Abstract
Recent advances in deep sequencing technologies have revealed that, while less than 2% of the human genome is transcribed into mRNA for protein synthesis, over 80% of the genome is transcribed, leading to the production of large amounts of noncoding RNAs (ncRNAs). It has been shown that ncRNAs, especially long non-coding RNAs (lncRNAs), may play crucial regulatory roles in gene expression. As one of the first isolated and reported lncRNAs, H19 has gained much attention due to its essential roles in regulating many physiological and/or pathological processes including embryogenesis, development, tumorigenesis, osteogenesis, and metabolism. Mechanistically, H19 mediates diverse regulatory functions by serving as competing endogenous RNAs (CeRNAs), Igf2/H19 imprinted tandem gene, modular scaffold, cooperating with H19 antisense, and acting directly with other mRNAs or lncRNAs. Here, we summarized the current understanding of H19 in embryogenesis and development, cancer development and progression, mesenchymal stem cell lineage-specific differentiation, and metabolic diseases. We discussed the potential regulatory mechanisms underlying H19's functions in those processes although more in-depth studies are warranted to delineate the exact molecular, cellular, epigenetic, and genomic regulatory mechanisms underlying the physiological and pathological roles of H19. Ultimately, these lines of investigation may lead to the development of novel therapeutics for human diseases by exploiting H19 functions.
Keywords: Cancer, Epigenetic regulation, H19, LncRNA, Long-noncoding RNA, Metabolic diseases, Stem cell differentiation
Introduction
Gene expression is the core process of all aspects of life. The process begins in the cell nucleus with genomic DNA, the template for transcription of messenger RNAs (mRNAs), after transcription, mRNAs translocate to the cytoplasm and act as blueprints for translation into proteins. In addition to mRNAs, there are many non-protein coding RNAs (ncRNAs), such as transfer RNAs, ribosomal RNAs, and small nuclear RNAs that are also essential for gene expression. Since the completion of the Human Genomic Project, it has been estimated that while over 80% of human genomic DNA is transcribed, only about 2% of the human genome is transcribed into mRNA and translation to proteins, indicating the pervasiveness of ncRNAs.1, 2, 3, 4, 5 Recently, ncRNAs have been characterized as having regulatory functions in many physiological and/or pathological processes and are regarded as the therapeutic targets for disease.2,6, 7, 8, 9, 10, 11 In particular, long non-coding RNAs (lncRNAs), non-protein coding transcripts longer than 200 nucleotides, are rapidly being identified as having crucial roles in regulatory control of gene expression,2,3,7,10,12 such as promoter-specific gene regulation,13, 14, 15 acting as competing endogenous RNAs (CeRNAs),15,16 X-chromosome inactivation,17, 18, 19, 20 imprinting,21, 22, 23, 24 maintenance of nuclear architecture,25, 26, 27 etc.
LncRNA H19, first isolated and reported in the 1980s by 4 different laboratories, is one of the first identified imprinted genes and lncRNAs.21, 22, 23,28, 29, 30, 31 In 1990, Brannan et al found that the H19 gene did not code for any protein even though it is transcribed by RNA polymerase II, spliced, and polyadenylated.29 In mice, H19 contains three reading frames, however, multiple translation termination signals were found in all three reading frames.29 In 1991, Bartolomei et al confirmed that H19 is only transcribed from the maternally inherited allele, the paternal H19 allele is not expressed in mice. Meanwhile, H19 and Igf2 genes are tightly linked and imprinted in opposite directions.21 The following year, Zhang et al identified that H19 is also maternally expressed in humans.32 Then, H19 was certified as a lncRNA since it does not contain the short introns that are characteristic of imprinted coding genes, complete absence of transcribed peptide, form secondary RNA structures and hairpin loops, localize in a cytoplasmic ribonucleoprotein particle, and may function as a riboregulator.23,29,33,34 In the past few decades, the regulatory functions of H19 have been increasingly clarified. The expression patterns of H19, H19 encoded-microRNAs and anti-sense, the interlinking expression of H19 and Igf2, etc. are characterized. Here, we summarize these diverse regulatory functions in numerous physiological and pathological processes.
Critical roles of H19 in embryogenesis and development
H19 is highly expressed in developing embryos, especially in mesoderm- and endoderm-derived tissues. However, H19 is strongly down-regulated after birth in all tissues including skeletal and cardiac muscle tissues,28,30 although not entirely turned off in skeletal and cardiac muscle tissues.35 Loss of H19 function in the liver and a few other endodermal tissues did not influence the survival of mice and instead resulted in an overgrowth phenotype similar to babies with Beckwith-Wiedemann syndrome, which may have been caused by overexpression of Igf2.34 However, the overexpression of H19 in the mouse zygote causes lethal effects between embryonic day 14 and birth, which indicates the importance of strict control of H19 during embryogenesis.36 On the other hand, mice lacking H19 showed an overgrowth phenotype, while mice with H19 enhancer overexpression had reduced postnatal growth.37 In addition, human imprinting control region 1 (IC1) can functionally replace mouse IC1on the maternal allele, but not paternally, which indicated the paternal imprinting at IC1 between mice and humans.38 These results indicated the fine-tuning role of H19 during both embryogenesis and development.
As a maternally imprinted gene, H19 expression is regulated by methylation during embryogenesis. H19 methylation regions differ according to parental inheritance, the paternal copy of H19 is methylated and silent while the maternal copy is either hypomethylated or unmethylated, resulting in different maternal H19 allele expression in offspring.39, 40, 41, 42, 43 Meanwhile, methylation of the H19 promoter is negatively correlated with H19 expression and H19 expression is negatively correlated with the neighboring gene Igf2 expression.42, 43, 44, 45 Han et al recently characterized that disruption of Igf2/H19 expression in parthenogenetic fetuses and placentas contributes to implantation failure and/or abortion in swine parthenogenesis, which might be associated with differential methylation patterns in the imprinting control region of imprinted genes.46 H19 and Igf2 shared several enhancers, which are tissue-specific.47 In other words, the mutual regulation of Igf2 and H19 could not be ignored during embryogenesis.41,48
Although H19 is strongly down-regulated after birth in most tissues, it still plays an important role in development. Since it was known that H19 was highly expressed in muscles after birth, the biological function of H19 was first explored in motor system development.37,49,50 H19-deficient mice displayed hyperplasia in the late fetal stage, hypertrophy at the postnatal stage in skeletal muscles, and had decreased number of mutant satellite cells.51 On the other hand, Martinet et al found that H19 deficiency decreased the number of mutant satellite cells; however, the self-renewing capacity of these cells was not affected, which may be due to the up-regulation of other genes in the imprinted gene network.51 During the myogenic differentiation process, H19 is highly activated by the key transcription factor MyoD, which is highly expressed during myoblast differentiation and muscle regeneration. What is more, H19 and Igf2 misexpression work separately, cooperatively, and antagonistically to establish the developmental phenotype through different signaling pathways.52 These results indicated the essential role of H19 in muscle development.49
As for osteogenic differentiation, H19 showed an increased expression pattern in mesenchymal stem cells (MSCs) during the initial period. H19 regulates several signaling pathways involved in MSC osteogenic differentiation.49,53, 54, 55, 56, 57 Similar to the function of H19 in embryogenesis, we found that adenovirus-mediated overexpression of H19 inhibited osteogenic differentiation of MSCs, while down-regulation of H19 alleviates MSC osteogenic differentiation.58,59 Meanwhile, it has been reported that H19 regulated chondrogenic differentiation by potentiating Sox9-mediated collagen type II formation, which indicates the regulatory function of H19 in cartilage formation.49,60 In addition, H19 was found to promote tenogenic differentiation and suppress adipogenic differentiation of MSCs.61, 62, 63 Overall, these results suggest that H19 plays an important role in motor system development.
Molecular mechanisms underlying H19 functions
Imprinted tandem gene Igf2-H19
As one of the firstly identified maternal imprinted genes, H19 and Igf2 belong to the same locus and are separated only by 90 kb which is located on chromosome 7 in mice and chromosome 11 in human.21,29,64,65 Most imprinted genes are methylated on maternally derived chromosomes; however, Igf2-H19 is methylated on paternally derived chromosomes. The expression balance of Igf2-H19 is one of the most important mechanisms underlying H19 functions.66, 67, 68 Loss of function of H19 and overexpression of Igf2 were associated with overgrowth syndrome called Beckwith-Wiedemann syndrome, on the contrary, will result in fetal and postnatal growth failure called Silver-Russell syndrome.52,69,70
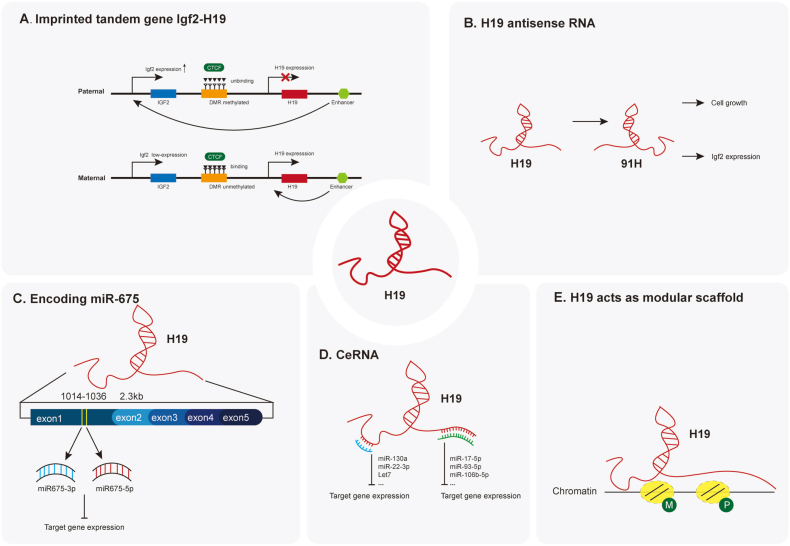
The imprinted tandem gene Igf2-H19 participates in several physiological and pathological processes, such as embryogenesis, tumorigenesis, and guardian of the proliferation of pluripotent stem cells, etc.48,71,72 The regulation of Igf2-H19 gene expression is one of the essential mechanisms. When the 3′ gene enhancer shared between H19 and Igf2 is deleted, both H19 and lfg2 expression are down-regulated. The 3′ enhancer has a more robust effect on the expression of H19 since H19 has a stronger promoter in this area than Igf2 and is physically closer to the 3′ enhancer.41,48,67,73 On the other hand, tissue-specific and parent-of-origin enhancers were also identified and proved to regulate the expression of Igf2-H1947 (Fig. 1A).
Figure 1.
Current models of action for H19. (A) The imprinted Igf2-H19 tandem gene participates in several physiological and pathological processes. (B) The antisense RNA 91H can regulate H19-Igf2 expression and hence impacts cell growth. (C)H19 can encode miR-675 which regulates gene expression. (D)H19 can act as CeRNA and regulate gene expressions. (E)H19 can act as modular scaffolds for epigenetic modifications.
The gap between H19 and Ifg2 contains differentially methylated regions (DMRs) in the chromosome, whose methylation state regulates the expression of H19 and Ifg2. Generally, DMRs are methylated in paternal chromosomes and unmethylated in the maternal chromosome. Methylated DMRs block the binding of DNA-binding zinc finger insulator protein, CTCF, which prevents the transcription of downstream H19 in the paternal chromosome.66, 67, 68 On the contrary, unmethylated DMRs bind with CTCF and prevent transcription of Igf2, and in this situation, only H19 is transcribed to RNA. This process ensures the maternal imprinted expression of H19 and maintains a proper balance of H19 and Igf2 genes.48,66, 67, 68,73 When the methylation of DMRs within the Igf2-H19 locus is erased, the expression of H19 increased and blocked the expression of Igf2, which prevents the uncontrolled proliferation and teratoma formation of cells.73, 74, 75 In contrast, loss of imprinting induces hypermethylation of DMRs within Igf2-H19 on both maternally and paternally derived chromosomes, which is associated with several malignancies and results in high expression of Igf248,73 (Fig. 1A).
