Abstract
Background: The prevention of lower extremity fractures and fracture-related morbidity and mortality is a critical component of health services for adults living with chronic spinal cord injury (SCI). Methods: Established best practices and guideline recommendations are articulated in recent international consensus documents from the International Society of Clinical Densitometry, the Paralyzed Veterans of America Consortium for Spinal Cord Medicine and the Orthopedic Trauma Association. Results: This review is a synthesis of the aforementioned consensus documents, which highlight the pathophysiology of lower extremity bone mineral density (BMD) decline after acute SCI. The role and actions treating clinicians should take to screen, diagnose and initiate the appropriate treatment of established low bone mass/osteoporosis of the hip, distal femur or proximal tibia regions associated with moderate or high fracture risk or diagnose and manage a lower extremity fracture among adults with chronic SCI are articulated. Guidance regarding the prescription of dietary calcium, vitamin D supplements, rehabilitation interventions (passive standing, functional electrical stimulation (FES) or neuromuscular electrical stimulation (NMES)) to modify bone mass and/or anti-resorptive drug therapy (Alendronate, Denosumab, or Zoledronic Acid) is provided. In the event of lower extremity fracture, the need for timely orthopedic consultation for fracture diagnosis and interprofessional care following definitive fracture management to prevent health complications (venous thromboembolism, pressure injury, and autonomic dysreflexia) and rehabilitation interventions to return the individual to his/her pre-fracture functional abilities is emphasized. Conclusions: Interprofessional care teams should use recent consensus publications to drive sustained practice change to mitigate fracture incidence and fracture-related morbidity and mortality among adults with chronic SCI.
Keywords: fractures, osteoporosis, spinal cord injuries, rehabilitation, drug therapy, dietary supplements
1. Introduction
Spinal cord injury (SCI) results in a myriad of motor, sensory and autonomic impairments [1]. The individual’s neurological level of injury and American Spinal Cord Injury Association (ASIA) Impairment Scale (AIS) [2] category predict neurological and functional recovery [3], with a lower frequency of AIS category conversion or motor recovery in those with AIS A injuries versus those with AIS category C or D. The presence of comorbid infections, specifically pneumonia [4], wound infection [4] or urinary tract infection with sepsis [5], contributes to variation in functional outcomes. Further, the AIS category predicts the degree of muscle atrophy from disruption in nerve activation or denervation and muscle atrophy related to disuse and changes in muscle fiber types [6] to a predominance of type II fast-twitch fibers. Inflammatory stress and oxidative stress are the main mechanisms of disuse muscle atrophy after SCI [7]. Wheelchair use, having motor complete paraplegia, with absent or limited lower extremity voluntary muscle function and no spasticity, is associated with lower muscle mass and higher lower extremity muscle density [8]. Muscle atrophy contributes to a reduction in metabolic rate and an increased risk of developing type II diabetes mellitus. SCI is also associated with a reduction in growth hormone, and Insulin-like growth factor 1 (IGF-1), deficiencies likely to exacerbate the further loss of muscles and bone [9]. The fates of muscle and bone are linked over the lifetime of an individual with SCI.
The enclosed review will assist clinicians in preventing lower extremity fractures or treating lower extremity fractures once they occur among adults with chronic SCI or disease (more than two years post injury/disease onset) and established low bone mass or osteoporosis and a moderate or high fracture risk. A succinct review of the etiology of the acute and subacute changes in the bone mass and bone architecture of the hip, distal femur (DF) and proximal tibia (PT) regions early after acute traumatic SCI is provided for context, acknowledging that changes in bone mass and bone architecture after non-traumatic SCI are poorly described in the literature. Although guidelines regarding the prevention of sublesional osteoporosis in the subacute setting [10] exist, this topic is beyond the scope of this review. The latter sections of this review (Section 3, Section 4 and Section 5) address how to assess fracture risk, diagnose osteoporosis and select safe and effective therapy to augment bone mass and practically or theoretically reduce fracture risk, recognizing that no study to date has had fracture risk reduction or fracture incidence as a primary outcome. Clinical considerations when selecting drug or rehab therapy for the treatment of osteoporosis or optimal fracture management and criteria for stopping ineffective therapy are discussed. The authors acknowledge that the majority of the data that comprise the scientific underpinning of the consensus documents summarized herein [10,11,12] are based on data stemming from patients with traumatic SCI and may not be universally applicable to patients with SCI or disease. Readers are encouraged to read this review and the related consensus documents [10,11,12] in entirety to have a full appreciation of the concepts and nuances of practice. Health services to prevent fracture among adults with chronic SCI are optimally provided by an interprofessional team with effective communication skills and a strong emphasis on patient and family caregiver education and shared decision making.
2. Overview of Bone Tissue and Remodeling
2.1. Pathophysiology of Osteoporosis in SCI
The early regional changes in bone mass and bone architecture after traumatic SCI result in a lifetime-increased propensity for lower extremity fracture. The etiology of these changes are multifactorial and include changes in mechanical loading and strain on bone, bone turnover and blood flow. Possible influential non-mechanical factors may include poor nutritional status, hypercortisolism (either therapeutic or stress related), hormone status or alterations in gonadal function, endocrine disorders and neural factors [13].
Bone tissue is in a state of constant remodeling, which is a highly complex process that is necessary for skeletal adaptation from external loading as well as fracture healing. The Mechanostat theory, a derivative of Wolff’s law, states that strains within bone are kept within certain limits by adding and removing bone tissue, resulting in improved bone strength according to the forces that are imposed. However, if force is applied below a certain set point, bone tissue will ultimately be lost [14]. This law is clearly dominant when studying SCI as a model of immobilization osteoporosis secondary to paralysis. At the time of SCI, the process of bone loss is initiated primarily from the unloading of the skeleton, with the magnitude of bone loss in proportion to the time and degree to which the forces of ambulation on long bones are removed.
Contrary to the occurrence of fractures in postmenopausal osteoporosis, a condition in which fractures predominantly occur at the wrist, femoral neck, hip and lumbar spine, in persons with chronic SCI, the epiphyses of the DF and PT are the most vulnerable anatomic regions to fracture [15,16]. Histomorphometric studies from bone biopsies immediately after SCI have revealed an increase in osteoblast and osteoclast activity, which shifts to an increase in osteoclastic activity and a suppression of osteoblastic activity [17,18]. This suppression of osteoblastic activity is present for the first few months after SCI and returns to pre-injury levels with continued osteoclastic activity and bone resorption noted in the chronic phase of SCI [19,20]. This uncoupling of the osteoblast/osteoclast relationship is supported by the clinical findings of hypercalcemia and hypercalciuria and dramatically elevated markers of bone resorption [21].
This depressed bone turnover leads to a rapid loss in bone mineral density (BMD) within the first two years after SCI [22,23] and the deterioration of the trabecular lattice in the chronic phase of injury [24]. Several imaging studies using dual energy X-ray absorptiometry (DXA) have demonstrated BMD values in the DF and PT regions, which are composed predominantly of trabecular bone, to lose as much as 1% per week in the initial weeks to months after paralysis, resulting in a BMD loss of 50% at these sites over the first two years [22,25], and into the chronic phase of SCI [26,27]. Contrasted against other well-recognized conditions of precipitous bone loss, the rate of bone loss after acute SCI is considerably higher than what has been documented in postmenopausal osteoporosis (3–5% annually) [28], long-term bed rest (0.1% per week) [29] and space flight (0.25% per week) [30]. In addition to the demineralization and deterioration of the trabecular lattice that results from SCI, age-related changes in the bone matrix proteins and the bone matrix structure can ultimately modify bone geometry to compensate for decreases in BMD [31]. These changes in bone geometry can lead to an increase in endosteal resorption (decreased cortical thickness), with a corresponding increase in bone diameter to maintain resistance to bending and torsional forces. The thinning of the endocortical envelope, with a corresponding increase in the periosteum to preserve total bone strength, is a finding from several peripheral quantitative computed tomography (pQCT) studies in persons with chronic SCI [32,33].
To date, there is a paucity of information regarding the molecular mechanisms underpinning the pathophysiology of osteoporosis; however, recent evidence suggests some plausible models. The remodeling of bone requires the resorption of bone by osteoclasts and the formation of new bone by osteoblasts. Thus, the initiation of the remodeling process requires the maturation and proliferation of osteoclasts [34]. Osteoclasts arise from the monocytic cell lineage and are stimulated by the release of receptor activator of nuclear factor-κꞵ ligand (RANKL), with RANKL produced by osteocytes after they become encased in bone. Cells from the osteoblast lineage also release an inhibitor of RANKL known as osteoprotegerin (OPG), which increases bone density by decreasing bone resorption. In summary, RANKL drives bone resorption and stimulates osteoclastogenesis after binding RANKL to its receptor RANK, present in the membrane of osteoclast precursors, while OPG, released by osteoblasts, acts as a “decoy” receptor down regulating osteoclast activity and function. Osteoblasts are derived from mesenchymal stem cells that have morphological characteristics of protein-synthesizing cells that are well recognized for their role in bone formation [35]. Primarily synthesized by osteocytes and essential for osteoblastogenesis is the Wingless (Wnt)/ꞵ-cantenin signaling pathway and its antagonists, sclerostin and Dickkopf (Dkk-1). The Wnt signaling pathway, when coupled with mechanical loading, stimulates the osteoblast to secrete collagenous and noncollagenous proteins such as osteocalcin, osteonectin and bone morphogenic proteins that are responsible for the mineralization of the bone matrix [34].
Several studies have demonstrated the changes in the RANKL/OPG system and Wnt signaling pathway after SCI. Work performed in a rodent SCI model suggests that there is a several-fold increase in RANKL expression within 56 days of acute SCI while the expression of OPG is unchanged, making the ratio of RANKL to OPG highly unfavorable after acute traumatic SCI. In this animal model, there was also an almost 2-fold increase in osteoclast differentiation markers; in contrast, osteoblast differentiation markers were markedly depressed [36]. The few human studies that have studied the RANKL/OPG system have reported that after adjusting for age, compared to the ambulatory group, OPG concentrations were significantly lower in those with cervical non-ambulatory SCI, supporting this unfavorable relationship in humans with SCI, as lower OPG concentrations would promote bone resorption and lower BMD values over time [37]. Supporting the findings of an adverse RANKL/OPG environment, in a prospective observational study performed by Gifre et al. [38], the effect of recent SCI (<6 months from SCI) on the serum levels of RANKL and OPG and the evolution of BMD in 23 patients with SCI and 27 healthy AB controls was determined. Compared to the AB control group, the authors found that the serum levels of RANKL were significantly higher in the SCI group at baseline with additional increases at the 6-month follow-up assessment. In addition to evidence of an unfavorable RANKL/OPG ratio causing increased bone resorption in persons with SCI, the effect of mechanical unloading on the Wnt/ꞵ-cantenin signaling pathway as a result of SCI has also been a topic of recent reports on human studies.
In a study by Morse et al. [39], sclerostin levels were found to be significantly lower in persons with chronic SCI (n = 39) when compared to an age-adjusted control group (n = 10) and was the only circulating biomarker of bone resorption and formation significantly correlated with the leg bone mineral content and BMD of the DF. Lower sclerostin levels were representative of maximal unloading and bone loss in the chronic SCI cohort and support the viability of sclerostin as a biomarker to diagnose bone loss in persons with SCI when more advanced densitometric methods are not available. This finding is supported by other studies that show sclerostin levels are highest in subjects with short-term SCI (≤5 years) and decrease significantly over the first 5 years post-injury as a result of the dramatic effect of mechanical unloading on sclerostin levels, an elevation that is sustained for as long as 5 years after injury [40]. In another study by Gifre and colleagues [41], the authors compared Wnt/ꞵ-cantenin signaling antagonists, sclerostin and Dkk-1, in a subacute SCI cohort (n = 42) to an age-matched control group (n = 10), with additional follow up 6 and 12 months after the initial assessment in the SCI group. Contrary to these findings of lower sclerostin levels in the chronic SCI cohort reported by Morse et al. [39], in this subacute SCI group, sclerostin levels were similar to those in the AB controls but the Dkk-1 levels were significantly higher. Furthermore, while sclerostin levels were relatively unchanged 6 and 12 months after the initial assessment, the concentrations of the antagonist Dkk-1 increased significantly a few months after SCI and remained elevated over the course of the study. Collectively, these findings provide some insight into the complexity of pathways influencing bone remodeling in patients with spinal cord injury and low bone mass.
2.2. Indexes of Bone Turnover after SCI
As a result of the invasiveness and difficulty of performing bone histomorphometry studies to measure bone formation and resorption in humans, more sensitive and specific biomarkers of bone resorption and formation have been developed, with several studies performed in persons with SCI. In an early cross-sectional report by Pietschmann et al. [42], the levels of osteocalcin (OC), a marker of bone formation, were in the low normal range in 41 SCI patients 1 month after injury that increased to a peak value 7 months after SCI. In addition to OC, another biomarker of bone formation, Procollagen 1 Intact N-terminal Propeptide (P1NP), followed the same trend as that of OC where a transient depression was followed by elevation back to the normal range [43]. Contrary to the markers of bone formation that demonstrated a temporary and mild suppression soon after SCI, C-terminal cross-linking telopeptide of type I collagen (CTX-1) and N-terminal cross-linking telopeptide of type I collagen (NTX-1), biomarkers of bone resorption, were dramatically elevated outside of the normal range and remained elevated in a majority of patients with chronic SCI [20,21].
