Abstract
BACKGROUND
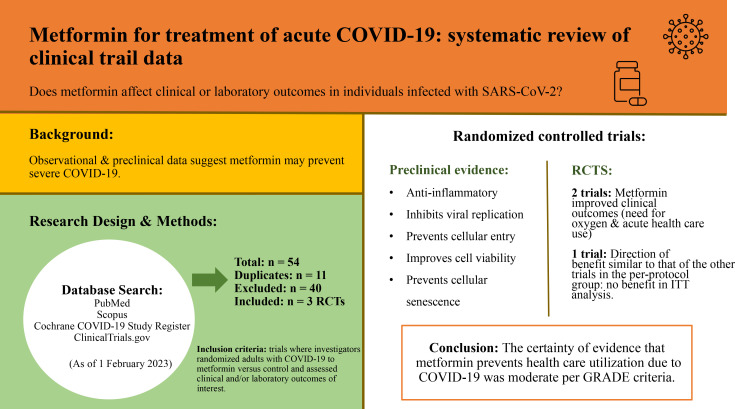
Observational and preclinical data suggest metformin may prevent severe coronavirus disease 2019 (COVID-19) outcomes.
PURPOSE
We conducted a systematic review of randomized, placebo-controlled clinical trials of metformin treatment for COVID-19 to determine whether metformin affects clinical or laboratory outcomes in individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and present a structured summary of preclinical data.
STUDY SELECTION
Two independent reviewers searched PubMed, Scopus, Cochrane COVID-19 Study Register, and ClinicalTrials.gov on 1 February 2023 with no date restrictions for trials where investigators randomized adults with COVID-19 to metformin versus control and assessed clinical and/or laboratory outcomes of interest. The Cochrane Risk of Bias 2 tool was used to assess bias.
DATA EXTRACTION
Two reviewers extracted data pertaining to prespecified outcomes of each interest from each included trial.
DATA SYNTHESIS
The synthesis plan was developed a priori and was guided by Synthesis Without Meta-analysis (SWiM) guidelines. Summary tables and narrative synthesis were used (PROSPERO, 2022, CRD42022349896). Three randomized trials met inclusion criteria. In two of the trials investigators found that metformin improved clinical outcomes (prevented need for oxygen and prevented need for acute health care use), and in the third trial a larger portion of adults with diabetes were enrolled but results did show a direction of benefit similar to that of the other trials in the per-protocol group. In the largest trial, subjects were enrolled during the delta and omicron waves and vaccinated individuals were included. The certainty of evidence that metformin prevents health care use due to COVID-19 was moderate per Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria. Many preclinical studies have shown metformin to be effective against SARS-CoV-2.
LIMITATIONS
Limitations include inclusion of only three trials and heterogeneity between trials.
CONCLUSIONS
Future trials will help define the role of metformin in COVID-19 treatment guidelines.
Graphical Abstract
Introduction
While vaccines remain highly effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (1), early outpatient treatment for coronavirus disease 2019 (COVID-19) is also needed. Wealthy countries have had disproportionate access to vaccines (2,3), uptake of vaccines remains variable, and ongoing transmission creates the opportunity for new variants (3). Moreover, geroscience-guided strategies such as metformin may also help improve immune resilience and outcomes in vulnerable older adults following infection with new and, at this time even unknown, pathogens (4).
Early in the pandemic, varied existing medications were promoted for use against SARS-CoV-2. While many repurposed therapies that lacked a sound biological rationale and preclinical evidence proved to be ineffective, a growing body of evidence appears to suggest that metformin may be a candidate for the treatment of COVID-19 (5).
A systematic review and meta-analysis of observational data in COVID-19 patients pointed to an association between metformin use and a significantly lower risk of hospitalization and mortality (6). In two recent retrospective studies of the National COVID Cohort Collaborative (N3C) database, with use of a prevalent user design of therapeutic equivalents, investigators found metformin use to be of benefit in preventing severe COVID-19 outcomes in subjects with prediabetes in comparison with placebo (7) and a lower risk of mechanical ventilation and mortality in women as compared with men when compared with sulfonylureas but not dipeptidyl peptidase 4 inhibitors, respectively (8). In addition to preventing hospitalization, metformin use may benefit hospitalized patients with COVID-19, as in one retrospective cohort study of patients with diabetes hospitalized with COVID-19 investigators found that metformin users had lower inflammatory markers, were hospitalized for fewer days, and had a lower rate of intubation (9).
Metformin acts through several mechanisms, including activation of AMP-activated protein kinase (AMPK) through liver kinase B1, activation of SIRT1 and peroxisome proliferator–activated receptor γ coactivator-1 α (PGC-1α), and inhibition of mitochondrial complex 1, nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), and mTORC1 (10,11). In addition to SARS-CoV-2, metformin has in vitro activity against other RNA viruses such as Zika and dengue (12) and may be beneficial in hepatitis B, hepatitis C, influenza (13), and HIV (4). In fact, metformin was found to be beneficial in the treatment of patients with influenza as far back as the 1940s (14). The ability of metformin to influence immune cell phenotype has also been suggested to be of potential use in COVID-19 (5).
Metformin has anti-inflammatory effects in people with and without diabetes (15). Anti-inflammatory actions of metformin that appear relevant to COVID-19 include decreased TNF-α, IL-1β, 1L-6, CXCL5, CXCL10, and MCP-1 (4,16) and inhibition of STAT3 (4), as well as reduction of neutrophil extracellular traps (14). Additionally, metformin may prevent activation of the NLRP3 inflammasome (13). Observational data suggest that pre–COVID-19 use of metformin is associated with a lower peak C-reactive protein (CRP) and admission and peak ferritin in a subgroup analysis of intensive care unit patients (17).
