Abstract
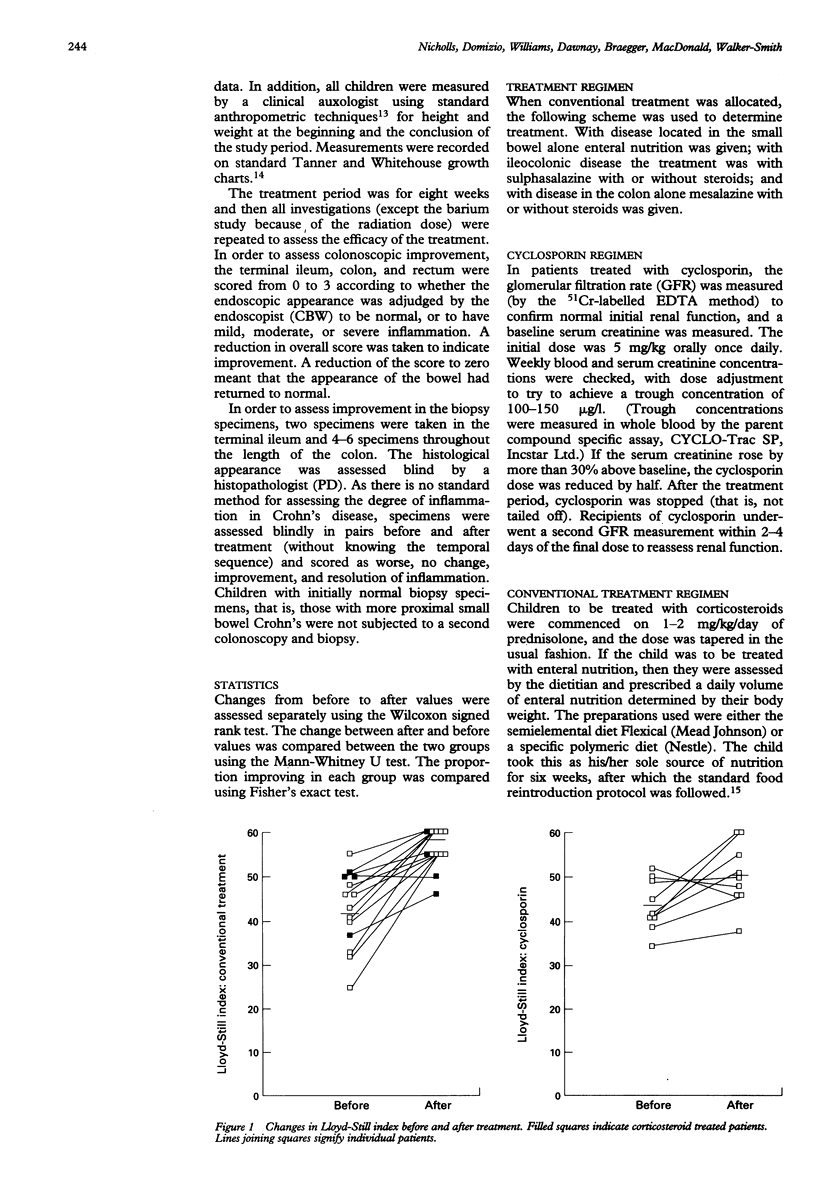
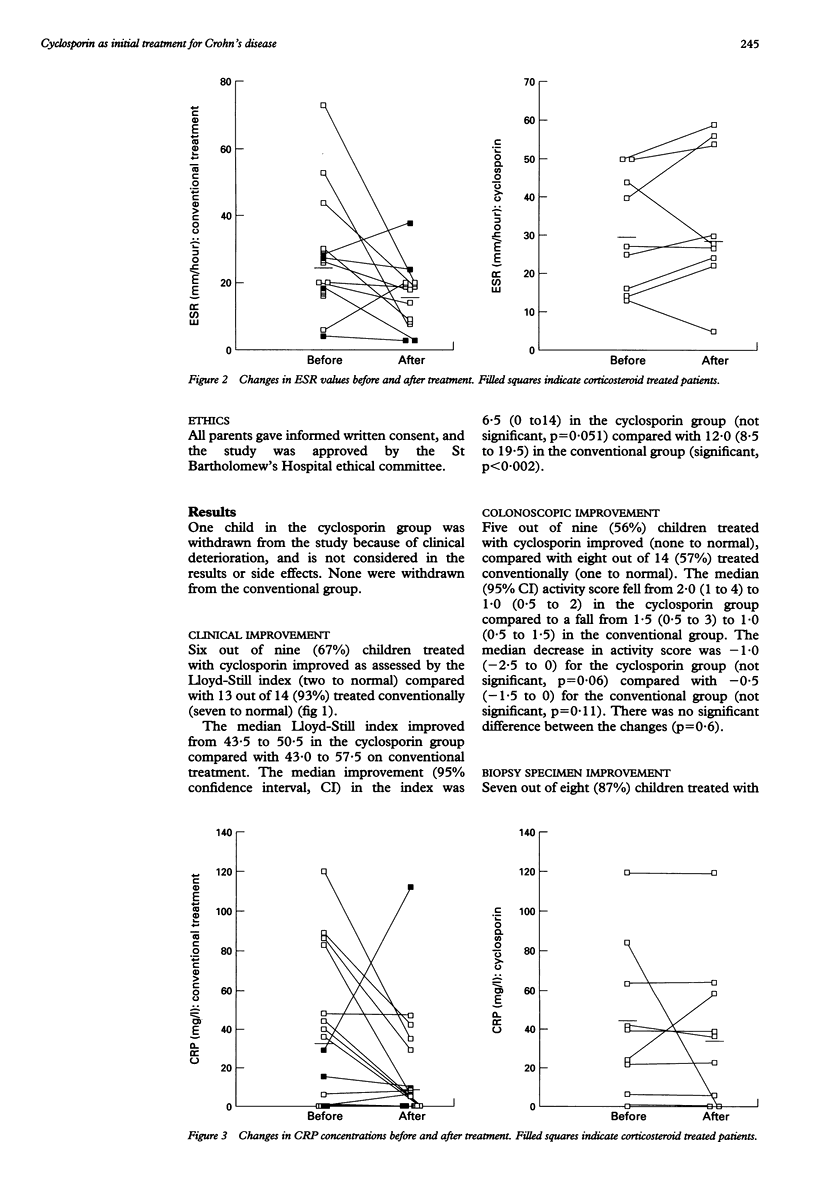
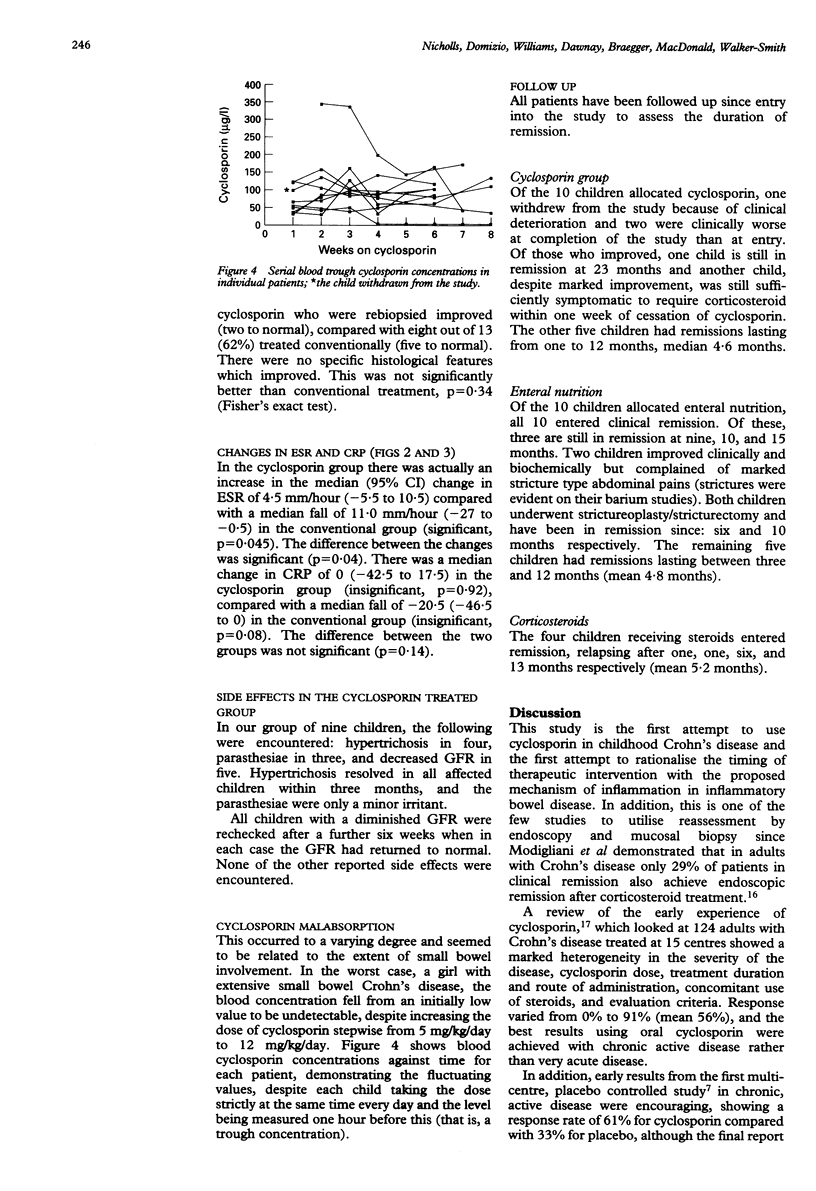
Childhood Crohn's disease may cause significant morbidity. T cell activation is considered to be central to Crohn's disease pathology, and as cyclosporin is a powerful inhibitor of T cell activation, and has been used in adult Crohn's disease with encouraging results, it may offer the prospect of remission if given early in the course of disease. Children with newly diagnosed Crohn's disease or those relapsing off treatment were therefore given cyclosporin or conventional treatment (enteral nutrition or corticosteroids) by random allocation. Evaluation was performed initially and at two months. Twenty four children were studied (10 on cyclosporin and 14 on conventional treatment; one child on cyclosporin withdrew). Significant clinical improvement occurred in the group on conventional treatment, but not in the cyclosporin group. Colonoscopic improvement was noted in 5/9 on cyclosporin and 8/14 on conventional treatment, but neither group produced a significant fall in median colonoscopic index. Histological improvement was seen in 7/8 on cyclosporin and 8/13 on conventional treatment, but cyclosporin was not significantly better. Cyclosporin produced improved clinical and histological appearance without matched improvement in blood disease indices. It was not better than conventional treatment, and simple oral administration is probably not suitable for newly diagnosed patients with Crohn's disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Pounder R. E. Cyclosporin for Crohn's disease. Aliment Pharmacol Ther. 1987 Feb;1(1):39–43. doi: 10.1111/j.1365-2036.1987.