Abstract
Simple Summary
Honey bees are of great importance because of their roles in pollination and the supply of bee products. However, the number of honey bee colonies is declining worldwide, and these colony losses mainly occur in winter. Colony loss surveys have been regarded as an efficient measure to protect managed honey bees, as they help identify potential risk factors for colony loss. They may also make beekeepers pay more attention to overwinter beekeeping management and thus reduce colony losses. We conducted surveys on the overwinter mortality of managed honey bee colonies in China from 2009 to 2021. The aim of the present study was to evaluate the health status of honey bee colonies in China and describe the risk factors for winter colony losses. We reported that colony losses were low, with variations among years, provinces, species (Apis mellifera and Apis cerana), and types of apiaries. The results showed that the queen problems (queenless colonies or drone-laying queens), operation size, migration, migration×species interaction, and species significantly affected winter colony losses. Our study contributes to improving the health of managed honey bees and provides useful strategies for colony overwintering.
Abstract
There is growing concern that massive loss of honey bees can cause serious negative effects on biodiversity and ecosystems. Surveys of colony losses have been performed worldwide to monitor the dynamic changes and health status of honey bee colonies. Here, we present the results of surveys regarding winter colony losses from 21 provinces in China from 2009 to 2021, with a total of 1,744,324 colonies managed by 13,704 beekeepers. The total colony losses were low (9.84%; 95% Confidence Interval (CI): 9.60–10.08%) but varied among years, provinces, and scales of apiaries. As little is known about the overwintering mortality of Apis cerana, in this study, we surveyed and compared the loss rates between Apis mellifera and A. cerana in China. We found colonies of A. mellifera suffered significantly lower losses than A. cerana in China. Larger apiaries resulted in higher losses in A. mellifera, whereas the opposite was observed in A. cerana. Furthermore, we used generalized linear mixed-effects models (GLMMs) to evaluate the effects of potential risk factors on winter colony losses and found that the operation size, species, migration, migration×species interaction, and queen problems were significantly related to the loss rates. New queens can increase their colony overwintering survival. Migratory beekeepers and large operations reported lower loss rates.
Keywords: colony winter losses, honey bee health, China, risk factor, Apis mellifera, Apis cerana
1. Introduction
As one of the major insect pollinators, honey bees are of vital significance to global food security and ecological balance [1,2]. However, pathogen invasion and pesticide abuse have led to a decline in honey bee colonies, resulting in severe threats to biodiversity, agriculture, and ecosystem [3,4,5]. Mounting evidence suggests that a decline in feral and managed honey bee colonies has occurred worldwide [6,7]. Consequently, colony losses have been surveyed across several continents in recent years [8,9,10,11]. Unexpectedly, the United States witnessed a sudden death of honey bee colonies in 2006, which raised great concern for colony losses. Since then, the overwintering mortality of honey bees has been investigated annually in the United States [12,13,14,15,16,17,18]. Soon after, many countries in Europe jointly surveyed winter colony losses in the efforts of Prevention of honey bee COlony LOSSes (COLOSS) [19,20,21,22,23,24]. Currently, colony losses are surveyed in many parts of the world, including Asia [25,26,27,28], Africa [29], the Americas [14,30,31,32,33,34,35], Europe [36,37,38], and Oceania [9,39], most of which present high loss rates. In China, continuous and systematic surveys on the winter mortality of managed honey bee colonies date back to 2009, even though a massive colony collapse has not been reported yet. To date, several colony loss surveys in China reported that the overall mortality of honey bees is maintained at a low level. Moreover, this loss rate is substantially influenced by risk factors such as queen problems, operation size, and the proportion of new queens [26,27,28].
Most areas of China lie in the northern temperate zone, which experiences cold winters. As honey bees cannot forage at low temperatures, overwintering is very important for honey bee colonies in temperate climates [40]. Colonies managed in northern China usually overwinter for about 5 months—from November to March. In Northeast China, where areas are of higher latitudes, the overwintering period is usually longer, extending by 10 to 30 days, and the minimum temperature during the winter period is lower. Some provinces in Central China, such as Anhui and Hubei, have a shorter wintering period (lasting for three months). Chinese beekeepers have adopted several management practices for the overwinter survival of honey bee colonies, including treatment against parasites (such as Varroa destructor), the construction of overwintering core colonies (strong colonies formed by merging colonies or supplementing capped brood) before winter, feeding sugar to the bees before winter, and keeping them warm during the winter. In China, honey bee colonies are mostly managed to provide bee products and pollination services, but a small part of the products are used for traditional Chinese medicines, such as propolis and bee venom [41,42]. Beekeeping is also considered an important industry in China for poverty alleviation and rural revitalization.
Two bee species exist in China—Apis mellifera and Apis cerana—and there is a long history of managed beekeeping of A. cerana, which is native to Asia [43]. In China, A. cerana was domesticated on a large scale more than 2000 years ago, and currently, there are approximately 2 million A. cerana colonies in the country [44]. A. mellifera was first introduced into China from Tsarist Russia in the late 1800s [45,46], and the first native A. mellifera subspecies was identified in China in 2016, as reported in our previous study [47]. A. mellifera and A. cerana exhibit some similarities and differences in their phenotypes, physiological characteristics, and biological habits [48,49,50]. For example, A. cerana is resistant to V. destructor and can forage under lower temperatures than A. mellifera [51,52]. Additionally, A. cerana outperforms A. mellifera with respect to the use of scattered nectar resources [51,52]. Recent studies on winter colony losses have focused on A. mellifera, and colony loss surveys regarding A. cerana are extremely rare worldwide. To our knowledge, this is the first national survey on the differences in winter colony losses between two different bee species—A. mellifera and A. cerana—in China.
Here, we report the results of a continued survey of winter colony losses from 2009 to 2021 and explore the possible factors that influence the overwintering mortality of honey bees using linear models. Specifically, we found significant differences in the loss rates of different honey bee species.
2. Materials and Methods
2.1. Survey Design and Data Validation
Survey questionnaires were designed in accordance with the COLOSS standardized questionnaire published by van der Zee et al., in 2013 [53] and modified for the apicultural context of China. The questionnaires were printed in Chinese, and all questions in the questionnaires were the same, regardless of the honey bee species. Questionnaires were shared with local beekeeping organizations as well as randomly distributed to local beekeepers by trained surveyors. These surveyors collected information using telephonic or face-to-face interviews and then submitted it using a website from the years 2009–2015 or by email correspondence.
Our survey questionnaire was divided into two parts. The first part focused on colony losses and included parts of the COLOSS questionnaire. These questions include the number of colonies which the beekeeper had on 1st October, the number of colonies lost between 1 October and 1 April, the number of the lost colonies without dead bees in the hive, and the number of the lost colonies with dead bees in cells. The number of colonies lost between 1 October and 1 April was regarded as the total number of colonies lost in this study. The second part of the questionnaire covered several kinds of questions on hive management practices, such as the proportion of wintered colonies with a new queen in the apiaries, the frequency of requeening in one year, the origin of the queen, the number of colonies that have unsolved queen problems (drone laying queens or no queen at all), the practices of migratory beekeeping, treatment practices against V. destructor, and renewal of honeycomb. Some of these operational factors were selected according to the COLOSS questionnaire and some additional options specific to Chinese apiculture were also included in our questionnaires. The loss data was collected from the October 1st of one year to April 1st of the next year.
All data were exported from the database and imported into a personal computer for data cleansing. First, the dataset was filtered to remove duplications and contradictory entries (number of losses > original colony number or number of overwintering colonies = 0). If the responses of one province for the entire study period were fewer than five, data were removed. The remaining data were used to calculate overall loss rates in the form of total losses, as recommended by van der Zee et al. [53]. The total loss is the total number of colonies that were lost divided by the total number of colonies in the sample before winter and is expressed as a percentage.
2.2. Statistical Analysis
Our final dataset included twelve years of data, part of which has been reported previously (2010–2017, [26,27,28]), while the remaining data were acquired for the present study. Apiaries were classified into three categories according to the actual situation of Chinese apiculture and our previous criteria [26]: hobby (1–50 colonies), side-line (51–200 colonies), and commercial (>200 colonies). Confidence intervals (CI) were calculated using an intercept-only generalized linear model (GLM) with a quasi-binomial distribution and logit link function using the standard methods [53]. The data and the transformed data are not normally distributed; therefore, they presented significant differences in terms of variance. Thus, the Kruskal–Wallis rank sum test using individual loss rates of beekeepers was conducted to explore the differences between subgroups of respondents, with FDR methods for adjustment of p-values for multiple comparisons (Dunn’s Multiple Comparison Test). Subgroups with less than ten data points were excluded from comparison. In addition, we used data from six provinces (Chongqing, Gansu, Guangdong, Guangxi, Jiangxi, and Zhejiang) to compare provincial losses across years. These provinces were selected as they contributed consistently to our survey each year, whereas other provinces lacked data for some years. The risk factors for winter colony losses were tested using generalized linear mixed models (GLMM) with a binomial distribution. The risk factors included the proportion of new queens, queen problems, migration, operation size, pollination, frequency of requeening, comb renewal, winter food, and treatment against V. destructor. We first tested the factors individually as fixed effects, with provinces, years, and beekeepers as random effects, and included all eight significant factors in the full model. Next, we tested the significance of the factors in the full model and deleted non-significant terms so that the simplified model could be achieved. The simplified model was further validated by comparing the BIC and AIC values of the models with all the possible combinations of the eight factors (28 = 256 models) to ensure the effectiveness of our model selection. Finally, possible interaction terms were tested, and a final model with an interaction term was formed based on BIC values.
