Abstract
Docosahexaenoic acid (DHA) is a polyunsaturated fatty acid that benefits the prevention of chronic diseases. Due to its high unsaturation, DHA is vulnerable to free radical oxidation, resulting in several unfavorable effects, including producing hazardous metabolites. However, in vitro and in vivo investigations suggest that the relationship between the chemical structure of DHA and its susceptibility to oxidation may not be as clear-cut as previously thought. Organisms have developed a balanced system of antioxidants to counteract the overproduction of oxidants, and the nuclear factor erythroid 2-related factor 2 (Nrf2) is the key transcription factor identified for transmitting the inducer signal to the antioxidant response element. Thus, DHA might preserve the cellular redox status promoting the transcriptional regulation of cellular antioxidants through Nrf2 activation. Here, we systematically summarize the research on the possible role of DHA in controlling cellular antioxidant enzymes. After the screening process, 43 records were selected and included in this review. Specifically, 29 studies related to the effects of DHA in cell cultures and 15 studies concerned the effects of consumption or treatment with DHA in animal. Despite DHA’s promising and encouraging effects at modulating the cellular antioxidant response in vitro/in vivo, some differences observed among the reviewed studies may be accounted for by the different experimental conditions adopted, including the time of supplementation/treatment, DHA concentration, and cell culture/tissue model. Moreover, this review offers potential molecular explanations for how DHA controls cellular antioxidant defenses, including involvement of transcription factors and the redox signaling pathway.
Keywords: docosahexaenoic acid, antioxidants, Nrf2
1. Introduction
Docosahexaenoic acid (DHA) is a highly polyunsaturated fatty acid (PUFA) of the n-3 series with 22 carbon atoms and 6 cis double bonds. DHA plays a crucial role in lipid metabolism [1], in membrane structure [2], in cell signaling [3], and in inflammation [4]. Epidemiological studies have demonstrated that diets rich in DHA have a positive effect against several types of disease [5,6,7]. Marine-based fish and fish oil are the most popular and well-known sources of DHA.
Plasma non-esterified DHA derived from chilomicrons and VLDLs enters the cells via passive diffusion or transporters such as fatty acid transport protein or fatty acid transporter CD36 [8]. Inside the cells, non-esterified DHA is converted by acylCoA synthases to DHA-CoAs, which are substrates for β-oxidation, desaturation/elongation and assimilation into complex lipids, i.e., phospholipids in the plasma membrane [9].
The physicochemical properties of the membrane bilayer and the chemical reactivity of the fatty acids that compose the membrane are two inherent traits of the membrane phospholipids that regulate their fluidity and determine their susceptibility to oxidative damage. The first property is related to the fact that oxygen and reactive species are more soluble in the fluid lipid bilayer than in the aqueous solution. Consequently, membrane lipids become primary targets of oxidative damage. The second and more significant property is related to the fact that PUFA residues of phospholipids are extremely sensitive to oxidation [10]. PUFAs are usually oxidized by a well-known mechanism called “free radical oxidation”. This theory involves an attack of oxygen at the allylic position with the formation of unsaturated hydroperoxides. These hydroperoxides also take part in the auto-oxidation and thus initiate a chain reaction [11].
Due to its high unsaturation, DHA susceptibility to free radical oxidation may represent the other side of the coin. This uncontrolled oxidation of DHA may have a variety of metabolic and physiological repercussions, such as altering the lipid bilayer’s structure and function [12] or producing harmful byproducts such malondialdehyde and alkenals [13]. Therefore, a theoretical concern remains on using DHA for preventing chronic diseases whenever oxidative stress is one of the underlying mechanisms.
The human body implemented several strategies to counteract the effects of excess free radicals based on antioxidant molecules [14]. Endogenous antioxidants, which are products of the body’s metabolism, may be enzymatic or non-enzymatic. Enzymatic antioxidants playing an essential role in the first line of defense are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPx), and peroxiredoxins (PRx) [15,16]. The second line of defense involves non-enzymatic antioxidants such as glutathione (GSH) and thioredoxin (TRx), characterized by the ability to rapidly inactivate ROS and oxidants [17].
This articulated mechanism is regulated at the cellular level through a cis-acting element called antioxidant or electrophile response elements (ARE/EpRE) [18]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is the key transcription factor for transmitting the inducer signal to AREs, and many food bioactive compounds were identified as Nrf2 inducers [18]. As DHA has been shown to modulate transcription of genes related to lipid metabolism, such as stearoyl-Coenzyme A desaturase 2 and 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase [19,20], by interacting with several nuclear receptors, such as peroxisome proliferator-activated receptor (PPAR) and sterol regulatory element binding protein (SREBP) [21,22,23], it is reasonable to assume that DHA could maintain the cellular redox status promoting the transcriptional regulation of antioxidant expression through Nrf2 activation.
In light of these considerations, we tried to overview studies on the potential effect of DHA in regulating cellular antioxidant enzymes. This review highlights DHA’s health-related potential and hypothesizes possible molecular scenarios between DHA and Nrf2 in regulating cellular antioxidant defenses.
2. Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [24]. The protocol for this systematic review was registered on INPLASY (INPLASY202360017) and is available in full at inplasy.com (https://inplasy.com/inplasy-2023-6-0017/). The search was carried out by using the PubMed database in December 2022, and was conducted using the following keywords and Boolean operators: “docosahexaenoic acid” OR “DHA” OR “C22:6” AND “antioxidant” NOT “review”. The initial search yielded 1063 hits. During the screening process (reviewing titles), 941 records were excluded. After abstract analysis, another 84 articles were ousted. Altogether, 43 records were selected and included in this review. Specifically, 29 studies related to the effects of DHA in cell cultures and 15 studies concerning the effects of consumption or treatment with DHA in animal models were deeply analyzed. One study concerned cell culture and animal models and was allocated to each group. Chosen studies were published between 1998 and 2021 without restriction regarding period or publication status. Exclusion criteria were: (i) titles irrelevant to the research topic; (ii) abstract inappropriate or not related to the research topic; (iii) studies that used n-3 PUFA rich oils which would not allow us to discriminate the effect of DHA from other n-3 PUFAs; (iv) studies that co-administrated DHA with other compounds; (v) studies that used DHA oxidation products to better reflect normal nutritional conditions; and (vi) studies or data with inadequate statistical analysis or inappropriate controls. Reviews, letters, abstracts, and articles without a complete text in the English language were also excluded. Two independent investigators (S.M.B and M.D.N.) checked the titles and abstracts of the studies, and disagreements between the two reviewers were resolved through a mediator (S.I.). Primary outcomes include the most relevant variables to answer the research question (the modulation of cellular antioxidant defenses by DHA), while secondary outcomes include additional variables to help the interpretation of the results of the primary outcomes. The detailed selection process is presented in Figure 1.
Figure 1.
Flow chart of papers included in this review.
3. Results and Discussion
3.1. Effect of DHA Supplementation on Antioxidant Defenses in Cultured Cells
Although studies on humans remain the gold standard for evaluating the relationship between nutrients and health, the development of reliable in vitro/ex vivo models allow the investigation of the cellular/molecular mechanisms and represents a first—and undoubtedly necessary—step when investigating the health-promoting properties of food components [25]. Table 1 summarizes the data published on the effect of DHA supplementation on antioxidant defenses in cultured cells (Table 1).
Table 1.
Summary of findings related to the effect of DHA supplementation on cell culture.
| Ref. | Cell/Tissue Model | DHA Concentration/Time of Incubation | Exogenous Treatment | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|
| [26] | Primary human peripheral blood mononuclear cells | Pretreatment with 5 µM for 1 h | Subsequent oxidative stress by 0.5, 1, 2, and 5 mM H2O2 for 10 min | Oxidative condition: ↓ GPx activity at 1 mM H2O2 | Not-oxidative condition: ↑ DHA content in membrane PLs |
| Oxidative condition: ↓ MDA content at 1 and 1.5 mM H2O2; ↑ cytosolic cAMP-PDE at 1, 2, and 5 mM H2O; ↑ cytosolic cGMP-PDE content at 1 and 5 mM H2O2 | |||||
| Primary rat thymocytes | Pretreatment with 5 µM for 1 h | Subsequent oxidative stress by 0.5, 1, 2, and 5 mM H2O2 for 10 min | Oxidative condition: ↔ GPx activity | Not-oxidative condition: ↑ DHA content in membrane PLs | |
| Oxidative condition: ↔ cytosolic cAMP-PDE and cGMP- PDE content | |||||
| [27] | Primary bovine retinal endothelial cells | 10 and 100 µM for 2 cell passages (approximately 10 d) | Not present | ↓ cGPx protein expression and activity at 10 µM | ↑ MDA content; ↔ αTC and γTC content; ↑ DHA content in PC and PE; ↔ GPx4 activity |
