Abstract
Hypertension is a major risk factor for heart attack, produce atherosclerosis (hardening of the arteries), congestive heart failure, stroke, kidney infection, blindness, end-stage renal infection, and cardiovascular diseases. Many mechanisms are involved in causing hypertension, i.e., via calcium channels, alpha and beta receptors, and the renin-angiotensin system (RAS). RAS has an important role in blood pressure control and is also involved in the metabolism of glucose, homeostasis, and balance of electrolytes in the body. The components of RAS that are involved in the regulation of blood pressure are angiotensinogen, Ang I (angiotensin I), Ang II (angiotensin II), ACE (angiotensin-converting enzyme), and ACE 2 (angiotensin-converting enzyme 2). These components provide for relevant therapeutic targets for the treatment of hypertension, and various drugs are commercially available that target individual components of RAS. Angiotensin receptor blockers (ARBs) and ACE inhibitors are the most popular among these drugs. ACE is chosen in this review as it makes an important target for blood pressure control because it converts Ang I into Ang II and also acts on the vasodilator, bradykinin, to degrade it into inactive peptides. This review highlights various aspects of blood pressure regulation in the body with a focus on ACE, drugs targeting the components involved in regulation, their associated side effects, and a need to shift to alternative therapy for putative hypertension treatment in the form of bioactive peptides from food.
1. Introduction
Hypertension or high blood pressure, generally called arterial hypertension, is defined by the American Heart Association as persistent high blood pressure consistently higher or above 140/90 mm Hg with systolic 130 mm Hg or higher and diastolic 80 mm Hg or higher affecting billions of people and is a common factor of death [1]. Hypertension is assessed based on many factors including genetic factors and hereditary factors and in most cases can also be attributed to obesity, diabetes, and depression [2]. Hypertension is also recognized as the permanent increase in systolic blood pressure in the arterial blood vessels [3]. Elevated pressure on the walls of blood vessels relies on several factors: pumping of the heart, accumulation of fluid, and the thickness of the vascular walls [4]. As an outcome of high blood pressure, the elasticity of the arteries is reduced, and also the accumulation of cholesterol in the walls occurs resulting in the blockage of vessels which is the raison d'etre for the organ damage that is caused as a result of hypertension. Hypertension accounts for 30–45% of the general population of which 60% are in the 60-65 years age group [5]. The World Health Organization (WHO) considers hypertension a significant preventable cause of worldwide death [6]. Hypertension is associated with an increased risk of cardiovascular pathologies and mortality in all age groups [7]. To treat high levels of blood pressure, an arsenal of antihypertensive drugs is available: calcium channel blockers, diuretics, beta blockers, ARBs, and ACE inhibitors [8, 9]. Some Indian studies showed an increase in the occurrence of hypertension about thirty times in the urban population in the 55-year-age group and about ten times in the rural population [6]. An important consideration of any systolic blood pressure analysis is whether the disorder is “primary” or “secondary.” Primary or secondary classifications for systolic blood pressure imply to the cause of high blood pressure and have nothing to do with the seriousness of the condition [10]. Primary or essential hypertension involves a rise in blood pressure without any known cause or medical condition, and some of the associated causes are lack of regular exercise, sedentary lifestyle, poor diet, and hereditary factors. Secondary hypertension, on the other hand, has a medical condition associated with it such as conditions affecting certain organs like the kidneys, endocrine system, and heart. It can be suspected in patients with an established history of familial hypertension and in women with pregnancy.
2. Mechanisms Involved in Hypertension
Different targets for regulating hypertension in the body include the renin-angiotensin system, calcium channels, and beta and α1-adrenergic receptors [11–13]. Calcium channel blockers (CCBs) are mainly utilized as antihypertensive drug targets. CCBs work by inhibiting the calcium ion flow into vascular smooth muscles, thereby dilating the arteries [13]. These blockers are mainly N-type, L-type, and T-type. Voltage-dependent calcium channels are present in the zona glomerulosa of the human adrenal gland. The drugs acting on Ca+ channels are listed (Table 1).
Table 1.
Commercially available drugs and their respective classes.
| S. no. | Class of drug | Commercial names | References |
|---|---|---|---|
| 1. | Ang II type 1 (AT 1) receptor antagonists | Candesartan, losartan, telmisartan, eprosartan, valsartan, olmesartan, irbesartan | [14] |
| 2. | Calcium channel blockers | Clevidipine, isradipine, amlodipine, aranidipine, benidipine, efonidipine, nimodipine, paradipine | [15] |
| 3. | Beta blockers | Atenolol, betaxolol, bisoprolol, metoprolol, nadolol, propranolol, timolol | [16] |
| 4. | ACE inhibitors | Captopril, moexipril, perindopril, ramipril, benzapril, fosinopril, lisinopril, trandolapril, quinapril | [17] |
| 5. | Renin inhibitors | Aliskiren | [18] |
These inhibitors have some side effects like constipation and dizziness. CCBs, apart from lowering blood pressure, also promote fibrinolysis by increasing the levels of tissue plasminogen activator (t-PA), decreasing inflammatory markers, inhibiting muscle proliferation, extracellular matrix formation, and inhibition of apoptosis of endothelial cells [19–22]. Amlodipine, a calcium channel blocker, also increases the levels of the vasodilatory agent, nitric oxide (NO), and restores the stability between Ang II and NO, thereby reducing hypertension [23]. Beta blockers include a number of drugs that are disparate in pharmacodynamic and pharmacokinetic properties and have shown tremendous success in reducing mortality and cardiac arrest when targeting populations with heart failure or myocardial infarction. Beta blockers can therefore be used as a primary medicine for hypertension [24]. However, the utilization of β-blockers as the first line of medicine is controversial as no convincing results are yet available [25]. The β-blockers were not as successful in preventing heart attacks, strokes, and deaths as other available drugs such as diuretics, CCBs, and RAS inhibitors [22, 26, 27]. Beta-blockers commercially available are listed (Table 2) [16].
Table 2.
