Summary
Background
Inappropriate use of antibiotics is a significant driver of antibiotic resistance in India. Largely unrestricted over-the-counter sales of most antibiotics, manufacturing and marketing of many fixed-dose combinations (FDC) and overlap in regulatory powers between national and state-level agencies complicate antibiotics availability, sales, and consumption in the country.
Methods
We analyzed cross-sectional data from PharmaTrac, a nationally representative private-sector drug sales dataset gathered from a panel of 9000 stockists across India. We used the AWaRe (Access, Watch, Reserve) classification and the defined daily dose (DDD) metrics to calculate the per capita private-sector consumption of systemic antibiotics across different categories: FDCs vs single formulations; approved vs unapproved; and listed vs not listed in the national list of essential medicines (NLEM).
Findings
The total DDDs consumed in 2019 was 5071 million (10.4 DDD/1000/day). Watch contributed 54.9% (2783 million) DDDs, while Access contributed 27.0% (1370 million). Formulations listed in the NLEM contributed 49.0% (2486 million DDDs); FDCs contributed 34.0% (1722 million), and unapproved formulations contributed 47.1% (2408 million DDDs). Watch antibiotics constituted 72.7% (1750 million DDDs) of unapproved products and combinations discouraged by the WHO constituted 48.7% (836 million DDDs) of FDCs.
Interpretation
Although the per-capita private-sector consumption rate of antibiotics in India is relatively low compared to many countries, India consumes a large volume of broad-spectrum antibiotics that should ideally be used sparingly. This, together with significant share of FDCs from formulations outside NLEM and a large volume of antibiotics not approved by the central drug regulators, call for significant policy and regulatory reform.
Funding
Not applicable.
Keywords: Antibiotics, Drug resistance, Antimicrobial stewardship, Drug combinations, Drug approval, Formulary, Antimicrobial consumption
Research in context.
Evidence before this study
India does not have a formal system to monitor antibiotic use. We searched PubMed with key words (“antimicrobial”[Title] OR “antibiotic”[Title]) AND (“consumption” [Title] OR “use”[Title] OR “usage”[Title]) AND (“India”[Title]) to look for studies on antibiotic use in India. We found 39 studies, most of them using geographically limited hospital-based survey data, and a few national level studies using large volume proprietary sales and prescription data. We found one systematic review that was limited to dental practice. Our literature review indicated an increase in antibiotic consumption especially of broad-spectrum molecules over the years and the presence of large number of fixed dose combinations in the Indian market.
Added value of this study
We used the WHO AWaRe classification system and the defined daily dose (DDD) metrics to analyze a large nationally representative private-sector drugs sales data. This study used the most recent data (2019) and examined the consumption of antibiotics using additional dimensions of essentiality (listed/not listed in national list of essential medicines), product type (fixed dose combinations, single formulations) and central regulatory approval status (approved/unapproved). We report a lower consumption rate compared to previous estimates but very high relative consumption of broad-spectrum antibiotics and unapproved formulations mostly outside the price-controlled essential medicines list.
Implications of all the available evidence
Using a standardized measure of defined daily doses, this study reports India's most recent private-sector antibiotic consumption estimates. The study provides evidence for policymakers to strengthen the existing policy measures and institute new efforts to achieve the global targets of appropriate use of antibiotics. Restricting the sale of unapproved formulations, expanding the national essential medicines list to include more antibiotics, strengthening antibiotic stewardship programs in private sector, expanding access to appropriate antibiotics through public health system, and tightening the legal regulation of over-the counter sales of antibiotics may help to address inappropriate use of antibiotics.
Alt-text: Unlabelled box
Introduction
Antibiotic resistance is a public health challenge with global ramifications. Although antibiotic resistance initially appeared in hospitals in the 1950s, it was the growing inappropriate use of antibiotics over the subsequent decades that contributed to the emergence of multi-drug resistant bacteria making the cure of many infections more expensive and, in some cases, impossible.1 The misuse of antibiotics as growth promoters in the poultry and animal industry also added to the problem. With very few new antibiotic classes entering the market, optimizing the appropriate use of available antibiotics is imperative.
Widespread inappropriate human use of antibiotics has been identified as a significant driver of antibiotic resistance in India.2 Even though India is the largest antibiotic consumer in absolute volume,3 the country does not have a formal system of antibiotic use surveillance to guide an antimicrobial stewardship program akin to those found in the US and the Europe. The latest global surveillance report from 65 countries—that examined the antibiotic consumption for the year 2015—did not have data from India.4 Like many countries, India's medicine sales and consumption data are primarily gathered by private commercial entities and cover mainly the private sector.
