Abstract
The rate of substance use is rising, especially among reproductive-age individuals. Emerging evidence suggests that paternal pre-conception and maternal prenatal substance use may alter offspring epigenetic regulation (changes to gene expression without modifying DNA) and outcomes later in life, including neurodevelopment and mental health. However, relatively little is known due to the complexities and limitations of existing studies, making causal interpretations challenging. This review examines the contributions and influence of parental substance use on the gametes and potential transmissibility to the offspring’s epigenome as possible areas to target public health warnings and healthcare provider counseling of individuals or couples in the pre-conception and prenatal periods to ultimately mitigate short- and long-term offspring morbidity and mortality.
Keywords: DNA methylation, epigenetic inheritance, histone modifications, intergenerational epigenetic inheritance, maternal drug use, paternal drug use, preconception drug use, prenatal drug use, transgenerational epigenetic inheritance
Plain language summary
More people, especially those of reproductive age, are using substances, and there is growing evidence to suggest that parental substance use before and during pregnancy may adversely affect offspring and result in issues later in life, including mental health challenges. Such relationships have been demonstrated with nicotine, alcohol, cannabis, opioids and illegal drugs (e.g., heroin, cocaine, methamphetamines). Some of these adverse impacts on offspring can potentially be passed down in families even after parents have quit using the substance. Because more individuals are using drugs, especially during the COVID-19 pandemic, it is important that families learn more about the potential impact of substance use on their future offspring before they try to get pregnant.
Tweetable abstract
Substance use before and during pregnancy can influence the offspring epigenome and later health outcomes. This is an area to target in public healthcare warnings and healthcare provider counseling to ultimately mitigate offspring morbidity and mortality.
Substance use is a public health issue that is continuing to rise worldwide. In 2018, approximately 269 million people worldwide used nonprescription drugs, which is one-third more than in 2009, and over 35 million individuals were affected by drug use disorders [1]. The COVID-19 crisis and subsequent economic downturn further compounded the existing drug problem. Of great concern is that the trends in drug use demonstrate that adolescents, young adults and those of reproductive age account for the largest group using drugs at this time [1].
‘Developmental origins of health and disease’ is a concept that originated in the 1990s [2] and postulates that environmental exposures can influence critical periods of offspring development and growth that may impact longer term health [3]. The literature supports that pregnancy is a window of susceptibility and that offspring exposure in utero to recreational or prescription drugs can perturb the fetal epigenome, resulting in adult disease phenotypes later in life [4]. Epigenetic regulation and modulation of gene expression are necessary for normal embryonic and fetal development. Because the epigenome is characterized by the reversible addition of specific chemical moieties to the DNA itself, or to the proteins that the DNA is wrapped around, it affords an inherent plasticity that can respond to the prevailing environment. This may provide for an adaptive response to natural changes in environmental signaling received early in life (e.g., nutritional deprivation or levels of stress) that capitalizes on the flexibility of the epigenome to ensure survival and preparation for postnatal life. However, any adaptive mechanism can become maladaptive when the environmental signaling comes from phenomena we have not evolutionarily become accustomed to encountering, including Western-style diet or drug use.
Maternal substance use is a public health crisis; the most frequently used substances in the prenatal period are alcohol, tobacco and cannabis [5]. There is substantial literature supporting that maternal substance use in pregnancy can adversely impact newborn outcomes, including an increased risk for premature birth, small for gestational age, congenital anomalies, attachment problems and withdrawal symptoms. Prenatal substance exposure can also impact offspring behavior, such as aggression and delinquency [6,7]. Maternal substance use also increases the risk of later life issues for the offspring, including a greater risk for mental health disorders and addiction vulnerability (e.g., earlier initiation and accelerated transition) [8–12]. Studies are now showing a relationship between maternal substance use and later behavioral and developmental sequelae in offspring, and that epigenetic responses to maternal psychoactive substance use may result in long-term molecular changes implicated in addiction and psychiatric disorders [13–15]. In addition, newer evidence suggests that synergistic interactions may result from exposure to multiple substances; maternal polysubstance use during pregnancy can increase the risk of attention-deficit hyperactivity disorder (ADHD) [16].
Substance use by the father prior to pregnancy may also impact the health of his progeny. Emerging preclinical studies suggest that substance use in males can alter the sperm epigenome and adversely affect offspring brain and neurobehavioral development via epigenetic mechanisms [17–20]. Additionally, there are several human studies investigating paternal health behaviors during pregnancy that suggest paternal tobacco and alcohol use are linked to worsened offspring mental health, in particular hyperactivity and ADHD [21].
Despite the growing evidence suggesting that prenatal and even pre-conception parental substance use may impact offspring epigenetic regulation and their outcomes later in life, including neurodevelopment and mental health, this is an area that is inadequately researched [4,15,17,21]. Existing research gaps are in part due to limitations of the epidemiological studies, making causal interpretations challenging. These limitations include variability between studies in sample size, offspring ages and outcomes studied, exposures examined, and adjustment for health behaviors and exposures in the other parent (e.g., studies of paternal smoking adjusting for maternal smoking) [21]. If appropriate adjustments for maternal versus paternal exposures are not performed, this results in a lack of certainty that associations observed with paternal exposures are not secondary to maternal contributions and vice versa.
The objective of this review is to examine the contributions and influence of maternal and paternal substance use on the offspring epigenome as potential areas to target public health warnings and to inform healthcare provider counseling of individuals or couples, during the pre-conception period or in pregnancy, to ultimately mitigate short- and long-term offspring mortality and morbidity.
Epigenetic regulatory mechanisms
DNA methylation and post-translational histone protein modifications through chromatin remodeling are the most common mechanisms of epigenetic regulation, thought to influence gene expression primarily through modifying DNA accessibility and transcription factor binding (Figure 1) [22,23]. There are also RNA-mediated processes sometimes referred to as epigenetic, including processes mediated by noncoding RNAs, and these have been reviewed elsewhere in the literature [24–26]. Epigenetic regulatory mechanisms do not impact the DNA nucleotide sequence but instead can alter its readiness for transcription.
Figure 1. . Epigenetic regulation through histone deacetylase.
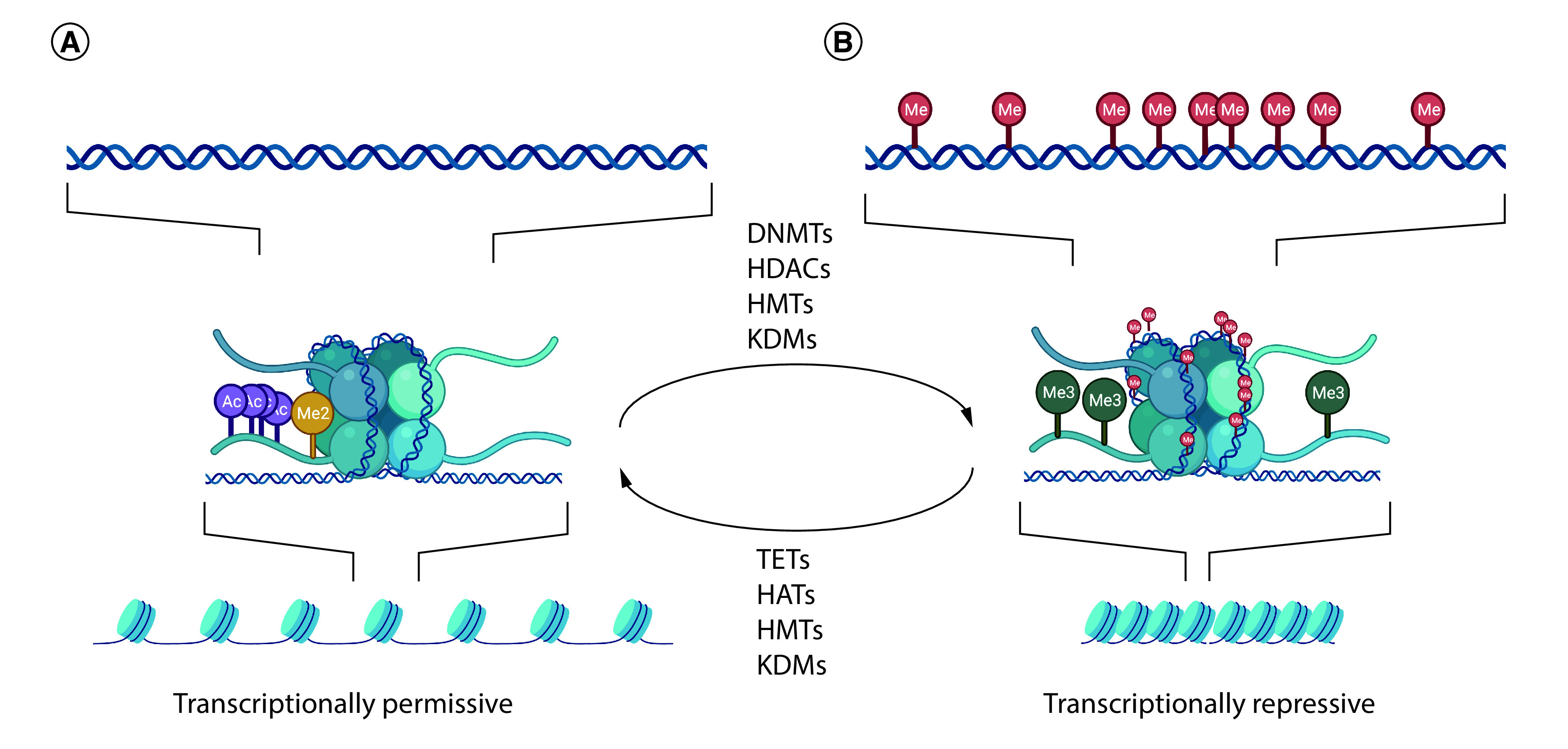
Chromatin remodeling occurs through histone modification. (A) Histone acetylation relaxes chromatin and allows transcription factors to bind. (B) Histone deacetylation causes chromatin to condense and represses transcription.
DNA methylation
CpG sites are dinucleotide sequences within the DNA where, in a linear sequence of bases, a cytosine nucleotide is followed by a guanine nucleotide along its 5′ to 3′ direction. CpG sites are the canonical target of DNA methylation. Genomic regions that have high CpG site frequency are called CpG islands. The promoter regions of nearly half the genes throughout the genome are associated with CpG islands. DNA methyltransferases can add a methyl group to the fifth carbon position of the cytosine ring to produce 5-methylcytosine, a covalent modification that can alter gene expression [27]. DNA methylation changes the characteristics of the DNA [28] and can either prevent or facilitate the recognition of and binding to DNA by proteins [29]. DNA methylation at promoter regions is often associated with gene repression [30,31], while intragenic methylation can be associated with transcriptional repression or enhanced transcription of the host gene [32]. In mammalian species, approximately 70–80% of CpG cytosines are normally methylated [33]. Methylated CpGs occur most commonly in regions outside of CpG islands, whereas CpGs located within CpG islands are usually unmethylated or have low levels of methylation [31,34,35].