List of various bioactive peptides and their sources.
| Source | Peptide/s | Reference |
|---|---|---|
| Whey | IW, VYPFPG, IPA, FP, VYP | [28, 29] |
| Milk | IPP, VPP | [28, 30, 31] |
| Rice | TQVY | [28] |
| Dried Bunito | LKPNM, LKP, IKP, IWH, IW, LYP | [29–31] |
| α S 1-Casein | FFVAPFPGVFGK, IAK, YAKPVA, WQVLPNAVPAK | [30] |
| Bovine casein | MKP, GPL, GPV | [29, 30] |
| Egg | YPI, IRW, RADHPFL, YAEERYPIL | [29, 30] |
| Soybean | VLIVP, IFL, WL | [29] |
| Royal jelly | IY, VY, IVY | [29] |
| Mushroom | GEP | [29] |
| Canola | VSV, FL | [29] |
| Alaska Pollack | FGASTRGA | [29] |
| Chicken | GFXGTXGLXGF (X-hydroxyproline) | [29] |
| Spinach | MRWRD, MRW, LRIPVA, IAYKPAG | [29] |
| Wheat germ | IVY | [29] |
| Mung bean | KDYRL, VTPALR, KLPAGTLF | [29] |
| Marine shrimp | FCVLRP, IFVPAF, KPPETV | [29] |
RAS is a central target for renal and cardiovascular defense and electrolyte balance [32]. Hormonal flow regulates blood quantity and arterial pressure to sustain sufficient organ perfusion. RAS begins with the release of renin into the circulation from the juxtaglomerular cells of the kidney. Renin converts angiotensinogen to a decapeptide, Ang I (Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10); this product is then cleaved by circulating and locally articulated ACE to an octapeptide, Ang II (Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8) [33, 34]. The predominant hormonal effects of Ang II are exerted by its binding to the Ang II type 1 (AT 1) and Ang II type 2 (AT 2) receptors [35]. Antihypertensive drugs that block RAS via diverse mechanisms include ACE inhibitors, direct renin inhibitors, and ARBs [36]. ARBs are an efficient group of antihypertensive agents and include drugs listed in Table 1 along with drugs used as ACE inhibitors [37]. The most widely used inhibitor is aliskerin for the curing of hypertension [38] and have demonstrated properties beyond blood pressure control [39].
Evidence suggests that chronic administration of ARBs to mice causes a significant reduction in vascular tone by activating eNOS-derived NO, even at doses below those that lower blood pressure. This effect was not seen after similar ACE inhibition or in eNOS-null animals. Telmisartan, an acute ex vivo pleiotropic ARB with AT1R-independent signalling properties, also inhibits aortic contractility in eNOS-dependent ways that are unrelated to traditional Ang II/AT1R signalling [40].
3. Angiotensin-Converting Enzyme (ACE)
The angiotensin-converting enzyme commission number is 3.4.15.1. ACE expression is found in the capillaries of the lungs and also in the endothelium of the kidney. It is necessary for the regulation of blood pressure and electrolyte homeostasis throughout the renin-angiotensin system [41]. RAS is the central monitor of arterial blood pressure, and ACE is one of its main regulators whose role is to convert the decapeptide angiotensin to an octapeptide angiotensin II [42], Ang-(1-9) to Ang-(1-7), and then other degrade its peptide to the inactive Ang-(1-5). ACE (kininase II) inactivates the vasodilators bradykinin-(1-9) and the kallikrein-kinin arrangement, by cleaving the C-terminal dipeptides. ACE ultimately cleaves its major metabolite bradykinin-(1-7) into the smaller fragment bradykinin-(1-5) [43]. Angiotensin-converting enzyme is recognized by several names: dipeptidyl carboxypeptidase I, kininase II, peptidase P, peptidyl-dipeptidase A, dipeptide hydrolase, peptidyl dipeptidase, and carboxycathepsin, based on its mode of action. The synthetic drugs for targeting ACE are mentioned above and are known as ACE inhibitors.
ACE inhibitors are usually administered orally, although intravenous formulations are available. The most common suffix for these medications is “-pril.” Some of the examples are lisinopril, ramipril, and captopril. According to the chemical composition, it is divided into three groups: sulfhydryl-containing ACE inhibitor (captopril), phosphorus-containing ACE inhibitor (fosinopril), and dicarboxylic-containing ACE inhibitors (benazepril, enalapril, lisinopril, moexipril, perindopril, quinapril, ramipril, and trandolapril) [44]. ACE inhibitors are a class of medicines that are used for the treatment of distinct disorders and are the first-class alternative for cardiovascular disease treatment [45]. ACE inhibitors do not depend on blood sugar levels and hence are the first choice for diabetic patients suffering from hypertension. ACE inhibition is the most recent advancement in the treatment of hypertension, and in the last recent years, it has become increasingly admired as the primary variety in the pharmacotherapy of this disease. As with additional classes of antihypertensive drugs, such as thiazide diuretics and 3-adrenergic blockers, the pharmacodynamics of this form of treatment has been most widely investigated using the first mediator of this class that became presented, captopril [46]. Some ACE inhibitor medicines are further part of a generic tablet by all of a calcium-channel blocker therapy or ‘water tablet' (diuretic) medicine. The main side effect associated with ACE inhibitors is low blood pressure (hypotension). Some uncommon side effects of ACE inhibitors include dry cough, inflammation of the eyes, throat, or tongue (angioedema), hives, irregular heartbeats (may be due to excessive potassium), dizziness or fainting, headache, and a decline in kidney function [47].
Inhibition of the ACE leads to bradykinin accumulation in the lung [47]. In human lung cancer tissue, bradykinin receptors [48–50] release vascular endothelial growth factor, which promotes angiogenesis [51] and matrix metalloproteinases [50, 52]. ACEIs also cause accumulation of the substance P (SP)/neurokinin (NK)-1 receptor, which is linked to tumour proliferation and angiogenesis [52, 53].
On the basis of decades of clinical observations on the use of ACEIs, a number of adverse effects have gained increasing attention. Interestingly, respiratory side effects have been a problem for a long time when ACEIs are used. A dry cough is a major side effect of respiratory side effects [54, 55]. Some studies have shown that taking ACEIs increases the risk of airway obstruction symptoms and makes the risk of bronchial asthma or asthma worse [56, 57]. Animal studies on carcinogenicity have shown negative results, but some studies have found that ACEIs are involved in angiogenesis, cell proliferation, and the growth of tumours [58, 59]. Recent research suggests that patients taking ACEIs may develop lung cancer [60–62]. ACEI inhibited tumour growth and metastasis in mice [63], according to various studies. As a result, the data on lung cancer and ACEIs are contradictory.
