Abstract
Testosterone deficiency (TD) is an increasingly common problem with significant health implications, but its diagnosis and management can be challenging. A multi-disciplinary panel from BSSM reviewed the available literature on TD and provide evidence-based statements for clinical practice. Evidence was derived from Medline, EMBASE and Cochrane searches on hypogonadism, testosterone therapy (T Therapy) and cardiovascular safety from May 2017 to September 2022. This revealed 1,714 articles, including 52 clinical trials and 32 placebo-controlled randomised controlled trials. A total of twenty-five statements are provided, relating to five key areas: screening, diagnosis, initiating T Therapy, benefits and risks of T Therapy, and follow-up. Seven statements are supported by level 1 evidence, eight by level 2, five by level 3, and five by level 4. Recent studies have demonstrated that low levels of testosterone in men are associated with increased risk of incident type 2 diabetes mellitus, worse outcomes in chronic kidney disease and COVID 19 infection with increased all-cause mortality, along with significant quality of life implications. These guidelines should help practitioners to effectively diagnose and manage primary and age-related TD.
Keywords: Erectile dysfunction, Hypogonadism, Testosterone deficiency, Testosterone therapy
INTRODUCTION
Testosterone is the principal androgen in men. The term ‘testosterone deficiency (TD)’ is used throughout, in preference to hypogonadism, which refers to underactivity of both endocrine and reproductive function of the testes. TD is a well-established and significant medical condition [1,2]. Testosterone is essential for the development and maintenance of secondary male characteristics [3]. When testosterone levels fall, patients may experience physical, psychological and metabolic effects, which can compromise their cardiovascular, metabolic and general health, well-being, sexuality and fertility [4,5].
These statements have been developed for UK practice and aim to address the widespread media and scientific concerns over the appropriate treatment of men with TD with testosterone therapy (T Therapy).
DEFINITION AND TERMINOLOGY
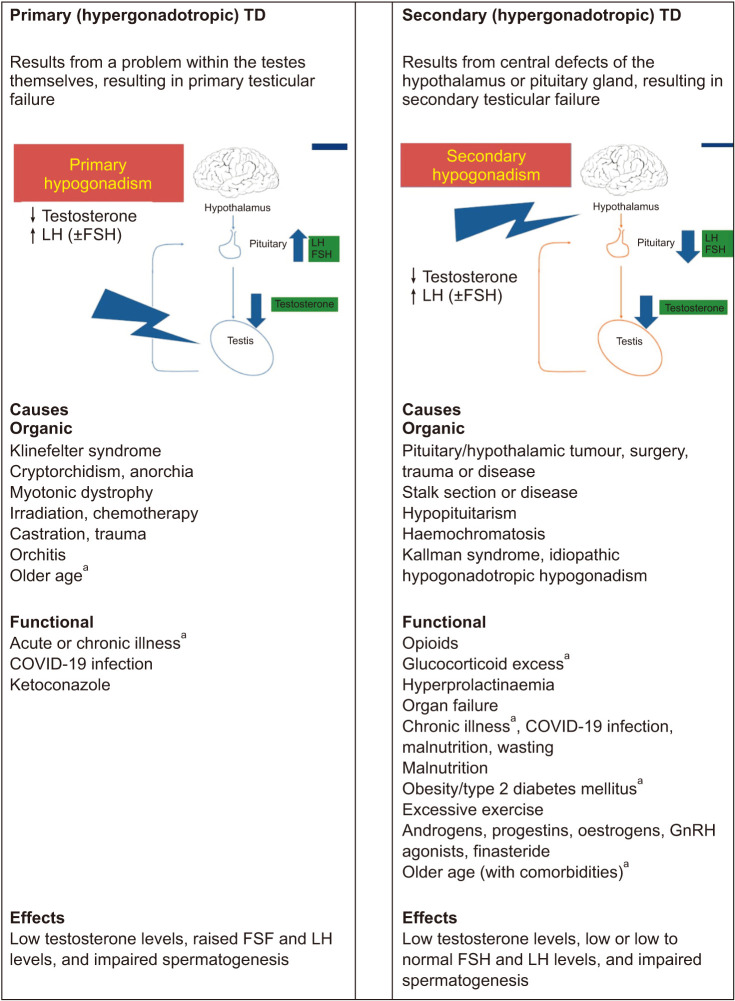
TD is a clinical and biochemical syndrome that stems from a reduced production of testosterone and spermatozoa by the testis. It can adversely affect multiple bodily systems and is associated with a marked decrease in quality of life. Hypogonadism is primarily classified according to it being a congenital or acquired disorder; the anatomical level of dysfunction: at testicular (primary hypogonadism), hypothalamic-pituitary (secondary hypogonadism) or combined levels and recognises affected men with idiopathic, metabolic or iatrogenic conditions resulting in TD. Treatment decisions should include lifestyle measures and a holistic review of the patient. Organic hypogonadism refers to conditions resulting in low testosterone where there is a proven dysfunction of the hypothalamic-pituitary-testicular (HPT) axis suppression rather than a functional gonadal axis suppression secondary to ill health (functional hypogonadism) [1].
It is defined as a clinical and biochemical syndrome associated with advancing age and comorbidities (LoE 2, Grade B) [6] characterised by a deficiency in serum androgen levels, (with or without reduced genomic sensitivity to androgens [1]), and relevant signs and symptoms [6,7].
TD can adversely affect multiple organ systems and result in significant reductions in quality of life, including changes in sexual function (LoE 2, Grade B) [4,6,7].
EPIDEMIOLOGY
Estimates regarding the prevalence of TD vary widely. The European Male Aging Study (EMAS) evaluated over 3,000 men aged 40–79 years according to biochemistry and symptoms. Results showed an overall prevalence of 2.1% in men aged 40–79 years and rates of 0.1% in 40–49 year olds, 0.6% in 50–59 year olds, 3.2% in 60–69 year olds and 5.1% in 70–79 year olds (where the syndrome of TD included at least 3 sexual symptoms associated with a total testosterone (TT) level <11 nmol/L and a free testosterone (FT) level <220 pmol/L (<0.22 nmol/L) [8]. However, 75% of men maintained normal testosterone levels into old age, suggesting that TD is not merely a function of aging. The prevalence of secondary TD was 11.8%, with 2% having primary TD and 9.5% having compensated (subclinical) TD, worthy of observation but not treatment with testosterone [9].
TD is more common in older men, especially those with obesity, type 2 diabetes, chronic kidney disease (CKD) and other comorbidities, including acute COVID-19 infection and its long term manifestations, plus general poor health status or medications, especially opiates, anabolic steroids, antipsychotics and anticonvulsants.
BASIC PHYSIOLOGY
In eugonadal men, the regulation of testosterone production is controlled by the hypothalamic-pituitary-gonadal (HPG) axis [3]. In the brain, the hypothalamus secretes gonadotropin-releasing hormone (GnRH), which stimulates the anterior pituitary gland to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In the testes, LH stimulates the Leydig cells to produce testosterone, while FSH stimulates the seminiferous tubules for sperm maturation. Via a negative feedback mechanism, testosterone inhibits GnRH and LH secretion, while the hormone inhibin B, secreted by the Sertoli cells, inhibits the surge of FSH [3,10].
Androgen receptors (ARs) are present in many body tissues. They allow the body to respond appropriately to testosterone by attaching (binding) to it. The resulting androgen-receptor complex then binds to DNA, regulating the activity of androgen-responsive genes. The AR gene contains a DNA segment known as CAG, which is repeated multiple times. In most people, the number of CAG repeats range from less than 10 to around 36 [11]. The length of these repeats in the AR gene may influence androgen sensitivity [12], androgen action [13], and androgenic phenotypical effects, even in the presence of normal testosterone levels [13].
A higher number of CAG repeats at the testosterone receptor gene associates with higher body mass index (BMI)/increased HbA1c. Heald et al [14] followed up 274 men over 15 years and reported a ‘u’ shaped relation between CAG repeat number and mortality rate. They suggested that CAG repeat number measurement may become part of cardiovascular risk assessment in type 2 diabetes mellitus (T2DM) men [14].
Testosterone has multiple effects on the body. In the brain, it stimulates libido and aggression, and aids cognition, memory and feelings. In the kidneys, it promotes erythropoiesis. In the skin, it supports collagen production, and stimulates hair growth and sebum production. In the heart, it affects cardiac output and coronary and peripheral blood flow, reduces the QTc interval and decreases reperfusion injury. Testosterone also affects muscle mass and strength, bone growth, density and erythropoiesis, growth of the sex organs, spermatogenesis and erectile function [15,16,17,18].
AETIOLOGY
TD occurs when the body is unable to produce enough testosterone in order to function normally. This may result from disruption of one or more levels of the HPG axis [6]:
• The testes (primary TD) [19]
• The hypothalamus and pituitary gland (secondary TD) (LoE 1) [20]
• The hypothalamus/pituitary and testes (combined primary and secondary TD) Grade A
Secondary TD is the most common form [9,21] The term ‘functional’ (late-onset [LOH], age-related, or adult onset) TD, has recently been introduced to describe androgen-deficiency-like features and low testosterone levels in men aged over 50 years. LOH is associated with conditions such as obesity and the metabolic syndrome, in the absence of both intrinsic, structural HPT axis pathology and specific pathological conditions suppressing the HPT axis (e.g., microprolactinoma or endogenous Cushing syndrome) [19] (see Fig. 1 for other causes of secondary TD). The British Society For Sexual Medicine (BSSM) however, are concerned that that this definition may lead to men with potentially important co-morbid conditions going untreated.
Fig. 1. Causes and effects of TD. TD: testosterone deficiency, LH: luteinizing hormone, FSH: follicle-stimulating hormone. aCombined primary and secondary. Data from Grossman et al (J Clin Endocrinol Metab 2017;102:1067-75) [19].
Combined primary and secondary TD, sometimes known as mixed TD, is associated with low testosterone levels, impaired spermatogenesis and variable FSH and LH levels, depending on whether primary or secondary testicular failure predominates [22] Combined primary and secondary TD occurs with certain conditions such as sickle cell disease, haemochromatosis, thalassemia, and mutations in the DAX-1 gene, as well as with glucocorticoid treatment and alcoholism [22,23].
SUBCLINICAL (COMPENSATED) TD (HYPOGONADISM)
This is a less studied type of hypogonadism, characterized by normal testosterone levels combined with elevated L for which the European Male Ageing study showed a prevalence of 10%. Whilst testosterone levels remain normal, there is the possibility that they declined from upper to lower normal. The high LH may reflect an adjustment of the hypothalamic-pituitary-testicular feedback loop in order to compensate. Moreover, subclinical hypogonadism shares some of the adverse health outcomes with overt hypogonadism, hence early identification and interventions such as lifestyle, are key. However, clinical experience tells us that advice alone is often not enough if testosterone is low partly because motivation and energy to change is poor in the presence of a low testosterone. However, motivation can increase via, for example, an improvement in mood, with help of testosterone replacement therapy. Long term follow-up is usually recommended but recent guidelines suggest early intervention [24].
TD may also result if the action of testosterone is impaired, due to reduced bioavailability of the hormone (resulting from variations in sex hormone binding globulin [SHBG]) [6] or because of AR changes that can affect androgen activity (LoE 2, Grade A) [6,14].
Certain medications may suppress testosterone levels, including oral glucocorticoids, opioids, anti-convulsants and antipsychotics [25,26,27,28] (see Table 1).
Table 1. Factors associated with an increased prevalence of TD.
| Andrological/endocrinological | Metabolic diseases associated with insulin resistance | Cardiovascular diseases | Other chronic diseases | Pharmacologic |
|---|---|---|---|---|
| Delayed puberty Cryptorchidism Pituitary disease Infertility Varicocele Testicular cancer plus associated therapy |
Obesity Metabolic syndrome Type 2 diabetes |
Hypertension Coronary artery disease Cerebrovascular disease Chronic heart failure Atrial fibrillation |
Chronic obstructive pulmonary disease Obstructive sleep apnoea End-stage renal disease Cirrhosis Osteoporosis Rheumatoid arthritis HIV infection Cancer, especially testicular cancer and associated therapies Anaemia Osteoporosis COVID 19 infection |
Oral glucocorticoid treatment Regular opioid use Antipsychotic medications Androgen deprivation therapy Finasteride/dutasteride Methadone maintenance therapy Antiretroviral therapy Chemotherapy & radiation Anticonvulsant therapy Roaccutane therapy for acne |
TD: testosterone deficiency.
Data from Khera et al (J Sex Med 2016;13:1787-804) [6].
PRESCRIBED OPIATE MEDICATIONS
Several studies have confirmed high levels of hypogonadism (25%–80%) in men on long-term prescribed opiates, dependent on the drug and dose prescribed [26]. We recommend routine screening of this population. Ideally reduced opiate prescribing and lifestyle change should be attempted, but often this is not practical in patients with chronic pain. We support the prescribing of testosterone therapy in conjunction with lifestyle change for patients with confirmed opiate induced hypogonadism [25].
POST FINASTERIDE SYNDROME
Finasteride and dutasteride are often prescribed to treat lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH) and male pattern hair loss in men with androgenic alopecia for prolonged periods of time [29]. It is postulated that men treated with these drugs are in a state of androgen deficiency and are at high risk of developing erectile dysfunction (ED), NAFLD, IR, T2DM, dry eye disease, potential kidney dysfunction, among other metabolic and psychiatric dysfunctions. Reviews suggest that greater attention is taken to assess patients at risk before prescribing long term. A similar syndrome of prolonged sexual dysfunction is seen with isotretinoin treatment for acne [30].
For these reasons, we believe that the clinical community should recognize these new potential health risks associated with these drugs and the need for assessment of sexual dysfunction, measurement of testosterone levels and testosterone and PDE5 inhibitor therapy for enduring symptoms associated with low testosterone levels, and LUTS/BPH.
