Epidemiology
Alopecia areata is an immune-mediated condition leading to non-scarring alopecia of the scalp and other hair-bearing areas of the body. It affects up to 2% of the global population. 1 It can affect all ages, but the prevalence appears higher in children compared to adults (1.92%, 1.47%). 2 A greater incidence has been reported in females than males, especially in patients with late-onset disease, defined as age greater than 50 years. 3
Pathogenesis
Dysregulation of several pathways immune-mediated pathways has been implicated in the development of AA, occurring in susceptible patients often with concurrent multigenetic predisposition and environmental triggers.
In unaffected individuals, hair follicles have an immune privilege that is protective against autoimmunity. In patients with AA, attack of the hair follicle is thought to be directed at autoantigens in the follicle, mediated by disruption of its immune privilege, with both melanocyte and keratinocyte-related autoantigens being proposed. 4 Immune attack has been attributed to activation of Th1, Th2 and Th17 cytokines, with Th1 cytokines (IL-2, TNF, IL-12) and Th17 (IL17 and IL17E) also correlating with disease activity. 5
A genetic predisposition to AA is supported by the frequent history of affected family members. Several genome-wide associated studies have identified potential polymorphisms associated with disease. The strongest evidence is for a single nucleoid polymorphism in PTPN22, with weaker data supporting polymorphisms in FAS, FASL, PTPN22, CTLA4, and IL2RA. 6
Several potential triggers of disease onset and flares have been identified. Viral infections associated with initial presentation and recurrence of AA include Epstein–Barr virus (EBV), hepatitis B and C viruses, and swine flu. 7 Vaccines are another reported trigger, with many different vaccines implicated including influenza, hepatitis, and coronavirus.8-10
Associations
Alopecia areata is associated with many different comorbidities, including atopic diseases, metabolic syndrome, Helicobacter pylori infection, lupus erythematosus, iron deficiency anemia, thyroid diseases, psychiatric diseases, and vitamin D deficiency. 11 Autoimmune thyroid disease is a more commonly reported association, with a pooled odds ratio (OR) of 1.66; (95% CI, 0.82-3.38; prevalence, 13.9%), and thyroid dysfunction also frequently reported (OR 4.36; 95% CI, 1.19-15.99; prevalence, 12.5%). Other notable comorbidities in the systematic review included atopic dermatitis (OR, 2.36; 95% CI, 1.80-3.09; prevalence, 9.6%), metabolic syndrome (OR, 3.68; 95% CI, 1.22-11.08; prevalence, 37.3%), Helicobacter pylori infection (OR, 2.03; 95% CI, 1.23-3.34; prevalence, 62.8%), Lupus erythematosus (OR, 4.73; 95% CI, 3.70-6.10; prevalence, 0.8%), iron deficiency anemia (OR, 2.78; 95% CI, 1.23-6.29; prevalence, 7.5%), and vitamin D deficiency (OR, 4.61; 95% CI, 2.33-9.10; prevalence, 65.4%). 11
With respect to psychiatric comorbidities, patients with AA appear to have a higher risk of both depression and anxiety. 12 The prevalence of anxiety is especially marked, with patients being reported to have 2.5-fold increased odds of having anxiety compared to control groups. Children and adolescents with AA have particularly high levels of anxiety. 13 A recent study reported that 51% of pediatric AA patients met criteria for an anxiety disorder. 14
Stress and anxiety have been implicated in triggering alopecia areata, and the burden and stigma associated with having alopecia has also been implicated in leading to anxiety in patients. Emotional stress can lead to increased catecholamines and decreased blood flow, and has been associated with upregulation of receptors for corticotropin-releasing hormone in skin around hair follicles. 15
Presentation
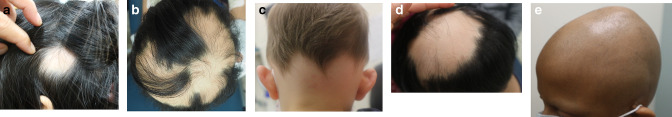
There are multiple different patterns of presentation of hair loss in AA (Figure 1a-e). The most common is patch, with circular patches recognizable on the scalp or beard areas which may coalesce with progression. Ophiasis describes a band of alopecia along the parietal and occipital areas, whereas sisaipho refers to an opposite pattern. More extensive hair loss is seen in diffuse patterns, with a decrease in density diffusely, and totalis, with complete scalp alopecia.
Figure 1.
Clinical Subtype Photos. a. Patch subtype with solitary patch. b. Patch subtype with multiple coalescing patches. c. Ophiasis Pattern. d. Sasaipho Pattern. e. Alopecia Totatalis
The presence of active disease can be assessed with the pull test. This involves grasping and firmly pulling on 50-60 hairs close to the scalp. A positive test, defined by >10% of hairs being pulled out, indicates active hair loss above normal shedding. 16
There are also several nail findings associated with AA, with an overall prevalence of up to 30%. 17 The most common changes include fine pitting in 0.6 to 11.4% of adults with AA, and trachyonychia in 8 to 14%. Less commonly reported findings include longitudinal ridging. Children may have a higher prevalence of nail pitting (13.2 to 18.8%), which is described as shallow pits in a grid-like distribution in contrast to the more irregularly distributed shallow pits seen with atopic dermatitis and the deeper irregular pits of psoriasis.
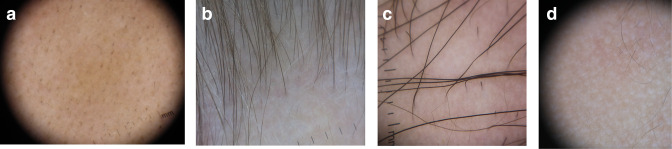
Trichoscopy
Many different signs have been described on examination of the scalp with a dermatoscope, which can aid in diagnosis and evaluation of disease activity (Table 1 and Figure 2a-d). The majority of these are not specific to AA, but some are sensitive, including yellow dots and short vellus hairs.18,19 Yellow dots correspond with distention of the affected follicular infundibulum with keratinous material and sebum, and can help demonstrate persistence of hair follicles which are typically destroyed in scarring alopecias. Short vellus hairs, which are thin and nonpigmented, correlate with regrowth when seen within AA patches. Conversely, exclamation mark hairs and black dots are considered signs of active disease. 20 Exclamation marks hairs are fairly specific for AA; they have a wide distal shaft diameter that thins proximally, as a result of the lymphocytic inflammatory infiltrate affecting the hair bulb. Black dots are the result of broken pigmented hair at the level of the scalp. An AA predictive score has been proposed based on 65 patients, with upright regrowing hairs and pigtail hairs given positive points for hair regrowth, while black dots, broken hairs, exclamation mark hairs, and tapered hairs are given negative points. 21
Table 1.
Trichoscopic Findings in AA and Their Significance
| Sign | Description/ Etiology | Significance a |
|---|---|---|
| Yellow Dots | Distention of the affected follicular infundibulum with keratinous material and sebum | Sensitive sign of AA Supports presence of non-scarring alopecia |
| Short Vellus Hairs | Thin and nonpigmented hairs | Sensitive sign of AA Supports regrowth when seen in AA |
| Black Dots | Broken pigmented hair at the level of the scalp | Specific for AA Supports presence of active disease Negative predictive marker for regrowth |
| Exclamation Mark Hairs | Pigmented short (1, 2mm) hairs with wide distal shaft diameter that thins proximally | Lymphocytic inflammatory infiltrate affecting the hair bulb Associated with active disease Negative predictive marker for regrowth |
| Tapered Hairs | Longer exclamation mark hairs (>2 mm) | Negative predictive marker for regrowth |
| Upright Regrowing hairs | Tapered end that thickens proximally, thicker than vellus hairs | Can be seen in many conditions Positive predictive marker for regrowth |
| Pigtail Hair | Short, regularly twisted hairs with short tapered end | Positive predictive marker for regrowth |
| Pohl-Pinkus Constrictions | Hairs with segmental thinning (constriction) or narrowing | Not specific for AA Presence of multiple segments supports cyclical disease activity |
aPredictive Markers based on Waskiel-Burnat et al
Figure 2.
Dermoscopy Photos. a. Black dots. b. Vellus Hairs in a regrowing patch. c. Upright regrowing hairs (right half) with a few exclamation mark hairs (left half). d. Empty Hair Follicles
Work-Up
Consensus guidelines have been published to provide some guidance on the approach to investigations and treatment in patients with AA.20,22 The diagnosis of AA can be made clinically in the vast majority of patients, with biopsies reserved for scenarios where a solitary patch is recalcitrant, when there is diffuse alopecia and when scarring alopecia cannot be excluded clinically. Biopsies should be performed at the edge of a patch and sectioned both horizontally and vertically for optimal interpretation.
Laboratory testing for comorbidities was not recommended uniformly in the consensus, with disagreement on the testing of thyroid and vitamin D. Tests to exclude potential mimickers of AA including fungal microscopy for tinea and capitis and viral serology for syphilis should be guided by clinical suspicion.
Assessment
Multiple assessment tools exist for objective scoring of alopecia. 23 The Severity of Alopecia Tool (SALT) Score was initially described in 1999 and now referred to as the SALT I. 24 The tool involves splitting the scalp into 4 quadrants and summing the percentage of scalp area devoid of terminal hairs in each quadrant and then the whole scalp to provide a total area affected. It does not account for loss of facial hair (eyelashes, eyebrows, beard) or body hair. The Alopecia Density and Extent (ALODEX) score is a modification of the SALT score which increases the divisions of the scalp area to provide a more precise estimate of scalp surface area involvement.
Intervals of severity have also been proposed, where S0 = no hair loss; S1 = 1 to 24% hair loss; S2 = 25 to 49% hair loss; S3 = 50 to 74% hair loss; S4 = 75 to 99% hair loss; and S5 = 100% hair loss. Tools based on advanced photographic images of the scalp may provide better objective assessment of hair density and loss. One such system, Hairmount, was recently described and tested in 66 patients with AA aged of different races. 25 It provides a provides a percentage hair loss at every pixel that can be visualized as a contour plot for a qualitative evaluation.
In order to standardize treatment response assessment in clinical trials, an Investigator’s Global assessment was developed based on consensus from 10 experts and patients. 26 The majority agreed that for patients with ≥50% scalp hairloss, a successful treatment could be defined as regrowth to a SALT score of 20% or less.
A scoring system has also been proposed for assessment of hair loss severity of eyelashes, eyebrows, and nail findings, based on qualitative analysis of interviews with expert dermatologists and patients (Table 2). 27
Table 2.