2.3. Calcium Metabolism and Systemic Factors Regulating Bone Metabolism
During the acute SCI phase, where skeletal resorption is the greatest (calcium released from bone), serum ionized calcium levels are elevated with an increase in the renal clearance of calcium (hypercalciuria and a high risk of renal stones). The hypercalciuria observed during the acute phase of injury begins within days of SCI and may stay elevated for several months after injury, returning to baseline levels within one year [44]. As a result of elevated ionized and serum calcium levels, there is a suppression of parathryroid hormone (PTH) release and the reduced conversion of 25 (OH) vitamin D (25 OH D) to the more biologically active 1,25-dihydroxyvitamin D (1,25(OH)2D), ultimately reducing intestinal calcium absorption [45]. During this acute period, calcium intake is often restricted by health care professionals due to the erroneous belief that increased calcium intake will increase the risk of renal stones. In chronic SCI, as in the general population, the secretion of PTH and the increase of circulating 1,25(OH)2D are subject to control by negative feedback mechanisms related to the level of serum calcium, which is, in turn, influenced by 25(OH)D levels [34].
Additional systemic factors that are important to normal bone metabolism include androgens and estrogens, which have particular significance in persons with chronic SCI. It is appreciated that androgens have a pivotal role in regulating bone homeostasis by inhibiting the osteoblastic release of local stimulating factors for osteoclastogenesis. There have been several reports of relative or absolute androgen deficiency in small cohorts of men with SCI [46,47], with a more recent retrospective analysis by Bauman et al. [48] demonstrating the prevalence of low total testosterone levels in 243 healthy men with chronic SCI. The authors demonstrated a 0.6%/year decline in serum total testosterone compared to a 0.4%/year decline in men in the Massachusetts Male Aging Study. In addition, low serum testosterone levels were observed at earlier decades of life with a higher prevalence in men with SCI than in non-disabled control subjects [49,50]. The role of estrogens in bone homeostasis is well-recognized, as the decrease in estrogen at menopause in women is the primary cause of osteoporosis. Estrogen has been observed to have anti-apoptotic effects leading to increased cell survival and a decreased inflammatory response—mechanisms of importance related to the maintenance of bone mass [51]. Several studies have shown that estrogen maintains bone homeostasis by inhibiting osteoblast and osteocyte apoptosis and preventing excessive bone resorption [52,53]. The ability of estrogens to prevent bone cell apoptosis may be just as significant in the model of immobilization osteoporosis. Animal models have shown that hind limb unloading results in immediate osteocyte and osteoblast apoptosis [54], as well as increased osteoclastic bone resorption [55]. In women with SCI, serum estrogen levels have been shown to be significantly lower than those in able-bodied (AB) controls and may be attributed to bone loss years after injury [56]. When compared to estrogen-free postmenopausal women, women with SCI demonstrate significant deterioration of trabecular bone [57]. Several reviews have summarized emerging therapies including anti-sclerostin antibodies, the mechanical loading of the lower extremity with electrical stimulation of muscle and the mechanical stimulation of bone via whole-body vibration therapy [58].
In the setting of chronic spinal cord injury/disease (SCI/D), the aforementioned changes in bone turnover, bone mass and bone architecture will have occurred prior to a clinician’s or interprofessional team’s initial assessment. The patient’s presentation may also be complicated by the presence of pre-morbid metabolic bone disease unrelated to their spinal cord impairments. Section 2 reviews the diagnostic criteria for sublesional osteoporosis, serum screening and fracture risk assessment.
3. Screening and Diagnosis of Sublesional Osteoporosis
3.1. Screening for Sublesional Osteoporosis
As highlighted previously, acute SCI results in rapid declines in hip, DF and PT bone mass, resulting in sublesional osteoporosis and increased fracture risk [58,59]. Fractures after SCI are most common at the PT followed by the DF [60], increase in incidence with time since injury and are most prevalent in those with motor complete (AIS A) injury [61,62,63]. Importantly, fracture-related deformity or limb length discrepancies or the impact of prior fracture on future fracture risk may limit the ability of patients to use emerging rehabilitative technologies such as body weight support treadmills, robotic-exoskeletons and brain computer interfaces or to benefit from advances in cure research.
Screening for sublesional osteoporosis is clinically indicated for all individuals with traumatic or nontraumatic SCI/D [11]. Screening includes a complete history and physical examination, laboratory evaluation to identify potentially treatable causes of secondary osteoporosis that may exacerbate bone loss (such as vitamin D deficiency) and the assessment of bone density and fracture risk by DXA or pQCT according to recently published SCI-specific position statements endorsed by the International Society for Clinical Densitometry [11].
The history and physical exam should focus on injury characteristics (date, age at injury, etiology of injury, neurological level and AIS category), nutrition status (protein, calcium and vitamin D intake), mobility and ambulatory status, lifestyle factors (physical activity including weight-bearing and loading activities, electrical stimulation, spinal cord stimulation, tobacco use and alcohol use), medication use, personal fracture history, parental fracture history, injurious fall history, menopausal status and menstrual status for women, spasticity assessment, body mass index and history of other comorbid conditions (such as kidney disease or diabetes), which may worsen the rate or severity of BMD decline [10]. Clinicians are encouraged to conduct fracture risk assessments on an annual basis or following a recent fall or incident (see Table 1).
3.2. Laboratory Screening
Regarding laboratory screening, vitamin D deficiency is common after SCI [64]. Because vitamin D is required for calcium absorption, vitamin D deficiency may lead to secondary hyperparathyroidism with increased osteoclastic bone resorption to liberate calcium from the skeleton, thereby maintaining adequate serum calcium levels. Similarly, hyperthyroidism [65], anemia [66], renal disease [67], liver disease [68,69] and diabetes mellitus [70,71] are all associated with bone loss or increased fracture risk. For this reason, all adults with SCI should have the following laboratory workup: serum 25-hydroxyvitamin D (25-(OH)D), complete blood cell count, ionized calcium or albumin-corrected serum calcium, phosphate, intact parathyroid hormone, creatinine (and estimated glomerular filtration rate), bone-specific alkaline phosphatase and transaminases, hemoglobin A1C, thyroid-stimulating hormone and 24-hour urine collection for calcium and creatinine excretion. The assessment of the hormonal status is also indicated for pre-menopausal women and all men. This includes the measurement of prolactin, follicle-stimulating hormone (FSH), luteinizing hormone (LH) and estradiol levels for both men and premenopausal women and morning fasting bioavailable testosterone for men. Additional laboratory testing is indicated to rule out other medical conditions. Serum protein electrophoresis should be considered for individuals who present with a vertebral compression fracture of unknown etiology to assess multiple myeloma or monoclonal gammopathy, a 24-hour urinary cortisol/overnight dexamethasone suppression test may be considered if Cushing’s disease is suspected and anti-tissue transglutaminase immunoglobulin A antibody levels may be measured if celiac disease is suspected. In addition to the history, physical exam and laboratory screening, bone density should be measured to screen for sublesional osteoporosis according to SCI-specific testing protocols [11].
3.3. Diagnostic Imaging for Sublesional Osteoporosis
Bone density may be assessed via established and reported pQCT protocols [11]. However, DXA is the current clinical “gold standard” based on cost, availability and evidence-based standards for osteoporosis diagnosis and fracture risk prediction. Because the prevalence of osteoporosis is much greater in individuals with SCI compared to the general population, irrespective of age, race, sex, or ambulatory status, all adults with SCI should have a baseline DXA of the total hip, DF and PT as soon as feasible after injury. The lumbar spine is not a recommended site for DXA assessment based on the technical difficulties associated with accurately determining bone density due to degenerative changes within the posterior elements, heterotopic ossification and/or hardware in the spine region of interest [72,73].
DXA testing should be repeated based on clinical indication such as a change in the individual’s functional abilities, interval fracture or at 1–2-year intervals to monitor response to therapy. SCI-specific protocols have been developed and previously reported for bone density determination by DXA, including acquisition protocols and normative BMD data for the DF and PT [74]. When possible, serial bone density measurements should be obtained on the same DXA machine with the same operational software and with a reported least significant change calculation for the DXA operator.
Once bone density is determined by DXA, osteoporosis is diagnosed in SCI according to the World Health Organization’s definitions using age-appropriate reference BMD to calculate T-scores or Z-scores (reported as standard deviations from the population mean). For post-menopausal women and men aged 50 or older, a T-score < −2.5 at any skeletal site tested (hip, DF or PT) is diagnosed as osteoporosis. For premenopausal women and men under the age of 50, a Z-score < −2 is diagnostic of low bone mass or secondary osteoporosis due to SCI. Additionally, an osteoporotic fracture that occurs after SCI, irrespective of bone density, is diagnostic of osteoporosis. Diagnostic criteria for sublesional osteoporosis are summarized in Table 1.
Table 1.
Definition of sublesional osteoporosis (SLOP).
| Age Range | Definition |
|---|---|
| Men ≥ 50 years or postmenopausal women |
Hip or knee region T score ≤ −2.5 |
| Men < 49 years or premenopausal women | Hip or knee region Z score < −2.0 with ≥3 risk factors for fracture |
| Men or women age 16–90 | Prior fragility fracture and no identifiable etiology of osteoporosis other than SCI |
Reprinted with permission from Topics in Spinal Cord Injury Rehabilitation; American Spinal Injury Association [75].
4. Treatment of Osteoporosis in SCI
4.1. Vitamin D Supplements
Vitamin D is a fat-soluable vitamin that helps the patient’s body absorb and retain calcium and phosphorus. An individual’s serum levels of vitamin D are dependent on the gut’s ability to absorb dietary fat [76]. Comorbid liver disease, celiac disease, Crohn’s disease and ulcerative colitis can impede vitamin D absorption [77]. Vitamin D can be found in cod liver oil, salmon, tuna, mackerel, sardines, eggs, mushrooms, milk and dairy products or orange juice fortified with vitamin D [78]. Most patients with chronic SCI cannot obtain an adequate vitamin D serum level from food sources. Vitamin D3 supplements (cholecalciferol) are usually required to ensure an adequate vitamin D intake [79].
Vitamin D insufficiency is common among those with SCI [80,81,82,83]; some estimate the prevalence of vitamin D insufficiency to be as high as 93%. A serum level of 50 nmol/L defines vitamin D deficiency, whereas a level of 75 nmol/L defines vitamin D insufficiency [84]. Low vitamin D (25-(OH)D) levels are associated with low serum testosterone levels [85], poor immune and respiratory system function, impaired balance and low leisure time physical activity [86]. There is a small amount of data regarding the optimal levels of vitamin D for bone and muscle health in individuals with chronic SCI. A validated assay should be used [87,88,89,90,91,92,93,94,95] to assess the vitamin D serum levels of adults with chronic SCI.
Vitamin D maintenance therapy with 1000–2000 IU or 25–50 mcg of vitamin D3 (cholecalciferol) per day is recommended [79,96], assuming that vitamin D serum levels are adequate. A reasonable target for a 25-(OH) D level is 100 nmol/day (40 ng/mL) [79,97]. Bauman et al. [88,98] reported that 2000 IU of vitamin D supplementation raised 25-OH vitamin D serum levels among individuals with chronic SCI.
4.2. Dietary Calcium and Nutritional Considerations
An adequate calcium intake is important to ensure patients with chronic SCI maintain their lower extremity BMD and reduce their fracture risk. A majority of patients with SCI (≈72% of one study) do not meet the Dietary Reference Intake for Calcium [99]. Patients with SCI and neurogenic bowel dysfunction often need to consume dairy and non-dairy sources of calcium to achieve an adequate intake. Clinicians are encouraged to have their patients complete a food frequency questionnaire such as the Calcium Assessment Tool [100] and to review their patient’s cumulative calcium intake through supplements and diet prior to making recommendations or initiating dietary counselling. An individual’s calcium intake should preferably be through diet, as opposed to supplements, and efforts should be made to ensure the patient’s intake meets the Dietary Reference Intake [101]. An individual’s recommended dietary calcium intake depends on his/her age, sex and history of renal and bladder stones (Table 2).
Table 2.
The following are recommendations for calcium intake as a combination of food and supplements (preference for dietary intake over supplements).
| Group and Age | Calcium Recommendation |
|---|---|
| Men & premenopausal women age 19–50 years | 1000 mg/day |
| Men 50–70 years | 1000 mg/day |
| Women 50–70 years | 1000–1200 mg/day |
| Men and women 71+ years | 1000–1200 mg/day |
Reprinted with permission from Paralyzed Veterans of America; Consortium for Spinal Cord Medicine Clinical Practice Guidelines [10].
Individuals with SCI, neurogenic bladder and calcium oxalate stones should have a lower calcium intake of 750 mg/day from food and/or supplements. Patients with chronic SCI taking vitamin D may experience hypercalciuria and are at an increased risk of developing renal stones. Patients with chronic SCI frequently have recurrent stones, staghorn calculi and bilateral stone disease [102]. The cumulative proportion of patients with chronic SCI who had renal calculi was 38% in a longitudinal study of 45 years [103]. An annual screening ultrasound of the bladder and kidneys can detect asymptomatic stone disease, particularly in patients with frequent urinary tract infections.
