Abstract
Adoptive cellular immunotherapy, mainly hematopoietic stem cell transplant and CAR-T cell therapy have revolutionized treatment of patients with acute leukemia. Indications and inclusion criteria for these treatments have expanded in recent years. While these therapies are associated with significant improvements in disease response and overall survival, patients may experience adverse events from associated chemotherapy conditioning, engraftment, cytokine storm, supportive medications, and post-transplant maintenance targeted therapies. Supportive oncodermatology is a growing specialty to manage cutaneous toxicities resulting from the anti-cancer therapies. In this review, we summarize diagnosis and management of the common cutaneous adverse events including drug eruptions, graft-versus-host disease, neoplastic and paraneoplastic complications in patients undergoing cellular therapies.
Keywords: Hematopoietic stem cell transplant, CAR-T cell therapy, drug eruptions, GVHD, dermatological complications
Drug Eruptions
CHEMOTHERAPY
Toxic erythema of Chemotherapy
Toxic erythema of chemotherapy (TEC) encompasses a wide array of non-allergic cutaneous eruptions to chemotherapeutic drugs. Historically, these eruptions went by many, often redundant, names, based primarily on clinical or histologic features. Some examples include hand foot syndrome (HFS), palmar plantar erythema/erythrodysesthesia, intertriginous eruptions associated with chemotherapy, and intertrigo dermatitis. TEC is a new term that broadly encompasses these, such that confusion among patients and providers is minimized [1].
TEC presents classically with painful erythema and edema, sometimes with a bullous component, and most often affects the hands, feet, and intertriginous zones and less frequently the elbows, knees, and ears [1, 2] (FIGURE 1). Atypical presentations may occur, especially in skin of color, and can manifest as hyperpigmentation [3]. A localized form at injection sites appears to be more likely when small distal vessels are used for chemotherapy [4]. In some cases, TEC may generalize and clinically mimic toxic epidermal necrolysis [5]. The mechanism behind TEC eruptions is not clearly elucidated but may relate to the accumulation of chemotherapy in eccrine glands, leading to local toxic effects [2].
Figure 1:

Toxic erythema of chemotherapy (TEC) in the groin.
TEC eruptions typically occur 2–3 weeks after administration of chemotherapy, although some may experience a delayed form months after chemotherapy initiation [1, 2]. In patients with AML, thiotepa, daunorubicin, cytarabine, and busulfan/fludarabine are often implicated in these eruptions [2, 6]. Eruptions are typically self-limited and resolve with desquamation and post inflammatory hyperpigmentation. Symptomatic treatment may be given including topical steroids, emollients, analgesics, topical antibiotics, and cold compresses [2].
Azacytidine
Azacytidine is a nucleoside inhibitor and hypomethylating agent with two forms – injectable, for the treatment of MDS and oral, which is approved for the treatment of AML after achieving first complete remission. One study assessing all adverse events to injectable azacytidine found that among AML patients, 7.2% and 19.9% experienced primarily low-grade skin/mucosal infections and injection site reactions, respectively [7]. Excessive bleeding at injection sites may also occur as azacytidine induced thrombocytopenia is common [8]. Azacytidine has been reported to precipitate Sweet syndrome. [9, 10].
Busulfan
Busulfan is an alkylating agent that is often used in combination with cyclophosphamide or fludarabine/clofarabine for conditioning prior to allogeneic hematopoietic stem cell transplantation (alloHCT). Dermatologic adverse events (dAEs) are common with this medication, with rash and pruritus occurring in 57% and 28% of patients in clinical trials, respectively. In addition, busulfan can cause generalized skin darkening which may involve the mucosal surfaces [11]. The use of busulfan as part of the conditioning regimen has also been identified as one of the primary risk factors for the development of post alloHCT permanent chemotherapy induced alopecia, which is defined as hair loss that persists for longer than 6 months [12, 13].
Cyclophosphamide
Cyclophosphamide is an alkylating agent that has many uses in the treatment of acute leukemias, including cytoreduction, conditioning prior to alloHCT, and prophylaxis against graft vs host disease (GvHD) [14]. Hyperpigmentation is the primary cutaneous toxicity and often presents on the palms, soles, and oral mucosa but may also affect the nails [15]. Cyclophosphamide can also induce hair loss or changes to hair texture or quality and may play a role in the development of late chemotherapy induced alopecia [16]. Hypersensitivity reactions may also occur and can manifest as urticaria, angioedema, or vasculitis [17]. These reactions typically begin shortly after cyclophosphamide infusion is begun, but may also have a delayed presentation if hypersensitivity is due to a cyclophosphamide metabolite [17].
Cyclosporine
Cyclosporine is a calcineurin inhibitor with immunosuppressive effects, most often given for GvHD prophylaxis. Most dAEs associated with this medication involve the pilosebaceous unit, including hypertrichosis, sebaceous hyperplasia, acne, folliculitis, epidermal cysts, and pilar keratosis [15]. Hypertrichosis is one of the most common dAEs, affecting up to 70% of patients, and is believed to be due to cyclosporine induction of the anagen phase of hair growth [18, 19].
Cytarabine and Daunorubicin
Cytarabine, a pyrimidine nucleoside analog, and daunorubicin, an anthracycline, are both cytotoxic chemotherapies used in the treatment of AML frequently as part of a “7+3” regimen [20]. Daunorubicin is often implicated in the development of TEC. Morbilliform eruptions or acral erythema, can occur in 50% of patients taking high-dose cytarabine [21] (FIGURE 2). Edema, rash, mucositis and pruritus have been reported in 60%, 52%, 46%, and 9% of patients on the “7+3” regimen, respectively [20].
Figure 2:
Painful rash secondary to cytarabine.
CPX-351 (Vyxeos®)
CPX-351 (Vyxeos®) is FDA approved for therapy related AML and AML with myelodysplasia related changes. This medication is a liposomal encapsulation of cytarabine and daunorubicin that is designed to deliver a synergistic 5:1 drug ratio [20]. DAEs to this therapy are common – clinical trials report rash, mucositis and pruritus with frequencies of 54%, 44%, and 15%, respectively [20]. In a study further characterizing these toxicities, investigators found that CPX-351 rash commonly presents as a purpuric maculopapular/papulonodular eruption [22]. Twenty-three percent developed this morphology of rash and of these, 35% had a high-grade form. Clinically, this is best categorized as a subtype of toxic erythema of chemotherapy. Fortunately, the rash appears to have a benign course and resolves spontaneously in most patients. Histology is nonspecific, most often showing superficial perivascular lymphocytic infiltrate and/or red blood extravasation. Notably, all patients in this study did not develop rash until after CPX-351 completion [22]. The mechanism of dAE development is not entirely clear, but may be due to the prolonged effects of the liposomal formulation of cytarabine and daunorubicin on the epidermis through upregulation of the CD95 and TNFαR cytotoxic receptors [23].
Decitabine
Decitabine, a nucleoside synthesis inhibitor and hypomethylating agent, which is often used for AML treatment off label in elderly patients. Decitabine has been considered a post alloHCT maintenance therapy option [24]. Common dAEs, include ecchymosis (22%), non-specific rash (19%), erythema (14%), petechiae of the oral mucosa (13%), stomatitis (12%), non-specified skin lesions (11%), pruritus (11%), alopecia (8%), urticaria (6%), and swelling of the face (6%) [25].
Fludarabine
In the context of alloHCT, fludarabine, a purine analogue, is most frequently used as part of the conditioning regimen [26]. Skin toxicity may occur and most often manifests as rash, including generalized maculopapular eruptions [15]. Less commonly, cases of erythema multiforme, Steven-Johnson syndrome/toxic epidermal necrolysis (SJS/TENS), and pemphigus have also been reported [27]. In addition, fludarabine has been associated with acceleration of both BCC and SCC growth; although the mechanism is not entirely clear, this may relate to its immunosuppressive effects [28, 29].
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an inosine monophosphate dehydrogenase inhibitor that is used as an immunosuppressive. Like the calcineurin inhibitors, this medication is used primarily for GvHD prophylaxis in patients undergoing alloHCT with acute leukemias. Cutaneous toxicities are fairly limited with mycophenolate mofetil. Patients may experience aphthous stomatitis, acne, edema, and worsening of dyshidrotic eczema [19]. While MMF use also appears to increase the risk of skin cancer [30], one study found that converting from calcineurin inhibitors to MMF as the maintenance immunosuppressant following alloHCT decreased non melanoma skin cancer risk [31].
Tacrolimus
Tacrolimus, a calcineurin inhibitor, has similar clinical effects and indications as cyclosporine. Compared to cyclosporine, tacrolimus has a relatively mild cutaneous toxicity profile. Telogen effluvium can occur frequently and is believed to be secondary to tacrolimus induced vasoconstriction of vessels supplying the hair follicles [32]. Increased risk of skin cancer has been well substantiated in the literature; as such, the FDA issued a black box warning for tacrolimus in 2005 [30, 33]. This risk of skin carcinoma appears to be increased with greater cumulative exposure to tacrolimus [34, 35].
Thiotepa
Thiotepa is an alkylating agent that is often used as part of the conditioning regimen prior to alloHCT. Development of dAEs is common – in one pediatric study, all patients developed skin reactions (TEC) to high-dose thiotepa. Morphologies included desquamation (97%), erythema (95%), hyperpigmentation (84%), and pruritus (68%), primarily in the intertriginous areas [36]. Thiotepa associated hair loss also occurs often, and increased exposure to thiotepa has been shown to increase risk of permanent chemotherapy induced alopecia [37].
