Abstract
Vitamin D and a healthy diet, based on World Cancer Research Fund (WCRF) recommendations, are considered key elements for colorectal cancer (CRC) prevention. In a CRC case-control study, we observed that CRC cases were often significantly Vitamin D deficient while subjects following WCRF recommendations significantly decreased their risk of developing CRC. We conducted a randomized phase-II trial (EudraCT number-2015-000467-14) where 74 CRC patients showed differences in response to Vitamin D supplementation, 2000 IU in average per day, according to gender and microbiota. The aim of this nested study is to correlate Vitamin D (supplementation, serum level and receptor polymorphisms), circulating biomarkers, and events (polyp/adenoma, CRC relapse and other cancers) in concomitant to WCRF recommendation adherence. Vitamin D supplementation did not modulate circulating biomarkers or follow-up events. FokI and TaqI VDR were associated with 25-hydroxyvitamin D (25OHD) levels. Patients following the WCRF recommendations had significantly lower leptin, significantly lower IL-6 (only in females), and significantly lower risk of events (HR = 0.41, 95%CI: 0.18–0.92; p = 0.03; median follow-up 2.6 years). Interestingly, no WCRF adherents had significantly more events if they were in the placebo (p < 0.0001), whereas no influence of WCRF was observed in the Vitamin D arm. While one-year Vitamin D supplementation might be too short to show significant preventive activity, a healthy diet and lifestyle should be the first step for preventive programs.
Keywords: Vitamin D, Vitamin D receptor, Vitamin D binding protein, colorectal cancer, adipokine, diet
1. Introduction
Colorectal cancer (CRC) is among the most common malignancies. The majority (60–65%) can be defined as sporadic cancer, and it has been suggested that inflammation plays a causative role in its pathogenesis together with mechanisms to escape immune surveillance [1,2]. Risk factors are strongly related to unhealthy behaviors. An unhealthy dietary pattern, a sedentary lifestyle, and obesity are well-established CRC risk factors. More than specific nutrients or single food, the dietary pattern should be considered. A recent meta-analysis showed a relative risk (RR) for CRC of 1.25 (95% CI 1.11–1.40) for Western diets compared to healthy dietary patterns, RR 0.81 (95% CI 0.73–0.91) [3].
Healthy dietary patterns include mostly plant-based foods such as vegetables, fruits, whole grains, nuts, legumes, and moderate consumption of animal products (poultry, fish, and seafood), as opposed to high intakes of red and processed meat, sugar-sweetened beverages, and refined grains (overall high glycemic index food) [4]. According to the World Cancer Research Fund (WCRF), the established risk factors for CRC are alcohol consumption, body fatness, and processed and red meat consumption; in contrast, physical activity and foods containing fiber and calcium are protective factors [5]. Moreover, the consumption of foods containing or fortified with Vitamin D can be a protective factor for CRC. According to two recent dose-response meta-analyses, foods containing Vitamin D and Vitamin D supplementation showed a significant decreased risk (RR 0.95; 95% CI 0.93–0.98) and (RR 0.93; 95% CI 0.88–0.98), respectively. No effect was found for plasma and serum Vitamin D after evaluating 12 studies. The dose-response meta-analysis showed a borderline effect per 30 nanomoles/L (RR 0.92; 95% CI 0.85–1.00), even stratifying by sex, geographical location, and cancer site (https://www.wcrf.org/wp-content/uploads/2021/02/Colorectal-cancer-report.pdf). 25OHD levels have been related to body fat, a higher body mass index (BMI) was significantly associated with lower serum 25OHD levels [6], and a low vitamin D (25-hydroxy vitamin D, or 25OHD) level has been associated with cancer risk and other diseases in several observational studies [7,8]. Nevertheless, its causality for pathological processes remains uncertain, especially the role of Vitamin D supplementation. In randomized controlled trials (RCT) with Vitamin D supplementation versus placebo, the benefit of vitamin D was significant only for cancer survival and mortality rather than incidence [9]. Interestingly, the secondary analyses of the VITAL study [10] found a significantly reduced risk of all-cancer incidence for those with a BMI < 25 kg/m2. Furthermore, it is important to remember that VITAL and most vitamin D RCTs are designed using guidelines for pharmaceutical drugs rather than nutrients. The vitamin D dose used in the RTCs is probably too low to have an impact on cancer prognosis, and the inclusion criteria should be more precisely defined to identify the patients who may really benefit from vitamin D supplementation [11,12,13].
We also showed that Vitamin D supplementation could be considered an intervention (tertiary prevention) to improve survival in cancer patients, but further investigations are warranted [14]. Interestingly, a secondary analysis of a randomized clinical trial with 25,871 patients found that supplementation with vitamin D3 reduced the risk of incidence of advanced (metastatic or fatal) cancer, in particular in subjects with normal weight, but no reduction was observed among overweight or obese individuals. These results suggest a potential interaction between Vitamin D and BMI [15].
Vitamin D activity is mediated by the vitamin D receptor (VDR), and its polymorphisms may impair the target cells’ response to the hormone. Indeed, VDR is expressed in many tissues supporting the role of Vitamin D beyond bone metabolisms [16]. VDR polymorphisms have been described as being correlated with cancer risk, and for CRC, the BsmI polymorphism has consistently been reported to be associated with a reduced CRC risk [17,18]. Moreover, other genetic polymorphisms of enzymes involved in the Vitamin D metabolism, such as the vitamin D binding protein (VDBP), may also affect the bioavailability of 25OHD [19,20]. Furthermore, Vitamin D has been shown to exert anti-inflammatory activity and to be inversely associated with serum levels of CRP and Interleukin-6 (IL-6) [21]. An inverse relationship between Vitamin D and IL6 was also evident in our recent CRC case-control study [22]. Several studies have reported a possible interaction between Vitamin D and gut microbiota, strongly correlated with obesity and inflammation. A possible inter-player between the two is the immune system [23].
