Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a definitive therapy for a variety of disorders. One of the complications is acute graft-versus-host disease (aGVHD), which has a high mortality rate. Patients can also develop chronic graft-versus-host disease (cGVHD), a more indolent yet afflicting condition that affects up to 70% of patients. Ocular involvement (oGVHD) is one of the most prevalent presentations of cGVHD and can manifest as dry eye disease, meibomian gland dysfunction, keratitis, and conjunctivitis. Early recognition of ocular involvement using regular clinical assessments as well as robust biomarkers can aid in better management and prevention. Currently, the therapeutic strategies for the management of cGVHD, and oGVHD in particular, have mainly focused on the control of symptoms. There is an unmet need for translating the preclinical and molecular understandings of oGVHD into clinical practice. Herein, we have comprehensively reviewed the pathophysiology, pathologic features, and clinical characteristics of oGVHD and summarized the therapeutic landscape available to combat it. We also discuss the direction of future research regarding a more directed delineation of pathophysiologic underpinnings of oGVHD and the development of preventive interventions.
Keywords: Allogeneic hematopoietic stem cell transplantation, graft-versus-host disease, ocular graft-versus-host disease, ocular surface, keratoconjunctivitis sicca, dry eye disease, meibomian gland dysfunction, GVHD
1. Introduction
Since the first successful report in 1968, allogeneic hematopoietic stem cell transplantation (allo-HSCT) has become a curative therapy for a variety of malignant and nonmalignant hematologic disorders and certain inherited metabolic diseases.42 Acute graft-versus-host disease (aGVHD) is a common lethal complication of allo-HSCT, with an incidence rate of 30–50%.158 Acute GVHD is an inflammatory condition that classically affects 3 target organs: the integumentary system, the gastrointestinal epithelium, and the liver and bile ducts. Chronic GVHD (cGVHD) is the most common persistent complication in allo-HSCT patients and is the leading cause of nonrelapse deaths. Although the presence of aGVHD is a risk factor for the development of cGVHD, it can also manifest de novo.39 According to the statistical estimates, the incidence of cGVHD among allo-HSCT patients is between 10% to 70%, and, likely due to increased survival rates there has been an increasing trend in its incidence.6 The signs and symptoms of cGVHD usually appear at least 100 days after the transplantation, and most patients will be diagnosed within the first year of transplantation.53 The criteria for other forms of GVHD (including the late onset aGVHD and the overlap syndrome, which has presentations of both aGVHD and cGVHD) have been developed in recent years; however, they are not as common as the two mentioned classic forms.53
Unlike aGVHD, cGVHD can affect any organ system, including skin, oral mucosa, gastrointestinal tract and hepatobiliary system, lungs, eyes, genitalia, and joints.53 Less than 10% of aGVHD patients will suffer from ocular involvement that manifests as pseudomembrane conjunctivitis and corneal involvement. Based on early reports, ocular involvement is associated with increased mortality risk.114 On the other hand, it has been reported that ocular involvement can be seen in 30 to 85% of cGVHD cases, making it a relatively common manifestation.50 Although ocular involvement usually manifests after the appearance of other signs of cGVHD, it can be the initial sign or symptom of the disease.121 According to the 2014 National Institutes of Health (NIH) criteria, new-onset signs of keratoconjunctivitis sicca (KCS); cicatricial conjunctivitis; confluent punctate keratopathy, or dry or painful eye--collectively known as dry eye disease (DED)--are cardinal manifestations of ocular GVHD (oGVHD).53 Progression of KCS to corneal infection, ulceration, and perforation may occur in severe cases.130 Nevertheless, the presence of DED by itself can significantly dwindle the vision,107 quality of life,27 and Karnofsky scores in patients.107
We review the pathophysiology, pathologic features, and clinical characteristics of oGVHD, as well as the therapeutic landscape available to combat it. We also discuss the direction of future research regarding a more directed delineation of pathophysiologic underpinnings of oGVHD and the development of preventive interventions.
2. Pathophysiology of aGVHD and cGVHD
There are fundamental differences between acute and chronic GVHD. The pathophysiology of acute GVHD has been extensively studied, and there is a general agreement that the inciting event is the donor T-cell activation against the recipient antigens.158 In the scenario of major histocompatibility complex (MHC) mismatch, the donor CD4+ T-cells can initiate the development of acute GVHD; however, for subjects with MHC match (almost all the currently encountered cases), the classic ‘three signals’ (e.g. antigen recognition, co-stimulation, and cytokine-mediated differentiation and expansion) are required for the donor T-cell activation.118 At the first step, antigen presenting cells (APC) are activated due to several factors such as underlying disease, prior infection, and tissue damage caused by the conditioning regimen (damage-associated molecular patterns [DAMPs] and pathogen-associated molecular patterns [PAMPs]).158 The second phase is activation, proliferation, and differentiation of donor T- cells. During this process, co-stimulation between T- cell and APC is established through interaction between CD28 of T-cells and CD80 or CD86 of APCs. After T-cell activation, cytokines such as IL- 2 and IFN- γ are released, IFN- γ, promoting aGVHD. The third phase is the effector phase, leading to cell death. During this phase, tissue damage occurs.118
Currently, the pathophysiological underpinnings of the development of cGVHD are not well-understood.40 Various cells such as Tregs and B-cells, effector T-cells, are implicated in the development of cGVHD.35, 149 Notably, it seems that patients with cGVHD have fewer Tregs compared to control patients.35 On the other hand, studies have reported that the number of B-cells is increased in cGVHD.55, 117 Overall, there is an unmet need to achieve more solid conclusions.
3. Pathologic features of oGVHD
3.1. Lacrimal glands
The most common manifestation of oGVHD is DED. In accordance with the fibrotic changes in different target tissues as the hallmark of cGVHD, lacrimal gland fibrosis is thought to be the main culprit for the development of DED.91 Fibrotic changes in the conjunctiva, leading to a decrease in tear production by accessory lacrimal glands and tear evaporation due to alterations in the lipid layer of the tear film are also implicated in this condition.91 Early immunohistochemical investigation of oGVHD-related lacrimal glands showed the presence of activated CD34+ fibroblasts.91 Ogawa and colleagues reported periductal infiltrations with T-cells (mostly CD8+ cells),92 thickening and multilayering of the vascular and ductal basal lamina, and a positive association between the bulk of fibrosis and the severity of oGVHD-related signs. They hypothesized that residing donor T-cells (mainly CD4+ cells) can induce fibroblasts activation,92 and more interestingly, such fibroblasts can act as APCs for T-cells, which creates a vicious feedback loop (Figure 1).91
Figure 1.
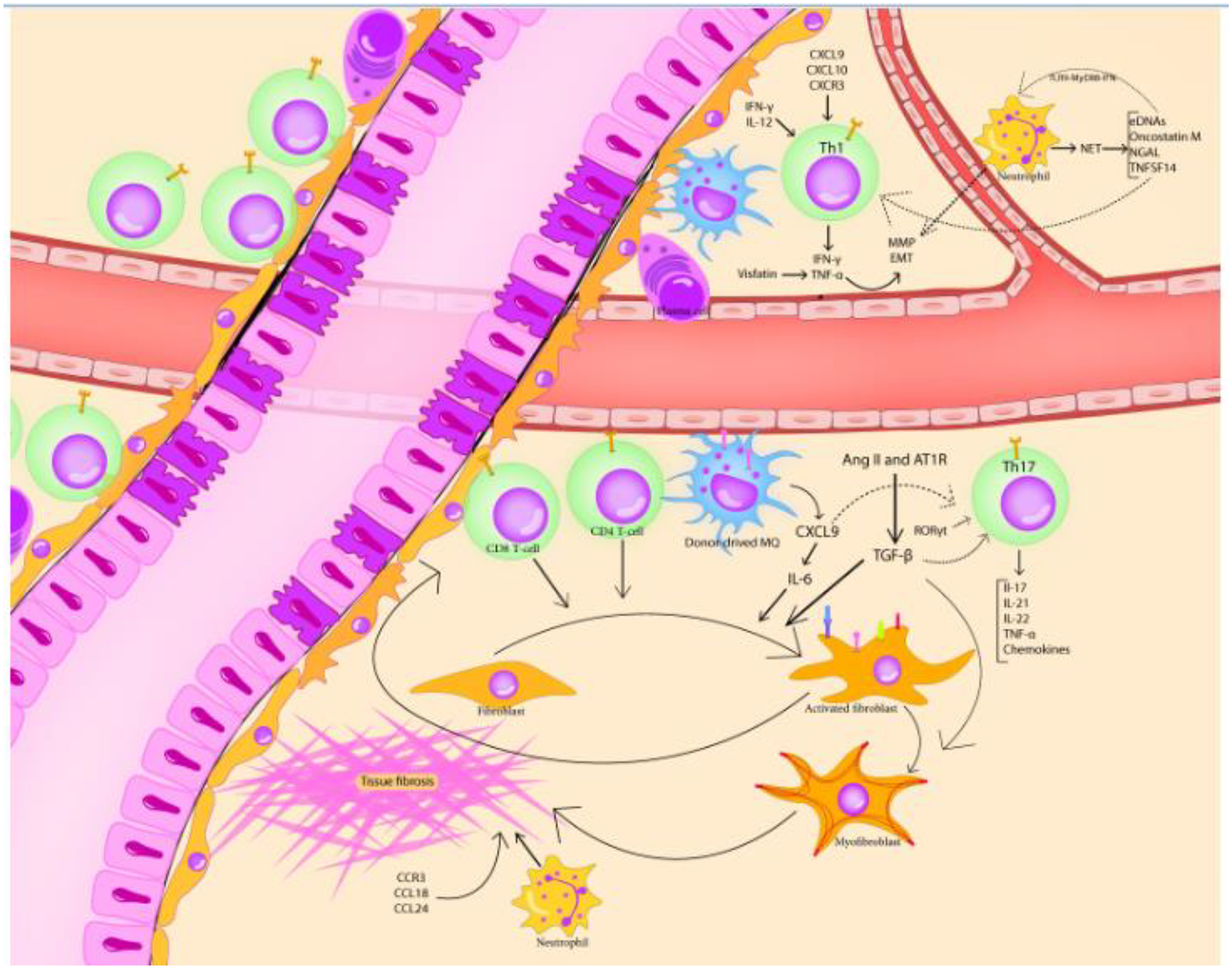
Pathological alterations in the lacrimal glands of oGVHD cases and its immunological underpinnings. The basement membrane of lacrimal ducts and lacrimal vasculature exhibits multilayering and thickening. The CD8+ and CD4+ T-cells, along with plasma cells, Th17 cells, macrophages, and neutrophils, infiltrate the peri-ductal area and contribute to fibroblast activation, myofibroblast emergence, and eventually, tissue fibrosis. In addition, activated fibroblasts perpetuate immune activation by acting as antigen-presenting cells (by expressing HLA-DR, CD34, CD40, CD54, CD80, and CD86). The involvement of other pro-fibrotic mediators and EMT-inducers, namely TGF-β, neutrophil extracellular traps, renin-angiotensin-aldosterone system, reactive oxygen species, and chemokines is also implicated in the oGVHD-related lacrimal gland destruction.
