Abstract
Objectives
To assess whether MR fingerprinting (MRF)–based relaxation properties exhibit cross-sectional and prospective correlations with patient outcome and compare the results with those from DTI.
Methods
Clinical imaging, MRF, and DTI were acquired in patients (24 ± 10 days after injury (timepoint 1) and 90 ± 17 days after injury (timepoint 2)) and once in controls. Patient outcome was assessed with global functioning, symptom profile, and neuropsychological testing. ADC and fractional anisotropy (FA) from DTI and T1 and T2 from MRF were compared in 12 gray and white matter regions with Mann–Whitney tests. Bivariate associations between MR measures and outcome were assessed using the Spearman correlation and logistic regression.
Results
Data from 22 patients (38 ± 12 years; 17 women) and 18 controls (32 ± 8 years; 12 women) were analyzed. Fourteen patients (37 ± 12 years; 11 women) returned for timepoint 2, while two patients provided only timepoint 2 clinical outcome data. At timepoint 1, there were no differences between patients and controls in T1, T2, and ADC, while FA was lower in mTBI frontal white matter. T1 at timepoint 1 and the change in T1 exhibited more (n = 18) moderate to strong correlations (|r|= 0.6–0.85) with clinical outcome at timepoint 2 than T2 (n = 3), FA (n = 7), and ADC (n = 2). High T1 at timepoint 1, and serially increasing T1, accounted for five of the six MR measures with the highest utility for identification of non-recovered patients at timepoint 2 (AUC > 0.80).
Conclusion
T1 derived from MRF was found to have higher utility than T2, FA, and ADC for predicting 3-month outcome after mTBI.
Keywords: Traumatic brain injury, Magnetic resonance fingerprinting, Relaxation, Clinical outcome
Introduction
Mild traumatic brain injury (mTBI) is a leading public health problem in civilian, as well as in military veteran, populations, amounting to an annual worldwide incidence of ~ 55 million [1, 2]. Although most patients recover completely, a subset develops persistent physical, psychiatric, emotional, or cognitive impairments [3]. Because their brain CT and qualitative MRI are most commonly negative [4, 5], there is a need for quantitative imaging-based markers to improve mTBI prognosis.
Diffuse axonal injury is thought to be the pathological process behind most TBI-related impairments [6]. Ionic imbalances cause axonal varicosities and other micro-structural damage to axonal tracts, which has been commonly studied using diffusion tensor imaging (DTI) [7, 8]. However, DTI findings are highly variable with respect to anatomical location, nature of alterations, and their correlation with clinical outcome [9]. Thus, there is a need for additional non-invasive markers that are sensitive to diffuse axonal injury and its related cellular changes. Changes in the local molecular environment of water are detectable with quantitative assessment of its longitudinal (T1) and transverse (T2) relaxation times, but despite promising results from TBI animal models [10–12], human studies are lacking. The only two in vivo investigations reported higher T2 in patients with predominantly moderate and severe TBI [13, 14]; while a recent ex vivo study linked T1 and T2 to diffuse axonal injury [15]. Further research has likely been hampered by the fact that most relaxometry approaches require long acquisition times limited to only a single property (T1 or T2). Fortunately, clinical applications of quantitative relaxometry have been made possible by magnetic resonance fingerprinting (MRF) [16], which enables fast reconstruction of different parameters from a single MRI acquisition [17, 18].
Given that (i) diffuse axonal injury is expected to change the molecular environment of water; and (ii) the previous animal studies [11, 12] in TBI found relaxation time increases correlating with outcomes, we hypothesized that higher relaxation times will be detectable in vivo by MRF in mTBI and that they will show cross-sectional and prospective correlations with patient outcome. These hypotheses were tested in gray matter (GM) and white matter (WM) regions of patients scanned at an average of 24 days after TBI (timepoint 1) and subsequently at a three-month follow-up (timepoint 2). The aims were to assess whether MRF-based relaxation properties (T1, T2) exhibit correlations with patient outcome and to compare the results to those obtained with the established DTI metrics of fractional anisotropy (FA) and apparent diffusion coefficient (ADC).
Methods
Study participants
The Institutional Review Board approved this Health Insurance Portability and Accountability Act–compliant study, and informed written consent was obtained prior to the examination. Between November 2018 and December 2019, we prospectively enrolled 31 patients (23 women, average age 37 ± 12 years) with mTBI to be scanned at an average of 1 month after injury, with a follow-up visit at an average 3 months after injury (Fig. 1). The inclusion criteria besides diagnosis of mTBI [19], along with exclusion criteria, are listed in the Supplemental Materials. Eighteen age- and sex-matched healthy volunteers (12 women, average age 32 ± 8 years) were included as controls and scanned once. This patient and control cohort is being studied with multimodal and multinuclear MRI as part of a larger project, which, at the time of publication, has produced one other manuscript [20]. Specific areas of data overlap between the two publications are reported in the Supplemental Materials.
Fig. 1.
