Abstract
During the past few decades, the incidence of thyroid cancer has increased substantially in many countries, including the USA. The rise in incidence seems to be attributable both to the growing use of diagnostic imaging and fine-needle aspiration biopsy, which has led to enhanced detection and diagnosis of subclinical thyroid cancers, and environmental factors. The latest American Thyroid Association (ATA) practice guidelines for the management of adult patients with thyroid nodules and differentiated thyroid cancer differ substantially from the previous ATA guidelines published in 2009. Specifically, the problems of overdiagnosis and overtreatment of a disease that is typically indolent, where treatment-related morbidity might not be justified by a survival benefit, now seem to be acknowledged. As few modifiable risk factors for thyroid cancer have been established, the specific environmental factors that have contributed to the rising incidence of thyroid cancer remain speculative. However, the findings of several large, well-designed epidemiological studies have provided new information about exposures (such as obesity) that might influence the development of thyroid cancer. In this Review, we describe the changing incidence of thyroid cancer, suggest potential explanations for these trends, emphasize the implications for patients and highlight ongoing and potential strategies to combat this growing clinical and public health issue.
Thyroid cancer is the most common endocrine malignancy, accounting for ~2.1% of all cancer diagnoses worldwide, with ~77% of these diagnoses occurring in women1. Approximately 90% of all thyroid cancers are differentiated, meaning that they arise from thyroid follicular cells and are generally iodine-avid (able to “take up” iodine). Papillary thyroid carcinoma (PTC) is the most common histological type of differentiated thyroid cancer, followed by follicular thyroid carcinoma2. Since the 1970s, rapidly rising incidence rates and comparatively stable mortality for thyroid cancer have been reported throughout much of the world3, including the USA4, Canada5,6, Europe7–10, Australia11, Asia12–15 and parts of South America16. Worldwide trends in thyroid cancer incidence have been largely driven by an increase in PTC as opposed to other major histological types3. In the USA, incidence rates of follicular and medullary thyroid cancer have also significantly increased (average annual percent change [APC] of 1.57% and 1.87%, respectively, between 1993 and 2012), albeit to a lesser extent than PTC (average APC of 6.26%; FIG. 1)17. Thyroid-cancer-specific mortality has also increased significantly17, with an average APC of 0.82% per year17 (FIG. 1).
FIG. 1 |.
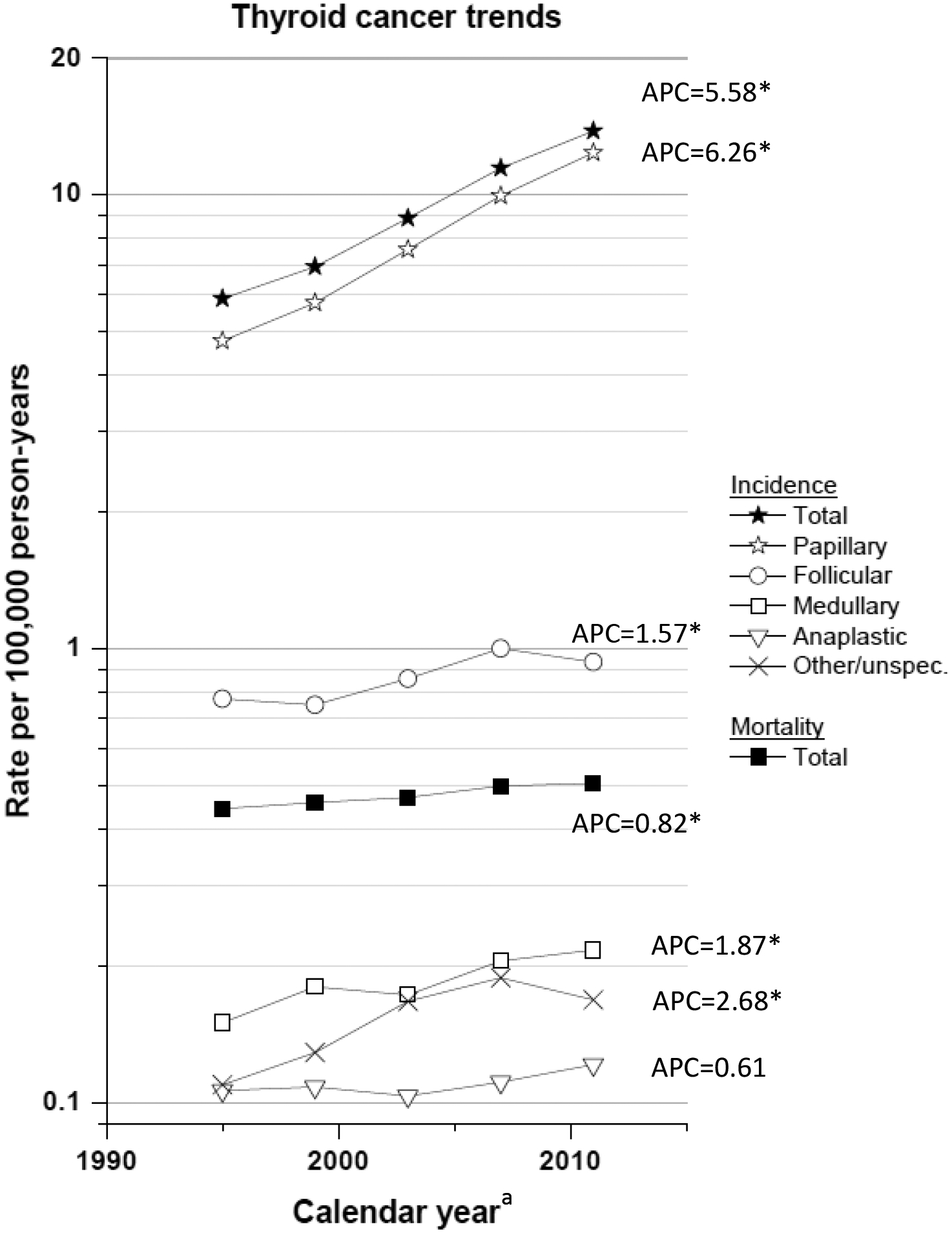
Trends in total and histology-specific thyroid cancer incidence (SEER 13) and total thyroid cancer mortality (USA) from 1993–2012. Rates are age-adjusted (2000 US standard population) and each point represents 4 years. Incidence data are from the SEER 13 areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, Rural Georgia, Los Angeles, San Jose-Monterey and the Alaska Native Registry); thyroid cancer cases are restricted to those microscopically confirmed and exclude cases identified only by autopsy or death certificates. Mortality data are from the US Mortality Files, National Center for Health Statistics, Center for Disease Control and Prevention. Annual percent change (APC) based on rates age-adjusted to the 2000 US standard population (19 age groups – Census P25–1130). *APC is significantly different from zero (P <0.05).
