INTRODUCTION
Cholangiocarcinoma (CCA), also known as bile duct cancer, is responsible for more than 7,000 deaths per year in the United States.1 Estimated mortality because of intrahepatic cholangiocarcinoma (ICC) has significantly increased both globally and in the United States in recent years, while the mortality for extrahepatic cholangiocarcinoma (ECC) has leveled off or decreased.2 Risk factors for biliary tract cancers (BTCs) include choledochal cysts, cholelithiasis, cirrhosis, and chronic inflammatory conditions of the bile ducts such as primary sclerosing cholangitis or liver fluke infection, but most cases are sporadic or emerge in the setting of weakly associated risk factors such as chronic hepatitis B or C, advanced age, alcohol use, inflammatory bowel disease, or type 2 diabetes.3
CONTEXT
Key Objective
To summarize recent advances in precision medicine and immunotherapy for patients with cholangiocarcinoma.
Knowledge Generated
Targetable alterations are found in up to 40%-50% of patients with intrahepatic cholangiocarcinoma and up to 15%-20% of patients with extrahepatic cholangiocarcinoma. Effective therapies exist for FGFR2 fusions, IDH1 mutations, BRAF V600E mutations, NTRK and RET fusions, HER2 amplification/overexpression, MSI-high tumors, and TMB-high tumors, and data continue to emerge for other potential targets such as KRAS G12C mutations, MDM2 amplifications, and DNA repair deficiencies.
Relevance
The emerging therapies outlined in this review are likely to reshape the treatment landscape for cholangiocarcinoma in the coming years.
Traditionally, BTCs have been divided anatomically into intrahepatic, perihilar, and distal bile duct CCAs as well as cancers of the gallbladder. ICC, defined as cancer arising distal to the left and right hepatic ducts, is responsible for approximately two thirds of the annual deaths from CCA in the United States.1,2
Although staging and surgical management vary by site, systemic chemotherapies have been developed in clinical trials that include all BTCs. For advanced BTCs, the combination of gemcitabine and cisplatin (GemCis) has been a frontline standard for more than a decade.4 Recently, the TOPAZ-1 study demonstrated improvements in objective response rate (ORR; 26.7% v 18.7%; P = .011), progression-free survival (PFS; median, 7.2 v 5.7 months; hazard ratio [HR], 0.75; P = .001), and overall survival (OS, median, 12.8 v 11.5 months; HR, 0.80; P = .021) with the addition of the PD-L1 inhibitor durvalumab to GemCis, resulting in US Food and Drug Administration (FDA) approval and establishing a new frontline standard.5
National Comprehensive Cancer Network (NCCN) guidelines also include the triplet regimen gemcitabine, cisplatin, and nab-paclitaxel (GAP) as an acceptable option for frontline treatment of advanced BTC on the basis of a median PFS of 11.8 months and an OS of 19.2 months in a single-arm phase II trial.6 The recently completed phase III SWOG S1815 study comparing GemCis and GAP did not demonstrate an OS advantage for GAP. Subset analyses suggested potential benefit with GAP in patients with locally-advanced tumors or gallbladder cancer.118
In the past decade, molecular profiling has shown that ICC is distinct from other BTCs, with rates of targetable driver mutations estimated as high as 40%-50%.7-12 ICCs arise from the peripheral bile ducts of the liver, and pathologists have further subclassified ICC on the basis of morphologic features into two subtypes—cholangiolar and bile duct—with isocitrate dehydrogenase (IDH) mutations and fibroblast growth factor receptor 2 (FGFR2) fusions being more common in the cholangiolar subtype and human epidermal growth factor receptor 2 (HER2) amplification/overexpression and KRAS mutations being more common in the bile duct subtype.13,14 The recognition of the potential of personalized medicine for patients with CCA has resulted in many novel treatments directed at molecular subsets (Table 1). In this review, we aim to summarize recent developments in therapeutics for CCA and discuss emerging targets and ongoing clinical trials (Fig 1).
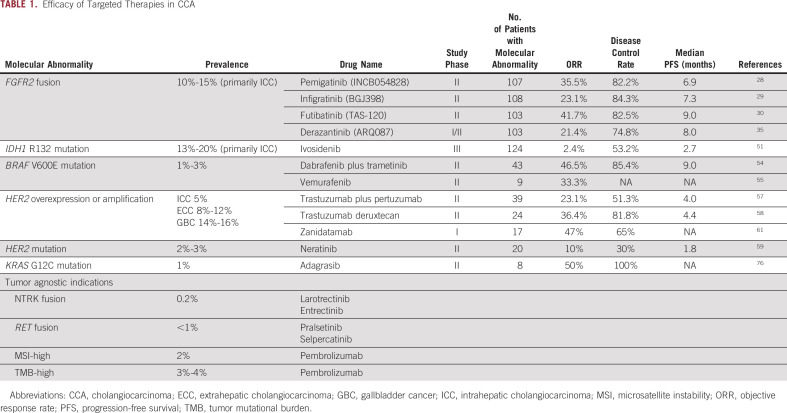
TABLE 1.
Efficacy of Targeted Therapies in CCA
FIG 1.
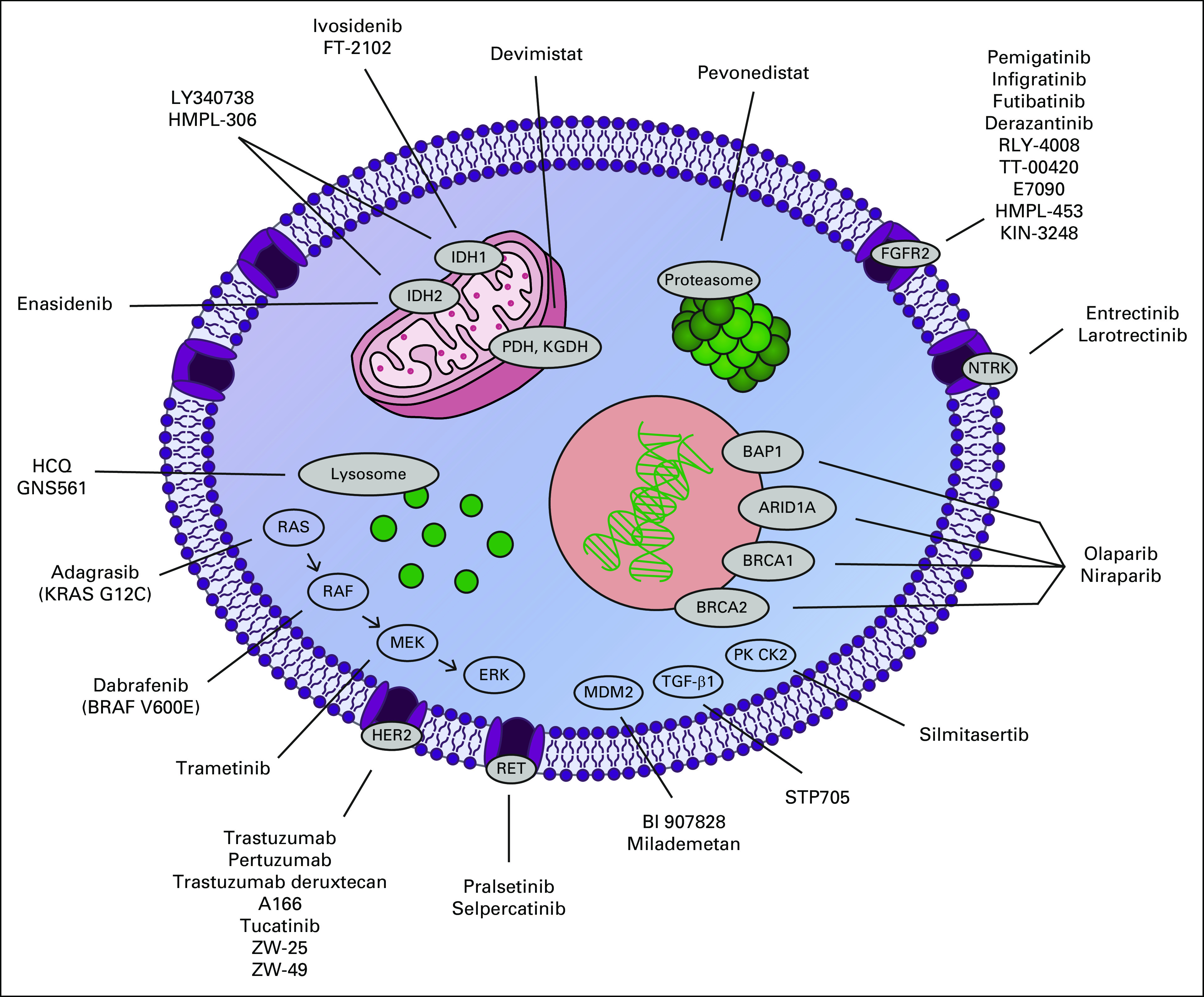
New and emerging targets in cholangiocarcinoma. ARID1A, AT-rich interaction domain 1A; BAP1, BRCA1-associated protein 1; BRAF, v-raf murine sarcoma viral oncogene homolog B1; BRCA, breast cancer gene; ERK, extracellular signal-regulated kinase; FGFR2, fibroblast growth factor receptor 2; HCQ, hydroxychloroquine; HER2, human epidermal growth factor receptor 2; IDH, isocitrate dehydrogenase; KGDH, alpha ketoglutarate dehydrogenase; KRAS, Kirsten rat sarcoma virus; MDM2, mouse double minute 2; MEK, mitogen-activated protein kinase; NTRK, neurotrophic tropomyosin receptor kinase; PDH, pyruvate dehydrogenase; PK CK2, protein kinase CK2; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma virus; RET, rearranged during transfection; TGF-β1, transforming growth factor beta 1.