We first conducted a case-control study with CRC patients at the time of cancer diagnosis [22]. This study showed that a beneficial microbiota ratio (Bifidobacteria/Escherichia genera ratio) attenuates CRC risk due to an unhealthy diet. Subsequently, a phase II clinical trial was developed to investigate the microbiome changes with Vitamin D supplementation in CRC patients [24]. Participants were randomized to vitamin D 2000 UI per day or placebo for one year. The trial showed that Vitamin D supplementation can shape gut microbiota and that microbiota mediates the effect of supplementation on final 25(OH)D levels. We observed gender differences within Vitamin D metabolism, underlining that sex can be a key variable in studies where the role of Vitamin D and/or microbiota is investigated. Moreover, we found a significant association of the FokI variant with CRC (p = 0.03).
Here we present the results of a prospective cohort study, nested within the randomized trial described above. We analyzed circulating biomarkers and events (polyps/adenoma, CRC relapse and other cancers) in relation to adherence to WCRF recommendations and Vitamin D supplementation in CRC patients. Furthermore, we investigated the association of single nucleotide polymorphisms (SNPs) involved in vitamin D bioactivity and baseline circulating levels of 25(OH)D and vitamin D binding protein (VDBP).
2. Materials and Methods
The main endpoint of the present trial was recently published [24]. Briefly, the study was conducted from 2016 to 2019. Participants had a CRC diagnosis (stage I–III) and were treated accordingly. After completion of their standard treatment, they were randomized to Vitamin D 2000 IU per day (7 drops) versus placebo (7 drops) in a 1:1 ratio for 12 months. The rationale for the choice of this dosage was that the safe Recommended Daily Allowance is 2000 IU [25,26,27]. Furthermore, it has been calculated that with 2000 IU daily, only 10–15% of persons remain with a concentration < 30 ng/mL [27,28] and the results from a meta-analysis on 25(OH)D serum levels showed that 30 ng/mL is associated with a significantly lower risk of CRC [8]. The study was approved by the Institutional Review Board (the European Institute of Oncology Ethical Committee IEO-223, EudraCT number 2015-000467-14), and all subjects gave their written informed consent.
Seventy-four participants were included in the study. The flow diagram and baseline characteristics details of the study population were described in the previous publication [24]. Briefly, after confirmation of eligibility criteria, the participant was randomized to Vitamin D supplementation vs. placebo and stratified by chemotherapy (yes vs. no). Eleven patients received neoadjuvant treatment with pelvic irradiation (total of 50 Gy) and concomitant chemotherapy (9 patients received capecitabine in monotherapy and 2 patients fluoropyrimidine with oxaliplatin). Adjuvant treatment was received by 38 participants (18 received capecitabine in monotherapy, and 20 received a fluoropyrimidine and oxaliplatin regime). After treatment completion and subsequent minimum 6 months wash-out, patients were invited to participate in the study. At the baseline visit, medical history, concomitant medications, food consumption, clinical examination, and anthropometric measurements were acquired. Fasting blood and fecal samples were collected. Follow-up was completed either through a clinical visit or a phone-call contact. After the year of study intervention, participants continued their annual oncology visits. Follow-up data were collected by their chart or by phone call contact for those who continued their follow-up visits in a different hospital.
2.1. Circulating Biomarkers
At baseline, serum 25(OH)D concentrations were determined by a commercially available chemiluminescent immunoassay designed for the IDS-iSYS automated instrument (Immunodiagnostic Systems, Pantec S.r.l., Turin, Italy). This method recognizes both metabolites of vitamin D (D2–D3), and correlates well with the isotope-dilution liquid chromatography-tandem mass spectrometry (ID–LC-MS/MS) method [29], without any statistically significant bias. Vitamin D Binding Protein (VDBP) was determined by ELISA (R&D Systems Europe, Ltd., Abingdon, UK), while IL-6, IL-10, leptin, and adiponectin were determined using an automated immunoassay platform called ELLA (ProteinSimple, Bio-techne, Minneapolis, MN, USA). All assay runs included pooled serum control samples to monitor the coefficient of inter-assay variability. This variability never exceeded 11%. To reduce the effect of technical variability, baseline and follow-up samples from each subject were processed next to each other.
2.2. Genotyping Biomarkers
Genomic DNA was extracted from whole blood samples with a QIAamp DNA blood kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions by the automated platform “QIAcube” (Qiagen, Valencia, CA, USA) and quantified using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). DNA samples were genotyped for a comprehensive set of single nucleotide polymorphisms (SNPs) by the use of TaqMan SNP genotyping assays run on an ABI PRISM 7500 FAST Real-Time PCR System (Thermo Fisher Scientific Wilmington, DE, USA).
We analyzed BsmI (rs1544410), TaqI (rs731236), FokI (rs2228570), ApaI (rs7975232), and CDX2 (rs11568820) in the VDR gene; CYP24A1-rs6013897, CYP27B1-rs10877012, CYP2R1-rs10741657, genes involved in Vitamin D metabolism; and rs2282679, and rs4588 in the GC gene coding for the VDBP. Briefly, nearly 10 ng of DNA in 2 μL was added to an 8-μL reaction well, together with 10 μL of reaction mix containing forward and reverse primers and two allele-specific fluorescent-labeled probes (one wild-type and one variant allele-specific).
