Abstract
Introduction
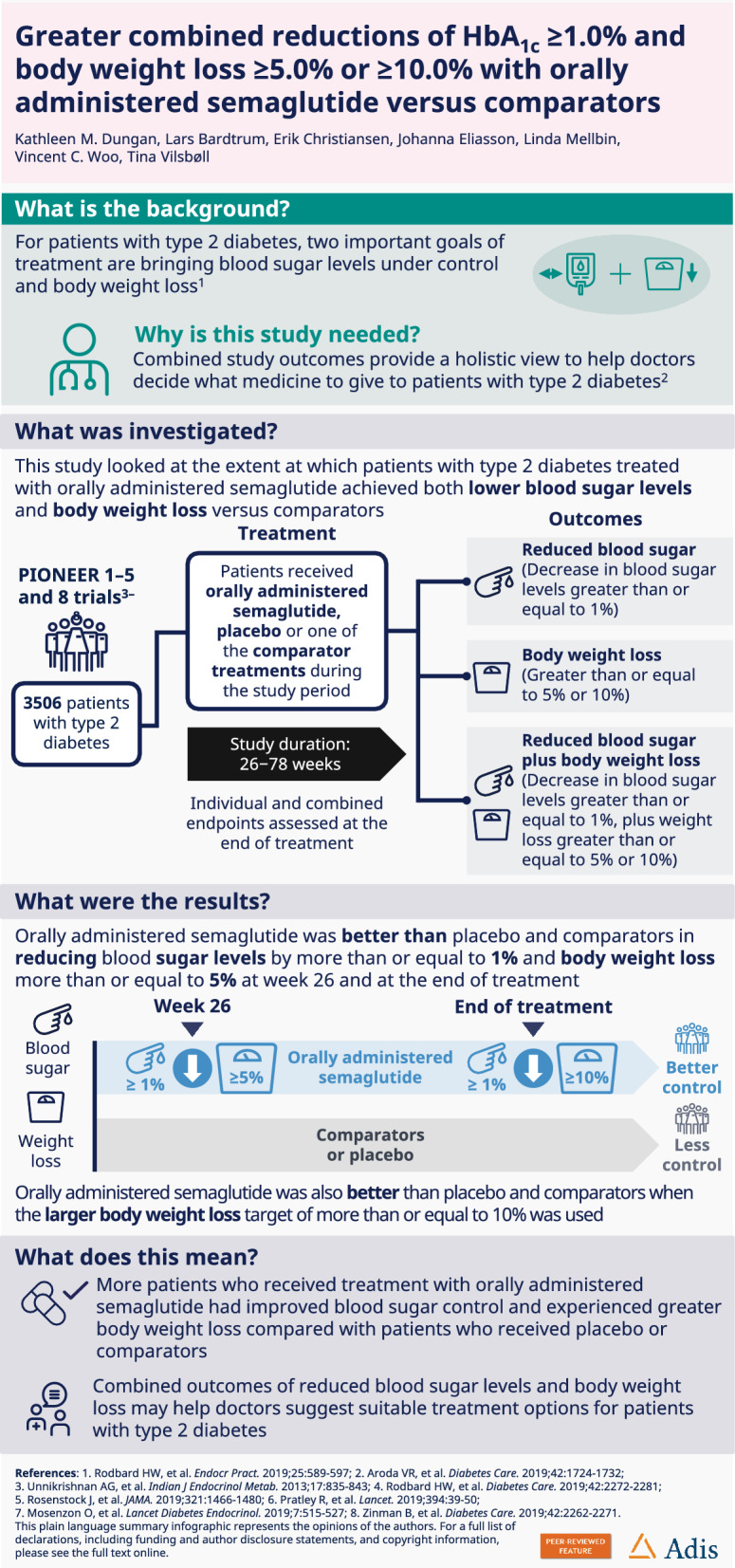
A post hoc analysis of the PIONEER 1–5 and 8 trials assessed the clinically relevant composite endpoints of HbA1c (glycated haemoglobin) reduction ≥ 1% and body weight loss of ≥ 5% or ≥ 10% with orally administered semaglutide versus comparators.
