Abstract:
Background: The diminishing antimicrobial options for the treatment of XDR and PDR Acinetobacter baumannii is an increasing concern. In this study, we assessed the in vitro synergy of the fosfomycin (FOS) with meropenem (MEM), amikacin (AK), tigecycline (TGC), and colistin (CL) in whole genome sequenced isolates. Methods: Non-replicate whole genome sequenced (illumina next-generation sequencing platform, Clevergene, India), A. baumanii (7 XDR, 1PDR) were subjected to in vitro synergy testing by checkerboard (CB) and time kill assay (TKA) after MIC determination, with glucose-6-phosphate being incorporated in all runs. FOS was used as a cornerstone drug in four combinations and colistin in one. ResFinder, MLST, PlasmidFinder, and CSIPhylogeny tools were used. Results: Mortality occurred in three patients. Diverse MLST were observed, ST-1962 (3 isolates) and one each of ST2062, ST2063, ST1816, ST1806, ST234. FOS MICs ranged from 32 to 128 mg/L, MEM MIC: 16–64 mg/L, TGC MIC: ≤2–≤4 mg/L and AK MIC: >512 mg/L. CL: MIC range, 0.25–≤2 mg/L, PDR MIC > 16 mg/L. Synergy results by CB: FOS-MEM: synergy in ⅞ (90%) isolates. Synergy lowered MEM MICs to susceptibility breakpoints in 6/8 cases. CL-MEM: Excellent synergy (3/3) isolates. FOS-AK: Indifference in ⅞, antagonism ⅛ (AK-susceptible isolate). FOS-TGC: Partial synergy (PS) in 8/8 (TGC MIC dropped to ≤0.25 mg/L in 3/8). In the PDR isolate, synergy was seen in FOS-MEM, CL-MEM, PS in FOS-CL, FOS-TGC, indifference in FOS-AK. TKA: Excellent synergy was observed with FOS-MEM from 4 h, while FOS-AK and FOS-TGC demonstrated synergy at 24 h. Synergy was achieved despite presence of widespread resistance markers against aminoglycosides (AacAad, AadA, AadB, Aph3″Ia, ArmA, Arr, StrA, StrB), beta-lactams (ADC, BlaA1, BlaA2, Zn-dependent_hydrolase, OXA-23, OXA-51, PER-1,TEM-1D, CARB-5, Mbl), sulphonamides (SulII, SulI), phenicols (CatBx, CmlA), macrolides (MphE, MsrE) and tetracycline (TetB) were widespread. Carbapenemase, CARB-5 was present in one isolate. Beta-lactamase genes OXA-23, OXA-51, BlaA2, Zn-dependent_hydrolase, ADC, Mbl and macrolide resistance genes MphE, MsrE were present in all 8 isolates. Conclusions: FOS-MEM and CL-MEM are promising combinations against A. baumannii. Synergy of FOS-MEM in intrinsically resistant A. baumannii shows that this antibiotic combination might be useful in treating such XDR and PDR pathogens.
Keywords: in vitro synergy, Acinetobacter baumannii, checkerboard assay, meropenem, time kill assay, fosfomycin
1. Introduction
Acinetobacter baumannii, an aerobic, Gram-negative, opportunistic coccobacilli, is frequently linked to nosocomial infections and is a predominant pathogen in the intensive care unit (ICU). It has the potential to cause difficult to treat bacteremia, ventilator-associated pneumonia, meningitis, and urinary tract infections due to the acquisition of multiple drug resistance [1]. Multiple pathways are responsible for the acquisition of antimicrobial resistance [2]. Conventional antimicrobials are frequently rendered ineffective by multiple inherent and acquired resistance mechanisms, which leads to the rise of extensively drug-resistant (XDR) strains [3]. An isolate is classified as XDR when it is non-susceptible to at least one agent in all but two or fewer antimicrobial categories [3]. Pan drug-resistance (PDR) implies resistance to all agents in all antimicrobial classes [3]. Due to the paucity of new antimicrobials, finding effective antibiotic combinations to treat XDR and PDR A. baumanii infections is crucial. Extended and pan drug-resistant (XDR and PDR) strains of A. baumannii are of particular concern as they are resistant to nearly all available antibiotics, leaving clinicians with limited treatment options. Exploring synergistic combinations of antibiotics is an important area of research for combating XDR and PDR A. baumannii strains. Synergistic combinations of antibiotics involve using two or more drugs that work together to enhance their effectiveness, allowing for better control of bacterial growth and reducing the likelihood of antibiotic resistance. This approach has the potential to improve treatment outcomes for patients with XDR and PDR A. baumannii infections and can help slow the spread of antibiotic-resistant strains [4]. Tests of in vitro antimicrobial synergy can provide important insight into which combinations will be successful in treating these challenging infections. Fosfomycin’s exceptional features, such as its ability to reach high plasma concentrations and penetrate tissues effectively, low cross-resistance [5], and absence of nephrotoxicity [6], make it a desirable primary medication to use in conjunction with other antibiotics. Moreover, in recent years, whole genome sequencing (WGS) has emerged as a powerful tool for identifying genetic mechanisms underlying antibiotic resistance and identifying potential new treatment strategies This study aimed to evaluate the in vitro synergistic interactions of fosfomycin with meropenem, colistin, tigecycline, and amikacin against XDR and PDR Acinetobacter baumannii using checkerboard and time-kill assays. We further assessed whether synergy would lead to lowering of the MICs to clinical breakpoints or below.