Metformin is known to inhibit mTOR, and targeting this pathway may be beneficial in SARS-CoV-2 infection (10,14). Metformin may also prevent entry of SARS-CoV-2 into cells via conformational changes in the ACE2 receptor, which is thought to be due to AMPK-mediated phosphorylation at S680 of the ACE2 protein (16). Other suggested beneficial effects of metformin in the context of SARS-CoV-2 infection include decreased reactive oxygen species secondary to mitochondrial electron transport chain complex 1 inhibition, decreased virus release from endosomes secondary to elevated endosomal pH (5), immune modulation, resolution of pulmonary fibrosis, prevention of mast cell activation, prevention of endothelial injury and thrombosis (14), decreased hypoxia inducible factor-1, decreased endoplasmic reticulum stress, and increased cell survival by inhibiting apoptosis, pyroptosis, ferroptosis, and the mitochondrial transition pore (13). Moreover, metformin targets aging hallmarks and may therefore potentially increase resilience to all pathogens with age (4).
Given the multiple hypothesized mechanisms of action by which metformin may be protective in the setting of SARS-CoV-2 infection, assessment of current clinical trial data of metformin as a treatment of COVID-19 is important. The objective of this review is systematic evaluation of clinical trials where individuals were randomized to metformin or placebo for testing metformin as treatment of SARS-CoV-2 infection, and to supplement this with narrative review of preclinical data. Metformin is safe, widely available, and inexpensive; has few contraindications; and thus, warrants further investigation.
Research Design and Methods
Review Question
We investigated whether metformin prevents clinical deterioration from COVID-19, inhibits growth of SARS-CoV-2, prevents pathological sequelae of infection, or improves host response to infection.
Data Sources and Searches
Two independent reviewers searched PubMed, Scopus, Cochrane COVID-19 Study Register, and ClinicalTrials.gov on 1 February 2023 for studies where adult patients with acute SARS-CoV-2 infection were randomized to metformin or placebo. We amended the protocol to focus our search terms and to expand our search to include additional databases. The full search strategy can be found in Supplementary Fig. 1.
Study Selection
We included randomized controlled trials (all phases) where investigators assessed the effect of metformin (any formulation) on pertinent clinical and laboratory outcomes in patients 18 years and older with confirmed acute SARS-CoV-2 infection.
Bias Assessment
Two independent reviewers used the Cochrane Risk of Bias 2 tool to assess the risk of bias in included studies (18). Differences were discussed between reviewers and resolved by a third coauthor.
Data Extraction and Quality Assessment
Two reviewers extracted data from included studies. For the clinical trials, the main outcome assessed was health care use, which we defined as hospitalization or death, mechanical ventilation, or emergency department (ED) visit. Additional outcomes assessed included other health care use (clinic visits, urgent care visits), hypoxia (O2 saturation ≤93%), viral load, time to clearance of virus, and other laboratory values. All results were assessed on the time frames available. Subgroups of interest were age, sex, vaccination status, variant wave (date within the pandemic), and time to initiation of drug post–symptom onset. For laboratory values, viral load, and time to clearance of virus, we reported measures of effect reported in the individual studies. Some data were extracted from the supplements of the included studies. We listed all subgroups of interest and summarized subgroup findings reported in at least two trials to assess heterogeneity of effect. No sensitivity analyses were conducted. We report point estimates and effect sizes of the main and additional outcomes of each trial in Table 3 and the text of Results and calculated the number needed to treat (NNT) for the health care use outcomes when possible. Additional data extracted included number of patients, demographic data, diabetes status, respiratory disease status, intervention with dosing information, placebo information, eligibility criteria, name of first author of each study, and year of publication (Table 2).
Table 3.
Health care use outcomes for prospective randomized controlled trials comparing metformin with placebo in individuals with COVID-19
| Need for ED visit | Need for hospitalization | Viral laboratory tests | |
|---|---|---|---|
| COVID-OUT (immediate-release metformin with a 6-day titration to 1,500 mg daily) | |||
| ITT | NNT = 69 | NNT = 53 | Not yet reported |
| OR 0.67 | OR 0.438 | ||
| mITT | NNT = 60 | NNT = 66 | Not yet reported |
| OR 0.636 | OR 0.446 | ||
| TOGETHER Trial (extended-release metformin initiated at 750 mg twice daily) | |||
| ITT | NNT = 61 OR 0.67 | NNT = 152 OR 0.94 | Time to viral clearance OR 0.99, P = 0.85 |
| Per protocol | NNT = 72 | NNT = 33 | Time to viral clearance OR 0.98, |
| OR 0.73 | OR 0.61 | P = 0.76 | |
| Need for oxygen† | Duration of hospitalization | Viral laboratory tests | |
| Ventura-López et al. (metformin glycinate 620 mg twice daily)* | Metformin 5.9 ± 4.6, placebo 10.6 ± 6.2, P = 0.03 | Metformin 8.8 ± 6.1 days, placebo 9.8 ± 5.4 days, P = 0.352 | Percent reduction in viral load: metformin 93.2 ± 15.4, placebo 78.3 ± 62.7, P = 0.013; days to negative viral load: metformin 3.3 ± 2.16, placebo 5.6 ± 0.89, P = 0.029; percent with negative viral load <3.3 days: metformin 40%, placebo 0, P = 0.043 |
| Health care use (ED visit, hospitalization, or death) | |||
| Overall | Symptom onset to drug initiation <4 days | Symptom onset to drug initiation ≥4 days | |
| COVID-OUT (mITT) | OR 0.58, 95% CI 0.35–0.94 | OR 0.45, 95% CI 0.22–0.93 | OR 0.75, 95% CI 0.38–1.47 |
| TOGETHER Trial | |||
| ITT | OR 1.14, 95% BCI 0.73–1.81 | OR 1.36, 95% CI 0.56–3.31 | OR 1.62, 95% CI 0.67–3.92 |
| Per protocol | Not reported | Not reported | Not reported |
| Ventura-López et al. | Not reported | Not reported | Not reported |
Data are means ± SD unless otherwise indicated. BCI, Bayesian credible interval.
