Abstract
Background and aims
The main causes of death in patients with severe Coronavirus disease-2019 (COVID-19) are acute respiratory distress syndrome (ARDS) and multiorgan failure caused by a severe inflammatory cascade. Novel treatment strategies, such as stem-cell-based therapy and their derivatives can be used to relieve inflammation in these cases. In this study, we aimed to evaluate the safety and efficacy of therapy using mesenchymal stromal cells (MSCs) and their derived extracellular vesicles in COVID-19 patients.
Materials and methods
COVID-19 patients with ARDS were included in this study and allocated into two study and control groups using block randomization. While all patients received recommended treatment based on guidelines from the national advisory committee for COVID-19 pandemic, the two intervention groups received two consecutive injections of MSCs (100 × 106 cells) or one dose of MSCs (100 × 106 cells) followed by one dose of MSC-derived extracellular vesicles (EVs). Patients were assessed for safety and efficacy by evaluating clinical symptoms, laboratory parameters, and inflammatory markers at baseline and 48 h after the second intervention.
Results
A total number of 43 patients (the MSC alone group = 11, MSC plus EV group = 8, and control group = 24) were included in the final analysis. Mortality was reported in three patients in the MSC alone group (RR: 0.49; 95% CI 0.14–1.11; P = 0.08); zero patient in the MSC plus EV group (RR: 0.08; 95% CI 0.005–1.26; P = 0.07) and eight patients in the control group. MSC infusion was associated with a decrease in inflammatory cytokines such as IL-6 (P = 0.015), TNF-α (P = 0.034), IFN-γ (P = 0.024), and CRP (P = 0.041).
Conclusion
MSCs and their extracellular vesicles can significantly reduce the serum levels of inflammatory markers in COVID-19 patients, with no serious adverse events.
Trial registration IRCT, IRCT registration number: IRCT20200217046526N2. Registered 13th April 2020, http://www.irct.ir/trial/47073.
Keywords: COVID-19, SARS-CoV-2, Acute respiratory distress syndrome, Cytokine release syndrome, Mesenchymal stromal cells, Cell therapy, Extracellular vesicles
Introduction
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), was first observed in December 2019 in Wuhan, Hubei province, China [1–3]. Subsequently, SARS-CoV-2 spread worldwide, and on March 11th, 2020, the World Health Organization (WHO) declared a global pandemic [4, 5]. As of June 7th, 2023, more than 767 million infected cases and almost 6.9 million related deaths have been reported and several hygiene measures and social limitations have been implemented in different countries [6]. Many patients experienced severe COVID-19, characterized by acute respiratory distress syndrome (ARDS), which requires oxygen therapy and intensive care unit (ICU) hospitalization [7, 8]. Additionally, the mortality rate in COVID-19 patients with ARDS is high, and prompt intervention is necessary [9].
SARS-CoV-2 could infect many organs, mainly through angiotensin-converting enzyme-2 receptor (ACE-2), and other potential receptors like glucose-regulated protein-78 (GRP-78), causing several symptoms in affected individuals [10–12]. Multiorgan failure is a serious consequence of severe COVID-19, due to acute inflammation [13]. COVID-19 pathogenesis is characterized by the inflammatory cascade, resulting from angiotensin II (Ang II) activation, which induces the production of pro-inflammatory cytokines [14–16]. According to current findings, extremely ill COVID-19 patients, like those with ARDS, have higher levels of pro-inflammatory cytokines like interleukin 6 (IL-6), in their serum [17]. This issue represents a poor prognosis and increases the mortality rate of COVID-19 [18].
In late 2020, vaccines were developed to prevent COVID-19 [19]. Vaccination has significantly reduced COVID-19 mortality rates; however, the best treatment approach is still being debated [20]. New treatment strategies, such as nanomedicine-based approach, cell-based therapy, and adoptive immunotherapy have emerged to treat COVID-19 patients [21–23]. Among these, cell-based therapies using mesenchymal stem cells (MSCs), have shown promising results in pilot studies and also in clinical trials [24–26]. Our previous study also observed positive effects of MSCs in severe COVID-19 cases [27]. Other studies have focused on the infusion of perinatal tissues MSC-derived extracellular vesicles (EVs) due to their feasibility, long-lasting effects and cost-effectiveness [28]. However, more supporting evidences for the positive impact of MSCs and MSC-derived EVs on clinical symptoms, laboratory parameters and inflammatory markers is needed. Therefore, this study aims to evaluate the safety and efficacy of perinatal tissue derived MSCs and MSC-derived EVs in COVID-19 patients, with ARDS.
Materials and methods
Study design
This phase II randomized, multicentric clinical trial was conducted on COVID-19 patients with acute respiratory distress syndrome referred to Masih Daneshvari and Shariati hospitals, two major referral centers for COVID-19 hospitalization in Tehran, Iran, during 2020. The inclusion criteria were: age between 18 and 65, confirmation of SARS-CoV-2 infection by qRT-PCR, diagnosis of ARDS according to the Berlin criteria [29], requiring supplemental oxygen therapy, confirmation of pneumonia based on chest radiography or high resolution computed tomography (HRCT) and progressive status (> 50% in 24–48 h), SPO2/FiO2 ≤ 300 mmHg, ICU admission < 48 h, and a Sequential Organ Failure Assessment (SOFA) score between 2 and 3. Patients with allergies or sensitivity to cell-based products, a history for malignancies, other viral respiratory co-infections, severe renal or liver failure, interstitial lung disease, underlying immunocompromised disease, and those on extracorporeal life support were excluded.
Randomization
The block randomization technique was used to perform the randomization procedure, using a randomized triple ABC blocking method based on a random number table. Patients were randomly divided into three study groups. All groups received conventional medical therapy according to national guidelines (Table 1), while the two intervention groups received either two consecutive doses of allogenic mesenchymal cells derived from perinatal tissue intravenously at a dose of 100 × 106 ± 10% over 10–12 min (MSC alone group), or one dose of allogenic MSCs intravenously at a dose of 100 × 106 ± 10% and one dose of MSC-derived EVs (isolated from the 200 × 106 ± 10% cells) through inhalation route (MSC plus EV group). We assumed that nebulized form of MSC (MSC + EV) may ameliorate localized respiratory syndrome. The second dose in both groups was administered 48 h after the first injection. Infusion speed was adjusted to 4–5 mL/minute for all injections.
Table 1.
