Abstract
We investigated ectoparasite diversity, interspecific infestation rates and host preference in roosting fruit bats, Eidolon helvum, from Bowen University, Southwest Nigeria. Fur of captured E. helvum were sampled monthly for ectoparasites from January 2021 to June 2022. We examined a total of 231 E. helvum and observed a significant female to male adult sex ratio (0.22:1); with 53.9% ectoparasitic infestation rate. We identified and enumerated the ectoparasite; and subjected its Cytochrome c oxidase subunit I (COI) gene to phylogenetic analysis with other nycteribiids. COI gene sequences obtained formed a distinct clade with other C. greeffi sequences. We recovered a total of 319 (149 female and 170 male) ectoparasites and observed a balanced C. greeffi female to male adult sex ratio of 0.88:1. Ectoparasitic sex distribution had no association with host sex and season. Prevalence was significantly higher during the wet season, but not between sexes of E. helvum. The intensity of infestation, 3.7 ± 0.4 individuals per fruit bat, was significantly higher during the wet season with a bimodal seasonal distribution. The strongly male-biased host adult sex ratio had no significant influence on C. greeffi metapopulation adult sex ratio.
Keywords: Cyclopodia greeffi, Eidolon helvum, COI gene, Nycteribiidae, Parasite intensity, Adult sex ratio
Graphical abstract
Highlights
-
•
The bat fly species, Cyclopodia greeffi, is ectoparasitic on Eidolon helvum.
-
•
Male biased adult sex ratio was observed for Eidolon helvum.
-
•
Prevalence and intensity of C. greeffi infestation showed seasonal significance.
-
•
There is no association between host and ectoparasite sex.
1. Introduction
Bat flies (Brachycera: Muscomorpha: Hippoboscoidea) include families of the wingless pupiparous Diptera, Nycteribiidae (Ogilvie et al., 2009)] parasitizing Eidolon helvum (Pteropodidae, Yinpterochiroptera) and the Streblidae. These bat flies exhibit morphological diversity and are obligate hematophagous (blood feeding) ectoparasites and potential disease vectors that live on the fur and flight membranes of bat hosts (Dick and Patterson, 2006). Although, the association of bat flies with their cavernicolous host bat species reveal a range of host specificity from monoxenous to stenoxenous to polyxenous (Poon et al., 2023), bat fly species from frugivorous bats have been reported to be monoxenous (Ramasindrazana et al., 2017), showing specific host preference. Cyclopodia greeffi (Diptera, Nycteribiidae) has been reported to specifically parasitize Eidolon helvum (Urich et al., 1922; Otubanjo, 1985; Billeter et al., 2012; Kamani et al., 2014; Wilkinson et al., 2016; Samabide and Lenga, 2018; Reeves et al., 2020), while C. dubia is reported to parasitize the cave-dwelling Madagascan fruit bat, E. dupreanum (Brook et al., 2015; Ramasindrazana et al., 2017).
Nycteribiid bat flies, as ectoparasites, are parasitized by other organisms, including fungi, protozoa, and bacteria. These apterous bat flies have been reported to be infested by ectoparasitic fungi of the order Laboulbeniales (Blackwell, 1980; Haelewaters et al., 2017, 2018; Walker et al., 2018) and regarded as hyperparasites; and are hosts to the apicomplexan haemosporidian parasite, Polychromophilus, that causes bat malaria (Gardner and Molyneux, 1988; Obame-Nkoghe et al., 2016; Sándor et al., 2021). P. murinus is suspected to be transmitted by the nycteribiid bat fly, Nycteribia kolenatii (Gardner and Molyneux, 1988). These bat flies are also infested with various bacteria species including the Gram-negative aerobic parasitic bacteria, Bartonella, with zoonotic potentials (Billeter et al., 2012; Trataris et al., 2012; Dietrich et al., 2016; Wilkinson et al., 2016; Lee et al., 2021). Bartonella strains have been isolated from E. helvum bats (Kosoy et al., 2010). Szentiványi et al. (2020) detected Polychromophilus and Bartonella in both bat hosts and their bat flies; and suggested the possibility of using hematophagous ectoparasites as replacement for the harmful invasive sampling of vector-borne microorganisms present in the blood of bat hosts. A nycteribiid bat fly has also been implicated as a reservoir of a novel rhabdovirus (Goldberg et al., 2017).
Pathogen infections in E. helvum (Ogawa et al., 2017; Olatimehin et al., 2018; Pernet et al., 2014) has been associated with C. greeffi in Africa, e.g., Kenya (Kosoy et al., 2010), Ghana (Billeter et al., 2012; Morse et al., 2012), Nigeria (Kamani et al., 2014, 2022), and Equatorial Guinea, Tanzania, and Uganda (Bai et al., 2015). The occurrence of C. greeffi on E. helvum could be a tool in host bat conservation during pathogen surveillance studies (Szentiványi et al., 2020); as this could provide secondary information and reduce harmful invasive method of blood sampling from the bat host.
Bowen University, Iwo in Nigeria houses tree roosting straw-coloured fruit bat (Eidolon helvum) colonies throughout the year. These bats are infested with nycteribiid bat flies of the genus Cyclopodia. Morphological keys for the identification of nycteribiids abound in literature (Theodor, 1957, 1959; Graciolli and Carvalho, 2001a, 2001b; Aguiar and Antonini, 2011); in recent times, however, molecular tools such as DNA barcoding, including nuclear and mitochondrial genes (Dittmar et al., 2006; Ramasindrazana et al., 2017; Poon et al., 2023) are used to authenticate and identify nycteribiids. Hence the aim of this study is to provide a phylogenetic relationship of Cytochrome c oxidase subunit I (COI) gene for C. greeffi and other related nycteribiids in the GenBank database; and to determine seasonal and sex prevalence and intensity of the ectoparasite on its bat host.
2. Materials and methods
2.1. Collection and morphological characterization of fruit bat ectoparasites
Monthly collection of fruit bat samples was conducted from January 2021 to June 2022. The months were categorised into dry season (November to March) and wet season (April to October). Bats were captured in the field by mist-netting, removed and kept in cotton bags; and then examined for ectoparasites in the laboratory. Observed bat flies on the fur and patagium were removed and placed in vials of 75% percent ethanol with tweezers. The fruit bats were sexed, and the body mass was determined using a weighing balance, and later released. The bat fly was identified by external morphological features with a dissecting microscope and photomicroscope (MicroCap V3.0) using (Theodor, 1957, 1959) as identification guides. The ectoparasite abundance (number of ectoparasite per fruit bat), ectoparasite prevalence (percentage of infested fruit bat), and mean ectoparasite intensity (mean number of ectoparasite per infested bat) were determined (Sharifi et al., 2013).