TARGETED THERAPIES
Alemtuzumab
Alemtuzumab is a monoclonal antibody that binds to CD52, an antigen that can be found on both normal and malignant mature lymphocytes. It is currently FDA approved for the treatment of chronic lymphocytic leukemia and has been used in some protocols as part of the preparative regimen for alloHCT. DAEs occur frequently among patients taking this medication – in clinical trials, patients experienced rash (52%), urticaria (16%), pruritus (14%), flushing (10%), dermatitis (8%), erythema (5%), and peripheral edema (5%) [38]. Anaphylactic infusion reactions may also occur [39].
Anti-thymocyte globulin
Anti-thymocyte globulin (ATG) is an equine or rabbit derived antibody that targets T cells and thymocytes, often used as GvHD prophylaxis in patients undergoing HCT. In clinical trials, dAEs reported by patients included rash (11%), edema (6%), pruritus (6%), and herpes simplex reactivation (4%) [40]. In addition, type III hypersensitivity reactions (serum sickness) are a well-recognized complication – these present in 0.25–8.5% of patients and typically begin one or two weeks after ATG infusion [41]. Rarely, immediate type I reactions including anaphylaxis may also occur [42].
Blinatumomab
Blinatumomab is used in relapsed and refractory ALL. Blinatumomab is a bispecific T cell antibody that engages CD19, which is expressed on over 90% of B cell precursor ALL blasts, and CD3 positive cytotoxic T cells. This interaction activates cytotoxic T cell driven lysis of CD19 positive ALL blasts [43, 44]. However, dermatologic toxicities occur frequently, with clinical trials reporting rash in 21–36%, edema in 30%, and stomatitis in 18.5% [43, 45] Fortunately, most cases of dAEs appear low grade, with only 1–2% of patients experiencing a high-grade form. Medium vessel vasculitis, possible secondary to blinatumomab induction of a type III hypersensitivity state, has also been described [46].
FLT3 inhibitors
Mutations in FMS-like tyrosine kinase 3 (FLT3) occur in about 35% of patients with AML [47]. Patients with these mutations are more likely to experience relapse of their disease. In recent years, therapies designed to target this mutation have been approved for usage in various cancers. There are several drugs in this class. Two commonly used ones, midostaurin and gilteritinib are used in the newly diagnosed and the relapsed/refractory setting, respectively. Clinical trials have shown that patients treated with these experienced greater overall and event free survival [48, 49]. Different FLT3 Inhibitors have been tried or are under trial for maintenance therapy after alloHCT [50].
DAEs occur frequently in these patients, although they have not been well characterized in the literature. Among patients on gilteritinib in clinical trials, edema, mucositis, and rash occurred in 41%, 40%, and 36% of patients, respectively. Cellulitis and skin infection were also reported, but less frequently [51]. Mucositis (66%), petechiae (36%), hyperhidrosis (14%), and rash (14%) were the most common dAEs among patients on midostaurin [52]. Cellulitis and skin infection were also reported, but less frequently [51].
In addition, cases of neutrophilic dermatoses, including Sweet syndrome and panniculitis, have been reported with these therapies [53, 54]. FLT3 inhibitors work by inducing terminal differentiation of myeloblasts and as a result may lead to differentiation syndrome and its associated neutrophilic dermatoses. If patients develop these toxicities, they made need to reduce, interrupt, or discontinue their therapeutic regimen.
IDH inhibitors
Mutations in isocitrate dehydrogenase (IDH) genes 1 and 2 occur in up to 30% of AML cases and lead to disruptions in epigenetic regulation, ultimately disrupting differentiation [55]. IDH1/2 inhibitors, including FDA approved medications enasidenib and ivosidenib, are a new class of therapies for use in patients with these mutations. They have demonstrated impressive efficacy, leading to complete remission in approximately 20% of patients [56, 57]. Enasidenib safety has been shown for maintenance therapy after alloHCT [58]. DAEs are common. In clinical trials, edema, rash, and pruritus were reported in 32%, 19–26% and 9–14% of patients, respectively [59, 60]. In one study, 32% of patients on enasidenib or ivosidenib experienced a dAE, with over 15% reporting high grade symptoms. Enasidenib associated toxicities occurred more frequently and tended to be higher grade. The most common dAEs were inflammatory dermatoses, with maculopapular rash occurring the most frequently. Infection, most frequently cellulitis, and vascular changes, most frequently edema, were also common in this cohort. Fortunately, dAEs appeared to be tolerable or manageable, with only 2 patients who experienced dAEs requiring interruption or discontinuation of their IDH inhibitor therapy [61].
Venetoclax
Venetoclax is an oral BCL2 inhibitor that is FDA approved for the treatment of AML in patients who are ≥ 75 years old or cannot undergo intensive chemotherapy due to comorbidities. Venetoclax has been tried as post-transplant maintenance therapy in AML patients at high relapse risk [62]. DAEs to this therapy have not been well characterized, but clinical trials reported rash in 13–18% and edema in 13–34% of patients [63, 64]. There are also cases in the literature of venetoclax induced vitiligo and venetoclax induced geographic tongue [65, 66]. These dAEs, while common, tend to be low grade [67].
TOTAL BODY IRRADIATION
Total body irradiation (TBI) is often given to patients undergoing HCT as part of the preparatory regimen. Administration of TBI can potentially treat malignancy in sites not penetrable by chemotherapy. TBI has been associated with numerous dAEs. In one single center analysis, 20% and 53% of patients experienced alopecia and oral mucositis, respectively [68]. These typically began within 90 days of undergoing TBI treatment. Permanent alopecia has also been reported [12, 69]. Dermatitis is well described in patients undergoing radiotherapy but occurs rarely in patients undergoing TBI due to lower and more fractionated doses [68]. An increased risk of basal cell carcinoma in alloHCT recipients with TBI exposure has been detected in some studies [70].
ANTIBIOTICS
Patients with acute leukemia have an increased risk for infection. The malignancy itself can lead to the production of defective lymphocytes with impaired cell-mediated immunity, and prolonged treatment with chemotherapy can cause severe neutropenia, ultimately increasing the risk of fungal, bacterial, and viral infections [41]. Oftentimes, antimicrobials are given prophylactically to these patients to prevent the development of severe infections. As a result, patients with acute leukemia are exposed to a wide array of antibiotics and other antimicrobials. This frequent use of antimicrobials carries a multitude of risks, including the development of cutaneous toxicities.
Trimethoprim-Sulfamethoxazole
Trimethoprim-sulfamethoxazole (TMP-SMX) is often used to prevent infection in alloHCT recipients [71]. TMP-SMX is commonly implicated in the development of drug hypersensitivity reactions; the active metabolites of sulfamethoxazole (SMX) are generally thought to be the causative agents [72]. Skin reactions occur in roughly 3–4% of patients receiving the medication and can vary in morphology – maculopapular eruptions, diffuse erythema, urticaria, erythema multiforme and purpura have all been reported [73]. Patients may also notice increased photosensitivity. Severe hypersensitivity reactions, including drug rash with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome/toxic epidermal necrosis (SJS/TEN), and anaphylaxis may also occur. These severe reactions occur rarely, but the SMX moiety and other sulfonamides appear to disproportionately increase risk of their development relative to other antibiotics [74]. In patients who develop hypersensitivity reactions (excluding SJS/TEN/DRESS) desensitization may allow for continued treatment with TMP-SMX and is generally quite successful [75].
Voriconazole
Voriconazole is an antifungal used to treat numerous fungal infections, including aspergillosis and candidiasis. It is commonly given prophylactically to patients who undergo alloHCT, as they are more predisposed to developing these fungal infections. Although effective, voriconazole has also been associated with a wide array of adverse effects, including visual changes, hallucinations and hepatic enzyme abnormalities [76]. Dermatologic toxicities have also been reported.
Phototoxicity occurs in approximately 8% of patients on voriconazole and often presents about 120 days following initiation of treatment [77]. Up to one third of children treated with voriconazole are affected [78]. Morphologies of these eruptions include cheilitis, exfoliative dermatitis, and pseudoporphyria [77]. Patients may also experience hypersensitivity to voriconazole, including severe reactions such as anaphylaxis and SJS/TEN [79, 80]. In addition, patients can develop chronic phototoxicity and accelerated photoaging, which often manifest as lentigines or actinic keratoses [81]. These symptoms possibly develop due to the generation of reactive oxygen species by voriconazole and its metabolite voriconazole N oxide, which leads to sensitization of keratinocytes to UVA light and resultant DNA damage [82].
By a similar mechanism of action, voriconazole also increases risk of squamous cell carcinoma. A systematic review found that patients were almost twice as likely to develop SCC if they had previously used voriconazole [83]. This relationship also appears to be dose dependent – one study reported that every 60-day exposure at standard dosing of 200 mg BID resulting in a 6% increased risk of developing SCC [84]. HPV may also be implicated in the pathogenesis, as many patients, regardless of immunosuppression status, have detectable HPV DNA within their voriconazole associated SCCs [85]. Cases of melanoma development following photosensitive reactions to voriconazole have also been reported, but the relationship between the two has not been well investigated [86]. Voriconazole does not display the same relationship with basal cell carcinoma [83].
