ABSTRACT
Unbiased in vivo selections of diverse capsid libraries can yield engineered capsids that overcome gene therapy delivery challenges like traversing the blood-brain barrier (BBB), but little is known about the parameters of capsid-receptor interactions that govern their improved activity. This hampers broader efforts in precision capsid engineering and is a practical impediment to ensuring the translatability of capsid properties between preclinical animal models and human clinical trials. In this work, we utilize the adeno-associated virus (AAV)-PHP.B-Ly6a model system to better understand the targeted delivery and BBB penetration properties of AAV vectors. This model offers a defined capsid-receptor pair that can be used to systematically define relationships between target receptor affinity and in vivo activity of engineered AAV vectors. Here, we report a high-throughput method for quantifying capsid-receptor affinity and demonstrate that direct binding assays can be used to organize a vector library into families with varied affinity for their target receptor. Our data indicate that efficient central nervous system transduction requires high levels of target receptor expression at the BBB, but it is not a requirement for receptor expression to be limited to the target tissue. We observed that enhanced receptor affinity leads to reduced transduction of off-target tissues but can negatively impact on-target cellular transduction and penetration of endothelial barriers. Together, this work provides a set of tools for defining vector-receptor affinities and demonstrates how receptor expression and affinity interact to impact the performance of engineered AAV vectors in targeting the central nervous system.
IMPORTANCE Novel methods for measuring adeno-associated virus (AAV)-receptor affinities, especially in relation to vector performance in vivo, would be useful to capsid engineers as they develop AAV vectors for gene therapy applications and characterize their interactions with native or engineered receptors. Here, we use the AAV-PHP.B-Ly6a model system to assess the impact of receptor affinity on the systemic delivery and endothelial penetration properties of AAV-PHP.B vectors. We discuss how receptor affinity analysis can be used to isolate vectors with optimized properties, improve the interpretation of library selections, and ultimately translate vector activities between preclinical animal models and humans.
KEYWORDS: Adeno-associated virus, Blood-brain barrier, Capsid engineering
INTRODUCTION
In May of 2019, the U.S. Food and Drug Administration granted final approval for Zolgensma, a genetic therapy for pediatric patients suffering from spinal muscular atrophy (1, 2). This marked the first time a gene therapy vector was approved for intravenous (i.v.) administration into the bloodstream, as opposed to a targeted injection into a tissue of interest such as muscle (3, 4) or retina (5, 6). Zolgensma uses the wild-type (WT) capsid sequence from adeno-associated virus 9 (AAV9), whose broad tropism results in the transduction of many cell types, in addition to the motor neurons affected by spinal muscular atrophy (7, 8). Off-target transduction in liver tissue can be problematic, however, as it may lead to undesirable immune reactions (9) and dose-limiting toxicity (10). Genetic therapies utilizing other naturally occurring capsids have faced similar challenges in the clinic, with severe or fatal adverse events occurring in several patients enrolled in clinical trials for muscular disorders such as Duchenne muscular dystrophy and X-linked myotubular myopathy (11–13). There is a need for AAV capsids with improved tissue specificity and transduction efficiency to expand the utility of systemically administered gene therapies.
To this end, protein engineers have leveraged AAV display. This technique involves rapidly screening large libraries of AAV vectors modified with unique peptide insertions to identify capsid modifications that confer binding interactions with novel cellular receptors and thereby direct AAV vectors to transduce target tissues and/or detarget undesired tissues (14–16). An important early success of this system came in the identification of the AAV-PHP.B vector, which is an engineered capsid capable of remarkably efficient blood-brain barrier (BBB) penetration and central nervous system (CNS) transduction following i.v. delivery to mice (17, 18). We and others have demonstrated that this BBB penetration property is conferred by Ly6a, an attachment receptor for AAV-PHP.B expressed on the surface of brain microvascular endothelial cells (19–21). This receptor is not conserved between mice and humans (22), however, meaning the translational relevance of AAV-PHP.B across species is significantly limited (23).
This discovery revealed an important pitfall associated with large empirical screens of AAV vectors, in that there is a risk of identifying lead molecules with activities that are specific to the biology of the animal models in which they were selected. To contend with this, researchers have taken additional steps in recent years to demonstrate that the capsids they select can maintain some level of enhanced activity in multiple strains of mice (24–27) or even in multiple species of model organisms (28, 29). While these approaches can select AAV vectors with broader biological activity, they are still limited in that they provide no mechanistic information about how vector function is achieved, sustaining concerns about the selected vectors’ eventual human translatability. This limitation has prompted additional efforts to obtain receptor binding information for promising AAV vectors, using CRISPR (clustered regularly interspaced short palindromic repeats)-mediated screens (30), rational testing of likely candidate receptors (29, 31), or the selection of AAV libraries on target receptors directly (32). As AAV-receptor pairings begin to emerge, the field requires a better understanding of how the parameters of capsid-receptor interactions govern an AAV vector’s tissue specificity, transduction efficiency, and optimum activity. This lack of knowledge hampers broader efforts in precision capsid engineering and impedes our ability to utilize receptor binding information to translate developments in capsid biology from preclinical animal models to human gene therapies.
In exploring the principles behind receptor-mediated AAV capsid targeting, we can draw on previous work regarding virus-host cell attachment and entry mechanisms. A distinguishing characteristic of virus attachment is that the highly multivalent virus capsid can combine multiple low-affinity monomeric receptor interactions in an avid binding context to enable binding to the cell membrane (33–36). Therefore, successful binding can be contingent on the expression level of a target receptor on the cell surface (37–39). Previous research demonstrated this principle with WT AAV9 vectors, where the in vivo tropism of this vector was modified by altering the systemic presentation of its native galactose receptor (40). In developing binding assays for our previous work (20, 41), we quickly determined that monovalent AAV-PHP.B-Ly6a interactions have a very low affinity and could only observe quantifiable binding of this vector-receptor pair when the vector was able to interact avidly with multiple Ly6a molecules on a receptor-coated surface. Another group recently reported this observation while attempting to solve a cryo-electron microscopy structure for this vector-receptor complex (42). Thus, the AAV-PHP.B-Ly6a model system presents a defined vector-receptor pair that follows our fundamental understanding of avidity-mediated vector binding and elicits a robust CNS transduction phenotype that can be interrogated to better understand the targeted delivery and BBB penetration properties of AAV vectors.
In this work, we systematically define relationships between target receptor expression level, target receptor affinity, and in vivo activity of engineered AAV vectors. We report a high-throughput method for quantifying individual vector-receptor affinities and demonstrate that direct binding assays can be used to separate a library of vectors into families with various affinities for their target receptor. Our data indicate that efficient CNS transduction requires high levels of target receptor expression at the BBB, but it is not a requirement for receptor expression to be limited to the target tissue. We observed that enhanced receptor affinity leads to reduced transduction of off-target tissues but can negatively impact on-target cellular transduction and penetration of endothelial barriers. Together, this work provides a set of tools for defining vector-receptor affinities, as well as a map of how receptor expression and affinity interact to impact the overall performance of engineered AAV vectors upon systemic administration.
RESULTS
A comprehensive set of point mutations introduced in the PHP.B peptide generates a library of mutants with enhanced and diminished Ly6a binding affinities.
Given that Ly6a confers AAV-PHP.B with its enhanced BBB penetration properties, we set out to determine how modulating the strength of this capsid-receptor interaction would influence the localization and transduction profile of systemically administered AAV-PHP.B vectors. We generated a single-site saturating mutagenesis library spanning the 7-amino-acid TLAVPFK peptide insertion found in the AAV-PHP.B capsid between amino acids 588 and 596 (Fig. 1A). This library would be expected to contain a broad spectrum of Ly6a affinities, while avoiding modifications to the native AAV9 capsid backbone that could impact interactions with endogenous cellular trafficking receptors such as AAVR (43). Each of the 127 peptides in the library was encoded by a set of three distinct codon sequences to increase the number of biological replicates for each capsid variant that could be detected in our sequencing-based vector activity measurements. We produced vectors from either an inverted terminal repeat (ITR)-containing cis plasmid backbone for pooled vector library production or an ITR-free trans plasmid backbone for colony PCR screening, selection of individual mutants, and production of individual preparations that would be used for Ly6a affinity measurements (Fig. 1A).
FIG 1.
A comprehensive set of point mutations introduced in the PHP.B peptide generates a library of mutants with enhanced and diminished Ly6a binding affinities. (A) A library of oligonucleotides encoding all nonnative and noncystine single-site mutations in the PHP.B peptide was ordered and cloned into position 588 of either an AAV9 trans plasmid backbone or an ITR-containing AAV9 cis plasmid backbone. The trans plasmid library was screened using colony PCR to select plasmids for producing individual vector preparations and obtaining individual vector affinity measurements. The pooled cis plasmid library was used to produce a purified vector library for use in various assays. Vector performance in the library format could then be ranked using Illumina sequencing and further contextualized via individual vector affinity measurements. (B) Representative SPR sensorgrams generated from individually produced vector preparations. Kinetic parameters obtained using fits to a global 1:1 binding model are shown below the charts. (C) Plot of the relationship between KD values obtained using kinetics fitting via SPR and surface-saturation Rmax values obtained using BLI on a log-linear axis. Linear regression was performed to establish a standard curve, calculate KD values for each variant in the vector library, and obtain an R2 value. (D) Heat map summary of the impact of specified amino acid substitutions on Ly6a affinity. Colors signify the log10 transform of each vector’s calculated KD value normalized to the KD value of the native AAV-PHP.B sequence. Dark blue squares indicate a 100-fold increase relative to the native affinity; dark red squares indicate a 100-fold reduction in affinity. Gray boxes show variants with no measurable Ly6a affinity, and gray boxes with an X represent vectors for which binding affinities could not be determined. AAV, adeno-associated virus; BLI, bio-layer interferometry; ITR, inverted terminal repeat; SPR, surface plasmon resonance.