Molecularly Targeted Agents for Cholangiocarcinoma
Large-scale genomic sequencing efforts have elucidated the mutational landscape of CCA and demonstrated distinct molecular profiles on the basis of site of origin.7,8,10-12,15-17 For example, FGFR2 fusions and IDH1 mutations nearly exclusively occur in ICC.10 Some alterations co-occur frequently in ICC, such as FGFR2 fusions and BAP1 mutations, while others tend to be mutually exclusive, such as FGFR2 fusions and KRAS mutations.8 Mutational frequency can vary on the basis of the underlying etiology (eg, liver-fluke–associated v non-fluke–associated) and geography, with lower frequencies of FGFR2 fusions and IDH1 mutations in Asian populations.16,18,19
The frequency of any given genomic alteration in CCA varies widely among published studies because of differences in sample size, sequencing platform, and proportion of resection versus metastatic samples. More recent analyses of commercial and academic sequencing databases have provided larger sample sizes enriched for advanced disease, which may further refine estimates of mutation prevalence in the relevant treatment population.8,10,12 A variety of assays exist for the molecular profiling of cholangiocarcinoma, and understanding their coverage and limitations is critical for optimizing selection or clinical use. A recent review by Saab et al20 summarizes these assays. Increased use of circulating tumor DNA analysis or liquid biopsy, especially in patients with tumor biopsies insufficient for next-generation sequencing, has provided an additional tool to expand molecular profiling and increase understanding of mutational changes during treatment.21-23
FGFR2 Fusions and Other Activating Alterations
The fibroblast growth factor (FGF) pathway is composed of four membrane tyrosine kinase receptors (FGFR1-4) and 22 FGF ligands involved in cellular growth and development.24 Wu and colleagues first reported the presence of FGFR2 fusions in two cases of ICC in 2013, and subsequent studies have shown the transforming potential and oncogenic activity of these alterations in ICC.25-27 Present in 10%-15% of ICCs, FGFR2 fusions have emerged as a druggable target in this disease with oral small-molecule FGFR inhibitors demonstrating an ORR of 20.7%-41.7% and a median PFS of 5.7-9.0 months.28-31 Additional activating FGFR2 alterations including extracellular domain in-frame deletions (indels) and activating point mutations such as C382R have also demonstrated responsiveness to FGFR inhibition.32,33
In April 2020, the reversible FGFR1-3 inhibitor pemigatinib gained accelerated FDA approval for patients with previously treated cholangiocarcinoma harboring an FGFR2 fusion or rearrangement.28 Among 107 patients with FGFR2 fusions or rearrangements, pemigatinib demonstrated an ORR of 35.5%, a median PFS of 6.9 months, and a median OS of 21.1 months. No responses were seen in 20 patients with other FGF/FGFR alterations or in 18 patients without an FGF/FGFR genetic alteration.34
Infigratinib, another reversible FGFR1-3 inhibitor, received accelerated FDA approval in May 2021. In 108 patients with an FGFR2 fusion or rearrangement, the ORR was 23.1%, with a median PFS of 7.3 months and a median OS of 12.2 months.29
Futibatinib, an irreversible pan-FGFR inhibitor, received accelerated FDA approval in September 2022. It is the first and only covalently binding FGFR inhibitor to receive an oncology indication. Futibatinib demonstrated an ORR of 41.7%, a median PFS of 9.0 months, and a median OS of 21.7 months in patients with FGFR2 fusion or rearrangement positive CCA.30
The reversible FGFR1-3 inhibitor derazantinib has shown preliminary efficacy in patients with cholangiocarcinoma harboring FGFR2 fusions (ORR, 20.7%; PFS, 5.7 months) or activating FGFR2 mutations or FGFR2 amplification (ORR, 8.7%; PFS, 7.3 months).31,35 More recently, RLY-4008, a highly selective irreversible inhibitor specific to FGFR2, showed early clinical activity in patients with FGFR2 fusion cholangiocarcinoma, with objective responses in 14 of 17 patients (82.4%) naive to FGFR inhibitors at the recommended phase II dose and activity in patients previously treated with FGFR inhibitors (RLY-4008: ClinicalTrials.gov identifier: NCT04526106).36-38 Additional FGFR inhibitors are under exploration in ICC, including the multikinase inhibitor TT-00420 (ClinicalTrials.gov identifier: NCT04919642), the bivalent FGFR1-3 inhibitor E7090 (ClinicalTrials.gov identifier: NCT04238715), the FGFR1-3 inhibitor HMPL-453 (ClinicalTrials.gov identifier: NCT04353375), and the irreversible pan-FGFR inhibitors gunagratinib (formerly ICP-192; ClinicalTrials.gov identifier: NCT04565275) and KIN-3248 (ClinicalTrials.gov identifier: NCT05242822).
Given the success of FGFR inhibitors in previously treated patients, an ongoing phase III trial aims to assess the superiority of pemigatinib to frontline GemCis (NCT03656536). The similarly designed phase III trials for infigratinib (NCT03773302) and futibatinib (NCT04093362) had modest accrual and are no longer actively recruiting. Combination studies with FGFR inhibitors are also ongoing in cholangiocarcinoma (GemCis plus pemigatinib or ivodesidenib: ClinicalTrials.gov identifier: NCT04088188, futibatinib plus binimetinib: ClinicalTrials.gov identifier: NCT04965818, derazantinib plus atezolizumab: ClinicalTrials.gov identifier: NCT05174650).