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Baker K., Jewell D. P. Cyclosporin for the treatment of severe inflammatory bowel disease. Aliment Pharmacol Ther. 1989 Apr;3(2):143–149. doi: 10.1111/j.1365-2036.1989.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Gunn H. C. Cyclosporine as a new approach to therapy of autoimmune diseases. Ann N Y Acad Sci. 1986;475:307–319. doi: 10.1111/j.1749-6632.1986.tb20879.x. [DOI] [PubMed] [Google Scholar]
- Brynskov J., Freund L., Nørby Rasmussen S., Lauritsen K., Schaffalitzky de Muckadell O., Williams C. N., MacDonald A. S., Tanton R., Molina F., Campanini M. C. Final report on a placebo-controlled, double-blind, randomized, multicentre trial of cyclosporin treatment in active chronic Crohn's disease. Scand J Gastroenterol. 1991 Jul;26(7):689–695. doi: 10.3109/00365529108998585. [DOI] [PubMed] [Google Scholar]
- Brynskov J., Freund L., Rasmussen S. N., Lauritsen K., de Muckadell O. S., Williams N., MacDonald A. S., Tanton R., Molina F., Campanini M. C. A placebo-controlled, double-blind, randomized trial of cyclosporine therapy in active chronic Crohn's disease. N Engl J Med. 1989 Sep 28;321(13):845–850. doi: 10.1056/NEJM198909283211301. [DOI] [PubMed] [Google Scholar]
- Feutren G., von Graffenried B. Pharmacology of cyclosporin A (Sandimmun) and clinical experience in inflammatory bowel diseases. Schweiz Med Wochenschr. 1991 May 18;121(20):748–753. [PubMed] [Google Scholar]