2.2. DNA extraction from bat fly specimens
DNA extraction was carried out by the method Dellaporta et al. (1983) with slight modification. Briefly, a portion of the body of each bat fly specimen was macerated in 1.5 ml Eppendorf tube using sterile plastic pestle and 200 μl of DNA Extraction Buffer (containing proteinase K - 0.05 mg/ml i.e., 1 μl) and 10 μl of 20% Sodium Dodecyl Sulphate (SDS) were added to each tube sequentially, and then, incubation was carried out in a water bath at 65 °C for 30 min. Each tube was allowed to cool to room temperature and thereafter, 20 μL of 7.5 M potassium acetate was added and mixed briefly. The mixture was centrifuged at 13,000 rpm for 10 min, and the supernatant obtained was transferred into new fresh autoclaved tubes. Two-third volume of cold isopropanol was added to the individual supernatants. The containing tubes were inverted 5 times to mix gently, and then incubated at −20 °C for 1 h. Centrifugation (at 13,000 rpm for 10 min) was carried out afterwards and then 200 μL of 70% ethanol was immediately added. Further centrifugation at 13,000 rpm for 5 min was done, and the supernatant obtained was carefully discarded, while keeping the DNA pellet intact. The DNA pellet was dried at 37 °C for 30 min, and then dissolved in 25 μL of sterile distilled water. DNA concentration and purity were determined using a Nanodrop spectrophotometer (ThermoScientific, model 2000) (Additional file 1: Table 1).
Table 1.
Number of infested Eidolon helvum and prevalence (%) of Cyclopodia greeffi relative to host sex and sampling seasons. Samples were collected from Iwo, Southwest Nigeria between January 2021, and June 2022.
| Sex | Dry Season |
Wet Season |
Total |
|||
|---|---|---|---|---|---|---|
| Number of Eidolon helvum examined | Prevalence (%) | Number of Eidolon helvum examined | Prevalence (%) | Number of Eidolon helvum examined | Prevalence (%) | |
| Female | 34 | 50.0 | 8 | 62.5 | 42 | 52.4 |
| Male | 131 | 55.0 | 58 | 72.4 | 189 | 60.3 |
| Total | 165 | 53.9 | 66 | 71.2 | 231 | 58.9 |
| Fruit bat sex ratio F:M = 0.22:1 | ||||||
2.3. PCR amplification and sequencing
Polymerase Chain Reaction (PCR) mix was prepared for each sample DNA and consisted of 10 μL of 5x GoTaq colourless reaction; 3 μL of 25 Mm MgCl2; 1 μL of 10 mM of dNTPs mix; 1 μL each of 10 pmol forward primer LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and reverse primer HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′); 0.24 μL of 0.3 units Taq DNA polymerase (Promega, USA); 42 μL of sterile distilled water; and 8 μL of working DNA template (Folmer et al., 1994). Amplification was carried out on a PCR system thermal cycler (Applied Biosystem Inc., USA) according to the following conditions: 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 90 s, and 72 °C for 10 min. The amplified DNA fragments of target sequences in PCR were ethanol purified to remove the PCR reagents. The presence of expected band size of amplified target cytochrome oxidase I (COI) gene fragments was confirmed by running 5 μL of the solution containing the purified fragments on a 2% agarose gel electrophoresis at a voltage of 120V for about 1 h and viewing under UV light. Sequencing of the purified fragments was carried out at the GenoScientific.
2.4. Phylogenetic and sequence analysis
Alignment of the forward and reverse sequence for each specimen was carried out using Geneious® v2022.2.2 (Kearse et al., 2012) for windows software. Observable misalignments were fixed through manual inspection. Sequences were aligned using the MUSCLE algorithm (Edgar, 2004) in Geneious® with available sequences of Eucampsipoda theodori, Nycteribia sp., N. pleuralis, N. allotopa, Cyclopodia dubia, C. horsfieldi and other C. greeffi isolates in the GenBank database. Thirty-eight gene fragment sequences in total were used for the Cytochrome c oxidase subunit I (COI) gene sequence phylogenetic analysis (Additional file 2: Table 2). The COI gene sequence data for each specimen was trimmed to 630 bp. Bayesian inference analysis (BI) was carried out on Geneious® software with MrBayes 3.2.6 (Huelsenbeck and Ronquist, 2001). The GTR + G substitution model (Tavaré, 1986) was used for the Bayesian posterior probability (BI) analyses. 2.0 × 106 generations, gamma category value of 5, sampling frequency of every 1000 generations, and burn-in value of 200 (Larsson et al., 2022) were used in the BI analysis. The sequences of the hippoboscid (Lipoptena cervi) and glossinid (Glossina morsitans) flies were used as outgroup.
Table 2.
Mean (± standard error, SE) abundance and intensity of Cyclopodia greeffi relative to host sex and sampling seasons. Samples were collected from Iwo, Southwest Nigeria between January 2021, and June 2022.
| Host Sex | Abundance Mean ± SE (n) |
Intensity Mean ± SE (n) |
||||
|---|---|---|---|---|---|---|
| Dry Season | Wet Season | Total | Dry Season | Wet Season | Total | |
| Female | 0.8 ± 0.2 (34) | 2.9 ± 1.3 (8) | 1.2 ± 0.3 (42) | 1.6 ± 0.3 (17) | 4.6 ± 1.6 (5) | 2.3 ± 0.5 (22) |
| Male | 0.9 ± 0.1 (131) | 2.6 ± 0.4 (n = 58) | 1.4 ± 0.1 (189) | 1.6 ± 0.1 (72) | 3.6 ± 0.4 (42) | 2.4 ± 0.2 (114) |
| Total | 0.9 ± 0.1 (165) | 2.6 ± 0.4 (66) | 1.4 ± 0.1 (231) | 1.6 ± 0.1 (89) | 3.7 ± 0.4 (47) | 2.3 ± 0.2 (136) |
| Range = | 1–6 | 1–14 | ||||
2.5. Data analysis
Ectoparasite prevalence was obtained by determining the proportion of infested bats out of the total examined and expressed as percentage (%); mean ectoparasite intensity was obtained by counting the number of ectoparasites and dividing by the number of infested bat and expressed as mean ± standard error (SE). Normal approximation method of Chi-square test was used to determine differences in prevalence among seasons and sex of host. Two sample t-test was used to determine difference between intensity of infestation for both season and sex after subjecting the data to two sample variance test. Statistical analyses and graphical presentation were performed using Minitab® version 20.4 software.
2.6. Ethics statement
This study was carried out in strict compliance with the standard operating procedure of the Institutional Animal Care and Use Committee (IACUC) for use of wild mammals in research (Sikes, 2016). Ethical approval for the study was given by Bowen University Research and Ethics Committee (BUREC/07a/20); efforts were made to minimise discomfort to the bats during capture, handling, and release.
