Abstract
Soybeans are rich in proteins and phytochemicals such as isoflavones and phenolic compounds. It is an excellent source of peptides with numerous biological functions, including anti-inflammatory, anticancer, and antidiabetic activities. Soy bioactive peptides are small building blocks of proteins that are released after fermentation or gastrointestinal digestion as well as by food processing through enzymatic hydrolysis, often in combination with novel food processing techniques (i.e., microwave, ultrasound, and high-pressure homogenization), which are associated with numerous health benefits. Various studies have reported the potential health benefits of soybean-derived functional peptides, which have made them a great substitute for many chemical-based functional elements in foods and pharmaceutical products for a healthy lifestyle. This review provides unprecedented and up-to-date insights into the role of soybean peptides in various diseases and metabolic disorders, ranging from diabetes and hypertension to neurodegenerative disorders and viral infections with mechanisms were discussed. In addition, we discuss all the known techniques, including conventional and emerging approaches, for the prediction of active soybean peptides. Finally, real-life applications of soybean peptides as functional entities in food and pharmaceutical products are discussed.
Keywords: soybean, functional peptides, plant food, health, fermentation
Introduction
Soybean (Glycine max) is a legume of the family Fabaceae (Leguminosae), which originated ~5,000 years ago in East Asia. Currently, the USA, Brazil, and Argentina account for ~34, 30, and 17% of the global soybean production, respectively. China, Japan, Mexico, and many European countries are the main soybean-importing countries, and their export products are oil, meal, and seed, which account for 80% of their known export value (1). Standard soybean seeds with 13% of moisture comprise 19% oil and 68% meal fractions (2, 3).
Soybean is a potent source of vegetable proteins and phytochemicals, such as isoflavones and phenolic compounds, because of its amino acid composition, high availability, and low cost, making it the second largest source of vegetable oil globally (4, 5). It consists of protein (30–35%), lipids (20%), dietary fiber (9%), and moisture (8.5%) based on the dry weight of soybean seeds (6). Various environmental conditions and genetic modifications affect the soybean protein composition which has an influence on soybean health-promoting properties for the production of functional soybean-based products (7). Albumins and globulins are the two main proteins in soybean, and the latter is considered the main protein component of soybeans. The two major storage proteins of soybean are β-conglycinin (βCG, 7S) and glycinin (11S), which account for 80–90% of the total protein in soybean (8–10). Soybean dietary proteins have been shown to have health benefits owing to their therapeutic nature as a result of the presence of functional peptides (11, 12). Functional motifs of a protein are termed peptides. Soybean-derived peptides have gained considerable interest because of their potential health benefits associated with metabolism, brain function, and cognitive ability, as well as their immunomodulatory, antioxidative, antithrombotic, anti-inflammatory, antihypertensive, antidiabetic, antiviral, and mineral-binding roles (13–17).
Soybean-derived peptides are also known to attenuate the severity of metabolic and age-related chronic disorders, such as cancer, hypocholesterolemia, obesity, and alcohol-induced liver injury (18–20). Several peptides derived from soybeans have been reported to exert multiple benefits and are termed multifunctional soybean peptides. Lunasin (5.5 kDa molecular weight), comprising 43 amino acids, is a multifunctional soybean peptide (21). This peptide has been reported to ameliorate several chronic diseases and metabolic conditions including cancer, hypertension, oxidative stress, and inflammation (22, 23). It is often utilized as a dietary supplement because of its high availability and heat stability.
Apart from the plethora of functional peptides in soybeans, isoflavones are also present as glycosides (24). They can lower the risk of oxidative damage to DNA and low-density lipoprotein (LDL) by free radical stress and increase the antioxidant function of enzymes that play a role in the defense system of the human body. They can bind to reactive oxidative species, improve glutathione production, and vitamins E and C, which also work effectively as antioxidants (25, 26). Moreover, these isoflavones can decrease cancer promoters that produce oxidative stress, such as xanthine and tetradecanoylphorbol-13-acetate (TPA) (27). Isoflavones are reported to act as potential antidepressants (28). Genistein is a widely existing and well-known isoflavone, which is associated with a decreased risk of various diseases that are linked to humans, such as tumors, by the enzymatic intervention (29). Genistein has been shown to be helpful in the regulation of gene transcription through DNA methylation and histone modification (30).
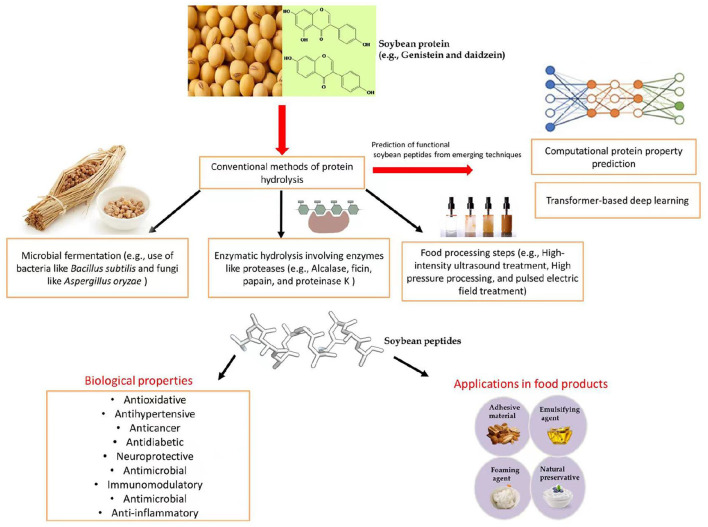
Soybeans are known to be a complete protein because they contain all the five key amino acids required for proper nutrition. The traditional methods of acquiring these peptides include the following techniques: (i) hydrolytic activities of enzymes (e.g., trypsin, pepsin, and papain) (31), (ii) microbial fermentation by Lactobacillus and yeast (32, 33), (iii) combined enzymatic and microbial treatment (34), and (iv) food processing by high pressure and enzymatic hydrolysis (35). Enzymatic hydrolysis is the most convenient and common method because of its high stability and production of many other useful molecules during fermentation, such as surfactants, bacteriocins, polysaccharides, amino acids, vitamins, and many other biomolecules (36). Lactic acid fermentation has proven to be the best method for bioactive peptide production and protein hydrolysis in soybean (37). Soybean fermentation causes the hydrolysis of soybean protein into specific bioactive peptides (38). The latest research trend is to predict functional peptides in different types of vegetable proteins using emerging techniques, such as machine learning algorithms, and to predict the potential of several peptides (39, 40).
The commercial use of soy protein in several food products is currently very popular, particularly in protein-based meat products for vegetarians (4). Figure 1 shows a general depiction of soybean-derived peptides in food products and their functional benefits.
Figure 1.
Functional applications of soybean-derived peptides.
However, the application of soybean peptides to food and feed still faces several challenges that hinder their effective use. The purpose of this review article is to provide recent updates on the functional and biological potential of soybean peptides and discuss conventional and emerging techniques to release these peptides from soybean. Finally, we discuss the practical applications of soybean peptides as functional ingredients in food and feed.
Functional roles of soybean-derived peptides
Many soybean components have shown biological activity, including but not limited to proteins, peptides, saponins, and isoflavones (41). Soybean peptides have received special attention because of their huge diversity and associated biological potential. Several studies have revealed its anti-atherosclerotic, anticancer, antioxidative, anti-inflammatory, antiviral, antidiabetic, anti-inflammatory, and cardioprotective effects (Table 1).
Table 1.
Health benefits of soybean-derived peptides.
| Soybean sources | Bioactive peptide sequence | Targeted model | Biological function | Outcomes | Reference |
|---|---|---|---|---|---|
| Soybean glycinin | IAVPGEVA IAVPTGVA LPYP | HepG2 cells | Anti-hypocholesterolemic | Inhibit 3-hydroxymethylglutaryl coenzyme A (HMGCoA) reductase and stimulate the LDL receptor pathway which in turn reduces the cholesterol | (19) |
| Mature and young soybean proteins hydrolysates | FPFPRPPHQ, FMYL, MMLM, SFFFPFELPRE | Pancreatic lipase (PL) and cholesterol esterase (C-Ease) enzymes | Anti-lipidemic | Compared to flavorzyme and alcalase, bromelain showed higher levels of protein degradation, and its hydrolysis resulted in enhanced PL and C-Ease inhibition effectiveness. FPFPRPPHQ, FMYL, MMLM, and SFFFPFELPRE were effective inhibitors of the C-Ease enzyme. FPFPRPPHQ and SFFFPFELPRE had inhibitory potencies against both PL and C-Ease | (42) |
| Soybean | VHVV | Rats | Anti-hypertensive | Reduced p-PI3K, p-AKT, anti-apoptosis proteins (Bcl2 and Bcl-XL), SIRT1, and FOXO3 in rats. Activated cell survival, AMPKα1, Sirt1, PGC1α, and FoX3α proteins | (43, 44) |
| Soy protein hydrolysates | ILL LLL VHVV | HepG2 cells | Anti-obesity | Hydrolysates of soybean may reduce serum levels of triglycerides by decreasing fatty acid synthase (13.6) than control (17.0) | (6) |
| Soybean meal fermented by Bacillus subtilis E20 | KHPHGRSYKTKLRILA LRFRAPAPVLRRIAKR HTSKALLDMLKRLGK | Shrimp | Anti-bacterial | Effective against microbes Vibrio alginolyticus and V. parahaemolyticus | (45, 46) |
| Soybean | PGTAVFK | In vitro | Anti-bacterial | At a concentration of 31 M, PGTAVFK also exhibits antibacterial action against E. coli and S. aureus | (47, 48) |
| Cheese peptidome | KFVPKQPNMIL | Glycoprotein receptor-binding domain (RBD) and main protease (3CLPro) | Anti-viral activity | Cheese peptidome could be used against the SARS-CoV-2 virus with binding energy values ranging from −8.45 to −26.8 kcal/mol and −15.22 to −22.85 kcal/mol to inhibit viruses' effect | (49) |
| Soy cheese fermented by Lactobacillus delbrueckii WS4 | KFVPKQPNMIL | Human red blood cells | Anti-viral | Soy cheese is made with Lb. delbrueckii WS4 could be potential meal for SARS-CoV-2 and related viruses' prophylaxis | (49, 50) |
| Glycinin | IAVPTGVA LPYP | Caco-2 cells in the intestine of humans and HK-2 cells in the kidney | ACE enzyme inhibition | The activity of the ACE enzyme of renal and intestinal get decreased enzyme activity with desired IC50 values of 14.7 ± 0.28 and 5.0 ± 0.28 μM for Caco-2 cells, and 6.0 ± 0.35 and 6.8 ± 0.20 μM for HK-2 cells | (51, 52) |
| Soybean | PGTAVFK IKAFKEATKVVVVLWTA | In vitro | Anti-bacterial | Inhibitory concentration of 37.2 M, the peptide with long chain (IKAFKEATKVVVVLWTA) is more efficient in opposing Listeria monocytogenes and Pseudomonas aeruginosa | (53, 54) |
| Soybean protein concentrate | FPLLVLLGTVFLASVCVSLKVREDE NNPFYFR FFEITPEKN- PQLRDLDIFLSSVDINEGALLLPHFNSK | In vitro | Antioxidant and inhibitory assay | High molecular mass peptides demonstrated maximum antioxidant activity than low molecular mass peptides, however in case of inhibitory activities, low molecular peptides resulted better effects for α -glucosidase inhibition (IC50 = 0.94) and lipases (IC50 = 4.06) than high molecular peptides inhibition of α-glucosidase (IC50 = 3.4) and of pancreatic lipase (IC50 = 2.02) | (55) |