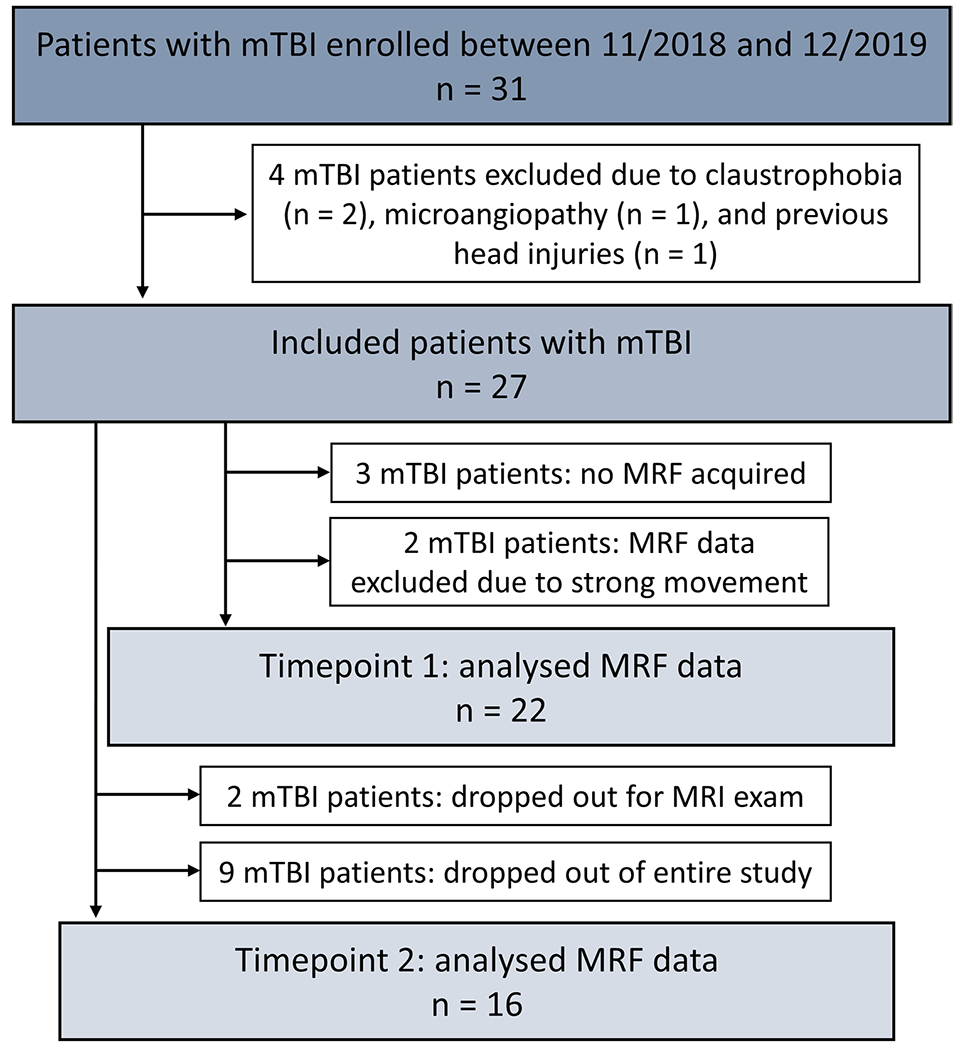
Flow diagram of study enrollment. From the initial 31 enrolled patients, four were excluded prior to data acquisition, and five were excluded due to strong movement or missing data, resulting in 22 MRF data sets at timepoint 1. At timepoint 2, two patients declined the MR exam but completed the outcome assessments, and nine patients dropped out of the entire study. mTBI, mild traumatic brain injury; MRF, magnetic resonance fingerprinting
Patient outcome assessment
At the time of each MRI visit, patients underwent clinical and neuropsychological assessment using the following tools from the National Institute of Neurological Disorders and Stroke TBI Common Data Elements database: the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) [21], which inquires about 16 symptoms frequently reported after mTBI; the Glasgow Outcome Scale – Extended (GOSE) [22], to assess the impact of an injury on daily life function; and the Brief Test of Adult Cognition by Telephone (BTACT) [23], to evaluate memory and executive functioning using performance-based cognitive testing. All assessments were performed by the research coordinator, who was unaware of any individual’s MRI data or results.
MRI acquisition
Imaging was performed on a 3-T scanner (Magnetom Prisma, Siemens Healthineers). The qualitative MRI protocol consisted of a 2D fluid-attenuation inversion recovery, a 3D T1-weighted gradient echo, and susceptibility-weighted imaging. DTI was acquired with a mono-polar gradient pulse echo-planar imaging sequence (nine b0 images with b = 250, 1000, and 2000s/mm2). For multiparametric mapping, we applied a multi-slice 2D radial MRF method to simultaneously measure T1 and T2, as previously described [24], with the following parameters: in-plane resolution of 1.25 × 1.25 mm2, 5-mm slice thickness, and a total scan time of less than 8 min. A dictionary with simulated MR fingerprints was created using extended-phase graphs with T1 and T2 entries in the ranges 150–4642 ms and 10–350 ms, respectively (step size of 2.5%) [24]. Imaging parameter details are provided in Table A.1 in the Supplemental Materials.
Image analysis
Qualitative images were evaluated by a neuroradiologist using the NIH Common Data Element guidelines [25]. MRI findings were manually outlined and excluded from the quantitative analysis to investigate only normal-appearing tissue. Twelve regions known for susceptibility to TBI and that are common sites of DTI abnormalities [25, 26] were extracted from the T1-weighted images to obtain average regional values of T1, T2, FA, and ADC (Fig. 2). Automatic brain segmentation was performed using FreeSurfer version 6.0.0 [27] to obtain global WM, global cortical GM, and four deep GM masks. Six regional WM masks were manually outlined using FireVoxel software [28, 29]. The global cortical GM and thalamus masks were eroded by one voxel, to reduce partial volume errors from neighboring cerebrospinal fluid.
Fig. 2.
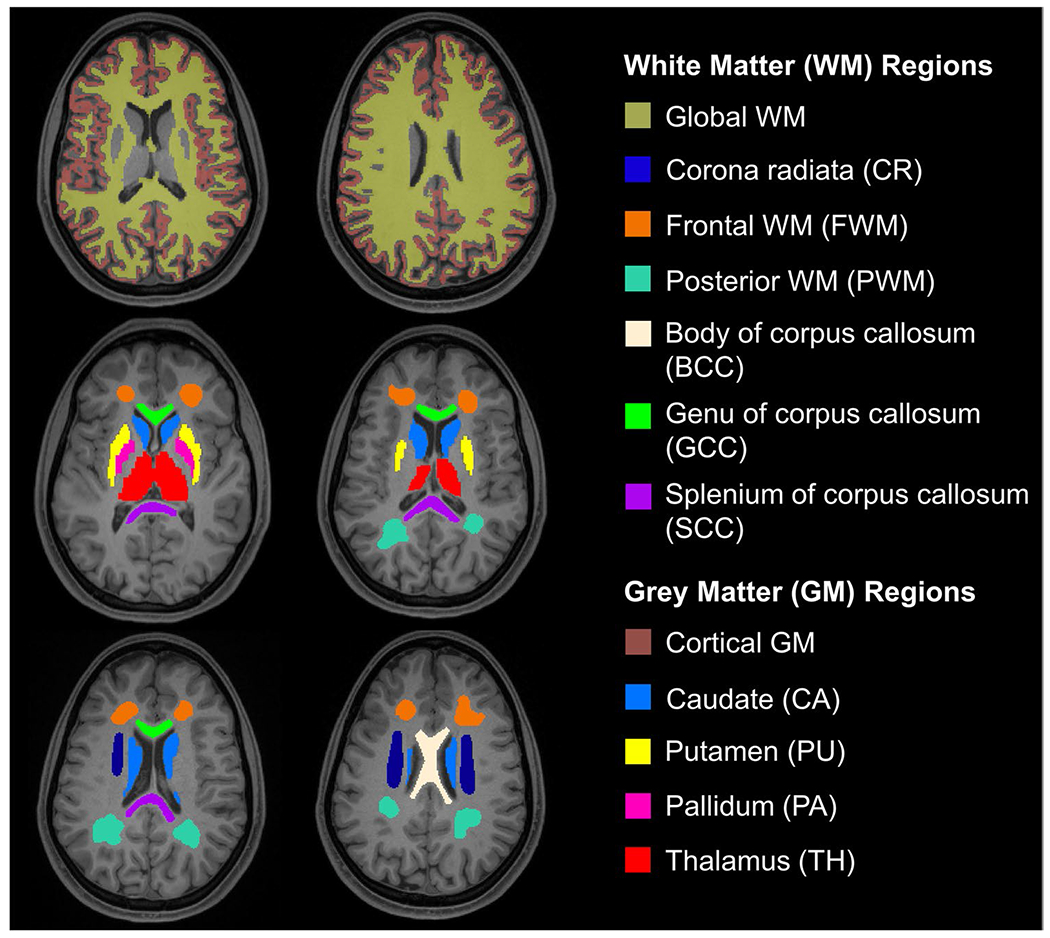
Regional analysis of the gray and white matter. T1-weighted axial MPRAGE images overlaid with the 12 ROIs in which T1, T2, FA, and ADC were quantified. Four GM structures (Thalamus, Caudate, Putamen, and Pallidum), global WM, and cortical GM were automatically segmented using FreeSurfer. Six WM regions (Frontal WM, Posterior WM, Corona Radiata, Body of Corpus Callosum, Splenium of Corpus Callosum, and Genu of Corpus Callosum) were manually outlined. The cortical GM and thalamus masks shown have been eroded by one voxel (to reduce CSF contributions)
Post-processing of diffusion data was performed offline using software tools from FSL [30] and MRTrix3.0 [31] to generate ADC and FA maps for each voxel. The MRF data was reconstructed offline with an in-house MATLAB script. Visual quality control ensured that only artifact-free slices were included in the analysis [32]. Slices with motion artifacts were completely excluded from the ROI analysis.