Due to the multivalent nature of vector-receptor interactions, measured equilibrium dissociation constant (KD) values obtained using techniques such as surface plasmon resonance (SPR) can be dependent on immobilized receptor levels on the sensor surface. Therefore, we took special care when performing our analyses to immobilize a consistent level of Ly6a in all experiments, so that we could reliably distinguish the relative receptor affinities of vectors selected from our library of mutants. Figure 1B shows representative sensorgrams for several of these affinity mutants, denoted as AAV-PB6, AAV-PB4, and AAV-PB1, with AAV-PHP.B as a control. The SPR data indicate that high-affinity vectors (AAV-PB6) were separated from low-affinity vectors (AAV-PB1) not only by their KD value, but also by their surface-saturating binding response, captured by the kinetic Rmax parameter. We suspected that this may arise from the multivalent nature of the AAV-PHP.B-Ly6a interaction and could be utilized as a relative ranking metric for receptor affinity. Thus, we adapted the SPR method to a bio-layer interferometry (BLI) platform, as this higher-throughput technique is better suited to the large number of individual affinity measurements needed for this study. The Rmax values obtained via BLI proved to be useful predictors of the relative KD measurements obtained by the SPR technique (Fig. 1C). We used this log-linear relationship as a type of standard curve to obtain calculated KD values for each of the Ly6a-binding variants in the vector library.
Figure 1D summarizes the relative Ly6a binding strength for 93.7% of the mutants in the vector library. Variants with improved or reduced affinity relative to AAV-PHP.B (44.1%) are indicated by blue or red shading, respectively, while nonbinding vectors (49.6%) are indicated by dark gray shading. Gray squares with an X (6.3%) indicate preparations for which the vector titer was too low to obtain meaningful affinity measurements. We observed that amino acids at the T, L, P, and F positions of the PHP.B peptide are the least tolerant of amino acid substitutions, whereas the A, V, and K residues are more amenable to modification. Grouping amino acid substitutions by size and charge properties also demonstrated some site-specific and site-independent trends. For the native V residue, substitutions of larger hydrophobic amino acids tended to improve receptor affinity, whereas acidic substitutions at any position were detrimental to receptor binding (Fig. 1D).
By individually assessing the impact of AAV amino acid substitutions on receptor binding, further lead-optimization efforts can be performed using focused combinatorial libraries designed to exclude irrelevant mutations, reducing library complexity and improving library activity in subsequent experiments.
A sequencing-based bead-binding assay can identify capsids with increased or decreased receptor binding affinity.
We next sought to develop an assay that can predict the relative receptor affinities of individual variants in a library of mutants using sequencing data alone. Such a method would offer a practical first step for lead optimization by filtering receptor binding from nonbinding sequences and could be validated using the comprehensive set of high-resolution individual affinity measurements we had collected using the BLI assay. We opted for a bead-based selection approach, wherein we incubated the AAV vector library with Ly6a-coated beads, performed several washes, and then allowed the bound library to dissociate from the beads in a neutral-pH solution. We generated amplicons from vector DNA in the (i) vector input, (ii) bead-bound, (iii) flowthrough, and (iv) elution fractions for Illumina sequencing to determine the number of reads per million (RPM) of each variant in each fraction. We calculated enrichment score metrics for bead association, supernatant depletion, and bead dissociation, each of which was successful in separating the vectors into broader-affinity subsets (see Fig. S1 in the supplemental material). The agreement between the sequencing and BLI data gave us confidence that our individual vector affinity measurements would be predictive of our library’s performance in a competitive binding context.
We found that the bead dissociation enrichment parameter provided the most consistent separation of vector variants into groups with differential affinity (Fig. 2). The top 50% of reads contained 75% of all Ly6a-binding variants, and 95% of the variants in the top 20% of reads had measurable Ly6a affinity. Furthermore, the top two deciles achieved statistically significant separations from each other at the P < 0.01 α level, with vectors in the top 10% having an average calculated KD of 5.72 × 10−11 M and vectors in the 10% to 20% bracket having an average calculated KD of 5.09 × 10−10 M.
FIG 2.
A sequencing-based bead-binding assay identifies capsids with increased or decreased receptor binding affinity. The pooled vector library was incubated with Ly6a-coated beads, and vector DNA was isolated from bead-bound and eluate fractions for analysis by Illumina sequencing. RPM values were used to calculate an enrichment score for each variant based on the mean of n = 3 sequencing replicates; scores were ranked from highest to lowest. Calculated vector KD values obtained using the BLI assay are plotted against the rank order of these enrichment values binned into deciles. Lines indicate population means for each decile, and error bars indicate the standard error of the mean. Enrichment scores corresponding to the native AAV-PHP.B sequence are indicated by an orange star and a horizontal dashed line. **, P < 0.01; *, P < 0.05; ns, not significant (P > 0.05). AAV, adeno-associated virus; BLI, bio-layer interferometry; RPM, reads per million.
Therefore, this sequencing-based approach was successful not only in separating receptor binding from nonbinding capsid mutants, but also in stratifying the binding sequences into groups with various overall affinities for the receptor target.
Vectors with receptor affinity tuned to the minimum level required for efficient cell surface binding elicit the most productive transgene expression.
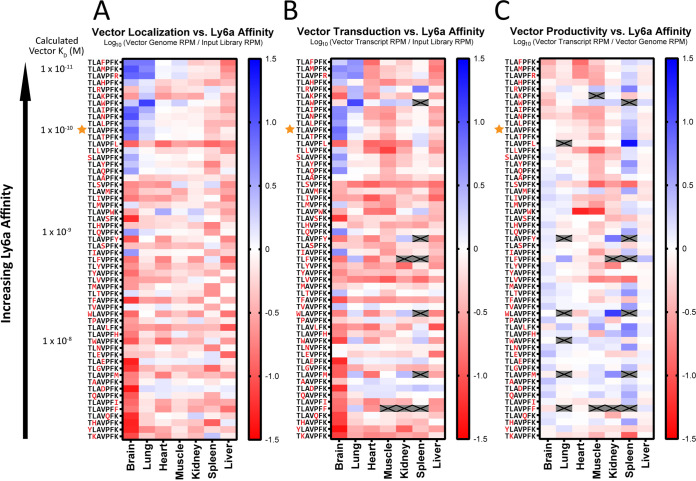
Having established that our individual affinity measurements would predict vector binding in a competitive context, we applied our vector library in vitro to assess the impact of receptor affinity on the cellular binding and transduction properties of AAV vectors. We incubated our library of AAV vectors with control HEK293 cells and HEK293 cells stably expressing the Ly6a receptor and generated amplicons from the (i) input vector library, (ii) cellular DNA, or (iii) cellular RNA for Illumina sequencing analysis. We plotted enrichment scores for Ly6a-binding vectors as a function of their calculated KD values, with Ly6a-nonbinding vectors plotted on a separate axis as negative controls (Fig. 3). We fit generalized trends in the enrichment data using three-parameter logistic regression with a 95% confidence interval (CI) (Fig. 3A and B) or linear regression with a 95% CI (Fig. 3C), depending on whether the results demonstrated a sigmoid or linear relationship with vector affinity. Logistic fits provided an R2 value to assess the goodness of fit, in addition to a value for the fitted curve’s inflection point, which we refer to as KD50. Linear fits provided an R2 value to assess the goodness of fit, in addition to a slope used to determine the statistical significance of the relationship between the calculated vector KD and vector performance.
FIG 3.

Vectors with receptor affinity tuned to the minimum level required for efficient cell surface binding elicit the most productive transgene expression. The pooled vector library was incubated with HEK293-Ly6a cells before the vector input DNA, cell-associated DNA, and cellular RNA fractions were isolated for analysis using Illumina sequencing. RPM values were used to calculate (A) DNA enrichment, (B) RNA enrichment, and (C) RNA/DNA enrichment as metrics for cell binding, cell transduction, and vector productivity, respectively. Vectors with measurable Ly6a affinity were plotted based on their calculated KD values, and nonbinding vectors were plotted as negative controls. For results demonstrating a sigmoid relationship (A and B), three-parameter logistic regression with a 95% CI was used to obtain R2 and KD50 values. For results demonstrating a linear relationship (C), linear regression with a 95% CI was used to obtain an R2 value, a slope, and a P value (F test). Points represent the means of n = 3 replicates; error bars indicate the standard error of the mean. Enrichment scores corresponding to the native AAV-PHP.B sequence are indicated by orange stars. Vertical dashed lines indicate the affinity threshold for optimum vector expression. AAV, adeno-associated virus; CI, confidence interval; RPM, reads per million.
We first assessed vector DNA enrichment in HEK293 cells expressing the Ly6a receptor as a metric for cell surface binding (Fig. 3A). We observed that increased vector affinity led to increased vector binding up to a calculated KD value of ~3 × 10−9 M; beyond this point, increased affinity produced no change in vector localization to the cell surface. We analyzed our RNA enrichment data as a metric for vector transduction (Fig. 3B) and observed a similar affinity relationship. Once again, increased receptor affinity led to increased cellular transduction up to a threshold value of ~3 × 10−9 M, but beyond this point, AAV transduction was increasingly negatively impacted. To highlight this relationship and control for the confounding variable of vector localization on vector transduction, we normalized vector transcript RPMs to vector genome RPMs to develop a vector productivity score (Fig. 3C). With this analysis, we observed a linear, statistically significant (P < 0.0001) negative relationship between Ly6a affinity and the productivity of vector expression. We expect that this trend results from high-affinity vectors having a reduced capacity to dissociate from their membrane attachment receptor and successfully navigate subsequent endosomal trafficking steps.