Methods
In the PIONEER trials, people with type 2 diabetes were randomised to orally administered semaglutide versus placebo (PIONEER 1, 4, 5 and 8), empagliflozin (PIONEER 2), sitagliptin (PIONEER 3) and liraglutide (PIONEER 4) for 26–78 weeks. This analysis assessed the proportion of people achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% at week 26 and at end of treatment, and the proportion of people achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 10% at end of treatment.
Results
Overall, 3506 people in PIONEER 1–5 and 8 were included. At week 26 and at end of treatment, odds of achieving the composite endpoint of an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% were significantly greater with orally administered semaglutide 14 mg than with placebo (PIONEER 1, 4, 5 and 8; all p < 0.0001), empagliflozin 25 mg (PIONEER 2, p < 0.0001), sitagliptin 100 mg (PIONEER 3, p < 0.0001) and liraglutide 1.8 mg (PIONEER 4, p < 0.0001). Odds of achieving the composite endpoint of HbA1c reduction of ≥ 1% and body weight loss of ≥ 10% at end of treatment were also significantly greater with orally administered semaglutide versus comparators.
Conclusion
In PIONEER 1–5 and 8, odds of achieving clinically relevant reductions in both HbA1c and body weight were significantly greater with orally administered semaglutide versus comparators.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-023-01413-5.
Keywords: Body weight loss, Composite endpoint, Glucagon-like peptide 1 analogue, Glycaemic control, Type 2 diabetes
Key Summary Points
| Treatment guidelines for type 2 diabetes (T2D) recommend a comprehensive, individualised approach with multiple therapeutic goals (including HbA1c reduction and body weight loss), as many people with T2D are associated with several comorbid conditions. |
| Composite endpoints provide physicians with a holistic view of the clinical benefit of treatments, and a foundation for clinical decision-making. |
| This post hoc analysis of the PIONEER 1–5 and 8 trials evaluated composite endpoints of ≥ 1% reduction in HbA1c and plus either a ≥ 5% or ≥ 10% reduction in body weight with orally administered semaglutide versus comparators in people with T2D. |
| The odds of achieving clinically relevant reductions in both HbA1c and body weight were significantly greater with orally administered semaglutide versus comparators. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.22645201.
Introduction
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) have been shown to reduce glycated haemoglobin (HbA1c) and body weight in people with type 2 diabetes (T2D), both of which are important therapeutic goals [1, 2]. The glucose and body weight lowering benefits and cardiovascular (CV) benefits of GLP-1RAs are becoming increasingly recognised, and GLP-1RAs are included in international T2D treatment recommendations and guidelines across the disease continuum [3]. Semaglutide is a long-acting GLP-1RA available as a once-weekly subcutaneous injection and a once-daily oral tablet for the treatment of people with inadequately controlled T2D [4]. The tablet formulation of semaglutide may remove the administrative burden on people and physicians (as compared to injectables) and facilitate initiation of GLP-1RAs earlier in the course of T2D [4, 5].
Treatment guidelines for T2D recommend a comprehensive, individualised approach with multiple therapeutic goals (including HbA1c reduction and body weight loss), as many people with T2D also have overweight or obesity, which is associated with several comorbid conditions [1, 3]. Composite endpoints are useful to differentiate between treatment options in terms of overall efficacy and safety, thereby providing physicians with a holistic view of the clinical benefit of different treatments [6, 7]. Additionally, composite endpoints can provide a foundation for clinical decision-making, addressing the limitation of basing clinical decisions on a single outcome, such as change in HbA1c [8].
HbA1c reductions of ≥ 1% and body weight loss of ≥ 5% in people with T2D are considered important indicators of a clinically meaningful response to treatment, and have been shown to reduce the risk of diabetes-related complications [1, 9, 10]. Furthermore, the incidence rate of diabetes-related complications has been reported to decrease with every 1% reduction in HbA1c, while a reduction in body weight of ≥ 5% can confer metabolic improvement and reduce the risk of cardiometabolic disease [3, 9, 11]. In this post hoc analysis, data from several trials included in the PIONEER programme were evaluated to determine to what extent people with T2D treated with orally administered semaglutide versus comparators achieved composite endpoints of ≥ 1% reduction in HbA1c plus either a ≥ 5% or ≥ 10% reduction in body weight, as well as separate endpoints of ≥ 1% decrease in HbA1c and a ≥ 5% or ≥ 10% decrease in body weight.
