This study demonstrates that increases in vasomotor symptoms, onset of a high frequency of vasomotor symptoms, and persistent vasomotor symptoms over time may precede weight gain in women.
Key Words: BMI, Hot flashes, Middle age, Night sweats, Sleep deprivation, Waist circumference
Abstract
Objective
The menopause transition is associated with weight gain in women. We examined whether changes in vasomotor symptom (VMS) frequency precede weight changes.
Methods
This longitudinal retrospective analysis included data from the multisite, multiethnic Study of Women's Health Across the Nation. Women in premenopause or perimenopause aged 42 to 52 years at baseline self-reported VMS frequency (hot flashes/night sweats) and sleep problems at up to 10 annual visits. Menopause status, weight, body mass index, and waist circumference were compared across visits. The primary objective was to measure the association between VMS frequency and weight gain using a lagged approach with first-difference regression models. Secondary objectives were to statistically quantify mediation by sleep problems and moderation by menopause status and explore the association between cumulative, 10-year VMS exposure and long-term weight gain.
Results
The primary analysis sample included 2,361 participants (12,030 visits; 1995-2008). Increased VMS frequency across visits was associated with subsequently increased weight (0.24 kg), body mass index (0.08 kg/m2), and waist circumference (0.20 cm). Cumulative exposure to a high frequency of VMS (≥6 d/2 wk) over 10 consecutive annual visits was associated with increases in weight measures, including a 3.0-cm increase in waist circumference. Contemporaneous sleep problems mediated no more than 27% of waist circumference increases. Menopause status was not a consistent moderator.
Conclusions
This study demonstrates that increases in VMS, onset of a high frequency of VMS, and persistent VMS symptoms over time may precede weight gain in women.
The menopause transition has long been associated with weight changes1; however, the contribution of factors, such as age, reduced physical activity, and hormonal changes, is not fully understood. During midlife, women gain a mean of 2.1 kg (3% increase) and experience a mean increase in waist circumference of 2.2 cm (2.8% increase) over 3 consecutive years.2 Some studies suggest that weight gain during menopause is associated with changes in body composition. This includes an increase in fat mass and a decrease in lean mass.3-5 In some midlife women, weight gain, particularly increased abdominal fat, is linked to cardiovascular and metabolic diseases, such as insulin resistance and type 2 diabetes.3,6 Therefore, an enhanced understanding of the underlying mechanisms that drive weight gain during midlife has the potential to inform helpful interventions and improve outcomes.
Weight changes, including changes in body mass index (BMI) and waist circumference, have been associated with the presence of symptoms of menopause, including vasomotor symptoms (VMS).7,8 VMS are commonly known as hot flashes (also called hot flushes) and night sweats. These symptoms are widespread, with 80% or more of US women reporting VMS during or after the menopause transition.9-11 VMS can reduce quality of life, in part by directly or indirectly causing sleep disturbances and leading to compromised concentration and memory.12-15 The high prevalence of VMS and the persistence of VMS for a median of 7.4 years overall and for up to 10.1 and 8.9 years in African American and Hispanic women, respectively,16 necessitates a greater understanding of the potential contributing factors and treatments that may decrease the burden of VMS. Prior research shows that greater body fat, BMI, and waist circumference are associated with increased VMS severity.17-19 These factors also increase the probability that women will experience VMS, particularly during perimenopause.17-19 However, there is a lack of research on the converse: whether increases in VMS frequency and cumulative exposure to VMS over time precede weight gain among midlife women.
In the current study, we use data from the Study of Women's Health Across the Nation (SWAN), an ongoing, multisite, longitudinal, epidemiologic cohort study capturing biopsychosocial information from women in the United States in midlife and beyond, to address this gap.20-23 We examined whether changes in VMS frequency were associated with weight gain in midlife women, taking into consideration the impact of concomitant sleep problems and menopause status. We also explored the extent to which cumulative exposure to VMS is associated with long-term weight gain. Characterizing the temporal association between VMS frequency and weight changes may help improve health outcomes in and after the menopause transition.
METHODS
Study design
This retrospective analysis used publicly available data from SWAN. All data in SWAN were collected in a standardized manner and included annual assessments conducted by trained interviewers in a clinic setting.21,24 Details of enrollment have been previously reported.24 Briefly, SWAN participants were in premenopause or perimenopause, were aged 42 to 52 years at study enrollment, and self-reported various ethnicities and races across seven sites in the United States.25 In the current analysis, we used data collected in annual surveys of enrolled women at baseline (n = 3,302) and for up to 10 annual follow-up visits between 1995 and 2008. The questionnaires assessed participants' medical history, use of medical services and medications, menstrual status, quality of life, psychosocial environment, work and lifestyle behaviors, and symptoms of menopause, including self-reported severity and frequency of VMS and quality of sleep.24
Participant-reported data used in the current analysis included frequency of VMS and changes in sleep quality. VMS were defined as hot flashes and/or night sweats. Frequency was self-reported as the number of days in the prior 2 weeks in which VMS were experienced (characterized as 0, 1-5 d, 6-8 d, 9-13 d, or 14 d). To ascertain sleep quality, participants self-reported three types of sleep problems: trouble falling asleep, waking up early, and/or waking up several times per night. Sleep problems were assigned values based on the number of days per week they were reported (from 1 [none] to 5 [≥5 per week]). For this analysis, sleep quality for each sleep problem was defined as binary indicators based on self-report of sleep problems occurring three times or more (yes) or less than three times (no) in the past 2 weeks.
