Abstract
In this Personal View, we discuss current knowledge on SARS-CoV-2 RNA or antigen persistence in children infected with SARS-CoV-2. Based on the evidence that the virus can persist in adults, we have done a literature review and analysed studies that looked for SARS-CoV-2 RNA or antigens in children undergoing autopsy, biopsy, or surgery for either death from COVID-19 or multisystem inflammatory syndrome, or assessments for long COVID-19 or other conditions. Our analysis suggests that in children, independent from disease severity, SARS-CoV-2 can spread systemically and persist for weeks to months. We discuss what is known about the biological effects of viral persistence for other viral infections and highlight new scenarios for clinical, pharmacological, and basic research exploration. Such an approach will improve the understanding and management of post-viral syndromes.
Introduction
SARS-CoV-2 was initially isolated in December, 2019. Since then, knowledge of this virus and its interaction with humans has advanced. Thanks to enormous efforts, researchers have been able to track the viru's variants, and develop vaccines, diagnostic tests, and pharmacological treatments that have improved management of the virus and the disease it causes—ie, COVID-19. However, several unknowns remain, which still have a major effect on the health of adults and children.
One of the major challenges for patients and researchers is post-COVID-19 condition (also known as long COVID), a term that describes the persistence of otherwise unexplained signs and symptoms, which begin after SARS-CoV-2 infection, that negatively affect peoples’ daily lives.1 A similar paediatric definition has also been released.2 Hundreds of studies have been published regarding long COVID in both adults and children. Several biological abnormalities have been linked to the development of this condition, however the exact pathogenesis is still unknown.3 Currently, attention is directed towards persistence of the virus, or parts thereof, in the human body after initial infection.
In this Personal View, we will discuss the current evidence on possible SARS-CoV-2 persistence in paediatric patients, how it might affect the patient, and how it might lead to long COVID. We will also discuss why these observations can inspire future research projects for both diagnostics and therapeutics. Personal experience from a doctor and patient are described in the (appendix p 1).
SARS-CoV-2 persistence in studies on adults
SARS-CoV-2 is known to invade both the respiratory and non-respiratory tissues, causing an infection varying in severity, from asymptomatic or mild, to severe and fatal. Several autopsy studies have documented the anatomopathological findings and related immune changes of multiple organs in patients with critical disease. These findings have shown that COVID-19 is not simply a respiratory infection, but that it potentially has major effects on the whole body,4, 5, 6 including the endothelial system.7 However, as there is evidence that SARS-CoV-2 causes a persistent illness in a subgroup of patients, researchers have looked for immunopathology and viral persistence in patients that died, for any reasons, weeks to months after the initial infection. The findings provided striking evidence that parts of the virus can persist in the body.8 A major study has been recently published by Stein and colleagues, who performed complete autopsies on 44 patients who died from COVID-19. They did extensive sampling of the CNS in 11 patients to map and quantify the distribution, replication, and cell-type specificity of SARS-CoV-2 across the human body, including the brain, from the moment of acute infection onset, to more than 7 months after symptom onset.8 In all patients who had died from COVID-19 several weeks to months after initial infection, SARS-CoV-2 RNA persistence was detected across multiple tissue groups, including in the CNS across several brain regions, despite being undetectable in the plasma. Stein and colleagues found subgenomic RNA in at least one tissue in 14 of 27 patients beyond day 14, indicating that viral replication might occur in non-respiratory tissues for several months. Although the viral RNA concentration was higher in respiratory versus non-respiratory tissue samples in the first days or weeks after initial infection, differences diminished in patients who died several weeks to months after initial infection. This observation suggests lower or less efficient viral clearance in non-respiratory tissues, leading the authors to speculate that “understanding how SARS-CoV-2 evades immune detection is essential to guide future therapeutic approaches to facilitate viral clearance”.8
Other anatomopathological and immunological studies in adults have also shown that SARS-CoV-2 RNA and antigens can persist in the lung and extra-pulmonary areas,9, 10 even in immunocompetent adults.11 For example, two patients with a clinical diagnosis of long COVID who underwent surgery for other reasons 175 and 426 days after initial infection had evidence of SARS-CoV-2 RNA in the breast, appendix, and skin.12 More recently, authors profiled the plasma of 181 individuals with or without long COVID and uninfected controls, showing persistence of viral protein in long COVID concomitant immunological perturbations, such as evidence of proinflammatory and pro-fibrotic cytokines.13 Viral persistence has also been detected in immunocompromised patients,14 with evidence that the persistent virus can replicate, evolve, and even generate new variants.
Similar findings have been documented also in non-human primates infected with SARS-CoV-2,15, 16 which further reinforces the evidence for SARS-CoV-2 RNA persistence in patients.
These events have been shown in patients infected with pre-omicron variants; therefore, these findings might not be translated to patients infected with the COVID-19 omicron variant. However, as there is evidence that even patients infected with omicron develop long COVID,16, 17, 18 viral persistence should be considered as a possible hypothesis even in the newly infected patients, until proven otherwise.
SARS-CoV-2 persistence in the paediatric population
To understand whether SARS-CoV-2 can spread throughout the body in children younger than 18 years infected with SARS-CoV-2 and how long it can persist in the body, we searched PubMed for clinical studies focused on finding SARS-CoV-2 RNA, proteins, or antigens in tissues obtained during autopsy or organ biopsy done more than 24 h after the initial diagnosis of SARS-CoV-2. The search strategy and selection criteria are given in the (appendix p 1).
After screening and selection of identified articles, 21 studies were included in the review.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 General characteristics of the included studies are reported in Table 1, Table 2, Table 3, Table 4 . Eight studies (38%) were done in the USA, five (23·8%) in Brazil, four (19%) in European countries (ie, Spain, Germany, and France), and two (9·5%) were multinational studies—one originated in (4·7%) in Thailand, and one (4·7%) in South Africa. The age of patients who underwent autopsies or tissue biopsies ranged from 1 day to 17 years.
Table 1.
Presence of SARS-CoV-2 in children with critical acute illness
| Country | Sample size | Age | Sex | Diagnosis of COVID-19 complication | Baseline severity of COVID-19 | Biopsy or autopsy | Organs analysed | Findings | Time between acute infection and biopsy or autopsy | Evidence of virus, viral fragments, or antibodies | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gomes et al (2021)19 | Brazil | 1 | 14 months | Female | Critical COVID-19 | Critical | Autopsy | CNS | SARS-CoV-2 infection of brain tissue confirmed by RT-qPCR in fragments of the choroid plexus, lateral ventricle, and cortex | Acute infection | Viral RNA |
| Poisson et al (2022)20 | USA | 1 | 8 years | Female | Critical COVID-19 | Critical | Autopsy | CNS | High SARS-CoV-2 IgM levels in the CSF | 15 days | Antibodies |
| Mabena et al (2021)21 | South Africa | 12 | 35 days (median age) | 58% males, 42% females | Critical COVID-19 | Critical | Autopsy | Lung, liver, heart, and brain | SARS-COV-2 N1 and N2 detected in blood of 4 patients | Acute infection | N1 and N2 targets of the nucleocapsid gene |
| Freij et al (2020)22 | USA | 1 | 5 years | Female | Critical COVID-19 | Critical | Autopsy | Brain | Cerebellar brain biopsy positive for SARS-CoV-2 RNA | 35 days | Viral RNA |
| Menger et al (2022)23 | Germany | 1 | 4 years | Female | Critical COVID-19 | Critical | Autopsy | Lung | Presence of SARS-CoV-2 RNA | 17 days | Viral RNA |
| Bhatnagar et al (2021)24 | USA | 4 | <1 year; 17 years | Not reported | Critical COVID-19 | Critical | Autopsy | Lung and trachea | Presence of SARS-CoV-2 RNA in lungs of all the patients and in the trachea of 1 patient | 1 −3 days | Viral RNA |
| Ninan et al (2021)25 | USA | 1 | 8 years | Female | Critical COVID-19 | Critical | Autopsy | CSF, brain, bilateral lungs, blood | SARS-CoV-2 RNA tested by RT-PCR in CSF, blood, brain tissue, and bilateral lungs was negative | Acute infection | Not found |
| Duarte-Neto et al (2021)26 | Brazil | 2 | 8–12 years | Female | Critical COVID-19 | Critical | Autopsy | Lungs, heart, liver, kidneys, spleen, brain, skin, and muscle | RT-PCR for SARS-CoV-2 positive in the lungs of all patients, and in the heart of 1 patient | Acute infection | Viral RNA |
CSF=cerebrospinal fluid.