Two predominant forms of ACE are articulated in humans; these are somatic form and germinal form. The somatic form is abundantly found on the endothelial surface of lung vessels and other cell types and also on the surface of monocytes, smooth muscle cells, T lymphocytes, and adipocytes, whereas the germinal form is present exclusively in the testis. Both forms of ACE are present on the outer cell surface as ectoenzymes, where they hydrolyze circulating peptides. The soluble form of ACE, different from the membrane-bound form, is formed due to the ACE secretase action and is found in serum and other body fluids. Testicular ACE is the ancestral form of the molecule with a particular active site; its crystal structure was published in 2003 [41]. Somatic ACE arose as an implication of gene duplication and contains two active sites (Terminal N- and C-domains). The polypeptide chain of somatic ACE is 1,277 amino acids, whereas the testicular ACE is smaller with 701 amino acid residues [63].
4. Somatic ACE
Somatic ACE (sACE) has a significant role in the blood pressure regulatory mechanism (renin-angiotensin system). In somatic ACE, the polypeptide chain is 1,277 amino acids in length [63]. It is involved in the Ang II formation which is a potent vasoconstrictor and also degrades the vasodilator bradykinin, thereby resulting in increased blood pressure [64]. The activity of somatic ACE is highly dependent on the chloride ion concentration and is immobile in its deficiency, whereas the N domain is completely active at relatively low concentrations or even in the absence of chloride ions [65]. Somatic ACE has two diverse domains C and D, and each contains an observant active site [41]. Both of these domains are involved in Ang I formation and bradykinin degradation; therefore, both of these domains are important in systolic blood pressure regulation and cardiac stroke.
5. Testicular/Germinal ACE
Testicular ACE has an important role in reproduction and is 701 amino acids in length [63]. It has the same gene sequence as somatic ACE but has a tissue-specific promoter located within intron 12 [66]. Germinal ACE relies on chloride to a slight extent as compared to the C domain of sACE [67]. As previously observed, sACE has two active sites, whereas testicular ACE has only one active site [68].
6. Angiotensin-Converting Enzyme 2
ACE 2 is a zinc metalloenzyme and carboxypeptidase situated as an ectoenzyme on the surface of endothelial and disparate cells. It has a great homology with ACE and catalyzes the cleavage of angiotensin I directed toward angiotensin 1-9, and angiotensin II into the vasodilator angiotensin 1-7 as demonstrated schematically (Figure 1). Although the main substrate for ACE 2 is Ang II, it has other physiological substrates as well. In addition to this, ACE 2 also has functions other than catalysis which include improving of intestinal impartial amino acid transport and acting as a receptor for a crucial respiratory disease-causing SARS virus [69]. ACE 2 negatively regulates the renin-angiotensin system and catalyzes the conversion of angiotensin II to angiotensin 1-7 that has vasodilatory effects, thereby counterbalancing ACE activity. Increasing evidence suggests that its enzymatic activity has a protective role in cardiovascular diseases [70]. Upregulation of ACE 2 expression is usually recognized as a therapeutic approach in conditions like acute myocardial infarction [71], diabetes [72], hypertension [73], lung grudge [74], fertility [75], and fibrotic disorders [76]. ACE 2 has been mainly localized in tissues like the liver, intestines, and lungs [77] and also found to be present in the brain [78] where it acts as an inner regulator of cardiovascular function [79].
Figure 1.
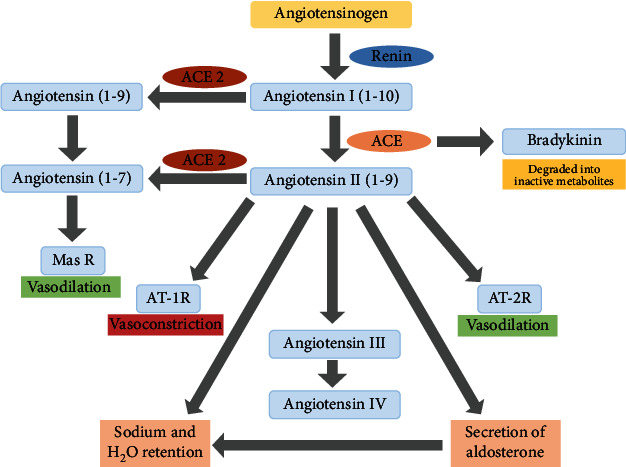
Schematic representation of the renin angiotensin aldosterone system. Abbreviations: Mas R: Mas Receptor; AT-1 R: angiotensin type 1 receptor; AT-2 R: angiotensin type 2 receptor; ACE: angiotensin-converting enzyme.
7. Polymorphisms in Renin-Angiotensin System
Numerous studies regarding polymorphisms in genes associated with RAS have been established. These polymorphisms have been found to be associated with various cardiovascular diseases [80]. Polymorphism is another extensive topic which is beyond the aspect of this review; hence, the focus here shall only be on polymorphism associated with ACE.
8. ACE Insertion/Deletion Polymorphism
Angiotensin converting enzyme is the main component of the renin-angiotensin system, and its insertion and deletion polymorphism is reported in intron 16 [81, 82]. Many researchers are working to study the involvement of genetic polymorphisms in ACE and its subsequent relation with hypertension [83]. ACE insertion and deletion polymorphism is associated with the occurrence of myocardial infarction, cardiovascular disease [84], left ventricular hypertrophy [85], microalbuminuria [86, 87], and pregnancy hypertensive disorders [88]. Furthermore, these polymorphisms are also associated with differences in blood pressure levels and organ damage, which is commonly reported in hypertensive patients [89].
9. Antihypertensive Drugs and Associated Side Effects
Commercially available antihypertensive drugs are given as a single drug or in combination of more than one drug so as to regulate high blood pressure. Although these drugs are very potent in controlling blood pressure in hypertensive patients, they have a number of side effects, some common and others rare. Some of these effects include low blood pressure or hypotension, kidney failure, liver failure, and decreased white blood cell count. These antihypertensives may also lead to birth defects in pregnant women and therefore should not be consumed during pregnancy. Studies have been conducted on the effects of antihypertensive drugs apart from their blood pressure-lowering effects.