| Germinated soybean protein digest | VVNPDNNEN QEPQESQQ SDESTESETEQA | Human colon cancer cells, mouse macrophages RAW 264.7 | Anticancer and anti-inflammatory effects | Fractions, 5–10 kDa peptides revealed maximum showed potency with IC50 = 11.70 mg/mL as compared to >10 kDa, which showed IC50 = 13.21 mg/mL values against cancer while 45% inhibition observed against inflammation | (56–58) |
| Soybean | SKWQHQQDSC RKQKQGVNLT PCEKHIMEKI QGRGDDDDDDD DD | Mice | Hypocholesterolemic | After 4 weeks of receiving lunasin with 0.125–0.5 μmol/kg·day dose mice had remarkably low PCSK9 and high amounts of LDLR levels in hepatic tissue, moreover, it decreased total-cholesterol (T-CHO), LDL-C in blood and up-regulated LDLR in HepG2 cells | (59) |
| β-conglycinin | YVVNPDNDEN | Mouse liver | Anti-obesity | Weight of adipose tissue reduced by increasing postprandial circulating FGF21 | (60) |
| Soybean protein | EKPQQQSSRRGS | Mice | Immunomodulatory effect | Enhanced phagocytosis, retard excessive inflammatory response, and induced macrophages M1 polarization in the spleen of mice | (61) |
| Soy protein isolate (bromelain and thermolysin) | - | Human oral squamous carcinoma cell line, HSC-3 | Anti-proliferative activity | After 72 h, both isolates showed inhibition against cancer cells which were 35.45–76.39% | (62) |
| Hydrolysates of soy protein Flavorzyme (F-SPIH) | VHVV | Hypertensive rats | Neuroprotective effect | A peptide that is bioactive VHVV had stimulated CREB-induced downstream proteins that might decrease the long-term memory loss mediated by hypertension and maintain the survival of neurons | (63, 64) |
| Black soy | – | In vivo | Blood pressure | Systolic blood pressure reduced clearly in peptide supplemented group (−9.69 ± 12.37 mm Hg) as compared to control (−2.91 ± 13.29 mm Hg) | (65) |
| Soy β-Conglycinin | YVVNPDNDEN YVVNPDNNEN | HepG2 Cells | Hypocholesterolemic | At 350 μM 1st peptide upregulates the mature SREBP2 protein level (134.0 ± 10.5%), enhanced the LDLR protein (152.0 ± 20.0%), and HMGCoA reductase protein (171 ± 29.9%), as compared to 2nd peptide where mature SREBP2 protein regulation was 158.0 ± 9.2%, the increase in LDL and HMGCoA reductase was 164.0 ± 17.9%, and 170 ± 50.0%, respectively | (66) |
| Glycinin | IAVPTGVA | Human intestinal Caco-2 cells and serum | Anti-diabetic | Stopped DPP-IV action in situ, with IC50 values 223.2 μM | (67, 68) |
| Glycinin and β-conglycinin, | – | Human intestinal Caco-2 cells | Hypocholesterolemic Anti-Diabetic | Peptides enhanced protein levels (51.5–63.0%, 55.2–85.8%) than control (0.5–1.0 mg/mL) moreover DPP-IV activity decreased (16.3–31.4% and 15.3–11.0%) than control (1.0 and 2.5 mg/mL) | (69) |
| Soybean protein isolate | FDPAL | Caenorhabditis elegans | Anti- oxidative | Caused a remarkably increase in resistance to oxidative stress, upregulated the specific expression of genes in Caenorhabditis elegans | (70) |
| Soybean glycinin | VAWWMY | Rats | Anti- hypocholesterolemic | Serum (0.03), liver (0.03), and intestine (0.2) detected low values of cholesterol | (71) |
| Soybean | QRPR | RAW 264.7 | Anti-inflammation | Soybean peptide reduced the cytokines levels such as TNF-α and IL-6 that are responsible for inflammation | (72) |
| Bacillus. licheniformis KN1G mediated soybean | KFVPKQPNMIL | Human ACE2 cell receptor | Anti-viral | Preventative measures against SARS-CoV-2 infection | (16) |
| Soybean fermented by Bacillus subtilis (KN2B and KN2M). | ALPEEVIQHTFNLKSQ | In silico | Anti-viral | Effective against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). | (16) |
| Soybean induced peptide | QRPR | RAW264.7 cell model | Anti-inflammation | Autophagy was activated by QRPR in the inflammatory cell; however, autophagy was inhibited by reducing QRPR inhibitory effect | (73, 74) |
| R95, N98-4445A, S03-543CR | – | Human blood, breast, and prostate | Anti-proliferative activity | Showed up to 68.0% inhibition of cancer lines | (75) |
| Glycinin, β-conglycinin and lectin from soy milk | HSYNLRQSQVSELKYEGNWGPLV NPESQQGSPRV | In vitro | Anti-oxidative and anti-hypertensive effects | Peptides (10 kDa) resulted maximum antioxidant and DDPH values as 1,831 ± 20.29 TEAC μm and 50.74 ± 0.27%, respectively while ace inhibition was 75.97 ± 1.5%. | (76) |
| Soybean | VHVV | Rats | Antihypertensive | Antioxidant defense induced, stabilized mitochondrial homeostasis, decreased renal damage, induced free radicals in rats | (77) |
| Alcalase and neutrase | QNGEQE RGASADGPR YGGGGE | Macrophage RAW264.7 cells | Immunoregulatory effect | The cell viabilities decreased from 97.61 to 115.01% to 86.65% | (78) |
| Soybean protein | DGWFR ALSWLR DGWFRL PNGPVWR | Mice model | Sleep effects | Mice provided with 0.65 g kg−1 soybean-derived peptides showed 59.21% sleep duration on 3rd day of observation and increased melatonin levels (95.31%) while with 2.60 g kg−1 peptides doubled the serotonin in the brain and increased the awaking situation | (79) |
| Alcalase low molecular weight fraction (SPH-I, MW < 3 kDa) | VVFVDRL VIYVVDLR IYVVDLR IYVFVR | Human intestinal Caco-2 cells | Anti-oxidative effect | Desirable results showed in the case of ORAC (143 ± 2.1–171 ± 4.8 μM TE/μM), FRAP (54.7 ± 1.2–79.0 ± 0.6 mM Fe2+/μM), radical-scavenging activity (3.42 ± 0.2–4.24 ± 0.4 mMTE/μM) and DPPH (16.5 ± 0.5–20.3 ± 1.0 μM (TE)/μM). SPH-IC (85.9%) and SPH-ID (96.2%) restrained maximum H2O2-induced ROS generation | (80) |
| Lunasin, lectin | SKWQHQQDSCRKQKQGVNLTPCEK HIMEKIQGRGDDDDDDDDD | Cancer cells of the human breast | Anti-cancer effect | MDA-MB-231 cells were inhibited with these bioactive peptides (glycitein, Genistein, β-sitosterol, and genistin) while 142.67 ± 5.88 μM, 93.75 ± 5.15 μM, 196.28 ± 4.45 μM and 127.82 ± 4.70 μM and values were detected | (81) |
Beyond its high nutritional value, soybean contains a large variety of useful substances, such as bioactive peptides. When soybean proteins break down, peptide pieces hidden inside their core assemblies are released. These peptide fragments exhibit a wide range of biological and functional properties. These peptides are produced from glycinin and beta-conglycinin (which includes three subunits, such as alpha, α′, and beta), the progenitors of the majority of isolated peptides (82). Fatty acid (FA) synthase (FAS) inhibition, triglyceride and cholesterol reduction, effectiveness against obesity and diabetes, and lipid metabolism enhancement are properties attributed to soybean peptides (83). Some studies have shown that soybean peptides have many functions, including hypocholesterolemia, outcomes against cancer, angiotensin-converting enzyme (ACE), hypertension, and regulation of the immune system, while additional uses and benefits are constantly being uncovered (84, 85). Immunomodulatory peptides belong to a complex class of biologically active peptides that contain substances with various mechanisms of action. Several studies have shown that soybean peptides exert immunoregulatory effects (Table 1). For instance, in the macrophage cell lines RAW 264.7 and THP-1 (86), lunasin displayed anti-inflammatory effects by reducing the generation of cyclooxygenase-2 (COX-2), E2 (PGE2), and nitric oxide (NO), as well as the expression of inducible NO synthase (iNOS). Additionally, interleukin-6 and interleukin-1 production was suppressed by lunasin, and its anti-inflammatory effects were linked to the repression of the NF-κB pathway. The ability of lunasin to inhibit integrins is likely responsible for its anti-inflammatory effects. The complete sequence is important for the anti-inflammatory benefits of lunasin fragments (21, 87). According to some studies, lunasin can reduce the production of tumor necrosis factor-alpha (TNF-α) which has anti-inflammatory effects. Lunasin demonstrates immunomodulatory activity against cancer by interacting with the cytokine's interleukin-2 (IL-2) and interleukin-12 (IL-12). This blend activates cells called natural killer (NK) cells to increase interferon-gamma (IFN-γ) production. Granzyme B (GZMB) and granulocyte-macrophage colony-stimulating factor (CSF2) expression were both elevated by a combination of lunasin/IL-2/IL-12, although transforming growth factor beta receptor 2 (TGFBR2) and transforming growth factor beta 1 (TGFB1) expression were decreased (88). As a result, the ability of lunasin to modulate gene expression is associated with its immunomodulatory properties. The combination of lunasin and IL-12 significantly increased H3 acetylation at the interferon gamma (IFNG) locus and decreased it at the TGFB1 locus, indicating an epigenetic mechanism (18). A peptide without either the RGD motif or the aspartic acid (D)-tail, however, had an impact comparable to that of full-length lunasin, indicating that they were not linked to a synergistic augmentation of IFN production. Therefore, the N-terminus and/or central regions of lunasin are linked to the immunomodulatory function of the compound. The reduction in cholesterol levels is attributed to lactostatin, which is β-lactoglobulin (IIAEK), which regulates these levels by controlling the channels of the calcium-associated signaling pathway of MAPK. Cholesterol 7α-hydroxylase (CYP7A1) is the limiting enzyme involved in the degradation of cholesterol and cells of HepG2, mediating transactivation, which is induced by lactostatin. The activity of this enzyme is controlled by calcium channels and the extracellular signal-regulated kinase (ERK) pathway (60). Various peptides have been shown to play important roles in lowering cholesterol and lipid content (Table 1). Glycinin hydrolysis with trypsin and pepsin revealed two peptides that were converted into IAVPTGVA (Soy1) and LPYP peptides, which were shown to be absorbed by Caco-2 cells (89). They either act as hypocholesterolemic or hypoglycemic agents and can inhibit HMGCoA reductase and stimulate the LDL receptor pathway, which, in turn, reduces cholesterol. Additionally, there was an increase in the intensity of phosphorylation on Ser 872 of HMGCoA reductase, which is the operative form of HMGCoA reductase, through the stimulation of the adenosine monophosphate-activated protein kinase (AMPK) pathway. Stimulation of AMPK and Akt/protein kinase B pathways is linked to the ability to regulate glucose metabolism and uptake (89). Inhibition of glycogen synthase (GS) and glycogen synthase kinase-3β (GSK3) is caused by the activation of Akt (phosphorylation at Ser 473), which further controls the activity of GS by modifying glycogen formation in hepatic cells. In addition, the upregulation effect of hepatocytes on extracellular free glucose is also determined by the expression level of glucose transporter type 1 (GLUT1) and glucose transporter 4 (GLUT4).