More recently, the importance of the renin-angiotensin-aldosterone system (RAAS) in the development of cGVHD-related fibrosis has been studied.151 Yaguchi and colleagues have shown that mouse lacrimal glands express mRNAs of angiotensinogen, prorenin, angiotensin-converting enzyme (ACE), and angiotensin II receptors type 1 (AT1R) and type 2 (AT2R), that collectively make the “tissue RAAS”.151 Interestingly, administration of an AT1R antagonist (valsartan) culminated in a lesser degree of fibrosis, decreased densities of CD45+ inflammatory cells and fibroblasts, and higher tear production in a cGVHD mouse model. It is presumed that the fibrogenic effects of angiotensin II are mediated via AT1R, which can subsequently trigger the upregulation of the expression of transforming growth factor-beta (TGF-β). TGF- β induces the activation and productivity of fibroblasts.152 α -SMA is a differentiation marker for activated myofibroblasts (MFBs), and its expression can be induced by the triggering effect of TGF-β.120 Shamloo and colleagues have further shown the co-localization of α-SMA and AT1R, which implicates another aspect of the role of RAAS in the induction of ocular fibrosis.120 Last but not least, the attachment of angiotensin II to the AT1R can also trigger the activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase and causes mitochondrial dysfunction which increases reactive oxygen species (ROS) generation.140
3.2. Meibomian glands
Meibomian gland dysfunction (MGD) is a common disorder that generally manifests as DED. MGD is the result of the morphological and functional changes in the meibomian glands.16 Clinically, meibomian gland involvement is present in 47.5 to 80% of patients with oGVHD and may be diagnosed even before the conduction of allo-HSCT.90 Studies on a mouse model of cGVHD, however, have shown endothelial damage, fibroblast activation, and immune cell infiltrations in the MGs, and necrosis and apoptosis of MG basal cells.155 Moreover, the eyelid blood vessels can exhibit several pathologic alterations, including tortuosity, dilation, neovascularization, and peri-vascular inflammatory cell infiltration and fibrosis.155 Clinically, MGD among oGVHD patients presents with gross abnormalities such as orifice obstruction (by hyperkeratinization), displacement of mucocutaneous (gray) line, vascular tortuosity, altered quality and quantity of sebum, and acinar cell atrophy.10 Compared to the non-dry eye/non-cGVHD cases, the mean meibomian gland acinar unit density (MGAUD), meibomian gland acinar longest diameter (MGALD), meibomian gland acinar shortest diameter (MGASD) values in confocal microscopy of cGVHD-related dry eye cases are significantly lower, meanwhile the mean fibrosis grade is significantly higher. Also, clinical findings on the lid margin, tear dynamics, and ocular surface findings were significantly worse.10
In a comparative study, Choi and colleagues aimed to assess and compare the features of meibomian glands of patients with oGVHD, Sjögren’s syndrome (SS), MGD, and healthy controls.17 They found that oGVHD cases have significantly more severe meibomian gland abnormalities (indicated by higher meibum expressibility score [MES], meibum quality score [MQS], lid margin abnormality score, ocular surface staining, and gland dropout). More importantly, they identified positive correlations between the mentioned scores and the severity of oGVHD.17
It has been proposed that early, pretransplantation loss of meibomian gland area (MGA) is a predictive indicator for the development of oGVHD.30 Nevertheless, there are controversies regarding this issue. Since the meibum is secreted via the holocrine pathway, meibocytes have a high turnover rate. As a result, chemotherapy and radiotherapy regimens might exert detrimental effects on the meibomian glands’ regeneration.106 In addition, meibum secretion is stimulated by circulating androgens, and there are reports of hormonal disturbances among allo-HSCT subjects.134 Hence, in addition to the importance of inflammation, the role of these hormonal factors should be investigated in future research.
3.3. Cornea
Corneal involvement is common among oGVHD patients (Figure 2). In one report, 66% of cases had superficial punctate keratopathy, and 29% showed evidence of filamentary keratitis.9 In another report, the prevalence of superficial punctate keratitis was as high as 91%.24 Persistent epithelial defects (PEDs) are reported to be seen in around 8% of oGVHD cases.126 PEDs are associated with limbal stem cell deficiency (LSCD), subconjunctival fibrosis, and filamentary keratitis. The most gruesome corneal complication of OGVHD, corneal perforation, occurs in 3.7% of cases.126 Superior limbic keratoconjunctivitis (SLK)-like involvement is not common among oGVHD patients, as its reported prevalence has been only 8.8%.66
Figure 2.
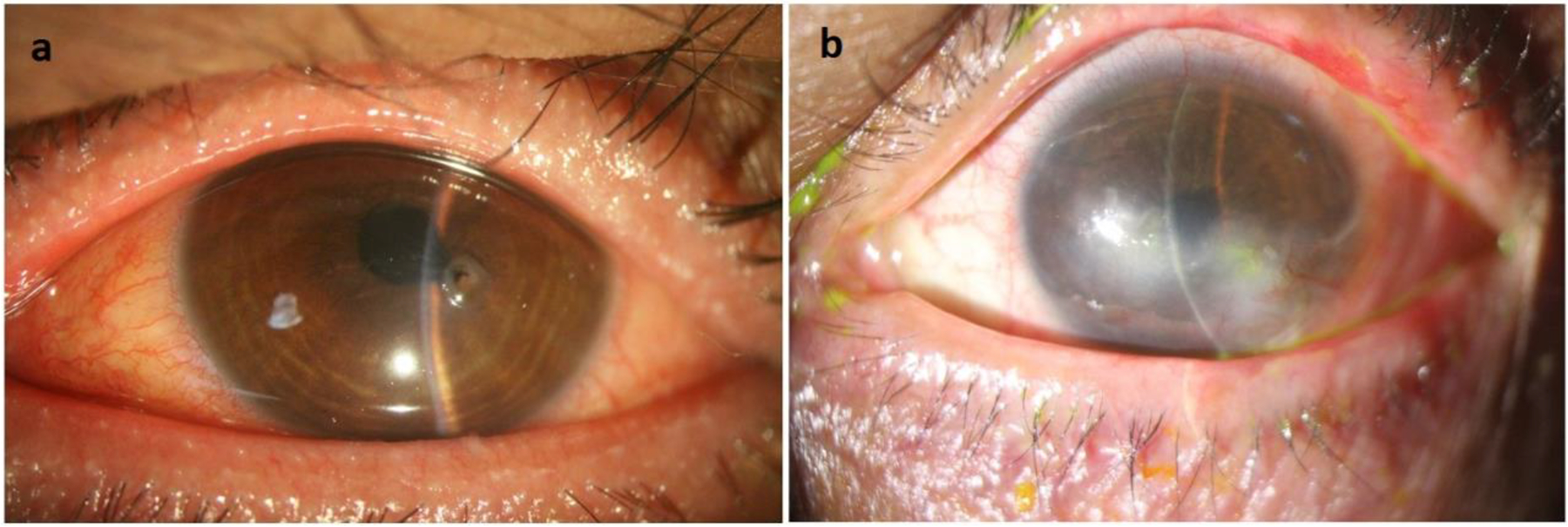
Corneal manifestations of oGVHD. a. A case of oGVHD which interestingly presented with corneal perforation. MGD, prominent eyelid margin vascularization, pupil distortion, and iris prolapse in the location of corneal thinning is evident. b. A case of oGVHD with chronic involvement of cornea including neovascularization and opacity.
Numerous pathological changes have been documented in the cornea and limbus of patients with cGVHD-related DED. An early study by Wang and colleagues showed that compared to healthy controls, post allo-HSCT patients demonstrated decreased corneal sensitivity (CS).144 Moreover, the number of inflammatory cells within the conjunctival brush cytology samples was significantly higher for oGVHD-related DED compared to healthy controls and post-HSCT cases without DED. It should be mentioned that, in this study, the decrease in CS was similar for all severity stratifications of DED.144 Therefore, the authors of this study speculated that the conditioning regimens that are being administered before the conduction of allo-HSCT might be responsible for this finding.144 In a retrospective study on oGVHD cases,125 14% showed evidences of neurotrophic keratopathy in the course of the disease.
In another attempt, DED cases with or without the underlying oGVHD were recruited and evaluated by in vivo confocal microscopy (IVCM). Both groups had a higher density of conjunctival immune cells and corneal dendritic cells (DCs) but showed decreases in the density of the corneal sub-basal nerves.10 The pathologic changes of corneal nerves are not limited to their altered densities; the other alterations include increased tortuosity of nerve fibers and DC densities and decreased corneal superficial epithelial cells, nerve fibers’ densities, and nerve reflectivity.10
Similarly, the corneal densities of DCs and their size and number of dendrites have been higher among patients with DED compared to healthy subjects.64 Intriguingly, one study documented significantly higher DC density, size, and related dendrites among those with SS and oGVHD-related DED, compared with non-immune causes.64 In addition, a recent study found that the size and dendrites of DCs increase as the DED becomes more severe.2
3.4. Conjunctiva
Conjunctival involvement among aGVHD patients may have different manifestations. A commonly used classification for the conjunctival involvement in aGVHD comprises four stages, i.e., conjunctival hyperemia (stage I), chemosis (stage II), pseudomembrane formation (stage III), and corneal epithelial sloughing (stage IV).51 Generally, conjunctival involvement is mostly seen among aGVHD cases. In a study on a series of post-HSCT subjects found that the prevalence rates of KCS and pseudomembrane formation are 10% and 9.2%, respectively.63 In another report, 14.2% of aGVHD cases have had acute conjunctivitis.90
Involvement in cGVHD can also be graded. In grade 1, conjunctival hyperemia is present. In grade 2, palpebral conjunctival fibrovascular changes occur with or without epithelial sloughing. In grade 3, these changes involve 25–75% of total surface area, and finally, in grade 4, involvement of 75% of total surface area with or without cicatricial entropion can be expected.51 In a retrospective study on chronic phase of 62 oGVHD cases, conjunctival hyperemia was detectable in 65% of cases, followed by chemosis in 29%, conjunctival ulceration and sloughing in 13%, subconjunctival fibrosis in 6%, and forniceal foreshortening and symblepharon in 5% of cases.9 The formation of fibrosis in the conjunctiva can further evolve into cicatricial changes, which eventually lead to eyelid abnormalities, including entropion and lagophthalmos, that will further perpetuate the vicious cycle of DED.9 From the molecular point of view, the conjunctiva of oGVHD eyes exhibits fibrosis, loss of secretory function, and metaplastic alterations.93 In one study, Ogawa and colleagues examined the conjunctival biopsies of oGVHD and healthy individuals with electron microscopy and immunohistochemical staining for epithelial and mesenchymal markers.93 Ocular GVHD samples showed fibrosis of subconjunctival stroma containing active fibroblasts, thinning of the conjunctival epithelial and basal lamina, and disruption of the basal epithelial cells.93 Along with these findings, basal epithelial cells exhibited reduced expression of E-cadherin and increased staining for Snail, α-SMA, heat shock protein (HSP) 47, and collagen bundles.93 E-cadherin is responsible for epithelial cell-to-cell adhesions, and its absence in the epithelial layer is seen before the initiation of epithelial to mesenchymal transition (EMT).93 Snail is a potent inhibitor of E-cadherin, and α-SMA, along with HSP47, are markers of mesenchymal cells.93 Moreover, the disruption and segmentation of the basal lamina in the stroma under disrupted segments were seen.93 Based on these observations and the interaction between p63/HSP47 positive cells and lymphocytes, the authors concluded that EMT occurs in the conjunctiva following cytokine release by the immune system and is partially responsible for the fibrotic changes. Furthermore, this study has found similar pathologic alterations in the lacrimal glands, which might be conducive to their fibrosis (Figure 3).93
Figure 3.
