Abstract
During our investigations of the microfungi on medicinal plants in Thailand, five isolates of Diaporthe were obtained. These isolates were identified and described using a multiproxy approach, viz. morphology, cultural characteristics, host association, the multiloci phylogeny of ITS, tef1-α, tub2, cal, and his3, and DNA comparisons. Five new species, Diaporthe afzeliae, D. bombacis, D. careyae, D. globoostiolata, and D. samaneae, are introduced as saprobes from the plant hosts, viz. Afzelia xylocarpa, Bombax ceiba, Careya sphaerica, a member of Fagaceae, and Samanea saman. Interestingly, this is the first report of Diaporthe species on these plants, except on the Fagaceae member. The morphological comparison, updated molecular phylogeny, and pairwise homoplasy index (PHI) analysis strongly support the establishment of novel species. Our phylogeny also revealed the close relationship between D. zhaoqingensis and D. chiangmaiensis; however, the evidence from the PHI test and DNA comparison indicated that they are distinct species. These findings improve the existing knowledge of taxonomy and host diversity of Diaporthe species as well as highlight the untapped potential of these medicinal plants for searching for new fungi.
Keywords: Asexual morph, classification, Diaporthaceae, new host records, saprobes
1. Introduction
Medicinal plants are essential for sustaining human health and livelihoods according to their ethnobotanical uses and therapeutic purposes [1,2]. They have also contributed to maintaining biodiversity in forest ecosystems and supporting natural recreation in urban ecosystems [1,2]. Fungi are usually encountered in medicinal plants, where they can affect their hosts in both beneficial and harmful manners [2,3,4]. As pathogens, they impair plant health and productivity [4]; whereas, as endophytes, they promote plant growth and produce a diverse array of secondary metabolites, which have been exploited for the development of new drugs and pharmaceutical products [2,3]. Thus, studies of fungi associated with medicinal plants represent a significant repository for the estimation of fungal diversity, the discovery of novel fungi and fungal–plant interactions, as well as the bioprospecting of new bioactive compounds and their biotechnological applications [5,6,7,8,9,10,11,12].
Diaporthe species are a large and diverse group of fungi known as endophytes, saprobes, and plant pathogens, with worldwide distribution and a broad range of host associations [13,14,15,16,17,18]. Pathogenic Diaporthe species cause various plant diseases, such as blight, cankers, diebacks, fruit rots, leaf spots, and wilts, on forest trees [19,20,21,22] and many agricultural crops such as citrus, grapevine, peach, soybean, sunflower, and tea [23,24,25,26,27,28]. Morphologically, Diaporthe is characterized by pseudostromatic ascomata that usually have black lines in the host substrate, along with elongated perithecial necks for the sexual morph [29], and asexual morph consisting of ostiolate conidiomata, aseptate, and polymorphic (alpha, gamma, and beta), and hyaline conidia [14]. However, identifying Diaporthe species based solely on morphological data is challenging due to their polyphyletic nature and the presence of numerous cryptic species [30,31,32]. Recent studies have used multilocus phylogeny, including internal transcribed spacers (ITS), the translation elongation factor 1-α (tef1-α), β-tubulin (tub2), calmodulin (cal), and histone H3 (his3), along with morphological characteristics, to accurately identify and classify Diaporthe species [15,19,23,26,31,33,34,35,36]. Norphanphoun et al. [32] classified Diaporthe into 13 species complexes based on a comprehensive sequence dataset of five loci (ITS, tef1-α, tub2, cal, and his3) to assist species delineation. The integrative approach based on cultural, ecological, morphological, and molecular characteristics is advantageous for accurately identifying Diaporthe species [22,27,28,35,36,37].
Taxonomic studies of Diaporthe revealed a variety of medicinal plants as their hosts [38]. However, most of these studies have been conducted in temperate zones (i.e., [15,16,17,21,24,26,28]). Knowledge of Diaporthe associated with medicinal plants in the tropics is still limited [31,32]. Therefore, this study aims to identify and describe isolates of Diaporthe associated with several medicinal plants in Thailand using both morphological and molecular analyses. To better illustrate the placements of the five new species, their morphological descriptions, micrographs, and updated phylogenetic trees are presented and discussed.
2. Materials and Methods
2.1. Sample Collection and Morphological Examination
Fresh fungal specimens were collected from the dead leaves and woody twigs of various medicinal plants in urban parks and forest areas in the Chiang Mai and Tak provinces of Thailand in 2019 and 2022. Collected samples were investigated for macro- and micro-morphological structures using a Nikon SMZ800N stereo microscope (Nikon Instruments Inc., Melville, NY, USA) and photomicrographed with a Nikon Eclipse Ni compound microscope attached to a Nikon DS-Ri2 camera system (Nikon Instruments Inc., Melville, NY, USA). The measurement of each structure (i.e., conidiomata, conidiomatal walls, conidiophores, conidiogenous cells, and conidia) was taken using the Tarosoft (R) Image Frame Work program. All figures were modified using Adobe Photoshop CS6 Extended version 10.0 software (Adobe Systems, San Jose, CA, USA).
2.2. Fungal Isolation and Preservation
Pure cultures were obtained from single spore isolation on 2% water agar (WA), and germinated conidia were aseptically transferred to potato dextrose agar (PDA) [39]. Fungal cultures were incubated at 25 °C for four to six weeks and then examined for colony morphology and spore production. Herbarium material and pure culture of Diaporthe globoostiolata were deposited in the herbarium of Mae Fah Luang University (MFLU) and the Mae Fah Luang University Culture Collection (MFLUCC), Chiang Rai Province, Thailand. Herbarium materials and pure cultures of D. afzeliae, D. bombacis, D. careyae, and D. samaneae were deposited in the Herbarium of the Department of Biology (CMUB) and the Culture Collection of Sustainable Development of Biological Resources Laboratory, Faculty of Science, Chiang Mai University (SDBR-CMU), Chiang Mai Province, Thailand. The numbers of Index Fungorum and Faces of Fungi were acquired as outlined in the Index Fungorum [40] and Jayasiri et al. [41].
2.3. DNA Extraction, PCR Amplification, and Sequencing
A DNA Extraction Mini Kit (FAVORGEN, Ping-Tung, Taiwan) was used to extract genomic DNA from fungal colonies grown on PDA for two weeks. Five phylogenetic markers including internal transcribed spacers (ITS), translation elongation factor 1-α (tef1-α), β-tubulin (tub2), calmodulin (cal), and histone H3 (his3) were amplified using the primer pairs ITS5/ITS4 [42], EF1-728F/EF1-986R [43], Bt2a/Bt2b [44], CAL228F/CAL737R [43], and CYLH3F/H3-1b [44,45], respectively. The PCR conditions for each gene region were carried out as described by Jiang et al. [21]. The purification of PCR products was processed using a PCR Clean-up Gel Extraction NucleoSpin® Gel and PCR Clean-up Kit (Macherey-Nagel, Düren, Germany). The sequence analysis was operated by the genetic analyzer at 1ST Base Company (Kembangan, Malaysia).
2.4. Phylogenetic Analyses
The sequences obtained in this study were submitted through a BLASTn search in GenBank (www.ncbi.nlm.nih.gov/blast/, assessed on 1 March 2023) to determine the most similar taxa. The initial phylogenetic analysis was conducted based on the ITS sequence dataset from Norphanphoun et al. [32] to identify the placement of our isolates within species complexes. The newly generated sequences and their related sequences were then selected for the concatenated ITS, tef1-α, tub2, cal, and his3 sequence dataset based on the BLASTn search results and updated literature [18,22,32,46,47,48] (Table 1). The alignment of a single locus dataset was performed using MAFFT v.7 (http://mafft.cbrc.jp/alignment/server/index.html, assessed on 1 March 2023) [49] and the ambiguous sites were manually adjusted using BioEdit 7.1.3.0 [50]. The phylogenetic trees of single locus and combined datasets were analyzed using maximum likelihood (ML) and Bayesian inference (BI) criteria. Tree topologies from single locus analyses were also compared and no conflicts were found.
Table 1.
List of taxa and their GenBank accession numbers included in the phylogenetic analyses.
| Taxa Names |
Culture Accession
No. |
GenBank Accession No. | ||||
| ITS | tef1-α | tub2 | cal | his3 | ||
| Cytospora disciformis | CBS 116827 T | KY051801 | KX965072 | KX964907 | N/A | N/A |
| C. leucostoma | SXYLt * | LKEB00000000 | N/A | N/A | N/A | N/A |
| Diaporthe acuta | CGMCC 3.19600 T | MK626957 | MK654802 | MK691225 | MK691124 | MK726161 |
| D. acuta | PSCG045 | MK626956 | MK654809 | MK691223 | MK691123 | MK726160 |