Studies from India, including analysis of antibiotics sales data, indicate that the use of newer classes of antibiotics is disproportionately high.2,5 However, very few studies have examined antibiotic consumption in India using the AWaRe classification—a stewardship framework advocated by the WHO to measure and improve appropriate antibiotic use—that categorizes antibiotics into Access, Watch, and Reserve groups. Access includes narrow-spectrum antibiotics recommended as first-line or second-line antibiotics; Watch includes broad-spectrum antibiotics with a high chance of resistance to be used only for specific indications; and Reserve includes antibiotics to be used only as a last resort. Even the few Indian studies that used the AWaRe framework examined only selected groups of antibiotics3 or used the data from a few years ago.5 Defined daily dose (DDD)—a globally accepted standard measure of antibiotic use—is used sparingly in Indian studies. DDD is defined as the average maintenance dose per day of a drug for its main indication in adults,6 and provides a unit of measurement independent of price, currencies, package size, and strength that helps in drug utilization studies across population groups and time. Of the three Indian studies that used AWaRe and DDD metrics concurrently, one was a single hospital-based study of 1000 prescriptions,7 and the other two were global analyses.8,9
Literature shows that worldwide human consumption of antibiotics increased by 36% between 2000 and 2010.10 Brazil, Russia, India, China, and South Africa (BRICS) accounted for three-quarters of the increase despite collectively representing only 40% of the world's population. Among these countries, 23% of the retail sales volume was attributable to India, where regulations to control over-the-counter sales of antibiotics are poorly enforced. The WHO and various national commissions and reports from India have advocated using the national list of essential medicines (NLEM) as a critical tool to achieve health equity.11 The last edition of India's NLEM published in 2015 contains 28 antibiotics. Examining antibiotic consumption against NLEM will help understand the affordability of antibiotics and the market response to price regulations in India, as the National Pharmaceutical Pricing Policy (2012) requires the price control of essential medicines with specified dosage and strength.12
Fixed-dose combinations (FDCs) are a hallmark of the Indian drug market.13 Some specific combinations such as trimethoprim-sulfamethoxazole are clinically and pharmacologically efficient and combinations are required to prevent the emergence of resistance in diseases like tuberculosis and leprosy. However, the widespread use of antibiotic FDCs is a significant concern due to the decreased therapeutic efficiency and difficulty in dose-scheduling, resulting in either under-dosing or over-dosing.14 Previous reports, including an Indian parliamentary standing committee report and an Indian Council of Medical Research expert committee report, highlighted the growing number of “irrational” FDCs as a public health issue.15 However, previous studies have not examined the consumption of FDCs in India using the AWaRe classification.
In India, the drug regulatory responsibilities are distributed between the Central Drugs Standard Control Organization (CDSCO)—the national regulatory body for pharmaceuticals and medical devices—under the national government and the State Drug Regulatory Authorities (SDRAs) under the respective state governments. This means each Indian state can provide market approval even without CDSCO approval. Although the SDRAs in most states have limited technical capacity, the capacity widely varies between SDRAs, thereby diminishing the regulatory effectiveness.16 An analysis of the Indian drug market during 2007-12 showed that a considerable proportion of antibiotics are unapproved by the CDSCO.14 However, we did not find studies that used the AWaRe classification to assess the sales and consumption of antibiotics against the approval status.
In summary, measuring antibiotic consumption using DDD metrics and AWaRe classification across product type (FDCs/single formulations [SF]), essentiality (listed in NLEM/not listed), and central regulatory approval status (CDSCO approved/unapproved) would provide a comprehensive understanding on the level of appropriateness of antibiotic use in India. This paper aims to describe the consumption of systemic antibiotics in the private sector in India in 2019 using nationally representative private-sector drug sales data. We present the consumption patterns across four dimensions: AWaRe classification, essentiality, product type, and approval status. (See Panel 1 for commonly used terminologies).
Panel 1.
Commonly used terminologies.
|
Systemic antibiotics: antibiotics administered orally or parenterally and classified under the J01 therapeutic subgroup as per the WHO ATC system. Formulation: a product in a specified form (e.g., solid/liquid) and route of administration (e.g., oral/injectables) containing one or more antibiotic molecules with specified strengths. Brands: formulations sold by a company under a specified trade name and represented by a unique product code. Antibiotic FDCs: formulations that contain two or more drugs in a fixed ratio, of which at least one is an antibiotic. Approved: refers to the approval of a formulation by India's national drug regulatory authority, CDSCO. |
Methods
Study design
We conducted a cross-sectional ecological analysis of private sector antibiotics sales in India during the calendar year 2019. We used PharmaTrac, a national-level private-sector drug sales audit dataset created by the All-Indian Origin Chemists and Distributors Ltd. (AIOCD Ltd.), used by government agencies for policy decisions, including price regulation, and by researchers.13,14,17, 18, 19
Outcomes
The primary outcome measures were absolute (DDDs) and per-capita rate of antibiotic use (DIDs-defined daily dose per 1000 inhabitants per day). Secondary outcomes included absolute and relative rates of consumption across categories in AWaRe groups (Access, Watch, Reserve), product types (FDCs/SF), listing in NLEM (listed/not listed), and central regulatory approval status (approved/unapproved).