Methods
Trial Designs and Patient Populations
Data from the PIONEER 1–5 and 8 trials were assessed in this post hoc analysis. PIONEER 6 and 7 were excluded due to the use of different trial designs. PIONEER 6 was a CV outcomes trial in a population with established CV disease or at high risk of CV events, while PIONEER 7 included a flexible dose adjustment approach where not all people received orally administered semaglutide 14 mg [12, 13].
Designs and patient populations of the PIONEER 1–5 and 8 trials have been reported previously [14–19]. In brief, the PIONEER 1–5 and 8 trials were conducted across 26–78 weeks, and assessed the efficacy and safety of orally administered semaglutide (3 mg, 7 mg or 14 mg) versus placebo or active comparators (a sodium-glucose co-transporter 2 inhibitor [empagliflozin 25 mg], a dipeptidyl peptidase 4 inhibitor [sitagliptin 100 mg] or a GLP-1RA [subcutaneously administered liraglutide 1.8 mg]) in adults with T2D across a variety of background regimens (diet and exercise alone, metformin, 1–2 oral antidiabetic drugs, basal insulin ± metformin or insulin ± metformin) [14–19]. The protocols for the PIONEER 1–5 and 8 trials were approved by local independent ethics committees/institutional review boards at each trial site and conform to the provisions of the Declaration of Helsinki; all people provided written informed consent to participate in the trials.
Assessments
All people who received orally administered semaglutide 14 mg or comparators (active or placebo) during the PIONEER 1–5 and 8 trials were included in this analysis. The following individual and composite endpoints were assessed at the end of treatment (EOT; week 26, 52 or 78): achievement of (1) an HbA1c reduction of ≥ 1%; (2) a body weight loss of ≥ 5%; (3) a body weight loss of ≥ 10%; (4) an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5%; and (5) an HbA1c reduction of ≥ 1% and body weight loss of ≥ 10%. In addition, the proportion of people achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% at week 26 in all trials was assessed to determine whether the results differed from the EOT.
Statistical Analysis
Data for the trial product estimand (on trial product without rescue medication) were analysed. Missing data were accounted for using an analysis of covariance-based sequential multiple imputation model. Separate logistic regression analyses were performed for complete data sets of each study. Endpoints were analysed with treatment, strata (background medication for PIONEER 3–5 and 8, renal function for PIONEER 5 and insulin regimen for PIONEER 8), region and interaction between strata (PIONEER 5 and 8) as categorical fixed effects, and continuous baseline value(s) as covariates. Results were combined by use of Rubin’s rule to draw inference [20].
Results
Baseline Characteristics
Overall, 3506 people with T2D in the PIONEER 1–5 and 8 trials were included. Baseline demographics and clinical characteristics grouped by trial and treatment arm are presented in Supplementary Table S1. Mean duration of diabetes was lowest in PIONEER 1 and highest in PIONEER 8. Likewise, the mean patient age was lowest in PIONEER 1 and highest in PIONEER 5 and 8. Baseline HbA1c and body weight were similar across trials.