Histone modification
Histone modifications occupy a key role in epigenetic regulation by influencing chromatin structure and gene transcription. These covalent post-translational modifications most commonly involve methylation, phosphorylation and acetylation of amino acids in the histone protein N-terminal tails. These modifications can impact chromatin structure and can alter gene expression. Other less common histone modifications include ubiquitination, sumolyation, ADP ribosylation and deamination and proline isomerization [36]. Chromatin is composed of DNA and proteins, mostly histones, which are tightly condensed to form the chromosomes [37]. Histones help package DNA to be contained in the nucleus; core histones are highly conserved basic proteins with globular domains wrapped with DNA and ‘tails’ protruding from the nucleosome [36]. Histone modifications are largely catalyzed by enzymes that add or remove specific chemical groups on the N-terminal histone tails at lysine, arginine, serine, threonine and tyrosine residues [38]. Histone acetylation by histone acetyltransferases is one of the most common modifications and its presence is linked to transcriptional permissiveness. Histone deacetylases, of which there are multiple isoforms, are critical mediators of normal placental [39] and fetal brain development [40]. Some histone modifications also interact with DNA methyltransferase to provide crosstalk between these epigenetic mechanisms [41,42].
Epigenetic inheritance
The cells within an individual share the exact same DNA blueprint, an identical genetic template that drives the development of multiple cell and tissue subtypes. How then does this same blueprint result in the generation of distinct cell types like a liver or muscle cell within a single individual? Much of this variability is known to arise from epigenetic changes to the DNA that drive cell lineage and sublineage differentiation. Although the stability of such epigenetic marks can vary and is susceptible to differentiation events and mutation, many of these marks are long-lasting, and if they affect the germline and are not reset during gametogenesis and embryo development, can be sustained in all subsequent cell lineages and potentially passed to the next generation. There are, in fact, several tissue-specific points in development when cells are particularly sensitive to epigenetic changes. For instance, the breast and brain undergo significant remodeling during puberty. It has been demonstrated that breast cells have a heightened susceptibility to epigenetic modifications in vitro when exposed to hormones and nutrient changes [43]. If these changes occur in stem cells or progenitor cells of the gland, duct or myoepithelial cells, they can be passed on to daughter cells and may be maintained in breast tissues in a manner that could affect future susceptibility to tumorigenesis. Likewise, developmental changes in the pubertal brain of rats can be adversely affected by exposure to stress conditions [44], a change that has been shown to be altered by paternal exposure to a commonly used fungicide several generations prior [45]. These stress-response modifications have been associated with epigenetic changes, especially those involving DNA methylation, in the toxin-exposed male. The periods of embryonic growth and of germ cell development are particularly sensitive to epigenetic changes, as both periods involve near-complete erasure and reacquisition of DNA methylation marks throughout the genome.
Intergenerational & transgenerational epigenetic inheritance
The transmission of epigenetic alterations from one generation to the next is referred to as ‘intergenerational epigenetic inheritance’ (Figure 2). Intergenerational transmission results from exposure of the mother or father and affects their gametes, with changes detectable in their offspring. Because the oocyte resides in the mother, and the sperm and its progenitor cells reside in the father, epigenetic changes can result from a direct impact at the time of the exposure on the gametes. ‘Transgenerational epigenetic inheritance’ occurs when the parent incurs an epigenetic alteration that undergoes intergenerational inheritance with continued heritability in subsequent generations, in the absence of any additional exposure. The key defining factor for transgenerational inheritance is that the epigenetic alteration is apparent in a generation that has not been directly exposed. Thus, for an exposed male or nonpregnant female (F0 generation), the first unexposed generation would be their grandchildren (F2 generation). For a pregnant female (F0), the embryo/fetus (F1) is exposed, as are the gametes (F2) within the gestating offspring. Transgenerational epigenetic inheritance is demonstrated if the great-grandchildren (F3) show the epigenetic alteration.
Figure 2. . Principles of intergenerational and transgenerational epigenetic inheritance.
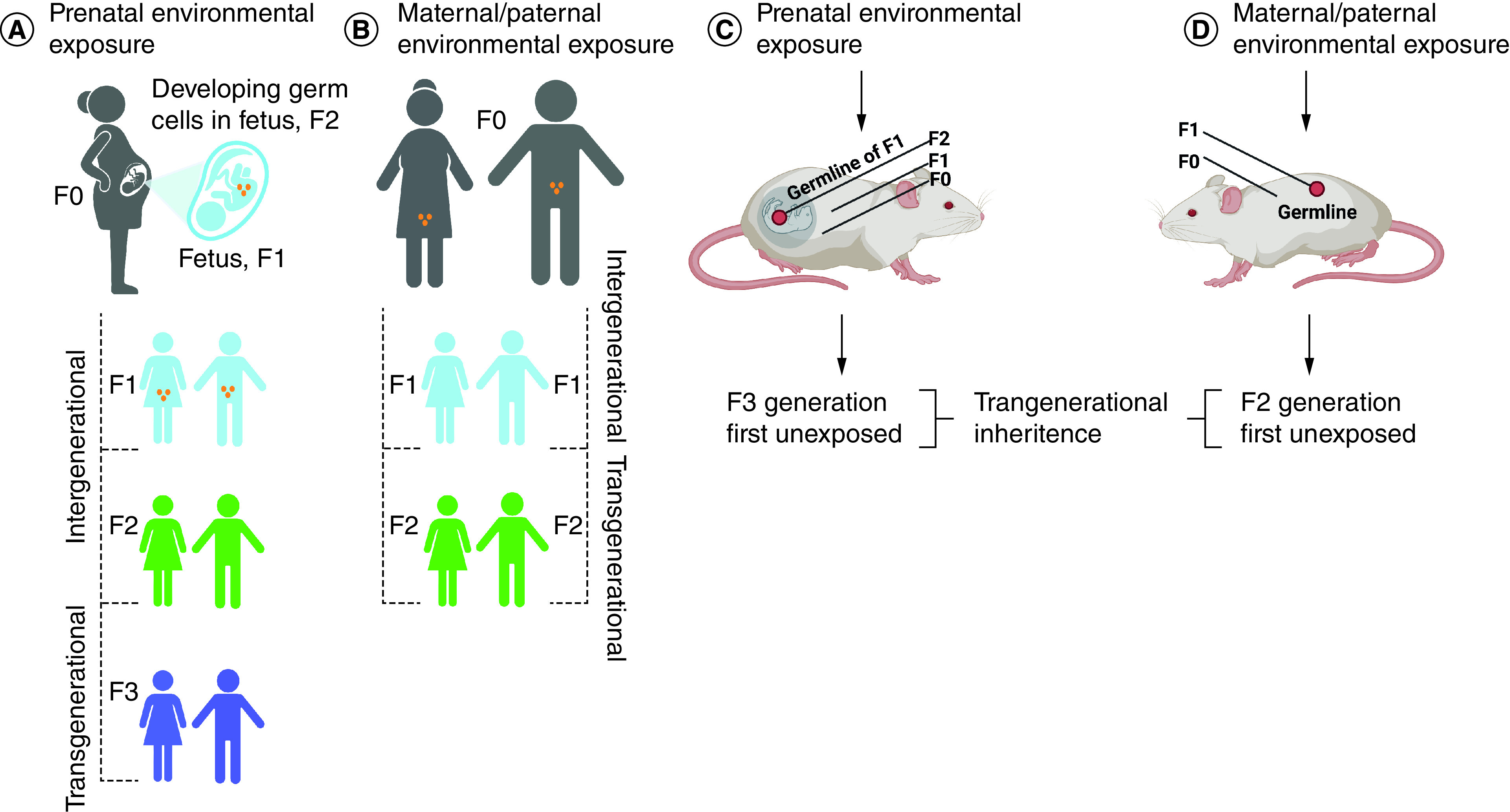
(A) If a pregnant individual (F0) is exposed to an environmental perturbation, the offspring (F1; blue), and their germ cells (orange) that form the F2 generation (green) are also directly exposed, potentially resulting in intergenerational effects. The third generation (F3; purple) is the first generation that can demonstrate transgenerational epigenetic inheritance. (B) If an individual (F0) and their germ cells (orange) that will form the F1 generation (blue) are exposed directly to an environmental perturbation, the F2 offspring (green) are the first generation that can demonstrate transgenerational epigenetic inheritance. The same principles hold for animal models. (C) For a pregnant dam (F0), an exposure would impact her, her F1 offspring and the germline of her offspring (F2), and detection in the F1 and F2 generations would represent intergenerational epigenetic inheritance. Transgenerational epigenetic inheritance requires observing a detectable epigenetic change in the first unexposed generation (F3). (D) Exposure of a nonpregnant dam or sire (F0) affects that individual and their gametes (F1). Detection of an epigenetic change in the F1 generation would be intergenerational epigenetic inheritance, while detection in the F2 generation would be regarded as transgenerational epigenetic inheritance.
As a concept, transgenerational epigenetic inheritance is relatively new. The first reports of environmentally induced transgenerational epigenetic inheritance appeared in a series of seminal works performed by Anway and Skinner et al. in 2006 [46,47]. In these experiments, index rats were exposed to an endocrine disruptor and fungicide (vinclozolin) during gonadal sex determination. No further exposures to the F1–F4 generations occurred. Several disease states – including testis, kidney and prostate disease, immune abnormalities and breast tumor development – were noted to occur at high frequency in F1–F4 offspring, and these changes were linked to epigenetic alterations in the male germline [48]. In retrospect, the phenomenon of environmentally induced transgenerational epigenetic inheritance was recognized many years earlier; perhaps first in 1918, when it was reported that decreased fertility and lifespan of guinea pigs exposed to ethanol vapor persisted for four generations despite no further ethanol vapor exposure [49]. In the intervening years, the term ‘epigenetic’ was first used by Waddington in 1942 when reporting on the inheritance of wing abnormalities in Drosophila melanogaster exposed to heat shock. Given that these morphological changes could be detected for up to seven generations after ancestral exposure despite removal of the stimulus, this too is an example of environmentally induced transgenerational epigenetic inheritance [50].
Epigenetic inheritance remains a much-debated topic. The discovery of postfertilization reprogramming of DNA methylation has led to the belief that any changes in the DNA methylation profile in the oocyte and sperm would effectively be erased, providing a fresh genomic palette for restoration of a normal methylation profile across the genome. If true, this would preclude the ability to transmit any methylation changes from one generation to the next. Further, the reprogramming that occurs during the process of gametogenesis also seemingly provides a mechanism to erase any alterations that were acquired in the prior generation. These reprogramming mechanisms likely provide a measure of protection against the inheritance of adverse epigenetic alterations. Supporting this, many studies have failed to provide evidence of epigenetic inheritance, and there are likely other studies unreported because the results are negative. Yet there are abundant examples of altered phenotypes being transmitted across generations – including with drug use, and particularly with cocaine and opioids [51] – in the absence of genetic mutations. Mechanistic evidence of intergenerational and transgenerational epigenetic transmission associated with drug use is emerging. For example, exposure of F0 male rats to tetrahydrocannabinol (THC) or to cannabis extract results in altered methylation in their sperm and to similar alterations for a small number of genes examined in tissues from the F1 generation [52]. For example, a ∼15% decrease in methylation in sperm of the exposed fathers at CpG sites associated with gene Pxylp1 was also present in the sperm of the F1 generation, but not in unexposed F0 controls or in their F1 offspring [52]. Other research has shown that adolescent exposure of F0 male and female rats to THC results in methylation changes in the nucleus accumbens of the F1 generation [53] and increased propensity of the offspring to self-administer heroin [54]. In this study, both parents were exposed to THC and the parents’ gamete methylation profiles were not obtained, so it is not clear what changes, if any, were heritable. However, a significant number of the methylation alterations in the brains of the rat offspring born to THC-exposed parents were also observed in an independent study of the sperm of male rats exposed to THC [55], suggesting that many of the methylation alterations in the offspring brains could have been intergenerationally transmitted from the father.