Different protein hydrolysates can be produced by enzymatic hydrolysis of mature and young soybean proteins using bromelain, alcalase, and flavourzyme at various hydrolysis reaction times (42). The antilipidemic qualities of the hydrolysates were assessed based on their capacity to inhibit pancreatic lipase (PL) and cholesterol esterase (C-Ease) enzymes. Using a liquid chromatography-mass spectrometry quadrupole time-of-flight system (LC-MS QTOF) and the prediction of molecular interaction mechanisms, additional analysis was performed on the chosen hydrolysates for peptide identification. The purpose of this study was to determine if the PL and C-Ease enzymes can be inhibited by mature soybean protein hydrolysates (MSPHs) and young soybean enzymatic protein hydrolysates (YSPHs). Compared to flavourzyme and alcalase, bromelain showed higher levels of protein degradation, and its hydrolysis resulted in enhanced PL and C-Ease inhibition effectiveness. Using LC-MS QTOF and molecular binding investigations, six PHs with strong antilipidemic effects were selected for sequencing. It was predicted that the peptides would have strong inhibitory effects on PL. Additionally, it was projected that these compounds would be effective against C-Ease. When tested against PL and C-Ease, they showed strong inhibitory potential. Soybean-derived protein hydrolysates from mature and young beans have been identified as a possible component in the treatment of hypercholesterolemia. Based on these findings, hydrolyzing MSPI and YSPI with alcalase, bromelain, and flavorzyme encouraged the production of powerful PHs rich in bioactive peptides that may inhibit PL and C-Ease. In contrast to YPHs, MSPHs produced by enzymes showed stronger PL inhibition; however, YSPHs produced by enzymes showed higher C-Ease inhibitory capabilities. Bioactive peptides produced from MSPHs and YSPHs were capable of suppressing PL and C-Ease, according to in silico molecular binding investigations. Overall, the peptides generated from the MSPHs and YSPHs showed strong inhibitory effects against PL. In addition, it was predicted that the peptides FPFPRPPHQ, FMYL, MMLM, and SFFFPFELPRE from MSPHs and YSPHs would be effective inhibitors of C-Ease. Furthermore, it was projected that the peptides FPFPRPPHQ and SFFFPFELPRE, which were generated from MSPHs and YSPHs, respectively, would have inhibitory potencies against both PL and C-Ease, indicating that these peptides would have dual functions against antilipidemic enzymes. The curative benefits of proteins obtained from soybean, which are mature and young, make it an intriguing candidate for use as an efficient component in the creation of anti-lipidemic functional products (42).
Superoxide anions (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals are examples of reactive oxygen species (ROS) that are essential for everyday bodily functions (OH). However, when ROS accumulates beyond the capacity of the cellular antioxidant defense system, metabolic disorders such as cardiovascular disease, Alzheimer's disease, type 2 diabetes (T2D), and certain types of cancer may develop (90, 91). Additionally, ROS are produced chemically or enzymatically in food systems, and their interactions with dietary ingredients result in unfavorable tastes and carcinogens. Therefore, soy protein hydrolysates and constitutive peptides have been employed to prevent food systems from degrading due to ROS and to shield the human body from harmful effects. The fraction with the lowest molecular weight (SPH-I, MW3 kDa) demonstrated the strongest radical scavenging ability and reducing power as well as the best potency in controlling H2O2-induced oxidative stress in Caco-2 cells (92). It would be interesting to isolate bioactive peptides with cytoprotective and antioxidant properties. These peptides also greatly reduced lipid peroxidation and intracellular ROS formation, which protected Caco-2 cells from H2O2-induced oxidative damage (p < 0.05). Furthermore, SPH-IC and SPH-ID significantly increased the ROS-mediated response to inflammation by preventing interleukin-8 release (p > 0.05) (80). The amino acid makeup of lunasin has been linked to its antioxidant action. In a study, lunasin was shown to decrease lipid peroxidation, shown 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity (ABTS•+), and prevent the production of ROS caused by lipopolysaccharide. After scavenging peroxyl and superoxide radicals, chelating ferrous ions, and reducing intracellular ROS levels, the antioxidant activity of lunasin was further proven (90, 93). According to Fernández-Tomé et al. (94), under oxidative stress, lunasin inhibits an increase in the activity of glutathione peroxidase and catalase, lowers intracellular ROS and protein carbonyl levels, and increases cytosolic glutathione levels. In some experiments, the inhibition of dipeptidyl peptidase-IV (DPP-IV) activity by Soy1 and LPYP is a favorable outcome for the prevention of diabetes (95). Using BIOPEP, an initial examination of their structures indicated that angiotensin-converting enzymes might be potent inhibitors. Consequently, a bottom-side-up approach was established to clarify hypotensive activity in vitro. Similarly, in another study using molecular modeling, their capacity to act as inhibitors was competitive with this enzyme (51). Production of low insulin or resistance to insulin causes dysregulation of the glucose balance which results in T2D. To manage T2D, a considerable focus is on natural remedies, especially food elements. DPP-IV, alpha-amylase, and alpha-glucosidase are essential for controlling blood glucose levels. It is believed that the suppression of these enzymes by bioactive peptides could be the most efficient method for managing T2D. The peptides obtained from food are being focused on as novel inhibitors because DPP-IV, which is present in the cell membrane and blood, is responsible for the inhibition of incretin hormones, such as stomach inhibitory polypeptide and glucagon-like peptide-1 (72). For the efficient control of T2D and other metabolic disorders, the inhibitory peptide DPP-IV (IAVPTGVA) obtained from soybean may be utilized.
Emerging tools and techniques for the exploration of functional peptides in soybean
Small protein fragments known as soy peptides are produced through enzymatic hydrolysis in vitro, fermentation (such as fermentation caused by bacteria containing lactic acid), processing of food such as modification of pH, heat treatment, isolation of protein, processing through ultra-high-pressure (96, 97), and GI digestion (specific and non-specific proteases from the pancreas, small intestine, and stomach, including pepsin, trypsin, and chymotrypsin). Table 2 shows the different methods used to produce soybean peptides. Soy protein also affects peptide composition through enzymatic hydrolysis or bacteria-mediated fermentation (6). Diverse functional features of soy peptides with various compositions have also been noted when producing tofu in terms of quality, yield, and texture (109). The digestion of food in the digestive tract can also produce peptides. Proteins are hydrolyzed by digestive enzymes, resulting in peptides of various lengths and free amino acids. Pepsin acts at the stomach level in in vitro systems, randomly hydrolyzing peptide bonds to create relatively large peptides and a mixture of acids from the pancreas and pancreatins. Trypsin, chymotrypsin, elastase, and carboxypeptidases are the only peptidases that constitute pancreatins. Except for trypsin, all enzymes hydrolyze peptide bonds, resulting in peptides with various amino acid sequences (110).
Table 2.
Various methods for soybean-based peptide generation.
| Methods | Species for hydrolysis | Findings | Peptide structure | Type of the trial | References |
|---|---|---|---|---|---|
| Microbial fermentation | Lactobacillus delbrueckii WS4 | The binding energy was −8.45 to −26.8 kcal/mol and −15.22 to −22.85 kcal/mol | KFVPKQPNMIL | In vitro | (49, 98) |
| Enzymatic hydrolysis | Corolase PP | The degree of hydrolysis enhanced to 18.9% ± 1.9 after 10 h, Significant increase in antioxidant and ACE inhibitory effects | FEITPEKNPQLRDLDIFLSI INAENNQRNFLAGSQDNVISQIPSQV FAIGINAENNQRNFLAGSQDNVISQIPSQV | In vitro | (99) |
| Enzymatic hydrolysis | Corolase PP | Showed protective effect against hypertension and potent antioxidant activity | IRHFNEGDVLVIPPGVPY, IRHFNEGDVLVIPPGVPYW, IYNFREGDLIAVPTG, VSIIDTNSLENQLDQMPRR, YRAELSEQDIFVIPAG | In silico | (100) |
| Enzymatic hydrolysis and lactic acid fermentation | Prozyme and Lactobacillus rhamnosus EBD1 | ACE inhibitory activity enhanced (60.8 ± 2.0%−88.24 ± 3.2%), moreover captopril showed an inhibitory effect (94.20 ± 5.4%) | PPNNNPASPSFSSSS GPKALPII IIRCTGC | In vitro | (101) |
| Microbial fermentation | Proteases and Enterococcus faecium | The ACE inhibitory ability increased from 15 ± 3% to 40 ± 2% by treating soybean with Prozyme 2000p while subsequent fermentation of the hydrolyzed samples by E. faecium further increased (80 ± 5%) and improved the antihypertensive peptides, phenolic compounds, gamma-aminobutyric acid, and antioxidants levels | ENPFNL EDEVSFSP RSPFNL SRPFNL | In vitro | (102) |
| Enzymatic hydrolysis and ultrasound pretreatment | Trypsin | Fraction < 5 kDa demonstrated the maximum inhibitory effect (0.27 mg/mL) and >5 kDa fraction resulted the least inhibitory activity (3.31 mg/mL) | Gly-Ser-Arg and Glu-Ala-Lys | In vitro | (103) |
| Pulsed electric field | 5 kV/cm, 2,400 Hz, and 2 h | DPPH remarkable enhanced (P < 0.05) to 94.35 ± 0.03%, while zeta potential decreased (0.59 ± 0.03 mV) | SHCMN | In vitro | (104) |
| Enzymatic hydrolysis | Corolase L10 (Cor), Promod 144MG (Prom) and Protamex (Prot) | IC50 amounts were 0.73 ± 0.11 to 3.54 ± 0.24 mg | LPQNIPPL | In vitro | (105) |
| Enzymatic hydrolysis | GI enzymes and Alcalase | Effectiveness in anti-proliferation against human blood, prostate cells, and breast cancer | KWKLFKKIPKFLHLAKKF | In silico | (75) |
| Ultrasound-assisted liquid-state fermentation | B.subtilis | Increase in ACE inhibitory, peptides content and biomass were 26.4, 36.2, and 55.0%, respectively | Gly-Gly-Tyr-Arg | In vitro | (106) |
| Microbial fermentation | B.licheniformis KN1G, Bacillus amyloliquefaciens KN2G and B.subtilis (KN2B and KN2M) | Effective inhibition of SARS-CoV-2 S1 receptor binding domain and the modulation of Toll-Like receptor 4 | ALPEEVIQHTFNLKSQ | In silico | (16) |
| Microbial fermentation | Lactobacillus plantarum strain C2 | Maximum peptide showed in 10 kDa fractions and resulted in highest ace inhibition (73.35 ± 1.5), ABTS (1,831 ± 20.29 TEAC μm) and DPPH (50.74 ± 0.27%) effects. Total of 51 peptides discovered | ESYFVDAQPKKKEEGNK SLKVREDENNPFYFRSSNS HSYNLRQSQVSELKYEG NWGPLVNPESQQGSPRV | In vitro | (76) |
| Microbial | Lacticaseibacillus casei (NK9) and Lacticaseibacillus fermentum (M2) | Higher ACE-inhibitory (48.44%) and proteolytic activity (0.67 OD) is seen in soy milk fermented with M2 than NK9 (proteolytic activity: 0.48 OD and ACE-inhibitory activity: 41.33%) | SGLGRGWIDGDIGHGK SMEDMM VPVVLGSKNEVDYIK GYHYVGTLSGHTK VREDGV YCEIVPFQK TPPASWSKLGYK | In vitro | (107) |
| Enzymatic hydrolysis | Alcalase | Protect Caco-2 cells from H2O2-induced oxidative damage, and reduced ROS-mediated inflammatory reactions by preventing the interleukin-8 release | VVFVDRL VIYVVDLR IYVVDLR IYVFVR | In silico | (80) |
| Enzymatic hydrolysis | Pepsin, chymotrypsin, and trypsin | Showed no cytotoxic effects on HEK293 with IC50 value of 3.95 ± 0.11 mM | DMG | In silico | (108) |
Chemical-based techniques and fermentation with microbes are the conventional methods used for hydrolyzing soybean peptides. There are a few examples of the chemical-based approaches mentioned above (Table 2). Advanced fermentation methods (fungus, yeast, or germ) and enzymatic treatments are two examples of processing technology (111). In addition to physical techniques, recent studies have highlighted the significance of enzymatic hydrolysis. Peptides, considered biowaste, are obtained from sources of protein by hydrolysis through enzymes and have been used as an assuring method. It has been reported that a meal of soybean contains two soybean lines that are high in oleic acid and one high-protein line when utilized from peptides are bioactive and are effective against cancer cells at multiple sites. GI enzymes and alcalase are used to resist GI to obtain fractions of soy peptides (75).