Assessment Scores for AA
| Score | Components |
|---|---|
| SALT score | S0: no hair loss S1: 1% to 24% hair loss S2: 25% to 49% hair loss S3: 50% to 74% hair loss S4: 75% to 99%% hair loss S4A: 75% to 90% hair loss S4B: 91% to 99% hair loss S5: 100% hair loss |
|
ClinRO Measure for Eyebrow Hair Loss (both eyebrows from 2 feet away) |
0 The eyebrows have full coverage and no areas of hair loss 1 There are minimal gaps in eyebrow hair and distribution is even 2 There are significant gaps in eyebrow hair or distribution is not even 3 No notable eyebrow hair |
|
ClinRO Measure for Eyelash Hair Loss (upper and lower eyelashes of both eyes) |
0 The eyelashes form a continuous line along the eyelids on both eyes 1 There are minimal gaps and the eyelashes are evenly spaced along the eyelids on both eyes 2 There are significant gaps along the eyelids or the eyelashes are not evenly spaced along the eyelids 3 No notable eyelashes |
|
ClinRO Measure for Nail Appearance (based on fingernails + toenails) |
0 Nails are not at all damaged (e.g., pitted, rough, brittle, split) 1 At least 1 nail is a little damaged (e.g., pitted, rough, brittle, split) 2 At least 1 nail is moderately damaged (e.g., pitted, rough, brittle, split) 3 At least 1 nail is very damaged (e.g., pitted, rough, brittle, split) or subject has lost at least 1 nail |
| Alopecia Areata Progression Index | [%AA(L) ×SL(L) ×.18] + [%AA(R) ×SL(R) ×.18] + [%AA(T) ×SL(T) ×.4] + [%AA(B) ×SL(B) ×.24] %AA= % of surface area on the scalp: L = left, R = right, T = Top, B = bottom SL = Score of hair loss over 5 times: 0 = negative pull test, 1 = 10 to 20%, 2 => 20% of grasped hairs |
Limitations of these scores include the lack of incorporation of disease activity or progression. One tool that incorporates the pull test is the Alopecia Areata Progression Index, where the surface area of active disease is multiplied by the degree of pull test positivity in each of 4 scalp quadrants (Table 2). 28
Quality of Life
There are several different assessment tools that can be used to qualify the impact of AA on quality of life (QOL). 29 Some challenges in characterizing impact include the frequent asymptomatic nature of the disease, in contrast to other dermatologic conditions such as atopic dermatitis, psoriasis, and hidradenitis suppurativa.
AA-specific Health-related Quality of Life Instruments in include the AA Patients QOL (AAQ, 7 items directed at the past month), the AA QOL Index (AA-QLI, 21 items directed at the past month), and the AA Symptom Impact Scale (AASIS, 13 items directed at the past week). Hair-disease specific instruments have also been created, including the hairdex (48 items), the Hair-specific Skindex-29 (29 items), and the Scalpdex (23 items).
Although a single optimal tool has not been identified, the EADV task force suggested the use of the dermatology-specific DLQI questionnaire, hair disease-specific Scalpdex and either the AASIS or AA-QLI for the time being. 29
Treatments
Regrowth in patches of alopecia in AA in the absence of active treatment is well recognized, and likely affected by subtype of disease. Estimates of spontaneous regrowth can be calculated based on data from placebo-controlled trials of patients with AA. 30 In patients with patchy AA (10 studies, n = 264), regrowth occurred in 0% to 30.0%, with a pooled rate of 6.4% (CI:1.4-13.8) over a period of 1.5 to 11 months. In patients with severe disease (10 studies, n = 274): regrowth was reported in 0% to 33.3%, with a pooled rate of 7.2% (CI: 2.6-13.2) by 12-24 weeks.
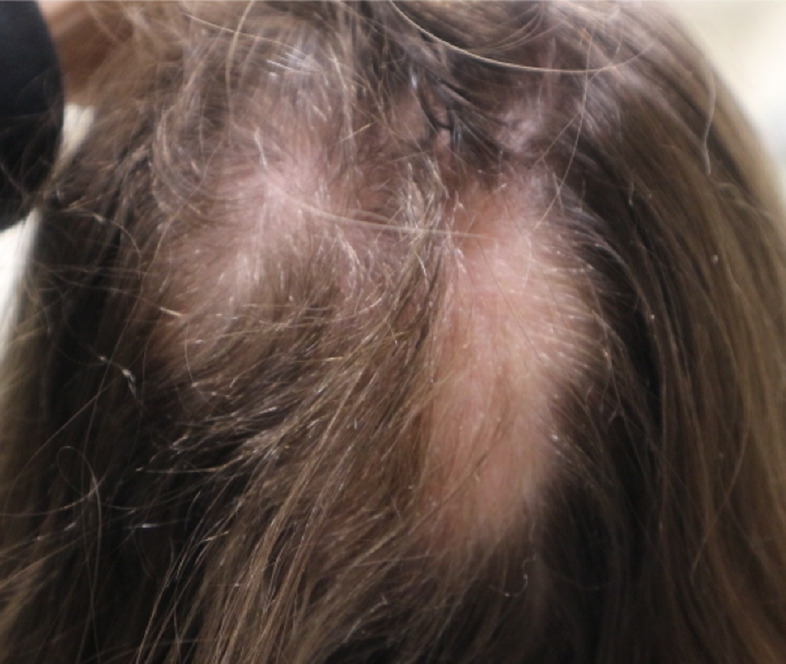
Hair may initially regrow without pigment (Figure 3). Electron microscopy analysis of these regrowing nonpigmented hairs reveals fewer melanocytes and decreased melanization. 31 This could be related to partial or incomplete melanocyte activation in early anagen of these follicles when there is still residual disease activity.
Figure 3.
Patch type with non-pigmented hair regrowth
Camouflage
There are many different types of camouflage that can be offered and used by patients with AA. These include hairpieces (wigs, demiwigs, wiglets, toupees, cascades), hair thickening fibers, concealing powders. 32 Semipermanent options include scalp micropigmentation which is a tattoo applied in a stippling pattern to mimic hair follicles. Realistic non-traumatic sticker tattoos can be useful for eyebrow regions and are a painless option in those who do not want a permanent option. 33
Microblading is an increasingly popular method of instilling pigment into the skin using a device and blade with stacked needles. 34 It is most commonly used to reproduce the appearance of eyebrows. Rare but concerning complications that have been reported include granulomatous reactions, sarcoid, allergic contact dermatitis, and preseptal cellulitis.35-38
Complementary and Alternative Medicines
A variety of different complementary and alternative treatments have been proposed to help treat AA as monotherapy or adjuncts. The best evidence exists for certain mixes of essential oils, topical garlic and oral glucosides of peony with compound glycyrrhizin. 39 Essential oils are thought to provide anti-oxidant and anti-inflammatory effects. Two double blinded randomized controlled trials evaluated the use of a mixture of essential oils in adults with patchy AA compared to carrier oils alone. In the first study (n = 84), the combination of Thyme oil, rosemary oil, lavender oil, and Atlas cedarwood oil was association with 44% efficacy compared to 15% in the control group at 7 months. 40 The second evaluated the same combination with the addition of evening primrose oil in 40 patients, with 60% achieving at least moderate regrowth at 12 weeks with the intervention compared to 30% in the carrier oil group. 41
Garlic may act as a topical irritant to trigger a contact dermatitis and distract the immune system from the hair follicle. 39 Its use in a 5% gel formulation in combination with betamethasone twice daily was reported to confer better efficacy as compared to betamethasone alone in a study of 40 children and adults with patchy AA at 3 months (95%, 5%, P = .001). 42 However, a subsequent trial of garlic alone in children was terminated early because of lack of efficacy, suggesting more data is needed to clarify the context in which it may work best. 43
Finally, Glycyrrhizin is a plant extract that has been demonstrated to lower serum levels of Th17 cells, IL-6, IL-17, IL-22 and TGF-beta. 44 Two RCTs evaluated its use with vitamin B2, with and without the addition of glucosides of peony, one in adults with AA and >75% loss and one in pediatric patients with >50% loss. Marked regrowth was reported in 68.2% versus 71.4% of adults after 3 months, with non-significant superior regrowth in the combination group. 45 In children, marked regrowth was reported in 35% vs 45% at 3 months, with the combination group having statistically significantly higher regrowth rates. 46 On average, adverse effects were reported in 15% of patients across all groups in both studies, the most common being edema and weight gain.
Micronutrient Supplementation
Many patients and providers advocate for supplementation of micronutrients in the treatment of AA. Although data supports an association between lower levels of serum vitamin D, zinc, and folate levels in patients with AA as compared to controls, there is a lack of data evaluating the benefit of supplementation of these. 47
Anthralin
Anthralin is a contact irritant that has been used for many years to treat AA (Table 3). Treatment regimen vary greatly, with initial frequencies ranging from twice weekly to daily, and concentrations of 0.5% or 1% used in different vehicles. Most regimens involve gradual uptitration of frequency or contact duration until a target of skin irritation is achieved. In 4 studies of children with AA, the use of anthralin was associated with complete response rates between 32% and 33.3% with relapse rates of 9.5% to 64%. 48 In a trial of 60 patients over 13 years with patchy AA, anthralin application for 12 weeks was associated with complete response in 56% compared to 53.3% of a comparator group treated with azelaic acid. 49 Side effects of anthralin include staining of skin, regional lymphadenopathy, itching, burning, oozing, and bullous eruptions. 48 Although targeted, irritation can be treatment limiting, resulting in discontinuation of up to 11% of patients in some cohorts. 50
Table 3.
Topical Treatments for AA: Highest Level of Evidence and Select Studies With Outcomes
| Medication | Highest level of evidence | Dose/Frequency | Efficacy | Side effects |
|---|---|---|---|---|
| Anthralin | LoE 4: 1 prospective study (n = 31) 2 case series (n = 67), case reports |
Anthralin 0.5 to 1% Titrated in frequency +contact time to target mild-moderate skin irritation |
Complete regrowth: 32 to 56% |
Staining of skin itching, burning, oozing bullous eruptions Lymphadenopathy |
| Contact Immunotherapy | LoE 1: Pooling of 45 studies (n = 2227) |
Diphenylcyclopropenone 2% in acetone to a 4 × 4 cm area on scalp then after 2 weeks: 0.001% dilution 1 x/week, increasing concentration to a goal of mild eczema |
Complete regrowth: 42.6% Major regrowth (>70%): 56.1% |
Severe eczema: 30.8% Lymphadenopathy: 25.7% Generalized eczema: 16% Hyperpigmentation: 12.7% Influenza-like illness: 11% Contact urticaria: 9% Folliculitis: 7.1% |
| Calcipotriol | LoE 2: 1 prospective study (n = 22) Retrospective review (n = 48) Combinations: Randomized open label trial (n = 60) Comparative study (n = 100) Comparative study (n = 60) |
0.005% lotion or cream twice daily 0.005% cream BID with clobetasol 0.005% cream BID with mometasone 0.005% / betamethasone diproprionate BID |
12 week: SALT50: 46.2% (6/22) SALT100: 9% (2/22), 27.1% (13/48) 3 month change in SALT: 4.5 (combo) vs 3.1 (clobetasol alone) 2.92 (combo) vs 2.42 (mometasone alone) 53.6% combo vs 48.6% (clobetasol alone) |
Irritation, erythema, pruritus, pigmentation, folliculitis: 31.8% (7/22) None Folliculitis (2%, 4%), erythema (2%), dermatitis (2%), atrophy (2%, 4%); Not specified (“fewer side effects” with calcipotriol) |
| Tacrolimus | LoE 3 Comparative studies and open label studies |
0.1% ointment twice daily | Good regrowth: 0 to 17% | None reported |
| Corticosteroids | LoE 2 RCT, prospective, single blind (n = 42) RCT (n = 70) |
Multiple options Clobetasol BID vs Hydrocortisone BID Desoximetasone 0.25% BID |
Response at wk 24: Clobetasol 85% (vs Hydrocortisone 33%) 3 month CR: 58% (vs placebo 39%) |
Clobetasol: Atrophy: 5% (n = 1/20) Irritation/acne: 12% |
| Prostaglandin Analogs | LoE 3 Retrospective review (n = 41) Prospective studies (n = 17, N = 71 72 Prospective unblinded study (n = 44) |
Bimatoprost 0.3 mg/ml solution daily or BID latanoprost 0.005% once daily | Eyelash growth at 1 yr: CR: 24% (10/41) Increased length/thickness: 2 year regrowth: CR: 17.5%, none: 25% |
Conjunctivitis: 7.3% |
Abbreviations: CR, complete regrowth; PR, good/moderate regrowth.