Additional SWAN data in the current analysis included proportional and absolute changes in weight (kg), BMI (kg/m2), and waist circumference (cm). These standardized measurements were collected by trained study staff at each annual visit. Smoking status, alcohol use, changes in social support, and changes in physical activity were also assessed as potential covariates. Menopause status was categorized as “early” or “late.” Early was defined as premenopause and early perimenopause (ie, menses in the prior 3 mo with or without changes in regularity in the prior year). Late was defined as late perimenopause and postmenopause (ie, no menses in the prior 3 mo but menses in the prior 11 mo, or no menses for ≥12 mo), as reported in Gold et al.18
Analytic sample
For the primary analysis, eligible participants had valid nonmissing exposure and outcome data from at least three consecutive annual visits (visit t, visit t − 1 [1 visit prior to visit t], and visit t − 2 [2 visits prior]). Each participant contributed data from as many visits as were available. Selection criteria were imposed at the person-visit level. Data from visits were excluded from these analyses if the participant reported undergoing an oophorectomy on or after the first visit; if the participant reported pregnancy or use of hormone therapy at visit t, t − 1, or t − 2; if the participant reported a cancer (not including skin cancer); and/or if there was a missing value for any of the three weight outcomes, duration of exposure to VMS, covariates (eg, smoking status, alcohol use, changes in social support or physical activity), or mediator variables (eg, changes in sleep quality) needed for model estimation.
For the secondary analysis population, separate inclusion criteria were applied to explore cumulative exposure to VMS frequency. The initial sample included visits from all participants, except for those who reported undergoing an oophorectomy on or after the first visit, were taking hormone therapy, were pregnant, or reported any cancer (except skin cancer) at any visit for up to 10 consecutive annual visits excluding the baseline visit at which accumulation of VMS was not measurable.
Study outcomes
The primary objective of this analysis was to quantify the impact of changes in VMS frequency on subsequent weight gain in midlife women. To accomplish this, changes in VMS frequency were examined from visits t − 2 and t − 1. Changes in weight, BMI, and waist circumference were examined from visits t − 1 to t. This lagged approach ensured that VMS frequency changes predated weight changes. Secondary outcomes were to assess the extent to which changes in sleep quality influenced the relationship between VMS frequency and subsequent weight gain, to quantify potential moderation of VMS frequency and subsequent weight gain by menopause status, and to determine whether cumulative exposure to VMS over 10 years was associated with long-term weight gain.
Statistical analysis
The analytic sample consisted of a panel of participants with visits occurring approximately annually. The visit was the unit of analysis. Participants could have multiple visits, each representing a single point in time. Descriptive statistics were calculated for demographics and all covariates stratified by visit. Proportional change in weight measures was calculated as percentage point (ppt) difference from visit t − 1 to visit t. Absolute change in weight was calculated as the value at visit t minus the value at visit t − 1.
For the primary analysis, associations between changes in VMS frequency between 2 consecutive visits (t − 2 to t − 1) and subsequent weight changes (t − 1 to t) were estimated using first-difference regression models, including both an unadjusted model and a model adjusting for time-varying covariates (eg, smoking status, alcohol use, changes in social support, changes in physical activity). These models control for all time-invariant characteristics (eg, race/ethnicity, age at baseline visit, site) by differencing them out; that is, by subtracting outcome and explanatory variables between consecutive visits, which removes all measures that do not vary across visits for a given respondent. The models were estimated using a mixed (generalized least squares) linear regression model with heteroskedastic robust variance-covariance matrix and a Toeplitz 1 error structure. Any missing values for the covariates of smoking status, alcohol use, social support, and physical activity were imputed from last observation carried forward for follow-up visits. Data from the first follow-up visit were used for missing baseline values for these four covariates. Proportional and absolute changes in weight measures (weight, BMI, and waist circumference) by VMS frequency were calculated for the primary analysis; change in VMS frequency was measured as the onset of new VMS. In sensitivity analyses, VMS frequency was measured as a change in frequency from low (0-6 d with VMS in the prior 2 wk) to high (≥6 d with VMS in the prior 2 wk) and as an increase in VMS frequency from visits t − 2 to t − 1.
To test the association between VMS frequency and weight change while controlling for the potential mediator of concomitant sleep problems, the estimated coefficient for VMS frequency was compared between model specifications with and without concomitant sleep problems. Moderation of VMS effects on weight was assessed by menopause status, for women in “early” and “late” menopause (as reported in Gold et al18) using a Wald test of whether the interaction terms between VMS frequency and early versus late menopause status were jointly equal to 0.