Data continue to emerge about resistance mechanisms to FGFR inhibitors in cholangiocarcinoma, with polyclonal secondary mutations in the FGFR2 kinase domain being a common form of acquired resistance.33,39-43 Many of these resistance mutations are either gatekeeper mutations (eg, V565F/L/I) that prevent binding of FGFR inhibitors through steric hindrance, or molecular brake mutations (eg, N550K/H/D/T) that lead to ligand-independent kinase activation.44,45 Futibatinib has shown potent preclinical and clinical activity against multiple of these mutations that arise at progression on reversible, ATP-competitive FGFR inhibitors.43,46,47
IDH Mutations
Isocitrate dehydrogenase 1 (IDH1) catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate in the cytoplasm.48 Several IDH1 R132 mutations result in neomorphic activity of the enzyme and consequent abnormal production of 2-hydroxyglutarate (2-HG), an oncometabolite that inhibits histone and DNA demethylases and results in widespread epigenetic alterations and oncogenesis.49,50 The reported frequency of IDH1 mutations in ICC versus ECC was 13.1% and 0.8%, respectively, in a systematic review.18
Ivosidenib, a specific inhibitor of mutated IDH1, gained FDA approval in August 2021 for patients with previously treated IDH1-mutated cholangiocarcinoma on the basis of an improvement in PFS versus placebo (HR, 0.37; median, 2.7 v 1.4 months; P < .0001) in the phase III ClarIDHy trial.51 Most patients with clinical benefit had stable disease as the ORR was 2%.
The IDH1 inhibitor BAY1436032 was evaluated in 12 patients with CCA, with stable disease in 42% of patients but no objective responses.52 No further clinical development of BAY1436032 in CCA is planned. Additional agents targeting IDH-mutated cholangiocarcinoma are under development in early-phase trials (FT 2102: ClinicalTrials.gov identifier: NCT03684811, LY3410738: ClinicalTrials.gov identifier: NCT04521686, HMPL-306: ClinicalTrials.gov identifier: NCT04762602)
Isocitrate dehydrogenase 2 (IDH2) is a mitochondrial protein which also promotes tumorigenesis via 2-HG through activating mutations in codons 140 and 172. Mutations in IDH2 occur less frequently in ICC (2%-5%) than mutations in IDH1, and the only reported study targeting IDH2 in patients with cholangiocarcinoma enrolled four patients and observed no objective responses with enasidenib.53
BRAF V600E
Activating V600E mutations in the oncogene BRAF are found in approximately 1%-3% of BTCs. In the phase II ROAR basket trial, 33 patients with previously treated BRAF V600E mutant BTC were treated with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib.54 Objective responses were seen in 20 of 43 evaluable patients (47%), with a median OS of 14.0 months. The FDA approved dabrafenib and trametinib for all noncolorectal solid tumors harboring a BRAF V600E mutation in June 2022. Data are limited with other BRAF inhibitors for BTCs, although vemurafenib monotherapy did demonstrate objective responses in three of nine patients with advanced BTCs.55
HER2
HER2 is a receptor tyrosine kinase overexpressed infrequently in ICC (4.8% per a meta-analysis) and more frequently in ECC (17.4%) and gallbladder cancer (19.1%).56 In the MyPathway basket trial, 39 patients with previously treated BTC with HER2 amplification and/or overexpression were treated with a combination of two anti-HER2 antibodies, trastuzumab and pertuzumab. Partial responses were seen in nine of 39 (23%) patients, and stable disease for at least 4 months was seen in 11 additional patients (28%).57 Median PFS was 4.0 months and median OS was 10.9 months. On the basis of this study, the regimen has been included within NCCN guidelines for advanced previously treated HER2-positive BTC.
The antibody-drug conjugate (ADC) trastuzumab deruxtecan demonstrated an ORR of 36.4% in patients with previously treated advanced HER2-positive BTC, with a median PFS of 4.4 months and a median OS of 7.1 months. One response was also seen among eight patients with HER2-low expression.58 An ongoing international basket study in HER2-expressing solid tumors will provide additional data about the efficacy of trastuzumab deruxtecan in BTC (ClinicalTrials.gov identifier: NCT04482309).
For patients with HER2-mutated BTC, the pan-HER irreversible tyrosine kinase inhibitor neratinib showed responses in two patients (10%) and stable disease of at least 16 weeks in an additional four patients (20%) in a subgroup of the SUMMIT trial.59 Trastuzumab in combination with chemotherapy has also demonstrated anecdotal benefit in case series in advanced HER2 overexpressing and/or amplified BTC.60
A variety of novel HER2-targeting agents are under development in BTCs. Zanidatamab, formerly ZW-25, a bispecific antibody targeting the same epitopes as trastuzumab and pertuzumab, demonstrated an interim ORR of 47% in 17 evaluable patients with advanced pretreated BTCs.61 Further monotherapy expansion in BTC is ongoing for zanidatamab (ClinicalTrials.gov identifier: NCT04466891), as are explorations in combinations with chemotherapy (ClinicalTrials.gov identifier: NCT03929666). The HER2 tyrosine kinase inhibitor tucatinib is being tested in combination with trastuzumab in HER2-positive BTC (ClinicalTrials.gov identifier: NCT04579380). Novel ADCs are also under development in HER2-overexpressing solid tumors including CCA (A166: ClinicalTrials.gov identifier: NCT03602079, ZW-49: ClinicalTrials.gov identifier: NCT03821233).
Additional Molecular Targets
Homologous DNA repair deficiencies.
Approximately 3%-4% of BTCs harbor mutations in BRCA1 (0.6%) or BRCA2 (3%).62 These mutations result in homologous repair deficiency, which limits cellular repair of double-stranded DNA breaks and connotes sensitivity to platinum chemotherapy and PARP inhibitors.63 Case reports have shown efficacy of PARP inhibitors in BRCA-mutant cholangiocarcinoma.64 An additional 10%-15% of ICCs have mutations in BRCA1 associated protein-1 (BAP1) and 15%-20% have mutations in ARID1A, both of which lead to alterations in homologous DNA repair and sensitivity to PARP inhibition in preclinical studies.65-67 A trial of niraparib for BAP1-mutated solid tumors including CCA was terminated early for lack of efficacy.68 Olaparib remains under evaluation in cholangiocarcinoma with DNA repair deficiencies (ClinicalTrials.gov identifier: NCT04042831).
NTRK and RET Fusions.
Oncogenic fusions involving the three tropomyosin receptor kinases TrkA, TrkB, and TrkC (encoded by genes NTRK1, NTRK2, and NTRK3, respectively) occur in approximately 0.2% of BTCs, and are effectively targeted by the NTRK inhibitors larotrectinib and entrectinib.69 Both agents received FDA approval agnostic of histology on the basis of efficacy across tumor types.70,71
RET fusions are also very rare in cholangiocarcinoma but appear sensitive to the RET inhibitors pralsetinib and selpercatinib.72,73 Pralsetinib is now recommended within NCCN guidelines for patients with previously treated BTC with a RET fusion, and selpercatinib is FDA approved for all solid tumors with a RET fusion.
KRAS G12C.
Activating KRAS mutations are present in approximately 12% of ICCs and 35%-40% of ECCs, but only 1% of cholangiocarcinomas harbor KRAS G12C mutations.10,23,74,75 In the initial phase I/II study of the KRAS G12C inhibitor adagrasib, a 100% disease control rate was observed in eight patients with BTCs, with four partial responses (50%).76 Further monotherapy expansion and exploration of combination approaches are ongoing with both adagrasib and sotorasib, and additional KRAS G12C inhibitors have entered clinical development (GDC-6036: ClinicalTrials.gov identifier: NCT04449874, JAB-21822: ClinicalTrials.gov identifier: NCT05002270).