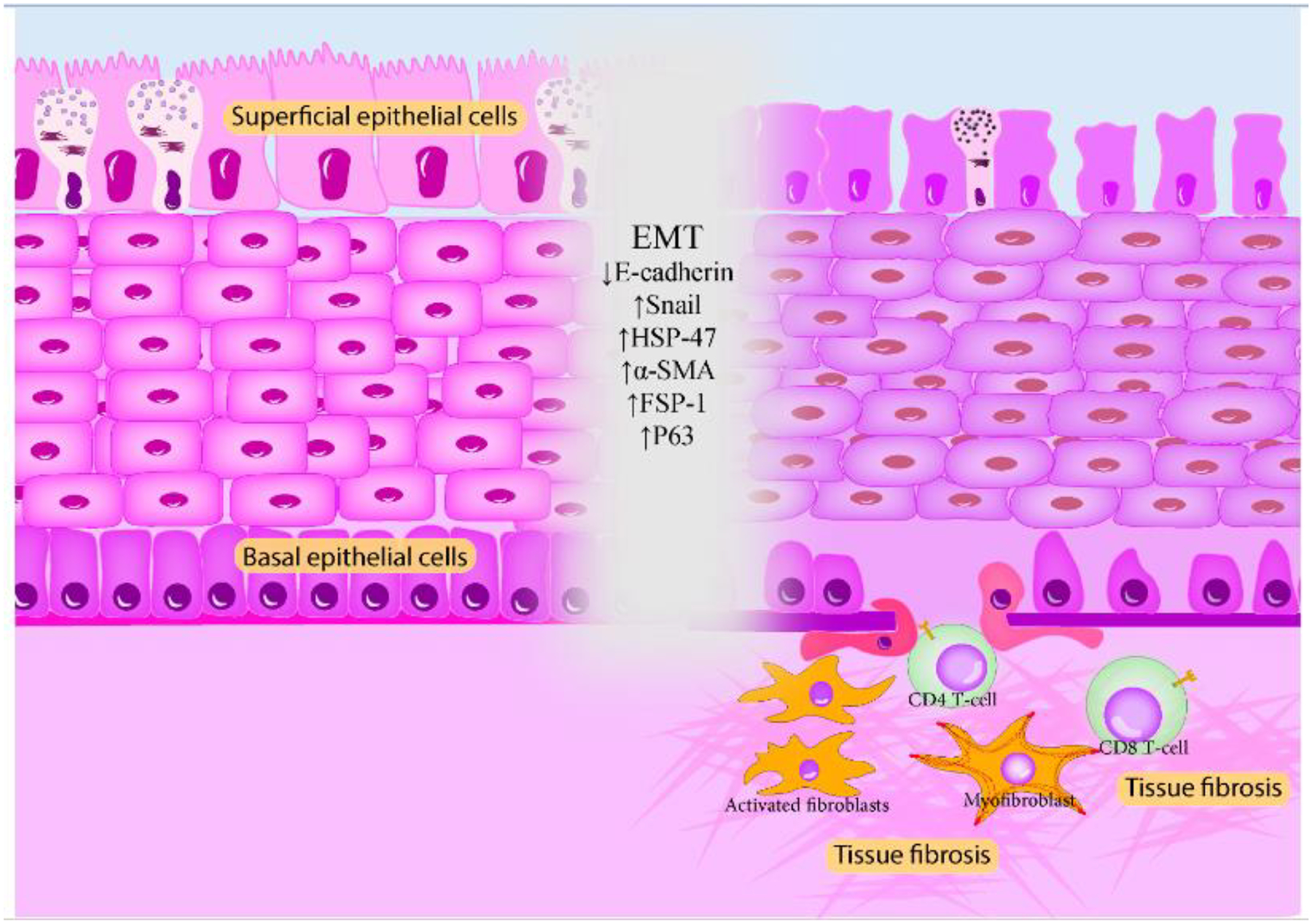
Pathological findings of the oGVHD conjunctiva. Thinning of epithelia, loss of goblet cell microvilli and their densities; thickening, multilayering, and disruption of the basal lamina; vacuolization of basal cells; CD8+ T-cell infiltration; and fibroblast activation and myofibroblast generation are seen in the conjunctival samples of oGVHD eyes. The most prominent pathologic feature is the EMT, which is the consequence of immune activation and cytokine release.
The microscopic characteristics of oGVHD-afflicted conjunctiva are not limited to the aforementioned findings. In fact, some studies8, 144 have documented squamous cell metaplasia (assessed by impression cytology) and decreased density of goblet cells in the superficial conjunctival epithelium, which has been considerably different from that of normal subjects and SS cases. Furthermore, the epithelial cell microvilli had a shorter height and wider width, as well as decreased densities. The epithelial cells of oGVHD and SS patients had a lower density of secreting vesicles, and more importantly, the oGVHD cases have shown thinner Mucin 1 (MUC1), MUC4, and MUC16, and also MUC5AC layers.143 Lubrication, hydration, and protection of the ocular surface against pathogen invasions are the main functions of these mucins.44 Goblet cells have also been proposed to have immunomodulatory functions. They produce TGF-β2, which can impede the IL-2-dependent growth of T-cells69 and maintain the immature, tolerogenic form of DCs.20 Other studies have shown the presence of intercellular adhesion molecule 1 (ICAM-1) on the oGVHD conjunctiva, which has been correlated with lower Schirmer’s test scores and goblet cell densities.7
3.5. Other ocular manifestations
Some studies have reported ocular hypertension and glaucoma as comorbidities of oGVHD. In one report, 33 out of 218 oGVHD patients had ocular hypertension, eight have had possible glaucoma, and one has had definite glaucoma.112 Of note, the authors of this study have mentioned the use of topical and systemic corticosteroids as the principal cause of increased ocular pressure.112 The formation of cataracts (mainly the posterior subcapsular form) generally follows the same pathway as ocular hypertension and glaucoma.112 In fact, it mainly develops as a complication of the administration of corticosteroids and radiation therapy, with a prevalence rate similar to that of glaucoma.130 Other conditions, including episcleritis, scleritis, uveitis, central serous chorioretinopathy, serous retinal detachment, microvascular retinopathy, infectious retinitis or endophthalmitis, optic nerve head edema, and intraocular lymphoma are rarely encountered in oGVHD cases.28
4. Microbiome and oGVHD
In recent years, the importance of commensal microflora in the development or prevention of a wide variety of disorders has gained unprecedented interest.137 The conjunctival epithelium is home to a variety of microbial agents, and some authors have attempted to investigate the alterations in their populations (concerning both numbers and species) before and after the development of oGVHD.
Using the traditional culture-based methods, Shimizua and colleagues123 observed significantly higher rates of positive cultures (75%) and also multiple detections (positive culture for more than 2 species, 45.8%) among the oGVHD cases compared to non-GVHD allo-HSCT cases (36% and 3.6%), and healthy subjects (25% and 0). They found Staphylococcus Epidermidis, α-hemolytic Streptococcus, Corynebacterium species, and Propionibacterium Acnes as the most common species among the oGVHD subjects.123
Using the DNA sequencing methods, another study aimed to evaluate the ocular microbiome of a variety of severe ocular surface disorders.160 Contrary to the findings of the previous report, this study found that 63% of healthy and 43% of oGVHD eyes have positive results (probably as the consequence of topical antibiotic usage). Furthermore, the authors found that one of the oGVHD eyes has Enterobacteriaceae species, which has been a unique finding in their study.160 In addition, five of six oGVHD-positive eyes exhibited evidence of Lactobacillus/Streptococcus species, similar to healthy eyes. Last but not least, in one patient with bilateral oGVHD, each eye showed a completely different microbiome.160 Another study74 used shotgun metagenomic sequencing to compare the diversity of the healthy and post-allo-HSCT eyes’ microbiome. This study found a significantly reduced microbiome diversity for post-allo-HSCT eyes. Besides, Gordonia bronchialis and Pseudomonas parafulva were the predominant species, showing a completely different microbiome in oGVHD eyes from normal ones.74
Animal studies are available evaluating the effects of the oral administration of antibiotics on the microbiome. It has been proposed that the interactions between the gut microbiome and the immune system might be an effective factor.115 In line with the importance of the microbiome and to evaluate the effects of oral administration of antibiotics, Sato and colleagues used a mouse model and gave them gentamicin, ampicillin, vancomycin, and fradiomycin two weeks before until four weeks after the transplantation and compared their clinicopathologic features with the control group. They observed that the gentamicin-treated group had lower corneal fluorescein staining (CFS) and higher tear film break-up time (TFBT) scores, less fibrosis and fewer inflammatory and α-SMA positive cell infiltration inside the target organs, lower mRNA levels of IL-6 and IL-17; fewer Th17 and higher Treg densities in the lacrimal glands, and lower systemic GVHD scores.115 The molecular and physiologic mechanisms behind such promising effects are yet to be elucidated; however, the investigators have proposed that the interactions between the gut microbiome and the immune system might play a leading role.
5. Tear biomarkers
With respect to the importance of delineating the pathologic processes contributing to the manifestation of oGVHD and diagnosing this condition more accurately and promptly, the cytokine and protein levels of the tear have been of interest to many researchers. Table 1 provides a brief summarization of the status of various cytokines, growth factors, and other immune-related molecules in the tear of oGVHD eyes.
Table 1.
Alteration in the tear levels of various cytokines, growth factors, and other immune-related molecules of oGVHD eyes.
| Tear molecule | Status | Reference(s) | Tear molecule | Status | Reference(s) |
|---|---|---|---|---|---|
| IL-1β | ↑ | 46 | CCL24 | ↑ | 18 |
| IL-1Rα | ↑ | 19 | CXCL9 | ↑ | 146 |
| IL-6 | ↑ | 18, 46, 86, 128 | CXCL10 | ↑ | 105, 146 |
| IL-8 | ↑ | 4, 19, 46, 86, 105 | CXCR3 | ↑ | 146 |
| IL-9 | ↑ | 18 | ICAM-1 | ↑ | 46 |
| IL-10 | ↑ | 18, 19, 86 | MMP-8 | ↑ | 5 |
| IL-12 | ↑ | 86 | MMP-9 | ↑ | 5, 86 |
| IL-17 | ↑ | 86 | VEGF | ↑ | 86 |
| IFN-γ | ↑ | 18, 86 | eDNA | ↑ | 4, 128 |
| CCL2 | ↑ | 18 | TNFSF14 | ↑ | 4 |
| CCL18 | ↑ | 18 | TNF-α | ↑ | 4, 105, 128 |
| Neutrophil elastase | ↑ | 4, 5, 128 | IL-7 | ↓ | 46 |
| Myeloperoxidase | ↑ | 4, 5 | EGF | ↓ | 19, 46 |
| NGAL | ↑ | 4 | EGFR | ↓ | 18 |
(oGVHD, ocular graft-versus-host disease; IL, interleukin; IFN, interferon; CCL, chemokine (C-C motif) ligand; NGAL, neutrophil gelatinase-associated lipocalin; CXCL, chemokine (C-X-C motif) ligand; CXCR, chemokine (C-X-C motif) receptor; ICAM-1, intercellular adhesion molecule 1; MMP-9, matrix metalloproteinase-9; VEGF, vascular endothelial growth factor; eDNA, extracellular DNA; TNFSF14, tumor necrosis factor superfamily 14; TNF- α, tumor necrosis factor-alpha; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor.)