2. Material and Methods
A ten-month study, spanning from September 2019 to June 2020, this study was carried out at the Sultan Qaboos University Hospital’s Department of Microbiology and Immunology in collaboration with the Sultan Qaboos University Hospital’s Department of Medicine in Muscat, Sultanate of Oman, the Department of Medical Microbiology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India. Prior to the start of the study, approval was obtained from the Medical Research Ethics Committee of the College of Medicine and Health Sciences at Sultan Qaboos University, Muscat, Oman.
Eight non-duplicate genotyped (7 XDR and 1 PDR) strains of Acinetobacter baumannii recovered from urinary tract, wound, respiratory tract, and bloodstream infections were assessed for synergy and molecular determinants of resistance. In vitro synergy testing by checkerboard (CB) and time kill assay (TKA) was performed after MIC determination, with glucose-6-phosphate being incorporated in all runs. FOS was used as a cornerstone drug in four combinations and colistin in one. Cepheid XpertCarba-R assay was used for preliminary genotypic characterization (Cepheid, Sunnyvale, CA, USA). The strains were cryopreserved and kept at −40 °C in sterile CryoBeads (Mast Diagnostics, UK) that included glycerol and a hypertonic additive along with a cryopreservative fluid.
2.1. Whole Genome Sequencing (WGS)
The genomic DNA of all 8 A. baumanii isolates was purified using a Qiagen kit (QIAquick PCR and Gel Cleaning Kit, 2018) in accordance with the manufacturer’s instructions after being isolated from 18 to 24 h old cultures using the traditional phenol-chloroform procedure [4]. For whole genome sequencing, extracted DNA was transferred to Clevergene Biocorp Pvt Ltd., Bangalore, India (Illumina next-generation sequencing). The Centre for Genomic Epidemiology, 2020 website’s tools were used to retrieve the sequences and analyze them. The acquired antimicrobial resistance genes, multilocus sequence typing (MLST), plasmids, and bacterial relatedness were all investigated using the ResFinder, MLST, PlasmidFinder, and CSIPhylogenyprogrammes, respectively. By locating and filtering high-quality SNPs, the CSIPhylogeny programme was able to detect differences between the generated sequence data (FASTA files) (z-score higher than 1.96 for all SNPs). The CARD bioinformatics, 2020 website was used to detect resistance genes.
2.2. MIC Determination
The following powders were purchased from Sigma-Aldrich Chemical Co., Saint Louis, MO, USA: fosfomycin (FOS), colistin sulphate (CL), amikacin (AK); meropenem (MEM), from the United States Pharmacopoeia, and tigecycline (TGC), from the European Pharmacopoeia, and stored at 4 °C or <−20 °C until use as per the manufacturers’ recommendations. The following strains ATCC 25922 (Escherichia coli), ATCC 27853 (Pseudomonas aeruginosa), and ATCC 29212 (Enterococcus faecalis) were used for quality control. The MICs were determined by two methods: the agar dilution method (AD) for fosfomycin on MHA plates (150-mm diameter) supplemented with 25 mg glucose-6-phosphate (G-6-P/L) and the broth microdilution (BMD) method (Becton Dickinson) against FOS, AK, MEM, TGC, and CL as described by CLSI guidelines (M07-A10, Vol.35 No.2, 2015) [7].
2.3. Antimicrobial Synergy Testing
Synergy testing was performed with FOS as the cornerstone drug: FOS-MEM, FOS-CL, FOS-TGC, and FOS-AK. We used the checkerboard assay for all combinations and the time-kill assay for representative strains for verifying our results as per Rizvi et al., 2013 [8].
2.4. Checkerboard Assay
The broth microdilution checkerboard (BMC) assay was conducted in duplicate. The MIC for each isolate determined the range of antibiotic concentrations to be utilised in the checkerboard assay. The concentration of the antibiotics ranged from ≤1/32 × MIC to 1 × MIC. FICI = FIC A in combination/MIC A alone, FIC B = MIC B in combination/MIC B alone, and FICI = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone) were used to calculate the type of interactions between the antimicrobials. FICI of 0.5 implied synergy, FICI > 0.5 to 1, partial synergism, and FICI > 1 to 4 indicated indifference, and FICI > 4 antagonism [7].
2.5. Time-Kill Assay
The time-kill assay is a dynamic assessment of the effect of the antimicrobial combination on the bacterial strain at several time periods (2, 4, 6, 24 h), while there is just a one-time assessment in checkerboard. It sheds light on whether the interaction is bactericidal, bacteriostatic, or if regrowth occurred at 24 h. To confirm the synergistic or additive reactions observed using the checkerboard approach as previously described, TKA was conducted in representative strains [7]. The time-kill curves were created by plotting the bacterial cell counts for a. growth control, b. each individual antibiotic, and c. the antimicrobial combination against time. A 2-log10 CFU/mL decrease in bacterial growth signified synergism, antagonism was defined as a 2-log10 CFU/mL increase in bacterial growth in the combination compared to the highest active single drug, while indifference was defined as a <2-log10 increase or decrease in colony count. A 3-log10 CFU/mL drop in bacterial counts was considered a bactericidal outcome [9].