Changes from Baseline in HbA1c and Body Weight
Individual Endpoints
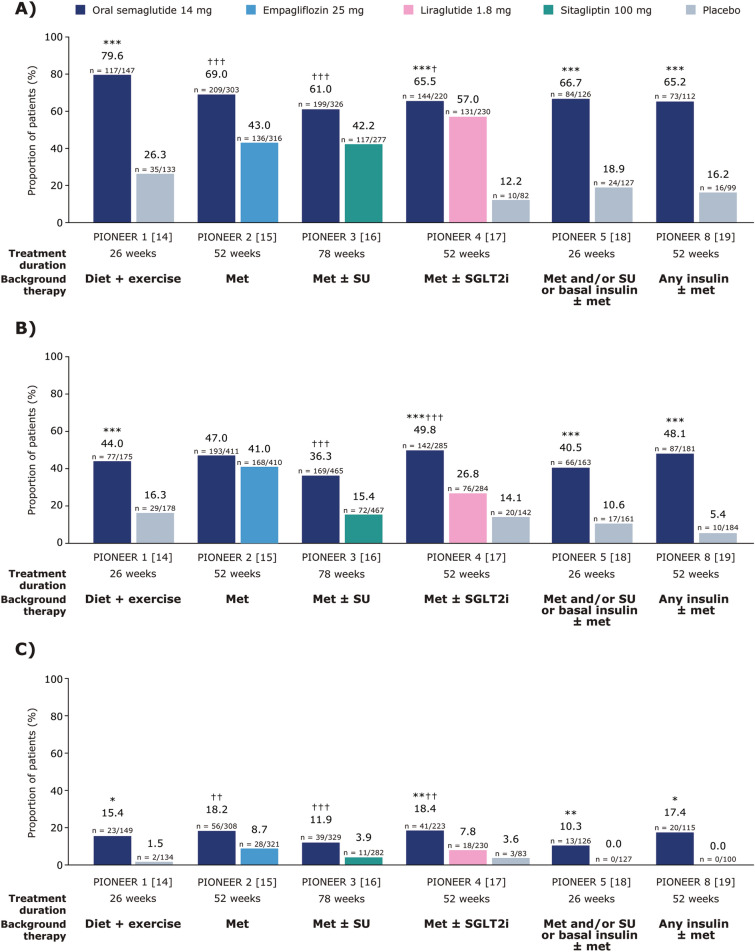
In the PIONEER 1–5 and 8 trials, the odds of achieving the HbA1c reduction of ≥ 1% at EOT were significantly greater with orally administered semaglutide 14 mg compared with placebo (PIONEER 1, 4, 5 and 8, all p < 0.0001) and the active comparators empagliflozin 25 mg (PIONEER 2, p < 0.0001), sitagliptin 100 mg (PIONEER 3, p < 0.0001) and liraglutide 1.8 mg (PIONEER 4, p < 0.05) (Fig. 1A).
Fig. 1.
Proportion of people in PIONEER 1–5 and 8 trials achieving an HbA1c reduction of ≥ 1% (A), achieving a body weight loss of ≥ 5% (B), or achieving a body weight loss of ≥ 10% (C) at EOT. Data are observed proportions for the trial product estimand (on trial product without rescue medication). In panels A and B and for PIONEER 1–4 in panel C, p values are for the EORs for orally administered semaglutide 14 mg versus placebo or the active comparator. For PIONEER 5 and 8 in panel C, EORs could not be calculated as a result of 0 events in the comparator arms; p values for the risk difference were calculated instead. *p < 0.01 versus placebo; **p < 0.001 versus placebo; ***p < 0.0001 versus placebo; †p < 0.05 versus the active comparator; ††p < 0.001 versus the active comparator; †††p < 0.0001 versus the active comparator. EOR estimated odds ratio, EOT end of treatment, HbA1c glycated haemoglobin, met metformin, SGLT2i sodium-glucose co-transporter 2 inhibitor, SU sulphonylurea
Similarly, the odds of achieving a body weight loss of ≥ 5% at EOT were significantly greater with orally administered semaglutide 14 mg than with placebo (PIONEER 1, 4, 5 and 8, all p < 0.0001), sitagliptin 100 mg (PIONEER 3, p < 0.0001) and liraglutide 1.8 mg (PIONEER 4, p < 0.0001), while the odds were comparable for orally administered semaglutide 14 mg and empagliflozin 25 mg in PIONEER 2 (Fig. 1B).
The odds of achieving a body weight loss of ≥ 10% at EOT were significantly greater with orally administered semaglutide 14 mg than with placebo (PIONEER 1, p < 0.01; PIONEER 4, p < 0.001; PIONEER 5, p < 0.001; PIONEER 8, p < 0.01), empagliflozin 25 mg (PIONEER 2, p < 0.001), sitagliptin 100 mg (PIONEER 3, p < 0.0001) and liraglutide 1.8 mg (PIONEER 4, p < 0.001) (Fig. 1C).