6. Tear proteomic of oGVHD
The investigators initiated to analyze the tear proteome in the early 2000s and identified more than 1500 proteins in the tears of healthy subjects.26, 159 Lactoferrin, lysozyme, lipocalin, and soluble immunoglobulin A (sIgA) are the most abundant proteins in the tears of healthy eyes.136 In recent years, researchers have aimed to identify similarities and differences between the tear proteome of oGVHD and healthy eyes, with similar purposes and methods as those of tear cytokine studies.
It is noteworthy that many of the mentioned changes in the tear proteome of oGVHD eyes are similar to those of DED.73 The reduction in the concentration of most of the mentioned proteins is the result of lacrimal gland destruction.23 Of note, the currently published studies on the tear proteome of oGVHD eyes have not enrolled a sufficient number of patients. Hence, prospective studies should focus on larger cohorts of post-allo-HSCT subjects to illustrate whether the tear proteome alterations occur before the onset of clinical manifestations of oGVHD. The functional roles and status of these proteins during oGVHD are summarized in Table 2.
Table 2.
Commonly reported tear proteins that exhibit alterations in their concentration after the development of oGVHD.
| Protein name | Functions | Status | Reference(s) |
|---|---|---|---|
| Histone H2B and H4 | Nucleosome formation; histone and its proteins get released from the dying cells77 and constitute a part of the NETs,4 which have substantial roles in the induction of ocular surface inflammation. | ↑ | 23, 38 |
| Periplakin | An intracellular scaffold protein that is involved in the cornification of epithelial cells.110 | ↑ | 38 |
| Ribosome binding protein 1 | Similar to histone proteins. | ↑ | 38 |
| Annexin A1 and A2 | Membrane trafficking, mediation of IFN-γ induced inflammation,141 and induction of epithelial cell migration during corneal wound healing.78 | ↑ | 23, 89 |
| Actin, cytoplasmic 1 | One of the cytoskeletal proteins. Its extracellular form is a sign of cell death and is an inducer of inflammation.71 | ↑ | 23, 89 |
| Prelamin-A/C | A nuclear membrane stabilizer.43 Its overexpression is related to the shortening telomerase, hence, linked to the senescence processes,47 which are implicated as one of the underpinnings of the oGVHD.154 | ↑ | 38 |
| S100 proteins A8 and A9 | S100 family contains calcium-binding proteins which are involved in the induction of inflammation, ROS production, and cornification of keratinocytes.29 | ↑ | 23, 38 |
| Ig gamma-1 and −3 chain C region | Complement activation, immune-cell chemoattractant, and antigen-binding capacities.38 | ↑ | 23, 38 |
| Lactotransferrin | Antimicrobial and anti-inflammatory functions via binding to and reducing the levels of free iron.33 | ↓ | 23, 38 |
| Proline-rich protein 1 | Unknown.38 Decreased in many studies on DED subjects.104 | ↓ | 23, 38 |
| Lipocalin-1 | An integral part of tear antimicrobial components and is also a stabilizer of the lipid layer of tear22 and, due to its endonuclease activities,157 an eliminator of extracellular DNAs. | ↓ | 23, 38 |
| Cystatin-S | Cysteine protease inhibitors | ↓ | 23, 38 |
| Lysozyme C | Antimicrobial activities via the hydrolysis of the bacterial cell wall.80 | ↓ | 23, 38 |
| Polymeric immunoglobulin receptor | Transportation of dimeric IgA through the apical surface of epithelial cells, also known as transmembrane secretory component.58 | ↓ | 23, 89 |
| Prolactin-inducible protein | Might have roles in maintaining tear quality and modifying tear evaporation.60 | ↓ | 23, 89 |
| Ig heavy constant α1 and α2 | - | ↓ | 23, 89 |
| Ig J chain | Formation of the binding site for polymeric Ig receptor within the polymeric IgA, which is essential for its secretion.57 | ↓ | 23, 89 |
(oGVHD, ocular graft-versus-host disease; NET, neutrophil extracellular trap; IFN-γ, interferon-gamma; ROS, reactive oxygen species; Ig, immunoglobulin; DNA, deoxyribonucleic acid)
7. The importance of senescence in the pathogenesis of oGVHD
Cellular senescence is defined as the arrest of the cell cycle, with the resultant loss of proliferative and regenerative capacities of cells.62 The DNA damage that is caused by conditioning chemo-radiotherapeutic regimens, as well as the inflammatory milieu, will induce the activation of senescence-associated molecular pathways.62
Stress-induced senescence can also instigate a “senescence-associated secretory phenotype” (SASP) in the affected cells, which is characterized by the secretion of several cytokines, including IL-1β, IL-6, IL-8, CXCL-1, and CXCL-9.109 A preclinical study on an oGVHD mouse model154 has confirmed the higher concentrations of the mentioned cytokines in the lacrimal gland of affected mice compared to healthy controls. In addition, immune cells, fibroblasts, and other cellular infiltration of the oGVHD-affected lacrimal glands have shown significantly higher levels of senescence-associated molecules (p16, p21, and osteopontin), markers of DNA damage response (53BP1), and also the proliferation biomarker Ki-67.154 Subsequently, the administration of the senolytic ABT-263 (navitoclax), an inhibitor of the Bcl-2 anti-apoptotic family and inducer of pro-apoptotic molecules Bax and Bak, has resulted in the alleviation of lacrimal gland fibrosis and immune-cells infiltration, decreased cytokine production, and enhanced tear secretion.154
8. Clinical risk factors for oGVHD
Apart from different underlying pathologic mechanisms, various cross-sectional and cohort studies have documented clinical parameters that are associated with an increased risk of developing oGVHD. In fact, there is considerable heterogeneity in the reports of studies regarding risk factors and epidemiologic characteristics of oGVHD. However, most of it stems from the lack of widely accepted criteria for the diagnosis of this disorder, as discussed earlier. Factors positively associated with the risk of developing oGVHD are listed in Table 3.
Table 3.
Signs, symptoms, and co-morbidities that are positively associated with the increased risk of oGVHD.
| Risk factor | Reference(s) | Risk factor | References(s) |
|---|---|---|---|
| Female-to-male allo-HSCT | 11, 52, 54, 61 | Chronic or acute myeloid leukemia (compared to aplastic anemia) | 72 |
| The female sex of recipients | 102 | Presence of malignant disease | 54 |
| Matched related donors | 11 | Higher age of recipients | 54, 102 |
| Matched unrelated donors | 54 | Age over 27 years of recipients | 72 |
| Diabetes mellitus | 85 | PBSCT | 54, 102 |
| Repeated allo-HSCT | 85 | PBSCT compared to BMT | 72 |
| History of aGVHD | 102 | PBSCT, compared to both BMT and CBT (only for severe oGVHD) | 138 |
| History of acute skin GVHD | 52 | Schirmer’s test value of <10 mm | 72 |
| Number of affected organs by stage I cGVHD | 85 | Schirmer’s test values of ≤10 mm/5 minute before transplantation | 54 |
| Grade III-IV aGVHD | 54 | Presence of dry mouth | 72 |
| Grade II/III overall cGVHD | 142 | Epstein-Barr virus-positive donor | 142 |
| Grade I-III oral and skin cGVHD | 142 | Conjunctival fibrosis and hyperemia | 11 |
| Presence of systemic cGVHD | 72 |
(oGVHD, ocular graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; PBSCT, peripheral blood stem cell transplantation; BMT, bone marrow stem cell transplantation; CBT, cord blood stem cell transplantation; mm, millimeters)
9. Diagnosis
Complete ophthalmological assessment should be performed including visual acuity testing, slit-lamp examination with vital dye staining (e.g., fluorescein dye, lissamine green, or Rose bengal), evaluation of tear breakup time (TBUT), Schirmer testing, and corneal sensitivity and also funduscopy.
The two widely acknowledged diagnostic criteria for oGVHD are as follows: NIH CC 2014 criteria: The diagnostic criteria were based on the Schirmer test and slit- lamp examination.53 The International Chronic oGVHD (ICCGVHD) consensus group diagnostic criteria are based on scores derived from the Ocular Surface Disease Index (OSDI), Schirmer’s test without anesthesia, CFS, conjunctival injection, and presence of systemic GVHD. The diagnostic categories included no oGVHD, probable oGVHD, and definite oGVHD87, 95 (Table 4).
Table 4:
Ocular GVHD diagnostic criteria and grading scale according to the NIH criteria (2014) and Ocular GVHD diagnostic criteria and grading scale according to the International Consensus Criteria on chronic ocular graft- versus- host disease (ICCGVHD)
| A. Ocular GVHD diagnostic criteria and grading scale according to the NIH criteria (2014) | ||||
|---|---|---|---|---|
| Severity Grade | Score 0 | Score 1 | Score 2 | Score 3 |
| Symptoms | KCS confirmed by an ophthalmologist in the absence of symptoms, the requirement of eye drops or ADL | Mild | Moderate | Severe |
| The requirement of lubricant eye drops | ≤3 times/day | >3 times/day or punctal plug | Special eyewear required to relieve pain | |
| ADL impairment | Not affected | Partially affected without new vision impairment due to KCS | Significantly affected or unable to work or loss of vision due to KCS | |
| B. Ocular GVHD diagnostic criteria and grading scale according to the International Consensus Criteria on chronic ocular graft-versus-host disease (ICCGVHD) | ||||
| Diagnosis | None (points) | Probable ocular GVHD (points) | Definite ocular GVHD (points) | |
| Systemic GVHD (+) | 0–3 | 4–5 | ≥6 | |
| Severity scale | Schirmer test (mm) | CFS (points) | OSDI (points) | Conjunctival injection |
| 3 | ≤5 | >4 (Severe staining) | ≥33 | - |
| oGVHD disease severity | None | Mild-Moderate | Severe | |
| The total score is obtained by adding the severity score for Schirmer test + CFS + OSDI + conjunctival injection | 0–4 | 5–8 | 9–11 | |
(KCS, keratoconjunctivitis sicca; ADL, activities of daily living; CFS, corneal fluorescein staining; OSDI, ocular surface disease index)
A moderate agreement between the two mentioned diagnostic criteria has been found.101 However, it seems that ICCGVHD is superior in differentiation between oGVHD patients and non- oGVHD DED.101 Regarding the agreement between ICCGVHD and Best Clinical Practices (BCPs), which is defined assessment of oGVHD cases by a highly trained single expert with extensive [>20 years] clinical experience on oGVHD, it has been reported that mild cases of oGVHD were over-diagnosed by BCPs while a stronger agreement was found in more severe cases.101 Besides, the Tear Film and Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) criteria and the Japanese Dry Eye Society criteria are the other diagnostic criteria, which have been used in studies on GVHD.41, 122, 133, 148 However, these criteria were originally meant for conventional DED.