Importantly, similar experiments using the negative-control cells (Fig. S2) demonstrated no overall trends in cell binding or transduction, increasing our confidence that the relationships we observed are due to the modulation of AAV-PHP.B-Ly6a affinity. Still, we noticed that a few Ly6a-binding and negative-control vectors did become specifically enriched on the surface of negative-control cells (Fig. S2A and B). These vectors often had basic substitutions that increase the net positive charge of the inserted peptide (data not shown), which suggests that the biophysical properties of the AAV capsid can also influence binding to the negatively charged cell membrane in a receptor-independent manner.
Taken together, these data establish a type of threshold effect for receptor-mediated AAV transduction, wherein vector expression increases with vector localization up to a threshold value, but beyond this point, additional receptor affinity can negatively impact vector transduction efficiency.
Ly6a expression is not restricted to the vascular beds of the CNS.
Before testing our library of affinity mutants in vivo, we set out to define the endothelial expression level of Ly6a in murine brain, lung, heart, muscle, kidney, spleen, and liver tissues. For these organs of interest, we examined several publicly available single-cell RNA sequencing data sets that quantitated Ly6a expression levels and provided the cell-type specificity of this expression in various murine tissues (44–46). Of these, we found the data set provided by the Tabula Muris Consortium (44) to be the most comprehensive, and these data were used to generate Table 1. Endothelial cells were the primary cell type expressing Ly6a in brain and lung tissue, and these organs had the highest levels of endothelial-specific Ly6a expression across our tissues of interest, with median normalized RPM values of ≥8.60. Heart, muscle, and kidney tissue had the next highest levels of endothelial Ly6a expression, with median normalized RPM values of 8.10, 7.74, and 8.29, respectively. Each of these tissues also had Ly6a expression detected at various levels in nonendothelial cell types, such as fibroblasts in heart tissue, mesenchymal stem cells in muscle tissue, and epithelial cells of the collecting ducts in kidney tissue. In spleen and liver tissue, Ly6a expression was primarily detected in immune cells, although liver tissue demonstrated some Ly6a expression in endothelial cells of the hepatic sinusoid. These tissues had the lowest level of endothelial Ly6a expression, however, with a median RPM value of 6.02 in liver tissue and undetectable Ly6a expression in the endothelium of spleen tissue.
TABLE 1.
Ly6a expression levels and cell specificity as reported in the Tabula Muris databasea
| Tissue | Cell type | Counts/million |
No. of cells | % of cells |
|||
|---|---|---|---|---|---|---|---|
| Mean | Median | Median positive | Negative | Positive | |||
| Brain | Endothelial cells | 8.42 | 8.60 | 8.62 | 715 | 1.12 | 98.88 |
| Brain pericytes | 0.65 | 0.00 | 2.29 | 156 | 74.36 | 25.64 | |
| Astrocytes | 0.37 | 0.00 | 1.22 | 432 | 76.62 | 23.38 | |
| Neurons | 0.33 | 0.00 | 1.07 | 281 | 76.87 | 23.13 | |
| Oligodendrocytes | 0.27 | 0.00 | 0.91 | 1,574 | 78.91 | 21.09 | |
| Oligodendrocyte precursor cells | 0.24 | 0.00 | 0.96 | 203 | 79.31 | 20.69 | |
| Bergmann glial cells | 0.21 | 0.00 | 0.87 | 40 | 80 | 20 | |
| Lung | Endothelial cells | 7.79 | 8.67 | 8.76 | 693 | 8.66 | 91.34 |
| B cells | 3.08 | 1.83 | 6.52 | 57 | 47.37 | 52.63 | |
| Stromal cells | 2.87 | 0.50 | 6.49 | 423 | 46.57 | 53.43 | |
| Ciliated columnar cells of tracheobronchial tree | 2.80 | 2.48 | 4.40 | 25 | 20.00 | 80.00 | |
| T cells | 2.03 | 0.00 | 5.64 | 53 | 54.72 | 45.28 | |
| Epithelial cells | 1.32 | 0.00 | 4.06 | 113 | 63.72 | 36.28 | |
| Natural killer cells | 0.91 | 0.00 | 2.59 | 37 | 72.97 | 27.03 | |
| Monocytes | 0.81 | 0.00 | 2.06 | 65 | 72.31 | 27.69 | |
| Classical monocytes | 0.71 | 0.00 | 0.88 | 90 | 58.89 | 41.11 | |
| Myeloid cells | 0.69 | 0.00 | 0.93 | 85 | 62.35 | 37.65 | |
| Leukocytes | 0.30 | 0.00 | 1.21 | 35 | 77.14 | 22.86 | |
| Heart | Endothelial cells | 7.69 | 8.10 | 8.12 | 1,177 | 2.46 | 97.54 |
| Fibroblasts | 3.13 | 1.23 | 5.94 | 2,119 | 38.60 | 61.40 | |
| Cardiac muscle cells | 1.36 | 0.00 | 3.66 | 133 | 61.65 | 38.35 | |
| Leukocytes | 0.77 | 0.00 | 1.09 | 523 | 69.79 | 30.21 | |
| Endocardial cells | 0.49 | 0.00 | 0.96 | 165 | 66.67 | 33.33 | |
| Myofibroblast cells | 0.45 | 0.00 | 1.30 | 178 | 78.09 | 21.91 | |
| Smooth muscle cells | 0.43 | 0.00 | 0.92 | 42 | 76.19 | 23.81 | |
| Muscle | Mesenchymal stem cells | 7.34 | 7.66 | 7.67 | 258 | 1.16 | 98.84 |
| Endothelial cells | 7.19 | 7.74 | 7.82 | 141 | 3.55 | 96.45 | |
| B cells | 3.21 | 1.88 | 6.15 | 71 | 38.03 | 61.97 | |
| T cells | 1.83 | 1.02 | 2.17 | 35 | 48.57 | 51.43 | |
| Macrophages | 1.22 | 0.50 | 1.19 | 45 | 44.44 | 55.56 | |
| Skeletal muscle satellite cells | 0.40 | 0.00 | 0.94 | 540 | 66.85 | 33.15 | |
| Kidney | Endothelial cells | 7.05 | 8.29 | 8.36 | 126 | 10.32 | 89.68 |
| Kidney collecting duct epithelial cells | 6.42 | 7.83 | 8.04 | 121 | 15.70 | 84.30 | |
| Epithelial cells of proximal tubule | 1.93 | 0.00 | 6.06 | 219 | 60.27 | 39.73 | |
| Leukocytes | 0.97 | 0.00 | 3.51 | 16 | 75.00 | 25 | |
| Macrophages | 0.75 | 0.00 | 1.12 | 37 | 70.27 | 29.73 | |
| Spleen | B cells | 3.92 | 5.30 | 6.83 | 1,297 | 35.54 | 64.46 |
| T cells | 2.31 | 0.00 | 6.03 | 352 | 51.99 | 48.01 | |
| Macrophages | 0.29 | 0.00 | 1.21 | 48 | 81.25 | 18.75 | |
| Liver | Natural killer cells | 4.80 | 6.21 | 6.70 | 39 | 23.08 | 76.92 |
| Endothelial cells of hepatic sinusoid | 4.38 | 6.02 | 6.73 | 182 | 29.12 | 70.88 | |
| Kupffer cells | 3.61 | 3.63 | 6.25 | 61 | 34.43 | 65.57 | |
| B cells | 3.56 | 5.02 | 6.47 | 41 | 41.46 | 58.54 | |
| Hepatocytes | 0.54 | 0.00 | 1.06 | 391 | 66.75 | 33.25 | |
Single-cell RNA sequencing data were reproduced from the Tabula Muris database for cell-type-specific expression of Ly6a in various tissues. The “Mean,” “Median,” and “Median positive” values represent natural log-transformed counts per million data for Ly6a in the specified tissue and cell type. Values in the “No. of cells,” “% of cells negative,” and “% of cells positive” columns are based on cell counts in the specified tissue and cell type and the percentage of cells that are positive or negative for Ly6a.
To corroborate these RNA sequencing data, we used immunofluorescence to detect Ly6a expression at the protein level in our tissues of interest using two histological techniques. We first performed Ly6a staining (green) in WT C57BL/6J mice and C57BL/6J Ly6a knockout mice with an antibody optimized for use in frozen sections (Fig. 4A), but due to technical limitations, we were unable to perform an endothelial costain in these samples. Using a different pairing of Ly6a (green) and PECAM-1 (red) antibodies in paraffin sections, we effectively demonstrated the colocalization of Ly6a with endothelial cells (Fig. 4B). To contend with the contributions of red blood cell autofluorescence to our colocalization signal, we also included a blank channel for each image (cyan). Therefore, in merged panels, true Ly6a–PECAM-1 colocalization is shown in yellow, whereas autofluorescence is shown in white.
FIG 4.
Ly6a expression is not restricted to the vascular beds of the CNS. (A) Fixed-frozen sections of the indicated tissues were prepared from C57BL/6J and C57BL/6J Ly6a−/− mice to assess Ly6a expression using immunofluorescence. Ly6a staining is shown in green. Images were acquired using a 20× objective and a 4-s exposure time. (B) Paraffin-embedded tissue sections of the indicated tissues were prepared from C57BL/6J mice to assess Ly6a colocalization with a PECAM-1 endothelial marker using coimmunofluorescence. Ly6a staining is shown in green, and PECAM-1 staining is shown in red. A blank channel is included in cyan to illustrate the contribution of autofluorescence to the merged images. Images were acquired using a 40× objective. Exposure times were optimized for each tissue independently to demonstrate Ly6a and PECAM-1 colocalization in a qualitative fashion. CNS, central nervous system; WT, wild type.