A viscozyme multi-enzyme complex was employed by de Figueiredo et al. (112) to pretreat okara (soybean waste product), which enhanced the conventional alkaline preparation procedure and increased the protein extraction rate. The major goal of this study was to suggest three techniques for producing low-molecular-weight peptides from okara. High-pressure homogenization and mixed enzyme hydrolysis were followed by alkaline protease hydrolysis and the alkali-dissolved acid precipitation method for alkaline protease hydrolysis and protein extraction, respectively. The findings of this study were an increase in the added value of okara and the production of biologically active peptides from soybean waste. In another study, a method for producing low-molecular-weight peptides (HPH-VAP) from okara was proposed using high-pressure homogenization-assisted double enzymes. To compare the effects of the various procedures, the rates of protein extraction, peptide structure, antioxidant capacity, and immunological characteristics were evaluated. The results demonstrated that the protein extraction rate of this method increased by 69 and 51%, compared to earlier methods. The results showed that it increased NO levels, cytokine production, and cell phagocytic capabilities (IL-6, IFN–γ, and TNF-α) (113).
Soybean fermentation breaks down soybean proteins into small peptides. The breakdown of proteins during soybean fermentation may be accelerated by enzyme hydrolysis, thereby improving peptide output. In a previous study, soy meal that had been fermented with Douchian B. subtilis was hydrolyzed using the enzyme thermolysin. Subsequently, four fractions of the extracted water were separated using ultrafiltration membranes. Following the evaluation of vasorelaxation capabilities, the most effective fraction was isolated and purified to obtain four peptides. Three peptides led to dose-dependent vasorelaxation (0.01–4.10 M) in the thoracic aorta ring of Sprague–Dawley rats (relaxation activities were all endothelium-independent), whereas one peptide led to vasoconstriction. Additionally, it was shown that the activities of ACE inhibition and vasorelaxation had a different causal relationship (114, 115). The goal of another study was to purify and identify antioxidant peptides from alcalase-hydrolyzed soybean (G. max L.) hydrolysate's low-molecular-weight fraction (SPH-I, MW3 kDa) and to further assess the peptides' ability to protect human intestinal Caco-2 cells from oxidative stress. The four main peptides were purified using reversed-phase HPLC and gel filtration chromatography, and their sequences were determined using nano-LC-ESI-MS/MS as follows: IYVVDLR (877 Da), VIYVVDLR (976 Da, SPH-IB), IYVFVR (795 Da, SPH-ID), and VVFVDRL (847 Da, SPH-IA and SPH-IC). The antioxidant peptides were created and showed promising antioxidant action against DPPH radicals [16.5 ± 0.5–20.3 ± 1.0 M Trolox equivalent (TE)/M], ABTS•+ radicals (3.42 ± 0.2–4.24 ± 0.4 mM TE/M), ORAC (143 ± 2.1–171 ± 4.8 M TE/M), and FRAP (54.7–79.0). Additionally, the peptides dramatically reduced lipid peroxidation and intracellular ROS formation, protecting Caco-2 cells from H2O2-induced oxidative damage. Additionally, SPH-IC and SPH-ID significantly increased total reduced glutathione synthesis, improved catalase, and glutathione reductase activities, and reduced ROS-mediated inflammatory reactions by preventing interleukin-8 release (80). In another experiment, antioxidant and antibacterial peptides were constantly produced from soybean milk in a membrane bioreactor. It was determined that using a static turbulence promoter in the membrane separation process resulted in a greater permeate flow and less energy being consumed in the filtering process. Using the static turbulence promoter throughout the membrane separation process at a constant operating transmembrane pressure of 3 bar and retentate flow rate of 100 L/h increased the permeate flux. The energy consumption was also reduced by the filtering process employed by a static turbulence booster. Both membrane permeate and enzyme-hydrolyzed milk have antioxidant and antibacterial activities against B. cereus (116). ACE-inhibitory tripeptides from soybean protein were determined using in vitro and in silico analyses. RP-HPLC was used to confirm the in vitro activity of the hypothetical ACE-inhibitory tripeptides. DMG was selected as a powerful ACE-inhibiting peptide. In a cell experiment, DMG was shown to have no cytotoxic effects on HEK293 cells. Furthermore, molecular docking results showed that DMG made good contact with the ACE active sites (His513, Ala354, Gln281, His353, Tyr520Glu384, and Lys511). Additionally, DMG showed significant anti-ACE activity with an IC50 value of 3.95 0.11 mM (108). Soybean protein hydrolysates were produced using two proteolytic enzymes (Alcalase and Protamex), and their functional and antioxidant properties, as well as the degree of hydrolysis (DH), were evaluated. The highest DH value was 20%, with a yield of 19.77% and protein content of 51.64%. More than 41% of the total amino acid composition of each protein was present in the hydrolysate. The protein hydrolysates from Protamex had excellent solubility, emulsifying activity, and foaming capacity at pH 2.0, with values of 83.83%, 95.03 m2/g, and 93.84%, respectively. The water-holding capacity of Alcalase was 4.52 g/g, whereas the oil-holding capacity of Protamex was 4.91 g/g. When samples were tested using DPPH (2, 2-diphenyl-1-picrylhydrazyl), Protamex showed the highest antioxidant activity (62.07%) and the lowest reducing power (0.27). Alcalase showed 70.21% ABTS activity. These findings show that soybean protein hydrolysates have substantial potential to improve the nutritional value, antioxidant activity, and functional properties of soybeans (117).
Different fermenting cultures have been used to hydrolyze soybean proteins (for example, Bacillus species). Soybean proteins are used extensively as functional and nutritional food ingredients. The effects of three factors [temperature, pH, and enzyme/substrate (E/S) ratio] on the production of soy protein isolate (SPI) hydrolysates using a microbial protease were estimated using a 23-central composite design. They evaluated the antioxidant activity, foaming, emulsifying potential, and soluble peptide content of the hydrolysates. The optimal conditions for producing soluble peptides were pH 6.5–9.5, 30–35°C, and E/S ratios of 1,650–6,300 UgL. The SPI hydrolysates produced showed a greater ability to chase the ABTS radical at pH 8.0–9.5, 30–45°C, and E/S ratios of 4,000–8,000 U g1p. These factors had no noticeable effect on the capacity of hydrolysates to scavenge the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical in the examined range. SPI hydrolysates also demonstrate the capacity to chelate iron and their ability to decrease. The hydrolysis temperature was important for the capacity of the hydrolysates to chelate Fe2+. The findings of this research suggest that precise hydrolysis conditions should be carefully selected to produce SPI hydrolysates with appropriate properties (118). While processing soybeans with subsequent fermentation and proteases, the release of bioactive substances that can be firmly bound to the food matrix increases. This study compared the capacity of raw soybean, Prozyme 2000p-hydrolyzed soybean (PSB), and Enterococcus faecium EBD1-fermented PSB to inhibit angiotensin-converting enzyme to create an antihypertensive functional food from soybean. When soybean was treated with Prozyme 2000p, the ACE inhibitory capacity increased from 15 to 40%. The hydrolyzed samples were then fermented, which further boosted their inhibitory ability to 80.5%. A discovery-based metabolomic method utilizing UHPLC Q-TOF MS/MS of the fermented sample demonstrated enhanced levels of gamma-aminobutyric acid, antihypertensive peptides, phenolic compounds, and antioxidants owing to E. faecium fermentation. Fermentation increased the concentrations of essential amino acids compared to the raw and enzyme-treated samples. High concentrations of putative antihypertensive chemicals in the fermented samples suggested that fermenting soybean treated with Prozyme with E. faecium EBD1 might be a good method for producing functional foods with these hypertensive qualities (102).
