Abstract
Traumatic brain injury (TBI) continues to be a major cause of death and disability worldwide. This study assessed the effectiveness of non-invasive vagus nerve stimulation (nVNS) in reducing brain lesion volume and improving neurobehavioral performance in a rat model of TBI. Animals were randomized into three experimental groups: (1) TBI with sham stimulation treatment (Control), (2) TBI treated with five lower doses (2-min) nVNS, and (3) TBI treated with five higher doses (2 × 2-min) nVNS. We used the gammaCore nVNS device to deliver stimulations. Magnetic resonance imaging studies were performed 1 and 7 days post-injury to confirm lesion volume. We observed smaller brain lesion volume in the lower dose nVNS group compared with the control group on days 1 and 7. The lesion volume for the higher dose nVNS group was significantly smaller than either the lower dose nVNS or the control groups on days 1 and 7 post-injury. The apparent diffusion coefficient differences between the ipsilateral and contralateral hemispheres on day 1 were significantly smaller for the higher dose (2 × 2 min) nVNS group than for the control group. Voxel-based morphometry analysis revealed an increase in the ipsilateral cortical volume in the control group caused by tissue deformation and swelling. On day 1, these abnormal volume changes were 13% and 55% smaller in the lower dose and higher dose nVNS groups, respectively, compared with the control group. By day 7, nVNS dampened cortical volume loss by 35% and 89% in the lower dose and higher dose nVNS groups, respectively, compared with the control group. Rotarod, beam walking, and anxiety performances were significantly improved in the higher-dose nVNS group on day 1 compared with the control group. The anxiety indices were also improved on day 7 post-injury compared with the control and the lower-dose nVNS groups. In conclusion, the higher dose nVNS (five 2 × 2-min stimulations) reduced brain lesion volume to a level that further refined the role of nVNS therapy for the acute treatment of TBI. Should nVNS prove effective in additional pre-clinical TBI models and later in clinical settings, it would have an enormous impact on the clinical practice of TBI in both civilian and military settings, as it can easily be adopted into routine clinical practice.
Keywords: brain lesion volume, magnetic resonance imaging, neurobehavioral outcomes, neuromodulation, traumatic brain injury, vagus nerve stimulation
Introduction
Traumatic brain injury (TBI) continues to be a significant cause of death and disability worldwide. The Centers for Disease Control estimates that ∼2,500,000 people sustain a TBI each year, resulting in 283,000 hospitalizations and 52,000 deaths with annual direct and indirect costs of $56 billion.1,2 It is estimated that 3,200,000 people in the United States live with long-term disabilities attributable to TBI, which is roughly 1.1% of the population.3 The pathogenesis of TBI triggers immune reactions driven by microgliosis and infiltrating peripheral monocyte-derived macrophages into the central nervous system, which leads to neuroinflammation and subsequent cell death.1 TBI-induced neuroinflammation has attracted significant attention as a potential target for therapy.1,4 Although the underlying pathophysiology of TBI has been extensively studied, no therapy has been able to significantly improve clinical outcomes. Therefore, developing novel and effective neuroprotective therapeutic strategies is a high priority.
Neuromodulation is a therapeutic technique that excites or inhibits neuronal activity by electrical stimulation of the nervous system. Vagus nerve stimulation (VNS) is an established form of neuromodulation primarily for treating refractory epilepsy, depression, neurorehabilitation applications, and, more recently, cluster and migraine headaches. It facilitates cerebral adrenergic and cholinergic neurotransmission, and increases neurotrophic factor and fibroblast growth factor gene expression, as well as norepinephrine concentration in the hippocampus and cerebral cortex.5–7 It is available in both implantable VNS (iVNS) and non-invasive VNS (nVNS) forms. VNS has been investigated as a treatment for TBI.8–12 iVNS therapy has been shown to improve motor and cognitive outcomes and reduce secondary neuronal damage.10,12–15 The use of iVNS and nVNS therapies in pre-clinical models has resulted in the reduction of nuclear factor kappa B (NF-κB)/NLRP3 inflammasome activation, suppression of proinflammatory cytokines, increased ghrelin release, reduction in matrix metalloproteinase 2 and 9, improvement in blood–brain barrier (BBB) function and reduction of brain edema, suppression of cyclooxygenase (COX)-2 upregulation in the cerebral cortex, and inhibition of cortical spreading depression.9,10,12,13,16–24 In this study, we assessed the efficacy of nVNS in a hyperacute setting using a controlled cortical impact (CCI) injury model in male Wistar rats. The study's primary hypothesis was that nVNS treatment may reduce brain lesion volume leading to improved functional and cognitive outcomes.
Methods
The study was approved and conducted according to the guidelines set by the Institutional Animal Care and Use Committee at the University of New Mexico. The results are reported based on the Animal Research: Reporting of In Vivo Experiments (ARRIVE) 2.0 Guidelines for Reporting Animal Research (Supplementary Checklist).25 Male Wistar rats (Charles River Laboratories, Wilmington, MA, USA) were used for the experiment. They were maintained under standard conditions (25 ± 2°C, humidity of 50 ± 6%, 12 h light/dark cycle) with unlimited access to standard food and water for at least 1 week, for acclimatization, prior to the neurobehavioral training. Prior to inducing TBI, animals were randomly assigned to three study groups: (1) control (TBI with sham stimulation treatment), (2) lower dose nVNS (TBI with five 2-min doses), and (3) higher dose nVNS (TBI with five 2 × 2-min [4 min] doses). For randomization, we used a publicly available algorithm (GraphPad Software Inc., La Jolla, CA, USA https://www.graphpad.com/quickcalcs/randomize1.cfm).
Induction of TBI
Rats were anesthetized with 3% isoflurane in an induction chamber and were maintained during the procedure with 2.0–2.5% isoflurane. Rats in each studied group were kept under anesthesia for the same duration based on the most prolonged required anesthesia (90 min post-injury) to avoid study bias. Buprenorphine (0.03 mg/kg), meloxicam (2 mg/kg), and 5 mL of normal saline were administered subcutaneously (SC) shortly before the injury to alleviate pain and dehydration. Prior to surgery, the scalp and neck areas were shaved. We used a standard CCI model, as previously described.26–30 Briefly, animals were placed in a standard rodent stereotaxic frame. The scalp was exposed, and the skull's fascia was retracted laterally to each side. Craniotomy was performed over the left motor cortex area using a micro drill with a 5-mm trephine. Next, a CCI device (Impact one, Leica Microsystem, Buffalo Grove, IL, USA) with a 3-mm flat impactor tip was centered at 2.5 mm lateral and 3.0 mm posterior from bregma to induce a moderate injury with the following parameters: 5 m/sec speed, 100 ms dwell time, and 2 mm deformation depth. After the injury was induced, the skin was sutured closed without replacing the skull flap. As part of post-operative care, administration of meloxicam (once daily) continued for 3 days to alleviate any possible pain and discomfort.
nVNS therapy protocol
A modified version of the commercial gammaCore (electroCore, Inc., NJ, USA) with miniaturized electrodes affixed to a Velcro collar was used to deliver non-invasive electrical stimulations (24 V, 60 mA, and 1 ms duration bursts of 5 kHz sinewaves, repeated at 25 Hz), see Figures 1 A and B. We initiated nVNS therapy 30 min post-TBI with five nVNS treatments at the highest intensity/amplitude (24 V) with two treatment durations (lower dose [five 2-min] or higher dose [five 2 × 2-min], 10 min apart); see Figure 1C. The device was attached but not turned on for the control group. The gammaCore device is factory programmed by the manufacturer to automatically deliver 2 min of stimulation. Therefore, for a 2 × 2-min stimulation, the device was turned on again within 15–30 sec to deliver the second 2-min stimulation. At the completion of the nVNS therapy, the rat was moved to an intensive care system for recovery from anesthesia (Water Jacketed Warmer ICU Base with Clear Dome Cover, DW-1, ThermoCare, Sparks, NV, USA).