Over the last 15+ years, multiple environmental exposures have been linked to intergenerational or transgenerational epigenetic inheritance in animals and/or humans. An inexhaustive list includes: tobacco smoke, lead, arsenic, organic pollutants, chromium, mercury, polycyclic aromatic hydrocarbons, aflatoxin B, air pollution, bisphenol A, cadmium, persistent organic pollutants, several nutritional factors and, pertinent to this review, THC [55–57]. The role of nutrition during pregnancy in epigenetic programming of offspring health gained considerable attention after a series of publications by Barker et al. demonstrating that infant birth weight and, more specifically, prenatal conditions that resulted in low birth weight were strongly associated with the development of major medical morbidities such as cardiovascular disease later in life [3,58–61]. These findings were instrumental in the genesis of the ‘developmental origins of health and disease’ hypothesis, also referred to as the ‘Barker Hypothesis’. In 2008 Heijmans et al. linked a specific mechanism to the Barker Hypothesis, showing that low birth weight and subsequent propensity for long-term medical morbidity were seen among men and women who were exposed prenatally to famine when their mothers endured the Dutch Hunger Winter of 1944–1945 [62]. More specifically, these investigators demonstrated a specific methylation alteration in the maternally imprinted IGF2 gene in the children of these pregnant women who experienced gestational famine when compared with same-sex siblings born outside of this period. This effect was particularly notable when exposure occurred in the periconceptional period and during early pregnancy. The decreased methylation at IGF2 was associated with lower birth weight in exposed offspring. While this report addresses intergenerational but not transgenerational epigenetic inheritance, together these findings have led to increasing attention on less tangible, but arguably more pervasive, environmental exposures to stress and other factors that accompany poverty and linkage to heritable epigenetic changes [63,64].
Challenges with demonstrating epigenetic inheritance
The demonstration of transgenerational epigenetic inheritance and, more pointedly, environmentally induced transgenerational epigenetic inheritance, can be challenging. As outlined in Figure 2, because a given exposure during pregnancy may affect the mother (F0), her germ cells, her fetus (F1) and the developing germ cells (F2) within that fetus, all are considered exposed. Therefore any demonstration that epigenetic changes are inherited in a transgenerational manner requires detection in the grandchildren (F2) of individuals exposed outside of pregnancy, and in the great-grandchildren (F3) when pregnant individuals are exposed. For species with a long generation time, such as humans, this often requires such a prolonged period of study that experimentation becomes impractical. It is much easier to study environmentally induced transgenerational epigenetic inheritance in animals with relatively short generation times, such as the fruit fly, mouse or rat. That said, there are several confounders that can affect transgenerational epigenetic inheritance that may complicate its study even in these models. For instance, epigenetic changes resulting from an environmental exposure may follow a nonmonotonic dose response. An example of this is the environmental endocrine-disrupting substance bisphenol A, which has been shown to exert both linear and nonlinear (nonmonotonic) dose–response effects on methylation in the livers of prenatally exposed fetuses [65]. As another example, the epigenetic effects of an environmental exposure to arsenic may be sex-specific for both prenatally exposed infants [66] and adults [67]. Further, environmentally induced epigenetic changes may be tissue-specific or even cell type-specific [68–70]. This can confound or obscure determination of such changes in a tissue biopsy that may contain several cell subtypes, some of which may be affected while others may not be. A final common potential confounder of all studies on transgenerational epigenetic inheritance is the fact that some epigenetic changes that are induced by environmental exposures may not be stable [71] and, even if transgenerationally inherited, could be affected by additional exposures.
In addition to the problem of long generation times, the study of transgenerational epigenetic inheritance in humans is plagued by the frequent inability to isolate a single exposure for study. For instance, real-world exposures can involve a complex mixture of chemicals, only one of which may cause epigenetic change – or, if there is more than one, the effects of each individual exposure may partially ameliorate the effects of the other. Even more problematic is the study of transgenerational epigenetic effects of such complex exposures as poverty or systemic racism, which likely involve stress responses, poor living conditions with mixed chemical and respiratory exposures, poor nutrition and a variety of related and possibly unknown factors.
Critical windows of development with increased susceptibility
There are distinct periods over the life course when cells differentiate into more specialized cell types or undergo intensive proliferation during periods of rapid growth. These dynamic phases of development are among the most important windows of epigenetic vulnerability [72–74]. During phases of cell proliferation, DNA methylation profiles must be faithfully propagated to daughter cells. Cellular differentiation is, in part, dependent on the establishment of epigenetic profiles, through de novo methylation or methylation erasure, that reflect the next step in the differentiation process. This is especially critical during gametogenesis and preimplantation development, where alterations that occur before germ-layer specification are expected to be mitotically heritable and thus present in the majority of somatic tissues derived thereafter (Figure 3) [75].
Figure 3. . Epigenetic reprogramming.
Periods of rapid growth during pregnancy, infancy and puberty are also windows of epigenetic vulnerability, although changes in the epigenome that occur during these windows are likely to be tissue-specific. Endocrine-disrupting agents are naturally occurring or synthetic chemicals that can interfere with the normal processes of hormonal signaling and have widely been associated with alterations in the epigenome, including DNA methylation [76,77]. Illicit substances can also function as endocrine disruptors, including cannabinoids like THC, which interfere with estrogen signaling [78–80]. Overall, exposures during any of these critical windows have the potential to disturb normal processes of epigenetic programming, with skewed methylation resulting in altered gene expression patterns.
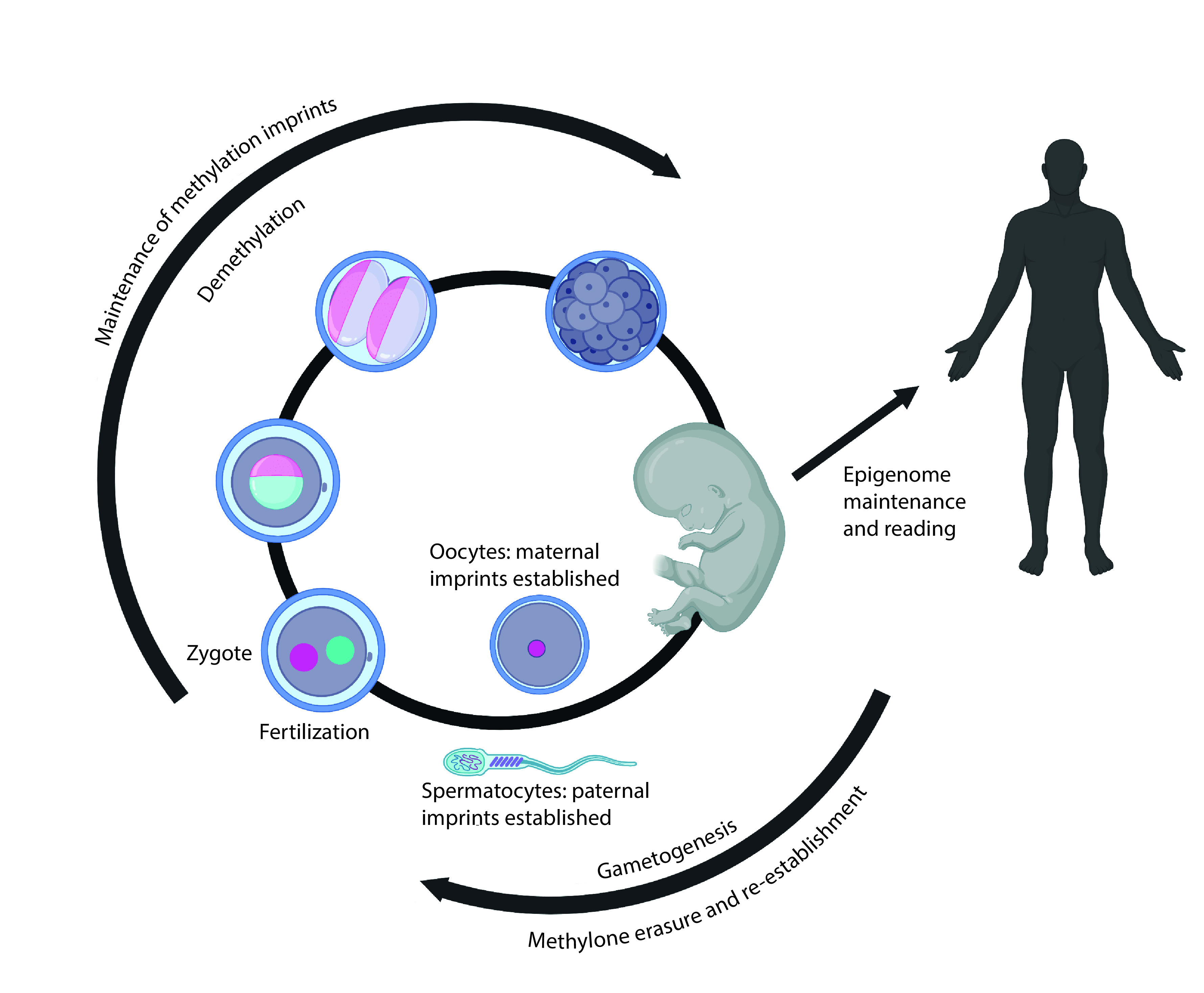
Gametic reprogramming
Formation of the germline begins in the early embryo when diploid primordial germ cells migrate to the genital ridge. The DNA methylation profile in these diploid cells undergoes erasure and is then re-established in a manner that depends on the sex of the embryo. The regions of the genome that exhibit such sexually dimorphic patterns of methylation include the regions that establish genomic imprinting. These regions are characterized by being fully methylated in the gametes of one sex but fully unmethylated in the gametes of the other. These differential methylation patterns, once established, are carried into the zygote and maintained in the diploid state of the embryo, and are transmitted to the somatic cells throughout the life course [81–84].
Postfertilization reprogramming
When the oocyte and sperm are united to form the zygote, the methylation profiles of each again undergo erasure. In mice, methylation on the paternally derived genome is actively and more rapidly erased, while the maternally derived methylation profiles are more passively lost over the course of early rounds of cell division. In humans, the paternally derived genome is also more rapidly demethylated than the maternally derived genome, but this process is largely complete at the two-cell stage of the developing embryo [82]. Around the time of implantation (∼10 days postfertilization in humans), methylation levels in the embryo increase as the diploid genome undergoes remethylation [85]. Importantly, the methylation at imprinted regions that was established in the individual gametes resists postfertilization reprogramming [84]. The retention of imprinted gene methylation means that any altered methylation that was established due to environmental exposures, including drug use, will be stably maintained after fertilization and transmitted to the somatic tissues during development.
There are compelling data to support that, in addition to retention of imprinted gene methylation during postfertilization reprogramming, there are numerous other genomic regions that are also resistant to demethylation during this process [86,87]. There are also some regions of the genome that resist reprogramming in primordial germ cells [88]. Exposures, including to cannabis and other drugs that can alter methylation, can therefore lead to heritable changes in the methylation status at these regions. Methylation changes that occur prior to germ-layer specification are expected to be stably maintained in all subsequent rounds of cell division and would theoretically be detectable in any tissue that has not undergone its own cell type-specific epigenetic remodeling. Methylation changes from exposures that occur in embryonic development during formation of the primordial germ cell lineage could also be carried forward into the next generation if they occur in regions that resist methylation reprogramming. Stability of skewed methylation profiles at regions resistant to both waves of methylation reprogramming could lead to transgenerational inheritance of epigenetic alterations.
Periods of rapid growth
Exposures that occur during puberty not only affect the individual but are also able to disrupt epigenetic patterns in the gametes of that individual. This may be most pertinent for males, given that the production of spermatozoa (spermatogenesis) is an ongoing process that initiates during puberty and continues throughout adult life.