Levels of Evidence (LoE):
Level 1 (systematic review of randomized controlled trials [RCTs] or high-quality randomized controlled trial)
Level 2 (lesser quality RCT or prospective cohort study) level 3 (case-control study, non-randomized controlled cohort or follow-up study)
Level 4 (case series), or level 5 (expert opinion, mechanism-based reasoning).
Contact Immunotherapy
Topical application of sensitizing chemicals on patches of alopecia has been used extensively to treat AA (Table 3). The outcome of induced contact dermatitis on applied areas has been shown to induce apoptosis of perifollicular lymphocytes, resulting in regrowth in AA patches. 51 The most commonly reported agents are diphenylcyprone and Squaric acid dibutyl ester. In a meta-analysis of 45 studies (n = 2227 patients), complete regrowth was reported in 42.6% of patients with patchy alopecia, with major regrowth defined by >70% in 56.1%. 52 Better outcomes were associated with SALT scores < 50, lack of atopic dermatitis, allergic rhinitis, or asthma), and lack of nail involvement. Adverse effects include severe or generalized eczema, lymphadenopathy, flu-like symptoms and contact urticaria (Table 2). Recurrences were reported in 48.5% of patients (n = 637).
Corticosteroids
Steroids are used in topical, injectable, and systemic formulations in the treatment of AA (Tables 3 and4). A large range of different topical corticosteroids are used in practice in varied regimens, and often in combination with other topical or systemic treatments. Few studies have formally evaluated the efficacy of topical steroids as monotherapy.48,53-55 In pediatric patients with AA, response is as high as 85% with clobetasol monotherapy after 24 weeks compared to 33% with hydrocortisone (n = 40), with atrophy reported in 5% (n = 1). 56 In 70 patients older than 15 years, dexosimetasone twice daily resulted in full regrowth in 58% at 3 months compared to 39% of patients applying placebo (n = 53). 55 Adverse effects included acneiform eruptions, burning and irritation in 12% in the corticosteroid group but no atrophy.
Table 4.
Systemic Treatments for AA With select studies, regimens and outcomes of interest.
| Medication | Highest level of evidence | Select dosing/ Frequency | Efficacy | Side effects |
|---|---|---|---|---|
| Minoxidil | LoE 2 retrospective study (n = 12 adults with tofacitinib) |
2.5 mg PO daily (F) 2.5 mg PO BID (Male) |
>75% SALT: 67% (combo with tofacitinib) |
Hypertrichosis 50% |
| Antihistamines |
LoE 2 1 RCT (fexofenadine) 3 clinical trials 3 retrospective reviews 2 case series 3 case reports |
Fexofenadine 60-120mg/day Ebastine 20 mg/da y Oxatamide 60 mg/day |
Hair regrowth Decreased erythema and pruritus |
Headache with fexofenadine (n = 2) Mild sedation with ebastine |
| Systemic Coticosteroids |
LoE 1 PULSE DOSING Any age: 41 studies (n = 1078) including 1 randomized placebo-controlled trial Children: 8 studies (n = 216) |
Dexamethasone 2.5-5mg x2days/wkMethylprednisolone 500 mg-1g x1-3 days/month Dexamethasone 1.5 mg/kg/day x1-3days/month Methylprednisolone 8-30mg/kg/day x1-3 days/month |
CR: 43% (466/1078) >50 regrowth:56% |
Total SEs: 21% (225/1078) Acne: 0.01% (3/216) GI discomfort (7/216) Headache: 8/216 Cushings: 0.01% (2/216) Allergic reaction |
| Methotrexate | LoE 1 Adults: 11 studies (n = 361) Children: At least 6 studies (n = 68) |
Adults: 15-25mg/week Children: 7.5-25mg/week (0.2, 0.4mg/kg/week) |
Complete response: 45.7% (165/361)Good/complete response: 70.3% (154/219) Good/complete response:50% (34/68) |
Total AEs: 24.2% (64/319) (Gastrointestinal symptoms, liver and hematologic abnormalities, all < 5%) Total AEs: 14.5% (6/44) |
| Cyclosporine | 14 studies (n = 340) | Cyclosporine monotherapy: 5-6mg/kg/day Cyclosporine with systemic steroid: 2-4mg/kg/day |
Response rate at end of treatment: CsA alone: 69.41%; (0 to 80%) CsA with steroid: 57.02% (6.67 to 100%) |
Overall AEs: 36.8% (125/340) Gastrointestinal: n≥28 Hypertrichosis: n≥20 Hypertension: n≥9 Dyslipidemia: n≥8 Headache: n≥7 |
| Azathioprine | LoE 1 Open-label pilot study (n = 20) Open-label, randomized comparative study (n = 50) |
Azathioprine 2 mg/kg/day Azathioprine (Aza) 300 mg weekly vs Betamethasone (BM) 5 mg/dy x 2 days/wk |
6 months: SALT < 25%: 50% 4 months: Change in SALT: Aza: 9.5 (0-13.5) BM: 14 (4-19) |
Transaminitis: 5% (n = 1) Leukopenia: 15% (n = 3) Nausea/Vomiting: 5% (n-1) Aza: nausea: 35% BM: facial puffiness: 76.2% wt gain 33%, GERD 24%, Acne 24%, striae 9.5% |
| Dupilumab (IL4/13 inhibitor) |
LoE 1 Randomized double blind placebo-controlled trial (n = 40) |
300 mg subcutaneous once weekly | Week 24 SALT £30: Dupilumab: 17.5% Placebo: 10% |
Week 24: Injection site reaction: 5% Conjunctivitis: 7.5% URTI: 5% |
| Tofacitinib (JAK1/3 inhibitor) |
LoE 2 Open label comparative study, Case series |
5 mg PO BID | Month 6 SALT50: 78.4% |
URTI 8.1% Folliculitis 10.8% Headache: 5.4% wt gain: 5.4% Triglyceride increase 5.4% |
| Baricitinib (JAK1/2 inhibitor) |
LoE 1 2 randomized placebo-controlled phase 3 trials (n = 654 + 546) |
2 or 4 mg PO daily | Week 36 SALT ≤20: 4 mg: 38.8% 2 mg: 22.8% Placebo: 6.2% |
Acne: 4.7%,5.8% Zoster: 0.9%, 1.9% HSV: 0%, 3.9% CK increase: 0%, 5.7% LDL increase: 20.5%, 30.3% |
| Beprocitinib (TYK2, JAK1/2 inhibitor) |
LoE 1 Phase 2 a RCT (n = 47) |
60 mg PO daily x 4 wks then 30 mg daily x 20 weeks | Week 24 SALT 50: 53.2% |
URTI/Nasopharyngitis 32% Acne 11% Headache 9% *Rhabdomyolysis in 2/47 |
| Ruxolitinib (JAK1/2 inhibitor) |
LoE 2 Open label comparative study |
2 ng PO BID | Month 6 SALT50: 84.2% |
Urinary tract infection 13.2% Headache 5.3% Folliculitis 2.6% wt gain 2.6% AST/ALT increase 7.9% |
| Deuroxolitinib (deuterated ruxolitinib) |
LoE 2 Phase 2 clinical trial in adults (n = 140) |
4 mg PO BID or 8mg PO BID or 12mg PO BID |
Week 24 SALT ≤50% 12 mg: 58% 8 mg: 47% 4 mg: 21% Placebo: 9% |
Acne: 13.48-16.7% Headache: 17.2- 19.4% CK increase: 10.3%, 5.3%, 2.8% LDL increase: 0, 10.5%, 0 |
| Ritlecitinib (JAK3 inhibitor) |
LoE 1 Phase 2 a RCT(n = 48) |
200 mg PO daily x 4 wks then 50 mg daily x 20 weeks | Week 24 SALT 50: 39.6% |
URTI/Nasopharyngitis 21% Acne 10% Headache 13% Folliculitis 6% |
Abbreviations: CR, complete regrowth; PR, good/moderate regrowth.
Levels of Evidence (LoE):
Level 1 (systematic review of randomized controlled trials [RCTs] or high-quality randomized controlled trial)
Level 2 (lesser quality RCT or prospective cohort study)
level 3 (case-control study, non-randomized controlled cohort or follow-up study)
Level 4 (case series), or level 5 (expert opinion, mechanism-based reasoning).
Intralesional injection of corticosteroids has been reported to lead to better responses compared to topical steroids.57,58 The most commonly used steroid for this purpose is triamcinolone acetonide, although betamethasone diproprionate use has been reported and may be associated with fewer adverse effects.59,60 Different concentrations have been used, but comparative studies reported no increased efficacy of higher concentrations (10 mg/ml) versus the more commonly used 5mg/mL. 61 Another method of triamcinolone administration is microneedling delivery, although this was not shown to offer any efficacy advantage over intradermal injections in a prospective randomized trial of 60 patients. 62
Systemic steroids have been used in a variety of regimens including intravenous or oral, and pulses or continuous administration. 63 A systematic review of pulse systemic steroids in adults and children with AA included 41 studies (1078 patients), of which only 1 was randomized controlled study. 63 Overall, complete response was reported in 43% (466/1078) of all patients, with relapses reported in 17%. In this review, pediatric patients had slightly higher response rates (51%) but also higher relapse rates (60%). Side effects were reported in 21% (n = 225) of all patients, and 12% of children (n = 8). In a subsequent pediatric review of 8 studies (216 children), 56% reported >50% regrowth of scalp hair. 64
Prostaglandin Analogs
Bimatoprost and latanoprost are prostaglandin analogs available in solutions for ophthalmic instillation that were noted to cause hypertrichosis when used to treat glaucoma. 65 This is likely related to their action on prostaglandin F receptors expressed in eyelash hair follicles in the dermal papilla and outer root sheath. 66
In prospective studies, latanoprost use on eyelids was associated with complete eyelash regrowth in 17.5% of 40 patients, and no response in 25% after 2 years. 67 On the scalp, monotherapy was less effective than betamethasone diproprionate in 50 adults with scalp AA after 16 weeks. 68 When added to clobetasol in a study of 30 patients with scalp AA, its use was associated with significantly increased hair density (37.2 +/- 26.1 vs. . 14.6 +/- 18.6) and regrowth (58.3 +/- 39.3 vs. . 21.6 +/- 24.1) compared to clobetasol alone at 12 weeks. 69 Similarly, the addition of latanoprost to betamethasone was associated with better SALT score reductions at a randomized controlled trial of 108 patients after just 2 weeks. 70 Of note, latanoprost is associated with irreversible iridial pigmentation at a high frequency (6.3% of patients at 1 month, 15.7% at 3 months, 37.8% at 6 months, and 56.5% at 12 months). 71
The efficacy of bimatoprost has been limited in individuals with AA. A prospective study of pediatric patients with eyelash loss secondary to AA or chemotherapy (n = 71) did not demonstrate a treatment benefit in the AA subgroup. 72 Another prospective study of adults with AA (n = 17) reported increase in length and thickness of eyelashes at 4 months, although there was no growth of new eyelashes. 73 Conversely, a retrospective study of 41 patients with AU reported complete regrowth in 24% after 1 year, with a mean time to regrowth of 4-8 months. 74 Application on patches on the scalp twice daily was associated with regrowth, with better response at a faster rate compared to mometasone in a study of 30 adults with S1 patchy disease. 75
Calcineurin Inhibitors
Topical tacrolimus, a calcineurin inhibitor, is associated with decreasing several cytokines including IL-2, IFN-gamma and TNF-alpha. 76 Its use has been evaluated in several studies and case reports in scalp AA with disappointing results overall. Good regrowth has been reported in 0 to 17% of treated patients, with inferior rates compared to topical steroids in several studies.58,77-79 It is typically administered twice daily. Side effects are minimal, with none reported in several studies (Table 3).
