Highlights
-
•
In this paper, we compared the detection targets, techniques, and methods of several common liquid biopsies, providing some insights for different study design chioces.
-
•
Previous liquid biopsy studies have been limited to the clinical application of single cancer types, or the detection techniques of pan-cancer. In this paper, we innovatively summarize the clinical application of pan-cancer.
-
•
The latest clinical trails of pan-cancer in liquid biopsy are discussed, and some constructive suggestions on what an ideal clinical trial should look like are put forward.
Keywords: Liquid biopsy, Pan-cancer, Clinical application, Colorectal cancer, Lung cancer
Abstract
Cancer morbidity and mortality are growing rapidly worldwide and it is urgent to develop a convenient and effective method that can identify cancer patients at an early stage and predict treatment outcomes. As a minimally invasive and reproducible tool, liquid biopsy (LB) offers the opportunity to detect, analyze and monitor cancer in any body fluids including blood, complementing the limitations of tissue biopsy. In liquid biopsy, circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) are the two most common biomarkers, displaying great potential in the clinical application of pan-cancer. In this review, we expound the samples, targets, and newest techniques in liquid biopsy and summarize current clinical applications in several specific cancers. Besides, we put forward a bright prospect for further exploring the emerging application of liquid biopsy in the field of pan-cancer precision medicine.
Graphical abstract
Introduction
According to the global cancer statistics in 2021, cancer morbidity and mortality are increasing continuously around the world, with 19.29 million new cases of cancer and 9.96 million cancer deaths [1]. This terrible data rings the bell to pay attention to the early diagnosis and treatment of cancer, because the stage of cancer diagnosis is one of the most significant predictors of survival. Patients with cancer at early stage have a better five-year overall survival rate than those with advanced cancer [2]. Therefore, finding a convenient and effective tool to identify patients at earlier stages and accessibly predict therapeutic effects is critical to the overall cancer survival.
In recent years, liquid biopsy has been widely studied and shown great potential in the application of disease [3]. Liquid biopsy is a minimally invasive, reproducible, and easily obtainable method sampled from body fluids, such as blood, saliva, urine, pleural effusion, cerebrospinal fluid, and so on [4]. It exactly complements the limitations of tissue biopsy and has the ability to early diagnose, evaluate efficacy [5] and monitor prognosis [6] which produces a promising clinical implication. Especially in the precision oncology area, through the detection and analysis of tumor-derived biomarkers in bodily fluids, liquid biopsy well characterizes tumors genetic and cellular phenotypes and provide dynamic, real-time monitoring of the condition of carcinoma patients.
Based on this, this paper focuses on exploring the current and potential application value of liquid biopsy in the early screening and perioperative prognostic monitoring of pan-cancer. Besides, we also summarize current innovation and progress of liquid biopsy methods and techniques.
Liquid biopsy is an emerging tool for cancer
Liquid biopsies refer to newly detective methods and techniques using any body fluids. The most common is peripheral blood analysis, others such as cerebrospinal fluid (CSF) for central nervous system tumors, saliva for head and neck tumors, pleural fluid for thoracic and metastatic cancers, ascites for abdominal and metastatic cancers, stool for gastrointestinal tract cancers and urine for urinary tract cancers [7]. At present, the clinical application of liquid biopsy is mostly based on peripheral blood detection, which is also the focus of this paper.
The selection of targets for liquid biopsy is also particularly important in the case of tumors. Generally speaking, tumor-derived biomarkers mainly include ctDNA, CTCs, exosomes and microrribonucleic acid (miRNA), circulating metabolites and tumor-educated blood platelets (TEPs), etc. [8]. Different target types have different detection characteristics and are suitable for various clinical scenarios (Table 1). CTCs and ctDNA are more mature and are widely used in present clinic with highly specific and interpretable, could monitor the progression and development of cancer subtype.
Table 1.
Comparison of liquid biopsy methodologies in oncology applications.
| Characteristics | CTCs | ctDNA | Exosomes | cfRNA | miRNA | Metabolites | TEPs | |
|---|---|---|---|---|---|---|---|---|
| Detective contents | Detecting somatic mutation, insertion deletion and copy number variant | YES | YES | YES | NO | NO | NO | NO |
| Evaluating methylation expression patterns | YES | YES | YES | NO | NO | NO | YES | |
| Analysing RNA expression or proteomics profile | YES | NO | YES | YES | YES | NO | YES | |
| Cell morphology and function | YES | NO | YES | NO | NO | NO | YES | |
| Application performance | Reflecting tumor heterogeneity over time and space | YES | YES | NO | YES | NO | NO | NO |
| High-throughput procedures available | NO | YES | NO | YES | NO | YES | YES | |
| Enrichment required for detection and isolation | YES | YES | YES | YES | YES | NO | NO | |
| Universal application biomarker present | YES | YES | NO | NO | NO | NO | NO | |
CTC, circulating tumor cell; ctDNA, circulating tumor DNA; cfDNA, cell free DNA; miRNA, micro RNA; TEPs, tumor-educated blood platelets.
The former are derived from primary tumors or metastases that gain the ability to escape from the basement membrane and invade tumor cells that enter blood vessels through tissue stroma [9]. And ctDNA refers to the release of somatic DNA from tumor cells into the circulation system after cell apoptosis or shedding [10]. It carries tumor mutation information, including gene mutation, indel, insertion, rearrangement, copy number abnormality, methylation and other abnormal DNA fragments [5,[11], [12], [13]]. And exosomes, as small vesicles released by cells (including cancer cells), contain various tumor-derived materials that provides clues regarding tissue-specific origins and intercellular communication. Analytes such as cfRNAs [14] and miRNA [15] are closely related to the occurrence and development of tumor and are the best candidates to advance the field of precision oncology. Circulating metabolites directly reflect pathological status of cancer patients and have the potential to serve as biomarkers for personalized cancer therapy [16]. TEP is widely recognized as a genuine mediator of malignant tumors, exerting significant influence on tumor progression, particularly in metastasis. Researches have revealed that platelets extracted from the blood from cancer patients frequently exhibit different RNA and protein expression profiles. Methods for isolating and analyzing spliced TEP messenger RNA (mRNA) have advanced rapidly and demonstrated remarkable precision in early pan-cancer detection [17], [18], [19]. In conclusion, selecting and combining information from multiple biosources for specific clinical purpose will play a key part in establishing liquid biopsies in pan-cancer.