2.3. Food Consumption
Food consumption was evaluated using a short questionnaire adapted from a validated questionnaire [30]. The questionnaire evaluates the main food groups commonly consumed by the Italian population. Moreover, a specific question was adapted to better discriminate food with a potential source of vitamin D. The frequency of the consumption of food is grouped into five levels, from “never or seldom” to “high frequency”, on a daily or weekly basis to assess average consumption. For each item, the standard portion size was indicated to obtain as accurate an answer as possible. To identify a protective pattern according to the WCRF’s recommendations (WCRF 2018 https://www.wcrf.org/diet-activity-and-cancer/cancer-prevention-recommendations), we built a score taking into account body weight (BMI), the level of physical activity, and dietary habits. The score inversely associated with CRC [22] was characterized by a high level of physical activity, a normal range of BMI, and a healthy pattern of high consumption of fruit and vegetables, or low consumption of meat or sweets, cakes, and pastries.
2.4. Statistical Analysis
A patient was considered to be adherent to the WCRF recommendations when he/she was in the normal range of baseline BMI (BMI < 25), practiced a high level of physical activity, and had a healthy diet (high consumption of fruit and vegetables, or low consumption of meat or sweets, cakes, and pastries). Differences in baseline serum biomarkers by WCRF adherence and gender and by VDR and VDBP variants were assessed through a non-parametric Wilcoxon rank test.
For the Event-Free Rate (EFR) analysis, the time-to-event was calculated as the difference between the date of the first event and the randomization date for those patients in whom at least one event occurred (colorectal adenoma, cancer relapse, or death), and as the difference between the date of last visit and the randomization date in those in whom no event of progression occurred. Comparisons in EFR by adherence to the WCRF’s recommendations were carried out using the Kaplan–Meier estimator and tested with the log-rank test. Multivariable Cox proportional-hazards models were employed to estimate the risk of the event in terms of hazard ratios (HRs); 95% confidence intervals (CI) were also provided.
3. Results
Seventy-four patients were enrolled in the ColoViD trial, 36 in the placebo group and 38 in the Vitamin D group, respectively. Their main characteristics were: average age was 62 years old, 53% were female, 60% of original cancers were stage II–III, and 55% were G2; 37 patients underwent chemotherapy (either as neoadjuvant or adjuvant treatment, for more details see the material section). Overall, the two arms were well balanced. Other descriptive features of the cohort can be found in the previous publication [24].
The Vitamin D supplementation did not significantly modulate leptin and adiponectin, nor the other analyzed circulating biomarkers (see Supplementary Table S1).
Based on the evaluation of the dietary pattern and lifestyle characteristics at baseline, we could categorize the population in two groups: “adherent” to WCRF recommendations versus “non-adherent”. Leptin and BMI were found to be significantly different between patients who adhered or did not adhere to the WCRF recommendations. Table 1 shows lower leptin (p = 0.001 and p = 0.003 for females and males, respectively) and lower BMI (p = 0.0003 and p = 0.0096 for females and males). Furthermore, we found borderline significantly higher 25(OH)D levels (p = 0.059 in males) in patients following WCRF indications. IL-6 was significantly higher in women who did not adhere to the WCRF recommendations (p = 0.046), while no significant differences were observed in men (p = 0.242).
Table 1.
Baseline serum biomarkers levels and BMI by dietary pattern and gender.
| No WCRF Adherents | WCRF Adherents | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Variable | n. | Median | 1st Q | 3rd Q | n | Median | 1st Q | 3rd Q | p-Values |
| Females | BMI | 12 | 28.87 | 26.64 | 30.62 | 22 | 23.32 | 20.70 | 24.24 | <0.001 |
| 25(OH)D ng/mL |
20.0 | 11.2 | 29.5 | 22.1 | 18.7 | 25.1 | 0.98 | |||
| VDBP µg/mL |
278 | 264 | 316 | 305 | 282 | 361 | 0.20 | |||
| Adiponectin µg/mL |
12.39 | 11.21 | 14.11 | 11.97 | 9.83 | 20.16 | 1.00 | |||
| Leptin ng/mL |
52.29 | 32.94 | 68.92 | 19.03 | 7.77 | 31.51 | 0.007 | |||
| IL-10 pg/mL |
2.18 | 1.99 | 2.70 | 2.12 | 1.90 | 3.21 | 0.97 | |||
| IL-6 pg/mL |
4.44 | 1.99 | 5.94 | 2.01 | 1.38 | 3.45 | 0.046 | |||
| Males | BMI | 15 | 29.01 | 26.59 | 32.51 | 25 | 25.88 | 24.22 | 27.36 | 0.011 |
| 25(OH)D ng/mL |
16.1 | 13.5 | 24.3 | 24.1 | 18.4 | 28.2 | 0.059 | |||
| VDBP µg/mL |
268 | 248 | 308 | 302 | 258 | 327 | 0.21 | |||
| Adiponectin µg/mL |
5.50 | 4.39 | 8.50 | 8.52 | 5.55 | 12.06 | 0.08 | |||
| Leptin ng/mL |
21.17 | 11.08 | 35.81 | 6.92 | 5.04 | 11.27 | 0.003 | |||
| IL-10 pg/mL |
2.41 | 1.69 | 2.89 | 2.33 | 1.86 | 2.65 | 0.88 | |||
| IL-6 pg/mL |
2.52 | 2.12 | 3.51 | 2.04 | 1.6 | 4.31 | 0.24 | |||
In bold significant or borderline significant p-values.
The circulating levels of VDBP were highly correlated with the SNPs of the GC gene and several SNPs of the VDR. Specifically, FokI and TaqI, were associated with 25(OH)D levels (Table 2 and Table 3). As we did not observe any associations of the enzymes involved in the 25(OH)D metabolism, we did not include this information in this table.
Table 2.