Composite Endpoints
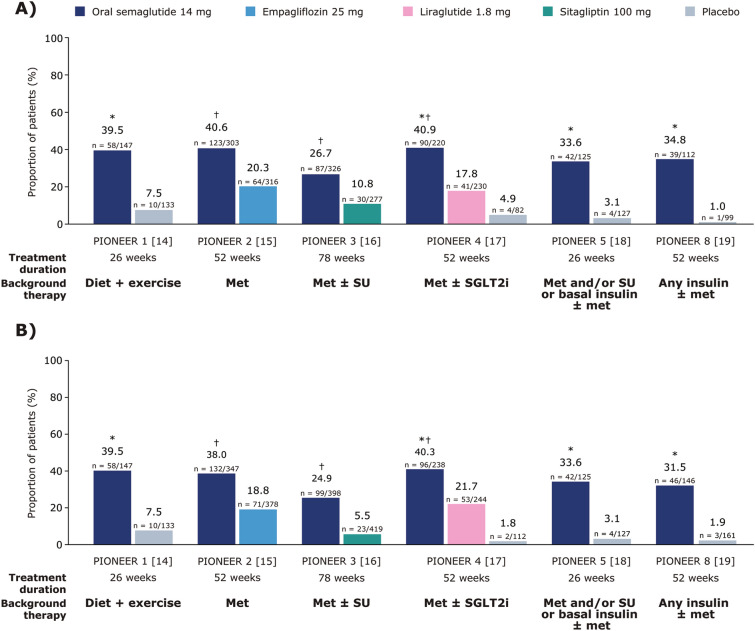
The odds of achieving the composite endpoint of an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% were significantly greater with orally administered semaglutide 14 mg than with any of the comparators in PIONEER 1–5 and 8, including empagliflozin in PIONEER 2, at week 26 and at EOT (all p < 0.0001; Fig. 2A, B).
Fig. 2.
Proportion of people achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% at EOT (A), or achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% at week 26 (B). Data are observed proportions for the trial product estimand (on trial product without rescue medication). *p < 0.0001 for the EOR with orally administered semaglutide 14 mg versus placebo; †p < 0.0001 for the EOR with orally administered semaglutide 14 mg versus the active comparator. EOR estimated odds ratio, EOT end of treatment, HbA1c glycated haemoglobin, met metformin, SGLT2i sodium-glucose co-transporter 2 inhibitor, SU sulphonylurea
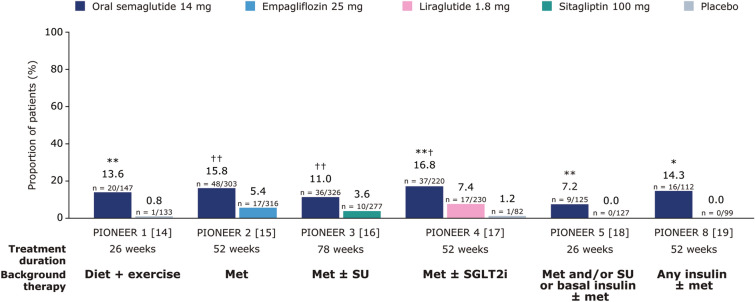
The odds of achieving both an HbA1c reduction of ≥ 1% and body weight loss of ≥ 10% at EOT were significantly greater with orally administered semaglutide 14 mg than with placebo (PIONEER 1, 4 and 5, all p < 0.01; PIONEER 8, p < 0.05), empagliflozin 25 mg (PIONEER 2, p < 0.0001), sitagliptin 100 mg (PIONEER 3, p < 0.0001) and liraglutide 1.8 mg (PIONEER 4, p < 0.001) (Fig. 3).
Fig. 3.