All statistical analyses were conducted using R statistical software (R Development Core Team, Vienna, Austria, 2022) (version 4.2.1) [54]. Adjustment of p-values for multiple comparisons for the Kruskal–Wallis rank sum test was conducted using the FSA package (version 0.9.3) [55]. GLMMs analysis was performed using the “lme4” package (version 1.1.30) [56]. Graphs were drawn using GraphPad Prism (version 8.3.1).
3. Results
3.1. Survey Sample
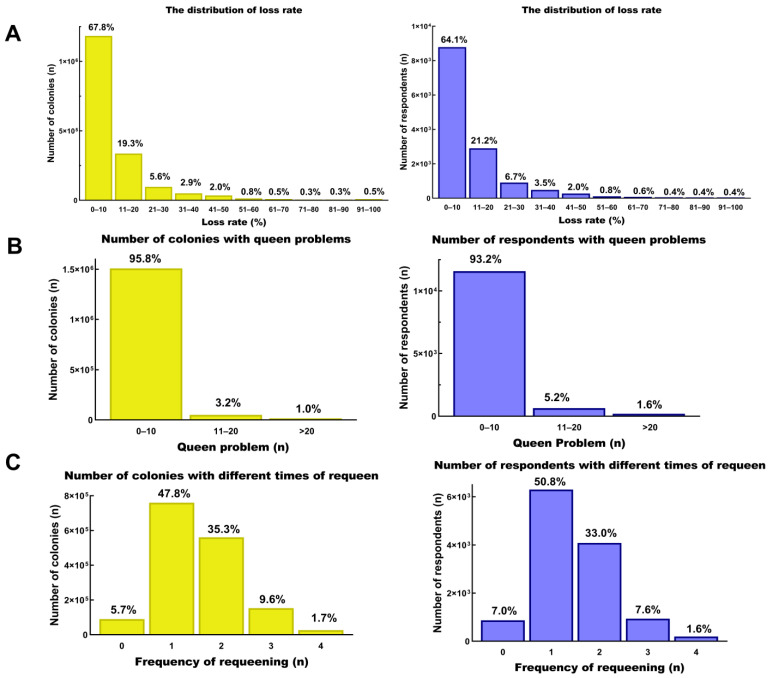
In China, the obligatory registration of beekeepers is not yet widely implemented, which considerably hindered our ability to reach a wider community of beekeepers. In our survey, the majority of the data was collected during 2020–2021, which included a total of 266,173 colonies (15.3% of the total colonies surveyed in the present study) managed by 1739 respondents (12.7% of the total respondents) (Figure S1). The distribution of colony losses showed that the majority of the beekeepers (N = 8876, 64.1%) managing a total of 1,183,386 colonies (67.8%) had loss rates between 0 and 10% (Figure 1A). The distribution of the number of colonies with unsolvable queen problems showed that most of the participating beekeepers (N = 11,589, 93.2%) who managed 95.8% of the colonies (N = 1,508,793) had between 0 and 10 disabled queens (Figure 1B). Of the total respondents, 50.8% (N = 6301) reported replacing the queens once every year, with 47.8% (N= 760,339) of the colonies having the queens replaced once every year (Figure 1C). Treatment against V. destructor was performed in 99.4% of the A. mellifera colonies (N = 699,920) by most beekeepers (N = 4779, 99.1%), but almost all colonies (N = 376,698, 94.3%) of A. cerana were not treated (Figure S2A). The distribution of the percentage of comb renewal showed that 23.5% of the colonies (N = 369,184) had 31–40% new combs managed by most beekeepers (N = 2860, 23.5%) (Figure S2B). Furthermore, 36% of the beekeepers (N = 1593) migrated about 43% of the total colonies (N = 226,793), whereas 12% of the beekeepers (N = 534) who managed a total of 69,787 colonies (13%) provided pollination services. About 9% of the beekeepers (N = 404) stated that they migrated the colonies as well as provided pollination services, which corresponded to 11% of the total colonies (N = 56,431) (Figure S2C). As shown in Figure S2D, the number of commercial beekeepers (N = 969, 72.8%) who introduced queens from outside was apparently higher than that of hobby beekeepers (N = 899, 41.6%). Most of the participating beekeepers (N =8303, 66.8%) who managed 57.0% of the colonies (N = 897,380), belonging to sideline beekeepers, had between 0 and 10 disabled queens (Figure S3).
Figure 1.
The distribution of colonies/respondents with loss rate interval, queen problems, and frequency of requeening. (A) The distribution of colonies/respondents with different loss rates. (B) The distribution of colonies/respondents with disabled queens. (C) The distribution of colonies/respondents with different frequency of requeening in one year.
3.2. Winter Colony Losses in China (2009–2021)
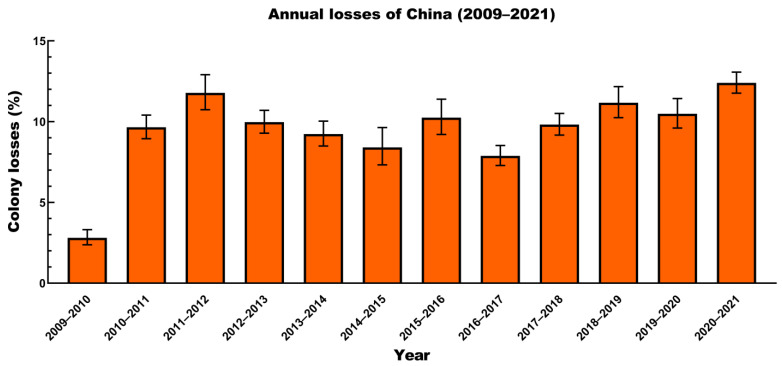
In total, we received 13,704 valid responses from 21 provinces in China, leading to a sample size of 1,744,324 colonies. Of all the valid responses, 32% respondents reported that they did not suffer colony losses in winter. The calculated total losses for 12 consecutive years were relatively low (9.84%; 95% CI: 9.60–10.08%) (Table 1) compared with other regions of the world [9,11,17,19,20,21].
Table 1.
Reported total colony losses of managed honey bees in China (2009–2021).
| Year | No. of Apiaries | No. of Colonies | Total Losses % (95% CI) |
|---|---|---|---|
| 2009–2021 | 13,704 | 1,744,324 | 9.84 (9.60–10.08) |
| 2009–2010 | 1016 | 116,547 | 2.81 (2.38–3.32) |
| 2010–2011 | 1586 | 188,580 | 9.65 (8.94–10.41) |
| 2011–2012 | 1419 | 174,009 | 11.78 (10.74–12.91) |
| 2012–2013 | 1509 | 176,171 | 9.97 (9.30–10.70) |
| 2013–2014 | 1536 | 216,690 | 9.23 (8.49–10.03) |
| 2014–2015 | 442 | 50,353 | 8.41 (7.32–9.64) |
| 2015–2016 | 800 | 110,540 | 10.25 (9.20–11.39) |
| 2016–2017 | 889 | 103,394 | 7.88 (7.29–8.52) |
| 2017–2018 | 1279 | 162,849 | 9.82 (9.17–10.51) |
| 2018–2019 | 668 | 80,427 | 11.17 (10.24–12.17) |
| 2019–2020 | 821 | 98,591 | 10.49 (9.61–11.43) |
| 2020–2021 | 1739 | 266,173 | 12.40 (11.76–13.06) |
3.3. Annual Losses (2009–2021)
The number of colonies for each year is shown in Table 1. As shown in Figure 2, the winter colony losses were differed by year, and the annual losses were generally low. The lowest annual loss (2.81%; 95% CI: 2.38–3.32%) in 2009–2010 was significantly different from the loss rate in other years (p < 0.00001), as validated by using Dunn’s multiple comparison test. The annual losses in 2020–2021 were significantly higher than those in the others (p < 0.0001). Again, this was validated using Dunn’s multiple comparison test.
Figure 2.
Winter colony losses (%, 95% CI) for different years (2009–2021).
