Abstract
Introduction:
Little attention has been paid to the influence of individually-measured social determinants of health (SDH) on cancer screening tests in the Medicaid population.
Methods:
Analysis was conducted on 2015 – 2020 claims data from a subgroup of Medicaid enrollees from the District of Columbia Medicaid Cohort Study (N=8,943) who were eligible for colorectal (N=2,131), breast (N=1,156) and cervical cancer (N=5,068) screening. Participants were grouped into four distinct SDH groups based on their responses to an SDH questionnaire. This study estimated the influence of the four SDH groups on the receipt of each screening test using log-binomial regression adjusted for demographics, illness severity, and neighborhood-level deprivation.
Results:
The receipt of cancer screening tests was 42%, 58%, and 66% for colorectal, cervical, and breast cancer, respectively. Those in the most disadvantaged SDH group were less likely to receive a colonoscopy/sigmoidoscopy than those in the least disadvantaged one (aRR = 0.70, 95% CI 0.54 – 0.92). The pattern for mammograms and Pap smears was similar (aRR=0.94, 95% CI 0.80 – 1.11 and aRR=0.90, 95% CI 0.81 – 1.00, respectively). In contrast, participants in the most disadvantaged SDH group were more likely to receive FOBT than those in the least disadvantaged one (aRR=1.52, 95% CI 1.09 – 2.12).
Conclusions:
Severe social determinants of health measured at the individual level are associated with lower cancer preventive screening. A targeted approach that addresses the social and economic adversities that affect cancer screening could result in higher preventive screening rates in this Medicaid population.
INTRODUCTION
Cancer is the second leading cause of death in the United States.1,2,3 Breast cancer is the most commonly diagnosed cancer and the second most common cause of cancer deaths, while colorectal cancer is the fourth most commonly diagnosed and the third cause of cancer deaths for both women and men nationwide.2,3,4,5,6,7,8 People with more education and higher incomes generally have lower cancer death rates than others but even so, they may experience disparities due to their race or ethnicity: Blacks have the highest cancer death rate of all racial groups regardless of socio-economic position.9,10
Broad consensus exists that early detection and treatment of breast, cervical and colorectal cancers can improve management and survival.11,12,13,14,15,16 Early detection can also reduce treatment and health care costs, including to the Medicaid program.17 Since 2010, public and private insurers must cover preventive services recommended by the United States Preventive Services Task Force (USPSTF) for which there is high or moderate certainty that the net benefit is substantial (i.e., Grade A) or moderate (i.e., Grade B).18,19 However, population-and state-based analyses of claims data show lower rates of cancer screening among Medicaid enrollees compared to commercially-insured individuals. 12,13,14,15 Lower screening rates among Medicaid enrollees are worrisome because they experience disproportionate cancer incidence and mortality.20
Multiple determinants contribute to individual cancer risk and survival, including biological/genetic, environmental, health care, and social determinants of health (SDH).21 SDH are the non-medical factors that influence health outcomes. SDH include the conditions in which people are born, grow, work, live and age as well as the wider set of economic policies and systems, social norms, social policies and political systems that shape them.22 While a number of studies have evaluated the role of health policies (i.e., expanded coverage, copayments, provider reimbursement) on cancer screening rates in the Medicaid population, few have examined the influence of SDH factors. The purpose of this paper is to estimate the influence of an aggregate set of individually-measured SDH factors on the cancer screening rates of a cohort of adults insured by the District of Columbia (DC) Medicaid program, controlling for demographics, disease severity, and area-level deprivation. Cancer screening test completion was hypothesized to be lower among Medicaid enrollees with the most social and economic adversities compared to those with the fewest.
METHODS
This study took advantage of data collected as part of a prospective cohort study, known as the DC Medicaid Cohort Study GW IRB#123456 (N=8,943 adult Medicaid enrollees),23 and examined the relationship between SDH grouped into four distinct social risk groups and the receipt of screening tests for three cancers. The study identified participants eligible for a colorectal (N=2,131), breast (N=1,156) and/or cervical (N=5,068) cancer screening test based on the USPSTF recommendations,24,25,26 and determined completion of a colorectal, breast and cervical cancer screening test as defined in the Healthcare Effectiveness Data and Information Set (HEDIS) guidelines.27 Separate models were developed using Medicaid claims over a 4-year period for the receipt of at least one colorectal, breast and cervical cancer screening test as a function of social risk group, age, sex, morbidity burden, having a regular medical provider, length of Medicaid coverage and neighborhood deprivation.