3. Results
3.1. Morphological characteristics of bat ectoparasite
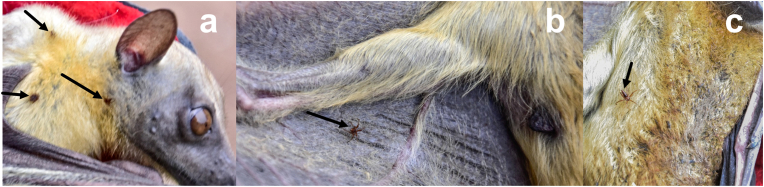
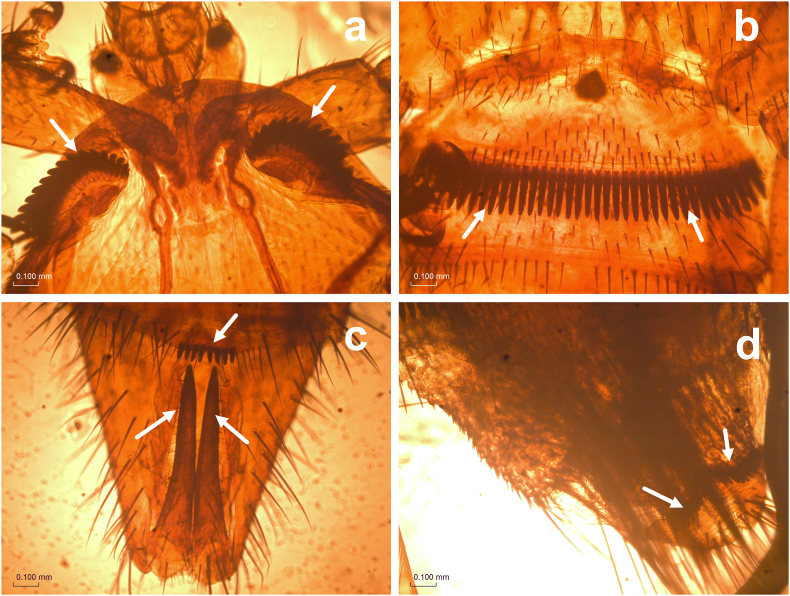
Bat fly ectoparasite Cyclopodia greeffi Karsch, 1884 was recovered from the fur and wing (patagium) regions of Eidolon helvum (Fig. 1). The bat flies are wingless and possess small spines on the sternal plate. The thorax bears two rows of ctenidia (one on either side of the anterior margin) with thick blunt teeth (Fig. 2a). The first abdominal sternite bears a ctenidium with about 40–44 blunt teeth (Fig. 2b). The male abdomen terminates in a pair of long slender claspers (genitalia) tapering to a long and pigmented point while the fifth abdominal sternite is convex with 6–10 spines in the middle (Fig. 2c). The abdominal region of the female is truncate, and the sternal region has 2 curved rows of spines (one in each half) around the posterior margin (Fig. 2d).
Fig. 1.
a, b, c. C. greeffi parasites on the straw-coloured fruit bat Eidolon helvum.a. fur around the right side of shoulder and neck region; b. ventral side of the wing (patagium) region below the right forearm; c. ventral side of the abdominal region. Arrows are pointing to the location of the bat flies.
Fig. 2.
Cyclopodia greeffi. a. Thorax, dorsal: ctenidia with thick blunt teeth. b, c, d. Abdomen ventral: b. sternite 1–2 bearing ctenidium, with about 40–44 blunt teeth; c. male, claspers long and slender, pigmented at the apex, fifth sternite with 8 spines; d. female, truncate abdomen, sternite with two curved rows of spine.
3.2. Molecular phylogenetic analysis of bat ectoparasites
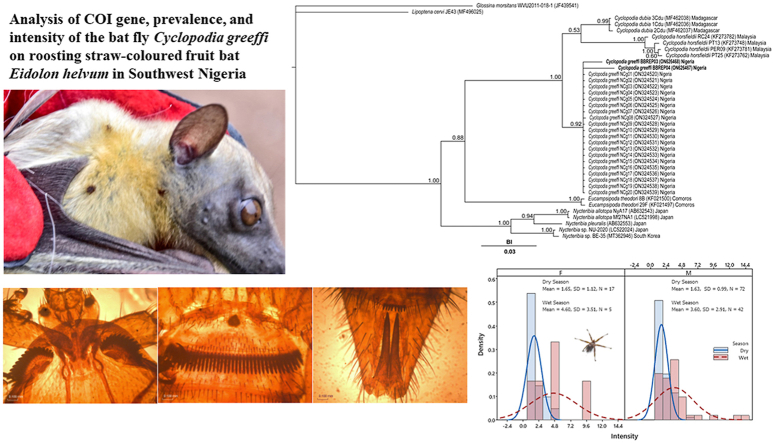
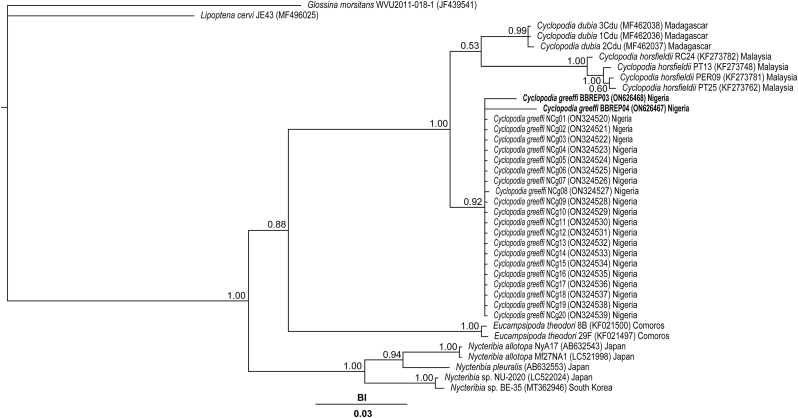
The Bayesian posterior probability (BI) phylogenetic analysis of the COI gene fragment sequence of the isolates of Cyclopodia greeffi (i.e., BBREP03 and BBREP04) reported in the current study and other locations within Nigeria in addition to those of other species of Cyclopodia and related genera (i.e., Nycteribia, Eucampsipoda, Lipoptena and Glossina) is presented in Fig. 3. Five distinct clades were observed aside from the individual Glossina morsitans and Lipoptena cervi outgroup sequences. Clade A contained a cluster of C. dubia sequences (BI value = 0.99). Clade B comprised of only C. horsfieldi sequences. One of these sequences (i.e., RC24 from Pteropus vampyrus bat) formed a sub-clade, while the other sequences (i.e., PT13, PER09 and PT25 from Pteropus hypomelanus bat) formed another sub-clade. Both sub-clades were strongly supported (BI value = 1.00 respectively). Clade C consisted mainly of C. greeffi sequences (BI value = 0.92). The sequences of Eucampsipoda theodori formed the fourth clade (Clade D), with a support value of 1.00. Clade E was constituted by sequences of Nycteribia (sub-clade 1 – N. allotopa; sub-clade 2 – N. pleuralis; sub-clade 3 – Nycteribia sp.). The three sub-clades in this clade had high support values of 1.00 respectively.