Prior research in rats has shown that adolescent exposure to THC can affect reward-related behavior and striatal gene expression in male offspring who were never postnatally exposed to the drug themselves. These changes arise as a consequence of parental germline exposure to the drug [54], with adult F1 offspring exhibiting increased self-administration of heroin [54]. Parental THC exposure during adolescence also resulted in altered mRNA expression of dopamine, cannabinoid and glutamatergic receptor genes in the striatum of the F1 generation which was correlated with decreased mRNA and protein levels, in addition to NMDA receptor binding within the dorsal striatum because of germline THC exposure [54]. Subsequent work by Szutorisz et al. in 2016 expanded upon these initial findings and found cross-generational consequences of parental adolescent THC exposure in both male and female offspring, with females exhibiting stronger correlation patterns between genes and locomotor alterations not observed in males [89].
The prepubertal period of development may also be a vulnerable period for the epigenome [90]. Several studies have reported that fathers who began smoking during prepuberty (defined as before 15 years of age) had children who were more likely to experience respiratory issues [91,92]. Prepubertal use of cannabis is associated with a higher risk of later cannabis dependence than is a postpubertal onset of cannabis use [93]. Taken together, these findings suggest that parental germline THC exposure during the periadolescent window of epigenetic vulnerability can impact offspring phenotype and may increase the risk of psychiatric disorders in the subsequent generation [54,89].
Aging
Existing studies support the role of substance use in aging processes and also suggest that accelerated biological aging largely contributes to adverse outcomes in patients with substance use disorders [94]. The evaluation of epigenetic clocks focuses on studying DNA methylation changes as an estimator of biological aging. A major goal of geroscience research, the study of the basic mechanisms driving aging, is to identify reliable biomarkers of aging that replace prediction of chronological age with prediction of a surrogate measure of ‘phenotypic age’ that helps differentiate morbidity and mortality risk among individuals of the same age [95]. DNAm PhenoAge is a recently described single epigenetic biomarker of aging that captures risks for a range of outcomes across different tissues and cells [95]. A prior study using DNAm PhenoAge to estimate DNA methylation in the blood of individuals with heavy, chronic alcohol consumption demonstrated epigenetic age acceleration in individuals with alcohol use disorder; disease severity was reported to further accelerate epigenetic aging, and potential recovery from this effect was observed with abstinence from alcohol [96]. Huang et al. recently used whole-genome bisulfite sequencing to study differential methylation in the nucleus accumbens and prefrontal cortex of deceased individuals who used illicit drugs, including heroin, amphetamine-type stimulants and ketamine [97]. This study also reported accelerated epigenetic clocks in both brain regions of illicit drug users, especially those who used ketamine, compared with nonusers [97].
Maternal substance use: epigenetic mechanisms & impact on offspring
Alcohol
Several rodent studies have demonstrated that pre-conception and prenatal alcohol use are associated with alterations in DNA methylation, gene expression and brain structure [12,98–101] that can impact short- and long-term offspring outcomes. It is well known that alcohol use in pregnancy can adversely impact the offspring’s neurodevelopment, including the increased risk of fetal alcohol spectrum disorder and the development of ADHD-like behavior [102]. Evidence suggests that prenatal alcohol-induced hyperactive, inattentive and impulsive offspring behavior may be in part due to global epigenetic changes, including decreased expression of the methyl CpG-binding protein gene MECP2 [102]. Maternal pre-conception or prenatal alcohol consumption has also been shown to affect the inheritance of stress-related diseases by potentially impacting epigenetic regulation in offspring, including altered methylation profiles of stress regulatory genes in different brain regions of offspring [100]. Prior research also supports that prenatal alcohol exposure can disrupt the fetal hypothalamic–pituitary–adrenal axis, resulting in hyper-responsiveness to stressors in adulthood [99]. One of the molecular mechanisms mediating these alterations has been shown to be a disturbance in one-carbon metabolism, a source of methyl donors for epigenetic regulation [99]. Ngai et al. have demonstrated that prenatal alcohol exposure in rats can impact methyl metabolism and influence serotonin transporter and glucocorticoid receptor expression in the brains of offspring [99].
Cannabis
The most common illicit drug used by pregnant individuals is cannabis [103,104], which targets the endocannabinoid system that contributes to organogenesis, neurogenesis and gliogenesis, in part through multiple epigenetic modifications [4,13]. Due to recent legalization in the USA, cannabis use in pregnancy has more than doubled in the past 10 years, with half of female cannabis users continuing to use throughout pregnancy, particularly in the first trimester for nausea – the period of organogenesis when the fetus is most vulnerable to adverse environmental perturbations [105–109]. The concern for detrimental fetal and offspring outcomes [110–112] stems primarily from the fact that THC, the main psychoactive component in cannabis, can cross the placenta and binds to endocannabinoid receptors in both the placenta and fetal central nervous system [109,113–116], leading to impacts throughout the entire perinatal period.
Although limited in number and mechanistic depth, studies suggest adverse effects of prenatal cannabis exposure that include preterm birth and infants who are small for gestational age [117–120]. Maternal cannabis use has also been associated with increased cognitive disabilities and behavioral issues in children, such as increased rates of autism spectrum disorder [121–125]. Preliminary data indicate that prenatal cannabis exposure results in epigenetic changes in both the placenta and the brains of exposed offspring [126–128] and that epigenetic marks are a potential mechanistic link between maternal cannabis use and associated negative offspring outcomes [15,129]. In vitro, THC treatment of a human trophoblast cell line has been shown to increase expression of HDAC3, indicating that THC use may also affect the placental epigenome [128]. This is concerning because the placenta has a critical role in early growth and development; the consequences of altered placental function include insufficient nutrition and oxygenation that can lead to fetal growth restriction and neurodevelopmental abnormalities. Prenatal cannabinoid exposure has resulted in sex-specific increased anxiety behavior and alterations to the brain epigenome in mice offspring [127]. Additionally, cannabinoid exposure alters developmental regulation of dopamine receptor D2 in rat offspring through epigenetic regulation of histone lysine methylation, which may contribute to increased addiction vulnerability later in life [126]. Although there are limited clinical data regarding the effects of cannabis exposure during pregnancy, the rising prevalence of prenatal cannabis use [130] is of significant concern as the potential short-term medicinal benefits for nausea and pain are outweighed by the possibility of adverse longer term impacts to the offspring.
Nicotine
Maternal use of nicotine products or tobacco during pregnancy is associated with adverse birth outcomes, including small for gestational age, premature birth, birth defects and increased risk of sudden infant death syndrome [131–134]. The effects of cigarette smoking during pregnancy on the offspring’s epigenome have been a recent focus of investigation, including large cohort studies and meta-analyses [135–138]. Fuemmeler et al. demonstrated that altered umbilical cord blood DNA methylation occurs even with second-hand smoke exposure during pregnancy [139]. Rat models of gestational exposure to tobacco smoke or nicotine, including levels mimicking secondhand smoke exposure, have revealed persistent cognitive and neurobehavioral deficits in the offspring, including ADHD-like behaviors [140–142]. Studies in humans have shown associations between smoking during pregnancy and deficits in executive function in the child [143,144], but other studies suggest these associations may be due to other confounding factors [145]. Gestational nicotine exposure has been shown, using a rat model, to induce changes in methylation-dependent masculinizing gene expression as well as alterations in normal DNA methylation profiles at the area of the sexually dimorphic nucleus of the preoptic area in the brains of offspring. These data suggest that prenatal nicotine exposure may induce masculinization of the rat preoptic area through epigenetic mechanisms [146].
Opioids
Maternal opioid-related diagnoses during pregnancy, including opioid use disorder (OUD), are a growing problem, increasing from 3.5 to 8.2 per 1000 delivery hospitalizations in the USA from 2010 to 2017 [147]. During that time, there was an associated increase in neonatal abstinence syndrome, more specifically neonatal opioid withdrawal syndrome (NOWS), which increased from 4.0 to 7.3 per 1000 birth hospitalizations [147]. Intrauterine exposure to opioids puts an infant at increased risk of developing NOWS and is also associated with preterm birth, low birth weight and stillbirth [148]. Genetic and epigenetic causes have been linked to the severity of NOWS following pregnancies complicated by OUD [149].
Epigenetic changes due to OUD have been previously demonstrated in DNA from nonpregnant individuals; for example, increased DNA methylation at CpG-rich DNA islands in the opioid receptor gene OPRM1 and at another global methylation site, LINE-1 [150]. Increased DNA methylation of OPRM1 has also been observed in pregnancies complicated by maternal OUD, and increasing methylation levels correlated with increasing NOWS severity [151,152]. Although a prior pilot study did not replicate these findings, genome-wide methylation scans further identified evidence of methylation dysregulation in placental tissue from neonates who developed NOWS following a pregnancy complicated by OUD [95,153]. More specifically, genome-wide methylation scans in a replication cohort using placental tissue identified several differentially methylated probes that annotated to OPRM1, PLD1, MGAM and KCNMA1 [154]. PLD1 regulates multiple cell functions such as cell growth, survival, differentiation, membrane trafficking and receptor endocytosis, and cytoskeletal organization [155]. More recently, PLD1 has been shown to have significant roles in regulating neuronal differentiation of neuronal stem cells and in regulating inflammation and oxidative stress in the context of cardiometabolic disorders [155,156]. MGAM is a digestive enzyme involved in starch digestion and production of glucose in the lumen of the small intestine [157]. KCNMA1 codes for a subunit of the calcium-activated large conductance channel involved in neurotransmitter releases and neural excitability [158,159]. Variants in this gene have been linked to duodenal atresia, fetal growth restriction, developmental delay, intellectual disability, mild ataxia and generalized epilepsy [158,159].
Our understanding of the epigenetic impact of maternal OUD is only just beginning. These early targeted genome-wide methylation scans focused on placental and saliva samples for DNA extraction and identified mechanistically plausible changes that correlate with neonatal outcomes such as NOWS. Future studies should continue to include genome-wide approaches with additional assays for histone modifications to obtain a more comprehensive understanding of the impact of maternal OUD on neonates while expanding the tissue types sampled. In addition, integrating RNA-sequencing analysis with epigenetics will further help us understand the impact of changes in chromatin structure and accessibility on cellular function and neonatal outcomes following maternal OUD in pregnancy.
Other illicit drug exposure in pregnancy
Prenatal illicit drug exposure, including to cocaine and methamphetamines, has been shown in rodent models to impair offspring’s cognitive function and social behavior through disruption of epigenetic reprogramming of gene expression [160,161]. A previously described mechanism for these outcomes associated with maternal cocaine use in pregnancy is through altered DNA methylation and gene expression in hippocampal neurons of offspring [162], in addition to epigenetic modification of IGF2 [160]. Similarly, prenatal methamphetamine exposure in a rat model demonstrated methamphetamine-induced behavioral sensitization and nucleus accumbens DNA methylation changes in male offspring [163]. Altered DNA methylation in the nucleus accumbens was associated with 86 annotated genes functionally enriched in the pathways of neurodevelopment and addiction, including Kirrel3, Lrpprc and Peg3 [163].