Chimeric antigen receptor T cell therapy (CAR-T)
Chimeric antigen receptor (CAR) T-cell therapy is a promising treatment modality with broadening scope due to its efficacy in the treatment of hematological malignancies. The CAR T-cell therapy is based on genetically engineering of T cells with selective targeting of tumor specific antigens which result in immune mediated destruction of tumor cells [87]. Given the expansion of CAR-T cell therapy indications, more patients will experience toxicities associated with these cytotoxic therapies due to the release of pro-inflammatory cytokines [88]. The cutaneous toxicities of various grade reported for FDA approved CAR T-cell products ranges from 9–31% with less than 5% of grade 3 or higher adverse events (AEs) [89–91]. The dAEs include erythematous maculopapular exanthemas as the most common one, petechial, pustular and bullous eruptions. The histopathology findings vary in correlation with etiopathogenesis and morphology. These include dermal infiltration by lymphocytes compatible with eruption of lymphocyte recovery, vacuolar interface changes and subepidermal bullae, papillary edema in conjunction with dense CD8+ and CD3+ CAR T cells predominance in the bulla in blistering reactions [92, 93]. The latency period for development of cutaneous toxicities with CAR T-cell therapy is highly variable and reported from day 1 to 19 months after CAR T-cell infusion. Clinicians should keep in mind the higher-than-expected short time frames of immunologic reactions when evaluating the patients with history of CAR-T therapy for a new eruption. Most of the dermatological toxicities associated with CAR T therapy are minor and resolve spontaneously with conservative management or topical steroids. Grade 3 or higher dermatological toxicities like blistering dermatoses occur less frequently, but may need prompt topical and systemic steroid therapy [94].
Graft-versus-Host Disease (GVHD)
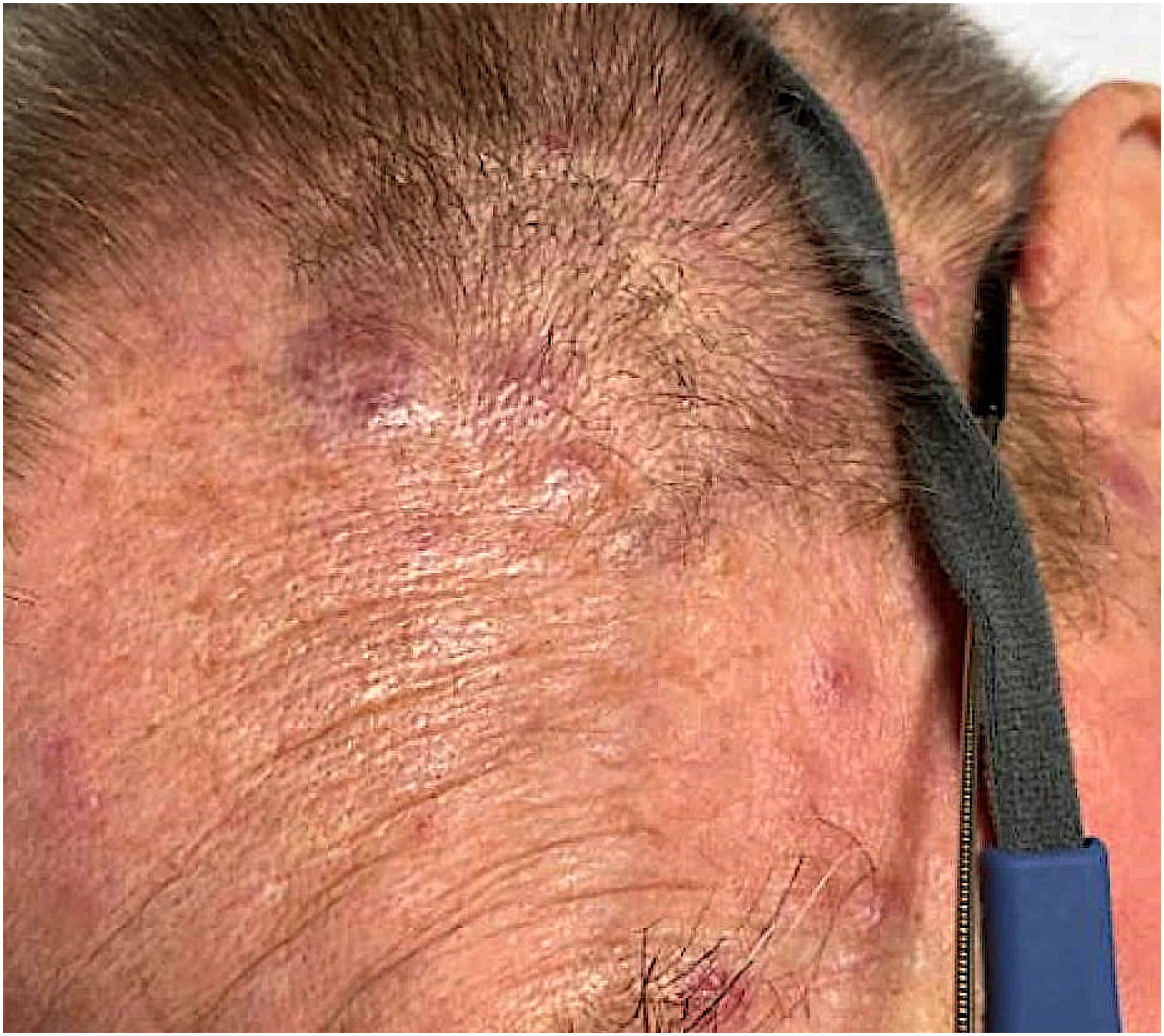
GVHD is an immune mediated multisystem disorder seen in 30–70% of patients who undergo allogenic HCT (alloHCT) [95]. It is associated with significant morbidity and mortality. Early recognition and management is important for improving quality of life and overall survival [96]. Acute GVHD is characterized by the activation of donor T cells with targeting of recipient tissues – mainly skin, liver and the gastrointestinal system [97]. Skin is the most frequently affected organ. GVHD is broadly classified into acute GVHD (aGVHD) and chronic GVHD (cGVHD) based on the timing of onset, using a cutoff of 100 days traditionally; however, the signs and symptoms of both aGVHD and cGVHD may overlap and can be seen outside the cutoff period. The clinical findings are predominantly used to distinguish and treat the two entities. Clinical manifestations of aGVHD of skin usually include a maculopapular rash which can accompany other organs involvement with persistent abdominal pain, diarrhea, and liver dysfunction with rising serum bilirubin [98]. The staging of skin aGVHD is based on the involvement of body surface area (BSA). Stage 1 with rash <25% of BSA, stage 2 with 25–50% of BSA, stage 3 > 50% of BSA and stage 4 having generalized erythroderma with bullae and/or desquamation [99]. The rash usually starts around the time of white blood cell engraftment and frequently involves the areas around the nape of the neck, ears, shoulders, and the palmar and plantar skin [100]. It is usually a pruritic maculopapular eruption (FIGURE 3) which is occasionally painful like sunburn. In severe cases, lesions can be more widespread with generalized erythroderma, bullae, and skin sloughing [101]. The skin biopsy has limited diagnostic utility for aGVHD due to histopathological similarities between the lesions related to aGVHD, drug eruption and viral exanthems [102]. The histopathological findings suggestive of aGVHD include dyskeratosis, interface dermatitis, vacuolar degeneration of basal layers and superficial infiltrates [103]. These histological findings are not specific to aGVHD and decision for diagnosis and treatment should be based mainly on the clinical judgement [104, 105]. A careful history, examination, investigation for extracutaneous organ involvement, and biopsies can aid in the diagnosis. The treatment of cutaneous aGVHD depends upon extent and severity of skin eruptions and other organ involvement. Topical steroids should be considered for stage 1–2 cutaneous aGVHD. For the trunk and extremities, moderate to high potency topical corticosteroids can be used. In patients with involvement of the face, groin and skin folds, low potency topical steroids and topical calcineurin inhibitors should be considered [106]. The patients with higher grade lesions (stage 3–4) should be treated with systemic steroids (prednisone 1–2 mg/kg) along with the support of topical corticosteroids. There is need for multi-disciplinary care in high grade skin lesions with involvement of the wound care team and aggressive skin care with application of burn sheets, topical and antibacterial emollients, non-adherent and impregnated dressings with regular gentle cleansing [107]. In the setting of refractory widespread disease, adding oral Janus Kinase 1/2 inhibitor ruxolitinib) should be considered [108]. There are other non-standard options available in steroid refractory disease which can be considered in a case-by case basis. These include clinical trials, extracorporeal photopheresis (ECP), tumor necrosis factor (TNF) antagonists (infliximab, etanercept), mycophenolate mofetil (MMF), antithymocyte globulin therapy (ATG), sirolimus (mTOR inhibitor) and pentostatin [109–114].
Figure 3:

Acute GVHD following alloSCT for AML.
Of note, some patients can present with aGVHD pictures outside of expected timeframe. Most notably, tapering immunosuppression which is routinely performed to prevent or treatment of relapse, elicits GVHD [115]. Donor lymphocyte infusion (DLI) which is used as a rescue ro treat the relapse or prevent it in stem-cell transplantation provokes GVHD [116].