All treated patients: patients who received at least one dose of the study drug and had both basal and later data recorded.
Oxygen need point scale. See Ventura-López et al. (28) for details on how this was calculated.
Table 2.
Basic study information and baseline patient characteristics of included studies
| COVID-OUT trial | TOGETHER Trial | Ventura-López et al. | |
|---|---|---|---|
| First author, year | Bramante et al., 2022 | Reis et al., 2022 | Ventura-López et al., 2022 |
| Type of study | Phase III RCT | Phase III RCT | Phase IIB RCT |
| No. of patients | 1,323 total; 663 metformin, 660 control (factorial design) | 418 total; 215 metformin, 203 placebo | 20; 10 metformin, 10 placebo |
| Country | U.S. | Brazil | Mexico |
| Cochrane risk of bias: R D Mi Me S O* | Low Low Low High Low High | Some High High Low Low High | Low High Low Low Low High |
| Age, years | |||
| Metformin group | 46 (median) | 52 (median) | 42.83 (mean) |
| Control group | 45 (median) | 52 (median) | 49.38 (mean) |
| Sex, % | |||
| Metformin group | 54.1 female | 55.3 female | 80 male |
| Control group | 57.9 female | 59.1 female | 90 male |
| Race/ethnicity, % | |||
| Metformin group | Not reported | ||
| White | 82.2 | 2.3 | |
| Black | 8.3 | 0.9 | |
| Asian | 3.8 | ||
| Native American | 1.5 | ||
| Native Hawaiian and Pacific Islander | 0.8 | ||
| Other | 6.5 | ||
| Latinx | 11.5 | ||
| Mixed | 90.7 | ||
| Unknown | 6.0 | ||
| Control group | Not reported | ||
| White | 83.7 | 1.5 | |
| Black | 6.8 | 2.0 | |
| Asian | 3.9 | ||
| NA | 2.6 | ||
| NHPI | 0.6 | ||
| Other | 5.6 | ||
| Latinx | 12.7 | ||
| Mixed | 91.6 | ||
| Unknown | 4.9 | ||
| Preexisting conditions, % | |||
| Metformin group | |||
| CVD | 26.8 | ||
| Chronic cardiac disease | 3.7 | ||
| Hypertension | 40.9 | 10 | |
| Dyslipidemia | 10 | ||
| Diabetes | 1.5 | 20 | |
| Type 1 diabetes | 2.3 | ||
| Type 2 diabetes | 14.4 | ||
| Respiratory disease | 20 | ||
| Chronic pulmonary disease | 1.4 | ||
| Asthma | 8.8 | ||
| Smoking | 5.1 | ||
| Tobacco use | 10 | ||
| CKD | 0.5 | ||
| Autoimmune disease | 0.9 | 10 | |
| Rheumatologic disorder | 0.9 | ||
| HIV/AIDS | 0.0 | ||
| Other comorbidities or risk factors | 11.6 | ||
| Control group | |||
| CVD | 26.5 | ||
| Chronic cardiac disease | 3.0 | ||
| Hypertension | 38.9 | 30 | |
| Dyslipidemia | 20 | ||
| Diabetes | 2.4 | 20 | |
| Type 1 diabetes | 3.0 | ||
| Type 2 diabetes | 9.4 | ||
| Respiratory disease | 0 | ||
| Chronic pulmonary disease | 1.0 | ||
| Asthma | 7.4 | ||
| Smoking | 6.9 | ||
| Tobacco use | 10 | ||
| CKD | 0.5 | ||
| Autoimmune disease | 1.0 | 10 | |
| Rheumatologic disorder | 0.0 | ||
| HIV/AIDS | 0.5 | ||
| Other comorbidities or risk factors | 10.3 | ||
| Vaccination status, % | |||
| Metformin group | Primary vaccine series: 54.1 | 0 (vaccination was an exclusion factor) | Not reported |
| Control group | Primary vaccine series: 50.2 | 0 (vaccination was an exclusion factor) | Not reported |
| BMI | |||
| Metformin group | 30 kg/m2 (median) | BMI ≥30 kg/m2, 43.7%;BMI <30 kg/m2, 56.3% | 29.1 kg/m2 (median) |
| Control group | 30 kg/m2 (median) | BMI ≥30 kg/m2, 45.3%;BMI <30 kg/m2, 53.2% | 27.7 kg/m2 (median) |
| Variant wave or date | |||
| Metformin group | Alpha (before 19 June 2021) 11.9%, delta (19 June 2021–12 December 2021) 66.4%, omicron (after 12 December 2021) 21.7% | Enrolled between 15 January 2021 and 3 April 2021 | Enrolled between 14 July 2020 and March 2021 |
| Control group | Alpha (before 19 June 2021) 12.1%, delta (19 June 2021–12 December 2021) 65.3%, omicron (after 12 December 2021) 22.6% | Enrolled between 15 January 2021 and 3 April 2021 | Enrolled between 14 July 2020 and March 2021 |
| Symptom duration, means ± SD | |||
| Metformin group | 4.8 ± 1.9 days | Not reported | Not reported |
| Control group | 4.8 ± 1.9 days | Not reported | Not reported |
| Inclusion criteria | Adults age 30–85 years with BMI ≥25 kg/m2 with confirmed SARS-CoV-2 infection within 3 days of enrollment and symptom onset ≤7 days prior to randomization | Individuals >18 years of age, acute illness resembling COVID-19 with ≤7 days of symptoms, positive antigen test, and at least one high risk criterion,† or age ≥50 years | Individuals >18 years old of both sexes, PCR-confirmed SARS-CoV-2 infection 4 days prior to randomization, and hospitalized with radiographs showing pulmonary infiltrates |
CKD, chronic kidney disease; CVD, cardiovascular disease; RCT, randomized controlled trial.
Risk of bias legend: R, bias arising from the randomization process; D, bias due to deviations for the intended interventions; Mi, bias due to missing outcome data; Me, bias in the measurement of the outcome; S, bias in the selection of the reported result; O, overall bias.
