Abstract
Cigarette smoke (CS) poses a significant risk factor for respiratory, vascular, and organ diseases owing to its high content of harmful chemicals and reactive oxygen species (ROS). These substances are known to induce oxidative stress, inflammation, apoptosis, and senescence due to their exposure to environmental pollutants and the presence of oxidative enzymes. The lung is particularly susceptible to oxidative stress. Persistent oxidative stress caused by chronic exposure to CS can lead to respiratory diseases such as chronic obstructive pulmonary disease (COPD), pulmonary fibrosis (PF), and lung cancer. Avoiding exposure to environmental pollutants, like cigarette smoke and air pollution, can help mitigate oxidative stress. A comprehensive understanding of oxidative stress and its impact on the lungs requires future research. This includes identifying strategies for preventing and treating lung diseases as well as investigating the underlying mechanisms behind oxidative stress. Thus, this review aims to investigate the cellular processes induced by CS, specifically inflammation, apoptosis, senescence, and their associated biomarkers. Furthermore, this review will delve into the alveolar response provoked by CS, emphasizing the roles of potential therapeutic target markers and strategies in inflammation and oxidative stress.
Keywords: cigarette smoke, oxidative stress, COPD, NF-κB, MAPK, RAGE, Nrf2, PF, cancer, ARDS, AE-COPD, CVD
1. Introduction
The World Health Organization (WHO) has estimated that smoking causes about 8 million deaths each year, with the majority occurring in low- and middle-income countries [1]. Despite estimates, more than 100 million people around the world continue to smoke regularly [2]. To combat this issue, various world health institutions have set agreements and goals to reduce the prevalence of smoking. Representatively, the WHO Framework Convention on Tobacco Control (FCTC) has come into effect since 2005, and 181 countries are participating as of 2020 [3]. The FCTC has implemented measures such as tobacco price and tax increases, packaging and labeling requirements, and health warning labels [4,5]. Due to these policies, the global average number of smokers is decreasing; however, smoking remains a significant cause of disease and death for individuals.
Cigarette smoke (CS) is a representative risk factor for vascular and various organ diseases, as well as respiratory illness [6]. With over 4000 chemicals, such as nicotine, acrolein, phenols, carbon monoxide, benzene, and formaldehyde [7,8]. Both active and passive inhalation of these harmful chemicals can induce oxidative stress in the body, leading to inflammation, apoptosis, and senescence. Acrolein, for example, causes mutations by forming acrolein-induced DNA adducts [9]. Acrylonitrile is a representative oxidant in cigarettes; it produces 8-oxo-2′-deoxyguanosine and contributes to oxidative stress [10].
CS contains a high level of reactive oxygen species (ROS), which can overwhelm the body’s antioxidant defense system and result in oxidative stress [11]. Chronic CS exposure causes persistent oxidative stress in the lung, damaging the respiratory system and increasing the risk of various diseases. The detrimental effects of smoking on the lung can be attributed to both the direct and indirect consequences of oxidative stress [12]. Direct effects include damage to cellular components such as lipids, proteins, and DNA [13]. Indirect effects involve the activation of inflammatory cells, leading to the release of pro-inflammatory cytokines and chemokines that further exacerbate oxidative stress and cause tissue damage [14].
The production of ROS is involved in various cells, and mitochondria and NADPH oxidase (NOX) are the main sources of ROS [15]. During ATP synthesis by oxidative phosphorylation, the main function of mitochondria, ROS is mainly produced, and the generated ROS is removed by superoxide dismutase, peroxiredoxin, and glutathione peroxidase [16,17]. NADPH oxidase is found in neutrophils and vascular cells and is involved in the generation of neutrophil extracellular traps (NETs) and vasoconstriction and vasodilation, depending on the cell [18,19].
Oxidative stress can inflict cellular damage through the oxidation of lipids, proteins, and DNA [20]. For example, lipid peroxidation leads to the formation of aldehydes and other toxic compounds that can disrupt cellular membranes [21]. The oxidative modification of lipids can lead to membrane permeability changes and cellular dysfunction, ultimately leading to cell death [22]. Protein oxidation may result in structural changes and loss of function, while DNA oxidation can lead to mutations and cell death [23]. In addition, this stress has been linked to a number of lung diseases, including chronic obstructive pulmonary disease (COPD) and lung cancer [24]. Oxidative stress induces inflammation and damage, leading to several respiratory diseases such as COPD, ARDS, lung cancer, and pulmonary fibrosis, as well as cardiovascular diseases [25]. Further research is necessary to comprehensively understand how oxidative stress affects the lung and to identify effective antioxidants for the treatment of lung diseases [26,27,28]. In addition, investigating the role of oxidative stress in the development of lung diseases and determining the most effective strategies for prevention and treatment are also crucial [29]. In this review, we aim to provide a comprehensive summary of the impacts of oxidative stress, primarily triggered by cigarette smoke, on cellular processes such as inflammation, apoptosis, aging, and autophagy. We will further explore the consequential diseases and potential therapeutic targets in the lungs and cardiovascular system.
2. Cellular Responses to Cigarette Smoke
2.1. Inflammation
Inflammation is a biological defense mechanism against harmful stimuli such as pathogens and damaged cells [30]. It can be acute or chronic, with CS often being a risk factor for chronic inflammation [31]. Smoking induces high concentrations of cigarette smoke particles to enter the lungs, and these stimuli are harmful factors that cause acute or chronic inflammation [32,33,34,35]. Oxidative stress is the primary cause of inflammation resulting from smoking [36]. During oxidative stress, cell damage leads to the release of damage-associated molecular patterns (DAMP) molecules [37]. The receptor for advanced glycation end products (RAGE) is a 35-kilodalton membrane receptor known as a receptor for DAMP molecules such as AGE, high mobility group box 1 (HMGB1), and S100 families [38]. RAGE, also known as a marker for type 1 lung epithelium, is closely related to lung disease [39]. RAGE has a soluble form (sRAGE) secreted out of the cell and a membrane-bound form (mRAGE). In general, the soluble form binds to DAMP instead of membrane-bound RAGE to act as an antagonist to inhibit the activation of membrane-bound RAGE due to cigarette smoke [40,41,42,43]. Toll-like receptors (TLRs) are also key receptors for DAMP and play a crucial role in the innate immune response [44]. TLRs are commonly found in various innate immune cells, such as macrophages and dendritic cells, and recognize pathogen-derived molecules [45]. TLRs are significant mediators in the inflammatory mechanism caused by smoking. Barua et al. reported that CS triggers inflammation through the activation of the histamine receptor-1 (H1R)-TLR2/4-cyclooxygenase2 (COX2) axis [46]. Moreover, Nadigel et al. confirmed the expression and inflammation of high TLR4/9 in COPD patients [47]. As such, oxidative stress and DAMP-induced inflammation by CS are essential mechanisms in the immune response.
And activation of DAMP receptors, such as RAGE and TLRs, typically activates the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light chain-enhancer of activated B cells (NF-κB) pathways [48]. NF-κB is a p50/p65 heterodimer that plays an important role in inflammation, immune response, and apoptosis [49,50]. IκB present in the cytoplasm under physiological conditions inhibits NF-κB, and phosphorylated IκB through activation of IκB kinase (IKK) promotes phosphorylation and translocation of NF-κB to induce it to act as a transcription factor [51]. These activated NF-κBs regulate the recruitment of various infectious cytokines and inflammatory cells [52]. MAPK is a family of protein kinases that regulate cellular responses to various stimuli, such as thermal shock, osmotic stress, and pro-inflammatory cytokines [53]. MAPK typically includes extracellular signal-regulated kinases (ERK1/2), p38, and c-Jun N-terminal Kinase (JNK1/2). Among them, ERK is activated by differentiation signals, and JNK and p38 are activated by inflammation and various stresses [53,54]. Each activated factor regulates the expression of pro-inflammatory cytokines. The secretion of inflammatory cytokines by activation of these factors activates the signal transducer and activator of transcription (STAT) pathway [55,56]. The phosphorylated STAT controls the cell cycle, apoptosis, and differentiation along with interferon regulatory factor 9 (IRF9), CREB-binding protein (CBP)/P300 [57,58,59].
Various cells are involved in immune responses, but macrophages, neutrophils, and T cells play a major role in innate and adaptive immunity. Macrophages are crucial components of the innate immune system that recruit and activate lymphocytes through phagocytosis and antigen presentation [60]. They can be polarized into M1 (pro-inflammatory) and M2 (anti-inflammatory) cells [61]. The relationship between CS and macrophage polarization has produced conflicting results. Generally, studies have shown that cigarette smoking upregulates the secretion of IL-8 in human macrophages [62,63] and releases pro-inflammatory cytokines such as IL-8 and TNF-α in rat lungs [64]. However, it has been reported that CS extract (CSE) inhibits the phagocytic response in COPD-derived alveolar macrophages [65] and that exposure to CS promotes M2 polarization in mouse macrophages [66]. Macrophage M1/M2 polarization progressively increases with the severity of smoking and disease [67]. Therefore, smoking affects the polarization of macrophages, but the direction of polarization varies depending on species and severity. Recently, Keshav et al. combined plant extracts of berberine and liquid crystalline nanoparticles to confirm antioxidant action in RAW 264.7 cells exposed to CSE [68]. As such, future research is needed to understand the impact of smoking on macrophage function.
Neutrophils are the most abundant granulocytes and play a pivotal role in innate immunity [69]. They are essential in the pathogenesis of COPD, as emerging studies support the hypothesis that neutrophil elastase breaks down alveolar elastin, leading to emphysema [70,71,72,73]. Guzik et al. reported that acute exposure to CS in vitro induces neutrophil necrosis, promoting phagocytosis in macrophages [74]. On the other hand, Noda et al. found that CS impaired alveolar macrophage phagocytosis of apoptotic neutrophils in COPD patients [75]. Furthermore, CS-induced COPD mouse models exhibited that necroptosis and DAMP release cause airway inflammation by neutrophils [76]. In other words, direct CS exposure to neutrophils induces apoptosis; however, the resulting DAMPs can be delivered as an inflammatory inducer for other neutrophils or macrophages. Therefore, inhibition of neutrophil activity in inflammation could be an effective therapeutic target.
T cells are a major subgroup of immune cells that mediate adaptive immunity. These T cells differentiate into helper, effector, memory, and regulatory T cells upon activation [77,78,79,80]. These T cells serve different functions depending on the specific antigen. Helper T cells’ inflammatory responses can be largely classified into Th1, Th2, and Th17 [81]. Th17 cells are predominantly regulated by CS. In COPD mice, CS exposure has been shown to upregulate Th17-related cytokines, such as IL-6, IL-17A, and IL-23 [82]. Additionally, Th1 and Th17 cells significantly increased in the bronchoalveolar lavage (BAL) fluid of mice exposed to CS for 6 months [83]. Th2 cells are associated with CS-induced airway inflammation. Hove et al. confirmed increased Th2 activity and expression of IL-13 in the airways of mice exposed to CS for 4–8 weeks [84]. It has also been reported that the expression of thymic stromal lymphopoietin, which is essential for Th2 activity, is increased in the nasal cavities of mice [85]. In summary, Th1 and Th17 develop an inflammatory response with several pro-inflammatory cytokines in lung diseases caused by CS, and in the nasal cavity, a Th2-induced allergic reaction can be caused by CS.
CD8+ T cells play a crucial role in the host immune response by eliminating infected or damaged cells. Typically, it has been reported that inflammation and emphysema caused by CS were suppressed in CD8 knockout mice [86]. CS also activates CD8+ T cells in COPD patients and increases expression of IL-1β, IL-6, IL-10, IL-12, TNF-α, and IFN-γ [47]. In addition, the activation of CD8+ T cells by smoking has been reported in autoimmune diseases such as rheumatoid arthritis [87]. In conclusion, most studies have shown that smoking increases the activation of CD8+ T cells. Therefore, inhibition of CD8+ T cells may be an effective therapeutic target for inhibiting cellular damage caused by smoking.
Most researchers are conducting inflammatory studies with immune cells as targets. However, there may be various mechanisms related to CS-induced inflammation. For example, CS-induced inflammation is also associated with the microbiome. Lingyue et al. reported that fermented black barley enriches probiotics such as Oscillospira and Ruminococcus, which can inhibit CS-induced lung inflammation [88]. These microbiome studies can be a new topic for the study of CS-induced inflammation. Therefore, many studies should be conducted on various targets for CS-induced inflammation.
2.2. Cigarette Smoke-Induced Cell Death
Apoptosis, or programmed cell death, is a highly regulated biological process crucial for maintaining tissue homeostasis and preventing disease development [89]. It features morphological changes, such as cell shrinkage, chromatin condensation, and apoptotic body formation, and is initiated by signals like cellular stress (e.g., oxidative stress) and extracellular signals (e.g., cytokines and growth factors) [90]. Apoptosis regulation is complex, involving multiple signaling pathways and target genes, including the B-cell lymphoma 2 (BCL-2) family of proteins, caspases, the tumor protein p53 (TP53), and inhibitors of apoptosis proteins (IAPs) [91]. In terms of the signaling pathways involved in apoptosis, there are two main pathways: the intrinsic pathway, initiated by signals, and the extrinsic pathway, initiated by extracellular signals [92]. The intrinsic pathway is regulated by the BCL-2 family of proteins and the activation of caspases, while the extrinsic pathway is regulated by signaling molecules such as TNF and Fas ligand [93]. The BCL-2 family of proteins plays a central role in regulating the intrinsic apoptotic pathway [94]. Pro-apoptotic members, including BAK and BAX, are responsible for releasing pro-apoptotic factors from the mitochondria in response to cellular stress, while anti-apoptotic members, such as BCL-2 and BCL-XL, inhibit apoptosis induction [95]. The activation of caspases, a family of proteases central to the execution of apoptosis, is another key step in the induction of apoptosis [96]. TP53 and the IAPs also play significant roles in apoptosis regulation, with TP53 regulating the cell cycle and the IAPs acting as inhibitors of caspase activity [97,98].
Caspase activation has been shown to increase in smokers, indicating an upregulation of apoptotic signaling pathways [99]. Cigarette smoke has been associated with various molecular and cellular changes in the lung tissue, including the upregulation of testis-specific serine/threonine kinase 4 (TSSK4) in alveolar epithelial type-II cells, the involvement of the SENP1-SIRT1 pathway in hyperoxia-induced alveolar epithelial cell injury, and the effects on the mouse alveolar epithelial cell line MLE 12. The upregulation of TSSK4 in alveolar epithelial type-II cells increases susceptibility to cigarette smoke-induced lung injury, as smoke exposure leads to oxidative stress, TSSK4 activation, and subsequent apoptosis of these cells, which collectively contribute to the development of lung disease-like emphysema [100]. Cigarette smoke exposure exacerbates hyperoxia-induced alveolar epithelial cell injury by dysregulating the SENP1-SIRT1 pathway, impairing the cells’ ability to cope with oxidative stress, and ultimately leading to increased cell death and lung tissue damage [101]. The dysregulation of long noncoding RNA uc.375 has been implicated in epithelial cell apoptosis and smoking-related lung diseases, including bronchopulmonary dysplasia [102]. In COPD, increased apoptosis levels in lung cells lead to the destruction of lung tissue and emphysema development [103]. In lung cancer, dysregulated apoptosis allows cancer cells to avoid programmed cell death and continue dividing and growing uncontrollably [104].
Apoptosis, along with necrosis and ferroptosis, three distinct forms of cell death, play crucial roles in lung injury and disease [105]. Necrosis, primarily triggered by injury or infection, leads to irreversible cellular damage [106]. This process can be mitigated by substances like ginkgolide C, which acts on the CD40/NF-κB pathway, is a significant mediator of necrosis-induced epithelial cell damage, and is considered a potential driver in COVID-19-induced acute respiratory distress syndrome [107]. On the other hand, ferroptosis is a regulated form of cell death involving iron-dependent lipid peroxides, closely linked to diseases instigated by cigarette smoke exposure [108]. Notably, several genes, such as NAD(P)H dehydrogenase [quinone] 1 (NQO1), aldo-keto reductase family 1 member C3 (AKR1C3), and glutathione peroxidase 2 (GPX2), identified through gene expression dataset analysis, have been implicated in ferroptosis and exhibit promising diagnostic potential [109].