Much attention has been given to the steep increases in PTC incidence, with considerable controversy surrounding the likely reasons for the observed epidemic18,19. Some researchers have concluded that the incidence patterns for thyroid cancer are entirely attributable to overdiagnosis15,19–22 or the detection and diagnosis of disease that would not otherwise have caused symptoms or death during an individual’s lifetime. Others have suggested that the explanation is probably multifactorial, with environmental factors also contributing to the trends in thyroid cancer incidence4,23. In this Review, we describe the changing incidence of thyroid cancer and suggest potential explanations for these trends. We also discuss the resultant health and financial implications for patients, and ongoing efforts and potential strategies to combat this growing clinical and public health issue.
Thyroid cancer
The role of (over)diagnosis
The argument that the incidence and mortality trends for thyroid cancer are solely the result of overdiagnosis gained particular traction with the publication of US and Canadian cancer registry data that demonstrated a shift towards an increased proportion of small versus large PTC diagnoses over time5,20. The introduction in the 1980s and the subsequent widespread use of ultrasonography and fine-needle aspiration biopsy, along with increased use of diagnostic imaging modalities, such as CT, MRI and PET, has led to increased detection of small thyroid nodules and diagnosis of thyroid cancer at an early stage5,20,24. These technological changes undoubtedly account for at least some of the increase in the number of new diagnoses of thyroid cancer since the 1990s. In particular, this potential for ‘surveillance bias’ could explain the increased incidence of small, localized tumours that are frequently subclinical. Overdiagnosis has undeniably contributed to the substantial increase in thyroid cancer incidence in countries, such as South Korea, that have encouraged and provided additional opportunity for thyroid cancer screening. Although South Korea introduced a national screening program for cancer and other common diseases in 1999, coverage for thyroid cancer screening by ultrasonography was not provided. Thyroid cancer screening was, however, heavily marketed by health-care providers and the media, and was often offered by providers for a small additional fee15. As a result, the incidence of thyroid cancer increased 15-fold in the country between 1993 and 2011, almost entirely due to an increase in PTC, while thyroid-cancer-related mortality remained largely stable15.
Studies with dedicated radiological review using a wide range of imaging modalities have identified undiagnosed thyroid nodules in 10–68% of adults, a large proportion of which are probably clinically irrelevant (that is, <1 cm in diameter, non-palpable and asymptomatic)25,26. However, a retrospective chart review at a tertiary care center in Chicago of 98,000 imaging studies involving the head or neck (obtained by ultrasonography, CT, MRI and PET) found a much lower prevalence (0.4%) of thyroid nodules that were incidentally detected and ultimately reported, which is a more accurate depiction of actual clinical practice26. About 7% of the incidentally-detected thyroid nodules were ultimately diagnosed as thyroid cancer by fine-needle aspiration biopsy26, a figure similar to the rate of malignancy among ultrasonography-detected thyroid nodules in other studies27. The findings from the Chicago study suggested that incidentally-detected thyroid nodules identified in clinical practice are much less common than has been suggested from studies based on dedicated radiological review26. Altogether, only 0.03% of the head or neck scans resulted in a diagnosis of thyroid cancer26. This figure is much lower than that suggested by autopsy studies, which found undiagnosed PTCs in 0.45–36% of adult patients who died of other causes27,28. However, the prevalence of papillary thyroid microcarcinomas (PTMCs) in autopsy series depends on the thoroughness of thyroid gland sectioning, histological criteria and population differences28. The increased prevalence estimates, thus, seem to vastly overestimate the actual proportion of undiagnosed PTCs in the general population that are detectable by radiological imaging29. On the basis of the above evidence, we conservatively estimate that the actual prevalence of undiagnosed PTC in the general population of countries such as the USA is <1%, a much lower estimate than had been suggested previously30.
In 2009, a comprehensive investigation of PTC incidence trends by stage and size at diagnosis in the USA from 1980 to 2005 was published4. Although the greatest increase in incidence of PTCs observed were those that were small (≤1 cm) and localized at the time of diagnosis, substantial increases were also observed for advanced stage and larger (>4 cm) size PTCs4. For overdiagnosis to be the only explanation for the increasing incidence of thyroid cancer, the incidence of larger and more aggressive PTCs would be expected to eventually stabilize or even decline as an increasing proportion of the prevalent PTCs in the general population are detected and diagnosed earlier. Overdiagnosis was estimated to explain, at most, ~50% of the increase in PTC incidence, assuming that all new diagnoses of PTCs ≤1 cm in diameter and none of the PTCs >1 cm in diameter were related to improved early detection4. This assumption might be an overestimate of the contribution of environmental factors, considering that a large proportion of nodules >1 cm in diameter are not palpable31. Nonetheless, data from other populations, including Australia27, have led to similar conclusions about the relative contributions of overdiagnosis and environmental factors. Furthermore, age-period-cohort models have identified effects of both calendar period and birth cohort on the increasing incidence of PTC in the USA, which provides additional support that overdiagnosis is not the only explanation for the increasing incidence of PTC32.
In California, USA, the incidence of PTCs increased regardless of size and stage at diagnosis between 1988 and 2009, with incidence of large/localized, large/advanced, smaller/advanced and smaller/localized tumours increasing at rates of 3.2%, 4.8%, 5.8% and 7.8% per year, respectively33. Furthermore, the rates of increase in incidence of PTC were similar across racial and ethnic groups, and socioeconomic status. The latter was based on census-tract data; specifically, similar patterns in rates of increase by tumour size and stage were observed for individuals of both high and low-socioeconomic status. In theory, if overdiagnosis was the only factor accounting for the increasing incidence of PTC, the increasing rates should have been restricted to smaller/localized PTCs and be most pronounced among population subgroups having greatest access to, and utilization of, medical care.