For the cumulative exposure analysis, exposure was measured as the cumulative number of annual visits at which a woman reported experiencing any VMS (≥1 d with VMS in the prior 2 wk, referred to herein as “any VMS”) or a high frequency of VMS (≥6 d with VMS in the prior 2 wk, referred to herein as “high frequency VMS”). The outcome was measured as the absolute or percentage change in weight from baseline to the current visit. Effect sizes were measured as the difference in an outcome between a participant with 10 annual visits with the exposure (“any VMS” or “high frequency VMS”) versus a participant with 0 visits with the same exposure. Binary exposures (yes/no) were summed across the follow-up visits for any VMS and high frequency VMS and analyzed using linear regression modeling accounting for multiple observations per person using the adjusted variance-covariance matrix.26 Covariates included categorical indicators of visit number and the outcome's value measured at the baseline visit.
RESULTS
Population
Of the 3,302 women who participated in the baseline survey, 2,361 composed the main analytic sample for this analysis, contributing data from 12,030 annual visits (Supplemental Table 1, http://links.lww.com/MENO/B150). The cumulative analytic sample included 1,743 participants contributing data at 12,182 visits (Supplemental Table 1, http://links.lww.com/MENO/B150). The mean (SD) age at the baseline visit for the 2,361 participants in the primary analysis sample was 51 (3.7) years. About half (47.5%) of participants in the analytic sample self-reported as White/non-Hispanic, and half (52.5%) self-reported as Black/African American, Japanese/Japanese American, Chinese/Chinese American, or Hispanic (Table 1). Participants were in late perimenopause or postmenopause during 45.5% of visits and in premenopause or early perimenopause at 54.4% of visits. At follow-up visit 2, just 13.3% of participants (n = 249) were in late menopause, but by visit 10, the majority (n = 1,074 [89.7%]) were in late menopause.
Table 1.
Characteristics of the overall study population (12,030 visits)a
| Characteristic | |
|---|---|
| Age at baseline visit (y), mean (SD) | 51.1 (3.7) |
| Visits by race/ethnicity, n (%) | |
| White non-Hispanic | 5,715 (47.5) |
| Black/African American | 3,187 (26.5) |
| Japanese/Japanese American | 1,429 (11.9) |
| Chinese/Chinese American | 1,304 (10.8) |
| Hispanic (Black or White) | 395 (3.3) |
| Visits by menopause status, n (%)b | |
| Premenopause/early perimenopause | 6,308 (54.4) |
| Late perimenopause/postmenopause | 5,279 (45.5) |
aA total of 12,030 visits from 2,361 participants.
bExcludes 443 visits at which participants reported menopause status other than classified here.
VMS frequency and covariates
VMS were prevalent. Among the 12,030 annual visits, 62.2% were with participants who had at least 1 day of VMS at either of the two preceding visits (visit t − 2 and t − 1), 38.4% were with participants who had at least 1 day of VMS at both the t − 2 and t − 1 visits, and 37.8% were with participants who had 0 days of VMS at either the t − 2 or t − 1 visit. At 59.7% of the visits, participants reported no change from the previous visit in the number of days with VMS (as determined for the prior 2 wk); the number of days with VMS increased at 22.0% of visits and decreased at 18.3% of visits (Table 2). Onset of new VMS since the prior visit was reported at 13.5% of the visits. High frequency VMS (≥6 d/2 wk) was reported at 27.2% of visits. High frequency VMS at two consecutive visits was reported for 10.3% of the visits. No/low VMS (<6 d/2 wk) at two consecutive visits was reported for 72.8% of visits. Of the 919 participants with 10 follow-up visits, 110 had 10 consecutive visits with any VMS, and 6 had 10 consecutive visits with high-frequency VMS.
Table 2.
Change from prior visit in VMS frequency and covariates
| Variable | No. visits (%) |
|---|---|
| Total no. visits | 12,030 |
| VMS frequency (from t − 2 to t − 1) | |
| Change in VMS days | |
| Decrease in days | 2,199 (18.3) |
| No change in days | 7,186 (59.7) |
| Increase in days | 2,645 (22.0) |
| Any VMS days | |
| Any new onset daysa | 1,619 (13.5) |
| No daysb | 4,542 (37.8) |
| Some days, both visitsc | 4,621 (38.4) |
| Loss of daysd | 1,248 (10.4) |
| High frequency VMS days | |
| High days, both visitse | 1,235 (10.3) |
| High days, new onsetf | 1,121 (9.3) |
| None (no high frequency VMS days), both visitsg | 8,760 (72.8) |
| Covariates (from t − 1 to t) | |
| Smoking | |
| No | 10,386 (86.3) |
| Yes | 1,427 (11.9) |
| Smoking onset | 96 (0.8) |
| Smoking cessation | 121 (1.0) |
| Change in alcohol use | |
| Decrease | 1,521 (12.6) |
| No change | 8,909 (74.1) |
| Increase | 1,600 (13.3) |
| Change in social support | |
| Decrease | 3,139 (26.1) |
| No change | 5,500 (45.7) |
| Increase | 3,391 (28.2) |
| Change in physical activity | |
| Decrease | 3,935 (32.7) |
| No change | 4,414 (36.7) |
| Increase | 3,681 (30.6) |
| Sleep problems | |
| Onset | 1,528 (12.7) |
| No | 6,329 (52.6) |
| Yes | 2,770 (23.0) |
| Loss | 1,403 (11.7) |
VMS, vasomotor symptoms.
aReported 0 days with VMS at t − 2 and ≥1 day with VMS at t − 1.
bReported no VMS at both visits t − 2 and t − 1.
cReported ≥1 day with VMS at both t − 2 and t − 1.
dReported ≥1 day with VMS at t − 2 and 0 days at t − 1.
eReported ≥6 days with VMS at both t − 2 and t − 1.
fReported <6 days with VMS at t − 2 and ≥6 days with VMS at t − 1.
gReported <6 days with VMS (including “no VMS”) at both t − 2 and t − 1.