All outlined regions were individually registered to the DTI and MRF maps in SPM12. The ROIs were overlaid on the maps, and voxel values within each ROI were averaged. To account for partial volume effects, voxels with a cerebrospinal fluid fraction greater than 0.3 were excluded from the analysis. For the estimation of the relaxation times, outliers defined by T1 > 2500 ms and T2 > 150 ms were also excluded (average ± two standard deviations of T1/T2 of cerebrospinal fluid).
All data analyses, including the radiologist’s readings, were done blinded to the patient’s clinical outcome data.
Statistical analysis
Sample size and statistical power calculations were performed as described in the Supplemental Materials. Nonparametric analyses were conducted due to their robustness against violations of the assumption of normality. The Mann–Whitney test was used to compare the control to the mTBI group. Since the T1 and T2 relationships with age are not well-characterized, the three oldest patients (ages 56, 59, and 60 years), who lacked age-matched controls, were omitted from the intergroup analyses comparing patients and controls but were included in the mTBI intragroup analyses (see Supplemental Materials). Bivariate associations were assessed using the nonparametric Spearman rank correlation. Logistic regression was used to identify measures that could discriminate patients who were recovered at timepoint 2 (GOSE = 8), from those who were not (GOSE < 8). The area under the receiver-operating-characteristic curve (AUC) assessed the performance of each imaging measure at timepoint 1 as a predictor of recovery. All statistical tests were conducted at the two-sided 5% significance level using SAS 9.4 software (SAS Institute, USA). p values are reported without multiple comparison corrections due to the exploratory nature of this study.
Results
Study population
Out of the 31 patients who signed informed consent, 22 contributed data at timepoint 1, 16 of which returned for a scan at timepoint 2. Reasons for exclusion or drop-out are shown in Fig. 1. Table 1 details the demographic and injury characteristics of the patients used in the analysis. The average time from injury at timepoint 1 was 24 ± 10 (average ± standard deviation) days (range: 8–53), and 90 ± 17 days (range: 82–142) at timepoint 2. Two patients scanned at timepoint 1 declined a scan at timepoint 2, but responded to the outcome questionnaires over the phone at 115 and 113 days after injury. Hemorrhagic diffuse axonal injury and non-axonal shear patterns of hemorrhages were observed on qualitative MRI in two patients.
Table 1.
Demographic and injury characteristics of study participants with mTBI at timepoint 1
| Characteristics | mTBI (n = 22) | Controls (n = 18) |
|---|---|---|
| Sex | 17 women (77%)/ 5 men (23%) | 12 women (67%)/ 6 men (33%) |
| Age | 38 ± 12 y (range: 23—60) | 32 ± 18 y (range: 23—54) |
| MRI findings | WM hyperintensities: 10 (45%) Diffuse axonal injury: 2 (9%) Hemorrhage: 1 (5%) |
WM hyperintensities: 6 (33%) |
| Time after injury (timepoint 1) | 24 ± 10 days (range: 8—53) | |
| Mechanism of injury | Falls: 7 (32%) Pedestrian-object: 5 (23%) Sports/recreation: 3 (14%) Motor vehicle accident: 2 (9%) Other: 5 (22%) |
|
| Loss of consciousness | No: 13 (59%), yes: 6 (27%), unknown: 3 (14%) | |
| Posttraumatic amnesia | No: 12 (55%), yes: 10 (45%) | |
| Alteration of consciousness | No: 1 (5%) < 1 min: 3 (14%) 1 – 59 min: 15 (68%) > 1 h: 3 (13%) |
Note. Data in parentheses are either group ranges or percentages. WM white matter
Patient outcome assessments
Results of the outcome assessments are compiled in Table 2. RPQ data was analyzed according to three classifications: (i) RPQ total score [21]; (ii) RPQ 3 and RPQ 13, which groups early- and late-onset symptoms, respectively [33]; and (iii) three-factor model with cognitive, somatic, and emotional subscales [34]. Concussion symptoms using all three RPQ methods improved over time (all p < 0.05), as did the GOSE (p = 0.005). Two BTACT subtests, namely word list recall (p = 0.005) and number series (p = 0.005), as well as the composite BTACT z-score (p = 0.001) improved over time, indicating improvement of memory and executive functioning.
Table 2.