Table 2.
Persistence of SARS-CoV-2 in children
| Country | Sample size | Age | Sex | Diagnosis of COVID-19 complication | Baseline severity of COVID-19 | Biopsy or autopsy | Organs analysed | Findings | Time between the acute infection and biopsy or autopsy | Evidence of virus, viral fragments, or antibodies | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Taweevisit et al (2022)27 | Thailand | 1 | 5 years; 7 months | Male | MIS-C | Asymptomatic | Autopsy | Heart, lungs, kidneys, liver, stomach, intestine, and brain | Viral particles detected in heart, kidney, and intestine | 2 months and 15 days | Viral RNA |
| Dolhnikoff et al (2020)28 | Brazil | 1 | 11 years | Female | MIS-C | Not reported | Autopsy | Heart, lungs, kidney, liver, stomach, brain, inguinal lymph nodes, muscle, and skin | SARS-CoV-2 RNA detected on a post-mortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR by use of primers and probes set for E (envelope) gene 2 | Not reported | Viral RNA |
| Duarte-Neto et al (2021)26 | Brazil | 3 | 8–12 years | 2 female, 1 male | MIS-C | Not reported | Autopsy | Lung, heart, liver, kidneys, spleen, brain, bone marrow, colon, skin, and muscle | RT-PCR for SARS-CoV-2 positive in lung and heart of patient 1; intestine and parotid of patient 2; viral particle detected in cardiomyocytes of patient 3 | Not reported | Viral RNA |
| Mayordomo-Colunga et al (2022)29 | Spain | 1 | 12 years | Male | MIS-C | Asymptomatc | Autopsy | Intestine, heart, lungs, and pericecal lymph nod | Spike protein detected by immunofluorescence in intestine | 6 weeks | Spike protein |
| Sigal et al (2022)30 | USA | 3 | Not reported | Not reported | MIS-C | Not reported | Blood sample | Blood | Antigens detected in 3 (5·7%) of 53 samples | >2 weeks | Antigen N or S |
| Arostegui et al (2022)31 | USA | 1 | 11 years | Female | Long COVID | Mild | Colonscopy | Intestine | SARS-CoV-2 nucleocapsid proteins detected in the intestinal lamina propria | 3 months | SARS-CoV-2 nucleocapsid proteins |
| Scottoni et al (2022)32 | Multinational | 2 | 1 month; 5 months | 1 male, 1 female | Intussusception | Mild | Biopsy | Lymph node and Ileum | Immunofluorescence staining revealed the presence of SARS-CoV-2 in both the mesenteric lymph node from patient 1 and ileum from patient 2 | 5 days | Virus |
| Colmenero et al (2020)33 | Spain | 7 | 11–17 years | 4 males; 3 females | Chiblains | Asymptomatic or mild | Biopsy | Skin of the toes | Cytoplasmic granular positivity for SARS-CoV-2 spike protein shown in endothelial cells of the capillary and postcapillary venules of the upper dermis, and in epithelial cells of the secretory portion of eccrine units in all cases | 4–30 days | Spike protein |
| Miura et al (2022)35 | Brazil | 48 | Not reported | Not reported | Asymptomatic | Asymptomatic | Tonsillectomy | Tonsils and adenoids | SARS-CoV-2 genome detection rate was 20% in the tonsils, 16·27% in the adenoids, 10·41% of nasal cytobrushes, and 6·25% of nasal washes. IHC confirmed the presence of SARS-CoV-2 nucleoprotein in 15 of 16 positive tonsils samples, both in epithelium and lymphoid compartment | Not reported | Viral RNA |
| Araùjo et al (2021)34 | Brazil | 1 | 17 years | Female | Guillain-Barrè syndrome | Mild | CSF | CSF | SARS-CoV-2 RNA in CSF | 8 days | Viral RNA |
| Xu et al (2022)36 | USA | 110 | 1·7–21 years | Female | No complication | Asymptomatic or mild | Biopsy | Tonsils and adenoids | Pharyngeal tissues from COVID-19-convalescent children showed persistent expansion of germinal centre and antiviral lymphocyte populations associated with interferon-γ-type responses, particularly in the adenoids, and viral RNA in both tissues | 25–303 days | Viral RNA |
IHC=immunohistochemistry. MIS-C=multisystem inflammatory syndrome in children. CSF= cerebrospinal fluid.
Table 3.
Presence of SARS-CoV-2 in neonates and fetuses
| Country | Sample size | Age (years) | Sex | Diagnosis of COVID-19 complication | Baseline severity of COVID-19 | Biopsy or autopsy | Organs analysed | Findings | Time between the acute infection and biopsy or autopsy | Evidence of virus or viral fragments or antibodies | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schwartz et al (2022)37 | International | 6 | Fetuses | No reported | Maternal COVID in pregnancy | Critical COVID-19 | Autopsy | Whole body | 4 of 6 autopsied stillborns babies had SARS-CoV-2 in internal organs (lung, brain, kidney, and heart) | Not reported | Viral RNA |
| Lesieur et al (2021)38 | France | 1 | Fetus | Female | Maternal COVID-19 in pregnancy | Mild | Autopsy | Thymus, lung, bronchial tree, stomach, spleen, adrenal gland, kidney, oesophagus, liver, heart, pancreas, and trachea | RNA and spike protein found in lungs, and liver; RNA found in spleen and thymus; spike protein found in the stomach, heart, and lymph nodes | 11 days | Viral RNA and spike protein |
| Reagan-Steiner et al (2022)39 | 2022 | 1 | 1 day | Male | Critical COVID-19 | Critical COVID-19 | Autopsy | Whole body | SARS-CoV-2 RNA detected in lung, airway, heart, liver, spleen, and kidney tissue by conventional RT-PCR; subgenomic RNA, suggesting SARS-CoV-2 replication, detected by subgenomic RT-PCR in lung, airway, heart, and liver tissue, but not in spleen or kidney tissue | 6 days from maternal infection | Viral RNA |
Table 4.