Procedure
The data were gathered from a panel of 9000 stockists who store (“stock”) products from approximately 5000 pharmaceutical companies. Through a massive network of distributors and sub-stockists, these stockists then distribute the medicines to healthcare “retailers,” including pharmacies, individual practitioners, clinics, and hospitals. PharmaTrac sample covers 60% of stockists in India, which corresponds to 18,000 distributors, 32,000 sub-stockists, and 500,000 retailers.13 The data collected were then extrapolated to represent the sales of medicines in the entire private retail sector,18 including the private pharmacy sales of antibiotics against prescriptions from public sector providers. However, these data did not include the drugs dispensed through public facilities, though this is less than 15–20% of all drug sales in the country as per studies and national health accounts estimates.18,20,21
The dataset provided the sales units (month-wise number of medicine packs sold) and the annual moving average total (MAT) for January 1 - December 31, 2019, for each product identified by a unique product code. In addition, the dataset included information on whether the product is a single formulation or an FDC, the company name, drug form (tablet, gel, suspension), categories (injectables, liquids, solids, inhalants, others), strength, pack size, and the price.
We defined systemic antibiotics as drugs classified under the J01 group in the WHO Anatomical Therapeutic Chemical (ATC) classification system,22 which comprises antibacterials for systemic use, except antimycobacterials. We excluded topical preparations, eye/ear drops, gel, pessaries, and suppositories as these preparations are not classified under the J01 subgroup as per the ATC system. Systemic antibiotics used in genitourinary systems infections are classified under J01 and have been included in our analysis, but the local use preparations (not J01) whose DDDs are based on the treatment of vaginal infections are excluded. For metronidazole, we included only the parenteral formulations classified under J01 to treat anaerobic bacterial infections. Oral FDCs of metronidazole used for Helicobacter pylori eradication classified under A02 and single formulations used for amoebiasis, trichomoniasis, and giardiasis classified under P01 are excluded. But we retained oral FDCs where metronidazole (or another imidazole) is a component, such as ofloxacin-tinidazole and ciprofloxacin-ornidazole.
We calculated the total DDD consumed per pack using the product and pack size information from the PharmaTrac data and the corresponding formulation's DDD information from the WHO ATC/DDD index 2019 list (https://aware.essentialmeds.org/groups). The DDD per pack was then multiplied by the total number of packs (MAT) consumed per year to obtain the total DDD of the product for the whole year. We then summed up the DDD at the molecule level and divided the sum by 365 and population to obtain DID values. We used the projected mid-year population for 2019 obtained from the National Population Commission (www.censusindia.gov.in). The detailed steps and the formulas are described in Supplement Box 1.
We used the following standards for molecules not included in the ATC/DDD index. Wherever DDD values were not available for oral formulations, we used DDD of parenteral formulation or vice-versa; examples include ceftazidime and clarithromycin. For those FDCs with one penicillin and an enzyme inhibitor (ATC (code J01CR), we applied the DDD value of the corresponding penicillin component (Supplement Table 1). In the case of FDCs with only one active antibiotic molecule (the other component being a non-antibiotic), we used the ATC code and DDD value of the antibiotic molecule, following the WHO 2020 guidelines. For FDCs of two or more penicillins with or without enzyme inhibitors (ATC code J01CR50) and other FDCs with two or more antibiotics, we added the DDDs of the individual antibiotics. For balofloxacin and levonadifloxacin, we used the DDD of levofloxacin as they belong to the same class (Supplement Table 2). We matched all the spellings from PharmaTrac with the WHO ATC database and retained the spellings from the latter. (Supplement Table 3) PharmaTrac did not specify the strengths of 85 brands in the database. For them, we used data from the Tata 1mg web portal (https://www.1mg.com) that provides curated and verified product information or from the respective pharmaceutical manufacturer's websites.
PharmaTrac database included the data on the NLEM listing. We used the updated WHO 2021 list to classify the antibiotics as per the AWaRe system (https://aware.essentialmeds.org/groups) and the data from the CDSCO (https://cdsco.gov.in/opencms) to determine approval status. We created reference files for AWaRe groups and approval status and added these variables as new columns to the already cleaned PharmaTrac data in Microsoft excel sheet.
We used the tidyverse, readxl, and openxlsx packages in R software version 4.1.1 (R Core Team, 2020),23 to clean and organize the data, add new variables, and analyze the data. We report our results following the STROBE cross-sectional checklist.
Role of the funding source
The authors have received no funding for this paper.