Some investigators have argued that if overdiagnosis was the sole explanation for the increase in thyroid cancer, then we should eventually observe a decline in thyroid-cancer-specific mortality due to the greater effectiveness of treatment for PTCs that are detected early, rather than later, in the disease process18. Smaller, localized PTCs are more amenable to treatment using surgery with or without radioactive iodine ablation than non-PTCs and advanced stage thyroid cancer. On the basis of the assumption that none of the increase in thyroid cancer incidence is ‘real’ (that is, environmental risk factors have no effect on incidence trends) and that all of the increase is due to early detection, a greater ability to detect and treat the more potentially aggressive thyroid cancers at earlier stages (including some types of PTMC) should eventually result in a decline in thyroid-cancer-related mortality. However, thyroid cancer mortality in the USA increased significantly between 1992 and 2012, with an average annual APC of 0.8% per year (FIG. 1)17. During this period, thyroid cancer mortality for white and black individuals in the USA increased at a similar rate (0.7% and 0.9% per year, respectively)17.
Demographic and environmental risk factors
Some modifiable risk factors for thyroid cancer have been identified, but it remains unclear which, if any, of these factors have contributed to the rising incidence of thyroid cancer and could be targeted in efforts aimed at primary prevention. Our limited understanding of thyroid cancer aetiology is due, in part, to limitations of most of the observational studies conducted, including small sample sizes and retrospective exposure assessment. However, findings from several large prospective studies34–46, including large pooled analyses of prospective studies47–50, have provided a much greater level of confirmation about exposures that have long been suspected to influence risk of thyroid cancer and provide new clues about exposures that might have a role in the development of thyroid cancer.
Most of the known or suspected risk factors for thyroid cancer are non-modifiable, with patient age, gender, race/ethnicity and family history of thyroid cancer serving as the strongest predictors of risk. Women have approximately a three-fold to four-fold higher incidence of thyroid cancer than men, a ratio consistently observed across countries and which has remained constant over time3. This difference is particularly pronounced for PTC compared with other major thyroid cancer histological subtypes3. The median age at diagnosis is younger for thyroid cancer than for most other major types of cancer, with a median age at diagnosis in the USA of 49 years for women and 54 years for men17. In the USA, non-Hispanic white individuals have the highest incidence of thyroid cancer, followed by Asian/Pacific Islanders, Hispanic individuals and non-Hispanic black individuals51.
Exposure to ionizing radiation in childhood and adolescence has long been considered to be the only established modifiable risk factor for thyroid cancer, and PTC in particular52. This risk has been shown to decrease with increasing age at exposure and time since exposure53–55, such that little radiation-related risk from exposures occurring after age 20 years exists. Ionizing radiation exposure among the general population has increased over time, doubling in the USA between 1980 and 2006, with much of the observed increase due to increased radiation from medical sources56. Some investigators have speculated that an increase in radiation exposure, particularly from medical sources, has contributed to the increased incidence of PTC4,57,58. However, for radiation to have any effect on thyroid cancer incidence, exposure must have specifically increased among children and we would expect to see some indication of an increase in incidence of radiation-induced thyroid tumors.
Radiation-related PTCs seem to have a different somatic mutation signature compared with most sporadic thyroid cancers, typically presenting with a higher frequency of RET chromosomal rearrangements54, 59–62. Sporadic PTCs, on the other hand, have a higher prevalence of either BRAFV600E or RAS point mutations than radiation-related PTCs63. BRAFV600E rarely occurs in radiation-induced tumours64. Approximately 70% of all PTCs harbour one of these mutually exclusive somatic mutations65. Studies across diverse populations have shown that PTCs with mutations indicative of radiation exposure (for example, RET rearrangements) have declined over time66,67, whereas PTCs harbouring a BRAF66,68,69 or RAS mutation67 have increased. Furthermore, a minority of patients with differentiated thyroid cancer report a history of radiation exposure in childhood18. Nonetheless, some researchers and medical organizations have called for increased surveillance of patients exposed to external irradiation to the head and neck during childhood, and recommend repeat ultrasound examinations of the thyroid gland every few years, and more frequently for childhood cancer survivors and those with a detected nodule70–72. Thus, even if radiation exposure in childhood accounts for a negligible proportion of the new cases of thyroid cancer diagnosed each year, some excess cases might have been identified due to prolonged, repeated surveillance among individuals exposed to high doses of radiation in early life.
Obesity has consistently been associated with an increased risk of thyroid cancer in epidemiological studies46. Trends in obesity prevalence also seem to have paralleled the trends in thyroid cancer incidence, with both increasing rapidly during the same time period and in the same geographical areas73. Between 1980 and 2013, the prevalence of obesity increased by 27.5% in adult individuals and 47.1% in children worldwide, with the fastest rate of increase between 1992 and 200273. This increase in obesity has slowed in the past decade, particularly in developed countries73. In a large, international pooled analysis of 22 prospective studies, a higher BMI at study baseline (hazard ratio [HR] per 5 kg/m2 = increase 1.06, 95% CI 1.02–1.10) and in young adulthood (recalled at baseline; HR per 5 kg/m2 = 1.13, 95% CI 1.02–1.25), greater waist circumference at baseline (HR per 5 cm increase = 1.03, 95% CI 1.01–1.05), and greater BMI gain between young adulthood and study baseline (HR per 5 kg/m2 increase = 1.07, 95% CI 1.00–1.15) were each associated with an increased risk of thyroid cancer50. These associations were observed for risk of all major histological types of thyroid cancer except medullary, and were stronger for thyroid cancer mortality than thyroid cancer incidence. Cross-sectional studies of patients with PTC have provided additional evidence that overweight (25–29 kg/m2) and obesity (≥30 kg/m2) are associated with aggressive clinical and pathological tumour characteristics74–76.