Detection techniques and SARS-CoV-2 fragments isolated in paediatric studies, including vaccination status of included children
| Detection techniques | RNA, antigens, or antibodies | Protein | Sample type | Cell type | Vaccination status | |
|---|---|---|---|---|---|---|
| Gomes et al (2021)19 | Immunofluorescence | NA | SARS-CoV-2 spike protein | Lung and brain tissue | Pulmonary parenchymal cells, apical region of the ChP epithelium, and ependyma of the lateral ventricle | Not vaccinated |
| Gomes et al (2021)19 | Immunofluorescence | Viral double-stranded RNA | NA | Brain | Lumina of ChP capillaries and medium size blood vessels | .. |
| Gomes et al (2021)19 | RT-qPCR | Nucleocapsid genes N1 and N2 | NA | Lung, brain (ChP, lateral ventricle, basal ganglia, and cerebellum), heart, kidney, liver, stomach, trachea, and larynx | .. | .. |
| Poisson et al (2022)20 | NA | SARS-CoV-2 IgM antibodies | NA | CSF | .. | Not vaccinated |
| Mabena et al (2021)21 | RT-PCR | N1 and N2 targets of the nucleocapsid gene | NA | Blood and lung | .. | Not vaccinated |
| Frejj et al (2020)22 | RT-PCR | SARS CoV-2 RNA | NA | Brain (cerebellum) | .. | Not vaccinated |
| Menger et al (2022)23 | RT-PCR | SARS CoV-2 RNA | NA | Lung | .. | Not vaccinated |
| Bhatnagar et al (2021)24 | RT-PCR | N gene and S gene | NA | Lung and trachea | .. | 3 patients not vaccinated, 1 unknown |
| Ninan et al (2021)25 | RT-PCR | Not found | NA | Blood, CSF, brain, and lung | .. | Not vaccinated |
| Duarte-Neto et al (2021)26 | Immunohistochemistry | NA | SARS-CoV-2 nucleocapsid protein and spike protein | Lung, heart, liver, kidney, spleen, brain, fat tissue, bone marrow, and parotid | Bronchiolar cells, type II pneumocytes, pulmonary megakaryocytes, cardiomyocytes, cardiac endothelial cells, hepatocytes and biliary tract epithelium, renal epithelial tubular cells, spleen (mononuclear cells in the red or white pulp), brain endothelial cells, perivascular astrocytes, sweat glands and subcutaneous nerves, microglia, bone marrow, mononuclear cells, parotid ductal, and acinar cells | Not vaccinated |
| Duarte-Neto et al (2021)26 | RT-PCR | SARS-CoV-2 RNA, nucleocapsid N gene, and envelope E gene | NA | Lung, heart, intestine, parotid | .. | .. |
| Taweevisit et al (2022)27 | Electron microscopy | Viral particles | NA | Heart, kidney, and small bowel | Cardiomyocytes, proximal tubular epithelial cells, and enterocytes | Not vaccinated |
| Dolhnikoff (2020)28 | Electron microscopy | Viral particles | NA | Heart | Cardiomyocytes, endocardial endothelial cells, fibroblasts, and neutrophils | Not vaccinated |
| Dolhnikoff (2020)28 | RT-PCR | SARS-CoV-2 RNA, envelope gene | NA | Heart, kidney, and intestine | .. | .. |
| Mayordomo-Colunga et al (2022)29 | Immunofluorescence | NA | SARS-CoV-2 spike protein | Intestine | Cecum cells | Not vaccinated |
| Sigal et al (2022)30 | Electrochemiluminescence immunoassay | NA | SARS-CoV-2 N and S Protein | Blood sample | .. | Not vaccinated |
| Arostegui et al (2022)31 | Immunohistochemistry | NA | SARS-CoV-2 nucleocapsid proteins | Colon (intestinal lamina propria) | .. | Not vaccinated |
| Scottoni et al (2022)32 | Immunofluorescence | Viral double-stranded RNA | Angiotensin-converting enzyme 2 SARS-CoV-2 nuclear protein | Mesenteric lymph node and ileum | .. | Not vaccinated |
| Colmenero et al (2020)33 | Immunohistochemistry | NA | SARS-CoV-2 spike protein | Skin | Endothelial cells of the capillary and post-capillary venules of the upper dermis, epithelial cells of the secretory portion of eccrine units | Not vaccinated |
| Miura et al (2022)35 | RT-PCR | SARS-CoV-2 RNA | .. | SARS-CoV-2 genome detection rate: 20% in the tonsils, 16·27% in the adenoids, 10·41% of nasal cytobrushes, and 6·25% of nasal washes | .. | Not reported |
| Miura et al (2022)35 | Immunohistochemistry | NA | SARS-CoV-2 nucleoprotein | Tonsils and adenoids | Epithelium and lymphoid compartment | As above |
| Araùjo et al (2021)34 | RT-PCR | SARS-CoV-2 RNA | NA | Cerebrospinal fluid | .. | Not vaccinated |
| Xu et al (2023)36 | Droplet digital PCR | SARS-CoV-2 nucleocapsid RNA | NA | Tonsils and adenoids | .. | Not vaccinated |
| Schwartz et al (2022)37 | RT-PCR | SARS-CoV-2 RNA | NA | Lung, brain, kidney, and heart | .. | Not vaccinated |
| Lesieur et al (2022)38 | Immunohistochemistry | NA | SARS-CoV-2 envelope protein | Lung and stomach | Desquamated cells in alveolar spaces, alveolar cells, fibroblasts, granulocytic cells, and desquamated cells | Not vaccinated |
| Lesieur et al (2022)38 | Immunohistochemistry | NA | SARS-CoV-2 spike protein | Stomach, liver, and heart | Cytoplasm of the stromal cells of the submucosae and the granulocytic cells, macrophages of the liver and lymph node, cytoplasm of pericardium, endothelial, and granulocytic cells | As above |
| Lesieur et al (2022)38 | RT-PCR | SARS-CoV-2 RNA | NA | Lung, spleen, liver, and trachea | .. | As above |
| Reagan-Steiner (2022)39 | Immunohistochemistry | NA | SARS-CoV-2 nucleocapsid and spike protein | Lung and trachea | Alveolar macrophages, type II pneumocytes, and hyaline membranes | Not vaccinated |
| Reagan-Steiner (2022)39 | In situ hybridisation | SARS-CoV-2 RNA | NA | Lung, heart, and liver | Alveolar macrophages and pneumocyte, bronchiolar and submucosal gland epithelium, macrophages in lymphoid follicles in airway submucosa, and endothelial cells in myocardium vessel walls | As above |
| Reagan-Steiner (2022)39 | RT-PCR | SARS-CoV-2 RNA | NA | Lung, airway, heart, and liver | .. | As above |
CFS=cerebrospinal fluid. ChP=choroid plexus. NA=not applicable.
