Abstract
Dendritic cells (DCs), as potent antigen presenting cells, are known to play a central role in the pathophysiology of asthma. Our understanding of DC biology has evolved over the years to include multiple subsets of DCs with distinct functions in the initiation and maintenance of asthma. Furthermore, asthma is increasingly recognized as a heterogenous disease with potentially diverse underlying mechanisms. The goal of this review is to summarize the role of DCs and the various subsets therein in the pathophysiology of asthma and highlight some of the crucial animal models shaping the field today. Potential future avenues of investigation to address existing gaps in knowledge are discussed.
Keywords: dendritic cells, DC subsets, asthma, atopic disease, allergic disease
GRAPHICAL ABSTRACT

Dendritic cells (DCs) play a central role in the pathophysiology of asthma. There are multiple subsets of DCs with distinct functions. Some subsets are involved in the induction and maintenance of eosinophilic asthma while others promote tolerance. There is also emerging evidence for a role of certain DC subsets in neutrophilic asthma.
1. Introduction
Asthma is a multifaceted disease that has been increasing in incidence and prevalence, particularly in the pediatric population, over the past 50 years.[1] Currently, asthma affects more than 300 million people worldwide and the impact to society has far reaching effects beyond the significant morbidity and mortality from the disease itself.[2] The financial burden of asthma in the United States alone is estimated at >$80 billion.[3]
Asthma is a chronic disease with airway hyperresponsiveness leading to reversible narrowing of the bronchial airways; if left unchecked, this can result in permanent airway remodeling. While the underlying pathology is heterogeneous and not fully understood, the most common and best studied cause is atopic or allergic asthma (~80% of all pediatric asthma and ~60% of asthma overall).[4–6] In atopic asthma, antigen exposure leads to eosinophilic lung inflammation, increased mucus production secondary to goblet cell hyperplasia, airway edema, changes in airway smooth muscle (hyperplasia, hypertrophy, and acute constriction) which serve to narrow the airways and cause the classic symptoms of shortness of breath, chest tightness, wheezing, and cough.
The understanding of asthma as a heterogenous disease process involving complex interactions among multiple cell types continues to evolve. Mechanistic mouse studies have elucidated many of the underlying cytokines, chemokines, and cell types responsible for individual symptoms /phenotypes of asthma (reviewed[7–9]). Our understanding of the influence of epigenetic changes, microbiome, and dietary modifications continues to expand.[10–16] In the midst of this complexity, dendritic cells (DCs) have emerged as critical players in the communication between the innate and adaptive immune system in initiation and maintenance of asthma.
The goal of this review is to summarize the role of DCs and the various subsets therein in the pathophysiology of asthma. Findings from mouse models of experimental asthma as well as human studies are individually presented and compared to provide an overall context of our understanding of DC subsets. Potential future avenues of investigation to address existing gaps in knowledge will be discussed.
2. Mouse models
Our understanding of the pathomechanisms of many human diseases is informed extensively by animal models. Asthma is no exception; however, it is important to understand the evolution and limitations of these models. Asthma is a heterogenous disease with potentially multiple unique underlying pathomechanisms which have recently been categorized into endotypes (reviewed[17]). Atopic asthma, which is predominantly Th2-mediated and characterized by lung eosinophilia and steroid-sensitivity, represents the bulk of the disease burden especially in the pediatric population. However, there is a group of non-atopic asthma endotypes characterized by neutrophilia, severe/refractory disease course, and absence of atopic disease which accounts for 5–10% of asthma.[17] Clinically, these endotypes are associated with late onset, steroid-resistant asthma which tends to have a more ominous disease course, be mediated by Th17 inflammatory responses, and is more likely to be associated with obesity, smoking, and/or advanced age which define the individual endotypes within this group[17] (Figure 1). There are other rare endotypes of asthma that fall on this continuum between Th2 and Th17 responses including aspirin-exacerbated respiratory disease (AERD) which has clinical features of a Th2 response including eosinophilia but is not steroid responsive and has a discrete underlying mechanism distinct from atopic asthma[17] (Figure 1). In order to learn more about these disease processes and design appropriate interventions, models for the various endotypes are necessary. Most current mouse models focus on Th2-mediated atopic asthma which is the focus of this review; however, the field is shifting to develop and include models that are representative of additional endotypes which has the potential to take the field in exciting new directions.
Figure 1.

Human asthma endotypes compared to existing mouse models of experimental asthma on a spectrum of Th2 vs Th17, steroid responsiveness, eosinophils vs neutrophil infiltration, and roles of DC subsets.
AERD- aspirin-exacerbated respiratory disease; Tg- transgenic mouse; OVA- Ovalbumin; HDM- house dust mite; DEP- diesel exhaust particles; NO2- nitric dioxide; LPS- lipopolysaccharide; CFA- complete Freund’s adjuvant ; RORγt- retinoid orphan receptor gamma t
There are two primary mouse strains used to study asthma: BALB/c and C57BL/6 mice. BALB/c have been reported to exhibit an innate Th2-biased immune response whereas C57BL/6 are reported to be Th1 predominate responders.[18–20] Despite this, C57BL/6 still develop experimental atopic asthma,[18, 21–23] but exhibit differences in disease phenotype. For example, BALB/c mice have a greater increase in airway reactivity to methacholine and increased numbers of mast cells in lung tissue than C57BL/6 mice.[18] Conversely, eosinophil and neutrophil counts in BAL fluid as well as peribronchial eosinophilia were greater in C57BL/6 mice.[18, 23] Cytokine levels also vary between the models and tend to be higher from whole lung extracts in BALB/c mice, whereas select cytokines (CCL11 and CCL5) levels were higher in BAL fluid of C57BL/6 mice.[18]
Regardless of which strain is used, inbred mice in specific-pathogen-free facilities do not develop asthma spontaneously and so models designed to mimic the pathophysiology have been introduced. Atopic asthma can be achieved by genetic modification, adoptive transfer of activated T cell or dendritic cells, or induction by exposure to allergen (reviewed[24–26]; Figure 1). The allergen-based models usually include 2 phases: sensitization (intraperitoneal injection of antigen+/− adjuvant, subdermal injection, or intranasal administration depending on allergen) and challenge (often inhaled but could also be intranasal or intratracheal). Advantages and disadvantages of each protocol including physiologic relevance of allergens to human disease are reviewed by Aun et al.[26] Protocols for inducing asthma in mouse models last up to 20 weeks and the resultant airway inflammation and airway hyperresponsiveness (AHR) resolve within weeks after the last allergen challenge. Animal models have also revealed a reproducible phenotype of allergen hyperresponsiveness in offspring of allergic mothers which has led to important investigations of contributions of the in utero environment to the development of the fetal/neonatal immune system.[21, 22, 27–30]
There are several allergens used in mouse models of allergic asthma, including house dust mite extract (HDM) and purified chicken egg ovalbumin (OVA). It is important to examine mechanisms of allergy with more than one type of allergen, including purified allergen proteins and complex allergen extracts. As a crude extract of an allergen implicated in human asthma, HDM contains multiple antigens and other immune stimulants (i.e. toll-like receptor agonists) and has intrinsic sensitization properties. OVA, while not a common airway allergen in human disease, is well characterized as an experimental asthma model and allows for the use of specialized tools for quantifying antigen-specific T cell activation and response such as OTII mice with OVA-specific T cell receptor expression.[31–33] Additionally, fungi (Aspergillus fumigatus and Alternatia alternata), cockroach extracts, Ascaris antigens, cotton dust ragweed and latex have also been used in murine models of asthma.[31] In order to stimulate robust allergic response, allergens (particularly those such as OVA which lack intrinsic sensitization properties) are sometimes used in conjunction with an adjuvant. Aluminum hydroxide (alum) is one of the most common adjuvants, but nitrogen dioxide,[34] diesel exhaust particles (DEP),[35–37] lipopolysaccharide (LPS),[38, 39] and complete Freund’s adjuvant (CFA)[40, 41] have also been used. Importantly, the choice of adjuvant affects the resultant immune response. For example, alum induces a potent Th2-mediated inflammatory response, LPS and CFA induce a Th17 neutrophilic response, and low dose LPS, nitrogen dioxide, and diesel exhaust particles induce a mixed response.[36, 37, 42] These various models can produce a spectrum of immune responses (similar to human asthma endotypes) which fall along a continuum of Th2- to Th17-mediated inflammation (Figure 1).