| Primary bovine aortic endothelial cells | 10 and 100 µM for 2 cell passages (approximately 10 d) | Not present | ↑ cGPx protein expression at 100 µM | ↑ MDA content; ↔ γTC and ↓ αTC content at 100 µM; ↑ DHA content in PC and PE; ↑ GPx4 activity at 100 µM | |
| [28] | Primary human fibroblasts | 5, 15, 30, and 60 µM for 48 h; 30 µM for 4, 8, 24, 48 h, and 7 d | Not present | ↑ GSH content at 30 µM and 60 µM for 48 h, and 30 µM for 7 d | ↑ γGCL and GST activity at 30 µM and 60 µM for 48 h; ↑ GR activity at 15 µM, 30 µM, and 60 µM for 48 h; ↑ γGCL mRNA expression at 30 µM for 4, 8, 24, and 48 h; ↑ GR mRNA expression at 30 µM for 24 and 48 h; ↑ HO-1 mRNA at 30 µM for 4 and 8 h; ↑ ROS formation at 30 µM for 4, 8, 24, and 48 h; ↑ cell DHA content at 30 µM for 48 h; ↔ lipoperoxides content at 30 µM for 48 h; ↑ γGCL, GR, and GST activity at 30 µM for 7 d |
| [29] | Primary rat hepatocytes | 5, 10, and 20 µM for 48 h | Concomitant oxidative stress by 5 and 10 µM TCDD for 48 h | Basal condition: ↑ TAC | Basal condition: ↔ TOS and 8-OHdG content |
| Oxidative condition: ↑ TAC | Oxidative condition: ↓ TOS content at 20 µM DHA with 5 and 10 µM TCDD; ↓ 8-OHdG content | ||||
| [30] | Primary rat hippocampal neurons | 5 µg/mL (15.2 µM), 30 µg/mL (91.3 µM), and 50 µg/mL (152 µM) for 24 h | Concomitant cytotoxic stress by 0.5 mM glutamate for 1 h | Excitotoxic condition: ↑ GPx activity and ↔ GSH content | Excitotoxic condition: ↑ GR activity; ↓ NO formation |
| [31] | Primary carp brain cells | Pretreatment with 30 nM for 48 h | Subsequent oxidative stress by 1 mg/L (1.39 µM) fullerene (C60) for 2 h | Basal condition: ↔ TAC and GSH content | Basal condition: ↓ ROS and TBARS content; ↔ Cys content |
| Oxidative condition: ↔ TAC and GSH content | Oxidative condition: ↓ ROS and TBARS content; ↔ Cys content | ||||
| [32] | Primary rat astrocytes | 5 µM for 24 h | Concomitant oxidative stress by 10 µM UCB for 20 h | Basal condition: ↑ SOD, CAT, and GPx activity | Basal condition: ↔ apoptosis |
| Oxidative condition: ↑ SOD, CAT, and GPx activity | Oxidative condition: ↓ apoptosis | ||||
| [33] | Primary rat astrocytes | Pretreatment with 10, 30, and 50 µM for 1, 2, and 3 h; 30 µM for 24 h | Subsequent oxidative stress by 0.5 mM for 2 h | Basal condition: ↑ GSH content at 30 µM for 24 h | Basal condition: ↓ ROS content at 30 and 50 µM; ↑ Nrf2 protein expression and binding activity; ↑ γGCL and GPx4 expression at 30 µM for 24 h; ↑ DHA content in mitochondrial and plasma membrane PL at 30 µM for 24 h |
| Oxidative condition: ↑ GSH content and GPx4 activity at 30 µM for 24 h | Oxidative condition: ↓ ROS content at 30 µM for 1, 2, and 3 h; ↓ and ↑ γGCL and GPx4 expression at 30 µM for 24 h, respectively | ||||
| [34] | Human ovarian cancer cell line (A2780) | 100 µM for 1, 4, and 20 h; 50, 100, and 150 µM per 4 h; 150 µM for 16 h | Not present | ↓ SOD1 promoter activity at 100 µM for 4 h and 20 h, and at 150 µM for 4 h | ↓ HRE-mediated gene transcription at 150 µM for 16 h |
| [35] | Mouse skeletal muscle cell line (C2C12) | 100 µM for 24 h | Not present | ↔ GPx, CAT, SOD1, and SOD2 protein expression; ↓ SOD activity; ↔ GPx and CAT activity | ↑ ROS production |
| [36] | Murine adipocyte cell line (3T3-L1) | Pretreatment with 100 µM for 8 h; 25, 50, and 100 µM for 8 h; 100 µM for 3, 6, 8, and 12 h; 100 µM for 24 h |
Subsequent oxidative stress by 0.5 mM for 24 h | Basal condition: ↔ SOD1, SOD2, CAT, and GPx mRNA expression at 100 µM for 8 h | Basal condition: ↑ HO-1 and NQO1 mRNA expression at 100 µM for 8 h; ↑ HO-1 mRNA expression at 50 and 100 µM for 8 h; ↑ protein expression at 100 µM for 24 h; ↔ ROS formation at 100 µM for 1 h |
| Oxidative condition: ↓ ROS formation at 100 µM for 1 h | |||||
| [37] | Human breast carcinoma cell line (MDA-MB-231) | 30 µM for 7 d | Concomitant oxidative stress by 5 nM doxorubicin for 7 d | Basal condition: ↓ GPx1 activity and ↑ GSH content; ↔ SOD and CAT activity; ↔ GPx1 mRNA and protein expression | Basal condition: ↔ γGCL and GR activity |
| Oxidative conditions: ↓ GPx1 activity and ↑ GSH content; ↔ SOD and CAT activity; ↔ and ↓ GPx1 mRNA and protein expression, respectively | Oxidative condition: ↑ γGCL and GR activity | ||||
| Human breast carcinoma cell line (MCF-7) | 30 µM for 7 d | Concomitant oxidative stress by 20 nM doxorubicin for 7 d | Basal condition: ↔ SOD, CAT, GPx1 activity, and GSH content | Basal conditions: ↑ and ↔ γGCL and GR activity, respectively | |
| Oxidative condition: ↔ SOD and CAT activity; ↑ GPx1 activity and ↓ GSH content | Oxidative conditions: ↑ and ↔ γGCL and GR activity, respectively | ||||
| [38] | Human hepatoma cell line (HepG2) | 60 µM DHA for 21 h | Not present | ↑ SOD and GPx activity; ↑ GSH content; ↔ CAT activity | ↑ GST activity; ↔ TAC and CD content |
| [39] | Human hepatoma cell line (HepG2) | 3.13, 6.25, 12.5, 25, and 50 µM for 24 h | Not present | ↑ TAC at 6.25 µM; ↑ GSH content at 25 and 50 µM | ↔ HO-1, NQO1, and GSTM2 mRNA expression; ↑ Nrf2 mRNA expression at 3.13 µM; ↑ ARE-mediated gene transcription at 6.25, 12.5, 25, and 50 µM |
| [40] | Human hepatoma cell line (HepG2) | Pretreatment with 60 µM for 21 h | Subsequent oxidative stress by 0.2 mM H2O2 for 20 min and 1 h | 20 min oxidative stress: ↔ SOD, CAT, and GPx activity | 20 min oxidative stress: ↔ GST activity |
| 1 h oxidative stress: ↑ SOD activity; ↑ TAC and GSH content; ↔ GPx and CAT activity | 1 h oxidative stress: ↔ GST activity; ↔ TBARS and CD content | ||||
| [41] | Human hepatoma cell line (HepG2) | 50 and 100 µM for 48 h | Not present | ↑ TAC; ↑ FRAP and GPx activity at 100 µM; ↑ SOD and CAT activity; ↑ SOD2 and GPx1 mRNA expression at 100 µM; ↑ CAT mRNA expression | ↓ mitochondrial ROS; ↓ ROS level at 100 µM; ↑ ADP/ATP ratio and ↓ ATP level at 100 µM; ↑ MMP level; ↔ ND1, ND5, and Nrf2; ↑ COX1, COX3, TFAM, TFB1M, TFB2M, Nrf1, ERRα, and PGC1α mRNA expression at 100 µM |
| [42] | Human hepatoma cell line (HepG2) | Pretreatment with 50 µM for 24 h | Subsequent oxidative stress by 6 mM H2O2 for 1 h | Oxidative condition: ↑ CAT and GPx activity; ↔ SOD activity | Oxidative condition: ↓ MDA content |
| [43] | Mouse hepatocyte cell line (AML12) | Pretreatment with 50 µM for 12 h | Subsequent oxidative stress by 0.4 mM H2O2 for 2 h | Oxidative condition: ↑ SOD1 protein expression | Oxidative condition: ↑ HO-1, total and mitochondrial LC3BI/II, beclin1, parkin, PINK1, PGC1α, and p-ERK/ERK protein expression; ↓ P62/SQSTM2 and Drp1 protein expression; ↔ p-AMPK/AMPK; ↓ ROS content and apoptosis; ↑ MMP |
| [44] | Mouse hippocampal nerve cell line (HT22) | 17.2 µM for 48 h and 54 h | Not present | ↔ SOD1, SOD2, CAT, GPX1, and PRDX5 mRNA expression at 48 h; ↑ PRDX2 and PRDX3 mRNA expression at 48 h; ↓ PRDX4 mRNA expression at 48 h; ↑ GPx activity; ↑ total GS and GSH at 54 h | ↔ TXNIP, TXNRD3, GLRX1, m/c GLRX2, and NXN mRNA expression at 48 h; ↑ TXN1, TXN2, TXNRD1, TXNRD2, GCLC, GR, mGPx4, m/cGPx4, nGPx4, and SRXN1 mRNA expression at 48 h; ↔ GST activity; ↑ TBARS content; ↑ GR and GPx4 activity; ↑ TrxR at 54 h; ↑ cell DHA content at 48 h |
| [45] | Rat glioblastoma cell line (C6) | 25, 50, and 75 µM for 24, 48, and 72 h | Not present | ↔ GSH content for 24 h; ↑ CAT activity at 50 µM and 75 µM for 48 h; ↑ GPx activity at 25 µM for 24 h and 48 h; ↑ and ↓ GPx activity at 50 µM for 24 h and 75 µM for 48 h, respectively | ↑ cell DHA and ROS content; ↓ TBARS content at 24 h and ↑ at 72 h; ↑ TBARS content at 75 µM for 48 h; ↔ G6PDH for 48 h |
| [46] | Human neuroblastoma cell line (SH-SY5Y) | Pretreatment with 0.01, 0.1, and 1 µM for 24 h | Subsequent oxidative stress by 3 µM MeHg for 2 h | Basal condition: ↔ GPx1 mRNA expression; ↑ CAT and SOD1 mRNA expression at 1 µM | Oxidative condition: ↓ ROS content |
| [47] | Mouse Schwann cell line (IMS32) | Pretreatment with 7.5 and 12.5 µM for 4 h; 7.5 µM for 3 h, 6 h, 12 h, and 24 h | Subsequent oxidative stress by 50 µM t-BHT for 1.5 h | Basal condition: ↑ CAT mRNA expression at 7.5 and 12.5 µM for 4 h; ↔ SOD and GPx mRNA expression at 7.5 and 12.5 µM for 4 h; ↑ CAT mRNA expression at 6 and 12 h; ↑ CAT activity at 12 h; ↑ GSH and ↔ GSSG content at 12 h | Basal condition: ↑ HO-1 and NQO1 mRNA expression at 7.5 and 12.5 µM for 4 h; ↑ HO-1 mRNA expression at 3, 6, and 12 h; ↑ NQO1 mRNA expression at 6, 12, and 24 h; ↑ HO-1 protein expression at 6, 12, and 24 h; ↑ NQO1 protein expression at 12 and 24 h; ↑ ARE activity at 12 h; ↑ and ↓ nuclear and cytoplasmic Nrf2 protein expression at 3 and 6 h, respectively |
| Oxidative condition: ↓ ROS production | |||||
| [48] | Rat pheochromocytoma cell line (PC12) | Pretreatment with 40 µg/mL PS-containing DHA for 24 h | Subsequent oxidative stress by 0.2 mM H2O2 and 0.15 mM t-BHT for 4 h | H2O2 oxidative condition: ↑ TAC and SOD activity | t-BHT oxidative condition: ↓ caspase 9 and caspase 3 mRNA expression; ↔ akt-2 and GSK-3β mRNA expression; ↑ bcl-2 mRNA expression; ↓ bax and ↑ bcl-2 protein expression |
| t-BHT oxidative condition: ↑ TAC and SOD activity | |||||
| [49] | Rat pheochromocytoma cell line (PC12) | Pretreatment with 12.5 µM for 4 h | Subsequent stress by 50 µM Aβ25−35 for 48 h | Cytotoxic condition: ↑ TAC; ↔ GSH content and GSSG/GSH ratio | Cytotoxic condition: ↓ MDA and ROS content; ↑ nucleus/cytoplasmic Nrf2 ratio; ↔ γGCLC mRNA and protein expression; ↓ and ↔ γGCLM mRNA and protein expression, respectively; ↔ and ↑ HO-1 mRNA and protein expression, respectively; ↑ and ↔ NQO1mRNA and protein expression, respectively; ↓ CD36 mRNA and protein expression; ↓ SRB1 and FABP5 mRNA expression; ↔ SRB1 and FABP5 protein expression |
| [50] | Rat pheochromocytoma cell line (PC12) | Pretreatment with 60 µM for 24 h | Subsequent oxidative stress by 0.3 mM H2O2 for 24 h | Basal condition: ↑ SOD and GPx activity; ↔ CAT activity; ↑ GSH content | Basal: ↔ ROS, MDA, ascorbic acid, nitrate and nitrite content; ↔ MMP; ↔ bax and bcl2 mRNA expression and protein content; ↔ Nrf2 and HO-1 mRNA expression; ↑ Nrf2 and HO-1 protein expression |