Study Sample
The parent study screened adult Medicaid enrollees at the time of a health care visit to an emergency department, primary care office, or obstetrics and gynecology office affiliated with one of two DC hospitals over a 16-month period starting in September 2017 and ending in December 2018.23 Patients had to be between 18 and 64 years old at the time of study enrollment, insured by the DC Medicaid program and with access to a telephone.23 Patients were deemed ineligible if unable to understand consent, non-English speaking, or also insured by Medicare.23
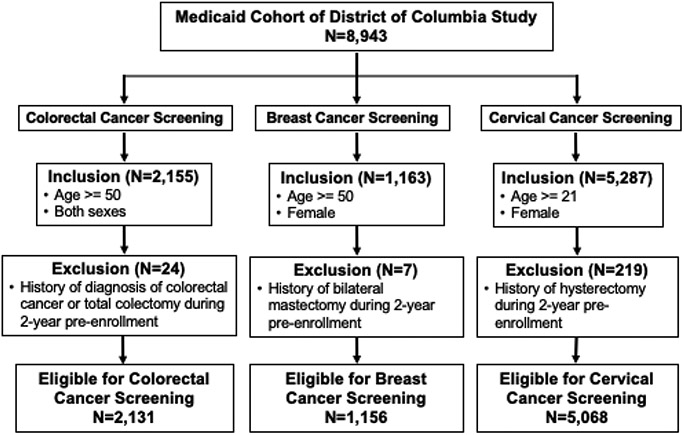
Eligibility for this analysis is based on the cancer screening test using sex, participant age two years prior to study enrollment, and medical history during a 2-year period prior to study enrollment (Appendix Table 1, medical exclusions). Participants had to be at least 50 years old without a prior history of colorectal cancer or total colectomy procedure, or female, aged 50 or older with no prior history of bilateral mastectomy, or female, at least 21 years of age with no prior history of a hysterectomy. Relatively few participants were excluded due to medical contraindications (Figure 1). The sample consists largely of Black Medicaid enrollees (91%).
Figure 1.
Derivation of Study Groups.
Measures
The receipt of at least one colorectal, breast or cervical cancer screening test during a 4-year study period (2-years pre-and 2-years post-study enrollment) was the main study outcome. It was identified using the Current Procedural Terminology (CPT)/Healthcare Common Procedure Coding System (HCPCS) codes detailed in the 2018 HEDIS resource guide (see Appendix Table 1).27
The recommended screening for colorectal cancer in adults aged 50 to 75 years includes stool-based (annual fecal occult blood test [FOBT]; annual fecal immunochemical test [FIT]); and direct visualization screening tests (colonoscopy every 10 years, CT colonography every 5 years, or flexible sigmoidoscopy every 5 years).24 The analysis excluded FIT or CT colonography as the study sample did not include any claims for these tests. Since only 0.3% percent of participants had a flexible sigmoidoscopy during the 4-year period, the analysis combined it with colonoscopy. For breast cancer, the recommended screening is a biennial mammogram for women between the ages of 50 and 75. For cervical cancer, the recommended screening is a Papanicolaou smear without HPV testing every three years for women under age 30 or a Pap with HPV testing every 5 years for women aged 30-65.25,26
The main covariate of interest was adverse SDH measured at the time of enrollment through a face-to-face interview with a research assistant. The World Health Organization model of SDH, which includes both structural determinants of health inequities and intermediary determinants of health, guided the SDH assessment.28. The structural determinants measured were race, education, and employment status; the intermediary determinants included material circumstances (e.g., residence type, housing instability, food insecurity, financial strain), health behaviors (e.g., smoking, illicit drug and alcohol use), and psychosocial factors (e.g., loneliness, marital status, living with children, history of being in jail/prison) (Appendix Figure 1, baseline SDH assessment).23
Since individual SDH factors are highly correlated with one another and evaluating them separately in a regression model may lead to important associations being missed, the study employed latent class analysis (LCA) to categorize participants into four social risk groups based on a similar response profile to the SDH assessment variables.23 All SDH factors were dichotomized to make them easier to interpret the class solution. LCA models with 2-6 class solutions were run due to the need for a relatively small number of latent classes for ease of interpretation. LCA assigns individuals to classes based on their probability of being in classes according to the pattern of scores they have on the indicator variables; here, the SDH factors.
The LCA identified four distinct social risk groups within the cohort according to fit, classification, diagnostics, and ease of interpretation.23 The lowest social risk group had the highest employment rate and reported the fewest social disadvantages (social risk group 1). Group 2 participants were also more employed but more likely to report trouble paying their bills. Group 3 consisted mostly of unemployed participants but not reporting financial strain whereas Group 4, the highest social risk group, had the highest unemployment rate and reported the most social hardships in terms of housing, food insecurity, financial strain and health behaviors.23
The analysis controlled for risk factors when examining the relationship between social risk group measured at the individual level and receipt of each cancer screening test, including socio-economic disadvantage assessed at the neighborhood level. All participants with a valid address were assigned a census block group and then linked to the 2019 Area Deprivation Index (ADI) - a weighted, composite score based on 17 census block-level indicators of poverty, educational attainment, housing, and employment.29 The ADI ranges from 1 to 100. The higher the score, the worse the area deprivation. This study grouped participants into four categories based on the ADI quartile distribution in the parent cohort.