3. Pathophysiology of Dendritic cells in Asthma
DC anatomic location combined with their antigen presenting expertise and ability to influence Th-type responses poise DCs as an essential component of atopic asthma. DCs have been shown to be necessary[43–48] and sufficient[49, 50] for both induction and maintenance of Th2-mediated asthma as well as for establishing allergic predisposition in offspring of allergic mothers.[21, 22, 27, 29, 51]
This has been extensively shown by deleting CD11c+ cells prior to sensitization or after sensitization but prior to challenge, both of which eliminate the clinical features of asthma.[49, 50] In another approach, administering CD11c+ allergen-pulsed DCs induces asthma in naïve mice.[49] Furthermore, transplantation of CD11c+ DCs isolated from pups of allergic mothers prior to antigen exposure into pups born from non-allergic mothers is sufficient to induce allergic predisposition.[27] The mechanism underlying these findings has been of considerable interest over the past 25 years.
DCs present on the basolateral side of epithelial cells in lungs sample antigen from the lumen of airways by extending dendrites through the epithelial barrier. This is regulated by interactions between epithelial cells and DCs (reviewed[8, 9]). Interestingly, both human and murine DCs express tight junction proteins which are hypothesized to functionally maintain the epithelial barrier during sampling. Once the antigen is acquired, the DC migrates to the draining lymph node. The DCs in the conducting airways have a rapid half-life of 2-days because of this migration.[52] In the lymph nodes, DCs carry out their professional antigen presenting cell role and present processed antigen to naïve T cells. In addition to their role in lymph nodes, DCs interact locally (in the lung mucosa) with primed T cells.[53–55] This then sets the adaptive immune system in motion to a Th2 pathway leading to production of IL-4, 5, and 13 as well as eosinophilic lung inflammation. Allergen exposure also stimulates generation of stem cell growth factors that induce bone marrow production of DCs and eosinophils which then circulate to the lungs and perpetuate the inflammatory process.[56, 57]
Allergic responses during pregnancy alter the in utero environment for offspring development of DCs. This altered in utero environment leads to changes in fetal DCs which predispose offspring to allergen hyperresponsiveness postnatally.[21, 22, 27–29, 51, 58] In particular, the immune-modulatory lipid beta glucosylceramide (βGlcCer) is elevated in allergic moms and crosses the placenta to alter fetal dendritic cell differentiation[29, 30] and has recently been shown to be both necessary and sufficient to establish allergic predisposition in offspring.[29]
In humans, levels of βGlcCer, in conjunction with other immune modulatory lipids present in cord blood, have been shown to correlate with incidence of childhood wheeze.[59, 60] These findings combined with the observation that a child born to an allergic mother is more likely to suffer from allergic disease than one born to an allergic father[61] implicate a role of the in utero environment in establishing allergic predisposition in offspring.
Given the relative inaccessibility of DC collection and study in humans, much less is definitively known about the specific mechanisms of DC involvement in humans. However, in humans, allergen exposure leads to increased DCs and eosinophils in the lungs and increases in Th2 cytokines and chemokines in conjunction with the clinical manifestations of airway hyperreactivity, goblet cell hypertrophy, and eosinophilic inflammation.[62] Furthermore, when human monocyte-derived DCs are exposed to allergen (HDM[63] or pollen[64]) in vitro, they acquire Th2 cell polarizing capacity similar to what is seen in mouse models. However, not all DCs are the same, and, in recent years, we have learned more about the specific contributions of individual DC subsets.
4. Specific roles of DC subsets
Dendritic cells differentiate from bone marrow-derived common dendritic cell progenitors (CDPs) and subsequently differentiate into plasmacytoid DCs (pDCs) and conventional DCs (cDCs) (Figure 2). cDCs are further subdivided into cDC1s (homologous subset in humans is also referred to as myeloid DC (mDC) type 1) and cDC2s (homologous subset in humans is also referred to as mDC type 2) (Figure 2). Additionally, there are monocyte-derived dendritic cells (moDCs) which have a prominent function in the pathophysiology of multiple diseases including atopic asthma. However, there are challenges identifying and defining moDCs more so than other DC subsets due to their plasticity and more complex lineage.[65–69] Each DC subset is defined based on their profiles of cytokine production, antigen processing and presentation efficiency, and capacity to induce different T cell responses—all of which help define the different physiologic roles DC subsets play in asthma. These subsets are differentiated via flow cytometry using a combination of markers (Table 1). The biggest caveat for all subsets is that the markers vary by location (including blood, lymphoid tissue, lung, or other primary organ tissue) and context (inflammation/contextual signals both locally and systemically as well as maturation and activation state) of the DCs.[8, 70–74] Furthermore, there is no standardized gating scheme to study DC subsets but there is ongoing work to develop techniques incorporating unsupervised computational analysis of flow cytometry data to improve reproducibility and allow for comparisons across various tissues and species.[70, 75] Characteristic markers for which there seems to be a consensus in the literature are bolded in Table 1. Localization within the lung is listed for murine subsets (Table 1) but there is little known about in situ localization in humans given the inaccessibility of tissue samples.
Figure 2.
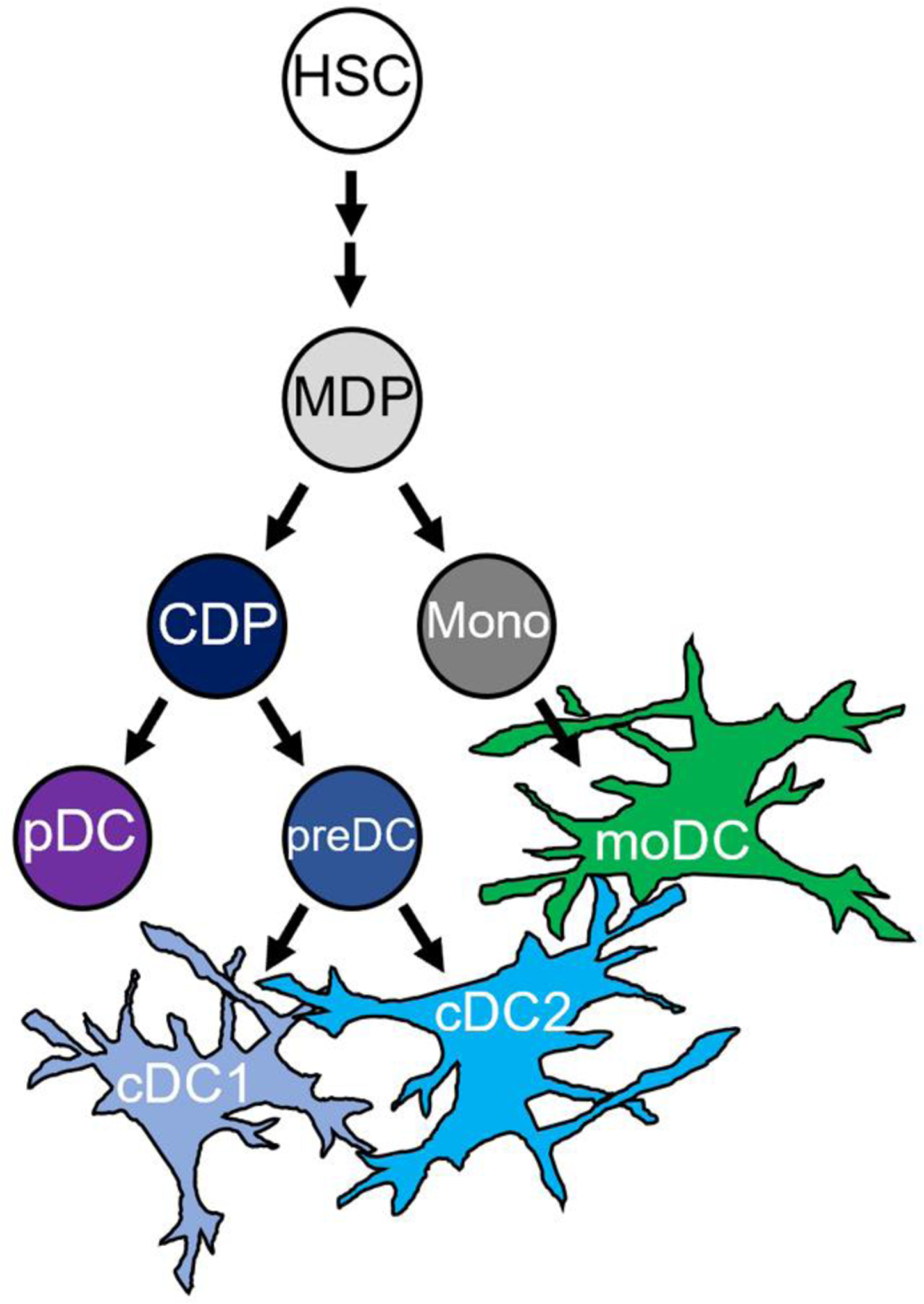
Ontological relationship of DC subsets during differentiation
HSC- hematopoietic stem cell; MDP- monocyte-macrophage DC progenitor r; CDP- common DC progenitor; Mono- monocyte; pDC- plasmacytoid DC; cDC- conventional DC; moDC- monocyte-derived DC
Adapted from Vroman, 2017[107]; Collin, 2018[106]; See, 2017[67])
Table 1.