High risk criteria: diabetes, hypertension requiring medication, cardiovascular disease, symptomatic asthma (requiring chronic medications for control), smoking, obesity (BMI >30 kg/m2), transplant, stage IV chronic kidney disease or dialysis, immunosuppressed or corticosteroid use at equivalent of ≥10 mg daily prednisone or immunosuppressive drugs, cancer within the past 5 years or currently treated.
Data Synthesis and Analysis
Due to significant heterogeneity between studies, we conducted a narrative synthesis using Synthesis Without Meta-analysis (SWiM) guidelines (19) to guide our synthesis plan. The full synthesis strategy and original protocol are available from International prospective register of systematic reviews (PROSPERO) (2022, CRD42022349896 [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=349896]) (20). We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (21) to assess certainty of evidence and described synthesized findings for each outcome of interest. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (22) were followed (Supplementary Fig. 2).
Results
Searches
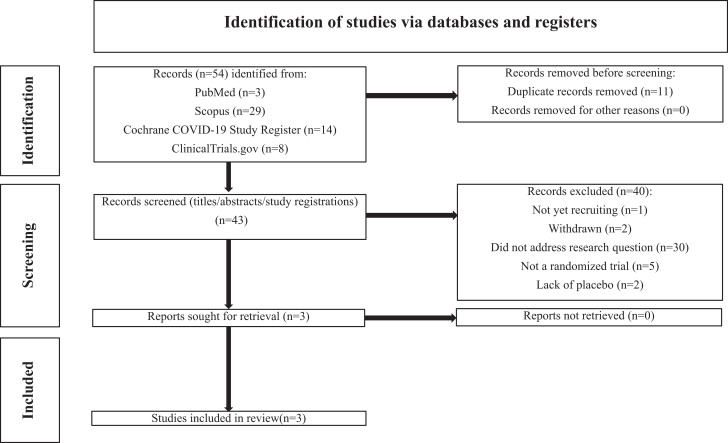
Our search returned a total of 54 results. N = 11 duplicates were removed. Titles and, if necessary, abstracts and full text articles of the remaining N = 43 studies were screened for inclusion and exclusion criteria. N = 1 was not yet recruiting. N = 2 were withdrawn. N = 30 did not address our research question. N = 5 were not randomized trials. N = 2 did not use a placebo. A total of N = 40 records were excluded. Full-text articles for the remaining N = 3 studies were retrieved and included in this systematic review. Figure 1 summarizes the results of our searches.
Figure 1.
PRISMA diagram: selection of included studies (22).
Key Findings in Preclinical Data
A large amount of preclinical data suggests potential benefits of metformin. As summarized in Table 1, direct experimental evidence of the benefits of metformin in models of SARS-CoV-2 infection includes prevention of viral replication, prevention of cellular entry, improved cell viability following infection, and prevention of an excessive inflammatory response. Gordon et al. (23) found that metformin inhibited viral growth and increased cell viability with in vitro assays against SARS-CoV-2. In human epithelial cells, metformin restored autophagic flux, inhibited cleavage of caspase-1 by nonstructural protein 6 (NSP6) and inhibited maturation and release of IL-1β, maturation of IL-18, pyroptosis, and formation of p30 N-terminal fragment of gasdermin D (GSDMD-NT) (24). However, it did not completely prevent nonpyroptotic cell death induced by NSP6 (24). Parthasarathy et al. (25) found that treatment before and after infection with SARS-CoV-2 with 10 mmol/L metformin significantly decreased infectious SARS-CoV-2 titers and viral RNA in SARS-CoV-2–infected Caco2 and Calu3 cells.
Table 1.
Basic science studies of metformin’s effect on models of SARS-CoV-2 infection
| First author, year (ref. no.) | Study design | Substrate | Relevant findings |
|---|---|---|---|
| Gordon, 2020 (23) | In vitro | Vero E6 cell line | Metformin inhibited viral growth and increased cell viability |
| Sun, 2022 (24) | In vitro | Human airway epithelial cell lines BEAS2B, A549, and 16HBE | Metformin restored autophagic flux, inhibited cleavage of caspase-1 by nonstructural protein 6 (NSP6), and inhibited maturation and release of interleukin (IL)-1β, maturation of IL-18, pyroptosis, and formation of p30 N-terminal fragment of gasdermin D (GSDMD-NT). However, it did not completely prevent nonpyroptotic cell death induced by NSP6 |
| Schaller, 2021 (35) | Ex vivo and in vitro assays | Cryopreserved bank of human lung tissue. Vero E6 cell line | Metformin decreased SARS-CoV-2 titers in a portion of donor lung tissues but not a Vero E6 cell line |
| Xian, 2021 (36) | In vitro and in vivo assays | Bone marrow–derived macrophages from nondiabetic mice | Metformin inhibited NLRP3 inflammasome activation, production of IL-1β, and secretion of IL-6 and ultimately led to attenuation of acute respiratory distress syndrome secondary to SARS-CoV-2 infection or lipopolysaccharide (LPS) exposure. Metformin had no effect on NF-κB or STAT3. Metformin treatment led to a 30–50% decreased induction of Il1b and 1l6 mRNA and increased Il10 in response to LPS exposure. Metformin inhibited mtDNA synthesis, prevented cytoplasmic oxidized mtDNA formation, and decreased POLγ activity |
| Cory, 2021 (37) | In vitro | Purified classical monocytes isolated from healthy human subjects | Metformin pretreatment of monocytes attenuated the glycolytic response and expression of IL1B, IL6, CXCL8, and TNF in response to recombinant SARS-CoV-2 spike protein subunit 1, as well as inhibited cellular respiration. Metformin pretreatment of monocytes exposed for 24 h to live SARS-CoV-2 strain WA1/2020 suppressed IL6 expression (other assays were not performed with live virus) |
| Chen, 2021 (38) | In vitro and in vivo assays | In vivo: midbrain dopaminergic neurons derived from H9 human embryonic stem cells injected into the anterior eye chambers of 6- to 8-week-old male NSG mice. In vitro: midbrain dopaminergic neuron cell line derived from human pluripotent stem cells | Metformin decreased viral RNA and prevented a senescence phenotype |