Other factors included disease burden (measured according to the Johns Hopkins Adjusted Clinical Groups (ACG) system (version 12.0)),30 regular medical providers, and Medicaid coverage duration during the 4-year study period, in addition to age and sex. The ACG system uses ICD-10 diagnosis and pharmacy codes from administrative billing data (here the Medicaid claims for a 2-year period prior to study enrollment) to quantify disease burden based on clinical factors, such as likelihood or persistence of a condition, severity of the condition, diagnostic certainty, etiology, and the types of healthcare services required.
An indicator variable of whether the participant had a regular doctor was included based on a question in the baseline SDH assessment. The Medicaid eligibility claims file was used to determine each participant’s Medicaid coverage status during the four-year study period.
Statistical Analysis
First, the relationship between the receipt of at least one colonoscopy and/or sigmoidoscopy, FOBT, mammogram, or Papanicolaou smear and adverse SDH (i.e., social risk group), age, sex, duration of Medicaid coverage, disease burden, having a regular medical provider, and ADI quartile was assessed using a chi-squared test of homogeneity. Second, the correlation between social risk group and the other risk factors was estimated. Third, the receipt of each cancer screening test was modeled separately as a function of social risk group, ADI quartile, disease burden, age, sex, duration of Medicaid coverage, and having a regular medical provider, using log-binomial regression. Because social risk group had a meaningfully different association with the receipt of colonoscopy/sigmoidoscopy versus FOBT, each was modeled separately.
Since there was a strong relationship between social risk group and having a regular medical provider, models were rerun without the regular medical provider variable to estimate the relationship between social risk group and receipt of the cancer screening tests less conservatively.
All analyses were completed using SAS 9.4 and R version 4.0.1.
RESULTS
During the 4-year study period, the receipt of a colonoscopy/sigmoidoscopy or FOBT was 42% among the 2,131 participants eligible for colorectal cancer screening. More participants received a colonoscopy/sigmoidoscopy (26%) than FOBT (13%) and few received both (4%). Among the 271 participants who only received an FOBT, 5 received an annual FOBT during the 4-year period.
Among the female participants, 66% of the 1,156 eligible participants received at least one mammogram during the study period and 39% received at least two mammograms. Slightly more than half (58%) of the 5,068 women eligible for cervical cancer screening received at least one Papanicolaou smear.
Younger women were more likely to receive a Papanicolaou smear than older women and men were less likely to receive a colonoscopy/sigmoidoscopy than women (Table 1). Participants who reported having a regular medical provider were more likely to receive a screening test than those who did not, except for FOBT. Those in the lowest social risk group (fewest disadvantages) were more likely to receive a colonoscopy/sigmoidoscopy or Papanicolaou smear compared to those in the highest social risk group. There was no significant association between the ADI quartile and receipt of the cancer screening tests.
Table 1.
Percent Distribution of Receipt of Preventive Services by Different Risk Factors.
| Risk Factorsa | Colonoscopy and/or Sigmoidoscopy (N=2131) |
FOBT (N=2131) |
Mammography (N=1156) |
Cervical Cytology (N=5068) |
||||
|---|---|---|---|---|---|---|---|---|
| 0 | ≥ 1 | 0 | ≥ 1 | 0 | ≥ 1 | 0 | ≥ 1 | |
| Social Risk Group | ||||||||
| Group 1 (fewest risks) | 366 (24%) | 186 (30%) | 472 (26%) | 80 (23%) | 101 (26%) | 249 (32%) | 959 (45%) | 1437 (49%) |