Median values and interquartile range of baseline serum levels of 25(OH)D (ng/mL) by variants of VDBP and VDR genes.
| SNPs | Variants | N. | Median of Serum 25(OH)D (ng/mL) | 1st Quartile | 3rd Quartile | p-Values |
|---|---|---|---|---|---|---|
| GC rs2282679 | GG | 6 | 21 | 15 | 24 | 0.73 |
| TG | 26 | 22 | 18 | 27 | ||
| TT | 42 | 23 | 14 | 26 | ||
| GC rs4588 | GG | 42 | 23 | 14 | 26 | 0.66 |
| GT | 28 | 23 | 19 | 27 | ||
| TT | 4 | 18 | 15 | 23 | ||
| VDR Fokl | AA | 9 | 22 | 16 | 24 | 0.01 |
| GA | 32 | 24 | 22 | 28 | ||
| GG | 33 | 19 | 14 | 24 | ||
| VDR Taql | AA | 25 | 20 | 14 | 24 | 0.04 |
| AG | 36 | 21 | 16 | 25 | ||
| GG | 13 | 26 | 24 | 28 | ||
| VDR CDX2 | CC | 38 | 21 | 14 | 25 | 0.43 |
| CT | 34 | 22 | 19 | 26 | ||
| TT | 2 | 24 | 22 | 27 | ||
| VDR BsmI | CC | 22 | 20 | 14 | 25 | 0.12 |
| CT | 38 | 22 | 16 | 25 | ||
| TT | 14 | 25.8 | 20 | 27.6 | ||
| VDR ApaI | AA | 26 | 24. | 19 | 28 | 0.23 |
| AC | 34 | 20 | 14 | 25 | ||
| CC | 14 | 22 | 14 | 26 |
p-values from Kruskal–Wallis Tests for the association between serum levels of 25(OH)D with VDR and VDBP genes variants. In bold significant or borderline significant p-values.
Table 3.
Median values and interquartile range of baseline serum levels of VDBP (µg/mL) by variants of VDBP and VDR genes.
| SNPs | Variants | N. | Median of Serum VDBP (µg/mL) | 1st Quartile | 3rd Quartile | p-Values |
|---|---|---|---|---|---|---|
| GC rs2282679 | GG | 6 | 217 | 197 | 234 | 0.0004 |
| TG | 26 | 287 | 263 | 325 | ||
| TT | 42 | 303 | 270 | 361 | ||
| GC rs4588 | GG | 42 | 303 | 270 | 361 | 0.002 |
| GT | 28 | 280 | 255 | 320 | ||
| TT | 4 | 201 | 197 | 219 | ||
| VDR Fokl | AA | 9 | 302 | 267 | 310 | 0.92 |
| GA | 32 | 285 | 268 | 333 | ||
| GG | 33 | 292 | 263 | 325 | ||
| VDR Taql | AA | 25 | 298 | 258 | 319 | 0.72 |
| AG | 36 | 289 | 266 | 328 | ||
| GG | 13 | 284 | 268 | 333 | ||
| VDR CDX2 | CC | 38 | 280 | 258 | 31 | 0.06 |
| CT | 34 | 305 | 266 | 375 | ||
| TT | 2 | 338 | 334 | 343 | ||
| VDR BsmI | CC | 22 | 290 | 241 | 310 | 0.44 |
| CT | 38 | 289 | 267 | 326 | ||
| TT | 14 | 294 | 268 | 334 | ||
| VDR ApaI | AA | 26 | 311 | 268 | 359 | 0.17 |
| AC | 34 | 284 | 267 | 319 | ||
| CC | 14 | 275 | 230 | 302 |
p-values from Kruskal–Wallis Tests for Vitamin D Binding Protein (VDBP) with VDR and VDBP genes variants. In bold, significant or borderline significant p-values.
The multivariable Cox proportional hazard model also confirmed a lower risk (HR = 0.41; 95% CI 0.18–0.92 p = 0.03, Table 4), adjusting for trial arms.
Table 4.
Hazard Ratio and 95% Confidence Interval for events from multivariable Cox proportional hazard model *.
| HR (95% CI) | p-Values | |
|---|---|---|
| Vitamin D vs. placebo arm | 1.20 (0.56; 2.57) | 0.64 |
| WCRF adherence (Yes vs. No) | 0.41 (0.18; 0.92) | 0.03 |
* EFR analysis includes: 6 relapses (8.1%), 21 adenoma/polyps (28.4%) and 4 other cancer sites (5.4%).
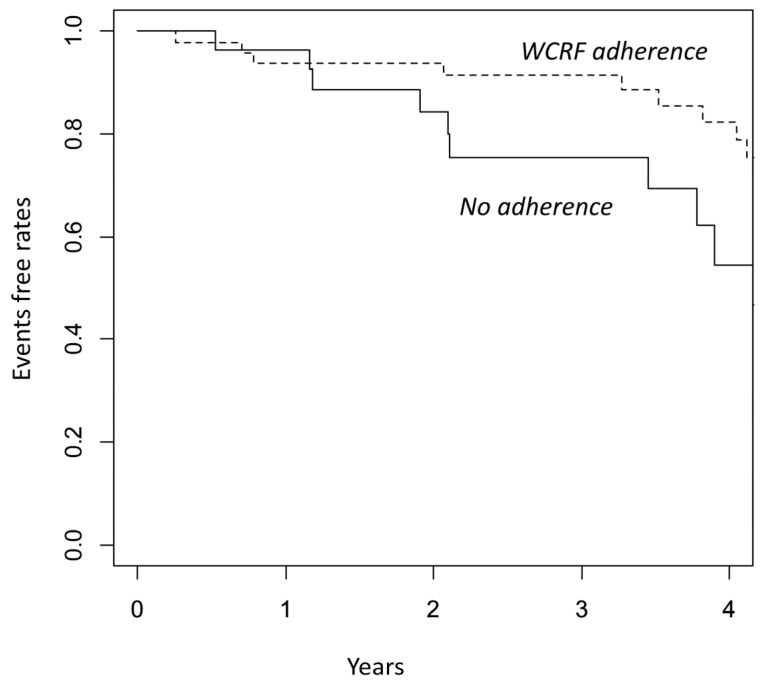
During a median follow-up of 2.6 years, we found 31 events: 6 CRC relapses (8.1%), 21 adenoma/polyps (28.4%), and 4 other cancers (5.4%). Adherence to WCRF was found to be associated with a significantly lower risk of any events (Figure 1 Kaplan–Meier curve for EFR; Log-rank p = 0.01).