Calcipotriol and Intralesional Vitamin D
Calcipotriol is a vitamin D analog that has been used topically with reported success in patients with AA. Vitamin D receptors (1,25-(OH)2) are known to be expressed in hair follicles, with a decrease in this expression reported in active alopecia areata patches. 80 Calcipotriol is also proposed to promote a shift in phenotype from Th1 to Th2. 81 In a prospective study of 22 adults with limited patchy AA, total regrowth was achieved in 9% at 12 weeks, and SALT50 achieved in 46.2% (6/22). 82 Better response was associated with lower baseline vitamin D levels. In a retrospective review of 48 patients with mild-moderate AA (S1 or S2) who had failed topical steroids, calcipotriol monotherapy was associated with total hair regrowth in 27.1% (13/36) at 12 weeks, with 10 patients remaining free of recurrence at 1 year. 83
There are also comparative studies reporting improved responses with the combination of calcipotriol with potent corticosteroids, including mometasone and clobetasol as compared to monotherapy with the same corticosteroids.77,84,85 Another study in 60 adults compared the combination of calcipotriol with betamethasone diproprionate to clobetasol, with a statistically small but significant improvement noted with the combination product after 3 months (53.6% mean SALT improvement vs 48.6%). The vast majority of patients in all groups in these studies were noted to have low vitamin D levels.
Intralesional Vitamin D has also been studied as a potential treatment for AA. In a randomized placebo-controlled trial of 60 adults with patchy AA, 1 ml of Vitamin D injected intralesionally every 4 weeks for up to 3 sessions was associated with a regrowth score of 4 in 53% (n = 16) compared to 0% in patients receiving saline injections. 86 Adverse effects included pain during injection (66.7%, 60%), pinpoint bleeding (33.3%, 43%) and vasovagal attack (4%, 0%) in patients treated with vitamin D vs controls.
Cyclosporine
Cyclosporine is a systemic calcineurin inhibitor usually given orally twice daily. Its use in AA was evaluated in a systematic review of 14 studies and 340 patients, excluding case reports 87 (Table 4). Overall, 65% of patients were reported to respond to cyclosporine, with increased response rates in patients receiving concurrent systemic corticosteroids compared to without (69.4%, 57.0%). Adverse effects were reported in 36.8%, including gastrointestinal symptoms, hypertrichosis, hypertension, dyslipidemia and headache. Factors associated with decreased incidence of relapse include concurrent corticosteroid treatment, increased duration of cyclosporine treatment more than 6 months, but not the daily dose. 88
Azathioprine
Azathioprine, a purine antagonist, is known to impair T-cell function and activation. In an open-label pilot study, 20 patients with AA affecting ≥20% of the scalp were treated with azathioprine monotherapy at 2mg/kg/day. 89 After 6 months, 2 patients had complete regrowth and 8 patients had <25% of the scalp affected. Side effects included increased liver enzymes in 1 patient requiring discontinuation, mild intermittent leukopenia in 3 patients, and nausea/vomiting in 1 patient. Subsequently, an open-label comparative clinical trial in 50 adults with AA and >10% scalp involvement evaluated the addition of azathioprine to twice weekly systemic betamethasone. After 4 months, the median percent scalp hair regrowth and the median change in SALT score was 44.52 and 9.5 in WAP group compared to 71.43 and 14 in betamethasone group. 90
Methotrexate
Methotrexate, a folic acid antagonist, has been demonstrated to inhibit upregulation of IL-15 and decrease levels of IFN-gamma and IL-2 in peripheral blood mononuclear cells. 91 There is evidence for the use of methotrexate as both monotherapy and combination with steroids in treating AA in children and adults (Table 4). In a systematic review that included 11 studies of adults with AA, complete response was reported in 45.7% (165/361) and good or complete response in 70.3% (154/219). 92 Combination treatment of methotrexate with corticosteroids resulted in good or complete response in 72.7% (56/77) compared to 48.6% (18/37) without. Onset of hair regrowth based on 6 studies was 3.13 (95% CI 2.3-4.0) months, and recurrence rate based on 9 studies was 52% (100/192). Adverse effects were reported in 24.2% (64/319), with gastrointestinal symptoms being the most common as well as liver enzyme or hematologic abnormalities in <5% of patients.
There have also been several studies in children treated with methotrexate. Overall, methotrexate has been associated with lower response rates compared to adults, but also fewer recurrences after stopping and fewer side effects. Pooled data from 5 studies in children treated with methotrexate reported good or complete response in 50% (34/68). 93 An additional retrospective review reported partial response in 4 of 7 children treated with MTX. 94 Recurrence rate in children based on 4 studies was 31.7% (9/30), and adverse effects were reported in 14.5% (6/44). 92
Janus Kinase (JAK) Inhibitors
JAK inhibitors are small molecules that interfere with the signaling of multiple cytokines that are implicated in AA including interferon-γ and interleukin-15. 95 Several JAK inhibitors have been used to treat AA in both topical and systemic formulations (Table 4).
Tofacitinib is a selective JAK1/3 inhibitor that has been used to treat patients with AA in multiple different topical formulations and concentrations. In general, results with topical formulation on scalp hair are unpredictable and do not commonly result in complete hair regrowth, likely related to suboptimal absorption and penetration to the level of the inflammatory infiltrate in AA. 96 The use of tofacitinib has also been reported for eyelash and facial hair growth. In a series of 119 patients, topical tofacitinib was associated with complete regrowth of eyebrows and eyelashes in 41% and 46% of patients respectively. 97 Systemic treatment has been reported in many case series in both adults and children.98-103 In a meta-analysis of 14 studies of patients treated with tofacitinib including 275 patients, pooled good/complete hair regrowth rate was reported in 54.0% (95% CI: 46.3% to 61.5%), and pooled partial response was 26.1% (20.7 to 32.2%). 104 Adverse effects from an open label study of 5 mg BID included folliculitis (10.8%), headache (5.4%) and weight gain (5.4%). 105 Weight increase has been postulated to result from leptin inhibitors wth some JAK inhibitors. 106 Relapse with discontinuation was reported in 25% of patients. Sublingual tofacitinib may offer increased absorption and decreased gastrointestinal side effects by bypassing hepatic first-pass metabolism. 107
Ruxolitinib is a selective JAK1/2 inhibitor available in a 1.5% cream formulation with diasppointing data for treatment of AA. Its efficacy was evaluated in 78 patients aged ≥18 years with AA characterized by a baseline SALT of ≥25. 108 After 24 weeks of twice daily application, there was no significant difference in rates of 50% improvement in the ruxolitinib group compared to the group applying vehicle alone; no significant adverse effects were noted. Conversely, systemic administration is effective. In a study of adults, 20 mg BID was associated with SALT50 in 84.2% of 38 participants, with main adverse events including urinary tract infections (13.2%), headache (5.3%), elevated liver enzymes (7.9%), folliculitis (2.6%) and weight gain (2.6%). 105
There is some data reporting inferiority of topical JAK inhibitors to topical steroids in AA. A double blind, placebo-controlled pilot study evaluated 1% topical ruxolitinib, 2% tofactinib, clobetasol and placebo to 4 different locations in 16 adult patients with alopecia universalis (bilateral temples and eyebrows). 109 After 12 weeks, there was good regrowth in 10 locations treated with topical clobetasol compared to 6 locations with tofacitinib, 5 with ruxolitinib and none with placebo application.
Baricitinib, a selective reversible inhibitor of JAK1 and 2, was recently approved by the FDA for adult patients with severe alopecia areata. The approved dose is 2 mg PO daily which can be increased to 4 mg. If patients have severe AA or involvement of eyelashes and eyebrows, treatment can be initiated with 4 mg and decreased to 2 mg once response has been achieved. The efficacy was demonstrated in a phase 3 randomized double blinded placebo-controlled trial in which more than 30% of patients achieved a SALT score ≤20 after 36 weeks of treatment. 110 Participants were aged 18 yrs or older with a baseline SALT of ≥50. Eyelash and Eyebrow ClinRO scores of 0-1 were reported in 36.2 to 36.8% and 35.2 to 38.9% of patients taking 4 mg of bariticinib daily at 36 weeks. Adverse effects included acne, infections, and LDL and CK elevations (Table 4). The likelihood of response has been shown to be influenced by the duration of alopecia, with patients who have not had growth in 10 years or more being less likely to respond.
Ritlecitinib, an oral JAK3 and TEC (tyrosine kinase expressed in hepatocellular carcinoma) inhibitor, and Beprocitinib, a TYK2/JAK1 inhibitor, have also been studied in AA patients. A phase 2 a study randomized adults with AA and a SALT of ≥50 to daily ritlecitinib (n = 48), beprocitinib (n = 47) or placebo (n = 47). At 24 weeks, a SALT score ≤30 was achieved by 50% of patients receiving ritlecitinib, 64% receiving brepocitinib, and 2% receiving placebo. 111 Subsequently, 5 dosing regimens of Ritlecitinib (30 mg or 50 mg daily with or without a 200 mg loading dose or 10 mg daily) were evaluated in a Phase 2b/3 placebo-controlled study. 112 After 24 weeks, efficacy was demonstrated in a dose-dependent manner, with the highest dosing regimen associated with a SALT score of ≤20 in 38% of participants at 24 weeks, compared to 1% of those receiving the lowest dose.
Interim results from the phase 3 open-label, multi-center long term study of ritlecitinib were presented orally at the EADV in September 2022 (ALLEGRO-LT), with 62.5% of 56 patients having a SALT ≤ 10 at 24 weeks, and 69.6% having a SALT ≤ 20. 113 There were 447 patients included in the safety analysis with mean duration of treatment of 487 days ± 167, with 85.7% of patients being treated for at least 12 months. The most common adverse effects were headache (16.3%), acne (11.6%), and nasopharyngitis (8.5%). Herpes zoster was reported in 0.9%, and 13.4% had a positive SARS-COV-2 test, with serious infection in 2 patients (0.4%).
Deuruxolitinib (CTP-543) is a deuterated form of ruxolitinib. A dose ranging phase 2 trial was performed in 140 adults with AA for 6 months or longer, which reported SALT score decrease of 50% or more in 58% of participants receiving the highest dose (12 mg BID) amd 21% of those receiving 4 mg BID. 114 Adverse effects included acne and headache in a dose dependent fashion (13.5 to 16.7% and 17.2 to 19.4%). More participants had CK increases in the lowest dose (10.3%) and LDL increased were highest with 8 mg BID (10.5%), but this was a relatively small study. There were no episodes of bleeding or thrombosis.
Minoxidil
Minoxidil is an antihypertensive that has been available in topical formulations and approved for androgenetic alopecia treatment since the 1990s. Several mechanisms of action in alopecia have been postulated including hair follicle aATP-dependent potassium channel activation, stimulation of adenosine dependent VEGF, prolongation of anagen and shortening telogen phases, and vasodilation leading to increase cutaneous delivery of oxygen and growth factors to hair follicles. 115 It may also suppress Tcell functioning. 116 It is activated to a sulfate form by a sulfotransferase in the hair follicle, thus patients with high activity of this enzyme may exhibit a higher response. 117 In support of this theory is the benefit afforded with use of a newer formula that increases the activity of this enzyme in regrowing hair for males with androgenetic alopecia. 118 Multiple studies support efficacy of the 5% formulation in children and adults with patchy AA. 119 In a meta-analysis that included 6 studies (n = 255), minoxidil use was associated with significant regrowth rates compared to placebo of 7.86 (95% 3.74,16.5). 119 Commercially available products include foam and solution vehicles with concentrations of 2 to 5%. The foam concentration has been associated with less hypertrichosis in men with androgenetic alopecia, and the propylene glycol in the solution has been associated with both irritant and allergic contact dermatitis.120-124 Much less common side effects reported include acquired trichorrhexis nodosa (possibly more notable in curly hair), headaches, edema, and rarely pericardial effusion.
