodent ischemic stroke model. Cell Physiol. Biochem. 2018;46:57–68. doi: 10.1159/000488409. [DOI] [PubMed] [Google Scholar]
- 317.Feng JF, et al. Electrical guidance of human stem cells in the rat brain. Stem Cell Rep. 2017;9:177–189. doi: 10.1016/j.stemcr.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sordini L, et al. Effect of electrical stimulation conditions on neural stem cells differentiation on cross-linked PEDOT:PSS films. Front. Bioeng. Biotechnol. 2021;9:591838. doi: 10.3389/fbioe.2021.591838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Petrella RA, et al. 3D bioprinter applied picosecond pulsed electric fields for targeted manipulation of proliferation and lineage specific gene expression in neural stem cells. J. Neural Eng. 2018;15:056021. doi: 10.1088/1741-2552/aac8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Dong ZY, et al. Ascl1 regulates electric field-induced neuronal differentiation through PI3K/Akt pathway. Neuroscience. 2019;404:141–152. doi: 10.1016/j.neuroscience.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 321.Fu C, et al. Effect of electrical stimulation combined with graphene-oxide-based membranes on neural stem cell proliferation and differentiation. Artif. Cells Nanomed. Biotechnol. 2019;47:1867–1876. doi: 10.1080/21691401.2019.1613422. [DOI] [PubMed] [Google Scholar]
- 322.Yang K, et al. Electroconductive nanoscale topography for enhanced neuronal differentiation and electrophysiological maturation of human neural stem cells. Nanoscale. 2017;9:18737–18752. doi: 10.1039/C7NR05446G. [DOI] [PubMed] [Google Scholar]
- 323.Heo DN, et al. Directly induced neural differentiation of human adipose-derived stem cells using three-dimensional culture system of conductive microwell with electrical stimulation. Tissue Eng. Part A. 2018;24:537–545. doi: 10.1089/ten.tea.2017.0150. [DOI] [PubMed] [Google Scholar]
- 324.Rahmani A, et al. Conductive electrospun scaffolds with electrical stimulation for neural differentiation of conjunctiva mesenchymal stem cells. Artif. Organs. 2019;43:780–790. doi: 10.1111/aor.13425. [DOI] [PubMed] [Google Scholar]
- 325.Anwar MA, et al. Inflammogenesis of secondary spinal cord injury. Front. Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Levi AD, et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82:562–575. doi: 10.1093/neuros/nyx250. [DOI] [PubMed] [Google Scholar]
- 327.Assinck P, et al. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 328.Bunge MB. Efficacy of Schwann cell transplantation for spinal cord repair is improved with combinatorial strategies. J. Physiol. 2016;594:3533–3538. doi: 10.1113/JP271531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Fouad K, et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Robinson J, et al. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp. Neurol. 2017;291:87–97. doi: 10.1016/j.expneurol.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 331.Sledge J, et al. A speculative pharmaceutical cocktail to treat spinal cord injury. Am. J. Phys. Med. Rehabil. 2016;95:e108–e110. doi: 10.1097/PHM.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 332.Liu Z, et al. High-dose methylprednisolone for acute traumatic spinal cord injury: a meta-analysis. Neurology. 2019;93:e841–e850. doi: 10.1212/WNL.0000000000007998. [DOI] [PubMed] [Google Scholar]
- 333.Bracken MB, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 334.Fehlings MG, et al. Efficacy and safety of methylprednisolone sodium succinate in acute spinal cord injury: a systematic review. Glob. Spine J. 2017;7:116S–137S. doi: 10.1177/2192568217706366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bracken MB, et al. Administration of methylprednisolone for 24 or 48 h or tirilazad mesylate for 48 h in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. J. Am. Med. Assoc. 1997;277:1597–1604. doi: 10.1001/jama.1997.03540440031029. [DOI] [PubMed] [Google Scholar]
- 336.Hurlbert RJ, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2015;76:S71–S83. doi: 10.1227/01.neu.0000462080.04196.f7. [DOI] [PubMed] [Google Scholar]
- 337.Fehlings MG, et al. A clinical practice guideline for the management of acute spinal cord injury: introduction, rationale, and scope. Glob. Spine J. 2017;7:84s–94s. doi: 10.1177/2192568217703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Nagoshi N, et al. Riluzole as a neuroprotective drug for spinal cord injury: from bench to bedside. Molecules. 2015;20:7775–7789. doi: 10.3390/molecules20057775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wu Y, et al. Riluzole improves outcome following ischemia-reperfusion injury to the spinal cord by preventing delayed paraplegia. Neuroscience. 2014;265:302–312. doi: 10.1016/j.neuroscience.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 340.Satkunendrarajah K, et al. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp. Neurol. 2016;276:59–71. doi: 10.1016/j.expneurol.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 341.Wu Q, et al. A single administration of riluzole applied acutely after spinal cord injury attenuates pro-inflammatory activity and improves long-term functional recovery in rats. J. Mol. Neurosci. 2022;72:730–740. doi: 10.1007/s12031-021-01947-y. [DOI] [PubMed] [Google Scholar]