In addition to the samples and targets, the techniques and methods in liquid biopsy exert an important influence on clinical application for cancer. Due to the high fragmentation degree and low abundance of ctDNA, improving the sensitivity of ctDNA detection has always been the direction of many researchers' efforts. With the development of second-generation sequencing, it is possible to detect multiple gene variants simultaneously and generate high-throughput data. At present, ctDNA detection technologies have been relatively mature, divided into two categories, targeted PCR-based approaches and nontargeted next-generation sequencing (NGS) methods. In the process of innovation and development of ctDNA detection technology, researches in recent years focus on finding a new method to balance test cost and efficiency. Blocker displacement amplification (BDA) Sanger is an innovative method that combines Sanger sequencing with BDA technology to detect ctDNA, which has been proved to be clinically effective and practical in the detection of EGFR in lung cancer clinical cohort [20]. And CyclomicsSeq is a nanopore sequencing technology based on sequence copies of a single DNA molecule that improves DNA copy detection consistency by approximately 60 times and has been successfully used to monitor tumor load in patients with head and neck cancer during treatment [21]. Besides, it is currently believed that ultra-deep sequencing can improve the sensitivity of different cancer types [22], and by this way limit of detection (LOD) can be as low as 0.001%. In cancer patients, ctDNA components vary with tumor characteristics, including tumor site, disease load, proliferation and apoptosis rates, degree of necrosis, inflammation, tumor microenvironment, and host-related state. At present, there is no single ctDNA detection assay that is suitable for all clinical applications in tumors, including early screening, minimal residual disease (MRD) analysis, detection of gene mutations, assessment of genetic heterogeneity, identification of molecular mechanisms for drug resistance, etc. [23] The key to the success of ctDNA application is to properly select the suitable techniques to solve specific scientific and clinical problems.
Similarly, the detection of CTCs remains difficult because of their low blood concentration and lack of cancer-specific markers, diminishing their value as a diagnostic tool [24]. In response to this dilemma, a number of CTCs separation and enrichment techniques have been developed to achieve high capture sensitivity. Current technologies extract CTCs based on their physical characteristics, biological characteristics, and the expression of different tumor markers [25]. One the one hand, based on epithelial cell adhesion molecule (EpCAM) on the cell surface, CellSearch remains the widely used gold standard since it was approved by the FDA in 2004, but its clinical application is limited due to high cost and low sensitivity [26]. The integration of immunomagnetic positive and negative selection based on a variety of cell surface antigens and the combination of microfluidic systems or nanotechnology can greatly improve CTCs capture efficiency and enrichment ratio. By targeting leukocyte surface antigens and sorting with ultra-high throughput microfluidic magnetic beads, LPCTC-iChip (leukapheresis product: circulating tumor cell-microfluidic chip) improves CTCs separation by two orders of magnitude [27]. In recent years, Chinese researchers developed Tumorfisher CTCs technology using polypeptide nanobead technology, which can conduct molecular typing and guide clinical treatment options. Based on this technique, we found that the level of Programmed cell death ligand 1(PD-L1) on CTC can be used as a marker to predict the efficacy of immunotherapy [28]. One the other hand, CTCs that express no or little antigen can be isolated by physical properties, such as cell size, density, stiffness, charge, and deformability. Cohen et al. developed a separation system (Parsortix) based on size and deformability to isolate and obtain CTCs clusters including heterogeneous population [29]. At the same time, the analysis method of CTCs after capture is also particularly important. Studies have shown that capture and sequencing based on individual circulating tumor cells can describe the genomic map and evolutionary history of cancer in detail, thus providing the potential for clinical stratification of cancer patients [30].
To sum up, the selection of samples, targets, techniques and methods in liquid biopsy has an impact on its clinical application in the field of cancers. With the advancement of technology and optimization of methods, liquid biopsy is increasingly applied in a variety of clinical medical scenarios, becoming a powerful tool for cancer diagnosis and prognosis.
Current application in liquid biopsy for pan-cancer
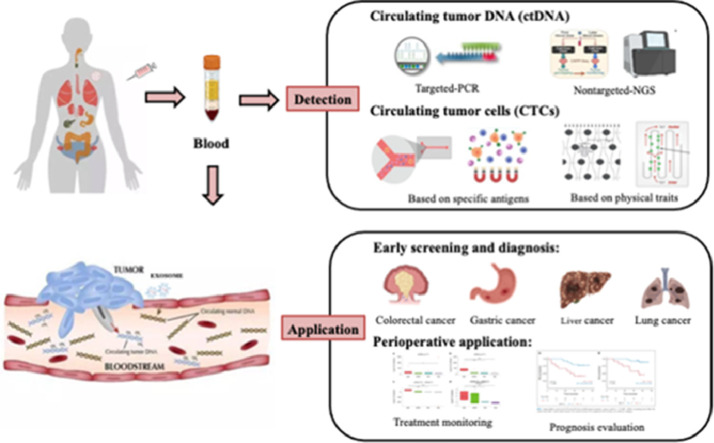
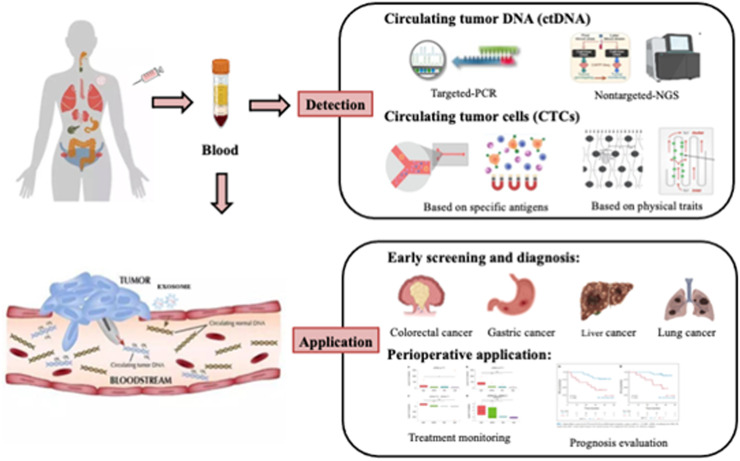
Liquid biopsy is currently a hot topic in the precision oncology area, attracting a large number of researchers to use this technique in the study of multiple types of cancer. Many studies have shown great evidence that liquid biopsy is related to early diagnosis, treatment and prognosis in various cancers. Next, we will describe the clinical application of liquid biopsy in individual cancer species by category, mainly focusing on cancer screening and perioperative prognostic evaluation (Fig. 1).
Fig. 1.
Phantom drawing of clinical applications of liquid biopsy in pan-cancer. Liquid biopsy is a new clinical method widely used in early screening of tumors, perioperative management and other aspects. The innovation of ctDNA and CTCs detection technology, and their potential applications in the field of pan-cancer are discussed.
Colorectal cancer
Colorectal cancer (CRC) is the second common cancer type and cancer-related mortality globally [1]. With the development of technology, liquid biopsy has made great progress in the diagnosis, treatment and prognosis assessment of colorectal cancer.