3.4. Provincial Losses
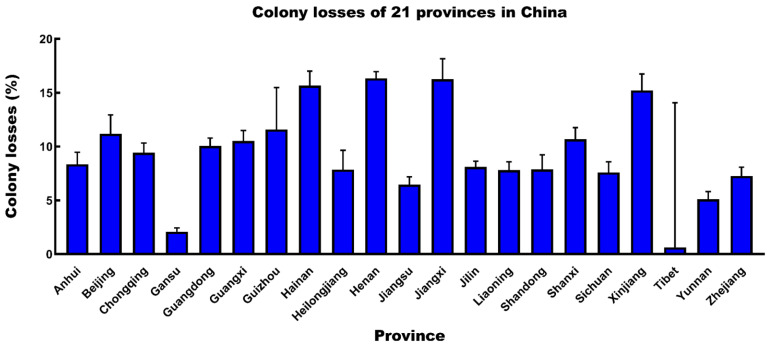
The number of apiaries/colonies and the total losses in all surveyed provinces were calculated during 2009–2021, and notable variations were observed among the 21 provinces in China (Table 2). The loss rates at the provincial level varied from 2.09% (95% CI: 1.80–2.44%) to 16.34% (95% CI: 15.74–16.96%) (Figure 3). The minimum winter loss occurred in Gansu, which was significantly different from that in all other provinces (p < 0.00001). Henan reported the highest colony losses and was significantly different from the loss rates of other provinces (p < 0.005), except for Guizhou (p = 1). The significant differences were shown by the Dunn’s multiple comparison test, using individual loss rates.
Table 2.
Provincial total winter losses (%, CI 95%) and number of apiaries in China.
| Province | No. of Apiaries | No. of Colonies | Total Losses % (95% CI) |
|---|---|---|---|
| Anhui | 144 | 18,699 | 8.35 (7.36–9.47) |
| Beijing | 174 | 17,352 | 11.19 (9.64–12.94) |
| Chongqing | 800 | 93,459 | 9.43 (8.60–10.33) |
| Gansu | 1134 | 87,483 | 2.09 (1.80–2.44) |
| Guangdong | 1099 | 146,351 | 10.06 (9.38–10.79) |
| Guangxi | 841 | 142,910 | 10.7 (8.5–12.8) |
| Guizhou | 100 | 24,770 | 11.58 (8.57–15.48) |
| Hainan | 574 | 46,293 | 15.67 (14.41–17.01) |
| Heilongjiang | 312 | 31,352 | 7.86 (6.37–9.65) |
| Henan | 870 | 70,543 | 16.34 (15.74–16.96) |
| Jiangsu | 541 | 62,879 | 6.47 (5.82–7.19) |
| Jiangxi | 337 | 41,263 | 16.25 (14.50–18.16) |
| Jilin | 1188 | 109,019 | 8.12 (7.63–8.63) |
| Liaoning | 996 | 91,527 | 7.80 (7.09–8.59) |
| Shandong | 281 | 32,485 | 7.87 (6.70–9.22) |
| Shanxi | 842 | 50,392 | 10.68 (9.70–11.75) |
| Sichuan | 615 | 134,297 | 7.59 (6.71–8.58) |
| Xinjiang | 878 | 272,405 | 15.22 (13.81–16.74) |
| Tibet 1 | 9 | 2,041 | 0.64 (0.03–14.07) |
| Yunnan | 826 | 107,503 | 5.11 (4.48–5.82) |
| Zhejiang | 1143 | 161,301 | 7.26 (6.53–8.07) |
1 Tibet not included in the Kruskal–Wallis Test (sample size < 10).
Figure 3.
Winter colony losses (%, 95% CI) for different provinces in China.
To assess the differences between provincial colony losses, we selected six representative beekeeping provinces and analyzed the differences in annual losses of these provinces during 2017–2021. These provinces include Chongqing, Gansu, Guangdong, Guangxi, Zhejiang, and Jiangxi. Significant differences in annual colony losses in the six provinces were tested using Dunn’s multiple comparison test. The results showed that colony losses in Gansu were maintained at a low level between 2017 and 2021, which was significantly lower than those of the other provinces in 2019–2020 (1.08%; 95% CI: 0.65–1.78%) and 2020–2021 (2.34%; 95% CI: 1.66–3.31%) (p < 0.00001) (Table S1). The highest annual loss in Gansu was observed in 2018–2019 (3.31%; 95% CI: 2.41–4.52%) and was significantly different from other years (p < 0.005). In 2017–2018, the losses reported in Zhejiang (p < 0.005), Jiangxi (p < 0.0001), and Gansu (p < 0.005) were significantly different from those in the other provinces. Similarly, significant differences were observed involving Gansu (p < 0.05), Jiangxi (p < 0.00001), and Zhejiang (p < 0.05) in 2018–2019 (Table S1).
3.5. Operation Sizes and Loss Rates
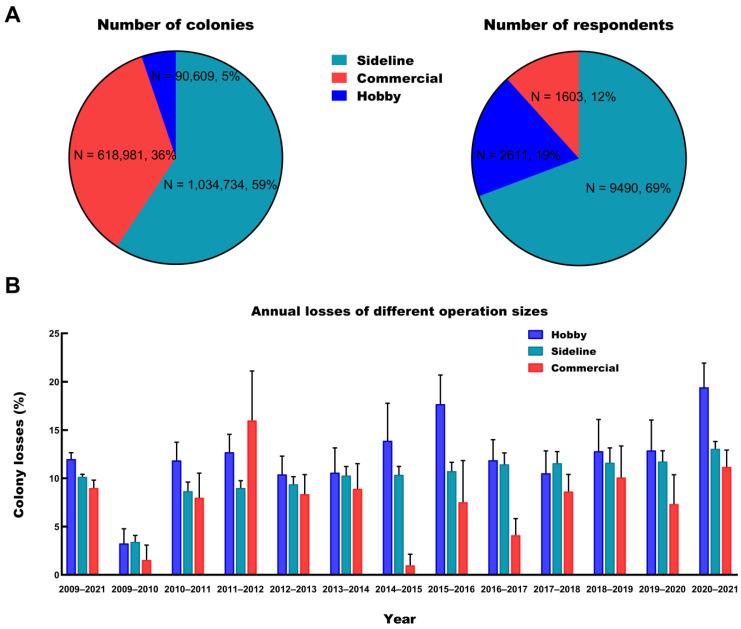
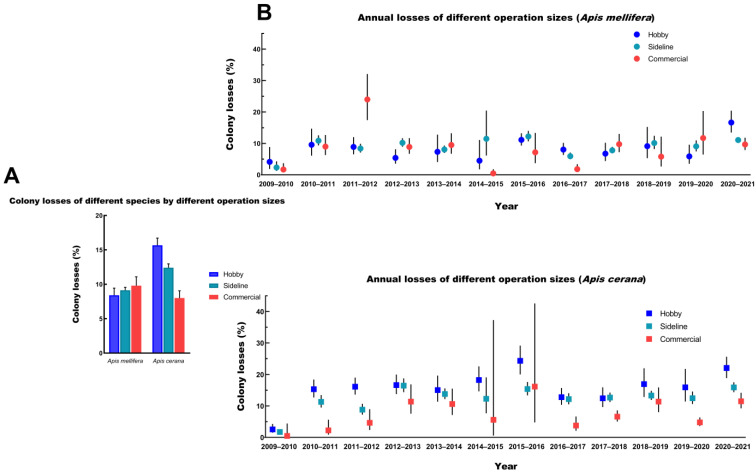
Our results demonstrated that most of the respondents (69%, N = 9490) were sideline beekeepers with a total of 1,034,734 (59%) colonies, and 36% of these sideline beekeepers reported no losses during winter (Figure 4A). Additionally, hobby beekeepers (19%, N = 2611) managed 5% of the colonies (N = 90,609), and 40% of them reported no losses. Commercial beekeepers (12%, N = 1603) managed 35% of the colonies (N = 618,981), and 36% of them reported no losses. The winter colony losses of hobby, sideline, and commercial beekeepers were 11.99% (95% CI: 11.36–12.65%), 10.15% (95% CI: 9.89–10.42%), and 8.99% (95% CI: 8.24–9.81%), respectively. Based on the colony loss data spanning 12 years, we concluded that the overwintering mortality of honey bees varied with the operation sizes, and larger-scale apiary owners reported lower mortality rates compared to small-scale apiary owners (Figure 4B). Only the loss rates of commercial beekeepers were significantly different from those of hobby/sideline beekeepers, according to Dunn’s multiple comparison test, which also considered the individual loss rates for the beekeepers (p < 0.00001). For the hobby beekeepers, the annual losses varied from 3.24% (95% CI: 2.19–4.77%) to 19.42% (95% CI: 17.15–21.92%). The lowest loss rate in 2009–2010 was significantly different from other annual losses, according to Dunn’s multiple comparison test (p < 0.00001). The mortality of sideline beekeepers was the highest in 2020–2021 (13.06%; 95% CI: 12.34–13.81%) and the lowest in 2009–2010 (3.41%; 95% CI: 2.85–4.08%). Both the highest and lowest values were significantly different from those of the other years, as determined by Dunn’s multiple comparison test (p < 0.001). For individual years, sideline beekeepers experienced significantly higher mortality than commercial and hobby beekeepers during 2009–2010 (p < 0.0001). The loss rates of commercial apiaries in 2014–2015 and 2020–2021 were significantly lower than those of the other two types of apiaries, according to Dunn’s multiple comparison test (p < 0.00001). There were significant differences in the loss rates among the three types of apiaries in 2015–2016 (p < 0.00001) and 2016–2017 (p < 0.005). Overall, with the exception of certain years, a trend of lower loss rates in larger operation sizes was observed.