Results
The total volume of anti-infectives sold in India in 2019 was 2807 million packs, of which systemic antibiotics constituted 2165 million packs (77.1%). We identified 163 antibiotic preparations involving 85 antibiotic molecules (see Supplement table 4 for antibiotic molecules included in these formulations and brands). The overall characteristics of systemic antibiotics used in 2019 are given in Table 1: (1) there were 1098 unique formulations and 10,100 unique products (brands) (2) 73.5% of systemic antibiotic brands were oral preparations (57.8% solids and 15.7% liquids), and 26.5% were parenteral preparations (3) 45.5% of brands (18.8% of formulations) belonged to CDSCO approved category, 33.7% (9.5% of formulations) belonged to the NLEM and 42.9% of formulations (53.3% of brands) belonged to the Watch category (4) 25.1% of formulations (19.4% of brands) belonged to the discouraged group.
Table 1.
Composition of the antibiotics market in India, 2019.
| Groups | Formulations$ | Brands$ | DDD in million | DID Consumed, %# |
|---|---|---|---|---|
| Total | 1098, 100% | 10,100, 100% | 5071, 100% | 10.4, 100% |
| Product type | ||||
| SF | 596, 54.3% | 6418, 63.5% | 3349 | 6.9, 66.0% |
| FDCs | 502, 45.7% | 3682, 36.5% | 1722 | 3.5, 34.0% |
| National list of essential medicines | ||||
| Listed | 104, 9.5% | 3405, 33.7% | 2486 | 5.1, 49.0% |
| Not listed | 994, 90.5% | 6695, 66.3% | 2585 | 5.3, 51.0% |
| CDSCO approval status | ||||
| Approved | 207, 18.9% | 4593, 45.5% | 2663 | 5.4, 52.9% |
| Unapproved | 891, 81.1% | 5507, 54.5% | 2408 | 5.0, 47.1% |
| AWaRe classification | ||||
| Access | 274, 24.9% | 2299, 22.8% | 1370 | 2.8, 27.0% |
| Watch | 472, 43.0% | 5384, 53.3% | 2783 | 5.7, 54.9% |
| Reserve | 48, 4.4% | 411, 4.1% | 53 | 0.11, 1.0% |
| Discouraged | 276, 25.1% | 1961, 19.4% | 836 | 1.7, 16.5% |
| Not classified | 28, 2.6% | 45, 0.4% | 30 | 0.06, 0.6% |
Note:
-number, %; DDD- Defined daily doses; DID- defined daily doses per 1000 population per day; SF- single formulation; FDCs- fixed dose combinations; CDSCO- Central Drugs Standard Control Organization.
-percentage applies for DDD and DID values.
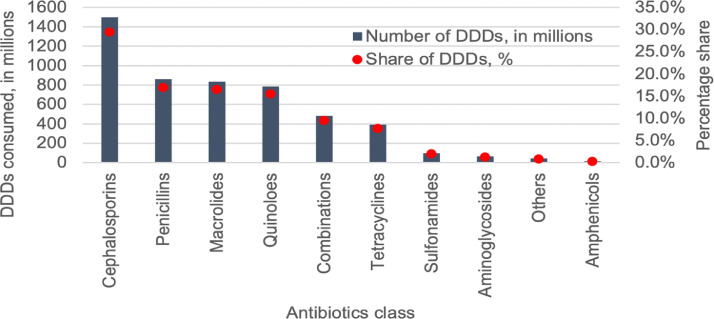
The total number of DDDs consumed was 5071 million, translating to 10.4 DDD per 1000 population per day (10.4 DID). Cephalosporins was the most used antibiotic class (1496 million DDDs, 29.5%), followed by penicillins (859 million, 17.0%) and macrolides (835 million, 16.5%). (Figure 1, Supplement Table 5) Twelve antibiotic molecules constituted 75% of the total consumption (Drug Utilization 75 (“DU75”)) (Supplement Figure 1 and Supplement Table 6). Azithromycin was the most consumed antibiotic molecule (640 million DDDs, 12.6%), followed by cefixime (516 million, 10.2%). Azithromycin 500mg tablet was the most consumed formulation (384 million DDDs, 7.6%), followed by cefixime 200 mg tablet (331 million DDDs, 6.5%). The top ten formulations are shown in Supplement Table 7.
Figure 1.
Volume and share of antibiotic classes consumed in the private sector in India, 2019.
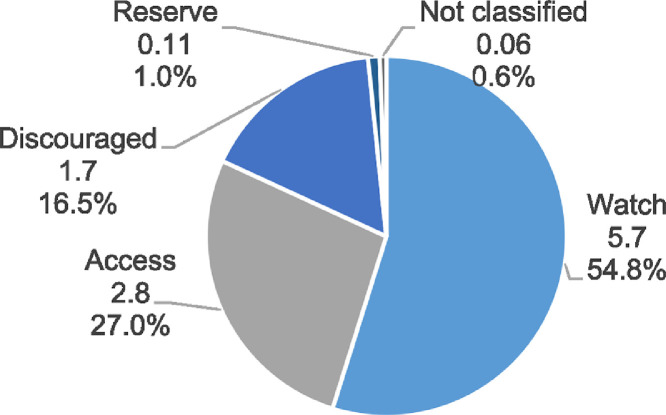
The Access group contributed only 27.0% of the total DDDs consumed (1370 million DDDs, 2.8 DID). The Watch group antibiotics contributed 54.9% (2783 million, 5.7 DID) and the Reserve group contributed 1.0% (53 million DDD, 0.1 DID). (Figure 2) Further, the discouraged groups contributed 16.5% (836 million DDDs, 1.7 DID). The Access-to-Watch ratio was 0.49. Azithromycin was the most consumed Watch antibiotic (640 million DDDs, 22.4% of Watch antibiotics) and linezolid was the most consumed Reserve antibiotic (41 million DDDs, 66.7% of Reserve antibiotics). Cefixime-ofloxacin was the most consumed discouraged FDC (310 million DDDs, 42.4% discouraged FDCs). See Supplement Table 8 for the top 10 antibiotics in each AWaRe category.