A study showed that CS extract increased oxidative stress and apoptosis in lung cells [110]. In particular, smoking has been shown to increase the levels of pro-apoptotic factors such as BAX and decrease the levels of anti-apoptotic factors such as BCL-2 in lung cells [111]. Another study found that CS extract caused mitochondrial dysfunction and the release of pro-apoptotic factors, leading to apoptosis in lung cells [112]. As mentioned, CS also activates the extrinsic apoptotic pathway through the upregulation of death receptor signaling molecules such as Fas and TNF [113]. In lung cancer, TP53 tumor suppressor gene mutations, which regulate apoptosis and cell cycle arrest, are common and can lead to dysregulation of apoptosis [114]. Lung cancer apoptosis dysregulation may also involve alterations in the expression of BCL-2 family proteins, such as the downregulation of the pro-apoptotic protein BAX and the upregulation of anti-apoptotic proteins such as BCL-2 [115]. These studies suggest that CS increases oxidative stress and dysregulates apoptosis in the lung, leading to the development of lung diseases such as COPD and lung cancer. Overall, smoking can dysregulate apoptosis signaling pathways, initiating lung cell apoptosis and contributing to the development of lung diseases. Understanding how smoking affects apoptosis is essential for developing effective strategies.
2.3. Effects of Cigarette Smoke on Cellular Senescence in the Lungs
Cigarette smoke (CS) plays a significant role in accelerating lung senescence and increasing the risk of respiratory diseases in the elderly [116]. CS contains a myriad of toxic chemicals that can induce oxidative stress, inflammation, and DNA damage, which contribute to the aging process [117].
One of the primary factors linking cigarette smoke to lung senescence is the production of reactive oxygen species (ROS) [118]. ROS is generated by the metabolism of toxic components in CS, subsequently leading to oxidative stress in lung cells [119]. Elevated ROS levels can damage cellular components such as proteins, lipids, and DNA, eventually resulting in cellular dysfunction and death [120]. In addition to oxidative stress, CS promotes inflammation in the lungs by increasing the levels of pro-inflammatory cytokines such as tumor necrosis factor and interleukin [121]. This chronic inflammation negatively impacts lung function and contributes to the development of respiratory diseases like COPD and interstitial lung disease (ILD) [122]. Cigarette smoke exposure can disrupt the balance of immune cells in the lungs, leading to chronic inflammation and impaired immune responses [123].
Cigarette smoke exposure can cause oxidative stress and inflammation in alveolar cells, leading to impaired surfactant production and reduced regenerative capacity [124]. Neutrophils, when activated by CS, can produce excessive ROS and release destructive enzymes, such as matrix metalloproteinases (MMPs), further contributing to lung tissue destruction and the progression of lung disease [72]. Cigarette smoke has also been found to affect key genes involved in lung senescence, such as fibronectin, MMPs, and sirtuin-1 (SIRT1) [125]. CS exposure leads to decreased fibronectin levels and increased MMP levels, which contribute to the loss of lung function and the development of respiratory diseases [126]. Furthermore, CS exposure results in the downregulation of SIRT1 in lung tissue, disrupting its protective role against lung injury and senescence [127].
Current therapeutic approaches for CS-induced lung diseases focus on managing symptoms, reducing inflammation, and improving lung function [128]. Inhaled corticosteroids, bronchodilators, and supplemental oxygen may be used for patients with COPD or ILD [129]. Enhancing the regenerative capacity of alveolar type II cells or modulating immune cell responses to reduce inflammation and oxidative stress could be potential strategies for treating CS-induced lung diseases [130]. Importantly, smoking cessation is the most effective way to prevent further lung damage and slow the progression of lung senescence [131].
Senescence and immunosenescence are interconnected concepts that all relate to aging and cellular function [132]. Cellular senescence refers to a state of permanent cell cycle arrest, which is a natural biological response to various types of stress, such as DNA damage, telomere shortening, or oncogenic stress [133]. While this process has a protective role against cancer, over time it can contribute to aging and age-related diseases [134]. Senescent cells exhibit a specific phenotype characterized by changes in morphology, gene expression, and secretion of pro-inflammatory factors, known as the senescence-associated secretory phenotype (SASP) [135]. SASP can influence the surrounding tissue microenvironment and contribute to inflammation and tissue dysfunction [136]. Senolytics are a class of drugs designed to selectively eliminate senescent cells by specifically targeting the survival pathways that these cells rely on [137]. By removing senescent cells, senolytics can alleviate SASP-induced inflammation and tissue dysfunction, potentially ameliorating the symptoms of aging and age-related diseases [132]. Preclinical studies have shown promising results, with senolytics improving health span in animal models of aging and age-related diseases, but their efficacy and safety in humans are still under investigation [138]. Immunosenescence refers to the gradual deterioration of the immune system with age, characterized by a decline in the function of the immune cells and an increase in systemic inflammation [139]. It is associated with increased susceptibility to infections, decreased response to vaccination, and a higher risk of autoimmunity and cancer in the elderly [140]. Senescent cells, through SASP, contribute to immunosenescence by creating a pro-inflammatory environment [141]. Interestingly, senescent immune cells themselves can also contribute to immunosenescence [142].
In conclusion, understanding the relationship between cigarette smoke, ROS, lung senescence, and the roles of key genes such as SIRT1 and MMPs is crucial for developing effective strategies to prevent and treat age-related lung diseases. Targeting the underlying mechanisms of senescence, including oxidative stress, inflammation, and the regulation of SIRT1 and MMPs, may lead to improved outcomes for elderly patients with respiratory diseases exacerbated by cigarette smoke exposure.
2.4. Understanding the Link between Cigarette Smoke and Autophagy
Autophagy is a spontaneous mechanism of decomposition that eliminates unnecessary or dysfunctional cellular components [143]. This process is crucial for maintaining cellular homeostasis through the orderly decomposition and recycling of intracellular elements [144,145]. Defects in autophagy have been linked to cancer and diabetes, with autophagy control in these diseases being studied as a potential treatment [146,147]. As autophagy is also considered an antioxidant mechanism for adapting to oxidative stress, the relationship between CS and autophagy is of particular interest [148,149]. Autophagy largely consists of five steps: initiation, elongation, autophagosome, autophagosome-lysosome fusion, and autolysosome formation [150,151]. Autophagy flux refers to the process from the autophagosome-lysosome fusion step, where degradation begins [152]. Recently, studies on the relationship between disease and disrupted autophagy flux have been actively conducted [153,154,155].
CS-induced autophagy mechanisms remain largely unknown. Recently, Wang et al. identified a link between oxidative stress caused by CS and autophagy in the human airway epithelium. They confirmed that the expression of oxidative stress-induced growth inhibitor (OSGIN1), an oxidative stress-induced growth inhibitor, was significantly increased in airway epithelial inflammation by smoking and was associated with activation of autophagy [156]. In addition, it has been reported that exposure of CS to the human alveolar epithelial cell line A549 accumulates LC3 and activates autophagy [157].
Conversely, there are reports that CS exposure impairs autophagy flux. Expression of Galectin-8, involved in initiating autophagosome engagement, increases due to activation of autophagy by CSE in macrophages. However, the accumulation of galectin-8 caused damage to autophagy flux [158]. However, the accumulation of Galectin-8 impairs autophagy flux. This accumulation is known to affect the immune response and induce cytokines and chemokines [159,160,161]. In addition, Monick et al. demonstrated that CSE treatment in human alveolar macrophages increased autophagosome production but impaired the binding process of autophagosomes and lysosomes through p62 accumulation [162].
As stated in numerous reports, autophagy is activates the production of autophagosomes to counteract the oxidative stress caused by CS. However, various factors can suppress the combination of the autophagosome and lysosome, which are crucial for degradation processes. For example, unlike normal oxidative stress, cadmium in cigarettes can cause the accumulation of autophagosomes [163]. This accumulation of autophagosomes results in cytotoxicity and ROS production [164]. In addition, mitophagy, which regulates the production of ROS in oxidative stress environments, is also associated with CS-induced damage [165]. Mitophagy is autophagy that occurs in mitochondria, and mitochondria are the main source of ROS, so mitophagy in oxidative stress environments is inevitable [166]. In alveolar type 2 cells, mitophagy is important for inhibiting CS-induced mitoROS that can be controlled by the expression of SIRT1 [167]. Therefore, further study of autophagy flux and mitophagy-related genes as potential treatment targets to mitigate damage to autophagy flux and mitophagy caused by CS is necessary.
3. CS-Induced Lung and Other Diseases
3.1. Chronic Obstructive Pulmonary Disease (COPD)
Chronic Obstructive Pulmonary Disease (COPD) is characterized by emphysema and chronic bronchitis [168]. Smoking is the most common cause of COPD, while other factors, such as exposure to cadmium or occupational smoke, are known to be contributors [169,170]. In addition, inorganic dust such as aluminum silicate or kaolinite in cigarettes can accumulate in alveolar macrophages and cause inflammation and pneumoconiosis [171]. Although COPD can be prevented by smoking cessation and reducing exposure to risk factors, it is not completely curable, and existing medications primarily alleviate symptoms [172].
Emphysema is a type of COPD that reduces oxygen supply to the blood by inhibiting gas exchange due to the destruction of the alveolar wall [173]. The involvement of MMPs in the destruction of alveolar walls is well known [174]. However, opinions are divided on which MMPs have a significant impact on COPD development. There are significant differences between human patients suffering from COPD and animal emphysema models [174,175]. For example, in mouse models, MMP-12 activates CXCL-5 to induce neutrophil infiltration, contributing to emphysema, but in humans, MMP12 performs the function of inhibiting the CXCL family [176]. MMP-2 has also been observed to have increased expression in the lungs and sputum of COPD patients, as well as in CS-exposed mice [177,178,179]. However, John V reported lowered MMP-2 gene expression in human lung tissue with the Global Initiative for Obstructive Lung Disease (GOLD) stage [180]. More studies are needed to resolve these discrepancies in results on the expression of MMPs and their mechanisms in COPD.
Bronchitis, a chronic mucous-producing disease in the airways, is one of the other aspects of COPD [181]. Smoking is the main cause of bronchitis, and the prevalence rate increases with years of smoking [182]. Excessive mucus resulting from inflammation in the airways can obstruct the airways. Mucus production-related genes MUC5B and MUC5AC are increasing representative factors in patients with chronic bronchitis [183,184,185]. It is difficult to conduct research on bronchitis despite the discovery of these target genes. Research on bronchitis is conducted using rat, canine, and monkey models, as the CS-induced bronchitis mouse model is nonexistent [186,187,188,189]. Therefore, the development and idea of a new disease modeling system for preclinical research on bronchitis are required.
Nuclear factor erythroid 2-related factor (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) regulating Nrf2 are regulatory redox proteins in cells [190,191]. Nrf2 and Keap1 separate in an oxidative stress environment, thereby activating Nrf2. The activated Nrf2 then translocates to the nucleus and enables expression of the Nrf2 target genes such as NQO1, heme oxygenase 1(HO1), and glutathione S-transferase (GST), which are able to eliminate ROS after binding to the promoter region of the antioxidant response elements (ARE) [192,193,194]. Since CS-induced oxidative stress and inflammation are the primary causes of COPD, Nrf2 is closely related to COPD. The importance of Nrf2 has been demonstrated in patients as well as in mouse models. It has been reported that exposure of Nrf2 knockout mice to CS made them more susceptible to emphysema [195]. Additionally, Kubo et al. reported that Nrf2 expression was observed to be lower in CS-induced emphysema mice, and treatment with astaxanthin restored the Nrf2 levels, alleviating emphysema [196]. Pasini et al. observed elevated expression of Nrf2 and HO-1 in the blood of COPD patients, accompanied by lower forced expiratory volume (FEV1) in the first second [197]. Furthermore, the genes NQO1, HO1, superoxide dismutase type 1 (SOD1), and thioredoxin reductase 1 (TXNRD1) were downregulated in COPD patients, with increased expression following treatment with Nrf2 activators [198]. These findings indicate that Nrf2 inhibition in patients or mice can result in an exacerbation of emphysema and inflammation due to an inadequate response to oxidative stress.
COPD can also be caused by the activation of various DAMPs and the subsequent inflammatory responses. Several studies have revealed that expression levels of AGEs and RAGEs are high in COPD patients. Smith et al. reported that sRAGE levels were lower in COPD patients in the presence of a negative association between FEV1 and sRAGE in a multiple linear regression analysis [199]. Hoonhorst et al. measured AGE and RAGE levels in young (18–40) as well as old (40–75) smokers, non-smokers, and COPD patients and found that the lowest sRAGE expression was seen in the plasma of COPD patients [200]. On the other hand, mRAGE is generally upregulated in COPD. Ferhani et al. reported significantly higher levels of high mobility group box 1 (HMGB1), a representative RAGE ligand, in COPD patients, with mRAGE overexpressed in airway epithelium and smooth muscle [201]. In addition, it has been reported that exposure to CS extract induces alveolar epithelial cell injury and results in an upregulation of mRAGE [42,202]. Furthermore, RAGE-/- mice exposed to cigarette smoke showed less emphysema compared to WT [203]. The differential expression of mRAGE and sRAGE in COPD patients or smokers may be genetic. In 2016, Miller et al. confirmed through single nucleotide polymorphism (SNP) analysis that a lower expression of sRAGE was observed in the UK smoker cohort, with individuals having the T allele of rs2070600, which is associated with pulmonary function [204,205,206].
RAGE-DAMP signaling is the initial point for various inflammatory and oxidative stress mechanisms, which then activate MAPK. It has been reported that exposure to CS results in increased inflammation, which is accompanied by increased activity of JNK and p38 in human bronchial epithelial cells [207]. Marumo et al. confirmed that CS exposure resulted in an increased mRNA expression of p38 in C57BL/6 mice, while no such effect was observed in the NZW mice (emphysema-resistant) [208]. Renda confirmed an increased expression of phosphorylated p38 in the alveoli of COPD patients [209]. Additionally, ERK expression in COPD patients induces endothelial cell apoptosis by upregulating MMP-1 and MUC5AC, leading to destruction of the alveolar wall and inflammation [210,211,212]. Taken together, RAGE-DAMP signaling can cause extensive alveolar damage and inflammation via MAPK. Therefore, inhibition of mRAGE or upregulation of sRAGE can serve as a potential target for preventing COPD.
The expression of NOX is closely related to COPD. It was confirmed that the protein expression of the NOX family in the tissues of COPD patients was significantly higher as compared to the non-smoker control group [213]. Xinjing et al. reported that NOX 1, 4, and 5 were detected at various sites such as lung epithelial cells, vascular endothelial cells, and macrophages, and NOX2 was mainly detected in lung macrophages and neutrophils [214]. Stanley et al. also reported that the NOX inhibitor apocynin inhibited lung inflammation and vascular injury caused by oxidative stress in the CS-induced mouse model [215]. In addition, expression of NOX in COPD has been reported in several studies [216,217,218]. Therefore, since NOX is a major source of ROS, targeting NOX to suppress overproduction of ROS might be an effective way to protect against oxidative stress. Dual oxidase (DUOX), a NOX homolog, is also important in COPD. Caspar et al. reported that DUOX1 is downregulated in airway epithelial cells of COPD patients and that a deficiency of DUOX1 in mice enhances emphysema [219]. In addition, Katsura Nagai et al. reported that DUOX1 was downregulated in airway epithelial cells of smokers compared to non-smokers and that both DUOX1 and DUOX2 were downregulated in bronchial epithelial cells of COPD patients [220]. Interestingly, DUOX is downregulated in COPD patients as opposed to NOX, which may be due to DUOX1 inhibiting epithelial damage and contributing to maintaining epithelial integrity [221]. In addition, in previous studies, DUOX1 is involved in epithelial damage response by MMP-9, and overexpression of MMP-9 can cause protease/antiprotease imbalance and lead to COPD [222,223]. Therefore, among NOX families, the functions of NOX series and DUOX series in COPD conflict with each other, so targeting them should be careful in the study.