Besides clinical examination, several diagnostic tools such as meibography, tear interferometry, confocal scanning, and tear film osmolarity are available to help ophthalmologists in the evaluation of oGVHD patients. Meibography provides the chance of in vivo evaluation of the structure of meibomian glands and ducts. A cutoff value of 40% of MG area has been adopted for diagnosing MGD in oGVHD patients.106 Also, the function of meibomian glands in oGVHD cases can be assessed by tear interferometry.10 As discussed earlier, structural alteration in the ocular surface of oGVHD patients can be studied by confocal microscopy at a cellular level.10 Regarding tear film osmolarity, cutoff value of >310 mOsm/L has sensitivity of 98.4% and specificity of 60.7% in diagnosing oGVHD.84
10. Prevention
GVHD prevention strategies are based on proper HLA-matching. Number, activity, and proliferation of donor T-cells can be targeted via prophylactic agents. For this purpose, several immunosuppressives can be applied, including calcineurin inhibitors (e.g., cyclosporine and tacrolimus), methotrexate, mycophenolate mofetil, Anti-thymocyte globulin (ATG), monoclonal antibody alemtuzumab (anti-CD52), and anti-TNF drugs. Currently, tacrolimus plus methotrexate, with or without ATG, is the standard prophylactic regimen.135 Also, it has been shown that targeting Aurora kinase A and JAK2 can prevent GVHD.12
11. Therapeutic strategies to combat oGVHD
Like most other immune system-related disorders, corticosteroids have been an integral part of the therapeutic approach for both aGVHD and cGVHD.103 The administration of systemic steroids is generally initiated for moderate and severe cGVHD,53, 147 and depending on the site of involvement, mild cGVHD can be managed by the application of topical immunosuppressants.147 Nevertheless, systemic steroids are not curative. In a cohort of 283 cGVHD patients, the 6-month overall response after the administration of systemic therapies (prednisone and/or a calcineurin inhibitor) was 32%. The response rate was even worse for ophthalmic involvement, as only 23% of patients showed a response.49 As a consequence, around 50% to 60% of cGVHD cases will eventually require second-line systemic therapies due to either steroid-refractory disease116 or the development of steroid-related debilitating complications.34 Until now, there was no accepted consensus on the agent(s) of choice for the second-line therapies. Tyrosine kinase inhibitors (TKIs, e.g., ibrutinib and nintedanib), Janus-associated kinase (JAK1/2) inhibitors (e.g., itacitinib and ruxolitinib), mammalian target of rapamycin (mTOR) inhibitors (e.g., sirolimus), Rho-associated coiled-coil-containing protein kinase-2 (ROCK2) inhibitor (Belumosudil), proteasome inhibitors (ixazomib), inhibitors of TGF-β and fibroblast proliferation (pirfenidone) and extracorporeal photopheresis (ECP) have been used, among other immunosuppressive agents.79 Early reports on such therapies revealed more favorable response rates, but at the expense of severe toxicities. The combination of methylprednisolone with tacrolimus and sirolimus led to an overall response rate of 63% (22/35) among cGVHD cases. Also, a response rate of 64% (7/11) was reported for oGVHD patients.21 The outcome of therapy with ruxolitinib was 26% (6/23) in one study81 although higher response rates are also reported.31
Due to the pathophysiologic underpinnings of oGVHD and the toxicity of systemic therapies, as well as the acceptable effectiveness of topical therapies and interventions, such formulas comprise the mainstay of the therapeutic approach for oGVHD.103 Topical therapies can be broadly categorized into those that prevent tear evaporation, reduce ocular surface inflammation, and increase surface lubrication.
11.1. Medical management
11.1.1. Lubrication
Preservative-free artificial tears and viscous eye drops reduce surface friction, dilute the concentration of inflammatory cytokines, and enhance the visual quality.14 The prescription of artificial tears might serve as the sole therapy for those with mild DED.14 The addition of acetylcysteine drops can be beneficial by enhancing TBUT and Schirmer test values among those with moderate/severe DED, probably by strengthening the adherence of the tear layer to the ocular surface.88 The administration of oral muscarinic agonists (namely pilocarpine and cevimeline) is effective in improving tear production among patients with SS,100 but their side effects and contraindications have prevented their routine prescription by physicians.
Diquafosol is another option for oGVHD DED. As a purinergic agonist of the P2Y2 receptor, diquafosol is dispersed on the ocular surface and, after stimulation, can induce mucin and aqueous tear secretion by the influx of calcium ions into the goblet cells.153 Treatment of mild-to-moderate oGVHD with 3.0% diquafosol drops has resulted in improved TBUT and CFS and reduced pain sensation, but without noticeable effects on the Schirmer’s test values.153 Rebamipide is another mucin secretagogue132 with anti-inflammatory properties as it inhibits IL-6, IL-8, and CCL-11 production and also attenuates the degradation of NF-κB inhibitor IκBα.36 Furthermore, it can stimulate the proliferation of conjunctival goblet cells.139 Rebamipide drop has shown favorable outcomes in studies and randomized trials enrolling patients with DED.67 In the case of oGVHD, rebamipide eye drop has been able to partially maintain tear film layer volume and decrease the corneal keratopathy score in a mouse model of cGVHD.119 Prospective trials are required to assess its efficacy for oGVHD patients. Finally, some studies have evidenced the efficacy of topical progesterone on enhanced tear production in mice.75 Based on such descriptions and the antinociceptive activities of progesterone, a randomized phase II trial aimed to evaluate the effects of the forehead application of progesterone 1% topical gel on oGVHD cases with moderate to severe ocular discomfort. This therapy resulted in significantly reduced severity and frequency of symptoms and central-inferior CFS.75
11.1.2. Control of inflammation
Owing to the inflammatory nature of oGVHD, topical anti-inflammatory agents should be administered for those who do not respond to the initial treatment with artificial tears, as well as those with moderate to severe disease.14 Topical corticosteroids might be used for cases with cicatricial108 and pseudomembranous conjunctivitis,66 acute exacerbations of both acute and chronic oGVHD,27 and moderate to severe disease.14 However, one study has documented a minimal response to low-dose topical steroids in moderate to severe oGVHD.156 Higher doses of these agents are also contraindicated in the presence of CEDs, ocular infections, and stromal thinning. Moreover, concerns about their debilitating side effects and the frequent use of systemic steroids in the situation of exacerbations has limited the use of topical steroids.14
Topical cyclosporine 0.05% is a well-known agent of this group, and various trials have supported its promising efficacy in enhancing tear secretion and TBUT and Schirmer test values, increasing the densities of goblet cells and MUC5AC mRNA expression, and diminishing the apoptosis and the number of inflammatory cells.143 Of note, the ocular response to calcineurin inhibitors might take weeks.27
Tacrolimus, a more potent calcineurin inhibitor than cyclosporine, might serve as a suitable substitute for steroids. The findings of a retrospective case series showed that the administration of topical tacrolimus 0.02% can significantly reduce ocular surface inflammation and steroid usage.59 In a prospective study on seven oGVHD cases with anterior segment inflammation refractory to topical steroids, the application of tacrolimus 0.03% ointment, along with infrequent steroid use, was able to significantly alleviate corneal and conjunctival inflammation.111 In one trial on patients with refractory oGVHD, a 10-week application of topical tacrolimus 0.05% resulted in a significant reduction in CFS, OSDI, and ICAM-1 and HLA-DR scores, while methylprednisolone 0.5% showed inferior efficacy in reducing HLA-DR score, CFS, and OSDI.1 However, these improvements have come at the cost of a severe burning sensation in 75% of the users, which has led to its withdrawal in three out of 24 cases.1
There are reports on the efficacy of several novel topical anti-inflammatory solutions for oGVHD. One study showed the potency of anakinra, an antagonist of IL-1Rα, in alleviating corneal epitheliopathy among patients with refractory DED and concomitant MGD.3 Lifitegrast is an antagonist of lymphocyte function antigen-1 (LFA-1), an integrin that is essential for the migration of lymphocytes.127 Several randomized trials have demonstrated its efficacy in reducing the eye dryness score (EDS), and to a lesser extent, the ocular discomfort score (ODS) of DED patients.45 Based on these results, the United States Food and Drug Administration (FDA) has approved lifitegrast for the treatment of DED.15, 45 Regarding oGVHD, a study on 18 cases showed the ability of lifitegrast to reduce the NIH severity score in 8 (44%) cases. However, it should be noted that 72% of the included cases have been receiving concurrent systemic immunosuppressives.15
Tranilast, an inhibitor of TGF-β and NF-κB, showed promising results in a few mild, early oGVHD patients94 and mice models of systemic cGVHD.83 In a pilot study70 enrolling DED patients with evidence of anti-citrullinated protein autoantibodies on the ocular surface, the administration of pooled human immune globulin-eye drops resulted in diminished OSDI, CFS, and SLK. Such outcomes are attributed to the ability of immune globulins to decrease cytokine release.70 Heavy chain-Hyaluronan/Pentraxin 3 is an amniotic membrane-derived compound with anti-inflammatory functions.96 In a mouse model of oGVHD, its subconjunctival and subcutaneous injection resulted in the reduced infiltration of T-cells and HSP47+ fibroblasts into the lacrimal glands, increased conjunctival goblet cell density, and preservation of tear layer volume.96 In another attempt to combat the pro-fibrogenic effects of HSP47, a vitamin A-coupled liposome that contains anti-HSP47 small interfering RNAs was administered to a mouse model of oGVHD.97 This compound could both prevent and reduce the collagen deposits in the lacrimal glands and increase the volume of the tear layer.97
Another study showed that the natural homeostatic glycoprotein clusterin (CLU) can inhibit MMPs and bind to and heal the damaged ocular surface.32 In another study, administration of 4-phenyl butyric acid (PBA) in a cGVHD mouse model showed reduced levels of inflammatory cytokines (namely, IL-6), HSP47, lacrimal gland fibrosis, and MGD.82 The role of nutritional supplement linoleic acid and omega-3 and omega-6 fatty acids in reducing the DED-related ocular surface inflammation are also implicated by some studies.25
Autologous serum eye drop (ASED) is another option for oGVHD cases, as it has both lubricating and anti-inflammatory advantages.65 The prominent feature of ASED is the containment of various nutrients, growth factors, inhibitors of MMPs, and electrolytes that make it similar to the natural tear.65 It should be mentioned that ASED has higher concentrations of fibronectin, vitamin A, lysozyme, and TGF-X, and lower concentrations of IgA, EGF, and vitamin C compared to natural tears.99 Despite anti-inflammatory features, a study demonstrated increased densities of HLA-DR positive conjunctival epithelial and Langerhans cells after the administration of ASED.56. In addition, the ocular surface HLA-DR density of SS cases has decreased after the administration of ASED.56 Finally, therapeutic levels of some systemically administered immunosuppressants can be found in ASED,131 but their potential impacts on the course of the disease must be determined by future studies. Regarding another blood-derived product, a phase I/II randomized trial aimed to assess the safety and effectiveness of fibrinogen-depleted human platelet lysate (FD hPL) eye drops for oGVHD cases. However, despite significant improvements in various ocular symptoms, TBUT and CFS showed no significant changes.129
Based on our experience, early start of anti-inflammatory agents such as tacrolimus and cyclosporine is so critical and can prevent progression of the disease and further damage. The practice of using anti-inflammatory agents is supported by the preponderance of literature on the topic.