Participants reported being nonsmokers at 86.3% of visits. There was little change from visit to visit in alcohol use, and social support availability was unchanged at 45.7% of visits. Increases and decreases in physical activity were reported at approximately one third of visits. Among the 12,030 visits overall, 52.6% of participants reported no sleep problems at one or both of the t − 2 and t − 1 visits, and onset of a sleep problem was reported at 12.7% of visits (Table 2).
Changes in weight measures
Differences in weight over 1 year were small and close to 0; however, cumulative increases in weight occurred over the 10-year period. The mean (SD) visit-to-visit change was a 0.57 (4.9) ppt increase in weight, 0.65 (5.1) ppt increase in BMI, and 0.64 (5.2) ppt increase in waist circumference. These changes were also reflected in mean (SD) visit-to-visit absolute increases of 0.33 (3.96) kg in weight, 0.15 (1.52) kg/m2 in BMI, and 0.44 (4.75) cm in waist circumference.
Association between change in VMS frequency and weight
The primary analysis found that an increase in the number of days with VMS was associated with subsequent weight increases. Compared with no changes in VMS frequency, increases in VMS frequency from visit t − 2 to visit t − 1 were associated with proportional increases in weight (0.31 ppt), BMI (0.29 ppt), and waist circumference (0.20 ppt). The onset of high frequency VMS during the previous visit was associated with proportional increases in weight (0.35 ppt [adjusted P = 0.029]), BMI (0.33 ppt [adjusted P = 0.035]), and waist circumference (0.30 ppt [adjusted P = 0.043]) (Fig. 1A). Onset of high frequency VMS was associated with an absolute increase of 0.23 kg (weight), 0.08 kg/m2 (BMI), and 0.26 cm (waist circumference), although none of these reached statistical significance (Fig. 1B). In addition, an increase in VMS days and onset of high frequency VMS were associated with subsequent proportional increases in waist circumference (adjusted P = 0.035 and adjusted P = 0.043, respectively) compared with days of no/low VMS (Fig. 1A and B; Supplemental Tables 2, http://links.lww.com/MENO/B150 and 3, http://links.lww.com/MENO/B150).
FIG. 1.
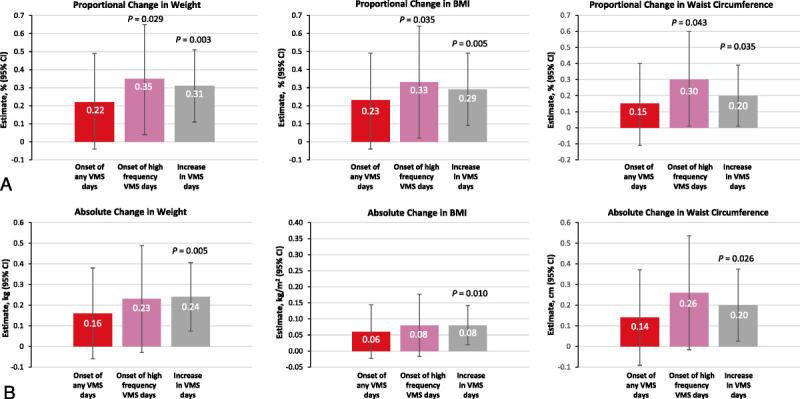
Proportional (A) and absolute (B) changes in weight, BMI, and waist circumference (visit t − 1 to t) by VMS status and frequency (visit t − 2 to t − 1).Adjusted P value for association between changes in weight over two consecutive visits using a first-difference mixed linear regression model, controlling for changes in smoking status, alcohol use, social support, and physical activity. BMI, body mass index; VMS, vasomotor symptoms.
Mediation by sleep
Reported sleep problems mediated the association between VMS frequency and subsequent weight gain by 7.3% to 15.6%. The onset of any sleep problem mediated 7.3% to 10.5% of the effect size of increased VMS frequency from visit to visit. Sleep problems were also responsible for 9.5% to 15.4% of the effect size of increase in days of high frequency VMS.
Moderation by menopause status
For participants who were in late perimenopause or postmenopause, the onset of ≥1 day with VMS was associated with statistically significant proportional increases (0.54 ppt; 95% CI, 0.1-1.0 ppt; moderation, P = 0.019) and absolute increases (0.41 kg; 95% CI, 0-0.82 kg; moderation, P = 0.005) in weight. Statistically significant evidence for moderation was not found for waist circumference. As such, evidence that menopause status affects the relationship between VMS frequency and weight gain was inconsistent.