Tsai et al. demonstrated that CTCs might be used for early cancer detection for the first time. In a CRC prospective study, CTC detection based on the Cellmax platform showed a sensitivity of 86.9%, specificity of 97.3%, and area under curve (AUC) of 0.88 [31]. However, CTCs are still difficult to detect because of their in vivo status and lack of cancer-specific markers as described above [9,24]. To this end, researchers have made many explorations in the enrichment, detection and analysis of CTCs. For example, Racila et al. used a ferrofluid-coupled antibody against epithelial cell adhesion molecules (EpCAM) and flow cytometry (FCM) to detect CTCs based on immunomagnetic enrichment [25]. Cell free DNA (cfDNA) detection of methylation is one of the most important and earliest epigenetic markers of CRC [32]. Promoter methylation markers [33] and Septin 9 (SEPT 9) [34] are highly sensitive (78% and 87%) and specific (100% to 90.6%), which are often used as markers to distinguish CRC patients from healthy controls. FDA has recently approved the first blood test specifically based on SEPT 9 methylation, which will greatly improve CRC screening [35,36]. Li et al. using the methylated CpG Tandem amplification and sequencing (MCTA-Seq) method identified a set of 80 methylated markers that were used to discriminate CRC stages [37]. Chen et al. studied gut microbiome associated serum metabolites (GMSM) and identified the elevated levels of different microbiome associated serum metabolites in CRC patients [38]. These findings demonstrate the possibility of combining liquid biopsy and metabolomics to improve accurate early diagnose.
In the treatment of CRC, microsatellite instability (MSI), PD-L1 expression, and tumor mutation burden (TMB) show predictive value of immune checkpointinhibition [39,40]. Recently, Lucrezia Raimondi et al. conducted a study to detect the expression level of PD-L1 on CTCs [41]. In a prospective, random, Phase III trial (VISN-1 trail), evaluated in 349 patients with metastatic colorectal cancer receiving different therapies, indicating the role of CTCs in treatment selection [42]. Cell free nucleic acids provide another dimension of predictive value for treatment and prognosis. A cohort study (Medoc-Create trail) [43] and the CIRCULATE-Japan clinical trial [44] confirmed that ctDNA can be used as a predictor of tumor recurrence and to monitor the effectiveness of adjuvant chemotherapy. In stage II and III colon cancer, we have the opportunity to increase treatment for those who will benefit (improve cure rates) and decrease treatment for those who will not benefit (reduce treatment-related toxicity) [45]. DANYMIC study found that in the ctDNA-guided management group, ctDNA test was received at the 4th and 7th week after surgery to guide the clinical use of adjuvant chemotherapy. These results indicate that ctDNA can reduce the use of adjuvant chemotherapy in the treatment of stage Ⅱ colon cancer without affecting the recurrence free survival [46]. Monitoring ctDNA after chemotherapy can reveal information about minimal residual lesions, treatment response, and recurrence in patients [47]. Patients whose ctDNA was detectable after surgery had a higher risk of recurrence at 5 years (38.6% vs. 85.5%) and a lower overall survival rate (64.6% vs. 89.4%) than patients whose ctDNA was not detectable after surgery [48]. Besides ctDNA also has a unique value in predicting curative effect, exploring drug resistance mechanism and predicting relapse.
Gastric cancer
The Cancer Genome Atlas (TCGA) Research Network have proposed a classification of gastric cancer (GC): (1) Tumors positive for EBV (Epstein-Barr Virus), (2) MSI-high tumors, (3) genomically stable tumors, and (4) tumors with chromosomal instability [49]. These characteristics allow identifying patients on the basis of the molecular features.
In a case-control study of 30 patients with gastric cancer and 34 healthy individuals, Kim et al. confirmed that the mean plasma cfDNA levels were shown to be lowest in healthy subjects and highest in patients with advanced gastric cancer [50]. Unexpectedly, one-third of the changes detected in gastrointestinal tumors were detected only in ctDNA, meanwhile not in tissue samples [51] The subtype of EBV- positive cancer is characterized by PIK3CA and ARID1A mutations, high expression of PD-L1 and PD-L2, and high DNA methylation, which are good candidate biomarkers for diagnosis and treatment [52]. With the deeper study of RNA, they are playing an increasingly important role in the process of tumor diagnosis and recognition. Souvick et al. established an 8-circRNA (circular RNA) biomarker panel that robustly identified patients with GC, including those with early stage of tumors (stage I & II), as well as the histological subtype (diffuse & enteric) [53].
Fluidomics biomarkers play an important role in detecting recurrence and metastasis of gastric cancer and exploring the mechanism of drug resistance. Yang et al. conducted a prospective study in 46 patients undergoing radical gastrectomy for stage Ⅰ to Ⅲ gastric cancer to explore the possibility of detecting MRD with ctDNA. ctDNA positivity occurred at a median of 6 months before radiological recurrence, and its positivity at any time after surgery was associated with worse disease-free survival and overall survival [54]. When CTCs are CD44 positive, they may have negative prognostic significance in epithelial tumors. A prospective trial of 228 patients with resectable gastric cancer found that distant metastases were observed in half of the 99 keratin-positive tumors compared with 19% in the CD44-negative group during long-term follow-up [55]. Detectable gastric cancer-derived clonal mutations or CNVs (Copy Number Variations) in ctDNA may lead to resistance to chemotherapy or HER2 inhibitors, respectively [56,57]. Dynamic ctDNA analysis can be used to determine personalized therapies that target tumor heterogeneity and evolution-driven resistance in the near future.
Liver cancer
Multiple pan-cancer studies have shown that liver cancer releases the strongest cfDNA signal into the blood in the early stage compared with other cancers [58], which makes cfDNA a potential biomarker for early liver cancer screening. In 2017, Xu et al. [59] selected ten methylation markers to establish a comprehensive diagnostic model for liver cancer. This model could effectively distinguish liver cancer from healthy people, with a sensitivity of 83.3% and specificity of 90.5%, and was better than AFP (Alpha fetoprotein) in diagnosing early liver cancer. To further distinguish patients with liver disease from liver cancer, Luo et al. [60] established another model based on cfDNA methylation markers with a sensitivity of 83.6% and a specificity of 96.0%, which effectively distinguish liver cancer, cirrhosis, and healthy people. As demonstrated by the above research, cfDNA offers sights for early warning, early diagnosis, and early treatment of high-risk groups of liver cancer. 5-hydroxymethylcytosine is closely related to gene regulation and tumor pathogenesis. By combining 5-hydroxymethylcytosine with terminal sequence, fragment distribution, and nucleosome imprinting characteristics, the sensitivity and specificity of the liver cancer diagnosis model were 95.4% and 98.0%, respectively [61]. Compared with single omic, the combined multidimensional omics model can be more effective in screening early liver cancer.
