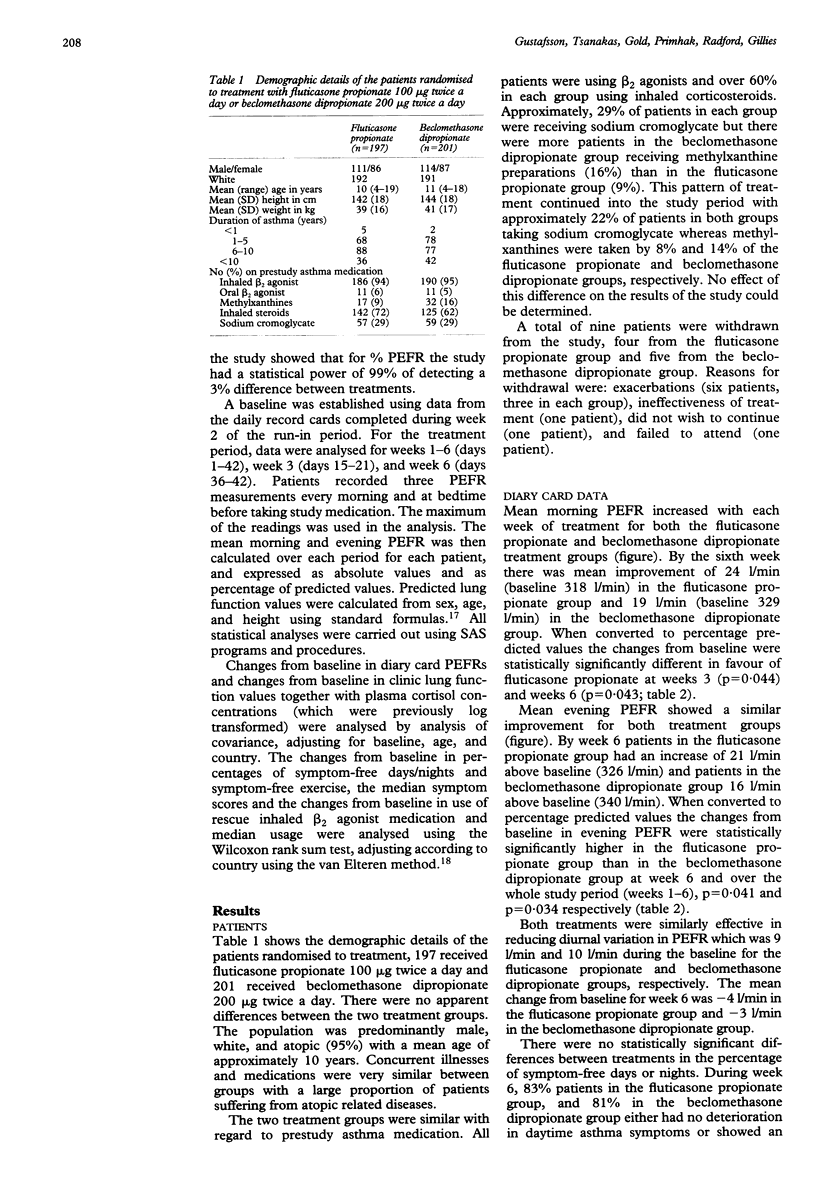
Abstract
This study was designed to compare the efficacy and safety of a new inhaled corticosteroid, fluticasone propionate at a total daily dose of 200 micrograms, with beclomethasone dipropionate 400 micrograms/day in childhood asthma. A total of 398 asthmatic children (aged 4-19 years) were randomised to receive either fluticasone propionate 200 micrograms daily or beclomethasone dipropionate 400 micrograms daily for six weeks inhaled via a spacer device from a metered dose inhaler. During the study the patients recorded morning and evening peak expiratory flow rate (PEFR), symptom scores, and use of beta 2 agonist rescue medication. In addition, clinic visit PEFR and forced expiratory volume in one second were measured. Safety was assessed by recording all adverse events and by performing routine biochemistry and haematology screens including plasma cortisol concentration before and after treatment. For the purposes of analysis the diary card data were grouped into three periods: week 3 (days 15-21), week 6 (days 36-42), and weeks 1-6 (days 1-42). The results showed no significant difference between treatments on most efficacy parameters. However, there were significant differences in changes from baseline in favour of fluticasone propionate for % predicted morning PEFR both at week 3 (fluticasone propionate 6.1%, beclomethasone dipropionate 3.9%) and at week 6 (fluticasone propionate 8.3%, beclomethasone dipropionate 5. 9%) and % predicted evening PEFR at week 6 (fluticasone propionate 7.3%, beclomethasone dipropionate 4.9% and over weeks 1-6 (fluticasone propionate 5.5%, beclomethasone dipropionate 3.6%. Comparison between groups showed that the group receiving fluticasone propionate had a lower % of days with symptom-free exercise at week 6 (fluticasone propionate 87%, beclomethasone dipropionate 81%) and % days without rescue medication at week 6 (fluticasone propionate 87%, beclomethasone dipropionate 80%) and over weeks 1-6 (fluticasone propionate 80%, beclomethasone dipropionate 73%). Except for a higher incidence of sore throat in the fluticasone propionate group, the two treatments did not differ with regard to safety. There was no evidence of adrenal suppression with either treatment. In conclusion, fluticasone propionate 200 microgram daily ws at least as effective and as well tolerated as beclomethasone dipropionate 400 microgram daily in childhood asthma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. R., Bailey P. A., Cooper J. S., Palmer J. C., West S. Morbidity and school absence caused by asthma and wheezing illness. Arch Dis Child. 1983 Oct;58(10):777–784. doi: 10.1136/adc.58.10.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. R. Epidemiology of asthma. 1992 Jan 23-Feb 4Br J Hosp Med. 47(2):99–104. [PubMed] [Google Scholar]