One additional approach under development in KRAS-mutant BTCs is the combination of the MEK inhibitor trametinib with the autophagy inhibitor hydroxychloroquine (ClinicalTrials.gov identifier: NCT04566133). This approach has shown preclinical and clinical activity in pancreatic adenocarcinoma and is being tested across a variety of tumor types.77
MDM2 Amplification.
Mouse double minute 2 (MDM2) amplification is a common driver of certain sarcomas, but has also been reported in up to 6% of ICCs, all bile duct subtype.78 MDM2 acts a negative regulator of TP53, and novel MDM2 inhibitors such as BI 907828 and milademetan have begun to demonstrate efficacy in TP53-wildtype, MDM2-amplified solid tumors79 (BI 907828: ClinicalTrials.gov identifier: NCT03449381; milademetan: ClinicalTrials.gov identifier: NCT05012397).
Immunotherapy for Cholangiocarcinoma
Like pancreatic cancer, CCA is characterized by cancer-associated fibroblasts that produce a desmoplastic stroma as well as a pauci-immune tumor microenvironment rich in immunosuppressive tumor-associated macrophages and myeloid-derived suppressor cells.80 The immune composition of the surrounding liver also plays an important role, with an immunotolerant environment rich in macrophages (Kupffer cells) and natural killer cells with an active innate immune system that is continually exposed to intestinal microbial products.81 Tumor agnostic indications for immunotherapy are uncommon in CCA, with approximately 2% being MSI-high and 3.5% having a high tumor mutational burden.10,82
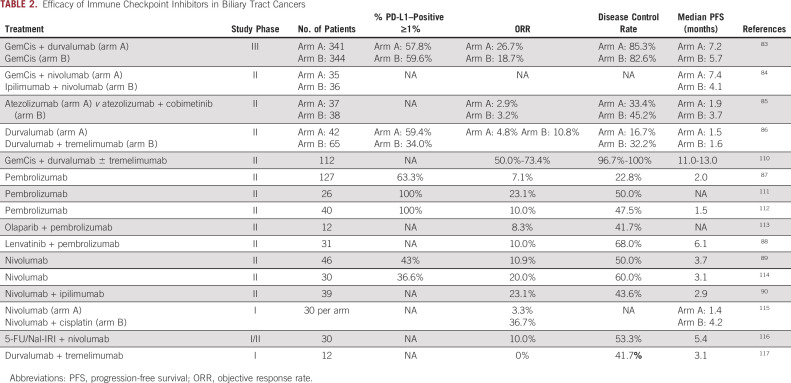
Trials of immune checkpoint inhibitors in refractory BTC have shown mixed results to date with response rates ranging from 5%-20% with single-agent PD-1 inhibitors (Table 2). Both nivolumab alone (ORR, 3.3-20%) and lenvatinib combined with pembrolizumab (ORR, 10%) are included within NCCN guidelines for refractory advanced BTCs on the basis of phase II studies.88,89 Pembrolizumab monotherapy, however, is not included, with an ORR of only 6.8% (eight of 118 patients).87 Small studies using dual checkpoint blockade have shown promising response rates in pretreated patients, with an ORR of 24% with nivolumab and ipilimumab and 11% with durvalumab and tremelimumab, although ipilimumab + nivolumab was inferior to GemCis + nivolumab for frontline treatment of advanced BTC.84,86,90
TABLE 2.
Efficacy of Immune Checkpoint Inhibitors in Biliary Tract Cancers
The global phase III TOPAZ-1 study demonstrated improvements in OS, PFS, and ORR with the addition of durvalumab to frontline GemCis, resulting in FDA approval of durvalumab in combination with chemotherapy for initial treatment of advanced BTC.83 Chemotherapy was stopped at 6 months in both arms, and a greater improvement in OS with durvalumab was seen after that point (HR, 0.91 up to 6 months; HR, 0.74 after 6 months). Subgroup analyses suggested greater benefit in patients in Asia versus the rest of the world (HR, 0.72 v 0.89) and no difference in outcome on the basis of PD-L1 expression. Recently, positive OS results were announced for the global phase III KEYNOTE-966 study, which compared GemCis plus pembrolizumab to GemCis plus placebo in advanced BTC and permitted the use of maintenance gemcitabine chemotherapy.119
Other novel immunotherapy agents have also been evaluated in advanced BTC. The bifunctional TGF-β trap and anti–PD-L1 fusion protein bintrafusp alfa demonstrated an ORR of 10.1% in 159 patients in the second-line setting.91-93 A phase III frontline trial combining bintrafusp alfa with GemCis was terminated early because of lack of efficacy.94 A phase I study combining bintrafusp alfa with hypofractioned radiation in refractory BTC remains ongoing (ClinicalTrials.gov identifier: NCT04708067).
In patients with previously treated advanced BTC, a phase II trial evaluating the combination of the MEK inhibitor cobimetinib with atezolizumab met its primary end point, with a significantly increased PFS (3.6 v 1.9 months; P = .027) with the doublet compared with atezolizumab monotherapy. The ORR was low (3%) in both arms.85 Further preclinical modeling demonstrated that MEK inhibition impaired T-cell activation that was rescued by the addition of either a 4-1BB or a CD27 agonist, and a next-generation trial combining atezolizumab plus the CD27 agonist varlilumab with or without cobimetinib is ongoing95 (ClinicalTrials.gov identifier: NCT04941287).
Novel targets in ongoing trials for BTCs in combination with immune checkpoint inhibitors include Dickkopf-related protein 1 (DKK1; DKN-01: ClinicalTrials.gov identifier: NCT04057365), CSF-1R (SNDX-6532: ClinicalTrials.gov identifier: NCT04301778), galectin 9 (LYT-200: ClinicalTrials.gov identifier: NCT04666688), and DNA-dependent protein kinase (nedisertib: ClinicalTrials.gov identifier: NCT04068194). In some studies, locoregional therapy such as transarterial embolization or external beam radiation is combined with checkpoint inhibition to promote antigen release and immune activation. The novel intratumoral injection INT230-6, consisting of an amphiphilic combination of cisplatin and vinblastine, similarly aims to increase tumor immunogenicity in combination with checkpoint inhibitors (ClinicalTrials.gov identifier: NCT03058289).
Additional Therapeutic Approaches
A variety of additional therapies have demonstrated preclinical and/or early clinical evidence of activity in cholangiocarcinoma. These agents target specific proteins or pathways involved in cholangiocarcinogenesis and are undergoing further clinical testing (Table 3).
TABLE 3.
Novel Targeted Agents for Biliary Tract Cancers
Antibodies and antibody-drug conjugates for CCA.
CCA expresses a variety of cell-surface proteins with limited expression in nontumor tissue, and a variety of new antibodies, ADCs, and cellular immunotherapies have been developed to engage these targets.96 Improvements in molecular biology have led to improved drug delivery and decreased systemic exposure with ADCs, allowing for combination with more potent cytotoxics.
Cancer antigen 19-9 (CA 19-9) is a sialylated Lewis antigen expressed on the surface of tumor cells as well as in the blood of approximately 80% of patients with advanced cholangiocarcinoma.97 MVT-5873, an IgG1 antibody targeting an epitope on CA 19-9, has demonstrated safety in combination with gemcitabine and nab-paclitaxel in patients with advanced pancreatic cancer, and is undergoing evaluation as a perioperative therapy in patients with resectable cholangiocarcinoma98 (ClinicalTrials.gov identifier: NCT03801915).