In the field of CTCs, EpCAM+ CTCs can be used as real-time parameters for monitoring hepatocellular carcinoma (HCC) recurrence. Preoperative CTCs were detected in 90% HCC patients and CTC count ≥16 and mesenchymal-CTC percentage ≥2% associated with recurrence and metastasis [62]. The CTCs subtype may be related to the therapeutic response and can be used as a complement to the HCC molecular subtype. Yan et al. confirmed that FGL1(Fibrinogen-like protein 1)-positive CTCS are associated with distant metastasis and poor prognosis of patients, suggesting that the expression of FGL1 in HCC cells may promote the development of epithelial–mesenchymal transition (EMT) in tumor cells, and the expression of FGL1 on CTCs may be related to the efficacy of PD-L1 immunotherapy [63]. And there is an ongoing clinical trial (NCT02973204) to investigate CTCs and ctDNA as clinical support tools in HCC.
Lung cancer
As the highest mortality rate, lung cancer possesses high heterogeneity and multiple genetic phenotypes, resulting in a high postoperative recurrence rate and a low five-year survival rate. In the current clinical lung cancer treatment, liquid biopsy techniques are relatively mature and mainly applied to postoperative MRD detection and auxiliary efficacy prediction.
Multi-dimensional circulating tumor DNA detection have been widely utilized in early diagnose of lung cancer, including genetic mutations and structural variations, fragmentomics characters, epigenetic changes, and other specific characteristics [64]. A series of studies shown liquid biopsy has the potential for the differentiation of lung cancer patients by peripheral blood multidimensional characteristics (Table 2). Besides, CTCs detection has good efficacy in the diagnosis of early non-small cell lung cancer (NSCLC), with an AUC of 0.76, a sensitivity of 67.2%, and a specificity of 80.8% in stage I lung cancer [65]. Combined with serum tumor markers can assist in the differentiation of small pulmonary nodules and early lung cancer, reducing the false positive rate of pulmonary nodules [66,67].
Table 2.
Peripheral blood multidimensional characteristics detection in lung cancer.
| Author | Years | Characteristic | Findings (Refs.) |
|---|---|---|---|
| Chabon et al. | 2020 | Mutations | Studies have shown that the differentiation of lung cancer patients and high-risk groups by cfDNA mutation characteristics shows that the sensitivity of stage I ∼ III is 41.0%∼67.0% with a specificity of 98.0% [68]. |
| Liang et al. | 2021 | Methylation | A classifier was established by screening lung cancer-related methylation characteristics and found that the sensitivity of stage ⅠA∼Ⅲ increased to 51.6%−81.4% when the specificity was 95.8% [69]. |
| Wang et al. | 2022 | Fragmentation size coverage and distribution, breakpoint and end motif | The multi-dimensional fragment feature model, showed excellent and stable performance in the screening prediction of lung cancer. Stage I lung cancer sensitivity reached 83.2%, and the predicted sensitivity reached 85% when the tumor was less than 1 cm [70]. |
| Guo et al. | 2022 | Motif profiling | A diagnostic model using the 6bp-breakpoint-motif feature has potential to distinguish stage I LUAD patients from healthy controls with 98.0% sensitivity, 94.7% specificity and 0.985 AUC [71]. |
| Martignano et al. | 2021 | Copy Number Variants | Using Nanopore sequencing, a customized analytical process was developed that could be used to detect changes in copy number in the plasma and identify common copy number variants associated with lung cancer [72]. |
| Fedyuk et al. | 2022 | Nucleosome Footprint | Through the development of the technique called plasmapheresis cosomal phenogenetics, it is used to distinguish and diagnose healthy people from cancer patients. The optimal prediction model obtained the AUC 0.96, 92% sensitivity, 85% specificity, and 92% accuracy [73]. |
| Li Y et al. | 2023 | End motifs, fragmentation, and Bincount | They developed a modified WGS -based HIFI method to enhanced the diagnostic performance of earlystage lung cancer and postoperative minimal residual disease [74]. |
cfDNA, cell-free DNA; LUAD, lung adenocarcinoma; AUC, area under curve; WGS, whole-genome sequencing; HIFI, High-performance Infrastructure for MultIomics.
Clinical perioperative period is the most common application scenario of ctDNA detection, including prognostic monitoring and treatment response evaluation. A series of studies have revealed that perioperative ctDNA as a reliable predictor for MRD can effectively predict postoperative recurrence in patients with NSCLC, and postoperative adjuvant therapy benefits survival in patients with ctDNA-based MRD detection [6,[75], [76], [77], [78]]. In addition, a growing body of evidence has shown that there was a correlation between pathologic responses and ctDNA responders for neoadjuvant therapy in lung cancer. During the process of neoadjuvant therapy, the percentage of patients with major pathological response (MPR) or pathologic complete response (pCR) was higher among those with ctDNA responder than among those without ctDNA responder [79], [80], [81]. One study [82] showed that the expression of CTCs was highly consistent with that of PD-L1 in tumor tissue, about 93%. On this basis, we detected CTCs continuously in patients treated with PD-1/PD-L1 inhibitors and found the number of PD-L1+ CTCs increased in drug-resistant patients after 3–5 courses of treatment. These results reveal the potential of CTCs assessment as a non-invasive, real-time biopsy to assess efficacy in patients with lung cancer.
Other cancers
In the aspect of breast cancer, CTCs and ctDNA are used for predicting survival prognosis, detecting MRD, monitoring treatment effect, and guiding the selection of treatment plan, etc. In the latest study, 465 patients with stage Ⅱ to stage Ⅲ triple negative breast cancer (TNBC) were enrolled in the PEARLY Stage Ⅲ trial and median follow-up was 16.8 months. It was found that baseline ctDNA before neoadjuvant chemotherapy could predict the risk of recurrence in patients with TNBC in stage Ⅱ to Ⅲ, and that ctDNA-score showed prognostic value independent of the pathological complete response [83]. In the study of head and neck tumors, Oliveira-Costa et al. [84] analyzed gene expression profiles of oral squamous cell carcinoma tumors to identify biomarkers that increased or decreased during tumor progression (from stage T1 to T4). The expression of four markers was evaluated in CTC, indicating PD-L1, HOXB9 and ZNF813 were increased, while BLNK expression was significantly decreased. Importantly, their expression patterns were consistent with those found in primary tumors. Currently, a variety of saliva molecules can be used as biomarkers for cancer diagnosis, prognosis, and therapeutic monitoring studies. In cases of HPV-associated oropharyngeal squamous cell carcinoma, ctHPVDNA deletion is associated with relapse-free survival, while ctHPVDNA testing has no specificity for relapsing disease [85]. This suggests a possible application of ctDNA in monitoring HPV positive head and neck squamous cell carcinoma recurrence. A study demonstrated that aqueous humor, the clear liquid in front of the eye, is an enriched source of tumor-derived cfDNA in retinoblastoma, which facilitates the analysis of tumor-derived cfDNA in the absence of tumor tissue [86].