In the pooled analysis, taller height also was associated with an increased risk of thyroid cancer (HR per 5 cm increase = 1.07, 95% 1.04–1.10)50, consistent with most other epidemiological studies45. Results from a large Danish registry linkage study suggested that height and weight measured in childhood (at age 7–13 years) are positively associated with thyroid cancer risk in adulthood, and that these associations are even stronger than those observed for adult height and weight77. The biological mechanisms underlying the associations for height and excess adiposity with increased risk of thyroid cancer are not fully understood. Unlike obesity, obesity-related factors, including level of physical activity, medical history of diabetes mellitus, and the metabolic syndrome have not been consistently associated with thyroid cancer risk40,48. However, increasing evidence from laboratory-based studies exists suggesting that obesity could promote the development of thyroid cancer via complex pathways involving both independent and synergistic effects of insulin resistance, insulin-like growth factor 1, adipokines (for example, leptin), oestrogen and thyroid-stimulating hormone78. Findings from a 2013 study showed, for the first time, a direct influence of diet-induced obesity on thyroid tumour aggressiveness and anaplastic change using a mouse model that spontaneously develops thyroid cancer79.
Another important factor that has been suspected to contribute to the increasing incidence of thyroid cancer is cigarette smoking, which has consistently been associated with an ~30–40% reduced risk of developing thyroid cancer49. Cigarette smoking prevalence has clearly declined over the same timeframe and in many of the same areas where thyroid cancer incidence has increased80. Some biological plausibility for this association exists, as current smoking has been associated with reduced levels of thyroid-stimulating hormone, T3 and T4, decreased prevalence of serum thyroid autoantibodies, increased risk of Graves’ hyperthyroidism and reduced levels of oestrogen49,81. Several lines of evidence support a relationship between iodine deficiency and follicular thyroid cancer, whereas weaker evidence suggests that iodine excess might increase the risk of PTC14,82,83. Changes in iodine fortification and supplementation might, thus, have had a role in the rising incidence of PTC. Endocrine-disrupting chemicals might also have contributed owing to their known effects on thyroid hormone synthesis, but few epidemiological studies have directly investigated the effect of these chemicals on thyroid cancer risk57.
For exposures to have an observable influence on population-level incidence rates (that is, a high population attributable risk), they either have to be strongly associated with risk or highly prevalent in the population84. Under the assumption that the observed associations of obesity (positive) and smoking (inverse) with thyroid cancer risk are causal, the population attributable fraction (PAF = ([prevalence(relative risk-1)]/[prevalence(relative risk-1)+1]x100), or the proportion of all thyroid cancers that can be attributed to a given exposure, would be approximately 15% and 29% for obesity and non-current smoking, respectively, in the USA. These estimates assume that the prevalence of obesity and non-current smoking are 35%85 and 83%86, respectively, and the relative risks for thyroid cancer associated with obesity and non-current smoking are both 1.546,49. Thus, the PAFs for obesity and non-current smoking will be higher for populations with higher prevalence and lower for populations with lower prevalence. However, the relative risks and, consequently, the PAFs for obesity and smoking might be overestimated if these exposures are associated with an increased likelihood of incidental thyroid cancer detection. At least for obesity, this scenario does not seem to be the case. One study based in New York found no differences in the mode of detection of differentiated thyroid cancers in patients with obesity compared with non-obese counterparts87. Furthermore, in cross-sectional studies of screened populations in which all patients had an equal opportunity for thyroid nodule detection and thyroid cancer diagnosis, obesity has generally been found to be positively associated with more aggressive features of PTC74–76.
Our understanding of thyroid cancer aetiology could benefit from improvements in the design of epidemiological studies that better distinguish factors involved in thyroid cancer aetiology from those that simply reflect increased access to health care and surveillance. One possible solution is to restrict thyroid cancer ‘events’ to those that are more clinically relevant or that have molecular or phenotypic characteristics that are predictive of aggressive behaviour, including large, advanced-stage PTCs and PTCs that are positive for the BRAFV600E mutation. Large case–control and, preferably, prospective cohort studies of thyroid cancer that incorporate additional tumour information, including clinical, pathological and molecular features, would enable the determination of relative risk estimates specific to the most clinically relevant thyroid cancers. These relative risk estimates combined with information about the prevalence of environmental, lifestyle, medical or other factors in the general population would enable more precise estimates of attributable risks (that is, the proportion of thyroid cancers diagnosed in a given period of time that are directly attributable to a particular factor). Factors with the highest attributable risks would make the best candidates to target in future thyroid cancer prevention efforts.
Clinical implications
From a clinical standpoint, the benefits of early detection, diagnosis and treatment of thyroid cancer need to be weighed against the potential risks. Overdiagnosis occurs when a tumour is detected that would not otherwise have been diagnosed during an individual’s lifetime. Related to this, overtreatment occurs when the benefits of treating an overdiagnosed case do not outweigh the unnecessary risks88. The ability to detect PTMCs with high-risk features, including the presence of clinical nodal and/or distant metastasis or extrathyroidal extension, can be seen as an advantage as extensive treatment of these cancers is justified to minimize further harm in these patients89. However, for the majority of patients with thyroid cancer and particularly those diagnosed with low-risk PTMC, the advantages of early detection, diagnosis and treatment might not outweigh the financial and health implications of treatment30. In South Korea, the situation is such that nearly all patients diagnosed with thyroid cancer have been treated using total or subtotal thyroidectomy, often with accompanying central lymph node dissection; nearly one quarter of patients undergoing surgery were diagnosed with a thyroid cancer that was <0.5 cm in diameter and more than half were diagnosed with a tumor <1 cm in diameter15. An analysis based on US SEER data between 1998 and 2010 showed that an increasing number of patients with PTMC underwent total thyroidectomy and radioactive iodine ablation in the USA, despite a lack of evidence suggesting that these treatments improve overall or disease-specific survival for the majority of patients with PTMCs that do not have aggressive prognostic features90.