| D. afzeliae | SDBR-CMU467 T | OQ600199 | OQ603502 | OQ678279 | OQ646882 | OQ646886 |
| D. ampelina | CBS 114016 T | AF230751 | AY745056 | JX275452 | AY745026 | N/A |
| D. anhuiensis | CNUCC 201901 T | MN219718 | MN224668 | MN227008 | MN224549 | MN224556 |
| D. anhuiensis | CNUCC 201902 | MN219727 | MN224669 | MN227009 | MN224550 | MN224557 |
| D. annellsiae | BRIP 59731a T | OM918687 | OM960596 | OM960614 | N/A | N/A |
| D. arecae | CBS 161.64 T | KC343032 | KC343758 | KC344000 | KC343274 | KC343516 |
| D. arecae | CBS 535.75 | KC343033 | KC343759 | KC344001 | KC343275 | KC343517 |
| D. arengae | CBS 114979 T | KC343034 | KC343760 | KC344002 | KC343276 | KC343518 |
| D. aseana | MFLUCC 12-0299a T | KT459414 | KT459448 | KT459432 | KT459464 | N/A |
| D. baccae | CPC 20585 | KJ160564 | KJ160596 | N/A | N/A | N/A |
| D. betulicola | CFCC 51128 T | KX024653 | KX024655 | KX024657 | KX024659 | KX024661 |
| D. betulicola | CFCC 51129 | KX024654 | KX024656 | KX024658 | KX024660 | KX024662 |
| D. bohemiae | CBS 143347 T | MG281015 | MG281536 | MG281188 | MG281710 | MG281361 |
| D. bohemiae | CBS 143348 | MG281016 | MG281537 | MG281189 | MG281711 | MG281362 |
| D. bombacis | SDBR-CMU468 T | OQ600198 | OQ603501 | OQ678278 | OQ646881 | OQ646885 |
| D. bounty | BRIP 59361a T | OM918690 | OM960599 | OM960617 | N/A | N/A |
| D. camelliae-oleiferae | HNZZ027 T | MZ509555 | MZ504707 | MZ504718 | MZ504685 | MZ504696 |
| D. camelliae-oleiferae | HNZZ030 | MZ509556 | MZ504708 | MZ504719 | MZ504686 | MZ504697 |
| D. camelliae-sinensis | SAUCC194.103 | MT822631 | MT855943 | MT855828 | MT855710 | MT855599 |
| D. camelliae-sinensis | SAUCC194.92 T | MT822620 | MT855932 | MT855817 | MT855699 | MT855588 |
| D. canthii | CBS 132533 T | JX069864 | KC843120 | KC843230 | KC843174 | N/A |
| D. careyae | SDBR-CMU469 T | OQ600196 | OQ603449 | OQ678276 | OQ646879 | OQ646883 |
| D. carpini | CBS 114437 | KC343044 | KC343770 | KC344012 | KC343286 | KC343528 |
| D. cercidis | CFCC 52565 T | MH121500 | MH121542 | MH121582 | MH121424 | MH121460 |
| D. chamaeropis | CBS 454.81 T | KC343048 | KC343774 | KC344016 | KC343290 | KC343532 |
| D. chamaeropis | CBS 753.70 | KC343049 | KC343775 | KC344017 | KC343291 | KC343533 |
| D. chiangmaiensis | MFLUCC 18-0544 T | OK393703 | OL439483 | N/A | N/A | N/A |
| D. chiangmaiensis | MFLUCC 18-0935 | OK393704 | OL439484 | N/A | N/A | N/A |
| D. chiangmaiensis | MFLUCC 21-0212 | OK393702 | OL439482 | OK490918 | N/A | N/A |
| D. cinerascens | CBS 719.96 | KC343050 | KC343776 | KC344018 | KC343292 | KC343534 |
| D. cissampeli | CBS 141331 T | KX228273 | N/A | KX228384 | N/A | KX228366 |
| D. corylicola | CFCC 53986 T | MW839880 | MW815894 | MW883977 | MW836684 | MW836717 |
| D. cytosporella | FAU461 T | KC843307 | KC843116 | KC843221 | KC843141 | MF418283 |
| D. decedens | CBS 109772 | KC343059 | KC343785 | KC344027 | KC343301 | KC343543 |
| D. decedens | CBS 114281 | KC343060 | KC343786 | KC344028 | KC343302 | KC343544 |
| Taxa Names |
Culture Accession
No. |
GenBank Accession No. | ||||
| ITS | tef1-α | tub2 | cal | his3 | ||
| D. decorticans | CBS 114200 | KC343169 | KC343895 | KC344137 | KC343411 | KC343653 |
| D. decorticans | CBS 114649 | KC343170 | KC343896 | KC344138 | KC343412 | KC343654 |
| D. delonicis | MFLU 16-1059 T | MT215490 | N/A | MT212209 | N/A | N/A |
| D. detrusa | CBS 109770 | KC343061 | KC343787 | KC344029 | KC343303 | KC343545 |
| D. detrusa | CBS 114652 | KC343062 | KC343788 | KC344030 | KC343304 | KC343546 |
| D. elaeagni-glabrae | CGMCC 3.18287 T | KX986779 | KX999171 | KX999212 | KX999281 | KX999251 |
| D. elaeagni-glabrae | LC4806 | KX986780 | KX999172 | KX999213 | KX999282 | KX999252 |
| D. endocitricola | ZHKUCC 20-0012 T | MT355682 | MT409336 | MT409290 | MT409312 | N/A |
| D. endocitricola | ZHKUCC 20-0013 | MT355683 | MT409337 | MT409291 | MT409313 | N/A |
| D. eugeniae | CBS 444.82 T | KC343098 | KC343824 | KC344066 | KC343340 | KC343582 |
| D. eugeniae | DPFT23 | MK110366 | MK117267 | MK122799 | N/A | N/A |
| D. fibrosa | CBS 109751 | KC343099 | KC343825 | KC344067 | KC343341 | KC343583 |
| D. fibrosa | CBS 113830 | KC343100 | KC343826 | KC344068 | KC343342 | KC343584 |
| D. foeniculina | CBS 111553 T | KC843295 | KC843104 | KC843209 | KC843129 | N/A |
| D. foeniculina (=D. foeniculacea) | CBS 123208 T | KC343104 | KC343830 | KC344072 | KC343346 | KC343588 |
| D. foeniculina (=D. rhoicola) | CBS 129528 T | JF951146 | KC843100 | KC843205 | KC843124 | N/A |
| D. forlicesenica | MFLUCC 17-1015 T | KY964215 | KY964171 | KY964099 | N/A | N/A |
| D. fraxini-angustifoliae | BRIP 54781 T | JX862528 | JX862534 | KF170920 | N/A | N/A |
| D. fraxini-angustifoliae | MFLUCC 15-0748 | KT459428 | KT459446 | KT459430 | KT459462 | N/A |
| D. fulvicolor | CGMCC 3.19601 T | MK626859 | MK654806 | MK691236 | MK691132 | MK726163 |
| D. fulvicolor | PSCG 057 | MK626858 | MK654810 | MK691233 | MK691131 | MK726164 |
| D. globoostiolata | MFLUCC 23-0025 T | OQ600200 | OQ603503 | OQ678280 | N/A | N/A |
| D. gossiae | BRIP 59730a T | OM918693 | OM960602 | OM960620 | N/A | N/A |
| D. guangxiensis | JZB320091 | MK335769 | MK523564 | MK500165 | MK736724 | N/A |
| D. guangxiensis | JZB320094 T | MK335772 | MK523566 | MK500168 | MK736727 | N/A |
| D. hickoriae | CBS 145.26 T | KC343118 | KC343844 | KC344086 | KC343360 | KC343602 |
| D. hispaniae | CBS 143351 T | MG281123 | MG281644 | MG281296 | MG281820 | MG281471 |
| D. hispaniae | CBS 143352 | MG281124 | MG281645 | MG281297 | MG281821 | MG281472 |
| D. hongkongensis | CBS 115448 T | KC343119 | KC343845 | KC344087 | KC343361 | KC343603 |
| D. howardiae | BRIP 59697a T | OM918695 | OM960604 | OM960622 | N/A | N/A |
| D. huangshanensis | CNUCC 201903 T | MN219729 | MN224670 | MN227010 | N/A | MN224558 |
| D. huangshanensis | CNUCC 201904 | MN219730 | MN224671 | MN227011 | N/A | MN224559 |
| D. hunanensis | HNZZ023 T | MZ509550 | MZ504702 | MZ504713 | MZ504680 | MZ504691 |
| D. hunanensis | HNZZ025 | MZ509551 | MZ504703 | MZ504714 | MZ504681 | MZ504692 |
| D. hungariae | CBS 143353 T | MG281126 | MG281647 | MG281299 | MG281823 | MG281474 |
| D. hungariae | CBS 143354 | MG281127 | MG281648 | MG281300 | MG281824 | MG281475 |
| Taxa Names |
Culture Accession
No. |
GenBank Accession No. | ||||
| ITS | tef1-α | tub2 | cal | his3 | ||
| D. impulsa | CBS 114434 | KC343121 | KC343847 | KC344089 | KC343363 | KC343605 |
| D. impulsa | CBS 141.27 | KC343122 | KC343848 | KC344090 | KC343364 | KC343606 |
| D. inconspicua | CBS 133813 T | KC343123 | KC343849 | KC344091 | KC343365 | KC343607 |
| D. inconspicua | URM7776 | MG696772 | MG710414 | MG710395 | MG710391 | MG710410 |
| D. isoberliniae | CPC 22549 T | KJ869133 | N/A | KJ869245 | N/A | N/A |
| D. juglandicola | CFCC 51134 T | KU985101 | KX024628 | KX024634 | KX024616 | KX024622 |
| D. juglandicola | CFCC 51135 | KU985102 | KX024629 | KX024635 | KX024617 | KX024623 |
| D. krabiensis | MFLUCC 17-2481 T | MN047101 | MN433215 | MN431495 | N/A | N/A |
| D. limonicola | CPC 27869 | MF418419 | MF418498 | MF418579 | MF418253 | MF418339 |
| D. imonicola | CPC 28200 T | NR_154980 | MF418501 | MF418582 | MF418256 | MF418342 |
| D. litchiicola | BRIP 54900 T | JX862533 | JX862539 | KF170925 | N/A | N/A |
| D. lithocarpi | CGMCC 3.15175 T | KC153104 | KC153095 | KF576311 | KF576236 | N/A |
| D. ithocarpi | CGMCC 3.17098 | KF576276 | KF576251 | KF576300 | KF576228 | N/A |
| D. lithocarpi | LC3079 | KP267851 | KP267925 | KP293431 | N/A | KP293505 |
| D. lutescens | SAUCC 194.36 T | MT822564 | MT855877 | MT855761 | MT855647 | MT855533 |
| D. macintoshii | BRIP 55064a T | KJ197289 | KJ197251 | KJ197269 | N/A | N/A |
| D. maytenicola | CPC 21896 T | KF777157 | N/A | KF777250 | N/A | N/A |
| D. melastomatis | SAUCC194.55 T | MT822583 | MT855896 | MT855780 | MT855664 | MT855551 |
| D. melastomatis | SAUCC194.80 | MT822608 | MT855920 | MT855805 | MT855687 | MT855576 |
| D. meliae | CFCC 53089 T | MK432657 | ON081654 | MK578057 | N/A | ON081662 |
| D. meliae | CFCC 53090 | MK432658 | ON081655 | MK578058 | N/A | ON081663 |
| D. melitensis | CPC 27873 T | MF418424 | MF418503 | MF418584 | MF418258 | MF418344 |
| D. melitensis | CPC 27875 | MF418425 | MF418504 | MF418585 | MF418259 | MF418345 |
| D. millettiae | GUCC9167 T | MK398674 | MK480609 | MK502089 | MK502086 | N/A |
| D. musigena | CBS 129519 T | KC343143 | KC343869 | KC344111 | KC343385 | KC343627 |
| D. musigena | HKFZL006 | MK050110 | MK054238 | MK079660 | N/A | N/A |
| D. nebulae | Phom240 | KY511315 | MH708543 | KY511346 | N/A | N/A |
| D. nebulae | PMM1681 T | KY511337 | MH708552 | KY511369 | N/A | N/A |
| D. nelumbonis | R. Kirschner 4114 T | KT821501 | N/A | LC086652 | N/A | N/A |
| D. nigra | JZB320170 T | MN653009 | MN892277 | MN887113 | N/A | N/A |
| D. norfolkensis | BRIP 59718a T | OM918699 | OM960608 | OM960626 | N/A | N/A |
| D. oculi | HHUF 30565 T | LC373514 | LC373516 | LC373518 | N/A | N/A |
| D. oncostoma | CBS 100454 | KC343160 | KC343886 | KC344128 | KC343402 | KC343644 |
| D. oncostoma | CBS 589.78 | KC343162 | KC343888 | KC344130 | KC343404 | KC343646 |