Figure 1.
Kaplan–Meier for events according to dietary pattern: adherence to WCRF versus non-adherence (p-value = 0.013, Log-rank test).
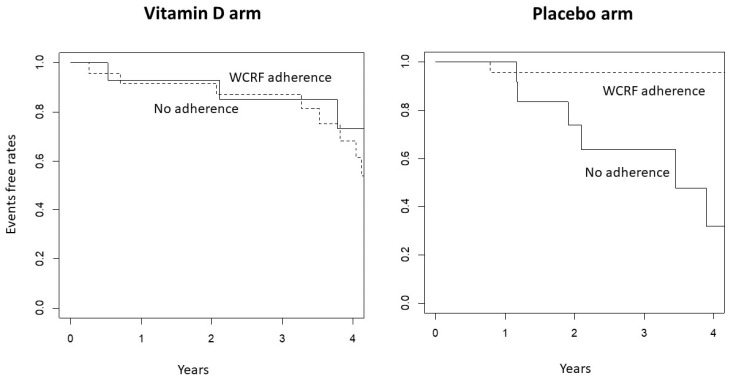
Interestingly, the analysis per study arms showed even greater reduced risk in the WCRF adherent participants within the placebo group. On the other hand, in the treatment arm no differences were seen in respect to diet adherence (Figure 2). These results suggest a possible interaction between Vitamin D and WRCF adherence: a lower risk of events is observed in the WCRF adherent group not taking vitamin D or in the vitamin D arm, independently of WCRF adherence.
Figure 2.
Kaplan–Meier for events according to dietary pattern: adherence to WCRF versus non-adherence by treatment arm (p-value = 0.97, in the Vitamin D arm and p < 0.0001 in the placebo arm Log-rank test).
4. Discussion
This exploratory study is nested within a phase II trial assessing the effect of Vitamin D supplementation on microbiome change [18]. Our data showed that CRC patients who adhere to WCRF recommendations had a significantly lower risk of events such as new polyps/adenoma, CRC relapse and other cancers compared to those with poor dietary habits. Interestingly, patients who follow WCRF recommendations have a significantly lower level of leptin; male participants have a greater level of 25OHD, and females have a lower level of IL-6. Adherence to such guidelines seems to produce a healthier profile of inflammation biomarkers and hormonal response, driven mainly by compliance with recommendations on body fatness, physical activity, and energy-dense foods and drinks as reported by other research [19].
The effect on colorectal events suggests a possible interaction between Vitamin D and diet: a lower risk of events is observed both in the WCRF adherent group not taking vitamin D and in the vitamin D arm, independently of WCRF adherence. Since the benefit due to the WCRF adherence was no longer evident in the Vitamin D arm, these data underline the role of both vitamin D supplementation and lifestyle indications for cancer prevention. It has been established that fat tissue that produces and secretes hormones, including leptin and adiponectin, is strongly involved in cancer risk [31,32]. Leptin is important in energy balance and appetite control and positively correlated with adipose tissues and nutritional status, and more recently has been extensively studied as a potential mediator of obesity-related cancer [33]. Moreover, leptin plays a key role in inflammation due to a large variety of metabolic effects, and increases both fatty acid oxidation [34] and glucose uptake [35]. However, chronic inflammation could downregulate immune system functions, producing homeostatic changes and affecting lipid metabolism [36].
Inflammation certainly has cancer-promoting effects. We found that IL-6 was significantly higher in women who did not adhere to the WCRF recommendations. IL-6 seems to be a valid marker of colorectal inflammation as reported by Kakourou et al. [37]. Diet can be pro-inflammatory, and it has been shown in a prospective cohort study that higher inflammatory dietary patterns in association with supplements increase the risk of adenoma recurrence and CRC incidence. These data would suggest that subjects with a history of adenoma should follow an anti-inflammatory diet and avoid unmotivated supplements [38]. Vitamin D supplementation has an effect on circulating cytokine and their modulation can be different based on the individual condition, being more pronounced in patients with inflammatory disorders compared to healthy subjects [39,40]. Furthermore, the trial by Fassio et al. did not show significant differences among the different Vitamin D dosages. To note, in Fassio’s study recruited subjects had vitamin D levels below 20 ng/mL (deficiency), while in our study the participants were below 30 ng/mL.
The impact of the Vitamin D treatment did not show any effects on the analyzed circulating biomarkers; however, we found that men with higher WCRF adherence have borderline significantly greater 25(OH)D serum levels. Several SNPs of the VDR gene have been associated with cancer, including CRC [41]. Different VDR domains are involved in several functions, including DNA binding, receptor dimerization, gene transactivation, and cofactor activation. The findings from our study regarding its association with 25(OH)D may play a role in cancer development. Low 25(OHD)D levels, its metabolisms, and VDR polymorphisms play a central role in Inflammatory Bowel Disease (IBD) pathogenesis [42], and IBD is a CRC risk factor. However, the effect of vitamin D supplementation to induce a therapeutic impact has still many open questions, including timing compared to the pathological project, duration, and optimal dosage [29]. Previous studies reported that plasma 25(OH)D concentrations seem to be affected by the food pattern followed by study participants. Crowe found a lower level of plasma 25(OH)D in vegetarians and vegans than in meat and fish eaters from the European Prospective Investigation into Cancer and Nutrition (EPIC)–Oxford cohort [43].