Mesothelin is a cell-surface protein expressed in mesothelial cells lining the peritoneum, pericardium, and pleural surface that is overexpressed in a variety of cancer types including pancreatic adenocarcinoma, mesothelioma, ovarian cancer, lung cancer, and cholangiocarcinoma.99 A variety of therapies targeting mesothelin overexpression are in development, including antibody-drug conjugates and cellular immunotherapies.100
Additional cell-surface targets found in CCA under investigation include claudin 18.2 (BNT141: ClinicalTrials.gov identifier: NCT04683939) and B7-H4 (AZD8205: ClinicalTrials.gov identifier: NCT05123482, SGN-B7H4V: ClinicalTrials.gov identifier: NCT05194072).
Novel molecular therapies.
Pevonedistat is an inhibitor of neddylation, a pathway of intracellular protein catabolism related to ubiquitination that is overactive in CCA.101 In a phase Ib trial combining pevonedistat with multiple chemotherapy regimens, both patients with previously treated cholangiocarcinoma had partial responses.102 Further evaluation of pevonedistat as monotherapy or in combination with carboplatin and paclitaxel in previously treated advanced ICC is ongoing (ClinicalTrials.gov identifier: NCT04175912).
Silmitasertib (CX-4945) is a small molecule inhibitor of protein kinase casein kinase II, which has been shown preclinically to inhibit growth of cholangiocarcinoma cell lines and induce lethal vacuolization.103 A phase Ib/II study in 87 patients with advanced CCA combining silmitasertib with GemCis showed a median PFS of 11.1 months, a median OS of 17.4 months, and an ORR of 32.1%, and a phase III trial is planned.104
GNS561 is a small lipophilic molecule that accumulates within lysosomes and causes dysregulation and apoptotic cell death. On the basis of preclinical efficacy in hepatocellular carcinoma and ICC models, a phase I/II trial was launched (ClinicalTrials.gov identifier: NCT03316222). In the initial 19 patients from the 3 + 3 dose escalation updated at ASCO in 2021, no dose-limiting toxicities were observed and two of nine patients with ICC experienced stable disease.105
BI 905711 is a novel tetravalent bispecific antibody targeting TRAILR2 and CDH17.106 TRAIL is a member of the TNFα superfamily, and activation of the TRAIL receptor results in apoptotic cell death, but prior antibodies have been limited by hepatic toxicity.107 The cell-surface marker CDH17 is expressed in a variety of gastrointestinal cancers but not in normal liver, allowing for specific targeting of tumor cells without engaging hepatocytes. A phase I trial is ongoing, with a planned expansion in cholangiocarcinoma (ClinicalTrials.gov identifier: NCT04137289).
Devimistat (formerly CPI-613) is a lipoate analog that inhibits pyruvate dehydrogenase and a-ketoglutarate dehydrogenase and alters mitochondrial metabolism. In the phase I portion of a phase I/II study of devimistat combined with GemCis in patients with untreated advanced BTC, the regimen demonstrated an ORR of 45% with a median PFS of 14.9 months without excess toxicity. The randomized phase II portion of the trial testing GemCis with or without devimistat is ongoing108 (ClinicalTrials.gov identifier: NCT04203160).
STP705 is an injectable combination of small interfering ribonucleic acid targeting TGF-β1 and cyclooxygenase-2 (COX-2) with histidine-lysine polypeptide (siRNA/HKP) in a nanoparticle formulation that has demonstrated efficacy with repeated intratumoral injections in cutaneous squamous cell carcinoma.109 A phase I study is evaluating serial liver tumor injections in cholangiocarcinoma and other liver tumors (ClinicalTrials.gov identifier: NCT04676633).
DISCUSSION
With the FDA approvals of pemigatinib, infigratinib, futibatinib, and ivosidenib, CCA has entered the era of molecular therapy, and it is likely that additional targeted agents will be approved in the next several years. Data strongly support targeting FGFR2 fusions, IDH1 mutations, HER2 overexpression/amplification, and tissue agnostic targets such as BRAF V600E mutations and NTRK and RET fusions. Among the most promising targeted therapies in development are irreversible FGFR2 inhibitors, which have been shown to overcome resistance to reversible FGFR2 inhibitors, HER2 ADCs such as trastuzumab deruxtecan, and KRAS G12C inhibitors.
Given that 40%-50% of ICCs and 15%-20% of ECCs will have actionable mutations, molecular testing should be conducted in all patients with advanced CCA early in the course of their treatment. CCA is often paucicellular and challenging to biopsy, and liquid biopsy can serve as a complementary approach for molecular profiling.120 Improved access to rapid biomarker profiling may also facilitate clinical trials in earlier-stage disease to test molecular therapies in the neoadjuvant and adjuvant settings. Serial sequencing of circulating tumor DNA will also elucidate tumor evolution and resistance mechanisms to targeted therapies and advance development of rational combinations and next-generation inhibitors.
The TOPAZ-1 study has led to a new frontline standard of GemCis with durvalumab for patients with advanced disease. Data from the similarly designed KEYNOTE-966 study are expected soon and will further clarify the benefit of frontline immunotherapy. No predictive biomarker for immunotherapy in cholangiocarcinoma has been identified to date, and further research in this area is sorely needed to better identify the subset of patients who experience significant benefit. Immunotherapeutic combinations may enhance the efficacy of PD-1 and PD-L1 inhibitors, but widespread use of durvalumab in the frontline setting will affect enrollment rates of immunotherapy-naive patients in refractory settings and will likely necessitate changes in trial design.
As additional therapies become available for CCA, questions about optimal selection and sequencing in those with targetable mutations will likely arise. No strong data yet exist to guide the choice between initial chemotherapy and highly effective therapies such as NTRK inhibitors for NTRK fusions or PD-1 inhibitors for MSI-high tumors. Randomized studies to assess the performance of frontline FGFR inhibitors against GemCis are commendable, but the results may be difficult to interpret with the evolving frontline standard for advanced BTC. With this growing treatment armamentarium, investment in biomarker development to optimize patient selection will enable even bigger strides in precision oncology in cholangiocarcinoma.
ACKNOWLEDGMENT
The authors would like to acknowledge support from Jacqui Lewis and the RARE Initiative, and from Joe and Katie Comeau. The authors would also like to acknowledge Nyah Yao for graphic design support.
Thomas B. Karasic
Honoraria: Incyte, Pfizer, Ipsen, AstraZeneca/MedImmune, Taiho Oncology
Consulting or Advisory Role: Nucorion, Incyte
Research Funding: Taiho Pharmaceutical (Inst), H3 Biomedicine (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Tempest Therapeutics (Inst), Xencor (Inst), Genentech (Inst)
Jennifer R. Eads
Employment: Bristol Myers Squibb, Janssen Oncology
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Advanced Accelerator Applications
Research Funding: Xencor (Inst), Tarveda Therapeutics (Inst), MedImmune (Inst), Incyte, Oncolys BioPharma (Inst), Seattle Genetics (Inst), Genentech (Inst), Hutchison MediPharma (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen Oncology
Lipika Goyal
Consulting or Advisory Role: Alentis Therapeutics, QED Therapeutics, AstraZeneca, Taiho Pharmaceutical, Incyte, Sirtex Medical, Genentech, Exelixis, TransThera Biosciences, Merck, Black Diamond Therapeutics, Synthekine, Eisai/H3 Biomedicine, Tyra Biosciences, Kinnate Biopharma, Compass Therapeutics, Blueprint Medicines, SERVIER
Uncompensated Relationships: Boehringer Ingelheim
No other potential conflicts of interest were reported.