Baseline characteristics of study variables between all patients in each group
| Variable | MSC group (n = 11) Mortality (n = 3) | MSC + EV group (n = 8) Mortality (n = 0) | Control group (n = 24) Mortality (n = 8) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 50 ± 12.48 | 47.75 ± 12.72 | 49.4 ± 11.87 | 0.993a |
| Male (%) | 10 (90.9) | 5 (62.5) | 16 (66.7) | 0.208b |
| Having comorbidities (%) | 5 (45.5) | 3 (37.5) | 11 (45.83) | 0.854b |
| CBC data | ||||
| WBC count (× 103) | 11.67 ± 4.58 | 13.04 ± 9.88 | 10.03 ± 9.26 | 0.176c |
| Lymphocyte (%) | 9.92 ± 3.9 | 8.17 ± 3.3 | 13.42 ± 8.88 | 0.221c |
| Hemoglobin (mg/dl) | 14.03 ± 1.87 | 13.15 ± 3.26 | 12.2 ± 2.18 | 0.109c |
| Platelet count (× 103) | 262 ± 95.95 | 215.87 ± 84.44 | 266 ± 178.7 | 0.657c |
| PT (s) | 12.93 ± 0.67 | 13.52 ± 1.67 | 14.42 ± 2.45 | 0.129c |
| PTT (s) | 42.11 ± 11.38 | 35.14 ± 8.43 | 40.2 ± 6.88 | 0.224c |
| Biochemistry data | ||||
| BUN (mg/dl) | 40.6 ± 18.6 | 33.57 ± 14.62 | 48.85 ± 32.47 | 0.402c |
| Creatinine (mg/dl) | 1.06 ± 0.22 | 0.9 ± 0.18 | 1.2 ± 0.55 | 0.224c |
| AST (U/L) | 39.7 ± 41.25 | 37.6 ± 7.02 | 56.12 ± 44.86 | 0.143c |
| ALT (U/L) | 59.3 ± 65.4 | 46.2 ± 33.6 | 48 ± 43.45 | 0.897c |
| Alkp (IU/L) | 169.67 ± 36.36 | 135.2 ± 38 | 206.43 ± 115.42 | 0.106c |
| Bilirubin (mg/dl) | 0.75 ± 0.22 | 0.68 ± 0.3 | 1 ± 0.8 | 0.803c |
| Arterial blood gas data | ||||
| pH | 7.4 ± 0.06 | 7.37 ± 0.12 | 7.37 ± 0.09 | 0.696c |
| PCO2 (mmHg) | 46.92 ± 11.15 | 42.8 ± 3.12 | 45.22 ± 15.2 | 0.739c |
| HCO3 (meq/L) | 28.02 ± 4.43 | 30.4 ± 4.72 | 26.43 ± 6.67 | 0.211c |
| O2 saturation (%) | 81.9 ± 10.94 | 75.7 ± 6.9 | 87.81 ± 8.4 | 0.017c |
| Inflammatory markers | ||||
| Interleukin-6 (pg/ml) | 184.53 ± 71.69 | 207.15 ± 38.93 | 128.2 ± 85.8 | 0.18c |
| TNF alpha (pg/ml) | 26.62 ± 11.1 | 24.2 ± 10.43 | 22.45 ± 9.54 | 0.792c |
| IFN-gamma (pg/ml) | 126.62 ± 66.9 | 177.85 ± 53.02 | 125.7 ± 27.5 | 0.183c |
| CRP (mg/L) | 36.11 ± 13.7 | 25.3 ± 10.2 | 30.2 ± 13.2 | 0.227c |
| Clinical symptoms | ||||
| Cough (%) | 6 (54.5) | 1 (12.5) | 22 (91.7) | < 0.001b |
| Dyspnea (%) | 11 (100) | 7 (87.5) | 22 (91.7) | 0.53b |
| Diarrhea (%) | 1 (9.1) | - | 3 (12.5) | 0.862b |
| Medications | ||||
| IV Dexamethasone (8 mg/day) | 8 (72.7) | 6 (75) | 19 (79.2) | 0.460b |
| Oral Prednisolone (0.5 mg/kg/day) | 3 (27.3) | 2 (25) | 5 (20.8) | 0.552b |
| Subcutaneous Enoxaparin (40 mg/day) | 7 (63.6) | 5 (62.5) | 17 (70.8) | 0.124b |
| Subcutaneous Heparin 5000UI (TDS) | 4 (36.4) | 3 (37.5) | 7 (29.2) | 0.206b |
| IV Remdesivir (200 mg at 1st and 100 mg at 2nd and 3rd day) | 2 (18.2) | 1 (12.5) | 5 (20.8) | 0.380b |
| Hospital information | ||||
| Hospitalization | 14.75 ± 6.79 | 20.75 ± 10.11 | 14.23 ± 19.55 | 0.634a |
| ICU stay | 7.75 ± 5.09 | 14.5 ± 10.55 | 10.9 ± 9.90 | 0.355a |
aOne-way ANOVA test
bChi-square test
cKruskal Wallis test
MSC and MSC-derived EVs
We used good manufacturing practice (GMP)-certified MSCs for this study, which underwent a panel of quality control tests as part of their certificate of analysis. The cell preparation protocol was previously described in our study [27]. To extract MSC-derived EVs, three batches of condition medium (CM) were collected from 1.6, 1.6 and 1.2 billion MSCs (viability > 92% at the time of CM collection), and were quarantined at 4 °C to be checked for mycoplasma, endotoxin and sterility tests. Then, the CM was concentrated by tangential flow filtration (TFF) by Sartorious VivaFlow® 200 and centrifuged at 3 K and 20 K G at 4°. The pellet was resuspended by PBS− and filtered by 0.2 µm of Amicon® (Merck Millipore, Darmstadt, Germany). The final pellet is dissolved in normal saline and aliquoted in vials. One vial from each batch of CM is processed for quality control and characterization of EVs according to the MISEV2018 guideline [30]. The protein concentration is determined using the Bicinchoninic acid (BCA) assay (Pierce™ BCA kit, Thermo Fisher), and confirmed by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The expressions of EV-specific proteins were assessed by western blotting. The size distribution and morphology of EVs were checked by dynamic light scattering and scanning electron microscopy, respectively. The average yield of EVs (after filtration) was 8 µg per one million cells.
Study endpoints
The primary endpoint in this study was assessment of adverse events, based on common terminology criteria for adverse events (CTCAE) version 4 [31]. In addition, improving the clinical symptoms of the patients and also the results of the complete blood count (CBC), arterial blood gas (ABG), biochemistry analysis, and inflammatory parameters were assumed as the secondary endpoints of this study. The follow-up time points for assessing endpoints were baseline, after first infusion, after second infusion, and 48 h after the second intervention. Also, the patients were followed for 28 days to assess possible adverse events.
Statistical analysis
For statistical analysis, the data was entered into version 26 of the Statistical Package for the Social Sciences (SPSS®). The normality distribution of data was assessed by the Kolmogorov-Smirnoff or Shapiro–Wilk tests. The quantitative variables were reported as mean ± standard deviation (SD), and the categorical variables were reported as number (percentage) data. The one-way ANOVA test or Kruskal Wallis test was used for analysis between the groups. Wilcoxon signed-rank test was administered for analyzing changes in each group. The graphs were drawn by GraphPad Prism (version 9.0). A P-value of < 0.05 is considered statistically significant.