| Parthasarathy, 2022 (25) | In vitro | Calu3 (respiratory epithelial cell line). Caco2 (gut epithelial cell line) | A nearly 90% decrease in viral RNA, a nearly 2-log decrease in infectious viral titers, and a 50% decrease in viral protein levels were observed in Calu3 cells pretreated with metformin and infected with SARS-CoV-2. A 2-log reduction in viral titers was observed in Caco2 cells. Metformin treatment postinfection caused a decrease in supernatant infectious viral titers (nearly 2-log decrease in Caco2 cells and nearly 1-log decrease in Calu3 cells) and decreased viral RNA and increased AMPK phosphorylation in both cell lines. A decrease in nucleocapsid expression was also seen in Calu3 cells |
| Ventura-López, 2022 (28) | In vitro | H1299 and Vero E6 cell lines | 98% reduction in viral load for cell-associated virus (SARS-CoV-2 MX/BC1/2020) after 48 h of exposure to metformin glycinate. For the supernatant medium, an 86% reduction in viral load was also observed after 48 h. No difference was observed after 24 h in either case. 100% survival in metformin-treated H1299 cells at 48 h post–SARS-CoV-2 infection. Significantly increased cell viability in metformin-treated Vero E6 cells at 96 h post–infection with SARS-CoV-2. Variants tested included B.1.387 (D614G), B.1.1.7 (alpha), B.1.617.2 (delta), and B.1.429 + B.1.427 (epsilon) |
| Mercado-Gómez, 2022 (39) | In vitro | Human primary hepatocytes, human upcyte second-generation hepatocytes, humanized ACE2 (hACE2) mice, and wild-type mice | Metformin decreased ACE2 and Nrp1 expression and protein levels in hACE2 hepatocytes, decreased Tmprs2 expression in hACE2 hepatocytes under steatotic conditions, and decreased expression of ACE2, Nrp1, and Tmprs2 in human primary hepatocytes under steatotic conditions. Metformin decreased the infection rate in steatotic hACE2 hepatocytes and human primary hepatocytes on exposure to pseudotyped lentiviral particles that expressed the full-length spike protein of SARS-CoV-2. In both human primary hepatocytes and hACE2 hepatocytes, metformin decreased levels of extracellular angiotensinogen 1–7 (ANG1–7) and decreased expression of TNF and IL-6 |
Overview of Randomized Trial Data
Baseline characteristics of patients, Cochrane Risk of Bias 2 rating, and details of each study are presented in Table 2. Key outcomes for all three included studies are summarized in Table 3. COVID-OUT: Early Outpatient Treatment for SARS-CoV-2 Infection (COVID-19) was a phase 3 randomized, placebo-controlled, double-blind trial conducted in the U.S. and had a sample size of 1,323 (26). The TOGETHER Trial was a phase 3, randomized, placebo-controlled, double-blind trial that occurred in Brazil and included 418 patients (27). Ventura-López et al. (28) report on a phase 2, randomized, placebo-controlled, double-blind trial that occurred in Mexico and enrolled 20 patients.
Primary Outcomes Differed Across the Three Randomized Trials
For trial of Ventura-López et al., the primary efficacy variables included days of hospitalization, oxygen need, and percent viral load reduction. COVID-OUT had a composite primary end point that included hypoxemia on home oximeter, ED visit, hospitalization, or death. The TOGETHER Trial had a primary end point of either >6 h in an emergency setting or transfer to a tertiary hospital 28 days after randomization for COVID-19.
Key Differences in Inclusion Criteria
Ventura-López et al. included only hospitalized patients. COVID-OUT and the TOGETHER Trial only enrolled nonhospitalized patients. COVID-OUT enrolled pregnant women.
Risk Factors for Severe Disease
Individuals at low risk (age <30 years and normal BMI) were excluded from COVID-OUT. Individuals were required to have at least one of various high risk factors, such as cardiovascular disease, diabetes, and obesity, or age of ≥50 years, for enrollment in the TOGETHER Trial.
Vaccination Status
Vaccination information was not reported by Ventura-López et al. Of COVID-OUT patients, >50% were vaccinated. Vaccination was an exclusion factor for the TOGETHER Trial.
Metformin Dose and Formulation
COVID-OUT used immediate-release metformin titrated to 1,500 mg daily over 6 days. The TOGETHER Trial used extended-release metformin at 750 mg twice daily with no titration. Ventura-López et al. used metformin glycinate 620 mg twice daily.
Overview of Results
In the trial by Ventura-López et al. (28), metformin treatment resulted in a significantly decreased need for supplementary oxygen, a greater decrease in percent viral load, and greater decrease in days required to reach an undetectable viral load but no difference in number of days hospitalized in comparison with placebo (Table 3). At the end of the study, AST (33.8 units/L basal to 31.5 units/L, P = 0.028), LDH (257.5 IU/L basal to 256.5 IU/L, P = 0.028), CRP (4.16 mg/L basal to 0.10 mg/L, P = 0.046), IgG (969.0 mg/L basal to 781.0 mg/dL, P = 0.028), and % neutrophils (85.2% basal to 76.2%, P = 0.046) all decreased from baseline in the metformin group. Increases in % lymphocytes (8.1% basal to 17.0%, P = 0.028), and d-dimer (480.0 ng/mL basal to 634.0 ng/mL, P = 0.028) were also seen in the metformin group. In the placebo group, ferritin (1709.0 ng/mL basal to 1381.0 ng/mL, P = 0.036), CRP (4.16 mg/L basal to 0.09 mg/L, P = 0.012), and IgG (969.0 mg/dL basal to 800.5 mg/dL, P = 0.017) were decreased at the end of the study in comparison with baseline, while ALT (44.0 units/L basal to 92.0 units/L, P = 0.017) increased. No changes were observed in levels of albumin, leukocytes, procalcitonin, IL-6, IgM, or any other laboratory value measured in either group.