Calcium apatite and oxalate stones are the most prevalent. SCI clinicians should work to differentiate calcium oxalate and hydroxyapatite stones [104] among patients with SCI. Some SCI patients may need to adhere to a low oxalate diet to reduce their stone disease risk. Foods to avoid that are high in oxalates include spinach, cranberries, rhubarb, beets, beans, berries, chocolate or coffee.
4.3. Fracture Risk and Rehabilitation Therapy
Prior to initiating rehabilitation therapy, clinicians should review with their patients any concurrent medical therapies with potential adverse events on bone density/quality and non-BMD risk factors for fracture (see Table 3, e.g., alcohol intake). Prior to patients with chronic injury engaging in rehabilitation therapy that requires loading, several factors should be considered by the clinician, including the patient’s hip, knee and ankle region range of motion, lower extremity alignment and BMD.
Table 3.
Fracture Risk Factor Checklist Prior to Rehabilitation Intervention (Adapted with permission [10]).
| Established Fracture Risk Factors | |
|---|---|
| □ | Alcohol intake > 5 servings per day |
| □ | Paraplegia |
| □ | Duration of SCI ≥ 10 years |
| □ | Motor complete injury (AIS A–B) |
| □ | Family history of fracture |
| □ | Hip fracture in the last year |
| □ | Routine use of benzodiazepines, anticonvulsants (i.e., carbamazepine, phenytoin), heparin, opioid analgesia (≥28 mg morphine for a 3-month period) |
Reprinted with permission from Paralyzed Veterans of America; Consortium for Spinal Cord Medicine Clinical Practice Guidelines [10].
As sublesional osteoporosis progresses, there is a reduction in trabecular volume and number as well as cortical thinning. Due to these changes, the femur and tibia become more susceptible to injury after a torsional stress or non-anatomic loading. There are currently no established thresholds of BMD that absolutely contraindicate patients with SCI from participating in weight-bearing activities [11]. However, it is critical that clinicians review with their patients the possible risk of fracture when participating in rehabilitation therapy and document the consent discussion regarding the risks and benefits of the planned rehabilitation intervention. In select cases, particularly among patients with chronic SCI, clinicians may consider radiographs of the patients’ forefoot, calcaneus and ankle in order to confirm the patient’s self-report of no pre-morbid fracture history. It is critical to report fractures and other musculoskeletal injuries that occur during rehabilitation therapy, although adverse event reporting during exercise interventions has typically been under-reported, inconsistent and suboptimal [105].
4.4. Rehabilitation Therapy: Standing and Walking
Bone is a dynamic tissue, which responds to increases and decreases in load. Standing and walking are common rehabilitation strategies to facilitate loading following SCI, provided these tasks are functionally appropriate for the individual based on their neurologic level of injury and AIS category, the individual’s therapeutic goals and the plan of care [106]. Passive standing, in which muscle activation is unlikely, may be performed with individuals with SCI in a standing frame, standing wheelchair, long leg braces, or other devices [10]. Passive standing for 1 h, five times per week, may reduce BMD decline, specifically in the hip and knee regions [107]. However, standing with an orthosis must be done longitudinally to measure the effects on lower extremity BMD. The high percentage of the force applied through the upper limb and shoulder when using forearm crutches (30–50% of body weight) and the energy cost of donning and doffing lower extremity orthoses or transferring in and out of a standing frame are important feasibility constraints that may impede longitudinal passive standing adherence [108].
Walk training may begin on a treadmill and can include supportive harnesses, orthoses, exoskeletons [109], or assistive devices [110] before progressing to overground walking with or without a gait aid. Despite the obvious progressions in loading available through the provisions of harnesses, orthoses/exoskeletons and supportive gait aids, walking over ground or on a treadmill is an insufficient stimulus to maintain or improve low bone mass in patients with chronic SCI. Walking [111] is a recommended therapeutic goal to maintain or increase bone strength in the general population [112]. Although the therapeutic value of walking as a rehabilitation intervention to improve functional independence and the patient’s physiological health is acknowledged [108], there is weak evidence and significant methodological limitations in treadmill, orthoses and walking training interventions for improving lower extremity BMD in the setting of chronic SCI [113,114,115].
4.5. Rehabilitation Therapy: Neuromuscular Electrical Stimulation (NMES) and Fucntional Electrical Stimulation (FES)
The recently published Paralyzed Veterans of America (PVA) guidelines distinguish neuromuscular electrical stimulation (NMES) and functional electrical stimulation (FES). NMES is defined as, “the application of an electrical current of sufficient intensity to elicit muscle contraction”, whereas FES refers to,”the process of pairing NMES simultaneously or intermittently with a functional task, such as cycling or rowing”. NMES and FES have the potential to mechanically load bone and influences bone mass and bone structure, and muscle contractions can produce physiological loads on bone in order to activate the muscle bone unit [36] through Wnt signalling molecules, Receptor activator of nuclear factor kappa-Β ligand (RANKL) and Osteoprotegerin (OPG). NMES or FES is recommended for treating patients with low bone mass or osteoporosis of the lower extremities in persons with a SCI [116,117]. FES cycling or FES standing for 30 mins per day or 3–5 h per week over one year may improve skeletal health [118,119,120,121]. Effective NMES or FES should (1) create a visibly strong contraction against incremental resistance when doing an isometric contraction, moving against gravity or weight bearing, (2) use a pulse duration of 200 us or higher and (3) use frequencies of 20–33 Hz and (4) amplitudes of up to 140 mA for at least 9 months. Ideally, rehabilitation interventions result in adaptive changes in bone architecture, which will produce reductions in fracture incidence. To maintain the effects of rehabilitation interventions on bone density, lower extremity muscle activation and load bearing need to continue in perpetuity, a major feasibility and cost constraint for most individuals. Further, there are many relative contraindications to the delivery of rehabilitation interventions among patients with chronic SCI, particularly those with a high fracture risk and prior fracture.
4.6. Drug Therapy: Alendronate, Zoledronic Acid and Densoumab
Along with optimizing vitamin D and calcium intake and stimulating bone formation with rehabilitation interventions, pharmacological interventions should be considered best practice for the management of patients with chronic SCI, established osteoporosis and moderate or high fracture risk. There are well-documented precipitous declines in trabecular BMD [32,122,123,124] during the first 2 years after SCI, after which BMD declines but to a far lesser degree over the remainder of the individual’s lifespan. The PVA clinical practice guidelines recommend that individuals with SCI and diagnosed low bone mass (Z-score of < −2.0) or osteoporosis (T-score of ≤ −2.5) be offered oral Alendronate, intravenous (IV) Zoledronic acid (ZA), or subcutaneous Denosumab to treat total hip, DF, or PT BMD [10]. After a discussion of the merits and potential adverse effects, the patient may want to pursue drug therapy to prevent further declines in hip and knee region BMD.
Although there are many oral bisphosphonates on the market, only Alendronate has been studied in the chronic SCI population [10]. Alendronate (10 mg daily or 70 mg weekly) may maintain or improve BMD and increase the cortical bone mineral content in the tibial epiphysis, tibial diaphysis, total hip [125], femoral neck, DF epiphysis, distal femoral metaphysis, PT epiphysis and spine [126]. ZA is a more potent bisphosphonate than Alendronate. ZA, given as a one-time infusion of 5 mg/100 mg solution infused over 15 min, has been reported to maintain BMD of the total hip, femoral neck and distal radius and produce increments in the cortical bone volume of the DF metaphysis and PT metaphysis when used with FES cycling [127]. Further, some studies suggest therapy with Denosumab for one year within 15 months of injury onset to increase total hip and femoral neck BMD [128]. It is important to note that the mechanisms of action and effects of bisphosphonates and Denosumab differ (Baron et al. 2011; Tu et al. 2018) [129,130], but a discussion of these pathways is not within the scope of this review.
4.6.1. Safety Considerations, Absolute and Relative Contraindications to Therapy
Prior to initiating drug therapy for sublesional osteoporosis, clinicians are encouraged to use shared decision making that accounts for the patient’s allergies, comorbidities, values and risk/adverse event tolerance. A detailed discussion of the relative and absolute contraindications of specific therapies must be considered as well as the patient’s sex and age before prescribing therapy, as there are unique contraindications for females of childbearing potential and unique contraindications based on age, allergies, prior cancer, dental history and the presence or absence of lower extremity edema or pressure injury. Alendronate, ZA and Denosumab are absolutely contraindicated in pregnancy [10]. Sexually active females of childbearing age choosing to take these medications will require birth control.
An important ZA relative contraindication is the patient’s renal function. Patients whose creatinine clearance is less than <30–35 mL/min should not be offered this therapy [10]. Likewise, a latex allergy, significant lower extremity edema or pressure sores [131] and hypocalcemia should be ruled out prior to prescribing Denosumab treatment [10], as Denosumab can increase the risk of lower extremity cellulitis.
Serious adverse events such as osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF) are rare and have been reported with long-term oral or IV bisphosphonate therapy in the non-SCI population. Patients who report a prior history of cancer and radiotherapy are most like to develop ONJ [132,133]. The reported incidence of ONJ in patients taking oral or IV bisphosphonates long-term varies from 1 per 10,000 to 1 per 100,000 patient-treatment years [134]. Prior to oral bisphosphonate prescription or IV ZA administration, clinicians are encouraged to examine the patient’s oral cavity for exposed gums, broken or abscessed teeth, or gum disease.
AFF is very rare and is often characterized by a transverse stress fracture of the femoral shaft in the sub trochanteric region, usually with a prodrome of groin pain originating laterally and extending through both cortices, with localized periosteal or endosteal cortical thickening [135], without a history of trauma. AFF incidence ranges from 1.8 per 100,000 per year after 2 years of bisphosphonate exposure to 113 per 100,000 per year after 8 to 9.9 years of drug exposure [136], suggesting AFF risk increases with the duration of bisphosphonate therapy. For primary postmenopausal osteoporosis, the benefit of bisphosphonate therapy from reduced fracture risk exceeds the risk of developing either ONJ or AFF [137]. AFF will typically result in the cessation of bisphosphonate therapy [138]. Fortunately, no definitive cases of ONJ or AFF have been reported following drug administration among patients with chronic SCI [139,140,141,142,143,144,145,146].
4.6.2. Monitoring and Cessation of Ineffective Therapy
The optimal duration of osteoporosis therapy for patients with chronic SCI is unknown. Prescribing drug therapy requires the treating physician to routinely assess treatment adherence and treatment effectiveness and screen for side effects. Physicians should have a low threshold for stopping ineffective therapy or therapy associated with persistent side effects. Bisphosphonate adherence is poor in general; patients with chronic SCI in the community report being on a mean of 11 (SD = 6) classes of drugs [147]. The most common drugs prescribed are laxatives, opioids and cardiovascular-related drugs. Thus, for patients with chronic SCI and polypharmacy assessment of osteoporosis, drug therapy adherence is important before determining whether therapy has been ineffective. The most commonly report reasons for poor adherence include gastrointestinal side effects [148].
It is critical that clinicians reassess and consider stopping, continuing or changing therapy on a routine basis, particularly if significant declines in hip, DF or PT BMD occur after two years of therapy adherence or if a lower extremity fracture occurs in an individual with chronic SCI who has been adherent to therapy for at least one year.
Clinicians can use the least significant change (LSC) to detect true biological change over time, defined as a gain or loss in BMD that exceeds the LSC of the densitometer and the local DXA technologist. LSC is specific to the diagnostic tool used and its accompanying precision. In some circumstances, drug holidays may also appropriate for individuals with moderate fracture risk following 5 consecutive years of oral Alendronate or 3 years of intravenous ZA. In the context of low bone mass or osteoporosis and moderate-to-high fracture risk, there is much uncertainty regarding the optimal duration of treatment and the circumstances or BMD thresholds for changing drug therapy. In the current scenario, clinicians are encouraged to follow the consensus opinions for therapy duration for post-menopausal and steroid-induced osteoporosis in the absence of evidence, recognizing an urgent and compelling need for better evidence to recommend actions regarding treatment duration [149,150]. Alternative treatment options may involve hormone therapy, although in the current review we are unable to discuss the role of estrogen and hormone therapy [151]. Likewise, there is a need to further explore the effects of hormone therapy on the maintenance of bone mass in SCI/D populations [152].
5. Fracture Management
This section of the review underscores the importance of an initial orthopedic consultation to confirm the presence of a fracture and discusses the merits of conservative versus operative fracture management. Specific medical and mobility considerations to reduce fracture-related morbidity and the current rates of fracture-related mortality are discussed.