Results
General information
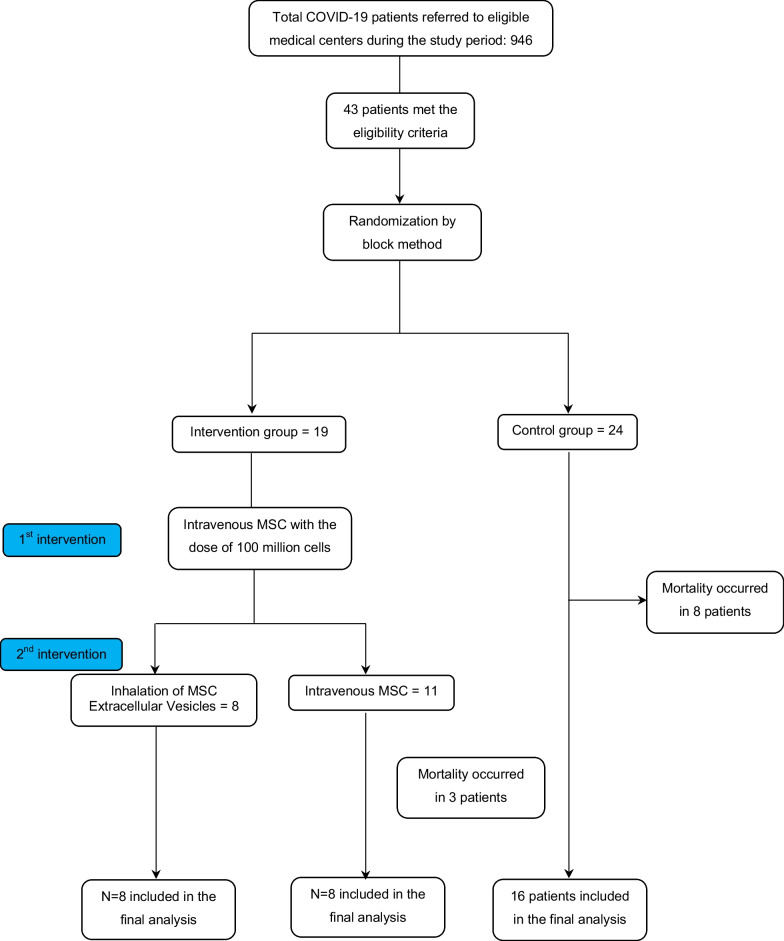
At baseline, 43 patients were enrolled in this study, of whom 24 were randomly placed in the control group, 11 were placed in the MSC alone group, and 8 were placed in the MSC plus EV group. The flowchart of the study is presented in Fig. 1. The mean age of patients was 49.3 ± 10.77 years and 12 of them were female (27.9%). The baseline characteristics of the patients are listed in Table 1. As seen, there were no significant differences, except for O2 saturation (P = 0.017) and having cough (P < 0.001), in the baseline information of the three studied groups.
Fig. 1.
Flowchart of the study
Adverse events
There was no adverse event (AE) or serious adverse event (SAE) linked to either type of intervention. Collectively, mortality occurred in three patients of the intervention groups (15.87%) and eight patients of the control group (33.4%). Mortality was reported in three patients of the MSC alone group (RR: 0.49; 95% CI 0.14–1.11; P = 0.08). There were no mortalities in the MSC plus EV group (RR: 0.08; 95% CI 0.005–1.26; P = 0.07). The causes for mortality were multifactorial and included several causes such as pulmonary dysfunction, multiorgan failure, myocardial infarction, congestive heart failure, septic shock, and acute ischemic cerebral stroke. In fact, it was not possible to find a single reason for the mortality of patients.
Clinical symptoms
In this study, various clinical symptoms were evaluated before and after the intervention. Specifically, symptoms closely associated with ARDS were examined in detail. The cough symptom showed improvement in both intervention groups compared to the control group. Additionally, all patients in the MSC group and nearly 90% of patients in the MSCs plus EVs group and control group had dyspnea at baseline, which decreased to below 10% in each group after the intervention (P = 0.54).
Laboratory parameters
The before-after results of laboratory parameters for three study groups are presented in Table 2. The PTT value decreased significantly in the MSC alone group (P = 0.018). Additionally, O2 saturation decreased significantly in all three groups (P < 0.05). When comparing the three studied groups (see Table 3), the BUN level was significantly decreased in the control group in comparison to the MSC group and MSC plus EV group (P = 0.019).
Table 2.
The characteristics of laboratory parameters after 2nd infusion among the survivors in each group
| Variable | MSC group (n = 8) | P-valuea | MSC + EV group (n = 8) | P-valuea | Control group (n = 16) | P-valuea | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 48 h after 2nd infusion | Baseline | 48 h after 2nd infusion | Baseline | 48 h after 2nd infusion | ||||
| WBC count (× 103) | 10.7 ± 4.6 | 10.8 ± 4.4 | 0.999 | 13.04 ± 9.9 | 12.68 ± 7 | 0.889 | 7.84 ± 3.06 | 8.8 ± 2.7 | 0.6 |
| Lymphocyte (%) | 10.7 ± 3.7 | 12.56 ± 7.05 | 0.612 | 8.17 ± 3.3 | 10 ± 7.2 | 0.999 | 15.8 ± 10.4 | 16.8 ± 13.2 | 0.534 |
| Hemoglobin (mg/dl) | 14.01 ± 2.2 | 13.72 ± 1.46 | 0.674 | 13.2 ± 3.3 | 12.85 ± 7 | 0.726 | 12.5 ± 2.5 | 12 ± 2.3 | 0.033 |
| Platelet count (× 103) | 255.6 ± 115 | 266 ± 94.8 | 0.499 | 215.9 ± 84.5 | 221.6 ± 126.2 | 0.998 | 288.3 ± 214.7 | 285.2 ± 152.9 | 0.625 |
| PT (s) | 12.71 ± 0.52 | 12.92 ± 0.62 | 0.610 | 13.52 ± 1.7 | 14.1 ± 1.7 | 0.779 | 13.7 ± 1.3 | 13.4 ± 0.9 | 0.859 |
| PTT (s) | 43.71 ± 12.6 | 35 ± 7.4 | 0.018 | 35.2 ± 8.5 | 32.4 ± 8.2 | 0.351 | 40.2 ± 7.4 | 36.9 ± 8.5 | 0.137 |
| BUN (mg/dl) | 38.9 ± 15 | 41.9 ± 19.8 | 0.779 | 33.6 ± 14.6 | 45.7 ± 33.3 | 0.128 | 49.2 ± 30.2 | 36.2 ± 16.5 | 0.028 |
| Creatinine (mg/dl) | 1.12 ± 0.23 | 1.06 ± 0.22 | 0.157 | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.786 | 1.2 ± 0.4 | 0.96 ± 0.2 | 0.228 |
| AST (U/L) | 43.12 ± 45.9 | 36.12 ± 18.8 | 0.779 | 37.6 ± 7.02 | 27.2 ± 7.9 | 0.057 | 60.7 ± 59.5 | 59.6 ± 32.9 | 0.575 |
| ALT (U/L) | 54.7 ± 66.9 | 64.5 ± 59.2 | 0.058 | 46.2 ± 33.7 | 40.4 ± 26 | 0.207 | 57.2 ± 53.4 | 58.6 ± 23 | 0.594 |
| Alkp (IU/L) | 172.9 ± 37.5 | 150 ± 35.9 | 0.063 | 135.2 ± 38 | 127.4 ± 45.3 | 0.344 | 197 ± 63.5 | 219.2 ± 92.2 | 0.327 |
| Bilirubin (mg/dl) | 0.76 ± 0.24 | 0.67 ± 0.2 | 0.084 | 0.68 ± 0.3 | 1.03 ± 0.2 | 0.058 | 1.2 ± 1.1 | 1.5 ± 1.4 | 0.270 |
| PH | 7.4 ± 0.07 | 7.4 ± 0.04 | 0.362 | 7.37 ± 0.12 | 7.4 ± 0.07 | 0.916 | 7.4 ± 0.1 | 7.44 ± 0.05 | 0.123 |
| PCO2 (mmHg) | 49.9 ± 10.26 | 46.6 ± 9.2 | 0.263 | 42.8 ± 3.2 | 43.2 ± 7.8 | 0.917 | 48.3 ± 18.7 | 50.3 ± 12.7 | 0.441 |
| HCO3 (meq/L) | 28.8 ± 4.3 | 30.3 ± 3.5 | 0.575 | 30.4 ± 4.7 | 30.4 ± 4.9 | 0.753 | 28.2 ± 8.3 | 33.5 ± 5.6 | 0.058 |
| O2 saturation (%) | 80.8 ± 11.4 | 91.2 ± 7.7 | 0.021 | 75.7 ± 7 | 93.5 ± 5 | 0.027 | 86.7 ± 8.3 | 93.5 ± 3.5 | 0.015 |
| Interleukin-6 (pg/ml) | 209.4 ± 56.4 | 70.9 ± 45 | 0.018 | 207.2 ± 38.9 | 56.7 ± 25.6 | 0.028 | 147.5 ± 56.2 | 105.8 ± 76 | 0.138 |
| TNF alpha (pg/ml) | 28.9 ± 11.5 | 5.72 ± 2.7 | 0.018 | 24.2 ± 10.4 | 5.47 ± 26 | 0.038 | 23.9 ± 10.6 | 14.9 ± 6.7 | 0.043 |
| IFN-gamma (pg/ml) | 121.1 ± 75.8 | 38.6 ± 26.6 | 0.028 | 177.85 ± 53 | 34.2 ± 8.7 | 0.018 | 129 ± 30.9 | 78.2 ± 51.7 | 0.043 |
| CRP (mg/L) | 36.9 ± 15.36 | 29.7 ± 11.5 | 0.02 | 25.3 ± 10.2 | 15.47 ± 5 | 0.024 | 30.2 ± 14.3 | 27.9 ± 13.8 | 0.043 |
aWilcoxon-Signed rank test
Table 3.