Metformin and astaxanthin are among the several treatments that have recently been studied to treat COPD. Metformin, originally a treatment for type 2 diabetes, has been reported not only to increase insulin sensitivity but also to suppress damage by controlling cell redox homeostasis [224,225]. Recently, Francesca et al. reported that metformin inhibits oxidative stress and apoptosis through regulation of adenosine monophosphate (AMP) kinase signaling in CS-induced emphysema mouse models [226].
Astaxanthin is a keto-carotenoid derived from Hematococcus pluvialis, which is used as a health binder with Sirtuin1 (SIRT1) supplements for improving muscle strength and also has therapeutic effects on atherosclerosis and macular degeneration [227,228,229,230]. Mingming et al. reported that Astaxanthin binds with SIRT1, inhibits Nrf2-modulated oxidative stress, and regulates NF-κB-related inflammatory responses in CS-induced emphysema mouse models and human bronchial epithelial cells [231].
Also, new therapeutic agents or transporter studies have been reported to treat COPD. For example, Noridzada et al. treated the human umbilical cord mesenchymal system cell (HUC-MSC)-derived extracellular vesicle, which resulted in the alleviation of airway inflammation in the CS-induced rat model [232]. In addition, Emanuela et al. used lipid-polymer hybrid nanoparticles (LPHNPs) to develop a drug delivery system for Roflumilast, a representative PDE4 inhibitor [233]. In addition, studies have been conducted using liposomes and nanoparticles targeting oxidative stress for treating COPD [234,235,236,237]. These therapeutic studies will be the steppingstones for obtaining treatment for COPD.
3.2. Pulmonary Fibrosis
Pulmonary fibrosis is a chronic lung disease characterized by excessive deposition of extracellular matrix and progressive scarring of lung tissue. Smoking is a major risk factor for pulmonary fibrosis. The genetic and molecular mechanisms underlying CS-induced pulmonary fibrosis involve oxidative stress, inflammation, fibroblast activation, and epithelial-mesenchymal transition (EMT) [238]. Genetic polymorphisms in SNPs related to ROS metabolism, such as cytochrome P450 Family 1 subfamily A Member 1 (CYP1A1) and cytochrome P450 Family 1 subfamily B Member 1 (CYP1B1), as well as antioxidant enzymes, such as GST and SOD, increase susceptibility to CS-induced pulmonary fibrosis [239,240]. Several studies have reported the overexpression of NADPH oxidases, particularly NOX4 [241], which is believed to contribute to the development and progression of the disease [242]. Specifically, NOX4-derived ROS has been implicated in fibroblast activation and myofibroblast transformation, which are the key processes in the pathogenesis of IPF [243]. Therefore, targeting NOX4 might offer therapeutic benefits in IPF [244].
Rage is one of the several molecular players implicated in the pathogenesis of PF [245]. RAGE contributes to the maintenance of alveolar structure and function in healthy lungs. However, the overall expression of RAGE is reported to be decreased in the lung tissues of patients with PF [246]. More specifically, RAGE expression has been observed to be significantly reduced in fibrotic areas of the lungs affected by PF [247]. This lowering of RAGE expression is associated with the loss of type I alveolar epithelial cells (AECs), which are replaced by type II AECs and fibroblasts during disease progression [248]. Additionally, levels of sRAGE in the serum of PF patients are often elevated [249], which might reflect increased cleavage and loss of membrane-bound RAGE from the injured alveolar epithelium in PF [250]. Further studies are needed to fully understand the role of RAGE in PF and its potential as a therapeutic target. Overexpression of RAGE leads to an exaggerated inflammatory response, including the release of pro-inflammatory cytokines [251]. In cigarette smoke exposure, it could exacerbate the inflammatory and oxidative stress responses, thereby contributing to the progression of lung disease [252]. In IPF, overexpression of RAGE could potentially worsen fibrotic responses, as RAGE signaling has been implicated in fibroblast activation and collagen production [247]. RAGE knock-out mice often show less severe disease phenotypes in response to harmful stimuli such as cigarette smoke [253]. The knock-out mice show less inflammation and oxidative stress in response to cigarette smoke and less fibrosis in models of IPF [254]. Nuclear RAGE interacts with specific DNA repair proteins, potentially regulating their activity [255]. As a result, it may influence the cellular response to DNA damage [42].
An increased risk of pulmonary fibrosis in smokers is associated with genetic variations in genes encoding inflammatory mediators such as IL-1, TNF-α, and TGF-β [256]. Additionally, polymorphisms in the genes encoding chemokine (C-C motif) ligand 18 (CCL18) and chemokine (C-X-C motif) receptor 3 (CXCR3) have been linked to CS-induced pulmonary fibrosis [257]. Fibroblast activation causes excessive deposition of extracellular matrix components, such as collagen and fibronectin. Activation of lung fibroblasts and extracellular matrix production, such as MMPs and tissue inhibitors of metalloproteinases (TIMPs), is also a result of CS exposure [258,259]. EMT, characterized by the transformation of epithelial cells into mesenchymal cells, is a critical process in pulmonary fibrosis, resulting in increased fibroblast activity and extracellular matrix deposition. Smoking upregulates TGF-β signaling, thereby inducing EMT in lung epithelial cells [260]. Susceptibility to CS-induced pulmonary fibrosis might be influenced by genetic variations in EMT-related genes, such as Snail Family Transcriptional Repressor 1 (SNAI1), Twist Family BHLH Transcription Factor 1 (TWIST1), and Zinc Finger E-Box Binding Homeobox 1 (ZEB1).
Antioxidant therapies, such as N-acetylcysteine, have shown potential for ameliorating oxidative stress in pulmonary fibrosis [261]. N-acetylcysteine replenishes the levels of the antioxidant glutathione and reduces reactive oxygen species, thereby mitigating the harmful effects of oxidative stress on lung tissue [262]. Anti-inflammatory agents targeting pro-inflammatory cytokines, such as TNF-α, IL-1, and TGF-β [263], and anti-fibrotic drugs, including pirfenidone and nintedanib [264,265] have been explored as potential treatments for pulmonary fibrosis in addition to antioxidant therapies.
Targeting EMT with TGF-β signaling inhibitors, such as galunisertib, has shown promising results in preclinical studies [266]. Modulating specific microRNAs (miRNAs) is emerging as a therapeutic strategy for CS-induced pulmonary fibrosis and EMT [267]. It would be therapeutically beneficial to target chemokine signaling pathways such as CCL2/CCR2 and CCL18/CCR8 [268].
Phosphodiesterase 4B (PDE4B) inhibitors can play a significant role in alleviating disease progression and function as a therapeutic target for PF. The inflammation and fibrotic processes, which are often exacerbated by CS exposure in normal human bronchial epithelial cells, are reduced by the PDE4B inhibitors [269]. With regards to CS-induced lung damage, inhibitors targeting PDE4B might help mitigate the harmful effects of smoke and contribute to the overall treatment of pulmonary fibrosis [270].
In conclusion, CS-induced pulmonary fibrosis is a complex disease with multiple genetic and molecular mechanisms. Currently, emerging therapeutic strategies targeting EMT, miRNAs, and chemokine signaling might provide more effective treatments for this debilitating lung disease.
3.3. Cancers
Lung cancer is the leading cause of cancer-related deaths worldwide, accounting for approximately 1.8 million deaths per year [271]. The primary cause of lung cancer is cigarette smoking, which accounts for approximately 80–90% of all cases [272] and 25% of all cancer deaths. Smokers are 15–30 times more likely to develop lung cancer than non-smokers, and the risk increases proportionally with the number of cigarettes smoked per day and the duration of smoking [273]. Additionally, antioxidants such as glutathione, which are crucial in protecting against oxidative stress, can be depleted by CS [274]. The imbalance between ROS and antioxidants can result in chronic oxidative stress, which has been linked to lung cancer development [14].
Cigarette smoking, which contains more than 70 carcinogens, including polycyclic aromatic hydrocarbons and nitrosamines, has been linked to an enhanced risk of various types of cancer. Studies have indicated an association between smoking and an increased risk of bladder cancer, pancreatic cancer, kidney cancer, esophageal cancer, and head and neck cancers [272,275,276,277,278,279,280]. Oxidative stress has also been implicated in the development of many types of cancer, including lung cancer, breast cancer, and prostate cancer [281,282,283,284], and is also involved in inducing epigenetic changes, such as DNA methylation and histone modifications, which can alter expression patterns of genes and contribute to tumorigenesis [285].
In cancer, the role of NADPH oxidases is complex and depends on the type of cancer [286]. Some cancers are associated with enhanced expression of certain NOX isoforms, which contribute to cancer progression by promoting cell proliferation, survival, angiogenesis, and metastasis [287]. For example, NOX1 is upregulated in colon cancer, while NOX4 and NOX5 are often overexpressed in breast cancer [288]. Furthermore, the reactive oxygen species produced by these enzymes can result in DNA damage, which can further lead to mutations and the development of cancer [289].
NF-κB is a key factor related to controlling inflammation caused by oxidative stress, which was caused by CS [290]. CS reportedly increases the expression of inducible nitric oxide synthase (iNOS) and activates NF-κB, causing inflammation in human lymphocytes [291]. Oxidative stress-induced activation of the NF-κB pathway is involved in tumorigenesis by enhancing c-Myc and cyclin D1 levels [292,293]. The DNA damage induced by ROS results in mutations and altered gene expression patterns that promote cell proliferation and survival, including the activation of oncogenes and the inactivation of tumor suppressor genes [294]. The hypoxia-inducible factor (HIF) pathway, which plays a critical role in tumor angiogenesis, metastasis, and resistance to chemotherapy, can also be activated by ROS [295]. The PI3K/AKT/mTOR pathway, which regulates cell survival and proliferation [296], and the dysregulation of which has been implicated in several cancers, including lung cancer, is activated by ROS [297]. The redox state imbalance is characteristic of cancer, and the role of the antioxidant factor Nrf2 is important [298]. Interestingly, Nrf2 plays a dual role in cancer. In general, in the early stages of cancer, NRF2 activates DNA damage, cell cycle arrest, and DNA repair [299,300], and with the progression of the tumor, the activity of NRF2 can contribute to protecting against oxidative stress in tumor cells [300,301]. Therefore, treatment targeting Nrf2 can be either effective or suppressive, depending on the progression of the cancer.
Animal models have been developed to determine key molecular pathways involved in lung cancer, such as the PI3K/AKT/mTOR pathway and hypoxia-inducible factors, and test the efficacy of various therapies. Nano-immunotherapy has been observed to enhance the antitumor immune response and enhance therapeutic outcome in preclinical lung cancer models [302,303]. Zhong et al. reported that apoptosis, along with inhibition of NF-κB, accumulation of IκBα, and reduction in the DNA binding activity of NF-κB, was observed in the lungs of cigarette-exposed mice [304]. Clinical trials demonstrated that targeted therapy with epidermal growth factor receptor (EGFR) inhibitors improved progression-free survival and overall survival in patients with non-small cell lung cancer with EGFR mutations [305]. Moreover, some patients achieved long-term remission when treated with immunotherapy with checkpoint inhibitors, such as pembrolizumab and nivolumab, in clinical trials [306,307].
The aim of cancer treatment strategies is to improve upon traditional therapies like dexamethasone, a steroid that is often used to reduce inflammation and suppress the immune response in various cancers [308]. PD-1/PD-L1 inhibitors act by potentially mitigating oxidative stress, thereby improving the therapeutic efficacy of traditional therapies. Reduced oxidative stress for PD-1/PD-L1 inhibitors may enhance the ability of the immune system to target cancer cells by preventing immunosuppressive effects in the tumor microenvironment. This can be achieved by combining antioxidant therapies with PD-1/PD-L1 inhibitors to lower ROS levels and restore immune cell functionality [309]. The advent of novel therapeutic modalities such as nanocarriers, antibodies, gene editing technologies, and exosome technologies has led to a significant evolution of the cancer treatment landscape. Liposomes are a type of nanocarrier that is widely used to safely transport drugs and deliver them to specific tissues [310]. Monoclonal antibodies (mAbs) [311], which can function by directly inhibiting cancer cell growth, inducing apoptosis, or stimulating the immune system to attack the cancer cells, have emerged as one of the most successful strategies for targeted cancer therapy [312]. Examples of monoclonal antibodies include trastuzumab for HER2-positive lung cancer and rituximab for B-cell malignancies [313,314]. Gene editing, particularly the CRISPR-Cas9 system, is a powerful tool in cancer research and therapy [315]. This technology can be used not only to modify or correct disease-causing mutations in cancer cells [316], but it can also be used to engineer immune cells, such as T cells, to enhance their ability to recognize and kill cancer cells—a strategy used in CAR-T cell therapy [317]. Combining CAR-T-cell therapy with antioxidant treatments enables the management of oxidative stress by neutralizing ROS and enhancing the immune response of tumors, thereby improving the survival and functionality of engineered T cells [318]. Exosomes are small vesicles secreted by cells that can carry proteins, lipids, and nucleic acids [319], which can be engineered to deliver therapeutic agents, such as drugs or RNA molecules, directly to cancer cells [320]. Exosomes derived from mesenchymal stem cells (MSCs) exert therapeutic effects in the lungs [321]. These novel therapeutic strategies, though promising, also face challenges such as potential side effects, difficulty in delivery, and issues with specificity and efficiency. Ongoing research is needed to address these challenges and enable the full realization of the potential of these technologies in cancer treatment.
3.4. Acute Respiratory Distress Syndrome and Acute Exacerbations of COPD
Acute respiratory distress syndrome (ARDS) is a type of respiratory failure characterized by extensive lung inflammation that is caused by sepsis, trauma, pneumonia, and aspiration damage [322]. Pathophysiology involves an inflammatory cascade and destruction of the air-blood barrier [323]. During this process, the infiltrated neutrophils produce neutrophil elastase (NE) and MMPs and damage the air-blood barrier, leading to a vicious cycle leading to more inflammation and edema [324,325,326].
Although a direct association between smoking and ARDS is not known, studies suggest that CS exposure in ARDS patients increases the risk and severity. Moazed et al. reported that patients with ARDS exposed to CS had elevated plasma IL-8 levels [327,328]. In addition, exposure of smokers or non-smokers to LPS resulted in elevated levels of IL-1β, IL-8, and more excess neutrophils in the BAL of smokers [329]. Liu et al. observed an increased expression of angiotensin-converting enzyme 2 (ACE2), the receptor for SARS-CoV-2, in human airway epithelial cells [330,331]. A meta-analysis conducted by Cai et al. confirmed higher ACE2 gene expression in the lungs of smokers than in non-smokers [332]. Voinsky et al. demonstrated an enhanced expression of transmembrane proteases serine 2 and 4 (TMPRSS2 and TMPRSS4), which are important for SARS-CoV-2 to enter the cell, as well as ACE2 in the bronchi of smokers [333]. Their findings suggest that smoking may increase the risk of severe COVID-19 [334].
Recently, it has been reported that vitamin C has a positive effect on ARDS. Intravenous injections of vitamin C regulate neutrophil extracellular traps (NETs) in ARDS patients, thereby attenuating the ARDS-related biomarker synndecan-1 [335]. Vitamin C is one of the leading antioxidants and is important for the functioning of the immune system [336]. Activation of NADPH oxidase via ROS causes NETs [337]. Furthermore, various studies have reported administering ARDS treatment by using nanocarrier drug delivery systems. Saiping et al. developed a nanostructured lipid carrier that combines the intercellular adhesion molecule 1 (ICAM-1) antibody (ICAM/NLC) and confirms low pro-inflammatory cytokine levels in the ARDS-mouse model [338]. The development of such treatment modalities is important for overcoming ARDS.