Using either histological technique, we detected Ly6a expression in the endothelium of brain tissue (Fig. 4A and B), consistent with previous reports (20, 21). In lung tissue, we detected endothelial Ly6a expression in both the microvasculature of the alveoli, which constitutes most of the lung parenchyma, and in larger PECAM-1-positive vessels (Fig. 4B). Thus, as in brain tissue, lung tissue exhibits endothelial Ly6a expression that is primed to interact with circulating AAV-PHP.B particles. In heart and muscle tissue, we could detect Ly6a expression at the protein level, depending on the histological technique used. In the frozen sections (Fig. 4A), Ly6a expression that appears endothelial by morphology can be observed, but this expression could not be confirmed via endothelial costaining in the paraffin sections (Fig. 4B). In kidney tissue, Ly6a expression was primarily detected in epithelial cells of the nephrons, separate from PECAM-1 staining in the microvasculature, but could also be found colocalized with PECAM-1 in larger vessels (Fig. 4B). In spleen tissue, Ly6a expression was observed in a cluster of Ly6a-positive cells but was once again distinct from the endothelial marker (Fig. 4B). Importantly, we did not detect Ly6a expression in the liver, the primary organ transduced by WT AAV9 vectors upon systemic delivery (7), using either technique (Fig. 4A and B). These results establish the liver as a true off-target organ for AAV-PHP.B vectors, where transduction is driven by Ly6a-independent effects.
By combining the results of these orthogonal techniques, we establish a profile for Ly6a expression in several tissues of therapeutic interest. In brain and lung tissue, sequencing data suggest that Ly6a is highly expressed in endothelial cells, and this expression can be readily detected at the protein level using either histological method. In heart and muscle tissue, sequencing data suggest that endothelial Ly6a expression occurs at an intermediate level, and this expression can be detected by histology, depending on the method used. In kidney tissue, costaining data demonstrate that Ly6a expression is primarily nonendothelial, with some expression in large vessels that is also captured in the RNA sequencing data. Spleen and liver tissues exhibit the lowest levels of endothelial Ly6a expression by all metrics.
Endothelial layers in different tissues have distinct affinity requirements for vector localization and expression.
We then applied our vector library in vivo to better understand the impact of receptor affinity on AAV localization and transduction in tissues with varied levels of endothelial Ly6a expression. We administered 1.27 × 1012 genome copies (GC) of our library to a group of C57BL/6J mice (n = 4) and isolated vector DNA and RNA from brain, lung, heart, muscle, kidney, spleen, and liver tissue after 14 days for amplicon generation and analysis via Illumina sequencing. Enrichment scores are summarized in heat maps to simplify comparisons of vector performance across tissues (Fig. 5) and are also provided as quantitative plots with logistic regression fits in the supplemental material (Fig. S3 to S5).
FIG 5.
Endothelial layers in different tissues have distinct affinity requirements for vector localization and expression. C57BL/6 mice (n = 4) were treated i.v. with a library of vectors at 1.27 × 1012 total vector genomes per mouse. At 14 days postinjection, mice were sacrificed and tissue DNA and RNA fractions were isolated from the indicated organs for analysis using Illumina sequencing. RPM values were used to calculate (A) DNA enrichment, (B) RNA enrichment, and (C) RNA/DNA enrichment as metrics for vector localization, vector transduction, and vector productivity, respectively. The heat map list vectors ordinally by Ly6a affinity, with the highest-affinity vectors at the top and the lowest-affinity vectors at the bottom. Colors signify the log10 transform of each enrichment score and represent the means of n = 12 replicates. Dark blue squares indicate a 30-fold increase in enrichment relative to the input vector library; dark red squares indicate a 30-fold decrease in enrichment relative to the input vector library. Gray boxes with an X indicate enrichment scores that could not be determined. Enrichment scores corresponding to the native AAV-PHP.B sequence are indicated by orange stars. AAV, adeno-associated virus; i.v., intravenous; RPM, reads per million.
We first assessed vector DNA enrichment in each tissue as a metric of vector localization (Fig. 5A). In brain tissue, we observed localization trends comparable to those observed in our cell binding data, wherein increased receptor affinity produces increases in vector enrichment up to a threshold value close to the native affinity of the PHP.B peptide, with no enhancement of receptor-mediated binding beyond the threshold. In lung tissue, this affinity threshold appeared to be higher than that of brain tissue, with a calculated KD value near 1 × 10−11 M. This finding was interesting given that single-cell data suggested both organs have a comparable level of endothelial Ly6a expression and indicates that other biological factors may work in combination with the receptor expression level to establish the localization threshold for a given tissue. In heart, muscle, and kidney tissue, where Ly6a may be expressed in the endothelium but at a reduced level relative to brain and lung tissue, our library of mutants did not contain vectors with sufficient affinity to impact tissue localization. Vector affinity and localization relationships were also absent in spleen tissue, which is simply explained by the lack of endothelial Ly6a expression detected in this organ. In liver tissue, we observed an inverse relationship between Ly6a affinity and liver transduction, wherein vectors with increasing receptor affinity become progressively liver detargeted. This relationship appears to follow a simple model, wherein increased receptor affinity leads to increased vector localization to on-target tissues, reducing the pool of vector available for eventual uptake by liver cells through a Ly6a-independent mechanism.
We next assessed RNA enrichment in each tissue using our established metrics for vector transduction (Fig. 5B) and vector productivity (Fig. 5C). The results in brain tissue mirror the findings from our in vitro cell-based assay, where vectors with receptor affinities beyond the localization threshold value demonstrate a progressive reduction in RNA expression. In lung tissue, most vectors in the library have receptor affinities below the lung localization threshold, where increases in localization are still well correlated with increases in transduction. In heart and muscle tissue, we observed that while endothelial Ly6a expression may not be sufficient to impact vector delivery, it may be sufficient to negatively impact vector expression. In our interpretation, vectors that would otherwise be delivered to muscle cells in these tissues are instead directed to Ly6a-expressing endothelial cells, where they establish comparatively less productive transduction events. In kidney, spleen, and liver tissue, biodistribution and transduction are generally well correlated, producing no trends in kidney or spleen tissue and an inverse relationship between receptor affinity and expression in liver tissue.
These results extend the concept of affinity thresholds for vector targeting from our in vitro experiments to our in vivo experiments, wherein tissues with different levels of endothelial Ly6a expression display different affinity thresholds for localization, and demonstrate that increasing receptor affinity beyond the threshold required for effective localization negatively impacts the efficiency of transgene expression.
Vectors dosed individually corroborate localization and transduction trends observed in library data.
Finally, we set out to determine whether the affinity relationships established using our vector library would be preserved when vectors are applied individually at a therapeutically relevant dose. We chose eight variants with mutations at the valine amino acid position and subnanomolar calculated KD values for testing alongside AAV9 and AAV-PHP.B controls. We produced purified preparations of these vectors, individually measured their KD values using SPR, and administered 1 × 1012 GC per mouse (n = 3 per group) i.v. After 21 days, we assessed the primary on-target (brain) and off-target (liver) organs for affinity trends in vector biodistribution by quantitative PCR (qPCR) and vector expression by native green fluorescent protein (GFP) fluorescence (Fig. 6A to C).
FIG 6.
Vectors dosed individually corroborate localization and transduction trends observed in library data. (A to C) C57BL/6 mice were treated i.v. with a panel of 10 affinity mutants at 1 × 1012 vector genomes per mouse. At 21 days postinjection, mice were sacrificed, and brain and liver tissues were analyzed by qPCR for vector genomes and native GFP fluorescence for vector expression. Biodistribution data are reported for (A) brain and (B) liver tissue, with values corresponding to AAV-PHP.B indicated by orange stars. Points represent an average of n = 3 replicates, and error bars indicate the standard error of the mean. (C) Representative images showing GFP expression in fixed-frozen sections for brain and liver tissue. Fluorescence images were acquired using a 4× objective and 2-s exposure. (D to F) C57BL/6 mice were treated i.v. with a reduced panel of affinity mutants at 1 × 1012 vector genomes per mouse. At 14 days postinjection, mice were sacrificed, and brain, lung, heart, muscle, and liver tissues were analyzed by qPCR, RT-qPCR, and anti-GFP IHC. Molecular data are reported in panel D as vector GC per diploid cell and in panel E as vector transcripts per microgram of RNA. Points represent the means of n = 3 replicates; error bars indicate the standard error of the mean. Numeric labels represent positive or negative fold changes relative to AAV9. **, P < 0.01; *, P < 0.05; ns, not significant. (F) Representative IHC for GFP expression using paraffin-embedded sections in lung tissue. Black arrowheads indicate large blood vessels as identified by morphology. Bright-field images were acquired with a 20× objective and a 20-ms exposure time. AAV, adeno-associated virus; BD, biodistribution; GC, genome copy; GFP, green fluorescent protein; IHC, immunohistochemistry; i.v., intravenous; qPCR, quantitative PCR; RT-qPCR, reverse transcription-quantitative PCR.
We found that the individual vector injections produced trends that mirrored those observed in the in vivo library data. AAV-PHP.B and each of the mutants over a range of subnanomolar affinities showed comparable localization to brain tissue (Fig. 6A), and on average, the biodistribution of these variants was improved 38.6-fold over AAV9. To address the possibility that these observations resulted from saturation of the Ly6a receptor at the BBB, we analyzed brain biodistribution data from an analogous experiment with the same set of vectors at 1 × 1011 GC/mouse (data not shown), which produced the same localization trends as in Fig. 6A. In liver tissue, we observed an inverse relationship between vector affinity and liver localization (Fig. 6B), with the highest-affinity variant achieving a 51.0-fold reduction in liver biodistribution compared with AAV9. Trends in native GFP fluorescence for vector expression also corroborated trends observed in the in vivo library data (Fig. 6C). Liver transduction correlated with vector biodistribution, with the highest-affinity vectors achieving the lowest levels of liver expression. At the BBB, high-affinity vectors were at a disadvantage relative to vectors closer to the affinity localization threshold in establishing productive transduction of the brain parenchyma. We hypothesize that at the BBB, vectors with high receptor affinity remain confined to endothelial cells, where they establish relatively unproductive transduction events, whereas vectors with lower affinity are better able to dissociate from attachment receptors, penetrate the BBB, and establish productive expression in CNS tissue.