Fig. 3.
Cytochrome c oxidase subunit I (COI) gene sequence phylogeny showing the relationship between Cyclopodia greeffi and other species of the same and different genera. Values obtained from Bayesian posterior are presented as supports at the nodes. BI – Bayesian posterior probability value.
3.3. Prevalence, abundance, intensity, and sex ratio of bat ectoparasite
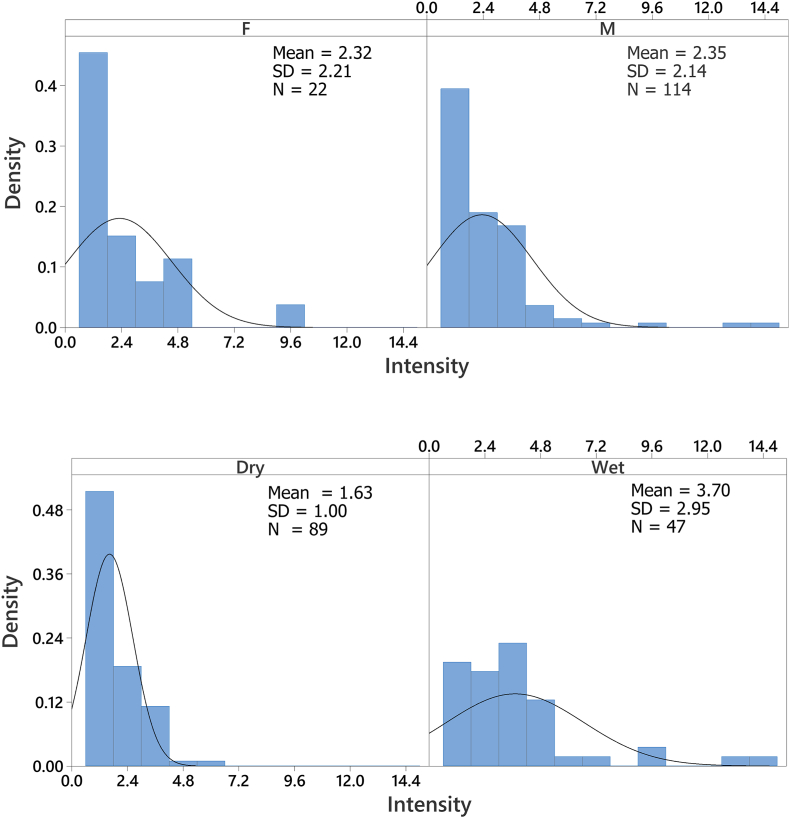
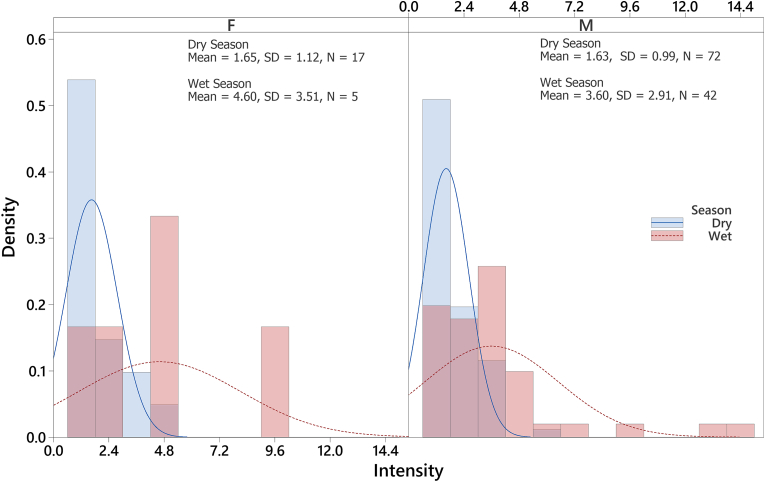
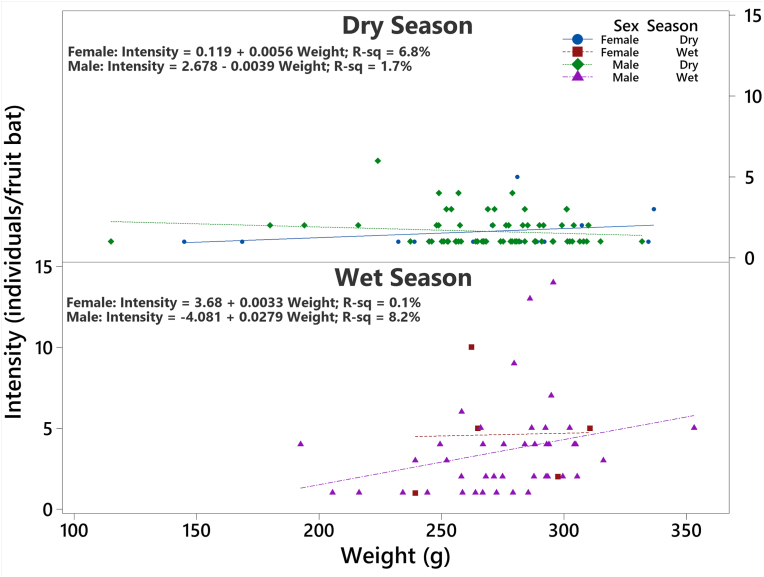
A total of 231 Eidolon helvum were captured and examined for ectoparasitic infestation. Three hundred and nineteen (319) Cyclopodia greeffi were recovered from 136 infested bats. Female to male sex ratio of captured E. helvum is 0.22:1 (Table 1), with the males significantly higher in number than the females . The overall prevalence of C. greeffi infestation during the study period is 58.9%. The prevalence of C. greeffi is not significantly different between the sexes, although higher prevalence (60.3%) was recorded for male E. helvum compared to females (52.4%); however, seasonal prevalence shows a significantly higher prevalence during the wet season (71.2%) compared to the dry season (53.9%). The overall abundance and intensity of infestation are 1.4 ± 0.1 individuals per fruit bat and 2.3 ± 0.2 individuals per fruit bat, respectively (Table 2). Although the distribution of infestation intensity is unimodal for both males and females, seasonal bimodal distribution of infestation intensity is observed for both sexes of E. helvum (Fig. 4, Fig. 5) with a larger spread during the wet season. The significant difference in intensity among seasons shows a higher mean value for the wet season (3.7 ± 0.4 individuals per fruit bat); however, the mean intensity between the sexes was not significant . There is no association between sex of E. helvum and sampling season for intensity of C. greeffi infestation . Regression equation shows a weak positive relationship (R2 = 0.1% and 8.2% for females and males respectively) between intensity of infestation and fruit bat weight during the wet season (Fig. 6). The infrapopulation bat fly size (for individual fruit bat) ranged from 0 to 6 individuals for males and 0–10 individuals for females. Although male C. greeffi recorded a higher value of 170 individuals with a female to male sex ratio of 0.88:1 (Table 3); there was no significant difference in sex abundance . Chi-square test of association shows no significant relationship between sex of C. greeffi infestation and host sex ; and between sex of C. greeffi infestation and season .