DC subsets and their defining characteristics
| DC Subset | Locationa) | Markersb) | Role in immune system |
|---|---|---|---|
| cDC1 (also mDC1 in humans) |
Associated with epithelium of large conducting airways above basement membrane 0.1% of mononuclear cells in blood |
Mouse: CD11b-, CD11c+, CD103+, Ly6c-, MHCII+, CLEC9A, XCR1e, IRF8+, CD24-, CD26+ Human: CD11b low/-, CD11c+/intermediate, MHCII+, BDCA3(CD141)+, CLEC9A, XCR1, IRF8+ |
Anti-viral immunity and pulmonary tolerance |
| cDC2 (also mDC2 in humans) |
Submucosa/lamina propria of conducting airways beneath basement membrane and lung parenchyma 1% of mononuclear cells in blood |
Mouse: CD11b+, CD11c+/high, Ly6c-, CD64-, MHCII+, CD103-, IRF4+, CD24+, CD26+, CD172a(SIRPα)+
Human: CD11c+/high, MHCII+, BDCA1(CD1c)+, CD172a+ (SIRPα), ESAM+, IRF4+ |
Highly implicated in allergic disease are major APCs in the uptake and processing of the antigens to prime and stimulate CD4+ T-lymphocytes |
| pDC | Large conducting airways beneath basement membrane <0.1% mononuclear cells in blood |
Mouse: CD317(PDCA1)+,CD11b-, CD11c-/low, MHCII+, siglec-H, Gr-1(Ly6G/C) intermediate, B220+ subgroups with CD8α-β-, α+β-, or α+β+ Human: CD123+, MHCII+, CD11c-/low, ILT7(CD85g)+, CD303(BCDA2)+, CD304(BDCA4)+, CD33- |
Anti-viral immunity and pulmonary tolerance; produce large amounts of IFN-α, may play a role in Th-17 mediated asthma |
| moDC (also mDC or inflammatory DC in mouse) |
No consensus location as is a highly variable subset induced by inflammation | Mouse: CD11b+, CD11c+, Ly6c+, MHCII+, CD64+, FcεR1 (MAR1)+, CX3CR1, CD209 (DC-SIGN), CD206, CD26- Human: CD14+, CD1c+, CD1a+,CD11c+, CD206, Sirpα+, FcεR1+,CD16- |
Thought to play a similar role to cDC2s in antigen presentation. Capable of inducing Th1, Th2, or Th17 responses. Role convoluted by plasticity and use of reliable markers |
Gaurav, 2013[72]; Lambrecht, 2009[8]; Bosteels, 2020[71]; Abdala-Valencia, 2014[21]; Merad, 2013[73]; Collin, 2013[74]; Guilliams, 2016[70]
Abbreviations: CD (cluster of differentiation molecule); Ly6c (lymphocyte antigen 6 complex, locus C1); MHCII (major histocompatibility complex); CLEC (C-type lectin-like receptor); XCR1 (X-C Motif Chemokine Receptor 1); IRF (interferon regulatory factor); BDCA (blood dendritic cell antigen); PDCA1 (bone marrow stromal antigen 2); SIRPa (signal regulatory protein alpha); ESAM (endothelial cell-specific adhesion molecule); siglec (sialic acid-binding immunoglobulin-like lectins); Gr-1 (Ly6G/C); B220 (CD45 receptor); ILT7 (immunoglobulin-like transcript 7); FcεR1 (high-affinity receptor for the Fc region of immunoglobulin E); CX3CR1 (C-X3-C Motif Chemokine Receptor 1)
Much work has been done to show that CD11c+ DCs (including all subsets other than pDCs) are necessary and sufficient for initiation and maintenance of atopic asthma as well as transmission of allergic predisposition in neonatal offspring of allergic mothers.[49, 50] This section will focus on the studies dedicated to understanding the roles of subsets of DCs as defined by the markers outlined in Table 1.
4.1. cDC1s
4.1.1. Mouse cDC1s
cDC1s are identified as the CD11b− CD103+ DCs in mice. More recently IRF8 has been used a marker for this subset as well; however, it is not specific for cDC1s as it is also expressed in pDCs, inflammatory cDC2s, and moDCs.[71, 76] cDC1s are generally thought to be less important in atopic asthma as their primary function lies in cross-presentation of intracellular antigens to CD8+ T cells and skewing CD4+ T cells toward Th1 and Th17 responses.[77] Furthermore, they do not increase in number in allergic mouse models[21, 22, 78] and have diminished capacity compared to other DC subsets to take up antigen.[30, 79] The bulk of the literature indicates that cDC1s play a role in pulmonary tolerance. Also, animal models demonstrate worse allergic airway inflammation in response to HDM or OVA in the absence of CD103+ DCs.[80, 81] Additionally, absence of cDC1s in the CD103 null mouse (on C57BL/6 background) results in loss of allergen tolerance in a tolerized mouse model.[82] While this seems to be the prevailing understanding in the field, there is some controversy as cDC1s have been shown to stimulate a Th2 response to HDM in vitro,[83, 84] and, more recently, a role for cDC1s in mediating eosinophil recruitment in a model of chronic asthma has been described.[85] Furthermore, some studies have shown decreased allergic airway inflammation in mouse models with decreased CD103+ DCs.[83, 84] These data are complicated by the use of multiple different mouse models and treatment conditions; however, this complexity is consistent with the findings in human studies.
4.1.2. Human cDC1s
In humans with asthma, cDC1s do not increase in bronchoalveolar lavage (BAL) fluid or bronchial tissue (from biopsy) after exposure to inhaled allergen.[86] There are mixed results when looking in the peripheral blood. Some studies show an increase in cDC1s[87] while others report a decrease.[88] Whether changes in circulating cDC1s are reflective of an absolute change in the number of cDC1s present or if it is more indicative of recruitment to the lung (or other tissue) remains to be seen. Functionally, there are reports of cDC1s inducing Th2 cell responses; however, these studies were either conducted with DCs expressing CD141 derived from peripheral blood monocytes[89] or cDC1s exposed to live-attenuated viral particles which would be expected to stimulate cDC1s disproportionally given their role in antigen cross-presentation.[90]
CD141 itself has a rich and convoluted history as a human DC marker. It has both been reported as a marker of cDC1s,[65, 70, 91–100] and in other studies is used as the definitive marker for cDC2s.[87, 89, 101–104] Most mechanistic studies conclude that CD141+ human DCs are functionally homologous to murine CD103+ DCs (cDC1s). Gene expression profile analysis confirm this conclusion.[65, 70, 92–97] However, it is worth noting that although pDCs are very similar between mouse and human; there is significantly less homology between cDC subsets of these two species.[92] CD141 can also be expressed on moDCs which can lead to further confusion.[74, 105, 106] In recent years there has been a push to use IRF8 as the functional marker of cDC1s.[88, 107, 108] However, this approach has been cautioned against as well because emerging data in mice indicate inducible IRF8 expression in cDC2s under inflammatory conditions.[71] Consensus is still lacking and further studies are required to definitively delineate cDC1s, cDC2s, and moDCs under both steady state and inflammatory conditions.
4.2. cDC2s
4.2.1. Mouse cDC2s
cDC2s are defined as CD11b+ CD11c high DCs and are differentiated from moDCs by their lack of monocyte markers such as Ly6c and CD64.[8, 21, 109] More recently, IRF4 and CD172a (SIRPα) have also become important markers, although these markers are also expressed on moDCs.[47, 48, 71, 76, 110–112] cDC2s are often considered the primary DC subset to mediate atopic asthma given their antigen processing and presenting efficiency combined with their propensity to induce a Th2 response in multiple different models of experimental asthma.[21, 22, 48, 50, 77, 79, 112–117] Adoptive transfers of cDC2s induce experimental asthma.[112] cDC2s are increased in the lung and BAL fluid in models of asthma,[21, 22, 50, 54–57, 78] in the fetal liver of fetuses gestating in allergic mothers, and in the lungs of offspring of allergic mothers after antigen exposure postnatally.[21, 22, 27] Not only do cDC2s increase under allergic conditions, they have also been shown to migrate to lung-draining lymph nodes and induce allergen-specific Th2 cells. This results in allergic inflammation in the lung,[48, 50, 79] thus fulfilling the primary role in the classic mechanism of induction of atopic asthma. They also have been hypothesized to activate local resident memory T cells leading to a robust Th2 response after re-exposure to allergen.[7] Overall, the data are convincing that cDC2s play a predominate role in the pathogenesis of experimental asthma.