- 342.Xu S, et al. Riluzole promotes neurite growth in rats after spinal cord injury through the GSK-3β/CRMP-2 pathway. Biol. Pharm. Bull. 2022;45:569–575. doi: 10.1248/bpb.b21-00693. [DOI] [PubMed] [Google Scholar]
- 343.Grossman RG, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J. Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kumarasamy D, et al. The role of riluzole in acute traumatic cervical spinal cord injury with incomplete neurological deficit: a prospective, randomised controlled study. Indian J. Orthop. 2022;56:2160–2168. doi: 10.1007/s43465-022-00758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Shultz RB, et al. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen. Res. 2017;12:702–713. doi: 10.4103/1673-5374.206633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sonmez E, et al. Minocycline treatment inhibits lipid peroxidation, preserves spinal cord ultrastructure, and improves functional outcome after traumatic spinal cord injury in the rat. Spine. 2013;38:1253–1259. doi: 10.1097/BRS.0b013e3182895587. [DOI] [PubMed] [Google Scholar]
- 347.Smith PD, et al. Preservation of motor function after spinal cord ischemia and reperfusion injury through microglial inhibition. Ann. Thorac. Surg. 2013;95:1647–1653. doi: 10.1016/j.athoracsur.2012.11.075. [DOI] [PubMed] [Google Scholar]
- 348.Wells JE, et al. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 349.Lee SM, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 350.Lee JH, et al. Lack of neuroprotective effects of simvastatin and minocycline in a model of cervical spinal cord injury. Exp. Neurol. 2010;225:219–230. doi: 10.1016/j.expneurol.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 351.Casha S, et al. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 352.Fiani B, et al. Current updates on various treatment approaches in the early management of acute spinal cord injury. Rev. Neurosci. 2021;32:513–530. doi: 10.1515/revneuro-2020-0148. [DOI] [PubMed] [Google Scholar]
- 353.Carlson GD, et al. Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J. Neurotrauma. 1997;14:951–962. doi: 10.1089/neu.1997.14.951. [DOI] [PubMed] [Google Scholar]
- 354.Fehlings MG, et al. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28–S35. doi: 10.1097/01.brs.0000217973.11402.7f. [DOI] [PubMed] [Google Scholar]
- 355.Fehlings MG, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS ONE. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Badhiwala JH, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20:117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 357.Telemacque D, et al. Effects of durotomy versus myelotomy in the repair of spinal cord injury. Neural Regen. Res. 2020;15:1814–1820. doi: 10.4103/1673-5374.280304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Yang CH, et al. Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target. Neural Regen. Res. 2022;17:1703–1710. doi: 10.4103/1673-5374.332203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Saadoun S, et al. Targeted perfusion therapy in spinal cord trauma. Neurotherapeutics. 2020;17:511–521. doi: 10.1007/s13311-019-00820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Phang I, et al. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: injured spinal cord pressure evaluation study. J. Neurotrauma. 2015;32:865–874. doi: 10.1089/neu.2014.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Awwad W, et al. Mitigating spinal cord distraction injuries: the effect of durotomy in decreasing cord interstitial pressure in vitro. Eur. J. Orthop. Surg. Traumatol. 2014;24:S261–S267. doi: 10.1007/s00590-013-1409-5. [DOI] [PubMed] [Google Scholar]
- 362.Zhang J, et al. Intrathecal decompression versus epidural decompression in the treatment of severe spinal cord injury in rat model: a randomized, controlled preclinical research. J. Orthop. Surg. Res. 2016;11:34. doi: 10.1186/s13018-016-0369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Khaing ZZ, et al. Effect of durotomy versus myelotomy on tissue sparing and functional outcome after spinal cord injury. J. Neurotrauma. 2021;38:746–755. doi: 10.1089/neu.2020.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Jeffery ND, et al. Extended durotomy to treat severe spinal cord injury after acute thoracolumbar disc herniation in dogs. Vet. Surg. 2020;49:884–893. doi: 10.1111/vsu.13423. [DOI] [PubMed] [Google Scholar]
- 365.Perkins PG, et al. Long-term follow-up of six patients with acute spinal injury following dural decompression. Injury. 1988;19:397–401. doi: 10.1016/0020-1383(88)90132-5. [DOI] [PubMed] [Google Scholar]
- 366.Zhu F, et al. Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: a novel concept and method of surgical decompression. Eur. Spine J. 2019;28:2275–2282. doi: 10.1007/s00586-019-06091-1. [DOI] [PubMed] [Google Scholar]