Fig. 4.
Density distribution plot of intensity of Cyclopodia greeffi infestation on Eidolon helvum for sexes and seasons.
Fig. 5.
Density distribution plot of intensity of infestation of Cyclopodia greeffi on Eidolon helvum showing seasonal bimodal distribution.
Fig. 6.
Regression distribution plot of Cyclopodia greeffi infestation intensity on Eidolon helvum weight for both sexes and seasons.
Table 3.
Chi-square test of association between number of male and female Cyclopodia greeffi relative to host sex and sampling seasons. Samples were collected from Iwo, Southwest Nigeria between January 2021, and June 2022.
| Parameter |
Cyclopodia greeffi |
Total | |
|---|---|---|---|
| Female | Male | ||
| Female Fruit bat (n = 22) | 20 | 31 | 51 |
| Male Fruit bat (n = 114) | 129 | 139 | 268 |
| Dry Season (n = 89) | 71 | 74 | 145 |
| Wet Season (n = 47) | 78 | 96 | 174 |
|
| |||
| Total | 149 | 170 | 319 |
| Bat fly sex ratio F:M = 0.88:1 | |||
4. Discussion
4.1. Bat host of Cyclopodia greeffi
Our study shows a significant female to male sex ratio of 0.22:1 (male bias) for the straw-coloured fruit bat roosting in Bowen University. High male to female sex ratios have been reported for several species of Myotis and Epstesicus fuscus (Adams and Hayes, 2018; Patriquin et al., 2019; Piksa, 2008); migratory noctule bat, Nyctalus noctula (Petit et al., 2001); and common vampire bats, Desmodus rotundus (Delpietro et al., 2017). The strong male bias may suggest strong sexual segregation and formation of bachelor E. helvum groups for this roosting location. Bachelor roosts have been reported for other fruit bats such as Rousettus aegyptiacus (Kwiecinski and Griffiths, 1999) and Pteropus mariannus mariannus(Wiles, 1987).
C. greeffi infests the tree-roosting fruit bat, Eidolon helvum as seen in this study although double infestation by ectoparasites (tick and insect) has been reported for E. helvum (Samabide and Lenga, 2018), where in addition to Cyclopodia, it was infested by the ixodid tick of the genus Antricola. In this study however, E. helvum was parasitized solely by C. greeffi which is similar to the findings from northern Nigerian towns in Bauchi, Benue, Nasarawa and Plateau States (Kamani et al., 2014, 2022) and Southwest Nigerian town of Ife in Osun State (Otubanjo, 1985). Monoxenous species parasitize only one host species (Olival et al., 2013). Cyclopodia greeffi and other nycteribiid bat flies exhibit host specificity. It is likely that C. greeffi is a specialist ectoparasite, being strictly monoxenous on the tree-roosting E. helvum.
4.2. Morphological characteristics of bat ectoparasites
The morphological features of the bat ectoparasites isolated in this study match with earlier reports by Theodor and Urich et al., (Theodor, 1957, 1959; Urich et al., 1922). C. greeffi differs from other closely related species of Cyclopodia based on size, the number of blunt teeth of the thoracic and abdominal ctenidia, number of spines on the abdominal plates and characteristics of the genitalia.
4.3. Molecular phylogenetic analysis of bat flies
As observed in the phylogenetic tree, the COI gene fragment sequences of C. greeffi from this study (southwest Nigeria) and those from northern Nigeria (Kamani et al., 2022) formed a single clade, and were clearly differentiated from those of other genera and related species with strong support values, hence, supporting its previous delineation as a species based on morphological features. Similarly, the clearly defined clusters formed by sequences of individual nycteribiid species, e.g., C. dubia, C. horsfieldi, E. theodori, Nycteribia sp., N. pleuralis and N. allotopa, supports their taxonomic description and the findings from other phylogenetic studies (Olival et al., 2013; Ramasindrazana et al., 2017).
4.4. Prevalence, infestation intensity and sex ratio of bat ectoparasites
The overall prevalence of 58.9% recorded for C. greeffi during this study is higher than 46.51% reported for the straw-coloured fruit bat from Brazzaville, Congo (Samabide and Lenga, 2018). However, the mean intensity of 2.3 ± 0.2 individuals for this study is lower than the 2.8 C. greeffi recorded from Brazzaville, Congo (Samabide and Lenga, 2018) for the straw-coloured fruit bat. An estimated average 1.79 nycteribiids is reported to parasitize Myotis daubentonii and Megaderma lyra bat hosts (Tendu et al., 2022). Large number of ectoparasites reported for a female E. helvum weighing 220g (Jiménez and Hazevoet, 2010) supports the broader range of ectoparasitic infestation (0–14 C. greeffi) recorded during this study as compared to the lower and narrower range (0–4 C. greeffi) per fruit bat reported earlier from Ife, Nigeria (Otubanjo, 1985). This study shows no significant difference in intensity of infestation between the sexes. Non-significant sexual differences in nycteribiid infestation of fruit bats was reported for two species of Rousettus and Thoopterus nigrescens in Indonesia (Nangoy et al., 2021). The significantly higher intensity of infestation and the wider spread of ectoparasitic infestation during the wet season is supported by the report of Otubanjo (1985), who recorded peak infestation abundance from July to September. However, in contrast to the latter author who did not record any infestation during dry season (January to March), this study recorded C. greeffi infestation throughout the period, although with a lower infestation intensity of 1.6 ± 0.1 individuals per fruit bat. C. greeffi recorded no significant difference in sex abundance with a female to male sex ratio of 0.88:1, suggesting a balanced ectoparasitic metapopulation adult sex ratio. Szentiványi et al. (2017) emphasized the importance of host and parasite sex ratio in better understanding host-parasite interactions. Reasons adduced for biased sex ratios include selective host grooming (Dick and Patterson, 2008), and parasite behaviour and sex-dependent mortality (Dittmar et al., 2011). This study shows no significant relationship between sex of C. greeffi infestation and host sex on one hand and between sex of C. greeffi infestation and season on the other hand. Barbier et al. (2019) opines that the ectoparasitic load of bat flies on bats is less correlated with environmental factors such as amount of rainfall and vegetation, however, Zarazúa-Carbajal et al. (2016) hinted that seasonality affect bat fly species richness.
The social habit of Eidolon helvum, implies close contact of individuals roosting together; and would suggest a higher prevalence of infestation by bat flies. The prevalence recorded for this study could be higher if some bat flies escaped during capture while the fruit bat was entangled in the mist net prior to removal into cloth bags; and also due to behavioural adaptation to reduce or eliminate bat fly transmission through auto- and allo-grooming activities (Ramanantsalama et al., 2018; Tendu et al., 2022).