Figure 2.
AWaRe composition of antibiotics consumed (DID), 2019.
Although only 9.5% of formulations in the market were listed in the NLEM, 49.0% of DDDs (5.1 DID, 2486 million DDDs) came from these formulations. Cefixime-ofloxacin 200/200 mg tablet was the most consumed formulation outside the NLEM (284 million DDDs, 11.0% of non-NLEM), followed by cefpodoxime 200 mg tablet (215 million DDDs, 8.3%). The top ten non-NLEM formulations accounted for 24.4% (1239 million) of total DDDs (Supplement Table 9).
FDCs constituted 33.7% of the total DDDs (1722 million). Amoxicillin-clavulanic acid 500/125mg tablet was the most consumed FDC (325 million DDDs, 6.4%). The top ten FDCs formed 23.7% (1202 million DDDs) of total DDDs consumed. (Supplement Table 10) 71.4% (1242 million DDDs) of FDCs and 40.6% (1343 million DDDs) of single formulations contained antibiotics not listed in the NLEM.
Centrally unapproved formulations accounted for 47.1% (2408 million) of total DDDs. Cephalosporins (917 million DDDs, 38.1%), macrolides (762 million, 31.7%), and penicillins (247 million, 10.3%) were the top three antibiotic classes among unapproved formulations. (Supplement Table 11) Further, 60.9% (2024 million DDDs) of single formulations were unapproved compared to only 22.9% (385 million DDDs) of FDCs. There was no substantial difference in approval rates across NLEM formulations (1275 million DDDs, 51.0% approved) and non-NLEM formulations (1388 million, 52.8%).
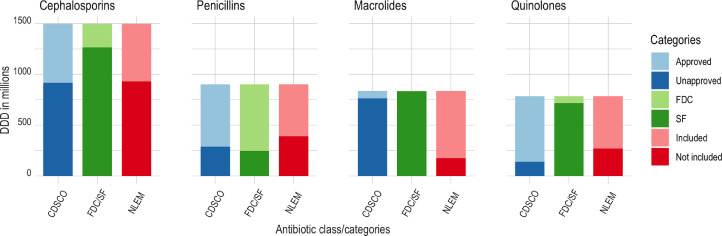
Figure 3 shows the distribution of DDD values of the top four antibiotic classes across product type, essentiality, and approval status. 762 million DDDs (91.2%) of macrolides and 917 million DDDs (61.3%) of cephalosporins came from products sold without the approval of the central agency. In addition, 655 million DDDs (72.7%) of penicillins came from FDCs, predominantly as penicillin-beta-lactamase inhibitor combinations, and 930 million DDDs (62.1%) of cephalosporins came from non-NLEM formulations.
Figure 3.
Characteristics of the top four antibiotic classes, 2019.
Note: DDD- defined daily doses; CDSCO- Central Drugs Standard Control Organization; FDC- fixed dose combination; SF- single drug formulation; NLEM- national list of essential medicines.
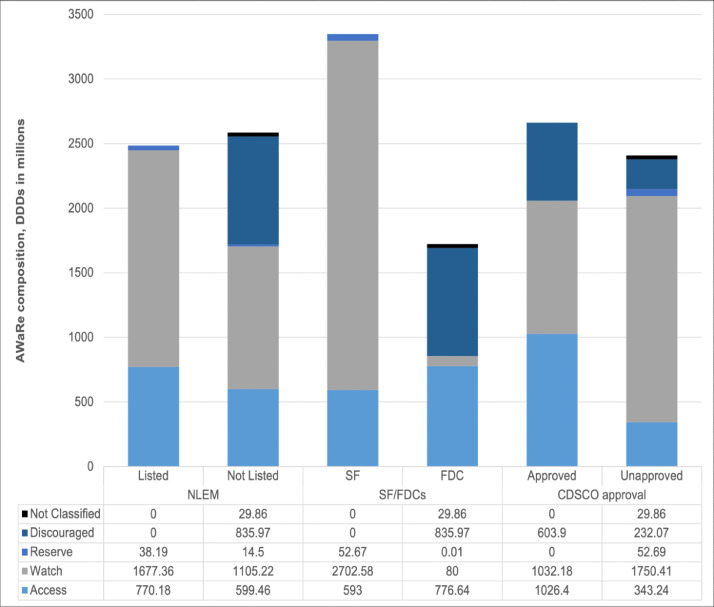
Figure 4 shows the overall distribution of AWaRe groups across other NLEM listing, product type, and approval status. SFs in the Indian market are mostly made up of Watch antibiotics (2703 million DDDs, 80.7%) and only 17.7% SFs (593 million DDDs) had Access antibiotics. On the other hand, FDCs contained similar proportion of Access (777 million DDDs, 45.1%) and discouraged formulations (836 million, 48.5%). Similarly, the proportions of approved products containing Access (1026 million) and Watch (1032 million) molecules were similar. Further, 604 million DDDs (22.7%) of approved formulations contained formulations discouraged by the WHO.