| Oxidative condition: ↑ SOD and GPx activity; ↔ CAT activity; ↑ GSH content | Oxidative condition: ↓ ROS and MDA content; ↔ nitrate, nitrite, and ascorbic acid content; ↑ MMP; ↓ bax mRNA expression and protein content; ↑ bcl2 mRNA expression and protein content; ↑ Nrf2 and HO-1 mRNA and protein expression | ||||
| [51] | Rat pheochromocytoma cell line (PC12) | Pretreatment with 20, 40, and 80 µM for 24 h | Subsequent oxidative stress by 0.4 mM H2O2 for 12 h | Oxidative condition: ↑ SOD activity | Oxidative condition: ↓ ROS and MDA content; ↑ Na+/K+-ATPase and ↔ Ca2+/Mg2+-ATPase; ↑ p-TrkB, p-PLCγ1, p-CaMKII, p-ERK 1/2, and p-CREB protein expression; ↓ AchE and ↑ ChAT activity; ↑ Ach and GABA content; ↓ Glu content |
| [52] | Rat pancreatic ß cell line (INS-1E) | 25, 50, 75, and 100 µM for 48 h | Not present | ↔ SOD1 and SOD2 protein expression; ↑ GPx1 protein expression | ↔ TXN1, GPR40, glycosylated, and unglycosilated gp 91 protein expression; ↓ and ↔ ROS content at 100 µM and NO content, respectively; ↔ secreted and ↑ intracellular insulin content |
| [53] | Rat pancreatic acinar cell line (AR42J) | Pretreatment with 20 and 50 µM for 2 h | Subsequent inflammatory/oxidative stress by 0.1 µM cerulein for 1, 4, and 24 h | Basal condition: ↔ CAT mRNA and protein expression | Basal condition: ↔ IL-6 mRNA and p-JAK2, p-STAT3, nPPARγ, and cPPARγ protein expression; ↔ IL-6 and ROS content |
| Inflammatory/oxidative condition: ↑ CAT mRNA and protein expression | Inflammatory/oxidative condition: ↓ IL-6 mRNA and p-JAK2, p-STAT3, and cPPARγ protein expression; ↓ IL-6 and ROS content; ↑ nPPARγ protein expression | ||||
| [54] | Human monocytic cell line (THP-1) | 10, 25, 50, and 100 µM for 24 h | Concomitant inflammatory stress by 100 µg/mL (0.53 mM) MSU for 24 h | Basal condition: ↔ SOD protein expression at 50 µM | Basal condition: ↔ NLRP3 mRNA expression and NLRP3, caspase-1, and IL-1β precursor protein expression at 50 µM; ↓ mitochondrial ROS content at 50 µM; ↑ TXNIP, HO-1, and NQO1 protein expression at 50 µM; ↔ total, ↑ nuclear, and ↓ cytosolic Nrf2 protein expression at 50 µM |
| Inflammatory condition: ↓, ↔, ↑, SOD, GPx, and CAT mRNA expression, respectively; ↔ SOD protein expression at 50 µM | Inflammatory condition: ↓ IL-1β and TNF-α mRNA and protein expression; ↓ and ↔ NLRP3 mRNA and protein expression at 50 µM, respectively; ↓ IL-1β precursor and ↔ caspase-1 protein expression at 50 µM; ↓ ROS content and mitochondrial ROS content at 50 µM; ↑ HO-1 and NQO1 mRNA expression; ↑ TXNIP, HO-1, and NQO1 protein expression at 50 µM; ↔ total, ↑ nuclear, and ↓ cytosolic Nrf2 protein expression at 50 µM |
Effects refer to respective unsupplemented control cells either in basal or stressed conditions. When DHA concentrations/times are not reported, the effects refer to all experimental conditions tested. Abbreviations: ↑: increase; ↓: decrease; ↔: no effect; 8-OHdG: 8-hydroxy-2-deoxyguanosine; MeHg: metilmercury; Ach: acetylcholine; AchE: acetylcholinesterase; ADP: adenosine diphosphate; Akt-2: serine/threonine kinase 2; AMP: adenosine monophosphate; AMPK: phospho-AMP-activated protein kinase; ARE: antioxidant response element; ATP: adenosine triphosphate; Aβ25−35: amyloid beta-peptide 25-35; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; cAMP: cyclic adenosine monophosphate; CAT: catalase; CD: conjugated dienes; CD36: cluster of differentiation 36; cGMP: cyclic guanosine monophosphate; cGPx: cytosolic glutathione peroxidase; ChAT: choline acetyltransferase; COX: cyclooxygenase; COX1: cytochrome c oxidase I; COX3: cytochrome c oxidase subunit III; cPPARγ: cytosolic peroxisome proliferator-activated receptor gamma; Cys: cysteine; DHA: docosahexaenoic acid; Drp1: dynamin-related protein 1; ERK: extracellular signal-regulated kinase; ERRα: estrogen-related receptor α; FABP5: fatty acid binding protein 5; FRAP: ferric reducing antioxidant power; G6PDH: glucose-6-phosphate dehydrogenase; GABA: gamma-aminobutyric acid; GCLC: glutamate cysteine ligase, catalytic subunit; GLRX1; glutaredoxin 1; Glu: glutamic acid; GPR40: G-protein-coupled receptor 40; GPx: glutathione peroxidase; GPx1: glutathione peroxidase 1; GPx4: phospholipid-hydroperoxide glutathione peroxidase 4; GR: glutathione reductase; GS: glutathione; GSH: reduced glutathione; GSK-3β: glycogen synthase kinase 3 beta; GSSG: glutathione oxidized; GST: glutathione S-transferase; GSTM2: glutathione S-transferase mu 2; H2O2: hydrogen peroxide; HO-1: heme oxygenase-1; HRE: hypoxia response element; IL-1β: interleukin-1β; LC3BI/II: microtubule-associated protein 1 light chain 3 isoform B; m/cGLRX2: mithocondrial/cytoplasmic glutaredoxin 2; m/cGPx4: mithocondrial/cytosolic phospholipid-hydroperoxide glutathione peroxidase 4; MDA: malondialdehyde; mGPx4: mithocondrial phospholipid-hydroperoxide glutathione peroxidase 4; MMP: mitochondrial membrane potential; MSU: monosodium urate; NADH: nicotinamide adenine dinucleotide; ND1: NADH-ubiquinone oxidoreductase chain 1; ND5: NADH-ubiquinone oxidoreductase chain 5; nGPx4: nuclear phospholipid-hydroperoxide glutathione peroxidase 4; NLRP3: NLR family pyrin domain containing 3; NO: nitric oxide; nPPARγ: nuclear peroxisome proliferator-activated receptor gamma; NQO1: NAD(P)H quinone dehydrogenase 1; Nrf1: nuclear respiratory factor 1; Nrf2: nuclear factor (erythroid-derived 2)-like 2; NXN: nucleoredoxin; P62: ubiquitin-binding protein p62; p-AMPK: phospho-AMP-activated protein kinase; PC: phosphatidylcholine; p-CaMKII: phospho-Ca2+/calmodulin-dependent protein kinase II; p-CREB: phospho-cyclic adenosine monophosphate response element-binding protein; PDE: phosphodiesterase; PE: phosphatidyletanolamine; p-ERK 1/2: phospho-extracellular signal-regulated kinases 1/2; p-ERK: phosphor-extracellular signal-regulated kinase; PGC1α: peroxisome proliferator-activated receptor gamma coactivator 1α; PINK1: phosphatase and tensin homolog-induced kinase 1; p-JAK2: phospho-janus kinase 2; PL: phospholipid; p-PLCγ1: phospho-phospholipase C, gamma 1; PRDX2: peroxiredoxin 2; PRDX3: peroxiredoxin 3; PRDX4: peroxiredoxin 4; PRDX5: peroxiredoxin 5; PS: phosphatidylserine; p-STAT3: phospho-signal transducer and activator of transcription 3; ROS: reactive oxygen species; SOD: superoxide dismutase; SOD1: superoxide dismutase 1; SOD2: superoxide dismutase 2; SQSTM2: sequestosome-2; SRB1: scavenger receptor, class B type 1; SRXN1: sulforedoxin 1; TAC: total antioxidant capacity; TBARS: thiobarbituric acid reactive substances; t-BHT: tert-butylhydroperoxide; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; TFAM: mitochondrial transcription factor A; TFB1M: dimethyladenosine transferase 1, mitochondrial; TFB2M: dimethyladenosine transferase 2, mitochondrial; TNF-α: tumor necrosis factor-α; TOS: total oxidative stress; TrkB: tropomyosin receptor kinase B; TXN1: thioredoxin 1; TXN2: thioredoxin 2; TXNIP: thioredoxin-interacting protein; TXNRD1: thioredoxin reductase 1; TXNRD2: thioredoxin reductase 2; TXNRD3: thioredoxin reductase 3; UCB: unconjugated bilirubin; α-TC: α-tocopherol; γGCL: γ-glutamate cysteine ligase; γGCLC: γ-glutamate cysteine ligase catalytic subunit; γGCLM: γ-glutamate cysteine ligase modifier subunit; and γTC: γ-tocopherol.
The number of available studies is limited to 29 cases. Eight of them used primary cells, while twenty-one relied on cell lines. Primary cells included human fibroblast [28] and peripheral blood mononuclear cells [26], bovine endothelial cells [27], carp brain cells [31], and rat thymocytes [26], hepatocytes [29], hippocampal neurons [30], and astrocytes [32,33]. Among cell culture studies, six were conducted on hepatocytes [38,39,40,41,42,43], four on nervous system cells [44,45,46,47], four in adrenal cells [48,49,50,51], two on pancreatic [52,53] and breast cells [37], and only one study was conducted on ovarian [34], skeletal muscle [35], adipocyte [36], and monocyte cells [54].
The studies listed above cover a wide variety of the effects of DHA supplementation on antioxidant defense systems. However, comparing the reported results is difficult because essential experimental conditions varied significantly between individual labs. Among the studies, the DHA concentration spanned a wide range of concentrations (from approximately 0.01 µM [46] to 150 µM [34]) as did supplementation times (from 1 h [26,33,34] to 10 days [27]). In addition, 10 studies were conducted in basal conditions [27,28,34,35,38,39,41,44,45,52], 8 studies with simultaneous or subsequent exogenous stress [26,30,40,42,43,48,49,51], and 11 studies included both conditions [29,31,32,33,36,37,46,47,50,53,54]. Among them, 20 studies measured the enzymatic activity [26,29,30,31,32,33,35,37,38,39,40,41,42,44,45,47,48,49,50,51] while 19 and 8 studies evaluated the antioxidant defenses at the post-transcriptional [27,28,30,31,33,35,37,38,39,40,43,44,45,47,49,50,52,53,54] and transcriptional level, respectively [36,37,41,44,46,47,53,54]. Furthermore, two studies evidenced an increase in cellular oxidative markers [27,45], eight studies found a decrease [26,29,31,42,45,49,50,51], and four studies reported no noticeable effects [29,38,40,50].
Despite these limitations, most of the studies (24) reported how DHA supplementation was able to positively regulate the inducible expression and/or activity of antioxidant species [27,28,29,30,32,33,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Four studies reported no noticeable outcomes [26,31,35,36] and only three reported a negative effect [26,27,34]. Taken together, the results summarized here indicate how DHA may represent an effective promoter of cellular antioxidant defenses. In any case, future studies should investigate a possible inhibitory effect of DHA in modulating antioxidant defenses as reported in some studies.
3.2. Effect of DHA Treatment on Antioxidant Defenses in Animal Models
Table 2 summarizes the studies based on the use of in vivo preclinical animal models employed to investigate the possible use of a DHA-rich diet or DHA treatment in the modulation of cellular antioxidant defenses.
Table 2.