Comparing the changes of study variables between three groups
| Variable | Changes in MSC group (n = 8) | Changes in MSC + EV group (n = 8) | Changes in control group (n = 16) | P-valuea |
|---|---|---|---|---|
| WBC count (× 103) | − 0.03 ± 4.21 | − 0.36 ± 8.85 | 0.43 ± 2.26 | 0.726 |
| Lymphocyte (%) | 1.78 ± 4.45 | 1.91 ± 6.93 | 0.97 ± 9.83 | 0.917 |
| Hemoglobin (mg/dl) | − 0.28 ± 1.54 | − 0.3 ± 1.58 | − 0.51 ± 0.9 | 0.828 |
| Platelet count (× 103) | 10.42 ± 68.68 | 5.75 ± 85.51 | − 3.15 ± 125.34 | 0.896 |
| PT (s) | 0.21 ± 0.82 | 0.57 ± 2.71 | − 0.24 ± 1.57 | 0.927 |
| PTT (s) | − 7 ± 8.56 | − 2.71 ± 8.8 | − 3.22 ± 5.56 | 0.971 |
| BUN (mg/dl) | 3 ± 17.77 | 12.14 ± 19.83 | − 13 ± 25.4 | 0.019 |
| Creatinine (mg/dl) | − 0.05 ± 0.09 | 0.03 ± 0.22 | − 0.2 ± 0.51 | 0.530 |
| AST (U/L) | − 7 ± 32.7 | − 10.42 ± 6.67 | − 1.12 ± 45.2 | 0.132 |
| ALT (U/L) | 9.75 ± 12.48 | − 5.71 ± 12.13 | 1.55 ± 49.77 | 0.138 |
| Alkp (IU/L) | − 22.87 ± 23.49 | − 7.83 ± 23.6 | 22.13 ± 67.3 | 0.228 |
| Bilirubin (mg/dl) | − 0.08 ± 0.12 | 0.35 ± 0.37 | 0.3 ± 1.04 | 0.037 |
| PH | 0.02 ± 0.07 | 0.03 ± 0.17 | 0.04 ± 0.08 | 0.670 |
| PCO2 (mmHg) | − 3.27 ± 7.73 | 0.35 ± 7.97 | 2 ± 8.48 | 0.468 |
| HCO3 (meq/L) | 1.5 ± 4.92 | 0.08 ± 8.2 | 5.36 ± 6.57 | 0.259 |
| O2 saturation (%) | 10.36 ± 8.63 | 17.83 ± 10.1 | 6.77 ± 6.96 | 0.07 |
| Interleukin-6 (pg/ml) | − 138.51 ± 55.55 | − 150.41 ± 45.73 | − 19.13 ± 68.31 | 0.015 |
| TNF alpha (pg/ml) | − 23.15 ± 10.95 | − 18.67 ± 8.92 | − 6.12 ± 9.43 | 0.034 |
| IFN-gamma (pg/ml) | − 82.5 ± 69.34 | − 143.73 ± 53.72 | − 43.94 ± 32.33 | 0.024 |
| CRP (mg/L) | − 7.27 ± 5.37 | − 9.83 ± 7.47 | − 2.26 ± 0.94 | 0.041 |
aOne Way Anova test
Inflammatory markers
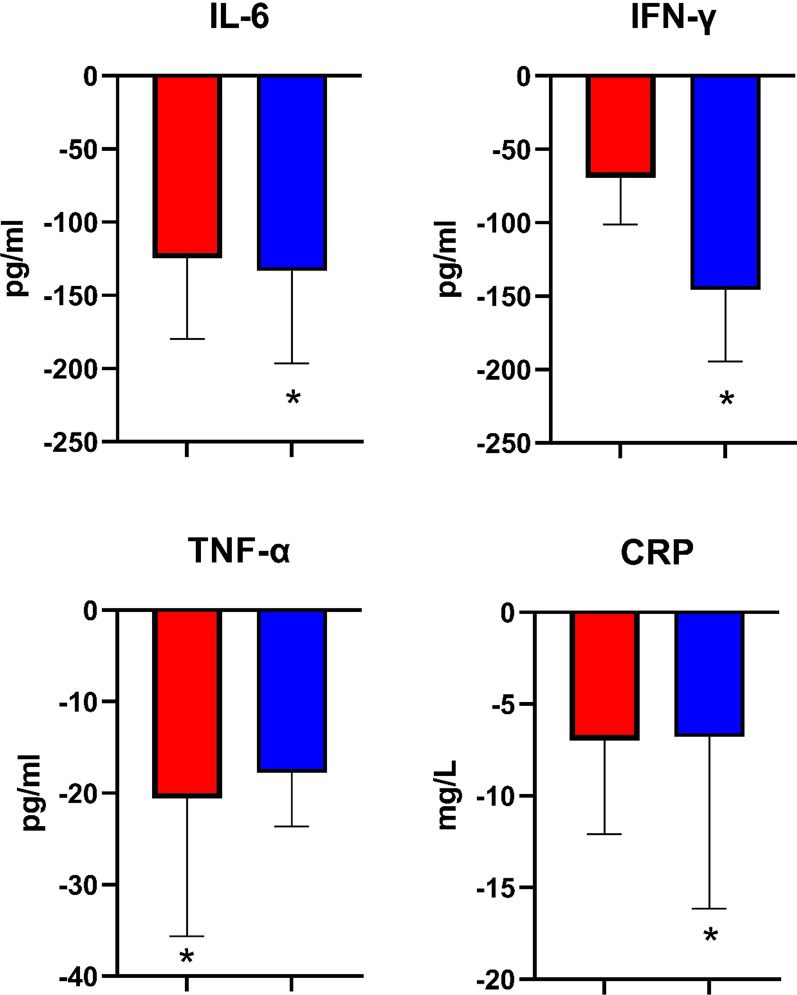
The serum levels of inflammatory markers before and after intervention are presented in Table 2. As shown, the concentration of inflammatory cytokines (IL-6, TNF-α, IFN-γ, and CRP) were significantly reduced in all studied group, except for IL-6 in the control group. However, group analysis (see Table 3) suggested that the cytokine level changes were more prominent in the intervention groups. Specifically, the changes in IL-6 (P = 0.015), IFN-γ (P = 0.024), and CRP (P = 0.041) were more significant in the MSC plus EV group, while TNF-α decreased more significantly in the MSC alone group (P = 0.034) (see Fig. 2).