| D. osmanthi | GUCC9165 T | MK398675 | MK480610 | MK502091 | MK502087 | N/A |
| D. pandanicola | MFLUCC 17-0607 T | MG646974 | N/A | MG646930 | N/A | N/A |
| D. parapterocarpi | CPC 22729 T | KJ869138 | N/A | KJ869248 | N/A | N/A |
| D. parvae | CGMCC 3.19599 T | MK626919 | MK654858 | MK691248 | N/A | MK726210 |
| D. parvae | PSCG035 | MK626920 | MK654859 | MK691249 | MK691169 | MK726211 |
| D. pascoei | BPPCA147 | MK111091 | MK117255 | MK122790 | N/A | N/A |
| Taxa Names |
Culture Accession
No. |
GenBank Accession No. | ||||
| ITS | tef1-α | tub2 | cal | his3 | ||
| D. pascoei | BRIP 54847 T | JX862532 | JX862538 | KF170924 | N/A | N/A |
| D. perseae | BPPCA257 | MK111098 | MK117256 | MK122791 | N/A | N/A |
| D. perseae | CBS 151.73 T | KC343173 | KC343899 | KC344141 | KC343415 | KC343657 |
| D. pescicola | MFLUCC 16-0105 T | KU557555 | KU557623 | KU557579 | KU557603 | N/A |
| D. pescicola | PSCG036 | MK626855 | MK654796 | MK691226 | MK691116 | MK726159 |
| D. phillipsii | CAA817 T | MK792305 | MK828076 | MN000351 | MK883831 | MK871445 |
| D. phillipsii | CAA818 | MK792307 | MK828078 | MN000352 | MK883833 | MK871447 |
| D. phoenicicola | CBS 161.64 T | MH858400 | GQ250349 | JX275440 | JX197432 | N/A |
| D. phoenicicola | KUC21217 | KT207733 | N/A | KT207633 | N/A | N/A |
| D. phoenicicola | KUC21243 | KT207761 | N/A | KT207659 | N/A | N/A |
| D. phoenicicola | PBMR340 | MK111086 | MK117271 | MK122805 | N/A | N/A |
| D. phoenicicola | PBMR345 | MK111088 | MK117275 | MK122810 | N/A | N/A |
| D. podocarpi-macrophylli | CGMCC 3.18281 T | KX986774 | KX999167 | KX999207 | KX999278 | KX999246 |
| D. podocarpi-macrophylli | LC6229 | KX986771 | KX999164 | KX999204 | KX999277 | KX999243 |
| D. poincianellae | URM 7932 T | MH989509 | MH989538 | MH989537 | MH989540 | MH989539 |
| D. portugallica | CPC 34247 T | MH063905 | MH063911 | MH063917 | MH063893 | MH063899 |
| D. portugallica | CPC 34248 | MH063906 | MH063912 | MH063918 | MH063894 | MH063900 |
| D. pseudoinconspicua | URM 7873 | MH122535 | MH122530 | MH122521 | MH122525 | MH122518 |
| D. pseudoinconspicua | URM 7874 T | MH122538 | MH122533 | MH122524 | MH122528 | MH122517 |
| D. pseudomangiferae | CBS 101339 T | KC343181 | KC343907 | KC344149 | KC343423 | KC343665 |
| D. pseudomangiferae | CBS 388.89 | KC343182 | KC343908 | KC344150 | KC343424 | KC343666 |
| D. pseudooculi | HHUF 30617 T | NR_161019 | LC373517 | LC373519 | N/A | N/A |
| D. pseudophoenicicola | CBS 462 69 T | KC343184 | KC343910 | KC344152 | KC343426 | KC343668 |
| D. pseudophoenicicola | HNQZ01 | MN424520 | MN424562 | MN424534 | MN424576 | MN424548 |
| D. psoraleae | CBS 136412 T | KF777158 | KF777245 | KF777251 | N/A | N/A |
| D. psoraleae pinnatae | CBS 136413 T | KF777159 | N/A | KF777252 | N/A | N/A |
| D. pterocarpi | MFLUCC 10-0571 T | JQ619899 | JX275416 | JX275460 | JX197451 | N/A |
| D. pterocarpi | MFLUCC 10-0588 | JQ619900 | JX275417 | JX275461 | JX197452 | N/A |
| D. pterocarpicola | MFLUCC 10-0580 T | JQ619887 | JX275403 | JX275441 | JX197433 | N/A |
| D. pungensis | SAUCC 194.112 T | MT822640 | MT855952 | MT855837 | MT855719 | MT855607 |
| D. pungensis | SAUCC 194.89 | MT822617 | MT855929 | MT855814 | MT855696 | MT855585 |
| D. ravennica | MFLUCC 15-0479 T | KU900335 | N/A | KX432254 | N/A | N/A |
| D. ravennica | MFLUCC 17-1029 | KY964191 | KY964147 | KY964075 | N/A | N/A |
| D. rhodomyrti | CFCC 53101 T | MK432643 | MK578119 | MK578046 | MK442965 | MK442990 |
| Taxa Names |
Culture Accession
No. |
GenBank Accession No. | ||||
| ITS | tef1-α | tub2 | cal | his3 | ||
| D. rhodomyrti | CFCC 53102 | MK432644 | MK578120 | MK578047 | MK442966 | MK442991 |
| D. rostrata | CFCC 50062 T | KP208847 | KP208853 | KP208855 | KP208849 | KP208851 |
| D. rostrata | CFCC 50063 | KP208848 | KP208854 | KP208856 | KP208850 | KP208852 |
| D. rumicicola | JZB320006 T | MK066126 | MK078545 | MK078546 | N/A | N/A |
| D. rumicicola | MFLUCC 18-0739 | MH846233 | MK049554 | MK049555 | N/A | N/A |
| D. saccarata | CBS 116311 T | KC343190 | KC343916 | KC344158 | KC343432 | KC343674 |
| D. salinicola | MFLU 17-2592 | MN047099 | MN077074 | N/A | N/A | N/A |
| D. salinicola | MFLU 18-0553 T | MN047098 | MN077073 | N/A | N/A | N/A |
| D. samaneae | SDBR-CMU470 T | OQ600197 | OQ603500 | OQ678277 | OQ646880 | OQ646884 |
| D. schimae | CFCC 53103 T | MK432640 | MK578116 | MK578043 | MK442962 | MK442987 |
| D. schimae | CFCC 53104 | MK432641 | MK578117 | MK578044 | MK442963 | MK442988 |
| D. schisandrae | CFCC 51988 T | KY852497 | KY852509 | KY852513 | KY852501 | KY852505 |
| D. schisandrae | CFCC 51989 | KY852498 | KY852510 | KY852514 | KY852502 | KY852506 |
| D. scobina | CBS 251.38 | KC343195 | KC343921 | KC344163 | KC343437 | KC343679 |
| D. searlei | CBS 146456 T | MN708231 | N/A | MN696540 | N/A | N/A |
| D. sennae | CFCC 51636 T | KY203724 | KY228885 | KY228891 | KY228875 | N/A |
| D. sennae | CFCC 51637 | KY203725 | KY228886 | KY228892 | KY228876 | N/A |
| D. spinosa | CGMCC 3.19602 T | MK626849 | MK654811 | MK691234 | MK691129 | MK726156 |
| D. spinosa | PSCG388 | MK626860 | MK654798 | MK691229 | MK691128 | MK726171 |
| D. stictica | CBS 370.54 T | KC343212 | KC343938 | KC344180 | KC343454 | KC343696 |
| D. taiwanensis | NTUCC 18-105-1 T | MT241257 | MT251199 | MT251202 | MT251196 | N/A |
| D. taiwanensis | NTUCC 18-105-2 | MT241258 | MT251200 | MT251203 | MT251197 | N/A |
| D. taoicola | MFLUCC 16-0117 T | KU557567 | KU557635 | KU557591 | N/A | N/A |
| D. taoicola | PSCG485 | MK626869 | MK654812 | MK691227 | MK691120 | MK726173 |
| D. tectonigena | LC6512 | KX986782 | KX999174 | KX999215 | KX999284 | KX999254 |
| D. tectonigena | MFLUCC 12-0767 T | KU712429 | KU749371 | KU743976 | KU749358 | N/A |
| D. thunbergiae | MFLUCC 10-0576a T | JQ619893 | JX275409 | JX275449 | JX197440 | N/A |
| D. thunbergiae | MFLUCC 10-0576b | JQ619894 | JX275410 | JX275450 | JX197441 | N/A |
| D. toxicodendri | FFPRI420990 | LC275193 | LC275217 | LC275225 | LC275201 | LC275209 |
| D. vangueriae | CPC 22703 T | KJ869137 | N/A | KJ869247 | N/A | N/A |
| D. velutina | CGMCC 3.18286 T | KX986790 | KX999182 | KX999223 | N/A | KX999261 |
| D. velutina | LC4419 | KX986789 | KX999181 | KX999222 | KX999286 | KX999260 |
| D. verniciicola | CFCC 53109 T | MK573944 | MK574619 | MK574639 | MK574583 | MK574599 |
| D. verniciicola | CFCC 53110 | MK573945 | MK574620 | MK574640 | MK574584 | MK574600 |
| D. viniferae | JZB320071 T | MK341550 | MK500107 | MK500112 | MK500119 | N/A |
| D. viniferae | JZB320072 | MK341551 | MK500108 | MK500113 | MK500120 | N/A |
| D. woolworthii | CBS 148.27 | KC343245 | KC343971 | KC344213 | KC343487 | KC343729 |
| D. xishuangbanica | CGMCC 3.18282 | KX986783 | KX999175 | KX999216 | N/A | KX999255 |
| D. xishuangbanica | CGMCC 3.18283 T | KX986784 | KX999176 | KX999217 | N/A | N/A |
| D. zaobaisu | CGMCC 3.19598 T | MK626922 | MK654855 | MK691245 | N/A | MK726207 |
| Taxa Names |
Culture Accession
No. |
GenBank Accession No. | ||||
| ITS | tef1-α | tub2 | cal | his3 | ||
| D. zaobaisu | PSCG032 | MK626923 | MK654856 | MK691246 | N/A | MK726208 |
| D. zhaoqingensis | ZHKUCC 22-0056 T | ON322885 | N/A | ON315074 | ON315000 | ON315015 |
| D. zhaoqingensis | ZHKUCC 22-0057 | ON322886 | ON315043 | ON315075 | N/A | ON315016 |
The ex-type cultures are indicated with the superscript “T” and the newly generated sequences are indicated in bold. “N/A” indicates the sequence is unavailable. “*” indicates a whole genomic DNA strain.
ML and BI analyses were performed using RAxML-HPC2 on XSEDE (v.8.2.12) [51] and MrBayes on XSEDE v.3.2.7a [52,53,54] in the CIPRES Science Platform V3.3 (https://www.phylo.org/portal2/home.action, assessed on 1 March 2023) [55]. The GTRGAMMA model of the bootstrapping phase with 1000 bootstrap iterations was set as the parameter for ML analysis [51]. The best nucleotide substitution model was determined using MrModeltest v.2.3 [56], and GTR + I + G was selected as the best-fitting model for the ITS, tef1-α, tub2, cal, and his3 datasets. For BI analysis, six simultaneous Markov chains were set to run 10,000,000 generations with a sampling frequency of 100 generations. The burn-in phase was set as 0.25, and the posterior probabilities (PP) were evaluated from the remaining trees. The phylogenetic trees resulting from the ML and BI analyses were visualized by the FigTree v1.4.0 program [57] and adjusted using Adobe Photoshop CS6 software (Adobe Systems, San Jose, CA, the USA). Novel obtained sequences were registered for GenBank accession numbers.
2.5. Genealogical Concordance Phylogenetic Species Recognition Analysis
The recombination level between new species and their most closely related taxa was examined using the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) model [58,59]. A pairwise homoplasy index (PHI) test was implemented by SplitsTree4 using the LogDet transformation and split decomposition options [60,61]. A PHI test result (Φw) above 0.05 indicated no significant recombination in the dataset. In addition, split graphs were generated for visualization of the relationship between closely related species.