Figure 4.
Comparison of the proportion and mortality of different apiaries. (A) The number of respondents/colonies by different types of apiaries. (B) Comparison of winter colony losses (%, 95% CI) by different types of apiaries for the years (2009–2021).
3.6. Differences in Colony Losses between A. mellifera and A. cerana in China
Our dataset included both species and compared the results. The survey recorded a total of 847,587 and 478,905 colonies for A. mellifera and A. cerana, respectively. These were separately managed by 5711 beekeepers (A. mellifera) and 4253 beekeepers (A. cerana). The 12-year loss rates of A. mellifera (9.38%; 95% CI: 8.98–9.79%) were significantly lower than those of A. cerana (11.13%; 95% CI: 10.72–11.55%), as shown by Dunn’s test (p < 0.00001) (Table 3). We also found that, with the exception of 2009–2010, A. mellifera had significantly different annual losses from A. cerana (p < 0.05). Furthermore, the annual losses of A. cerana in 2009–2010 (1.56%; 95% CI: 1.10–2.21%) were significantly lower than those in the other years (p < 0.00001). For A. mellifera, the annual losses in 2009–2010 (p < 0.005) and in 2020–2021 (p < 0.0001) were significantly different from that in the other years, as tested by Dunn’s multiple comparison test. Our data showed that the two honey bee species had significant differences in winter colony losses among all types of apiaries, as tested by Dunn’s multiple comparison test (p < 0.00001). Meanwhile, the loss rates of A. cerana managed by different operation sizes were significantly different (p < 0.0005). However, for A. mellifera, only the loss rates of sideline beekeepers differed significantly from those of hobby and commercial beekeepers (p < 0.00001). When comparing the overall losses of the two honey bee species managed by different operation sizes, we found that the larger apiaries of A. cerana suffered lower losses, but the opposite trend was observed for A. mellifera (Figure 5A). When examined for each individual year, the pattern was generally consistent with the annual losses for A. cerana. In contrast, the annual losses of A. mellifera from apiaries of different sizes fluctuated wildly, with different years showing different patterns (Figure 5B). Migratory beekeepers who managed A. cerana (9.76%; 95% CI: 8.95–10.62%) had lower losses than their stationary counterparts (13.58%; 95% CI: 12.95–14.24%) (p = 8.64 × 10−8). Migrated A. mellifera colonies (8.42%; 95% CI: 7.91–8.96%) suffered a lower loss rate than migrated A. cerana (9.76%; 95% CI: 8.95–10.62%) (p = 1.23 × 10−8). A. mellifera colonies without pollination (11.09%; 95% CI: 10.29–11.94%) lost nearly more than twice the number of colonies for pollination (6.62%; 95% CI: 5.80–7.54%) (p = 0.0034).
Table 3.
Total colony losses by different honey bee species in China (2009–2021).
| Year | Species | No. of Apiaries | No. of Colonies | Total Losses % (95% CI) |
|---|---|---|---|---|
| 2009–2021 | A. cerana | 4253 | 478,905 | 11.13 (10.73–11.55) |
| 2009–2010 |
A. mellifera
A. cerana |
5711 330 |
847,587 30,973 |
9.38 (8.98–9.79) 1.56 (1.10–2.21) |
| 2010–2011 |
A. mellifera
A. cerana |
345 446 |
56,575 39,779 |
2.06 (1.33–3.19) 9.85 (8.52–11.38) |
| 2011–2012 |
A. mellifera
A. cerana |
545 463 |
86,287 39,625 |
10.02 (8.80–11.39) |
| 8.99 (7.79–10.36) | ||||
| A. mellifera | 602 | 84,881 | 15.33 (13.41–17.48) | |
| 2012–2013 | A. cerana | 388 | 33,138 | 15.67 (14.11–17.37) |
| A. mellifera | 701 | 94,612 | 9.45 (8.45–10.55) | |
| 2013–2014 | A. cerana | 341 | 35,056 | 12.92 (11.54–14.43) |
| A. mellifera | 721 | 127,645 | 8.75 (7.57–10.09) | |
| 2014–2015 | A. cerana | 80 | 5554 | 12.69 (9.69–16.47) |
| A. mellifera | 75 | 12,976 | 4.08 (1.91–8.52) | |
| 2015–2016 | A. cerana | 207 | 14,981 | 16.89 (14.88–19.10) |
| A. mellifera | 452 | 71,759 | 10.14 (8.69–11.80) | |
| 2016–2017 | A. cerana | 308 | 37,090 | 8.75 (7.58–10.07) |
| A. mellifera | 282 | 32,229 | 5.32 (4.52–6.24) | |
| 2017–2018 | A. cerana | 391 | 56,232 | 9.82 (9.17–10.51) |
| A. mellifera | 660 | 85,578 | 8.28 (7.42–9.23) | |
| 2018–2019 | A. cerana | 320 | 40,435 | 12.83 (11.54–14.24) |
| A. mellifera | 198 | 24,231 | 9.46 (7.83–11.39) | |
| 2019–2020 | A. cerana | 304 | 43,774 | 9.17 (7.90–10.61) |
| A. mellifera | 276 | 34,023 | 9.53 (8.03–11.26) | |
| 2020–2021 | A. cerana | 675 | 102,268 | 14.02 (12.92–15.20) |
| A. mellifera | 854 | 136,791 | 10.56 (9.82–11.34) |
Figure 5.
Variation of winter colony losses (%, 95% CI) by different species, types of apiaries, and years (A) Total winter colony losses (%, 95% CI) of different species in different types of apiaries. (B) Apis mellifera winter colony losses (%, 95% CI) by different types of apiaries for the years (2009–2021), upper panel. Apis cerana winter colony losses (%, 95% CI) by different types of apiaries for the years (2009–2021), lower panel.
3.7. Risk Factors Attributed to Winter Colony Losses
Our analysis of risk factors comprised a total of 6825 responses, and the potential risk factors included the proportion of new queens, frequency of requeening, honey bee species, comb renewal, treatment against V. destructor, queen problems, operation size, winter food, origin of queen, migration, pollination service, nectar source, and so on. GLMMs analysis was used to evaluate the influence of risk factors on winter colony losses. Consistent with previous studies, provinces, beekeepers, and years were added to the null generalized linear mixed model as random factors. After dropping non-significant terms, a simplified model with operation size, queen problems, migration, and species was formed (Table 4 and Supplementary Material S2). Among the four factors, species×migration and species×size showed signs of interaction. As a result, we added the interaction terms and, based on BIC values, reached a final model that includes one interaction term species×migration. In Section 3.5, it is clear that the operation size significantly affected the winter loss rate. Migration was also a significant influencing factor, as those who migrated their colonies had lower loss rates. In China, colonies of A. mellifera had lower losses than colonies of A. cerana. Young queens had a positive effect on colony survival. Our analysis revealed that treatment against V. destructor was not a significant variable for colony loss, which may be caused by insufficient data. It is well known that V. destructor control failure has a significant negative effect on colony loss.
Table 4.
Risk factors for winter colony losses in the final model.
| Risk Factors | Estimate (SE) | Z Value | p |
|---|---|---|---|
| Intercept | −3.313 (0.215) | −15.373 | <2 × 10−16 *** |
| Operation Size (sideline) | 0.754 (0.081) | 9.335 | <2 × 10−16 *** |
| Operation Size (hobby) | 0.969 (0.009) | 9.793 | <2 × 10−16 *** |
| Species (A. mellifera) | −0.915 (0.084) | −10.902 | <2 × 10−16 *** |
| Migration | −0.729 (0.090) | −8.068 | 7.17 × 10−16 *** |
| Queen Problem | 0.111 (0.004) | 24.678 | <2 × 10−16 *** |
| Species (A. mellifera) × Migration | 0.436 (0.108) | 4.042 | 5.29 × 10−5 *** |
SE = Standard error; Significant codes: 0.0001 ‘***’.
4. Discussion
As one of the major beekeeping countries, China has a total of approximately 9 million managed colonies of two honey bee species, namely A. mellifera and A. cerana. Data from the Food and Agriculture Organization of the United Nations (FAO) show that the total number of honey bee colonies in China (2009–2021) is increasing [57]. In the present study, the number of responding colonies was 109,362,501, cumulatively covering 11.66% of the total honey bee colonies in China. The response rate in the present study was lower than that of other surveys, but this could be attributed to the difficulties in reaching beekeepers [9,15,16]. As Chinese beekeepers are not required to register, the availability of contact details of beekeepers is scarce. Moreover, only a small number of beekeepers were aware of the importance of the surveys and actively participated in our survey. Therefore, we acknowledge that convenient access to the questionnaires and providing rewards for participation could aid our investigations in the future.