H19 antisense RNA
Since noncoding antisense transcripts have been suggested to constitute a new epigenetic regulatory system, H19 antisense (91H RNA) was identified with varying levels of expression in both the estrogen-sensitive T47D and the estrogen-insensitive BT20 breast cancer cell lines.76 These results indicate the existence and regulatory function of 91H. In addition, through nucleic acid analysis, Berteaux et al76 confirmed 91H is a single 120-kb transcript generated from the H19/Igf2 locus. Next, it was confirmed that, rather than affecting H19 expression, 91H influence Igf2 expression.76 Moreover, although 91H, H19, and Igf2 are overexpressed in breast tumors, 91H was demonstrated to promote breast tumor cell growth, migration, and invasion by preventing histone and DNA methylation on the maternal allele at the H19/Igf2 locus.77
In most tumor cells except breast tumor cells, 91H was identified to promote Ifg2 transcription by activating the Igf2 promoter in mouse myoblast.78 Gao et al79 found that 91H was down-regulated in esophageal squamous cell carcinoma with higher depth of invasion, neoplastic grading, and TNM. Lower 91H expression was associated with an increased risk for the occurrence, progression, and prognosis of esophageal squamous cell carcinoma. Recently, the regulatory function of 91H was also reported in colorectal cancer, osteosarcoma, and hepatocellular carcinoma, etc.80, 81, 82, 83 On the other hand, H19 antisense encodes a protein, H19 opposite tumor suppressor, and could inhibit Wilms, rhabdoid, rhabdomyosarcoma, and choriocarcinoma tumor cell growth, which indicates that H19 locus harbors an imprinted gene encoding a tumor suppressor protein.84 Taken together, these results suggested a new mechanism of H19/Igf2-associated regulation (Fig. 1B).
H19: encoding miR-675
Exon 1 of H19 encodes two distinct miRNAs, miR-675-5p and miR-675-3p, which are highly conserved and confer functionality to H1985,86. MiR-675-5p and miR-675-3p could synergistically regulate the same or different physiological or pathologic processes with different mechanisms87, 88, 89 (Fig. 1C). To prove H19 encodes miR-675, Cai et al90 first identified the sequence in H19 located from 1014 to 1036 that encoded miR-675. They then identified that miR-675 was generated from longer pri-miRNA precursors by the RNase III enzymes Drosha and Dicer in the 293 T cell line, which does not normally express H19. In addition, the 293 T cell line readily expressed miR-675 at high levels after being transfected with human or mouse H19 plasmid, but not in mock-transfected cells. These results indicated that miR-675 is encoded by H19 and positively correlated with the expression of H19.41,49,90,91
As an indispensable part of the H19-miR-675 axis, miR-675 has been reported to regulate myogenesis, chondrogenesis, adipogenesis, tenogenesis, and osteogenesis, etc.41,49,60,92 Dudek et al identified that miR675 expression level was positively correlated with the expression level of Sox9 and regulated type II collagen expression in human articular chondrocytes.60 Dey93 et al found that H19 encoded-miR-675-3p and miR-675-5p, which promoted skeletal muscle differentiation and regeneration by directly targeting and down-regulating the anti-differentiation Smad transcription factors. Similarly, miR-675 was reported to down-regulate some functional genes and regulate signaling pathways during tumor progression.86,94,95
H19: competing endogenous RNA (CeRNA)
MicroRNAs (miRNAs) have important regulatory functions in many physiological and pathological processes. One of the most essential roles of miRNAs is post-transcriptional regulation, in which miRNAs bind to specific recognition sequences (“seed sequences”) located in the 3′ untranslated regions (3′ UTRs) of target mRNAs. In this event, miRNAs inhibit the expression of target proteins by enhancing mRNA degradation and/or by directly interfering with protein translation.7,96,97 As mentioned above, lncRNA can generate miRNAs, simultaneously, lncRNA can act as CeRNA, in which specific lncRNA can impair miRNA activity through sequestration and finally up-regulate miRNA target gene expression.7,98, 99, 100
It is reported that H19 contains functional miRNAs let-7 interaction sites and can inhibit let-7 function by acting as a molecular sponge. In addition, this competitive binding can accelerate muscle differentiation and regulate muscle glucose metabolism in muscle cells, which indicates that H19's function as CeRNA is one of its essential mechanisms of the regulatory function of H19.101,102 By preventing H19 from interacting with miR-let-7, Park et al identified cardiac phenotypes were associated with the interaction between H19 and miR-let-7.35 At the same time, H19 can bind with miR-17–5p,103 miR-93–5p,104 miRNA-106 b-5p,105 miR-130a,106 miR-22–3p,107 etc. respectively, and regulates the specific biological process. Significantly, H19 may regulate a specific biological process by simultaneously binding with several miRNAs. These results indicate the flexibility and diversity of H19 in regulating gene expression by acting as a CeRNA58,108,109 (Fig. 1D).
H19 acts as a modular scaffold
It was known that lncRNAs regulate chromatin states and epigenetic inheritance. Tsai et al identified that lnRNA HOTAIR serves as a modular scaffold for two histone modification complexes, in which the 5′ domain of HOTAIR binds to the polycomb repressive complex 2 and the 3′ domain binds to the LSD1/CoREST/REST complex.110 Different from HOTAIR, it is reported that hypoxia regulates H19 expression and function by regulating allele-specific histone modification.111 On the other hand, we found that H19 could act as a modular scaffold in the cytoplasm and promote specific protein phosphorylation, then it can regulate the activation of the protein and downstream signaling pathway112,113 (Fig. 1E). The main mechanisms of H19 mediated regulation are shown in Figure 1.
Complex roles of H19 in cancer development and progression
Abnormal expression of H19 has been demonstrated in a variety of different types of cancer cells.85 As H19-mediated regulatory function can occur through several different mechanisms, H19 was found to be associated with different types of cancer and played different roles in cancer development and cancer progression.85,114
H19 in osteosarcoma (OS)
The disruption of H19 and Igf2 expression was associated with the development of rhabdomyosarcoma, which was identified in 1997, and H19 was regarded as the tumor suppressor of rhabdomyosarcoma.115,116 Recently, defective osteogenic differentiation has been characterized to play an important role in the development of OS.117 With the use of induced pluripotent stem cells, Lee118 et al found that impaired osteoblast osteogenic differentiation resulted in Li-Fraumeni syndrome -associated OS. The mechanism underlying this is the low expression of H19 and dysregulation of H19 IGN. Aside from participating in the development of OS, H19 also regulates the migration and invasion of OS. It was reported that H19 has higher expression in OS tumor tissue than in adjacent healthy tissue.119 Furthermore, H19 expression level in OS is positively associated with tumor migration and invasion, which is mediated by the down-regulation of the nuclear factor-κB pathway (NF κB).120,121 By acting as a CeRNA, H19 can regulate OS development, invasion, and migration by suppressing functional miRNA expression.85,122 Taken together, the down-regulation of H19 increases the risk of OS development and up-regulated H19 is associated with a poor prognosis of OS.
H19 in breast cancer
Breast cancer is one of the most common cancers among women worldwide. Since H19 is an estrogen-regulated transcript and regulates cell proliferation and differentiation, several studies have been carried out to clarify the effects and mechanisms of H19 in regulating breast cancer development, progression, and metastasis.85,123 Firstly, H19 expression levels were positively associated with breast cancer cell proliferation, invasion, and angiogenesis, and ectopic overexpression of H19 promotes tumorigenic properties of breast cancer cells.124, 125, 126 Secondly, a recent clinical case-control study showed that H19-associated single nucleotide polymorphisms were associated with breast cancer risk and have the potential for breast cancer diagnosis and estimating prognosis.127,128 In addition, it was identified that H19 can lead to breast tumorigenesis by altering DNA methylation.126 Simultaneously, as mentioned above, H19 antisense 91H also regulates breast cancer development by epigenetic modifications.76, 77, 78 In summary, it can be concluded that H19 is an oncogene in breast tumorigenesis.
Additionally, as the precursor of miR-675, it was reported that miR-675 expression was significantly higher in breast cancer tissue compared with the control group, which demonstrates regulation of H19-miR576 axis.123,129 Further studies identified that ectopic expression of H19-miR675 reinforced breast cancer cell proliferation, migration, metastasis, and drug resistance.85,95,123,125,129 As for the ceRNA network of H19, by differently sponging miR-200b/c and let-7b, H19 regulates the epithelial-to-mesenchymal transition in breast cancer cells. Similarly, H19 can bind with miR-152, miR-93-5p, let-7a/b, miR-324-5p, miR-29b-3p, etc. in breast cancer progression.85,123,130
H19 in gastrointestinal cancers
The digestive system includes the gastrointestinal tract (the esophagus, stomach, intestine, and colorectum) and the accessory organs of digestion (the pancreas, liver, and gallbladder). Recently, H19 has been reported to regulate digestive system cancers on multiple levels, both as an oncogene or regulating tumor-suppressing gene expression.93,129,131, 132, 133, 134
Firstly, H19 is found to be up-regulated in gastric cancers,135, 136, 137 pancreatic cancers,138, 139, 140 hepatocellular carcinoma,141, 142, 143 esophageal squamous cell carcinoma,144,145 colorectal cancer,132,146, 147, 148 etc., which indicates that H19 may act as an oncogene in digestive system cancers. Simultaneously, H19 polymorphisms were identified to be a potential marker for the diagnosis of digestive system cancers,149, 150, 151 and H19 expression levels were reported to have a positive correlation with the proliferation, invasion, and metastasis of digestive cancer cells.131,137, 138, 139,152 In addition, Wang et al found that H19 could function as CeRNA, bind with miR-194–5p and influence SIRT1-mediated autophagy, and finally promote 5-Fu resistance in colorectal cancer.153 Wu et al demonstrated that H19 promotes methotrexate resistance in colorectal cancer by activating Wnt/beta-catenin signaling.154 In hepatocellular carcinoma, Xu et al155 identified that H19 participated in sorafenib resistance by the H19-miR675 axis. In summary, H19 may act as an oncogene in digestive system cancers and hold the potential for early diagnosis, acting as a target of treatment and working as prognosis markers.135,140,146,156,157
H19 in lung cancer
There are two main types of lung cancer, non-small cell lung cancer, which occupies 80%–84% of all lung cancer cases, and small cell lung cancer, which occupies 10%–15%. Although these two types of lung cancer are treated very differently, H19 was found to be up-regulated in both non-small and small cell lung cancer with different mechanisms.158, 159, 160, 161 Studies conducted on ethnic Chinese populations showed that the polymorphism rs2107425 in the H19 gene was associated with the risk of lung cancer among females whom never smoked,162 and the single nucleotide polymorphism rs217727 in lncRNA H19 was significantly associated with susceptibility to lung cancer, particularly in squamous cell carcinoma and adenocarcinoma in smokers.163 In non-small cell lung cancer, Zheng et al164 identified that up-regulated H19/miR675 promoted hypoxia-induced cancer progression by inhibiting p53 signaling. Ren et al165 reported that H19 sponged miR-196b, which targeted a conserved RNA binding protein LIN28B, and accelerated lung cancer growth. Simultaneously, by acting as a CeRNA, H19 was found to sponge miR-200a,166 miR-107,161 miR-17,167 miR-6515-3p,168 miR-138,169 miR-203, etc, and promoted lung cancer cell proliferation, invasion, metastasis, or epithelial-mesenchymal transition. In small cell lung cancer, Li et al170 found that H19 promoted small cell lung cancer tumorigenesis by regulating the miR-140-5p/FGF9 axis by acting as CeRNA.
In addition, several studies identified that H19 expression was associated with lung cancer drug resistance.171, 172, 173, 174 Zhou et al174 characterized that inhibition of H19 could enhance gefitinib sensitivity and the effectiveness of chemotherapy, which indicated the function of H19 in lung cancer drug resistance. Pan et al173 found that H19 was highly expressed in erlotinib resistance lung cancer cell lines via exosomes and regulated drug resistance via targeting miR-615-3p to regulate ATG7 expression. Lei et al171 also proved that H19 packaged into exosomes and mediated lung cancer cell resistance to gefitinib. In human lung adenocarcinoma cells, H19-mediated cisplatin resistance potentially serves as a molecular marker to predict the prognosis of lung adenocarcinoma.172
In summary, H19 gene acts as an oncogene in lung cancer and participates in tumorigenesis, progression, metastasis, and drug resistance, which indicates that H19 can be a molecular marker or target for lung cancer diagnosis and treatment.