11.1.3. Control of evaporation
Meibomian gland dysfunction and blepharitis are associated with the loss of the tear layer as a result of increased evaporation. Along with the beneficial effects of topical anti-inflammatory drugs and regular application of warm compresses,98 the usage of topical antibiotic ointments (e.g., tetracycline or doxycycline), drops (e.g., azithromycin), and also systemic antibiotics (e.g., tetracycline or doxycycline) can combat the superimposed infections.27, 65 Of note, the latter has the advantage of alleviating MGD. Using swim goggles to reduce tear evaporation can aid in diminishing the experienced discomfort by DED patients.68 Placement of fluid-ventilated, gas-permeable scleral lenses can diminish tear evaporation and is another therapeutic option for severe DED, including oGVHD cases.76 Such devices are able to attenuate pain and photophobia, enhance OSDI, and improve visual acuity in oGVHD eyes refractory to conventional therapies.76
11.2. Surgical management
Obstruction of canalicular puncta with silicone or collagen plugs may be conducive to retaining the tear layer, and their potential side effect of increasing the concentrations of inflammatory cytokines does not seem to be a major drawback.113, 124 For oGVHD-related KCS, punctal plugs have been able to improve subjective symptoms, CFS, and TBUT;113, 124 however, their extrusion is common,124 and the next steps to reduce the lacrimal duct drainage are surgical ligation or cauterization of puncta.37 Punctal thermal cauterization has been effective in improving subjective symptoms, CFS, and TBUT,150 and stepping down the severity of DED, but recanalization can occur in as many as 21% of cases.145
Tarsorrhaphy is both a prophylactic and therapeutic approach to various ocular surface disorders, including CED and DED. Partial tarsorrhaphy is proposed as an option for severe oGVHD cases.14, 27
Amniotic membrane transplantation serves as an appropriate intervention for those with refractory disease resulting in persistent CED, SLK, corneal perforation, and symblepharon.48 The amniotic membrane is rich in growth factors and cytokines and will provide a physical barrier to the ocular surface and diminish the eyelid friction forces, which, along with the lack of HLA antigens, makes it a promising option for such severe cases. Nevertheless, keratoplasty might be needed as the final resort for the advanced form of the disease.48 Conjunctival-limbal stem cell transplantation is another option for severe cases, although its efficacy is only supported by case reports.13
12. Conclusion
Ocular GVHD is a common disorder in those who are affected by cGVHD. Its detrimental effects on the quality of life have been well established, and serious complications including corneal perforation and loss of vision can occur. Although the pathological mechanisms that lead to oGVHD are not fully understood, there is now solid evidence regarding the pathologic alterations that occur after the initiation of the disease. Such data can provide insight into the development of novel agents that can inhibit crucial signaling cascades during the development of oGVHD. Future research should focus on translating preclinical data into large clinical studies. In addition, it is prudent to amass and summarize the currently available diagnostic criteria and reach well-accepted robust criteria.
13. Methods of literature search
A PubMed search was performed to identify relevant articles (with no time restrictions) using the search terms: “graft-versus host disease” or “GVHD” in combination with “dry eye” or “DED” or “ophthalmology” or “lacrimal” or “keratoconjunctivitis sicca” or “tear” or “eye” or “ocular”. Searches were performed on August 1, 2022. Articles are excluded if they were not referenced in English.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This work was supported by R01 EY024349 (ARD), UH3 EY031809 (ARD): Core Grant for Vision Research EY01792 all from NEI/NIH; Vision Research Program – Congressionally Directed Medical Research Program VR170180 from the Department of Defense, Unrestricted Grant to the department and Physician-Scientist Award both from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Abud TB, Amparo F, Saboo US, et al. : A clinical trial comparing the safety and efficacy of topical tacrolimus versus methylprednisolone in ocular graft-versus-host disease. Ophthalmology 123:1449–1457, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. : Correlation of corneal immune cell changes with clinical severity in dry eye disease: An in vivo confocal microscopy study. The ocular surface 19:183–189, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amparo F, Dastjerdi MH, Okanobo A, et al. : Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA ophthalmology 131:715–723, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An S, Raju I, Surenkhuu B, et al. : Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: Implications for novel biomarkers and therapeutic strategies. Ocul Surf 17:589–614, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arafat SN, Robert MC, Abud T, et al. : Elevated Neutrophil Elastase in Tears of Ocular Graft-Versus-Host Disease Patients. Am J Ophthalmol 176:46–52, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Arai S, Arora M, Wang T, et al. : Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biology of Blood and Marrow Transplantation 21:266–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronni S, Cortes M, Sacchetti M, et al. : Upregulation of ICAM-1 expression in the conjunctiva of patients with chronic graft-versus-host disease. Eur J Ophthalmol 16:17–23, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Aronni S, Cortes M, Sacchetti M, et al. : Upregulation of ICAM-1 expression in the conjunctiva of patients with chronic graft-versus-host disease. European journal of ophthalmology 16:17–23, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Balaram M, Rashid S, Dana R: Chronic ocular surface disease after allogeneic bone marrow transplantation. The Ocular Surface 3:203–210, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ban Y, Ogawa Y, Ibrahim OM, et al. : Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Molecular vision 17:2533, 2011 [PMC free article] [PubMed] [Google Scholar]
- 11.Berchicci L, Rabiolo A, Marchese A, et al. : Ocular chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation in an Italian referral center. Ocul Surf 16:314–321, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Betts BC, Veerapathran A, Pidala J, et al. : Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Science Translational Medicine 9:8269, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busin M, Giannaccare G, Sapigni L, et al. : Conjunctival and Limbal Transplantation From the Same Living-Related Bone Marrow Donor to Patients With Severe Ocular Graft-vs-Host Disease. JAMA Ophthalmol 135:1123–1125, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Carpenter PA, Kitko CL, Elad S, et al. : National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 ancillary therapy and supportive care working group report. Biology of Blood and Marrow Transplantation 21:1167–1187, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra S, Jerkins JH, Conto JE, et al. : Lifitegrast ophthalmic solution for treatment of ocular chronic graft-versus-host disease. Leukemia & lymphoma 61:869–874, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Chhadva P, Goldhardt R, Galor A: Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology 124:S20–S26, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi W, Ha JY, Li Y, et al. : Comparison of the meibomian gland dysfunction in patients with chronic ocular graft-versus-host disease and Sjögren’s syndrome. International journal of ophthalmology 12:393, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocho L, Fernández I, Calonge M, et al. : Gene Expression-Based Predictive Models of Graft Versus Host Disease-Associated Dry Eye. Invest Ophthalmol Vis Sci 56:4570–4581, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Cocho L, Fernández I, Calonge M, et al. : Biomarkers in Ocular Chronic Graft Versus Host Disease: Tear Cytokine- and Chemokine-Based Predictive Model. Invest Ophthalmol Vis Sci 57:746–758, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Contreras-Ruiz L, Masli S: Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 10:e0120284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couriel D, Saliba R, Escalon M, et al. : Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft- versus- host disease. British journal of haematology 130:409–417, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Dartt DA: Tear lipocalin: structure and function. Ocul Surf 9:126–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Almeida Borges D, Alborghetti MR, Franco Paes Leme A, et al. : Tear proteomic profile in three distinct ocular surface diseases: keratoconus, pterygium, and dry eye related to graft-versus-host disease. Clin Proteomics 17:42, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De la Parra-Colín P, Agahan A, Pérez-Simón J, et al. : Dry eye disease in chronic graft-versus-host disease: results from a Spanish retrospective cohort study: Transplantation proceedings, Vol. 43, Elsevier, 2011, pp. 1934–1938 [DOI] [PubMed] [Google Scholar]
- 25.De Paiva C, Pflugfelder S: Rationale for anti-inflammatory therapy in dry eye syndrome. Arquivos Brasileiros de Oftalmologia 71:89–95, 2008 [DOI] [PubMed] [Google Scholar]
- 26.de Souza GA, Godoy LM, Mann M: Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol 7:R72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich-Ntoukas T, Cursiefen C, Westekemper H, et al. : Diagnosis and treatment of ocular chronic graft-versus-host disease: Report from the German–Austrian–Swiss Consensus Conference on Clinical Practice in Chronic GVHD. Cornea 31:299–310, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Dietrich-Ntoukas T, Cursiefen C, Westekemper H, et al. : Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea 31:299–310, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Donato R, Cannon BR, Sorci G, et al. : Functions of S100 proteins. Curr Mol Med 13:24–57, 2013 [PMC free article] [PubMed] [Google Scholar]
- 30.Engel L, Wittig S, Bock F, et al. : Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplantation 50:961–967, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Ferreira AM, Pontes da Silva CA, Pereira AD, et al. : Ruxolitinib in steroid-refractory chronic graft-versus-host disease: experience of a single center. Bone Marrow Transplantation 53:503–506, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Fini ME, Jeong S, Wilson MR: Therapeutic potential of the molecular chaperone and matrix metalloproteinase inhibitor clusterin for dry eye. International Journal of Molecular Sciences 22:116, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flanagan JL, Willcox MD: Role of lactoferrin in the tear film. Biochimie 91:35–43, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Flowers ME, Martin PJ: How we treat chronic graft-versus-host disease. Blood, The Journal of the American Society of Hematology 125:606–615, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn R, Du J, Veenstra RG, et al. : Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood, The Journal of the American Society of Hematology 123:3988–3998, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda K, Ishida W, Tanaka H, et al. : Inhibition by rebamipide of cytokine-induced or lipopolysaccharide-induced chemokine synthesis in human corneal fibroblasts. British Journal of Ophthalmology 98:1751–1755, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Geerling G, Tost FHW: Surgical occlusion of the lacrimal drainage system. Dev Ophthalmol 41:213–229, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Gerber-Hollbach N, Plattner K, O’Leary OE, et al. : Tear Film Proteomics Reveal Important Differences Between Patients With and Without Ocular GvHD After Allogeneic Hematopoietic Cell Transplantation. Invest Ophthalmol Vis Sci 59:3521–3530, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Ghimire S, Weber D, Mavin E, et al. : Pathophysiology of GvHD and other HSCT-related major complications. Frontiers in immunology 8:79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghimire S, Weber D, Mavin E, et al. : Pathophysiology of GvHD and Other HSCT-Related Major Complications. Front Immunol 8:79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannaccare G, Versura P, Bonifazi F, et al. : Comparison among different diagnostic criteria for chronic ocular graft-versus-host disease applied with and without pre-transplant ophthalmological examination. Eye 33:154–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyurkocza B, Rezvani A, Storb RF: Allogeneic hematopoietic cell transplantation: the state of the art. Expert review of hematology 3:285–299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho CY, Lammerding J: Lamins at a glance. J Cell Sci 125:2087–2093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodges RR, Dartt DA: Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Experimental eye research 117:62–78, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holland EJ, Jackson MA, Donnenfeld E, et al. : Efficacy of Lifitegrast Ophthalmic Solution, 5.0%, in Patients With Moderate to Severe Dry Eye Disease: A Post Hoc Analysis of 2 Randomized Clinical Trials. JAMA ophthalmology 139:1200–1208, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu B, Qiu Y, Hong J: Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease(GVHD). Ocul Surf 18:298–304, 2020 [DOI] [PubMed] [Google Scholar]