Summary of patient outcome assessments at timepoints 1 and 2, as well as the change for each measure (value at timepoint 2 minus the value at timepoint 1, Δ 2–1). Only the participants providing data at both time points were included in the change computations. Median, percentage, and range are presented in parentheses. Each p value is from the Wilcoxon signed-rank test assessing whether the change was different from zero. The RPQ was analyzed according to different classification methods: RPQ total score (italic bold), RPQ 3 / RPQ 13, and the three-factor model. The BTACT sub-scores are summarized in a composite z-score of cognitive function (italic bold). Note the higher proportion of recovered patients (GOSE), reduction of post-concussion symptoms severity (RPQ), and the improvement of cognitive scores (BTACT) over time
| Characteristics | mTBI timepoint 1 (n = 22) | mTBI timepoint 2 (n = 16) | Change (Δ 2–1) (n = 13) | ||
|---|---|---|---|---|---|
| Glasgow Outcome Scale – Extended (GOSE) | 5 (lower moderate disability) | 1 (5%) | 0 (0%) | n/a | |
| 6 (upper moderate disability) | 11 (50%) | 2 (13%) | n/a | ||
| 7 (lower good recovery) | 3 (14%) | 6 (38%) | n/a | ||
| 8 (upper good recovery) | 7 (32%) | 8 (50%) | n/a | ||
| Average | 6.7 ± 1 (6) | 7.4 ± 0.7 (7.5) | 0.76 ± 0.9 (p = 0.005) | ||
| Rivermead Post-Concussion Symptoms Questionnaire (RPQ) | Total | 21.5 ± 11.9 (0 – 47) | 12.1 ± 14 (0 – 46) | −11.12 ± 13.97 (p = 0.007) | |
| 3-factor model | Somatic | 11.9 ± 6.9 (0 – 30) | 6.4 ± 7.8 (0 – 28) | −6.94 ± 9.26 (p = 0.007) | |
| Emotional | 4.2 ± 14.1 (0 – 15) | 2.5 ± 3.8 (0 – 14) | −2.06 ± 3.11 (p = 0.045) | ||
| Cognitive | 5.4 ± 3.9 (0 – 12) | 3.2 ± 3.5 (0 – 9) | −2.12 ± 3.43 (p = 0.02) | ||
| RPQ 3/13 | RPQ 3 | 3.4 ± 2.5 (0 – 10) | 1.4 ± 2.2 (0 – 8) | −2.47 ± 2.43 (p = 0.002) | |
| RPQ 13 | 18.1 ± 10.1 (0 – 37) | 10.9 ± 12.8 (0 – 42) | −8.41 ± 12.65 (p = 0.009) | ||
| Brief Test of Adult Cognition by Telephone (BTACT) | BTACT composite z-score | −0.38 ± 0.67 (−1.44 – 0.86) | −0.08 ± 0.77 (−1.31 – 1.47) | 0.43 ± 0.45 (p = 0.001) | n/a |
| Subtest z-scores | Word list recall | −0.52 ± 1.07 (−2.67 – 2.06) | 0.0 ± 0.85 (−1.13 – 1.77) | 0.58 ± 0.81 (p = 0.005) | |
| Short – delay word list recall | −0.60 ± 0.79 (−1.75 – 0.76) | −0.18 ± 0.95 (−1.77 – 1.78) | 0.27 ± 0.79 (p = 0.1637) | ||
| Backward digit | −0.11 ± 0.90 (−1.39 – 1.64) | −0.17 ± 1.04 (−2.15 – 1.68) | 0.17 ± 0.71 (p = 0.65) | ||
| Category fluency | 0.0 ± 1.27 (−1.87 – 2.92) | −0.02 ± 1.07 (−2.07 – 1.95) | 0.04 ± 0.69 (p = 0.723) | ||
| Number series | −0.70 ± 1.52 (−3.74 – 1.36) | −0.08 ± 1.02 (−1.79 – 1.65) | 0.9 ± 1.03 (p = 0.005) | ||
| Backward counting | −0.46 ± 0.91 (−2.05 – 1.95) | −0.05 ± 1.77 (−2.86 – 4.30) | 0.78 ± 1.61 (p = 0.052) | ||
Note. n/a not applicable
Quantitative MRI measures at timepoint 1
Figure 3 shows example T1, T2, ADC, and FA maps next to qualitative images from a patient and an age-matched control. In general, GM exhibited higher T1 and T2 values than WM, producing high gray-white matter contrast. Figure 4 shows the distributions of T1, T2, ADC, and FA in all ROIs in patients and controls at timepoint 1. Numerical values are provided in Table A.2 and A.3. FA in frontal WM was lower in mTBI compared to controls (p = 0.017). There were no statistically significant differences in T1 and T2 between patients and controls at timepoint 1 (Table A.4).
Fig. 3.
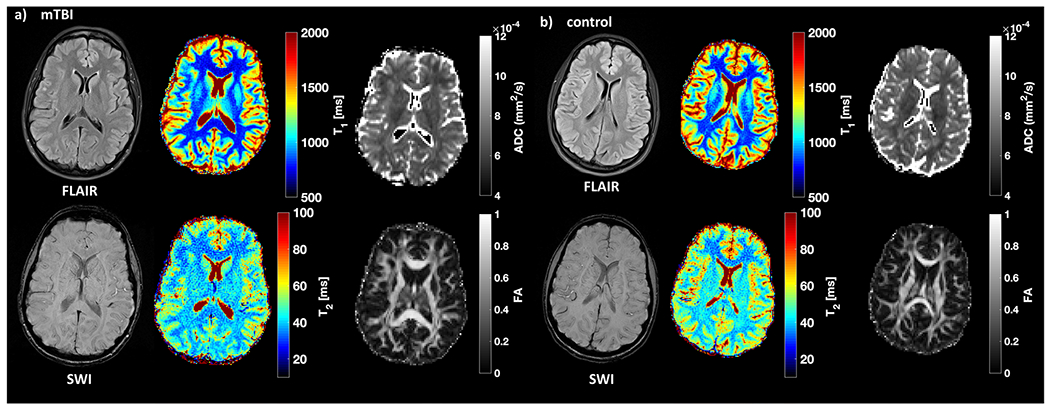
Examples of qualitative and quantitative images from a patient with mTBI and a matched control. Fluid-attenuation inversion recovery (FLAIR) and susceptibility-weighted images (SWI) as well as T1, T2, ADC, and FA maps from a 34-year-old female patient (a) at timepoint 1 and a 30-year-old female (b) control. Note the stark T1 and T2 difference between gray and white matter
Fig. 4.