A study carried out by Schunkert et al. showed that patients consuming antihypertensive like diuretics, beta blockers, ACE inhibitors, and ARBs are at a high risk of gout. Beta blockers and diuretics also increase the serum levels of uric acid which is in turn associated with gout [90]. Another report by Choi et al. reported that there are experimental and epidemiological findings which are suggestive of the fact that there is a link between antihypertensive drug-induced photosensitivity and skin cancer that may be due to DNA damage induced in predisposed individuals [91]. Another study reported the association of various antihypertensive drugs with the risk of cancer, for example, long-term use of angiotensin receptor blockers was found to be linked to malignant melanoma and long-term use of diuretics was linked to squamous cell carcinoma [92].
10. Alternate/Complimentary Treatment for Hypertension
Drugs extensively used for the treatment of hypertension cause a plethora of side effects, some rare and others serious, as mentioned in the above section. The need therefore arises to consider other sources of therapy for hypertension in the form of herbal medicines that have few or no known side effects [85] and are natural, safe, and effective [93]. There are studies suggesting the efficacy of various natural and herbal sources in controlling hypertension which include medicinal plants that are utilized for their antihypertensive properties [93] and include Allium sativum (garlic), Andrographis paniculata (king of bitter), Apium graveolens (celery), Bidens pilosa L. (beggar's tick, black-jack, etc.), Camellia sinensis (tea), Coptis chinensis (goldthread), Coriandrum sativum (cilantro or coriander), Crataegus spp. (hawthorns), Crocus sativus (saffron), Cymbopogon citratus (lemongrass), Hibiscus sabdariffa (roselle), Nigella sativa (black cumin; seed of blessing), Panax (ginseng), Salviae miltiorrhizae (chinese sage), and Zingiber officinale (ginger) [94]. A study also reports the use of Stevia leaf for treating diabetes and hypertension [95]. These antihypertensive plants have a variety of phytochemicals that have protective effects against hypertension [96].
Apart from the antihypertensive plants mentioned above, honey has also been shown to have therapeutic effects on cardiovascular diseases. People have been using honey since time immemorial for treating a plethora of ailments, and researchers have just started reconsidering its role in nutrition and therapeutics [97]. The high content of flavonoids as well as phenolic acids in natural honey is of significance for human health including protection against cardiovascular diseases [98]. A study on animal models using Malaysian honey has shown beneficial effects in protecting against hypertension [99]. The use of honey has various limitations including contamination with pesticides and antibiotics; therefore, these limitations need to be considered before using it for therapeutic purposes, although there is significant evidence that proves its health benefits [100].
11. Bioactive Peptides from Natural Sources as ACE Inhibitors
Bioactive peptides that are derived from food are compounds originating from animals and plants that are produced by food processing and fermentation [31]. Apart from nutrition, these have other regulatory functions in the human system including antimicrobial, antidiabetic, antiinflammatory, immune-modulatory, antioxidative, and renin and ACE 1 inhibitory bioactivities. Some of the food products that act as sources of ACE-I inhibitory peptides include dairy products such as fermented cheese and yogurt, collagen from fish skins, soy, meat by-products, hemp seed, broccoli, traditional medicines of Chinese and Iranian origin, cereals, micro and macroalgae, sardine, sesame, corn milk, and eggs [32]. These food products are under the “Food for Specified Health Uses” regulatory framework [32].
The length of inhibitory peptides is usually short, about 12 amino acids in length. This short length has its advantages for the inhibitory activity of the peptide such as helping in absorption and thereby ensuring its bioavailability. Some studies have reported that the presence of certain conditions enhance the ACE binding ability of the peptides. These include the presence of certain amino acids (hydrophobic) at the C-terminal along with the presence of proline, leucine, tryptophan, phenylalanine, and tyrosine at the C-terminal of the peptide. ACE inhibitory bioactive peptides have three classifications, namely, inhibitor type, substrate type, and prodrug type on the basis of their mode of action [32]. In the case of inhibitory type peptide, no cleavage of the peptide occurs, and hence its activity remains unchanged. The substrate-type peptide, on the other hand, is cleaved by ACE, and hence its activity decreases gradually. In the prodrug type, the larger peptide undergoes cleavage into smaller potent ACE inhibitory peptides by ACE itself [32]. Numerous clinical studies have suggested that food nutrients like proteins, carbohydrates, and fats have a significant role in blood pressure regulation. Substitution of carbohydrates with proteins and monounsaturated fats has a positive effect on maintaining blood pressure and lowering the risk of heart diseases. This positive effect of protein intake is associated with the presence of bioactive peptide fragments that are known to have antihypertensive effects. ACE-inhibiting antihypertensive peptides were first isolated from snake venom, and this led to further research to isolate such peptides from food in the hope to find an alternative to traditional antihypertensive medicines [31]. A list of some of the food-derived antihypertensive peptides is given in Table 2 having ACE inhibitory effects in both animal models and human subjects.
An interesting study on human subjects showed that oral administration of milk-derived peptides, IPP and VPP, significantly reduced blood pressure levels in individuals of Finnish and Japanese racial origin, but the same peptides failed to do so in individuals of Danish and Dutch racial origin. When these studies are performed in animal models, the animals usually belong to a specific strain, whereas human subjects may be of different racial and genetic origin. This leads to discrepancies in the results and efficacy of bioactive peptides. Therefore, this creates a need for more and more clinical trials on human subjects of varied ethnicities to establish a solid ground for the antihypertensive nature of bioactive peptides [31].
12. Conclusion
The present review highlights the role of the angiotensin-converting enzyme in blood pressure regulation and how its inhibitors are used in the control of hypertension. From our review, we can safely conclude that commercially available synthetic ACE inhibitors pose numerous side effects in patients, and since hypertension is a chronic disease and leads to various cardiovascular disorders, the long-term consumption of these synthetic inhibitors can be harmful.