- 367.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J. Am. Med. Assoc. 1911;1911:878–880. doi: 10.1001/jama.1911.04260090100008. [DOI] [Google Scholar]
- 368.Hu AM, et al. Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord injury. Spinal Cord. 2015;53:98–102. doi: 10.1038/sc.2014.209. [DOI] [PubMed] [Google Scholar]
- 369.Koyanagi I, et al. Myelotomy for acute cervical cord injury. Report of four cases. Neurol. Med. Chir. 1989;29:302–306. doi: 10.2176/nmc.29.302. [DOI] [PubMed] [Google Scholar]
- 370.Fox JL, et al. Central spinal cord injury: magnetic resonance imaging confirmation and operative considerations. Neurosurgery. 1988;22:340–347. doi: 10.1227/00006123-198802000-00011. [DOI] [PubMed] [Google Scholar]
- 371.Meyer C, et al. The effect of myelotomy following low thoracic spinal cord compression injury in rats. Exp. Neurol. 2018;306:10–21. doi: 10.1016/j.expneurol.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 372.Patel NP, et al. Hyperbaric oxygen therapy of spinal cord injury. Med Gas. Res. 2017;7:133–143. doi: 10.4103/2045-9912.208520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Tan JW, et al. Hyperbaric oxygen ameliorated the lesion scope and nerve function in acute spinal cord injury patients: a retrospective study. Clin. Biochem. 2018;53:1–7. doi: 10.1016/j.clinbiochem.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 374.Zhang Z, et al. Effects of hyperbaric oxygen therapy on postoperative recovery after incomplete cervical spinal cord injury. Spinal Cord. 2021;60:129–134. doi: 10.1038/s41393-021-00674-w. [DOI] [PubMed] [Google Scholar]
- 375.Asamoto S, et al. Hyperbaric oxygen (HBO) therapy for acute traumatic cervical spinal cord injury. Spinal Cord. 2000;38:538–540. doi: 10.1038/sj.sc.3101023. [DOI] [PubMed] [Google Scholar]
- 376.Sandrow-Feinberg HR, et al. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015;1619:12–21. doi: 10.1016/j.brainres.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Gaspar R, et al. Physical exercise for individuals with spinal cord injury: systematic review based on the international classification of functioning, disability, and health. J. Sport Rehabil. 2019;28:505–516. doi: 10.1123/jsr.2017-0185. [DOI] [PubMed] [Google Scholar]
- 378.Nightingale TE, et al. Home-based exercise enhances health-related quality of life in persons with spinal cord injury: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2018;99:1998–2006. doi: 10.1016/j.apmr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 379.Anderson KD, et al. Multicentre, single-blind randomised controlled trial comparing MyndMove neuromodulation therapy with conventional therapy in traumatic spinal cord injury: a protocol study. BMJ Open. 2020;10:e039650. doi: 10.1136/bmjopen-2020-039650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Ganzer PD, et al. Restoring the sense of touch using a sensorimotor demultiplexing neural interface. Cell. 2020;181:763–773. doi: 10.1016/j.cell.2020.03.054. [DOI] [PubMed] [Google Scholar]
- 381.Davis KC, et al. Design-development of an at-home modular brain-computer interface (BCI) platform in a case study of cervical spinal cord injury. J. Neuroeng. Rehabil. 2022;19:53. doi: 10.1186/s12984-022-01026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Capogrosso M, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Willett FR, et al. High-performance brain-to-text communication via handwriting. Nature. 2021;593:249–254. doi: 10.1038/s41586-021-03506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Löfvenmark I, et al. Traumatic spinal cord injury in Botswana: characteristics, aetiology and mortality. Spinal Cord. 2015;53:150–154. doi: 10.1038/sc.2014.203. [DOI] [PubMed] [Google Scholar]
- 385.Lenehan B, et al. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine. 2012;37:321–329. doi: 10.1097/BRS.0b013e31822e5ff8. [DOI] [PubMed] [Google Scholar]
- 386.Ruiz IA, et al. Incidence and natural progression of neurogenic shock after traumatic spinal cord injury. J. Neurotrauma. 2018;35:461–466. doi: 10.1089/neu.2016.4947. [DOI] [PubMed] [Google Scholar]
- 387.Li HL, et al. Epidemiology of traumatic spinal cord injury in Tianjin, China: an 18-year retrospective study of 735 cases. J. Spinal Cord Med. 2019;42:778–785. doi: 10.1080/10790268.2017.1415418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Wu Q, et al. Epidemiology of traumatic cervical spinal cord injury in Tianjin, China. Spinal Cord. 2012;50:740–744. doi: 10.1038/sc.2012.42. [DOI] [PubMed] [Google Scholar]
- 389.Felix ER, et al. Prevalence and impact of neuropathic and nonneuropathic pain in chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2022;103:729–737. doi: 10.1016/j.apmr.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 390.Cowan H, et al. Autonomic dysreflexia in spinal cord injury. BMJ. 2020;371:m3596. doi: 10.1136/bmj.m3596. [DOI] [PubMed] [Google Scholar]
- 391.Kennedy P, et al. The relationship between pain and mood following spinal cord injury. J. Spinal Cord. Med. 2017;40:275–279. doi: 10.1080/10790268.2016.1147680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kuris EO, et al. Bowel and bladder care in patients with spinal cord injury. J. Am. Acad. Orthop. Surg. 2022;30:263–272. doi: 10.5435/JAAOS-D-21-00873. [DOI] [PubMed] [Google Scholar]