We performed a second experiment to examine how receptor affinity tuning impacts delivery to peripheral organs such as lung, heart, and muscle tissue, where endothelial Ly6a had been detected and we had observed some affinity trends in our in vivo library analysis. We further reduced the panel of AAV vectors to include AAV9 and AAV-PHP.B controls alongside AAV-PB6, the highest-affinity variant in our library. We performed a 14-day study with 1 × 1012 GC i.v. per mouse and assessed vector biodistribution by qPCR and vector expression by reverse transcription (RT)-qPCR and anti-GFP immunohistochemistry (IHC) (Fig. 6D to F).
In control brain and liver tissues, biodistribution data (Fig. 6D) and RT-qPCR data (Fig. 6E) generally reproduced the localization and expression trends reported in Fig. 6A to C. In lung tissue, our qPCR data also corroborated trends observed in our in vivo library data, indicating that AAV-PB6, but not AAV-PHP.B, can achieve improved vector localization to this organ. This improvement in localization was modest, however, producing a 4-fold improvement over AAV9 delivery that did not lead to meaningful increases in vector RNA expression, as was suggested by the library data. We also confirmed the observed library trends in heart and muscle tissue, where the Ly6a-binding vectors did not achieve enhanced delivery to either tissue, but AAV-PB6 saw a significant decrease in heart transduction, and both Ly6a-binding vectors had reduced expression in muscle. This finding suggests that unlike in brain tissue, where interactions with endothelial cells are a requirement for penetrating the BBB, engineering endothelial-targeted vectors for heart and muscle transduction may not be necessary and could in fact be counterproductive.
Given that we had seen a positive relationship between Ly6a targeting and vector localization to lung tissue, we sought to further assess vector transduction in this organ using IHC (Fig. 6F). We observed an increase in transgene expression in the endothelial cells of large blood vessels for AAV-PHP.B and a further increase in expression within these vessels for AAV9-PB6 variants. Notably, these large vessels produced some of the brightest immunofluorescence staining for Ly6a in the histology shown in Fig. 4. We conclude that while increasing receptor affinity may improve vector delivery to lung tissue via endothelium-expressed Ly6a, this increased receptor binding may also cause an unproductive retention of vectors within endothelial cells rather than supporting transduction of the lung parenchyma.
DISCUSSION
In this work, we took a systematic and quantitative approach to analyze how the strength of attachment receptor binding impacts the biodistribution, endothelial penetration, and transduction properties of AAV vectors after systemic administration. In so doing, we developed methods of practical utility for quantitatively ranking the affinities of individual AAV-receptor interactions and selecting vectors with a range of receptor affinities from a single-site evaluation library. We used a combination of histological and single-cell RNA sequencing data to define Ly6a expression level and cell type specificity in various tissues and found that it is not simply the presence or absence of a target receptor, but its relative endothelial expression level, that influences effective AAV localization to a tissue of interest. We demonstrated that affinity tuning of a defined vector-receptor interaction can produce significant changes in a vector’s systemic transduction profile, with the counterintuitive finding that in vivo activity optima and receptor affinity optima are not necessarily coincident.
We propose that systemic AAV targeting follows a threshold affinity model, wherein vectors with receptor affinities greater than or equal to a tissue-specific threshold value localize to target tissues equivalently. It has been suggested in the literature that the affinity threshold effect we observe in our experiments is an intrinsic property associated with multivalent receptor engagement (35, 39). We posit that the most important factor in determining this affinity threshold is the expression level of attachment receptors found in the endothelium of each organ, where direct interactions with circulating AAV vectors are most likely. This is evidenced by high-affinity AAV-PHP.B vectors attaining improved localization to brain and lung tissue, where endothelial Ly6a is highly expressed, but not heart, muscle, and kidney tissue, where Ly6a is found at a reduced endothelial level or predominantly in nonendothelial cell types. The observed difference in affinity thresholds between brain and lung tissue was somewhat surprising, given our histology data and our analysis of several single-cell RNA sequencing data sets suggesting that the levels of Ly6a expression per endothelial cell was comparable in both tissues (44–46). It is possible that capillaries in lung tissue, which receive the entirety of the cardiac output in each heartbeat (47), experience higher linear flow rates than the capillaries of the CNS. This feature has been shown to increase the receptor affinity threshold of multivalent particle-receptor interactions (38). Differences in luminal versus apical Ly6a presentation in brain and lung tissue may also account for these different thresholds, as could differences in Ly6a-associated transport pathway activities between the two tissue types. While further research may be required to definitively establish the mechanism, we believe that the simplest explanation for the systemic biodistribution data presented herein is that the receptor expression level in combination with other biophysical parameters dictates the localization properties of AAV particles.
We also demonstrated that the endothelial penetration and overall transduction activities of AAV vectors are optimized when receptor affinity is tuned to the minimum level required for efficient localization to a tissue of interest. In our cell transduction experiments, we found a steady decrease in the productivity of AAV transgene expression as the target receptor affinity increased, and our in vivo data demonstrated an analogous relationship between affinity and a vectors’ BBB penetration properties. We expect that in both cases, increases in receptor affinity result in a lower off-rate from the membrane attachment receptor, which negatively impacts subsequent endosomal trafficking steps. This model may also explain the transduction patterns of AAV9-PB6 we observed in lung tissue, wherein high-affinity vectors achieved improved lung localization, but this increased affinity also resulted in the unproductive retention of these vectors within endothelial cells. Other groups studying the delivery of antibody therapeutics (48) or nanoparticles (49) through the BBB have reported the most success when using antibodies with reduced affinity for their transferrin receptor target and have posed a similar explanation for their findings. It may also be possible that the avid engagement of multiple Ly6a receptors could produce receptor clustering that is important for AAV-PHP.B internalization and that low-affinity vectors are better suited to initiating this response than their high-affinity counterparts. Regardless of the mechanism(s) at play, our data indicate that increasing AAV-receptor affinity beyond what is necessary for tissue localization only serves to negatively impact the transduction efficiency of AAV vectors in on-target tissues.
Given clinical observations of liver toxicity associated with systemically administered AAVs, development of methods for liver detargeting has become an area of focus in the field (11–13). Intuitively, AAV targeting to novel receptors in peripheral organs should reduce liver transduction, but our results are the first to define how the strength of this detargeting correlates with receptor affinity. Importantly, we demonstrated that the receptor affinity optimum for liver detargeting is distinct from the affinity optimum for CNS transduction and that researchers may need to consider these properties independently when performing library selections or capsid optimizations. Depending on the therapeutic application, capsid engineers may seek to optimize BBB penetration at the expense of increased liver transduction or accept a reduced level of on-target activity to improve tissue specificity. In the end, utilizing receptor affinity tuning in combination with liver-detargeting capsid mutations (50–52) and tissue-specific transgene cassettes (53–55) may be necessary to obtain capsids with simultaneously optimized on-target and off-target activities.
Given these observations, we argue that it is unrealistic to expect an AAV capsid optimized for a complex quality such as BBB penetration in a particular model organism to demonstrate the same optimized activity in a separate model organism with distinct biology. Even for cases in which an AAV’s target receptor is expressed in both models, species-specific differences in vascular biology, target receptor amino acid sequence, and target receptor expression level are likely to impact the affinity of a given AAV-receptor interaction, influencing vector performance. To improve translatability, hits identified in preliminary animal models should be linked to a target receptor as quickly as possible, through either CRISPR-mediated screens (30, 56, 57) or other rational approaches (29, 31, 32). Using the methods described here, hit sequences can then be diversified and tested for binding affinity to murine, macaque, and human versions of target receptors. Candidate vectors spanning a range of receptor binding affinities and with comparable binding activities across species can then be tested in model organisms with improved confidence in their eventual translatability. Affinity data introduce an explanatory variable that aids in the interpretation of on-target and off-target activities, ensures that vectors with a diversity of properties are tested, and can be used to define vector activity optima. An interesting area of future research would be to apply these methods to the study of native capsid interactions with AAVR (57) or GPR108 (56) as well as engineered capsid interactions with integrins (29), major histocompatibility complex class I (MHC-I) (30), CA4 (31), or Ly6c1 (32) to determine whether the affinity relationships we observe with the Ly6a receptor are maintained or vary based on target receptor type.
In this work, we have provided a set of tools that can be used to optimize vector candidates based on the quantifiable biophysical property of receptor affinity, in addition to providing a first look at how this property impacts systemic AAV vector delivery. By improving our understanding of the mechanistic relationships at play in AAV targeting, we believe that this study represents a crucial step toward finally translating the gains of precision AAV engineering to humans.
MATERIALS AND METHODS
Vector library production.
A library of AAV9 capsid insert sequences was designed in silico, obtained as single-stranded oligonucleotides from Integrated DNA Technologies, and introduced to linearized and gel-purified AAV9 cis or trans plasmids at the amino acid 588 position. We submitted pooled cis plasmids to the Penn Vector Core at the University of Pennsylvania for AAV library production. This method uses a triple-transfection technique described previously (58) with a modified pAdΔF6/pRep (encoding AAV2 Rep gene)/library DNA plasmid ratio (weight) of 2:1:0.001. We submitted pooled trans plasmids to Azenta Life Sciences for colony PCR screening and for the preparation of individual plasmids encoding capsid sequences for individual library mutants.
AAV vector production.
All AAV vectors for in vivo studies and SPR measurements were produced by the Penn Vector Core at the University of Pennsylvania as previously described (59). Briefly, trans plasmids encoding capsid sequences for specific library mutants were used to generate vectors packaging a cis plasmid expressing enhanced GFP (eGFP) from the chicken β-actin (CB7) promoter, including the WPRE sequence. Individual researchers produced unpurified preparations of AAV vectors for BLI measurements in 15-cm dishes using a similar protocol.