2.6. Statistical Analysis
The IBM Statistical Package for the Social Sciences (SPSS) was used to analyse the data. Paired sample t-test was used to determine whether the MIC reduction in the combination interaction (alone vs combined) was significant. A p-value of ≤0.05 was considered statistically significant.
3. Results
The XDR and PDR A. baumannii were isolated from the respiratory tract (n = 5), wound (n = 3), and bloodstream (n = 1) infections. Male patients predominated (n = 6/8), of which three expired.
3.1. Minimal Inhibitory Concentrations (MIC) of Fosfomycin, Meropenem, Tigecycline, Colistin and Amikacin in Acinetobacter baumannii
Fosfomycin MIC ranged from 32 to ≥128 mg/L (Table 1), MEM MIC: 16–64 mg/L, TGC MIC: ≤2 to ≤4 mg/L and AK MIC: >512 mg/L. CL: MIC range, 0.25 to ≤2 mg/L, PDR MIC > 16 mg/L. As per EUCAST, the MICs of fosfomycin for all but two isolates were in the resistant range (>32 mg/L) by agar dilution. All isolates were resistant to MEM (16–64 mg/L). All isolates except one were resistant to TGC (2 to ≥4 mg/L) and AK (MIC >512 mg/L). All isolates were susceptible to colistin (MIC range, 0.25 to ≤2 mg/L) except for the PDR isolate (16 mg/L).
Table 1.
Minimum inhibitory concentrations (MICs) of fosfomycin, meropenem, tigecycline, colistin, and amikacin against A. baumannii isolates (n = 8).
| Isolate | MIC Value (mg/L) | |||||
|---|---|---|---|---|---|---|
| Fosfomycin | Meropenem | Tigecycline | Amikacin | Colistin | ||
| AD * | BMD # | BMD # | BMD # | BMD # | BMD # | |
| Ab1 (PDR) | 128 | 128 | 16 | 2 | >512 | 16 |
| Ab 2 | 128 | 64 | 32 | 2 | >512 | 0.5 |
| Ab 3 | 128 | ≤128 | 32 | 4 | >512 | 1 |
| Ab 4 | 32 | 64 | 32 | 4 | >512 | 1 |
| Ab 5 | 32 | 64 | 32 | 2 | >512 | 1 |
| Ab 6 | ≤128 | 128 | 32 | 4 | >512 | 1 |
| Ab 7 | 64 | 32 | 32 | 4 | >512 | 0.25 |
| Ab 8 | 64 | 64 | 64 | ≤0.5 | ≤8 | ≤1 |
* AD: agar dilution, # BMD: broth microdilution, Ab: A. baumannii.
3.2. Synergy Outcomes
Synergy (SY), partial synergy (PS), indifference (IN), and antagonism (AN) against fosfomycin in different antimicrobial combinations is shown in Table 2. FOS-MEM combination showed synergy in ⅞ (90%) isolates. Synergy lowered MEM MICs to susceptibility breakpoints in 6/8 cases. CL-MEM combination showed excellent synergy (3/3) isolates. FOS-AK combination showed indifference in ⅞ and antagonism in an AK-susceptible isolate. FOS-TGC combination showed partial synergy in all isolates (TGC MIC dropped to ≤0.25 mg/L in 3/8 isolates).
Table 2.
Outcomes of various antibiotic combinations using the Checkerboard assay in XDR and PDR A. baumannii isolates (n = 8).