FIG. 1.
Experiment setup configuration. (A) Modified gammaCore device for rat studies, (B) Miniaturized electrodes, (C) the non-invasive vagus nerve stimulation (nVNS) protocols used for the experiment: lower dose (five 2-min) stimulations and higher dose (five 2 × 2-min) stimulations. There are 10 min intervals between each stimulation, (D) Modified beam-walking apparatus (MBW), and (E) Standard elevated plus maze (EPM) apparatus. Color image is available online.
Motor function assessments
The neurobehavioral assessments were conducted by an investigator (H.A.I.) blinded to the treatment groups. Motor function training and assessment procedures used in our laboratory have been described previously.31–34 Rats were trained on the beam walking (see Fig. 1D) and rotarod apparatuses for 5 days prior to the injury. Assessments were performed on days 1 and 7 post-TBI. For the beam walking, the time to cross the 1-m distance, number of footfalls, and falling from the beam (if applicable) were recorded and scored. For post-TBI assessments, an arbitrary 15 sec was assigned to the crossing time if the rats fell from the beam before completing the task.
Balance and motor function were also assessed using a rotarod test (Rotamex-5; Columbus Instruments, Columbus, OH).31–34 The rats were trained on the apparatus until they could hold their balance on the rotating rod for 120 sec in multiple trials with a rotational speed between 0 and 20 rpm and an acceleration of 2 rpm every 4 sec (cutoff time at 120 sec). For post-TBI assessments, the assessment continued until the cutoff time of 120 sec or until the animal fell, where the latency to falling was recorded. Some of the rats would not stay on the rotating rod for 120 sec, which may have been the result of increased anxiety and not the motor deficit. Therefore, rats were given two trials, 30 min apart, for post-TBI assessments. We used the best time from the two trials as the result.
Anxiety assessments
An elevated plus maze (EPM) was used to assess anxiety in animals before and after TBI, see Figure 1E.32–34 The 5-min follow-up assessments were videotaped on days 1 and 7 post-TBI. We used the EPM anxiety index ranging from 0 to 1, where an increased value expresses increased anxiety-like behavior:32–34
To assess anxiety, we previously reported a novel paradigm using a modified beam walking (MBW) test that can provide information on anxiety level changes in rodents, which can potentially be a substitute for the EPM.34 For evaluation of anxiety on MBW, we defined two behaviors: peeking and full-body emergence from the safe box during a 5-min recording from the time the rats crosses the beam and enters the safe box on the apparatus. To obtain a single value that integrates anxiety-related behaviors on the MBW, a novel MBW anxiety index value (modified from our previously reported equation34) was calculated and ranged on a scale from 0 to 1 (mimicking the EPM anxiety index range), where total time on MBW refers to 5 min:
Magnetic resonance imaging (MRI) studies
MRI studies were conducted by the investigators (C.M.W., E.T., and Y.Y.) blinded to the treatment groups. The rats, anesthetized with 2.5% isoflurane, were placed in a dedicated holder and positioned in the isocenter of a 7-T MRI scanner with a 30-cm bore (Bruker Corporation, Billerica, MA, USA) equipped with a 20-cm gradient with a strength of 660 mT/m and shim systems.
MRIs of the rats with T2-weighted (T2W) and diffusion-weighted imaging (DWI) were conducted on days 1 and 7 post-TBI, along with additional baseline MRI conducted in 7 intact normal rats for comparison. A fast spin-echo sequence (i.e., rapid acquisition with relaxation enhancement [RARE]) was used to acquire T2W MRI with the following parameters: repetition time (TR)/echo time (TE) = 5000 ms/50 ms, field of view (FOV) = 40 mm × 40 mm, slice thickness = 0.5 mm, inter-slice thickness = 0 mm, number of slices = 32, matrix = 256 × 256, number of averages = 4. Those parameters provided high resolution and high signal-to-noise ratio imaging.
DWI was implemented based on echo planar imaging (EPI) with parameters: TR/TE = 3000 ms/20 ms, FOV = 40 mm × 40 mm, slice thickness = 1 mm, inter-slice distance = 0 mm, number of slices = 16, matrix = 128 × 128, number of averages = 4. An apparent diffusion coefficient (ADC) map was generated in Paravision 6 (Bruker Corporation).
Brain lesion analysis
We used medical image processing, analysis, and visualization (MIPAV, National Institute of Health) software for performing planimetry measurements, as reported previously.35–39 Briefly, the lesion comprising the hyperintense region (caused by edema) and tissue rupture/swelling observed on each MRI slice was outlined and multiplied by the slice thickness (0.5 mm). The total lesion volume was the sum of the volumes for each slice.
Analysis of lesion ADC was performed using Vivoquant software (version 2021, Invicro, Needham, MA, USA). ADC maps (voxel size: 0.31 × 0.31 × 1.00 mm, dimensions: 128 × 128 × 16 mm) were re-sampled to match the structural T2 image voxel size (0.16 × 0.16 × 0.50 mm) and image dimensions (256 × 256 × 32 mm). Volumes-of-interest (VOIs) were drawn on the transverse view of the structural T2 image and applied to the co-registered ADC maps. Specifically, a two-dimensional (2D) circular region of interest (ROI) was placed within the ipsilateral cortical lesion on the slice of maximal injury and on two adjacent slices to obtain a lesion VOI. The same procedure was performed in the contralateral cortex to obtain a normal tissue VOI. The ipsilateral lesion and contralateral cortex VOIs were equal in volume (2.23 mm3). ADC values were determined as the % difference between the ipsilateral and the contralateral cortical tissues.
Brain voxel-based morphometry
Voxel-based brain volumetric changes were assessed using the T2W images with Advanced Normalization Tools in Python (ANTsPy)40 and the FMRIB Software Library (FSL, Oxford, UK41). Brain scans were exported from Bruker to NIFTI.42 Initial bias-field correction was performed, followed by skull stripping using a 2D U-Net deep learning method,43 a manual skull contour correction where necessary, and a second bias-field correction. As previously published by Pagani and coworkers,44 voxel-based morphometry (VBM) analysis was performed following skull stripping. Briefly, a template from seven intact rats was created in ANTsPy, whereby individual brains of each subject were then non-linearly co-registered, and the Jacobian determinant map quantifying the amplitude of the local brain tissue shrinkage or expansion was determined. VBM was then performed using the FSL “Randomise” tool for non-parametric permutation inference on neuroimaging data with one-way between-subjects analysis of variance (ANOVA) and threshold-free cluster enhancement for image-based p value determination. For better 2D visualization, projections from four MRI slices were created as either the mean with Jacobian determinants in the range of -0.4 to +0.4 for each studied group or the maximum projection of the image-based ANOVA p value (threshold as 1- p < 0.05), with this overlaid onto the gray scale T2W MRI intact baseline template, unless otherwise indicated.
Histopathological assessments
Histopathological studies were conducted by the investigators (O.A.B. and K.S.S) blinded to the treatment groups. Rats were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS; their brains were harvested, fixed for 24 h in 4% PFA in PBS, cryoprotected in 30% sucrose in PBS, and frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) using dry ice for hematoxylin and eosin (H&E) staining. Sample rat brains from each treatment group were sectioned coronally using cryostat Leica CM1850 (Leica Biosystems, Wetzlar, Germany). Tissue morphology was displayed by H&E staining protocols (Anatech LTD Harris hematoxylin and eosin-Y) for 10 μm sections. Images were taken using NanoZoomer 2.0-HT slide scanner and analyzed using Hamamatsu NDP.view2 software (Hamamatsu Photonics K.K., Hamamatsu City, Japan).