| Group 2 | 254 (17%) | 101 (16%) | 303 (17%) | 52 (15%) | 84 (22%) | 143 (19%) | 479 (23%) | 711 (24%) |
| Group 3 | 677 (45%) | 283 (45%) | 794 (45%) | 166 (48%) | 155 (40%) | 304 (40%) | 501 (24%) | 609 (21%) |
| Group 4 (most risks) | 211 (14%) | 53 (9%) | 214 (12%) | 50 (14%) | 48 (12%) | 72 (9%) | 186 (9%) | 186 (6%) |
| Ageb | ||||||||
| 20 – 29 years old | - | - | - | - | - | - | 685 (32%) | 1333 (45%) |
| 30 – 39 years old | - | - | - | - | - | - | 482 (23%) | 697 (24%) |
| 40 – 49 years old | - | - | - | - | - | - | 377 (18%) | 416 (14%) |
| >=50 (50-54 years old) | 695 (46%) | 285 (46%) | 803 (45%) | 177 (51%) | 171 (44%) | 359 (47%) | 581 (27%) | 497 (17%) |
| >=50 (55-59 years old) | 578 (38%) | 259 (42%) | 711 (40%) | 126 (36%) | 152 (39%) | 298 (39%) | ||
| >=50 (60-62 years old) | 235 (16%) | 79 (13%) | 269 (15%) | 45 (13%) | 65 (17%) | 111 (14%) | ||
| Sex | ||||||||
| Male | 738 (49%) | 242 (39%) | 821 (46%) | 159 (46%) | - | - | - | - |
| Female | 770 (51%) | 381 (61%) | 962 (54%) | 189 (54%) | 388 (100%) | 768 (100%) | 2125 (100%) | 2943 (100%) |
| Medicaid Coverage During Study Period | ||||||||
| < 4 years | 475 (32%) | 145 (23%) | 544 (31%) | 76 (22%) | 144 (37%) | 155 (20%) | 738 (35%) | 729 (25%) |
| 4 years | 1033 (69%) | 478 (77%) | 1239 (69%) | 272 (78%) | 244 (63%) | 613 (80%) | 1387 (65%) | 2214 (75%) |
| Regular Medical Provider | ||||||||
| No | 278 (18%) | 52 (8%) | 286 (16%) | 44 (13%) | 57 (15%) | 57 (7%) | 404 (19%) | 480 (16%) |
| Yes | 1223 (81%) | 569 (91%) | 1490 (84%) | 302 (87%) | 329 (85%) | 708 (92%) | 1713 (81%) | 2452 (83%) |
| Adjusted Clinical Groups | ||||||||
| 1st quartile (healthiest) | 182 (12%) | 51 (8%) | 203 (11%) | 30 (9%) | 47 (12%) | 64 (8%) | 511 (24%) | 681 (23%) |
| 2nd quartile | 253 (17%) | 103 (17%) | 295 (17%) | 61 (18%) | 51 (13%) | 146 (19%) | 547 (26%) | 833 (28%) |
| 3rd quartile | 353 (23%) | 176 (28%) | 423 (24%) | 106 (30%) | 97 (25%) | 223 (29%) | 541 (25%) | 831 (28%) |
| 4th quartile (sickest) | 720 (48%) | 293 (47%) | 862 (48%) | 151 (43%) | 193 (50%) | 335 (44%) | 526 (25%) | 598 (20%) |
| Area Deprivation Indexc | ||||||||
| 1st quartile (least) | 405 (27%) | 152 (24%) | 458 (26%) | 99 (28%) | 93 (24%) | 184 (24%) | 437 (21%) | 647 (22%) |
| 2nd quartile | 365 (24%) | 151 (24%) | 430 (24%) | 86 (25%) | 95 (24%) | 154 (20%) | 517 (24%) | 627 (21%) |
| 3rd quartile | 323 (21%) | 152 (24%) | 399 (22%) | 76 (22%) | 84 (22%) | 199 (26%) | 563 (26%) | 787 (27%) |
| 4th quartile (most) | 335 (22%) | 140 (22%) | 404 (23%) | 71 (20%) | 90 (23%) | 193 (25%) | 525 (25%) | 780 (27%) |
Boldface indicates statistical significance (p < 0.05) between each risk factor and the receipt of the specific cancer screening test.
Age measured 2 years prior to study enrollment.
Area Deprivation Index (ADI) missing for 4-6% depending on specific preventive service test.
Table 2 shows the relationship between social risk group and the other covariates for participants eligible for a colorectal screening test. (Appendix Table 2 for results for breast and cervical cancer subgroups.) Participants in social risk group 1 were significantly more likely to report having a medical provider (88%) than those in social risk group 4 (74%). The higher the social risk group, the more severe the disease burden. There was significant variation within each social risk group by ADI quartile and vice versa. The patterns observed in Table 2 for participants eligible for a colorectal screening test were qualitatively the same for the other two cancer screening subgroups (Appendix Table 2) apart from age. For those eligible for a cervical cancer screening test, participants in social risk group 1 were younger than those in social risk group 4.
Table 2.
Percent Distribution Of Social Risk Group By Different Risk Factors For Colorectal Screening Group (N=2,131).