CLINICAL DIAGNOSIS OF TD
The diagnosis of symptomatic TD requires the presence of characteristic signs and symptoms (Level 2, Grade A), PLUS reduced serum concentrations of TT or FT (Level 2, Grade A) [8].
The assessment of gonadotropins is required to determine the origin of the TD (see the section ‘LABORATORY DIAGNOSIS’).
SIGNS, SYMPTOMS AND COMORBIDITIES
TD has well established symptoms [1]. The Fourth International Consultation for Sexual Medicine (ICSM) [6] made the following recommendations for the clinical diagnosis of TD, based on the signs and symptoms:
• Sexual dysfunction, especially low sexual desire, reduced morning and night-time erections, and ED are prominent, commonly presenting symptoms, particularly suggestive of TD when associated with each other (LoE 1, Grade A) [6]. As the link between morning erections and cardiovascular disease (CVD) is well established, it is important that the question is asked to patients not currently involved in physical relationships [1].
• Less-specific symptoms such as fatigue, sleep disturbance, loss of physical strength, reduced energy and motivation, and depressed mood are often present (LoE 1, Grade B) [6].
• Visceral obesity, and reductions in muscle mass and bone mineral density (BMD), are commonly observed (LoE 1, Grade A) [6].
• Hot flushes, and changes in cognition and memory, can be associated with TD (LoE 3, Grade C) [6].
• On physical examination, features suggestive of TD include reduced body hair, decreased testicular size and gynaecomastia, but these are not always present (LoE 1, Grade B) [6]. Fine wrinkling of the skin, especially around the mouth, may also be apparent.
TD is often associated with an increased waist circumference, obesity, metabolic syndrome and impaired health status [1]. In a primary care population of men aged 45 years and older, the reported odds ratios for TD with comorbid conditions were obesity 2.38, T2DM 2.09, hypertension 1.84, dyslipidaemia 1.47, chronic obstructive pulmonary disease (COPD) 1.40 and LUTS/BPH 1.20 [31,32,33].
Significant TD is associated with an increased risk of chronic anaemia and osteoporosis. See Table 1 for a list of the conditions associated with an increased prevalence of TD.
Many signs and symptoms of TD are multifactorial in origin and associated with various lifestyle and psychological factors, as well as with normal ageing, so they may be found in men with normal testosterone levels [4,34].
Although it is commonly stated that testosterone levels decline with age [34,35] over 80% of men maintain normal testosterone levels into old age [9]. The age-related rise in SHBG does cause a fall in FT and bioavailable testosterone, but the increased rates of TD seen in older men are primarily related to an increased prevalence of obesity, T2DM and chronic illness [36].
The signs and symptoms of TD vary depending on age of onset, duration and severity [1,4]. While the prevalence of TD increases with advancing age, it can occur in adult men of any age (LoE 1, Grade B) [6].The more signs/symptoms a man has, the greater the probability that he has genuine TD [36,37].
The prevalence of hypogonadal symptoms increases when TT levels fall below 12.1 nmo/L (Level 2b, Grade A) [26,36,37]. However, they are also seen at levels higher than this. A cross-sectional cohort study investigating complaints structures in 434 hypogonadal men aged 50–86 years, reported loss of erections with a TT of around 8 nmol/L, diabetes and depression at 10 nmol/L, obesity at 12 nmol/L and reduced vigour at 15 nmol/L. They found no evidence for a uniform structure of testosterone concentrations and complaints though, instead concluding that in aging men, psychosomatic complaints and metabolic risk relate to testosterone in a symptom-specific manner [37].
LIFESTYLE CHANGE AND TESTOSTERONE LEVELS
Armamento-Villareal et al [38] reported that dietary interventions, with and without exercise, resulted in significant increases in testosterone level. Moran et al [39] reported on 68 men participating in a weight-loss diet. Men lost a mean of 10.3–10.8±1.2 kg over the 52-week study period and experienced significant increases in total and FT. Several studies have suggested that significant weight loss can improve testosterone levels in Male Obesity Secondary Hypogonadism (MOSH) [38]. In contrast EMAS [8] showed that a 20% reduction in BMI was required to produce a significant increase in FT level, due to the associated rise in SHBG, or meaningful increase in EF score.
Rigon [41] and colleagues evaluated bariatric surgery in 29 men with a mean baseline weight of 155.26±25.88 kg with significant improvements in TT levels from 229.53±96.45 ng/dL to 388.38±160.91 ng/dL (P<0.001).
ORAL SUPPLEMENTS
Many compounds are being marketed as oral dietary supplements or adjuncts to “boost” testosterone. However, studies suggest that oral formulations of testosterone fail to improve symptoms of TD and do not raise serum testosterone levels above placebo due to a lack of bioavailability with an oral formulation [42]. Despite this, there is continued interest in oral over-the-counter supplements to treat symptoms of TD [43].
A new generation of lipid based native testosterone (NT) preparations have been launched in the US [44]. These newer agents avoid first pass liver metabolism due to intestinal lymphatic absorption. These may become available in the UK.
THE RATIONALE FOR T THERAPY
Because many adult men with TD are sick and/or obese, weight reduction if overweight, lifestyle modification and optimal management of comorbidities are important first steps [45].
However, a 4.3 year follow-up from EMAS [46] found that biochemical reversal of secondary hypogonadism was not associated with an improvement in symptoms. A randomized controlled trial (RCT) in obese hypogonadal men treated with severe weight reduction and either long-acting testosterone undecanoate (TU) injections or placebo for 12 months, found that although weight and BMI loss was achieved in both groups, only the TU group preserved lean muscle and achieved symptomatic benefit [47,48]. We therefore feel that current evidence does not support the recommendation for lifestyle intervention alone as first-line therapy. Patients with low testosterone present with distressing symptoms and therapy should be relevant to the effective relief of those symptoms. We therefore endorse lifestyle modification in conjunction with T Therapy in men with symptomatic TD (LoE 2, Grade A).
T Therapy is indicated for men with symptoms of TD supported by low biochemical levels. As the 3 most common symptoms of deficiency are ED, loss of early morning erections and low sexual desire [40,46], most candidates for T Therapy present with sexual dysfunction and a desire for treatment. The increasing realisation that ED and especially loss of morning erections are strong markers for coronary heart disease (CHD) risk, combined with recent recommendations on obesity and diabetes by the American Association of Clinical Endocrinologists and the American College of Endocrinology [49,50] are likely to increase the number of men detected by routine screening. Against this recognition of the importance of testosterone in cardiometabolic disease, it is inevitable that the last 20 years has seen a large increase in men being treated [49].
Multiple studies have shown that low testosterone levels are associated with increased risk of type 2 diabetes [51,52]. TTh in men with pre-diabetes and T levels of 14 nmol/L or below (n=1,007) has been shown to reduce progression to T2DM by 40% over 2 years, in conjunction with improvement in lean muscle mass and sexual symptoms, compared with men on active lifestyle intervention plus placebo [53]. Despite weight loss of 3.85 kg in the placebo arm, there was no improvement in FT level and worsening of sexual function after 2 years suggesting that lifestyle alone is unlikely to be successful in such patients. Despite 4.45 kg weight loss and 7 cm waist circumference in the testosterone replacement therapy (TRT) cohort, there was only a 1-point improvement in EF score. The prevalence of significant LUTS in both groups approached 40% and did not improve after 2 years. These findings confirm the importance of consideration of co-administration of a PDE5 inhibitor, preferably daily tadalafil 5 mg daily, licensed to treat both ED and LUTS. This is the largest and longest RCT of TRT to date and the important finding of diabetes prevention is of such importance that, on the basis of this evidence, the BSSM feel that the threshold for TRT should be adjusted from 12 to 14 nmol/L in men with pre-diabetes [53].
Multiple symptoms such as loss of vitality, tiredness, diminished strength and tiredness may also demand attention. In men with no other co-morbidities, T Therapy alone may reverse symptoms [54,55], but where other co-morbidities exist, ED-specific therapy (usually a PDE5i) will often be required [56]. Studies suggest that the best responses in terms of sexual symptoms are seen with baseline levels below 8 nmol/L, and that sexual desire improves by 6 weeks, but ED improvement may take longer [54,55]. Benefits in terms of loss of fat mass and increase of lean muscle mass may take 12 months or more. Longer-term studies suggest that symptoms improve progressively over many years [57,58]. As most men will only receive one trial of T Therapy in their lifetime, we recommend a minimum of 6 months, assuming effective levels are achieved. At that point, continuation should be discussed once the patient has received sufficient accurate information for an informed decision.
The result from a meta-analysis showed an increased risk of all-cause dementia with decreasing TT (4,572 participants; hazard ratio [HR], 1.14; 95% confidence interval [CI], 1.04–1.26). Some studies also found an increased risk of AD with a lower level of total-T, FT, and bioavailable testosterone. Testosterone supplement treatment may improve general cognitive function and motor response in the short term as measured by the Developmental Test of Visual-Motor Integration (mean difference [MD], 4.4; 95% CI, 1.20–7.59) and the Mini-Mental State Examination (MD, 3.4; 95% CI, 0.83–5.97) and verbal memory as measured by story recall delay at 3 months (MD, 8.4; 95% CI, 0.49–16.3) [59].
A UK biobank study involving 159,411, mean age 61, for 7 years and found that the highest versus the lowest testosterone quintiles was associated with significant reduction in risk of dementia and Alzheimer’s disease (AD). Higher SHBG was independently associated with increased risk of dementia and AD [60].
Men who have failed despite multiple doses (>8) of a PDE5i can frequently be salvaged by correcting low testosterone levels with significance only below 10.4 nmol/L [56]. Testosterone has been shown to upregulate PDE5 and most regulatory trials of oral therapy excluded men with untreated TD [61]. Failure of oral therapy in these men may also be related to previously undetected low desire, ejaculatory or orgasmic function, or dislike of on-demand therapy by the couple [56]. With the availability of generic PDE5is, avoiding the progression to expensive second- and third-line therapies will become increasingly cost-effective [54].
TESTOSTERONE AND FERTILITY
TD is commonly associated with reduced fertility and men presenting with infertility should be screened for low testosterone levels [4]. TD and subfertility often co-exist, and given the negative impact of exogenous testosterone on sperm production, there is often a need to look at alternative therapeutic approaches for men wishing to both start a family and see an improvement in their hypogonadal symptoms.
There often co-existing negative lifestyle factors which can be modified in a safe and cost-effective way, and therefore, this should be the starting point. It is also important to manage underlying contributary comorbidities and reduce the use of drugs that may interfere with the HPG axis.
In younger men, T Therapy reduces LH and FSH levels and frequently causes infertility after 6-12 months; but this is reversible in 60%–70% of men within 12 months [61,62].
Gonadotrophins such as hCG or FSH are effective in increasing testosterone levels and semen parameters, but are costly and require administration via injection. They currently require approval and prescription and follow-up from specialist centres. Pulsatile GnRH is a less attractive option due to both cost and the impracticality of a continuous infusion.
The selective oestrogen receptor modulators (SERMs) such as clomiphene citrate have been shown to increase testosterone levels without negative impact on fertility, though there is a lack of long-term data regarding their impact on hypogonadal symptoms. The aromatase inhibitors (AIs) may also raise testosterone levels, but are associated with reduced oestradiol levels and BMD, and their use requires close long-term monitoring.
Further studies of clomiphene and AIs in conjunction with testosterone therapy are required to confirm whether these agents can be used in a synergistic way. This might mitigate the risk of adverse events of TRT in terms of reduced fertility and symptoms associated with increased oestradiol levels.
THE BENEFITS OF T THERAPY IN MEN WITH TD
In the TIMES2 [18] and BLAST [63,64] studies, which recruited patients with T2DM and/or the metabolic syndrome, T Therapy was associated with small benefits in lipid metabolism, but blood pressure was unaffected. T Therapy appeared to improve insulin sensitivity most markedly in poorly controlled patients. These benefits were amplified when combined with lifestyle modification.
A long-term registry study lasting over 5 years, showed progressive weight loss with T Therapy, as well as reductions in waist circumference and BMI, total and low density lipoprotein (LDL) cholesterol, triglycerides, systolic and diastolic blood pressure, blood glucose, improved insulin sensitivity and C reactive protein, and an increase in high density lipoprotein (HDL) cholesterol [58].
An Australian study evaluated severely obese men with hypogonadism in a double-blind RCT involving long-acting TU or placebo PLUS intense dieting in the first 10 weeks. At 12 months, there was no significant difference in weight loss, BMI and waist circumference, but the men on TU preserved all their lean muscle, whereas men on diet alone lost an additional 3.5 kg of lean muscle compared with TU. The active group also showed significant loss of visceral and appendicular fat plus increased grip strength [53].
The Testosterone Trials were a set of 7 coordinated, double-blind, placebo-controlled trials conducted at 12 sites [55] involving 790 men aged 65 years and over with serum testosterone concentrations <275 ng/dL (10 nmol/L). Participants received testosterone- or placebo-gel for 1 year. Dosing was adjusted to maintain testosterone concentrations within the normal range for young men (age 19–40 y). Efficacy was assessed at baseline, then at 3, 6, 9, and 12 months [56]. In the sexual function, the physical function, and the vitality trials, T Therapy was associated with significant improvements in sexual desire, sexual activity and erectile function, and modest improvements in 6-minute walking test, anaemia, mood and depression [55].
The bone trial [65] found that T Therapy significantly increased volumetric BMD and estimated bone strength. These changes were greater in trabecular than peripheral bone, and in the spine than the hip. Larger and longer trials are required to determine whether this treatment also reduces fracture risk. Earlier evidence had shown that T Therapy consistently improved BMD in the lumbar spine, but was not associated with significant improvements in hip scores [66].
The T4DM study investigated 1,007 men with prediabetes and basal TT of less than 14 nmol/L showing a 40% reduction in progression to type 2 diabetes over 2 years, with additional benefit in BMI waist circumference and grip strength compared with placebo [53]. Based on this high level evidence, BSSM recommend treatment of TT levels of 14 nmol or below in men with pre-diabetes.