Numerous potential health and financial implications arise from the diagnosis and treatment of thyroid cancer. Patients with thyroid cancer are generally diagnosed at a younger age than patients with other types of cancer, and they have a high long-term survival given the indolent behaviour of the majority of the disease17. As a result, these patients have many years to experience the negative effects of thyroid cancer diagnosis and treatment options, which include potential remedial surgery, radioactive iodine, and now small molecule therapy and clinical trial enrollment for the minority of patients who go on to develop locally advanced and metastatic disease that is iodine-resistant. Complications associated with thyroid surgery, including hypoparathyroidism and recurrent and superior laryngeal nerve injury, can have profound implications for patients’ quality of life91. Other costs of living with a thyroid cancer diagnosis include lifelong thyroid-hormone supplementation or replacement therapy, an increased risk of developing secondary malignancies and the psychological burden of living with a cancer diagnosis and having to undergo biochemical and imaging surveillance for potential recurrence92. At least in the USA, patients diagnosed with thyroid cancer have an increased likelihood of bankruptcy compared with patients without cancer; this is particularly true for younger female patients (<35 years old) who are less likely to have access to high-quality health insurance and who are more financially vulnerable93. One study estimated that the overall societal costs of well-differentiated thyroid cancer in the USA in 2013 for all patients diagnosed since 1985 was US$1.6 billion, and predicted that these costs would continue to rise substantially due to the increasing incidence of the disease and enhanced disease-specific survival94. Another study projected a much higher financial burden (US$18–21 billion) in the USA by 2019, specifically for the care of patients with PTC95. Perhaps not too surprisingly, thyroid cancer survivors have reported a similar quality of life as that of survivors of cancers having a worse prognosis, including colon, breast and gynaecological cancers92. Patients who are diagnosed with benign nodules but are not found to have thyroid cancer still experience many of the same consequences, including the need for repeat follow-up and, sometimes, repeat biopsy due to the possibilities of an indeterminate cytological result or a false-negative test result with fine-needle aspiration biopsy and potential for nodular growth96.
Changes in clinical recommendations
The Incidental Thyroid Findings Committee of the American College of Radiology convened in 2013 to provide consensus recommendations for the radiological handling of thyroid nodules detected incidentally in imaging studies of the neck97. The guidelines include decision trees that health-care providers can follow according to the method by which the nodule was detected, and other prognostic factors, including age and life expectancy of the patient, nodule size and presence or absence of suspicious features in the nodule. The purpose of these guidelines was to reduce the proportion of patients with incidentally-detected nodules that undergo further workup, including surveillance, repeat biopsy and diagnostic surgery.
The ATA practice guidelines released earlier this year98 differ significantly from the previous version in 200999, seeming now to acknowledge the problem of overdiagnosis and overtreatment of a disease that is typically indolent, where treatment-related morbidity might not be justified by a survival benefit. The new guidelines recommend a more cautious approach to diagnostic evaluation and treatment of thyroid nodules and patients with differentiated thyroid cancer. This approach includes a higher threshold for fine-needle aspiration biopsy to begin the diagnostic work-up of a thyroid nodule, in which both nodule size and index of radiologic suspicion are taken into account (for instance, the nodule should be ≥1 cm for high and intermediate suspicion, ≥1.5 for low suspicion, and ≥2 cm for very low suspicion), consideration for less extensive surgery (thyroid lobectomy versus total thyroidectomy), and reduced use of radioactive iodine with a lower dose of 131I for the purpose of ablation of low-risk differentiated thyroid cancers. For the first time, the guidelines discuss active surveillance management as a safe and effective alternative to immediate surgical resection in properly selected patients with PTMC. This development was in response to studies suggesting a very low likelihood of progression in patients having low-risk, asymptomatic PTMC, particularly in older patients (≥60 years)89,100,101.
In patients for whom surgery is indicated, the guidelines support consideration of ipsilateral thyroid lobectomy as an alternative to total thyroidectomy for low-risk differentiated thyroid cancers. Regarding radioactive iodine ablation, the guidelines advocate less frequent use and, if indicated, use of lower doses. Molecular testing was discussed as an adjunct to ultrasound-guided fine needle aspiration biopsy to potentially better triage the risk of malignancy in the setting of cytologically indeterminate thyroid nodules102, thereby potentially reducing the need for diagnostic surgery with its attendant morbidity. Next-generation sequencing using multigene panels also was discussed for its ability to predict tumour aggressiveness103. In determining the course of treatment, the guidelines emphasize the importance of patient preference and patient-centered decision making so that patients understand and can balance the potential risks and benefits of each diagnostic and treatment option.
The ATA clinical practice guidelines also address future research needs to further improve risk stratification for treatment decisions and clinical management98. These needs include research that identifies prognostic factors that could be incorporated into the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM (tumor/node/metastasis) staging system104. For instance, current AJCC/UICC TNM staging classifies young patients with PTC (that is, those aged <45 years) with cervical lymph node metastases as having stage I PTC, with no recognition that nodal metastases seem to be associated with reduced survival105. An improved understanding is, thus, needed regarding patient groups that might be undertreated as a result of the current staging system and recommended guidelines.
The more cautious management of thyroid cancers, as advocated in recent guidelines98, might already be reflected in a recent reduction in the rate of increase of thyroid cancer incidence (delay-adjusted) among white women between 2009 and 201217. Conversely, with more cautious management of thyroid nodules and cancer treatment, some small, seemingly indolent cancers might progress rapidly, as size and stage at diagnosis are not always perfectly predictive of aggressive behaviour. If seemingly lower-risk thyroid cancer patients are treated less aggressively now and in the future compared to the recent past, we might begin to see subtle declines in relative survival rates and increases in thyroid cancer mortality. Monitoring trends in thyroid cancer incidence and mortality trends before and after the implementation of the latest ATA guidelines will be important.
A 2016 publication proposed a change to the terminology used when describing the noninvasive encapsulated follicular variant of PTC that seems to have a low risk of adverse outcomes106. The investigators suggested reclassifying this subgroup of tumours as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features,” or NIFTP, and estimated that implementation of the nomenclature change could result in a modest reduction in thyroid cancer incidence. The supporting data, based on a small sample size of 109 patients, will probably require replication, preferably in a larger sample with long-term follow-up.