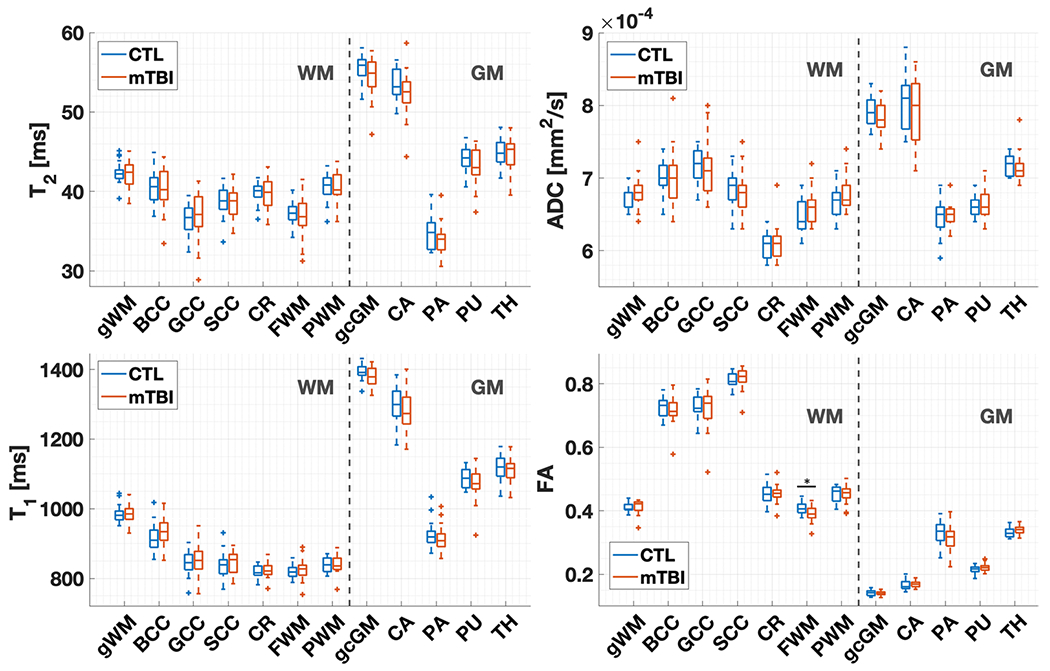
T2, T1, ADC, and FA distributions in mTBI (n = 19) and controls (n = 18) (CTL) at timepoint 1. As in the statistical analysis, the boxplots exclude the three non-age-matched patients. Note that T1 and T2 in mTBI did not differ from controls for any region. Compared to controls, FA in patients was lower in the frontal white matter (Mann–Whitney test, p < 0.05). The middle line in the boxes depicts the median value, and the boxes’ top and bottom edges the 25th and 75th percentiles of the data, respectively. The whiskers extend to the most extreme data points not considering outliers (95% of data), which are depicted by a plus sign. BCC body of corpus callosum, SCC splenium of the corpus callosum, GCC genu of the corpus callosum, FWM frontal white matter, PWM posterior white matter, CR corona radiata, CA caudate, PA pallidum, PU putamen, TH thalamus
Quantitative MRI measures at timepoint 2 and changes compared to timepoint 1
At timepoint 2, ADC and T1 in caudate and in global cortical GM were lower in mTBI compared to controls (all p < 0.05) (Table A.4). Statistically significant within-subject changes (value at timepoint 2 minus the value at timepoint 1, Δ 2–1) in MRI measures among patients who provided data at both timepoints are compiled in Table 3. FA in the body of the corpus callosum, corona radiata, thalamus, and global cortical GM dropped over time, while the T2 in the pallidum increased (all p < 0.05).
Table 3.
The mean, standard deviation (SD), median, and interquartile range (IQR) of the within-subject change in MRI measures among mTBI patients. The change was computed for each subject as the value at timepoint 2 minus the value at timepoint 1. Only patients providing data at both times were included in the computations. p-values are from the matched-pair Wilcoxon signed-rank test (WSRT) assessing whether the change was different from zero. Only measures with p < 0.05 are presented
| Measure | Region | Change (Δ 2–1) |
WSRT | |||
|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | p value | ||
| FA | Body of corpus callosum | −0.039 | 0.095 | −0.018 | 0.047 | 0.019 |
| Corona radiata | −0.008 | 0.014 | −0.01 | 0.017 | 0.020 | |
| Thalamus | −0.006 | 0.006 | −0.004 | 0.009 | 0.004 | |
| Global cortical GM | −0.013 | 0.028 | −0.005 | 0.014 | 0.014 | |
| T2 (ms) | Pallidum | 1.7 | 5.1 | 0.7 | 0.7 | 0.025 |
Cross-sectional relationships between MRI measures and patient outcome
All statistically significant correlations are presented in Tables A.5 and A.6, while a graphical summary of those for MRF is shown in Fig. 5.
Fig. 5.
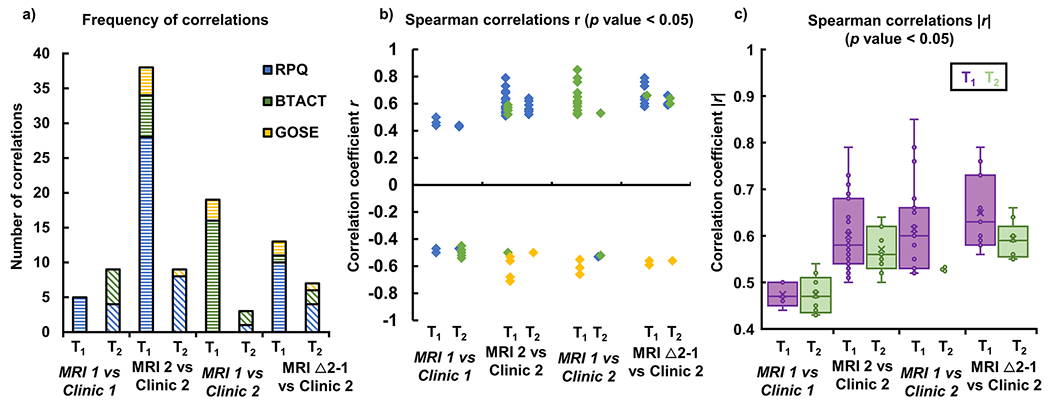
Frequency (a), directionality (b), and strength (c) of the correlations between T1 and T2 with patient outcome at timepoints 1 and 2. Shown are the statistically significant (p < 0.05) T1 and T2 Spearman correlations with subtests and/or total scores of the three assessments (RPQ, BTACT, GOSE): cross-sectional associations at timepoint 1 (MRI 1 vs clinic 1) and timepoint 2 (MRI 2 vs clinic 2); and predictive associations for clinical outcome at timepoint 2 of MRI at visit 1 (MRI 1 vs clinic 2), and the change in T1 and T2 from timepoint 1 to timepoint 2 (Δ 2–1) (MRI Δ 2–1 vs clinic 2). The associations in (a) are plotted with (b) the correlation coefficient (r) and (c) the absolute value of the correlation coefficient (|r|) on the y axis. Note that, in general, (i) T1 shows a higher frequency of correlations with clinical outcome, compared to T2; (ii) these correlations are positive for RPQ and BTACT, and negative for GOSE; and (iii) they are stronger than those seen for T2
At timepoint 1, the number of correlations and their average strength were similar amongst all MRI metrics (Table A.5). At timepoint 2, T1 showed the largest number of correlations, but their average strength was comparable to that of DTI (Table A.6). All MRI measures showed higher number, and stronger correlations with clinical outcome at timepoint 2, than at timepoint 1 (Table A.5 vs A.6).
While there was the same amount of T2 correlations at both timepoints, there were almost eight times more correlations for T1 at timepoint 2, compared to timepoint 1 (Fig. 5a). T1 and T2 correlated with the RPQ at both timepoints, and with the GOSE only at the second timepoint. Correlations with the BTACT were detected with T2 at timepoint 1, and with T1 at timepoint 2 (Fig. 5a). Apart from RPQ at timepoint 1, the directionality of these associations was consistent (Fig. 5b). Both relaxation times showed stronger associations with clinical measures at timepoint 2 than at timepoint 1 (Fig. 5c). The strongest associations (r > |0.7|) were between genu T1 and RPQ (Table A.6).
Predictive relationships between MRI measures and patient clinical outcome
The statistically significant correlations between MRI values at timepoint 1 and clinical outcome at timepoint 2, as well as those between the longitudinal change in MRI values (Δ 2–1) and clinical outcome at timepoint 2, are compiled in Table A.7. A graphical summary of the MRF correlations is shown in Fig. 5.