4.2.2. Human cDC2s
In humans with asthma, cDC2s are increased in peripheral blood, BAL fluid, and bronchial tissue both at baseline and after exposure to inhaled antigen.[86, 88, 108, 118, 119] Functionally, cDC2s isolated from patients with asthma, when primed with HDM, skew CD4+ T cells isolated from non-asthmatic, non-allergic donors to a Th2 phenotype.[120] Lung cDC2s are implicated in Th2 airway inflammation in humans as they express the high-affinity receptor FcεRI[121, 122] and express increased CD86 and OX-40L.[88] Interestingly, there is differential expression of markers on cDC2s of patients who experience few asthma exacerbations compared to those with multiple exacerbations per year indicating that there may be biomarkers of disease severity specific to cDC2s. Furthermore, the increase in number of cDC2s was more prominent in patients with increased exacerbations.[88] Taken together these data further emphasize that greater understanding of DC subsets and their context may inform clinical practice either through targeted treatments or by triaging/prognosticating disease severity.
4.3. pDCs
4.3.1. Mouse pDCs
pDCs are identified among DC subsets by their lack of expression of CD11b and low expression of CD11c as well as the presence of CD317+, siglec-H, and B220 (Table 1). They can be further subdivided into 3 populations based on expression of CD8α and β. Adoptive transfers indicate that CD8α-β- pDCs enhance allergic lung inflammation and the other two (α+β− and α+β+) contribute to tolerance by inducing FOXP3+ regulatory T cells.[123] pDCs are functionally distinct from cDCs in that they have a decreased capacity to uptake, process, and present antigens.[30, 124, 125] The primary cytokine pDCs produce is interferon-alpha (IFN-α) and they play a role in antiviral immunity (reviewed[126–129]) and allergen tolerance through the induction of regulatory T cells.[123, 129–132] In the context of allergy and asthma, pDCs serve to inhibit airway hyperreactivity and allergic lung inflammation through IFN-α-mediated suppression of group 2 innate lymphoid cell (ILC2) survival and function[133] and to restrain sensitization to otherwise harmless antigens.[134] Furthermore, increasing the number of pDCs through Fms-like tyrosine kinase receptor-3 ligand (Flt3L) administration or administering antigen pulsed pDCs prior to sensitization decreases allergic lung inflammation whereas depletion of pDCs prior to OVA sensitization worsens allergic lung inflammation.[135–140] Interestingly, pDC depletion via 120G8 (pDC specific antibody) after OVA sensitization reduces AHR and eosinophils in BAL.[140] Therefore, pDCs seem to be protective during allergy/asthma initiation and may play a role in maintenance/worsening of disease after sensitization, but this requires further study. Interestingly, there may be age-specific effects as study of neonatal mice which naturally lack pDCs or depletion of pDCs in young mice predisposed to development of severe asthma whereas older mice were protected.[129, 141] Furthermore, this predisposition in neonatal mice was rescued by adoptive transfer of pDCs or administration of IFN-α.[141] Others have shown in models of neutrophilic asthma (more severe and refractory to steroid treatments) that pDCs are the primary DC subset involved as they are markedly increased in BAL fluid of mice with neutrophilic asthma in contrast to the eosinophilic asthma.[142] Furthermore, neutrophils have been shown to activate pDCs in both humans and mouse models.[143, 144] Taken together, these data indicate that the role of pDCs in disease may depend on the underlying pathophysiology of the disease (neutrophilic > eosinophilic asthma and chronic>acute) as well as potential functional differences among subsets.
4.3.2. Human pDCs
In humans, pDCs are classically identified by expression of CD123 or CD303+ (blood DC antigen 2 (BCDA2))(Table 1). While pDCs more classically promote a Th1 response,[145] HDM exposure can elicit pDCs to promote Th2 responses.[63, 146, 147] Some propose that this result is due to contamination of preDC cells which were recently discovered to share classical markers of pDCs and can be differentiated by their expression of CD33 which pDCs lack.[67] Regardless, the physiologic significance of ability of pDCs to induce a Th2 response is unclear as the clinical data tends to support a protective role for pDCs in the initiation of Th2-mediated disease via induction of regulatory T cells (similar to that seen in mouse models).[60, 148–151] For example, increased numbers of circulating pDCs in children are protective from a diagnosis of asthma in the first 5 years of life whereas decreased pDCs is a risk factor for wheezing, diagnosis of asthma, and increased number and severity of respiratory tract infections.[152, 153] Furthermore, children with atopic asthma tend to have decreased pDCs in circulation.[154, 155] Conversely, in adults with asthma, circulating pDCs are increased compared to healthy controls[156, 157] as are pDCs in BAL fluid collected 24 hrs post allergen exposure.[152, 158] This is mediated by an increase in bone marrow production of pDC and cDC precursors which are then recruited to the lungs.[86] Even in the absence of allergen challenge, pDCs in the sputum of adults with atopic asthma are increased compared to controls and drastically increase from baseline (up to 4-fold increase) during an asthma exacerbation.[140] Clinically, pDC lung infiltration has been associated with refractory, non-atopic asthma[159] and may serve as a biomarker for severe disease. Given the growing focus on asthma endotypes (particularly separating atopic from non-atopic asthma), the mouse data showing increased role for pDCs in non-atopic asthma, and reports in humans of pDC numbers correlating with severity of inflammation and increased risk for asthma exacerbations in some populations, there is compelling reason for further investigation of pDCs in asthma. They may play a more active role particularly in the non-atopic endotype than previously recognized.
4.4. moDCs
4.4.1. Mouse moDCs
moDCs have a considerable heterogeneity and thus are the most difficult to definitively characterize as a distinct population independent of macrophages.[65] There is considerable heterogeneity between moDCs even within a single organ/tissue under the same conditions and the markers used to define this subset has been subject to frequent revisions/updates.[7, 8, 68–73, 160] There is, indeed, a population of monocyte-derived cells which are considered functionally similar to cDC2s: they express CD11b, CD11c, and MHCII (characteristic of cDC2s) while simultaneously expressing what are considered to be monocyte-specific markers (i.e Ly6c or CD64).[8, 71, 161–163] Functionally, moDCs have been shown to process and present antigen to activate T cells in the context of allergic disease.[30, 44, 79, 164] moDCs play important roles in Th1, Th17, and Th2-mediated inflammation.[112, 165–172] In the context of asthma, the number of moDCs present in the lung and BAL fluid of allergic animals is increased.[21, 22] They have been shown to be both necessary (by depletion with FcεRI antibody[173]) and sufficient (adoptive transfer of moDCs) for the development of allergic lung inflammation.[112, 171, 172, 174]
Recently, these results have been called into question by a report indicating that inflammatory cDC2s (which have now been shown to share many of the same markers as moDCs including FcεRI) may be present in previous studies of moDCs and that properties such as antigen presentation and migration may have been misattributed to moDCs.[7, 71] Indeed, when CD26+ cells (indicative of cDC lineage) are removed from moDC cultures, moDCs are no longer able to process and present antigens to either CD4+ or CD8+ T cells.[71] Further studies are needed to understand the differentiation, function and full impact that this finding will have on the field.
4.4.2. Human moDCs
Human moDCs are a versatile subset capable of stimulating CD4+T cells, cross-presenting antigen to CD8+ T cells and producing many cytokines including IL-1, 6, 12, 23, and TNF-α.[175] Similar to their murine counterparts, human moDCs share many markers and functional roles with cDC2s. These similarities make differentiating the two subsets challenging, but CD14 is often used to identify cells of monocyte lineage and thus differentiate moDCs from cDC2s (Table 1).[67, 176, 177] moDCs cultured from peripheral blood of allergic patients exhibit recruitment to epithelial cells exposed to HDM in in vitro migration assays[178] as well as increased capacity to induce HDM-specific T cell proliferation.[179] moDCs, similar to other DC subsets, also undergo complex interactions with the epithelium which differentially regulates moDC gene expression.[180, 181] When exposed to allergen, the DCs also secrete more IL-10 and less IL-12 shifting the immune response to the Th2 response classically seen in allergic disease.[63, 146, 147, 182] Although human moDCs have been studied in culture (derived from peripheral blood monocytes), few studies have looked at moDCs present in the lung and their biology. However, there appear to be links to disease severity because moDCs are increased in the outer wall of the large airways in patients who succumb to fatal asthma exacerbation.[183] Given the similarities in markers expressed, it is likely that moDCs are included in studies that separate cDCs and pDCs without additional markers to exclude moDCs;[71] however, there are few human studies dedicated to studying this subset in situ. Given the plasticity of moDCs and their context-dependence, in vitro studies may represent only a fraction of their behavior in situ. We do not yet fully understand the breadth of the roles this subset may play and more work dedicated to moDCs is needed.