Therefore, based on evidences from existing literature, it can be concluded that natural remedies in the form of medicinal plants, bioactive peptides from food, and honey can be used as putative treatment option for hypertension and other cardiovascular diseases. Further research is definitely required in this direction to accept or reject these remedies for their antihypertensive nature.
Acknowledgments
We would like to acknowledge the support from President-Prof. Ismail Matalka; Dean Dr. K.L. Bairy; RAK Medical and Health Sciences University, U.A.E.; Shoolini University, Solan, India; and Feinstein Institute of Medical Research, New York, for extending their support for the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Whelton P. K., Carey R. M., Aronow W. S., et al. Correction to: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Journal of the American College of Cardiology . 2018;71(6):e127–e248. doi: 10.1161/HYP.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 2.Meretskyi V., Meretska I., Kulyanda O., Kamtoh G. Direct renin inhibitors in arterial hypertension treatment. Scientific Journal of Polonia University . 2017;25(6):146–154. doi: 10.23856/2515. [DOI] [Google Scholar]
- 3.Kjeldsen S., Feldman R. D., Lisheng L., et al. Updated national and international hypertension guidelines: a review of current recommendations. Drugs . 2014;74(17):2033–2051. doi: 10.1007/s40265-014-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddouks M., Maghrani M., Lemhadri A., Ouahidi M. L., Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) Journal of Ethnopharmacology . 2002;82(2-3):97–103. doi: 10.1016/S0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- 5.Giles T. D., Materson B. J., Cohn J. N., Kostis J. B. Definition and classification of hypertension: an update. The Journal of Clinical Hypertension . 2009;11(11):611–614. doi: 10.1111/j.1751-7176.2009.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancia G., De Backer G., Dominiczak A., et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) European Heart Journal . 2007;28(12):1462–1536. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 7.Weber M. A., Schiffrin E. L., White W. B., et al. Clinical practice guidelines for the management of hypertension in the community. The Journal of Clinical Hypertension . 2014;16(1):14–26. doi: 10.1111/jch.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens S. L., Wood S., Koshiaris C., et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ . 2016;354, article i4098 doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chazova I. E., Dongre N., Vigdorchik A. V. Real-life safety and effectiveness of amlodipine/valsartan combination in the treatment of hypertension. Advances in Therapy . 2011;28(2):134–149. doi: 10.1007/s12325-010-0099-1. [DOI] [PubMed] [Google Scholar]
- 10.Williams B., Poulter N. R., Brown M. J., et al. British hypertension society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ . 2004;328(7440):634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baradaran A., Nasri H., Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. Journal of Research in Medical Sciences . 2014;19(4):358–367. [PMC free article] [PubMed] [Google Scholar]
- 12.Dzau V. J. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation . 1988;77, 6 Part 2:I4–13. [PubMed] [Google Scholar]
- 13.Kobori H., Nangaku M., Navar L. G., Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacological Reviews . 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 14.Elliott W. J., Ram C. V. S. Calcium channel blockers. The Journal of Clinical Hypertension . 2011;13(9):687–689. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsoi B., Akioyamen L. E., Bonner A., et al. Comparative efficacy of angiotensin II antagonists in essential hypertension: systematic review and network meta-analysis of randomised controlled trials. Heart, Lung & Circulation . 2018;27(6):666–682. doi: 10.1016/j.hlc.2017.06.721. [DOI] [PubMed] [Google Scholar]
- 16.Seegobin K., Maharaj S., Deosaran A., Reddy P. Severe beta blocker and calcium channel blocker overdose: role of high dose insulin. The American Journal of Emergency Medicine . 2018;36(4):736.e5–736.e6. doi: 10.1016/j.ajem.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Wiysonge C. S., Bradley H. A., Volmink J., Mayosi B. M. Cochrane corner: beta-blockers for hypertension. Heart . 2018;104(4):282–283. doi: 10.1136/heartjnl-2017-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkerson R. G., Martinelli A. N., Oliver W. D. Treatment of angioedema induced by angiotensin-converting enzyme inhibitor. Journal of Emergency Medicine . 2018;55(1):132–133. doi: 10.1016/j.jemermed.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Upadhya B., Brubaker P. H., Morgan T. M., et al. The effect of Aliskiren on exercise capacity in older patients with heart failure and preserved ejection fraction: a randomized, placebo-controlled, double-blind trial. American Heart Journal . 2018;201:164–167. doi: 10.1016/j.ahj.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogari R., Mugellini A., Zoppi A., et al. Influence of losartan and atenolol on memory function in very elderly hypertensive patients. Journal of Human Hypertension . 2003;17(11):781–785. doi: 10.1038/sj.jhh.1001613. [DOI] [PubMed] [Google Scholar]
- 21.Mason R. P., Marche P., Hintze T. H. Novel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipine. Arteriosclerosis, Thrombosis, and Vascular Biology . 2003;23(12):2155–2163. doi: 10.1161/01.ATV.0000097770.66965.2A. [DOI] [PubMed] [Google Scholar]
- 22.Mason J. M., Dickinson H. O., Nicolson D. J., Campbell F., Ford G. A., Williams B. The diabetogenic potential of thiazide-type diuretic and beta-blocker combinations in patients with hypertension. Journal of Hypertension . 2005;23(10):1777–1781. doi: 10.1097/01.hjh.0000177537.91527.09. [DOI] [PubMed] [Google Scholar]