H19 in genitourinary cancer
By the function of H19 in other systems, H19 participates in tumorigenesis in genitourinary cancer.71,129,175,176 As a rare kidney cancer, Wilms tumor primarily affects children, H19 was identified to be an imprinted tumor-suppressor gene of Wilms tumor.177 Meanwhile, H19 could interact with another anti-oncogene to suppress Wilms tumor development.177, 178, 179, 180 Except for breast cancer,181 genomic variants with H19 were identified to hold the potential to be diagnosis markers in bladder cancer,182,183 epithelial ovarian cancer,184 Wilms tumor,185 etc. In prostate cancer, H19 is regarded as the essential regulator of cell fate, plasticity, and treatment resistance and holds the potential to be a diagnosis marker.186 Meanwhile, H19 acts as an estrogen- and hypoxia-response regulator and promotes prostate cancer invasion through the epithelial-to-mesenchymal transition to a β integrin pathway.187 By acting as CeRNA, H19 was reported to regulate prostate cancer, bladder cancer,188 ovarian cancer,189 seminoma,105 etc. In addition, impaired Igf2-H19 imprinting was found in prostate cancer,190 ovarian cancer,184,191 bladder cancer,192 Wilm's disease,193 malignant endometrium,194 etc. Simultaneously, H19 regulates several genitourinary cancer cell proliferation, invasion, metastasis, and drug resistance through different mechanisms.184,186,188,189
It is worth mentioning that, as an essential oncogene, targeting H19--mediated tumor treatment has been explored in various studies.195 Sidi et al196 investigated the effects of intravesical injection of a DNA plasmid that contains H19 gene regulatory sequences which drive the expression of an intracellular toxin in a phase I/II marker lesion study. They found an ablation effect on the bladder, which indicated the potential of this method. A fragment of diphtheria toxin (DTA) or herpes simplex virus thymidine kinase (HSV-tk), under the control of an 814-bp 5′-flanking region of the H19 gene, was applied for regulating the expression of H19.195 A phase 1/2a clinical study found that intraperitoneal use of H19-DTA was a safe and effective method for recurrent ovarian/peritoneal cancer.195 A phase 2 b marker lesion trial also confirmed the effect of H19-DTA in nonmusical invasive bladder cancer. These results indicated the treatment potential of targeting H19.
H19 in MSC lineage-specific differentiation
H19 in osteogenic differentiation
As we mentioned before, H19 plays an important role in motor system development. As the key process of bone development and regeneration, osteogenic differentiation of MSCs is closely involved in the regulation of H19.53,197, 198, 199, 200
Like the function of H19 in development, we identified that the expression balance of H19 is essential for the osteogenic differentiation of MSCs. Our in vitro and in vivo studies found that overexpression of H19 blocked BMP9-induced osteogenic differentiation of MSCs and kept MSCs in undifferentiation status. However, down-regulation of H19 in BMP9 induced osteogenic differentiation of MSCs inhibited osteogenic differentiation and relatively promoted adipogenic differentiation.58,109 Wang et al further found that H19 regulates osteogenic and adipogenic differentiation of BMSCs through ligand-dependent corepressor by sponging miR-188.201 Furthermore, Huang et al proved that the H19-miR 675 axis regulated the balance between osteogenesis and adipogenesis of MSCs by targeting the 3′ UTRs of the histone deacetylase (HDAC) 4–6 transcripts.62 Simultaneously, H19 was reported to hold the potential to induce stem cell osteogenic differentiation. Li et al202 found that H19 promoted osteogenic differentiation of bone marrow MSCs by sponging miR-149, which increased the expression of stromal cell-derived factor-1. Huang et al54 identified that both H19 and miR-675 promoted osteogenic differentiation of MSCs by activating the TGF-beta1/Smad3/HDAC signaling pathways. In addition, H19 mediated CeRNA mechanism was proved to regulate several signaling, including the ERK1/2 and p38 MAPK signaling pathways,203 Runx2,204 focal adhesion protein-tyrosine kinases,199 etc. Meanwhile, as an imprinted gene, H19-IFG2 also regulated osteogenic differentiation.205 Loss of imprinting of Igf2 could improve the osteogenic potential of human deciduous teeth MSCs, which could be the main difference compared with human adipose tissues.206
Crosstalk between H19 and Wnt signaling was identified recently. Wnt signaling is essential for the osteogenic differentiation of MSCs.207 As an enhancer of Wnt4, forkhead box C2 (Foxc2) binds to the promoter area of Wnt4 and then activates the Wnt-β-catenin pathway, and H19 synergistically promotes this process by directly binding with Foxc2.208 On the other hand, H19-encoded miR675-5p was proved to be a negative regulator of BMSC osteogenic differentiation, as the H19-miR675-5p axis regulates osteogenic differentiation through Wnt-β-catenin pathway.209 However, Ma et al found that H19-derived miR-675 targeted the adenomatous polyposis coli protein and promoted osteogenic differentiation of MSCs by activating Wnt signaling.210 In addition, Liang et al56 found that H19 acted as CeRNA and sponged miR-22 and miR-141 which targeted Wnt/beta-catenin signaling, and then regulated osteogenic differentiation of human MSCs. Similar results were detected by Gong et al211 in ectomesenchyme stem cells.
Moreover, H19 was reported to be activated by estrogen and regulated osteogenic differentiation of BMSCs, which could reduce osteoporosis via the miR-532-30/SIRT1 axis.197 Simultaneously, H19 was also identified to regulate different types of body cells including osteoblasts, osteoclasts, and osteocytes in the osteogenic differentiation process which hold the potential to be a target for osteoporosis treatment.203,212, 213, 214
H19 in endochondral ossification and angiogenesis
H19 was identified to regulate chondrogenic differentiation and maintain the anabolic and catabolic activities of chondrocytes and endochondral ossification.60,92,215 In mouse limbs, we detected that the expression level of H19 is high in the proliferation zone and decreases gradually from the prehypertrophic zone to the hypertrophic zone. In addition, we characterized that H19 is essential for chondrogenic differentiation and maintaining the proliferation of chondrocytes, and down-regulation of H19 promoted hypertrophic differentiation of chondrocytes and followed endochondral ossification. In mechanism, we proved that H19 facilitated Runx2 phosphorylation.112 In chondrocytes, Steck et al found that H19 expression levels were not correlated with Igf2 expression levels, but positively correlated with miR-675 expression levels; a further study confirmed that H19 could be an attractive marker for chondrocyte anabolism and be a potential target for cartilage recovery.92
Angiogenesis is a key process of endochondral ossification and osteogenesis. Notch signaling regulates both endothelial cell generation and angiogenesis during both bone development and regeneration.216, 217, 218, 219, 220, 221, 222 We characterized that H19 functions as CeRNA that sponges miRNAs targeted to Notch signaling, which indicated the regulatory function of H19 in Notch signaling activation.58 As reported by Shang et al,223 Notch signaling promoted the hypertrophic differentiation process. Our previous work also proved that the down-regulation of H19 promoted hypertrophic differentiation of MSCs.112 Furthermore, our recent work found that H19 binds with p53 and promotes the phosphorylation of p53, phosphorylated p53 promoted Notch1 transcription by binding on the promoter area of Notch1, and then the activated Notch1 promoted endothelial cell generation.113 These results indicated the regulation axis of H19-Notch in endochondral ossification.
Roles of H19 in metabolic cardiovascular diseases
H19 and its variant expression alteration were associated with cardiovascular diseases, endocrine system disease, liver disease, nephrotic syndrome, etc., which indicated the functional effects of H19 in metabolism.224, 225, 226
H19 in atherosclerosis and cardiovascular diseases
Atherosclerosis is one of the main cardiometabolic diseases and drives cardiovascular disease, which is one of the main causes of mortality worldwide.225 Atherosclerosis is mechanistically involved in numerous biological processes including lipid metabolism, adipogenesis, angiogenesis, inflammatory response, cellular proliferation, and apoptosis, etc.225,227 Foam cell-mediated lipid accumulation and inflammatory response occupy an important position in the progression of atherosclerosis. It was reported that H19 was highly expressed in atherosclerosis foam cells, and down-regulation of H19 counteracted the increase of triglycerides/total cholesterol/low-density lipoprotein-cholesterol and down-regulated the level of high-density lipoprotein-cholesterol.228 Meanwhile, down-regulation of H19 decreased the expression of pro-inflammatory factors (TNF-α and IL-β) and increased the expression of anti-inflammatory factors (IL-4 and IL-10).228 In mechanism, these effects were mediated by the H19-miR-130 b axis. In addition, the expression level of miR-130b was correlated with the expression levels of triglycerides, LDL, serum creatinine, and blood urea nitrogen and inflammatory response in diabetic nephropathy, which also indicated the regulation of miR-130b in lipid metabolism.229
H19 has also been identified to regulate the proliferation and apoptosis of vascular smooth muscle cells and endothelial cells, which contribute to the development of atherosclerosis. Park et al identified that H19 interacting with miR-let-7 and regulating the expression of Igf2 were associated with cardiac phenotypes.35 Huang et al found that H19 promoted the proliferation of vascular smooth muscle cells and endothelial cells and suppressed apoptosis through acid phosphatase 5, which promoted the development of atherosclerosis.230 Sun et al identified that H19 sponging miR-let-7a, which targets cyclin D1, promoted vascular remodeling by regulating the cell cycle.231 Meanwhile, it is reported that H19 regulates vascular smooth muscle cell proliferation by miR-148b/Wnt/beta-catenin singling. Overall, H19 regulates atherosclerosis development through lipid metabolism, inflammatory response, cell proliferation, apoptosis, etc. Meanwhile, in myocardial infarction, H19 was found to ameliorate myocardial infarction-induced myocardial injury and maladaptive cardiac remodeling by acting as CeRNA, which sponges miRNA-22-3p and promotes the expression of KDM3A.232 Moreover, by acting as CeRNA, H19 was reported to inhibit myocardial infarction and myocardial ischemia/reperfusion by sponging different miRs.225 Recently, Viereck et al found that H19 is an anti-hypertrophic lncRNA and represents a promising therapeutic target to combat pathological cardiac remodelling.233
H19 in hepatic lipid metabolism, non-alcoholic fatty liver disease, and liver fibrosis
H19 was also proved to play an important role in liver diseases.234,235 In hepatic stellate cells, H19 was up-regulated by hypoxia-inducible factor-1alpha through binding to the lncRNA-H19 promoter at two hypoxia response element sites located at 492–499 and 515–522 bp. Up-regulated H19 activated hepatic stellate cells and promoted the development of liver fibrosis through the AMPKα signaling pathway.236 Inhibition of H19-AMPKα signaling by dihydroartemisinin holds the potential for improving lipid droplet metabolism and the prevention and treatment of liver fibrosis.237 The liver plays an essential role in lipid metabolism. It was reported that up-regulated H19 was associated with non-alcoholic fatty liver disease. Mechanistically, H19 induced lipid accumulation and up-regulated the expression of lipid synthesis-, storage-, and breakdown-associated genes by up-regulating both the mTORC1 signaling axis and MLXIPL transcriptional network.226 As a lncRNA, H19 was also identified to promote lipogenesis and triglyceride secretion in hepatocytes by sponging miR130a, which targets PPARγ directly.106 In addition, the expression level of H19 was positively associated with the expression levels of lipids, which served as a lipid sensor. By synergizing with the RNA-binding polypyrimidine tract-binding protein 1, H19 regulated hepatic metabolic homeostasis and exacerbated the development of fatty liver.238
H19 in insulin sensitivity, obesity, and diabetes
As H19 is highly expressed in skeletal muscles, which is essential for glucose and lipid metabolism. It was reported that H19 was down-regulated in the muscle of both db/db mice and high-fat diet-induced obese mice and that over-expression of H19 ameliorated glucose intolerance and insulin resistance in db/db mice and decreased ectopic lipid accumulation in the skeletal muscle and liver. In terms of mechanism, H19 promotes the oxidation of fatty acids by targeting heterogeneous nuclear ribonucleoprotein.239 As a cellular energy sensor, AMPK enhanced muscle insulin sensitivity and whole-body energy metabolism by regulating glucose uptake, lipid oxidation, and mitochondrial biogenesis. Geng et al240 found that H19 sponged miR-let7 and increased the expression of DUSP27, which activated AMPK signaling and enhanced muscle insulin sensitivity. Meanwhile, H19 variants were identified to have a significant association with T2MD.241
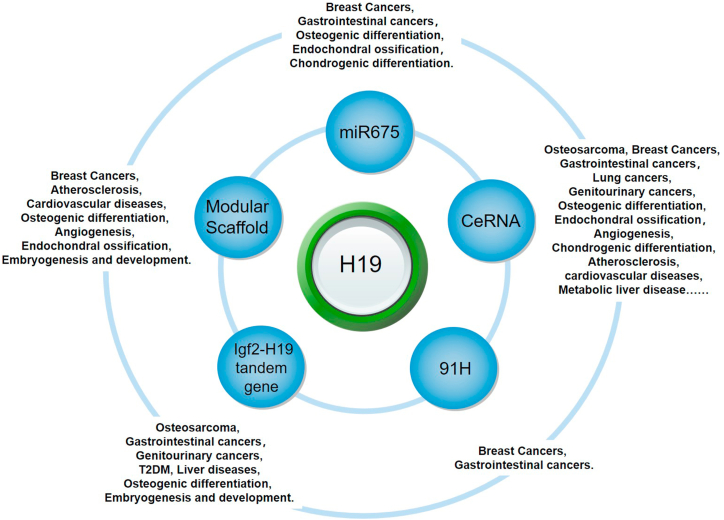
In addition, an intrauterine hyperinsulinemia environment may cause hepatic Igf2/H19 epigenetic alteration and induce glucose intolerance in gestational diabetes mellitus. Mechanistically, an intrauterine hyperinsulinemia environment could increase hepatic FoxO1 levels, which subsequently increases the expression of DNMT3A and epigenetic alterations on Igf2/H19 DMRs.242 In other words, H19 may be a potential target for the treatment of type 2 diabetes mellitus.243,244 The function of H19 in different diseases, physiological processes, and pathological processes are summarized in Figure 2.