Effect of extended exposure to VMS
In the cumulative analytic sample comprising 1,743 participants with 12,182 visits, extended exposure to a high frequency of VMS over 10 years led to significant increases in weight, BMI, and waist circumference (Table 3; Fig. 2A, B). Compared with no reports of a high frequency of VMS, 10 consecutive high frequency VMS visits was associated with a 3.0-ppt greater increase in weight (after adjusting for baseline weight), a 3.5-ppt increase in BMI (after adjusting for baseline BMI), and a 3.5-ppt increase in waist circumference (P < 0.05 after adjusting for baseline waist circumference). These changes corresponded to increases of 2.1 kg, 0.93 kg/m2, and 3.01 cm, respectively. In addition, compared with no VMS at any visit, 10 consecutive visits with any VMS was associated with significant increases in waist circumference of 2.6 ppt and 2.1 cm (adjusted, both P < 0.005), but nonsignificant relative increases in weight of 1.5 ppt and 0.97 kg and BMI of 1.7 ppt and 0.42 kg/m2 (all P > 0.05) over 10 years. In the cumulative analytic sample, contemporaneous cumulative exposure to sleep problems mediated 20% to 27% of the effect sizes on waist circumference.
Table 3.
Adjusted and unadjusted effects of cumulative exposure to VMS on weight and body composition measuresa
| Cumulative exposure | Outcome | Unadjusted | Adjustedb | ||
|---|---|---|---|---|---|
| Estimate (95% CI) |
P | Estimate (95% CI) |
P | ||
| 10 y with high frequency VMS daysc | Percentage change in weight | 0.021 (−0.007 to 0.050) |
0.146 | 0.030 (0.001 to 0.058) |
0.040 |
| Percentage change in BMI | 0.027 (−0.002 to 0.056) |
0.069 | 0.035 (0.007 to 0.064) |
0.015 | |
| Percentage change in waist circumference | 0.027 (0.002 to 0.052) |
0.034 | 0.035 (0.010 to 0.060) |
0.006 | |
| Absolute change in weight (kg) | 1.593 (−0.490 to 3.68) |
0.134 | 2.056 (−0.026 to 4.14) |
0.053 | |
| Absolute change in BMI (kg/m2) | 0.760 (−0.022 to 1.54) |
0.057 | 0.929 (0.142 to 1.72) |
0.021 | |
| Absolute change in waist circumference (cm) | 2.595 (0.463 to 4.73) |
0.017 | 3.011 (0.858 to 5.16) |
0.006 | |
| 10 y with any VMS daysd | Percentage change in weight | 0.007 (−0.011 to 0.025) |
0.472 | 0.015 (−0.003 to 0.033) |
0.097 |
| Percentage change in BMI | 0.009 (−0.009 to 0.027) |
0.335 | 0.017 (−0.001 to 0.036) |
0.060 | |
| Percentage change in waist circumference | 0.018 (0.002 to 0.034) |
0.030 | 0.026 (0.009 to 0.042) |
0.002 | |
| Absolute change in weight (kg) | 0.492 (−0.902 to 1.89) |
0.489 | 0.973 (−0.441 to 2.39) |
0.178 | |
| Absolute change in BMI (kg/m2) | 0.248 (−0.276 to 0.772) |
0.354 | 0.416 (−0.119 to 0.952) |
0.128 | |
| Absolute change in waist circumference (cm) | 1.7474 (0.343 to 3.14) |
0.015 | 2.1414 (0.718 to 3.56) |
0.003 | |
BMI, body mass index; VMS, vasomotor symptoms.
aIncludes data from 12,182 visits by 1,743 participants.
bAdjusted for covariates of visit number and outcome value measured at baseline visit.
cHigh frequency VMS days are ≥6 days of VMS for 10 consecutive visits.
dAny VMS days are ≥1 day of VMS for 10 consecutive visits.
FIG. 2.
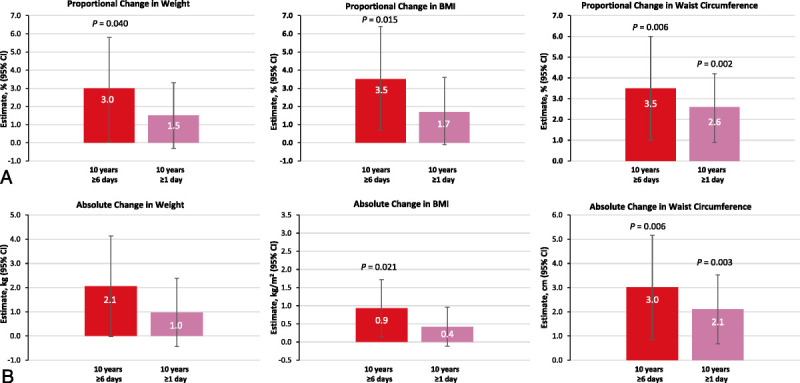
Proportional (A) and absolute (B) changes in weight, BMI, and waist circumference by cumulative exposure to VMS over 10 years (adjusted model). P value adjusted for multiple observations using the variance-covariance matrix, controlling for covariates of visit number and outcome value measured at baseline visit. BMI, body mass index; VMS, vasomotor symptoms.