Summary of findings related to the effect of DHA feeding in animal models.
| Ref. | Animal Model | DHA Somministration | Treatment | Primary Outcomes | Secondary Metabolic Outcomes |
|---|---|---|---|---|---|
| [55] | Male Wistar rats | 250 and 1500 mg/d/kg bw for 5 d by gastric intubation | Not present | 250 mg/d/kg bw: ↔ plasmatic and hepatic GSH content | 250 mg/d/kg bw: ↔ plasmatic VE, VA, MDA, Cys, and Cys-Gly content; ↔ hepatic VE, VA, MDA, Cys, and HC content; ↔ hepatic XOX, XDH, and ACO activity |
| 1500 mg/d/kg bw: ↔ hepatic SOD1, SOD2 mRNA expression; hepatic GPx and SOD activity; ↔ plasmatic and hepatic GSH | 1500 mg/d/kg bw: ↔ plasmatic VA, Cys, Cys-Gly, and HC; ↑ and ↓ plasmatic MDA and VE; ↔ hepatic VE, VA, AsA, and Cys content; ↑ hepatic MDA content and ACO activity; ↔ hepatic XOX, GR, GST, and XDH activity | ||||
| [56] | Male AsA-requiring ODS rats | DHA-rich diet (5 g/kg diet), ad libitum for 12 h/d for 32 d | Not present | ↑ and ↔ hepatic and renal GSH content, respectively | ↑ hepatic CD, TBARS, and DHA content; ↓ hepatic α-TC and AsA content; ↑ renal TBARS and DHA content; ↓ renal AsA content; ↔ testicular TBARS level and AsA content; ↑ testicular DHA content |
| [57] | Male Wister rats | 13.3 mg/d/kg bw for 12w by oral gavage | STZ-induced diabetes (single dose at 65 mg/kg bw) | Normal rats: ↔ retinal GSH content and GPx activity | Normal rats: ↔ retinal MDA and nitrotyrosine content; ↔ total retinal, INL, and ONL thickness; ↔ n° of cells in retinal GC layer, n° of row in retinal ONL, and n° of row in retinal INL; ↔ apoptosis in retinal cells |
| Diabetic rats: ↑ retinal GSH content and GPx activity | Diabetic rats: ↔ and ↓ retinal nitrotyrosine and MDA content, respectively; ↔ total retinal, INL, and ONL thickness; ↔ n° of ganglion cells, n° of row in ONL, and n° of row in INL; ↓ apoptosis in retinal cells | ||||
| [58] | Male Wistar rats | 13.3 mg/d/kg bw for 12w by oral gavage | STZ-induced diabetes (single dose at 65 mg/kg bw) | Normal rats: ↔ cortexal GSH content and GPx activity | Normal rats: ↔ cortexal MDA content and n° of 4-HNE positive cells |
| Diabetic rats: ↑ cortexal GSH content and GPx activity | Diabetic rats: ↓ cortexal MDA content and n° of 4-HNE positive cells | ||||
| [59] | Sprague-Dawley rats | 2.5 mg/d/kg bw for 36 d by oral gavage | PTZ-induced epilepsy (repetitive doses ranging from 45 to 10 mg/kg bw) | Epileptic rats: ↑ cerebral SOD and CAT activity; ↑ and ↔ hepatic CAT and SOD activity, respectively | Epileptic rats: ↓ and ↔ hepatic and cerebral NO content, respectively |
| [60] | Sprague-Dawley rats | Intraperitoneally 250 mg/d/kg bw for 21 d | TCDD-induced toxicity (8 µg/d/kg bw for 21 d) | Normal rats: ↑ hepatic GSH content and SOD, CAT, and GPx activity | Normal rats: ↔ MNHEP |
| Stressed rats: ↑ hepatic GSH content and SOD, CAT, and GPx activity | Stressed rats: ↓ MNHEP | ||||
| [61] | Sprague-Dawley rats | 2.5 mg/d/kg bw for 36 d by oral gavage | Not present | ↑ cerebral CAT and SOD activity; ↑ hepatic GPx and SOD activity; ↔ hepatic CAT and cerebral GPx activity | ↔ cerebral and hepatic NO content |
| [62] | Male Wistar rats | 100 mg/d/kg bw for 90 d by oral gavage | AlCl3-induced neurotoxicity (100 mg/d/kg bw for 90 d) | Normal rats: ↔ cerebral SOD, CAT, and GPx activity | Normal rats: ↔ cerebral GR activity; ↔ cerebral lipoperoxides and protein carbonyl content |
| Stressed rats: ↑ cerebral SOD, CAT, and GPx activity | Stressed rats: ↔ cerebral GR activity; ↓ cerebral lipoperoxides and protein carbonyl content | ||||
| [63] | Male offspring Wistar rats | 75, 150, and 300 mg/d/kg for 21 d by oral gavage | VPA-induced autism (single dose to pregnant rats at 600 mg/kg bw) | Autistic rats: ↑ hippocampal GSH content and SOD and GPx activity at 150 and 300 mg/d/kg bw | Autistic rats: ↓ hippocampal MDA content at 150 and 300 mg/d/kg bw; ↓ hippocampal caspase-3 protein expression and activity, and TUNEL-positive cells at 150 and 300 mg/d/kg bw; ↑ hippocampal BDNF, p-Akt/Akt, p-CaMKII/CaMKII, and p-CREB/CREB protein expression at 150 and 300 mg/d/kg bw; ↑ and ↓ hippocampal Bcl-2 and Bax protein expression, respectively; ↑ hippocampal and plasmatic DHA content at 150 and 300 mg/d/kg bw |
| [64] | Male Wistar rats | Single administration of 555 mg/kg bw by intragastric gavage | Fluid percussion-induced brain traumatic injury | Brain injured rats: ↑ hippocampal SOD and GPx activity | Brain injured rats: ↓ hippocampal MDA content; ↓ hippocampal caspase-3, and Bax protein expression; ↑ and ↓ hippocampal Bcl-2 protein expression and TUNEL-positive cells, respectively; ↑ and ↓ hippocampal nuclear and cytoplasmic Nrf2 protein expression, respectively; ↑ hippocampal HO-1 and NQO1 mRNA and protein expression |
| [65] | Sprague-Dawley rats | DHA-rich diet (10 g/kg diet) ad libitum for 24 d | Not present | ↑ plasmatic TAC and erythrocytic SOD activity; ↔ whole blood GPx activity | ↔ plasmatic TG, TC, CE, HDL-C, non HDL-C, ASAT, ALAT, MDA, TNF-α, IL-2, and IL-6 content; ↑ DHA content in liver, heart, lung, spleen, kidney, and red blood cells |
| [66] | Sprague-Dawley newborn rats | 300 mg/d/kg bw for 21 d | Pregnant intrauterine growth restriction | Normal newborn rat: ↔ spleenic GPx and CAT mRNA expression | Normal newborn rat: ↔ spleenic IL-4 and INF-γ mRNA expression; ↔ spleenic Bax and Bcl2 protein expression |
| Newborn rat from intrauterine growth restricted pregnant: ↑ spleenic GPx and CAT mRNA expression | Newborn rat from intrauterine growth restricted pregnant: ↑ spleenic IL-4 and INF-γ mRNA expression; ↓ and ↑ spleenic Bax and Bcl2 protein expression, respectively | ||||
| [67] | Male BALB-c mice | Single administration of 100 mg/kg bw by oral gavage | Indomethacin-induced gastric lesion (single dose at 30 mg/kg bw) | Normal mice: ↑ gastric GSH content; ↔ gastric SOD and CAT activity | Normal mice: ↔ gastric MDA, protein carbonyl, MPO, LTB4, ICAM-1, RvD1, and NF-kB content; ↓ TNF-α content; ↔ gastric FFA4 protein expression |
| Gastric injured mice: ↑ gastric GSH content; ↔ gastric SOD and CAT activity | Gastric injured mice: ↓ gastric MDA, protein carbonyl, MPO, LTB4, ICAM-1, TNF-a, and NF-kB content; ↑ gastric RvD1 content and FFA4 protein expression | ||||
| [68] | Male C57BL/6J mice | 50 mg/d/kg bw for 12 weeks | HFD-induced steatosis (60% fat, 20% protein, and 20% carbohydrate), ad libitum for 12 weeks | Normal mice: ↔ hepatic GSH content and GPx, SOD, and CAT activity; ↔ serum TAC | Normal mice: ↔ hepatic SREBP-1c, PPAR-α, NF-kB, and Nrf2 DNA binding activity; ↔ hepatic SREBP-1c, PPAR-α, NF-kB, Nrf2, ACC, FAS, SCD-1, CPT-1α, ACOX-1, TNF-α, IL-6, and IL-1β mRNA expression; ↔ hepatic ACC, FAS, GR, GST, GGT, NQO1, CPT-1α, and ACO activity; ↔ hepatic protein carbonyl, F-8 isoprostane, and TBARS content; ↑ hepatic RvD1 and RvD2 content; ↔ hepatic RvE1 and RvE2; ↔ serum TNF-α, IL-6, and IL-1β content |
| Steatotic mice: ↑ hepatic GSH content and GPx, SOD, and CAT activity; ↔ serum TAC | Steatotic mice: ↑ hepatic PPAR-α and Nrf2 DNA binding activity; ↓ hepatic SREBP-1c and NF-kB DNA binding activity; ↑ hepatic PPAR-α, CPT-1α, ACOX-1, and Nrf2 mRNA expression; ↓ hepatic SREBP-1c, ACC, FAS SCD-1, NF-kB, TNF-α, IL-6, and IL-1β mRNA expression; ↑ hepatic GR, GST, GGT, NQO1, CPT-1α, and ACO activity; ↓ hepatic ACC and FAS activity; ↓ hepatic protein carbonyl, F-8 isoprostane, and TBARS content; ↔ hepatic RvE1 and RvE2 content; ↑ hepatic RvD1 and RvD2 content; ↓ serum TNF-α, IL-6, and IL-1β content | ||||
| [43] | Male C57BL/6J mice | 50 mg/d/kg bw for 7 d by intragastric gavage | CCl4-induced liver toxicity (single dose at 0.2 mL/kg bw) | Liver injured mice: ↑ hepatic SOD activity and GSH content | Liver injured mice: ↓ serum LDH and ALAT activity; ↔ serum ASAT activity; ↑ hepatic LC3BI/II, beclin1, and P62/SQSTM2 protein expression; ↔ hepatic MDA content |
Effects refer to respective unsupplemented or placebo control animals either in normal or injured conditions. When DHA concentrations are not reported, the effects refer to all experimental conditions. In the presence of more than one concentration, only statistically significant changes are reported. Abbreviations: ↑: increase; ↓: decrease; ↔: no effect; 4-HNE: 4-hydroxy-2-nonenal; ACC: acetyl-CoA carboxylase; ACO: acyl-CoA oxidase; ACOX-1: peroxisomal acyl-coenzyme A oxidase 1; Akt: serine/threonine kinase; ALAT: alanine aminotransferase; AsA: ascorbic acid; ASAT: aspartate transaminase; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; BDNF: brain-derived neurotrophic factor; Bw: body weight; CaMKII: Ca2+/calmodulin-dependent protein kinase II; CAT: catalase; CD: conjugated dienes; CE: cholesterol esters; CPT-1α: carnitine-palmitoyl transferase 1α; CREB: cyclic adenosine monophosphate response element-binding protein; Cys: cysteine; Cys-gly: cysteinylglycine; DHA: docosahexaenoic acid; dUTP: deoxyuridine triphosphate; EtOH: ethanol; FAS: fatty acid synthase; FFA4: free fatty acid receptor 4; GGT: γ-glutamyltransferase; GPx: glutathione peroxidase; GPx1: glutathione peroxidase 1; GR: glutathione reductase; GSH: reduced glutathione; GST: glutathione-S-transferase; HC: homocysteine; HDL-C: high density lipoproteins cholesterol; HFD: high-fat diet; HO-1: heme oxygenase-1; ICAM-1: intercellular adhesion molecule 1; IL-1β: interleukin 1β; IL-2: interleukin 2; IL-4: interleukin 4; IL-6: interleukin 6; INF-γ: interferone-γ; INL: inner nuclear layer; LC3BI/II: microtubule-associated protein 1 light chain 3 isoform B; LDH: lactic dehydrogenase; LTB4: leukotriene B4; MDA: malondialdehyde; MNHEP: micronucleated hepatocytes; MPO: myeloperoxidase; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO: nitric monoxide; NQO1: NAD(P)H quinone dehydrogenase 1; Nrf2: nuclear factor (erythroid-derived 2)-like 2; ODS: osteogenic disorder-Shionogi; ONL: outer nuclear layer; P62: ubiquitin-binding protein p62; p-Akt: phospho-serine/threonine kinase; p-CaMKII: phospho-Ca2+/calmodulin-dependent protein kinase II; p-CREB: phosphor-cyclic adenosine monophosphate response element-binding protein; PPAR-α: Peroxisome proliferator-activated receptor α; PTZ: pentylenetetrazol; RvD1: resolvin D1; RvD2: resolvin D2; RvE1: resolvin E1; RvE2: resolvin E2; SCD-1: stearoyl-CoA-desaturase-1; SOD: superoxide dismutase; SOD1: superoxide dismutase1; SOD2: superoxide dismutase2; SQSTM2: sequestosome-2; SREBP-1c: sterol regulatory element-binding transcription factor 1; STZ: streptozotocin; TAC: total antioxidant capacity; TBARS: thiobarbituric acid reactive substances; TC: total cholesterol; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; TG: triacylglycerol; TNF-α: tumor necrosis factor α; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; VA: vitamin A; VE: vitamin E; VPA: valproic acid; XDH: xanthine dehydrogenase; XOX: xanthine oxidase; and α-TC: α-tocopherol.