- 23.Abadir P. M., Periasamy A., Carey R. M., Siragy H. M. Angiotensin II type 2 receptor–bradykinin B2 receptor functional heterodimerization. Hypertension . 2006;48(2):316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 24.Weir M. R., Bush C., Anderson D. R., Zhang J., Keefe D., Satlin A. Antihypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren in patients with hypertension: a pooled analysis. Journal of the American Society of Hypertension . 2007;1(4):264–277. doi: 10.1016/j.jash.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Esunge P. M. From blood pressure to hypertension: the history of research. Journal of the Royal Society of Medicine . 1991;84(10):p. 621. doi: 10.1177/014107689108401019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangalore S., Messerli F. H. β-Blockers as fourth-line therapy for hypertension: stay the course. International Journal of Clinical Practice . 2008;62(11):1643–1646. doi: 10.1111/j.1742-1241.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 27.Nuttall S. L., Routledge H. C., Kendall M. J. A comparison of the β1-selectivity of three β1-selective β-blockers. Journal of Clinical Pharmacy and Therapeutics . 2003;28(3):179–186. doi: 10.1046/j.1365-2710.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 28.Weber M. A. The role of the new β-blockers in treating cardiovascular disease. American Journal of Hypertension . 2005;18(12):169–176. doi: 10.1016/j.amjhyper.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Michelke L., Deussen A., Dieterich P., Martin M. Effects of bioactive peptides encrypted in whey-, soy- and rice protein on local and systemic angiotensin-converting enzyme activity. Journal of Functional Foods . 2017;28:299–305. doi: 10.1016/j.jff.2016.11.026. [DOI] [Google Scholar]
- 30.Barbosa-Filho J. M., Martins V. K., Rabelo L. A., et al. Natural products inhibitors of the angiotensin converting enzyme (ACE): a review between 1980-2000. Revista Brasileira de Farmacognosia . 2006;16(3):421–446. doi: 10.1590/S0102-695X2006000300021. [DOI] [Google Scholar]
- 31.Majumder K., Wu J. Molecular targets of antihypertensive peptides: understanding the mechanisms of action based on the pathophysiology of hypertension. International Journal of Molecular Sciences . 2015;16(1):256–283. doi: 10.3390/ijms16010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganguly A., Sharma K., Majumder K. Food-derived bioactive peptides and their role in ameliorating hypertension and associated cardiovascular diseases. Advances in Food and Nutrition Research . 2019;89:165–207. doi: 10.1016/bs.afnr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Yim H. E., Yoo K. H. Renin-angiotensin system-considerations for hypertension and kidney. Electrolytes & Blood Pressure . 2008;6(1):42–50. doi: 10.5049/EBP.2008.6.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao D. Y., Saito S., Veiras L. C., et al. Role of angiotensin-converting enzyme in myeloid cell immune responses. Cellular & Molecular Biology Letters . 2020;25(1):1–12. doi: 10.1186/s11658-020-00225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmermans P. B., Wong P. C., Chiu A. T., et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacological Reviews . 1993;45(2):205–251. [PubMed] [Google Scholar]
- 36.Gandhi S. K., Gainer J., King D., Brown N. J. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension . 1998;31(1):90–96. doi: 10.1161/01.HYP.31.1.90. [DOI] [PubMed] [Google Scholar]
- 37.Pettinger W. A., Campbell W. B., Keeton K. Adrenergic component of renin release induced by vasodilating antihypertensive drugs in the rat. Circulation Research . 1973;33(1):82–86. doi: 10.1161/01.RES.33.1.82. [DOI] [PubMed] [Google Scholar]
- 38.Brown N. J., Vaughan D. E. Angiotensin-converting enzyme inhibitors. Circulation . 1998;97(14):1411–1420. doi: 10.1161/01.CIR.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 39.Lévy S. Drug Insight: angiotensin-converting-enzyme inhibitors and atrial fibrillation--indications and contraindications. Nature Clinical Practice. Cardiovascular Medicine . 2006;3(4):220–225. doi: 10.1038/ncpcardio0480. [DOI] [PubMed] [Google Scholar]
- 40.Robles N. R., Cerezo I., Hernandez-Gallego R. Renin–angiotensin system blocking drugs. Journal of Cardiovascular Pharmacology and Therapeutics . 2014;19(1):14–33. doi: 10.1177/1074248413501018. [DOI] [PubMed] [Google Scholar]
- 41.Tehrani A. Y., White Z., Tung L. W., et al. Pleiotropic activation of endothelial function by angiotensin II receptor blockers is crucial to their protective anti-vascular remodeling effects. Scientific Reports . 2022;12(1):p. 9771. doi: 10.1038/s41598-022-13772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natesh R., Schwager S. L., Sturrock E. D., Acharya K. R. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature . 2003;421(6922):551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- 43.Santhamma K. R., Sadhukhan R., Kinter M., Chattopadhyay S., McCue B., Sen I. Role of tyrosine phosphorylation in the regulation of cleavage secretion of angiotensin-converting enzyme. Journal of Biological Chemistry . 2004;279(38):40227–40236. doi: 10.1074/jbc.M407176200. [DOI] [PubMed] [Google Scholar]
- 44.Guang C., Phillips R. D., Jiang B., Milani F. Proteases cles du systeme renine-angiotensine: enzyme de conversion de l'angiotensine 1 et 2 et renine. Archives of Cardiovascular Diseases . 2012;105(6-7):373–385. doi: 10.1016/j.acvd.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyal A., Cusick A. S., Thielemier B. Stat Pearls . Stat Pearls Publishing, Treasure Island (FL); 2022. ACE inhibitors. [PubMed] [Google Scholar]
- 46.Julius S., Kjeldsen S. E., Weber M., et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. The Lancet . 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 47.Ondetti M. A., Rubin B., Cushman D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science . 1977;196(4288):441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 48.Ramalingam L., Menikdiwela K., LeMieux M., et al. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease . 2017;1863(5):1106–1114. doi: 10.1016/j.bbadis.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Campbell D. J., Kladis A., Duncan A. M. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension . 1994;23(4):439–449. doi: 10.1161/01.HYP.23.4.439. [DOI] [PubMed] [Google Scholar]
- 50.Golias C., Charalabopoulos A., Stagikas D., Charalabopoulos K., Batistatou A. The kinin system–bradykinin: biological effects and clinical implications. Multiple role of the kinin system–bradykinin. Hippokratia . 2007;11(3):124–128. [PMC free article] [PubMed] [Google Scholar]