| Risk Factorsa | Social Risk Group | ||||
|---|---|---|---|---|---|
| Overall | Group 1 | Group 2 | Group 3 | Group 4 | |
| Ageb | |||||
| 50-54 years old | 980 (46%) | 265 (48%) | 178 (50%) | 405 (42%) | 132 (50%) |
| 55-59 years old | 837 (39%) | 198 (36%) | 127 (36%) | 404 (42%) | 108 (41%) |
| 60 -62 years old | 314 (15%) | 89 (16%) | 50 (14%) | 151 (16%) | 24 (9%) |
| Sex | |||||
| Male | 980 (46%) | 205 (37%) | 126 (35%) | 505 (53%) | 144 (55%) |
| Female | 1151 (54%) | 347 (63%) | 229 (65%) | 455 (47%) | 120 (45%) |
| Medicaid Coverage During Study Period | |||||
| < 4 years | 620 (29%) | 154 (28%) | 121 (34%) | 268 (28%) | 77 (29%) |
| 4 years | 1511 (71%) | 398 (72%) | 234 (66%) | 692 (72%) | 187 (71%) |
| Regular Medical Provider | |||||
| No | 330 (15%) | 67 (12%) | 54 (15%) | 141 (15%) | 68 (26%) |
| Yes | 1792 (84%) | 483 (88%) | 300 (85%) | 813 (85%) | 196 (74%) |
| Adjusted Clinical Groups | |||||
| 1st quartile (healthiest) | 233 (11%) | 88 (16%) | 44 (12%) | 85 (9%) | 16 (6%) |
| 2nd quartile | 356 (17%) | 119 (22%) | 69 (19%) | 143 (15%) | 25 (9%) |
| 3rd quartile | 529 (25%) | 171 (31%) | 108 (30%) | 200 (21%) | 50 (19%) |
| 4th quartile (sickest) | 1013 (48%) | 174 (32%) | 134 (38%) | 532 (55%) | 173 (66%) |
| Area Deprivation Index (ADI) c | |||||
| 1st quartile (least) | 557 (26%) | 173 (31%) | 66 (19%) | 247 (26%) | 71 (27%) |
| 2nd quartile | 516 (24%) | 112 (20%) | 98 (28%) | 237 (25%) | 69 (26%) |
| 3rd quartile | 475 (22%) | 128 (23%) | 89 (25%) | 202 (21%) | 56 (21%) |
| 4th quartile (most) | 475 (22%) | 117 (21%) | 84 (24%) | 225 (23%) | 49 (19%) |
Boldface indicates statistical significance (p < 0.05) between each risk factor and social risk group.
Age measured 2 years prior to study enrollment.
Area Deprivation Index (ADI) is missing on 5% of sample due to invalid address.
The likelihood of receiving a colonoscopy/sigmoidoscopy was 30% lower for participants in the highest social risk group (most disadvantaged) compared to the lowest group (aRR=0.70; 95% CI 0.54 to 0.92) after adjusting for the other covariates (Table 3). In contrast to colonoscopy/sigmoidoscopy, participants in the highest social risk group were more likely to receive an FOBT compared to those in the lowest social risk group (aRR=1.52, 95% CI 1.09 to 2.12). For mammograms and Pap smears, the pattern for social risk group was similar to colonoscopy/sigmoidoscopy. When the models excluded regular medical providers, participants in social risk group 4 were less likely to receive a Pap smear (aRR=0.89, 95% CI 0.80 to 0.99).
Table 3.
Adjusted Relative Risk (95% CI) Of Receiving Cancer Screening Test During 4-Year Study Period.
| Risk Factora,b | Colonoscopy and/or Sigmoidoscopy (N=2131) |
FOBT (N=2131) | Mammography (N=1156) |
Cervical Cytology (N=5068) |
|---|---|---|---|---|
| Model Including Regular Medical Provider c | ||||
| Social Risk Group (Group 1 least risks) | ||||
| Group 2 | 0.82 (0.67,1.01) | 1.04 (0.75,1.44) | 0.93 (0.83,1.05) | 1.02 (0.96,1.07) |
| Group 3 | 0.92 (0.78,1.07) | 1.24 (0.96,1.61) | 0.98 (0.89,1.07) | 1.00 (0.94,1.07) |
| Group 4 (most risks) | 0.70 (0.54,0.92) | 1.52 (1.09,2.12) | 0.94 (0.80,1.11) | 0.90 (0.81,1.00) |
| Model Excluding Regular Medical Provider d | ||||
| Social Risk Group (Group 1 least risks) | ||||
| Group 2 | 0.81 (0.65,0.99) | 1.03 (0.74,1.43) | 0.92 (0.81,1.03) | 1.01 (0.95,1.07) |
| Group 3 | 0.90 (0.77,1.06) | 1.24 (0.96,1.6) | 0.97 (0.88,1.06) | 0.99 (0.93,1.06) |
| Group 4 (most risks) | 0.65 (0.50,0.86) | 1.46 (1.05,2.04) | 0.91 (0.77,1.07) | 0.89 (0.80,0.99) |
Reference group is in parenthesis.
Boldface indicates statistical significance (p < 0.05) between social risk group and receipt of cancer screening test.
Log-binomial model adjusted for age, sex, morbidity burden, having a regular medical provider, the length of Medicaid coverage, Area Deprivation Index quartile and social risk group.
Log-binomial model adjusted for age, sex, morbidity burden, the length of Medicaid coverage, Area Deprivation Index quartile and social risk group.