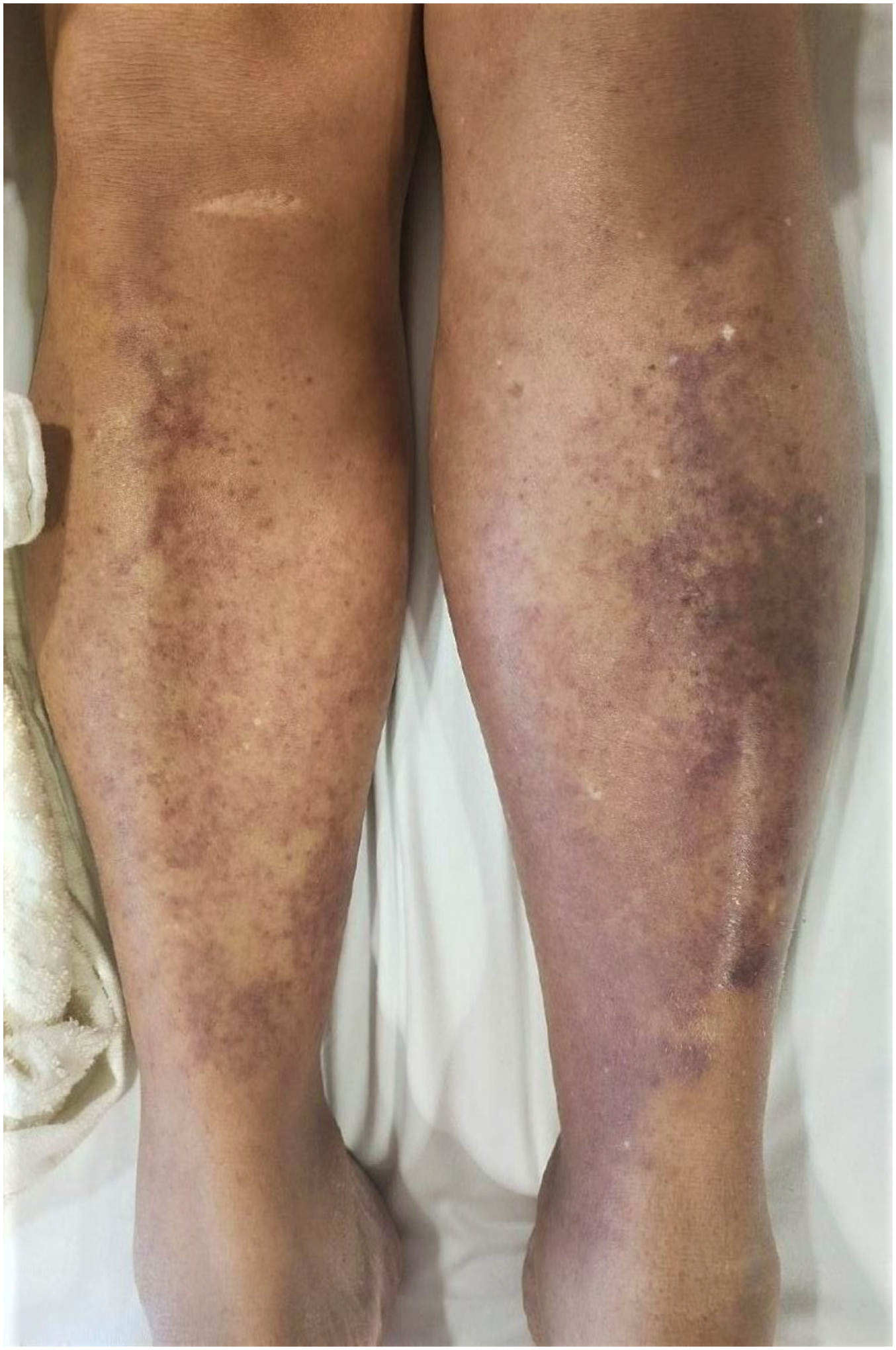
cGVHD usually affects 30–50% of alloHCT patients with approximately 30% of cases occurring de-novo without any previous history of aGVHD. It is the leading cause of non-relapse mortality in patients otherwise cured of their underlying primary malignant disease [117, 118]. The development of cGVHD is characterized by chronic inflammation in the setting of repeated tissue injury with loss of central immune tolerance and aberrant tissue repair mechanisms [119]. cGVHD is characterized by skin involvement with lichen planus or scleroderma like cutaneous lesions, dry oral mucosa, oral ulcerations with sclerosis of gastrointestinal tract and liver dysfunction with elevated serum bilirubin level [120]. The most common non-sclerotic cutaneous features in cGVHD are lichen planus or poikiloderma-like cGVHD [121]. The lichen planus lesions are flat-topped erythematous papules or plaques mainly on the dorsal aspects of hands, feet, forearms and trunk. Poikiloderma like GVHD is characterized by skin atrophy and pigmentary and telangiectatic changes [100]. Sclerotic cGVHD usually develops in the later stage of disease and can affect the skin areas similar to lichen planus-like lesions. It can present as a firm skin-colored, shiny, hyper- or hypo-pigmented plaques, hair loss, lichen sclerosis-like white plaques, scleroderma-like lesions with contractures, progressive loss of range of motion and joint dysfunction. In some cases, chronic sclerotic changes and skin atrophy due to prolonged steroids can lead to chronic non-healing ulcers (FIGURE 4) [122, 123]. The 2014 NIH cGVHD consensus criteria and scoring system is the most commonly used method for evaluating the severity of mucocutaneous cGVHD [124]. The skin lesions are scored from 1 to 3 based on the degree of skin involvement with 1–18%, 19–50% and >50% BSA, respectively. A sclerosis severity score of 2 is given for superficial sclerotic features (no hidebound, lesion able to pinch) and a score of 3 is given for deep sclerotic features (hidebound, unable to pinch) or impaired mobility and ulceration. Oral disease is recorded with presence or absence of lichen planus like features and scored based on the severity of symptoms and limitation in oral intake from 1 to 3 for no, partial, or major limitations, respectively [124]. The diagnosis is usually made by 4-mm punch biopsy in sclerotic cGVHD. [125]. The mainstay of therapy for cutaneous cGVHD is the use of medium-to-high-potency corticosteroids, topical calcineurin inhibitors (pimecrolimus, tacrolimus) and ultraviolet (UV) phototherapy. Given the chronic nature of cutaneous changes, the combination of topical and systemic therapy is usually administrated in moderate to severe cases for accelerated response and to prevent irreversible effects. Similar to aGVHD, the choice and strength of topical corticosteroids or calcineurin inhibitors depend upon the involved area of skin. The standard first-line systematic therapy is prednisone (1mg/kg daily) [126]. In steroid-refractory disease, oral ruxolitinib, oral rho-kinase inhibitor belumosudil, and/or Bruton’s tyrosine kinase inhibitor (BTK) ibrutinib are approved as a subsequent line of therapy [127]. For cases refractory to initial FDA-approved therapies, clinical trials, ECP, MMF, rituximab, methotrexate (MTX), pentostatin, sirolimus, imatinib and hydroxychloroquine have been used with variable efficacy [128–134].
Figure 4:

Early inflammatory phase of sclerodermoid GVHD (left), fully developed sclerodermoid GVHD complicated by progressive ulceration (right, top and bottom).
Paraneoplastic Dermatological Manifestations
Paraneoplastic skin lesions are sometimes the first manifestation of underlying occult disease or disease recurrence in which prompt recognition and diagnosis can help in the early diagnosis of the primary malignant process [135, 136]. The most common cutaneous paraneoplastic diseases seen in patients with leukemia are neutrophilic dermatoses including Sweet syndrome and pyoderma gangrenosum, paraneoplastic pemphigus and vasculitis [137].
Sweet syndrome (acute febrile neutrophilic dermatosis) is characterized by the abrupt onset of painful erythematous papules, plaques or nodules along with fever and leukocytosis. Malignancy-associated Sweet syndrome is most often hematological and is most commonly seen alongside AML followed by MDS [138, 139]. The underlying pathogenesis may be related to a hypersensitivity reaction to cancer antigens and/or cytokine dysregulation. The inflammatory process leads to dermal edema, pustule formation and development of targetoid lesion [139]. The upper extremity is usually the most common site of involvement (FIGURE 5), followed by the trunk, lower extremities, and head and neck. In bullous Sweet syndrome, there are vesicles and flaccid bullae along with erythematous to violaceous plaques with occasional ulceration [140]. In a less common variant, subcutaneous Sweet syndrome, there is neutrophilic infiltration of subcutaneous fat rather than dermis with development of subcutaneous nodules without significant dermal changes [141]. The diagnosis of Sweet syndrome is based on an abrupt onset of painful erythematous plaques or nodules, histopathologic evidence of a dense neutrophilic infiltrate with two minor criteria including fever, underlying malignancy, abnormal laboratory values at presentation (elevated ESR, CRP) and excellent response to therapy with systemic steroids or potassium iodide [142, 143]. Topical and systemic steroids are the first line of therapy for patients with Sweet syndrome. Topical corticosteroids are preferable for patients with localized skin involvement. Systemic steroids usually start at a dose of 0.5–1 mg/kg per day and usually taper over the period of 4–6 weeks after clinical response [144]. In patients with a contraindication to systematic glucocorticoids, colchicine, dapsone and potassium iodide are some of the options [145]. In selected case of severe disease, pulse high dose IV steroids can be used for 3–5 days followed oral steroid therapy with a gradual taper [144, 146]. If refractory to first line agents, patients can be treated with cyclosporine, methotrexate, oral retinoids such as etretinate, anakinra and TNF antagonists on a case-by-case basis [147–153].
Figure 5:

Sweet’s syndrome in an AML patient on induction chemotherapy.
Pyoderma gangerosum (PG) is another neutrophilic dermatosis associated with underlying hematological malignancy [154]. It is an ulcerative disorder with formation of inflammatory papules or pustules that progress to a painful ulcer (FIGURE 6) with a violaceous undermined border and a purulent base [155]. PG has four major subtypes: ulcerative (classic), bullous, pustular and vegetative [156]. The development of lesions is usually rapid, and patients’ reported pain score is much higher than expected based on the degree of skin lesion [157]. The most common site of skin involvement is the lower extremities, but lesions can occur in any other area of body. Bullous PG is the less common form and commonly seen in patients with hematological malignancy. The diagnosis of PG requires detailed history and physical examination along with skin biopsy for histopathological confirmation and to rule out other more common etiology of skin lesions like infection. The biopsy should include both the inflamed border and ulcer area along with subcutaneous fat [158]. Overall, PG is a diagnosis of exclusion and can be challenging [159]. The multi-disciplinary approach with local wound care and in some cases plastic surgery is very important for wound healing [160]. The initial therapy in patients with mild and localized PG is usually high potency topical steroids (e.g., clobetasol) and topical calcineurin inhibitors (tacrolimus 0.1%) [161]. The initial clinical improvement can be seen within a couple of days to weeks of treatment, but wound healing of ulcerated lesion may take several weeks to months. In cases of extensive or rapidly progressive disease, systemic corticosteroids (prednisone 0.5 to 1.5 mg/kg per day) can be used as initial therapy. After initial clinical response, steroids can be tapered in 4–10 weeks with close monitoring. In patients with intolerance or lack of response to glucocorticoids, cyclosporine, TNF antagonists, MMF, azathioprine, MTX, and dapsone are some of the options [160, 162, 163].