This is an exploratory study describing the results of secondary endpoints of a study presented previously [24]. Its main limitation is the small sample size, which does not allow enough statistical power for our results, especially for gender subgroup analysis. Another issue is the choice of dose of 2000 IU/day that it may be too low [44]. However, all participants receiving the active treatment reached the sufficient level of vitamin D (>30 ng/mL). Moreover, one-year Vitamin D supplementation might be too short to have a clinical impact on the events since it takes time to reach a sufficient plasma level and make its beneficial effects explicit [44,45]. The dietary pattern of the WCRF adherence group was a personal choice that could reasonably be adopted for a longer time compared to the Vitamin D supplementation, leading to a clinical effect. This study is exploratory and did not evaluate a specific intervention on lifestyle, except for some general indications at baseline. For these reasons, the results of this study need to be confirmed in a randomized trial on lifestyle interventions, considering also gender differences.
The increasing cancer incidence and the number of long-term survivors underscore the need to promote and improve prevention projects. Cancer prevention programs have to meet the challenge of overcoming the lack of validated biomarkers as the readout of the intervention’s efficacy. Adipokines could be promising biomarkers, and adiponectin in particular showed an inverse correlation with breast cancer risk and relapse, specifically in a cohort study of premenopausal women [46] and in a meta-analysis [47]. Adiponectin was also found to be inversely associated with CRC risk [48] and relapse [49]. On the other hand, leptin was found to be associated with a higher risk of CRC, specifically in a meta-analysis of prospective studies [50].
5. Conclusions
This study supports the implementation of lifestyle intervention programs as approximately 45% of CRCs in Western countries can be related to modifiable lifestyle risk factors [51]. To expand the knowledge in cancer prevention, lifestyle educational and gender-oriented programs are needed in cancer and screening centers and should be offered by family doctors. Moreover, vitamin D supplementation confirms its potential as a preventive agent.
Acknowledgments
Samuel William Russell Edu for his English supervision. Giorgia Bollani, Darina Tamayo, and Cristina Mazzon were study data managers. Federica Bellerba is a PhD student at the European School of Molecular Medicine (SEMM), Milan, Italy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11061766/s1.
Author Contributions
D.S.: protocol design, patient recruitment and manuscript preparation; F.B., bioinformatics analysis and manuscript preparation; H.J., biomarkers analysis and results interpretation; D.M., biomarkers analysis; V.A., biomarkers analysis; C.A.A. research nurse; A.G.-G., protocol design and database preparation; C.M.T., patient follow up and endoscopy; M.G.Z., patient recruitment and follow up; E.O.S., pharmacist (vitamin D and matching placebo); B.B., protocol and manuscript supervisor; S.G.: protocol design, statistical analysis and manuscript preparation; P.G., diet analysis and manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was identified as IEO #223 and was approved by the Institutional Review Board, namely the European Institute of Oncology Ethical Committee.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data on serum biomarkers and dietary information are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no conflict of interest to declare.
Funding Statement
This work was supported by “Associazione Italiana per la Ricerca sul Cancro” (Grant No. AIRC14-IG-GANDINI) and partially by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Itzkowitz S.H., Yio X. Inflammation and Cancer IV. Colorectal Cancer in Inflammatory Bowel Disease: The Role of Inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G., Zeuthen J., Kiessling R. Escape from Host-Antitumor Immunity. Crit. Rev. Oncog. 1997;8:111–142. doi: 10.1615/CritRevOncog.v8.i2-3.10. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Larsen V., Morton V., Norat T., Moreira A., Potts J.F., Reeves T., Bakolis I. Dietary Patterns Derived from Principal Component Analysis (PCA) and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2019;73:366–386. doi: 10.1038/s41430-018-0234-7. [DOI] [PubMed] [Google Scholar]
- 4.Clinton S.K., Giovannucci E.L., Hursting S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020;150:663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abar L., Vieira A.R., Aune D., Sobiecki J.G., Vingeliene S., Polemiti E., Stevens C., Greenwood D.C., Chan D.S.M., Schlesinger S., et al. Height and Body Fatness and Colorectal Cancer Risk: An Update of the WCRF-AICR Systematic Review of Published Prospective Studies. Eur. J. Nutr. 2018;57:1701–1720. doi: 10.1007/s00394-017-1557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliai Araghi S., van Dijk S.C., Ham A.C., Brouwer-Brolsma E.M., Enneman A.W., Sohl E., Swart K.M.A., van der Zwaluw N.L., van Wijngaarden J.P., Dhonukshe-Rutten R.A.M., et al. BMI and Body Fat Mass Is Inversely Associated with Vitamin D Levels in Older Individuals. J. Nutr. Health Aging. 2015;19:980–985. doi: 10.1007/s12603-015-0657-y. [DOI] [PubMed] [Google Scholar]