Trials involving liquid biopsy to screen for pan-cancer are urgently awaited, and high-level clinical evidence is highly anticipated (Table 3). We look forward to incorporating more sample analysis and further optimization of the model to provide additional data support for early detection of multiple cancers.
Table 3.
Emerging application in liquid biopsy for pan-cancer.
| ID | Name | Study type | Enrollment (Patients number) | Cancer type | Detection content | Clinical application | Endpoint |
|---|---|---|---|---|---|---|---|
| NCT03832569 [88] | Study of Pembrolizumab Following Surgery in Patients With Microsatellite Instability High (MSI-H) Solid Tumors | Interventional | 10 | Colorectal cancer | ctDNA | Efficacy monitoring and relapse monitoring | Proportion of patients with ctDNA clearance and DFS. |
| NCT03803553 [89] | Identification and Treatment of Micrometastatic Disease in Stage III Colon Cancer | Interventional | 500 | Colorectal cancer | ctDNA | Micrometastatic indentification and recurrence screening | DFS and clearance rate of ctDNA. |
| NCT04457297 [90] | Initial Attack on Latent Metastasis Using TAS-102 for ct DNA Identified Colorectal Cancer Patients After Curative Resection (ALTAIR) | Interventional | 240 | Colorectal cancer | ctDNA | Selection of treatment | DFS |
| NCT04576858 [91] | Clinical Utility of Circulating Tumor DNA in Gastro-Esophageal Cancer (CURE) | Observational | 1950 | Esophageal cancer, Gastric cancer | ctDNA | Treatment effect evaluation, predictive and prognostic factors exploration | Time to recurrence. |
| NCT04000425 [92] | Potential Clinical Utilities of Circulating Tumor DNA in Gastric Cancer | Observational | 55 | Gastric cancer | ctDNA | MRD monitoring or adjuvant chemotherapy response prediction | Description of disease recurrence risk according to first positive ctDNA detection. Description of ctDNA changing to adjuvant chemotherapy response. |
| NCT04665687 [93] | Clinical Application of Genetic Sequencing of Early Gastric Cancer and Gastric Adenoma Patients | Observational | 1730 | Gastric cancer | ctDNA, immunohistochemistry | Early detection and prognosis monitoring | Identify biomarkers for differential diagnosis and predicting recurrence between early gastric cancer and precancerous adenoma. |
| NCT03588442 [94] | Prospective Surveillance for Very Early Hepatocellular Carcinoma (PRECAR) | Observational | 10,000 | Liver cancer | ctDNA | Early diagnosis and stratification of risk population | OS and liver-related disease progression. |
| NCT02973204 [95] | Circulating Tumor Cells and Tumor DNA in HCC and NET | Observational | 167 | Liver cancer, Neuroendocrine Tumors | CTCs and cfDNA | Prognostic monitoring and individualized therapy | Through digital droplet PCR and targeted sequencing to explore concordance with specific DNA mutations found in patient biopsies and ctDNA. |
| NCT04817046 [96] | Assessment of Early-detection Based on Liquid Biopsy in Lung Cancer (ASCEND-LUNG) | Observational | 467 | Lung cancer | cfDNA | Early detection | Accuracy of the multi-omics early-stage lung cancer diagnosis model. |
| NCT03317080 [97] | Dynamic Monitoring Circulating Tumor DNA in Surgical Patients With Lung Cancer (LUNGCA) | Observational | 400 | Lung cancer | ctDNA | Cancer diagnosis, drug efficacy, surgical effect evaluation, recurrence monitoring, prognosis judgment, medication guidance, and molecular classification differentiation | Recurrence-free survival. |
| NCT04790682 [98] | LIquid Biopsy to prEdict Responses To First-line immunotherapY in Metastatic Non-small Cell LUNG Cancer. LIBERTY LUNG (LIBERTYLUNG) | Interventional | 300 | Lung cancer | ctDNA | Immunotherapy treatment prediction | ctDNA variation of the prominent mutant allele variation |
| NCT04822792 [99] | Pan-canceR Early-Stage deteCtion by lIquid Biopsy tEchNique projecT (PRESCIENT) | Observational | 11,879 | Pan-cancer | Serum protein markers, cfDNA, blood RNA markers | Early-stage detection | Sensitivity and specificity of early detection of 22 types of cancers and TOO accuracy of a cfDNA methylation-based model, in combination with serum tumor markers. |
| NCT04972201 [100] | A Proof of Concept Study of Pan-cancer Early Detection by Liquid Biopsy (PROMISE) | Observational | 2305 | Pan-cancer | cfDNA and miRNA | Early detection | Sensitivity of early detection of cancers and TOO accuracy of 3 assays of cfDNA mutation, cfDNA methylation and miRNA expression when specificity was 90%, 95% or 98% in healthy participants. |
ctDNA, circulating tumor DNA; DFS, disease-free survival; OS, overall survival; HCC, hepatocellular carcinoma; NET, neuro-endocrine tumor; cfDNA, cell free DNA; TOO, tissue of origin.
Future perspectives and challenges
The development of next-generation sequencing methods as well as technological advances in molecular biology has sparked interest in liquid biopsies as an important tool for cancers. However, how to apply liquid biopsies to clinical precision medicine still needs to be addressed. Thus, further exploration is required before the liquid biopsy technology actually entered clinical practice. Many challenges of ctDNA remain as described below:
First, lack of uniform standards. Present different experimental studies have great heterogeneity and lack scientific comparability among them. Therefore, we need to establish unified standards, including accurate and stable library construction technology, powerful bioinformatics analysis platform and database. Standardization requirements for pre-analysis procedures include selection of collection vessels, timing and procedures used between blood drawing and plasma processing, and extraction/separation procedures for liquid biopsy components. The characterization and quantification of standard procedures should also be verified [59,87]. In addition, standardization should also focus on maximizing the yield of liquid biopsy markers.
Secondly, detection complexity and accuracy. One the one hand, tumor heterogeneity lead detection complexity, such as histological and hematological inconsonant results, different variations in tumor progression, and so on. On the other hand, specific clinical application scenarios have different requirements. For example, drug-resistant mutations detection requires a higher sensitivity; the precise evaluation of tumor mutation load requires comprehensive detection of master clone and subclone; the predictive effect of drug efficacy requires a precise definition of ctDNA clearance. Besides, the exclusion of clonal hematopoietic and other background noise is also significant.