The low regression coefficient (R2) between ectoparasite intensity and fruit bat body mass indicates that the body mass of E. helvum has no apparent influence on intensity of C. greeffi infestation. Variations of ectoparasitic prevalence and intensity in bat hosts have been reported to be mediated by behaviour, sex and age (Webber et al., 2015); roost group size, grooming efficiency and energy budgets (Czenze and Broders, 2011) in a highly gregarious mammals of the genus Myotis. Ramanantsalama et al. (2018) reported similarity in the grooming rates for both sexes of the endemic Madagascan fruit bat, Rousettus madagascariensis. Reports show that ectoparasitic load has no apparent effect on bat's health (Sharifi et al., 2013).
5. Conclusion
This study reports the cytochrome c oxidase I (COI) gene sequence, prevalence, and intensity of the bat fly, Cyclopodia greeffi, infestation of the straw-coloured fruit bat, Eidolon helvum, roosting on a University campus in southwest Nigeria throughout the year. The data for phylogenetic relationship between C. greeffi from our study location and other nycteribiids is limited as it utilised a single genetic marker, Cytochrome c oxidase I gene fragment. Significant seasonal influence on the mean prevalence and intensity is reported for the wet season.
Ethics statement
Ethical approval for the study was given by Bowen University Research and Ethics Committee (BUREC/07a/20).
Funding
Funding was provided by Bowen University Grant (Grant reference number: BRE/2020/001).
Author contributions
OEA conceptualised and designed the study. All authors participated in field and laboratory work including data collection. Data analysis, interpretation, writing, preparation and editing of manuscript were performed by OEA, IVO and OGO: All authors read and approved the final manuscript.
Data availability
Gene fragment sequence data generated during the current study are available in GenBank (accession numbers ON626457 – 626459, ON629806).
Declaration of competing interest
The authors declare no competing interest.
Acknowledgements
We thank Capt. Raymond Bitanis Tangle of the Directorate of Security Service and his team for assistance during field work. We also thank Toluwase Adebayo for the photographs of the straw-coloured fruit bat infested with bat fly in Fig. 1.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2023.06.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- Adams R.A., Hayes M.A. Assemblage-level analysis of sex-ratios in Coloradan bats in relation to climate variables: a model for future expectations. Glob. Ecol. Conserv. 2018;14(e00379):1–11. doi: 10.1016/j.gecco.2018.e00379. [DOI] [Google Scholar]
- Aguiar L.M. de S., Antonini Y. Descriptive ecology of bat flies (Diptera: Hippoboscoidea)associated with vampire bats (Chiroptera: phyllostomidae) in the cerrado of central Brazil. Mem. Inst. Oswaldo Cruz. 2011;106(2):170–176. doi: 10.1590/S0074-02762011000200009. [DOI] [PubMed] [Google Scholar]
- Bai Y., Hayman D.T.S., McKee C.D., Kosoy M.Y. Classification of bartonella strains associated with straw-colored fruit bats (Eidolon helvum) across africa using a multi-locus sequence typing platform. PLoS Neglected Trop. Dis. 2015;9(1):1–16. doi: 10.1371/journal.pntd.0003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E., Graciolli G., Bernard E. Structure and composition of Nycteribiidae and Streblidae flies on bats along an environmental gradient in northeastern Brazil. Can. J. Zool. 2019;97:409–418. [Google Scholar]
- Billeter S.A., Hayman D.T.S., Peel A.J., Baker K., Wood J.L.N., Cunningham A., Suu-Ire R., Dittmar K., Kosoy M.Y. Bartonella species in bat flies (Diptera: nycteribiidae) from western Africa. Parasitology. 2012;139(3):324–329. doi: 10.1017/S0031182011002113. [DOI] [PubMed] [Google Scholar]
- Blackwell M. Incidence, host specificity, distribution, and morphological variation in arthrorhynchus nycteribiae and A. Eucampsipodae (Laboulbeniomycetes) Mycol. 1980;72(1):143–158. doi: 10.1080/00275514.1980.12021163. [DOI] [Google Scholar]
- Brook C.E., Bai Y., Dobson A.P., Osikowicz L.M., Ranaivoson H.C., Zhu Q., Kosoy M.Y., Dittmar K. Bartonella spp. in fruit bats and blood-feeding ectoparasites in Madagascar. PLoS Neglected Trop. Dis. 2015;9(2):1–9. doi: 10.1371/journal.pntd.0003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czenze Z.J., Broders H.G. Ectoparasite community structure of two bats (Myotis lucifugus and M. septentrionalis) from the maritimes of Canada. J. Parasitol. Res. 2011;1–10 doi: 10.1155/2011/341535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta S.L., Wood J., Hicks J.B. A plant DNA minipreparation: version II. Plant Mol. Biol. Rep. 1983;1(4):19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- Delpietro H.A., Russo R.G., Carter G.G., Lord R.D., Delpietro G.L. Reproductive seasonality, sex ratio and philopatry in Argentina's common vampire bats. R. Soc. Open Sci. 2017;4(4):1–14. doi: 10.1098/rsos.160959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick C.W., Patterson B.D. In: Micromammals And Macroparasites: from Evolutionary Ecology To Management (Issue January 2006. (Professor) R.P., Morand Serge, Krasnov Boris R., editors. Springer Tokyo; 2006. Bat flies: obligate ectoparasites of bats; pp. 179–194. [DOI] [Google Scholar]
- Dick C.W., Patterson B.D. An excess of males: skewed sex ratios in bat flies (Diptera: streblidae) Evol. Ecol. 2008;22(6):757–769. doi: 10.1007/s10682-007-9201-9. [DOI] [Google Scholar]
- Dietrich M., Tjale M.A., Weyer J., Kearney T., Seamark E.C.J., Nel L.H., Monadjem A., Markotter W. Diversity of bartonella and Rickettsia spp. in bats and their blood-feeding ectoparasites from South Africa and Swaziland. PLoS One. 2016;11(3):1–9. doi: 10.1371/journal.pone.0152077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K., Morse S., Gruwell M., Mayberry J., DiBlasi E. Spatial and temporal complexities of reproductive behavior and sex ratios: a case from parasitic insects. PLoS One. 2011;6(5):1–9. doi: 10.1371/journal.pone.0019438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K., Porter M.L., Murray S., Whiting M.F. Molecular phylogenetic analysis of nycteribiid and streblid bat flies (Diptera: brachycera, Calyptratae): implications for host associations and phylogeographic origins. Mol. Phylogenet. Evol. 2006;38(1):155–170. doi: 10.1016/j.ympev.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer 0., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3(5):294–299. doi: 10.1071/ZO9660275. [DOI] [PubMed] [Google Scholar]