DISCUSSION
This study, using longitudinal multisite SWAN data, provides the first evidence that frequent VMS (≥6 d over 2 wk) are associated with subsequent weight gain in midlife women. Increases in VMS frequency and onset of high frequency VMS from visit to visit were followed by increases of approximately 0.31 (any VMS) and 0.35 ppt (high frequency VMS) in weight, increases of 0.29 (any VMS) and 0.33 ppt (high frequency VMS) in BMI, and increases of 0.20 (any VMS) and 0.30 ppt (high frequency VMS) in waist circumference. The current study reports a link between VMS frequency and subsequent weight gain and central weight distribution. Notably, long-term exposure to high frequency VMS over a 10-year period was associated with an increase in waist circumference of 3.5 ppt, and exposure to any VMS over this time period resulted in an increase of 2.6 ppt in waist circumference. Waist circumference may be more indicative of weight distribution than weight gain alone.
Sleep problems accounted for 20% to 27% of the observed effect of VMS on the weight measure of waist circumference. Menopause status did not consistently moderate the VMS-associated weight gain relationship. A prior cross-sectional study of midlife women who were experiencing ≥35 moderate to severe VMS per week reported that the physical discomfort caused by VMS often led to sleep disturbances, which were reported as the most bothersome symptom of VMS among 75% of US women and 50% of European women surveyed.12 Given the well-established association between lack of sleep and weight gain,27,28 a larger mediating effect of sleep problems on VMS-associated weight gain may have been expected. Our findings suggest that sleep is not the only mediator of VMS-associated weight gain and that other factors contribute as well.
These findings add new perspectives and build on prior studies that have linked VMS and weight and body composition (including BMI). A retrospective subanalysis of data from 430 Chinese women who took part in a prospective, community-based, longitudinal study showed that women who experienced more frequent (≥10 hot flashes per day) and more severe VMS had a significantly greater likelihood of being obese. Furthermore, there was a significant positive correlation between abdominal obesity and VMS severity.19 Data reported in a pooled analysis of more than 21,000 women from 8 studies found that being overweight or obese was associated with a greater frequency and severity of VMS and that women who were obese and smoked had a high risk of frequent or severe VMS.17 In addition, menopause status moderated the effect of weight on VMS risk, with an increased risk of VMS early in the menopause transition but diminished risk in postmenopause.17 This is consistent with previous SWAN findings that showed an increased risk of VMS in women with higher concurrent BMI and waist circumference in premenopause/early perimenopause but not late perimenopause or postmenopause.18 In the current analysis, there was insufficient evidence to support an association between weight gain and either early or late menopause stage. Interestingly, a large cohort study that examined the effect of weight loss on VMS in more than 48,000 women in postmenopause showed that women who were assigned to a dietary intervention and lost weight were more likely to eliminate VMS within 1 year compared with control women, who maintained their weight.29 The effect of weight loss on diminished VMS was most pronounced for women who reported mild rather than moderate or severe symptoms at baseline.29 This further underscores the bidirectional relationship between VMS and weight gain, highlighting the importance of treating VMS to potentially reduce subsequent weight gain and suggesting that weight loss may reduce VMS.
Clinical implications
Although the current study reports a link between VMS frequency and subsequent weight gain, the associations between VMS and weight gain are likely multifactorial. The mechanisms that underlie this association are unclear. Adipokines, such as leptin, adiponectin, high-molecular-weight (HMW) adiponectin, and soluble leptin receptor may be involved. Lower adiponectin, lower HMW adiponectin, and higher leptin have been shown to be associated with increased risk of VMS at early but not later stages of menopause.30 However, additional studies are needed to examine this effect. Importantly, adiponectin and leptin levels differ between women with certain racial backgrounds. For example, a previous analysis from SWAN reported that African American women had lower adiponectin and HMW adiponectin and higher leptin levels than White women.31 Furthermore, reduced melatonin associated with sleep disturbances may also be involved in weight gain, as others report that melatonin supplementation reduces body weight in women who are in postmenopause.32 A deeper understanding of adipokines, melatonin, and other mechanisms driving the physiologic association between VMS and weight could elucidate helpful screening methods and targeted therapeutics.
The associations found between VMS and weight gain suggest a need for appropriate counseling about health risks, as well as potential weight loss interventions for some of those experiencing VMS. For example, educational initiatives before the menopause transition may be helpful. Physician-led diet and activity consultations could potentially mitigate weight gain for patients who are experiencing VMS. Psychological support around identifying potential obstacles to weight loss and developing strategies to initiate weight loss and maintain a healthy weight may also help.6 Other interventions may be prescribed, as weight gain often poses undue risks. The finding that cumulative exposure to VMS (any or high frequency VMS) is associated with increased waist circumference is especially concerning because central obesity is related to increased health risks, regardless of BMI.3,33,34 Those with cardiovascular disease, diabetes, and other health risks may be particularly vulnerable. Overall, an enhanced understanding of the relationship between VMS and weight increases provides critical information that can help improve women's health outcomes and quality of life.