5.1. Fracture Incidence
Long bone fractures are common, with an annual incidence of 2–3 lower extremity fractures/100 person years [15,20,61,153]. The tibia/fibula, femur and hip are among the most common sites fractured [61,154]. The majority of these fractures result from wheelchair or transfer-related falls [60,155,156]. Other lower extremity sites, including fractures of the talus [157] and calcaneus [158], have been reported to occur while using exoskeletons [159]. Upper extremity fractures are less common, with one study reporting that approximately 83% of all long bone fractures are in the lower and only 17% in the upper extremity, with the most common upper fracture site being the humerus [160]. The risk of mortality following a lower extremity fracture is greater in men older than 50 years of age (hazard ratio [HR] 3.42, 95% confidence interval [CI] 2.75–4.25), in men with motor complete injury (HR 3.13, 95% CI 2.19–4.45) and in men with a high Charlson Comorbidity Index [161]. Women with SCI over age 50 are at a higher risk of fracture than are younger women or men of any age [HR 1.56, (95% CI 1.18–2.06)]. Both the fracture itself and chronic comorbid conditions contribute to mortality in older men with SCI.
5.2. Operative or Non-Operative Management of Fractures
The goal for the treatment of osteoporotic fractures, whether done operatively or non-operatively, is to return the individual to their pre-fracture level of mobility and independence [162,163]. Treatment choices should be guided by an ascertainment of the risks and benefits of operative versus non-operative approaches, the person’s premorbid level of function and consideration for the impact of fracture management strategies on the ability to use both current and future mobility technologies [12]. Regarding the latter, the effects of the fracture treatment modality on the range of motion are important to consider as current robotic exoskeleton devices require a specific range of motion for hip and knee extension and neutral ankle-joint positions [159,164,165,166,167,168,169,170,171,172]. Education on the risks and benefits of operative compared with non-operative approaches should be provided, and shared decision-making should form the basis for the fracture treatment [12]. However, the extent to which this is happening in clinical practice is not clear. In one study including Veterans with a lower extremity fracture, some felt that they would have preferred operative treatments but were not offered these [173].
Non-operative treatment of fractures includes circular [15] or bivalve [174] casts, padded [175] or pillow [174] splints, leg lengthening devices [176] plaster casts or shells [177,178], traction [15,174,175,178,179,180,181,182], external fixation [176] and observation [183]. Such non-operative strategies have traditionally been considered the treatment of choice for fractures, as historically surgical treatment of fractures was associated with high complication rates [180,184]. A CPG for the management of acute fractures concluded that if non-operative therapies are chosen, these should be well padded with attention to neutral rotation alignment and should allow for frequent skin inspection [12]. No specific recommendations for particular immobilization devices were given [12].
However, non-operative therapies for fracture treatment have risks; these largely stem from a longer time for fracture healing with potential complications from immobilization and subsequent impact on the activities of daily living [185]. In patients with lower extremity fractures in which the treatment was fracture immobilization with a splint, more than 80% were admitted for prolonged inpatient stays, principally because of inadequate support at home or inaccessibility of the home environment for the patient [186]. These concerns have led to an interest in operative strategies for fracture management. Such strategies include open reduction with internal fixation (ORIF) [15,183], intramedullary rodding and/or nailing [155,174,175,176,177,178,187,188,189], plate and screw fixation [182,188], resections [182], compression screw devices [187] and total or hemi arthroplasties [175,183,190]. Amputations are most often done after the failure of non-operative treatments [183,191].
There are a few definite indications for the primary operative management of extremity fractures. Open fractures and fractures that an orthopedic surgeon determines would not reliably heal in a position that would restore the patient to their pre-fracture function status are two such situations [12]. Other indications for surgical intervention stem from the failure of primary non-operative therapy, including the development of a nonunion or malunion associated with residual deformity that impairs functional ability. Conversion to an open fracture after primary non-operative management is another indication for surgery. Surgical treatment should also be considered for a National Pressure Ulcer Advisory Panel Stage-3 or 4 pressure injury that has failed to heal with conservative therapy [12].
ORIF has historically been the most common operative strategy for long bone fracture repair [175,181,183,189,192]. The Orthopaedic Trauma Association provides operative strategies by fracture site. These include internal fixation for nondisplaced hip and femur fractures, arthroplasty for displaced femoral neck fractures and internal or external fixation for tibia/fibula and ankle fractures. Percutaneous fixation is considered for foot fractures. In view of the increasing use of exoskeleton devices, the authors suggested that patients should avoid early return to weight-bearing activities after the healing of ankle/foot fractures because of the high risk of re-fracture with upright activities such as the exoskeleton [12].
Few reports have compared outcomes following non-operative versus operative fracture treatments, and there are no randomized clinical trials addressing this. Several studies have suggested improved outcomes following the operative management of fractures [155,177,188,189]. Some [155,177], but not all [182,193], have noted fewer fracture nonunion with operative compared with non-operative therapies. No differences in malunion rates by type of fracture treatment were noted in one report [194]. A better range of motion was achieved in a cohort managed surgically compared to those managed non-operatively [177]. Amputations resulting from fracture-related complications are more than twice as common when nonsurgical compared to surgical treatments are used as the initial management for lower extremity fractures [191]; diabetes mellitus [183,191,195] and peripheral vascular disease [191,195] are additional risk factors [191]. Mortality is increased following lower extremity fractures [161]; however, no differences in mortality based on operative or non-operative treatment have been reported [183,185].
5.3. Rehabilitation Post Fracture
Physical and occupational therapists and, for wheelchair users, seating specialists, should be involved in fracture care to assist with range of motion exercises, provide recommendations for braces and devices to prevent pressure injuries and assess needs for patient equipment, skills training and/or temporary increases in caregiver support [196].
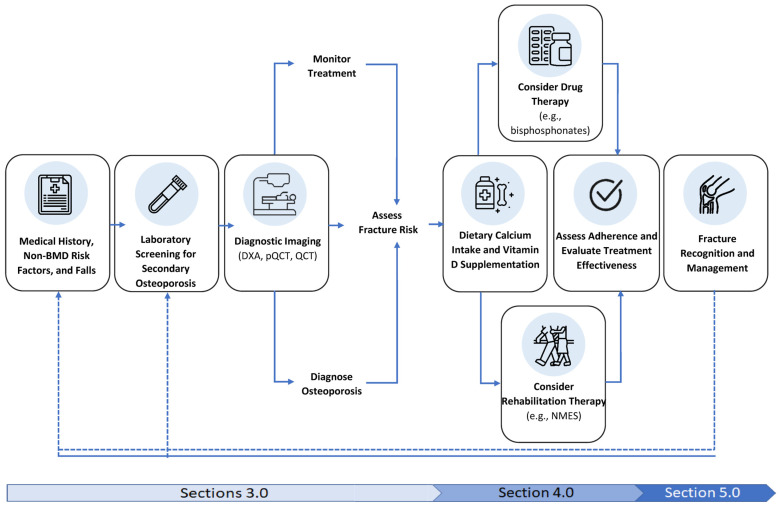
Upwards of half of patients with chronic SCI and a lower extremity fracture [60,61] may develop fracture-related complications. These include infections [15,182,190], compartment syndrome from a poor-fitting device for immobilization [197], heterotopic ossification [198], pressure injuries [60,61,185,199], fracture nonunion [60,155,193], venous thromboembolism [199], pain [12], autonomic dysreflexia and spasticity [60]. The extent to which depression and anxiety occur after a long bone fracture in patients with a SCI is not clear; affective disorders commonly complicate open lower-limb fractures in the AB population [200]. For individuals with a hip, DF or PT fracture, thromboprophylaxis should start as soon as is feasible, with therapy continuing for 2–4 weeks or until the person returns to their premorbid mobility status for non-wheelchair users. Patients awaiting definitive fracture management require monitoring for signs and symptoms of autonomic dysreflexia. As shown in Figure 1, the evaluation of a patient’s BMD and fracture risk and initiation of appropriate therapy should begin as soon as feasible following definitive fracture management. Wheelchair users with a visible limb length discrepancy or change in their seated posture require seating intervention and a graded return to their premorbid wheelchair skills, while patients who walk or use gait aids who have a limb length discrepancy or residual deformity often require orthoses, functional upgrading, gait and balance training to return to their premorbid functional abilities.
Figure 1.
Flow diagram of the process of identifying, treating, and managing sublesional osteoporosis and fractures in SCI (Figure adapted with permission from Paralyzed Veterans of America; Consortium for Spinal Cord Medicine Clinical Practice Guidelines [10]).
Many gaps still remain in our understanding of what constitutes optimal fracture repair in persons with a SCI. Future studies should also address which patients benefit most from operative compared with non-operative fracture repair with the goal of any repair being to return to the patient to their pre-fracture functional level as quickly as possible.
Acknowledgments
The authors wish to acknowledge our colleague and friend David Gater who inspired many throughout his career. The authors acknowledge the support of Curtis Black with manuscript preparation and the Paralyzed Veteran’s of America Bone Health and Osteoporosis Management Guideline Panel members (Anthony Burns, Kristine Cowley, Janice Eng, Gail Forrest, Therese E Johnston, B Jenny Kiratli, Sarah Morgan, Karen L. Troy, Frances M. Weaver, Tomas Cervinka, William Geerts) for their contributions to the work summarized in the enclosed review.
Abbreviations
| Acronym | Definition |
| AB | Able-bodied |
| AFF | Atypical Femoral Fracture |
| AIS | ASIA Impairment Scale |
| ASIA | American Spinal Cord Injury Association |
| BMD | Bone Mineral Density |
| CI | Confidence Interval |
| CPG | Clinical Practice Guideline |
| CTX-1 | C-terminal Cross-linking Telopeptide of Type I Collagen |
| DXA | Dual Energy X-ray Absorptiometry |
| DF | Distal Femur |
| FES | Functional Electrical Stimulation |
| FSH | Follicle-Stimulating Hormone |
| HR | Hazard Ratio |
| IGF-1 | Insulin-like Growth Factor 1 |
| IV | Intravenous |
| LH | Luteinizing Hormone |
| NMES | Neuromuscular Electrical Stimulation |
| NTX-1 | N-terminal Cross-linking Telopeptide of Type I Collagen |
| OC | Osteocalcin |
| ONJ | Osteonecrosis of the Jaw |
| OPG | Osteoprotegerin |
| ORIF | Open Reduction with Internal Fixation |
| PTH | Parathryroid Hormone |
| P1NP | Procollagen 1 Intact N-Terminal Propeptide |
| pQCT | Peripheral Quantitative Computed Tomography |
| PT | Proximal Tibia |
| PVA | Paralyzed Veterans of America |
| RANKL | Receptor Activator of Nuclear factor kappa-Β Ligand |
| SCI | Spinal Cord Injury |
| SCI/D | Spinal Cord Injury/Disease |
| ZA | Zolendronic Acid |
Author Contributions
The conceptualization, conduct of this review and the original drafts of this article and the related key consensus documents from ISCD, PVA and OTA were completed by B.C.C., C.M.C., L.R.M. and L.D.C. alone and in concert with one another. P.T. contributed to the identification of key concepts for the review and writing of some sections. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this review are openly available in References [8,9,10].
Conflicts of Interest
Craven acknowledges grant support from CIHR, Ontario Ministry of Health and Long-Term Care, PVA Education Foundation, Praxis Spinal Cord Institute and Craig H Nielsen Foundation. Craven was the panel chair for the PVA Bone Health Guideline and is the current Chair of the Canadian Spinal Cord Injury- Rehabilitation Institute and a member of the Osteoporosis Canada Scientific Advisory Committee. Morse reports no conflict of interest and is currently receiving funding from MN State Department of Education, NIDILRR, PVA, and CHNF.