Fig. 2.
The inflammatory markers change in study groups. Red: MSC group; blue: MSC + EV group. Data is presented based on median and interquartile range and compared to the control group. *Refers to P value < 0.05
Discussion
In this study, we administered allogenic MSCs to COVID-19 patients who were in the progressive phase of ARDS. We observed that systemic administration of MSC and local administration of MSC-EVs in COVID-19 patients is safe, with minimal adverse events. Furthermore, MSCs had significant effects on inflammatory markers compared to the control group.
Evaluating laboratory outcomes of COVID-19 patients after administering MSCs was one of the goals of this study. The unpredictable clinical course of SARS-CoV-2 infection presents a challenging issue in managing COVID-19 [32]. COVID-19 can rapidly progress from a mild/moderate status to a severe condition with irreversible outcomes [33]. Routine laboratory parameters, such as hematologic and biochemical biomarker abnormalities can be used to predict the severity and mortality of COVID-19 patients [34]. Platelet level, WBC count, lymphocytes proportion and hemoglobin concentration have all been found to be linked to the severity of COVID-19 infection [35]. Thrombocytopenia, lymphopenia, and anemia have been associated with the worse clinical outcomes in COVID-19 [36]. Biochemical parameters such as creatinine, urea, lactate dehydrogenase (LDH), liver function tests (LFTs), and creatine kinase (CK) are linked to the severity of COVID-19, and higher values of these parameters reflect a worse outcome [37]. In this study, we suggest that the administration of MSCs alone or with their EVs is not associated with significant alterations in laboratory outcomes. However, it is important to consider that patients in the severe phase of COVID-19 may have several abnormalities in their laboratory results, which may be confounders to these findings.
Assessing the laboratory markers of coagulation was another goal of this study. Multiorgan failure due to cytokine storm is usually reported in severe cases of COVID-19 patients, particularly in patients with ARDS [38]. COVID-19 associated coagulopathy (CAC) is one of the predisposing factors for multiorgan failure, which can affect the outcomes of affected patients [39]. CAC is usually associated with micro- and macro- thrombosis in COVID-19 patients and also increases the risk of disseminated intravascular coagulation (DIC) [40]. In a systematic review by Jenner and colleagues, the incidence of thrombotic events was reported to be almost 34% in ICU hospitalized COVID-19 patients [41]. In addition, a meta-analysis in 2021 suggested that almost 3% of COVID-19 patients may develop DIC and this condition may increase the likelihood of mortality by more than 2.4 times [42]. Therefore, monitoring and diagnosis of CAC is a major challenge in the management of COVID-19 patients [43]. Reports have revealed that COVID-19 patients, particularly with ARDS, may represent hypercoagulability state, i.e., higher levels of D-dimer, mild prolongation of PT and PTT, and thrombocytopenia [44, 45]. We assessed platelet levels, PT, and PTT in our patients, and the results suggest that PTT values were significantly reduced after the administration of systemic MSC alone. This phenomenon could be associated with a lower risk of thrombotic events and DIC in the MSC group compared to the control group. However, to have a clear understanding of the coagulation status, other laboratory parameters such as D-dimer concentration, fibrinogen concentration, and clotting times should also be assessed in future studies [46]. Additionally, MSCs can reverse CAC by affecting the inflammatory phase of the disease [47]. Moreover, endothelial dysfunction, which is commonly seen in COVID-19, can be improved after the administration of MSCs [48]. This improvement plays a key role in preventing coagulopathy and thrombotic disorders [49]. It could be explained as an angiogenesis process that is stimulated by MSC-secreted growth factors, which can enhance the endothelial cell survival rate, support vascular remodeling, and stabilize the endothelial barriers by increasing the expression of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) [50]. However, we observed no significant changes in the coagulopathy parameters in between-group analysis, and further studies are needed to confirm these results.
In this study, we also evaluated the impact of MSCs on inflammatory markers. We observed that MSCs significantly decreased the concentration of inflammatory markers in COVID-19 patients. Cytokine release syndrome (CRS), which is a rigorous systemic inflammatory response, may be responsible for severe COVID-19 pathogenesis and extensive lung damage [51]. Anti-inflammatory agents such as corticosteroids, NSAIDs, monoclonal antibodies against as IL-6 (Tocilizumab), IL-1 (Canakinumab), and TNF-α (Adalimumab and Golimumab), and Interferon-based immunotherapy can be applied to treat COVID-19 patients [52–56]. However, monoclonal antibodies are expensive [57], not accessible in every country [58], and also not free of side effects [59], same as corticosteroids and NSAIDs [60]. MSCs, which have a promising immunomodula-tory role, could be a suitable alternative to these medications since immunomodulation has fewer side effects than immunosuppression. We observed that the levels of inflammatory cytokines, such as IL-6, TNF-α, and IFN-γ, decreased significantly after MSCs infusion. This result was in association with previous studies. In a study in 2020 in China, the administration of allogenic MSCs caused a significant decrement in IL-6 level [61]. In another study, transplantation of MSCs in COVID-19 patients was associated with a significant increase in anti-inflammatory cytokines (IL-10, IL-13) and also a significant decrease in pro-inflammatory cytokines, i.e., IFN-γ, IL-6 [62]. Similar results were observed in other recent studies [63–65]. We also assessed the CRP level after the administration of the MSCs. Current evidence suggests a significant increase (average 20–50 mg/L) in CRP in almost 86% of COVID-19 patients [66]. This marker is assumed as the prognostic factor in mortality and helps in the management and care planning of COVID-19 patients [67]. Moreover, high CRP is linked to severe inflammatory conditions, including major cardiac events and probability of stroke in the COVID-19 patients [68]. As observed in our results, MSCs significantly reduced the CRP level in COVID-19 patients. This result is also in line with previous studies [69, 70]. An interesting finding in this study was the superiority of MSCs plus EVs in comparison to MSCs alone. We observed that MSC plus EV group was more capable to decrease the inflammatory markers (except for TNF-α). In fact, it seems that MSC plus EV can be used as a suitable and accessible approach for relieving the inflammatory cascade in COVID-19 patients. It might be explained as when the MSC-EVs were administered through inhalation, they could suppress the inflammatory cascade in lungs during COVID-19 induced ARDS, locally and effectively. This superiority in decrement of the inflammatory markers was associated with zero number of mortalities in the patients receiving MSC plus EV, compared to MSC alone and control groups.
The decrement of inflammatory cytokines was more pronounced in the case of IL-6, which was reduced by both MSCs and MSCs plus EV administration. However, routine treatment according to national guidelines for COVID-19 did not change IL-6 values in the control group. This finding is particularly valuable considering that IL-6 is a key mediator of cytokine storm and strongly correlates with complicated COVID-19 patients with adverse clinical outcomes [71]. Therefore, clinical procedures that reduce the serum level of IL-6 could be more effective in alleviating disease progression [72].