This study evaluated the impact of fermentation on the nutritional value of food and food-grade soybeans. Both were fermented for 48 h by Aspergillus oryzae GB-107 in a bed-packed solid fermenter. After fermentation, nutritional and trypsin inhibitor levels were compared to those of soybean meal and raw soybeans. Compared to raw soybeans and soybean meals, fermented soybeans and soybean meals contained 10% higher crude protein (P < 0.05). The essential amino acid profile remained unchanged during the fermentation. Most trypsin inhibitors were removed by fermentation of both soybeans and soybean meal (P < 0.05). Although fermentation significantly reduced the number of large peptides (60 kDa) compared to raw soybeans, it significantly increased the number of small peptides (20 kDa) (P < 0.05). Fermented soybean meal contained more small peptides (20 kDa) than soybean meal (P < 0.01); however, soybean meal contained 22.1% larger peptides (60 kDa) than fermented soybean meal. In general, fermentation reduces the peptide size in soybeans and soybean meals, eliminates trypsin inhibitors, and increases protein content (108). The purpose of this study was to assess the bioactivities, such as galactosidase and glucosidase activities, and the growth behavior of Lactobacillus cultures in the soymilk medium. Among the 10 Lactobacillus cultures in the soymilk medium used in this study, L. casei (NK9) and L. fermentum (M2) were chosen because of their improved growth patterns and higher levels of glucosidase and galactosidase activities during fermentation. Additionally, soymilk fermented with M2 had stronger ACE-inhibitory (48.44%) proteolytic activity (0.67 OD) than NK9 (proteolytic activity: 0.48 OD and ACE-inhibitory activity:41.33%) (9). Using specific Lactobacillus cultures during the fermentation of soy milk, peptides were produced that were recognized by MALDI-TOF spectrometry as having effective ACE-inhibitory activity. Using the BIOPEP database, the identified ACE-inhibitory peptide arrangements from fermented soymilk were characterized (107).
IAVPTGVA (Soy1) and LPYP, two soybean peptides with multiple behavioral functions, have shown hypoglycemic and hypocholesterolemic effects in vitro. According to a preliminary structural screening conducted using BIOPEP, they may be significant ACE inhibitors. As a result, a bottom-up method was established to explain the in vitro hypotensive activity. With IC50 values of 14.7 ± 0.28 and 5.0 ± 0.28 M (Caco-2 cells) and 6.0 ± 0.35 and 6.8 ± 0.20 M (HK-2 cells), correspondingly, LPYP and Soy1 decreased the renal and intestine ACE enzyme activity. In addition, molecular modeling studies have suggested that they have the potential to function as competitive inhibitors of this enzyme. To improve stability and hypotensive qualities, a viable method for the non-toxic regulation of their release from a nanomaterial was devised by encapsulation into a RADA16-assembling peptide (51). AHTPs have been perceived in various organisms, and their anti-hypertensive activity in the laboratory for the identification of peptides is time- and resource-consuming. Before verification through experiments, computational techniques that comprise the stout learning of machines can recognize capable AHTPs. The research proposed Ensemble-AHTPpred, a collective learning of a machine-containing algorithm comprising maximum gradient boosting (XGB), a support vector machine (SVM), and a random forest (RF), to enhance the robustness of the final predictive model and incorporate various heterogeneous algorithms. To analyze or explain the characteristics of the predicted peptide, computed features such as transitions, n-grams, various physicochemical properties, secondary structure-related information, and amino acid composition (AACs) were used. Above 90%, was achieved on the liberated inspection data using the Ensemble-AHTPpred tool. Furthermore, based on the latest studies, the method was practical for innovative empirically authorized AHTPs that were not overlaid with the test and datasets that are based on training, and these AHTPs might specifically be predicted by the tool (119).
Functional applications of soybean peptides in food and feed
Soybean-derived peptides have gained great popularity as one of the most economical and easily accessible peptides, with a plethora of functional applications in the food, feed, and pharmaceutical industries. Thermal and gastrointestinal stability (including pH) are crucial parameters for the practical application of soybean peptides in food and feed (120). A study reported that the interfacial and emulsifying characteristics of soy peptides varied based on the degree of hydrolysis. A peptide with the lowest degree of hydrolysis was found to be an excellent functional agent for the emulsification of not only silicon oil and liquid paraffin but also soybean oil (121). Novel nanoparticles derived from soy peptides have shown great potential as oil–water emulsion stabilizers and effective food-grade emulsifiers, with the potential to replace surfactants and polymers (122). These studies indicate that soy peptides had previously unappreciated emulsifying activity and great interfacial properties, which make them useful for the food processing industry. Soy peptides have exceptional assimilating properties, and there is a need to determine whether the foaming properties of egg white powder can be enhanced by using soy peptides as foaming agents. There is some evidence that 9–12% of soy peptides at pH 7 improve the foaming properties of egg white powder by conferring it a more flexible secondary structure, uniform size, more surface hydrophobicity, and foam elasticity (123).
The effect of soybean-derived peptides on the quality attributes (physicochemical, sensory, and microbiological) of yogurt was evaluated under storage (3 weeks) conditions. The enzymatic hydrolysis of soy whey protein was performed using trypsin at 45°C for 4 h. Various concentrations of soy peptides (6.5, 13, and 17 mg/mL) were incorporated into yogurt. Increasing the peptide content enhanced antioxidant activity; however, viscosity and syneresis were reduced. During 3 weeks of storage, acidity (from 1.04 to 1.14%), syneresis (from 15.23 to 19.84%), and viscosity (from 5.31 to 8.04) of yogurt increased while pH (from 4.54 to 4.37) and antioxidant activity (from 12.55 to 9.32) decreased. The incorporation of 13 mg/mL of peptide showed a maximum decline in Escherichia coli (0.25 CFU/mL) and Staphylococcus aureus (0.79 CFU/mL) levels. Therefore, we conclude that soy whey-derived peptides can be used as a natural preservative of yogurt (124).
A previous study determined the thermal and pH stability of antioxidative and ACE-inhibitory peptides derived from soybean peptides after fermentation with L. plantarum strain C2. It was concluded that the peptides were thermally stable over a wide temperature range (25–121°C) and pH range (4, 6–8, 13, 14). These stability features indicate the potential thermal resistance of soy peptides in food processing and gastrointestinal digestion at low pH (76). Soy-derived bioactive peptides or soy protein isolates are relatively more economical, even when compared to peptides derived from different dairy proteins (125). They have been reported to promote the growth of some probiotic bacteria, making them a potential prebiotic in food and feed. For instance, Lacticaseibacillus rhamnosus has been shown to utilize both hydrophobic (with 3–5 amino acid residues) and hydrophilic peptides (with >5 amino acid residues) (126). Similarly, soy peptides and proteins have been reported to promote growth and confer competitive advantages to Lactobacillus rhamnosus over Escherichia coli (127, 128). Furthermore, the antimicrobial potential of soybean peptides (53, 128) makes them a great additive to enhance the shelf-life of food and various animal feeds. Kefir is fermented milk that has been consumed for thousands of years. It originated in parts of Eastern Europe and the lush regions of the Caucasus Mountains. Isoflavone biotransformation and flavor production during soymilk kefir fermentation were found to be cultivar- and culture-specific (129–131).
Conclusion
The increasing trend in scientists exploring soybean, their proteins, the synthesis of bioactive peptides, and the discovery of new health benefits associated with soybean will surely help improve human health. The consumption of soy products is promising for reducing various chronic diseases, such as cancer and diabetes. These health benefits are associated with the raw soy protein or derived soy bioactive peptides obtained by processing. Bioactive peptides are primarily generated by enzymes, fermentation, and gastrointestinal digestion. Soy peptides' bioregulatory mechanisms, structural configuration, and mechanism identification are in the developing stage; therefore, research should be conducted on a large scale to identify all of these aspects. This research will lead to the identification of new bioactive peptides and new roles in health, and hence, will improve public health.
Author contributions
YZ drafted the manuscript. GC collected the data and results. CW and JD modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was supported by the Heilongjiang Major Science and Technology Project (2021ZX12B06) and the Central Guidance on Local Science and Technology Development Fund (DQKJJYD0001).
Conflict of interest
YZ and GC were employed by Hangzhou Joyoung Soymilk & Food Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.FAOSTAT . (2017). Available online at: http://www.fao.org/faostat (accessed November 20, 2017).
- 2.Akyuz FA, Kandel H, Morlock D. Developing a growing degree day model for North Dakota and Northern Minnesota soybean. Agric For Meteorol. (2017) 239:134–40. 10.1016/j.agrformet.2017.02.027 [DOI] [Google Scholar]
- 3.Grassini P, Cafaro La Menza N, Rattalino Edreira JI, Monzón JP, Tenorio FA, Specht JE. Soybean. Crop Physiology Case Histories for Major Crops. (2021). p. 282–319. [Google Scholar]
- 4.Qin P, Wang T, Luo Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J Agric Food Res. (2022) 7:100265. 10.1016/j.jafr.2021.10026532370073 [DOI] [Google Scholar]
- 5.Miyake Y, Tanaka K, Okubo H, Sasaki S, Furukawa S, Arakawa M. Soy isoflavone intake and prevalence of depressive symptoms during pregnancy in Japan: baseline data from the Kyushu Okinawa Maternal and Child Health Study. Eur J Nutr. (2018) 57:441–50. 10.1007/s00394-016-1327-5 [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee C, Gleddie S, Xiao CW. Soybean bioactive peptides and their functional properties. Nutrients. (2018) 10:1211. 10.3390/nu10091211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebollo-Hernanz M, Kusumah J, Bringe NA, Shen Y, Gonzalez de Mejia E. Peptide release, radical scavenging capacity, and antioxidant responses in intestinal cells are determined by soybean variety and gastrointestinal digestion under simulated conditions. Food Chem. (2023) 405:134929. 10.1016/j.foodchem.2022.134929 [DOI] [Google Scholar]