- 51.Bunn P. A., Jr., Chan D., Dienhart D. G., Tolley R., Tagawa M., Jewett P. B. Neuropeptide signal transduction in lung cancer: clinical implications of bradykinin sensitivity and overall heterogeneity. Cancer Research . 1992;52(1):24–31. [PubMed] [Google Scholar]
- 52.Ishihara K., Hayash I., Yamashina S., Majima M. A potential role of bradykinin in angiogenesis and growth of S-180 mouse tumors. Japanese Journal of Pharmacology . 2001;87(4):318–326. doi: 10.1254/jjp.87.318. [DOI] [PubMed] [Google Scholar]
- 53.Stewart J. M. Bradykinin antagonists as anti-cancer agents. Current Pharmaceutical Design . 2003;9(25):2036–2042. doi: 10.2174/1381612033454171. [DOI] [PubMed] [Google Scholar]
- 54.Munoz M., Covenas R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides . 2013;48:1–9. doi: 10.1016/j.peptides.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Sanchis-Gomar F., Lavie C. J., Perez-Quilis C., Henry B. M., Lippi G. Angiotensin converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clinic Proceedings . 2020;95(6):1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Regulski M., Regulska K., Stanisz J. B., et al. Chemistry and pharmacology of angiotensin-converting enzyme inhibitors. Current Pharmaceutical Design . 2015;21(13):1764–1775. doi: 10.2174/1381612820666141112160013. [DOI] [PubMed] [Google Scholar]
- 57.McDowell S. E., Coleman J. J., Ferner R. E. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ . 2006;332(7551):1177–1181. doi: 10.1136/bmj.38803.528113.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yılmaz İ., Türk M., Baran Ketencioğlu B., et al. The presence of underlying asthma should be investigated in patients diagnosed with ACE inhibitor induced cough. The Clinical Respiratory Journal . 2020;14(4):382–388. doi: 10.1111/crj.13143. [DOI] [PubMed] [Google Scholar]
- 59.Rosenthal T., Gavras I. Angiotensin inhibition and malignancies: a review. Journal of Human Hypertension . 2009;23(10):623–635. doi: 10.1038/jhh.2009.21. [DOI] [PubMed] [Google Scholar]
- 60.Xu K., Han H., Luo Y., Ye H., Lin H., Ni L. The angiotensin-converting enzyme inhibitory state promotes the transformation of non-small cell lung cancer blood supply pattern toward vasculogenic mimicry formation. Frontiers in Oncology . 2021;11, article 663671 doi: 10.3389/fonc.2021.663671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prontera C., Mariani B., Rossi C., Poggi A., Rotilio D. Inhibition of gelatinase a (MMP-2) by batimastat and captopril reduces tumor growth and lung metastases in mice bearing Lewis lung carcinoma. International Journal of Cancer . 1999;81(5):761–766. doi: 10.1002/(SICI)1097-0215(19990531)81:5<761::AID-IJC16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 62.Sipahi I., Chou J., Mishra P., Debanne S. M., Simon D. I., Fang J. C. Meta-analysis of randomized controlled trials on effect of angiotensin-converting enzyme inhibitors on cancer risk. The American Journal of Cardiology . 2011;108(2):294–301. doi: 10.1016/j.amjcard.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 63.Wu Z., Yao T., Wang Z., et al. Association between angiotensin-converting enzyme inhibitors and risk of lung cancer: a systematic review and meta-analysis. British Journal of Cancer . 2022;17:1–9. doi: 10.2139/ssrn.4113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohlstedt K., Busse R., Fleming I. Signaling via the angiotensin-converting enzyme enhances the expression of cyclooxygenase-2 in endothelial cells. Hypertension . 2005;45(1):126–132. doi: 10.1161/01.HYP.0000150159.48992.11. [DOI] [PubMed] [Google Scholar]
- 65.Costerousse O., Allegrini J., Huang W., Alhenc-Gelas F. Angiotensin I-converting enzyme (kininase II) in cardiovascular and renal regulations and diseases. Biological Research . 1998;31(3):161–167. [PubMed] [Google Scholar]
- 66.Fleming I. Signaling by the angiotensin-converting enzyme. Circulation Research . 2006;98(7):887–896. doi: 10.1161/01.RES.0000217340.40936.53. [DOI] [PubMed] [Google Scholar]
- 67.Acharya K., Sturrock E., Riordan J., Ehlers M. R. W. Ace revisited: a new target for structure-based drug design. Nature Reviews. Drug Discovery . 2003;2(11):891–902. doi: 10.1038/nrd1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riordan J. F. Angiotensin-I-converting enzyme and its relatives. Genome Biology . 2003;4(8):225–225. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cozier G. E., Arendse L. B., Schwager S. L., Sturrock E. D., Acharya K. R. Molecular basis for multiple omapatrilat binding sites within the ACE C-domain: implications for drug design. Journal of Medicinal Chemistry . 2018;61(22):10141–10154. doi: 10.1021/acs.jmedchem.8b01309. [DOI] [PubMed] [Google Scholar]
- 70.Villela D., Leonhardt J., Patel N., et al. Angiotensin type 2 receptor (AT2R) and receptor mas: a complex liaison. Clinical Science . 2015;128(4):227–234. doi: 10.1042/CS20130515. [DOI] [PubMed] [Google Scholar]
- 71.Kuba K., Imai Y., Penninger J. M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circulation Journal . 2013;77(2):301–308. doi: 10.1253/circj.CJ-12-1544. [DOI] [PubMed] [Google Scholar]
- 72.Der Sarkissian S., Huentelman M. J., Stewart J., Katovich M. J., Raizada M. K. ACE2: a novel therapeutic target for cardiovascular diseases. Progress in Biophysics and Molecular Biology . 2006;91(1-2):163–198. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 73.Soler M. J., Barrios C., Oliva R., Batlle D. Pharmacologic modulation of ACE2 expression. Current Hypertension Reports . 2008;10(5):410–414. doi: 10.1007/s11906-008-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arendse L. B., Danser A. J., Poglitsch M., et al. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacological Reviews . 2019;71(4):539–570. doi: 10.1124/pr.118.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole-Jeffrey C. T., Liu M., Katovich M. J., Raizada M. K., Shenoy V. ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. Journal of Cardiovascular Pharmacology . 2015;66(6):540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner A. J., Nalivaeva N. N. Angiotensin-converting enzyme 2 (ACE2): two decades of revelations and re-evaluation. Peptides . 2022;151, article 170766 doi: 10.1016/j.peptides.2022.170766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner A. J. The Protective Arm of the Renin Angiotensin System (RAS) Elsevier; 2015. ACE2 cell biology, regulation, and physiological functions; p. p. 185. [Google Scholar]