| Isolate | Fos MIC (mg/L) | Fold Decline | MEM MIC (mg/L) | Fold Decline | FICI (x̄) | ||
|---|---|---|---|---|---|---|---|
| Alone | Combined with MEM | Alone | Combined with FOS | ||||
| Ab1 | 128 | 32 | 4 | 16 | 4 | 4 | 0.50 (S) |
| Ab2 | 64 | 1 | ≥64 | 32 | 16 | 2 | 0.52 (PS) |
| Ab3 | 64 | 16 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab4 | 64 | 16 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab5 | 64 | 16 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab6 | 128 | 32 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab7 | 32 | 8 | 4 | 32 | 8 | 4 | 0.50 (S) |
| Ab8 | 64 | 16 | 4 | 64 | 16 | 4 | 0.50 (S) |
| FOS MIC (mg/L) | AK MIC (mg/L) | ||||||
| alone | Combined with AK | alone | Combined with FOS | ||||
| Ab1 | 128 | 128 | 0 | >1024 | ≤8 | ≥256 | 1.00 (IN) |
| Ab2 | 128 | 128 | 0 | >1024 | ≤8 | ≥256 | 1.00 (IN) |
| Ab3 | 128 | 128 | 0 | >1024 | ≤8 | ≥256 | 1.00 (IN) |
| Ab4 | 64 | 64 | 0 | 1024 | ≤4 | ≥256 | 1.00 (IN) |
| Ab5 | 64 | 64 | 0 | 1024 | ≤4 | ≥256 | 1.00 (IN) |
| Ab6 | 128 | 128 | 0 | 1024 | ≤4 | ≥256 | 1.00 (IN) |
| Ab7 | 32 | 32 | 0 | >1024 | ≤4 | ≥512 | 1.00 (IN) |
| MEM MIC (mg/L) | CL MIC (mg/L) | ||||||
| alone | Combined with COL | alone | Combined with MEM | ||||
| Ab 1 | 16 | 0.5 | 32 | 8 | ≤1 | ≥8 | 0.16 (S) |
| Ab 2 | 32 | 4 | 8 | ≤1 | 0.25 | 4 | 0.37 (S) |
| Ab 3 | 32 | 8 | 4 | 1 | 0.25 | 4 | 0.50 (S) |
| FOS MIC (mg/L) | TGC MIC (mg/L) | ||||||
| alone | Combined with TGC | alone | Combined with FOS | ||||
| Ab 1 | 128 | ≤2 | ≥64 | 2 | 1 | 2 | 0.52 |
| Ab 2 | 128 | ≤4 | ≥32 | 2 | 1 | 2 | 0.53 |
| Ab 3 | 128 | ≤4 | ≥32 | 4 | 2 | 2 | 0.53 |
| Ab 4 | 64 64 |
≤1 32 |
≥64 2 |
4 4 |
2 0.25 |
2 16 |
0.52 0.56 |
| Ab 5 | 64 64 |
≤1 32 |
≥64 2 |
2 2 |
1 ≤0.06 |
2 ≥32 |
0.52 0.53 |
| Ab 6 | 128 128 |
≤1 64 |
≥128 2 |
4 4 |
2 ≤0.06 |
2 ≥64 |
0.51 0.52 |
| Ab 7 | 32 | ≤1 | ≥32 | 4 | 2 | 2 | 0.53 |
Abbreviation: Ab = A. baumannii, FICI = fractional inhibition concentration index, x̄ = mean value, S = synergy, PS = partial synergy, MIC = minimal inhibition concentration, x̄ = mean value. FOS, fosfomycin; IMI, imipenem; MEM, meropenem; CL, colistin; AK, amikacin; TGC, tigecycline; synergy (SY), partial synergy (PS), indifference (IN) and antagonism (AN).
3.3. Outcome of Fosfomycin-Meropenem Combination by Checkerboard Assay
Out of 8 isolates, 7 (88%) showed effective synergistic interactions at 0.25 MIC FOS + 0.25 MIC MEM (Table 2). Although the synergistic interactions could not bring down the MEM MICs to the susceptible range, i.e., ≤2 mg/L, it did drop to 8–16 mg/L.
3.4. Assessment of Fosfomycin-Meropenem Interactions of A. baumannii by Time-Kill Assay
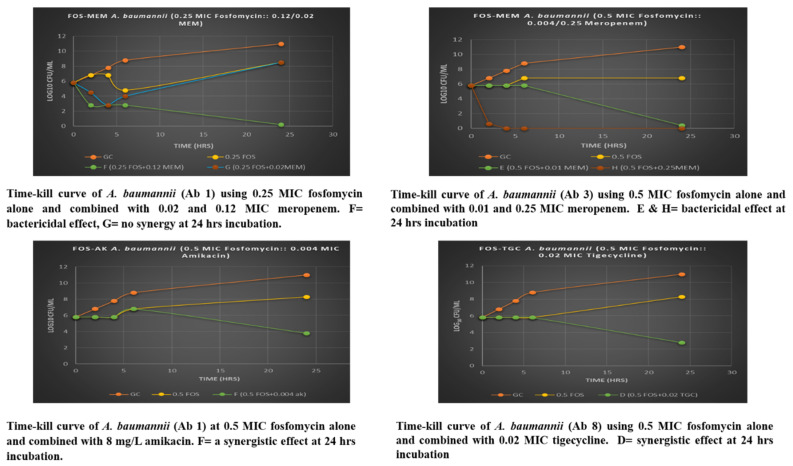
On assessing FOS-MEM by TKA in the representative strain, excellent synergy was observed with FOS-MEM from 4 h onwards, while synergy was observed from 24 h onwards with FOS-AK and FOS-TGC (Figure 1). Synergy was observed despite the widespread presence of resistance markers. Time-kill assay was conducted to confirm the observed synergy at 0.25 MIC FOS and partial synergy at 0.5 MIC FOS with extremely low meropenem (0.5 mg/L). Bacterial count in the growth control increased gradually by one log10 every 2 h through 6-h incubation and more than five log10 at 24 h. At 1 MIC, fosfomycin alone and in combination with 0.5 MIC meropenem (64 mg/L) reduced the bacterial growth to zero counts at all time intervals.
Figure 1.
Time Kill Analysis of A. baumannii with fosfomycin alone and in combination with meropenem, amikacin and tigecycline.