Recombinant Ly6a-hIgG1 expression.
Ly6a-hIgG1 recombinant protein (amino acids 1 to 111 of the Ly6a protein fused via a tobacco etch virus [TEV] protease-cleavable linker to the human immunoglobulin G1 Fc chain) was produced by Genscript Biotech Corporation following their protocols. The recombinant protein product was purified by affinity chromatography using MabSelect SuRe LX (Cytiva Life Sciences), and the final protein concentration was determined by a bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific).
SPR binding assays.
We performed SPR binding analysis on a Biacore T200 instrument (GE Healthcare) at room temperature in HBS-EP(+) buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, and 0.05% P20 surfactant) (Cytiva) using a CM5 sensor chip (Cytiva) and a human antibody capture kit (Cytiva). After immobilization of the capture antibody, Ly6a-hIgG1 was diluted to 90 nM in HBS-EP(+) and introduced to the flow cells at 10 μL/min for 5 min to capture ~2,800 to 3,000 response units of Ly6a-hIgG1 on the sensor surface in each cycle. We measured AAV affinity mutant binding to this surface at concentrations ranging from 1.25 nM (7.53 × 1011 GC/mL) to 156 pM (9.39 × 1010 GC/mL). Regeneration between binding cycles used 10 mM glycine (pH 1.5) injected at a flow rate of 60 μL/min for 1 min. We performed kinetics fits using a global 1:1 binding model.
BLI binding assays.
We performed BLI binding analysis on an Octet HTX instrument (Sartorius) at room temperature in Octet kinetics buffer (Sartorius) using anti-human Fc capture biosensors (Sartorius). Ly6a-hIgG1 was diluted to 50 nM in kinetics buffer and loaded onto sensor tips for 15 min at 1,000 RPM to produce a 1 nM shift in sensor binding. We measured the binding of crude preparations of AAV affinity mutants to this surface using an 8-point series of 2-fold dilutions in kinetics buffer starting at each vector’s neat concentration. Starting concentrations ranged from 470 pM (2.83 × 1011 GC/mL) to 21.4 pM (1.29 × 1010 GC/mL), depending on the titer of the vector preparation. Vectors with titers of <1 × 1010 GC/mL were excluded from analysis. We performed kinetics fits to determine Rmax values using a global 1:1 binding model.
Bead binding assay.
The HaloTag protein purification system was used to generate Ly6a-coated beads for the competitive library binding assay (Promega). We generated a Ly6A-HaloTag expression vector by cloning amino acids 1 to 111 of the native Ly6a sequence into pHTC HaloTag CMV-neo (Promega). This vector was transfected into HEK293 cells, and Ly6a-HaloTag protein was expressed and loaded onto magnetic HaloTag beads (Promega) as per the manufacturer’s protocol. We incubated the Ly6a-coated beads with the vector library at a concentration of 1.27 × 1012 GC/mL for 2 h at room temperature. After 2 h, the flowthrough was collected, and vector-bound beads were washed three times with HaloTag purification buffer. Vectors were then allowed to dissociate from the beads in HaloTag purification buffer at room temperature for 1 h, and the bead and eluate fractions were collected. We then isolated vector DNA from the input vector library, flowthrough, bead, and eluate fractions using the Purelink viral RNA/DNA minikit (Invitrogen) according to the manufacturer’s protocols.
Cell lines.
We produced HEK293 cells stably expressing Ly6a (HEK293-Ly6a) by transfection with a plasmid containing the Ly6a complementary DNA (cDNA) sequence under the control of the cytomegalovirus (CMV) promoter and a neomycin resistance gene. Stably transfected cells were selected and maintained using growth medium supplemented with 600 μg/mL G418 sulfate solution (Millipore Sigma). We screened cells for Ly6a expression by Western blotting using a Ly6a primary antibody (Thermo Fisher; 701919).
Cell binding and transduction assay.
The pooled library of affinity mutants was incubated with HEK293 and HEK293-Ly6a cells at a concentration of 1.27 × 1012 GC/mL for 2 h at 37°C. After the incubation period, we collected the supernatant fractions to isolate vector DNA using the Purelink viral RNA/DNA minikit (Invitrogen) according to the manufacturer’s protocols. Cells were then either washed three times with phosphate-buffered saline (PBS) and processed directly to obtain cell-associated DNA as described below or replenished with fresh medium and allowed to incubate for an additional 48 h before being processed to obtain total RNA as described below.
Mice.
All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. We purchased male C57BL/6J mice from The Jackson Laboratory. We housed the mice in standard caging, with 3 to 4 animals per cage. We maintained an automatic 12-h light/dark cycle and provided water and irradiated laboratory rodent food ad libitum.
In vivo studies.
In studies using the AAV vector library, male C57BL/6 mice 6 to 8 weeks of age received 1.27 × 1012 GC (5.1 × 1013 GC/kg of body weight) of a pooled library of AAV-PHP.B mutants via tail vein injection. In studies using individual vectors, male C57BL/6 mice 6 to 8 weeks of age received 1 × 1012 GC (4 × 1013 GC/kg) of a single capsid vector encoding CMV-eGFP via tail vein injection. All mice were necropsied, and tissues were harvested 14 or 21 days postinjection.
Vector DNA and mRNA isolation from cells and in vivo tissues for amplicon generation, biodistribution, and RT-qPCR analysis.
Cell samples were freeze-thawed three times in G2 buffer (Qiagen) for DNA isolation or lysed directly in TRIzol reagent (Thermo Fisher) for RNA isolation. Tissue samples were snap-frozen at the time of necropsy. We extracted DNA using either the QIAamp DNA minikit for brain, lung, kidney, spleen, and liver tissues or the 20/G Qiagen Blood & Cell Culture DNA kit for heart and muscle tissues and cell samples (Qiagen). DNase-treated total RNA was isolated from cells or 100 mg of tissue using TRIzol reagent (Thermo Fisher). We quantified RNA by spectrophotometry, and aliquots were reverse transcribed to cDNA using random primers. We detected and quantified vector GCs in extracted DNA and relative transgene transcript expression in cDNA by real-time PCR, as previously described (60, 61). Briefly, we quantified vector GC and RNA levels using primers and a probe designed against a vector-specific sequence.
Amplicon generation.
We generated amplicons to peptide insertions at position 588 of the AAV9 capsid sequence using amplicon sequencing in a manner similar to that described previously (62–64). Briefly, the region of interest was amplified by PCR. We then generated next-generation sequencing libraries from the PCR product, which were sequenced on a MiSeq instrument (Illumina). We used these sequences to generate counts of individual vector variants in various DNA or cDNA samples expressed as RPM.
Histology.
We fixed tissue samples overnight in 10% neutral buffered formalin. After a brief rinse in PBS, the samples were transferred to 15% sucrose in PBS for 1 to 2 h at 4°C, followed by incubation in 30% sucrose in PBS overnight at 4°C. Tissues for fixed-frozen sectioning were then frozen in OCT (optimum-cutting temperature) embedding medium (Tissue-Tek) and sectioned at 30 μm. Tissues for paraffin-embedded sectioning were embedded in paraffin and sectioned at 6 μm.
Fixed-frozen cryosections for native GFP fluorescence required no further processing and were mounted directly with Fluoromount G plus DAPI (4′,6-diamidino-2-phenylindole) (Electron Microscopy Services). We acquired images using a 4× objective and a 2-s exposure time.
Fixed-frozen cryosections for Ly6a immunofluorescence were air dried, washed twice for 5 min with PBST (0.02% Tween 20 in PBS), and incubated in blocking buffer (1% normal donkey serum in PBST) for 1 h. Ly6a primary antibody (Abcam; ab51317) was diluted 1:200 in blocking buffer and applied to the tissue sections overnight at 4°C. After washing with PBST (three 5-min washes), we incubated the sections with a secondary fluorescein isothiocyanate (FITC)-labeled antibody (Jackson ImmunoResearch; 712-095-153) diluted 1:100 in blocking buffer. After washing the sections with PBST (three 5-min washes), we mounted the sections with Fluoromount G plus DAPI. Images were acquired using a 20× objective and a 4-s exposure time.
Paraffin-embedded sections for anti-GFP IHC were processed directly using a Leica Bond Rx autostainer with Leica kit reagents following the standard instrument protocol for IHC. Primary antibody (Novus; NB100-1770) was used at 1:500 dilution with a 30-min incubation time and citrate buffer for antigen retrieval. We acquired images using a 20× objective and a 30-ms exposure time.
Paraffin-embedded sections for Ly6a–PECAM-1 coimmunofluorescence were dried by incubation at 60°C before treatment with xylenes and an ethanol gradient to rehydrate the samples. We then treated the slides using an IHC protocol with tyramide signal amplification according to references 65 and 66.
Briefly, we incubated the slides with 1× EDTA solution (Vector Labs) for 20 min at 100°C in a pressure cooker for antigen retrieval. Antigen-retrieved slides were blocked with Opal antibody diluent solution (OADS) (Akoya Biosciences) for 1 h at room temperature before an overnight treatment with Ly6a primary antibody (Thermo Scientific; 701919) diluted 1:500 in OADS in a humid chamber at 4°C. Slides were then washed with PBST (5 min) and treated with a horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch; 711-035-152) diluted 1:500 in OADS for 30 min at room temperature. This step was followed by a PBST wash (three 5-min washes) and a 30-min incubation with the Akoya Opal 520 fluorescent reagent diluted 1:500 in tyramide signal amplification buffer (Akoya).
The steps for antigen retrieval through signal development described in the above paragraph were then repeated using a PECAM-1 primary antibody (DiaNOVA; DIA-310) diluted 1:100, a horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch; 712-035-153) diluted 1:500, and the Akoya Opal 570 fluorescent reagent diluted 1:500. This procedure denatures previous antibodies used for detection but preserves the fluorophores that have been produced in the tissue. We acquired images using a 40× objective and optimized exposure times for each tissue to qualitatively display Ly6a–PECAM-1 colocalization.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism (version 9.0.2).