- 393.Parent S, et al. The impact of specialized centers of care for spinal cord injury on length of stay, complications, and mortality: a systematic review of the literature. J. Neurotrauma. 2011;28:1363–1370. doi: 10.1089/neu.2009.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Walters BC, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60:82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- 395.Kirshblum S, et al. Updates of the international standards for neurologic classification of spinal cord injury: 2015 and 2019. Phys. Med. Rehabil. Clin. N. Am. 2020;31:319–330. doi: 10.1016/j.pmr.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 396.Huang H, et al. Spinal cord injury or dysfunction quality of life rating scale (SCIDQLRS) (IANR 2022 version) J. Neurorestoratol. 2022;10:100016. doi: 10.1016/j.jnrt.2022.100016. [DOI] [Google Scholar]
- 397.Le CT, et al. Survival from spinal cord injury. J. Chronic Dis. 1982;35:487–492. doi: 10.1016/0021-9681(82)90063-7. [DOI] [PubMed] [Google Scholar]
- 398.McDonald JW, et al. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 399.Penrod LE, et al. Age effect on prognosis for functional recovery in acute, traumatic central cord syndrome. Arch. Phys. Med. Rehabil. 1990;71:963–968. [PubMed] [Google Scholar]
- 400.Roth EJ, et al. Traumatic central cord syndrome: clinical features and functional outcomes. Arch. Phys. Med. Rehabil. 1990;71:18–23. [PubMed] [Google Scholar]
- 401.Teles AR, et al. Surgical timing in traumatic spinal cord injury: current practice and obstacles to early surgery in Latin America. Spinal Cord. 2022;60:368–374. doi: 10.1038/s41393-022-00789-8. [DOI] [PubMed] [Google Scholar]
- 402.Furlan JC, et al. Motor and sensory assessment of patients in clinical trials for pharmacological therapy of acute spinal cord injury: psychometric properties of the ASIA Standards. J. Neurotrauma. 2008;25:1273–1301. doi: 10.1089/neu.2008.0617. [DOI] [PubMed] [Google Scholar]
- 403.Kuzu D, et al. Spinal cord injury/disorder function, affiliate stigma, and caregiver burden in Turkey. PMR. 2021;13:1376–1384. doi: 10.1002/pmrj.12548. [DOI] [PubMed] [Google Scholar]
- 404.Oh SK, et al. Current concept of stem cell therapy for spinal cord injury: a review. Korean J. Neurotrauma. 2016;12:40–46. doi: 10.13004/kjnt.2016.12.2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ruzicka J, et al. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transpl. 2017;26:585–603. doi: 10.3727/096368916X693671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Yamazaki K, et al. Clinical trials of stem cell treatment for spinal cord injury. Int. J. Mol. Sci. 2020;21:3994. doi: 10.3390/ijms21113994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Shang Z, et al. Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022;20:284. doi: 10.1186/s12916-022-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Tsai SHL, et al. Cannabinoid use for pain reduction in spinal cord injuries: a meta-analysis of randomized controlled trials. Front. Pharm. 2022;13:866235. doi: 10.3389/fphar.2022.866235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Vaquero J, et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy. 2018;20:796–805. doi: 10.1016/j.jcyt.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 410.Allahdadi KJ, et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res. Ther. 2019;10:146. doi: 10.1186/s13287-019-1223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ruzicka J, et al. The effect of iPS-derived neural progenitors seeded on laminin-coated pHEMA-MOETACl hydrogel with dual porosity in a rat model of chronic spinal cord injury. Cell Transpl. 2019;28:400–412. doi: 10.1177/0963689718823705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Fessler RG, et al. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J. Neurosurg. Spine. 2022;37:812–820. doi: 10.3171/2022.5.SPINE22167. [DOI] [PubMed] [Google Scholar]
- 413.Sugai K, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen. Ther. 2021;18:321–333. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Shin JC, et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. doi: 10.1155/2015/630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.AD L, et al. Clinical outcomes from a multi-center study of human neural stem cell transplantation in chronic cervical spinal cord injury. J. Neurotrauma. 2019;36:891–902. doi: 10.1089/neu.2018.5843. [DOI] [PubMed] [Google Scholar]
- 416.Curtis E, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 417.Chen WC, et al. Transplantation of mesenchymal stem cells for spinal cord injury: a systematic review and network meta-analysis. J. Transl. Med. 2021;19:178. doi: 10.1186/s12967-021-02843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Satti HS, et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a Phase I pilot study. Cytotherapy. 2016;18:518–522. doi: 10.1016/j.jcyt.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 419.Yang Y, et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study. Cytotherapy. 2021;23:57–64. doi: 10.1016/j.jcyt.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 420.Tahmasebi F, et al. Effects of mesenchymal stem cell transplantation on spinal cord injury patients. Cell Tissue Res. 2022;389:373–384. doi: 10.1007/s00441-022-03648-3. [DOI] [PubMed] [Google Scholar]