- Coughlin S. P. Sport and the asthmatic child: a study of exercise-induced asthma and the resultant handicap. J R Coll Gen Pract. 1988 Jun;38(311):253–255. [PMC free article] [PubMed] [Google Scholar]
- Davies D. S. Pharmacokinetics of inhaled substances. Postgrad Med J. 1975;51(7 Suppl):69–75. [PubMed] [Google Scholar]
- Harding S. M. The human pharmacology of fluticasone propionate. Respir Med. 1990 Nov;84 (Suppl A):25–29. doi: 10.1016/s0954-6111(08)80004-2. [DOI] [PubMed] [Google Scholar]
- Hauspie R., Susanne C., Alexander F. Maturational delay and temporal growth retardation in asthmatic boys. J Allergy Clin Immunol. 1977 Mar;59(3):200–206. doi: 10.1016/0091-6749(77)90150-6. [DOI] [PubMed] [Google Scholar]
- Larsen G. L. Asthma in children. N Engl J Med. 1992 Jun 4;326(23):1540–1545. doi: 10.1056/NEJM199206043262306. [DOI] [PubMed] [Google Scholar]
- McNichol K. N., Williams H. E. Spectrum of asthma in children. II. Allergic components. Br Med J. 1973 Oct 6;4(5883):12–16. doi: 10.1136/bmj.4.5883.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnicol K. N., Macnicol K. N., Williams H. B. Spectrum of asthma in children. I. Clinical and physiological components. Br Med J. 1973 Oct 6;4(5883):7–11. doi: 10.1136/bmj.4.5883.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipps G. H. Structure-activity relationships of topically active steroids: the selection of fluticasone propionate. Respir Med. 1990 Nov;84 (Suppl A):19–23. doi: 10.1016/s0954-6111(08)80003-0. [DOI] [PubMed] [Google Scholar]
- Priftis K., Milner A. D., Conway E., Honour J. W. Adrenal function in asthma. Arch Dis Child. 1990 Aug;65(8):838–840. doi: 10.1136/adc.65.8.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. O., Götz M., Landau L. I., Levison H., Milner A. D., Pedersen S., Silverman M. Management of asthma: a consensus statement. Arch Dis Child. 1989 Jul;64(7):1065–1079. doi: 10.1136/adc.64.7.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H., McNicol K. N. Prevalence, natural history, and relationship of wheezy bronchitis and asthma in children. An epidemiological study. Br Med J. 1969 Nov 8;4(5679):321–325. doi: 10.1136/bmj.4.5679.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R., Waschek J., Weinberger M., Sherman B. Effects of inhaled beclomethasone dipropionate and alternate-day prednisone on pituitary-adrenal function in children with chronic asthma. N Engl J Med. 1978 Dec 21;299(25):1387–1392. doi: 10.1056/NEJM197812212992504. [DOI] [PubMed] [Google Scholar]