There were six times more correlations between T1 at timepoint 1 and clinical outcomes at timepoint 2, than between T2 at timepoint 1 and clinical outcomes at timepoint 2 (Fig. 5a). The longitudinal change in T1 showed two times more correlations with clinical outcome at timepoint 2, compared to the longitudinal change in T2 (Fig. 5a). Correlations with RPQ were dominant in the comparisons between the longitudinal change of relaxation times and clinical outcome at timepoint 2, while correlations with the BTACT were dominant in the comparisons between relaxation times at timepoint 1 and clinical outcome at timepoint 2 (Fig. 5a). The directionality of these associations was consistent: positive for RPQ and BTACT, and negative for GOSE (Fig. 5b). As shown in Fig. 5c, predictive relationships between MRF and clinical outcome were stronger for T1.
T1 showed the largest number of both types of correlations, and their average strength across all outcome measures was always higher than that of DTI (Table A.7). Out of the four strongest associations of any metric three were for genu T1 (Fig. 6).
Fig. 6.
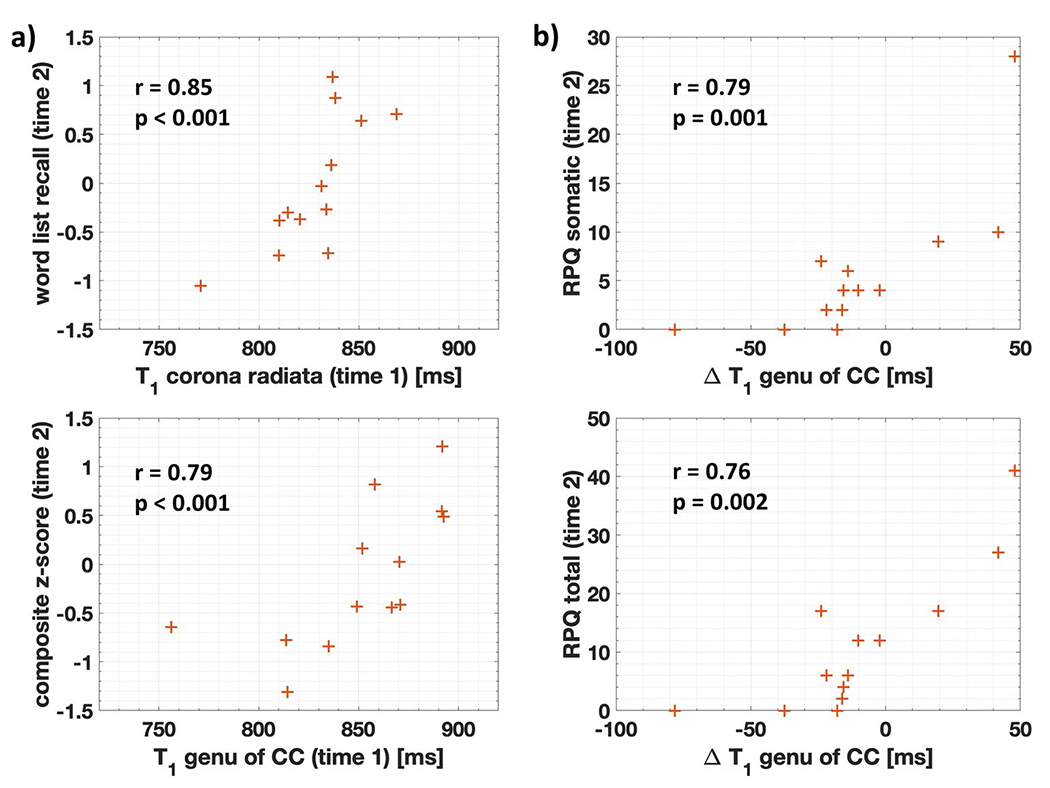
Highest predictive correlations with patient outcome at timepoint 2. (a) The two highest Spearman correlations r (shown with their p-values) for the association at timepoint 1 with clinical outcomes at timepoint 2 were found for T1. (b) The two highest Spearman correlations r (shown with their p-values) for the association of the change from timepoint 1 to timepoint 2 with clinical outcomes at timepoint 2 were also found for T1. Note that: (i) T1 at timepoint 1 correlates strongly with BTACT, while changes in T1 from timepoint 1 to timepoint 2 correlate strongly with RPQ; (ii) three of the correlations are for genu T1. CC corpus callosum
Patients were dichotomized according to their GOSE at timepoint 2 to recovered (n = 8) or non-recovered (n = 11). As shown in Table 4, the following measures obtained at timepoint 1 were found to be good discriminators (defined as AUC > 0.80) between recovered and non-recovered individuals at timepoint 2: T1 of the corona radiata, posterior WM, and splenium, as well as ADC of the corona radiata. The change from timepoint 1 to timepoint 2 as a predictor of recovery at timepoint 2 was a good discriminator for the T1 of the frontal WM and the genu.
Table 4.
MRI measures at timepoint 1, and within-subject change in MRI measures, with the highest utility for predicting timepoint 2 recovered and non-recovered status as assessed by GOSE. The p values from the logistic regression and the area under the receiver operating characteristic curve (AUC) to assess the utility of imaging measure at timepoint 1 and of the within-subject change as a predictor of recovery at timepoint 2 are presented, followed by the mean and standard deviation (SD) of both groups and p values from the Mann–Whitney (MW) test. Only measures in regions with an AUC ≥0.80 are presented
| Measure | Region | Logistic regression p value | AUC | No recovery (n = 8) |
Recovery (n = 11) |
MW p value | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| ADC (mm2/s) | Corona radiata | 0.049 | 0.80 | 0.62 | 0.01 | 0.6 | 0.01 | 0.023 |
| T1 (ms) | Corona radiata | 0.123 | 0.80 | 837 | 18 | 817 | 023 | 0.063 |
| Posterior WM | 0.067 | 0.83 | 859 | 19 | 831 | 026 | 0.035 | |
| Splenium of corpus callosum | 0.04 | 0.88 | 864 | 26 | 822 | 025 | 0.018 | |
| Change (Δ 2–1) in T1 (ms) | Frontal WM | 0.135 | 0.81 | 0.116 | 0.138 | −0.122 | 0.268 | 0.074 |
| Genu of corpus callosum | 0.176 | 0.83 | 0.113 | 0.3 | −0.28 | 0.238 | 0.051 | |
Discussion
Pre-clinical and limited human data suggest that quantification of relaxation times is a promising marker for TBI pathology and clinical outcomes. Here we evaluated the potential of quantitative T1 and T2 to predict patient outcome following mTBI. The major findings were as follows. MRF differences between patients and controls were absent at timepoint 1 and were limited at timepoint 2 to two regions of abnormal T1. DTI differences were found at the same frequency at timepoint 2 (two regions with abnormal ADC), while at timepoint 1, one region showed a difference in FA. The cross-sectional correlations of T1 and T2 with clinical outcome were similar in number and strength to those observed with DTI. However, both T1 at timepoint 1 and the serial change in T1 revealed more and stronger predictive correlations with clinical outcome 3 months after injury than did T2, ADC, or FA. Moreover, T1 of five regions enabled the identification of non-recovered patients at the follow-up visit with AUC > 0.80. ADC showed one association in the same AUC range, while T2 and FA showed none. There are three notable aspects of these predictive properties. First, they arose from T1 values, while T2 performed on par with DTI. Second, higher or serially increasing relaxation times were consistently linked to worse global functional (GOSE) and symptomatology (RPQ) outcome; the association of relaxation times with neuropsychological testing (BTACT) scores was clear only for T1, with low values linked to a worse score. Third, the prediction was largely related to relaxation times originating from WM, as expected given the putative effects of diffuse axonal injury in post-concussive symptomatology. T1 of the genu showed some of the strongest cross-sectional and predictive correlations with RPQ and BTACT, and a high AUC for the GOSE-defined outcome.