- 421.Oh SK, et al. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78:436–447. doi: 10.1227/NEU.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 422.Oraee-Yazdani S, et al. Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: safety considerations and possible outcomes. Stem Cell Res. Ther. 2021;12:445. doi: 10.1186/s13287-021-02515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Xiao Z, et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with neuroregen scaffolds and mesenchymal stem cells. Cell Transpl. 2018;27:907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zhao Y, et al. Study of the diffusion tensor imaging for preclinical therapeutic efficacy of umbilical cord mesenchymal stem cell transplantation in the treatment of spinal cord injury. Int J. Gen. Med. 2021;14:9721–9732. doi: 10.2147/IJGM.S326023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Zamani H, et al. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. 2022;60:63–70. doi: 10.1038/s41393-021-00687-5. [DOI] [PubMed] [Google Scholar]
- 426.Suzuki Y, et al. Bone marrow-derived mononuclear cell transplantation in spinal cord injury patients by lumbar puncture. Restor. Neurol. Neurosci. 2014;32:473–482. doi: 10.3233/RNN-130363. [DOI] [PubMed] [Google Scholar]
- 427.Zhu H, et al. Phase I-II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transpl. 2016;25:1925–1943. doi: 10.3727/096368916X691411. [DOI] [PubMed] [Google Scholar]
- 428.Geissler SA, et al. Biomimetic hydrogels direct spinal progenitor cell differentiation and promote functional recovery after spinal cord injury. J. Neural Eng. 2018;15:025004. doi: 10.1088/1741-2552/aaa55c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Santi S, et al. Injectable scaffold-systems for the regeneration of spinal cord: advances of the past decade. ACS Biomater. Sci. Eng. 2021;7:983–999. doi: 10.1021/acsbiomaterials.0c01779. [DOI] [PubMed] [Google Scholar]
- 430.Luo J, et al. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021;6:4816–4829. doi: 10.1016/j.bioactmat.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Hong JY, et al. Self-assembling peptide gels promote angiogenesis and functional recovery after spinal cord injury in rats. J. Tissue Eng. 2022;13:20417314221086491. doi: 10.1177/20417314221086491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Abdolahi S, et al. Improvement of rat spinal cord injury following lentiviral vector-transduced neural stem/progenitor cells derived from human epileptic brain tissue transplantation with a self-assembling peptide scaffold. Mol. Neurobiol. 2021;58:2481–2493. doi: 10.1007/s12035-020-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Li Y, et al. Co-transplantation of Schwann cells and neural stem cells in the laminin-chitosan-PLGA nerve conduit to repair the injured recurrent laryngeal nerve in SD rats. J. Mater. Sci. Mater. Med. 2020;31:99. doi: 10.1007/s10856-020-06436-z. [DOI] [PubMed] [Google Scholar]
- 434.Zhou P, et al. Promoting 3D neuronal differentiation in hydrogel for spinal cord regeneration. Colloids Surf. B Biointerfaces. 2020;194:111214. doi: 10.1016/j.colsurfb.2020.111214. [DOI] [PubMed] [Google Scholar]
- 435.Huang CT, et al. A graphene-polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B. 2017;5:8854–8864. doi: 10.1039/C7TB01594A. [DOI] [PubMed] [Google Scholar]
- 436.Li H, et al. A hydrogel bridge incorporating immobilized growth factors and neural stem/progenitor cells to treat spinal cord injury. Adv. Health. Mater. 2016;5:802–812. doi: 10.1002/adhm.201500810. [DOI] [PubMed] [Google Scholar]
- 437.Liu S, et al. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017;60:167–180. doi: 10.1016/j.actbio.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 438.Chen W, et al. NeuroRegen scaffolds combined with autologous bone marrow mononuclear cells for the repair of acute complete spinal cord injury: a 3-year clinical study. Cell Transpl. 2020;29:963689720950637. doi: 10.1177/0963689720950637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Nussbaum EL, et al. Comparison of ultrasound/ultraviolet-C and laser for treatment of pressure ulcers in patients with spinal cord injury. Phys. Ther. 1994;74:812–823. doi: 10.1093/ptj/74.9.812. [DOI] [PubMed] [Google Scholar]
- 440.Hogaboom N, et al. A pilot study to evaluate micro-fragmented adipose tissue injection under ultrasound guidance for the treatment of refractory rotator cuff disease in wheelchair users with spinal cord injury. J. Spinal Cord Med. 2021;44:886–895. doi: 10.1080/10790268.2021.1903140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Azadvari M, et al. Ultrasound-guided versus blind subacromial bursa corticosteroid injection for paraplegic spinal cord injury patients with rotator cuff tendinopathy: a randomized, single-blind clinical trial. Int. J. Neurosci. 2021;131:445–452. doi: 10.1080/00207454.2020.1748620. [DOI] [PubMed] [Google Scholar]