The antimicrobial effect of the combination was proportionally related to a higher meropenem concentration. The bactericidal activity increased greatly at 0.25 MIC fosfomycin with a higher dose of meropenem (0.12 MIC MEM = 16 mg/L) and demonstrated bactericidal effect at 2–24 h incubation (Table 3). The ratio of 0.25 MIC fosfomycin with low meropenem concentration (2 mg/L) had a synergistic effect at 2 h and bactericidal at 4 h; however, the growth increased significantly to 2–3 log10 at 24 h. The combination was more potent and its effect was further augmented in the ratio of 0.5 MIC FOS and a high MEM dose (0.25 MEM MIC= 32 mg/L), which brought down the bacterial population to constant zero counts at 4–24 h incubation. At an extremely low meropenem concentration (0.01 MIC MEM= 0.5 mg/L), it was interesting to observe that there was bactericidal activity at 24 h incubation.
Table 3.
Summary of time-kill and checkerboard assessments of different combinations in different bacteria (n = 8).
| Combination | [(FOS) + (MEM)] mg/L | 6-h Effect | 24-h Effect | 24-h log10 Killing ∆ 24 h | Checkerboard |
|---|---|---|---|---|---|
| Fosfomycin + Meropenem | Isolate Ab 3 | ||||
| 0.25 FOS + 0.02 MEM | (32, 2) | Synergy | None | >+2 | Growth |
| 0.25 FOS + 0.12 MEM | (32, 8) | Bactericidal | Bactericidal | >−5 | Synergy |
| 0.5 FOS + 0.01 MEM | (64, 0.5) | None | Bactericidal | >−5 | Growth |
| 0.5 FOS + 0.25 MEM | (64, 16) | Bactericidal | Bactericidal | −6 | Partial synergy |
| Fosfomycin + Amikacin | Isolate Ab 1 | ||||
| 0.5 FOS + 0.004 AK | (64, 8) | None | Synergy | −2 | Growth |
| Fosfomycin + Tigecycline | Isolate Ab 4 | ||||
| 0.5 FOS + 0.02 TGC | (64, 0.06) | None | Bactericidal | −3 | Partial synergy |
Abbreviation: FOS = fosfomycin, MEM = meropenem, TGC = tigecycline, AK = amikacin, Ab = A. baumannii, Bactericidal effect = ≥3 log10 reduction in CFU/mL after 24 h compared with the starting inoculum (0 h). Synergy = ≥2 log10 reduction in CFU/mL after 24 h compared with in the antibiotic alone. Difference (∆) in bacterial concentration in log10 CFU/mL at 24 h compared with the starting inoculum. Bactericidal effect (≥3 log10 reduction in CFU/mL after 24 h) and synergy (≥2 log10 reduction in CFU/mL at 24 h with the combination as compared with the most active single drug). Checkerboard FICI values of x ≤ 0.5 (synergism) and 0.5 < x < 1 (partial synergy).
3.5. Synergy in PDR Strains
PDR strain (Ab1) demonstrated excellent synergy in FOS-MEM, CL-MEM, PS in FOS-CL, FOS-TGC and indifference in FOS-AK (Table 2).
3.6. Identification of STs
The isolates surprisingly exhibited varying MLSTs as seen in Table 4, except for two isolates, which belonged to ST-1962; the other five had a different MLST (ST2062, ST2063, ST1816, ST1806, ST234).
Table 4.
Multi-Locus Sequence Typing (MLST) of A. baumannii isolates (n = 8).
| Sample | ST | gltA | gyrB | gdhB | recA | cpn60 | gpi | rpoD | Mismatches | Uncertainty | Depth | maxMAF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab1 | 2062 | 1 | 17 | 189 | 2 | 2 | 108 | 3 | 0 | - | 197.8256 | 0.016667 |
| Ab2 | 2063 | 1 | 17 | 3 | 2 | 2 | 108 | 3 | 0 | - | 187.7057 | 0.013793 |
| Ab3 | 1816 | 1 | 3 | 189 | 2 | 2 | 96 | 3 | 0 | - | 161.3086 | 0.015625 |
| Ab4 | 1962 | 1 | 3 | 189 | 2 | 2 | 140 | 3 | 0 | - | 234.2391 | 0.00885 |
| Ab5 | 1962 | 1 | 3 | 189 | 2 | 2 | 140 | 3 | 0 | - | 210.5916 | 0.011364 |
| Ab6 | 1806 | 1 | 3 | 189 | 2 | 2 | 97 | 3 | 0 | - | 179.503 | 0.012422 |
| Ab7 | 234 | 21 | 48 | 58 | 42 | 36 | 109 | 4 | 0 | - | 258.423 | 0.013263 |
3.7. Antibiotic Resistance Genes
We performed whole genome sequencing on XDR and PDR A. baumannii isolates and identified several antibiotic resistance genes. Resistance to aminoglycosides (AacAad, AadA, AadB, Aph3″Ia, ArmA, Arr, StrA, StrB), beta-lactams (ADC, BlaA1, BlaA2, Zn-dependent_hydrolase, OXA-23, OXA-51, PER-1, TEM-1D, CARB-5, Mbl), sulphonamides (SulII, SulI), phenicols (CatBx, CmlA), macrolides (MphE, MsrE), and tetracycline (TetB) was widespread. Carbapenemase, CARB-5 was present in one isolate. Beta-lactamase genes OXA-23, OXA-51, BlaA2, Zn-dependent_hydrolase, ADC, Mbl and macrolide resistance genes MphE, MsrE were present in all isolates.