3. Results
3.1. Molecular Phylogeny
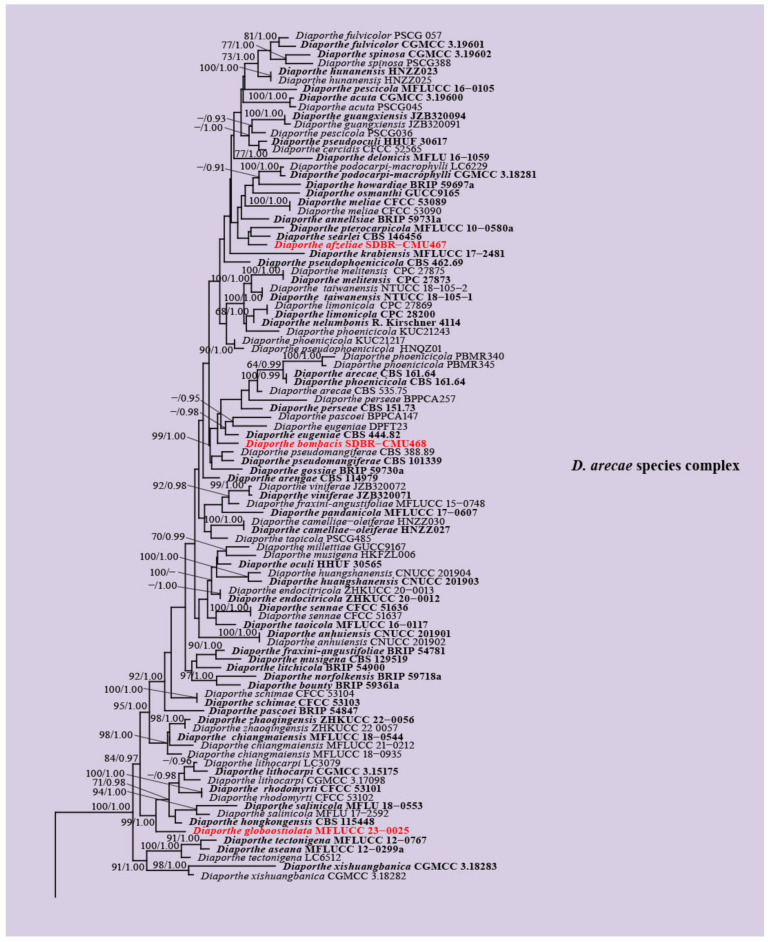
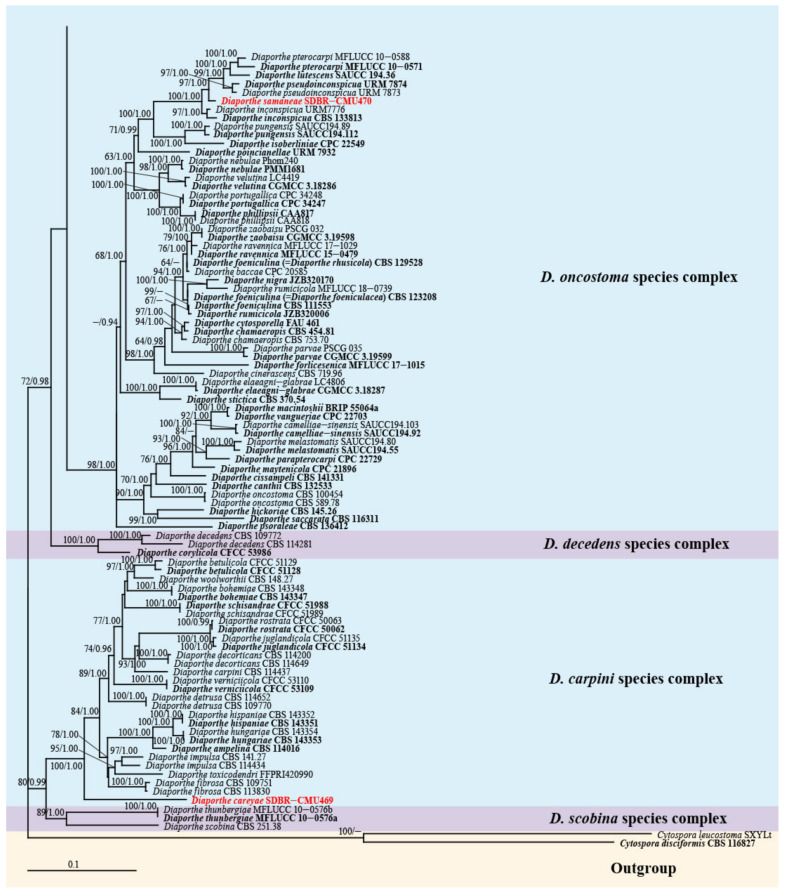
The combination of the ITS, tef1-α, tub2, cal, and his3 sequence datasets comprised 191 Diaporthe strains, with Cytospora disciformis CBS 116,827 and C. leucostoma SXYLt as the outgroups. The aligned sequence dataset contained a total of 3020 characters with gaps in the order of ITS (1–588), tef1-α (589–992), tub2 (993–1800), cal (1801–2522), and his3 (2523–3020). The final RAxML analysis resulted in the best scoring tree with a final optimization likelihood value of -42610.603037. The matrix comprised 2000 distinct alignment patterns, with 33.63% undetermined characters or gaps. The estimated base frequencies were as follows: A = 0.218896, C = 0.324616, G = 0.235283, and T = 0.221205; substitution rates AC = 1.207023, AG = 3.073601, AT = 1.095752, CG = 0.816932, CT = 4.008593, and GT = 1.000000; and gamma distribution shape parameter of 0.398901. The phylogenetic trees generated from the ML and BI analyses revealed similar topologies. The newly recovered isolates formed five monophyletic lineages within three species complexes as follows: D. afzeliae, D. bombacis, and D. globoostiolata were clustered within the D. arecae species complex; D. samaneae was grouped in the D. oncostoma species complex; and D. careyae was associated with the D. carpini species complex (Figure 1).
Figure 1.
Phylogenetic tree obtained from the RAxML analysis of the combined ITS, tef1-α, tub2, cal, and his3 sequence data. Bootstrap support values for ML equal to or greater than 60% and Bayesian posterior probabilities equal to or greater than 0.90 PP are indicated at the nodes as ML/PP. The ex-type strains are in black, and the new isolates obtained in this study are in red. The tree is rooted in Cytospora disciformis (CBS 116827) and C. leucostoma (SXYLt).
3.2. Genealogical Concordance Phylogenetic Species Recognition Analysis
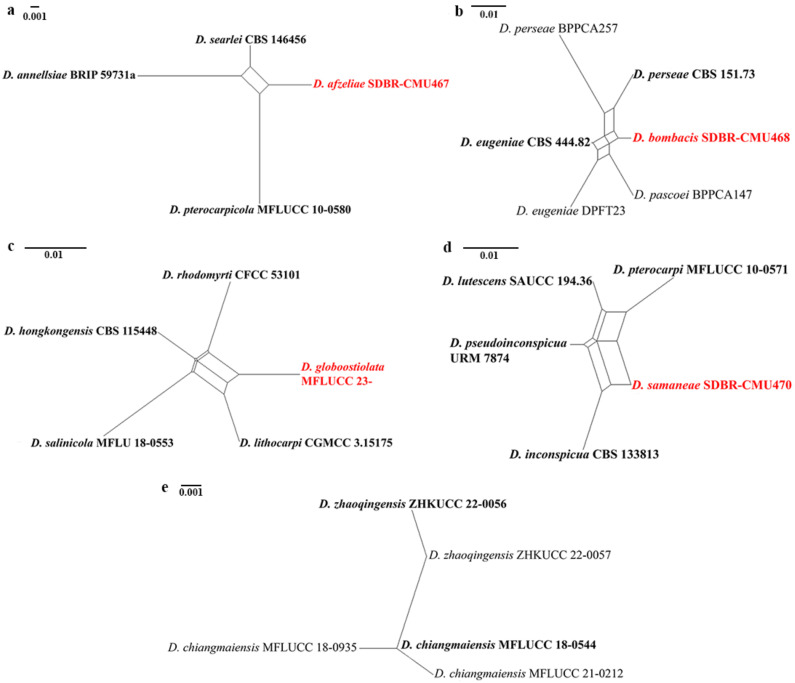
In the PHI analysis, there was no evidence of significant recombination (Φw > 0.05) between each new species (Diaporthe afzeliae, D. bombacis, D. globoostiolata, and D. samaneae) and their closely related taxa in the combined ITS, tef1-α, tub2, cal, and his3 sequence dataset (Figure 2a–d). The results of PHI analysis also revealed no significant recombination (Φw > 0.05) between D. zhaoqingensis and D. chiangmaiensis (Figure 2e). This evidence confirms that they are distinct species.
Figure 2.
The split graphs of a PHI test result of (a) Diaporthe afzeliae, (b) D. bombacis, (c) D. globoostiolata, and (d) D. samaneae with their closely related taxa, and (e) D. zhaoqingensis and D. chiangmaiensis using the LogDet transformation and split decomposition options. New species in each graph are indicated in red.
3.3. Taxonomy
Diaporthe afzeliae Monkai and S. Lumyong, sp. nov.
Index Fungorum number: IF900377; Faces of fungi number: FoF 14091; Figure 3
Etymology: Refers to the host genus, Afzelia, from which the holotype was collected.
Holotype: CMUB39998
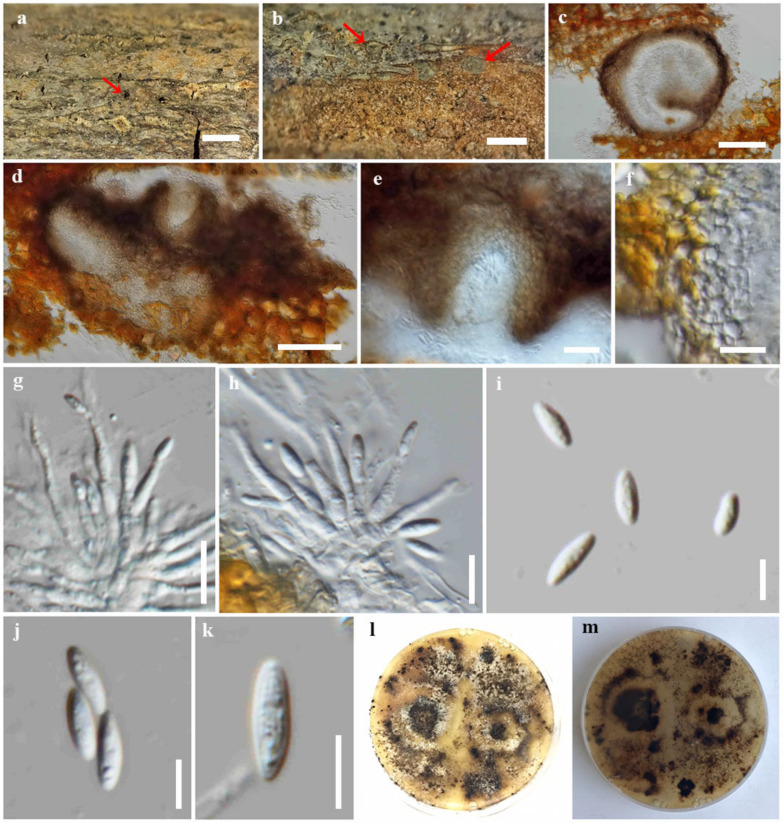
Saprobic on dead wood of Afzelia xylocarpa. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata: 200–300 high × 450–850 μm diam., pycnidial, stromatic, subepidermal, immersed, clustered, ovoid to subconical, elongated, dark brown to black, ostiolate, and multi-loculate. Ostioles: up to 120 μm wide, subglobose or conical, dark brown, and papillate. Conidiomatal wall: up to 40 μm wide, comprising several layers of thin-walled cells, arranged in textura angularis, with dark brown outer layers and hyaline to pale brown inner layers. Conidiophores: 9–26.7 × 1.7–3 μm (x = 15.8 × 2.3 μm, n = 30), tightly aggregated, subcylindrical, hyaline, septate, branched, and straight to sinuous. Conidiogenous cells: 8.2–18 × 1.4–2.7 μm (x = 12 × 2 μm, n = 30), subcylindrical to ampulliform, tapering towards apex, hyaline, phialidic, and terminal, with visible periclinal thickening and a prominent collarette. Alpha conidi:a 5.6–10.4 × 2.3–2.8 μm (x = 8.5 × 2.3 μm, n = 30), ellipsoid to elongate fusiform, obtuse at apex, subtruncate at base, sometimes with a denticle attached to the base, aseptate, hyaline, smooth-walled, and eguttulate. Beta conidia: not observed.
Culture characteristics: Colonies on PDA reached 5 cm diam. after 10 days at 25 °C, effuse, fluffy, lobate margin, originally white, becoming grey and yellow grey mycelium with age, yellowish to pale brown in reverse, with numerous black dots developing as the fruiting bodies (conidial production not seen).
Material examined: Thailand, Chiang Mai Province, Kanjanapisak Park, on dead wood of Afzelia xylocarpa (Kurz) Craib (Fabaceae), 4 April 2022, J. Monkai, KJ32 (CMUB39998, holotype), ex-type living culture, SDBR-CMU467.
Notes: Diaporthe afzeliae formed a sister clade to D. searlei and D. pterocarpicola (Figure 1). Diaporthe afzeliae can be distinguished from D. searlei CBS 146,456 by 0.84% and 2.22% base pair differences in ITS (5/598 bp) and tef1-α (11/495 bp) and D. pterocarpicola MFLUCC 10-0580 in 3.5%, 0.8%, 1.84%, and 3.79% base pair differences in ITS (18/515 bp), tef1-α (3/373 bp), tub2 (8/435 bp), and cal (17/448 bp). Diaporthe afzeliae is different from D. searlei by its wider conidia {5.6–10.4 × 2.3–2.8 vs. 5–9 × 1.5–2 μm} [62] and D. pterocarpicola by its narrower conidia {5.6–10.4 × 2.3–2.8 vs. (5–)6–7(–8) × (2–)2.5(–3.5) μm} [33]. Moreover, D. afzeliae was isolated as a saprobe from Afzelia xylocarpa, while D. searlei was associated with the husk rot of Macadamia sp. [62] and D. pterocarpicola infected leaves of Pterocarpus indicus [33].