To our knowledge, this is among the largest surveys of winter colony losses in China. A total of 13,704 Chinese beekeepers from 21 provinces reported a comparatively low overwintering mortality rate of 9.84% between 2009 and 2021 (Table 1) [58,59]. Compared with previous results, the calculated total colony losses (2009–2021) were higher than the losses reported in 2020 [28] and lower than those reported in 2016 [26] and 2017 [27]. A total loss of 12.8% was considered acceptable, and the loss rate in this survey was within the acceptable level [14]. In addition to differences in climate, management practices by Chinese beekeepers may explain the low mortality of colonies during winter. Based on the data (Figure 1C), 94% of the colonies were replaced with new queens at least once every year by 93% of beekeepers in this survey. With the high frequency and proportion of requeening by Chinese beekeepers, the stability and survival of honey bee colonies can be greatly improved by the emergence of young queens [60]. The health status of the queen can affect the development of existing colonies, including colony strength and pathogen resistance [61]. Increasing the proportion of young queens can improve colony health and increase the brood and oviposition numbers of the colonies [62].
The winter colony losses also varied among years, provinces, and operation sizes. Our results show that the annual losses in 2009–2010 and 2020–2021 were significantly different from those in other years. These differences may be caused by multiple factors, such as climate, precipitation, and nectar source, with the interacting effects of these factors. The minimum value, a 2.81% loss, was reported in 2009–2010 (our first year of the survey), and we suspect that inexperienced surveyors also affected the loss rate.
For provincial losses, different regions exhibited contrasting loss rates. The differences may be attributed to the landscapes, beekeeping practices, weather, and climate, and must be investigated in future [63,64,65]. In the present study, we received responses from Tibet for the first time, with only nine responses. Compared to other provinces, beekeeping in Tibet started late. The apiculture of Tibet is relatively backward, with a small number of managed honey bee colonies. The provincial loss of Gansu was always kept at a low level and was greatly associated with high-level beekeeping practices, which was referred to in previous surveys [26,28]. The colony loss in Gansu was significantly lower than that in the other provinces. The annual losses of six representative provinces demonstrated that the pattern of colony losses in one province remained stable during the surveyed years, which is also consistent with the findings of our previous studies [26,27]. Some provinces with low mortality, such as Gansu, tend to experience low mortality in all years in China. Such a pattern was not observed in Europe or the United States, where places with high/low colony losses varied among years [14,15,16,19,20,22]. The low mortality of Gansu may be due to its long apiculture history and excellent beekeeping skills [28].
In the present survey, most participants belonged to sideline beekeepers (69%; N = 9490), and the colonies of the hobby beekeepers only accounted for 5% of the colonies. Overall, the size of apiaries has increased in recent years across China but is still relatively small (Figure 4A). Although there were inconsistent phenomena in the annual losses of different operation sizes, the trend that a larger apiary had a lower loss can be observed, and the loss rate of commercial beekeepers was significantly lower than that of the other two types of beekeepers (Figure 4B). Colonies from large operations had a higher probability of overwinter survival, which has often been found in many other studies [23,38,66]. The following factors are likely to contribute to this trend: First, Chinese commercial beekeepers preferred to introduce good-quality queens from professional breeding institutions instead of breeding by themselves and 73% of beekeeper did this (Figure S2D). As a result, the performance of the whole population in apiaries could be adequately improved on a large scale [67,68,69]. Second, commercial beekeepers usually have excellent skills. More advanced beekeeping practices and anti-epidemic measures have been applied to commercial apiaries, reducing the risk of colony losses to some extent [11,28]. Further investigation into the management differences between small and large apiaries may help us find a way to reduce colony losses.
Considering that winter colony losses regarding A. cerana have been poorly studied, we first explored the differences in winter colony losses between A. mellifera and A. cerana. A long time ago, A. mellifera was introduced into China, and as a result, it has been tolerant to the climate of China and has become the dominant species in Chinese beekeeping [70,71]. Recently, many studies on the influence of alien honey bee species (A. mellifera) on local species (A. cerana) in food competition, disease transmission, and reproduction interference have been performed in China [72,73,74,75,76]. Meanwhile, the population size of A. cerana in China has decreased in recent years [46,51]. A. cerana may be even more threatened than A. mellifera in China. Our data showed that the total loss in A. cerana was significantly higher than that in A. mellifera. The annual losses of A. mellifera and A. cerana both differed from year to year. Regarding the loss rates of A. mellifera and A. cerana in different apiaries, colonies of A. mellifera with a large operation size had high losses, whereas colonies of A. cerana with a large operation size had low losses. These patterns were consistent with our previous results but contrasted with our previous study [28], which only covered a fraction of the data on A. mellifera honey bee colony losses used in the current study. Extending the survey to more years showed that the pattern of A. mellifera varied among years (Figure 5). The underlying reasons for the different patterns of colony winter loss between A. mellifera and A. cerana need to be studied further and identified. Additionally, beekeepers who migrated had significantly lower losses, and A. mellifera colonies used for pollination had significantly lower losses. Migration can increase the probability of colony overwinter survival, and pollination can increase the probability of colony overwinter survival [21,24], perhaps because these two operations contribute to acquiring sufficient and/or diverse food for honey bee colonies. Simone-Finstrom et al. showed that migrating colonies to agricultural areas with good nutrition can reduce oxidative stress in honey bees [77]. However, many studies have demonstrated that migratory pollination practices have varying health effects on honey bee colonies [78]. More research is necessary to explore the impact of migratory beekeeping on bee health, not only on pollinator health but also on food security [79,80]. As for the pollination, previous work found that the effect of pollination on honey bee health was heterogeneous, probably due to several uncontrolled underlying factors such as beekeeping management, species of pollinated crops, parasites, and pathogens [81,82,83]. It is a pity that detailed information about pollination was not included in our questionnaires, which limited our survey. In the future, we will make more of an effort to investigate pollination.
It was estimated by the GLMMs analysis that the significant risk factors were the operation size, species, migration, migration×species interaction, and queen problems. As mentioned previously, Chinese apiaries with large operation sizes showed significantly lower winter mortality. This is in accordance with the survey results of other countries [11,28,66]. Large operation sizes represent high-level beekeeping management practices and strong honey bee colonies, contributing to the overwintering of colonies. Many previous results have demonstrated that queen problems are significantly associated with winter colony losses, which was also observed in this survey [37,84,85]. A healthy queen was the most important factor affecting colony winter survival. A healthy queen can maintain the health status of the colonies and provide better control over swarming. Different honey bee species also had a significant influence on colony loss. Significantly lower losses were observed for A. mellifera in China. A. mellifera and A. cerana have some similarities but strongly differ in many aspects, such as their resistance to V. destructor [49,86], which may underlie the differences in colony survival [29]. Consistent with previous studies [21,23,66], our analysis revealed that migratory beekeepers experienced a lower loss rate. Comparing the percentage of migratory beekeepers with the percentage of beekeepers for pollination, we found that Chinese beekeepers migrated to their colonies mainly for honey harvest—not for pollination. Although migration increases the possibility of exposure to pathogens and pesticides, it also gives the colonies more access to better foraging sources. In addition, migratory beekeepers are often more well-trained and experienced. In many previous studies, migration was not always recognized as a significant factor in reducing colony losses [13]. However, this effect has been reported in several surveys [21,23,67]. Migration and species interaction also exists, possibly because A. cerana beekeeping is mainly stationary. It is well known that V. destructor can seriously lower the chance of colony survival, especially A. mellifera, and sometimes the damage is devastating [10,87,88]. However, treatment with V. destructor did not significantly correlate with colony loss in our GLMMs analysis. As shown in Figure S2A, 99% of Chinese beekeepers who managed A. mellifera treated their colonies against Varroa mites, and the number of colonies untreated in the survey may be insufficient to show the statistical significance of the effect of Varroa treatment on colony losses.
As for A. cerana, the Chinese COLOSS questionnaires were not designed specifically for A. cerana. Many questions about the unique traits of this species were not designed to be specific to our study. As a result, some important information may have been missed, and this something which we need to improve on in future surveys. Some questions about beekeeping styles (traditional or movable-frame hive), sacbrood disease, and honey yield could be included into our future COLOSS questionnaires specific to A. cerana.
5. Conclusions
Honey bee decline has been widely reported in recent years, raising concerns worldwide. Our China-based colony loss survey involved the highest number of colonies to date. Our survey showed the overall losses of managed honey bee colonies from 21 provinces in China between 2009 and 2021, and a relatively low mortality (below the world average level) was reported. Colony losses varied among years, provinces, and types of apiaries. Apiaries with larger operation sizes suffered lower colony losses. Additionally, to the best of our knowledge, this is the first national survey to reveal the differences in the pattern of winter colony losses between A. mellifera and A. cerana. Using GLMMs analysis, we explored the effects of risk factors on winter colony losses and found that queen problems, operation size, species, migration, and migration×species interaction were the primary risk factors that significantly affected the overwintering mortality of honey bees in China. Although treatment against V. destructor was not identified in our analysis because of insufficient data, V. destructor control is likely to be important for A. mellifera survival during winter. Future studies should increase response rates and include more refined information, and more efforts are needed to further investigate honey bee colony loss. The findings of this surveillance study provide more reliable and abundant insights into the health status of honey bee colonies in China and the pattern of winter colony losses in China.