5. Conclusion
With asthma being increasingly recognized as a heterogenous disease composed of multiple endotypes with distinct underlying pathomechanisms, it becomes increasingly important to have mouse models for each of the endotypes to facilitate design of targeted interventions (reviewed[25, 184]). Clinicians strive to allow the asthma endotype of the patients to help inform treatment; however, there currently exists a dearth of targeted therapies to address the clinical heterogeneity present.
The basis of current asthma treatment is symptom control with inhaled beta-adrenergic agonists and steroids. There are disease modifying treatments focused at Th2 inflammation such as monoclonal antibodies against IL-4R, IL-5/5R, and IgE.[16, 185] In the past few years, DC targeted therapies are being developed which show promise in mouse models and are a focus of ongoing investigation but none are yet approved for use in humans.[102, 185–190] Understanding of DC biology, the role of DC subsets, and utilization of mouse models which phenocopy specific endotypes of human disease will allow for generation and testing of targeted therapeutics to improve the lives of patients.
Funding Acknowledgements
NIH U01 AI131337 (Cook-Mills)
NIH R01AI127695 (Cook-Mills)
Marshall Klaus Perinatal Research Award (Lajiness)
Pediatric Scientist Development Program (Lajiness)- Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K12HD000850. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Dr. Jacquelyn D. Lajiness, Indiana University School of Medicine, Department of Pediatrics, Division of Neonatology, Neonatal-Perinatal Medicine Fellow, 1030 West Michigan Street, Suite C 4600, Indianapolis, IN 46202-5201
Prof. Joan M. Cook-Mills, Indiana University School of Medicine, Department of Pediatrics, Department of Microbiology and Immunology, Director, Pediatric Pulmonary, Asthma, and Allergy Basic Research Program, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W. Walnut Street, R4-202A, Indianapolis, IN 46202, USA
References
- [1].Theofani E, Semitekolou M, Morianos I, Samitas K, and Xanthou G, J Clin Med 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].GINA. Global Initiative for Asthma (GINA): Global strategy for asthma management and prevention Update 2014 and Online Appendix. . [cited 2014 12 Nov]; Available from: http://www.ginasthma.org.
- [3].Nurmagambetov T, Kuwahara R, and Garbe P, Ann Am Thorac Soc 2018, 15, 348. [DOI] [PubMed] [Google Scholar]
- [4].Ross MK, Romero T, Sim MS, and Szilagyi PG, J Asthma 2019, 56, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pakkasela J, Ilmarinen P, Honkamäki J, Tuomisto LE, Andersén H, Piirilä P, Hisinger-Mölkänen H, Sovijärvi A, Backman H, Lundbäck B, Rönmark E, Kankaanranta H, and Lehtimäki L, BMC Pulm Med 2020, 20, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Akar-Ghibril N, Casale T, Custovic A, and Phipatanakul W, J Allergy Clin Immunol Pract 2020, 8, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hammad H and Lambrecht BN, Cell 2021, 184, 1469. [DOI] [PubMed] [Google Scholar]
- [8].Lambrecht BN and Hammad H, Immunity 2009, 31, 412. [DOI] [PubMed] [Google Scholar]
- [9].Lambrecht BN and Hammad H, Ann Am Thorac Soc 2014, 11 Suppl 5, S236. [DOI] [PubMed] [Google Scholar]
- [10].Frati F, Salvatori C, Incorvaia C, Bellucci A, Di Cara G, Marcucci F, and Esposito S, Int J Mol Sci 2018, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hufnagl K, Pali-Schöll I, Roth-Walter F, and Jensen-Jarolim E, Semin Immunopathol 2020, 42, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim YJ, Womble JT, Gunsch CK, and Ingram JL, Obesity (Silver Spring) 2021, 29, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peters U, Dixon AE, and Forno E, J Allergy Clin Immunol 2018, 141, 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, and Wood LG, Nutrients 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sheikhpour M, Maleki M, Ebrahimi Vargoorani M, and Amiri V, Clin Epigenetics 2021, 13, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Agache I, Eguiluz-Gracia I, Cojanu C, Laculiceanu A, Del Giacco S, Zemelka-Wiacek M, Kosowska A, Akdis CA, and Jutel M, Allergy 2021, 76, 3390. [DOI] [PubMed] [Google Scholar]
- [17].Kuruvilla ME, Lee FE, and Lee GB, Clin Rev Allergy Immunol 2019, 56, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noël A, and Cataldo DD, Inflamm Res 2009, 58, 845. [DOI] [PubMed] [Google Scholar]
- [19].Trunova GV, Makarova OV, Diatroptov ME, Bogdanova IM, Mikchailova LP, and Abdulaeva SO, Bull Exp Biol Med 2011, 151, 99. [DOI] [PubMed] [Google Scholar]
- [20].Bleul T, Zhuang X, Hildebrand A, Lange C, Böhringer D, Schlunck G, Reinhard T, and Lapp T, Journal of Innate Immunity 2021, 13, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abdala-Valencia H, Berdnikovs S, Soveg FW, and Cook-Mills JM, American journal of physiology. Lung cellular and molecular physiology 2014, 307, L482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abdala-Valencia H, Soveg F, and Cook-Mills JM, American journal of physiology. Lung cellular and molecular physiology 2016, 310, L759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morokata T, Ishikawa J, Ida K, and Yamada T, Immunology 1999, 98, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alessandrini F, Musiol S, Schneider E, Blanco-Pérez F, and Albrecht M, Frontiers in Immunology 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martin RA, Hodgkins SR, Dixon AE, and Poynter ME, Respirology 2014, 19, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, and Giavina-Bianchi P, J Asthma Allergy 2017, 10, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fedulov AV and Kobzik L, American journal of respiratory cell and molecular biology 2011, 44, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mikhaylova L, Zhang Y, Kobzik L, and Fedulov AV, PloS one 2013, 8, e70387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Walker MT, Ferrie RP, Hoji A, Schroeder-Carter LM, Cohen JD, Schnaar RL, and Cook-Mills JM, Front. Allergy 2021, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lajiness JD, Amarsaikhan N, Tat K, Tsoggerel A, and Cook-Mills JM, J Immunol 2022. [DOI] [PMC free article] [PubMed]
- [31].Zosky GR and Sly PD, Clin Exp Allergy 2007, 37, 973. [DOI] [PubMed] [Google Scholar]
- [32].Barnden MJ, Allison J, Heath WR, and Carbone FR, Immunol Cell Biol 1998, 76, 34. [DOI] [PubMed] [Google Scholar]
- [33].Hyde EJ, Wakelin KA, Daniels NJ, Ghosh S, and Ronchese F, BMC Immunol 2019, 20, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Poynter ME, Persinger RL, Irvin CG, Butnor KJ, van Hirtum H, Blay W, Heintz NH, Robbins J, Hemenway D, Taatjes DJ, and Janssen-Heininger Y, Am J Physiol Lung Cell Mol Physiol 2006, 290, L144. [DOI] [PubMed] [Google Scholar]
- [35].Acciani TH, Brandt EB, Khurana Hershey GK, and Le Cras TD, Clin Exp Allergy 2013, 43, 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Manners S, Alam R, Schwartz DA, and Gorska MM, J Allergy Clin Immunol 2014, 134, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, and Khurana Hershey GK, J Allergy Clin Immunol 2013, 132, 1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, and Cook DN, Am J Respir Crit Care Med 2009, 180, 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Whitehead GS, Thomas SY, and Cook DN, Environ Health Perspect 2014, 122, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bogaert P, Naessens T, De Koker S, Hennuy B, Hacha J, Smet M, Cataldo D, Di Valentin E, Piette J, Tournoy KG, and Grooten J, Am J Physiol Lung Cell Mol Physiol 2011, 300, L679. [DOI] [PubMed] [Google Scholar]