- 47.Huang S, Risques RA, Martin GM, et al. : Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A. Exp Cell Res 314:82–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikarashi H, Aketa N: Two case reports of continued progression of chronic ocular graft-versus-host disease without concurrent systemic comorbidities treated by amniotic membrane transplantation. 21:164, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inamoto Y, Martin PJ, Chai X, et al. : Clinical benefit of response in chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation 18:1517–1524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanir Y, Shimoni A, Ezra-Nimni O, Barequet IS: Prevalence of dry eye syndrome after allogeneic hematopoietic stem cell transplantation. Cornea 32:e97–e101, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Jabs DA, Hirst LW, Green WR, et al. : The eye in bone marrow transplantation. II. Histopathology. Arch Ophthalmol 101:585–590, 1983 [DOI] [PubMed] [Google Scholar]
- 52.Jacobs R, Tran U, Chen H, et al. : Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant 47:1470–1473, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Jagasia MH, Greinix HT, Arora M, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation 21:389–401. e381, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeppesen H, Sengeløv H, Eriksson F, et al. : Chronic ocular graft-versus-host disease after allogeneic haematopoietic stem cell transplantation in Denmark–factors associated with risks and rates in adults according to conditioning regimen. Bone marrow transplantation 56:144–154, 2021 [DOI] [PubMed] [Google Scholar]
- 55.Jin H, Ni X, Deng R, et al. : Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood, The Journal of the American Society of Hematology 127:2249–2260, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jirsova K, Seidler Stangova P, Palos M, et al. : Aberrant HLA-DR expression in the conjunctival epithelium after autologous serum treatment in patients with graft-versus-host disease or Sjögren’s syndrome. PloS one 15:e0231473, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansen FE, Braathen R, Brandtzaeg P: Role of J chain in secretory immunoglobulin formation. Scand J Immunol 52:240–248, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Johansen FE, Kaetzel CS: Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol 4:598–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung JW, Lee YJ, Yoon SC, et al. : Long-term result of maintenance treatment with tacrolimus ointment in chronic ocular graft-versus-host disease. American journal of ophthalmology 159:519–527. e511, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Jüngert K, Paulsen F, Jacobi C, et al. : Prolactin Inducible Protein, but Not Prolactin, Is Present in Human Tears, Is Involved in Tear Film Quality, and Influences Evaporative Dry Eye Disease. Front Med (Lausanne) 9:892831, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamoi M, Ogawa Y, Uchino M, et al. : Donor-recipient gender difference affects severity of dry eye after hematopoietic stem cell transplantation. Eye (Lond) 25:860–865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawai M, Ogawa Y, Shimmura S, et al. : Expression and localization of aging markers in lacrimal gland of chronic graft-versus-host disease. Sci Rep 3:2455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerty El, Vigander K, Flage T, Brinch L: Ocular findings in allogeneic stem cell transplantation without total body irradiation. Ophthalmology 106:1334–1338, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Kheirkhah A, Darabad RR, Cruzat A, et al. : Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Investigative ophthalmology & visual science 56:7179–7185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim M, Lee Y, Mehra D, et al. : Dry eye: why artificial tears are not always the answer. BMJ open ophthalmology 6:e000697, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S, Couriel D, Ghosh S, Champlin R: Ocular graft vs. host disease experience from MD Anderson Cancer Center: Newly described clinical spectrum and new approach to the management of stage III and IV ocular GVHD. Biology of Blood and Marrow Transplantation 12:49–50, 2006 [Google Scholar]
- 67.Kinoshita S, Awamura S, Oshiden K, et al. : Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology 119:2471–2478, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Korb DR, Blackie CA: Using goggles to increase periocular humidity and reduce dry eye symptoms. Eye Contact Lens 39:273–276, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S: TGF-β - an excellent servant but a bad master. J Transl Med 10:183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon J, Surenkhuu B, Raju I, et al. : Pathological consequences of anti-citrullinated protein antibodies in tear fluid and therapeutic potential of pooled human immune globulin-eye drops in dry eye disease. The Ocular Surface 18:80–97, 2020 [DOI] [PubMed] [Google Scholar]
- 71.Lee WM, Galbraith RM: The extracellular actin-scavenger system and actin toxicity. N Engl J Med 326:1335–1341, 1992 [DOI] [PubMed] [Google Scholar]
- 72.Leite SC, de Castro RS, Alves M, et al. : Risk factors and characteristics of ocular complications, and efficacy of autologous serum tears after haematopoietic progenitor cell transplantation. Bone Marrow Transplant 38:223–227, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Li B, Sheng M, Li J, et al. : Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci Rep 4:5772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Liang Q, Huang F, et al. : Metagenomic Profiling of the Ocular Surface Microbiome in Patients After Allogeneic Hematopoietic Stem Cell Transplantation. Am J Ophthalmol 242:144–155, 2022 [DOI] [PubMed] [Google Scholar]
- 75.Luo ZK, Domenech-Estarellas EA, Han A, et al. : Efficacy and Safety of 1% Progesterone Gel to the Forehead for Ocular Chronic Graft-versus-Host Disease. Transplantation and Cellular Therapy 27:433. e431–433. e438, 2021 [DOI] [PubMed] [Google Scholar]
- 76.Magro L, Gauthier J, Richet M, et al. : Scleral lenses for severe chronic GvHD-related keratoconjunctivitis sicca: a retrospective study by the SFGM-TC. 52:878–882, 2017 [DOI] [PubMed] [Google Scholar]
- 77.Marsman G, Zeerleder S, Luken BM: Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 7:e2518, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsuda A, Tagawa Y, Yamamoto K, et al. : Identification and immunohistochemical localization of annexin II in rat cornea. Curr Eye Res 19:368–375, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Matthaiou EI, Sharifi H, O’Donnell C, et al. : The safety and tolerability of pirfenidone for bronchiolitis obliterans syndrome after hematopoietic cell transplant (STOP-BOS) trial. Bone Marrow Transplantation:1–8, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McDermott AM: Antimicrobial compounds in tears. Exp Eye Res 117:53–61, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Modi B, Hernandez-Henderson M, Yang D, et al. : Ruxolitinib as salvage therapy for chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation 25:265–269, 2019 [DOI] [PubMed] [Google Scholar]
- 82.Mukai S, Ogawa Y, Urano F, et al. : Novel treatment of chronic graft-versus-host disease in mice using the ER stress reducer 4-phenylbutyric acid. Scientific reports 7:1–13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukai S, Ogawa Y, Saya H, et al. : Therapeutic potential of tranilast for the treatment of chronic graft-versus-host disease in mice. PloS one 13:e0203742, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Na K-S, Yoo Y-s, Hwang K-y, et al. : Tear osmolarity and ocular surface parameters as diagnostic markers of ocular graft-versus-host disease. American Journal of Ophthalmology 160:143–149. e141, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Na KS, Yoo YS, Mok JW, et al. : Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 50:1459–1464, 2015 [DOI] [PubMed] [Google Scholar]
- 86.Nair S, Vanathi M, Mahapatra M, et al. : Tear inflammatory mediators and protein in eyes of post allogenic hematopoeitic stem cell transplant patients. Ocul Surf 16:352–367, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Nair S, Vanathi M, Mukhija R, et al. : Update on ocular graft-versus-host disease. Indian Journal of Ophthalmology 69:1038, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nepp J, Knoetzl W, Prinz A, et al. : Management of moderate-to-severe dry eye disease using chitosan-N-acetylcysteine (Lacrimera®) eye drops: a retrospective case series. International Ophthalmology 40:1547–1552, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Leary OE, Schoetzau A, Amruthalingam L, et al. : Tear Proteomic Predictive Biomarker Model for Ocular Graft Versus Host Disease Classification. Transl Vis Sci Technol 9:3, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogawa Y, Okamoto S, Wakui M, et al. : Dry eye after haematopoietic stem cell transplantation. British Journal of Ophthalmology 83:1125–1130, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ogawa Y, Yamazaki K, Kuwana M, et al. : A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Investigative ophthalmology & visual science 42:111–119, 2001 [PubMed] [Google Scholar]
- 92.Ogawa Y, Kuwana M, Yamazaki K, et al. : Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease. Investigative ophthalmology & visual science 44:1888–1896, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Ogawa Y, Shimmura S, Kawakita T, et al. : Epithelial mesenchymal transition in human ocular chronic graft-versus-host disease. The American journal of pathology 175:2372–2381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ogawa Y, Dogru M, Uchino M, et al. : Topical tranilast for treatment of the early stage of mild dry eye associated with chronic GVHD. Bone marrow transplantation 45:565–569, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Ogawa Y, Kim SK, Dana R, et al. : International chronic ocular graft-vs-host-disease (GVHD) consensus group: proposed diagnostic criteria for chronic GVHD (Part I). Scientific reports 3:1–6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogawa Y, He H, Mukai S, et al. : Heavy chain-hyaluronan/pentraxin 3 from amniotic membrane suppresses inflammation and scarring in murine lacrimal gland and conjunctiva of chronic graft-versus-host disease. Scientific reports 7:1–9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohigashi H, Hashimoto D, Hayase E, et al. : Ocular instillation of vitamin A–coupled liposomes containing HSP47 siRNA ameliorates dry eye syndrome in chronic GVHD. Blood advances 3:1003–1010, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olson MC, Korb DR, Greiner JV: Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye & contact lens 29:96–99, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Pan Q, Angelina A, Marrone M, et al. : Autologous serum eye drops for dry eye. Cochrane Database of Systematic Reviews, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papas AS, Sherrer YS, Charney M, et al. : Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. JCR: Journal of Clinical Rheumatology 10:169–177, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Pathak M, Diep PP, Lai X, et al. : Ocular findings and ocular graft-versus-host disease after allogeneic stem cell transplantation without total body irradiation. Bone Marrow Transplantation 53:863–872, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pellegrini M, Bernabei F, Barbato F, et al. : Incidence, risk factors and complications of ocular graft-versus-host disease following hematopoietic stem cell transplantation. American Journal of Ophthalmology 227:25–34, 2021 [DOI] [PubMed] [Google Scholar]