Acknowledgments
We appreciate all the beekeepers and surveyors for providing the original data for this survey. We also thank Hongxia Zhao, Yuexiong Luo, Zhaluo, Shidong Liu, Wenhua Luo, Qingsheng Niu, Junjun Hu, Wenzhong Qi, Chunying Yuan, Yingsheng Zhang, Xuewen Zhang, Ting Ji, Zhongyin Zhang, Shunhai Wang, Fuchao Gao, Jinglin Gao, Rongguo Dai, and other members of the beekeeping organizations and apicultural management agencies of various provinces for their participation.
Abbreviations
| COLOSS | prevention of honey bee COlony LOSSes. |
| FAO | the Food and Agriculture Organization of the United Nations |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14060554/s1, the file Supplementary Materials S1 (S1), Figure S1: The proportion of respondents/colonies surveyed in different years; Figure S2: (A) The proportion of respondents/colonies with V. destructor control or without V. destructor control. (B) Distribution of respondents/colonies with new combs, with every 10% of new combs divided into one group. (C) Proportion of migrated and pollinated respondents/colonies in the survey. (D) The proportion of respondents in different types of apiaries with three origins of queens; Table S1: Annual winter colony losses (%, CI: 95%) in Chongqing, Gansu, Guangdong, Guangxi, Jiangxi, and Zhejiang provinces of China (2017–2021). Figure S3: The proportion of respondents/colonies with queen problem in different types of apiaries. Supplementary Material S2 (S2) and GLMM model selection results.
Author Contributions
Conceptualization, J.T.; Methodology, C.C.; Validation, C.J., W.S., X.C. and Y.Z.; Formal analysis, J.T., C.J. and C.C.; Investigation, W.S., S.S., Y.X., J.X., X.C. and Y.Z.; Data curation, C.J., S.S., Y.X. and J.X.; Writing—original draft, J.T.; Visualization, C.C.; Project administration, W.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors. The data are not publicly available due to privacy concerns and potential political sensitivities.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the National Natural Science Foundation of China (Grant Nos. 32202740 and 31902219) and the earmarked fund for the China Agriculture Research System (Grant No. CARS-44-KXJ1), and Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2022-IAR).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Khalifa S.A.M., Elshafiey E.H., Shetaia A.A., El-Wahed A.A.A., Algethami A.F., Musharraf S.G., AlAjmi M.F., Zhao C., Masry S.H.D., Abdel-Daim M.M., et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects. 2021;12:688. doi: 10.3390/insects12080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein A.M., Vaissiere B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C., Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dicks L.V., Breeze T.D., Ngo H.T., Senapathi D., An J., Aizen M.A., Basu P., Buchori D., Galetto L., Garibaldi L.A., et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 2021;5:1453–1461. doi: 10.1038/s41559-021-01534-9. [DOI] [PubMed] [Google Scholar]
- 4.Goulson D., Nicholls E., Botias C., Rotheray E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 5.Mashilingi S.K., Zhang H., Garibaldi L.A., An J. Honeybees are far too insufficient to supply optimum pollination services in agricultural systems worldwide. Agric. Ecosyst. Environ. 2022;335:108003. doi: 10.1016/j.agee.2022.108003. [DOI] [Google Scholar]
- 6.Ratnieks F.L., Carreck N.L. Ecology. Clarity on honey bee collapse? Science. 2010;327:152–153. doi: 10.1126/science.1185563. [DOI] [PubMed] [Google Scholar]
- 7.Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Johannesen J., Wohl S., Berg S., Otten C. Annual Fluctuations in Winter Colony Losses of Apis mellifera L. Are Predicted by Honey Flow Dynamics of the Preceding Year. Insects. 2022;13:829. doi: 10.3390/insects13090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahlmann-Brown P., Hall R.J., Pragert H., Robertson T. Varroa Appears to Drive Persistent Increases in New Zealand Colony Losses. Insects. 2022;13:589. doi: 10.3390/insects13070589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Zee R., Gray A., Pisa L., de Rijk T. An Observational Study of Honey Bee Colony Winter Losses and Their Association with Varroa destructor, Neonicotinoids and Other Risk Factors. PLoS ONE. 2015;10:e0131611. doi: 10.1371/journal.pone.0131611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morawetz L., Koglberger H., Griesbacher A., Derakhshifar I., Crailsheim K., Brodschneider R., Moosbeckhofer R. Health status of honey bee colonies (Apis mellifera) and disease-related risk factors for colony losses in Austria. PLoS ONE. 2019;14:e0219293. doi: 10.1371/journal.pone.0219293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Engelsdorp D., Underwood R., Caron D., Hayes J. An Estimate of Managed Colony Losses in the Winter of 2006–2007 A Report Commissioned by the Apiary Inspectors of America. Am. Bee J. 2007;147:599–603. [Google Scholar]
- 13.van Engelsdorp D., Hayes J., Jr., Underwood R.M., Pettis J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE. 2008;3:e4071. doi: 10.1371/journal.pone.0004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhauer N.A., Rennich K., Wilson M.E., Caron D.M., Lengerich E.J., Pettis J.S., Rose R., Skinner J.A., Tarpy D.R., Wilkes J.T., et al. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2014;53:1–18. doi: 10.3896/IBRA.1.53.1.01. [DOI] [Google Scholar]
- 15.Lee K.V., Steinhauer N., Rennich K., Wilson M.E., Tarpy D.R., Caron D.M., Rose R., Delaplane K.S., Baylis K., Lengerich E.J., et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie. 2015;46:292–305. doi: 10.1007/s13592-015-0356-z. [DOI] [Google Scholar]
- 16.Kulhanek K., Steinhauer N., Rennich K., Caron D.M., Sagili R.R., Pettis J.S., Ellis J.D., Wilson M.E., Wilkes J.T., Tarpy D.R., et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017;56:328–340. doi: 10.1080/00218839.2017.1344496. [DOI] [Google Scholar]
- 17.Steinhauer N.A.D., Bruckner S., Wilson M., Rennich K. United States Honey Bee Colony Losses 2020–2021: Preliminary Results Embargoed until Wednesday, 23 June 2021, 12.00 PM Noon CST. [(accessed on 13 May 2022)]. Available online: https://beeinformed.org/2021/06/21/united-states-honey-bee-colony-losses-2020-2021-preliminary-results/
- 18.Ellis J.D., Evans J.D., Pettis J. Colony losses, managed colony population decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 2010;49:134–136. doi: 10.3896/IBRA.1.49.1.30. [DOI] [Google Scholar]
- 19.Brodschneider R., Gray A., Adjlane N., Ballis A., Brusbardis V., Charrière J.-D., Chlebo R., Coffey M.F., Dahle B., de Graaf D.C., et al. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apic. Res. 2018;57:452–457. doi: 10.1080/00218839.2018.1460911. [DOI] [Google Scholar]
- 20.Brodschneider R., Gray A., van der Zee R., Adjlane N., Brusbardis V., Charrière J.-D., Chlebo R., Coffey M.F., Crailsheim K., Dahle B., et al. Preliminary analysis of loss rates of honey bee colonies during winter 2015/16 from the COLOSS survey. J. Apic. Res. 2016;55:375–378. doi: 10.1080/00218839.2016.1260240. [DOI] [Google Scholar]
- 21.Gray A., Adjlane N., Arab A., Ballis A., Brusbardis V., Bugeja Douglas A., Cadahía L., Charrière J.-D., Chlebo R., Coffey M.F., et al. Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: The combined effects of operation size, migration and queen replacement. J. Apic. Res. 2022;62:204–210. doi: 10.1080/00218839.2022.2113329. [DOI] [Google Scholar]
- 22.Gray A., Adjlane N., Arab A., Ballis A., Brusbardis V., Charrière J.-D., Chlebo R., Coffey M.F., Cornelissen B., Amaro da Costa C., et al. Honey bee colony winter loss rates for 35 countries participating in the COLOSS survey for winter 2018–2019, and the effects of a new queen on the risk of colony winter loss. J. Apic. Res. 2020;59:744–751. doi: 10.1080/00218839.2020.1797272. [DOI] [Google Scholar]
- 23.Gray A., Brodschneider R., Adjlane N., Ballis A., Brusbardis V., Charrière J.-D., Chlebo R., Coffey M.F., Cornelissen B., Amaro da Costa C., et al. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apic. Res. 2019;58:479–485. doi: 10.1080/00218839.2019.1615661. [DOI] [Google Scholar]