A limitation of some of the previous RCTs, is that they were of relatively short duration, usually 3–12 months. The evidence strongly suggests that trials of T Therapy should last at least 6 months [53].
The anaemia trial [65] found that T Therapy significantly increased haemoglobin levels in men with anaemia from unexplained or known causes, but the cognitive function trial [66] found no effect on memory or other cognitive function.
Several meta-analyses of RCTs, notably by Corona et al in 2017 [67] concluded that T Therapy in men with TD, significantly improves sexual desire and erectile function (particularly in men below 8 nmol/L), and increases sexual activity, satisfaction and orgasm.
In a meta-analysis of 59 RCTs involving 5,078 subjects, T Therapy was not associated with any significant changes in body weight, BMI or waist circumference, but it consistently improved lean muscle and decreased fat mass [68].
No evidence from long-term studies, and various reviews and meta-analyses, have provided evidence to support the association between TD and increased cardiovascular and all-cause mortality [69,70,71,72].
Two systematic reviews and meta-analyses evaluating the association between low endogenous testosterone and all-cause [52,73] and CVD mortality reported a protective effect of increased TT. Research examining the data from 1954 subjects, in terms of several statistical models, found that even after strict adjustment for comorbidities, there was a consistent link between low testosterone level and mortality risk throughout, without proving causation [52].
Recent meta-analyses concluded that low testosterone levels were associated with higher rates of incident diabetes [73] and increased disease progression and mortality in men with CKD [74], progressive over 7 years [75] especially those on haemodialysis and that this was largely overlooked by renal physicians [74].
In a prospective study involving 581 men with T2DM, patients were followed up for a mean of 5.81 years. Low testosterone was defined as TT <10.4 nmol/L. Fifty-one men received T Therapy for at least 2 years. The mortality rates were 20% in the low testosterone group versus 9.1% in the normal testosterone group, independent of comorbidities and therapies, and 9.4% in those with TD in the treated group [76].
In a 10-year Australian study involving 3,690 older men, TT and FT levels in the normal range were associated with reduced all-cause and cardiovascular mortality. This was the first evidence to suggest that both low and high levels are associated with all-cause mortality, and higher levels of dihydrotestosterone (DHT) reduce cardiovascular risk [77].
A study from Sweden involving 1,109 subjects aged 40 years and over, with a mean follow up of 14.1 years, suggested a strong association between low baseline testosterone and incident myocardial infarction (MI) [72]. Although evidence for a pathogenic link is lacking, as low testosterone could be considered a marker of chronic illness.
POSSIBLE INCREASED CARDIOVASCULAR RISK WITH T THERAPY
Although it has been proposed that serum DHT may increase the risk of CVD through mechanisms involving inflammation, coagulation and vasoreactivity [72,78,79]. The results of studies investigating this association have been conflicting.
Concerns regarding T Therapy in elderly men stem initially from the premature discontinuation of the Testosterone in Older Men (TOM) trial [79] which involved 209 frail, elderly men with a mean age of 74 years. This study was not powered to detect differences in cardiovascular events, but it was stopped early due to 23 cardiovascular-related events (two deaths) in the 106 men in the testosterone group versus five in the placebo group, despite positive results in study endpoints. The primary limitation of interpreting cardiovascular risk from this trial, is that the majority of adverse cardiovascular events were of questionable significance (e.g., palpitations and non-specific ECG changes) and were not systematically queried. It also involved treatment initiation with 100 mg of topical testosterone gel (twice the recommended starting dose), rapid escalation up to 150 mg per day (above the manufacturer’s recommended dose), and many of the events were reported with inadequate validation and mainly occurred in those subjects receiving the higher doses [80]. Similar dosing regimens used several years later in 790 men in the T Trials failed to reproduce these findings and in the 12 month study, there were 3 deaths reported on testosterone and 7 on placebo [55].
A retrospective US study [81] involved 8,709 men with a baseline TT of ≤10.4 nmol/L who were undergoing angiography. During a mean follow-up of 840 days, 681 of the 7,486 patients not receiving T Therapy died, 420 suffered MIs and 486 experienced strokes. Of the 1,223 patients receiving T Therapy, 67 died, 23 suffered MIs and 33 experienced strokes. Complex statistical analysis, using more than 50 covariates, concluded that there was a greater risk in the T Therapy group. However, there were concerns regarding the exclusion of 1,132 patients who experienced events because they were prescribed T Therapy after the event, when they should have been included in the untreated group, increasing the events by 70%. When challenged, the authors revised the number to 132, but admitted that 104 women had been mistakenly included in the results [75]. There were also no data confirming a correct diagnosis of TD syndrome before T Therapy and none on compliance with therapy. Treated patients had baseline T levels 1.2 nmol/L below the untreated group and would therefore have been at increased risk, especially if undertreated, a possibility confirmed by the authors.
A study analysing prescribing data in men treated with T Therapy, without records of blood results or symptoms, defined non-fatal coronary events as the major endpoint, assessed in the 12 months before and 3 months after therapy [82]. However, the benefits of T Therapy take longer than this to appear and other studies have excluded the first 3 months treatment from analysis due to the likelihood of events relating to the pre-existing condition. Crucially, data on fatal cardiovascular events and all-cause mortality data was not collected, despite T Therapy in other studies having a major impact on mortality rather than event numbers. Twelve-month post-treatment data were collected but not presented. Prior to treatment, the event rates within the groups were strangely identical. There was a small increase in non-fatal cardiac events in men taking T Therapy, which was more marked in those with increased risk. Overall, events were lower than predicted from comparable research. The lack of mortality data demonstrates a failing to realise that a treatment that reduced mortality was likely to increase non-fatal events. In addition, the study design was not prospective, which casts doubts on the validity of retrospective assessment for the 12-month pre-treatment period. While this study has been widely quoted in public media, it is discredited by several design flaws and statistical analyses [75].
In the cardiovascular arm of the testosterone trials [83], 170 men were assessed by cardiac computed tomography angiography (CCTA) for baseline and 1 year plaque burden. As compared with placebo, T Therapy was associated with a significantly greater increase in noncalcified plaque volume over 12 months, despite previous studies showing no link between plague size and cardiovascular events. Unfortunately, there was a significantly greater baseline plaque level in the placebo group that resulted in multiple interventions including stents and bypass grafting. However, the lack of change in coronary calcium in this study, raises questions as to the reliability of the result of non-calcified plaque. The authors reported no coronary events or significant drug changes in either cohort throughout the study and conceded that testosterone might actually stabilize plaque and promote re-modelling.
The 3 studies above led the FDA [83,84] to express concerns over TTh prescribing in the US but an European Medicines Agency (EMA) review found no grounds for concern in Europe [84,85].
A systematic review and meta-analysis evaluated whether T Therapy was associated with an increased risk of serious cardiovascular events compared to other treatments or placebo. It included 39 RCTS and 10 observational studies. The meta-analysis used data from 30 RCTS. As compared to placebo, T Therapy was not associated with any significant risk in MI, stroke or mortality. However, the strength of the evidence for the 3 outcomes, was classed as low, because of the risk of bias in the included RCTs and imprecision. These results reflect those of several other systematic reviews and meta-analyses, that have yielded inconclusive findings, representing the many limitations of the individual studies.
Patient level data could not be used, many studies did not specify the reasons for study withdrawal and whether the adverse events were identified using prespecified criteria for safety end points, potentially biasing the results, and the impact of the pharmaceutical industry funding was not explored. The results from the observational studies were very varied and thus not pooled statistically. The strengths of this study included rigorous and comprehensive screening of several databases, the inclusion of both trials and observational studies, focusing on specific disaggregated cardiovascular endpoints of clinical interests, the use of meta-analytic approaches suitable for sparse data, evaluation of the stability of the results in several sensitivity analyses, and in contrast to previous studies, the provision of strength-of evidence ratings for the evidence. Until the results of prospective trials become available, rigorously designed observational evidence will continue to expand the growing evidence base regarding the risks and benefits of T Therapy, and its optimal clinical application [86].
STUDIES SUGGESTING DECREASED CARDIOVASCULAR RISK WITH T THERAPY
In a retrospective study involving 1,031 hypogonadal men, 372 of whom took T Therapy, the cumulative mortality was 21% in the untreated group versus 10% in the treated group. The greatest effect was observed in younger men and those with T2DM [87].
In a prospective study involving 581 men with T2DM, patients were followed up for a mean of 5.81 years. Low testosterone was defined as TT <10.4 nmol/L. Fifty-one men received T Therapy for at least 2 years. The mortality rates were 20% in the low testosterone group versus 9.1% in the normal testosterone group, independent of comorbidities and therapies, and 9.4% in those with TD in the treated group [88].
The same authors also reported that TTh improved angina symptoms and reduced time to ST depression in angina patients and improvements in moderate heart failure.
A real-life observational registry study [89] assessed the long-term effectiveness and safety of parenteral TU used for up to 10 years in 656 men with a mean age of 60.7 years. The conclusions were that long-term treatment was well tolerated, with excellent adherence. Furthermore, mortality related to CVD was significantly reduced in the group taking testosterone versus the untreated group.
A retrospective study followed up 857 men with T2DM for 4 years after baseline testosterone measurement. Patients were randomised to either long-acting TU or placebo. Results showed that low baseline TT and FT were associated with increased all-cause mortality. T Therapy and the use of PDE5is were independently associated with reduced all-cause mortality, with the greatest benefit from both treatments being seen in older men [90].
Sharma et al [91] retrospectively evaluated 83,010 male veterans with recorded low TT levels. The subjects were categorised into three groups: group 1 - T Therapy with resulting normalisation of TT levels; group 2 - T Therapy without normalisation of TT levels; and group 3 - did not receive T Therapy. The all-cause mortality (HR, 0.53; 95% CI, 0.50–0.55), risk of MI (HR, 0.82; 95% CI, 0.71–0.95) and stroke (HR, 0.70; 95% CI, 0.51–0.96) were significantly lower in group 1 versus group 2 (n=25,701, median age 66 y, mean follow-up 4.6 y).
A study comparing acute MI rates in 6355 men receiving at least one testosterone injection, compared with a matched placebo group over 8 years, found no overall increase in events. In those at greatest risk, there was a significant reduction in events and mortality. There was no increased risk from venous thromboembolism (VTE) [92].
In a virtual controlled study, researchers examined electronic medical records between 1996 and 2011 to identify 5,695 men with a low initial TT level, a subsequent testosterone level, and up to 3 years of follow-up. Testosterone levels were correlated with the use of testosterone supplementation. The primary outcomes were a composite of death, nonfatal MI, and stroke (major adverse coronary events, MACE) and death alone. Testosterone supplementation in men with low testosterone levels was associated with a reduced incidence of MACE and death over 3 years compared with no or ineffective supplementation. The results suggest that the positive impact of T Therapy was mainly on mortality as opposed to the number of events, and the benefits were associated with the achievement of therapeutic levels of testosterone. There was no suggestion of increased risk with sustained higher serum levels [93].
The same group have more recently, shown significant reduction in cardiovascular events in a cohort of hypogonadal men with angiographically diagnosed coronary artery disease [94].
In a study of 10,311 testosterone-treated men compared with 28,029 controls, Wallis et al [95], demonstrated a reduction in all-cause and cardiovascular mortality with T Therapy achieved and maintained in the normal range, with an increase in mortality in the first 6 months compared with normal, most likely due to the impact of underlying undertreated TD. This study also reported a 40% reduction in new diagnoses of prostate cancer (PCa) in the treated group compared with the control group.
Cheetham et al [96] retrospectively reported on 8,808 testosterone-treated and 35,527 untreated men with low testosterone and found a 33% reduction in cardiac events associated with T Therapy.
These studies present the most compelling evidence to date for the safety of T Therapy in patients with reduction in mortality in clearly defined TD treated to the therapeutic range; suggesting that studies with negative outcomes usually included inadequate diagnosis and little evidence of effective therapeutic levels or adequate follow-up, and failed to exclude the possibility that increased risk is related to TD and not its treatment.
Registry studies have published data collected over 6 years of follow-up, with no suggestion of increased mortality [97].
However, a study of 1,470 men over 14 years with previous MI and low testosterone level [98] found a mortality rate of 101/1,000 patient years in the normalised T treated group (n=755), 137/1,000 (n=542) in the treated but non-normalised group and 163/1,000 (n=175) in the untreated group (p=0.002 between group 1 and 2). Interestingly regression analysis did not reveal significant reduction in risk of recurrent MI [98].
Sharma et al [73] reported 38,706 men with end stage CKD with baseline low T subsequently normalized with TRT and followed up for 10 years, compared with 9,755 who were not normalised. They found that TRT to normal levels was associated with a delay of progression to end stage disease of 284 days (p<0.0001) and an increase delay in mortality of 324 days (p<0.05) [73].
Based on these studies, we recommend screening for low testosterone in men post MI and those with CKD. We also suggest that future studies on TRT need to distinguish between treatment to normal levels and non-normalised patients, especially as recent studies show higher mortality rates on men not achieving normal levels when TRT is prescribed [73].
FINDINGS FROM META-ANALYSES
A 2022 meta-analysis of 109 peer reviewed publications T Therapy lasting longer than 12 weeks, concluded that there was no increased risk of CHD, MI, heart failure or arrythmia [99]. Findings from earlier studies were heavily skewed by over-reliance on the heavily criticised TOM [79], Vigen [81], and Finkle [82] studies. Another meta-analysis concluded that T Therapy was not associated with increased risk, and in certain cohorts there was evidence of reduced events, especially in men with cardio-metabolic disease [100].
In response to early publications and US media publicity, in 2015, the FDA issued a request for more information on the safety of testosterone from future studies [84] whereas a bulletin from the EMA expressed no concerns [85].
T THERAPY AND RISK OF pCa
Concerns about T Therapy and pCa date back to 1941, when Huggins and Hodges [101] reported that marked reductions in testosterone by castration or oestrogen treatment caused metastatic cancer to regress, but administration of exogenous testosterone caused PCa to grow.