- 8.Sui X, Zhang T, Jiang L. Soy protein: molecular structure revisited and recent advances in processing technologies. Annu Rev Food Sci Technol. (2021) 12:119–47. 10.1146/annurev-food-062220-104405 [DOI] [PubMed] [Google Scholar]
- 9.Ahsan S, Zahoor T, Shehzad A, Zia MA. Valuation of co-culture soymilk as a pragmatic approach on hyperglycemia and hypercholesterolemia in sprague - dawley rats. J Anim Plant Sci. (2019) 3:674–83. [Google Scholar]
- 10.Amigo-Benavent M, Clemente A, Caira S, Stiuso P, Ferranti P, Del Castillo MD. Use of phytochemomics to evaluate the bioavailability and bioactivity of antioxidant peptides of soybean β-conglycinin. Electrophoresis. (2014) 35:1582–9. 10.1002/elps.201300527 [DOI] [PubMed] [Google Scholar]
- 11.Henchion M, Hayes M, Mullen AM, Fenelon M, Tiwari B. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods. (2017) 6:53. 10.3390/foods6070053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agyei D. Bioactive proteins and peptides from soybeans. Recent Pat Food Nutr Agric. (2015) 7:100–7. 10.2174/2212798407666150629134141 [DOI] [PubMed] [Google Scholar]
- 13.Das D, Kabir ME, Sarkar S, Wann SB, Kalita J, Manna P. Antidiabetic potential of soy protein/peptide: a therapeutic insight. Int J Biol Macromol. (2022) 194:276–88. 10.1016/j.ijbiomac.2021.11.131 [DOI] [PubMed] [Google Scholar]
- 14.Kim IS, Yang WS, Kim CH. Beneficial effects of soybean-derived bioactive peptides. Int J Mol Sci. (2021) 22, 8570. 10.3390/ijms22168570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T, Zhang X, Ren Y, Zeng Y, Huang Q, Wang C. Antihypertensive effect of soybean bioactive peptides: a review. Curr Opin Pharmacol. (2022) 62:74–81. 10.1016/j.coph.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 16.Padhi S, Sanjukta S, Chourasia R, Labala RK, Singh SP, Rai AK. A multifunctional peptide from bacillus fermented soybean for effective inhibition of SARS-CoV-2 S1 receptor binding domain and modulation of toll like receptor 4: a molecular docking study. Front Mol Bioscie. (2021) 8:1–14. 10.3389/fmolb.2021.636647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang YC, Cheng MC, Lee CC, Chiou TY, Tsai TY. Effect of ethanol extract from Lactobacillus plantarum TWK10-fermented soymilk on wound healing in streptozotocin-induced diabetic rat. AMB Exp. (2019) 9:163. 10.1186/s13568-019-0886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alves de Souza SM, Hernández-Ledesma B, de Souza TLF. Lunasin as a promising plant-derived peptide for cancer therapy. Int J Mol Sci. (2022) 23:9548. 10.3390/ijms23179548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aiello G, Ferruzza S, Ranaldi G, Sambuy Y, Arnoldi A, Vistoli G, et al. Behavior of three hypocholesterolemic peptides from soy protein in an intestinal model based on differentiated Caco-2 cell. J Funct Foods. (2018) 45:363–70. 10.1016/j.jff.2018.04.023 [DOI] [Google Scholar]
- 20.Ren J, Li S, Song C, Sun X, Liu X. Black soybean-derived peptides exerted protective effect against alcohol-induced liver injury in mice. J Funct Foods. (2021) 87:104828. 10.1016/j.jff.2021.104828 [DOI] [Google Scholar]
- 21.Indiano-Romacho P, Fernández-Tomé S, Amigo L, Hernández-Ledesma B. Multifunctionality of lunasin and peptides released during its simulated gastrointestinal digestion. Food Res Int. (2019) 125:108513. 10.1016/j.foodres.2019.108513 [DOI] [PubMed] [Google Scholar]
- 22.Serra A, Gallart-Palau X, See-Toh RSE, Hemu X, Tam JP, Sze SK. Commercial processed soy-based food product contains glycated and glycoxidated lunasin proteoforms. Sci Rep. (2016) 6:1–12. 10.1038/srep26106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjeldsen SE. Hypertension and cardiovascular risk: General aspects. Pharmacol Res. (2018) 129:95–9. 10.1016/j.phrs.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 24.Kim IS. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants. (2021) 10:1064. 10.3390/antiox10071064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goh YX, Jalil J, Lam KW, Husain K, Premakumar CM. Genistein: a review on its anti-inflammatory properties. Front Pharmacol. (2022) 13:28. 10.3389/fphar.2022.820969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Wu Y, Yang C, Xu X, Meng Y. Antioxidant and hypolipidemic effects of soymilk fermented via Lactococcus acidophilus MF204. Food Funct. (2017) 8:4414–20. 10.1039/C7FO00701A [DOI] [PubMed] [Google Scholar]
- 27.Matoša Kočar M, Sudari, ć A, Sudar R, Duvnjak T, Zdunić Z. Ankstyvosios brandos sojos genotipu atranka siekiant pagaminti aukštos kokybes maistini alieju. Zemdirbyste. (2018) 105:55–62. 10.13080/z-a.2018.105.008 [DOI] [Google Scholar]
- 28.Zhang T, Jiang G, Li F, Gu X, Zhai Y, Xu L, et al. Soy product consumption and the risk of major depressive disorder in older adults: evidence from a cohort study. Front Psychiatry. (2022) 13:1929. 10.3389/fpsyt.2022.888667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganai AA, Farooqi H. Bioactivity of genistein: a review of in vitro and in vivo studies. Biomed Pharmacother. (2015) 76:30–8. 10.1016/j.biopha.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 30.Sahin CB, Işler N. Foliar applied zinc and iron effects on yield and yield components of soybean: determination by PCA analysis. Commun Soil Sci Plant Anal. (2020) 52:212–21. 10.1080/00103624.2020.1854297 [DOI] [Google Scholar]
- 31.Coscueta ER, Campos DA, Osório H, Nerli BB, Pintado M. Enzymatic soy protein hydrolysis: a tool for biofunctional food ingredient production. Food Chem X. (2019) 1:100006. 10.1016/j.fochx.2019.100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanjukta S, Rai AK. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci Technol. (2016) 50:1–10. 10.1016/j.tifs.2016.01.01036014024 [DOI] [Google Scholar]
- 33.Fakri EFM, Lim SM, Musa NH, Hasan MH, Adam A, Ramasamy K. Lactobacillus fermentum LAB 9-fermented soymilk with enriched isoflavones and antioxidants improved memory in vivo. Sains Malaysiana. (2016) 9:1289–97. [Google Scholar]
- 34.Cruz-Casas DE, Aguilar CN, Ascacio-Valdés JA, Rodríguez-Herrera R, Chávez-González ML, Flores-Gallegos AC. Enzymatic hydrolysis and microbial fermentation: the most favorable biotechnological methods for the release of bioactive peptides. Food Chem Mol Sci. (2021) 3:100047. 10.1016/j.fochms.2021.100047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen TH, Yesiltas B, Marin FI, Pertseva M, García-Moreno PJ, Gregersen S, et al. AnOxPePred: using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. (2020) 10:1–10. 10.1038/s41598-020-78319-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manikkam V, Vasiljevic T, Donkor ON, Mathai ML. A review of potential marine-derived hypotensive and anti-obesity peptides. Crit Rev Food Sci Nutr. (2016) 56:92–112. 10.1080/10408398.2012.753866 [DOI] [PubMed] [Google Scholar]
- 37.Fukuda M, Kobayashi M, Honda Y. Functional components and health benefits of fermented soymilk. Soft Chem Food Ferment. (2017) 6:145–178. 10.1016/B978-0-12-811412-4.00006-0 [DOI] [Google Scholar]
- 38.Ahn SB, Wu WH, Lee JH, Jun DW, Kim J, Kim R, et al. Fermented soymilk alleviates lipid accumulation by inhibition of SREBP-1 and activation of NRF-2 in the hepatocellular steatosis model. J Microbiol Biotechnol. (2018) 28:236–45. 10.4014/jmb.1707.07061 [DOI] [PubMed] [Google Scholar]
- 39.Dee W. LMPred: predicting antimicrobial peptides using pre-trained language models and deep learning. Bioinformat Adv. (2022) 2:1–7. 10.1093/bioadv/vbac021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lule VK, Garg S, Pophaly SD, Hitesh, Tomar SK. Potential health benefits of lunasin: a multifaceted soy-derived bioactive peptide. J Food Sci. (2015) 80:R485–94. 10.1111/1750-3841.12786 [DOI] [PubMed] [Google Scholar]
- 41.Albert Dhayakaran RP, Neethirajan S, Xue J, Shi J. Characterization of antimicrobial efficacy of soy isoflavones against pathogenic biofilms. LWT Food Sci Technol. (2015) 63:859–65. 10.1016/j.lwt.2015.04.053 [DOI] [Google Scholar]
- 42.Alnuaimi A, Fisayo Ajayi F, Hamdi M, Mudgil P, Kamal H, Yuen Gan C, et al. A comparative analysis of anti-lipidemic potential of soybean (Glycine max) protein hydrolysates obtained from different ripening stages: Identification, and molecular interaction mechanisms of novel bioactive peptides. Food Chem. (2023) 402:. 10.1016/j.foodchem.2022.134192 [DOI] [PubMed] [Google Scholar]
- 43.Baskaran R, Balasubramanian B, Ho JH, Wang MF, Abomughaid MM, Yang HS, et al. VH-4-A bioactive peptide from soybean and exercise training constrict hypertension in rats through activating cell survival and AMPKα1, Sirt1, PGC1α, and FoX3 α. Molecules. (2022) 27:7705. 10.3390/molecules27227705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mcclean S, Beggs LB, Welch RW. Antimicrobial activity of antihypertensive food-derived peptides and selected alanine analogues. Food Chem. (2014) 146:443–7. 10.1016/j.foodchem.2013.09.094 [DOI] [PubMed] [Google Scholar]
- 45.Cheng AC, Lin HL, Shiu YL, Tyan YC, Liu CH. Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture. Fish Shellf Immunol. (2017) 67:270–9. 10.1016/j.fsi.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 46.Eom JS, Lee SY, Choi HS. Bacillus subtilis HJ18-4 from traditional fermented soybean food inhibits Bacillus cereus growth and toxin-related genes. J Food Sci. (2014) 79:M2279–87. 10.1111/1750-3841.12569 [DOI] [PubMed] [Google Scholar]
- 47.Chi CF, Wang B, Wang YM, Zhang B, Deng SG. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J Funct Foods. (2015) 12:1–10. 10.1016/j.jff.2014.10.027 [DOI] [Google Scholar]
- 48.Mora-Escobedo R, Robles-Ramírez MD, Román-Gutiérrez AD, Castro-Rosas J, Muñoz-Llandes CB, Guzmán-Ortiz FA. Peptides and microorganisms isolated from soybean sources with antimicrobial activity. Soybean Biomass Yield Prod. (2018) 10.5772/intechopen.81243 [DOI] [Google Scholar]