- 24.Van Der Zee R., Brodschneider R., Brusbardis V., Charrière J.-D., Chlebo R., Coffey M.F., Dahle B., Drazic M.M., Kauko L., Kretavicius J., et al. Results of international standardised beekeeper surveys of colony losses for winter 2012–2013: Analysis of winter loss rates and mixed effects modelling of risk factors for winter loss. J. Apic. Res. 2014;53:19–34. doi: 10.3896/IBRA.1.53.1.02. [DOI] [Google Scholar]
- 25.van der Zee R., Pisa L., Andonov S., Brodschneider R., Charrière J.-D., Chlebo R., Coffey M.F., Crailsheim K., Dahle B., Gajda A., et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–9 and 2009–10. J. Apic. Res. 2012;51:100–114. doi: 10.3896/IBRA.1.51.1.12. [DOI] [Google Scholar]
- 26.Liu Z., Chen C., Niu Q., Qi W., Yuan C., Su S., Liu S., Zhang Y., Zhang X., Ji T., et al. Survey results of honey bee (Apis mellifera) colony losses in China (2010–2013) J. Apic. Res. 2016;55:29–37. doi: 10.1080/00218839.2016.1193375. [DOI] [Google Scholar]
- 27.Chen C., Liu Z., Luo Y., Xu Z., Wang S., Zhang X., Dai R., Gao J., Chen X., Guo H., et al. Managed honeybee colony losses of the Eastern honeybee (Apis cerana) in China (2011–2014) Apidologie. 2017;48:692–702. doi: 10.1007/s13592-017-0514-6. [DOI] [Google Scholar]
- 28.Tang J., Ma C., Shi W., Chen X., Liu Z., Wang H., Chen C. A National Survey of Managed Honey Bee Colony Winter Losses (Apis mellifera) in China (2013–2017) Diversity. 2020;12:318. doi: 10.3390/d12090318. [DOI] [Google Scholar]
- 29.Pirk C.W.W., Human H., Crewe R.M., vanEngelsdorp D. A survey of managed honey bee colony losses in the Republic of South Africa–2009 to 2011. J. Apic. Res. 2014;53:35–42. doi: 10.3896/IBRA.1.53.1.03. [DOI] [Google Scholar]
- 30.Spleen A.M., Lengerich E.J., Rennich K., Caron D., Rose R., Pettis J.S., Henson M., Wilkes J.T., Wilson M., Stitzinger J., et al. A national survey of managed honey bee 2011–12 winter colony losses in the United States: Results from the Bee Informed Partnership. J. Apic. Res. 2013;52:44–53. doi: 10.3896/IBRA.1.52.2.07. [DOI] [Google Scholar]
- 31.vanEngelsdorp D., Caron D., Hayes J., Underwood R., Henson M., Rennich K., Spleen A., Andree M., Snyder R., Lee K., et al. A national survey of managed honey bee 2010–11 winter colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2012;51:115–124. doi: 10.3896/IBRA.1.51.1.14. [DOI] [Google Scholar]
- 32.Currie R.W., Pernal S.F., Guzmán-Novoa E. Honey bee colony losses in Canada. J. Apic. Res. 2010;49:104–106. doi: 10.3896/IBRA.1.49.1.18. [DOI] [Google Scholar]
- 33.Castilhos D., Bergamo G.C., Gramacho K.P., Gonçalves L.S. Bee colony losses in Brazil: A 5-year online survey. Apidologie. 2019;50:263–272. doi: 10.1007/s13592-019-00642-7. [DOI] [Google Scholar]
- 34.Requier F., Antúnez K., Morales C.L., Aldea Sánchez P., Castilhos D., Garrido P.M., Giacobino A., Reynaldi F.J., Rosso Londoño J.M., Santos E., et al. Trends in beekeeping and honey bee colony losses in Latin America. J. Apic. Res. 2018;57:657–662. doi: 10.1080/00218839.2018.1494919. [DOI] [Google Scholar]
- 35.Requier F., Antúnez K., Aldea P., Castilhos D., Garrido M., Giacobino A., Morales C., Reynaldi F., Rosso J., Santos E., et al. Honey bee colony losses in Latin America over the last seven years; Proceedings of the 13th COLOSS Conference; Athens, Greece. 2–3 November 2017. [Google Scholar]
- 36.Genersch E., von der Ohe W., Kaatz H., Schroeder A., Otten C., Büchler R., Berg S., Ritter W., Mühlen W., Gisder S., et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie. 2010;41:332–352. doi: 10.1051/apido/2010014. [DOI] [Google Scholar]
- 37.Brodschneider R., Moosbeckhofer R., Crailsheim K. Surveys as a tool to record winter losses of honey bee colonies: A two year case study in Austria and South Tyrol. J. Apic. Res. 2010;49:23–30. doi: 10.3896/IBRA.1.49.1.04. [DOI] [Google Scholar]
- 38.Porrini C., Mutinelli F., Bortolotti L., Granato A., Laurenson L., Roberts K., Gallina A., Silvester N., Medrzycki P., Renzi T., et al. The Status of Honey Bee Health in Italy: Results from the Nationwide Bee Monitoring Network. PLoS ONE. 2016;11:e015541110. doi: 10.1371/journal.pone.0155411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown P., Newstrom-Lloyd L.E., Foster B.J., Badger P.H., McLean J.A. Winter 2016 honey bee colony losses in New Zealand. J. Apic. Res. 2018;57:278–291. doi: 10.1080/00218839.2018.1430980. [DOI] [Google Scholar]
- 40.Döke M.A., Frazier M., Grozinger C.M. Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 2015;10:185–193. doi: 10.1016/j.cois.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Giampieri F., Quiles J.L., Cianciosi D., Forbes-Hernandez T.Y., Orantes-Bermejo F.J., Alvarez-Suarez J.M., Battino M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022;70:6833–6848. doi: 10.1021/acs.jafc.1c05822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tauber J.P., Collins W.R., Schwarz R.S., Chen Y., Grubbs K., Huang Q., Lopez D., Peterson R., Evans J.D. Natural Product Medicines for Honey Bees: Perspective and Protocols. Insects. 2019;10:356. doi: 10.3390/insects10100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su S.K., Chen S.L. The beekeeping and ecology. J. Bee. 2009;1:8–10. [Google Scholar]
- 44.Yancan L., Tianle C., Yunhan F., Delong L., Guizhi W. Population genomics and morphological features underlying the adaptive evolution of the eastern honey bee (Apis cerana) BMC Genomics. 2019;20:869. doi: 10.1186/s12864-019-6246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The National Animal Genetic Resources Committee . Animal Genetic Resources in China Bees. China Agriculture Press; Beijing, China: 2011. pp. 8–9. [Google Scholar]
- 46.Theisen-Jones H., Bienefeld K. The Asian Honey Bee (Apis cerana) is significantly in Decline. Bee World. 2016;93:90–97. doi: 10.1080/0005772X.2017.1284973. [DOI] [Google Scholar]
- 47.Chen C., Liu Z., Pan Q., Chen X., Wang H., Guo H., Liu S., Lu H., Tian S., Li R., et al. Genomic Analyses Reveal Demographic History and Temperate Adaptation of the Newly Discovered Honey Bee Subspecies Apis mellifera sinisxinyuan n. ssp. Mol. Biol. Evol. 2016;33:1337–1348. doi: 10.1093/molbev/msw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan K., Yang S., Wang Z.-W., Radloff S.E., Oldroyd B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie. 2012;43:618–623. doi: 10.1007/s13592-012-0136-y. [DOI] [Google Scholar]
- 49.Park D., Jung J.W., Choi B.S., Jayakodi M., Lee J., Lim J., Yu Y., Choi Y.S., Lee M.L., Park Y., et al. Uncovering the novel characteristics of Asian honey bee, Apis cerana, by whole genome sequencing. BMC Genom. 2015;16:1. doi: 10.1186/1471-2164-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Ma W., Shen J., Long D., Feng Y., Su W., Xu K., Du Y., Jiang Y. Tolerance and response of two honeybee species Apis cerana and Apis mellifera to high temperature and relative humidity. PLoS ONE. 2019;14:e0217921. doi: 10.1371/journal.pone.0217921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou C., Li B., Luo Y., Deng S., Diao Q. First detection of Apis mellifera filamentous virus in Apis cerana cerana in China. J. Invertebr. Pathol. 2016;138:112–115. doi: 10.1016/j.jip.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Chen F., Chen W., Xie Y., Tang X. Visiting behavior of Apis cerana on rape and characteristics if flowering and seed setting in the early spring. Southwest China J. Agric. Sci. 2015;2:498–503. [Google Scholar]
- 53.van der Zee R., Gray A., Holzmann C., Pisa L., Brodschneider R., Chlebo R., Coffey M.F., Kence A., Kristiansen P., Mutinelli F., et al. Standard survey methods for estimating colony losses and explanatory risk factors in Apis mellifera. J. Apic. Res. 2013;52:1–36. doi: 10.3896/IBRA.1.52.4.18. [DOI] [Google Scholar]
- 54.Team R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Google Scholar]
- 55.Ogle D.H., Doll J.C., Wheeler P., Dinno A. FSA: Fisheries Stock Analysis; R package version 0.9.3.9000. 2022. [(accessed on 15 January 2023)]. Available online: https://fishr-core-team.github.io/FSA/
- 56.Bates D., Machler M., Bolker B.M., Walker S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 57.FAOSTAT Food and Agriculture Organization Corporate Statistical Database. 2022. [(accessed on 15 January 2023)]. Available online: http://www.fao.org/faostat/en/#home.