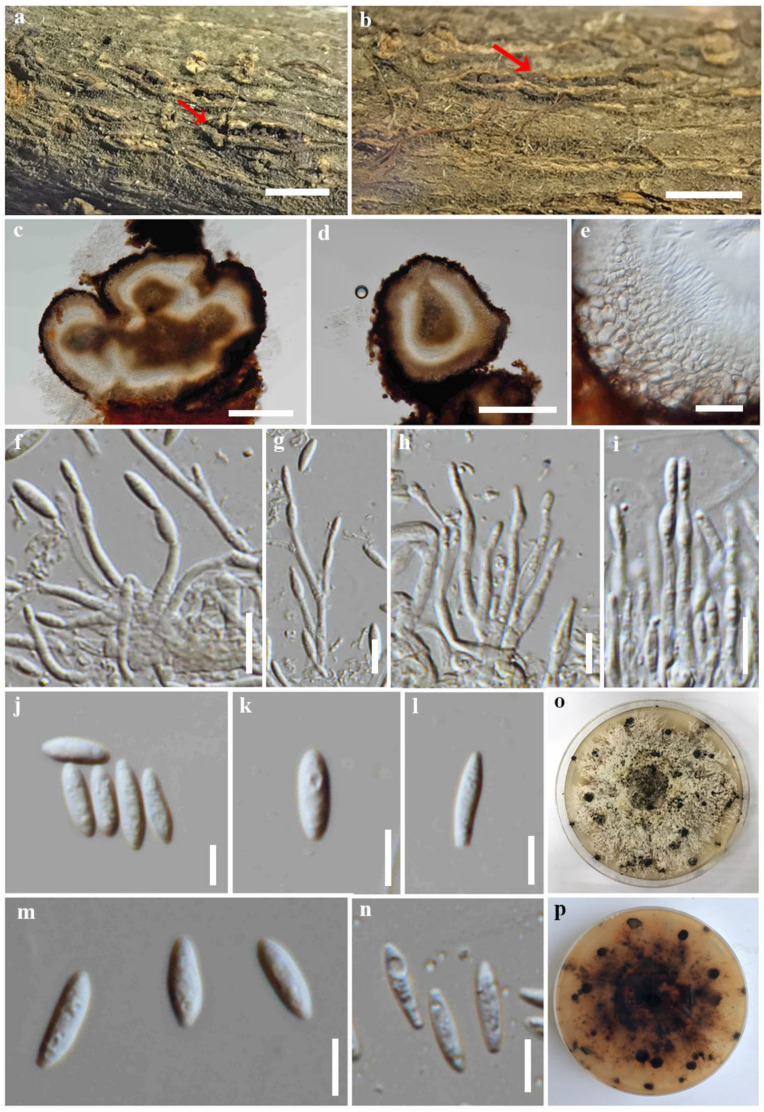
Diaporthe bombacis Monkai and S. Lumyong, sp. nov.
Index Fungorum number: IF900378; Faces of fungi number: FoF 14092; Figure 4
Etymology: Refers to the host genus, Bombax, from which the holotype was collected.
Holotype: CMUB39995
Saprobic on dead wood of Bombax ceiba. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata: 220–330 high × 270–430 μm diam., pycnidial, stromatic, subepidermal, immersed, clustered, subglobose to ovoid, dark brown to brown, ostiolate, and uni-to multi-loculate. Ostioles: up to 90 μm wide, central, subglobose, dark brown. Conidiomatal wall: up to 40 μm wide, comprising a few layers of thin-walled cells, arranged in textura angularis, with dark brown outer layers and hyaline to pale brown inner layers. Conidiophores: 6.2–24 × 1.5–2.8 μm (x = 15.8 × 2 μm, n = 30), tightly aggregated, subcylindrical, hyaline, septate, branched, and straight to sinuous. Conidiogenous cells: 4.5–12 × 1.4–2.4 μm (x = 8 × 2 μm, n = 30), subcylindrical to ampulliform, tapering towards the apex, hyaline, phialidic, and terminal, with visible periclinal thickening; collarette not observed. Alpha conidia: 6–9.4 × 1.7–3 μm (x = 7.6 × 2.4 μm, n = 30), ellipsoid to elongate fusiform, obtuse at apex, subtruncate at base, aseptate, hyaline, smooth-walled, and eguttulate. Beta conidia: not observed.
Culture characteristics: Colonies on PDA reached 5 cm diam. after 10 days at 25 °C, effuse, fluffy, lobate at the margin, originally white, becoming yellowish to pale brown mycelium with age, yellowish to pale brown in reverse, with numerous black dots developing as the fruiting bodies (conidial production not seen).
Material examined: Thailand, Chiang Mai Province, Kanjanapisak Park, on dead wood of Bombax ceiba L. (Bombacaceae), 4 April 2022, J. Monkai, KJ12 (CMUB39995, holotype), ex-type living culture, SDBR-CMU468.
Notes: Diaporthe bombacis formed a distinct clade adjacent to D. eugeniae (Figure 1). Diaporthe bombacis can be distinguished from D. eugeniae CBS 444.82 in 0.7%, 0.85%, 4.98%, 2.68%, and 1.92% base pair differences in ITS (4/571 bp), tef1-α (6/352 bp), tub2 (20/402 bp), cal (13/485 bp), and his3 (9/469 bp). Diaporthe bombacis resembles D. eugeniae in having stromatic and uni-to multi-loculate conidiomata with ostioles [63]. However, D. bombacis differs from D. eugeniae in having longer alpha conidia {6–9.4 × 1.7–3 vs. 6 × 2–3 μm} and the absence of beta conidia [63]. Diaporthe eugeniae was reported from Eugenia aromatica [63], while D. bombacis was found on Bombax ceiba.
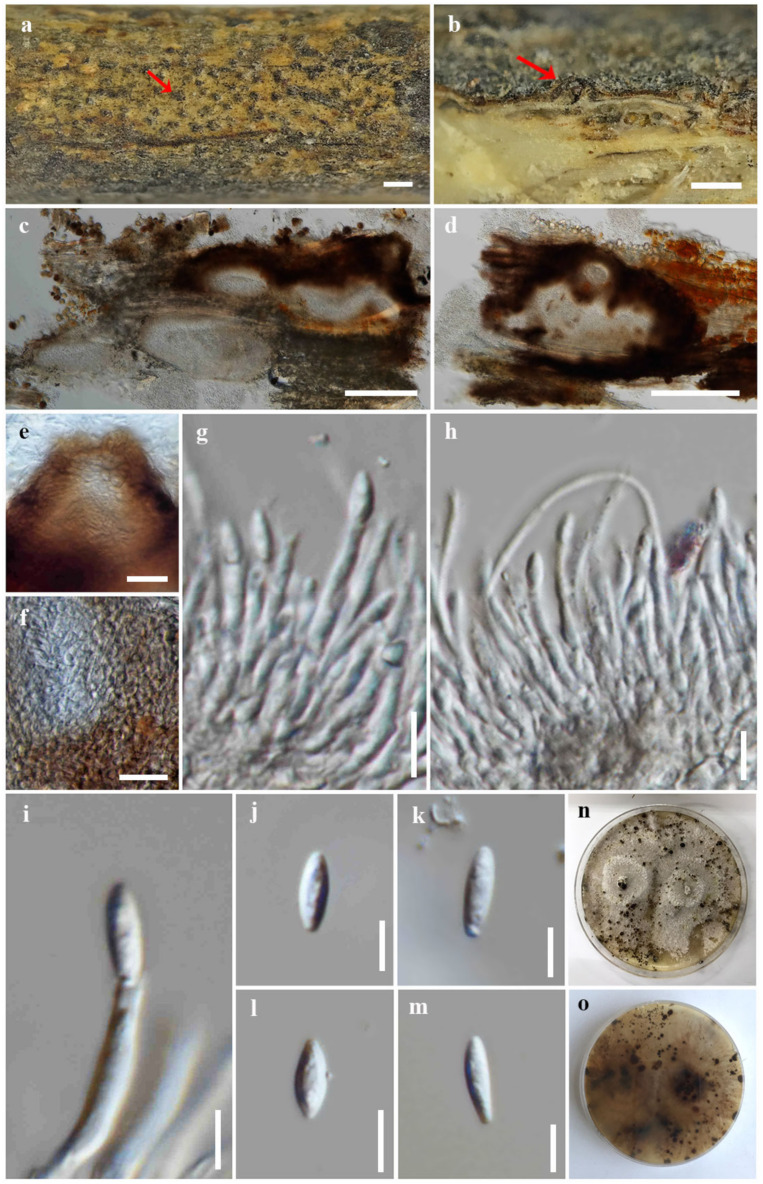
Figure 3.
Diaporthe afzeliae (CMUB39998, holotype). (a) Conidiomata on host substrate (indicated with the red arrow). (b–d) Section through conidiomata (indicated with the red arrow). (e) Ostiole. (f) Conidiomatal walls. (g–i) Conidiogenous cells giving rise to conidia. (j–m) Alpha conidia. (n,o) Colonies on PDA, (n) from above and (o) from reverse. Scale bars: (a) = 500 μm, (b–d) = 200 μm, (e,f) = 20 μm, (g,h) = 10 μm, and (i–m) = 5 μm.
Figure 4.
Diaporthe bombacis (CMUB39995, holotype). (a,b) Conidiomata on host substrate (indicated with the red arrow). (c,d) Section through conidioma. (e) Ostiole. (f) Conidiomatal walls. (g,h) Conidiogenous cells giving rise to conidia. (i–k) Alpha conidia. (l,m) Colonies on PDA, (l) from above and (m) from reverse. Scale bars: (a,b) = 500 μm, (c,d) = 100 μm, (e,f) = 20 μm, (g,h) = 10 μm, and (i–k) = 5 μm.
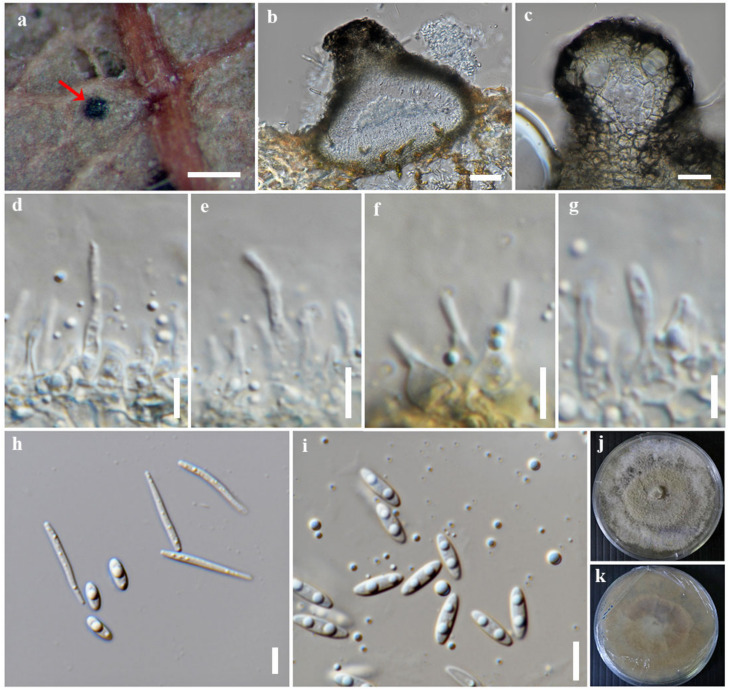
Diaporthe careyae Monkai and S. Lumyong, sp. nov.
Index Fungorum number: IF900379; Faces of fungi number: FoF 14093; Figure 5
Etymology: Refers to the host genus, Careya, from which the holotype was collected.