Low dose systemic (oral) minoxidil has been used with increasing frequency in the past few years to treat a large range of alopecias (Table 4). In a case series of patients with AA, efficacy of low dose oral minoxidil ranged from 18 to 82.4%. 116 Side effects reported with the use of minoxidil use for many different types of alopecia include hypertrichosis in up to 24%, pedal edema in 2%, postural hypotension in 1.1% and heart rate alterations in 1.3% of patients. 125 Many have adopted the practice of giving minoxidil in combination with other systemic agents, a clear advantage being the lack of added immunosuppression in these cases. A small retrospective review of 12 patients with AA who were prescribed minoxidil 2.5 mg daily (females) or BID (men) with tofacitinib, 67% achieved 75% scalp regrowth, with the most common side effect being hypertrichosis in 50%. 126 Pediatric use has also been described: in a series of 63 children aged 0-12yrs treated with 0.025-0.5mg daily, side effects included facial/back hypertrichosis (n = 13), postural hypotension (n = 4) and headaches (n = 2). 127 Accidental exposure to higher doses (mean dose 0.9 mg/kg/day) to 20 children aged 2 months-13 years through contaminated omeprazole has also allowed review of safety. 128 In this cohort, systemic side effects were reported in 3 patients (diarrhea/anxiety in 1, headache/facial edema in 1, and asthenia in 1), and hypertrichosis in 13 patients (65%).
Antihistamines
Several antihistamines have been reported to help patients with AA (Table 4). Proposed mechanisms include targeting perifollicular mast cells that are increased in subacute AA and stimulated by CD8 +tcells and IFNgamma. 129 A recent systematic review included 343 patients treated with various antihistamines: all reported, decreased pruritus and erythema, but only fexofenadine, oxatamide, and ebastine were associated with hair regrowth. 130
Hydroxychloroquine
Hydroxychloroquine is a systemic antimalarial known to affect the immune system via interference with Toll-like receptors and prevents activation of interferon-1. 131 Its efficacy as treatment of AA was reported in 9 children who received doses of 50-400mg daily for 4-24 months, with partial regrowth in 56%, and none achieving full regrowth. 132 Adverse effects included abdominal pain and headache.
Apremilast
Apremilast inhibits phosphodiesterase 4, resulting in a decrease in the production of multiple pro-inflammatory cytokines. Its lack of immunosuppressive effects confer a favorable side effect profile when compared to traditional immunosuppressants. Unfortunately, it was not effective in a small pilot study of 30 patients who were randomized to receive apremilast (n = 20) or placebo (n = 10) orally for 24 weeks. 133 Only 1 patient achieved a 50% reduction in SALT at 24 weeks, and 8 patients withdrew from the apremilast arm due to lack of effect or side effects.
HMG-CoA Reductase Inhibitors
HMG-CoA Reductase Inhibitors (statins) have been demonstrated to decrease proinflammatory cytokines, including IFN-g, TNF-a, IL-1b, and IL-6, which contribute to AA disease. 134 A combination of simvastatin and ezetimibe in a series of patients with AA affecting 40 to 70% of the scalp reported regrowth in 14 of 19 patients who completed 24 weeks of treatment. 135 However, in 2 other subsequent studies, there was no significant regrowth reported, calling into question the potential efficacy of this combination treatment.136,137
Dupilumab
Dupilumab is a monoclonal antibody targeting IL-4 and IL-13, leading to downregulation of Th2-mediated inflammation. The role of Dupilumab in the treatment or triggering of AA continues to be studied, with cases of regrowth of AA in patients with atopic dermatitis contrasted with cases of new onset AA in others. 130 One possible but unproven explanation for the divergent responses to dupilumab involves considering AA in four classifications described by Ikeda. 138 In patients with severe atopic dermatitis and AA that is being perpetuated by massive interferon release, control of the dermatitis may be enough to allow for resolution of the alopecia. Conversely, in patients without preexisting AA, treatment with dupilumab may lead to a shift in the immune system, triggering alopecia areata mediated by autoreactive CD8 +T cells.
A randomized placebo-controlled trial evaluated the effect of weekly dupilumab for 48 weeks in 40 patients aged 18 years or older with alopecia areata with a minimum of 30% hair loss for 6 months. 139 Patients with increased baseline IgE and a personal or family history of atopy were more likely to regrow compared to controls (Table 4). Side effects included conjunctivitis (7.5%, 0%), injection site reactions (5%, 0%), and Upper respiratory tract infections (5%, 5%) in dupilumab compared to placebo groups at 24 weeks. In children, a recent case series of 16 patients with both atopic dermatitis and AA who were treated with dupilumab reported good regrowth in 4 of 8 children who had follow-up at 4 months. 140
Secukinumab
Secukinumab is a fully human monoclonal IgG Antibody that inhibits IL-17A and F. Its use to treat AA was evaluated in a double-blinded, randomized prospective pilot study of 11 subjects, but none of the 7 participants achieved the primary outcome of SALT50 at week 24. 141 No adverse events were reported.
Platelet Rich Plasma
Platelet Rich Plasma is a product that is prepared from whole blood that contains platelets, growth factors and cytokines. It is produced by centrifuging whole blood from a patient and is injected into affected alopecia skin. Its efficacy in the treatment of AA has been evaluated in multiple studies, using a large range of different protocols. 142 Pooled results from 4 studies (n = 201 adults with AA), PRP was associated with nonsignficantly different decreases in SALT scores compared to traimcinolone acetonide injections (mean difference −2, CI −4.7-0.65, P: 0.14). Recurrence rates with PRP were lower than triamcinolone in 1 study (0%, 38% at 6 months, and 31% vs 71% at 12 months). 143 The most common cited adverse effect is pain, reported in 12% of PRP patients vs 0% of triamcinolone patients in a study of 50 adults. 144
Laser
Laser and Light therapies are another treatment modality that have been used in patients with AA. Types of laser reported include the fractional CO2 laser, the He-Ne laser, the 308 nm excimer laser, as well as light therapy with narrow-band UVB. 145
Fractional CO2 is thought to lead to apoptosis of the perifollicular lymphocytes, with effects including prolongation of anagen phase and increased blood flow to the dermal papilla. Five studies (n = 372) evaluating the use of this laser with minoxidil compared to minoxidil alone all reported superior response with fractional CO2, with a pooled response rate ratio of 1.29 (95% CI 1.14, 1.46). 145 Adverse effects include burning, irritation and hyperpigmentation.
The 308 nm excimer laser is also proposed to target and decrease the perifollicular lymphocyte infiltration, although depth of penetration may not be sufficient in most cases. Pooled response rate was greater in patients receiving the laser based on 3 studies (total N = 224, rate ratio 1.32, 95% CI 1.12, 1.55). Phototherapy with narrow band UVB (311 nm) has a similar proposed mechanism of action and limitations. Its use was not associated with statistically superior responses compared to topical calcipotriol in study of 60 patients, and monotherapy was inferior to combination with intramuscular steroids in a retrospective reivew of 25 patients.146,147
Prognosis
In a study of 75 patients with AA diagnosed in childhood or adolescence, 34.7% had full-hair regrowth over a period of >10 years, and 32.0% had no hair regrowth or aggravation. 148 Severity of AA at time of first consultation is an important prognostic factor, demonstrated in a study of 191 patient in which lack of disease at a mean follow-up of 17.74 years was present in 68.3% of patients with S1 at initial consultation compared to 9.1% of patients with S4. 149 A review of 9 studies (689 patients) with long-term outcome data reported recovery rates of 17.9% for AT and 9.3% for AU. 150
Factors associated with more progression to extensive disease (AU or AT) were identified in a survey study of 531 patients, including younger age of onset (P < .001), concurrent autoimmune or atopic disease (P = .047), atopic dermatitis (P = .021) and thyroid disease (P = .012). 151
Conclusion
This review highlights the recent increase in important research on pathogenesis, associated comorbidities, and treatment modalities for AA. Given how prevalent AA is in the general population and its potential impacts on quality of life, continuation of these research efforts are needed to define optimal approaches to evaluation and management of those affected by this condition.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Cathryn Sibbald https://orcid.org/0000-0001-9288-1121
References
- 1.Zhou C., Li X., Wang C., Zhang J. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. 2021;61(3):403-423. 10.1007/s12016-021-08883-0 [DOI] [PubMed] [Google Scholar]
- 2.Lee HH., Gwillim E., Patel KRet al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82(3):675-682. 10.1016/j.jaad.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 3.Wu MC., Yang C-C., Tsai RY., Chen WC. Late-onset alopecia areata: a retrospective study of 73 patients from Taiwan. J Eur Acad Dermatol Venereol. 2013;27(4):468-472. 10.1111/j.1468-3083.2012.04467.x [DOI] [PubMed] [Google Scholar]
- 4.Jadeja SD., Tobin DJ. Autoantigen discovery in the hair loss disorder, alopecia areata: implication of post-translational modifications. Front Immunol. 2022;13:890027. 10.3389/fimmu.2022.890027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waśkiel-Burnat A., Osińska M., Salińska Aet al. The role of serum Th1, Th2, and Th17 cytokines in patients with alopecia areata: clinical implications. Cells. 2021;10(12):3397. 10.3390/cells10123397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil-Quiñones SR., Sepúlveda-Pachón IT., Sánchez Vanegas G., Gutierrez-Castañeda LD. Effect of PTPN22, Fas/FasL, IL2RA and CTLA4 genetic polymorphisms on the risk of developing alopecia areata: a systematic review of the literature and meta-analysis. PLoS One. 2021;16(11):e0258499. 10.1371/journal.pone.0258499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuyama M., Ito T., Ohyama M. Alopecia areata: current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese dermatological association guidelines. J Dermatol. 2022;49(1):19-36. 10.1111/1346-8138.16207 [DOI] [PubMed] [Google Scholar]
- 8.Sundberg JP., Silva KA., Zhang Wet al. Recombinant human hepatitis B vaccine initiating alopecia areata: testing the hypothesis using the C3H/HeJ mouse model. Vet Dermatol. 2009;20(2):99-104. 10.1111/j.1365-3164.2008.00692.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu C-H., Cheng Y-P., Chan J-YL. Alopecia areata after vaccination: recurrence with rechallenge. Pediatr Dermatol. 2016;33(3):e218-e219. 10.1111/pde.12849 [DOI] [PubMed] [Google Scholar]
- 10.Scollan ME., Breneman A., Kinariwalla Net al. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022;20:1-5. 10.1016/j.jdcr.2021.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S., Lee H., Lee CH., Lee W-S. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):e16:466-477. 10.1016/j.jaad.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 12.Okhovat J-P., Marks DH., Manatis-Lornell A., Hagigeorges D., Locascio JJ., Senna MM. Association between alopecia areata, anxiety, and depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2019 10.1016/j.jaad.2019.05.08601 Jun 2019. [DOI] [PubMed] [Google Scholar]