Conclusions
Thyroid cancer has become an increasingly important public health concern in much of the world during the past few decades. Increasing use of diagnostic imaging and fine-needle aspiration biopsy have led to greater opportunities for detection and diagnosis of latent, mostly small, thyroid cancers that would otherwise not have been detected through symptoms or palpation. Data from the USA showing a rising incidence of PTCs of all sizes and stages at diagnosis, together with emerging data from experimental and epidemiological studies, suggest that changes in the prevalence of environmental risk factors might, at least in some populations, explain a substantial proportion of the increasing incidence of thyroid cancer. A combination of clinical and public health approaches to the problem might be the most effective means of curbing the incidence of thyroid cancer and reducing unnecessary risks to patients. The most recent ATA guidelines have already taken a step in this direction by calling for more parsimonious evaluation of thyroid nodules and more judicious use of thyroid cancer treatment to minimize risks and maximize benefits to patients98. Over the coming years, monitoring trends in thyroid cancer incidence and mortality to understand the effect of these guidelines and others at the population level will be important. Increased attention and priority should be given to large-scale, prospective epidemiological studies and laboratory-based studies for identifying modifiable risk factors for thyroid cancer and promising targets for thyroid cancer prevention.
Key points.
The incidence of thyroid cancer has increased over the past several decades and, in many countries around the world, has been largely driven by new cases of papillary thyroid cancer
Increased opportunities for detection and diagnosis of small, indolent thyroid cancers seem to explain much, but not all, of the patterns in thyroid cancer incidence
Results from epidemiological studies suggest that a substantial proportion of thyroid cancer diagnoses (>40% in the USA) could be attributable to environmental factors, such as obesity and cigarette smoking
Clinical practice guidelines have recently changed in response to an increasing awareness of the potential for unnecessary diagnosis and treatment in a subset of patients
Large-scale, prospective epidemiological studies and laboratory-based investigations are needed to identify modifiable risk factors for thyroid cancer and promising targets for thyroid cancer prevention
Acknowledgements
C.M.K. and J.A.S. acknowledge S. Devesa and D. Check of the Biostatistics Branch and H. Lim of the Radiation Epidemiology Branch of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, for assistance with the figure in the article and analysis of SEER and US mortality data.
Glossary terms
- Thyroid cancer
- epidemiology
- trends
- incidence
- public health
- clinical guidelines
- risk factors
Biographies
Cari Meinhold Kitahara, PhD MHS is a cancer epidemiology and an Investigator in the Radiation Epidemiology Branch of the National Cancer Institute’s Division of Cancer Epidemiology and Genetics. Her research focuses on the epidemiology of thyroid cancer and on risks of cancer following occupational exposure to radiation from medical sources. She received her BS from the University of Michigan and her MHS and PhD from the Johns Hopkins Bloomberg School of Public Health.
Julie Ann Sosa, MD MA FACS is Professor of Surgery and Medicine (Oncology) at Duke University, where she serves as Chief of Endocrine Surgery and Director of the Surgical Center for Outcomes Research, as well as Leader of the Endocrine Neoplasia Diseases Group, a large transdisciplinary group of clinicians and researchers. Her clinical practice is in endocrine surgery, with a focus in thyroid cancer. She is an NIH-funded investigator focused on outcomes research and clinical trials. She received her AB at Princeton, her MA at Oxford, and her MD at Johns Hopkins, where she completed the Halsted residency and a fellowship.
Footnotes
Competing interests
C.M.K. declares no competing interests. J.A.S. is on the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry, which is sponsored by NovoNordisk, GlaxoSmithKline, Astra Zeneca and Eli Lilly.
REFERENCES
- 1.Ferlay J, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; (2013). Available from: http://globocan.iarc.fr, accessed on 5/6/2016. [Google Scholar]
- 2.Ron E, Schneider AB in Cancer Epidemiology and Prevention (ed. Schottenfeld D, Fraumeni JF Jr.) (Oxford University Press, 2006). Chapter 50, pp 975–94. [Google Scholar]
- 3.Kilfoy BA et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20, 525–31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; Increases in the incidence of thyroid cancer were found in most of the countries with available, high-quality registry data, and no differences were observed by region of the world or underlying thyroid cancer rates.
- 4.Enewold L et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18, 784–91 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This comprehensive descriptive analysis of thyroid cancer trends in the U.S. described increases in incidence of papillary thyroid cancer of all stages and sizes at diagnosis, including similar rates of increase for large (>5 cm) and small (≤1 cm) tumors, suggesting that medical surveillance cannot completely explain these patterns.
- 5.Kent WD et al. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 177, 1357–61 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]; Pathology reports obtained from the Ontario Cancer Registry showed an increasing incidence of differentiated thyroid cancer and a disproportionate increase in the number of smaller (≤ 2cm) versus larger tumors between 1990 and 2001.
- 6.Liu S, Semenciw R, Ugnat AM & Mao Y Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer 85, 1335–9 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhry Z et al. Estimating infra-national and national thyroid cancer incidence in France from cancer registries data and national hospital discharge database. Eur J Epidemiol 22, 607–14 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Colonna M et al. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol 39, 511–8 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Reynolds RM et al. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf) 62, 156–62 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Smailyte G, Miseikyte-Kaubriene E & Kurtinaitis J Increasing thyroid cancer incidence in Lithuania in 1978–2003. BMC Cancer 6, 284 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandeya N et al. Increasing thyroid cancer incidence in Queensland, Australia 1982–2008 - true increase or overdiagnosis? Clin Endocrinol (Oxf) (2015). [DOI] [PubMed] [Google Scholar]
- 12.Keinan-Boker L & Silverman BG Trends of Thyroid Cancer in Israel: 1980–2012. Rambam Maimonides Med J 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubina A et al. Time trends of incidence rates of thyroid cancer in Israel: what might explain the sharp increase. Thyroid 16, 1033–40 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Wang Y & Wang W Increasing incidence of thyroid cancer in Shanghai, China, 1983–2007. Asia Pac J Public Health 27, NP223–9 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ahn HS, Kim HJ & Welch HG Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med 371, 1765–7 (2014). [DOI] [PubMed] [Google Scholar]; This commentary describes the rapid increase in thyroid cancer incidence in South Korea following a government-initiated national screening program for cancer and other common diseases.