- [41].Dejager L, Dendoncker K, Eggermont M, Souffriau J, Van Hauwermeiren F, Willart M, Van Wonterghem E, Naessens T, Ballegeer M, Vandevyver S, Hammad H, Lambrecht B, De Bosscher K, Grooten J, and Libert C, Mucosal Immunol 2015, 8, 1212. [DOI] [PubMed] [Google Scholar]
- [42].Yu QL and Chen Z, Exp Ther Med 2018, 15, 2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, and Lambrecht BN, J Exp Med 2008, 205, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, and Tschopp J, J Immunol 2008, 181, 3755. [DOI] [PubMed] [Google Scholar]
- [45].Sokolovska A, Hem SL, and HogenEsch H, Vaccine 2007, 25, 4575. [DOI] [PubMed] [Google Scholar]
- [46].Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hämmerling GJ, Maizels RM, and MacDonald AS, The Journal of experimental medicine 2010, 207, 2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Camacho DF, Velez TE, Hollinger MK, Wang E, Howard CL, Darnell EP, Kennedy DE, Krishack PA, Hrusch CL, Clark MR, Moon JJ, and Sperling AI, JCI Insight 2022. [DOI] [PMC free article] [PubMed]
- [48].Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, and Sperling AI, Nat Commun 2013, 4, 2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, and Lambrecht BN, J Exp Med 2005, 201, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lambrecht BN, Salomon B, Klatzmann D, and Pauwels RA, J Immunol 1998, 160, 4090. [PubMed] [Google Scholar]
- [51].Lim RH and Kobzik L, American journal of reproductive immunology (New York, N.Y. : 1989) 2009, 61, 1. [DOI] [PubMed] [Google Scholar]
- [52].Holt PG, Haining S, Nelson DJ, and Sedgwick JD, J Immunol 1994, 153, 256. [PubMed] [Google Scholar]
- [53].Huh JC, Strickland DH, Jahnsen FL, Turner DJ, Thomas JA, Napoli S, Tobagus I, Stumbles PA, Sly PD, and Holt PG, J Exp Med 2003, 198, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vermaelen KY, Cataldo D, Tournoy K, Maes T, Dhulst A, Louis R, Foidart JM, Noël A, and Pauwels R, J Immunol 2003, 171, 1016. [DOI] [PubMed] [Google Scholar]
- [55].Vermaelen K and Pauwels R, Am J Respir Cell Mol Biol 2003, 29, 405. [DOI] [PubMed] [Google Scholar]
- [56].van Rijt LS, Prins JB, Leenen PJ, Thielemans K, de Vries VC, Hoogsteden HC, and Lambrecht BN, Blood 2002, 100, 3663. [DOI] [PubMed] [Google Scholar]
- [57].Lambrecht BN, Carro-Muino I, Vermaelen K, and Pauwels RA, Am J Respir Cell Mol Biol 1999, 20, 1165. [DOI] [PubMed] [Google Scholar]
- [58].Fedulov A, Silverman E, Xiang Y, Leme A, and Kobzik L, J Immunol 2005, 175, 4292. [DOI] [PubMed] [Google Scholar]
- [59].James BN, Oyeniran C, Sturgill JL, Newton J, Martin RK, Bieberich E, Weigel C, Maczis MA, Palladino END, Lownik JC, Trudeau JB, Cook-Mills JM, Wenzel S, Milstien S, and Spiegel S, J Allergy Clin Immunol 2021, 147, 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hoji A, Kumar R, Gern JE, Bendixsen CG, Seroogy CM, and Cook-Mills JM, J Allergy Clin Immunol Glob 2022, 1, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lim RH, Kobzik L, and Dahl M, PloS one 2010, 5, e10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Maddox L and Schwartz DA, Annu Rev Med 2002, 53, 477. [DOI] [PubMed] [Google Scholar]
- [63].Hammad H, Charbonnier AS, Duez C, Jacquet A, Stewart GA, Tonnel AB, and Pestel J, Blood 2001, 98, 1135. [DOI] [PubMed] [Google Scholar]
- [64].Gilles S, Mariani V, Bryce M, Mueller MJ, Ring J, Behrendt H, Jakob T, and Traidl-Hoffmann C, Allergy Asthma Clin Immunol 2009, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, and Yona S, Nat Rev Immunol 2014, 14, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, Rogers NC, Moncaut N, Carvajal JJ, and Reis e Sousa C, Cell 2013, 154, 843. [DOI] [PubMed] [Google Scholar]
- [67].See P, Dutertre CA, Chen J, Günther P, McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, Sumatoh HRB, Ruffin N, Jouve M, Gea-Mallorquí E, Hennekam RCM, Lim T, Yip CC, Wen M, Malleret B, Low I, Shadan NB, Fen CFS, Tay A, Lum J, Zolezzi F, Larbi A, Poidinger M, Chan JKY, Chen Q, Rénia L, Haniffa M, Benaroch P, Schlitzer A, Schultze JL, Newell EW, and Ginhoux F, Science 2017, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Weinreb C, Rodriguez-Fraticelli A, Camargo FD, and Klein AM, Science 2020, 367. [DOI] [PMC free article] [PubMed]
- [69].Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, Shin A, Huang W, Dress RJ, Dutertre CA, Schlitzer A, Chen J, Ng LG, Wang H, Liu Z, Su B, and Ginhoux F, Cell 2019, 178, 1509. [DOI] [PubMed] [Google Scholar]
- [70].Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GHL, Chung TJK, Chan DKH, Tan KK, Hon TLK, Fossum E, Bogen B, Choolani M, Chan JKY, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, and Ginhoux F, Immunity 2016, 45, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bosteels C, Neyt K, Vanheerswynghels M, van Helden MJ, Sichien D, Debeuf N, De Prijck S, Bosteels V, Vandamme N, Martens L, Saeys Y, Louagie E, Lesage M, Williams DL, Tang SC, Mayer JU, Ronchese F, Scott CL, Hammad H, Guilliams M, and Lambrecht BN, Immunity 2020, 52, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gaurav R and Agrawal DK, Expert Rev Clin Immunol 2013, 9, 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Merad M, Sathe P, Helft J, Miller J, and Mortha A, Annu Rev Immunol 2013, 31, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Collin M, McGovern N, and Haniffa M, Immunology 2013, 140, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saeys Y, Van Gassen S, and Lambrecht BN, Nature Reviews Immunology 2016, 16, 449. [DOI] [PubMed] [Google Scholar]
- [76].Bajaña S, Turner S, Paul J, Ainsua-Enrich E, and Kovats S, J Immunol 2016, 196, 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Furuhashi K, Suda T, Hasegawa H, Suzuki Y, Hashimoto D, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Shibata K, Nakamura H, and Chida K, Am J Respir Cell Mol Biol 2012, 46, 165. [DOI] [PubMed] [Google Scholar]
- [78].Sakurai S, Furuhashi K, Horiguchi R, Nihashi F, Yasui H, Karayama M, Suzuki Y, Hozumi H, Enomoto N, Fujisawa T, Nakamura Y, Inui N, and Suda T, Allergol Int 2021, 70, 351. [DOI] [PubMed] [Google Scholar]
- [79].Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, and Lambrecht BN, Immunity 2013, 38, 322. [DOI] [PubMed] [Google Scholar]
- [80].Bernatchez E, Gold MJ, Langlois A, Lemay AM, Brassard J, Flamand N, Marsolais D, McNagny KM, and Blanchet MR, Am J Physiol Lung Cell Mol Physiol 2015, 308, L816. [DOI] [PubMed] [Google Scholar]
- [81].Conejero L, Khouili SC, Martínez-Cano S, Izquierdo HM, Brandi P, and Sancho D, JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, and Ray A, J Immunol 2013, 191, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, and Cook DN, Mucosal Immunol 2012, 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fear VS, Lai SP, Zosky GR, Perks KL, Gorman S, Blank F, von Garnier C, Stumbles PA, and Strickland DH, Physiol Rep 2016, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yi S, Zhai J, Niu R, Zhu G, Wang M, Liu J, Huang H, Wang Y, Jing X, Kang L, Song W, Shi Y, and Tang H, Nat Commun 2018, 9, 3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].El-Gammal A, Oliveria JP, Howie K, Watson R, Mitchell P, Chen R, Baatjes A, Smith S, Al-Sajee D, Hawke TJ, Killian KJ, Gauvreau GM, and O'Byrne PM, Am J Respir Crit Care Med 2016, 194, 169. [DOI] [PubMed] [Google Scholar]
- [87].Hayashi Y, Ishii Y, Hata-Suzuki M, Arai R, Chibana K, Takemasa A, and Fukuda T, Respir Res 2013, 14, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vroman H, Tindemans I, Lukkes M, van Nimwegen M, de Boer GM, Tramper-Stranders GA, Braunstahl GJ, Hendriks RW, and Kool M, Eur Respir J 2020, 55. [DOI] [PubMed]
- [89].Yerkovich ST, Roponen M, Smith ME, McKenna K, Bosco A, Subrata LS, Mamessier E, Wikström ME, Le Souef P, Sly PD, Holt PG, and Upham JW, J Allergy Clin Immunol 2009, 123, 209. [DOI] [PubMed] [Google Scholar]