The paradox of why lowering testosterone causes PCa to regress, but raising testosterone fails to cause PCa to grow, may be explained by the saturation model, which posits a finite ability of androgens to stimulate PCa growth [102]. The AR becomes saturated in human prostate tissue at approximately 8 nmol/L in vivo [103,104] and there appears to be no further appreciable growth with increasing serum testosterone concentrations beyond a saturation point of approximately 8 to 8.7 nmol/L [105]. This explains why men with testosterone below the saturation point of approximately 8.7 nmol/L at baseline, are likely to see a rise in PSA upon treatment initiation, whereas PSA is unlikely to rise in men with testosterone above this level [105].
The REDUCE trial, which investigated dutasteride for preventing PCa and included results from 3,255 men who had prostate biopsies at 2 and 4 years, showed no association between serum testosterone or DHT and PCa risk. The risk of PCa was no greater in men with high testosterone levels than those with low testosterone levels [106].
Guidelines from the European Association of Urology (EAU) [4], the BSSM [5], the ICSM [6], the International Society of Sexual Medicine (ISSM) [7], and the Endocrine Society (ES) [22] all conclude that there is no compelling evidence that T Therapy is associated with an increased risk of PCa (LoE 2, Grade B), and the ICSM [6] states that there is no compelling evidence that T Therapy increases the risk of PCa or is associated with PCa progression [LoE 1, Grade C].
Recent research suggests that lower testosterone levels are associated with a risk of poorly differentiated cancers and greater risk of positive biopsy [106,107] and there is evidence linking low T concentrations to aggressive, high grade PCa, higher rates of positive biopsy, biochemical recurrence and disease progression in men under active surveillance [107,108].
However, estimates suggest that in order to detect a 30% difference in PCa rates between testosterone- and placebo-treated men, approximately 6,000 men aged 65–80 years with low testosterone levels would need to be randomised to testosterone- or placebo-treatment for an average of 5 years [109]. Because such an RCT would probably include relatively healthy, older men, with unequivocally low testosterone levels, but without PCa or PSA levels >4 ng/mL, it would require screening ofasignificantly greater number of older men in order to enrol the required number of eligible participants. As it seems unlikely that a study of this size will be funded, it will remain uncertain whether long-term T Therapy affects the incidence of clinically overt PCa [110].
Careful assessment of the prostate prior to starting T Therapy, and regular monitoring for prostate disease once the patient is on T Therapy, remains essential. Early rises in PSA after initiation of T therapy may unmask an occult prostate carcinoma which was undetected at baseline [110].
ROUTINE MEASUREMENT OF TESTOSTERONE
EAU [111] and BSSM [5] guidelines on male sexual dysfunction, recommend that all men with ED should have an initial measurement of testosterone as minimal standard, and re-checking is indicated in the event of poor response to PDE5 inhibitors.
NICE guidance [112] recommends that men with T2DM are asked about ED annually, and assessed, educated and supported as necessary. As the expected prevalence of ED in men with T2DM is over 70%, following NICE guidance should detect hypogonadism (below 12 nmol/L in at least 40%, in line with other studies.
In 2016, the American Association of Clinical Endocrinologists and American College of Endocrinology guidelines on the management of obesity [49] recommended that all men with a waist circumference >102 cm or BMI >30 kg/m2 should be assessed for TD by history, clinical examination and measurement of testosterone. They recommend measurement of testosterone in all men with diabetes. They point out that weight loss and lifestyle change should always form part of the management, but that significant elevation in testosterone is not usually seen unless more than 5%–10% of weight loss is achieved. They also recommend testosterone measurement in men with HIV and chronic renal disease.
Screening for low testosterone (Table 2) is recommended, especially in the presence of TD symptoms in all other populations (including those with CVD, chronic pulmonary diseases, cirrhosis, rheumatoid arthritis and cancer), because although such conditions are potentially associated with an increased prevalence of low testosterone, there is a lack of evidence for benefit of T Therapy in asymptomatic individuals (LoE 3, Grade B) [6].
Table 2. Recommendations – screening.
| Target populations to screen for tesosterone deficiency | LoE | Grade |
|---|---|---|
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED or loss of spontaneous erections, or reduced sexual desire | 1 | A |
| Screen for TD in all men with type 2 diabetes, BMI >30 kg/m2 or waist circumference >102 cm | 2 | A |
| Consider screening for TD in all men post covid-19 infection, cirrhosis, rheumatoid arthritis and testicular cancer or associated therapy | 2 | C |
| Screen for TD in all men with CKD | 2 | A |
| Screen for TD in all men on long term prescribed opiate analgesics, antipsychotic, anticonvulsant, Roaccutane of finasteride therapy | 2 | B |
| Screen for TD in men with anaemia | 2 | A |
| Screen for TD in men with osteoporosis | 2 | A |
TD: testosterone deficiency, BMI: body amss index, CKD: chronic kidney disease.
HISTORY TAKING AND QUESTIONNAIRES
History taking should include signs and symptoms suggestive of TD (see Table 3), pharmacological treatment with corticosteroids or opiates, abuse of drugs such as marijuana, alcohol and anabolic steroids, and previous treatment or use of testosterone. It is important to assess and exclude systemic illness such as COVID infection, ongoing acute disease, malabsorption and malnutrition [4].
Table 3. Clinical signs and symptoms suggestive of TD.
| Sexual | Physical |
|---|---|
| Erectile dysfunction Reduced sexual desire and activity Reduced or absent morning/night-time erections Delayed puberty Small testes Infertility Reduced frequency of sexual thoughts Delayed ejaculation Reduced volume of ejaculate |
Reduced body hair Gynaecomastia Reduced muscle mass and strength Hot flushes/sweats Sleep disturbances Fatigue Osteoporosis/height loss/low trauma fractures |
| Cardiometabolic | Psychological |
| Increased BMI/obesity Visceral obesity Metabolic syndrome Insulin resistance, pre-diabetes and type 2 diabetes |
Changes in mood (e.g., anger, irritability, sadness, depression) Reduced well-being/poor self-rated health Diminished cognitive function (including impaired concentration, verbal memory and spatial performance) |
TD: testosterone deficiency, BMI: body mass index.
Data from Hackett et al (Int J Clin Pract 2017;71:e12901) [1], Dohle et al (http://uroweb.org/guideline/male-hypogonadism/) [4], and Khera et al (J Sex Med 2016;13:1787-804) [6].
Clinical assessment can be supported by the use of validated questionnaires such as the Aging Male Symptoms (AMS) Scale (available at http://www.issam.ch/AMS_English.pdf and http://www.issam.ch/AMS_English_Evaluation.pdf) and the ANDROTEST [113] to provide a quantitative baseline assessment of baseline symptoms and evaluate the clinical response to treatment. We endorse the use of validated questionnaires to demonstrate changes in symptoms with T Therapy, especially when multiple physicians are involved in the patients’ care.
Because the signs and symptoms may be non-specific [4], patients at risk of, or suspected of having TD, should receive a thorough physical and biochemical work-up (LoE 2, Grade A) [5,26].
PHYSICAL EXAMINATION
Physical examination in men with suspected TD should include measurement of height, weight, BMI and waist circumference [22] assessment of the degree and distribution of body hair (including facial and pubic), and examination for the presence of acanthosis nigricans associated with insulin resistance [6] presence and degree of breast enlargement, appearance of the penis, presence of subcutaneous plaque, testicular size and consistency, and scrotum size and abnormalities [4,26]. We recommend that the prostate should ideally also be examined via digital rectal examination (DRE) or via PSA measurement [4].
DIGITAL RECTAL EXAMINATION
We express concern that current EAU guidelines [111] suggest 3 routine DREs within 12 months of initiation of T Therapy and that the Society for Endocrinology Guidelines [114] suggest that endocrinologists are not sufficiently skilled and that there are possible risks of frequent DRE. Given the lack of evidence linking T Therapy with PCa [110,114], BSSM do not feel that frequent DREs by specialists are realistic or cost-effective, especially following the recent global pandemic and advise an initial DRE to exclude significant benign enlargement or possible malignancy plus baseline PSA, with follow up PSA on an annual basis.
LABORATORY DIAGNOSIS
Because the diurnal rhythm of serum testosterone means levels are highest in the early morning, testosterone measurement before 11am is particularly important for men under 40 years of age [6]. Although diurnal variation is substantially blunted in older men and men with lower levels [115] it may still be evident, even in the elderly, supporting the recommendation of fasting testosterone measurement to the morning hours for all age groups [116].
Serum testosterone should be measured between 7 am and 11 am (LoE 2a, Grade A), on at least 2 occasions with a reliable method (LoE 1, Grade A) [26], preferably 4 weeks apart and, if possible, not during an acute illness (Table 1). As testosterone levels are influenced by insulin, 75G of glucose was shown to lower testosterone by 25% [116].
Although there is a lack of evidence supporting the necessity of evaluating fasting testosterone, the EAU recommend that testosterone is measured in the fasting state [4] which creates difficulty as the routine blood testing for dyslipidemia, hypertension and diabetes no longer require fasting samples. Until further evidence is accumulated, we also feel that fasting testing is appropriate for a first test. Fasting levels were found to be up to 30% higher than non-fasting levels in healthy volunteers [116,117] and most RCTS involved fasting levels. In contrast, a UK review of laboratory samples of 213 patients with suspected hypogonadism showed no significant effect of fasting [118]. We recommend that patients should “ideally fast” but recommend a pragmatic approach. Clinicians should be aware that patients, especially those with type 2 diabetes do not routinely fast and insistence on fasting samples is unreasonable.
Assays of TT, performed by most laboratories in the everyday clinical setting, are readily available and inexpensive [6]. Although widely recommended as the standard for research, equilibrium dialysis is most widely used clinically, most notably in the EMAS studies, where the results of equilibrium dialysis diagnosis were found to correlate well with isotope dilution gas chromatography mass spectrometry (ID-GCMS) [119].
Assays of FT based on analogue displacement immunoassays give unpredictable results and are not recommended. In obese men, the free androgen index (FAI) calculated by TT/SHBG ×100 is used by some laboratories but we recommend the calculated FT derived from the SHBG and albumen via the Vermeulen equation [120]. An online FT calculator and downloadable app, sponsored by the Primary Care Testosterone Advisory Group (PCTAG), can be found at https://www.pctag.uk/testosterone-calculator/. The ISSAM also provide an online FT calculator available at http://www.issam.ch/freetesto.htm [121].
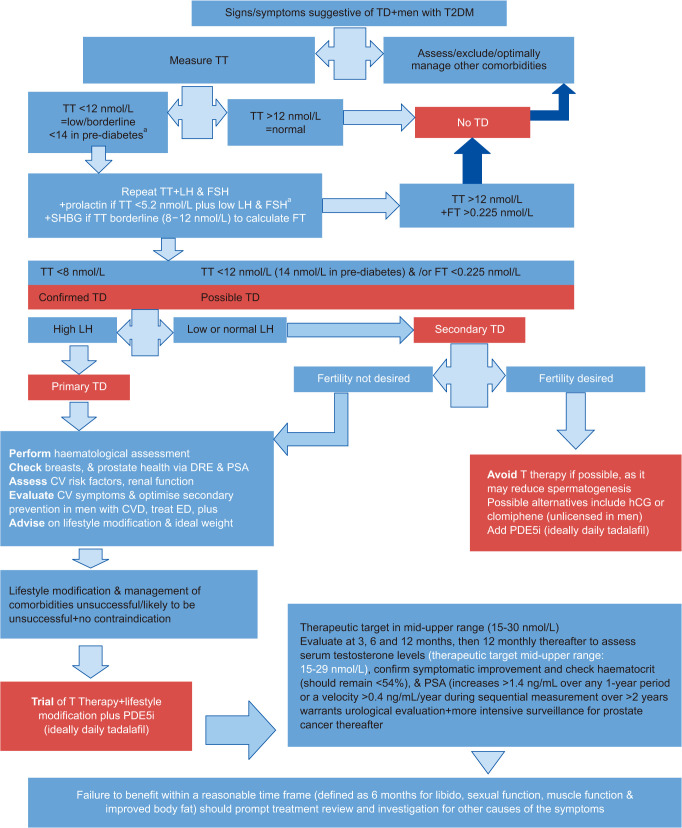
It is important that clinicians familiarise themselves with their local laboratories. TD is a diagnosis made from clinical symptoms and abnormal biochemistry. Clinicians need to appreciate that “reference ranges” quoted by laboratories represent the normal population and that the “action levels” recommended by BSSM refer to men with clinical symptoms of TD (Fig. 2). Just as reference ranges on normal lipids are inappropriate for men with T2DM and for secondary prevention in CHD, reference ranges for testosterone are not designed to replace evidence-based guidelines. Wide variations in laboratory practice for testosterone testing in the UK may lead to variations in patient care and potentially result in some patients with TD not being identified. Improvements in the standardisation of testosterone assays and the consistency of reporting between laboratories is required.
Fig. 2. BSSM Guidelines 2022. Diagnosing and managing TD in adult men. BSSM: British Society for Sexual Medicie, TD: testosterone deficiency, TT: total testosterone, LH: luteinizing hormone, FSH: follicle-stimulating hormone, SHBG: sex-hormone-binding globulin, FT: free testosterone, DRE: digital rectal examination, PSA: prostate specific antigen, CV: cardiovascular, CVD: cardiovascular disease, ED: erectile dysfunction, hCG: human chorionic gonadotropin, T Therapy: testosterone therapy. aFor men with TT levels <5.2 nmol/L plus low LH and FSH or increased prolactin levels, refer to endocrinology or arrange a pituitary MRI to exclude a pituitary adenoma [2,15]. The T4DM study [53] showed a 21% reduction in progression to T2DM by treating T <14 nmol/L in men with pre-diabetes.