Figure 6:

Early stage of pyoderma gangrenosum (biopsy proven prior to deep ulceration) in a MDS patient on azacytdine (left) with relatively rapid resolution after 2 weeks on systemic steroid (right).
Infections
Skin infections are seen in about 8–10 % of patients with acute leukemia due to the impaired immune responses related to disease itself and associated treatments [164]. However, infections in the setting of HCT occur in the context of multiple interplaying factors including but not limited to the conditioning regimen, GVHD status and use of immunosuppressive regimens [165]. Invasive fungal infections with cutaneous manifestations are a major cause of morbidity and mortality in acute leukemia and alloHCT patients, in particular with a history of prolonged neutropenia. Aspergillus, Fusarium and Zygomycetes are some notable examples [166]. Herpes simplex virus (HSV) commonly affects the mucocutaneous orofacial region (85–90%) and genital areas (10–15%). Primary infection is not common and the majority of lesions are associated with reactivation of latent virus [167]. Varicella-zoster virus (VZV) causes varicella (chickenpox) and reactivation results in herpes zoster (shingles) which appear as grouped painful, pruritic, vesicular lesions in a dermatomal distribution [167]. Demodex folliculorum has recently been more scrutinized in hematological malignancies and alloHCT patients. It usually presents as an itching, redness and inflammation of affected area [168]. Facial involvement is the most common site and can easily be diagnosed as GVHD. Cutoff sign on the scalp described as a sharp demarcation between erythematous skin and spared scalp at the frontal and temporal hairline is one of the differentiating features [169]. A notable cutaneous bacterial infection in immunocompromised patients is ecthyma, associated with Pseudomonas aeruginosa, staphylococci, or group A beta-hemolytic streptococci and usually presents as a deep, painful erythematous or hemorrhagic nodules or vesicles that can progress to ulcers and crusty sores [170].
Leukemia Cutis
Leukemia cutis (LC), also known as cutaneous myeloid sarcoma, is most common in AML although it can be seen in other types of leukemia. It has been associated with an unfavorable prognosis. The morphology varies and includes classic papules (FIGURE 7), nodules, plaques or rare manifestations with erythroderma, blistering or ulceration [171].
Figure 7:
Leukemia cutis as the first presenation in aucte monocytic leukemia.
Although LC is not a direct complication of cellular therapy, it demands high attention in HCT candidates. LC can develop anytime in the course of the disease including- in the author’s experience-immediately pre-HCT. The chemotherapy that can induce sustainable marrow remission and prepares the patient for HCT may not eliminate the LC component. Indeed, skin can become a sanctuary for leukemia cells [172]. If missed prior to the HCT, the patient would undergo HCT while not in remission.
Skin cancers
HCT survivors have an increased risk of UV-induced skin cancers including squamous cell carcinoma (SCC), basal cell carcinoma (BCC) and melanoma. Male sex, clinically photodamaged skin, history of skin cancer, history of chronic lymphocytic leukemia, and acute and chronic GVHD are risk factors for cutaneous SCC post alloHCT [173, 174]. Total-body irradiation is a major contributor to BCC development [175]. Some of the medications used in the setting of HCT including voriconazole and lenalidomide increase the risk of developing skin cancers. (Refs) Sun protection measures and early detection of precancerous and cancerous lesions with regular periodic surveillance are recommended. Annual dermatologic exams generally are appropriate; however, among patients with cGVHD or a history of skin cancer, surveillance every 6 months is preferred [176].
Practice Points
Becoming familiar with acute cutaneous complications of cellular therapies in patients with acute leukemia.
Early diagnosis of the skin toxicities to intervene at earlier stages.
Preventive measures regarding increased risk of future cutaneous malignancies in patients
Research Agenda
Review Article; Regarding the broad nature of the topic, the authors tried to provide a concise and at the same time comprehensive coverage for the readers regarding the most important and widely seen cutaneous complications of cellular therapies.
Dr Markova Disclosures:
Research funding from Incyte Corporation and Amryt Pharma; Consults for ADC Therapeutics, Blueprint Medicines, Alira Health, Protagonist Therapeutics, OnQuality, and Janssen; Royalties from UpToDate
This study was supported by the NIH/NCI Cancer Center Support Grant P30-CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr Babakoohi, Stepahnie Gu and Dr Ehsan have no disclosures.
References
- [1].Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524–9. [DOI] [PubMed] [Google Scholar]
- [2].Wolf R, Tüzün Y. Baboon syndrome and toxic erythema of chemotherapy: Fold (intertriginous) dermatoses. Clin Dermatol. 2015;33:462–5. [DOI] [PubMed] [Google Scholar]
- [3].Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, Hashmi SK, Bridges AG, Lehman JS, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19–25. [DOI] [PubMed] [Google Scholar]
- [4].Ríos-Viñuela E, Bernia E, Toledo-Pastrana T, Requena C, Diago A, Serra-Guillén C, et al. Localized Injection-site Toxic Erythema of Chemotherapy: An Under-recognized Acquaintance Revisited. Acta Derm Venereol. 2021;101:adv00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mehta H, Mete UK, Gupta P, Ranjan KR, Nahar Saikia U, Mahajan R. Toxic epidermal necrolysis-like presentation of toxic erythema of chemotherapy. Clin Exp Dermatol. 2022;47:1201–3. [DOI] [PubMed] [Google Scholar]
- [6].Parker TL, Cooper DL, Seropian SE, Bolognia JL. Toxic erythema of chemotherapy following i.v. BU plus fludarabine for allogeneic PBSC transplant. Bone Marrow Transplant. 2013;48:646–50. [DOI] [PubMed] [Google Scholar]
- [7].Leisch M, Pfeilstöcker M, Stauder R, Heibl S, Sill H, Girschikofsky M, et al. Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents-A Prospective Cohort Study of the AGMT Study-Group. Cancers (Basel). 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martínez-Francés A. Adverse effects of azacitidine: onset, duration, and treatment. Adv Ther. 2011;28 Suppl 4:1–5. [DOI] [PubMed] [Google Scholar]
- [9].Verma P, Chandra U, Shukla P, Verma SP, Suvirya S. Reticular Skin Rash as an Adverse Effect of 5-Azacitidine. Cureus. 2022;14:e24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Trickett HB, Cumpston A, Craig M. Azacitidine-associated Sweet’s syndrome. Am J Health Syst Pharm. 2012;69:869–71. [DOI] [PubMed] [Google Scholar]
- [11].Sprunt JG, Rizza CR. Pigmentation and busulphan therapy. Br Med J. 1966;1:736–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bresters D, Wanders DCM, Louwerens M, Ball LM, Fiocco M, van Doorn R. Permanent diffuse alopecia after haematopoietic stem cell transplantation in childhood. Bone Marrow Transplant. 2017;52:984–8. [DOI] [PubMed] [Google Scholar]
- [13].Ljungman P, Hassan M, Békássy AN, Ringdén O, Oberg G. Busulfan concentration in relation to permanent alopecia in recipients of bone marrow transplants. Bone Marrow Transplant. 1995;15:869–71. [PubMed] [Google Scholar]
- [14].Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–47. [DOI] [PubMed] [Google Scholar]
- [15].Deutsch A, Leboeuf NR, Lacouture ME, McLellan BN. Dermatologic Adverse Events of Systemic Anticancer Therapies: Cytotoxic Chemotherapy, Targeted Therapy, and Immunotherapy. Am Soc Clin Oncol Educ Book. 2020;40:485–500. [DOI] [PubMed] [Google Scholar]
- [16].Kim GM, Kim S, Park HS, Kim JY, Nam S, Park S, et al. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast Cancer Res Treat. 2017;163:527–33. [DOI] [PubMed] [Google Scholar]
- [17].Popescu NA, Sheehan MG, Kouides PA, Loughner JE, Condemi JJ, Looney RJ, et al. Allergic reactions to cyclophosphamide: delayed clinical expression associated with positive immediate skin tests to drug metabolites in five patients. J Allergy Clin Immunol. 1996;97:26–33. [DOI] [PubMed] [Google Scholar]
- [18].Xu W, Fan W, Yao K. Cyclosporine A stimulated hair growth from mouse vibrissae follicles in an organ culture model. J Biomed Res. 2012;26:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ilyas M, Colegio OR, Kaplan B, Sharma A. Cutaneous Toxicities From Transplantation-Related Medications. Am J Transplant. 2017;17:2782–9. [DOI] [PubMed] [Google Scholar]
- [20].VYXEOS™ (daunorubicin and cytarabine) liposome for injection, for intravenous use. Initial U.S. Approval: 2017. [Google Scholar]
- [21].Cetkovská P, Pizinger K, Cetkovský P. High-dose cytosine arabinoside-induced cutaneous reactions. J Eur Acad Dermatol Venereol. 2002;16:481–5. [DOI] [PubMed] [Google Scholar]
- [22].Stoll JR, Battle L, Moy A, Dusza SW, Park JH, Tallman MS, et al. Liposomal cytarabine and daunorubicin (CPX-351/Vyxeos)-associated distinct purpuric subtype of toxic erythema of chemotherapy: A retrospective review of 54 patients. J Am Acad Dermatol. 2022;86:232–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Urbantat RM, Popper V, Menschel E, Pfeilstöcker M, Forjan E, Nader A, et al. CPX-351 (Vyxeos(®)) can cause severe rash in acute myeloid leukemia-A case report. Clin Case Rep. 2021;9:1933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2015;21:1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DACOGEN® (decitabine) for injection, for intravenous use. Initial U.S. Approval: 2006 [Google Scholar]
- [26].Pastore D, Specchia G, Carluccio P, Liso A, Mestice A, Rizzi R, et al. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience. Ann Hematol. 2003;82:231–5. [DOI] [PubMed] [Google Scholar]
- [27]. Fludara®(fludarabine phosphate)
- [28].Rashid K, Ng R, Mastan A, Sager D, Hirschman R. Accelerated growth of skin carcinoma following fludarabine therapy for chronic lymphocytic leukemia. Leuk Lymphoma. 2005;46:1051–5. [DOI] [PubMed] [Google Scholar]
- [29].Larsen CR, Hansen PB, Clausen NT. Aggressive growth of epithelial carcinomas following treatment with nucleoside analogues. Am J Hematol. 2002;70:48–50. [DOI] [PubMed] [Google Scholar]