Paternal substance use: epigenetic mechanisms & impact on offspring
Alcohol
Emerging literature suggests that paternal pre-conception alcohol exposure may influence intergenerational alcohol-related behaviors and exert cross-generational effects akin to chronic stress [164]. Studies are beginning to delineate genomic loci and RNAs in sperm that can be impacted by alcohol use and are related to cross-generational effects. Alcohol has been shown to act directly on DNA methylation mechanisms, decreasing levels of cytosine methyltransferase DNMT1 and S-adenosyl methionine (the methyl group donor in DNA methylation reactions) in somatic cells, with similar effects on DNA methylation in the male germline [164]. There have also been studies demonstrating the effects of alcohol on post-translational histone modifications [165] and small noncoding RNAs [166] in sperm, although reports of DNA methylation changes in offspring resulting from paternal alcohol exposure are mixed. A prior murine study showed that paternal ethanol consumption impacts DNA integrity in the testicular germline and sperm, with alterations that result in deleterious effects on embryonic development and cause testicular and spermatic changes in the offspring [167]. Nieto et al. reported phenotypic effects in the offspring of alcohol-exposed male rats but no differences in global 5-methylcytosine levels in the offsprings’ nucleus accumbens or prefrontal cortex [168]. Similarly, Chang et al. reported phenotypic effects of paternal alcohol exposure, including effects on the placenta and lipid transport, as well as differences in males, but not females, in the expression of genes involved in liver fibrosis. They reported no differences in the methylation of imprinted genes, but there were differences in the expression of some of these genes in the placenta, with no evidence for loss of imprinted expression. They also reported no differences in S-adenosylmethionine and, using a stringent threshold of a 25% difference in methylation, only two regions in sperm were identified and then validated as significantly differentially methylated [169]. Knezovich et al. found that pre-conception paternal alcohol exposure impacts DNA methylation at two paternally methylated imprinting control regions (H19 and Rasgrf1) in the sperm of exposed male mice and the somatic DNA of sired offspring [170]. Additionally, this study observed significant reductions in methylation at two of the H19 CTCF binding sites in the offspring of ethanol-treated sires [170]. These methylation changes were apparently not transmitted from the sperm, because no difference in DNA methylation was observed in the sperm of alcohol-exposed males relative to controls. These results indicate that there must be another epigenetic mechanism (e.g., noncoding RNAs, histone modifications) that transmits information about the father’s exposure and results in a postfertilization loss of DNA methylation at the normally paternally methylated CTCF binding sites.
Cannabis
Prior studies have examined the effects of paternal exposure to cannabis or THC on the epigenetic profile of the gametes and offspring behavior [171]. These human and rat studies have highlighted the significant impact to the sperm DNA methylome that occurs with cannabis exposure [171]. Murphy et al. reported that cannabis use in male humans and THC exposure in rats significantly impacts both the human and rat sperm DNA methylomes using reduced-representation bisulfite sequencing [55]. Affected genes were enriched among the ascorbate and aldarate metabolism, Hippo signaling, MAPK signaling and circadian entrainment pathways [55]. Most recently, Schrott et al. extended these findings using whole-genome bisulfite sequencing on an independent cohort and demonstrated that cannabis use in humans and exposure to cannabis extract in rats were not only associated with methylation changes across the sperm methylome, but that discontinuation of the exposure for the period of one spermatogenic cycle (∼74 days in humans and ∼56 days in rats) mitigated many, but not all, of the significant alterations [52,172]. Genes associated with the altered methylation in sperm were enriched for developmental processes [52,173,174] and functionally related to modifications in gene expression and to a cardiomegaly phenotype that was most evident in the F1 females [52]. Targeted analyses of several of the genes showing altered methylation in the F0 sperm in rats showed evidence of intergenerational heritability, because the same methylation changes were detectable in tissues from the F1 generation (brain and sperm). In a mouse model, Innocenzi et al. studied the effect of male CB2 activation using a selective CB2 agonist, JWH-133. They found that when male mice were exposed to JWH-133, they had reduced sperm counts, and this manifested in placental function impairments and reduced offspring growth. These phenotypic changes were associated with altered methylation and hydroxymethylation at several imprinted genes (Peg 10 and Plagl1) both in F0 sperm and in the F1 placentas [175]. These findings provide insight into the role that pre-conception paternal cannabis use might have on influencing short- and long-term offspring health.
Nicotine
Although few studies have focused on the impact of paternal nicotine use on the subsequent generation, the existing literature suggests that tobacco or nicotine exposure can alter sperm DNA methylation profiles [176–178] and can influence offspring behavior and neurodevelopment [179–182]. Unlike paternal cocaine exposure, paternal nicotine use does not induce nicotine-specific reward responses in offspring [183]. In mice, Dai et al. demonstrated that nicotine exposure resulted in the exposed fathers exhibiting depression [179]. Pre-conception paternal nicotine exposure resulted in methylation-mediated repression of mmu-miR-15b expression in F0 sperm, which was maintained in the thalamus of subsequent F1 offspring [179]. As a result, expression of Wnt4, the target gene of mmu-miR-15b, was elevated in the thalamus of F1 mice secondary to inherited DNA methylation patterns from the paternal generation [179]. Hypermethylation of mmu-miR-15b in F1 offspring was associated with greater hyperactivity and less depression-like behavior [179]. These intergenerational effects were not observed in the F2 generation. Similarly, Hawkey et al. demonstrated that paternal nicotine exposure in male rats resulted in male offspring with increased locomotor activity, impaired habituation and altered behavior [184]. Another study examining the effects of paternal nicotine exposure in rats reported altered sperm DNA methylation at genes involved in neurodevelopment, including autism candidate genes [173].
Opioids
Emerging evidence demonstrates that paternal opioid exposure may impact developmental trajectories spanning multiple generations [183,185–187]. Paternal opioid use, even a single exposure, may impact behavioral and neurobiological characteristics in subsequent generations, including increased withdrawal-like behaviors and synaptic plasticity deficits [180]. Additionally, paternal opioid use has been suggested to increase the risk of offspring vulnerability to opioid abuse in a sex-dependent manner [180]. The literature is conflicting regarding whether paternal opioid exposure results in increased anxiety and depression-like behavior in offspring [183], but does support changes in organ weights (e.g., adrenal gland, thymus) [188], synaptic activity [189,190] and hormone levels (e.g., luteinizing hormone, testosterone) [186] that are associated with growth regulation and neurotransmitter function [183].
There are no existing studies demonstrating transgenerational inheritance of epigenome modifications from opioid exposure, but some have shown epigenetic alterations within a single generation. Animal studies have reported that morphine exposure can result in histone modifications in the brain, including the nucleus accumbens, that may mediate the rewarding effects of morphine [191–193]. Aside from altered histone acetylation or methylation, morphine exposure can also result in methylation changes to DNA [150,194–196]. In humans, opioid use has been linked with elevated levels of DNA methylation at the OPRM1 promoter in both blood and sperm of male opioid addicts [196]. Although these findings suggest the possibility of epigenetic heritability of opioid-abuse or -dependence phenotypes, there are currently no existing studies that have determined whether these epigenetic modifications are inherited by offspring.
Other illicit drug use
Methamphetamines and cocaine increase adrenergic and dopaminergic signaling through different mechanisms, but with similar physiological effects. Despite methamphetamine use being greater than that of cocaine or opiates, little is known about the prenatal effects of methamphetamine use on neonatal epigenetic changes [197]. A single study evaluated DNA methylation in mice following prenatal exposure of both parents to methamphetamine compared with saline-exposed controls, whereas most other studies evaluated only maternal methamphetamine exposure. The authors showed male offspring with prenatal parental methamphetamine exposure had increased cocaine-induced conditioned place preference testing and hyperactivity [161]. Hippocampal DNA methylation analysis focused on differentially methylated promoter regions and identified 62 elevated and 35 reduced promoter regions with DNA methylation following prenatal methamphetamine exposure [161]. There is a need for further studies that isolate the effect of paternal methamphetamine use on neonatal outcomes and epigenetic changes.
Vassoler et al. previously described a heritable phenotype secondary to epigenetic reprogramming of the germline from voluntary paternal ingestion of cocaine in rats [19]. Male offspring of sires that self-administered cocaine had observed resistance to cocaine reinforcement and profound effects on medial prefrontal cortex gene expression [19]. BDNF mRNA and protein were increased in the prefrontal cortex of male offspring of cocaine-experienced sires and there was an association with acetylated histone H3 of BDNF promoters in sperm of sires that self-administered cocaine [19].
There are no well-controlled studies investigating the contribution of paternal prenatal hallucinogen use on epigenetic changes in the offspring. Prenatal use of hallucinogenic compounds is an emerging problem due to increasing decriminalization of psilocybin in US cities and states. Further studies are needed to determine the safety of paternal psilocybin use and maternal use in pregnancy and any subsequent epigenetic changes.
Conclusion
The prevalence of substance use is rising, predominantly among individuals of reproductive age. Although there are accumulating data on the potential epigenetic consequences of pre-conception substance use that can adversely impact offspring, a large gap in knowledge remains as there are only a limited number of drugs with known measured epigenetic effects. This is a nascent field of study, and much work remains to be done. Larger studies are needed to verify reported findings and to evaluate the methylome more comprehensively, in addition to other epigenetic modifications. It will be important to determine whether methylation changes are functionally related to changes in gene expression and phenotypic changes or if they are bystander effects of the exposure. The use of relevant translational animal models with care to include physiologically relevant levels of exposure will permit tissue and biological sampling to directly link epigenetic changes with tissue-specific mRNA and proteins. However, there remains the challenge of distinguishing the influence of programming from that of the offspring environment (e.g., affected maternal care from drug use) impacting the offspring epigenome from the direct intergenerational substance use effects on the methylome. Larger longitudinal human studies and the use of strong translational preclinical models are necessary to bridge this gap and facilitate more comprehensive counseling for individuals and couples interested in conceiving regarding how their substance use may affect not only their own health, but also the health of their offspring.
Future perspective
As the landscape for substance use is changing and polysubstance use is prevalent, future directions of the field include exploration of the underlying epigenetic mechanisms of co-use (e.g., nicotine and cannabis, or nicotine, cannabis and alcohol) and different drug formulations and delivery methods. Advances in technology and reductions in cost to allow for detailed characterization of the canonical epigenetic modifications across the spermatozoa and oocyte genomes with drug use, including histone modifications, will provide more comprehensive understanding of how target loci are impacted. It will be important to determine the specific effects of each substance on the epigenome, because it has been shown that different substances can induce methylation changes at the same CpG sites but in different directions [173]. Use of artificial intelligence primed with growing information about the epigenetic effects of single drugs and those co-used may provide tools for predicting the risk of epigenetic alterations that are transmissible to the next generation. It is essential that we obtain a much more detailed understanding of the nonimprinted loci that resist postfertilization epigenetic reprogramming, because it is these loci that will convey the epigenetic signal coming from the prior generation’s drug use to the next generation. Lastly, tantalizing evidence suggests that methylation changes in human sperm resulting from the use of cannabis can be greatly reduced by stopping use for the duration of a spermatogenic cycle, and these results are supported by rat [52,172] and rhesus macaque models [198]. This is likely due to a ‘washing out’ effect that eliminates the altered sperm with time. This also suggests that it is the developing spermatozoa that are the targets of these changes rather than the spermatogonia, at least for THC or cannabis. It will be important to test longer periods of withdrawal from use to see whether a complete restoration to a pre-substance-use profile is possible, or if not, to catalog those loci that exhibit permanent changes – as well as to determine whether this applies to other types of substance use. Such improved understanding will inform the development of public health measures and interventions to help mitigate the epigenetic consequences of parental substance use.
Executive summary.
Growing evidence suggests that paternal pre-conception and maternal prenatal substance use may influence offspring epigenetic regulation and outcomes later in life, including neurodevelopment and mental health.
Parental substance use can alter the epigenetic regulation of genes that are genomically imprinted; such changes are intergenerationally transmissible.