In COVID-OUT (26), metformin did not prevent the primary composite end point of hypoxemia, ED visit, hospitalization, or death (odds ratio [OR] 0.84, P = 0.19, 95% CI 0.66–1.09). However, results of a prespecified secondary analysis of the primary composite end point sequentially removing the component least associated with severe disease showed a reduced odds for ED visit, hospitalization, or death (OR 0.58, 95% CI 0.35–0.94) and for hospitalization or death (OR 0.47, 95% CI 0.20–1.11). No difference was observed with respect to adverse events, overall symptoms, or COVID-19–specific symptoms as compared with placebo. All analyses were performed only with concurrently enrolled control subjects in a 2 × 3 factorial design that also included ivermectin and fluvoxamine. No laboratory outcomes were reported in the manuscript included in this systematic review, but the clinical trial registration for this trial (clinical trial reg. no. NCT04510194, ClinicalTrials.gov) lists several planned substudies patients could opt into, including measurement of viral load and stool sample microbiome analysis.
In the TOGETHER Trial (27), metformin did not significantly reduce hospitalizations due to COVID-19, defined as retention in a COVID-19 emergency setting for >6 h or transfer to a tertiary care hospital 28 days after randomization (relative risk 1.14, 95% credible interval 0.73–1.81); viral clearance at day 7 (OR 0.99, 95% credible interval 0.88–1.11); time to hospitalization; or clinical improvement 28 days postrandomization (OR 1.05, 95% credible interval 0.71–1.56). However, in the per-protocol sample (83% of participants), there was a lower odds for ED visits (OR 0.73) and hospitalizations (OR 0.61), with absolute risk reduction 1.4% and 3.1%, respectively.
The NNT to prevent an ED visit was similar for COVID-OUT (NNT = 60 for the intention-to-treat (ITT) analysis and NNT = 69 for the modified ITT (mITT) analysis) and the TOGETHER Trial (NNT = 60 for the ITT analysis and NNT = 72 for the per-protocol analysis). For COVID-OUT, the NNT to prevent hospitalization was 53 in the ITT analysis and 66 in the mITT analysis. For the TOGETHER Trial, the NNT to prevent hospitalization was 152 in the ITT analysis and 33 in the per-protocol analysis (8 of 168 patients in the metformin group were hospitalized [4.8%], and 14 of 179 [7.8%] in the control group).
Heterogeneity
For COVID-OUT, metformin’s effect for preventing health care use was consistent across subgroups. In a notable subgroup, the OR was 0.45 (95% CI 0.22–0.93) for those who initiated study drugs after <4 days of symptoms and 0.75 (95% CI 0.38–1.47) after ≥4 days of symptoms. No differences were observed in any subgroup in the TOGETHER Trial. Subgroup analyses were not done in the trial by Ventura-López et al. due to small sample size.
Risk of Bias
All three trials had a high risk of bias for their respective primary outcomes per the Cochrane Risk of Bias 2 tool. The trial by Ventura-López et al. had a high risk of bias due to use of an “all treated patients” analysis in which randomized patients were included for analysis if they took at least one dose of the study drug and had initial and later data recorded. COVID-OUT had a high risk of bias due to low internal validity of the hypoxia component of the composite primary end point. The TOGETHER Trial had a high risk of bias due to use of a nonidentically matched placebo and missingness of outcome data.
GRADE Evidence Rating
In weighing all the evidence presented for included studies, the certainty of evidence of benefit of metformin for preventing a health care use outcome in COVID-19 is moderate. For all other outcomes we assessed, the certainty of evidence is low at this time. All three studies were randomized trials; however, the TOGETHER Trial and the trial by Ventura-López et al. both had significant sources of bias per the Cochrane Risk of Bias 2 tool. The trial by Ventura-López et al. had a small sample size, thus limiting generalizability of results from this trial. The differing estimates of effect between the included trials are likely due to bias, differences in populations studied, metformin dosing and formulation used, and unique outcome measures used in each trial. Most of the weight in this synthesis was given to COVID-OUT due to its much larger sample size, which showed a large effect size in reduction of health care use and the fact that the hypoxia component was the only major source of bias in the trial.
Discussion
Three randomized controlled trials testing metformin in adult patients with SARS-CoV-2 infection met our inclusion criteria. Differences in methodology, patient populations, and predominant variants during enrollment of each trial make direct comparison of outcomes challenging. The 42% decrease in ED visits, hospitalization, or death observed in a prespecified secondary analysis of COVID-OUT (26), the decrease in supplementary oxygen requirement and more rapid decline in viral load compared with placebo observed in the study by Ventura-López et al. (28), as well as the direction of benefit in the per-protocol analyses of the TOGETHER Trial collectively suggests that metformin may have a role in the treatment of SARS-CoV-2 infection. Metformin is contraindicated in patients with acute renal failure or hypoxemia (16), so the use of metformin in acute SARS-CoV-2 infection is most supported for use early in the infection, before severe COVID-19 develops (16). Additionally, a distinction must be made regarding beneficial effects observed in retrospective cohort studies of patients taking metformin prior to SARS-CoV-2 infection (16) and the trials described herein in which patients were randomized to metformin or placebo after infection but before severe COVID-19 developed. The COVID-OUT trial results were reported by time from symptom onset, and those subgroups suggest that early initiation may be critical for metformin altering the course of COVID-19 (26).
While the TOGETHER Trial did not meet the primary or any secondary end points, the per-protocol data presented in the supplement of that article suggest that metformin did prevent hospitalizations in ∼40% of the per-protocol sample. The per-protocol sample may actually be very informative in this situation because >12% of the sample in the trial had type 2 diabetes (27). The goal of quickly achieving a dose of 1,500 mg was understandable, but the lack of titration in the TOGETHER Trial may also have caused participants to not tolerate the intervention. The use of a nonidentically matched placebo introduced bias (27), which may be more influential because immediate-release metformin is over-the-counter in Brazil.