Proportion of people achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 10% at EOT. Data are observed proportions for the trial product estimand (on trial product without rescue medication). For PIONEER 1–4, p values are for the EORs for orally administered semaglutide 14 mg versus placebo or the active comparator. For PIONEER 5 and 8, EORs could not be calculated as a result of 0 events in the comparator arms; p values for the risk difference were calculated instead. *p < 0.05 versus placebo; **p < 0.01 versus placebo; ***p < 0.001 versus placebo; †p < 0.001 versus the active comparator; ††p < 0.0001 versus the active comparator. EOR estimated odds ratio, EOT end of treatment, HbA1c glycated haemoglobin, met metformin, SGLT2i sodium-glucose co-transporter 2 inhibitor, SU sulphonylurea
Discussion
This post hoc analysis evaluated the composite endpoint of a body weight loss of ≥ 5% or ≥ 10% together with an HbA1c reduction of ≥ 1%, arguably a more clinically relevant endpoint than that reported in the individual trials [14–19]. Indeed, current T2D treatment guidelines recommend multiple therapeutic goals, including HbA1c < 7% with low or no incidence of hypoglycaemia and ≥ 5% weight loss in people with T2D with overweight or obesity [3, 10, 21]. Composite endpoints are therefore preferred over individual endpoints for their ability to assess the net clinical benefit of an intervention by combining two or more events in one outcome. The extent of information achieved on the efficacy and safety of an intervention by analysing the composite endpoints may prove helpful to physicians and patients as a foundation for clinical decision-making [6].
Orally administered semaglutide 14 mg was better than placebo and active comparators in achieving a combined HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% at week 26 and EOT. Orally administered semaglutide was also better than placebo and active comparators when a larger body weight loss target of ≥ 10% was applied. The odds of achieving each target were also statistically significantly greater with orally administered semaglutide than with comparator.
Studies have previously shown that HbA1c reductions of ≥ 1% and body weight loss of ≥ 5%, measured separately as individual endpoints, are associated with clinically meaningful health benefits [1, 2, 22]. Indeed, the UK Prospective Diabetes Study (UKPDS) of patients with T2D reported that each 1% reduction in HbA1c has the potential to reduce the risk of microvascular complications by 37%, myocardial infarction by 14% and diabetes-related deaths by 21% [9]. Reductions of body weight of ≥ 5% improve glycaemic control, lipid levels and blood pressure in people with overweight/obesity and T2D [1], and are recommended in the American Diabetes Association (ADA) guidelines. The guidelines also recommend considering the effect of medications on weight when selecting a glucose-lowering medication [3]. Furthermore, HbA1c and body weight are considered surrogate endpoints and powerful predictors of CV disease and chronic kidney disease in T2D management. A meta-analysis of five randomised controlled trials (ACCORD, ADVANCE, PROactive, UKPDS and VADT) showed that a reduction in HbA1c of approximately 1% can lead to a 17% relative risk reduction in non-fatal myocardial infarction, while during the UKPDS study, a 33% risk reduction in incident microalbuminuria was reported for patients who achieved glycaemic control [23, 24]. Similarly, weight loss > 5% has been associated with improvements in lipid levels and blood pressure [3, 11, 25]. Evaluating these surrogate endpoints can help physicians draw clinically important conclusions about a therapeutic intervention because of their proven association with these outcomes. The composite endpoint of HbA1c reduction of ≥ 1% and body weight loss of ≥ 5% could therefore be used in clinical practice to differentiate between T2D treatment options by comparing overall efficacy, as part of a holistic treatment approach. Furthermore, orally administered semaglutide provides an option for people who prefer an oral medication over an injectable agent.
A key limitation of this study is that the individual PIONEER trials were not powered for this analysis; therefore, further investigation is warranted to determine whether this composite endpoint can be used as a basis for clinical decision-making in a real-world setting.
Conclusion
Significantly more people achieved clinically relevant reductions in both HbA1c and body weight with orally administered semaglutide versus comparators in the PIONEER 1–5 and 8 trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Emisphere Technologies is acknowledged for providing a license to Eligen Technology, the SNAC component of orally administered semaglutide. Novo Nordisk A/S designed the trials, monitored sites, and collected and analysed the data. The authors thank the people participating in these trials, the investigators, all trial sites staff and all Novo Nordisk employees involved in these trials.
Funding
These trials, and the journal’s Rapid Service Fee, were funded by Novo Nordisk A/S, Søborg, Denmark.