Funding Statement
Craven wishes to acknowledge the UHN Foundation’s support as the University of Toronto/Toronto Rehabilitation Institute Chair in SCI Rehabilitation Research. Morse wishes to acknowledge the funding from NIDILRR, Department of Health and Human Services, 90SIMS0008-01-00 (Morse PD). Tsang wishes to acknowledge fellowship funding from UHN Foundation/SCI Ontario. Carbone wishes to acknowledge funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (VA IIR 15-294: Best Practices for Management of Fractures in Spinal Cord Injuries and Disorders).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hou S., Rabchevsky A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014;4:1419–1453. doi: 10.1002/cphy.c130045. [DOI] [PubMed] [Google Scholar]
- 2.Bourguignon L., Tong B., Geisler F., Schubert M., Röhrich F., Saur M., Weidner N., Rupp R., Kalke Y.-B.B., Abel R., et al. International surveillance study in acute spinal cord injury confirms viability of multinational clinical trials. BMC Med. 2022;20:225. doi: 10.1186/s12916-022-02395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson J.R., Cadotte D.W., Fehlings M.G. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: A systematic review. J. Neurosurg. Spine. 2012;17((Suppl. S1)):11–26. doi: 10.3171/2012.4.AOSPINE1245. [DOI] [PubMed] [Google Scholar]
- 4.Kopp M.A., Watzlawick R., Martus P., Failli V., Finkenstaedt F.W., Chen Y., DeVivo M.J., Dirnagl U., Schwab J.M. Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology. 2017;88:892–900. doi: 10.1212/WNL.0000000000003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stampas A., Dominick E., Zhu L. Evaluation of functional outcomes in traumatic spinal cord injury with rehabilitation-acquired urinary tract infections: A retrospective study. J. Spinal. Cord. Med. 2019;42:579–585. doi: 10.1080/10790268.2018.1452389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelletier C.A., Hicks A.L. Muscle characteristics and fatigue properties after spinal cord injury. Crit. Rev. Biomed. Eng. 2009;37:139–164. doi: 10.1615/CritRevBiomedEng.v37.i1-2.40. [DOI] [PubMed] [Google Scholar]
- 7.Xu X., Talifu Z., Zhang C.J., Gao F., Ke H., Pan Y.-Z., Gong H., Du H.-Y., Yu Y., Jing Y.-L., et al. Mechanism of skeletal muscle atrophy after spinal cord injury: A narrative review. Front. Nutr. 2023;10:1099143. doi: 10.3389/fnut.2023.1099143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore C.D., Craven B.C., Thabane L., Laing A., Frank-Wilson A., Kontulainen S., Papaioannou A., Adachi J., Giangregorio L. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal. Interact. 2015;15:32–41. [PMC free article] [PubMed] [Google Scholar]
- 9.Qin W., Bauman W.A., Cardozo C. Bone and muscle loss after spinal cord injury: Organ interactions. Ann. N. Y. Acad. Sci. 2010;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x. [DOI] [PubMed] [Google Scholar]
- 10.Craven B.C., Burns A.S., Carbone L., Cirnigliaro C.M., Cowley K., Eng J., Forrest G., Johnston T.E., Kiratli B.J., Morgan S., et al. Bone Health and Osteoporosis Management in Individuals with Spinal Cord Injury. Paralyzed Veterans of America; Washington, DC, USA: 2022. [(accessed on 15 May 2023)]. Clinical Practice Guideline. Available online: https://pva.org/wp-content/uploads/2022/05/CPG_Spinal-Cord-Medicine_2022_FINAL.pdf. [Google Scholar]
- 11.Morse L.R., Biering-Soerensen F., Carbone L.D., Cervinka T., Cirnigliaro C.M., Johnston T.E., Liu N., Troy K., Weaver F.M., Shuhart C., et al. Bone Mineral Density Testing in Spinal Cord Injury: 2019 ISCD Official Position. J. Clin. Densitom. 2019;22:554–566. doi: 10.1016/j.jocd.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Carbone L.D., Ahn J., Adler R.A., Cervinka T., Craven C., Geerts W., Hsu J., Huang D., Karunakar M., Kiratli B., et al. Acute Lower Extremity Fracture Management in Chronic Spinal Cord Injury: 2022 Delphi Consensus Recommendations. JBJS Open Access. 2022;7:e21.00152. doi: 10.2106/JBJS.OA.21.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S.D., Dai L.Y., Jiang L.S. Osteoporosis after spinal cord injury. Osteoporos. Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 14.Frost H.M. The mechanostat: A proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 15.Ragnarsson K.T., Sell G.H. Lower extremity fractures after spinal cord injury: A retrospective study. Arch. Phys. Med. Rehabil. 1981;62:418–423. [PubMed] [Google Scholar]
- 16.Abderhalden L., Weaver F.M., Bethel M., Demirtas H., Burns S., Svircev J., Hoenig H., Lyles K., Miskevics S., Carbone L.D. Dual-energy X-ray absorptiometry and fracture prediction in patients with spinal cord injuries and disorders. Osteoporos. Int. 2017;28:925–934. doi: 10.1007/s00198-016-3841-y. [DOI] [PubMed] [Google Scholar]
- 17.Chantraine A., Nusgens B., Lapiere C.M. Bone remodeling during the development of osteoporosis in paraplegia. Calcif. Tissue Int. 1986;38:323–327. doi: 10.1007/BF02555744. [DOI] [PubMed] [Google Scholar]
- 18.Minaire P., Neunier P., Edouard C., Bernard J., Courpron P., Bourret J. Quantitative histological data on disuse osteoporosis: Comparison with biological data. Calcif. Tissue Res. 1974;17:57–73. doi: 10.1007/BF02547214. [DOI] [PubMed] [Google Scholar]
- 19.Szollar S.M., Martin E.M., Sartoris D.J., Parthemore J.G., Deftos L.J. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am. J. Phys. Med. Rehabil. 1998;77:28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Zehnder Y., Luthi M., Michel D., Knecht H., Perrelet R., Neto I., Kraenzlin M., Lippuner K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: A cross-sectional observational study in 100 paraplegic men. Osteoporos. Int. 2004;15:180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- 21.Roberts D., Lee W., Cuneo R.C., Wittmann J., Ward G., Flatman R., McWhinney B., Hickman P.E. Longitudinal study of bone turnover after acute spinal cord injury. J. Clin. Endocrinol. Metab. 1998;83:415–422. doi: 10.1210/jc.83.2.415. [DOI] [PubMed] [Google Scholar]
- 22.Biering-Sorensen F., Bohr H.H., Schaadt O.P. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur. J. Clin. Investig. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 23.Dauty M., Perrouin Verbe B., Maugars Y., Dubois C., Mathe J.F. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–309. doi: 10.1016/S8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 24.Shields R.K., Dudley-Javoroski S., Boaldin K.M., Corey T.A., Fog D.B., Ruen J.M. Peripheral quantitative computed tomography: Measurement sensitivity in persons with and without spinal cord injury. Arch. Phys. Med. Rehabil. 2006;87:1376–1381. doi: 10.1016/j.apmr.2006.07.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garland D., Adkins R., Stewar C. Fracture Threshold and Risk for Osteoporosis and Pathologic Fractures in Individuals with Spinal Cord Injury. Top. Spinal Cord. Inj. 2005;11:61–69. doi: 10.1310/G6TD-HPGC-XM3Q-7YJH. [DOI] [Google Scholar]
- 26.Cirnigliaro C.M., Myslinski M.J., Asselin P., Hobson J.C., Specht A., La Fountaine M.F., Kirshblum S.C., Forrest G.F., Dyson-Hudson T., Spungen A.M., et al. Progressive Sublesional Bone Loss Extends into the Second Decade after Spinal Cord Injury. J. Clin. Densitom. 2019;22:185–194. doi: 10.1016/j.jocd.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Garland D.E., Adkins R.H., Stewart C.A. Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J. Spinal Cord. Med. 2008;31:543–550. doi: 10.1080/10790268.2008.11753650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recker R., Lappe J., Davies K., Heaney R. Characterization of perimenopausal bone loss: A prospective study. J. Bone Miner. Res. 2000;15:1965–1973. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 29.Leblanc A.D., Schneider V.S., Evans H.J., Engelbretson D.A., Krebs J.M. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 30.Vico L., Collet P., Guignandon A., Lafage-Proust M.-H., Thomas T., Rehailia M., Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/S0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 31.Bouxsein M.L., Seeman E. Quantifying the material and structural determinants of bone strength. Best. Pract. Res. Clin. Rheumatol. 2009;23:741–753. doi: 10.1016/j.berh.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Eser P., Frotzler A., Zehnder Y., Wick L., Knecht H., Denoth J., Schiessl H. Relationship between the duration of paralysis and bone structure: A pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Rittweger J., Goosey-Tolfrey V.L., Cointry G., Ferretti J.L. Structural analysis of the human tibia in men with spinal cord injury by tomographic (pQCT) serial scans. Bone. 2010;47:511–518. doi: 10.1016/j.bone.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Florencio-Silva R., Sasso G.R., Sasso-Cerri E., Simoes M.J., Cerri P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang S.D., Jiang L.S., Dai L.Y. Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. 2006;65:555–565. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 36.Qin W., Sun L., Cao J., Peng Y., Collier L., Wu Y., Creasey G., Li J., Qin Y., Jarvis J., et al. The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J. Biol. Chem. 2013;288:13511–13521. doi: 10.1074/jbc.M113.454892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morse L.R., Nguyen H.P., Jain N., Williams S., Tun C.G., Battaglino R.A., Stashenko P., Garshick E. Age and motor score predict osteoprotegerin level in chronic spinal cord injury. J. Musculoskelet. Neuronal. Interact. 2008;8:50–57. [PMC free article] [PubMed] [Google Scholar]
- 38.Gifre L., Ruiz-Gaspa S., Carrasco J.L., Portell E., Vidal J., Muxi A., Monegal A., Guañabens N., Peris P. Effect of recent spinal cord injury on the OPG/RANKL system and its relationship with bone loss and the response to denosumab therapy. Osteoporos. Int. 2017;28:2707–2715. doi: 10.1007/s00198-017-4090-4. [DOI] [PubMed] [Google Scholar]
- 39.Morse L.R., Sudhakar S., Lazzari A.A., Tun C., Garshick E., Zafonte R., Battaglino R.A. Sclerostin: A candidate biomarker of SCI-induced osteoporosis. Osteoporos. Int. 2013;24:961–968. doi: 10.1007/s00198-012-2072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglino R.A., Sudhakar S., Lazzari A.A., Garshick E., Zafonte R., Morse L.R. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone. 2012;51:600–605. doi: 10.1016/j.bone.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gifre L., Vidal J., Carrasco J.L., Filella X., Ruiz-Gaspà S., Muxi A., Portell E., Monegal A., Guañabens N., Peris P. Effect of recent spinal cord injury on wnt signaling antagonists (sclerostin and dkk-1) and their relationship with bone loss. A 12-month prospective study. J. Bone Miner. Res. 2015;30:1014–1021. doi: 10.1002/jbmr.2423. [DOI] [PubMed] [Google Scholar]
- 42.Pietschmann P., Pils P., Woloszczuk W., Maerk R., Lessan D., Stipicic J. Increased serum osteocalcin levels in patients with paraplegia. Paraplegia. 1992;30:204–209. doi: 10.1038/sc.1992.56. [DOI] [PubMed] [Google Scholar]
- 43.Uebelhart D., Hartmann D., Vuagnat H., Castanier M., Hachen H.J., Chantraine A. Early modifications of biochemical markers of bone metabolism in spinal cord injury patients. A preliminary study. Scand. J. Rehabil. Med. 1994;26:197–202. doi: 10.2340/165019771994264197202. [DOI] [PubMed] [Google Scholar]
- 44.Stewart A.F., Adler M., Byers C.M., Segre G.V., Broadus A.E. Calcium homeostasis in immobilization: An example of resorptive hypercalciuria. N. Engl. J. Med. 1982;306:1136–1140. doi: 10.1056/NEJM198205133061903. [DOI] [PubMed] [Google Scholar]
- 45.Heaney R.P. Radiocalcium metabolism in disuse osteoporosis in man. Am. J. Med. 1962;33:188–200. doi: 10.1016/0002-9343(62)90017-7. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y.H., Huang T.S., Lien I.N. Hormone changes in men with spinal cord injuries. Am. J. Phys. Med. Rehabil. 1992;71:328–332. doi: 10.1097/00002060-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Tsitouras P.D., Zhong Y.G., Spungen A.M., Bauman W.A. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm. Metab. Res. 1995;27:287–292. doi: 10.1055/s-2007-979961. [DOI] [PubMed] [Google Scholar]
- 48.Bauman W.A., La Fountaine M.F., Spungen A.M. Age-related prevalence of low testosterone in men with spinal cord injury. J. Spinal Cord. Med. 2014;37:32–39. doi: 10.1179/2045772313Y.0000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauman W.A., La Fountaine M.F., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. Testicular responses to hCG stimulation at varying doses in men with spinal cord injury. Spinal Cord. 2017;55:659–663. doi: 10.1038/sc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauman W.A., La Fountaine M.F., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. Administration of increasing doses of gonadotropin-releasing hormone in men with spinal cord injury to investigate dysfunction of the hypothalamic-pituitary-gonadal axis. Spinal Cord. 2018;56:247–258. doi: 10.1038/s41393-017-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song R.X., Santen R.J. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/A:1021649019025. [DOI] [PubMed] [Google Scholar]
- 52.Tomkinson A., Reeve J., Shaw R.W., Noble B.S. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 53.Emerton K.B., Hu B., Woo A.A., Sinofsky A., Hernandez C., Majeska R., Jepsen K., Schaffler M. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46:577–583. doi: 10.1016/j.bone.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum. Mol. Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 55.Li X., Ominsky M.S., Niu Q.T., Sun N., Daugherty B., D’Agostin D., Kurahara C., Gao Y., Cao J., Gong J., et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 56.Rosenquist R.C. Evaluation of 17-ketosteroid, estrogen and gonadotrophin excretion in patients with spinal cord injury. Am. J. Med. 1950;8:534–535. doi: 10.1016/0002-9343(50)90262-2. [DOI] [PubMed] [Google Scholar]
- 57.Slade J.M., Bickel C.S., Modlesky C.M., Majumdar S., Dudley G.A. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos. Int. 2005;16:263–272. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- 58.Battaglino R.A., Lazzari A.A., Garshick E., Morse L.R. Spinal cord injury-induced osteoporosis: Pathogenesis and emerging therapies. Curr. Osteoporos. Rep. 2012;10:278–285. doi: 10.1007/s11914-012-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozan Tan C. Spinal Cord Injury and Osteoporosis: Causes, Mechanisms, and Rehabilitation Strategies. Int. J. Phys. Rehabil. Med. 2013;1 doi: 10.4172/2329-9096.1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morse L.R., Battaglino R.A., Stolzmann K.L., Hallett L.D., Waddimba A., Gagnon D., Lazzari A.A., Garshick E. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos. Int. 2009;20:385–392. doi: 10.1007/s00198-008-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gifre L., Vidal J., Carrasco J., Portell E., Puig J., Monegal A., Guañabens N., Peris P. Incidence of skeletal fractures after traumatic spinal cord injury: A 10-year follow-up study. Clin. Rehabil. 2014;28:361–369. doi: 10.1177/0269215513501905. [DOI] [PubMed] [Google Scholar]
- 62.Lala D., Craven B.C., Thabane L., Papaioannou A., Adachi J.D., Popovic M.R., Giangregorio L.M. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos. Int. 2014;25:177–185. doi: 10.1007/s00198-013-2419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelletier C.A., Dumont F.S., Leblond J., Noreau L., Giangregorio L., Craven B.C. Self-report of one-year fracture incidence and osteoporosis prevalence in a community cohort of canadians with spinal cord injury. Top. Spinal Cord. Inj. Rehabil. 2014;20:302–309. doi: 10.1310/sci2004-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauman W.A., Zhong Y.G., Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44:1612–1616. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 65.Vestergaard P., Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk—A meta-analysis. Thyroid. 2003;13:585–593. doi: 10.1089/105072503322238854. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z., Thomson C.A., Aickin M., Nicholas J.S., Van Wyck D., Lewis C.E., Cauley J.A., Bassford T. The relationship between incidence of fractures and anemia in older multiethnic women. J. Am. Geriatr. Soc. 2010;58:2337–2344. doi: 10.1111/j.1532-5415.2010.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller P.D. Chronic kidney disease and the skeleton. Bone Res. 2014;2:14044. doi: 10.1038/boneres.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upala S., Jaruvongvanich V., Wijarnpreecha K., Sanguankeo A. Nonalcoholic fatty liver disease and osteoporosis: A systematic review and meta-analysis. J. Bone Miner. Metab. 2017;35:685–693. doi: 10.1007/s00774-016-0807-2. [DOI] [PubMed] [Google Scholar]
- 69.Bang C.S., Shin I.S., Lee S.W., Kim J.B., Baik G.H., Suk K.T., Yoon J.H., Kim Y.S., Kim D.J. Osteoporosis and bone fractures in alcoholic liver disease: A meta-analysis. World J. Gastroenterol. 2015;21:4038–4047. doi: 10.3748/wjg.v21.i13.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valderrabano R.J., Linares M.I. Diabetes mellitus and bone health: Epidemiology, etiology and implications for fracture risk stratification. Clin. Diabetes Endocrinol. 2018;4:9. doi: 10.1186/s40842-018-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnaldi G., Angeli A., Atkinson A.B., Bertagna X., Cavagnini F., Chrousos G.P., Fava G.A., Findling J.W., Gaillard R.C., Grossman A.B., et al. Diagnosis and complications of Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2003;88:5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 72.Bauman W.A., Kirshblum S., Cirnigliaro C., Forrest G.F., Spungen A.M. Underestimation of bone loss of the spine with posterior-anterior dual-energy X-ray absorptiometry in patients with spinal cord injury. J. Spinal Cord. Med. 2010;33:214–220. doi: 10.1080/10790268.2010.11689698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giangregorio L.M., Gibbs J.C., Craven B.C. Measuring muscle and bone in individuals with neurologic impairment; lessons learned about participant selection and pQCT scan acquisition and analysis. Osteoporos. Int. 2016;27:2433–2446. doi: 10.1007/s00198-016-3572-0. [DOI] [PubMed] [Google Scholar]
- 74.Craven B.C., Moreno J.C., Brown A., Raja N., Wong L. Acquisition and Analysis of Bone Mineral Density of the Distal Femur and Proximal Tibia; 2019. [(accessed on 19 April 2023)]. Available online: https://kite-uhn-contents.s3.ca-central-1.amazonaws.com/resourceMaterial/KneeDXAProtocol2019_+06_27_V5.0.pdf.