The largest limitation of the trial by Ventura-López et al. is the small sample size. In addition, the authors used an “all treated patients” analysis, which has the potential to introduce bias, similar to per-protocol analyses (28,29).
The largest limitation in COVID-OUT is the internal validity of the hypoxia component of the primary outcome. Participants self-reported SpO2 values using pulse oximeters and a daily symptom log sheet. Sources of bias in the oxygen value likely did not reflect severe COVID-19, meaning that this component of the composite outcome introduced significant noise into the primary composite outcome of hypoxemia, ED visit, hospitalization, and death (29). The U.S. Food and Drug Administration issued a warning about the questionable accuracy of pulse oximeters after the trial had begun (30). A prespecified secondary analysis with exclusion of the hypoxia component of the primary outcome showed an OR of 0.58 for metformin preventing ED visits/hospitalization/death, with CIs that do not cross 1. The point estimate for preventing hospitalization was even smaller, but the CI did include 1. A point estimate that decreases as the outcome is more closely associated with severe disease is generally an indication of effect. COVID-OUT was also the only trial to enroll pregnant women, although this subgroup was underpowered.
Temporal trends in the pandemic affect the generalizability of findings of each trial. COVID-OUT may be temporally more relevant because it enrolled during the delta and omicron waves of the virus. Additionally, COVID-OUT included individuals who had been vaccinated, so the results of this trial may be more generalizable to communities with ∼50% uptake of vaccines. The trial by Ventura-López et al. included only hospitalized patients. Thus, these patients were likely at a different point in the progression of their illness, which complicates comparison with the other two trials.
As metformin glycinate has shown efficacy in a small trial and has a slightly different mechanism of action than metformin (28), in future studies investigators should seek to characterize the significance of how this formulation compares with older formulations of metformin with respect to properties beyond glycemic control. While its action in the gut is critical for glycemic control (31), systemic action may be important for its anti-inflammatory properties in the context of COVID-19, as its primary cellular transporter, the organic cation transporter 1, is expressed on adipocytes (11). This is relevant to our findings of patients with obesity benefitting more from metformin, as obese individuals have greater expression of the gene for this transporter (32).
Preclinical Data
Direction of effect for metformin preventing severe COVID-19 is consistent with preclinical data (Table 1).
In vitro work by Parthasarathy et al. (25) that showed that metformin may prevent SARS-CoV-2 replication at a physiologically relevant dose is consistent with the results of the trial by Ventura-López et al. (28) where patients in the metformin group reached an undetectable viral load in fewer days and had a greater decrease in percent viral load reduction in comparison with placebo. However, it is not clear whether the improved viral clearance observed was due to direct antiviral properties of metformin or an improved host response.
Metformin has been shown to beneficially affect the metabolism of T cells to improve the cellular immune response to viral infection in obese mice (33). In COVID-OUT, the direction of effect for metformin preventing health care use was strongest in vaccinated individuals, which may point toward improved T cell immunity (33). A recent study suggested an association with lower influenza mortality in a retrospective cohort of vaccinated obese patients taking metformin (34), suggesting metformin may have a beneficial effect on humoral responses. Thus, the impact of metformin on humoral immunity in SARS-CoV-2 infection deserves further study.
Strengths
This is the first systematic review of the literature of randomized controlled trials testing the effect of metformin in adults with SARS-CoV-2 infection. This review also summarizes recent preclinical studies of metformin with in vitro models of SARS-CoV-2 infection.
Limitations
Limitations of this systematic review are inclusion of only three randomized controlled trials and use of narrative synthesis in place of meta-analysis due to significant heterogeneity between trials. Each trial was conducted in different areas of the world (U.S., Brazil, and Mexico) and over different time periods, which played a role in which predominant strains were circulating and differing risks of COVID-19 exposure due to country-wide restrictions and precautions in place. Each study used different methods, including different dosing and formulations of metformin; had different inclusion criteria; and included significantly different primary outcomes. Thus, direct comparisons of outcomes between trials are challenging. Additionally, all three trials had a relatively young median patient age, which means the sample populations may not have been representative of the oldest and most vulnerable older adults with multiple morbidities and frailty who might be expected to benefit the most from metformin.
Conclusions
This was a systematic review of randomized controlled trials of metformin for early treatment of COVID-19 in adults and a narrative review of the preclinical data of metformin against SARS-CoV-2. In all three clinical trials, metformin demonstrated some efficacy in preventing severe COVID-19. While none of this effect was statistically significant for a primary outcome in the phase 3 trials, there are examples of secondary outcomes changing clinical practice (29). A meta-analysis was not conducted because individual-level data were not available for all trials. Any future meta-analysis should correct for misclassification of the exposure in the Together Trial (27) and of the outcome for the COVID-OUT trial (26). The scientific community must further investigate metformin for treatment of COVID-19. Metformin’s safety has been well described, including in children and pregnancy. Further research on the effects of metformin as a COVID-19 therapeutic in broad populations should be pursued.
Article Information
Funding. C.T.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, grant K23 DK124654-01-A1.
Duality of Interest. C.T.B. is the principal investigator of a clinical trial investigating metformin for use in COVID-19 patients (clinical trial reg. no. NCT04510194, ClinicalTrials.gov) but did not receive compensation from any pharmaceutical company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.M.E., S.L.F., and C.T.B. developed the systematic review protocol and wrote the initial draft of the manuscript. S.M.E and S.L.F performed the searches, screening of studies against inclusion and exclusion criteria, and bias assessment, with differences resolved by C.T.B. All authors helped conceptualize the study and critically revised the manuscript.
Prior Presentation. Parts of this study were presented in abstract form at the Society of General Internal Medicine Annual Meeting, Aurora, CO, 10–13 May 2023.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22265911.