- [90].Yu CI, Becker C, Metang P, Marches F, Wang Y, Toshiyuki H, Banchereau J, Merad M, and Palucka AK, J Immunol 2014, 193, 4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, and Harrison LC, J Immunol 2011, 186, 6207. [DOI] [PubMed] [Google Scholar]
- [92].Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, and Dalod M, Genome Biol 2008, 9, R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, and Ginhoux F, Immunity 2012, 37, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, Boudinot P, Hosmalin A, Schwartz-Cornil I, and Dalod M, J Exp Med 2010, 207, 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, Malissen B, and Dalod M, Immunol Rev 2010, 234, 177. [DOI] [PubMed] [Google Scholar]
- [96].Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, and Kroczek RA, J Exp Med 2010, 207, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, and Reis e Sousa C, J Exp Med 2010, 207, 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Galibert L, Diemer GS, Liu Z, Johnson RS, Smith JL, Walzer T, Comeau MR, Rauch CT, Wolfson MF, Sorensen RA, Van der Vuurst de Vries AR, Branstetter DG, Koelling RM, Scholler J, Fanslow WC, Baum PR, Derry JM, and Yan W, J Biol Chem 2005, 280, 21955. [DOI] [PubMed] [Google Scholar]
- [99].Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, and Radford KJ, J Exp Med 2010, 207, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, Miron M, Kumar BV, Griesemer A, Ho SH, Lerner H, Thome JJC, Connors T, Reizis B, and Farber DL, Immunity 2017, 46, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Johnson TR, Johnson CN, Corbett KS, Edwards GC, and Graham BS, PLoS One 2011, 6, e16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Morianos I and Semitekolou M, Int J Mol Sci 2020, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Demedts IK, Brusselle GG, Vermaelen KY, and Pauwels RA, Am J Respir Cell Mol Biol 2005, 32, 177. [DOI] [PubMed] [Google Scholar]
- [104].Gill MA, J Allergy Clin Immunol 2012, 129, 889. [DOI] [PubMed] [Google Scholar]
- [105].Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, Barinaga G, Grys K, Sharif-Paghaleh E, Karagiannis SN, Peakman M, Lombardi G, and Nestle FO, J Exp Med 2012, 209, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Collin M and Bigley V, Immunology 2018, 154, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vroman H, Hendriks RW, and Kool M, Front Immunol 2017, 8, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Peters MC, Ringel L, Dyjack N, Herrin R, Woodruff PG, Rios C, O'Connor B, Fahy JV, and Seibold MA, Am J Respir Crit Care Med 2019, 199, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Min J, Yang D, Kim M, Haam K, Yoo A, Choi J-H, Schraml BU, Kim YS, Kim D, and Kang S-J, Experimental & Molecular Medicine 2018, 50, e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, and Ginhoux F, Immunity 2013, 38, 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gao Y, Nish SA, Jiang R, Hou L, Licona-Limón P, Weinstein JS, Zhao H, and Medzhitov R, Immunity 2013, 39, 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Raymond M, Rubio M, Fortin G, Shalaby KH, Hammad H, Lambrecht BN, and Sarfati M, J Allergy Clin Immunol 2009, 124, 1333. [DOI] [PubMed] [Google Scholar]
- [113].Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, and Nussenzweig MC, Science 2007, 315, 107. [DOI] [PubMed] [Google Scholar]
- [114].Suzuki Y, Suda T, Furuhashi K, Shibata K, Hashimoto D, Enomto N, Fujisawa T, Nakamura Y, Inui N, Nakamura H, and Chida K, Am J Respir Cell Mol Biol 2012, 46, 773. [DOI] [PubMed] [Google Scholar]
- [115].Norimoto A, Hirose K, Iwata A, Tamachi T, Yokota M, Takahashi K, Saijo S, Iwakura Y, and Nakajima H, Am J Respir Cell Mol Biol 2014, 51, 201. [DOI] [PubMed] [Google Scholar]
- [116].Mishra A, Brown AL, Yao X, Yang S, Park SJ, Liu C, Dagur PK, McCoy JP, Keeran KJ, Nugent GZ, Jeffries KR, Qu X, Yu ZX, Levine SJ, and Chung JH, Nat Commun 2015, 6, 6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, Kuperman DA, Erle DJ, and Luster AD, J Immunol 2009, 182, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Greer AM, Matthay MA, Kukreja J, Bhakta NR, Nguyen CP, Wolters PJ, Woodruff PG, Fahy JV, and Shin JS, PLoS One 2014, 9, e99084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Dua B, Tang W, Watson R, Gauvreau G, and O'Byrne PM, Clin Exp Allergy 2014, 44, 921. [DOI] [PubMed] [Google Scholar]
- [120].Froidure A, Shen C, Gras D, Van Snick J, Chanez P, and Pilette C, Allergy 2014, 69, 1068. [DOI] [PubMed] [Google Scholar]
- [121].Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, Ng PY, van den Hoogen LL, Leong JY, Lee B, Chevrier M, Zhang XM, Yong PJA, Koh G, Lum J, Howland SW, Mok E, Chen J, Larbi A, Tan HKK, Lim TKH, Karagianni P, Tzioufas AG, Malleret B, Brody J, Albani S, van Roon J, Radstake T, Newell EW, and Ginhoux F, Immunity 2019, 51, 573. [DOI] [PubMed] [Google Scholar]
- [122].Naessens T, Morias Y, Hamrud E, Gehrmann U, Budida R, Mattsson J, Baker T, Skogberg G, Israelsson E, Thörn K, Schuijs MJ, Angermann B, Melville F, Staples KJ, Cunoosamy DM, and Lambrecht BN, Am J Respir Crit Care Med 2020, 202, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lombardi V, Speak AO, Kerzerho J, Szely N, and Akbari O, Mucosal Immunol 2012, 5, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, and Wills-Karp M, PLoS One 2008, 3, e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kool M, Geurtsvankessel C, Muskens F, Madeira FB, van Nimwegen M, Kuipers H, Thielemans K, Hoogsteden HC, Hammad H, and Lambrecht BN, J Leukoc Biol 2011, 90, 1177. [DOI] [PubMed] [Google Scholar]
- [126].Reizis B, Bunin A, Ghosh HS, Lewis KL, and Sisirak V, Annu Rev Immunol 2011, 29, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, and Reizis B, Proc Natl Acad Sci U S A 2012, 109, 3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lynch JP, Mazzone SB, Rogers MJ, Arikkatt JJ, Loh Z, Pritchard AL, Upham JW, and Phipps S, Eur Respir J 2014, 43, 264. [DOI] [PubMed] [Google Scholar]
- [129].Lynch JP, Werder RB, Loh Z, Sikder MAA, Curren B, Zhang V, Rogers MJ, Lane K, Simpson J, Mazzone SB, Spann K, Hayball J, Diener K, Everard ML, Blyth CC, Forstner C, Dennis PG, Murtaza N, Morrison M, Ó.C. P, Zhang P, Haque A, Hill GR, Sly PD, Upham JW, and Phipps S, J Exp Med 2018, 215, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ouabed A, Hubert FX, Chabannes D, Gautreau L, Heslan M, and Josien R, J Immunol 2008, 180, 5862. [DOI] [PubMed] [Google Scholar]
- [131].Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Brière F, and Trinchieri G, Nat Immunol 2001, 2, 1144. [DOI] [PubMed] [Google Scholar]
- [132].Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, and Gilliet M, J Exp Med 2007, 204, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Maazi H, Banie H, Aleman Muench GR, Patel N, Wang B, Sankaranarayanan I, Bhargava V, Sato T, Lewis G, Cesaroni M, Karras J, Das A, Soroosh P, and Akbari O, J Allergy Clin Immunol 2018, 141, 893. [DOI] [PubMed] [Google Scholar]
- [134].de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, and Lambrecht BN, J Exp Med 2004, 200, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Agrawal DK, Hopfenspirger MT, Chavez J, and Talmadge JE, Int Immunopharmacol 2001, 1, 2081. [DOI] [PubMed] [Google Scholar]
- [136].Edwan JH, Perry G, Talmadge JE, and Agrawal DK, J Immunol 2004, 172, 5016. [DOI] [PubMed] [Google Scholar]
- [137].Edwan JH, Talmadge JE, and Agrawal DK, Int Immunopharmacol 2005, 5, 345. [DOI] [PubMed] [Google Scholar]
- [138].Edwan JH and Agrawal DK, Immunol Res 2007, 37, 147. [DOI] [PubMed] [Google Scholar]
- [139].Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, and Hammad H, J Immunol 2009, 183, 1074. [DOI] [PubMed] [Google Scholar]