Where TT levels are close to the lower normal range (8–12 nmol/L), the FT level should also be checked (LoE 1, Grade A) [4,5]. Where the TT levels are <12 nmol/L, serum LH and FSH levels should also be measured. Where TT is borderline, or low to normal, particularly in obese and older men, SHBG levels should also be checked (LoE 2, Grade C) [6]. In the case of known or suspected abnormal SHBG levels, FT should also be measured (LoE 1, Grade A). LH levels should be assessed to differentiate between primary and secondary TD (LoE 2, Grade A) [4]. Serum prolactin levels should be measured when both LH and FSH levels are low. A very low TT (<5.2 nmol/L), and low LH and FSH are more likely to be associated with hyperprolactinaemia, or when secondary TD due to a pituitary tumour or other pituitary disease is suspected.
Regarding other investigations, in men with testosterone levels <5.2 nmol/L and increased prolactin levels or reduced LH and FSH levels, pituitary magnetic resonance imaging should be performed to exclude a pituitary adenoma [6,22].
The EAU [111] recommend screening for concomitant osteoporosis in men aged over 50 years with established TD (LoE 2, Grade B).
THRESHOLDS FOR T THERAPY
Regarding the thresholds for treatment intervention in symptomatic men, BSSM [1,5] and ISSM guidelines recommend the following:
• TT level <12 nmol/L or FT <225 pmol/L (<0.225 nmol/L) (based on two separate 8–11 am levels), usually requires T Therapy (LoE=1 Grade A) [1].
• TT level >12 nmol/L, or FT of >225 pmol/L (>0.225 nmol/L), does not require T Therapy (LoE=Grade 1 Grade A) [1].
• Levels between 8–14 nmol/L may require a trial of T Therapy for a minimum of 6 months, based on symptom (LoE = 3, Grade C) [117].
-
The BSSM also says (Table 4):
• Evidence supports the treatment of men with TT levels <14 nmol/L in symptomatic men with prediabetes to prevent progression to overt type 2 diabetes (LoE=2 Grade B) [53].
• A FT level <225 pmol/L (0.225 nmol/L) provides supportive evidence for T Therapy in the presence of appropriate symptoms (LoE=1 Grade A) [122].
Table 4. Recommendations – initiating T Therapy.
| Criteria for appropriate initiation of testosterone therapy | LoE | Grade |
|---|---|---|
| Perform cardiovascular, prostate, breast and haematological assessment before the start of treatment | 1a | A |
| TT level <12 nmol/L or FT <225 pmol/L (<0.225 nmol/L) (based on two separate 8–11 am levels), usually requires T Therapy [1]. | 1 | A |
| TT level >12 nmol/L, or FT of >225 pmol/L (>0.225 nmol/L), does not require T Therapy [1]. | 1 | A |
| Evidence supports the treatment of men with TT levels <14 nmol/L in symptomatic men with pre-diabetes to prevent progression to overt type 2 diabetes [53]. | 2 | B |
| Offer T Therapy to symptomatic men with TD syndrome for treated localised low risk prostate cancera and without evidence of active disease (based on measurable PSA, DRE and evidence of metastatic disease) | 3 | B |
| Assess cardiovascular risk factors commencing T Therapy and optimise secondary prevention in men with established disease | 1a | A |
T Therapy: testosterone therapy, TT: total testosterone, FT: free testosterone, TD: testosterone deficiency, PSA: prostate specific antigen, DRE: digital rectal examination.
a<8 Gleason score, stage 1–2, pre-op PSA <10 ng/mL and not starting before 1 year of follow-up.
Additional recommendations include:
• Raised LH levels and testosterone below normal or in the lower quartile range, indicates testicular failure, so T Therapy should be considered based on symptom severity [123].
• Raised LH levels in men with normal testosterone levels but symptoms of TD, should be considered as TD [26].
• Data from EMAS [123] found that clinical symptoms and all-cause mortality were more closely related to calculated FT (LoE 2 Grade=B) [124].
TESTOSTERONE THERAPY
There are many different testosterone preparations available (Table 5), which differ in their formulations, routes of administration, dosage intervals and safety profiles. Aromatizable testosterone is recommended for replacement therapy [26].
Table 5. T Therapy options.
| Formulation | Route of administration | Frequency of administration | Advantages | Disadvantages |
|---|---|---|---|---|
| Testosterone 1% 1.62% and 2% gel available | Transdermal | Applied daily to upper arms or lower abdomen requires dose titration Daily |
Fast onset. Levels peak 2–4 hours after application then gradually fall to very low levels before the next application | Skin irritation at application site Potential for interpersonal transfer, partner or child Non-compliance long-term especially with hot climate, swimming etc. |
| 5% testosterone cream | Small volume applied to scrotum application | Licensed in Australia and for import into UK. | ||
| Testosterone undecanoate | Oral capsules | Once or twice daily | Lymphatic absorption reduces liver involvement | Levels fluctuate, lower efficacy. Normal serum T level attained for only up to 3–5 hours Daily/twice daily commitment Must be taken with food |
| Testosterone undecanoate | Intramuscular injection. Injected slowly deep into the gluteal muscle. The second injection (loading dose) is given at 6 weeks, and the third dose 12 weeks after the second. Injection interval is adjusted based on trough total T level just before the third injection, aiming for the lower end of normal reference range level. Monitor trough total T and FBC every 3–5 injections or annually | Every 10–14 weeks adjusted to maintain trough T >12 nmol/L | Steady state levels Reduced frequency of administration improves compliance. Patient compliance less of a problem |
Long duration of action prevents drug withdrawal in the event of adverse side-effects. Pain, discomfort and adverse reaction at injection site. Requires large muscle bulk for injection. Lifestyle restrictions as it cannot be self‐administered. Rare AE of POME presenting with severe coughing episode during injection. Longer recovery period after therapy cessation |
| Testosterone enanthate | Intramuscular injection | Every 2–3 weeks. Dose flexibility and convenient administration, relatively inexpensive. Improves symptoms of androgen deficiency; mostly noticeable in the first days after the injection. SC injection has comparable pharmacokinetics, safety and tolerability to IM injection and can be self‐injected. | Short duration of action allows drug withdrawal if there are adverse side effects | Levels fluctuate potentially unpleasant “peak & trough” symptoms due to supraphysiological T levels post-injection which decline to hypogonadal range prior to the next injection. Polycythaemia due to supraphysiological T levels. Pain, discomfort at injection site. Lifestyle restrictions for patients not self‐injecting |
| Testosterone cypionate | Intramuscular injection (also subcutaneous micro-dosing) | Every 1–4 weeks | Medium/short duration | Little long term published data |
| Testosterone propionate | Intramuscular injection | Currently available as one of four testosterone esters used in Sustanon 250, which is usually administered every 3 weeks. Sustanon’s long duration of action prevents drug withdrawal in the event of adverse side-effects |
||
T: testosterone, FBC: full blood count, POME: pulmonary micro-embolism.
Data from Hackett et al (Int J Clin Pract 2017;71:e12901) and Jayasena et al (Clin Endocrinol [Oxf] 2022;96:200-19) [114].
Oral therapies are rarely used in the UK because of the need for multiple dosing regimens and fears over hepatotoxicity. Two oral formulations (Jatenzo and Tlando) are available in the US as is a twice or three times daily nasal formulation (Natesto) [124]. The choice of licensed products in the UK usually lies between the transdermal route and long-acting TU.
Unlicensed short acting injections (enanthate and cypionate) are marketed by commercial websites as being superior to gel and long-acting TU. Whilst these preparations may have a prompter onset of action, multiple studies report a higher rate of polycythaemia [125,126] Recently subcutaneous micro-dosing with short acting testosterone has become fashionable, but long-term studies are not available [46,127,128]. The BSSM position is that pivotal long-term trials have been conducted with licensed gels and long-acting TU and the findings from these studies in terms of efficacy and safety cannot be extrapolated to unlicensed formulations.
The features of each method should be discussed with the patient. Based on current evidence we can see no justification for selecting one licensed formulation over another except for patient choice. Some patients may be hesitant about starting testosterone treatment as they are unaware of all the treatment benefits, time scales and expectations. They may be concerned about perceived side effects often resulting from internet sources. These can have a negative impact on adherence to treatment. Clinicians are advised to make patients aware of all possible testosterone treatments and to support them to select the most suitable treatment for their needs.
The BSSM recommend initiating T Therapy only in men with bothersome symptoms, in conjunction with weight loss advice, lifestyle modification and treatment of comorbidities (LoE 2, Grade A) (Fig. 2). Weight loss and lifestyle modification alone in the EMAS study failed to demonstrate effective improvement in clinical symptoms, especially sexual desire and ED or significant increase in FT, even after >4 years, and patients need to be informed of this [124].
THE IMPORTANCE OF CO-ADMINISTRATION OF DAILY PDE5 INHIBITORS IN HYPOGONADISM
The 3 sexual symptoms (ED, loss of EMEs and reduced sexual desire) are the most common reasons for initiating treatment [124] and patients expect prompt resolution of distressing symptoms.
As most trials of testosterone therapy involve patients with multiple co-morbidities, especially endothelial dysfunction, TTh only produces a modest, 1–3 point improvement in EF score, leading to discontinuation in many men who do not see a prompt response in their primary symptom [53,54]. Pivotal long term studies such as the T trials and T4DM, show baseline EF scores of 11–14/30, with improvements of only 1–2 EF points over 12–24 months. In the BLAST extension study, at 18 months, men taking frequent doses of PDE5 inhibitors showed 9 point EF improvements at 18 months, likely to be highly meaningful. The mechanism of the ED in men with diabetes or pre-diabetes is predominantly through endothelial dysfunction and not low testosterone.
A combination of testosterone and PDE5i therapy should be considered in patients with TD and ED, especially in those with multiple risk factors for ED, in addition to TD (LoE 2, Grade A) [63]. Daily dosing with tadalafil has been found to have greater efficacy than on demand medication in multiple long term meta-analyses and salvage up to 50% of men who fail ondemand medication [129,130,131]. It has been found to be more acceptable to patients (80%) and partners (79%) in preference studies [132,133]. We need to be aware that the major reason for sexual activity in the adult population is to provide pleasure for the patient and their partner. Daily tadalafil, now available generically at low cost is the preferred option to restore regular morning erections and in men with low sex desire, who might not be motivated to take on-demand medication [132].
Daily PDE5 inhibitors have also been reported to increase testosterone production through a direct effect on the testes [134,135,136] enhance insulin sensitivity [137] improve, co-existing LUTS [138] especially as bothersome LUTS was detected in 40% of men with prediabetes in testosterone for diabetes mellitus study [53]. Daily PDE5 inhibitors have been shown to reduce all-cause mortality in men with type 2 diabetes [89] and previous MI [139]. A Recent US study showed a 40% reduction in major adverse coronary events (MACE) and VTE compared with alpha-blockers in men with LUTS [140].
As men with TD are only likely to receive one trial of testosterone therapy, BSSM believes that a daily PDE5 inhibitor, along with full explanation of the potential benefits, is essential, especially in men with cardio-metabolic disease. As sexual dysfunction is often multi-faceted in nature, therapy involving physical, additional behavioural, psychological, interpersonal and contextual aspects of psychosexual therapy may also be indicated [1].
T Therapy has been shown to be appropriate for treating ED, particularly at TT levels <8 nmol/L [5,7] or calculated free testosterone (cFT) <180 pmol/L and for salvaging ED treatment failures with oral medication, particularly at TT levels <10.4 nmol/L [47]. Furthermore, appropriate intervention with T Therapy and PDE5 inhibitor reduces the need for more invasive and expensive second- and third-line treatments in these patients [123].
Critical review of the AUA [134] EAU [111] and Society for Endocrinology guidelines [114] suggest that men are assessed at 3 months and T Therapy discontinuation discussed if there has not been meaningful improvement. Strict interpretation of this guidance will lead to high rates of discontinuation, due to unrealistic expectations especially as increase in sexual desire in the absence of improved erection is likely to lead to frustration for the man and his partner. BSSM therefore recommends efficacy assessment at 6 months. EAU guidance [111] recommends 3 DREs within the first 12 months, whereas the Society for Endocrinology [114] state that routine DRE is not necessary and may be harmful.
ALTERNATIVES FOR MEN WITH HYPOGONADISM WHO DESIRE FUTURE FERTILITY
Although TD is often associated with reduced fertility, treatment with exogenous testosterone reduces endogenous testosterone production though negative feedback on the HPG axis [4] resulting in decreased LH and FSH levels, and reduced spermatogenesis. If a man wishes to father children in the near future, then the risk of T Therapy on fertility needs to be discussed, as long-term use is associated with azoospermia. A wish for paternity is one of the main contraindications to T Therapy [4]. Younger patients may not perceive this as an issue as they cannot see beyond the immediate problem. Be aware that 7% of men may be infertile for other reasons. Fertility may take 12 months to recover after cessation of T Therapy and patients can be reassured that they will usually subsequently respond to HCG [141]. For secondary TD, possible alternatives include human chorionic gonadotropin (HCG), off-label [142] SERMs (e.g., clomiphene) [143] and AIs [144]. SERMs and AIs [144] are particularly useful in men with metabolic disturbances. However, although these drugs appear safe [145], they should not be used when pituitary function is compromised and data on long-term safety in men are lacking.
There is no scientific basis for withholding T Therapy from men with TD on the basis of age [1].
The clinical and physiological responses to T Therapy, particularly the benefits of increased muscle mass and strength [54] may be more clinically and economically significant in older men, since reduced muscle mass and lower limb strength are strongly related to frailty and an increased rate of falls [125].
CARDIOVASCULAR RISK AND TESTOSTERONE THERAPY
Recent research suggests a greater reduction in all-cause mortality in hypogonadal men over 75 years treated with testosterone [90,91]. The traditional view that younger men experience greater benefit from improvement in sexual symptoms associated with T Therapy, has not been supported by recent studies [146].