There are nonimprinted regions of the genome that withstand methylation erasure in primordial germ cells and during postfertilization reprogramming, providing an additional means for intergenerational and transgenerational transmission of epigenetic alterations.
Demonstration of epigenetic heritability in human studies is difficult due to the multitude of confounding factors inherent in studies of a group of individuals.
Studies in animal models, though limited by species differences, are valuable for determining the effects of exposure to single or combined agents, the impact on gametes and the impact on the development of the next and subsequent generations, without the potential masking of results by the confounding factors common in human studies.
There are specific periods of development that are windows of epigenetic vulnerability, including gametogenesis, the periconceptional period, prenatal development, early postnatal development and puberty.
Substance use may lead to acceleration of age determined by the ‘epigenetic clock’, to reflect an age that is older than chronological age.
Maternal alcohol use in pregnancy increases the risk of fetal alcohol spectrum disorder, altered behavior in the offspring and lifelong changes in stress responsiveness.
Paternal pre-conception alcohol use can impact the sperm epigenome, with changes in DNA methylation observed at several imprinted genes in offspring that are normally paternally methylated.
Cannabinoid use is associated with changes in sperm DNA methylation at many genes involved in neurodevelopment and neurobehavior; these changes show evidence of intergenerational transmission along with neurobehavioral and cognitive deficits and can be partially mitigated by withdrawal from use for at least the duration of one spermatogenic cycle.
Paternal tobacco or nicotine use is associated with altered sperm DNA methylation, while maternal use of tobacco or secondhand tobacco smoke exposure is associated with altered DNA methylation in the offspring. Nicotine and THC exhibit divergent methylation changes at some of the same CpG sites in sperm. The effects of co-use are unknown.
Opioid use during pregnancy leads to altered methylation in the placenta, coincident with adverse pregnancy outcomes and neonatal opioid withdrawal syndrome.
Current trends in the expansion of drug use underscore a critical need to better understand the consequences of pre-conception and prenatal substance use to allow individuals to make informed decisions about their use of these substances as it relates to their procreation.
Footnotes
Author contributions
J Lo: conceptualization, investigation, writing (original draft; review and editing). R D’Mello: investigation, writing (original draft; review and editing). L Watch: investigation, writing (original draft; review and editing). D Schust: investigation, writing (original draft; review and editing). S Murphy: conceptualization, investigation, writing (original draft; review and editing).
Financial & competing interests disclosure
The authors received grant support from the National Institutes of Health (DP1 DA056493) and the John Templeton Foundation (60957). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.UN Office on Drugs and Crime. UNODC World Drug Report 2020. United Nations Office on Drugs and Crime, Vienna, Austria: (2020). [Google Scholar]
- 2.Hales CN, Barker DJ, Clark PM et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303(6809), 1019–1022 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ. The fetal and infant origins of adult disease. BMJ 301(6761), 1111 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanner NM, Colwell ML, Faulk C. The epigenetic legacy of illicit drugs: developmental exposures and late-life phenotypes. Environ. Epigenet. 5(4), dvz022 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang G. Maternal substance use: consequences, identification, and interventions. Alcohol Res. 40(2), 06 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodge NC, Jacobson JL, Jacobson SW. Effects of fetal substance exposure on offspring substance use. Pediatr. Clin. North Am. 66(6), 1149–1161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruisch IH, Dietrich A, Glennon JC, Buitelaar JK, Hoekstra PJ. Maternal substance use during pregnancy and offspring conduct problems: a meta-analysis. Neurosci. Biobehav. Rev. 84, 325–336 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Chassin L, Curran PJ, Hussong AM, Colder CR. The relation of parent alcoholism to adolescent substance use: a longitudinal follow-up study. J. Abnorm. Psychol. 105(1), 70–80 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Hussong AM, Curran PJ, Chassin L. Pathways of risk for accelerated heavy alcohol use among adolescent children of alcoholic parents. J. Abnorm. Child Psychol. 26(6), 453–466 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Obot IS, Wagner FA, Anthony JC. Early onset and recent drug use among children of parents with alcohol problems: data from a national epidemiologic survey. Drug Alcohol Depend. 65(1), 1–8 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Vucinovic M, Roje D, Vucinovic Z, Capkun V, Bucat M, Banovic I. Maternal and neonatal effects of substance abuse during pregnancy: our ten-year experience. Yonsei Med. J. 49(5), 705–713 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang SS, Diop H, Liu CL et al. Maternal substance use disorders and infant outcomes in the first year of life among massachusetts singletons, 2003-2010. J. Pediatr. 191, 69–75 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Szutorisz H, Hurd YL. Epigenetic effects of cannabis exposure. Biol. Psychiatry 79(7), 586–594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith A, Kaufman F, Sandy MS, Cardenas A. Cannabis exposure during critical windows of development: epigenetic and molecular pathways implicated in neuropsychiatric disease. Curr. Environ. Health Rep. 7(3), 325–342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Pre-conception and prenatal cannabinoid exposure can impact epigenetic processes with functional gene consequences that may be heritable and associated with genes and molecular pathways critical for offspring brain development.
- 15.Knopik VS, Marceau K, Bidwell LC, Rolan E. Prenatal substance exposure and offspring development: does DNA methylation play a role? Neurotoxicol. Teratol. 71, 50–63 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrison-Desany HM, Hong X, Maher BS et al. Individual and combined association between prenatal polysubstance exposure and childhood risk of attention-deficit/hyperactivity disorder. JAMA Netw. Open 5(3), e221957 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology 40(1), 61–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killinger CE, Robinson S, Stanwood GD. Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse 66(10), 902–908 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 16(1), 42–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microrna content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33(21), 9003–9012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Easey KE, Sharp GC. The impact of paternal alcohol, tobacco, caffeine use and physical activity on offspring mental health: a systematic review and meta-analysis. Reprod. Health 18(1), 214 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaluscha S, Domcke S, Wirbelauer C et al. Evidence that direct inhibition of transcription factor binding is the prevailing mode of gene and repeat repression by DNA methylation. Nat. Genet. 54(12), 1895–1906 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg MVC, Bourc'his D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 20(10), 590–607 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Eidem TM, Kugel JF, Goodrich JA. Noncoding RNAs: regulators of the mammalian transcription machinery. J. Mol. Biol. 428(12), 2652–2659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurokawa R, Rosenfeld MG, Glass CK. Transcriptional regulation through noncoding RNAs and epigenetic modifications. RNA Biol. 6(3), 233–236 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics (review). Oncol. Rep. 37(1), 3–9 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Newell-Price J, Clark AJ, King P. DNA methylation and silencing of gene expression. Trends Endocrinol. Metab. 11(4), 142–148 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Li S, Peng Y, Panchenko AR. DNA methylation: precise modulation of chromatin structure and dynamics. Curr. Opin. Struct. Biol. 75, 102430 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Prokhortchouk E, Defossez PA. The cell biology of DNA methylation in mammals. Biochim. Biophys. Acta 1783(11), 2167–2173 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Weber M, Schübeler D. Genomic patterns of DNA methylation: targets and function of an epigenetic mark. Curr. Opin. Cell Biol. 19(3), 273–280 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J. Cell. Physiol. 213(2), 384–390 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Cain JA, Montibus B, Oakey RJ. Intragenic CpG islands and their impact on gene regulation. Front. Cell Dev. Biol. 10, 832348 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabbari K, Bernardi G. Cytosine methylation and CpG, TpG (CpA) and TpA frequencies. Gene 333, 143–149 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Delcuve GP, Rastegar M, Davie JR. Epigenetic control. J. Cell. Physiol. 219(2), 243–250 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Nafee TM, Farrell WE, Carroll WD, Fryer AA, Ismail KM. Epigenetic control of fetal gene expression. BJOG 115(2), 158–168 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity 105(1), 4–13 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Prado F, Jimeno-González S, Reyes JC. Histone availability as a strategy to control gene expression. RNA Biol. 14(3), 281–286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alaskhar Alhamwe B, Khalaila R, Wolf J et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy, Asthma Clin. Immunol. 14(1), 39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaju Bhattad G, Jeyarajah MJ, Mcgill MG et al. Histone deacetylase 1 and 2 drive differentiation and fusion of progenitor cells in human placental trophoblasts. Cell Death Dis. 11(5), 311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc. Natl Acad. Sci. USA 106(19), 7876–7881 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren W, Fan H, Grimm SA et al. Direct readout of heterochromatic H3K9me3 regulates DNMT1-mediated maintenance DNA methylation. Proc. Natl Acad. Sci. USA 117(31), 18439–18447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren W, Fan H, Grimm SA et al. DNMT1 reads heterochromatic H4K20me3 to reinforce line-1 DNA methylation. Nat. Commun. 12(1), 2490 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Ann. NY Acad. Sci. 1089, 14–35 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal–juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14(5), 636–648 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Rogers MS, Boyartchuk V, Rohan RM, Birsner AE, Dietrich WF, D’Amato RJ. The classical pink-eyed dilution mutation affects angiogenic responsiveness. PLOS ONE 7(5), e35237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J. Androl. 27(6), 868–879 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derick CL, Hitchcock CH, Swift HF. The effect of anti-rheumatic drugs on the arthritis and immune body production in serum disease. J. Clin. Invest. 5(3), 427–440 (1928). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727), 1466–1469 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Using a rat model, this study demonstrated that transient exposure to an environmental factor (e.g., endocrine disruptor) can result in altered DNA methylation patterns in the germline that has a transgenerational impact.
- 49.Stockard CR, Papanicolaou GN. Further studies on the modification of the germ-cells in mammals: the effect of alcohol on treated guinea pigs and their descendants. J. Exp. Zool. 26, 119–226 (1918). [Google Scholar]
- 50.Waddington CH. Canalisation of development and the inheritance of acquired characters. Nature 150(3811), 563–565 (1942). [Google Scholar]
- 51.Sadat-Shirazi MS, Sadeghi-Adl M, Akbarabadi A, Ashabi G, Mokri A, Zarrindast MR. Inter/transgenerational effects of drugs of abuse: a scoping review. CNS Neurol. Disord. Drug Targets 22(4), 512–538 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Schrott R, Modliszewski JL, Hawkey AB et al. Sperm DNA methylation alterations from cannabis extract exposure are evident in offspring. Epigenetics Chromatin 15(1), 33 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson CT, Szutorisz H, Garg P et al. Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology 40(13), 2993–3005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In a rat model, adolescent tetrahydrocannabinol exposure altered DNA methylation in the nucleus accumbens resulting in changes to the epigenome that were inherited cross-generation.