4. Discussion
The diminishing antimicrobial options for the treatment of XDR and PDR A. baumanii are a huge challenge. As we inexorably inch towards the post-antibiotic age, when treating critically ill patients, one has to resort to drugs of last resort such as fosfomycin, colistin, tigecycline, and carbapenems. Ceftazidime-avibactam was introduced in our facility subsequent to our study and was therefore not tested. It is also a harsh reality that a large population group does not have access to the newly introduced beta-lactam/beta-lactamase inhibitors, cefiderocol, eravacycline, and plazomycin. In such populations, the drug combinations that we have tested are more accessible. Empirical monotherapy with these antimicrobials is usually not advisable in XDR A. baumanii, as there are chances of the development of resistance and a possibility of treatment failure [10]. Management of PDR, on the other hand, undoubtedly entails a cocktail of antimicrobials [11]. Several studies have pointed to the utility of using combinations of antimicrobial agents to arrive at a synergistic effect, thus substantially reducing the risk of inappropriate empirical therapy on the one hand and the development of resistance on the other [12,13].
All the XDR isolates in this study were susceptible to only one (colistin) or a maximum of two antibiotic classes, except the MDR A. baumannii (Ab 8), which was sensitive to colistin, amikacin, and tigecycline. In this study, excellent synergy was observed with FOS-MEM and CL + MEM combinations against A. baumannii XDR and PDR isolates despite the widespread presence of resistance markers. Fosfomycin alone has excellent bactericidal activity (zero colonies) against XDR and PDR A. baumanii isolates, which began as early as 2 h and the activity continued as late as 24 h. A study from Australia [14] also reported that the in vitro activity of FOS against the carbapenem-resistant A. baumannii(CR-AB) isolates was enhanced by the addition of MEM. Overall, our results suggest that the fosfomycin-meropenem combination may have potential as a treatment option for XDR and PDR A. baumannii infections, and further studies are needed to validate this finding in clinical settings. Similarly, Singkham-in and Chatsuwan, 2018 reported the synergism of fosfomycin-imipenem in 65.2% of OXA-23-producing CR A. baumannii [15].
Beta-lactamase genes OXA-23, OXA-51, BlaA2, Zn-dependent_hydrolase, ADC, Mbl, and macrolide resistance genes MphE, MsrE were present in all 8/8 isolates. Other resistance genes were against aminoglycosides (Aac, Aad, AadA, AadB, Aph3″Ia, ArmA, Arr, StrA, StrB), beta-lactams (ADC, BlaA1, BlaA2, Zn-dependent_hydrolase, OXA-23, OXA-51, PER-1, TEM-1D, CARB-5, Mbl), sulphonamides (SulII, SulI), phenicols (CatBx, CmlA), macrolides (MphE, MsrE), and tetracycline (TetB). Interestingly, one isolate, Ab 8, was found to harbour the carbapenemase gene CARB-5, which is a cause for concern as carbapenems are often used as a last-resort treatment for A. baumannii infections. Overall, our study highlights the extent of antibiotic resistance in XDR and PDR A. baumannii isolates and emphasises the urgent need for novel treatment strategies to combat this public health threat.
FOS-AK combination displayed a disappointing lack of synergy against A. baumannii isolates. Indifferent interactions were seen against all isolates by checkerboard assay. The low activity of this combination could be attributed to high-level aminoglycoside resistance (HLAR) in A. baumannii, [16] the prevalence of aminoglycoside-modifying enzymes, and the over-expression of efflux pumps such as adeABC [17,18]. A Study by Leite et al., 2016 [19] demonstrated the synergistic effects of this combination in both colistin-susceptible and resistant OXA-23-like A. baumannii isolates using the time-kill assay. Similar to our findings, indifferent outcomes were observed by the checkerboard. The colistin-resistant isolates in their study displayed the same CL MIC range (8–64 mg/L) and FOS MIC90 of 128 mg/L. However, they did not report the individual strain MIC after combination, which would have been useful to understand when to expect synergy in this combination. In our study, the observed antagonistic interactions in all amikacin susceptible A. baumannii isolates suggests that the monotherapy by either antibiotic would be more helpful for patient management, though other studies have reported the synergistic effect of fosfomycin-amikacin combination [20,21].
The MIC50 of tigecycline in the XDR A. baumannii was 4 mg/L, while MIC90 of fosfomycin was higher at 128 mg/L. Despite the high MIC90, all isolates (100%) of A. baumannii displayed partial synergistic interactions at ≤0.03 MIC FOS + 0.5 MIC TGC (1–2 mg/L). Compared to fosfomycin-amikacin, a high rate of antagonism was observed in the fosfomycin-tigecycline combination against A. baumannii isolates. The time-kill assay confirmed the synergistic interaction at this ratio in the representative isolate and showed synergy (>2 log10 decline) at 24 h incubation. Thus, 0.5 MIC fosfomycin combined with very low tigecycline concentration (0.06 mg/L) had a substantial synergistic effect over 0.5 MIC fosfomycin alone at 24 h incubation but no bactericidal activity was observed.