The clinical response to T Therapy appears unrelated to the underlying aetiology [55] as recent studies have shown benefits in men without ‘classical TD’. It is really a matter of clinical judgement and patient expectation as to whether the underlying conditions should be addressed first [1] but because many men with TD are sick and/or obese, evidence suggests that better outcomes may be achieved if lifestyle modifications, including weight reduction, and T Therapy are combined [4,40].
Evidence suggests that responsiveness to T Therapy is not only dependent on serum testosterone concentration, but also the length of CAG repeats on the AR [147]. Longer CAG repeats are associated with more severe symptoms and reduced response to standard-dose therapy. A 14 year follow-up study of 423 men showed that those with 21 CAG repeats had a 58% reduced mortality compared with those with 22 or more or 20 or less [148]. The wide variations in CAG repeats within different ethnic groups is particularly important in the ethnically-diverse UK population [14]. AR sensitivity was found to be less marked in men with raised BMI and waist circumference [147]. When T Therapy is used in men with BPH, symptomatic improvement of LUTS has been reported but no improvement was seen in 2 years of T Therapy in T4DM, where bothersome LUTS prevalence was around 40% [53] Daily tadalafil is recommended as first line treatment by the EAU guidelines in men with ED and bothersome LUTS [117]. There is some evidence that alpha-blockers may precipitate heart failure in high risk patients [14]. BSSM strongly recommends that therapeutic decisions need to consider multiple co-morbidities and not simply flow rates in LUTS patients.
Regarding treatment duration, in RCTs involving 447 men aged between 45–90 years, cessation of T Therapy resulted in relapse and reversal of benefits within 6 months, so therapy is likely to be required life-long [99].
ADVERSE EFFECTS OF T THERAPY
Serious adverse events related to T Therapy are relatively rare (Table 6). They are more significant in elderly patients and often dependent on the method of delivery. Some adverse events are related to supraphysiological levels and can be reduced or stopped altogether by adjusting the dose or switching to a different formulation [6].
Table 6. Recommendations – diagnosis.
| Recommendatios for the diagnosis of restosterone deficiency | LoE | Grade |
|---|---|---|
| Restrict the diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low testosterone | 3 | C |
| Measure (ideally fasting) testosterone levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels may be up to 30% lower | 2 | A |
| Repeat total testosterone on at least 2 occasions by a reliable method. In addition, measure free testosterone in men with levels close to the lower normal range (8–14 nmol/L) or those with suspected or known abnormal SHBG | 1 | A |
| Measure LH serum levels to differentiate between primary and secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
TD: testosterone deficiency, SHBG: sex hormone binding globulin, LH: luteinizing hormone.
The intramuscular injections are associated with pain at the injection site, changes in mood, energy and sexual desire, and rarely, coughing straight after the injection (pulmonary micro-embolism, POME), this risk is reduced if the injection is given over several minutes [149].
Transdermal gels carry a risk of interpersonal transfer, skin irritation and fluctuations in absorption [1].
The 17 α-alkylated formulations are associated with a risk of hepatotoxicity, cholestasis, peliosis hepatis, hepatic tumours and a marked reduction in HDL cholesterol [1]. These treatments should no longer be prescribed [5].
Other side effects seen with T Therapy include polycythaemia (which is more common in older men treated with short acting injectable testosterone preparations) [125,126] with increases in haematocrit and haemoglobin. Long-acting TU and transdermal gels have the least effect on haematocrit [126]. Change of formulation or reduction in dose may resolve the issue. Some guidelines recommend caution in commencing T Therapy with baseline haematocrit of 48 or 52%, but a recent study has suggested better outcomes in patients treated into the upper normal range [150] (Table 7).
Table 7. Recommendations – benefits and risks of T Therapy.
| Benefits and risks of testosterone therapy in men with testosterone deficiency | LoE | Grade |
|---|---|---|
| Beyond 6 months there is evidence of benefit for T Therapy in body composition, bone mineralisation and features of metabolic syndrome | 3 | A |
| Testosterone Therapy improves sexual desire, erectile function and sexual satisfaction | 1 | A |
| A reduction in BMI, waist size, improved glycaemic control and lipid profile are observed in hypogonadal men receiving T Therapy | 2 | A |
| Trials of T Therapy should be at least 6 months and maximal benefit is often seen beyond 12 months | 2 | A |
| Fully inform the patient about expected benefits and side-effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse impact of T Therapy and its reversibility for future fertility and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when T Therapy is prescribed, offer additional weight loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels below 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
T Therapy: testosterone therapy, BMI: body mass index, TD: testosterone deficiency, TT: total testosterone.
Obese men are more likely to suffer adverse effects from T Therapy than men of normal weight [5].
CONTRAINDICATIONS TO T THERAPY
The main contraindications to T Therapy are [4]:
• Locally advanced/metastatic PCa
• Male breast cancer
• An active desire to have children
• Haematocrit >54%
• Severe chronic heart failure (NYHA class IV)
Additional contraindications, include unevaluated prostate nodule or induration, PSA >4 ng/mL [6,22] (or >3 ng/mL in men at high risk of PCa, such as African-Americans and those with first-degree relatives who have PCa) [22], severe, untreated sleep apnoea [26] and severe LUTS associated with BPH as indicated by the AUA symptom index/IPSS >19 [6,22].
A critical update of the ES guidelines published in 2015 [129] which examined the body of level 1 evidence literature published since 2010, states that patients with elevated PSA levels should be referred for urologic consultation prior to initiating TRT, and untreated sleep apnoea and severe LUTS may not be absolute contraindications to T Therapy.
FOLLOW-UP AND MONITORING
Before initiating T Therapy, haematological assessment should be performed, prostate health should be assessed, ideally by DRE and PSA, the man’s breasts should be checked, cardiovascular risk factors should be assessed, and men with CVD should receive evaluation of cardiovascular symptoms and optimisation of secondary prevention [4].
Following initiation of T Therapy, we recommend evaluating patients at 3, 6 to 12 months, then every 12 months thereafter, to assess serum testosterone levels, confirm symptomatic improvement, and check for any changes in haematocrit, PSA and DRE (according to symptoms). In line with the current Society for Endocrinology Guidelines [118], we feel that current evidence does not support the need for frequent DREs in addition to PSA, especially with the number of specialist referrals required [117] (Fig. 1).
Monitoring levels of TT, SHBG and albumin (to calculate bioavailable or FT) is required to ensure normal testosterone levels are being achieved. There is currently insufficient data to define optimal serum testosterone levels during treatment. The aim of therapy is to restore serum testosterone levels to the mid-normal range for specific age groups [4]. Impairment of sexual function and nocturnal erections are usually associated with TT levels <7 nmol/L, with the maximal benefit of testosterone appearing to result with levels ≥12–16 nmol/L [6,130].
For monitoring purposes, we recommend a therapeutic target in the mid to upper range (15–30 nmol/L), making reference ranges of little value. For patients on transdermal preparations, blood samples should be taken 2–4 hours after application of gel or lotion, being careful to avoid any skin contamination. In the case of injections, the timing for blood testing is irrelevant, as the trough levels which assess the duration of effect of the formulation is all that is required.
Haematocrit levels should remain below 54% (LoE 2, Grade B) [4,6,26]. Dose adjustments and/or periodic venesection may be required in order to achieve this [5].
PSA increases >1.4 ng/mL during any 1-year period after initiation of T Therapy, or a PSA velocity >0.4 ng/mL per year during sequential PSA measurements over more than two years, warrants a urological evaluation and more intensive surveillance for PCa thereafter [5]. Due to an early physiological increase in PSA with T Therapy, a level 6 months after commencing T Therapy should be taken as baseline, with an annual check thereafter (Table 8).
Table 8. Recommendations – follow-up.
| Follow-up on men on testosterone therapy | LoE | Grade |
|---|---|---|
| Assess the response to therapy at 3, 6 to 12 months, and every 12 months thereafter | 4 | C |
| Aim for a target level of total testosterone 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor haematocrit before treatment, at 3–6 months, 12 months and every 12 months thereafter. Decrease dosage, or switch preparation, if haematocrit >0.54. If haematocrit remains elevated, consider stopping and re-introduce at a lower dose | 4 | C |
| Assess prostate health by PSA and, ideally, DRE before commencing TRT followed by PSA at 3–6 months, 12 months and every 12 months thereafter | 4 | C |
| Assess cardiovascular risk before TRT is initiated and monitor cardiovascular risk factors throughout therapy | 1b | A |
PSA: prostate specific antigen, DRE: digital rectal examination, TRT: testosterone replacement therapy.
Men with CVD should be monitored carefully, with clinical assessment and measurement of testosterone and haematocrit performed regularly [4].
BMD need only be monitored in men with abnormal BMD prior to initiating T Therapy [4,6] and repeat dual energy X-ray absorptiometry (DEXA scanning) is recommended after 1-2 years of T Therapy [6].
While monitoring of serum lipids and glycaemia is not required for safety during T Therapy, it may be necessary for monitoring the efficacy of other aspects of treatment (LoE 2, Grade A) [6].
Assessment of treatment outcome, and decisions regarding continuation of T Therapy, should be based on improvement in signs and symptoms of TD. Failure to benefit within a reasonable time frame (defined as 6 months for libido, sexual function, muscle function and improved body fat), should prompt discontinuation of treatment. Further investigation for other causes of the symptoms is then essential [5].
BSSM POSITION STATEMENT ON COVID-19 INFECTION
A large-scale global statistical analysis showed that whilst males and females are at equivalent risk of COVID-19 infection, male sex is associated with the development of severe disease:
• ITU admission (OR=2.84; 95% CI=2.06–3.92; p=1.86×10-10)
• Death (OR=1.39; 95% CI=1.31–1.47; p=5.00×10-30).
Several studies from Italy, Germany and the UK [99,151,152] have shown higher prevalence of COVID-19 infection in men with obesity, T2DM and COPD, all conditions associated with higher rates of TD [99]. One study of patients with pre-covid testosterone assessment showed 3–4 nmol/L mean drop in TT in men requiring ventilation with acute infection [153]. Inflammatory markers and mortality levels were consistently related to TT levels [151,152]. There is evidence of acute and longer term primary and secondary hypogonadism [99,150,151,152,153]. In survivors following intensive care therapy, 50% still had low TT levels 7 months later and in 10% of cases, levels were lower than on admission [154]. It is therefore likely that COVID-19 infection has become a major cause of hypogonadism, metabolic disease and sexual dysfunction. However, one study suggested that men on androgen deprivation therapy had lower mortality rates [155]. Currently however, topical TRT and oral PDE5 inhibitors [156] are likely to be beneficial but, paradoxically, are likely to be discontinued on acute hospital admission with suspected COVID-19 infection, whereas long-acting TU injection might remain active for several weeks.
Based on these findings BSSM recommend measurement of testosterone levels in men with acute covid infection and treatment according to current guidelines during acute illness and for levels that have not recovered within 3 months.
CONCLUSIONS
TD is a well-established and significant medical condition, encompassing somatic, sexual and psychological effects. It is also associated with increased risk of type 2 diabetes and cardiovascular and all-cause mortality. Clearly defined clinical symptoms, especially ED, loss of morning erections and reduced sexual desire should prompt swift evaluation of these, together with modification of underlying risk factors, and further investigations performed where appropriate.
In order to identify individuals who should be screened for TD, consideration should be given to routinely asking men if they have any sexual concerns, particularly those at high risk. These include men with diabetes, osteoporosis/fragility fractures, CVD, CKD, anaemia, ED, depression, COVID-19 infection and those on long-term opiate/oral glucocorticoid therapy.
T Therapy is evidence-based, effective and safe, and evidence suggests that treatment-related sustained normalisation of serum testosterone levels is associated with reduced morbidity and mortality.
However, whilst the multiple benefits of T Therapy are highly relevant to the patient, they are often underestimated by specialist physicians focused on specific outcomes related to their particular discipline. Because sexual dysfunction is often multi-faceted in nature, the treatment of men with sexual desire, arousal and ejaculation problems should be individualised and may include T Therapy, erectogenic agents and psychosexual therapy.
Until a definitive well-powered long-term study is published, treatment of TD with T Therapy should be guided by the best available evidence.
Footnotes
Conflict of Interest: Geoffrey Hackett: Medical adviser to Teds Health, Besins, Sanofi. Sudarshan Ramachandran: Travel grants from Besins Healthcare.
Funding: Grant from British Society for Sexual Medicine.
- Conceptualisation: GH, MK.
- Data Curation: GH, MK.
- Formal analysis: GH.
- Funding acquisition: GH, MK.
- Investigation: GH, MK.
- Methodology: GH, MK.
- Resources: GH, MK.
- Software: GH.
- Supervision: GH, MK, AM.
- Validation: GH, MK, AM, JG, JF, RR, THJ, ML, NOG, JD, PAK, SR.
- Visualisation: GH, MK, AM, JG, JF, RR.
- Writing – Review & Editing: GH, MK.
- Writing – Original Draft: GH, MK.