Our focus on the relaxation properties of water stems from previous work showing that they can be affected by cytoskeletal damage, inflammation, and edema [35, 36]. T1 and T2 relaxation depend on the concentration of macromolecules and the level of binding between them and water. Specifically, water protons in the vicinity of macromolecules have short T1 and T2 [37]. Previous pre-clinical studies [10–12] reported elevated T2, which was explained by vasogenic edema. In addition, elevated T1ρ and T2 following resolution of edema were predictive of long-term outcome, possibly due to progressive neuronal loss [11]. The two previous investigations in human TBI also reported elevated T2, including in normal-appearing WM [13, 14]. Our study did not find conclusive evidence for altered relaxation times in normal-appearing tissue, but our cohort consisted only of mTBI patients, while the previous studies consisted of mostly moderate and severe TBI [13, 14]. Importantly, while the prior literature reports change exclusively in T2, we found stronger correlations with clinical outcome for T1. The directionality of these associations was consistent, with higher, or increasing values associated with worse outcome in symptoms and global functioning, and lower values associated with worse neuropsychological testing scores. The question therefore arises, whether, similarly to FA, higher and lower relaxation values reflect different pathologies, which in turn can be linked to different functional domains. Conjecturing any further based on our data, however, including as to what biological processes higher and lower values may represent, is speculative, especially given the lack of convincing evidence that relaxation times are altered compared to controls.
The results must be viewed in the context of the following limitations. First, multiple comparison corrections were not performed. Due to the lack of previous relaxation time studies in mTBI, this project qualified as exploratory, and therefore, we aimed to uncover the most true findings, with the understanding that type I errors were not controlled, and the results require replication. We therefore report on overall trends and avoid giving disproportionate weight to individual findings. Our statistical approach, however, may not be appropriate for DTI, given the vast body of prior literature. Therefore, we use the DTI results solely for comparison to MRF and refrain from comparing them to past DTI studies. Second, DTI did not show widespread changes, and only two patients had findings on qualitative MR images. It is possible that MRF shows better utility in differentiating controls from patients who show more macro- and micro-structural injury. Third, SWI at 7 T would be better suited to answer the question of whether relaxation times are related to subtle micro-bleeds, which are more readily identified at ultra-high field [38, 39]. As more 7 T systems become available for clinical use, quantitative MR modalities, such as MRF, should also be utilized in TBI to test whether they show increased sensitivity. Fourth, scanning occurred within a wide range of time around the 1- and 3-month timepoints. The pathological process post-TBI is dynamic [40], which could have potentially decreased the sensitivity of MRF and DTI. Finally, the findings of this study should be interpreted in the context of the study design, the patient population, and the limited sample size. The findings that MRF is prognostically better than DTI needs further validation on a larger population.
Conclusion
MRF-based relaxometry, which enables collection of water relaxation times within clinical scan timeframes, revealed no widespread differences in T1 and T2 between patients and controls at 1 and at 3 months post-mTBI. However, WM T1 at 1 month and the change of WM T1 from 1 to 3 months were linked to clinical and neurocognitive impairments at 3 months after injury. These correlations were stronger than those for T2 and stronger than those for FA and ADC from DTI and motivate studies to determine whether MRF can provide a differential increase in prognostic accuracy beyond qualitative MRI and DTI.
Supplementary Material
Key Points.
In a region-of-interest approach, FA, ADC, and T1 and T2 all showed limited utility in differentiating patients from controls at an average of 24 and 90 days post-mild traumatic brain injury.
T1 at 24 days, and the serial change in T1, revealed more and stronger predictive correlations with clinical outcome at 90 days than did T2, ADC, or FA.
T1 showed better prospective identification of non-recovered patients at 90 days than ADC, T2, and FA.
Acknowledgements
We thank all participants for their time and interest in this study.
Funding
This study has received funding by the United States’ National Institutes of Health (NIH) through grants numbers R01NS097494 and R01EB026456.
Abbreviations
- BTACT
Brief Test of Adult Cognition by Telephone
- DTI
Diffusion tensor imaging
- GM
Gray matter
- GOSE
Glasgow outcome scale – extended
- MRF
Magnetic resonance fingerprinting
- mTBI
Mild traumatic brain injury
- RPQ
Rivermead post-concussion symptoms questionnaire
- T1
Longitudinal relaxation times
- T2
Transverse relaxation times
- WM
White matter
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00330-021-08235-8.
Guarantor The scientific guarantor of this publication is Ivan Kirov, Ph.D.
Conflict of interest The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry One of the authors has significant statistical expertise (James Babb, Ph.D.).
Informed consent Written informed consent was obtained from all subjects in this study.
Ethical approval Institutional Review Board approval was obtained for this study.
Study subjects or cohorts overlap The subject cohorts have been previously reported on in the following publication: Gerhalter et al, Global decrease in brain sodium concentration after mild traumatic brain injury. Brain Communications, Volume 3, Issue 2, 2021, https://doi.org/10.1093/braincomms/fcab051.
The overlap is mentioned in the manuscript and the details are thoroughly described in the Supplemental Materials section.
Methodology
• prospective
• cross-sectional study/diagnostic or prognostic study.
• performed at one institution.