Limitations
A limitation of this study is that SWAN is not a true national probability sample, and there may be selection bias, as recruitment occurred at only seven research sites, and inclusion bias due to nonrandom sampling. These analyses were limited to participants experiencing natural menopause and not taking hormone therapy for VMS during the study period. Therefore, these findings may not be generalizable to women with surgical menopause and/or hormone therapy use. In general, epidemiologic cohort data are inherently limited because they rely on self-report, although it should be noted that the period of self-reported data in the current analysis did not extend past 2 weeks before the visit, which may have minimized recall bias. Despite advantages of including a longitudinal sample, there is also an inherent limitation based on the year-long gap between visits. The lagged first-difference estimate is useful in that it ensured that exposure to VMS occurred before weight gain; however, this could have introduced bias against the true causal process. Other limitations include possible additional variables, such as the use of medications that may impact sleep, VMS, and weight gain, and/or potential sociologic changes in eating habits that may have occurred in the United States in the long study window that were not considered or adjusted for in the statistical models. Some of these factors could have increased bias in the results. These possible limitations should be considered while interpreting these data.
CONCLUSIONS
This is the first known study demonstrating that increases in VMS, onset of high frequency VMS, and cumulative exposure to VMS over time may be independently associated with weight gain among midlife women. Sustained accrual of VMS was associated with a significant increase in waist circumference over 10 years. These novel findings build on previous study findings linking weight to VMS. Taken together, this body of research highlights the need for future studies elucidating the mechanisms that underlie this relationship and contributes information that may help improve appropriate counseling and potential interventions for women with VMS.
Supplementary Material
Acknowledgments
Medical writing and editorial support were provided by Hannah L. Mayberry, PhD, and Traci A. Stuve, MA, of Echelon Brand Communications, LLC (Parsippany, NJ), an OPEN Health company, and funded by Astellas Pharma, Inc. Research assistance was provided by Julia Bond, MPH, of Medicus Economics, LLC (Milton, MA).
Footnotes
This paper was presented at the Annual Meeting of The North American Menopause Society, Atlanta, GA, October 12–15, 2022.
Funding/support: This study was sponsored by Astellas Pharma, Inc. Medical writing and editorial support were provided by Hannah L. Mayberry, PhD, and Traci A. Stuve, MA, of Echelon Brand Communications, LLC (Parsippany, NJ), an OPEN Health company, and funded by Astellas Pharma, Inc.
Financial disclosure/conflicts of interest: A.J.E. is an employee of Medicus Economics, LLC, which received payment from Astellas to participate in this research project. A.S., W.H., and S.M. are employees of Astellas Pharma, Inc. C.J.G. has no disclosures to report.
Inclusive language: In our report, we use gender-specific language, as reflected in the referenced publications and protocols. However, we recognize that some individuals who experience vasomotor symptoms related to menopause may identify differently from gender and pronouns used in this article.
Author contributions: A.S., A.J.E., C.J.G., and S.M. contributed in the study design. A.S. and A.J.E. were the study investigators. A.J.E. contributed in collection and assembly of data. A.S., A.J.E., W.H., and S.M. contributed in the data analysis. A.S., C.J.G., A.J.E., W.H., and S.M. contributed in the data interpretation. A.S. and A.J.E. contributed in the manuscript preparation. All authors contributed in the manuscript review and revisions as well as the final approval of manuscript.
Data sharing statement: Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Contributor Information
Aki Shiozawa, Email: aki.shiozawa@astellas.com.
Andrew J. Epstein, Email: andrew.epstein@medicuseconomics.com.
Wei Han, Email: wei.han@astellas.com.
Shayna Mancuso, Email: Shayna.mancuso@astellas.com.
REFERENCES
- 1.Dhanoya T Sievert LL Muttukrishna S, et al. Hot flushes and reproductive hormone levels during the menopausal transition. Maturitas 2016;89:43–51. doi: 10.1016/j.maturitas.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 2.Sternfeld B Wang H Quesenberry CP Jr., et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol 2004;160:912–922. doi: 10.1093/aje/kwh299 [DOI] [PubMed] [Google Scholar]
- 3.Fenton A. Weight, shape, and body composition changes at menopause. J Midlife Health 2021;12:187–192. doi: 10.4103/jmh.jmh_123_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greendale GA Sternfeld B Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight 2019;4:e124865. doi: 10.1172/jci.insight.124865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juppi HK Sipilä S Cronin NJ, et al. Role of menopausal transition and physical activity in loss of lean and muscle mass: a follow-up study in middle-aged Finnish women. J Clin Med 2020;9:9. doi: 10.3390/jcm9051588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor E, Collazo-Clavell ML, Faubion SS. Weight gain in women at midlife: a concise review of the pathophysiology and strategies for management. Mayo Clin Proc 2017;92:1552–1558. doi: 10.1016/j.mayocp.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Ziv-Gal A, Flaws JA. Factors that may influence the experience of hot flushes by healthy middle-aged women. J Womens Health (Larchmt) 2010;19:1905–1914. doi: 10.1089/jwh.2009.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston RC Sowers MR Chang Y, et al. Adiposity and reporting of vasomotor symptoms among midlife women: the Study of Women's Health Across the Nation. Am J Epidemiol 2008;167:78–85. doi: 10.1093/aje/kwm244 [DOI] [PubMed] [Google Scholar]