Eight autopsy studies19, 20, 21, 22, 23, 24, 25, 26 described the anatomopathological findings and the laboratory tests done to detect SARS-CoV-2 in tissues of children and adolescents who died because of acute illness caused by COVID-19. Five studies (23·8%)2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 reported post-mortem findings in paediatric patients diagnosed with multisystem inflammatory syndrome in children. We included two articles (9·5%)31, 32 describing the presence of virus or its fragments in gastrointestinal systems of two patients who developed two different COVID-related complications—long-COVID and intussusception. One original article (4·7%)34 described a paediatric case of Guillain-Barrè syndrome with detection of SARS-CoV-2 in cerebrospinal fluid (CSF). Two autopsy studies (9·5%)37, 38 were done on stillborn babies who died because of maternal COVID-19 infection during pregnancy, and one (4·7%)39 was done on a neonate. According to two studies (4·7%),35, 36 the persistence of viral SARS-CoV-2 RNA was found in tonsils and adenoids of children who had asymptomatic acute infection and recovered.
Presence of SARS-CoV-2 in children with critical acute illness
Eight publications discussed the post-mortem histopathological findings and the detection of SARS-CoV-2 RNA by RT-PCR in tissues of children who underwent autopsy because of complications of fatal acute infection.19, 20, 21, 22, 23, 24, 25, 26 In six studies viral RNA was detected in various organs and tissues, including the CNS.19, 24 Gomes and colleagues19 described SARS-CoV-2 RNA-positive cells in fragments of choroid plexus, lateral ventricle, and cortex of a child aged 14 months who died of COVID-19 pneumonitis. Brain tissue infection was also reported by Freij and colleagues,22 who described the case of a girl aged 5 years with SARS-CoV-2 RNA and Mycobacterium tuberculosis complex DNA found in a cerebellar biopsy. Poisson and colleagues20 reported the case of a paediatric patient who developed a cerebral vasculitis secondary to SARS-CoV-2 infection; this hypothesis finding was supported by the anatomopathological evidence of parenchymal infarct, multifocal haemorrhages, and perivascular inflammatory infiltrates along with the presence of SARS-CoV-2 IgM in the CSF. We included a case report describing the post-mortem examination of a paediatric patient who died because of acute fulminant cerebral oedema in the setting of acute SARS-CoV-2 infection.25 In the case of this girl patient, no viral RNA was identified in samples of the CSF, blood, brain tissue, or lungs. Three studies documented viral infection of respiratory tissues, in particular lung and trachea, confirmed by RT-PCR in paediatric patients with COVID-19-related pneumonia.23, 24, 29
Persistence of SARS-CoV-2 in children
11 studies reported the persistence of virus, or parts of it, in children's tissues or biological fluids for weeks to months after the acute SARS-CoV-2 infection.26, 27 Time between the initial infection and the anatomopathological and microbiological examination ranged from 4 to 303 days.33, 36 Three studies described the post-mortem detection of SARS-CoV-2 RNA in tissues of paediatric patients diagnosed with multisystem inflammatory syndrome.26, 27, 28 In two studies on children diagnosed with multisystem inflammatory syndrome the laboratory tests done could detect only viral fragments: Mayordomo-Colunga and colleagues identified spike protein through immunofluorescence in the intestine of a boy aged 12 years; Sigal and colleagues29, 30 detected SARS-CoV-2 nucleocapsid and spike antigen in plasma of three patients, corresponding to 5·7% of the samples of multisystem inflammatory syndrome in paediatric patients analysed.
We included one case report31 showing the presence of SARS-CoV-2 nucleocapsid proteins in the intestinal lamina propria of a girl with persistent gastrointestinal symptoms 3 months after the acute SARS-CoV-2 infection. In this patient, the mucosal biopsies of the colon identified a widespread lymphocytic infiltrate that could be related to the persistent viral infection. According to Scottoni and colleagues,32 SARS-CoV-2 was identified through immunofluorescence in a mesenteric lymph node and in the ileum of two young patients who underwent surgery for ileocaecal and ileocolic intussusception.
Two studies described the persistence of SARS-CoV-2, confirmed by RT-PCR, in palatine tonsils and adenoids of children diagnosed with asymptomatic or mild acute infection.35, 36 Miura and colleagues35 reported that SARS-CoV-2 RNA was detected in 20% of the tonsils analysed, 16·27% of the adenoids, 10·4% of nasal cytobrushes, and 6·2% of nasal washes. They also did immunohistochemistry, neutralisation assay, and flow cytometry, which revealed CD123+ dendritic cells as the most common infected cells.
Araùjo and colleagues reported the detection of SARS-CoV-2 RNA by RT-PCR in the CSF of a girl aged 17 years diagnosed with Guillain-Barrè syndrome related to the acute infection.34
Presence of SARS-CoV-2 in neonates and stillborn babies
In two included studies,37, 38 the authors looked for SARS-CoV-2 RNA in tissues of stillborn fetuses who died after maternal SARS-CoV-2 infection during pregnancy. In a multinational case-based retrospective study,37 in four of six autopsied stillborn babies the viral RNA was present in their organs. In these stillborn babies, the most common anatomopathological findings were related to intrauterine hypoxia and asphyxia. Lesieur and colleagues38 described an in-utero fetal death at 24 weeks of gestation that occurred 7 days after the diagnosis of symptomatic acute infection in the mother. The anatomopathological examination revealed hepatocellular damage and hemosiderosis. Microbiological tests confirmed the presence of viral RNA in lung tissue, liver, spleen, and trachea. Furthermore, the immunohistochemistry for spike protein on stomach, liver, lymph node, and heart sample gave positive results.
Steiner and colleagues39 reported autopsy findings from an extremely premature neonate who died 4 days after birth whose mother had severe acute COVID-19. Viral RNA was detected in neonatal heart and liver vascular endothelium through in-situ hybridisation and detected in different neonatal and placental samples by RT-PCR. The subgenomic RT-PCR positivity was suggestive of viral replication in the lungs, heart, and liver of the baby.
Persistence of SARS-CoV-2 or its fragments and its possible biological effects
Whether the persistence of SARS-CoV-2 RNA has biological effects is unknown; however, there is preliminary evidence that these particles can stimulate immune responses. Xu and colleagues36 collected blood, tonsils, and adenoids from 110 children who underwent tonsillectomy or adenoidectomy between September, 2020, and January, 2021, and had negative SARS-CoV-2 PCR results. They found expanded populations of lymphocytes expressing CXCR5 , which were located in the germinal centres. The populations included CXCR5+ CD8+ T cells and were similar to the progenitor cells that maintain antiviral responses in chronic viral infections.40, 41 The authors also found enrichment of various CD57+ T-cell populations, which are developed after repeated antigen exposure in chronic infections.42 Researchers have also shown that SARS-CoV-2 can infect monocytes and monocyte-derived macrophages without production of the infectious virus but preserving its infectivity.43, 44 These findings led the authors to speculate that these cells might act as spreaders for the virus in different body areas concealing the virus,43, 44 or that these infected immune cells might be a source of inflammation in long COVID.45
These findings raise the intriguing hypothesis that SARS-CoV-2 fragments can chronically stimulate local immune responses46 and, through unknown mechanisms, contribute to or be the major pathological event leading to symptoms, including myalgic encephalomyelitis or chronic fatigue syndrome, pains, and other symptoms that characterise long COVID, or even lead to uncontrolled inflammatory events of multisystem inflammatory syndrome in children.36 These data, although preliminary, are in line with the mounting evidence of dysregulated and persistent multiorgan inflammation in long COVID.47, 48 Studies to date have documented: highly activated innate immune signatures and higher expression of both type I and type III interferons, as well as CXCL9 and CXCL10 8 months after non-severe SARS-CoV-2 infection;49 a predictive signature of long COVID chronicity included elevated concentrations of plasma type II interferon and IL-2;50 self-reactive immune responses;51 and clonal and mutational maturation of memory B cells.10, 52 Chronic inflammatory events, which are subtle and undetected by routine laboratory tests such as those measuring C-reactive protein, might lead to tissue damage (including of the CNS), hypoxia-induced injury, abnormal activation of immune cells, and endothelial dysregulation.47, 53 Several studies by Pretorius and colleagues have shown the presence of circulating microclots entrapped with pro-inflammatory molecules in patients with long COVID which might play a key role in the severe spectrum of long COVID.54 Many other infections (including Ebola, Lassa, chikungunya, and influenza viruses) have been linked to long lasting immune stimulation; therefore, similar events might occur following infection with SARS-CoV-2.55
In children, data about immunological profile in long COVID compared with recovered groups are more scarce; however, we have published one study on this topic.56 In this pilot study, we documented that a subgroup of children with long COVID had a compromised ability to switch from the innate to adaptive immune response, which was shown by a contraction of the naive and switched B-cell compartments, and an unstable balance of regulatory T lymphocytes. Additionally, the expression of pro-inflammatory cytokines was not significantly different between children with long COVID and recovered children. In this cohort, viral persistence was not investigated, although the described immunological features could not allow us to rule out the presence of chronic immunological stimuli.