- 75.Craven B.C., Robertson L.A., McGillivray C.F., Adachi J.D. Detection and Treatment of Sublesional Osteoporosis among Patients with Chronic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2009;14:1–22. doi: 10.1310/sci1404-1. [DOI] [Google Scholar]
- 76.Silva M.C., Furlanetto T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2018;76:60–76. doi: 10.1093/nutrit/nux034. [DOI] [PubMed] [Google Scholar]
- 77.Pappa H.M., Bern E., Kamin D., Grand R.J. Vitamin D status in gastrointestinal and liver disease. Curr. Opin. Gastroenterol. 2008;24:176–183. doi: 10.1097/MOG.0b013e3282f4d2f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Institutes of Health: Office of Dietary Supplements Vitamin D Fact Sheet for Consumers. Internet. [(accessed on 19 April 2023)]; Available online: https://ods.od.nih.gov/factsheets/VitaminD-Consumer/
- 79.Camacho P.M., Petak S.M., Binkley N., Clarke B.L., Harris S.T., Hurley D.L., Kleerekoper M., Lewiecki E.M., Miller P.D., Narula H.S., et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016—Executive Summary. Endocr. Pract. 2016;22:1111–1118. doi: 10.4158/EP161435.ESGL. [DOI] [PubMed] [Google Scholar]
- 80.Farkas G.J., Pitot M.A., Berg A.S., Gater D.R. Nutritional status in chronic spinal cord injury: A systematic review and meta-analysis. Spinal Cord. 2019;57:3–17. doi: 10.1038/s41393-018-0218-4. [DOI] [PubMed] [Google Scholar]
- 81.Javidan A.N., Sabour H., Latifi S., Vafa M., Shidfar F., Khazaeipour Z., Shahbazi F., Rahimi A., Razavi S.-H.E. Calcium and vitamin D plasma concentration and nutritional intake status in patients with chronic spinal cord injury: A referral center report. J. Res. Med. Sci. 2014;19:881–884. [PMC free article] [PubMed] [Google Scholar]
- 82.Koutrakis N.E., Goldstein R.L., Walia P., Polak M.M., Lazzari A.A., Tun C.G., Hart J.E., Garshick E. Vitamin D, diet, and lifestyle in a chronic SCI population. Spinal Cord. 2019;57:117–127. doi: 10.1038/s41393-018-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walters J.L., Buchholz A.C., Martin Ginis K.A. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord. 2009;47:318–322. doi: 10.1038/sc.2008.134. [DOI] [PubMed] [Google Scholar]
- 84.Flueck J.L., Perret C. Vitamin D deficiency in individuals with a spinal cord injury: A literature review. Spinal Cord. 2017;55:428–434. doi: 10.1038/sc.2016.155. [DOI] [PubMed] [Google Scholar]
- 85.Barbonetti A., Vassallo M.R., Felzani G., Francavilla S., Francavilla F. Association between 25(OH)-vitamin D and testosterone levels: Evidence from men with chronic spinal cord injury. J. Spinal Cord. Med. 2016;39:246–252. doi: 10.1179/2045772315Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barbonetti A., D’Andrea S., Martorella A., Felzani G., Francavilla S., Francavilla F. Low vitamin D levels are independent predictors of 1-year worsening in physical function in people with chronic spinal cord injury: A longitudinal study. Spinal Cord. 2018;56:494–501. doi: 10.1038/s41393-017-0058-7. [DOI] [PubMed] [Google Scholar]
- 87.Binkley N., Carter G.D. Toward Clarity in Clinical Vitamin D Status Assessment: 25(OH)D Assay Standardization. Endocrinol. Metab. Clin. N. Am. 2017;46:885–899. doi: 10.1016/j.ecl.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Sempos C.T., Heijboer A.C., Bikle D.D., Bollerslev J., Bouillon R., Brannon P.M., DeLuca H.F., Jones G., Munns C.F., Bilezikian J.P., et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018;84:2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sempos C.T., Betz J.M., Camara J.E., Carter G.D., Cavalier E., Clarke M., Dowling K.G., Durazo-Arvizu R.A., Hoofnagle A.N., Liu A., et al. General Steps to Standardize the Laboratory Measurement of Serum Total 25-Hydroxyvitamin, D.J. AOAC Int. 2017;100:1230–1233. doi: 10.5740/jaoacint.17-0259. [DOI] [PubMed] [Google Scholar]
- 90.Binkley N., Dawson-Hughes B., Durazo-Arvizu R., Thamm M., Tian L., Merkel J., Jones J., Carter G., Sempos C. Vitamin D measurement standardization: The way out of the chaos. J. Steroid Biochem. Mol. Biol. 2017;173:117–121. doi: 10.1016/j.jsbmb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Binkley N., Sempos C.T. Vitamin D Standardization Program. Standardizing vitamin D assays: The way forward. J. Bone Miner. Res. 2014;29:1709–1714. doi: 10.1002/jbmr.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carter G.D., Berry J., Durazo-Arvizu R., Gunter E., Jones G., Jones J., Makin H., Pattni P., Phinney K., Sempos C., et al. Quality assessment of vitamin D metabolite assays used by clinical and research laboratories. J. Steroid Biochem. Mol. Biol. 2017;173:100–104. doi: 10.1016/j.jsbmb.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 93.Durazo-Arvizu R.A., Tian L., Brooks S.P.J., Sarafin K., Cashman K.D., Kiely M., Merkel J., Myers G.L., Coates P.M., Sempos C.T. The Vitamin D Standardization Program (VDSP) Manual for Retrospective Laboratory Standardization of Serum 25-Hydroxyvitamin D Data. J. AOAC Int. 2017;100:1234–1243. doi: 10.5740/jaoacint.17-0196. [DOI] [PubMed] [Google Scholar]
- 94.Wise S.A., Phinney K.W., Tai S.S., Camara J.E., Myers G.L., Durazo-Arvizu R., Tian L., Hoofnagle A.N., Bachmann L.M., Young I., et al. Baseline Assessment of 25-Hydroxyvitamin D Assay Performance: A Vitamin D Standardization Program (VDSP) Interlaboratory Comparison Study. J. AOAC Int. 2017;100:1244–1252. doi: 10.5740/jaoacint.17-0258. [DOI] [PubMed] [Google Scholar]
- 95.Wise S.A., Tai S.S., Burdette C.Q., Camara J.E., Bedner M., Lippa K.A., Nelson M.A., Nalin F., Phinney K.W., Sander L.C., et al. Role of the National Institute of Standards and Technology (NIST) in Support of the Vitamin D Initiative of the National Institutes of Health, Office of Dietary Supplements. J. AOAC Int. 2017;100:1260–1276. doi: 10.5740/jaoacint.17-0305. [DOI] [PubMed] [Google Scholar]
- 96.Heaney R.P. Toward a physiological referent for the vitamin D requirement. J. Endocrinol. Investig. 2014;37:1127–1130. doi: 10.1007/s40618-014-0190-6. [DOI] [PubMed] [Google Scholar]
- 97.Binkley N., Lewiecki E.M. Vitamin D and common sense. J. Clin. Densitom. 2011;14:95–99. doi: 10.1016/j.jocd.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 98.Bauman W.A., Emmons R.R., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. An effective oral vitamin D replacement therapy in persons with spinal cord injury. J. Spinal Cord. Med. 2011;34:455–460. doi: 10.1179/2045772311Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miyatani M., Craven B., Loewenberger E., McGillivray C., Adachi J. The Dietary Intakes of Calcium and Bone Health Related Nutrients among Individuals with and without Spinal Cord Injury. J. Nutr. Ther. 2014;3:103–113. doi: 10.6000/1929-5634.2014.03.02.9. [DOI] [Google Scholar]
- 100.University Health Network Osteoporosis Program Calcium Assessment Tool. Internet. [(accessed on 19 April 2023)]. Available online: https://osteoconnection.files.wordpress.com/2014/04/cat_patient-version-apr2014.pdf.
- 101.National Institutes of Health: Office of Dietary Supplements Calcium Fact Sheet for Health Professionals. Internet. [(accessed on 19 April 2023)]; Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/#:~:text=Calcium%20Intakes%20and%20Status,-A%20substantial%20proportion&text=Average%20daily%20intakes%20of%20calcium,to%201%2C015%20mg%20%5B18%5D.