Statistical analysis
Median and inter-quartile ranges (IQR) were used to present weight, lesion volumes, and neurobehavioral results. We used the Shapiro–Wilk test for normality, which has been recognized as the most powerful and commonly used normality test.45 Kruskal–Wallis tests were used for overall inter-group differences at each time point (day 1 and day 7), followed by post-hoc Conover tests for paired group comparisons. Effect sizes were measured for the lesion volumes using the common Cohen's d (with pooled standard deviation [SD]). Following the common interpretation, Cohen's d cutoff values 0.20, 0.50, 0.80, and 1.2 were chosen for interpreting small, medium, large, or very large effect sizes, respectively. Multivariate days 1 and 7 treatment group effects for lesion volumes, neurobehavioral test performances, and anxiety indices were assessed using repeated-measures ANOVA. The Spearman Rho correlations were computed between lesion volumes and anxiety indices. A partial linear (Pearson) correlation was computed to assess the correlation between the two anxiety indices while controlling for the effect of the lesion volume. The effect of the treatment group on the footfall incidence for the MBW test was tested with Mann–Whitney tests and a Poisson regression model, assuming a Poisson distribution for the fall count data. The statistical analysis was performed with a custom R code and MedCalc (v20.014).
For the ADC map % difference values, two outliers (far out values according to Tukey definition46) in the control group and one outlier in the lower dose nVNS group for day 7 were removed. These outliers were also removed for day 1 for applying the Wilcoxon paired tests between days 1 and 7. Intergroup differences for ADC % difference values on day 1 and day 7 were tested with Mann–Whitney tests (independent samples). The homogeneity of variances among the three rat groups was tested using the Levene test. For VBM statistics, the FSL Randomize function with one-way between-subjects ANOVA and threshold-free cluster enhancement for image-based p value determination was used. A p value of 0.05 was considered statistically significant.
Results
Male Wistar rats (n = 42, median weight = 386 g [IQR: 369–416], median age = 12.43 weeks [IQR: 11.7–13.29]) were allocated to three study groups: the control group (n = 14), the lower dose nVNS group (n = 14), and the higher dose nVNS (n = 14).
Brain lesion volume measured from MRI on days 1 and 7 post-injury
Sample T2-weighted images obtained on days 1 and 7 post-injury for the studied groups are shown in Figure 2. The median lesion volumes on day 1 post-injury were 30.10 mL (IQR: 19.31–35.75 mL) for the control (n = 14), 23.36 mL (IQR: 16.85–25.37 mL) for the lower dose nVNS (n = 13), and 5.30 mL (IQR: 3.0–7.24 mL) for the higher dose nVNS (n = 14) groups. The overall difference in the brain lesion volume (log10) between the treatment groups was significant (Kruskal–Wallis tests 25.58, 2 df, p < 0.0001). The post-hoc Conover tests also showed significantly reduced brain lesion volume: (1) for the lower dose nVNS group compared with the control group (p = 0.0221), (2) for the higher dose nVNS group compared with the control group (p < 0.0001), and (3) for the higher dose nVNS group compared with the lower dose nVNS group (p < 0.0001). The effect sizes (Cohen's d statistics) on day 1 for the difference in mean brain lesion volumes between the lower dose nVNS group and the control group was -0.60 (95% confidence interval [CI]: [-1.15, 0.06]) and between the higher dose nVNS group and the control group -3.23 (95% CI: [-4.37, -1.83]), indicating a medium and a very large lesion volume reduction for the lower dose nVNS and the higher dose nVNS treatment groups, respectively.
FIG. 2.
Sample T2-weighted images. Panels A & B are the control group on days 1 and 7, respectively. Panels C & D are the lower dose (2-min) non-invasive vagus nerve stimulation (nVNS) group on days 1 and 7, respectively. Panels E & F are the higher dose (2 × 2-min) nVNS group on days 1 and 7, respectively. Arrows point to the site of the injury. Panel G shows mean ± standard error of the mean (SEM) of brain lesion volume for the studied group on days 1 and 7 post-injury. Significant p values from the post-hoc Conover tests are shown for each time point.
Similarly, on day 7 post-injury, the median volume was 22.16 mL (IQR: 16.59–25.43 mL) for the control (n = 13), 9.34 mL (IQR: 6.20–10.52 mL) for the lower dose nVNS (n = 13), and 3.53 mL (IQR: 2.36–4.99 mL) for the higher dose nVNS (n = 14) groups. The overall difference in the lesion volume between the treatment groups was highly significant (Kruskal–Wallis tests 23.85, 2 df, p < 0.0001). The post-hoc conover tests also showed significantly reduced lesion volume (1) for the lower dose nVNS group compared with the control group (p = 0.0185), (2) for the higher dose nVNS group compared with the control group (p < 0.0001), and (3) for the higher dose nVNS group compared with the lower dose nVNS group (p < 0.0001). The effect size (Cohen's d) on day 7 for the difference in mean lesion volume between the lower dose nVNS and the control groups was -1.09 (95% CI: [-2.28, -0.08]) and -2.49 (95% CI: [-3.48, -1.39]) between the lower dose nVNS and the control groups, indicating a large and a very large lesion volume reduction for the lower dose nVNS and the higher dose nVNS treatment arms respectively.
Overall brain lesion volume results (time × treatment effect)
The effect of time (day 1 vs. day 7) and treatment on the lesion volume (log-transformed) were jointly tested with repeated measures ANOVA (n = 40, Control = 13, lower dose nVNS = 13, and higher dose nVNS = 14). There was a significant effect of the between-subject factor “treatment group” on the lesion volume (F1,37 = 42.80, p < 0.001) and a significant effect of “time” (within-subject factor) on the lesion volume (F1,37 = 32.27, p < 0.001), showing an overall lesion volume reduction between day 1 and day 7 and an early (day 1) volume reduction for the higher dose nVNS group (see Fig. 2G). Distinct from lesion volume, in MRI sequences we frequently observed lesion extension into the ipsilateral hippocampus and, to some extent, into the thalamus regions in the control group that were not evident in the higher dose nVNS group.
Histopathological observations
Brain samples from 7 days post-TBI stained with H&E are shown in Figure 3. The injury involved the somatosensory cortex. The observed injuries on H&E slides for the treatment groups confirmed brain damage observed on MRI.
FIG. 3.
Sample hematoxylin and eosin (H&E) staining shows the damaged area on the somatosensory cortex on day 7. (A) Control, (B) lower dose (2-min) non-invasive vagus nerve stimulation (nVNS), and (C) higher dose (2 × 2-min) nVNS. The H&E slides confirm the level of damage observed on the magnetic resonance image (MRI). Arrows point to the site of the injury. Color image is available online.