Sudden deterioration of respiratory function in COPD patients is referred to as acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) [339]. This exacerbation worsens respiratory failure, necessitating mechanical ventilation [340]. Bacterial infections are commonly associated with AE-COPD [341,342,343,344] and COPD patients due to their compromised airways. Wang et al. categorized AE-COPD patients as smokers or non-smokers and conducted an analysis of the hematological parameters, which demonstrated that smokers exhibited higher counts of eosinophils and basophils in BAL [345]. Moreover, Li and colleagues reported that, as compared to smokers, non-smokers with AE-COPD had higher FEV1/forced vital capacity (FVC) and experienced less wheezing and phlegm production [346]. Thus, it is evident that CS-induced lung damage negatively impacts both chronic and acute inflammatory lung diseases, both before and after their onset.
3.5. Cardiovascular Disease
Cardiovascular disease (CVD) encompasses various heart and vascular conditions, such as angina, myocardial infarction, coronary artery disease, and heart failure [347]. Numerous reports have reported CS-induced adverse effects on cardiovascular disease [348,349,350,351,352]. However, the specific mechanisms that exist between CS and cardiovascular disease have not been fully elucidated.
CS exposure increases the risk of atherosclerosis and, consequently, coronary artery syndrome and stroke, as it causes vascular dysfunction and inflammation. Several reports have shown that CS exposure impairs endothelial-dependent vasodilation in humans [353,354]. It has also been found that CS reduces the synthesis of nitrogen oxide (NO) [355,356]. In general, NO, along with guanosine monophosphate (GMP) and calcium channels, is involved in vasodilation [357]. In vitro studies have demonstrated lower endothelial NOS (eNOS) activity and decreased production of NO when endothelial cells of the human coronary artery were treated with the serum of smokers [358]. Furthermore, CS-induced ROS activates the NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3), inducing the expression of IL-1β and IL-18, resulting in autophagy, apoptosis, and endothelial cell dysfunction [359,360]. In addition, CS can secrete several cytokines, thereby affecting white blood cell recruitment. Mazzone reported that elevated levels of soluble intercellular adhesion molecule 1 (sICAM-1) and soluble vascular cell adhesion molecule 1 (sVCAM-1), which could contribute to hypertension, were identified in the plasma of smokers [361]. Smoking can also increase low-density lipoprotein (LDL) levels in the blood, leading to the formation of oxidized LDLs (oxLDL), which in turn leads to the production of pro-inflammatory cytokines and foam cells [362,363,364]. Foam cells are generated when oxLDL functions as a ligand in the lectin-like oxidized LDL receptor-1 (LOX-1), which is a macrophage-expressing receptor. Smoking increases the expression of LOX-1 [365,366]. The accumulation of foam cells can lead to atherosclerosis [367].
4. Conclusions
In this review, we explored the cigarette smoke-mediated cellular pathways, focusing on NF-κB, MAPK, Nrf2, and RAGE. Furthermore, HIF, mTOR, TGF-β, and NLRP3 play key roles in respiratory diseases such as cancer, pulmonary fibrosis, and cardiovascular diseases. These factors are influenced by oxidative stress within the body, resulting in a variety of harmful effects. These complex and pathogenic cellular processes negatively impact various diseases, including multiple types of cancer, chronic and acute lung diseases, and cardiovascular diseases. Recently, focus has shifted beyond traditional methods, and novel treatment modalities such as nanocarriers, drug delivery systems, mAbs, gene editing, and exosomes have gained attention. We have summarized the various therapy modalities for CS-induced lung diseases in Figure 1 and Table 1. Future research targeting these damage mechanisms may pave the way for the development of therapeutic modalities to mitigate the effects of these diseases.
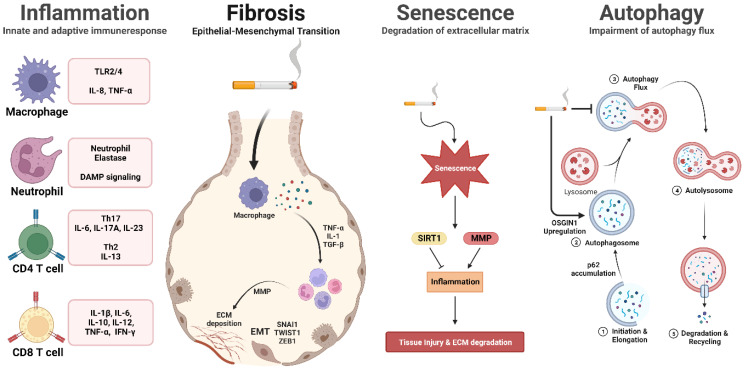
Figure 1.
Cigarette smoke-mediated cellular pathways in inflammation, fibrosis, senescence, and autophagy. Cigarette smoke exposure initiates inflammation pathways, leading to the recruitment of immune cells and the subsequent production of pro-inflammatory cytokines. Prolonged exposure to cigarette smoke activates fibrosis pathways, resulting in extracellular matrix deposition and fibrosis formation. Moreover, cellular senescence is expedited, and the autophagy pathway is modified in response to cigarette smoke, contributing to the onset of age-related diseases. This illustration depicts the cellular pathways influenced by cigarette smoke exposure.
Table 1.
Treatment approaches for cigarette smoke-induced lung diseases.
| Respiratory Disease | Therapy | CS-Induced Mechanism | Reference |
|---|---|---|---|
| COPD | Metformin | Inhibition of apoptosis through regulation of AMP kinase. | [226] |
| Astaxanthin | Inhibition of Nrf2-modulated oxidative stress and regulation of NF-κB-related inflammatory responses through binding with SIRT1. | [231] | |
| HUC-MSC- derived EVs |
Alleviation of airway inflammation in the CS-induced rat model. | [232] | |
| LPHNPs | Upregulation of cytocompatibility toward bronchial epithelial cells and macrophages. | [233] | |
| Pulmonary Fibrosis |
N-acetylcysteine | Replenish the levels of the antioxidant glutathione and reduce reactive oxygen species by targeting pro-inflammatory cytokines such as TNF-α, IL-1, and TGF-β. | [262,263] |
| PDE4B inhibitors | Reducing inflammation and fibrotic processes by inhibiting the degradation of cAMP. | [269] | |
| Cancer | PD-1/PD-L1 inhibitors | Enhance the immune system’s ability to target cancer cells and restore immune cell functionality by combining PD-L1 from cancer and PD-1 from T cells. | [309] |
| Trastuzumab | Durable anticancer activity in patients with previously treated HER2-mutant of NSCLC. | [291] | |
| Rituximab | Depletes CD20-positive B cells in lung tumors. | [292] | |
| CRISPR-Cas9 | Modify or correct disease-related genes causing mutations in cancer cells. | [316] | |
| CAR-T-cell | improve the survival and functionality of engineered T-cells by reducing ROS and eliminating cancer cells. | [318] | |
| MSCs-exosome | Cell-to-cell communication within the tumor microenvironment and suppression of angiogenesis. | [321] | |
| ARDS and AE-COPD |
Vitamin C | Antioxidant vitamin C inhibits ROS-mediated NET by inactivating NADPH oxidase. | [335] |
| ICAM/NLC | Decrease pro-inflammatory cytokines in the ARDS mouse model. | [338] |
Abbreviations
| Abbreviation | Definitions |
| CS | cigarette smoke |
| ROS | reactive oxygen species |
| COPD | chronic obstructive pulmonary disease |
| PF | pulmonary fibrosis |
| WHO | World Health Organization |
| FCTC | Framework Convention on Tobacco Control |
| NOX | NADPH oxidase |
| NETs | neutrophil extracellular traps |
| DAMP | damage-associated molecular patterns |
| RAGE | receptor for advanced glycation end products |
| HMGB1 | high mobility group box 1 |
| sRAGE | soluble form RAGE |
| mRAGE | membrane-bound form RAGE |
| TLRs | toll-like receptors |
| H1R | histamine receptor-1 |
| COX2 | cyclooxygenase2 |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor kappa-light chain-enhancer of activated B cells |
| IKK | IκB kinase |
| ERK1/2 | extracellular signal-regulated kinases |
| JNK1/2 | c-Jun N-terminal Kinase |
| STAT | signal transducer and activator of transcription |
| IRF9 | interferon regulatory factor 9 |
| CBP | CREB-binding protein |
| CSE | cigarette smoke extract |
| BAL | bronchoalveolar lavage |
| BCL-2 | B-cell lymphoma 2 |
| TP53 | tumor protein p53 |
| IAPs | inhibitor of apoptosis proteins |
| TSSK4 | testis-specific serine/threonine kinase 4 |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
| AKR1C3 | aldo-keto reductase family 1 member C3 |
| GPX2 | glutathione peroxidase 2 |
| ILD | interstitial lung disease |
| MMPs | matrix metalloproteinases |
| SIRT1 | sirtuin-1 |
| SASP | senescence-associated secretory phenotype |
| OSGIN | oxidative stress-induced growth inhibitor |
| Nrf2 | nuclear factor erythroid 2-related factor |
| Keap1 | Kelch-like ECH-associated protein 1 |
| ARE | antioxidant response elements |
| HO1 | heme oxygenase 1 |
| GST | glutathione S-transferase |
| FEV1 | forced expiratory volume |
| SOD1 | superoxide dismutase type 1 |
| TXNRD1 | thioredoxin reductase 1 |
| GOLD | Global Initiative for Obstructive Lung Disease |
| HUC-MSC | Human umbilical cord mesenchymal system cell |
| LPHNPs | lipid-polymer hybrid nanoparticles |
| EMT | epithelial-mesenchymal transition |
| SNPs | single nucleotide polymorphisms |
| CYP1A1 | cytochrome P450 Family 1 Subfamily A Member 1 |
| CYP1B1 | cytochrome P450 Family 1 Subfamily B Member 1 |
| CCL18 | chemokine (C-C motif) ligand 18 |
| CXCR3 | chemokine (C-X-C motif) receptor 3 |
| TIMPs | tissue inhibitors of metalloproteinases |
| miRNAs | microRNAs |
| PDE4B | phosphodiesterase 4B |
| iNOS | inducible nitric oxide synthase |
| HIF | hypoxia-inducible factor |
| NSCLC | non-small-cell lung cancer |
| mAbs | monoclonal antibodies |
| MSCs | mesenchymal stem cells |
| PAHs | polycyclic aromatic hydrocarbons |
| ARDS | acute respiratory distress syndrome |
| NE | neutrophil elastase |
| ACE2 | angiotensin-converting enzyme 2 |
| TMPRSS2/4 | transmembrane protease, serine 2 and 4 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| AE-COPD | acute exacerbations of chronic obstructive pulmonary disease |
| FVC | forced vital capacity |
| CVD | cardiovascular disease |
| GMP | guanosine monophosphate |
| eNOS | endothelial NOS |
| NLR | NOD-like receptor |
| NLRP3 | NLR family pyrin domain containing 3 |
| sVCAM-1 | soluble vascular cell adhesion molecule 1 |
| LDL | low-density lipoprotein |
| oxLDL | oxidized LDLs |
| LOX-1 | lectin-like oxidized LDL receptor-1 |
Author Contributions
Conceptualization, S.-R.C., J.J. and S.-R.Y.; writing—original draft preparation, S.-R.C. and J.J.; writing—review and editing, S.-M.P., S.M.R. and S.-J.C.; supervision, S.-R.Y.; funding acquisition, S.-R.Y. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
National Research Foundation of Korea: 2020R1A2C2010712, 2020R1A5A8019180, Ministry of Education (MOE): 2022RIS-005.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025. 2nd ed. WHO; Geneva, Switzerland: 2018. [Google Scholar]
- 2.Dai X., Gakidou E., Lopez A.D. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob. Control. 2022;31:129–137. doi: 10.1136/tobaccocontrol-2021-056535. [DOI] [PubMed] [Google Scholar]
- 3.Hiilamo H., Glantz S. Global Implementation of Tobacco Demand Reduction Measures Specified in Framework Convention on Tobacco Control. Nicotine Tob. Res. 2022;24:503–510. doi: 10.1093/ntr/ntab216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong G.T., Chung-Hall J., Craig L., Group W.F.I.A.E. Impact assessment of the WHO FCTC over its first decade: Methodology of the expert group. Tob. Control. 2019;28:s84–s88. doi: 10.1136/tobaccocontrol-2018-054374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung-Hall J., Craig L., Gravely S., Sansone N., Fong G.T. Impact of the WHO FCTC over the first decade: A global evidence review prepared for the Impact Assessment Expert Group. Tob. Control. 2019;28:s119–s128. doi: 10.1136/tobaccocontrol-2018-054389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamceva G., Arsova-Sarafinovska Z., Ruskovska T., Zdravkovska M., Kamceva-Panova L., Stikova E. Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced. J. Med. Sci. 2016;4:636–640. doi: 10.3889/oamjms.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan J.C., Byron M.J., Baig S.A., Stepanov I., Brewer N.T. How people think about the chemicals in cigarette smoke: A systematic review. J. Behav. Med. 2017;40:553–564. doi: 10.1007/s10865-017-9823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koul A., Bhatia V., Bansal M.P. Effect of alpha-tocopherol on pulmonary antioxidant defence system and lipid peroxidation in cigarette smoke inhaling mice. BMC Biochem. 2001;2:14. doi: 10.1186/1471-2091-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X.Y., Zhu M.X., Xie J.P. Mutagenicity of acrolein and acrolein-induced DNA adducts. Toxicol. Mech. Methods. 2010;20:36–44. doi: 10.3109/15376510903530845. [DOI] [PubMed] [Google Scholar]
- 10.Pu X., Kamendulis L.M., Klaunig J.E. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats. Toxicol. Sci. 2009;111:64–71. doi: 10.1093/toxsci/kfp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Rahman I., MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 14.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Almyroudis N.G., Grimm M.J., Davidson B.A., Rohm M., Urban C.F., Segal B.H. NETosis and NADPH oxidase: At the intersection of host defense, inflammation, and injury. Front. Immunol. 2013;4:45. doi: 10.3389/fimmu.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dusting G.J., Selemidis S., Jiang F. Mechanisms for suppressing NADPH oxidase in the vascular wall. Mem. Inst. Oswaldo Cruz. 2005;100((Suppl. S1)):97–103. doi: 10.1590/S0074-02762005000900016. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 21.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman I., Biswas S.K., Jimenez L.A., Torres M., Forman H.J. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 23.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman I., Biswas S.K., Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 25.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 26.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 27.Comhair S.A., Erzurum S.C. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer. 2012;131:2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valavanidis A., Vlachogianni T., Fiotakis K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J., Taneja V., Vassallo R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberdorster G., Finkelstein J.N., Johnston C., Gelein R., Cox C., Baggs R., Elder A.C. Acute pulmonary effects of ultrafine particles in rats and mice. Res. Rep. Health Eff. Inst. 2000;5–74:75–86. [PubMed] [Google Scholar]
- 33.Salvi S., Holgate S.T. Mechanisms of particulate matter toxicity. Clin. Exp. Allergy. 1999;29:1187–1194. doi: 10.1046/j.1365-2222.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 34.Pappas R.S., Halstead M.M., Watson C.H. Electron Microscopic Analysis of Silicate and Calcium Particles in Cigarette Smoke Tar. Int. J. Respir. Pulm. Med. 2016;3:39. doi: 10.23937/2378-3516/1410039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borm P.J., Schins R.P., Albrecht C. Inhaled particles and lung cancer, part B: Paradigms and risk assessment. Int. J. Cancer. 2004;110:3–14. doi: 10.1002/ijc.20064. [DOI] [PubMed] [Google Scholar]