Our study suggested that the administration of MSCs is safe in COVID-19 patients. We observed lower rates of mortality in intervention groups compared to the control group, although this difference was not significant. Previous studies have also demonstrated no adverse events of MSCs in COVID-19 patients [73, 74]. A study in 2021 reported a significantly lower mortality rate in the MSC-treated group compared to the control group [62]. Additionally, another study also observed a better 28-day survival in COVID-19 patients treated with MSCs [75].
In summary, stem cell therapy, particularly the use of MSCs has emerged as a promising treatment option for severe COVID-19 cases. Several clinical studies have been conducted so far in this regard. Clinical studies have demonstrated that MSC therapy is generally safe and well-tolerated in severe COVID-19 patients, associated with no significant adverse events [76–78]. As mentioned above, MSCs possess potent immunomodulatory properties, which can help regulate the immune response in COVID-19 patients through reducing the cytokine storm and also facilitate tissue repair [79]. Furthermore, MSCs have been shown to exert antiviral effects by producing soluble factors that can inhibit viral replication and promote the clearance of infected cells [80]. This can help reduce the viral load in COVID-19 patients and improve their clinical outcomes. Moreover, several clinical studies have reported improved clinical outcomes in severe COVID-19 patients treated with MSCs [81, 82]. These include reduced mortality rates, shorter hospital stays, and improved lung function. The promising results from early clinical studies have led to an increased interest in the clinical development of stem cell therapy for severe COVID-19. Several clinical trials are currently underway to further evaluate the safety and efficacy of MSCs in treating COVID-19 patients with severe symptoms. If these trials yield positive results, MSC therapy could become an important treatment option for severe COVID-19 cases, particularly for patients who do not respond well to conventional treatments.
This study has certain remarkable advantages. It has one of the largest sample sizes in the national population, providing a suitable view of MSCs administration in Iranian COVID-19 patients. In addition, we assessed basic laboratory parameters and reported their improvement in the MSC treated COVID-19 patients. Another positive aspect of this study is the administration of MSC-EVs through a nebulizing device, which can be an accessible method for COVID-19 patients. However, the current study has some limitations. Many COVID-19 patients with ARDS had been treated with corticosteroids and antiviral drugs before MSCs transfusion, according to the national guideline for treating COVID-19 induced ARDS. This is a confounding variable in assessing the inflammatory markers. Besides, the levels of routine laboratory outcomes in many patients had some abnormality due to the severe condition and we couldn’t assess the exact impact of MSC or MSC-EV on laboratory outcomes. Our medical records did not include any other critical laboratory parameters to include in the study and also, we cannot assess advance laboratory parameters, such as flow cytometric analysis of lymphocytes due to high costs. Furthermore, given the pandemic situation, we could not register their complete laboratory data and this issue was one of the pitfalls of this study. We started the MSC-based at the critical stage of COVID-19 and earlier infusion of MSCs might be associated with better outcomes. Besides, lower sample size of the intervention group was challenging for subgroup analysis. These issues should be addressed in the future studies. In addition, many other molecules and signaling pathways that are essential in the pathogenesis of COVID-19 were not evaluated in this study.
Conclusion
The systemic administration of MSCs and respiratory inhalation of MSC-EVs in COVID-19 patients are safe and associated with improvement in inflammatory markers. The immune modulatory impact of MSCs and MSC-EVs can alleviate cytokine storm and its related consequences in COVID-19 patients. However, further studies with larger sample sizes should be conducted to verify and validate these results.
Acknowledgements
The authors deeply appreciate the efforts by their collaborators at Royan ATMP Technology Development Center (ATMP-TDC), Regenerative Medicine Department at Royan institute, QA, QC, and production departments at Cell Tech Pharmed Co., Royan Stem Cell Technology Co., and Royan Ati Tech Pharmed Co., clinicians and nurses at Shariati and Masih Daneshvari hospitals.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease-2019
- MSCs
Mesenchymal stromal cells
- EV
Extracellular vesicles
- SARS-CoV-2
Severe acute respiratory syndrome-coronavirus-2
- ICU
Intensive care unit
- ACE-2
Angiotensin-converting enzyme-2 receptor
- GRP-78
Glucose-regulated protein-78
- Ang II
Angiotensin II
- IL-6
Interleukin 6
- HRCT
High resolution computed tomography
- SOFA
Sequential organ failure assessment
- GMP
Good manufacturing practice
- CM
Condition medium
- TFF
Tangential flow filtration
- BCA
Bicinchoninic acid
- SDS-PAGE
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- CTCAE
Common terminology criteria for adverse events
- CBC
Complete blood count
- ABG
Arterial blood gas
- AE
Adverse event
- SAE
Serious adverse event
- LDH
Lactate dehydrogenase
- LFT
Liver function tests
- CK
Creatine kinase
- CAC
COVID-19 associated coagulopathy
- DIC
Disseminated intravascular coagulation
- HGF
Hepatocyte growth factor
- VEGF
Vascular endothelial growth factor
- CRS
Cytokine release syndrome
Author contributions
MZ, MAS, MN, FS, and SHE contributed in drafting manuscript, data collection, and performing follow-up of the patients. SMH, RA, HJ, NK, HA, and HM contributed in the patient’s selection, conducting medical practice and follow-up. AN, AA, SNH, FA, NJ, NF, LT, and MG contributed in quality assurance, quality control, and production. EHS, MV, and HB contributed to the study design, analyzing data, editing the manuscript and final approval of the manuscript.
Funding
Grants from the Royesh Venture Capital Fund and CellTech Pharmed supported this work. The funding resources provided cell products and covered the costs of hospitalization and laboratory parameters of patients. The funding sources had no responsibilities in the study design, data collection, analysis, and interpretation, manuscript writing, or in the decision to submit this paper for publication.
Availability of data and materials
The datasets generated and analyzed during the current study are available upon reasonable request from the corresponding author, after obtaining permission from the national institutional review board of COVID-19 in Iran. All materials used in this study are either commercially available or can be obtained from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
We conducted this study in accordance with the Helsinki declaration [83]. The informed consent was received from all patients. The researchers ensured that patient information remained confidential. The study was approved in the ethical committee of the National Institute for Medical Research Development (NIMAD), Tehran, Iran, on March 12th 2020, with the title of “Mesenchymal stem cell therapy for ARDS in Coronavirus infection with two protocols: A randomized, placebo-controlled Phase 1 and 2 clinical trial” and the registration number of IR.NIMAD.REC.1398.412. The registration can be found at https://ethics.research.ac.ir/EthicsProposalView.php?id=124475. Also, the clinical trial protocol was registered by the Iranian Registry of Clinical Trials (IRCT), on April 13th 2020, with the title of “Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome in Coronavirus Infection: A Phase 2–3 Clinical Trial” and trial ID code of IRCT20200217046526N2. The registration can be found at http://www.irct.ir/trial/47073.
Consent for publication
Not applicable.
Competing interests
EHS and MV were collaborating for the industrial manufacturing of the products used in this work, and they declare no financial interest that could have appeared to influence the data reported in this paper. The remaining authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Morteza Zarrabi, Mohammad Amin Shahrbaf, Masoumeh Nouri, Faezeh Shekari and Seyedeh-Esmat Hosseini contributed equally and considered as first authors.