ADC map percent-difference values
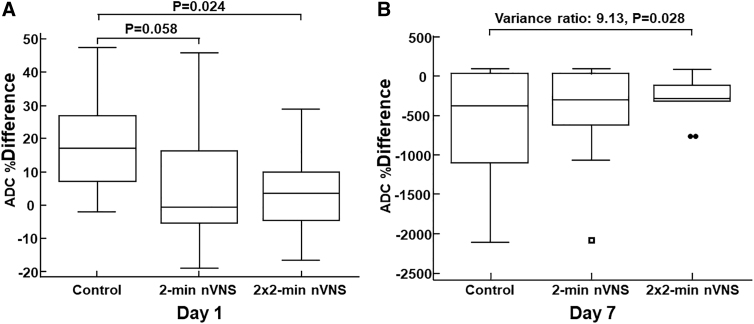
On day 1, the median ADC % difference was 17.22% (IQR: 7.33–26.84%) for the control group (n = 14), -0.48% (IQR: -5.40–16.50%) for the lower dose nVNS group (n = 13) and 3.62% (IQR: -4.63–10.0%) for the higher dose nVNS group (n = 14). The ADC % difference on day 1 was significantly smaller for the higher dose (2 × 2-min) nVNS group than for the control group (Mann–Whitney test, p = 0.024). The ADC % difference on day 1 for the lower dose (2-min) nVNS group was almost but not significantly different from the control group (p = 0.058); see Figure 4A. Similarly, the ADC % difference on day 1 for the lower dose nVNS group was not significantly different from those of the higher dose nVNS group (p = 0.80). Considering the variances of ADC % difference on day 1, no significant difference was found between variances of: (1) the control group versus the higher dose nVNS group (Levene test, p = 0.99), (2) the control group versus the lower dose nVNS group (Levene test, p = 0.27), and (3) the lower dose nVNS group versus the higher dose nVNS group (Levene test, p = 0.28).
FIG. 4.
Apparent diffusion coefficient (ADC) % difference values. (A) On day 1, the ADC % difference is significantly reduced for the 2 × 2-min non-invasive vagus nerve stimulation (nVNS) group compared with the control group. Alhough not significant, a trend is also observed for the 2-min nVNS versus the control group and (B) On day 7, even though no significant intergroup reduction is observed for the % difference in ADC (not shown), the variance of the 2 × 2-min nVNS group is significantly reduced compared with the control group (Levene test: p = 0.028).
On day 1, the effect size (Cohen's d) for the mean ADC % difference between the control group and the higher dose nVNS group was -1.00 95% CI (-1.99 to -0.22) , indicating a large effect size reduction of ADC % difference for the higher dose nVNS group (Cohen's d cutoff values 0.20, 0.50, 0.80, and 1.2 were chosen for interpreting small, medium, large, or very large effect sizes).
On day 7, the median ADC % difference was -370.68% (IQR: -1089.44%–43.16%) for the control group (n = 11), -292.20% (IQR: -620.98–34.43%) for the lower dose nVNS group (n = 12), and -283.85% (IQR: -316.90% to -117.56%) for the higher dose nVNS group (n = 14).
We observed no inter-group difference for the ADC % difference values on day 7. As shown in Figure 4B, the variances of ADC % difference on day 7 were only significantly different between the control group and the higher dose nVNS group (Levene test, variance ratio: 9.13, p = 0.028), although a trend was observed for the higher dose nVNS group versus the lower dose nVNS group (Levene test, p = 0.077).
Brain voxel-based morphometry results
Significant volumetric changes in the whole brain with TBI were identified in T2W MRI using VBM analysis. Mean intensity projections of deformation from all subjects in respective groups are shown in Figure 5 (red represents increased volume and blue represents decreased volume, relative to intact brain template). The composite of the baseline template (gray scale) overlaid with ANOVA statistical analysis (regions determined as p < 0.05; red color) is shown in the right-most column. On day 1 (top row), volume significantly increased on the primary somatosensory cortex surface of the control group, while deep tissue (below the cortex) volume decreased in the ipsilateral corpus callosum area, as indicated by the image-based ANOVA p value. The lower dose nVNS dampened the surface cortex volume increase as measured by the mean Jacobian determinate map by 13%, and the higher dose nVNS dampened cortex volume increase by 55% compared with the control group. Further, on day 1, the higher dose nVNS reduced deep tissue volume loss in the corpus callosum by 96% (values obtained from four consecutive MRI slices in the respective brain regions). On day 7 (bottom row), the lower dose nVNS dampened deep tissue volume loss by 35%, while the higher dose nVNS dampened the deep tissue volume loss by 89% compared with the control group.
FIG. 5.
Voxel-based morphometry (VBM) statistical comparisons of brain volume changes without (control) and with non-invasive vagus nerve stimulation (nVNS) treatments (n = 40). Group means are displayed with red color (positive volume change) and blue color (negative volume change) compared with the intact baseline template. In composite images (overlaying the seven baseline T2W magnetic resonance imaging [MRI] templates with the analysis of variance [ANOVA] results) the regions of p < 0.05 are shown in red. The arrow in the day 1 composite coincides with the injury site. Color image is available online.
Rotarod results
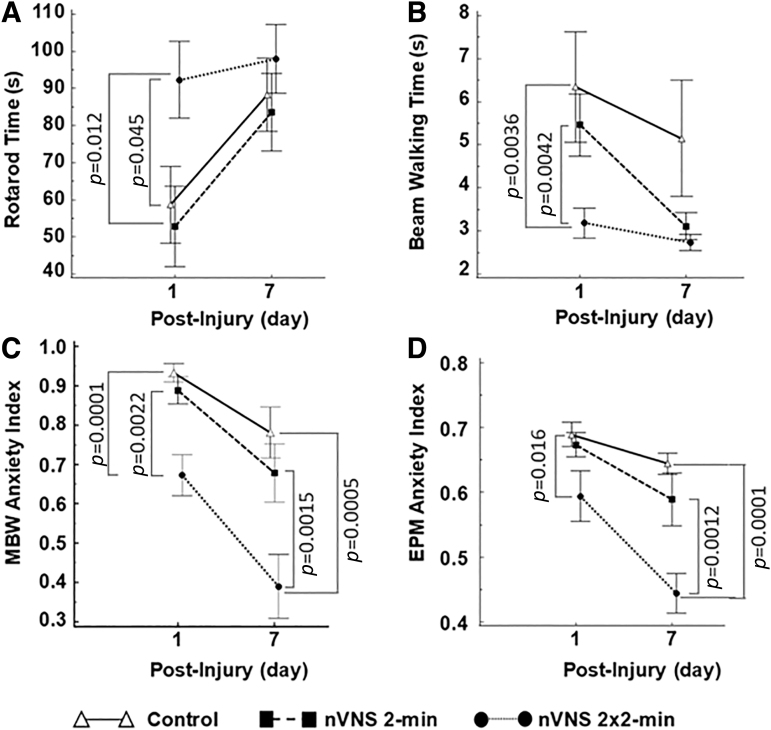
The accelerating rotarod was used to assess the motor deficits post-TBI on days 1 and 7 (Fig. 5A). The median rotarod times were 43.5 sec (IQR: 29.3–88.5 sec) for the control (n = 14), 35.3 sec (IQR: 27.9–86.9 sec) for the lower dose nVNS (n = 14), and 120 sec (IQR: 47–120 sec) for the higher dose nVNS (n = 14) groups on day 1 post-TBI. The overall rotarod time difference between the groups was significant (Kruskal–Wallis test: 6.72, 2 df, p < 0.030). The post-hoc Conover tests showed (1) a significant increase in rotarod time for the higher dose nVNS group compared with the control group (p = 0.0445) and (2) a significant increase of rotarod time for the higher dose nVNS group compared to the lower dose nVNS group (p = 0.0124). The mean difference rotarod time effect size between the higher dose nVNS and the control groups was 0.87 (95 % CI: [0.056, 1.82]) and between the lower dose nVNS and the higher dose nVNS groups was 0.99 (95% CI: [0.40, 2.01]), indicating a large increase in rotarod time for the higher dose nVNS group compared with the control and the lower dose nVNS groups on day 1 post-TBI.