- 7.Zittermann A., Iodice S., Pilz S., Grant W.B., Bagnardi V., Gandini S. Vitamin D Deficiency and Mortality Risk in the General Population: A Meta-Analysis of Prospective Cohort Studies. Am. J. Clin. Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 8.Gandini S., Boniol M., Haukka J., Byrnes G., Cox B., Sneyd M.J., Mullie P., Autier P. Meta-Analysis of Observational Studies of Serum 25-Hydroxyvitamin D Levels and Colorectal, Breast and Prostate Cancer and Colorectal Adenoma. Int. J. Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 9.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Krstic G., Wetterslev J., Gluud C. Vitamin D Supplementation for Prevention of Cancer in Adults. Cochrane Database Syst. Rev. 2014:CD007469. doi: 10.1002/14651858.CD007469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manson J.E., Cook N.R., Lee I.-M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilz S., Trummer C., Theiler-Schwetz V., Grübler M.R., Verheyen N.D., Odler B., Karras S.N., Zittermann A., März W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients. 2022;14:303. doi: 10.3390/nu14020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaney R.P. Guidelines for Optimizing Design and Analysis of Clinical Studies of Nutrient Effects. Nutr. Rev. 2014;72:48–54. doi: 10.1111/nure.12090. [DOI] [PubMed] [Google Scholar]
- 13.Dawson-Hughes B., Nelson J., Pittas A.G. Response to Comment on Dawson-Hughes et al. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults with Prediabetes: A Secondary Analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916–2922. doi: 10.2337/dc20-1765. Erratum in Diabetes Care 2021, 44, e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnagnarella P., Muzio V., Caini S., Raimondi S., Martinoli C., Chiocca S., Miccolo C., Bossi P., Cortinovis D., Chiaradonna F., et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients. 2021;13:3285. doi: 10.3390/nu13093285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler P.D., Chen W.Y., Ajala O.N., Hazra A., Cook N., Bubes V., Lee I.-M., Giovannucci E.L., Willett W., Buring J.E., et al. Effect of Vitamin D 3 Supplements on Development of Advanced Cancer. JAMA Netw. Open. 2020;3:e2025850. doi: 10.1001/jamanetworkopen.2020.25850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Zhu J., DeLuca H.F. Where Is the Vitamin D Receptor? Arch. Biochem. Biophys. 2012;523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y.-H. Vitamin D Receptor Gene Polymorphisms and Colorectal Cancer Risk: A Systematic Meta-Analysis. World J. Gastroenterol. 2012;18:1672. doi: 10.3748/wjg.v18.i14.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raimondi S., Pasquali E., Gnagnarella P., Serrano D., Disalvatore D., Johansson H.A., Gandini S. BsmI Polymorphism of Vitamin D Receptor Gene and Cancer Risk: A Comprehensive Meta-Analysis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014;769:17–34. doi: 10.1016/j.mrfmmm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang T.J., Zhang F., Richards J.B., Kestenbaum B., van Meurs J.B., Berry D., Kiel D.P., Streeten E.A., Ohlsson C., Koller D.L., et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikle D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020;4:bvz038. doi: 10.1210/jendso/bvz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouvari M., Panagiotakos D.B., Chrysohoou C., Yannakoulia M., Georgousopoulou E.N., Tousoulis D., Pitsavos C. Dietary Vitamin D Intake, Cardiovascular Disease and Cardiometabolic Risk Factors: A Sex-based Analysis from the ATTICA Cohort Study. J. Hum. Nutr. Diet. 2020;33:708–717. doi: 10.1111/jhn.12748. [DOI] [PubMed] [Google Scholar]
- 22.Serrano D., Pozzi C., Guglietta S., Fosso B., Suppa M., Gnagnarella P., Corso F., Bellerba F., Macis D., Aristarco V., et al. Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines. Nutrients. 2021;13:363. doi: 10.3390/nu13020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinninella E., Mele M.C., Raoul P., Cintoni M., Gasbarrini A. Vitamin D and Colorectal Cancer: Chemopreventive Perspectives through the Gut Microbiota and the Immune System. BioFactors. 2022;48:285–293. doi: 10.1002/biof.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellerba F., Serrano D., Harriet J., Pozzi C., Segata N., NabiNejad A., Piperni E., Gnagnarella P., Macis D., Aristarco V., et al. Colorectal Cancer, Vitamin D and Microbiota: A Double-Blind Phase II Randomized Trial (ColoViD) in Colorectal Cancer Patients. Neoplasia. 2022;34:100842. doi: 10.1016/j.neo.2022.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieth R., Kimball S., Hu A., Walfish P.G. Randomized Comparison of the Effects of the Vitamin D3 Adequate Intake versus 100 Mcg (4000 IU) per Day on Biochemical Responses and the Wellbeing of Patients. Nutr. J. 2004;3:8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieth R. Why the Optimal Requirement for Vitamin D3 Is Probably Much Higher than What Is Officially Recommended for Adults. J. Steroid Biochem. Mol. Biol. 2004;89–90:575–579. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari H.A., Giovannucci E., Willett W.C., Dietrich T., Dawson-Hughes B. Estimation of Optimal Serum Concentrations of 25-Hydroxyvitamin D for Multiple Health Outcomes. Am. J. Clin. Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Gorham E.D., Garland C.F., Garland F.C., Grant W.B., Mohr S.B., Lipkin M., Newmark H.L., Giovannucci E., Wei M., Holick M.F. Vitamin D and Prevention of Colorectal Cancer. J. Steroid Biochem. Mol. Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Janssen M.J.W., Wielders J.P.M., Bekker C.C., Boesten L.S.M., Buijs M.M., Heijboer A.C., van der Horst F.A.L., Loupatty F.J., van den Ouweland J.M.W. Multicenter Comparison Study of Current Methods to Measure 25-Hydroxyvitamin D in Serum. Steroids. 2012;77:1366–1372. doi: 10.1016/j.steroids.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Gnagnarella P., Dragà D., Misotti A.M., Sieri S., Spaggiari L., Cassano E., Baldini F., Soldati L., Maisonneuve P. Validation of a Short Questionnaire to Record Adherence to the Mediterranean Diet: An Italian Experience. Nutr. Metab. Cardiovasc. Dis. 2018;28:1140–1147. doi: 10.1016/j.numecd.