Contributor Information
Ensiyeh Hajizadeh-Saffar, Email: hajizadeh.ehs@gmail.com.
Massoud Vosough, Email: masvos@royaninstitute.org.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezaei FS, Hezavehei M, Shokoohian B, Barekat M, Hosseini R, Ramezankhani R, et al. Metabolic syndrome and COVID-19; clinical complications and challenges. J Isfahan Med Sch. 2021;38(607):1021–1030. [Google Scholar]
- 3.Shahrbaf MA, Hassan M, Vosough M. COVID-19 and hygiene hypothesis: increment of the inflammatory bowel diseases in next generation? Taylor & Francis; 2022. p. 1–3. [DOI] [PubMed]
- 4.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahrbaf MA, Nasr DS, Langroudi ZT. COVID-19 and health promoting hospitals in Iran; what do we stand? Int J Prev Med. 2022;13:125. doi: 10.4103/ijpvm.ijpvm_492_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Coronavirus (COVID-19) Dashboard 7 June 2023 [Available from: https://covid19.who.int/.
- 7.Dries DJ. Coronavirus disease 2019: from intensive care unit to the long Haul-part 2. Air Med J. 2021;40(5):298–302. doi: 10.1016/j.amj.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masoumbeigi H, Mirshafiee A, Ghanizadeh G, Raei M, Saffarri M, Yousefi Arfaei R, et al. Evaluation of the effect of educational interventions on knowledge, attitude, and practice against COVID-19 in a residential complex in tehran: a prospective cross-sectional study. Med J Islam Repub Iran (MJIRI) 2023;37(1):439–445. doi: 10.47176/mjiri.37.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant HA, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahrbaf MA, Tabary M, Khaheshi I. The right ventricle in COVID-19 patients. Oxford University Press; 2021. [DOI] [PMC free article] [PubMed]
- 11.Shahrbaf MA, Tabary M, Khaheshi I. Cardiovascular considerations of Remdesivir and Favipiravir in the treatment of COVID-19. Cardiovasc Haematol Disord Drug Targets. 2021;21(2):88–90. doi: 10.2174/1871529X21666210812103535. [DOI] [PubMed] [Google Scholar]
- 12.Felordi MS, Memarnejadian A, Najimi M, Vosough M. Is there any alternative receptor for SARS-CoV-2? Cell J (Yakhteh) 2021;23(2):247. doi: 10.22074/cellj.2021.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barekat M, Shahrbaf MA, Rahi K, Vosough M. Hypertension in COVID-19, a risk factor for infection or a late consequence? Cell J (Yakhteh) 2022;24(7):424. doi: 10.22074/cellj.2022.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robat-Jazi B, Ghorban K, Gholami M, Samizadeh E, Aghazadeh Z, Shahrbaf MA, et al. β-D-mannuronic acid (M2000) and inflammatory cytokines in COVID-19; an in vitro study. Iran J Allergy Asthma Immunol. 2022;21(6):677–686. doi: 10.18502/ijaai.v21i6.11528. [DOI] [PubMed] [Google Scholar]
- 17.Mortaz E, Bassir A, Dalil Roofchayee N, Dezfuli NK, Jamaati H, Tabarsi P, et al. Serum cytokine levels of COVID-19 patients after 7 days of treatment with Favipiravir or Kaletra. Int Immunopharmacol. 2021;93:107407. doi: 10.1016/j.intimp.2021.107407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. An update to “novel therapeutic approaches for treatment of COVID-19”. J Mol Med. 2021;99(2):303–310. doi: 10.1007/s00109-020-02027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabavi SM, Mehrabani M, Ghalichi L, Nahayati MA, Ghaffari M, Ashtari F, et al. COVID-19 vaccination willingness and acceptability in multiple sclerosis patients: a cross sectional study in Iran. Vaccines. 2022;10(1):135. doi: 10.3390/vaccines10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID-19. J Mol Med. 2020;98(6):789–803. doi: 10.1007/s00109-020-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramezankhani R, Solhi R, Memarnejadian A, Nami F, Hashemian SMR, Tricot T, et al. Therapeutic modalities and novel approaches in regenerative medicine for COVID-19. Int J Antimicrob Agents. 2020;56(6):106208. doi: 10.1016/j.ijantimicag.2020.106208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghasemzad M, Hashemian SMR, Memarnejadian A, Akbarzadeh I, Hossein-Khannazer N, Vosough M. The nano-based theranostics for respiratory complications of COVID-19. Drug Dev Ind Pharm. 2021;47(9):1353–1361. doi: 10.1080/03639045.2021.1994989. [DOI] [PubMed] [Google Scholar]
- 24.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A Double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng FP, Xu RN, Wang SY, Xu Z, Zhang C, Li YY, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambrano GMT, Ahmed YM, Carmenate YV, Elsadawy ME. Stem cell nebulization therapy for COVID-19 infection: radiological and clinical outcomes. Egypt J Radiol Nucl Med. 2021;52(1):115. doi: 10.1186/s43055-021-00492-3. [DOI] [Google Scholar]
- 27.Hashemian S-MR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini S-E, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Li X, Yang L. Mesenchymal stem cells and their derived small extracellular vesicles for COVID-19 treatment. Stem Cell Res Ther. 2022;13(1):410. doi: 10.1186/s13287-022-03034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5(3):326–334. doi: 10.3978/j.issn.2072-1439.2013.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller TP, Fisher BT, Getz KD, Sack L, Razzaghi H, Seif AE, et al. Unintended consequences of evolution of the common terminology criteria for adverse events. Pediatr Blood Cancer. 2019;66(7):e27747. doi: 10.1002/pbc.27747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold DT, Attwood M, Barratt S, Morley A, Elvers KT, McKernon J, et al. Predicting outcomes of COVID-19 from admission biomarkers: a prospective UK cohort study. Emerg Med J. 2021;38(7):543–548. doi: 10.1136/emermed-2020-210380. [DOI] [PubMed] [Google Scholar]
- 33.Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from Coronavirus disease 2019 (COVID-19) infection. Ann Acad Med Singap. 2020;49(3):108–118. doi: 10.47102/annals-acadmedsg.202057. [DOI] [PubMed] [Google Scholar]
- 34.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 35.Urbano M, Costa E, Geraldes C. Hematological changes in SARS-COV-2 positive patients. Hematol Transfus Cell Ther. 2022;44(2):218–224. doi: 10.1016/j.htct.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdullah I, Cornelissen HM, Musekwa E, Zemlin A, Jalavu T, Mashigo N, et al. Hematological findings in adult patients with SARS CoV-2 infection at Tygerberg Hospital Cape Town South Africa. Health Sci Rep. 2022;5(3):e550. doi: 10.1002/hsr2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mir SM, Tahamtan A, Nikoo HR, Arabi MS, Moradi AW, Ardakanian S, et al. Evaluation of biochemical characteristics of 183 COVID-19 patients: A retrospective study. Gene Rep. 2022;26:101448. doi: 10.1016/j.genrep.2021.101448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin S, Jin Y, Xu B, Hong J, Yang X. Prevalence and impact of coagulation dysfunction in COVID-19 in China: a meta-analysis. Thromb Haemost. 2020;120(11):1524–1535. doi: 10.1055/s-0040-1714369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenner WJ, Kanji R, Mirsadraee S, Gue YX, Price S, Prasad S, et al. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;51(3):595–607. doi: 10.1007/s11239-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Cheng Z, Luo L, Zhu Y, Lin W, Ming Z, et al. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb Res. 2021;201:23–29. doi: 10.1016/j.thromres.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsay PJ, Rosovsky R, Bittner EA, Chang MG. Nuts and bolts of COVID-19 associated coagulopathy: the essentials for management and treatment. Postgrad Med. 2021;133(8):899–911. doi: 10.1080/00325481.2021.1974212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aggarwal M, Dass J, Mahapatra M. Hemostatic abnormalities in COVID-19: an update. Indian J Hematol Blood Transfus. 