Holotype: CMUB39996
Saprobic on dead wood of Careya sphaerica. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata: 100–180 high × 150–320 μm diam., pycnidial, immersed to semi-immersed, erumpent, solitary to gregarious, subglobose to ovoid, dark brown to reddish-brown, uni-to bi-loculate, ostiolate, and lacking necks. Conidiomatal wall: up to 20 μm wide, comprising a few layers of thick-walled cells, arranged in textura angularis, with reddish-brown outer layers and hyaline to brown inner layers. Conidiophores: reduced to conidiogenous cells. Conidiogenous cells: 4.8–10.7 × 1.4–2.5 μm (x = 8 × 2 μm, n = 30), subcylindrical, tapering towards apex, producing 1–2 conidia, hyaline, phialidic, terminal, with visible periclinal thickening and a prominent collarette. Alpha conidia: 7–12 × 1.8–3 μm (x = 9.4 × 2.6 μm, n = 30), oblong to ellipsoid, obtuse at apex, subtruncate at base, straight to slightly curved or asymmetrical, 0–1(–2) septate, hyaline, smooth-walled, and bi- to multi-guttulate. Beta conidia: not observed.
Culture characteristics: Colonies on PDA reached 9 cm diam. after 10 days at 25 °C, effuse, sparse hyphae, filiform margin, originally white, becoming grey with age, yellowish to light brown in reverse.
Material examined: Thailand, Chiang Mai Province, Chiang Mai University, near Angkaew Reservoir, on dead wood of Careya sphaerica Roxb. (Lecythidaceae), 16 March 2022, J. Monkai, AK02 (CMUB39996, holotype), ex-type living culture, SDBR-CMU469.
Notes: Diaporthe careyae formed a well-supported monophyletic lineage basal to species in the D. carpini species complex (100% ML, 1.00 PP, Figure 1). Phylogenetically, this species was not clustered with any Diaporthe species, and the base pair difference between closely related species was not possible to compare. The morphological characteristics of D. careyae are distinct from other Diaporthe species in having septate and oblong alpha conidia. Thus, D. careyae was proposed as a new species based on its distinctive morphology and phylogenetic placement.
Figure 5.
Diaporthe careyae (CMUB39996, holotype). (a) Conidiomata on host substrate (indicated with the red arrow). (b,c) Section through conidioma. (d–f) Conidiogenous cells giving rise to conidia. (g–l) Alpha conidia. (m,n) Colonies on PDA, (m) from above and (n) from reverse. Scale bars: (a) = 500 μm, (b,c) = 50 μm, (d–f) = 10 μm, and (g–l) = 5 μm.
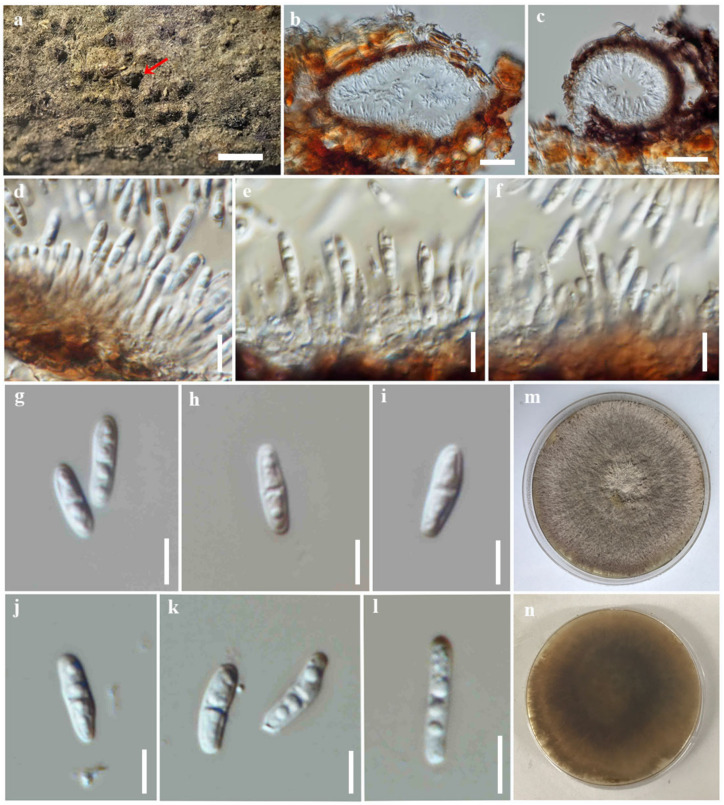
Diaporthe globoostiolata Monkai and S. Lumyong, sp. nov.
Index Fungorum number: IF900380; Faces of fungi number: FoF 14094; Figure 6
Etymology: Refers to the globular shape of the ostiole.
Holotype: MFLU 23-0063
Saprobic on dead leaves of a member of Fagaceae. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata: 90–120 high × 110–180 μm diam., pycnidial, semi-immersed, partly erumpent, solitary, subconical to subglobose, dark brown to black, uni-loculate, with ostiolar necks protruding through host surface. Ostioles: up to 80 μm wide, central, globose, black, papillate. Conidiomatal wall: up to 20 μm wide, comprising a few layers of thin-walled cells, arranged in textura angularis, with dark brown outer layers and hyaline to pale brown inner layers. Conidiophores: reduced to conidiogenous cells. Conidiogenous cells: 3.5–11.4 × 1.4–3.7 μm (x = 6.5 × 2.2 μm, n = 30), subcylindrical to ampulliform, slightly tapering towards the apex, hyaline, monophialidic, terminal, with visible periclinal thickening and a prominent collarette. Alpha conidia: 6–9.6 × 1.8–2.8 μm (x = 7.6 × 2.2 μm, n = 30), fusiform to ellipsoid, obtuse at both ends, aseptate, hyaline, smooth-walled, and mono- to bi-guttulate. Beta conidia: 13.2–22 × 1–1.8 μm (x = 16.8 ×1.4 μm, n = 30), filiform, tapering towards apex, truncate at base, straight to slightly curved, hyaline, smooth-walled, and eguttulate.
Culture characteristics: Colonies on PDA reached 9 cm diam. after 10 days at 25 °C, effuse, fluffy, lobate margin, originally white, becoming pale yellowish mycelium with age, yellowish to pale brown in reverse.
Material examined: Thailand, Tak Province, Tambon Chiang Tong, Wang Chao District, on dead leaves of a member of Fagaceae, 22 August 2019, P. Sysouphanthong, TS1-5 (MFLU 23-0063, holotype), ex-type living culture, MFLUCC 23-0025.
Notes: Diaporthe globoostiolata formed a well-supported clade basal to D. hongkongensis (99% ML, 1.00 PP, Figure 1). Diaporthe globoostiolata can be distinguished from D. hongkongensis CBS 115,448 in 1.23%, 3.55%, and 3.67% base pair differences in ITS (7/571 bp), tef1-α (12/338 bp), and tub2 (16/436 bp). Diaporthe globoostiolata and D. hongkongensis have overlapping sizes of alpha conidia {6–9.6 × 1.8–2.8 vs. (5–)6–7(–8) × (2–)2.5(–3) μm} [15]. However, the beta conidia of D. globoostiolata are slightly shorter than those of D. hongkongensis {13.2–22 × 1–1.8 vs. 18–22 × 1.5–2 μm} [15]. Our isolate and its closely related taxa, which are D. hongkongensis and D. lithocarpi, were found on the same host (member of the family, Fagaceae) [30,64,65].
Diaporthe samaneae Monkai and S. Lumyong, sp. nov.
Index Fungorum number: IF900381; Faces of fungi number: FoF 14095; Figure 7
Etymology: Refers to the host genus, Samanea, from which the holotype was collected.
Holotype: CMUB39997
Saprobic on dead wood of Samanea saman. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata: 300–480 high × 290–740 μm diam., pycnidial, stromatic, superficial to semi-immersed, erumpent, clustered, subglobose to ovoid, elongate, dark brown to brown, multi-loculate, and ostiolate. Conidiomatal wall: up to 50 μm wide, comprising a few layers of thin-walled cells, arranged in textura angularis, with brown outer layers and hyaline to pale brown inner layers. Conidiophores: 7.5–31.7 × 1.5–2.7 μm (x = 19 × 2 μm, n = 30), tightly aggregated, subcylindrical, hyaline to pale brown, septate, branched, straight to sinuous, and smooth. Conidiogenous cells: 5.2–14.3 × 1.5–2.7 μm (x = 9.7 × 2 μm, n = 30), subcylindrical to ampulliform, tapering towards apex, hyaline, phialidic, terminal, with visible periclinal thickening and a prominent collarette. Alpha conidia: 7–11 × 1.8–2.8 μm (x = 8.4 × 2.4 μm, n = 30), ellipsoid to elongate fusiform, obtuse at apex, subtruncate at base, aseptate, hyaline, smooth-walled, eguttulate, forming basipetal chains of two or more conidia on phialidic neck. Beta conidia: not observed.
Culture characteristics: Colonies on PDA reached 9 cm diam. after 10 days at 25 °C, effuse, sparse hyphae, filiform margin, originally white, becoming pale yellowish mycelium with age, yellowish to pale brown in reverse, with numerous black dots developing as the fruiting bodies (conidial production not seen).
Material examined: Thailand, Chiang Mai Province, Charoen Prathet Public Park, on dead wood of Samanea saman (Jacq.) Merr. (Fabaceae), 27 March 2022, J. Monkai, JS01 (CMUB39997, holotype), ex-type living culture, SDBR-CMU470.
Notes: Diaporthe samaneae formed an independent lineage and are closely related to D. inconspicua and D. pseudoinconspicua (97% ML, 1.00 PP, Figure 1). Diaporthe samaneae can be distinguished from D. inconspicua CBS 133,813 in 3%, 1.68%, 0%, 2.71%, and 0.84% base pair differences in ITS (17/567 bp), tef1-α (5/298 bp), tub2 (0/423 bp), cal (11/406 bp), and his3 (4/479 bp) and D. pseudoinconspicua URM 7874 in 3.93%, 1.08%, 0.64%, 2.47%, and 1.01% base pair differences in ITS (18/458 bp), tef1-α (3/278 bp), tub2 (3/467 bp), cal (10/405 bp), and his3 (5/496 bp). Diaporthe samaneae differs from D. inconspicua and D. pseudoinconspicua in having longer alpha conidia {7–11 × 1.8–2.8 vs. 5.5–6.5 × 1.5–2 μm and 5–7.5(–8.5) ×2–2.5(–3.5) μm} [66,67]. The host preference of D. inconspicua is the species of Maytenus, Poincianella, and Spondias [15,66], while D. pseudoinconspicua was associated with Poincianella [67]. Both species, D. inconspicua and D. pseudoinconspicua, were reported as endophytes, while D. samaneae was reported as a saprobe from Samanea.
Figure 6.
Diaporthe globoostiolata (MFLU 23-0063, holotype). (a) Conidioma on host substrate (indicated with the red arrow). (b) Section through conidioma. (c) Ostiole. (d–g) Conidiogenous cells giving rise to conidia. (h,i) Alpha and beta conidia. (j,k) Colonies on PDA, (j) from above and (k) from reverse. Scale bars: (a) = 200 μm, (b,c) = 20 μm, and (d–i) = 5 μm.
Figure 7.
Diaporthe samaneae (CMUB39997, holotype). (a,b) Conidiomata on host substrate (indicated with the red arrow). (c,d) Section through conidiomata. (e) Conidiomatal walls. (f–i) Conidiogenous cells giving rise to conidia. (j–n) Alpha conidia. (o,p) Colonies on PDA, (o) from above and (p) from reverse. Scale bars: (a,b) = 500 μm, (c,d) = 200 μm, (e) = 20 μm, (f–i) = 10 μm, and (j–n) = 5 μm.