References
- 1.Hackett G, Kirby M, Edwards D, Jones TH, Rees J, Muneer A. UK policy statements on testosterone deficiency. Int J Clin Pract. 2017;71:e12901. doi: 10.1111/ijcp.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine: levels of evidence (March 2009) [Internet] Oxford: Centre for Evidence Based Medicine; c2009. [cited 2022 May 21]. Available from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ [Google Scholar]
- 3.Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract. 2010;64:682–696. doi: 10.1111/j.1742-1241.2010.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S. Male hypogonadism [Internet] Arnhem: European Association of Urology; c2017. [cited 2022 Apr 16]. Available from: http://uroweb.org/guideline/male-hypogonadism/ [Google Scholar]
- 5.British Society for Sexual Medicine. Management of sexual problems in men: the role of Androgens [Internet] Lichfield: The British Society for Sexual Medicine; c2010. [cited 2017 Jan 28]. Available from: http://bssm.org.uk/ [Google Scholar]
- 6.Khera M, Adaikan G, Buvat J, Carrier S, El-Meliegy A, Hatzimouratidis K, et al. Diagnosis and treatment of testosterone deficiency: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:1787–1804. doi: 10.1016/j.jsxm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Dean JD, McMahon CG, Guay AT, Morgentaler A, Althof SE, Becher EF, et al. The International Society for Sexual Medicine's process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med. 2015;12:1660–1686. doi: 10.1111/jsm.12952. [DOI] [PubMed] [Google Scholar]
- 8.Antonio L, Wu FCW, Moors H, Matheï C, Huhtaniemi IT, Rastrelli G, et al. EMAS Study Group. Erectile dysfunction predicts mortality in middle-aged and older men independent of their sex steroid status. Age Ageing. 2022;51:afac094. doi: 10.1093/ageing/afac094. [DOI] [PubMed] [Google Scholar]
- 9.Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, Finn JD, et al. EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 10.Costanzo LS. Physiology. 3rd ed. Baltimore (MD): Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 11.MedlinePlus. AR gene [Internet] Bethesda (MD): National Library of Medicine; c2017. [cited 2022 May 5]. Available from: https://ghr.nlm.nih.gov/gene/AR. [Google Scholar]
- 12.Zitzmann M. Mechanisms of disease: pharmacogenetics of testosterone therapy in hypogonadal men. Nat Clin Pract Urol. 2007;4:161–166. doi: 10.1038/ncpuro0706. [DOI] [PubMed] [Google Scholar]
- 13.Rajender S, Singh L, Thangaraj K. Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J Androl. 2007;9:147–179. doi: 10.1111/j.1745-7262.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 14.Heald AH, Yadegar Far G, Livingston M, Fachim H, Lunt M, Narayanan RP, et al. Androgen receptor-reduced sensitivity is associated with increased mortality and poorer glycaemia in men with type 2 diabetes mellitus: a prospective cohort study. Cardiovasc Endocrinol Metab. 2020;10:37–44. doi: 10.1097/XCE.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloner RA, Carson C, 3rd, Dobs A, Kopecky S, Mohler ER., 3rd Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545–557. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. Erratum in: JAMA 2008;299:634. [DOI] [PubMed] [Google Scholar]
- 17.Aversa A, Bruzziches R, Francomano D, Greco EA, Fornari R, Di Luigi L, et al. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15:96–102. doi: 10.3109/13685538.2011.631230. [DOI] [PubMed] [Google Scholar]
- 18.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, et al. TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102:1067–1075. doi: 10.1210/jc.2016-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto AM. Testosterone administration in older men. Endocrinol Metab Clin North Am. 2013;42:271–286. doi: 10.1016/j.ecl.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Corona G, Maseroli E, Rastrelli G, Sforza A, Forti G, Mannucci E, et al. Characteristics of compensated hypogonadism in patients with sexual dysfunction. J Sex Med. 2014;11:1823–1834. doi: 10.1111/jsm.12549. [DOI] [PubMed] [Google Scholar]
- 22.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. Erratum in: J Clin Endocrinol Metab 2021;106:e2848. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto AM. In: Endocrinology & metabolism. 4th ed. Felig P, Frohman LA, editors. New York (NY): McGraw-Hill; 2001. The testis; pp. 635–705. [Google Scholar]
- 24.Schartau PES. In: Testosterone in cardiometabolic and other diseases. Hackett GI, Kirby M, editors. Torino: Minerva Medica; 2022. Diagnosis and terminology in hypogonadism. [Google Scholar]
- 25.Glintborg D, Vaegter HB, Christensen LL, Bendix E, Graven-Nielsen T, Andersen PG, et al. Testosterone replacement therapy of opioid-induced male hypogonadism improved body composition but not pain perception: a double-blind, randomized, and placebo-controlled trial. Eur J Endocrinol. 2020;182:539–548. doi: 10.1530/EJE-19-0979. [DOI] [PubMed] [Google Scholar]
- 26.Kirby M, David J. In: Testosterone in cardiometabolic and other diseases. Hackett GI, Kirby M, editors. Torino: Minerva Medica; 2022. Opiate induced Hypogonadism. [Google Scholar]
- 27.Morrison D, Capewell S, Reynolds SP, Thomas J, Ali NJ, Read GF, et al. Testosterone levels during systemic and inhaled corticosteroid therapy. Respir Med. 1994;88:659–663. doi: 10.1016/s0954-6111(05)80062-9. [DOI] [PubMed] [Google Scholar]
- 28.Raheem OA, Patel SH, Sisul D, Furnish TJ, Hsieh TC. The role of testosterone supplemental therapy in opioid-induced hypogonadism: a retrospective pilot analysis. Am J Mens Health. 2017;11:1208–1213. doi: 10.1177/1557988316672396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhetheli GI, Alrashidi FH, Alhammad SH. Post-finasteride syndrome: current views and where do we stand? Ann Med Health Sci Res. 2022;12:12–18. [Google Scholar]
- 30.Traish AM. Post-finasteride syndrome: a surmountable challenge for clinicians. Fertil Steril. 2020;113:21–50. doi: 10.1016/j.fertnstert.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan J, Barkin J. Testosterone deficiency syndrome: benefits, risks, and realities associated with testosterone replacement therapy. Can J Urol. 2016;23(Suppl 1):20–30. [PubMed] [Google Scholar]
- 34.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 35.Gencer B, Mach F. Testosterone: a hormone preventing cardiovascular disease or a therapy increasing cardiovascular events? Eur Heart J. 2016;37:3569–3575. doi: 10.1093/eurheartj/ehv439. [DOI] [PubMed] [Google Scholar]
- 36.McBride JA, Carson CC, 3rd, Coward RM. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8:47–60. doi: 10.1177/1756287215612961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 38.Armamento-Villareal R, Aguirre LE, Qualls C, Villareal DT. Effect of lifestyle intervention on the hormonal profile of frail, obese older men. J Nutr Health Aging. 2016;20:334–340. doi: 10.1007/s12603-016-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran LJ, Brinkworth GD, Martin S, Wycherley TP, Stuckey B, Lutze J, et al. Long-term effects of a randomised controlled trial comparing high protein or high carbohydrate weight loss diets on testosterone, SHBG, erectile and urinary function in overweight and obese men. PLoS One. 2016;11:e0161297. doi: 10.1371/journal.pone.0161297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Vincenzo A, Busetto L, Vettor R, Rossato M. Obesity, male reproductive function and bariatric surgery. Front Endocrinol (Lausanne) 2018;9:769. doi: 10.3389/fendo.2018.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rigon FA, Ronsoni MF, Hohl A, van de Sande-Lee S. Effects of bariatric surgery in male obesity-associated hypogonadism. Obes Surg. 2019;29:2115–2125. doi: 10.1007/s11695-019-03829-0. [DOI] [PubMed] [Google Scholar]
- 42.Morales A, Black A, Emerson L, Barkin J, Kuzmarov I, Day A. Androgens and sexual function: a placebo-controlled, randomized, double-blind study of testosterone vs. dehydroepiandrosterone in men with sexual dysfunction and androgen deficiency. Aging Male. 2009;12:104–112. doi: 10.3109/13685530903294388. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramanian A, Thirumavalavan N, Srivatsav A, Yu J, Lipshultz LI, Pastuszak AW. Testosterone imposters: an analysis of popular online testosterone boosting supplements. J Sex Med. 2019;16:203–212. doi: 10.1016/j.jsxm.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newell-Price J, Huatan H, Quirke J, Porter J, Daniel E, Mumdzic E, et al. An oral lipidic native testosterone formulation that is absorbed independent of food. Eur J Endocrinol. 2021;185:607–615. doi: 10.1530/EJE-21-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumagai H, Zempo-Miyaki A, Yoshikawa T, Tsujimoto T, Tanaka K, Maeda S. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62:423–430. doi: 10.1507/endocrj.EJ14-0555. [DOI] [PubMed] [Google Scholar]
- 46.Rastrelli G, Carter EL, Ahern T, Finn JD, Antonio L, O'Neill TW, et al. EMAS Study Group. Development of and recovery from secondary hypogonadism in aging men: prospective results from the EMAS. J Clin Endocrinol Metab. 2015;100:3172–3182. doi: 10.1210/jc.2015-1571. [DOI] [PubMed] [Google Scholar]
- 47.Ng Tang Fui M, Hoermann R, Prendergast LA, Zajac JD, Grossmann M. Symptomatic response to testosterone treatment in dieting obese men with low testosterone levels in a randomized, placebo-controlled clinical trial. Int J Obes (Lond) 2017;41:420–426. doi: 10.1038/ijo.2016.242. [DOI] [PubMed] [Google Scholar]
- 48.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14:153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 50.Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, et al. American Association of Clinical Endocrinologists and American College of Endocrinology-clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(Suppl 1):1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL Rancho Bernardo Study. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 52.Yao QM, Wang B, An XF, Zhang JA, Ding L. Testosterone level and risk of type 2 diabetes in men: a systematic review and meta-analysis. Endocr Connect. 2018;7:220–231. doi: 10.1530/EC-17-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9:32–45. doi: 10.1016/S2213-8587(20)30367-3. [DOI] [PubMed] [Google Scholar]
- 54.Hackett G, Cole N, Saghir A, Jones P, Strange RC, Ramachandran S. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 2016;118:804–813. doi: 10.1111/bju.13516. [DOI] [PubMed] [Google Scholar]
- 55.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Testosterone Trials Investigators. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–1981. doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 57.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Kang D, Li H. Testosterone and cognitive impairment or dementia in middle-aged or aging males: causation and intervention, a systematic review and meta-analysis. J Geriatr Psychiatry Neurol. 2021;34:405–417. doi: 10.1177/0891988720933351. [DOI] [PubMed] [Google Scholar]
- 59.Marriott RJ, Murray K, Flicker L, Hankey GJ, Matsumoto AM, Dwivedi G, et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: the UK Biobank prospective cohort study. Alzheimers Dement. 2022;18:1907–1918. doi: 10.1002/alz.12529. [DOI] [PubMed] [Google Scholar]
- 60.Lowe G, Bahnson R. Non-invasive management of primary phosphodiesterase type 5 inhibitor failure in patients with erectile dysfunction. Ther Adv Urol. 2009;1:235–242. doi: 10.1177/1756287210362069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minhas S, Mulhall J. Male sexual dysfunction: a clinical guide. Chichester: Wiley-Blackwell; 2017. pp. 133–142. [Google Scholar]
- 62.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C Hormonal Male Contraception Summit Group. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412–1420. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 63.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P, et al. Blast Study Group. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study) Int J Clin Pract. 2014;68:203–215. doi: 10.1111/ijcp.12235. [DOI] [PubMed] [Google Scholar]
- 64.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177:471–479. doi: 10.1001/jamainternmed.2016.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy CN, Snyder PJ, Stephens-Shields AJ, Artz AS, Bhasin S, Cohen HJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177:480–490. doi: 10.1001/jamainternmed.2016.9540. Erratum in: JAMA Intern Med 2019;179:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA. 2017;317:717–727. doi: 10.1001/jama.2016.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chedrawe E, Sathe A, White J, Ory J, Ramasamy R. Testosterone therapy in advanced prostate cancer. Androg Clin Res Ther. 2022;3:180–186. doi: 10.1089/andro.2021.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97:870–875. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 70.Haring R, Völzke H, Steveling A, Krebs A, Felix SB, Schöfl C, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31:1494–1501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 71.Daka B, Langer RD, Larsson CA, Rosén T, Jansson PA, Råstam L, et al. Low concentrations of serum testosterone predict acute myocardial infarction in men with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:35. doi: 10.1186/s12902-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2:e000272. doi: 10.1161/JAHA.113.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma R, Oni O, Wiegmann P, Sharma M, Garcia-Touza M, Goel A, et al. Testosterone replacement therapy (TRT) is associated with delayed progression of chronic kidney disease: a retrospective analysis of testosterone normalization in US veterans. Ann Nephrol. 2020;5:51–59. [Google Scholar]
- 74.Alwani M, Al-Zoubi RM, Al-Qudimat A, Yassin A, Aboumarzouk O, Al-Rumaihi K, et al. The impact of long-term Testosterone Therapy (TTh) in renal function (RF) among hypogonadal men: an observational cohort study. Ann Med Surg (Lond) 2021;69:102748. doi: 10.1016/j.amsu.2021.102748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 76.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–E18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 77.Li S, Li X, Li Y. Regulation of atherosclerotic plaque growth and stability by testosterone and its receptor via influence of inflammatory reaction. Vascul Pharmacol. 2008;49:14–18. doi: 10.1016/j.vph.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11:624–629. doi: 10.1111/jsm.12464. [DOI] [PubMed] [Google Scholar]
- 80.Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. Erratum in: JAMA 2014;311:967. [DOI] [PubMed] [Google Scholar]
- 81.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, 3rd, Wenger NK, Bhasin S, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.FDA. FDA drug safety communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use [Internet] Silver Spring (MD): FDA; c2015. [cited 2022 May 31]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. [DOI] [PubMed] [Google Scholar]
- 84.European Medicines Agency. No consistent evidence of an increased risk of heart problems with testosterone medicines [Internet] London: European Medicines Agency; c2014. [cited 2022 May 31]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Testosterone_31/Position_provided_by_CMDh/WC500177617.pdf. [Google Scholar]