The number of studies conducted on animal models is limited to 15 cases. Twelve of them involved rats [55,56,57,58,59,60,61,62,63,64,65,66], whereas three were conducted in mice [43,67,68]. Seven studies were focused on the liver [43,55,56,59,60,61,68], six on the nervous system [58,59,61,62,63,64], three on plasma/serum [55,65,68], and only one study was reported for the kidney [56], retina [57], spleen [66], stomach [67], and erythrocytes [65]. In addition, four studies were carried out in normal conditions [55,56,61,65], four studies in association with injuries [43,59,63,64], and seven studies in both conditions [57,58,60,62,66,67,68].
Again, the DHA treatment spanned a range from 2.5 [59,61] to 1500 mg/d/kg bw [55] or a diet containing DHA from 5 [56] to 10 g/kg diet [65], with a time of somministration ranging from 1 day (single administration) [64,67] to 90 days [62].
Among them, 13 studies measured the enzymatic activity [43,55,57,58,59,60,61,62,63,64,65,67,68], while 9 and 2 studies evaluated the antioxidant defenses at the post-transcriptional [43,55,56,57,58,60,63,67,68] and transcriptional level, respectively [55,66]. Furthermore, one study evidenced an increase in cellular oxidative markers [56], four studies found a decrease [57,63,64,67], and nine studies reported no noticeable effects [43,55,56,57,58,62,65,67,68].
Despite these limitations, 14 studies reported a promoting effect of DHA supplementation on at least one expression or activity of antioxidant molecules [43,56,57,58,59,60,61,62,63,64,65,66,67,68], whereas only an individual study reported no effects [55].
3.3. Role of DHA in the Intracellular Redox Homeostasis Mechanism
To maintain their energy metabolism, mammalian cells have evolved to use oxygen as a final electron acceptor. Consequently, they must deal with a collection of undesired oxygenated byproducts produced due to these oxygen-dependent metabolic processes. These oxygenated byproducts are collectively referred to as ROS [69]. At low levels, ROS can undergo reactions with biological macromolecules contributing to redox signaling and biological function [69], but—at supraphysiological concentrations—they may undergo aspecific reactions that generate other reactive species with potentially toxic consequences [70].
The susceptibility of fatty acids to oxidation is thought to depend directly on their degree of unsaturation. Reportedly, the oxidation rate of a fatty acid or its esters is typically increased by at least one or two factors for every additional double bond in a fatty acid [71], thus placing DHA in the highest ranks among oxidizable species. However, various in vitro [26,31,40,42,49,50,51] and in vivo studies [43,57,58,63,64,67,68] suggest that the relation between the chemical structure of DHA and, even from a theoretical standpoint, its vulnerability to ROS oxidation is not as easy to predict.
Most of the selected in vitro and in vivo studies considered in this review reported a general promoting effect at the transcriptional or post-transcriptional level. Given the evidence for the role of DHA in the development of chronic diseases, it appears necessary to assess the molecular mechanism at the basis of the protective role of DHA.
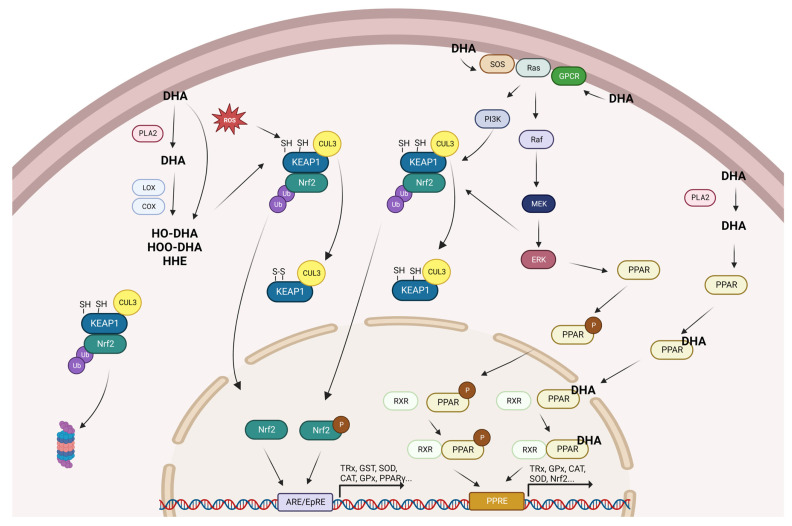
The cap’n’collar basic-region leucine zipper transcription factor Nrf2, encoded by NFE2L2, is a master regulator of intracellular redox homeostasis because, in response to oxidative stress, it orchestrates induction of a battery of genes such as SOD, NAD(P)H quinone dehydrogenase 1, and heme oxygenase-1 that serve to increase the antioxidant and detoxifying capacity of the cell [72]. This tight control of Nrf2 is achieved by a repressor protein Keap1, a cysteine redox-sensitive factor, that, under normal conditions, serves as a Nrf2-specific adaptor protein for the Cullin-3 ubiquitin ligase complex and perpetually targets Nrf2 in the cytoplasm for degradation by the 26S proteasome [73]. Dissociation of Keap1/Nrf2 complex by oxidants leads to transportation and accumulation of Nrf2 to the nucleus where it binds ARE/EpRE sequences in the promotor region of several genes related to phase II drug conjugation, scavenging of H2O2, and GSH- and Trx-based antioxidant systems [74]. Since oxidative stress is associated with several diseases [75,76,77] and many of these ailments have been demonstrated to be prevented by DHA [78,79,80], it might not surprising that DHA could reversibly activate Nrf2 and promote induction of cellular antioxidant defenses. DHA itself is not a ligand for Nrf2, so one crucial issue that remains to be determined is the endogenous activation underlying the transcriptional responses elicited by DHA.
Under physiological conditions, DHA can be oxidized enzymatically or non-enzymatically. In the enzymatic oxidation pathway, cyclooxygenase and lipooxygenase catalyze the conversion of DHA to produce a large variety of oxidation metabolites, including hydroperoxide and hydroxide positional isomers [81]. In addition, ROS can oxidize DHA through non-enzymatic reactions that release highly electrophilic species, including neuroprostane and hydroperoxide break-down products such as 4-hydroxy-2-hexenal (HHE) [82,83]. As a result, oxidized DHA, or its derived electrophilic species, may react with Keap1 sulfhydryls, altering Keap1 secondary structure which is followed by a loss of association between Keap1 and Cullin-3. This in turn inhibits Nrf2 ubiquitination, leading to stabilization and nuclear translocation of Nrf2 and to the subsequent induction of Nrf2 target genes [84]. In support of this hypothesis, one recent observation indicates that low but significant levels of HHE are generated upon DHA supplementation [85] just before changes in gene expression are observed. Furthermore, 15-deoxy-Δ12,14-prostagandin [86], F4 neuroprostanes [83], and HHE [87] have been demonstrated to be activators of Nrf2 and to induce expression of cytoprotective enzymes [88].
After incorporation in the plasma membrane, DHA may profoundly influence cellular membrane composition affecting membrane fluidity, phase behavior, permeability, fusion, flip-flop, and protein function [89]. DHA acyl chains have been shown to affect bilayer properties including lateral pressure, microviscosity, curvature, permeability, elasticity, microdomain formation, and hydrophobic match due its high conformational flexibility arising from the low potential energy barriers to rotation about the single carbon–carbon bonds [90]. Moreover, DHA infiltrates rafts and non-raft membrane microdomains, disrupts raft clustering, and increases the size of rafts [91]. The rearrangement of membrane microdomains may have implications for the raft platform signaling and collocation of the transmembrane protein into or out of rafts. Membrane physical properties are mainly affected by lipid composition, and as previous studies have indicated, the activity of G protein-coupled receptors (GPCRs) located in plasma membranes are influenced by the surrounding fluidic membranes [92,93]. Recently, Yoshida et al. demonstrated, using nanodisc technology to control membrane properties, that increased membrane fluidity shifted the equilibrium toward an active form of the receptor through conformational changes [94]. After activation, GPCR transmits signals by downstream pathways leading to the regulation of physiological processes, including antioxidant response [95]. DHA-containing lipids enhance the function of the prototypical GPCR rhodopsin, which simulation studies have explained takes place as a result of the high conformational flexibility of DHA chains [96]. This provides hybrid lipids with a high affinity for the rough surface of GPCRs, further promoting protein–protein interactions.
The Ras/Raf/MAP/ERK kinase (MEK)/ERK cascade couples signals from cell surface receptors to transcription factors [97] involving the MAPK cascade [98]. Nicolini et al. reported that N-Ras prefers a liquid-disordered lipid phase, regardless of the lipid anchor system [99]. Furthermore, several protein kinases such as protein kinase C (PKC), mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), c-Jun N-terminal kinase (JNK), and extracellular-signal-regulated kinase (ERK) have been found to phosphorylate Nrf2 [100,101,102] blocking the KEAP1–Nrf2 interaction and the subsequent KEAP1-dependent proteasomal degradation of Nrf2 [103]. In this complicated but intriguing scenario, DHA-enriched membranes may facilitate Ras binding with the guanine nucleotide exchange factor SOS activating the Ras/Raf/MEK/ERK downstream signaling pathway, causing subsequent phosphorylation of Nrf2 and, thereby, its nuclear accumulation and ARE-driven transcription.
PPARs, including α, δ, and γ isoforms, comprise a subfamily of the nuclear receptor superfamily that is highly expressed in mammalian tissues [104]. Each PPAR subtype is located in the cytoplasm [105] and, after activation by specific ligands, enters to the nucleus heterodimerizing with the retinoid X receptor (RXR) before binding to the PPAR responsive element (PPRE) of specific target genes [106]. Natural products, including DHA and its metabolites, serve as endogenous PPARs ligands, which exert adaptive metabolic responses to changes in metabolic status in various tissues [107,108]. Several studies have shown that PPARs ligands can transcriptionally modulate antioxidants such as TRx [109], GPx [110,111], CAT [112], and SOD [21] due the presence of a PPRE in the promotor regions of the coding gene sequence [113,114]. In addition, evidence indicates that PPARs may be phosphorylated by several kinases such as protein kinase A, MEK/ERK, and p38 kinase [115,116,117,118], all of which affect the A/B domain of the receptor and modulate its ligand-independent AF-1 transactivating function [119]. Several studies also strongly support a reciprocal regulation of the Nrf2 and PPARγ pathways to reinforce the expression of one another [120,121,122,123]. In this sense, Nrf2 and PPARγ pathways seem to be connected by a positive collaborative feedback loop, which maintains the expression of both transcription factors and their target antioxidant genes in a simultaneous manner [124].
4. Conclusions
Considering the results obtained in the present review and the extensive search of relevant information available in the literature, we have proposed a scheme to give a logical explanation regarding the potential mechanisms of action of DHA in the modulation of cellular antioxidant defenses (Figure 2).
Figure 2.