- 78.Mendoza-Torres E., Oyarzún A., Mondaca-Ruff D., et al. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Therapeutic Advances in Cardiovascular Disease . 2015;9(4):217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 79.Xu P., Sriramula S., Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology . 2011;300(4):R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paizis G., Tikellis C., Cooper M. E., et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut . 2005;54(12):1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Losito A., Kalidas K., Santoni S., Ceccarelli L., Jeffery S. Polymorphism of renin–angiotensin system genes in dialysis patients--association with cerebrovascular disease. Nephrology, Dialysis, Transplantation . 2002;17(12):2184–2188. doi: 10.1093/ndt/17.12.2184. [DOI] [PubMed] [Google Scholar]
- 82.Sayed-Tabatabaei F. A., Oostra B. A., Isaacs A., Van Duijn C. M., Witteman J. C. M. ACE polymorphisms. Circulation Research . 2006;98(9):1123–1133. doi: 10.1161/01.RES.0000223145.74217.e7. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L., Miyaki K., Araki J., et al. Interaction of angiotensin i–converting enzyme insertion-deletion polymorphism and daily salt intake influences hypertension in Japanese men. Hypertension Research . 2006;29(10):751–758. doi: 10.1291/hypres.29.751. [DOI] [PubMed] [Google Scholar]
- 84.Zhou T. B., Qin Y. H., Su L. N., Lei F. Y., Huang W. F., Zhao Y. J. ACE I/D gene polymorphism can't predict the steroid responsiveness in Asian children with idiopathic nephrotic syndrome: a meta-analysis. PLoS One . 2011;6(5, article e19599) doi: 10.1371/journal.pone.0019599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zintzaras E., Raman G., Kitsios G., Lau J. Angiotensin-converting enzyme insertion/deletion gene polymorphic variant as a marker of coronary artery disease: a meta-analysis. Archives of Internal Medicine . 2008;168(10):1077–1089. doi: 10.1001/archinte.168.10.1077. [DOI] [PubMed] [Google Scholar]
- 86.Fedor R., Asztalos L., Lőcsey L., et al. Insertion/deletion polymorphism of the angiotensin-converting enzyme predicts left ventricular hypertrophy after renal transplantation. Transplantation Proceedings . 2011;43(4):1259–1260. doi: 10.1016/j.transproceed.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 87.Ng D. P. K., Tai B. C., Koh D., Tan K. W., Chia K. S. Angiotensin-I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: a meta-analysis of studies reported between 1994 and 2004 and comprising 14, 727 subjects. Diabetologia . 2005;48(5):1008–1016. doi: 10.1007/s00125-005-1726-2. [DOI] [PubMed] [Google Scholar]
- 88.Foffa I., Murzi M., Mariani M., et al. Angiotensin-converting enzyme insertion/deletion polymorphism is a risk factor for thoracic aortic aneurysm in patients with bicuspid or tricuspid aortic valves. The Journal of Thoracic and Cardiovascular Surgery . 2012;144(2):390–395. doi: 10.1016/j.jtcvs.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 89.Reshetnikov E. A., Akulova L. Y., Dobrodomova I. S., Dvornyk V. Y., Polonikov A. V., Churnosov M. I. The insertion-deletion polymorphism of the ACE gene is associated with increased blood pressure in women at the end of pregnancy. System . 2015;16(3):623–632. doi: 10.1177/1470320313501217. [DOI] [PubMed] [Google Scholar]
- 90.Schunkert H., Hense H. W., Holmer S. R., et al. Association between a deletion polymorphism of the angiotensin-converting-enzyme gene and left ventricular hypertrophy. New England Journal of Medicine . 1994;330(23):1634–1638. doi: 10.1056/NEJM199406093302302. [DOI] [PubMed] [Google Scholar]
- 91.Choi H. K., Soriano L. C., Zhang Y., Rodríguez L. A. G. Antihypertensive drugs and risk of incident gout among patients with hypertension: population-based case-control study. BMJ . 2012;344(jan12 1, article d8190) doi: 10.1136/bmj.d8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cassano N., Di Stefani A., Vena G. A., Peris K. Antihypertensive drugs and risk of skin cancer. Giornale Italiano di Dermatologia e Venereologia: Organo Ufficiale, Societa Italiana di Dermatologia e Sifilografia . 2018;153(5):672–684. doi: 10.23736/S0392-0488.18.05870-4. [DOI] [PubMed] [Google Scholar]
- 93.Schmidt S. A. J., Schmidt M., Mehnert F., Lemeshow S., Sørensen H. T. Use of antihypertensive drugs and risk of skin cancer. Journal of the European Academy of Dermatology and Venereology . 2015;29(8):1545–1554. doi: 10.1111/jdv.12921. [DOI] [PubMed] [Google Scholar]
- 94.Afolayan A. J., Wintola O. A. Dietary supplements in the management of hypertension and diabetes-a review. African Journal of Traditional, Complementary, and Alternative Medicines . 2014;11(3):248–258. doi: 10.4314/ajtcam.v11i3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chrysant S. G., Chrysant G. S. Herbs used for the treatment of hypertension and their mechanism of action. Current Hypertension Reports . 2017;19(9):1–10. doi: 10.1007/s11906-017-0775-5. [DOI] [PubMed] [Google Scholar]
- 96.Tomasulo P. LactMed-new NLM database on drugs and lactation. Medical Reference Services Quarterly . 2007;26(1):51–58. doi: 10.1300/J115v26S01_04. [DOI] [PubMed] [Google Scholar]
- 97.Al Disi S. S., Anwar M. A., Eid A. H. Anti-hypertensive herbs and their mechanisms of action: part I. Frontiers in Pharmacology . 2015;6:p. 323. doi: 10.3389/fphar.2015.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmed S., Othman N. H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. The Malaysian Journal of Medical Sciences . 2013;20(3):6–13. [PMC free article] [PubMed] [Google Scholar]
- 99.Cianciosi D., Forbes-Hernández T. Y., Afrin S., et al. Phenolic compounds in honey and their associated health benefits: a review. Molecules . 2018;23(9):p. 2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ajibola A. Novel insights into the health importance of natural honey. The Malaysian Journal of Medical Sciences . 2015;22(5):7–22. [PMC free article] [PubMed] [Google Scholar]