Figure 2.
Summary of the functions of H19 in different physiological and/or pathological processes.
Conclusions and future directions
As one of the first identified noncoding RNAs, H19 was identified to play essential roles in several physiological and pathological processes. Dandolo et al found that H19-Igf2 locus interact with Myod and regulated diaphragm formation,245 and they also identified that H19 acted as a trans regulator on imprinted gene network and controlling growth and development.37,41,51,246, 247, 248 On the other hand, Hochberg and Adriaenssens et al found that H19 was associated with cancer development, invasion, and metastasis, and implied that H19 was a potential target for cancer treatment.77,129,194,249, 250, 251, 252, 253, 254, 255, 256, 257, 258, 259, 260 These studies indicated the multiple functions of H19 in different cell types. In mechanism, it has been shown that H19 mediates diverse regulatory functions by serving as CeRNA, Igf2/H19 imprinted tandem gene, modular scaffold, cooperating with H19 antisense, acting directly with other mRNAs or lncRNAs, etc. Biologically, H19 participates in embryogenesis, development, tumorigenesis, osteogenesis, and metabolism, which are associated with the development of many diseases. Therefore, H19 holds the potential for disease diagnosis, tissue regeneration, and the development of targeted therapeutics. Recently, a plasmid DNA encoding the A chain of the diphtheria toxin (DTA-H19) driven by the transcriptional regulatory sequences of human H19, was tested as a monotherapy or in combinations with other chemotherapeutics in treating some cancers, such as pancreatic cancer and ovarian cancer, while more clinical trials are being conducted.261, 262, 263, 264, 265 However, H19 was regarded as a double-edged sword in cancer therapy.266 Thus, more in-depth studies are warranted to delineate the exact molecular, cellular, and genomic mechanisms underlying the physiological and pathological roles of H19. Ultimately, these lines of investigation may lead to the development of novel therapeutics for human diseases by exploiting H19 functions.
Conflict of interests
The authors declare no conflict of interests.
Funding
The reported work was supported by the National Natural Science Foundation of China (NSFC) (No. 82002312, 81972069). The reported work was supported in part by research grants from the National Institutes of Health, USA (No. CA226303 to TCH and No. DE030480 to RRR). This project was also supported by the Science and Technology Research Program of Chongqing Education Commission, China (No. KJQN202100431, KJZD-M202100401), the Top Talent Award from The First Affiliated Hospital of Chongqing Medical University, China (No. BJRC2021-04) and Cultivation Program of Postdoctoral Research of The First Affiliated Hospital of Chongqing Medical University, China (No. CYYY-BSHPYXM-202202). JY was supported by a postdoctoral fellowship from Chongqing Medical University and rewarded by China Postdoctoral Science Foundation (No. 2022M720605). JF was supported in part by research grants from the 2019 Science and Technology Research Plan Project of Chongqing Education Commission, China (No. KJQN201900410), the 2019 Funding for Postdoctoral Research (Chongqing Human Resources and Social Security Bureau No. 298), and the Natural Science Foundation of China (No. 82102696). WW was supported by the Medical Scientist Training Program of the National Institutes of Health, USA (No. T32 GM007281). This project was also supported in part by The University of Chicago Cancer Center Support Grant, USA (No. P30CA014599) and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health, USA (No. 5UL1TR002389). TCH was supported by the Mabel Green Myers Research Endowment Fund and The University of Chicago Orthopedics Alumni Fund. Funding sources were not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Junyi Liao, Email: liaojunyi@cqmu.edu.cn.
Ning Hu, Email: 1276321387@qq.com.
Wei Huang, Email: huagnwei68@263.net.
References
- 1.Kim T.K., Shiekhattar R. Diverse regulatory interactions of long noncoding RNAs. Curr Opin Genet Dev. 2016;36:73–82. doi: 10.1016/j.gde.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris K.V., Mattick J.S. The rise of regulatory RNA. Nat Rev Genet. 2014;15(6):423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacquier A. The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nat Rev Genet. 2009;10(12):833–844. doi: 10.1038/nrg2683. [DOI] [PubMed] [Google Scholar]
- 4.Morozova O., Marra M.A. Applications of next-generation sequencing technologies in functional genomics. Genomics. 2008;92(5):255–264. doi: 10.1016/j.ygeno.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Clark M.B., Amaral P.P., Schlesinger F.J., et al. The reality of pervasive transcription. PLoS Biol. 2011;9(7) doi: 10.1371/journal.pbio.1000625. discussion e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adnane S., Marino A., Leucci E. LncRNAs in human cancers: signal from noise. Trends Cell Biol. 2022;32(7):565–573. doi: 10.1016/j.tcb.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Hombach S., Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 8.Khan M.R., Wellinger R.J., Laurent B. Exploring the alternative splicing of long noncoding RNAs. Trends Genet. 2021;37(8):695–698. doi: 10.1016/j.tig.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Li Y.P., Wang Y. Large noncoding RNAs are promising regulators in embryonic stem cells. J Genet Genom. 2015;42(3):99–105. doi: 10.1016/j.jgg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y.H. Crosstalk of lncRNA and cellular metabolism and their regulatory mechanism in cancer. Int J Mol Sci. 2020;21(8):2947. doi: 10.3390/ijms21082947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boriack-Sjodin P.A., Ribich S., Copeland R.A. RNA-modifying proteins as anticancer drug targets. Nat Rev Drug Discovery. 2018;17(6):435–453. doi: 10.1038/nrd.2018.71. [DOI] [PubMed] [Google Scholar]
- 12.Cleary J.D., Ranum L.P. New developments in RAN translation: insights from multiple diseases. Curr Opin Genet Dev. 2017;44:125–134. doi: 10.1016/j.gde.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martianov I., Ramadass A., Serra Barros A., et al. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445(7128):666–670. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 14.Bond A.M., Vangompel M.J.W., Sametsky E.A., et al. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12(8):1020–1027. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia T., Liao Q., Jiang X., et al. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep. 2014;4:6088. doi: 10.1038/srep06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Li Y., Zhang Y., et al. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother. 2017;89:1178–1186. doi: 10.1016/j.biopha.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 17.Tian D., Sun S., Lee J.T. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell. 2010;143(3):390–403. doi: 10.1016/j.cell.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata S., Yokota T., Wutz A. Synergy of eed and Tsix in the repression of Xist gene and X-chromosome inactivation. EMBO J. 2008;27(13):1816–1826. doi: 10.1038/emboj.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue M., Charles Richard J.L., Ogawa Y. Dynamic interplay and function of multiple noncoding genes governing X chromosome inactivation. Biochim Biophys Acta. 2016;1859(1):112–120. doi: 10.1016/j.bbagrm.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J., Bartolomei M. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152(6):1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Bartolomei M.S., Zemel S., Tilghman S.M. Parental imprinting of the mouse H19 gene. Nature. 1991;351(6322):153–155. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 22.Hemberger M., Redies C., Krause R., et al. H19 and Igf2 are expressed and differentially imprinted in neuroectoderm-derived cells in the mouse brain. Dev Gene Evol. 1998;208(7):393–402. doi: 10.1007/s004270050195. [DOI] [PubMed] [Google Scholar]
- 23.Ripoche M.A., Kress C., Poirier F., et al. Deletion of the H19 transcription unit reveals the existence of a putative imprinting control element. Genes Dev. 1997;11(12):1596–1604. doi: 10.1101/gad.11.12.1596. [DOI] [PubMed] [Google Scholar]
- 24.Sleutels F., Zwart R., Barlow D.P. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415(6873):810–813. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 25.Iurlaro M., von Meyenn F., Reik W. DNA methylation homeostasis in human and mouse development. Curr Opin Genet Dev. 2017;43:101–109. doi: 10.1016/j.gde.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Yang F., Deng X., Ma W., et al. The lncRNA Firre anchors the inactive X chromosome to the nucleolus by binding CTCF and maintains H3K27me3 methylation. Genome Biol. 2015;16(1):52. doi: 10.1186/s13059-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hacisuleyman E., Goff L.A., Trapnell C., et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21(2):198–206. doi: 10.1038/nsmb.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirier F., Chan C.T., Timmons P.M., et al. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development. 1991;113(4):1105–1114. doi: 10.1242/dev.113.4.1105. [DOI] [PubMed] [Google Scholar]
- 29.Brannan C.I., Dees E.C., Ingram R.S., et al. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10(1):28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pachnis V., Brannan C.I., Tilghman S.M. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988;7(3):673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pachnis V., Belayew A., Tilghman S.M. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci U S A. 1984;81(17):5523–5527. doi: 10.1073/pnas.81.17.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Tycko B. Monoallelic expression of the human H19 gene. Nat Genet. 1992;1(1):40–44. doi: 10.1038/ng0492-40. [DOI] [PubMed] [Google Scholar]
- 33.Moulton T., Crenshaw T., Hao Y., et al. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- 34.Leighton P.A., Saam J.R., Ingram R.S., et al. An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 1995;9(17):2079–2089. doi: 10.1101/gad.9.17.2079. [DOI] [PubMed] [Google Scholar]
- 35.Park K.S., Rahat B., Lee H.C., et al. Cardiac pathologies in mouse loss of imprinting models are due to misexpression of H19 long noncoding RNA. Elife. 2021;10 doi: 10.7554/eLife.67250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunkow M.E., Tilghman S.M. Ectopic expression of the H19 gene in mice causes prenatal lethality. Genes Dev. 1991;5(6):1092–1101. doi: 10.1101/gad.5.6.1092. [DOI] [PubMed] [Google Scholar]
- 37.Gabory A., Ripoche M.A., Le Digarcher A., et al. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development. 2009;136(20):3413–3421. doi: 10.1242/dev.036061. [DOI] [PubMed] [Google Scholar]
- 38.Hur S.K., Freschi A., Ideraabdullah F., et al. Humanized H19/Igf2 locus reveals diverged imprinting mechanism between mouse and human and reflects Silver-Russell syndrome phenotypes. Proc Natl Acad Sci U S A. 2016;113(39):10938–10943. doi: 10.1073/pnas.1603066113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes S., Lewis A., Hajkova P., et al. Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet. 2003;12(3):295–305. doi: 10.1093/hmg/ddg022. [DOI] [PubMed] [Google Scholar]
- 40.Arney K.L. H19 and Igf2–enhancing the confusion? Trends Genet. 2003;19(1):17–23. doi: 10.1016/s0168-9525(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 41.Gabory A., Jammes H., Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays. 2010;32(6):473–480. doi: 10.1002/bies.200900170. [DOI] [PubMed] [Google Scholar]
- 42.Bartolomei M.S., Webber A.L., Brunkow M.E., et al. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 1993;7(9):1663–1673. doi: 10.1101/gad.7.9.1663. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson-Smith A.C., Sasaki H., Cattanach B.M., et al. Parental-origin-specific epigenetic modification of the mouse H19 gene. Nature. 1993;362(6422):751–755. doi: 10.1038/362751a0. [DOI] [PubMed] [Google Scholar]
- 44.Arima T., Matsuda T., Takagi N., et al. Association of IGF2 and H19 imprinting with choriocarcinoma development. Cancer Genet Cytogenet. 1997;93(1):39–47. doi: 10.1016/s0165-4608(96)00221-x. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki H., Ishihara K., Kato R. Mechanisms of Igf2/H19 imprinting: DNA methylation, chromatin and long-distance gene regulation. J Biochem. 2000;127(5):711–715. doi: 10.1093/oxfordjournals.jbchem.a022661. [DOI] [PubMed] [Google Scholar]