Figure 4.
Distribution of AWaRe categories across NLEM listing, product type, and approval status, 2019.
Note: DDD- defined daily doses; CDSCO- Central Drugs Standard Control Organization; FDC- fixed dose combination; SF- single drug formulation; NLEM- national list of essential medicines.
Discussion
This is the first published study analyzing private-sector consumption of systemic antibiotics in India using the DDD metrics and the WHO AWaRe classification system. The key findings include: (1) low per-capita consumption of antibiotics compared to previously reported rates, (2) high consumption rate of broad-spectrum antibiotics, (3) high consumption rate of FDCs discouraged by WHO, (4) large share of non-NLEM formulations in FDCs, and (5) significant use of unapproved formulations.
First, we report a per-capita consumption rate (systemic antibiotics, J01) of 10.4 DIDs which is lower compared to the rate in 2015 (13.6 DID) reported by Klein and colleagues, and the rate (15.7) in the 2021 WHO report.24,25 Notwithstanding the slightly different methods, antibiotics groups included in the estimates, and differences in the datasets used, the lower rate needs to be examined further. This may include time trend analysis to see the effect of regulatory changes to restrict the sale of certain medicines,18 and increased availability of drugs in public facilities. In global comparison, Europe reported an average DID of 19.4 (country range: 9.5 to 34.1) for 2019,26 but this is the sum of private and public sector consumption. On the other hand, among BRICS countries, Brazil (22.75) and Russia (14.82) reported higher rates.4 Among countries from various WHO regions that reported consumption rates for 2016 (most recent data), Côte d'Ivoire (10.68) in Africa, Peru in the Americas (10.26), and Armenia (10.31) in Europe had rates comparable to ours.4 In the Asian context, Sri Lanka registered a higher DID of 16.3 in 2017,27 and China and Pakistan reported 8.4 and 19.6 respectively in 2015.24 The lower rate in India compared to other countries with similar infectious disease burden may indicate persisting issues of lack of access to antibiotics or better availability of drugs in the public sector.
Second, the consumption of broad-spectrum antibiotics categorized under the Watch list is proportionately very high (55%). While the global goal is to have at least a 60% share for Access antibiotics, we found reversed Access-to-Watch ratio. Our finding agrees with previous studies, including a systematic review and spatial modeling.8,9,28 In our analysis, the broad-spectrum third-generation cephalosporins alone constituted 22.5% of all antibiotics. This may reflect inappropriate prescription or over-the-counter antibiotic dispensing and indicates the need for stricter regulations and stewardship programs. Further, we found significant use of newer Reserve antibiotics like linezolid (41 million DDDs, 0.8% of total DDDs) and carbapenems (3.7 million DDDs, 0.1% of entire DDDs). We need more granular data to understand the healthcare settings where these medicines are used, prescription patterns across different categories of providers, and the indications for which these drugs are used, including microbiological evidence to develop appropriate strategies.
Third, FDCs discouraged by WHO are consumed in high volume. A previous study had shown that while there were 118 antibiotic FDC formulations on the market in India from 2007 to 2012, there were only four FDCs in the UK and US.14 A recent analysis using data for the year 2015 from 75 countries showed that India had the highest number of antibiotic FDCs (n = 80), followed by China (n = 25) and Vietnam (n = 19).29 The same study also found that India sold the highest number of “not US FDA-approved” antibiotic FDCs, followed by China, Francophone West Africa, and Vietnam. Our study also found that 9.5% of DDDs came from combinations containing antibiotics belonging to two or more different antibiotic classes.