The colistin-meropenem combination displayed excellent synergism in all A. baumannii (3/3), with the best outcomes in the PDR (Ab 1). Effective synergistic interactions with a relatively low meropenem MIC (≤32 mg/L) of OXA-51/23 XDR-A. baumannii strains were demonstrated in vivo using a Murine Thigh-Infection Model by Fan et al. 2016 [22]. This could explain the notable outcome in the PDR Ab isolate, which had a meropenem MIC of 16 mg/L compared to other isolates (MIC 32 mg/L), although it must be noted that the colistin MIC in this case was higher (8 mg/L). A study from China also demonstrated the superiority of colistin-meropenem against CR A. baumannii with an MIC of ≥32 mg/L by checkerboard and static time-kill assays [23]. In their study, an effective dosage regimen of 2 g meropenem daily via 3-h infusion combined with steady-state 1 mg/L colistin suppressed bacterial growth at 24 h with a 2-log10 decrease. This finding is quite similar to our partial synergistic outcomes in the checkerboard at 0.5–1 mg/L MIC MEM + 0.25–1 mg/L MIC CL. The possible mechanism behind this synergism is that colistin interferes with the outer membrane, causing disruption to its permeability and subsequently allowing higher concentrations of meropenem (inhibition of peptidoglycan) to enter the bacterial cells so that the resistant bacteria become more susceptible [24]. Maifiah et al. 2017 discovered that colistin-doripenem combination kills A. baumannii synergistically in a time-dependent manner by perturbing different key metabolic pathways necessary for bacterial survival using liquid-chromatography mass spectrometry (LC-MS) [25].
FOS-CL combination demonstrated disappointingly indifferent interactions in the PDR isolates. On the other hand, excellent synergy was observed in PDRs with FOS-MEM (FICI ≤ 0.40) and FOS-AK (FICI ≤ 0.50), while antagonism was noted with FOS-TGC combination. Further studies are needed to confirm the efficacy of these antibiotic combinations in vivo and to determine the optimal dosages and treatment regimens.
The variation in MLSTs among the isolates in this study is an interesting finding and could suggest the presence of diverse sources of infection or the acquisition of resistance genes through horizontal gene transfer. MLST is a widely used method for molecular typing of A. baumannii and helps in identifying the clonal relatedness among the isolates [26]. The fact that two isolates belonged to the same ST, ST-1962, indicates a possible clonal outbreak. However, the presence of different STs among the other isolates suggests the possibility of multiple sources of infection or acquisition of resistance genes through horizontal gene transfer. Further studies are required to determine the epidemiology and genetic relatedness among the isolates.
5. Conclusions
Our study demonstrated that the FOS and MEM combination could be a useful option to treat XDR and PDR A. baumannii. Synergy of FOS-MEM in intrinsically resistant A. baumannii shows that this antibiotic combination might be useful in treating CR-AB isolates. To further support the findings, a larger preclinical or clinical investigation is essential.
Author Contributions
Conceptualization, M.R.; Methodology, M.A.Q. and M.R.; Formal analysis, J.M., N.T. and M.R.; Investigation, M.A.Q.; Resources, Z.A.J. and I.A.B.; Data curation, M.A.Q. and N.T.; Writing–original draft, H.S.; Writing–review & editing, H.S. and M.R.; Visualization, H.S.; Supervision, Z.A.M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Internal Grant of College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman. The funding agency had no role in the study design, collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Antunes L.C.S., Visca P., Towner K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014;71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 2.Al Samawi M.S., Khan F.Y., Eldeeb Y., Almaslamani M., Alkhal A., Alsoub H., Ghadban W., Howady F., Hashim S. Acinetobacter Infections among Adult Patients in Qatar: A 2-Year Hospital-Based Study. Can. J. Infect. Dis. Med. Microbiol. 2016;2016:e6873689. doi: 10.1155/2016/6873689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 4.Ju Y.G., Lee H.J., Yim H.S., Lee M.G., Sohn J.W., Yoon Y.K. In vitro synergistic antimicrobial activity of a combination of meropenem, colistin, tigecycline, rifampin, and ceftolozane/tazobactam against carbapenem-resistant Acinetobacter baumannii. Sci. Rep. 2022;12:7541. doi: 10.1038/s41598-022-11464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas M.E., Vouloumanou E.K., Samonis G., Vardakas K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016;29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sastry S., Clarke L.G., Alrowais H., Querry A.M., Shutt K.A., Doi Y. Clinical Appraisal of Fosfomycin in the Era of Antimicrobial Resistance. Antimicrob. Agents Chemother. 2015;59:7355–7361. doi: 10.1128/AAC.01071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI . Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically. 10th ed. CLSI document M07-A10; Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. [Google Scholar]