Summary of the proposed mechanisms for the promotion of antioxidant gene expression by DHA. CAT: catalase; Cox: ciclooxygenase; Cul3: cullin 3; DHA: docosahexaenoic acid; ERK: extracellular signal-regulated kinases; GPCR: G protein-coupled receptors; GPx: glutathione peroxidase; GST: glutathione S-transferases; HO-DHA: hydroxy-DHA; HOO-DHA: hydroperoxy-DHA; Keap1: kelch-like ECH-associated protein 1; Lox: lipoxygenase; MEK: mitogen-activated protein kinase kinase; Nrf2: nuclear factor erythroid 2-related factor 2; P: phosphorylated; PI3K: phosphoinositide 3-kinase; PLA2: phospholipase A2; PPAR: peroxisome proliferator-activated receptor; ROS: reactive oxygen species; RXR: retinoid X receptor; SOD: superoxide dismutase; SOS: son of sevenless; and TRx: thioredoxin.
Despite DHA’s promising and encouraging effects at modulating the cellular antioxidant response, differences observed among the reviewed studies may be accounted for by the different experimental conditions adopted. In fact, unlike the genetically predetermined protein profile, the diet profoundly influences the acyl composition, and several studies have shown a time- and concentration-dependent effect on incorporating DHA into cellular lipids [125,126]. In this line, a very recent paper conducted in football players has demonstrated a dose–response incorporation of DHA into red blood cell membranes up to 6 g·d−1, which can be used to rapidly achieve a desired omega-3 index (>8%) in only 8 weeks [127].
In addition, although various cells readily take up DHA, its accumulation is organ-specific, with a higher content in the brain, liver, and heart respecting plasma, pancreas, and erythrocytes [128,129]. At a cellular level, the uptake of long chain fatty acids is mainly regulated by the fatty acid transporter CD36, a transmembrane glycoprotein highly expressed in tissue with high fatty acid uptake [130] with a pivotal role in cellular lipid homeostasis [131].
Moreover, DHA bioavailability depends on the chemical form it conveys. Recent evidence indicates that DHA esterified in phospholipid and triglyceride is more readily absorbed by the body than in ethyl ester form [132]. Taken together, discrepancies in these terms in the studies selected in this review may have determined substantial differences in DHA accumulation and should be deeply considered in future studies to evaluate the minimum effective treatment times and concentrations of DHA according to the cellular models adopted. In any case, further studies including pharmacokinetic/pharmacodynamic modeling and human trials which consider not only DHA in particular but n-3 PUFA in general should be taken into deep consideration.
To conclude, the identification of molecular mechanisms involved in redox metabolism is an important issue for the development of therapies for chronic disorders, and although further studies are needed, the comprehensive vision offered here may help to address future studies toward specific pathways and to provide molecular-based support to any recommendation pertaining the food/health relationship.
Author Contributions
Conceptualization, S.M.B. and M.D.N.; methodology, S.M.B., S.I. and M.D.N.; validation, S.M.B., S.I. and M.D.N.; formal analysis, S.M.B., S.I. and M.D.N.; investigation, S.M.B., S.I. and M.D.N.; data curation, S.M.B., S.I. and M.D.N.; writing—original draft preparation, M.D.N.; writing—review and editing, S.M.B., S.I. and M.D.N.; supervision, M.D.N.; funding acquisition, M.D.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partially supported by the Italian Piano di Sostegno della Ricerca 2021-Azione A (Linea 2), University of Milan (M.D.N.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ghini V., Di Nunzio M., Tenori L., Valli V., Danesi F., Capozzi F., Luchinat C., Bordoni A. Evidence of a DHA Signature in the Lipidome and Metabolome of Human Hepatocytes. Int. J. Mol. Sci. 2017;18:359. doi: 10.3390/ijms18020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bie N., Han L., Meng M., Yan Z., Wang C. The immunomodulatory effect of docosahexaenoic acid (DHA) on the RAW264.7 cells by modification of the membrane structure and function. Food Funct. 2020;11:2603–2616. doi: 10.1039/C9FO02618E. [DOI] [PubMed] [Google Scholar]
- 3.Sung N.J., Kim N.H., Bae N.Y., Jo H.S., Park S.A. DHA inhibits Gremlin-1-induced epithelial-to-mesenchymal transition via ERK suppression in human breast cancer cells. Biosci. Rep. 2020;40:BSR20200164. doi: 10.1042/BSR20200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira I., Falcato F., Bandarra N., Rauter A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules. 2022;27:1677. doi: 10.3390/molecules27051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai X., Shao J., Zhou S., Zhao Z., Li F., Xiang R., Zhao A.Z., Pan J. Inhibition of lung cancer growth and metastasis by DHA and its metabolite, RvD1, through miR-138-5p/FOXC1 pathway. J. Exp. Clin. Cancer Res. 2019;38:479. doi: 10.1186/s13046-019-1478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira I., Rauter A.P., Bandarra N.M. Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect. Mar. Drugs. 2022;20:662. doi: 10.3390/md20110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asztalos I.B., Gleason J.A., Sever S., Gedik R., Asztalos B.F., Horvath K.V., Dansinger M.L., Lamon-Fava S., Schaefer E.J. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism. 2016;65:1636–1645. doi: 10.1016/j.metabol.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Jiménez A., Zavala-Lira R.A., Moreno-Brito V., González-Rodríguez E. FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review. J. Clin. Med. 2023;12:318. doi: 10.3390/jcm12010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Righi V., Di Nunzio M., Danesi F., Schenetti L., Mucci A., Boschetti E., Biagi P., Bonora S., Tugnoli V., Bordoni A. EPA or DHA supplementation increases triacylglycerol, but not phospholipid, levels in isolated rat cardiomyocytes. Lipids. 2011;46:627–636. doi: 10.1007/s11745-011-3562-0. [DOI] [PubMed] [Google Scholar]
- 10.Naudí A., Jové M., Ayala V., Portero-Otín M., Barja G., Pamplona R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front Physiol. 2013;4:372. doi: 10.3389/fphys.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeb A. Chemistry and liquid chromatography methods for the analyses of primary oxidation products of triacylglycerols. Free Radic. Res. 2015;49:549–564. doi: 10.3109/10715762.2015.1022540. [DOI] [PubMed] [Google Scholar]
- 12.Bacellar I.O.L., Baptista M.S. Mechanisms of Photosensitized Lipid Oxidation and Membrane Permeabilization. ACS Omega. 2019;4:21636–21646. doi: 10.1021/acsomega.9b03244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aranda-Rivera A.K., Cruz-Gregorio A., Arancibia-Hernández Y.L., Hernández-Cruz E.Y., Pedraza-Chaverri J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen. 2022;2:437–478. doi: 10.3390/oxygen2040030. [DOI] [Google Scholar]
- 16.Islam M., Rauf A., Islam Fahad F., Emran T., Mitra S., Olatunde A., Shariati M.A., Rebezov M., Rengasamy K., Mubarak M. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2021;62:7282–7300. doi: 10.1080/10408398.2021.1913400. [DOI] [PubMed] [Google Scholar]
- 17.Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Raghunath A., Sundarraj K., Nagarajan R., Arfuso F., Bian J., Kumar A.P., Sethi G., Perumal E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018;17:297–314. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danesi F., Larsen B.D., Di Nunzio M., Nielsen R., de Biase D., Valli V., Mandrup S., Bordoni A. Co-Administration of Propionate or Protocatechuic Acid Does Not Affect DHA-Specific Transcriptional Effects on Lipid Metabolism in Cultured Hepatic Cells. Nutrients. 2020;12:2952. doi: 10.3390/nu12102952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordoni A., Astolfi A., Morandi L., Pession A., Danesi F., Di Nunzio M., Franzoni M., Biagi P., Pession A. N-3 PUFAs modulate global gene expression profile in cultured rat cardiomyocytes. Implications in cardiac hypertrophy and heart failure. FEBS Lett. 2007;581:923–929. doi: 10.1016/j.febslet.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 21.Korbecki J., Bobiński R., Dutka M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019;68:443–458. doi: 10.1007/s00011-019-01231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nunzio M., van Deursen D., Verhoeven A.J., Bordoni A. n-3 and n-6 Polyunsaturated fatty acids suppress sterol regulatory element binding protein activity and increase flow of non-esterified cholesterol in HepG2 cells. Br. J. Nutr. 2010;103:161–167. doi: 10.1017/S000711450999167X. [DOI] [PubMed] [Google Scholar]
- 23.Di Nunzio M., Danesi F., Bordoni A. n-3 PUFA as regulators of cardiac gene transcription: A new link between PPAR activation and fatty acid composition. Lipids. 2009;44:1073–1079. doi: 10.1007/s11745-009-3362-y. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iametti S., Bonomi F., Di Nunzio M. Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review. Int. J. Mol. Sci. 2022;23:14355. doi: 10.3390/ijms232214355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bechoua S., Dubois M., Dominguez Z., Goncalves A., Némoz G., Lagarde M., Prigent A.F. Protective effect of docosahexaenoic acid against hydrogen peroxide-induced oxidative stress in human lymphocytes. Biochem. Pharm. 1999;57:1021–1030. doi: 10.1016/S0006-2952(99)00012-X. [DOI] [PubMed] [Google Scholar]
- 27.Delton-Vandenbroucke I., Véricel E., Januel C., Carreras M., Lecomte M., Lagarde M. Dual regulation of glutathione peroxidase by docosahexaenoic acid in endothelial cells depending on concentration and vascular bed origin. Free Radic. Biol. Med. 2001;30:895–904. doi: 10.1016/S0891-5849(01)00482-8. [DOI] [PubMed] [Google Scholar]
- 28.Arab K., Rossary A., Flourié F., Tourneur Y., Steghens J.P. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating gamma-glutamyl-cysteinyl ligase and glutathione reductase. Br. J. Nutr. 2006;95:18–26. doi: 10.1079/BJN20051626. [DOI] [PubMed] [Google Scholar]
- 29.Turkez H., Geyikoglu F., Yousef M.I. Ameliorative effects of docosahexaenoic acid on the toxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in cultured rat hepatocytes. Toxicol. Ind. Health. 2016;32:1074–1085. doi: 10.1177/0748233714547382. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Zhao X., Mao Z.Y., Wang X.M., Liu Z.L. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14:2457–2461. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 31.da Silva Acosta D., Kneip F.C., Alves de Almeida E., Ventura-Lima J., Monserrat J.M., Geracitano L.A. Fullerene and omega-3 and omega-6 fatty acids on fish brain antioxidant status. Fish Physiol. Biochem. 2012;38:1477–1485. doi: 10.1007/s10695-012-9635-z. [DOI] [PubMed] [Google Scholar]
- 32.Becerir C., Kılıç İ., Sahin Ö., Özdemir Ö., Tokgün O., Özdemir B., Akca H. The protective effect of docosahexaenoic acid on the bilirubin neurotoxicity. J. Enzym. Inhib. Med. Chem. 2013;28:801–807. doi: 10.3109/14756366.2012.684053. [DOI] [PubMed] [Google Scholar]
- 33.Zgórzyńska E., Dziedzic B., Gorzkiewicz A., Stulczewski D., Bielawska K., Su K.P., Walczewska A. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharm. Rep. 2017;69:935–942. doi: 10.1016/j.pharep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Tuller E.R., Beavers C.T., Lou J.R., Ihnat M.A., Benbrook D.M., Ding W.Q. Docosahexaenoic acid inhibits superoxide dismutase 1 gene transcription in human cancer cells: The involvement of peroxisome proliferator-activated receptor alpha and hypoxia-inducible factor-2alpha signaling. Mol. Pharm. 2009;76:588–595. doi: 10.1124/mol.109.057430. [DOI] [PubMed] [Google Scholar]