- 13.Bilgiç Ö., Bilgiç A., Bahalı K., Bahali AG., Gürkan A., Yılmaz S. Psychiatric symptomatology and health-related quality of life in children and adolescents with alopecia areata. J Eur Acad Dermatol Venereol. 2014;28(11):1463-1468. 10.1111/jdv.12315 [DOI] [PubMed] [Google Scholar]
- 14.Altunisik N., Ucuz I., Turkmen D. Psychiatric basics of alopecia areata in pediatric patients: evaluation of emotion dysregulation, somatization, depression, and anxiety levels. J Cosmet Dermatol. 2022;21(2):770-775. 10.1111/jocd.14122 [DOI] [PubMed] [Google Scholar]
- 15.Katsarou-Katsari A., Singh LK., Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203(2):157-161. 10.1159/000051732 [DOI] [PubMed] [Google Scholar]
- 16.McDonald KA., Shelley AJ., Colantonio S., Beecker J. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76(3):472-477. 10.1016/j.jaad.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 17.Chelidze K., Lipner SR. Nail changes in alopecia areata: an update and review. Int J Dermatol. 2018;57(7):776-783. 10.1111/ijd.13866 [DOI] [PubMed] [Google Scholar]
- 18.Hegde SP., Naveen KN., Athanikar SB., Reshme P. Clinical and dermatoscopic patterns of alopecia areata: a tertiary care centre experience. Int J Trichology. 2013;5(3):132-136. 10.4103/0974-7753.125608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inui S., Nakajima T., Nakagawa K., Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008;47(7):688-693. 10.1111/j.1365-4632.2008.03692.x [DOI] [PubMed] [Google Scholar]
- 20.Meah N., Wall D., York Ket al. The alopecia areata consensus of experts (ACE) study: results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. 2020;83(1):123-130. 10.1016/j.jaad.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 21.Waśkiel-Burnat A., Rakowska A., Sikora M., Olszewska M., Rudnicka L. Alopecia areata predictive score: a new trichoscopy-based tool to predict treatment outcome in patients with patchy alopecia areata. J Cosmet Dermatol. 2020;19(3):746-751. 10.1111/jocd.13064 [DOI] [PubMed] [Google Scholar]
- 22.Meah N., Wall D., York Ket al. The alopecia areata consensus of experts (ACE) study part II: results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J Am Acad Dermatol. 2021;84(6):1594-1601. 10.1016/j.jaad.2020.09.028 [DOI] [PubMed] [Google Scholar]
- 23.Olsen EA., Roberts J., Sperling Let al. Objective outcome measures: collecting meaningful data on alopecia areata. J Am Acad Dermatol. 2018;79(3):470-478. 10.1016/j.jaad.2017.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen E., Hordinsky M., McDonald-Hull Set al. Alopecia areata investigational assessment guidelines. National alopecia areata Foundation. J Am Acad Dermatol. 1999;40(2 Pt 1):242-246. 10.1016/s0190-9622(99)70195-7 [DOI] [PubMed] [Google Scholar]
- 25.Gudobba C., Mane T., Bayramova Aet al. Automating hair loss labels for universally scoring alopecia from images: rethinking alopecia scores. JAMA Dermatol. 2022. 10.1001/jamadermatol.2022.5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyrwich KW., Kitchen H., Knight Set al. The alopecia areata investigator global assessment scale: a measure for evaluating clinically meaningful success in clinical trials. Br J Dermatol. 2020;183(4):702-709. 10.1111/bjd.18883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyrwich KW., Kitchen H., Knight Set al. Development of clinician-reported outcome (ClinRO) and patient-reported outcome (pro) measures for eyebrow, Eyelash and nail assessment in alopecia areata. Am J Clin Dermatol. 2020;21(5):725-732. 10.1007/s40257-020-00545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang YH., Moon SY., Lee WJet al. Alopecia areata progression index, a scoring system for evaluating overall hair loss activity in alopecia areata patients with pigmented hair: a development and reliability assessment. Dermatology. 2016;232(2):143-149. 10.1159/000442816 [DOI] [PubMed] [Google Scholar]
- 29.Chernyshov PV., Tomas-Aragones L., Finlay AYet al. Quality of life measurement in alopecia areata. position statement of the European Academy of dermatology and venereology task force on quality of life and patient oriented outcomes. J Eur Acad Dermatol Venereol. 2021;35(8):1614-1621. 10.1111/jdv.17370 [DOI] [PubMed] [Google Scholar]
- 30.Han JJ., Desai S., Li SJet al. Placebo group regrowth rate in alopecia areata clinical trials: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87(2):389-390. 10.1016/j.jaad.2021.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Messenger AG., Bleehen SS. Alopecia areata: light and electron microscopic pathology of the regrowing white hair. Br J Dermatol. 1984;110(2):155-162. 10.1111/j.1365-2133.1984.tb07461.x [DOI] [PubMed] [Google Scholar]
- 32.Daruwalla SB., Dhurat RS., Hamid SAT. All that a dermatotrichologist needs to know about hair camouflage: a comprehensive review. Int J Trichology. 2022;14(3):77-83. 10.4103/ijt.ijt_6_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García Castro R., Domínguez Santás M., Luna Bastante L., Lobato Izagirre A., Krasnovska Zayets K., Feito-Rodríguez M. Painless, inexpensive and non-permanent tattoo sticker for the eyebrow alopecia in children. Pediatr Dermatol. 2021;38(3):699-700. 10.1111/pde.14546 [DOI] [PubMed] [Google Scholar]
- 34.Marwah MK., Kerure AS., Marwah GS. Microblading and the science behind it. Indian Dermatol Online J. 2021;12(1):6-11. 10.4103/idoj.IDOJ_230_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spurr A., Hanna N., Colantonio S. Cutaneous sarcoidosis in eyebrows cosmetically pigmented with microblading method: a case report and review of the literature. SAGE Open Med Case Rep. 2022;10:2050313X221117720. 10.1177/2050313X221117720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahouel I., Salah NB., Machghoul S., Belhadjali H., Youssef M., Zili J. Allergic contact dermatitis post-microblading eyebrow tattooing: a manifestation of pre-existing nickel sensitization. J Cosmet Dermatol. 2022;21(10):5270-5271. 10.1111/jocd.14920 [DOI] [PubMed] [Google Scholar]
- 37.Kluger N. Delayed granulomatous reaction after eyebrow Microblading. Dermatol Surg. 2022;48(4):472-473. 10.1097/DSS.0000000000003361 [DOI] [PubMed] [Google Scholar]
- 38.Akoh CC., Akintilo L., Shankar S., Lo Sicco K. A rare case of microblading-induced preseptal cellulitis. JAAD Case Rep. 2021;16:98-100. 10.1016/j.jdcr.2021.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tkachenko E., Okhovat J-P., Manjaly P., Huang KP., Senna MM., Mostaghimi A. Complementary and alternative medicine for alopecia areata: a systematic review. J Am Acad Dermatol. 2023;88(1):131-143. 10.1016/j.jaad.2019.12.027 [DOI] [PubMed] [Google Scholar]
- 40.Hay IC., Jamieson M., Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134(11):1349-1352. 10.1001/archderm.134.11.1349 [DOI] [PubMed] [Google Scholar]
- 41.Ozmen I., Caliskan E., Arca E., Acikgoz G., Koc E. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Medical Journal. 2015;57(3):233. 10.5455/gulhane.38258 [DOI] [Google Scholar]
- 42.Hajheydari Z., Jamshidi M., Akbari J., Mohammadpour R. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73(1):29-32. 10.4103/0378-6323.30648 [DOI] [PubMed] [Google Scholar]
- 43.E P. Topical Garlic Concentrate for Alopecia Areata in Children. NCT02691117. 2023. https://clinicaltrials.gov/ct2/show/NCT02691117?term=garlic&cond=Areata+Alopecia&draw=2&rank=1
- 44.Wu W-Z., Zhang F-R. Glycyrrhizin combined with acitretin improve clinical symptom of psoriasis via reducing Th17 cell differentiation and related serum cytokine concentrations. Int J Clin Exp Med. 2015;8(9):16266-16272. [PMC free article] [PubMed] [Google Scholar]
- 45.Yang D-Q., You L-P., Song P-H., Zhang L-X., Bai Y-P. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18(8):621-625. 10.1007/s11655-012-1173-0 [DOI] [PubMed] [Google Scholar]
- 46.Yang D., Zheng J., Zhang Y., Jin Y., Gan C., Bai Y. Total glucosides of paeony capsule plus compound glycyrrhizin tablets for the treatment of severe alopecia areata in children: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:1-5. 10.1155/2013/378219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson JM., Mirza MA., Park MK., Qureshi AA., Cho E. The role of micronutrients in alopecia areata: a review. Am J Clin Dermatol. 2017;18(5):663-679. 10.1007/s40257-017-0285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barton VR., Toussi A., Awasthi S., Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol. 2022;86(6):1318-1334. 10.1016/j.jaad.2021.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasmaz S., Arican O. Comparison of azelaic acid and anthralin for the therapy of patchy alopecia areata: a pilot study. Am J Clin Dermatol. 2005;6(6):403-406. 10.2165/00128071-200506060-00007 [DOI] [PubMed] [Google Scholar]
- 50.Wu SZ., Wang S., Ratnaparkhi R., Bergfeld WF. Treatment of pediatric alopecia areata with anthralin: a retrospective study of 37 patients. Pediatr Dermatol. 2018;35(6):817-820. 10.1111/pde.13703 [DOI] [PubMed] [Google Scholar]
- 51.Herbst V., Zöller M., Kissling S., Wenzel E., Stutz N., Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16(5):537-542. [PubMed] [Google Scholar]
- 52.Lee S., Kim BJ., Lee YB., Lee W-S. Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(10):1145-1151. 10.1001/jamadermatol.2018.2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukumoto T., Fukumoto R., Magno E., Oka M., Nishigori C., Horita N. Treatments for alopecia areata: a systematic review and network meta-analysis. Dermatol Ther. 2021;34(3):e14916. 10.1111/dth.14916 [DOI] [PubMed] [Google Scholar]
- 54.Waśkiel-Burnat A., Kołodziejak M., Sikora Met al. Therapeutic management in paediatric alopecia areata: a systematic review. J Eur Acad Dermatol Venereol. 2021;35(6):1299-1308. 10.1111/jdv.17187 [DOI] [PubMed] [Google Scholar]
- 55.Charuwichitratana S., Wattanakrai P., Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136(10):1276-1277. 10.1001/archderm.136.10.1276 [DOI] [PubMed] [Google Scholar]
- 56.Lenane P., Macarthur C., Parkin PCet al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol. 2014;150(1):47-50. 10.1001/jamadermatol.2013.5764 [DOI] [PubMed] [Google Scholar]
- 57.Devi M., Rashid A., Ghafoor R. Intralesional triamcinolone acetonide versus topical betamethasone valearate in the management of localized alopecia areata. J Coll Physicians Surg Pak. 2015;25(12):860-862. [PubMed] [Google Scholar]
- 58.Kuldeep C., Singhal H., Khare AK., Mittal A., Gupta LK., Garg A. Randomized comparison of topical betamethasone valerate foam, intralesional triamcinolone acetonide and tacrolimus ointment in management of localized alopecia areata. Int J Trichology. 2011;3(1):20-24. 10.4103/0974-7753.82123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ustuner P., Balevi A., Özdemir M. Best dilution of the best corticosteroid for intralesional injection in the treatment of localized alopecia areata in adults. J Dermatolog Treat. 2017;28(8):753-761. 10.1080/09546634.2017.1329497 [DOI] [PubMed] [Google Scholar]
- 60.de Sousa VB., Arcanjo FP., Aguiar Fet al. Intralesional betamethasone versus triamcinolone acetonide in the treatment of localized alopecia areata: a within-patient randomized controlled trial. J Dermatolog Treat. 2022;33(2):875-877. 10.1080/09546634.2020.1788703 [DOI] [PubMed] [Google Scholar]
- 61.Muhaidat JM., Al-Qarqaz F., Khader Y., Alshiyab DM., Alkofahi H., Almalekh M. A retrospective comparative study of two concentrations of intralesional triamcinolone acetonide in the treatment of patchy alopecia areata on the scalp. Clin Cosmet Investig Dermatol. 2020;13:795-803. 10.2147/CCID.S280855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arora A., Bhalla M., Thami GP. Comparative efficacy of injection triamcinolone acetonide given intralesionally and through microneedling in Alopecia areata. Int J Trichology. 2022;14(5):156-161. 10.4103/ijt.ijt_140_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shreberk-Hassidim R., Ramot Y., Gilula Z., Zlotogorski A. A systematic review of pulse steroid therapy for alopecia areata. J Am Acad Dermatol. 2016;74(2):372-374. 10.1016/j.jaad.2015.09.045 [DOI] [PubMed] [Google Scholar]