SUPPORT
L.G. receives funding from the American Cancer Society Clinical Scientist Development Grant 134,013‐CSDG‐19‐163‐01‐T.B.G., the NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003, V Foundation for Cancer Research Translational Grant, and the Cholangiocarcinoma Foundation Andrea Marie Fuquay Research Fellowship.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Thomas B. Karasic, Lipika Goyal
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas B. Karasic
Honoraria: Incyte, Pfizer, Ipsen, AstraZeneca/MedImmune, Taiho Oncology
Consulting or Advisory Role: Nucorion, Incyte
Research Funding: Taiho Pharmaceutical (Inst), H3 Biomedicine (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Tempest Therapeutics (Inst), Xencor (Inst), Genentech (Inst)
Jennifer R. Eads
Employment: Bristol Myers Squibb, Janssen Oncology
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Advanced Accelerator Applications
Research Funding: Xencor (Inst), Tarveda Therapeutics (Inst), MedImmune (Inst), Incyte, Oncolys BioPharma (Inst), Seattle Genetics (Inst), Genentech (Inst), Hutchison MediPharma (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen Oncology
Lipika Goyal
Consulting or Advisory Role: Alentis Therapeutics, QED Therapeutics, AstraZeneca, Taiho Pharmaceutical, Incyte, Sirtex Medical, Genentech, Exelixis, TransThera Biosciences, Merck, Black Diamond Therapeutics, Synthekine, Eisai/H3 Biomedicine, Tyra Biosciences, Kinnate Biopharma, Compass Therapeutics, Blueprint Medicines, SERVIER
Uncompensated Relationships: Boehringer Ingelheim
No other potential conflicts of interest were reported.
REFERENCES
- 1. Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: An analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16:117. doi: 10.1186/s12876-016-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 3. Clements O, Eliahoo J, Kim JU, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95–103. doi: 10.1016/j.jhep.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 4. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 5. Oh DY, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1:EVIDoa2200015. doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 6. Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial. JAMA Oncol. 2019;5:824–830. doi: 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahn DH, Javle M, Ahn CW, et al. Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer. 2016;122:3657–3666. doi: 10.1002/cncr.30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for Intervention. Clin Cancer Res. 2018;24:4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valle JW, Lamarca A, Goyal L, et al. New Horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7:943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinberg BA, Xiu J, Lindberg MR, et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J Gastrointest Oncol. 2019;10:652–662. doi: 10.21037/jgo.2018.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wardell CP, Fujita M, Yamada T, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959–969. doi: 10.1016/j.jhep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 12. Lee H, Ross JS. The potential role of comprehensive genomic profiling to guide targeted therapy for patients with biliary cancer. Ther Adv Gastroenterol. 2017;10:507–520. doi: 10.1177/1756283X17698090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brackett DG, Neyaz A, Arora K, et al. Cholangiolar pattern and albumin in situ hybridisation enable a diagnosis of intrahepatic cholangiocarcinoma. J Clin Pathol. 2020;73:23–29. doi: 10.1136/jclinpath-2019-206055. [DOI] [PubMed] [Google Scholar]
- 14. Liau JY, Tsai JH, Yuan RH, et al. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27:1163–1173. doi: 10.1038/modpathol.2013.241. [DOI] [PubMed] [Google Scholar]
- 15. Farshidfar F, Zheng S, Gingras MC, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;19:2878–2880. doi: 10.1016/j.celrep.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7:1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 18. Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J Gastrointest Oncol. 2019;10:751–765. doi: 10.21037/jgo.2019.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaisaingmongkol J, Budhu A, Dang H, et al. Common molecular subtypes among asian Hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell. 2017;32:57–70.e3. doi: 10.1016/j.ccell.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol. 2021;32:1111–1126. doi: 10.1016/j.annonc.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 21. Ettrich TJ, Schwerdel D, Dolnik A, et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep. 2019;9:13261. doi: 10.1038/s41598-019-49860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mody K, Kasi PM, Yang J, et al. Circulating tumor DNA profiling of advanced biliary tract cancers. JCO Precision Oncol. 2019;3:1–9. doi: 10.1200/PO.18.00324. [DOI] [PubMed] [Google Scholar]
- 23. Berchuck JE, Facchinetti F, DiToro DF, et al. The clinical landscape of cell-free DNA alterations in 1,671 patients with advanced biliary tract cancer. Ann Oncol Off J Eur Soc Med Oncol. 2022;33:1269–1283. doi: 10.1016/j.annonc.2022.09.150. [DOI] [PubMed] [Google Scholar]
- 24. Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 25. Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 27. Sia D, Losic B, Moeini A, et al. Massive parallel sequencing uncovers actionable FGFR2 – PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- 28. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. The Lancet Gastroenterol Hepatol. 2021;6:803–815. doi: 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 30. Goyal L, Meric-Bernstam F, Hollebecque A, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388:228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 31. Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cleary JM, Raghavan S, Wu Q, et al. FGFR2 extracellular domain in-frame deletions are therapeutically targetable genomic alterations that function as oncogenic drivers in cholangiocarcinoma. Cancer Discov. 2021;11:2488–2505. doi: 10.1158/2159-8290.CD-20-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silverman IM, Hollebecque A, Friboulet L, et al. Clinicogenomic analysis of FGFR2 -rearranged cholangiocarcinoma identifies Correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11:326–339. doi: 10.1158/2159-8290.CD-20-0766. [DOI] [PubMed] [Google Scholar]
- 34. Vogel A, Sahai V, Hollebecque A, et al. FIGHT-202: A phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA) Ann Oncol. 2019;30:v876. [Google Scholar]
- 35. Javle MM, Abou-Alfa GK, Macarulla T, et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01 J Clin Oncol 40 427 2022. 34793250 [Google Scholar]
- 36. Casaletto J, Maglic D, Toure BB, et al. Abstract 1455: RLY-4008, a novel precision therapy for FGFR2-driven cancers designed to potently and selectively inhibit FGFR2 and FGFR2 resistance mutations. Cancer Res. 2021;81:1455. [Google Scholar]
- 37. Goyal L, Borad M, Subbiah V, et al. Abstract P02-02: First results of RLY-4008, a potent and highly selective FGFR2 inhibitor in a first-in-human study in patients with FGFR2-altered cholangiocarcinoma and multiple solid tumors. Mol Cancer Ther. 2021;20:P02-02. [Google Scholar]
- 38. Hollebecque A, Borad M, Goyal L, et al. LBA12 efficacy of RLY-4008, a highly selective FGFR2 inhibitor in patients (pts) with an FGFR2-fusion or rearrangement (f/r), FGFR inhibitor (FGFRi)-naïve cholangiocarcinoma (CCA): ReFocus trial. Ann Oncol. 2022;33:S1381. [Google Scholar]