- Gardner D.H., Molyneux R.A. Polychromophilus murinus: a malarial parasite of bats: Life-history and ultrastructural studies. Parasitology. 1988;96(3):591–605. doi: 10.1017/S0031182000080215. [DOI] [PubMed] [Google Scholar]
- Goldberg T.L., Bennett A.J., Kityo R., Kuhn J.H., Chapman C.A. Kanyawara virus: a novel rhabdovirus infecting newly discovered nycteribiid bat flies infesting previously unknown pteropodid bats in Uganda. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graciolli G., Carvalho C. Batflies (Diptera, Hippoboscoidea, nycteribiidae) from paraná state, Brazil. I. Basilia, taxonomy and pictorial key to species. Rev. Bras. Zool. 2001;18(July):33–49. [Google Scholar]
- Graciolli G., Carvalho C.J.B. Morcegos (mammalia , Chiroptera) do Estado do paraná. Rev. Bras. Zool. 2001;18(3):907–960. [Google Scholar]
- Haelewaters D., Hiller T., Dick C.W. Bats, bat flies, and fungi: a case of hyperparasitism. Trends Parasitol. 2018;34(9):784–799. doi: 10.1016/j.pt.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Haelewaters D., Pfliegler W.P., Szentiványi T., Földvári M., Sándor A.D., Barti L., Camacho J.J., Gort G., Estók P., Hiller T., Dick C.W., Pfister D.H. Parasites of parasites of bats: Laboulbeniales (fungi: ascomycota) on bat flies (Diptera: nycteribiidae) in central europe. Parasites Vectors. 2017;10(1):1–14. doi: 10.1186/s13071-017-2022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jiménez S., Hazevoet C.J. First record of straw-coloured fruit bat Eidolon helvum (Kerr, 1792) for the Cape Verde islands. Zool. Caboverdiana. 2010;1(2):116–118. [Google Scholar]
- Kamani J., Baneth G., Mitchell M., Mumcuoglu K.Y., Gutiérrez R., Harrus S. Bartonella species in bats (Chiroptera) and bat flies (nycteribiidae) from Nigeria, west africa. Vector Borne Zoonotic Dis. 2014;14(9):625–632. doi: 10.1089/vbz.2013.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamani J., González-Miguel J., Msheliza E.G., Goldberg T.L. Straw-Colored fruit bats (Eidolon helvum) and their bat flies (cyclopodia greefi) in Nigeria host viruses with multifarious modes of transmission. Vector Borne Zoonotic Dis. 2022;22(11):545–552. doi: 10.1089/vbz.2022.0025. [DOI] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M., Bai Y., Lynch T., Kuzmin I.V., Niezgoda M., Franka R., Agwanda B., Breiman R.F., Rupprecht C.E. Bartonella spp. in bats, Kenya. Emerg. Infect. Dis. 2010;16(12):1875–1881. doi: 10.3201/eid1612.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecinski G., Griffiths T.A. Rousettus egyptiacus. Mamm. Species. 1999;611:1–9. [Google Scholar]
- Larsson M.E., Bramucci A.R., Collins S., Hallegraeff G., Kahlke T., Raina J.B., Seymour J.R., Doblin M.A. Mucospheres produced by a mixotrophic protist impact ocean carbon cycling. Nat. Commun. 2022;13(1):1–15. doi: 10.1038/s41467-022-28867-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Seo M.-G., Lee S.-H., Oem J.-K., Kim S.-H., Jeong H., Kim Y., Jheong W.-H., Kwon O.-D., Kwak D. Relationship among bats , parasitic bat flies , and associated pathogens in Korea. Parasites Vectors. 2021;14(503):1–11. doi: 10.1186/s13071-021-05016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S.F., Olival K.J., Kosoy M., Billeter S., Patterson B.D., Dick C.W., Dittmar K. Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae) Infect. Genet. Evol. 2012;12(8):1717–1723. doi: 10.1016/j.meegid.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Nangoy M., Ransaleleh T., Lengkong H., Koneri R., Latinne A., Kyes R.C. Diversity of fruit bats (pteropodidae) and their ectoparasites in batuputih nature tourism park , sulawesi , Indonesia. Biodiversitas. 2021;22(6):3075–3082. doi: 10.13057/biodiv/d220609. [DOI] [Google Scholar]
- Obame-Nkoghe J., Rahola N., Bourgarel M., Yangari P., Prugnolle F., Maganga G.D., Leroy E.M., Fontenille D., Ayala D., Paupy C. Bat flies (Diptera: nycteribiidae and Streblidae) infesting cave-dwelling bats in Gabon: diversity, dynamics and potential role in Polychromophilus melanipherus transmission. Parasites Vectors. 2016;9(1):1–12. doi: 10.1186/s13071-016-1625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Kajihara M., Nao N., Shigeno A., Fujikura D., Hang'Ombe B.M., Mweene A.S., Mutemwa A., Squarre D., Yamada M., Higashi H., Sawa H., Takada A. Characterization of a novel bat adenovirus isolated from straw-colored fruit bat (Eidolon helvum) Viruses. 2017;9(12):1–16. doi: 10.3390/v9120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie P.W., Ogilvie M.B., Journal S., May N. American Society of mammalogists Observations of a Roost of Yellow or giant fruit-Eating bats , Eidolon helvum published by. Am. Soc. Mammalogists. 2009;45(2):309–311. [Google Scholar]
- Olatimehin A., Shittu A.O., Onwugamba F.C., Mellmann A., Becker K., Schaumburg F. Staphylococcus aureus complex in the straw-colored fruit bat (Eidolon helvum) in Nigeria. Front. Microbiol. 2018;9(FEB):1–7. doi: 10.3389/fmicb.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival K.J., Dick C.W., Simmons N.B., Morales J.C., Melnick D.J., Dittmar K., Perkins S.L., Daszak P., DeSalle R. Lack of population genetic structure and host specificity in the bat fly, Cyclopodia horsfieldi, across species of Pteropus bats in Southeast Asia. Parasites Vectors. 2013;6(231):1–18. doi: 10.1186/1756-3305-6-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otubanjo O.A. Concinnum epomopis sandground, 1937 (dicrocoeliidae) from the pancreas of the west african fruit bat Eidolon helvum in Nigeria. Z. Parasitenkd Parasitol. Res. 1985;71(4):485–494. doi: 10.1007/BF00928351. [DOI] [Google Scholar]
- Patriquin K.J., Guy C., Hinds J., Ratcliffe J.M. Male and female bats differ in their use of a large urban park. J. Urban Econ. 2019;5(1):1–13. doi: 10.1093/jue/juz015. [DOI] [Google Scholar]