On day 7, the median rotarod times were 109.2 sec (IQR: 53.8–120 sec) for the control, 93.5 sec (IQR: 40.3–120 sec) for the lower dose nVNS, and 120 sec (IQR: 72–120 sec) for the higher dose nVNS groups. No significant inter-group difference in rotarod times was observed (Kruskal–Wallis test: 0.92, 2 df, p = 0.63).
Time × treatment effect
The effect of time (day 1 vs. day 7) and treatment on the rotarod time were jointly tested with repeated measures ANOVA (n = 42 with control = 14, lower dose nVNS = 14, and higher dose nVNS = 14). The between-subject effect of the “treatment group” was not significant (F2,39 = 3.03, p = 0.060), while the within-subject effect of “time” was significant (F1,39 = 9.83, p = 0.003), showing an overall rotarod time increase between days 1 and 7 (see Fig. 6A).
FIG. 6.
Neurobehavioral assessments for the studied groups on days 1 and 7 post-injury. (A) Rotarod time, (B) beam-walking time, (C) modified beam walking (MBW) anxiety index, and (D) Elevated plus maze (EPM) anxiety index. Error bars represent the standard error of the mean (SEM). Significant p values from the post-hoc Conover tests are shown for each time point.
Beam-walking results
Four rats from the control group did not finish the test, and three fell from the beam on either day 1 or day 7 post-injury. To avoid study bias by excluding these cases that represent the worst-case scenario in performance from the analysis because they all belonged to the control group, an arbitrary 15-sec (three to four times longer than the average time required to cross the beam post-injury as a marker for not being able to cross the beam) was assigned to cases that did not finish the task.
The overall inter-group beam-walking time difference on day 1 was significant (Kruskal–Wallis test: 10.0, 2 df, p = 0.010). The post-hoc tests revealed a reduced beam-walking time for the higher dose nVNS group compared with the control group (p = 0.0036) and the lower dose nVNS group (p = 0.0042). The overall inter-group beam-walking time difference on day 7 was not significant (Kruskal–Wallis test: 1.40, 2 df, p = 0.50).
A repeated-measures ANOVA (control = 14, lower dose nVNS = 14, and higher dose nVNS = 14) revealed a significant effect for the “treatment group” (F2,39 = 3.54, p = 0.039) and a significant effect of “time” for the beam-walking time (F1,39 = 8.5, p = 0.006), showing an overall beam-walking time reduction between days 1 and 7 and an early (day 1) reduction of the beam-waking time for the higher dose nVNS group, see Figure 6B.
The median footfall counts for the beam-walking test on day 1 were 2.5 (IQR: 0–4) for the control group (n = 14), 1.5 (IQR: 1–6) for the lower dose nVNS group (n = 14), and 0 (IQR: 0–1) for the higher dose nVNS group (n = 14). There were significantly fewer footfalls in the higher dose nVNS group on day 1 than in the control group (p = 0.043) and the lower dose nVNS group (Mann–Whitney tests p = 0.036). On day 7, the median number of footfalls was 0 (IQR: 0–1) for all three groups, the mean number of footfalls was 1.1 (SD: 2.2) for the control group (n = 14), 0.6 (SD: 0.8) for the lower dose nVNS group (n = 14), and 0.21 (SD: 0.42) for the higher dose nVNS group (n = 14). Even though there were fewer footfalls for the higher dose nVNS group on day 7, the number of falls per rat was considered negligible (median count = 0 for each group), and we were unable to perform a statistical test.
MBW anxiety index
The median MBW anxiety index on day 1 was 0.96 (IQR: 0.89–1) for the control group (n = 14), 0.95 (0.86–0.98) for the lower dose nVNS group (n = 14), and 0.67 ± 0.052 for the higher dose nVNS group (n = 14). The overall difference in the MBW anxiety index between the groups was significant (Kruskal–Wallis test: 14.45, 2 df, p = 0.0001). Post-hoc Conover tests showed a significantly reduced MBW anxiety index in the VNS higher dose group compared with the control group (p = 0.0001) and the lower dose nVNS group (p = 0.0022).
The median MBW anxiety index on day 7 was 0.72 (IQR: 0.67–0.74) for the control group (n = 14), 0.70 (0.64–0.72) for the lower dose nVNS group (n = 14), and 0.63 (IQR: 0.54–0.71) for the higher dose nVNS group (n = 14). The overall difference in MBW anxiety index on day 7 between the treatment groups was significant (Kruskal–Wallis test: 11.25, 2 df, p = 0.0001). Post-hoc Conover tests showed a significantly reduced MBW anxiety index for the higher dose nVNS group compared with the control (p = 0.0005) and the lower dose nVNS groups (p = 0.0155).
Repeated measures ANOVA (control = 14, lower dose nVNS = 14, and higher dose nVNS = 14) revealed a significant effect for the “treatment group” (F2,39 = 12.33, p < 0.001) and “time” effect (F1,39 = 31.14, p = 0.001), showing an overall MBW anxiety reduction between days 1 and 7 and an early (day 1) reduction of MBW anxiety for the higher dose nVNS group (see Fig. 5C).
EPM anxiety index
The median EPM anxiety index on day 1 was 0.72 (IQR: 0.67–0.74) for the control group (n = 14), 0.70 (IQR: 0.64–0.72) for the lower dose nVNS group (n = 14), and 0.63 (IQR: 0.54–0.71) for the higher dose nVNS group (n = 14). The inter-group difference was marginally significant (Kruskal–Wallis test: 5.8, 2 df, p = 0.050). Post-hoc Conover tests showed a significantly reduced EPM anxiety index for the higher dose nVNS group compared with the control group (p = 0.0165).
The median EPM anxiety index on day 7 was 0.65 (IQR: 0.58–0.68) for the control group (n = 14), 0.63 (IQR: 0.55–0.70) for the lower dose nVNS group (n = 14), and 0.43 (IQR: 0.33–0.53) for the higher dose nVNS group (n = 14). The overall difference in the EPM anxiety index on day 7 between the treatment groups was highly significant (Kruskal–Wallis test: 14.68, 2 df, p < 0.0001). Post-hoc Conover tests showed a significantly reduced EPM anxiety index for the higher dose nVNS group compared with the control group (p = 0.0001) and the lower dose nVNS group (p = 0.0012).
A repeated measures ANOVA (control = 14, lower dose nVNS = 14, and higher dose nVNS = 14) revealed a significant “treatment group” effect (F2,39 = 11.64, p < 0.001) and a significant “time” effect (F1,39 = 19.8, p < 0.001), suggesting an overall EPM anxiety reduction between days 1 and 7 and an early (day 1) reduced EPM anxiety index for the higher dose nVNS group (see Fig. 6D).
Anxiety correlation analysis
Noticeable correlations (Spearman Rho) were found in EPM anxiety versus brain lesion volume on day 1 (Rho = 0.46 95% CI: [0.18, 0.68], p = 0.0027, n = 40) and day 7 (Rho = 0.53 95% CI: [0.27, 0.72], p = 0.0004, n = 40) and MBW anxiety versus brain lesion volume on day 1 (Rho = 0.62 95% CI: [0.38, 0.78], p < 0.0001, n = 41) and day 7 (Rho = 0.50 95% CI: [0.23, 0.70], p = 0.0009, n = 40). Moreover, a moderate correlation (Spearman Rho) was found between the MBW anxiety index and the EPM anxiety index on day 1 (Rho = 0.57 95% CI: [0.32, 0.74], p = 0.0001, n = 42) and a strong correlation on day 7 (Rho = 0.63 95% CI: [0.40, 0.78], p = 0.0001, n = 42). The partial linear correlation (Pearson) between the EPM anxiety index and the MBW anxiety index, adjusted for the covariate: brain lesion (log), were also computed at day 1 (r = 0.45, p = 0.0039) and at day 7 (r = 0.49, p = 0.0015) showing a moderate but significant association between these anxiety indices for both time points.