- 36.Caliri A.W., Tommasi S., Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021;787:108365. doi: 10.1016/j.mrrev.2021.108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carta S., Castellani P., Delfino L., Tassi S., Vene R., Rubartelli A. DAMPs and inflammatory processes: The role of redox in the different outcomes. J. Leukoc. Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- 38.Logan S.M., Storey K.B. Pro-inflammatory AGE-RAGE signaling is activated during arousal from hibernation in ground squirrel adipose. PeerJ. 2018;6:e4911. doi: 10.7717/peerj.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirasawa M., Fujiwara N., Hirabayashi S., Ohno H., Iida J., Makita K., Hata Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9:165–174. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirschi-Budge K.M., Tsai K.Y.F., Curtis K.L., Davis G.S., Theurer B.K., Kruyer A.M.M., Homer K.W., Chang A., Van Ry P.M., Arroyo J.A., et al. RAGE signaling during tobacco smoke-induced lung inflammation and potential therapeutic utility of SAGEs. BMC Pulm. Med. 2022;22:160. doi: 10.1186/s12890-022-01935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson A.B., Stogsdill J.A., Lewis J.B., Wood T.T., Reynolds P.R. RAGE and tobacco smoke: Insights into modeling chronic obstructive pulmonary disease. Front. Physiol. 2012;3:301. doi: 10.3389/fphys.2012.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H., Lee J., Hong S.H., Rahman I., Yang S.R. Inhibition of RAGE Attenuates Cigarette Smoke-Induced Lung Epithelial Cell Damage via RAGE-Mediated Nrf2/DAMP Signaling. Front. Pharmacol. 2018;9:684. doi: 10.3389/fphar.2018.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders K.A., Delker D.A., Huecksteadt T., Beck E., Wuren T., Chen Y., Zhang Y., Hazel M.W., Hoidal J.R. RAGE is a Critical Mediator of Pulmonary Oxidative Stress, Alveolar Macrophage Activation and Emphysema in Response to Cigarette Smoke. Sci. Rep. 2019;9:231. doi: 10.1038/s41598-018-36163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 45.Krutzik S.R., Tan B., Li H., Ochoa M.T., Liu P.T., Sharfstein S.E., Graeber T.G., Sieling P.A., Liu Y.J., Rea T.H., et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barua R.S., Sharma M., Dileepan K.N. Cigarette Smoke Amplifies Inflammatory Response and Atherosclerosis Progression Through Activation of the H1R-TLR2/4-COX2 Axis. Front. Immunol. 2015;6:572. doi: 10.3389/fimmu.2015.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadigel J., Prefontaine D., Baglole C.J., Maltais F., Bourbeau J., Eidelman D.H., Hamid Q. Cigarette smoke increases TLR4 and TLR9 expression and induces cytokine production from CD8+ T cells in chronic obstructive pulmonary disease. Respir. Res. 2011;12:149. doi: 10.1186/1465-9921-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girard S., Kadhim H., Roy M., Lavoie K., Brochu M.E., Larouche A., Sebire G. Role of perinatal inflammation in cerebral palsy. Pediatr. Neurol. 2009;40:168–174. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Moynagh P.N. The NF-kappaB pathway. J. Cell Sci. 2005;118:4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayden M.S., Ghosh S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Sabio G., Davis R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Shea J.J., Schwartz D.M., Villarino A.V., Gadina M., McInnes I.B., Laurence A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wojciak J.M., Martinez-Yamout M.A., Dyson H.J., Wright P.E. Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. EMBO J. 2009;28:948–958. doi: 10.1038/emboj.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tesoriere A., Dinarello A., Argenton F. The Roles of Post-Translational Modifications in STAT3 Biological Activities and Functions. Biomedicines. 2021;9:956. doi: 10.3390/biomedicines9080956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen K.L., Brockwell N.K., Parker B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers. 2019;11:2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cutolo M., Campitiello R., Gotelli E., Soldano S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022;13:867260. doi: 10.3389/fimmu.2022.867260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang S.R., Chida A.S., Bauter M.R., Shafiq N., Seweryniak K., Maggirwar S.B., Kilty I., Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 63.Ko H.K., Lee H.F., Lin A.H., Liu M.H., Liu C.I., Lee T.S., Kou Y.R. Regulation of Cigarette Smoke Induction of IL-8 in Macrophages by AMP-activated Protein Kinase Signaling. J. Cell Physiol. 2015;230:1781–1793. doi: 10.1002/jcp.24881. [DOI] [PubMed] [Google Scholar]
- 64.Yang S.R., Wright J., Bauter M., Seweryniak K., Kode A., Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: Implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 65.Metcalfe H.J., Lea S., Hughes D., Khalaf R., Abbott-Banner K., Singh D. Effects of cigarette smoke on Toll-like receptor (TLR) activation of chronic obstructive pulmonary disease (COPD) macrophages. Clin. Exp. Immunol. 2014;176:461–472. doi: 10.1111/cei.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H., Yang T., Ning Q., Li F., Chen T., Yao Y., Sun Z. Cigarette smoke extract-treated mast cells promote alveolar macrophage infiltration and polarization in experimental chronic obstructive pulmonary disease. Inhal. Toxicol. 2015;27:822–831. doi: 10.3109/08958378.2015.1116644. [DOI] [PubMed] [Google Scholar]
- 67.Bazzan E., Turato G., Tine M., Radu C.M., Balestro E., Rigobello C., Biondini D., Schiavon M., Lunardi F., Baraldo S., et al. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir. Res. 2017;18:40. doi: 10.1186/s12931-017-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paudel K.R., Panth N., Manandhar B., Singh S.K., Gupta G., Wich P.R., Nammi S., MacLoughlin R., Adams J., Warkiani M.E., et al. Attenuation of Cigarette-Smoke-Induced Oxidative Stress, Senescence, and Inflammation by Berberine-Loaded Liquid Crystalline Nanoparticles: In Vitro Study in 16HBE and RAW264. 7 cells. Antioxidants. 2022;11:873. doi: 10.3390/antiox11050873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ermert D., Niemiec M.J., Rohm M., Glenthoj A., Borregaard N., Urban C.F. Candida albicans escapes from mouse neutrophils. J. Leukoc. Biol. 2013;94:223–236. doi: 10.1189/jlb.0213063. [DOI] [PubMed] [Google Scholar]
- 70.Hoenderdos K., Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2013;48:531–539. doi: 10.1165/rcmb.2012-0492TR. [DOI] [PubMed] [Google Scholar]
- 71.Thulborn S.J., Mistry V., Brightling C.E., Moffitt K.L., Ribeiro D., Bafadhel M. Neutrophil elastase as a biomarker for bacterial infection in COPD. Respir. Res. 2019;20:170. doi: 10.1186/s12931-019-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voynow J.A., Shinbashi M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules. 2021;11:65. doi: 10.3390/biom11081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee K.Y., Ho S.C., Lin H.C., Lin S.M., Liu C.Y., Huang C.D., Wang C.H., Chung K.F., Kuo H.P. Neutrophil-derived elastase induces TGF-beta1 secretion in human airway smooth muscle via NF-kappaB pathway. Am. J. Respir. Cell Mol. Biol. 2006;35:407–414. doi: 10.1165/rcmb.2006-0012OC. [DOI] [PubMed] [Google Scholar]
- 74.Guzik K., Skret J., Smagur J., Bzowska M., Gajkowska B., Scott D.A., Potempa J.S. Cigarette smoke-exposed neutrophils die unconventionally but are rapidly phagocytosed by macrophages. Cell Death Dis. 2011;2:e131. doi: 10.1038/cddis.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noda N., Matsumoto K., Fukuyama S., Asai Y., Kitajima H., Seki N., Matsunaga Y., Kan O.K., Moriwaki A., Morimoto K., et al. Cigarette smoke impairs phagocytosis of apoptotic neutrophils by alveolar macrophages via inhibition of the histone deacetylase/Rac/CD9 pathways. Int. Immunol. 2013;25:643–650. doi: 10.1093/intimm/dxt033. [DOI] [PubMed] [Google Scholar]
- 76.Pouwels S.D., Zijlstra G.J., van der Toorn M., Hesse L., Gras R., Ten Hacken N.H., Krysko D.V., Vandenabeele P., de Vries M., van Oosterhout A.J., et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L377–L386. doi: 10.1152/ajplung.00174.2015. [DOI] [PubMed] [Google Scholar]
- 77.Kaech S.M., Wherry E.J., Ahmed R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 78.Zhou L., Chong M.M., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Raphael I., Nalawade S., Eagar T.N., Forsthuber T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. doi: 10.4049/jimmunol.155.3.1151. [DOI] [PubMed] [Google Scholar]
- 81.Kaiko G.E., Horvat J.C., Beagley K.W., Hansbro P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Peng W., Weng Y., Ying H., Li H., Xia D., Yu W. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int. Immunopharmacol. 2012;14:504–512. doi: 10.1016/j.intimp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Harrison O.J., Foley J., Bolognese B.J., Long E., 3rd, Podolin P.L., Walsh P.T. Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol. Lett. 2008;121:13–21. doi: 10.1016/j.imlet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 84.Van Hove C.L., Moerloose K., Maes T., Joos G.F., Tournoy K.G. Cigarette smoke enhances Th-2 driven airway inflammation and delays inhalational tolerance. Respir. Res. 2008;9:42. doi: 10.1186/1465-9921-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura Y., Miyata M., Ohba T., Ando T., Hatsushika K., Suenaga F., Shimokawa N., Ohnuma Y., Katoh R., Ogawa H., et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J. Allergy Clin. Immunol. 2008;122:1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 86.Maeno T., Houghton A.M., Quintero P.A., Grumelli S., Owen C.A., Shapiro S.D. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J. Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 87.Wasen C., Turkkila M., Bossios A., Erlandsson M., Andersson K.M., Ekerljung L., Malmhall C., Brisslert M., Toyra Silfversward S., Lundback B., et al. Smoking activates cytotoxic CD8+ T cells and causes survivin release in rheumatoid arthritis. J. Autoimmun. 2017;78:101–110. doi: 10.1016/j.jaut.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 88.Zhong L., Qin L., Ding X., Ma L., Wang Y., Liu M., Chen H., Yan H., Song L. The regulatory effect of fermented black barley on the gut microbiota and metabolic dysbiosis in mice exposed to cigarette smoke. Food Res. Int. 2022;157:111465. doi: 10.1016/j.foodres.2022.111465. [DOI] [PubMed] [Google Scholar]
- 89.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fulda S., Debatin K.M. Targeting apoptosis pathways in cancer therapy. Curr. Cancer Drug Targets. 2004;4:569–576. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- 92.Krammer P.H., Arnold R., Lavrik I.N. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 93.Cory S., Adams J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 94.Delbridge A.R., Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuentes-Prior P., Salvesen G.S. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vousden K.H., Lane D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 97.Silke J., Meier P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb. Perspect. Biol. 2013;5:8730. doi: 10.1101/cshperspect.a008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 99.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 100.Chen H., He A., Li H., Chen H., Xie H., Luo L., Huang Y., Chen J., Guan J., He Q., et al. TSSK4 upregulation in alveolar epithelial type-II cells facilitates pulmonary fibrosis through HSP90-AKT signaling restriction and AT-II apoptosis. Cell Death Dis. 2021;12:938. doi: 10.1038/s41419-021-04232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong W., Zhu X., Liu X., Zhao X., Lei X., Kang L., Liu L. Role of the SENP1-SIRT1 pathway in hyperoxia-induced alveolar epithelial cell injury. Free Radic. Biol. Med. 2021;173:142–150. doi: 10.1016/j.freeradbiomed.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 102.Bao T., Zhu H., Zheng Y., Hu J., Wang H., Cheng H., Zhang Y., Tian Z. Expression of long noncoding RNA uc.375 in bronchopulmonary dysplasia and its function in the proliferation and apoptosis of mouse alveolar epithelial cell line MLE 12. Front. Physiol. 2022;13:971732. doi: 10.3389/fphys.2022.971732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krysko D.V., Vanden Berghe T., D’Herde K., Vandenabeele P. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Rahman I., MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic. Biol. Med. 1996;21:669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 105.Ma T.L., Zhou Y., Wang C., Wang L., Chen J.X., Yang H.H., Zhang C.Y., Zhou Y., Guan C.X. Targeting Ferroptosis for Lung Diseases: Exploring Novel Strategies in Ferroptosis-Associated Mechanisms. Oxid. Med. Cell Longev. 2021;2021:1098970. doi: 10.1155/2021/1098970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Batista Napotnik T., Polajzer T., Miklavcic D. Cell death due to electroporation-A review. Bioelectrochemistry. 2021;141:107871. doi: 10.1016/j.bioelechem.2021.107871. [DOI] [PubMed] [Google Scholar]
- 107.Tojo K., Yamamoto N., Tamada N., Mihara T., Abe M., Nishii M., Takeuchi I., Goto T. Early alveolar epithelial cell necrosis is a potential driver of COVID-19-induced acute respiratory distress syndrome. iScience. 2023;26:105748. doi: 10.1016/j.isci.2022.105748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin Z., Xu Y., Guan L., Qin L., Ding J., Zhang Q., Zhou L. Seven ferroptosis-specific expressed genes are considered as potential biomarkers for the diagnosis and treatment of cigarette smoke-induced chronic obstructive pulmonary disease. Ann. Transl. Med. 2022;10:331. doi: 10.21037/atm-22-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mercado N., Ito K., Barnes P.J. Accelerated ageing of the lung in COPD: New concepts. Thorax. 2015;70:482–489. doi: 10.1136/thoraxjnl-2014-206084. [DOI] [PubMed] [Google Scholar]
- 111.Brereton C.F., Blander J.M. Responding to infection and apoptosis—A task for TH17 cells. Ann. N. Y. Acad. Sci. 2010;1209:56–67. doi: 10.1111/j.1749-6632.2010.05747.x. [DOI] [PubMed] [Google Scholar]
- 112.Gaillat J. Should patients with chronic obstructive pulmonary disease be vaccinated against pneumococcal diseases? Expert Rev. Respir. Med. 2009;3:585–596. doi: 10.1586/ers.09.53. [DOI] [PubMed] [Google Scholar]
- 113.Haegele J.A., Yessick A., Zhu X. Females with Visual Impairments in Physical Education: Exploring the Intersection between Disability and Gender Identities. Res. Q. Exerc. Sport. 2018;89:298–308. doi: 10.1080/02701367.2018.1484067. [DOI] [PubMed] [Google Scholar]
- 114.Muradian K., Schachtschabel D.O. The role of apoptosis in aging and age-related disease: Update. Z. Gerontol. Geriatr. 2001;34:441–446. doi: 10.1007/s003910170015. [DOI] [PubMed] [Google Scholar]
- 115.Yao H., Yang S.R., Kode A., Rajendrasozhan S., Caito S., Adenuga D., Henry R., Edirisinghe I., Rahman I. Redox regulation of lung inflammation: Role of NADPH oxidase and NF-kappaB signalling. Biochem. Soc. Trans. 2007;35:1151–1155. doi: 10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]
- 116.Vij N., Chandramani-Shivalingappa P., Van Westphal C., Hole R., Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol. Cell Physiol. 2018;314:C73–C87. doi: 10.1152/ajpcell.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leyane T.S., Jere S.W., Houreld N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022;23:7273. doi: 10.3390/ijms23137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morsch A., Wisniewski E., Luciano T.F., Comin V.H., Silveira G.B., Marques S.O., Thirupathi A., Silveira Lock P.C., De Souza C.T. Cigarette smoke exposure induces ROS-mediated autophagy by regulating sestrin, AMPK, and mTOR level in mice. Redox Rep. 2019;24:27–33. doi: 10.1080/13510002.2019.1601448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Su L.J., Zhang J.H., Gomez H., Murugan R., Hong X., Xu D., Jiang F., Peng Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vella G., Ritzmann F., Wolf L., Kamyschnikov A., Stodden H., Herr C., Slevogt H., Bals R., Beisswenger C. IL-17C contributes to NTHi-induced inflammation and lung damage in experimental COPD and is present in sputum during acute exacerbations. PLoS ONE. 2021;16:e0243484. doi: 10.1371/journal.pone.0243484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strzelak A., Ratajczak A., Adamiec A., Feleszko W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health. 2018;15:1033. doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scott J.E. The pulmonary surfactant: Impact of tobacco smoke and related compounds on surfactant and lung development. Tob. Induc. Dis. 2004;2:3–25. doi: 10.1186/1617-9625-2-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yao H., Sundar I.K., Ahmad T., Lerner C., Gerloff J., Friedman A.E., Phipps R.P., Sime P.J., McBurney M.W., Guarente L., et al. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L816–L828. doi: 10.1152/ajplung.00323.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Christopoulou M.E., Papakonstantinou E., Stolz D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023;24:3786. doi: 10.3390/ijms24043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao R., Ma Z., Hu Y., Chen J., Shetty S., Fu J. Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L847–L853. doi: 10.1152/ajplung.00274.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang M.Y., Zhou T.Y., Zhang Z.D., Liu H.Y., Zheng Z.Y., Xie H.Q. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. MedComm. 2021;2:351–380. doi: 10.1002/mco2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Babu K.S., Kastelik J.A., Morjaria J.B. Inhaled corticosteroids in chronic obstructive pulmonary disease: A pro-con perspective. Br. J. Clin. Pharmacol. 2014;78:282–300. doi: 10.1111/bcp.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jerkic M., Szaszi K., Laffey J.G., Rotstein O., Zhang H. Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury. Int. J. Mol. Sci. 2023;24:3376. doi: 10.3390/ijms24043376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Eerd E.A., van der Meer R.M., van Schayck O.C., Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2016;2016:CD010744. doi: 10.1002/14651858.CD010744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaib S., Tchkonia T., Kirkland J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022;28:1556–1568. doi: 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumari R., Jat P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z., Zhang Z., Ren Y., Wang Y., Fang J., Yue H., Ma S., Guan F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology. 2021;22:165–187. doi: 10.1007/s10522-021-09910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takasugi M., Yoshida Y., Ohtani N. Cellular senescence and the tumour microenvironment. Mol. Oncol. 2022;16:3333–3351. doi: 10.1002/1878-0261.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kirkland J.L., Tchkonia T. Senolytic drugs: From discovery to translation. J. Intern Med. 2020;288:518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., Inman C.L., Ogrodnik M.B., Hachfeld C.M., Fraser D.G., et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaur G., Muthumalage T., Rahman I. Clearance of senescent cells reverts the cigarette smoke-induced lung senescence and airspace enlargement in p16-3MR mice. Aging Cell. 2023:e13850. doi: 10.1111/acel.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lord J.M. The effect of ageing of the immune system on vaccination responses. Hum. Vaccin Immunother. 2013;9:1364–1367. doi: 10.4161/hv.24696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Birch J., Gil J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee K.A., Flores R.R., Jang I.H., Saathoff A., Robbins P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging. 2022;3:900028. doi: 10.3389/fragi.2022.900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klionsky D.J. Autophagy revisited: A conversation with Christian de Duve. Autophagy. 2008;4:740–743. doi: 10.4161/auto.6398. [DOI] [PubMed] [Google Scholar]