- 49.Chourasia R, Padhi S, Chiring Phukon L, Abedin MM, Singh SP, Rai AK. A potential peptide from soy cheese produced using Lactobacillus delbrueckii WS4 for effective inhibition of SARS-CoV-2 main protease and S1 glycoprotein. Front Mol Biosci. (2020) 7:1–12. 10.3389/fmolb.2020.601753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babashahi M, Mirlohi M, Torki-Baghbadorani S, Ghiasvand R, Azadbakht L, Mosharaf L. Effects of probiotic soy milk fermented by Lactobacillus plantarum A7 (KC 355240) added with cuminum cyminum essential oil on fasting blood glucose levels, serum lipid profile and body weight in diabetic wistar rats. Int J Prev Med. (2020) 11:8. 10.4103/ijpvm.IJPVM_541_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dellafiora L, Pugliese R, Bollati C, Gelain F, Galaverna G, Arnoldi A, et al. “Bottom-Up” strategy for the identification of novel soybean peptides with angiotensin-converting enzyme inhibitory activity. J Agric Food Chem. (2020) 68:2082–90. 10.1021/acs.jafc.9b07361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devries MC, Sithamparapillai A, Brimble KS, Banfield L, Morton RW, Phillips SM. Changes in kidney function do not differ between healthy adults consuming higher- compared with lower- or normal-protein diets: a systematic review and meta-analysis. J Nutr. (2018) 148:1760–75. 10.1093/jn/nxy197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhayakaran R, Neethirajan S, Weng X. Investigation of the antimicrobial activity of soy peptides by developing a high throughput drug screening assay. Biochem Biophys Rep. (2016) 6:149. 10.1016/j.bbrep.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitagawa M, Shiraishi T, Yamamoto S, Kutomi R, Ohkoshi Y, Sato T, et al. Novel antimicrobial activities of a peptide derived from a Japanese soybean fermented food, Natto, against Streptococcus pneumoniae and Bacillus subtilis group strains. AMB Exp. (2017) 7:1–11. 10.1186/s13568-017-0430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farias TC, Abreu JP, Oliveira JPS, Macedo AF, Rodríguez-Vega A, Tonin AP, et al. Bioactive properties of peptide fractions from Brazilian soy protein hydrolysates: in silico evaluation and experimental evidence. Food Hydrocoll Health. (2023) 3:100112. 10.1016/j.fhfh.2022.100112 [DOI] [Google Scholar]
- 56.González-Montoya M, Hernández-Ledesma B, Silván JM, Mora-Escobedo R, Martínez-Villaluenga C. Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem. (2018) 242:75–82. 10.1016/j.foodchem.2017.09.035 [DOI] [PubMed] [Google Scholar]
- 57.Kiarie EG, Parenteau IA, Zhu C, Ward NE, Cowieson AJ. Digestibility of amino acids, energy, and minerals in roasted full-fat soybean and expelled-extruded soybean meal fed to growing pigs without or with multienzyme supplement containing fiber-degrading enzymes, protease, and phytase. J Anim Sci. (2020) 98:1–10. 10.1093/jas/skaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kojima-Yuasa A, Huang X, Matsui-Yuasa I. Synergistic anticancer activities of natural substances in human hepatocellular carcinoma. Diseases. (2015) 3:260–81. 10.3390/diseases3040260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu L, Wang Y, Xu Y, Tian Q, Lei G, Zhao C, et al. Lunasin functionally enhances LDL uptake via inhibiting PCSK9 and enhancing LDLR expression in vitro and in vivo. Oncotarget. (2017) 8:80826. 10.18632/oncotarget.20590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hashidume T, Kato A, Tanaka T, Miyoshi S, Itoh N, Nakata R, et al. Single ingestion of soy β-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci Rep. (2016) 6:1–15. 10.1038/srep28183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsieh LS, Lu MS, Chiang WD. Identification and characterization of immunomodulatory peptides from pepsin–soy protein hydrolysates. Bioresour Bioprocess. (2022) 9:1–13. 10.1186/s40643-022-00526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsieh CH, Wang TY, Tung BC, Liu HP, Yeh L, Hsu KC. The hydrolytic peptides of soybean protein induce cell cycle arrest and apoptosis on human oral cancer cell line HSC-3. Molecules. (2022) 27:2839. 10.3390/molecules27092839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ju DT, Ashok Kumar K, Kuo WW, Ho TJ, Chang RL, Lin WT, et al. Bioactive peptide VHVV upregulates the long-term memory-related biomarkers in adult spontaneously hypertensive rats. Int J Mol Sci. (2019) 20:3069. 10.3390/ijms20123069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jang CH, Oh J, Lim JS, Kim HJ, Kim JS. Fermented soy products: beneficial potential in neurodegenerative diseases. Foods. (2021) 10:638. 10.3390/foods10030636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwak JH, Kim M, Lee E, Lee SH, Ahn CW, Lee JH. Effects of black soy peptide supplementation on blood pressure and oxidative stress: a randomized controlled trial. Hypertens Res. (2013) 36:1060–6. 10.1038/hr.2013.79 [DOI] [PubMed] [Google Scholar]
- 66.Lammi C, Zanoni C, Arnoldi A, Vistoli G. Two peptides from soy β-conglycinin induce a hypocholesterolemic effect in HepG2 cells by a statin-like mechanism: comparative in vitro and in silico modeling studies. J Agric Food Chem. (2015) 63:7945–51. 10.1021/acs.jafc.5b03497 [DOI] [PubMed] [Google Scholar]
- 67.Lammi C, Bollati C, Ferruzza S, Ranaldi G, Sambuy Y, Arnoldi A. Soybean- and lupin-derived peptides inhibit DPP-IV activity on in situ human intestinal caco-2 cells and ex vivo human serum. Nutrients. (2018) 10:1082. 10.3390/nu10081082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu TH, Lin WJ, Cheng MC, Tsai TY. Lactobacillus plantarum TWK10-fermented soymilk improves cognitive function in type 2 diabetic rats. J Sci Food Agric. (2020) 100:5152–61. 10.1002/jsfa.10564 [DOI] [PubMed] [Google Scholar]
- 69.Lammi C, Arnoldi A, Aiello G. Soybean peptides exert multifunctional bioactivity modulating 3-hydroxy-3-methylglutaryl-CoA reductase and dipeptidyl peptidase-IV targets in vitro. J Agric Food Chem. (2019) 17:4824–30. 10.1021/acs.jafc.9b01199 [DOI] [PubMed] [Google Scholar]
- 70.Ma H, Liu R, Zhao Z, Zhang Z, Cao Y, Ma Y, et al. A novel peptide from soybean protein isolate significantly enhances resistance of the organism under oxidative stress. PLoS ONE. (2016) 11:e0159938. 10.1371/journal.pone.0159938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagaoka S. Mystery of cholesterol-lowering peptides, lactostatin and soystatin. J Agric Food Chem. (2018) 66:3993–4. 10.1021/acs.jafc.8b01025 [DOI] [PubMed] [Google Scholar]
- 72.Nagaoka S, Takeuchi A, Banno A. Plant-derived peptides improving lipid and glucose metabolism. Peptides. (2021) 142:170577. 10.1016/j.peptides.2021.170577 [DOI] [PubMed] [Google Scholar]
- 73.Pan F, Wang L, Cai Z, Wang Y, Wang Y, Guo J, et al. Soybean peptide QRPR activates autophagy and attenuates the inflammatory response in the RAW264.7 cell model. Protein Pept Lett. (2019) 26:301–12. 10.2174/0929866526666190124150555 [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Chen S, Zong X, Wang C, Shi C, Wang F, et al. Peptides derived from fermented soybean meal suppresses intestinal inflammation and enhances epithelial barrier function in piglets. Food Agric Immunol. (2020) 31:120–35. 10.1080/09540105.2019.1705766 [DOI] [Google Scholar]
- 75.Rayaprolu SJ, Hettiarachchy NS, Horax R, Phillips GK, Mahendran M, Chen P. Soybean peptide fractions inhibit human blood, breast and prostate cancer cell proliferation. J Food Sci Technol. (2017) 54:38–44. 10.1007/s13197-016-2426-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh BP, Vij S. Growth and bioactive peptides production potential of Lactobacillus plantarum strain C2 in soy milk: A LC-MS/MS based revelation for peptides biofunctionality. LWT. (2017) 86:293–301. 10.1016/j.lwt.2017.08.013 [DOI] [Google Scholar]
- 77.Tsai BCK, Kuo WW, Day CH, Hsieh DJY, Kuo CH, Daddam J, et al. The soybean bioactive peptide VHVV alleviates hypertension-induced renal damage in hypertensive rats via the SIRT1-PGC1α/Nrf2 pathway. J Funct Foods. (2020) 75:104255. 10.1016/j.jff.2020.104255 [DOI] [Google Scholar]
- 78.Wen L, Bi H, Zhou X, Zhu H, Jiang Y, Ramadan NS, et al. Structure and activity of bioactive peptides produced from soybean proteins by enzymatic hydrolysis. Food Chem Adv. (2022) 1:100089. 10.1016/j.focha.2022.100089 [DOI] [Google Scholar]
- 79.Yi G, Safdar B, Zhang Y, Li Y, Liu X. A study of the mechanism of small-molecule soybean-protein-derived peptide supplement to promote sleep in a mouse model. RSC Adv. (2020) 10:11264–73. 10.1039/D0RA00389A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Q, Tong X, Li Y, Wang H, Wang Z, Qi B, et al. Purification and characterization of antioxidant peptides from alcalase-hydrolyzed Soybean (Glycine max L.) hydrolysate and their cytoprotective effects in human intestinal Caco-2 cells. J Agric Food Chem. (2019) 67:5772–81. 10.1021/acs.jafc.9b01235 [DOI] [PubMed] [Google Scholar]
- 81.Zhu Y, Yao Y, Shi Z, Everaert N, Ren G. Synergistic effect of bioactive anticarcinogens from soybean on anti-proliferative activity in MDA-MB-231 and MCF-7 human breast cancer cells in vitro. Molecule. (2018) 23:1557. 10.3390/molecules23071557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh BP, Vij S, Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. (2014) 54:171–9. 10.1016/j.peptides.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 83.Nagaoka S. Structure-function properties of hypolipidemic peptides. J Food Biochem. (2019) 43:e12539. 10.1111/jfbc.12539 [DOI] [PubMed] [Google Scholar]
- 84.Cabanos C, Matsuoka Y, Maruyama N. Soybean proteins/peptides: a review on their importance, biosynthesis, vacuolar sorting, and accumulation in seeds. Peptides. (2021) 143:170598. 10.1016/j.peptides.2021.170598 [DOI] [PubMed] [Google Scholar]
- 85.Escudero E, Mora L, Toldrá F. Stability of ACE inhibitory ham peptides against heat treatment and in vitro digestion. Food Chem. (2014) 161:305–11. 10.1016/j.foodchem.2014.03.117 [DOI] [PubMed] [Google Scholar]
- 86.Cam A, de Mejia EG. RGD-peptide lunasin inhibits Akt-mediated NF-κB activation in human macrophages through interaction with the αVβ3 integrin. Mol Nutr Food Res. (2012) 56:1569–81. 10.1002/mnfr.201200301 [DOI] [PubMed] [Google Scholar]
- 87.Mohammadi Sartang M, Mazloomi SM, Tanideh N, Rezaian Zadeh A. The effects of probiotic soymilk fortified with omega-3 on blood glucose, lipid profile, haematological and oxidative stress, and inflammatory parameters in streptozotocin nicotinamide-induced diabetic rats. J Diabetes Res. (2015) 2015:696372. 10.1155/2015/696372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang HC, Lewis D, Tung CY, Han L, Henriquez SMP, Voiles L, et al. Soypeptide lunasin in cytokine immunotherapy for lymphoma. Cancer Immunol Immunother. (2014) 63:283–95. 10.1007/s00262-013-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lammi C, Zanoni C, Arnoldi A, Vistoli G. Peptides derived from soy and lupin protein as dipeptidyl-peptidase IV inhibitors: in vitro biochemical screening and in silico molecular modeling study. J Agric Food Chem. (2016) 64:9601–6. 10.1021/acs.jafc.6b04041 [DOI] [PubMed] [Google Scholar]