Discussion
In this study, we used a modified version of the commercially available gammaCore (nVNS) in our rat model of TBI. The Food and Drug Administration (FDA) initially approved gammaCore for cluster headaches in 2017 and has subsequently been cleared for most forms of primary headache, including migraine in adults and adolescents.
We observed a statistically significant reduction in post-TBI lesion volume among both nVNS groups (2-min and 2 × 2-min). The brain damage reduction in the higher dose (2 × 2-min) nVNS group was large enough to translate into a significant improvement in the neurobehavioral outcome measures (motor function and anxiety).
Parasympathetic activation by VNS has been shown to reduce proinflammatory responses, increase norepinephrine concentrations, attenuate glutamate-mediated excitotoxicity, and improve BBB function following TBI, leading to improved cognitive and motor functions.10,12–15 Published data on the use of iVNS in TBI models indicate its effect on different brain areas, which suggests that more than one mechanism may be involved.10,12–15 Multiple studies have shown that both iVNS and nVNS increases brain concentrations of norepinephrine by upregulation of endogenous noradrenergic activity by activating locus coeruleus (LC).19,23,24,47–49 VNS is associated with the release of norepinephrine in the amygdala,50 and this leads to an increase in norepinephrine concentration in the cortex and hippocampus.51
Different mechanisms, such as BBB breakdown, cell swelling caused by ionic pumps and fluid hemostasis dysfunction, and inflammatory responses, contribute to cerebral vasogenic and cytotoxic edema formation that is interrelated, leading to increased intracranial pressure (ICP). Elevated ICP and imaging evidence of cerebral edema have been associated with unfavorable outcomes in TBI.52 VNS therapy has been shown to reduce brain edema and decrease BBB disruption in rat models of TBI. Clough and coworkers13 have shown that iVNS (0.5 mA, 30 sec train of 0.5 ms biphasic pulses delivered at 20 Hz per 30 min for 48 h) reduces cortical edema. In a study conducted by Zhou and coworkers,12 rabbits with induced TBI that received VNS therapy (10 V, 5 Hz, and 5 ms pulses for 20 min) 1 h after the injury had a significant reduction in brain water content 24 h after the injury. Our data support those of Zhou and colleagues in that the acute injury period may be the most effective for the prevention of brain edema. However, investigations on the effect of nVNS therapy at later time intervals post-injury on brain edema is warranted. Lopez and coworkers10 investigated the effect of VNS on BBB permeability in a mouse model of TBI. The investigators showed a decrease in both cerebral vascular permeability and upregulation of AQP-4 post-injury in the VNS-treated mice. Our studies did not directly test for BBB disruption, but future studies will include investigation on the BBB function following our stimulation paradigm.
In our study, analysis of the difference in edema between the ipsilateral and contralateral edema for the studied groups revealed significantly smaller edema for the higher dose nVNS group compared with the control with a large effect size on day 1 post-injury. The amount of edema was also smaller for the lower dose nVNS than the control but did not reach a statistically significant level. We observed no statistically significant inter-group differences in ADC values, possibly because of the natural course of edema absorption by day 7. We did, however, observe a statistically significant difference in ADC values variances between the higher dose nVNS and the control group, indicating that the reduction in edema in the higher dose group was homogenous whereas the variation in reduction of edema was higher in the control group.
Alhough the results of iVNS in pre-clinical models of TBI are promising, its clinical study has been limited because of the invasive nature of the treatment that requires surgery to place electrodes around the vagus nerve and implant a battery pack in the chest cavity. These limitations have prevented the utilization of iVNS in clinical research, and would be impractical in clinical practice in situations in which a rapid application is needed to limit TBI-induced damage in the hyperacute and acute phases. To overcome the clinical challenges associated with iVNS therapy for TBI, nVNS has been proposed.
Previously, nVNS has been used in rat models of ischemic stroke studies.22,53 Five 2-min nVNS treatments (10 min apart) initiated 30 min after ischemia has shown to improve BBB function, reduce inflammation, and reduce the ischemic-induced immune response post-ischemia. In our study, we observed reductions in brain lesion volume for five 2-min nVNS applied 30 min post-TBI. However, improvements in motor function and anxiety were not significant at this dose, but reached significance when we used a higher dose nVNS (five 2 × 2 min stimulations). This highlights the need for neurobehavioral assessments vis-à-vis immunohistochemistry and neurochemical assessments to determine the optimum treatment that can improve the outcome.
Previous clinical studies have found significant changes in brain volume for different severities post-TBI, particularly reduced volume of the gray and white matter, with neurocognitive measures of memory, attention, and various post-concussive symptoms significantly associated with regional changes in brain volume after TBI.54–56 Brain atrophy has been observed as early as 8 weeks post-TBI.56 The mechanism for such an observation, however, is not fully understood. Furthermore, there are no reports in the literature that assess and discuss brain volumetric measurements in acute settings (either pre-clinical or clinical) using VBM measurements. Further studies are warranted to investigate the cause of such an observation in acute and subacute settings.
Here we have reported the use of previously designed MBW and EPM indices with modification to the MBW index.34 Both our previous report34 and our current results reveal a similar trend between the anxiety-related behavior obtained from EPM and our novel approach of using MBW. In particular, the current study found a moderate but significant partial linear correlation between the EPM anxiety index and the MBW anxiety index at day 1 (r = 0.45, p = 0.0039) and at day 7 (r = 0.49, p = 0.0015), after adjustment for the effect of the lesion volume. Therefore, the MBW index may serve as a substitute for EPM. This is important when optimizing resource allocation for rodents' neurobehavioral assessments.57
Study limitations and future direction
This pilot study used male Wistar rats to induce TBI using the CCI model and initiated different nVNS dose paradigms 30 min post-injury. The CCI model has been used in large pre-clinical TBI studies.26,29,30 The model is considered relevant as it reproduces many aspects of TBI observed in clinical settings.29 At the same time, the CCI model shares similar limitations with other commonly used TBI models, such as the fact that the injury is administered with the animal under anesthesia and requires a craniotomy. Therefore, like other TBI models, the CCI model cannot capture the full extent of TBI characteristics in clinical settings. Each model mimics certain histopathological and functional outcomes of clinical TBI, but no current model can fully represent the human condition.
Liu and coworkers19 tested the effect of varying stimulation paradigms (dose and intensity) on the suppression of KCl-induced cortical spreading depression (CSD) susceptibility using the gammaCore device in a rat model. They obtained statistically similar results in CSD suppression by using a medium-intensity (11.4 V) versus high-intensity (24.4 V) stimulation. Therefore, future TBI studies should also include different paradigms, including different stimulation intensities, multiple-day therapy, and a time-response study beyond 30 min post-injury to arrive at the optimum therapy protocol for TBI. Even though there were statistically significant differences in the amount of brain damage for the lower dose (2 min) and higher dose (2 × 2 min) groups on days 1 and 7 post-injury compared with the control group, we only observed a statistically significant improvement in the neurobehavioral assessments in the higher dose nVNS group. Significance was not reached in only one outcome measure (rotarod test) on day 7, likely because of insufficient sample size and rodents' natural ability to recover from brain injury. Thus, a larger sample size would allow the stratification of the rat groups (for example, male vs. female, additional time points, the effect of nVNS on sham TBI injury, or additional treatment options). Further studies encompassing different TBI models (such as fluid percussion injury and blast-TBI), different TBI severities, different species, the inclusion of both sexes and other age groups, using more detailed neurobehavioral assessments such as Morris water maze, and relevant histopathological studies can shed additional light on the effectiveness of nVNS for improving outcomes in TBI.