- 35.da Silva E.P., Jr., Nachbar R.T., Levada-Pires A.C., Hirabara S.M., Lambertucci R.H. Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress Chaperones. 2016;21:87–95. doi: 10.1007/s12192-015-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusunoki C., Yang L., Yoshizaki T., Nakagawa F., Ishikado A., Kondo M., Morino K., Sekine O., Ugi S., Nishio Y., et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013;430:225–230. doi: 10.1016/j.bbrc.2012.10.115. [DOI] [PubMed] [Google Scholar]
- 37.Vibet S., Goupille C., Bougnoux P., Steghens J.P., Goré J., Mahéo K. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic. Biol. Med. 2008;44:1483–1491. doi: 10.1016/j.freeradbiomed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Di Nunzio M., Valli V., Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids. 2011;85:121–127. doi: 10.1016/j.plefa.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Saw C.L.L., Yang A.Y., Guo Y., Kong A.-N.T. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2–ARE pathway. Food Chem. Toxicol. 2013;62:869–875. doi: 10.1016/j.fct.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Di Nunzio M., Valli V., Bordoni A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016;67:834–843. doi: 10.1080/09637486.2016.1201790. [DOI] [PubMed] [Google Scholar]
- 41.Li G., Li Y., Xiao B., Cui D., Lin Y., Zeng J., Li J., Cao M.-J., Liu J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J. Agric. Food Chem. 2021;69:1647–1655. doi: 10.1021/acs.jafc.0c07751. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Bian Y., Luo X., Wang C., Mu D., Pan G., Wu J., Shi H. Synergistic effect of docosahexaenoic acid or conjugated linoleic acid with caffeic acid on ameliorating oxidative stress of HepG2 cells. J. Food Sci. 2021;86:3240–3251. doi: 10.1111/1750-3841.15775. [DOI] [PubMed] [Google Scholar]
- 43.Chen J., Wang D., Zong Y., Yang X. DHA Protects Hepatocytes from Oxidative Injury through GPR120/ERK-Mediated Mitophagy. Int. J. Mol. Sci. 2021;22:5675. doi: 10.3390/ijms22115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casañas-Sánchez V., Pérez J.A., Fabelo N., Herrera-Herrera A.V., Fernández C., Marín R., González-Montelongo M.C., Díaz M. Addition of docosahexaenoic acid, but not arachidonic acid, activates glutathione and thioredoxin antioxidant systems in murine hippocampal HT22 cells: Potential implications in neuroprotection. J. Neurochem. 2014;131:470–483. doi: 10.1111/jnc.12833. [DOI] [PubMed] [Google Scholar]
- 45.Leonardi F., Attorri L., Benedetto R.D., Biase A.D., Sanchez M., Tregno F.P., Nardini M., Salvati S. Docosahexaenoic acid supplementation induces dose and time dependent oxidative changes in C6 glioma cells. Free Radic. Res. 2007;41:748–756. doi: 10.1080/10715760701324067. [DOI] [PubMed] [Google Scholar]
- 46.Oguro A., Fujita K., Ishihara Y., Yamamoto M., Yamazaki T. DHA and Its Metabolites Have a Protective Role against Methylmercury-Induced Neurotoxicity in Mouse Primary Neuron and SH-SY5Y Cells. Int. J. Mol. Sci. 2021;22:3213. doi: 10.3390/ijms22063213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tatsumi Y., Kato A., Sango K., Himeno T., Kondo M., Kato Y., Kamiya H., Nakamura J., Kato K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2019;10:602–612. doi: 10.1111/jdi.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Che H., Fu X., Zhang L., Gao X., Wen M., Du L., Xue C., Xu J., Wang Y. Neuroprotective Effects of n-3 Polyunsaturated Fatty Acid-Enriched Phosphatidylserine Against Oxidative Damage in PC12 Cells. Cell Mol. Neurobiol. 2018;38:657–668. doi: 10.1007/s10571-017-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang X., Zhen J., Dong S., Zhang H., Van Halm-Lutterodt N., Yuan L. DHA and vitamin E antagonized the Aβ25–35-mediated neuron oxidative damage through activation of Nrf2 signaling pathways and regulation of CD36, SRB1 and FABP5 expression in PC12 cells. Food Funct. 2019;10:1049–1061. doi: 10.1039/C8FO01713A. [DOI] [PubMed] [Google Scholar]
- 50.Clementi M.E., Lazzarino G., Sampaolese B., Brancato A., Tringali G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019;43:2523–2531. doi: 10.3892/ijmm.2019.4170. [DOI] [PubMed] [Google Scholar]
- 51.Bie N., Feng X., Li C., Meng M., Wang C. The Protective Effect of Docosahexaenoic Acid on PC12 Cells in Oxidative Stress Induced by H(2)O(2) through the TrkB-Erk1/2-CREB Pathway. ACS Chem. Neurosci. 2021;12:3433–3444. doi: 10.1021/acschemneuro.1c00421. [DOI] [PubMed] [Google Scholar]
- 52.Graciano M.F., Leonelli M., Curi R., Carpinelli A. Omega-3 fatty acids control productions of superoxide and nitrogen oxide and insulin content in INS-1E cells. J. Physiol. Biochem. 2016;72:699–710. doi: 10.1007/s13105-016-0509-1. [DOI] [PubMed] [Google Scholar]
- 53.Song E.A., Lim J.W., Kim H. Docosahexaenoic acid inhibits IL-6 expression via PPARγ-mediated expression of catalase in cerulein-stimulated pancreatic acinar cells. Int. J. Biochem. Cell Biol. 2017;88:60–68. doi: 10.1016/j.biocel.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., Liu L., Sun D., He Y., Jiang Y., Cheng K.-W., Chen F. DHA protects against monosodium urate-induced inflammation through modulation of oxidative stress. Food Funct. 2019;10:4010–4021. doi: 10.1039/C9FO00573K. [DOI] [PubMed] [Google Scholar]
- 55.Vaagenes H., Muna Z.A., Madsen L., Berge R.K. Low doses of eicosapentaenoic acid, docosahexaenoic acid, and hypolipidemic eicosapentaenoic acid derivatives have no effect on lipid peroxidation in plasma. Lipids. 1998;33:1131–1137. doi: 10.1007/s11745-998-0315-6. [DOI] [PubMed] [Google Scholar]
- 56.Sekine S., Kubo K., Tadokoro T., Saito M. Dietary docosahexaenoic acid-induced generation of liver lipid peroxides is not suppressed further by elevated levels of glutathione in ODS rats. Nutrition. 2006;22:385–394. doi: 10.1016/j.nut.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Arnal E., Miranda M., Johnsen-Soriano S., Alvarez-Nölting R., Díaz-Llopis M., Araiz J., Cervera E., Bosch-Morell F., Romero F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009;34:928–938. doi: 10.3109/02713680903205238. [DOI] [PubMed] [Google Scholar]
- 58.Arnal E., Miranda M., Barcia J., Bosch-Morell F., Romero F.J. Lutein and docosahexaenoic acid prevent cortex lipid peroxidation in streptozotocin-induced diabetic rat cerebral cortex. Neuroscience. 2010;166:271–278. doi: 10.1016/j.neuroscience.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 59.Liu S.H., Chang C.D., Chen P.H., Su J.R., Chen C.C., Chaung H.C. Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res. 2012;1451:19–26. doi: 10.1016/j.brainres.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 60.Türkez H., Geyikoglu F., Yousef M.I. Ameliorative effect of docosahexaenoic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress, and DNA damage in rat liver. Toxicol. Ind. Health. 2012;28:687–696. doi: 10.1177/0748233711420475. [DOI] [PubMed] [Google Scholar]
- 61.Chaung H.C., Chang C.D., Chen P.H., Chang C.J., Liu S.H., Chen C.C. Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem. 2013;138:342–347. doi: 10.1016/j.foodchem.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 62.Chaudhary M., Joshi D.K., Tripathi S., Kulshrestha S., Mahdi A.A. Docosahexaenoic acid ameliorates aluminum induced biochemical and morphological alteration in rat cerebellum. Ann. Neurosci. 2014;21:5–9. doi: 10.5214/ans.0972.7531.210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao J., Wang X., Sun H., Cao Y., Liang S., Wang H., Wang Y., Yang F., Zhang F., Wu L. Neuroprotective effects of docosahexaenoic acid on hippocampal cell death and learning and memory impairments in a valproic acid-induced rat autism model. Int. J. Dev. Neurosci. 2016;49:67–78. doi: 10.1016/j.ijdevneu.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Zhu W., Ding Y., Kong W., Li T., Chen H. Docosahexaenoic Acid (DHA) Provides Neuroprotection in Traumatic Brain Injury Models via Activating Nrf2-ARE Signaling. Inflammation. 2018;41:1182–1193. doi: 10.1007/s10753-018-0765-z. [DOI] [PubMed] [Google Scholar]
- 65.Drouin G., Catheline D., Guillocheau E., Gueret P., Baudry C., Le Ruyet P., Rioux V., Legrand P. Comparative effects of dietary n-3 docosapentaenoic acid (DPA), DHA and EPA on plasma lipid parameters, oxidative status and fatty acid tissue composition. J. Nutr. Biochem. 2019;63:186–196. doi: 10.1016/j.jnutbio.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 66.Jiang W., Wan L., Chen P., Lu W. Docosahexaenoic acid activates the Nrf2 signaling pathway to alleviate impairment of spleen cellular immunity in intrauterine growth restricted rat pups. Saudi J. Biol. Sci. 2021;28:4987–4993. doi: 10.1016/j.sjbs.2021.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pineda-Peña E.A., Martínez-Pérez Y., Galicia-Moreno M., Navarrete A., Segovia J., Muriel P., Favari L., Castañeda-Hernández G., Chávez-Piña A.E. Participation of the anti-inflammatory and antioxidative activity of docosahexaenoic acid on indomethacin-induced gastric injury model. Eur. J. Pharmacol. 2018;818:585–592. doi: 10.1016/j.ejphar.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 68.Soto-Alarcón S.A., Ortiz M., Orellana P., Echeverría F., Bustamante A., Espinosa A., Illesca P., Gonzalez-Mañán D., Valenzuela R., Videla L.A. Docosahexaenoic acid and hydroxytyrosol co-administration fully prevents liver steatosis and related parameters in mice subjected to high-fat diet: A molecular approach. Biofactors. 2019;45:930–943. doi: 10.1002/biof.1556. [DOI] [PubMed] [Google Scholar]
- 69.Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., Murphy M.P., Yamamoto M., Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23:499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- 70.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holman R.T., Elmer O.C. The rates of oxidation of unsaturated fatty acids and esters. J. Am. Oil Chem. Soc. 1947;24:127–129. doi: 10.1007/BF02643258. [DOI] [Google Scholar]
- 72.Yu C., Xiao J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell Longev. 2021;2021:6635460. doi: 10.1155/2021/6635460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ulasov A.V., Rosenkranz A.A., Georgiev G.P., Sobolev A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022;291:120111. doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertson H., Dinkova-Kostova A.T., Hayes J.D. NRF2 and the Ambiguous Consequences of Its Activation during Initiation and the Subsequent Stages of Tumourigenesis. Cancers. 2020;12:3609. doi: 10.3390/cancers12123609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bourgonje A.R., Feelisch M., Faber K.N., Pasch A., Dijkstra G., van Goor H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020;26:1034–1046. doi: 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Dubois-Deruy E., Peugnet V., Turkieh A., Pinet F. Oxidative Stress in Cardiovascular Diseases. Antioxidants. 2020;9:864. doi: 10.3390/antiox9090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative Stress in Cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv W., Xu D. Docosahexaenoic Acid Delivery Systems, Bioavailability, Functionality, and Applications: A Review. Foods. 2022;11:2685. doi: 10.3390/foods11172685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tułowiecka N., Kotlęga D., Prowans P., Szczuko M. The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. Int. J. Mol. Sci. 2020;21:7628. doi: 10.3390/ijms21207628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao H., Wu S., Luo Z., Liu H., Sun J., Jin X. The association between circulating docosahexaenoic acid and lung cancer: A Mendelian randomization study. Clin. Nutr. 2022;41:2529–2536. doi: 10.1016/j.clnu.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Derogis P.B., Freitas F.P., Marques A.S., Cunha D., Appolinário P.P., de Paula F., Lourenço T.C., Murgu M., Di Mascio P., Medeiros M.H., et al. The development of a specific and sensitive LC-MS-based method for the detection and quantification of hydroperoxy- and hydroxydocosahexaenoic acids as a tool for lipidomic analysis. PLoS ONE. 2013;8:e77561. doi: 10.1371/journal.pone.0077561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma L., Liu G., Liu X. Amounts of malondialdehyde do not accurately represent the real oxidative level of all vegetable oils: A kinetic study of malondialdehyde formation. Int. J. Food Sci. Technol. 2019;54:412–423. doi: 10.1111/ijfs.13952. [DOI] [Google Scholar]