- 102.Welk B., Fuller A., Razvi H., Denstedt J. Renal stone disease in spinal-cord-injured patients. J. Endourol. 2012;26:954–959. doi: 10.1089/end.2012.0063. [DOI] [PubMed] [Google Scholar]
- 103.Hansen R.B., Biering-Sorensen F., Kristensen J.K. Urinary calculi following traumatic spinal cord injury. Scand. J. Urol. Nephrol. 2007;41:115–119. doi: 10.1080/00365590600991383. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y., Qu M., Carter R.E., Leng S., Ramirez-Giraldo J.C., Jaramillo G., Krambeck A.E., Lieske J.C., Vrtiska T.J., McCollough C.H. Differentiating calcium oxalate and hydroxyapatite stones in vivo using dual-energy CT and urine supersaturation and pH values. Acad. Radiol. 2013;20:1521–1525. doi: 10.1016/j.acra.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Kotob R., Pagcanlungan J.R., Craven B.C., Sherrington C., Mourtzakis M., Giangregorio L.M. Researchers’ perspectives on adverse event reporting in resistance training trials: A qualitative study. Appl. Physiol. Nutr. Metab. 2022;47:893–902. doi: 10.1139/apnm-2022-0012. [DOI] [PubMed] [Google Scholar]
- 106.Schenkman M., Deutsch J.E., Gill-Body K.M. An integrated framework for decision making in neurologic physical therapist practice. Phys. Ther. 2006;86:1681–1702. doi: 10.2522/ptj.20050260. [DOI] [PubMed] [Google Scholar]
- 107.Alekna V., Tamulaitiene M., Sinevicius T., Juocevicius A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord. 2008;46:727–732. doi: 10.1038/sc.2008.36. [DOI] [PubMed] [Google Scholar]
- 108.Karimi T.M. The Physiological Benefits and Problems Associated with using Standing and Walking Orthoses in Individuals with Spinal Cord Injury—A Meta-analytic Review. J. Orthop. Trauma. Rehabil. 2012;16:37–40. doi: 10.1016/j.jotr.2011.07.008. [DOI] [Google Scholar]
- 109.Esquenazi A., Talaty M., Jayaraman A. Powered Exoskeletons for Walking Assistance in Persons with Central Nervous System Injuries: A Narrative Review. PM R. 2017;9:46–62. doi: 10.1016/j.pmrj.2016.07.534. [DOI] [PubMed] [Google Scholar]
- 110.Dobkin B., Apple D., Barbeau H., Basso M., Behrman A., Deforge D., Ditunno J., Dudley G., Elashoff R., Fugate L., et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Worthen L.C., Kim C.M., Kautz S.A., Lew H.L., Kiratli B.J., Beaupre G.S. Key characteristics of walking correlate with bone density in individuals with chronic stroke. J. Rehabil. Res. Dev. 2005;42:761–768. doi: 10.1682/JRRD.2005.02.0036. [DOI] [PubMed] [Google Scholar]
- 112.National Institutes of Health: Osteoporosis and Related Bone Diseases National Resource Centre Exercise for your Bone Health. Internet. [(accessed on 19 April 2023)]; Available online: https://www.niams.nih.gov/health-topics/exercise-your-bone-health.
- 113.Giangregorio L.M., Webber C.E., Phillips S.M., Hicks A.L., Craven B.C., Bugaresti J.M., McCartney N. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl. Physiol. Nutr. Metab. 2006;31:283–291. doi: 10.1139/h05-036. [DOI] [PubMed] [Google Scholar]
- 114.Ogilvie C., Bowker P., Rowley D.I. The physiological benefits of paraplegic orthotically aided walking. Paraplegia. 1993;31:111–115. doi: 10.1038/sc.1993.19. [DOI] [PubMed] [Google Scholar]
- 115.Thoumie P., Le Claire G., Beillot J., Dassonville J., Chevalier T., Perrouin-Verbe B., Bedoiseau M., Busnel M., Cormerais A., Courtillon A., et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia. 1995;33:654–659. doi: 10.1038/sc.1995.137. [DOI] [PubMed] [Google Scholar]
- 116.Belanger M., Stein R.B., Wheeler G.D., Gordon T., Leduc B. Electrical stimulation: Can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch. Phys. Med. Rehabil. 2000;81:1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- 117.Shields R.K., Dudley-Javoroski S. Musculoskeletal adaptations in chronic spinal cord injury: Effects of long-term soleus electrical stimulation training. Neurorehabil. Neural Repair. 2007;21:169–179. doi: 10.1177/1545968306293447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dudley-Javoroski S., Saha P.K., Liang G., Li C., Gao Z., Shields R.K. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos. Int. 2012;23:2335–2346. doi: 10.1007/s00198-011-1879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frotzler A., Coupaud S., Perret C., Kakebeeke T.H., Hunt K.J., Donaldson N.D.N., Eser P. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43:169–176. doi: 10.1016/j.bone.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 120.Chen S.C., Lai C.H., Chan W.P., Huang M.H., Tsai H.W., Chen J.J. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil. Rehabil. 2005;27:1337–1341. doi: 10.1080/09638280500164032. [DOI] [PubMed] [Google Scholar]
- 121.Mohr T., Podenphant J., Biering-Sorensen F., Galbo H., Thamsborg G., Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif. Tissue Int. 1997;61:22–25. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- 122.Coupaud S., McLean A.N., Allan D.B. Role of peripheral quantitative computed tomography in identifying disuse osteoporosis in paraplegia. Skelet. Radiol. 2009;38:989–995. doi: 10.1007/s00256-009-0674-1. [DOI] [PubMed] [Google Scholar]
- 123.Coupaud S., McLean A.N., Lloyd S., Allan D.B. Predicting patient-specific rates of bone loss at fracture-prone sites after spinal cord injury. Disabil. Rehabil. 2012;34:2242–2250. doi: 10.3109/09638288.2012.681831. [DOI] [PubMed] [Google Scholar]
- 124.Edwards W.B., Schnitzer T.J., Troy K.L. Bone mineral and stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporos. Int. 2014;25:1005–1015. doi: 10.1007/s00198-013-2557-5. [DOI] [PubMed] [Google Scholar]
- 125.Zehnder Y., Risi S., Michel D., Knecht H., Perrelet R., Kraenzlin M., Zäch G.A., Lippuner K. Prevention of bone loss in paraplegics over 2 years with alendronate. J. Bone Miner. Res. 2004;19:1067–1074. doi: 10.1359/JBMR.040313. [DOI] [PubMed] [Google Scholar]
- 126.Haider I.T., Simonian N., Saini A.S., Leung F.M., Edwards W.B., Schnitzer T.J. Open-label clinical trial of alendronate after teriparatide therapy in people with spinal cord injury and low bone mineral density. Spinal Cord. 2019;57:832–842. doi: 10.1038/s41393-019-0303-3. [DOI] [PubMed] [Google Scholar]
- 127.Morse L.R., Troy K.L., Fang Y., Nguyen N., Battaglino R., Goldstein R.F., Gupta R., Taylor J.A. Combination Therapy with Zoledronic Acid and FES-Row Training Mitigates Bone Loss in Paralyzed Legs: Results of a Randomized Comparative Clinical Trial. JBMR Plus. 2019;3:e10167. doi: 10.1002/jbm4.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gifre L., Vidal J., Carrasco J.L., Muxi A., Portell E., Monegal A., Guanabens N., Peris P. Denosumab increases sublesional bone mass in osteoporotic individuals with recent spinal cord injury. Osteoporos. Int. 2016;27:405–410. doi: 10.1007/s00198-015-3333-5. [DOI] [PubMed] [Google Scholar]
- 129.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 130.Tu K.N., Lie J.D., Wan C.K.V., Cameron M., Austel A.G., Nguyen J.K., Van K., Hyun D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018;43:92–104. [PMC free article] [PubMed] [Google Scholar]
- 131.Watts N.B., Roux C., Modlin J.F., Brown J.P., Daniels A., Jackson S., Smith S., Zack D.J., Zhou L., Grauer A., et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: Coincidence or causal association? Osteoporos. Int. 2012;23:327–337. doi: 10.1007/s00198-011-1755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kajizono M., Sada H., Sugiura Y., Soga Y., Kitamura Y., Matsuoka J., Sendo T. Incidence and Risk Factors of Osteonecrosis of the Jaw in Advanced Cancer Patients after Treatment with Zoledronic Acid or Denosumab: A Retrospective Cohort Study. Biol. Pharm. Bull. 2015;38:1850–1855. doi: 10.1248/bpb.b15-00385. [DOI] [PubMed] [Google Scholar]
- 133.Khan A.A., Morrison A., Hanley D.A., Felsenberg D., McCauley L.K., O’Ryan F., Reid I.R., Ruggiero S.L., Taguchi A., Tetradis S., et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 134.Khosla S., Burr D., Cauley J., Dempster D.W., Ebeling P.R., Felsenberg D., Gagel R.F., Gilsanz V., Guise T., Koka S., et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 135.Shane E., Burr D., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D.W., et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 136.Dell R.M., Adams A.L., Greene D.F., Funahashi T.T., Silverman S.L., Eisemon E.O., Zhou H., Burchette R.J., Ott S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012;27:2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 137.Adler R.A., El-Hajj Fuleihan G., Bauer D.C., Camacho P.M., Clarke B.L., Clines G.A., Compston J.E., Drake M.T., Edwards B.J., Favus M.J., et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Begin M.J., Audet M.C., Chevalley T., Portela M., Padlina I., Hannouche D., Lorenzini K.I., Meier R., Peter R., Uebelhart B., et al. Fracture Risk Following an Atypical Femoral Fracture. J. Bone Miner. Res. 2022;37:87–94. doi: 10.1002/jbmr.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goenka S., Sethi S., Pandey N., Joshi M., Jindal R. Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: A randomized controlled trial. Spinal Cord. 2018;56:1207–1211. doi: 10.1038/s41393-018-0195-7. [DOI] [PubMed] [Google Scholar]
- 140.Bauman W.A., Cirnigliaro C.M., La Fountaine M.F., Martinez L., Kirshblum S.C., Spungen A.M. Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: An observational cohort study. J. Bone Miner. Metab. 2015;33:410–421. doi: 10.1007/s00774-014-0602-x. [DOI] [PubMed] [Google Scholar]
- 141.Bauman W.A., Wecht J.M., Kirshblum S., Spungen A.M., Morrison N., Cirnigliaro C., Schwartz E. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J. Rehabil. Res. Dev. 2005;42:305–313. doi: 10.1682/JRRD.2004.05.0062. [DOI] [PubMed] [Google Scholar]
- 142.Bubbear J.S., Gall A., Middleton F.R., Ferguson-Pell M., Swaminathan R., Keen R.W. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos. Int. 2011;22:271–279. doi: 10.1007/s00198-010-1221-6. [DOI] [PubMed] [Google Scholar]
- 143.Nance P.W., Schryvers O., Leslie W., Ludwig S.J., Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch. Phys. Med. Rehabil. 1999;80:243–251. doi: 10.1016/S0003-9993(99)90133-8. [DOI] [PubMed] [Google Scholar]
- 144.Shapiro J., Smith B., Beck T., Ballard P., Dapthary M., BrintzenhofeSzoc K., Caminis J. Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif. Tissue Int. 2007;80:316–322. doi: 10.1007/s00223-007-9012-6. [DOI] [PubMed] [Google Scholar]
- 145.Gilchrist N.L., Frampton C.M., Acland R.H., Nicholls M.G., March R.L., Maguire P., Heard A., Reilly P., Marshall K. Alendronate prevents bone loss in patients with acute spinal cord injury: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007;92:1385–1390. doi: 10.1210/jc.2006-2013. [DOI] [PubMed] [Google Scholar]
- 146.Schnitzer T.J., Kim K., Marks J., Yeasted R., Simonian N., Chen D. Zoledronic Acid Treatment after Acute Spinal Cord Injury: Results of a Randomized, Placebo-Controlled Pilot Trial. PM R. 2016;8:833–843. doi: 10.1016/j.pmrj.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 147.Guilcher S.J.T., Hogan M.E., Calzavara A., Nicholls M.G., March R.L., Maguire P., Heard A., Reilly P., Marshall K. Prescription drug claims following a traumatic spinal cord injury for older adults: A retrospective population-based study in Ontario, Canada. Spinal Cord. 2018;56:1059–1068. doi: 10.1038/s41393-018-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Le B., Ray C., Gonzalez B., Miskevics S., Weaver F.M., Priebe M., Carbone L.D. Reasons for Initiation and Discontinuation of Pharmacological Therapies for Osteoporosis in Veterans with Spinal Cord Injury and Disorders. J. Clin. Densitom. 2021;24:67–77. doi: 10.1016/j.jocd.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 149.Papaioannou A., Morin S., Cheung A.M., Atkinson S., Brown J.P., Feldman S., Hanley D.A., Hodsman A., Jamal S.A., Kaiser S.M., et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schneider D.L. Long-term bisphosphonate use: When to stop? when to restart. TodayLs Geriatr. Med. 2015;8:10–12. [Google Scholar]
- 151.Ott S.M. Osteoporosis in Women with Spinal Cord Injuries. Phys. Med. Rehabil. Clin. N. Am. 2001;12:111–131. doi: 10.1016/S1047-9651(18)30086-X. [DOI] [PubMed] [Google Scholar]
- 152.Ludwig P.E., Patil A.A., Chamczuk A.J., Agrawal D.K. Hormonal therapy in traumatic spinal cord injury. Am. J. Transl. Res. 2017;9:3881–3895. [PMC free article] [PubMed] [Google Scholar]