4. Discussion
This study describes five novel species of Diaporthe in Thailand. Aside from the phenotypic traits, phylogenetic and PHI analyses based on the combined sequence datasets of ITS, tef1-α, tub2, cal, and his3 were successfully applied to justify the novel species. In particular, tub2, cal, and his3 have a high discrimination power for distinguishing species in Diaporthe, and this is consistent with the results from other studies [15,18,22,35,36,37].
Our study also gains better insight into the phylogenetic relationships within Diaporthe, especially in the D. arecae species complex. Diaporthe zhaoqingensis and D. chiangmaiensis were clustered together in the same clade (98% ML, 1.00 PP) and not so well separated (Figure 1). Therefore, we compared the base pair differences between the type strains of D. zhaoqingensis ZHKUCC 22-0056 and D. chiangmaiensis MFLUCC 18-0544. There are 1.38% base pair differences in ITS (7/508 bp) between the ex-type of both strains. In the tef1-α gene region, there are 0.33% base pair differences (1/300 bp) between the type strains of D. chiangmaiensis MFLUCC 18-0544 and D. zhaoqingensis ZHKUCC 22-0057. There are 4.94% base pair differences (19/385 bp) in the tub2 gene region, between D. chiangmaiensis MFLUCC 21-0212 and the type strain of D. zhaoqingensis ZHKUCC 22-0056. However, some genes from the type strains were not available to compare. The PHI test result also showed that D. zhaoqingensis and D. chiangmaiensis were not conspecific, indicating that they are different species (Figure 2e). Diaporthe zhaoqingensis was isolated as an endophyte on Morinda officinalis [18], and D. chiangmaiensis was isolated from Magnolia lilifera as an endophyte and saprobe [47]. However, the morphological characteristics of these two species could not be compared as only gamma conidia were observed in D. zhaoqingensis while alpha conidia were observed in D. chiangmaiensis [18,47]. Therefore, more sequence data such as the tub2, cal, and his3 of the type strain of D. chiangmaiensis are needed to resolve their taxonomic placements and confirm whether they are distinct species.
Furthermore, the new species, D. careyae, was shown to be distinct from other Diaporthe species based on its morphology and phylogeny. The conidia of D. careyae were 0–1(–2) septate, whereas aseptate conidia were a typical characteristic of Diaporthe. The septation of conidia has been reported in some Diaporthe species (e.g., D. foeniculina and D. saccarata) [17,68], however, their phylogenetic placements were not closely related to D. careyae. It is noteworthy that there are some singleton species that were not grouped into any species complex, and their taxonomic positions remain unclear [32]. In addition, most species of Diaporthe lack sequence data and have incomplete morphological descriptions [31,32]; therefore, further extensive sampling is needed in order to unravel the taxonomic circumscription of this genus.
The newly introduced species of Diaporthe were associated with different medicinal plants, comprising D. afzeliae on Afzelia xylocarpa, D. bombacis on Bombax ceiba, D. careyae on Careya sphaerica, and D. samaneae on Samanea saman. These plant species have been used as traditional medicines in tropical countries, including Thailand, and have been reported on concerning their various phytochemicals and pharmacological activities [69,70,71,72,73,74,75]. To the best of our knowledge, none of the Diaporthe species have been isolated from these host genera, making this the first report of such a host association [38]. Moreover, a new species, D. globoostiolata, was found on a member of Fagaceae. Some plant genera in Fagaceae, such as Castanopsis, Quercus, and Lithocarpus, have also been reported on regarding their medicinal usage and pharmacological properties [76,77,78,79]. Furthermore, more than 30 Diaporthe species have been recorded from the host family Fagaceae [38]. This study reflects the high genetic diversity and phenotypic variation within Diaporthe and expands our understanding of the diversity and host relationships of the Diaporthe species associated with medicinal plants in tropical regions. However, future studies are necessary to investigate the disease symptoms and evaluate the pathogenicity of these Diaporthe isolates as they are important for tree health assessments and management.
Acknowledgments
J.M., S.H. and S.L. are thankful for the partial support of Chiang Mai University, Thailand. J.M. is grateful to Phongeun Sysouphanthong and Areerat Manowong for their assistance during this research. D.J.B. and T.M.D. gratefully acknowledge the financial support under the Distinguished Scientist Fellowship Programme (DSFP), at King Saud University, Riyadh, Saudi Arabia. Shaun Pennycook from Landcare Research, Auckland, New Zealand, is thanked for advising on the taxon name.
Author Contributions
Conceptualization, J.M. and S.H.; methodology, J.M. and S.H.; software, J.M. and S.H.; validation, S.H., D.J.B. and S.L.; formal analysis, J.M. and S.H.; investigation, J.M.; resources, J.M.; data curation, J.M.; writing—original draft preparation, J.M.; writing—review and editing, J.M., S.H., D.J.B., T.M.D. and S.L.; visualization, J.M.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences generated in this study were submitted to GenBank (https://www.ncbi.nlm.nih.gov, accessed on 1 April 2023).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Research Center of Thailand (No. 42A650198).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen S.L., Yu H., Luo H.M., Wu Q., Li C.F., Steinmetz A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes M.J.R., Quave C.L., Collemare J., Tatsis E.C., Twilley D., Lulekal E., Farlow A., Li L.P., Cazar M.-E., Leaman D.J., et al. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet. 2020;2:463–481. doi: 10.1002/ppp3.10138. [DOI] [Google Scholar]
- 3.Kaul S., Gupta S., Ahmed M., Dhar M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012;11:487–505. doi: 10.1007/s11101-012-9260-6. [DOI] [Google Scholar]
- 4.Abtahi F., Nourani S.L. The most important fungal diseases associated with some useful medicinal plants. In: Ghorbanpour M., Varma A., editors. Medicinal Plants and Environmental Challenges. Springer International Publishing; Cham, Switzerland: 2017. pp. 279–293. [Google Scholar]
- 5.Bungihan M.E., Tan M.A., Kitajima M., Kogure N., Franzblau S.G., Cruz T.E.E., Takayama H., Nonato M.G. Bioactive metabolites of Diaporthe sp. P133, an endophytic fungus isolated from Pandanus amaryllifolius. J. Nat. Med. 2011;65:606–609. doi: 10.1007/s11418-011-0518-x. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho C.R., Gonçalves V.N., Pereira C.B., Johann S., Galliza I.V., Alves T.M.A., Rabello A., Sobral M.E.G., Zani C.L., Rosa C.A., et al. The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (Mart.) Coville (Fabaceae) from the Brazilian savannah. Symbiosis. 2012;57:95–107. doi: 10.1007/s13199-012-0182-2. [DOI] [Google Scholar]
- 7.Chowdhary L.K., Kaushik N. Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS ONE. 2015;10:e0141444. doi: 10.1371/journal.pone.0141444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares D.A., De Oliveira D.P., Dos Santos T.T., Marson P.G., Pimenta R.S. Multiloci identification of Diaporthe fungi isolated from the medicinal plant Costus spiralis (Jacq.) Roscoe (Costaceae) J. Appl. Microbiol. 2018;125:172–180. doi: 10.1111/jam.13769. [DOI] [PubMed] [Google Scholar]
- 9.Keshri P.K., Rai N., Verma A., Kamble S.C., Barik S., Mishra P., Singh S.K., Salvi P., Gautam V. Biological potential of bioactive metabolites derived from fungal endophytes associated with medicinal plants. Mycol. Prog. 2021;20:577–594. doi: 10.1007/s11557-021-01695-8. [DOI] [Google Scholar]
- 10.Lu L., Karunarathna S.C., Hyde K.D., Suwannarach N., Elgorban A.M., Stephenson S.L., Al-Rejaie S., Jayawardena R.S., Tibpromma S. Endophytic fungi associated with coffee leaves in China exhibited in vitro antagonism against fungal and bacterial pathogens. J. Fungi. 2022;8:698. doi: 10.3390/jof8070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibpromma S., Karunarathna S.C., Bhat J.D., Suwannarach N., Stephenson S.L., Elgorban A.M., Al-Rejaie S., Xu J., Mortimer P.E. Using culture-dependent and molecular techniques to identify endophytic fungi associated with tea leaves (Camellia spp.) in Yunnan Province, China. Diversity. 2022;14:287. doi: 10.3390/d14040287. [DOI] [Google Scholar]
- 12.Sun Y.R., Jayawardena R.S., Sun J.E., Wang Y. Pestalotioid species associated with medicinal plants in southwest China and Thailand. Microbiol. Spectr. 2023;11:e03987-22. doi: 10.1128/spectrum.03987-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossman A.Y., Farr D.F., Castlebury L.A. A review of the phylogeny and biology of the Diaporthales. Mycoscience. 2007;48:135–144. doi: 10.47371/mycosci.MYC48135. [DOI] [Google Scholar]
- 14.Udayanga D., Liu X.Z., McKenzie E.H.C., Chukeatirote E., Bahkali A.H. The genus Phomopsis: Biology, applications, species concepts and names of common pathogens. Fungal Divers. 2011;50:189–225. doi: 10.1007/s13225-011-0126-9. [DOI] [Google Scholar]
- 15.Gomes R.R., Glienke C., Videira S.I.R., Lombard L., Groenewald J.Z. Diaporthe; a genus of endophytic; saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin-Felix Y., Hernández-Restrepo M., Wingfield M.J., Akulov A., Carnegie A.J., Cheewangkoon R., Gramaje D., Groenewald J.Z., Guarnaccia V., Halleen F., et al. Genera of phytopathogenic fungi: GOPHY 2. Stud. Mycol. 2019;92:47–133. doi: 10.1016/j.simyco.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abeywickrama P.D., Camporesi E., Jayawardena R.S., Hyde K.D., Yan J., Zhang W., Li X. Novel and surprising host associations of Diaporthe (Diaporthaceae, Diaporthales) species from Italy. Chiang Mai J. Sci. 2022;49:223–247. doi: 10.12982/CMJS.2022.028. [DOI] [Google Scholar]
- 18.Luo M., Guo W., Zhao M., Manawasinghe I.S., Guarnaccia V., Liu J., Hyde K.D., Dong Z., You C. Endophytic Diaporthe associated with Morinda officinalis in China. J. Fungi. 2022;8:806. doi: 10.3390/jof8080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q., Fan X.L., Guarnaccia V., Tian C.M. High diversity of Diaporthe species associated with twelve new species described. MycoKeys. 2018;39:97–149. doi: 10.3897/mycokeys.39.26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S., Xia J., Zhang X., Sun W. Morphological and phylogenetic analyses reveal three new species of Diaporthe from Yunnan, China. MycoKeys. 2021;78:49–77. doi: 10.3897/mycokeys.78.60878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang N., Voglmayr H., Piao C.G., Li Y. Two new species of Diaporthe (Diaporthaceae, Diaporthales) associated with tree cankers in the Netherlands. MycoKeys. 2021;85:31. doi: 10.3897/mycokeys.85.73107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao L., Luo D., Lin W., Yang Q., Deng X. Four new species of Diaporthe (Diaporthaceae, Diaporthales) from forest plants in China. MycoKeys. 2022;91:25–47. doi: 10.3897/mycokeys.91.84970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udayanga D., Castlebury L.A., Rossman A.Y., Chukeatirote E., Hyde K.D. The Diaporthe sojae species complex: Phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol. 2015;119:383–407. doi: 10.1016/j.funbio.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Guarnaccia V., Crous P.W. Emerging citrus diseases in Europe caused by Diaporthe spp. IMA Fungus. 2017;8:317–334. doi: 10.5598/imafungus.2017.08.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dissanayake A.J., Zhang W., Liu M., Hyde K.D., Zhao W.S., Li X.H., Yan J.Y. Diaporthe species associated with peach tree dieback in Hubei, China. Mycosphere. 2017;8:533–549. doi: 10.5943/mycosphere/8/5/2. [DOI] [Google Scholar]