- 103.Penack O, Marchetti M, Ruutu T, et al. : Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. The Lancet Haematology 7:e157–e167, 2020 [DOI] [PubMed] [Google Scholar]
- 104.Perumal N, Funke S, Pfeiffer N, Grus FH: Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci Rep 6:29629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pietraszkiewicz AA, Payne D, Abraham M, et al. : Ocular surface indicators and biomarkers in chronic ocular graft-versus-host disease: a prospective cohort study. 56:1850–1858, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Que L, Zhang X, Li M: Single-center retrospective study on meibomian gland loss in patients with ocular chronic graft-versus-host disease. Eye & Contact Lens 44:S169–S175, 2018 [DOI] [PubMed] [Google Scholar]
- 107.Riemens A, Te Boome LC, Kalinina Ayuso V, et al. : Impact of ocular graft- versus- host disease on visual quality of life in patients after allogeneic stem cell transplantation: questionnaire study. Acta ophthalmologica 92:82–87, 2014 [DOI] [PubMed] [Google Scholar]
- 108.Robinson M, Lee S, Rubin B, et al. : Topical corticosteroid therapy for cicatricial conjunctivitis associated with chronic graft-versus-host disease. Bone marrow transplantation 33:1031–1035, 2004 [DOI] [PubMed] [Google Scholar]
- 109.Rodier F, Coppé JP, Patil CK, et al. : Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11:973–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruhrberg C, Hajibagheri MA, Parry DA, Watt FM: Periplakin, a novel component of cornified envelopes and desmosomes that belongs to the plakin family and forms complexes with envoplakin. J Cell Biol 139:1835–1849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryu EH, Kim JM, Laddha PM, et al. : Therapeutic effect of 0.03% tacrolimus ointment for ocular graft versus host disease and vernal keratoconjunctivitis. Korean Journal of Ophthalmology 26:241–247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saboo US, Amparo F, Shikari H, Dana R: Prevalence of ocular hypertension and glaucoma in patients with chronic ocular graft-versus-host disease. Graefes Arch Clin Exp Ophthalmol 254:923–928, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sabti S, Halter JP, Braun Fränkl BC, Goldblum D: Punctal occlusion is safe and efficient for the treatment of keratoconjunctivitis sicca in patients with ocular GvHD. Bone Marrow Transplant 47:981–984, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Saito T, Shinagawa K, Takenaka K, et al. : Ocular manifestation of acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. International journal of hematology 75:332–334, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Sato S, Shimizu E: Positive Effects of Oral Antibiotic Administration in Murine Chronic Graft-Versus-Host Disease. 22, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schoemans HM, Lee SJ, Ferrara JL, et al. : EBMT− NIH− CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone marrow transplantation 53:1401–1415, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwab L, Goroncy L, Palaniyandi S, et al. : Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nature medicine 20:648–654, 2014 [DOI] [PubMed] [Google Scholar]
- 118.Sckisel GD, Bouchlaka MN, Monjazeb AM, et al. : Out-of-sequence signal 3 paralyzes primary CD4+ T-cell-dependent immunity. Immunity 43:240–250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shamloo K, Barbarino A, Alfuraih S, Sharma A: Graft versus host disease-associated dry eye: role of ocular surface mucins and the effect of rebamipide, a mucin secretagogue. Investigative Ophthalmology & Visual Science 60:4511–4519, 2019 [DOI] [PubMed] [Google Scholar]
- 120.Shamloo K, Weng J, Ross C, et al. : Local Renin-Angiotensin System Activation and Myofibroblast Formation in Graft Versus Host Disease–Associated Conjunctival Fibrosis. Investigative Ophthalmology & Visual Science 62:10–10, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shikari H, Amparo F, Saboo U, Dana R: Onset of ocular graft-versus-host disease symptoms after allogeneic hematopoietic stem cell transplantation. Cornea 34:243–247, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimazaki J: Definition and diagnostic criteria of dry eye disease: historical overview and future directions. Investigative ophthalmology & visual science 59:DES7–DES12, 2018 [DOI] [PubMed] [Google Scholar]
- 123.Shimizu E, Ogawa Y, Saijo Y, et al. : Commensal microflora in human conjunctiva; characteristics of microflora in the patients with chronic ocular graft-versus-host disease. Ocul Surf 17:265–271, 2019 [DOI] [PubMed] [Google Scholar]
- 124.Singh RB, Yung A, Coco G, et al. : Efficacy and retention of silicone punctal plugs for treatment of dry eye in patients with and without ocular graft-versus-host-disease. Ocul Surf 18:731–735, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh RB, Yuksel E, Sinha S, et al. : Prevalence of neurotrophic keratopathy in patients with chronic ocular graft-versus-host disease. The Ocular Surface 26:13–18, 2022 [DOI] [PubMed] [Google Scholar]
- 126.Sinha S, Singh RB, Dohlman TH, et al. : Prevalence and risk factors associated with corneal perforation in chronic ocular graft-versus-host-disease. Cornea 40:877–882, 2021 [DOI] [PubMed] [Google Scholar]
- 127.Smith A, Stanley P, Jones K, et al. : The role of the integrin LFA- 1 in T- lymphocyte migration. Immunological reviews 218:135–146, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Sonawane S, Khanolkar V, Namavari A, et al. : Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci 53:8253–8263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sugar A, Hussain M, Chamberlain W, et al. : A Randomized Trial of Topical Fibrinogen-Depleted Human Platelet Lysate Treatment of Dry Eye Secondary to Chronic Graft-versus-Host Disease. Ophthalmology science 2:100176, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tabbara KF, Al-Ghamdi A, Al-Mohareb F, et al. : Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology 116:1624–1629, 2009 [DOI] [PubMed] [Google Scholar]
- 131.Tahmaz V, Wiesen MH, Gehlsen U, et al. : Detection of systemic immunosuppressants in autologous serum eye drops (ASED) in patients with severe chronic ocular graft versus host disease. Graefe’s Archive for Clinical and Experimental Ophthalmology 259:121–128, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takeji Y, Urashima H, Aoki A, Shinohara H: Rebamipide increases the mucin-like glycoprotein production in corneal epithelial cells. Journal of ocular pharmacology and therapeutics 28:259–263, 2012 [DOI] [PubMed] [Google Scholar]
- 133.Tatematsu Y, Ogawa Y, Abe T, et al. : Grading criteria for chronic ocular graft-versus-host disease: Comparing the NIH eye score, Japanese dry eye score and DEWS 2007 score. Scientific reports 4:1–6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tauchmanovà L, Selleri C, Rosa GD, et al. : High prevalence of endocrine dysfunction in long- term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer 95:1076–1084, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Tegla C, Choi J, Abdul-Hay M, et al. : Current Status and Future Directions in Graft-Versus-Host Disease Prevention Following Allogeneic Blood and Marrow Transplantation in Adults. Clinical Hematology International 2:5, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tiffany JM: Tears in health and disease. Eye (Lond) 17:923–926, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, et al. : The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 8:110–120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Uchino M, Ogawa Y, Uchino Y, et al. : Comparison of stem cell sources in the severity of dry eye after allogeneic haematopoietic stem cell transplantation. Br J Ophthalmol 96:34–37, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Urashima H, Takeji Y, Okamoto T, et al. : Rebamipide increases mucin-like substance contents and periodic acid Schiff reagent-positive cells density in normal rabbits. Journal of ocular pharmacology and therapeutics 28:264–270, 2012 [DOI] [PubMed] [Google Scholar]
- 140.Vajapey R, Rini D, Walston J, Abadir P: The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Frontiers in physiology 5:439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang CY, Lin CF: Annexin A2: its molecular regulation and cellular expression in cancer development. Dis Markers 2014:308976, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang JC, Teichman JC, Mustafa M, et al. : Risk factors for the development of ocular graft-versus-host disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol 99:1514–1518, 2015 [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Ogawa Y, Dogru M, et al. : Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone marrow transplantation 41:293–302, 2008 [DOI] [PubMed] [Google Scholar]
- 144.Wang Y, Ogawa Y, Dogru M, et al. : Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone marrow transplantation 45:1077–1083, 2010 [DOI] [PubMed] [Google Scholar]
- 145.Wang Y, Carreno-Galeano JT, Singh RB, et al. : Long-term Outcomes of Punctal Cauterization in the Management of Ocular Surface Diseases. Cornea 40:168–171, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Westekemper H, Meller S, Citak S, et al. : Differential chemokine expression in chronic GVHD of the conjunctiva. Bone Marrow Transplant 45:1340–1346, 2010 [DOI] [PubMed] [Google Scholar]
- 147.Wolff D, Gerbitz A, Ayuk F, et al. : Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biology of Blood and Marrow Transplantation 16:1611–1628, 2010 [DOI] [PubMed] [Google Scholar]
- 148.Wolffsohn JS, Arita R, Chalmers R, et al. : TFOS DEWS II diagnostic methodology report. The ocular surface 15:539–574, 2017 [DOI] [PubMed] [Google Scholar]
- 149.Wu T, Young JS, Johnston H, et al. : Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. The Journal of Immunology 191:488–499, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yaguchi S, Ogawa Y, Kamoi M, et al. : Surgical management of lacrimal punctal cauterization in chronic GVHD-related dry eye with recurrent punctal plug extrusion. Bone Marrow Transplant 47:1465–1469, 2012 [DOI] [PubMed] [Google Scholar]
- 151.Yaguchi S, Ogawa Y, Shimmura S, et al. : Presence and physiologic function of the renin–angiotensin system in mouse lacrimal gland. Investigative Ophthalmology & Visual Science 53:5416–5425, 2012 [DOI] [PubMed] [Google Scholar]
- 152.Yaguchi S, Ogawa Y, Kawakita T, et al. : Tissue renin–angiotensin system in lacrimal gland fibrosis in a murine model of chronic graft-versus-host disease. Cornea 34:S142–S152, 2015 [DOI] [PubMed] [Google Scholar]
- 153.Yamane M, Ogawa Y, Fukui M, et al. : Long-term topical diquafosol tetrasodium treatment of dry eye disease caused by chronic graft-versus-host disease: a retrospective study. Eye & contact lens 44:S215–S220, 2018 [DOI] [PubMed] [Google Scholar]
- 154.Yamane M, Sato S, Shimizu E, et al. : Senescence-associated secretory phenotype promotes chronic ocular graft-vs-host disease in mice and humans. Faseb j 34:10778–10800, 2020 [DOI] [PubMed] [Google Scholar]
- 155.Yang F, Hayashi I, Sato S, et al. : Eyelid blood vessel and meibomian gland changes in a sclerodermatous chronic GVHD mouse model. The Ocular Surface, 2021 [DOI] [PubMed] [Google Scholar]
- 156.Yin J, Kheirkhah A, Dohlman T, et al. : Reduced efficacy of low-dose topical steroids in dry eye disease associated with graft-versus-host disease. American journal of ophthalmology 190:17–23, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yusifov TN, Abduragimov AR, Narsinh K, et al. : Tear lipocalin is the major endonuclease in tears. Mol Vis 14:180–188, 2008 [PMC free article] [PubMed] [Google Scholar]
- 158.Zeiser R, Blazar BR: Acute graft-versus-host disease—biologic process, prevention, and therapy. New England Journal of Medicine 377:2167–2179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou L, Zhao SZ, Koh SK, et al. : In-depth analysis of the human tear proteome. J Proteomics 75:3877–3885, 2012 [DOI] [PubMed] [Google Scholar]
- 160.Zilliox MJ, Gange WS, Kuffel G, et al. : Assessing the ocular surface microbiome in severe ocular surface diseases. Ocul Surf 18:706–712, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