- 153.Vestergaard P., Krogh K., Rejnmark L., Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 154.Frotzler A., Cheikh-Sarraf B., Pourtehrani M., Krebs J., Lippuner K. Long-bone fractures in persons with spinal cord injury. Spinal Cord. 2015;53:701–704. doi: 10.1038/sc.2015.74. [DOI] [PubMed] [Google Scholar]
- 155.Grassner L., Klein B., Maier D., Buhren V., Vogel M. Lower extremity fractures in patients with spinal cord injury characteristics, outcome and risk factors for non-unions. J. Spinal Cord. Med. 2018;41:676–683. doi: 10.1080/10790268.2017.1329915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Akhigbe T., Chin A.S., Svircev J.N., Hoenig H., Burns S.P., Weaver F.M., Bailey L., Carbone L. A retrospective review of lower extremity fracture care in patients with spinal cord injury. J. Spinal Cord. Med. 2015;38:2–9. doi: 10.1179/2045772313Y.0000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Benson I., Hart K., Tussler D., van Middendorp J.J. Lower-limb exoskeletons for individuals with chronic spinal cord injury: Findings from a feasibility study. Clin. Rehabil. 2016;30:73–84. doi: 10.1177/0269215515575166. [DOI] [PubMed] [Google Scholar]
- 158.Bass A., Morin S.N., Vermette M., Aubertin-Leheudre M., Gagnon D.H. Incidental bilateral calcaneal fractures following overground walking with a wearable robotic exoskeleton in a wheelchair user with a chronic spinal cord injury: Is zero risk possible? Osteoporos. Int. 2020;31:1007–1011. doi: 10.1007/s00198-020-05277-4. [DOI] [PubMed] [Google Scholar]
- 159.Miller L.E., Zimmermann A.K., Herbert W.G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices. 2016;9:455–466. doi: 10.2147/MDER.S103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bethel M., Bailey L., Weaver F., Harmon R.L., Priebe M.M., Le B., Aslam H., Fausel Z., Hoenig H., Carbone L.D. A historical study of appendicular fractures in veterans with traumatic chronic spinal cord injury: 2002–2007. J. Spinal Cord. Med. 2016;39:686–692. doi: 10.1080/10790268.2016.1149930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carbone L.D., Chin A.S., Burns S.P., Svircev J.N., Hoenig H., Heggeness M., Bailey L., Weaver F. Mortality after lower extremity fractures in men with spinal cord injury. J. Bone Miner. Res. 2014;29:432–439. doi: 10.1002/jbmr.2050. [DOI] [PubMed] [Google Scholar]
- 162.Lems W.F., Dreinhofer K.E., Bischoff-Ferrari H., Blauth M., Czerwinski E., Da Silva J., Herrera A., Hoffmeyer P., Kvien T., Maalouf G., et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann. Rheum. Dis. 2017;76:802–810. doi: 10.1136/annrheumdis-2016-210289. [DOI] [PubMed] [Google Scholar]
- 163.Barlehner C., Bohm V., Flieger R., Meiners T. Surgery for fractures of the lower extremities in cases of chronic spinal cord injury. Orthopade. 2005;34:137–143. doi: 10.1007/s00132-004-0757-6. [DOI] [PubMed] [Google Scholar]
- 164.Asselin P., Knezevic S., Kornfeld S., Cirnigliaro C., Agranova-Breyter I., Bauman W.A., Spungen A.M. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J. Rehabil. Res. Dev. 2015;52:147–158. doi: 10.1682/JRRD.2014.02.0060. [DOI] [PubMed] [Google Scholar]
- 165.Bach Baunsgaard C., Vig Nissen U., Katrin Brust A., Frotzler A., Ribeill C., Kalke Y.-B., León N., Gómez B., Samuelsson K., Antepohl W., et al. Gait training after spinal cord injury: Safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord. 2018;56:106–116. doi: 10.1038/s41393-017-0013-7. [DOI] [PubMed] [Google Scholar]
- 166.Evans N., Hartigan C., Kandilakis C., Pharo E., Clesson I. Acute Cardiorespiratory and Metabolic Responses During Exoskeleton-Assisted Walking Overground Among Persons with Chronic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2015;21:122–132. doi: 10.1310/sci2102-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Federici S., Meloni F., Bracalenti M., De Filippis M.L. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: A systematic review. NeuroRehabilitation. 2015;37:321–340. doi: 10.3233/NRE-151265. [DOI] [PubMed] [Google Scholar]
- 168.Gorgey A.S., Sumrell R., Goetz L.L. 44—Exoskeletal Assisted Rehabilitation after Spinal Cord Injury. In: Webster J.B., Murphy D.P., editors. Atlas of Orthoses and Assistive Devices. 5th ed. Elsevier; Amsterdam, The Netherlands: 2019. pp. 440–447. [Google Scholar]
- 169.Gorgey A.S. Robotic exoskeletons: The current pros and cons. World J. Orthop. 2018;9:112–119. doi: 10.5312/wjo.v9.i9.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gorgey A.S., Wade R., Sumrell R., Villadelgado L., Khalil R.E., Lavis T. Exoskeleton Training May Improve Level of Physical Activity after Spinal Cord Injury: A Case Series. Top. Spinal Cord. Inj. Rehabil. 2017;23:245–255. doi: 10.1310/sci16-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kressler J., Thomas C.K., Field-Fote E.C., Sanchez J., Widerstrom-Noga E., Cilien D.C., Gant K., Ginnety K., González H., Martinez A., et al. Understanding therapeutic benefits of overground bionic ambulation: Exploratory case series in persons with chronic, complete spinal cord injury. Arch. Phys. Med. Rehabil. 2014;95:1878–1887.e4. doi: 10.1016/j.apmr.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 172.Louie D.R., Eng J.J., Lam T. Spinal Cord Injury Research Evidence Research, T. Gait speed using powered robotic exoskeletons after spinal cord injury: A systematic review and correlational study. J. Neuroeng. Rehabil. 2015;12:82. doi: 10.1186/s12984-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Etingen B., Carbone L.D., Guihan M., Ray C., Aslam H., Elam R., Weaver F.M. Lower extremity fracture prevention and management in persons with spinal cord injuries and disorders: The patient perspective. J. Spinal Cord. Med. 2022;45:946–956. doi: 10.1080/10790268.2021.1907675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Comarr A.E., Hutchinson R.H., Bors E. Extremity fractures of patients with spinal cord injuries. Am. J. Surg. 1962;103:732–739. doi: 10.1016/0002-9610(62)90256-8. [DOI] [PubMed] [Google Scholar]
- 175.Ingram R.R., Suman R.K., Freeman P.A. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia. 1989;27:133–139. doi: 10.1038/sc.1989.20. [DOI] [PubMed] [Google Scholar]
- 176.Baird R.A., Kreitenberg A., Eltorai I. External fixation of femoral shaft fractures in spinal cord injury patients. Paraplegia. 1986;24:183–190. doi: 10.1038/sc.1986.25. [DOI] [PubMed] [Google Scholar]
- 177.Barrera-Ochoa S., Haddad S., Rodriguez-Alabau S., Teixidor J., Tomas J., Molero V. Should lower limb fractures be treated surgically in patients with chronic spinal injuries? Experience in a reference centre. Rev. Esp. Cir. Ortop. Traumatol. 2017;61:19–27. doi: 10.1016/j.recote.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 178.Eichenholtz S.N. Management of Long-Bone Fractures in Paraplegic Patients. J. Bone Jt. Surg. 1963;45:299–310. doi: 10.2106/00004623-196345020-00008. [DOI] [Google Scholar]
- 179.Freehafer A.A., Mast W.A. Lower Extremity Fractures in Patients with Spinal-Cord Injury. J. Bone Jt. Surg. 1965;47:683–694. doi: 10.2106/00004623-196547040-00003. [DOI] [PubMed] [Google Scholar]
- 180.McMaster W.C., Stauffer E.S. The Management of Long Bone Fracture in the Spinal Cord Injured Patient. Clin. Orthop. Relat. Res. 1975;112:44–52. [PubMed] [Google Scholar]
- 181.Nottage W.M. A Review of Long-Bone Fractures in Patients with Spinal Cord Injuries. Clin. Orthop. Relat. Res. 1981;155:65–70. doi: 10.1097/00003086-198103000-00012. [DOI] [PubMed] [Google Scholar]
- 182.Perkins C., Buck J.S., Karunakar M.A. Outcomes in the treatment of femur fractures in patients with pre-existing spinal cord injury. Bull. NYU Hosp. Jt. Dis. 2019;77:211–216. [PubMed] [Google Scholar]
- 183.Bethel M., Bailey L., Weaver F., Le B., Burns S.P., Svircev J.N., Heggeness M.H., Carbone L.D. Surgical compared with nonsurgical management of fractures in male veterans with chronic spinal cord injury. Spinal Cord. 2015;53:402–407. doi: 10.1038/sc.2015.5. [DOI] [PubMed] [Google Scholar]
- 184.Freehafer A.A. Limb fractures in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 1995;76:823–827. doi: 10.1016/S0003-9993(95)80546-X. [DOI] [PubMed] [Google Scholar]
- 185.Bishop J.A., Suarez P., DiPonio L., Ota D., Curtin C.M. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: An administrative analysis of risks. Arch. Phys. Med. Rehabil. 2013;94:2357–2364. doi: 10.1016/j.apmr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 186.Nelson A., Ahmed S., Harrow J., Fitzgerald S., Sanchez-Anguiano A., Gavin-Dreschnack D. Fall-related fractures in persons with spinal cord impairment: A descriptive analysis. SCI Nurs. 2003;20:30–37. [PubMed] [Google Scholar]
- 187.Cochran T.P., Bayley J.C., Smith M. Lower Extremity Fractures in Paraplegics: Pattern, Treatment, and Functional Results. Clin. Spine Surg. 1988;1:219–223. doi: 10.1097/00002517-198803000-00007. [DOI] [PubMed] [Google Scholar]
- 188.Fouasson-Chailloux A., Gross R., Dauty M., Gadbled G., Touchais S., Le Fort M., Perrouin-Verbe B. Surgical management of lower limb fractures in patients with spinal cord injury less associated with complications than non-operative management: A retrospective series of cases. J. Spinal Cord. Med. 2019;42:39–44. doi: 10.1080/10790268.2017.1325560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sugi M.T., Davidovitch R., Montero N., Nobel T., Egol K.A. Treatment of lower-extremity long-bone fractures in active, nonambulatory, wheelchair-bound patients. Orthopedics. 2012;35:e1376–e1382. doi: 10.3928/01477447-20120822-25. [DOI] [PubMed] [Google Scholar]
- 190.Freehafer A.A., Hazel C.M., Becker C.L. Lower extremity fractures in patients with spinal cord injury. Paraplegia. 1981;19:367–372. doi: 10.1038/sc.1981.69. [DOI] [PubMed] [Google Scholar]
- 191.Elam R.E., Ray C.E., Miskevics S., Weaver F.M., Gonzalez B., Obremskey W., Carbone L.D. Predictors of lower extremity fracture-related amputation in persons with traumatic spinal cord injury: A case-control study. Spinal Cord. 2023;61:260–268. doi: 10.1038/s41393-023-00879-1. [DOI] [PubMed] [Google Scholar]
- 192.Garland D.E., Saucedo T., Reiser T.V. The Management of Tibial Fractures in Acute Spinal Cord Injury Patients. Clin. Orthop. Relat. Res. 1986;213:237–240. doi: 10.1097/00003086-198612000-00034. [DOI] [PubMed] [Google Scholar]
- 193.Sinnott B., Ray C., Weaver F., Gonzalez B., Chu E., Premji S., Raiford M., Elam R., Miskevics S., Parada S., et al. Risk Factors and Consequences of Lower Extremity Fracture Nonunions in Veterans with Spinal Cord Injury. JBMR Plus. 2022;6:e10595. doi: 10.1002/jbm4.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Le B., Gonzalez B., Weaver F., Sinnott B., Ray C., Chu E., Premji S., Raiford M., Mayur O., Carbone L. Malunions following lower extremity fractures in veterans with a spinal cord injury/disorder. J. Spinal Cord. Med. 2023:1–7. doi: 10.1080/10790268.2023.2188391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Svircev J., Tan D., Garrison A., Pennelly B., Burns S.P. Limb loss in individuals with chronic spinal cord injury. J. Spinal Cord. Med. 2022;45:420–425. doi: 10.1080/10790268.2020.1800964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guihan M., Roddick K., Cervinka T., Ray C., Sutton C., Carbone L., Weaver F.M. Physical and occupational therapist rehabilitation of lower extremity fractures in veterans with spinal cord injuries and disorders. J. Spinal Cord. Med. 2022;45:33–41. doi: 10.1080/10790268.2021.1890680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Halanski M., Noonan K.J. Cast and splint immobilization: Complications. J. Am. Acad. Orthop. Surg. 2008;16:30–40. doi: 10.5435/00124635-200801000-00005. [DOI] [PubMed] [Google Scholar]
- 198.Ung L., Ohlmeier M., Jettkant B., Grasmücke D., Aach M., Meindl R., Nicolas V., Schildhauer T., Citak M. Clinical and Radiological Outcomes after Surgical Treatment of Lower Limb Fractures in Patients with Spinal Cord Injury. Glob. Spine J. 2020;10:715–719. doi: 10.1177/2192568219871019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carbone L.D., Chin A.S., Burns S.P., Svircev J.N., Hoenig H., Heggeness M., Weaver F. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos. Int. 2013;24:2261–2267. doi: 10.1007/s00198-013-2295-8. [DOI] [PubMed] [Google Scholar]
- 200.Abukhder M., Dobbs T., Shaw J., Whelan R., Jones E. A systematic literature review and narrative synthesis on the risk factors for developing affective disorders in open lower-limb fracture patients. Ann. Med. Surg. 2022;80:104190. doi: 10.1016/j.amsu.2022.104190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this review are openly available in References [8,9,10].