- 442.Leister I, et al. The effect of extracorporeal shock wave therapy in acute traumatic spinal cord injury on motor and sensory function within 6 months post-injury: a study protocol for a two-arm three-stage adaptive, prospective, multi-center, randomized, blinded, placebo-controlled clinical trial. Trials. 2022;23:245. doi: 10.1186/s13063-022-06161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Kumru H, et al. Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabil. Neural Repair. 2013;27:421–429. doi: 10.1177/1545968312471901. [DOI] [PubMed] [Google Scholar]
- 444.de Araújo AVL, et al. Effects of high-frequency transcranial magnetic stimulation on functional performance in individuals with incomplete spinal cord injury: study protocol for a randomized controlled trial. Trials. 2017;18:522. doi: 10.1186/s13063-017-2280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zhao CG, et al. Analgesic effects of directed repetitive transcranial magnetic stimulation in acute neuropathic pain after spinal cord injury. Pain. Med. 2020;21:1216–1223. doi: 10.1093/pm/pnz290. [DOI] [PubMed] [Google Scholar]
- 446.Sun X, et al. Analgesia-enhancing effects of repetitive transcranial magnetic stimulation on neuropathic pain after spinal cord injury: an fNIRS study. Restor. Neurol. Neurosci. 2019;37:497–507. doi: 10.3233/RNN-190934. [DOI] [PubMed] [Google Scholar]
- 447.Krogh S, et al. Effects of repetitive transcranial magnetic stimulation on recovery in lower limb muscle strength and gait function following spinal cord injury: a randomized controlled trial. Spinal Cord. 2022;60:135–141. doi: 10.1038/s41393-021-00703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Xu L, et al. Efficacy of biofeedback, repetitive transcranial magnetic stimulation and pelvic floor muscle training for female neurogenic bladder dysfunction after spinal cord injury: a study protocol for a randomised controlled trial. BMJ Open. 2020;10:e034582. doi: 10.1136/bmjopen-2019-034582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Rattay F, et al. Mechanisms of electrical stimulation with neural prostheses. Neuromodulation. 2003;6:42–56. doi: 10.1046/j.1525-1403.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 450.Sivaramakrishnan A, et al. Comparison of transcutaneous electrical nerve stimulation (TENS) and functional electrical stimulation (FES) for spasticity in spinal cord injury—a pilot randomized cross-over trial. J. Spinal Cord Med. 2018;41:397–406. doi: 10.1080/10790268.2017.1390930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Rahimi M, et al. Advanced weight-bearing mat exercises combined with functional electrical stimulation to improve the ability of wheelchair-dependent people with spinal cord injury to transfer and attain independence in activities of daily living: a randomized controlled trial. Spinal Cord. 2020;58:78–85. doi: 10.1038/s41393-019-0328-7. [DOI] [PubMed] [Google Scholar]
- 452.Gorgey AS, et al. Neuromuscular electrical stimulation resistance training enhances oxygen uptake and ventilatory efficiency independent of mitochondrial complexes after spinal cord injury: a randomized clinical trial. J. Appl Physiol. 2021;131:265–276. doi: 10.1152/japplphysiol.01029.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Levin MF, et al. Conventional and acupuncture-like transcutaneous electrical nerve stimulation excite similar afferent fibers. Arch. Phys. Med. Rehabil. 1993;74:54–60. [PubMed] [Google Scholar]
- 454.Keller A, et al. Noninvasive spinal stimulation safely enables upright posture in children with spinal cord injury. Nat. Commun. 2021;12:5850. doi: 10.1038/s41467-021-26026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Huang R, et al. Minimal handgrip force is needed for transcutaneous electrical stimulation to improve hand functions of patients with severe spinal cord injury. Sci. Rep. 2022;12:7733. doi: 10.1038/s41598-022-11306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Sayenko DG, et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J. Neurotrauma. 2019;36:1435–1450. doi: 10.1089/neu.2018.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Harkema S, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Wagner FB, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 459.Squair JW, et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature. 2021;590:308–314. doi: 10.1038/s41586-020-03180-w. [DOI] [PubMed] [Google Scholar]
- 460.Rowald A, et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 2022;28:260–271. doi: 10.1038/s41591-021-01663-5. [DOI] [PubMed] [Google Scholar]