S.M.E. and S.L.F. contributed equally.
This article is part of a special article collection available at diabetesjournals.org/journals/collection/52/Diabetes-and-COVID-19.
References
- 1. Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med 2022;20:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharun K, Dhama K. COVID-19 vaccine diplomacy and equitable access to vaccines amid ongoing pandemic. Arch Med Res 2021;52:761–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haque A, Pant AB. Mitigating Covid-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J Autoimmun 2022;127:102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Justice JN, Gubbi S, Kulkarni AS, Bartley JM, Kuchel GA, Barzilai N. A geroscience perspective on immune resilience and infectious diseases: a potential case for metformin. Geroscience 2021;43:1093–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamyshnyi O, Matskevych V, Lenchuk T, Strilbytska O, Storey K, Lushchak O. Metformin to decrease COVID-19 severity and mortality: Molecular mechanisms and therapeutic potential. Biomed Pharmacother 2021;144:112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Yang X, Yan P, Sun T, Zeng Z, Li S. Metformin in patients with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:704666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan LE, Casiraghi E, Laraway B, et al. N3C consortium . Metformin is associated with reduced COVID-19 severity in patients with prediabetes. Diabetes Res Clin Pract 2022;194:110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bramante CT, Johnson SG, Garcia V, et al. N3C core authors . Diabetes medications and associations with Covid-19 outcomes in the N3C database: a national retrospective cohort study. PLoS One 2022;17:e0271574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Usman A, Bliden KP, Cho A, et al. Metformin use in patients hospitalized with COVID-19: lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. J Thromb Thrombolysis 2022;53:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Triggle CR, Mohammed I, Bshesh K, et al. Metformin: is it a drug for all reasons and diseases? Metabolism 2022;133:155223. [DOI] [PubMed] [Google Scholar]
- 11. Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab 2020;32:15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farfan-Morales CN, Cordero-Rivera CD, Osuna-Ramos JF, et al. The antiviral effect of metformin on zika and dengue virus infection. Sci Rep 2021;11:8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiernsperger N, Al-Salameh A, Cariou B, Lalau JD. Protection by metformin against severe Covid-19: An in-depth mechanistic analysis. Diabetes Metab 2022;48:101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibrahim S, Lowe JR, Bramante CT, et al. Metformin and Covid-19: focused review of mechanisms and current literature suggesting benefit. Front Endocrinol (Lausanne) 2021;12:587801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cameron AR, Morrison VL, Levin D, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res 2016;119:652–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bailey CJ, Gwilt M. Diabetes, metformin and the clinical course of Covid-19: outcomes, mechanisms and suggestions on the therapeutic use of metformin. Front Pharmacol 2022;13:784459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma Z, Patel N, Vemparala P, Krishnamurthy M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci Rep 2022;12:5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 19. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erickson S, Bramante C, Fenno S. Metformin for treatment of Covid-19: a systematic review of clinical trial data against SARS-Cov-2. Accessed 30 December 2022. Available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022349896 [DOI] [PMC free article] [PubMed]
- 21. Guyatt GH, Oxman AD, Vist GE, et al.; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020;583:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun X, Liu Y, Huang Z, et al. SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1. Cell Death Differ 2022;29:1240–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parthasarathy H, Tandel D, Siddiqui AH, Harshan KH. Metformin suppresses SARS-CoV-2 in cell culture. Virus Res 2022;323:199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bramante CT, Huling JD, Tignanelli CJ, et al. COVID-OUT Trial Team . Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med 2022;387:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial. Lancet Reg Health Am 2022;6:100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ventura-López C, Cervantes-Luevano K, Aguirre-Sánchez JS, et al. Treatment with metformin glycinate reduces SARS-CoV-2 viral load: an in vitro model and randomized, double-blind, phase IIb clinical trial. Biomed Pharmacother 2022;152:113223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pocock SJ, Stone GW. The primary outcome fails - what next? N Engl J Med 2016;375:861–870 [DOI] [PubMed] [Google Scholar]
- 30. U.S. Food and Drug Administration . Pulse Oximeter Accuracy and Limitations: FDA Safety Communication, 2021. Accessed 12 January 2023. Available from https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-andlimitations-fda-safety-communication
- 31. Buse JB, DeFronzo RA, Rosenstock J, et al. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes Care 2016;39:198–205 [DOI] [PubMed] [Google Scholar]
- 32. Moreno-Navarrete JM, Ortega FJ, Rodríguez-Hermosa JI, et al. OCT1 expression in adipocytes could contribute to increased metformin action in obese subjects. Diabetes 2011;60:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greene E, MacIver NJ. Targeting T cell (oxidative) metabolism to improve immunity to viral infection in the context of obesity. Front Immunol 2022;13:1025495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cummings TH, Magagnoli J, Hardin JW, Sutton SS. Patients with obesity and a history of metformin treatment have lower influenza mortality: a retrospective cohort study. Pathogens 2022;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schaller MA, Sharma Y, Dupee Z, et al. Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses. JCI Insight 2021;6:e148003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xian H, Liu Y, Rundberg Nilsson A, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 2021;54:1463–1477.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cory TJ, Emmons RS, Yarbro JR, Davis KL, Pence BD. Metformin suppresses monocyte immunometabolic activation by SARS-CoV-2 spike protein subunit 1. Front Immunol 2021;12:733921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen S, Han Y, Yang L, et al. SARS-CoV-2 infection causes dopaminergic neuron senescence. Res Sq. 21 May 2021 [preprint]. DOI: 10.21203/rs.3.rs-513461/v1 [DOI] [Google Scholar]
- 39. Mercado-Gómez M, Prieto-Fernández E, Goikoetxea-Usandizaga N, et al. The spike of SARS-CoV-2 promotes metabolic rewiring in hepatocytes. Commun Biol 2022;5:827. [DOI] [PMC free article] [PubMed] [Google Scholar]