- 39. Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7:252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krook MA, Bonneville R, Chen HZ, et al. Tumor heterogeneity and acquired drug resistance in FGFR2-fusion-positive cholangiocarcinoma through rapid research autopsy. Cold Spring Harb Mol Case Stud. 2019;5:a004002. doi: 10.1101/mcs.a004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krook MA, Lenyo A, Wilberding M, et al. Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma. Mol Cancer Ther. 2020;19:847–857. doi: 10.1158/1535-7163.MCT-19-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varghese AM, Patel J, Janjigian YY, et al. Noninvasive detection of polyclonal acquired resistance to FGFR inhibition in patients with cholangiocarcinoma harboring FGFR2 alterations. JCO Precision Oncol. 2021;5:44–50. doi: 10.1200/PO.20.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goyal L, Shi L, Liu LY, et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion–positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9:1064–1079. doi: 10.1158/2159-8290.CD-19-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen H, Ma J, Li W, et al. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol Cell. 2007;27:717–730. doi: 10.1016/j.molcel.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tan L, Wang J, Tanizaki J, et al. Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors. Proc Natl Acad Sci USA. 2014;111:E4869–E4877. doi: 10.1073/pnas.1403438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sootome H, Fujita H, Ito K, et al. Futibatinib is a novel irreversible FGFR 1–4 inhibitor that Shows selective antitumor activity against FGFR-deregulated tumors. Cancer Res. 2020;80:4986–4997. doi: 10.1158/0008-5472.CAN-19-2568. [DOI] [PubMed] [Google Scholar]
- 47.Meric-Bernstam F, Bahleda R, Hierro C, et al. Futibatinib, an irreversible FGFR1–4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: A phase I dose-expansion study Cancer Discov 12402-415, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metab. 2012;16:9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. The Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wick A, Bähr O, Schuler M, et al. Phase I assessment of safety and therapeutic activity of BAY1436032 in patients with IDH1-mutant solid tumors. Clin Cancer Res. 2021;27:2723–2733. doi: 10.1158/1078-0432.CCR-20-4256. [DOI] [PubMed] [Google Scholar]
- 53.CelgeneStudy of Orally Administered Enasidenib (AG-221) in Adults With Advanced Solid Tumors, Including Glioma, or Angioimmunoblastic T-Cell Lymphoma, With an IDH2 Mutation 2016. https://clinicaltrials.gov/ct2/show/NCT02273739 [Google Scholar]
- 54. Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 55. Subbiah V, Puzanov I, Blay JY, et al. Pan-cancer efficacy of vemurafenib in BRAF V600 -mutant non-melanoma cancers. Cancer Discov. 2020;10:657–663. doi: 10.1158/2159-8290.CD-19-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Galdy S, Lamarca A, McNamara MG, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017;36:141–157. doi: 10.1007/s10555-016-9645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Javle M, Borad MJ, Azad NS, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22:1290–1300. doi: 10.1016/S1470-2045(21)00336-3. [DOI] [PubMed] [Google Scholar]
- 58. Ohba A, Morizane C, Kawamoto Y, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial) J Clin Oncol. 2022;40:4006. [Google Scholar]
- 59. Harding J, Cleary J, Shapiro G, et al. Treating HER2-mutant advanced biliary tract cancer with neratinib: Benefits of HER2-directed targeted therapy in the phase 2 SUMMIT ‘basket’ trial. Ann Oncol. 2019;30:iv127. [Google Scholar]
- 60. Mondaca S, Razavi P, Xu C, et al. Genomic characterization of ERBB2-driven biliary cancer and a case of response to ado-trastuzumab emtansine. JCO Precis Oncol. 2019 doi: 10.1200/PO.19.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meric-Bernstam F, Hanna DL, El-Khoueiry AB, et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J Clin Oncol. 2021;39:299. [Google Scholar]
- 62. Spizzo G, Puccini A, Xiu J, et al. Frequency of BRCA mutation in biliary tract cancer and its correlation with tumor mutational burden (TMB) and microsatellite instability (MSI) J Clin Oncol. 2019;37:4085. [Google Scholar]
- 63. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Golan T, Raitses-Gurevich M, Kelley RK, et al. Overall Survival and clinical Characteristics of BRCA-associated cholangiocarcinoma: A multicenter retrospective study. Oncologist. 2017;22:804–810. doi: 10.1634/theoncologist.2016-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Inoue S, Li WY, Tseng A, et al. Mutant IDH1 downregulates ATM and alters DNA repair and sensitivity to DNA damage independent of TET2. Cancer Cell. 2016;30:337–348. doi: 10.1016/j.ccell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017;9:eaal2463. doi: 10.1126/scitranslmed.aal2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shen J, Peng Y, Wei L, et al. ARID1A deficiency Impairs the DNA damage checkpoint and Sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5:752–767. doi: 10.1158/2159-8290.CD-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. George TJ, Lee JH, Hosein PJ, et al. Results of a phase II trial of the PARP inhibitor, niraparib, in BAP1 and other DNA damage response pathway deficient neoplasms. J Clin Oncol. 2022;40:3122. [Google Scholar]
- 69. Westphalen CB, Krebs MG, Le Tourneau C, et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precision Oncol. 2021;5:69–9. doi: 10.1038/s41698-021-00206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and Children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Subbiah V, Wolf J, Konda B, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23:1261–1273. doi: 10.1016/S1470-2045(22)00541-1. [DOI] [PubMed] [Google Scholar]
- 73. Subbiah V, Cassier PA, Siena S, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28:1640–1645. doi: 10.1038/s41591-022-01931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou SL, Xin HY, Sun RQ, et al. Association of KRAS variant subtypes with Survival and recurrence in patients with surgically treated intrahepatic cholangiocarcinoma. JAMA Surg. 2022;157:59–65. doi: 10.1001/jamasurg.2021.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73:315–327. doi: 10.1016/j.jhep.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bekaii-Saab TS, Spira AI, Yaeger R, et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation J Clin Oncol 40 519 2022. 34878805 [Google Scholar]
- 77. Kinsey CG, Camolotto SA, Boespflug AM, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620–627. doi: 10.1038/s41591-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim SJ, Akita M, Sung YN, et al. MDM2 amplification in intrahepatic cholangiocarcinomas: Its relationship with large-duct type morphology and uncommon KRAS mutations. Am J Surg Pathol. 2018;42:512–521. doi: 10.1097/PAS.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 79. LoRusso P, Gounder MM, Patel MR, et al. A phase I dose-escalation study of the MDM2-p53 antagonist BI 907828 in patients (pts) with advanced solid tumors. J Clin Oncol. 2021;39:3016. [Google Scholar]
- 80. Loeuillard E, Conboy CB, Gores GJ, Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019;1:297–311. doi: 10.1016/j.jhepr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fabris L, Perugorria MJ, Mertens J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39:63–78. doi: 10.1111/liv.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oh DY, He AR, Qin S, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol. 2022;40:378. [Google Scholar]
- 84. Sahai V, Griffith KA, Beg MS, et al. A multicenter randomized phase II study of nivolumab in combination with gemcitabine/cisplatin or ipilimumab as first-line therapy for patients with advanced unresectable biliary tract cancer (BilT-01) J Clin Oncol. 2020;38:4582. [Google Scholar]
- 85. Yarchoan M, Cope L, Ruggieri AN, et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J Clin Invest. 2021;131:e152670. doi: 10.1172/JCI152670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ioka T, Ueno M, Oh DY, et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC) J Clin Oncol. 2019;37:387. [Google Scholar]