- 16.Veiga LH, Neta G, Aschebrook-Kilfoy B, Ron E & Devesa SS Thyroid cancer incidence patterns in Sao Paulo, Brazil, and the U.S. SEER program, 1997–2008. Thyroid 23, 748–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlader N et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. (National Cancer Institute, Bethesda, MD, 2015). [Google Scholar]; The SEER program is an authoritative source of information on cancer incidence and survival in the U.S. and includes data collected from population-based cancer registries that cover approximately 30% of the U.S. population.
- 18.Ito Y, Nikiforov YE, Schlumberger M & Vigneri R Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol 9, 178–84 (2013). [DOI] [PubMed] [Google Scholar]; This article presents interview questions and answers with four experts regarding the potential reasons for the rising incidence of thyroid cancer in many regions of the world.
- 19.Brito JP & Davies L Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes 21, 405–8 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Davies L & Welch HG Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295, 2164–7 (2006). [DOI] [PubMed] [Google Scholar]; In this descriptive analysis of thyroid cancer incidence in the U.S., the authors show a marked increase in thyroid cancer incidence and stable rate of thyroid cancer mortality over time and attribute these trends entirely to “increased diagnostic scrutiny.”
- 21.Franceschi S & Vaccarella S Thyroid cancer: an epidemic of disease or an epidemic of diagnosis? Int J Cancer 136, 2738–9 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Morris LG, Tuttle RM & Davies L Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol Head Neck Surg (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aschebrook-Kilfoy B & Grogan RH Re: Brito et al., overdiagnosis of thyroid cancer and Graves’ disease. Thyroid 24, 403–4 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Sosa JA, Hanna JW, Robinson KA & Lanman RB Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery 154, 1420–6; discussion 1426–7 (2013). [DOI] [PubMed] [Google Scholar]; This study demonstrated the rapid increase in use of thyroid fine-needle aspiration biopsies in the U.S. between 2006 and 2011, and showed that thyroidectomies were more commonly performed than lobectomies and that the use of thyroidectomies increased at a faster rate.
- 25.Guth S, Theune U, Aberle J, Galach A & Bamberger CM Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 39, 699–706 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Uppal A et al. Benign and Malignant Thyroid Incidentalomas Are Rare in Routine Clinical Practice: A Review of 97,908 Imaging Studies. Cancer Epidemiol Biomarkers Prev 24, 1327–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; A retrospective chart review of all reports for scans of the head, neck, and chest in a single tertiary care center revealed much lower rate of incidental thyroid nodule reporting than was found on dedicated review, suggesting that the contribution of incidentalomas to the rising incidence of thyroid cancer in the U.S. has been greatly overestimated.
- 27.Burgess JR Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982–1997). Thyroid 12, 141–9 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Piersanti M, Ezzat S & Asa SL Controversies in papillary microcarcinoma of the thyroid. Endocr Pathol 14, 183–91 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Tello FJ, Martinez-Cabruja R, Fernandez-Martin J, Lasso-Oria C & Ballestin-Carcavilla C Occult carcinoma of the thyroid. A systematic autopsy study from Spain of two series performed with two different methods. Cancer 71, 4022–9 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Ross DS & Tuttle RM Observing micopapillary thyroid cancers. Thyroid 24, 3–6 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Schneider AB et al. Thyroid nodules in the follow-up of irradiated individuals: comparison of thyroid ultrasound with scanning and palpation. J Clin Endocrinol Metab 82, 4020–7 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Zhu C et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid 19, 1061–6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn-Ross PL et al. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 23, 1067–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meinhold CL, et al. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol 171, 242–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen A, et al. Baseline and lifetime alcohol consumption and risk of differentiated thyroid carcinoma in the EPIC study. Br J Cancer 113, 840–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamora-Ros R, et al. Energy and macronutrient intake and risk of differentiated thyroid carcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer 138, 65–73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinaldi S, et al. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J Natl Cancer Inst 106, dj097 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Braganza MZ, Potischman N, Park Y, Thompson FE, Hollenbeck AR, Kitahara CM Adolescent and mid-life diet and subsequent risk of thyroid cancer in the NIH-AARP Diet and Health Study. Int J Cancer 137, 2413–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balasubramaniam S, Ron E, Gridley G, Schneider AB, Brenner AV Association between benign thyroid and endocrine disorders and subsequent risk of thyroid cancer among 4.5 million U.S. male veterans. J Clin Endocrinol Metab 97, 2661–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almquist M et al. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control 22, 743–51 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Luo J, Phillips L, Liu S, Wactawski-Wende J, Margolis KL Diabetes, diabetes treatment, and risk of thyroid cancer. J Clin Endocrinol Metab 101, 1243–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol 28, 945–58 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Cho YA, Kim J Thyroid cancer risk and smoking status: a meta-analysis. Cancer Causes Control 25, 1187–95 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Caini S, Gibelli B, Palli D, Saieva C, Ruscica M, Gandini S Menstrual and reproductive history and use of exogenous sex hormones and risk of thyroid cancer among women: a meta-analysis of prospective studies. Cancer Causes Control 26, 511–8 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Jing Z, Hou X, Liu Y, Wang R, Zhao S, Wang Y Association between height and thyroid cancer risk: a meta-analysis of prospective cohort studies. Int J Cancer 137, 1484–90 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Schmid D, Ricci C, Behrens G, Leitzmann MF Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 16, 1042–54 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Patel D, et al. Thyroid cancer and nonsteroidal anti-inflammatory drug use: a pooled analysis of patients older than 40 years of age. Thyroid 25, 1355–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitahara CM et al. Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitahara CM et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control 23, 1615–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitahara CM et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid 26, 306–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive evaluation the associations of height and several indicators of adiposity, including body mass index in younger and middle-to-older adulthood, with thyroid cancer risk by histologic type and thyroid cancer-specific mortality using a compilation of data from 22 large prospective studies from North America, Europe, Australia, and Asia. The findings support etiologic differences in thyroid cancer according to histologic type and tumor aggressiveness.
- 51.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P & Grogan RH The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol 20, 2746–53 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Dal Maso L, Bosetti C, La Vecchia C & Franceschi S Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control 20, 75–86 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Furukawa K et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer 132, 1222–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinnott B, Ron E & Schneider AB Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev 31, 756–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ron E et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 141, 259–77 (1995). [PubMed] [Google Scholar]; Data from five cohort studies and two case-control studies were pooled to quantify the radiation dose-response association for thyroid cancer and revealed a much stronger association for radiation exposure at younger (particularly <5 years) versus older ages.