- Pernet O., Schneider B.S., Beaty S.M., Lebreton M., Yun T.E., Park A., Zachariah T.T., Bowden T.A., Hitchens P., Ramirez C.M., Daszak P., Mazet J., Freiberg A.N., Wolfe N.D., Lee B. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 2014;5:1–10. doi: 10.1038/ncomms6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit E., Balloux F., Goudet J. Sex-Biased Dispersal in a Migratory Bat : A Characterization Using Sex-Specific Demographic Parameters. 2001;55(3):635–640. doi: 10.1554/0014-3820(2001)055[0635:sbdiam]2.0.co;2. http://www.jstor.org/stable/2640524 Published by : Society for the Study of Evolution Stable URL : 21 : 04 UTC Your use of the JST. Evolution. [DOI] [PubMed] [Google Scholar]
- Piksa K. Swarming of Myotis mystacinus and other bat species at high elevation in the Tatra Mountains, southern Poland. Acta Chir. 2008;10(1):69–79. doi: 10.3161/150811008X331108. [DOI] [Google Scholar]
- Poon E.S.K., Chen G., Tsang H.Y., Shek C.T., Tsui W.C., Zhao H., Guénard B., Sin S.Y.W. Species richness of bat flies and their associations with host bats in a subtropical East Asian region. Parasites Vectors. 2023;16(37):1–15. doi: 10.1186/s13071-023-05663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsalama R.V., Andrianarimisa A., Raselimanana A.P., Goodman S.M. Rates of hematophagous ectoparasite consumption during grooming by an endemic Madagascar fruit bat. Parasites Vectors. 2018;11(1):330. doi: 10.1186/s13071-018-2918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasindrazana B., Goodman S.M., Gomard Y., Dick C.W., Tortosa P. Hidden diversity of Nycteribiidae (Diptera) bat flies from the Malagasy region and insights on host-parasite interactions. Parasites Vectors. 2017;10(1):1–8. doi: 10.1186/s13071-017-2582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W.K., Laverty T.M., Gratton E.M., Mushabati L.M., Eiseb S.J. New national records for cyclopodia greeffi greeffi (diptera: nycteribiidae) from the kunene region, Namibia, africa. Entomol. News. 2020;129(3):327–329. doi: 10.3157/021.129.0311. [DOI] [Google Scholar]
- Samabide K.T., Lenga A. Actualisation des ectoparasites inféodés à deux espèces de chiroptères trouvés dans les forêts relictuelles voisines de la ville de Brazzaville , Congo Résumé. Afr. Sci. 2018;14(1):402–414. [Google Scholar]
- Sándor A.D., Péter Á., Corduneanu A., Barti L., Csősz I., Kalmár Z., Hornok S., Kontschán J., Mihalca A.D. Wide distribution and diversity of malaria-related haemosporidian parasites (Polychromophilus spp.) in bats and their ectoparasites in eastern europe. Microorganisms. 2021;9(2):1–12. doi: 10.3390/microorganisms9020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi M., Taghinezhad N., Mozafari F., Vaissi S. Variation in ectoparasite load in the Mehely's horseshoe bat, Rhinolophus mehelyi (Chiroptera: Rhinolophidae) in a nursery colony in western Iran. Acta Parasitol. 2013;58(2):180–184. doi: 10.2478/s11686-013-0122-1. [DOI] [PubMed] [Google Scholar]
- Sikes R.S. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016;97(3):663–688. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentiványi T., Markotter W., Dietrich M., Clément L., Ançay L., Brun L., Genzoni E., Kearney T., Seamark E., Estók P., Christe P., Glaizot O. Host conservation through their parasites: molecular surveillance of vector-borne microorganisms in bats using ectoparasitic bat flies. Parasite. 2020;27(72):1–10. doi: 10.1051/parasite/2020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentiványi T., Vincze O., Estók P. Density-dependent sex ratio and sex-specific preference for host traits in parasitic bat flies. Parasites Vectors. 2017;10(1):1–9. doi: 10.1186/s13071-017-2340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré S. Some probabilistic and statistical problems in the analysis of DNA sequences. Some Mathematical Questions in Biology-DNA Sequence Analysis (Am. Math. Soc.) 1986;17:57–86. [Google Scholar]
- Tendu A., Hughes A.C., Berthet N., Wong G. Viral hyperparasitism in bat ectoparasites: implications for pathogen maintenance and transmission. Microorganisms. 2022;10(6):1–20. doi: 10.3390/microorganisms10061230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodor O. The nycteribiidae of the ethiopian region and Madagascar. Parasitology. 1957;47(3–4):457–543. doi: 10.1017/S0031182000022162. [DOI] [PubMed] [Google Scholar]
- Theodor O. A Revision of the genus cyclopodia (nycteribiidae, Diptera) Parasitology. 1959;49(1–2):242–308. doi: 10.1017/S0031182000026858. [DOI] [PubMed] [Google Scholar]
- Trataris A.N., Rossouw J., Arntzen L., Karstaedt A., Frean J. Bartonella spp . in human and animal populations in Gauteng , South Africa , from 2007 to 2009. Onderstepoort J. Vet. Res. 2012;79(2):1–8. doi: 10.4102/ojvr.v79i2.452. [DOI] [PubMed] [Google Scholar]
- Urich F.W., Scott H., Waterston J. Note on the dipterous bat‐parasite cyclopodia greeffi Karsch, and on a new species of Hymenopterous (Chalcid) parasite bred from it. Proc. Zool. Soc. Lond. 1922;92(2):471–477. doi: 10.1111/j.1096-3642.1922.tb02153.x. [DOI] [Google Scholar]
- Walker M.J., Dorrestein A., Camacho J.J., Meckler L.A., Silas K.A., Hiller T., Haelewaters D. A tripartite survey of hyperparasitic fungi associated with ectoparasitic flies on bats (Mammalia: Chiroptera) in a neotropical cloud forest in Panama. Parasite. 2018;25(19):1–21. doi: 10.1051/parasite/2018017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber Q.M.R., Mcguire L.P., Smith S.B., Willis C.K.R. Host behaviour, age and sex correlate with ectoparasite prevalence and intensity in a colonial mammal, the little brown bat. Behaviour. 2015;152(1):83–105. doi: 10.1163/1568539X-00003233. [DOI] [Google Scholar]
- Wiles G.J. The status of fruit bats on Guam. Pac. Sci. 1987;41(1–4):148–157. [Google Scholar]
- Wilkinson D.A., Duron O., Cordonin C., Gomard Y., Ramasindrazana B., Mavingui P., Goodman S.M., Tortosaa P. The bacteriome of bat flies (nycteribiidae) from the Malagasy region : a community shaped by host ecology , bacterial transmission mode, host-vector specificity. Appl. Environ. Microbiol. 2016;82(6):1778–1788. doi: 10.1128/AEM.03505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarazúa-Carbajal M., Saldaña-Vázquez R.A., Sandoval-Ruiz C.A., Stoner K.E., Benitez-Malvido J. The specificity of host-bat fly interaction networks across vegetation and seasonal variation. Parasitol. Res. 2016;115(10):4037–4044. doi: 10.1007/s00436-016-5176-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene fragment sequence data generated during the current study are available in GenBank (accession numbers ON626457 – 626459, ON629806).