- 461.Stieglitz LH, et al. Deep brain stimulation for locomotion in incomplete human spinal cord injury (DBS-SCI): protocol of a prospective one-armed multi-centre study. BMJ Open. 2021;11:e047670. doi: 10.1136/bmjopen-2020-047670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Degenhart AD, et al. Remapping cortical modulation for electrocorticographic brain-computer interfaces: a somatotopy-based approach in individuals with upper-limb paralysis. J. Neural Eng. 2018;15:026021. doi: 10.1088/1741-2552/aa9bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Stenudd M, et al. Identification of a discrete subpopulation of spinal cord ependymal cells with neural stem cell properties. Cell Rep. 2022;38:110440. doi: 10.1016/j.celrep.2022.110440. [DOI] [PubMed] [Google Scholar]
- 464.Wallace DJ, et al. Spinal cord injury and the human microbiome: beyond the brain-gut axis. Neurosurg. Focus. 2019;46:E11. doi: 10.3171/2018.12.FOCUS18206. [DOI] [PubMed] [Google Scholar]
- 465.Yousefifard M, et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment: a systematic review and meta-analysis. Neuroscience. 2016;322:377–397. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 466.Zhang HA, et al. Neural stem cell transplantation alleviates functional cognitive deficits in a mouse model of tauopathy. Neural Regen. Res. 2022;17:152–162. doi: 10.4103/1673-5374.314324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Paredes-Espinosa MB, et al. Human stem cell-derived neurons and neural circuitry therapeutics: Next frontier in spinal cord injury repair. Exp. Biol. Med. 2022;247:2142–2151. doi: 10.1177/15353702221114099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Fischer I, et al. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020;21:366–383. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Li X, et al. Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo. ACS Appl Mater. Interfaces. 2015;7:13960–13971. doi: 10.1021/acsami.5b03879. [DOI] [PubMed] [Google Scholar]
- 470.Agarwal G, et al. Highly elastic, electroconductive, immunomodulatory graphene crosslinked collagen cryogel for spinal cord regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021;118:111518. doi: 10.1016/j.msec.2020.111518. [DOI] [PubMed] [Google Scholar]
- 471.Liu S, et al. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen. Res. 2021;16:2284–2292. doi: 10.4103/1673-5374.310698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Koffler J, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Joung D, et al. 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Adv. Funct. Mater. 2018;28:1801850. doi: 10.1002/adfm.201801850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Maclean FL, et al. A programmed anti-inflammatory nanoscaffold (PAIN) as a 3D tool to understand the brain injury response. Adv. Mater. 2018;30:e1805209. doi: 10.1002/adma.201805209. [DOI] [PubMed] [Google Scholar]
- 475.Faustino Martins JM, et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. 2020;26:172–186. doi: 10.1016/j.stem.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 476.Zhang N, et al. A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv. Sci. 2021;8:e2100805. doi: 10.1002/advs.202100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Liu X, et al. Release of O-GlcNAc transferase inhibitor promotes neuronal differentiation of neural stem cells in 3D bioprinted supramolecular hydrogel scaffold for spinal cord injury repair. Acta Biomater. 2022;151:148–162. doi: 10.1016/j.actbio.2022.08.031. [DOI] [PubMed] [Google Scholar]
- 478.Kiyotake EA, et al. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022;139:43–64. doi: 10.1016/j.actbio.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 479.Wang Y, et al. Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil. Med. Res. 2022;9:16. doi: 10.1186/s40779-022-00376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Minev IR, et al. Biomaterials. Electronic dura mater for long-term multimodal neural interfaces. Science. 2015;347:159–163. doi: 10.1126/science.1260318. [DOI] [PubMed] [Google Scholar]
- 481.Majdan M, et al. Epidemiology of traumatic spinal cord injuries in Austria 2002-2012. Eur. Spine J. 2016;25:62–73. doi: 10.1007/s00586-015-3985-z. [DOI] [PubMed] [Google Scholar]
- 482.Santos EA, et al. Epidemiology of severe cervical spinal trauma in the north area of São Paulo City: a 10-year prospective study. Clinical article. J. Neurosurg. Spine. 2009;11:34–41. doi: 10.3171/2009.3.SPINE08325. [DOI] [PubMed] [Google Scholar]
- 483.Choi JH, et al. Epidemiology and clinical management of traumatic spine injuries at a major government hospital in Cambodia. Asian Spine J. 2017;11:908–916. doi: 10.4184/asj.2017.11.6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Thompson C, et al. The changing demographics of traumatic spinal cord injury: An 11-year study of 831 patients. J. Spinal Cord Med. 2015;38:214–223. doi: 10.1179/2045772314Y.0000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.El Tallawy HN, et al. Prevalence of spinal cord disorders in Al-Quseir City, Red Sea Governorate, Egypt. Neuroepidemiology. 2013;41:42–47. doi: 10.1159/000348320. [DOI] [PubMed] [Google Scholar]
- 486.Sabre L, et al. High incidence of traumatic spinal cord injury in Estonia. Spinal Cord. 2012;50:755–759. doi: 10.1038/sc.2012.54. [DOI] [PubMed] [Google Scholar]