- [30].Hao X, Lai W, Xia X, Xu J, Wu Y, Lv C, et al. Skin cancer outcomes and risk factors in renal transplant recipients: Analysis of organ procurement and transplantation network data from 2000 to 2021. Front Oncol. 2022;12:1017498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aguiar D, Martínez-Urbistondo D, D’Avola D, Iñarrairaegui M, Pardo F, Rotellar F, et al. Conversion from Calcineurin Inhibitor-Based Immunosuppression to Mycophenolate Mofetil in Monotherapy Reduces Risk of De Novo Malignancies After Liver Transplantation. Ann Transplant. 2017;22:141–7. [DOI] [PubMed] [Google Scholar]
- [32].Tricot L, Lebbé C, Pillebout E, Martinez F, Legendre C, Thervet E. Tacrolimus-induced alopecia in female kidney-pancreas transplant recipients. Transplantation. 2005;80:1546–9. [DOI] [PubMed] [Google Scholar]
- [33].Gibson JAG, Cordaro A, Dobbs TD, Griffiths R, Akbari A, Whitaker S, et al. The association between immunosuppression and skin cancer in solid organ transplant recipients: a control-matched cohort study of 2,852 patients. Eur J Dermatol. 2021;31:712–21. [DOI] [PubMed] [Google Scholar]
- [34].Rodríguez-Perálvarez M, Colmenero J, González A, Gastaca M, Curell A, Caballero-Marcos A, et al. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am J Transplant. 2022;22:1671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Castellsague J, Kuiper JG, Pottegård A, Anveden Berglind I, Dedman D, Gutierrez L, et al. A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study). Clin Epidemiol 2018;10:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosman IS, Lloyd BM, Hayashi RJ, Bayliss SJ. Cutaneous effects of thiotepa in pediatric patients receiving high-dose chemotherapy with autologous stem cell transplantation. J Am Acad Dermatol. 2008;58:575–8. [DOI] [PubMed] [Google Scholar]
- [37].de Jonge ME, Mathôt RA, Dalesio O, Huitema AD, Rodenhuis S, Beijnen JH. Relationship between irreversible alopecia and exposure to cyclophosphamide, thiotepa and carboplatin (CTC) in high-dose chemotherapy. Bone Marrow Transplant. 2002;30:593–7. [DOI] [PubMed] [Google Scholar]
- [38].LEMTRADA® (alemtuzumab) injection, for intravenous use. Initial U.S. Approval: 2001 [Google Scholar]
- [39].Nye CJS, Wagner A, Kousin-Ezewu O, Jones JL, Coles AJ. A case of anaphylaxis to alemtuzumab. J Neurol. 2019;266:780–1. [DOI] [PubMed] [Google Scholar]
- [40].THYMOGLOBULIN (anti-thymocyte globulin [rabbit]) for intravenous use. Initial U.S. Approval: 1998. [Google Scholar]
- [41].Logan C, Koura D, Taplitz R. Updates in infection risk and management in acute leukemia. Hematology Am Soc Hematol Educ Program. 2020;2020:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brabant S, Facon A, Provôt F, Labalette M, Wallaert B, Chenivesse C. An avoidable cause of thymoglobulin anaphylaxis. Allergy Asthma Clin Immunol. 2017;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. Jama. 2021;325:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of Postreinduction Therapy Consolidation With Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. Jama. 2021;325:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].BLINCYTO® (blinatumomab) for injection, for intravenous use. Initial U.S. Approval: 2014. [Google Scholar]
- [46].Liau MM, Long V, Huang J, Jaffar H. Blinatumomab-associated vasculitis. JAAD Case Rep. 2017;3:395–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhao JC, Agarwal S, Ahmad H, Amin K, Bewersdorf JP, Zeidan AM. A review of FLT3 inhibitors in acute myeloid leukemia. Blood Rev. 2022;52:100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017;377:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med. 2019;381:1728–40. [DOI] [PubMed] [Google Scholar]
- [50].Blackmon A, Aldoss I, Ball BJ. FLT3 Inhibitors as Maintenance Therapy after Allogeneic Stem-Cell Transplantation. Blood Lymphat Cancer. 2022;12:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].XOSPATA® (gilteritinib) tablets, for oral use. Initial U.S. Approval: 2018 [Google Scholar]
- [52].RYDAPT® (midostaurin) capsules, for oral use. Initial U.S. Approval: 2017. [Google Scholar]
- [53].Paudel A, Dhital R, Areoye G, Basnet S, Tachamo N. Sweet’s syndrome in a granulocytopenic patient with acute myeloid leukemia on FLT3 inhibitor. J Community Hosp Intern Med Perspect. 2020;10:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yasin H, Laytem T, Sutamtewagul G, Ayyappan S. A Rare Case of Midostaurin-Associated Sweet’s Syndrome. Case Rep Hematol. 2022;2022:1099005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pirozzi CJ, Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol. 2021;18:645–61. [DOI] [PubMed] [Google Scholar]
- [56].Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med. 2018;378:2386–98. [DOI] [PubMed] [Google Scholar]
- [58].Fathi AT, Kim HT, Soiffer RJ, Levis MJ, Li S, Kim AS, et al. Enasidenib as maintenance following allogeneic hematopoietic cell transplantation for IDH2-mutated myeloid malignancies. Blood Adv. 2022;6:5857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].TIBSOVO® (ivosidenib tablets), for oral use. Initial U.S. Approval: 2018. [Google Scholar]
- [60].IDHIFA® (enasidenib) tablets, for oral use. Initial U.S. Approval: 2017 [Google Scholar]
- [61].Parisi R, Cowen EA, Stoll JR, Zhu H, Dusza S, Pulitzer MP, et al. Dermatologic adverse events associated with IDH inhibitors ivosidenib and enasidenib for the treatment of acute myeloid leukemia. Leuk Res. 2022;123:106970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kent A, Pollyea DA, Winters A, Jordan CT, Smith C, Gutman JA. Venetoclax Is Safe and Tolerable As Post-Transplant Maintenance Therapy for AML Patients at High Risk for Relapse. Blood. 2020;136:11–2. [DOI] [PubMed] [Google Scholar]
- [63].DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Baddam S, Diaz Castro J. Does Venetoclax Cause Vitiligo? Blood. 2019;134:5139-. [Google Scholar]
- [66].Krispinsky AJ, Rzepka PV, Awan F, Kaffenberger BH. GEOGRAPHIC TONGUE INDUCED BY VENETOCLAX IN A PATIENT WITH CHRONIC LYMPHOCYTIC LEUKEMIA. J Clin Aesthet Dermatol. 2019;12:11. [PMC free article] [PubMed] [Google Scholar]
- [67].VENCLEXTA® (venetoclax tablets) for oral use. Initial U.S. Approval: 2016 [Google Scholar]
- [68].Pearlman R, Hanna R, Burmeister J, Abrams J, Dominello M. Adverse Effects of Total Body Irradiation: A Two-Decade, Single Institution Analysis. Adv Radiat Oncol. 2021;6:100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979–82. [DOI] [PubMed] [Google Scholar]
- [70].Omland SH, Gniadecki R, Hædersdal M, Helweg-Larsen J, Omland LH. Skin Cancer Risk in Hematopoietic Stem-Cell Transplant Recipients Compared With Background Population and Renal Transplant Recipients: A Population-Based Cohort Study. JAMA Dermatol. 2016;152:177–83. [DOI] [PubMed] [Google Scholar]
- [71].Gaffney KJ, Urban TA, Lucena M, Rybicki L, Majhail NS, Mossad SB. Prophylactic Trimethoprim-Sulfamethoxazole for Allogeneic Hematopoietic Stem Cell Transplant Recipients During the Pre-engraftment Period. Clin Hematol Int. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Meyer C, Behm N, Brown E, Copeland NK, Sklar MJ. An Adverse Drug Reaction to Trimethoprim-Sulfamethoxazole Revealing Primary HIV: A Case Report and Literature Review. Case Rep Infect Dis. 2015;2015:691010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163:402–10. [DOI] [PubMed] [Google Scholar]
- [74].Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. [DOI] [PubMed] [Google Scholar]
- [75].Negishi S, Miyao K, Ohara F, Motegi K, Wakabayashi H, Yokota H, et al. Feasibility of trimethoprim/sulfamethoxazole desensitization therapy in hematological diseases. Clin Exp Med. 2022. [DOI] [PubMed] [Google Scholar]
- [76].Zonios D, Yamazaki H, Murayama N, Natarajan V, Palmore T, Childs R, et al. Voriconazole metabolism, toxicity, and the effect of cytochrome P450 2C19 genotype. J Infect Dis. 2014;209:1941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Epaulard O, Leccia MT, Blanche S, Chosidow O, Mamzer-Bruneel MF, Ravaud P, et al. Phototoxicity and photocarcinogenesis associated with voriconazole. Med Mal Infect. 2011;41:639–45. [DOI] [PubMed] [Google Scholar]
- [78].Sheu J, Hawryluk EB, Guo D, London WB, Huang JT. Voriconazole phototoxicity in children: a retrospective review. J Am Acad Dermatol. 2015;72:314–20. [DOI] [PubMed] [Google Scholar]
- [79].Chen J, Song X, Yang P, Wang J. Appearance of anaphylactic shock after long-term intravenous itraconazole treatment. Ann Pharmacother. 2009;43:537–41. [DOI] [PubMed] [Google Scholar]
- [80].Gomulka J, Wilson BD, Joyce JC. Toxic epidermal necrolysis due to voriconazole: case report and review. Dermatol Online J. 2014;20. [PubMed] [Google Scholar]
- [81].Cowen EW, Nguyen JC, Miller DD, McShane D, Arron ST, Prose NS, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ona K, Oh DH. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br J Dermatol. 2015;173:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tang H, Shi W, Song Y, Han J. Voriconazole exposure and risk of cutaneous squamous cell carcinoma among lung or hematopoietic cell transplant patients: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:500–7.e10. [DOI] [PubMed] [Google Scholar]
- [84].Singer JP, Boker A, Metchnikoff C, Binstock M, Boettger R, Golden JA, et al. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transplant. 2012;31:694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nguyen CV, He Q, Rady PL, Tyring SK, Miller DD, Rubin N, et al. Presence of human papillomavirus DNA in voriconazole-associated cutaneous squamous cell carcinoma. Int J Dermatol. 2020;59:595–8. [DOI] [PubMed] [Google Scholar]
- [86].Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014;58:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Siddiqi HF, Staser KW, Nambudiri VE. Research techniques made simple: CAR T-cell therapy. Journal of Investigative Dermatology. 2018;138:2501–4. e1. [DOI] [PubMed] [Google Scholar]
- [88].Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nature Reviews Cancer. 2021;21:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang B. Kymriah: FDA Approves First Gene Therapy in the United States.