- 56.National Council on Radiation Protection and Measurements. Ionizing Radiation Exposure of the Population of the United States. NCRP Report 160 (Bethesda, MD, 2009). http://ncrponline.org/publications/reports/ncrp-report-160/. [Google Scholar]
- 57.Pellegriti G, Frasca F, Regalbuto C, Squatrito S & Vigneri R Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013, 965212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schonfeld SJ, Lee C & Berrington de Gonzalez A Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol) 23, 244–50 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Hamatani K et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res 68, 7176–82 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H & Fagin JA Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 57, 1690–4 (1997). [PubMed] [Google Scholar]
- 61.Ciampi R & Nikiforov YE RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology 148, 936–41 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Fugazzola L et al. Oncogenic rearrangements of the RET proto-oncogene in papillary thyroid carcinomas from children exposed to the Chernobyl nuclear accident. Cancer Res 55, 5617–20 (1995). [PubMed] [Google Scholar]
- 63.Elisei R et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93, 3943–9 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Collins BJ, Schneider AB, Prinz RA & Xu X Low frequency of BRAF mutations in adult patients with papillary thyroid cancers following childhood radiation exposure. Thyroid 16, 61–6 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Xing M BRAF mutation in thyroid cancer. Endocr Relat Cancer 12, 245–62 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Romei C et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab 97, E1758–65 (2012). [DOI] [PubMed] [Google Scholar]; This study demonstrated changes in the molecular profile of PTC, with increases in BRAF mutations and decreases in RET/PTC rearrangements. The authors discuss several possible explanations, including environmental exposures, that could account for these trends.
- 67.Jung CK et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 99, E276–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated changes in the molecular profile of PTC, with increases in RAS mutations and decreases in RET/PTC rearrangements. The authors discuss several possible explanations, including environmental exposures, that could account for these trends.
- 68.Kebebew E et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246, 466–70; discussion 470–1 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathur A et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer 117, 4390–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated changes in the molecular profile of PTC, with increases in BRAFV600E mutations over time. The authors discuss several possible explanations, including environmental exposures, that could account for this trend.
- 70.Brignardello E et al. Ultrasound screening for thyroid carcinoma in childhood cancer survivors: a case series. J Clin Endocrinol Metab 93, 4840–3 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Acharya S et al. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer 97, 2397–403 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Crom DB et al. Ultrasonography for thyroid screening after head and neck irradiation in childhood cancer survivors. Med Pediatr Oncol 28, 15–21 (1997). [DOI] [PubMed] [Google Scholar]
- 73.Ng M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tresallet C et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery 156, 1145–52 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Kim HJ et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol (Oxf) 78, 134–40 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Choi JS, Kim EK, Moon HJ & Kwak JY Higher body mass index may be a predictor of extrathyroidal extension in patients with papillary thyroid microcarcinoma. Endocrine 48, 264–71 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Kitahara CM, Gamborg M, Berrington de Gonzalez A, Sorensen TI & Baker JL Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res 74, 235–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pazaitou-Panayiotou K, Polyzos SA & Mantzoros CS Obesity and thyroid cancer: epidemiologic associations and underlying mechanisms. Obes Rev 14, 1006–22 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Kim WG, Park JW, Willingham MC & Cheng SY Diet-induced obesity increases tumor growth and promotes anaplastic change in thyroid cancer in a mouse model. Endocrinology 154, 2936–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ng M et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 311, 183–92 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Wiersinga WM Smoking and thyroid. Clin Endocrinol (Oxf) 79, 145–51 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Zimmermann MB & Galetti V Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 8, 8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blomberg M, Feldt-Rasmussen U, Andersen KK & Kjaer SK Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer 131, 2360–6 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Levin ML The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum 9, 531–41 (1953). [PubMed] [Google Scholar]
- 85.Ogden CL, Carroll MD, Kit BK & Flegal KM Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311, 806–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.National Center for Health Statistics. Centers for Disease Control and Prevention. Early release of selected estimates based on data from the National Health Interview Survey, 2014. http://www.cdc.gov/nchs/fastats/smoking.htm. Last accessed on 5/4/2016. [Google Scholar]
- 87.Zagzag J et al. Method of Detection of Well-Differentiated Thyroid Cancers in Obese and Non-Obese Patients. PLoS One 11, e0152768 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loeb S et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 65, 1046–55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugitani I et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34, 1222–31 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Wang TS, Goffredo P, Sosa JA & Roman SA Papillary thyroid microcarcinoma: an over-treated malignancy? World J Surg 38, 2297–303 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Giordano D et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 22, 911–7 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Applewhite MK et al. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J Surg 40, 551–61 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Ramsey S et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 32, 1143–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lubitz CC et al. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer 120, 1345–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aschebrook-Kilfoy B et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev 22, 1252–9 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Cooper DS et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16, 109–42 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Hoang JK et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol 12, 143–50 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Haugen BR et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the most recent set of evidence-based guidelines from the American Thyroid Association for clinical decision-making in the management of thyroid nodules and differentiated thyroid cancer.
- 99.Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–214 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Ito Y et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34, 28–35 (2010). [DOI] [PubMed] [Google Scholar]; The results of this study provide support for observation as a viable treatment option for patients with papillary thyroid microcarcinomas without unfavorable features.
- 101.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A Patient age is significantly related to the progrsesion of papillary microcarcinoma of the thyroid under observation. Thyroid 24, 27–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shrestha RT, Karunamurthy A, Amin K, Nikiforov YE & Caramori ML Multiple Mutations Detected Preoperatively May Predict Aggressive Behavior of Papillary Thyroid Cancer and Guide Management-A Case Report. Thyroid 25, 1375–8 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Nikiforova MN, Wald AI, Roy S, Durso MB & Nikiforov YE Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 98, E1852–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A Thyroid cancer staging. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds). AJCC Cancer Staging Manual. 7th edition. Springer-Verlag, New York, pp 59–64 (2010). [Google Scholar]
- 105.Adam MA et al. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J Clin Oncol 33, 2370–5 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Nikiforov YE et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