- 487.Hasler RM, et al. Epidemiology and predictors of spinal injury in adult major trauma patients: European cohort study. Eur. Spine J. 2011;20:2174–2180. doi: 10.1007/s00586-011-1866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Niemi-Nikkola V, et al. Traumatic spinal injuries in Northern Finland. Spine. 2018;43:e45–e51. doi: 10.1097/BRS.0000000000002214. [DOI] [PubMed] [Google Scholar]
- 489.Knútsdóttir S, et al. Epidemiology of traumatic spinal cord injuries in Iceland from 1975 to 2009. Spinal Cord. 2012;50:123–126. doi: 10.1038/sc.2011.105. [DOI] [PubMed] [Google Scholar]
- 490.Mathur N, et al. Spinal cord injury: scenario in an Indian state. Spinal Cord. 2015;53:349–352. doi: 10.1038/sc.2014.153. [DOI] [PubMed] [Google Scholar]
- 491.Rahimi-Movaghar V, et al. Prevalence of spinal cord injury in Tehran, Iran. J. Spinal Cord Med. 2009;32:428–431. doi: 10.1080/10790268.2009.11754572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.O’Connor RJ, et al. Review of spinal cord injuries in Ireland. Spinal Cord. 2006;44:445–448. doi: 10.1038/sj.sc.3101856. [DOI] [PubMed] [Google Scholar]
- 493.Barbiellini Amidei C, et al. Epidemiology of traumatic spinal cord injury: a large population-based study. Spinal Cord. 2022;60:812–819. doi: 10.1038/s41393-022-00795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Katoh S, et al. High incidence of acute traumatic spinal cord injury in a rural population in Japan in 2011 and 2012: an epidemiological study. Spinal Cord. 2014;52:264–267. doi: 10.1038/sc.2014.13. [DOI] [PubMed] [Google Scholar]
- 495.Shin JC, et al. Epidemiologic change of patients with spinal cord injury. Ann. Rehabil. Med. 2013;37:50–56. doi: 10.5535/arm.2013.37.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Prasad L, et al. Epidemiological profile of spinal cord injuries at a tertiary rehabilitation center in Kuwait. Spinal Cord Ser. Cases. 2018;4:7. doi: 10.1038/s41394-017-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Singh G, et al. Spinal cord injury in organizational setup: a hospital based descriptive study. J. Mar. Med. Soc. 2019;21:46. doi: 10.4103/jmms.jmms_67_18. [DOI] [Google Scholar]
- 498.Ibrahim A, et al. Epidemiology of spinal cord injury in Hospital Kuala Lumpur. Spine. 2013;38:419–424. doi: 10.1097/BRS.0b013e31826ef594. [DOI] [PubMed] [Google Scholar]
- 499.Shrestha P, et al. Retrospective study of spinal cord injury patients admitted to spinal injury rehabilitation center, Sanga, Banepa, Nepal. Nepal Med. Coll. J. 2014;16:169–172. [PubMed] [Google Scholar]
- 500.Nwankwo OE, et al. Epidemiological and treatment profiles of spinal cord injury in southeast Nigeria. Spinal Cord. 2013;51:448–452. doi: 10.1038/sc.2013.10. [DOI] [PubMed] [Google Scholar]
- 501.Fredø HL, et al. Incidence of traumatic cervical spine fractures in the Norwegian population: a national registry study. Scand. J. Trauma Resusc. Emerg. Med. 2014;22:78. doi: 10.1186/s13049-014-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Bilal H, et al. The Incidence of traumatic spinal cord injury in Khyber Pukhtunkhwa, Pakistan from 2008 to 2012. J. Riphah Coll. Rehabil. Sci. 2016;4:30–34. [Google Scholar]
- 503.Mirzaeva L, et al. Incidence of adult traumatic spinal cord injury in Saint Petersburg, Russia. Spinal Cord. 2019;57:692–699. doi: 10.1038/s41393-019-0266-4. [DOI] [PubMed] [Google Scholar]
- 504.Alshahri SS, et al. Traumatic spinal cord injury in Saudi Arabia: an epidemiological estimate from Riyadh. Spinal Cord. 2012;50:882–884. doi: 10.1038/sc.2012.65. [DOI] [PubMed] [Google Scholar]
- 505.Bárbara-Bataller E, et al. Epidemiology of traumatic spinal cord injury in Gran Canaria. Neurocirugia. 2017;28:15–21. doi: 10.1016/j.neucir.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 506.Chamberlain JD, et al. Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj. Epidemiol. 2015;2:28. doi: 10.1186/s40621-015-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Moshi H, et al. Traumatic spinal cord injury in the north-east Tanzania: describing incidence, etiology and clinical outcomes retrospectively. Glob. Health Action. 2017;10:1355604. doi: 10.1080/16549716.2017.1355604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Mendonça MV, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.El-Kheir WA, et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transpl. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 510.Ra JC, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 511.Cheng H, et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Yang Y, et al. Human umbilical cord mesenchymal stem cells to treat spinal cord injury in the early chronic phase: study protocol for a prospective, multicenter, randomized, placebo-controlled, single-blinded clinical trial. Neural Regen. Res. 2020;15:1532–1538. doi: 10.4103/1673-5374.274347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Jin D, et al. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun. 2015;6:8074. doi: 10.1038/ncomms9074. [DOI] [PMC free article] [PubMed] [Google Scholar]