Other discoveries made from 2022, to 2023, in children with multisystem inflammatory syndrome might further reinforce a possible biological role of SARS-CoV-2 persistence. Multisystem inflammatory syndrome in children is a well-defined, delayed-onset, COVID-19-related hyperinflammatory condition.57 Boribond and colleagues found that children with this condition have neutrophils characterised by a distinct granulocytic myeloid-derived suppressor cell signature with highly altered metabolism. These children also have extensive spontaneous neutrophil extracellular trap formation with neutrophil activation and degranulation signatures, all triggered by SARS-CoV-2 immune complexes. These findings suggest that persistent SARS-CoV-2 antigenaemia can trigger hyperinflammatory presentation during multi-system inflammatory syndrome in children57 and, as a consequence, possibly also in long COVID. This hypothesis is coherent with observation of myeloid-derived suppressor cells as part of the dysregulated immune responses observed in children with severe COVID-19,58 in other inflammatory conditions and viral respiratory infections,59 and also detected in mild and asymptomatic COVID-19 convalescents.60
In addition to previous immunological studies, observational studies have found an increase in newly onset immune-mediated diseases in children previously infected with SARS-CoV-2, such as type 1 diabetes.61 Although a clear link and causal effect between COVID-19 and type 1 diabetes has not been clearly proven, there is clinical plausibility for a diabetogenic effect of COVID-19. Therefore, further reinforcing a possible chronic effect of SARS-CoV-2 infection on the immune system needs further investigation.
Consideration of antivirals for pharmacological trials
Nirmatrelvir is an orally administered antiviral agent targeting the SARS-CoV-2 3-chymotrypsin-like cysteine protease enzyme, Mpro,62 which plays a pivotal role in the viral replication cycle (ie, in processing viral polyproteins into functional units).62 A placebo-controlled randomised trial documented an 89% risk reduction of progression to severe COVID-19, compared with placebo, in non-hospitalised patients at high risk. This finding led to the approval of nirmatrelvir in the USA in December, 2021. Since then, the medication has been used for millions of patients with acute SARS-CoV-2 infection. However, given the medication's antiviral activity, authors have questioned whether nirmatrelvir might affect development of long COVID, given the growing evidence of possible SARS-CoV-2 RNA spread and persistence in the body after acute infection. Although studies to address this question have not yet been done, the large numbers of patients already treated during acute SARS-CoV-2 infection are providing early indirect evidence of the plausibility of this hypothesis. Xie and colleagues in 2023, published the estimates of the effect of nirmatrelvir on a prespecified panel of 12 long COVID outcomes in 9217 treated patients versus 47 123 untreated controls. Both groups had risk factors for progression to severe disease.63 Treatment with nirmatrelvir was associated with reduced risk of long COVID (hazard ratio 0·74, 95% CI 0·69–0·81; absolute risk reduction 2·32, 1·73–2·91) including reduced risk of ten of 12 long COVID sequelae (including cardiovascular, coagulation, and haematological disorders, fatigue, liver disease, acute kidney disease, muscle pain, neurocognitive impairment, and shortness of breath). This study reinforces the hypothesis that better clearance of initial viral infection would be linked to lower long-term sequelae.
Similarly, other authors have speculated that even in patients who have already developed long COVID (who were infected months before) it would be worth investigating the role of nirmatrelvir in the elimination of possible viral reservoir should be investigated. Peluso and colleagues published a case series of four patients whose symptoms improved with nirmatrelvir, suggesting that systematic study of antiviral therapy for long COVID is warranted.64 However, Peluso and colleague's finding should be interpreted with caution, because although RNA molecules are likely to be present months after infection, replicating virus is not. A study of patients at high risk of developing long COVID should be given priority over a systematic study of antiviral therapy. A trial with early treatment of those patients at higher risk of developing long COVID has a stronger rationale.
Other treatment options are also promising, such as immune modulatory agents aimed at reducing chronic inflammation. The GNC-501 study (NCT05497089) will enrol 200 patients from Swiss and EU study centres, who have severe neuropsychiatric syndromes after COVID-19. After SARS-CoV-2 infection, some patients might have a chronic expression of the pathogenic W-ENV protein triggered by the SARS-CoV-2 infection. This expression is suspected to have a major role in the persistence of inflammation in many patients with long COVID patients (NCT05497089). Immunoglobulin G4 monoclonal antibody that targets the MSRV-Env protein can neutralise its action. The treatment has already shown promising results in a trial on multiple sclerosis, which is a well known disease, in which immunity in the CNS—and between 2021, and 2023, researchers showed that previous Epstein-Barr virus infection—plays a pathogenetic role. Although the immunoglobulin G4 monoclonal antibodies role in post-acute sequelae of SARS-CoV-2 infection is still preliminary, if proved successful, the use of immune-modulating agents for treating virus-induced chronic inflammation would further reinforce the hypothesis that viral persistence (or persistence of viral fragments) might play a pathogenetic role in long COVID. Other options including steroids,65 immunoglobulins,66 and plasmapheresis, have been tested in case series or are under investigations, although the evidence behind their effectiveness is weak and highly debated. Although vaccination might not be considered an immune modulatory treatment, it is worth nothing that a 2023 systematic review found that COVID-19 vaccines might have therapeutic effects on long COVID by boosting and rebalancing the immune system.67 However, the results of this study were based on low-quality studies and there are currently no studies addressing this possible role of COVID-19 vaccines in children.
No studies reporting similar effects in children have been published. However, there are two publications describing three children with long COVID and chronic gastrointestinal symptoms, with evidence of persistent viral shedding. In two children, a gastrointestinal anti-inflammatory and immune stimulating agent was successful in eliminating the virus in the stool and symptoms resolution.29, 68 Even children with multisystem inflammatory syndrome, who are expected to have been infected with SARS-CoV-2 1–3 months before diagnosis, have been found to have persistent fecal SARS-CoV-2 positivity.69 These findings are not conclusive, but reinforce the hypothesis on the possible role of viral persistence and highlight the need for future studies on the topic.