- 26.Guarnaccia V., Groenewald J.Z., Woodhall J., Armengol J., Cinelli T., Eichmeier A. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia. 2018;40:135–153. doi: 10.3767/persoonia.2018.40.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manawasinghe I.S., Dissanayake A.J., Li X., Liu M., Wanasinghe D.N., Xu J., Zhao W., Zhang W., Zhou Y., Hyde K.D., et al. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front. Microbiol. 2019;10:e1936. doi: 10.3389/fmicb.2019.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomzhina M.M., Gannibal P.B. Diaporthe species infecting sunflower (Helianthus annuus) in Russia, with the description of two new species. Mycologia. 2022;114:556–574. doi: 10.1080/00275514.2022.2040285. [DOI] [PubMed] [Google Scholar]
- 29.Wehmeyer L.E. The genus Diaporthe Nitschke and its segregates. Ann. N. Y. Acad. Sci. 1933;215:89–97. [Google Scholar]
- 30.Gao Y., Liu F., Duan W., Crous P.W., Cai L. Diaporthe is paraphyletic. IMA Fungus. 2017;8:153–187. doi: 10.5598/imafungus.2017.08.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dissanayake A.J., Phillips A.J.L., Yan J.Y., Li X.H., Hyde K.D. The current status of species in Diaporthe. Mycosphere. 2017;8:1106–1156. doi: 10.5943/mycosphere/8/5/5. [DOI] [Google Scholar]
- 32.Norphanphoun C., Gentekaki E., Hongsanan S., Jayawardena R., Senanayake S.C., Manawasinghe I.S., Abeywickrama P.D., Bhunjun C.S., Hyde K.D. Diaporthe: Formalizing the species-group concept. Mycosphere. 2022;13:752–819. doi: 10.5943/mycosphere/13/1/9. [DOI] [Google Scholar]
- 33.Udayanga D., Liu X.Z., MCkenzie E.H.C., Chukeatirote E., Hyde K.D. Multi-locus phylogeny reveals three new species of Diaporthe from Thailand. Cryptogam. Mycol. 2012;33:295–309. doi: 10.7872/crym.v33.iss3.2012.295. [DOI] [Google Scholar]
- 34.Santos L., Phillips A.J.L., Crous P.W., Alves A. Diaporthe species on Rosaceae with descriptions of D. pyracanthae sp. nov. and D. malorum sp. nov. Mycosphere. 2017;8:485–511. doi: 10.5943/mycosphere/8/5/1. [DOI] [Google Scholar]
- 35.Dissanayake A.J., Chen Y.Y., Liu J.K. Unravelling Diaporthe species associated with woody hosts from karst formations (Guizhou) in China. J. Fungi. 2020;6:251. doi: 10.3390/jof6040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaisiri C., Liu X., Lin Y., Fu Y., Zhu F., Luo C. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology. 2021;10:179. doi: 10.3390/biology10030179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zapata M., Palma M.A., Aninat M.J., Piontelli E. Polyphasic studies of new species of Diaporthe from native forest in Chile, with descriptions of Diaporthe araucanorum sp. nov., Diaporthe foikelawen sp. nov. and Diaporthe patagonica sp. nov. Int. J. Syst. Evol. Microbiol. 2020;70:3379–3390. doi: 10.1099/ijsem.0.004183. [DOI] [PubMed] [Google Scholar]
- 38.Farr D.F., Rossman A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. [(accessed on 1 March 2023)]; Available online: https://nt.ars-grin.gov/fungaldatabases/
- 39.Senanayake I., Rathnayaka A., Marasinghe D., Calabon M., Gentekaki E., Lee H., Hurdeal V., Pem D., Dissanayake L., Wijesinghe S.N., et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere. 2020;11:2678–2754. doi: 10.5943/mycosphere/11/1/20. [DOI] [Google Scholar]
- 40.Index Fungorum. 2023. [(accessed on 1 April 2023)]. Available online: http://www.indexfungorum.org.
- 41.Jayasiri S.C., Hyde K.D., Ariyawansa H.A., Bhat J., Buyck B., Cai L., Dai Y.C., Abd-Elsalam K.A., Ertz D., Hidayat I., et al. The Faces of Fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015;74:3–18. doi: 10.1007/s13225-015-0351-8. [DOI] [Google Scholar]
- 42.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Volume 18. Academic Press; Cambridge, MA, USA: 1990. p. 7. [Google Scholar]
- 43.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 44.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crous P.W., Gams W., Stalpers J.A. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004;50:19–22. [Google Scholar]
- 46.Perera R.H., Hyde K.D., Maharachchikumbura S.S.N., Jones E.B.G., McKenzie E.H.C., Stadler M., Lee H.B., Samarakoon M.C., Ekanayaka A.H., Camporesi E., et al. Fungi on wild seeds and fruits. Mycosphere. 2020;11:2108–2480. doi: 10.5943/mycosphere/11/1/14. [DOI] [Google Scholar]
- 47.De Silva N.I., Hyde K.D., Lumyong S., Phillips A.J.L., Bhat D.J., Maharachchikumbura S.S.N., Thambugala K.M., Tennakoon D.S., Suwannarach N., Karunarathna S.C. Morphology, phylogeny, host association and geography of fungi associated with plants of Annonaceae, Apocynaceae and Magnoliaceae. Mycosphere. 2022;13:955–1076. doi: 10.5943/mycosphere/13/1/12. [DOI] [Google Scholar]
- 48.Tan Y.P., Shivas R.G. Nomenclatural novelties of microfungi from Australia. Index Aust. Fungi. 2022;2:1–12. [Google Scholar]
- 49.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT; Proceedings of the Nucleic Acids Symposium Series; London, UK. 2–6 September 1999; pp. 95–98. [Google Scholar]
- 51.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 53.Zhaxybayeva O., Gogarten J.P. Bootstrap, Bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genom. 2002;3:4. doi: 10.1186/1471-2164-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Hoehna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees; Proceedings of the 2010 Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; New Orleans, LA, USA: IEEE; 2010. pp. 1–8. [Google Scholar]
- 56.Nylander J.A. MrModeltest 2. Program Distributed by the Author. Department of Systematic Zoology; Evolutionary Biology Centre, Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- 57.Rambaut A., Drummond A.J. FigTree: Tree Figure Drawing Tool. Institute of Evolutionary Biology, University of Edinburgh; Edinburgh, UK: 2012. [Google Scholar]
- 58.Bruen T.C., Philippe H., Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics. 2006;172:2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quaedvlieg W., Binder M., Groenewald J., Summerell B., Carnegie A., Burgess T., Crous P. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia-Mol. Phylogeny Evol. Fungi. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huson D.H. Splits Tree: Analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- 61.Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2005;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 62.Wrona C.J., Mohankumar V., Schoeman M.H., Tan Y.P., Shivas R.G., Jeff-Ego O.S., Akinsanmi O.A. Phomopsis husk rot of macadamia in Australia and South Africa caused by novel Diaporthe species. Plant Pathol. J. 2020;69:911–921. doi: 10.1111/ppa.13170. [DOI] [Google Scholar]
- 63.Punithalingam E. New species of Phomopsis. Trans. Br. Mycol. Soc. 1974;63:229–236. doi: 10.1016/S0007-1536(74)80167-1. [DOI] [Google Scholar]
- 64.Gao Y.H., Sun W., Su Y.Y., Cai L. Three new species of Phomopsis in Gutianshan nature reserve in China. Mycol. Prog. 2014;13:111–121. doi: 10.1007/s11557-013-0898-2. [DOI] [Google Scholar]
- 65.Gao Y.H., Liu F., Cai L. Unravelling Diaporthe species associated with Camellia. Syst. Biodivers. 2016;14:102–117. doi: 10.1080/14772000.2015.1101027. [DOI] [Google Scholar]
- 66.Bezerra J.D.P., Machado A.R., Firmino A.L., Rosado A.W.C., Souza C.A.F.D., Souza-Motta C.M.D., Freire K.T.L.D.S., Paiva L.M., Magalhães C.M.O., Pereira O.L., et al. Mycological diversity description I. Acta Bot. Bras. 2018;32:656–666. doi: 10.1590/0102-33062018abb0154. [DOI] [Google Scholar]
- 67.Crous P.W., Wingfield M.J., Burgess T.I., Hardy G.S.J., Gené J., Guarro J., Baseia I.G., García D., Gusmão L.F.P., Souza-Motta C.M., et al. Fungal Planet description sheets: 716–784. Persoonia-Mol. Phylogeny Evol. Fungi. 2018;40:240. doi: 10.3767/persoonia.2018.40.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mostert L., Kang J.C., Crous P.W., Denman S. Phomopsis saccharata sp. nov., causing a canker and die-back disease of Protea repens in South Africa. Sydowia-Horn. 2001;53:227–260. [Google Scholar]
- 69.Cai X., Liu J.X., Song Q.S., Chen K.L., Lu Z.Y., Zhang Y.M. Chemical constituents of Afzelia xylocarpa. Chem. Nat. Compd. 2018;54:764–765. doi: 10.1007/s10600-018-2467-z. [DOI] [Google Scholar]
- 70.Chakraborty D.D., Ravi V., Chakraborty P. Phytochemical evaluation and TLC protocol of various extracts of Bombax ceiba Linn. Int. J. Pharm. Sci. Rev. Res. 2010;1:63–71. [Google Scholar]
- 71.Daduang J., Vichitphan S., Daduang S., Hongsprabhas P., Boonsiri P. High phenolics and antioxidants of some tropical vegetables related to antibacterial and anticancer activities. Afr. J. Pharm. Pharmacol. 2011;5:608–615. doi: 10.5897/AJPP10.243. [DOI] [Google Scholar]
- 72.Maisuthisakul P. Phenolic constituents and antioxidant properties of some Thai plants. In: Rao V., editor. Phytochemicals: A Global Perspective of their role in Nutrition and Health. InTech; Rijeka, Croatia: 2012. pp. 187–212. [Google Scholar]
- 73.Phuong D.L., Thuy N.T., Long P.Q., Kuo P.C., Thang T.D. Composition of fatty acids, tocopherols, sterols, total phenolics, and antioxidant activity of seed oils of Afzelia xylocarpa and Cassia fistula. Chem. Nat. Compd. 2019;55:242–246. doi: 10.1007/s10600-019-02659-x. [DOI] [Google Scholar]
- 74.Rani S., Rahman K., Sultana A. Ethnomedicinal and pharmacological activities of Mochrus (Bombax ceiba Linn.): An overview. CELLMED. 2016;6:2.1–2.9. doi: 10.5667/tang.2015.0025. [DOI] [Google Scholar]
- 75.Vinodhini S. Review on ethnomedical uses, pharmacological activity and phytochemical constituents of Samanea saman (jacq.) Merr. rain tree. Phcog. J. 2018;10:202–209. doi: 10.5530/pj.2018.2.35. [DOI] [Google Scholar]
- 76.Khuankaew S., Srithi K., Tiansawat P., Jampeetong A., Inta A., Wangpakapattanawong P. Ethnobotanical study of medicinal plants used by Tai Yai in Northern Thailand. J. Ethnopharmacol. 2014;151:829–838. doi: 10.1016/j.jep.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 77.Prakash S., Bindu M., Prasad A.S., Timilsina H., Ram B. Total phenolic and flavonoids contents, antioxidant activities, phytochemical and nutritional analysis of Castanopsis indica (Indian chestnut) Nat. Resour. Sustain. Dev. 2020;10:175–187. [Google Scholar]
- 78.Taib M., Rezzak Y., Bouyazza L., Lyoussi B. Medicinal uses, phytochemistry, and pharmacological activities of Quercus species. Evid. Based Complement. Altern. Med. 2020;2020:1920683. doi: 10.1155/2020/1920683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie Y., Ma G., Wei H., Yuan J., Wu H., Zhou X., Yang J., Xu X. Three new phenolics and other constituents from the seeds of Lithocarpus pachylepis. Molecules. 2013;18:10397–10403. doi: 10.3390/molecules180910397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences generated in this study were submitted to GenBank (https://www.ncbi.nlm.nih.gov, accessed on 1 April 2023).