Conclusion
To date, clinical trials have failed to produce effective treatments to combat the devastating effects of TBI. Safe and effective treatments are desperately needed to improve the quality of life of the military service members and civilian populations affected by TBI and promote better recovery and community reintegration after their injury. In our study, we observed that the higher dose (five 2 × 2-min) nVNS group had a statistically significant improvement in motor function and anxiety compared with the control group. The reduction in the neurobehavioral deficits was not seen in our lower dose (2-min) nVNS group, suggesting a possible dose-response. Should nVNS therapy be proven effective in clinical settings, it would dramatically change the landscape and potential for TBI therapy in civilian and military populations. The advantage of the nVNS treatment is that it can be potentially applied to a person with TBI (because of its small portable nature) at an earlier stage (as early as when the paramedic attends the patient) than is currently possible.
Transparency, Rigor, and Reproducibility Summary
A sample size of 14 Wistar rats per group (n = 42) was used for the study, determined for significant between-factors differences on a three-group, two time point repeated measures ANOVA and considering a conservative 0.50 Cohen's d effect size based on our prior pilot data, 0.80 power, 0.05 alpha error rate. The sample size calculation was performed with G*Power v3.1.9.7 software.58 We excluded two rats for technical reasons, so we added two more rats because of attrition. Rats were randomly assigned to groups using a random number generator. All 42 rats received the allocated treatment. Because of a scheduling conflict, we could not perform the MRI studies for one rat. Another rat died on day 5 from peritonitis unrelated to the procedure, so we could not perform the MRI study on day 7. Therefore, complete data were obtained for 40 rats. Investigators who performed behavioral outcome assessments and MRI data analysis were blinded to treatment groups. The nVNS therapy was applied 30 min post-injury. A dose-response relationship was performed using two doses (2-min nVNS and 2 × 2-min nVNS).
Supplementary Material
Acknowledgments
The authors thank electroCore, Inc. for providing the gammaCore devices for this study. Ms. Justyna Blake and Dr. Bruce Simon from electroCore provided valuable technical support for this study. We also thank the medical students (Remy L. Link, Hoi D. Doan, Victoria L. Carlson, Vineet Narayanan, and Allae N. Abdelrahman) who helped with the voxel-based morphometry analysis for this study.
Authors' Contributions
Afshin A. Divani was responsible for conceptualization, methodology, resources, data curation, visualization, supervision, writing-review and editing; Pascal Salazar: was responsible for formal analysis, visualization, writing-review and editing; Hafiz A. Ikram: was responsible for investigation, data curation; Erik Taylor: was responsible for investigation, visualization, writing-review and editing; Colin W. Wilson: was responsible for investigation, visualization, writing-review and editing; Yirong Yang: was responsible for investigation, data curation; Javad Mahmoudi: was responsible for methodology, writing-review and editing; Alina Seletska: was responsible for writing-review and editing; Karen S. SantaCruz: was responsible for methodology, writing-review and editing; Eric J. Liebler: was responsible for writing-review and editing; Olga A. Bragina: was responsible for data curation, visualization, writing; Russel A. Morton: was responsible for Data Curation, Visualization, Writing-Review and Editing; Denis Bragin: was responsible for Writing-Review and Editing; Michel Torbey: was responsible for Writing-Review and Editing
Funding Information
This project was supported by research grants from the UNM Center for Brain Recovery and Repair Center of Biomedical Research Excellence through Grant Numbers (NIH P20GM109089, Pilot PI: Divani; W81XWH-17-2-0053, PI: Divani; 1R21NS130423-01, PI: Divani; and NIH R01 NS112808, PI: Bragin).
Author Disclosure Statement
Eric Liebler is an employee of electroCore, Inc. Pascal Salazar is an employee of Canon Medical Informatics, Inc. The other authors have nothing to disclose.
Supplementary Material
References
- 1. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 2015;72(3):355–362; doi: 10.1001/jamaneurol.2014.3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. Atlanta: National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention; 2014. [Google Scholar]
- 3. Summers CR, Ivins B, Schwab KA. Traumatic brain injury in the United States: an epidemiologic overview. Mt Sinai J Med, New York 2009;76(2):105–110; doi: 10.1002/msj.20100 [DOI] [PubMed] [Google Scholar]
- 4. Kim CC, Nakamura MC, Hsieh CL. Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation 2016;13(1):117; doi: 10.1186/s12974-016-0581-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben-Menachem E, Revesz D, Simon BJ, et al. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol 2015;22(9):1260–1268; doi: 10.1111/ene.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 2007;1179:28–34; doi: 10.1016/j.brainres.2007.08.045 [DOI] [PubMed] [Google Scholar]
- 7. Hays SA. Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics 2016;13(2):382–394; doi: 10.1007/s13311-015-0417-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol 2010;27(2):130–138; doi: 10.1097/WNP.0b013e3181d64d8a [DOI] [PubMed] [Google Scholar]
- 9. Lopez NE, Krzyzaniak M, Costantini TW, et al. Vagal nerve stimulation blocks peritoneal macrophage inflammatory responsiveness after severe burn injury. Shock 2012;38(3):294–300; doi: 10.1097/SHK.0b013e31825f5fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez NE, Krzyzaniak MJ, Costantini TW, et al. Vagal nerve stimulation decreases blood–brain barrier disruption after traumatic brain injury. J Trauma Acute Care Surg 2012;72(6):1562–1566; doi: 10.1097/TA.0b013e3182569875 [DOI] [PubMed] [Google Scholar]
- 11. Smith DC, Tan AA, Duke A, et al. Recovery of function after vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. J Neurotrauma 2006;23(10):1549–1560; doi: 10.1089/neu.2006.23.1549 [DOI] [PubMed] [Google Scholar]
- 12. Zhou L, Lin J, Kui G, et al. Neuroprotective effects of vagus nerve stimulation on traumatic brain injury. Neural Regen Res 2014;9(17):1585–1591; doi: 10.4103/1673-5374.141783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clough RW, Neese SL, Sherill LK, et al. Cortical edema in moderate fluid percussion brain injury is attenuated by vagus nerve stimulation. Neuroscience 2007;147(2):286–293; doi: 10.1016/j.neuroscience.2007.04.043 [DOI] [PubMed] [Google Scholar]
- 14. Neese SL, Sherill LK, Tan AA, et al. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: an immunocytochemical study. Brain Res 2007;1128(1):157–163; doi: 10.1016/j.brainres.2006.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith DC, Modglin AA, Roosevelt RW, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma 2005;22(12):1485–1502; doi: 10.1089/neu.2005.22.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stone JR, Okonkwo DO, Singleton RH, et al. Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid Beta peptide in traumatic axonal injury. J Neurotrauma 2002;19(5):601–614; doi: 10.1089/089771502753754073 [DOI] [PubMed] [Google Scholar]
- 17. Tang Y, Dong X, Chen G, et al. Vagus nerve stimulation attenuates early traumatic brain injury by regulating the NF-kappaB/NLRP3 signaling pathway. Neurorehabil Neural Repair 2020;34(9):831–843; doi: 10.1177/1545968320948065 [DOI] [PubMed] [Google Scholar]