- 58.Antúnez K., Invernizzi C., Mendoza Y., vanEngelsdorp D., Zunino P. Honeybee colony losses in Uruguay during 2013–2014. Apidologie. 2016;48:364–370. doi: 10.1007/s13592-016-0482-2. [DOI] [Google Scholar]
- 59.Brodschneider R., Brus J., Danihlik J. Comparison of apiculture and winter mortality of honey bee colonies (Apis mellifera) in Austria and Czechia. Agric. Ecosyst. Environ. 2019;274:24–32. doi: 10.1016/j.agee.2019.01.002. [DOI] [Google Scholar]
- 60.Ricigliano V.A., Mott B.M., Floyd A.S., Copeland D.C., Carroll M.J., Anderson K.E. Honey bees overwintering in a southern climate: Longitudinal effects of nutrition and queen age on colony-level molecular physiology and performance. Sci. Rep. 2018;8:10475. doi: 10.1038/s41598-018-28732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akyol E., Yeninar H., Karatepe M., Karatepe B., Ozkok D. Effects of queen ages on Varroa (Varroa destructor) infestationlevel in honey bee (Apis mellifera caucasica) colonies and colony performance. Ital. J. Anim. Sci. 2016;6:143–149. doi: 10.4081/ijas.2007.143. [DOI] [Google Scholar]
- 62.Simeunovic P., Stevanovic E., Cirkovic D., Radojicic S., Lakic N., Stanisic L., Stanimirovic Z. Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. J. Apic. Res. 2015;53:545–554. doi: 10.3896/IBRA.1.53.5.09. [DOI] [Google Scholar]
- 63.Buchler R., Costa C., Hatjina F., Andonov S., Meixner M.D., Le Conte Y., Uzunov A., Berg S., Bienkowska M., Bouga M., et al. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J. Apic. Res. 2014;53:205–214. doi: 10.3896/IBRA.1.53.2.03. [DOI] [Google Scholar]
- 64.El Agrebi N., Steinhauer N., Tosi S., Leinartz L., de Graaf D.C., Saegerman C. Risk and protective indicators of beekeeping management practices. Sci. Total Environ. 2021;799:149381. doi: 10.1016/j.scitotenv.2021.149381. [DOI] [PubMed] [Google Scholar]
- 65.Belsky J., Joshi N.K. Impact of Biotic and Abiotic Stressors on Managed and Feral Bees. Insects. 2019;10:233. doi: 10.3390/insects10080233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oberreiter H., Brodschneider R. Austrian COLOSS Survey of Honey Bee Colony Winter Losses 2018/19 and Analysis of Hive Management Practices. Diversity. 2020;12:99. doi: 10.3390/d12030099. [DOI] [Google Scholar]
- 67.Steinhauer N., Kulhanek K., Antunez K., Human H., Chantawannakul P., Chauzat M.P., vanEngelsdorp D. Drivers of colony losses. Curr. Opin. Insect Sci. 2018;26:142–148. doi: 10.1016/j.cois.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Amiri E., Strand M.K., Rueppell O., Tarpy D.R. Queen Quality and the Impact of Honey Bee Diseases on Queen Health: Potential for Interactions between Two Major Threats to Colony Health. Insects. 2017;8:48. doi: 10.3390/insects8020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattila H.R., Seeley T.D. Genetic diversity in honey bee colonies enhances productivity and fitness. Science. 2007;317:362–364. doi: 10.1126/science.1143046. [DOI] [PubMed] [Google Scholar]
- 70.Ma W., Li X., Shen J., Du Y., Xu K., Jiang Y. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotoxicol. Environ. Saf. 2019;184:109599. doi: 10.1016/j.ecoenv.2019.109599. [DOI] [PubMed] [Google Scholar]
- 71.Yang G. The effect of introducing the western honey bee Apis mellifera L. to the Chinese honey bee Apis cerana F. and its ecological impact. Acta Entomol. Sin. 2005;3:401–406. [Google Scholar]
- 72.Yang W., Kuang H., Wang S., Wang J., Liu W., Wu Z., Tian Y., Huang Z.Y., Miao X. Comparative sucrose responsiveness in Apis mellifera and A. cerana foragers. PLoS ONE. 2013;8:e79026. doi: 10.1371/journal.pone.0079026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Remnant E.J., Koetz A., Tan K., Hinson E., Beekman M., Oldroyd B.P. Reproductive interference between honeybee species in artificial sympatry. Mol. Ecol. 2014;23:1096–1107. doi: 10.1111/mec.12669. [DOI] [PubMed] [Google Scholar]
- 74.Gong H.R., Chen X.X., Chen Y.P., Hu F.L., Zhang J.L., Lin Z.G., Yu J.W., Zheng H.Q. Evidence of Apis cerana Sacbrood virus Infection in Apis mellifera. Appl. Environ. Microbiol. 2016;82:2256–2262. doi: 10.1128/AEM.03292-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang J.C., Chang Z.T., Ko C.Y., Scotty Yang C.C., Chen Y.W., Nai Y.S. Sacbrood viruses cross-infection between Apis cerana and Apis mellifera: Rapid detection, viral dynamics, evolution and spillover risk assessment. J. Invertebr. Pathol. 2021;186:107687. doi: 10.1016/j.jip.2021.107687. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y., Ma S., Yan Z., Liu F., Diao Q., Dai P. Effects of three common pesticides on survival, food consumption and midgut bacterial communities of adult workers Apis cerana and Apis mellifera. Environ. Pollut. 2019;249:860–867. doi: 10.1016/j.envpol.2019.03.077. [DOI] [PubMed] [Google Scholar]
- 77.Simone-Finstrom M., Li-Byarlay H., Huang M.H., Strand M.K., Rueppell O., Tarpy D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016;6:32023. doi: 10.1038/srep32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simone-Finstrom M., Strand M.K., Tarpy D.R., Rueppell O. Impact of Honey Bee Migratory Management on Pathogen Loads and Immune Gene Expression is Affected by Complex Interactions With Environment, Worker Life History, and Season. J. Insect Sci. 2022;22:1–10. doi: 10.1093/jisesa/ieab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alger S.A., Burnham P.A., Lamas Z.S., Brody A.K., Richardson L.L. Home sick: Impacts of migratory beekeeping on honey bee (Apis mellifera) pests, pathogens, and colony size. PeerJ. 2018;6:e5812. doi: 10.7717/peerj.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Lopez V., Ruiz C., De la Rua P. Migratory beekeeping and its influence on the prevalence and dispersal of pathogens to managed and wild bees. Int. J. Parasitol. Parasites Wildl. 2022;18:184–193. doi: 10.1016/j.ijppaw.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dufour C., Fournier V., Giovenazzo P. The impact of lowbush blueberry (Vaccinium angustifolium Ait.) and cranberry (Vaccinium macrocarpon Ait.) pollination on honey bee (Apis mellifera L.) colony health status. PLoS ONE. 2020;15:e0227970. doi: 10.1371/journal.pone.0227970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Seedi H.R., Ahmed H.R., El-Wahed A.A.A., Saeed A., Algethami A.F., Attia N.F., Guo Z., Musharraf S.G., Khatib A., Alsharif S.M., et al. Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health. Vet. Sci. 2022;9:199. doi: 10.3390/vetsci9050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ricigliano V.A., Mott B.M., Maes P.W., Floyd A.S., Fitz W., Copeland D.C., Meikle W.G., Anderson K.E. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019;9:4894. doi: 10.1038/s41598-019-41281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.vanEngelsdorp D., Tarpy D.R., Lengerich E.J., Pettis J.S. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 2013;108:225–233. doi: 10.1016/j.prevetmed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 85.Gajger I.T., Tomljanović Z., Petrinec Z. Monitoring health status of Croatian honey bee colonies and possible reasons for winter losses. J. Apic. Res. 2010;49:107–108. doi: 10.3896/IBRA.1.49.1.19. [DOI] [Google Scholar]
- 86.Ji T., Yin L., Liu Z., Liang Q., Luo Y., Shen J., Shen F. Transcriptional responses in eastern honeybees (Apis cerana) infected with mites. Varroa Destructor. Genet. Mol. Res. 2014;13:8888–8900. doi: 10.4238/2014.October.31.4. [DOI] [PubMed] [Google Scholar]
- 87.Beyer M., Junk J., Eickermann M., Clermont A., Kraus F., Georges C., Reichart A., Hoffmann L. Winter honey bee colony losses, Varroa destructor control strategies, and the role of weather conditions: Results from a survey among beekeepers. Res. Vet. Sci. 2018;118:52–60. doi: 10.1016/j.rvsc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 88.Brodschneider R., Schlagbauer J., Arakelyan I., Ballis A., Brus J., Brusbardis V., Cadahía L., Charrière J.-D., Chlebo R., Coffey M.F., et al. Spatial clusters of Varroa destructor control strategies in Europe. J. Pest Sci. 2022;96:759–783. doi: 10.1007/s10340-022-01523-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors. The data are not publicly available due to privacy concerns and potential political sensitivities.