- 54.Szutorisz H, Dinieri JA, Sweet E et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 39(6), 1315–1323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy SK, Itchon-Ramos N, Visco Z et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 13(12), 1208–1221 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin EM, Fry RC. Environmental influences on the epigenome: exposure-associated DNA methylation in human populations. Annu. Rev. Public Health 39, 309–333 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Lo JO, Hedges JC, Girardi G. Impact of cannabinoids on pregnancy, reproductive health, and offspring outcomes. Am. J. Obstet. Gynecol. 227(4), 571–581 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker DJ. Mothers, Babies, and Disease in Later Life. BMJ Publishing Group, London, England: (1994). [Google Scholar]
- 59.Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ 315(7112), 837–840 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 301(6746), 259–262 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 2(8663), 577–580 (1989). [DOI] [PubMed] [Google Scholar]
- 62.Heijmans BT, Tobi EW, Stein AD et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA 105(44), 17046–17049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Needham BL, Smith JA, Zhao W et al. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics 10(10), 958–969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borghol N, Suderman M, Mcardle W et al. Associations with early-life socio-economic position in adult DNA methylation. Int. J. Epidemiol. 41(1), 62–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faulk C, Kim JH, Jones TR et al. Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver. Environ. Epigenet. 1(1), dvv006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilsner JR, Hall MN, Liu X et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLOS ONE 7(5), e37147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hossain K, Suzuki T, Hasibuzzaman MM et al. Chronic exposure to arsenic, LINE-1 hypomethylation, and blood pressure: a cross-sectional study in Bangladesh. Environ. Health 16(1), 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Begum G, Davies A, Stevens A et al. Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology 154(12), 4560–9 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Seifuddin F, Wand G, Cox O et al. Genome-wide Methyl-Seq analysis of blood-brain targets of glucocorticoid exposure. Epigenetics 12(8), 637–652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novakovic B, Ryan J, Pereira N, Boughton B, Craig JM, Saffery R. Postnatal stability, tissue, and time specific effects of AHRR methylation change in response to maternal smoking in pregnancy. Epigenetics 9(3), 377–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herman JJ, Spencer HG, Donohue K, Sultan SE. How stable ‘should’ epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution 68(3), 632–643 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Natarajan R, Aljaber D, Au D et al. Environmental exposures during puberty: window of breast cancer risk and epigenetic damage. Int. J. Environ. Res. Public Health 17(2), 493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Safi-Stibler S, Gabory A. Epigenetics and the developmental origins of health and disease: parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin. Cell Dev. Biol. 97, 172–180 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr. Rev. 75(12), 951–970 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Loyfer N, Magenheim J, Peretz A et al. A DNA methylation atlas of normal human cell types. Nature 613(7943), 355–364 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez-Rodriguez D, Franssen D, Bakker J, Lomniczi A, Parent AS. Cellular and molecular features of EDC exposure: consequences for the GNRH network. Nat. Rev. Endocrinol. 17(2), 83–96 (2021). [DOI] [PubMed] [Google Scholar]
- 77.Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell. Endocrinol. 398(1–2), 4–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takeda S, Yoshida K, Nishimura H et al. Δ(9)-tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ). Chem. Res. Toxicol. 26(7), 1073–1079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takeda S. Δ9-tetrahydrocannabinol targeting estrogen receptor signaling: the possible mechanism of action coupled with endocrine disruption. Biol. Pharm. Bull. 37(9), 1435–1438 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Maia J, Almada M, Midao L et al. The cannabinoid delta-9-tetrahydrocannabinol disrupts estrogen signaling in human placenta. Toxicol. Sci. 177(2), 420–430 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Hackett JA, Surani MA. DNA methylation dynamics during the mammalian life cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368(1609), 20110328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo H, Zhu P, Yan L et al. The DNA methylation landscape of human early embryos. Nature 511(7511), 606–610 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Wasserzug-Pash P, Klutstein M. Epigenetic changes in mammalian gametes throughout their lifetime: the four seasons metaphor. Chromosoma 128(3), 423–441 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Monk D. Germline-derived DNA methylation and early embryo epigenetic reprogramming: the selected survival of imprints. Int. J. Biochem. Cell Biol. 67, 128–138 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Marcho C, Cui W, Mager J. Epigenetic dynamics during preimplantation development. Reproduction 150(3), R109–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borgel J, Guibert S, Li Y et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nat. Genet. 42(12), 1093–1100 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Tang WW, Dietmann S, Irie N et al. A unique gene regulatory network resets the human germline epigenome for development. Cell 161(6), 1453–1467 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hackett JA, Sengupta R, Zylicz JJ et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 339(6118), 448–452 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Imprint erasure of CpG methylation in mouse primordial germ cells occurs via conversion to 5-hydroxymethylcytosine and may contribute to transgenerational epigenetic inheritance.
- 89.Szutorisz H, Egervári G, Sperry J, Carter JM, Hurd YL. Cross-generational THC exposure alters the developmental sensitivity of ventral and dorsal striatal gene expression in male and female offspring. Neurotoxicol. Teratol. 58, 107–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perobelli JE. The male peripubertal phase as a developmental window for reproductive toxicology studies. Curr. Pharm. Des. 20(34), 5398–5415 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Accordini S, Calciano L, Johannessen A et al. Prenatal and prepubertal exposures to tobacco smoke in men may cause lower lung function in future offspring: a three-generation study using a causal modelling approach. Eur. Respir. J. 58(4), 2002791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Svanes C, Koplin J, Skulstad SM et al. Father’s environment before conception and asthma risk in his children: a multi-generation analysis of the respiratory health in northern europe study. Int. J. Epidemiol. 46(1), 235–245 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict. Biol. 13(2), 253–263 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Cabrera-Mendoza B, Stertz L, Najera K et al. Within subject cross-tissue analyzes of epigenetic clocks in substance use disorder postmortem brain and blood. Am J. Med. Genet. B Neuropsychiatr. Genet. 192(1–2), 13–27 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levine ME, Lu AT, Quach A et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10(4), 573–591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luo A, Jung J, Longley M et al. Epigenetic aging is accelerated in alcohol use disorder and regulated by genetic variation in APOL2. Neuropsychopharmacology 45(2), 327–336 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang C-H, Chang M-C, Lai Y-C et al. Mitochondrial DNA methylation profiling of the human prefrontal cortex and nucleus accumbens: correlations with aging and drug use. Clin. Epigenetics 14(1), 79 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marjonen H, Sierra A, Nyman A et al. Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model. PLOS ONE 10(5), e0124931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ngai YF, Sulistyoningrum DC, O’Neill R, Innis SM, Weinberg J, Devlin AM. Prenatal alcohol exposure alters methyl metabolism and programs serotonin transporter and glucocorticoid receptor expression in brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309(5), R613–622 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jabbar S, Chastain LG, Gangisetty O, Cabrera MA, Sochacki K, Sarkar DK. Preconception alcohol increases offspring vulnerability to stress. Neuropsychopharmacology 41(11), 2782–2793 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haycock PC, Ramsay M. Exposure of mouse embryos to ethanol during preimplantation development: effect on DNA methylation in the H19 imprinting control region. Biol. Reprod. 81(4), 618–627 (2009). [DOI] [PubMed] [Google Scholar]
- 102.Kim P, Park JH, Choi CS et al. Effects of ethanol exposure during early pregnancy in hyperactive, inattentive and impulsive behaviors and mecp2 expression in rodent offspring. Neurochem. Res. 38(3), 620–631 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Martin CE, Longinaker N, Mark K, Chisolm MS, Terplan M. Recent trends in treatment admissions for marijuana use during pregnancy. J. Addict. Med. 9(2), 99–104 (2015). [DOI] [PubMed] [Google Scholar]
- 104.National Academies of Sciences Engineering AM. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Press, WA, USA: (2017). [PubMed] [Google Scholar]
- 105.Passey ME, Sanson-Fisher RW, D’Este CA, Stirling JM. Tobacco, alcohol and cannabis use during pregnancy: clustering of risks. Drug Alcohol Depend. 134, 44–50 (2014). [DOI] [PubMed] [Google Scholar]
- 106.(. Beatty JR, Svikis DS, Ondersma SJ. Prevalence and perceived financial costs of marijuana versus tobacco use among urban low-income pregnant women. J. Addict. Res. Ther. 3(4), 1000135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore DG, Turner JD, Parrott AC et al. During pregnancy, recreational drug-using women stop taking ecstasy (3,4-methylenedioxy-n-methylamphetamine) and reduce alcohol consumption, but continue to smoke tobacco and cannabis: initial findings from the development and infancy study. J. Psychopharmacol. 24(9), 1403–1410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA 317(2), 207–209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. JAMA 317(2), 129–130 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG 109(1), 21–27 (2002). [DOI] [PubMed] [Google Scholar]
- 111.El Marroun H, Tiemeier H, Steegers EA et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R study. J. Am. Acad. Child Adolesc. Psychiatry 48(12), 1173–1181 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Hurd YL, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 27(2), 221–229 (2005). [DOI] [PubMed] [Google Scholar]
- 113.Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 44(11), 697–701 (1989). [DOI] [PubMed] [Google Scholar]
- 114.Cristino L, Di Marzo V. Fetal cannabinoid receptors and the ‘dis-joint-ed’ brain. EMBO J. 33(7), 665–667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kenney SP, Kekuda R, Prasad PD, Leibach FH, Devoe LD, Ganapathy V. Cannabinoid receptors and their role in the regulation of the serotonin transporter in human placenta. Am. J. Obstet. Gynecol. 181(2), 491–497 (1999). [DOI] [PubMed] [Google Scholar]
- 116.Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr. Pharm. Des. 20(13), 2138–2167 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Varner MW, Silver RM, Rowland Hogue CJ et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet. Gynecol. 123(1), 113–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet. Gynecol. 128(4), 713–723 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Baía I, Domingues R. The effects of cannabis use during pregnancy on low birth weight and preterm birth: a systematic review and meta-analysis. Am. J. Perinatol. (2022) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 120.Marchand G, Masoud AT, Govindan M et al. Birth outcomes of neonates exposed to marijuana in utero: a systematic review and meta-analysis. JAMA Netw. Open 5(1), e2145653 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Corsi DJ, Donelle J, Sucha E et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 26(10), 1536–1540 (2020). [DOI] [PubMed] [Google Scholar]; • Large, retrospective population-based birth cohort study demonstrating an association between maternal cannabis use in pregnancy and the incidence of autism spectrum disorder in the offspring.
- 122.Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction 101(9), 1313–1322 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Leech SL, Larkby CA, Day R, Day NL. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J. Am. Acad. Child Adolesc. Psychiatry 45(2), 223–230 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol. Teratol. 33(1), 129–136 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol. Teratol. 22(3), 325–336 (2000). [DOI] [PubMed] [Google Scholar]
- 126.Dinieri JA, Wang X, Szutorisz H et al. Maternal cannabis use alters ventral striatal dopamine d2 gene regulation in the offspring. Biol. Psychiatry 70(8), 763–769 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wanner NM, Colwell M, Drown C, Faulk C. Developmental cannabidiol exposure increases anxiety and modifies genome-wide brain DNA methylation in adult female mice. Clin. Epigenetics 13(1), 4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khare M, Taylor AH, Konje JC, Bell SC. Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Mol. Hum. Reprod. 12(5), 321–333 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Gillies R, Lee K, Vanin S et al. Maternal exposure to δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod. Toxicol. 94, 84–91 (2020). [DOI] [PubMed] [Google Scholar]
- 130.Young-Wolff KC, Sarovar V, Tucker LY et al. Self-reported daily, weekly, and monthly cannabis use among women before and during pregnancy. JAMA Netw. Open 2(7), e196471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am. J. Prev. Med. 49(2), 286–293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Havard A, Chandran JJ, Oei JL. Tobacco use during pregnancy. Addiction 117(6), 1801–1810 (2022). [DOI] [PubMed] [Google Scholar]
- 133.Di HK, Gan Y, Lu K et al. Maternal smoking status during pregnancy and low birth weight in offspring: systematic review and meta-analysis of 55 cohort studies published from 1986 to 2020. World J. Pediatr. 18(3), 176–185 (2022). [DOI] [PubMed] [Google Scholar]
- 134.Ren M, Lotfipour S, Leslie F. Unique effects of nicotine across the lifespan. Pharmacol. Biochem. Behav. 214, 173343 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joubert BR, Haberg SE, Nilsen RM et al. 450k epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ. Health Perspect. 120(10), 1425–1431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joubert BR, Felix JF, Yousefi P et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am. J. Hum. Genet. 98(4), 680–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Everson TM, Vives-Usano M, Seyve E et al. Placental DNA methylation signatures of maternal smoking during pregnancy and potential impacts on fetal growth. Nat. Commun. 12(1), 5095 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blostein FA, Fisher J, Dou J et al. Polymethylation scores for prenatal maternal smoke exposure persist until age 15 and are detected in saliva in the Fragile Families and Child Wellbeing cohort. Epigenetics 17(13), 2223–2240 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fuemmeler BF, Dozmorov MG, Do EK et al. DNA methylation in babies born to nonsmoking mothers exposed to secondhand smoke during pregnancy: an epigenome-wide association study. Environ. Health Perspect. 129(5), 057010-1–057010-13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hall BJ, Cauley M, Burke DA, Kiany A, Slotkin TA, Levin ED. Cognitive and behavioral impairments evoked by low-level exposure to tobacco smoke components: comparison with nicotine alone. Toxicol. Sci. 151(2), 236–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cauley M, Hall BJ, Abreu-Villaca Y et al. Critical developmental periods for effects of low-level tobacco smoke exposure on behavioral performance. Neurotoxicology 68, 81–87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hawkey A, Junaid S, Yao L et al. Gestational exposure to nicotine and/or benzo[a]pyrene causes long-lasting neurobehavioral consequences. Birth Defects Res. 111(17), 1248–1258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fuemmeler BF, Glasgow TE, Schechter JC et al. Prenatal and childhood smoke exposure associations with cognition, language, and attention-deficit/hyperactivity disorder. J. Pediatr. 256, 77–84.e1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oh K, Xu Y, Terrizzi BF et al. Associations between early low-level tobacco smoke exposure and executive function at age 8 years. J. Pediatr. 221, 174–180.e1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Tobacco exposure during pregnancy and childhood is associated with adverse impacts on children’s executive function, especially task intitiation and metacognition.