- 46.Han X., Ouyang H., Chen X., et al. Aberrant expression of Igf2/H19 in porcine parthenogenetic fetuses and placentas. Anim Reprod Sci. 2013;139(1–4):101–108. doi: 10.1016/j.anireprosci.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Kaffer C.R., Grinberg A., Pfeifer K. Regulatory mechanisms at the mouse Igf2/H19 locus. Mol Cell Biol. 2001;21(23):8189–8196. doi: 10.1128/MCB.21.23.8189-8196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratajczak M.Z. Igf2-H19, an imprinted tandem gene, is an important regulator of embryonic development, a guardian of proliferation of adult pluripotent stem cells, a regulator of longevity, and a 'passkey' to cancerogenesis. Folia Histochem Cytobiol. 2012;50(2):171–179. doi: 10.5603/fhc.2012.0026. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y., Li G., Zhang J.F. The role of long non-coding RNA H19 in musculoskeletal system: a new player in an old game. Exp Cell Res. 2017;360(2):61–65. doi: 10.1016/j.yexcr.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Qin C.Y., Cai H., Qing H.R., et al. Recent advances on the role of long non-coding RNA H19 in regulating mammalian muscle growth and development. Yi Chuan. 2017;39(12):1150–1157. doi: 10.16288/j.yczz.17-193. [DOI] [PubMed] [Google Scholar]
- 51.Martinet C., Monnier P., Louault Y., et al. H19 controls reactivation of the imprinted gene network during muscle regeneration. Development. 2016;143(6):962–971. doi: 10.1242/dev.131771. [DOI] [PubMed] [Google Scholar]
- 52.Park K.S., Mitra A., Rahat B., et al. Loss of imprinting mutations define both distinct and overlapping roles for misexpression of IGF2 and of H19 lncRNA. Nucleic Acids Res. 2017;45(22):12766–12779. doi: 10.1093/nar/gkx896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Z., Hossain M.S., Liu D. Involvement of the long noncoding RNA H19 in osteogenic differentiation and bone regeneration. Stem Cell Res Ther. 2021;12:74. doi: 10.1186/s13287-021-02149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y., Zheng Y., Jia L., et al. Long noncoding RNA H19 promotes osteoblast differentiation via TGF-β1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cell. 2015;33(12):3481–3492. doi: 10.1002/stem.2225. [DOI] [PubMed] [Google Scholar]
- 55.Hupkes M., Sotoca A.M., Hendriks J.M., et al. microRNA miR-378 promotes BMP2-induced osteogenic differentiation of mesenchymal progenitor cells. BMC Mol Biol. 2014;15:1. doi: 10.1186/1471-2199-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang W.C., Fu W.M., Wang Y.B., et al. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci Rep. 2016;6:20121. doi: 10.1038/srep20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen T.B., Jensen T.I., Clausen B.H., et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 58.Liao J., Yu X., Hu X., et al. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget. 2017;8(32):53581–53601. doi: 10.18632/oncotarget.18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao J., Xiao H., Dai G., et al. Recombinant adenovirus (AdEasy system) mediated exogenous expression of long non-coding RNA H19 (lncRNA H19) biphasic regulating osteogenic differentiation of mesenchymal stem cells (MSCs) Am J Transl Res. 2020;12(5):1700–1713. [PMC free article] [PubMed] [Google Scholar]
- 60.Dudek K.A., Lafont J.E., Martinez-Sanchez A., et al. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285(32):24381–24387. doi: 10.1074/jbc.M110.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Y.F., Liu Y., Fu W.M., et al. Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-β1 signaling. Faseb J. 2017;31(3):954–964. doi: 10.1096/fj.201600722R. [DOI] [PubMed] [Google Scholar]
- 62.Huang Y., Zheng Y., Jin C., et al. Long non-coding RNA H19 inhibits adipocyte differentiation of bone marrow mesenchymal stem cells through epigenetic modulation of histone deacetylases. Sci Rep. 2016;6:28897. doi: 10.1038/srep28897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Squillaro T., Peluso G., Galderisi U., et al. Long non-coding RNAs in regulation of adipogenesis and adipose tissue function. Elife. 2020;9 doi: 10.7554/eLife.59053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeChiara T.M., Robertson E.J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64(4):849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 65.Barlow D.P., Stöger R., Herrmann B.G., et al. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991;349(6304):84–87. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- 66.Reik W., Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2(1):21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 67.Bartolomei M.S., Ferguson-Smith A.C. Mammalian genomic imprinting. Cold Spring Harbor Perspect Biol. 2011;3(7):a002592. doi: 10.1101/cshperspect.a002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tucci V., Isles A.R., Kelsey G., et al. Genomic imprinting and physiological processes in mammals. Cell. 2019;176(5):952–965. doi: 10.1016/j.cell.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 69.Abi Habib W., Brioude F., Azzi S., et al. 11p15 ICR1 partial deletions associated with IGF2/H19 DMR hypomethylation and silver-Russell syndrome. Hum Mutat. 2017;38(1):105–111. doi: 10.1002/humu.23131. [DOI] [PubMed] [Google Scholar]
- 70.Alders M., Bliek J., Lip K.V., et al. Determination of KCNQ1OT1 and H19 methylation levels in BWS and SRS patients using methylation-sensitive high-resolution melting analysis. Eur J Hum Genet. 2009;17(4):467–473. doi: 10.1038/ejhg.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghafouri-Fard S., Esmaeili M., Taheri M. H19 lncRNA: roles in tumorigenesis. Biomed Pharmacother. 2020;123:109774. doi: 10.1016/j.biopha.2019.109774. [DOI] [PubMed] [Google Scholar]
- 72.Ulaner G.A., Vu T.H., Li T., et al. Loss of imprinting of IGF2 and H19 in osteosarcoma is accompanied by reciprocal methylation changes of a CTCF-binding site. Hum Mol Genet. 2003;12(5):535–549. doi: 10.1093/hmg/ddg034. [DOI] [PubMed] [Google Scholar]
- 73.Fu V.X., Schwarze S.R., Kenowski M.L., et al. A loss of insulin-like growth factor-2 imprinting is modulated by CCCTC-binding factor down-regulation at senescence in human epithelial cells. J Biol Chem. 2004;279(50):52218–52226. doi: 10.1074/jbc.M405015200. [DOI] [PubMed] [Google Scholar]
- 74.Shin D.M., Zuba-Surma E.K., Wu W., et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4+ very small embryonic-like stem cells. Leukemia. 2009;23(11):2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shin D.M., Liu R., Klich I., et al. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24(8):1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- 76.Berteaux N., Aptel N., Cathala G., et al. A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression. Mol Cell Biol. 2008;28(22):6731–6745. doi: 10.1128/MCB.02103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vennin C., Spruyt N., Robin Y.M., et al. The long non-coding RNA 91H increases aggressive phenotype of breast cancer cells and up-regulates H19/IGF2 expression through epigenetic modifications. Cancer Lett. 2017;385:198–206. doi: 10.1016/j.canlet.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 78.Tran V.G., Court F., Duputié A., et al. H19 antisense RNA can up-regulate Igf2 transcription by activation of a novel promoter in mouse myoblasts. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao T., He B., Pan Y., et al. Long non-coding RNA 91H contributes to the occurrence and progression of esophageal squamous cell carcinoma by inhibiting IGF2 expression. Mol Carcinog. 2015;54(5):359–367. doi: 10.1002/mc.22106. [DOI] [PubMed] [Google Scholar]
- 80.Gao T., Liu X., He B., et al. Exosomal lncRNA 91H is associated with poor development in colorectal cancer by modifying HNRNPK expression. Cancer Cell Int. 2018;18:11. doi: 10.1186/s12935-018-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao T., Liu X., He B., et al. Long non-coding RNA 91H regulates IGF2 expression by interacting with IGF2BP2 and promotes tumorigenesis in colorectal cancer. Artif Cells, Nanomed Biotechnol. 2020;48(1):664–671. doi: 10.1080/21691401.2020.1727491. [DOI] [PubMed] [Google Scholar]
- 82.Xia W.K., Lin Q.F., Shen D., et al. Clinical implication of long noncoding RNA 91H expression profile in osteosarcoma patients. Onco Targets Ther. 2016;9:4645–4652. doi: 10.2147/OTT.S103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yi T., Wang T., Shi Y., et al. Long noncoding RNA 91H overexpression contributes to the growth and metastasis of HCC by epigenetically positively regulating IGF2 expression. Liver Int. 2020;40(2):456–467. doi: 10.1111/liv.14300. [DOI] [PubMed] [Google Scholar]
- 84.Onyango P., Feinberg A.P. A nucleolar protein, H19 opposite tumor suppressor (HOTS), is a tumor growth inhibitor encoded by a human imprinted H19 antisense transcript. Proc Natl Acad Sci U S A. 2011;108(40):16759–16764. doi: 10.1073/pnas.1110904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alipoor B., Parvar S.N., Sabati Z., et al. An updated review of the H19 lncRNA in human cancer: molecular mechanism and diagnostic and therapeutic importance. Mol Biol Rep. 2020;47(8):6357–6374. doi: 10.1007/s11033-020-05695-x. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W., Zhou K., Zhang X., et al. Roles of the H19/microRNA-675 axis in the proliferation and epithelial-mesenchymal transition of human cutaneous squamous cell carcinoma cells. Oncol Rep. 2021;45(4):39. doi: 10.3892/or.2021.7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dey B.K., Pfeifer K., Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28(5):491–501. doi: 10.1101/gad.234419.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo Dico A., Costa V., Martelli C., et al. MiR675-5p acts on HIF-1α to sustain hypoxic responses: a new therapeutic strategy for glioma. Theranostics. 2016;6(8):1105–1118. doi: 10.7150/thno.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cen B., Lang J.D., Du Y., et al. Prostaglandin E2 induces miR675-5p to promote colorectal tumor metastasis via modulation of p53 expression. Gastroenterology. 2020;158(4):971–984.e10. doi: 10.1053/j.gastro.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cai X., Cullen B.R. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13(3):313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao W.L., Liu M., Yang Y., et al. The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets Nodal Modulator 1 (NOMO1) RNA Biol. 2012;9(7):1002–1010. doi: 10.4161/rna.20807. [DOI] [PubMed] [Google Scholar]
- 92.Steck E., Boeuf S., Gabler J., et al. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med. 2012;90(10):1185–1195. doi: 10.1007/s00109-012-0895-y. [DOI] [PubMed] [Google Scholar]
- 93.Zhuang M., Gao W., Xu J., et al. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448(3):315–322. doi: 10.1016/j.bbrc.2013.12.126. [DOI] [PubMed] [Google Scholar]
- 94.Liu G., Xiang T., Wu Q.F., et al. Long noncoding RNA H19-derived miR-675 enhances proliferation and invasion via RUNX1 in gastric cancer cells. Oncol Res. 2016;23(3):99–107. doi: 10.3727/096504015X14496932933575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Müller V., Oliveira-Ferrer L., Steinbach B., et al. Interplay of lncRNA H19/miR-675 and lncRNA NEAT1/miR-204 in breast cancer. Mol Oncol. 2019;13(5):1137–1149. doi: 10.1002/1878-0261.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Wijnen A.J., van de Peppel J., van Leeuwen J.P., et al. microRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep. 2013;11(2):72–82. doi: 10.1007/s11914-013-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mok G.F., Lozano-Velasco E., Münsterberg A. microRNAs in skeletal muscle development. Semin Cell Dev Biol. 2017;72:67–76. doi: 10.1016/j.semcdb.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 98.Leone S., Santoro R. Challenges in the analysis of long noncoding RNA functionality. FEBS Lett. 2016;590(15):2342–2353. doi: 10.1002/1873-3468.12308. [DOI] [PubMed] [Google Scholar]
- 99.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 100.Yao T., Chen Q., Fu L., et al. Circular RNAs: biogenesis, properties, roles, and their relationships with liver diseases. Hepatol Res. 2017;47(6):497–504. doi: 10.1111/hepr.12871. [DOI] [PubMed] [Google Scholar]
- 101.Kallen A., Zhou X.B., Xu J., et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52(1):101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao Y., Wu F., Zhou J., et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 2014;42(22):13799–13811. doi: 10.1093/nar/gku1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L., Yang J., Zhu X., et al. Long noncoding RNA H19 competitively binds miR-17-5p to regulate YES1 expression in thyroid cancer. FEBS J. 2016;283(12):2326–2339. doi: 10.1111/febs.13741. [DOI] [PubMed] [Google Scholar]
- 104.Li J.P., Xiang Y., Fan L.J., et al. Long noncoding RNA H19 competitively binds miR-93-5p to regulate STAT3 expression in breast cancer. J Cell Biochem. 2019;120(3):3137–3148. doi: 10.1002/jcb.27578. [DOI] [PubMed] [Google Scholar]