- 87. Piha-Paul SA, Oh DY, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 88. Villanueva L, Lwin Z, Chung HC, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021;39:321. [Google Scholar]
- 89. Kim RD, Chung V, Alese OB, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6:888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klein O, Kee D, Nagrial A, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: Subgroup analysis of a phase 2 Nonrandomized clinical trial. JAMA Oncol. 2020;6:1405. doi: 10.1001/jamaoncol.2020.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Borad M, Javle M, Furuse J, et al. AB052. P-20. Phase 2, open-label study of second-line M7824 treatment in patients with locally advanced or metastatic biliary tract cancer. Hepatobiliary Surg Nutr. 2019;8:AB052. [Google Scholar]
- 92. Yoo C, Oh DY, Choi HJ, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8:e000564. doi: 10.1136/jitc-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merck KGaA M7824 Monotherapy in Locally Advanced or Metastatic Second Line (2L) Biliary Tract Cancer (Cholangiocarcinoma and Gallbladder Cancer) 2020. https://www.merckgroup.com/en/news/bintrafusp-topline-data-biliary-tract-cancer-16-03-2021 [Google Scholar]
- 94.Merck KGaA Gemcitabine Plus Cisplatin with or without Bintrafusp Alfa (M7824) in Participants with 1L BTC 2021. https://www.emdgroup.com/en/news/bintrafusp-alfa-037-update-20-01-2021.html [Google Scholar]
- 95. Dennison L, Ruggieri A, Mohan A, et al. Context-dependent Immunomodulatory effects of MEK inhibition are enhanced with T-cell agonist therapy. Cancer Immunol Res. 2021;9:1187–1201. doi: 10.1158/2326-6066.CIR-21-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stephenson B, Shimwell N, Humphreys E, et al. Quantitative assessment of the cell surface proteome to identify novel therapeutic targets in cholangiocarcinoma. Lancet. 2015;385:S94. doi: 10.1016/S0140-6736(15)60409-3. [DOI] [PubMed] [Google Scholar]
- 97. Qin XL, Wang ZR, Shi JS, et al. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J Gastroenterol. 2004;10:427–432. doi: 10.3748/wjg.v10.i3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. O’Reilly EM, Wang JSZ, Yu KH, et al. Abstract LB-B25: Preliminary phase I data comparing HuMab-5B1 (MVT-5873), a monoclonal antibody targeting sLea, as a single agent and in combination with first line nab-paclitaxel and gemcitabine in patients with CA19-9 positive pancreatic cancer. Mol Cancer Ther. 2018;17:LB-B25. [Google Scholar]
- 99. Hassan R, Thomas A, Alewine C, et al. Mesothelin immunotherapy for cancer: Ready for prime time? J Clin Oncol. 2016;34:4171–4179. doi: 10.1200/JCO.2016.68.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rottey S, Clarke J, Aung K, et al. Phase I/IIa trial of BMS-986148, an anti-mesothelin antibody–drug conjugate, alone or in combination with nivolumab in patients with advanced solid tumors. Clin Cancer Res. 2022;28:95–105. doi: 10.1158/1078-0432.CCR-21-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gao Q, Yu GY, Shi JY, et al. Neddylation pathway is up-regulated in human intrahepatic cholangiocarcinoma and serves as a potential therapeutic target. Oncotarget. 2014;5:7820–7832. doi: 10.18632/oncotarget.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bauer TM, Harvey RD, Lee CB, et al. Investigational NEDD8-activating enzyme inhibitor pevonedistat (Pev) plus chemotherapy in patients (Pts) with solid tumors (Phase 1b study): Antitumor activity of pev plus carboplatin (Carbo)/paclitaxel (Pac) J Clin Oncol. 2016;34:2580. [Google Scholar]
- 103. Lertsuwan J, Lertsuwan K, Sawasdichai A, et al. CX-4945 induces methuosis in cholangiocarcinoma cell lines by a CK2-independent mechanism. Cancers. 2018;10:283. doi: 10.3390/cancers10090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Borad MJ, Bai LY, Chen MH, et al. Silmitasertib (CX-4945) in combination with gemcitabine and cisplatin as first-line treatment for patients with locally advanced or metastatic cholangiocarcinoma: A phase Ib/II study. J Clin Oncol. 2021;39:312. [Google Scholar]
- 105. Harding JJ, Awada A, Decaens T, et al. First-in-human phase I, pharmacokinetic (PK), and pharmacodynamic (PD) study of oral GNS561, a palmitoyl-protein thioesterase 1 (PPT1) inhibitor, in patients with primary and secondary liver malignancies. J Clin Oncol. 2021;39:e16175. [Google Scholar]
- 106. García-Martínez JM, Wang S, Weishaeupl C, et al. Selective tumor cell apoptosis and tumor regression in CDH17-positive colorectal cancer models using BI 905711, a novel liver-Sparing TRAILR2 agonist. Mol Cancer Ther. 2021;20:96–108. doi: 10.1158/1535-7163.MCT-20-0253. [DOI] [PubMed] [Google Scholar]
- 107. Papadopoulos KP, Isaacs R, Bilic S, et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody® targeting the DR5 receptor. Cancer Chemother Pharmacol. 2015;75:887–895. doi: 10.1007/s00280-015-2712-0. [DOI] [PubMed] [Google Scholar]
- 108. Sahai V, Zhen DB, Crysler OV, et al. Phase 1b results of a multicenter, randomized phase 1b/2 study of gemcitabine and cisplatin +/- CPI-613 as first-line therapy for patients with advanced biliary tract cancer (BilT-04) J Clin Oncol. 2022;40:4094. [Google Scholar]
- 109. Nestor M, Berman B, Lu P, et al. Safety and efficacy of TGF-β1/COX-2 Silencing therapeutic in adults with cutaneous squamous cell carcinoma in situ. J Drugs Dermatol. 2022;21:472–477. doi: 10.36849/JDD.6384. [DOI] [PubMed] [Google Scholar]
- 110. Oh DY, Lee KH, Lee DW, et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC) J Clin Oncol. 2020;38:4520. supp 15; abstr 4520. [Google Scholar]
- 111. Ahn S, Lee JC, Shin DW, et al. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 2020;10:12348. doi: 10.1038/s41598-020-69366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kang J, Jeong JH, Hwang HS, et al. Efficacy and safety of pembrolizumab in patients with refractory advanced biliary tract cancer: Tumor proportion score as a potential biomarker for response. Cancer Res Treat. 2020;52:594–603. doi: 10.4143/crt.2019.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yin C, Armstrong SA, Agarwal S, et al. Phase II study of combination pembrolizumab and olaparib in patients with advanced cholangiocarcinoma: Interim results. J Clin Oncol. 2022;40:452. supp 4; abstr 452. [Google Scholar]
- 114. Gou M, Zhang Y, Si H, Dai G. Efficacy and safety of nivolumab for metastatic biliary tract cancer. OncoTargets Ther. 2019;12:861–7. doi: 10.2147/OTT.S195537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:611–621. doi: 10.1016/S2468-1253(19)30086-X. [DOI] [PubMed] [Google Scholar]
- 116. Sahai V, Griffith KA, Lin BSL, et al. A multicenter phase Ib/II study of liposomal-irinotecan, 5-fluorouracil (5-FU), and leucovorin (LV) with nivolumab as second-line therapy for patients with advanced biliary tract cancer (BilT-03) J Clin Oncol 40 438 2022. supp 4; abstr 438 34890214 [Google Scholar]
- 117. Floudas CS, Xie C, Brar G, et al. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC) J Clin Oncol 37 336 2019. supp 4; abstr 336 30707056 [Google Scholar]
- 118. Shroff RT, Guthrie KA, Scott AJ, et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023;41 suppl; abstr LBA490. [Google Scholar]
- 119.Merck Merck's Keytruda (pembrtolizumab) plus chemotherapy significantly improved overall survival versus chemotherapy in first-line advanced or unresectable biliary tract cancer in KEYNOTE-966 trial. https://www.merck.com/news/mercks-keytruda-pembrolizumab-plus-chemotherapy-significantly-improved-overall-survival-versus-chemotherapy-in-first-line-advanced-or-unresectable-biliary-tract-cancer-in-keynote-966
- 120. Berchuck JE, Facchinetti F, DiToro DF, et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann Oncol. 2022;33:1269–1283. doi: 10.1016/j.annonc.2022.09.150. [DOI] [PubMed] [Google Scholar]