- 8.Rizvi M., Ahmed J., Khan F., Shukla I., Malik A. Assessment of combination therapy by time kill curve analysis and chequerboard assay for treatment of multi-drug resistant Pseudomonas aeruginosa isolates. J. Glob. Antimicrob. Resist. 2013;1:103–108. doi: 10.1016/j.jgar.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Garcia L. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Clinical Microbiology Procedures Handbook: Update 2007. American Society for Microbiology; Washington, DC, USA: 2012. [Google Scholar]
- 10.Zaidan N., Hornak J.P., Reynoso D. Extensively Drug-Resistant Acinetobacter baumannii Nosocomial Pneumonia Successfully Treated with a Novel Antibiotic Combination. Antimicrob. Agents Chemother. 2021;65:e00924-21. doi: 10.1128/AAC.00924-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oo C., Sy S.K.B. Fixed-dose combinations: A potential means to boost drug development for selected drugs. Drug Discov. Today. 2018;23:457–459. doi: 10.1016/j.drudis.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 12.AL-Quraini M., Rizvi M., AL-Jabri Z., Sami H., AL-Muzahmi M., AL-Muharrmi Z., Taneja N., Al-Busaidi I., Soman R. Assessment of In-Vitro Synergy of Fosfomycin with Meropenem, Amikacin and Tigecycline in Whole Genome Sequenced Extended and Pan Drug Resistant Klebsiella Pneumoniae: Exploring A Colistin Sparing Protocol. Antibiotics. 2022;11:153. doi: 10.3390/antibiotics11020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karakonstantis S., Ioannou P., Kofteridis D.D. In search for a synergistic combination against pandrug-resistant A. baumannii; methodological considerations. Infection. 2022;50:569–581. doi: 10.1007/s15010-021-01748-w. [DOI] [PubMed] [Google Scholar]
- 14.Mohd Sazlly Lim S., Heffernan A.J., Roberts J.A., Sime F.B. Pharmacodynamic Analysis of Meropenem and Fosfomycin Combination Against Carbapenem-Resistant Acinetobacter baumannii in Patients with Normal Renal Clearance: Can It Be a Treatment Option? Microb. Drug Resist. 2021;27:546–552. doi: 10.1089/mdr.2020.0197. [DOI] [PubMed] [Google Scholar]
- 15.Singkham-In U., Chatsuwan T. In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 2018;91:169–174. doi: 10.1016/j.diagmicrobio.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Nie L., Lv Y., Yuan M., Hu X., Nie T., Yang X., Li G., Pang J., Zhang J., Li C., et al. Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China. Acta Pharm. Sin. B. 2014;4:295–300. doi: 10.1016/j.apsb.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishk R., Soliman N., Nemr N., Eldesouki R., Mahrous N., Gobouri A., Azab E., Anani M. Prevalence of Aminoglycoside Resistance and Aminoglycoside Modifying Enzymes in Acinetobacter baumannii Among Intensive Care Unit Patients, Ismailia, Egypt. Infect. Drug Resist. 2021;14:143–150. doi: 10.2147/IDR.S290584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C., Bilya S.R., Xu W. adeABC efflux gene in Acinetobacter baumannii. New Microbes New Infect. 2019;30:100549. doi: 10.1016/j.nmni.2019.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leite G.C., Oliveira M.S., Perdigão-Neto L.V., Rocha C.K.D., Guimarães T., Rizek C., Levin A.S., Costa S.F. Antimicrobial Combinations against Pan-Resistant Acinetobacter baumannii Isolates with Different Resistance Mechanisms. PLoS ONE. 2016;11:e0151270. doi: 10.1371/journal.pone.0151270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery A.B., Rhomberg P.R., Abuan T., Walters K.A., Flamm R.K. Potentiation Effects of Amikacin and Fosfomycin against Selected Amikacin-Nonsusceptible Gram-Negative Respiratory Tract Pathogens. Antimicrob. Agents Chemother. 2014;58:3714–3719. doi: 10.1128/AAC.02780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Martinez L., Rodriguez G., Pascual A., Suárez A.I., Perea E.J. In-vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii. J. Antimicrob. Chemother. 1996;38:1107–1108. doi: 10.1093/jac/38.6.1107. [DOI] [PubMed] [Google Scholar]
- 22.Fan B., Guan J., Wang X., Cong Y. Activity of Colistin in Combination with Meropenem, Tigecycline, Fosfomycin, Fusidic Acid, Rifampin or Sulbactam against Extensively Drug-Resistant Acinetobacter baumannii in a Murine Thigh-Infection Model. PLoS ONE. 2016;11:e0157757. doi: 10.1371/journal.pone.0157757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian X., Liu X., Chen Y., Chen D., Li J., Zhang J. Dose Optimization of Colistin Combinations against Carbapenem-Resistant Acinetobacter baumannii from Patients with Hospital-Acquired Pneumonia in China by Using an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2019;63:e01989-18. doi: 10.1128/AAC.01989-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann G.R., Lehár J., Keith C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Maifiah M.H.M., Creek D.J., Nation R.L., Forrest A., Tsuji B.T., Velkov T., Li J. Untargeted metabolomics analysis reveals key pathways responsible for the synergistic killing of colistin and doripenem combination against Acinetobacter baumannii. Sci. Rep. 2017;7:45527. doi: 10.1038/srep45527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaiarsa S., Batisti Biffignandi G., Esposito E.P., Castelli M., Jolley K.A., Brisse S., Sassera D., Zarrilli R. Comparative Analysis of the Two Acinetobacter baumanniiMultilocus Sequence Typing (MLST) Schemes. Front. Microbiol. 2019;10:930. doi: 10.3389/fmicb.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.