- 144.Mizushima N., Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 145.Kobayashi S. Choose Delicately and Reuse Adequately: The Newly Revealed Process of Autophagy. Biol. Pharm. Bull. 2015;38:1098–1103. doi: 10.1248/bpb.b15-00096. [DOI] [PubMed] [Google Scholar]
- 146.Meijer A.J., Codogno P. Autophagy: Regulation and role in disease. Crit. Rev. Clin. Lab. Sci. 2009;46:210–240. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- 147.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yun H.R., Jo Y.H., Kim J., Shin Y., Kim S.S., Choi T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020;21:3289. doi: 10.3390/ijms21093289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ndoye A., Weeraratna A.T. Autophagy—An emerging target for melanoma therapy. F1000Res. 2016;5:8347. doi: 10.12688/f1000research.8347.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang X., Yu D.D., Yan F., Jing Y.Y., Han Z.P., Sun K., Liang L., Hou J., Wei L.X. The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci. 2015;5:14. doi: 10.1186/s13578-015-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loos B., du Toit A., Hofmeyr J.H. Defining and measuring autophagosome flux-concept and reality. Autophagy. 2014;10:2087–2096. doi: 10.4161/15548627.2014.973338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X.J., Chen S., Huang K.X., Le W.D. Why should autophagic flux be assessed? Acta Pharmacol. Sin. 2013;34:595–599. doi: 10.1038/aps.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shoubridge A.P., Fourrier C., Choo J.M., Proud C.G., Sargeant T.J., Rogers G.B. Gut Microbiome Regulation of Autophagic Flux and Neurodegenerative Disease Risks. Front. Microbiol. 2021;12:817433. doi: 10.3389/fmicb.2021.817433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klionsky D.J., Petroni G., Amaravadi R.K., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cadwell K., Cecconi F., Choi A.M.K., et al. Autophagy in major human diseases. EMBO J. 2021;40:e108863. doi: 10.15252/embj.2021108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang G., Zhou H., Strulovici-Barel Y., Al-Hijji M., Ou X., Salit J., Walters M.S., Staudt M.R., Kaner R.J., Crystal R.G. Role of OSGIN1 in mediating smoking-induced autophagy in the human airway epithelium. Autophagy. 2017;13:1205–1220. doi: 10.1080/15548627.2017.1301327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szoka P., Lachowicz J., Cwiklinska M., Lukaszewicz A., Rybak A., Baranowska U., Holownia A. Cigarette Smoke-Induced Oxidative Stress and Autophagy in Human Alveolar Epithelial Cell Line (A549 Cells) Adv. Exp. Med. Biol. 2019;1176:63–69. doi: 10.1007/5584_2019_373. [DOI] [PubMed] [Google Scholar]
- 158.Kono Y., Colley T., To M., Papaioannou A.I., Mercado N., Baker J.R., To Y., Abe S., Haruki K., Ito K., et al. Cigarette smoke-induced impairment of autophagy in macrophages increases galectin-8 and inflammation. Sci. Rep. 2021;11:335. doi: 10.1038/s41598-020-79848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tribulatti M.V., Carabelli J., Prato C.A., Campetella O. Galectin-8 in the onset of the immune response and inflammation. Glycobiology. 2020;30:134–142. doi: 10.1093/glycob/cwz077. [DOI] [PubMed] [Google Scholar]
- 160.Sampson J.F., Suryawanshi A., Chen W.S., Rabinovich G.A., Panjwani N. Galectin-8 promotes regulatory T-cell differentiation by modulating IL-2 and TGFbeta signaling. Immunol. Cell Biol. 2016;94:213–219. doi: 10.1038/icb.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eckardt V., Miller M.C., Blanchet X., Duan R., Leberzammer J., Duchene J., Soehnlein O., Megens R.T., Ludwig A.K., Dregni A., et al. Chemokines and galectins form heterodimers to modulate inflammation. EMBO Rep. 2020;21:e47852. doi: 10.15252/embr.201947852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Monick M.M., Powers L.S., Walters K., Lovan N., Zhang M., Gerke A., Hansdottir S., Hunninghake G.W. Identification of an autophagy defect in smokers’ alveolar macrophages. J. Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang H., Dong X., Zhao R., Zhang R., Xu C., Wang X., Liu C., Hu X., Huang S., Chen L. Cadmium results in accumulation of autophagosomes-dependent apoptosis through activating Akt-impaired autophagic flux in neuronal cells. Cell Signal. 2019;55:26–39. doi: 10.1016/j.cellsig.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Button R.W., Roberts S.L., Willis T.L., Hanemann C.O., Luo S. Accumulation of autophagosomes confers cytotoxicity. J. Biol. Chem. 2017;292:13599–13614. doi: 10.1074/jbc.M117.782276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ahmad T., Sundar I.K., Lerner C.A., Gerloff J., Tormos A.M., Yao H., Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: Implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scott S.V., Klionsky D.J. Delivery of proteins and organelles to the vacuole from the cytoplasm. Curr. Opin. Cell Biol. 1998;10:523–529. doi: 10.1016/S0955-0674(98)80068-9. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Y., Huang W., Zheng Z., Wang W., Yuan Y., Hong Q., Lin J., Li X., Meng Y. Cigarette smoke-inactivated SIRT1 promotes autophagy-dependent senescence of alveolar epithelial type 2 cells to induce pulmonary fibrosis. Free Radic. Biol. Med. 2021;166:116–127. doi: 10.1016/j.freeradbiomed.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 168.Myc L.A., Shim Y.M., Laubach V.E., Dimastromatteo J. Role of medical and molecular imaging in COPD. Clin. Transl. Med. 2019;8:12. doi: 10.1186/s40169-019-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Devine J.F. Chronic obstructive pulmonary disease: An overview. Am. Health Drug Benefits. 2008;1:34–42. [PMC free article] [PubMed] [Google Scholar]
- 170.Oh C.M., Oh I.H., Lee J.K., Park Y.H., Choe B.K., Yoon T.Y., Choi J.M. Blood cadmium levels are associated with a decline in lung function in males. Environ. Res. 2014;132:119–125. doi: 10.1016/j.envres.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 171.Girod C.E., King T.E., Jr. COPD: A dust-induced disease? Chest. 2005;128:3055–3064. doi: 10.1378/chest.128.4.3055. [DOI] [PubMed] [Google Scholar]
- 172.Devillier P. Limitations of drug prescriptions in patients with chronic obstructive pulmonary disease. Rev. Pneumol. Clin. 2004;60:203–208. doi: 10.1016/S0761-8417(04)72100-8. [DOI] [PubMed] [Google Scholar]
- 173.Laniado-Laborin R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int. J. Environ. Res. Public Health. 2009;6:209–224. doi: 10.3390/ijerph6010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gharib S.A., Manicone A.M., Parks W.C. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51. doi: 10.1016/j.matbio.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Churg A., Zhou S., Wright J.L. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur. Respir. J. 2012;39:197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 176.Dean R.A., Cox J.H., Bellac C.L., Doucet A., Starr A.E., Overall C.M. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: Potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 177.Gueders M.M., Foidart J.M., Noel A., Cataldo D.D. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: Potential implications in asthma and other lung diseases. Eur. J. Pharmacol. 2006;533:133–144. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 178.Churg A., Wang R.D., Tai H., Wang X., Xie C., Wright J.L. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am. J. Respir. Crit. Care Med. 2004;170:492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 179.March T.H., Wilder J.A., Esparza D.C., Cossey P.Y., Blair L.F., Herrera L.K., McDonald J.D., Campen M.J., Mauderly J.L., Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol. Sci. 2006;92:545–559. doi: 10.1093/toxsci/kfl016. [DOI] [PubMed] [Google Scholar]
- 180.Gosselink J.V., Hayashi S., Elliott W.M., Xing L., Chan B., Yang L., Wright C., Sin D., Pare P.D., Pierce J.A., et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;181:1329–1335. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Decramer M., Janssens W., Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pelkonen M. Smoking: Relationship to chronic bronchitis, chronic obstructive pulmonary disease and mortality. Curr. Opin. Pulm. Med. 2008;14:105–109. doi: 10.1097/MCP.0b013e3282f379e9. [DOI] [PubMed] [Google Scholar]
- 183.Kesimer M., Ford A.A., Ceppe A., Radicioni G., Cao R., Davis C.W., Doerschuk C.M., Alexis N.E., Anderson W.H., Henderson A.G., et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N. Engl. J. Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Radicioni G., Ceppe A., Ford A.A., Alexis N.E., Barr R.G., Bleecker E.R., Christenson S.A., Cooper C.B., Han M.K., Hansel N.N., et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2021;9:1241–1254. doi: 10.1016/S2213-2600(21)00079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li J., Ye Z. The Potential Role and Regulatory Mechanisms of MUC5AC in Chronic Obstructive Pulmonary Disease. Molecules. 2020;25:4437. doi: 10.3390/molecules25194437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ghorani V., Boskabady M.H., Khazdair M.R., Kianmeher M. Experimental animal models for COPD: A methodological review. Tob. Induc. Dis. 2017;15:25. doi: 10.1186/s12971-017-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chapman R.W. Canine models of asthma and COPD. Pulm. Pharmacol. Ther. 2008;21:731–742. doi: 10.1016/j.pupt.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 188.Park S.S., Kikkawa Y., Goldring I.P., Daly M.M., Zelefsky M., Shim C., Spierer M., Morita T. An animal model of cigarette smoking in beagle dogs: Correlative evaluation of effects on pulmonary function, defense, and morphology. Am. Rev. Respir. Dis. 1977;115:971–979. doi: 10.1164/arrd.1977.115.6.971. [DOI] [PubMed] [Google Scholar]
- 189.Plopper C.G., Hyde D.M. The non-human primate as a model for studying COPD and asthma. Pulm. Pharmacol. Ther. 2008;21:755–766. doi: 10.1016/j.pupt.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 190.Gold R., Kappos L., Arnold D.L., Bar-Or A., Giovannoni G., Selmaj K., Tornatore C., Sweetser M.T., Yang M., Sheikh S.I., et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 191.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ross D., Siegel D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021;41:101950. doi: 10.1016/j.redox.2021.101950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chiang S.K., Chen S.E., Chang L.C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells. 2021;10:2401. doi: 10.3390/cells10092401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Deshmukh P., Unni S., Krishnappa G., Padmanabhan B. The Keap1-Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017;9:41–56. doi: 10.1007/s12551-016-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W., Yamamoto M., Petrache I., Tuder R.M., Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Investig. 2004;114:1248–1259. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kubo H., Asai K., Kojima K., Sugitani A., Kyomoto Y., Okamoto A., Yamada K., Ijiri N., Watanabe T., Hirata K., et al. Astaxanthin Suppresses Cigarette Smoke-Induced Emphysema through Nrf2 Activation in Mice. Mar. Drugs. 2019;17:673. doi: 10.3390/md17120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fratta Pasini A.M., Stranieri C., Ferrari M., Garbin U., Cazzoletti L., Mozzini C., Spelta F., Peserico D., Cominacini L. Oxidative stress and Nrf2 expression in peripheral blood mononuclear cells derived from COPD patients: An observational longitudinal study. Respir. Res. 2020;21:37. doi: 10.1186/s12931-020-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li J., Baker J., Higham A., Shah R., Montero-Fernandez A., Murray C., Cooper N., Lucas C., Fox C., Singh D., et al. COPD lung studies of Nrf2 expression and the effects of Nrf2 activators. Inflammopharmacology. 2022;30:1431–1443. doi: 10.1007/s10787-022-00967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smith D.J., Yerkovich S.T., Towers M.A., Carroll M.L., Thomas R., Upham J.W. Reduced soluble receptor for advanced glycation end-products in COPD. Eur. Respir. J. 2011;37:516–522. doi: 10.1183/09031936.00029310. [DOI] [PubMed] [Google Scholar]
- 200.Hoonhorst S.J., Lo Tam Loi A.T., Pouwels S.D., Faiz A., Telenga E.D., van den Berge M., Koenderman L., Lammers J.W., Boezen H.M., van Oosterhout A.J., et al. Advanced glycation endproducts and their receptor in different body compartments in COPD. Respir. Res. 2016;17:46. doi: 10.1186/s12931-016-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ferhani N., Letuve S., Kozhich A., Thibaudeau O., Grandsaigne M., Maret M., Dombret M.C., Sims G.P., Kolbeck R., Coyle A.J., et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;181:917–927. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 202.Lee H., Park J.R., Kim W.J., Sundar I.K., Rahman I., Park S.M., Yang S.R. Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signaling. FASEB J. 2017;31:2076–2089. doi: 10.1096/fj.201601155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sambamurthy N., Leme A.S., Oury T.D., Shapiro S.D. The receptor for advanced glycation end products (RAGE) contributes to the progression of emphysema in mice. PLoS ONE. 2015;10:e0118979. doi: 10.1371/journal.pone.0118979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miller S., Henry A.P., Hodge E., Kheirallah A.K., Billington C.K., Rimington T.L., Bhaker S.K., Obeidat M., Melen E., Merid S.K., et al. The Ser82 RAGE Variant Affects Lung Function and Serum RAGE in Smokers and sRAGE Production In Vitro. PLoS ONE. 2016;11:e0164041. doi: 10.1371/journal.pone.0164041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cheng D.T., Kim D.K., Cockayne D.A., Belousov A., Bitter H., Cho M.H., Duvoix A., Edwards L.D., Lomas D.A., Miller B.E., et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 206.Gaens K.H., Ferreira I., van der Kallen C.J., van Greevenbroek M.M., Blaak E.E., Feskens E.J., Dekker J.M., Nijpels G., Heine R.J., Hart L.M., et al. Association of polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating RAGE levels. J. Clin. Endocrinol. Metab. 2009;94:5174–5180. doi: 10.1210/jc.2009-1067. [DOI] [PubMed] [Google Scholar]
- 207.Xu L., Li X., Wang H., Xie F., Liu H., Xie J. Cigarette smoke triggers inflammation mediated by autophagy in BEAS-2B cells. Ecotoxicol. Environ. Saf. 2019;184:109617. doi: 10.1016/j.ecoenv.2019.109617. [DOI] [PubMed] [Google Scholar]