Conclusions from other viruses
Chronic, unexplained persistence of signs and symptoms that negatively affect daily life of infected people are well known. Long-term effects of infetctions with several viruses, have been described long before COVID-19 emergence.70 Choutka and colleagues have analysed in detail the similarities between symptoms reported by patients with long COVID and those who survived other infections, including Lyme, Ebola, influenza, chikungunya, and many others. All patients reported a higher number of symptoms compared with control groups.70 These clinical observations provide evidence that the health of millions of people who have survived infections can be negatively affected for years, decades, or lifelong. Nevertheless, these patients were historically labelled as having psychiatric or psychosomatic conditions, with the negative consequences of negated access to care or research and absence of attention of funding agencies and companies. This situation continues, despite increasing evidence supporting the pathogenic role of neuroinflammation, which might be triggered by viral infections, in psychiatric diseases.71 Hopefully, long COVID will serve as a model to understand many of the currently unexplained chronic post-viral conditions.
In addition to these subtle and uncharacterised post-viral conditions, there are several other better characterised complications, including Guillain-Barré syndrome, multisystem inflammatory syndrome in children, post-measles immune deficiency, and subacute sclerosing panencephalitis.
Although subacute sclerosing panencephalitis has a very severe course and, so far, does not seem to share any neurological complications described after SARS-CoV-2 infection, the model of subacute scleoring panencephalitis is interesting and worthy of consideration. There is overwhelming evidence that most patients infected with measles do recover.72 Measles RNA has been detected in the peripheral blood mononuclear cells, urine, and the respiratory secretions in naturally infected children and animal models for several months after initial infection.72 These molecules can stimulate the immune system contributing to both life-long immunity after natural infection and immune system dysfunction.72 Persistent but anomalous measles particles have been detected in patients with subacute sclerosing panencephalitis—a condition that can be diagnosed years after initial measles infection.72 Measles virus is widely distributed in neurons and inflammation has been incontrovertibly documented in patients with subacute sclerosing panencephalitis.72 Evidence of neuronal loss can be seen on MRI and PET, and signs of focal metabolic abnormalities can also be identified with PET, even when conventional imaging is normal.73 However, measles persistence is also hypothesised to promote maturation of the immune response and development of life-long immunity.72
There is no evidence that SARS-CoV-2 infection can lead to consequences as severe as post-measles subacute sclerosing panencephalitis. However, we believe that researchers should not negate some similarities between measles and SARS-CoV-2. As in measles, SARS-CoV-2 RNA persistence has been documented, as has been its ability to stimulate the immune system. Also, brain MRI changes before and after infection, as well as PET abnormalities, have been shown in adults and children infected with SARS-CoV-2.73 Such similarities cannot be ignored, including the potential positive role of viral persistence in immune development and maturation, and should inspire future research—from long-term clinical follow-up to studying mechanisms of pathogenesis.
Future perspectives
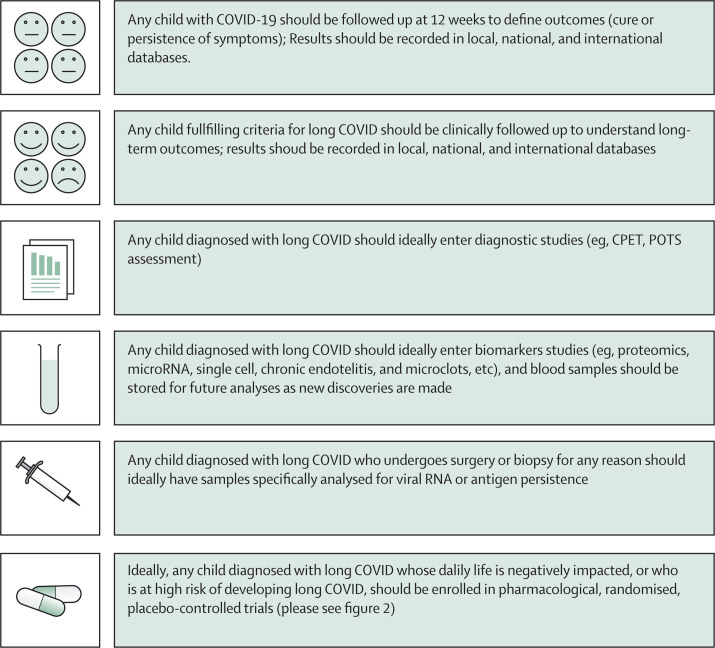
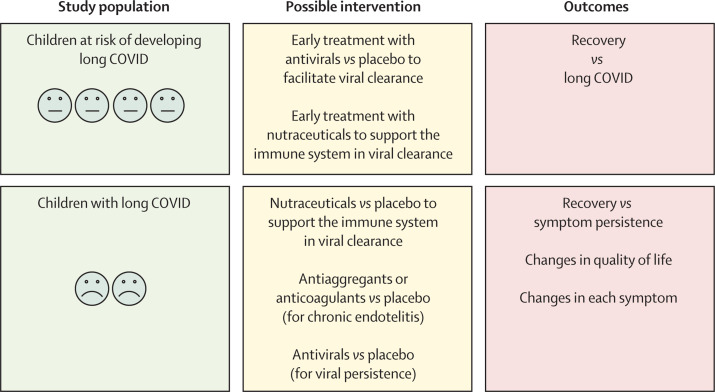
RNA persistence after viral infection is well documented;55, 74 however, its contribution to health, immunity, and chronic diseases has not been fully elucidated. The immense scientific interest in SARS-CoV-2, including from funding agencies and companies, gives a unique opportunity to better understand the effect of viral persistence on humans from biological, clinical, and therapeutic perspectives (Figure 1, Figure 2 ).
Figure 1.
Proposed basic follow-up and clinical or diagnostic studies for children with SARS-CoV-2 infection or long COVID
CPET=cardiopulmonary exercise testing. POTS= postural ortostatic tachicardia syndrome.
Figure 2.
Proposed placebo-controlled trials to treat or prevent long COVID in children, according to the viral persistence hypothesis
Trials are ongoing in the USA. Patients can be treated if they have developed long COVID, or they have acute SARS-CoV-2 infection and risk factors to develop long COVID.
From a clinical perspective, a randomised, placebo-controlled trial of early antivirals in adults and children at risk of long COVID seems justified by both available scientific evidence and the severe negative effect of long COVID on daily life of patients. Trials for children who have developed severe life-limiting long COVID would also be justified (currently registered trials in adults and children with PASC are available in a WHO platform).75
From a biological perspective, preclinical models should be developed to better understand the possible long-term effects of SARS-CoV-2 RNA persistence in the human body, and how these molecules can drive subtle, chronic inflammatory responses leading to disease. Only between 2021 and 2023, a strong link between Epstein-Barr virus and multiple sclerosis has been found, probably through reprogramming of latently infected B lymphocytes and the chronic presentation of viral antigens.76 The Epstein-Barr virus infected cells, the free virus, and its gene products have been found in the CNS,76 providing a good model for basic scientific research in the field of SARS-CoV-2 and PASC. Another key question is why only a small portion of people infected with common viruses are not able to recover from the virus and can develop long term sequelae.