- 18. Bansal V, Ryu SY, Lopez N, et al. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation 2012;35(1):214–220; doi: 10.1007/s10753-011-9307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu TT, Morais A, Takizawa T, et al. Efficacy profile of noninvasive vagus nerve stimulation on cortical spreading depression susceptibility and the tissue response in a rat model. J Headache Pain 2022;23(1):12; doi: 10.1186/s10194-022-01384-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405(6785):458–462; doi: 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- 21. Yang LY, Bhaskar K, Thompson J, et al. Non-invasive vagus nerve stimulation reduced neuron-derived IL-1β and neuroinflammation in acute ischemic rat brain. Brain Hemorrhages 2022;3(2):45–56, doi: 10.1016/j.hest.2021.06.003 [DOI] [Google Scholar]
- 22. Yang Y, Yang LY, Orban L, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain stimulation 2018;11(4):689–698, doi: 10.1016/j.brs.2018.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen SP, Ay I, de Morais AL, et al. Vagus nerve stimulation inhibits cortical spreading depression. Pain 2016;157(4):797–805; doi: 10.1097/j.pain.0000000000000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morais A, Liu TT, Qin T, et al. Vagus nerve stimulation inhibits cortical spreading depression exclusively through central mechanisms. Pain 2020;161(7):1661–1669; doi: 10.1097/j.pain.0000000000001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020;40(9):1769–1777; doi: 10.1177/0271678X20943823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brody DL, Mac Donald C, Kessens CC, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma 2007;24(4):657–673; doi: 10.1089/neu.2006.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tran HT, LaFerla FM, Holtzman DM, et al. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci 2011;31(26):9513–9525; doi: 10.1523/JNEUROSCI.0858-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osier ND, Dixon CE. The controlled cortical impact model: applications, considerations for researchers, and future directions. Front Neurol 2016;7:134; doi: 10.3389/fneur.2016.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osier N, Dixon CE. The controlled cortical impact model of experimental brain trauma: overview, research applications, and protocol. Methods Mol Biol 2016;1462:177–192; doi: 10.1007/978-1-4939-3816-2_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brabazon F, Wilson CM, Jaiswal S, et al. Intranasal insulin treatment of an experimental model of moderate traumatic brain injury. J Cereb Blood Flow Metab 2017;37(9):3203–3218; doi: 10.1177/0271678X16685106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhatia PM, Chamberlain R, Luo X, et al. Elevated blood pressure causes larger hematoma in a rat model of intracerebral hemorrhage. Transl Stroke Res 2012;3(4):428–434; doi: 10.1007/s12975-012-0199-0 [DOI] [PubMed] [Google Scholar]
- 32. Divani AA, Murphy AJ, Meints J, et al. A novel preclinical model of moderate primary blast-induced traumatic brain injury. J Neurotrauma 2015;32(14):1109–1116; doi: 10.1089/neu.2014.3686 [DOI] [PubMed] [Google Scholar]
- 33. Divani AA, Salazar P, Monga M, et al. Inducing different brain injury levels using shock wave lithotripsy. J Ultrasound Med 2018;37: 2925–2933; doi: 10.1002/jum.14656 [DOI] [PubMed] [Google Scholar]
- 34. Sweis BM, Bachour SP, Brekke JA, et al. A modified beam-walking apparatus for assessment of anxiety in a rodent model of blast traumatic brain injury. Behav Brain Res 2016;296:149–156; doi: 10.1016/j.bbr.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 35. Divani AA, Hevesi M, Pulivarthi S, et al. Predictors of nosocomial pneumonia in intracerebral hemorrhage patients: a multi-center observational study. Neurocrit Care 2015;22(2):234–242; doi: 10.1007/s12028-014-0065-x [DOI] [PubMed] [Google Scholar]
- 36. Divani AA, Majidi S, Luo X, et al. The ABCs of accurate volumetric measurement of cerebral hematoma. Stroke 2011;42(6):1569–1574; doi: 10.1161/STROKEAHA.110.607861 [DOI] [PubMed] [Google Scholar]
- 37. Hevesi M, Bershad EM, Jafari M, et al. Untreated hypertension as predictor of in-hospital mortality in intracerebral hemorrhage: a multi-center study. J Crit Care 2018;43:235–239: doi: 10.1016/j.jcrc.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 38. Divani AA, Liu X, Petersen A, et al. The magnitude of blood pressure reduction predicts poor in-hospital outcome in acute intracerebral hemorrhage. Neurocrit Care 2020;33(2):389–398; doi: 10.1007/s12028-020-01016-z [DOI] [PubMed] [Google Scholar]
- 39. Divani AA, Liu X, Di Napoli M, et al. Blood pressure variability predicts poor in-hospital outcome in spontaneous intracerebral hemorrhage. Stroke 2019;50(8):2023–2029; doi: 10.1161/STROKEAHA.119.025514 [DOI] [PubMed] [Google Scholar]
- 40. Tustison NJ, Cook PA, Holbrook AJ, et al. The ANTsX ecosystem for quantitative biological and medical imaging. Sci Rep 2021;11(1):9068; doi: 10.1038/s41598-021-87564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. NeuroImage 2014;92:381–397; doi: 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrais S, Shakir DI, Van Der Merwe J, et al. Bruker2nifti: magnetic resonance images converter from Bruker ParaVision to Nifti format. J Open Source Softw 2017;2(16):354; doi: 10.21105/joss.00354 [DOI] [Google Scholar]
- 43. Hsu LM, Wang S, Ranadive P, et al. Automatic skull stripping of rat and mouse brain MRI data using U-Net. Front Neurosci 2020;14:568614; doi: 10.3389/fnins.2020.568614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pagani M, Damiano M, Galbusera A, et al. Semi–automated registration-based anatomical labelling, voxel based morphometry and cortical thickness mapping of the mouse brain. J Neurosci Meth 2016;267:62–73; doi: 10.1016/j.jneumeth.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 45. Razali NM, Wah YB. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Statistical Modeling Analytics 2011;2(1):21–33. [Google Scholar]
- 46. Tukey J. Exploratory Data Analysis. Addison-Wesley: Reading, MA; 1977. [Google Scholar]
- 47. Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev 2005;29(3):493–500; doi: 10.1016/j.neubiorev.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 48. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 1998;39(7):709–714; doi: 10.1111/j.1528-1157.1998.tb01155.x [DOI] [PubMed] [Google Scholar]
- 49. Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res 1995;22(1):53–62; doi: 10.1016/0920-1211(95)00035-9 [DOI] [PubMed] [Google Scholar]
- 50. Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 2004;118(1):79–88; doi: 10.1037/0735-7044.118.1.79 [DOI] [PubMed] [Google Scholar]
- 51. Roosevelt RW, Smith DC, Clough RW, et al. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 2006;1119(1):124–132; doi: 10.1016/j.brainres.2006.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2019;145(Pt B):230–246; doi: 10.1016/j.neuropharm.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ay I, Nasser R, Simon B, et al. Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stim 2016;9(2):166–173; doi: 10.1016/j.brs.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dean PJA, Sato JR, Vieira G, et al. Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Inj 2015;29(10):1211–121; doi: 10.3109/02699052.2015.1035334 [DOI] [PubMed] [Google Scholar]
- 55. Kim E, Seo HG, Lee HH, et al. Reduced brainstem volume after mild traumatic brain injury. Am J Phys Med Rehabil 2021;100(5):473–482; doi: 10.1097/PHM.0000000000001580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sidaros A, Skimminge A, Liptrot MG, et al. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. NeuroImage 2009;44(1):1–8; doi: 10.1016/j.neuroimage.2008.08.030 [DOI] [PubMed] [Google Scholar]
- 57. Bachour SP, Hevesi M, Bachour O, et al. Comparisons between Garcia, Modo, and Longa rodent stroke scales: optimizing resource allocation in rat models of focal middle cerebral artery occlusion. J Neurol Sci 2016;364:136–140: doi: 10.1016/j.jns.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 58. Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175–191; doi: 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.