Neighborhood socio-economic disadvantage as measured by the ADI was not associated with the receipt of cancer screening except for cervical cancer (data not shown). Female participants who lived in the second and third ADI quartiles were less likely to receive a Pap smear compared to women living in the least disadvantaged neighborhood quartile (aRR=0.90, 95% CI 0.84 to 0.97 and aRR=0.94, 95% CI 0.88 to 0.99 respectively).
DISCUSSION
This study evaluated the influence of a comprehensive set of adverse SDH measured at the individual level on the receipt of colorectal, breast, and cancer screening tests in an adult sample of Medicaid enrollees. Participants with worse SDH were less likely to receive the recommended cancer screening tests compared to those with the fewest social risks.
The proportion of participants who completed cancer screening tests in the sample was low but higher than prior research using Medicaid claims for mammograms11,13,31 and Pap smears.11 The percentage of participants who completed colonoscopy or FOBT was higher than in several studies11,14,15 but lower than in at least one study.13 Findings demonstrate that access to comprehensive health insurance coverage is not enough to eliminate disparities in the receipt of cancer screening tests among individuals of low-income. Social risk group significantly influenced both types of colorectal cancer tests and was borderline significant for breast and cervical cancer screening.
Results suggest that a wide array of adverse SDH typically measured (i.e., poverty, lower education, and race/ethnicity) negatively affect the receipt of cancer screening tests. For example, a few studies have found that food insecurity,32 low health literacy,33,34 limited English proficiency,33 and homelessness35 are associated with a lower proportion of individuals completing a cancer screening test within the recommended period. While this study did not evaluate the impact of each SDH factor separately, the four social risk groups represent individuals who vary meaningfully in terms of structural determinants of health inequities (e.g., educational level, employment status) and intermediary determinants (e.g., smoking, food insecurity, housing instability, financial strain).
This study did not find that SDH measured at the neighborhood-level were associated with completion of cancer screening. Prior research reported inconsistent results of area-level SDH on cancer screening tests after controlling for individual-level SDH measures.36 One reason for the inconsistent results may be that most past studies measure SDH at one level but not both. For example, Kurani et al. reported that the odds of completing cancer screening tests were significantly lower among individuals living in the most deprived census block quintile compared with the least deprived.16 However, they did not control for individual-level SDH factors except for age, sex, and race/ethnicity.16 In contrast, Akinlotan and colleagues found that once they controlled for individual-level household income, education, and race-ethnicity, area-level measures of poverty and race were not significantly associated with cervical cancer screening.37 This study results imply that SDH factors measured at the individual level are the more important determinants of completion of cancer screening tests.
This is the first study to report that the influence of SDH on colorectal cancer screening completion varied by type of test. Participants with the most social disadvantages were more likely to do a FOBT whereas participants with the least adversities were more likely to complete a colonoscopy. Unlike colonoscopy, FOBT is less invasive and more convenient as it does not require people to complete laxative preparations, take the day off work, undergo sedation, and have another adult drive them home. At least one managed care company contracting with the DC Medicaid program has implemented a targeted outreach program accompanied by mail-in FOBT kits,19 an approach found to be cost-effective.38 However, it should be emphasized that very few participants completed the FOBT test annually. If the criteria had been annual FOBT, the relationship between FOBT and social risk group could not have been examined.
Limitations
This study has limitations. First, the four-year study period is short, particularly for colorectal cancer screening. Second, not all participants were covered by Medicaid for the entire period, which may have resulted in underestimating adherence. This study may also have overestimated cancer screening test completion if some procedures were conducted for diagnostic rather than screening purposes. Third, claims do not include information on the extent to which low cancer screenings resulted from the absence of specialist referrals. Fourth, the study may have failed to exclude (a likely small number of) participants who had a disqualifying medical procedure before the 2-year pre-study enrollment period. Fifth, the SDH information was not collected annually, which may have resulted in some misclassification since circumstances can change over the 4-year period. Furthermore, this study evaluated the influence of an aggregated set of SDH rather than specific determinants. Sixth, the study sample was identified at the time of a healthcare encounter, consisting primarily of Black enrollees who were very sick, so generalizability is limited. Finally, data are pre-COVID, precluding an assessment of COVID on cancer screening test completion.
CONCLUSIONS
Among a sample of adults covered by the same public insurance program, social and economic disadvantages were associated with receipt of cancer screening tests, signifying additional barriers to accessing recommended preventive care. To reduce cancer-related disparities, state Medicaid agencies need to systematically assess SDH among their members and use the results to seek solutions in collaboration with their enrollees, the healthcare system, and other service sectors. Medicaid must provide more infrastructure, resources, and support for more targeted action informed by these assessments to achieve health equity.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge Lydia Abma, MPH, who provided research assistance to this project while she was enrolled in the MPH in Health Policy program in the Department of Health Policy and Management at The George Washington University Milken Institute School of Public Health.