Last, multidimensional and multiomics combination. More and more studies indicated that a single class of tumor markers (such as ctDNA mutation or DNA methylation) has significant limitations in sensitivity and specificity, thus the combination of multidimensional markers and multiple omics provide new direction for the application of liquid biopsy in cancer [101,102]. Multi-markers combinational analysis may provide a more complete understanding of cancer specificity. But the complementarity of several components of liquid biopsy, which may be derived from different cancer cell populations, remains to be investigated, and the combination of ctDNA testing with methylation, exosomes, circulating miRNAs, circulating tumor cells, metabolomics, and molecular imaging approaches should be considered.
Above all, although there are still some challenges in liquid biopsy, the relatively mature CTC and ctDNA have been universally applied in the area of pan-cancer clinical management. More and more researches are conducting various liquid biopsy clinical trials for multiple cancers to evaluate diagnostic and prognostic values of liquid biopsy, including early screening and, lead time analysis, MRD detection, treatment response evaluation, and so on. A meta-analysis studying clinical utility of liquid biopsy in early lung cancer showed that the estimated diagnostic values of different common liquid biopsy biomarkers with AUCs ranged from 0.84 to 0.87 and circulating biomarkers positive were also associated with significantly inferior recurrence-free survival (RFS) and overall survival (OS) [103]. Most of the current studies focus on specific single cancer species, while pan-cancer studies are relatively fewer and most of them are ongoing without clear results published. We believe that with the improvement of liquid biopsy techniques, detection approaches and platforms, liquid biopsy will be increasingly and widely studied and become a powerful tool for pan-cancer clinical application.
An ideal liquid biopsy clinical trial should identify specific scientific questions and clear research objectives based on clinical experience and patient demands, and estimate the number of patients enrolled and the duration of follow-up according to statistics and epidemiology. As for end point measurement, it needs to be formulated according to specific goals. For evaluating diagnostic values of biomarkers, specify, sensitivity and AUC are supposed to listed and calculated. For prognostic analysis, we are more concerned about the time point of disease relapse, inferior overall survival (OS) and disease-free survival (DFS), as well as average lead time between molecular recurrence and radiographic progression. For treatment response evaluation, it is more important which indicator is more accurate in assessing pathological response and therapy benefit and predicting longer survival after neoadjuvant and/or adjuvant treatment. Higher-quality trials are required to provide more rigorous evidence for routine clinical application of liquid biopsy.
Conclusion
The field of liquid biopsy has grown rapidly over the past decade, with many new breakthroughs. However, there are still some shortcomings that need to be improved. In this paper, we focus on the application of liquid biopsy in pan-cancer, where both CTCs and ctDNA show great potential in the diagnosis and treatment of single cancer types. And, the innovation and progress of liquid biopsy methods and techniques bring opportunities and challenges to the application of liquid biopsy. The highlight of this review is to expounding the application of ctDNA and CTCs, two mature liquid biopsy techniques, in various cancer species, mainly focusing on cancer screening and perioperative prognosis. To sum up, liquid biopsy is an important part of precision medicine for cancer. The future applications of liquid biopsy require further research and multi-omics data support. It is believed that this will become a clinical reality soon.
Ethical approval and consent to participate
Not applicapable.
Consent for publication
Not applicable.
Availability of data and materials
No supporting data.
Funding
This work was supported by Research Unit of Intelligence Diagnosis and Treatment in Early Non-small Cell Lung Cancer, Chinese Academy of Medical Science (2021RU002), CAMS Innovation Fund for Medical Sciences(CIFMS) 2022-I2M-C&T-B-120, National Natural Science Foundation of China (No. 82072566 and No. 92059203), Beijing Natural Science Foundation (L222021) and Peking University People's Hospital Research and Development Funds (RZ2022-03). And it also sponsored by Clinical Medicine Plus X - Young Scholars Project, Peking University, the Fundamental Research Funds for the CentralUniversities (PKU2023LCXQ008) .
CRediT authorship contribution statement
Wenxiang Wang: Conceptualization, Writing – review & editing. Yue He: Conceptualization, Writing – review & editing. Fan Yang: Writing – review & editing. Kezhong Chen: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Alix-Panabieres C. The future of liquid biopsy. Nature. 2020;579:S9. doi: 10.1038/d41586-020-00844-5. [DOI] [PubMed] [Google Scholar]
- 4.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 5.Tie J. Tailoring immunotherapy with liquid biopsy. Nat. Cancer. 2020;1:857–859. doi: 10.1038/s43018-020-00113-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen K., et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC) Clin. Cancer Res. 2019;25:7058–7067. doi: 10.1158/1078-0432.CCR-19-1213. [DOI] [PubMed] [Google Scholar]
- 7.Corcoran R.B., Chabner B.A. Application of cell-free DNA analysis to cancer treatment. N. Engl. J. Med. 2018;379:1754–1765. doi: 10.1056/NEJMra1706174. [DOI] [PubMed] [Google Scholar]
- 8.Heitzer E., Haque I.S., Roberts C.E.S., Speicher M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019;20:71–88. doi: 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 9.Meng S., et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 10.Gorgannezhad L., Umer M., Islam M.N., Nguyen N.T., Shiddiky M.J.A. Circulating tumor DNA and liquid biopsy: opportunities, challenges, and recent advances in detection technologies. Lab Chip. 2018;18:1174–1196. doi: 10.1039/C8LC00100F. [DOI] [PubMed] [Google Scholar]
- 11.Tzanikou E., et al. PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: a direct comparison study. Mol. Oncol. 2019;13:2515–2530. doi: 10.1002/1878-0261.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misale S., et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murtaza M., et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa N., et al. Highly sensitive fusion detection using plasma cell-free RNA in non-small-cell lung cancers. Cancer Sci. 2021;112:4393–4403. doi: 10.1111/cas.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue J., Inazawa J. Cancer-associated miRNAs and their therapeutic potential. J. Hum. Genet. 2021;66:937–945. doi: 10.1038/s10038-021-00938-6. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt D.R., et al. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021;71:333–358. doi: 10.3322/caac.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best M.G., et al. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Best M.G., et al. Swarm intelligence-enhanced detection of non-small-cell lung cancer using tumor-educated platelets. Cancer Cell. 2017;32:238–252. doi: 10.1016/j.ccell.2017.07.004. e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roweth H.G., Battinelli E.M. Lessons to learn from tumor-educated platelets. Blood. 2021;137:3174–3180. doi: 10.1182/blood.2019003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H., et al. Validation of a highly sensitive Sanger sequencing in detecting EGFR mutations from circulating tumor DNA in patients with lung cancers. Clin. Chim. Acta. 2022;536:98–103. doi: 10.1016/j.cca.2022.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Marcozzi A., et al. Accurate detection of circulating tumor DNA using nanopore consensus sequencing. NPJ Genom. Med. 2021;6:106. doi: 10.1038/s41525-021-00272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razavi P., et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 2019;25:1928–1937. doi: 10.1038/s41591-019-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual J., et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022;33:750–768. doi: 10.1016/j.annonc.2022.05.520. [DOI] [PubMed] [Google Scholar]