Fourth, two-thirds of FDCs came from formulations not listed in the NLEM. This is an improvement compared to the findings by Barbara et al. that more than 80% of FDCs sold in 2015 were not compatible with the 2017 WHO Essential Medicines List.29 Considering that the drugs listed in NLEM are subject to a price control much more than the drugs not listed, our results indicate loop-holes in the policy that incentivize manufacturers to shift to inappropriate FDCs with formulations outside of NLEM. Further, the government has recently increased the price of NLEM medicines including antibiotics by more than 10%.30
Finally, there is a significant volume of antibiotics in the market that the central drug regulators do not approve. More than 90% of macrolides and 61% of cephalosporins are sold in the market without the approval of the central agency. These products are permitted by the state government agencies that have limited technical capacity to decide on the merit of approval.29 Studies have also shown that pharmacologically incompatible combinations are widely used in India.29 In 2015, the national government had banned 16 unapproved systemic antibiotics FDCs that accounted for 14% of antibiotic FDC sales following the Kokate Committee recommendations.31
Our study has four critical limitations. The first relates to inherent limitations of the WHO DDD method8 including using adult antibiotic dosage to prepare DDD unit values. This makes it challenging to differentiate consumption among adults and children and compare countries with different population-age structures. Second, the dataset covers only the private sector sales of antibiotics and will not reflect the antibiotics dispensed through the public system. However, previous studies18,20 and national health accounts estimates for India21 have shown that 85-90% of all drug prescriptions happen in the private sector, though this rate may vary between states. Therefore, our analysis might have underestimated the total consumption in a few states with a robust public system for free medicine provision. Third, the data do not differentiate between community and hospital use as the data are collected at the stockist level. However, 85% of data in PharmaTrac represents drugs sold through retail pharmacies, and 15% of data represents drugs sold through hospitals and dispensing doctors,13 as per the PharmaTrac sampling design. Finally, as the data are not available at the individual patient level, we could not analyze the appropriateness of prescription at the patient level. However, analysis across AWaRe and FDC metrics provides valuable broad insights regarding the appropriateness of use.
Conclusion
We examined the private sector antibiotic use, which contributes to 85–90% of the total consumption in India. We found that even with a relatively low overall rate of consumption indicating access issues, a high proportion of broad-spectrum antibiotic use in India is a public health concern. With antibiotic resistance emerging as a global public health problem, it is crucial to institute new regulations and strengthen existing ones to monitor and regulate the sale and use of antibiotics while improving access to appropriate antibiotics through the public health system.
Contributors
S.F.K. conceptualized and designed the study with the guidance of S.S., S.G., V.J.W., and P.C.R. S.F.K. cleaned and prepared the data with the assistance of Se.G. S.F.K. prepared the first draft of the manuscript. S.S., S.G., V.J.W., and P.C.R. had a guiding role throughout this. All authors discussed and reviewed the results. All authors reviewed the draft manuscript and approved the final version for publication. The corresponding author (SFK) and SS had full access to all the data used in the study.
Data sharing statement
The PharmaTrac data used in this study are available from the AIOCD Pharma soft tech AWACS Pvt. Ltd. Restrictions apply to the availability of these data, which were used under license for this study. Permission can be obtained at https://aiocdawacs.com.
Declaration of interests
V.J.W. served as co‐leader [unpaid] of Health Systems Global Technical Working Group “Medicines in Health Systems” during 2019‐2021 and leader [unpaid] of Special Interest Group “Drug Utilization and Health Service Research” during 2018‐2019. V.J.W. reported research grants [outside the submitted work] from Gilead Sciences, Management sciences for health, Sandoz, Fleming Fund, Roche, Amgen, United States Pharmacopeia (USP), and International Volkswagen/Novonordis kfondon/Caixa: Mobility. P.C.R. reported research grants [outside the submitted work] from Gilead Sciences, F. Hoffmann-La Roche, Amgen, Novartis, and International Federation for Pharmaceutical Manufacturers and Associations during the past 36 months. S.F.K.’s salary from Boston University is partly supported by the 3-D Commission project under the Rockefeller Foundation grant (2019 HTH 024).
Acknowledgments
We acknowledge the inputs of Aashna Mehta, Public health foundation of India, New Delhi, in the early phase of the project. The authors would like to thank the reviewers of the manuscript and the editors for their careful consideration of the manuscript and their suggested changes.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lansea.2022.100025.
Appendix. Supplementary materials
References
- 1.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12):S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Dixit A, Kumar N, Kumar S, Trigun V. Antimicrobial resistance: progress in the decade since emergence of New Delhi Metallo-β-Lactamase in India. Indian J Commun Med Off Publ Indian Assoc Prev Soc Med. 2019;44(1):4–8. doi: 10.4103/ijcm.IJCM_217_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulis G, Batomen B, Kotwani A, Pai M, Gandra S. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: an interrupted time series analysis. PLoS Med. 2021;18(7) doi: 10.1371/journal.pmed.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Report on Surveillance of Antibiotic Consumption. https://www.who.int/publications-detail-redirect/who-report-on-surveillance-of-antibiotic-consumption. Accessed 12 October 2021
- 5.Farooqui HH, Selvaraj S, Mehta A, Heymann DL. Community level antibiotic utilization in India and its comparison vis-à-vis European countries: evidence from pharmaceutical sales data. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHOCC - Definition and General Considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Accessed 12 October 2021.