- 90.García-Nebot MJ, Recio I, Hernández-Ledesma B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food Chem Toxicol. (2014) 65:155–61. 10.1016/j.fct.2013.12.021 [DOI] [PubMed] [Google Scholar]
- 91.Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. (2020) 27:1–13. 10.1186/s12929-019-0609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Q, Tong X, Sui X, Wang Z, Qi B, Li Y, et al. Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res Int. (2018) 111:256–64. 10.1016/j.foodres.2018.05.046 [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto N, Shoji M, Hoshigami H, Watanabe K, Watanabe K, Takatsuzu T, et al. Antioxidant capacity of soymilk yogurt and exopolysaccharides produced by lactic acid bacteria. Biosci Microbiota Food Health. (2019) 38:97–104. 10.12938/bmfh.18-017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernández-Tomé S, Ramos S, Cordero-Herrera I, Recio I, Goya L, Hernández-Ledesma B. In vitro chemo-protective effect of bioactive peptide lunasin against oxidative stress in human HepG2 cells. Food Res Int. (2014) 62:793–800. 10.1016/j.foodres.2014.04.054 [DOI] [Google Scholar]
- 95.Lammi C, Zanoni C, Arnoldi A. Three peptides from soy glycinin modulate glucose metabolism in human hepatic HepG2 Cells. Int J Mol Sci. (2015) 16:27362. 10.3390/ijms161126029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barati M, Javanmardi F, Mousavi Jazayeri SMH, Jabbari M, Rahmani J, Barati F, et al. Techniques, perspectives, and challenges of bioactive peptide generation: a comprehensive systematic review. Comprehens Rev Food Sci Food Saf. (2020) 19:1488–520. 10.1111/1541-4337.12578 [DOI] [PubMed] [Google Scholar]
- 97.Tu Z, Chen L, Wang H, Ruan C, Zhang L, Kou Y. Effect of fermentation and dynamic high pressure microfluidization on dietary fibre of soybean residue. J Food Sci Technol. (2014) 51:3285–92. 10.1007/s13197-012-0838-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagino T, Kaga C, Kano M, Masuoka N, Anbe M, Moriyama K, et al. Effects of fermented soymilk with Lactobacillus casei Shirota on skin condition and the gut microbiota: a randomised clinical pilot trial. Benef Microbes. (2018) 9:209–18. 10.3920/BM2017.0091 [DOI] [PubMed] [Google Scholar]
- 99.Coscueta ER, Amorim MM, Voss GB, Nerli BB, Picó GA, Pintado ME. Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chem. (2016) 198:36–44. 10.1016/j.foodchem.2015.11.068 [DOI] [PubMed] [Google Scholar]
- 100.Coscueta ER, Pintado ME, Picó GA, Knobel G, Boschetti CE, Malpiedi LP, et al. Continuous method to determine the trypsin inhibitor activity in soybean flour. Food Chem. (2017) 214:156–61. 10.1016/j.foodchem.2016.07.056 [DOI] [PubMed] [Google Scholar]
- 101.Daliri EBM, Ofosu FK, Chelliah R, Park MH, Kim JH, Oh DH. Development of a soy protein hydrolysate with an antihypertensive effect. Int J Mol Sci. (2019) 20:1496. 10.3390/ijms20061496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daliri EBM, Tyagi A, Ofosu FK, Chelliah R, Kim JH, Kim JR, et al. A discovery-based metabolomic approach using UHPLC Q-TOF MS/MS unveils a plethora of prospective antihypertensive compounds in Korean fermented soybeans. Lwt. (2021) 137:110399. 10.1016/j.lwt.2020.110399 [DOI] [Google Scholar]
- 103.Jiang M, Yan H, He R, Ma Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur Food Res Technol. (2018) 244:1995–2005. 10.1007/s00217-018-3111-7 [DOI] [Google Scholar]
- 104.Lin S, Liang R, Li X, Xing J, Yuan Y. Effect of pulsed electric field (PEF) on structures and antioxidant activity of soybean source peptides-SHCMN. Food Chem. (2016) 213:588–94. 10.1016/j.foodchem.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 105.Nongonierma AB, FitzGerald RJ. Investigation of the potential of hemp, pea, rice and soy protein hydrolysates as a source of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Digest. (2015) 6:19–29. 10.1007/s13228-015-0039-2 [DOI] [Google Scholar]
- 106.Ruan S, Luo J, Li Y, Wang Y, Huang S, Lu F, et al. Ultrasound-assisted liquid-state fermentation of soybean meal with Bacillus subtilis: Effects on peptides content, ACE inhibitory activity and biomass. Process Biochem. (2020) 91:73–82. 10.1016/j.procbio.2019.11.035 [DOI] [Google Scholar]
- 107.Undhad Trupti J, Das S, Solanki D, Kinariwala D, Hati S. Bioactivities and ACEinhibitory peptides releasing potential of lactic acid bacteria in fermented soy milk. Food Prod Process Nutr. (2021) 3:10. 10.1186/s43014-021-00056-y [DOI] [Google Scholar]
- 108.Zhao W, Xue S, Yu Z, Ding L, Li J, Liu J. Novel ACE inhibitors derived from soybean proteins using in silico and in vitro studies. J Food Biochem. (2019) 43:1–10. 10.1111/jfbc.12975 [DOI] [PubMed] [Google Scholar]
- 109.Rekha CR, Vijayalakshmi G. Influence of processing parameters on the quality of soycurd (tofu). J Food Sci Technol. (2013) 50:176–80. 10.1007/s13197-011-0245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Capriotti AL, Caruso G, Cavaliere C, Samperi R, Ventura S, Zenezini Chiozzi R, et al. Identification of potential bioactive peptides generated by simulated gastrointestinal digestion of soybean seeds and soy milk proteins. J Food Compos Anal. (2015) 44:205–13. 10.1016/j.jfca.2015.08.007 [DOI] [Google Scholar]
- 111.Yang LC, Fu TJ, Yang FC. Biovalorization of soybean residue (okara) via fermentation with Ganoderma lucidum and Lentinus edodes to attain products with high anti-osteoporotic effects. J Biosci Bioeng. (2020) 129:514–8. 10.1016/j.jbiosc.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 112.de Figueiredo VRG, Yamashita F, Vanzela ALL, Ida EI, Kurozawa LE. Action of multi-enzyme complex on protein extraction to obtain a protein concentrate from okara. J Food Sci Technol. (2018) 55:1508–17. 10.1007/s13197-018-3067-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fang J, Lu J, Zhang Y, Wang J, Wang S, Fan H, et al. Structural properties, antioxidant and immune activities of low molecular weight peptides from soybean dregs (Okara). Food Chem X. (2021) 12:100175. 10.1016/j.fochx.2021.100175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z, Cui Y, Liu P, Zhao Y, Wang L, Liu Y, et al. Small peptides isolated from enzymatic hydrolyzate of fermented soybean meal promote endothelium-independent vasorelaxation and ACE inhibition. J Agric Food Chem. (2017) 65:10844–50. 10.1021/acs.jafc.7b05026 [DOI] [PubMed] [Google Scholar]
- 115.Jayachandran M, Xu B. An insight into the health benefits of fermented soy products. Food Chem. (2019) 271:362–71. 10.1016/j.foodchem.2018.07.158 [DOI] [PubMed] [Google Scholar]
- 116.Nath A, Kailo GG, Mednyánszky Z, Kiskó G, Csehi B, Pásztorné-Huszár K, et al. Antioxidant and antibacterial peptides from soybean milk through enzymatic-and membrane-based technologies. Bioengineering. (2020) 7:1–18. 10.3390/bioengineering7010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Islam M, Huang Y, Islam S, Fan B, Tong L, Wang F. Influence of the degree of hydrolysis on functional properties and antioxidant activity of enzymatic soybean protein hydrolysates. Molecules. (2022) 27:6110. 10.3390/molecules27186110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Oliveira CF, Corrêa APF, Coletto D, Daroit DJ, Cladera-Olivera F, Brandelli A. Soy protein hydrolysis with microbial protease to improve antioxidant and functional properties. J Food Sci Technol. (2015) 52:2668–78. 10.1007/s13197-014-1317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lertampaiporn S, Hongsthong A, Wattanapornprom W, Thammarongtham C. Ensemble-AHTPpred: a robust ensemble machine learning model integrated with a new composite feature for identifying antihypertensive peptides. Front Genet. (2022) 13:1–16. 10.3389/fgene.2022.883766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J, Yadav V, Smart AL, Tajiri S, Basit AW. Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol Pharm. (2015) 12:966–73. 10.1021/mp500809f [DOI] [PubMed] [Google Scholar]
- 121.Imura T, Nakayama M, Taira T, Sakai H, Abe M, Kitamoto D. Interfacial and emulsifying properties of soybean peptides with different degrees of hydrolysis. J Oleo Sci. (2015) 64:183–9. 10.5650/jos.ess14167 [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Zhou F, Zhao M, Lin L, Ning Z, Sun B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. (2018) 74:62–71. 10.1016/j.foodhyd.2017.07.021 [DOI] [Google Scholar]
- 123.Tang T, Liu J, Tang S, Xiao N, Jiang Y, Tu Y, et al. Effects of soy peptides and pH on foaming and physicochemical properties of egg white powder. Lwt. (2022) 153:112503. 10.1016/j.lwt.2021.112503 [DOI] [Google Scholar]
- 124.Mashayekh F, Pourahmad R, Eshaghi MR, Akbari-Adergani B. Improving effect of soy whey-derived peptide on the quality characteristics of functional yogurt. Food Sci Nutr. (2023) 1–10. 10.1002/fsn3.3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zheng L, Regenstein JM, Teng F, Li Y. Tofu products: A review of their raw materials, processing conditions, and packaging. Comprehens Rev Food Sci Food Saf. (2020) 19:3683–714. 10.1111/1541-4337.12640 [DOI] [PubMed] [Google Scholar]
- 126.Zhang Y, C, Zhang S, Zhu J, Wang H, Li, Liu X. Identification and characterization of soybean peptides and their fractions used by Lacticaseibacillus rhamnosus Lra05. Food Chem. (2023) 401:134195. 10.1016/j.foodchem.2022.134195 [DOI] [PubMed] [Google Scholar]
- 127.Zhang CY, Zhang G, Liu W, Li S, Xia H, Li X, et al. Effects of soybean protein isolates and peptides on the growth and metabolism of Lactobacillus rhamnosus. J Funct Foods. (2021) 77:104335. 10.1016/j.jff.2020.10433530934634 [DOI] [Google Scholar]
- 128.Dai J, Ou W, Yu G, Ai Q, Zhang W, Mai K, et al. The antimicrobial peptide cecropin AD supplement alleviated soybean meal-induced intestinal inflammation, barrier damage, and microbial dysbiosis in juvenile turbot, Scophthalmus maximus. Front Mar Sci. (2020) 7:1–11. 10.3389/fmars.2020.58448232802822 [DOI] [Google Scholar]
- 129.Yang M, Yang X, Chen X, Wang J, Liao Z, Wang L, et al. Effect of kefir on soybean isoflavone aglycone content in soymilk kefir. Front Nutr. (2020) 7:587665. 10.3389/fnut.2020.587665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Freitas CS, Vericimo MA, da Silva ML, da Costa GCV, Pereira PR, Paschoalin VMF, et al. Encrypted antimicrobial and antitumoral peptides recovered from a protein-rich soybean (Glycine max) by-product. J Funct Foods. (2019) 54:187–98. 10.1016/j.jff.2019.01.024 [DOI] [Google Scholar]
- 131.He W, Chung HY. Exploring core functional microbiota related with flavor compounds involved in the fermentation of a natural fermented plain sufu (Chinese fermented soybean curd). Food Microbiol. (2020) 90:103408. 10.1016/j.fm.2019.103408 [DOI] [PubMed] [Google Scholar]