- 24.Kowalik A., Kowalewska M., Gozdz S. Current approaches for avoiding the limitations of circulating tumor cells detection methods-implications for diagnosis and treatment of patients with solid tumors. Transl. Res. 2017;185:58–84. doi: 10.1016/j.trsl.2017.04.002. e15. [DOI] [PubMed] [Google Scholar]
- 25.Rushton A.J., Nteliopoulos G., Shaw J.A., Coombes R.C. A review of circulating tumour cell enrichment technologies. Cancers. 2021;13 doi: 10.3390/cancers13050970. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tieng F.Y.F., Abu N., Nasir S.N., Lee L.H., Ab Mutalib N.S. Liquid biopsy-based colorectal cancer screening via surface markers of circulating tumor cells. Diagnostics. 2021;11 doi: 10.3390/diagnostics11112136. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra A., et al. Ultrahigh-throughput magnetic sorting of large blood volumes for epitope-agnostic isolation of circulating tumor cells. Proc. Natl. Acad. Sci. USA. 2020;117:16839–16847. doi: 10.1073/pnas.2006388117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Z., et al. Assessment of PD-L1 expression on circulating tumor cells for predicting clinical outcomes in patients with cancer receiving PD-1/PD-L1 blockade therapies. Oncologist. 2021;26:e2227–e2238. doi: 10.1002/onco.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen E.N., et al. Antigen-agnostic microfluidics-based circulating tumor cell enrichment and downstream molecular characterization. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0241123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Z., et al. Inferring the evolution and progression of small-cell lung cancer by single-cell sequencing of circulating tumor cells. Clin. Cancer Res. 2019;25:5049–5060. doi: 10.1158/1078-0432.CCR-18-3571. [DOI] [PubMed] [Google Scholar]
- 31.Tsai W.S., et al. Novel circulating tumor cell assay for detection of colorectal adenomas and cancer. Clin. Transl. Gastroenterol. 2019;10:e00088. doi: 10.14309/ctg.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerachian M.A., et al. Crosstalk between DNA methylation and gene expression in colorectal cancer, a potential plasma biomarker for tracing this tumor. Sci. Rep. 2020;10:2813. doi: 10.1038/s41598-020-59690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., et al. Development of a liquid biopsy based purely quantitative digital droplet PCR assay for detection of MLH1 promoter methylation in colorectal cancer patients. BMC Cancer. 2021;21:797. doi: 10.1186/s12885-021-08497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui J., et al. Discovery and validation of methylation signatures in blood-based circulating tumor cell-free DNA in early detection of colorectal carcinoma: a case-control study. Clin Epigenet. 2021;13:26. doi: 10.1186/s13148-020-00985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren J.D., et al. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. doi: 10.1186/1741-7015-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Church T.R., et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., et al. Detection of colorectal cancer in circulating cell-free DNA by methylated CpG tandem amplification and sequencing. Clin. Chem. 2019;65:916–926. doi: 10.1373/clinchem.2019.301804. [DOI] [PubMed] [Google Scholar]
- 38.Chen F., et al. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut. 2022;71:1315–1325. doi: 10.1136/gutjnl-2020-323476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le D.T., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandara D.R., et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 41.Raimondi L., Raimondi F.M., Di Benedetto L., Cimino G., Spinelli G.P. PD-L1 expression on circulating tumour cells may be predictive of response to regorafenib in patients diagnosed with chemorefractory metastatic colorectal cancer. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21186907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troncarelli Flores B.C., et al. Molecular and kinetic analyses of circulating tumor cells as predictive markers of treatment response in locally advanced rectal cancer patients. Cells. 2019;8 doi: 10.3390/cells8070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schraa S.J., et al. Circulating tumor DNA guided adjuvant chemotherapy in stage II colon cancer (MEDOCC-CrEATE): study protocol for a trial within a cohort study. BMC Cancer. 2020;20:790. doi: 10.1186/s12885-020-07252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi H., et al. CIRCULATE-Japan: circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112:2915–2920. doi: 10.1111/cas.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tie J., et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016;8:346ra392. doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tie J., et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N. Engl. J. Med. 2022;386:2261–2272. doi: 10.1056/NEJMoa2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tie J., et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. 2019;5:1710–1717. doi: 10.1001/jamaoncol.2019.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tie J., et al. Prognostic significance of postsurgery circulating tumor DNA in nonmetastatic colorectal cancer: individual patient pooled analysis of three cohort studies. Int. J. Cancer. 2021;148:1014–1026. doi: 10.1002/ijc.33312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim K., et al. Circulating cell-free DNA as a promising biomarker in patients with gastric cancer: diagnostic validity and significant reduction of cfDNA after surgical resection. Ann. Surg. Treat. Res. 2014;86:136–142. doi: 10.4174/astr.2014.86.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura Y., et al. Characteristics of genomic alterations in circulating tumor DNA (ctDNA) in patients (Pts) with advanced gastrointestinal (GI) cancers in nationwide large-scale ctDNA screening: SCRUM-Japan Monstar-Screen. J. Clin. Oncol. 2021;39 doi: 10.1200/JCO.2021.39.3_suppl.106. [DOI] [Google Scholar]
- 52.Matsuoka T., Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J. Gastroenterol. 2018;24:2818–2832. doi: 10.3748/wjg.v24.i26.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy S., et al. Diagnostic efficacy of circular RNAs as noninvasive, liquid biopsy biomarkers for early detection of gastric cancer. Mol. Cancer. 2022;21:42. doi: 10.1186/s12943-022-01527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J., et al. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis. 2020;11:346. doi: 10.1038/s41419-020-2531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szczepanik A., et al. CD44(+) cytokeratin-positive tumor cells in blood and bone marrow are associated with poor prognosis of patients with gastric cancer. Gastric Cancer. 2019;22:264–272. doi: 10.1007/s10120-018-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., et al. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab-resistant mechanism in advanced gastric cancer. EBioMedicine. 2019;43:261–269. doi: 10.1016/j.ebiom.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D.S., et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68:1152–1161. doi: 10.1136/gutjnl-2018-316522. [DOI] [PubMed] [Google Scholar]