- Jewell D. P., Patel C. Immunology of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1985;114:119–126. doi: 10.3109/00365528509093772. [DOI] [PubMed] [Google Scholar]
- Lloyd-Still J. D., Green O. C. A clinical scoring system for chronic inflammatory bowel disease in children. Dig Dis Sci. 1979 Aug;24(8):620–624. doi: 10.1007/BF01333706. [DOI] [PubMed] [Google Scholar]
- Maglinte D. D., Burney B. T., Miller R. E. Lesions missed on small-bowel follow-through: analysis and recommendations. Radiology. 1982 Sep;144(4):737–739. doi: 10.1148/radiology.144.4.7111717. [DOI] [PubMed] [Google Scholar]
- Masuda K., Nakajima A., Urayama A., Nakae K., Kogure M., Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet's disease. Lancet. 1989 May 20;1(8647):1093–1096. doi: 10.1016/s0140-6736(89)92381-7. [DOI] [PubMed] [Google Scholar]
- Modigliani R., Mary J. Y., Simon J. F., Cortot A., Soule J. C., Gendre J. P., Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990 Apr;98(4):811–818. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Parrott N. R., Taylor R. M., Venables C. W., Record C. O. Treatment of Crohn's disease in relapse with cyclosporin A. Br J Surg. 1988 Dec;75(12):1185–1188. doi: 10.1002/bjs.1800751213. [DOI] [PubMed] [Google Scholar]
- Strober W., James S. P. The immunologic basis of inflammatory bowel disease. J Clin Immunol. 1986 Nov;6(6):415–432. doi: 10.1007/BF00915248. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966 Dec;41(220):613–635. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]