- 208.Marumo S., Hoshino Y., Kiyokawa H., Tanabe N., Sato A., Ogawa E., Muro S., Hirai T., Mishima M. p38 mitogen-activated protein kinase determines the susceptibility to cigarette smoke-induced emphysema in mice. BMC Pulm. Med. 2014;14:79. doi: 10.1186/1471-2466-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Renda T., Baraldo S., Pelaia G., Bazzan E., Turato G., Papi A., Maestrelli P., Maselli R., Vatrella A., Fabbri L.M., et al. Increased activation of p38 MAPK in COPD. Eur. Respir. J. 2008;31:62–69. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 210.Mercer B.A., Kolesnikova N., Sonett J., D’Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J. Biol. Chem. 2004;279:17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- 211.Zong D., Li J., Cai S., He S., Liu Q., Jiang J., Chen S., Long Y., Chen Y., Chen P., et al. Notch1 regulates endothelial apoptosis via the ERK pathway in chronic obstructive pulmonary disease. Am. J. Physiol. Cell Physiol. 2018;315:C330–C340. doi: 10.1152/ajpcell.00182.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Deshmukh H.S., Case L.M., Wesselkamper S.C., Borchers M.T., Martin L.D., Shertzer H.G., Nadel J.A., Leikauf G.D. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am. J. Respir. Crit. Care Med. 2005;171:305–314. doi: 10.1164/rccm.200408-1003OC. [DOI] [PubMed] [Google Scholar]
- 213.Bernard K., Hecker L., Luckhardt T.R., Cheng G., Thannickal V.J. NADPH oxidases in lung health and disease. Antioxid. Redox Signal. 2014;20:2838–2853. doi: 10.1089/ars.2013.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang X., Murugesan P., Zhang P., Xu S., Peng L., Wang C., Cai H. NADPH Oxidase Isoforms in COPD Patients and Acute Cigarette Smoke-Exposed Mice: Induction of Oxidative Stress and Lung Inflammation. Antioxidants. 2022;11:1539. doi: 10.3390/antiox11081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chan S.M.H., Brassington K., Almerdasi S.A., Dobric A., De Luca S.N., Coward-Smith M., Wang H., Mou K., Akhtar A., Alateeq R.A., et al. Inhibition of oxidative stress by apocynin attenuated chronic obstructive pulmonary disease progression and vascular injury by cigarette smoke exposure. Br. J. Pharmacol. 2023:16068. doi: 10.1111/bph.16068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schiffers C., Reynaert N.L., Wouters E.F.M., van der Vliet A. Redox Dysregulation in Aging and COPD: Role of NOX Enzymes and Implications for Antioxidant Strategies. Antioxidants. 2021;10:11799. doi: 10.3390/antiox10111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hollins F., Sutcliffe A., Gomez E., Berair R., Russell R., Szyndralewiez C., Saunders R., Brightling C. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respir. Res. 2016;17:84. doi: 10.1186/s12931-016-0403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Seimetz M., Sommer N., Bednorz M., Pak O., Veith C., Hadzic S., Gredic M., Parajuli N., Kojonazarov B., Kraut S., et al. NADPH oxidase subunit NOXO1 is a target for emphysema treatment in COPD. Nat. Metab. 2020;2:532–546. doi: 10.1038/s42255-020-0215-8. [DOI] [PubMed] [Google Scholar]
- 219.Schiffers C., van de Wetering C., Bauer R.A., Habibovic A., Hristova M., Dustin C.M., Lambrichts S., Vacek P.M., Wouters E.F., Reynaert N.L., et al. Downregulation of epithelial DUOX1 in chronic obstructive pulmonary disease. JCI Insight. 2021;6:142189. doi: 10.1172/jci.insight.142189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nagai K., Betsuyaku T., Suzuki M., Nasuhara Y., Kaga K., Kondo S., Nishimura M. Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid. Redox Signal. 2008;10:705–714. doi: 10.1089/ars.2007.1941. [DOI] [PubMed] [Google Scholar]
- 221.van der Vliet A., Danyal K., Heppner D.E. Dual oxidase: A novel therapeutic target in allergic disease. Br. J. Pharmacol. 2018;175:1401–1418. doi: 10.1111/bph.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wesley U.V., Bove P.F., Hristova M., McCarthy S., van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J. Biol. Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 223.Atkinson J.J., Senior R.M. Matrix metalloproteinase-9 in lung remodeling. Am. J. Respir. Cell Mol. Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 224.Libby G., Donnelly L.A., Donnan P.T., Alessi D.R., Morris A.D., Evans J.M. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nath N., Khan M., Paintlia M.K., Singh I., Hoda M.N., Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 2009;182:8005–8014. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Polverino F., Wu T.D., Rojas-Quintero J., Wang X., Mayo J., Tomchaney M., Tram J., Packard S., Zhang D., Cleveland K.H., et al. Metformin: Experimental and Clinical Evidence for a Potential Role in Emphysema Treatment. Am. J. Respir. Crit. Care Med. 2021;204:651–666. doi: 10.1164/rccm.202012-4510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oslan S.N.H., Tan J.S., Oslan S.N., Matanjun P., Mokhtar R.A.M., Shapawi R., Huda N. Haematococcus pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules. 2021;26:6470. doi: 10.3390/molecules26216470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kishimoto Y., Yoshida H., Kondo K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs. 2016;14:35. doi: 10.3390/md14020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Piermarocchi S., Saviano S., Parisi V., Tedeschi M., Panozzo G., Scarpa G., Boschi G., Lo Giudice G., Carmis Study G. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): Two-year results of a randomized study. Eur. J. Ophthalmol. 2012;22:216–225. doi: 10.5301/ejo.5000069. [DOI] [PubMed] [Google Scholar]
- 230.Liu S.Z., Ali A.S., Campbell M.D., Kilroy K., Shankland E.G., Roshanravan B., Marcinek D.J., Conley K.E. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachexia Sarcopenia Muscle. 2018;9:826–833. doi: 10.1002/jcsm.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Deng M., Tong R., Bian Y., Hou G. Astaxanthin attenuates cigarette smoking-induced oxidative stress and inflammation in a sirtuin 1-dependent manner. Biomed. Pharmacother. 2023;159:114230. doi: 10.1016/j.biopha.2023.114230. [DOI] [PubMed] [Google Scholar]
- 232.Ridzuan N., Zakaria N., Widera D., Sheard J., Morimoto M., Kiyokawa H., Mohd Isa S.A., Chatar Singh G.K., Then K.Y., Ooi G.C., et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD) Stem Cell Res. Ther. 2021;12:54. doi: 10.1186/s13287-020-02088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Craparo E.F., Cabibbo M., Scialabba C., Giammona G., Cavallaro G. Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast. Biomacromolecules. 2022;23:3439–3451. doi: 10.1021/acs.biomac.2c00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu Y., Liu H., Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: A review. J. Nanobiotechnol. 2020;18:145. doi: 10.1186/s12951-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Barjaktarevic I.Z., Arredondo A.F., Cooper C.B. Positioning new pharmacotherapies for COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2015;10:1427–1442. doi: 10.2147/COPD.S83758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Paranjpe M., Muller-Goymann C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014;15:5852–5873. doi: 10.3390/ijms15045852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Geiser M., Quaile O., Wenk A., Wigge C., Eigeldinger-Berthou S., Hirn S., Schaffler M., Schleh C., Moller W., Mall M.A., et al. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part Fibre Toxicol. 2013;10:19. doi: 10.1186/1743-8977-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Barrera L., Mendoza F., Zuniga J., Estrada A., Zamora A.C., Melendro E.I., Ramirez R., Pardo A., Selman M. Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2008;177:44–55. doi: 10.1164/rccm.200701-093OC. [DOI] [PubMed] [Google Scholar]
- 239.Carcoforo P., Feo C., Sortini D., Pozza E., Carrella G., Sortini A. Localization of pulmonary nodules. Chest. 2004;125:796; author reply 796–797. doi: 10.1378/chest.125.2.796. [DOI] [PubMed] [Google Scholar]
- 240.Kanoh S., Kobayashi H., Motoyoshi K. Exhaled ethane: An in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest. 2005;128:2387–2392. doi: 10.1378/chest.128.4.2387. [DOI] [PubMed] [Google Scholar]
- 241.Amara N., Goven D., Prost F., Muloway R., Crestani B., Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zheng H., Jiang W.H., Tian T., Tan H.S., Chen Y., Qiao G.L., Han J., Huang S.Y., Yang Y., Li S., et al. CBX6 overexpression contributes to tumor progression and is predictive of a poor prognosis in hepatocellular carcinoma. Oncotarget. 2017;8:18872–18884. doi: 10.18632/oncotarget.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jarman E.R., Khambata V.S., Cope C., Jones P., Roger J., Ye L.Y., Duggan N., Head D., Pearce A., Press N.J., et al. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am. J. Respir. Cell Mol. Biol. 2014;50:158–169. doi: 10.1165/rcmb.2013-0174OC. [DOI] [PubMed] [Google Scholar]
- 245.Inui N., Sakai S., Kitagawa M. Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-beta and the Ubiquitin-Proteasome Pathway. Int. J. Mol. Sci. 2021;22:6107. doi: 10.3390/ijms22116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Oczypok E.A., Perkins T.N., Oury T.D. All the "RAGE" in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Perkins T.N., Oury T.D. The perplexing role of RAGE in pulmonary fibrosis: Causality or casualty? Ther. Adv. Respir. Dis. 2021;15:17534666211016071. doi: 10.1177/17534666211016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Queisser M.A., Kouri F.M., Konigshoff M., Wygrecka M., Schubert U., Eickelberg O., Preissner K.T. Loss of RAGE in pulmonary fibrosis: Molecular relations to functional changes in pulmonary cell types. Am. J. Respir. Cell Mol. Biol. 2008;39:337–345. doi: 10.1165/rcmb.2007-0244OC. [DOI] [PubMed] [Google Scholar]
- 249.Dozio E., Sitzia C., Pistelli L., Cardani R., Rigolini R., Ranucci M., Corsi Romanelli M.M. Soluble Receptor for Advanced Glycation End Products and Its Forms in COVID-19 Patients with and without Diabetes Mellitus: A Pilot Study on Their Role as Disease Biomarkers. J. Clin. Med. 2020;9:3785. doi: 10.3390/jcm9113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Buckley S.T., Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J. Biomed. Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Weinhage T., Wirth T., Schutz P., Becker P., Lueken A., Skryabin B.V., Wittkowski H., Foell D. The Receptor for Advanced Glycation Endproducts (RAGE) Contributes to Severe Inflammatory Liver Injury in Mice. Front. Immunol. 2020;11:1157. doi: 10.3389/fimmu.2020.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lugade A.A., Bogner P.N., Thatcher T.H., Sime P.J., Phipps R.P., Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J. Immunol. 2014;192:5226–5235. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen M., Wang T., Shen Y., Xu D., Li X., An J., Dong J., Li D., Wen F., Chen L. Knockout of RAGE ameliorates mainstream cigarette smoke-induced airway inflammation in mice. Int. Immunopharmacol. 2017;50:230–235. doi: 10.1016/j.intimp.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 254.Re S.L., Giordano G., Yakoub Y., Devosse R., Uwambayinema F., Couillin I., Ryffel B., Marbaix E., Lison D., Huaux F. Uncoupling between inflammatory and fibrotic responses to silica: Evidence from MyD88 knockout mice. PLoS ONE. 2014;9:e99383. doi: 10.1371/journal.pone.0099383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kumar V., Fleming T., Terjung S., Gorzelanny C., Gebhardt C., Agrawal R., Mall M.A., Ranzinger J., Zeier M., Madhusudhan T., et al. Homeostatic nuclear RAGE-ATM interaction is essential for efficient DNA repair. Nucleic Acids Res. 2017;45:10595–10613. doi: 10.1093/nar/gkx705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schupp J.C., Binder H., Jager B., Cillis G., Zissel G., Muller-Quernheim J., Prasse A. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS ONE. 2015;10:e0116775. doi: 10.1371/journal.pone.0116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu T., De Los Santos F.G., Phan S.H. The Bleomycin Model of Pulmonary Fibrosis. Methods Mol. Biol. 2017;1627:27–42. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 258.Selman M., Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: From innocent targets to serial killers. Proc. Am. Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 259.Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: Potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 2005;166:1321–1332. doi: 10.1016/S0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Meyer A., Buhl R., Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur. Respir. J. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 261.Comeau M.R., Ziegler S.F. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–147. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- 262.Zhitkovich A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019;32:1318–1319. doi: 10.1021/acs.chemrestox.9b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 264.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 265.Zhang Y.E. Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb. Perspect. Biol. 2017;9:22129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cushing L., Kuang P.P., Qian J., Shao F., Wu J., Little F., Thannickal V.J., Cardoso W.V., Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ask K., Bonniaud P., Maass K., Eickelberg O., Margetts P.J., Warburton D., Groffen J., Gauldie J., Kolb M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int. J. Biochem. Cell Biol. 2008;40:484–495. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Prasse A., Probst C., Bargagli E., Zissel G., Toews G.B., Flaherty K.R., Olschewski M., Rottoli P., Muller-Quernheim J. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2009;179:717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 269.Fortin M., D’Anjou H., Higgins M.E., Gougeon J., Aube P., Moktefi K., Mouissi S., Seguin S., Seguin R., Renzi P.M., et al. A multi-target antisense approach against PDE4 and PDE7 reduces smoke-induced lung inflammation in mice. Respir. Res. 2009;10:39. doi: 10.1186/1465-9921-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Richeldi L., Azuma A., Cottin V., Hesslinger C., Stowasser S., Valenzuela C., Wijsenbeek M.S., Zoz D.F., Voss F., Maher T.M., et al. Trial of a Preferential Phosphodiesterase 4B Inhibitor for Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2022;386:2178–2187. doi: 10.1056/NEJMoa2201737. [DOI] [PubMed] [Google Scholar]
- 271.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 272.Hecht S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 273.Goldenberg M., Danovitch I., IsHak W.W. Quality of life and smoking. Am. J. Addict. 2014;23:540–562. doi: 10.1111/j.1521-0391.2014.12148.x. [DOI] [PubMed] [Google Scholar]
- 274.Pryor W.A., Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993;686:12–27; discussion 18–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 275.Freedman N.D., Silverman D.T., Hollenbeck A.R., Schatzkin A., Abnet C.C. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ji B.T., Chow W.H., Gridley G., McLaughlin J.K., Dai Q., Wacholder S., Hatch M.C., Gao Y.T., Fraumeni J.F., Jr. Dietary factors and the risk of pancreatic cancer: A case-control study in Shanghai China. Cancer Epidemiol. Biomark. Prev. 1995;4:885–893. [PubMed] [Google Scholar]
- 277.Hunt J.D., van der Hel O.L., McMillan G.P., Boffetta P., Brennan P. Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int. J. Cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 278.Lagergren J., Bergstrom R., Lindgren A., Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 279.Hashibe M., Brennan P., Chuang S.C., Boccia S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Phillips D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999;443:139–147. doi: 10.1016/S1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 281.Toyokuni S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9–16. doi: 10.1111/j.1349-7006.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mengozzi A., Pugliese N.R., Chiriaco M., Masi S., Virdis A., Taddei S. Microvascular Ageing Links Metabolic Disease to Age-Related Disorders: The Role of Oxidative Stress and Inflammation in Promoting Microvascular Dysfunction. J. Cardiovasc. Pharmacol. 2021;78:S78–S87. doi: 10.1097/FJC.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 283.Fleshner N.E., Klotz L.H. Diet, androgens, oxidative stress and prostate cancer susceptibility. Cancer Metastasis Rev. 1998;17:325–330. doi: 10.1023/A:1006118628183. [DOI] [PubMed] [Google Scholar]