Medical Writing Assistance
Medical writing support was provided by Ceilidh McConnachie of Axis, a division of Spirit Medical Communications Group Limited, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Author Contributions
Erik Christiansen and Johanna Eliasson were involved in the concept and design of the analysis. Kathleen M. Dungan, Linda Mellbin, Vincent C. Woo and Tina Vilsbøll were involved in conducting the studies included in the analysis and data collection. Lars Bardtrum was involved in analysing the data. All authors were involved in the interpretation of the data and critically reviewing, editing and approving the manuscript.
Disclosures
Kathleen M. Dungan reports research support from Abbott, Dexcom, Sanofi and ViaCyte; consulting fees from Boehringer Ingelheim, Dexcom and Eli Lilly; and honoraria from the Academy for Continued Healthcare Learning, Elsevier, Integritas Healthcare, Med Learning Group, Medscape and UpToDate. Lars Bardtrum and Erik Christiansen are employees and shareholders in Novo Nordisk A/S. Johanna Eliasson is an employee of Novo Nordisk A/S. Linda Mellbin reports research support from Amgen AB and Bayer AG, and lecture/consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Novo Nordisk and Sanofi. Vincent C. Woo has served on advisory boards, participated in clinical trials and received speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GSK, Janssen, Novo Nordisk and Pfizer. Tina Vilsbøll has served on scientific advisory panels, participated in speaker bureaus and served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, GSK, MSD/Merck, Mundipharma, Novo Nordisk, Sanofi and Sun Pharmaceuticals.
Compliance with Ethics Guidelines
The protocols for the PIONEER 1–5 and 8 trials were approved by independent ethics committees/institutional review boards at each trial site, and the trials were conducted in accordance with International Council on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
Data Availability
Data will be shared with researchers submitting a research proposal approved by the independent review board. Access request proposals can be found on the Novo Nordisk Trials website (novonordisk-trials.com). Data will be made available after research completion and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de-identified and anonymised format, with no limitations on how the data can be used.
References
- 1.Rodbard HW, Bellary S, Hramiak I, et al. Greater combined reductions in HbA1c ≥1.0% and weight ≥5.0% with semaglutide versus comparators in type 2 diabetes. Endocr Pract. 2019;25:589–597. doi: 10.4158/EP-2018-0444. [DOI] [PubMed] [Google Scholar]
- 2.Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22:1263–1277. doi: 10.1111/dom.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2022;65:1925–1966. doi: 10.1007/s00125-022-05787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallwitz B, Giorgino F. Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Front Endocrinol (Lausanne) 2021;12:645507. doi: 10.3389/fendo.2021.645507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 6.Unnikrishnan AG, Bhattacharyya A, Baruah MP, Sinha B, Dharmalingam M, Rao PV. Importance of achieving the composite endpoints in diabetes. Indian J Endocrinol Metab. 2013;17:835–843. doi: 10.4103/2230-8210.117225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einarson TR, Garg M, Kaur V, Hemels ME. Composite endpoints in trials of type-2 diabetes. Diabetes Obes Metab. 2014;16:492–499. doi: 10.1111/dom.12226. [DOI] [PubMed] [Google Scholar]
- 8.McCoy CE. Understanding the use of composite endpoints in clinical trials. West J Emerg Med. 2018;19:631–634. doi: 10.5811/westjem.2018.4.38383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American diabetes association professional practice committee. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S113–24. [DOI] [PubMed]
- 11.Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399:394–405. doi: 10.1016/S0140-6736(21)01919-X. [DOI] [PubMed] [Google Scholar]
- 12.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528–539. doi: 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed] [Google Scholar]
- 13.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 14.Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–1732. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 15.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 18.Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–527. doi: 10.1016/S2213-8587(19)30192-5. [DOI] [PubMed] [Google Scholar]
- 19.Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42:2262–2271. doi: 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little RJ, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 21.American diabetes association professional practice committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2021;45(Suppl 1):S83–96. [DOI] [PubMed]
- 22.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 24.Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med. 2008;25(Suppl 2):25–29. doi: 10.1111/j.1464-5491.2008.02496.x. [DOI] [PubMed] [Google Scholar]
- 25.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared with researchers submitting a research proposal approved by the independent review board. Access request proposals can be found on the Novo Nordisk Trials website (novonordisk-trials.com). Data will be made available after research completion and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de-identified and anonymised format, with no limitations on how the data can be used.