- 105.Wei J., Gan Y., Peng D., et al. Long non-coding RNA H19 promotes TDRG1 expression and cisplatin resistance by sequestering miRNA-106b-5p in seminoma. Cancer Med. 2018;7(12):6247–6257. doi: 10.1002/cam4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J., Tang T., Wang G.D., et al. LncRNA-H19 promotes hepatic lipogenesis by directly regulating miR-130a/PPARγ axis in non-alcoholic fatty liver disease. Biosci Rep. 2019;39(7) doi: 10.1042/BSR20181722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu X.T., Xing W., Zhao R.S., et al. HDAC2 inhibits EMT-mediated cancer metastasis by downregulating the long noncoding RNA H19 in colorectal cancer. J Exp Clin Cancer Res. 2020;39:270. doi: 10.1186/s13046-020-01783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu B., Zhang Y., Yu Y., et al. Long noncoding RNA H19:a novel therapeutic target emerging in oncology via regulating oncogenic signaling pathways. Front Cell Dev Biol. 2021;9:796740. doi: 10.3389/fcell.2021.796740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Martino M., Forzati F., Marfella M., et al. HMGA1P7-pseudogene regulates H19 and Igf2 expression by a competitive endogenous RNA mechanism. Sci Rep. 2016;6:37622. doi: 10.1038/srep37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsai M.C., Manor O., Wan Y., et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moon Y., Kim I., Chang S., et al. Hypoxia regulates allele-specific histone modification of the imprinted H19 gene. Biochim Biophys Acta Gene Regul Mech. 2020;1863(11):194643. doi: 10.1016/j.bbagrm.2020.194643. [DOI] [PubMed] [Google Scholar]
- 112.Dai G., Xiao H., Zhao C., et al. LncRNA H19 regulates BMP2-induced hypertrophic differentiation of mesenchymal stem cells by promoting Runx2 phosphorylation. Front Cell Dev Biol. 2020;8:580. doi: 10.3389/fcell.2020.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du C., Cheng Q., Zhao P., et al. LncRNA H19 mediates BMP9-induced angiogenesis in mesenchymal stem cells by promoting the p53-Notch1 angiogenic signaling axis. Genes Dis. 2023;10(3):1040–1054. doi: 10.1016/j.gendis.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gibb E.A., Brown C.J., Lam W.L. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderson J., Gordon A., McManus A., et al. Disruption of imprinted genes at chromosome region 11p15.5 in paediatric rhabdomyosarcoma. Neoplasia. 1999;1(4):340–348. doi: 10.1038/sj.neo.7900052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Casola S., Pedone P.V., Cavazzana A.O., et al. Expression and parental imprinting of the H19 gene in human rhabdomyosarcoma. Oncogene. 1997;14(12):1503–1510. doi: 10.1038/sj.onc.1200956. [DOI] [PubMed] [Google Scholar]
- 117.Wagner E.R., Luther G., Zhu G., et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma. 2011;2011:325238. doi: 10.1155/2011/325238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee D.F., Su J., Kim H., et al. Modeling familial cancer with induced pluripotent stem cells. Cell. 2015;161(2):240–254. doi: 10.1016/j.cell.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan L.H., Wang W., Yeung W., et al. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33(40):4857–4866. doi: 10.1038/onc.2013.433. [DOI] [PubMed] [Google Scholar]
- 120.Zhao J., Ma S.T. Downregulation of lncRNA H19 inhibits migration and invasion of human osteosarcoma through the NF-κB pathway. Mol Med Rep. 2018;17(5):7388–7394. doi: 10.3892/mmr.2018.8746. [DOI] [PubMed] [Google Scholar]
- 121.He P., Zhang Z., Huang G., et al. miR-141 modulates osteoblastic cell proliferation by regulating the target gene of lncRNA H19 and lncRNA H19-derived miR-675. Am J Transl Res. 2016;8(4):1780–1788. [PMC free article] [PubMed] [Google Scholar]
- 122.He T.D., Xu D., Sui T., et al. Association between H19 polymorphisms and osteosarcoma risk. Eur Rev Med Pharmacol Sci. 2017;21(17):3775–3780. [PubMed] [Google Scholar]
- 123.Wang J., Sun J., Yang F. The role of long non-coding RNA H19 in breast cancer. Oncol Lett. 2020;19(1):7–16. doi: 10.3892/ol.2019.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshimura H., Matsuda Y., Yamamoto M., et al. Expression and role of long non-coding RNA H19 in carcinogenesis. Front Biosci. 2018;23(4):614–625. doi: 10.2741/4608. [DOI] [PubMed] [Google Scholar]
- 125.Doyle L.A., Yang W., Rishi A.K., et al. H19 gene overexpression in atypical multidrug-resistant cells associated with expression of a 95-kilodalton membrane glycoprotein. Cancer Res. 1996;56(13):2904–2907. [PubMed] [Google Scholar]
- 126.Lottin S., Adriaenssens E., Dupressoir T., et al. Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis. 2002;23(11):1885–1895. doi: 10.1093/carcin/23.11.1885. [DOI] [PubMed] [Google Scholar]
- 127.Cui P., Zhao Y., Chu X., et al. SNP rs2071095 in LincRNA H19 is associated with breast cancer risk. Breast Cancer Res Treat. 2018;171(1):161–171. doi: 10.1007/s10549-018-4814-y. [DOI] [PubMed] [Google Scholar]
- 128.Lin Y., Fu F., Chen Y., et al. Genetic variants in long noncoding RNA H19 contribute to the risk of breast cancer in a southeast China Han population. Onco Targets Ther. 2017;10:4369–4378. doi: 10.2147/OTT.S127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raveh E., Matouk I.J., Gilon M., et al. The H19 Long non-coding RNA in cancer initiation, progression and metastasis - a proposed unifying theory. Mol Cancer. 2015;14:184. doi: 10.1186/s12943-015-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collette J., Le Bourhis X., Adriaenssens E. Regulation of human breast cancer by the long non-coding RNA H19. Int J Mol Sci. 2017;18(11):2319. doi: 10.3390/ijms18112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin Y., Xu L., Wei W., et al. Long noncoding RNA H19 in digestive system cancers: a meta-analysis of its association with pathological features. BioMed Res Int. 2016;2016 doi: 10.1155/2016/4863609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen S., Shen X. Long noncoding RNAs: functions and mechanisms in colon cancer. Mol Cancer. 2020;19(1):167. doi: 10.1186/s12943-020-01287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fattahi S., Kosari-Monfared M., Golpour M., et al. LncRNAs as potential diagnostic and prognostic biomarkers in gastric cancer: a novel approach to personalized medicine. J Cell Physiol. 2020;235(4):3189–3206. doi: 10.1002/jcp.29260. [DOI] [PubMed] [Google Scholar]
- 134.Wang J., Song Y.X., Wang Z.N. Non-coding RNAs in gastric cancer. Gene. 2015;560(1):1–8. doi: 10.1016/j.gene.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 135.Zhang L., Zhou Y., Huang T., et al. The interplay of LncRNA-H19 and its binding partners in physiological process and gastric carcinogenesis. Int J Mol Sci. 2017;18(2):450. doi: 10.3390/ijms18020450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Virgilio E., Giarnieri E., Giovagnoli M.R., et al. Long non-coding RNAs in the gastric juice of gastric cancer patients. Pathol Res Pract. 2018;214(9):1239–1246. doi: 10.1016/j.prp.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 137.Zhou X., Ye F., Yin C., et al. The interaction between miR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell Physiol Biochem. 2015;36(4):1440–1452. doi: 10.1159/000430309. [DOI] [PubMed] [Google Scholar]
- 138.Yoshimura H., Matsuda Y., Yamamoto M., et al. Reduced expression of the H19 long non-coding RNA inhibits pancreatic cancer metastasis. Lab Invest. 2018;98(6):814–824. doi: 10.1038/s41374-018-0048-1. [DOI] [PubMed] [Google Scholar]
- 139.Sun Y., Zhu Q., Yang W., et al. LncRNA H19/miR-194/PFTK1 axis modulates the cell proliferation and migration of pancreatic cancer. J Cell Biochem. 2019;120(3):3874–3886. doi: 10.1002/jcb.27669. [DOI] [PubMed] [Google Scholar]
- 140.Wang J., Zhao L., Shang K., et al. Long non-coding RNA H19, a novel therapeutic target for pancreatic cancer. Mol Med. 2020;26(1):30. doi: 10.1186/s10020-020-00156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang J., Han C., Ungerleider N., et al. A transforming growth factor-β and H19 signaling axis in tumor-initiating hepatocytes that regulates hepatic carcinogenesis. Hepatology. 2019;69(4):1549–1563. doi: 10.1002/hep.30153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan F.F., Zheng Y.B., Shi C.J., et al. H19-Wnt/β-catenin regulatory axis mediates the suppressive effects of apigenin on tumor growth in hepatocellular carcinoma. Eur J Pharmacol. 2021;893:173810. doi: 10.1016/j.ejphar.2020.173810. [DOI] [PubMed] [Google Scholar]
- 143.Ye Y., Guo J., Xiao P., et al. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma. Cancer Lett. 2020;469:310–322. doi: 10.1016/j.canlet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 144.Wang G., Ye M., Zheng S., et al. Cigarette smoke extract induces H19 in esophageal squamous cell carcinoma in smoking patients: based on A chronic exposed cell model. Toxicol Lett. 2020;333:62–70. doi: 10.1016/j.toxlet.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 145.Tan D., Wu Y., Hu L., et al. Long noncoding RNA H19 is up-regulated in esophageal squamous cell carcinoma and promotes cell proliferation and metastasis. Dis Esophagus. 2017;30(1):1–9. doi: 10.1111/dote.12481. [DOI] [PubMed] [Google Scholar]
- 146.Zhao M., Wang H., Chen J., et al. Expression of long non-coding RNA H19 in colorectal cancer patients with type 2 diabetes. Arch Physiol Biochem. 2021;127(3):228–234. doi: 10.1080/13813455.2019.1628068. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y., Huang W., Yuan Y., et al. Long non-coding RNA H19 promotes colorectal cancer metastasis via binding to hnRNPA2B1. J Exp Clin Cancer Res. 2020;39(1):141. doi: 10.1186/s13046-020-01619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liang W., Zou Y., Qin F., et al. sTLR4/MD-2 complex inhibits colorectal cancer migration and invasiveness in vitro and in vivo by lncRNA H19 down-regulation. Acta Biochim Biophys Sin. 2017;49(11):1035–1041. doi: 10.1093/abbs/gmx105. [DOI] [PubMed] [Google Scholar]
- 149.Nacarkahya G., Borazan E., Horozoglu C., et al. Investigation of long non-coding RNAs H19 and LINC00675 in colorectal cancers in terms of histopathological features and correlations with plasma markers. Anticancer Res. 2022;42(3):1301–1306. doi: 10.21873/anticanres.15597. [DOI] [PubMed] [Google Scholar]
- 150.Li S., Hua Y., Jin J., et al. Association of genetic variants in lncRNA H19 with risk of colorectal cancer in a Chinese population. Oncotarget. 2016;7(18):25470–25477. doi: 10.18632/oncotarget.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chu M., Yuan W., Wu S., et al. Quantitative assessment of polymorphisms in H19 lncRNA and cancer risk: a meta-analysis of 13, 392 cases and 18, 893 controls. Oncotarget. 2016;7(48):78631–78639. doi: 10.18632/oncotarget.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang Z., Chu L., Liang J., et al. H19 promotes HCC bone metastasis through reducing osteoprotegerin expression in a protein phosphatase 1 catalytic subunit alpha/p38 mitogen-activated protein kinase-dependent manner and sponging microRNA 200b-3p. Hepatology. 2021;74(1):214–232. doi: 10.1002/hep.31673. [DOI] [PubMed] [Google Scholar]
- 153.Wang M., Han D., Yuan Z., et al. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018;9(12):1149. doi: 10.1038/s41419-018-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu K.F., Liang W.C., Feng L., et al. H19 mediates methotrexate resistance in colorectal cancer through activating Wnt/β-catenin pathway. Exp Cell Res. 2017;350(2):312–317. doi: 10.1016/j.yexcr.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 155.Xu Y., Liu Y., Li Z., et al. Long non-coding RNA H19 is involved in sorafenib resistance in hepatocellular carcinoma by upregulating miR-675. Oncol Rep. 2020;44(1):165–173. doi: 10.3892/or.2020.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li T., Mo X., Fu L., et al. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016;7(8):8601–8612. doi: 10.18632/oncotarget.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J., Ma X., Si H., et al. Role of long non-coding RNA H19 in therapy resistance of digestive system cancers. Mol Med. 2021;27:1. doi: 10.1186/s10020-020-00255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cui J., Mo J., Luo M., et al. c-Myc-activated long non-coding RNA H19 downregulates miR-107 and promotes cell cycle progression of non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(10):12400–12409. [PMC free article] [PubMed] [Google Scholar]