References
- 1.Dang B, Chen W, He W, Chen G (2017) Rehabilitation treatment and progress of traumatic brain injury dysfunction. Neural Plast 2017:1–6. 10.1155/2017/1582182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beran R, Bhaskar S (2018) Concussion within the military. J Mil Veterans Health 26:20–27 [Google Scholar]
- 3.Maas AIR, Menon DK, Adelson PD et al. (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16:987–1048. 10.1016/S1474-4422(17)30371-X [DOI] [PubMed] [Google Scholar]
- 4.Wang KK, Yang Z, Zhu T et al. (2018) An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn 18:165–180. 10.1080/14737159.2018.1428089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blennow K, Brody DL, Kochanek PM et al. (2016) Traumatic brain injuries. Nat Rev Dis Prim 2:16084. 10.1038/nrdp.2016.84 [DOI] [PubMed] [Google Scholar]
- 6.Bešsenski N (2002) Traumatic injuries: imaging of head injuries. Eur Radiol 12:1237–1252. 10.1007/s00330-002-1355-9 [DOI] [PubMed] [Google Scholar]
- 7.Shenton ME, Hamoda HM, Schneiderman JS et al. (2012) A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 6:137–192. 10.1007/sll682-012-9156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hergan K, Schaefer P, Sorensen A et al. (2002) Diffusion-weighted MRI in diffuse axonal injury of the brain. Eur Radiol 12:2536–2541. 10.1007/s00330-002-1333-2 [DOI] [PubMed] [Google Scholar]
- 9.Wallace EJ, Mathias JL, Ward L (2018) The relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: a meta-analysis. Neurosci Biobehav Rev 92:93–103. 10.1016/j.neubiorev.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 10.Li W, Watts L, Long J et al. (2016) Spatiotemporal changes in blood-brain barrier permeability, cerebral blood flow, T2 and diffusion following mild traumatic brain injury. Brain Res 1646:53–61. 10.1016/j.brainres.2016.05.036 [DOI] [PubMed] [Google Scholar]
- 11.Immonen RJ, Kharatishvili I, Gröhn H et al. (2009) Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage 45:1–9. 10.1016/j.neuroimage.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 12.Kharatishvili I, Sierra A, Immonen RJ et al. (2009) Quantitative T2 mapping as a potential marker for the initial assessment of the severity of damage after traumatic brain injury in rat. Exp Neurol 217:154–164. 10.1016/j.expneurol.2009.01.026 [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Gupta RK, Rao SB et al. (2003) Magnetization transfer and T2 quantitation in normal appearing cortical gray matter and white matter adjacent to focal abnormality in patients with traumatic brain injury. Magn Reson Imaging 21:893–899. 10.1016/S0730-725X(03)00189-9 [DOI] [PubMed] [Google Scholar]
- 14.Mamere AE, Saraiva LAL, Matos ALM et al. (2009) Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques. Am J Neuroradiol 30:947–952. 10.3174/ajnr.A1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini D, Iacono D, Komlosh ME et al. (2021) Diffuse axonal injury has a characteristic multidimensional MRI signature in the human brain. Brain 144:800–816. 10.1093/brain/awaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma D, Gulani V, Seiberlich N et al. (2013) Magnetic resonance fingerprinting. Nature 495:187–192. 10.1038/naturell971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badve C, Yu A, Dastmalchian S et al. (2017) MR fingerprinting of adult brain tumors: initial experience. Am J Neuroradiol 38:492–499. 10.3174/ajnr.A5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keil VC, Bakoeva SP, Jurcoane A et al. (2019) MR fingerprinting as a diagnostic tool in patients with frontotemporal lobe degeneration: a pilot study. NMR Biomed 32:1–13. 10.1002/nbm.4157 [DOI] [PubMed] [Google Scholar]
- 19.Kay T, Harrington DE, Adams R (1993) American Congress of Rehabilitation Medicine, Head Injury Interdisciplinary Special Interest Group. Definition of mild traumatic brain injury. J Head Trauma Rehabil 8:86–87 [Google Scholar]
- 20.Gerhalter T, Chen AM, Dehkharghani S et al. (2021) Global decrease in brain sodium concentration after mild traumatic brain injury. Brain Commun 3. 10.1093/braincomms/fcab051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King NS, Crawford S, Wenden FJ et al. (1995) The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242:587–592. 10.1007/BF00868811 [DOI] [PubMed] [Google Scholar]
- 22.Wilson JT, Pettigrew LE, Teasdale GM (1998) Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 15:573–585. 10.1089/neu.1998.15.573 [DOI] [PubMed] [Google Scholar]
- 23.Tun PA, Lachman ME (2006) Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing 35:629–632. 10.1093/ageing/af1095 [DOI] [PubMed] [Google Scholar]
- 24.Cloos MA, Assländer J, Abbas B et al. (2019) Rapid Radial T1 and T2 Mapping of the Hip Articular Cartilage With Magnetic Resonance Fingerprinting. J Magn Reson Imaging 50:810–815. 10.1002/jmri.26615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haacke EM, Duhaime AC, Gean AD et al. (2010) Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging 32:516–543. 10.1002/jmri.22259 [DOI] [PubMed] [Google Scholar]
- 26.Hulkower MB, Poliak DB, Rosenbaum SB et al. (2013) A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am J Neuroradiol 34:2064–2074. 10.3174/ajnr.A3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B (2012) FreeSurfer. Neuroimage 62:774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusinek H, Glodzik L, Mikheev A et al. (2013) Fully automatic segmentation of white matter lesions: error analysis and validation of a new tool. Intern J Comput Assist Radiol Surg 8:289–291 [Google Scholar]
- 29.Mikheev A, Nevsky G, Govindan S et al. (2008) Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J Magn Reson Imaging 27:1235–1241. 10.1002/jmri.2f372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkinson M, Beckmann CF, Behrens TEJ et al. (2012) FSF Neuroimage 62:782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 31.Tournier J-D, Smith R, Raffelt D et al. (2019) MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- 32.Yu Z, Zhao T, Assländer J et al. (2018) Exploring the sensitivity of magnetic resonance fingerprinting to motion. Magn Reson Imaging 54:241–248. 10.1016/j.mri.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyres S, Carey A, Gilworth G et al. (2005) Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin Rehabil 19:878–887. 10.1191/0269215505cr905oa [DOI] [PubMed] [Google Scholar]
- 34.Smith-Seemiller L, Fow NR, Kant R, Franzen MD (2003) Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj 17:199–206. 10.1080/0269905021000030823 [DOI] [PubMed] [Google Scholar]
- 35.Giza CC, Hovda DA (2014) The new neurometabolic cascade of concussion. Neurosurgery 75:S24–S33. 10.1227/NEU.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang D, Bhatta S, Gerzanich V, Simard JM (2007) Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg Focus 22:1–9. 10.3171/foc.2007.22.5.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edzes HT, Samulski ET (1977) Cross relaxation and spin diffusion in the proton NMR or hydrated collagen. Nature 265:521–523. 10.1038/265521a0 [DOI] [PubMed] [Google Scholar]
- 38.Moenninghoff C, Kraff O, Maderwald S et al. (2015) Diffuse axonal injury at ultra-high field MRI. PLoS ONE 10:e0122329. 10.1371/journal.pone.0122329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hütter BO, Altmeppen J, Kraft O et al. (2020) Higher sensitivity for traumatic cerebral microbleeds at 7 T ultra-high field MRI: is it clinically significant for the acute state of the patients and later quality of life? Ther Adv Neurol Disord 13:1–12. 10.1177/1756286420911295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Povlishock JT, Katz DI (2005) Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 20:76–94. 10.1097/00001199-200501000-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