- 58.van der Pol Y., Mouliere F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell. 2019;36:350–368. doi: 10.1016/j.ccell.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Xu R.H., et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017;16:1155–1161. doi: 10.1038/nmat4997. [DOI] [PubMed] [Google Scholar]
- 60.Luo B., et al. Cell-free DNA methylation markers for differential diagnosis of hepatocellular carcinoma. BMC Med. 2022;20:8. doi: 10.1186/s12916-021-02201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L., et al. Genome-scale profiling of circulating cell-free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients. Cell Res. 2021;31:589–592. doi: 10.1038/s41422-020-00457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi L.N., et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018;78:4731–4744. doi: 10.1158/0008-5472.CAN-17-2459. [DOI] [PubMed] [Google Scholar]
- 63.Yan Q., et al. Immune checkpoint FGL1 expression of circulating tumor cells is associated with poor survival in curatively resected hepatocellular carcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.810269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin Y.C., Wu M.Q., Chen K.Z., Wang J. Role of circulating tumor DNA in the management of patients with non-small cell lung cancer. Glob. Transl. Med. 2022;1:1–13. doi: 10.36922/gtm.v1i1.96. [DOI] [Google Scholar]
- 65.Liu W.R., et al. Detection of circulating genetically abnormal cells in peripheral blood for early diagnosis of non-small cell lung cancer. Thorac. Cancer. 2020;11:3234–3242. doi: 10.1111/1759-7714.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye M., et al. Circulating genetically abnormal cells add non-invasive diagnosis value to discriminate lung cancer in patients with pulmonary nodules </=10 mm. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.638223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C., et al. The value of circulating tumor cells with positive centromere probe 8 in the diagnosis of small pulmonary nodules. Transl. Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chabon J.J., et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580:245–251. doi: 10.1038/s41586-020-2140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang N., et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat. Biomed. Eng. 2021;5:586–599. doi: 10.1038/s41551-021-00746-5. [DOI] [PubMed] [Google Scholar]
- 70.Wang S., et al. Multi-dimensional cell-free DNA fragmentomic assay for detection of early-stage lung cancer. Am. J. Respir. Crit. Care Med. 2022 doi: 10.1164/rccm.202109-2019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo W., et al. Sensitive detection of stage I lung adenocarcinoma using plasma cell-free DNA breakpoint motif profiling. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martignano F., et al. Nanopore sequencing from liquid biopsy: analysis of copy number variations from cell-free DNA of lung cancer patients. Mol. Cancer. 2021;20:32. doi: 10.1186/s12943-021-01327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fedyuk V., et al. Multiplexed, single-molecule, epigenetic analysis of plasma-isolated nucleosomes for cancer diagnostics. Nat. Biotechnol. 2022 doi: 10.1038/s41587-022-01447-3. [DOI] [PubMed] [Google Scholar]
- 74.Li Y., et al. Multi-omics integrated circulating cell-free DNA genomic signatures enhanced the diagnostic performance of early-stage lung cancer and postoperative minimal residual disease. EBioMedicine. 2023;91 doi: 10.1016/j.ebiom.2023.104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia L., et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1) Clin. Cancer Res. 2022;28:3308–3317. doi: 10.1158/1078-0432.CCR-21-3044. [DOI] [PubMed] [Google Scholar]
- 76.Abbosh C., et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhuri A.A., et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.CD-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zviran A., et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat. Med. 2020;26:1114–1124. doi: 10.1038/s41591-020-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yue D., et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl. Lung Cancer Res. 2022;11:263–276. doi: 10.21037/tlcr-22-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou C., et al. IMpower010: biomarkers of disease-free survival (DFS) in a phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann. Oncol. 2021;32:S1374. doi: 10.1016/j.annonc.2021.10.018. -S1374. [DOI] [Google Scholar]
- 81.Forde P.M., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022;386:1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ilie M., et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018;29:193–199. doi: 10.1093/annonc/mdx636. [DOI] [PubMed] [Google Scholar]
- 83.Kim M.H., et al. Copy number aberration burden on circulating tumor DNA predicts recurrence risk after neoadjuvant chemotherapy in patients with triple-negative breast cancer: post-hoc analysis of phase III PEARLY trial. J. Clin. Oncol. 2022;40:603. doi: 10.1200/jco.2022.40.16_suppl.603. [DOI] [Google Scholar]
- 84.Oliveira-Costa J.P., et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget. 2015;6:20902–20920. doi: 10.18632/oncotarget.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chera B.S., et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J. Clin. Oncol. 2020;38:1050–1058. doi: 10.1200/JCO.19.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitazawa K., et al. Safety of anterior chamber paracentesis using a 30-gauge needle integrated with a specially designed disposable pipette. Br. J. Ophthalmol. 2017;101:548–550. doi: 10.1136/bjophthalmol-2016-309650. [DOI] [PubMed] [Google Scholar]
- 87.Wu H.C., Yang H.I., Wang Q., Chen C.J., Santella R.M. Plasma DNA methylation marker and hepatocellular carcinoma risk prediction model for the general population. Carcinogenesis. 2017;38:1021–1028. doi: 10.1093/carcin/bgx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Study of Pembrolizumab Following Surgery in Patients With Microsatellite Instability High (MSI-H) Solid Tumors 2023.
- 89.Identification and Treatment Of Micrometastatic Disease in Stage III Colon Cancer 2023.
- 90.Initial Attack on Latent Metastasis Using TAS-102 for ct DNA Identified Colorectal Cancer Patients After Curative Resection 2023.
- 91.Clinical Utility of Circulating Tumor DNA in Gastro-Esophageal Cancer (CURE) 2023.
- 92.Potential Clinical Utilities of Circulating Tumor DNA in Gastric Cancer 2023.
- 93.Clinical Application of Genetic Sequencing of Early Gastric Cancer and Gastric Adenoma Patients 2023.
- 94.Prospective Surveillance for Very Early Hepatocellular Carcinoma (PRECAR) 2023.
- 95.Circulating Tumor Cells and Tumor DNA in HCC and NET 2023.
- 96.Assessment of Early-detection Based on Liquid Biopsy in Lung Cancer (ASCEND-LUNG) 2023.
- 97.Dynamic Monitoring Circulating Tumor DNA in Surgical Patients With Lung Cancer (LUNGCA) 2023.
- 98.LIquid Biopsy to prEdict Responses To First-line immunotherapY in Metastatic Non-small Cell LUNG Cancer. LIBERTY LUNG 2023.
- 99.Pan-canceR Early-Stage deteCtion by lIquid Biopsy tEchNique projecT (PRESCIENT) 2023.
- 100.A Proof of Concept Study of Pan-cancer Early Detection by Liquid Biopsy (PROMISE) 2023.
- 101.Chen K., et al. Spatiotemporal genomic analysis reveals distinct molecular features in recurrent stage I non-small cell lung cancers. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111047. [DOI] [PubMed] [Google Scholar]
- 102.Chen K., et al. Non-invasive lung cancer diagnosis and prognosis based on multi-analyte liquid biopsy. Mol. Cancer. 2021;20:23. doi: 10.1186/s12943-021-01323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen H., et al. Potential clinical utility of liquid biopsy in early-stage non-small cell lung cancer. BMC Med. 2022;20:480. doi: 10.1186/s12916-022-02681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No supporting data.