- 9.Freeman EW, Sammel MD, Gross SA, Pien GW. Poor sleep in relation to natural menopause: a population-based 14-year follow-up of midlife women. Menopause 2015;22:719–726. doi: 10.1097/gme.0000000000000392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold EB Colvin A Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women's Health Across the Nation. Am J Public Health 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RE Kalilani L DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric 2008;11:32–43. doi: 10.1080/13697130701744696 [DOI] [PubMed] [Google Scholar]
- 12.English M Stoykova B Slota C, et al. Qualitative study: burden of menopause-associated vasomotor symptoms (VMS) and validation of PROMIS sleep disturbance and sleep-related impairment measures for assessment of VMS impact on sleep. J Patient Rep Outcomes 2021;5:37. doi: 10.1186/s41687-021-00289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinkerton JV, Abraham L, Bushmakin AG, Cappelleri JC, Komm BS. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause 2016;23:1060–1066. doi: 10.1097/gme.0000000000000678 [DOI] [PubMed] [Google Scholar]
- 14.Savolainen-Peltonen H, Hautamaki H, Tuomikoski P, Ylikorkala O, Mikkola TS. Health-related quality of life in women with or without hot flashes: a randomized placebo-controlled trial with hormone therapy. Menopause 2014;21:732–739. doi: 10.1097/GME.0000000000000120 [DOI] [PubMed] [Google Scholar]
- 15.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005;3:47. doi: 10.1186/1477-7525-3-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avis NE Crawford SL Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015;175:531–539. doi: 10.1001/jamainternmed.2014.8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson DJ Chung HF Seib CA, et al. Obesity, smoking, and risk of vasomotor menopausal symptoms: a pooled analysis of eight cohort studies. Am J Obstet Gynecol 2020;222:478.e1–478.e17. doi: 10.1016/j.ajog.2019.10.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold EB Crawford SL Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Women's Health Across the Nation (SWAN). Menopause 2017;24:9–26. doi: 10.1097/gme.0000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang R Fan Y Luo M, et al. General and central obesity are associated with increased severity of the VMS and sexual symptoms of menopause among Chinese women: a longitudinal study. Front Endocrinol (Lausanne) 2022;13:814872. doi: 10.3389/fendo.2022.814872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan R, Shiozawa A, Epstein AJ, Espinosa R. Impact of sleep disturbances on employment and work productivity among midlife women in the US SWAN database: a brief report. Menopause 2021;28:1176–1180. doi: 10.1097/GME.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avis NE Colvin A Karlamangla AS, et al. Change in sexual functioning over the menopausal transition: results from the Study of Women's Health Across the Nation. Menopause 2017;24:379–390. doi: 10.1097/gme.0000000000000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromberger JT, Kravitz HM. Mood and menopause: findings from the Study of Women's Health Across the Nation (SWAN) over 10 years. Obstet Gynecol Clin North Am 2011;38:609–625. doi: 10.1016/j.ogc.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bromberger JT Kravitz HM Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Study of Women's Health Across the Nation. Menopause 2013;20:488–495. doi: 10.1097/GME.0b013e3182730599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sowers M, Crawford S, Sternfeld B. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R. (eds). Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000:175–188. [Google Scholar]
- 25.Study of Women's Health Across the Nation (SWAN) . Available at: https://www.swanstudy.org/about/about-swan/. Accessed December 7, 2022.
- 26.Cameron AC, Miller DL. A practitioner's guide to cluster-robust inference. J Hum Resour 2015;50:317–372. doi: 10.3368/jhr.50.2.317 [DOI] [Google Scholar]
- 27.Anic GM, Titus-Ernstoff L, Newcomb PA, Trentham-Dietz A, Egan KM. Sleep duration and obesity in a population-based study. Sleep Med 2010;11:447–451. doi: 10.1016/j.sleep.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care 2011;14:402–412. doi: 10.1097/MCO.0b013e3283479109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke CH Caan BJ Stefanick ML, et al. Effects of a dietary intervention and weight change on vasomotor symptoms in the Women's Health Initiative. Menopause 2012;19:980–988. doi: 10.1097/gme.0b013e31824f606e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston RC, Chang Y, Mancuso P, Matthews KA. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women's Health Across the Nation. Fertil Steril 2013;100:793–800. doi: 10.1016/j.fertnstert.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan UI Wang D Sowers MR, et al. Race-ethnic differences in adipokine levels: the Study of Women's Health Across the Nation (SWAN). Metabolism 2012;61:1261–1269. doi: 10.1016/j.metabol.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walecka-Kapica E, Klupińska G, Chojnacki J, Tomaszewska-Warda K, Błońska A, Chojnacki C. The effect of melatonin supplementation on the quality of sleep and weight status in postmenopausal women. Prz Menopauzalny 2014;13:334–338. doi: 10.5114/pm.2014.47986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodama S Horikawa C Fujihara K, et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol 2012;176:959–969. doi: 10.1093/aje/kws172 [DOI] [PubMed] [Google Scholar]
- 34.de Hollander EL Bemelmans WJ Boshuizen HC, et al. The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: a meta-analysis of 29 cohorts involving more than 58 000 elderly persons. Int J Epidemiol 2012;41:805–817. doi: 10.1093/ije/dys008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