- 145.Gustavson K, Ystrom E, Stoltenberg C et al. Smoking in pregnancy and child ADHD. Pediatrics 139(2), e20162509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Joglekar R, Cauley M, Lipsich T et al. Developmental nicotine exposure and masculinization of the rat preoptic area. Neurotoxicology 89, 41–54 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA 325(2), 146–155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Corsi DJ, Hsu H, Fell DB, Wen SW, Walker M. Association of maternal opioid use in pregnancy with adverse perinatal outcomes in Ontario, Canada, from 2012 to 2018. JAMA Netw. Open 3(7), e208256 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Wachman EM, Farrer LA. The genetics and epigenetics of neonatal abstinence syndrome. Semin. Fetal Neonatal Med. 24(2), 105–110 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Doehring A, Oertel BG, Sittl R, Lötsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain 154(1), 15–23 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Wachman EM, Hayes MJ, Lester BM et al. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J. Pediatr. 165(3), 472–478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wachman EM, Hayes MJ, Shrestha H et al. Epigenetic variation in OPRM1 gene in opioid-exposed mother–infant dyads. Genes Brain Behav. 17(7), e12476 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Radhakrishna U, Vishweswaraiah S, Uppala LV et al. Placental DNA methylation profiles in opioid-exposed pregnancies and associations with the neonatal opioid withdrawal syndrome. Genomics 113(3), 1127–1135 (2021). [DOI] [PubMed] [Google Scholar]
- 154.Borrelli KN, Wachman EM, Beierle JA et al. Effect of prenatal opioid exposure on the human placental methylome. Biomedicines 10(5), 1150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park SY, Han JS. Phospholipase d1 signaling: essential roles in neural stem cell differentiation. J. Mol. Neurosci. 64(3), 333–340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Auclair N, Sané AT, Delvin E, Spahis S, Levy E. Phospholipase D as a potential modulator of metabolic syndrome: impact of functional foods. Antioxid. Redox Signal. 34(3), 252–278 (2021). [DOI] [PubMed] [Google Scholar]
- 157.Ren L, Qin X, Cao X et al. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2(10), 827–836 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liang L, Liu H, Bartholdi D et al. Identification and functional analysis of two new de novo KCNMA1 variants associated with Liang–Wang syndrome. Acta Physiol. 235(1), e13800 (2022). [DOI] [PubMed] [Google Scholar]
- 159.Liang L, Li X, Moutton S et al. De novo loss-of-function KCNMA1 variants are associated with a new multiple malformation syndrome and a broad spectrum of developmental and neurological phenotypes. Hum. Mol. Genet. 28(17), 2937–2951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao Q, Hou J, Chen B et al. Prenatal cocaine exposure impairs cognitive function of progeny via insulin growth factor II epigenetic regulation. Neurobiol. Dis. 82, 54–65 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Itzhak Y, Ergui I, Young JI. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. Mol. Psychiatry 20(2), 232–239 (2015). [DOI] [PubMed] [Google Scholar]
- 162.Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLOS ONE 3(4), e1919 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dong N, Zhu J, Wang R et al. Maternal methamphetamine exposure influences behavioral sensitization and nucleus accumbens DNA methylation in subsequent generation. Front. Pharmacol. 13, 940798 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rompala GR, Homanics GE. Intergenerational effects of alcohol: a review of paternal preconception ethanol exposure studies and epigenetic mechanisms in the male germline. Alcohol. Clin. Exp. Res. 43(6), 1032–1045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rahimipour M, Talebi AR, Anvari M, Sarcheshmeh AA, Omidi M. Effects of different doses of ethanol on sperm parameters, chromatin structure and apoptosis in adult mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 170(2), 423–428 (2013). [DOI] [PubMed] [Google Scholar]
- 166.Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, Homanics GE. Heavy chronic intermittent ethanol exposure alters small noncoding RNAs in mouse sperm and epididymosomes. Front. Genet. 9, 32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cambiasso MY, Gotfryd L, Stinson MG et al. Paternal alcohol consumption has intergenerational consequences in male offspring. J. Assist. Reprod. Genet. 39(2), 441–459 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nieto SJ, Harding MJ, Nielsen DA, Kosten TA. Paternal alcohol exposure has task- and sex-dependent behavioral effect in offspring. Alcohol. Clin. Exp. Res. 46(12), 2191–2202 (2022). [DOI] [PubMed] [Google Scholar]
- 169.Chang RC, Skiles WM, Chronister SS et al. DNA methylation-independent growth restriction and altered developmental programming in a mouse model of preconception male alcohol exposure. Epigenetics 12(10), 841–853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Knezovich J, Ramsay M. The effect of preconception paternal alcohol exposure on epigenetic remodeling of the H19 and Rasgrf1 imprinting control regions in mouse offspring. Front. Genet. 3, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schrott R, Murphy SK. Cannabis use and the sperm epigenome: a budding concern? Environ. Epigenet. 6(1), dvaa002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schrott R, Murphy SK, Modliszewski JL et al. Refraining from use diminishes cannabis-associated epigenetic changes in human sperm. Environ. Epigenet. 7(1), dvab009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schrott R, Rajavel M, Acharya K et al. Sperm DNA methylation altered by THC and nicotine: vulnerability of neurodevelopmental genes with bivalent chromatin. Sci. Rep. 10(1), 16022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schrott R, Acharya K, Itchon-Ramos N et al. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 15(1–2), 161–173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Innocenzi E, De Domenico E, Ciccarone F et al. Paternal activation of CB(2) cannabinoid receptor impairs placental and embryonic growth via an epigenetic mechanism. Sci. Rep. 9(1), 17034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Paternal selective activation of cannabinoid receptor type 2 may alter the sperm epigenome in a way that can be transmitted to the next generation and compromise offspring growth.
- 176.Jenkins TG, James ER, Alonso DF et al. Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology 5(6), 1089–1099 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Laqqan MM, Yassin MM. Cigarette heavy smoking alters DNA methylation patterns and gene transcription levels in humans spermatozoa. Environ. Sci. Pollut. Res. Int. 29(18), 26835–26849 (2022). [DOI] [PubMed] [Google Scholar]
- 178.Liu Y, Chen S, Pang D et al. Effects of paternal exposure to cigarette smoke on sperm DNA methylation and long-term metabolic syndrome in offspring. Epigenetics Chromatin 15(1), 3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dai J, Wang Z, Xu W et al. Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-mir-15b. Sci. Rep. 7(1), 7286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goldberg LR, Gould TJ. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur. J. Neurosci. 50(3), 2453–2466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vallaster MP, Kukreja S, Bing XY et al. Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. Elife 6, e24771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McCarthy DM, Morgan TJ Jr, Lowe SE et al. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol. 16(10), e2006497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nieto SJ, Kosten TA. Who’s your daddy? Behavioral and epigenetic consequences of paternal drug exposure. Int. J. Dev. Neurosci. 78, 109–121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hawkey AB, White H, Pippen E et al. Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol. 74, 106808 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jalali Z, Bahrampour S, Khalili P, Khademalhosseini M, Esmaeili Nadimi A. Cohort-based analysis of paternal opioid use in relation to offspring’s BMI and plasma lipid profile. Sci. Rep. 11(1), 9462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cicero TJ, Adams ML, Giordano A, Miller BT, O’Connor L, Nock B. Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. J. Pharmacol. Exp. Ther. 256(3), 1086–1093 (1991). [PubMed] [Google Scholar]
- 187.Cicero TJ, Nock B, O’Connor L, Adams M, Meyer ER. Adverse effects of paternal opiate exposure on offspring development and sensitivity to morphine-induced analgesia. J. Pharmacol. Exp. Ther. 273(1), 386–392 (1995). [PubMed] [Google Scholar]
- 188.Joffe JM, Peruzović M, Milković K. Progeny of male rats treated with methadone: physiological and behavioural effects. Mutat. Res. 229(2), 201–211 (1990). [DOI] [PubMed] [Google Scholar]
- 189.Li CQ, Luo YW, Bi FF et al. Development of anxiety-like behavior via hippocampal IGF-2 signaling in the offspring of parental morphine exposure: effect of enriched environment. Neuropsychopharmacology 39(12), 2777–2787 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sarkaki A, Assaei R, Motamedi F, Badavi M, Pajouhi N. Effect of parental morphine addiction on hippocampal long-term potentiation in rats offspring. Behav. Brain Res. 186(1), 72–77 (2008). [DOI] [PubMed] [Google Scholar]
- 191.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann. NY Acad. Sci. 1216, 99–113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sun H, Maze I, Dietz DM et al. Morphine epigenomically regulates behavior through alterations in histone h3 lysine 9 dimethylation in the nucleus accumbens. J. Neurosci. 32(48), 17454–17464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rice JC, Allis CD. Code of silence. Nature 414(6861), 258–261 (2001). [DOI] [PubMed] [Google Scholar]
- 194.Nielsen DA, Yuferov V, Hamon S et al. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology 34(4), 867–873 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Trivedi M, Shah J, Hodgson N, Byun HM, Deth R. Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino acid transporter type 3-mediated cysteine uptake. Mol. Pharmacol. 85(5), 747–757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chorbov VM, Todorov AA, Lynskey MT, Cicero TJ. Elevated levels of DNA methylation at the OPRM1 promoter in blood and sperm from male opioid addicts. J. Opioid Manag. 7(4), 258–264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rorabaugh BR. Does prenatal exposure to cns stimulants increase the risk of cardiovascular disease in adult offspring? Front. Cardiovasc. Med. 8, 652634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hedges JC, Hanna CB, Shorey-Kendrick LE et al. Cessation of chronic delta-9-tetrahydrocannabinol use partially reverses impacts on male fertility and the sperm epigenome in rhesus macaques. Fertil Steril. 24, S0015-0282(23)00167-X . (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]