- 7.Mugada V, Mahato V, Andhavaram VSM. Evaluation of prescribing patterns of antibiotics using selected indicators for antimicrobial use in hospitals and the access, watch, reserve (AWaRe) classification by the World Health Organization. Turk J Pharm Sci. 2021;18(3):282–288. doi: 10.4274/tjps.galenos.2020.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein EY, Milkowska-Shibata M, Tseng KK, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1):107–115. doi: 10.1016/S1473-3099(20)30332-7. [DOI] [PubMed] [Google Scholar]
- 9.Browne AJ, Chipeta MG, Haines-Woodhouse G, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health. 2021;5(12):e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 11.Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet Lond Engl. 2017;389(10067):403–476. doi: 10.1016/S0140-6736(16)31599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad A, Khan MU, Patel I. Drug pricing policies in one of the largest drug manufacturing nations in the world: are affordability and access a cause for concern? J Res Pharm Pract. 2015;4(1):1–3. doi: 10.4103/2279-042X.150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGettigan P, Roderick P, Mahajan R, Kadam A, Pollock AM. Use of fixed dose combination (FDC) drugs in India: central regulatory approval and sales of FDCs containing non-steroidal anti-inflammatory drugs (NSAIDs), metformin, or psychotropic drugs. PLoS Med. 2015;12(5) doi: 10.1371/journal.pmed.1001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGettigan P, Roderick P, Kadam A, Pollock A. Threats to global antimicrobial resistance control: centrally approved and unapproved antibiotic formulations sold in India. Br J Clin Pharmacol. 2019;85(1):59–70. doi: 10.1111/bcp.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICMR. Drug Technical Advisory Board Sub-Committee Report and Recommendations. ICMR. https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/Committee/dtab_sub_report.pdf. Accessed 15 November 2021
- 16.Chowdhury N., Joshi P., Patnaik A., Saraswathy B. Indian Council For Research On International Economic Relations; New Delhi, India: 2015. Administrative Structure and Functions of Drug Regulatory Authorities in India. [Google Scholar]
- 17.Mehta A, Farooqui HH, Selvaraj S. A critical analysis of concentration and competition in the Indian pharmaceutical market. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqui HH, Selvaraj S, Mehta A, Mathur MR. The impact of stringent prescription-only antimicrobial sale regulation (Schedule H1) in India: an interrupted time series analysis, 2008–18. JAC-Antimicrob Resist. 2020;2(3):dlaa076. doi: 10.1093/jacamr/dlaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talreja P, Kalra R. Pharmaceutical cost analysis of dental diseases: an Indian scenario. Int J Risk Saf Med. 2020;31(3):165–177. doi: 10.3233/JRS-191033. [DOI] [PubMed] [Google Scholar]
- 20.Farooqui HH, Mehta A, Selvaraj S. Outpatient antibiotic prescription rate and pattern in the private sector in India: evidence from medical audit data. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0224848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.2018. National Health Accounts: Estimates for India.https://nhsrcindia.org/sites/default/files/2021-06/NHA% 20Estimates%20Report%20-2015-16.pdf Financial Year 2015-16. Accessed 3 May 2022. [Google Scholar]
- 22.Anatomical Therapeutic Chemical (ATC) Classification. https://www.who.int/tools/atc-ddd-toolkit/atc-classification. Accessed 12 October 2021
- 23.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43) doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 24.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The State of the World's Antibiotics in 2021. Center for Disease Dynamics, Economics & Policy (CDDEP). https://cddep.org/publications/the-state-of-the-worlds-antibiotic-in-2021/. Accessed 16 March 2022
- 26.ECDC . 2020. Antimicrobial Consumption in the EU/EEA – Annual Epidemiological Report 2019.https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf Accessed 23 November 2021. [Google Scholar]
- 27.Ranganathan SS, Wanigatunge C, Senadheera GPSG, Beneragama BVSH. A national survey of antibacterial consumption in Sri Lanka. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhuvaraghan A, King R, Larvin H, Aggarwal VR. Antibiotic use and misuse in dentistry in India-a systematic review. Antibiot Basel Switz. 2021;10(12):1459. doi: 10.3390/antibiotics10121459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bortone B, Jackson C, Hsia Y, Bielicki J, Magrini N, Sharland M. High global consumption of potentially inappropriate fixed dose combination antibiotics: Analysis of data from 75 countries. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0241899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thacker T. Essential medicines to get expensive by more than 10 per cent from April. The Economic Times. https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/ess ential-medicines-to-get-expensive-from-april/articleshow/904463 47.cms?from=mdr. Published April 1, 2022. Accessed 9 May 2022.
- 31.Ministry of health . Directorate General of Health Services; 2015. Kokate Committee Report.https://www.cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCommitteeFiles/fdc16.04.2015.pdf Accessed 28 November 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.