- 83.Lee Y.Y., Galano J.-M., Leung H.H., Balas L., Oger C., Durand T., Lee J.C.-Y. Nonenzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F4t-neuroprostane, acts as a bioactive lipid molecule in neuronal cells. FEBS Lett. 2020;594:1797–1808. doi: 10.1002/1873-3468.13774. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Díaz M., Mesa-Herrera F., Marín R. DHA and Its Elaborated Modulation of Antioxidant Defenses of the Brain: Implications in Aging and AD Neurodegeneration. Antioxidants. 2021;10:907. doi: 10.3390/antiox10060907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim E.-H., Kim S.-J., Na H.-K., Han W., Kim N.-J., Suh Y.-G., Surh Y.-J. 15-Deoxy-Δ12,14-prostaglandin J2 Upregulates VEGF Expression via NRF2 and Heme Oxygenase-1 in Human Breast Cancer Cells. Cells. 2021;10:526. doi: 10.3390/cells10030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikado A., Nishio Y., Morino K., Ugi S., Kondo H., Makino T., Kashiwagi A., Maegawa H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010;402:99–104. doi: 10.1016/j.bbrc.2010.09.124. [DOI] [PubMed] [Google Scholar]
- 88.Bang H.-Y., Park S.-A., Saeidi S., Na H.-K., Surh Y.-J. Docosahexaenoic Acid Induces Expression of Heme Oxygenase-1 and NAD(P)H:quinone Oxidoreductase through Activation of Nrf2 in Human Mammary Epithelial Cells. Molecules. 2017;22:969. doi: 10.3390/molecules22060969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turk H.F., Barhoumi R., Chapkin R.S. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS ONE. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaikh S.R., Edidin M. Polyunsaturated fatty acids and membrane organization: Elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem. Phys. Lipids. 2008;153:24–33. doi: 10.1016/j.chemphyslip.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shaikh S.R., Rockett B.D., Salameh M., Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 92.Wootten D., Christopoulos A., Marti-Solano M., Babu M.M., Sexton P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018;19:638–653. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 93.Latorraca N.R., Venkatakrishnan A.J., Dror R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017;117:139–155. doi: 10.1021/acs.chemrev.6b00177. [DOI] [PubMed] [Google Scholar]
- 94.Yoshida K., Nagatoishi S., Kuroda D., Suzuki N., Murata T., Tsumoto K. Phospholipid Membrane Fluidity Alters Ligand Binding Activity of a G Protein-Coupled Receptor by Shifting the Conformational Equilibrium. Biochemistry. 2019;58:504–508. doi: 10.1021/acs.biochem.8b01194. [DOI] [PubMed] [Google Scholar]
- 95.Hamdi Y., Kaddour H., Vaudry D., Douiri S., Bahdoudi S., Leprince J., Castel H., Vaudry H., Amri M., Tonon M.C., et al. The stimulatory effect of the octadecaneuropeptide ODN on astroglial antioxidant enzyme systems is mediated through a GPCR. Front Endocrinol. 2012;3:138. doi: 10.3389/fendo.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Javanainen M., Enkavi G., Guixà-Gonzaléz R., Kulig W., Martinez-Seara H., Levental I., Vattulainen I. Reduced level of docosahexaenoic acid shifts GPCR neuroreceptors to less ordered membrane regions. PLoS Comput. Biol. 2019;15:e1007033. doi: 10.1371/journal.pcbi.1007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang F., Steelman L.S., Lee J.T., Shelton J.G., Navolanic P.M., Blalock W.L., Franklin R.A., McCubrey J.A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 98.Yang S., Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017;13:1041–1047. doi: 10.3892/ol.2017.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicolini C., Baranski J., Schlummer S., Palomo J., Lumbierres-Burgues M., Kahms M., Kuhlmann J., Sanchez S., Gratton E., Waldmann H., et al. Visualizing association of N-ras in lipid microdomains: Influence of domain structure and interfacial adsorption. J. Am. Chem. Soc. 2006;128:192–201. doi: 10.1021/ja055779x. [DOI] [PubMed] [Google Scholar]
- 100.Feng J., Zhang P., Chen X., He G. PI3K and ERK/Nrf2 Pathways Are Involved in Oleanolic Acid-Induced Heme Oxygenase-1 Expression in Rat Vascular Smooth Muscle Cells. J. Cell. Biochem. 2011;112:1524–1531. doi: 10.1002/jcb.23065. [DOI] [PubMed] [Google Scholar]
- 101.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by Protein Kinase C Regulates Antioxidant Response Element-mediated Transcription*. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 102.Yu R., Chen C., Mo Y.-Y., Hebbar V., Owuor E.D., Tan T.-H., Kong A.N.T. Activation of Mitogen-activated Protein Kinase Pathways Induces Antioxidant Response Element-mediated Gene Expression via a Nrf2-dependent Mechanism*. J. Biol. Chem. 2000;275:39907–39913. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- 103.Liu T., Lv Y.-F., Zhao J.-L., You Q.-D., Jiang Z.-Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic. Biol. Med. 2021;168:129–141. doi: 10.1016/j.freeradbiomed.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 104.Mirza A.Z., Althagafi I.I., Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 105.Ciccocioppo R., Ubaldi M. Nuclear peroxisome proliferator activated receptor-gamma (PPARγ) as a therapeutic target to treat neurodegeneration and dependence elicited by drugs of abuse. Neural Regen. Res. 2021;16:984–985. doi: 10.4103/1673-5374.297072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Almeida N.R., Conda-Sheridan M. A review of the molecular design and biological activities of RXR agonists. Med. Res. Rev. 2019;39:1372–1397. doi: 10.1002/med.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carvalho M.V., Gonçalves-de-Albuquerque C.F., Silva A.R. PPAR Gamma: From Definition to Molecular Targets and Therapy of Lung Diseases. Int. J. Mol. Sci. 2021;22:805. doi: 10.3390/ijms22020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song J., Li C., Lv Y., Zhang Y., Amakye W.K., Mao L. DHA increases adiponectin expression more effectively than EPA at relative low concentrations by regulating PPARγ and its phosphorylation at Ser273 in 3T3-L1 adipocytes. Nutr. Metab. 2017;14:52. doi: 10.1186/s12986-017-0209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hawkes H.J., Karlenius T.C., Tonissen K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta. 2014;1840:303–314. doi: 10.1016/j.bbagen.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 110.O’Brien M.L., Cunningham M.L., Spear B.T., Glauert H.P. Effects of peroxisome proliferators on glutathione and glutathione-related enzymes in rats and hamsters. Toxicol. Appl. Pharm. 2001;171:27–37. doi: 10.1006/taap.2000.9111. [DOI] [PubMed] [Google Scholar]
- 111.Chung S.S., Kim M., Youn B.S., Lee N.S., Park J.W., Lee I.K., Lee Y.S., Kim J.B., Cho Y.M., Lee H.K., et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol. Cell Biol. 2009;29:20–30. doi: 10.1128/MCB.00544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khoo N.K.H., Hebbar S., Zhao W., Moore S.A., Domann F.E., Robbins M.E. Differential activation of catalase expression and activity by PPAR agonists: Implications for astrocyte protection in anti-glioma therapy. Redox. Biol. 2013;1:70–79. doi: 10.1016/j.redox.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ding G., Fu M., Qin Q., Lewis W., Kim H.W., Fukai T., Bacanamwo M., Chen Y.E., Schneider M.D., Mangelsdorf D.J., et al. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc. Res. 2007;76:269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 114.Girnun G.D., Domann F.E., Moore S.A., Robbins M.E. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol. Endocrinol. 2002;16:2793–2801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- 115.Lazennec G., Canaple L., Saugy D., Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol. Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Camp H.S., Tafuri S.R. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J. Biol. Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 117.Barger P.M., Browning A.C., Garner A.N., Kelly D.P. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: A potential role in the cardiac metabolic stress response. J. Biol. Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 118.Prusty D., Park B.H., Davis K.E., Farmer S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002;277:46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 119.Gelman L., Michalik L., Desvergne B., Wahli W. Kinase signaling cascades that modulate peroxisome proliferator-activated receptors. Curr. Opin. Cell Biol. 2005;17:216–222. doi: 10.1016/j.ceb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 120.Cho H.Y., Gladwell W., Wang X., Chorley B., Bell D., Reddy S.P., Kleeberger S.R. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gendy A.M., El-Gazar A.A., Ragab G.M., Al-Mokaddem A.K., El-Haddad A.E., Selim H., Yousef E.M., Hamed N.O., Ibrahim S.S.A. Possible Implication of Nrf2, PPAR-γ and MAPKs Signaling in the Protective Role of Mangiferin against Renal Ischemia/Reperfusion in Rats. Pharmaceuticals. 2022;16:6. doi: 10.3390/ph16010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mannan A., Garg N., Singh T.G., Kang H.K. Peroxisome Proliferator-Activated Receptor-Gamma (PPAR-ɣ): Molecular Effects and Its Importance as a Novel Therapeutic Target for Cerebral Ischemic Injury. Neurochem. Res. 2021;46:2800–2831. doi: 10.1007/s11064-021-03402-1. [DOI] [PubMed] [Google Scholar]
- 123.Reddy R.C., Standiford T.J. Nrf2 and PPAR{gamma}: PPARtnering against oxidant-induced lung injury. Am. J. Respir. Crit. Care Med. 2010;182:134–135. doi: 10.1164/rccm.201004-0457ED. [DOI] [PubMed] [Google Scholar]
- 124.Lee C. Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell Longev. 2017;2017:1378175. doi: 10.1155/2017/1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cao J., Schwichtenberg K.A., Hanson N.Q., Tsai M.Y. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin. Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 126.Flock M.R., Skulas-Ray A.C., Harris W.S., Etherton T.D., Fleming J.A., Kris-Etherton P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose-response randomized controlled trial. J. Am. Heart. Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lust C.A.C., Burns J.L., Jones M.T., Smith S.B., Choi S.H., Krk M., Gable D.A., Oliver J.M., Ma D.W.L. The Dose-Response Effect of Docosahexaenoic Acid on the Omega-3 Index in American Football Athletes. Med. Sci. Sport. Exerc. 2023;55:865–872. doi: 10.1249/MSS.0000000000003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salem N.M., Lin Y.H., Moriguchi T., Lim S.Y., Salem N., Jr., Hibbeln J.R. Distribution of omega-6 and omega-3 polyunsaturated fatty acids in the whole rat body and 25 compartments. Prostaglandins Leukot. Essent. Fat. Acids. 2015;100:13–20. doi: 10.1016/j.plefa.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boschetti E., Di Nunzio M., Danesi F., Tugnoli V., Bordoni A. Influence of genotype on the modulation of gene and protein expression by n-3 LC-PUFA in rats. Genes Nutr. 2013;8:589–600. doi: 10.1007/s12263-013-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y., Zhang J., Cui W., Silverstein R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022;219:e20211314. doi: 10.1084/jem.20211314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Glatz J.F.C., Nabben M., Luiken J.J.F.P. CD36 (SR-B2) as master regulator of cellular fatty acid homeostasis. Curr. Opin. Lipidol. 2022;33:103–111. doi: 10.1097/MOL.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li J., Pora B.L.R., Dong K., Hasjim J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021;9:5229–5243. doi: 10.1002/fsn3.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.