The lead author, Anne Markus, conceived the study, co-designed it with the senior author, Melissa McCarthy, and contributed significantly to the interpretation of the data analysis. Yixuan Li conducted the statistical analyses needed for the paper. Marceé Wilder and Jillian Catalanotti contributed their clinical expertise to the design of the study and the statistical analyses. Anne Markus, Melissa McCarthy, and Yixuan Li co-led the writing of the article and all authors revised it significantly from an intellectual standpoint. Finally, all authors read and approved the final version of the submitted manuscript.
Study Funding
This analysis was made possible by the National Institute on Minority Health and Health Disparities (NIMHD), grant number R01MD011607.
Footnotes
Conflict of Interest Statement
The authors do not have any conflicts of interest to report.
Individual Financial Disclosures
No financial disclosures have been reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne R. Markus, Department of Health Policy and Management, Milken Institute School of Public Health, The George Washington University, Washington, DC.
Yixuan Li, Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, The George Washington University, Washington, DC.
Marceé E. Wilder, Department of Emergency Medicine, School of Medicine and Health Sciences, The George Washington University, Washington, DC.
Jillian Catalanotti, Department of Emergency Medicine, School of Medicine and Health Sciences, The George Washington University, Washington, DC.
Melissa L. McCarthy, Department of Health Policy and Management, Milken Institute School of Public Health, The George Washington University, Washington, DC.
REFERENCES
- 1.National Center for Health Statistics. Deaths and mortality. Hyattsville, MD: Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated January 13, 2022. Accessed July 20, 2022. [Google Scholar]
- 2.Centers for Disease Control and Prevention. An update on cancer deaths in the United States. Atlanta, GA: Division of Cancer Prevention and Control. https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm#:~:text=What%20were%20the%20leading%20causes,intrahepatic%20bile%20duct%20(5%25). Updated February 28, 2022. Accessed August 22, 2022. [Google Scholar]
- 3.DC Health. Cancer in the District (2018). Washington, DC. https://dchealth.dc.gov/sites/default/files/dc/sites/doh/service_content/attachments/2018%20All%20Cancers%20%5BFinal%5D%20%281%29.pdf. Accessed July 20, 2022. [Google Scholar]
- 4.National Cancer Institute. Common cancer types. Bethesda, MD. https://www.cancer.gov/types/common-cancers#:~:text=The%20most%20common%20type%20of,are%20combined%20for%20the%20list. Accessed August 22, 2022. [Google Scholar]
- 5.Islami F, Ward E, Sung H et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. JNCI. 2021; 113(12): 1648–1669. 10.1093/jnci/djab131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD: National Institutes of Health, National Cancer Institute. https://seer.cancer.gov/statfacts/html/breast.html. Accessed August 22, 2022. [Google Scholar]
- 7.Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. Bethesda, MD: National Institutes of Health, National Cancer Institute. https://seer.cancer.gov/statfacts/html/cervix.html. Accessed August 22, 2022. [Google Scholar]
- 8.Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD: National Institutes of Health, National Cancer Institute. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed August 22, 2022. [Google Scholar]
- 9.National Cancer Institute. Cancer disparities. Bethesda, MD. https://www.cancer.gov/about-cancer/understanding/disparities Accessed December 10, 2022. [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cancer disparities. Bethesda, MD: National Institutes of Health, National Cancer Institute. https://seer.cancer.gov/statfacts/html/disparities.html. Accessed August 22, 2022. [Google Scholar]
- 11.Murphy KA, Daumit GL, McGinty EE et al. Predictors of cancer screening among Black and White Maryland Medicaid enrollees with serious mental illness. Psychooncology. 2021; 30(12): 2092–2098. doi: 10.1002/pon.5815. Epub 2021 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goudie A, Martin B, Li C et al. Higher rates of preventive health care with commercial insurance compared with Medicaid findings from the Arkansas Health Care Independence “Private Option” Program. Med Care. 2020; 58(2): 120–127. doi: 10.1097/MLR.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 13.Bonafede MM, Miller JD, Pohlman SK et al. Breast, cervical, and colorectal cancer screening: patterns among women with Medicaid and commercial insurance. Am J Prev Med. 2019; 57(3): 394–402. doi: 10.1016/j.amepre.2019.04.010. Epub 2019 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atherly A, Mortensen K. Medicaid primary care physician fees and the use of preventive services among Medicaid enrollees. Health Serv Res. 2014; 49(4): 1306–1328. 10.1111/1475-6773.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis M, Renfro S, Pham R et al. Geographic and population-level disparities in colorectal cancer testing: a multilevel analysis of Medicaid and commercial claims data. Prev Med 2017: 101:44–52. doi: 10.1016/j.ypmed.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurani S, McCoy R, Lampman M et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open. 2020: 3(3): e200618. doi: 10.1001/jamanetworkopen.2020.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homan S, Yun S, Bouras A et al. Breast cancer population screening program results in early detection and reduced treatment and health care costs for Medicaid. J Public Health Manag Pract. 2021; 27(1): 70–79. doi: 10.1097/PHH.0000000000001041 [DOI] [PubMed] [Google Scholar]
- 18.Section 2713 of the Affordable Care Act. https://www.law.cornell.edu/uscode/text/42/300gg-13. Accessed August 9, 2022.