However, what is needed most urgently is a paradigm shift. Long COVID has been considered a psychosomatic condition just as several other conditions, including myalgic encephalomyelitis and chronic fatigue syndrome. The evidence of biological events in patients with long COVID is overwhelming and cannot be ignored. Rather, this evidence should inspire future research and a scientific and medical revolution in the whole field of post-viral chronic conditions. Recognition of long COVID should have practical consequences and be translated in real-world clinical settings, with patients being able to receive a formal diagnosis of long COVID by their family doctor or specialist. This step should be straightforward as a formal definition of PASC has been released by the WHO for both adults1 and children.2 Long COVID diagnosis might support patients to gain access to specialised centres, but also provide social support in terms of access to dedicated insurance policies or specific support for young people (eg, school certificates for personalised schedules for children with neurocognitive problems who struggle to return to their pre-COVID-19 performance and attendance). In our paediatric long COVID unit in Rome, we issue formal diagnosis of long COVID as well as certificates for school directors, teachers, and all other extracurricular activities of our patients. This is a pivotal step in the care of children with long COVID according to our experience, since having a supportive social environment is currently most important to support a child living with a chronic condition.
Conclusions
Evidence exists for the possible spread of SARS-CoV-2 spread into different organs and persistence for weeks to months after initial infection, even in children independently from severity of the acute disease. Viral RNA has been documented in children who have died from critical acute disease, but also in paediatric patients diagnosed with multisystem inflammatory syndrome weeks to months after previous asymptomatic or mild infection with SARS-CoV-2. Whether these events can also occur with new variants of SARS-CoV-2 or in previously vaccinated children is still unknown. Although the biological significance of the possibility for viral spread and persistence in children is unknown, the substantial evidence for it should not be neglected, and should inform future clinical, biological, and pharmacological studies.
Declaration of interests
DB has received grants from Pfizer and Roche Italy to study long COVID in children. DB has participated in a peer-to-peer teaching programme on COVID-19 vaccines and long COVID in children, sponsored by Pfizer. All other authors declare no competing interests.
Contributors
DB conceptualised the report. FM implemented the research strategy. LM and RM did the literature review. PV supervised the study team. KF was the doctor with living long COVID experience, wrote her own perspective of living with long COVID, and did the language corrections. DB, PV, and LM wrote the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Material
References
- 1.WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 2.WHO A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus. 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1
- 3.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar J, Gary J, Regan-Steiner S, et al. Evidence of severe acute respiratory syndrome coronavirus 2 replication and tropism in the lungs, airways, and vascular endothelium of patients with fatal coronavirus disease 2019: an autopsy case series. J Infect Dis. 2021;223:752–764. doi: 10.1093/infdis/jiab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorward DA, Russel CD, Un IH, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med. 2021;203:192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Cleemput J, van Snippenberg W, Lambrechts L, et al. Organ-specific genome diversity of replication-competent SARS-CoV-2. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceulemans LJ, Khan M, Yoo S-J, et al. Persistence of SARS-CoV-2 RNA in lung tissue after mild COVID-19. Lancet Respir Med. 2021;9:e78–e79. doi: 10.1016/S2213-2600(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh D, Lim JCT, Fernaındez SB, et al. Case report: persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.939989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultheiß C, Willscher E, Paschold L, et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J Med Virol. 2023;95 doi: 10.1002/jmv.28364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hettle D, Hutchings S, Muir P, Moran E. Persistent SARS-CoV-2 infection in immunocompromised patients facilitates rapid viral evolution: retrospective cohort study and literature review. Clin Infect Pract. 2022;16 doi: 10.1016/j.clinpr.2022.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkai I, Mayer MG, Hellmers LM, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. 2022;13 doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morioka S, Tsuzuki S, Suzuki M, et al. Post COVID-19 condition of the Omicron variant of SARS-CoV-2. J Infect Chemother. 2022;28:1546–1551. doi: 10.1016/j.jiac.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9:815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnusson K, Kristoffersen DT, Dell'Isola A, et al. Post-covid medical complaints following infection with SARS-CoV-2 Omicron vs Delta variants. Nat Commun. 2022;13 doi: 10.1038/s41467-022-35240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes I, Karmirian K, Oliveira JT, et al. SARS-CoV-2 infection of the central nervous system in a 14-month-old child: a case report of a complete autopsy. Lancet Reg Health Am. 2021;2 doi: 10.1016/j.lana.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poisson KE, Zygmunt A, Leino D, et al. Lethal pediatric cerebral vasculitis triggered by severe acute respiratory syndrome coronavirus 2. Pediatr Neurol. 2022;127:1–5. doi: 10.1016/j.pediatrneurol.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mabena FC, Baillie VL, Hale MJ, et al. Clinical characteristics and histopathology of coronavirus disease 2019–related deaths in African children. Pediatr Infect Dis J. 2021;40:e323–e332. doi: 10.1097/INF.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 22.Freij BJ, Gebara BM, Tariq R, et al. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. 2020;20:429. doi: 10.1186/s12887-020-02308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menger J, Apostolidou S, Edler C, et al. Fatal outcome of SARS-CoV-2 infection (B1.1.7) in a 4-year-old child. Int J Legal Med. 2022;136:189–192. doi: 10.1007/s00414-021-02687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatnagar J, Gary J, Reagan-Steiner S, et al. Evidence of severe acute respiratory syndrome coronavirus 2 replication and tropism in the lungs, airways, and vascular endothelium of patients with fatal coronavirus disease 2019: an autopsy case series. J Infect Dis. 2021;223:752–764. doi: 10.1093/infdis/jiab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ninan S, Thompson P, Gershon T, et al. Fatal pediatric COVID-19 case with seizures and fulminant cerebral edema. Child Neurol Open. 2021;8 doi: 10.1177/2329048X211022532. 2329048X21102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte-Neto AN, Caldini E, Gomes-Gouvea MS, et al. An autopsy study of the spectrum of severe COVID-19 in children: from SARS to different phenotypes of MIS-C. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taweevisit M, Chindamporn A, Sujjavorakul K, Samransamruajkit R, Thorner PS. Multisystem inflammatory syndrome in children (MIS-C) showing disseminated aspergillosis, cytomegalovirus reactivation and persistent SARS-CoV-2: case report with autopsy review. Pathol Res Pract. 2022;238 doi: 10.1016/j.prp.2022.154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayordomo-Colunga J, Vivanco-Allende A, Lopez-Alonso S, et al. SARS-CoV-2 spike protein in intestinal cells of a patient with coronavirus disease 2019 multisystem inflammatory syndrome. J Pediatr. 2022;243:214–18e215. doi: 10.1016/j.jpeds.2021.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigal GB, Novak T, Mathew A, et al. Overcoming COVID-19 investigators. Measurement of severe acute respiratory syndrome coronavirus 2 antigens in plasma of pediatric patients with acute coronavirus disease 2019 or multisystem inflammatory syndrome in children using an ultrasensitive and quantitative immunoassay. Clin Infect Dis. 2022;75:1351–1358. doi: 10.1093/cid/ciac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arostegui D, Castro K, Schwarz S, Vaidy K, Rabinowitz S, Wallach T. Persistent SARS-CoV-2 nucleocapsid protein presence in the intestinal epithelium of a pediatric patient 3 months after acute infection. JPGN Reports. 2022;3:e152. doi: 10.1097/PG9.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scottoni F, Giobbe GG, Zambaiti E, et al. Intussusception and COVID-19 in infants: evidence for an etiopathologic correlation. Pediatrics. 2022;149 doi: 10.1542/peds.2021-054644. [DOI] [PubMed] [Google Scholar]