- 85.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Am J Med. 2017;130:293–305. doi: 10.1016/j.amjmed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 86.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 87.Muraleedharan V, Jones TH. Testosterone and mortality. Clin Endocrinol (Oxf) 2014;81:477–487. doi: 10.1111/cen.12503. [DOI] [PubMed] [Google Scholar]
- 88.Jones TH, Kelly DM. Randomized controlled trials - mechanistic studies of testosterone and the cardiovascular system. Asian J Androl. 2018;20:120–130. doi: 10.4103/aja.aja_6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–433. doi: 10.1177/1074248417691136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hackett G, Heald AH, Sinclair A, Jones PW, Strange RC, Ramachandran S. Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016;70:244–253. doi: 10.1111/ijcp.12779. [DOI] [PubMed] [Google Scholar]
- 91.Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 92.Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48:1138–1144. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson J, May H, Lappe D. Abstract 13220: cardiovascular impact of testosterone therapy in men with low testosterone levels. Circulation. 2014;130:A13220 [Google Scholar]
- 94.Anderson JL, May HT, Lappé DL, Bair T, Le V, Carlquist JF, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016;117:794–799. doi: 10.1016/j.amjcard.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 95.Wallis CJ, Lo K, Lee Y, Krakowsky Y, Garbens A, Satkunasivam R, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498–506. doi: 10.1016/S2213-8587(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 96.Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, et al. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–499. doi: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 97.Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with "diabesity": results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515. doi: 10.1155/2014/683515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oni OA, Dehkordi SHH, Jazayeri MA, Sharma R, Sharma M, Masoomi R, et al. Relation of testosterone normalization to mortality and myocardial infarction in men with previous myocardial infarction. Am J Cardiol. 2019;124:1171–1178. doi: 10.1016/j.amjcard.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 99.Hudson J, Cruickshank M, Quinton R, Aucott L, Aceves-Martins M, Gillies K, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. 2022;3:e381–e393. doi: 10.1016/S2666-7568(22)00096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27:557–579. doi: 10.1016/j.beem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Huggins C, Hodges CV. Studies on prostatic cancer. I. The Effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 102.Kaplan AL, Hu JC, Morgentaler A, Mulhall JP, Schulman CC, Montorsi F. Testosterone therapy in men with prostate cancer. Eur Urol. 2016;69:894–903. doi: 10.1016/j.eururo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Traish AM, Williams DF, Hoffman ND, Wotiz HH. Validation of the exchange assay for the measurement of androgen receptors in human and dog prostates. Prog Clin Biol Res. 1988;262:145–160. [PubMed] [Google Scholar]
- 104.Traish AM, Muller RE, Wotiz HH. A new procedure for the quantitation of nuclear and cytoplasmic androgen receptors. J Biol Chem. 1981;256:12028–12033. [PubMed] [Google Scholar]
- 105.Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Changes in prostate specific antigen in hypogonadal men after 12 months of testosterone replacement therapy: support for the prostate saturation theory. J Urol. 2011;186:1005–1011. doi: 10.1016/j.juro.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 106.Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by dutasteride of prostate cancer events trial. Eur Urol. 2012;62:757–764. doi: 10.1016/j.eururo.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 107.San Francisco IF, Rojas PA, DeWolf WC, Morgentaler A. Low free testosterone levels predict disease reclassification in men with prostate cancer undergoing active surveillance. BJU Int. 2014;114:229–235. doi: 10.1111/bju.12682. [DOI] [PubMed] [Google Scholar]
- 108.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17:132–143. doi: 10.1038/pcan.2013.60. [DOI] [PubMed] [Google Scholar]
- 110.Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl. 2003;24:299–311. doi: 10.1002/j.1939-4640.2003.tb02676.x. [DOI] [PubMed] [Google Scholar]
- 111.Hatzimouratidis K, Giuliano F, Moncada I, Muneer A, Salonia A, Verze P. EAU guidelines on male sexual dysfunction [Internet] Arnhem: EAU; c2016. [cited 2022 May 5]. Available from: http://uroweb.org/individual-guidelines/non-oncology-guidelines/ [Google Scholar]
- 112.National Institute for Health and Care Excellence (NICE) Type 2 diabetes in adults: management [Internet] London: NICE; c2015. [cited 2022 May 12]. Available from: https://www.nice.org.uk/guidance/ng28. [PubMed] [Google Scholar]
- 113.Corona G, Mannucci E, Petrone L, Balercia G, Fisher AD, Chiarini V, et al. ANDROTEST: a structured interview for the screening of hypogonadism in patients with sexual dysfunction. J Sex Med. 2006;3:706–715. doi: 10.1111/j.1743-6109.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- 114.Jayasena CN, Anderson RA, Llahana S, Barth JH, MacKenzie F, Wilkes S, et al. Society for Endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clin Endocrinol (Oxf) 2022;96:200–219. doi: 10.1111/cen.14633. [DOI] [PubMed] [Google Scholar]
- 115.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caronia LM, Dwyer AA, Hayden D, Amati F, Pitteloud N, Hayes FJ. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin Endocrinol (Oxf) 2013;78:291–296. doi: 10.1111/j.1365-2265.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- 117.Lehtihet M, Arver S, Bartuseviciene I, Pousette A. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia. 2012;44:405–410. doi: 10.1111/j.1439-0272.2012.01296.x. [DOI] [PubMed] [Google Scholar]
- 118.Livingston M, Hackett G, Ramachandran S, Heald A. Is a fasting testosterone level really necessary for the determination of androgen status in men? Clin Chim Acta. 2021;521:64–69. doi: 10.1016/j.cca.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 119.Huhtaniemi IT, Tajar A, Lee DM, O'Neill TW, Finn JD, Bartfai G, et al. EMAS Group. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166:983–991. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- 120.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 121.ISSM. ISSM patient information sheet on premature ejaculation [Internet] Wormerveer: ISSM; c2015. [cited 2022 Mar 12]. Available from: http://www.issm.info/education/clinical-guidelines/ [Google Scholar]
- 122.Antonio L, Wu FC, O'Neill TW, Pye SR, Ahern TB, Laurent MR, et al. European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab. 2016;101:2647–2657. doi: 10.1210/jc.2015-4106. [DOI] [PubMed] [Google Scholar]
- 123.Tajar A, McBeth J, Lee DM, Macfarlane GJ, Huhtaniemi IT, Finn JD, et al. European Male Aging Study Group. Elevated levels of gonadotrophins but not sex steroids are associated with musculoskeletal pain in middle-aged and older European men. Pain. 2011;152:1495–1501. doi: 10.1016/j.pain.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu FCW. Ageing male (part 2): management of functional hypogonadism in older men, a patient-centric holistic approach. Best Pract Res Clin Endocrinol Metab. 2022;36:101626. doi: 10.1016/j.beem.2022.101626. [DOI] [PubMed] [Google Scholar]
- 125.Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6:77–85. doi: 10.1016/j.sxmr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nackeeran S, Kohn T, Gonzalez D, White J, Ory J, Ramasamy R. The effect of route of testosterone on changes in hematocrit: a systematic review and Bayesian network meta-analysis of randomized trials. J Urol. 2022;207:44–51. doi: 10.1097/JU.0000000000002188. [DOI] [PubMed] [Google Scholar]
- 127.Choi EJ, Xu P, Barham D, El-Khatib FM, Yafi FA, Kavoussi PK. Comparison of outcomes for hypogonadal men treated with intramuscular testosterone cypionate versus subcutaneous testosterone enanthate. J Urol. 2022;207:677–683. doi: 10.1097/JU.0000000000002301. [DOI] [PubMed] [Google Scholar]
- 128.Aspen. Sustanon 250 summary of product characteristics [Internet] Leatherhead: EMC; c2016. [cited 2022 May 18]. Available from: http://www.medicines.org.uk/emc/medicine/28840 . [Google Scholar]
- 129.Bansal UK, Jones C, Fuller TW, Wessel C, Jackman SV. The efficacy of tadalafil daily vs on demand in the treatment of erectile dysfunction: a systematic review and meta-analysis. Urology. 2018 doi: 10.1016/j.urology.2017.08.031. [Epub] [DOI] [PubMed] [Google Scholar]
- 130.Zhou Z, Chen H, Wu J, Wang J, Zhang X, Ma J, et al. Meta-analysis of the long-term efficacy and tolerance of tadalafil daily compared with tadalafil on-demand in treating men with erectile dysfunction. Sex Med. 2019;7:282–291. doi: 10.1016/j.esxm.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prasetyo DT, Raharja PAR, Mantiri BJ, Ringoringo DRL, Rahman IA, Felizio J, et al. Tadalafil once a day for men with erectile dysfunction: is it superior than on-demand administration? Acta Med Indones. 2019;51:275–281. [PubMed] [Google Scholar]
- 132.Peng Z, Yang L, Dong Q, Wei Q, Liu L, Yang B. Efficacy and safety of tadalafil once-a-day versus tadalafil on-demand in patients with erectile dysfunction: a systematic review and meta-analyses. Urol Int. 2017;99:343–352. doi: 10.1159/000477496. [DOI] [PubMed] [Google Scholar]
- 133.Park HJ, Hyun JS, Park NC. 602 A comparison of baseline erectile function after on-demand 20 mg tadalafil vs daily 5 mg tadalafil in men with erectile dysfunction and diabetes: a prospective, observational 2-year study. Eur Urol Suppl. 2014;13:e602 [Google Scholar]
- 134.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 135.Ozcan L, Polat EC, Kocaaslan R, Onen E, Otunctemur A, Ozbek E. Effects of taking tadalafil 5 mg once daily on erectile function and total testosterone levels in patients with metabolic syndrome. Andrologia. 2017;49:e12751. doi: 10.1111/and.12751. [DOI] [PubMed] [Google Scholar]
- 136.Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, et al. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med. 2012;157:681–691. doi: 10.7326/0003-4819-157-10-201211200-00004. [DOI] [PubMed] [Google Scholar]
- 137.Santi D, Giannetta E, Isidori AM, Vitale C, Aversa A, Simoni M. Therapy of endocrine disease. Effects of chronic use of phosphodiesterase inhibitors on endothelial markers in type 2 diabetes mellitus: a meta-analysis. Eur J Endocrinol. 2015;172:R103–R114. doi: 10.1530/EJE-14-0700. [DOI] [PubMed] [Google Scholar]
- 138.Guo B, Chen X, Wang M, Hou H, Zhang Z, Liu M. Comparative effectiveness of tadalafil versus tamsulosin in treating lower urinary tract symptoms suggestive of benign prostate hyperplasia: a meta-analysis of randomized controlled trials. Med Sci Monit. 2020;26:e923179. doi: 10.12659/MSM.923179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anderson SG, Hutchings DC, Woodward M, Rahimi K, Rutter MK, Kirby M, et al. Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality. Heart. 2016;102:1750–1756. doi: 10.1136/heartjnl-2015-309223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andersson DP, Trolle Lagerros Y, Grotta A, Bellocco R, Lehtihet M, Holzmann MJ. Association between treatment for erectile dysfunction and death or cardiovascular outcomes after myocardial infarction. Heart. 2017;103:1264–1270. doi: 10.1136/heartjnl-2016-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goberdhan S, Blachman-Braun R, Nackeeran S, Masterson TA, 3rd, Ramasamy R. Is tadalafil associated with decreased risk of major adverse cardiac events or venous thromboembolism in men with lower urinary tract symptoms? World J Urol. 2022;40:1799–1803. doi: 10.1007/s00345-022-04005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu PY, Wishart SM, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab. 2002;87:3125–3135. doi: 10.1210/jcem.87.7.8630. [DOI] [PubMed] [Google Scholar]
- 143.Wheeler KM, Sharma D, Kavoussi PK, Smith RP, Costabile R. Clomiphene citrate for the treatment of hypogonadism. Sex Med Rev. 2019;7:272–276. doi: 10.1016/j.sxmr.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 144.Helo S, Ellen J, Mechlin C, Feustel P, Grossman M, Ditkoff E, et al. A randomized prospective double-blind comparison trial of clomiphene citrate and anastrozole in raising testosterone in hypogonadal infertile men. J Sex Med. 2015;12:1761–1769. doi: 10.1111/jsm.12944. [DOI] [PubMed] [Google Scholar]
- 145.Surampudi P, Swerdloff RS, Wang C. An update on male hypogonadism therapy. Expert Opin Pharmacother. 2014;15:1247–1264. doi: 10.1517/14656566.2014.913022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saad F, Yassin A, Haider A, Doros G, Gooren L. Elderly men over 65 years of age with late-onset hypogonadism benefit as much from testosterone treatment as do younger men. Korean J Urol. 2015;56:310–317. doi: 10.4111/kju.2015.56.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lusty A, Siemens DR, Tohidi M, Whitehead M, Tranmer J, Nickel JC. Cardiac failure associated with medical therapy of benign prostatic hyperplasia: a population based study. J Urol. 2021;205:1430–1437. doi: 10.1097/JU.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 148.Heald A, Cook M, Antonio L, Vanderschueren D, Javed A, Fachim H, et al. The number of androgen receptor CAG repeats and mortality in men. Aging Male. 2022;25:167–172. doi: 10.1080/13685538.2022.2061452. [DOI] [PubMed] [Google Scholar]
- 149.Adam JA, Pastuszak AW, Christensen MB, Spencer R, Sandberg A, Hotaling JM, et al. Occurrence of pulmonary oil microembolism (POME) with intramuscular testosterone undecanoate injection: literature review. Int J Impot Res. 2022 doi: 10.1038/s41443-022-00585-1. [Epub] [DOI] [PubMed] [Google Scholar]
- 150.Strange RC, König CS, Ahmed A, Hackett G, Haider A, Haider KS, et al. Testosterone therapy: increase in hematocrit is associated with decreased mortality. Androg Clin Res Ther. 2021;2:150–159. [Google Scholar]
- 151.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pozzilli P, Lenzi A. Commentary: testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108:154252. doi: 10.1016/j.metabol.2020.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Karkin K, Gürlen G. Does COVID-19 cause testicular damage? A cross-sectional study comparing hormonal parameters. Eur Rev Med Pharmacol Sci. 2022;26:3745–3750. doi: 10.26355/eurrev_202205_28871. [DOI] [PubMed] [Google Scholar]
- 154.Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology. 2021;9:1043–1052. doi: 10.1111/andr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mostafa T. Could oral phosphodiesterase 5 inhibitors have a potential adjuvant role in combating COVID-19 infection? Sex Med Rev. 2021;9:15–22. doi: 10.1016/j.sxmr.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]