- 284.Hecht S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 285.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome-biological and translational implications. Nat. Rev. Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Skonieczna M., Hejmo T., Poterala-Hejmo A., Cieslar-Pobuda A., Buldak R.J. NADPH Oxidases: Insights into Selected Functions and Mechanisms of Action in Cancer and Stem Cells. Oxid. Med. Cell Longev. 2017;2017:9420539. doi: 10.1155/2017/9420539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Konate M.M., Antony S., Doroshow J.H. Inhibiting the Activity of NADPH Oxidase in Cancer. Antioxid. Redox Signal. 2020;33:435–454. doi: 10.1089/ars.2020.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang R., Dashwood W.M., Nian H., Lohr C.V., Fischer K.A., Tsuchiya N., Nakagama H., Ashktorab H., Dashwood R.H. NADPH oxidase overexpression in human colon cancers and rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) Int. J. Cancer. 2011;128:2581–2590. doi: 10.1002/ijc.25610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Waris G., Ahsan H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ahn K.S., Aggarwal B.B. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 291.Hasnis E., Bar-Shai M., Burbea Z., Reznick A.Z. Mechanisms underlying cigarette smoke-induced NF-kappaB activation in human lymphocytes: The role of reactive nitrogen species. J. Physiol. Pharmacol. 2007;58((Suppl. S5)):275–287. [PubMed] [Google Scholar]
- 292.Hoesel B., Schmid J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Karin M., Cao Y., Greten F.R., Li Z.W. NF-kappaB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 294.Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 295.Semenza G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Fresno Vara J.A., Casado E., de Castro J., Cejas P., Belda-Iniesta C., Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 297.Courtney K.D., Corcoran R.B., Engelman J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sanchez-Ortega M., Carrera A.C., Garrido A. Role of NRF2 in Lung Cancer. Cells. 2021;10:1879. doi: 10.3390/cells10081879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sporn M.B., Liby K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.DeBlasi J.M., DeNicola G.M. Dissecting the Crosstalk between NRF2 Signaling and Metabolic Processes in Cancer. Cancers. 2020;12:23. doi: 10.3390/cancers12103023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hammad A., Namani A., Elshaer M., Wang X.J., Tang X. “NRF2 addiction” in lung cancer cells and its impact on cancer therapy. Cancer Lett. 2019;467:40–49. doi: 10.1016/j.canlet.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 302.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control Release. 2000;65:271–284. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 303.Heidel J.D., Davis M.E. Clinical developments in nanotechnology for cancer therapy. Pharm. Res. 2011;28:187–199. doi: 10.1007/s11095-010-0178-7. [DOI] [PubMed] [Google Scholar]
- 304.Zhong C.Y., Zhou Y.M., Pinkerton K.E. NF-kappaB inhibition is involved in tobacco smoke-induced apoptosis in the lungs of rats. Toxicol. Appl. Pharmacol. 2008;230:150–158. doi: 10.1016/j.taap.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 306.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 307.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Janowitz T., Kleeman S., Vonderheide R.H. Reconsidering Dexamethasone for Antiemesis when Combining Chemotherapy and Immunotherapy. Oncologist. 2021;26:269–273. doi: 10.1002/onco.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Geng Y., Zhang Q., Feng S., Li C., Wang L., Zhao X., Yang Z., Li Z., Luo H., Liu R., et al. Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Med. 2021;10:1222–1239. doi: 10.1002/cam4.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Su S., Kang P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics. 2020;12:837. doi: 10.3390/pharmaceutics12090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zahavi D., Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies. 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Joly-Battaglini A., Hammarstrom C., Stankovic B., Aamodt H., Stjarne J., Brustugun O.T., Helland A., Oynebraten I., Corthay A. Rituximab efficiently depletes B cells in lung tumors and normal lung tissue. F1000Res. 2016;5:38. doi: 10.12688/f1000research.7599.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li B.T., Smit E.F., Goto Y., Nakagawa K., Udagawa H., Mazieres J., Nagasaka M., Bazhenova L., Saltos A.N., Felip E., et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhang H., Qin C., An C., Zheng X., Wen S., Chen W., Liu X., Lv Z., Yang P., Xu W., et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol. Cancer. 2021;20:126. doi: 10.1186/s12943-021-01431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Li H., Yang Y., Hong W., Huang M., Wu M., Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target Ther. 2020;5:1. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Milone M.C., Xu J., Chen S.J., Collins M.A., Zhou J., Powell D.J., Jr., Melenhorst J.J. Engineering enhanced CAR T-cells for improved cancer therapy. Nat. Cancer. 2021;2:780–793. doi: 10.1038/s43018-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Qu J., Mei Q., Chen L., Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): Current status and future perspectives. Cancer Immunol. Immunother. 2021;70:619–631. doi: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sadeghi S., Tehrani F.R., Tahmasebi S., Shafiee A., Hashemi S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology. 2023;31:145–169. doi: 10.1007/s10787-022-01115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Raghav A., Khan Z.A., Upadhayay V.K., Tripathi P., Gautam K.A., Mishra B.K., Ahmad J., Jeong G.B. Mesenchymal Stem Cell-Derived Exosomes Exhibit Promising Potential for Treating SARS-CoV-2-Infected Patients. Cells. 2021;10:587. doi: 10.3390/cells10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Fan E., Brodie D., Slutsky A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 323.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Thompson B.T., Chambers R.C., Liu K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 325.Matthay M.A., Zemans R.L. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu. Rev. Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Qiao Q., Liu X., Yang T., Cui K., Kong L., Yang C., Zhang Z. Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design. Acta Pharm. Sin. B. 2021;11:3060–3091. doi: 10.1016/j.apsb.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Moazed F., Hendrickson C., Jauregui A., Gotts J., Conroy A., Delucchi K., Zhuo H., Arambulo M., Vessel K., Ke S., et al. Cigarette Smoke Exposure and Acute Respiratory Distress Syndrome in Sepsis: Epidemiology, Clinical Features, and Biologic Markers. Am. J. Respir. Crit. Care Med. 2022;205:927–935. doi: 10.1164/rccm.202105-1098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cesta M.C., Zippoli M., Marsiglia C., Gavioli E.M., Mantelli F., Allegretti M., Balk R.A. The Role of Interleukin-8 in Lung Inflammation and Injury: Implications for the Management of COVID-19 and Hyperinflammatory Acute Respiratory Distress Syndrome. Front. Pharmacol. 2021;12:808797. doi: 10.3389/fphar.2021.808797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Moazed F., Burnham E.L., Vandivier R.W., O’Kane C.M., Shyamsundar M., Hamid U., Abbott J., Thickett D.R., Matthay M.A., McAuley D.F., et al. Cigarette smokers have exaggerated alveolar barrier disruption in response to lipopolysaccharide inhalation. Thorax. 2016;71:1130–1136. doi: 10.1136/thoraxjnl-2015-207886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Aslan A., Aslan C., Zolbanin N.M., Jafari R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia. 2021;13:14. doi: 10.1186/s41479-021-00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu A., Zhang X., Li R., Zheng M., Yang S., Dai L., Wu A., Hu C., Huang Y., Xie M., et al. Overexpression of the SARS-CoV-2 receptor ACE2 is induced by cigarette smoke in bronchial and alveolar epithelia. J. Pathol. 2021;253:17–30. doi: 10.1002/path.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cai G., Bosse Y., Xiao F., Kheradmand F., Amos C.I. Tobacco Smoking Increases the Lung Gene Expression of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;201:1557–1559. doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Voinsky I., Gurwitz D. Smoking and COVID-19: Similar bronchial ACE2 and TMPRSS2 expression and higher TMPRSS4 expression in current versus never smokers. Drug Dev. Res. 2020;81:1073–1080. doi: 10.1002/ddr.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nawijn M.C., Timens W. Can ACE2 expression explain SARS-CoV-2 infection of the respiratory epithelia in COVID-19? Mol. Syst. Biol. 2020;16:e9841. doi: 10.15252/msb.20209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Qiao X., Kashiouris M.G., L’Heureux M., Fisher B.J., Leichtle S.W., Truwit J.D., Nanchal R., Hite R.D., Morris P.E., Martin G.S., et al. Biological Effects of Intravenous Vitamin C on Neutrophil Extracellular Traps and the Endothelial Glycocalyx in Patients with Sepsis-Induced ARDS. Nutrients. 2022;14:4415. doi: 10.3390/nu14204415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds; Washington, DC, USA: 2000. [Google Scholar]
- 337.Azzouz D., Khan M.A., Palaniyar N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021;7:113. doi: 10.1038/s41420-021-00491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Jiang S., Li S., Hu J., Xu X., Wang X., Kang X., Qi J., Lu X., Wu J., Du Y., et al. Combined delivery of angiopoietin-1 gene and simvastatin mediated by anti-intercellular adhesion molecule-1 antibody-conjugated ternary nanoparticles for acute lung injury therapy. Nanomedicine. 2019;15:25–36. doi: 10.1016/j.nano.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 339.MacIntyre N., Huang Y.C. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc. Am. Thorac Soc. 2008;5:530–535. doi: 10.1513/pats.200707-088ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bach P.B., Brown C., Gelfand S.E., McCrory D.C., American College of Physicians-American Society of Internal Medicine Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann. Intern Med. 2001;134:600–620. doi: 10.7326/0003-4819-134-7-200104030-00016. [DOI] [PubMed] [Google Scholar]
- 341.Papi A., Bellettato C.M., Braccioni F., Romagnoli M., Casolari P., Caramori G., Fabbri L.M., Johnston S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 342.Fagon J.Y., Chastre J., Trouillet J.L., Domart Y., Dombret M.C., Bornet M., Gibert C. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patients. Am. Rev. Respir. Dis. 1990;142:1004–1008. doi: 10.1164/ajrccm/142.5.1004. [DOI] [PubMed] [Google Scholar]
- 343.Monso E., Ruiz J., Rosell A., Manterola J., Fiz J., Morera J., Ausina V. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 344.Sethi S., Evans N., Grant B.J., Murphy T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 345.Wang G., Ma A., Zhang L., Guo J., Liu Q., Petersen F., Wang Z., Yu X. Acute exacerbations of chronic obstructive pulmonary disease in a cohort of Chinese never smokers goes along with decreased risks of recurrent acute exacerbation, emphysema and comorbidity of lung cancer as well as decreased levels of circulating eosinophils and basophils. Front. Med. 2022;9:907893. doi: 10.3389/fmed.2022.907893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Li X., Wu Z., Xue M., Du W. Smoking status affects clinical characteristics and disease course of acute exacerbation of chronic obstructive pulmonary disease: A prospectively observational study. Chron. Respir. Dis. 2020;17:1479973120916184. doi: 10.1177/1479973120916184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Mortality G.B.D., Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Messner B., Bernhard D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 349.Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 350.Kondo T., Nakano Y., Adachi S., Murohara T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019;83:1980–1985. doi: 10.1253/circj.CJ-19-0323. [DOI] [PubMed] [Google Scholar]
- 351.Dahdah A., Jaggers R.M., Sreejit G., Johnson J., Kanuri B., Murphy A.J., Nagareddy P.R. Immunological Insights into Cigarette Smoking-Induced Cardiovascular Disease Risk. Cells. 2022;11:3190. doi: 10.3390/cells11203190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Morris P.B., Ference B.A., Jahangir E., Feldman D.N., Ryan J.J., Bahrami H., El-Chami M.F., Bhakta S., Winchester D.E., Al-Mallah M.H., et al. Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes: Clinical Perspectives from the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J. Am. Coll. Cardiol. 2015;66:1378–1391. doi: 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 353.Celermajer D.S., Sorensen K.E., Georgakopoulos D., Bull C., Thomas O., Robinson J., Deanfield J.E. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.CIR.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 354.Barua R.S., Ambrose J.A., Eales-Reynolds L.J., DeVoe M.C., Zervas J.G., Saha D.C. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104:1905–1910. doi: 10.1161/hc4101.097525. [DOI] [PubMed] [Google Scholar]
- 355.Su Y., Han W., Giraldo C., De Li Y., Block E.R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol. 1998;19:819–825. doi: 10.1165/ajrcmb.19.5.3091. [DOI] [PubMed] [Google Scholar]
- 356.Toda N., Toda H. Nitric oxide-mediated blood flow regulation as affected by smoking and nicotine. Eur. J. Pharmacol. 2010;649:1–13. doi: 10.1016/j.ejphar.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 357.Cohen R.A., Weisbrod R.M., Gericke M., Yaghoubi M., Bierl C., Bolotina V.M. Mechanism of nitric oxide-induced vasodilatation: Refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 1999;84:210–219. doi: 10.1161/01.RES.84.2.210. [DOI] [PubMed] [Google Scholar]
- 358.Barua R.S., Ambrose J.A., Srivastava S., DeVoe M.C., Eales-Reynolds L.J. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–2347. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 359.Ismaeel A., Brumberg R.S., Kirk J.S., Papoutsi E., Farmer P.J., Bohannon W.T., Smith R.S., Eidson J.L., Sawicki I., Koutakis P. Oxidative Stress and Arterial Dysfunction in Peripheral Artery Disease. Antioxidants. 2018;7:145. doi: 10.3390/antiox7100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wang X., Bian Y., Zhang R., Liu X., Ni L., Ma B., Zeng R., Zhao Z., Song X., Liu C. Melatonin alleviates cigarette smoke-induced endothelial cell pyroptosis through inhibiting ROS/NLRP3 axis. Biochem. Biophys. Res. Commun. 2019;519:402–408. doi: 10.1016/j.bbrc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 361.Mazzone A., Cusa C., Mazzucchelli I., Vezzoli M., Ottini E., Ghio S., Tossini G., Pacifici R., Zuccaro P. Cigarette smoking and hypertension influence nitric oxide release and plasma levels of adhesion molecules. Clin. Chem. Lab. Med. 2001;39:822–826. doi: 10.1515/CCLM.2001.136. [DOI] [PubMed] [Google Scholar]
- 362.Craig W.Y., Palomaki G.E., Haddow J.E. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. BMJ. 1989;298:784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Heitzer T., Yla-Herttuala S., Luoma J., Kurz S., Munzel T., Just H., Olschewski M., Drexler H. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation. 1996;93:1346–1353. doi: 10.1161/01.CIR.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 364.Bekkering S., Quintin J., Joosten L.A., van der Meer J.W., Netea M.G., Riksen N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014;34:1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 365.Maiolino G., Rossitto G., Caielli P., Bisogni V., Rossi G.P., Calo L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013;2013:714653. doi: 10.1155/2013/714653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Komiyama M., Wada H., Ono K., Yamakage H., Satoh-Asahara N., Shimada S., Akao M., Morimoto T., Shimatsu A., Takahashi Y., et al. Smoking cessation reduces the lectin-like low-density lipoprotein receptor index, an independent cardiovascular risk marker of vascular inflammation. Heart Vessels. 2018;33:9–16. doi: 10.1007/s00380-017-1026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Yu X.H., Fu Y.C., Zhang D.W., Yin K., Tang C.K. Foam cells in atherosclerosis. Clin. Chim. Acta. 2013;424:245–252. doi: 10.1016/j.cca.2013.06.006. [DOI] [PubMed] [Google Scholar]