- 33.Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araújo NM, Ferreira LC, Dantas DP, et al. First report of SARS-CoV-2 detection in cerebrospinal fluid in a child with Guillain-Barré syndrome. Pediatr Infect Dis J. 2021;40:e274–e276. doi: 10.1097/INF.0000000000003146. [DOI] [PubMed] [Google Scholar]
- 35.Miura CS, Lima TM, Martins RB, et al. Asymptomatic SARS-CoV-2 infection in children's tonsils. Rev Bras Otorrinolaringol. 2022;88:9. [Google Scholar]
- 36.Xu Q, Milanez-Almeida P, Martins AJ, et al. Adaptive immune responses to SARS-CoV-2 persist in the pharyngeal lymphoid tissue of children. Nat Immunol. 2023;24:186–199. doi: 10.1038/s41590-022-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz DA, Avvad-Portari E, Babál P, et al. placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch Pathol Lab Med. 2022;146:660–676. doi: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 38.Lesieur E, Torrents J, Fina F, et al. Congenital infection of severe acute respiratory syndrome coronavirus 2 with intrauterine fetal death: a clinicopathological study with molecular analysis. Clin Infect Dis. 2022;75:e1092–e1100. doi: 10.1093/cid/ciab840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reagan-Steiner S, Bhatnagar J, Martines RB, et al. Detection of SARS-CoV-2 in neonatal autopsy tissues and placenta. Emerg Infect Dis. 2022;28:510–517. doi: 10.3201/eid2803.211735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He R, Hou S, Liu C, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537:412–416. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 41.Yu D, Ye L. A portrait of CXCR5+ follicular cytotoxic CD8+ T cells. Trends Immunol. 2018;39:965–979. doi: 10.1016/j.it.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 43.Percivalle E, Sammartino JC, Cassaniti I, et al. Macrophages and monocytes: “Trojan Horses” in COVID-19. Viruses. 2021;13 doi: 10.3390/v13112178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matveeva O, Nechipurenko Y, Lagutkin D, Yegorov YE, Kzhyshkowska J. SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: antibody-dependent as well as independent cell entry. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1050478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson BK, Francisco EB, Yogendra R, et al. Persistence of SARS-CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol. 2022;12 doi: 10.3389/fimmu.2021.746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newell KL, Waickman AT. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infection. Curr Opin Immunol. 2022;77 doi: 10.1016/j.coi.2022.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peluso MJ, Lu S, Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224:1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 50.Patterson BK, Guevara-Coto J, Yogendra R, et al. Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son K, Jamil R, Chowdhury A, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J. 2023;61 doi: 10.1183/13993003.00970-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poon MML, Rybkina K, Kato Y, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chioh FW, Fong S-W, Young BE, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife. 2021;10 doi: 10.7554/eLife.64909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruger A, Vlok M, Turner S, et al. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 2022;21:190. doi: 10.1186/s12933-022-01623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirschenberger M, Hunszinger V, Sparrer KMJ. Implications of innate immunity in post-acute sequelae of non-persistent viral infections. Cells. 2021;10 doi: 10.3390/cells10082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buonsenso D, Valentini P, De Rose C, et al. Recovering or persisting: the immunopathological features of SARS-CoV-2 infection in children. J Clin Med. 2022;11 doi: 10.3390/jcm11154363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boribong BP, LaSalle TJ, Bartsch YC, et al. Neutrophil profiles of pediatric COVID-19 and multisystem inflammatory syndrome in children. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bline K, Andrews A, Moore-Clingenpeel M, et al. Myeloid-derived suppressor cells and clinical outcomes in children with COVID-19. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.893045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koushki K, Salemi M, Miri SM, Arjeini Y, Keshavarz M, Ghaemi A. Role of myeloid-derived suppressor cells in viral respiratory infections; hints for discovering therapeutic targets for COVID-19. Biomed Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siemińska I, Węglarczyk K, Surmiak M, et al. Mild and asymptomatic COVID-19 convalescents present long-term endotype of immunosuppression associated with neutrophil subsets possessing regulatory functions. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.748097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 63.Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med. 2023 doi: 10.1001/jamainternmed.2023.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peluso MJ, Anglin K, Durstenfeld MS, et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun. 2022;7:95–103. doi: 10.20411/pai.v7i1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goel N, Goyal N, Nagaraja R, Kumar R. Systemic corticosteroids for management of ‘long-COVID’: an evaluation after 3 months of treatment. Monaldi Arch Chest Dis. 2021;92 doi: 10.4081/monaldi.2021.1981. [DOI] [PubMed] [Google Scholar]
- 66.Thompson JS, Thornton AC, Ainger T, Garvy BA. Long-term high-dose immunoglobulin successfully treats Long COVID patients with pulmonary, neurologic, and cardiologic symptoms. Front Immunol. 2023;13 doi: 10.3389/fimmu.2022.1033651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byambasuren O, Stehlik P, Clark J, Alcorn K, Glasziou P. Effect of covid-19 vaccination on long covid: systematic review. BMJ Med. 2023;2 doi: 10.1136/bmjmed-2022-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morello R, De Rose C, Cardinali S, Valentini P, Buonsenso D. Lactoferrin as possible treatment for chronic gastrointestinal symptoms in children with long COVID: case series and literature review. Children. 2022;9 doi: 10.3390/children9101446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parodi E, Carpino A, Franchitti E, et al. Detection of faecal SARS-CoV-2 RNA in a prospective cohort of children with multisystem inflammatory syndrome (MIS-C) Epidemiol Prev. 2021;45:522–527. doi: 10.19191/EP21.6.120. [DOI] [PubMed] [Google Scholar]
- 70.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911–923. doi: 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]
- 71.Yao XP, Ye J, Feng T, et al. Adaptor protein MyD88 confers the susceptibility to stress via amplifying immune danger signals. Brain Behav Immun. 2023;108:204–220. doi: 10.1016/j.bbi.2022.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Griffin DE. Measles virus persistence and its consequences. Curr Opin Virol. 2020;41:46–51. doi: 10.1016/j.coviro.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cocciolillo F, Di Giuda D, Morello R, De Rose C, Valentini P, Buonsenso D. Orbito-frontal cortex hypometabolism in children with post-COVID condition (long COVID): a preliminary experience. Pediatr Infect Dis J. 2022;41:663–665. doi: 10.1097/INF.0000000000003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffin DE. Why does viral RNA sometimes persist after recovery from acute infections? PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fawzy NA, Shaar BA, Taha R, et al. A systematic review of trials currently investigating therapeutic modalities for post-acute COVID-19 syndrome and registered on World Health Organization international clinical trials platform. Clin Microbiol Infect. 2023;29:570–577. doi: 10.1016/j.cmi.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2023;21:51–64. doi: 10.1038/s41579-022-00770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.