- 64.Gallaga NM., Carrillo B., Good A., Munoz-Gonzalez A., Ross L. Pediatric pulse dose corticosteroid therapy dosing and administration in the treatment of alopecia areata: a review of literature. Pediatr Dermatol. 2023;40(2):276-281. 10.1111/pde.15209 [DOI] [PubMed] [Google Scholar]
- 65.Johnstone MA., Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(Suppl 1):S185-202. 10.1016/s0039-6257(02)00307-7 [DOI] [PubMed] [Google Scholar]
- 66.Schlötzer-Schrehardt U., Zenkel M., Nüsing RM. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest Ophthalmol Vis Sci. 2002;43(5):1475-1487. [PubMed] [Google Scholar]
- 67.Coronel-Pérez IM., Rodríguez-Rey EM., Camacho-Martínez FM. Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J Eur Acad Dermatol Venereol. 2010;24(4):481-485. 10.1111/j.1468-3083.2009.03543.x [DOI] [PubMed] [Google Scholar]
- 68.Bhat S., Handa S., De D. A randomized comparative study of the efficacy of topical latanoprost versus topical betamethasone diproprionate lotion in the treatment of localized alopecia areata. Indian J Dermatol Venereol Leprol. 2021;87(1):42-48. 10.25259/IJDVL_787_19 [DOI] [PubMed] [Google Scholar]
- 69.Rafati M., Mahmoudian R., Golpour M., Kazeminejad A., Saeedi M., Nekoukar Z. The effect of latanoprost 0.005% solution in the management of scalp alopecia areata, a randomized double-blind placebo-controlled trial. Dermatol Ther. 2022;35(6):e15450. 10.1111/dth.15450 [DOI] [PubMed] [Google Scholar]
- 70.Ghassemi M., Yazdanian N., Behrangi E., Jafari M., Goodarzi A. Comparison of efficacy, safety and satisfaction of latanoprost versus minoxidil, betamethasone and in combination in patients with alopecia areata: a blinded multiple group randomized controlled trial. Dermatol Ther. 2022;35(12):e15943. 10.1111/dth.15943 [DOI] [PubMed] [Google Scholar]
- 71.Chiba T., Kashiwagi K., Ishijima Ket al. A prospective study of iridial pigmentation and eyelash changes due to ophthalmic treatment with latanoprost. Jpn J Ophthalmol. 2004;48(2):141-147. 10.1007/s10384-003-0039-6 [DOI] [PubMed] [Google Scholar]
- 72.Borchert M., Bruce S., Wirta Det al. An evaluation of the safety and efficacy of bimatoprost for eyelash growth in pediatric subjects. Clin Ophthalmol. 2016;10:419-429. 10.2147/OPTH.S89561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ricar J., Cetkovska P., Hordinsky M., Ricarova R. Topical bimatoprost in the treatment of eyelash loss in alopecia totalis and universalis: a prospective, open-label study. Dermatol Ther. 2022;35(6):e15438. 10.1111/dth.15438 [DOI] [PubMed] [Google Scholar]
- 74.Vila TO., Camacho Martinez FM. Bimatoprost in the treatment of eyelash universalis alopecia areata. Int J Trichology. 2010;2(2):86-88. 10.4103/0974-7753.77511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaher H., Gawdat HI., Hegazy RA., Hassan M. Bimatoprost versus mometasone furoate in the treatment of scalp alopecia areata: a pilot study. Dermatology. 2015;230(4):308-313. 10.1159/000371416 [DOI] [PubMed] [Google Scholar]
- 76.Homey B., Assmann T., Vohr HWet al. Topical FK506 suppresses cytokine and costimulatory molecule expression in epidermal and local draining lymph node cells during primary skin immune responses. J Immunol. 1998;160(11):5331-5340. 10.4049/jimmunol.160.11.5331 [DOI] [PubMed] [Google Scholar]
- 77.Nassar A., Elradi M., Radwan M., Albalat W. Comparative evaluation of the efficacy of topical tacrolimus 0.03% and topical calcipotriol 0.005% mixed with betamethasone dipropionate versus topical clobetasol 0.05% in treatment of alopecia areata: a clinical and trichoscopic study. J Cosmet Dermatol. 2022. [DOI] [PubMed] [Google Scholar]
- 78.Jung KE., Gye JW., Park MK., Park BC. Comparison of the topical FK506 and clobetasol propionate as first-line therapy in the treatment of early alopecia areata. Int J Dermatol. 2017;56(12):1487-1488. 10.1111/ijd.13676 [DOI] [PubMed] [Google Scholar]
- 79.Price VH., Willey A., Chen BK. Topical tacrolimus in alopecia areata. J Am Acad Dermatol. 2005;52(1):138-139. 10.1016/j.jaad.2004.05.019 [DOI] [PubMed] [Google Scholar]
- 80.Xie Z., Komuves L., Yu Q-Cet al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118(1):11-16. 10.1046/j.1523-1747.2002.01644.x [DOI] [PubMed] [Google Scholar]
- 81.Nancy A-L., Yehuda S. Prediction and prevention of autoimmune skin disorders. Arch Dermatol Res. 2009;301(1):57-64. 10.1007/s00403-008-0889-3 [DOI] [PubMed] [Google Scholar]
- 82.Narang T., Daroach M., Kumaran MS. Efficacy and safety of topical calcipotriol in management of alopecia areata: a pilot study. Dermatol Ther. 2017;30(3) 10.1111/dth.1246430 01 2017. [DOI] [PubMed] [Google Scholar]
- 83.Çerman AA., Solak SS., Altunay İlknur., Küçükünal NA. Topical calcipotriol therapy for mild-to-moderate alopecia areata: a retrospective study. J Drugs Dermatol. 2015;14(6):616-620. [PubMed] [Google Scholar]
- 84.Gupta M., Singh S., Khan BH. Comparative evaluation of efficacy between topical calcipotriol used along with topical clobetasol and topical clobetasol monotherapy in treatment of alopecia areata: a randomised clinical trial. Journal Of Clinical And Diagnostic Research. 2021:15. 10.7860/JCDR/2021/48438.14823 [DOI] [Google Scholar]
- 85.Alam M., Amin SS., Adil M., Arif T., Zahra FT., Varshney I. Comparative study of efficacy of topical mometasone with calcipotriol versus mometasone alone in the treatment of alopecia areata. Int J Trichology. 2019;11(3):123-127. 10.4103/ijt.ijt_18_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rashad AF., Elgamal E., Fouda I. Intralesional vitamin D3 in treatment of alopecia areata: a randomized controlled clinical trial. J Cosmet Dermatol. 2022;21(10):4617-4622. 10.1111/jocd.14844 [DOI] [PubMed] [Google Scholar]
- 87.Nowaczyk J., Makowska K., Rakowska A., Sikora M., Rudnicka L. Cyclosporine with and without systemic corticosteroids in treatment of alopecia areata: a systematic review. Dermatol Ther. 2020;10(3):387-399. 10.1007/s13555-020-00370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Husein-ElAhmed H., Steinhoff M. Efficacy and predictive factors of cyclosporine A in alopecia areata: a systematic review with meta-analysis. J Dermatolog Treat. 2022;33(3):1643-1651. 10.1080/09546634.2021.1886230 [DOI] [PubMed] [Google Scholar]
- 89.Farshi S., Mansouri P., Safar F., Khiabanloo SR. Could azathioprine be considered as a therapeutic alternative in the treatment of alopecia areata? A pilot study. Int J Dermatol. 2010;49(10):1188-1193. 10.1111/j.1365-4632.2010.04576.x [DOI] [PubMed] [Google Scholar]
- 90.Gupta P., Verma KK., Khandpur S., Bhari N. Weekly azathioprine pulse versus betamethasone oral Mini-Pulse in the treatment of moderate-to-severe alopecia areata. Indian J Dermatol. 2019;64(4):292-298. 10.4103/ijd.IJD_481_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bedoui Y., Guillot X., Sélambarom Jet al. Methotrexate an old drug with new tricks. Int J Mol Sci. 2019;20(20):5023 10.3390/ijms20205023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phan K., Ramachandran V., Sebaratnam DF. Methotrexate for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(1):120-127. 10.1016/j.jaad.2018.06.064 [DOI] [PubMed] [Google Scholar]
- 93.Phan K., Lee G., Fischer G. Methotrexate in the treatment of paediatric alopecia areata: retrospective case series and updated meta-analysis. Australas J Dermatol. 2020;61(2):119-124. 10.1111/ajd.13206 [DOI] [PubMed] [Google Scholar]
- 94.Kinoshita-Ise M., Sachdeva M., Martinez-Cabriales SA., Shear NH., Lansang P. Oral methotrexate monotherapy for severe alopecia areata: a single center retrospective case series. J Cutan Med Surg. 2021;25(5):490-497. 10.1177/1203475421995712 [DOI] [PubMed] [Google Scholar]
- 95.Xing L., Dai Z., Jabbari Aet al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043-1049. 10.1038/nm.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phan K., Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(5):850-856. 10.1111/jdv.15489 [DOI] [PubMed] [Google Scholar]
- 97.Liu LY., King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80(6):1778-1779. 10.1016/j.jaad.2018.11.037 [DOI] [PubMed] [Google Scholar]
- 98.Castelo-Soccio L. Experience with oral tofacitinib in 8 adolescent patients with alopecia universalis. J Am Acad Dermatol. 2017;76(4):754-755. 10.1016/j.jaad.2016.11.038 [DOI] [PubMed] [Google Scholar]
- 99.Craiglow BG., King BA. Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol. 2019;80(2):568-570. 10.1016/j.jaad.2018.08.041 [DOI] [PubMed] [Google Scholar]
- 100.Craiglow BG., Liu LY., King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76(1):29-32. 10.1016/j.jaad.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 101.Dai Y-X., Chen C-C. Tofacitinib therapy for children with severe alopecia areata. J Am Acad Dermatol. 2019;80(4):1164-1166. 10.1016/j.jaad.2018.12.041 [DOI] [PubMed] [Google Scholar]
- 102.Jerjen R., Meah N., Trindade de Carvalho L., Wall D., Eisman S., Sinclair R. Treatment of alopecia areata in pre-adolescent children with oral tofacitinib: a retrospective study. Pediatr Dermatol. 2021;38(1):103-108. 10.1111/pde.14422 [DOI] [PubMed] [Google Scholar]
- 103.Kibbie J., Kines K., Norris D., Dunnick CA. Oral tofacitinib for the treatment of alopecia areata in pediatric patients. Pediatr Dermatol. 2022;39(1):31-34. 10.1111/pde.14855 [DOI] [PubMed] [Google Scholar]
- 104.Guo L., Feng S., Sun B., Jiang X., Liu Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34(1):192-201. 10.1111/jdv.15937 [DOI] [PubMed] [Google Scholar]
- 105.Almutairi N., Nour TM., Hussain NH. Janus kinase inhibitors for the treatment of severe alopecia areata: an open-label comparative study. Dermatology. 2019;235(2):130-136. 10.1159/000494613 [DOI] [PubMed] [Google Scholar]
- 106.Mollé N., Krichevsky S., Kermani P., Silver RT., Ritchie E., Scandura JM. Ruxolitinib can cause weight gain by blocking leptin signaling in the brain via JAK2/STAT3. Blood. 2020;135(13):1062-1066. 10.1182/blood.2019003050 [DOI] [PubMed] [Google Scholar]
- 107.Lai VWY., Bokhari L., Sinclair R. Sublingual tofacitinib for alopecia areata: a roll-over pilot clinical trial and analysis of pharmacokinetics. Int J Dermatol. 2021;60(9):1135-1139. 10.1111/ijd.15657 [DOI] [PubMed] [Google Scholar]
- 108.Olsen EA., Kornacki D., Sun K., Hordinsky MK. Ruxolitinib cream for the treatment of patients with alopecia areata: a 2-part, double-blind, randomized, vehicle-controlled phase 2 study. J Am Acad Dermatol. 2020;82(2):412-419. 10.1016/j.jaad.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 109.Bokhari L., Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors - a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57(12):1464-1470. 10.1111/ijd.14192 [DOI] [PubMed] [Google Scholar]
- 110.King B., Ohyama M., Kwon Oet al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687-1699. 10.1056/NEJMoa2110343 [DOI] [PubMed] [Google Scholar]
- 111.King B., Guttman-Yassky E., Peeva Eet al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol. 2021;85(2):379-387. 10.1016/j.jaad.2021.03.050 [DOI] [PubMed] [Google Scholar]
- 112.ClinicalTrials.gov . PF-06651600 for the treatment of alopecia areata (ALLEGRO-2b/3). https://clinicaltrials.gov/ct2/show/study/NCT03732807?term=ritlecitinib+alopecia+areata&draw=2&rank=2
- 113.. A. T. Long-term safety and efficacy of ritlecitinib in adults and adolescents with alopecia areata: interim results from the ALLEGRO-LT phase 3, open-label trial. D3T01.1G. presented at: European Academy of Dermatology and Venereology Congress; 07–10 Sept 2022 2022; Milan, Italy.