- 159.Gao L.M., Xu S.F., Zheng Y., et al. Long non-coding RNA H19 is responsible for the progression of lung adenocarcinoma by mediating methylation-dependent repression of CDH1 promoter. J Cell Mol Med. 2019;23(9):6411–6428. doi: 10.1111/jcmm.14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moradi S., Sharifi-Zarchi A., Ahmadi A., et al. Small RNA sequencing reveals Dlk1-Dio3 locus-embedded microRNAs as major drivers of ground-state pluripotency. Stem Cell Rep. 2017;9(6):2081–2096. doi: 10.1016/j.stemcr.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qian B., Wang D.M., Gu X.S., et al. LncRNA H19 serves as a ceRNA and participates in non-small cell lung cancer development by regulating microRNA-107. Eur Rev Med Pharmacol Sci. 2018;22(18):5946–5953. doi: 10.26355/eurrev_201809_15925. [DOI] [PubMed] [Google Scholar]
- 162.Yin Z., Cui Z., Li H., et al. Polymorphisms in the H19 gene and the risk of lung Cancer among female never smokers in Shenyang, China. BMC Cancer. 2018;18(1):893. doi: 10.1186/s12885-018-4795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li L., Guo G., Zhang H., et al. Association between H19 SNP rs217727 and lung cancer risk in a Chinese population: a case control study. BMC Med Genet. 2018;19(1):136. doi: 10.1186/s12881-018-0573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng Z.H., Wu D.M., Fan S.H., et al. Upregulation of miR-675-5p induced by lncRNA H19 was associated with tumor progression and development by targeting tumor suppressor p53 in non-small cell lung cancer. J Cell Biochem. 2019;120(11):18724–18735. doi: 10.1002/jcb.29182. [DOI] [PubMed] [Google Scholar]
- 165.Ren J., Fu J., Ma T., et al. LncRNA H19-elevated LIN28B promotes lung cancer progression through sequestering miR-196b. Cell Cycle. 2018;17(11):1372–1380. doi: 10.1080/15384101.2018.1482137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao Y., Feng C., Li Y., et al. LncRNA H19 promotes lung cancer proliferation and metastasis by inhibiting miR-200a function. Mol Cell Biochem. 2019;460(1):1–8. doi: 10.1007/s11010-019-03564-1. [DOI] [PubMed] [Google Scholar]
- 167.Huang Z., Lei W., Hu H.B., et al. H19 promotes non-small-cell lung cancer (NSCLC) development through STAT3 signaling via sponging miR-17. J Cell Physiol. 2018;233(10):6768–6776. doi: 10.1002/jcp.26530. [DOI] [PubMed] [Google Scholar]
- 168.Xu Y., Lin J., Jin Y., et al. The miRNA hsa-miR-6515-3p potentially contributes to lncRNA H19-mediated-lung cancer metastasis. J Cell Biochem. 2019;120(10):17413–17421. doi: 10.1002/jcb.29006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang T., Wen Y., Peng B., et al. Upregulated lncRNA H19 promotes non-small cell lung cancer cell proliferation through miR-138/PDK1 axis. Int J Clin Exp Pathol. 2017;10(8):9012–9020. [PMC free article] [PubMed] [Google Scholar]
- 170.Li X., Lv F., Li F., et al. Long noncoding RNA H19 facilitates small cell lung cancer tumorigenesis through miR-140-5p/FGF9 axis. OncoTargets Ther. 2020;13:3525–3534. doi: 10.2147/OTT.S245710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lei Y., Guo W., Chen B., et al. Tumor-released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non-small cell lung cancer. Oncol Rep. 2018;40(6):3438–3446. doi: 10.3892/or.2018.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Q., Cheng N., Li X., et al. Correlation of long non-coding RNA H19 expression with cisplatin-resistance and clinical outcome in lung adenocarcinoma. Oncotarget. 2017;8(2):2558–2567. doi: 10.18632/oncotarget.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pan R., Zhou H. Exosomal transfer of lncRNA H19 promotes erlotinib resistance in non-small cell lung cancer via miR-615-3p/ATG7 axis. Cancer Manag Res. 2020;12:4283–4297. doi: 10.2147/CMAR.S241095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou Y., Zhang Y. Inhibition of LncRNAH19 has the effect of anti-tumour and enhancing sensitivity to Gefitinib and Chemotherapy in Non-small-cell lung cancer in vivo. J Cell Mol Med. 2020;24(10):5811–5816. doi: 10.1111/jcmm.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Deng H., Zhang J., Shi J., et al. Role of long non-coding RNA in tumor drug resistance. Tumour Biol. 2016;37(9):11623–11631. doi: 10.1007/s13277-016-5125-8. [DOI] [PubMed] [Google Scholar]
- 176.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 177.Zhang Y., Shields T., Crenshaw T., et al. Imprinting of human H19:allele-specific CpG methylation, loss of the active allele in Wilms tumor, and potential for somatic allele switching. Am J Hum Genet. 1993;53(1):113–124. [PMC free article] [PubMed] [Google Scholar]
- 178.Gaston V., Le Bouc Y., Soupre V., et al. Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2001;9(6):409–418. doi: 10.1038/sj.ejhg.5200649. [DOI] [PubMed] [Google Scholar]
- 179.Dao D., Walsh C.P., Yuan L., et al. Multipoint analysis of human chromosome 11p15/mouse distal chromosome 7:inclusion of H19/IGF2 in the minimal WT2 region, gene specificity of H19 silencing in Wilms' tumorigenesis and methylation hyper-dependence of H19 imprinting. Hum Mol Genet. 1999;8(7):1337–1352. doi: 10.1093/hmg/8.7.1337. [DOI] [PubMed] [Google Scholar]
- 180.Chung W.Y., Yuan L., Feng L., et al. Chromosome 11p15.5 regional imprinting: comparative analysis of KIP2 and H19 in human tissues and Wilms' tumors. Hum Mol Genet. 1996;5(8):1101–1108. doi: 10.1093/hmg/5.8.1101. [DOI] [PubMed] [Google Scholar]
- 181.Safari M.R., Mohammad Rezaei F., Dehghan A., et al. Genomic variants within the long non-coding RNA H19 confer risk of breast cancer in Iranian population. Gene. 2019;701:121–124. doi: 10.1016/j.gene.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 182.Li Z., Niu Y. Association between lncRNA H19 (rs217727, rs2735971 and rs3024270) polymorphisms and the risk of bladder cancer in Chinese population. Minerva Urol Nefrol. 2019;71(2):161–167. doi: 10.23736/S0393-2249.18.03004-7. [DOI] [PubMed] [Google Scholar]
- 183.Hua Q., Lv X., Gu X., et al. Genetic variants in lncRNA H19 are associated with the risk of bladder cancer in a Chinese population. Mutagenesis. 2016;31(5):531–538. doi: 10.1093/mutage/gew018. [DOI] [PubMed] [Google Scholar]
- 184.Zhang H.B., Zeng Y., Li T.L., et al. Correlation between polymorphisms in IGF2/H19 gene locus and epithelial ovarian cancer risk in Chinese population. Genomics. 2020;112(3):2510–2515. doi: 10.1016/j.ygeno.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 185.Li W., Hua R.X., Wang M., et al. H19 gene polymorphisms and Wilms tumor risk in Chinese children: a four-center case-control study. Mol Genet Genomic Med. 2021;9(2):e1584. doi: 10.1002/mgg3.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Singh N., Ramnarine V.R., Song J.H., et al. The long noncoding RNA H19 regulates tumor plasticity in neuroendocrine prostate cancer. Nat Commun. 2021;12(1):7349. doi: 10.1038/s41467-021-26901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bacci L., Aiello A., Ripoli C., et al. H19-dependent transcriptional regulation of β3 and β4 integrins upon estrogen and hypoxia favors metastatic potential in prostate cancer. Int J Mol Sci. 2019;20(16):4012. doi: 10.3390/ijms20164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lv M., Zhong Z., Huang M., et al. lncRNA H19 regulates epithelial–mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res. 2017;1864(10):1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 189.Xu H., Ding Y., Yang X. Overexpression of long noncoding RNA H19 downregulates miR-140-5p and activates PI3K/AKT signaling pathway to promote invasion, migration and epithelial-mesenchymal transition of ovarian cancer cells. BioMed Res Int. 2021;2021 doi: 10.1155/2021/6619730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schagdarsurengin U., Lammert A., Schunk N., et al. Impairment of IGF2 gene expression in prostate cancer is triggered by epigenetic dysregulation of IGF2-DMR0 and its interaction with KLF4. Cell Commun Signal. 2017;15(1):40. doi: 10.1186/s12964-017-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zeng Y., Li T.L., Zhang H.B., et al. Polymorphisms in IGF2/H19 gene locus are associated with platinum-based chemotherapeutic response in Chinese patients with epithelial ovarian cancer. Pharmacogenomics. 2019;20(3):179–188. doi: 10.2217/pgs-2018-0153. [DOI] [PubMed] [Google Scholar]
- 192.Byun H.M., Wong H.L., Birnstein E.A., et al. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67(22):10753–10758. doi: 10.1158/0008-5472.CAN-07-0329. [DOI] [PubMed] [Google Scholar]
- 193.Riccio A., Sparago A., Verde G., et al. Inherited and sporadic epimutations at the IGF2-H19 locus in beckwith-Wiedemann syndrome and Wilms' tumor. Endocr Dev. 2009;14:1–9. doi: 10.1159/000207461. [DOI] [PubMed] [Google Scholar]
- 194.Tanos V., Ariel I., Prus D., et al. H19 and IGF2 gene expression in human normal, hyperplastic, and malignant endometrium. Int J Gynecol Cancer. 2004;14(3):521–525. doi: 10.1111/j.1048-891x.2004.014314.x. [DOI] [PubMed] [Google Scholar]
- 195.Ohana P., Bibi O., Matouk I., et al. Use of H19 regulatory sequences for targeted gene therapy in cancer. Int J Cancer. 2002;98(5):645–650. doi: 10.1002/ijc.10243. [DOI] [PubMed] [Google Scholar]
- 196.Sidi A.A., Ohana P., Benjamin S., et al. Phase I/II marker lesion study of intravesical BC-819 DNA plasmid in H19 over expressing superficial bladder cancer refractory to Bacillus calmette-Guerin. J Urol. 2008;180(6):2379–2383. doi: 10.1016/j.juro.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 197.Li T., Jiang H., Li Y., et al. Estrogen promotes lncRNA H19 expression to regulate osteogenic differentiation of BMSCs and reduce osteoporosis via miR-532-3p/SIRT1 axis. Mol Cell Endocrinol. 2021;527:111171. doi: 10.1016/j.mce.2021.111171. [DOI] [PubMed] [Google Scholar]
- 198.Wang L., Qi L. The role and mechanism of long non-coding RNA H19 in stem cell osteogenic differentiation. Mol Med. 2021;27(1):86. doi: 10.1186/s10020-021-00350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu J., Zhao J., Sun L., et al. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone. 2018;108:62–70. doi: 10.1016/j.bone.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 200.Zheng X., Gan S., Su C., et al. Screening and preliminary identification of long non-coding RNAs critical for osteogenic differentiation of human umbilical cord mesenchymal stem cells. Bioengineered. 2022;13(3):6880–6894. doi: 10.1080/21655979.2022.2044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang Y., Liu W., Liu Y., et al. Long noncoding RNA H19 mediates LCoR to impact the osteogenic and adipogenic differentiation of mBMSCs in mice through sponging miR-188. J Cell Physiol. 2018;233(9):7435–7446. doi: 10.1002/jcp.26589. [DOI] [PubMed] [Google Scholar]
- 202.Li G., Yun X., Ye K., et al. Long non-coding RNA-H19 stimulates osteogenic differentiation of bone marrow mesenchymal stem cells via the microRNA-149/SDF-1 axis. J Cell Mol Med. 2020;24(9):4944–4955. doi: 10.1111/jcmm.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu F., Zhong J.Y., Guo B., et al. H19 promotes osteoblastic transition by acting as ceRNA of miR-140-5p in vascular smooth muscle cells. Front Cell Dev Biol. 2022;10:774363. doi: 10.3389/fcell.2022.774363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhou W., Feng Q., Cheng M., et al. LncRNA H19 sponges miR-103-3p to promote the high phosphorus-induced osteoblast phenotypic transition of vascular smooth muscle cells by upregulating Runx2. Cell Signal. 2022;91:110220. doi: 10.1016/j.cellsig.2021.110220. [DOI] [PubMed] [Google Scholar]
- 205.Giabicani E., Pham A., Sélénou C., et al. Dental pulp stem cells as a promising model to study imprinting diseases. Int J Oral Sci. 2022;14(1):19. doi: 10.1038/s41368-022-00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fanganiello R.D., Ishiy F.A.A., Kobayashi G.S., et al. Increased in vitro osteopotential in SHED associated with higher IGF2 expression when compared with hASCs. Stem Cell Rev Rep. 2015;11(4):635–644. doi: 10.1007/s12015-015-9592-x. [DOI] [PubMed] [Google Scholar]
- 207.Dudakovic A., Camilleri E., Riester S.M., et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem. 2014;115(10):1816–1828. doi: 10.1002/jcb.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhou P., Li Y., Di R., et al. H19 and Foxc2 synergistically promotes osteogenic differentiation of BMSCs via Wnt-β-catenin pathway. J Cell Physiol. 2019;234(8):13799–13806. doi: 10.1002/jcp.28060. [DOI] [PubMed] [Google Scholar]
- 209.Bian W., Xiao S., Yang L., et al. Quercetin promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through the H19/miR-625-5p axis to activate the Wnt/β-catenin pathway. BMC Complement Med Ther. 2021;21(1):243. doi: 10.1186/s12906-021-03418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ma X., Bian Y., Yuan H., et al. Human amnion-derived mesenchymal stem cells promote osteogenic differentiation of human bone marrow mesenchymal stem cells via H19/miR-675/APC axis. Aging. 2020;12(11):10527–10543. doi: 10.18632/aging.103277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gong Y.Y., Peng M.Y., Yin D.Q., et al. Long non-coding RNA H19 promotes the osteogenic differentiation of rat ectomesenchymal stem cells via Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(24):8805–8813. doi: 10.26355/eurrev_201812_16648. [DOI] [PubMed] [Google Scholar]