- 19.Markus A, Gerstein M, Gunsalus R. An Analysis of Disparities in Reported Cancer Screening Behaviors in the DC Region by Insurance Status, Age, and Income – Final Report. Washington, DC: The George Washington University Cancer Center; 2019. Available upon request from the lead author. [Google Scholar]
- 20.Pan H, Walker G, Grant S et al. Insurance status and racial disparities in cancer-specific mortality in the United States: a population-based analysis. Cancer Epidemiol Biomarkers Prev. 2017; 26(6): 869–875. doi: 10.1158/1055-9965.EPI-16-0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcaraz K, Wiedt T, Daniels E et al. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020; 70(1): 31–46. doi: 10.3322/caac.21856 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Social determinants of health. Geneva, Switzerland. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1. Accessed September 21, 2022. [Google Scholar]
- 23.McCarthy ML, Zheng Z, Wilder ME et al. The influence of social determinants of health on emergency departments visits in a Medicaid sample. Ann Emerg Med. 2021; 77(5): 511–522. doi: 10.1016/j.annemergmed.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Preventive Services Task Force. Final recommendation statement – colorectal cancer: screening. Rockville, MD. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening. Updated May 18, 2021. Accessed August 9, 2022. [Google Scholar]
- 25.U.S. Preventive Services Task Force. final recommendation statement – breast cancer: screening. Rockville, MD. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening. Updated January 11, 2016. Accessed August 9, 2022. [Google Scholar]
- 26.U.S. Preventive Services Task Force. Final recommendation statement – cervical cancer: screening. Rockville, MD. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Updated March 10, 2022. Accessed August 9, 2022. [Google Scholar]
- 27.National Committee for Quality Assurance. HEDIS measures and technical resources. Washington, DC. https://www.ncqa.org/hedis/measures/. Accessed August 9, 2022. [Google Scholar]
- 28.Solar O, Irwin A. A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice). Geneva, Switzerland: World Health Organization; 2010. https://apps.who.int/iris/bitstream/handle/10665/44489/?sequence=1. Published April 5, 2011. Accessed August 12, 2022. [Google Scholar]
- 29.Singh G. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003; 93(7): 1137–1143. doi: 10.2105/ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johns Hopkins Medicine. ACG® System Version 12.0 System Documentation (all guides). Baltimore, MD: Johns Hopkins University. https://www.hopkinsacg.org/document/acg-system-version-12-0-system-documentation-all-guides/. Updated March 13, 2019. Accessed August 9, 2022. [Google Scholar]
- 31.Mobley L, Subramanian S, Tangka F et al. Breast cancer screening among women with Medicaid, 2006-2008: a multilevel analysis. J Racial Ethn Health Disparities. 2017; 4(3): 446–454. doi: 10.1007/s40615-016-0245-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza J, Miller C, Martin K et al. Examining the association of food insecurity and being up-to-date for breast and colorectal cancer screenings. Cancer Epidemiol Biomarkers Prev. 2022:31:1017–25. doi: 10.1158/1055-9965.EPI-21-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sentell T, Braun K, Davis J, Davis T. Colorectal cancer screening: low health literacy and limited English proficiency among Asians and whites in California. J Health Commun. 2013; 18: 242–255. doi: 10.1080/10810730.2013.825669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sentell T, Tsoh J, Davis T et al. Low health literacy and cancer screening among Chinese Americans in California: A cross-sectional analysis. BMJ Open. 2015:5:e006104. doi: 10.1136/bmjopen-2014-006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asgary R, Garland V, Jakubowski A, Sckell B. Colorectal cancer screening among the homeless population of New York City shelter-based clinics. Am J Public Health. 2014; 104(7): 1307–1313. doi: 10.2105/AJPH.2013.301792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruitt S, Shim M, Mullen P et al. Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009; 18(10): 2579–2599. doi: 10.1158/1055-9965.EPI-09-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinlotan M, Weston C, Bolin J. Individual-and county-level predictors of cervical cancer screening: a multi-level analysis. Public Health. 2018; 160: 116–124. doi: 10.1016/j.puhe.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 38.Mohan G, Chattopadyay S. Cost-effectiveness of leveraging social determinants of health to improve breast, cervical, and colorectal cancer screening a systematic review. JAMA Oncol. 2020; 6(9): 1434–1444. doi: 10.1001/jamaoncol.2020.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.