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Tabung F.K., Fung T.T., Chavarro J.E., Smith-Warner S.A., Willett W.C., Giovannucci E.L. Associations between Adherence to the World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Biomarkers of Inflammation, Hormonal, and Insulin Response. Int. J. Cancer. 2017;140:764–776. doi: 10.1002/ijc.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiménez-Cortegana C., López-Saavedra A., Sánchez-Jiménez F., Pérez-Pérez A., Castiñeiras J., Virizuela-Echaburu J.A., de la Cruz-Merino L.d.l., Sánchez-Margalet V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules. 2021;11:913. doi: 10.3390/biom11060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vucenik I., Stains J.P. Obesity and Cancer Risk: Evidence, Mechanisms, and Recommendations. Ann. N. Y. Acad. Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern J.H., Rutkowski J.M., Scherer P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell. Metab. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meek T.H., Morton G.J. The Role of Leptin in Diabetes: Metabolic Effects. Diabetologia. 2016;59:928–932. doi: 10.1007/s00125-016-3898-3. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Pérez A., Sánchez-Jiménez F., Vilariño-García T., Sánchez-Margalet V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020;21:5887. doi: 10.3390/ijms21165887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakourou A., Koutsioumpa C., Lopez D.S., Hoffman-Bolton J., Bradwin G., Rifai N., Helzlsouer K.J., Platz E.A., Tsilidis K.K. Interleukin-6 and Risk of Colorectal Cancer: Results from the CLUE II Cohort and a Meta-Analysis of Prospective Studies. Cancer Causes Control. 2015;26:1449–1460. doi: 10.1007/s10552-015-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z., Wang K., Shivappa N., Hébert J.R., Chen H., Liu H., Jiang X. Inflammatory Potential of Diet and Colorectal Carcinogenesis: A Prospective Longitudinal Cohort. Br. J. Cancer. 2022;126:1735–1743. doi: 10.1038/s41416-022-01731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fassio A., Gatti D., Rossini M., Bertelle D., Bixio R., Viapiana O., Milleri S., Benini C., Pistillo F., Zanetti G., et al. Effects on Serum Inflammatory Cytokines of Cholecalciferol Supplementation in Healthy Subjects with Vitamin D Deficiency. Nutrients. 2022;14:4823. doi: 10.3390/nu14224823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bader D.A., Abed A., Mohammad B.A., Aljaberi A., Sundookah A., Habash M., Alsayed A.R., Abusamak M., Al-Shakhshir S., Abu-Samak M. The Effect of Weekly 50,000 IU Vitamin D3 Supplements on the Serum Levels of Selected Cytokines Involved in Cytokine Storm: A Randomized Clinical Trial in Adults with Vitamin D Deficiency. Nutrients. 2023;15:1188. doi: 10.3390/nu15051188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gnagnarella P., Raimondi S., Aristarco V., Johansson H.A., Bellerba F., Corso F., Gandini S. Vitamin D Receptor Polymorphisms and Cancer. Adv. Exp. Med. Biol. 2020;1268:53–114. doi: 10.1007/978-3-030-46227-7_4. [DOI] [PubMed] [Google Scholar]
- 42.Vernia F., Valvano M., Longo S., Cesaro N., Viscido A., Latella G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients. 2022;14:269. doi: 10.3390/nu14020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe F.L., Steur M., Allen N.E., Appleby P.N., Travis R.C., Key T.J. Plasma Concentrations of 25-Hydroxyvitamin D in Meat Eaters, Fish Eaters, Vegetarians and Vegans: Results from the EPIC–Oxford Study. Public Health Nutr. 2011;14:340–346. doi: 10.1017/S1368980010002454. [DOI] [PubMed] [Google Scholar]
- 44.Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Barger-Lux M.J. Human Serum 25-Hydroxycholecalciferol Response to Extended Oral Dosing with Cholecalciferol. Am. J. Clin. Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 45.Lappe J.M., Travers-Gustafson D., Davies K.M., Recker R.R., Heaney R.P. Vitamin D and Calcium Supplementation Reduces Cancer Risk: Results of a Randomized Trial. Am. J. Clin. Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 46.Macis D., Gandini S., Guerrieri-Gonzaga A., Johansson H., Magni P., Ruscica M., Lazzeroni M., Serrano D., Cazzaniga M., Mora S., et al. Prognostic Effect of Circulating Adiponectin in a Randomized 2 × 2 Trial of Low-Dose Tamoxifen and Fenretinide in Premenopausal Women at Risk for Breast Cancer. J. Clin. Oncol. 2012;30:151–157. doi: 10.1200/JCO.2011.35.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macis D., Guerrieri-Gonzaga A., Gandini S. Circulating Adiponectin and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Int. J. Epidemiol. 2014;43:1226–1236. doi: 10.1093/ije/dyu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An W., Bai Y., Deng S.-X., Gao J., Ben Q.-W., Cai Q.-C., Zhang H.-G., Li Z.-S. Adiponectin Levels in Patients with Colorectal Cancer and Adenoma. Eur. J. Cancer Prev. 2012;21:126–133. doi: 10.1097/CEJ.0b013e32834c9b55. [DOI] [PubMed] [Google Scholar]
- 49.Ferroni P., Palmirotta R., Spila A., Martini F., Raparelli V., Fossile E., Mariotti S., del Monte G., Buonomo O., Roselli M., et al. Prognostic Significance of Adiponectin Levels in Non-Metastatic Colorectal Cancer. Anticancer Res. 2007;27:483–489. [PubMed] [Google Scholar]
- 50.Joshi R.K., Kim W.J., Lee S.A. Association between Obesity-Related Adipokines and Colorectal Cancer: A Case-Control Study and Meta-Analysis. World J. Gastroenterol. 2014;20:7941–7949. doi: 10.3748/wjg.v20.i24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keum N.N., Giovannucci E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data on serum biomarkers and dietary information are available from the corresponding author on reasonable request.