- 212.Chen S., Liu D., Zhou Z., et al. Role of long non-coding RNA H19 in the development of osteoporosis. Mol Med. 2021;27(1):122. doi: 10.1186/s10020-021-00386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tang W., Liu Q., Tan W., et al. LncRNA expression profile analysis of Mg2+-induced osteogenesis by RNA-seq and bioinformatics. Genes Genom. 2021;43(11):1247–1257. doi: 10.1007/s13258-021-01140-w. [DOI] [PubMed] [Google Scholar]
- 214.Zhang Y., Gao Y., Cai L., et al. microRNA-221 is involved in the regulation of osteoporosis through regulates RUNX2 protein expression and osteoblast differentiation. Am J Transl Res. 2017;9(1):126–135. [PMC free article] [PubMed] [Google Scholar]
- 215.Dong S., Yang B., Guo H., et al. microRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys Res Commun. 2012;418(4):587–591. doi: 10.1016/j.bbrc.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 216.Liao J., Wei Q., Zou Y., et al. Notch signaling augments BMP9-induced bone formation by promoting the osteogenesis-angiogenesis coupling process in mesenchymal stem cells (MSCs) Cell Physiol Biochem. 2017;41(5):1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]
- 217.Roca C., Adams R.H. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 218.Pitulescu M.E., Schmidt I., Giaimo B.D., et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol. 2017;19(8):915–927. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- 219.Lobov I.B., Renard R.A., Papadopoulos N., et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hellström M., Phng L.K., Hofmann J.J., et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 221.Cui J., Zhang W., Huang E., et al. BMP9-induced osteoblastic differentiation requires functional Notch signaling in mesenchymal stem cells. Lab Investig. 2019;99(1):58–71. doi: 10.1038/s41374-018-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Benedito R., Roca C., Sörensen I., et al. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 223.Shang X., Wang J., Luo Z., et al. Notch signaling indirectly promotes chondrocyte hypertrophy via regulation of BMP signaling and cell cycle arrest. Sci Rep. 2016;6:25594. doi: 10.1038/srep25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang J., Li M., Li J., et al. LncRNA H19 rs4929984 variant is associated with coronary artery disease susceptibility in Han Chinese female population. Biochem Genet. 2021;59(6):1359–1380. doi: 10.1007/s10528-021-10055-w. [DOI] [PubMed] [Google Scholar]
- 225.Shi X., Wei Y.T., Li H., et al. Long non-coding RNA H19 in atherosclerosis: what role? Mol Med. 2020;26(1):72. doi: 10.1186/s10020-020-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang H., Cao Y., Shu L., et al. Long non-coding RNA (lncRNA) H19 induces hepatic steatosis through activating MLXIPL and mTORC1 networks in hepatocytes. J Cell Mol Med. 2020;24(2):1399–1412. doi: 10.1111/jcmm.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Camaré C., Pucelle M., Nègre-Salvayre A., et al. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Han Y., Ma J., Wang J., et al. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol Immunol. 2018;93:107–114. doi: 10.1016/j.molimm.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 229.Lv C., Zhou Y.H., Wu C., et al. The changes in miR-130b levels in human serum and the correlation with the severity of diabetic nephropathy. Diabetes Metab Res Rev. 2015;31(7):717–724. doi: 10.1002/dmrr.2659. [DOI] [PubMed] [Google Scholar]
- 230.Huang Y., Wang L., Mao Y., et al. Long noncoding RNA-H19 contributes to atherosclerosis and induces ischemic stroke via the upregulation of acid phosphatase 5. Front Neurol. 2019;10:32. doi: 10.3389/fneur.2019.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sun W., Lv J., Duan L., et al. Long noncoding RNA H19 promotes vascular remodeling by sponging let-7a to upregulate the expression of cyclin D1. Biochem Biophys Res Commun. 2019;508(4):1038–1042. doi: 10.1016/j.bbrc.2018.11.185. [DOI] [PubMed] [Google Scholar]
- 232.Zhang B.F., Jiang H., Chen J., et al. LncRNA H19 ameliorates myocardial infarction-induced myocardial injury and maladaptive cardiac remodelling by regulating KDM3A. J Cell Mol Med. 2020;24(1):1099–1115. doi: 10.1111/jcmm.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Viereck J., Bührke A., Foinquinos A., et al. Targeting muscle-enriched long non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur Heart J. 2020;41(36):3462–3474. doi: 10.1093/eurheartj/ehaa519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang Z., Zhang T., Han S., et al. Long noncoding RNA H19 - a new player in the pathogenesis of liver diseases. Transl Res. 2021;230:139–150. doi: 10.1016/j.trsl.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao Y., Wu J., Liangpunsakul S., et al. Long non-coding RNA in liver metabolism and disease: current status. Liver Res. 2017;1(3):163–167. doi: 10.1016/j.livres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang Z., Yang X., Kai J., et al. HIF-1α-upregulated lncRNA-H19 regulates lipid droplet metabolism through the AMPKα pathway in hepatic stellate cells. Life Sci. 2020;255:117818. doi: 10.1016/j.lfs.2020.117818. [DOI] [PubMed] [Google Scholar]
- 237.Xia S., Wang Z., Chen L., et al. Dihydroartemisinin regulates lipid droplet metabolism in hepatic stellate cells by inhibiting lncRNA-H19-induced AMPK signal. Biochem Pharmacol. 2021;192:114730. doi: 10.1016/j.bcp.2021.114730. [DOI] [PubMed] [Google Scholar]
- 238.Liu C., Yang Z., Wu J., et al. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology. 2018;67(5):1768–1783. doi: 10.1002/hep.29654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gui W., Zhu W.F., Zhu Y., et al. LncRNAH19 improves insulin resistance in skeletal muscle by regulating heterogeneous nuclear ribonucleoprotein A1. Cell Commun Signal. 2020;18(1):173. doi: 10.1186/s12964-020-00654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Geng T., Liu Y., Xu Y., et al. H19 lncRNA promotes skeletal muscle insulin sensitivity in part by targeting AMPK. Diabetes. 2018;67(11):2183–2198. doi: 10.2337/db18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ghaedi H., Zare A., Omrani M.D., et al. Genetic variants in long noncoding RNA H19 and MEG3 confer risk of type 2 diabetes in an Iranian population. Gene. 2018;675:265–271. doi: 10.1016/j.gene.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 242.Jiang Y., Zhu H., Chen Z., et al. Hepatic IGF2/H19 epigenetic alteration induced glucose intolerance in gestational diabetes mellitus offspring via FoxO1 mediation. Front Endocrinol. 2022;13:844707. doi: 10.3389/fendo.2022.844707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tello-Flores V.A., Beltrán-Anaya F.O., Ramírez-Vargas M.A., et al. Role of long non-coding RNAs and the molecular mechanisms involved in insulin resistance. Int J Mol Sci. 2021;22(14):7256. doi: 10.3390/ijms22147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hernández-Aguilar A.I., Luciano-Villa C.A., Tello-Flores V.A., et al. Dysregulation of lncRNA-H19 in cardiometabolic diseases and the molecular mechanism involved: a systematic review. Expert Rev Mol Diagn. 2021;21(8):809–821. doi: 10.1080/14737159.2021.1944808. [DOI] [PubMed] [Google Scholar]
- 245.Borensztein M., Monnier P., Court F., et al. Myod and H19-Igf2 locus interactions are required for diaphragm formation in the mouse. Development. 2013;140(6):1231–1239. doi: 10.1242/dev.084665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Monnier P., Martinet C., Pontis J., et al. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc Natl Acad Sci U S A. 2013;110(51):20693–20698. doi: 10.1073/pnas.1310201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fauque P., Jouannet P., Lesaffre C., et al. Assisted Reproductive Technology affects developmental kinetics, H19 Imprinting Control Region methylation and H19 gene expression in individual mouse embryos. BMC Dev Biol. 2007;7:116. doi: 10.1186/1471-213X-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Keniry A., Oxley D., Monnier P., et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14(7):659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lecerf C., Le Bourhis X., Adriaenssens E. The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell Mol Life Sci. 2019;76(23):4673–4687. doi: 10.1007/s00018-019-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Adriaenssens E., Lottin S., Berteaux N., et al. Cross-talk between mesenchyme and epithelium increases H19 gene expression during scattering and morphogenesis of epithelial cells. Exp Cell Res. 2002;275(2):215–229. doi: 10.1006/excr.2002.5500. [DOI] [PubMed] [Google Scholar]
- 251.Vennin C., Spruyt N., Dahmani F., et al. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-B. Oncotarget. 2015;6(30):29209–29223. doi: 10.18632/oncotarget.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Peperstraete E., Lecerf C., Collette J., et al. Enhancement of breast cancer cell aggressiveness by lncRNA H19 and its mir-675 derivative: insight into shared and different actions. Cancers. 2020;12(7):1730. doi: 10.3390/cancers12071730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lecerf C., Peperstraete E., Le Bourhis X., et al. Propagation and maintenance of cancer stem cells: a major influence of the long non-coding RNA H19. Cells. 2020;9(12):2613. doi: 10.3390/cells9122613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lustig-Yariv O., Schulze E., Komitowski D., et al. The expression of the imprinted genes H19 and IGF-2 in choriocarcinoma cell lines. Is H19 a tumor suppressor gene? Oncogene. 1997;15(2):169–177. doi: 10.1038/sj.onc.1201175. [DOI] [PubMed] [Google Scholar]
- 255.Lavie O., Edelman D., Levy T., et al. A phase 1/2a, dose-escalation, safety, pharmacokinetic, and preliminary efficacy study of intraperitoneal administration of BC-819 (H19-DTA) in subjects with recurrent ovarian/peritoneal cancer. Arch Gynecol Obstet. 2017;295(3):751–761. doi: 10.1007/s00404-017-4293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Amit D., Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J Transl Med. 2010;8:134. doi: 10.1186/1479-5876-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Matouk I.J., Halle D., Gilon M., et al. The non-coding RNAs of the H19-IGF2 imprinted loci: a focus on biological roles and therapeutic potential in Lung Cancer. J Transl Med. 2015;13:113. doi: 10.1186/s12967-015-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ariel I., de Groot N., Hochberg A. Imprinted H19 gene expression in embryogenesis and human cancer: the oncofetal connection. Am J Med Genet. 2000;91(1):46–50. doi: 10.1002/(sici)1096-8628(20000306)91:1<46::aid-ajmg8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 259.Matouk I., Raveh E., Ohana P., et al. The increasing complexity of the oncofetal h19 gene locus: functional dissection and therapeutic intervention. Int J Mol Sci. 2013;14(2):4298–4316. doi: 10.3390/ijms14024298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Amit D., Hochberg A. Development of targeted therapy for a broad spectrum of cancers (pancreatic cancer, ovarian cancer, glioblastoma and HCC) mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. Int J Clin Exp Med. 2012;5(4):296–305. [PMC free article] [PubMed] [Google Scholar]
- 261.Scaiewicz V., Sorin V., Fellig Y., et al. Use of H19 gene regulatory sequences in DNA-based therapy for pancreatic cancer. J Oncol. 2010;2010:178174. doi: 10.1155/2010/178174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Smaldone M.C., Davies B.J. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther. 2010;12(5):607–616. [PubMed] [Google Scholar]
- 263.Mizrahi A., Czerniak A., Levy T., et al. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med. 2009;7:69. doi: 10.1186/1479-5876-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sorin V., Ohana P., Gallula J., et al. H19-promoter-targeted therapy combined with gemcitabine in the treatment of pancreatic cancer. ISRN Oncol. 2012;2012:351750. doi: 10.5402/2012/351750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sorin V., Ohana P., Mizrahi A., et al. Regional therapy with DTA-H19 vector suppresses growth of colon adenocarcinoma metastases in the rat liver. Int J Oncol. 2011;39(6):1407–1412. doi: 10.3892/ijo.2011.1171. [DOI] [PubMed] [Google Scholar]
- 266.Shermane Lim Y.W., Xiang X., Garg M., et al. The double-edged sword of H19 lncRNA: insights into cancer therapy. Cancer Lett. 2021;500:253–262. doi: 10.1016/j.canlet.2020.11.006. [DOI] [PubMed] [Google Scholar]