We performed between-group comparisons using a two-sample two-sided t test, with statistical significance assessed at the 0.05 level. A correction for multiple comparisons was performed using the two-stage step-up method of Benjamini, Krieger, and Yekutieli with a false-discovery rate of 1%.
Linear regression analysis was performed using GraphPad Prism’s straight-line, semilog line, or log-log-line model as necessary. We assessed the statistical significance of linear relationships at the 0.05 level using an additional sum-of-squares F test and plotted the resulting models with 95% confidence bands.
We performed logistic regression analysis using GraphPad Prism’s three-parameter (inhibitor) versus response or three-parameter (agonist) versus response models as necessary. The Hill slope was held constant at −1.0, and the baseline parameter was restricted to be greater than −2.0. Models returned a value for the fitted curve’s inflection point, which we refer to as KD50, and were plotted with 95% confidence bands.
Data availability.
All relevant data are provided within the article and supplemental material.
ACKNOWLEDGMENTS
We thank Joel Cassel at the Wistar Institute’s Molecular Screening and Protein Expression Facility for help with our SPR assays. Within the Gene Therapy Program at the University of Pennsylvania, we thank the Program for Comparative Medicine for research study support and the Histopathology Core, including Jing-Xu Zhu, for helping us prepare samples for analysis. We also thank the Nucleic Acid Technologies Core for assistance with sequencing library preparation and data collection. All vector libraries and vectors for in vivo studies were produced by the Penn Vector Core (RRID: SCR_022432). We thank Mingyao Li and Hanying Yan for advice regarding statistical analysis and Nathan Denton for extensive efforts in facilitating figure and manuscript preparation.
This research was supported by research agreements with Amicus Therapeutics and Passage Bio.
R.A.M. contributed Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – Original Draft Preparation, Writing – Review & Editing; Q.W. contributed Data Curation, Formal Analysis, Investigation, Methodology, Resources; H.X. contributed Data Curation, Formal Analysis, Investigation, Methodology; G.H. contributed Investigation; P.B. contributed Data Curation, Formal Analysis, Investigation, Methodology; E.J.A. contributed Data Curation, Formal Analysis, Investigation, Methodology; J.J.S. contributed Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Supervision, Visualization, Writing – Review & Editing; J.M.W. contributed Conceptualization, Funding Acquisition, Project Administration, Supervision, Writing – Review and Editing.
J.M.W. is a paid advisor to and holds equity in iECURE, Scout Bio, Passage Bio, and the Center for Breakthrough Medicines (CBM). He also holds equity in the G2 Bio-associated asset companies. He has sponsored research agreements with Amicus Therapeutics, CBM, Elaaj Bio, FA212, G2 Bio, G2 Bio-associated asset companies, iECURE, Passage Bio, and Scout Bio, which are licensees of Penn technology. J.M.W. and J.J.S. are inventors on patents that have been licensed to various biopharmaceutical companies and for which they may receive payments.
Footnotes
Supplemental material is available online only.
Contributor Information
James M. Wilson, Email: wilsonjm@upenn.edu.
Lawrence Banks, International Centre for Genetic Engineering and Biotechnology.
REFERENCES
- 1.Al-Zaidy SA, Kolb SJ, Lowes L, Alfano LN, Shell R, Church KR, Nagendran S, Sproule DM, Feltner DE, Wells C, Ogrinc F, Menier M, L'Italien J, Arnold WD, Kissel JT, Kaspar BK, Mendell JR. 2019. AVXS-101 (onasemnogene abeparvovec) for SMA1: comparative study with a prospective natural history cohort. J Neuromuscul Dis 6:307–317. doi: 10.3233/JND-190403. [DOI] [PubMed] [Google Scholar]
- 2.Hoy SM. 2019. Onasemnogene abeparvovec: first global approval. Drugs 79:1255–1262. doi: 10.1007/s40265-019-01162-5. [DOI] [PubMed] [Google Scholar]
- 3.Haddley K. 2013. Alipogene tiparvovec for the treatment of lipoprotein lipase deficiency. Drugs Today (Barc) 49:161–170. doi: 10.1358/dot.2013.49.3.1937398. [DOI] [PubMed] [Google Scholar]
- 4.Burnett JR, Hooper AJ. 2009. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther 11:681–691. [PubMed] [Google Scholar]
- 5.Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, Zhu X, Haller JA, Sohn EH, Mahajan VB, Pfeifer W, Weckmann M, Johnson C, Gewaily D, Drack A, Stone E, Wachtel K, Simonelli F, Leroy BP, Wright JF, High KA, Maguire AM. 2017. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel U, Boucher M, de Léséleuc L, Visintini S. 1 March 2018. Voretigene neparvovec: an emerging gene therapy for the treatment of inherited blindness, p 169. In CADTH issues in emerging health technologies. Issue 169. Canadian Agency for Drugs and Technologies in Health, Ottawa, Ontario, Canada. [PubMed] [Google Scholar]
- 7.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. 2008. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 8.Lang JF, Toulmin SA, Brida KL, Eisenlohr LC, Davidson BL. 2019. Standard screening methods underreport AAV-mediated transduction and gene editing. Nat Commun 10:3415. doi: 10.1038/s41467-019-11321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L, Vandenberghe LH, Grant R, Sanmiguel J, Furth EE, Wilson JM. 2009. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther 20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, Richman LK, Wilson JM. 2018. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishimoto TK, Samulski RJ. 2022. Addressing high dose AAV toxicity—'one and done' or 'slower and lower'? Expert Opin Biol Ther 22:1067–1071. doi: 10.1080/14712598.2022.2060737. [DOI] [PubMed] [Google Scholar]
- 12.Morales L, Gambhir Y, Bennett J, Stedman HH. 2020. Broader implications of progressive liver dysfunction and lethal sepsis in two boys following systemic high-dose AAV. Mol Ther 28:1753–1755. doi: 10.1016/j.ymthe.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullard A. 2021. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat Rev Drug Discov 20:804–805. doi: 10.1038/d41573-021-00164-x. [DOI] [PubMed] [Google Scholar]
- 14.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. 2013. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 15.Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, Trepel M. 2003. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat Biotechnol 21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 16.Perabo L, Buning H, Kofler DM, Ried MU, Girod A, Wendtner CM, Enssle J, Hallek M. 2003. In vitro selection of viral vectors with modified tropism: the adeno-associated virus display. Mol Ther 8:151–157. doi: 10.1016/s1525-0016(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 17.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V. 2017. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V. 2016. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista AR, King OD, Reardon CP, Davis C, Shankaracharya, Philip V, Gray-Edwards H, Aronin N, Lutz C, Landers J, Sena-Esteves M. 2020. Ly6a differential expression in blood-brain barrier is responsible for strain specific central nervous system transduction profile of AAV-PHP.B. Hum Gene Ther 31:90–102. doi: 10.1089/hum.2019.186. [DOI] [PubMed] [Google Scholar]
- 20.Hordeaux J, Yuan Y, Clark PM, Wang Q, Martino RA, Sims JJ, Bell P, Raymond A, Stanford WL, Wilson JM. 2019. The GPI-linked protein LY6A drives AAV-PHP.B transport across the blood-brain barrier. Mol Ther 27:912–921. doi: 10.1016/j.ymthe.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Q, Chan KY, Tobey IG, Chan YA, Poterba T, Boutros CL, Balazs AB, Daneman R, Bloom JM, Seed C, Deverman BE. 2019. Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS One 14:e0225206. doi: 10.1371/journal.pone.0225206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loughner CL, Bruford EA, McAndrews MS, Delp EE, Swamynathan S, Swamynathan SK. 2016. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics 10:10. doi: 10.1186/s40246-016-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM. 2018. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol Ther 26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanlon KS, Meltzer JC, Buzhdygan T, Cheng MJ, Sena-Esteves M, Bennett RE, Sullivan TP, Razmpour R, Gong Y, Ng C, Nammour J, Maiz D, Dujardin S, Ramirez SH, Hudry E, Maguire CA. 2019. Selection of an efficient AAV vector for robust CNS transgene expression. Mol Ther Methods Clin Dev 15:320–332. doi: 10.1016/j.omtm.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonnenmacher M, Wang W, Child MA, Ren XQ, Huang C, Ren AZ, Tocci J, Chen Q, Bittner K, Tyson K, Pande N, Chung CH, Paul SM, Hou J. 2021. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol Ther Methods Clin Dev 20:366–378. doi: 10.1016/j.omtm.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravindra Kumar S, Miles TF, Chen X, Brown D, Dobreva T, Huang Q, Ding X, Luo Y, Einarsson PH, Greenbaum A, Jang MJ, Deverman BE, Gradinaru V. 2020. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat Methods 17:541–550. doi: 10.1038/s41592-020-0799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinmann J, Weis S, Sippel J, Tulalamba W, Remes A, El Andari J, Herrmann AK, Pham QH, Borowski C, Hille S, Schonberger T, Frey N, Lenter M, VandenDriessche T, Muller OJ, Chuah MK, Lamla T, Grimm D. 2020. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat Commun 11:5432. doi: 10.1038/s41467-020-19230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Ravindra Kumar S, Adams CD, Yang D, Wang T, Wolfe DA, Arokiaraj CM, Ngo V, Campos LJ, Griffiths JA, Ichiki T, Mazmanian SK, Osborne PB, Keast JR, Miller CT, Fox AS, Chiu IM, Gradinaru V. 2022. Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems. Neuron 110:2242–2257.e6. doi: 10.1016/j.neuron.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabebordbar M, Lagerborg KA, Stanton A, King EM, Ye S, Tellez L, Krunnfusz A, Tavakoli S, Widrick JJ, Messemer KA, Troiano EC, Moghadaszadeh B, Peacker BL, Leacock KA, Horwitz N, Beggs AH, Wagers AJ, Sabeti PC. 2021. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184:4919–4938.e22. doi: 10.1016/j.cell.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyberg WA, Ark J, To A, Clouden S, Reeder G, Muldoon JJ, Chung JY, Xie WH, Allain V, Steinhart Z, Chang C, Talbot A, Kim S, Rosales A, Havlik LP, Pimentel H, Asokan A, Eyquem J. 2023. An evolved AAV variant enables efficient genetic engineering of murine T cells. Cell 186:446–460.e19. doi: 10.1016/j.cell.2022.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shay TF, Sullivan EE, Ding X, Chen X, Kumar SR, Goertsen D, Brown D, Vielmetter J, Borsos M, Lam AW, Gradinaru V. 2023. Primate-conserved carbonic anhydrase IV and murine-restricted Ly6c1 are new targets for crossing the blood-brain barrier. https://www.biorxiv.org/content/10.1101/2023.01.12.523632v1. [DOI] [PMC free article] [PubMed]