- [90].Food, Administration D. YESCARTA package insert. 2018.
- [91].Food U, Administration D. Package insert: Breyanzi. WWW-document: https://wwwfdagov/media/145711/download Date of access. 2021:05–21.
- [92].Rubin CB, Elenitsas R, Taylor L, Lacey SF, Kulikovskaya I, Gupta M, et al. Evaluating the skin in patients undergoing chimeric antigen receptor modified T-cell therapy. Journal of the American Academy of Dermatology. 2016;75:1054–7. [DOI] [PubMed] [Google Scholar]
- [93].Hu Y, Zheng W, Qiao J, Cui Q, Zhu Y, Chang AH, et al. Bullous and exanthematous lesions associated with chimeric antigen receptor T-cell therapy in a patient with diffuse large B-cell lymphoma. JAMA dermatology. 2020;156:1026–8. [DOI] [PubMed] [Google Scholar]
- [94].Nusbaum KB, Dulmage B, Choi JN, Jaglowski SM, Korman AM. Cutaneous manifestations of chimeric antigen receptor T-cell therapy: An introduction for dermatologists. Journal of the American Academy of Dermatology. 2022;87:597–604. [DOI] [PubMed] [Google Scholar]
- [95].Elsabbagh EM, Shubert S, Leung K, Naik S, Gottschalk S, Allen C, et al. Acute GvHD incidence and outcome: single center experience. Biology of Blood and Marrow Transplantation. 2017;23:S231. [Google Scholar]
- [96].Jamani K, Russell J, Daly A, Stewart D, Savoie L, Duggan P, et al. Prognosis of grade 3–4 acute GVHD continues to be dismal. Bone marrow transplantation. 2013;48:1359–61. [DOI] [PubMed] [Google Scholar]
- [97].Ghimire S, Weber D, Mavin E, Wang XN, Dickinson AM, Holler E. Pathophysiology of GvHD and other HSCT-related major complications. Frontiers in immunology. 2017;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Strong Rodrigues K, Oliveira-Ribeiro C, de Abreu Fiuza Gomes S, Knobler R. Cutaneous Graft-Versus-Host Disease: Diagnosis and Treatment. Am J Clin Dermatol. 2018;19:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. The Lancet. 2009;373:1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhou Y, Barnett MJ, Rivers JK. Clinical significance of skin biopsies in the diagnosis and management of graft-vs-host disease in early postallogeneic bone marrow transplantation. Archives of dermatology. 2000;136:717–21. [DOI] [PubMed] [Google Scholar]
- [103].Lerner K. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplantation Proc 1974. p. 367–71. [PubMed] [Google Scholar]
- [104].Kohler S, Hendrickson MR, Chao NJ, Smoller BR. Value of skin biopsies in assessing prognosis and progression of acute graft-versus-host disease. The American journal of surgical pathology. 1997;21:988–96. [DOI] [PubMed] [Google Scholar]
- [105].Barksdale SK, Oberlender SA, Barnhill RL. “Rush” skin biopsy specimens in a tertiary medical center: diagnostic yield and clinical utility. Journal of the American Academy of Dermatology. 1998;38:548–54. [DOI] [PubMed] [Google Scholar]
- [106].Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. D iagnosis and management of acute graft-versus-host disease. British journal of haematology. 2012;158:30–45. [DOI] [PubMed] [Google Scholar]
- [107].Vaidya T, Menzer C, Ponce DM, Markova A. Inpatient Management of Mucocutaneous GVHD. Curr Dermatol Rep. 2019;8:258–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jagasia M, Ali H, Schroeder MA, Shah NN, Chen Y-B, Dawkins F, et al. Ruxolitinib in combination with corticosteroids for the treatment of steroid-refractory acute graft-vs-host disease: results from the phase 2 REACH1 trial. Biology of Blood and Marrow Transplantation. 2019;25:S52. [Google Scholar]
- [109].Alfred A, Taylor PC, Dignan F, El-Ghariani K, Griffin J, Gennery AR, et al. The role of extracorporeal photopheresis in the management of cutaneous T-cell lymphoma, graft-versus-host disease and organ transplant rejection: a consensus statement update from the UK Photopheresis Society. British journal of haematology. 2017;177:287–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nogueira M, Azevedo A, Pereira S, Ferreira J, Lerner D, Lobo A, et al. Anti-tumor necrosis factor-a for the treatment of steroid-refractory acute graft-versus-host disease. Brazilian Journal of Medical and Biological Research. 2007;40:1623–9. [DOI] [PubMed] [Google Scholar]
- [111].Hattori K, Doki N, Kurosawa S, Hino Y, Yamamoto K, Sakaguchi M, et al. Mycophenolate mofetil is effective only for involved skin in the treatment for steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Annals of hematology. 2017;96:319–21. [DOI] [PubMed] [Google Scholar]
- [112].MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NK, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2002;8:40–6. [DOI] [PubMed] [Google Scholar]
- [113].Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease1. Transplantation. 2001;72:1924–9. [DOI] [PubMed] [Google Scholar]
- [114].Bolanos-Meade J, Jacobsohn DA, Margolis J, Ogden A, Wientjes MG, Byrd JC, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. Journal of clinical oncology. 2005;23:2661–8. [DOI] [PubMed] [Google Scholar]
- [115].Kekre N, Kim HT, Thanarajasingam G, Armand P, Antin JH, Cutler C, et al. Efficacy of immune suppression tapering in treating relapse after reduced intensity allogeneic stem cell transplantation. Haematologica. 2015;100:1222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. 2008;21:205–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Journal of clinical oncology. 2011;29:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biology of Blood and Marrow Transplantation. 2015;21:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation. 2017;23:211–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biology of Blood and Marrow Transplantation. 2015;21:389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Peñas PF, Jones-Caballero M, Aragüés M, Fernández-Herrera J, Fraga J, García-Díez A. Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Archives of dermatology. 2002;138:924–34. [DOI] [PubMed] [Google Scholar]
- [122].Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood, The Journal of the American Society of Hematology. 2013;121:5098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jachiet M, Masson A, Peffault de Latour R, Rybojad M, Robin M, Bourhis JH, et al. Skin ulcers related to chronic graft-versus-host disease: clinical findings and associated morbidity. British Journal of Dermatology. 2014;171:63–8. [DOI] [PubMed] [Google Scholar]
- [124].Carpenter PA, Kitko CL, Elad S, Flowers ME, Gea-Banacloche JC, Halter JP, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 ancillary therapy and supportive care working group report. Biology of Blood and Marrow Transplantation. 2015;21:1167–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hillen U, Häusermann P, Massi D, Janin A, Wolff D, Lawitschka A, et al. Consensus on performing skin biopsies, laboratory workup, evaluation of tissue samples and reporting of the results in patients with suspected cutaneous graft-versus-host disease. Journal of the European Academy of Dermatology and Venereology. 2015;29:948–54. [DOI] [PubMed] [Google Scholar]
- [126].Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood, The Journal of the American Society of Hematology. 2015;125:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood, The Journal of the American Society of Hematology. 2017;130:2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Richet C, Huynh A, Dimeglio C, Borel C, Lepage B, Boulinguez S, et al. Extracorporeal photopheresis: an efficacious and well-tolerated treatment for cutaneous and oral mucosal chronic graft-versus-host disease. Dermatology. 2018;234:23–30. [DOI] [PubMed] [Google Scholar]
- [129].Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29:2062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Iida M, Fukuda T, Uchida N, Murata M, Aotsuka N, Minagawa K, et al. Mycophenolate mofetil use after unrelated hematopoietic stem cell transplantation for prophylaxis and treatment of graft- vs. - host disease in adult patients in J apan. Clinical transplantation. 2014;28:980–9. [DOI] [PubMed] [Google Scholar]
- [131].Wang Y, Xu L-p, Liu D-h, Chen H, Chen Y-h, Han W, et al. First-line therapy for chronic graft-versus-host disease that includes low-dose methotrexate is associated with a high response rate. Biology of Blood and Marrow Transplantation. 2009;15:505–11. [DOI] [PubMed] [Google Scholar]