- 114.King B., Mesinkovska N., Mirmirani Pet al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus Kinase inhibitor, in moderate-to-severe alopecia areata. J Am Acad Dermatol. 2022;87(2):306-313. 10.1016/j.jaad.2022.03.045 [DOI] [PubMed] [Google Scholar]
- 115.Messenger AG., Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186-194. 10.1111/j.1365-2133.2004.05785.x [DOI] [PubMed] [Google Scholar]
- 116.Sharma AN., Michelle L., Juhasz M., Muller Ramos P., Atanaskova Mesinkovska N. Low-dose oral minoxidil as treatment for non-scarring alopecia: a systematic review. Int J Dermatol. 2020;59(8):1013-1019. 10.1111/ijd.14933 [DOI] [PubMed] [Google Scholar]
- 117.Gupta AK., Talukder M., Venkataraman M., Bamimore MA. Minoxidil: a comprehensive review. J Dermatolog Treat. 2022;33(4):1896-1906. 10.1080/09546634.2021.1945527 [DOI] [PubMed] [Google Scholar]
- 118.Dhurat R., Daruwalla S., Pai Set al. SULT1A1 (minoxidil sulfotransferase) enzyme booster significantly improves response to topical minoxidil for hair regrowth. J Cosmet Dermatol. 2022;21(1):343-346. 10.1111/jocd.14299 [DOI] [PubMed] [Google Scholar]
- 119.Freire PCB., Riera R., Martimbianco ALC., Petri V., Atallah AN. Minoxidil for patchy alopecia areata: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(9):1792-1799. 10.1111/jdv.15545 [DOI] [PubMed] [Google Scholar]
- 120.Olsen EA., Dunlap FE., Funicella Tet al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377-385. 10.1067/mjd.2002.124088 [DOI] [PubMed] [Google Scholar]
- 121.Lucky AW., Piacquadio DJ., Ditre CMet al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50(4):541-553. 10.1016/j.jaad.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 122.Olsen EA., Whiting D., Bergfeld Wet al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2007;57(5):767-774. 10.1016/j.jaad.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 123.Blume-Peytavi U., Hillmann K., Dietz E., Canfield D., Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65(6):1126-1134. 10.1016/j.jaad.2010.09.724 [DOI] [PubMed] [Google Scholar]
- 124.Friedman ES., Friedman PM., Cohen DE., Washenik K. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46(2):309-312. 10.1067/mjd.2002.119104 [DOI] [PubMed] [Google Scholar]
- 125.Jimenez-Cauhe J., Saceda-Corralo D., Rodrigues-Barata Ret al. Safety of low-dose oral minoxidil treatment for hair loss. A systematic review and pooled-analysis of individual patient data. Dermatol Ther. 2020;33(6):e14106. 10.1111/dth.14106 [DOI] [PubMed] [Google Scholar]
- 126.Wambier CG., Craiglow BG., King BA. Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J Am Acad Dermatol. 2021;85(3):743-745. 10.1016/j.jaad.2019.08.080 [DOI] [PubMed] [Google Scholar]
- 127.John JM., Sinclair RD. Systemic minoxidil for hair disorders in pediatric patients: a safety and tolerability review. Int J Dermatol. 2023;62(2):257-259. 10.1111/ijd.16373 [DOI] [PubMed] [Google Scholar]
- 128.Sánchez-Díaz M., López-Delgado D., Montero-Vílchez Tet al. Systemic minoxidil accidental exposure in a paediatric population: a case series study of cutaneous and systemic side effects. J Clin Med. 2021;10(18):4257. 10.3390/jcm10184257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bertolini M., Zilio F., Rossi Aet al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One. 2014;9(5):e94260. 10.1371/journal.pone.0094260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pham C., Sung C., Juhasz Met al. The role of antihistamines and Dupilumab in the management of alopecia areata: a systematic review. J Drugs Dermatol. 2022;21(10):1070-1083. 10.36849/JDD.6553 [DOI] [PubMed] [Google Scholar]
- 131.Gies V., Bekaddour N., Dieudonné Yet al. Beyond anti-viral effects of Chloroquine/Hydroxychloroquine. Front Immunol. 2020;11:1409. 10.3389/fimmu.2020.01409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yun D., Silverberg NB., Stein SL. Alopecia areata treated with hydroxychloroquine: a retrospective study of nine pediatric cases. Pediatr Dermatol. 2018;35(3):361-365. 10.1111/pde.13451 [DOI] [PubMed] [Google Scholar]
- 133.Mikhaylov D., Pavel A., Yao Cet al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. 10.1007/s00403-018-1876-y [DOI] [PubMed] [Google Scholar]
- 134.Cervantes J., Jimenez JJ., DelCanto GM., Tosti A. Treatment of alopecia areata with Simvastatin/Ezetimibe. J Investig Dermatol Symp Proc. 2018;19(1):S25-S31. 10.1016/j.jisp.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 135.Lattouf C., Jimenez JJ., Tosti Aet al. Treatment of alopecia areata with simvastatin/ezetimibe. J Am Acad Dermatol. 2015;72(2):359-361. 10.1016/j.jaad.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 136.Freitas Gouveia M., Trüeb RM. Unsuccessful treatment of alopecia areata with Simvastatin/Ezetimibe: experience in 12 patients. Skin Appendage Disord. 2017;3(3):156-160. 10.1159/000468991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loi C., Starace M., Piraccini BM. Alopecia areata (AA) and treatment with simvastatin/ezetimibe: experience of 20 patients. J Am Acad Dermatol. 2016;74(5):e99-e100. 10.1016/j.jaad.2015.09.071 [DOI] [PubMed] [Google Scholar]
- 138.Ikeda T. A new classification of alopecia areata. Dermatologica. 1965;131(6):421-445. 10.1159/000254503 [DOI] [PubMed] [Google Scholar]
- 139.Guttman-Yassky E., Renert-Yuval Y., Bares Jet al. Phase 2A randomized clinical trial of dupilumab (anti-IL-4Ralpha) for alopecia areata patients. Allergy. 2022;77(3):897-906. 10.1111/all.15071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McKenzie PL., Castelo-Soccio L. Dupilumab therapy for alopecia areata in pediatric patients with concomitant atopic dermatitis. J Am Acad Dermatol. 2021;84(6):1691-1694. 10.1016/j.jaad.2021.01.046 [DOI] [PubMed] [Google Scholar]
- 141.Guttman-Yassky E., Nia JK., Hashim PWet al. Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res. 2018;310(8):607-614. 10.1007/s00403-018-1853-5 [DOI] [PubMed] [Google Scholar]
- 142.Meznerics FA., Illés K., Dembrovszky Fet al. Platelet-rich plasma in alopecia Areata-A steroid-free treatment modality: a systematic review and meta-analysis of randomized clinical trials. Biomedicines. 2022;10(8):1829. 10.3390/biomedicines10081829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trink A., Sorbellini E., Bezzola Pet al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169(3):690-694. 10.1111/bjd.12397 [DOI] [PubMed] [Google Scholar]
- 144.Hegde P., Relhan V., Sahoo B., Garg VK. A randomized, placebo and active controlled, split scalp study to evaluate the efficacy of platelet-rich plasma in patchy alopecia areata of the scalp. Dermatol Ther. 2020;33(6):e14388. 10.1111/dth.14388 [DOI] [PubMed] [Google Scholar]
- 145.Zhang J., Lin P., Lin Het al. Laser and light therapy combined with topical minoxidil for alopecia areata: a systematic review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2023;38(1):74. 10.1007/s10103-023-03734-0 [DOI] [PubMed] [Google Scholar]
- 146.Bayramgürler D., Demirsoy EO., Aktürk AŞ., Kıran R. Narrowband ultraviolet B phototherapy for alopecia areata. Photodermatol Photoimmunol Photomed. 2011;27(6):325-327. 10.1111/j.1600-0781.2011.00612.x [DOI] [PubMed] [Google Scholar]
- 147.El Taieb MA., Hegazy EM., Ibrahim HM., Osman AB., Abualhamd M. Topical calcipotriol vs narrowband ultraviolet B in treatment of alopecia areata: a randomized-controlled trial. Arch Dermatol Res. 2019;311(8):629-636. 10.1007/s00403-019-01943-8 [DOI] [PubMed] [Google Scholar]
- 148.Jang YH., Eun DH., Kim DW. Long-term prognosis of alopecia areata in children and adolescents. Ann Dermatol. 2019;31(2):231-234. 10.5021/ad.2019.31.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tosti A., Bellavista S., Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55(3):438-441. 10.1016/j.jaad.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 150.Burroway B., Griggs J., Tosti A. Alopecia totalis and universalis long-term outcomes: a review. J Eur Acad Dermatol Venereol. 2020;34(4):709-715. 10.1111/jdv.15994 [DOI] [PubMed] [Google Scholar]
- 151.Goh C., Finkel M., Christos PJ., Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20(9):1055-1060. 10.1111/j.1468-3083.2006.01676.x [DOI] [PubMed] [Google Scholar]