- [140].Chairakaki AD, Saridaki MI, Pyrillou K, Mouratis MA, Koltsida O, Walton RP, Bartlett NW, Stavropoulos A, Boon L, Rovina N, Papadopoulos NG, Johnston SL, and Andreakos E, J Allergy Clin Immunol 2018, 142, 542. [DOI] [PubMed] [Google Scholar]
- [141].Wu M, Gao L, He M, Liu H, Jiang H, Shi K, Shang R, Liu B, Gao S, Chen H, Gong F, Gelfand EW, Huang Y, and Han J, Cell Mol Immunol 2020, 17, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Özkan M, Eskiocak YC, and Wingender G, PLoS One 2021, 16, e0250533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Xia M, Xu F, Ni H, Wang Q, Zhang R, Lou Y, and Zhou J, Respiratory Research 2022, 23, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Qiu SL, Zhang H, Tang QY, Bai J, He ZY, Zhang JQ, Li MH, Deng JM, Liu GN, and Zhong XN, Thorax 2017, 72, 1084. [DOI] [PubMed] [Google Scholar]
- [145].Cella M, Facchetti F, Lanzavecchia A, and Colonna M, Nat Immunol 2000, 1, 305. [DOI] [PubMed] [Google Scholar]
- [146].Charbonnier AS, Hammad H, Gosset P, Stewart GA, Alkan S, Tonnel AB, and Pestel J, J Leukoc Biol 2003, 73, 91. [DOI] [PubMed] [Google Scholar]
- [147].Hammad H, Smits HH, Ratajczak C, Nithiananthan A, Wierenga EA, Stewart GA, Jacquet A, Tonnel AB, and Pestel J, Eur Cytokine Netw 2003, 14, 219. [PubMed] [Google Scholar]
- [148].Boor PP, Metselaar HJ, Jonge S, Mancham S, van der Laan LJ, and Kwekkeboom J, Eur J Immunol 2011, 41, 1663. [DOI] [PubMed] [Google Scholar]
- [149].Kavousanaki M, Makrigiannakis A, Boumpas D, and Verginis P, Arthritis Rheum 2010, 62, 53. [DOI] [PubMed] [Google Scholar]
- [150].Martín-Gayo E, Sierra-Filardi E, Corbí AL, and Toribio ML, Blood 2010, 115, 5366. [DOI] [PubMed] [Google Scholar]
- [151].Palomares O, Rückert B, Jartti T, Kücüksezer UC, Puhakka T, Gomez E, Fahrner HB, Speiser A, Jung A, Kwok WW, Kalogjera L, Akdis M, and Akdis CA, J Allergy Clin Immunol 2012, 129, 510. [DOI] [PubMed] [Google Scholar]
- [152].Bratke K, Lommatzsch M, Julius P, Kuepper M, Kleine HD, Luttmann W, and Christian Virchow J, Thorax 2007, 62, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Upham JW, Zhang G, Rate A, Yerkovich ST, Kusel M, Sly PD, and Holt PG, J Allergy Clin Immunol 2009, 124, 707. [DOI] [PubMed] [Google Scholar]
- [154].Hagendorens MM, Ebo DG, Schuerwegh AJ, Huybrechs A, Van Bever HP, Bridts CH, De Clerck LS, and Stevens WJ, Clin Exp Allergy 2003, 33, 633. [DOI] [PubMed] [Google Scholar]
- [155].Silver E, Yin-DeClue H, Schechtman KB, Grayson MH, Bacharier LB, and Castro M, Pediatr Allergy Immunol 2009, 20, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Matsuda H, Suda T, Hashizume H, Yokomura K, Asada K, Suzuki K, Chida K, and Nakamura H, Am J Respir Crit Care Med 2002, 166, 1050. [DOI] [PubMed] [Google Scholar]
- [157].Spears M, McSharry C, Donnelly I, Jolly L, Brannigan M, Thomson J, Lafferty J, Chaudhuri R, Shepherd M, Cameron E, and Thomson NC, Clin Exp Allergy 2011, 41, 665. [DOI] [PubMed] [Google Scholar]
- [158].Dua B, Watson RM, Gauvreau GM, and O'Byrne PM, J Allergy Clin Immunol 2010, 126, 133. [DOI] [PubMed] [Google Scholar]
- [159].Lin S, PREPRINT (Version 1) available at Research Square 2022.
- [160].Rodriguez-Fraticelli AE and Camargo F, Curr Opin Hematol 2021, 28, 18. [DOI] [PubMed] [Google Scholar]
- [161].Yang J, Zhang L, Yu C, Yang X-F, and Wang H, Biomarker Research 2014, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Segura E and Amigorena S, Trends Immunol 2013, 34, 440. [DOI] [PubMed] [Google Scholar]
- [163].Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, Malissen B, and Guilliams M, J Immunol 2012, 188, 1751. [DOI] [PubMed] [Google Scholar]
- [164].Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, and Steinman RM, Cell 2010, 143, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hespel C and Moser M, Eur J Immunol 2012, 42, 2535. [DOI] [PubMed] [Google Scholar]
- [166].De Trez C, Magez S, Akira S, Ryffel B, Carlier Y, and Muraille E, PLoS Pathog 2009, 5, e1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Copin R, De Baetselier P, Carlier Y, Letesson JJ, and Muraille E, J Immunol 2007, 178, 5182. [DOI] [PubMed] [Google Scholar]
- [168].Serbina NV, Jia T, Hohl TM, and Pamer EG, Annu Rev Immunol 2008, 26, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, and Pamer EG, Immunity 2003, 19, 59. [DOI] [PubMed] [Google Scholar]
- [170].Guilliams M, Movahedi K, Bosschaerts T, VandenDriessche T, Chuah MK, Hérin M, Acosta-Sanchez A, Ma L, Moser M, Van Ginderachter JA, Brys L, De Baetselier P, and Beschin A, J Immunol 2009, 182, 1107. [DOI] [PubMed] [Google Scholar]
- [171].Hammad H, Lambrecht BN, Pochard P, Gosset P, Marquillies P, Tonnel AB, and Pestel J, J Immunol 2002, 169, 1524. [DOI] [PubMed] [Google Scholar]
- [172].Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, and Lambrecht BN, J Exp Med 2010, 207, 2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, Muskens F, and Lambrecht BN, The Journal of experimental medicine 2010, 207, 2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, and Lambrecht BN, Immunity 2011, 34, 527. [DOI] [PubMed] [Google Scholar]
- [175].Ebner S, Ratzinger G, Krösbacher B, Schmuth M, Weiss A, Reider D, Kroczek RA, Herold M, Heufler C, Fritsch P, and Romani N, J Immunol 2001, 166, 633. [DOI] [PubMed] [Google Scholar]
- [176].Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, and Hacohen N, Science 2017, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lee J, Zhou YJ, Ma W, Zhang W, Aljoufi A, Luh T, Lucero K, Liang D, Thomsen M, Bhagat G, Shen Y, and Liu K, Nat Immunol 2017, 18, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Pichavant M, Charbonnier AS, Taront S, Brichet A, Wallaert B, Pestel J, Tonnel AB, and Gosset P, J Allergy Clin Immunol 2005, 115, 771. [DOI] [PubMed] [Google Scholar]
- [179].van den Heuvel MM, Vanhee DD, Postmus PE, Hoefsmit EC, and Beelen RH, J Allergy Clin Immunol 1998, 101, 90. [DOI] [PubMed] [Google Scholar]
- [180].Paplinska-Goryca M, Misiukiewicz-Stepien P, Proboszcz M, Nejman-Gryz P, Gorska K, and Krenke R, Cells 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Paplinska-Goryca M, Misiukiewicz-Stepien P, Nejman-Gryz P, Proboszcz M, Mlacki M, Gorska K, and Krenke R, Clin Immunol 2020, 215, 108421. [DOI] [PubMed] [Google Scholar]
- [182].Bellinghausen I, Brand U, Knop J, and Saloga J, J Allergy Clin Immunol 2000, 105, 988. [DOI] [PubMed] [Google Scholar]
- [183].Cagnoni EF, Ferreira DS, Ferraz da Silva LF, Nicoletti Carvalho Petry AL, Gomes dos Santos AB, Rodrigues Medeiros MC, Dolhnikoff M, Rabe KF, and Mauad T, J Allergy Clin Immunol 2015, 135, 1352. [DOI] [PubMed] [Google Scholar]
- [184].Lombardi V, Singh AK, and Akbari O, Int Arch Allergy Immunol 2010, 151, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Chinn AM and Insel PA, Br J Pharmacol 2020, 177, 3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Jaiswal AK, Sandey M, Suryawanshi A, Cattley RC, and Mishra A, Immun Inflamm Dis 2019, 7, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Jaiswal AK, Yadav J, Makhija S, Mazumder S, Mitra AK, Suryawanshi A, Sandey M, and Mishra A, Mucosal Immunol 2022, 15, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sun S, Yao M, Yuan L, and Qiao J, Allergol Immunopathol (Madr) 2021, 49, 100. [DOI] [PubMed] [Google Scholar]
- [189].Pivniouk V, Gimenes-Junior JA, Ezeh P, Michael A, Pivniouk O, Hahn S, VanLinden SR, Malone SP, Abidov A, Anderson D, Gozdz J, DeVries A, Martinez FD, Pasquali C, and Vercelli D, J Allergy Clin Immunol 2022, 149, 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Zhang F, Su X, Huang G, Xin X-F, Cao EH, Shi Y, and Song Y, Scientific Reports 2017, 7, 14268. [DOI] [PMC free article] [PubMed] [Google Scholar]