- [132].Jacobsohn DA, Chen AR, Zahurak M, Piantadosi S, Anders V, Bolanos-Meade J, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. Journal of Clinical Oncology. 2007;25:4255–61. [DOI] [PubMed] [Google Scholar]
- [133].Couriel D, Saliba R, Escalon M, Hsu Y, Ghosh S, Ippoliti C, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. British journal of haematology. 2005;130:409–17. [DOI] [PubMed] [Google Scholar]
- [134].Baird K, Comis LE, Joe GO, Steinberg SM, Hakim FT, Rose JJ, et al. Imatinib mesylate for the treatment of steroid-refractory sclerotic-type cutaneous chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2015;21:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85:838–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Raza S, Kirkland RS, Patel AA, Shortridge JR, Freter C. Insight into Sweet’s syndrome and associated-malignancy: a review of the current literature. Int J Oncol. 2013;42:1516–22. [DOI] [PubMed] [Google Scholar]
- [137].Nelson CA, Noe MH, McMahon CM, Gowda A, Wu B, Ashchyan HJ, et al. Sweet syndrome in patients with and without malignancy: A retrospective analysis of 83 patients from a tertiary academic referral center. J Am Acad Dermatol. 2018;78:303–9.e4. [DOI] [PubMed] [Google Scholar]
- [138].Cohen PR. Sweet’s syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rochet NM, Chavan RN, Cappel MA, Wada DA, Gibson LE. Sweet syndrome: clinical presentation, associations, and response to treatment in 77 patients. J Am Acad Dermatol. 2013;69:557–64. [DOI] [PubMed] [Google Scholar]
- [140].Voelter-Mahlknecht S, Bauer J, Metzler G, Fierlbeck G, Rassner G. Bullous variant of Sweet’s syndrome. Int J Dermatol. 2005;44:946–7. [DOI] [PubMed] [Google Scholar]
- [141].Cohen PR. Subcutaneous Sweet’s syndrome: a variant of acute febrile neutrophilic dermatosis that is included in the histopathologic differential diagnosis of neutrophilic panniculitis. J Am Acad Dermatol. 2005;52:927–8. [DOI] [PubMed] [Google Scholar]
- [142].von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535–56; quiz 57–60. [DOI] [PubMed] [Google Scholar]
- [143].Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167–74. [PubMed] [Google Scholar]
- [144].Cohen PR, Kurzrock R. Sweet’s syndrome: a review of current treatment options. Am J Clin Dermatol. 2002;3:117–31. [DOI] [PubMed] [Google Scholar]
- [145].Amouri M, Masmoudi A, Ammar M, Boudaya S, Khabir A, Boudawara T, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033–9. [DOI] [PubMed] [Google Scholar]
- [146].Case JD, Smith SZ, Callen JP. The use of pulse methylprednisolone and chlorambucil in the treatment of Sweet’s syndrome. Cutis. 1989;44:125–9. [PubMed] [Google Scholar]
- [147].Rujiwetpongstorn R, Chuamanochan M, Tovanabutra N, Chaiwarith R, Chiewchanvit S. Efficacy of acitretin in the treatment of reactive neutrophilic dermatoses in adult-onset immunodeficiency due to interferon-gamma autoantibody. J Dermatol. 2020;47:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Altomare G, Capella GL, Frigerio E. Sweet’s syndrome in a patient with idiopathic myelofibrosis and thymoma-myasthenia gravis-immunodeficiency complex: efficacy of treatment with etretinate. Haematologica. 1996;81:54–8. [PubMed] [Google Scholar]
- [149].von den Driesch P, Steffan C, Zöbe A, Hornstein OP. Sweet’s syndrome--therapy with cyclosporin. Clin Exp Dermatol. 1994;19:274–7. [DOI] [PubMed] [Google Scholar]
- [150].Hrin ML, Williams J, Bowers NL, Pichardo RO, Jorizzo JL, Feldman SR, et al. Evaluation of Methotrexate in the Management of Sweet Syndrome. J Cutan Med Surg. 2022;26:532–3. [DOI] [PubMed] [Google Scholar]
- [151].Browning CE, Dixon JE, Malone JC, Callen JP. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53:S135–8. [DOI] [PubMed] [Google Scholar]
- [152].Kluger N, Gil-Bistes D, Guillot B, Bessis D. Efficacy of anti-interleukin-1 receptor antagonist anakinra (Kineret®) in a case of refractory Sweet’s syndrome. Dermatology. 2011;222:123–7. [DOI] [PubMed] [Google Scholar]
- [153].Joshi TP, Friske SK, Hsiou DA, Duvic M. New Practical Aspects of Sweet Syndrome. Am J Clin Dermatol. 2022;23:301–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244–50. [DOI] [PubMed] [Google Scholar]
- [155].Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008–17. [DOI] [PubMed] [Google Scholar]
- [156].Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB Jr., White WL, Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore). 2000;79:37–46. [DOI] [PubMed] [Google Scholar]
- [157].Wong WW, Machado GR, Hill ME. Pyoderma gangrenosum: the great pretender and a challenging diagnosis. J Cutan Med Surg. 2011;15:322–8. [DOI] [PubMed] [Google Scholar]
- [158].Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790–800. [DOI] [PubMed] [Google Scholar]
- [159].Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412–8. [DOI] [PubMed] [Google Scholar]
- [160].Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191–211. [DOI] [PubMed] [Google Scholar]
- [161].Thomas KS, Ormerod AD, Craig FE, Greenlaw N, Norrie J, Mitchell E, et al. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: A prospective cohort study. J Am Acad Dermatol. 2016;75:940–9. [DOI] [PubMed] [Google Scholar]
- [162].Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hughes AP, Jackson JM, Callen JP. Clinical features and treatment of peristomal pyoderma gangrenosum. Jama. 2000;284:1546–8. [DOI] [PubMed] [Google Scholar]
- [164].Rolston KV. Infections in patients with acute leukemia. Infections in Hematology. 2015:3–23. [Google Scholar]
- [165].Marty FM, Baden LR. Infection in the Hematopoietic Stem Cell Transplant Recipient. Hematopoietic Stem Cell Transplantation. 2008:421–48. [Google Scholar]
- [166].Rahi MS, Jindal V, Pednekar P, Parekh J, Gunasekaran K, Sharma S, et al. Fungal infections in hematopoietic stem-cell transplant patients: a review of epidemiology, diagnosis, and management. Ther Adv Infect Dis. 2021;8:20499361211039050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–70. [DOI] [PubMed] [Google Scholar]
- [168].Seyhan ME, Karincaoğlu Y, Bayram N, Aycan O, Kuku I. Density of Demodex folliculorum in haematological malignancies. J Int Med Res. 2004;32:411–5. [DOI] [PubMed] [Google Scholar]
- [169].Chen C, Timerman D, Finnin CY, Gallitano SM. Sparing of the scalp in severe Demodex folliculitis after stem cell transplantation. JAAD Case Reports. 2018;4:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hadano Y, Yoshida-Sakai N, Imamura Y, Inoue T, Koga H. Acute myeloid leukaemia presenting with ecthyma gangrenosum as the first manifestation: A case report. Medicine (Baltimore). 2021;100:e25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wagner G, Fenchel K, Back W, Schulz A, Sachse MM. Leukemia cutis–epidemiology, clinical presentation, and differential diagnoses. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2012;10:27–36. [DOI] [PubMed] [Google Scholar]
- [172].Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA dermatology. 2019;155:826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].de Masson A, Bouaziz J-D, Socié G. Who needs a skin exam? Skin cancers in allogeneic hematopoietic stem cell transplant recipients in the contemporary era. Journal of Investigative Dermatology. 2019;139:512–4. [DOI] [PubMed] [Google Scholar]
- [174].Scott JF, Brough KR, Grigoryan KV, Muzic JG, Kim GY, Conic RRZ, et al. Risk Factors for Keratinocyte Carcinoma in Recipients of Allogeneic Hematopoietic Cell Transplants. JAMA Dermatol. 2020;156:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Omland SH, Gniadecki R, Hædersdal M, Helweg-Larsen J, Omland LH. Skin cancer risk in hematopoietic stem-cell transplant recipients compared with background population and renal transplant recipients: a population-based cohort study. JAMA dermatology. 2016;152:177–83. [DOI] [PubMed] [Google Scholar]
- [176].Szlauer-Stefańska A, Kamińska-Winciorek G, Giebel S, Bagłaj M. Secondary skin neoplasms in patients after autologous and allogeneic hematopoietic stem cell transplantation procedures. Advances in Clinical and Experimental Medicine. 2020;29:1221–30. [DOI] [PubMed] [Google Scholar]