2020;36(4):1–11. doi: 10.1007/s12288-020-01328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan BE, Ng J, Chan SSW, Christopher D, Tso ACY, Ling LM, et al. COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J Thromb Thrombolysis. 2021;51(3):663–674. doi: 10.1007/s11239-020-02318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin C, Chen Y, Chen B, Zheng K, Luo X, Lin F. D-dimer combined with fibrinogen predicts the risk of venous thrombosis in fracture patients. Emerg Med Int. 2020;2020:1930405. doi: 10.1155/2020/1930405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahani P, Datta I. Mesenchymal stromal cell therapy for coronavirus disease 2019: Which? When? And how much? Cytotherapy. 2021;23(10):861–873. doi: 10.1016/j.jcyt.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu SW, Ilyas I, Weng JP. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44(4):695–709. doi: 10.1038/s41401-022-00998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruhl L, Pink I, Kühne JF, Beushausen K, Keil J, Christoph S, et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct Target Ther. 2021;6(1):418. doi: 10.1038/s41392-021-00819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paris GC, Azevedo AA, Ferreira AL, Azevedo YMA, Rainho MA, Oliveira GP, et al. Therapeutic potential of mesenchymal stem cells in multiple organs affected by COVID-19. Life Sci. 2021;278:119510. doi: 10.1016/j.lfs.2021.119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darif D, Hammi I, Kihel A, El Idrissi SI, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb Pathog. 2021;153:104799. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katia F, Myriam DP, Ucciferri C, Auricchio A, Di Nicola M, Marchioni M, et al. Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun Inflamm Dis. 2021;9(2):399–405. doi: 10.1002/iid3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fakharian A, Barati S, Mirenayat M, Rezaei M, Haseli S, Torkaman P, et al. Evaluation of adalimumab effects in managing severe cases of COVID-19: a randomized controlled trial. Int Immunopharmacol. 2021;99:107961. doi: 10.1016/j.intimp.2021.107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore N, Bosco-Levy P, Thurin N, Blin P, Droz-Perroteau C. NSAIDs and COVID-19: a systematic review and meta-analysis. Drug Saf. 2021;44(9):929–938. doi: 10.1007/s40264-021-01089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–45.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubin R. Monoclonal antibodies for COVID-19 preexposure prophylaxis can’t come fast enough for some people. JAMA. 2021;326(19):1895–1897. doi: 10.1001/jama.2021.19534. [DOI] [PubMed] [Google Scholar]
- 58.Elgundi Z, Reslan M, Cruz E, Sifniotis V, Kayser V. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev. 2017;122:2–19. doi: 10.1016/j.addr.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 60.Mattos-Silva P, Felix NS, Silva PL, Robba C, Battaglini D, Pelosi P, et al. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492. doi: 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transpl. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10(9):1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Şahin AS, Kaya E, Turgut G, Dolay K, Kocataş A. Mesenchymal stem cell therapy in COVID-19 pneumonia: a prospective, randomized clinical research. Turk Klin J Med Sci. 2022;42(1):5–13. [Google Scholar]
- 65.Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, Stimamiglio MA, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13(1):1–22. doi: 10.1186/s13287-022-02796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92(11):2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stringer D, Braude P, Myint PK, Evans L, Collins JT, Verduri A, et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol. 2021;50(2):420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luan Y-Y, Yin C-H, Yao Y-M. Update advances on C-reactive protein in COVID-19 and other viral infections. Front Immunol. 2021;12:3153. doi: 10.3389/fimmu.2021.720363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu R, Yan T, Feng Y, Liu Y, Cao H, Peng G, et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021;31(12):1244–1262. doi: 10.1038/s41422-021-00573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasamineh S, Kalajahi HG, Yasamineh P, Gholizadeh O, Youshanlouei HR, Matloub SK, et al. Spotlight on therapeutic efficiency of mesenchymal stem cells in viral infections with a focus on COVID-19. Stem Cell Res Ther. 2022;13(1):257. doi: 10.1186/s13287-022-02944-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vatansever HS, Becer E. Relationship between IL-6 and COVID-19: to be considered during treatment. Futur Virol. 2020;15(12):817–822. doi: 10.2217/fvl-2020-0168. [DOI] [Google Scholar]
- 73.Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei FT, Kong DX, Li T, Li A, Tan Y, Fang JF, et al. Efficacy and safety of umbilical cord mesenchymal stem cells for the treatment of patients with COVID-19. Clinics. 2021;76. [DOI] [PMC free article] [PubMed]
- 75.Fathi-Kazerooni M, Fattah-Ghazi S, Darzi M, Makarem J, Nasiri R, Salahshour F, et al. Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID-19 patients: clinical trial phase I & II. Stem Cell Res Ther. 2022;13(1):96. doi: 10.1186/s13287-022-02771-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi L, Wang L, Xu R, Zhang C, Xie Y, Liu K, et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther. 2021;6(1):339. doi: 10.1038/s41392-021-00754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu R, Feng Z, Wang F-S. Mesenchymal stem cell treatment for COVID-19. EBioMedicine. 2022;77:103920. doi: 10.1016/j.ebiom.2022.103920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao W, Shi L, Zhang Y, Dong H, Zhang Y. Mesenchymal stem/stromal cell therapy for COVID-19 pneumonia: potential mechanisms, current clinical evidence, and future perspectives. Stem Cell Res Ther. 2022;13(1):124. doi: 10.1186/s13287-022-02810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jayaramayya K, Mahalaxmi I, Subramaniam MD, Raj N, Dayem AA, Lim KM, et al. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep. 2020;53(8):400–412. doi: 10.5483/BMBRep.2020.53.8.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rocha JLM, de Oliveira WCF, Noronha NC, Dos Santos NCD, Covas DT, Picanço-Castro V, et al. Mesenchymal stromal cells in viral infections: implications for COVID-19. Stem Cell Rev Rep. 2021;17(1):71–93. doi: 10.1007/s12015-020-10032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grumet M, Sherman J, Dorf BS. Efficacy of MSC in patients with severe COVID-19: analysis of the literature and a case study. Stem Cells Transl Med. 2022;11(11):1103–1112. doi: 10.1093/stcltm/szac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossello-Gelabert M, Gonzalez-Pujana A, Igartua M, Santos-Vizcaino E, Hernandez RM. Clinical progress in MSC-based therapies for the management of severe COVID-19. Cytokine Growth Factor Rev. 2022;68:25–36. doi: 10.1016/j.cytogfr.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191-4. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available upon reasonable request from the corresponding author, after obtaining permission from the national institutional review board of COVID-19 in Iran. All materials used in this study are either commercially available or can be obtained from the corresponding author upon reasonable request.