- 32.Huang Q, Chen AT, Chan KY, Sorensen H, Barry AJ, Azari B, Beddow T, Zheng Q, Zhao B, Tobey IG, Eid F-E, Chan YA, Deverman BE. 2022. Targeting AAV vectors to the CNS via de novo engineered capsid-receptor interactions. https://www.biorxiv.org/content/10.1101/2022.10.31.514553v1. [DOI] [PMC free article] [PubMed]
- 33.Xiong X, Coombs PJ, Martin SR, Liu J, Xiao H, McCauley JW, Locher K, Walker PA, Collins PJ, Kawaoka Y, Skehel JJ, Gamblin SJ. 2013. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 497:392–396. doi: 10.1038/nature12144. [DOI] [PubMed] [Google Scholar]
- 34.Sauter NK, Bednarski MD, Wurzburg BA, Hanson JE, Whitesides GM, Skehel JJ, Wiley DC. 1989. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry 28:8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- 35.Muller M, Lauster D, Wildenauer HHK, Herrmann A, Block S. 2019. Mobility-based quantification of multivalent virus-receptor interactions: new insights into influenza A virus binding mode. Nano Lett 19:1875–1882. doi: 10.1021/acs.nanolett.8b04969. [DOI] [PubMed] [Google Scholar]
- 36.Mammen M, Choi SK, Whitesides GM. 1998. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed Engl 37:2754–2794. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Szklarczyk OM, Gonzalez-Segredo N, Kukura P, Oppenheim A, Choquet D, Sandoghdar V, Helenius A, Sbalzarini IF, Ewers H. 2013. Receptor concentration and diffusivity control multivalent binding of SV40 to membrane bilayers. PLoS Comput Biol 9:e1003310. doi: 10.1371/journal.pcbi.1003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overeem NJ, Hamming PHE, Tieke M, van der Vries E, Huskens J. 2021. Multivalent affinity profiling: direct visualization of the superselective binding of influenza viruses. ACS Nano 15:8525–8536. doi: 10.1021/acsnano.1c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Veracoechea FJ, Frenkel D. 2011. Designing super selectivity in multivalent nano-particle binding. Proc Natl Acad Sci USA 108:10963–10968. doi: 10.1073/pnas.1105351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen S, Bryant KD, Sun J, Brown SM, Troupes A, Pulicherla N, Asokan A. 2012. Glycan binding avidity determines the systemic fate of adeno-associated virus type 9. J Virol 86:10408–10417. doi: 10.1128/JVI.01155-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martino RA, Fluck EC, III, Murphy J, Wang Q, Hoff H, Pumroy RA, Lee CY, Sims JJ, Roy S, Moiseenkova-Bell VY, Wilson JM. 2021. Context-specific function of the engineered peptide domain of PHP.B. J Virol 95:e01164-21. doi: 10.1128/JVI.01164-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu G, Zhang R, Li H, Yin K, Ma X, Lou Z. 2022. Structural basis for the neurotropic AAV9 and the engineered AAVPHP.eB recognition with cellular receptors. Mol Ther Methods Clin Dev 26:52–60. doi: 10.1016/j.omtm.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang S, Shen HK, Ding X, Miles TF, Gradinaru V. 2022. Structural basis of receptor usage by the engineered capsid AAV-PHP.eB. Mol Ther Methods Clin Dev 26:343–354. doi: 10.1016/j.omtm.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabula Muris Consortium. 2018. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562:367–372. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan S, Taverna F, Rohlenova K, Treps L, Geldhof V, de Rooij L, Sokol L, Pircher A, Conradi LC, Kalucka J, Schoonjans L, Eelen G, Dewerchin M, Karakach T, Li X, Goveia J, Carmeliet P. 2019. EndoDB: a database of endothelial cell transcriptomics data. Nucleic Acids Res 47:D736–D744. doi: 10.1093/nar/gky997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, Garcia-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA, Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X, Luo Y, Carmeliet P. 2020. Single-cell transcriptome atlas of murine endothelial cells. Cell 180:764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Fagard R, Conway J. 1990. Measurement of cardiac output: Fick principle using catheterization. Eur Heart J 11(Suppl I):1–5. doi: 10.1093/eurheartj/11.suppl_i.1. [DOI] [PubMed] [Google Scholar]
- 48.Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y, Luk W, Lu Y, Dennis MS, Weimer RM, Chung I, Watts RJ. 2014. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med 211:233–244. doi: 10.1084/jem.20131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnsen KB, Bak M, Kempen PJ, Melander F, Burkhart A, Thomsen MS, Nielsen MS, Moos T, Andresen TL. 2018. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 8:3416–3436. doi: 10.7150/thno.25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Li S, Gessler DJ, Xie J, Zhong L, Li J, Tran K, Van Vliet K, Ren L, Su Q, He R, Goetzmann JE, Flotte TR, Agbandje-McKenna M, Gao G. 2018. A rationally engineered capsid variant of AAV9 for systemic CNS-directed and peripheral tissue-detargeted gene delivery in neonates. Mol Ther Methods Clin Dev 9:234–246. doi: 10.1016/j.omtm.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiles KM, Frenk EZ, Kaminsky SM, Crystal RG. 2022. Genetic modification of the AAV5 capsid with lysine residues results in a lung-tropic liver-detargeted gene transfer vector. Hum Gene Ther 33:148–154. doi: 10.1089/hum.2021.200. [DOI] [PubMed] [Google Scholar]
- 52.Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M, Asokan A. 2011. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther 19:1070–1078. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiao C, Yuan Z, Li J, He B, Zheng H, Mayer C, Li J, Xiao X. 2011. Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene Ther 18:403–410. doi: 10.1038/gt.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hordeaux J, Buza EL, Jeffrey B, Song C, Jahan T, Yuan Y, Zhu Y, Bell P, Li M, Chichester JA, Calcedo R, Wilson JM. 2020. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci Transl Med 12:eaba9188. doi: 10.1126/scitranslmed.aba9188. [DOI] [PubMed] [Google Scholar]
- 55.Dhungel B, Ramlogan-Steel CA, Steel JC. 2018. Synergistic and independent action of endogenous microRNAs 122a and 199a for post-transcriptional liver detargeting of gene vectors. Sci Rep 8:15539. doi: 10.1038/s41598-018-33801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudek AM, Zabaleta N, Zinn E, Pillay S, Zengel J, Porter C, Franceschini JS, Estelien R, Carette JE, Zhou GL, Vandenberghe LH. 2020. GPR108 is a highly conserved AAV entry factor. Mol Ther 28:367–381. doi: 10.1016/j.ymthe.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, Carette JE. 2016. An essential receptor for adeno-associated virus infection. Nature 530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. 2010. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, Figueredo J, Lock M, Wilson JM. 2006. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther 13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A, Sanmiguel JC, Sun X, Wivel NA, Raper SE, Furth EE, Batshaw ML, Wilson JM. 2006. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther 14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Greig JA, Peng H, Ohlstein J, Medina-Jaszek CA, Ahonkhai O, Mentzinger A, Grant RL, Roy S, Chen SJ, Bell P, Tretiakova AP, Wilson JM. 2014. Intramuscular injection of AAV8 in mice and macaques is associated with substantial hepatic targeting and transgene expression. PLoS One 9:e112268. doi: 10.1371/journal.pone.0112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Breton C, Warzecha CC, Bell P, Yan H, He Z, White J, Zhu Y, Li M, Buza EL, Jantz D, Wilson JM. 2021. Long-term stable reduction of low-density lipoprotein in nonhuman primates following in vivo genome editing of PCSK9. Mol Ther 29:2019–2029. doi: 10.1016/j.ymthe.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breton C, Furmanak T, Avitto AN, Smith MK, Latshaw C, Yan H, Greig JA, Wilson JM. 2021. Increasing the specificity of AAV-based gene editing through self-targeting and short-promoter strategies. Mol Ther 29:1047–1056. doi: 10.1016/j.ymthe.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Smith J, Breton C, Clark P, Zhang J, Ying L, Che Y, Lape J, Bell P, Calcedo R, Buza EL, Saveliev A, Bartsevich VV, He Z, White J, Li M, Jantz D, Wilson JM. 2018. Meganuclease targeting of PCSK9 in macaque liver leads to stable reduction in serum cholesterol. Nat Biotechnol 36:717–725. doi: 10.1038/nbt.4182. [DOI] [PubMed] [Google Scholar]
- 65.Toth ZE, Mezey E. 2007. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem 55:545–554. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- 66.Stack EC, Wang C, Roman KA, Hoyt CC. 2014. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70:46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5. Download jvi.00174-23-s0001.docx, DOCX file, 2.1 MB (2.1MB, docx)
Data Availability Statement
All relevant data are provided within the article and supplemental material.